User login
Heroes: Nurses’ sacrifice in the age of COVID-19
This past year, the referrals to my private practice have taken a noticeable shift and caused me to pause.
More calls have come from nurses, many who work directly with COVID-19 patients, understandably seeking mental health treatment, or support. Especially in this time, nurses are facing trauma and stress that is unimaginable to many, myself included. Despite the collective efforts we have made as a society to recognize their work, I do not think we have given enough consideration to the enormous sacrifice nurses are currently undertaking to save our collective psyche.
As physicians and mental health providers, we have a glimpse into the complexities and stressors of medical treatment. In our line of work, we support patients with trauma on a regular basis. We feel deeply connected to patients, some of whom we have treated until the end of their lives. Despite that, I am not sure that I, or anyone, can truly comprehend what nurses face in today’s climate of care.
There is no denying that doctors are of value to our system, but our service has limits; nurses and doctors operate as two sides to a shared coin. As doctors, we diagnose and prescribe, while nurses explain and dispense. As doctors, we talk to patients, while nurses comfort them. Imagine spending an entire year working in a hospital diligently wiping endotracheal tubes that are responsible for maintaining someone’s life. Imagine spending an entire year laboring through the heavy task of lifting patients to prone them in a position that may save their lives. Imagine spending an entire year holding the hands of comatose patients in hopes of maintaining a sense of humanity.
And this only begins to describe the tasks bestowed upon nurses. While doctors answer pagers or complete insurance authorization forms, nurses empathize and reassure scared and isolated patients. Imagine spending an entire year updating crying family members who cannot see their loved ones. Imagine spending an entire year explaining and pleading to the outside world that wearing a mask and washing hands would reduce the suffering that takes place inside the hospital walls.
Despite the uncertainties, pressures, and demands, nurses have continued, and will continue, to show up for their patients, shift by shift. It takes a tragic number of deaths for the nurses I see in my practice to share that they have lost count. These numbers reflect people they held to feed, carried to prevent ulcers, wiped for decency, caressed for compassion, probed with IVs and tubes, monitored for signs of life, and warmed with blankets. If love were in any job description, it would fall under that of a nurse.
And we can’t ignore the fact that all the lives lost by COVID-19 had family. Family members who, without ever stepping foot in the hospital, needed a place to be heard, a place to receive explanation, and a place for reassurance. This invaluable place is cultivated by nurses. Through Zoom and phone calls, nurses share messages of hope, love, and fear between patients and family. Through Zoom and phone calls, nurses orchestrate visits and last goodbyes.
There is no denying that we have all been affected by this shared human experience. But the pause we owe our nurses feels long overdue, and of great importance. Nurses need a space to be heard, to be comforted, to be recognized. They come to our practices, trying to contain the world’s angst, while also navigating for themselves what it means to go through what they are going through. They hope that by coming to see us, they will find the strength to go back another day, another week, another month. Sometimes, they come to talk about everything but the job, in hopes that by talking about more mundane problems, they will feel “normal” and reconnected.
I hope that our empathy, congruence, and unconditional positive regard will allow them to feel heard.1 I hope that our warmth, concern, and hopefulness provide a welcoming place to voice sadness, anger, and fears.2 I hope that our processing of traumatic memory, our challenge to avoid inaccurate self-blaming beliefs, and our encouragement to create more thought-out conclusions will allow them to understand what is happening more accurately.3
Yet, I worry. I worry that society hasn’t been particularly successful with helping prior generations of heroes. From war veterans, to Sept. 11, 2001, firefighters, it seems that we have repeated mistakes. My experience with veterans in particular has taught me that for many who are suffering, it feels like society has broken its very fabric by being bystanders to the pain.
But suffering and tragedy are an inevitable part of the human experience that we share. What we can keep sight of is this: As physicians, we work with nurses. We are witnessing firsthand the impossible sacrifice they are taking and the limits of resilience. Let us not be too busy to stop and give recognition where and when it is due. Let us listen and learn from our past, and present, heroes. And let us never forget to extend our own hand to those who make a living extending theirs.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Rogers CR. J Consult Psychol. 1957;21(2):95-103.
2. Mallo CJ, Mintz DL. Psychodyn Psychiatry. 2013 Mar;41(1):13-37.
3. Resick PA et al. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. Guilford Publications, 2016.
This past year, the referrals to my private practice have taken a noticeable shift and caused me to pause.
More calls have come from nurses, many who work directly with COVID-19 patients, understandably seeking mental health treatment, or support. Especially in this time, nurses are facing trauma and stress that is unimaginable to many, myself included. Despite the collective efforts we have made as a society to recognize their work, I do not think we have given enough consideration to the enormous sacrifice nurses are currently undertaking to save our collective psyche.
As physicians and mental health providers, we have a glimpse into the complexities and stressors of medical treatment. In our line of work, we support patients with trauma on a regular basis. We feel deeply connected to patients, some of whom we have treated until the end of their lives. Despite that, I am not sure that I, or anyone, can truly comprehend what nurses face in today’s climate of care.
There is no denying that doctors are of value to our system, but our service has limits; nurses and doctors operate as two sides to a shared coin. As doctors, we diagnose and prescribe, while nurses explain and dispense. As doctors, we talk to patients, while nurses comfort them. Imagine spending an entire year working in a hospital diligently wiping endotracheal tubes that are responsible for maintaining someone’s life. Imagine spending an entire year laboring through the heavy task of lifting patients to prone them in a position that may save their lives. Imagine spending an entire year holding the hands of comatose patients in hopes of maintaining a sense of humanity.
And this only begins to describe the tasks bestowed upon nurses. While doctors answer pagers or complete insurance authorization forms, nurses empathize and reassure scared and isolated patients. Imagine spending an entire year updating crying family members who cannot see their loved ones. Imagine spending an entire year explaining and pleading to the outside world that wearing a mask and washing hands would reduce the suffering that takes place inside the hospital walls.
Despite the uncertainties, pressures, and demands, nurses have continued, and will continue, to show up for their patients, shift by shift. It takes a tragic number of deaths for the nurses I see in my practice to share that they have lost count. These numbers reflect people they held to feed, carried to prevent ulcers, wiped for decency, caressed for compassion, probed with IVs and tubes, monitored for signs of life, and warmed with blankets. If love were in any job description, it would fall under that of a nurse.
And we can’t ignore the fact that all the lives lost by COVID-19 had family. Family members who, without ever stepping foot in the hospital, needed a place to be heard, a place to receive explanation, and a place for reassurance. This invaluable place is cultivated by nurses. Through Zoom and phone calls, nurses share messages of hope, love, and fear between patients and family. Through Zoom and phone calls, nurses orchestrate visits and last goodbyes.
There is no denying that we have all been affected by this shared human experience. But the pause we owe our nurses feels long overdue, and of great importance. Nurses need a space to be heard, to be comforted, to be recognized. They come to our practices, trying to contain the world’s angst, while also navigating for themselves what it means to go through what they are going through. They hope that by coming to see us, they will find the strength to go back another day, another week, another month. Sometimes, they come to talk about everything but the job, in hopes that by talking about more mundane problems, they will feel “normal” and reconnected.
I hope that our empathy, congruence, and unconditional positive regard will allow them to feel heard.1 I hope that our warmth, concern, and hopefulness provide a welcoming place to voice sadness, anger, and fears.2 I hope that our processing of traumatic memory, our challenge to avoid inaccurate self-blaming beliefs, and our encouragement to create more thought-out conclusions will allow them to understand what is happening more accurately.3
Yet, I worry. I worry that society hasn’t been particularly successful with helping prior generations of heroes. From war veterans, to Sept. 11, 2001, firefighters, it seems that we have repeated mistakes. My experience with veterans in particular has taught me that for many who are suffering, it feels like society has broken its very fabric by being bystanders to the pain.
But suffering and tragedy are an inevitable part of the human experience that we share. What we can keep sight of is this: As physicians, we work with nurses. We are witnessing firsthand the impossible sacrifice they are taking and the limits of resilience. Let us not be too busy to stop and give recognition where and when it is due. Let us listen and learn from our past, and present, heroes. And let us never forget to extend our own hand to those who make a living extending theirs.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Rogers CR. J Consult Psychol. 1957;21(2):95-103.
2. Mallo CJ, Mintz DL. Psychodyn Psychiatry. 2013 Mar;41(1):13-37.
3. Resick PA et al. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. Guilford Publications, 2016.
This past year, the referrals to my private practice have taken a noticeable shift and caused me to pause.
More calls have come from nurses, many who work directly with COVID-19 patients, understandably seeking mental health treatment, or support. Especially in this time, nurses are facing trauma and stress that is unimaginable to many, myself included. Despite the collective efforts we have made as a society to recognize their work, I do not think we have given enough consideration to the enormous sacrifice nurses are currently undertaking to save our collective psyche.
As physicians and mental health providers, we have a glimpse into the complexities and stressors of medical treatment. In our line of work, we support patients with trauma on a regular basis. We feel deeply connected to patients, some of whom we have treated until the end of their lives. Despite that, I am not sure that I, or anyone, can truly comprehend what nurses face in today’s climate of care.
There is no denying that doctors are of value to our system, but our service has limits; nurses and doctors operate as two sides to a shared coin. As doctors, we diagnose and prescribe, while nurses explain and dispense. As doctors, we talk to patients, while nurses comfort them. Imagine spending an entire year working in a hospital diligently wiping endotracheal tubes that are responsible for maintaining someone’s life. Imagine spending an entire year laboring through the heavy task of lifting patients to prone them in a position that may save their lives. Imagine spending an entire year holding the hands of comatose patients in hopes of maintaining a sense of humanity.
And this only begins to describe the tasks bestowed upon nurses. While doctors answer pagers or complete insurance authorization forms, nurses empathize and reassure scared and isolated patients. Imagine spending an entire year updating crying family members who cannot see their loved ones. Imagine spending an entire year explaining and pleading to the outside world that wearing a mask and washing hands would reduce the suffering that takes place inside the hospital walls.
Despite the uncertainties, pressures, and demands, nurses have continued, and will continue, to show up for their patients, shift by shift. It takes a tragic number of deaths for the nurses I see in my practice to share that they have lost count. These numbers reflect people they held to feed, carried to prevent ulcers, wiped for decency, caressed for compassion, probed with IVs and tubes, monitored for signs of life, and warmed with blankets. If love were in any job description, it would fall under that of a nurse.
And we can’t ignore the fact that all the lives lost by COVID-19 had family. Family members who, without ever stepping foot in the hospital, needed a place to be heard, a place to receive explanation, and a place for reassurance. This invaluable place is cultivated by nurses. Through Zoom and phone calls, nurses share messages of hope, love, and fear between patients and family. Through Zoom and phone calls, nurses orchestrate visits and last goodbyes.
There is no denying that we have all been affected by this shared human experience. But the pause we owe our nurses feels long overdue, and of great importance. Nurses need a space to be heard, to be comforted, to be recognized. They come to our practices, trying to contain the world’s angst, while also navigating for themselves what it means to go through what they are going through. They hope that by coming to see us, they will find the strength to go back another day, another week, another month. Sometimes, they come to talk about everything but the job, in hopes that by talking about more mundane problems, they will feel “normal” and reconnected.
