User login
T2D Medications II
Buzz kill: Lung damage looks worse in pot smokers
Scans of the lungs of pot users have turned up an alarming surprise:
“There’s a public perception that marijuana is safe,” said Giselle Revah, MD, a radiologist at the University of Ottawa. “This study is raising concern that this might not be true.”
Dr. Revah said she can often tell immediately if a CT scan is from a heavy or long-time cigarette smoker. But with the legalization and increased use of marijuana in Canada and many U.S. states, she began to wonder what cannabis use does to the lungs and whether she would be able to differentiate its effects from those of cigarette smoking.
She and her colleagues retrospectively examined chest CT scans from 56 marijuana smokers and compared them to scans of 57 nonsmokers and 33 users of tobacco alone.
Emphysema was significantly more common among marijuana smokers (75%) than among nonsmokers (5%). When matched for age and sex, 93% of marijuana smokers had emphysema, vs. 67% of those who smoked tobacco only (P = .009).
Without age matching, rates of emphysema remained slightly higher among the marijuana users (75% vs. 67%), although the difference was no longer statistically significant. Yet more than 40% of the marijuana group was younger than 50 years, and all of the tobacco-only users were 50 or older – meaning that marijuana smokers may develop lung damage earlier or with less exposure, Dr. Revah said.
Dr. Revah added that her colleagues in family medicine have said the findings match their clinical experience. “In their practices, they have younger patients with emphysema,” she said.
Marijuana smokers also showed higher rates of airway inflammation, including bronchial thickening, bronchiectasis, and mucoid impaction, with and without sex- and age-matching, the researchers found.
The findings are “not even a little bit surprising,” according to Alan Kaplan, MD, a family physician in Ontario who has expertise in respiratory health. He is the author of a 2021 review on cannabis and lung health.
In an editorial accompanying the journal article by Dr. Revah and colleagues , pulmonary experts noted that the new data give context to a recent uptick in referrals for nontraumatic pneumothorax. The authors said they had received 22 of these referrals during the past 2 years but that they had received only 6 between 2012 and 2020. “Many, but not all, of these patients have a documented history of marijuana use,” they wrote.
One reason for the additional damage may be the way marijuana is inhaled, Dr. Kaplan said. Marijuana smokers “take a big breath in, and they really push it into lungs and hold pressure on it, which may actually cause alveoli to distend over time.”
Because most marijuana smokers in the study also smoked cigarettes, whether the observed damage was caused by marijuana alone or occurred through a synergy with tobacco is impossible to discern, Dr. Revah said.
Still, the results are striking, she said, because the marijuana group was compared to tobacco users who had an extensive smoking history – 25 to 100 pack-years – and who were from a high-risk lung cancer screening program.
Dr. Revah and her colleagues are now conducting a larger, prospective study to see whether they can confirm their findings.
“The message to physicians is to ask about cannabis smoking,” Dr. Kaplan said. In the past, people have been reluctant to admit to using cannabis. Even with legalization, they may be slow to tell their physicians. But clinicians should still try to identify frequent users, especially those who are predisposed for lung conditions. If they intend to use the drug, the advice should be, “There are safer ways to use cannabis,” he said.
Dr. Revah and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Scans of the lungs of pot users have turned up an alarming surprise:
“There’s a public perception that marijuana is safe,” said Giselle Revah, MD, a radiologist at the University of Ottawa. “This study is raising concern that this might not be true.”
Dr. Revah said she can often tell immediately if a CT scan is from a heavy or long-time cigarette smoker. But with the legalization and increased use of marijuana in Canada and many U.S. states, she began to wonder what cannabis use does to the lungs and whether she would be able to differentiate its effects from those of cigarette smoking.
She and her colleagues retrospectively examined chest CT scans from 56 marijuana smokers and compared them to scans of 57 nonsmokers and 33 users of tobacco alone.
Emphysema was significantly more common among marijuana smokers (75%) than among nonsmokers (5%). When matched for age and sex, 93% of marijuana smokers had emphysema, vs. 67% of those who smoked tobacco only (P = .009).
Without age matching, rates of emphysema remained slightly higher among the marijuana users (75% vs. 67%), although the difference was no longer statistically significant. Yet more than 40% of the marijuana group was younger than 50 years, and all of the tobacco-only users were 50 or older – meaning that marijuana smokers may develop lung damage earlier or with less exposure, Dr. Revah said.
Dr. Revah added that her colleagues in family medicine have said the findings match their clinical experience. “In their practices, they have younger patients with emphysema,” she said.
Marijuana smokers also showed higher rates of airway inflammation, including bronchial thickening, bronchiectasis, and mucoid impaction, with and without sex- and age-matching, the researchers found.
The findings are “not even a little bit surprising,” according to Alan Kaplan, MD, a family physician in Ontario who has expertise in respiratory health. He is the author of a 2021 review on cannabis and lung health.
In an editorial accompanying the journal article by Dr. Revah and colleagues , pulmonary experts noted that the new data give context to a recent uptick in referrals for nontraumatic pneumothorax. The authors said they had received 22 of these referrals during the past 2 years but that they had received only 6 between 2012 and 2020. “Many, but not all, of these patients have a documented history of marijuana use,” they wrote.
One reason for the additional damage may be the way marijuana is inhaled, Dr. Kaplan said. Marijuana smokers “take a big breath in, and they really push it into lungs and hold pressure on it, which may actually cause alveoli to distend over time.”
Because most marijuana smokers in the study also smoked cigarettes, whether the observed damage was caused by marijuana alone or occurred through a synergy with tobacco is impossible to discern, Dr. Revah said.
Still, the results are striking, she said, because the marijuana group was compared to tobacco users who had an extensive smoking history – 25 to 100 pack-years – and who were from a high-risk lung cancer screening program.
Dr. Revah and her colleagues are now conducting a larger, prospective study to see whether they can confirm their findings.
“The message to physicians is to ask about cannabis smoking,” Dr. Kaplan said. In the past, people have been reluctant to admit to using cannabis. Even with legalization, they may be slow to tell their physicians. But clinicians should still try to identify frequent users, especially those who are predisposed for lung conditions. If they intend to use the drug, the advice should be, “There are safer ways to use cannabis,” he said.
Dr. Revah and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Scans of the lungs of pot users have turned up an alarming surprise:
“There’s a public perception that marijuana is safe,” said Giselle Revah, MD, a radiologist at the University of Ottawa. “This study is raising concern that this might not be true.”
Dr. Revah said she can often tell immediately if a CT scan is from a heavy or long-time cigarette smoker. But with the legalization and increased use of marijuana in Canada and many U.S. states, she began to wonder what cannabis use does to the lungs and whether she would be able to differentiate its effects from those of cigarette smoking.
She and her colleagues retrospectively examined chest CT scans from 56 marijuana smokers and compared them to scans of 57 nonsmokers and 33 users of tobacco alone.
Emphysema was significantly more common among marijuana smokers (75%) than among nonsmokers (5%). When matched for age and sex, 93% of marijuana smokers had emphysema, vs. 67% of those who smoked tobacco only (P = .009).
Without age matching, rates of emphysema remained slightly higher among the marijuana users (75% vs. 67%), although the difference was no longer statistically significant. Yet more than 40% of the marijuana group was younger than 50 years, and all of the tobacco-only users were 50 or older – meaning that marijuana smokers may develop lung damage earlier or with less exposure, Dr. Revah said.
Dr. Revah added that her colleagues in family medicine have said the findings match their clinical experience. “In their practices, they have younger patients with emphysema,” she said.
Marijuana smokers also showed higher rates of airway inflammation, including bronchial thickening, bronchiectasis, and mucoid impaction, with and without sex- and age-matching, the researchers found.
The findings are “not even a little bit surprising,” according to Alan Kaplan, MD, a family physician in Ontario who has expertise in respiratory health. He is the author of a 2021 review on cannabis and lung health.
In an editorial accompanying the journal article by Dr. Revah and colleagues , pulmonary experts noted that the new data give context to a recent uptick in referrals for nontraumatic pneumothorax. The authors said they had received 22 of these referrals during the past 2 years but that they had received only 6 between 2012 and 2020. “Many, but not all, of these patients have a documented history of marijuana use,” they wrote.
One reason for the additional damage may be the way marijuana is inhaled, Dr. Kaplan said. Marijuana smokers “take a big breath in, and they really push it into lungs and hold pressure on it, which may actually cause alveoli to distend over time.”
Because most marijuana smokers in the study also smoked cigarettes, whether the observed damage was caused by marijuana alone or occurred through a synergy with tobacco is impossible to discern, Dr. Revah said.
Still, the results are striking, she said, because the marijuana group was compared to tobacco users who had an extensive smoking history – 25 to 100 pack-years – and who were from a high-risk lung cancer screening program.
Dr. Revah and her colleagues are now conducting a larger, prospective study to see whether they can confirm their findings.
“The message to physicians is to ask about cannabis smoking,” Dr. Kaplan said. In the past, people have been reluctant to admit to using cannabis. Even with legalization, they may be slow to tell their physicians. But clinicians should still try to identify frequent users, especially those who are predisposed for lung conditions. If they intend to use the drug, the advice should be, “There are safer ways to use cannabis,” he said.
Dr. Revah and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM RADIOLOGY
Is there a doctor on the plane? Tips for providing in-flight assistance
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.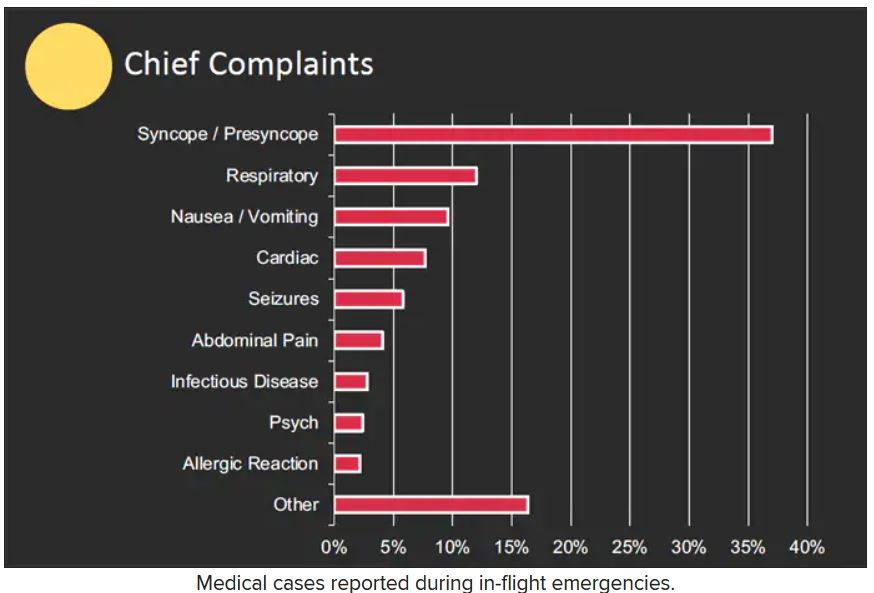
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
34-year-old man • chronic lower back pain • peripheral neuropathy • leg spasms with increasing weakness • Dx?
THE CASE
A 34-year-old man was referred to the sports medicine clinic for evaluation of lumbar radiculopathy. He had a 2-year history of chronic lower back pain that started while he was working on power line towers in Puerto Rico. The back pain was achy, burning, shooting, and stabbing in nature. He had been treated with anti-inflammatories by a company health care provider while in Puerto Rico, but he did not have any imaging done.
At that time, he had tingling and burning that radiated down his left leg to his ankle. The patient also had leg spasms—in his left leg more than his right—and needed a cane when walking. His symptoms did not worsen at any particular time of day or with activity. He had no history of eating exotic foods or sustaining any venomous bites/stings. Ultimately, the back pain and leg spasms forced him to leave his job and return home to Louisiana.
Upon presentation to the sports medicine clinic, he explained that things had worsened since his return home. The pain and burning in his left leg had increased and were now present in his right leg, as well (bilateral paresthesias). In addition, he said he was feeling anxious (and described symptoms of forgetfulness, confusion, and agitation), was sleeping less, and was experiencing worsening fatigue.
Work-ups over the course of the previous 2 years had shed little light on the cause of his symptoms. X-rays of his lumbar spine revealed moderate degenerative changes at L5-S1. A lab work-up was negative and included a complete blood count, testing for HIV and herpes, a hepatitis panel, an antinuclear antibody screen, a C-reactive protein test, and a comprehensive metabolic panel. Thyroid-stimulating hormone, creatine kinase, rapid plasma reagin, and human leukocyte antigen B27 tests were also normal.
Magnetic resonance imaging (MRI) revealed a cystic lesion in the right ilium near the sacroiliac joint. A more recent follow-up MRI and computed tomography scan of the pelvis found the cyst to be stable and well marginalized, with no cortical erosion. Attempts at physical therapy had been unsuccessful because of the pain and decreasing muscle strength in his lower extremities. The patient’s primary care provider was treating him with meloxicam 15 mg/d and duloxetine 60 mg/d, but that had not provided any relief.
Our physical examination revealed a patient who was in mild distress and had limited lumbar spine range of motion (secondary to pain in all planes) and significant paraspinal spasms on the right side in both the lumbar and thoracic regions. The patient had reduced vibratory sensation on his left side vs the right, with a 256-Hz tuning fork at the great toe, as well as reduced sensation to fine touch with a cotton swab and a positive Babinski sign bilaterally. Lower extremity reflexes were hyperreflexic on the left compared with the right. He had no pronator drift; Trendelenburg, straight leg raise, Hoover sign, and slump tests were all negative. His gait was antalgic with a cane, as he described bilateral paresthesias.
