User login
Tactile stimulation for inadequate neonatal respiration at birth
Recently, I encountered a study in Pediatrics that hoped to answer the question of whether there was any benefit to tactile stimulation in those nerve-rattling moments when a newborn didn’t seem to take much interest in breathing: “Tactile stimulation in newborn infants with inadequate respiration at birth: A systematic review.” Now there is a title that grabs the attention of every frontline pediatrician who has sweated through those minutes that seemed like hours in the delivery room when some little rascal has decided that breathing isn’t a priority.
Of course, your great grandmother and everyone else knew what needed to be done – the obstetrician hung the baby by his or her ankles and slapped it on the bottom a couple of times. But you went to medical school and learned that was barbaric. Instead, you modeled the behavior of the residents and delivery room nurses who had more refined techniques such as heel flicking and vigorous spine rubbing. You never thought to ask if there was any science behind those activities because everyone did them.
Well, the authors of the article in Pediatrics, writing on behalf of the International Liaison Committee on Resuscitation and Neonatal Life Support Task Force, thought the time had come to turn over a few stones and see if tactile stimulation was a benefit in resuscitation. Beginning with 2,455 possibly relevant articles, they quickly (I suspect they would quibble with the “quickly” part) winnowed these down to two observational studies, one of which was rejected because of “critical risk of bias.” The surviving study showed a reduction in tracheal intubation in infants who had received tactile stimulation. However, the authors felt that the “certainty of evidence was very low.”
So, there you have it. Aren’t you glad you didn’t invest 15 or 20 minutes discovering what you probably had guessed already? You can thank me later.
You already suspected that it may not help. However, like any good physician, what you really wanted to know is whether were you doing any harm by heel flicking and spine rubbing. And I bet you already had an opinion about the answer to that question. During your training, you may have seen delivery room personnel who were clearly too vigorous in their tactile stimulation and/or too persistent in their heel flicking and spine rubbing when the next steps in resuscitation needed to be taken. That’s the next study that needs to be done. I hope that study finds that tactile stimulation may not help but as long as it is done using specific techniques and within certain temporal parameters it does no harm.
I was never much for heel flicking. My favorite tactile stimulation was encircling the pokey infant’s chest in my hand, gently compressing and then quickly releasing a couple of times. My hope was that by mimicking the birth process the sensors in the infant’s chest wall would remind him it was time to breathe. That, and a silent plea to Mother Nature, worked most of the time.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Recently, I encountered a study in Pediatrics that hoped to answer the question of whether there was any benefit to tactile stimulation in those nerve-rattling moments when a newborn didn’t seem to take much interest in breathing: “Tactile stimulation in newborn infants with inadequate respiration at birth: A systematic review.” Now there is a title that grabs the attention of every frontline pediatrician who has sweated through those minutes that seemed like hours in the delivery room when some little rascal has decided that breathing isn’t a priority.
Of course, your great grandmother and everyone else knew what needed to be done – the obstetrician hung the baby by his or her ankles and slapped it on the bottom a couple of times. But you went to medical school and learned that was barbaric. Instead, you modeled the behavior of the residents and delivery room nurses who had more refined techniques such as heel flicking and vigorous spine rubbing. You never thought to ask if there was any science behind those activities because everyone did them.
Well, the authors of the article in Pediatrics, writing on behalf of the International Liaison Committee on Resuscitation and Neonatal Life Support Task Force, thought the time had come to turn over a few stones and see if tactile stimulation was a benefit in resuscitation. Beginning with 2,455 possibly relevant articles, they quickly (I suspect they would quibble with the “quickly” part) winnowed these down to two observational studies, one of which was rejected because of “critical risk of bias.” The surviving study showed a reduction in tracheal intubation in infants who had received tactile stimulation. However, the authors felt that the “certainty of evidence was very low.”
So, there you have it. Aren’t you glad you didn’t invest 15 or 20 minutes discovering what you probably had guessed already? You can thank me later.
You already suspected that it may not help. However, like any good physician, what you really wanted to know is whether were you doing any harm by heel flicking and spine rubbing. And I bet you already had an opinion about the answer to that question. During your training, you may have seen delivery room personnel who were clearly too vigorous in their tactile stimulation and/or too persistent in their heel flicking and spine rubbing when the next steps in resuscitation needed to be taken. That’s the next study that needs to be done. I hope that study finds that tactile stimulation may not help but as long as it is done using specific techniques and within certain temporal parameters it does no harm.
I was never much for heel flicking. My favorite tactile stimulation was encircling the pokey infant’s chest in my hand, gently compressing and then quickly releasing a couple of times. My hope was that by mimicking the birth process the sensors in the infant’s chest wall would remind him it was time to breathe. That, and a silent plea to Mother Nature, worked most of the time.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Recently, I encountered a study in Pediatrics that hoped to answer the question of whether there was any benefit to tactile stimulation in those nerve-rattling moments when a newborn didn’t seem to take much interest in breathing: “Tactile stimulation in newborn infants with inadequate respiration at birth: A systematic review.” Now there is a title that grabs the attention of every frontline pediatrician who has sweated through those minutes that seemed like hours in the delivery room when some little rascal has decided that breathing isn’t a priority.
Of course, your great grandmother and everyone else knew what needed to be done – the obstetrician hung the baby by his or her ankles and slapped it on the bottom a couple of times. But you went to medical school and learned that was barbaric. Instead, you modeled the behavior of the residents and delivery room nurses who had more refined techniques such as heel flicking and vigorous spine rubbing. You never thought to ask if there was any science behind those activities because everyone did them.
Well, the authors of the article in Pediatrics, writing on behalf of the International Liaison Committee on Resuscitation and Neonatal Life Support Task Force, thought the time had come to turn over a few stones and see if tactile stimulation was a benefit in resuscitation. Beginning with 2,455 possibly relevant articles, they quickly (I suspect they would quibble with the “quickly” part) winnowed these down to two observational studies, one of which was rejected because of “critical risk of bias.” The surviving study showed a reduction in tracheal intubation in infants who had received tactile stimulation. However, the authors felt that the “certainty of evidence was very low.”
So, there you have it. Aren’t you glad you didn’t invest 15 or 20 minutes discovering what you probably had guessed already? You can thank me later.
You already suspected that it may not help. However, like any good physician, what you really wanted to know is whether were you doing any harm by heel flicking and spine rubbing. And I bet you already had an opinion about the answer to that question. During your training, you may have seen delivery room personnel who were clearly too vigorous in their tactile stimulation and/or too persistent in their heel flicking and spine rubbing when the next steps in resuscitation needed to be taken. That’s the next study that needs to be done. I hope that study finds that tactile stimulation may not help but as long as it is done using specific techniques and within certain temporal parameters it does no harm.
