User login
Dogs show potential as medical detectives in breast cancer
Breast cancer screening using urine samples based on the volatile organic compounds (VOCs) sensed by a trained dog is feasible, according to a preliminary study published in the journal Biology June 10.
“The extrapolation of our results to widespread implementation is still uncertain,” wrote Shoko Kure, MD, PhD, of Nippon Medical School in Tokyo, and colleagues. “However, even if few dogs could be trained to detect breast cancer, the result may open the door to a robust and inexpensive way to detect breast cancer.” They added that “dog cancer detection is entirely noninvasive, safe and easy for both patients and everyone.”
Early detection of breast cancer, which is the leading cause of death globally, is essential for more efficient treatment. While mammography can detect asymptomatic breast cancer and reduce mortality, it has a poor compliance, is less sensitive in dense breast tissue, detects nonmalignant lesions, and has not been shown to reduce mortality in women younger than 40. VOCs are emitted in the breath, blood, and urine, with different volatile patterns correlated with a variety of diseases including cancers, which dogs can be trained to detect. Breast cancer screening by dog sniffing of the VOCs in urine samples has not been attempted.
Dogs have been used as medical detectives for several cancers and conditions. A study published in 2018 showed that trained dogs who were able to differentiate the specific odor from the metabolic waste of breast cancer in vitro could identify that of colorectal cancer, and vice versa. More recently, research showed that trained dogs could detect advanced prostate cancer in urine samples with high specificity and sensitivity. In this double-blinded pilot study, two dogs were trained to detect Gleason 9 prostate cancer in urine collected from biopsy-confirmed patients. The canine olfaction system was 71% sensitive and as much as 76% specific at detecting Gleason 9 cancer. Along with cancer, trained dogs have been shown to identify people with COVID-19, even those who were asymptomatic. In this study, dogs who sniffed swab samples of armpit sweat could identify which samples came from patients infected with COVID-19 with up to 100% accuracy, while ruling out infection with up to 99% accuracy.
The double-blind study by Dr. Kure aimed to assess the potential of VOCs in urine samples for breast cancer screening by using a single trained sniffer dog – in this case a 9-year-old female Labrador retriever. Urine samples from 40 patients with primary breast cancer and 142 patients with non-breast malignant diseases were included along with samples from 18 healthy volunteers. In 40 times out of 40 runs of the double-blind test, the dog correctly identified urine samples of patients with breast cancer, with 100% sensitivity and 100% specificity.
“The dog in this test successfully differentiated breast cancer from non-breast malignancies and healthy controls,” the authors wrote. “This is the first, preliminary study indicating the feasibility of developing a new breast cancer screening method using urine samples based on VOCs.”
While the authors noted that the study was limited as it relied on one trained dog, they suggested that this method has potential in low-income countries where access to mammography is inadequate.
“Some well-trained sniffing dogs traveling around medically underserved [countries] all over the world could save many lives. Even when a healthy control was indicated by a trained dog, there would be a suspicion of undiagnosed/early-stage cancer, and the person would be advised to undergo medical screening,” the authors wrote.
The authors declared no conflicts of interest.
Breast cancer screening using urine samples based on the volatile organic compounds (VOCs) sensed by a trained dog is feasible, according to a preliminary study published in the journal Biology June 10.
“The extrapolation of our results to widespread implementation is still uncertain,” wrote Shoko Kure, MD, PhD, of Nippon Medical School in Tokyo, and colleagues. “However, even if few dogs could be trained to detect breast cancer, the result may open the door to a robust and inexpensive way to detect breast cancer.” They added that “dog cancer detection is entirely noninvasive, safe and easy for both patients and everyone.”
Early detection of breast cancer, which is the leading cause of death globally, is essential for more efficient treatment. While mammography can detect asymptomatic breast cancer and reduce mortality, it has a poor compliance, is less sensitive in dense breast tissue, detects nonmalignant lesions, and has not been shown to reduce mortality in women younger than 40. VOCs are emitted in the breath, blood, and urine, with different volatile patterns correlated with a variety of diseases including cancers, which dogs can be trained to detect. Breast cancer screening by dog sniffing of the VOCs in urine samples has not been attempted.
Dogs have been used as medical detectives for several cancers and conditions. A study published in 2018 showed that trained dogs who were able to differentiate the specific odor from the metabolic waste of breast cancer in vitro could identify that of colorectal cancer, and vice versa. More recently, research showed that trained dogs could detect advanced prostate cancer in urine samples with high specificity and sensitivity. In this double-blinded pilot study, two dogs were trained to detect Gleason 9 prostate cancer in urine collected from biopsy-confirmed patients. The canine olfaction system was 71% sensitive and as much as 76% specific at detecting Gleason 9 cancer. Along with cancer, trained dogs have been shown to identify people with COVID-19, even those who were asymptomatic. In this study, dogs who sniffed swab samples of armpit sweat could identify which samples came from patients infected with COVID-19 with up to 100% accuracy, while ruling out infection with up to 99% accuracy.
The double-blind study by Dr. Kure aimed to assess the potential of VOCs in urine samples for breast cancer screening by using a single trained sniffer dog – in this case a 9-year-old female Labrador retriever. Urine samples from 40 patients with primary breast cancer and 142 patients with non-breast malignant diseases were included along with samples from 18 healthy volunteers. In 40 times out of 40 runs of the double-blind test, the dog correctly identified urine samples of patients with breast cancer, with 100% sensitivity and 100% specificity.
“The dog in this test successfully differentiated breast cancer from non-breast malignancies and healthy controls,” the authors wrote. “This is the first, preliminary study indicating the feasibility of developing a new breast cancer screening method using urine samples based on VOCs.”
While the authors noted that the study was limited as it relied on one trained dog, they suggested that this method has potential in low-income countries where access to mammography is inadequate.
“Some well-trained sniffing dogs traveling around medically underserved [countries] all over the world could save many lives. Even when a healthy control was indicated by a trained dog, there would be a suspicion of undiagnosed/early-stage cancer, and the person would be advised to undergo medical screening,” the authors wrote.
The authors declared no conflicts of interest.
Breast cancer screening using urine samples based on the volatile organic compounds (VOCs) sensed by a trained dog is feasible, according to a preliminary study published in the journal Biology June 10.
“The extrapolation of our results to widespread implementation is still uncertain,” wrote Shoko Kure, MD, PhD, of Nippon Medical School in Tokyo, and colleagues. “However, even if few dogs could be trained to detect breast cancer, the result may open the door to a robust and inexpensive way to detect breast cancer.” They added that “dog cancer detection is entirely noninvasive, safe and easy for both patients and everyone.”
Early detection of breast cancer, which is the leading cause of death globally, is essential for more efficient treatment. While mammography can detect asymptomatic breast cancer and reduce mortality, it has a poor compliance, is less sensitive in dense breast tissue, detects nonmalignant lesions, and has not been shown to reduce mortality in women younger than 40. VOCs are emitted in the breath, blood, and urine, with different volatile patterns correlated with a variety of diseases including cancers, which dogs can be trained to detect. Breast cancer screening by dog sniffing of the VOCs in urine samples has not been attempted.
Dogs have been used as medical detectives for several cancers and conditions. A study published in 2018 showed that trained dogs who were able to differentiate the specific odor from the metabolic waste of breast cancer in vitro could identify that of colorectal cancer, and vice versa. More recently, research showed that trained dogs could detect advanced prostate cancer in urine samples with high specificity and sensitivity. In this double-blinded pilot study, two dogs were trained to detect Gleason 9 prostate cancer in urine collected from biopsy-confirmed patients. The canine olfaction system was 71% sensitive and as much as 76% specific at detecting Gleason 9 cancer. Along with cancer, trained dogs have been shown to identify people with COVID-19, even those who were asymptomatic. In this study, dogs who sniffed swab samples of armpit sweat could identify which samples came from patients infected with COVID-19 with up to 100% accuracy, while ruling out infection with up to 99% accuracy.
The double-blind study by Dr. Kure aimed to assess the potential of VOCs in urine samples for breast cancer screening by using a single trained sniffer dog – in this case a 9-year-old female Labrador retriever. Urine samples from 40 patients with primary breast cancer and 142 patients with non-breast malignant diseases were included along with samples from 18 healthy volunteers. In 40 times out of 40 runs of the double-blind test, the dog correctly identified urine samples of patients with breast cancer, with 100% sensitivity and 100% specificity.
“The dog in this test successfully differentiated breast cancer from non-breast malignancies and healthy controls,” the authors wrote. “This is the first, preliminary study indicating the feasibility of developing a new breast cancer screening method using urine samples based on VOCs.”
While the authors noted that the study was limited as it relied on one trained dog, they suggested that this method has potential in low-income countries where access to mammography is inadequate.
“Some well-trained sniffing dogs traveling around medically underserved [countries] all over the world could save many lives. Even when a healthy control was indicated by a trained dog, there would be a suspicion of undiagnosed/early-stage cancer, and the person would be advised to undergo medical screening,” the authors wrote.
The authors declared no conflicts of interest.
FROM BIOLOGY
SUGAR trial finds superior stent for those with diabetes and CAD
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Superiority shown on TLF endpoint
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021
COVID-19 has brought more complex, longer office visits
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
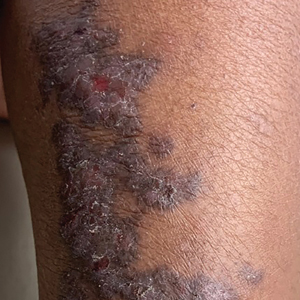
Linear Violaceous Papules in a Child
The Diagnosis: Linear Lichen Planus
The patient was clinically diagnosed with linear lichen planus and was started on betamethasone dipropionate ointment 0.05% applied once daily with improvement in both the pruritus and appearance at 4-month follow-up. A biopsy was deferred based on the parents’ wishes.