THE DIAGNOSIS
The differential diagnosis for low back pain is quite extensive and includes simple mechanical low back pain, lumbar radiculopathy, facet arthritis, spinal stenosis, spondylolysis/spondylolisthesis, and referred pain from the hip, knee, or upper back. It can also be caused by referred pain from visceral organs such as the liver, colon, or kidneys. Low back pain can also signal primary or metastatic disease. However, most of these potential diagnoses had been ruled out with imaging and lab tests.
Two things caught our attention. First: Mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain. Our patient had weakness in gait and pain and burning in both of his legs. Second: Our patient described decreased sleep and feeling anxious, with symptoms of forgetfulness, confusion, and agitation. These factors prompted us to look beyond the normal differential and consider a potential toxicity. A heavy metal screen was ordered, and the results were positive for arsenic toxicity.
DISCUSSION
Arsenic toxicity is a global health problem that affects millions of people.1,2 Arsenic has been used for centuries in depilatories, cosmetics, poisons, and therapeutic agents. Today it is used as a treatment for leukemia and in several ayurvedic and homeopathic remedies.3-7 It is a common earth element found in ground water and a waste product from mining and the manufacturing of glass, computer chips, wood preservatives, and various pesticides.2,3,7,8
A great masquerader. Once in the body, arsenic can cause many serious ailments ranging from urinary tract, liver, and skin cancers to various peripheral and central nervous system disorders.2 Arsenic can cause symmetrical peripheral neuropathy characterized by sensory nerves being more sensitive than motor nerves.2,3,5,6 Clinically, it causes numbness and paresthesias of the distal extremities, with the lower extremities more severely affected.3,6 Symptoms can develop within 2 hours to 2 years of exposure, with vomiting, diarrhea, or both preceding the onset of the neuropathy.2,3,5,6 Arsenic is linked to forgetfulness, confusion, visual distortion, sleep disturbances, decreased concentration, disorientation, severe agitation, paranoid ideation, emotional lability, and decreases in locomotor activity.3,5,6
Testing and treatment. Arsenic levels in the body are measured by blood and urine testing. Blood arsenic levels are typically detectable immediately after exposure and with continued exposure, but quickly normalize as the metal integrates into the nonvascular tissues. Urine arsenic levels can be detected for weeks. Normal levels for arsenic in both urine and blood are ≤ 12 µg/L.3 Anything greater than 12 µg/L is considered high; critically high values are those above 50 µg/L.3,5 Our patient’s blood arsenic level was 13 µg/L.
Treatment involves removing the source of the arsenic. Chelation therapy should be pursued when urine arsenic levels are greater than 50 µg/L or when removing the source of the arsenic fails to reduce arsenic levels. Chelation therapy should be continued until urine arsenic levels are below 20 µg/L.5,6
Continue to: After discussing potential sources of exposure
After discussing potential sources of exposure, our patient decided to move out of the house he shared with his ex-wife. He started to recover soon after moving out. Two weeks after his clinic visit, he no longer needed a cane to walk, and his blood arsenic level had dropped to 6 µg/L. Two months after his clinic visit, the patient’s blood arsenic level was undetectable. The patient’s peripheral neuropathy symptoms continued to improve.
The source of this patient’s arsenic exposure was never confirmed. The exposure could have occurred in Puerto Rico or in Louisiana. Even though no one else in the Louisiana home became ill, the patient was instructed to contact the local health department and water department to have the water tested. However, when he returned to the clinic for follow-up, he had not followed through.
THE TAKEAWAY
When evaluating causes of peripheral neuropathy, consider the possibility of heavy metal toxicity, which can be easily overlooked by the busy clinician. In this case, the patient initially experienced asymmetric paresthesia that gradually increased to burning pain and weakness, with reduced motor control bilaterally. This was significant because mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain.
Our patient’s leg symptoms, the constellation of forgetfulness, confusion, and agitation, and his sleep issues prompted us to look outside our normal differential. Fortunately, once arsenic exposure ceases, patients will gradually improve because arsenic is rapidly cleared from the bloodstream.3,6
CORRESPONDENCE
Charles W. Webb, DO, CAQSM, FAMSSM, FAAFP, Department of Family Medicine, 1501 Kings Highway, PO Box 33932, Shreveport, LA 71130-3932; [email protected]
1. Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Health Policy. 2018;11:251-261. doi: 10.2147/RMHP.S153188
2. Roh T, Steinmaus C, Marshall G, et al. Age at exposure to arsenic in water and mortality 30-40 years after exposure cessation. Am J Epidemiol. 2018;187:2297-2305. doi: 10.1093/aje/kwy159
3. Baker BA, Cassano VA, Murray C, ACOEM Task Force on Arsenic Exposure. Arsenic exposure, assessment, toxicity, diagnosis, and management. J Occup Environ Med. 2018;60:634-639. doi: 10.1097/JOM.0000000000001485
4. Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18-21. doi: 10.1289/ehp.6407
5. Lindenmeyer G, Hoggett K, Burrow J, et al. A sickening tale. N Engl J Med. 2018;379:75-80. doi: 10.1056/NEJMcps1716775
6. Rodríguez VM, Jímenez-Capdevill ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145: 1-18. doi: 10.1016/s0378-4274(03)00262-5
7. Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian- manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915-923. doi: 10.1001/jama.300.8.915
8. Rose M, Lewis J, Langford N, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263-1267. doi: 10.1016/j.fct.2007.01.007
THE CASE
A 34-year-old man was referred to the sports medicine clinic for evaluation of lumbar radiculopathy. He had a 2-year history of chronic lower back pain that started while he was working on power line towers in Puerto Rico. The back pain was achy, burning, shooting, and stabbing in nature. He had been treated with anti-inflammatories by a company health care provider while in Puerto Rico, but he did not have any imaging done.
At that time, he had tingling and burning that radiated down his left leg to his ankle. The patient also had leg spasms—in his left leg more than his right—and needed a cane when walking. His symptoms did not worsen at any particular time of day or with activity. He had no history of eating exotic foods or sustaining any venomous bites/stings. Ultimately, the back pain and leg spasms forced him to leave his job and return home to Louisiana.
Upon presentation to the sports medicine clinic, he explained that things had worsened since his return home. The pain and burning in his left leg had increased and were now present in his right leg, as well (bilateral paresthesias). In addition, he said he was feeling anxious (and described symptoms of forgetfulness, confusion, and agitation), was sleeping less, and was experiencing worsening fatigue.
Work-ups over the course of the previous 2 years had shed little light on the cause of his symptoms. X-rays of his lumbar spine revealed moderate degenerative changes at L5-S1. A lab work-up was negative and included a complete blood count, testing for HIV and herpes, a hepatitis panel, an antinuclear antibody screen, a C-reactive protein test, and a comprehensive metabolic panel. Thyroid-stimulating hormone, creatine kinase, rapid plasma reagin, and human leukocyte antigen B27 tests were also normal.
Magnetic resonance imaging (MRI) revealed a cystic lesion in the right ilium near the sacroiliac joint. A more recent follow-up MRI and computed tomography scan of the pelvis found the cyst to be stable and well marginalized, with no cortical erosion. Attempts at physical therapy had been unsuccessful because of the pain and decreasing muscle strength in his lower extremities. The patient’s primary care provider was treating him with meloxicam 15 mg/d and duloxetine 60 mg/d, but that had not provided any relief.
Our physical examination revealed a patient who was in mild distress and had limited lumbar spine range of motion (secondary to pain in all planes) and significant paraspinal spasms on the right side in both the lumbar and thoracic regions. The patient had reduced vibratory sensation on his left side vs the right, with a 256-Hz tuning fork at the great toe, as well as reduced sensation to fine touch with a cotton swab and a positive Babinski sign bilaterally. Lower extremity reflexes were hyperreflexic on the left compared with the right. He had no pronator drift; Trendelenburg, straight leg raise, Hoover sign, and slump tests were all negative. His gait was antalgic with a cane, as he described bilateral paresthesias.
THE DIAGNOSIS
The differential diagnosis for low back pain is quite extensive and includes simple mechanical low back pain, lumbar radiculopathy, facet arthritis, spinal stenosis, spondylolysis/spondylolisthesis, and referred pain from the hip, knee, or upper back. It can also be caused by referred pain from visceral organs such as the liver, colon, or kidneys. Low back pain can also signal primary or metastatic disease. However, most of these potential diagnoses had been ruled out with imaging and lab tests.
Two things caught our attention. First: Mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain. Our patient had weakness in gait and pain and burning in both of his legs. Second: Our patient described decreased sleep and feeling anxious, with symptoms of forgetfulness, confusion, and agitation. These factors prompted us to look beyond the normal differential and consider a potential toxicity. A heavy metal screen was ordered, and the results were positive for arsenic toxicity.
DISCUSSION
Arsenic toxicity is a global health problem that affects millions of people.1,2 Arsenic has been used for centuries in depilatories, cosmetics, poisons, and therapeutic agents. Today it is used as a treatment for leukemia and in several ayurvedic and homeopathic remedies.3-7 It is a common earth element found in ground water and a waste product from mining and the manufacturing of glass, computer chips, wood preservatives, and various pesticides.2,3,7,8
A great masquerader. Once in the body, arsenic can cause many serious ailments ranging from urinary tract, liver, and skin cancers to various peripheral and central nervous system disorders.2 Arsenic can cause symmetrical peripheral neuropathy characterized by sensory nerves being more sensitive than motor nerves.2,3,5,6 Clinically, it causes numbness and paresthesias of the distal extremities, with the lower extremities more severely affected.3,6 Symptoms can develop within 2 hours to 2 years of exposure, with vomiting, diarrhea, or both preceding the onset of the neuropathy.2,3,5,6 Arsenic is linked to forgetfulness, confusion, visual distortion, sleep disturbances, decreased concentration, disorientation, severe agitation, paranoid ideation, emotional lability, and decreases in locomotor activity.3,5,6
Testing and treatment. Arsenic levels in the body are measured by blood and urine testing. Blood arsenic levels are typically detectable immediately after exposure and with continued exposure, but quickly normalize as the metal integrates into the nonvascular tissues. Urine arsenic levels can be detected for weeks. Normal levels for arsenic in both urine and blood are ≤ 12 µg/L.3 Anything greater than 12 µg/L is considered high; critically high values are those above 50 µg/L.3,5 Our patient’s blood arsenic level was 13 µg/L.
Treatment involves removing the source of the arsenic. Chelation therapy should be pursued when urine arsenic levels are greater than 50 µg/L or when removing the source of the arsenic fails to reduce arsenic levels. Chelation therapy should be continued until urine arsenic levels are below 20 µg/L.5,6
Continue to: After discussing potential sources of exposure
After discussing potential sources of exposure, our patient decided to move out of the house he shared with his ex-wife. He started to recover soon after moving out. Two weeks after his clinic visit, he no longer needed a cane to walk, and his blood arsenic level had dropped to 6 µg/L. Two months after his clinic visit, the patient’s blood arsenic level was undetectable. The patient’s peripheral neuropathy symptoms continued to improve.
The source of this patient’s arsenic exposure was never confirmed. The exposure could have occurred in Puerto Rico or in Louisiana. Even though no one else in the Louisiana home became ill, the patient was instructed to contact the local health department and water department to have the water tested. However, when he returned to the clinic for follow-up, he had not followed through.
THE TAKEAWAY
When evaluating causes of peripheral neuropathy, consider the possibility of heavy metal toxicity, which can be easily overlooked by the busy clinician. In this case, the patient initially experienced asymmetric paresthesia that gradually increased to burning pain and weakness, with reduced motor control bilaterally. This was significant because mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain.
Our patient’s leg symptoms, the constellation of forgetfulness, confusion, and agitation, and his sleep issues prompted us to look outside our normal differential. Fortunately, once arsenic exposure ceases, patients will gradually improve because arsenic is rapidly cleared from the bloodstream.3,6
CORRESPONDENCE
Charles W. Webb, DO, CAQSM, FAMSSM, FAAFP, Department of Family Medicine, 1501 Kings Highway, PO Box 33932, Shreveport, LA 71130-3932; [email protected]
THE CASE
A 34-year-old man was referred to the sports medicine clinic for evaluation of lumbar radiculopathy. He had a 2-year history of chronic lower back pain that started while he was working on power line towers in Puerto Rico. The back pain was achy, burning, shooting, and stabbing in nature. He had been treated with anti-inflammatories by a company health care provider while in Puerto Rico, but he did not have any imaging done.
At that time, he had tingling and burning that radiated down his left leg to his ankle. The patient also had leg spasms—in his left leg more than his right—and needed a cane when walking. His symptoms did not worsen at any particular time of day or with activity. He had no history of eating exotic foods or sustaining any venomous bites/stings. Ultimately, the back pain and leg spasms forced him to leave his job and return home to Louisiana.
Upon presentation to the sports medicine clinic, he explained that things had worsened since his return home. The pain and burning in his left leg had increased and were now present in his right leg, as well (bilateral paresthesias). In addition, he said he was feeling anxious (and described symptoms of forgetfulness, confusion, and agitation), was sleeping less, and was experiencing worsening fatigue.
Work-ups over the course of the previous 2 years had shed little light on the cause of his symptoms. X-rays of his lumbar spine revealed moderate degenerative changes at L5-S1. A lab work-up was negative and included a complete blood count, testing for HIV and herpes, a hepatitis panel, an antinuclear antibody screen, a C-reactive protein test, and a comprehensive metabolic panel. Thyroid-stimulating hormone, creatine kinase, rapid plasma reagin, and human leukocyte antigen B27 tests were also normal.
Magnetic resonance imaging (MRI) revealed a cystic lesion in the right ilium near the sacroiliac joint. A more recent follow-up MRI and computed tomography scan of the pelvis found the cyst to be stable and well marginalized, with no cortical erosion. Attempts at physical therapy had been unsuccessful because of the pain and decreasing muscle strength in his lower extremities. The patient’s primary care provider was treating him with meloxicam 15 mg/d and duloxetine 60 mg/d, but that had not provided any relief.
