User login
Resident doctor who attempted suicide three times fights for change
In early 2020, Justin Bullock, MD, MPH, did what few, if any, resident physicians have done: He published an honest account in the New England Journal of Medicine of a would-be suicide attempt during medical training.
In the article, Dr. Bullock matter-of-factly laid out how, in 2019, intern-year night shifts contributed to a depressive episode. For Dr. Bullock, who has a bipolar disorder, sleep dysregulation can be deadly. He had a plan for completing suicide, and this wouldn’t have been his first attempt. Thanks to his history and openness about his condition, Dr. Bullock had an experienced care team that helped him get to a psychiatric hospital before anything happened. While there for around 5 days, he wrote the bulk of the NEJM article.
The article took Dr. Bullock’s impact nationwide. On Twitter and in interviews, Dr. Bullock is an unapologetic advocate for accommodations for people in medicine with mental illness. “One of the things that inspired me to speak out early on is that I feel I stand in a place of so much privilege,” Dr. Bullock told this news organization. “I often feel this sense of ... ‘you have to speak up, Justin; no one else can.’ ”
Dr. Bullock’s activism is especially noteworthy, given that he is still establishing his career. In August, while an internal medicine resident at the University of California, San Francisco, he received a lifetime teaching award from UCSF because he had received three prior teaching awards; a recognition like this is considered rare someone so early in their career. Now in his final year of residency, he actively researches medical education, advocates for mental health support, and is working to become a leading voice on related issues.
“It seems to be working,” his older sister, Jacquis Mahoney, RN, said during a visit to the UCSF campus. Instead of any awkwardness, everyone is thrilled to learn that she is Justin’s sister. “There’s a lot of pride and excitement.”
Suicide attempts during medical training
Now 28, Dr. Bullock grew up in Detroit, with his mom and two older sisters. His father was incarcerated for much of Dr. Bullock’s childhood, in part because of his own bipolar disorder not being well controlled, Dr. Bullock said.
When he was younger, Dr. Bullock was the peacekeeper in the house between his two sisters, said Ms. Mahoney: “Justin was always very delicate and kind.”
He played soccer and ran track but also loved math and science. While outwardly accumulating an impressive resume, Dr. Bullock was internally struggling. In high school, he made what he now calls an “immature” attempt at suicide after coming out as gay to his family. While Dr. Bullock said he doesn’t necessarily dwell on the discrimination he has faced as a gay, Black man, his awareness of how others perceive and treat him because of his identity increases the background stress present in his daily life.
After high school, Dr. Bullock went to MIT in Boston, where he continued running and studied chemical-biological engineering. During college, Dr. Bullock thought he was going to have to withdraw from MIT because of his depression. Thankfully, he received counseling from student services and advice from a track coach who sat him down and talked about pragmatic solutions, like medication. “That was life-changing,” said Dr. Bullock.
When trying to decide between engineering and medicine, Dr. Bullock realized he preferred contemplating medical problems to engineering ones. So he applied to medical school. Dr. Bullock eventually ended up at UCSF, where he was selected to participate in the Program in Medical Education for the Urban Underserved, a 5-year track at the college for students committed to working with underserved communities.
By the time Dr. Bullock got to medical school, he was feeling good. In consultation with his psychiatrist, he thought it worthwhile to take a break from his medications. At that time, his diagnosis was major depressive disorder and he had only had one serious depressive episode, which didn’t necessarily indicate that he would need medication long-term, he said.
Dr. Bullock loved everything about medical school. “One day when I was in my first year of med school, I called my mom and said: ‘It’s like science summer camp but every day!’” he recalled.
Despite his enthusiasm, though, he began feeling something troubling. Recognizing the symptoms of early depression, Dr. Bullock restarted his medication. But this time, the same SSRI only made things worse. He went from sleeping 8 hours to 90 minutes a night. He felt angry. One day, he went on a furious 22-mile run. Plus, within the first 6 months of moving to San Francisco, Dr. Bullock was stopped by the police three different times while riding his bike. He attributes this to his race, which has only further added to his stress. In September 2015, during his second year of medical school, Dr. Bullock attempted suicide again. This time, he was intubated in the ED and rushed to the ICU.
He was given a new diagnosis: bipolar disorder. He changed medications and lived for a time with Ms. Mahoney and his other sister, who moved from Chicago to California to be with him. “My family has helped me a lot,” he said.
Dr. Bullock was initially not sure whether he would be able to return to school after his attempted suicide. Overall, UCSF was extremely supportive, he said. That came as a relief. Medical school was a grounding force in his life, not a destabilizing one: “If I had been pushed out, it would have been really harmful to me.”
Then Dr. Bullock started residency. The sleep disruption that comes with the night shift – the resident rite of passage – triggered another episode. At first, Dr. Bullock was overly productive; his mind was active and alert after staying up all night. He worked on new research during the day instead of sleeping.
Sleep disturbance is a hallmark symptom of bipolar disorder. “Justin should never be on a 24-hour call,” said Lisa Meeks, PhD, associate professor of psychiatry and family medicine at the University of Colorado at Denver, Aurora, and a leading scholar on disability advocacy for medical trainees. When he started residency, Dr. Bullock was open with his program director about his diagnosis and sought accommodations to go to therapy each week. But he didn’t try to get out of night shifts or 24-hour calls, despite his care team urging him to do so. “I have this sense of wanting to tough it out,” he said. He also felt guilty making his peers take on his share of those challenging shifts.
In December 2019, Dr. Bullock was voluntarily hospitalized for a few days and started writing the article that would later appear in NEJM. In January, a friend and UCSF medical student completed suicide. In March, the same month his NEJM article came out, Dr. Bullock attempted suicide again. This time, he quickly recognized that he was making a mistake and called an ambulance. “For me, as far as suicide attempts go, it’s the most positive one.”
Advocating for changes in medical training
Throughout his medical training, Dr. Bullock was always open about his struggles with his peers and with the administration. He shared his suicidal thoughts at a Mental Illness Among Us event during medical school. His story resonated with peers who were surprised that Dr. Bullock, who was thriving academically, could be struggling emotionally.
During residency, he led small group discussions and gave lectures at the medical school, including a talk about his attempts to create institutional change at UCSF, such as his public fight against the college’s Fitness for Duty (FFD) assessment process. That discussion earned him an Outstanding Lecturer award. Because it was the third award he had received from the medical school, Dr. Bullock also automatically earned a lifetime teaching award. When he told his mom, a teacher herself, about the award, she joked: “Are you old enough for ‘lifetime’ anything?”
Dr. Bullock has also spoken out and actively fought against the processes within the medical community that prevent people from coming forward until it is too late. Physicians and trainees often fear that if they seek mental health treatment, they will have to disclose that treatment to a potential employer or licensing board and then be barred from practicing medicine. Because he has been open about his mental health for so long, Dr. Bullock feels that he is in a position to push back against these norms. For example, in June he coauthored another article, this time for the Journal of Hospital Medicine, describing the traumatizing FFD assessment that followed his March 2020 suicide attempt.
In that article, Dr. Bullock wrote how no mental health professional served on the UCSF Physician Well Being Committee – comprising physicians and lawyers who evaluate physician impairment or potential physician impairment – that evaluated him. Dr. Bullock was referred to an outside psychiatrist. He also describes how he was forced to release all of his psychiatric records and undergo extensive drug testing, despite having no history of substance abuse. To return to work, he had to sign a contract, agreeing to be monitored and to attend a specific kind of therapy.
While steps like these can, in the right circumstances, protect both the public and doctors-in-training in important ways, they can also “be very punitive and isolating for someone going through a mental health crisis,” said Dr. Meeks. There were also no Black physicians or lawyers on the committee evaluating Dr. Bullock. “That was really egregious, when you look back.” Dr. Meeks is a coauthor on Dr. Bullock’s JHM article and a mentor and previous student disability officer at UCSF.
Dr. Bullock raised objections to UCSF administrators about how he felt that the committee was discriminating against him because of his mental illness despite assurances from the director of his program that there have never been any performance or professionalism concerns with him. He said the administrators told him he was the first person to question the FFD process. This isn’t surprising, given that all the power in such situations usually lies with the hospital and the administrators, whereas the resident or physician is worried about losing their job and their license, said Dr. Meeks.
Dr. Bullock contends that he’s in a unique position to speak out, considering his stellar academic and work records, openness about his mental illness before a crisis, access to quality mental health care, and extensive personal network among the UCSF administration. “I know that I hold power within my institution; I spoke out because I could,” Dr. Bullock said. In addition to writing an article about his experience, Dr. Bullock shared his story with a task force appointed by the medical staff president to review the Physician Well-Being Committee and the overall FFD process. Even before Dr. Bullock shared his story with the public, the task force had already been appointed as a result of the increased concern about physician mental health during the ongoing COVID-19 pandemic, Michelle Guy, MD, clinical professor of medicine at UCSF, told this news organization.
Elizabeth Fernandez, a UCSF senior public information representative, declined to comment on Dr. Bullock’s specific experience as reported in the JHM. “As with every hospital accredited by the Joint Commission, UCSF Medical Center has a Physician Well Being Committee that provides resources for physicians who may need help with chemical dependency or mental illness,” Ms. Fernandez said.
“Our goal through this program is always, first, to provide the compassion and assistance our physicians need to address the issues they face and continue to pursue their careers. This program is entirely voluntary and is bound by federal and state laws and regulations to protect the confidentiality of its participants, while ensuring that – first and foremost – no one is harmed by the situation, including the participant.”
Overcoming stigma to change the system
All of the attention – from national media outlets such as Vox to struggling peers and others – is fulfilling, Dr. Bullock said. But it can also be overwhelming. “I have definitely been praised as ‘Black excellence,’ and that definitely has added to the pressure to keep going ... to keep pushing at times,” he said.
Ms. Mahoney added: “He’s willing to sacrifice himself in order to make a difference. He would be a sacrificial lamb” for the Black community, the gay community, or any minority community.
Despite these concerns and his past suicide attempts, colleagues feel that Dr. Bullock is in a strong place to make decisions. “I trust Justin to put the boundaries up when they are needed and to engage in a way that feels comfortable for him,” said Ms. Meeks. “He is someone who has incredible self-awareness.”
Dr. Bullock’s history isn’t just something he overcame: It’s something that makes him a better, more empathetic doctor, said Ms. Mahoney. He knows what it’s like to be hospitalized, to deal with the frustration of insurance, to navigate the complexity of the health care system as a patient, or to be facing a deep internal darkness. He “can genuinely hold that person’s hand and say: ‘I know what you’re going through and we’re going to work through this day by day,’ ” she said. “That is something he can bring that no other physician can bring.”
In his advocacy on Twitter, in lectures, and in conversations with UCSF administrators, Dr. Bullock is pushing for board licensing questions to be reformed so physicians are no longer penalized for seeking mental health treatment. He would also like residency programs to make it easier and less stigmatizing for trainees to receive accommodations for a disability or mental illness.
“They say one person can’t change a system,” said Dr. Meeks, “but I do think Justin is calling an awful lot of attention to the system and I do think there will be changes because of his advocacy.”
A version of this article first appeared on Medscape.com.
In early 2020, Justin Bullock, MD, MPH, did what few, if any, resident physicians have done: He published an honest account in the New England Journal of Medicine of a would-be suicide attempt during medical training.
In the article, Dr. Bullock matter-of-factly laid out how, in 2019, intern-year night shifts contributed to a depressive episode. For Dr. Bullock, who has a bipolar disorder, sleep dysregulation can be deadly. He had a plan for completing suicide, and this wouldn’t have been his first attempt. Thanks to his history and openness about his condition, Dr. Bullock had an experienced care team that helped him get to a psychiatric hospital before anything happened. While there for around 5 days, he wrote the bulk of the NEJM article.
The article took Dr. Bullock’s impact nationwide. On Twitter and in interviews, Dr. Bullock is an unapologetic advocate for accommodations for people in medicine with mental illness. “One of the things that inspired me to speak out early on is that I feel I stand in a place of so much privilege,” Dr. Bullock told this news organization. “I often feel this sense of ... ‘you have to speak up, Justin; no one else can.’ ”
Dr. Bullock’s activism is especially noteworthy, given that he is still establishing his career. In August, while an internal medicine resident at the University of California, San Francisco, he received a lifetime teaching award from UCSF because he had received three prior teaching awards; a recognition like this is considered rare someone so early in their career. Now in his final year of residency, he actively researches medical education, advocates for mental health support, and is working to become a leading voice on related issues.
“It seems to be working,” his older sister, Jacquis Mahoney, RN, said during a visit to the UCSF campus. Instead of any awkwardness, everyone is thrilled to learn that she is Justin’s sister. “There’s a lot of pride and excitement.”
