User login
Open notes in health care: The good, the bad, and the ugly of the Cures Act
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
On April 5, 2021, a requirement of the 21st Century Cures Act went into effect: Patients must be able to access information in their EHRs “without delay.” (This requirement does not apply to paper records.) The Cures Act prohibition against information blocking, often referred to as an “open notes” provision, provides patients with transparency in the outcomes of their health care via convenient access to information in their EHR, which can positively or negatively impact the patient-doctor relationship.
Patient access to records is not new, and neither is the Cures Act, which dates to 2016. What is new is the requirement that patients have electronic records access that is fast and easy. This requirement is expected to result in more patients – still a small proportion overall, but more patients – accessing additional EHR information, including providers’ notes.
The requirement to provide patients with EHR access raises questions for health care practices. Some questions are logistical, and some are relational. Concerns include the potential for increased time for patient education, or patient requests for changes to their records that the clinician cannot support.
Health care providers should understand the good, bad, and ugly implications of the Cures Act open notes provisions so they can meet the requirements and reap their benefits, while avoiding the potential for fines or sanctions based on noncompliance, or other negative impacts.
Good news about open notes
Many patients feel better about their provider after reading a note. Positive effects on the patient-provider relationship may be most significant among vulnerable patients, such as those with fewer years of formal education.
Further, open notes have positive impacts on patient engagement and understanding. Patients report that reading notes is a way to better understand and feel more in control of their health care. They also say it builds trust with their provider. The nonprofit organization OpenNotes (not a part of the Cures Act) cites helping laypeople maintain trust in scientific medicine as one benefit of the transparency created by the Cures Act open notes provisions.
Bad news about open notes
Concerns about open notes mainly revolve around the potential for conflicts with patients and potential time conflicts.
Concerns include:
- Timing: The originally planned implementation date for the open notes provisions in the Cures Act was November 2020. Because of the COVID-19 pandemic, this was pushed back to April 2021. However, many providers and practices are still feeling the pandemic’s effects, leading to the question: “Will new demands never end?”
- Uncertainty about the documentation process: Most patients will not understand clinical shorthand, and providers may need added time for explanation. Providers are wondering: “How can I make my notes comprehensible to patients while still writing them quickly?”
- Technology: Some EHR vendors are still racing to provide services that allow practices to remain in compliance with the Cures Act. It may be necessary for a provider to call their EHR vendor and say: “What are you doing to ensure my interoperability compliance?” Meanwhile, secure drop box options for records requests provide a workaround.
Ugly news about open notes
Some patient requests for record amendment are legitimate and easily handled. Some patients, however, will request removal of material they find embarrassing, even though it is accurate.
More frequent requests for records changes from patients could increase already weighty administrative burdens on providers. Worse, some of these requests will be for changes providers cannot support, and making time for careful conversations with patients and providing written responses for requests that are rejected will be a challenge. Inevitably, some of these conversations will not go well, whether through the patient feeling the provider did not adequately respond to their concerns, or through the patient insisting on unreasonable demands. These negative relationship outcomes will add emotional stress on both the patient and the provider, as well as a reputational threat to providers from angry patients posting negative reviews online.
More tangibly, noncompliance with the open notes requirement carries the potential for fines, penalties, and/or sanctions from medical boards. The specifics of potential penalties are not yet known – there are more changes coming with the Cures Act.
Making changes in open notes
Patients will ask providers to amend their medical records. Be familiar with what the patient has the right to ask, what the provider can grant and/or refuse, and how to amend notes.
Here are some highlights:
- Patients have the right to request amendments to their medical records: HIPAA requires a signed, dated request from the patient regarding what they want changed and why.
- Providers have the right to determine whether the requested amendment will be made: The provider must respond, in writing, within 60 days of receipt of the patient’s request.
- Common reasons to deny a patient’s request include that the provider who received the request did not create the record entry, or that the medical record is accurate as is.
- The patient’s request and the provider’s response both become part of the patient’s medical record.
Strategies for success
When composing notes, certain simple strategies will raise the odds that notes will be well understood and well received. Beyond being clear and succinct, strategies for success include composing at least a portion of the note as instructions directly addressed to the patient – “Start taking lisinopril and check your blood pressure twice a week” versus “Initiated lisinopril and instructed her to check her blood pressure twice a week” – and providing a list of commonly used medical terms and abbreviations.
For an in-depth review of strategies for success when composing notes, see “12 Strategies for Success With Open Notes in Healthcare: The Cures Act.”
Exceptions
Unless an exception applies, clinical notes must not be blocked, but the Cures Act allows for a fairly long list of specific, well-delineated exceptions. For instance, a record can be blocked if a provider believes that viewing a note presents a substantial risk of harm to the physical safety of the patient or someone else. The Cures Act also recognizes exemptions that apply to certain caregiving situations, such as when parents attempt to access confidential parts of an adolescent child’s records.
For information regarding exceptions to open notes, please see “What Open Notes Exceptions Does the Cures Act Allow?”
Seeing open notes as part of high-touch, high-value care
While many physicians and other providers have anticipated open notes with dread, most outcomes so far have been positive. Patients have reacted well to clarity. They have used open notes as a tool to improve their own understanding of and adherence to care instructions. When patients have noted valid issues or miscommunications, they have appreciated being able to quickly clear them up. More than an administrative burden, open notes present an opportunity to improve documentation, patient-provider relationships, and patient safety. By improving patient adherence to treatment plans, open notes have the potential to improve provider satisfaction, as well.
Chad Anguilm, MBA, is vice president, in-practice technology services, Medical Advantage, part of TDC Group. Richard F. Cahill, JD, is vice president and associate general counsel, The Doctors Company, part of TDC Group. Kathleen Stillwell, MPA/HSA, RN, is senior patient safety risk manager, The Doctors Company, part of TDC Group.
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
On April 5, 2021, a requirement of the 21st Century Cures Act went into effect: Patients must be able to access information in their EHRs “without delay.” (This requirement does not apply to paper records.) The Cures Act prohibition against information blocking, often referred to as an “open notes” provision, provides patients with transparency in the outcomes of their health care via convenient access to information in their EHR, which can positively or negatively impact the patient-doctor relationship.
Patient access to records is not new, and neither is the Cures Act, which dates to 2016. What is new is the requirement that patients have electronic records access that is fast and easy. This requirement is expected to result in more patients – still a small proportion overall, but more patients – accessing additional EHR information, including providers’ notes.
The requirement to provide patients with EHR access raises questions for health care practices. Some questions are logistical, and some are relational. Concerns include the potential for increased time for patient education, or patient requests for changes to their records that the clinician cannot support.