Our physical examination revealed a patient who was in mild distress and had limited lumbar spine range of motion (secondary to pain in all planes) and significant paraspinal spasms on the right side in both the lumbar and thoracic regions. The patient had reduced vibratory sensation on his left side vs the right, with a 256-Hz tuning fork at the great toe, as well as reduced sensation to fine touch with a cotton swab and a positive Babinski sign bilaterally. Lower extremity reflexes were hyperreflexic on the left compared with the right. He had no pronator drift; Trendelenburg, straight leg raise, Hoover sign, and slump tests were all negative. His gait was antalgic with a cane, as he described bilateral paresthesias.
THE DIAGNOSIS
The differential diagnosis for low back pain is quite extensive and includes simple mechanical low back pain, lumbar radiculopathy, facet arthritis, spinal stenosis, spondylolysis/spondylolisthesis, and referred pain from the hip, knee, or upper back. It can also be caused by referred pain from visceral organs such as the liver, colon, or kidneys. Low back pain can also signal primary or metastatic disease. However, most of these potential diagnoses had been ruled out with imaging and lab tests.
Two things caught our attention. First: Mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain. Our patient had weakness in gait and pain and burning in both of his legs. Second: Our patient described decreased sleep and feeling anxious, with symptoms of forgetfulness, confusion, and agitation. These factors prompted us to look beyond the normal differential and consider a potential toxicity. A heavy metal screen was ordered, and the results were positive for arsenic toxicity.
DISCUSSION
Arsenic toxicity is a global health problem that affects millions of people.1,2 Arsenic has been used for centuries in depilatories, cosmetics, poisons, and therapeutic agents. Today it is used as a treatment for leukemia and in several ayurvedic and homeopathic remedies.3-7 It is a common earth element found in ground water and a waste product from mining and the manufacturing of glass, computer chips, wood preservatives, and various pesticides.2,3,7,8
A great masquerader. Once in the body, arsenic can cause many serious ailments ranging from urinary tract, liver, and skin cancers to various peripheral and central nervous system disorders.2 Arsenic can cause symmetrical peripheral neuropathy characterized by sensory nerves being more sensitive than motor nerves.2,3,5,6 Clinically, it causes numbness and paresthesias of the distal extremities, with the lower extremities more severely affected.3,6 Symptoms can develop within 2 hours to 2 years of exposure, with vomiting, diarrhea, or both preceding the onset of the neuropathy.2,3,5,6 Arsenic is linked to forgetfulness, confusion, visual distortion, sleep disturbances, decreased concentration, disorientation, severe agitation, paranoid ideation, emotional lability, and decreases in locomotor activity.3,5,6
Testing and treatment. Arsenic levels in the body are measured by blood and urine testing. Blood arsenic levels are typically detectable immediately after exposure and with continued exposure, but quickly normalize as the metal integrates into the nonvascular tissues. Urine arsenic levels can be detected for weeks. Normal levels for arsenic in both urine and blood are ≤ 12 µg/L.3 Anything greater than 12 µg/L is considered high; critically high values are those above 50 µg/L.3,5 Our patient’s blood arsenic level was 13 µg/L.
Treatment involves removing the source of the arsenic. Chelation therapy should be pursued when urine arsenic levels are greater than 50 µg/L or when removing the source of the arsenic fails to reduce arsenic levels. Chelation therapy should be continued until urine arsenic levels are below 20 µg/L.5,6
Continue to: After discussing potential sources of exposure
After discussing potential sources of exposure, our patient decided to move out of the house he shared with his ex-wife. He started to recover soon after moving out. Two weeks after his clinic visit, he no longer needed a cane to walk, and his blood arsenic level had dropped to 6 µg/L. Two months after his clinic visit, the patient’s blood arsenic level was undetectable. The patient’s peripheral neuropathy symptoms continued to improve.
The source of this patient’s arsenic exposure was never confirmed. The exposure could have occurred in Puerto Rico or in Louisiana. Even though no one else in the Louisiana home became ill, the patient was instructed to contact the local health department and water department to have the water tested. However, when he returned to the clinic for follow-up, he had not followed through.
THE TAKEAWAY
When evaluating causes of peripheral neuropathy, consider the possibility of heavy metal toxicity, which can be easily overlooked by the busy clinician. In this case, the patient initially experienced asymmetric paresthesia that gradually increased to burning pain and weakness, with reduced motor control bilaterally. This was significant because mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain.
Our patient’s leg symptoms, the constellation of forgetfulness, confusion, and agitation, and his sleep issues prompted us to look outside our normal differential. Fortunately, once arsenic exposure ceases, patients will gradually improve because arsenic is rapidly cleared from the bloodstream.3,6
CORRESPONDENCE
Charles W. Webb, DO, CAQSM, FAMSSM, FAAFP, Department of Family Medicine, 1501 Kings Highway, PO Box 33932, Shreveport, LA 71130-3932; [email protected]
1. Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Health Policy. 2018;11:251-261. doi: 10.2147/RMHP.S153188
2. Roh T, Steinmaus C, Marshall G, et al. Age at exposure to arsenic in water and mortality 30-40 years after exposure cessation. Am J Epidemiol. 2018;187:2297-2305. doi: 10.1093/aje/kwy159
3. Baker BA, Cassano VA, Murray C, ACOEM Task Force on Arsenic Exposure. Arsenic exposure, assessment, toxicity, diagnosis, and management. J Occup Environ Med. 2018;60:634-639. doi: 10.1097/JOM.0000000000001485
4. Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18-21. doi: 10.1289/ehp.6407
5. Lindenmeyer G, Hoggett K, Burrow J, et al. A sickening tale. N Engl J Med. 2018;379:75-80. doi: 10.1056/NEJMcps1716775
6. Rodríguez VM, Jímenez-Capdevill ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145: 1-18. doi: 10.1016/s0378-4274(03)00262-5
7. Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian- manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915-923. doi: 10.1001/jama.300.8.915
8. Rose M, Lewis J, Langford N, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263-1267. doi: 10.1016/j.fct.2007.01.007
1. Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Health Policy. 2018;11:251-261. doi: 10.2147/RMHP.S153188
2. Roh T, Steinmaus C, Marshall G, et al. Age at exposure to arsenic in water and mortality 30-40 years after exposure cessation. Am J Epidemiol. 2018;187:2297-2305. doi: 10.1093/aje/kwy159
3. Baker BA, Cassano VA, Murray C, ACOEM Task Force on Arsenic Exposure. Arsenic exposure, assessment, toxicity, diagnosis, and management. J Occup Environ Med. 2018;60:634-639. doi: 10.1097/JOM.0000000000001485
4. Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18-21. doi: 10.1289/ehp.6407
5. Lindenmeyer G, Hoggett K, Burrow J, et al. A sickening tale. N Engl J Med. 2018;379:75-80. doi: 10.1056/NEJMcps1716775
6. Rodríguez VM, Jímenez-Capdevill ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145: 1-18. doi: 10.1016/s0378-4274(03)00262-5
7. Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian- manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915-923. doi: 10.1001/jama.300.8.915
8. Rose M, Lewis J, Langford N, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263-1267. doi: 10.1016/j.fct.2007.01.007
Severe pediatric oral mucositis
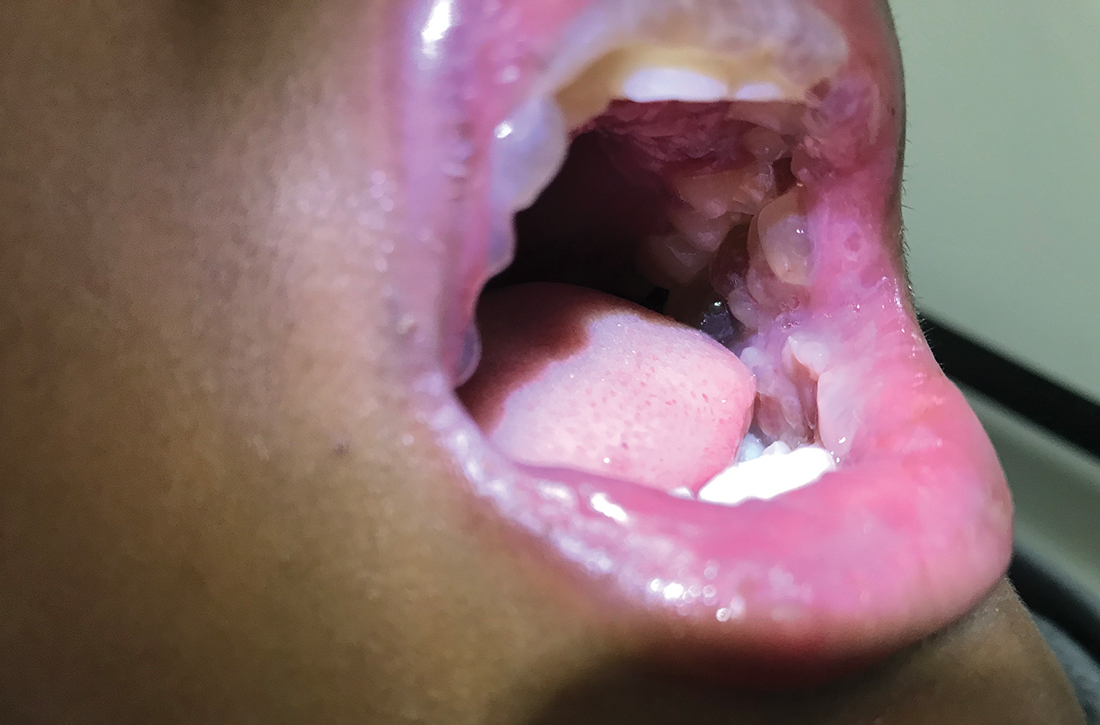
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).
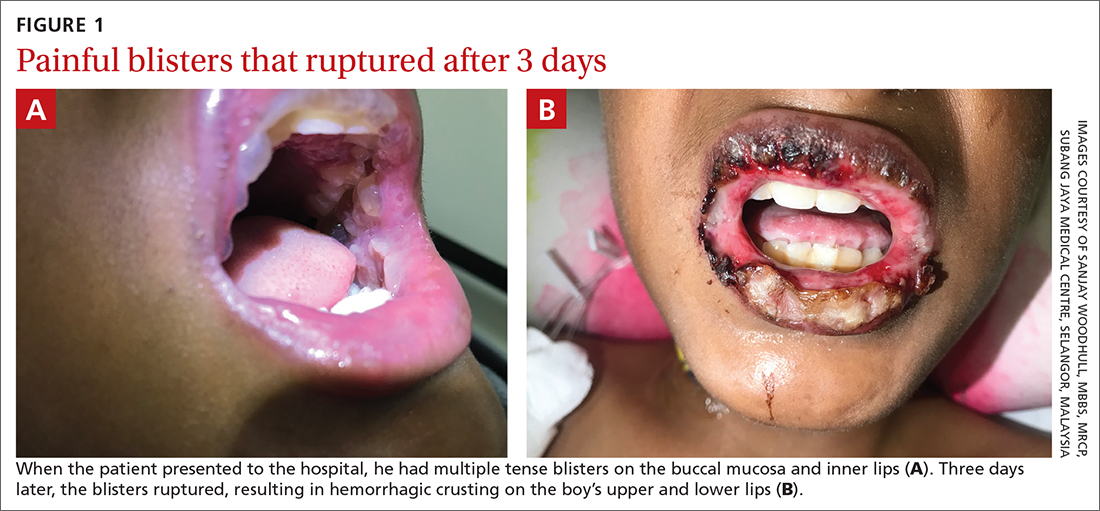
The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
A 12-YEAR-OLD BOY presented to the hospital with a 2-day history of fever, cough, and painful blisters on swollen lips. On examination, he had multiple tense blisters with clear fluid on the buccal mucosa and inner lips (FIGURE 1A), as well as multiple discrete ulcers on his posterior pharynx. The patient had no other skin, eye, or urogenital involvement, but he was dehydrated. Respiratory examination was unremarkable. A complete blood count and metabolic panel were normal, as was a C-reactive protein (CRP) test (0.8 mg/L).

The preliminary diagnosis was primary herpetic gingivostomatitis, and treatment was initiated with intravenous (IV) acyclovir (10 mg/kg every 8 hours), IV fluids, and topical lidocaine gel and topical steroids for analgesia. However, the patient’s fever persisted over the next 4 days, with his temperature fluctuating between 101.3 °F and 104 °F, and he had a worsening productive cough. The blisters ruptured on Day 6 of illness, leaving hemorrhagic crusting on his lips (FIGURE 1B). Herpes simplex virus types 1 and 2 and polymerase chain reaction (PCR) testing were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Mycoplasma pneumoniae–induced rash and mucositis
Further follow-up on Day 6 of illness revealed bibasilar crepitations along with an elevated CRP level of 40.5 mg/L and a positive mycoplasma antibody serology (titer > 1:1280; normal, < 1:80). The patient was given a diagnosis of pneumonia (due to infection with Mycoplasma pneumoniae) and M pneumoniae–induced rash and mucositis (MIRM).
MIRM was first proposed as a distinct clinical entity in 2015 to distinguish it from Stevens-Johnson syndrome and erythema multiforme.1 MIRM is seen more commonly in children and young adults, with a male preponderance.1
A small longitudinal study found that approximately 22.7% of children who have M pneumoniae infections present with mucocutaneous lesions, and of those cases, 6.8% are MIRM.2 Chlamydia pneumoniae is another potential causal organism of mucositis resembling MIRM.3
Pathogenesis. The commonly accepted mechanism of MIRM is an immune response triggered by a distant infection. This leads to tissue damage via polyclonal B cell proliferation and subsequent immune complex deposition, complement activation, and cytokine overproduction. Molecular mimicry between M pneumoniae P1-adhesion molecules and keratinocyte antigens may also contribute to this pathway.