I hope that our empathy, congruence, and unconditional positive regard will allow them to feel heard.1 I hope that our warmth, concern, and hopefulness provide a welcoming place to voice sadness, anger, and fears.2 I hope that our processing of traumatic memory, our challenge to avoid inaccurate self-blaming beliefs, and our encouragement to create more thought-out conclusions will allow them to understand what is happening more accurately.3
Yet, I worry. I worry that society hasn’t been particularly successful with helping prior generations of heroes. From war veterans, to Sept. 11, 2001, firefighters, it seems that we have repeated mistakes. My experience with veterans in particular has taught me that for many who are suffering, it feels like society has broken its very fabric by being bystanders to the pain.
But suffering and tragedy are an inevitable part of the human experience that we share. What we can keep sight of is this: As physicians, we work with nurses. We are witnessing firsthand the impossible sacrifice they are taking and the limits of resilience. Let us not be too busy to stop and give recognition where and when it is due. Let us listen and learn from our past, and present, heroes. And let us never forget to extend our own hand to those who make a living extending theirs.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Rogers CR. J Consult Psychol. 1957;21(2):95-103.
2. Mallo CJ, Mintz DL. Psychodyn Psychiatry. 2013 Mar;41(1):13-37.
3. Resick PA et al. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. Guilford Publications, 2016.
Inflammatory immune findings likely in acute schizophrenia, MDD, bipolar
Researchers have come a long way in understanding the link between acute inflammation and treatment-resistant depression, but more work needs to be done, according to Mark Hyman Rapaport, MD.
“Inflammation has been a hot topic in the past decade, both because of its impact in medical disorders and in psychiatric disorders,” Dr. Rapaport, CEO of the Huntsman Mental Health Institute in Salt Lake City, Utah, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “We run into difficulty with chronic inflammation, which we see with rheumatic disorders, and when we think of metabolic syndrome and obesity.”
The immune system helps to control energy regulation and neuroendocrine function in acute inflammation and chronic inflammatory diseases. “We see a variety of effects on the central nervous system or liver function or on homeostasis of the body,” said Dr. Rapaport, who also chairs the department of psychiatry at the University of Utah, also in Salt Lake City. “These are all normal and necessary to channel energy to the immune system in order to fight what’s necessary in acute inflammatory response.”
A chronic state of inflammation can result in prolonged allocation of fuels to the immune system, tissue inflammation, and a chronically aberrant immune reaction, he continued. This can cause depressive symptoms/fatigue, anorexia, malnutrition, muscle wasting, cachectic obesity, insulin resistance, dyslipidemia, increased adipose tissue in the proximity of inflammatory lesion, alterations of steroid hormone axes, elevated sympathetic tone, hypertension, decreased parasympathetic tone, inflammation-related anemia, and osteopenia. “So, chronic inflammation has a lot of long-term sequelae that are detrimental,” he said.
Both physical stress and psychological stress also cause an inflammatory state. After looking at the medical literature, Dr. Rapaport and colleagues began to wonder whether inflammation and immune activation associated with psychiatric disorders are attributable to the stress of acute illness. To find out, they performed a meta-analysis of blood cytokine network alterations in psychiatric patients and evaluated comparisons between schizophrenia, bipolar disorder, and depression. A total of three meta-analyses were performed: one of acute/inpatient studies, one on the impact of acute treatment, and one of outpatient studies. The researchers hypothesized that inflammatory and immune findings in psychiatric illnesses were tied to two distinct etiologies: the acute stress of illness and intrinsic immune dysfunction.
The meta-analyses included 68 studies: 40 involving patients with schizophrenia, 18 involving those with major depressive disorder (MDD) and 10 involving those with bipolar disorder. The researchers found that levels of four cytokines were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD, compared with controls: interleukin-6, tumor necrosis factor–alpha (TNF-alpha), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA). “There has not been a consistent blood panel used across studies, be it within a disorder itself like depression, or across disorders,” Dr. Rapaport noted. “This is a challenge that we face in looking at these data.”
Following treatment of acute illness, IL-6 levels significantly decreased in schizophrenia and MDD, but no significant changes in TNF-alpha levels were observed in patients with schizophrenia or MDD. In addition, sIL-2R levels increase in schizophrenia but remained unchanged in bipolar and MDD, while IL-1RA levels in bipolar mania decreased but remained unchanged in MDD. Meanwhile, assessment of the study’s 24 outpatient studies revealed that levels of IL-6 were significantly increased in outpatients with schizophrenia, euthymic bipolar disorder, and MDD, compared with controls (P < .01 for each). In addition, levels of IL-1 beta and sIL-2R were significantly increased in outpatients with schizophrenia and bipolar disorder.
According to Dr. Rapaport, these meta-analyses suggest that there are likely inflammatory immune findings present in acutely ill patients with MDD, schizophrenia, and bipolar disorder.
“Some of this activation decreases with effective acute treatment of the disorder,” he said. “The data suggest that immune changes are present in a subset of patients with all three disorders.”
“We also need to understand the regulatory role that microglia and astroglia play within the brain,” he said. “We need to identify changes in brain circuitry and function associated with inflammation and other immune changes. We also need to carefully scrutinize publications, understand the assumptions behind the statistics, and carry out more research beyond the protein level.”
He concluded his presentation by calling for research to help clinicians differentiate acute from chronic inflammation. “The study of both is important,” he said. “We need to understand the pathophysiology of immune changes in psychiatric disorders. We need to study both the triggers and pathways to resolution.”
Dr. Rapaport disclosed that he has received research support from the National Institutes of Health, the National Institute of Mental Health, and the National Center for Complementary and Integrative Health.
Researchers have come a long way in understanding the link between acute inflammation and treatment-resistant depression, but more work needs to be done, according to Mark Hyman Rapaport, MD.
“Inflammation has been a hot topic in the past decade, both because of its impact in medical disorders and in psychiatric disorders,” Dr. Rapaport, CEO of the Huntsman Mental Health Institute in Salt Lake City, Utah, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “We run into difficulty with chronic inflammation, which we see with rheumatic disorders, and when we think of metabolic syndrome and obesity.”
The immune system helps to control energy regulation and neuroendocrine function in acute inflammation and chronic inflammatory diseases. “We see a variety of effects on the central nervous system or liver function or on homeostasis of the body,” said Dr. Rapaport, who also chairs the department of psychiatry at the University of Utah, also in Salt Lake City. “These are all normal and necessary to channel energy to the immune system in order to fight what’s necessary in acute inflammatory response.”
A chronic state of inflammation can result in prolonged allocation of fuels to the immune system, tissue inflammation, and a chronically aberrant immune reaction, he continued. This can cause depressive symptoms/fatigue, anorexia, malnutrition, muscle wasting, cachectic obesity, insulin resistance, dyslipidemia, increased adipose tissue in the proximity of inflammatory lesion, alterations of steroid hormone axes, elevated sympathetic tone, hypertension, decreased parasympathetic tone, inflammation-related anemia, and osteopenia. “So, chronic inflammation has a lot of long-term sequelae that are detrimental,” he said.
Both physical stress and psychological stress also cause an inflammatory state. After looking at the medical literature, Dr. Rapaport and colleagues began to wonder whether inflammation and immune activation associated with psychiatric disorders are attributable to the stress of acute illness. To find out, they performed a meta-analysis of blood cytokine network alterations in psychiatric patients and evaluated comparisons between schizophrenia, bipolar disorder, and depression. A total of three meta-analyses were performed: one of acute/inpatient studies, one on the impact of acute treatment, and one of outpatient studies. The researchers hypothesized that inflammatory and immune findings in psychiatric illnesses were tied to two distinct etiologies: the acute stress of illness and intrinsic immune dysfunction.
The meta-analyses included 68 studies: 40 involving patients with schizophrenia, 18 involving those with major depressive disorder (MDD) and 10 involving those with bipolar disorder. The researchers found that levels of four cytokines were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD, compared with controls: interleukin-6, tumor necrosis factor–alpha (TNF-alpha), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA). “There has not been a consistent blood panel used across studies, be it within a disorder itself like depression, or across disorders,” Dr. Rapaport noted. “This is a challenge that we face in looking at these data.”
Following treatment of acute illness, IL-6 levels significantly decreased in schizophrenia and MDD, but no significant changes in TNF-alpha levels were observed in patients with schizophrenia or MDD. In addition, sIL-2R levels increase in schizophrenia but remained unchanged in bipolar and MDD, while IL-1RA levels in bipolar mania decreased but remained unchanged in MDD. Meanwhile, assessment of the study’s 24 outpatient studies revealed that levels of IL-6 were significantly increased in outpatients with schizophrenia, euthymic bipolar disorder, and MDD, compared with controls (P < .01 for each). In addition, levels of IL-1 beta and sIL-2R were significantly increased in outpatients with schizophrenia and bipolar disorder.
According to Dr. Rapaport, these meta-analyses suggest that there are likely inflammatory immune findings present in acutely ill patients with MDD, schizophrenia, and bipolar disorder.
“Some of this activation decreases with effective acute treatment of the disorder,” he said. “The data suggest that immune changes are present in a subset of patients with all three disorders.”
“We also need to understand the regulatory role that microglia and astroglia play within the brain,” he said. “We need to identify changes in brain circuitry and function associated with inflammation and other immune changes. We also need to carefully scrutinize publications, understand the assumptions behind the statistics, and carry out more research beyond the protein level.”
He concluded his presentation by calling for research to help clinicians differentiate acute from chronic inflammation. “The study of both is important,” he said. “We need to understand the pathophysiology of immune changes in psychiatric disorders. We need to study both the triggers and pathways to resolution.”
Dr. Rapaport disclosed that he has received research support from the National Institutes of Health, the National Institute of Mental Health, and the National Center for Complementary and Integrative Health.
Researchers have come a long way in understanding the link between acute inflammation and treatment-resistant depression, but more work needs to be done, according to Mark Hyman Rapaport, MD.
“Inflammation has been a hot topic in the past decade, both because of its impact in medical disorders and in psychiatric disorders,” Dr. Rapaport, CEO of the Huntsman Mental Health Institute in Salt Lake City, Utah, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “We run into difficulty with chronic inflammation, which we see with rheumatic disorders, and when we think of metabolic syndrome and obesity.”
The immune system helps to control energy regulation and neuroendocrine function in acute inflammation and chronic inflammatory diseases. “We see a variety of effects on the central nervous system or liver function or on homeostasis of the body,” said Dr. Rapaport, who also chairs the department of psychiatry at the University of Utah, also in Salt Lake City. “These are all normal and necessary to channel energy to the immune system in order to fight what’s necessary in acute inflammatory response.”