Lichen planus is an inflammatory disorder involving the skin and oral mucosa. Cutaneous lichen planus classically presents as flat-topped, violaceous, pruritic, polygonal papules with overlying fine white or grey lines known as Wickham striae.1 Postinflammatory hyperpigmentation is common, especially in patients with darker skin tones. Expected histologic findings include orthokeratosis, apoptotic keratinocytes, and bandlike lymphocytic infiltration at the dermoepidermal junction.1
An estimated 5% of cases of cutaneous lichen planus occur in children.2 A study of 316 children with lichen planus demonstrated that the classic morphology remained the most common presentation, while the linear variant was present in only 6.9% of pediatric cases.3 Linear lichen planus appears to be more common among children than adults. A study of 36 pediatric cases showed a greater representation of lichen planus in Black children (67% affected vs 21% cohort).2
Cutaneous lichen planus often clears spontaneously in approximately 1 year.4 Treatment in children primarily is focused on shortening the time to resolution and relieving pruritus, with topical corticosteroids as firstline therapy.3,4 Oral corticosteroids have a faster clinical response; greater efficacy; and more effectively prevent residual hyperpigmentation, which is especially relevant in individuals with darker skin.3 Nonetheless, oral corticosteroids are considered a second-line treatment due to their unfavorable side-effect profile. Additional treatment options include oral aromatic retinoids (acitretin) and phototherapy.3
Incontinentia pigmenti is characterized by a defect in the inhibitor of nuclear factor–κB kinase regulatory subunit gamma, IKBKG, gene on the X chromosome. Incontinentia pigmenti usually is lethal in males; in females, it leads to ectodermal dysplasia associated with skin findings in a blaschkoid distribution occurring in 4 stages.5 The verrucous stage is preceded by the vesicular stage and expected to occur within the first few months of life, making it unlikely in our 5-year-old patient. Inflammatory linear verrucous epidermal nevus usually occurs in children younger than 5 years and is characterized by psoriasiform papules coalescing into a plaque with substantial scale instead of Wickham striae, as seen in our patient.6 Lichen striatus consists of smaller, pink to flesh-colored papules that rarely are pruritic.7 It is more common among atopic individuals and is associated with postinflammatory hypopigmentation.8 Linear psoriasis presents similarly to inflammatory linear verrucous epidermal nevus, with greater erythema and scale compared to the fine lacy Wickham striae that were seen in our patient.8
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Walton KE, Bowers EV, Drolet BA, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27:34-38.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31:59-67.
- Le Cleach L, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;366:723-732.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:E45-E52.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Payette MJ, Weston G, Humphrey S, et al. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33:631-643.
- Moss C, Browne F. Mosaicism and linear lesions. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
The Diagnosis: Linear Lichen Planus
The patient was clinically diagnosed with linear lichen planus and was started on betamethasone dipropionate ointment 0.05% applied once daily with improvement in both the pruritus and appearance at 4-month follow-up. A biopsy was deferred based on the parents’ wishes.
Lichen planus is an inflammatory disorder involving the skin and oral mucosa. Cutaneous lichen planus classically presents as flat-topped, violaceous, pruritic, polygonal papules with overlying fine white or grey lines known as Wickham striae.1 Postinflammatory hyperpigmentation is common, especially in patients with darker skin tones. Expected histologic findings include orthokeratosis, apoptotic keratinocytes, and bandlike lymphocytic infiltration at the dermoepidermal junction.1
An estimated 5% of cases of cutaneous lichen planus occur in children.2 A study of 316 children with lichen planus demonstrated that the classic morphology remained the most common presentation, while the linear variant was present in only 6.9% of pediatric cases.3 Linear lichen planus appears to be more common among children than adults. A study of 36 pediatric cases showed a greater representation of lichen planus in Black children (67% affected vs 21% cohort).2
Cutaneous lichen planus often clears spontaneously in approximately 1 year.4 Treatment in children primarily is focused on shortening the time to resolution and relieving pruritus, with topical corticosteroids as firstline therapy.3,4 Oral corticosteroids have a faster clinical response; greater efficacy; and more effectively prevent residual hyperpigmentation, which is especially relevant in individuals with darker skin.3 Nonetheless, oral corticosteroids are considered a second-line treatment due to their unfavorable side-effect profile. Additional treatment options include oral aromatic retinoids (acitretin) and phototherapy.3
Incontinentia pigmenti is characterized by a defect in the inhibitor of nuclear factor–κB kinase regulatory subunit gamma, IKBKG, gene on the X chromosome. Incontinentia pigmenti usually is lethal in males; in females, it leads to ectodermal dysplasia associated with skin findings in a blaschkoid distribution occurring in 4 stages.5 The verrucous stage is preceded by the vesicular stage and expected to occur within the first few months of life, making it unlikely in our 5-year-old patient. Inflammatory linear verrucous epidermal nevus usually occurs in children younger than 5 years and is characterized by psoriasiform papules coalescing into a plaque with substantial scale instead of Wickham striae, as seen in our patient.6 Lichen striatus consists of smaller, pink to flesh-colored papules that rarely are pruritic.7 It is more common among atopic individuals and is associated with postinflammatory hypopigmentation.8 Linear psoriasis presents similarly to inflammatory linear verrucous epidermal nevus, with greater erythema and scale compared to the fine lacy Wickham striae that were seen in our patient.8
The Diagnosis: Linear Lichen Planus
The patient was clinically diagnosed with linear lichen planus and was started on betamethasone dipropionate ointment 0.05% applied once daily with improvement in both the pruritus and appearance at 4-month follow-up. A biopsy was deferred based on the parents’ wishes.
Lichen planus is an inflammatory disorder involving the skin and oral mucosa. Cutaneous lichen planus classically presents as flat-topped, violaceous, pruritic, polygonal papules with overlying fine white or grey lines known as Wickham striae.1 Postinflammatory hyperpigmentation is common, especially in patients with darker skin tones. Expected histologic findings include orthokeratosis, apoptotic keratinocytes, and bandlike lymphocytic infiltration at the dermoepidermal junction.1
An estimated 5% of cases of cutaneous lichen planus occur in children.2 A study of 316 children with lichen planus demonstrated that the classic morphology remained the most common presentation, while the linear variant was present in only 6.9% of pediatric cases.3 Linear lichen planus appears to be more common among children than adults. A study of 36 pediatric cases showed a greater representation of lichen planus in Black children (67% affected vs 21% cohort).2
Cutaneous lichen planus often clears spontaneously in approximately 1 year.4 Treatment in children primarily is focused on shortening the time to resolution and relieving pruritus, with topical corticosteroids as firstline therapy.3,4 Oral corticosteroids have a faster clinical response; greater efficacy; and more effectively prevent residual hyperpigmentation, which is especially relevant in individuals with darker skin.3 Nonetheless, oral corticosteroids are considered a second-line treatment due to their unfavorable side-effect profile. Additional treatment options include oral aromatic retinoids (acitretin) and phototherapy.3
Incontinentia pigmenti is characterized by a defect in the inhibitor of nuclear factor–κB kinase regulatory subunit gamma, IKBKG, gene on the X chromosome. Incontinentia pigmenti usually is lethal in males; in females, it leads to ectodermal dysplasia associated with skin findings in a blaschkoid distribution occurring in 4 stages.5 The verrucous stage is preceded by the vesicular stage and expected to occur within the first few months of life, making it unlikely in our 5-year-old patient. Inflammatory linear verrucous epidermal nevus usually occurs in children younger than 5 years and is characterized by psoriasiform papules coalescing into a plaque with substantial scale instead of Wickham striae, as seen in our patient.6 Lichen striatus consists of smaller, pink to flesh-colored papules that rarely are pruritic.7 It is more common among atopic individuals and is associated with postinflammatory hypopigmentation.8 Linear psoriasis presents similarly to inflammatory linear verrucous epidermal nevus, with greater erythema and scale compared to the fine lacy Wickham striae that were seen in our patient.8
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Walton KE, Bowers EV, Drolet BA, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27:34-38.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31:59-67.
- Le Cleach L, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;366:723-732.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:E45-E52.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Payette MJ, Weston G, Humphrey S, et al. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33:631-643.
- Moss C, Browne F. Mosaicism and linear lesions. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Walton KE, Bowers EV, Drolet BA, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27:34-38.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31:59-67.
- Le Cleach L, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;366:723-732.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:E45-E52.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Payette MJ, Weston G, Humphrey S, et al. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33:631-643.