3 criteria to make the diagnosis
Canavan et al1 have proposed the following criteria for the diagnosis of MIRM:
- Clinical symptoms, such as fever and cough, and laboratory findings of M pneumoniae infection (elevated M pneumoniae immunoglobulin M antibodies, positive cultures or PCR for M pneumoniae from oropharyngeal samples or bullae, and/or serial cold agglutinins) AND
- a rash to the mucosa that usually affects ≥ 2 sites (although rare cases may have fewer than 2 mucosal sites involved) AND
- skin detachment of less than 10% of the body surface area.
Continue to: The 3 variants of MIRM include...
The 3 variants of MIRM include:
- Classic MIRM has evidence of all 3 diagnostic criteria plus a nonmucosal rash, such as vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).4
- MIRM sine rash includes all 3 criteria but there is no significant cutaneous, nonmucosal rash. There may be “few fleeting morbilliform lesions or a few vesicles.”4
- Severe MIRM includes the first 2 criteria listed, but the cutaneous rash is extensive, with widespread nonmucosal blisters or flat atypical target lesions.4
Our patient had definitive clinical symptoms, laboratory evidence, and severe oral mucositis without significant cutaneous rash, thereby fulfilling the criteria for a diagnosis of MIRM sine rash variant.
These skin conditions were considered in the differential
The differential diagnosis for sudden onset of severe oral mucosal blisters in children includes herpes gingivostomatitis; hand, foot, and mouth disease
Herpes gingivostomatitis would involve numerous ulcerations of the oral mucosa and tongue, as well as gum hypertrophy.
Hand, foot, and mouth disease is characterized by
Continue to: Erythema multiforme
Erythema multiforme appears as cutaneous target lesions on the limbs that spread in a centripetal manner following herpes simplex virus infection.
SJS/TEN manifests with severe mucositis and is commonly triggered by medications (eg, sulphonamides, beta-lactams, nonsteroidal anti-inflammatory drugs, and antiepileptics).
With antibiotics, the prognosis is good
There are no established guidelines for the treatment of MIRM. Antibiotics and supportive care are universally accepted. Immunosuppressive therapy (eg, systemic steroids) is frequently used in patients with MIRM who have extensive mucosal involvement, in an attempt to decrease inflammation and pain; however, evidence for such an approach is lacking. The hyperimmune reactions of the host to M pneumoniae infection include cytokine overproduction and T-cell activation, which promote both pulmonary and extrapulmonary manifestations. This forms the basis of immunosuppressive therapy, such as systemic corticosteroids, IV immunoglobulin, and cyclosporin A, particularly when MIRM is associated with pneumonia caused by infection with M pneumoniae.1,5,6
The overall prognosis of MIRM is good. Recurrence has been reported in up to 8% of cases, the treatment of which remains the same. Mucocutaneous and ocular sequelae (oral or genital synechiae, corneal ulcerations, dry eyes, loss of eye lashes) have been reported in less than 9% of patients.1 Other rare reported complications following the occurrence of MIRM include persistent cutaneous lesions, B cell lymphopenia, and restrictive lung disease or chronic obliterative bronchitis.
Our patient was started on IV ceftriaxone (50 mg/kg/d), azithromycin (10 mg/kg/d on the first day, then 5 mg/kg/d on the subsequent 5 days), and methylprednisolone (3 mg/kg/d) on Day 6 of illness. Within 3 days, there was marked improvement of mucositis and respiratory symptoms with resolution of fever. He was discharged on Day 10. At his outpatient follow-up 2 weeks later, the patient had made a complete recovery.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol 2015;72:239-245. doi: 10.1016/j.jaad.2014.06.026
2. Sauteur PMM, Theiler M, Buettcher M, et al. Frequency and clinical presentation of mucocutaneous disease due to mycoplasma pneumoniae infection in children with community-acquired pneumonia. JAMA Dermatol. 2020;156:144-150. doi: 10.1001/jamadermatol.2019.3602
3. Mayor-Ibarguren A, Feito-Rodriguez M, González-Ramos J, et al. Mucositis secondary to chlamydia pneumoniae infection: expanding the mycoplasma pneumoniae-induced rash and mucositis concept. Pediatr Dermatol 2017;34:465-472. doi: 10.1111/pde.13140
4. Frantz GF, McAninch SA. Mycoplasma mucositis. StatPearls [Internet]. Updated August 8, 2022. Accessed November 1, 2022. www.ncbi.nlm.nih.gov/books/NBK525960/
5. Yang EA, Kang HM, Rhim JW, et al. Early corticosteroid therapy for Mycoplasma pneumoniae pneumonia irrespective of used antibiotics in children. J Clin Med. 2019;8:726. doi: 10.3390/jcm8050726
6. Li HOY, Colantonio S, Ramien ML. Treatment of Mycoplasma pneumoniae-induced rash and mucositis with cyclosporine. J Cutan Med Surg. 2019;23:608-612. doi: 10.1177/1203475419874444
Keeping up with the evidence (and the residents)
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
I work with medical students nearly every day that I see patients. I recently mentioned to a student that I have a limited working knowledge of the brand names of diabetes medications released in the past 10 years. Just like the M3s, I need the full generic name to know whether a medication is a GLP-1 inhibitor or a DPP-4 inhibitor, because I know that “flozins” are SGLT-2 inhibitors and “glutides” are GLP-1 agonists. The combined efforts of an ambulatory care pharmacist and some flashcards have helped me to better understand how they work and which ones to prescribe when. Meanwhile, the residents are capably counseling on the adverse effects of the latest diabetes agent, while I am googling its generic name.
The premise of science is continuous discovery. In the first 10 months of 2022, the US Food & Drug Administration approved more than 2 dozen new medications, almost 100 new generics, and new indications for dozens more.1,2 The US Preventive Services Task Force (USPSTF) issued 13 new or reaffirmed recommendations in the first 10 months of 2022, and it is just one of dozens of bodies that issue guidelines relevant to primary care.3 PubMed indexes more than a million new articles each year. Learning new information and changing practice are crucial to being an effective clinician.
In this edition of JFP, Covey and Cagle4 write about updates to the USPSTF’s lung cancer screening guidelines. The authors reference changing evidence that led to the revised recommendations. When the original guideline was released in 2013, it drew on the best available evidence at the time.5 The National Lung Screening Trial, which looked at CT scanning compared with chest x-rays as screening tests for lung cancer, was groundbreaking in its methods and results.6 However, it was not without its flaws. It enrolled < 5% Black patients, and so the recommendations for age cutoffs and pack-year cutoffs were made based on the majority White population from the trial.
Black patients experience a higher mortality from lung cancer and are diagnosed at an earlier age and a lower cumulative pack-year exposure than White patients.7 Other studies have explored the social and political factors that lead to these disparities, which range from access to care to racial segregation of neighborhoods and tobacco marketing practices.7 When the USPSTF performed its periodic update of the guideline, it had access to additional research. The updates reflect the new information.
Every physician has a responsibility to find a way to adapt to important new information in medicine. Not using SGLT-2 inhibitors in the management of diabetes would be substandard care, and my patients would suffer for it. Not adopting the new lung cancer screening recommendations would exclude patients most at risk of lung cancer and allow disparities in lung cancer morbidity and mortality to grow.7,8Understanding the evidence behind the recommendations also reminds me that the guidelines will change again. These recommendations are no more static than the first guidelines were. I’ll be ready when the next update comes, and I’ll have the medical students and residents to keep me sharp.
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
1. US Food & Drug Administration. Novel drug approvals for 2022. Accessed October 27. 2022. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2022
2. US Food & Drug Administration. First generic drug approvals. Accessed October 27. 2022. www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/first-generic-drug-approvals
3. US Preventive Services Task Force. Recommendations. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P
4. Covey CL, Cagle SD. Lung cancer screening: New evidence, updated guidance. J Fam Pract. 2022;71:398-402;415.
5. US Preventive Services Task Force. Lung cancer: screening. December 31, 2013. Accessed October 27, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
6. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
7. Pinheiro LC, Groner L, Soroka O, et al. Analysis of eligibility for lung cancer screening by race after 2021 changes to US Preventive Services Task Force screening guidelines. JAMA network open. 2022;5:e2229741. doi: 10.1001/jamanetworkopen.2022.29741
8. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
Which anticoagulant is safest for frail elderly patients with nonvalvular A-fib?
ILLUSTRATIVE CASE
A frail 76-year-old woman with a history of hypertension and hyperlipidemia presents for evaluation of palpitations. An in-office electrocardiogram reveals that the patient is in AF. Her CHA2DS2-VASc score is 4 and her HAS-BLED score is 2.2,3 Using shared decision making, you decide to start medications for her AF. You plan to initiate a beta-blocker for rate control and must now decide on anticoagulation. Which oral anticoagulant would you prescribe for this patient’s AF, given her frail status?
Frailty is defined as a state of vulnerability with a decreased ability to recover from an acute stressful event.4 The prevalence of frailty varies by the measurements used and the population studied. A 2021 meta-analysis found that frailty prevalence ranges from 12% to 24% worldwide in patients older than 50 years5 and may increase to > 30% among those ages 85 years and older.6 Frailty increases rates of AEs such as falls7 and fracture,8 leading to disability,9 decreased quality of life,10 increased utilization of health care,11 and increased mortality.12 A number of validated approaches are available to screen for and measure frailty.13-18
Given the association with negative health outcomes and high health care utilization, frailty is an important clinical factor for physicians to consider when treating elderly patients. Frailty assessment may allow for more tailored treatment choices for patients, with a potential reduction in complications. Although CHA2DS2-VASc and HAS-BLED scores assist in the decision-making process of whether to start anticoagulation, these tools do not take frailty into consideration or guide anticoagulant choice.2,3 The purpose of this study was to analyze how levels of frailty affect the association of 3 different direct oral anticoagulants (DOACs) vs warfarin with various AEs (death, stroke, or major bleeding).
STUDY SUMMARY
This DOAC rose above the others
This retrospective cohort study compared the safety of 3 DOACs—dabigatran, rivaroxaban, and apixaban—vs warfarin in Medicare beneficiaries with AF, using 1:1 propensity score (PS)–matched analysis. Eligible patients were ages 65 years or older, with a filled prescription for a DOAC or warfarin, no prior oral anticoagulant exposure in the previous 183 days, a diagnostic code of AF, and continuous enrollment in Medicare Parts A, B, and D only. Patients were excluded if they had missing demographic data, received hospice care, resided in a nursing facility at drug initiation, had another indication for anticoagulation, or had a contraindication to either a DOAC or warfarin.
Frailty was measured using a claims-based frailty index (CFI), which applies health care utilization data to estimate a frailty index, with cut points for nonfrailty, prefrailty, and frailty. The CFI score has 93 claims-based variables, including wheelchairs and durable medical equipment, open wounds, diseases such as chronic obstructive pulmonary disease and ischemic heart disease, and transportation services.15-17 In this study, nonfrailty was defined as a CFI < 0.15, prefrailty as a CFI of 0.15 to 0.24, and frailty as a CFI ≥ 0.25.
The primary outcome—a composite endpoint of death, ischemic stroke, or major bleeding—was measured for each of the DOAC–warfarin cohorts in the overall population and stratified by frailty classification. Patients were followed until the occurrence of a study outcome, Medicare disenrollment, the end of the study period, discontinuation of the index drug (defined as > 5 days), change to a different anticoagulant, admission to a nursing facility, enrollment in hospice, initiation of dialysis, or kidney transplant. The authors conducted a PS-matched analysis to reduce any imbalances in clinical characteristics between the DOAC- and warfarin-treated groups, as well as a sensitivity analysis to assess the strength of the data findings using different assumptions.
The authors created 3 DOAC–warfarin cohorts: dabigatran (n = 81,863) vs warfarin (n = 256,722), rivaroxaban (n = 185,011) vs warfarin (n = 228,028), and apixaban (n = 222,478) vs warfarin (n = 206,031). After PS matching, the mean age in all cohorts was 76 to 77 years, about 50% were female, and 91% were White. The mean HAS-BLED score was 2 and the mean CHA2DS2-VASc score was 4. The mean CFI was 0.19 to 0.20, defined as prefrail. Patients classified as frail were older, more likely to be female, and more likely to have greater comorbidities, higher scores on CHA2DS2-VASc and HAS-BLED, and higher health care utilization.
Continue to: In the dabigatran-warfarin...
In the dabigatran–warfarin cohort (median follow-up, 72 days), the event rate of the composite endpoint per 1000 person-years (PY) was 63.5 for dabigatran and 65.6 for warfarin (hazard ratio [HR] = 0.98; 95% CI, 0.92 to 1.05; rate difference [RD] per 1000 PY = –2.2; 95% CI, –6.5 to 2.1). A lower rate of the composite endpoint was associated with dabigatran than warfarin for the nonfrail subgroup but not the prefrail or frail groups.
In the rivaroxaban–warfarin cohort (median follow-up, 82 days), the composite endpoint rate per 1000 PY was 77.8 for rivaroxaban and 83.7 for warfarin (HR = 0.98; 95% CI, 0.94 to 1.02; RD per 1000 PY = –5.9; 95% CI, –9.4 to –2.4). When stratifying by frailty category, both dabigatran and rivaroxaban were associated with a lower composite endpoint rate than warfarin for the nonfrail population only (HR = 0.81; 95% CI, 0.68 to 0.97, and HR = 0.88; 95% CI, 0.77 to 0.99, respectively).
