User login
One in four NSCLC patients respond poorly to COVID-19 vaccine
according to a new study.
The study was published in the Journal of Clinical Oncology.
“Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant,” wrote the authors, who were led by Rafi Ahmed, PhD, Emory University, Atlanta.
Researchers found that 18% had no detectable antibody at all and active treatment type had no association with vaccine response.
Researchers examined antibody titers among 82 NSCLC patients and 53 healthy volunteers. They collected blood samples longitudinally for analysis. While most patients had binding and neutralizing antibody titers that were comparable with healthy volunteers, 25% had poor responses, which led to six- to sevenfold lower titers than healthy controls. There was no association between worse vaccine responses and history of programmed death–1 monotherapy, chemotherapy, or both in combination. Receipt of a booster vaccine improved binding and neutralizing antibody titers to both the wild type and the Omicron variant, but 2-4 months after the booster there was a five- to sevenfold decrease in neutralizing titers to both the wild type and Omicron variant.
“This study indicates both the need to monitor our patients with lung cancer for response to COVID-19 mRNA vaccines, identify the nonresponders for follow-up and further attempts at immunization, and continue collecting and analyzing clinicodemographic information and biospecimens from our patients,” wrote the authors of an accompanying editorial.
Although the findings reveal potential concerns, the good news is that most patients NSCLC patients do respond normally to COVID-19 vaccination, said John D. Minna, MD, University of Texas Southwestern Medical Center, Dallas, lead author of the editorial.
He offered some advice to physicians. “You can test your patients using currently available [Clinical Laboratory Improvement Amendments]–approved lab tests to determine what their antibody titers are. This should be done after boosting since titers will go down after time. We know that if a patient has lung cancer and they do get infected with SARS-CoV-2 that potentially they could develop serious COVID-19 disease. Besides giving antiviral treatment, it is important that they be closely monitored for symptoms of progression so if they need to be hospitalized it can be done in a prudent manner,” said Dr. Minna, who is director of the Hamon Center for Therapeutic Oncology Research at the University of Texas Southwestern Medical Center.
No clinical details have emerged that might predict which patients have an insufficient response to vaccination. “When we started these studies, a lot of us thought that anyone who did not develop a good antibody response would be weak or sicker. For example, [patients with] late-stage disease, or having had a lot of therapy, or perhaps immune checkpoint blockade. However, none of these things are correlated. The main advice we are giving our lung cancer patients are to get vaccinated, get boosted (double boosted), and just do the smart thing to protect yourself from exposure,” he said.
For example, when traveling on a plane, patients should wear a mask. They should also avoid large indoor events. He also recommended that, following vaccination and boosters, patients seek out CLIA-certified tests to get their titer checked.
“Upon any COVID infection, even if their titer is at or above 80%, patients should see their physician to consider treatment with Paxlovid (nirmatrelvir/ritonavir), which has emergency use authorization. For patients with a lower titer, it’s important to seek out a physician and consider Paxlovid and possibly antibody therapy. But these are individual decisions to be made with your doctor,” Dr. Minna said.
The next important research question is what happens to T-cell immune response following vaccination. “We know that a good cellular immune response is also important in preventing infection and severe infection, but we don’t yet know which persons (with or without cancer) have good T-cell responses. This information will also likely impact what we tell our patients and will add to the antibody data,” he said.
Studies are ongoing to determine specific T-cell responses to mRNA vaccines, and how well those T-cell responses respond to SARS-CoV-2 infection in the laboratory.
according to a new study.
The study was published in the Journal of Clinical Oncology.
“Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant,” wrote the authors, who were led by Rafi Ahmed, PhD, Emory University, Atlanta.
Researchers found that 18% had no detectable antibody at all and active treatment type had no association with vaccine response.
Researchers examined antibody titers among 82 NSCLC patients and 53 healthy volunteers. They collected blood samples longitudinally for analysis. While most patients had binding and neutralizing antibody titers that were comparable with healthy volunteers, 25% had poor responses, which led to six- to sevenfold lower titers than healthy controls. There was no association between worse vaccine responses and history of programmed death–1 monotherapy, chemotherapy, or both in combination. Receipt of a booster vaccine improved binding and neutralizing antibody titers to both the wild type and the Omicron variant, but 2-4 months after the booster there was a five- to sevenfold decrease in neutralizing titers to both the wild type and Omicron variant.
“This study indicates both the need to monitor our patients with lung cancer for response to COVID-19 mRNA vaccines, identify the nonresponders for follow-up and further attempts at immunization, and continue collecting and analyzing clinicodemographic information and biospecimens from our patients,” wrote the authors of an accompanying editorial.
Although the findings reveal potential concerns, the good news is that most patients NSCLC patients do respond normally to COVID-19 vaccination, said John D. Minna, MD, University of Texas Southwestern Medical Center, Dallas, lead author of the editorial.
He offered some advice to physicians. “You can test your patients using currently available [Clinical Laboratory Improvement Amendments]–approved lab tests to determine what their antibody titers are. This should be done after boosting since titers will go down after time. We know that if a patient has lung cancer and they do get infected with SARS-CoV-2 that potentially they could develop serious COVID-19 disease. Besides giving antiviral treatment, it is important that they be closely monitored for symptoms of progression so if they need to be hospitalized it can be done in a prudent manner,” said Dr. Minna, who is director of the Hamon Center for Therapeutic Oncology Research at the University of Texas Southwestern Medical Center.
No clinical details have emerged that might predict which patients have an insufficient response to vaccination. “When we started these studies, a lot of us thought that anyone who did not develop a good antibody response would be weak or sicker. For example, [patients with] late-stage disease, or having had a lot of therapy, or perhaps immune checkpoint blockade. However, none of these things are correlated. The main advice we are giving our lung cancer patients are to get vaccinated, get boosted (double boosted), and just do the smart thing to protect yourself from exposure,” he said.
For example, when traveling on a plane, patients should wear a mask. They should also avoid large indoor events. He also recommended that, following vaccination and boosters, patients seek out CLIA-certified tests to get their titer checked.
“Upon any COVID infection, even if their titer is at or above 80%, patients should see their physician to consider treatment with Paxlovid (nirmatrelvir/ritonavir), which has emergency use authorization. For patients with a lower titer, it’s important to seek out a physician and consider Paxlovid and possibly antibody therapy. But these are individual decisions to be made with your doctor,” Dr. Minna said.
The next important research question is what happens to T-cell immune response following vaccination. “We know that a good cellular immune response is also important in preventing infection and severe infection, but we don’t yet know which persons (with or without cancer) have good T-cell responses. This information will also likely impact what we tell our patients and will add to the antibody data,” he said.
Studies are ongoing to determine specific T-cell responses to mRNA vaccines, and how well those T-cell responses respond to SARS-CoV-2 infection in the laboratory.
according to a new study.
The study was published in the Journal of Clinical Oncology.
“Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant,” wrote the authors, who were led by Rafi Ahmed, PhD, Emory University, Atlanta.
Researchers found that 18% had no detectable antibody at all and active treatment type had no association with vaccine response.
Researchers examined antibody titers among 82 NSCLC patients and 53 healthy volunteers. They collected blood samples longitudinally for analysis. While most patients had binding and neutralizing antibody titers that were comparable with healthy volunteers, 25% had poor responses, which led to six- to sevenfold lower titers than healthy controls. There was no association between worse vaccine responses and history of programmed death–1 monotherapy, chemotherapy, or both in combination. Receipt of a booster vaccine improved binding and neutralizing antibody titers to both the wild type and the Omicron variant, but 2-4 months after the booster there was a five- to sevenfold decrease in neutralizing titers to both the wild type and Omicron variant.
“This study indicates both the need to monitor our patients with lung cancer for response to COVID-19 mRNA vaccines, identify the nonresponders for follow-up and further attempts at immunization, and continue collecting and analyzing clinicodemographic information and biospecimens from our patients,” wrote the authors of an accompanying editorial.
Although the findings reveal potential concerns, the good news is that most patients NSCLC patients do respond normally to COVID-19 vaccination, said John D. Minna, MD, University of Texas Southwestern Medical Center, Dallas, lead author of the editorial.
He offered some advice to physicians. “You can test your patients using currently available [Clinical Laboratory Improvement Amendments]–approved lab tests to determine what their antibody titers are. This should be done after boosting since titers will go down after time. We know that if a patient has lung cancer and they do get infected with SARS-CoV-2 that potentially they could develop serious COVID-19 disease. Besides giving antiviral treatment, it is important that they be closely monitored for symptoms of progression so if they need to be hospitalized it can be done in a prudent manner,” said Dr. Minna, who is director of the Hamon Center for Therapeutic Oncology Research at the University of Texas Southwestern Medical Center.
No clinical details have emerged that might predict which patients have an insufficient response to vaccination. “When we started these studies, a lot of us thought that anyone who did not develop a good antibody response would be weak or sicker. For example, [patients with] late-stage disease, or having had a lot of therapy, or perhaps immune checkpoint blockade. However, none of these things are correlated. The main advice we are giving our lung cancer patients are to get vaccinated, get boosted (double boosted), and just do the smart thing to protect yourself from exposure,” he said.
For example, when traveling on a plane, patients should wear a mask. They should also avoid large indoor events. He also recommended that, following vaccination and boosters, patients seek out CLIA-certified tests to get their titer checked.
“Upon any COVID infection, even if their titer is at or above 80%, patients should see their physician to consider treatment with Paxlovid (nirmatrelvir/ritonavir), which has emergency use authorization. For patients with a lower titer, it’s important to seek out a physician and consider Paxlovid and possibly antibody therapy. But these are individual decisions to be made with your doctor,” Dr. Minna said.
The next important research question is what happens to T-cell immune response following vaccination. “We know that a good cellular immune response is also important in preventing infection and severe infection, but we don’t yet know which persons (with or without cancer) have good T-cell responses. This information will also likely impact what we tell our patients and will add to the antibody data,” he said.
Studies are ongoing to determine specific T-cell responses to mRNA vaccines, and how well those T-cell responses respond to SARS-CoV-2 infection in the laboratory.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Summer flu, RSV in July, ‘super colds?’
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Treatment combo shows ‘clinical benefit’ in liver cancer trial
in patients with hepatocellular carcinoma, shows a new study.
While the combination has been shown to be beneficial in renal cell carcinoma and other solid tumor types, it has never before been tested in a phase 3 clinical trial for hepatocellular carcinoma until now.
The new study, published in The Lancet Oncology, included 837 patients from 178 hospital in 32 countries who were enrolled in the study (called COSMIC-312) between December 2018 and August 2020. 432 patients were randomly assigned to receive a combination of cabozantinib (Cabometyx, Exelixis), a tyrosine kinase inhibitor (TKI), and atezolizumab (Tecentriq, Genentech), a PD-L1 inhibitor. While 217 patients were treated with sorafenib (Nexavar, Bayer) alone and 188 patients were treated with cabozantinib.
Clinically meaningful improvements in progression-free survival, increased disease control and lower primary progression were seen in patients who received the cabozantinib and atezolizumab combination therapy over patients who were treated with sorafenib. However, there was no improvement in overall survival.
“The improvement in progression-free survival with cabozantinib plus atezolizumab in this study shows that the combination confers clinical benefit for patients with advanced hepatocellular carcinoma previously untreated with systemic anticancer therapy,” wrote the authors of the study, led by Robin Kate Kelley, MD, a gastrointestinal oncologist with the University of California, San Francisco, and Lorenza Rimassa, MD, a gastrointestinal oncologist with Humanitas University, Milan. “The absence of a benefit in overall survival, along with the availability of atezolizumab in combination with bevacizumab, indicates the need for additional studies to determine if cabozantinib plus atezolizumab would be an appropriate first-line treatment option in select patient populations.”
For symptomatic patients with high disease burden or main portal vein occlusion who are at risk for impending complications, controlling the disease as quickly as possible is vital, the authors wrote. “Underlying chronic liver disease is nearly universal in patients with hepatocellular carcinoma and the risk of gastrointestinal bleeding is high in this population, particularly if portal vein tumor thrombus is present.”
