User login
Review of Ethnoracial Representation in Clinical Trials (Phases 1 Through 4) of Atopic Dermatitis Therapies
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).
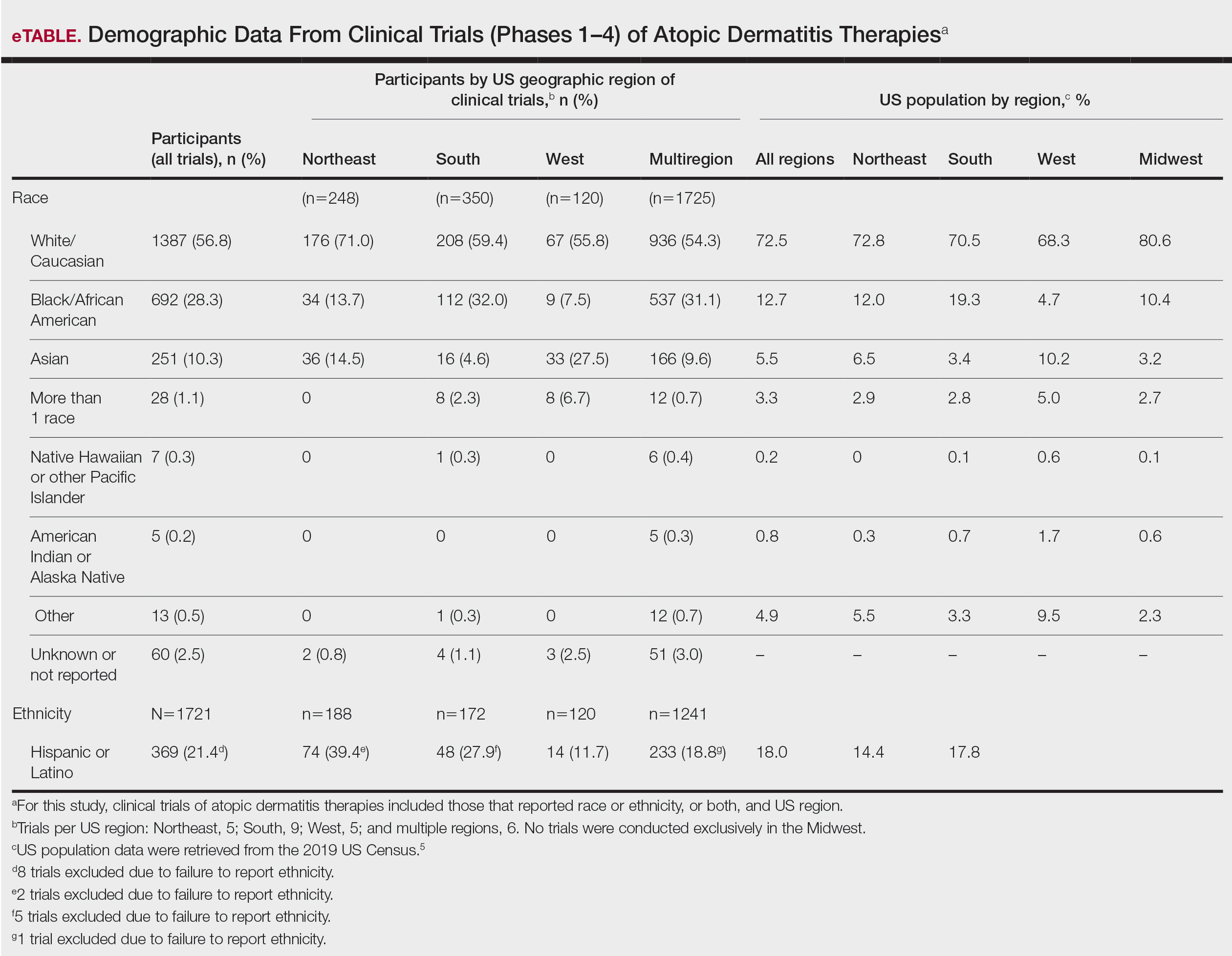
Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).

Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).

Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
Practice Points
- Although minority groups are disproportionally affected by atopic dermatitis (AD), they may be underrepresented in clinical trials for AD in the United States.
- Equal representation among ethnoracial groups in clinical trials is important to allow for a more thorough investigation of the efficacy of treatments for AD.
When Are Inpatient and Emergency Dermatologic Consultations Appropriate?
There are limited clinical data concerning inpatient and emergency department (ED) dermatologic consultations. The indications for these consultations vary widely, but in one study (N=271), it was found that 21% of inpatient consultations were for contact dermatitis and 10% were for drug eruptions.1 In the same study, 77% of patients who required a dermatology consultation eventually were given a different diagnosis or change in treatment after consultation. For example, of all consultations for suspected cellulitis, only 10% were confirmed after dermatology evaluation.1
Hospitalists and emergency physicians continue to struggle with the assessment of dermatologic conditions, often consulting dermatology whenever a patient has a “rash” or skin concern. Dermatology is still not emphasized in medical education and often is taught to most medical students in an abbreviated fashion, which results in physicians feeling ill-equipped to deal with any dermatologic condition—either mundane or potentially life-threatening. A study in 2016 showed that a monthly lecture series given to hospitalists over the course of 5 years did not improve diagnostic accuracy in patients who were admitted with skin manifestations.2 This further shows that there is a need for dermatologic experts in the hospital.
We need to develop better guidelines for physicians in the ED and on inpatient units to guide them on appropriate use of dermatologic consultation outside the ambulatory office and the clinic. A 2013 study showed that patients often were discharged immediately after a dermatologic consultation, furthering our hypothesis that many inpatient consultations can be delayed until after discharge.3
In an era in which medical costs are soaring and there is constant surveillance for ways to reduce costs without impairing quality of care, limiting unnecessary specialty consultations should be embraced. In 2009, $1.8 billion in Medicare claims was paid for dermatology-related admissions.3 A substantial savings to Medicare consulting fees for certain diagnoses, such as cellulitis or contact dermatitis, could be realized if patients were referred for outpatient assessment and treatment. In a study of 271 consultations, 54 patients also had a skin biopsy, which further increases dollars spent on inpatient care and is (usually) something that can be performed in the outpatient setting.1 In another study, the more common recommended treatments were topical corticosteroids and supportive educational measures for patients and hospital staff,3 which further substantiates that most dermatology consultations are not truly emergent and can wait for outpatient consultation.
In addition, we are dealing with the COVID-19 pandemic in our hospitals and EDs. Many physicians, including dermatologists, would prefer to avoid unnecessary exposure to SARS-CoV-2 on inpatient units and in the ED. It certainly would be preferable to require consultants to come in to evaluate patients only when they truly need to be seen while in the hospital.
There also is limited dermatology training in other specialties, and the dermatology team can help fill this gap with educational programs and one-on-one teaching. Hospital teams have signaled this need, but there has been limited success with multiple teaching opportunities.4
We believe that this need for inpatient dermatology services can be filled with the newer subspecialty of hospital dermatology, which is not commonly present at most hospitals; a reason why the subspecialty has not been more popular is that there are few available data in the form of randomized clinical trials that can guide inpatient dermatologists with the care of rare hospital skin diseases.5 Having a dermatologic hospitalist available might allow for patients to be seen more readily, which ultimately will save lives and health care dollars and would increase real-time teaching and education for house staff, nursing, and attendings at the bedside.
In a 2018 article,6 it was postulated that quicker diagnosis of pseudocellulitis and initiation of antibiotics to treat this condition would save the US health care system $210 million annually. We believe that pseudocellulitis would be best evaluated by inpatient dermatology teams, thereby avoiding costly dermatologic consultations, at an average rate of $138.89.6
Morbilliform drug eruptions are among the most common skin conditions seen in the hospital; approximately 95% of cases are an uncomplicated reaction to a medication or virus. Data show that many of these consultations might be unnecessary.7
Our institution (Hackensack University Medical Center, New Jersey) is a tertiary hospital that also is connected with a major cancer center. Given this connection, skin eruptions due to chemotherapy and radiation are common. The treatment of drug eruptions, graft-vs-host disease, and other oncologic or drug-related eruptions should be within the scope of practice of our hospitalists, but these cases frequently involve dermatologic consultation.
We constructed a consultation flowchart (Figure) to help guide the triage of patients in need of dermatologic evaluation by inpatient teams and possibly to avoid unnecessary consultation fees. The manner in which this—or any flowchart or teaching aid—is conveyed to hospital personnel is critical so that these tools are not perceived as patronizing or confrontational. In our flowchart, we list emergent dermatologic conditions that we believe are appropriate for dermatology consultation including erythrodermic psoriasis, bullous pemphigoid flare, and Stevens-Johnson syndrome/toxic epidermal necrolysis.
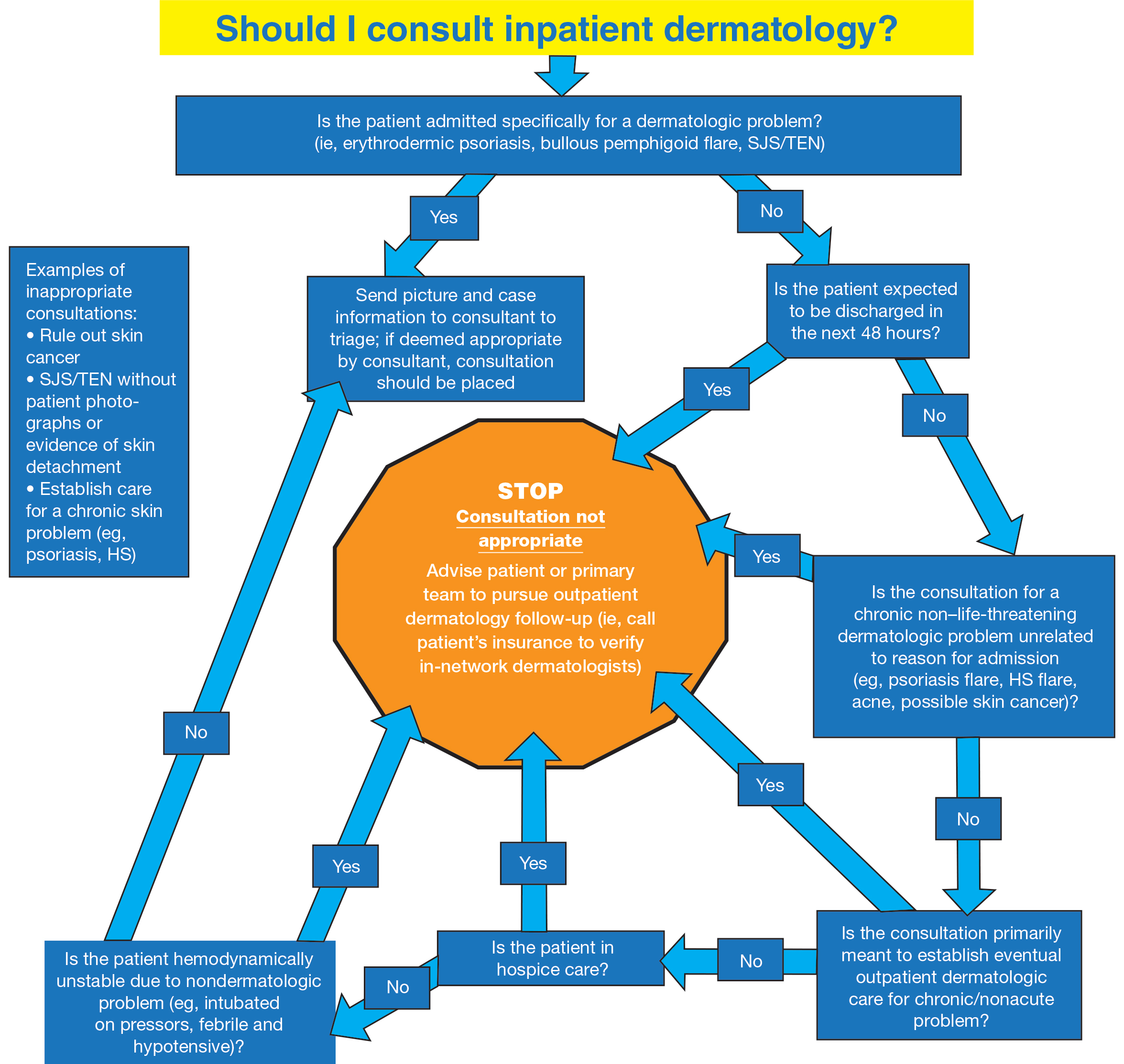
We believe that the flowchart can educate inpatient medical teams about appropriate dermatology consultation. Use of the flowchart also may decrease unnecessary consultations, which ultimately will lower health care spending overall.
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Beshay A, Liu M, Fox L, et al. Inpatient dermatology consultative programs: a continued need, tools for needs assessment for curriculum development, and a call for new methods of teaching. J Am Acad Dermatol. 2016;74:769-771. doi:10.1016/j.jaad.2015.11.017
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Faletsky A, Han JJ, Mostaghimi A. Inpatient dermatology best practice strategies for educating and relaying findings to colleagues. Curr Dermatol Rep. 2020;9:256-260. doi:10.1007/s13671-020-00317-y
- Fox LP. Hospital dermatology, introduction. Semin Cutan Med Surg. 2017;36:1-2. doi:10.12788/j.sder.2017.015
- Li D, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364. doi:10.1016/j.mcna.2015.06.004
There are limited clinical data concerning inpatient and emergency department (ED) dermatologic consultations. The indications for these consultations vary widely, but in one study (N=271), it was found that 21% of inpatient consultations were for contact dermatitis and 10% were for drug eruptions.1 In the same study, 77% of patients who required a dermatology consultation eventually were given a different diagnosis or change in treatment after consultation. For example, of all consultations for suspected cellulitis, only 10% were confirmed after dermatology evaluation.1
Hospitalists and emergency physicians continue to struggle with the assessment of dermatologic conditions, often consulting dermatology whenever a patient has a “rash” or skin concern. Dermatology is still not emphasized in medical education and often is taught to most medical students in an abbreviated fashion, which results in physicians feeling ill-equipped to deal with any dermatologic condition—either mundane or potentially life-threatening. A study in 2016 showed that a monthly lecture series given to hospitalists over the course of 5 years did not improve diagnostic accuracy in patients who were admitted with skin manifestations.2 This further shows that there is a need for dermatologic experts in the hospital.
We need to develop better guidelines for physicians in the ED and on inpatient units to guide them on appropriate use of dermatologic consultation outside the ambulatory office and the clinic. A 2013 study showed that patients often were discharged immediately after a dermatologic consultation, furthering our hypothesis that many inpatient consultations can be delayed until after discharge.3
In an era in which medical costs are soaring and there is constant surveillance for ways to reduce costs without impairing quality of care, limiting unnecessary specialty consultations should be embraced. In 2009, $1.8 billion in Medicare claims was paid for dermatology-related admissions.3 A substantial savings to Medicare consulting fees for certain diagnoses, such as cellulitis or contact dermatitis, could be realized if patients were referred for outpatient assessment and treatment. In a study of 271 consultations, 54 patients also had a skin biopsy, which further increases dollars spent on inpatient care and is (usually) something that can be performed in the outpatient setting.1 In another study, the more common recommended treatments were topical corticosteroids and supportive educational measures for patients and hospital staff,3 which further substantiates that most dermatology consultations are not truly emergent and can wait for outpatient consultation.
In addition, we are dealing with the COVID-19 pandemic in our hospitals and EDs. Many physicians, including dermatologists, would prefer to avoid unnecessary exposure to SARS-CoV-2 on inpatient units and in the ED. It certainly would be preferable to require consultants to come in to evaluate patients only when they truly need to be seen while in the hospital.
There also is limited dermatology training in other specialties, and the dermatology team can help fill this gap with educational programs and one-on-one teaching. Hospital teams have signaled this need, but there has been limited success with multiple teaching opportunities.4
We believe that this need for inpatient dermatology services can be filled with the newer subspecialty of hospital dermatology, which is not commonly present at most hospitals; a reason why the subspecialty has not been more popular is that there are few available data in the form of randomized clinical trials that can guide inpatient dermatologists with the care of rare hospital skin diseases.5 Having a dermatologic hospitalist available might allow for patients to be seen more readily, which ultimately will save lives and health care dollars and would increase real-time teaching and education for house staff, nursing, and attendings at the bedside.
In a 2018 article,6 it was postulated that quicker diagnosis of pseudocellulitis and initiation of antibiotics to treat this condition would save the US health care system $210 million annually. We believe that pseudocellulitis would be best evaluated by inpatient dermatology teams, thereby avoiding costly dermatologic consultations, at an average rate of $138.89.6
Morbilliform drug eruptions are among the most common skin conditions seen in the hospital; approximately 95% of cases are an uncomplicated reaction to a medication or virus. Data show that many of these consultations might be unnecessary.7
Our institution (Hackensack University Medical Center, New Jersey) is a tertiary hospital that also is connected with a major cancer center. Given this connection, skin eruptions due to chemotherapy and radiation are common. The treatment of drug eruptions, graft-vs-host disease, and other oncologic or drug-related eruptions should be within the scope of practice of our hospitalists, but these cases frequently involve dermatologic consultation.
We constructed a consultation flowchart (Figure) to help guide the triage of patients in need of dermatologic evaluation by inpatient teams and possibly to avoid unnecessary consultation fees. The manner in which this—or any flowchart or teaching aid—is conveyed to hospital personnel is critical so that these tools are not perceived as patronizing or confrontational. In our flowchart, we list emergent dermatologic conditions that we believe are appropriate for dermatology consultation including erythrodermic psoriasis, bullous pemphigoid flare, and Stevens-Johnson syndrome/toxic epidermal necrolysis.

