User login
COPD screening for asymptomatic adults? USPSTF weighs in, again
Screening for chronic obstructive pulmonary disease (COPD) in asymptomatic adults has no net benefit, according to a U.S. Preventive Services Task Force (USPSTF) reassessment of its 2016 screening recommendations. The new recommendation is in line with the previous one and is made with moderate certainty (grade D evidence).
The USPSTF recommendation applies to adults who do not recognize or report respiratory symptoms. It does not apply to people with symptoms such as chronic cough, sputum production, difficulty breathing, or wheezing, or those known to be at very high risk for COPD. These latter include people with alpha-1 antitrypsin deficiency or workers exposed to certain toxins at their jobs, according to the report published in JAMA.
“Considering that the outcomes of several other chronic conditions, including cardiovascular disease and cancer, have been improved over the years with early detection and intervention, it is logical to ask whether screening to achieve early detection of COPD might also lead to better outcomes,” Surya P. Bhatt, MD, of the University of Alabama at Birmingham, and George T. O’Connor, MD, of the Boston University, explained in an editorial.
Task force assessment
The task force examined relevant publications after the 2016 deliberations and found no new studies that directly assessed the effects of screening for COPD in asymptomatic adults on morbidity, mortality, or health-related quality of life.
Although, as in their previous review, serious harms from treatment trials were not consistently reported, more recent large observational studies in screen-relevant populations suggested possible harms from the initiation of long-acting beta-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and the use of inhaled corticosteroids.
“In addition to potential treatment harms, there are opportunity costs to screening that may include time spent on counseling and providing services and patient referrals for diagnostic testing,” the task force stated.
Because cigarette smoking is the leading cause of COPD, the USPSTF has reiterated its recommendations for physicians to address tobacco smoking cessation in adults, including pregnant persons, as well as tobacco use in children and adolescents.
Not the whole story?
“Truly asymptomatic individuals with airflow obstruction do not meet criteria for COPD therapy, but sensitive questionnaires may detect symptoms not previously reported by the patient. It may be more effective to redirect the focus from screening for asymptomatic COPD to case finding using sensitive and cost-effective tools,” Dr. Bhatt and Dr. O’Connor suggested in their editorial.
“Even though available data may not support screening asymptomatic adults for COPD, there is substantial rationale for further investigation of strategies to enhance earlier detection of this condition,” they concluded.
More research needed
Despite the recommendation, the USPSTF indicated that further studies are needed to fill in research gaps, including:
- The effectiveness of screening asymptomatic adults for COPD to reduce morbidity or mortality or improve health-related quality of life, with long-term follow-up.
- The effectiveness of early treatment for asymptomatic, minimally symptomatic, or screen-detected populations to slow disease progression and improve health outcomes, with long-term follow-up.
- The harms of screening in and treatment of persons with asymptomatic or minimally symptomatic COPD.
The USPSTF is an independent, voluntary body, and potential conflicts of interest of the members are on file with the organization. Dr. Bhatt reported serving on an advisory board for Boehringer Ingelheim and receiving consulting fees from Sanofi/Regeneron; and Dr. O’Connor reported receiving consulting fees from Grupo Menarini and Dicerna Pharmaceuticals.
Screening for chronic obstructive pulmonary disease (COPD) in asymptomatic adults has no net benefit, according to a U.S. Preventive Services Task Force (USPSTF) reassessment of its 2016 screening recommendations. The new recommendation is in line with the previous one and is made with moderate certainty (grade D evidence).
The USPSTF recommendation applies to adults who do not recognize or report respiratory symptoms. It does not apply to people with symptoms such as chronic cough, sputum production, difficulty breathing, or wheezing, or those known to be at very high risk for COPD. These latter include people with alpha-1 antitrypsin deficiency or workers exposed to certain toxins at their jobs, according to the report published in JAMA.
“Considering that the outcomes of several other chronic conditions, including cardiovascular disease and cancer, have been improved over the years with early detection and intervention, it is logical to ask whether screening to achieve early detection of COPD might also lead to better outcomes,” Surya P. Bhatt, MD, of the University of Alabama at Birmingham, and George T. O’Connor, MD, of the Boston University, explained in an editorial.
Task force assessment
The task force examined relevant publications after the 2016 deliberations and found no new studies that directly assessed the effects of screening for COPD in asymptomatic adults on morbidity, mortality, or health-related quality of life.
Although, as in their previous review, serious harms from treatment trials were not consistently reported, more recent large observational studies in screen-relevant populations suggested possible harms from the initiation of long-acting beta-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and the use of inhaled corticosteroids.
“In addition to potential treatment harms, there are opportunity costs to screening that may include time spent on counseling and providing services and patient referrals for diagnostic testing,” the task force stated.
Because cigarette smoking is the leading cause of COPD, the USPSTF has reiterated its recommendations for physicians to address tobacco smoking cessation in adults, including pregnant persons, as well as tobacco use in children and adolescents.
Not the whole story?
“Truly asymptomatic individuals with airflow obstruction do not meet criteria for COPD therapy, but sensitive questionnaires may detect symptoms not previously reported by the patient. It may be more effective to redirect the focus from screening for asymptomatic COPD to case finding using sensitive and cost-effective tools,” Dr. Bhatt and Dr. O’Connor suggested in their editorial.
“Even though available data may not support screening asymptomatic adults for COPD, there is substantial rationale for further investigation of strategies to enhance earlier detection of this condition,” they concluded.
More research needed
Despite the recommendation, the USPSTF indicated that further studies are needed to fill in research gaps, including:
- The effectiveness of screening asymptomatic adults for COPD to reduce morbidity or mortality or improve health-related quality of life, with long-term follow-up.
- The effectiveness of early treatment for asymptomatic, minimally symptomatic, or screen-detected populations to slow disease progression and improve health outcomes, with long-term follow-up.
- The harms of screening in and treatment of persons with asymptomatic or minimally symptomatic COPD.
The USPSTF is an independent, voluntary body, and potential conflicts of interest of the members are on file with the organization. Dr. Bhatt reported serving on an advisory board for Boehringer Ingelheim and receiving consulting fees from Sanofi/Regeneron; and Dr. O’Connor reported receiving consulting fees from Grupo Menarini and Dicerna Pharmaceuticals.
Screening for chronic obstructive pulmonary disease (COPD) in asymptomatic adults has no net benefit, according to a U.S. Preventive Services Task Force (USPSTF) reassessment of its 2016 screening recommendations. The new recommendation is in line with the previous one and is made with moderate certainty (grade D evidence).
The USPSTF recommendation applies to adults who do not recognize or report respiratory symptoms. It does not apply to people with symptoms such as chronic cough, sputum production, difficulty breathing, or wheezing, or those known to be at very high risk for COPD. These latter include people with alpha-1 antitrypsin deficiency or workers exposed to certain toxins at their jobs, according to the report published in JAMA.
“Considering that the outcomes of several other chronic conditions, including cardiovascular disease and cancer, have been improved over the years with early detection and intervention, it is logical to ask whether screening to achieve early detection of COPD might also lead to better outcomes,” Surya P. Bhatt, MD, of the University of Alabama at Birmingham, and George T. O’Connor, MD, of the Boston University, explained in an editorial.
Task force assessment
The task force examined relevant publications after the 2016 deliberations and found no new studies that directly assessed the effects of screening for COPD in asymptomatic adults on morbidity, mortality, or health-related quality of life.
Although, as in their previous review, serious harms from treatment trials were not consistently reported, more recent large observational studies in screen-relevant populations suggested possible harms from the initiation of long-acting beta-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), and the use of inhaled corticosteroids.
“In addition to potential treatment harms, there are opportunity costs to screening that may include time spent on counseling and providing services and patient referrals for diagnostic testing,” the task force stated.
Because cigarette smoking is the leading cause of COPD, the USPSTF has reiterated its recommendations for physicians to address tobacco smoking cessation in adults, including pregnant persons, as well as tobacco use in children and adolescents.
Not the whole story?
“Truly asymptomatic individuals with airflow obstruction do not meet criteria for COPD therapy, but sensitive questionnaires may detect symptoms not previously reported by the patient. It may be more effective to redirect the focus from screening for asymptomatic COPD to case finding using sensitive and cost-effective tools,” Dr. Bhatt and Dr. O’Connor suggested in their editorial.
“Even though available data may not support screening asymptomatic adults for COPD, there is substantial rationale for further investigation of strategies to enhance earlier detection of this condition,” they concluded.
More research needed
Despite the recommendation, the USPSTF indicated that further studies are needed to fill in research gaps, including:
- The effectiveness of screening asymptomatic adults for COPD to reduce morbidity or mortality or improve health-related quality of life, with long-term follow-up.
- The effectiveness of early treatment for asymptomatic, minimally symptomatic, or screen-detected populations to slow disease progression and improve health outcomes, with long-term follow-up.
- The harms of screening in and treatment of persons with asymptomatic or minimally symptomatic COPD.
The USPSTF is an independent, voluntary body, and potential conflicts of interest of the members are on file with the organization. Dr. Bhatt reported serving on an advisory board for Boehringer Ingelheim and receiving consulting fees from Sanofi/Regeneron; and Dr. O’Connor reported receiving consulting fees from Grupo Menarini and Dicerna Pharmaceuticals.
FROM JAMA
Mental Health Support of Frontline Medical Personnel in the Javits New York Medical Station Federal COVID-19 Treatment Center
New York City (NYC) was the early epicenter of the COVID-19 pandemic in the United States. By late March 2020, NYC hospitals were overwhelmed, leading to the development of a 452-bed field hospital that became the Javits New York Medical Station (JNYMS).1,2 More than 600 uniformed and other federal personnel, including medical personnel from US Army, Navy, and Public Health Service Commissioned Corps, mobilized to provide medical support to the JNYMS in late March 2020, leading to the treatment of more than 1000 patients with COVID-19 within a 30-day period.1
Literature from the SARS, Ebola, and HIV epidemics indicate that adverse mental health consequences, including burnout, depression, and posttraumatic stress disorder symptoms are common in frontline medical workers.3,4 Emerging data shows a similar trend occurring during the COVID-19 pandemic.5 A recent publication detailed the role of a federal force health protection program created to enhance resiliency of deployed officers during the COVID-19 pandemic, but this focused primarily on providing remote services to frontline workers.6 Another report addressed mental health interventions for health care workers in an academic health care system in NYC during COVID-19.7 However, there has been little published on real-time mental health support for deployed personnel during the pandemic.
Prior publications have described the patient flow, infection control measures, and development of a Consultation-Liaison Psychiatry Service in the JNYMS.2,8,9 Here, we detail the establishment of preventative and responsive mental health services for frontline workers at the JNYMS and explore lessons learned through the outpatient and general support experiences.
Development of Outpatient Mental Health Support Services
At the end of March 2020, the Jacob K. Javits Convention Center was repurposed into the 452-bed JNYMS field hospital, where exposition rooms were transformed into a medical unit and intensive care unit.2 While the majority of personnel providing direct clinical care were specialists, the station also was staffed with uniformed and other federal mental health clinicians, including 5 licensed clinical social workers (LCSWs), 3 psychiatrists, 1 dual-trained internal medicine–psychiatry physician, 1 psychiatric nurse, and 2 behavioral health technicians. To standardize processes early in the deployment, standard operating procedures for behavioral health support of personnel were developed and disseminated within the first few days of the deployment.
The initial mission of the behavioral health team was to establish comprehensive mental health services, as the rapidly shifting mission and unfamiliar environment increased the risk of new-onset stress responses and exacerbating pre-existing stressors in personnel. Behavioral health leadership established operations in conference rooms within the convention center, focusing on identifying, prioritizing, and staffing high-traffic areas. A resiliency center was also established adjacent to the changing room, where all staff would enter and leave the units, and to the dining facility, further increasing traffic. This center was staffed 24 hours a day by at least 1 LCSW and a behavioral health technician with 2 shifts: one from 0630 to 1830 and another from 1830 to 0630. Psychiatrists were available during the day for psychiatry intervention and evaluations, and an on-call schedule was developed for off-hours to provide time-sensitive responses.
The resiliency center was developed to provide a welcoming atmosphere to meet basic needs, including nourishment, healthy social interaction, and a calm environment. Water and food were made available free to personnel, bolstering morale for staff working 12-hour shifts in a pandemic treatment floor where personal protective equipment prevented intake of food or water. Mental health staff were also available to counsel and provide social support to personnel. If personnel wished to discuss stressors or appeared to be in distress, a mental health clinician would provide a real-time intervention or schedule an appointment with the behavioral health team. Resources were made available, including brochures and other reading materials on resilience, stress management, and other mental health topics. Uniformed services and state and federal JNYMS leadership were encouraged to visit the resiliency center to normalize interactions and encourage participation in a behavioral health environment. Signage was placed throughout JNYMS to direct personnel to behavioral health services.
The behavioral health interventions and influence spread from the resiliency center nexus. Initially, therapeutic interventions occurred where and when necessary. One psychiatrist provided crisis intervention to a bereaved soldier in the stairwell within 2 hours of arrival to the JNYMS. Leadership and the behavioral health team recognized that the need for privacy was essential for timely therapeutic interventions, leading to the development of a private individual counseling room. As the area became generally accepted as the central hub of behavioral health activity, space was provided to establish a quiet space and a meditation room. The quiet area provided a cool dark space for personnel to sit quietly in solitude; many were grateful for this reprieve after an overstimulating medical shift. The meditation room supplied sterilized yoga mats for personal mindfulness interventions. The behavioral health team also liaised with military chaplains, who established a spiritual service room near the resiliency center. The chaplains held regular religious services and were available 24 hours a day for timely spiritual interventions.
Rapid notification and movement of uniformed personnel to JNYMS resulted in limited ability for personnel to schedule medical appointments and refill medications. Psychiatrists also had limited access to relevant electronic health record systems. This led to a delay in nonurgent care to evaluate personnel records and confirm prescriptions, especially controlled medications. Local pharmacies filled prescriptions, psychiatrists placed electronic health profiles, and command teams were notified in accordance with US Army and federal regulations.
Medical Unit Support Services
Although a robust outpatient behavioral health service was laid out in the JNYMS, the behavioral health team recognized the need to provide mental health interventions within the main patient care areas as well. The intention was to maximize availability and support while minimizing interference to patient care. As previously described, a psychiatric consultation-liaison (CL) team was organized and operated 24 hours a day by early April 2020.9 Indeed, CL psychiatrists have played a valuable role in supporting the unique patient and staff needs in other COVID-19 treatment environments.10 The CL team at JNYMS observed that medical staff were exposed to multiple stressors, including fear of acquiring COVID-19, treating patients with significant medical comorbidities, practicing outside of clinical specialty, working with unfamiliar and limited equipment, and adjusting to frequently shifting changes in personnel and work schedules. Moreover, psychological stress was compounded by long shifts, jetlag, and continuous wear of extensive personal protective equipment, as has been documented in other COVID-19 treatment centers.11
The team of psychiatrists conducted informal rounds to nursing stations to evaluate the morale and develop relationships with the medical team, including nurses, physicians, medics, and other personnel. Areas of high stress and increased interpersonal conflict were identified for more frequent check-ins by mental health clinicians. The psychiatrists and LCSWs were available for informal walk-in therapy when requested by personnel. When the acuity increased, personnel could be accompanied to the individual counseling room for rapid therapeutic interventions. The CL psychiatrists developed professional relationships with the command and medical leadership teams. Through these relationships and sensitive awareness of morale in the medical work environment, psychiatrists were able to advocate for alterations in the nursing work schedule. Leadership was receptive and resultant changes decreased the hours per shift and number of shifts for most nurses. Morale quickly improved, likely resulting in improved quality of patient care and prevention of burnout.
Mental Health Care Beyond JNYMS
Uniformed services and other federal personnel further supplemented health care operations beyond JYNMS. In April 2020, Urban Augmentation Medical Task Forces were organized and distributed throughout regions where COVID-19–related hospitalizations had significantly overwhelmed the local health care force. Urban Augmentation Medical Task Forces often included a psychiatrist, psychologist, and behavioral health technician with the mission to provide mental health support and interventions to patients and medical staff. Combat Operational Stress Control units from US Army medical brigades operated in NYC and the greater northeast region, providing mental health support and resiliency training to military personnel working in civilian hospitals, medical centers, and other health care or support environments. In addition, a LCSW and behavioral health technician worked with New York Army Reserve personnel assigned to mortuary affairs, providing point-of-care interventions at or near the worksite.
A collaborative federal, uniformed services, and state operation led to the development of the HERO-NY: Healing, Education, Resilience, and Opportunity for New York’s Frontline Workforce “Train the Trainer” Series.12 The series was intended to use uniformed services expertise to address mental health challenges related to the COVID-19 epidemic. Psychiatrists and mental health clinicians from JNYMS modeled small group trainings for future medical trainers. In lieu of traditional unidirectional lecturing, which yields limited retention and learning, the panelists demonstrated how to lead interactive small group training with resiliency topics, including goal setting, communication, anger management, and sleep hygiene.
Transition
After the last patient was discharged from JNYMS in May 2020, personnel were quickly redeployed to their duty stations. At the time of mission completion, the JNYMS behavioral health team had been supplemented with psychiatrists, social workers, behavioral health technicians, psychiatric nurse practitioners, psychiatric nurses, and psychologists representing US Public Health Service Commissioned Corps, Army, Air Force, and Navy, and provided comprehensive support to the nearly 1100 patients with COVID-19 and 600 deployed federal and state medical and support personnel.
Lessons Learned and Future Considerations
Behavioral health care provided at JNYMS offers insight into support of frontline workers in pandemic settings, as literature is limited in this area.13 TheJNYMS behavioral health team used strategies similar to military medical interventions in limited and unpredictable environments, such as rapid formalization of team structure and establishment of standard operating procedures to facilitate uniformity across interventions. Physical space was necessary to create an environment conducive to productive mental health interventions, including therapy rooms and quiet and spiritual spaces. Placing behavioral resources in high-traffic areas normalized mental health and maximized accessibility to interventions. Mental health personnel also addressed issues in the work environment, such as providing informal support and crisis interventions to frontline workers. Finally, Urban Augmentation Medical Task Forces mental health personnel and Combat Operational Stress Control units provided therapeutic interventions and resiliency training for military and civilian personnel throughout burdened medical systems beyond JNYMS.
Future operations should consider what equipment and logistic access are necessary to provide psychiatric and psychological care to mobilized federal and uniformed personnel, such as access to frontline worker electronic health records. Given that prior work has found that provision of resources alone is inadequate, frontline medical workers must be aware of where resources are available (eg, signage) and have easy access to material (eg, brochures) focusing on resiliency and psychological health.14 The spaces can be used for formal psychiatric and psychological interventions, such as assessment, therapy, and medication management. Equally important, these spaces serve as a safe place for healthy social interaction and fulfillment of basic needs (eg, nourishment) and a peaceful environment free of stimulation.
Since mental health personnel provide varied services ranging from basic human interaction to complex crisis interventions, mental health personnel should supplement pandemic medical operations. Evidence supports the notion that effective communication and cohesion throughout the entire leadership and health care team structure can improve resilience and implementation of mental health interventions.15 Incorporating mental health personnel into leadership planning meetings would allow for timely recommendations to improve medical logistics and planning of deployment of behavioral health resources. As a general rule, providing behavioral health experts with a seat at the table enhances advocacy and command awareness of the morale and mental health of frontline personnel.
Conclusions
We present the experience of developing mental health support services for deployed personnel during the COVID-19 pandemic and address the real-time mental health treatment and support of deployed uniformed services and federal personnel in the COVID-19 response environment. Timely and effective interventions included securing safe therapeutic space in high-traffic areas, developing relationships with leadership and frontline workers in their own work environments, and disseminating such services throughout the civilian medical system.
Mental health supplementation during the medical response mission strengthened morale in frontline workers in a disaster scenario. We hope that this report and others like it will provide information to improve mental health responses, reinforce mental health support, and encourage research in evidence-based interventions in challenging pandemic and disaster settings.
Acknowledgments
We would like to acknowledge and thank those serving on the frontlines of the COVID-19 pandemic.