- Moss C, Browne F. Mosaicism and linear lesions. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
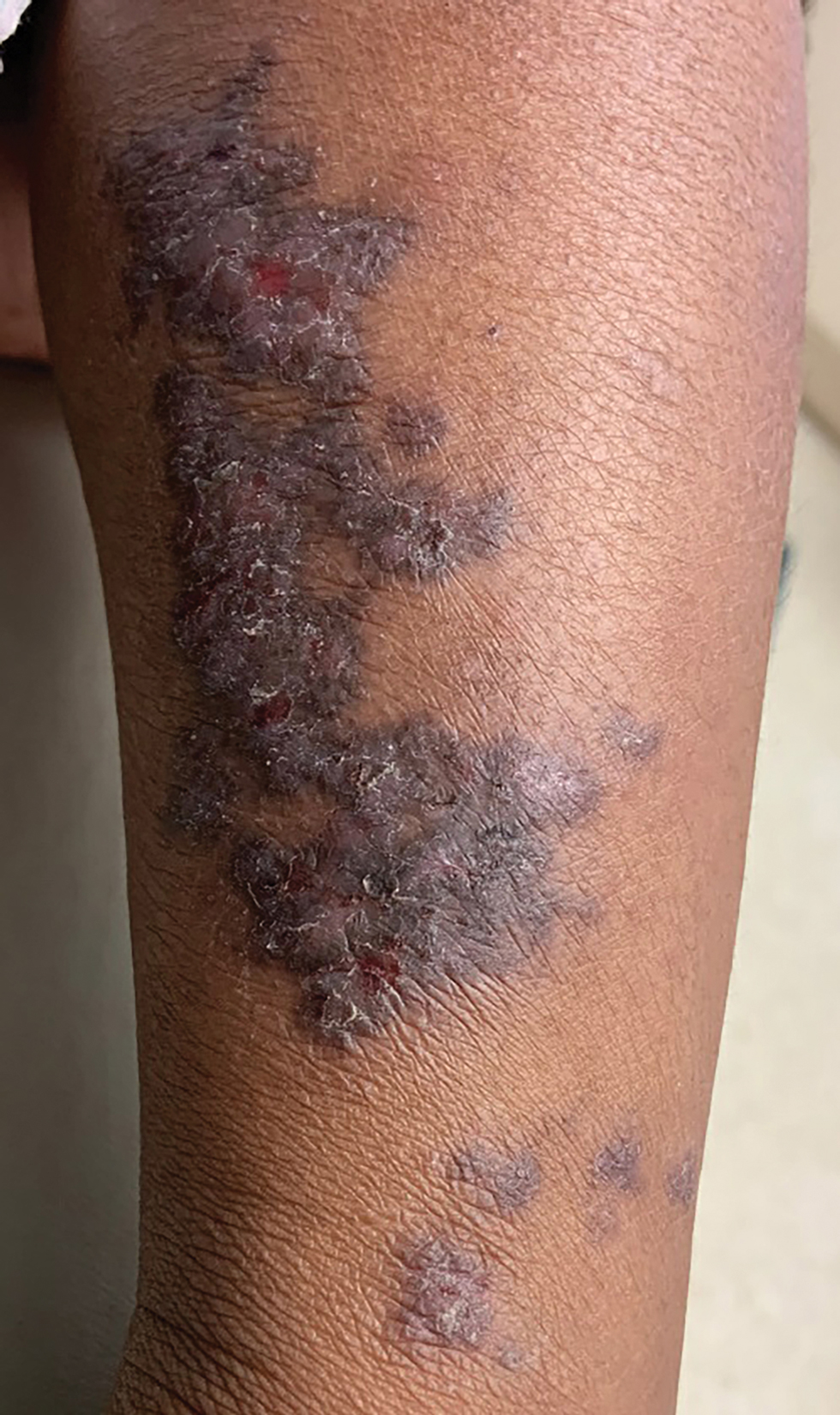
A 5-year-old Black girl presented to the dermatology clinic with a stable pruritic eruption on the right leg of 1 month’s duration. Over-the-counter hydrocortisone cream was applied for 3 days with no response. Physical examination revealed grouped, flat-topped, violaceous papules coalescing into plaques with overlying lacy white striae along the right lower leg, wrapping around to the right dorsal foot in a blaschkoid distribution. The patient was otherwise healthy and up-to-date on immunizations and had an unremarkable birth history.
Filtering pulmonary function tests through race/ethnicity may add to biased care
The use of race/ethnicity in medicine to explain and interpret pulmonary function test (PFT) differences between individuals may contribute to biased medical care and research. Furthermore, it may perpetuate health disparities and structural racism, according to a study published in the journal CHEST®.
Current practices of PFT measurement and interpretation, are imperfect in their ability to accurately describe the relationship between function and health outcomes, according to Nirav R. Bhakta, MD, University of California,San Francisco, and colleagues.
The authors summarized arguments against using race-specific equations, while voicing genuine concerns about removing race from PFT interpretations, and described knowledge gaps and critical questions needing to be addressed for remediation of health disparities.
“Leaving out the perspectives of practicing pulmonologists and physiologists has global relevance for increasingly multicultural communities in which the range of values that represent normal lung function is uncertain,” Dr. Bhakta said in an interview.
A lesson in history
Tracing the history of spirometry, the authors stated that observations about vital lung capacity showing differences attributable to height, age, sex, and occupation (e.g., typesetter vs. firefighter) were then extended to include social classes and ultimately race. Whites showed greater average vital capacity for the same sex, height, and age than non-Whites.
While some investigators pointed to environmental sources (such as early life nutrition, respiratory illness, air pollution, exercise, and altitude), research into their mechanisms and magnitudes of effect was not pursued, but rather “a narrative of innate differences took hold,” Dr. Bhakta and colleagues reported.
That sort of narrative risks comparison with those used to uphold slavery and structural racism in the past. More recently, such a narrative was used to deny disability claims of Welsh versus English White miners, and was expanded to interpret algorithms designed to predict expected lung function.
Use of standing height questioned
The current practice of using normalized standard height for lung function comparisons misses racial and ethnic differences in the proportion of sitting height to standing height shown in multiple studies, the authors stated. These comparisons may ignore effects on standing height of early-life nutrition, genetics, lung-specific factors such as respiratory infections and exposures to indoor and outdoor pollution, physical activity, and high altitude. Using sitting height instead of standing height reduces lung volume differences up to 50% between White and Black populations, they noted, and socioeconomic variables, such as poverty and immigration status, accounted further for the differences seen. Population differences disappeared by as much as 90% when chest measurements used to estimate surface area or volume were more finely detailed.
The researchers warned, however, that, “because current clinical and policy algorithms rely so heavily on the comparison of an individual’s observed lung function to that which is expected for similar people without typical respiratory disease, an abrupt change to not using race/ethnicity, if not paired with education and a reform of existing algorithms and policies, is also expected to have risks on average to groups of non-White individuals.”
That could lead to potential challenges for some groups ranging from the ability to obtain employment in certain occupations, to being considered for potentially curative lung resections, or having access to home assisted ventilation and rehabilitation programs. “An abrupt change to not using race/ethnicity and taking a society’s overall average as the reference range also has the potential to lead to delayed care, denial of disability benefits, and higher life insurance premiums to White individuals.”
Evidence base is limited
“Although evidence demonstrates differences in lung function between racial/ethnic groups, the premise that dividing lung function interpretation up by racial/ethnic background is helpful in the clinical setting is not a proven one.” The authors cited some evidence that lung function interpretation without consideration of race/ethnicity has superior prognostic ability. In addition, research has shown only a weak relationship between lung function and work ability, according to the authors. More appropriate ways of assessing expected lung function for an individual in the absence of a diagnoses are under study.
Offering an alternative
As an alternative to race, Dr. Bhakta and colleagues proposed using a range of values that include individuals across many global populations while still adjusting for sex, age, and height. The resultant value would represent a diverse population average and widen the limits of normal that can be expected in otherwise-healthy people.
The approach would include PFTs with other factors for clinical decision-making, but would allow clinicians and patients to appreciate the limitations of interpretation based on comparison to reference values. However, such an approach may miss pathophysiologically reduced lung function in some individuals, in which case lifesaving therapies, such as chemotherapy, lung cancer resection, and bone marrow transplantation could be withheld. In other instances the consequence would be overtesting and diagnosis, they acknowledged.
The authors further discussed general concerns about the use of race in interpretation of PFTs, addressing limits/considerations as well as knowledge and practice gaps.
For example, one particular concern involves the fact that race does not capture acculturation and mixed ancestry. The limit/consideration is the need to discover mechanisms for differences and to suggest societal interventions, and the knowledge gap pertains to ignorance regarding mechanisms leading to differences in lung function.
For the concern that race is not a proxy for an individual’s genetics, the limit/consideration is that race captures only some genetics and the gap is the need for better genetic information. As an antidote to over reliance on lung function thresholds (without supporting data), they urged outcomes-based standards rather than comparisons with reference populations.
New thinking needed
Dr. Bhakta and colleagues pointed out that the forced expiratory volume in 1 second/forced vital capacity ratios important for diagnosis of obstructive lung disease are similar between racial/ethnic categories, underscoring the need for education about limitations of thresholds and reference values with regard to race, particularly as they are used to detect mild disease.
Ignoring race, on the other hand, can lead to unnecessary testing and treatment (with concomitant side effects), and anxiety.
“Reporting through race-based algorithms in the PFT laboratory risks portraying racial disparities as innate and immutable. By anchoring on the improved prediction of lung function from racial/ethnic-specific reference equations, we miss how the significant residual variation still leaves much uncertainty about the expected value for an individual,” the authors concluded. “Given their origin and historical and current use in society, these racial/ethnic labels are better used to identify the effects of structural racism on respiratory health in research and ensure adequate representation in research, rather than in clinical algorithms.”
One of the authors is a speaker for MGC Diagnostics. The others indicated that they had no relevant disclosures.
The use of race/ethnicity in medicine to explain and interpret pulmonary function test (PFT) differences between individuals may contribute to biased medical care and research. Furthermore, it may perpetuate health disparities and structural racism, according to a study published in the journal CHEST®.
Current practices of PFT measurement and interpretation, are imperfect in their ability to accurately describe the relationship between function and health outcomes, according to Nirav R. Bhakta, MD, University of California,San Francisco, and colleagues.
The authors summarized arguments against using race-specific equations, while voicing genuine concerns about removing race from PFT interpretations, and described knowledge gaps and critical questions needing to be addressed for remediation of health disparities.
“Leaving out the perspectives of practicing pulmonologists and physiologists has global relevance for increasingly multicultural communities in which the range of values that represent normal lung function is uncertain,” Dr. Bhakta said in an interview.
A lesson in history
Tracing the history of spirometry, the authors stated that observations about vital lung capacity showing differences attributable to height, age, sex, and occupation (e.g., typesetter vs. firefighter) were then extended to include social classes and ultimately race. Whites showed greater average vital capacity for the same sex, height, and age than non-Whites.
While some investigators pointed to environmental sources (such as early life nutrition, respiratory illness, air pollution, exercise, and altitude), research into their mechanisms and magnitudes of effect was not pursued, but rather “a narrative of innate differences took hold,” Dr. Bhakta and colleagues reported.
That sort of narrative risks comparison with those used to uphold slavery and structural racism in the past. More recently, such a narrative was used to deny disability claims of Welsh versus English White miners, and was expanded to interpret algorithms designed to predict expected lung function.
Use of standing height questioned
The current practice of using normalized standard height for lung function comparisons misses racial and ethnic differences in the proportion of sitting height to standing height shown in multiple studies, the authors stated. These comparisons may ignore effects on standing height of early-life nutrition, genetics, lung-specific factors such as respiratory infections and exposures to indoor and outdoor pollution, physical activity, and high altitude. Using sitting height instead of standing height reduces lung volume differences up to 50% between White and Black populations, they noted, and socioeconomic variables, such as poverty and immigration status, accounted further for the differences seen. Population differences disappeared by as much as 90% when chest measurements used to estimate surface area or volume were more finely detailed.