Health care providers should understand the good, bad, and ugly implications of the Cures Act open notes provisions so they can meet the requirements and reap their benefits, while avoiding the potential for fines or sanctions based on noncompliance, or other negative impacts.
Good news about open notes
Many patients feel better about their provider after reading a note. Positive effects on the patient-provider relationship may be most significant among vulnerable patients, such as those with fewer years of formal education.
Further, open notes have positive impacts on patient engagement and understanding. Patients report that reading notes is a way to better understand and feel more in control of their health care. They also say it builds trust with their provider. The nonprofit organization OpenNotes (not a part of the Cures Act) cites helping laypeople maintain trust in scientific medicine as one benefit of the transparency created by the Cures Act open notes provisions.
Bad news about open notes
Concerns about open notes mainly revolve around the potential for conflicts with patients and potential time conflicts.
Concerns include:
- Timing: The originally planned implementation date for the open notes provisions in the Cures Act was November 2020. Because of the COVID-19 pandemic, this was pushed back to April 2021. However, many providers and practices are still feeling the pandemic’s effects, leading to the question: “Will new demands never end?”
- Uncertainty about the documentation process: Most patients will not understand clinical shorthand, and providers may need added time for explanation. Providers are wondering: “How can I make my notes comprehensible to patients while still writing them quickly?”
- Technology: Some EHR vendors are still racing to provide services that allow practices to remain in compliance with the Cures Act. It may be necessary for a provider to call their EHR vendor and say: “What are you doing to ensure my interoperability compliance?” Meanwhile, secure drop box options for records requests provide a workaround.
Ugly news about open notes
Some patient requests for record amendment are legitimate and easily handled. Some patients, however, will request removal of material they find embarrassing, even though it is accurate.
More frequent requests for records changes from patients could increase already weighty administrative burdens on providers. Worse, some of these requests will be for changes providers cannot support, and making time for careful conversations with patients and providing written responses for requests that are rejected will be a challenge. Inevitably, some of these conversations will not go well, whether through the patient feeling the provider did not adequately respond to their concerns, or through the patient insisting on unreasonable demands. These negative relationship outcomes will add emotional stress on both the patient and the provider, as well as a reputational threat to providers from angry patients posting negative reviews online.
More tangibly, noncompliance with the open notes requirement carries the potential for fines, penalties, and/or sanctions from medical boards. The specifics of potential penalties are not yet known – there are more changes coming with the Cures Act.
Making changes in open notes
Patients will ask providers to amend their medical records. Be familiar with what the patient has the right to ask, what the provider can grant and/or refuse, and how to amend notes.
Here are some highlights:
- Patients have the right to request amendments to their medical records: HIPAA requires a signed, dated request from the patient regarding what they want changed and why.
- Providers have the right to determine whether the requested amendment will be made: The provider must respond, in writing, within 60 days of receipt of the patient’s request.
- Common reasons to deny a patient’s request include that the provider who received the request did not create the record entry, or that the medical record is accurate as is.
- The patient’s request and the provider’s response both become part of the patient’s medical record.
Strategies for success
When composing notes, certain simple strategies will raise the odds that notes will be well understood and well received. Beyond being clear and succinct, strategies for success include composing at least a portion of the note as instructions directly addressed to the patient – “Start taking lisinopril and check your blood pressure twice a week” versus “Initiated lisinopril and instructed her to check her blood pressure twice a week” – and providing a list of commonly used medical terms and abbreviations.
For an in-depth review of strategies for success when composing notes, see “12 Strategies for Success With Open Notes in Healthcare: The Cures Act.”
Exceptions
Unless an exception applies, clinical notes must not be blocked, but the Cures Act allows for a fairly long list of specific, well-delineated exceptions. For instance, a record can be blocked if a provider believes that viewing a note presents a substantial risk of harm to the physical safety of the patient or someone else. The Cures Act also recognizes exemptions that apply to certain caregiving situations, such as when parents attempt to access confidential parts of an adolescent child’s records.
For information regarding exceptions to open notes, please see “What Open Notes Exceptions Does the Cures Act Allow?”
Seeing open notes as part of high-touch, high-value care
While many physicians and other providers have anticipated open notes with dread, most outcomes so far have been positive. Patients have reacted well to clarity. They have used open notes as a tool to improve their own understanding of and adherence to care instructions. When patients have noted valid issues or miscommunications, they have appreciated being able to quickly clear them up. More than an administrative burden, open notes present an opportunity to improve documentation, patient-provider relationships, and patient safety. By improving patient adherence to treatment plans, open notes have the potential to improve provider satisfaction, as well.
Chad Anguilm, MBA, is vice president, in-practice technology services, Medical Advantage, part of TDC Group. Richard F. Cahill, JD, is vice president and associate general counsel, The Doctors Company, part of TDC Group. Kathleen Stillwell, MPA/HSA, RN, is senior patient safety risk manager, The Doctors Company, part of TDC Group.
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
On April 5, 2021, a requirement of the 21st Century Cures Act went into effect: Patients must be able to access information in their EHRs “without delay.” (This requirement does not apply to paper records.) The Cures Act prohibition against information blocking, often referred to as an “open notes” provision, provides patients with transparency in the outcomes of their health care via convenient access to information in their EHR, which can positively or negatively impact the patient-doctor relationship.
Patient access to records is not new, and neither is the Cures Act, which dates to 2016. What is new is the requirement that patients have electronic records access that is fast and easy. This requirement is expected to result in more patients – still a small proportion overall, but more patients – accessing additional EHR information, including providers’ notes.
The requirement to provide patients with EHR access raises questions for health care practices. Some questions are logistical, and some are relational. Concerns include the potential for increased time for patient education, or patient requests for changes to their records that the clinician cannot support.
Health care providers should understand the good, bad, and ugly implications of the Cures Act open notes provisions so they can meet the requirements and reap their benefits, while avoiding the potential for fines or sanctions based on noncompliance, or other negative impacts.
Good news about open notes
Many patients feel better about their provider after reading a note. Positive effects on the patient-provider relationship may be most significant among vulnerable patients, such as those with fewer years of formal education.
Further, open notes have positive impacts on patient engagement and understanding. Patients report that reading notes is a way to better understand and feel more in control of their health care. They also say it builds trust with their provider. The nonprofit organization OpenNotes (not a part of the Cures Act) cites helping laypeople maintain trust in scientific medicine as one benefit of the transparency created by the Cures Act open notes provisions.
Bad news about open notes
Concerns about open notes mainly revolve around the potential for conflicts with patients and potential time conflicts.
Concerns include:
- Timing: The originally planned implementation date for the open notes provisions in the Cures Act was November 2020. Because of the COVID-19 pandemic, this was pushed back to April 2021. However, many providers and practices are still feeling the pandemic’s effects, leading to the question: “Will new demands never end?”