1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. Published 2020 Apr 17. doi:10.15585/mmwr.mm6915e4
2. Brady K, Milzman D, Walton E, Sommer D, Neustadtl A, Napoli A. Uniformed services and the field hospital experience during Coronovirus Disease 2019 (SARS-CoV-2) pandemic: open to closure in 30 days with 1,100 patients: the Javits New York Medical Station [published online ahead of print, 2021 Feb 13]. Mil Med. 2021;usab003. doi:10.1093/milmed/usab003
3. Tucci V, Moukaddam N, Meadows J, Shah S, Galwankar SC, Kapur GB. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151-156. doi:10.4103/jgid.jgid_66_17
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311. doi:10.1177/070674370905400504
5. Panchal N, Kamal R, Cox C, Garfield R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed April 7, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
6. Myles IA, Johnson DR, Pham H, et al. USPHS Corps Care: force health protection for public health officers during the Ebola and COVID-19 responses. Public Health Rep. 2021;136(2):148-153. doi:10.1177/0033354920984775
7. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. 2020;95(8):1136-1139. doi:10.1097/ACM.0000000000003414
8. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):308-311. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009a3
9. Kaplan A, Smith CM, Toukolehto O, van Schalkwyk G. Psychiatric care in a novel federal COVID-19 treatment center: development of a consultation-liaison psychiatry service at the Javits New York Medical Station. Mil Med. 2021;186(5-6):129-131. doi:10.1093/milmed/usaa557
10. Shalev D, Shapiro PA. Epidemic psychiatry: The opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68-71. doi:10.1016/j.genhosppsych.2020.03.009
11. Horn M, Granon B, Vaiva G, Fovet T, Amad A. Role and importance of consultation-liaison psychiatry during the Covid-19 epidemic [published online ahead of print, 2020 Aug 5]. J Psychosom Res. 2020;137:110214. doi:10.1016/j.jpsychores.2020.110214
12. Wei EK, Segall J, Linn-Walton R, et al. Combat stress management and resilience: adapting department of defense combat lessons learned to civilian healthcare during the COVID-19 pandemic [published online ahead of print, 2020 Jul 17]. Health Secur. 2020;10.1089/hs.2020.0091. doi:10.1089/hs.2020.0091
13. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11(11):CD013779. Published 2020 Nov 5. doi:10.1002/14651858.CD013779
14. Schreiber M, Cates DS, Formanski S, King M. Maximizing the Resilience of Healthcare Workers in Multi-hazard Events: Lessons from the 2014-2015 Ebola Response in Africa. Mil Med. 2019;184(suppl 1):114-120. doi:10.1093/milmed/usy400
15. Klomp RW, Jones L, Watanabe E, Thompson WW. CDC’s multiple approaches to safeguard the health, safety, and resilience of Ebola responders. Prehosp Disaster Med. 2020;35(1):69-75. doi:10.1017/S1049023X19005144
New York City (NYC) was the early epicenter of the COVID-19 pandemic in the United States. By late March 2020, NYC hospitals were overwhelmed, leading to the development of a 452-bed field hospital that became the Javits New York Medical Station (JNYMS).1,2 More than 600 uniformed and other federal personnel, including medical personnel from US Army, Navy, and Public Health Service Commissioned Corps, mobilized to provide medical support to the JNYMS in late March 2020, leading to the treatment of more than 1000 patients with COVID-19 within a 30-day period.1
Literature from the SARS, Ebola, and HIV epidemics indicate that adverse mental health consequences, including burnout, depression, and posttraumatic stress disorder symptoms are common in frontline medical workers.3,4 Emerging data shows a similar trend occurring during the COVID-19 pandemic.5 A recent publication detailed the role of a federal force health protection program created to enhance resiliency of deployed officers during the COVID-19 pandemic, but this focused primarily on providing remote services to frontline workers.6 Another report addressed mental health interventions for health care workers in an academic health care system in NYC during COVID-19.7 However, there has been little published on real-time mental health support for deployed personnel during the pandemic.
Prior publications have described the patient flow, infection control measures, and development of a Consultation-Liaison Psychiatry Service in the JNYMS.2,8,9 Here, we detail the establishment of preventative and responsive mental health services for frontline workers at the JNYMS and explore lessons learned through the outpatient and general support experiences.
Development of Outpatient Mental Health Support Services
At the end of March 2020, the Jacob K. Javits Convention Center was repurposed into the 452-bed JNYMS field hospital, where exposition rooms were transformed into a medical unit and intensive care unit.2 While the majority of personnel providing direct clinical care were specialists, the station also was staffed with uniformed and other federal mental health clinicians, including 5 licensed clinical social workers (LCSWs), 3 psychiatrists, 1 dual-trained internal medicine–psychiatry physician, 1 psychiatric nurse, and 2 behavioral health technicians. To standardize processes early in the deployment, standard operating procedures for behavioral health support of personnel were developed and disseminated within the first few days of the deployment.
The initial mission of the behavioral health team was to establish comprehensive mental health services, as the rapidly shifting mission and unfamiliar environment increased the risk of new-onset stress responses and exacerbating pre-existing stressors in personnel. Behavioral health leadership established operations in conference rooms within the convention center, focusing on identifying, prioritizing, and staffing high-traffic areas. A resiliency center was also established adjacent to the changing room, where all staff would enter and leave the units, and to the dining facility, further increasing traffic. This center was staffed 24 hours a day by at least 1 LCSW and a behavioral health technician with 2 shifts: one from 0630 to 1830 and another from 1830 to 0630. Psychiatrists were available during the day for psychiatry intervention and evaluations, and an on-call schedule was developed for off-hours to provide time-sensitive responses.
The resiliency center was developed to provide a welcoming atmosphere to meet basic needs, including nourishment, healthy social interaction, and a calm environment. Water and food were made available free to personnel, bolstering morale for staff working 12-hour shifts in a pandemic treatment floor where personal protective equipment prevented intake of food or water. Mental health staff were also available to counsel and provide social support to personnel. If personnel wished to discuss stressors or appeared to be in distress, a mental health clinician would provide a real-time intervention or schedule an appointment with the behavioral health team. Resources were made available, including brochures and other reading materials on resilience, stress management, and other mental health topics. Uniformed services and state and federal JNYMS leadership were encouraged to visit the resiliency center to normalize interactions and encourage participation in a behavioral health environment. Signage was placed throughout JNYMS to direct personnel to behavioral health services.
The behavioral health interventions and influence spread from the resiliency center nexus. Initially, therapeutic interventions occurred where and when necessary. One psychiatrist provided crisis intervention to a bereaved soldier in the stairwell within 2 hours of arrival to the JNYMS. Leadership and the behavioral health team recognized that the need for privacy was essential for timely therapeutic interventions, leading to the development of a private individual counseling room. As the area became generally accepted as the central hub of behavioral health activity, space was provided to establish a quiet space and a meditation room. The quiet area provided a cool dark space for personnel to sit quietly in solitude; many were grateful for this reprieve after an overstimulating medical shift. The meditation room supplied sterilized yoga mats for personal mindfulness interventions. The behavioral health team also liaised with military chaplains, who established a spiritual service room near the resiliency center. The chaplains held regular religious services and were available 24 hours a day for timely spiritual interventions.
Rapid notification and movement of uniformed personnel to JNYMS resulted in limited ability for personnel to schedule medical appointments and refill medications. Psychiatrists also had limited access to relevant electronic health record systems. This led to a delay in nonurgent care to evaluate personnel records and confirm prescriptions, especially controlled medications. Local pharmacies filled prescriptions, psychiatrists placed electronic health profiles, and command teams were notified in accordance with US Army and federal regulations.
Medical Unit Support Services
Although a robust outpatient behavioral health service was laid out in the JNYMS, the behavioral health team recognized the need to provide mental health interventions within the main patient care areas as well. The intention was to maximize availability and support while minimizing interference to patient care. As previously described, a psychiatric consultation-liaison (CL) team was organized and operated 24 hours a day by early April 2020.9 Indeed, CL psychiatrists have played a valuable role in supporting the unique patient and staff needs in other COVID-19 treatment environments.10 The CL team at JNYMS observed that medical staff were exposed to multiple stressors, including fear of acquiring COVID-19, treating patients with significant medical comorbidities, practicing outside of clinical specialty, working with unfamiliar and limited equipment, and adjusting to frequently shifting changes in personnel and work schedules. Moreover, psychological stress was compounded by long shifts, jetlag, and continuous wear of extensive personal protective equipment, as has been documented in other COVID-19 treatment centers.11
The team of psychiatrists conducted informal rounds to nursing stations to evaluate the morale and develop relationships with the medical team, including nurses, physicians, medics, and other personnel. Areas of high stress and increased interpersonal conflict were identified for more frequent check-ins by mental health clinicians. The psychiatrists and LCSWs were available for informal walk-in therapy when requested by personnel. When the acuity increased, personnel could be accompanied to the individual counseling room for rapid therapeutic interventions. The CL psychiatrists developed professional relationships with the command and medical leadership teams. Through these relationships and sensitive awareness of morale in the medical work environment, psychiatrists were able to advocate for alterations in the nursing work schedule. Leadership was receptive and resultant changes decreased the hours per shift and number of shifts for most nurses. Morale quickly improved, likely resulting in improved quality of patient care and prevention of burnout.
Mental Health Care Beyond JNYMS
Uniformed services and other federal personnel further supplemented health care operations beyond JYNMS. In April 2020, Urban Augmentation Medical Task Forces were organized and distributed throughout regions where COVID-19–related hospitalizations had significantly overwhelmed the local health care force. Urban Augmentation Medical Task Forces often included a psychiatrist, psychologist, and behavioral health technician with the mission to provide mental health support and interventions to patients and medical staff. Combat Operational Stress Control units from US Army medical brigades operated in NYC and the greater northeast region, providing mental health support and resiliency training to military personnel working in civilian hospitals, medical centers, and other health care or support environments. In addition, a LCSW and behavioral health technician worked with New York Army Reserve personnel assigned to mortuary affairs, providing point-of-care interventions at or near the worksite.
A collaborative federal, uniformed services, and state operation led to the development of the HERO-NY: Healing, Education, Resilience, and Opportunity for New York’s Frontline Workforce “Train the Trainer” Series.12 The series was intended to use uniformed services expertise to address mental health challenges related to the COVID-19 epidemic. Psychiatrists and mental health clinicians from JNYMS modeled small group trainings for future medical trainers. In lieu of traditional unidirectional lecturing, which yields limited retention and learning, the panelists demonstrated how to lead interactive small group training with resiliency topics, including goal setting, communication, anger management, and sleep hygiene.
Transition
After the last patient was discharged from JNYMS in May 2020, personnel were quickly redeployed to their duty stations. At the time of mission completion, the JNYMS behavioral health team had been supplemented with psychiatrists, social workers, behavioral health technicians, psychiatric nurse practitioners, psychiatric nurses, and psychologists representing US Public Health Service Commissioned Corps, Army, Air Force, and Navy, and provided comprehensive support to the nearly 1100 patients with COVID-19 and 600 deployed federal and state medical and support personnel.
Lessons Learned and Future Considerations
Behavioral health care provided at JNYMS offers insight into support of frontline workers in pandemic settings, as literature is limited in this area.13 TheJNYMS behavioral health team used strategies similar to military medical interventions in limited and unpredictable environments, such as rapid formalization of team structure and establishment of standard operating procedures to facilitate uniformity across interventions. Physical space was necessary to create an environment conducive to productive mental health interventions, including therapy rooms and quiet and spiritual spaces. Placing behavioral resources in high-traffic areas normalized mental health and maximized accessibility to interventions. Mental health personnel also addressed issues in the work environment, such as providing informal support and crisis interventions to frontline workers. Finally, Urban Augmentation Medical Task Forces mental health personnel and Combat Operational Stress Control units provided therapeutic interventions and resiliency training for military and civilian personnel throughout burdened medical systems beyond JNYMS.
Future operations should consider what equipment and logistic access are necessary to provide psychiatric and psychological care to mobilized federal and uniformed personnel, such as access to frontline worker electronic health records. Given that prior work has found that provision of resources alone is inadequate, frontline medical workers must be aware of where resources are available (eg, signage) and have easy access to material (eg, brochures) focusing on resiliency and psychological health.14 The spaces can be used for formal psychiatric and psychological interventions, such as assessment, therapy, and medication management. Equally important, these spaces serve as a safe place for healthy social interaction and fulfillment of basic needs (eg, nourishment) and a peaceful environment free of stimulation.
Since mental health personnel provide varied services ranging from basic human interaction to complex crisis interventions, mental health personnel should supplement pandemic medical operations. Evidence supports the notion that effective communication and cohesion throughout the entire leadership and health care team structure can improve resilience and implementation of mental health interventions.15 Incorporating mental health personnel into leadership planning meetings would allow for timely recommendations to improve medical logistics and planning of deployment of behavioral health resources. As a general rule, providing behavioral health experts with a seat at the table enhances advocacy and command awareness of the morale and mental health of frontline personnel.
Conclusions
We present the experience of developing mental health support services for deployed personnel during the COVID-19 pandemic and address the real-time mental health treatment and support of deployed uniformed services and federal personnel in the COVID-19 response environment. Timely and effective interventions included securing safe therapeutic space in high-traffic areas, developing relationships with leadership and frontline workers in their own work environments, and disseminating such services throughout the civilian medical system.
Mental health supplementation during the medical response mission strengthened morale in frontline workers in a disaster scenario. We hope that this report and others like it will provide information to improve mental health responses, reinforce mental health support, and encourage research in evidence-based interventions in challenging pandemic and disaster settings.
Acknowledgments
We would like to acknowledge and thank those serving on the frontlines of the COVID-19 pandemic.
New York City (NYC) was the early epicenter of the COVID-19 pandemic in the United States. By late March 2020, NYC hospitals were overwhelmed, leading to the development of a 452-bed field hospital that became the Javits New York Medical Station (JNYMS).1,2 More than 600 uniformed and other federal personnel, including medical personnel from US Army, Navy, and Public Health Service Commissioned Corps, mobilized to provide medical support to the JNYMS in late March 2020, leading to the treatment of more than 1000 patients with COVID-19 within a 30-day period.1
Literature from the SARS, Ebola, and HIV epidemics indicate that adverse mental health consequences, including burnout, depression, and posttraumatic stress disorder symptoms are common in frontline medical workers.3,4 Emerging data shows a similar trend occurring during the COVID-19 pandemic.5 A recent publication detailed the role of a federal force health protection program created to enhance resiliency of deployed officers during the COVID-19 pandemic, but this focused primarily on providing remote services to frontline workers.6 Another report addressed mental health interventions for health care workers in an academic health care system in NYC during COVID-19.7 However, there has been little published on real-time mental health support for deployed personnel during the pandemic.
Prior publications have described the patient flow, infection control measures, and development of a Consultation-Liaison Psychiatry Service in the JNYMS.2,8,9 Here, we detail the establishment of preventative and responsive mental health services for frontline workers at the JNYMS and explore lessons learned through the outpatient and general support experiences.
Development of Outpatient Mental Health Support Services
At the end of March 2020, the Jacob K. Javits Convention Center was repurposed into the 452-bed JNYMS field hospital, where exposition rooms were transformed into a medical unit and intensive care unit.2 While the majority of personnel providing direct clinical care were specialists, the station also was staffed with uniformed and other federal mental health clinicians, including 5 licensed clinical social workers (LCSWs), 3 psychiatrists, 1 dual-trained internal medicine–psychiatry physician, 1 psychiatric nurse, and 2 behavioral health technicians. To standardize processes early in the deployment, standard operating procedures for behavioral health support of personnel were developed and disseminated within the first few days of the deployment.
The initial mission of the behavioral health team was to establish comprehensive mental health services, as the rapidly shifting mission and unfamiliar environment increased the risk of new-onset stress responses and exacerbating pre-existing stressors in personnel. Behavioral health leadership established operations in conference rooms within the convention center, focusing on identifying, prioritizing, and staffing high-traffic areas. A resiliency center was also established adjacent to the changing room, where all staff would enter and leave the units, and to the dining facility, further increasing traffic. This center was staffed 24 hours a day by at least 1 LCSW and a behavioral health technician with 2 shifts: one from 0630 to 1830 and another from 1830 to 0630. Psychiatrists were available during the day for psychiatry intervention and evaluations, and an on-call schedule was developed for off-hours to provide time-sensitive responses.
The resiliency center was developed to provide a welcoming atmosphere to meet basic needs, including nourishment, healthy social interaction, and a calm environment. Water and food were made available free to personnel, bolstering morale for staff working 12-hour shifts in a pandemic treatment floor where personal protective equipment prevented intake of food or water. Mental health staff were also available to counsel and provide social support to personnel. If personnel wished to discuss stressors or appeared to be in distress, a mental health clinician would provide a real-time intervention or schedule an appointment with the behavioral health team. Resources were made available, including brochures and other reading materials on resilience, stress management, and other mental health topics. Uniformed services and state and federal JNYMS leadership were encouraged to visit the resiliency center to normalize interactions and encourage participation in a behavioral health environment. Signage was placed throughout JNYMS to direct personnel to behavioral health services.
The behavioral health interventions and influence spread from the resiliency center nexus. Initially, therapeutic interventions occurred where and when necessary. One psychiatrist provided crisis intervention to a bereaved soldier in the stairwell within 2 hours of arrival to the JNYMS. Leadership and the behavioral health team recognized that the need for privacy was essential for timely therapeutic interventions, leading to the development of a private individual counseling room. As the area became generally accepted as the central hub of behavioral health activity, space was provided to establish a quiet space and a meditation room. The quiet area provided a cool dark space for personnel to sit quietly in solitude; many were grateful for this reprieve after an overstimulating medical shift. The meditation room supplied sterilized yoga mats for personal mindfulness interventions. The behavioral health team also liaised with military chaplains, who established a spiritual service room near the resiliency center. The chaplains held regular religious services and were available 24 hours a day for timely spiritual interventions.
Rapid notification and movement of uniformed personnel to JNYMS resulted in limited ability for personnel to schedule medical appointments and refill medications. Psychiatrists also had limited access to relevant electronic health record systems. This led to a delay in nonurgent care to evaluate personnel records and confirm prescriptions, especially controlled medications. Local pharmacies filled prescriptions, psychiatrists placed electronic health profiles, and command teams were notified in accordance with US Army and federal regulations.
Medical Unit Support Services
Although a robust outpatient behavioral health service was laid out in the JNYMS, the behavioral health team recognized the need to provide mental health interventions within the main patient care areas as well. The intention was to maximize availability and support while minimizing interference to patient care. As previously described, a psychiatric consultation-liaison (CL) team was organized and operated 24 hours a day by early April 2020.9 Indeed, CL psychiatrists have played a valuable role in supporting the unique patient and staff needs in other COVID-19 treatment environments.10 The CL team at JNYMS observed that medical staff were exposed to multiple stressors, including fear of acquiring COVID-19, treating patients with significant medical comorbidities, practicing outside of clinical specialty, working with unfamiliar and limited equipment, and adjusting to frequently shifting changes in personnel and work schedules. Moreover, psychological stress was compounded by long shifts, jetlag, and continuous wear of extensive personal protective equipment, as has been documented in other COVID-19 treatment centers.11
The team of psychiatrists conducted informal rounds to nursing stations to evaluate the morale and develop relationships with the medical team, including nurses, physicians, medics, and other personnel. Areas of high stress and increased interpersonal conflict were identified for more frequent check-ins by mental health clinicians. The psychiatrists and LCSWs were available for informal walk-in therapy when requested by personnel. When the acuity increased, personnel could be accompanied to the individual counseling room for rapid therapeutic interventions. The CL psychiatrists developed professional relationships with the command and medical leadership teams. Through these relationships and sensitive awareness of morale in the medical work environment, psychiatrists were able to advocate for alterations in the nursing work schedule. Leadership was receptive and resultant changes decreased the hours per shift and number of shifts for most nurses. Morale quickly improved, likely resulting in improved quality of patient care and prevention of burnout.
Mental Health Care Beyond JNYMS
Uniformed services and other federal personnel further supplemented health care operations beyond JYNMS. In April 2020, Urban Augmentation Medical Task Forces were organized and distributed throughout regions where COVID-19–related hospitalizations had significantly overwhelmed the local health care force. Urban Augmentation Medical Task Forces often included a psychiatrist, psychologist, and behavioral health technician with the mission to provide mental health support and interventions to patients and medical staff. Combat Operational Stress Control units from US Army medical brigades operated in NYC and the greater northeast region, providing mental health support and resiliency training to military personnel working in civilian hospitals, medical centers, and other health care or support environments. In addition, a LCSW and behavioral health technician worked with New York Army Reserve personnel assigned to mortuary affairs, providing point-of-care interventions at or near the worksite.
A collaborative federal, uniformed services, and state operation led to the development of the HERO-NY: Healing, Education, Resilience, and Opportunity for New York’s Frontline Workforce “Train the Trainer” Series.12 The series was intended to use uniformed services expertise to address mental health challenges related to the COVID-19 epidemic. Psychiatrists and mental health clinicians from JNYMS modeled small group trainings for future medical trainers. In lieu of traditional unidirectional lecturing, which yields limited retention and learning, the panelists demonstrated how to lead interactive small group training with resiliency topics, including goal setting, communication, anger management, and sleep hygiene.