Suicide attempts during medical training
Now 28, Dr. Bullock grew up in Detroit, with his mom and two older sisters. His father was incarcerated for much of Dr. Bullock’s childhood, in part because of his own bipolar disorder not being well controlled, Dr. Bullock said.
When he was younger, Dr. Bullock was the peacekeeper in the house between his two sisters, said Ms. Mahoney: “Justin was always very delicate and kind.”
He played soccer and ran track but also loved math and science. While outwardly accumulating an impressive resume, Dr. Bullock was internally struggling. In high school, he made what he now calls an “immature” attempt at suicide after coming out as gay to his family. While Dr. Bullock said he doesn’t necessarily dwell on the discrimination he has faced as a gay, Black man, his awareness of how others perceive and treat him because of his identity increases the background stress present in his daily life.
After high school, Dr. Bullock went to MIT in Boston, where he continued running and studied chemical-biological engineering. During college, Dr. Bullock thought he was going to have to withdraw from MIT because of his depression. Thankfully, he received counseling from student services and advice from a track coach who sat him down and talked about pragmatic solutions, like medication. “That was life-changing,” said Dr. Bullock.
When trying to decide between engineering and medicine, Dr. Bullock realized he preferred contemplating medical problems to engineering ones. So he applied to medical school. Dr. Bullock eventually ended up at UCSF, where he was selected to participate in the Program in Medical Education for the Urban Underserved, a 5-year track at the college for students committed to working with underserved communities.
By the time Dr. Bullock got to medical school, he was feeling good. In consultation with his psychiatrist, he thought it worthwhile to take a break from his medications. At that time, his diagnosis was major depressive disorder and he had only had one serious depressive episode, which didn’t necessarily indicate that he would need medication long-term, he said.
Dr. Bullock loved everything about medical school. “One day when I was in my first year of med school, I called my mom and said: ‘It’s like science summer camp but every day!’” he recalled.
Despite his enthusiasm, though, he began feeling something troubling. Recognizing the symptoms of early depression, Dr. Bullock restarted his medication. But this time, the same SSRI only made things worse. He went from sleeping 8 hours to 90 minutes a night. He felt angry. One day, he went on a furious 22-mile run. Plus, within the first 6 months of moving to San Francisco, Dr. Bullock was stopped by the police three different times while riding his bike. He attributes this to his race, which has only further added to his stress. In September 2015, during his second year of medical school, Dr. Bullock attempted suicide again. This time, he was intubated in the ED and rushed to the ICU.
He was given a new diagnosis: bipolar disorder. He changed medications and lived for a time with Ms. Mahoney and his other sister, who moved from Chicago to California to be with him. “My family has helped me a lot,” he said.
Dr. Bullock was initially not sure whether he would be able to return to school after his attempted suicide. Overall, UCSF was extremely supportive, he said. That came as a relief. Medical school was a grounding force in his life, not a destabilizing one: “If I had been pushed out, it would have been really harmful to me.”
Then Dr. Bullock started residency. The sleep disruption that comes with the night shift – the resident rite of passage – triggered another episode. At first, Dr. Bullock was overly productive; his mind was active and alert after staying up all night. He worked on new research during the day instead of sleeping.
Sleep disturbance is a hallmark symptom of bipolar disorder. “Justin should never be on a 24-hour call,” said Lisa Meeks, PhD, associate professor of psychiatry and family medicine at the University of Colorado at Denver, Aurora, and a leading scholar on disability advocacy for medical trainees. When he started residency, Dr. Bullock was open with his program director about his diagnosis and sought accommodations to go to therapy each week. But he didn’t try to get out of night shifts or 24-hour calls, despite his care team urging him to do so. “I have this sense of wanting to tough it out,” he said. He also felt guilty making his peers take on his share of those challenging shifts.
In December 2019, Dr. Bullock was voluntarily hospitalized for a few days and started writing the article that would later appear in NEJM. In January, a friend and UCSF medical student completed suicide. In March, the same month his NEJM article came out, Dr. Bullock attempted suicide again. This time, he quickly recognized that he was making a mistake and called an ambulance. “For me, as far as suicide attempts go, it’s the most positive one.”
Advocating for changes in medical training
Throughout his medical training, Dr. Bullock was always open about his struggles with his peers and with the administration. He shared his suicidal thoughts at a Mental Illness Among Us event during medical school. His story resonated with peers who were surprised that Dr. Bullock, who was thriving academically, could be struggling emotionally.
During residency, he led small group discussions and gave lectures at the medical school, including a talk about his attempts to create institutional change at UCSF, such as his public fight against the college’s Fitness for Duty (FFD) assessment process. That discussion earned him an Outstanding Lecturer award. Because it was the third award he had received from the medical school, Dr. Bullock also automatically earned a lifetime teaching award. When he told his mom, a teacher herself, about the award, she joked: “Are you old enough for ‘lifetime’ anything?”
Dr. Bullock has also spoken out and actively fought against the processes within the medical community that prevent people from coming forward until it is too late. Physicians and trainees often fear that if they seek mental health treatment, they will have to disclose that treatment to a potential employer or licensing board and then be barred from practicing medicine. Because he has been open about his mental health for so long, Dr. Bullock feels that he is in a position to push back against these norms. For example, in June he coauthored another article, this time for the Journal of Hospital Medicine, describing the traumatizing FFD assessment that followed his March 2020 suicide attempt.
In that article, Dr. Bullock wrote how no mental health professional served on the UCSF Physician Well Being Committee – comprising physicians and lawyers who evaluate physician impairment or potential physician impairment – that evaluated him. Dr. Bullock was referred to an outside psychiatrist. He also describes how he was forced to release all of his psychiatric records and undergo extensive drug testing, despite having no history of substance abuse. To return to work, he had to sign a contract, agreeing to be monitored and to attend a specific kind of therapy.
While steps like these can, in the right circumstances, protect both the public and doctors-in-training in important ways, they can also “be very punitive and isolating for someone going through a mental health crisis,” said Dr. Meeks. There were also no Black physicians or lawyers on the committee evaluating Dr. Bullock. “That was really egregious, when you look back.” Dr. Meeks is a coauthor on Dr. Bullock’s JHM article and a mentor and previous student disability officer at UCSF.
Dr. Bullock raised objections to UCSF administrators about how he felt that the committee was discriminating against him because of his mental illness despite assurances from the director of his program that there have never been any performance or professionalism concerns with him. He said the administrators told him he was the first person to question the FFD process. This isn’t surprising, given that all the power in such situations usually lies with the hospital and the administrators, whereas the resident or physician is worried about losing their job and their license, said Dr. Meeks.
Dr. Bullock contends that he’s in a unique position to speak out, considering his stellar academic and work records, openness about his mental illness before a crisis, access to quality mental health care, and extensive personal network among the UCSF administration. “I know that I hold power within my institution; I spoke out because I could,” Dr. Bullock said. In addition to writing an article about his experience, Dr. Bullock shared his story with a task force appointed by the medical staff president to review the Physician Well-Being Committee and the overall FFD process. Even before Dr. Bullock shared his story with the public, the task force had already been appointed as a result of the increased concern about physician mental health during the ongoing COVID-19 pandemic, Michelle Guy, MD, clinical professor of medicine at UCSF, told this news organization.
Elizabeth Fernandez, a UCSF senior public information representative, declined to comment on Dr. Bullock’s specific experience as reported in the JHM. “As with every hospital accredited by the Joint Commission, UCSF Medical Center has a Physician Well Being Committee that provides resources for physicians who may need help with chemical dependency or mental illness,” Ms. Fernandez said.
“Our goal through this program is always, first, to provide the compassion and assistance our physicians need to address the issues they face and continue to pursue their careers. This program is entirely voluntary and is bound by federal and state laws and regulations to protect the confidentiality of its participants, while ensuring that – first and foremost – no one is harmed by the situation, including the participant.”
Overcoming stigma to change the system
All of the attention – from national media outlets such as Vox to struggling peers and others – is fulfilling, Dr. Bullock said. But it can also be overwhelming. “I have definitely been praised as ‘Black excellence,’ and that definitely has added to the pressure to keep going ... to keep pushing at times,” he said.
Ms. Mahoney added: “He’s willing to sacrifice himself in order to make a difference. He would be a sacrificial lamb” for the Black community, the gay community, or any minority community.
Despite these concerns and his past suicide attempts, colleagues feel that Dr. Bullock is in a strong place to make decisions. “I trust Justin to put the boundaries up when they are needed and to engage in a way that feels comfortable for him,” said Ms. Meeks. “He is someone who has incredible self-awareness.”
Dr. Bullock’s history isn’t just something he overcame: It’s something that makes him a better, more empathetic doctor, said Ms. Mahoney. He knows what it’s like to be hospitalized, to deal with the frustration of insurance, to navigate the complexity of the health care system as a patient, or to be facing a deep internal darkness. He “can genuinely hold that person’s hand and say: ‘I know what you’re going through and we’re going to work through this day by day,’ ” she said. “That is something he can bring that no other physician can bring.”
In his advocacy on Twitter, in lectures, and in conversations with UCSF administrators, Dr. Bullock is pushing for board licensing questions to be reformed so physicians are no longer penalized for seeking mental health treatment. He would also like residency programs to make it easier and less stigmatizing for trainees to receive accommodations for a disability or mental illness.
“They say one person can’t change a system,” said Dr. Meeks, “but I do think Justin is calling an awful lot of attention to the system and I do think there will be changes because of his advocacy.”
A version of this article first appeared on Medscape.com.
In early 2020, Justin Bullock, MD, MPH, did what few, if any, resident physicians have done: He published an honest account in the New England Journal of Medicine of a would-be suicide attempt during medical training.
In the article, Dr. Bullock matter-of-factly laid out how, in 2019, intern-year night shifts contributed to a depressive episode. For Dr. Bullock, who has a bipolar disorder, sleep dysregulation can be deadly. He had a plan for completing suicide, and this wouldn’t have been his first attempt. Thanks to his history and openness about his condition, Dr. Bullock had an experienced care team that helped him get to a psychiatric hospital before anything happened. While there for around 5 days, he wrote the bulk of the NEJM article.
The article took Dr. Bullock’s impact nationwide. On Twitter and in interviews, Dr. Bullock is an unapologetic advocate for accommodations for people in medicine with mental illness. “One of the things that inspired me to speak out early on is that I feel I stand in a place of so much privilege,” Dr. Bullock told this news organization. “I often feel this sense of ... ‘you have to speak up, Justin; no one else can.’ ”
Dr. Bullock’s activism is especially noteworthy, given that he is still establishing his career. In August, while an internal medicine resident at the University of California, San Francisco, he received a lifetime teaching award from UCSF because he had received three prior teaching awards; a recognition like this is considered rare someone so early in their career. Now in his final year of residency, he actively researches medical education, advocates for mental health support, and is working to become a leading voice on related issues.
“It seems to be working,” his older sister, Jacquis Mahoney, RN, said during a visit to the UCSF campus. Instead of any awkwardness, everyone is thrilled to learn that she is Justin’s sister. “There’s a lot of pride and excitement.”
Suicide attempts during medical training
Now 28, Dr. Bullock grew up in Detroit, with his mom and two older sisters. His father was incarcerated for much of Dr. Bullock’s childhood, in part because of his own bipolar disorder not being well controlled, Dr. Bullock said.
When he was younger, Dr. Bullock was the peacekeeper in the house between his two sisters, said Ms. Mahoney: “Justin was always very delicate and kind.”
He played soccer and ran track but also loved math and science. While outwardly accumulating an impressive resume, Dr. Bullock was internally struggling. In high school, he made what he now calls an “immature” attempt at suicide after coming out as gay to his family. While Dr. Bullock said he doesn’t necessarily dwell on the discrimination he has faced as a gay, Black man, his awareness of how others perceive and treat him because of his identity increases the background stress present in his daily life.
After high school, Dr. Bullock went to MIT in Boston, where he continued running and studied chemical-biological engineering. During college, Dr. Bullock thought he was going to have to withdraw from MIT because of his depression. Thankfully, he received counseling from student services and advice from a track coach who sat him down and talked about pragmatic solutions, like medication. “That was life-changing,” said Dr. Bullock.
When trying to decide between engineering and medicine, Dr. Bullock realized he preferred contemplating medical problems to engineering ones. So he applied to medical school. Dr. Bullock eventually ended up at UCSF, where he was selected to participate in the Program in Medical Education for the Urban Underserved, a 5-year track at the college for students committed to working with underserved communities.
By the time Dr. Bullock got to medical school, he was feeling good. In consultation with his psychiatrist, he thought it worthwhile to take a break from his medications. At that time, his diagnosis was major depressive disorder and he had only had one serious depressive episode, which didn’t necessarily indicate that he would need medication long-term, he said.