- Uncertainty about the documentation process: Most patients will not understand clinical shorthand, and providers may need added time for explanation. Providers are wondering: “How can I make my notes comprehensible to patients while still writing them quickly?”
- Technology: Some EHR vendors are still racing to provide services that allow practices to remain in compliance with the Cures Act. It may be necessary for a provider to call their EHR vendor and say: “What are you doing to ensure my interoperability compliance?” Meanwhile, secure drop box options for records requests provide a workaround.
Ugly news about open notes
Some patient requests for record amendment are legitimate and easily handled. Some patients, however, will request removal of material they find embarrassing, even though it is accurate.
More frequent requests for records changes from patients could increase already weighty administrative burdens on providers. Worse, some of these requests will be for changes providers cannot support, and making time for careful conversations with patients and providing written responses for requests that are rejected will be a challenge. Inevitably, some of these conversations will not go well, whether through the patient feeling the provider did not adequately respond to their concerns, or through the patient insisting on unreasonable demands. These negative relationship outcomes will add emotional stress on both the patient and the provider, as well as a reputational threat to providers from angry patients posting negative reviews online.
More tangibly, noncompliance with the open notes requirement carries the potential for fines, penalties, and/or sanctions from medical boards. The specifics of potential penalties are not yet known – there are more changes coming with the Cures Act.
Making changes in open notes
Patients will ask providers to amend their medical records. Be familiar with what the patient has the right to ask, what the provider can grant and/or refuse, and how to amend notes.
Here are some highlights:
- Patients have the right to request amendments to their medical records: HIPAA requires a signed, dated request from the patient regarding what they want changed and why.
- Providers have the right to determine whether the requested amendment will be made: The provider must respond, in writing, within 60 days of receipt of the patient’s request.
- Common reasons to deny a patient’s request include that the provider who received the request did not create the record entry, or that the medical record is accurate as is.
- The patient’s request and the provider’s response both become part of the patient’s medical record.
Strategies for success
When composing notes, certain simple strategies will raise the odds that notes will be well understood and well received. Beyond being clear and succinct, strategies for success include composing at least a portion of the note as instructions directly addressed to the patient – “Start taking lisinopril and check your blood pressure twice a week” versus “Initiated lisinopril and instructed her to check her blood pressure twice a week” – and providing a list of commonly used medical terms and abbreviations.
For an in-depth review of strategies for success when composing notes, see “12 Strategies for Success With Open Notes in Healthcare: The Cures Act.”
Exceptions
Unless an exception applies, clinical notes must not be blocked, but the Cures Act allows for a fairly long list of specific, well-delineated exceptions. For instance, a record can be blocked if a provider believes that viewing a note presents a substantial risk of harm to the physical safety of the patient or someone else. The Cures Act also recognizes exemptions that apply to certain caregiving situations, such as when parents attempt to access confidential parts of an adolescent child’s records.
For information regarding exceptions to open notes, please see “What Open Notes Exceptions Does the Cures Act Allow?”
Seeing open notes as part of high-touch, high-value care
While many physicians and other providers have anticipated open notes with dread, most outcomes so far have been positive. Patients have reacted well to clarity. They have used open notes as a tool to improve their own understanding of and adherence to care instructions. When patients have noted valid issues or miscommunications, they have appreciated being able to quickly clear them up. More than an administrative burden, open notes present an opportunity to improve documentation, patient-provider relationships, and patient safety. By improving patient adherence to treatment plans, open notes have the potential to improve provider satisfaction, as well.
Chad Anguilm, MBA, is vice president, in-practice technology services, Medical Advantage, part of TDC Group. Richard F. Cahill, JD, is vice president and associate general counsel, The Doctors Company, part of TDC Group. Kathleen Stillwell, MPA/HSA, RN, is senior patient safety risk manager, The Doctors Company, part of TDC Group.
Ulcerated and Verrucous Plaque on the Chest
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
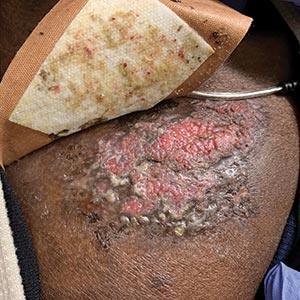
A 36-year-old man presented to an emergency department in the southwestern United States with a cough, fatigue, and worsening back pain associated with night sweats of 1 month’s duration. He experienced a 9.07-kg weight loss, as well as development of a rough, nontender, nonpruritic rash along the left upper chest over the prior month. The patient was born in West Africa and reported that he had moved to the southwestern United States from the eastern United States approximately 6 years prior to presentation. Physical examination on admission revealed a 5×3-cm, purple-brown, verrucous plaque with a central pink cobblestone appearance and ulceration. Chest radiography was notable for perihilar adenopathy with no focal infiltrates or cavitary lesions. Computed tomography and magnetic resonance imaging of the chest were notable for miliary nodules throughout the lungs; extensive lytic spine lesions of cervical, thoracic, and lumbar vertebral bodies and left twelfth rib; and a left paraspinal thoracic epidural soft tissue phlegmon. Initial laboratory investigations revealed peripheral eosinophilia without absolute leukocytosis and a microcytic anemia.
FDA approves rapid-acting insulin, Lyumjev, for pump use
The Food and Drug Administration has expanded the label for Eli Lilly’s ultra–rapid-acting insulin lispro-aabc injection 100 units/mL (Lyumjev) for use in insulin pumps.
Lyumjev (insulin lispro-aabc injection 100 and 200 units/mL) was initially approved in June 2020 to improve glycemic control in adults with type 1 or type 2 diabetes. That formulation is administered by injection from a pen or syringe. Now, the 100 units/mL formulation can also be delivered via continuous subcutaneous insulin infusion with an insulin pump.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp). Fiasp had a head start: it was approved for use in adults in the United States in September 2017. It was approved for use in insulin pumps in October 2019 and for use in children with diabetes in January 2020.
The new approval for Lyumjev was based on data from a phase 3 trial, PRONTO-Pump-2. That trial, which included 432 participants with type 1 diabetes, confirmed the drug’s safety and efficacy when used in pumps.
The study met the primary endpoint of noninferiority in reduction of hemoglobin A1c from baseline to week 16, compared with insulin lispro (Humalog 100 units/mL). It was superior in both 1-hour and 2-hour postprandial glucose reduction when delivered 0-2 minutes before meals, according to a Lilly statement.
Patients who cannot afford the drug can go to www.insulinaffordability.com for assistance. Those with commercial insurance can also visit www.Lyumjev.com to access the Lyumjev Savings Card.