Transition
After the last patient was discharged from JNYMS in May 2020, personnel were quickly redeployed to their duty stations. At the time of mission completion, the JNYMS behavioral health team had been supplemented with psychiatrists, social workers, behavioral health technicians, psychiatric nurse practitioners, psychiatric nurses, and psychologists representing US Public Health Service Commissioned Corps, Army, Air Force, and Navy, and provided comprehensive support to the nearly 1100 patients with COVID-19 and 600 deployed federal and state medical and support personnel.
Lessons Learned and Future Considerations
Behavioral health care provided at JNYMS offers insight into support of frontline workers in pandemic settings, as literature is limited in this area.13 TheJNYMS behavioral health team used strategies similar to military medical interventions in limited and unpredictable environments, such as rapid formalization of team structure and establishment of standard operating procedures to facilitate uniformity across interventions. Physical space was necessary to create an environment conducive to productive mental health interventions, including therapy rooms and quiet and spiritual spaces. Placing behavioral resources in high-traffic areas normalized mental health and maximized accessibility to interventions. Mental health personnel also addressed issues in the work environment, such as providing informal support and crisis interventions to frontline workers. Finally, Urban Augmentation Medical Task Forces mental health personnel and Combat Operational Stress Control units provided therapeutic interventions and resiliency training for military and civilian personnel throughout burdened medical systems beyond JNYMS.
Future operations should consider what equipment and logistic access are necessary to provide psychiatric and psychological care to mobilized federal and uniformed personnel, such as access to frontline worker electronic health records. Given that prior work has found that provision of resources alone is inadequate, frontline medical workers must be aware of where resources are available (eg, signage) and have easy access to material (eg, brochures) focusing on resiliency and psychological health.14 The spaces can be used for formal psychiatric and psychological interventions, such as assessment, therapy, and medication management. Equally important, these spaces serve as a safe place for healthy social interaction and fulfillment of basic needs (eg, nourishment) and a peaceful environment free of stimulation.
Since mental health personnel provide varied services ranging from basic human interaction to complex crisis interventions, mental health personnel should supplement pandemic medical operations. Evidence supports the notion that effective communication and cohesion throughout the entire leadership and health care team structure can improve resilience and implementation of mental health interventions.15 Incorporating mental health personnel into leadership planning meetings would allow for timely recommendations to improve medical logistics and planning of deployment of behavioral health resources. As a general rule, providing behavioral health experts with a seat at the table enhances advocacy and command awareness of the morale and mental health of frontline personnel.
Conclusions
We present the experience of developing mental health support services for deployed personnel during the COVID-19 pandemic and address the real-time mental health treatment and support of deployed uniformed services and federal personnel in the COVID-19 response environment. Timely and effective interventions included securing safe therapeutic space in high-traffic areas, developing relationships with leadership and frontline workers in their own work environments, and disseminating such services throughout the civilian medical system.
Mental health supplementation during the medical response mission strengthened morale in frontline workers in a disaster scenario. We hope that this report and others like it will provide information to improve mental health responses, reinforce mental health support, and encourage research in evidence-based interventions in challenging pandemic and disaster settings.
Acknowledgments
We would like to acknowledge and thank those serving on the frontlines of the COVID-19 pandemic.
1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. Published 2020 Apr 17. doi:10.15585/mmwr.mm6915e4
2. Brady K, Milzman D, Walton E, Sommer D, Neustadtl A, Napoli A. Uniformed services and the field hospital experience during Coronovirus Disease 2019 (SARS-CoV-2) pandemic: open to closure in 30 days with 1,100 patients: the Javits New York Medical Station [published online ahead of print, 2021 Feb 13]. Mil Med. 2021;usab003. doi:10.1093/milmed/usab003
3. Tucci V, Moukaddam N, Meadows J, Shah S, Galwankar SC, Kapur GB. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151-156. doi:10.4103/jgid.jgid_66_17
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311. doi:10.1177/070674370905400504
5. Panchal N, Kamal R, Cox C, Garfield R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed April 7, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
6. Myles IA, Johnson DR, Pham H, et al. USPHS Corps Care: force health protection for public health officers during the Ebola and COVID-19 responses. Public Health Rep. 2021;136(2):148-153. doi:10.1177/0033354920984775
7. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. 2020;95(8):1136-1139. doi:10.1097/ACM.0000000000003414
8. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):308-311. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009a3
9. Kaplan A, Smith CM, Toukolehto O, van Schalkwyk G. Psychiatric care in a novel federal COVID-19 treatment center: development of a consultation-liaison psychiatry service at the Javits New York Medical Station. Mil Med. 2021;186(5-6):129-131. doi:10.1093/milmed/usaa557
10. Shalev D, Shapiro PA. Epidemic psychiatry: The opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68-71. doi:10.1016/j.genhosppsych.2020.03.009
11. Horn M, Granon B, Vaiva G, Fovet T, Amad A. Role and importance of consultation-liaison psychiatry during the Covid-19 epidemic [published online ahead of print, 2020 Aug 5]. J Psychosom Res. 2020;137:110214. doi:10.1016/j.jpsychores.2020.110214
12. Wei EK, Segall J, Linn-Walton R, et al. Combat stress management and resilience: adapting department of defense combat lessons learned to civilian healthcare during the COVID-19 pandemic [published online ahead of print, 2020 Jul 17]. Health Secur. 2020;10.1089/hs.2020.0091. doi:10.1089/hs.2020.0091
13. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11(11):CD013779. Published 2020 Nov 5. doi:10.1002/14651858.CD013779
14. Schreiber M, Cates DS, Formanski S, King M. Maximizing the Resilience of Healthcare Workers in Multi-hazard Events: Lessons from the 2014-2015 Ebola Response in Africa. Mil Med. 2019;184(suppl 1):114-120. doi:10.1093/milmed/usy400
15. Klomp RW, Jones L, Watanabe E, Thompson WW. CDC’s multiple approaches to safeguard the health, safety, and resilience of Ebola responders. Prehosp Disaster Med. 2020;35(1):69-75. doi:10.1017/S1049023X19005144
1. CDC COVID-19 Response Team. Geographic differences in COVID-19 cases, deaths, and incidence - United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. Published 2020 Apr 17. doi:10.15585/mmwr.mm6915e4
2. Brady K, Milzman D, Walton E, Sommer D, Neustadtl A, Napoli A. Uniformed services and the field hospital experience during Coronovirus Disease 2019 (SARS-CoV-2) pandemic: open to closure in 30 days with 1,100 patients: the Javits New York Medical Station [published online ahead of print, 2021 Feb 13]. Mil Med. 2021;usab003. doi:10.1093/milmed/usab003
3. Tucci V, Moukaddam N, Meadows J, Shah S, Galwankar SC, Kapur GB. The forgotten plague: psychiatric manifestations of Ebola, Zika, and emerging infectious diseases. J Glob Infect Dis. 2017;9(4):151-156. doi:10.4103/jgid.jgid_66_17
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311. doi:10.1177/070674370905400504
5. Panchal N, Kamal R, Cox C, Garfield R. The implications of COVID-19 for mental health and substance use. Published February 10, 2021. Accessed April 7, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/the-implications-of-covid-19-for-mental-health-and-substance-use/
6. Myles IA, Johnson DR, Pham H, et al. USPHS Corps Care: force health protection for public health officers during the Ebola and COVID-19 responses. Public Health Rep. 2021;136(2):148-153. doi:10.1177/0033354920984775
7. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. 2020;95(8):1136-1139. doi:10.1097/ACM.0000000000003414
8. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):308-311. Published 2021 Mar 5. doi:10.15585/mmwr.mm7009a3
9. Kaplan A, Smith CM, Toukolehto O, van Schalkwyk G. Psychiatric care in a novel federal COVID-19 treatment center: development of a consultation-liaison psychiatry service at the Javits New York Medical Station. Mil Med. 2021;186(5-6):129-131. doi:10.1093/milmed/usaa557
10. Shalev D, Shapiro PA. Epidemic psychiatry: The opportunities and challenges of COVID-19. Gen Hosp Psychiatry. 2020;64:68-71. doi:10.1016/j.genhosppsych.2020.03.009
11. Horn M, Granon B, Vaiva G, Fovet T, Amad A. Role and importance of consultation-liaison psychiatry during the Covid-19 epidemic [published online ahead of print, 2020 Aug 5]. J Psychosom Res. 2020;137:110214. doi:10.1016/j.jpsychores.2020.110214
12. Wei EK, Segall J, Linn-Walton R, et al. Combat stress management and resilience: adapting department of defense combat lessons learned to civilian healthcare during the COVID-19 pandemic [published online ahead of print, 2020 Jul 17]. Health Secur. 2020;10.1089/hs.2020.0091. doi:10.1089/hs.2020.0091
13. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Syst Rev. 2020;11(11):CD013779. Published 2020 Nov 5. doi:10.1002/14651858.CD013779
14. Schreiber M, Cates DS, Formanski S, King M. Maximizing the Resilience of Healthcare Workers in Multi-hazard Events: Lessons from the 2014-2015 Ebola Response in Africa. Mil Med. 2019;184(suppl 1):114-120. doi:10.1093/milmed/usy400
15. Klomp RW, Jones L, Watanabe E, Thompson WW. CDC’s multiple approaches to safeguard the health, safety, and resilience of Ebola responders. Prehosp Disaster Med. 2020;35(1):69-75. doi:10.1017/S1049023X19005144
Higher industriousness reduces risk of predementia syndrome in older adults
Higher industriousness was associated with a 25% reduced risk of concurrent motoric cognitive risk syndrome (MCR), based on data from approximately 6,000 individuals.
Previous research supports an association between conscientiousness and a lower risk of MCR, a form of predementia that involves slow gait speed and cognitive complaints, wrote Yannick Stephan, PhD, of the University of Montpellier (France), and colleagues. However, the specific facets of conscientiousness that impact MCR have not been examined.
In a study published in the Journal of Psychiatric Research, the authors reviewed data from 6,001 dementia-free adults aged 65-99 years who were enrolled in the Health and Retirement Study, a nationally representative longitudinal study of adults aged 50 years and older in the United States.
Baseline data were collected between 2008 and 2010, and participants were assessed for MCR at follow-up points during 2012-2014 and 2016-2018. Six facets of conscientiousness were assessed using a 24-item scale that has been used in previous studies. The six facets were industriousness, self-control, order, traditionalism, virtue, and responsibility. The researchers controlled for variables including demographic factors, cognition, physical activity, disease burden, depressive symptoms, and body mass index.
Overall, increased industriousness was significantly associated with a lower likelihood of concurrent MCR (odds ratio, 0.75) and a reduced risk of incident MCR (hazard ratio, 0.63,; P < .001 for both).
The conscientiousness facets of order, self-control, and responsibility also were associated with a lower likelihood of both concurrent and incident MCR, with ORs ranging from 0.82-0.88 for concurrent and HRs ranging from 0.72-0.82 for incident.
Traditionalism and virtue were significantly associated with a lower risk of incident MCR, but not concurrent MCR (HR, 0.84; P < .01 for both).
The mechanism of action for the association may be explained by several cognitive, health-related, behavioral, and psychological pathways, the researchers wrote. With regard to industriousness, the relationship could be partly explained by cognition, physical activity, disease burden, BMI, and depressive symptoms. However, industriousness also has been associated with a reduced risk of systemic inflammation, which may in turn reduce MCR risk. Also, data suggest that industriousness and MCR share a common genetic cause.
The study findings were limited by several factors including the observational design and the positive selection effect from patients with complete follow-up data, as these patients likely have higher levels of order, industriousness, and responsibility, the researchers noted. However, the results support those from previous studies and were strengthened by the large sample and examination of six facets of conscientiousness.
“This study thus provides a more detailed understanding of the specific components of conscientiousness that are associated with risk of MCR among older adults,” and the facets could be targeted in interventions to reduce both MCR and dementia, they concluded.
The Health and Retirement Study is supported by the National Institute on Aging and conducted by the University of Michigan. The current study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Higher industriousness was associated with a 25% reduced risk of concurrent motoric cognitive risk syndrome (MCR), based on data from approximately 6,000 individuals.
Previous research supports an association between conscientiousness and a lower risk of MCR, a form of predementia that involves slow gait speed and cognitive complaints, wrote Yannick Stephan, PhD, of the University of Montpellier (France), and colleagues. However, the specific facets of conscientiousness that impact MCR have not been examined.
In a study published in the Journal of Psychiatric Research, the authors reviewed data from 6,001 dementia-free adults aged 65-99 years who were enrolled in the Health and Retirement Study, a nationally representative longitudinal study of adults aged 50 years and older in the United States.
Baseline data were collected between 2008 and 2010, and participants were assessed for MCR at follow-up points during 2012-2014 and 2016-2018. Six facets of conscientiousness were assessed using a 24-item scale that has been used in previous studies. The six facets were industriousness, self-control, order, traditionalism, virtue, and responsibility. The researchers controlled for variables including demographic factors, cognition, physical activity, disease burden, depressive symptoms, and body mass index.
Overall, increased industriousness was significantly associated with a lower likelihood of concurrent MCR (odds ratio, 0.75) and a reduced risk of incident MCR (hazard ratio, 0.63,; P < .001 for both).
The conscientiousness facets of order, self-control, and responsibility also were associated with a lower likelihood of both concurrent and incident MCR, with ORs ranging from 0.82-0.88 for concurrent and HRs ranging from 0.72-0.82 for incident.
Traditionalism and virtue were significantly associated with a lower risk of incident MCR, but not concurrent MCR (HR, 0.84; P < .01 for both).
The mechanism of action for the association may be explained by several cognitive, health-related, behavioral, and psychological pathways, the researchers wrote. With regard to industriousness, the relationship could be partly explained by cognition, physical activity, disease burden, BMI, and depressive symptoms. However, industriousness also has been associated with a reduced risk of systemic inflammation, which may in turn reduce MCR risk. Also, data suggest that industriousness and MCR share a common genetic cause.
The study findings were limited by several factors including the observational design and the positive selection effect from patients with complete follow-up data, as these patients likely have higher levels of order, industriousness, and responsibility, the researchers noted. However, the results support those from previous studies and were strengthened by the large sample and examination of six facets of conscientiousness.
“This study thus provides a more detailed understanding of the specific components of conscientiousness that are associated with risk of MCR among older adults,” and the facets could be targeted in interventions to reduce both MCR and dementia, they concluded.
The Health and Retirement Study is supported by the National Institute on Aging and conducted by the University of Michigan. The current study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Higher industriousness was associated with a 25% reduced risk of concurrent motoric cognitive risk syndrome (MCR), based on data from approximately 6,000 individuals.
Previous research supports an association between conscientiousness and a lower risk of MCR, a form of predementia that involves slow gait speed and cognitive complaints, wrote Yannick Stephan, PhD, of the University of Montpellier (France), and colleagues. However, the specific facets of conscientiousness that impact MCR have not been examined.
In a study published in the Journal of Psychiatric Research, the authors reviewed data from 6,001 dementia-free adults aged 65-99 years who were enrolled in the Health and Retirement Study, a nationally representative longitudinal study of adults aged 50 years and older in the United States.
Baseline data were collected between 2008 and 2010, and participants were assessed for MCR at follow-up points during 2012-2014 and 2016-2018. Six facets of conscientiousness were assessed using a 24-item scale that has been used in previous studies. The six facets were industriousness, self-control, order, traditionalism, virtue, and responsibility. The researchers controlled for variables including demographic factors, cognition, physical activity, disease burden, depressive symptoms, and body mass index.
Overall, increased industriousness was significantly associated with a lower likelihood of concurrent MCR (odds ratio, 0.75) and a reduced risk of incident MCR (hazard ratio, 0.63,; P < .001 for both).
The conscientiousness facets of order, self-control, and responsibility also were associated with a lower likelihood of both concurrent and incident MCR, with ORs ranging from 0.82-0.88 for concurrent and HRs ranging from 0.72-0.82 for incident.
Traditionalism and virtue were significantly associated with a lower risk of incident MCR, but not concurrent MCR (HR, 0.84; P < .01 for both).
The mechanism of action for the association may be explained by several cognitive, health-related, behavioral, and psychological pathways, the researchers wrote. With regard to industriousness, the relationship could be partly explained by cognition, physical activity, disease burden, BMI, and depressive symptoms. However, industriousness also has been associated with a reduced risk of systemic inflammation, which may in turn reduce MCR risk. Also, data suggest that industriousness and MCR share a common genetic cause.
The study findings were limited by several factors including the observational design and the positive selection effect from patients with complete follow-up data, as these patients likely have higher levels of order, industriousness, and responsibility, the researchers noted. However, the results support those from previous studies and were strengthened by the large sample and examination of six facets of conscientiousness.
“This study thus provides a more detailed understanding of the specific components of conscientiousness that are associated with risk of MCR among older adults,” and the facets could be targeted in interventions to reduce both MCR and dementia, they concluded.
The Health and Retirement Study is supported by the National Institute on Aging and conducted by the University of Michigan. The current study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRIC RESEARCH
Endovascular benefit finally confirmed for basilar artery stroke
The benefit of endovascular therapy in the treatment of stroke caused by an occlusion of the basilar artery has finally been confirmed in the ATTENTION randomized trial.
The study, conducted in China, showed that endovascular therapy for basilar artery occlusion is associated with higher rates of favorable and independent outcomes, as well as lower overall disability and lower mortality at 90 days, than best medical management alone.
The results were presented by Raul Nogueira, MD, professor of neurology at the University of Pittsburgh School of Medicine, at the European Stroke Organisation Conference (ESOC) 2022, where they were greeted with applause from the audience.
“We can finally say that we have conquered the basilar artery territory. It is about time. We can finally confirm that the benefit of endovascular therapy persists in the posterior circulation,” Dr. Nogueira said.
“The disability reduction benefit of endovascular therapy for basilar artery occlusion appears to be within the same range as that observed in the anterior circulation. However, in contrast to most anterior circulation endovascular trials, the ATTENTION trial also demonstrated a potential benefit in terms of mortality,” he added.
Dr. Nogueira explained that the first series of endovascular treatment for stroke in the modern era was published in 1988, and this was in the basilar artery occlusion territory, but almost 35 years later, although there has been overwhelming proof of benefit of endovascular treatment in the antiterror circulation, it remains unknown whether endovascular treatment is beneficial to treat acute basilar artery occlusion. This is despite efforts in conducting two trials – the BEST and BASICS trials – which showed a direction of benefit but failed to show real significance.
“Having said that, these trials paved the way for the current trial, specifically by demonstrating the importance of consecutive recruitment, fast enrollment, and the minimalization of crossover. They also confirmed the ideal target population for this procedure in an individual patient level meta-analysis of these two trials,” he said.
In addition, there have also been two large Chinese registries suggesting significant benefits.
The ATTENTION trial was conducted to evaluate the hypothesis that endovascular therapy is superior to best medical management alone in achieving more favorable outcomes (mRS, 0-3) at 90 days in subjects presenting with acute basilar artery stroke within 12 hours of the estimated time of onset.
The study enrolled 342 patients at 36 comprehensive stroke centers in China. All patients had occlusion of the basilar artery confirmed on vascular imaging within 12 hours of stroke onset, and they had severe symptoms at presentation, with an NIHSS score of at least 10. They were randomized in a 2:1 ratio to endovascular treatment or best medical management alone.
“It took us less than a year to enroll 342 patients,” Dr. Nogueira noted. “To put this into perspective, it took the BASICS trial over 8 years to enroll 300 patients, so these are very high-volume centers.”
He reported that two patients withdrew consent, and there were three patient crossovers on each side, comparing favorably with BASICS, leaving 226 patients in the intervention group and 114 in the control group.
Baseline characteristics were similar between the two groups: median age was 67 years, median NIHSS score was 24, about 25% received thrombolysis, and median time from stroke onset to randomization was 5 hours.
Results showed that the primary outcome – a favorable functional outcome (mRS, 0-3) at 90 days – was achieved in 22.8% of the control group and in 46% of the endovascular group, giving an adjusted risk ratio of 2.1 (P < .001).
The number needed to treat was just four.
“There were no surprises with secondary endpoints; everything was highly statistically significant,” Dr. Nogueira said.
Specifically, there was a lower rate of overall disability in the shift analysis, with a common odds ratio of 2.8 favoring the intervention.
Safety results showed an increased risk for symptomatic ICH in the endovascular group (5.3% vs. 0.0%) but, despite that, 90-day mortality was significantly lower in the endovascular group (36.7% vs. 55.3%).
Dr. Nogueira noted a limitation of the study was that it was conducted in China.
“This was a Chinese study and, as Asians are known to have higher rates of intracranial atherosclerotic disease, the overall degree of generalizability of our findings to Western countries needs to be considered,” he commented.
However, subgroup analysis showed no treatment effect modification based on the presence of intracranial atherosclerotic disease, he noted.