We believe that the flowchart can educate inpatient medical teams about appropriate dermatology consultation. Use of the flowchart also may decrease unnecessary consultations, which ultimately will lower health care spending overall.
There are limited clinical data concerning inpatient and emergency department (ED) dermatologic consultations. The indications for these consultations vary widely, but in one study (N=271), it was found that 21% of inpatient consultations were for contact dermatitis and 10% were for drug eruptions.1 In the same study, 77% of patients who required a dermatology consultation eventually were given a different diagnosis or change in treatment after consultation. For example, of all consultations for suspected cellulitis, only 10% were confirmed after dermatology evaluation.1
Hospitalists and emergency physicians continue to struggle with the assessment of dermatologic conditions, often consulting dermatology whenever a patient has a “rash” or skin concern. Dermatology is still not emphasized in medical education and often is taught to most medical students in an abbreviated fashion, which results in physicians feeling ill-equipped to deal with any dermatologic condition—either mundane or potentially life-threatening. A study in 2016 showed that a monthly lecture series given to hospitalists over the course of 5 years did not improve diagnostic accuracy in patients who were admitted with skin manifestations.2 This further shows that there is a need for dermatologic experts in the hospital.
We need to develop better guidelines for physicians in the ED and on inpatient units to guide them on appropriate use of dermatologic consultation outside the ambulatory office and the clinic. A 2013 study showed that patients often were discharged immediately after a dermatologic consultation, furthering our hypothesis that many inpatient consultations can be delayed until after discharge.3
In an era in which medical costs are soaring and there is constant surveillance for ways to reduce costs without impairing quality of care, limiting unnecessary specialty consultations should be embraced. In 2009, $1.8 billion in Medicare claims was paid for dermatology-related admissions.3 A substantial savings to Medicare consulting fees for certain diagnoses, such as cellulitis or contact dermatitis, could be realized if patients were referred for outpatient assessment and treatment. In a study of 271 consultations, 54 patients also had a skin biopsy, which further increases dollars spent on inpatient care and is (usually) something that can be performed in the outpatient setting.1 In another study, the more common recommended treatments were topical corticosteroids and supportive educational measures for patients and hospital staff,3 which further substantiates that most dermatology consultations are not truly emergent and can wait for outpatient consultation.
In addition, we are dealing with the COVID-19 pandemic in our hospitals and EDs. Many physicians, including dermatologists, would prefer to avoid unnecessary exposure to SARS-CoV-2 on inpatient units and in the ED. It certainly would be preferable to require consultants to come in to evaluate patients only when they truly need to be seen while in the hospital.
There also is limited dermatology training in other specialties, and the dermatology team can help fill this gap with educational programs and one-on-one teaching. Hospital teams have signaled this need, but there has been limited success with multiple teaching opportunities.4
We believe that this need for inpatient dermatology services can be filled with the newer subspecialty of hospital dermatology, which is not commonly present at most hospitals; a reason why the subspecialty has not been more popular is that there are few available data in the form of randomized clinical trials that can guide inpatient dermatologists with the care of rare hospital skin diseases.5 Having a dermatologic hospitalist available might allow for patients to be seen more readily, which ultimately will save lives and health care dollars and would increase real-time teaching and education for house staff, nursing, and attendings at the bedside.
In a 2018 article,6 it was postulated that quicker diagnosis of pseudocellulitis and initiation of antibiotics to treat this condition would save the US health care system $210 million annually. We believe that pseudocellulitis would be best evaluated by inpatient dermatology teams, thereby avoiding costly dermatologic consultations, at an average rate of $138.89.6
Morbilliform drug eruptions are among the most common skin conditions seen in the hospital; approximately 95% of cases are an uncomplicated reaction to a medication or virus. Data show that many of these consultations might be unnecessary.7
Our institution (Hackensack University Medical Center, New Jersey) is a tertiary hospital that also is connected with a major cancer center. Given this connection, skin eruptions due to chemotherapy and radiation are common. The treatment of drug eruptions, graft-vs-host disease, and other oncologic or drug-related eruptions should be within the scope of practice of our hospitalists, but these cases frequently involve dermatologic consultation.
We constructed a consultation flowchart (Figure) to help guide the triage of patients in need of dermatologic evaluation by inpatient teams and possibly to avoid unnecessary consultation fees. The manner in which this—or any flowchart or teaching aid—is conveyed to hospital personnel is critical so that these tools are not perceived as patronizing or confrontational. In our flowchart, we list emergent dermatologic conditions that we believe are appropriate for dermatology consultation including erythrodermic psoriasis, bullous pemphigoid flare, and Stevens-Johnson syndrome/toxic epidermal necrolysis.

We believe that the flowchart can educate inpatient medical teams about appropriate dermatology consultation. Use of the flowchart also may decrease unnecessary consultations, which ultimately will lower health care spending overall.
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Beshay A, Liu M, Fox L, et al. Inpatient dermatology consultative programs: a continued need, tools for needs assessment for curriculum development, and a call for new methods of teaching. J Am Acad Dermatol. 2016;74:769-771. doi:10.1016/j.jaad.2015.11.017
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Faletsky A, Han JJ, Mostaghimi A. Inpatient dermatology best practice strategies for educating and relaying findings to colleagues. Curr Dermatol Rep. 2020;9:256-260. doi:10.1007/s13671-020-00317-y
- Fox LP. Hospital dermatology, introduction. Semin Cutan Med Surg. 2017;36:1-2. doi:10.12788/j.sder.2017.015
- Li D, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364. doi:10.1016/j.mcna.2015.06.004
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Beshay A, Liu M, Fox L, et al. Inpatient dermatology consultative programs: a continued need, tools for needs assessment for curriculum development, and a call for new methods of teaching. J Am Acad Dermatol. 2016;74:769-771. doi:10.1016/j.jaad.2015.11.017
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Faletsky A, Han JJ, Mostaghimi A. Inpatient dermatology best practice strategies for educating and relaying findings to colleagues. Curr Dermatol Rep. 2020;9:256-260. doi:10.1007/s13671-020-00317-y
- Fox LP. Hospital dermatology, introduction. Semin Cutan Med Surg. 2017;36:1-2. doi:10.12788/j.sder.2017.015
- Li D, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364. doi:10.1016/j.mcna.2015.06.004
Practice Points
- Primary inpatient teams should call patients’ insurance companies to verify in-network dermatologists for eventual outpatient follow-up.
- Chronic skin problems (eg, psoriasis, hidradenitis suppurativa) are better cared for in an outpatient setting due to the necessity for follow-up reassessments.
- There remains a need to fill knowledge gaps for common inpatient dermatologic problems that do not necessitate consultations, such as morbilliform drug rash and other chronic and unchanged dermatoses.
Tinted Sunscreens: Consumer Preferences Based on Light, Medium, and Dark Skin Tones
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.

Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
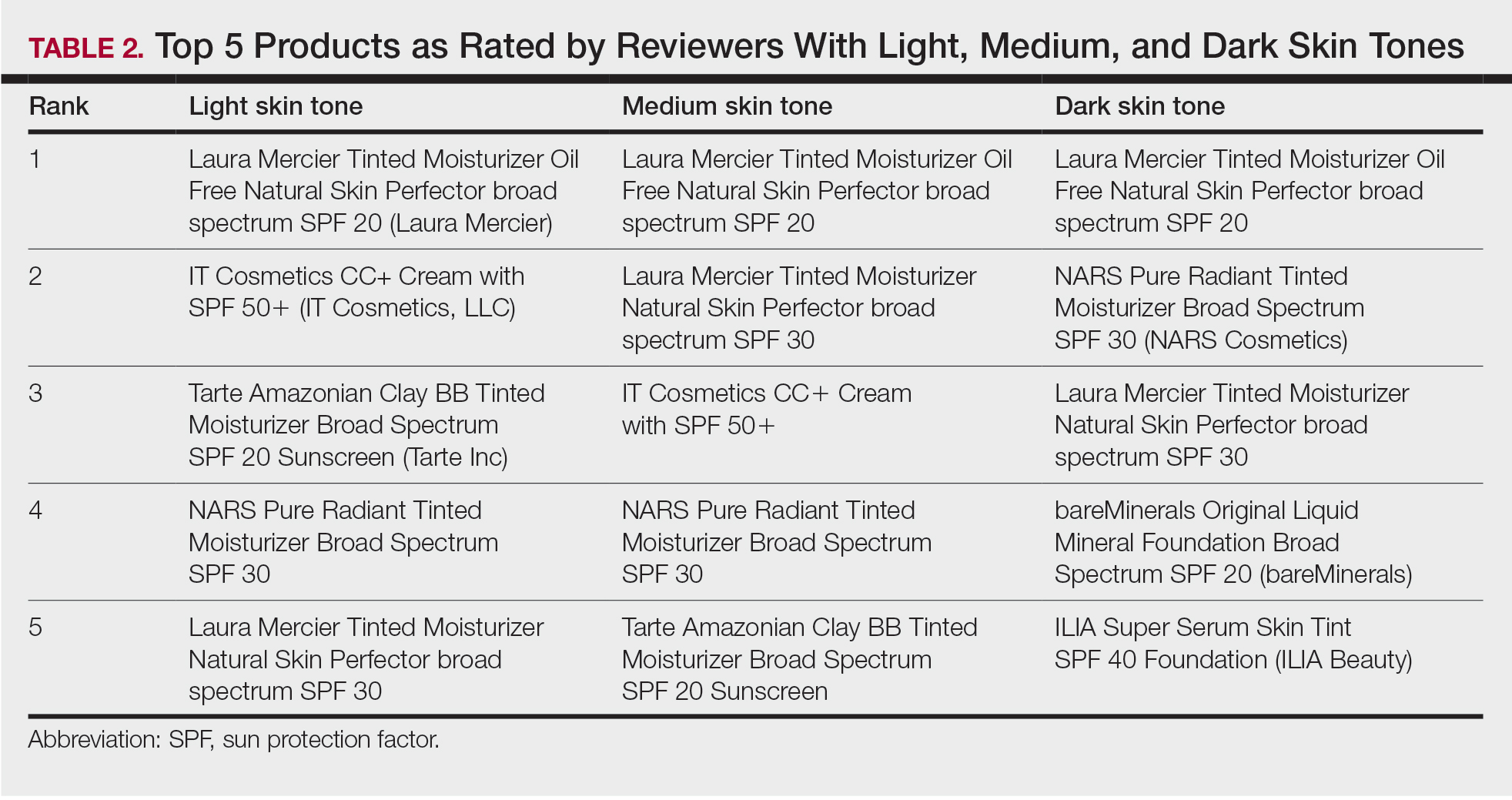
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.
Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.

Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.

Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.

Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.

Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Practice Points
- Visible light has been shown to increase tyrosinase activity and induce immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.
- The formulation of sunscreens with iron oxides and pigmentary titanium dioxide are a safe and effective way to protect against high-energy visible light, especially when combined with zinc oxide.
- Physicians should be aware of sunscreen characteristics that patients like and dislike to tailor recommendations that are appropriate for each individual to enhance adherence.
- Cosmetic elegance and tone compatibility are the most important criteria for individuals seeking tinted sunscreens.
Apremilast has neutral effect on vascular inflammation in psoriasis study
BOSTON – Treatment with , and glucose metabolism, in a study presented at the 2022 American Academy of Dermatology annual meeting.
In the phase 4, open-label, single arm trial, participants also lost subcutaneous and visceral fat after 16 weeks on the oral medication, a phosphodiesterase 4 (PDE4) inhibitor, and maintained that loss at 52 weeks.
People with psoriasis have an increased risk of obesity, type 2 diabetes, and cardiovascular events. Patients with more significant psoriasis “tend to die about 5 years younger than they should, based on their risk factors for mortality,” Joel Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
He led the research and presented the findings at the AAD meeting March 26. “As a result, there has been a keen interest in understanding how psoriasis therapies impact cardiovascular risk, the idea being that by controlling inflammation, you may lower the risk of these patients developing cardiovascular disease over time,” he said.
Previous trials looking at the effect of psoriasis therapies on vascular inflammation “have been, for the most part, inconclusive,” Michael Garshick, MD, a cardiologist at NYU Langone Health, told this news organization. Dr. Garshick was not involved with the research. A 2021 systematic review of psoriasis clinicals trials reported that the tumor necrosis factor (TNF) blocker adalimumab (Humira) and phototherapy had the greatest effect on cardiometabolic markers, while ustekinumab (Stelara), an interleukin (IL)-12 and IL-23 antagonist, was the only treatment that improved vascular inflammation. These variable findings make this area “ripe for study,” noted Dr. Garshick.
To observe how apremilast, which is approved by the FDA for treating psoriasis and psoriatic arthritis, affected vascular inflammation, adiposity, and blood-based cardiometabolic markers, Dr. Gelfand organized an open-label study in adults with moderate-to-severe psoriasis. All participants were 18 years or older, had psoriasis for at least 6 months, and were candidates for systemic therapy. All patients underwent FDG PET/CT scans to assess aortic vascular inflammation and had blood work at baseline. Of the 70 patients originally enrolled in the study, 60 remained in the study at week 16, including 57 who underwent imaging for the second time. Thirty-nine participants remained in the study until week 52, and all except one had another scan.
The average age of participants was 47 years, and their mean BMI was 30. More than 80% of participants were White (83%) and 77% were male. The study population had lived with psoriasis for an average of 16 years and 8 patients also had psoriatic arthritis. At baseline, on average, participants had a Psoriasis Area and Severity Index (PASI) score of 18.62, a dermatology life quality index (DLQI) score of 11.60, and 22% of participants’ BSA (body surface area) were affected. The mean TBRmax, the marker for vascular inflammation, was 1.61.
Treatment responses were as expected for apremilast, with 35% of patients achieving PASI 75 and 65% of participants reporting DLQI scores of 5 or less by 16 weeks. At 52 weeks, 31% of the cohort had achieved PASI 75, and 67% reported DLQI score of 5 or higher. All psoriasis endpoints had improved since baseline (P = .001).
Throughout the study period, there was no significant change in TBRmax. However, in a sensitivity analysis, the 16 patients with a baseline TBRmax of 1.6 or higher had an absolute reduction of 0.21 in TBR by week 52. “That suggests that maybe a subset of people who have higher levels of aortic inflammation at baseline may experience some reduction that portend, potentially, some health benefits over time,” Dr. Gelfand said. “Ultimately, I wouldn’t hang my hat on the finding,” he said, noting that additional research comparing the treatment to placebo is necessary.
Both visceral and subcutaneous adipose tissue (VAT and SAT) decreased by week 16, and this reduction was maintained through week 52. In the first 16 weeks of the study, VAT decreased by 5.32% (P = .0009), and SAT decreased by 5.53% (P = .0005). From baseline to 52 weeks, VAT decreased by 5.52% (P = .0148), and SAT decreased by 5.50% (P = .0096). There were no significant differences between week 16 and week 52 in VAT or SAT.
Of the 68 blood biomarkers analyzed, there were significant decreases in the inflammatory markers ferritin (P = .015) and IL-beta (P = .006), the lipid metabolism biomarker HDL-cholesterol efflux (P = .008), and ketone bodies (P = .006). There were also increases in the inflammatory marker IL-8 (P = .003), the lipid metabolism marker ApoA (P = .05), and insulin (P = .05). Ferritin was the only biomarker that was reduced on both week 16 and week 52.
“If you want to be a purist, this was a negative trial,” said Dr. Garshick, because apremilast was not found to decrease vascular inflammation; however, he noted that the biomarker changes “were hopeful secondary endpoints.” It could be, he said, that another outcome measure may be better able to show changes in vascular inflammation compared with FDG. “It’s always hard to figure out what a good surrogate endpoint is in cardiovascular trials,” he noted, “so it may be that FDG/PET is too noisy or not reliable enough to see the outcome that we want to see.”
Dr. Gelfand reports consulting fees/grants from Amgen, AbbVie, BMS, Boehringer Ingelheim, Janssen Biologics, Novartis Corp, Pfizer, and UCB (DSMB). He serves as the Deputy Editor for the Journal of Investigative Dermatology and the Chief Medical Editor at Healio Psoriatic Disease and receives honoraria for both roles. Dr. Garshick has received consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
BOSTON – Treatment with , and glucose metabolism, in a study presented at the 2022 American Academy of Dermatology annual meeting.
In the phase 4, open-label, single arm trial, participants also lost subcutaneous and visceral fat after 16 weeks on the oral medication, a phosphodiesterase 4 (PDE4) inhibitor, and maintained that loss at 52 weeks.
People with psoriasis have an increased risk of obesity, type 2 diabetes, and cardiovascular events. Patients with more significant psoriasis “tend to die about 5 years younger than they should, based on their risk factors for mortality,” Joel Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
He led the research and presented the findings at the AAD meeting March 26. “As a result, there has been a keen interest in understanding how psoriasis therapies impact cardiovascular risk, the idea being that by controlling inflammation, you may lower the risk of these patients developing cardiovascular disease over time,” he said.
Previous trials looking at the effect of psoriasis therapies on vascular inflammation “have been, for the most part, inconclusive,” Michael Garshick, MD, a cardiologist at NYU Langone Health, told this news organization. Dr. Garshick was not involved with the research. A 2021 systematic review of psoriasis clinicals trials reported that the tumor necrosis factor (TNF) blocker adalimumab (Humira) and phototherapy had the greatest effect on cardiometabolic markers, while ustekinumab (Stelara), an interleukin (IL)-12 and IL-23 antagonist, was the only treatment that improved vascular inflammation. These variable findings make this area “ripe for study,” noted Dr. Garshick.
To observe how apremilast, which is approved by the FDA for treating psoriasis and psoriatic arthritis, affected vascular inflammation, adiposity, and blood-based cardiometabolic markers, Dr. Gelfand organized an open-label study in adults with moderate-to-severe psoriasis. All participants were 18 years or older, had psoriasis for at least 6 months, and were candidates for systemic therapy. All patients underwent FDG PET/CT scans to assess aortic vascular inflammation and had blood work at baseline. Of the 70 patients originally enrolled in the study, 60 remained in the study at week 16, including 57 who underwent imaging for the second time. Thirty-nine participants remained in the study until week 52, and all except one had another scan.
The average age of participants was 47 years, and their mean BMI was 30. More than 80% of participants were White (83%) and 77% were male. The study population had lived with psoriasis for an average of 16 years and 8 patients also had psoriatic arthritis. At baseline, on average, participants had a Psoriasis Area and Severity Index (PASI) score of 18.62, a dermatology life quality index (DLQI) score of 11.60, and 22% of participants’ BSA (body surface area) were affected. The mean TBRmax, the marker for vascular inflammation, was 1.61.
Treatment responses were as expected for apremilast, with 35% of patients achieving PASI 75 and 65% of participants reporting DLQI scores of 5 or less by 16 weeks. At 52 weeks, 31% of the cohort had achieved PASI 75, and 67% reported DLQI score of 5 or higher. All psoriasis endpoints had improved since baseline (P = .001).
Throughout the study period, there was no significant change in TBRmax. However, in a sensitivity analysis, the 16 patients with a baseline TBRmax of 1.6 or higher had an absolute reduction of 0.21 in TBR by week 52. “That suggests that maybe a subset of people who have higher levels of aortic inflammation at baseline may experience some reduction that portend, potentially, some health benefits over time,” Dr. Gelfand said. “Ultimately, I wouldn’t hang my hat on the finding,” he said, noting that additional research comparing the treatment to placebo is necessary.
Both visceral and subcutaneous adipose tissue (VAT and SAT) decreased by week 16, and this reduction was maintained through week 52. In the first 16 weeks of the study, VAT decreased by 5.32% (P = .0009), and SAT decreased by 5.53% (P = .0005). From baseline to 52 weeks, VAT decreased by 5.52% (P = .0148), and SAT decreased by 5.50% (P = .0096). There were no significant differences between week 16 and week 52 in VAT or SAT.
Of the 68 blood biomarkers analyzed, there were significant decreases in the inflammatory markers ferritin (P = .015) and IL-beta (P = .006), the lipid metabolism biomarker HDL-cholesterol efflux (P = .008), and ketone bodies (P = .006). There were also increases in the inflammatory marker IL-8 (P = .003), the lipid metabolism marker ApoA (P = .05), and insulin (P = .05). Ferritin was the only biomarker that was reduced on both week 16 and week 52.
“If you want to be a purist, this was a negative trial,” said Dr. Garshick, because apremilast was not found to decrease vascular inflammation; however, he noted that the biomarker changes “were hopeful secondary endpoints.” It could be, he said, that another outcome measure may be better able to show changes in vascular inflammation compared with FDG. “It’s always hard to figure out what a good surrogate endpoint is in cardiovascular trials,” he noted, “so it may be that FDG/PET is too noisy or not reliable enough to see the outcome that we want to see.”
Dr. Gelfand reports consulting fees/grants from Amgen, AbbVie, BMS, Boehringer Ingelheim, Janssen Biologics, Novartis Corp, Pfizer, and UCB (DSMB). He serves as the Deputy Editor for the Journal of Investigative Dermatology and the Chief Medical Editor at Healio Psoriatic Disease and receives honoraria for both roles. Dr. Garshick has received consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
BOSTON – Treatment with , and glucose metabolism, in a study presented at the 2022 American Academy of Dermatology annual meeting.
In the phase 4, open-label, single arm trial, participants also lost subcutaneous and visceral fat after 16 weeks on the oral medication, a phosphodiesterase 4 (PDE4) inhibitor, and maintained that loss at 52 weeks.
People with psoriasis have an increased risk of obesity, type 2 diabetes, and cardiovascular events. Patients with more significant psoriasis “tend to die about 5 years younger than they should, based on their risk factors for mortality,” Joel Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
He led the research and presented the findings at the AAD meeting March 26. “As a result, there has been a keen interest in understanding how psoriasis therapies impact cardiovascular risk, the idea being that by controlling inflammation, you may lower the risk of these patients developing cardiovascular disease over time,” he said.
Previous trials looking at the effect of psoriasis therapies on vascular inflammation “have been, for the most part, inconclusive,” Michael Garshick, MD, a cardiologist at NYU Langone Health, told this news organization. Dr. Garshick was not involved with the research. A 2021 systematic review of psoriasis clinicals trials reported that the tumor necrosis factor (TNF) blocker adalimumab (Humira) and phototherapy had the greatest effect on cardiometabolic markers, while ustekinumab (Stelara), an interleukin (IL)-12 and IL-23 antagonist, was the only treatment that improved vascular inflammation. These variable findings make this area “ripe for study,” noted Dr. Garshick.
To observe how apremilast, which is approved by the FDA for treating psoriasis and psoriatic arthritis, affected vascular inflammation, adiposity, and blood-based cardiometabolic markers, Dr. Gelfand organized an open-label study in adults with moderate-to-severe psoriasis. All participants were 18 years or older, had psoriasis for at least 6 months, and were candidates for systemic therapy. All patients underwent FDG PET/CT scans to assess aortic vascular inflammation and had blood work at baseline. Of the 70 patients originally enrolled in the study, 60 remained in the study at week 16, including 57 who underwent imaging for the second time. Thirty-nine participants remained in the study until week 52, and all except one had another scan.
The average age of participants was 47 years, and their mean BMI was 30. More than 80% of participants were White (83%) and 77% were male. The study population had lived with psoriasis for an average of 16 years and 8 patients also had psoriatic arthritis. At baseline, on average, participants had a Psoriasis Area and Severity Index (PASI) score of 18.62, a dermatology life quality index (DLQI) score of 11.60, and 22% of participants’ BSA (body surface area) were affected. The mean TBRmax, the marker for vascular inflammation, was 1.61.
Treatment responses were as expected for apremilast, with 35% of patients achieving PASI 75 and 65% of participants reporting DLQI scores of 5 or less by 16 weeks. At 52 weeks, 31% of the cohort had achieved PASI 75, and 67% reported DLQI score of 5 or higher. All psoriasis endpoints had improved since baseline (P = .001).
Throughout the study period, there was no significant change in TBRmax. However, in a sensitivity analysis, the 16 patients with a baseline TBRmax of 1.6 or higher had an absolute reduction of 0.21 in TBR by week 52. “That suggests that maybe a subset of people who have higher levels of aortic inflammation at baseline may experience some reduction that portend, potentially, some health benefits over time,” Dr. Gelfand said. “Ultimately, I wouldn’t hang my hat on the finding,” he said, noting that additional research comparing the treatment to placebo is necessary.