Lyumjev is available in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the label for Eli Lilly’s ultra–rapid-acting insulin lispro-aabc injection 100 units/mL (Lyumjev) for use in insulin pumps.
Lyumjev (insulin lispro-aabc injection 100 and 200 units/mL) was initially approved in June 2020 to improve glycemic control in adults with type 1 or type 2 diabetes. That formulation is administered by injection from a pen or syringe. Now, the 100 units/mL formulation can also be delivered via continuous subcutaneous insulin infusion with an insulin pump.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp). Fiasp had a head start: it was approved for use in adults in the United States in September 2017. It was approved for use in insulin pumps in October 2019 and for use in children with diabetes in January 2020.
The new approval for Lyumjev was based on data from a phase 3 trial, PRONTO-Pump-2. That trial, which included 432 participants with type 1 diabetes, confirmed the drug’s safety and efficacy when used in pumps.
The study met the primary endpoint of noninferiority in reduction of hemoglobin A1c from baseline to week 16, compared with insulin lispro (Humalog 100 units/mL). It was superior in both 1-hour and 2-hour postprandial glucose reduction when delivered 0-2 minutes before meals, according to a Lilly statement.
Patients who cannot afford the drug can go to www.insulinaffordability.com for assistance. Those with commercial insurance can also visit www.Lyumjev.com to access the Lyumjev Savings Card.
Lyumjev is available in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the label for Eli Lilly’s ultra–rapid-acting insulin lispro-aabc injection 100 units/mL (Lyumjev) for use in insulin pumps.
Lyumjev (insulin lispro-aabc injection 100 and 200 units/mL) was initially approved in June 2020 to improve glycemic control in adults with type 1 or type 2 diabetes. That formulation is administered by injection from a pen or syringe. Now, the 100 units/mL formulation can also be delivered via continuous subcutaneous insulin infusion with an insulin pump.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp). Fiasp had a head start: it was approved for use in adults in the United States in September 2017. It was approved for use in insulin pumps in October 2019 and for use in children with diabetes in January 2020.
The new approval for Lyumjev was based on data from a phase 3 trial, PRONTO-Pump-2. That trial, which included 432 participants with type 1 diabetes, confirmed the drug’s safety and efficacy when used in pumps.
The study met the primary endpoint of noninferiority in reduction of hemoglobin A1c from baseline to week 16, compared with insulin lispro (Humalog 100 units/mL). It was superior in both 1-hour and 2-hour postprandial glucose reduction when delivered 0-2 minutes before meals, according to a Lilly statement.
Patients who cannot afford the drug can go to www.insulinaffordability.com for assistance. Those with commercial insurance can also visit www.Lyumjev.com to access the Lyumjev Savings Card.
Lyumjev is available in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps.
A version of this article first appeared on Medscape.com.
U.S. reports record COVID-19 hospitalizations of children
The number of children hospitalized with COVID-19 in the U.S. hit a record high on Aug. 14, with more than 1,900 in hospitals.
Hospitals across the South are running out of beds as the contagious Delta variant spreads, mostly among unvaccinated people. Children make up about 2.4% of the country’s COVID-19 hospitalizations, and those under 12 are particularly vulnerable since they’re not eligible to receive a vaccine.
“This is not last year’s COVID,” Sally Goza, MD, former president of the American Academy of Pediatrics, told CNN on Aug. 14.
“This one is worse, and our children are the ones that are going to be affected by it the most,” she said.
The number of newly hospitalized COVID-19 patients for ages 18-49 also hit record highs during the week of Aug. 9. A fifth of the nation’s hospitalizations are in Florida, where the number of COVID-19 patients hit a record high of 16,100 on Aug. 14. More than 90% of the state’s intensive care unit beds are filled.
More than 90% of the ICU beds in Texas are full as well. On Aug. 13, there were no pediatric ICU beds available in Dallas or the 19 surrounding counties, which means that young patients would be transported father away for care – even Oklahoma City.
“That means if your child’s in a car wreck, if your child has a congenital heart defect or something and needs an ICU bed, or more likely, if they have COVID and need an ICU bed, we don’t have one,” Clay Jenkins, a Dallas County judge, said on Aug. 13.
“Your child will wait for another child to die,” he said.
As children return to classes, educators are talking about the possibility of vaccine mandates. The National Education Association announced its support of mandatory vaccination for its members.
“Our students under 12 can’t get vaccinated,” Becky Pringle, president of the association, told CNN.
“It’s our responsibility to keep them safe,” she said. “Keeping them safe means that everyone who can be vaccinated should be vaccinated.”
The U.S. now has an average of about 129,000 new COVID-19 cases per day, Reuters reported, which has doubled in about 2 weeks. The number of hospitalized patients is at a 6-month high, and about 600 people are dying each day.
Arkansas, Florida, Louisiana, Mississippi, and Oregon have reported record numbers of COVID-19 hospitalizations.
In addition, eight states make up half of all the COVID-19 hospitalizations in the U.S. but only 24% of the nation’s population – Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Nevada, and Texas. These states have vaccination rates lower than the national average, and their COVID-19 patients account for at least 15% of their overall hospitalizations.
To address the surge in hospitalizations, Oregon Gov. Kate Brown has ordered the deployment of up to 1,500 Oregon National Guard members to help health care workers.
“I know this is not the summer many of us envisioned,” Gov. Brown said Aug. 13. “The harsh and frustrating reality is that the Delta variant has changed everything. Delta is highly contagious, and we must take action now.”
A version of this article first appeared on WebMD.com.
The number of children hospitalized with COVID-19 in the U.S. hit a record high on Aug. 14, with more than 1,900 in hospitals.
Hospitals across the South are running out of beds as the contagious Delta variant spreads, mostly among unvaccinated people. Children make up about 2.4% of the country’s COVID-19 hospitalizations, and those under 12 are particularly vulnerable since they’re not eligible to receive a vaccine.
“This is not last year’s COVID,” Sally Goza, MD, former president of the American Academy of Pediatrics, told CNN on Aug. 14.
“This one is worse, and our children are the ones that are going to be affected by it the most,” she said.
The number of newly hospitalized COVID-19 patients for ages 18-49 also hit record highs during the week of Aug. 9. A fifth of the nation’s hospitalizations are in Florida, where the number of COVID-19 patients hit a record high of 16,100 on Aug. 14. More than 90% of the state’s intensive care unit beds are filled.
More than 90% of the ICU beds in Texas are full as well. On Aug. 13, there were no pediatric ICU beds available in Dallas or the 19 surrounding counties, which means that young patients would be transported father away for care – even Oklahoma City.
“That means if your child’s in a car wreck, if your child has a congenital heart defect or something and needs an ICU bed, or more likely, if they have COVID and need an ICU bed, we don’t have one,” Clay Jenkins, a Dallas County judge, said on Aug. 13.