A chronic state of inflammation can result in prolonged allocation of fuels to the immune system, tissue inflammation, and a chronically aberrant immune reaction, he continued. This can cause depressive symptoms/fatigue, anorexia, malnutrition, muscle wasting, cachectic obesity, insulin resistance, dyslipidemia, increased adipose tissue in the proximity of inflammatory lesion, alterations of steroid hormone axes, elevated sympathetic tone, hypertension, decreased parasympathetic tone, inflammation-related anemia, and osteopenia. “So, chronic inflammation has a lot of long-term sequelae that are detrimental,” he said.
Both physical stress and psychological stress also cause an inflammatory state. After looking at the medical literature, Dr. Rapaport and colleagues began to wonder whether inflammation and immune activation associated with psychiatric disorders are attributable to the stress of acute illness. To find out, they performed a meta-analysis of blood cytokine network alterations in psychiatric patients and evaluated comparisons between schizophrenia, bipolar disorder, and depression. A total of three meta-analyses were performed: one of acute/inpatient studies, one on the impact of acute treatment, and one of outpatient studies. The researchers hypothesized that inflammatory and immune findings in psychiatric illnesses were tied to two distinct etiologies: the acute stress of illness and intrinsic immune dysfunction.
The meta-analyses included 68 studies: 40 involving patients with schizophrenia, 18 involving those with major depressive disorder (MDD) and 10 involving those with bipolar disorder. The researchers found that levels of four cytokines were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD, compared with controls: interleukin-6, tumor necrosis factor–alpha (TNF-alpha), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA). “There has not been a consistent blood panel used across studies, be it within a disorder itself like depression, or across disorders,” Dr. Rapaport noted. “This is a challenge that we face in looking at these data.”
Following treatment of acute illness, IL-6 levels significantly decreased in schizophrenia and MDD, but no significant changes in TNF-alpha levels were observed in patients with schizophrenia or MDD. In addition, sIL-2R levels increase in schizophrenia but remained unchanged in bipolar and MDD, while IL-1RA levels in bipolar mania decreased but remained unchanged in MDD. Meanwhile, assessment of the study’s 24 outpatient studies revealed that levels of IL-6 were significantly increased in outpatients with schizophrenia, euthymic bipolar disorder, and MDD, compared with controls (P < .01 for each). In addition, levels of IL-1 beta and sIL-2R were significantly increased in outpatients with schizophrenia and bipolar disorder.
According to Dr. Rapaport, these meta-analyses suggest that there are likely inflammatory immune findings present in acutely ill patients with MDD, schizophrenia, and bipolar disorder.
“Some of this activation decreases with effective acute treatment of the disorder,” he said. “The data suggest that immune changes are present in a subset of patients with all three disorders.”
“We also need to understand the regulatory role that microglia and astroglia play within the brain,” he said. “We need to identify changes in brain circuitry and function associated with inflammation and other immune changes. We also need to carefully scrutinize publications, understand the assumptions behind the statistics, and carry out more research beyond the protein level.”
He concluded his presentation by calling for research to help clinicians differentiate acute from chronic inflammation. “The study of both is important,” he said. “We need to understand the pathophysiology of immune changes in psychiatric disorders. We need to study both the triggers and pathways to resolution.”
Dr. Rapaport disclosed that he has received research support from the National Institutes of Health, the National Institute of Mental Health, and the National Center for Complementary and Integrative Health.
FROM NPA 2021
Myocardial injury seen on MRI in 54% of recovered COVID-19 patients
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
Study: Central sleep apnea is common in ticagrelor users post ACS
The prevalence of asymptomatic central sleep apnea after acute coronary syndrome is high and may be associated with the use of ticagrelor, a new study finds.
Prior studies have suggested that ticagrelor is associated with an increased likelihood of central sleep apnea. The drug’s label notes that two respiratory conditions – central sleep apnea and Cheyne-Stokes respiration – are adverse reactions that were identified after the drug’s approval in the United States in 2011. “Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the label says.
Among 80 patients receiving ticagrelor, 24 had central sleep apnea hypopnea syndrome (CSAHS), whereas of 41 patients not taking ticagrelor, 3 had this condition (30% vs. 7.3%, P = .004), in the new study published online Jan. 20, 2021, in Sleep Medicine. A multivariable analysis included in the paper found that age and ticagrelor administration were the only two factors associated with the occurrence of CSAHS.
Findings are ‘striking’
The different rates of central sleep apnea in the study are striking, but it is not clear that asymptomatic central sleep apnea in patients taking ticagrelor is a concern, Ofer Jacobowitz, MD, PhD, associate professor of otolaryngology at Hofstra University, Hempstead, N.Y, said in an interview.
“Whether this particular drug-induced central sleep apnea is consequential” is an open question, noted Dr. Jacobowitz. “There is no evidence that shows that this is definitely harmful.”
“The different types of central sleep apnea are caused by different mechanisms and this one, we don’t know,” Dr. Jacobwitz added.
Study author continues to prescribe ticagrelor
One of the study authors, Philippe Meurin, MD, said that he continues to prescribe ticagrelor every day and that the side effect is not necessarily important.
It is possible that central sleep apnea may resolve, although further studies would need to examine central sleep apnea over time to establish the duration of the condition, he added. Nevertheless, awareness of the association could have implications for clinical practice, Dr. Meurin said.
Central sleep apnea is rare, and if doctors detect it during a sleep study, they may perform extensive tests to assess for possible neurologic diseases, for example, when the cause may be attributed to the medication, he said. In addition, if a patient who is taking ticagrelor has dyspnea, the presence of central sleep apnea may suggest that dyspnea could be related to the drug, although this possibility needs further study, he noted.
Study included patients with ACS history, but no heart failure
Dr. Meurin, of Centre de Réadaptation Cardiaque de La Brie, Les Grands Prés, Villeneuve-Saint-Denis, France, and colleagues included in their study patients between 1 week and 1 year after acute coronary syndrome who did not have heart failure or a history of sleep apnea.
After an overnight sleep study, they classified patients as normal, as having CSAHS (i.e., an apnea-hypopnea index of 15 or greater, mostly with central sleep apneas), or as having obstructive sleep apnea hypopnea syndrome (OSAHS; i.e., an apnea-hypopnea index of 15 or greater, mostly with obstructive sleep apneas).
The prospective study included 121 consecutive patients between January 2018 and March 2020. Patients had a mean age of 56.8, and 88% were men.
Switching to another P2Y12 inhibitor ‘does not seem appropriate’
“CSAHS could be promoted by the use of ticagrelor, a relatively new drug that modifies the apneic threshold,” the study authors wrote. “Regarding underlying mechanisms, the most probable explanation seems to be increased chemosensitivity to hypercapnia by a direct P2Y12 inhibitory effect on the central nervous system.”
Doctors should not overestimate the severity of the adverse reaction or consider it the same way they do OSASH, they added.
Among patients with acute coronary syndrome in the PLATO study, ticagrelor, compared with clopidogrel, “significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke,” Dr. Meurin and colleagues said. “Because in this study more than 9,000 patients received ticagrelor for 12 months, CSAHS (even if it seems frequent in our study) did not seem to impair the good efficacy/tolerance balance of the drug. Therefore, in asymptomatic CSAHS patients, switching from ticagrelor to another P2Y12 inhibitor does not seem appropriate.”
A recent analysis of data from randomized, controlled trials with ticagrelor did not find excess cases of sleep apnea with the drug. But an asymptomatic adverse event such as central sleep apnea “cannot emerge from a post hoc analysis,” Dr. Meurin and colleagues said.
The analysis of randomized trial data was conducted by Marc S. Sabatine, MD, MPH, chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital, and coauthors. It was published in JACC: Cardiovascular Interventions in April 2020.
They “used the gold standard for medical evidence (randomized, placebo-controlled trials) and found 158 cases of sleep apnea reported, with absolutely no difference between ticagrelor and placebo,” Dr. Sabatine said in an interview. Their analysis examined clinically overt apnea, he noted.
“It is quite clear that when looking at large numbers in placebo-controlled trials, there is no excess,” Dr. Sabatine said. “Meurin et al. are examining a different outcome: the results of a lab test in what may be entirely asymptomatic patients.”
A randomized trial could confirm the association, he said.
“The association may be real, but also may be play of chance or confounded,” said Dr. Sabatine. “To convince the medical community, the next step would be for the investigators to do a randomized trial and test whether ticagrelor increases the risk of central sleep apnea.”
Dr. Meurin and the study coauthors had no disclosures. The analysis of randomized, controlled trial data by Dr. Sabatine and colleagues was funded by AstraZeneca, which distributes ticagrelor under the trade name Brilinta. Dr. Sabatine has been a consultant for AstraZeneca and received research grants through Brigham and Women’s Hospital from AstraZeneca. He has consulted for and received grants through the hospital from other companies as well. Dr. Jacobowitz had no relevant disclosures.
[email protected]
The prevalence of asymptomatic central sleep apnea after acute coronary syndrome is high and may be associated with the use of ticagrelor, a new study finds.
Prior studies have suggested that ticagrelor is associated with an increased likelihood of central sleep apnea. The drug’s label notes that two respiratory conditions – central sleep apnea and Cheyne-Stokes respiration – are adverse reactions that were identified after the drug’s approval in the United States in 2011. “Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the label says.
Among 80 patients receiving ticagrelor, 24 had central sleep apnea hypopnea syndrome (CSAHS), whereas of 41 patients not taking ticagrelor, 3 had this condition (30% vs. 7.3%, P = .004), in the new study published online Jan. 20, 2021, in Sleep Medicine. A multivariable analysis included in the paper found that age and ticagrelor administration were the only two factors associated with the occurrence of CSAHS.
Findings are ‘striking’
The different rates of central sleep apnea in the study are striking, but it is not clear that asymptomatic central sleep apnea in patients taking ticagrelor is a concern, Ofer Jacobowitz, MD, PhD, associate professor of otolaryngology at Hofstra University, Hempstead, N.Y, said in an interview.
“Whether this particular drug-induced central sleep apnea is consequential” is an open question, noted Dr. Jacobowitz. “There is no evidence that shows that this is definitely harmful.”
“The different types of central sleep apnea are caused by different mechanisms and this one, we don’t know,” Dr. Jacobwitz added.
Study author continues to prescribe ticagrelor
One of the study authors, Philippe Meurin, MD, said that he continues to prescribe ticagrelor every day and that the side effect is not necessarily important.
It is possible that central sleep apnea may resolve, although further studies would need to examine central sleep apnea over time to establish the duration of the condition, he added. Nevertheless, awareness of the association could have implications for clinical practice, Dr. Meurin said.
Central sleep apnea is rare, and if doctors detect it during a sleep study, they may perform extensive tests to assess for possible neurologic diseases, for example, when the cause may be attributed to the medication, he said. In addition, if a patient who is taking ticagrelor has dyspnea, the presence of central sleep apnea may suggest that dyspnea could be related to the drug, although this possibility needs further study, he noted.
Study included patients with ACS history, but no heart failure
Dr. Meurin, of Centre de Réadaptation Cardiaque de La Brie, Les Grands Prés, Villeneuve-Saint-Denis, France, and colleagues included in their study patients between 1 week and 1 year after acute coronary syndrome who did not have heart failure or a history of sleep apnea.