The researchers warned, however, that, “because current clinical and policy algorithms rely so heavily on the comparison of an individual’s observed lung function to that which is expected for similar people without typical respiratory disease, an abrupt change to not using race/ethnicity, if not paired with education and a reform of existing algorithms and policies, is also expected to have risks on average to groups of non-White individuals.”
That could lead to potential challenges for some groups ranging from the ability to obtain employment in certain occupations, to being considered for potentially curative lung resections, or having access to home assisted ventilation and rehabilitation programs. “An abrupt change to not using race/ethnicity and taking a society’s overall average as the reference range also has the potential to lead to delayed care, denial of disability benefits, and higher life insurance premiums to White individuals.”
Evidence base is limited
“Although evidence demonstrates differences in lung function between racial/ethnic groups, the premise that dividing lung function interpretation up by racial/ethnic background is helpful in the clinical setting is not a proven one.” The authors cited some evidence that lung function interpretation without consideration of race/ethnicity has superior prognostic ability. In addition, research has shown only a weak relationship between lung function and work ability, according to the authors. More appropriate ways of assessing expected lung function for an individual in the absence of a diagnoses are under study.
Offering an alternative
As an alternative to race, Dr. Bhakta and colleagues proposed using a range of values that include individuals across many global populations while still adjusting for sex, age, and height. The resultant value would represent a diverse population average and widen the limits of normal that can be expected in otherwise-healthy people.
The approach would include PFTs with other factors for clinical decision-making, but would allow clinicians and patients to appreciate the limitations of interpretation based on comparison to reference values. However, such an approach may miss pathophysiologically reduced lung function in some individuals, in which case lifesaving therapies, such as chemotherapy, lung cancer resection, and bone marrow transplantation could be withheld. In other instances the consequence would be overtesting and diagnosis, they acknowledged.
The authors further discussed general concerns about the use of race in interpretation of PFTs, addressing limits/considerations as well as knowledge and practice gaps.
For example, one particular concern involves the fact that race does not capture acculturation and mixed ancestry. The limit/consideration is the need to discover mechanisms for differences and to suggest societal interventions, and the knowledge gap pertains to ignorance regarding mechanisms leading to differences in lung function.
For the concern that race is not a proxy for an individual’s genetics, the limit/consideration is that race captures only some genetics and the gap is the need for better genetic information. As an antidote to over reliance on lung function thresholds (without supporting data), they urged outcomes-based standards rather than comparisons with reference populations.
New thinking needed
Dr. Bhakta and colleagues pointed out that the forced expiratory volume in 1 second/forced vital capacity ratios important for diagnosis of obstructive lung disease are similar between racial/ethnic categories, underscoring the need for education about limitations of thresholds and reference values with regard to race, particularly as they are used to detect mild disease.
Ignoring race, on the other hand, can lead to unnecessary testing and treatment (with concomitant side effects), and anxiety.
“Reporting through race-based algorithms in the PFT laboratory risks portraying racial disparities as innate and immutable. By anchoring on the improved prediction of lung function from racial/ethnic-specific reference equations, we miss how the significant residual variation still leaves much uncertainty about the expected value for an individual,” the authors concluded. “Given their origin and historical and current use in society, these racial/ethnic labels are better used to identify the effects of structural racism on respiratory health in research and ensure adequate representation in research, rather than in clinical algorithms.”
One of the authors is a speaker for MGC Diagnostics. The others indicated that they had no relevant disclosures.
The use of race/ethnicity in medicine to explain and interpret pulmonary function test (PFT) differences between individuals may contribute to biased medical care and research. Furthermore, it may perpetuate health disparities and structural racism, according to a study published in the journal CHEST®.
Current practices of PFT measurement and interpretation, are imperfect in their ability to accurately describe the relationship between function and health outcomes, according to Nirav R. Bhakta, MD, University of California,San Francisco, and colleagues.
The authors summarized arguments against using race-specific equations, while voicing genuine concerns about removing race from PFT interpretations, and described knowledge gaps and critical questions needing to be addressed for remediation of health disparities.
“Leaving out the perspectives of practicing pulmonologists and physiologists has global relevance for increasingly multicultural communities in which the range of values that represent normal lung function is uncertain,” Dr. Bhakta said in an interview.
A lesson in history
Tracing the history of spirometry, the authors stated that observations about vital lung capacity showing differences attributable to height, age, sex, and occupation (e.g., typesetter vs. firefighter) were then extended to include social classes and ultimately race. Whites showed greater average vital capacity for the same sex, height, and age than non-Whites.
While some investigators pointed to environmental sources (such as early life nutrition, respiratory illness, air pollution, exercise, and altitude), research into their mechanisms and magnitudes of effect was not pursued, but rather “a narrative of innate differences took hold,” Dr. Bhakta and colleagues reported.
That sort of narrative risks comparison with those used to uphold slavery and structural racism in the past. More recently, such a narrative was used to deny disability claims of Welsh versus English White miners, and was expanded to interpret algorithms designed to predict expected lung function.
Use of standing height questioned
The current practice of using normalized standard height for lung function comparisons misses racial and ethnic differences in the proportion of sitting height to standing height shown in multiple studies, the authors stated. These comparisons may ignore effects on standing height of early-life nutrition, genetics, lung-specific factors such as respiratory infections and exposures to indoor and outdoor pollution, physical activity, and high altitude. Using sitting height instead of standing height reduces lung volume differences up to 50% between White and Black populations, they noted, and socioeconomic variables, such as poverty and immigration status, accounted further for the differences seen. Population differences disappeared by as much as 90% when chest measurements used to estimate surface area or volume were more finely detailed.
The researchers warned, however, that, “because current clinical and policy algorithms rely so heavily on the comparison of an individual’s observed lung function to that which is expected for similar people without typical respiratory disease, an abrupt change to not using race/ethnicity, if not paired with education and a reform of existing algorithms and policies, is also expected to have risks on average to groups of non-White individuals.”
That could lead to potential challenges for some groups ranging from the ability to obtain employment in certain occupations, to being considered for potentially curative lung resections, or having access to home assisted ventilation and rehabilitation programs. “An abrupt change to not using race/ethnicity and taking a society’s overall average as the reference range also has the potential to lead to delayed care, denial of disability benefits, and higher life insurance premiums to White individuals.”
Evidence base is limited
“Although evidence demonstrates differences in lung function between racial/ethnic groups, the premise that dividing lung function interpretation up by racial/ethnic background is helpful in the clinical setting is not a proven one.” The authors cited some evidence that lung function interpretation without consideration of race/ethnicity has superior prognostic ability. In addition, research has shown only a weak relationship between lung function and work ability, according to the authors. More appropriate ways of assessing expected lung function for an individual in the absence of a diagnoses are under study.
Offering an alternative
As an alternative to race, Dr. Bhakta and colleagues proposed using a range of values that include individuals across many global populations while still adjusting for sex, age, and height. The resultant value would represent a diverse population average and widen the limits of normal that can be expected in otherwise-healthy people.
The approach would include PFTs with other factors for clinical decision-making, but would allow clinicians and patients to appreciate the limitations of interpretation based on comparison to reference values. However, such an approach may miss pathophysiologically reduced lung function in some individuals, in which case lifesaving therapies, such as chemotherapy, lung cancer resection, and bone marrow transplantation could be withheld. In other instances the consequence would be overtesting and diagnosis, they acknowledged.
The authors further discussed general concerns about the use of race in interpretation of PFTs, addressing limits/considerations as well as knowledge and practice gaps.
For example, one particular concern involves the fact that race does not capture acculturation and mixed ancestry. The limit/consideration is the need to discover mechanisms for differences and to suggest societal interventions, and the knowledge gap pertains to ignorance regarding mechanisms leading to differences in lung function.
For the concern that race is not a proxy for an individual’s genetics, the limit/consideration is that race captures only some genetics and the gap is the need for better genetic information. As an antidote to over reliance on lung function thresholds (without supporting data), they urged outcomes-based standards rather than comparisons with reference populations.
New thinking needed
Dr. Bhakta and colleagues pointed out that the forced expiratory volume in 1 second/forced vital capacity ratios important for diagnosis of obstructive lung disease are similar between racial/ethnic categories, underscoring the need for education about limitations of thresholds and reference values with regard to race, particularly as they are used to detect mild disease.
Ignoring race, on the other hand, can lead to unnecessary testing and treatment (with concomitant side effects), and anxiety.
“Reporting through race-based algorithms in the PFT laboratory risks portraying racial disparities as innate and immutable. By anchoring on the improved prediction of lung function from racial/ethnic-specific reference equations, we miss how the significant residual variation still leaves much uncertainty about the expected value for an individual,” the authors concluded. “Given their origin and historical and current use in society, these racial/ethnic labels are better used to identify the effects of structural racism on respiratory health in research and ensure adequate representation in research, rather than in clinical algorithms.”
One of the authors is a speaker for MGC Diagnostics. The others indicated that they had no relevant disclosures.
FROM THE JOURNAL CHEST®
Pharma rep admits to money laundering, obstruction of justice
Pharma rep admits to health care fraud, money laundering, and more
Mr. Camarda pleaded guilty in federal court to one count of conspiracy to commit healthcare fraud and one count of conspiracy to obstruct justice and engage in money laundering.
Mr. Camarda, 39, of Holmdel, N. J., created a side business called Dynasty Capital LLC to independently market medical products and services for other companies, including compounded prescription medications for specialty pharmacies, according to the U.S. Department of Justice.
Mr. Camarda learned that certain local government employees had insurance coverage for these compounded medications and discovered that certain compounded medications were reimbursed up to thousands of dollars for a 1-month supply. Mr. Camarda recruited individuals with insurance coverage to fraudulently obtain medically unnecessary compounded medications.
He marketed compounded medications for several pharmacies. As part of his arrangements with the compounding pharmacies and his conspirators, Mr. Camarda was paid a percentage of the insurance payments received for prescriptions arranged by him and those working for him.
Mr. Camarda received more than $2.2 million in payments for the prescriptions he and those working with him arranged. Mr. Camarda and his recruits caused more than $3.4 million in fraudulent claims to be submitted to the pharmacy benefits administrator for compounded medications.
He is due to be sentenced in November and faces up to 15 years in prison plus $500,000 in fines.