Hepatocellular carcinoma (HCC) is an angiogenic tumor, making it a logical target for TKIs that target vascular endothelial growth factor. The TKI sorafenib was the first to be approved as a first-line treatment for HCC, and since then immune checkpoint inhibitors have been shown to induce durable responses in the first-line setting, but have not improved overall survival in randomized trials.
Study methodology
In the study, after a median follow-up of 15.8 months, median progression-free survival was 6.8 months in the combination group and 4.2 months in the sorafenib group (hazard ratio, 0.63; P = .0012). The median overall survival was 15.4 months in the combination group and 15.5 months in the sorafenib group (not significant). Grade 3-4 adverse events included an increase in ALT, which occurred in 9% of the combination group, 3% of the sorafenib group, and 6% of the cabozantinib only group; hypertension (9%, 8%, and 12%, respectively); an increase in AST increase (9%, 4%, 10%); and palmar-plantar erythrodysesthesia (8%, 8%, 9%). Serious treatment-related adverse events occurred in 18% of patients in the combination arm, 8% in the sorafenib arm, and 13% in the cabozantinib arm.
There were no excess serious bleeding events in the treatment groups containing cabozantinib, compared with sorafenib which is noteworthy because HCC patients are at high risk for gastrointestinal bleeding.
Treatment-related grade 5 events were rare, occurring in 1% (six patients) of the combination group, and in just one patient in both the sorafenib and cabozantinib groups.
Although the results suggest promising clinical benefit, the lack of overall survival benefit limit the implications of these findings. Since atezolizumab combined with bevacizumab is also available for this patient population, more research is needed to determine if cabozantinib plus atezolizumab can become a first-line option.
The study had some limitations: Participants had to have a Child-Pugh class of A, though there was no requirement to assess for fibrosis or cirrhosis. Otherwise there were few barriers to study entry.
The study was sponsored by Exelixis (Alameda) and Ipsen (Boulogne-Billancourt, France).
in patients with hepatocellular carcinoma, shows a new study.
While the combination has been shown to be beneficial in renal cell carcinoma and other solid tumor types, it has never before been tested in a phase 3 clinical trial for hepatocellular carcinoma until now.
The new study, published in The Lancet Oncology, included 837 patients from 178 hospital in 32 countries who were enrolled in the study (called COSMIC-312) between December 2018 and August 2020. 432 patients were randomly assigned to receive a combination of cabozantinib (Cabometyx, Exelixis), a tyrosine kinase inhibitor (TKI), and atezolizumab (Tecentriq, Genentech), a PD-L1 inhibitor. While 217 patients were treated with sorafenib (Nexavar, Bayer) alone and 188 patients were treated with cabozantinib.
Clinically meaningful improvements in progression-free survival, increased disease control and lower primary progression were seen in patients who received the cabozantinib and atezolizumab combination therapy over patients who were treated with sorafenib. However, there was no improvement in overall survival.
“The improvement in progression-free survival with cabozantinib plus atezolizumab in this study shows that the combination confers clinical benefit for patients with advanced hepatocellular carcinoma previously untreated with systemic anticancer therapy,” wrote the authors of the study, led by Robin Kate Kelley, MD, a gastrointestinal oncologist with the University of California, San Francisco, and Lorenza Rimassa, MD, a gastrointestinal oncologist with Humanitas University, Milan. “The absence of a benefit in overall survival, along with the availability of atezolizumab in combination with bevacizumab, indicates the need for additional studies to determine if cabozantinib plus atezolizumab would be an appropriate first-line treatment option in select patient populations.”
For symptomatic patients with high disease burden or main portal vein occlusion who are at risk for impending complications, controlling the disease as quickly as possible is vital, the authors wrote. “Underlying chronic liver disease is nearly universal in patients with hepatocellular carcinoma and the risk of gastrointestinal bleeding is high in this population, particularly if portal vein tumor thrombus is present.”
Hepatocellular carcinoma (HCC) is an angiogenic tumor, making it a logical target for TKIs that target vascular endothelial growth factor. The TKI sorafenib was the first to be approved as a first-line treatment for HCC, and since then immune checkpoint inhibitors have been shown to induce durable responses in the first-line setting, but have not improved overall survival in randomized trials.
Study methodology
In the study, after a median follow-up of 15.8 months, median progression-free survival was 6.8 months in the combination group and 4.2 months in the sorafenib group (hazard ratio, 0.63; P = .0012). The median overall survival was 15.4 months in the combination group and 15.5 months in the sorafenib group (not significant). Grade 3-4 adverse events included an increase in ALT, which occurred in 9% of the combination group, 3% of the sorafenib group, and 6% of the cabozantinib only group; hypertension (9%, 8%, and 12%, respectively); an increase in AST increase (9%, 4%, 10%); and palmar-plantar erythrodysesthesia (8%, 8%, 9%). Serious treatment-related adverse events occurred in 18% of patients in the combination arm, 8% in the sorafenib arm, and 13% in the cabozantinib arm.
There were no excess serious bleeding events in the treatment groups containing cabozantinib, compared with sorafenib which is noteworthy because HCC patients are at high risk for gastrointestinal bleeding.
Treatment-related grade 5 events were rare, occurring in 1% (six patients) of the combination group, and in just one patient in both the sorafenib and cabozantinib groups.
Although the results suggest promising clinical benefit, the lack of overall survival benefit limit the implications of these findings. Since atezolizumab combined with bevacizumab is also available for this patient population, more research is needed to determine if cabozantinib plus atezolizumab can become a first-line option.
The study had some limitations: Participants had to have a Child-Pugh class of A, though there was no requirement to assess for fibrosis or cirrhosis. Otherwise there were few barriers to study entry.
The study was sponsored by Exelixis (Alameda) and Ipsen (Boulogne-Billancourt, France).
in patients with hepatocellular carcinoma, shows a new study.
While the combination has been shown to be beneficial in renal cell carcinoma and other solid tumor types, it has never before been tested in a phase 3 clinical trial for hepatocellular carcinoma until now.
The new study, published in The Lancet Oncology, included 837 patients from 178 hospital in 32 countries who were enrolled in the study (called COSMIC-312) between December 2018 and August 2020. 432 patients were randomly assigned to receive a combination of cabozantinib (Cabometyx, Exelixis), a tyrosine kinase inhibitor (TKI), and atezolizumab (Tecentriq, Genentech), a PD-L1 inhibitor. While 217 patients were treated with sorafenib (Nexavar, Bayer) alone and 188 patients were treated with cabozantinib.
Clinically meaningful improvements in progression-free survival, increased disease control and lower primary progression were seen in patients who received the cabozantinib and atezolizumab combination therapy over patients who were treated with sorafenib. However, there was no improvement in overall survival.
“The improvement in progression-free survival with cabozantinib plus atezolizumab in this study shows that the combination confers clinical benefit for patients with advanced hepatocellular carcinoma previously untreated with systemic anticancer therapy,” wrote the authors of the study, led by Robin Kate Kelley, MD, a gastrointestinal oncologist with the University of California, San Francisco, and Lorenza Rimassa, MD, a gastrointestinal oncologist with Humanitas University, Milan. “The absence of a benefit in overall survival, along with the availability of atezolizumab in combination with bevacizumab, indicates the need for additional studies to determine if cabozantinib plus atezolizumab would be an appropriate first-line treatment option in select patient populations.”
For symptomatic patients with high disease burden or main portal vein occlusion who are at risk for impending complications, controlling the disease as quickly as possible is vital, the authors wrote. “Underlying chronic liver disease is nearly universal in patients with hepatocellular carcinoma and the risk of gastrointestinal bleeding is high in this population, particularly if portal vein tumor thrombus is present.”
Hepatocellular carcinoma (HCC) is an angiogenic tumor, making it a logical target for TKIs that target vascular endothelial growth factor. The TKI sorafenib was the first to be approved as a first-line treatment for HCC, and since then immune checkpoint inhibitors have been shown to induce durable responses in the first-line setting, but have not improved overall survival in randomized trials.
Study methodology
In the study, after a median follow-up of 15.8 months, median progression-free survival was 6.8 months in the combination group and 4.2 months in the sorafenib group (hazard ratio, 0.63; P = .0012). The median overall survival was 15.4 months in the combination group and 15.5 months in the sorafenib group (not significant). Grade 3-4 adverse events included an increase in ALT, which occurred in 9% of the combination group, 3% of the sorafenib group, and 6% of the cabozantinib only group; hypertension (9%, 8%, and 12%, respectively); an increase in AST increase (9%, 4%, 10%); and palmar-plantar erythrodysesthesia (8%, 8%, 9%). Serious treatment-related adverse events occurred in 18% of patients in the combination arm, 8% in the sorafenib arm, and 13% in the cabozantinib arm.
There were no excess serious bleeding events in the treatment groups containing cabozantinib, compared with sorafenib which is noteworthy because HCC patients are at high risk for gastrointestinal bleeding.
Treatment-related grade 5 events were rare, occurring in 1% (six patients) of the combination group, and in just one patient in both the sorafenib and cabozantinib groups.
Although the results suggest promising clinical benefit, the lack of overall survival benefit limit the implications of these findings. Since atezolizumab combined with bevacizumab is also available for this patient population, more research is needed to determine if cabozantinib plus atezolizumab can become a first-line option.
The study had some limitations: Participants had to have a Child-Pugh class of A, though there was no requirement to assess for fibrosis or cirrhosis. Otherwise there were few barriers to study entry.
The study was sponsored by Exelixis (Alameda) and Ipsen (Boulogne-Billancourt, France).
FROM THE LANCET
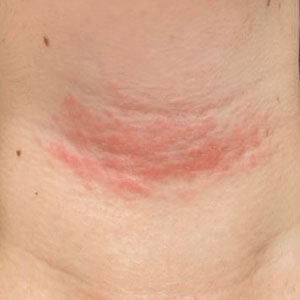
Solitary Pink Plaque on the Neck
The Diagnosis: Plaque-type Syringoma
A biopsy demonstrated multiple basaloid islands of tumor cells in the reticular dermis with ductal differentiation, some with a commalike tail. The ducts were lined by 2 to 3 layers of small uniform cuboidal cells without atypia and contained inspissated secretions within the lumina of scattered ducts. There was an associated fibrotic collagenous stroma. There was no evidence of perineural invasion and no deep dermal or subcutaneous extension (Figure 1). Additional cytokeratin immunohistochemical staining highlighted the adnexal proliferation (Figure 2). A diagnosis of plaque-type syringoma (PTS) was made.

Syringomas are benign dermal sweat gland tumors that typically present as flesh-colored papules on the cheeks or periorbital area of young females. Plaque-type tumors as well as papulonodular, eruptive, disseminated, urticaria pigmentosa–like, lichen planus–like, or milialike syringomas also have been reported. Syringomas may be associated with certain medical conditions such as Down syndrome, Nicolau-Balus syndrome, and both scarring and nonscarring alopecias.1 The clear cell variant of syringoma often is associated with diabetes mellitus.2 Kikuchi et al3 first described PTS in 1979. Plaque-type syringomas rarely are reported in the literature, and sites of involvement include the head and neck region, upper lip, chest, upper extremities, vulva, penis, and scrotum.4-6

Histologically, syringomatous lesions are composed of multiple small ducts lined by 2 to 3 layers of cuboidal epithelium. The ducts may be arranged in nests or strands of basaloid cells surrounded by a dense fibrotic stroma. Occasionally, the ducts will form a comma- or teardropshaped tail; however, this also may be observed in desmoplastic trichoepithelioma (DTE).7 Perineural invasion is absent in syringomas. Syringomas exhibit a lateral growth pattern that typically is limited to the upper half of the reticular dermis and spares the underlying subcutis, muscle, and bone. The growth pattern may be discontinuous with proliferations juxtaposed by normal-appearing skin.8 Syringomas usually express progesterone receptors and are known to proliferate at puberty, suggesting that these neoplasms are under hormonal control.9 Although syringomas are benign, various treatment options that may be pursued for cosmetic purposes include radiofrequency, staged excision, laser ablation, and oral isotretinoin.8,10 If only a superficial biopsy is obtained, syringomas may display features of other adnexal neoplasms, including microcystic adnexal carcinoma (MAC), DTE, morpheaform basal cell carcinoma (BCC), and inflammatory linear verrucous epidermal nevus (ILVEN).