After an overnight sleep study, they classified patients as normal, as having CSAHS (i.e., an apnea-hypopnea index of 15 or greater, mostly with central sleep apneas), or as having obstructive sleep apnea hypopnea syndrome (OSAHS; i.e., an apnea-hypopnea index of 15 or greater, mostly with obstructive sleep apneas).
The prospective study included 121 consecutive patients between January 2018 and March 2020. Patients had a mean age of 56.8, and 88% were men.
Switching to another P2Y12 inhibitor ‘does not seem appropriate’
“CSAHS could be promoted by the use of ticagrelor, a relatively new drug that modifies the apneic threshold,” the study authors wrote. “Regarding underlying mechanisms, the most probable explanation seems to be increased chemosensitivity to hypercapnia by a direct P2Y12 inhibitory effect on the central nervous system.”
Doctors should not overestimate the severity of the adverse reaction or consider it the same way they do OSASH, they added.
Among patients with acute coronary syndrome in the PLATO study, ticagrelor, compared with clopidogrel, “significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke,” Dr. Meurin and colleagues said. “Because in this study more than 9,000 patients received ticagrelor for 12 months, CSAHS (even if it seems frequent in our study) did not seem to impair the good efficacy/tolerance balance of the drug. Therefore, in asymptomatic CSAHS patients, switching from ticagrelor to another P2Y12 inhibitor does not seem appropriate.”
A recent analysis of data from randomized, controlled trials with ticagrelor did not find excess cases of sleep apnea with the drug. But an asymptomatic adverse event such as central sleep apnea “cannot emerge from a post hoc analysis,” Dr. Meurin and colleagues said.
The analysis of randomized trial data was conducted by Marc S. Sabatine, MD, MPH, chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital, and coauthors. It was published in JACC: Cardiovascular Interventions in April 2020.
They “used the gold standard for medical evidence (randomized, placebo-controlled trials) and found 158 cases of sleep apnea reported, with absolutely no difference between ticagrelor and placebo,” Dr. Sabatine said in an interview. Their analysis examined clinically overt apnea, he noted.
“It is quite clear that when looking at large numbers in placebo-controlled trials, there is no excess,” Dr. Sabatine said. “Meurin et al. are examining a different outcome: the results of a lab test in what may be entirely asymptomatic patients.”
A randomized trial could confirm the association, he said.
“The association may be real, but also may be play of chance or confounded,” said Dr. Sabatine. “To convince the medical community, the next step would be for the investigators to do a randomized trial and test whether ticagrelor increases the risk of central sleep apnea.”
Dr. Meurin and the study coauthors had no disclosures. The analysis of randomized, controlled trial data by Dr. Sabatine and colleagues was funded by AstraZeneca, which distributes ticagrelor under the trade name Brilinta. Dr. Sabatine has been a consultant for AstraZeneca and received research grants through Brigham and Women’s Hospital from AstraZeneca. He has consulted for and received grants through the hospital from other companies as well. Dr. Jacobowitz had no relevant disclosures.
[email protected]
The prevalence of asymptomatic central sleep apnea after acute coronary syndrome is high and may be associated with the use of ticagrelor, a new study finds.
Prior studies have suggested that ticagrelor is associated with an increased likelihood of central sleep apnea. The drug’s label notes that two respiratory conditions – central sleep apnea and Cheyne-Stokes respiration – are adverse reactions that were identified after the drug’s approval in the United States in 2011. “Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the label says.
Among 80 patients receiving ticagrelor, 24 had central sleep apnea hypopnea syndrome (CSAHS), whereas of 41 patients not taking ticagrelor, 3 had this condition (30% vs. 7.3%, P = .004), in the new study published online Jan. 20, 2021, in Sleep Medicine. A multivariable analysis included in the paper found that age and ticagrelor administration were the only two factors associated with the occurrence of CSAHS.
Findings are ‘striking’
The different rates of central sleep apnea in the study are striking, but it is not clear that asymptomatic central sleep apnea in patients taking ticagrelor is a concern, Ofer Jacobowitz, MD, PhD, associate professor of otolaryngology at Hofstra University, Hempstead, N.Y, said in an interview.
“Whether this particular drug-induced central sleep apnea is consequential” is an open question, noted Dr. Jacobowitz. “There is no evidence that shows that this is definitely harmful.”
“The different types of central sleep apnea are caused by different mechanisms and this one, we don’t know,” Dr. Jacobwitz added.
Study author continues to prescribe ticagrelor
One of the study authors, Philippe Meurin, MD, said that he continues to prescribe ticagrelor every day and that the side effect is not necessarily important.
It is possible that central sleep apnea may resolve, although further studies would need to examine central sleep apnea over time to establish the duration of the condition, he added. Nevertheless, awareness of the association could have implications for clinical practice, Dr. Meurin said.
Central sleep apnea is rare, and if doctors detect it during a sleep study, they may perform extensive tests to assess for possible neurologic diseases, for example, when the cause may be attributed to the medication, he said. In addition, if a patient who is taking ticagrelor has dyspnea, the presence of central sleep apnea may suggest that dyspnea could be related to the drug, although this possibility needs further study, he noted.
Study included patients with ACS history, but no heart failure
Dr. Meurin, of Centre de Réadaptation Cardiaque de La Brie, Les Grands Prés, Villeneuve-Saint-Denis, France, and colleagues included in their study patients between 1 week and 1 year after acute coronary syndrome who did not have heart failure or a history of sleep apnea.
After an overnight sleep study, they classified patients as normal, as having CSAHS (i.e., an apnea-hypopnea index of 15 or greater, mostly with central sleep apneas), or as having obstructive sleep apnea hypopnea syndrome (OSAHS; i.e., an apnea-hypopnea index of 15 or greater, mostly with obstructive sleep apneas).
The prospective study included 121 consecutive patients between January 2018 and March 2020. Patients had a mean age of 56.8, and 88% were men.
Switching to another P2Y12 inhibitor ‘does not seem appropriate’
“CSAHS could be promoted by the use of ticagrelor, a relatively new drug that modifies the apneic threshold,” the study authors wrote. “Regarding underlying mechanisms, the most probable explanation seems to be increased chemosensitivity to hypercapnia by a direct P2Y12 inhibitory effect on the central nervous system.”
Doctors should not overestimate the severity of the adverse reaction or consider it the same way they do OSASH, they added.
Among patients with acute coronary syndrome in the PLATO study, ticagrelor, compared with clopidogrel, “significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke,” Dr. Meurin and colleagues said. “Because in this study more than 9,000 patients received ticagrelor for 12 months, CSAHS (even if it seems frequent in our study) did not seem to impair the good efficacy/tolerance balance of the drug. Therefore, in asymptomatic CSAHS patients, switching from ticagrelor to another P2Y12 inhibitor does not seem appropriate.”
A recent analysis of data from randomized, controlled trials with ticagrelor did not find excess cases of sleep apnea with the drug. But an asymptomatic adverse event such as central sleep apnea “cannot emerge from a post hoc analysis,” Dr. Meurin and colleagues said.
The analysis of randomized trial data was conducted by Marc S. Sabatine, MD, MPH, chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital, and coauthors. It was published in JACC: Cardiovascular Interventions in April 2020.
They “used the gold standard for medical evidence (randomized, placebo-controlled trials) and found 158 cases of sleep apnea reported, with absolutely no difference between ticagrelor and placebo,” Dr. Sabatine said in an interview. Their analysis examined clinically overt apnea, he noted.
“It is quite clear that when looking at large numbers in placebo-controlled trials, there is no excess,” Dr. Sabatine said. “Meurin et al. are examining a different outcome: the results of a lab test in what may be entirely asymptomatic patients.”
A randomized trial could confirm the association, he said.
“The association may be real, but also may be play of chance or confounded,” said Dr. Sabatine. “To convince the medical community, the next step would be for the investigators to do a randomized trial and test whether ticagrelor increases the risk of central sleep apnea.”
Dr. Meurin and the study coauthors had no disclosures. The analysis of randomized, controlled trial data by Dr. Sabatine and colleagues was funded by AstraZeneca, which distributes ticagrelor under the trade name Brilinta. Dr. Sabatine has been a consultant for AstraZeneca and received research grants through Brigham and Women’s Hospital from AstraZeneca. He has consulted for and received grants through the hospital from other companies as well. Dr. Jacobowitz had no relevant disclosures.
[email protected]
FROM SLEEP MEDICINE
EEG data may help aid diagnosis, treatment of focal epilepsy
, new research suggests. Findings from a large longitudinal study show that seizure onset in patients with focal epilepsy follows circadian, multiday, and annual cycles.
“Although daily and multiday rhythms have previously been identified, the extent to which these nonrandom rhythms exist in a larger cohort has been unclear,” said study investigator Joline Marie Fan, MD, a clinical fellow at the University of California, San Francisco. “This means that a patient with epilepsy may have a unique combination of seizure rhythms that can inform the days and timing of his or her highest seizure risk,” she added.
The study was published online Feb. 8 in JAMA Neurology.
Distinct chronotypes
Clinicians and patients alike have long observed cyclical patterns in the onset of epileptic seizures. However, such patterns have rarely been measured in a quantitative way.
Previous studies have examined seizure cycles using inpatient seizure monitoring and patients’ seizure diaries, but the duration of these recordings and their accuracy have been limited. Within the past decade, the advent of cEEG has allowed researchers to observe the cyclical pattern of interictal epileptiform activity, but the numbers of patients involved in such studies have been limited.
To investigate seizure chronotypes in greater detail, the researchers examined retrospective data for 222 adults with medically refractory focal epilepsy who took part in clinical trials of the NeuroPace responsive neurostimulation (RNS) system.
After implantation in the brain, this system monitors the seizure focus or foci continuously and delivers stimulation to stop seizures. Participants also kept seizure diaries and classified their seizures as simple motor, simple other, complex partial, and generalized tonic-clonic.
Dr. Fan’s group examined three subpopulations of patients to investigate three durations of seizure cycles. They examined self-reported disabling seizures, electrographic seizures, and interictal epileptiform activity. Because patients did not record the time of their disabling seizures, the investigators examined them only in multidien and circannual cycles.
To examine circannual seizure cycles, the investigators included 194 patients who kept continuous seizure diaries for 2 or more years and who reported 24 or more days in which disabling seizures occurred.
To examine multidien seizure cycles, they included 186 participants who reported 24 or more days with disabling seizures over a period of 6 or more months during which the RNS system collected cEEG data. They included 85 patients who had 48 hours or more in which electrographic seizure counts were above zero during 6 or more months of cEEG data collection to examine circadian seizure cycles.
Phase-locking value (PLV) was used to determine the strength of a cycle (i.e., the degree of consistency with which seizures occur during certain phases of a cycle). A PLV of 0 represents a uniform distribution of events during various phases of a cycle; a PLV of 1 indicates that all events occur exactly at the same phase of a cycle.