Also, the proportion of comorbidities in the ATTENTION trial was similar to that in the BASICS trial, with the same degree of diabetes and atrial fibrillation.
Dr. Nogueira concluded that, in contrast to previous randomized trials of endovascular treatment for basilar artery occlusion, the ATTENTION trial was able to reinforce consecutive enrollment, resulting in a fast recruitment while minimizing crossovers.
Furthermore, he pointed out that the overall results are consistent with modern era observational studies, large registries, and meta-analysis.
Commenting on the study, Joanna Wardlaw, MD, professor of applied neuroimaging at the University of Edinburgh (Scotland), and chair of the ESOC Planning Group, said: “This is a very important result, since it provides confirmation beyond doubt the benefit of thrombectomy versus medical therapy for basilar artery occlusion stroke up to 12 hours after onset.”
Dr. Wardlaw added: “The trial was large enough to provide clear results and to enable subgroup analyses; no subgroup did not benefit from thrombectomy.”
In a discussion after the presentation, Urs Fischer, MD, chair of the department of neurology at the University Hospital Basel, Switzerland, said he was not surprised by the results of the ATTENTION trial.
“We have been doing thrombectomy in patients with basilar artery occlusion now for 20 years, although trials are extremely important to answer these questions, so now we have some clear evidence,” Dr. Fischer said. “Nevertheless, there are some caveats, as this is an Asian population, but this is a proof of concept, and it is going in the right direction.”
The ATTENTION trial was sponsored by the First Affiliated Hospital of University of Science and Technology of China.
A version of this article first appeared on Medscape.com.
The benefit of endovascular therapy in the treatment of stroke caused by an occlusion of the basilar artery has finally been confirmed in the ATTENTION randomized trial.
The study, conducted in China, showed that endovascular therapy for basilar artery occlusion is associated with higher rates of favorable and independent outcomes, as well as lower overall disability and lower mortality at 90 days, than best medical management alone.
The results were presented by Raul Nogueira, MD, professor of neurology at the University of Pittsburgh School of Medicine, at the European Stroke Organisation Conference (ESOC) 2022, where they were greeted with applause from the audience.
“We can finally say that we have conquered the basilar artery territory. It is about time. We can finally confirm that the benefit of endovascular therapy persists in the posterior circulation,” Dr. Nogueira said.
“The disability reduction benefit of endovascular therapy for basilar artery occlusion appears to be within the same range as that observed in the anterior circulation. However, in contrast to most anterior circulation endovascular trials, the ATTENTION trial also demonstrated a potential benefit in terms of mortality,” he added.
Dr. Nogueira explained that the first series of endovascular treatment for stroke in the modern era was published in 1988, and this was in the basilar artery occlusion territory, but almost 35 years later, although there has been overwhelming proof of benefit of endovascular treatment in the antiterror circulation, it remains unknown whether endovascular treatment is beneficial to treat acute basilar artery occlusion. This is despite efforts in conducting two trials – the BEST and BASICS trials – which showed a direction of benefit but failed to show real significance.
“Having said that, these trials paved the way for the current trial, specifically by demonstrating the importance of consecutive recruitment, fast enrollment, and the minimalization of crossover. They also confirmed the ideal target population for this procedure in an individual patient level meta-analysis of these two trials,” he said.
In addition, there have also been two large Chinese registries suggesting significant benefits.
The ATTENTION trial was conducted to evaluate the hypothesis that endovascular therapy is superior to best medical management alone in achieving more favorable outcomes (mRS, 0-3) at 90 days in subjects presenting with acute basilar artery stroke within 12 hours of the estimated time of onset.
The study enrolled 342 patients at 36 comprehensive stroke centers in China. All patients had occlusion of the basilar artery confirmed on vascular imaging within 12 hours of stroke onset, and they had severe symptoms at presentation, with an NIHSS score of at least 10. They were randomized in a 2:1 ratio to endovascular treatment or best medical management alone.
“It took us less than a year to enroll 342 patients,” Dr. Nogueira noted. “To put this into perspective, it took the BASICS trial over 8 years to enroll 300 patients, so these are very high-volume centers.”
He reported that two patients withdrew consent, and there were three patient crossovers on each side, comparing favorably with BASICS, leaving 226 patients in the intervention group and 114 in the control group.
Baseline characteristics were similar between the two groups: median age was 67 years, median NIHSS score was 24, about 25% received thrombolysis, and median time from stroke onset to randomization was 5 hours.
Results showed that the primary outcome – a favorable functional outcome (mRS, 0-3) at 90 days – was achieved in 22.8% of the control group and in 46% of the endovascular group, giving an adjusted risk ratio of 2.1 (P < .001).
The number needed to treat was just four.
“There were no surprises with secondary endpoints; everything was highly statistically significant,” Dr. Nogueira said.
Specifically, there was a lower rate of overall disability in the shift analysis, with a common odds ratio of 2.8 favoring the intervention.
Safety results showed an increased risk for symptomatic ICH in the endovascular group (5.3% vs. 0.0%) but, despite that, 90-day mortality was significantly lower in the endovascular group (36.7% vs. 55.3%).
Dr. Nogueira noted a limitation of the study was that it was conducted in China.
“This was a Chinese study and, as Asians are known to have higher rates of intracranial atherosclerotic disease, the overall degree of generalizability of our findings to Western countries needs to be considered,” he commented.
However, subgroup analysis showed no treatment effect modification based on the presence of intracranial atherosclerotic disease, he noted.
Also, the proportion of comorbidities in the ATTENTION trial was similar to that in the BASICS trial, with the same degree of diabetes and atrial fibrillation.
Dr. Nogueira concluded that, in contrast to previous randomized trials of endovascular treatment for basilar artery occlusion, the ATTENTION trial was able to reinforce consecutive enrollment, resulting in a fast recruitment while minimizing crossovers.
Furthermore, he pointed out that the overall results are consistent with modern era observational studies, large registries, and meta-analysis.
Commenting on the study, Joanna Wardlaw, MD, professor of applied neuroimaging at the University of Edinburgh (Scotland), and chair of the ESOC Planning Group, said: “This is a very important result, since it provides confirmation beyond doubt the benefit of thrombectomy versus medical therapy for basilar artery occlusion stroke up to 12 hours after onset.”
Dr. Wardlaw added: “The trial was large enough to provide clear results and to enable subgroup analyses; no subgroup did not benefit from thrombectomy.”
In a discussion after the presentation, Urs Fischer, MD, chair of the department of neurology at the University Hospital Basel, Switzerland, said he was not surprised by the results of the ATTENTION trial.
“We have been doing thrombectomy in patients with basilar artery occlusion now for 20 years, although trials are extremely important to answer these questions, so now we have some clear evidence,” Dr. Fischer said. “Nevertheless, there are some caveats, as this is an Asian population, but this is a proof of concept, and it is going in the right direction.”
The ATTENTION trial was sponsored by the First Affiliated Hospital of University of Science and Technology of China.
A version of this article first appeared on Medscape.com.
The benefit of endovascular therapy in the treatment of stroke caused by an occlusion of the basilar artery has finally been confirmed in the ATTENTION randomized trial.
The study, conducted in China, showed that endovascular therapy for basilar artery occlusion is associated with higher rates of favorable and independent outcomes, as well as lower overall disability and lower mortality at 90 days, than best medical management alone.
The results were presented by Raul Nogueira, MD, professor of neurology at the University of Pittsburgh School of Medicine, at the European Stroke Organisation Conference (ESOC) 2022, where they were greeted with applause from the audience.
“We can finally say that we have conquered the basilar artery territory. It is about time. We can finally confirm that the benefit of endovascular therapy persists in the posterior circulation,” Dr. Nogueira said.
“The disability reduction benefit of endovascular therapy for basilar artery occlusion appears to be within the same range as that observed in the anterior circulation. However, in contrast to most anterior circulation endovascular trials, the ATTENTION trial also demonstrated a potential benefit in terms of mortality,” he added.
Dr. Nogueira explained that the first series of endovascular treatment for stroke in the modern era was published in 1988, and this was in the basilar artery occlusion territory, but almost 35 years later, although there has been overwhelming proof of benefit of endovascular treatment in the antiterror circulation, it remains unknown whether endovascular treatment is beneficial to treat acute basilar artery occlusion. This is despite efforts in conducting two trials – the BEST and BASICS trials – which showed a direction of benefit but failed to show real significance.
“Having said that, these trials paved the way for the current trial, specifically by demonstrating the importance of consecutive recruitment, fast enrollment, and the minimalization of crossover. They also confirmed the ideal target population for this procedure in an individual patient level meta-analysis of these two trials,” he said.
In addition, there have also been two large Chinese registries suggesting significant benefits.
The ATTENTION trial was conducted to evaluate the hypothesis that endovascular therapy is superior to best medical management alone in achieving more favorable outcomes (mRS, 0-3) at 90 days in subjects presenting with acute basilar artery stroke within 12 hours of the estimated time of onset.
The study enrolled 342 patients at 36 comprehensive stroke centers in China. All patients had occlusion of the basilar artery confirmed on vascular imaging within 12 hours of stroke onset, and they had severe symptoms at presentation, with an NIHSS score of at least 10. They were randomized in a 2:1 ratio to endovascular treatment or best medical management alone.
“It took us less than a year to enroll 342 patients,” Dr. Nogueira noted. “To put this into perspective, it took the BASICS trial over 8 years to enroll 300 patients, so these are very high-volume centers.”
He reported that two patients withdrew consent, and there were three patient crossovers on each side, comparing favorably with BASICS, leaving 226 patients in the intervention group and 114 in the control group.
Baseline characteristics were similar between the two groups: median age was 67 years, median NIHSS score was 24, about 25% received thrombolysis, and median time from stroke onset to randomization was 5 hours.
Results showed that the primary outcome – a favorable functional outcome (mRS, 0-3) at 90 days – was achieved in 22.8% of the control group and in 46% of the endovascular group, giving an adjusted risk ratio of 2.1 (P < .001).
The number needed to treat was just four.
“There were no surprises with secondary endpoints; everything was highly statistically significant,” Dr. Nogueira said.
Specifically, there was a lower rate of overall disability in the shift analysis, with a common odds ratio of 2.8 favoring the intervention.
Safety results showed an increased risk for symptomatic ICH in the endovascular group (5.3% vs. 0.0%) but, despite that, 90-day mortality was significantly lower in the endovascular group (36.7% vs. 55.3%).
Dr. Nogueira noted a limitation of the study was that it was conducted in China.
“This was a Chinese study and, as Asians are known to have higher rates of intracranial atherosclerotic disease, the overall degree of generalizability of our findings to Western countries needs to be considered,” he commented.
However, subgroup analysis showed no treatment effect modification based on the presence of intracranial atherosclerotic disease, he noted.
Also, the proportion of comorbidities in the ATTENTION trial was similar to that in the BASICS trial, with the same degree of diabetes and atrial fibrillation.
Dr. Nogueira concluded that, in contrast to previous randomized trials of endovascular treatment for basilar artery occlusion, the ATTENTION trial was able to reinforce consecutive enrollment, resulting in a fast recruitment while minimizing crossovers.
Furthermore, he pointed out that the overall results are consistent with modern era observational studies, large registries, and meta-analysis.
Commenting on the study, Joanna Wardlaw, MD, professor of applied neuroimaging at the University of Edinburgh (Scotland), and chair of the ESOC Planning Group, said: “This is a very important result, since it provides confirmation beyond doubt the benefit of thrombectomy versus medical therapy for basilar artery occlusion stroke up to 12 hours after onset.”
Dr. Wardlaw added: “The trial was large enough to provide clear results and to enable subgroup analyses; no subgroup did not benefit from thrombectomy.”
In a discussion after the presentation, Urs Fischer, MD, chair of the department of neurology at the University Hospital Basel, Switzerland, said he was not surprised by the results of the ATTENTION trial.
“We have been doing thrombectomy in patients with basilar artery occlusion now for 20 years, although trials are extremely important to answer these questions, so now we have some clear evidence,” Dr. Fischer said. “Nevertheless, there are some caveats, as this is an Asian population, but this is a proof of concept, and it is going in the right direction.”
The ATTENTION trial was sponsored by the First Affiliated Hospital of University of Science and Technology of China.
A version of this article first appeared on Medscape.com.
Alarming global rise in pediatric hepatitis: Expert Q&A
This spring, global health advisories have been issued regarding an alarming – and as-yet unexplained – uptick of hepatitis in children. Currently, over 200 cases have been reported worldwide, a relatively small amount that nonetheless belies a considerable toll, including several deaths and the need for liver transplantation in a number of patients. The long-term implications are not yet known. Global health officials are working hard to determine a cause, with many focusing on the underlying cases of adenovirus that several patients have presented with.
To understand more, this news organization reached out to frequent contributor William F. Balistreri, MD, a specialist in pediatric gastroenterology and hepatology at Cincinnati Children’s Hospital Medical Center, where to date they have treated at least six cases of hepatitis in otherwise healthy young children, with one requiring a liver transplant. Dr. Balistreri discussed how the outbreak has developed to date, his advice to hepatologists and pediatricians, and where we stand now in this fast-evolving crisis.
Tracing the outbreak in the United States
How has this outbreak played out thus far in the United States, and what have we learned from that?
Sporadic reports of cases in multiple states are appearing. On April 21, 2022, a health alert was issued by the Centers for Disease Control and Prevention, recommending testing for adenovirus in children with acute hepatitis of an unknown etiology.
Baker and colleagues recently described five children with severe hepatitis and adenovirus viremia who were admitted to a children’s hospital in Birmingham, Ala., between October and November 2021. In collaboration with local and state officials, the CDC reviewed clinical records in order to identify patients with hepatitis and concomitant adenovirus infection, confirmed by polymerase chain reaction (PCR).
By February 2022, a total of nine children were identified. There was no epidemiologic linkage among these nine patients; all were well and immunocompetent. The prodromal features were somewhat similar: upper respiratory infection, vomiting, diarrhea, and jaundice. All children had markedly elevated aminotransferase levels and variably elevated total bilirubin levels. Extensive workup for other causes of acute liver injury (for example, other viruses, toxins/drugs, metabolic and autoimmune diseases) was unrevealing.
Specifically, none had documented SARS-CoV-2 infection. However, in all nine children, adenovirus was detected in whole blood samples. In the six children who underwent liver biopsy, there was nonspecific hepatitis, without inclusions or immunohistochemical detection of viral agents, including adenovirus. In three patients, the liver injury progressed, and despite the administration of antiviral agents, two underwent liver transplantation.
Baker and colleagues also suggested that measurement of adenovirus titers in whole blood (rather than plasma) may be more sensitive.
The CDC has recommended monitoring and surveillance in order to more fully understand the nature of the illness.
European and global cases
What has been the experience with this in Europe and elsewhere globally?
In mid-to-late 2021, several cases of acute hepatitis of unknown nature in children were identified in Europe. Public health officials in the United Kingdom investigated the high number of cases seen in children from England, Scotland, and Wales. They noted approximately 60 cases in England, mostly in children aged 2-5 years.
Marsh and colleagues reported a cluster of cases of severe hepatitis of unknown origin in Scotland affecting children aged 3-5 years. In Scotland, admitted cases were routinely tested for SARS-CoV-2. Of the 13 cases, five had a recent positive test. They discussed the possibility of increased severity of disease following infection with Omicron BA.2 (the dominant SARS-CoV-2 virus circulating in Scotland at that time) or infection by an uncharacterized SARS-CoV-2 variant. None of the children had been vaccinated for SARS-CoV-2.
On April 15, 2022, the World Health Organization Disease Outbreak News published a report of acute hepatitis of unknown etiology occurring in Great Britain and Northern Ireland. By April 21, 2022, 169 cases of acute hepatitis of unknown origin in children younger than 16 years had been reported from 11 countries in the WHO European region and 1 country in the WHO region of the Americas. Approximately 10% required a liver transplantation and at least one death was reported.
What has been established about the possible connection to the SARS-CoV-2 virus, particularly as it relates to coinfection with adenovirus?
In that WHO report of 169 cases, adenovirus was detected in 74 and SARS-CoV-2 in 20. Of note, 19 cases had a SARS-CoV-2 and adenovirus coinfection.
The report’s authors emphasized that, “while adenovirus is a possible hypothesis, investigations are ongoing for the causative agent.” The authors questioned whether this represents a continuing increase in cases of hepatitis or reflects an increased awareness.
The stated priority of the WHO is to determine the cause and to further refine control and prevention actions.
Given the worldwide nature of this outbreak, have connections between any of the cases been made yet?
Not to my knowledge.
What clinicians need to know
What makes this outbreak of hepatitis cases particularly concerning to the health care community, in comparison to other childhood diseases that occur globally? Is it because the cause is unknown or is it for other reasons?
It may be a collective heightened concern following the emergence of COVID.
Whether it represents a new form of acute hepatitis, a continuing increase in cases of hepatitis, or an increased awareness because of the well-publicized alerts remains to be determined. We certainly saw “viral-induced hepatitis” in the past.
Young patients may first be brought to pediatricians. What, if anything, should pediatricians be on the lookout for? Do they need a heightened index of suspicion or are the cases too rare at this point?
An awareness of the “outbreak” may allow the clinician to extend the typical workup of a child presenting with an undefined, presumably viral illness.
In the cases reported, the prodromal and/or presenting symptoms were respiratory and gastrointestinal in nature. They include nausea, vomiting, diarrhea, and abdominal pain.
Specifically, if jaundice and/or scleral icterus is noted, then hepatitis should be suspected.
Should pediatricians consider early referral to a pediatric gastroenterologist or hepatologist?
Yes, because there is the potential for finding a treatable cause (for example, autoimmune hepatitis or a specific metabolic disease) in a patient presenting in this fashion.
In addition, the potential for progression to acute liver failure (with coagulopathy and encephalopathy), albeit rare, exists.
What do hepatologists need to be doing when presented with suspected cases?
The typical clinical picture holds and the workup is standard. The one new key, given the recent data, is to test for adenovirus, using whole blood versus plasma, as the former may be more sensitive.
In addition, it is prudent to check for SARS-CoV-2 by PCR.
What are the major questions that remain and that you’d like to see elucidated going forward?
There are many. Is this a new disease? A new variant of adenovirus? A synergy or susceptibility related to SARS-CoV-2? Is it related to a variant of SARS-CoV-2? Is it triggering an adverse immune response? Are there other epigenetic factors involved? And finally, is this an increase, or is it related to a collective heightened concern following the pandemic?
Dr. Balistreri is the Dorothy M.M. Kersten Professor of Pediatrics, director emeritus of the Pediatric Liver Care Center, medical director emeritus of liver transplantation, and professor at the University of Cincinnati; he is also with the department of pediatrics at Cincinnati Children’s Hospital Medical Center.
A version of this article first appeared on Medscape.com.
This spring, global health advisories have been issued regarding an alarming – and as-yet unexplained – uptick of hepatitis in children. Currently, over 200 cases have been reported worldwide, a relatively small amount that nonetheless belies a considerable toll, including several deaths and the need for liver transplantation in a number of patients. The long-term implications are not yet known. Global health officials are working hard to determine a cause, with many focusing on the underlying cases of adenovirus that several patients have presented with.
To understand more, this news organization reached out to frequent contributor William F. Balistreri, MD, a specialist in pediatric gastroenterology and hepatology at Cincinnati Children’s Hospital Medical Center, where to date they have treated at least six cases of hepatitis in otherwise healthy young children, with one requiring a liver transplant. Dr. Balistreri discussed how the outbreak has developed to date, his advice to hepatologists and pediatricians, and where we stand now in this fast-evolving crisis.
Tracing the outbreak in the United States
How has this outbreak played out thus far in the United States, and what have we learned from that?
Sporadic reports of cases in multiple states are appearing. On April 21, 2022, a health alert was issued by the Centers for Disease Control and Prevention, recommending testing for adenovirus in children with acute hepatitis of an unknown etiology.
Baker and colleagues recently described five children with severe hepatitis and adenovirus viremia who were admitted to a children’s hospital in Birmingham, Ala., between October and November 2021. In collaboration with local and state officials, the CDC reviewed clinical records in order to identify patients with hepatitis and concomitant adenovirus infection, confirmed by polymerase chain reaction (PCR).
By February 2022, a total of nine children were identified. There was no epidemiologic linkage among these nine patients; all were well and immunocompetent. The prodromal features were somewhat similar: upper respiratory infection, vomiting, diarrhea, and jaundice. All children had markedly elevated aminotransferase levels and variably elevated total bilirubin levels. Extensive workup for other causes of acute liver injury (for example, other viruses, toxins/drugs, metabolic and autoimmune diseases) was unrevealing.