Microcystic adnexal carcinoma is a locally aggressive neoplasm first described by Goldstein et al11 in 1982 an indurated, ill-defined plaque or nodule on the face with a predilection for the upper and lower lip. Prior radiation therapy and immunosuppression are risk factors for the development of MAC.12 Histologically, the superficial portion displays small cornifying cysts interspersed with islands of basaloid cells and may mimic a syringoma. However, the deeper portions demonstrate ducts lined by a single layer of cells with a background of hyalinized and sclerotic stroma. The tumor cells may occupy the deep dermis and underlying subcutis, muscle, or bone and demonstrate an infiltrative growth pattern and perineural invasion. Treatment includes Mohs micrographic surgery.
Desmoplastic trichoepitheliomas most commonly present as solitary white to yellowish annular papules or plaques with a central dell located on sun-exposed areas of the face, cheeks, or chin. This benign neoplasm has a bimodal age distribution, primarily affecting females either in childhood or adulthood.13 Histologically, strands and nests of basaloid epithelial cells proliferate in a dense eosinophilic desmoplastic stroma. The basaloid islands are narrow and cordlike with growth parallel to the surface epidermis and do not dive deeply into the deep dermis or subcutis. Ductal differentiation with associated secretions typically is not seen in DTE.1 Calcifications and foreign body granulomatous infiltrates may be present. Merkel cells also are present in this tumor and may be highlighted by immunohistochemistry with cytokeratin 20.14 Rarely, desmoplastic trichoepitheliomas may transform into trichoblastic carcinomas. Treatment may consist of surgical excision or Mohs micrographic surgery.
Morpheaform BCC also is included in the clinical and histopathologic differential diagnosis of infiltrative basaloid neoplasms. It is one of the more aggressive variants of BCC. The use of immunohistochemical staining may aid in differentiating between these sclerosing adnexal neoplasms.15 For example, pleckstrin homologylike domain family A member 1 (PHLDA1) is a stem cell marker that is heavily expressed in DTE as a specific follicular bulge marker but is not present in a morpheaform BCC. This highlights the follicular nature of DTEs at the molecular level. BerEP4 is a monoclonal antibody that serves as an epithelial marker for 2 glycopolypeptides: 34 and 39 kDa. This antibody may demonstrate positivity in morpheaform BCC but does not stain cells of interest in MAC.
Inflammatory linear verrucous epidermal nevus clinically presents with erythematous and warty papules in a linear distribution following the Blaschko lines. The papules often are reported to be intensely pruritic and usually are localized to one extremity.16 Although adultonset forms of ILVEN have been described,17 it most commonly is diagnosed in young children. Histologically, ILVEN consists of psoriasiform epidermal hyperplasia with alternating areas of parakeratosis and orthokeratosis with underlying agranulosis and hypergranulosis, respectively.18 The upper dermis contains a perivascular lymphocytic infiltrate. Treatment with laser therapy and surgical excision has led to both symptomatic and clinical improvement of ILVEN.16
Plaque-type syringomas are a rare variant of syringomas that clinically may mimic other common inflammatory and neoplastic conditions. An adequate biopsy is imperative to differentiate between adnexal neoplasms, as a small superficial biopsy of a syringoma may demonstrate features observed in other malignant or locally aggressive neoplasms. In our patient, the small ducts lined by cuboidal epithelium with no cellular atypia and no deep dermal growth or perineural invasion allowed for the diagnosis of PTS. Therapeutic options were reviewed with our patient, including oral isotretinoin, laser therapy, and staged excision. Ultimately, our patient elected not to pursue treatment, and she is being monitored clinically for any changes in appearance or symptoms.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma [published online November 12, 2007]. J Cutan Pathol. 2008;35:570-574.
- Furue M, Hori Y, Nakabayashi Y. Clear-cell syringoma. association with diabetes mellitus. Am J Dermatopathol. 1984;6:131-138.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Okuda H, Tei N, Shimizu K, et al. Chondroid syringoma of the scrotum. Int J Urol. 2008;15:944-945.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaquetype syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Mainitz M, Schmidt JB, Gebhart W. Response of multiple syringomas to isotretinoin. Acta Derm Venereol. 1986;66:51-55.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Pujol RM, LeBoit PE, Su WP. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol. 1997;19:358-362.
- Rahman J, Tahir M, Arekemase H, et al. Desmoplastic trichoepithelioma: histopathologic and immunohistochemical criteria for differentiation of a rare benign hair follicle tumor from other cutaneous adnexal tumors. Cureus. 2020;12:E9703.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Gianfaldoni S, Tchernev G, Gianfaldoni R, et al. A case of “inflammatory linear verrucous epidermal nevus” (ILVEN) treated with CO2 laser ablation. Open Access Maced J Med Sci. 2017;5:454-457.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus [published online October 27, 1999]. J Dermatol. 1999;26:599-602.
- Patterson JW, Hosler GA, Prenshaw KL, et al. The psoriasiform reaction pattern. In: Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:99-120.
The Diagnosis: Plaque-type Syringoma
A biopsy demonstrated multiple basaloid islands of tumor cells in the reticular dermis with ductal differentiation, some with a commalike tail. The ducts were lined by 2 to 3 layers of small uniform cuboidal cells without atypia and contained inspissated secretions within the lumina of scattered ducts. There was an associated fibrotic collagenous stroma. There was no evidence of perineural invasion and no deep dermal or subcutaneous extension (Figure 1). Additional cytokeratin immunohistochemical staining highlighted the adnexal proliferation (Figure 2). A diagnosis of plaque-type syringoma (PTS) was made.

Syringomas are benign dermal sweat gland tumors that typically present as flesh-colored papules on the cheeks or periorbital area of young females. Plaque-type tumors as well as papulonodular, eruptive, disseminated, urticaria pigmentosa–like, lichen planus–like, or milialike syringomas also have been reported. Syringomas may be associated with certain medical conditions such as Down syndrome, Nicolau-Balus syndrome, and both scarring and nonscarring alopecias.1 The clear cell variant of syringoma often is associated with diabetes mellitus.2 Kikuchi et al3 first described PTS in 1979. Plaque-type syringomas rarely are reported in the literature, and sites of involvement include the head and neck region, upper lip, chest, upper extremities, vulva, penis, and scrotum.4-6

Histologically, syringomatous lesions are composed of multiple small ducts lined by 2 to 3 layers of cuboidal epithelium. The ducts may be arranged in nests or strands of basaloid cells surrounded by a dense fibrotic stroma. Occasionally, the ducts will form a comma- or teardropshaped tail; however, this also may be observed in desmoplastic trichoepithelioma (DTE).7 Perineural invasion is absent in syringomas. Syringomas exhibit a lateral growth pattern that typically is limited to the upper half of the reticular dermis and spares the underlying subcutis, muscle, and bone. The growth pattern may be discontinuous with proliferations juxtaposed by normal-appearing skin.8 Syringomas usually express progesterone receptors and are known to proliferate at puberty, suggesting that these neoplasms are under hormonal control.9 Although syringomas are benign, various treatment options that may be pursued for cosmetic purposes include radiofrequency, staged excision, laser ablation, and oral isotretinoin.8,10 If only a superficial biopsy is obtained, syringomas may display features of other adnexal neoplasms, including microcystic adnexal carcinoma (MAC), DTE, morpheaform basal cell carcinoma (BCC), and inflammatory linear verrucous epidermal nevus (ILVEN).
Microcystic adnexal carcinoma is a locally aggressive neoplasm first described by Goldstein et al11 in 1982 an indurated, ill-defined plaque or nodule on the face with a predilection for the upper and lower lip. Prior radiation therapy and immunosuppression are risk factors for the development of MAC.12 Histologically, the superficial portion displays small cornifying cysts interspersed with islands of basaloid cells and may mimic a syringoma. However, the deeper portions demonstrate ducts lined by a single layer of cells with a background of hyalinized and sclerotic stroma. The tumor cells may occupy the deep dermis and underlying subcutis, muscle, or bone and demonstrate an infiltrative growth pattern and perineural invasion. Treatment includes Mohs micrographic surgery.
Desmoplastic trichoepitheliomas most commonly present as solitary white to yellowish annular papules or plaques with a central dell located on sun-exposed areas of the face, cheeks, or chin. This benign neoplasm has a bimodal age distribution, primarily affecting females either in childhood or adulthood.13 Histologically, strands and nests of basaloid epithelial cells proliferate in a dense eosinophilic desmoplastic stroma. The basaloid islands are narrow and cordlike with growth parallel to the surface epidermis and do not dive deeply into the deep dermis or subcutis. Ductal differentiation with associated secretions typically is not seen in DTE.1 Calcifications and foreign body granulomatous infiltrates may be present. Merkel cells also are present in this tumor and may be highlighted by immunohistochemistry with cytokeratin 20.14 Rarely, desmoplastic trichoepitheliomas may transform into trichoblastic carcinomas. Treatment may consist of surgical excision or Mohs micrographic surgery.
Morpheaform BCC also is included in the clinical and histopathologic differential diagnosis of infiltrative basaloid neoplasms. It is one of the more aggressive variants of BCC. The use of immunohistochemical staining may aid in differentiating between these sclerosing adnexal neoplasms.15 For example, pleckstrin homologylike domain family A member 1 (PHLDA1) is a stem cell marker that is heavily expressed in DTE as a specific follicular bulge marker but is not present in a morpheaform BCC. This highlights the follicular nature of DTEs at the molecular level. BerEP4 is a monoclonal antibody that serves as an epithelial marker for 2 glycopolypeptides: 34 and 39 kDa. This antibody may demonstrate positivity in morpheaform BCC but does not stain cells of interest in MAC.
Inflammatory linear verrucous epidermal nevus clinically presents with erythematous and warty papules in a linear distribution following the Blaschko lines. The papules often are reported to be intensely pruritic and usually are localized to one extremity.16 Although adultonset forms of ILVEN have been described,17 it most commonly is diagnosed in young children. Histologically, ILVEN consists of psoriasiform epidermal hyperplasia with alternating areas of parakeratosis and orthokeratosis with underlying agranulosis and hypergranulosis, respectively.18 The upper dermis contains a perivascular lymphocytic infiltrate. Treatment with laser therapy and surgical excision has led to both symptomatic and clinical improvement of ILVEN.16
Plaque-type syringomas are a rare variant of syringomas that clinically may mimic other common inflammatory and neoplastic conditions. An adequate biopsy is imperative to differentiate between adnexal neoplasms, as a small superficial biopsy of a syringoma may demonstrate features observed in other malignant or locally aggressive neoplasms. In our patient, the small ducts lined by cuboidal epithelium with no cellular atypia and no deep dermal growth or perineural invasion allowed for the diagnosis of PTS. Therapeutic options were reviewed with our patient, including oral isotretinoin, laser therapy, and staged excision. Ultimately, our patient elected not to pursue treatment, and she is being monitored clinically for any changes in appearance or symptoms.
The Diagnosis: Plaque-type Syringoma
A biopsy demonstrated multiple basaloid islands of tumor cells in the reticular dermis with ductal differentiation, some with a commalike tail. The ducts were lined by 2 to 3 layers of small uniform cuboidal cells without atypia and contained inspissated secretions within the lumina of scattered ducts. There was an associated fibrotic collagenous stroma. There was no evidence of perineural invasion and no deep dermal or subcutaneous extension (Figure 1). Additional cytokeratin immunohistochemical staining highlighted the adnexal proliferation (Figure 2). A diagnosis of plaque-type syringoma (PTS) was made.