The population’s median age was 35 years, and the sample included approximately equal numbers of men and women. Patients’ focal epilepsies included mesiotemporal (57.2%), frontal (14.0%), neocortical-temporal (9.9%), parietal (4.1%), occipital (1.4%), and multifocal (13.5%). The data included 1,118 patient-years of cEEG, 754,108 electrographic seizures, and 313,995 self-reported seizures.
The prevalence of statistically significant circannual seizure cycles in this population was 12%. The prevalence of multidien seizure cycles was 60%, and the prevalence of circadian seizure cycles was 89%. Multidien cycles (mean PLV, 0.34) and circadian cycles (mean PLV, 0.34) were stronger than were circannual cycles (mean PLV, 0.17).
Among patients with circannual seizure cycles, there was a weak to moderate tendency for seizures to occur during one of the four seasons. There was no overall trend toward seizure onset in one season among this group.
Among patients with multidien seizure cycles, investigators identified five patterns of interictal epileptiform activity fluctuations. One pattern had irregular periodicity, and the others reached peak periodicity at 7, 15, 20, and 30 days. For some patients, one or more periodicities occurred. For most patients, electrographic or self-reported seizures tended to occur on the rising phase of the interictal epileptiform activity cycle. Interictal epileptiform activity increased on days around seizures.
Results showed there were five main seizure peak times among patients with circadian seizure cycles: midnight, 3:00 a.m., 9:00 a.m., 2:00 p.m., and 6:00 p.m. These findings corroborate the observations of previous investigations, the researchers noted. Hourly interictal epileptiform activity peaked during the night, regardless of peak seizure time.
“Although the neurostimulation device offers us a unique opportunity to investigate electrographic seizure activity quantitatively, the generalizability of our study is limited to the patient cohort that we studied,” said Dr. Fan. “The study findings are limited to patients with neurostimulation devices used for intractable focal epilepsies.”
The results support patients’ impressions that their seizures occur in a cyclical pattern.
“Ultimately, these findings will be helpful for developing models to aid with seizure forecasting and prediction in order to help reduce the uncertainty of seizure timing for patients with epilepsy,” said Dr. Fan.
“Other implications include optimizing the timing for patients to be admitted into the hospital for seizure characterization based on their seizure chronotype, or possibly tailoring a medication regimen in accordance with a patient’s seizure cycles,” she added.
Need for more research
Commenting on the findings, Tobias Loddenkemper, MD, professor of neurology at Harvard Medical School, Boston, noted that the study is “one of the largest longitudinal seizure pattern analyses, based on the gold standard of intracranially recorded epileptic seizures.”
The research, he added, extends neurologists’ understanding of seizure patterns over time, expands knowledge about seizure chronotypes, and emphasizes a relationship between interictal epileptiform activity and seizures.
The strengths of the study include the recording of seizures with intracranial EEG, its large number of participants, and the long duration of recordings, Dr. Loddenkemper said.
However, he said, it is important to note that self-reports are not always reliable. The results may also reflect the influence of potential confounders of seizure patterns, such as seizure triggers, treatment, stimulation, or sleep-wake, circadian, or hormonal cycles, he added.
“In the short term, validation studies, as well as confirmatory studies with less invasive sensors, may be needed,” said Dr. Loddenkemper.
“This could potentially include a trial that confirms findings prospectively, utilizing results from video EEG monitoring admissions. In the long term, seizure detection and prediction, as well as interventional chronotherapeutic trials, may be enabled, predicting seizures in individual patients and treating at times of greatest seizure susceptibility.”
The study was supported by grants to some of the authors from the Wyss Center for Bio and Neuroengineering, the Ernest Gallo Foundation, the Swiss National Science Foundation, and the Velux Stiftung. Dr. Fan has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Findings from a large longitudinal study show that seizure onset in patients with focal epilepsy follows circadian, multiday, and annual cycles.
“Although daily and multiday rhythms have previously been identified, the extent to which these nonrandom rhythms exist in a larger cohort has been unclear,” said study investigator Joline Marie Fan, MD, a clinical fellow at the University of California, San Francisco. “This means that a patient with epilepsy may have a unique combination of seizure rhythms that can inform the days and timing of his or her highest seizure risk,” she added.
The study was published online Feb. 8 in JAMA Neurology.
Distinct chronotypes
Clinicians and patients alike have long observed cyclical patterns in the onset of epileptic seizures. However, such patterns have rarely been measured in a quantitative way.
Previous studies have examined seizure cycles using inpatient seizure monitoring and patients’ seizure diaries, but the duration of these recordings and their accuracy have been limited. Within the past decade, the advent of cEEG has allowed researchers to observe the cyclical pattern of interictal epileptiform activity, but the numbers of patients involved in such studies have been limited.
To investigate seizure chronotypes in greater detail, the researchers examined retrospective data for 222 adults with medically refractory focal epilepsy who took part in clinical trials of the NeuroPace responsive neurostimulation (RNS) system.
After implantation in the brain, this system monitors the seizure focus or foci continuously and delivers stimulation to stop seizures. Participants also kept seizure diaries and classified their seizures as simple motor, simple other, complex partial, and generalized tonic-clonic.
Dr. Fan’s group examined three subpopulations of patients to investigate three durations of seizure cycles. They examined self-reported disabling seizures, electrographic seizures, and interictal epileptiform activity. Because patients did not record the time of their disabling seizures, the investigators examined them only in multidien and circannual cycles.
To examine circannual seizure cycles, the investigators included 194 patients who kept continuous seizure diaries for 2 or more years and who reported 24 or more days in which disabling seizures occurred.
To examine multidien seizure cycles, they included 186 participants who reported 24 or more days with disabling seizures over a period of 6 or more months during which the RNS system collected cEEG data. They included 85 patients who had 48 hours or more in which electrographic seizure counts were above zero during 6 or more months of cEEG data collection to examine circadian seizure cycles.
Phase-locking value (PLV) was used to determine the strength of a cycle (i.e., the degree of consistency with which seizures occur during certain phases of a cycle). A PLV of 0 represents a uniform distribution of events during various phases of a cycle; a PLV of 1 indicates that all events occur exactly at the same phase of a cycle.
The population’s median age was 35 years, and the sample included approximately equal numbers of men and women. Patients’ focal epilepsies included mesiotemporal (57.2%), frontal (14.0%), neocortical-temporal (9.9%), parietal (4.1%), occipital (1.4%), and multifocal (13.5%). The data included 1,118 patient-years of cEEG, 754,108 electrographic seizures, and 313,995 self-reported seizures.
The prevalence of statistically significant circannual seizure cycles in this population was 12%. The prevalence of multidien seizure cycles was 60%, and the prevalence of circadian seizure cycles was 89%. Multidien cycles (mean PLV, 0.34) and circadian cycles (mean PLV, 0.34) were stronger than were circannual cycles (mean PLV, 0.17).
Among patients with circannual seizure cycles, there was a weak to moderate tendency for seizures to occur during one of the four seasons. There was no overall trend toward seizure onset in one season among this group.
Among patients with multidien seizure cycles, investigators identified five patterns of interictal epileptiform activity fluctuations. One pattern had irregular periodicity, and the others reached peak periodicity at 7, 15, 20, and 30 days. For some patients, one or more periodicities occurred. For most patients, electrographic or self-reported seizures tended to occur on the rising phase of the interictal epileptiform activity cycle. Interictal epileptiform activity increased on days around seizures.
Results showed there were five main seizure peak times among patients with circadian seizure cycles: midnight, 3:00 a.m., 9:00 a.m., 2:00 p.m., and 6:00 p.m. These findings corroborate the observations of previous investigations, the researchers noted. Hourly interictal epileptiform activity peaked during the night, regardless of peak seizure time.
“Although the neurostimulation device offers us a unique opportunity to investigate electrographic seizure activity quantitatively, the generalizability of our study is limited to the patient cohort that we studied,” said Dr. Fan. “The study findings are limited to patients with neurostimulation devices used for intractable focal epilepsies.”
The results support patients’ impressions that their seizures occur in a cyclical pattern.
“Ultimately, these findings will be helpful for developing models to aid with seizure forecasting and prediction in order to help reduce the uncertainty of seizure timing for patients with epilepsy,” said Dr. Fan.
“Other implications include optimizing the timing for patients to be admitted into the hospital for seizure characterization based on their seizure chronotype, or possibly tailoring a medication regimen in accordance with a patient’s seizure cycles,” she added.
Need for more research
Commenting on the findings, Tobias Loddenkemper, MD, professor of neurology at Harvard Medical School, Boston, noted that the study is “one of the largest longitudinal seizure pattern analyses, based on the gold standard of intracranially recorded epileptic seizures.”
The research, he added, extends neurologists’ understanding of seizure patterns over time, expands knowledge about seizure chronotypes, and emphasizes a relationship between interictal epileptiform activity and seizures.
The strengths of the study include the recording of seizures with intracranial EEG, its large number of participants, and the long duration of recordings, Dr. Loddenkemper said.
However, he said, it is important to note that self-reports are not always reliable. The results may also reflect the influence of potential confounders of seizure patterns, such as seizure triggers, treatment, stimulation, or sleep-wake, circadian, or hormonal cycles, he added.
“In the short term, validation studies, as well as confirmatory studies with less invasive sensors, may be needed,” said Dr. Loddenkemper.
“This could potentially include a trial that confirms findings prospectively, utilizing results from video EEG monitoring admissions. In the long term, seizure detection and prediction, as well as interventional chronotherapeutic trials, may be enabled, predicting seizures in individual patients and treating at times of greatest seizure susceptibility.”
The study was supported by grants to some of the authors from the Wyss Center for Bio and Neuroengineering, the Ernest Gallo Foundation, the Swiss National Science Foundation, and the Velux Stiftung. Dr. Fan has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Findings from a large longitudinal study show that seizure onset in patients with focal epilepsy follows circadian, multiday, and annual cycles.
“Although daily and multiday rhythms have previously been identified, the extent to which these nonrandom rhythms exist in a larger cohort has been unclear,” said study investigator Joline Marie Fan, MD, a clinical fellow at the University of California, San Francisco. “This means that a patient with epilepsy may have a unique combination of seizure rhythms that can inform the days and timing of his or her highest seizure risk,” she added.
The study was published online Feb. 8 in JAMA Neurology.
Distinct chronotypes
Clinicians and patients alike have long observed cyclical patterns in the onset of epileptic seizures. However, such patterns have rarely been measured in a quantitative way.
Previous studies have examined seizure cycles using inpatient seizure monitoring and patients’ seizure diaries, but the duration of these recordings and their accuracy have been limited. Within the past decade, the advent of cEEG has allowed researchers to observe the cyclical pattern of interictal epileptiform activity, but the numbers of patients involved in such studies have been limited.
To investigate seizure chronotypes in greater detail, the researchers examined retrospective data for 222 adults with medically refractory focal epilepsy who took part in clinical trials of the NeuroPace responsive neurostimulation (RNS) system.
After implantation in the brain, this system monitors the seizure focus or foci continuously and delivers stimulation to stop seizures. Participants also kept seizure diaries and classified their seizures as simple motor, simple other, complex partial, and generalized tonic-clonic.