Specifically, none had documented SARS-CoV-2 infection. However, in all nine children, adenovirus was detected in whole blood samples. In the six children who underwent liver biopsy, there was nonspecific hepatitis, without inclusions or immunohistochemical detection of viral agents, including adenovirus. In three patients, the liver injury progressed, and despite the administration of antiviral agents, two underwent liver transplantation.
Baker and colleagues also suggested that measurement of adenovirus titers in whole blood (rather than plasma) may be more sensitive.
The CDC has recommended monitoring and surveillance in order to more fully understand the nature of the illness.
European and global cases
What has been the experience with this in Europe and elsewhere globally?
In mid-to-late 2021, several cases of acute hepatitis of unknown nature in children were identified in Europe. Public health officials in the United Kingdom investigated the high number of cases seen in children from England, Scotland, and Wales. They noted approximately 60 cases in England, mostly in children aged 2-5 years.
Marsh and colleagues reported a cluster of cases of severe hepatitis of unknown origin in Scotland affecting children aged 3-5 years. In Scotland, admitted cases were routinely tested for SARS-CoV-2. Of the 13 cases, five had a recent positive test. They discussed the possibility of increased severity of disease following infection with Omicron BA.2 (the dominant SARS-CoV-2 virus circulating in Scotland at that time) or infection by an uncharacterized SARS-CoV-2 variant. None of the children had been vaccinated for SARS-CoV-2.
On April 15, 2022, the World Health Organization Disease Outbreak News published a report of acute hepatitis of unknown etiology occurring in Great Britain and Northern Ireland. By April 21, 2022, 169 cases of acute hepatitis of unknown origin in children younger than 16 years had been reported from 11 countries in the WHO European region and 1 country in the WHO region of the Americas. Approximately 10% required a liver transplantation and at least one death was reported.
What has been established about the possible connection to the SARS-CoV-2 virus, particularly as it relates to coinfection with adenovirus?
In that WHO report of 169 cases, adenovirus was detected in 74 and SARS-CoV-2 in 20. Of note, 19 cases had a SARS-CoV-2 and adenovirus coinfection.
The report’s authors emphasized that, “while adenovirus is a possible hypothesis, investigations are ongoing for the causative agent.” The authors questioned whether this represents a continuing increase in cases of hepatitis or reflects an increased awareness.
The stated priority of the WHO is to determine the cause and to further refine control and prevention actions.
Given the worldwide nature of this outbreak, have connections between any of the cases been made yet?
Not to my knowledge.
What clinicians need to know
What makes this outbreak of hepatitis cases particularly concerning to the health care community, in comparison to other childhood diseases that occur globally? Is it because the cause is unknown or is it for other reasons?
It may be a collective heightened concern following the emergence of COVID.
Whether it represents a new form of acute hepatitis, a continuing increase in cases of hepatitis, or an increased awareness because of the well-publicized alerts remains to be determined. We certainly saw “viral-induced hepatitis” in the past.
Young patients may first be brought to pediatricians. What, if anything, should pediatricians be on the lookout for? Do they need a heightened index of suspicion or are the cases too rare at this point?
An awareness of the “outbreak” may allow the clinician to extend the typical workup of a child presenting with an undefined, presumably viral illness.
In the cases reported, the prodromal and/or presenting symptoms were respiratory and gastrointestinal in nature. They include nausea, vomiting, diarrhea, and abdominal pain.
Specifically, if jaundice and/or scleral icterus is noted, then hepatitis should be suspected.
Should pediatricians consider early referral to a pediatric gastroenterologist or hepatologist?
Yes, because there is the potential for finding a treatable cause (for example, autoimmune hepatitis or a specific metabolic disease) in a patient presenting in this fashion.
In addition, the potential for progression to acute liver failure (with coagulopathy and encephalopathy), albeit rare, exists.
What do hepatologists need to be doing when presented with suspected cases?
The typical clinical picture holds and the workup is standard. The one new key, given the recent data, is to test for adenovirus, using whole blood versus plasma, as the former may be more sensitive.
In addition, it is prudent to check for SARS-CoV-2 by PCR.
What are the major questions that remain and that you’d like to see elucidated going forward?
There are many. Is this a new disease? A new variant of adenovirus? A synergy or susceptibility related to SARS-CoV-2? Is it related to a variant of SARS-CoV-2? Is it triggering an adverse immune response? Are there other epigenetic factors involved? And finally, is this an increase, or is it related to a collective heightened concern following the pandemic?
Dr. Balistreri is the Dorothy M.M. Kersten Professor of Pediatrics, director emeritus of the Pediatric Liver Care Center, medical director emeritus of liver transplantation, and professor at the University of Cincinnati; he is also with the department of pediatrics at Cincinnati Children’s Hospital Medical Center.
A version of this article first appeared on Medscape.com.
This spring, global health advisories have been issued regarding an alarming – and as-yet unexplained – uptick of hepatitis in children. Currently, over 200 cases have been reported worldwide, a relatively small amount that nonetheless belies a considerable toll, including several deaths and the need for liver transplantation in a number of patients. The long-term implications are not yet known. Global health officials are working hard to determine a cause, with many focusing on the underlying cases of adenovirus that several patients have presented with.
To understand more, this news organization reached out to frequent contributor William F. Balistreri, MD, a specialist in pediatric gastroenterology and hepatology at Cincinnati Children’s Hospital Medical Center, where to date they have treated at least six cases of hepatitis in otherwise healthy young children, with one requiring a liver transplant. Dr. Balistreri discussed how the outbreak has developed to date, his advice to hepatologists and pediatricians, and where we stand now in this fast-evolving crisis.
Tracing the outbreak in the United States
How has this outbreak played out thus far in the United States, and what have we learned from that?
Sporadic reports of cases in multiple states are appearing. On April 21, 2022, a health alert was issued by the Centers for Disease Control and Prevention, recommending testing for adenovirus in children with acute hepatitis of an unknown etiology.
Baker and colleagues recently described five children with severe hepatitis and adenovirus viremia who were admitted to a children’s hospital in Birmingham, Ala., between October and November 2021. In collaboration with local and state officials, the CDC reviewed clinical records in order to identify patients with hepatitis and concomitant adenovirus infection, confirmed by polymerase chain reaction (PCR).
By February 2022, a total of nine children were identified. There was no epidemiologic linkage among these nine patients; all were well and immunocompetent. The prodromal features were somewhat similar: upper respiratory infection, vomiting, diarrhea, and jaundice. All children had markedly elevated aminotransferase levels and variably elevated total bilirubin levels. Extensive workup for other causes of acute liver injury (for example, other viruses, toxins/drugs, metabolic and autoimmune diseases) was unrevealing.
Specifically, none had documented SARS-CoV-2 infection. However, in all nine children, adenovirus was detected in whole blood samples. In the six children who underwent liver biopsy, there was nonspecific hepatitis, without inclusions or immunohistochemical detection of viral agents, including adenovirus. In three patients, the liver injury progressed, and despite the administration of antiviral agents, two underwent liver transplantation.
Baker and colleagues also suggested that measurement of adenovirus titers in whole blood (rather than plasma) may be more sensitive.
The CDC has recommended monitoring and surveillance in order to more fully understand the nature of the illness.
European and global cases
What has been the experience with this in Europe and elsewhere globally?
In mid-to-late 2021, several cases of acute hepatitis of unknown nature in children were identified in Europe. Public health officials in the United Kingdom investigated the high number of cases seen in children from England, Scotland, and Wales. They noted approximately 60 cases in England, mostly in children aged 2-5 years.
Marsh and colleagues reported a cluster of cases of severe hepatitis of unknown origin in Scotland affecting children aged 3-5 years. In Scotland, admitted cases were routinely tested for SARS-CoV-2. Of the 13 cases, five had a recent positive test. They discussed the possibility of increased severity of disease following infection with Omicron BA.2 (the dominant SARS-CoV-2 virus circulating in Scotland at that time) or infection by an uncharacterized SARS-CoV-2 variant. None of the children had been vaccinated for SARS-CoV-2.
On April 15, 2022, the World Health Organization Disease Outbreak News published a report of acute hepatitis of unknown etiology occurring in Great Britain and Northern Ireland. By April 21, 2022, 169 cases of acute hepatitis of unknown origin in children younger than 16 years had been reported from 11 countries in the WHO European region and 1 country in the WHO region of the Americas. Approximately 10% required a liver transplantation and at least one death was reported.
What has been established about the possible connection to the SARS-CoV-2 virus, particularly as it relates to coinfection with adenovirus?
In that WHO report of 169 cases, adenovirus was detected in 74 and SARS-CoV-2 in 20. Of note, 19 cases had a SARS-CoV-2 and adenovirus coinfection.
The report’s authors emphasized that, “while adenovirus is a possible hypothesis, investigations are ongoing for the causative agent.” The authors questioned whether this represents a continuing increase in cases of hepatitis or reflects an increased awareness.
The stated priority of the WHO is to determine the cause and to further refine control and prevention actions.
Given the worldwide nature of this outbreak, have connections between any of the cases been made yet?
Not to my knowledge.
What clinicians need to know
What makes this outbreak of hepatitis cases particularly concerning to the health care community, in comparison to other childhood diseases that occur globally? Is it because the cause is unknown or is it for other reasons?
It may be a collective heightened concern following the emergence of COVID.
Whether it represents a new form of acute hepatitis, a continuing increase in cases of hepatitis, or an increased awareness because of the well-publicized alerts remains to be determined. We certainly saw “viral-induced hepatitis” in the past.
Young patients may first be brought to pediatricians. What, if anything, should pediatricians be on the lookout for? Do they need a heightened index of suspicion or are the cases too rare at this point?
An awareness of the “outbreak” may allow the clinician to extend the typical workup of a child presenting with an undefined, presumably viral illness.
In the cases reported, the prodromal and/or presenting symptoms were respiratory and gastrointestinal in nature. They include nausea, vomiting, diarrhea, and abdominal pain.
Specifically, if jaundice and/or scleral icterus is noted, then hepatitis should be suspected.
Should pediatricians consider early referral to a pediatric gastroenterologist or hepatologist?
Yes, because there is the potential for finding a treatable cause (for example, autoimmune hepatitis or a specific metabolic disease) in a patient presenting in this fashion.
In addition, the potential for progression to acute liver failure (with coagulopathy and encephalopathy), albeit rare, exists.
What do hepatologists need to be doing when presented with suspected cases?
The typical clinical picture holds and the workup is standard. The one new key, given the recent data, is to test for adenovirus, using whole blood versus plasma, as the former may be more sensitive.
In addition, it is prudent to check for SARS-CoV-2 by PCR.
What are the major questions that remain and that you’d like to see elucidated going forward?
There are many. Is this a new disease? A new variant of adenovirus? A synergy or susceptibility related to SARS-CoV-2? Is it related to a variant of SARS-CoV-2? Is it triggering an adverse immune response? Are there other epigenetic factors involved? And finally, is this an increase, or is it related to a collective heightened concern following the pandemic?
Dr. Balistreri is the Dorothy M.M. Kersten Professor of Pediatrics, director emeritus of the Pediatric Liver Care Center, medical director emeritus of liver transplantation, and professor at the University of Cincinnati; he is also with the department of pediatrics at Cincinnati Children’s Hospital Medical Center.
A version of this article first appeared on Medscape.com.
Antithrombotic therapies shifting for Watchman LAA occlusion
A new study finds clinicians are shifting away from the U.S. Food and Drug Administration–approved combination of warfarin and aspirin after left atrial appendage occlusion (LAAO) with the Watchman device and that adverse events, particularly bleeding, are lower when aspirin is dropped.
Of 31,994 patients successfully implanted with the Watchman 2.5 device in the 3 years after its March 2015 approval, only 1 in 10 received the full postprocedure protocol studied in pivotal trials and codified into the FDA-device approval.
The protocol consisted of aspirin (81-325 mg) indefinitely and warfarin for 45 days. Following transesophageal echocardiography, patients were then maintained on warfarin and aspirin if there was a peridevice leak greater than 5 mm or switched to clopidogrel 75 mg for 6 months if a peridevice leak was ruled out or was 5 mm or less.
Based on the results, drawn from the National Cardiovascular Data Registry (NCDR) LAAO Registry, the most common discharge medications were warfarin and aspirin in 36.9% of patients, followed by a direct oral anticoagulant (DOAC) and aspirin (20.8%), warfarin alone (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (5%).
“There’s a little bit of practice leading the science in this space,” lead author James V. Freeman, MD, MPH, Yale School of Medicine, New Haven, Conn., told this news organization.
Patients who couldn’t tolerate long-term anticoagulation were excluded from the pivotal trials but are now the patients in whom the device is most often used, because of the Centers for Medicare & Medicaid reimbursement mandate for a relative or absolute contraindication to long-term anticoagulation, he noted.
Not surprisingly, 70% of patients in the registry had history of clinically relevant bleeding, the mean CHA2DS2-VASc score was 4.6, and mean HAS-BLED score was 3. At an average age of 76, they were also older, by years, than those in the clinical trials.
Secular trends at the time also saw the ascendancy of the DOACs relative to warfarin, observed Dr. Freeman. “So I think it’s pretty reasonable for physicians to be considering DOACs rather than warfarin in this context.”
Aspirin takes another hit
Results, published May 2 in the Journal of the American College of Cardiology, showed that any adverse event occurred at 45 days in 5.7% of patients discharged on warfarin and aspirin, 4% on warfarin alone, 5.2% on DOAC and aspirin, 3.8% on DOAC only, and 5.5% on DAPT.
Rates of any major adverse event were 4.4%, 3.3%, 4.3%, 3.1%, and 4.2% respectively, and for major bleeding were 3%, 1.8%, 2.8%, 1.7%, and 2.2% respectively. Although patients were similar across treatment groups, those treated with DAPT were slightly older and had more comorbidities, Dr. Freeman said.
In Cox frailty regression, the adjusted risk of any adverse event at 45 days was significantly lower when patients were discharged on warfarin alone (hazard ratio, 0.692; 95% confidence interval, 0.56-0.84) and a DOAC alone (HR, 0.731; 95% CI, 0.57-0.93), compared with warfarin and aspirin. There were no differences among the other groups.
The risk of any major adverse event was also significantly lower with warfarin alone (HR, 0.658; 95% CI, 0.53-0.80) and DOAC alone (HR, 0.767; 95% CI, 0.59-0.98).
At 6 months, rates of any adverse event (HR, 0.814; 95% CI, 0.72-0.93) and any major adverse event (HR, 0.840; 95% CI, 0.73-0.95) were significantly lower only in patients treated with warfarin alone.
“I think if there’s a take-home [message] here, it’s that for a lot of patients there’s good data now to suggest getting rid of the aspirin is a very reasonable thing to do,” Dr. Freeman said.
Further studies are needed in the space, but the results are consistent with those from transcatheter aortic valve replacement studies showing discharge on warfarin or DOAC anticoagulation alone reduces major adverse events without increasing thrombotic events, he said.
“I do think if there’s a strong indication for aspirin – someone has terrible coronary disease – there may be a role for using it,” Dr. Freeman said. But for a lot of these patients, anticoagulation alone without aspirin “may present a big opportunity to mitigate morbidity associated with this procedure.”
Dr. Freeman said he doesn’t expect the findings would be dramatically different with the second-generation Watchman FLX device but noted that randomized data will be forthcoming, as Boston Scientific changed the CHAMPION-AF trial protocol to include DOAC alone without aspirin.
Commenting for this news organization, Domenico Della Rocca, MD, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the study is a useful overview of post-LAAO therapies in a large population – but not surprising.
“Practice has changed over the years. More and more we are adopting and trusting the DOACs,” he said. “And, we are realizing that dual antiplatelet therapy is so aggressive and antiplatelet therapy alone maybe is not the best choice based on data on activation of coagulation.”
Commenting further, he said “I think it’s too early to suggest being too keen to completely drop aspirin,” noting that 20%-25% of patients have clopidogrel resistance and that the combination of two antiplatelets may be too aggressive a strategy for others.
Dr. Della Rocca and colleagues recently reported favorable long-term results with half-dose DOAC therapy after Watchman implantation and said the team is launching a randomized trial in more than 500 LAAO patients in the United States and Europe later this year. The trial will be comparing a DOAC-based strategy with low-dose apixaban long-term versus clopidogrel and aspirin initially and then switching to 100 mg aspirin long-term.
“We hope that in the next 2-3 years we will have some better answers, but at this point I would say that clopidogrel is kind of an obsolete strategy for appendage closure,” Dr. Della Rocca said.
In an accompanying editorial, David R. Holmes Jr., MD, Mayo Clinic, Rochester, Minn., says “the cornucopia of these specific strategies can be expected to change as practices evolve, as instructions for use broaden and, hopefully, with the results of well-done, scientifically performed trials. This current LAAO Registry report, however, serves as a useful benchmark.”
He cautioned that this is an observational cohort study and that unmeasured imbalances still may affect the ability to identify an unbiased treatment signal. The use of DAPT was also infrequent during the study and “conclusions based on this information are soft.”
The study was funded by the American College of Cardiology National Cardiovascular Data Registry (NCDR), and the National Heart, Lung, and Blood Institute (NHLBI) grants. Dr. Freeman has received salary support from the ACC NCDR and the NHLBI and has received consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster.
A version of this article first appeared on Medscape.com.
A new study finds clinicians are shifting away from the U.S. Food and Drug Administration–approved combination of warfarin and aspirin after left atrial appendage occlusion (LAAO) with the Watchman device and that adverse events, particularly bleeding, are lower when aspirin is dropped.
Of 31,994 patients successfully implanted with the Watchman 2.5 device in the 3 years after its March 2015 approval, only 1 in 10 received the full postprocedure protocol studied in pivotal trials and codified into the FDA-device approval.
The protocol consisted of aspirin (81-325 mg) indefinitely and warfarin for 45 days. Following transesophageal echocardiography, patients were then maintained on warfarin and aspirin if there was a peridevice leak greater than 5 mm or switched to clopidogrel 75 mg for 6 months if a peridevice leak was ruled out or was 5 mm or less.
Based on the results, drawn from the National Cardiovascular Data Registry (NCDR) LAAO Registry, the most common discharge medications were warfarin and aspirin in 36.9% of patients, followed by a direct oral anticoagulant (DOAC) and aspirin (20.8%), warfarin alone (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (5%).
“There’s a little bit of practice leading the science in this space,” lead author James V. Freeman, MD, MPH, Yale School of Medicine, New Haven, Conn., told this news organization.
Patients who couldn’t tolerate long-term anticoagulation were excluded from the pivotal trials but are now the patients in whom the device is most often used, because of the Centers for Medicare & Medicaid reimbursement mandate for a relative or absolute contraindication to long-term anticoagulation, he noted.
Not surprisingly, 70% of patients in the registry had history of clinically relevant bleeding, the mean CHA2DS2-VASc score was 4.6, and mean HAS-BLED score was 3. At an average age of 76, they were also older, by years, than those in the clinical trials.
Secular trends at the time also saw the ascendancy of the DOACs relative to warfarin, observed Dr. Freeman. “So I think it’s pretty reasonable for physicians to be considering DOACs rather than warfarin in this context.”
Aspirin takes another hit
Results, published May 2 in the Journal of the American College of Cardiology, showed that any adverse event occurred at 45 days in 5.7% of patients discharged on warfarin and aspirin, 4% on warfarin alone, 5.2% on DOAC and aspirin, 3.8% on DOAC only, and 5.5% on DAPT.
Rates of any major adverse event were 4.4%, 3.3%, 4.3%, 3.1%, and 4.2% respectively, and for major bleeding were 3%, 1.8%, 2.8%, 1.7%, and 2.2% respectively. Although patients were similar across treatment groups, those treated with DAPT were slightly older and had more comorbidities, Dr. Freeman said.
In Cox frailty regression, the adjusted risk of any adverse event at 45 days was significantly lower when patients were discharged on warfarin alone (hazard ratio, 0.692; 95% confidence interval, 0.56-0.84) and a DOAC alone (HR, 0.731; 95% CI, 0.57-0.93), compared with warfarin and aspirin. There were no differences among the other groups.
The risk of any major adverse event was also significantly lower with warfarin alone (HR, 0.658; 95% CI, 0.53-0.80) and DOAC alone (HR, 0.767; 95% CI, 0.59-0.98).
At 6 months, rates of any adverse event (HR, 0.814; 95% CI, 0.72-0.93) and any major adverse event (HR, 0.840; 95% CI, 0.73-0.95) were significantly lower only in patients treated with warfarin alone.
“I think if there’s a take-home [message] here, it’s that for a lot of patients there’s good data now to suggest getting rid of the aspirin is a very reasonable thing to do,” Dr. Freeman said.
Further studies are needed in the space, but the results are consistent with those from transcatheter aortic valve replacement studies showing discharge on warfarin or DOAC anticoagulation alone reduces major adverse events without increasing thrombotic events, he said.