In the apixaban–warfarin cohort (median follow-up, 84 days), the rate of the composite endpoint per 1000 PY was 60.1 for apixaban and 92.3 for warfarin (HR = 0.68; 95% CI, 0.65 to 0.72; RD per 1000 PY = –32.2; 95% CI, –36.1 to –28.3). The beneficial association for apixaban was present in all frailty categories, with an HR of 0.61 (95% CI, 0.52 to 0.71) for nonfrail patients, 0.66 (95% CI, 0.61 to 0.70) for prefrail patients, and 0.73 (95% CI, 0.67 to 0.80) for frail patients. Apixaban was the only DOAC with a relative reduction in the hazard of death, ischemic stroke, or major bleeding among all frailty groups.
WHAT’S NEW
Only apixaban had lower AE rates vs warfarin across frailty levels
Three DOACs (dabigatran, rivaroxaban, and apixaban) reduced the risk of death, ischemic stroke, or major bleeding compared with warfarin in older adults with AF, but only apixaban was associated with a relative reduction of these adverse outcomes in patients of all frailty classifications.
CAVEATS
Important data but RCTs are needed
The power of this observational study is considerable. However, it remains a retrospective observational study. The authors attempted to account for these limitations and potential confounders by performing a PS-matched analysis and sensitivity analysis; however, these findings should be confirmed with randomized controlled trials.
Continue to: Additionally, the study...
Additionally, the study collected data on each of the DOAC–warfarin cohorts for < 90 days. Trials to address long-term outcomes are warranted.
Finally, there was no control group in comparison with anticoagulation. It is possible that choosing not to use an anticoagulant is the best choice for frail elderly patients.
CHALLENGES TO IMPLEMENTATION
Doctors need a practical frailty scale, patients need an affordable Rx
Frailty is not often considered a measurable trait. The approach used in the study to determine the CFI is not a practical clinical tool. Studies comparing a frailty calculation software application or an easily implementable survey may help bring this clinically impactful information to the hands of primary care physicians. The Clinical Frailty Scale—a brief, 7-point scale based on the physician’s clinical impression of the patient—has been found to correlate with other established frailty measures18 and might be an option for busy clinicians. However, the current study did not utilize this measurement, and the validity of its use by primary care physicians in the outpatient setting requires further study.
In addition, cost may be a barrier for patients younger than 65 years or for those older than 65 years who do not qualify for Medicare or do not have Medicare Part D. The average monthly cost of the DOACs ranges from $560 for dabigatran19 to $600 for rivaroxaban20 and $623 for apixaban.21 As always, the choice of anticoagulant therapy is a clinical judgment and a joint decision of the patient and physician.
1. Kim DH, Pawar A, Gagne JJ, et al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med. 2021;174:1214-1223. doi: 10.7326/M20-7141
2. Zhu W, He W, Guo L, et al. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561. doi: 10.1002/clc.22435
3. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124
4. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15. doi: 10.1016/j.cger.2010.08.009
5. O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96-104. doi: 10.1093/ageing/afaa219
6. Campitelli MA, Bronskill SE, Hogan DB, et al. The prevalence and health consequences of frailty in a population-based older home care cohort: a comparison of different measures. BMC Geriatr. 2016;16:133. doi: 10.1186/s12877-016-0309-z
7. Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16:1027-1033. doi: 10.1016/j.jamda. 2015.06.018
8. Kojima G. Frailty as a predictor of fractures among community-dwelling older people: a systematic review and meta-analysis. Bone. 2016;90:116-122. doi: 10.1016/j.bone.2016.06.009
9. Kojima G. Quick and simple FRAIL scale predicts incident activities of daily living (ADL) and instrumental ADL (IADL) disabilities: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:1063-1068. doi: 10.1016/j.jamda.2018.07.019
10. Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23-30. doi: 10.2147/RMHP.S168750
11. Roe L, Normand C, Wren MA, et al. The impact of frailty on healthcare utilisation in Ireland: evidence from The Irish Longitudinal Study on Ageing. BMC Geriatr. 2017;17:203. doi: 10.1186/s12877-017-0579-0
12. Hao Q, Zhou L, Dong B, et al. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9:1207. doi: 10.1038/s41598-018-38072-7
13. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
14. Ryan J, Espinoza S, Ernst ME, et al. Validation of a deficit-accumulation frailty Index in the ASPirin in Reducing Events in the Elderly study and its predictive capacity for disability-free survival. J Gerontol A Biol Sci Med Sci. 2022;77:19-26. doi: 10.1093/gerona/glab225
15. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74:1271-1276. doi: 10.1093/gerona/gly197
16. Kim DH, Schneeweiss S, Glynn RJ, et al. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73:980-987. doi: 10.1093/gerona/glx229
17. Claims-based frailty index. Harvard Dataverse website. 2022. Accessed April 5, 2022. https://dataverse.harvard.edu/dataverse/cfi
18. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-95. doi: 10.1503/cmaj.050051
19. Dabigatran. GoodRx. Accessed September 26, 2022. www.goodrx.com/dabigatran
20. Rivaroxaban. GoodRx. Accessed September 26, 2022. www.goodrx.com/rivaroxaban
21. Apixaban (Eliquis). GoodRx. Accessed September 26, 2022. www.goodrx.com/eliquis
ILLUSTRATIVE CASE
A frail 76-year-old woman with a history of hypertension and hyperlipidemia presents for evaluation of palpitations. An in-office electrocardiogram reveals that the patient is in AF. Her CHA2DS2-VASc score is 4 and her HAS-BLED score is 2.2,3 Using shared decision making, you decide to start medications for her AF. You plan to initiate a beta-blocker for rate control and must now decide on anticoagulation. Which oral anticoagulant would you prescribe for this patient’s AF, given her frail status?
Frailty is defined as a state of vulnerability with a decreased ability to recover from an acute stressful event.4 The prevalence of frailty varies by the measurements used and the population studied. A 2021 meta-analysis found that frailty prevalence ranges from 12% to 24% worldwide in patients older than 50 years5 and may increase to > 30% among those ages 85 years and older.6 Frailty increases rates of AEs such as falls7 and fracture,8 leading to disability,9 decreased quality of life,10 increased utilization of health care,11 and increased mortality.12 A number of validated approaches are available to screen for and measure frailty.13-18
Given the association with negative health outcomes and high health care utilization, frailty is an important clinical factor for physicians to consider when treating elderly patients. Frailty assessment may allow for more tailored treatment choices for patients, with a potential reduction in complications. Although CHA2DS2-VASc and HAS-BLED scores assist in the decision-making process of whether to start anticoagulation, these tools do not take frailty into consideration or guide anticoagulant choice.2,3 The purpose of this study was to analyze how levels of frailty affect the association of 3 different direct oral anticoagulants (DOACs) vs warfarin with various AEs (death, stroke, or major bleeding).
STUDY SUMMARY
This DOAC rose above the others
This retrospective cohort study compared the safety of 3 DOACs—dabigatran, rivaroxaban, and apixaban—vs warfarin in Medicare beneficiaries with AF, using 1:1 propensity score (PS)–matched analysis. Eligible patients were ages 65 years or older, with a filled prescription for a DOAC or warfarin, no prior oral anticoagulant exposure in the previous 183 days, a diagnostic code of AF, and continuous enrollment in Medicare Parts A, B, and D only. Patients were excluded if they had missing demographic data, received hospice care, resided in a nursing facility at drug initiation, had another indication for anticoagulation, or had a contraindication to either a DOAC or warfarin.
Frailty was measured using a claims-based frailty index (CFI), which applies health care utilization data to estimate a frailty index, with cut points for nonfrailty, prefrailty, and frailty. The CFI score has 93 claims-based variables, including wheelchairs and durable medical equipment, open wounds, diseases such as chronic obstructive pulmonary disease and ischemic heart disease, and transportation services.15-17 In this study, nonfrailty was defined as a CFI < 0.15, prefrailty as a CFI of 0.15 to 0.24, and frailty as a CFI ≥ 0.25.
The primary outcome—a composite endpoint of death, ischemic stroke, or major bleeding—was measured for each of the DOAC–warfarin cohorts in the overall population and stratified by frailty classification. Patients were followed until the occurrence of a study outcome, Medicare disenrollment, the end of the study period, discontinuation of the index drug (defined as > 5 days), change to a different anticoagulant, admission to a nursing facility, enrollment in hospice, initiation of dialysis, or kidney transplant. The authors conducted a PS-matched analysis to reduce any imbalances in clinical characteristics between the DOAC- and warfarin-treated groups, as well as a sensitivity analysis to assess the strength of the data findings using different assumptions.
The authors created 3 DOAC–warfarin cohorts: dabigatran (n = 81,863) vs warfarin (n = 256,722), rivaroxaban (n = 185,011) vs warfarin (n = 228,028), and apixaban (n = 222,478) vs warfarin (n = 206,031). After PS matching, the mean age in all cohorts was 76 to 77 years, about 50% were female, and 91% were White. The mean HAS-BLED score was 2 and the mean CHA2DS2-VASc score was 4. The mean CFI was 0.19 to 0.20, defined as prefrail. Patients classified as frail were older, more likely to be female, and more likely to have greater comorbidities, higher scores on CHA2DS2-VASc and HAS-BLED, and higher health care utilization.
Continue to: In the dabigatran-warfarin...
In the dabigatran–warfarin cohort (median follow-up, 72 days), the event rate of the composite endpoint per 1000 person-years (PY) was 63.5 for dabigatran and 65.6 for warfarin (hazard ratio [HR] = 0.98; 95% CI, 0.92 to 1.05; rate difference [RD] per 1000 PY = –2.2; 95% CI, –6.5 to 2.1). A lower rate of the composite endpoint was associated with dabigatran than warfarin for the nonfrail subgroup but not the prefrail or frail groups.
In the rivaroxaban–warfarin cohort (median follow-up, 82 days), the composite endpoint rate per 1000 PY was 77.8 for rivaroxaban and 83.7 for warfarin (HR = 0.98; 95% CI, 0.94 to 1.02; RD per 1000 PY = –5.9; 95% CI, –9.4 to –2.4). When stratifying by frailty category, both dabigatran and rivaroxaban were associated with a lower composite endpoint rate than warfarin for the nonfrail population only (HR = 0.81; 95% CI, 0.68 to 0.97, and HR = 0.88; 95% CI, 0.77 to 0.99, respectively).
In the apixaban–warfarin cohort (median follow-up, 84 days), the rate of the composite endpoint per 1000 PY was 60.1 for apixaban and 92.3 for warfarin (HR = 0.68; 95% CI, 0.65 to 0.72; RD per 1000 PY = –32.2; 95% CI, –36.1 to –28.3). The beneficial association for apixaban was present in all frailty categories, with an HR of 0.61 (95% CI, 0.52 to 0.71) for nonfrail patients, 0.66 (95% CI, 0.61 to 0.70) for prefrail patients, and 0.73 (95% CI, 0.67 to 0.80) for frail patients. Apixaban was the only DOAC with a relative reduction in the hazard of death, ischemic stroke, or major bleeding among all frailty groups.
WHAT’S NEW
Only apixaban had lower AE rates vs warfarin across frailty levels
Three DOACs (dabigatran, rivaroxaban, and apixaban) reduced the risk of death, ischemic stroke, or major bleeding compared with warfarin in older adults with AF, but only apixaban was associated with a relative reduction of these adverse outcomes in patients of all frailty classifications.
CAVEATS
Important data but RCTs are needed
The power of this observational study is considerable. However, it remains a retrospective observational study. The authors attempted to account for these limitations and potential confounders by performing a PS-matched analysis and sensitivity analysis; however, these findings should be confirmed with randomized controlled trials.
Continue to: Additionally, the study...
Additionally, the study collected data on each of the DOAC–warfarin cohorts for < 90 days. Trials to address long-term outcomes are warranted.
Finally, there was no control group in comparison with anticoagulation. It is possible that choosing not to use an anticoagulant is the best choice for frail elderly patients.
CHALLENGES TO IMPLEMENTATION
Doctors need a practical frailty scale, patients need an affordable Rx
Frailty is not often considered a measurable trait. The approach used in the study to determine the CFI is not a practical clinical tool. Studies comparing a frailty calculation software application or an easily implementable survey may help bring this clinically impactful information to the hands of primary care physicians. The Clinical Frailty Scale—a brief, 7-point scale based on the physician’s clinical impression of the patient—has been found to correlate with other established frailty measures18 and might be an option for busy clinicians. However, the current study did not utilize this measurement, and the validity of its use by primary care physicians in the outpatient setting requires further study.
In addition, cost may be a barrier for patients younger than 65 years or for those older than 65 years who do not qualify for Medicare or do not have Medicare Part D. The average monthly cost of the DOACs ranges from $560 for dabigatran19 to $600 for rivaroxaban20 and $623 for apixaban.21 As always, the choice of anticoagulant therapy is a clinical judgment and a joint decision of the patient and physician.
ILLUSTRATIVE CASE
A frail 76-year-old woman with a history of hypertension and hyperlipidemia presents for evaluation of palpitations. An in-office electrocardiogram reveals that the patient is in AF. Her CHA2DS2-VASc score is 4 and her HAS-BLED score is 2.2,3 Using shared decision making, you decide to start medications for her AF. You plan to initiate a beta-blocker for rate control and must now decide on anticoagulation. Which oral anticoagulant would you prescribe for this patient’s AF, given her frail status?