I was never much for heel flicking. My favorite tactile stimulation was encircling the pokey infant’s chest in my hand, gently compressing and then quickly releasing a couple of times. My hope was that by mimicking the birth process the sensors in the infant’s chest wall would remind him it was time to breathe. That, and a silent plea to Mother Nature, worked most of the time.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Screening for diabetes at normal BMIs could cut racial disparities
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Home BP monitoring is essential
I believe that the most important recommendation from the American Heart Association in recent years is to confirm office blood pressure (BP) readings with repeated home BP measurements, for both diagnosis and management of hypertension. Office BPs are notoriously inaccurate, because it is exceedingly difficult to measure BP properly in a busy office setting. Even when measured correctly, the office BP does not accurately reflect a person’s BP throughout the day, which is the best predictor of cardiovascular damage from hypertension.
Among the problems with relying on office BP readings:We would treat many people for hypertension who are not hypertensive, because 15% to 30% of those with elevated office BP readings have “white-coat” hypertension, which does not require medication.1 White-coat hypertension can only be diagnosed with home BP readings or 24-hour ambulatory BP monitoring.
We would miss the diagnosis of hypertension in patients with “masked” hypertension—that is, people who have normal BP in the office but elevated ambulatory BP. It is estimated that 12% of US adults have masked hypertension.2
We would overtreat some patients who have hypertension and undertreat others, since office BP measurements can underestimate BP by an average of 24/14 mm Hg and overestimate BP by an average of 33/23 mm Hg.3
In this issue of JFP, Spaulding and colleagues4 provide an extensive summary of the research that supports the recommendation for home BP measurements. Here are 3 key takeaways:
- Use an automated BP monitor to measure BP in the office. Automated BP monitors that take repeated BPs over the course of about 5 minutes and average the results provide a much better estimate of 24-hour BP. It is worth the extra time and may be the only basis for making decisions about medications if a patient is unwilling or unable to take home BP readings.
- Provide training to patients who are willing to monitor their BP at home. Explain how to take their BP properly and instruct them to record at least 12 readings over the course of 3 days prior to office visits.
- Recommend patients use a validated BP monitor that uses the brachial artery for measurement, not the wrist (visit www.stridebp.org/bp-monitors and choose “Home”).
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
3. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
4. Spaulding J, Kasper RE, Viera AJ. Hypertension—or not? Looking beyond office BP readings. J Fam Pract. 2022;71:151-158. doi: 10.12788/jfp.0399
I believe that the most important recommendation from the American Heart Association in recent years is to confirm office blood pressure (BP) readings with repeated home BP measurements, for both diagnosis and management of hypertension. Office BPs are notoriously inaccurate, because it is exceedingly difficult to measure BP properly in a busy office setting. Even when measured correctly, the office BP does not accurately reflect a person’s BP throughout the day, which is the best predictor of cardiovascular damage from hypertension.
Among the problems with relying on office BP readings:We would treat many people for hypertension who are not hypertensive, because 15% to 30% of those with elevated office BP readings have “white-coat” hypertension, which does not require medication.1 White-coat hypertension can only be diagnosed with home BP readings or 24-hour ambulatory BP monitoring.
We would miss the diagnosis of hypertension in patients with “masked” hypertension—that is, people who have normal BP in the office but elevated ambulatory BP. It is estimated that 12% of US adults have masked hypertension.2
We would overtreat some patients who have hypertension and undertreat others, since office BP measurements can underestimate BP by an average of 24/14 mm Hg and overestimate BP by an average of 33/23 mm Hg.3
In this issue of JFP, Spaulding and colleagues4 provide an extensive summary of the research that supports the recommendation for home BP measurements. Here are 3 key takeaways:
- Use an automated BP monitor to measure BP in the office. Automated BP monitors that take repeated BPs over the course of about 5 minutes and average the results provide a much better estimate of 24-hour BP. It is worth the extra time and may be the only basis for making decisions about medications if a patient is unwilling or unable to take home BP readings.
- Provide training to patients who are willing to monitor their BP at home. Explain how to take their BP properly and instruct them to record at least 12 readings over the course of 3 days prior to office visits.
- Recommend patients use a validated BP monitor that uses the brachial artery for measurement, not the wrist (visit www.stridebp.org/bp-monitors and choose “Home”).
I believe that the most important recommendation from the American Heart Association in recent years is to confirm office blood pressure (BP) readings with repeated home BP measurements, for both diagnosis and management of hypertension. Office BPs are notoriously inaccurate, because it is exceedingly difficult to measure BP properly in a busy office setting. Even when measured correctly, the office BP does not accurately reflect a person’s BP throughout the day, which is the best predictor of cardiovascular damage from hypertension.
Among the problems with relying on office BP readings:We would treat many people for hypertension who are not hypertensive, because 15% to 30% of those with elevated office BP readings have “white-coat” hypertension, which does not require medication.1 White-coat hypertension can only be diagnosed with home BP readings or 24-hour ambulatory BP monitoring.
We would miss the diagnosis of hypertension in patients with “masked” hypertension—that is, people who have normal BP in the office but elevated ambulatory BP. It is estimated that 12% of US adults have masked hypertension.2
We would overtreat some patients who have hypertension and undertreat others, since office BP measurements can underestimate BP by an average of 24/14 mm Hg and overestimate BP by an average of 33/23 mm Hg.3
In this issue of JFP, Spaulding and colleagues4 provide an extensive summary of the research that supports the recommendation for home BP measurements. Here are 3 key takeaways:
- Use an automated BP monitor to measure BP in the office. Automated BP monitors that take repeated BPs over the course of about 5 minutes and average the results provide a much better estimate of 24-hour BP. It is worth the extra time and may be the only basis for making decisions about medications if a patient is unwilling or unable to take home BP readings.
- Provide training to patients who are willing to monitor their BP at home. Explain how to take their BP properly and instruct them to record at least 12 readings over the course of 3 days prior to office visits.
- Recommend patients use a validated BP monitor that uses the brachial artery for measurement, not the wrist (visit www.stridebp.org/bp-monitors and choose “Home”).
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
3. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
4. Spaulding J, Kasper RE, Viera AJ. Hypertension—or not? Looking beyond office BP readings. J Fam Pract. 2022;71:151-158. doi: 10.12788/jfp.0399
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
3. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
4. Spaulding J, Kasper RE, Viera AJ. Hypertension—or not? Looking beyond office BP readings. J Fam Pract. 2022;71:151-158. doi: 10.12788/jfp.0399
USPSTF recommendation roundup
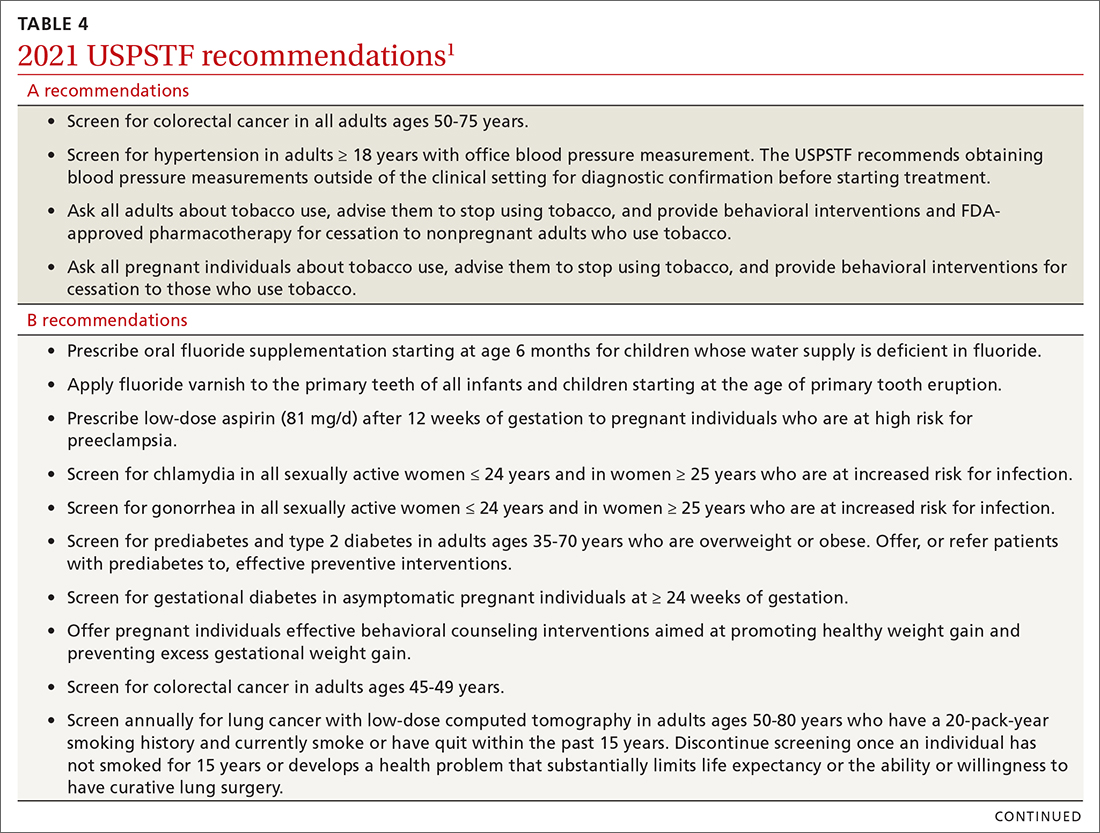
In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2
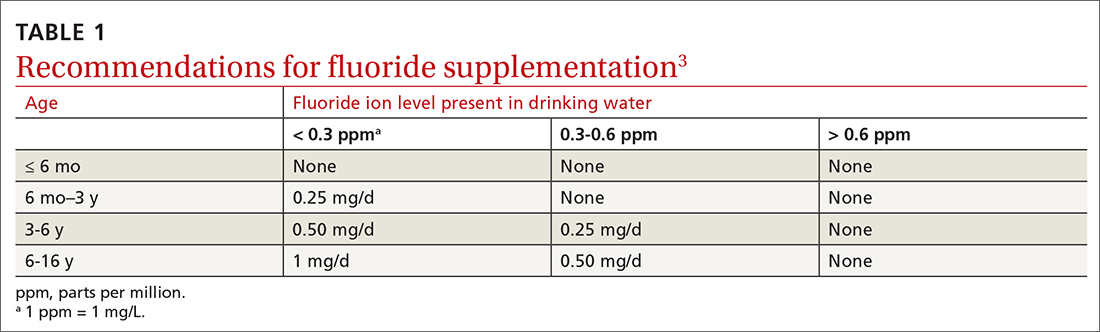
In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
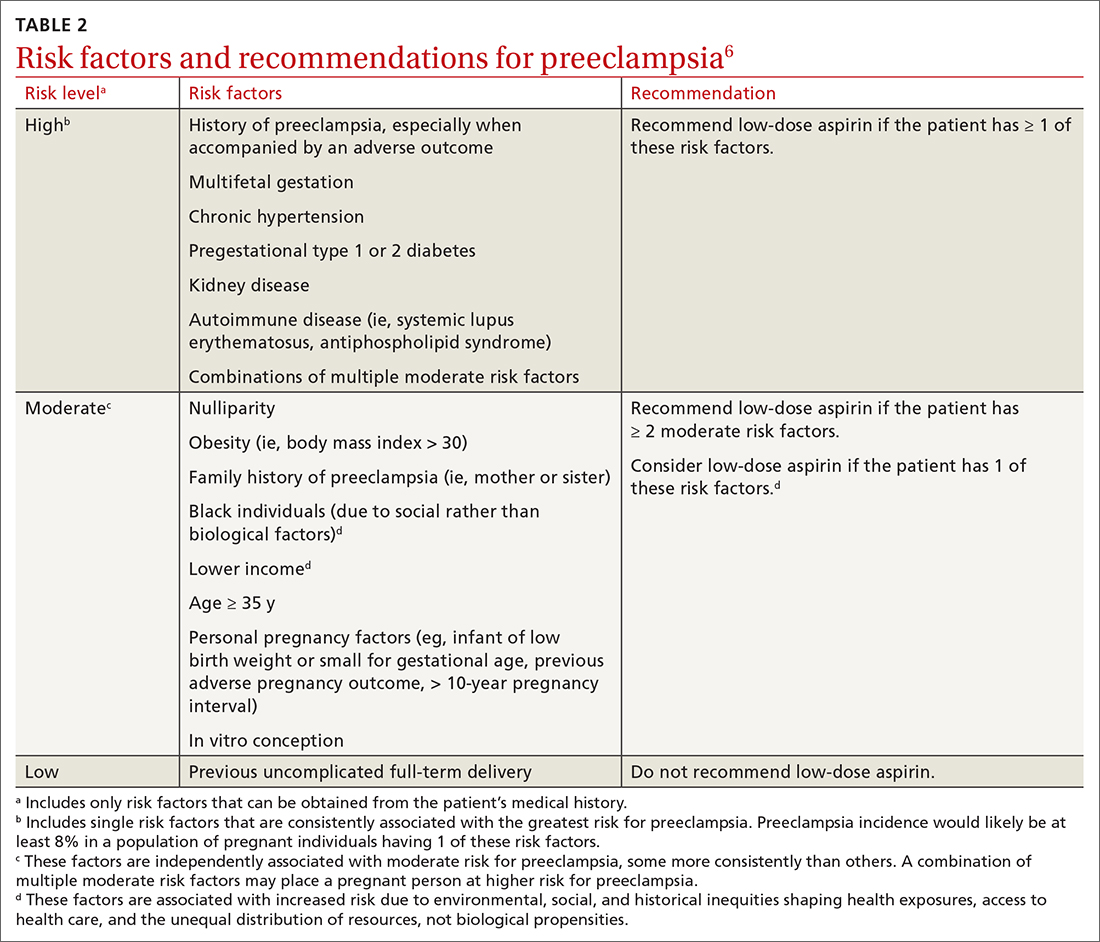
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.
Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
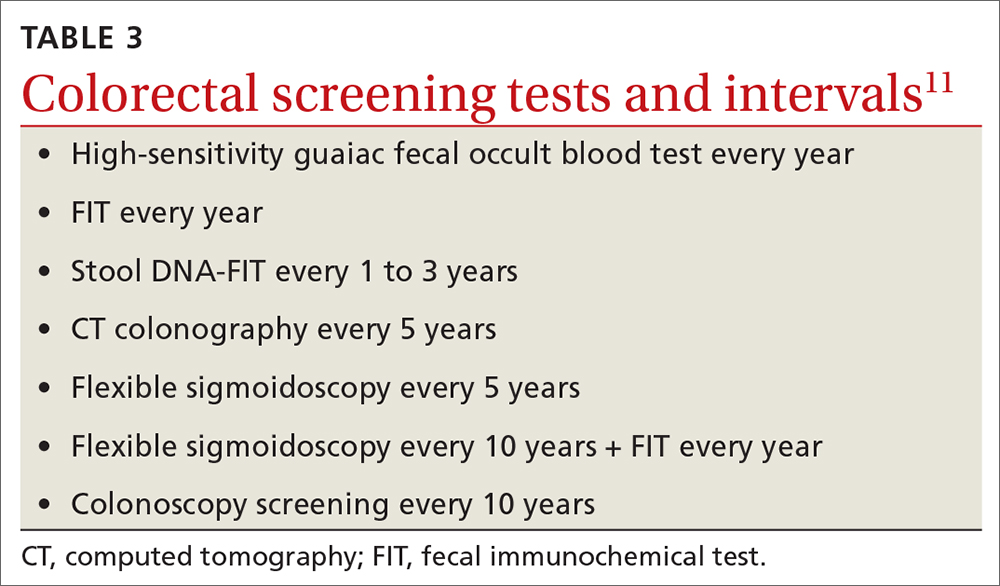
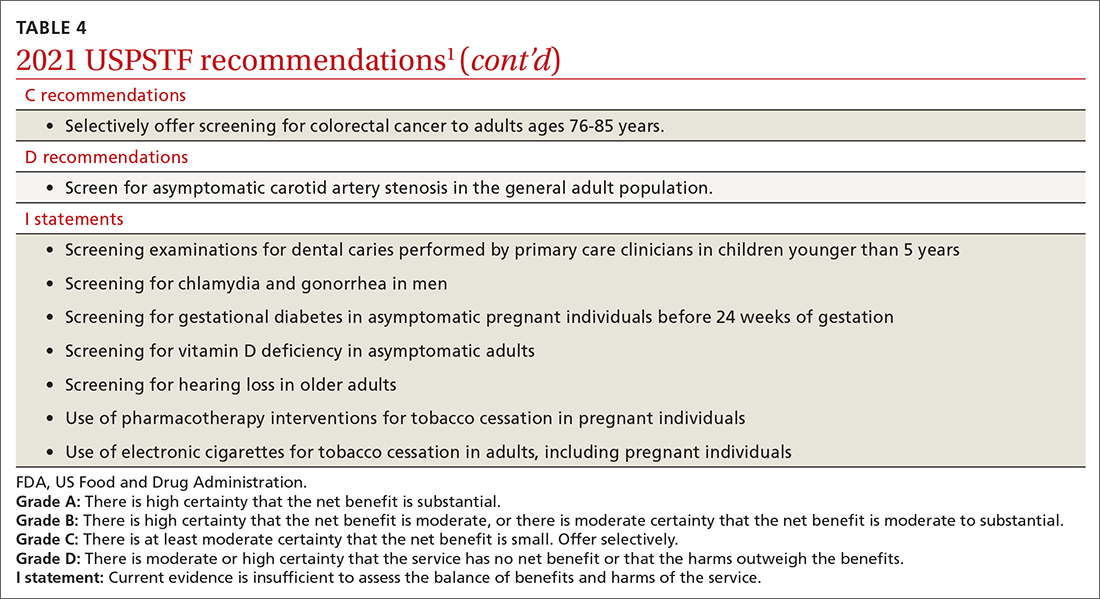
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11
For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1
1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2