“I do think if there’s a strong indication for aspirin – someone has terrible coronary disease – there may be a role for using it,” Dr. Freeman said. But for a lot of these patients, anticoagulation alone without aspirin “may present a big opportunity to mitigate morbidity associated with this procedure.”
Dr. Freeman said he doesn’t expect the findings would be dramatically different with the second-generation Watchman FLX device but noted that randomized data will be forthcoming, as Boston Scientific changed the CHAMPION-AF trial protocol to include DOAC alone without aspirin.
Commenting for this news organization, Domenico Della Rocca, MD, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the study is a useful overview of post-LAAO therapies in a large population – but not surprising.
“Practice has changed over the years. More and more we are adopting and trusting the DOACs,” he said. “And, we are realizing that dual antiplatelet therapy is so aggressive and antiplatelet therapy alone maybe is not the best choice based on data on activation of coagulation.”
Commenting further, he said “I think it’s too early to suggest being too keen to completely drop aspirin,” noting that 20%-25% of patients have clopidogrel resistance and that the combination of two antiplatelets may be too aggressive a strategy for others.
Dr. Della Rocca and colleagues recently reported favorable long-term results with half-dose DOAC therapy after Watchman implantation and said the team is launching a randomized trial in more than 500 LAAO patients in the United States and Europe later this year. The trial will be comparing a DOAC-based strategy with low-dose apixaban long-term versus clopidogrel and aspirin initially and then switching to 100 mg aspirin long-term.
“We hope that in the next 2-3 years we will have some better answers, but at this point I would say that clopidogrel is kind of an obsolete strategy for appendage closure,” Dr. Della Rocca said.
In an accompanying editorial, David R. Holmes Jr., MD, Mayo Clinic, Rochester, Minn., says “the cornucopia of these specific strategies can be expected to change as practices evolve, as instructions for use broaden and, hopefully, with the results of well-done, scientifically performed trials. This current LAAO Registry report, however, serves as a useful benchmark.”
He cautioned that this is an observational cohort study and that unmeasured imbalances still may affect the ability to identify an unbiased treatment signal. The use of DAPT was also infrequent during the study and “conclusions based on this information are soft.”
The study was funded by the American College of Cardiology National Cardiovascular Data Registry (NCDR), and the National Heart, Lung, and Blood Institute (NHLBI) grants. Dr. Freeman has received salary support from the ACC NCDR and the NHLBI and has received consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster.
A version of this article first appeared on Medscape.com.
A new study finds clinicians are shifting away from the U.S. Food and Drug Administration–approved combination of warfarin and aspirin after left atrial appendage occlusion (LAAO) with the Watchman device and that adverse events, particularly bleeding, are lower when aspirin is dropped.
Of 31,994 patients successfully implanted with the Watchman 2.5 device in the 3 years after its March 2015 approval, only 1 in 10 received the full postprocedure protocol studied in pivotal trials and codified into the FDA-device approval.
The protocol consisted of aspirin (81-325 mg) indefinitely and warfarin for 45 days. Following transesophageal echocardiography, patients were then maintained on warfarin and aspirin if there was a peridevice leak greater than 5 mm or switched to clopidogrel 75 mg for 6 months if a peridevice leak was ruled out or was 5 mm or less.
Based on the results, drawn from the National Cardiovascular Data Registry (NCDR) LAAO Registry, the most common discharge medications were warfarin and aspirin in 36.9% of patients, followed by a direct oral anticoagulant (DOAC) and aspirin (20.8%), warfarin alone (13.5%), DOAC only (12.3%), and dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor (5%).
“There’s a little bit of practice leading the science in this space,” lead author James V. Freeman, MD, MPH, Yale School of Medicine, New Haven, Conn., told this news organization.
Patients who couldn’t tolerate long-term anticoagulation were excluded from the pivotal trials but are now the patients in whom the device is most often used, because of the Centers for Medicare & Medicaid reimbursement mandate for a relative or absolute contraindication to long-term anticoagulation, he noted.
Not surprisingly, 70% of patients in the registry had history of clinically relevant bleeding, the mean CHA2DS2-VASc score was 4.6, and mean HAS-BLED score was 3. At an average age of 76, they were also older, by years, than those in the clinical trials.
Secular trends at the time also saw the ascendancy of the DOACs relative to warfarin, observed Dr. Freeman. “So I think it’s pretty reasonable for physicians to be considering DOACs rather than warfarin in this context.”
Aspirin takes another hit
Results, published May 2 in the Journal of the American College of Cardiology, showed that any adverse event occurred at 45 days in 5.7% of patients discharged on warfarin and aspirin, 4% on warfarin alone, 5.2% on DOAC and aspirin, 3.8% on DOAC only, and 5.5% on DAPT.
Rates of any major adverse event were 4.4%, 3.3%, 4.3%, 3.1%, and 4.2% respectively, and for major bleeding were 3%, 1.8%, 2.8%, 1.7%, and 2.2% respectively. Although patients were similar across treatment groups, those treated with DAPT were slightly older and had more comorbidities, Dr. Freeman said.
In Cox frailty regression, the adjusted risk of any adverse event at 45 days was significantly lower when patients were discharged on warfarin alone (hazard ratio, 0.692; 95% confidence interval, 0.56-0.84) and a DOAC alone (HR, 0.731; 95% CI, 0.57-0.93), compared with warfarin and aspirin. There were no differences among the other groups.
The risk of any major adverse event was also significantly lower with warfarin alone (HR, 0.658; 95% CI, 0.53-0.80) and DOAC alone (HR, 0.767; 95% CI, 0.59-0.98).
At 6 months, rates of any adverse event (HR, 0.814; 95% CI, 0.72-0.93) and any major adverse event (HR, 0.840; 95% CI, 0.73-0.95) were significantly lower only in patients treated with warfarin alone.
“I think if there’s a take-home [message] here, it’s that for a lot of patients there’s good data now to suggest getting rid of the aspirin is a very reasonable thing to do,” Dr. Freeman said.
Further studies are needed in the space, but the results are consistent with those from transcatheter aortic valve replacement studies showing discharge on warfarin or DOAC anticoagulation alone reduces major adverse events without increasing thrombotic events, he said.
“I do think if there’s a strong indication for aspirin – someone has terrible coronary disease – there may be a role for using it,” Dr. Freeman said. But for a lot of these patients, anticoagulation alone without aspirin “may present a big opportunity to mitigate morbidity associated with this procedure.”
Dr. Freeman said he doesn’t expect the findings would be dramatically different with the second-generation Watchman FLX device but noted that randomized data will be forthcoming, as Boston Scientific changed the CHAMPION-AF trial protocol to include DOAC alone without aspirin.
Commenting for this news organization, Domenico Della Rocca, MD, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Austin, said the study is a useful overview of post-LAAO therapies in a large population – but not surprising.
“Practice has changed over the years. More and more we are adopting and trusting the DOACs,” he said. “And, we are realizing that dual antiplatelet therapy is so aggressive and antiplatelet therapy alone maybe is not the best choice based on data on activation of coagulation.”
Commenting further, he said “I think it’s too early to suggest being too keen to completely drop aspirin,” noting that 20%-25% of patients have clopidogrel resistance and that the combination of two antiplatelets may be too aggressive a strategy for others.
Dr. Della Rocca and colleagues recently reported favorable long-term results with half-dose DOAC therapy after Watchman implantation and said the team is launching a randomized trial in more than 500 LAAO patients in the United States and Europe later this year. The trial will be comparing a DOAC-based strategy with low-dose apixaban long-term versus clopidogrel and aspirin initially and then switching to 100 mg aspirin long-term.
“We hope that in the next 2-3 years we will have some better answers, but at this point I would say that clopidogrel is kind of an obsolete strategy for appendage closure,” Dr. Della Rocca said.
In an accompanying editorial, David R. Holmes Jr., MD, Mayo Clinic, Rochester, Minn., says “the cornucopia of these specific strategies can be expected to change as practices evolve, as instructions for use broaden and, hopefully, with the results of well-done, scientifically performed trials. This current LAAO Registry report, however, serves as a useful benchmark.”
He cautioned that this is an observational cohort study and that unmeasured imbalances still may affect the ability to identify an unbiased treatment signal. The use of DAPT was also infrequent during the study and “conclusions based on this information are soft.”
The study was funded by the American College of Cardiology National Cardiovascular Data Registry (NCDR), and the National Heart, Lung, and Blood Institute (NHLBI) grants. Dr. Freeman has received salary support from the ACC NCDR and the NHLBI and has received consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster.
A version of this article first appeared on Medscape.com.
‘Agony of choice’ for clinicians treating leukemia
“Targeted therapies have outnumbered chemoimmunotherapy-based treatment approaches, demonstrating superior efficacy and tolerability profiles across nearly all CLL patient subgroups in the frontline and relapsed disease treatment setting,” author Jan-Paul Bohn, MD, PhD, of the department of internal medicine V, hematology and oncology, at Medical University of Innsbruck (Austria), reported in the review published in Memo, the Magazine of European Medical Oncology.
The options leave clinicians “spoilt for choice when selecting optimal therapy,” he said.
The three major drug classes to emerge – inhibitors of Bruton tyrosine kinase (BTK), antiapoptotic protein B-cell lymphoma 2 (BCL2) and phosphoinositide 3’-kinase (PI3K) – all appear similar in efficacy and tolerability.
Particularly in high-risk patients, the drugs have been so effective that the less desirable previous standard of “chemoimmunotherapy has widely faded into the background in the Western hemisphere,” Dr. Bohn wrote.
However, with caveats of the newer drugs including acquired resistances and potential toxicities, challenges have shifted to determining how to best juggle and/or combine the agents.
Frontline therapy
In terms of frontline options for CLL therapy, the BTK inhibitors, along with the BCL2 inhibitor venetoclax have been key in negating the need for chemotherapy, with some of the latest data showing superiority of venetoclax in combination with obinutuzumab (GVe) over chemotherapy even in the higher-risk subset of patients with mutated IGHV status and without TP53 disruption.
Hence, “chemoimmunotherapy may now even be questioned in the remaining subset of CLL patients with mutated IGHV status and without TP53 disruption,” Dr. Bohn reported.
That being said, the criteria for treatment choices in the frontline setting among the newer drug classes can often come down to the key issues of patients’ comorbidities and treatment preferences.
For example, in terms of patients who have higher risk because of tumor lysis syndrome (TLS), or issues including declining renal function, continuous BTK inhibitor treatment may be the preferred choice over the combination of venetoclax plus obinutuzumab (GVe), Dr. Bohn noted.
Conversely, for patients with cardiac comorbidities or a higher risk of bleeding, the GVe combination may be preferred over ibrutinib, with recent findings showing ibrutinib to be associated with as much as an 18-times higher risk of sudden unexplained death or cardiac death in young and fit patients who had preexisting arterial hypertension and/or a history of cardiac disorders requiring therapy.
For those with cardiac comorbidities, the more selective second-generation BTK inhibitor acalabrutinib is a potentially favorable alternative, as the drug is “at least similarly effective and more favorable in terms of tolerability, compared with ibrutinib, particularly as far as cardiac and bleeding side effects are considered,” Dr. Bohn said.
And in higher-risk cases involving TP53 dysfunction, a BTK inhibitor may be superior to GVe for frontline treatment, Dr. Bohn noted, with data showing progression-free survival in patients with and without deletion 17p to be significantly reduced with GVe versus the BTK inhibitor ibrutinib.
Relapsed and refractory disease
With similarly high efficacy observed with the new drug classes among relapsed and/or refractory patients, chemoimmunotherapy has likewise “become obsolete in nearly all patients naive to novel agents at relapse who typically present with genetically high-risk disease,” Dr. Bohn noted.
He wrote that most of the recommendations for frontline therapy hold true in the relapsed and refractory patients, with comorbidities and personal preferences again key drivers of treatment choices.
While data is currently limited regarding benefits of venetoclax-based regimens over BTK inhibitors in relapsed/refractory patients, there is “growing evidence suggesting similar clinical outcomes achievable with these agents in either order,” Dr. Bohn wrote.
Further recommendations regarding relapsed or refractory patients include:
- Among patients who do experience disease progression while on continuous treatment with BTK inhibitors, venetoclax-based regimes seem most effective. However, with relapse after venetoclax-based regimes, some growing evidence supports retreatment with the drug “depending on depth and duration of response achieved after first venetoclax exposure,” Dr. Bohn noted.
- For patients with deletion 17p, venetoclax shows promising efficacy during relapse when given as monotherapy until disease progression or occurrence of unacceptable toxicity.
- And for patients with TP53 abnormalities, the considerations are the same as for frontline therapy, with venetoclax showing promising efficacy when given in monotherapy until disease progression or occurrence of unacceptable toxicity.
Of note, PI3K inhibitors are generally not used in CLL patients naive to BTK and BCL2 inhibitors because of the higher risk of immune-mediated toxicities and infectious complications associated with the currently approved PI3K inhibitors idelalisib and duvelisib, he reported.
Nevertheless, “PI3K inhibitors remain a valuable therapeutic addition in patients refractory or intolerant to BTK inhibitors and venetoclax-based regimens,” Dr. Bohn said.
Newer agents, fixed duration
Commenting on the review, hematologist Seema A. Bhat, MD, an assistant professor with the Ohio State University Comprehensive Cancer Center, Columbus, said that the advances with targeted therapies in CLL are paying off with improved survival.
“With these recent advances in the treatment of CLL, especially the availability of targeted therapies, there has been an improvement in survival of patients with CLL, as the CLL-related death rate steadily reduced by approximately 3% per year between 2006 and 2015,” she said in an interview.
She added that even-newer agents in development, including the reversibly binding BTK inhibitor–like pirtobrutinib and nemtabrutinib, when approved, will further add to the treatment choices for patients.
Meanwhile, a key area of focus is the combination of BTK inhibitors and BCL2 inhibitors, specifically for a fixed duration of time to obtain a deeper response and hence possibility a time-limited therapy, she noted. “We are also excited about the possibility of having more fixed-duration treatments available for our patients, which will make their treatment journey less troublesome, both physically as well as financially.”
Dr. Bohn reported receiving personal fees from AbbVie, AstraZeneca and Janssen for advisory board participation. Dr. Bhat has served on advisory board for AstraZeneca and received honorarium from them.
“Targeted therapies have outnumbered chemoimmunotherapy-based treatment approaches, demonstrating superior efficacy and tolerability profiles across nearly all CLL patient subgroups in the frontline and relapsed disease treatment setting,” author Jan-Paul Bohn, MD, PhD, of the department of internal medicine V, hematology and oncology, at Medical University of Innsbruck (Austria), reported in the review published in Memo, the Magazine of European Medical Oncology.
The options leave clinicians “spoilt for choice when selecting optimal therapy,” he said.
The three major drug classes to emerge – inhibitors of Bruton tyrosine kinase (BTK), antiapoptotic protein B-cell lymphoma 2 (BCL2) and phosphoinositide 3’-kinase (PI3K) – all appear similar in efficacy and tolerability.
Particularly in high-risk patients, the drugs have been so effective that the less desirable previous standard of “chemoimmunotherapy has widely faded into the background in the Western hemisphere,” Dr. Bohn wrote.
However, with caveats of the newer drugs including acquired resistances and potential toxicities, challenges have shifted to determining how to best juggle and/or combine the agents.
Frontline therapy
In terms of frontline options for CLL therapy, the BTK inhibitors, along with the BCL2 inhibitor venetoclax have been key in negating the need for chemotherapy, with some of the latest data showing superiority of venetoclax in combination with obinutuzumab (GVe) over chemotherapy even in the higher-risk subset of patients with mutated IGHV status and without TP53 disruption.
Hence, “chemoimmunotherapy may now even be questioned in the remaining subset of CLL patients with mutated IGHV status and without TP53 disruption,” Dr. Bohn reported.
That being said, the criteria for treatment choices in the frontline setting among the newer drug classes can often come down to the key issues of patients’ comorbidities and treatment preferences.
For example, in terms of patients who have higher risk because of tumor lysis syndrome (TLS), or issues including declining renal function, continuous BTK inhibitor treatment may be the preferred choice over the combination of venetoclax plus obinutuzumab (GVe), Dr. Bohn noted.
Conversely, for patients with cardiac comorbidities or a higher risk of bleeding, the GVe combination may be preferred over ibrutinib, with recent findings showing ibrutinib to be associated with as much as an 18-times higher risk of sudden unexplained death or cardiac death in young and fit patients who had preexisting arterial hypertension and/or a history of cardiac disorders requiring therapy.
For those with cardiac comorbidities, the more selective second-generation BTK inhibitor acalabrutinib is a potentially favorable alternative, as the drug is “at least similarly effective and more favorable in terms of tolerability, compared with ibrutinib, particularly as far as cardiac and bleeding side effects are considered,” Dr. Bohn said.
And in higher-risk cases involving TP53 dysfunction, a BTK inhibitor may be superior to GVe for frontline treatment, Dr. Bohn noted, with data showing progression-free survival in patients with and without deletion 17p to be significantly reduced with GVe versus the BTK inhibitor ibrutinib.
Relapsed and refractory disease
With similarly high efficacy observed with the new drug classes among relapsed and/or refractory patients, chemoimmunotherapy has likewise “become obsolete in nearly all patients naive to novel agents at relapse who typically present with genetically high-risk disease,” Dr. Bohn noted.
He wrote that most of the recommendations for frontline therapy hold true in the relapsed and refractory patients, with comorbidities and personal preferences again key drivers of treatment choices.
While data is currently limited regarding benefits of venetoclax-based regimens over BTK inhibitors in relapsed/refractory patients, there is “growing evidence suggesting similar clinical outcomes achievable with these agents in either order,” Dr. Bohn wrote.
Further recommendations regarding relapsed or refractory patients include:
- Among patients who do experience disease progression while on continuous treatment with BTK inhibitors, venetoclax-based regimes seem most effective. However, with relapse after venetoclax-based regimes, some growing evidence supports retreatment with the drug “depending on depth and duration of response achieved after first venetoclax exposure,” Dr. Bohn noted.
- For patients with deletion 17p, venetoclax shows promising efficacy during relapse when given as monotherapy until disease progression or occurrence of unacceptable toxicity.
- And for patients with TP53 abnormalities, the considerations are the same as for frontline therapy, with venetoclax showing promising efficacy when given in monotherapy until disease progression or occurrence of unacceptable toxicity.
Of note, PI3K inhibitors are generally not used in CLL patients naive to BTK and BCL2 inhibitors because of the higher risk of immune-mediated toxicities and infectious complications associated with the currently approved PI3K inhibitors idelalisib and duvelisib, he reported.
Nevertheless, “PI3K inhibitors remain a valuable therapeutic addition in patients refractory or intolerant to BTK inhibitors and venetoclax-based regimens,” Dr. Bohn said.
Newer agents, fixed duration
Commenting on the review, hematologist Seema A. Bhat, MD, an assistant professor with the Ohio State University Comprehensive Cancer Center, Columbus, said that the advances with targeted therapies in CLL are paying off with improved survival.
“With these recent advances in the treatment of CLL, especially the availability of targeted therapies, there has been an improvement in survival of patients with CLL, as the CLL-related death rate steadily reduced by approximately 3% per year between 2006 and 2015,” she said in an interview.
She added that even-newer agents in development, including the reversibly binding BTK inhibitor–like pirtobrutinib and nemtabrutinib, when approved, will further add to the treatment choices for patients.
Meanwhile, a key area of focus is the combination of BTK inhibitors and BCL2 inhibitors, specifically for a fixed duration of time to obtain a deeper response and hence possibility a time-limited therapy, she noted. “We are also excited about the possibility of having more fixed-duration treatments available for our patients, which will make their treatment journey less troublesome, both physically as well as financially.”
Dr. Bohn reported receiving personal fees from AbbVie, AstraZeneca and Janssen for advisory board participation. Dr. Bhat has served on advisory board for AstraZeneca and received honorarium from them.
“Targeted therapies have outnumbered chemoimmunotherapy-based treatment approaches, demonstrating superior efficacy and tolerability profiles across nearly all CLL patient subgroups in the frontline and relapsed disease treatment setting,” author Jan-Paul Bohn, MD, PhD, of the department of internal medicine V, hematology and oncology, at Medical University of Innsbruck (Austria), reported in the review published in Memo, the Magazine of European Medical Oncology.
The options leave clinicians “spoilt for choice when selecting optimal therapy,” he said.
The three major drug classes to emerge – inhibitors of Bruton tyrosine kinase (BTK), antiapoptotic protein B-cell lymphoma 2 (BCL2) and phosphoinositide 3’-kinase (PI3K) – all appear similar in efficacy and tolerability.