Frailty is defined as a state of vulnerability with a decreased ability to recover from an acute stressful event.4 The prevalence of frailty varies by the measurements used and the population studied. A 2021 meta-analysis found that frailty prevalence ranges from 12% to 24% worldwide in patients older than 50 years5 and may increase to > 30% among those ages 85 years and older.6 Frailty increases rates of AEs such as falls7 and fracture,8 leading to disability,9 decreased quality of life,10 increased utilization of health care,11 and increased mortality.12 A number of validated approaches are available to screen for and measure frailty.13-18
Given the association with negative health outcomes and high health care utilization, frailty is an important clinical factor for physicians to consider when treating elderly patients. Frailty assessment may allow for more tailored treatment choices for patients, with a potential reduction in complications. Although CHA2DS2-VASc and HAS-BLED scores assist in the decision-making process of whether to start anticoagulation, these tools do not take frailty into consideration or guide anticoagulant choice.2,3 The purpose of this study was to analyze how levels of frailty affect the association of 3 different direct oral anticoagulants (DOACs) vs warfarin with various AEs (death, stroke, or major bleeding).
STUDY SUMMARY
This DOAC rose above the others
This retrospective cohort study compared the safety of 3 DOACs—dabigatran, rivaroxaban, and apixaban—vs warfarin in Medicare beneficiaries with AF, using 1:1 propensity score (PS)–matched analysis. Eligible patients were ages 65 years or older, with a filled prescription for a DOAC or warfarin, no prior oral anticoagulant exposure in the previous 183 days, a diagnostic code of AF, and continuous enrollment in Medicare Parts A, B, and D only. Patients were excluded if they had missing demographic data, received hospice care, resided in a nursing facility at drug initiation, had another indication for anticoagulation, or had a contraindication to either a DOAC or warfarin.
Frailty was measured using a claims-based frailty index (CFI), which applies health care utilization data to estimate a frailty index, with cut points for nonfrailty, prefrailty, and frailty. The CFI score has 93 claims-based variables, including wheelchairs and durable medical equipment, open wounds, diseases such as chronic obstructive pulmonary disease and ischemic heart disease, and transportation services.15-17 In this study, nonfrailty was defined as a CFI < 0.15, prefrailty as a CFI of 0.15 to 0.24, and frailty as a CFI ≥ 0.25.
The primary outcome—a composite endpoint of death, ischemic stroke, or major bleeding—was measured for each of the DOAC–warfarin cohorts in the overall population and stratified by frailty classification. Patients were followed until the occurrence of a study outcome, Medicare disenrollment, the end of the study period, discontinuation of the index drug (defined as > 5 days), change to a different anticoagulant, admission to a nursing facility, enrollment in hospice, initiation of dialysis, or kidney transplant. The authors conducted a PS-matched analysis to reduce any imbalances in clinical characteristics between the DOAC- and warfarin-treated groups, as well as a sensitivity analysis to assess the strength of the data findings using different assumptions.
The authors created 3 DOAC–warfarin cohorts: dabigatran (n = 81,863) vs warfarin (n = 256,722), rivaroxaban (n = 185,011) vs warfarin (n = 228,028), and apixaban (n = 222,478) vs warfarin (n = 206,031). After PS matching, the mean age in all cohorts was 76 to 77 years, about 50% were female, and 91% were White. The mean HAS-BLED score was 2 and the mean CHA2DS2-VASc score was 4. The mean CFI was 0.19 to 0.20, defined as prefrail. Patients classified as frail were older, more likely to be female, and more likely to have greater comorbidities, higher scores on CHA2DS2-VASc and HAS-BLED, and higher health care utilization.
Continue to: In the dabigatran-warfarin...
In the dabigatran–warfarin cohort (median follow-up, 72 days), the event rate of the composite endpoint per 1000 person-years (PY) was 63.5 for dabigatran and 65.6 for warfarin (hazard ratio [HR] = 0.98; 95% CI, 0.92 to 1.05; rate difference [RD] per 1000 PY = –2.2; 95% CI, –6.5 to 2.1). A lower rate of the composite endpoint was associated with dabigatran than warfarin for the nonfrail subgroup but not the prefrail or frail groups.
In the rivaroxaban–warfarin cohort (median follow-up, 82 days), the composite endpoint rate per 1000 PY was 77.8 for rivaroxaban and 83.7 for warfarin (HR = 0.98; 95% CI, 0.94 to 1.02; RD per 1000 PY = –5.9; 95% CI, –9.4 to –2.4). When stratifying by frailty category, both dabigatran and rivaroxaban were associated with a lower composite endpoint rate than warfarin for the nonfrail population only (HR = 0.81; 95% CI, 0.68 to 0.97, and HR = 0.88; 95% CI, 0.77 to 0.99, respectively).
In the apixaban–warfarin cohort (median follow-up, 84 days), the rate of the composite endpoint per 1000 PY was 60.1 for apixaban and 92.3 for warfarin (HR = 0.68; 95% CI, 0.65 to 0.72; RD per 1000 PY = –32.2; 95% CI, –36.1 to –28.3). The beneficial association for apixaban was present in all frailty categories, with an HR of 0.61 (95% CI, 0.52 to 0.71) for nonfrail patients, 0.66 (95% CI, 0.61 to 0.70) for prefrail patients, and 0.73 (95% CI, 0.67 to 0.80) for frail patients. Apixaban was the only DOAC with a relative reduction in the hazard of death, ischemic stroke, or major bleeding among all frailty groups.
WHAT’S NEW
Only apixaban had lower AE rates vs warfarin across frailty levels
Three DOACs (dabigatran, rivaroxaban, and apixaban) reduced the risk of death, ischemic stroke, or major bleeding compared with warfarin in older adults with AF, but only apixaban was associated with a relative reduction of these adverse outcomes in patients of all frailty classifications.
CAVEATS
Important data but RCTs are needed
The power of this observational study is considerable. However, it remains a retrospective observational study. The authors attempted to account for these limitations and potential confounders by performing a PS-matched analysis and sensitivity analysis; however, these findings should be confirmed with randomized controlled trials.
Continue to: Additionally, the study...
Additionally, the study collected data on each of the DOAC–warfarin cohorts for < 90 days. Trials to address long-term outcomes are warranted.
Finally, there was no control group in comparison with anticoagulation. It is possible that choosing not to use an anticoagulant is the best choice for frail elderly patients.
CHALLENGES TO IMPLEMENTATION
Doctors need a practical frailty scale, patients need an affordable Rx
Frailty is not often considered a measurable trait. The approach used in the study to determine the CFI is not a practical clinical tool. Studies comparing a frailty calculation software application or an easily implementable survey may help bring this clinically impactful information to the hands of primary care physicians. The Clinical Frailty Scale—a brief, 7-point scale based on the physician’s clinical impression of the patient—has been found to correlate with other established frailty measures18 and might be an option for busy clinicians. However, the current study did not utilize this measurement, and the validity of its use by primary care physicians in the outpatient setting requires further study.
In addition, cost may be a barrier for patients younger than 65 years or for those older than 65 years who do not qualify for Medicare or do not have Medicare Part D. The average monthly cost of the DOACs ranges from $560 for dabigatran19 to $600 for rivaroxaban20 and $623 for apixaban.21 As always, the choice of anticoagulant therapy is a clinical judgment and a joint decision of the patient and physician.
1. Kim DH, Pawar A, Gagne JJ, et al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med. 2021;174:1214-1223. doi: 10.7326/M20-7141
2. Zhu W, He W, Guo L, et al. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561. doi: 10.1002/clc.22435
3. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124
4. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15. doi: 10.1016/j.cger.2010.08.009
5. O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96-104. doi: 10.1093/ageing/afaa219
6. Campitelli MA, Bronskill SE, Hogan DB, et al. The prevalence and health consequences of frailty in a population-based older home care cohort: a comparison of different measures. BMC Geriatr. 2016;16:133. doi: 10.1186/s12877-016-0309-z
7. Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16:1027-1033. doi: 10.1016/j.jamda. 2015.06.018
8. Kojima G. Frailty as a predictor of fractures among community-dwelling older people: a systematic review and meta-analysis. Bone. 2016;90:116-122. doi: 10.1016/j.bone.2016.06.009
9. Kojima G. Quick and simple FRAIL scale predicts incident activities of daily living (ADL) and instrumental ADL (IADL) disabilities: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:1063-1068. doi: 10.1016/j.jamda.2018.07.019
10. Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23-30. doi: 10.2147/RMHP.S168750
11. Roe L, Normand C, Wren MA, et al. The impact of frailty on healthcare utilisation in Ireland: evidence from The Irish Longitudinal Study on Ageing. BMC Geriatr. 2017;17:203. doi: 10.1186/s12877-017-0579-0
12. Hao Q, Zhou L, Dong B, et al. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9:1207. doi: 10.1038/s41598-018-38072-7
13. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
14. Ryan J, Espinoza S, Ernst ME, et al. Validation of a deficit-accumulation frailty Index in the ASPirin in Reducing Events in the Elderly study and its predictive capacity for disability-free survival. J Gerontol A Biol Sci Med Sci. 2022;77:19-26. doi: 10.1093/gerona/glab225
15. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74:1271-1276. doi: 10.1093/gerona/gly197
16. Kim DH, Schneeweiss S, Glynn RJ, et al. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73:980-987. doi: 10.1093/gerona/glx229
17. Claims-based frailty index. Harvard Dataverse website. 2022. Accessed April 5, 2022. https://dataverse.harvard.edu/dataverse/cfi
18. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-95. doi: 10.1503/cmaj.050051
19. Dabigatran. GoodRx. Accessed September 26, 2022. www.goodrx.com/dabigatran
20. Rivaroxaban. GoodRx. Accessed September 26, 2022. www.goodrx.com/rivaroxaban
21. Apixaban (Eliquis). GoodRx. Accessed September 26, 2022. www.goodrx.com/eliquis
1. Kim DH, Pawar A, Gagne JJ, et al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med. 2021;174:1214-1223. doi: 10.7326/M20-7141
2. Zhu W, He W, Guo L, et al. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561. doi: 10.1002/clc.22435
3. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124
4. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15. doi: 10.1016/j.cger.2010.08.009
5. O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96-104. doi: 10.1093/ageing/afaa219
6. Campitelli MA, Bronskill SE, Hogan DB, et al. The prevalence and health consequences of frailty in a population-based older home care cohort: a comparison of different measures. BMC Geriatr. 2016;16:133. doi: 10.1186/s12877-016-0309-z
7. Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16:1027-1033. doi: 10.1016/j.jamda. 2015.06.018
8. Kojima G. Frailty as a predictor of fractures among community-dwelling older people: a systematic review and meta-analysis. Bone. 2016;90:116-122. doi: 10.1016/j.bone.2016.06.009
9. Kojima G. Quick and simple FRAIL scale predicts incident activities of daily living (ADL) and instrumental ADL (IADL) disabilities: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:1063-1068. doi: 10.1016/j.jamda.2018.07.019
10. Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23-30. doi: 10.2147/RMHP.S168750
11. Roe L, Normand C, Wren MA, et al. The impact of frailty on healthcare utilisation in Ireland: evidence from The Irish Longitudinal Study on Ageing. BMC Geriatr. 2017;17:203. doi: 10.1186/s12877-017-0579-0
12. Hao Q, Zhou L, Dong B, et al. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9:1207. doi: 10.1038/s41598-018-38072-7
13. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. doi: 10.1093/gerona/56.3.m146
14. Ryan J, Espinoza S, Ernst ME, et al. Validation of a deficit-accumulation frailty Index in the ASPirin in Reducing Events in the Elderly study and its predictive capacity for disability-free survival. J Gerontol A Biol Sci Med Sci. 2022;77:19-26. doi: 10.1093/gerona/glab225
15. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74:1271-1276. doi: 10.1093/gerona/gly197
16. Kim DH, Schneeweiss S, Glynn RJ, et al. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73:980-987. doi: 10.1093/gerona/glx229
17. Claims-based frailty index. Harvard Dataverse website. 2022. Accessed April 5, 2022. https://dataverse.harvard.edu/dataverse/cfi
18. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-95. doi: 10.1503/cmaj.050051
19. Dabigatran. GoodRx. Accessed September 26, 2022. www.goodrx.com/dabigatran
20. Rivaroxaban. GoodRx. Accessed September 26, 2022. www.goodrx.com/rivaroxaban
21. Apixaban (Eliquis). GoodRx. Accessed September 26, 2022. www.goodrx.com/eliquis
PRACTICE CHANGER
Consider apixaban, which demonstrated a lower adverse event (AE) rate than warfarin regardless of frailty status, for anticoagulation treatment of older patients with nonvalvular atrial fibrillation (AF); by comparison, AE rates for dabigatran and rivaroxaban were lower vs warfarin only among nonfrail individuals.
STRENGTH OF RECOMMENDATION
C: Based on a retrospective observational cohort study.1
Kim DH, Pawar A, Gagne JJ, et al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med. 2021;174:1214-1223. doi: 10.7326/M20-7141
Lung cancer screening: New evidence, updated guidance
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.
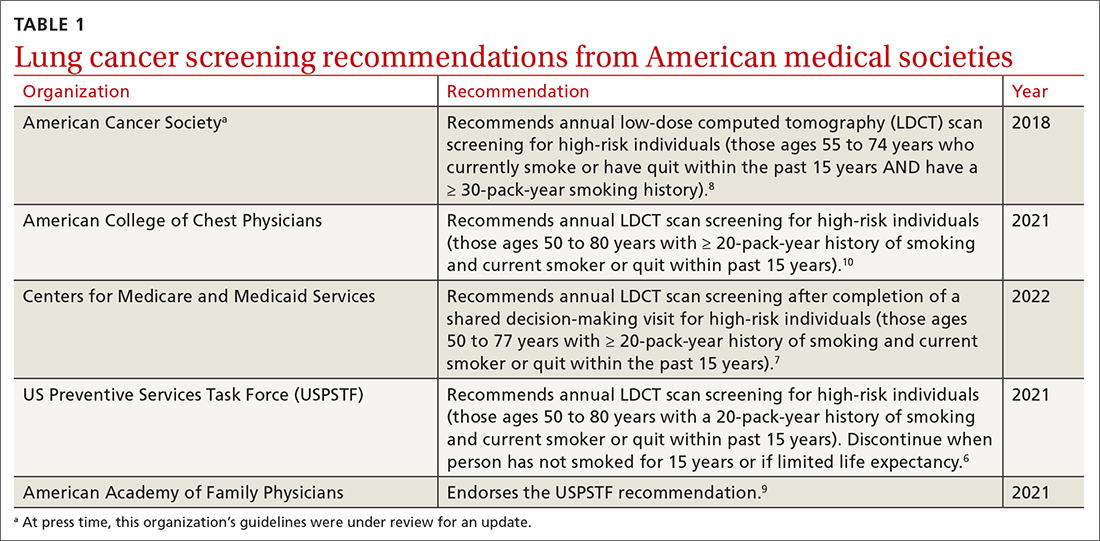
The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14 TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSON trials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19 TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.