Dr. Bullock loved everything about medical school. “One day when I was in my first year of med school, I called my mom and said: ‘It’s like science summer camp but every day!’” he recalled.
Despite his enthusiasm, though, he began feeling something troubling. Recognizing the symptoms of early depression, Dr. Bullock restarted his medication. But this time, the same SSRI only made things worse. He went from sleeping 8 hours to 90 minutes a night. He felt angry. One day, he went on a furious 22-mile run. Plus, within the first 6 months of moving to San Francisco, Dr. Bullock was stopped by the police three different times while riding his bike. He attributes this to his race, which has only further added to his stress. In September 2015, during his second year of medical school, Dr. Bullock attempted suicide again. This time, he was intubated in the ED and rushed to the ICU.
He was given a new diagnosis: bipolar disorder. He changed medications and lived for a time with Ms. Mahoney and his other sister, who moved from Chicago to California to be with him. “My family has helped me a lot,” he said.
Dr. Bullock was initially not sure whether he would be able to return to school after his attempted suicide. Overall, UCSF was extremely supportive, he said. That came as a relief. Medical school was a grounding force in his life, not a destabilizing one: “If I had been pushed out, it would have been really harmful to me.”
Then Dr. Bullock started residency. The sleep disruption that comes with the night shift – the resident rite of passage – triggered another episode. At first, Dr. Bullock was overly productive; his mind was active and alert after staying up all night. He worked on new research during the day instead of sleeping.
Sleep disturbance is a hallmark symptom of bipolar disorder. “Justin should never be on a 24-hour call,” said Lisa Meeks, PhD, associate professor of psychiatry and family medicine at the University of Colorado at Denver, Aurora, and a leading scholar on disability advocacy for medical trainees. When he started residency, Dr. Bullock was open with his program director about his diagnosis and sought accommodations to go to therapy each week. But he didn’t try to get out of night shifts or 24-hour calls, despite his care team urging him to do so. “I have this sense of wanting to tough it out,” he said. He also felt guilty making his peers take on his share of those challenging shifts.
In December 2019, Dr. Bullock was voluntarily hospitalized for a few days and started writing the article that would later appear in NEJM. In January, a friend and UCSF medical student completed suicide. In March, the same month his NEJM article came out, Dr. Bullock attempted suicide again. This time, he quickly recognized that he was making a mistake and called an ambulance. “For me, as far as suicide attempts go, it’s the most positive one.”
Advocating for changes in medical training
Throughout his medical training, Dr. Bullock was always open about his struggles with his peers and with the administration. He shared his suicidal thoughts at a Mental Illness Among Us event during medical school. His story resonated with peers who were surprised that Dr. Bullock, who was thriving academically, could be struggling emotionally.
During residency, he led small group discussions and gave lectures at the medical school, including a talk about his attempts to create institutional change at UCSF, such as his public fight against the college’s Fitness for Duty (FFD) assessment process. That discussion earned him an Outstanding Lecturer award. Because it was the third award he had received from the medical school, Dr. Bullock also automatically earned a lifetime teaching award. When he told his mom, a teacher herself, about the award, she joked: “Are you old enough for ‘lifetime’ anything?”
Dr. Bullock has also spoken out and actively fought against the processes within the medical community that prevent people from coming forward until it is too late. Physicians and trainees often fear that if they seek mental health treatment, they will have to disclose that treatment to a potential employer or licensing board and then be barred from practicing medicine. Because he has been open about his mental health for so long, Dr. Bullock feels that he is in a position to push back against these norms. For example, in June he coauthored another article, this time for the Journal of Hospital Medicine, describing the traumatizing FFD assessment that followed his March 2020 suicide attempt.
In that article, Dr. Bullock wrote how no mental health professional served on the UCSF Physician Well Being Committee – comprising physicians and lawyers who evaluate physician impairment or potential physician impairment – that evaluated him. Dr. Bullock was referred to an outside psychiatrist. He also describes how he was forced to release all of his psychiatric records and undergo extensive drug testing, despite having no history of substance abuse. To return to work, he had to sign a contract, agreeing to be monitored and to attend a specific kind of therapy.
While steps like these can, in the right circumstances, protect both the public and doctors-in-training in important ways, they can also “be very punitive and isolating for someone going through a mental health crisis,” said Dr. Meeks. There were also no Black physicians or lawyers on the committee evaluating Dr. Bullock. “That was really egregious, when you look back.” Dr. Meeks is a coauthor on Dr. Bullock’s JHM article and a mentor and previous student disability officer at UCSF.
Dr. Bullock raised objections to UCSF administrators about how he felt that the committee was discriminating against him because of his mental illness despite assurances from the director of his program that there have never been any performance or professionalism concerns with him. He said the administrators told him he was the first person to question the FFD process. This isn’t surprising, given that all the power in such situations usually lies with the hospital and the administrators, whereas the resident or physician is worried about losing their job and their license, said Dr. Meeks.
Dr. Bullock contends that he’s in a unique position to speak out, considering his stellar academic and work records, openness about his mental illness before a crisis, access to quality mental health care, and extensive personal network among the UCSF administration. “I know that I hold power within my institution; I spoke out because I could,” Dr. Bullock said. In addition to writing an article about his experience, Dr. Bullock shared his story with a task force appointed by the medical staff president to review the Physician Well-Being Committee and the overall FFD process. Even before Dr. Bullock shared his story with the public, the task force had already been appointed as a result of the increased concern about physician mental health during the ongoing COVID-19 pandemic, Michelle Guy, MD, clinical professor of medicine at UCSF, told this news organization.
Elizabeth Fernandez, a UCSF senior public information representative, declined to comment on Dr. Bullock’s specific experience as reported in the JHM. “As with every hospital accredited by the Joint Commission, UCSF Medical Center has a Physician Well Being Committee that provides resources for physicians who may need help with chemical dependency or mental illness,” Ms. Fernandez said.
“Our goal through this program is always, first, to provide the compassion and assistance our physicians need to address the issues they face and continue to pursue their careers. This program is entirely voluntary and is bound by federal and state laws and regulations to protect the confidentiality of its participants, while ensuring that – first and foremost – no one is harmed by the situation, including the participant.”
Overcoming stigma to change the system
All of the attention – from national media outlets such as Vox to struggling peers and others – is fulfilling, Dr. Bullock said. But it can also be overwhelming. “I have definitely been praised as ‘Black excellence,’ and that definitely has added to the pressure to keep going ... to keep pushing at times,” he said.
Ms. Mahoney added: “He’s willing to sacrifice himself in order to make a difference. He would be a sacrificial lamb” for the Black community, the gay community, or any minority community.
Despite these concerns and his past suicide attempts, colleagues feel that Dr. Bullock is in a strong place to make decisions. “I trust Justin to put the boundaries up when they are needed and to engage in a way that feels comfortable for him,” said Ms. Meeks. “He is someone who has incredible self-awareness.”
Dr. Bullock’s history isn’t just something he overcame: It’s something that makes him a better, more empathetic doctor, said Ms. Mahoney. He knows what it’s like to be hospitalized, to deal with the frustration of insurance, to navigate the complexity of the health care system as a patient, or to be facing a deep internal darkness. He “can genuinely hold that person’s hand and say: ‘I know what you’re going through and we’re going to work through this day by day,’ ” she said. “That is something he can bring that no other physician can bring.”
In his advocacy on Twitter, in lectures, and in conversations with UCSF administrators, Dr. Bullock is pushing for board licensing questions to be reformed so physicians are no longer penalized for seeking mental health treatment. He would also like residency programs to make it easier and less stigmatizing for trainees to receive accommodations for a disability or mental illness.
“They say one person can’t change a system,” said Dr. Meeks, “but I do think Justin is calling an awful lot of attention to the system and I do think there will be changes because of his advocacy.”
A version of this article first appeared on Medscape.com.
Feds launch COVID-19 worker vaccine mandates
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
How to screen for prediabetes and type 2 diabetes in an ObGyn practice
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
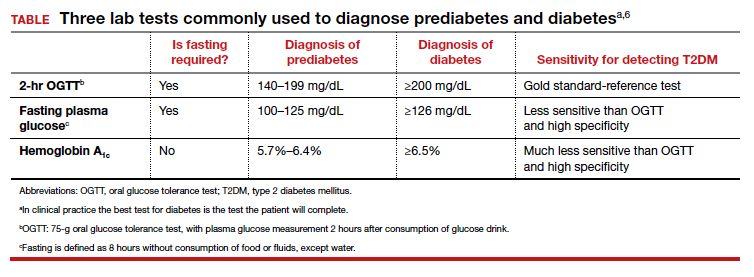
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5
Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5

Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5

Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
Is vaginal laser therapy more efficacious in improving vaginal menopausal symptoms compared with sham therapy?
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
Remote and in-home prenatal care: Safe, inclusive, and here to stay

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.
Infectious disease pop quiz: Clinical challenges for the ObGyn
In this question-and-answer article (the first in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
1. What are the best tests for the diagnosis of congenital cytomegalovirus (CMV) infection?
When congenital CMV is suspected, if the patient is at least 15 weeks’ gestation, an amniocentesis should be performed to test for CMV DNA in the amniotic fluid using polymerase chain reaction (PCR) methodology. If the initial test is negative, amniocentesis should be repeated in approximately 4 weeks. Coincident with amniocentesis, a detailed ultrasound examination should be performed to search for findings suggestive of fetal injury, such as growth restriction, microcephaly, periventricular calcifications, hepatosplenomegaly, echogenic bowel, and serous effusions in the pleural space or abdomen.
2. Which major organisms cause urinary tract infections (UTIs) in women?
The most common causative organism is Escherichia coli, which is responsible for approximately 70% of all UTIs. Klebsiella pneumoniae and Proteus species are the 2 other aerobic gram-negative bacilli that are common uropathogens. In addition, 3 gram-positive cocci are important: enterococci, Staphylococcus saprophyticus, and group B streptococcus.
3. What are the major complications of pyelonephritis in pregnancy?
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
4. What is the most ominous manifestation of congenital parvovirus infection, and what is the cause of this abnormality?
Hydrops fetalis is the most ominous complication of congenital parvovirus infection. The virus crosses the placenta and attacks red cell progenitor cells, resulting in an aplastic anemia. In addition, the virus may cause myocarditis that, in turn, may result in cardiac failure in the fetus.
5. What are the major manifestations of congenital rubella syndrome?
Rubella is one of the most highly teratogenic of all the viral infections, particularly when maternal infection occurs in the first trimester. Manifestations of congenital rubella include hearing deficits, cataracts, glaucoma, microcephaly, mental retardation, cardiac malformations such as patent ductus arteriosus and pulmonic stenosis, and growth restriction.
6. Which vaccines are contraindicated in pregnancy?
Live virus vaccines should not be used in pregnancy because of the possibility of teratogenic effects. Live agents include the measles, mumps, and rubella (MMR) vaccine; live influenza vaccine (FluMist); oral polio vaccine; BCG (bacille Calmette-Guerin) vaccine; yellow fever vaccine; and smallpox vaccine.
7. What is the most appropriate treatment for trichomonas infection in pregnancy?
Trichomonas infection should be treated with oral metronidazole 500 mg twice daily for 7 days. Metronidazole also can be given as a single oral 2-g dose. This treatment is not quite as effective as the multidose regimen, but it may be appropriate for patients who are not likely to be adherent with the longer course of treatment.
Resistance to metronidazole is rare; in such instances, oral tinidazole 2 g in a single dose may be effective.
8. For uncomplicated gonorrhea in a pregnant woman, what is the most appropriate treatment?
The current recommendation from the Centers for Disease Control and Prevention for treatment of uncomplicated gonorrhea is a single 500-mg intramuscular dose of ceftriaxone. For the patient who is opposed to an intramuscular injection, an alternative treatment is cefixime 800 mg orally. With either of these regimens, if chlamydia infection cannot be excluded, the pregnant patient also should receive azithromycin 1,000 mg orally in a single dose. In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days should be used to cover for concurrent chlamydia infection.
In a patient with an allergy to β-lactam antibiotics, an alternative regimen for treatment of uncomplicated gonorrhea is intramuscular gentamicin 240 mg plus a single 2,000-mg dose of oral azithromycin. (St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.) ●
1. Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
2. Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
In this question-and-answer article (the first in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
1. What are the best tests for the diagnosis of congenital cytomegalovirus (CMV) infection?
When congenital CMV is suspected, if the patient is at least 15 weeks’ gestation, an amniocentesis should be performed to test for CMV DNA in the amniotic fluid using polymerase chain reaction (PCR) methodology. If the initial test is negative, amniocentesis should be repeated in approximately 4 weeks. Coincident with amniocentesis, a detailed ultrasound examination should be performed to search for findings suggestive of fetal injury, such as growth restriction, microcephaly, periventricular calcifications, hepatosplenomegaly, echogenic bowel, and serous effusions in the pleural space or abdomen.