Dr. Fan’s group examined three subpopulations of patients to investigate three durations of seizure cycles. They examined self-reported disabling seizures, electrographic seizures, and interictal epileptiform activity. Because patients did not record the time of their disabling seizures, the investigators examined them only in multidien and circannual cycles.
To examine circannual seizure cycles, the investigators included 194 patients who kept continuous seizure diaries for 2 or more years and who reported 24 or more days in which disabling seizures occurred.
To examine multidien seizure cycles, they included 186 participants who reported 24 or more days with disabling seizures over a period of 6 or more months during which the RNS system collected cEEG data. They included 85 patients who had 48 hours or more in which electrographic seizure counts were above zero during 6 or more months of cEEG data collection to examine circadian seizure cycles.
Phase-locking value (PLV) was used to determine the strength of a cycle (i.e., the degree of consistency with which seizures occur during certain phases of a cycle). A PLV of 0 represents a uniform distribution of events during various phases of a cycle; a PLV of 1 indicates that all events occur exactly at the same phase of a cycle.
The population’s median age was 35 years, and the sample included approximately equal numbers of men and women. Patients’ focal epilepsies included mesiotemporal (57.2%), frontal (14.0%), neocortical-temporal (9.9%), parietal (4.1%), occipital (1.4%), and multifocal (13.5%). The data included 1,118 patient-years of cEEG, 754,108 electrographic seizures, and 313,995 self-reported seizures.
The prevalence of statistically significant circannual seizure cycles in this population was 12%. The prevalence of multidien seizure cycles was 60%, and the prevalence of circadian seizure cycles was 89%. Multidien cycles (mean PLV, 0.34) and circadian cycles (mean PLV, 0.34) were stronger than were circannual cycles (mean PLV, 0.17).
Among patients with circannual seizure cycles, there was a weak to moderate tendency for seizures to occur during one of the four seasons. There was no overall trend toward seizure onset in one season among this group.
Among patients with multidien seizure cycles, investigators identified five patterns of interictal epileptiform activity fluctuations. One pattern had irregular periodicity, and the others reached peak periodicity at 7, 15, 20, and 30 days. For some patients, one or more periodicities occurred. For most patients, electrographic or self-reported seizures tended to occur on the rising phase of the interictal epileptiform activity cycle. Interictal epileptiform activity increased on days around seizures.
Results showed there were five main seizure peak times among patients with circadian seizure cycles: midnight, 3:00 a.m., 9:00 a.m., 2:00 p.m., and 6:00 p.m. These findings corroborate the observations of previous investigations, the researchers noted. Hourly interictal epileptiform activity peaked during the night, regardless of peak seizure time.
“Although the neurostimulation device offers us a unique opportunity to investigate electrographic seizure activity quantitatively, the generalizability of our study is limited to the patient cohort that we studied,” said Dr. Fan. “The study findings are limited to patients with neurostimulation devices used for intractable focal epilepsies.”
The results support patients’ impressions that their seizures occur in a cyclical pattern.
“Ultimately, these findings will be helpful for developing models to aid with seizure forecasting and prediction in order to help reduce the uncertainty of seizure timing for patients with epilepsy,” said Dr. Fan.
“Other implications include optimizing the timing for patients to be admitted into the hospital for seizure characterization based on their seizure chronotype, or possibly tailoring a medication regimen in accordance with a patient’s seizure cycles,” she added.
Need for more research
Commenting on the findings, Tobias Loddenkemper, MD, professor of neurology at Harvard Medical School, Boston, noted that the study is “one of the largest longitudinal seizure pattern analyses, based on the gold standard of intracranially recorded epileptic seizures.”
The research, he added, extends neurologists’ understanding of seizure patterns over time, expands knowledge about seizure chronotypes, and emphasizes a relationship between interictal epileptiform activity and seizures.
The strengths of the study include the recording of seizures with intracranial EEG, its large number of participants, and the long duration of recordings, Dr. Loddenkemper said.
However, he said, it is important to note that self-reports are not always reliable. The results may also reflect the influence of potential confounders of seizure patterns, such as seizure triggers, treatment, stimulation, or sleep-wake, circadian, or hormonal cycles, he added.
“In the short term, validation studies, as well as confirmatory studies with less invasive sensors, may be needed,” said Dr. Loddenkemper.
“This could potentially include a trial that confirms findings prospectively, utilizing results from video EEG monitoring admissions. In the long term, seizure detection and prediction, as well as interventional chronotherapeutic trials, may be enabled, predicting seizures in individual patients and treating at times of greatest seizure susceptibility.”
The study was supported by grants to some of the authors from the Wyss Center for Bio and Neuroengineering, the Ernest Gallo Foundation, the Swiss National Science Foundation, and the Velux Stiftung. Dr. Fan has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Innovation requires experimentation
A call for more health care trials
Successful innovation requires experimentation, according to a recent editorial in BMJ Quality & Safety – that’s why health systems should engage in more experimenting, more systematically, to improve health care.
“Most health systems implement interventions without testing them against other designs,” said co-author Mitesh S. Patel, MD, MBA, MS, of the University of Pennsylvania, Philadelphia. “This means that good ideas are often not spread (because we don’t know how impactful they are) and bad ones persist (because we don’t realize they don’t work).”
Dr. Patel, who is director of the Penn Medicine Nudge Unit at the Perelman Center for Advanced Medicine, encourages health systems and clinicians to implement new interventions in testable ways such as through a randomized trial, so that we can learn what works and why. A more systematic approach could help to expand programs that work and improve workflow and patient care.
“First, we must embed research teams within health systems in order to create the capacity for this kind of work. Expertise is required to identify a promising intervention, design the conceptual approach, conduct the technical implementation and rigorously evaluate the trial. These teams are also able to design interventions within the context of existing workflows in order to ensure that successful projects can be quickly scaled and that ineffective initiatives can be seamlessly terminated.” the authors wrote.
“Second, we must take advantage of existing data systems. The field of health care is ripe with detailed and reliable administrative data and electronic medical record data. These data offer the potential to do high-quality, low-cost, rapid trials. Third, we must measure a wide range of meaningful outcomes. We should examine the effect of interventions on health care costs, health care utilization and health outcomes.”
Next steps could be focused on thinking about the key priority areas and how can experiments be used to generate new knowledge on what works and what does not. “Luckily, the complex world of health care provides endless opportunities for rapid-cycle, randomized trials that target health care costs and outcomes,” Dr. Patel said.
Reference
1. Oakes AH, Patel MS. A nudge towards increased experimentation to more rapidly improve healthcare. BMJ Qual Saf. 2020;29:179-181. doi: 10.1136/bmjqs-2019-009948. Accessed Dec 3, 2019.
A call for more health care trials
A call for more health care trials
Successful innovation requires experimentation, according to a recent editorial in BMJ Quality & Safety – that’s why health systems should engage in more experimenting, more systematically, to improve health care.
“Most health systems implement interventions without testing them against other designs,” said co-author Mitesh S. Patel, MD, MBA, MS, of the University of Pennsylvania, Philadelphia. “This means that good ideas are often not spread (because we don’t know how impactful they are) and bad ones persist (because we don’t realize they don’t work).”
Dr. Patel, who is director of the Penn Medicine Nudge Unit at the Perelman Center for Advanced Medicine, encourages health systems and clinicians to implement new interventions in testable ways such as through a randomized trial, so that we can learn what works and why. A more systematic approach could help to expand programs that work and improve workflow and patient care.
“First, we must embed research teams within health systems in order to create the capacity for this kind of work. Expertise is required to identify a promising intervention, design the conceptual approach, conduct the technical implementation and rigorously evaluate the trial. These teams are also able to design interventions within the context of existing workflows in order to ensure that successful projects can be quickly scaled and that ineffective initiatives can be seamlessly terminated.” the authors wrote.
“Second, we must take advantage of existing data systems. The field of health care is ripe with detailed and reliable administrative data and electronic medical record data. These data offer the potential to do high-quality, low-cost, rapid trials. Third, we must measure a wide range of meaningful outcomes. We should examine the effect of interventions on health care costs, health care utilization and health outcomes.”
Next steps could be focused on thinking about the key priority areas and how can experiments be used to generate new knowledge on what works and what does not. “Luckily, the complex world of health care provides endless opportunities for rapid-cycle, randomized trials that target health care costs and outcomes,” Dr. Patel said.
Reference
1. Oakes AH, Patel MS. A nudge towards increased experimentation to more rapidly improve healthcare. BMJ Qual Saf. 2020;29:179-181. doi: 10.1136/bmjqs-2019-009948. Accessed Dec 3, 2019.
Successful innovation requires experimentation, according to a recent editorial in BMJ Quality & Safety – that’s why health systems should engage in more experimenting, more systematically, to improve health care.
“Most health systems implement interventions without testing them against other designs,” said co-author Mitesh S. Patel, MD, MBA, MS, of the University of Pennsylvania, Philadelphia. “This means that good ideas are often not spread (because we don’t know how impactful they are) and bad ones persist (because we don’t realize they don’t work).”
Dr. Patel, who is director of the Penn Medicine Nudge Unit at the Perelman Center for Advanced Medicine, encourages health systems and clinicians to implement new interventions in testable ways such as through a randomized trial, so that we can learn what works and why. A more systematic approach could help to expand programs that work and improve workflow and patient care.
“First, we must embed research teams within health systems in order to create the capacity for this kind of work. Expertise is required to identify a promising intervention, design the conceptual approach, conduct the technical implementation and rigorously evaluate the trial. These teams are also able to design interventions within the context of existing workflows in order to ensure that successful projects can be quickly scaled and that ineffective initiatives can be seamlessly terminated.” the authors wrote.
“Second, we must take advantage of existing data systems. The field of health care is ripe with detailed and reliable administrative data and electronic medical record data. These data offer the potential to do high-quality, low-cost, rapid trials. Third, we must measure a wide range of meaningful outcomes. We should examine the effect of interventions on health care costs, health care utilization and health outcomes.”
Next steps could be focused on thinking about the key priority areas and how can experiments be used to generate new knowledge on what works and what does not. “Luckily, the complex world of health care provides endless opportunities for rapid-cycle, randomized trials that target health care costs and outcomes,” Dr. Patel said.
Reference
1. Oakes AH, Patel MS. A nudge towards increased experimentation to more rapidly improve healthcare. BMJ Qual Saf. 2020;29:179-181. doi: 10.1136/bmjqs-2019-009948. Accessed Dec 3, 2019.
Methotrexate-associated hepatotoxicity risk differs between psoriasis, PsA, and RA patients
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Rise of JAK inhibitors highlights axial spondyloarthritis year in review
Rheumatologists can look forward to the likely regulatory approval of the oral Janus kinase inhibitors tofacitinib and upadacitinib for the treatment of axial spondyloarthritis in the first half of 2021, speakers predicted at the 2021 Rheumatology Winter Clinical Symposium.
This will be a major advance in the treatment of axial spondyloarthritis (axSpA) and promises to be one of the overall highlights of the coming year in rheumatology, according to the speakers. Both medications are now under Food and Drug Administration review for the proposed new indication.