Home health care and hospice agency owner defrauds Medicare for $31 million
Akop Atoyan, 48, of Glendale, Calif., pleaded guilty to one count of conspiracy to commit healthcare fraud and one count of conspiracy to pay and receive healthcare kickbacks.
According to the U.S. Department of Justice, Mr. Atoyan and his wife, Liana Karapetyan, owned and controlled home healthcare and hospice agencies in the greater Sacramento area: ANG Health Care Inc, Excel Home Healthcare Inc, and Excel Hospice Inc. Mr. Atoyan and Ms. Karapetyan certified to Medicare that their agencies would not pay kickbacks in exchange for Medicare beneficiary referrals.
Officials claim Mr. Atoyan and Ms. Karapetyan paid and directed others to pay kickbacks to multiple individuals for beneficiary referrals, including employees of healthcare facilities, as well as employees’ spouses. In total, Mr. Atoyan, Ms. Karapetyan, and others caused the agencies to submit over 8,000 claims to Medicare for the cost of home healthcare and hospice services. Medicare was billed about $31 million.
As part of his guilty plea, Mr. Atoyan agreed to pay about $2.5 million in restitution to the U.S. Department of Health and Human Services. He also agreed to forfeit that amount to the United States.
Medical clinic owner sentenced to jail for Medicaid fraud
Larry Lance Crawford, 49, of Las Vegas, was sentenced in a Medicaid fraud case involving the failure to maintain adequate records to substantiate claims submitted to Nevada Medicaid.
The Nevada Attorney General’s Office announced that Mr. Crawford was given 364 days in jail and was ordered to pay $50,000.00 in restitution.
The Medicaid Fraud Control Unit received information that Mr. Crawford, the owner of Dynamic Future, was using his business to submit false claims for services that were never provided to Medicaid recipients. The investigation revealed that Mr. Crawford failed to maintain records to support the services that were allegedly provided.
A version of this article first appeared on Medscape.com.
Pharma rep admits to health care fraud, money laundering, and more
Mr. Camarda pleaded guilty in federal court to one count of conspiracy to commit healthcare fraud and one count of conspiracy to obstruct justice and engage in money laundering.
Mr. Camarda, 39, of Holmdel, N. J., created a side business called Dynasty Capital LLC to independently market medical products and services for other companies, including compounded prescription medications for specialty pharmacies, according to the U.S. Department of Justice.
Mr. Camarda learned that certain local government employees had insurance coverage for these compounded medications and discovered that certain compounded medications were reimbursed up to thousands of dollars for a 1-month supply. Mr. Camarda recruited individuals with insurance coverage to fraudulently obtain medically unnecessary compounded medications.
He marketed compounded medications for several pharmacies. As part of his arrangements with the compounding pharmacies and his conspirators, Mr. Camarda was paid a percentage of the insurance payments received for prescriptions arranged by him and those working for him.
Mr. Camarda received more than $2.2 million in payments for the prescriptions he and those working with him arranged. Mr. Camarda and his recruits caused more than $3.4 million in fraudulent claims to be submitted to the pharmacy benefits administrator for compounded medications.
He is due to be sentenced in November and faces up to 15 years in prison plus $500,000 in fines.
Home health care and hospice agency owner defrauds Medicare for $31 million
Akop Atoyan, 48, of Glendale, Calif., pleaded guilty to one count of conspiracy to commit healthcare fraud and one count of conspiracy to pay and receive healthcare kickbacks.
According to the U.S. Department of Justice, Mr. Atoyan and his wife, Liana Karapetyan, owned and controlled home healthcare and hospice agencies in the greater Sacramento area: ANG Health Care Inc, Excel Home Healthcare Inc, and Excel Hospice Inc. Mr. Atoyan and Ms. Karapetyan certified to Medicare that their agencies would not pay kickbacks in exchange for Medicare beneficiary referrals.
Officials claim Mr. Atoyan and Ms. Karapetyan paid and directed others to pay kickbacks to multiple individuals for beneficiary referrals, including employees of healthcare facilities, as well as employees’ spouses. In total, Mr. Atoyan, Ms. Karapetyan, and others caused the agencies to submit over 8,000 claims to Medicare for the cost of home healthcare and hospice services. Medicare was billed about $31 million.
As part of his guilty plea, Mr. Atoyan agreed to pay about $2.5 million in restitution to the U.S. Department of Health and Human Services. He also agreed to forfeit that amount to the United States.
Medical clinic owner sentenced to jail for Medicaid fraud
Larry Lance Crawford, 49, of Las Vegas, was sentenced in a Medicaid fraud case involving the failure to maintain adequate records to substantiate claims submitted to Nevada Medicaid.
The Nevada Attorney General’s Office announced that Mr. Crawford was given 364 days in jail and was ordered to pay $50,000.00 in restitution.
The Medicaid Fraud Control Unit received information that Mr. Crawford, the owner of Dynamic Future, was using his business to submit false claims for services that were never provided to Medicaid recipients. The investigation revealed that Mr. Crawford failed to maintain records to support the services that were allegedly provided.
A version of this article first appeared on Medscape.com.
Pharma rep admits to health care fraud, money laundering, and more
Mr. Camarda pleaded guilty in federal court to one count of conspiracy to commit healthcare fraud and one count of conspiracy to obstruct justice and engage in money laundering.
Mr. Camarda, 39, of Holmdel, N. J., created a side business called Dynasty Capital LLC to independently market medical products and services for other companies, including compounded prescription medications for specialty pharmacies, according to the U.S. Department of Justice.
Mr. Camarda learned that certain local government employees had insurance coverage for these compounded medications and discovered that certain compounded medications were reimbursed up to thousands of dollars for a 1-month supply. Mr. Camarda recruited individuals with insurance coverage to fraudulently obtain medically unnecessary compounded medications.
He marketed compounded medications for several pharmacies. As part of his arrangements with the compounding pharmacies and his conspirators, Mr. Camarda was paid a percentage of the insurance payments received for prescriptions arranged by him and those working for him.
Mr. Camarda received more than $2.2 million in payments for the prescriptions he and those working with him arranged. Mr. Camarda and his recruits caused more than $3.4 million in fraudulent claims to be submitted to the pharmacy benefits administrator for compounded medications.
He is due to be sentenced in November and faces up to 15 years in prison plus $500,000 in fines.
Home health care and hospice agency owner defrauds Medicare for $31 million
Akop Atoyan, 48, of Glendale, Calif., pleaded guilty to one count of conspiracy to commit healthcare fraud and one count of conspiracy to pay and receive healthcare kickbacks.
According to the U.S. Department of Justice, Mr. Atoyan and his wife, Liana Karapetyan, owned and controlled home healthcare and hospice agencies in the greater Sacramento area: ANG Health Care Inc, Excel Home Healthcare Inc, and Excel Hospice Inc. Mr. Atoyan and Ms. Karapetyan certified to Medicare that their agencies would not pay kickbacks in exchange for Medicare beneficiary referrals.
Officials claim Mr. Atoyan and Ms. Karapetyan paid and directed others to pay kickbacks to multiple individuals for beneficiary referrals, including employees of healthcare facilities, as well as employees’ spouses. In total, Mr. Atoyan, Ms. Karapetyan, and others caused the agencies to submit over 8,000 claims to Medicare for the cost of home healthcare and hospice services. Medicare was billed about $31 million.
As part of his guilty plea, Mr. Atoyan agreed to pay about $2.5 million in restitution to the U.S. Department of Health and Human Services. He also agreed to forfeit that amount to the United States.
Medical clinic owner sentenced to jail for Medicaid fraud
Larry Lance Crawford, 49, of Las Vegas, was sentenced in a Medicaid fraud case involving the failure to maintain adequate records to substantiate claims submitted to Nevada Medicaid.
The Nevada Attorney General’s Office announced that Mr. Crawford was given 364 days in jail and was ordered to pay $50,000.00 in restitution.
The Medicaid Fraud Control Unit received information that Mr. Crawford, the owner of Dynamic Future, was using his business to submit false claims for services that were never provided to Medicaid recipients. The investigation revealed that Mr. Crawford failed to maintain records to support the services that were allegedly provided.
A version of this article first appeared on Medscape.com.
ECTRIMS 2021: Disease-Modifying Therapies for Relapsing-Remitting Multiple Sclerosis
Dr Joseph Berger of the Perelman School of Medicine in Philadelphia discusses abstracts from ECTRIMS 2021 focusing on the use of disease-modifying therapies (DMTs) in patients with relapsing-remitting multiple sclerosis.
Dr Berger discusses ULTIMATE I and ULTIMATE II results, in which ublituximab — a novel monoclonal antibody — improved annualized relapse rates, Multiple Sclerosis Functional Composite scores, and percentages of patients with no evidence of disease activity compared to teriflunomide.
Dr Berger also highlights a study that examined the association between serum neurofilament light (NfL) levels and disease progression in patients on natalizumab. Although NfL levels were significantly reduced after initiation of therapy, no differences were evident between progressors and nonprogressors.
Next, he examines 3-year data from the CASTING study, which assessed ocrelizumab in patients who had a suboptimal response to one or two previous DMTs. Follow-up analysis showed that patients who received ocrelizumab had consistently low disease activity throughout the study period; mean Expanded Disability Status Scale (EDSS) scores, annualized relapse rates, and no evidence of disease activity were also stable.
Dr Berger concludes with a comparative analysis of patients who started on or switched to dimethyl fumarate or teriflunomide. Dimethyl fumarate showed more favorable outcomes in time to relapse, time to EDSS worsening, and sensitivity analysis.
--
Joseph R. Berger, MD, Professor; Associate Chief, Department of Neurology, Multiple Sclerosis Division, University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania
Joseph R. Berger, MD, has disclosed the following relevant financial relationships:
Received research grant from: Biogen; Roche/Genentech
Received income in an amount equal to or greater than $250 from: Amgen; Biogen; Bristol-Myers Squibb; Celgene; Genzyme; Excision Bio; Dr. Reddy; Serono; Morphic; Novartis; Inhibikase; Morphic; Encycle; Merck; Mapi
Dr Joseph Berger of the Perelman School of Medicine in Philadelphia discusses abstracts from ECTRIMS 2021 focusing on the use of disease-modifying therapies (DMTs) in patients with relapsing-remitting multiple sclerosis.