Syringomas are benign dermal sweat gland tumors that typically present as flesh-colored papules on the cheeks or periorbital area of young females. Plaque-type tumors as well as papulonodular, eruptive, disseminated, urticaria pigmentosa–like, lichen planus–like, or milialike syringomas also have been reported. Syringomas may be associated with certain medical conditions such as Down syndrome, Nicolau-Balus syndrome, and both scarring and nonscarring alopecias.1 The clear cell variant of syringoma often is associated with diabetes mellitus.2 Kikuchi et al3 first described PTS in 1979. Plaque-type syringomas rarely are reported in the literature, and sites of involvement include the head and neck region, upper lip, chest, upper extremities, vulva, penis, and scrotum.4-6

Histologically, syringomatous lesions are composed of multiple small ducts lined by 2 to 3 layers of cuboidal epithelium. The ducts may be arranged in nests or strands of basaloid cells surrounded by a dense fibrotic stroma. Occasionally, the ducts will form a comma- or teardropshaped tail; however, this also may be observed in desmoplastic trichoepithelioma (DTE).7 Perineural invasion is absent in syringomas. Syringomas exhibit a lateral growth pattern that typically is limited to the upper half of the reticular dermis and spares the underlying subcutis, muscle, and bone. The growth pattern may be discontinuous with proliferations juxtaposed by normal-appearing skin.8 Syringomas usually express progesterone receptors and are known to proliferate at puberty, suggesting that these neoplasms are under hormonal control.9 Although syringomas are benign, various treatment options that may be pursued for cosmetic purposes include radiofrequency, staged excision, laser ablation, and oral isotretinoin.8,10 If only a superficial biopsy is obtained, syringomas may display features of other adnexal neoplasms, including microcystic adnexal carcinoma (MAC), DTE, morpheaform basal cell carcinoma (BCC), and inflammatory linear verrucous epidermal nevus (ILVEN).
Microcystic adnexal carcinoma is a locally aggressive neoplasm first described by Goldstein et al11 in 1982 an indurated, ill-defined plaque or nodule on the face with a predilection for the upper and lower lip. Prior radiation therapy and immunosuppression are risk factors for the development of MAC.12 Histologically, the superficial portion displays small cornifying cysts interspersed with islands of basaloid cells and may mimic a syringoma. However, the deeper portions demonstrate ducts lined by a single layer of cells with a background of hyalinized and sclerotic stroma. The tumor cells may occupy the deep dermis and underlying subcutis, muscle, or bone and demonstrate an infiltrative growth pattern and perineural invasion. Treatment includes Mohs micrographic surgery.
Desmoplastic trichoepitheliomas most commonly present as solitary white to yellowish annular papules or plaques with a central dell located on sun-exposed areas of the face, cheeks, or chin. This benign neoplasm has a bimodal age distribution, primarily affecting females either in childhood or adulthood.13 Histologically, strands and nests of basaloid epithelial cells proliferate in a dense eosinophilic desmoplastic stroma. The basaloid islands are narrow and cordlike with growth parallel to the surface epidermis and do not dive deeply into the deep dermis or subcutis. Ductal differentiation with associated secretions typically is not seen in DTE.1 Calcifications and foreign body granulomatous infiltrates may be present. Merkel cells also are present in this tumor and may be highlighted by immunohistochemistry with cytokeratin 20.14 Rarely, desmoplastic trichoepitheliomas may transform into trichoblastic carcinomas. Treatment may consist of surgical excision or Mohs micrographic surgery.
Morpheaform BCC also is included in the clinical and histopathologic differential diagnosis of infiltrative basaloid neoplasms. It is one of the more aggressive variants of BCC. The use of immunohistochemical staining may aid in differentiating between these sclerosing adnexal neoplasms.15 For example, pleckstrin homologylike domain family A member 1 (PHLDA1) is a stem cell marker that is heavily expressed in DTE as a specific follicular bulge marker but is not present in a morpheaform BCC. This highlights the follicular nature of DTEs at the molecular level. BerEP4 is a monoclonal antibody that serves as an epithelial marker for 2 glycopolypeptides: 34 and 39 kDa. This antibody may demonstrate positivity in morpheaform BCC but does not stain cells of interest in MAC.
Inflammatory linear verrucous epidermal nevus clinically presents with erythematous and warty papules in a linear distribution following the Blaschko lines. The papules often are reported to be intensely pruritic and usually are localized to one extremity.16 Although adultonset forms of ILVEN have been described,17 it most commonly is diagnosed in young children. Histologically, ILVEN consists of psoriasiform epidermal hyperplasia with alternating areas of parakeratosis and orthokeratosis with underlying agranulosis and hypergranulosis, respectively.18 The upper dermis contains a perivascular lymphocytic infiltrate. Treatment with laser therapy and surgical excision has led to both symptomatic and clinical improvement of ILVEN.16
Plaque-type syringomas are a rare variant of syringomas that clinically may mimic other common inflammatory and neoplastic conditions. An adequate biopsy is imperative to differentiate between adnexal neoplasms, as a small superficial biopsy of a syringoma may demonstrate features observed in other malignant or locally aggressive neoplasms. In our patient, the small ducts lined by cuboidal epithelium with no cellular atypia and no deep dermal growth or perineural invasion allowed for the diagnosis of PTS. Therapeutic options were reviewed with our patient, including oral isotretinoin, laser therapy, and staged excision. Ultimately, our patient elected not to pursue treatment, and she is being monitored clinically for any changes in appearance or symptoms.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma [published online November 12, 2007]. J Cutan Pathol. 2008;35:570-574.
- Furue M, Hori Y, Nakabayashi Y. Clear-cell syringoma. association with diabetes mellitus. Am J Dermatopathol. 1984;6:131-138.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Okuda H, Tei N, Shimizu K, et al. Chondroid syringoma of the scrotum. Int J Urol. 2008;15:944-945.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaquetype syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Mainitz M, Schmidt JB, Gebhart W. Response of multiple syringomas to isotretinoin. Acta Derm Venereol. 1986;66:51-55.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Pujol RM, LeBoit PE, Su WP. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol. 1997;19:358-362.
- Rahman J, Tahir M, Arekemase H, et al. Desmoplastic trichoepithelioma: histopathologic and immunohistochemical criteria for differentiation of a rare benign hair follicle tumor from other cutaneous adnexal tumors. Cureus. 2020;12:E9703.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Gianfaldoni S, Tchernev G, Gianfaldoni R, et al. A case of “inflammatory linear verrucous epidermal nevus” (ILVEN) treated with CO2 laser ablation. Open Access Maced J Med Sci. 2017;5:454-457.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus [published online October 27, 1999]. J Dermatol. 1999;26:599-602.
- Patterson JW, Hosler GA, Prenshaw KL, et al. The psoriasiform reaction pattern. In: Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:99-120.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma [published online November 12, 2007]. J Cutan Pathol. 2008;35:570-574.
- Furue M, Hori Y, Nakabayashi Y. Clear-cell syringoma. association with diabetes mellitus. Am J Dermatopathol. 1984;6:131-138.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Okuda H, Tei N, Shimizu K, et al. Chondroid syringoma of the scrotum. Int J Urol. 2008;15:944-945.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaquetype syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Mainitz M, Schmidt JB, Gebhart W. Response of multiple syringomas to isotretinoin. Acta Derm Venereol. 1986;66:51-55.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Pujol RM, LeBoit PE, Su WP. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol. 1997;19:358-362.
- Rahman J, Tahir M, Arekemase H, et al. Desmoplastic trichoepithelioma: histopathologic and immunohistochemical criteria for differentiation of a rare benign hair follicle tumor from other cutaneous adnexal tumors. Cureus. 2020;12:E9703.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Gianfaldoni S, Tchernev G, Gianfaldoni R, et al. A case of “inflammatory linear verrucous epidermal nevus” (ILVEN) treated with CO2 laser ablation. Open Access Maced J Med Sci. 2017;5:454-457.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus [published online October 27, 1999]. J Dermatol. 1999;26:599-602.
- Patterson JW, Hosler GA, Prenshaw KL, et al. The psoriasiform reaction pattern. In: Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:99-120.
A 17-year-old adolescent girl presented with a solitary, 8-cm, pink plaque on the anterior aspect of the neck of 5 years’ duration. No similar skin findings were present elsewhere on the body. The rash was not painful or pruritic, and she denied prior trauma to the site. The patient previously had tried a salicylic acid bodywash as well as mupirocin cream 2% and mometasone ointment with no improvement. Her medical history was unremarkable, and she had no known allergies. There was no family history of a similar rash. Physical examination revealed no palpable subcutaneous lumps or masses and no lymphadenopathy of the head or neck. An incisional biopsy was performed.
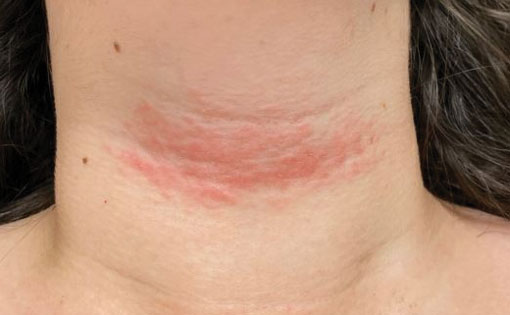
Gout flares linked to transient jump in MI, stroke risk
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
FROM JAMA
Taste dysfunction in head and neck cancer due to radiation dose
finds a new study from JAMA Otolaryngology–Head & Neck Surgery.
Taste dysfunction can affect up to 90% of patients undergoing radiotherapy for head and neck cancer. While the ability to taste usually returns after the treatment concludes, some patients can still feel the lingering effects of radiotherapy on taste function long after the treatment concludes. It can lead to weight loss and dry mouth which can, in turn, negatively affect quality of life.
“Taste dysfunction has profound effects on quality of life in patients with head and neck cancer, and the oral cavity dose could be significantly lower with modern radiotherapy techniques,” wrote the researchers, who were led by Miao-Fen Chen, MD, PhD, of Chang Gung University, Taoyuan City, Taiwan. “This study provides useful dose constraints of the oral cavity that may be associated with reduced taste dysfunction.”
Degradation of taste is an important quality of life factor for head and neck cancer patients. A 2021 systematic review published in the journal Radiotherapy and Oncology found that acute taste dysfunction affected 96% of patients as measured objectively, and 79% as measured subjectively. While most patients recover an estimated 23-53% of patients experience long-term dysfunction.
In 2019, a study published in the journal Chemical Senses found that 31% of head and neck cancer patients had long-term changes to taste at 27 months after intensity-modulated radiotherapy (IMRT), with dysfunction associated with glossectomy and oral cavity radiation doses greater than 50 Gy, but the study only used one quality of life subjective measure to evaluate taste function.
In the new JAMA study, researchers reported the results of a longitudinal using the whole-mouth solution method for basic tastes, including salt, sweet, sour, and bitter.
Study methodology
The study included 87 patients (mean age, 58 years; 90% men) who were enrolled between 2017 and 2020 from a single hospital. 45 patients received primary intensity-modulated radiotherapy and 42 received postoperative radiotherapy. 78 patients received volumetric arc therapy, and 9 received intensity-modulated radiotherapy. The radiotherapy was directed to minimize the effect on the parotid glands and oral cavity.
Researchers measured taste dysfunction according to detection thresholds based on solutions with different concentrations. After moving the solution around the mouth and spitting it out, patients were asked to identify taste components. Following a water rinse, they tested a solution with another concentration of taste components. A number was assigned based on the concentration level they were able to detect, with nigher numbers indicating greater sensitivity.
Two to four weeks after initiation of radiotherapy, there were drops in taste scores for salt (4.7 to 1.4), sweet (4.2 to 1.8), sour (4.5 to 2.3), and bitter (4.7 to 1.2). 1 week after radiotherapy, those mean scores increased to 2.6, 2.6, 2.9, and 2.3 respectively. Over the following 3 months, mean scores reflected general recovery to near preradiotherapy levels (4.2, 3.9, 4.1, and 4.0, respectively). At 6 months and 1 year, the scores were equivalent to preradiotherapy levels.