“Your child will wait for another child to die,” he said.
As children return to classes, educators are talking about the possibility of vaccine mandates. The National Education Association announced its support of mandatory vaccination for its members.
“Our students under 12 can’t get vaccinated,” Becky Pringle, president of the association, told CNN.
“It’s our responsibility to keep them safe,” she said. “Keeping them safe means that everyone who can be vaccinated should be vaccinated.”
The U.S. now has an average of about 129,000 new COVID-19 cases per day, Reuters reported, which has doubled in about 2 weeks. The number of hospitalized patients is at a 6-month high, and about 600 people are dying each day.
Arkansas, Florida, Louisiana, Mississippi, and Oregon have reported record numbers of COVID-19 hospitalizations.
In addition, eight states make up half of all the COVID-19 hospitalizations in the U.S. but only 24% of the nation’s population – Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Nevada, and Texas. These states have vaccination rates lower than the national average, and their COVID-19 patients account for at least 15% of their overall hospitalizations.
To address the surge in hospitalizations, Oregon Gov. Kate Brown has ordered the deployment of up to 1,500 Oregon National Guard members to help health care workers.
“I know this is not the summer many of us envisioned,” Gov. Brown said Aug. 13. “The harsh and frustrating reality is that the Delta variant has changed everything. Delta is highly contagious, and we must take action now.”
A version of this article first appeared on WebMD.com.
The number of children hospitalized with COVID-19 in the U.S. hit a record high on Aug. 14, with more than 1,900 in hospitals.
Hospitals across the South are running out of beds as the contagious Delta variant spreads, mostly among unvaccinated people. Children make up about 2.4% of the country’s COVID-19 hospitalizations, and those under 12 are particularly vulnerable since they’re not eligible to receive a vaccine.
“This is not last year’s COVID,” Sally Goza, MD, former president of the American Academy of Pediatrics, told CNN on Aug. 14.
“This one is worse, and our children are the ones that are going to be affected by it the most,” she said.
The number of newly hospitalized COVID-19 patients for ages 18-49 also hit record highs during the week of Aug. 9. A fifth of the nation’s hospitalizations are in Florida, where the number of COVID-19 patients hit a record high of 16,100 on Aug. 14. More than 90% of the state’s intensive care unit beds are filled.
More than 90% of the ICU beds in Texas are full as well. On Aug. 13, there were no pediatric ICU beds available in Dallas or the 19 surrounding counties, which means that young patients would be transported father away for care – even Oklahoma City.
“That means if your child’s in a car wreck, if your child has a congenital heart defect or something and needs an ICU bed, or more likely, if they have COVID and need an ICU bed, we don’t have one,” Clay Jenkins, a Dallas County judge, said on Aug. 13.
“Your child will wait for another child to die,” he said.
As children return to classes, educators are talking about the possibility of vaccine mandates. The National Education Association announced its support of mandatory vaccination for its members.
“Our students under 12 can’t get vaccinated,” Becky Pringle, president of the association, told CNN.
“It’s our responsibility to keep them safe,” she said. “Keeping them safe means that everyone who can be vaccinated should be vaccinated.”
The U.S. now has an average of about 129,000 new COVID-19 cases per day, Reuters reported, which has doubled in about 2 weeks. The number of hospitalized patients is at a 6-month high, and about 600 people are dying each day.
Arkansas, Florida, Louisiana, Mississippi, and Oregon have reported record numbers of COVID-19 hospitalizations.
In addition, eight states make up half of all the COVID-19 hospitalizations in the U.S. but only 24% of the nation’s population – Alabama, Arkansas, Florida, Georgia, Louisiana, Mississippi, Nevada, and Texas. These states have vaccination rates lower than the national average, and their COVID-19 patients account for at least 15% of their overall hospitalizations.
To address the surge in hospitalizations, Oregon Gov. Kate Brown has ordered the deployment of up to 1,500 Oregon National Guard members to help health care workers.
“I know this is not the summer many of us envisioned,” Gov. Brown said Aug. 13. “The harsh and frustrating reality is that the Delta variant has changed everything. Delta is highly contagious, and we must take action now.”
A version of this article first appeared on WebMD.com.
Shedding the super-doctor myth requires an honest look at systemic racism
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
An overwhelmingly loud and high-pitched screech rattles against your hip. You startle and groan into the pillow as your thoughts settle into conscious awareness. It is 3 a.m. You are a 2nd-year resident trudging through the night shift, alerted to the presence of a new patient awaiting an emergency assessment. You are the only in-house physician. Walking steadfastly toward the emergency unit, you enter and greet the patient. Immediately, you observe a look of surprise followed immediately by a scowl.
You extend a hand, but your greeting is abruptly cut short with: “I want to see a doctor!” You pace your breaths to quell annoyance and resume your introduction, asserting that you are a doctor and indeed the only doctor on duty. After moments of deep sighs and questions regarding your credentials, you persuade the patient to start the interview.
It is now 8 a.m. The frustration of the night starts to ease as you prepare to leave. While gathering your things, a visitor is overheard inquiring the whereabouts of a hospital unit. Volunteering as a guide, you walk the person toward the opposite end of the hospital. Bleary eyed, muscle laxed, and bone weary, you point out the entrance, then turn to leave. The steady rhythm of your steps suddenly halts as you hear from behind: “Thank you! You speak English really well!” Blankly, you stare. Your voice remains mute while your brain screams: “What is that supposed to mean?” But you do not utter a sound, because intuitively, you know the answer.
While reading this scenario, what did you feel? Pride in knowing that the physician was able to successfully navigate a busy night? Relief in the physician’s ability to maintain a professional demeanor despite belittling microaggressions? Are you angry? Would you replay those moments like reruns of a bad TV show? Can you imagine entering your home and collapsing onto the bed as your tears of fury pool over your rumpled sheets?
The emotional release of that morning is seared into my memory. Over the years, I questioned my reactions. Was I too passive? Should I have schooled them on their ignorance? Had I done so, would I have incurred reprimands? Would standing up for myself cause years of hard work to fall away? Moreover, had I defended myself, would I forever have been viewed as “The Angry Black Woman?”
This story is more than a vignette. For me, it is another reminder that, despite how far we have come, we have much further to go. As a Black woman in a professional sphere, I stand upon the shoulders of those who sacrificed for a dream, a greater purpose. My foremothers and forefathers fought bravely and tirelessly so that we could attain levels of success that were only once but a dream. Despite this progress, a grimace, carelessly spoken words, or a mindless gesture remind me that, no matter how much I toil and what levels of success I achieve, when I meet someone for the first time or encounter someone from my past, I find myself wondering whether I am remembered for me or because I am “The Black One.”