Particularly in high-risk patients, the drugs have been so effective that the less desirable previous standard of “chemoimmunotherapy has widely faded into the background in the Western hemisphere,” Dr. Bohn wrote.
However, with caveats of the newer drugs including acquired resistances and potential toxicities, challenges have shifted to determining how to best juggle and/or combine the agents.
Frontline therapy
In terms of frontline options for CLL therapy, the BTK inhibitors, along with the BCL2 inhibitor venetoclax have been key in negating the need for chemotherapy, with some of the latest data showing superiority of venetoclax in combination with obinutuzumab (GVe) over chemotherapy even in the higher-risk subset of patients with mutated IGHV status and without TP53 disruption.
Hence, “chemoimmunotherapy may now even be questioned in the remaining subset of CLL patients with mutated IGHV status and without TP53 disruption,” Dr. Bohn reported.
That being said, the criteria for treatment choices in the frontline setting among the newer drug classes can often come down to the key issues of patients’ comorbidities and treatment preferences.
For example, in terms of patients who have higher risk because of tumor lysis syndrome (TLS), or issues including declining renal function, continuous BTK inhibitor treatment may be the preferred choice over the combination of venetoclax plus obinutuzumab (GVe), Dr. Bohn noted.
Conversely, for patients with cardiac comorbidities or a higher risk of bleeding, the GVe combination may be preferred over ibrutinib, with recent findings showing ibrutinib to be associated with as much as an 18-times higher risk of sudden unexplained death or cardiac death in young and fit patients who had preexisting arterial hypertension and/or a history of cardiac disorders requiring therapy.
For those with cardiac comorbidities, the more selective second-generation BTK inhibitor acalabrutinib is a potentially favorable alternative, as the drug is “at least similarly effective and more favorable in terms of tolerability, compared with ibrutinib, particularly as far as cardiac and bleeding side effects are considered,” Dr. Bohn said.
And in higher-risk cases involving TP53 dysfunction, a BTK inhibitor may be superior to GVe for frontline treatment, Dr. Bohn noted, with data showing progression-free survival in patients with and without deletion 17p to be significantly reduced with GVe versus the BTK inhibitor ibrutinib.
Relapsed and refractory disease
With similarly high efficacy observed with the new drug classes among relapsed and/or refractory patients, chemoimmunotherapy has likewise “become obsolete in nearly all patients naive to novel agents at relapse who typically present with genetically high-risk disease,” Dr. Bohn noted.
He wrote that most of the recommendations for frontline therapy hold true in the relapsed and refractory patients, with comorbidities and personal preferences again key drivers of treatment choices.
While data is currently limited regarding benefits of venetoclax-based regimens over BTK inhibitors in relapsed/refractory patients, there is “growing evidence suggesting similar clinical outcomes achievable with these agents in either order,” Dr. Bohn wrote.
Further recommendations regarding relapsed or refractory patients include:
- Among patients who do experience disease progression while on continuous treatment with BTK inhibitors, venetoclax-based regimes seem most effective. However, with relapse after venetoclax-based regimes, some growing evidence supports retreatment with the drug “depending on depth and duration of response achieved after first venetoclax exposure,” Dr. Bohn noted.
- For patients with deletion 17p, venetoclax shows promising efficacy during relapse when given as monotherapy until disease progression or occurrence of unacceptable toxicity.
- And for patients with TP53 abnormalities, the considerations are the same as for frontline therapy, with venetoclax showing promising efficacy when given in monotherapy until disease progression or occurrence of unacceptable toxicity.
Of note, PI3K inhibitors are generally not used in CLL patients naive to BTK and BCL2 inhibitors because of the higher risk of immune-mediated toxicities and infectious complications associated with the currently approved PI3K inhibitors idelalisib and duvelisib, he reported.
Nevertheless, “PI3K inhibitors remain a valuable therapeutic addition in patients refractory or intolerant to BTK inhibitors and venetoclax-based regimens,” Dr. Bohn said.
Newer agents, fixed duration
Commenting on the review, hematologist Seema A. Bhat, MD, an assistant professor with the Ohio State University Comprehensive Cancer Center, Columbus, said that the advances with targeted therapies in CLL are paying off with improved survival.
“With these recent advances in the treatment of CLL, especially the availability of targeted therapies, there has been an improvement in survival of patients with CLL, as the CLL-related death rate steadily reduced by approximately 3% per year between 2006 and 2015,” she said in an interview.
She added that even-newer agents in development, including the reversibly binding BTK inhibitor–like pirtobrutinib and nemtabrutinib, when approved, will further add to the treatment choices for patients.
Meanwhile, a key area of focus is the combination of BTK inhibitors and BCL2 inhibitors, specifically for a fixed duration of time to obtain a deeper response and hence possibility a time-limited therapy, she noted. “We are also excited about the possibility of having more fixed-duration treatments available for our patients, which will make their treatment journey less troublesome, both physically as well as financially.”
Dr. Bohn reported receiving personal fees from AbbVie, AstraZeneca and Janssen for advisory board participation. Dr. Bhat has served on advisory board for AstraZeneca and received honorarium from them.
FROM MEMO – MAGAZINE OF EUROPEAN MEDICAL ONCOLOGY
Dermatology attracts more than its share of physician assistants
Dermatology added PAs at a mean rate of 11.6% annually over that 6-year period, compared with a mean of 7.8% for all other specialties (P <.001), as the National Commission on Certification of Physician Assistants (NCCPA) tallied 2,324 working in dermatology and 64,490 in all other specialties in 2013 and 3,938/94,616, respectively, in 2018, Justin D. Arnold, MD, of the University of California, Irvine, and associates reported in JAMA Dermatology.
“There is, however, a lack of racial and ethnic diversity within the dermatology PA workforce,” they noted. A detailed comparison using the 2018 data showed that only 1.6% of dermatology PAs identified as Black, compared with 3.7% of those in all other specialties (P <.001), although “similar rates of Hispanic ethnicity were observed” in dermatology PAs (6.0%) and PAs in other fields (6.5%), the investigators added.
That was not the case for women in the profession, as 82% of PAs in dermatology were female in 2018, compared with 67% in the other specialties. Dermatology PAs also were significantly more likely to work in office-based practices than their nondermatology peers (93% vs. 37%, P < .001) and to reside in metropolitan areas (95% vs. 92%, P < .001), Dr. Arnold and associates said in the research letter.
The dermatology PAs also were more likely to work part time (30 or fewer hours per week) than those outside dermatology, 19.1% vs. 12.9% (P < .001). Despite that, the dermatology PAs reported seeing more patients per week (a mean of 119) than those in all of the other specialties (a mean of 71), the investigators said.
The total number of certified PAs was over 131,000 in 2018, but about 25% had not selected a principal specialty in their PA Professional Profiles and were not included in the study, they explained.
“Although this study did not assess the reasons for the substantial increase of dermatology PAs, numerous factors, such as a potential physician shortage or the expansion of private equity–owned practices, may contribute to the accelerating use of PAs within the field,” they wrote.
Dermatology added PAs at a mean rate of 11.6% annually over that 6-year period, compared with a mean of 7.8% for all other specialties (P <.001), as the National Commission on Certification of Physician Assistants (NCCPA) tallied 2,324 working in dermatology and 64,490 in all other specialties in 2013 and 3,938/94,616, respectively, in 2018, Justin D. Arnold, MD, of the University of California, Irvine, and associates reported in JAMA Dermatology.
“There is, however, a lack of racial and ethnic diversity within the dermatology PA workforce,” they noted. A detailed comparison using the 2018 data showed that only 1.6% of dermatology PAs identified as Black, compared with 3.7% of those in all other specialties (P <.001), although “similar rates of Hispanic ethnicity were observed” in dermatology PAs (6.0%) and PAs in other fields (6.5%), the investigators added.
That was not the case for women in the profession, as 82% of PAs in dermatology were female in 2018, compared with 67% in the other specialties. Dermatology PAs also were significantly more likely to work in office-based practices than their nondermatology peers (93% vs. 37%, P < .001) and to reside in metropolitan areas (95% vs. 92%, P < .001), Dr. Arnold and associates said in the research letter.
The dermatology PAs also were more likely to work part time (30 or fewer hours per week) than those outside dermatology, 19.1% vs. 12.9% (P < .001). Despite that, the dermatology PAs reported seeing more patients per week (a mean of 119) than those in all of the other specialties (a mean of 71), the investigators said.
The total number of certified PAs was over 131,000 in 2018, but about 25% had not selected a principal specialty in their PA Professional Profiles and were not included in the study, they explained.
“Although this study did not assess the reasons for the substantial increase of dermatology PAs, numerous factors, such as a potential physician shortage or the expansion of private equity–owned practices, may contribute to the accelerating use of PAs within the field,” they wrote.
Dermatology added PAs at a mean rate of 11.6% annually over that 6-year period, compared with a mean of 7.8% for all other specialties (P <.001), as the National Commission on Certification of Physician Assistants (NCCPA) tallied 2,324 working in dermatology and 64,490 in all other specialties in 2013 and 3,938/94,616, respectively, in 2018, Justin D. Arnold, MD, of the University of California, Irvine, and associates reported in JAMA Dermatology.
“There is, however, a lack of racial and ethnic diversity within the dermatology PA workforce,” they noted. A detailed comparison using the 2018 data showed that only 1.6% of dermatology PAs identified as Black, compared with 3.7% of those in all other specialties (P <.001), although “similar rates of Hispanic ethnicity were observed” in dermatology PAs (6.0%) and PAs in other fields (6.5%), the investigators added.
That was not the case for women in the profession, as 82% of PAs in dermatology were female in 2018, compared with 67% in the other specialties. Dermatology PAs also were significantly more likely to work in office-based practices than their nondermatology peers (93% vs. 37%, P < .001) and to reside in metropolitan areas (95% vs. 92%, P < .001), Dr. Arnold and associates said in the research letter.
The dermatology PAs also were more likely to work part time (30 or fewer hours per week) than those outside dermatology, 19.1% vs. 12.9% (P < .001). Despite that, the dermatology PAs reported seeing more patients per week (a mean of 119) than those in all of the other specialties (a mean of 71), the investigators said.
The total number of certified PAs was over 131,000 in 2018, but about 25% had not selected a principal specialty in their PA Professional Profiles and were not included in the study, they explained.
“Although this study did not assess the reasons for the substantial increase of dermatology PAs, numerous factors, such as a potential physician shortage or the expansion of private equity–owned practices, may contribute to the accelerating use of PAs within the field,” they wrote.
FROM JAMA DERMATOLOGY
43-year-old male • fatigue • unintentional weight loss • pancytopenia • Dx?
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.
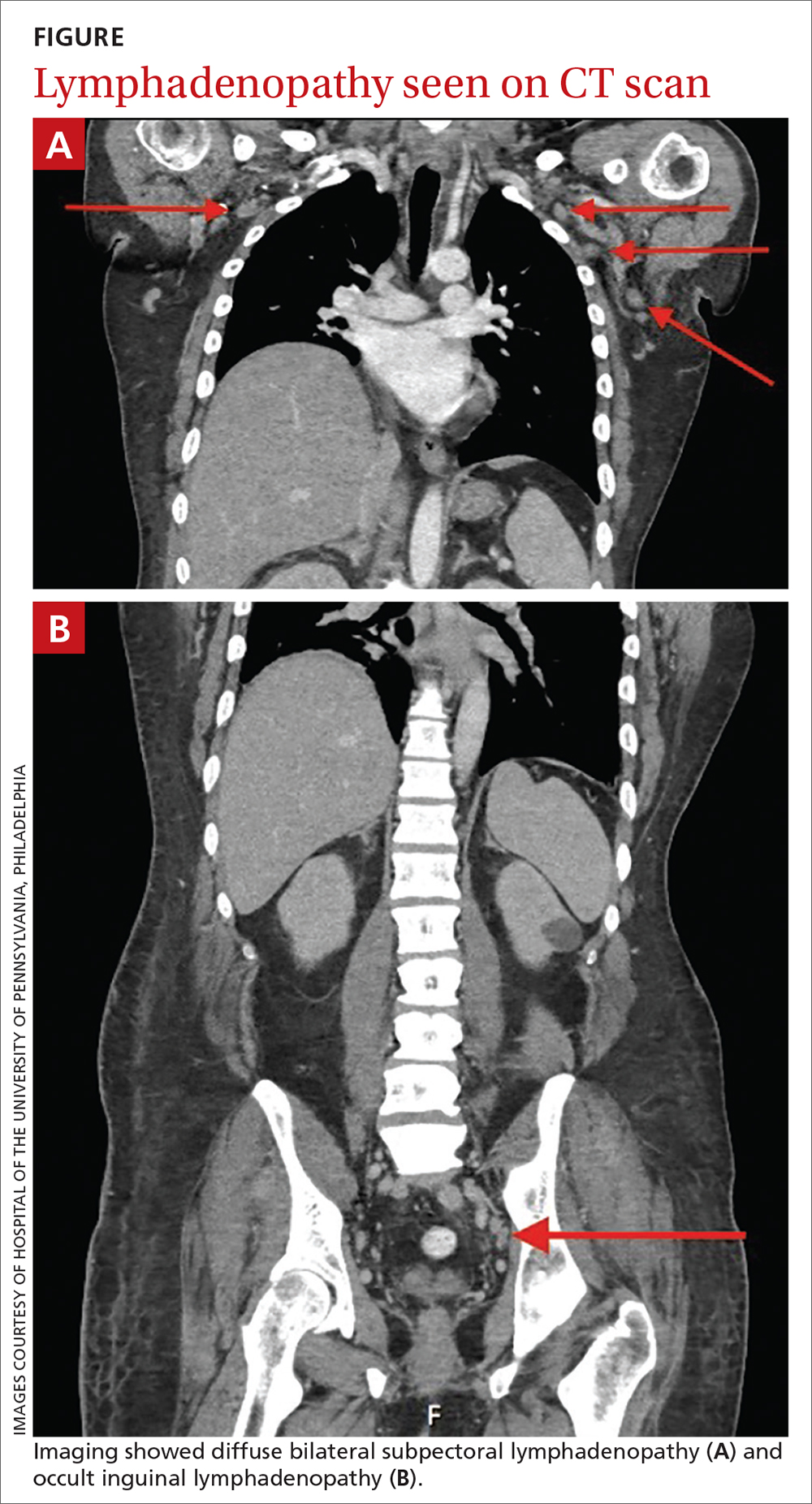
Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.

Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
THE CASE
A 43-year-old Black male presented to his primary care physician with an 8-month history of progressive fatigue, weakness, and unintentional weight loss. The patient’s history also included antiphospholipid antibody syndrome (APS) with prior deep venous thrombosis/pulmonary embolism for which he was taking warfarin.
At the time of presentation, he reported profound dyspnea on exertion, lightheadedness, dry mouth, low back pain, and worsening nocturia. The remainder of the review of systems was negative. He denied tobacco, alcohol, or illicit drug use or recent travel. His personal and family histories were negative for cancer.
Laboratory data collected during the outpatient visit were notable for a white blood cell count of 2300/mcL (reference range, 4000-11,000/mcL); hemoglobin, 8.6 g/dL (13.5-17.5 g/dL); and platelets, 44,000/mcL (150,000-400,000/mcL). Proteinuria was indicated by a measurement > 500 mg/dL on urine dipstick.
The patient was admitted to the hospital for further work-up of new pancytopenia. His vital signs on admission were notable for tachycardia and a weight of 237 lbs, decreased from 283 lbs 8 months prior. His physical exam revealed dry mucous membranes, bruising of fingertips, and marked lower extremity weakness with preserved sensation. No lymphadenopathy was noted on the admission physical exam.
THE DIAGNOSIS
Inpatient laboratory studies showed elevated inflammatory markers and a positive Coombs test with low haptoglobin. There was no evidence of bacterial or viral infection.

Autoimmune laboratory data included a positive antiphospholipid antibody (ANA) test (1:10,240, diffuse; reference < 1:160), an elevated dsDNA antibody level (800 IU/mL; reference range, 0-99 IU/mL), low complement levels, and antibody titers consistent with the patient’s known APS. Based on these findings, the patient was given a diagnosis of systemic lupus erythematosus (SLE).
DISCUSSION
Lymphadenopathy, revealed by exam or by imaging, in combination with systemic symptoms such as weight loss and fatigue, elicits an extensive differential diagnosis. In the absence of recent exposures, travel, or risk factors for infectious causes, our patient’s work-up was appropriately narrowed to noninfectious etiologies of pancytopenia and lymphadenopathy. At the top of this differential are malignancies—in particular, multiple myeloma and lymphoma—and rheumatologic processes, such as sarcoidosis, connective tissue disease, and SLE.1,2 Ultimately, the combination of autoimmune markers with the pancytopenia and a negative work-up for malignancy confirmed a diagnosis of SLE.
Continue to: SLE classification and generalized lymphadenopathy
SLE classification and generalized lymphadenopathy. SLE is a multisystem inflammatory process with a wide spectrum of clinical presentations. The American College of Rheumatology (ACR) has established validated criteria to aid in the diagnosis of SLE,3 which were most recently updated in 2012 to improve clinical utility. For a diagnosis to be made, at least 1 clinical and 1 immunologic criterion must be present or a renal biopsy must show lupus nephritis.3
Notably, lymphadenopathy is not included in this validated model, despite its occurrence in 25% to 50% of patients with SLE.1,3,4 With this in mind, SLE should be considered in the work-up of generalized lymphadenopathy.
ANA and SLE. Although it is estimated that 30% to 40% of patients with SLE test positive for ANA,5 the presence of ANA also is not part of the diagnostic criteria for SLE. Interestingly, the co-occurrence of the 2 has clinical implications for patients. In particular, patients with SLE and a positive ANA have higher prevalence of thrombosis, valvular disease, thrombocytopenia, and hemolytic anemia, among other complications.5 Although our patient’s presentation of thrombocytopenia and hemolysis clouded the initial work-up, such a combination is consistent with co-presentation of SLE and APS.
Differences in sex, age, and race. SLE is more common in women than in men, with a prevalence ratio of 7:1.6 It is estimated that 65% of patients with SLE experience disease onset between the ages of 16 and 55 years.7
The median age of diagnosis also differs based on sex and race: According to Rus et al,8 the typical age ranges are 37 to 50 years for White women; 50 to 59 for White men; 15 to 44 for Black women; and 45 to 64 for Black men. These estimates of incidence stratified by race, sex, and age can be helpful when evaluating patients with confusing clinical presentations. Our patient’s age was consistent with the median for his sex and race.
Continue to: Our patient
Our patient was started on oral prednisone 60 mg/d with plans for a prolonged taper over 6 months under the close supervision of Rheumatology. His weakness and polyuria began to improve within a month, and lupus-related symptoms resolved within 3 months. His cytopenia also significantly improved, with the exception of refractory thrombocytopenia.
THE TAKEAWAY
SLE is a common diagnosis with multiple presentations. Although lymphadenopathy is not part of the clinical criteria for the diagnosis of SLE, multiple case studies have highlighted its prevalence among affected patients.1,2,4,9-17 APS and antiphospholipid antibodies are also absent in the diagnostic criteria despite being highly associated with SLE. Thus, co-presentation (as well as age and sex) can be helpful with both disease stratification and risk assessment once a diagnosis is made.
CORRESPONDENCE
Isabella Buzzo Bellon Brout, MD, 409 West Broadway, Boston, MA 02127; [email protected]
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
1. Afzal W, Arab T, Ullah T, et al. Generalized lymphadenopathy as presenting features of systemic lupus erythematosus: case report and review of literature. J Clin Med Res. 2016;8:819-823. doi: 10.14740/jocmr2717w
2. Smith LW, Petri M. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 2013;19:397-399. doi: 10.1097/RHU.0b013e3182a6a924
3. Petri M, Orbai A, Graciela S, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi: 10.1002/art.34473
4. Kitsanou M, Adreopoulou E, Bai MK, et al. Extensive lymphadenopathy as the first clinical manifestation in systemic lupus erythematosus. Lupus. 2000;9:140-143. doi: 10.1191/096120300678828037
5. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. 2016;3:75-84. doi: 10.5152/eurjrheum.2015.0085
6. Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352-356. doi: 10.1097/00002281-199909000-00005
7. Rothfield N. Clinical features of systemic lupus erythematosus. In: Kelley WN, Harris ED, Ruddy S, Sledge CB (eds). Textbook of Rheumatology. WB Saunders; 1981.
8. Rus V, Maury EE, Hochberg MC. The epidemiology of systemic lupus erythematosus. In: Wallace DJ, Hahn BH (eds). Dubois’ Lupus Erythematosus. Lippincott Williams and Wilkins; 2002.
9. Biner B, Acunas B, Karasalihoglu S, et al. Systemic lupus erythematosus presenting with generalized lymphadenopathy: a case report. Turk J Pediatr. 2001;43:94-96.