Objective taste tests were performed on 81 participants. 33.3% had taste dysfunction 6 months after radiotherapy. 6 months after, 8.9% had taste dysfunction. At 3 months following radiotherapy, taste dysfunction was associated with an oral cavity mean dose of 4,000 cGy or higher (relative risk, 2.87; 95% confidence interval, 1.21-6.81) or 5,000 cGy or higher (RR, 2.04; 95% CI, 1.12-3.72). At 6 months, taste dysfunction was predicted by glossectomy (RR, 5.63; 95% CI, 1.12-28.15) and oral cavity mean dose 5,000 cGy or greater (RR, 7.79; 95% CI, 0.93-64.92).
The researchers quantified the relationship between mean oral cavity dose and probability of developing taste dysfunction at 3 and 6 months. 3 months after radiotherapy, 25 Gy predicted a 15% chance, 38 Gy predicted a 25% chance, and 60 Gy predicted a 50% chance. At 6 months, the numbers were 57, 60, and 64 Gy.
The study was limited by being conducted at a single center and its small sample size, and it recruited patients varied significantly in treatment modality and disease subtype.
finds a new study from JAMA Otolaryngology–Head & Neck Surgery.
Taste dysfunction can affect up to 90% of patients undergoing radiotherapy for head and neck cancer. While the ability to taste usually returns after the treatment concludes, some patients can still feel the lingering effects of radiotherapy on taste function long after the treatment concludes. It can lead to weight loss and dry mouth which can, in turn, negatively affect quality of life.
“Taste dysfunction has profound effects on quality of life in patients with head and neck cancer, and the oral cavity dose could be significantly lower with modern radiotherapy techniques,” wrote the researchers, who were led by Miao-Fen Chen, MD, PhD, of Chang Gung University, Taoyuan City, Taiwan. “This study provides useful dose constraints of the oral cavity that may be associated with reduced taste dysfunction.”
Degradation of taste is an important quality of life factor for head and neck cancer patients. A 2021 systematic review published in the journal Radiotherapy and Oncology found that acute taste dysfunction affected 96% of patients as measured objectively, and 79% as measured subjectively. While most patients recover an estimated 23-53% of patients experience long-term dysfunction.
In 2019, a study published in the journal Chemical Senses found that 31% of head and neck cancer patients had long-term changes to taste at 27 months after intensity-modulated radiotherapy (IMRT), with dysfunction associated with glossectomy and oral cavity radiation doses greater than 50 Gy, but the study only used one quality of life subjective measure to evaluate taste function.
In the new JAMA study, researchers reported the results of a longitudinal using the whole-mouth solution method for basic tastes, including salt, sweet, sour, and bitter.
Study methodology
The study included 87 patients (mean age, 58 years; 90% men) who were enrolled between 2017 and 2020 from a single hospital. 45 patients received primary intensity-modulated radiotherapy and 42 received postoperative radiotherapy. 78 patients received volumetric arc therapy, and 9 received intensity-modulated radiotherapy. The radiotherapy was directed to minimize the effect on the parotid glands and oral cavity.
Researchers measured taste dysfunction according to detection thresholds based on solutions with different concentrations. After moving the solution around the mouth and spitting it out, patients were asked to identify taste components. Following a water rinse, they tested a solution with another concentration of taste components. A number was assigned based on the concentration level they were able to detect, with nigher numbers indicating greater sensitivity.
Two to four weeks after initiation of radiotherapy, there were drops in taste scores for salt (4.7 to 1.4), sweet (4.2 to 1.8), sour (4.5 to 2.3), and bitter (4.7 to 1.2). 1 week after radiotherapy, those mean scores increased to 2.6, 2.6, 2.9, and 2.3 respectively. Over the following 3 months, mean scores reflected general recovery to near preradiotherapy levels (4.2, 3.9, 4.1, and 4.0, respectively). At 6 months and 1 year, the scores were equivalent to preradiotherapy levels.
Objective taste tests were performed on 81 participants. 33.3% had taste dysfunction 6 months after radiotherapy. 6 months after, 8.9% had taste dysfunction. At 3 months following radiotherapy, taste dysfunction was associated with an oral cavity mean dose of 4,000 cGy or higher (relative risk, 2.87; 95% confidence interval, 1.21-6.81) or 5,000 cGy or higher (RR, 2.04; 95% CI, 1.12-3.72). At 6 months, taste dysfunction was predicted by glossectomy (RR, 5.63; 95% CI, 1.12-28.15) and oral cavity mean dose 5,000 cGy or greater (RR, 7.79; 95% CI, 0.93-64.92).
The researchers quantified the relationship between mean oral cavity dose and probability of developing taste dysfunction at 3 and 6 months. 3 months after radiotherapy, 25 Gy predicted a 15% chance, 38 Gy predicted a 25% chance, and 60 Gy predicted a 50% chance. At 6 months, the numbers were 57, 60, and 64 Gy.
The study was limited by being conducted at a single center and its small sample size, and it recruited patients varied significantly in treatment modality and disease subtype.
finds a new study from JAMA Otolaryngology–Head & Neck Surgery.
Taste dysfunction can affect up to 90% of patients undergoing radiotherapy for head and neck cancer. While the ability to taste usually returns after the treatment concludes, some patients can still feel the lingering effects of radiotherapy on taste function long after the treatment concludes. It can lead to weight loss and dry mouth which can, in turn, negatively affect quality of life.
“Taste dysfunction has profound effects on quality of life in patients with head and neck cancer, and the oral cavity dose could be significantly lower with modern radiotherapy techniques,” wrote the researchers, who were led by Miao-Fen Chen, MD, PhD, of Chang Gung University, Taoyuan City, Taiwan. “This study provides useful dose constraints of the oral cavity that may be associated with reduced taste dysfunction.”
Degradation of taste is an important quality of life factor for head and neck cancer patients. A 2021 systematic review published in the journal Radiotherapy and Oncology found that acute taste dysfunction affected 96% of patients as measured objectively, and 79% as measured subjectively. While most patients recover an estimated 23-53% of patients experience long-term dysfunction.
In 2019, a study published in the journal Chemical Senses found that 31% of head and neck cancer patients had long-term changes to taste at 27 months after intensity-modulated radiotherapy (IMRT), with dysfunction associated with glossectomy and oral cavity radiation doses greater than 50 Gy, but the study only used one quality of life subjective measure to evaluate taste function.
In the new JAMA study, researchers reported the results of a longitudinal using the whole-mouth solution method for basic tastes, including salt, sweet, sour, and bitter.
Study methodology
The study included 87 patients (mean age, 58 years; 90% men) who were enrolled between 2017 and 2020 from a single hospital. 45 patients received primary intensity-modulated radiotherapy and 42 received postoperative radiotherapy. 78 patients received volumetric arc therapy, and 9 received intensity-modulated radiotherapy. The radiotherapy was directed to minimize the effect on the parotid glands and oral cavity.
Researchers measured taste dysfunction according to detection thresholds based on solutions with different concentrations. After moving the solution around the mouth and spitting it out, patients were asked to identify taste components. Following a water rinse, they tested a solution with another concentration of taste components. A number was assigned based on the concentration level they were able to detect, with nigher numbers indicating greater sensitivity.
Two to four weeks after initiation of radiotherapy, there were drops in taste scores for salt (4.7 to 1.4), sweet (4.2 to 1.8), sour (4.5 to 2.3), and bitter (4.7 to 1.2). 1 week after radiotherapy, those mean scores increased to 2.6, 2.6, 2.9, and 2.3 respectively. Over the following 3 months, mean scores reflected general recovery to near preradiotherapy levels (4.2, 3.9, 4.1, and 4.0, respectively). At 6 months and 1 year, the scores were equivalent to preradiotherapy levels.
Objective taste tests were performed on 81 participants. 33.3% had taste dysfunction 6 months after radiotherapy. 6 months after, 8.9% had taste dysfunction. At 3 months following radiotherapy, taste dysfunction was associated with an oral cavity mean dose of 4,000 cGy or higher (relative risk, 2.87; 95% confidence interval, 1.21-6.81) or 5,000 cGy or higher (RR, 2.04; 95% CI, 1.12-3.72). At 6 months, taste dysfunction was predicted by glossectomy (RR, 5.63; 95% CI, 1.12-28.15) and oral cavity mean dose 5,000 cGy or greater (RR, 7.79; 95% CI, 0.93-64.92).
The researchers quantified the relationship between mean oral cavity dose and probability of developing taste dysfunction at 3 and 6 months. 3 months after radiotherapy, 25 Gy predicted a 15% chance, 38 Gy predicted a 25% chance, and 60 Gy predicted a 50% chance. At 6 months, the numbers were 57, 60, and 64 Gy.
The study was limited by being conducted at a single center and its small sample size, and it recruited patients varied significantly in treatment modality and disease subtype.
FROM JAMA OTOLARYNGOLOGY–HEAD AND NECK SURGERY
Ustekinumab becomes second biologic approved for PsA in kids
The Food and Drug Administration has approved the dual interleukin-12 and IL-23 inhibitor ustekinumab (Stelara) for the treatment of juvenile psoriatic arthritis (jPsA) in patients aged 6 years and older, according to an Aug. 1 announcement from its manufacturer, Janssen.
The approval makes jPsA the sixth approved indication for ustekinumab, which include active psoriatic arthritis in adults, moderate to severe plaque psoriasis in both adults and children aged 6 years or older who are candidates for phototherapy or systemic therapy, moderately to severely active Crohn’s disease in adults, and moderately to severely active ulcerative colitis in adults.
In addition, ustekinumab is now the second biologic to be approved for jPsA, following the agency’s December 2021 approval of secukinumab (Cosentyx) to treat jPsA in children and adolescents aged 2 years and older as well as enthesitis-related arthritis in children and adolescents aged 4 years and older.
In pediatric patients, ustekinumab is administered as a subcutaneous injection dosed four times per year after two starter doses.
Ustekinumab’s approval is based on “an extrapolation of the established data and existing safety profile” of ustekinumab in multiple phase 3 studies in adult and pediatric patients with moderate to severe plaque psoriasis and adult patients with active PsA, according to Janssen.
“With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population,” according to the October 2021 announcement from the company that the Biologics License Application had been submitted to the FDA.
Juvenile arthritis occurs in an estimated 20-45 children per 100,000 in the United States, with about 5% of those children having jPsA, according to the National Psoriasis Foundation.
The prescribing information for ustekinumab includes specific warnings and areas of concern. The drug should not be administered to individuals with known hypersensitivity to ustekinumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, sometimes serious, and a test for tuberculosis infection should be given before administration.
Patients taking ustekinumab should not be given a live vaccine, and their doctors should be informed if anyone in their household needs a live vaccine. They also should not receive the BCG vaccine during the 1 year before receiving the drug or 1 year after they stop taking it, according to Johnson & Johnson.
The most common adverse effects include nasal congestion, sore throat, runny nose, upper respiratory infections, fever, headache, tiredness, itching, nausea and vomiting, redness at the injection site, vaginal yeast infections, urinary tract infections, sinus infection, bronchitis, diarrhea, stomach pain, and joint pain.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the dual interleukin-12 and IL-23 inhibitor ustekinumab (Stelara) for the treatment of juvenile psoriatic arthritis (jPsA) in patients aged 6 years and older, according to an Aug. 1 announcement from its manufacturer, Janssen.
The approval makes jPsA the sixth approved indication for ustekinumab, which include active psoriatic arthritis in adults, moderate to severe plaque psoriasis in both adults and children aged 6 years or older who are candidates for phototherapy or systemic therapy, moderately to severely active Crohn’s disease in adults, and moderately to severely active ulcerative colitis in adults.
In addition, ustekinumab is now the second biologic to be approved for jPsA, following the agency’s December 2021 approval of secukinumab (Cosentyx) to treat jPsA in children and adolescents aged 2 years and older as well as enthesitis-related arthritis in children and adolescents aged 4 years and older.
In pediatric patients, ustekinumab is administered as a subcutaneous injection dosed four times per year after two starter doses.