Dr Berger discusses ULTIMATE I and ULTIMATE II results, in which ublituximab — a novel monoclonal antibody — improved annualized relapse rates, Multiple Sclerosis Functional Composite scores, and percentages of patients with no evidence of disease activity compared to teriflunomide.
Dr Berger also highlights a study that examined the association between serum neurofilament light (NfL) levels and disease progression in patients on natalizumab. Although NfL levels were significantly reduced after initiation of therapy, no differences were evident between progressors and nonprogressors.
Next, he examines 3-year data from the CASTING study, which assessed ocrelizumab in patients who had a suboptimal response to one or two previous DMTs. Follow-up analysis showed that patients who received ocrelizumab had consistently low disease activity throughout the study period; mean Expanded Disability Status Scale (EDSS) scores, annualized relapse rates, and no evidence of disease activity were also stable.
Dr Berger concludes with a comparative analysis of patients who started on or switched to dimethyl fumarate or teriflunomide. Dimethyl fumarate showed more favorable outcomes in time to relapse, time to EDSS worsening, and sensitivity analysis.
--
Joseph R. Berger, MD, Professor; Associate Chief, Department of Neurology, Multiple Sclerosis Division, University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania
Joseph R. Berger, MD, has disclosed the following relevant financial relationships:
Received research grant from: Biogen; Roche/Genentech
Received income in an amount equal to or greater than $250 from: Amgen; Biogen; Bristol-Myers Squibb; Celgene; Genzyme; Excision Bio; Dr. Reddy; Serono; Morphic; Novartis; Inhibikase; Morphic; Encycle; Merck; Mapi
Dr Joseph Berger of the Perelman School of Medicine in Philadelphia discusses abstracts from ECTRIMS 2021 focusing on the use of disease-modifying therapies (DMTs) in patients with relapsing-remitting multiple sclerosis.
Dr Berger discusses ULTIMATE I and ULTIMATE II results, in which ublituximab — a novel monoclonal antibody — improved annualized relapse rates, Multiple Sclerosis Functional Composite scores, and percentages of patients with no evidence of disease activity compared to teriflunomide.
Dr Berger also highlights a study that examined the association between serum neurofilament light (NfL) levels and disease progression in patients on natalizumab. Although NfL levels were significantly reduced after initiation of therapy, no differences were evident between progressors and nonprogressors.
Next, he examines 3-year data from the CASTING study, which assessed ocrelizumab in patients who had a suboptimal response to one or two previous DMTs. Follow-up analysis showed that patients who received ocrelizumab had consistently low disease activity throughout the study period; mean Expanded Disability Status Scale (EDSS) scores, annualized relapse rates, and no evidence of disease activity were also stable.
Dr Berger concludes with a comparative analysis of patients who started on or switched to dimethyl fumarate or teriflunomide. Dimethyl fumarate showed more favorable outcomes in time to relapse, time to EDSS worsening, and sensitivity analysis.
--
Joseph R. Berger, MD, Professor; Associate Chief, Department of Neurology, Multiple Sclerosis Division, University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania
Joseph R. Berger, MD, has disclosed the following relevant financial relationships:
Received research grant from: Biogen; Roche/Genentech
Received income in an amount equal to or greater than $250 from: Amgen; Biogen; Bristol-Myers Squibb; Celgene; Genzyme; Excision Bio; Dr. Reddy; Serono; Morphic; Novartis; Inhibikase; Morphic; Encycle; Merck; Mapi

‘Residents’ Viewpoint’ revisited
We are currently republishing an installment of this column as part of our continuing celebration of Family Practice News’s 50th anniversary.
Bruce A. Bagley, MD, wrote the first batch of these columns, when he was chief resident in family medicine at St. Joseph’s Hospital, Syracuse, N.Y. Joseph E. Scherger, MD, was the second writer for Family Practice News’s monthly “Residents’ Viewpoint.” At the time Dr. Scherger became a columnist, he was a 26-year-old, 2nd-year family practice resident at the Family Medical Center, University Hospital, University of Washington, Seattle.
Dr. Scherger’s first column was published on Feb. 5, 1977. We are republishing his “Residents’ Viewpoint” from June 15, 1977 (see below) and a new column by Victoria Persampiere, DO, who is currently a 2nd-year resident in the family medicine program at Abington Jefferson Health. (See “My experience as a family medicine resident in 2021” after Dr. Scherger’s column.).
We hope you will enjoy comparing and contrasting the experiences of a resident practicing family medicine today to those of a resident practicing family medicine nearly 4½ decades ago.To learn about Dr. Scherger’s current practice and long career, you can read his profile on the cover of the September 2021 issue of Family Practice News or on MDedge.com/FamilyMedicine in our “Family Practice News 50th Anniversary” section.
Art of medicine or deception?
Originally published in Family Practice News on June 15, 1977.
In medical school I learned the science of medicine. There I diligently studied the basic sciences and gained a thorough understanding of the pathophysiology of disease. In the clinical years I learned to apply this knowledge to a wide variety of interesting patients who came to the academic center.
Yet, when I started my family practice residency, I lacked the ability to care for patients. Though I could take a thorough history, perform a complete physical examination, and diagnose and treat specific illnesses, I had little idea how to satisfy patients by meeting their needs.
The art of medicine is the nonscientific part of a successful doctor-patient interaction. For a doctor-patient interaction to be successful, not only must the illness be appropriately addressed, but both patient and physician must be satisfied.
In the university environment, the art of medicine often gets inadequate attention. Indeed, most academic physicians think that only scientific medicine exists and that patients should be satisfied with a sophisticated approach to their problems. Some patients are satisfied, but many are disgruntled. It is not unusual for a patient, after a $1,000 work-up, to go to a family physician or chiropractor for satisfaction.
I was eager to discover the art of medicine at its finest during my rotation away from the university in a rural community. During these 2 months I looked for the pearls of wisdom that allowed community physicians to be so successful. I found that a very explicit technique was used by some physicians to achieve not only satisfaction but adoration from their patients. Unfortunately, this technique is dishonest.
Early in my community experience I was impressed by how often patients told me a doctor had saved them. I heard such statements as “Dr. X saved my leg,” or “Dr. X saved my life.” I know that it does occur, but not as often as I was hearing it.
Investigating these statements I found such stories as, “One day l twisted my ankle very badly, and it became quite swollen. My doctor told me 1 could lose my leg from this but that he would take x-rays, put my leg in an Ace bandage, and give me crutches. In 3 days I was well. I am so thankful he saved my leg.”
And, “One day I had a temperature of 104. All of my muscles ached, my head hurt, and I had a terrible sore throat and cough. My doctor told me l could die from this, but he gave me a medicine and made me stay home. I was sick for about 2 weeks, but I got better. He saved my life.”
Is the art of medicine the art of deception? This horrifying thought actually came to me after hearing several such stories, but I learned that most of the physicians involved in such stories were not well respected by their colleagues.
I learned many honest techniques for successfully caring for patients. The several family physicians with whom I worked, all clinical instructors associated with my residency, were impeccably honest and taught me to combine compassion and efficiency.
Despite learning many positive techniques and having good role models, I left the community experience somewhat saddened by the lack of integrity that can exist in the profession. I was naive in believing that all the nonscientific aspects of medicine that made patients happy must be good.
By experiencing deception, I learned why quackery continues to flourish despite the widespread availability of honest medical care. Most significantly, I learned the importance of a sometimes frustrating humility; my patients with sprained ankles and influenza will not believe I saved their lives.
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19 era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome, which was strengthened by every “there is nothing else we can offer your loved one at this time” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today; you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician. ■
Dr. Persampiere is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
We are currently republishing an installment of this column as part of our continuing celebration of Family Practice News’s 50th anniversary.
Bruce A. Bagley, MD, wrote the first batch of these columns, when he was chief resident in family medicine at St. Joseph’s Hospital, Syracuse, N.Y. Joseph E. Scherger, MD, was the second writer for Family Practice News’s monthly “Residents’ Viewpoint.” At the time Dr. Scherger became a columnist, he was a 26-year-old, 2nd-year family practice resident at the Family Medical Center, University Hospital, University of Washington, Seattle.
Dr. Scherger’s first column was published on Feb. 5, 1977. We are republishing his “Residents’ Viewpoint” from June 15, 1977 (see below) and a new column by Victoria Persampiere, DO, who is currently a 2nd-year resident in the family medicine program at Abington Jefferson Health. (See “My experience as a family medicine resident in 2021” after Dr. Scherger’s column.).
We hope you will enjoy comparing and contrasting the experiences of a resident practicing family medicine today to those of a resident practicing family medicine nearly 4½ decades ago.To learn about Dr. Scherger’s current practice and long career, you can read his profile on the cover of the September 2021 issue of Family Practice News or on MDedge.com/FamilyMedicine in our “Family Practice News 50th Anniversary” section.
Art of medicine or deception?
Originally published in Family Practice News on June 15, 1977.
In medical school I learned the science of medicine. There I diligently studied the basic sciences and gained a thorough understanding of the pathophysiology of disease. In the clinical years I learned to apply this knowledge to a wide variety of interesting patients who came to the academic center.
Yet, when I started my family practice residency, I lacked the ability to care for patients. Though I could take a thorough history, perform a complete physical examination, and diagnose and treat specific illnesses, I had little idea how to satisfy patients by meeting their needs.
The art of medicine is the nonscientific part of a successful doctor-patient interaction. For a doctor-patient interaction to be successful, not only must the illness be appropriately addressed, but both patient and physician must be satisfied.
In the university environment, the art of medicine often gets inadequate attention. Indeed, most academic physicians think that only scientific medicine exists and that patients should be satisfied with a sophisticated approach to their problems. Some patients are satisfied, but many are disgruntled. It is not unusual for a patient, after a $1,000 work-up, to go to a family physician or chiropractor for satisfaction.
I was eager to discover the art of medicine at its finest during my rotation away from the university in a rural community. During these 2 months I looked for the pearls of wisdom that allowed community physicians to be so successful. I found that a very explicit technique was used by some physicians to achieve not only satisfaction but adoration from their patients. Unfortunately, this technique is dishonest.