2. Which major organisms cause urinary tract infections (UTIs) in women?
The most common causative organism is Escherichia coli, which is responsible for approximately 70% of all UTIs. Klebsiella pneumoniae and Proteus species are the 2 other aerobic gram-negative bacilli that are common uropathogens. In addition, 3 gram-positive cocci are important: enterococci, Staphylococcus saprophyticus, and group B streptococcus.
3. What are the major complications of pyelonephritis in pregnancy?
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
4. What is the most ominous manifestation of congenital parvovirus infection, and what is the cause of this abnormality?
Hydrops fetalis is the most ominous complication of congenital parvovirus infection. The virus crosses the placenta and attacks red cell progenitor cells, resulting in an aplastic anemia. In addition, the virus may cause myocarditis that, in turn, may result in cardiac failure in the fetus.
5. What are the major manifestations of congenital rubella syndrome?
Rubella is one of the most highly teratogenic of all the viral infections, particularly when maternal infection occurs in the first trimester. Manifestations of congenital rubella include hearing deficits, cataracts, glaucoma, microcephaly, mental retardation, cardiac malformations such as patent ductus arteriosus and pulmonic stenosis, and growth restriction.
6. Which vaccines are contraindicated in pregnancy?
Live virus vaccines should not be used in pregnancy because of the possibility of teratogenic effects. Live agents include the measles, mumps, and rubella (MMR) vaccine; live influenza vaccine (FluMist); oral polio vaccine; BCG (bacille Calmette-Guerin) vaccine; yellow fever vaccine; and smallpox vaccine.
7. What is the most appropriate treatment for trichomonas infection in pregnancy?
Trichomonas infection should be treated with oral metronidazole 500 mg twice daily for 7 days. Metronidazole also can be given as a single oral 2-g dose. This treatment is not quite as effective as the multidose regimen, but it may be appropriate for patients who are not likely to be adherent with the longer course of treatment.
Resistance to metronidazole is rare; in such instances, oral tinidazole 2 g in a single dose may be effective.
8. For uncomplicated gonorrhea in a pregnant woman, what is the most appropriate treatment?
The current recommendation from the Centers for Disease Control and Prevention for treatment of uncomplicated gonorrhea is a single 500-mg intramuscular dose of ceftriaxone. For the patient who is opposed to an intramuscular injection, an alternative treatment is cefixime 800 mg orally. With either of these regimens, if chlamydia infection cannot be excluded, the pregnant patient also should receive azithromycin 1,000 mg orally in a single dose. In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days should be used to cover for concurrent chlamydia infection.
In a patient with an allergy to β-lactam antibiotics, an alternative regimen for treatment of uncomplicated gonorrhea is intramuscular gentamicin 240 mg plus a single 2,000-mg dose of oral azithromycin. (St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.) ●
In this question-and-answer article (the first in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
1. What are the best tests for the diagnosis of congenital cytomegalovirus (CMV) infection?
When congenital CMV is suspected, if the patient is at least 15 weeks’ gestation, an amniocentesis should be performed to test for CMV DNA in the amniotic fluid using polymerase chain reaction (PCR) methodology. If the initial test is negative, amniocentesis should be repeated in approximately 4 weeks. Coincident with amniocentesis, a detailed ultrasound examination should be performed to search for findings suggestive of fetal injury, such as growth restriction, microcephaly, periventricular calcifications, hepatosplenomegaly, echogenic bowel, and serous effusions in the pleural space or abdomen.
2. Which major organisms cause urinary tract infections (UTIs) in women?
The most common causative organism is Escherichia coli, which is responsible for approximately 70% of all UTIs. Klebsiella pneumoniae and Proteus species are the 2 other aerobic gram-negative bacilli that are common uropathogens. In addition, 3 gram-positive cocci are important: enterococci, Staphylococcus saprophyticus, and group B streptococcus.
3. What are the major complications of pyelonephritis in pregnancy?
Pyelonephritis is an important cause of preterm labor, sepsis, and adult respiratory distress syndrome. Most cases of pyelonephritis develop as a result of an untreated or inadequately treated lower urinary tract infection.
4. What is the most ominous manifestation of congenital parvovirus infection, and what is the cause of this abnormality?
Hydrops fetalis is the most ominous complication of congenital parvovirus infection. The virus crosses the placenta and attacks red cell progenitor cells, resulting in an aplastic anemia. In addition, the virus may cause myocarditis that, in turn, may result in cardiac failure in the fetus.
5. What are the major manifestations of congenital rubella syndrome?
Rubella is one of the most highly teratogenic of all the viral infections, particularly when maternal infection occurs in the first trimester. Manifestations of congenital rubella include hearing deficits, cataracts, glaucoma, microcephaly, mental retardation, cardiac malformations such as patent ductus arteriosus and pulmonic stenosis, and growth restriction.
6. Which vaccines are contraindicated in pregnancy?
Live virus vaccines should not be used in pregnancy because of the possibility of teratogenic effects. Live agents include the measles, mumps, and rubella (MMR) vaccine; live influenza vaccine (FluMist); oral polio vaccine; BCG (bacille Calmette-Guerin) vaccine; yellow fever vaccine; and smallpox vaccine.
7. What is the most appropriate treatment for trichomonas infection in pregnancy?
Trichomonas infection should be treated with oral metronidazole 500 mg twice daily for 7 days. Metronidazole also can be given as a single oral 2-g dose. This treatment is not quite as effective as the multidose regimen, but it may be appropriate for patients who are not likely to be adherent with the longer course of treatment.
Resistance to metronidazole is rare; in such instances, oral tinidazole 2 g in a single dose may be effective.
8. For uncomplicated gonorrhea in a pregnant woman, what is the most appropriate treatment?
The current recommendation from the Centers for Disease Control and Prevention for treatment of uncomplicated gonorrhea is a single 500-mg intramuscular dose of ceftriaxone. For the patient who is opposed to an intramuscular injection, an alternative treatment is cefixime 800 mg orally. With either of these regimens, if chlamydia infection cannot be excluded, the pregnant patient also should receive azithromycin 1,000 mg orally in a single dose. In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days should be used to cover for concurrent chlamydia infection.
In a patient with an allergy to β-lactam antibiotics, an alternative regimen for treatment of uncomplicated gonorrhea is intramuscular gentamicin 240 mg plus a single 2,000-mg dose of oral azithromycin. (St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.) ●
1. Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
2. Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
1. Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
2. Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Time to retire race- and ethnicity-based carrier screening
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.
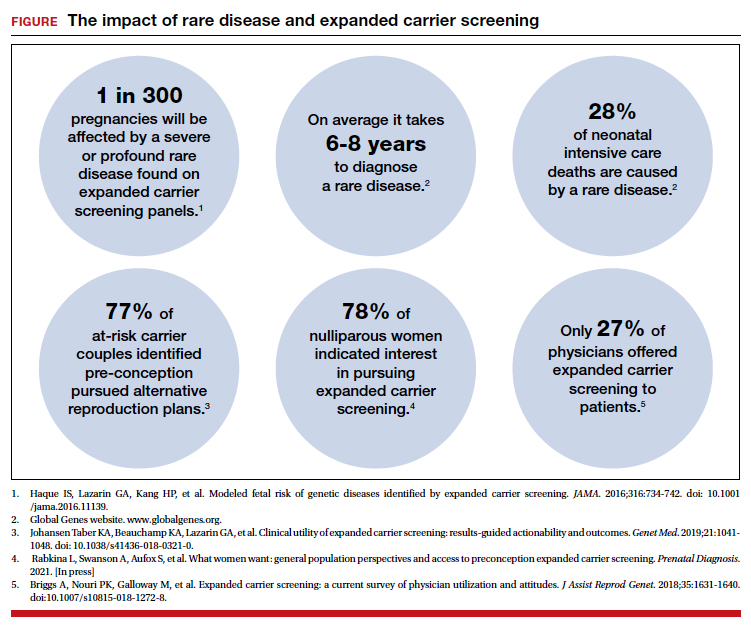
Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
4 new short-acting hormonal contraceptives offer enhancement over earlier options
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).
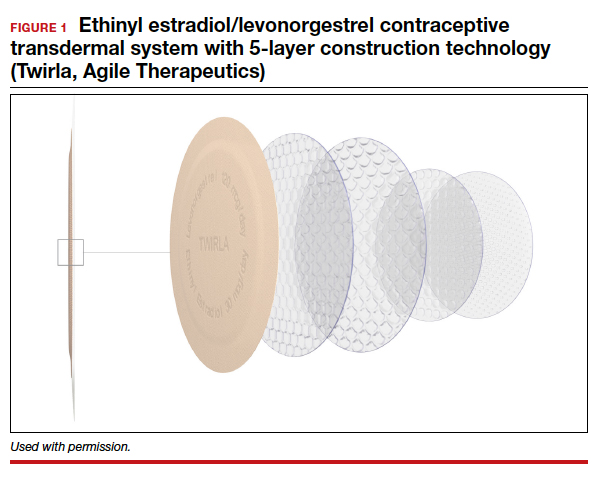
Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4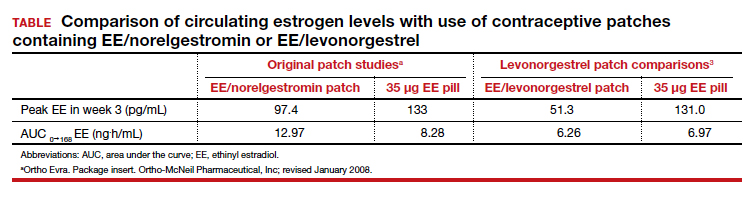
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6
Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).

Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6

Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
Short-term hormonal contraceptives remain the most popular class of reversible contraceptives in the United States, despite the availability of longer-acting methods. Oral contraceptives (OCs), contraceptive patches, and contraceptive vaginal rings are extensively used not only because these methods are easy to initiate but also because their ongoing use remains under the control of the woman herself and also provides her with a wide range of important noncontraceptive benefits.
Despite the more than 60 years of innovation that have made hormonal contraceptives safer, more tolerable, and more convenient, there has been room for improvement. Over the last few years, 4 new hormonal methods have been introduced, and each addresses different limitations and problems associated with the existing, often generic, products.
Compared with the traditional norethindrone pill (Micronor and generics), a new drospirenone progestin-only pill (POP) increases ovulation suppression, offers an improved cyclical bleeding profile, and relaxes the tight missed-pill rules that are usually associated with POPs.
In contrast with the older norelgestromin patch (Evra, Xulane), a new contraceptive transdermal patch significantly decreases total estrogen exposure and pairs its estrogen with levonorgestrel, the progestin associated with the lowest venous thromboembolism (VTE) risk in combined hormonal pills.
While existing combination OCs are formulated with the potent estrogen ethinyl estradiol (EE), a new combination pill, formulated with estetrol (E4) and drospirenone, introduces the first new estrogen (estetrol) used in a contraceptive in more than 50 years. Estetrol, a native estrogen, has selective tissue activity with minimal hepatic and breast impacts. Combined with drospirenone, this formulation offers women good contraceptive efficacy and bleeding patterns.
A new contraceptive vaginal ring introduces a new long-acting, specific progestin (segesterone acetate) and pairs it with low-dose EE. These hormones are packaged in a soft vaginal ring that provides up to 13 cycles of contraceptive protection (3 weeks in/1 week out) with one ring, greatly increasing convenience for women.
Each of these new products represents important incremental improvement over existing options.
Continue to: 1. The drospirenone-only OC...
1. The drospirenone-only OC
The new POP with drospirenone 4 mg (Slynd), which received US Food and Drug Administration (FDA) approval in 2019, is packaged in a 24/4 formulation (24 hormonally active tablets followed by 4 inactive tablets). This formulation results in more predictable bleeding than does the 0.35-mg norethindrone POP, which contains 28 hormonally active tablets in each pack. In the US clinical trials of drospirenone 4 mg, scheduled bleeding decreased from 81% in cycle 1 to 20% in cycle 13. Unscheduled spotting and bleeding decreased from 61% to 40% in the same timeframe. Notably, this bleeding pattern was well tolerated; only 0.4% of trial participants discontinued this drospirenone POP due to problems with irregular bleeding or amenorrhea.