Ustekinumab’s approval is based on “an extrapolation of the established data and existing safety profile” of ustekinumab in multiple phase 3 studies in adult and pediatric patients with moderate to severe plaque psoriasis and adult patients with active PsA, according to Janssen.
“With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population,” according to the October 2021 announcement from the company that the Biologics License Application had been submitted to the FDA.
Juvenile arthritis occurs in an estimated 20-45 children per 100,000 in the United States, with about 5% of those children having jPsA, according to the National Psoriasis Foundation.
The prescribing information for ustekinumab includes specific warnings and areas of concern. The drug should not be administered to individuals with known hypersensitivity to ustekinumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, sometimes serious, and a test for tuberculosis infection should be given before administration.
Patients taking ustekinumab should not be given a live vaccine, and their doctors should be informed if anyone in their household needs a live vaccine. They also should not receive the BCG vaccine during the 1 year before receiving the drug or 1 year after they stop taking it, according to Johnson & Johnson.
The most common adverse effects include nasal congestion, sore throat, runny nose, upper respiratory infections, fever, headache, tiredness, itching, nausea and vomiting, redness at the injection site, vaginal yeast infections, urinary tract infections, sinus infection, bronchitis, diarrhea, stomach pain, and joint pain.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the dual interleukin-12 and IL-23 inhibitor ustekinumab (Stelara) for the treatment of juvenile psoriatic arthritis (jPsA) in patients aged 6 years and older, according to an Aug. 1 announcement from its manufacturer, Janssen.
The approval makes jPsA the sixth approved indication for ustekinumab, which include active psoriatic arthritis in adults, moderate to severe plaque psoriasis in both adults and children aged 6 years or older who are candidates for phototherapy or systemic therapy, moderately to severely active Crohn’s disease in adults, and moderately to severely active ulcerative colitis in adults.
In addition, ustekinumab is now the second biologic to be approved for jPsA, following the agency’s December 2021 approval of secukinumab (Cosentyx) to treat jPsA in children and adolescents aged 2 years and older as well as enthesitis-related arthritis in children and adolescents aged 4 years and older.
In pediatric patients, ustekinumab is administered as a subcutaneous injection dosed four times per year after two starter doses.
Ustekinumab’s approval is based on “an extrapolation of the established data and existing safety profile” of ustekinumab in multiple phase 3 studies in adult and pediatric patients with moderate to severe plaque psoriasis and adult patients with active PsA, according to Janssen.
“With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population,” according to the October 2021 announcement from the company that the Biologics License Application had been submitted to the FDA.
Juvenile arthritis occurs in an estimated 20-45 children per 100,000 in the United States, with about 5% of those children having jPsA, according to the National Psoriasis Foundation.
The prescribing information for ustekinumab includes specific warnings and areas of concern. The drug should not be administered to individuals with known hypersensitivity to ustekinumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, sometimes serious, and a test for tuberculosis infection should be given before administration.
Patients taking ustekinumab should not be given a live vaccine, and their doctors should be informed if anyone in their household needs a live vaccine. They also should not receive the BCG vaccine during the 1 year before receiving the drug or 1 year after they stop taking it, according to Johnson & Johnson.
The most common adverse effects include nasal congestion, sore throat, runny nose, upper respiratory infections, fever, headache, tiredness, itching, nausea and vomiting, redness at the injection site, vaginal yeast infections, urinary tract infections, sinus infection, bronchitis, diarrhea, stomach pain, and joint pain.
A version of this article first appeared on Medscape.com.
Commentary: Treating Gastric Cancer Subtypes, August 2022
Patients with stage II or III gastric cancer are treated with surgical resection and perioperative chemotherapy. Platinum agents have established activity in this disease. Combination chemotherapy FLOT (5-fluorouracil, oxaliplatin, and docetaxel) is now standard perioperative treatment for resectable gastric cancer.1 The study by Slagter and colleagues evaluated whether cisplatin was noninferior to oxaliplatin when used in the treatment of early-stage gastric cancer. Prior to the incorporation of FLOT into standard treatment practice, patients were treated with ECX (epirubicin, cisplatin, and docetaxel), as per the MAGIC trial.2 In the metastatic setting, chemotherapy regimens with either cisplatin or oxaliplatin as a choice of platinum agent have comparable activity against these tumors. Oxaliplatin activity has been shown to be noninferior to cisplatin in the randomized REAL2 trial in metastatic setting.3
The study by Slagter and colleagues is a post hoc analysis of 781 patients with resectable gastric cancer who were enrolled in the CRITICS trial. This analysis demonstrated that chemotherapy regimens containing oxaliplatin and cisplatin had comparable 5-year overall survival rates. Not surprisingly, oxaliplatin was associated with higher neurotoxicity. Based on this analysis, it is likely safe to conclude that, just as in the advanced setting, cisplatin and oxaliplatin have similar activity in early-stage disease.
Mismatch repair protein deficient or microsatellite unstable gastric cancer (MSI-H) represent unique subtypes of gastric cancer, with distinct biologic behaviors and treatment responses. The efficacy of chemotherapy in patients with early-stage MSI-H tumors has been questioned previously. Similar to MSI-H colorectal cancers, the benefit of chemotherapy in resectable MSI-H gastric and esophagogastric junction tumors appears to be less robust than in microsatellite stable (MSS) tumors. In the exploratory analysis of patients with MSI-H tumors enrolled in the perioperative MAGIC trial, patients with MSI-H tumors had better prognosis when treated with surgery alone and potentially experienced detrimental effects from chemotherapy.4 The retrospective analysis by Vos and colleagues adds to the body of knowledge about early-stage MSI-H gastric cancers. They evaluated 535 patients with early-stage disease who were treated with surgery alone or surgery plus perioperative therapy between 2000 and 2018. The overall survival in 82 patients with MSI-H tumors was 20% better than in those with MSS disease. This favorable outcome was seen irrespective of whether chemotherapy was given. Though these results suggest that chemotherapy may not be necessary in the treatment of these tumors and there are emerging data regarding the activity of immune checkpoint inhibitors in this setting, these results should definitely be investigated further in prospective studies.5 However, in the absence of prospective randomized data, it is difficult to recommend deviating from the established standard of care with FLOT, especially for patients undergoing curative intent treatment.
A study by Yukami and colleagues evaluated whether the presence of liver metastasis, which have been shown to be enriched in immunosuppressive cells in the preclinical setting, had any bearing on the activity of immune checkpoint inhibitors alone or in combination with multi-tyrosine kinase inhibitors. The analysis included 54 patients enrolled in a phase 1b trial of REGONIVO (regorafenib and nivolumab) and a phase 2 trial of LENPEM (lenvatinib and pembrolizumab). With a median follow up of 14 months, there was no significant difference in the efficacy of the above regimens (overall survival, progression-free survival, and objective response rate) between patients with and without liver metastasis. The promising activity of these combinations is continuing with longer follow-up. The above regimens should be investigated further in larger prospective studies irrespective of metastatic sites.
Additional References
1. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. Doi: 10.1016/S0140-6736(18)32557-1
2. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. Doi: 10.1056/NEJMoa055531
3. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. Doi: 10.1056/NEJMoa073149
4. Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3:1197-1203. Doi: 10.1001/jamaoncol.2016.6762
5. Andre T, Tougeron D, Piessen G, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in patients (pts) with localized microsatellite instability-high (MSI)/mismatch repair deficient (dMMR) oeso-gastric adenocarcinoma (OGA): the GERCOR NEONIPIGA phase II study. J Clin Oncol. 2022;40:244-244. Doi: 10.1200/JCO.2022.40.4_suppl.244
Patients with stage II or III gastric cancer are treated with surgical resection and perioperative chemotherapy. Platinum agents have established activity in this disease. Combination chemotherapy FLOT (5-fluorouracil, oxaliplatin, and docetaxel) is now standard perioperative treatment for resectable gastric cancer.1 The study by Slagter and colleagues evaluated whether cisplatin was noninferior to oxaliplatin when used in the treatment of early-stage gastric cancer. Prior to the incorporation of FLOT into standard treatment practice, patients were treated with ECX (epirubicin, cisplatin, and docetaxel), as per the MAGIC trial.2 In the metastatic setting, chemotherapy regimens with either cisplatin or oxaliplatin as a choice of platinum agent have comparable activity against these tumors. Oxaliplatin activity has been shown to be noninferior to cisplatin in the randomized REAL2 trial in metastatic setting.3
The study by Slagter and colleagues is a post hoc analysis of 781 patients with resectable gastric cancer who were enrolled in the CRITICS trial. This analysis demonstrated that chemotherapy regimens containing oxaliplatin and cisplatin had comparable 5-year overall survival rates. Not surprisingly, oxaliplatin was associated with higher neurotoxicity. Based on this analysis, it is likely safe to conclude that, just as in the advanced setting, cisplatin and oxaliplatin have similar activity in early-stage disease.
Mismatch repair protein deficient or microsatellite unstable gastric cancer (MSI-H) represent unique subtypes of gastric cancer, with distinct biologic behaviors and treatment responses. The efficacy of chemotherapy in patients with early-stage MSI-H tumors has been questioned previously. Similar to MSI-H colorectal cancers, the benefit of chemotherapy in resectable MSI-H gastric and esophagogastric junction tumors appears to be less robust than in microsatellite stable (MSS) tumors. In the exploratory analysis of patients with MSI-H tumors enrolled in the perioperative MAGIC trial, patients with MSI-H tumors had better prognosis when treated with surgery alone and potentially experienced detrimental effects from chemotherapy.4 The retrospective analysis by Vos and colleagues adds to the body of knowledge about early-stage MSI-H gastric cancers. They evaluated 535 patients with early-stage disease who were treated with surgery alone or surgery plus perioperative therapy between 2000 and 2018. The overall survival in 82 patients with MSI-H tumors was 20% better than in those with MSS disease. This favorable outcome was seen irrespective of whether chemotherapy was given. Though these results suggest that chemotherapy may not be necessary in the treatment of these tumors and there are emerging data regarding the activity of immune checkpoint inhibitors in this setting, these results should definitely be investigated further in prospective studies.5 However, in the absence of prospective randomized data, it is difficult to recommend deviating from the established standard of care with FLOT, especially for patients undergoing curative intent treatment.
A study by Yukami and colleagues evaluated whether the presence of liver metastasis, which have been shown to be enriched in immunosuppressive cells in the preclinical setting, had any bearing on the activity of immune checkpoint inhibitors alone or in combination with multi-tyrosine kinase inhibitors. The analysis included 54 patients enrolled in a phase 1b trial of REGONIVO (regorafenib and nivolumab) and a phase 2 trial of LENPEM (lenvatinib and pembrolizumab). With a median follow up of 14 months, there was no significant difference in the efficacy of the above regimens (overall survival, progression-free survival, and objective response rate) between patients with and without liver metastasis. The promising activity of these combinations is continuing with longer follow-up. The above regimens should be investigated further in larger prospective studies irrespective of metastatic sites.
Additional References
1. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. Doi: 10.1016/S0140-6736(18)32557-1
2. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. Doi: 10.1056/NEJMoa055531
3. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. Doi: 10.1056/NEJMoa073149
4. Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3:1197-1203. Doi: 10.1001/jamaoncol.2016.6762
5. Andre T, Tougeron D, Piessen G, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in patients (pts) with localized microsatellite instability-high (MSI)/mismatch repair deficient (dMMR) oeso-gastric adenocarcinoma (OGA): the GERCOR NEONIPIGA phase II study. J Clin Oncol. 2022;40:244-244. Doi: 10.1200/JCO.2022.40.4_suppl.244
Patients with stage II or III gastric cancer are treated with surgical resection and perioperative chemotherapy. Platinum agents have established activity in this disease. Combination chemotherapy FLOT (5-fluorouracil, oxaliplatin, and docetaxel) is now standard perioperative treatment for resectable gastric cancer.1 The study by Slagter and colleagues evaluated whether cisplatin was noninferior to oxaliplatin when used in the treatment of early-stage gastric cancer. Prior to the incorporation of FLOT into standard treatment practice, patients were treated with ECX (epirubicin, cisplatin, and docetaxel), as per the MAGIC trial.2 In the metastatic setting, chemotherapy regimens with either cisplatin or oxaliplatin as a choice of platinum agent have comparable activity against these tumors. Oxaliplatin activity has been shown to be noninferior to cisplatin in the randomized REAL2 trial in metastatic setting.3
The study by Slagter and colleagues is a post hoc analysis of 781 patients with resectable gastric cancer who were enrolled in the CRITICS trial. This analysis demonstrated that chemotherapy regimens containing oxaliplatin and cisplatin had comparable 5-year overall survival rates. Not surprisingly, oxaliplatin was associated with higher neurotoxicity. Based on this analysis, it is likely safe to conclude that, just as in the advanced setting, cisplatin and oxaliplatin have similar activity in early-stage disease.