Early in my community experience I was impressed by how often patients told me a doctor had saved them. I heard such statements as “Dr. X saved my leg,” or “Dr. X saved my life.” I know that it does occur, but not as often as I was hearing it.
Investigating these statements I found such stories as, “One day l twisted my ankle very badly, and it became quite swollen. My doctor told me 1 could lose my leg from this but that he would take x-rays, put my leg in an Ace bandage, and give me crutches. In 3 days I was well. I am so thankful he saved my leg.”
And, “One day I had a temperature of 104. All of my muscles ached, my head hurt, and I had a terrible sore throat and cough. My doctor told me l could die from this, but he gave me a medicine and made me stay home. I was sick for about 2 weeks, but I got better. He saved my life.”
Is the art of medicine the art of deception? This horrifying thought actually came to me after hearing several such stories, but I learned that most of the physicians involved in such stories were not well respected by their colleagues.
I learned many honest techniques for successfully caring for patients. The several family physicians with whom I worked, all clinical instructors associated with my residency, were impeccably honest and taught me to combine compassion and efficiency.
Despite learning many positive techniques and having good role models, I left the community experience somewhat saddened by the lack of integrity that can exist in the profession. I was naive in believing that all the nonscientific aspects of medicine that made patients happy must be good.
By experiencing deception, I learned why quackery continues to flourish despite the widespread availability of honest medical care. Most significantly, I learned the importance of a sometimes frustrating humility; my patients with sprained ankles and influenza will not believe I saved their lives.
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19 era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome, which was strengthened by every “there is nothing else we can offer your loved one at this time” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today; you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician. ■
Dr. Persampiere is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
We are currently republishing an installment of this column as part of our continuing celebration of Family Practice News’s 50th anniversary.
Bruce A. Bagley, MD, wrote the first batch of these columns, when he was chief resident in family medicine at St. Joseph’s Hospital, Syracuse, N.Y. Joseph E. Scherger, MD, was the second writer for Family Practice News’s monthly “Residents’ Viewpoint.” At the time Dr. Scherger became a columnist, he was a 26-year-old, 2nd-year family practice resident at the Family Medical Center, University Hospital, University of Washington, Seattle.
Dr. Scherger’s first column was published on Feb. 5, 1977. We are republishing his “Residents’ Viewpoint” from June 15, 1977 (see below) and a new column by Victoria Persampiere, DO, who is currently a 2nd-year resident in the family medicine program at Abington Jefferson Health. (See “My experience as a family medicine resident in 2021” after Dr. Scherger’s column.).
We hope you will enjoy comparing and contrasting the experiences of a resident practicing family medicine today to those of a resident practicing family medicine nearly 4½ decades ago.To learn about Dr. Scherger’s current practice and long career, you can read his profile on the cover of the September 2021 issue of Family Practice News or on MDedge.com/FamilyMedicine in our “Family Practice News 50th Anniversary” section.
Art of medicine or deception?
Originally published in Family Practice News on June 15, 1977.
In medical school I learned the science of medicine. There I diligently studied the basic sciences and gained a thorough understanding of the pathophysiology of disease. In the clinical years I learned to apply this knowledge to a wide variety of interesting patients who came to the academic center.
Yet, when I started my family practice residency, I lacked the ability to care for patients. Though I could take a thorough history, perform a complete physical examination, and diagnose and treat specific illnesses, I had little idea how to satisfy patients by meeting their needs.
The art of medicine is the nonscientific part of a successful doctor-patient interaction. For a doctor-patient interaction to be successful, not only must the illness be appropriately addressed, but both patient and physician must be satisfied.
In the university environment, the art of medicine often gets inadequate attention. Indeed, most academic physicians think that only scientific medicine exists and that patients should be satisfied with a sophisticated approach to their problems. Some patients are satisfied, but many are disgruntled. It is not unusual for a patient, after a $1,000 work-up, to go to a family physician or chiropractor for satisfaction.
I was eager to discover the art of medicine at its finest during my rotation away from the university in a rural community. During these 2 months I looked for the pearls of wisdom that allowed community physicians to be so successful. I found that a very explicit technique was used by some physicians to achieve not only satisfaction but adoration from their patients. Unfortunately, this technique is dishonest.
Early in my community experience I was impressed by how often patients told me a doctor had saved them. I heard such statements as “Dr. X saved my leg,” or “Dr. X saved my life.” I know that it does occur, but not as often as I was hearing it.
Investigating these statements I found such stories as, “One day l twisted my ankle very badly, and it became quite swollen. My doctor told me 1 could lose my leg from this but that he would take x-rays, put my leg in an Ace bandage, and give me crutches. In 3 days I was well. I am so thankful he saved my leg.”
And, “One day I had a temperature of 104. All of my muscles ached, my head hurt, and I had a terrible sore throat and cough. My doctor told me l could die from this, but he gave me a medicine and made me stay home. I was sick for about 2 weeks, but I got better. He saved my life.”
Is the art of medicine the art of deception? This horrifying thought actually came to me after hearing several such stories, but I learned that most of the physicians involved in such stories were not well respected by their colleagues.
I learned many honest techniques for successfully caring for patients. The several family physicians with whom I worked, all clinical instructors associated with my residency, were impeccably honest and taught me to combine compassion and efficiency.
Despite learning many positive techniques and having good role models, I left the community experience somewhat saddened by the lack of integrity that can exist in the profession. I was naive in believing that all the nonscientific aspects of medicine that made patients happy must be good.
By experiencing deception, I learned why quackery continues to flourish despite the widespread availability of honest medical care. Most significantly, I learned the importance of a sometimes frustrating humility; my patients with sprained ankles and influenza will not believe I saved their lives.
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19 era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome, which was strengthened by every “there is nothing else we can offer your loved one at this time” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today; you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician. ■
Dr. Persampiere is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
Small fiber neuropathy is rising in the U.S., but why is a mystery
The exact reason for the increase in isolated SFN “remains unclear,” said Christopher J. Klein, MD, of the Mayo Clinic in Rochester, Minn. However, “we noted during the study period the population has had increased BMI, which appears to be a risk factor for this disorder, with many (50%) developing either glucose impairment or frank diabetes during the study period even if not present at first small fiber neuropathy presentation, also with associated higher triglyceride levels,” he explained.
The study was published online October 27 in Neurology.
Significant upward trend
Investigators reviewed the records of all 94 adults diagnosed with pure SFN (no large fiber involvement) between 1998 and 2017 in Olmsted and adjacent counties in Minnesota – and compared them with 282 adults of similar age and gender who did not have neuropathy.
The incidence of SFN over the entire study period was 1.3 per 100,000 per year and the prevalence was 13.3 per 100,000.
There was a “significant upward trend” in SFN incidence over the study period that could not be attributed to the availability of intraepidermal nerve fiber density testing, the authors reported.
The median age of onset of SFN was 54 years and two-thirds were women (67%).
Diabetes, obesity, and hypertriglyceridemia were significantly more common in patients with SFN compared with matched controls. These metabolic risk factors are also associated with peripheral neuropathy regardless of fiber type.
Autonomic symptoms were common and generally mild, affecting 85% of patients with SFN, and included male erectile dysfunction, constipation, light-headedness and palpitations, urinary symptoms, diarrhea, dry eyes and mouth, sweat abnormalities, and gastroparesis.
Insomnia and use of opioid pain medication were more common in those with SFN than matched controls.
More than one-third (36%) of patients with SFN developed large fiber neuropathy an average of 5.3 years after developing SFN.
During an average follow-up of 6.1 years, adults with SFN were significantly more likely to suffer myocardial infarction (46% vs. 27%; odds ratio, 2.0; 95% CI, 1.8-4.9), congestive heart failure (27% vs. 12%; OR, 2.6; 95% CI, 1.4-4.8), peripheral vascular disease (22% vs. 6%; OR, 4.0; 95% CI, 1.9-8.1), stroke (24% vs. 10%; OR, 2.8; 95% CI, 1.5-5.3), diabetes (51% vs. 22%; OR, 4.6; 95% CI, 2.8-7.6) and rheumatologic disease (30% vs. 7%; OR, 5.3; 95% CI, 2.8-10.4).
For 70% of patients, no cause for SFN could be determined. Diabetes (15%) was the most common cause identified. Other less common causes included Sjögren syndrome, lupus, amyloidosis, and Fabry disease.
“It is important to quantitatively diagnose patients with SFN as many non-neurological musculoskeletal causes can mimic the disorder,” said Dr. Klein.
“If rates of progression are rapid, sinister causes such as out-of-control diabetes, hereditary [transthyretin] TTR amyloidosis, and Fabry disease can be responsible. For other patients, rates of progression are slow and generally do not lead to significant neurologic impairments,” he added.
“However,” he said, “internal medicine follow-up is important for all as this disorder associates with development with higher risk of cardiovascular disease, including commonly heart attacks.”
Of note, although mean age at death was not significantly different in patients with SFN than controls (70 vs. 73 years), there was a significantly higher number of deaths in patients with SFN (n = 18; 19%) than in matched controls (n = 35; 12%) from the time of symptom onset, the researchers reported.
Important research
This “important” study sheds light on the comorbidities and longitudinal consequences of SFN, wrote Brian Callaghan, MD, with the University of Michigan, Ann Arbor, and J. Robinson Singleton, MD, with the University of Utah, Salt Lake City, in an accompanying editorial in Neurology.
The study demonstrates clearly that SFN has “metabolic risk factors similar to those seen for sensory predominant peripheral neuropathies affecting a broader range of fiber types. As a result, therapies that address metabolic risk factors are likely to help prevent or treat both conditions,” they wrote.
Dr. Callaghan and Dr. Singleton added that a key strength of the study is the detailed follow-up that examines SFN progression over time. “The authors found that patients with SFN do not report high disability and that progression tends to be slow. Therefore, patients with SFN can be counseled that progression and disability are likely to be modest in most cases. However, when patients do progress quickly, uncommon etiologies should be sought,” the editorialists wrote.