In contrast to the continuous norethindrone POP, which is not sufficiently dosed to consistently suppress ovulation, the 4-mg daily dose of drospirenone in this new POP is higher than the 3 mg used in commonly prescribed combination OCs that contain EE and drospirenone. This results in a POP that has more consistent ovulation suppression. Because this drospirenone POP is appropriately dosed and based on a longer-acting progestin, it is more forgiving of inconsistent pill taking. Accordingly, the missed-pill rules for this pill are the same as with combination estrogen-progestin OCs.1 The package labeling cites a first-year failure rate of 4%, but this includes unconfirmed pregnancies. The Pearl Index from the North American trials, based on confirmed pregnancies in nonbreastfeeding women, was 2.9.2
The package labeling for this drospirenone POP includes few contraindications. Conditions that preclude use include the US Medical Eligibility Criteria for contraception Category 4 condition (breast cancer in the last 5 years), renal impairment, and adrenal insufficiency. Other standard contraindications are listed in the prescribing information. Serum potassium levels should be checked (one time only) in the first cycle only for women who chronically use medications that could cause hyperkalemia, such as nonsteroidal anti-inflammatory drugs.
Given the ovulation suppression associated with this drospirenone POP, the safety of a progestin-only method, and the persistent popularity of OC pills, this pill should greatly increase the use of POPs beyond their traditional niche of postpartum and breastfeeding women. The advent of the drospirenone POP means that clinicians now have better options for women who have contraindications to estrogen and desire to control their own contraceptive use. It would be a logical consideration for over-the-counter accessibility.
2. Transdermal patch with ethinyl estradiol/levonorgestrel
The new EE/levonorgestrel transdermal contraceptive patch (Twirla) is soft and flexible, about the same size as other contraceptive patches, and contains EE 2.3 mg/levonorgestrel 2.6 mg. It provides total estrogen exposure that is similar to that of OCs with EE 30 µg and distinctly lower than estrogen levels seen with the original norelgestromin-containing patch or its 2 subsequent generic versions.3 This EE/levonorgestrel patch uses a new 5-layer drug delivery system that focuses the steroids for absorption beneath the patch; there is no peripheral spread of drug around the patch (FIGURE 1).

Transdermal patches offer the convenience of once-a-week dosing. One patch is used each week for 3 consecutive weeks followed by a patch-free week. Patches can be worn on the abdomen, buttock, or trunk (except breasts). Patches should not be placed consecutively on the same site; after a week’s rest, however, the first site can be reused. All transdermal contraceptive products are indicated for use only by women with a body mass index (BMI) <30 kg/m2.4
While no head-to-head trials have compared this new lower-dose patch with older patches, each patch was compared against a standardized pill, so meaningful comparisons can be made.
In each case, the circulating estrogen levels associated with use of the EE/levonorgestrel patch were considerably lower than those of the comparator pill, while the older norelgestromin patch consistently delivered higher total estrogen levels than its 35-µg comparator pill (TABLE).3 Along these lines, no VTE events occurred in women in the clinical trial of the new patch among women with a BMI <30 kg/m2.4
Women with a BMI <25 kg/m2 experienced lower Pearl Index (PI) pregnancy rates (3.5%) compared with women with a BMI between 25 and 30 kg/m2 (5.7%), according to clinical trial data cited in the package labeling. All the modern PI criteria were used to calculate these failure rates. Cycles in which no coitus occurred were excluded. Similarly, cycles in which another contraceptive method (for example, condoms) was added (even once) were excluded. Frequent pregnancy testing was done in the study centers and by the women at home. Bleeding patterns were well accepted; only 2.2% of study participants exited the study early due to menstrual disorders of any kind. Similarly, 3.1% of women discontinued use because of application site disorders. Women should be advised to press down on the patch edges after emerging from water exposure. Replacement patches are rapidly available from the manufacturer should permanent complete patch detachment occur.
Larger-scale phase 4 trials will be conducted to study the impact of this lower-dose patch on VTE rates.
Continue to: 3. A 1-year contraceptive vaginal ring...
3. A 1-year contraceptive vaginal ring
The need to obtain new supplies every month or every 3 months contributes to high rates of contraceptive failure and unintended pregnancy among women using short-acting hormonal contraceptives (pills, patches, and vaginal rings).5 A woman-controlled contraceptive that would provide 1 year of protection against unintended pregnancy represents a step forward. A contraceptive vaginal ring (CVR) that releases the novel progestin segesterone acetate and EE provides woman-controlled contraception for up to 1 year. This CVR (Annovera) received FDA approval in 2018 and has been marketed in the United States since 2020.
The segesterone acetate/EE CVR is a soft, flexible ring that is opaque white in color and fabricated from nonbiodegradable silicone (FIGURE 2). The outside diameter is 5.6 cm, compared with the 5.4-cm outer diameter of the etonogestrel/EE vaginal ring (NuvaRing). The segesterone acetate/EE CVR has 2 channels: one releases segesterone acetate only and the other releases segesterone acetate and EE. In contrast with the etonogestrel/EE CVR, the segesterone acetate/EE CVR does not need to be refrigerated when stored.6

Segesterone is a 19-nor-progesterone derivative that binds in a highly selective fashion to progesterone receptors, and it is potent in suppressing ovulation. During use of the segesterone acetate/EE CVR, mean levels of EE are incrementally higher than those observed with use of the etonogestrel/EE CVR.
Two 13-cycle (1 year) phase 3 clinical trials conducted from 2006 to 2009 enrolled 2,308 women aged 18 to 40 years, including 2,265 women aged 18 to 35 (the age group the FDA considers for efficacy analysis). Trial participants placed the ring vaginally on cycle days 2 to 5 and were asked to keep the ring in place for 21 days, then to remove the CVR for 7 days, during which scheduled bleeding was anticipated. For sexual intercourse, rings could be removed, depending on patient/couple preference, for up to 2 hours.
In the combined trials, the PI was 2.98 per 100 woman-years, a pregnancy rate comparable to those seen in other recent trials of combination estrogen-progestin contraceptives. The incidence of contraceptive failure did not increase over time during the 1-year trials, indicating that contraceptive efficacy of the segesterone acetate/EE was maintained during 1 year of use. While the pregnancy rate was lower in participants who did not report any instances of CVR removal during the 21-day periods of use, the rate was substantially higher among those who reported prolonged episodes of CVR removal.
In the 2 trials, bleeding patterns were similar to those observed with other combination estrogen-progestin contraceptives. Fewer than 2% of trial participants discontinued the trial early due to what they considered unacceptable bleeding.
More than one-half of trial participants reported at least 1 episode of complete or partial CVR expulsion. Most expulsions occurred in the first cycle, suggesting a learning curve with CVR use. Fewer than 2% of participants discontinued trial participation due to expulsions.
Almost 90% of participants reported that they were “highly satisfied” or “satisfied” with the CVR. Although more than two-thirds of participants reported that they never felt the ring during intercourse, if a couple did report feeling the ring during sex, the likelihood of dissatisfaction with the CVR doubled. In addition, feeling the CVR at other times was strongly associated with dissatisfaction. Because a deeply positioned CVR is less likely to be felt by users, these observations underscore the importance of counseling users to place the ring into the upper vagina. Of note, neither prior ring use nor tampon use was associated with CVR satisfaction.
One other important counseling point regarding CVR use relates to the discoloration of the ring that occurs over time. The initially white ring tends to become dark brown during the 1-year usage period. Although this discoloration does not indicate hygiene problems, women who are not advised about this in advance may be put off by the color change.
Four nonfatal VTE events occurred, all in the US trial sites. The overall VTE incidence was higher than expected, particularly among participants with a BMI of 29 kg/m2 or higher. After this association was noted, participants with a BMI >29 kg/m2 were discontinued from the trials. The package labeling for the segesterone acetate/EE CVR states that “Limited data are available in females with a BMI >29.0 kg/m2 because this subpopulation was excluded from the clinical trials after VTEs were reported.”6
A 1-year CVR raises the possibility that users could use their rings in an experimental extended fashion to reduce the frequency of withdrawal bleeding or continuously so as to eliminate withdrawal bleeding. In a randomly chosen sample of CVRs that had been used in the 13-cycle clinical trials, residual steroids in the CVRs were assessed. Sixty percent of segesterone acetate and 80% of EE remained. Using these observations as well as pharmacokinetic data collected from phase 3 trial participants, predicted segesterone acetate levels after 1 year of hypothetical continuous use appear to be sufficient to provide effective contraception.7 These observations suggest that performing clinical trials of extended as well as continuous segesterone acetate/EE CVR use is warranted.
Continue to: 4. An OC with a novel estrogen...
4. An OC with a novel estrogen
Even as use of intrauterine devices and contraceptive implants continues to grow, OCs remain the reversible contraceptive most used by US women. While OCs have been widely studied and represent a safe method of contraception for most reproductive-age women, combination estrogen-progestin OCs are well recognized to increase the risk of VTE. Although the primary role of the progestin component of combination OCs is to suppress ovulation, estrogen is included in combination OCs to stimulate endometrial proliferation, thereby causing predictable bleeding. EE, the potent synthetic estrogen used in the great majority of current OC formulations, induces hepatic production of prothrombotic proteins while inhibiting synthesis of antithrombotic proteins. While the lower EE doses (10–35 µg) in today’s OC formulations are associated with a lower VTE risk than older OCs that contained higher doses of estrogen, VTE continues to represent the principal health risk associated with use of combination OCs. Accordingly, development of a combination OC that has less impact on risk of VTE would be appealing.
In April 2021, the FDA approved an OC formulation that combines 15 mg of the novel estrogen estetrol with 3 mg of drospirenone (Nextstellis). This dose of drospirenone is the same as that used in commonly prescribed EE/drospirenone OC formulations. Also known as E4, estetrol is a natural estrogen synthesized by the fetal liver. Plant-derived E4 is used in this new OC.
Depending on the tissue, E4 acts differently than other estrogens. Similar to other estrogens, E4 acts as an agonist on the nuclear receptor to produce beneficial effects in bone, vaginal mucosa, and heart.8 Unlike other estrogens, E4 inhibits proliferation of mammary gland cells and has a neutral impact on the liver.9
In contrast with EE, E4 is not inhibited by the liver’s P450 enzymes; accordingly, the risk of drug-drug interactions is reduced. Because E4 is primarily excreted through the urine and not through the biliary tract, the risk of gallstone formation may be lower than with an EE OC. Likewise, E4 has substantially less impact on triglycerides, which are increased with EE. Finally, because of E4’s reduced effect on the liver, the impact on clotting parameters is less than that observed with an OC formulated with EE.10 This latter observation raises the possibility that VTE risk is lower with the E4/drospirenone OC than an OC formulated with EE.
A 13-cycle phase 3 trial of the E4/drospirenone OC conducted in the United States and Canada enrolled 1,864 women aged 16 to 50 years, including 1,674 who were aged 16 to 35 years.11 Among women in this latter age group, the PI was 2.65 per 100 woman-years. Bleeding/cycle control patterns were similar to those observed in recent trials of other combination contraceptives. Likewise, the proportion of trial participants who discontinued the study due to adverse effects was similar to or lower than that noted in recent trials of other combination contraceptives. Of particular note, no cases of VTE were noted among trial participants of any BMI, a finding which contrasts with recent phase 3 trials of other combination contraceptives. The result of this pivotal trial suggests that the theoretic advantages of E4 when used in a combination OC formulation may translate into a safer, effective, and well-tolerated contraceptive.
Refinements in hormonal contraceptives continue
The 4 new short-acting hormonal contraceptives we reviewed represent enhancements on existing pills, patches, and rings. We hope that, financially, women will have access to these innovative methods and, in particular, that third-party payers will facilitate women’s access to these enhanced short-acting hormonal contraceptives. ●
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Archer DF, Stanczyk FZ, Rubin A, et al. Ethinyl estradiol and levonorgestrel pharmacokinetics with a low-dose transdermal contraceptive delivery system, AG200-15: a randomized controlled trial. Contraception. 2012;85:595-601.
- Nelson AL, Kaunitz AM, Kroll R, et al; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1-6; discussion 412.e6-7.
- Nelson AL. Comprehensive overview of the recently FDAapproved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: a new year-long, patient controlled, reversible birth control method. Expert Rev Clin Pharmacol. 2019;12:953-963.
- Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception. 2021;104:229-234.
- Mawet M, Maillard C, Klipping C, et al. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463-475.
- Gérard C, Blacher S, Communal L, et al. Estetrol is a weak estrogen antagonizing estradiol-dependent mammary gland proliferation. J Endocrinol. 2015;224:85-95.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetroldrospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
2021 Update on minimally invasive gynecologic surgery

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5
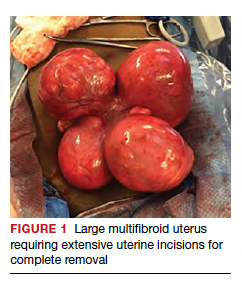
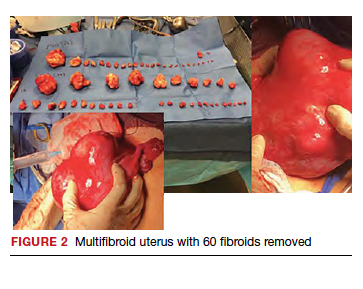
For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.