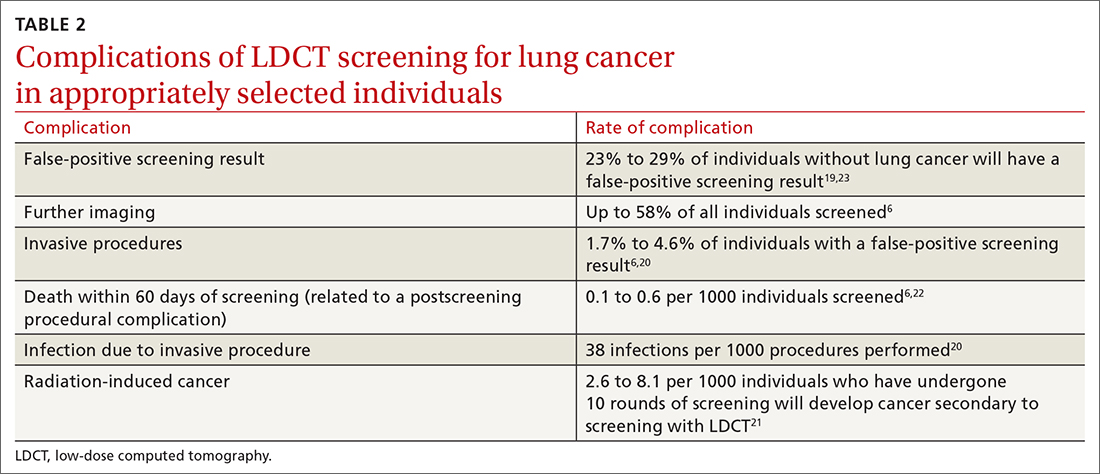
The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14 TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSON trials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19 TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
CASE
A 51-year-old man presents to your office to discuss lung cancer screening. He has a history of hypertension and prediabetes. His father died of lung cancer 5 years ago, at age 77. The patient stopped smoking soon thereafter; prior to that, he smoked 1 pack of cigarettes per day for 20 years. He wants to know if he should be screened for lung cancer.
The relative lack of symptoms during the early stages of lung cancer frequently results in a delayed diagnosis. This, and the speed at which the disease progresses, underscores the need for an effective screening modality. More than half of people with lung cancer die within 1 year of diagnosis.1 Excluding skin cancer, lung cancer is the second most commonly diagnosed cancer, and more people die of lung cancer than of colon, breast, and prostate cancers combined.2 In 2022, it was estimated that there would be 236,740 new cases of lung cancer and 130,180 deaths from lung cancer.1,2 The average age at diagnosis is 70 years.2

Screening modalities: Only 1 has demonstrated mortality benefit
In 1968, Wilson and Junger3 outlined the characteristics of the ideal screening test for the World Health Organization: it should limit risk to the patient, be sensitive for detecting the disease early in its course, limit false-positive results, be acceptable to the patient, and be inexpensive to the health system.3 For decades, several screening modalities for lung cancer were trialed to fit the above guidance, but many of them fell short of the most important outcome: the impact on mortality.
Sputum cytology. The use of sputum cytology, either in combination with or without chest radiography, is not recommended. Several randomized controlled trials (RCTs) have failed to demonstrate improved lung cancer detection or mortality reduction in patients screened with this modality.4
Chest radiography (CXR). Several studies have assessed the efficacy of CXR as a screening modality. The best known was the Prostate, Lung, Colon, Ovarian (PLCO) Trial.5 This multicenter RCT enrolled more than 154,000 participants, half of whom received CXR at baseline and then annually for 3 years; the other half continued usual care (no screening). After 13 years of follow-up, there were no significant differences in lung cancer detection or mortality rates between the 2 groups.5
Low-dose computed tomography (LDCT). Several major medical societies recommend LDCT to screen high-risk individuals for lung cancer (TABLE 16-10). Results from 2 major RCTs have guided these recommendations.

The National Lung Screening Trial (NLST) was a multicenter RCT comparing 2 screening tests for lung cancer.11 Approximately 54,000 high-risk participants were enrolled between 2002 and 2004 and were randomized to receive annual screening with either LDCT or single-view CXR. The trial was discontinued prematurely when investigators noted a 20% reduction in lung cancer mortality in the LDCT group vs the CXR group.12 This equates to 3 fewer deaths for every 1000 people screened with LDCT vs CXR. There was also a 6% reduction in all-cause mortality noted in the LDCT vs the CXR group.12
Continue to: The NELSON trial...
The NELSON trial, conducted between 2005 and 2015, studied more than 15,000 current or former smokers ages 50 to 74 years and compared LDCT screening at various intervals to no screening.13 After 10 years, lung cancer–related mortality was reduced by 24% (or 1 less death per 1000 person-years) in men who were screened vs their unscreened counterparts.13 In contrast to the NLST, in the NELSON trial, no significant difference in all-cause mortality was observed. Subgroup analysis of the relatively small population of women included in the NELSON trial suggested a 33% reduction in 10-year mortality; however, the difference was nonsignificant between the screened and unscreened groups.13
Each of these landmark studies had characteristics that could limit the results' generalizability to the US population. In the NELSON trial, more than 80% of the study participants were male. In both trials, there was significant underrepresentation of Black, Asian, Hispanic, and other non-White people.12,13 Furthermore, participants in these studies were of higher socioeconomic status than the general US screening-eligible population.
At this time, LDCT is the only lung cancer screening modality that has shown benefit for both disease-related and all-cause mortality, in the populations that were studied. Based on the NLST, the number needed to screen (NNS) with LDCT to prevent 1 lung cancer–related death is 308. The NNS to prevent 1 death from any cause is 219.6
Updated evidence has led to a consensus on screening criteria
Many national societies endorse annual screening with LDCT in high-risk individuals (TABLE 16-10). Risk assessment for the purpose of lung cancer screening includes a detailed review of smoking history and age. The risk of lung cancer increases with advancing age and with cumulative quantity and duration of smoking, but decreases with increasing time since quitting. Therefore, a detailed smoking history should include total number of pack-years, current smoking status, and, if applicable, when smoking cessation occurred.
In 2021, the US Preventive Services Task Force (USPSTF) updated their 2013 lung cancer screening recommendations, expanding the screening age range and lowering the smoking history threshold for triggering initiation of screening.6 The impetus for the update was emerging evidence from systematic reviews, RCTs, and the Cancer Intervention and Surveillance Modeling Network (CISNET) that could help to determine the optimal age for screening and identify high-risk groups. For example, the NELSON trial, combined with results from CISNET modeling data, showed an empirical benefit for screening those ages 50 to 55 years.6
Continue to: As a result...
As a result, the USPSTF now recommends annual lung cancer screening with LDCT for any adult ages 50 to 80 years who has a 20-pack-year smoking history and currently smokes or has quit within the past 15 years.6 Screening should be discontinued once a person has not smoked for 15 years, develops a health problem that substantially limits life expectancy, or is not willing to have curative lung surgery.6
Expanding the screening eligibility may also address racial and gender disparities in health care. Black people and women who smoke have a higher risk for lung cancer at a lower intensity of smoking.6
Following the USPSTF update, the American College of Chest Physicians and the Centers for Medicare and Medicaid Services published updated guidance that aligns with USPSTF’s recommendations to lower the age and pack-year qualifications for initiating screening.7,10 The American Cancer Society is currently reviewing its 2018 guidelines on lung cancer screening.14 TABLE 16-10 summarizes the guidance on lung cancer screening from these medical societies.
Effective screening could save lives (and money)
A smoker’s risk for lung cancer is 20 times higher than that of a nonsmoker15,16; 55% of lung cancer deaths in women and 70% in men are attributed to smoking.17 Once diagnosed with lung cancer, more than 50% of people will die within 1 year.1 This underpins the need for a lung cancer screening modality that reduces mortality. Large RCTs, including the NLST and NELSON trials, have shown that screening high-risk individuals with LDCT can significantly reduce lung cancer–related death when compared to no screening or screening with CXR alone.11,13
There is controversy surrounding the cost benefit of implementing a nationwide lung cancer screening program. However, recent use of microsimulation models has shown LDCT to be a cost-effective strategy, with an average cost of $81,000 per quality-adjusted life-year, which is below the threshold of $100,000 to be considered cost effective.18 Expanding the upper age limit for screening leads to a greater reduction in mortality but increases treatment costs and overdiagnosis rates, and overall does not improve quality-adjusted life-years.18
Continue to: Potential harms
Potential harms: False-positives and related complications
Screening for lung cancer is not without its risks. Harms from screening typically result from false-positive test results leading to overdiagnosis, anxiety and distress, unnecessary invasive tests or procedures, and increased costs.19 TABLE 26,19-23 lists specific complications from lung cancer screening with LDCT.

The false-positive rate is not trivial. For every 1000 patients screened, 250 people will have a positive LDCT finding but will not have lung cancer.19 Furthermore, about 1 in every 2000 individuals who screen positive, but who do not have lung cancer, die as a result of complications from the ensuing work-up.6
Annual LDCT screening increases the risk of radiation-induced cancer by approximately 0.05% over 10 years.21 The absolute risk is generally low but not insignificant. However, the mortality benefits previously outlined are significantly more robust in both absolute and relative terms vs the 10-year risk of radiation-induced cancer.
Lastly, it is important to note that the NELSON trial and NLST included a limited number of LDCT scans. Current guidelines for lung cancer screening with LDCT, including those from the USPSTF, recommend screening annually. We do not know the cumulative harm of annual LDCT over a 20- or 30-year period for those who would qualify (ie, current smokers).
If you screen, you must be able to act on the results
Effective screening programs should extend beyond the LDCT scan itself. The studies that have shown a benefit of LDCT were done at large academic centers that had the appropriate radiologic, pathologic, and surgical infrastructure to interpret and act on results and offer further diagnostic or treatment procedures.
Continue to: Prior to screening...
Prior to screening for lung cancer with LDCT, documentation of shared decision-making between the patient and the clinician is necessary.7 This discussion should include the potential benefits and harms of screening, potential results and likelihood of follow-up diagnostic testing, the false-positive rate of LDCT lung cancer screening, and cumulative radiation exposure. In addition, screening should be considered only if the patient is willing to be screened annually, is willing to pursue follow-up scans and procedures (including lung biopsy) if deemed necessary, and does not have comorbid conditions that significantly limit life expectancy.
Smoking cessation: The most important change to make
Smoking cessation is the single most important risk-modifying behavior to reduce one’s chance of developing lung cancer. At age 40, smokers have a 2-fold increase in all-cause mortality compared to age-matched nonsmokers. This rises to a 3-fold increase by the age of 70.16
Smoking cessation reduces the risk of lung cancer by 20% after 5 years, 30% to 50% after 10 years, and up to 70% after 15 years.24 In its guidelines, the American Thoracic Society recommends varenicline (Chantix) for all smokers to assist with smoking cessation.25
CASE
This 51-year-old patient with at least a 20-pack-year history of smoking should be commended for giving up smoking. Based on the USPSTF recommendations, he should be screened annually with LDCT for the next 10 years.
Screening to save more lives
The results of 2 large multicenter RCTs have led to the recent recommendation for lung cancer screening of high-risk adults with the use of LDCT. Screening with LDCT has been shown to reduce disease-related mortality and likely be cost effective in the long term.
Screening with LDCT should be part of a multidisciplinary system that has the infrastructure not only to perform the screening, but also to diagnose and appropriately follow up and treat patients whose results are concerning. The risk of false-positive results leading to increased anxiety, overdiagnosis, and unnecessary procedures points to the importance of proper patient selection, counseling, and shared decision-making. Smoking cessation remains the most important disease-modifying behavior one can make to reduce their risk for lung cancer.