Both visceral and subcutaneous adipose tissue (VAT and SAT) decreased by week 16, and this reduction was maintained through week 52. In the first 16 weeks of the study, VAT decreased by 5.32% (P = .0009), and SAT decreased by 5.53% (P = .0005). From baseline to 52 weeks, VAT decreased by 5.52% (P = .0148), and SAT decreased by 5.50% (P = .0096). There were no significant differences between week 16 and week 52 in VAT or SAT.
Of the 68 blood biomarkers analyzed, there were significant decreases in the inflammatory markers ferritin (P = .015) and IL-beta (P = .006), the lipid metabolism biomarker HDL-cholesterol efflux (P = .008), and ketone bodies (P = .006). There were also increases in the inflammatory marker IL-8 (P = .003), the lipid metabolism marker ApoA (P = .05), and insulin (P = .05). Ferritin was the only biomarker that was reduced on both week 16 and week 52.
“If you want to be a purist, this was a negative trial,” said Dr. Garshick, because apremilast was not found to decrease vascular inflammation; however, he noted that the biomarker changes “were hopeful secondary endpoints.” It could be, he said, that another outcome measure may be better able to show changes in vascular inflammation compared with FDG. “It’s always hard to figure out what a good surrogate endpoint is in cardiovascular trials,” he noted, “so it may be that FDG/PET is too noisy or not reliable enough to see the outcome that we want to see.”
Dr. Gelfand reports consulting fees/grants from Amgen, AbbVie, BMS, Boehringer Ingelheim, Janssen Biologics, Novartis Corp, Pfizer, and UCB (DSMB). He serves as the Deputy Editor for the Journal of Investigative Dermatology and the Chief Medical Editor at Healio Psoriatic Disease and receives honoraria for both roles. Dr. Garshick has received consulting fees from AbbVie.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Protease inhibitors increase small-for-gestational-age but not other pregnancy risks
Pregnant women with HIV can be reassured that protease inhibitors are safer than previously thought in terms of risk to the fetus, according to research from the National Perinatal Epidemiology Unit (NPEU) at Oxford Population Health, a research institute based at the University of Oxford (England).
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV and plays a crucial role both in improving maternal health and in reducing transmission of HIV from mother to child. However, there has been a critical lack of evidence about the effects of ART on the risk of adverse pregnancy outcomes, with particular concern about protease inhibitors.
Current guidelines recommend that protease inhibitor-based therapies should be used in pregnancy only if first-line treatments (such as integrase and reverse-transcriptase based treatments) are either unsuitable or unavailable. These guidelines also often advise against the use of a specific protease inhibitor, lopinavir/ritonavir, citing an increased risk of preterm birth. However, such advice may restrict treatment options for pregnant women with HIV on the basis of limited evidence.
Largest review to date
The NPEU researchers, therefore, conducted the largest systematic review to date of adverse perinatal outcomes after a range of antiretroviral therapies. It included 34 cohort studies published between 1980 and 2020 and involving over 57,000 pregnant women with HIV in 22 different countries. The review, published in eClinicalMedicine, looked for evidence of 11 perinatal outcomes:
- Preterm birth, very preterm birth, and spontaneous preterm birth
- Low birth weight, very low birth weight, term low birth weight, and preterm low birth weight
- Small for gestational age and very small for gestational age
- Stillbirth, and neonatal death
Using pairwise random-effects meta-analyses, researchers compared protease inhibitor versus non-protease inhibitor-based ART, as well as specifically looking at the comparative risks associated with different protease inhibitor regimens.
They found that protease inhibitor-based ART significantly increased the risk of small or very small for gestational age babies, with relative risks of 1.24 (95% confidence interval, 1.08-1.43; I2 = 66.7%) and 1.40 (95% CI, 1.09-1.81; I2 = 0.0%), respectively. However there were no significant differences in other adverse pregnancy outcomes for protease inhibitors, compared with other therapies.
In addition, researchers found no significant differences in perinatal outcomes between ART regimens containing lopinavir/ritonavir, atazanavir/ritonavir, or darunavir/ritonavir, which are the most frequently used protease inhibitors.
No increased risk of preterm birth
Senior author Dr. Joris Hemelaar, senior clinical research fellow at the NPEU and honorary consultant in obstetrics at the John Radcliffe Hospital, Oxford (England), said: “Antiretroviral therapy in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but our study has shown for the first time that protease inhibitors are associated with babies being small or very small for their gestational age.”
“However, there was no increased risk of preterm birth, or any other adverse pregnancy outcomes. This means protease inhibitors remain an important option for pregnant women living with HIV if other treatments are unsuitable, for example due to drug resistance, or unavailable. The evidence presented here indicates that the commonly used protease inhibitors atazanavir, lopinavir, and darunavir are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.”
Over 70% of the studies assessed were conducted in high-income countries, and Dr. Hemelaar added that there is an urgent need for more research on pregnancy outcomes after different ART in low- to middle-income countries, where the burden of HIV is highest.
Professor Yvonne Gilleece, a spokesperson for the British HIV Association (BHIVA) and immediate past chair of the BHIVA guidelines on the management of HIV in pregnancy and the postpartum period commented: “Pregnancy is a unique life situation in which we must consider the safety of both the birthing parent and the baby. Due to ongoing under-representation of all women in clinical trials, but particularly pregnant women, we do not have enough evidence on which to base all our management decisions. This systematic review includes large numbers of pregnant women living with HIV and can, therefore, improve an informed discussion regarding the safety of the use of protease inhibitors during pregnancy.”
Dr. Hemelaar told Medscape UK: “Many international treatment guidelines cite adverse pregnancy outcomes, in particular preterm birth, associated with protease inhibitor (PI)-drugs as a reason for caution for their use in pregnancy. However, PI drugs are not associated with preterm birth in our analysis. This suggests that PI drugs may not be as detrimental as previously thought (and we found no differences between different PI drugs used), and, hence, these drugs may have a more favourable profile for use in pregnancy.
“However, many other aspects of treatment, including the extent to which the virus can be suppressed, adverse drug effects, adherence to drug prescriptions, antiretroviral drug resistance, drug interactions, drug cost, and availability, should also be taken into account by clinicians and guideline development committees.”
A version of this article first appeared on Medscape UK.
Pregnant women with HIV can be reassured that protease inhibitors are safer than previously thought in terms of risk to the fetus, according to research from the National Perinatal Epidemiology Unit (NPEU) at Oxford Population Health, a research institute based at the University of Oxford (England).
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV and plays a crucial role both in improving maternal health and in reducing transmission of HIV from mother to child. However, there has been a critical lack of evidence about the effects of ART on the risk of adverse pregnancy outcomes, with particular concern about protease inhibitors.
Current guidelines recommend that protease inhibitor-based therapies should be used in pregnancy only if first-line treatments (such as integrase and reverse-transcriptase based treatments) are either unsuitable or unavailable. These guidelines also often advise against the use of a specific protease inhibitor, lopinavir/ritonavir, citing an increased risk of preterm birth. However, such advice may restrict treatment options for pregnant women with HIV on the basis of limited evidence.
Largest review to date
The NPEU researchers, therefore, conducted the largest systematic review to date of adverse perinatal outcomes after a range of antiretroviral therapies. It included 34 cohort studies published between 1980 and 2020 and involving over 57,000 pregnant women with HIV in 22 different countries. The review, published in eClinicalMedicine, looked for evidence of 11 perinatal outcomes:
- Preterm birth, very preterm birth, and spontaneous preterm birth
- Low birth weight, very low birth weight, term low birth weight, and preterm low birth weight
- Small for gestational age and very small for gestational age
- Stillbirth, and neonatal death
Using pairwise random-effects meta-analyses, researchers compared protease inhibitor versus non-protease inhibitor-based ART, as well as specifically looking at the comparative risks associated with different protease inhibitor regimens.
They found that protease inhibitor-based ART significantly increased the risk of small or very small for gestational age babies, with relative risks of 1.24 (95% confidence interval, 1.08-1.43; I2 = 66.7%) and 1.40 (95% CI, 1.09-1.81; I2 = 0.0%), respectively. However there were no significant differences in other adverse pregnancy outcomes for protease inhibitors, compared with other therapies.
In addition, researchers found no significant differences in perinatal outcomes between ART regimens containing lopinavir/ritonavir, atazanavir/ritonavir, or darunavir/ritonavir, which are the most frequently used protease inhibitors.
No increased risk of preterm birth
Senior author Dr. Joris Hemelaar, senior clinical research fellow at the NPEU and honorary consultant in obstetrics at the John Radcliffe Hospital, Oxford (England), said: “Antiretroviral therapy in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but our study has shown for the first time that protease inhibitors are associated with babies being small or very small for their gestational age.”
“However, there was no increased risk of preterm birth, or any other adverse pregnancy outcomes. This means protease inhibitors remain an important option for pregnant women living with HIV if other treatments are unsuitable, for example due to drug resistance, or unavailable. The evidence presented here indicates that the commonly used protease inhibitors atazanavir, lopinavir, and darunavir are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.”
Over 70% of the studies assessed were conducted in high-income countries, and Dr. Hemelaar added that there is an urgent need for more research on pregnancy outcomes after different ART in low- to middle-income countries, where the burden of HIV is highest.
Professor Yvonne Gilleece, a spokesperson for the British HIV Association (BHIVA) and immediate past chair of the BHIVA guidelines on the management of HIV in pregnancy and the postpartum period commented: “Pregnancy is a unique life situation in which we must consider the safety of both the birthing parent and the baby. Due to ongoing under-representation of all women in clinical trials, but particularly pregnant women, we do not have enough evidence on which to base all our management decisions. This systematic review includes large numbers of pregnant women living with HIV and can, therefore, improve an informed discussion regarding the safety of the use of protease inhibitors during pregnancy.”
Dr. Hemelaar told Medscape UK: “Many international treatment guidelines cite adverse pregnancy outcomes, in particular preterm birth, associated with protease inhibitor (PI)-drugs as a reason for caution for their use in pregnancy. However, PI drugs are not associated with preterm birth in our analysis. This suggests that PI drugs may not be as detrimental as previously thought (and we found no differences between different PI drugs used), and, hence, these drugs may have a more favourable profile for use in pregnancy.
“However, many other aspects of treatment, including the extent to which the virus can be suppressed, adverse drug effects, adherence to drug prescriptions, antiretroviral drug resistance, drug interactions, drug cost, and availability, should also be taken into account by clinicians and guideline development committees.”
A version of this article first appeared on Medscape UK.
Pregnant women with HIV can be reassured that protease inhibitors are safer than previously thought in terms of risk to the fetus, according to research from the National Perinatal Epidemiology Unit (NPEU) at Oxford Population Health, a research institute based at the University of Oxford (England).
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV and plays a crucial role both in improving maternal health and in reducing transmission of HIV from mother to child. However, there has been a critical lack of evidence about the effects of ART on the risk of adverse pregnancy outcomes, with particular concern about protease inhibitors.
Current guidelines recommend that protease inhibitor-based therapies should be used in pregnancy only if first-line treatments (such as integrase and reverse-transcriptase based treatments) are either unsuitable or unavailable. These guidelines also often advise against the use of a specific protease inhibitor, lopinavir/ritonavir, citing an increased risk of preterm birth. However, such advice may restrict treatment options for pregnant women with HIV on the basis of limited evidence.
Largest review to date
The NPEU researchers, therefore, conducted the largest systematic review to date of adverse perinatal outcomes after a range of antiretroviral therapies. It included 34 cohort studies published between 1980 and 2020 and involving over 57,000 pregnant women with HIV in 22 different countries. The review, published in eClinicalMedicine, looked for evidence of 11 perinatal outcomes:
- Preterm birth, very preterm birth, and spontaneous preterm birth
- Low birth weight, very low birth weight, term low birth weight, and preterm low birth weight
- Small for gestational age and very small for gestational age
- Stillbirth, and neonatal death
Using pairwise random-effects meta-analyses, researchers compared protease inhibitor versus non-protease inhibitor-based ART, as well as specifically looking at the comparative risks associated with different protease inhibitor regimens.
They found that protease inhibitor-based ART significantly increased the risk of small or very small for gestational age babies, with relative risks of 1.24 (95% confidence interval, 1.08-1.43; I2 = 66.7%) and 1.40 (95% CI, 1.09-1.81; I2 = 0.0%), respectively. However there were no significant differences in other adverse pregnancy outcomes for protease inhibitors, compared with other therapies.
In addition, researchers found no significant differences in perinatal outcomes between ART regimens containing lopinavir/ritonavir, atazanavir/ritonavir, or darunavir/ritonavir, which are the most frequently used protease inhibitors.
No increased risk of preterm birth
Senior author Dr. Joris Hemelaar, senior clinical research fellow at the NPEU and honorary consultant in obstetrics at the John Radcliffe Hospital, Oxford (England), said: “Antiretroviral therapy in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but our study has shown for the first time that protease inhibitors are associated with babies being small or very small for their gestational age.”