Honest look at medicine is imperative
It is important to consider multiple facets of the super-doctor myth. We are dedicated, fearless, authoritative, ambitious individuals. We do not yield to sickness, family obligations, or fatigue. Medicine is a calling, and the patient deserves the utmost respect and professional behavior. Impervious to ethnicity, race, nationality, or creed, we are unbiased and always in service of the greater good. Often, however, I wonder how the expectations of patient-focused, patient-centered care can prevail without an honest look at the vicissitudes facing medicine.
We find ourselves amid a tumultuous year overshadowed by a devastating pandemic that skews heavily toward Black and Brown communities, in addition to political turmoil and racial reckoning that sprang forth from fear, anger, and determination ignited by the murders of Breonna Taylor and George Floyd – communities united in outrage lamenting the cries of Black Lives Matter.
I remember the tears briskly falling upon my blouse as I watched Mr. Floyd’s life violently ripped from this Earth. Shortly thereafter, I remember the phone calls, emails, and texts from close friends, acquaintances, and colleagues offering support, listening ears, pledging to learn and endeavoring to understand the struggle for recognition and the fight for human rights. Even so, the deafening support was clouded by the preternatural silence of some medical organizations. Within the Black physician community, outrage was palpable. We reflected upon years of sacrifice and perseverance despite the challenge of bigotry, ignorance, and racism – not only from patients and their families – but also colleagues and administrators. Yet, in our time of horror and need, in those moments of vulnerability ... silence. Eventually, lengthy proclamations of support were expressed through various media. However, it felt too safe, too corporate, and too generic and inauthentic. As a result, an exodus of Black physicians from leadership positions and academic medicine took hold as the blatant continuation of rhetoric – coupled with ineffective outreach and support – finally took its toll.
Frequently, I question how the obstacles of medical school, residency, and beyond are expected to be traversed while living in a world that consistently affords additional challenges to those who look, act, or speak in a manner that varies from the perceived standard. In a culture where the myth of the super doctor reigns, how do we reconcile attainment of a false and detrimental narrative while the overarching pressure acutely felt by Black physicians magnifies in the setting of stereotypes, sociopolitical turbulence, bigotry, and racism? How can one sacrifice for an entity that is unwilling to acknowledge the psychological implications of that sacrifice?
For instance, while in medical school, I transitioned my hair to its natural state but was counseled against doing so because of the risk of losing residency opportunities as a direct result of my “unprofessional” appearance. Throughout residency, multiple incidents come to mind, including frequent demands to see my hospital badge despite the same not being of asked of my White cohorts; denial of entry into physician entrance within the residency building because, despite my professional attire, I was presumed to be a member of the custodial staff; and patients being confused and asking for a doctor despite my long white coat and clear introductions.
Furthermore, the fluency of my speech and the absence of regional dialect or vernacular are quite often lauded by patients. Inquiries to touch my hair as well as hypotheses regarding my nationality or degree of “blackness” with respect to the shape of my nose, eyes, and lips are openly questioned. Unfortunately, those uncomfortable incidents have not been limited to patient encounters.
In one instance, while presenting a patient in the presence of my attending and a 3rd-year medical student, I was sternly admonished for disclosing the race of the patient. I sat still and resolute as this doctor spoke on increased risk of bias in diagnosis and treatment when race is identified. Outwardly, I projected patience but inside, I seethed. In that moment, I realized that I would never have the luxury of ignorance or denial. Although I desire to be valued for my prowess in medicine, the mythical status was not created with my skin color in mind. For is avoidance not but a reflection of denial?
In these chaotic and uncertain times, how can we continue to promote a pathological ideal when the roads traveled are so fundamentally skewed? If a White physician faces a belligerent and argumentative patient, there is opportunity for debriefing both individually and among a larger cohort via classes, conferences, and supervisions. Conversely, when a Black physician is derided with racist sentiment, will they have the same opportunity for reflection and support? Despite identical expectations of professionalism and growth, how can one be successful in a system that either directly or indirectly encourages the opposite?
As we try to shed the super-doctor myth, we must recognize that this unattainable and detrimental persona hinders progress. This myth undermines our ability to understand our fragility, the limitations of our capabilities, and the strength of our vulnerability. We must take an honest look at the manner in which our individual biases and the deeply ingrained (and potentially unconscious) systemic biases are counterintuitive to the success and support of physicians of color.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
Outstanding medical bills: Dealing with deadbeats
Since the COVID-19 pandemic began, I have received a growing number of inquiries about collection issues. For a variety of reasons, many patients seem increasingly reluctant to pay their medical bills. I’ve written many columns on keeping credit card numbers on file, and other techniques for keeping your accounts receivable in check; but despite your best efforts, there will always be a few deadbeats that you will need to pursue.
For the record, I am not speaking about patients who lost income due to the pandemic and are now struggling with debts, or otherwise have fallen on hard times and are unable to pay.
The worst kinds of deadbeats are the ones who rob you twice; they accept payments from insurance companies and keep them. Such crooks must be pursued aggressively, with all the means at your disposal; but to reiterate the point I’ve tried to drive home repeatedly, the best cure is prevention.
You already know that you should collect as many fees as possible at the time of service. For cosmetic procedures you should require a substantial deposit in advance, with the balance due at the time of service. When that is impossible, maximize the chances you will be paid by making sure all available payment mechanisms are in place.
With my credit-card-on-file system that I’ve described many times, patients who fail to pay their credit card bill are the credit card company’s problem, not yours. In cases where you suspect fees might exceed credit card limits, you can arrange a realistic payment schedule in advance and have the patient fill out a credit application. You can find forms for this online at formswift.com, templates.office.com, and many other websites.
In some cases, it may be worth the trouble to run a background check. There are easy and affordable ways to do this. Dunn & Bradstreet, for example, will furnish a report containing payment records and details of any lawsuits, liens, and other legal actions for a nominal fee. The more financial information you have on file, the more leverage you have if a patient later balks at paying his or her balance.
For cosmetic work, always take before and after photos, and have all patients sign a written consent giving permission for the procedure, assuming full financial responsibility, and acknowledging that no guarantees have been given or implied. This defuses the common deadbeat tactics of claiming ignorance of personal financial obligations and professing dissatisfaction with the results.
Despite all your precautions, a deadbeat will inevitably slip through on occasion; but even then, you have options for extracting payment. Collection agencies are the traditional first line of attack for most medical practices. Ideally, your agency should specialize in handling medical accounts, so it will know exactly how much pressure to exert to avoid charges of harassment. Delinquent accounts should be submitted earlier rather than later to maximize the chances of success; my manager never allows an account to age more than 90 days, and if circumstances dictate, she refers them sooner than that.