In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.

Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11

For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1


In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2

In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.

Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11

For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1


1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
Hypertension—or not? Looking beyond office BP readings
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1
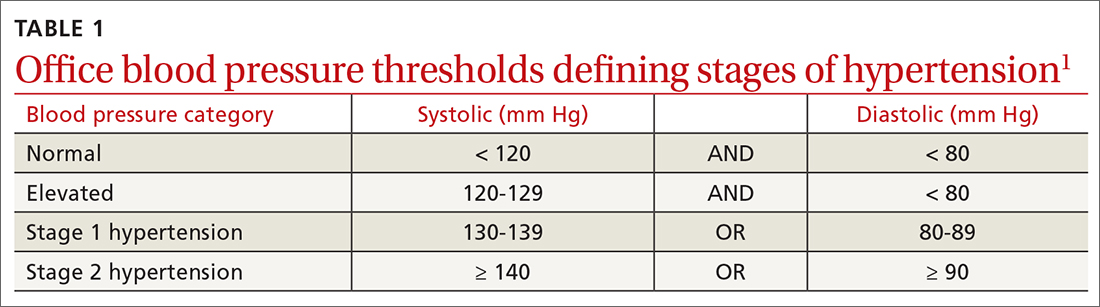
Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.
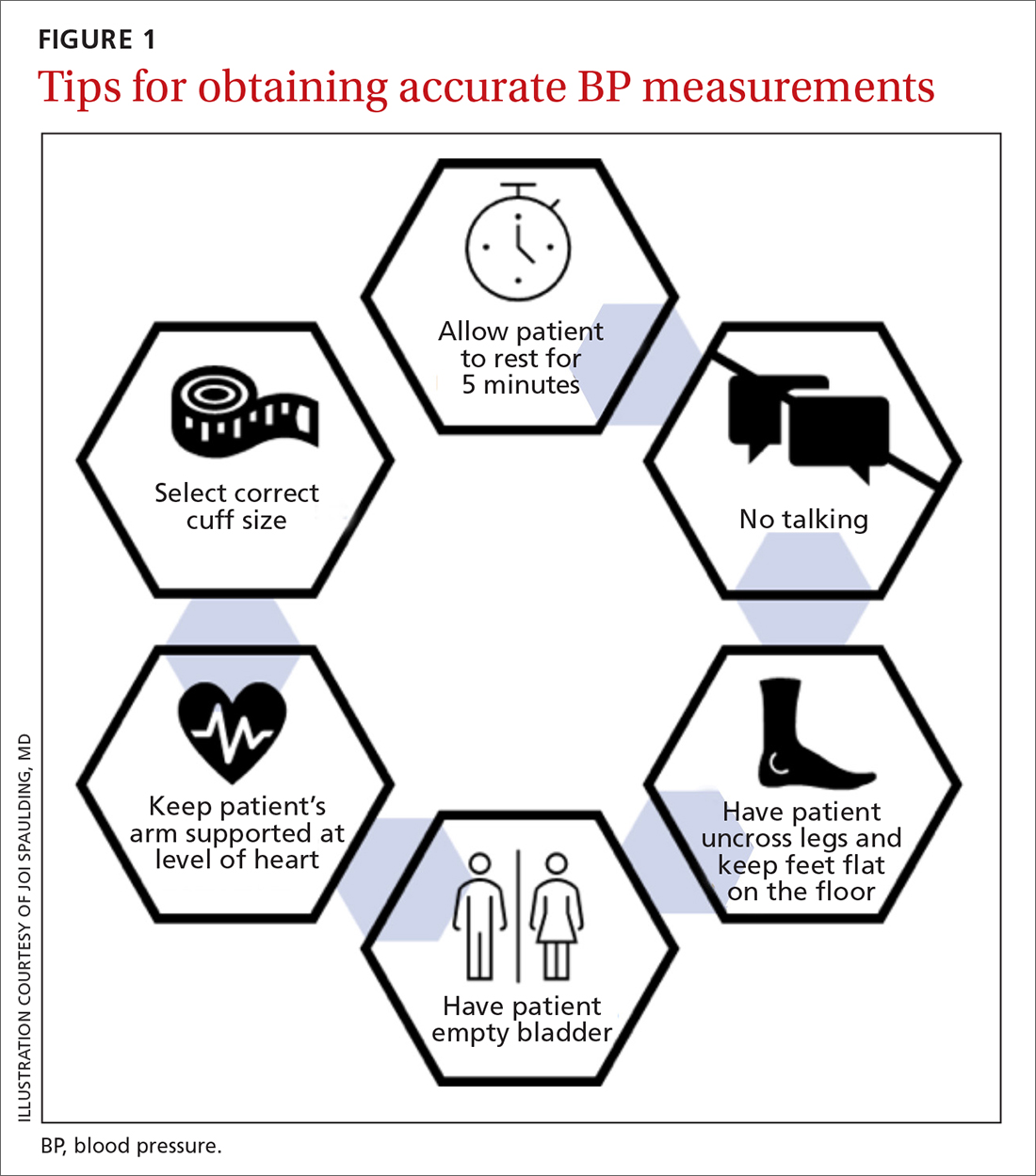
Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).
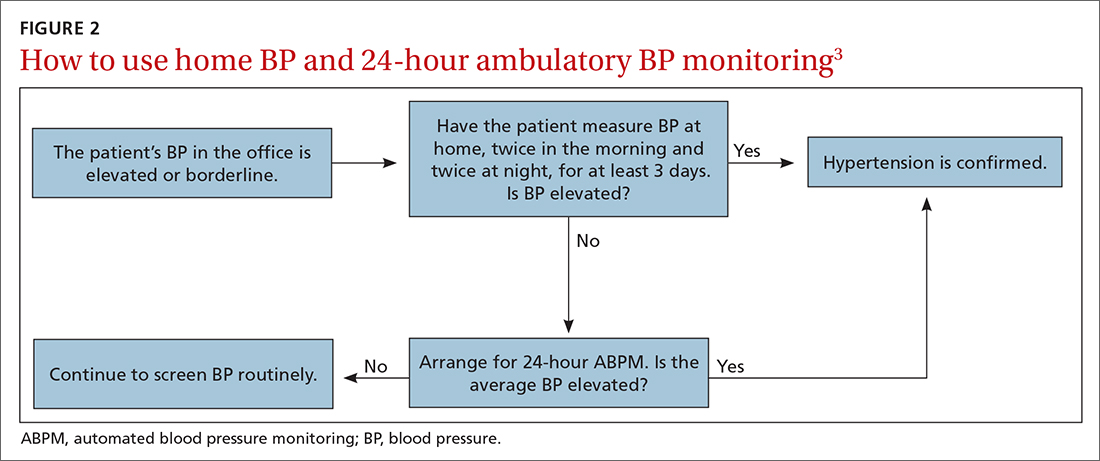
Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.
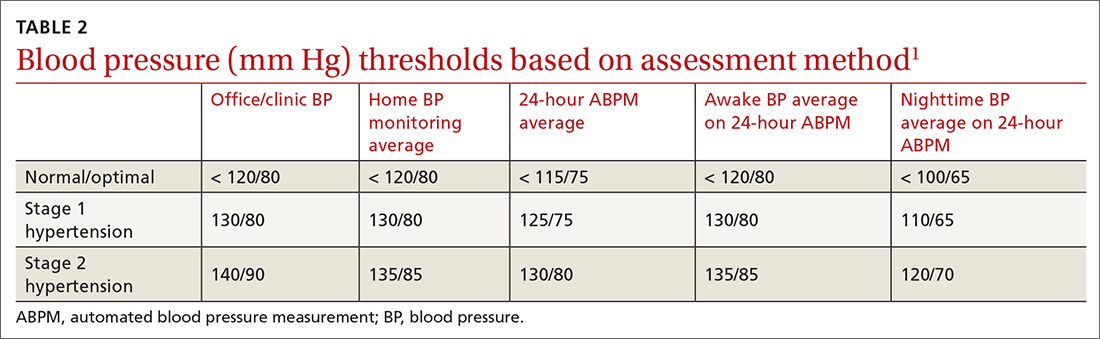
Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.
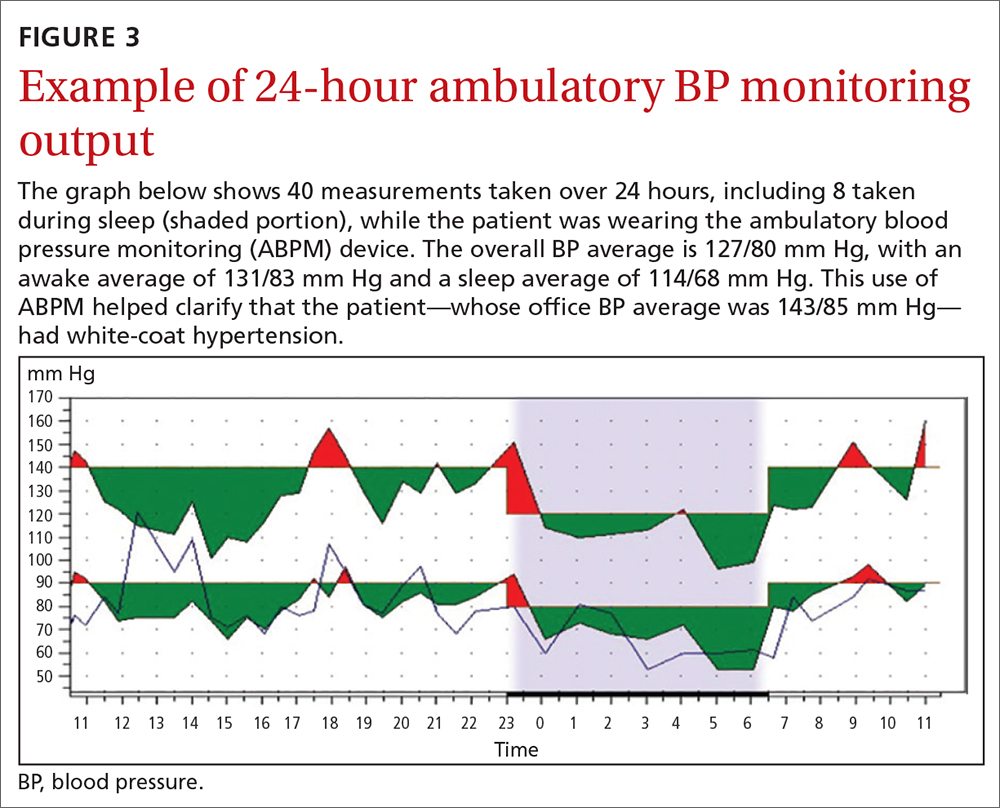
Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.
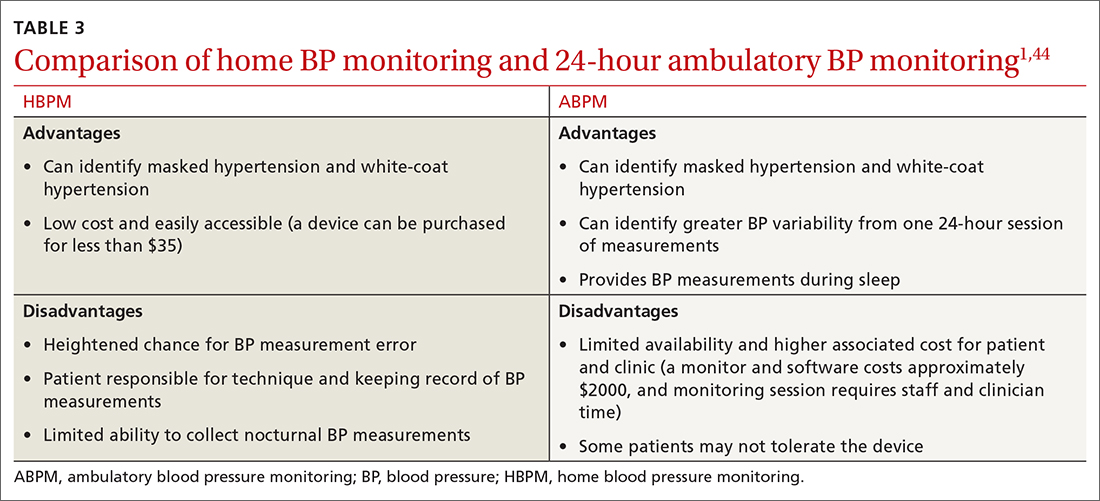
Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; [email protected]
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1

Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.

Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).

Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.

Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.

Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.

Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; [email protected]
Normal blood pressure (BP) is defined as systolic BP (SBP) < 120 mm Hg and diastolic BP (DBP) < 80 mm Hg.1 The thresholds for hypertension (HTN) are shown in TABLE 1.1 These thresholds must be met on at least 2 separate occasions to merit a diagnosis of HTN.1

Given the high prevalence of HTN and its associated comorbidities, the US Preventive Services Task Force (USPSTF) recently reaffirmed its recommendation that every adult be screened for HTN, regardless of risk factors.2 Patients 40 years of age and older and those with risk factors (obesity, family history of HTN, diabetes) should have their BP checked at least annually. Individuals ages 18 to 39 years without risk factors who are initially normotensive should be rescreened within 3 to 5 years.2
Patients are most commonly screened for HTN in the outpatient setting. However, office BP measurements may be inaccurate and are of limited diagnostic utility when taken as a single reading.1,3,4 As will be described later, office BP measurements are subject to multiple sources of error that can result in a mean underestimation of 24 mm Hg to a mean overestimation of 33 mm Hg for SBP, and a mean underestimation of 14 mm Hg to a mean overestimation of 23 mm Hg for DBP.4
Differences to this degree between true BP and measured BP can have important implications for the diagnosis, surveillance, and management of HTN. To diminish this potential for error, the American Heart Association HTN guideline and USPSTF recommendation advise clinicians to obtain out-of-office BP measurements to confirm a diagnosis of HTN before initiating treatment.1,2 The preferred methods for out-of-office BP assessment are home BP monitoring (HBPM) and 24-hour ambulatory BP monitoring (ABPM).
Limitations of office BP measurement
Multiple sources of error can lead to wide variability in the measurement of office BP, whether taken via the traditional sphygmomanometer auscultatory approach or with an oscillometric monitor.1,4 Measurement error can be patient related (eg, talking during the reading, or eating or using tobacco prior to measurement), device related (eg, device has not been calibrated or validated), or procedure related (eg, miscuffing, improper patient positioning).
Although use of validated oscillometric monitors eliminates some sources of error such as terminal digit bias, rapid cuff deflation, and missed Korotkoff sounds, their use does not eliminate other sources of error. For example, a patient’s use of tobacco 30 to 60 minutes prior to measurement can raise SBP by 2.8 to 25 mm Hg and DBP 2 to 18 mm Hg.4 Having a full bladder can elevate SBP by 4.2 to 33 mm Hg and DBP by 2.8 to 18.5 mm Hg.4 If the patient is talking during measurement, is crossing one leg over the opposite knee, or has an unsupported arm below the level of the heart, SBP and DBP can rise, respectively, by an estimated mean 2 to 23 mm Hg and 2 to 14 mm Hg.4
Although many sources of BP measurement error can be reduced or eliminated through standardization of technique across office staff, some sources of inaccuracy will persist. Even if all variables are optimized, relying solely on office BP monitoring will still misclassify BP phenotypes, which require out-of-office BP assessments.1,3FIGURE 1 reviews key tips for maximizing the accuracy of BP measurement, regardless of where the measurement is done.

Continue to: Automated office BP
Automated office BP (AOBP) lessens some of the limitations inherent with the traditional sphygmomanometer auscultatory and single-measurement oscillometric devices. AOBP combines oscillometric technology with the capacity to record multiple BP readings within a single activation, thereby providing an average of these readings.1 The total time required for AOBP is 4 to 6 minutes, including a brief rest period before the measurement starts. Studies have reported comparable readings between staff-attended and unattended AOBP, which is an encouraging way to eliminate some measurement error (eg, talking with the patient) and to improve efficiency.5,6
Waiting several minutes per patient to record BP may not be practical in a busy office setting and may require an alteration of workflow. There is a paucity of literature evaluating practice realities, which makes it difficult to know how many patients are getting their BP checked in this manner. Several studies have shown that BP measured with AOBP is closer to awake out-of-office BP as measured with ABPM (discussed in a bit),5-8 largely through mitigation of white-coat effect. Canada now recommends AOBP as the preferred method for diagnosing HTN and monitoring BP.9
Home blood pressure monitoring
HBPM refers to individuals measuring their own BP at home. It is important to remember this definition,
There is strong evidence that HBPM adds value over and above office measurements in predicting end-organ damage and cardiovascular disease (CVD) outcomes, and it has a stronger relationship with CVD risk than office BP.1 Compared with office BP measurement, HBPM is a better predictor of echocardiographic left ventricular mass index, urinary albumin-to-creatinine ratio, proteinuria, silent cerebrovascular disease, nonfatal cardiovascular outcomes, cardiovascular mortality, and all-cause mortality.15,16 There is no strong evidence demonstrating the superiority of HBPM over ABPM, or vice versa, for predicting CVD events or mortality.17 Both ABPM and HBPM have important roles in out-of-office monitoring (FIGURE 23).

Clinical indications for HBPM
HBPM can facilitate diagnosis of white-coat HTN or effect (if already on BP-lowering medication) as well as masked uncontrolled HTN and masked HTN. Importantly, masked HTN is associated with nearly the same risk of target organ damage and cardiovascular events as sustained HTN. In one meta-analysis the overall adjusted hazard ratio for CVD events was 2.00 (95% CI, 1.58-2.52) for masked HTN and 2.28 (95% CI, 1.87-2.78) for sustained HTN, compared with normotensive individuals.18 Other studies support these results, demonstrating that masked HTN confers risk similar to sustained HTN.19,20
Even treated subjects with masked uncontrolled HTN (normal office and high home BP) have higher CVD risk, likely due to undertreatment given lower BP in the office setting. Among 1451 treated patients in a large cohort study who were followed for a median of 8.3 years, CVD was higher in those with masked uncontrolled HTN (adjusted hazard ratio = 1.76; 95% CI, 1.23-2.53) compared to treated controlled patients (normal office and home BP).21
HBPM also can be used to monitor BP levels over time, to increase patient involvement in chronic disease management, and to improve adherence with medications. Since 2008, several meta-analyses have been published showing improved BP control when HBPM is combined with other interventions and patient education.22-25 Particularly relevant in the age of increased telehealth, several meta-analyses demonstrate improvement in BP control when HBPM is combined with web- or phone-based support, systematic medication titration, patient education, and provider counseling.22-25 A comprehensive systematic review found HBPM with this kind of ongoing support (compared with usual care) led to clinic SBP reductions of 3.2 mm Hg (95% CI, 1.6-4.9) at 12 months.22
Continue to: HBPM nuts and bolts
HBPM nuts and bolts
When using HBPM to obtain a BP average either for confirming a diagnosis or assessing HTN control, patients should be instructed to record their BP measurements twice in the morning and twice at night for a minimum of 3 days (ie, 12 readings).26,27 For each monitoring period, both SBP and DBP readings should be recorded, although protocols differ as to whether to discard the initial reading of each day, or the entire first day of readings.26-29 Consecutive days of monitoring are preferred, although nonconsecutive days also are likely to provide valid data. Once BP stabilizes, monitoring 1 to 3 days a week is likely sufficient.
Most guidelines cite a mean BP of ≥ 135/85 mm Hg as the indication of high BP on HBPM.1,28,29 This value corresponds to an office BP average of 140/90 mm Hg. TABLE 21 shows the comparison of home, ambulatory, and office BP thresholds.

Device selection and validation
As with any BP device, validation and proper technique are important. Recommend only upper-arm cuff devices that have passed validation protocols.30 To eliminate the burden on patients to accurately record and store their BP readings, and to eliminate this step as a source of bias, additionally recommend devices with built-in memory. Although easy-to-use wrist and finger monitors have become popular, there are important limitations in terms of accurate positioning and a lack of validated protocols.31,32
The brachial artery is still the recommended measurement location, unless otherwise precluded due to arm size (the largest size for most validated upper-arm cuffs is 42 cm), patient discomfort, medical contraindication (eg, lymphedema), or immobility (eg, due to injury). Arm size limitation is particularly important as obesity rates continue to rise. Data from the National Health and Nutrition Examination Survey indicate that 52% of men and 38% of women with HTN need a different cuff size than the US standard.33 If the brachial artery is not an option, there are no definitive data to recommend finger over wrist devices, as both are limited by lack of validated protocols.
The website www.stridebp.org maintains a current list of validated and preferred BP devices, and is supported by the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League. There are more than 4000 devices on the global market, but only 8% have been validated according to StrideBP.
Advances in HBPM that offset previous limitations
The usefulness of HBPM depends on patient factors such as a commitment to monitoring, applying standardized technique, and accurately recording measurements. Discuss these matters with patients before recommending HBPM. Until recently, HBPM devices could not measure BP during sleep. However, a device that assesses BP during sleep has now come on the US market, with preliminary data suggesting the BP measurements are similar to those obtained with ABPM.34 Advances in device memory and data storage and increased availability of electronic health record connection continue to improve the standardization and reliability of HBPM. In fact, there is a growing list of electronic health portals that can be synced with apps for direct transfer of HBPM data.
Ambulatory blood pressure monitoring
ABPM involves wearing a small device connected to an arm BP cuff that measures BP at pre-programmed intervals over a 24-hour period, during sleep and wakefulness. ABPM is the standard against which HBPM and office BP are compared.1-3
Continue to: Clinical indications for ABPM
Clinical indications for ABPM
Compared with office-based BP measurements, ABPM has a stronger positive correlation with clinical CVD outcomes and HTN-related organ damage.1 ABPM has the advantage of being able to provide a large number of measurements over the course of a patient’s daily activities, including sleep. It is useful to evaluate for a wide spectrum of hypertensive or hypotensive patterns, including nocturnal, postprandial, and drug-related patterns. ABPM also is used to assess for white-coat HTN and masked HTN.1
Among these BP phenotypes, an estimated 15% to 30% of adults in the United States exhibit white-coat HTN.1 Most evidence suggests that white-coat HTN confers similar cardiovascular risk as normotension, and it therefore does not require treatment.35 Confirming this diagnosis saves the individual and the health care system the cost of unnecessary diagnosis and treatment.
One cost-effectiveness study using ABPM for annual screening with subsequent treatment for those confirmed to be hypertensive found that ABPM reduced treatment-years by correctly identifying white-coat HTN, and also delayed treatment for those who would eventually develop HTN with advancing age.36 The estimates in savings were 3% to 14% for total cost of care for hypertension and 10% to 23% reduction in treatment days.36 An Australian study showed similar cost reductions.37 A more recent analysis demonstrated that compared with clinic BP measurement alone, incorporation of ABPM is associated with lifetime cost-savings ranging from $77 to $5013, depending on the age and sex of the patients modeled.38
ABPM can also be used to rule out white-coat effect in patients being evaluated for resistant HTN. Several studies demonstrate that among patients with apparent resistant HTN, approximately one-third have controlled BP when assessed by ABPM.39-41 Thus, it is recommended to conduct an out-of-office BP assessment in patients with apparent resistant HTN prior to adding another medication.41Twelve percent of US adults have masked HTN.42 As described earlier, these patients, unrecognized without out-of-office BP assessment, are twice as likely to experience a CVD event compared with normotensive patients.1,42,43
ABPM nuts and bolts
ABPM devices are typically worn for 24 hours and with little interruption to daily routines. Prior to BP capture, the device will alert the patient to ensure the patient’s arm can be held still while the BP measurement is being captured.44 At the completion of 24 hours, specific software uses the stored data to calculate the BP and heart rate averages, as well as minimums and maximums throughout the monitoring period. Clinical decision-making should be driven by the average BP measurements during times of sleep and wakefulness.1,14,44FIGURE 3 is an example of output from an ABPM session. TABLE 31,44 offers a comparison of HBPM and ABPM.