10. Gilmore R, Sin WY. Systemic lupus erythematosus mimicking lymphoma: the relevance of the clinical background in interpreting imaging studies. BMJ Case Rep. 2014;2014:bcr2013201802. doi: 10.1136/bcr-2013-201802
11. Shrestha D, Dhakal AK, Shiva RK, et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 2013;13:179. doi: 10.1186/1471-2431-13-179
12. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Rheumatol Port. 2008;33:402-406.
13. Tamaki K, Morishima S, Nakachi S, et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 2017;105:526-531. doi: 10.1007/s12185-016-2126-8
14. Hyami T, Kato T, Moritani S, et al. Systemic lupus erythematosus with abdominal lymphadenopathy. Eur J Dermatol. 2019;29:342-344. doi: 10.1684/ejd.2019.3589
15. Mull ES, Aranez V, Pierce D, et al. Newly diagnosed systemic lupus erythematosus: atypical presentation with focal seizures and long-standing lymphadenopathy. J Clin Rheumatol. 2019;25:e109-e113. doi: 10.1097/RHU.0000000000000681
16. Kassan SS, Moss ML, Reddick RL. Progressive hilar and mediastinal lymphadenopathy in systemic lupus erythematosus on corticosteroid therapy. N Engl J Med. 1976;294:1382-1383. doi: 10.1056/NEJM197606172942506
17. Tuinman PR, Nieuwenhuis MB, Groen E, et al. A young woman with generalized lymphadenopathy. Systemic lupus erythematosus. Neth J Med. 2011;69:284-288.
Atypical knee pain
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.
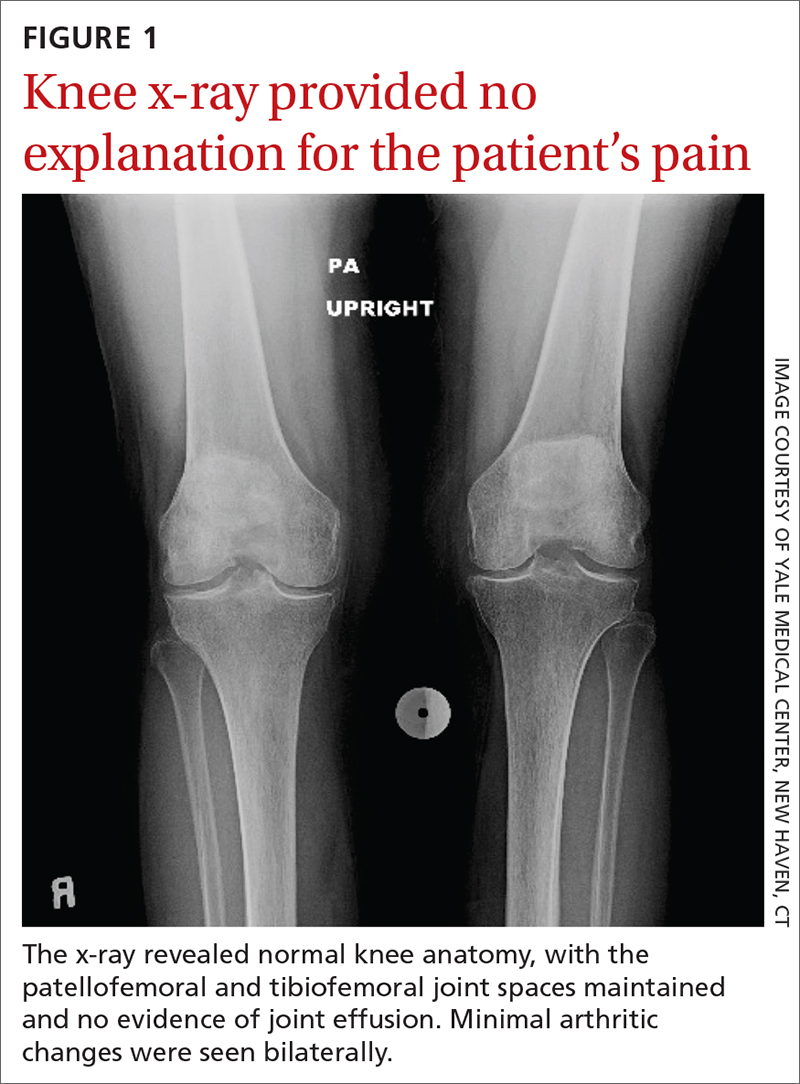
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.
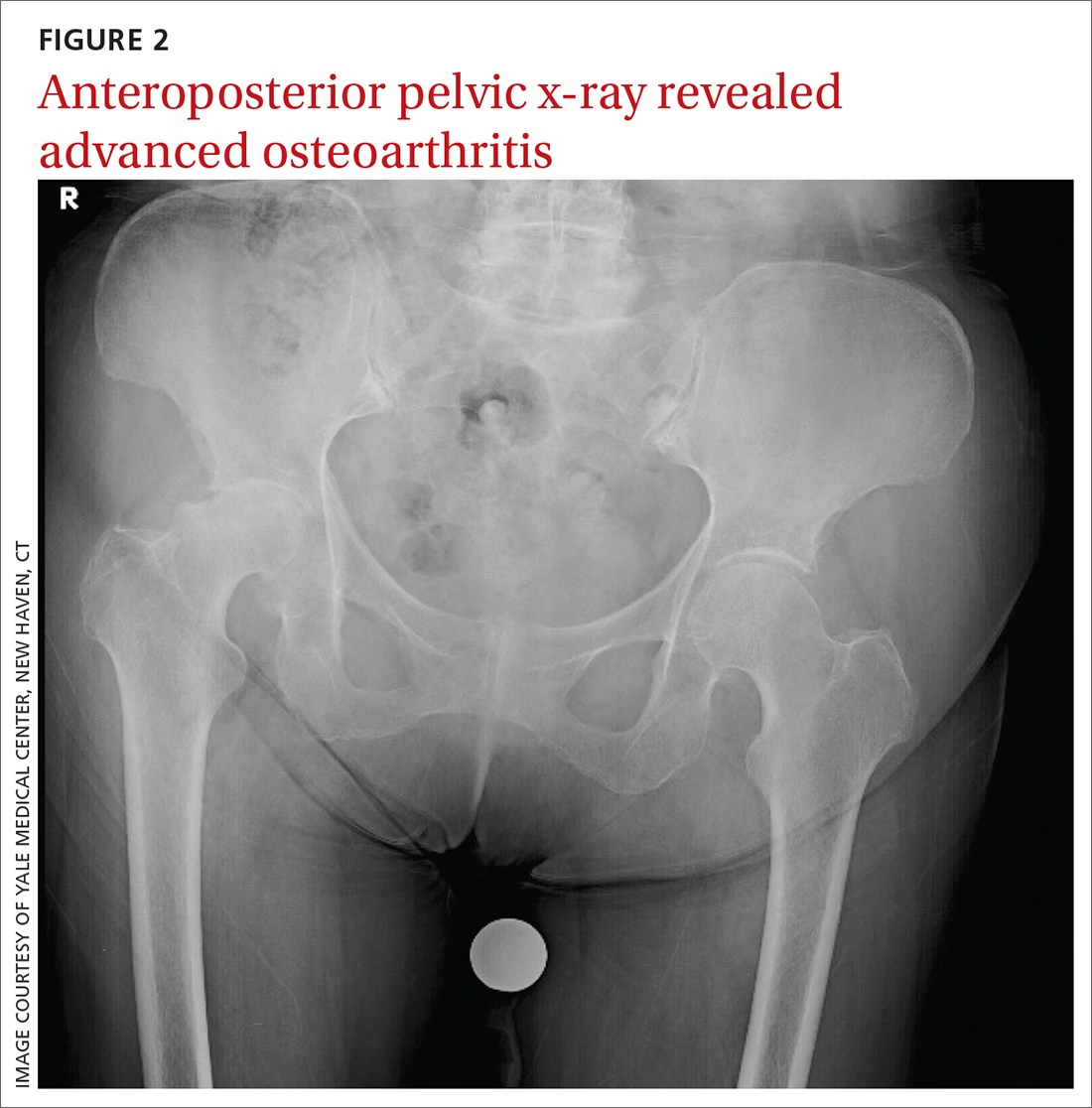
Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.
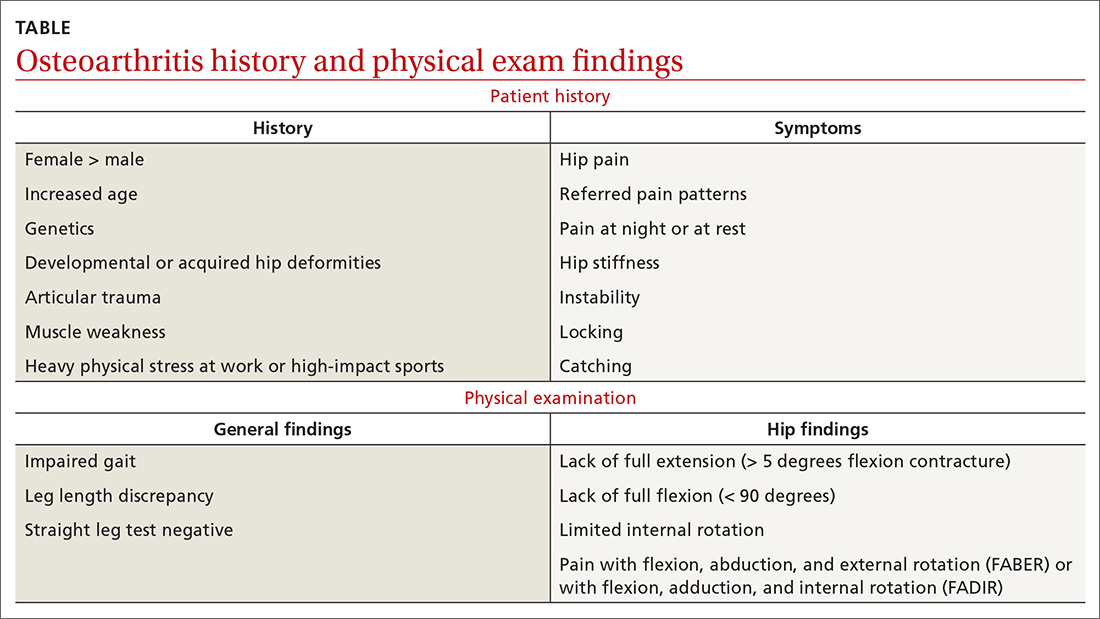
The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.
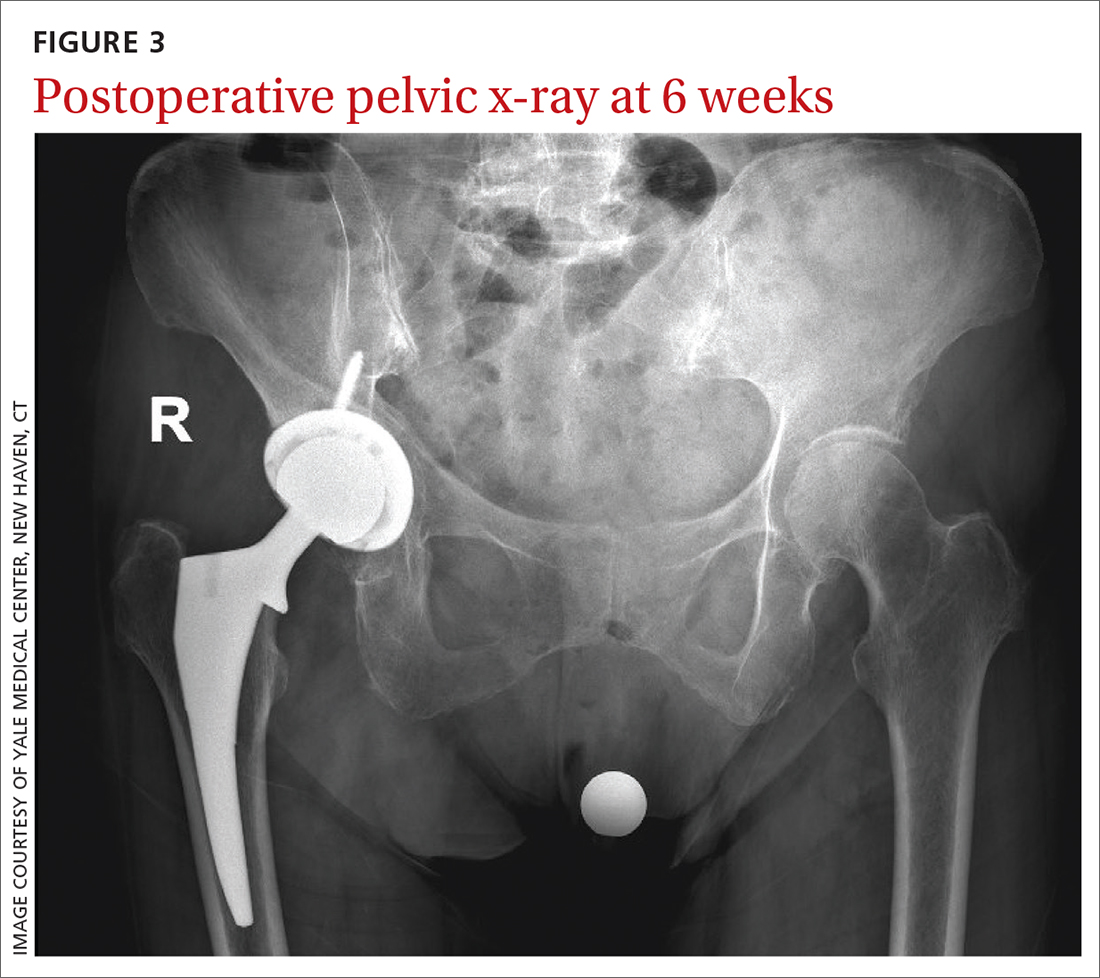
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.

Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.

The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.

An 83-year-old woman, with an otherwise noncontributory past medical history, presented with chronic right knee pain. Over the prior 4 years, she had undergone evaluation by an outside physician and received several corticosteroid and hyaluronic acid intra-articular injections, without symptom resolution. She described the pain as a 4/10 at rest and as “severe” when climbing stairs and exercising. The pain was localized to her lower back and right groin and extended to her right knee. She also said that she found it difficult to put on her socks. An outside orthopedic surgeon recommended right total knee arthroplasty, prompting her to seek a second opinion.
Examination of her right knee was unrevealing. However, during the hip examination, there was a pronounced loss of range of motion and concordant pain reproduction with the FABER (combined flexion, abduction, external rotation) and FADIR (combined flexion, adduction, and internal rotation) maneuvers.
The patient’s extensive clinical and diagnostic history, combined with benign knee examination and imaging (FIGURE 1), ruled out isolated knee pathology.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Right hip OA with referred knee pain
The patient’s history and physical exam prompted us to suspect right hip osteoarthritis (OA) with referred pain to the right knee. This suspicion was confirmed with hip radiographs (FIGURE 2), which revealed significant OA of the right hip, as evidenced by marked joint space narrowing, subchondral sclerosis, and osteophytes. There was also superior migration of the right femoral head relative to the acetabulum. Additionally, there was loss of sphericity of the right femoral head, suggesting avascular necrosis with collapse.

Hip and knee OA are among the most common causes of disability worldwide. Knee and hip pain are estimated to affect up to 27% and 15% of the general population, respectively.1,2 Referred knee pain secondary to hip pathology, also known as atypical knee pain, has been cited at highly variable rates, ranging from 2% to 27%.3
Eighty-six percent of patients with atypical knee pain experience a delay in diagnosis of more than 1 year.4 Half of these patients require the use of a wheelchair or walker for community navigation.4 These findings highlight the impact that a delay in diagnosis can have on the day-to-day quality of life for these patients. Also, delayed or missed diagnoses may have contributed to the doubling in the rate of knee replacement surgery from 2000 to 2010 and the reports that up to one-third of knee replacement surgeries did not meet appropriate criteria to be performed.5,6
Convergence confusion
Referred pain is likely explained by the convergence of nociceptive and non-nociceptive nerve fibers.7 Both of these fiber types conduct action potentials that terminate at second order neurons. Occasionally, nociceptive nerve fibers from different parts of the body (ie, knee and hip) terminate at the same second order fiber. At this point of convergence, higher brain centers lose their ability to discriminate the anatomic location of origin. This results in the perception of pain in a different location, where there is no intrinsic pathology.
Patients with hip OA report that the most common locations of pain are the groin, anterior thigh, buttock, anterior knee, and greater trochanter.3 One small study revealed that 85% of patients with referred pain who underwent total hip arthroplasty (THA) reported complete resolution of pain symptoms within 4 days of the procedure.3
Continue to: A comprehensive exam can reveal a different origin of pain
A comprehensive exam can reveal a different origin of pain
As with any musculoskeletal complaint, history and physical examination should include a focus on the joints proximal and distal to the purported joint of concern. When the hip is in consideration, historical inquiry should focus on degree and timeline of pain, stiffness, and traumatic history. Our patient reported difficulty donning socks, an excellent screening question to evaluate loss of range of motion in the hip. On physical examination, the FABER and FADIR maneuvers are quite specific to hip OA. A comprehensive list of history and physical examination findings can be found in the TABLE.

The differential includes a broad range of musculoskeletal diagnoses
The differential diagnosis for knee pain includes knee OA, spinopelvic pathology, infection, and rheumatologic disease.
Knee OA can be confirmed with knee radiographs, but one must also assess the joint above and below, as with all musculoskeletal complaints.
Spinopelvic pathology may be established with radiographs and a thorough nervous system exam.
Infection, such as septic arthritis or gout, can be diagnosed through radiographs, physical exam, and lab tests to evaluate white blood cell count, erythrocyte sedimentation rate, and C-reactive protein levels. High clinical suspicion may warrant a joint aspiration.
Continue to: Rheumatologic disease
Rheumatologic disease can be evaluated with a comprehensive physical exam, as well as lab work.
Management includes both surgical and nonsurgical options
Hip OA can be managed much like OA in other areas of the body. The Osteoarthritis Research Society International guidelines provide direction and insight concerning outpatient nonsurgical management.8 Weight loss and land-based, low-impact exercise programs are excellent first-line options. Second-line therapies include symptomatic management with systemic nonsteroidal anti-inflammatory drugs (NSAIDs) in patients without contraindications. (Topical NSAIDs, while useful in the treatment of knee OA, are not as effective for hip OA due to thickness of soft tissue in this area of the body.)
Patients who do not achieve symptomatic relief with these first- and second-line therapies may benefit from other nonoperative measures, such as intra-articular corticosteroid injections. If pain persists, patients may need a referral to an orthopedic surgeon to discuss surgical candidacy.
Following the x-ray, our patient received a fluoroscopic guided intra-articular hip joint anesthetic and corticosteroid injection. Her pain level went from a reported6/10 prior to the procedure to complete pain relief after it.
However, at her follow-up visit 4 weeks later, the patient reported return of functionally limiting pain. The orthopedic surgeon talked to the patient about the potential risks and benefits of THA. She elected to proceed with a right THA.
Six weeks after the surgery, the patient presented for follow-up with minimal hip pain and complete resolution of her knee pain (FIGURE 3). Functionally, she found it much easier to stand straight, and she was able to climb the stairs in her house independently.

1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011
1. Fernandes GS, Parekh SM, Moses J, et al. Prevalence of knee pain, radiographic osteoarthritis and arthroplasty in retired professional footballers compared with men in the general population: a cross-sectional study. Br J Sports Med. 2018;52:678-683. doi: 10.1136/bjsports-2017-097503
2. Christmas C, Crespo CJ, Franckowiak SC, et al. How common is hip pain among older adults? Results from the Third National Health and Nutrition Examination Survey. J Fam Pract. 2002;51:345-348.
3. Hsieh PH, Chang Y, Chen DW, et al. Pain distribution and response to total hip arthroplasty: a prospective observational study in 113 patients with end-stage hip disease. J Orthop Sci. 2012;17:213-218. doi: 10.1007/s00776-012-0204-1
4. Dibra FF, Prietao HA, Gray CF, et al. Don’t forget the hip! Hip arthritis masquerading as knee pain. Arthroplast Today. 2017;4:118-124. doi: 10.1016/j.artd.2017.06.008
5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323-1330. doi: 10.1136/annrheumdis-2013-204763
6. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. doi: 10.2106/JBJS.N.01141
7. Sessle BJ. Central mechanisms of craniofacial musculoskeletal pain: a review. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, eds. Fundamentals of musculoskeletal pain. 1st ed. IASP Press; 2008:87-103.
8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578-1589. doi: 10.1016/j.joca.2019.06.011