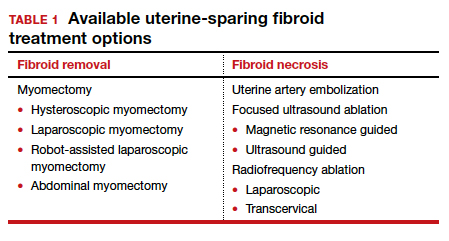
Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.
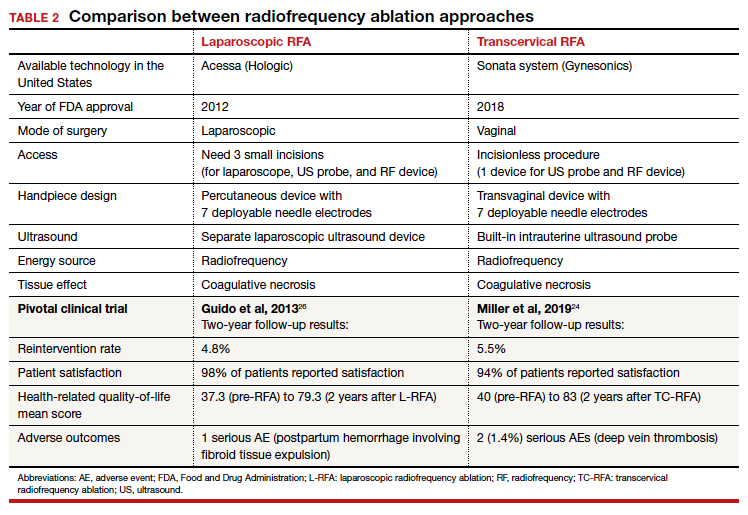
Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
An MD’s nightmare began with reporting her manic episode to the medical board
Susan Haney, MD, a board-certified emergency physician in Coos Bay, Ore., was 2 years into her career when she had her first manic episode, likely a side effect of the steroid prednisone, which she had been prescribed for an asthma flare-up. Her boss at Bay Area Hospital told her that if she wanted to return to work, she would need to have written clearance from the medical board.
In retrospect, Dr. Haney says, “I don’t think they had any idea of what they would set in motion.”
Dr. Haney says the Oregon Medical Board posted her name and the nondisciplinary action on their website and in their newsletter. Her local newspaper read it and ran a story about her. “They effectively announced my mental illness to the general public despite my objections,” she says.
During the next decade, she had two more manic episodes, and more board investigations and actions followed. Despite being cleared for work each time, Dr. Haney says the board actions decimated her career in emergency medicine and her income, which is about half of what she would have earned by now. She is frustrated, sad, and angry about what happened but considers herself lucky to be practicing medicine in urgent care.
Being investigated is scary
After her first manic episode in 2006, Dr. Haney contacted the board’s medical director, a retired general surgeon, who told her the only way the board would authorize her return to work was if she agreed to open a board investigation.
She gave them the green light because she thought she had nothing to fear – she was cooperating fully and wasn’t impaired. Now Dr. Haney says she was naive. “The board is not your friend,” she says.
Dr. Haney was also anxious to return to work. She worked in a seven-person emergency department, and two colleagues were on maternity leave or medical leave.
“My colleagues kept calling asking me when I was going to return to work, and I kept saying, ‘I don’t know because the board won’t tell me,’ “ she says.
She was also feeling a lot of financial pressure. She was 2 years out of residency, owed $100,000 in student loans, and had just bought a house.
“I was really scared – I didn’t know how long this would last or if they would let me return to work. Early on, I even got a fitness for duty evaluation from the state’s consulting psychiatrist, who cleared me for work, and the board still wouldn’t let me return. They told me I had to go through their bureaucracy and a board meeting, which didn’t make sense to me.”
Dr. Haney consented to give the board’s investigative staff access to her medical records because she feared that if she challenged them, they would suspend or revoke her license immediately.
After investigating her for 4 months, the board cleared Dr. Haney to return to work at Bay Area Hospital. She agreed to the board’s “corrective action” terms: She would continue to receive psychiatric care, maintain a physician-patient relationship with a primary care physician, and enroll in the Health Physicians Program (HPP) for substance abuse monitoring.
Dr. Haney suspects that the board investigation damaged her reputation at work. “Before this, my work evaluations were consistently excellent. Afterwards, they were all adequate. I don’t think that was a coincidence.”
Worst time of her life
Five years later, after taking prednisone for another asthma flare-up, Dr. Haney had a more severe manic episode and was hospitalized.
The consulting psychiatrist who evaluated her reported her case to the medical board, stating she had bipolar disorder, was mentally incompetent, and shouldn’t be practicing medicine. The board opened a second investigation of her in 2012, which lasted 4 months.
Dr. Haney had quit her job at Bay Area Hospital in 2011 because she was pregnant and was planning to take a year off to care for the baby at home.
“That was the worst time of my life. I lost the baby at 4 months, I wasn’t working, and now I was under investigation by the board again,” she says.
The board issued an “interim stipulated order” that required that she be monitored regularly for mental illness and substance abuse by the Health Professionals Services Program (HPSP) for 2 years. “The board accused me of abusing prednisone, which I wasn’t. I was using it as prescribed and medically indicated,” she said.
The board order was reported to the National Practitioner Databank and is now permanently in her record. Although the board cleared her to work, she could not find a permanent job in a hospital emergency department.
“The repeated ‘nondisciplinary’ public board orders have had the same net impact on my career as if I had been disciplined for killing or harming my patients. For all intents and purposes, people treat it as a disciplinary action for the rest of your career,” she said.
To keep afloat financially, she found locum tenens work in local emergency departments until 2019.
Mental health toll
Dr. Haney feels that the stress of repeated board investigations has affected her mental health. “Both times this happened, it made my mental health worse, made the mania worse, and subsequent depression worse.”
Particularly distressing to her was the fact that the administrative staff who investigated her were attorneys and persons in law enforcement, rather than medical professionals with mental health training.
“I was required to disclose intimate personal details of my psychological and psychiatric history to anybody at the board who requested them. These investigators were asking me about my childhood history. That was traumatic and none of their business!”
Dr. Haney had quietly managed episodes of major depression since she was in her early 20s with the help of a psychiatrist. Her third episode of mania, which occurred in 2014, triggered a more severe depression, which she says deepened when she learned that the HPSP had notified the board about her manic symptoms and that she would not be released from the 2-year monitoring contract. When the board notified her 2 weeks later that they were opening another investigation, Dr. Haney says she had an emotional crisis, attempted suicide, and was briefly hospitalized. Several weeks later, she decided to take a mood stabilizer, which she continues to take.
The board’s 2015 corrective action agreement required Dr. Haney to practice medicine only in settings that the board’s medical director preapproved and to obtain a preapproved monitoring health care provider who would send quarterly reports to the medical director. Dr. Haney says the “nondisciplinary” action agreement was also reported to the National Practitioner Data Bank.
She also agreed to ongoing monitoring by the HPSP for mental illness and substance abuse, which involved random drug testing. When she didn’t call in one day in 2019 and missed a scheduled test, the board opened another investigation on her that lasted 7 months until July 2020. Dr. Haney said this was despite three subsequent negative tests.
Dr. Haney believes that the “open investigation” doomed a job offer from a hospital emergency department in the Virgin Islands. “I had passed all the required credentialing and explained previous board orders. They pulled the rug from under me 1 week before I was supposed to move there,” says Dr. Haney.
Her license was inactivated again because she hadn’t practiced medicine for a year, which she says was a new board policy. Although Dr. Haney says the medical director reactivated her license after talking with her, “By the time I was able to apply emergency medicine jobs, no one was interested in me anymore.”
Financial toll
Dr. Haney started her medical career when she was 42 as a second career. She says the board investigations and actions have resulted in a significant loss of work and income. “I have only worked 14 of the past 17 years as a doctor. I live cheaply because I never know how much longer my career will last,” says Dr. Haney.
The ordeal has devastated her finances. She has shelled out at least $200,000 in legal fees – she hired an attorney in 2007 and filed a lawsuit against the board in Oregon district court alleging that members had violated several of her rights. The district judge sided with the state medical board, and it was upheld on appeal in 2012, referring to state laws that gave the board absolute immunity from civil lawsuits. “I had no legal recourse to contest their decisions, no matter how injurious or unjust,” says Dr. Haney.
She has also shelled out at least $100,000 to be evaluated and monitored by the health physician program (now HPSP) for several years. Physicians who agree to be monitored by these health programs have to pay their fees. The board finally agreed last July to end her HPSP participation.
Dr. Haney also filed a complaint in 2007 with the federal Department of Health & Human Services Office for Civil Rights, alleging that the board violated her civil rights under the Americans with Disabilities Act. She says that her lawsuit and the OCR investigation of the board enabled her to withdraw from the HPP in good standing in 2008..
What would she have done differently?
She regrets not hiring an attorney earlier because “most likely the board action would not have been made public. It snowballed after that -- any mistake I made in my career was viewed in the lens of potential impairment.”
She also regrets telling her employer about the nature of her illness and reporting it to the board. A psychiatrist she saw later shared advice he gives to other patients who want to remain anonymous: get help but go out of town, use a false name, and pay cash.
“I wish I had that advice when all this started. That was the best way to protect my career,” says Dr. Haney.
Protecting the public?
The Oregon Medical Board declined to comment on Dr. Haney’s experience because investigations are confidential, but the executive director, Nicole Krishnaswami, JD, answered questions in an email about how the current board operates.
She says the board has 11 medical professionals and employs a medical director and expert consultants in specialty-specific fields. MDs with mental health training are involved in investigating/reviewing cases involving doctors with mental illnesses.
“State medical boards have a responsibility to protect and inform the public. State laws further require state agencies to provide access and transparency regarding the board’s official actions. If the board receives a complaint that a licensee is impaired and thus unable to safely practice, the board has a responsibility to investigate and ensure the licensee is practicing medicine safely,” Ms. Krishnaswami said.
The HPSP is the monitoring program established by state law to provide oversight in order to ensure that licensees are not practicing while impaired. HPSP is separate from the board and the board adopted a statement outlining its perspective on the program in support of doctors with substance abuse and mental health disorder.
The board also founded the Oregon Wellness Program, which provides free, confidential counseling to all Oregon-licensed physicians and physician assistants.
Stigma continues
Dr. Haney feels there is huge stigma associated with mental illness in the medical profession. “If I had cancer twice, I wouldn’t have been put in this position and would be at the peak of my career,” she says.
Nearly half of the 862 emergency medicine physicians surveyed last October said they were reluctant to seek mental health treatment. The reasons included fear of professional repercussions and stigma in the workplace. Several physicians said they were concerned about potentially having to report the treatment on medical license applications in the future, according to a survey by the American College of Emergency Physicians.
In addition, 26% of the more than 12,000 physicians who responded to a Medscape survey last year said they didn’t want to risk disclosure (20%) or that they distrusted mental health professionals (6%).
Another physician fights back
Steven Miles, MD, an award-winning professor emeritus of medicine and bioethics at the Center for Bioethics at the University of Minnesota, in Minneapolis, understands their reluctance. In 1996, he disclosed on his license renewal application that he had recently been diagnosed with a mainly depressive type of bipolar disorder and was in treatment. He had already told his employer, who was supportive.
That set off a 14-month investigation of him by the Minnesota Board of Medical Practice. Dr. Miles and his psychiatrist refused to release his confidential records to a panel of physicians, most of whom had no expertise in mental health care. He also filed a federal claim that the board’s requests violated the ADA, and he won the case.
“Had the board given me evidence of impaired ability to practice with ordinary skill and safety, I would have cooperated. Instead, they proposed a course of action, which would have degraded the privacy of my relationship with my psychiatrist and arguably increased the barrier to getting proper care and the risk of impairment,” he said.
The board kept renewing his license, and Dr. Miles continued to work full time. “I was empowered and protected by my stature in the field at the time my mental illness was diagnosed. Early-career physicians do not yet have that protection and should be very careful of disclosing, given the still widespread stigma of mental illnesses,” he said.
His advocacy led to changes
Dr. Miles went public to mobilize support for his ADA claim. He wrote editorials that were published in JAMA and Minnesota Medicine that refer to the American Psychiatric Association’s 1984 position paper, which says that the mandatory disclosure of the physician’s confidential medical record is without merit. Dr. Miles adds that major newspapers ran stories based on his editorials.
The board backed down after Dr. Miles won his ADA case, and it met with him. “I said this is not good stewardship of the medical profession; you are injuring doctors by keeping them from psychiatric care, which is out of line with the medical view of the treatability of depression and that needs to change,” he says.