Limitations of ABPM
While ABPM has been designed to be almost effortless to use, some may find it inconvenient to wear. The repeated cuff inflations can cause discomfort or bruising, and the device can interfere with sleep.45 Inconsistent or incorrect wear of ABPM can diminish the quality of BP measurements, which can potentially affect interpretation and subsequent clinical decision-making. Therefore, consider the likelihood of correct and complete usage before ordering ABPM for your patient. Such deliberation is particularly relevant when there is concern for BP phenotypes such as nocturnal nondipping (failure of BP to fall appropriately during sleep) and postprandial HTN and hypotension.

Trained personnel are needed to oversee coordination of the ABPM service within the clinic and to educate patients about proper wear. Additionally, ABPM has not been widely used in US clinical practices to date, in part because this diagnostic strategy is not favorably reimbursed. Based on geographic region, Medicare currently pays between $56 and $122 per 24-hour ABPM session, and only for suspected white-coat HTN.38 Discrepancies remain between commercial and Medicaid/Medicare coverage.44
Continue to: Other modes of monitoring BP
Other modes of monitoring BP
The COVID pandemic has changed health care in many ways, including the frequency of in-person visits. As clinics come to rely more on virtual visits and telehealth, accurate monitoring of out-of-office BP has become more important. Kiosks and smart technology offer the opportunity to supplement traditional in-office BP readings. Kiosks are commonly found in pharmacies and grocery stores. These stations facilitate BP monitoring, as long as the device is appropriately validated and calibrated. Unfortunately, most kiosks have only one cuff size that is too small for many US adults, and some do not have a back support.46,47 Additionally, despite US Food and Drug Administration clearance, many kiosks do not have validated protocols, and the reproducibility of kiosk-measured BP is questionable.46,47
Mobile health technology is increasingly being examined as an effective means of providing health information, support, and management in chronic disease. Smartphone technology, wearable sensors, and cuffless BP monitors offer promise for providing BP data in more convenient ways. However, as with kiosk devices, very few of these have been validated, and several have been shown to have poor accuracy compared with oscillometric devices.48-50 For these reasons, kiosk and smart technology for BP monitoring are not recommended at this time, unless no alternatives are available to the patient.
CORRESPONDENCE
Anthony J. Viera, MD, Department of Family Medicine and Community Health, Duke University School of Medicine, 2200 West Main Street, Suite 400, Durham, NC 27705; [email protected]
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
1. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66. doi: 10.1161/HYP.0000000000000087
2. Krist AH, Davidson KW, Mangione CM, et al; U.S. Preventive Services Task Force. Screening for hypertension in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
3. Viera AJ, Yano Y, Lin FC, et al. Does this adult patient have hypertension?: the Rational Clinical Examination systematic review. JAMA. 2021;326:339-347. doi: 10.1001/jama.2021.4533
4. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017; 35:421-441. doi: 10.1097/HJH.0000000000001197
5. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204-208. doi: 10.1097/MBP.0000000000000133
6. Myers MG, Valdivieso M, Kiss A. Consistent relationship between automated office blood pressure recorded in different settings. Blood Press Monit. 2009;14:108-111. doi: 10.1097/MBP.0b013e32832c5167
7. Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomized parallel design controlled trial. BMJ. 2011;342:d286. doi: 10.1136/bmj.d286
8. Ringrose JS, Cena J, Ip S, et al. Comparability of automated office blood pressure to daytime 24-hour ambulatory blood pressure. Can J Cardiol. 2018;34:61-65. doi: 10.1016/j.cjca.2017.09.022
9. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33:557-576. doi: 10.1016/j.cjca.2017.03.005
10. Sakuma M, Imai Y, Nagai K, et al. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10:798-803. doi: 10.1016/s0895-7061(97)00117-9
11. Brody S, Veit R, Rau H. Four-year test-retest reliability of self-measured blood pressure. Arch Intern Med. 1999;159:1007-1008. doi: 10.1001/archinte.159.9.1007
12. Calvo-Vargas C, Padilla Rios V, Troyo-Sanromán R, et al. Reproducibility and cost of blood pressure self-measurement using the ‘Loaned Self-measurement Equipment Model.’ Blood Press Monit. 2001;6:225-232. doi: 10.1097/00126097-200110000-00001
13. Scisney-Matlock M, Grand A, Steigerwalt SP, et al. Reliability and reproducibility of clinic and home blood pressure measurements in hypertensive women according to age and ethnicity. Blood Press Monit. 2009;14:49-57. doi: 10.1097/MBP.0b013e3283263064
14. Shimbo D, Abdalla M, Falzon L, et al. Role of ambulatory and home blood pressure monitoring in clinical practice: a narrative review. Ann Intern Med. 2015;163:691-700. doi: 10.7326/M15-1270
15. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30:1289-1299. doi: 10.1097/HJH.0b013e3283531eaf
16. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep.2013;15:413. doi: 10.1007/s11886-013-0413-z
17. Shimbo D, Abdalla M, Falzon L, et al. Studies comparing ambulatory blood pressure and home blood pressure on cardiovascular disease and mortality outcomes: a systematic review. J Am Soc Hypertens. 2016;10:224-234. doi: 10.1016/j.jash.2015.12.013
18. Fagard RH, Cornelessen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198. doi: 10.1097/HJH.0b013e3282ef6185
19. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta-analysis. Am J Hypertens. 2011;24:52-58. doi: 10.1038/ajh.2010.203
20. Ohkubo T, Kikuya M, Metoki H, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508-515. doi: 10.1016/j.jacc.2005.03.070
21. Stergiou GS, Asayama K, Thijs L, et al; on behalf of the International Database on Home blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675-682. doi: 10.1161/HYPERTENSIONAHA.113.02741
22. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389. doi: 10.1371/journal.pmed.1002389
23. Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371-386. doi: 10.3109/07853890.2010.489567
24. Glynn LG, Murphy AW, Smith SM, et al. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2010;60:e476-e488. doi: 10.3399/bjgp10X544113
25. Agarwal R, Bills JE, Hecht TJ, et al. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011;57:29-38. doi: 10.1161/HYPERTENSIONAHA.110.160911
26. Stergiou GS, Skeva II, Zourbaki AS, et al. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16:725-773. doi: 10.1097/00004872-199816060-00002
27. Stergiou GS, Nasothimiou EG, Kalogeropoulos PG, et al. The optimal home blood pressure monitoring schedule based on the Didima outcome study. J Hum Hypertens. 2010;24:158-164. doi: 10.1038/jhh.2009.54
28. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785. doi: 10.1038/jhh.2010.54
29. Imai Y, Kario K, Shimada K, et al; Japanese Society of Hypertension Committee for Guidelines for Self-monitoring of Blood Pressure at Home. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res.2012;35:777-795. doi: 10.1038/hr.2012.56
30. O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010; 15:23-38. doi: 10.1097/MBP.0b013e3283360e98
31. Casiglia E, Tikhonoff V, Albertini F, et al. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68:896-903. doi: 10.1161/HYPERTENSIONAHA.116.07961
32. Harju J, Vehkaoja A, Kumpulainen P, et al. Comparison of non-invasive blood pressure monitoring using modified arterial applanation tonometry with intra-arterial measurement. J Clin Monit Comput. 2018;32:13-22. doi: 10.1007/s10877-017-9984-3
33. Ostchega Y, Hughes JP, Zhang G, et al. Mean mid-arm circumference and blood pressure cuff sizes for U.S. adults: National Health and Nutrition Examination Survey, 1999-2010. Blood Press Monit. 2013;18:138-143. doi: 10.1097/MBP.0b013e3283617606
34. White WB, Barber V. Ambulatory monitoring of blood pressure: an overview of devices, analyses, and clinical utility. In: White WB, ed. Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics. Springer International Publishing; 2016:55-76.
35. Franklin SS, Thijs L, Asayama K, et al; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033-2043. doi: 10.1016/j.jacc.2016.08.035
36. Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29-34. doi: 10.1161/01.HYP.0000197195.84725.66
37. Ewald B, Pekarsky B. Cost analysis of ambulatory blood pressure monitoring in initiating antihypertensive drug treatment in Australian general practice. Med J Aust. 2002;176:580-583. doi: 10.5694/j.1326-5377.2002.tb04588.x
38. Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension. 2019;73:121-131. doi: 10.1161/HYPERTENSIONAHA.118.11715
39. De la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898-902. doi: 10.1161/HYPERTENSIONAHA.110.168948
40. Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263-1269. doi: 10.1016/s0895-7061(01)02193-8
41. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
42. Wang YC, Shimbo D, Muntner P, et al. Prevalence of masked hypertension among US adults with non-elevated clinic blood pressure. Am J Epidemiol. 2017;185:194-202. doi: 10.1093/aje/kww237
43. Thakkar HV, Pope A, Anpalahan M. Masked hypertension: a systematic review. Heart Lung Circ. 2020;29:102-111. doi: 10.1016/j.hlc.2019.08.006
44. Kronish IM, Hughes C, Quispe K, et al. Implementing ambulatory blood pressure monitoring in primary care practice. Fam Pract Manag. 2020;27:19-25.
45. Viera AJ, Lingley K, Hinderliter AL. Tolerability of the Oscar 2 ambulatory blood pressure monitor among research participants: a cross-sectional repeated measures study. BMC Med Res Methodol. 2011;11:59. doi: 10.1186/1471-2288-11-59
46. Alpert BS, Dart RA, Sica DA. Public-use blood pressure measurement: the kiosk quandary. J Am Soc Hypertens. 2014;8:739-742. doi: 10.1016/j.jash.2014.07.034
47. Al Hamarneh YN, Houle SK, Chatterley P, et al. The validity of blood pressure kiosk validation studies: a systematic review. Blood Press Monit. 2013;18:167-172. doi: 10.1097/MBP.0b013e328360fb85
48. Kumar N, Khunger M, Gupta A, et al. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens. 2015;9:130-136. doi: 10.1016/j.jash.2014.12.001
49. Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the Task Force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21(suppl 2):4-13. doi: 10.1177/2047487314552604
50. Chandrasekaran V, Dantu R, Jonnada S, et al. Cuffless differential blood pressure estimation using smart phones. IEEE Trans Biomed Eng. 2013;60:1080-1089. doi: 10.1109/TBME.2012.2211078
PRACTICE RECOMMENDATIONS
› Use home blood pressure measurement (HBPM) for initial out-of-office evaluation to confirm hypertension. A
› Use 24-hour ambulatory measurement only when the results between office and HBPM are discordant. A
› Instruct patients to record their home BP measurements twice in the morning and twice at night for a minimum of 3 days. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Obesity interactions complex in acute pancreatitis
Obesity, in combination with other risk factors, is associated with increased morbidity and mortality in acute pancreatitis (AP); however, body mass index (BMI) alone is not a successful predictor of disease severity, new research shows.
“As there was no agreement or consistency between BMI and AP severity, it can be concluded that AP severity cannot be predicted successfully by examining BMI only,” reported the authors in research published recently in Pancreatology.
The course of acute pancreatitis is typically mild in the majority (80%-85%) of cases; however, in severe cases, permanent organ failure can occur, with much worse outcomes and mortality rates of up to 35%.
Research has previously shown not only a link between obesity and acute pancreatitis but also an increased risk for complications and in-hospital mortality in obese patients with severe cases of acute pancreatitis – though a wide range of factors and comorbidities may complicate the association.
To more closely evaluate the course and outcomes of acute pancreatitis based on BMI classification, study authors led by Ali Tuzun Ince, MD, of the department of internal medicine, Gastroenterology Clinic of Bezmialem Vakif University, Istanbul, analyzed retrospective data from 2010 to 2020 on 1,334 adult patients (720 female, 614 male) who were diagnosed with acute pancreatitis per the Revised Atlanta Classification (RAC) criteria.
The patients were stratified based on their BMI as normal weight, overweight, or obese and whether they had mild, moderate, or severe (with permanent organ failure) acute pancreatitis.
In terms of acute pancreatitis severity, based on RAC criteria, 57.1% of patients had mild disease, 20.4% had moderate disease, and 22.5% had severe disease.
The overall mortality rate was 9.9% (n = 132); half of these patients were obese, and 87% had severe acute pancreatitis.
The overall rate of complications was 42.9%, including 20.8% in the normal weight group, 40.6% in the overweight group, and 38.6% in the obese group.
Patients in the overweight and obese groups also had higher mortality rates (3.7% and 4.9%, respectively), interventional procedures (36% and 39%, respectively), and length of hospital stay (11.6% and 9.8%, respectively), compared with the normal-weight group.
Other factors that were significantly associated with an increased mortality risk, in addition to obesity (P = .046), included old age (P = .000), male sex (P = .05), alcohol use (P = .014), low hematocrit (P = .044), high C-reactive protein (P = .024), moderate to severe and severe acute pancreatitis (P = .02 and P < .001, respectively), and any complications (P < .001).
Risk factors associated with increased admission to the ICU differed from those for mortality, and included female gender (P = .024), smoking (P = .021), hypertriglyceridemia (P = .047), idiopathic etiology (P = .023), and moderate to severe and severe acute pancreatitis (P < .001).
Of note, there were no significant associations between BMI and either the RAC score or Balthazar CT severity index (Balthazar CTSI) groups.
Specifically, among patients considered to have severe acute pancreatitis per Balthazar CTSI, 6.3% were of normal weight, 5% were overweight, and 7.1% were obese.
“In addition, since agreement and consistency between BMI and Balthazar score cannot be determined, the Balthazar score cannot be estimated from BMI,” the authors reported.
While the prediction of prognosis in acute pancreatitis is gaining interest, the findings underscore the role of combined factors, they added.