The study was supported by the Mayo Clinic Foundation, Mayo Clinic Center for Individualized Medicine, and Mayo Clinic Center of MS and Autoimmune Neurology. Dr. Klein has received teaching honorarium from Ackea pharmaceuticals for lectures on hereditary transthyretin amyloidosis and Fabry disease, consulted for Pfizer regarding tafamidis (all compensation for consulting activities is paid directly to Mayo Clinic), and participated in the clinical trials for inotersen and patisiran but received no personal compensation for his participation. Dr. Callaghan consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Singleton has consulted for Regenacy.
A version of this article first appeared on Medscape.com.
The exact reason for the increase in isolated SFN “remains unclear,” said Christopher J. Klein, MD, of the Mayo Clinic in Rochester, Minn. However, “we noted during the study period the population has had increased BMI, which appears to be a risk factor for this disorder, with many (50%) developing either glucose impairment or frank diabetes during the study period even if not present at first small fiber neuropathy presentation, also with associated higher triglyceride levels,” he explained.
The study was published online October 27 in Neurology.
Significant upward trend
Investigators reviewed the records of all 94 adults diagnosed with pure SFN (no large fiber involvement) between 1998 and 2017 in Olmsted and adjacent counties in Minnesota – and compared them with 282 adults of similar age and gender who did not have neuropathy.
The incidence of SFN over the entire study period was 1.3 per 100,000 per year and the prevalence was 13.3 per 100,000.
There was a “significant upward trend” in SFN incidence over the study period that could not be attributed to the availability of intraepidermal nerve fiber density testing, the authors reported.
The median age of onset of SFN was 54 years and two-thirds were women (67%).
Diabetes, obesity, and hypertriglyceridemia were significantly more common in patients with SFN compared with matched controls. These metabolic risk factors are also associated with peripheral neuropathy regardless of fiber type.
Autonomic symptoms were common and generally mild, affecting 85% of patients with SFN, and included male erectile dysfunction, constipation, light-headedness and palpitations, urinary symptoms, diarrhea, dry eyes and mouth, sweat abnormalities, and gastroparesis.
Insomnia and use of opioid pain medication were more common in those with SFN than matched controls.
More than one-third (36%) of patients with SFN developed large fiber neuropathy an average of 5.3 years after developing SFN.
During an average follow-up of 6.1 years, adults with SFN were significantly more likely to suffer myocardial infarction (46% vs. 27%; odds ratio, 2.0; 95% CI, 1.8-4.9), congestive heart failure (27% vs. 12%; OR, 2.6; 95% CI, 1.4-4.8), peripheral vascular disease (22% vs. 6%; OR, 4.0; 95% CI, 1.9-8.1), stroke (24% vs. 10%; OR, 2.8; 95% CI, 1.5-5.3), diabetes (51% vs. 22%; OR, 4.6; 95% CI, 2.8-7.6) and rheumatologic disease (30% vs. 7%; OR, 5.3; 95% CI, 2.8-10.4).
For 70% of patients, no cause for SFN could be determined. Diabetes (15%) was the most common cause identified. Other less common causes included Sjögren syndrome, lupus, amyloidosis, and Fabry disease.
“It is important to quantitatively diagnose patients with SFN as many non-neurological musculoskeletal causes can mimic the disorder,” said Dr. Klein.
“If rates of progression are rapid, sinister causes such as out-of-control diabetes, hereditary [transthyretin] TTR amyloidosis, and Fabry disease can be responsible. For other patients, rates of progression are slow and generally do not lead to significant neurologic impairments,” he added.
“However,” he said, “internal medicine follow-up is important for all as this disorder associates with development with higher risk of cardiovascular disease, including commonly heart attacks.”
Of note, although mean age at death was not significantly different in patients with SFN than controls (70 vs. 73 years), there was a significantly higher number of deaths in patients with SFN (n = 18; 19%) than in matched controls (n = 35; 12%) from the time of symptom onset, the researchers reported.
Important research
This “important” study sheds light on the comorbidities and longitudinal consequences of SFN, wrote Brian Callaghan, MD, with the University of Michigan, Ann Arbor, and J. Robinson Singleton, MD, with the University of Utah, Salt Lake City, in an accompanying editorial in Neurology.
The study demonstrates clearly that SFN has “metabolic risk factors similar to those seen for sensory predominant peripheral neuropathies affecting a broader range of fiber types. As a result, therapies that address metabolic risk factors are likely to help prevent or treat both conditions,” they wrote.
Dr. Callaghan and Dr. Singleton added that a key strength of the study is the detailed follow-up that examines SFN progression over time. “The authors found that patients with SFN do not report high disability and that progression tends to be slow. Therefore, patients with SFN can be counseled that progression and disability are likely to be modest in most cases. However, when patients do progress quickly, uncommon etiologies should be sought,” the editorialists wrote.
The study was supported by the Mayo Clinic Foundation, Mayo Clinic Center for Individualized Medicine, and Mayo Clinic Center of MS and Autoimmune Neurology. Dr. Klein has received teaching honorarium from Ackea pharmaceuticals for lectures on hereditary transthyretin amyloidosis and Fabry disease, consulted for Pfizer regarding tafamidis (all compensation for consulting activities is paid directly to Mayo Clinic), and participated in the clinical trials for inotersen and patisiran but received no personal compensation for his participation. Dr. Callaghan consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Singleton has consulted for Regenacy.
A version of this article first appeared on Medscape.com.
The exact reason for the increase in isolated SFN “remains unclear,” said Christopher J. Klein, MD, of the Mayo Clinic in Rochester, Minn. However, “we noted during the study period the population has had increased BMI, which appears to be a risk factor for this disorder, with many (50%) developing either glucose impairment or frank diabetes during the study period even if not present at first small fiber neuropathy presentation, also with associated higher triglyceride levels,” he explained.
The study was published online October 27 in Neurology.
Significant upward trend
Investigators reviewed the records of all 94 adults diagnosed with pure SFN (no large fiber involvement) between 1998 and 2017 in Olmsted and adjacent counties in Minnesota – and compared them with 282 adults of similar age and gender who did not have neuropathy.
The incidence of SFN over the entire study period was 1.3 per 100,000 per year and the prevalence was 13.3 per 100,000.
There was a “significant upward trend” in SFN incidence over the study period that could not be attributed to the availability of intraepidermal nerve fiber density testing, the authors reported.
The median age of onset of SFN was 54 years and two-thirds were women (67%).
Diabetes, obesity, and hypertriglyceridemia were significantly more common in patients with SFN compared with matched controls. These metabolic risk factors are also associated with peripheral neuropathy regardless of fiber type.
Autonomic symptoms were common and generally mild, affecting 85% of patients with SFN, and included male erectile dysfunction, constipation, light-headedness and palpitations, urinary symptoms, diarrhea, dry eyes and mouth, sweat abnormalities, and gastroparesis.
Insomnia and use of opioid pain medication were more common in those with SFN than matched controls.
More than one-third (36%) of patients with SFN developed large fiber neuropathy an average of 5.3 years after developing SFN.
During an average follow-up of 6.1 years, adults with SFN were significantly more likely to suffer myocardial infarction (46% vs. 27%; odds ratio, 2.0; 95% CI, 1.8-4.9), congestive heart failure (27% vs. 12%; OR, 2.6; 95% CI, 1.4-4.8), peripheral vascular disease (22% vs. 6%; OR, 4.0; 95% CI, 1.9-8.1), stroke (24% vs. 10%; OR, 2.8; 95% CI, 1.5-5.3), diabetes (51% vs. 22%; OR, 4.6; 95% CI, 2.8-7.6) and rheumatologic disease (30% vs. 7%; OR, 5.3; 95% CI, 2.8-10.4).
For 70% of patients, no cause for SFN could be determined. Diabetes (15%) was the most common cause identified. Other less common causes included Sjögren syndrome, lupus, amyloidosis, and Fabry disease.
“It is important to quantitatively diagnose patients with SFN as many non-neurological musculoskeletal causes can mimic the disorder,” said Dr. Klein.
“If rates of progression are rapid, sinister causes such as out-of-control diabetes, hereditary [transthyretin] TTR amyloidosis, and Fabry disease can be responsible. For other patients, rates of progression are slow and generally do not lead to significant neurologic impairments,” he added.
“However,” he said, “internal medicine follow-up is important for all as this disorder associates with development with higher risk of cardiovascular disease, including commonly heart attacks.”
Of note, although mean age at death was not significantly different in patients with SFN than controls (70 vs. 73 years), there was a significantly higher number of deaths in patients with SFN (n = 18; 19%) than in matched controls (n = 35; 12%) from the time of symptom onset, the researchers reported.
Important research
This “important” study sheds light on the comorbidities and longitudinal consequences of SFN, wrote Brian Callaghan, MD, with the University of Michigan, Ann Arbor, and J. Robinson Singleton, MD, with the University of Utah, Salt Lake City, in an accompanying editorial in Neurology.
The study demonstrates clearly that SFN has “metabolic risk factors similar to those seen for sensory predominant peripheral neuropathies affecting a broader range of fiber types. As a result, therapies that address metabolic risk factors are likely to help prevent or treat both conditions,” they wrote.
Dr. Callaghan and Dr. Singleton added that a key strength of the study is the detailed follow-up that examines SFN progression over time. “The authors found that patients with SFN do not report high disability and that progression tends to be slow. Therefore, patients with SFN can be counseled that progression and disability are likely to be modest in most cases. However, when patients do progress quickly, uncommon etiologies should be sought,” the editorialists wrote.
The study was supported by the Mayo Clinic Foundation, Mayo Clinic Center for Individualized Medicine, and Mayo Clinic Center of MS and Autoimmune Neurology. Dr. Klein has received teaching honorarium from Ackea pharmaceuticals for lectures on hereditary transthyretin amyloidosis and Fabry disease, consulted for Pfizer regarding tafamidis (all compensation for consulting activities is paid directly to Mayo Clinic), and participated in the clinical trials for inotersen and patisiran but received no personal compensation for his participation. Dr. Callaghan consults for DynaMed, performs medical legal consultations, including consultations for the Vaccine Injury Compensation Program, and receives research support from the American Academy of Neurology. Dr. Singleton has consulted for Regenacy.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Success of HPV vaccination: ‘Dramatic’ reduction in cervical cancer
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.