Dr. Miles says he won a victory because his practice continued. “I also won a victory in the way the board was handling these questions, which was an opening salvo in a process that continues to this day.”
The original form asked whether he had ever been diagnosed with or treated for manic depression, schizophrenia, compulsive gambling, or other psychiatric conditions.
The revised form asks, “Do you have a physical or mental condition that would affect your ability, with or without reasonable accommodation, to provide appropriate care to patients and otherwise perform the essential functions of a practitioner in your area of practice without posing a health or safety risk to your patients? If yes, what accommodations would help you provide appropriate care to patients and perform other essential functions?”
Dr. Miles says that the final wording wasn’t ideal and that it was confusing to physicians. He says this prompted additional changes in wording by the board. Starting in January, applicants will be asked, “Do you currently have any condition that is not being appropriately treated that is likely to impair or adversely affect your ability to practice medicine with reasonable skill and safety in a competent, ethical, and professional manner?” the medical board’s executive director, Ruth M. Martinez, said in an email.
When asked whether the board still investigates physicians who reveal mental illnesses on licensing applications, Ms. Martinez responded, “All disclosures are evaluated to assure that the practitioner is qualified and safe to practice.”
This article was updated 11/4/21.
A version of this article first appeared on Medscape.com.
Susan Haney, MD, a board-certified emergency physician in Coos Bay, Ore., was 2 years into her career when she had her first manic episode, likely a side effect of the steroid prednisone, which she had been prescribed for an asthma flare-up. Her boss at Bay Area Hospital told her that if she wanted to return to work, she would need to have written clearance from the medical board.
In retrospect, Dr. Haney says, “I don’t think they had any idea of what they would set in motion.”
Dr. Haney says the Oregon Medical Board posted her name and the nondisciplinary action on their website and in their newsletter. Her local newspaper read it and ran a story about her. “They effectively announced my mental illness to the general public despite my objections,” she says.
During the next decade, she had two more manic episodes, and more board investigations and actions followed. Despite being cleared for work each time, Dr. Haney says the board actions decimated her career in emergency medicine and her income, which is about half of what she would have earned by now. She is frustrated, sad, and angry about what happened but considers herself lucky to be practicing medicine in urgent care.
Being investigated is scary
After her first manic episode in 2006, Dr. Haney contacted the board’s medical director, a retired general surgeon, who told her the only way the board would authorize her return to work was if she agreed to open a board investigation.
She gave them the green light because she thought she had nothing to fear – she was cooperating fully and wasn’t impaired. Now Dr. Haney says she was naive. “The board is not your friend,” she says.
Dr. Haney was also anxious to return to work. She worked in a seven-person emergency department, and two colleagues were on maternity leave or medical leave.
“My colleagues kept calling asking me when I was going to return to work, and I kept saying, ‘I don’t know because the board won’t tell me,’ “ she says.
She was also feeling a lot of financial pressure. She was 2 years out of residency, owed $100,000 in student loans, and had just bought a house.
“I was really scared – I didn’t know how long this would last or if they would let me return to work. Early on, I even got a fitness for duty evaluation from the state’s consulting psychiatrist, who cleared me for work, and the board still wouldn’t let me return. They told me I had to go through their bureaucracy and a board meeting, which didn’t make sense to me.”
Dr. Haney consented to give the board’s investigative staff access to her medical records because she feared that if she challenged them, they would suspend or revoke her license immediately.
After investigating her for 4 months, the board cleared Dr. Haney to return to work at Bay Area Hospital. She agreed to the board’s “corrective action” terms: She would continue to receive psychiatric care, maintain a physician-patient relationship with a primary care physician, and enroll in the Health Physicians Program (HPP) for substance abuse monitoring.
Dr. Haney suspects that the board investigation damaged her reputation at work. “Before this, my work evaluations were consistently excellent. Afterwards, they were all adequate. I don’t think that was a coincidence.”
Worst time of her life
Five years later, after taking prednisone for another asthma flare-up, Dr. Haney had a more severe manic episode and was hospitalized.
The consulting psychiatrist who evaluated her reported her case to the medical board, stating she had bipolar disorder, was mentally incompetent, and shouldn’t be practicing medicine. The board opened a second investigation of her in 2012, which lasted 4 months.
Dr. Haney had quit her job at Bay Area Hospital in 2011 because she was pregnant and was planning to take a year off to care for the baby at home.
“That was the worst time of my life. I lost the baby at 4 months, I wasn’t working, and now I was under investigation by the board again,” she says.
The board issued an “interim stipulated order” that required that she be monitored regularly for mental illness and substance abuse by the Health Professionals Services Program (HPSP) for 2 years. “The board accused me of abusing prednisone, which I wasn’t. I was using it as prescribed and medically indicated,” she said.
The board order was reported to the National Practitioner Databank and is now permanently in her record. Although the board cleared her to work, she could not find a permanent job in a hospital emergency department.
“The repeated ‘nondisciplinary’ public board orders have had the same net impact on my career as if I had been disciplined for killing or harming my patients. For all intents and purposes, people treat it as a disciplinary action for the rest of your career,” she said.
To keep afloat financially, she found locum tenens work in local emergency departments until 2019.
Mental health toll
Dr. Haney feels that the stress of repeated board investigations has affected her mental health. “Both times this happened, it made my mental health worse, made the mania worse, and subsequent depression worse.”
Particularly distressing to her was the fact that the administrative staff who investigated her were attorneys and persons in law enforcement, rather than medical professionals with mental health training.
“I was required to disclose intimate personal details of my psychological and psychiatric history to anybody at the board who requested them. These investigators were asking me about my childhood history. That was traumatic and none of their business!”
Dr. Haney had quietly managed episodes of major depression since she was in her early 20s with the help of a psychiatrist. Her third episode of mania, which occurred in 2014, triggered a more severe depression, which she says deepened when she learned that the HPSP had notified the board about her manic symptoms and that she would not be released from the 2-year monitoring contract. When the board notified her 2 weeks later that they were opening another investigation, Dr. Haney says she had an emotional crisis, attempted suicide, and was briefly hospitalized. Several weeks later, she decided to take a mood stabilizer, which she continues to take.
The board’s 2015 corrective action agreement required Dr. Haney to practice medicine only in settings that the board’s medical director preapproved and to obtain a preapproved monitoring health care provider who would send quarterly reports to the medical director. Dr. Haney says the “nondisciplinary” action agreement was also reported to the National Practitioner Data Bank.
She also agreed to ongoing monitoring by the HPSP for mental illness and substance abuse, which involved random drug testing. When she didn’t call in one day in 2019 and missed a scheduled test, the board opened another investigation on her that lasted 7 months until July 2020. Dr. Haney said this was despite three subsequent negative tests.
Dr. Haney believes that the “open investigation” doomed a job offer from a hospital emergency department in the Virgin Islands. “I had passed all the required credentialing and explained previous board orders. They pulled the rug from under me 1 week before I was supposed to move there,” says Dr. Haney.
Her license was inactivated again because she hadn’t practiced medicine for a year, which she says was a new board policy. Although Dr. Haney says the medical director reactivated her license after talking with her, “By the time I was able to apply emergency medicine jobs, no one was interested in me anymore.”
Financial toll
Dr. Haney started her medical career when she was 42 as a second career. She says the board investigations and actions have resulted in a significant loss of work and income. “I have only worked 14 of the past 17 years as a doctor. I live cheaply because I never know how much longer my career will last,” says Dr. Haney.
The ordeal has devastated her finances. She has shelled out at least $200,000 in legal fees – she hired an attorney in 2007 and filed a lawsuit against the board in Oregon district court alleging that members had violated several of her rights. The district judge sided with the state medical board, and it was upheld on appeal in 2012, referring to state laws that gave the board absolute immunity from civil lawsuits. “I had no legal recourse to contest their decisions, no matter how injurious or unjust,” says Dr. Haney.
She has also shelled out at least $100,000 to be evaluated and monitored by the health physician program (now HPSP) for several years. Physicians who agree to be monitored by these health programs have to pay their fees. The board finally agreed last July to end her HPSP participation.
Dr. Haney also filed a complaint in 2007 with the federal Department of Health & Human Services Office for Civil Rights, alleging that the board violated her civil rights under the Americans with Disabilities Act. She says that her lawsuit and the OCR investigation of the board enabled her to withdraw from the HPP in good standing in 2008..
What would she have done differently?
She regrets not hiring an attorney earlier because “most likely the board action would not have been made public. It snowballed after that -- any mistake I made in my career was viewed in the lens of potential impairment.”
She also regrets telling her employer about the nature of her illness and reporting it to the board. A psychiatrist she saw later shared advice he gives to other patients who want to remain anonymous: get help but go out of town, use a false name, and pay cash.
“I wish I had that advice when all this started. That was the best way to protect my career,” says Dr. Haney.
Protecting the public?
The Oregon Medical Board declined to comment on Dr. Haney’s experience because investigations are confidential, but the executive director, Nicole Krishnaswami, JD, answered questions in an email about how the current board operates.
She says the board has 11 medical professionals and employs a medical director and expert consultants in specialty-specific fields. MDs with mental health training are involved in investigating/reviewing cases involving doctors with mental illnesses.
“State medical boards have a responsibility to protect and inform the public. State laws further require state agencies to provide access and transparency regarding the board’s official actions. If the board receives a complaint that a licensee is impaired and thus unable to safely practice, the board has a responsibility to investigate and ensure the licensee is practicing medicine safely,” Ms. Krishnaswami said.
The HPSP is the monitoring program established by state law to provide oversight in order to ensure that licensees are not practicing while impaired. HPSP is separate from the board and the board adopted a statement outlining its perspective on the program in support of doctors with substance abuse and mental health disorder.
The board also founded the Oregon Wellness Program, which provides free, confidential counseling to all Oregon-licensed physicians and physician assistants.
Stigma continues
Dr. Haney feels there is huge stigma associated with mental illness in the medical profession. “If I had cancer twice, I wouldn’t have been put in this position and would be at the peak of my career,” she says.
Nearly half of the 862 emergency medicine physicians surveyed last October said they were reluctant to seek mental health treatment. The reasons included fear of professional repercussions and stigma in the workplace. Several physicians said they were concerned about potentially having to report the treatment on medical license applications in the future, according to a survey by the American College of Emergency Physicians.
In addition, 26% of the more than 12,000 physicians who responded to a Medscape survey last year said they didn’t want to risk disclosure (20%) or that they distrusted mental health professionals (6%).
Another physician fights back
Steven Miles, MD, an award-winning professor emeritus of medicine and bioethics at the Center for Bioethics at the University of Minnesota, in Minneapolis, understands their reluctance. In 1996, he disclosed on his license renewal application that he had recently been diagnosed with a mainly depressive type of bipolar disorder and was in treatment. He had already told his employer, who was supportive.
That set off a 14-month investigation of him by the Minnesota Board of Medical Practice. Dr. Miles and his psychiatrist refused to release his confidential records to a panel of physicians, most of whom had no expertise in mental health care. He also filed a federal claim that the board’s requests violated the ADA, and he won the case.
“Had the board given me evidence of impaired ability to practice with ordinary skill and safety, I would have cooperated. Instead, they proposed a course of action, which would have degraded the privacy of my relationship with my psychiatrist and arguably increased the barrier to getting proper care and the risk of impairment,” he said.
The board kept renewing his license, and Dr. Miles continued to work full time. “I was empowered and protected by my stature in the field at the time my mental illness was diagnosed. Early-career physicians do not yet have that protection and should be very careful of disclosing, given the still widespread stigma of mental illnesses,” he said.
His advocacy led to changes
Dr. Miles went public to mobilize support for his ADA claim. He wrote editorials that were published in JAMA and Minnesota Medicine that refer to the American Psychiatric Association’s 1984 position paper, which says that the mandatory disclosure of the physician’s confidential medical record is without merit. Dr. Miles adds that major newspapers ran stories based on his editorials.
The board backed down after Dr. Miles won his ADA case, and it met with him. “I said this is not good stewardship of the medical profession; you are injuring doctors by keeping them from psychiatric care, which is out of line with the medical view of the treatability of depression and that needs to change,” he says.
Dr. Miles says he won a victory because his practice continued. “I also won a victory in the way the board was handling these questions, which was an opening salvo in a process that continues to this day.”
The original form asked whether he had ever been diagnosed with or treated for manic depression, schizophrenia, compulsive gambling, or other psychiatric conditions.
The revised form asks, “Do you have a physical or mental condition that would affect your ability, with or without reasonable accommodation, to provide appropriate care to patients and otherwise perform the essential functions of a practitioner in your area of practice without posing a health or safety risk to your patients? If yes, what accommodations would help you provide appropriate care to patients and perform other essential functions?”