“Although many scoring systems are currently in use attempt to estimate the severity [in acute pancreatitis], none is 100% accurate yet,” the authors noted. “Each risk factor exacerbates the course of disease. Therefore, it would be better to consider the combined effects of risk factors.”
That being said, the findings show “mortality is increased significantly by the combined presence of risk factors such as male sex, OB [obesity], alcohol, MSAP [moderate to severe acute pancreatitis] and SAP [severe acute pancreatitis], all kinds of complications, old age, low Hct, and high CRP,” they wrote.
Obesity’s complex interactions
Commenting on the study, Vijay P. Singh, MD, a professor of medicine in the division of gastroenterology and hepatology at the Mayo Clinic in Scottsdale, Ariz., agreed that the complexities risk factors, particularly with obesity, can be tricky to detangle.
“Broadly, the study confirms several previous reports from different parts of the world that obesity was associated with increased mortality in acute pancreatitis,” he said in an interview.
“However, obesity had two complex interactions, the first that obesity is also associated with increased diabetes, and hypertriglyceridemia, which may themselves be risk factors for severity,” he explained.
“The second one is that intermediary severity markers [e.g., Balthazar score on imaging] did not correlate with the BMI categories.”
Dr. Singh noted that is “likely because therapies like IV fluids that may get more intense in predicted severe disease alter the natural course of pancreatitis.”
The findings are a reminder that “BMI is only a number that attempts to quantify fat,” Dr. Singh said, noting that BMI doesn’t address either the location of fat, such as being in close proximity to the pancreas, or fat composition, such as the proportion of unsaturated fat.
“When the unsaturated fat proportion is higher, the pancreatitis is worse, even at smaller total fat amounts [for example, at a lower BMI],” he said. “Taking these aspects into account may help in risk assessment.”
The authors and Dr. Singh had no disclosures to report.
Obesity, in combination with other risk factors, is associated with increased morbidity and mortality in acute pancreatitis (AP); however, body mass index (BMI) alone is not a successful predictor of disease severity, new research shows.
“As there was no agreement or consistency between BMI and AP severity, it can be concluded that AP severity cannot be predicted successfully by examining BMI only,” reported the authors in research published recently in Pancreatology.
The course of acute pancreatitis is typically mild in the majority (80%-85%) of cases; however, in severe cases, permanent organ failure can occur, with much worse outcomes and mortality rates of up to 35%.
Research has previously shown not only a link between obesity and acute pancreatitis but also an increased risk for complications and in-hospital mortality in obese patients with severe cases of acute pancreatitis – though a wide range of factors and comorbidities may complicate the association.
To more closely evaluate the course and outcomes of acute pancreatitis based on BMI classification, study authors led by Ali Tuzun Ince, MD, of the department of internal medicine, Gastroenterology Clinic of Bezmialem Vakif University, Istanbul, analyzed retrospective data from 2010 to 2020 on 1,334 adult patients (720 female, 614 male) who were diagnosed with acute pancreatitis per the Revised Atlanta Classification (RAC) criteria.
The patients were stratified based on their BMI as normal weight, overweight, or obese and whether they had mild, moderate, or severe (with permanent organ failure) acute pancreatitis.
In terms of acute pancreatitis severity, based on RAC criteria, 57.1% of patients had mild disease, 20.4% had moderate disease, and 22.5% had severe disease.
The overall mortality rate was 9.9% (n = 132); half of these patients were obese, and 87% had severe acute pancreatitis.
The overall rate of complications was 42.9%, including 20.8% in the normal weight group, 40.6% in the overweight group, and 38.6% in the obese group.
Patients in the overweight and obese groups also had higher mortality rates (3.7% and 4.9%, respectively), interventional procedures (36% and 39%, respectively), and length of hospital stay (11.6% and 9.8%, respectively), compared with the normal-weight group.
Other factors that were significantly associated with an increased mortality risk, in addition to obesity (P = .046), included old age (P = .000), male sex (P = .05), alcohol use (P = .014), low hematocrit (P = .044), high C-reactive protein (P = .024), moderate to severe and severe acute pancreatitis (P = .02 and P < .001, respectively), and any complications (P < .001).
Risk factors associated with increased admission to the ICU differed from those for mortality, and included female gender (P = .024), smoking (P = .021), hypertriglyceridemia (P = .047), idiopathic etiology (P = .023), and moderate to severe and severe acute pancreatitis (P < .001).
Of note, there were no significant associations between BMI and either the RAC score or Balthazar CT severity index (Balthazar CTSI) groups.
Specifically, among patients considered to have severe acute pancreatitis per Balthazar CTSI, 6.3% were of normal weight, 5% were overweight, and 7.1% were obese.
“In addition, since agreement and consistency between BMI and Balthazar score cannot be determined, the Balthazar score cannot be estimated from BMI,” the authors reported.
While the prediction of prognosis in acute pancreatitis is gaining interest, the findings underscore the role of combined factors, they added.
“Although many scoring systems are currently in use attempt to estimate the severity [in acute pancreatitis], none is 100% accurate yet,” the authors noted. “Each risk factor exacerbates the course of disease. Therefore, it would be better to consider the combined effects of risk factors.”
That being said, the findings show “mortality is increased significantly by the combined presence of risk factors such as male sex, OB [obesity], alcohol, MSAP [moderate to severe acute pancreatitis] and SAP [severe acute pancreatitis], all kinds of complications, old age, low Hct, and high CRP,” they wrote.
Obesity’s complex interactions
Commenting on the study, Vijay P. Singh, MD, a professor of medicine in the division of gastroenterology and hepatology at the Mayo Clinic in Scottsdale, Ariz., agreed that the complexities risk factors, particularly with obesity, can be tricky to detangle.
“Broadly, the study confirms several previous reports from different parts of the world that obesity was associated with increased mortality in acute pancreatitis,” he said in an interview.
“However, obesity had two complex interactions, the first that obesity is also associated with increased diabetes, and hypertriglyceridemia, which may themselves be risk factors for severity,” he explained.
“The second one is that intermediary severity markers [e.g., Balthazar score on imaging] did not correlate with the BMI categories.”
Dr. Singh noted that is “likely because therapies like IV fluids that may get more intense in predicted severe disease alter the natural course of pancreatitis.”
The findings are a reminder that “BMI is only a number that attempts to quantify fat,” Dr. Singh said, noting that BMI doesn’t address either the location of fat, such as being in close proximity to the pancreas, or fat composition, such as the proportion of unsaturated fat.
“When the unsaturated fat proportion is higher, the pancreatitis is worse, even at smaller total fat amounts [for example, at a lower BMI],” he said. “Taking these aspects into account may help in risk assessment.”
The authors and Dr. Singh had no disclosures to report.
Obesity, in combination with other risk factors, is associated with increased morbidity and mortality in acute pancreatitis (AP); however, body mass index (BMI) alone is not a successful predictor of disease severity, new research shows.
“As there was no agreement or consistency between BMI and AP severity, it can be concluded that AP severity cannot be predicted successfully by examining BMI only,” reported the authors in research published recently in Pancreatology.
The course of acute pancreatitis is typically mild in the majority (80%-85%) of cases; however, in severe cases, permanent organ failure can occur, with much worse outcomes and mortality rates of up to 35%.
Research has previously shown not only a link between obesity and acute pancreatitis but also an increased risk for complications and in-hospital mortality in obese patients with severe cases of acute pancreatitis – though a wide range of factors and comorbidities may complicate the association.
To more closely evaluate the course and outcomes of acute pancreatitis based on BMI classification, study authors led by Ali Tuzun Ince, MD, of the department of internal medicine, Gastroenterology Clinic of Bezmialem Vakif University, Istanbul, analyzed retrospective data from 2010 to 2020 on 1,334 adult patients (720 female, 614 male) who were diagnosed with acute pancreatitis per the Revised Atlanta Classification (RAC) criteria.
The patients were stratified based on their BMI as normal weight, overweight, or obese and whether they had mild, moderate, or severe (with permanent organ failure) acute pancreatitis.
In terms of acute pancreatitis severity, based on RAC criteria, 57.1% of patients had mild disease, 20.4% had moderate disease, and 22.5% had severe disease.
The overall mortality rate was 9.9% (n = 132); half of these patients were obese, and 87% had severe acute pancreatitis.
The overall rate of complications was 42.9%, including 20.8% in the normal weight group, 40.6% in the overweight group, and 38.6% in the obese group.
Patients in the overweight and obese groups also had higher mortality rates (3.7% and 4.9%, respectively), interventional procedures (36% and 39%, respectively), and length of hospital stay (11.6% and 9.8%, respectively), compared with the normal-weight group.
Other factors that were significantly associated with an increased mortality risk, in addition to obesity (P = .046), included old age (P = .000), male sex (P = .05), alcohol use (P = .014), low hematocrit (P = .044), high C-reactive protein (P = .024), moderate to severe and severe acute pancreatitis (P = .02 and P < .001, respectively), and any complications (P < .001).
Risk factors associated with increased admission to the ICU differed from those for mortality, and included female gender (P = .024), smoking (P = .021), hypertriglyceridemia (P = .047), idiopathic etiology (P = .023), and moderate to severe and severe acute pancreatitis (P < .001).
Of note, there were no significant associations between BMI and either the RAC score or Balthazar CT severity index (Balthazar CTSI) groups.
Specifically, among patients considered to have severe acute pancreatitis per Balthazar CTSI, 6.3% were of normal weight, 5% were overweight, and 7.1% were obese.
“In addition, since agreement and consistency between BMI and Balthazar score cannot be determined, the Balthazar score cannot be estimated from BMI,” the authors reported.
While the prediction of prognosis in acute pancreatitis is gaining interest, the findings underscore the role of combined factors, they added.
“Although many scoring systems are currently in use attempt to estimate the severity [in acute pancreatitis], none is 100% accurate yet,” the authors noted. “Each risk factor exacerbates the course of disease. Therefore, it would be better to consider the combined effects of risk factors.”
That being said, the findings show “mortality is increased significantly by the combined presence of risk factors such as male sex, OB [obesity], alcohol, MSAP [moderate to severe acute pancreatitis] and SAP [severe acute pancreatitis], all kinds of complications, old age, low Hct, and high CRP,” they wrote.
Obesity’s complex interactions
Commenting on the study, Vijay P. Singh, MD, a professor of medicine in the division of gastroenterology and hepatology at the Mayo Clinic in Scottsdale, Ariz., agreed that the complexities risk factors, particularly with obesity, can be tricky to detangle.
“Broadly, the study confirms several previous reports from different parts of the world that obesity was associated with increased mortality in acute pancreatitis,” he said in an interview.
“However, obesity had two complex interactions, the first that obesity is also associated with increased diabetes, and hypertriglyceridemia, which may themselves be risk factors for severity,” he explained.
“The second one is that intermediary severity markers [e.g., Balthazar score on imaging] did not correlate with the BMI categories.”
Dr. Singh noted that is “likely because therapies like IV fluids that may get more intense in predicted severe disease alter the natural course of pancreatitis.”
The findings are a reminder that “BMI is only a number that attempts to quantify fat,” Dr. Singh said, noting that BMI doesn’t address either the location of fat, such as being in close proximity to the pancreas, or fat composition, such as the proportion of unsaturated fat.
“When the unsaturated fat proportion is higher, the pancreatitis is worse, even at smaller total fat amounts [for example, at a lower BMI],” he said. “Taking these aspects into account may help in risk assessment.”
The authors and Dr. Singh had no disclosures to report.
FROM PANCREATOLOGY
Retiform Purpura on the Legs
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2
Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
A 70-year-old woman with a medical history of Takayasu arteritis, end-stage renal disease on peritoneal dialysis, coronary artery disease, hypertension, hypothyroidism, and anemia of chronic disease presented to the emergency department with enlarging painful stellate eschars of the legs with associated edema of 3 weeks’ duration. She denied a history of similar-appearing skin lesions. She initially thought the lesions were burns secondary to frequent hot showers for relief of uremic pruritus. For the treatment of these suspected burns prior to hospitalization, she had been applying over-the-counter antibiotic ointments to the affected areas and had completed a 2-week course of oral cephalexin without notable improvement. Physical examination revealed retiform purpura of the legs with large stellate eschars overlying the anteromedial thighs and right medial calf. Computed tomography angiogram of the abdomen and pelvis demonstrated diffuse calcifications of the aortic wall and its associated branches that were most pronounced in the legs without evidence of vessel wall thickening. Punch biopsies were performed, and nephrology, rheumatology, and wound care services were consulted.
Melanoma
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.

Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.

Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
More selective antibiotic shows promise for C. diff. infection
An investigational, novel oral antibiotic with greater selectivity than vancomycin, metronidazole, and even fidaxomicin may offer improved protection of healthy gut bacteria during the treatment of Clostridium difficile infection (CDI), according to ongoing research.
“CDI treatment has historically been dominated by metronidazole and vancomycin,” said Katherine Johnson, DO, from the Western Infectious Disease Consultants, P.C., Denver. However, these broad-spectrum drugs negatively affect healthy bacteria in the gut and increase the risk of CDI recurrence.
This is also a problem for drugs in the CDI antibiotic pipeline: Many candidate drugs have failed because of their broad-spectrum activity, she added during a session at the Peggy Lillis Foundation 2022 National C. diff. Advocacy Summit.
“An ideal CDI therapy would be a very narrow-spectrum antibiotic that has a minimal effect on normal gut bacteria,” she said.
Dr. Johnson is currently working on a phase 2 clinical trial that is evaluating the novel antibiotic, dubbed CRS3123, for the treatment of primary CDI and first-recurrence CDI. The investigational agent targets and inhibits a form of the methionyl tRNA synthetase enzyme, which is strictly required for protein biosynthesis in C. diff. and is therefore an ideal target for treatment of primary and recurrent CDI.
In her session, Dr. Johnson reported that CRS3123 inhibits the damaging toxins produced by C. diff., potentially resulting in more rapid symptom resolution. Additionally, owing to its novel mode of action, no strains are currently resistant to CRS3123.
She presented findings from an animal study that showed that CRS3123 was superior to vancomycin in terms of prolonging survival. She also presented findings from phase 1 clinical trials that showed that most adverse events (AEs) associated with CRS3123 were mild. No serious AEs were reported.
A ‘huge infectious burden’
If successful in further research, CRS3123 could offer significant value to patients with C. diff., especially those with recurrent infection, given the sometimes extreme clinical, quality-of-life, and economic burdens associated with CDI.
“CDI is a huge infectious burden to the U.S. health care system and globally has been listed by the Centers for Disease Control and Prevention as an urgent threat,” Byron Vaughn, MD, from the University of Minnesota, told this news organization.
“Despite a number of antibiotic stewardship and infection control and prevention efforts, we haven’t seen much of a change in the incidence of CDI,” he said. He said that the risk of recurrence can be as high as 30%.
While oral vancomycin is effective for treating C. diff., Dr. Vaughn noted that the antibiotic lacks selectivity and destroys healthy gut bacteria, resulting in substantial dysbiosis. “Dysbiosis is really the key to getting recurrent C. diff.,” he explained, “because if you have healthy gut bacteria, you will inherently resist CDI.”
Dr. Vaughn stated that his center is in the startup phase for being a site for a clinical trial of CRS3123. The hope is that CRS3123, because its spectrum is narrower than that of fidaxomicin and vancomycin, doesn’t induce intestinal dysbiosis. “It really just treats the C. diff. and leaves every other bug alone so that your gut bacteria can recover while the C. diff. is being treated,” he said. “And then when you stop CRS3123, you have healthy gut bacteria already present to prevent recurrence.”
If this is confirmed in large-scale trials, there could be a “dramatic decrease” in the rates of recurrent C. diff., said Dr. Vaughn.
Aside from the potential clinical impact, the economic implications of a novel selective antibiotic that preserves healthy gut bacteria could be significant, he added. “Depending on exactly what population you’re looking at, probably about a third of the cost of C. diff. is actually attributable to recurrence. That’s a huge economic burden that could be improved.”
Dr. Johnson is an employee of Crestone, which is developing CRS3123. Dr. Vaughn reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An investigational, novel oral antibiotic with greater selectivity than vancomycin, metronidazole, and even fidaxomicin may offer improved protection of healthy gut bacteria during the treatment of Clostridium difficile infection (CDI), according to ongoing research.
“CDI treatment has historically been dominated by metronidazole and vancomycin,” said Katherine Johnson, DO, from the Western Infectious Disease Consultants, P.C., Denver. However, these broad-spectrum drugs negatively affect healthy bacteria in the gut and increase the risk of CDI recurrence.
This is also a problem for drugs in the CDI antibiotic pipeline: Many candidate drugs have failed because of their broad-spectrum activity, she added during a session at the Peggy Lillis Foundation 2022 National C. diff. Advocacy Summit.
“An ideal CDI therapy would be a very narrow-spectrum antibiotic that has a minimal effect on normal gut bacteria,” she said.
Dr. Johnson is currently working on a phase 2 clinical trial that is evaluating the novel antibiotic, dubbed CRS3123, for the treatment of primary CDI and first-recurrence CDI. The investigational agent targets and inhibits a form of the methionyl tRNA synthetase enzyme, which is strictly required for protein biosynthesis in C. diff. and is therefore an ideal target for treatment of primary and recurrent CDI.
In her session, Dr. Johnson reported that CRS3123 inhibits the damaging toxins produced by C. diff., potentially resulting in more rapid symptom resolution. Additionally, owing to its novel mode of action, no strains are currently resistant to CRS3123.
She presented findings from an animal study that showed that CRS3123 was superior to vancomycin in terms of prolonging survival. She also presented findings from phase 1 clinical trials that showed that most adverse events (AEs) associated with CRS3123 were mild. No serious AEs were reported.
A ‘huge infectious burden’
If successful in further research, CRS3123 could offer significant value to patients with C. diff., especially those with recurrent infection, given the sometimes extreme clinical, quality-of-life, and economic burdens associated with CDI.
“CDI is a huge infectious burden to the U.S. health care system and globally has been listed by the Centers for Disease Control and Prevention as an urgent threat,” Byron Vaughn, MD, from the University of Minnesota, told this news organization.
“Despite a number of antibiotic stewardship and infection control and prevention efforts, we haven’t seen much of a change in the incidence of CDI,” he said. He said that the risk of recurrence can be as high as 30%.
While oral vancomycin is effective for treating C. diff., Dr. Vaughn noted that the antibiotic lacks selectivity and destroys healthy gut bacteria, resulting in substantial dysbiosis. “Dysbiosis is really the key to getting recurrent C. diff.,” he explained, “because if you have healthy gut bacteria, you will inherently resist CDI.”
Dr. Vaughn stated that his center is in the startup phase for being a site for a clinical trial of CRS3123. The hope is that CRS3123, because its spectrum is narrower than that of fidaxomicin and vancomycin, doesn’t induce intestinal dysbiosis. “It really just treats the C. diff. and leaves every other bug alone so that your gut bacteria can recover while the C. diff. is being treated,” he said. “And then when you stop CRS3123, you have healthy gut bacteria already present to prevent recurrence.”
If this is confirmed in large-scale trials, there could be a “dramatic decrease” in the rates of recurrent C. diff., said Dr. Vaughn.
Aside from the potential clinical impact, the economic implications of a novel selective antibiotic that preserves healthy gut bacteria could be significant, he added. “Depending on exactly what population you’re looking at, probably about a third of the cost of C. diff. is actually attributable to recurrence. That’s a huge economic burden that could be improved.”
Dr. Johnson is an employee of Crestone, which is developing CRS3123. Dr. Vaughn reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An investigational, novel oral antibiotic with greater selectivity than vancomycin, metronidazole, and even fidaxomicin may offer improved protection of healthy gut bacteria during the treatment of Clostridium difficile infection (CDI), according to ongoing research.
“CDI treatment has historically been dominated by metronidazole and vancomycin,” said Katherine Johnson, DO, from the Western Infectious Disease Consultants, P.C., Denver. However, these broad-spectrum drugs negatively affect healthy bacteria in the gut and increase the risk of CDI recurrence.
This is also a problem for drugs in the CDI antibiotic pipeline: Many candidate drugs have failed because of their broad-spectrum activity, she added during a session at the Peggy Lillis Foundation 2022 National C. diff. Advocacy Summit.
“An ideal CDI therapy would be a very narrow-spectrum antibiotic that has a minimal effect on normal gut bacteria,” she said.
Dr. Johnson is currently working on a phase 2 clinical trial that is evaluating the novel antibiotic, dubbed CRS3123, for the treatment of primary CDI and first-recurrence CDI. The investigational agent targets and inhibits a form of the methionyl tRNA synthetase enzyme, which is strictly required for protein biosynthesis in C. diff. and is therefore an ideal target for treatment of primary and recurrent CDI.
In her session, Dr. Johnson reported that CRS3123 inhibits the damaging toxins produced by C. diff., potentially resulting in more rapid symptom resolution. Additionally, owing to its novel mode of action, no strains are currently resistant to CRS3123.
She presented findings from an animal study that showed that CRS3123 was superior to vancomycin in terms of prolonging survival. She also presented findings from phase 1 clinical trials that showed that most adverse events (AEs) associated with CRS3123 were mild. No serious AEs were reported.
A ‘huge infectious burden’
If successful in further research, CRS3123 could offer significant value to patients with C. diff., especially those with recurrent infection, given the sometimes extreme clinical, quality-of-life, and economic burdens associated with CDI.
“CDI is a huge infectious burden to the U.S. health care system and globally has been listed by the Centers for Disease Control and Prevention as an urgent threat,” Byron Vaughn, MD, from the University of Minnesota, told this news organization.
“Despite a number of antibiotic stewardship and infection control and prevention efforts, we haven’t seen much of a change in the incidence of CDI,” he said. He said that the risk of recurrence can be as high as 30%.
While oral vancomycin is effective for treating C. diff., Dr. Vaughn noted that the antibiotic lacks selectivity and destroys healthy gut bacteria, resulting in substantial dysbiosis. “Dysbiosis is really the key to getting recurrent C. diff.,” he explained, “because if you have healthy gut bacteria, you will inherently resist CDI.”