“However, there was no increased risk of preterm birth, or any other adverse pregnancy outcomes. This means protease inhibitors remain an important option for pregnant women living with HIV if other treatments are unsuitable, for example due to drug resistance, or unavailable. The evidence presented here indicates that the commonly used protease inhibitors atazanavir, lopinavir, and darunavir are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.”
Over 70% of the studies assessed were conducted in high-income countries, and Dr. Hemelaar added that there is an urgent need for more research on pregnancy outcomes after different ART in low- to middle-income countries, where the burden of HIV is highest.
Professor Yvonne Gilleece, a spokesperson for the British HIV Association (BHIVA) and immediate past chair of the BHIVA guidelines on the management of HIV in pregnancy and the postpartum period commented: “Pregnancy is a unique life situation in which we must consider the safety of both the birthing parent and the baby. Due to ongoing under-representation of all women in clinical trials, but particularly pregnant women, we do not have enough evidence on which to base all our management decisions. This systematic review includes large numbers of pregnant women living with HIV and can, therefore, improve an informed discussion regarding the safety of the use of protease inhibitors during pregnancy.”
Dr. Hemelaar told Medscape UK: “Many international treatment guidelines cite adverse pregnancy outcomes, in particular preterm birth, associated with protease inhibitor (PI)-drugs as a reason for caution for their use in pregnancy. However, PI drugs are not associated with preterm birth in our analysis. This suggests that PI drugs may not be as detrimental as previously thought (and we found no differences between different PI drugs used), and, hence, these drugs may have a more favourable profile for use in pregnancy.
“However, many other aspects of treatment, including the extent to which the virus can be suppressed, adverse drug effects, adherence to drug prescriptions, antiretroviral drug resistance, drug interactions, drug cost, and availability, should also be taken into account by clinicians and guideline development committees.”
A version of this article first appeared on Medscape UK.
FROM ECLINICALMEDICINE
Mutation testing recommended for advanced and refractory thyroid cancer
A focuses on a definition of advanced thyroid cancer and outlines strategies for mutation testing and targeted treatment.
Mutation testing should not be pursued if cancer burden and disease threat is low, since most thyroid cancers have a very good prognosis and are highly treatable. But 15% of differentiated thyroid cancer cases are locally advanced, and radioiodine refractory differentiated thyroid cancer has a 10-year survival below 50%.
More generally, advanced thyroid cancer has not been well defined clinically. Physicians with experience diagnosing advanced disease may recognize it, but there is no widely accepted definition. “This may be the first time that an expert group of physicians has attempted to define what advanced thyroid cancer is,” said David Shonka, MD, who is a coauthor of the consensus statement, which was published online in Head & Neck. He is an associate professor of otolaryngology/head and neck surgery at the University of Virginia, Charlottesville.
“All patients with advanced thyroid disease and most patients with incurable radioiodine refractory differentiated thyroid cancer should undergo somatic mutational testing,” the authors wrote. “Next-generation sequencing can reveal targetable mutations and potentially give patients affected by advanced thyroid carcinoma systemic treatment options that can prolong survival. These new innovative approaches are changing the landscape of clinical care for patients with advanced thyroid cancer.”
For differentiated thyroid cancer and medullary thyroid carcinoma, the authors created a definition that combines structural factors on imaging, along with surgical findings, and biochemical, histologic, and molecular factors. Anaplastic thyroid cancer should always be considered advanced, even after a complete resection and incidental pathological identification.
The statement also summarizes recent advances in thyroid cancer that have revealed molecular markers which contribute to oncogenesis. Initially, those approaches were applied to indeterminate fine needle biopsies to improve diagnosis. More recent studies used them to match patients to targeted therapies. There are Food and Drug Administration–approved therapies targeting the BRAF and RET mutations, but advanced thyroid cancer is also included in some “basket” trials that test targeted agents against driver mutations across multiple tumor types.
Radioiodine refractory differentiated thyroid cancer had few treatments as recently as 10 years ago. But recent research has shown that multikinase inhibitors improve outcomes, and a range of mutations have been found in this type of thyroid cancer, including BRAF V600E, RET, PIK3CA, and PTEN, and fusions involving RET, NTRK, and ALK. Other mutations have been linked to more aggressive disease. Efforts to personalize treatment also include microsatellite stability status, tumor mutational burden, and programmed death–ligand 1 status as indicators for immunotherapy. “With discovery of many other molecular targets, and emerging literature showcasing promise of matched targeted therapies, we recommend that all patients with advanced thyroid cancer have comprehensive genomic profiling on tumor tissue through (next generation sequencing),” the authors wrote.
These newer and novel therapies have presented physicians with options outside of surgery, chemotherapy, or radiotherapy, which have low efficacy against advanced thyroid cancer. “It is an area in which there has been substantial change. Even 5-7 years ago, patients with advanced thyroid cancer that was not responsive to radioactive iodine or surgery really didn’t have a lot of options. This is a really an exciting and growing field,” Dr. Shonka said.
He specifically cited anaplastic thyroid cancer, which like radioiodine refractory differentiated thyroid cancer has had few treatment options until recently. “Now, if you see a patient with anaplastic thyroid cancer, your knee-jerk reaction should be ‘let’s do molecular testing on this, this is definitely advanced disease.’ If they have a BRAF mutation, that’s targetable, and we can treat this patient with combination therapy that actually improves their survival. So, there’s some exciting stuff happening and probably more coming down the road as we develop new drugs that can target these mutations that we’re identifying.”
Dr. Shonka has no relevant financial disclosures.
A focuses on a definition of advanced thyroid cancer and outlines strategies for mutation testing and targeted treatment.
Mutation testing should not be pursued if cancer burden and disease threat is low, since most thyroid cancers have a very good prognosis and are highly treatable. But 15% of differentiated thyroid cancer cases are locally advanced, and radioiodine refractory differentiated thyroid cancer has a 10-year survival below 50%.
More generally, advanced thyroid cancer has not been well defined clinically. Physicians with experience diagnosing advanced disease may recognize it, but there is no widely accepted definition. “This may be the first time that an expert group of physicians has attempted to define what advanced thyroid cancer is,” said David Shonka, MD, who is a coauthor of the consensus statement, which was published online in Head & Neck. He is an associate professor of otolaryngology/head and neck surgery at the University of Virginia, Charlottesville.
“All patients with advanced thyroid disease and most patients with incurable radioiodine refractory differentiated thyroid cancer should undergo somatic mutational testing,” the authors wrote. “Next-generation sequencing can reveal targetable mutations and potentially give patients affected by advanced thyroid carcinoma systemic treatment options that can prolong survival. These new innovative approaches are changing the landscape of clinical care for patients with advanced thyroid cancer.”
For differentiated thyroid cancer and medullary thyroid carcinoma, the authors created a definition that combines structural factors on imaging, along with surgical findings, and biochemical, histologic, and molecular factors. Anaplastic thyroid cancer should always be considered advanced, even after a complete resection and incidental pathological identification.
The statement also summarizes recent advances in thyroid cancer that have revealed molecular markers which contribute to oncogenesis. Initially, those approaches were applied to indeterminate fine needle biopsies to improve diagnosis. More recent studies used them to match patients to targeted therapies. There are Food and Drug Administration–approved therapies targeting the BRAF and RET mutations, but advanced thyroid cancer is also included in some “basket” trials that test targeted agents against driver mutations across multiple tumor types.
Radioiodine refractory differentiated thyroid cancer had few treatments as recently as 10 years ago. But recent research has shown that multikinase inhibitors improve outcomes, and a range of mutations have been found in this type of thyroid cancer, including BRAF V600E, RET, PIK3CA, and PTEN, and fusions involving RET, NTRK, and ALK. Other mutations have been linked to more aggressive disease. Efforts to personalize treatment also include microsatellite stability status, tumor mutational burden, and programmed death–ligand 1 status as indicators for immunotherapy. “With discovery of many other molecular targets, and emerging literature showcasing promise of matched targeted therapies, we recommend that all patients with advanced thyroid cancer have comprehensive genomic profiling on tumor tissue through (next generation sequencing),” the authors wrote.
These newer and novel therapies have presented physicians with options outside of surgery, chemotherapy, or radiotherapy, which have low efficacy against advanced thyroid cancer. “It is an area in which there has been substantial change. Even 5-7 years ago, patients with advanced thyroid cancer that was not responsive to radioactive iodine or surgery really didn’t have a lot of options. This is a really an exciting and growing field,” Dr. Shonka said.
He specifically cited anaplastic thyroid cancer, which like radioiodine refractory differentiated thyroid cancer has had few treatment options until recently. “Now, if you see a patient with anaplastic thyroid cancer, your knee-jerk reaction should be ‘let’s do molecular testing on this, this is definitely advanced disease.’ If they have a BRAF mutation, that’s targetable, and we can treat this patient with combination therapy that actually improves their survival. So, there’s some exciting stuff happening and probably more coming down the road as we develop new drugs that can target these mutations that we’re identifying.”
Dr. Shonka has no relevant financial disclosures.
A focuses on a definition of advanced thyroid cancer and outlines strategies for mutation testing and targeted treatment.
Mutation testing should not be pursued if cancer burden and disease threat is low, since most thyroid cancers have a very good prognosis and are highly treatable. But 15% of differentiated thyroid cancer cases are locally advanced, and radioiodine refractory differentiated thyroid cancer has a 10-year survival below 50%.
More generally, advanced thyroid cancer has not been well defined clinically. Physicians with experience diagnosing advanced disease may recognize it, but there is no widely accepted definition. “This may be the first time that an expert group of physicians has attempted to define what advanced thyroid cancer is,” said David Shonka, MD, who is a coauthor of the consensus statement, which was published online in Head & Neck. He is an associate professor of otolaryngology/head and neck surgery at the University of Virginia, Charlottesville.
“All patients with advanced thyroid disease and most patients with incurable radioiodine refractory differentiated thyroid cancer should undergo somatic mutational testing,” the authors wrote. “Next-generation sequencing can reveal targetable mutations and potentially give patients affected by advanced thyroid carcinoma systemic treatment options that can prolong survival. These new innovative approaches are changing the landscape of clinical care for patients with advanced thyroid cancer.”
For differentiated thyroid cancer and medullary thyroid carcinoma, the authors created a definition that combines structural factors on imaging, along with surgical findings, and biochemical, histologic, and molecular factors. Anaplastic thyroid cancer should always be considered advanced, even after a complete resection and incidental pathological identification.
The statement also summarizes recent advances in thyroid cancer that have revealed molecular markers which contribute to oncogenesis. Initially, those approaches were applied to indeterminate fine needle biopsies to improve diagnosis. More recent studies used them to match patients to targeted therapies. There are Food and Drug Administration–approved therapies targeting the BRAF and RET mutations, but advanced thyroid cancer is also included in some “basket” trials that test targeted agents against driver mutations across multiple tumor types.
Radioiodine refractory differentiated thyroid cancer had few treatments as recently as 10 years ago. But recent research has shown that multikinase inhibitors improve outcomes, and a range of mutations have been found in this type of thyroid cancer, including BRAF V600E, RET, PIK3CA, and PTEN, and fusions involving RET, NTRK, and ALK. Other mutations have been linked to more aggressive disease. Efforts to personalize treatment also include microsatellite stability status, tumor mutational burden, and programmed death–ligand 1 status as indicators for immunotherapy. “With discovery of many other molecular targets, and emerging literature showcasing promise of matched targeted therapies, we recommend that all patients with advanced thyroid cancer have comprehensive genomic profiling on tumor tissue through (next generation sequencing),” the authors wrote.
These newer and novel therapies have presented physicians with options outside of surgery, chemotherapy, or radiotherapy, which have low efficacy against advanced thyroid cancer. “It is an area in which there has been substantial change. Even 5-7 years ago, patients with advanced thyroid cancer that was not responsive to radioactive iodine or surgery really didn’t have a lot of options. This is a really an exciting and growing field,” Dr. Shonka said.
He specifically cited anaplastic thyroid cancer, which like radioiodine refractory differentiated thyroid cancer has had few treatment options until recently. “Now, if you see a patient with anaplastic thyroid cancer, your knee-jerk reaction should be ‘let’s do molecular testing on this, this is definitely advanced disease.’ If they have a BRAF mutation, that’s targetable, and we can treat this patient with combination therapy that actually improves their survival. So, there’s some exciting stuff happening and probably more coming down the road as we develop new drugs that can target these mutations that we’re identifying.”
Dr. Shonka has no relevant financial disclosures.
FROM HEAD & NECK
Trichotillomania: What you should know about this common hair-pulling disorder
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