When collection agencies fail, think about small claims court. You will need to learn the rules in your state, but in most states there is a small filing fee and a limit of $5,000 or so on claims. No attorneys are involved. If your paperwork is in order, the court will nearly always rule in your favor, but it will not provide the means for actual collection. In other words, you will still have to persuade the deadbeat to pay up. However, in many states a court order will give you the authority to attach a lien to property, or garnish wages, which often provides enough leverage to force payment.
What about those double-deadbeats who keep the insurance checks for themselves? First, check your third-party contract; sometimes the insurance company or HMO will be compelled to pay you directly and then go after the patient to get back its money. (They won’t volunteer this service, however – you’ll have to ask for it.)
If that’s not an option, consider reporting the misdirected payment to the Internal Revenue Service as income to the patient, by submitting a 1099 Miscellaneous Income form. Be sure to notify the deadbeat that you will be doing this. Sometimes the threat of such action will convince the individual to pay up; if not, at least you’ll have the satisfaction of knowing he or she will have to pay taxes on the money.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Since the COVID-19 pandemic began, I have received a growing number of inquiries about collection issues. For a variety of reasons, many patients seem increasingly reluctant to pay their medical bills. I’ve written many columns on keeping credit card numbers on file, and other techniques for keeping your accounts receivable in check; but despite your best efforts, there will always be a few deadbeats that you will need to pursue.
For the record, I am not speaking about patients who lost income due to the pandemic and are now struggling with debts, or otherwise have fallen on hard times and are unable to pay.
The worst kinds of deadbeats are the ones who rob you twice; they accept payments from insurance companies and keep them. Such crooks must be pursued aggressively, with all the means at your disposal; but to reiterate the point I’ve tried to drive home repeatedly, the best cure is prevention.
You already know that you should collect as many fees as possible at the time of service. For cosmetic procedures you should require a substantial deposit in advance, with the balance due at the time of service. When that is impossible, maximize the chances you will be paid by making sure all available payment mechanisms are in place.
With my credit-card-on-file system that I’ve described many times, patients who fail to pay their credit card bill are the credit card company’s problem, not yours. In cases where you suspect fees might exceed credit card limits, you can arrange a realistic payment schedule in advance and have the patient fill out a credit application. You can find forms for this online at formswift.com, templates.office.com, and many other websites.
In some cases, it may be worth the trouble to run a background check. There are easy and affordable ways to do this. Dunn & Bradstreet, for example, will furnish a report containing payment records and details of any lawsuits, liens, and other legal actions for a nominal fee. The more financial information you have on file, the more leverage you have if a patient later balks at paying his or her balance.
For cosmetic work, always take before and after photos, and have all patients sign a written consent giving permission for the procedure, assuming full financial responsibility, and acknowledging that no guarantees have been given or implied. This defuses the common deadbeat tactics of claiming ignorance of personal financial obligations and professing dissatisfaction with the results.
Despite all your precautions, a deadbeat will inevitably slip through on occasion; but even then, you have options for extracting payment. Collection agencies are the traditional first line of attack for most medical practices. Ideally, your agency should specialize in handling medical accounts, so it will know exactly how much pressure to exert to avoid charges of harassment. Delinquent accounts should be submitted earlier rather than later to maximize the chances of success; my manager never allows an account to age more than 90 days, and if circumstances dictate, she refers them sooner than that.
When collection agencies fail, think about small claims court. You will need to learn the rules in your state, but in most states there is a small filing fee and a limit of $5,000 or so on claims. No attorneys are involved. If your paperwork is in order, the court will nearly always rule in your favor, but it will not provide the means for actual collection. In other words, you will still have to persuade the deadbeat to pay up. However, in many states a court order will give you the authority to attach a lien to property, or garnish wages, which often provides enough leverage to force payment.
What about those double-deadbeats who keep the insurance checks for themselves? First, check your third-party contract; sometimes the insurance company or HMO will be compelled to pay you directly and then go after the patient to get back its money. (They won’t volunteer this service, however – you’ll have to ask for it.)
If that’s not an option, consider reporting the misdirected payment to the Internal Revenue Service as income to the patient, by submitting a 1099 Miscellaneous Income form. Be sure to notify the deadbeat that you will be doing this. Sometimes the threat of such action will convince the individual to pay up; if not, at least you’ll have the satisfaction of knowing he or she will have to pay taxes on the money.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Since the COVID-19 pandemic began, I have received a growing number of inquiries about collection issues. For a variety of reasons, many patients seem increasingly reluctant to pay their medical bills. I’ve written many columns on keeping credit card numbers on file, and other techniques for keeping your accounts receivable in check; but despite your best efforts, there will always be a few deadbeats that you will need to pursue.
For the record, I am not speaking about patients who lost income due to the pandemic and are now struggling with debts, or otherwise have fallen on hard times and are unable to pay.
The worst kinds of deadbeats are the ones who rob you twice; they accept payments from insurance companies and keep them. Such crooks must be pursued aggressively, with all the means at your disposal; but to reiterate the point I’ve tried to drive home repeatedly, the best cure is prevention.
You already know that you should collect as many fees as possible at the time of service. For cosmetic procedures you should require a substantial deposit in advance, with the balance due at the time of service. When that is impossible, maximize the chances you will be paid by making sure all available payment mechanisms are in place.
With my credit-card-on-file system that I’ve described many times, patients who fail to pay their credit card bill are the credit card company’s problem, not yours. In cases where you suspect fees might exceed credit card limits, you can arrange a realistic payment schedule in advance and have the patient fill out a credit application. You can find forms for this online at formswift.com, templates.office.com, and many other websites.
In some cases, it may be worth the trouble to run a background check. There are easy and affordable ways to do this. Dunn & Bradstreet, for example, will furnish a report containing payment records and details of any lawsuits, liens, and other legal actions for a nominal fee. The more financial information you have on file, the more leverage you have if a patient later balks at paying his or her balance.
For cosmetic work, always take before and after photos, and have all patients sign a written consent giving permission for the procedure, assuming full financial responsibility, and acknowledging that no guarantees have been given or implied. This defuses the common deadbeat tactics of claiming ignorance of personal financial obligations and professing dissatisfaction with the results.
Despite all your precautions, a deadbeat will inevitably slip through on occasion; but even then, you have options for extracting payment. Collection agencies are the traditional first line of attack for most medical practices. Ideally, your agency should specialize in handling medical accounts, so it will know exactly how much pressure to exert to avoid charges of harassment. Delinquent accounts should be submitted earlier rather than later to maximize the chances of success; my manager never allows an account to age more than 90 days, and if circumstances dictate, she refers them sooner than that.