Dr. Vaughn stated that his center is in the startup phase for being a site for a clinical trial of CRS3123. The hope is that CRS3123, because its spectrum is narrower than that of fidaxomicin and vancomycin, doesn’t induce intestinal dysbiosis. “It really just treats the C. diff. and leaves every other bug alone so that your gut bacteria can recover while the C. diff. is being treated,” he said. “And then when you stop CRS3123, you have healthy gut bacteria already present to prevent recurrence.”
If this is confirmed in large-scale trials, there could be a “dramatic decrease” in the rates of recurrent C. diff., said Dr. Vaughn.
Aside from the potential clinical impact, the economic implications of a novel selective antibiotic that preserves healthy gut bacteria could be significant, he added. “Depending on exactly what population you’re looking at, probably about a third of the cost of C. diff. is actually attributable to recurrence. That’s a huge economic burden that could be improved.”
Dr. Johnson is an employee of Crestone, which is developing CRS3123. Dr. Vaughn reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The importance of toxin testing in C. difficile infection: Understanding the results
Clostridioides difficile infection is often confirmed through toxin testing, yet toxin tests alone may not be sufficient for diagnosing and guiding treatment decisions for patients with CDI.
“The presence of a toxigenic strain does not always equal disease,” said David Lyerly, PhD, during a session on C. difficile toxin testing at the Peggy Lillis Foundation 2022 National C. diff Advocacy Summit.
Dr. Lyerly, the chief science officer at Techlab, explained that exotoxins A and B are produced by specific strains of C. difficile and are involved in infections, but some patients who test positive for these toxins by polymerase chain reaction or other tests do not have CDI or they are not appropriate candidates for CDI treatment.
Several studies conducted during the past decade, however, support the importance of toxin detection. Some research has suggested that toxin-positive patients tend to have more clinically severe disease than those who test negative, he noted.
Although its use is limited when it is used alone, toxin testing is needed to confirm a CDI diagnosis and to ensure antibiotic stewardship, Dr. Lyerly said.
He suggested that, in addition to toxin testing, there is a need for molecular measures and other improved diagnostics to identify candidates most likely to benefit from CDI treatment.
“Because we generally detect toxin genes instead of toxin proteins, you can identify persons colonized with toxigenic C. difficile who do not actually have CDI,” Kevin W. Garey, PharmD, from the University of Houston, said in an interview.
Dr. Garey added that a person could likewise have low levels of toxins that aren’t detected by toxin tests but could still have CDI.
“Given this, better diagnostics that incorporate active toxin production and your body’s response to those toxins are needed,” he said, especially since C. difficile toxins are responsible for disease sequelae, including gastroenteritis, colonic perforation, sepsis, and death.
Toxin testing a ‘controversial area’
“C. difficile toxin testing has been a controversial area for almost a decade or more,” Shruti K. Gohil, MD, from University of California, Irvine, Health Epidemiology and Infection Prevention, said in an interview.
Dr. Gohil noted that toxin testing is a better test for clinical C. difficile colitis but by itself can miss C. difficile. “So, we are in this conundrum nationally,” she said.
“Many facilities will use a double- or triple-test strategy to make sure that you have a true C. difficile case mandating the use of antibiotics,” she explained. “The reason we test specifically with the enzyme immunoassay or toxin test is to know whether or not you have real C. difficile that’s actively producing the toxin for colitis.”
A patient with C. difficile who has been treated and is in recovery may still test positive on a C. difficile toxin test, added Dr. Gohil. “It would be great if we had a test that could really judge an active, clinical C. difficile infection. This [test] would help in identifying the right patients who need treatment and would also be able to tell if a patient has been cleared of C. difficile.”
Dr. Lyerly is an employee of Techlab. Dr. Garey and Dr. Gohil reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clostridioides difficile infection is often confirmed through toxin testing, yet toxin tests alone may not be sufficient for diagnosing and guiding treatment decisions for patients with CDI.
“The presence of a toxigenic strain does not always equal disease,” said David Lyerly, PhD, during a session on C. difficile toxin testing at the Peggy Lillis Foundation 2022 National C. diff Advocacy Summit.
Dr. Lyerly, the chief science officer at Techlab, explained that exotoxins A and B are produced by specific strains of C. difficile and are involved in infections, but some patients who test positive for these toxins by polymerase chain reaction or other tests do not have CDI or they are not appropriate candidates for CDI treatment.
Several studies conducted during the past decade, however, support the importance of toxin detection. Some research has suggested that toxin-positive patients tend to have more clinically severe disease than those who test negative, he noted.
Although its use is limited when it is used alone, toxin testing is needed to confirm a CDI diagnosis and to ensure antibiotic stewardship, Dr. Lyerly said.
He suggested that, in addition to toxin testing, there is a need for molecular measures and other improved diagnostics to identify candidates most likely to benefit from CDI treatment.
“Because we generally detect toxin genes instead of toxin proteins, you can identify persons colonized with toxigenic C. difficile who do not actually have CDI,” Kevin W. Garey, PharmD, from the University of Houston, said in an interview.
Dr. Garey added that a person could likewise have low levels of toxins that aren’t detected by toxin tests but could still have CDI.
“Given this, better diagnostics that incorporate active toxin production and your body’s response to those toxins are needed,” he said, especially since C. difficile toxins are responsible for disease sequelae, including gastroenteritis, colonic perforation, sepsis, and death.
Toxin testing a ‘controversial area’
“C. difficile toxin testing has been a controversial area for almost a decade or more,” Shruti K. Gohil, MD, from University of California, Irvine, Health Epidemiology and Infection Prevention, said in an interview.
Dr. Gohil noted that toxin testing is a better test for clinical C. difficile colitis but by itself can miss C. difficile. “So, we are in this conundrum nationally,” she said.
“Many facilities will use a double- or triple-test strategy to make sure that you have a true C. difficile case mandating the use of antibiotics,” she explained. “The reason we test specifically with the enzyme immunoassay or toxin test is to know whether or not you have real C. difficile that’s actively producing the toxin for colitis.”
A patient with C. difficile who has been treated and is in recovery may still test positive on a C. difficile toxin test, added Dr. Gohil. “It would be great if we had a test that could really judge an active, clinical C. difficile infection. This [test] would help in identifying the right patients who need treatment and would also be able to tell if a patient has been cleared of C. difficile.”
Dr. Lyerly is an employee of Techlab. Dr. Garey and Dr. Gohil reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clostridioides difficile infection is often confirmed through toxin testing, yet toxin tests alone may not be sufficient for diagnosing and guiding treatment decisions for patients with CDI.
“The presence of a toxigenic strain does not always equal disease,” said David Lyerly, PhD, during a session on C. difficile toxin testing at the Peggy Lillis Foundation 2022 National C. diff Advocacy Summit.
Dr. Lyerly, the chief science officer at Techlab, explained that exotoxins A and B are produced by specific strains of C. difficile and are involved in infections, but some patients who test positive for these toxins by polymerase chain reaction or other tests do not have CDI or they are not appropriate candidates for CDI treatment.
Several studies conducted during the past decade, however, support the importance of toxin detection. Some research has suggested that toxin-positive patients tend to have more clinically severe disease than those who test negative, he noted.
Although its use is limited when it is used alone, toxin testing is needed to confirm a CDI diagnosis and to ensure antibiotic stewardship, Dr. Lyerly said.
He suggested that, in addition to toxin testing, there is a need for molecular measures and other improved diagnostics to identify candidates most likely to benefit from CDI treatment.
“Because we generally detect toxin genes instead of toxin proteins, you can identify persons colonized with toxigenic C. difficile who do not actually have CDI,” Kevin W. Garey, PharmD, from the University of Houston, said in an interview.
Dr. Garey added that a person could likewise have low levels of toxins that aren’t detected by toxin tests but could still have CDI.
“Given this, better diagnostics that incorporate active toxin production and your body’s response to those toxins are needed,” he said, especially since C. difficile toxins are responsible for disease sequelae, including gastroenteritis, colonic perforation, sepsis, and death.
Toxin testing a ‘controversial area’
“C. difficile toxin testing has been a controversial area for almost a decade or more,” Shruti K. Gohil, MD, from University of California, Irvine, Health Epidemiology and Infection Prevention, said in an interview.
Dr. Gohil noted that toxin testing is a better test for clinical C. difficile colitis but by itself can miss C. difficile. “So, we are in this conundrum nationally,” she said.
“Many facilities will use a double- or triple-test strategy to make sure that you have a true C. difficile case mandating the use of antibiotics,” she explained. “The reason we test specifically with the enzyme immunoassay or toxin test is to know whether or not you have real C. difficile that’s actively producing the toxin for colitis.”
A patient with C. difficile who has been treated and is in recovery may still test positive on a C. difficile toxin test, added Dr. Gohil. “It would be great if we had a test that could really judge an active, clinical C. difficile infection. This [test] would help in identifying the right patients who need treatment and would also be able to tell if a patient has been cleared of C. difficile.”
Dr. Lyerly is an employee of Techlab. Dr. Garey and Dr. Gohil reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.