Trichotillomania is a chronic psychiatric disorder that causes people to repeatedly pull out their own hair. Not only does it result in alopecia with no other underlying causes but it can have significant psychosocial ramifications and rare, but serious, complications. Though the reported prevalence rates are up to approximately 2%, it’s probable that you’ll come upon a patient suffering with this disorder at your practice, if you haven’t already.
To find out more about the best methods for diagnosing and treating this disorder, we spoke with Jon E. Grant, JD, MD, MPH, a leading trichotillomania researcher and part of the department of psychiatry and behavioral neuroscience at the University of Chicago.
Defining trichotillomania
What were the earliest descriptions of trichotillomania in medical literature?
The first real discussion of it probably goes back to Hippocrates, but from a modern medical perspective, discussion began in the 19th century with reports from the French dermatologist François Hallopeau.
They didn’t really call them disorders then – it was long before the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) – but they described this in young men who kept pulling their hair for unclear reasons. These early case reports don’t provide a lot of psychological perspective, but they seem consistent with what we see now.
What are the diagnostic criteria for trichotillomania?
The current DSM-5 criteria are recurrent pulling out of hair, an inability to stop it, the pulling resulting in some noticeable thinning or hair loss, and that it causes some level of distress or some type of impairment in functioning.
At what age do most people experience an onset of symptoms?
Generally speaking, it’s in early adolescence, post puberty, around 12-15 years of age. Having said that, we do see children as young as 1-2 years who are pulling their hair, and we occasionally see somebody far older who is doing it for the first time, a sort of geriatric onset.
Overlap and differences with other disorders
You’ve written that although trichotillomania is grouped with obsessive-compulsive disorder (OCD) in the DSM-5, the thinking around that has recently shifted. Why is that?
At first, it was noticed that many of these people pulled their hair repetitively in an almost ritualized manner, perhaps every night before bed. That looked like a compulsion of OCD.
When DSM-5 came out in 2013, they grouped it with OCD. Yet people shifted to thinking that it’s kind of a cousin of OCD because it has this compulsive quality but doesn’t really have obsessive thinking that drives it. Many people just pull their hair. They’re not even always aware of it: sometimes yes, sometimes no.
We know that it has some links to OCD. You’ll see more OCD in folks with trichotillomania, but it clearly is not just the same as OCD. One of the biggest pieces of evidence for that is that our first-line treatment for OCD – a selective serotonin reuptake inhibitor antidepressant – does not really help hair pulling.
Having said that, if people are looking for help with trichotillomania, they often are best served by therapists and doctors who have a familiarity with OCD and have kept it on their radar over the past couple of decades.
How does trichotillomania overlap with skin picking disorder, which is another condition that you’ve closely researched?
It does have some overlap with skin picking in the sense that it often seems familial. For example, the mother may pull her hair and child picks their skin.
It also has a fair amount of comorbidity with skin picking. Many people who pull will pick a little bit or did at some point. Many people who pick pulled their hair at some point. It seems closely related to nail biting as well.
Studies have also shown that one of the things that runs in the histories of most families of people with trichotillomania might be substance abuse – alcohol or drug addiction.
All of this has led people to believe that there might be subtypes of trichotillomania: one that’s more like an OCD and one that’s more like an addiction. That’s similar to the debate with other mental health conditions, that there are probably multiple types of depression, multiple types of schizophrenia.
Is there a component of this that could be defined as self-harm?
That’s been its own debate. It doesn’t seem to have the same developmental trajectory that we see with self-harm, or even some of the personality features.
However, there may be a small segment of folks with trichotillomania that might more appropriately fit that category. For example, those with family histories of trauma, higher rates of posttraumatic stress disorder, or borderline personality. But it wouldn’t be the majority.
The problem is, if you look at some of the pediatrician data, they often group picking, pulling, and cutting. I think that’s far too all-inclusive.
A gap in clinician education
Are adolescent patients likely to self-report this behavior, or is it something that physicians need to suss out for themselves?
Clearly, if child psychologists, psychiatrists, or pediatricians see young people with patches of alopecia – eyebrows or eyelashes missing, head hair with spots – in addition to a dermatologic assessment, they should simply ask, “Do you pull your hair?”
But it’s interesting that with the internet, young people are much more likely to disclose and actually come forward and tell their parents that they think they have trichotillomania.
I also hear from a lot of the adolescents that they have to educate their doctors about trichotillomania because so often physicians don’t know much about it and will assume that it’s self-injury or just a symptom of anxiety. It’s a little bit of a flip from what we might have seen 20 years ago.
I’ve seen several patients who’ve said, basically, “I’m tired of no professionals seeming to know about this. I shouldn’t have to be educating my doctors about this.” I tell them that I completely agree. It’s a shame because if a doctor doesn’t know about it, then how can they get the appropriate care?
What are the complications that accompany trichotillomania?
A small percentage, maybe about 10%, will ingest their hair, much like people who bite and swallow their fingernails. The concern there is that because hair is nondigestible, it could create an intestinal plug that could rupture and be potentially life-threatening. That makes it all the more important to ask those who pull their hair what they do with the hair once they pull it.
However, with most people, the real problem is with self-esteem. Young people may not want to socialize, go on dates, or do other things they would normally do because of it. In adults, you may find that they’re far more educated than their job allows but don’t want to go to an interview because they don’t want to have somebody sit there and look at them and notice that perhaps they don’t have any eyebrows, or that they’re wearing a wig. Those psychosocial implications are huge for so many people.
Treatment options
In a 2021 study, you showed that nearly one-quarter of people with trichotillomania do naturally recover from it. What characteristics do they seem to have?
It’s interesting because we see natural recovery across many mental health problems: alcohol addition, gambling, OCD. The question then becomes why is that some people can seemingly just stop doing a behavior? Can we learn from those people?
We did see that those who naturally recovered were less likely to have some other mental health comorbidities. It seems like when you have other things such as skin picking or OCD plus trichotillomania, that it probably speaks to something that perhaps synergistically is keeping it going. But this is just a first study; learning how to harness and understand it is the next step.
What’s the goal of treating trichotillomania?
The desired goal is zero pulling. The realistic goal is more likely significantly reduced pulling that then leads to greater function in life, greater quality-of-life.
One doesn’t have to go from 100 to 0 in order to do that. I always tell people that maybe every now and then, every few months, when something is going on in life, you might find yourself pulling a hair or two. That’s okay. If you’re not pulling every day and it’s significantly reduced, we’ll call that a success. I think that setting reasonable goals at this point is really important.
And what would the treatment pathway look like for most patients?
The standard approach is probably some type of habit-reversal therapy, of which there have been many variants over the years. It involves doing something different with your hand, identifying the triggers that may set you off, and then doing something in response to those triggers that is not pulling and might neutralize whatever that anxious or stressed feeling is. That could be different with each person.
At this point, there is no drug approved by the U.S. Food and Drug Administration for trichotillomania. Our best approaches have included N-acetylcysteine, a glutamate modulator, which we’ve done research in.
That’s kind of a go-to option for people because its side-effect profile is generally innocuous. The data show that it could be beneficial in many people with very few, if any, side effects. That would be one “medication,” although it’s actually an over-the-counter vitamin. But we’re constantly looking for better and better treatments.
Do you have any final advice for clinicians or researchers?
Given how common it is, I don’t think clinicians should just see it as an innocuous little habit that people should be able to stop on their own. Clinicians should educate themselves about trichotillomania and know where the person should get the appropriate care.
From the research perspective, given the fact that we see this in animals of multiple species – that they overgroom – this seems to be deeply ingrained in us as animals. So when it comes to the underlying neuroscience, people should pay more attention because it probably has a lot to do with our understanding of habit and compulsive behaviors. It arguably can cut across a lot of different behaviors.
A version of this article first appeared on Medscape.com.
The Molting Man: Anasarca-Induced Full-Body Desquamation
Edema blisters are a common but often underreported entity most commonly seen on the lower extremities in the setting of acute edema. 1 Reported risk factors and associations include chronic venous insufficiency, congestive heart failure, hereditary angioedema, and medications (eg, amlodipine). 1,2 We report a newly described variant that we have termed anasarca-induced desquamation in which a patient sloughed the entire cutaneous surface of the body after gaining almost 40 pounds over 5 days.
Case Report
A 50-year-old man without a home was found minimally responsive in a yard. His core body temperature was 25.5 °C. He was profoundly acidotic (pH, <6.733 [reference range, 7.35–7.45]; lactic acid, 20.5 mmol/L [reference range, 0.5–2.2 mmol/L]) at admission. His medical history was notable for diabetes mellitus, hypertension, alcohol abuse, and pulmonary embolism. The patient was resuscitated with rewarming and intravenous fluids in the setting of acute renal insufficiency. By day 5 of the hospital stay, he had a net positive intake of 21.8 L and an 18-kg (39.7-lb) weight gain.
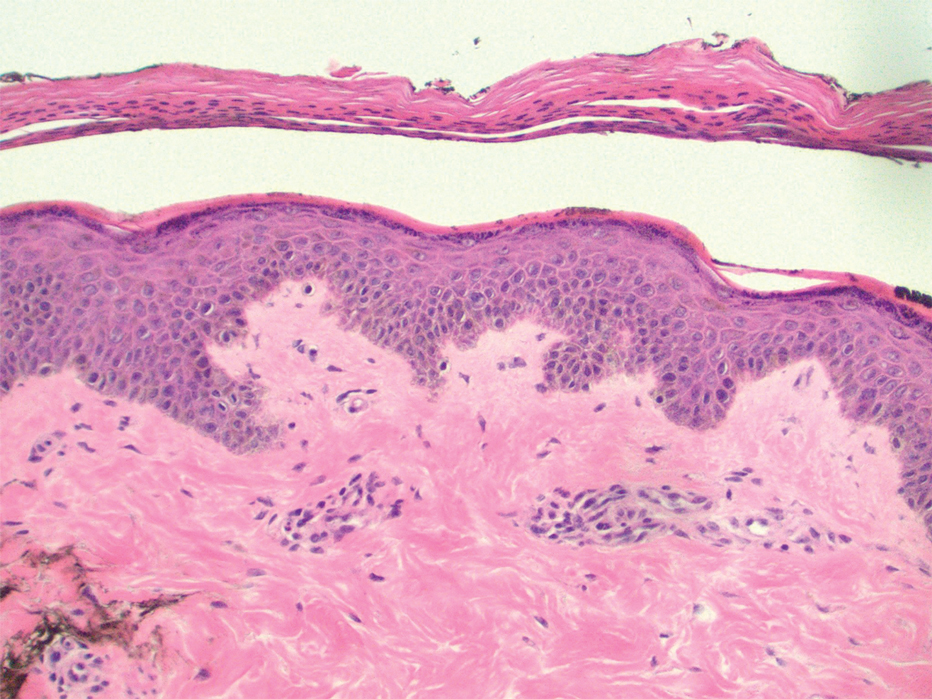
Dermatology was consulted for skin sloughing. Physical examination revealed nonpainful desquamation of the vermilion lip, periorbital skin, right shoulder, and hips without notable mucosal changes. Two 4-mm punch biopsies of the shoulder revealed an intracorneal split with desquamation of the stratum corneum and a mild dermal lymphocytic infiltrate, consistent with exfoliation secondary to edema or staphylococcal scalded skin syndrome (Figure 1). No staphylococcal growth was noted on blood, urine, nasal, wound, and ocular cultures throughout the hospital stay.
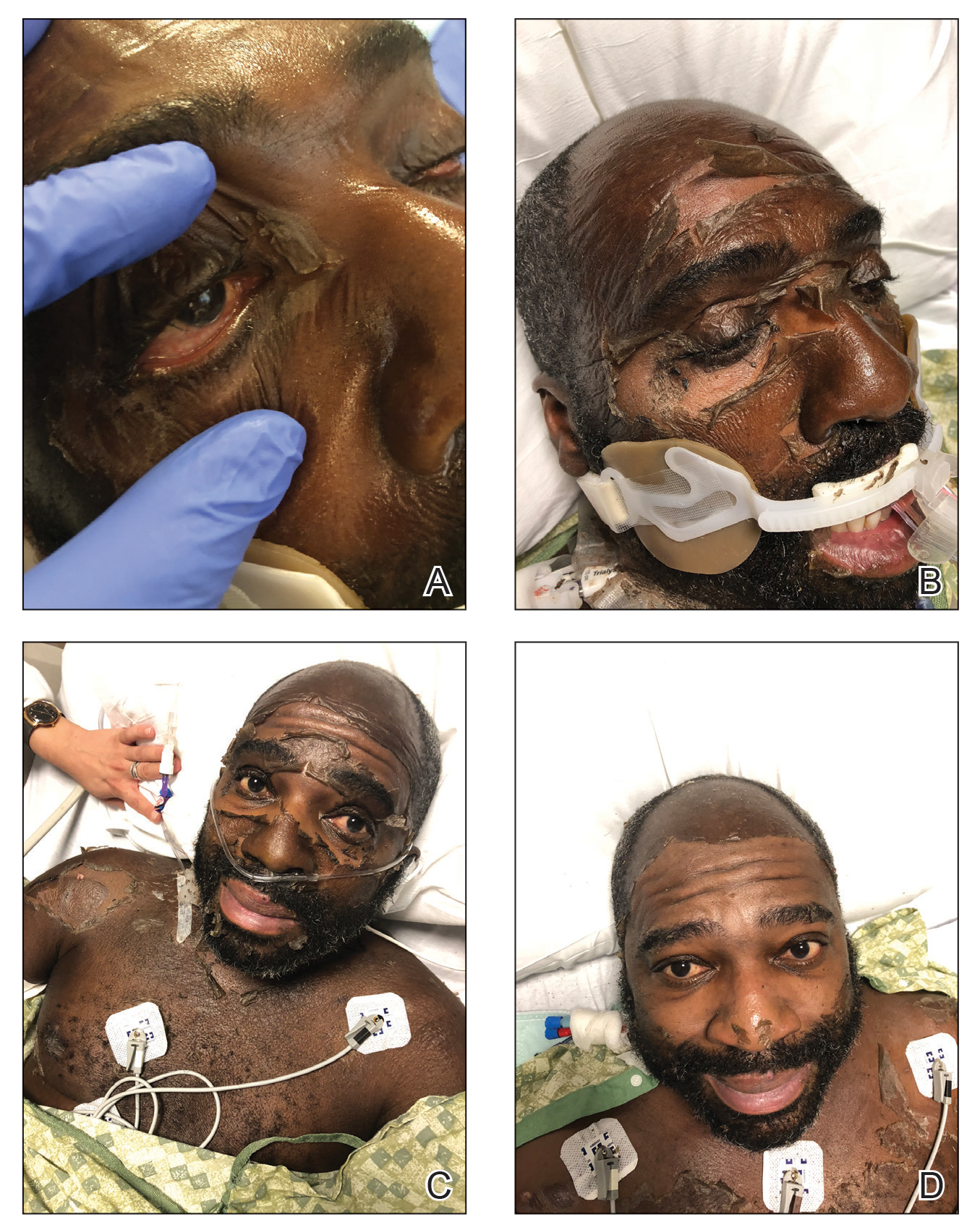
As the patient’s anasarca improved with diuretics and continuous renal replacement therapy, the entire cutaneous surface—head to toe—underwent desquamation, including the palms and soles. He was managed with supportive skin care. The anasarca healed completely with residual hypopigmentation (Figures 2 and 3).
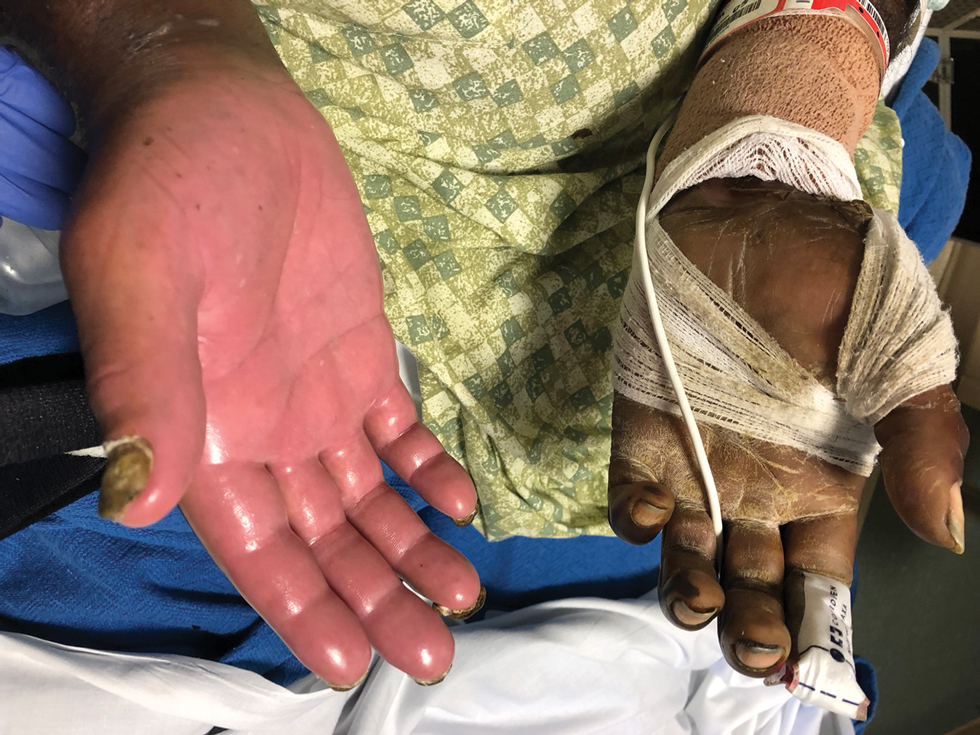
Comment
Anasarca-induced desquamation represents a more diffuse form of a known entity: edema blisters. Occurring most commonly in the setting of acute exacerbation of chronic venous insufficiency, edema blisters can mimic other vesiculobullous conditions, such as bullous pemphigoid and herpes zoster.3
Pathogenesis of Edema Blisters—Edema develops in the skin when the capillary filtration rate, determined by the hydrostatic and oncotic pressures of the capillaries and interstitium, exceeds venous and lymphatic drainage. The appearance of edema blisters in the acute setting likely is related to the speed at which edema develops in skin.1 Although edema blisters often are described as tense, there is a paucity of histologic data at the anatomical level of split in the skin.In our patient, desquamation was within the stratum corneum and likely multifactorial. His weight gain of nearly 40 lb, the result of intravenous instillation of fluids and low urine output, was undeniably a contributing factor. The anasarca was aggravated by hypoalbuminemia (2.1 g/dL) in the setting of known liver disease. Other possible contributing factors were hypotension, which required vasopressor therapy that led to hypoperfusion of the skin, and treatment of hypothermia, with resulting reactive vasodilation and capillary leak.
Management—Treatment of acute edema blisters is focused on the underlying cause of the edema. In a study of 13 patients with edema blisters, all had blisters on the legs that resolved with treatment, such as diuretics or compression therapy.1
Anasarca-induced desquamation is an inherently benign condition that mimics potentially fatal disorders, such as Stevens-Johnson syndrome, staphylococcal scalded skin syndrome, and toxic shock syndrome. Therefore, patients presenting with diffuse superficial desquamation should be assessed for the mucosal changes of Stevens-Johnson syndrome and a history of acute edema in the affected areas to avoid potentially harmful empiric treatments, such as corticosteroids and intravenous antibiotics.
Conclusion
Anasarca-induced desquamation represents a more diffuse form of edema blisters. This desquamation can mimic a potentially fatal rash, such as Stevens-Johnson syndrome and staphylococcal scalded skin syndrome.
- Bhushan M, Chalmers RJ, Cox NH. Acute oedema blisters: a report of 13 cases. Br J Dermatol. 2001;144:580-582. doi:10.1046/j.1365-2133.2001.04087.x
- Fabiani J, Bork K. Acute edema blisters on a skin swelling: an unusual manifestation of hereditary angioedema. Acta Derm Venereol. 2016;96:556-557. doi:10.2340/00015555-2252
- Chen SX, Cohen PR. Edema bullae mimicking disseminated herpes zoster. Cureus. 2017;9:E1780. doi:10.7759/cureus.1780
Edema blisters are a common but often underreported entity most commonly seen on the lower extremities in the setting of acute edema. 1 Reported risk factors and associations include chronic venous insufficiency, congestive heart failure, hereditary angioedema, and medications (eg, amlodipine). 1,2 We report a newly described variant that we have termed anasarca-induced desquamation in which a patient sloughed the entire cutaneous surface of the body after gaining almost 40 pounds over 5 days.
Case Report
A 50-year-old man without a home was found minimally responsive in a yard. His core body temperature was 25.5 °C. He was profoundly acidotic (pH, <6.733 [reference range, 7.35–7.45]; lactic acid, 20.5 mmol/L [reference range, 0.5–2.2 mmol/L]) at admission. His medical history was notable for diabetes mellitus, hypertension, alcohol abuse, and pulmonary embolism. The patient was resuscitated with rewarming and intravenous fluids in the setting of acute renal insufficiency. By day 5 of the hospital stay, he had a net positive intake of 21.8 L and an 18-kg (39.7-lb) weight gain.