“My sense is within the next 6 months we’re going to have two different oral JAK inhibitors that offer a new option for our ankylosing spondylitis and axial spondyloarthritis patients,” predicted Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Alexis R. Ogdie, MD, MSCE, noted that, at present, only two classes of potent medications are available for treatment of axial spondyloarthritis: tumor necrosis factor (TNF) inhibitors and anti–interleukin-17 biologics.
“I think it would be so exciting to have more treatment options. To have only two classes of drugs you can use for this disease is not enough,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
She and her fellow panelists also highlighted other recent key developments in axSpA, including epidemiologic evidence that case numbers are climbing sharply, identification of two previously unrecognized common comorbidities, a successful biologic remission induction and maintenance dose–reduction strategy, data on the best and worst biologics for patients with anterior uveitis, and evidence regarding next-step therapy in axSpA patients who’ve had an inadequate response to a TNF inhibitor.
The JAK inhibitors are coming
Oral tofacitinib (Xeljanz) at 5 mg twice daily was the focus of a pivotal phase 3, double-blind, 16-week, placebo-controlled clinical trial including 269 patients with axSpA. The results were presented at the 2020 annual meeting of the American College of Rheumatology. The primary endpoint was at least a 20% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 20). This was accomplished in 56.4% of patients on tofacitinib and 29.4% of placebo-treated controls. The ASAS 40 response rate was even more impressive: 40.6% with the JAK inhibitor, compared with 12.5% with placebo. There was one serious infection in the tofacitinib group, but no cases of venous thromboembolism, interstitial lung disease, opportunistic infection, major adverse cardiovascular events, or malignancy in this brief 4-month study.
Also now under FDA review are data from SELECT-AXIS 1, a phase 2/3, double-blind trial in which 187 biologic-naive patients with ankylosing spondylitis were randomized to 14 weeks of upadacitinib (Rinvoq) at 15 mg once daily or placebo. The primary endpoint, an ASAS 40 response, occurred in 51.6% of patients on the JAK inhibitor and half as many controls.
“They saw improvement in MRI scores with upadacitinib, so there’s biologic plausibility to this,” Dr. Ruderman noted.
He predicted the JAK inhibitors are going to have a big impact in clinical practice, especially in men.
“I have a lot of ankylosing spondylitis and axial spondyloarthritis patients on NSAIDs who I’m not convinced are doing as well as they could, but they push back every time I raise the possibility of going on a biologic,” the rheumatologist said. “I suspect that, given the rapid response with JAK inhibitors here, as in rheumatoid arthritis, it might be a little bit easier to persuade these people to give this a try for 4-6 weeks and then see how much better they are. It’s a pill. You don’t have to give yourself a shot.”
Dr. Ogdie predicted the new oral agents will bring more axSpA patients into rheumatologists’ offices.
“I think it’ll be kind of like the apremilast effect in psoriasis, where the drug got a lot more people into the market,” she said.
U.S. ankylosing spondylitis prevalence rising
The diagnostic prevalence of axSpA in the United States increased by 86% between 2006 and 2014 in a retrospective analysis based on Medicare fee-for-service claims data. A separate analysis using IBM MarketScan data for the same years was confirmatory, showing a 56% increase, Jeffrey R. Curtis, MD, MS, MPH, of the University of Alabama at Birmingham reported at the 2020 annual meeting of the European League Against Rheumatism (EULAR), now known as the European Alliance of Associations for Rheumatology.
“I think the take home is we’re seeing more of this. Some of this is likely due to increased awareness and inclusion of nonradiographic disease,” according to Dr. Ruderman.
Two previously overlooked comorbidities
It’s well recognized that 5%-10% of patients with axSpA have concurrent inflammatory bowel disease. But how about irritable bowel syndrome (IBS)?
A study of 186 Swedish patients with axSpA in the population-based SPARTAKUS registry, none with inflammatory bowel disease, concluded that 30% of them met ROME III diagnostic criteria for IBS, compared with 16% of healthy controls. Of note, the axSpA patients with comorbid IBS had significantly worse axSpA disease outcomes, compared with those without IBS as measured by pain, fatigue, and quality-of-life scores, as well as significantly greater disease activity on the Bath Ankylosing Spondylitis Disease Activity Index.
New-onset inflammatory back pain occurred in a hefty 24% of 513 Saudi patients placed on isotretinoin for acne. About 42% of those with inflammatory back pain displayed evidence of sacroiliitis on MRI. Moreover, 52% of patients with MRI-proven sacroiliitis fulfilled ASAS criteria for axSpA. In this longitudinal study, the MRI abnormalities and back pain symptoms completely resolved after isotretinoin discontinuation, but it took a long time: up to 9 months.
“When you see these people with inflammatory back pain on isotretinoin, I think it’s important that before you saddle them with a diagnosis that they have axSpA – a diagnosis that will go with them forever – give them time off drug, because this can look like the real thing. It’s something to think about as these pretty young kids come in to see you with back pain: Always ask about their medication history because it could be important,” Dr. Ruderman said.
A successful biologic remission induction-and-maintenance strategy
The phase 3b, multicenter C-OPTIMISE study sought to determine the best strategy for avoiding axSpA flares once sustained clinical remission has been achieved with a TNF inhibitor, in this case certolizumab pegol (Cimzia). The first part of the trial involved 736 patients with early axSpA, including 329 with nonradiographic disease. During the 48-week open-label induction period, 43.9% of patients achieved sustained clinical remission at the approved dose of 200 mg every 2 weeks, with similar success rates in radiographic and nonradiographic axSpA.
Those in sustained remission were then randomized double blind to one of three groups: an additional 48 weeks of certolizumab pegol at the full maintenance dose of 200 mg every 2 weeks, reduced maintenance dosing at 200 mg every 4 weeks, or placebo. During this period, 83.7% of the group who continued on full-dose certolizumab remained flare free, as did 79% of those on the reduced maintenance dose. In contrast, only 20.2% of patients in whom the biologic was completely withdrawn remained flare free. The investigators concluded that certolizumab dose reduction is a winning strategy for maintenance of clinical remission, as it reduces costs and limits long-term exposure to immunosuppressive therapy while maintaining clinical benefits.
All biologics aren’t equal when it comes to anterior uveitis risk
An analysis of Swedish Rheumatology Quality Register data presented at the 2020 EULAR meeting concluded that, among 3,568 patients started on one of four biologics for treatment of spondyloarthritis, the incidence of anterior uveitis was 2.9 per 100 patient-years in those on infliximab (Remicade), 4.0 per 100 patient-years with adalimumab (Humira), 6.8 per 100 patient-years with secukinumab (Cosentyx), and 7.5 per 100 patient-years with etanercept (Enbrel).
“This is important information for us in the clinic. The big question has been, do we see a reduced risk of anterior uveitis with secukinumab, an interleukin-17 inhibitor,” observed RWCS director Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy in the division of rheumatology, allergy, and immunology at the University of California, San Diego.
“When I’ve switched people with uveitis to secukinumab or ixekizumab [Taltz], I do see it come back. So I think it’s important to have these data out there,” Dr. Ogdie said.
Certolizumab pegol markedly reduced the incidence of anterior uveitis flares in patients with radiographic and nonradiographic axSpA and a history of recurrent uveitis flares in the ongoing phase 4 C-VIEW study. In the 48 weeks prior to going on certolizumab pegol, the 89 participants included in this analysis had an acute anterior uveitis incidence of 1.5 episodes per patient; during their first 48 weeks on the TNF inhibitor, the rate plunged to 0.2 episodes, representing an 87% reduction.
Secukinumab ‘not the obvious choice’ after inadequate response to a TNF inhibitor
While it might seem logical to turn to an IL-17 inhibitor in patients with an inadequate response to one or more TNF inhibitors, two recently published studies suggest that starting secukinumab is not more effective than trying yet another TNF inhibitor.
A retrospective analysis of Swiss registry data on next-step therapy in 390 axSpA patients who had withdrawn from one or more TNF inhibitors concluded that efficacy at 1 year in the 106 who switched to secukinumab wasn’t significantly different than in the 284 who moved on to another TNF inhibitor.
Similarly, an analysis of 10,583 courses of biologic therapy in 8,050 axSpA patients in five Nordic registries concluded that secukinumab and adalimumab as second-line therapy in patients with inadequate response to an initial TNF inhibitor performed similarly through 1 year of follow-up. However, in patients who’d previously failed to respond to two or three different biologic agents, adalimumab proved superior to the interleukin-17 inhibitor.
“These are two studies that don’t support the intuitive notion of trying a drug with a different mechanism of action when a patient has an inadequate response to a TNF inhibitor. It’s not clear that’s going to make a difference. It doesn’t mean secukinumab can’t work, but it means secukinumab is not the obvious choice,” Dr. Ruderman commented.
All three speakers reported financial relationships with numerous pharmaceutical companies.
Rheumatologists can look forward to the likely regulatory approval of the oral Janus kinase inhibitors tofacitinib and upadacitinib for the treatment of axial spondyloarthritis in the first half of 2021, speakers predicted at the 2021 Rheumatology Winter Clinical Symposium.
This will be a major advance in the treatment of axial spondyloarthritis (axSpA) and promises to be one of the overall highlights of the coming year in rheumatology, according to the speakers. Both medications are now under Food and Drug Administration review for the proposed new indication.
“My sense is within the next 6 months we’re going to have two different oral JAK inhibitors that offer a new option for our ankylosing spondylitis and axial spondyloarthritis patients,” predicted Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Alexis R. Ogdie, MD, MSCE, noted that, at present, only two classes of potent medications are available for treatment of axial spondyloarthritis: tumor necrosis factor (TNF) inhibitors and anti–interleukin-17 biologics.
“I think it would be so exciting to have more treatment options. To have only two classes of drugs you can use for this disease is not enough,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
She and her fellow panelists also highlighted other recent key developments in axSpA, including epidemiologic evidence that case numbers are climbing sharply, identification of two previously unrecognized common comorbidities, a successful biologic remission induction and maintenance dose–reduction strategy, data on the best and worst biologics for patients with anterior uveitis, and evidence regarding next-step therapy in axSpA patients who’ve had an inadequate response to a TNF inhibitor.
The JAK inhibitors are coming
Oral tofacitinib (Xeljanz) at 5 mg twice daily was the focus of a pivotal phase 3, double-blind, 16-week, placebo-controlled clinical trial including 269 patients with axSpA. The results were presented at the 2020 annual meeting of the American College of Rheumatology. The primary endpoint was at least a 20% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 20). This was accomplished in 56.4% of patients on tofacitinib and 29.4% of placebo-treated controls. The ASAS 40 response rate was even more impressive: 40.6% with the JAK inhibitor, compared with 12.5% with placebo. There was one serious infection in the tofacitinib group, but no cases of venous thromboembolism, interstitial lung disease, opportunistic infection, major adverse cardiovascular events, or malignancy in this brief 4-month study.