Mismatch repair protein deficient or microsatellite unstable gastric cancer (MSI-H) represent unique subtypes of gastric cancer, with distinct biologic behaviors and treatment responses. The efficacy of chemotherapy in patients with early-stage MSI-H tumors has been questioned previously. Similar to MSI-H colorectal cancers, the benefit of chemotherapy in resectable MSI-H gastric and esophagogastric junction tumors appears to be less robust than in microsatellite stable (MSS) tumors. In the exploratory analysis of patients with MSI-H tumors enrolled in the perioperative MAGIC trial, patients with MSI-H tumors had better prognosis when treated with surgery alone and potentially experienced detrimental effects from chemotherapy.4 The retrospective analysis by Vos and colleagues adds to the body of knowledge about early-stage MSI-H gastric cancers. They evaluated 535 patients with early-stage disease who were treated with surgery alone or surgery plus perioperative therapy between 2000 and 2018. The overall survival in 82 patients with MSI-H tumors was 20% better than in those with MSS disease. This favorable outcome was seen irrespective of whether chemotherapy was given. Though these results suggest that chemotherapy may not be necessary in the treatment of these tumors and there are emerging data regarding the activity of immune checkpoint inhibitors in this setting, these results should definitely be investigated further in prospective studies.5 However, in the absence of prospective randomized data, it is difficult to recommend deviating from the established standard of care with FLOT, especially for patients undergoing curative intent treatment.
A study by Yukami and colleagues evaluated whether the presence of liver metastasis, which have been shown to be enriched in immunosuppressive cells in the preclinical setting, had any bearing on the activity of immune checkpoint inhibitors alone or in combination with multi-tyrosine kinase inhibitors. The analysis included 54 patients enrolled in a phase 1b trial of REGONIVO (regorafenib and nivolumab) and a phase 2 trial of LENPEM (lenvatinib and pembrolizumab). With a median follow up of 14 months, there was no significant difference in the efficacy of the above regimens (overall survival, progression-free survival, and objective response rate) between patients with and without liver metastasis. The promising activity of these combinations is continuing with longer follow-up. The above regimens should be investigated further in larger prospective studies irrespective of metastatic sites.
Additional References
1. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. Doi: 10.1016/S0140-6736(18)32557-1
2. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. Doi: 10.1056/NEJMoa055531
3. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. Doi: 10.1056/NEJMoa073149
4. Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. JAMA Oncol. 2017;3:1197-1203. Doi: 10.1001/jamaoncol.2016.6762
5. Andre T, Tougeron D, Piessen G, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in patients (pts) with localized microsatellite instability-high (MSI)/mismatch repair deficient (dMMR) oeso-gastric adenocarcinoma (OGA): the GERCOR NEONIPIGA phase II study. J Clin Oncol. 2022;40:244-244. Doi: 10.1200/JCO.2022.40.4_suppl.244
Commentary: Diabetes Drug Comparisons, August 2022
Meta-analyses of sodium-glucose cotransporter 2 inhibitor (SGLT2i) outcome trials have shown reductions in all-cause and cardiovascular mortality, but dipeptidyl peptidase 4 inhibitors (DPP4i) have been neutral for these outcomes. In a Taiwanese retrospective cohort study, Chung and colleagues compared 53,264 pairs of propensity-matched patients with type 2 diabetes who were treated with either an SGLT2i or DPP4i. They not only reported relative risk reductions of 34% and 32% for all-cause death and cardiovascular death, respectively, but also a reduction in cancer death of 27% and a reduction in noncancer, noncardiovascular death of 38%. Although limited by its retrospective, observational design, the finding of a benefit of SGLT2i treatment on both cancer death and noncancer, noncardiovascular death would benefit from further research.
In another large Taiwanese retrospective cohort study with propensity matching, Chan and colleagues compared patients treated with SGLT2i, DPP4i, and glucagon-like peptide 1 receptor agonists (GLP-1RA), with a main study outcome of new-onset atrial fibrillation (AF). They noted that SGLT2i treatment was associated with a 10% and 36% lower risk for AF compared with DPP4i and GLP-1RA treatment, respectively. These results are consistent with meta-analyses of SGLT2i outcome trials that have demonstrated reductions in AF with SGLT2i vs placebo. Perhaps it is time to recognize that another clinical benefit of the SGLT2i class is the reduction in risk for AF.
Although GLP-1RA have been linked to an increased risk for gallbladder-related events in many studies, there has been little data suggesting an increased risk with DPP4i. He and colleagues have published a pairwise meta-analysis of 82 randomized clinical trials and found that DPP4i compared with placebo or nonincretin drugs increased the risk for gallbladder or biliary diseases by 1.22-fold, with 11 more events per 10,000 person years. In a network meta-analysis of 184 randomized trials, they also found that DPP4i treatment increased the risk for gallbladder or biliary diseases compared with SGLT2i but not compared with GLP-1RA. This was the first meta-analysis to systematically study the association between DPP4i and gallbladder-related diseases. Although the absolute risk is small, clinicians need to be aware of this link and consider this adverse effect when deciding about the risks vs benefits of DPP4i treatment.
Individuals with obesity and prediabetes are at greater risk for type 2 diabetes. Trials of lifestyle modification and antiobesity agents have shown that restoration to normoglycemia can occur with weight loss. Phase 3A studies of the antiobesity agent semaglutide (2.4 mg/week) included 3375 individuals with prediabetes across three trials. In a post hoc analysis of these patients with prediabetes, Perreault and colleagues found that after 68 weeks of treatment, there was a much higher likelihood of normoglycemia with 2.4 mg/week of semaglutide compared with placebo. Though definite conclusions are limited owing to the post hoc nature of this analysis, these results make it very likely that the ongoing STEP 10 trial of 2.4 mg semaglutide vs placebo (201 participants with obesity and prediabetes) will probably show a significant benefit on the primary outcome of change to normoglycemia.
Meta-analyses of sodium-glucose cotransporter 2 inhibitor (SGLT2i) outcome trials have shown reductions in all-cause and cardiovascular mortality, but dipeptidyl peptidase 4 inhibitors (DPP4i) have been neutral for these outcomes. In a Taiwanese retrospective cohort study, Chung and colleagues compared 53,264 pairs of propensity-matched patients with type 2 diabetes who were treated with either an SGLT2i or DPP4i. They not only reported relative risk reductions of 34% and 32% for all-cause death and cardiovascular death, respectively, but also a reduction in cancer death of 27% and a reduction in noncancer, noncardiovascular death of 38%. Although limited by its retrospective, observational design, the finding of a benefit of SGLT2i treatment on both cancer death and noncancer, noncardiovascular death would benefit from further research.
In another large Taiwanese retrospective cohort study with propensity matching, Chan and colleagues compared patients treated with SGLT2i, DPP4i, and glucagon-like peptide 1 receptor agonists (GLP-1RA), with a main study outcome of new-onset atrial fibrillation (AF). They noted that SGLT2i treatment was associated with a 10% and 36% lower risk for AF compared with DPP4i and GLP-1RA treatment, respectively. These results are consistent with meta-analyses of SGLT2i outcome trials that have demonstrated reductions in AF with SGLT2i vs placebo. Perhaps it is time to recognize that another clinical benefit of the SGLT2i class is the reduction in risk for AF.
Although GLP-1RA have been linked to an increased risk for gallbladder-related events in many studies, there has been little data suggesting an increased risk with DPP4i. He and colleagues have published a pairwise meta-analysis of 82 randomized clinical trials and found that DPP4i compared with placebo or nonincretin drugs increased the risk for gallbladder or biliary diseases by 1.22-fold, with 11 more events per 10,000 person years. In a network meta-analysis of 184 randomized trials, they also found that DPP4i treatment increased the risk for gallbladder or biliary diseases compared with SGLT2i but not compared with GLP-1RA. This was the first meta-analysis to systematically study the association between DPP4i and gallbladder-related diseases. Although the absolute risk is small, clinicians need to be aware of this link and consider this adverse effect when deciding about the risks vs benefits of DPP4i treatment.
Individuals with obesity and prediabetes are at greater risk for type 2 diabetes. Trials of lifestyle modification and antiobesity agents have shown that restoration to normoglycemia can occur with weight loss. Phase 3A studies of the antiobesity agent semaglutide (2.4 mg/week) included 3375 individuals with prediabetes across three trials. In a post hoc analysis of these patients with prediabetes, Perreault and colleagues found that after 68 weeks of treatment, there was a much higher likelihood of normoglycemia with 2.4 mg/week of semaglutide compared with placebo. Though definite conclusions are limited owing to the post hoc nature of this analysis, these results make it very likely that the ongoing STEP 10 trial of 2.4 mg semaglutide vs placebo (201 participants with obesity and prediabetes) will probably show a significant benefit on the primary outcome of change to normoglycemia.
Meta-analyses of sodium-glucose cotransporter 2 inhibitor (SGLT2i) outcome trials have shown reductions in all-cause and cardiovascular mortality, but dipeptidyl peptidase 4 inhibitors (DPP4i) have been neutral for these outcomes. In a Taiwanese retrospective cohort study, Chung and colleagues compared 53,264 pairs of propensity-matched patients with type 2 diabetes who were treated with either an SGLT2i or DPP4i. They not only reported relative risk reductions of 34% and 32% for all-cause death and cardiovascular death, respectively, but also a reduction in cancer death of 27% and a reduction in noncancer, noncardiovascular death of 38%. Although limited by its retrospective, observational design, the finding of a benefit of SGLT2i treatment on both cancer death and noncancer, noncardiovascular death would benefit from further research.
In another large Taiwanese retrospective cohort study with propensity matching, Chan and colleagues compared patients treated with SGLT2i, DPP4i, and glucagon-like peptide 1 receptor agonists (GLP-1RA), with a main study outcome of new-onset atrial fibrillation (AF). They noted that SGLT2i treatment was associated with a 10% and 36% lower risk for AF compared with DPP4i and GLP-1RA treatment, respectively. These results are consistent with meta-analyses of SGLT2i outcome trials that have demonstrated reductions in AF with SGLT2i vs placebo. Perhaps it is time to recognize that another clinical benefit of the SGLT2i class is the reduction in risk for AF.
Although GLP-1RA have been linked to an increased risk for gallbladder-related events in many studies, there has been little data suggesting an increased risk with DPP4i. He and colleagues have published a pairwise meta-analysis of 82 randomized clinical trials and found that DPP4i compared with placebo or nonincretin drugs increased the risk for gallbladder or biliary diseases by 1.22-fold, with 11 more events per 10,000 person years. In a network meta-analysis of 184 randomized trials, they also found that DPP4i treatment increased the risk for gallbladder or biliary diseases compared with SGLT2i but not compared with GLP-1RA. This was the first meta-analysis to systematically study the association between DPP4i and gallbladder-related diseases. Although the absolute risk is small, clinicians need to be aware of this link and consider this adverse effect when deciding about the risks vs benefits of DPP4i treatment.