When collection agencies fail, think about small claims court. You will need to learn the rules in your state, but in most states there is a small filing fee and a limit of $5,000 or so on claims. No attorneys are involved. If your paperwork is in order, the court will nearly always rule in your favor, but it will not provide the means for actual collection. In other words, you will still have to persuade the deadbeat to pay up. However, in many states a court order will give you the authority to attach a lien to property, or garnish wages, which often provides enough leverage to force payment.
What about those double-deadbeats who keep the insurance checks for themselves? First, check your third-party contract; sometimes the insurance company or HMO will be compelled to pay you directly and then go after the patient to get back its money. (They won’t volunteer this service, however – you’ll have to ask for it.)
If that’s not an option, consider reporting the misdirected payment to the Internal Revenue Service as income to the patient, by submitting a 1099 Miscellaneous Income form. Be sure to notify the deadbeat that you will be doing this. Sometimes the threat of such action will convince the individual to pay up; if not, at least you’ll have the satisfaction of knowing he or she will have to pay taxes on the money.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Hypertension in adults: USPSTF reaffirms this screening protocol
REFERENCES
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
- US Preventive Services Task Force. Screening for hypertension in adults: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
REFERENCES
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
- US Preventive Services Task Force. Screening for hypertension in adults: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
REFERENCES
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
- US Preventive Services Task Force. Screening for hypertension in adults: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
‘No justification’ for suicide warning on all antiseizure meds
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Universal masking is the key to safe school attendance
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
Walking through time
In the Phoenix summer days of 1998 I did a lot of walking. It wasn’t for exercise, though it was pretty good for that, I guess.
I had privileges at three hospitals, and used their staff directories to make a map of every medical office building in the area I was trying to start my practice in. I was 32, idealistic, married for a year, a child on the way, and we’d just bought our first house. So I had a lot of incentive.
The Phoenix summer isn’t conducive to walking, especially in standard medical office attire (I didn’t give that up until 2006). But I did it. I went into one office after another, introduced myself, gave them my CV, some business cards, and my pager number (yeah, I had a pager). I cooled off and drank water in my car as I drove to the next building – wash, rinse, repeat.
Occasionally the doctors I met would have a few minutes to meet me, which I appreciated. One of them, who’d been in the same boat a few years earlier himself, invited me back to his office, and we chatted for maybe 10 minutes.
We got along, and worked well together for several years. We tended to round at the same times of day and so ran into each other a lot. He sent me patients, I sent him patients, and when we met on rounds we’d talk about nothing in particular for a few minutes.
After I cut back on my hospital work I didn’t see him as much, though we still referred patients back and forth and occasionally crossed paths while covering weekends.
I found out that he retired recently.
It gave me an odd pause. I thought of our first encounter 23 years ago, me trying to get started in my profession, him established, but close enough to recall what it was like to be starting out that he spared a few minutes for me. Remembering that, I still try to make an effort to meet new physicians who come by for the same reason. Hell, they might end up taking care of me someday. Assuming a medical career is 30-40 years, I’m past the halfway point.
Not today, not tomorrow, but in the years to come my generation of physicians will start to retire, walking away from a role that has defined both our personal and professional lives.
I both am and am not looking forward to it. This was just another reminder that .
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the Phoenix summer days of 1998 I did a lot of walking. It wasn’t for exercise, though it was pretty good for that, I guess.
I had privileges at three hospitals, and used their staff directories to make a map of every medical office building in the area I was trying to start my practice in. I was 32, idealistic, married for a year, a child on the way, and we’d just bought our first house. So I had a lot of incentive.
The Phoenix summer isn’t conducive to walking, especially in standard medical office attire (I didn’t give that up until 2006). But I did it. I went into one office after another, introduced myself, gave them my CV, some business cards, and my pager number (yeah, I had a pager). I cooled off and drank water in my car as I drove to the next building – wash, rinse, repeat.
Occasionally the doctors I met would have a few minutes to meet me, which I appreciated. One of them, who’d been in the same boat a few years earlier himself, invited me back to his office, and we chatted for maybe 10 minutes.
We got along, and worked well together for several years. We tended to round at the same times of day and so ran into each other a lot. He sent me patients, I sent him patients, and when we met on rounds we’d talk about nothing in particular for a few minutes.
After I cut back on my hospital work I didn’t see him as much, though we still referred patients back and forth and occasionally crossed paths while covering weekends.
I found out that he retired recently.
It gave me an odd pause. I thought of our first encounter 23 years ago, me trying to get started in my profession, him established, but close enough to recall what it was like to be starting out that he spared a few minutes for me. Remembering that, I still try to make an effort to meet new physicians who come by for the same reason. Hell, they might end up taking care of me someday. Assuming a medical career is 30-40 years, I’m past the halfway point.
Not today, not tomorrow, but in the years to come my generation of physicians will start to retire, walking away from a role that has defined both our personal and professional lives.
I both am and am not looking forward to it. This was just another reminder that .
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the Phoenix summer days of 1998 I did a lot of walking. It wasn’t for exercise, though it was pretty good for that, I guess.
I had privileges at three hospitals, and used their staff directories to make a map of every medical office building in the area I was trying to start my practice in. I was 32, idealistic, married for a year, a child on the way, and we’d just bought our first house. So I had a lot of incentive.
The Phoenix summer isn’t conducive to walking, especially in standard medical office attire (I didn’t give that up until 2006). But I did it. I went into one office after another, introduced myself, gave them my CV, some business cards, and my pager number (yeah, I had a pager). I cooled off and drank water in my car as I drove to the next building – wash, rinse, repeat.
Occasionally the doctors I met would have a few minutes to meet me, which I appreciated. One of them, who’d been in the same boat a few years earlier himself, invited me back to his office, and we chatted for maybe 10 minutes.
We got along, and worked well together for several years. We tended to round at the same times of day and so ran into each other a lot. He sent me patients, I sent him patients, and when we met on rounds we’d talk about nothing in particular for a few minutes.
After I cut back on my hospital work I didn’t see him as much, though we still referred patients back and forth and occasionally crossed paths while covering weekends.
I found out that he retired recently.
It gave me an odd pause. I thought of our first encounter 23 years ago, me trying to get started in my profession, him established, but close enough to recall what it was like to be starting out that he spared a few minutes for me. Remembering that, I still try to make an effort to meet new physicians who come by for the same reason. Hell, they might end up taking care of me someday. Assuming a medical career is 30-40 years, I’m past the halfway point.
Not today, not tomorrow, but in the years to come my generation of physicians will start to retire, walking away from a role that has defined both our personal and professional lives.
I both am and am not looking forward to it. This was just another reminder that .
Dr. Block has a solo neurology practice in Scottsdale, Ariz.