Dermatology was consulted for skin sloughing. Physical examination revealed nonpainful desquamation of the vermilion lip, periorbital skin, right shoulder, and hips without notable mucosal changes. Two 4-mm punch biopsies of the shoulder revealed an intracorneal split with desquamation of the stratum corneum and a mild dermal lymphocytic infiltrate, consistent with exfoliation secondary to edema or staphylococcal scalded skin syndrome (Figure 1). No staphylococcal growth was noted on blood, urine, nasal, wound, and ocular cultures throughout the hospital stay.

As the patient’s anasarca improved with diuretics and continuous renal replacement therapy, the entire cutaneous surface—head to toe—underwent desquamation, including the palms and soles. He was managed with supportive skin care. The anasarca healed completely with residual hypopigmentation (Figures 2 and 3).

Comment
Anasarca-induced desquamation represents a more diffuse form of a known entity: edema blisters. Occurring most commonly in the setting of acute exacerbation of chronic venous insufficiency, edema blisters can mimic other vesiculobullous conditions, such as bullous pemphigoid and herpes zoster.3
Pathogenesis of Edema Blisters—Edema develops in the skin when the capillary filtration rate, determined by the hydrostatic and oncotic pressures of the capillaries and interstitium, exceeds venous and lymphatic drainage. The appearance of edema blisters in the acute setting likely is related to the speed at which edema develops in skin.1 Although edema blisters often are described as tense, there is a paucity of histologic data at the anatomical level of split in the skin.In our patient, desquamation was within the stratum corneum and likely multifactorial. His weight gain of nearly 40 lb, the result of intravenous instillation of fluids and low urine output, was undeniably a contributing factor. The anasarca was aggravated by hypoalbuminemia (2.1 g/dL) in the setting of known liver disease. Other possible contributing factors were hypotension, which required vasopressor therapy that led to hypoperfusion of the skin, and treatment of hypothermia, with resulting reactive vasodilation and capillary leak.
Management—Treatment of acute edema blisters is focused on the underlying cause of the edema. In a study of 13 patients with edema blisters, all had blisters on the legs that resolved with treatment, such as diuretics or compression therapy.1
Anasarca-induced desquamation is an inherently benign condition that mimics potentially fatal disorders, such as Stevens-Johnson syndrome, staphylococcal scalded skin syndrome, and toxic shock syndrome. Therefore, patients presenting with diffuse superficial desquamation should be assessed for the mucosal changes of Stevens-Johnson syndrome and a history of acute edema in the affected areas to avoid potentially harmful empiric treatments, such as corticosteroids and intravenous antibiotics.
Conclusion
Anasarca-induced desquamation represents a more diffuse form of edema blisters. This desquamation can mimic a potentially fatal rash, such as Stevens-Johnson syndrome and staphylococcal scalded skin syndrome.
Edema blisters are a common but often underreported entity most commonly seen on the lower extremities in the setting of acute edema. 1 Reported risk factors and associations include chronic venous insufficiency, congestive heart failure, hereditary angioedema, and medications (eg, amlodipine). 1,2 We report a newly described variant that we have termed anasarca-induced desquamation in which a patient sloughed the entire cutaneous surface of the body after gaining almost 40 pounds over 5 days.
Case Report
A 50-year-old man without a home was found minimally responsive in a yard. His core body temperature was 25.5 °C. He was profoundly acidotic (pH, <6.733 [reference range, 7.35–7.45]; lactic acid, 20.5 mmol/L [reference range, 0.5–2.2 mmol/L]) at admission. His medical history was notable for diabetes mellitus, hypertension, alcohol abuse, and pulmonary embolism. The patient was resuscitated with rewarming and intravenous fluids in the setting of acute renal insufficiency. By day 5 of the hospital stay, he had a net positive intake of 21.8 L and an 18-kg (39.7-lb) weight gain.

Dermatology was consulted for skin sloughing. Physical examination revealed nonpainful desquamation of the vermilion lip, periorbital skin, right shoulder, and hips without notable mucosal changes. Two 4-mm punch biopsies of the shoulder revealed an intracorneal split with desquamation of the stratum corneum and a mild dermal lymphocytic infiltrate, consistent with exfoliation secondary to edema or staphylococcal scalded skin syndrome (Figure 1). No staphylococcal growth was noted on blood, urine, nasal, wound, and ocular cultures throughout the hospital stay.

As the patient’s anasarca improved with diuretics and continuous renal replacement therapy, the entire cutaneous surface—head to toe—underwent desquamation, including the palms and soles. He was managed with supportive skin care. The anasarca healed completely with residual hypopigmentation (Figures 2 and 3).