Individuals with obesity and prediabetes are at greater risk for type 2 diabetes. Trials of lifestyle modification and antiobesity agents have shown that restoration to normoglycemia can occur with weight loss. Phase 3A studies of the antiobesity agent semaglutide (2.4 mg/week) included 3375 individuals with prediabetes across three trials. In a post hoc analysis of these patients with prediabetes, Perreault and colleagues found that after 68 weeks of treatment, there was a much higher likelihood of normoglycemia with 2.4 mg/week of semaglutide compared with placebo. Though definite conclusions are limited owing to the post hoc nature of this analysis, these results make it very likely that the ongoing STEP 10 trial of 2.4 mg semaglutide vs placebo (201 participants with obesity and prediabetes) will probably show a significant benefit on the primary outcome of change to normoglycemia.
Commentary: Concomitant Lung Disease, Drug Efficacy, and Potential Misdiagnosis in RA, August 2022
Interstitial lung disease (ILD) is a serious and not infrequent complication of rheumatoid arthritis (RA). Despite the use of several immunosuppressive medications for ILD as well as others for RA, the most effective treatment for both is yet unclear. Prior cross-sectional, retrospective, and open-label registry studies have suggested that abatacept can be used in patients with RA-ILD, with stability or improvement in pulmonary parameters in a majority of patients. Mena-Vazquez and colleagues present the results of a prospective, observational cohort study with 57 patients from multiple centers in Spain. Similar to previously published results, this study found stability or improvement in pulmonary function tests in 70% of patients as well as improvement in RA disease activity. A relatively high proportion of patients (25 in 57) experienced infections. The study lends further weight to the proposal that abatacept is a reasonable choice in patients with RA-ILD for treatment of both joint and pulmonary inflammation, given the lack of randomized controlled trials.
Lauper and colleagues published the results of a large cohort study of more than 30,000 treatment courses in patients with RA, looking at the efficacy of different biologics and a Janus kinase (JAK) inhibitor. Discontinuation of therapy was used as the primary efficacy outcome, and one secondary outcome was low disease activity based on Clinical Disease Activity Index (CDAI) at 12 months. Over 17,000 courses were anti–tumor necrosis factor (TNF) therapy, with about 7000 JAK-inhibitor therapy courses and the remainder an interleukin 6 (IL-6) inhibitor or abatacept therapy; individual data was only available from 13 to 17 registries (depending on the parameter of interest). Overall, IL-6 inhibitors and JAK inhibitors were less frequently stopped for ineffectiveness compared with anti-TNF agents, but were more frequently stopped owing to adverse events. Drug retention rates also varied between different countries, suggesting that prescription pattern differences may affect the primary outcome. In terms of CDAI, response rates at 1 year were similar between anti-TNF agents, JAK inhibitors, and IL-6 inhibitors and was slightly lower for abatacept. This real-world study does support similar efficacy between these classes of medications, though further conclusions are somewhat hampered by the lack of individual data.
A study in Japan by Mori and colleagues looked at biologic disease-modifying antirheumatic drugs (bDMARD) (TNF inhibitors and an IL-6 inhibitor) and tofacitinib discontinuation in a cohort of 97 patients with RA . Patients were required to initially be in a high or moderate disease activity state prior to treatment, then in remission or a low disease activity state with treatment for more than 48 weeks. Mean follow-up was 2.1 years and disease flare occurred in about 75% of patients at about 1.6 years after medication discontinuation. Though bDMARD- or targeted synthetic DMARD (tsDMARD)–free remission was not "durable" for most patients, the majority of those patients who experienced flares improved with resumption of their previous medication. Though it is reassuring that most study patients were able to discontinue their bDMARD or tsDMARD medication for a period of time, the fact that most experienced flares within 2 years suggests that discontinuation of these medications in patients with high disease activity is not a viable long-term approach.
Krekeler and colleagues performed a retrospective analysis of about 500 patients seen in a single rheumatology clinic to evaluate possible misdiagnosis of RA. The diagnosis of calcium pyrophosphate deposition disease (CPPD), as well as the presence of radiographic chondrocalcinosis, were more frequently found among patients diagnosed with seronegative RA vs those diagnosed with seropositive RA, particularly RA in the wrists. The CPPD diagnosis was made by rheumatologists on the basis of the presence of radiographic chondrocalcinosis along with typical joint swelling and signs of inflammation. Because chondrocalcinosis was part of the CPPD diagnosis, it is unsurprising that both followed similar patterns. Whether patients with CPPD were actually misdiagnosed as having seronegative RA is unclear from this retrospective study; as the authors note, chondrocalcinosis itself has been found to be associated with older age and osteoarthritis in prior studies, particularly in the knee. However, the study confirms that alternative diagnoses in seronegative RA should be considered.
Interstitial lung disease (ILD) is a serious and not infrequent complication of rheumatoid arthritis (RA). Despite the use of several immunosuppressive medications for ILD as well as others for RA, the most effective treatment for both is yet unclear. Prior cross-sectional, retrospective, and open-label registry studies have suggested that abatacept can be used in patients with RA-ILD, with stability or improvement in pulmonary parameters in a majority of patients. Mena-Vazquez and colleagues present the results of a prospective, observational cohort study with 57 patients from multiple centers in Spain. Similar to previously published results, this study found stability or improvement in pulmonary function tests in 70% of patients as well as improvement in RA disease activity. A relatively high proportion of patients (25 in 57) experienced infections. The study lends further weight to the proposal that abatacept is a reasonable choice in patients with RA-ILD for treatment of both joint and pulmonary inflammation, given the lack of randomized controlled trials.
Lauper and colleagues published the results of a large cohort study of more than 30,000 treatment courses in patients with RA, looking at the efficacy of different biologics and a Janus kinase (JAK) inhibitor. Discontinuation of therapy was used as the primary efficacy outcome, and one secondary outcome was low disease activity based on Clinical Disease Activity Index (CDAI) at 12 months. Over 17,000 courses were anti–tumor necrosis factor (TNF) therapy, with about 7000 JAK-inhibitor therapy courses and the remainder an interleukin 6 (IL-6) inhibitor or abatacept therapy; individual data was only available from 13 to 17 registries (depending on the parameter of interest). Overall, IL-6 inhibitors and JAK inhibitors were less frequently stopped for ineffectiveness compared with anti-TNF agents, but were more frequently stopped owing to adverse events. Drug retention rates also varied between different countries, suggesting that prescription pattern differences may affect the primary outcome. In terms of CDAI, response rates at 1 year were similar between anti-TNF agents, JAK inhibitors, and IL-6 inhibitors and was slightly lower for abatacept. This real-world study does support similar efficacy between these classes of medications, though further conclusions are somewhat hampered by the lack of individual data.
A study in Japan by Mori and colleagues looked at biologic disease-modifying antirheumatic drugs (bDMARD) (TNF inhibitors and an IL-6 inhibitor) and tofacitinib discontinuation in a cohort of 97 patients with RA . Patients were required to initially be in a high or moderate disease activity state prior to treatment, then in remission or a low disease activity state with treatment for more than 48 weeks. Mean follow-up was 2.1 years and disease flare occurred in about 75% of patients at about 1.6 years after medication discontinuation. Though bDMARD- or targeted synthetic DMARD (tsDMARD)–free remission was not "durable" for most patients, the majority of those patients who experienced flares improved with resumption of their previous medication. Though it is reassuring that most study patients were able to discontinue their bDMARD or tsDMARD medication for a period of time, the fact that most experienced flares within 2 years suggests that discontinuation of these medications in patients with high disease activity is not a viable long-term approach.
Krekeler and colleagues performed a retrospective analysis of about 500 patients seen in a single rheumatology clinic to evaluate possible misdiagnosis of RA. The diagnosis of calcium pyrophosphate deposition disease (CPPD), as well as the presence of radiographic chondrocalcinosis, were more frequently found among patients diagnosed with seronegative RA vs those diagnosed with seropositive RA, particularly RA in the wrists. The CPPD diagnosis was made by rheumatologists on the basis of the presence of radiographic chondrocalcinosis along with typical joint swelling and signs of inflammation. Because chondrocalcinosis was part of the CPPD diagnosis, it is unsurprising that both followed similar patterns. Whether patients with CPPD were actually misdiagnosed as having seronegative RA is unclear from this retrospective study; as the authors note, chondrocalcinosis itself has been found to be associated with older age and osteoarthritis in prior studies, particularly in the knee. However, the study confirms that alternative diagnoses in seronegative RA should be considered.
Interstitial lung disease (ILD) is a serious and not infrequent complication of rheumatoid arthritis (RA). Despite the use of several immunosuppressive medications for ILD as well as others for RA, the most effective treatment for both is yet unclear. Prior cross-sectional, retrospective, and open-label registry studies have suggested that abatacept can be used in patients with RA-ILD, with stability or improvement in pulmonary parameters in a majority of patients. Mena-Vazquez and colleagues present the results of a prospective, observational cohort study with 57 patients from multiple centers in Spain. Similar to previously published results, this study found stability or improvement in pulmonary function tests in 70% of patients as well as improvement in RA disease activity. A relatively high proportion of patients (25 in 57) experienced infections. The study lends further weight to the proposal that abatacept is a reasonable choice in patients with RA-ILD for treatment of both joint and pulmonary inflammation, given the lack of randomized controlled trials.
Lauper and colleagues published the results of a large cohort study of more than 30,000 treatment courses in patients with RA, looking at the efficacy of different biologics and a Janus kinase (JAK) inhibitor. Discontinuation of therapy was used as the primary efficacy outcome, and one secondary outcome was low disease activity based on Clinical Disease Activity Index (CDAI) at 12 months. Over 17,000 courses were anti–tumor necrosis factor (TNF) therapy, with about 7000 JAK-inhibitor therapy courses and the remainder an interleukin 6 (IL-6) inhibitor or abatacept therapy; individual data was only available from 13 to 17 registries (depending on the parameter of interest). Overall, IL-6 inhibitors and JAK inhibitors were less frequently stopped for ineffectiveness compared with anti-TNF agents, but were more frequently stopped owing to adverse events. Drug retention rates also varied between different countries, suggesting that prescription pattern differences may affect the primary outcome. In terms of CDAI, response rates at 1 year were similar between anti-TNF agents, JAK inhibitors, and IL-6 inhibitors and was slightly lower for abatacept. This real-world study does support similar efficacy between these classes of medications, though further conclusions are somewhat hampered by the lack of individual data.
A study in Japan by Mori and colleagues looked at biologic disease-modifying antirheumatic drugs (bDMARD) (TNF inhibitors and an IL-6 inhibitor) and tofacitinib discontinuation in a cohort of 97 patients with RA . Patients were required to initially be in a high or moderate disease activity state prior to treatment, then in remission or a low disease activity state with treatment for more than 48 weeks. Mean follow-up was 2.1 years and disease flare occurred in about 75% of patients at about 1.6 years after medication discontinuation. Though bDMARD- or targeted synthetic DMARD (tsDMARD)–free remission was not "durable" for most patients, the majority of those patients who experienced flares improved with resumption of their previous medication. Though it is reassuring that most study patients were able to discontinue their bDMARD or tsDMARD medication for a period of time, the fact that most experienced flares within 2 years suggests that discontinuation of these medications in patients with high disease activity is not a viable long-term approach.
Krekeler and colleagues performed a retrospective analysis of about 500 patients seen in a single rheumatology clinic to evaluate possible misdiagnosis of RA. The diagnosis of calcium pyrophosphate deposition disease (CPPD), as well as the presence of radiographic chondrocalcinosis, were more frequently found among patients diagnosed with seronegative RA vs those diagnosed with seropositive RA, particularly RA in the wrists. The CPPD diagnosis was made by rheumatologists on the basis of the presence of radiographic chondrocalcinosis along with typical joint swelling and signs of inflammation. Because chondrocalcinosis was part of the CPPD diagnosis, it is unsurprising that both followed similar patterns. Whether patients with CPPD were actually misdiagnosed as having seronegative RA is unclear from this retrospective study; as the authors note, chondrocalcinosis itself has been found to be associated with older age and osteoarthritis in prior studies, particularly in the knee. However, the study confirms that alternative diagnoses in seronegative RA should be considered.