Dr. Miles says that the final wording wasn’t ideal and that it was confusing to physicians. He says this prompted additional changes in wording by the board. Starting in January, applicants will be asked, “Do you currently have any condition that is not being appropriately treated that is likely to impair or adversely affect your ability to practice medicine with reasonable skill and safety in a competent, ethical, and professional manner?” the medical board’s executive director, Ruth M. Martinez, said in an email.
When asked whether the board still investigates physicians who reveal mental illnesses on licensing applications, Ms. Martinez responded, “All disclosures are evaluated to assure that the practitioner is qualified and safe to practice.”
This article was updated 11/4/21.
A version of this article first appeared on Medscape.com.
Susan Haney, MD, a board-certified emergency physician in Coos Bay, Ore., was 2 years into her career when she had her first manic episode, likely a side effect of the steroid prednisone, which she had been prescribed for an asthma flare-up. Her boss at Bay Area Hospital told her that if she wanted to return to work, she would need to have written clearance from the medical board.
In retrospect, Dr. Haney says, “I don’t think they had any idea of what they would set in motion.”
Dr. Haney says the Oregon Medical Board posted her name and the nondisciplinary action on their website and in their newsletter. Her local newspaper read it and ran a story about her. “They effectively announced my mental illness to the general public despite my objections,” she says.
During the next decade, she had two more manic episodes, and more board investigations and actions followed. Despite being cleared for work each time, Dr. Haney says the board actions decimated her career in emergency medicine and her income, which is about half of what she would have earned by now. She is frustrated, sad, and angry about what happened but considers herself lucky to be practicing medicine in urgent care.
Being investigated is scary
After her first manic episode in 2006, Dr. Haney contacted the board’s medical director, a retired general surgeon, who told her the only way the board would authorize her return to work was if she agreed to open a board investigation.
She gave them the green light because she thought she had nothing to fear – she was cooperating fully and wasn’t impaired. Now Dr. Haney says she was naive. “The board is not your friend,” she says.
Dr. Haney was also anxious to return to work. She worked in a seven-person emergency department, and two colleagues were on maternity leave or medical leave.
“My colleagues kept calling asking me when I was going to return to work, and I kept saying, ‘I don’t know because the board won’t tell me,’ “ she says.
She was also feeling a lot of financial pressure. She was 2 years out of residency, owed $100,000 in student loans, and had just bought a house.
“I was really scared – I didn’t know how long this would last or if they would let me return to work. Early on, I even got a fitness for duty evaluation from the state’s consulting psychiatrist, who cleared me for work, and the board still wouldn’t let me return. They told me I had to go through their bureaucracy and a board meeting, which didn’t make sense to me.”
Dr. Haney consented to give the board’s investigative staff access to her medical records because she feared that if she challenged them, they would suspend or revoke her license immediately.
After investigating her for 4 months, the board cleared Dr. Haney to return to work at Bay Area Hospital. She agreed to the board’s “corrective action” terms: She would continue to receive psychiatric care, maintain a physician-patient relationship with a primary care physician, and enroll in the Health Physicians Program (HPP) for substance abuse monitoring.
Dr. Haney suspects that the board investigation damaged her reputation at work. “Before this, my work evaluations were consistently excellent. Afterwards, they were all adequate. I don’t think that was a coincidence.”
Worst time of her life
Five years later, after taking prednisone for another asthma flare-up, Dr. Haney had a more severe manic episode and was hospitalized.
The consulting psychiatrist who evaluated her reported her case to the medical board, stating she had bipolar disorder, was mentally incompetent, and shouldn’t be practicing medicine. The board opened a second investigation of her in 2012, which lasted 4 months.
Dr. Haney had quit her job at Bay Area Hospital in 2011 because she was pregnant and was planning to take a year off to care for the baby at home.
“That was the worst time of my life. I lost the baby at 4 months, I wasn’t working, and now I was under investigation by the board again,” she says.
The board issued an “interim stipulated order” that required that she be monitored regularly for mental illness and substance abuse by the Health Professionals Services Program (HPSP) for 2 years. “The board accused me of abusing prednisone, which I wasn’t. I was using it as prescribed and medically indicated,” she said.
The board order was reported to the National Practitioner Databank and is now permanently in her record. Although the board cleared her to work, she could not find a permanent job in a hospital emergency department.
“The repeated ‘nondisciplinary’ public board orders have had the same net impact on my career as if I had been disciplined for killing or harming my patients. For all intents and purposes, people treat it as a disciplinary action for the rest of your career,” she said.
To keep afloat financially, she found locum tenens work in local emergency departments until 2019.
Mental health toll
Dr. Haney feels that the stress of repeated board investigations has affected her mental health. “Both times this happened, it made my mental health worse, made the mania worse, and subsequent depression worse.”
Particularly distressing to her was the fact that the administrative staff who investigated her were attorneys and persons in law enforcement, rather than medical professionals with mental health training.
“I was required to disclose intimate personal details of my psychological and psychiatric history to anybody at the board who requested them. These investigators were asking me about my childhood history. That was traumatic and none of their business!”
Dr. Haney had quietly managed episodes of major depression since she was in her early 20s with the help of a psychiatrist. Her third episode of mania, which occurred in 2014, triggered a more severe depression, which she says deepened when she learned that the HPSP had notified the board about her manic symptoms and that she would not be released from the 2-year monitoring contract. When the board notified her 2 weeks later that they were opening another investigation, Dr. Haney says she had an emotional crisis, attempted suicide, and was briefly hospitalized. Several weeks later, she decided to take a mood stabilizer, which she continues to take.
The board’s 2015 corrective action agreement required Dr. Haney to practice medicine only in settings that the board’s medical director preapproved and to obtain a preapproved monitoring health care provider who would send quarterly reports to the medical director. Dr. Haney says the “nondisciplinary” action agreement was also reported to the National Practitioner Data Bank.
She also agreed to ongoing monitoring by the HPSP for mental illness and substance abuse, which involved random drug testing. When she didn’t call in one day in 2019 and missed a scheduled test, the board opened another investigation on her that lasted 7 months until July 2020. Dr. Haney said this was despite three subsequent negative tests.
Dr. Haney believes that the “open investigation” doomed a job offer from a hospital emergency department in the Virgin Islands. “I had passed all the required credentialing and explained previous board orders. They pulled the rug from under me 1 week before I was supposed to move there,” says Dr. Haney.
Her license was inactivated again because she hadn’t practiced medicine for a year, which she says was a new board policy. Although Dr. Haney says the medical director reactivated her license after talking with her, “By the time I was able to apply emergency medicine jobs, no one was interested in me anymore.”
Financial toll
Dr. Haney started her medical career when she was 42 as a second career. She says the board investigations and actions have resulted in a significant loss of work and income. “I have only worked 14 of the past 17 years as a doctor. I live cheaply because I never know how much longer my career will last,” says Dr. Haney.
The ordeal has devastated her finances. She has shelled out at least $200,000 in legal fees – she hired an attorney in 2007 and filed a lawsuit against the board in Oregon district court alleging that members had violated several of her rights. The district judge sided with the state medical board, and it was upheld on appeal in 2012, referring to state laws that gave the board absolute immunity from civil lawsuits. “I had no legal recourse to contest their decisions, no matter how injurious or unjust,” says Dr. Haney.
She has also shelled out at least $100,000 to be evaluated and monitored by the health physician program (now HPSP) for several years. Physicians who agree to be monitored by these health programs have to pay their fees. The board finally agreed last July to end her HPSP participation.
Dr. Haney also filed a complaint in 2007 with the federal Department of Health & Human Services Office for Civil Rights, alleging that the board violated her civil rights under the Americans with Disabilities Act. She says that her lawsuit and the OCR investigation of the board enabled her to withdraw from the HPP in good standing in 2008..
What would she have done differently?
She regrets not hiring an attorney earlier because “most likely the board action would not have been made public. It snowballed after that -- any mistake I made in my career was viewed in the lens of potential impairment.”
She also regrets telling her employer about the nature of her illness and reporting it to the board. A psychiatrist she saw later shared advice he gives to other patients who want to remain anonymous: get help but go out of town, use a false name, and pay cash.
“I wish I had that advice when all this started. That was the best way to protect my career,” says Dr. Haney.
Protecting the public?
The Oregon Medical Board declined to comment on Dr. Haney’s experience because investigations are confidential, but the executive director, Nicole Krishnaswami, JD, answered questions in an email about how the current board operates.
She says the board has 11 medical professionals and employs a medical director and expert consultants in specialty-specific fields. MDs with mental health training are involved in investigating/reviewing cases involving doctors with mental illnesses.
“State medical boards have a responsibility to protect and inform the public. State laws further require state agencies to provide access and transparency regarding the board’s official actions. If the board receives a complaint that a licensee is impaired and thus unable to safely practice, the board has a responsibility to investigate and ensure the licensee is practicing medicine safely,” Ms. Krishnaswami said.
The HPSP is the monitoring program established by state law to provide oversight in order to ensure that licensees are not practicing while impaired. HPSP is separate from the board and the board adopted a statement outlining its perspective on the program in support of doctors with substance abuse and mental health disorder.
The board also founded the Oregon Wellness Program, which provides free, confidential counseling to all Oregon-licensed physicians and physician assistants.
Stigma continues
Dr. Haney feels there is huge stigma associated with mental illness in the medical profession. “If I had cancer twice, I wouldn’t have been put in this position and would be at the peak of my career,” she says.
Nearly half of the 862 emergency medicine physicians surveyed last October said they were reluctant to seek mental health treatment. The reasons included fear of professional repercussions and stigma in the workplace. Several physicians said they were concerned about potentially having to report the treatment on medical license applications in the future, according to a survey by the American College of Emergency Physicians.
In addition, 26% of the more than 12,000 physicians who responded to a Medscape survey last year said they didn’t want to risk disclosure (20%) or that they distrusted mental health professionals (6%).
Another physician fights back
Steven Miles, MD, an award-winning professor emeritus of medicine and bioethics at the Center for Bioethics at the University of Minnesota, in Minneapolis, understands their reluctance. In 1996, he disclosed on his license renewal application that he had recently been diagnosed with a mainly depressive type of bipolar disorder and was in treatment. He had already told his employer, who was supportive.
That set off a 14-month investigation of him by the Minnesota Board of Medical Practice. Dr. Miles and his psychiatrist refused to release his confidential records to a panel of physicians, most of whom had no expertise in mental health care. He also filed a federal claim that the board’s requests violated the ADA, and he won the case.
“Had the board given me evidence of impaired ability to practice with ordinary skill and safety, I would have cooperated. Instead, they proposed a course of action, which would have degraded the privacy of my relationship with my psychiatrist and arguably increased the barrier to getting proper care and the risk of impairment,” he said.
The board kept renewing his license, and Dr. Miles continued to work full time. “I was empowered and protected by my stature in the field at the time my mental illness was diagnosed. Early-career physicians do not yet have that protection and should be very careful of disclosing, given the still widespread stigma of mental illnesses,” he said.
His advocacy led to changes
Dr. Miles went public to mobilize support for his ADA claim. He wrote editorials that were published in JAMA and Minnesota Medicine that refer to the American Psychiatric Association’s 1984 position paper, which says that the mandatory disclosure of the physician’s confidential medical record is without merit. Dr. Miles adds that major newspapers ran stories based on his editorials.
The board backed down after Dr. Miles won his ADA case, and it met with him. “I said this is not good stewardship of the medical profession; you are injuring doctors by keeping them from psychiatric care, which is out of line with the medical view of the treatability of depression and that needs to change,” he says.
Dr. Miles says he won a victory because his practice continued. “I also won a victory in the way the board was handling these questions, which was an opening salvo in a process that continues to this day.”
The original form asked whether he had ever been diagnosed with or treated for manic depression, schizophrenia, compulsive gambling, or other psychiatric conditions.
The revised form asks, “Do you have a physical or mental condition that would affect your ability, with or without reasonable accommodation, to provide appropriate care to patients and otherwise perform the essential functions of a practitioner in your area of practice without posing a health or safety risk to your patients? If yes, what accommodations would help you provide appropriate care to patients and perform other essential functions?”
Dr. Miles says that the final wording wasn’t ideal and that it was confusing to physicians. He says this prompted additional changes in wording by the board. Starting in January, applicants will be asked, “Do you currently have any condition that is not being appropriately treated that is likely to impair or adversely affect your ability to practice medicine with reasonable skill and safety in a competent, ethical, and professional manner?” the medical board’s executive director, Ruth M. Martinez, said in an email.
When asked whether the board still investigates physicians who reveal mental illnesses on licensing applications, Ms. Martinez responded, “All disclosures are evaluated to assure that the practitioner is qualified and safe to practice.”
This article was updated 11/4/21.
A version of this article first appeared on Medscape.com.