Comment
Anasarca-induced desquamation represents a more diffuse form of a known entity: edema blisters. Occurring most commonly in the setting of acute exacerbation of chronic venous insufficiency, edema blisters can mimic other vesiculobullous conditions, such as bullous pemphigoid and herpes zoster.3
Pathogenesis of Edema Blisters—Edema develops in the skin when the capillary filtration rate, determined by the hydrostatic and oncotic pressures of the capillaries and interstitium, exceeds venous and lymphatic drainage. The appearance of edema blisters in the acute setting likely is related to the speed at which edema develops in skin.1 Although edema blisters often are described as tense, there is a paucity of histologic data at the anatomical level of split in the skin.In our patient, desquamation was within the stratum corneum and likely multifactorial. His weight gain of nearly 40 lb, the result of intravenous instillation of fluids and low urine output, was undeniably a contributing factor. The anasarca was aggravated by hypoalbuminemia (2.1 g/dL) in the setting of known liver disease. Other possible contributing factors were hypotension, which required vasopressor therapy that led to hypoperfusion of the skin, and treatment of hypothermia, with resulting reactive vasodilation and capillary leak.
Management—Treatment of acute edema blisters is focused on the underlying cause of the edema. In a study of 13 patients with edema blisters, all had blisters on the legs that resolved with treatment, such as diuretics or compression therapy.1
Anasarca-induced desquamation is an inherently benign condition that mimics potentially fatal disorders, such as Stevens-Johnson syndrome, staphylococcal scalded skin syndrome, and toxic shock syndrome. Therefore, patients presenting with diffuse superficial desquamation should be assessed for the mucosal changes of Stevens-Johnson syndrome and a history of acute edema in the affected areas to avoid potentially harmful empiric treatments, such as corticosteroids and intravenous antibiotics.
Conclusion
Anasarca-induced desquamation represents a more diffuse form of edema blisters. This desquamation can mimic a potentially fatal rash, such as Stevens-Johnson syndrome and staphylococcal scalded skin syndrome.
- Bhushan M, Chalmers RJ, Cox NH. Acute oedema blisters: a report of 13 cases. Br J Dermatol. 2001;144:580-582. doi:10.1046/j.1365-2133.2001.04087.x
- Fabiani J, Bork K. Acute edema blisters on a skin swelling: an unusual manifestation of hereditary angioedema. Acta Derm Venereol. 2016;96:556-557. doi:10.2340/00015555-2252
- Chen SX, Cohen PR. Edema bullae mimicking disseminated herpes zoster. Cureus. 2017;9:E1780. doi:10.7759/cureus.1780
- Bhushan M, Chalmers RJ, Cox NH. Acute oedema blisters: a report of 13 cases. Br J Dermatol. 2001;144:580-582. doi:10.1046/j.1365-2133.2001.04087.x
- Fabiani J, Bork K. Acute edema blisters on a skin swelling: an unusual manifestation of hereditary angioedema. Acta Derm Venereol. 2016;96:556-557. doi:10.2340/00015555-2252
- Chen SX, Cohen PR. Edema bullae mimicking disseminated herpes zoster. Cureus. 2017;9:E1780. doi:10.7759/cureus.1780
Practice Points
- The appearance of anasarca-induced desquamation can be similar to staphylococcal scalded skin syndrome and Stevens-Johnson syndrome.
- Histopathologic evaluation of this condition shows desquamation localized to the stratum corneum without epidermal necrosis.
- Careful evaluation, including bacterial culture, is required to rule out an infectious cause.
- Early diagnosis of anasarca-induced desquamation reduces the potential for providing harmful empiric treatment, such as systemic steroids and intravenous antibiotics, especially in patients known to have comorbidities.
Study suggests keto diet increases tumor growth in ovarian cancer
A ketogenic diet fed to mice with epithelial ovarian cancer led to significantly increased tumor growth and gut microbiome alterations, according to study recently presented at the annual meeting of the Society of Gynecologic Oncology.
“The keto diet is very popular, especially among patients who believe it may treat cancer by starving tumors of the fuel they need to grow, altering the immune system, and other anticancer effects,” said study leader Mariam AlHilli, MD, of the Cleveland Clinic.
The findings are surprising because in other studies the high-fat, zero-carb ketogenic diet has demonstrated tumor-suppressing effects. It has been under study as a possible adjuvant therapy for other cancers, such as glioblastoma, colon cancer, prostate cancer, and pancreatic cancer.
“While we don’t know yet whether these findings extend to patients, the results in animals indicate that instead of being protective, the keto diet appears to promote ovarian cancer growth and progression,” Dr. AlHilli said. In the present study, tumor bearing mice were fed a keto diet consisting of 10% protein, 0% carbohydrates, and 90% fat, while the high-fat diet was 10% protein, 15% carbohydrates, and 75% fat. The control diet consisted of 10% protein, 77% carbohydrates, and 13% fat. Epithelial ovarian cancer tumor growth was monitored weekly.
Over the 6- to 10-week course of study, a 9.1-fold increase from baseline in tumor growth was observed in the keto diet-fed mice (n = 20). Among mice fed a high-fat diet (n = 20) that included some carbohydrates, tumor growth increased 2.0-fold from baseline, and among control group mice (n = 20) fed a low-fat, high carbohydrate diet, tumor growth increased 3.1-fold.
The investigators observed several hallmarks of tumor progression: tumor associated macrophages were enriched significantly, activated lymphoid cells (natural killer cells) were significantly reduced (P < .001), and M2:M1 polarization trended higher. Also, in keto diet–fed mice, gene set enrichment analysis revealed that epithelial ovarian cancer tumors had increased angiogenesis and inflammatory responses, enhanced epithelial-to-mesenchymal transition phenotype, and altered lipid metabolism. Compared with high-fat diet–fed mice, the keto-fed mice had increases in lipid catalytic activity and catabolism, as well as decreases in lipid synthesis.
“The tumor increase could be mediated by the gut microbiome or by gene alterations or by metabolite levels that influence tumor growth. It’s possible that each cancer type is different. The composition of the diet may be a factor, as well as how tumors metabolize fat and ketones,” Dr. AlHilli said.
The results need to be confirmed in preclinical animal studies and in additional models, she added.
The study was funded by a K12 Grant and internal funding from Cleveland Clinic. Dr. AlHilli declared no relevant disclosures.
A ketogenic diet fed to mice with epithelial ovarian cancer led to significantly increased tumor growth and gut microbiome alterations, according to study recently presented at the annual meeting of the Society of Gynecologic Oncology.
“The keto diet is very popular, especially among patients who believe it may treat cancer by starving tumors of the fuel they need to grow, altering the immune system, and other anticancer effects,” said study leader Mariam AlHilli, MD, of the Cleveland Clinic.
The findings are surprising because in other studies the high-fat, zero-carb ketogenic diet has demonstrated tumor-suppressing effects. It has been under study as a possible adjuvant therapy for other cancers, such as glioblastoma, colon cancer, prostate cancer, and pancreatic cancer.
“While we don’t know yet whether these findings extend to patients, the results in animals indicate that instead of being protective, the keto diet appears to promote ovarian cancer growth and progression,” Dr. AlHilli said. In the present study, tumor bearing mice were fed a keto diet consisting of 10% protein, 0% carbohydrates, and 90% fat, while the high-fat diet was 10% protein, 15% carbohydrates, and 75% fat. The control diet consisted of 10% protein, 77% carbohydrates, and 13% fat. Epithelial ovarian cancer tumor growth was monitored weekly.
Over the 6- to 10-week course of study, a 9.1-fold increase from baseline in tumor growth was observed in the keto diet-fed mice (n = 20). Among mice fed a high-fat diet (n = 20) that included some carbohydrates, tumor growth increased 2.0-fold from baseline, and among control group mice (n = 20) fed a low-fat, high carbohydrate diet, tumor growth increased 3.1-fold.
The investigators observed several hallmarks of tumor progression: tumor associated macrophages were enriched significantly, activated lymphoid cells (natural killer cells) were significantly reduced (P < .001), and M2:M1 polarization trended higher. Also, in keto diet–fed mice, gene set enrichment analysis revealed that epithelial ovarian cancer tumors had increased angiogenesis and inflammatory responses, enhanced epithelial-to-mesenchymal transition phenotype, and altered lipid metabolism. Compared with high-fat diet–fed mice, the keto-fed mice had increases in lipid catalytic activity and catabolism, as well as decreases in lipid synthesis.
“The tumor increase could be mediated by the gut microbiome or by gene alterations or by metabolite levels that influence tumor growth. It’s possible that each cancer type is different. The composition of the diet may be a factor, as well as how tumors metabolize fat and ketones,” Dr. AlHilli said.
The results need to be confirmed in preclinical animal studies and in additional models, she added.
The study was funded by a K12 Grant and internal funding from Cleveland Clinic. Dr. AlHilli declared no relevant disclosures.
A ketogenic diet fed to mice with epithelial ovarian cancer led to significantly increased tumor growth and gut microbiome alterations, according to study recently presented at the annual meeting of the Society of Gynecologic Oncology.
“The keto diet is very popular, especially among patients who believe it may treat cancer by starving tumors of the fuel they need to grow, altering the immune system, and other anticancer effects,” said study leader Mariam AlHilli, MD, of the Cleveland Clinic.
The findings are surprising because in other studies the high-fat, zero-carb ketogenic diet has demonstrated tumor-suppressing effects. It has been under study as a possible adjuvant therapy for other cancers, such as glioblastoma, colon cancer, prostate cancer, and pancreatic cancer.
“While we don’t know yet whether these findings extend to patients, the results in animals indicate that instead of being protective, the keto diet appears to promote ovarian cancer growth and progression,” Dr. AlHilli said. In the present study, tumor bearing mice were fed a keto diet consisting of 10% protein, 0% carbohydrates, and 90% fat, while the high-fat diet was 10% protein, 15% carbohydrates, and 75% fat. The control diet consisted of 10% protein, 77% carbohydrates, and 13% fat. Epithelial ovarian cancer tumor growth was monitored weekly.
Over the 6- to 10-week course of study, a 9.1-fold increase from baseline in tumor growth was observed in the keto diet-fed mice (n = 20). Among mice fed a high-fat diet (n = 20) that included some carbohydrates, tumor growth increased 2.0-fold from baseline, and among control group mice (n = 20) fed a low-fat, high carbohydrate diet, tumor growth increased 3.1-fold.
The investigators observed several hallmarks of tumor progression: tumor associated macrophages were enriched significantly, activated lymphoid cells (natural killer cells) were significantly reduced (P < .001), and M2:M1 polarization trended higher. Also, in keto diet–fed mice, gene set enrichment analysis revealed that epithelial ovarian cancer tumors had increased angiogenesis and inflammatory responses, enhanced epithelial-to-mesenchymal transition phenotype, and altered lipid metabolism. Compared with high-fat diet–fed mice, the keto-fed mice had increases in lipid catalytic activity and catabolism, as well as decreases in lipid synthesis.
“The tumor increase could be mediated by the gut microbiome or by gene alterations or by metabolite levels that influence tumor growth. It’s possible that each cancer type is different. The composition of the diet may be a factor, as well as how tumors metabolize fat and ketones,” Dr. AlHilli said.
The results need to be confirmed in preclinical animal studies and in additional models, she added.
The study was funded by a K12 Grant and internal funding from Cleveland Clinic. Dr. AlHilli declared no relevant disclosures.
FROM SGO 2022
AI model predicts ovarian cancer responses
The model, using still-frame images from pretreatment laparoscopic surgical videos, had an overall accuracy rate of 93%, according to the pilot study’s first author, Deanna Glassman, MD, an oncologic fellow at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Glassman described her research in a presentation given at the annual meeting of the Society of Gynecologic Oncology.
While the AI model successfully identified all excellent-response patients, it did classify about a third of patients with poor responses as excellent responses. The smaller number of images in the poor-response category, Dr. Glassman speculated, may explain the misclassification.
Researchers took 435 representative still-frame images from pretreatment laparoscopic surgical videos of 113 patients with pathologically proven high-grade serous ovarian cancer. Using 70% of the images to train the model, they used 10% for validation and 20% for the actual testing. They developed the AI model with images from four anatomical locations (diaphragm, omentum, peritoneum, and pelvis), training it using deep learning and neural networks to extract morphological disease patterns for correlation with either of two outcomes: excellent response or poor response. An excellent response was defined as progression-free survival of 12 months or more, and poor response as PFS of 6 months or less. In the retrospective study of images, after excluding 32 gray-zone patients, 75 patients (66%) had durable responses to therapy and 6 (5%) had poor responses.
The PFS was 19 months in the excellent-response group and 3 months in the poor-response group.
Clinicians have often observed differences in gross morphology within the single histologic diagnosis of high-grade serous ovarian cancer. The research intent was to determine if AI could detect these distinct morphological patterns in the still frame images taken at the time of laparoscopy, and correlate them with the eventual clinical outcomes. Dr. Glassman and colleagues are currently validating the model with a much larger cohort, and will look into clinical testing.
“The big-picture goal,” Dr. Glassman said in an interview, “would be to utilize the model to predict which patients would do well with traditional standard of care treatments and those who wouldn’t do well so that we can personalize the treatment plan for those patients with alternative agents and therapies.”
Once validated, the model could also be employed to identify patterns of disease in other gynecologic cancers or distinguish between viable and necrosed malignant tissue.
The study’s predominant limitation was the small sample size which is being addressed in a larger ongoing study.
Funding was provided by a T32 grant, MD Anderson Cancer Center Support Grant, MD Anderson Ovarian Cancer Moon Shot, SPORE in Ovarian Cancer, the American Cancer Society, and the Ovarian Cancer Research Alliance. Dr. Glassman declared no relevant financial relationships.
The model, using still-frame images from pretreatment laparoscopic surgical videos, had an overall accuracy rate of 93%, according to the pilot study’s first author, Deanna Glassman, MD, an oncologic fellow at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Glassman described her research in a presentation given at the annual meeting of the Society of Gynecologic Oncology.
While the AI model successfully identified all excellent-response patients, it did classify about a third of patients with poor responses as excellent responses. The smaller number of images in the poor-response category, Dr. Glassman speculated, may explain the misclassification.
Researchers took 435 representative still-frame images from pretreatment laparoscopic surgical videos of 113 patients with pathologically proven high-grade serous ovarian cancer. Using 70% of the images to train the model, they used 10% for validation and 20% for the actual testing. They developed the AI model with images from four anatomical locations (diaphragm, omentum, peritoneum, and pelvis), training it using deep learning and neural networks to extract morphological disease patterns for correlation with either of two outcomes: excellent response or poor response. An excellent response was defined as progression-free survival of 12 months or more, and poor response as PFS of 6 months or less. In the retrospective study of images, after excluding 32 gray-zone patients, 75 patients (66%) had durable responses to therapy and 6 (5%) had poor responses.
The PFS was 19 months in the excellent-response group and 3 months in the poor-response group.
Clinicians have often observed differences in gross morphology within the single histologic diagnosis of high-grade serous ovarian cancer. The research intent was to determine if AI could detect these distinct morphological patterns in the still frame images taken at the time of laparoscopy, and correlate them with the eventual clinical outcomes. Dr. Glassman and colleagues are currently validating the model with a much larger cohort, and will look into clinical testing.
“The big-picture goal,” Dr. Glassman said in an interview, “would be to utilize the model to predict which patients would do well with traditional standard of care treatments and those who wouldn’t do well so that we can personalize the treatment plan for those patients with alternative agents and therapies.”
Once validated, the model could also be employed to identify patterns of disease in other gynecologic cancers or distinguish between viable and necrosed malignant tissue.
The study’s predominant limitation was the small sample size which is being addressed in a larger ongoing study.
Funding was provided by a T32 grant, MD Anderson Cancer Center Support Grant, MD Anderson Ovarian Cancer Moon Shot, SPORE in Ovarian Cancer, the American Cancer Society, and the Ovarian Cancer Research Alliance. Dr. Glassman declared no relevant financial relationships.
The model, using still-frame images from pretreatment laparoscopic surgical videos, had an overall accuracy rate of 93%, according to the pilot study’s first author, Deanna Glassman, MD, an oncologic fellow at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Glassman described her research in a presentation given at the annual meeting of the Society of Gynecologic Oncology.
While the AI model successfully identified all excellent-response patients, it did classify about a third of patients with poor responses as excellent responses. The smaller number of images in the poor-response category, Dr. Glassman speculated, may explain the misclassification.
Researchers took 435 representative still-frame images from pretreatment laparoscopic surgical videos of 113 patients with pathologically proven high-grade serous ovarian cancer. Using 70% of the images to train the model, they used 10% for validation and 20% for the actual testing. They developed the AI model with images from four anatomical locations (diaphragm, omentum, peritoneum, and pelvis), training it using deep learning and neural networks to extract morphological disease patterns for correlation with either of two outcomes: excellent response or poor response. An excellent response was defined as progression-free survival of 12 months or more, and poor response as PFS of 6 months or less. In the retrospective study of images, after excluding 32 gray-zone patients, 75 patients (66%) had durable responses to therapy and 6 (5%) had poor responses.
The PFS was 19 months in the excellent-response group and 3 months in the poor-response group.
Clinicians have often observed differences in gross morphology within the single histologic diagnosis of high-grade serous ovarian cancer. The research intent was to determine if AI could detect these distinct morphological patterns in the still frame images taken at the time of laparoscopy, and correlate them with the eventual clinical outcomes. Dr. Glassman and colleagues are currently validating the model with a much larger cohort, and will look into clinical testing.
“The big-picture goal,” Dr. Glassman said in an interview, “would be to utilize the model to predict which patients would do well with traditional standard of care treatments and those who wouldn’t do well so that we can personalize the treatment plan for those patients with alternative agents and therapies.”
Once validated, the model could also be employed to identify patterns of disease in other gynecologic cancers or distinguish between viable and necrosed malignant tissue.
The study’s predominant limitation was the small sample size which is being addressed in a larger ongoing study.
Funding was provided by a T32 grant, MD Anderson Cancer Center Support Grant, MD Anderson Ovarian Cancer Moon Shot, SPORE in Ovarian Cancer, the American Cancer Society, and the Ovarian Cancer Research Alliance. Dr. Glassman declared no relevant financial relationships.
FROM SGO 2022