CORRESPONDENCE
Carlton J. Covey, MD, 101 Bodin Circle, David Grant Medical Center, Travis Air Force Base, Fairfield, CA, 94545; [email protected]
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
1. National Cancer Institute. Cancer Stat Facts: lung and bronchus cancer. Accessed October 12, 2022. https://seer.cancer.gov/statfacts/html/lungb.html
2. American Cancer Society. Key statistics for lung cancer. Accessed October 12, 2022. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
3. Wilson JMG, Junger G. Principles and Practice of Screening for Disease. World Health Organization; 1968:21-25, 100. https://apps.who.int/iris/handle/10665/37650
4. Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the United States preventive services task force. Ann Intern Med. 2004;140:740-753. doi: 10.7326/0003-4819-140-9-200405040-00015
5. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865-1873. doi: 10.1001/jama.2011.1591
6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. doi: 10.1001/jama.2021.1117
7. Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439R). Accessed October 14, 2022. www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
8. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297-316. doi: 10.3322/caac.21446
9. American Academy of Family Physicians. AAFP updates recommendation on lung cancer screening. Published April 6, 2021. Accessed October 12, 2022. www.aafp.org/news/health-of-the-public/20210406lungcancer.html
10. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. CHEST. 2021;160:E427-E494. doi: 10.1016/j.chest.2021.06.063
11. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi: 10.1056/NEJMoa1102873
12. The National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980-1991. doi: 10.1056/NEJMoa1209120
13. de Koning HJ, van der Aalst CM, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503-513. doi: 10.1056/NEJMoa1911793
14. American Cancer Society. Lung cancer screening guidelines. Accessed October 14, 2022. www.cancer.org/health-care-professionals/american-cancer-society-prevention-early-detection-guidelines/lung-cancer-screening-guidelines.html
15. Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133-141. doi: 10.1016/S0140-6736(12)61720-6
16. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE
17. O’Keefe LM, Gemma T, Huxley R, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open. 2018;8:e021611. doi: 10.1136/bmjopen-2018-021611
18. Criss SD, Pianpian C, Bastani M, et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171:796-805. doi: 10.7326/M19-0322
19. Lazris A, Roth RA. Lung cancer screening: pros and cons. Am Fam Physician. 2019;99:740-742.
20. Ali MU, Miller J, Peirson L, et al. Screening for lung cancer: a systematic review and meta-analysis. Prev Med. 2016;89:301-314. doi: 10.1016/j.ypmed.2016.04.015
21. Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: 10.1136/bmj.j347
22. Manser RL, Lethaby A, Irving LB, et al. Screening for lung cancer. Cochrane Database Syst Rev. 2013;CD001991. doi: 10.1002/14651858.CD001991.pub3
23. Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. CHEST. 2018;153:954-985. doi: 10.1016/j.chest.2018.01.016
24. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking. and Health. Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services; 2020. www.ncbi.nlm.nih.gov/books/NBK555591/
25. Leone FT, Zhang Y, Evers-Casey S, et al, on behalf of the American Thoracic Society Assembly on Clinical Problems. Initiating pharmacologic treatment in tobacco-dependent adults: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e5-e31. doi: 10.1164/rccm.202005-1982ST
PRACTICE RECOMMENDATIONS
› Recommend annual lung cancer screening for all highrisk adults ages 50 to 80 years using low-dose computed tomography. A
› Do not pursue lung cancer screening in patients who quit smoking ≥ 15 years ago, have a health problem that limits their life expectancy, or are unwilling to undergo lung surgery. A
› Recommend varenicline as first-line pharmacotherapy for smokers who would like to quit. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Clear toe lesion
This is a digital mucous cyst, also known as a myxoid cyst. The clear to translucent appearance over a finger or toe joint is usually diagnosed clinically. If uncertain, a biopsy can confirm the diagnosis.
Digital mucous cysts are a type of ganglion cyst that is associated with trauma or arthritis in the toe joint. A microscopic opening in the joint capsule results in a fluid filled cyst in the surrounding tissue. If the cyst is ruptured, thick, gelatinous (sometimes blood-tinged) hyaluronic acid–rich fluid may escape. Sometimes, the cyst applies pressure to the nail matrix, causing a scooped out longitudinal nail deformity.
Digital mucous cysts more commonly affect the fingers than the toes. Although benign, patients may be bothered by the appearance of these cysts and their effect on nails. Observation is a reasonable approach. Rarely, digital mucous cysts resolve spontaneously.
Treatment options include cryotherapy, needle draining and scarification, and surgical excision with flap repair. Surgical excision may be performed quickly in the office and offers the highest cure rate of 95% in 1 study on fingers.1 Cryotherapy is successful in 70% of cases and needle drainage is successful in 39% of cases, but these modalities are quick and require minimal downtime.1
In this case, the patient was not significantly bothered by the lesion and was happy to forego treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Jabbour S, Kechichian E, Haber R, et al. Management of digital mucous cysts: a systematic review and treatment algorithm. Int J Dermatol. 2017;56:701-708. doi: 10.1111/ijd.13583

This is a digital mucous cyst, also known as a myxoid cyst. The clear to translucent appearance over a finger or toe joint is usually diagnosed clinically. If uncertain, a biopsy can confirm the diagnosis.
Digital mucous cysts are a type of ganglion cyst that is associated with trauma or arthritis in the toe joint. A microscopic opening in the joint capsule results in a fluid filled cyst in the surrounding tissue. If the cyst is ruptured, thick, gelatinous (sometimes blood-tinged) hyaluronic acid–rich fluid may escape. Sometimes, the cyst applies pressure to the nail matrix, causing a scooped out longitudinal nail deformity.
Digital mucous cysts more commonly affect the fingers than the toes. Although benign, patients may be bothered by the appearance of these cysts and their effect on nails. Observation is a reasonable approach. Rarely, digital mucous cysts resolve spontaneously.
Treatment options include cryotherapy, needle draining and scarification, and surgical excision with flap repair. Surgical excision may be performed quickly in the office and offers the highest cure rate of 95% in 1 study on fingers.1 Cryotherapy is successful in 70% of cases and needle drainage is successful in 39% of cases, but these modalities are quick and require minimal downtime.1
In this case, the patient was not significantly bothered by the lesion and was happy to forego treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This is a digital mucous cyst, also known as a myxoid cyst. The clear to translucent appearance over a finger or toe joint is usually diagnosed clinically. If uncertain, a biopsy can confirm the diagnosis.
Digital mucous cysts are a type of ganglion cyst that is associated with trauma or arthritis in the toe joint. A microscopic opening in the joint capsule results in a fluid filled cyst in the surrounding tissue. If the cyst is ruptured, thick, gelatinous (sometimes blood-tinged) hyaluronic acid–rich fluid may escape. Sometimes, the cyst applies pressure to the nail matrix, causing a scooped out longitudinal nail deformity.
Digital mucous cysts more commonly affect the fingers than the toes. Although benign, patients may be bothered by the appearance of these cysts and their effect on nails. Observation is a reasonable approach. Rarely, digital mucous cysts resolve spontaneously.
Treatment options include cryotherapy, needle draining and scarification, and surgical excision with flap repair. Surgical excision may be performed quickly in the office and offers the highest cure rate of 95% in 1 study on fingers.1 Cryotherapy is successful in 70% of cases and needle drainage is successful in 39% of cases, but these modalities are quick and require minimal downtime.1
In this case, the patient was not significantly bothered by the lesion and was happy to forego treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Jabbour S, Kechichian E, Haber R, et al. Management of digital mucous cysts: a systematic review and treatment algorithm. Int J Dermatol. 2017;56:701-708. doi: 10.1111/ijd.13583
1. Jabbour S, Kechichian E, Haber R, et al. Management of digital mucous cysts: a systematic review and treatment algorithm. Int J Dermatol. 2017;56:701-708. doi: 10.1111/ijd.13583
OSA raises risk of atrial fibrillation and stroke
compared with controls, based on data from 303 individuals.
OSA has become a common chronic disease, and cardiovascular diseases including AFib also are known independent risk factors associated with OSA, Anna Hojager, MD, of Zealand University Hospital, Roskilde, Denmark, and colleagues wrote. Previous studies have shown a significant increase in AFib risk in OSA patients with severe disease, but the prevalence of undiagnosed AFib in OSA patients has not been explored.
In a study published in Sleep Medicine, the researchers enrolled 238 adults with severe OSA (based on apnea-hypopnea index of 15 or higher) and 65 with mild or no OSA (based on an AHI of less than 15). The mean AHI across all participants was 34.2, and ranged from 0.2 to 115.8.
Participants underwent heart rhythm monitoring using a home system or standard ECG for 7 days; they were instructed to carry the device at all times except when showering or sweating heavily. The primary outcome was the detection of AFib, defined as at least one period of 30 seconds or longer with an irregular heart rhythm but without detectable evidence of another diagnosis. Sleep was assessed for one night using a portable sleep monitoring device. All participants were examined at baseline and measured for blood pressure, body mass index, waist-to-hip ratio, and ECG.
Overall, AFib occurred in 21 patients with moderate to severe OSA and 1 patient with mild/no OSA (8.8% vs. 1.5%, P = .045). The majority of patients across both groups had hypertension (66%) and dyslipidemia (77.6%), but the severe OSA group was more likely to be dysregulated and to have unknown prediabetes. Participants who were deemed candidates for anticoagulation therapy were referred for additional treatment. None of the 22 total patients with AFib had heart failure with reduced ejection fraction, and 68.2% had normal ejection fraction and ventricle function.
The researchers noted that no guidelines currently exist for systematic opportunistic screening for comorbidities in OSA patients, although the American Academy of Sleep Medicine recommends patient education as part of a multidisciplinary chronic disease management strategy. The high prevalence of AFib in OSA patients, as seen in the current study, “might warrant a recommendation of screening for paroxysmal [AFib] and could be valuable in the management of modifiable cardiovascular risk factors in patients with OSA,” they wrote.
The study findings were limited by several factors including the observational design and absence of polysomnography to assess OSA, the researchers noted. However, the study has the highest known prevalence of silent AFib in patients with moderate to severe OSA, and supports the value of screening and management for known comorbidities of OSA.
The study received no outside funding. The researchers had no financial conflicts to disclose.
compared with controls, based on data from 303 individuals.
OSA has become a common chronic disease, and cardiovascular diseases including AFib also are known independent risk factors associated with OSA, Anna Hojager, MD, of Zealand University Hospital, Roskilde, Denmark, and colleagues wrote. Previous studies have shown a significant increase in AFib risk in OSA patients with severe disease, but the prevalence of undiagnosed AFib in OSA patients has not been explored.
In a study published in Sleep Medicine, the researchers enrolled 238 adults with severe OSA (based on apnea-hypopnea index of 15 or higher) and 65 with mild or no OSA (based on an AHI of less than 15). The mean AHI across all participants was 34.2, and ranged from 0.2 to 115.8.
Participants underwent heart rhythm monitoring using a home system or standard ECG for 7 days; they were instructed to carry the device at all times except when showering or sweating heavily. The primary outcome was the detection of AFib, defined as at least one period of 30 seconds or longer with an irregular heart rhythm but without detectable evidence of another diagnosis. Sleep was assessed for one night using a portable sleep monitoring device. All participants were examined at baseline and measured for blood pressure, body mass index, waist-to-hip ratio, and ECG.
Overall, AFib occurred in 21 patients with moderate to severe OSA and 1 patient with mild/no OSA (8.8% vs. 1.5%, P = .045). The majority of patients across both groups had hypertension (66%) and dyslipidemia (77.6%), but the severe OSA group was more likely to be dysregulated and to have unknown prediabetes. Participants who were deemed candidates for anticoagulation therapy were referred for additional treatment. None of the 22 total patients with AFib had heart failure with reduced ejection fraction, and 68.2% had normal ejection fraction and ventricle function.
The researchers noted that no guidelines currently exist for systematic opportunistic screening for comorbidities in OSA patients, although the American Academy of Sleep Medicine recommends patient education as part of a multidisciplinary chronic disease management strategy. The high prevalence of AFib in OSA patients, as seen in the current study, “might warrant a recommendation of screening for paroxysmal [AFib] and could be valuable in the management of modifiable cardiovascular risk factors in patients with OSA,” they wrote.
The study findings were limited by several factors including the observational design and absence of polysomnography to assess OSA, the researchers noted. However, the study has the highest known prevalence of silent AFib in patients with moderate to severe OSA, and supports the value of screening and management for known comorbidities of OSA.
The study received no outside funding. The researchers had no financial conflicts to disclose.
compared with controls, based on data from 303 individuals.
OSA has become a common chronic disease, and cardiovascular diseases including AFib also are known independent risk factors associated with OSA, Anna Hojager, MD, of Zealand University Hospital, Roskilde, Denmark, and colleagues wrote. Previous studies have shown a significant increase in AFib risk in OSA patients with severe disease, but the prevalence of undiagnosed AFib in OSA patients has not been explored.
In a study published in Sleep Medicine, the researchers enrolled 238 adults with severe OSA (based on apnea-hypopnea index of 15 or higher) and 65 with mild or no OSA (based on an AHI of less than 15). The mean AHI across all participants was 34.2, and ranged from 0.2 to 115.8.
Participants underwent heart rhythm monitoring using a home system or standard ECG for 7 days; they were instructed to carry the device at all times except when showering or sweating heavily. The primary outcome was the detection of AFib, defined as at least one period of 30 seconds or longer with an irregular heart rhythm but without detectable evidence of another diagnosis. Sleep was assessed for one night using a portable sleep monitoring device. All participants were examined at baseline and measured for blood pressure, body mass index, waist-to-hip ratio, and ECG.
Overall, AFib occurred in 21 patients with moderate to severe OSA and 1 patient with mild/no OSA (8.8% vs. 1.5%, P = .045). The majority of patients across both groups had hypertension (66%) and dyslipidemia (77.6%), but the severe OSA group was more likely to be dysregulated and to have unknown prediabetes. Participants who were deemed candidates for anticoagulation therapy were referred for additional treatment. None of the 22 total patients with AFib had heart failure with reduced ejection fraction, and 68.2% had normal ejection fraction and ventricle function.
The researchers noted that no guidelines currently exist for systematic opportunistic screening for comorbidities in OSA patients, although the American Academy of Sleep Medicine recommends patient education as part of a multidisciplinary chronic disease management strategy. The high prevalence of AFib in OSA patients, as seen in the current study, “might warrant a recommendation of screening for paroxysmal [AFib] and could be valuable in the management of modifiable cardiovascular risk factors in patients with OSA,” they wrote.
The study findings were limited by several factors including the observational design and absence of polysomnography to assess OSA, the researchers noted. However, the study has the highest known prevalence of silent AFib in patients with moderate to severe OSA, and supports the value of screening and management for known comorbidities of OSA.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM SLEEP MEDICINE