Also now under FDA review are data from SELECT-AXIS 1, a phase 2/3, double-blind trial in which 187 biologic-naive patients with ankylosing spondylitis were randomized to 14 weeks of upadacitinib (Rinvoq) at 15 mg once daily or placebo. The primary endpoint, an ASAS 40 response, occurred in 51.6% of patients on the JAK inhibitor and half as many controls.
“They saw improvement in MRI scores with upadacitinib, so there’s biologic plausibility to this,” Dr. Ruderman noted.
He predicted the JAK inhibitors are going to have a big impact in clinical practice, especially in men.
“I have a lot of ankylosing spondylitis and axial spondyloarthritis patients on NSAIDs who I’m not convinced are doing as well as they could, but they push back every time I raise the possibility of going on a biologic,” the rheumatologist said. “I suspect that, given the rapid response with JAK inhibitors here, as in rheumatoid arthritis, it might be a little bit easier to persuade these people to give this a try for 4-6 weeks and then see how much better they are. It’s a pill. You don’t have to give yourself a shot.”
Dr. Ogdie predicted the new oral agents will bring more axSpA patients into rheumatologists’ offices.
“I think it’ll be kind of like the apremilast effect in psoriasis, where the drug got a lot more people into the market,” she said.
U.S. ankylosing spondylitis prevalence rising
The diagnostic prevalence of axSpA in the United States increased by 86% between 2006 and 2014 in a retrospective analysis based on Medicare fee-for-service claims data. A separate analysis using IBM MarketScan data for the same years was confirmatory, showing a 56% increase, Jeffrey R. Curtis, MD, MS, MPH, of the University of Alabama at Birmingham reported at the 2020 annual meeting of the European League Against Rheumatism (EULAR), now known as the European Alliance of Associations for Rheumatology.
“I think the take home is we’re seeing more of this. Some of this is likely due to increased awareness and inclusion of nonradiographic disease,” according to Dr. Ruderman.
Two previously overlooked comorbidities
It’s well recognized that 5%-10% of patients with axSpA have concurrent inflammatory bowel disease. But how about irritable bowel syndrome (IBS)?
A study of 186 Swedish patients with axSpA in the population-based SPARTAKUS registry, none with inflammatory bowel disease, concluded that 30% of them met ROME III diagnostic criteria for IBS, compared with 16% of healthy controls. Of note, the axSpA patients with comorbid IBS had significantly worse axSpA disease outcomes, compared with those without IBS as measured by pain, fatigue, and quality-of-life scores, as well as significantly greater disease activity on the Bath Ankylosing Spondylitis Disease Activity Index.
New-onset inflammatory back pain occurred in a hefty 24% of 513 Saudi patients placed on isotretinoin for acne. About 42% of those with inflammatory back pain displayed evidence of sacroiliitis on MRI. Moreover, 52% of patients with MRI-proven sacroiliitis fulfilled ASAS criteria for axSpA. In this longitudinal study, the MRI abnormalities and back pain symptoms completely resolved after isotretinoin discontinuation, but it took a long time: up to 9 months.
“When you see these people with inflammatory back pain on isotretinoin, I think it’s important that before you saddle them with a diagnosis that they have axSpA – a diagnosis that will go with them forever – give them time off drug, because this can look like the real thing. It’s something to think about as these pretty young kids come in to see you with back pain: Always ask about their medication history because it could be important,” Dr. Ruderman said.
A successful biologic remission induction-and-maintenance strategy
The phase 3b, multicenter C-OPTIMISE study sought to determine the best strategy for avoiding axSpA flares once sustained clinical remission has been achieved with a TNF inhibitor, in this case certolizumab pegol (Cimzia). The first part of the trial involved 736 patients with early axSpA, including 329 with nonradiographic disease. During the 48-week open-label induction period, 43.9% of patients achieved sustained clinical remission at the approved dose of 200 mg every 2 weeks, with similar success rates in radiographic and nonradiographic axSpA.
Those in sustained remission were then randomized double blind to one of three groups: an additional 48 weeks of certolizumab pegol at the full maintenance dose of 200 mg every 2 weeks, reduced maintenance dosing at 200 mg every 4 weeks, or placebo. During this period, 83.7% of the group who continued on full-dose certolizumab remained flare free, as did 79% of those on the reduced maintenance dose. In contrast, only 20.2% of patients in whom the biologic was completely withdrawn remained flare free. The investigators concluded that certolizumab dose reduction is a winning strategy for maintenance of clinical remission, as it reduces costs and limits long-term exposure to immunosuppressive therapy while maintaining clinical benefits.
All biologics aren’t equal when it comes to anterior uveitis risk
An analysis of Swedish Rheumatology Quality Register data presented at the 2020 EULAR meeting concluded that, among 3,568 patients started on one of four biologics for treatment of spondyloarthritis, the incidence of anterior uveitis was 2.9 per 100 patient-years in those on infliximab (Remicade), 4.0 per 100 patient-years with adalimumab (Humira), 6.8 per 100 patient-years with secukinumab (Cosentyx), and 7.5 per 100 patient-years with etanercept (Enbrel).
“This is important information for us in the clinic. The big question has been, do we see a reduced risk of anterior uveitis with secukinumab, an interleukin-17 inhibitor,” observed RWCS director Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy in the division of rheumatology, allergy, and immunology at the University of California, San Diego.
“When I’ve switched people with uveitis to secukinumab or ixekizumab [Taltz], I do see it come back. So I think it’s important to have these data out there,” Dr. Ogdie said.
Certolizumab pegol markedly reduced the incidence of anterior uveitis flares in patients with radiographic and nonradiographic axSpA and a history of recurrent uveitis flares in the ongoing phase 4 C-VIEW study. In the 48 weeks prior to going on certolizumab pegol, the 89 participants included in this analysis had an acute anterior uveitis incidence of 1.5 episodes per patient; during their first 48 weeks on the TNF inhibitor, the rate plunged to 0.2 episodes, representing an 87% reduction.
Secukinumab ‘not the obvious choice’ after inadequate response to a TNF inhibitor
While it might seem logical to turn to an IL-17 inhibitor in patients with an inadequate response to one or more TNF inhibitors, two recently published studies suggest that starting secukinumab is not more effective than trying yet another TNF inhibitor.
A retrospective analysis of Swiss registry data on next-step therapy in 390 axSpA patients who had withdrawn from one or more TNF inhibitors concluded that efficacy at 1 year in the 106 who switched to secukinumab wasn’t significantly different than in the 284 who moved on to another TNF inhibitor.
Similarly, an analysis of 10,583 courses of biologic therapy in 8,050 axSpA patients in five Nordic registries concluded that secukinumab and adalimumab as second-line therapy in patients with inadequate response to an initial TNF inhibitor performed similarly through 1 year of follow-up. However, in patients who’d previously failed to respond to two or three different biologic agents, adalimumab proved superior to the interleukin-17 inhibitor.
“These are two studies that don’t support the intuitive notion of trying a drug with a different mechanism of action when a patient has an inadequate response to a TNF inhibitor. It’s not clear that’s going to make a difference. It doesn’t mean secukinumab can’t work, but it means secukinumab is not the obvious choice,” Dr. Ruderman commented.
All three speakers reported financial relationships with numerous pharmaceutical companies.
Rheumatologists can look forward to the likely regulatory approval of the oral Janus kinase inhibitors tofacitinib and upadacitinib for the treatment of axial spondyloarthritis in the first half of 2021, speakers predicted at the 2021 Rheumatology Winter Clinical Symposium.
This will be a major advance in the treatment of axial spondyloarthritis (axSpA) and promises to be one of the overall highlights of the coming year in rheumatology, according to the speakers. Both medications are now under Food and Drug Administration review for the proposed new indication.
“My sense is within the next 6 months we’re going to have two different oral JAK inhibitors that offer a new option for our ankylosing spondylitis and axial spondyloarthritis patients,” predicted Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Alexis R. Ogdie, MD, MSCE, noted that, at present, only two classes of potent medications are available for treatment of axial spondyloarthritis: tumor necrosis factor (TNF) inhibitors and anti–interleukin-17 biologics.
“I think it would be so exciting to have more treatment options. To have only two classes of drugs you can use for this disease is not enough,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
She and her fellow panelists also highlighted other recent key developments in axSpA, including epidemiologic evidence that case numbers are climbing sharply, identification of two previously unrecognized common comorbidities, a successful biologic remission induction and maintenance dose–reduction strategy, data on the best and worst biologics for patients with anterior uveitis, and evidence regarding next-step therapy in axSpA patients who’ve had an inadequate response to a TNF inhibitor.
The JAK inhibitors are coming
Oral tofacitinib (Xeljanz) at 5 mg twice daily was the focus of a pivotal phase 3, double-blind, 16-week, placebo-controlled clinical trial including 269 patients with axSpA. The results were presented at the 2020 annual meeting of the American College of Rheumatology. The primary endpoint was at least a 20% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 20). This was accomplished in 56.4% of patients on tofacitinib and 29.4% of placebo-treated controls. The ASAS 40 response rate was even more impressive: 40.6% with the JAK inhibitor, compared with 12.5% with placebo. There was one serious infection in the tofacitinib group, but no cases of venous thromboembolism, interstitial lung disease, opportunistic infection, major adverse cardiovascular events, or malignancy in this brief 4-month study.
Also now under FDA review are data from SELECT-AXIS 1, a phase 2/3, double-blind trial in which 187 biologic-naive patients with ankylosing spondylitis were randomized to 14 weeks of upadacitinib (Rinvoq) at 15 mg once daily or placebo. The primary endpoint, an ASAS 40 response, occurred in 51.6% of patients on the JAK inhibitor and half as many controls.
“They saw improvement in MRI scores with upadacitinib, so there’s biologic plausibility to this,” Dr. Ruderman noted.
He predicted the JAK inhibitors are going to have a big impact in clinical practice, especially in men.
“I have a lot of ankylosing spondylitis and axial spondyloarthritis patients on NSAIDs who I’m not convinced are doing as well as they could, but they push back every time I raise the possibility of going on a biologic,” the rheumatologist said. “I suspect that, given the rapid response with JAK inhibitors here, as in rheumatoid arthritis, it might be a little bit easier to persuade these people to give this a try for 4-6 weeks and then see how much better they are. It’s a pill. You don’t have to give yourself a shot.”
Dr. Ogdie predicted the new oral agents will bring more axSpA patients into rheumatologists’ offices.
“I think it’ll be kind of like the apremilast effect in psoriasis, where the drug got a lot more people into the market,” she said.
U.S. ankylosing spondylitis prevalence rising
The diagnostic prevalence of axSpA in the United States increased by 86% between 2006 and 2014 in a retrospective analysis based on Medicare fee-for-service claims data. A separate analysis using IBM MarketScan data for the same years was confirmatory, showing a 56% increase, Jeffrey R. Curtis, MD, MS, MPH, of the University of Alabama at Birmingham reported at the 2020 annual meeting of the European League Against Rheumatism (EULAR), now known as the European Alliance of Associations for Rheumatology.
“I think the take home is we’re seeing more of this. Some of this is likely due to increased awareness and inclusion of nonradiographic disease,” according to Dr. Ruderman.
Two previously overlooked comorbidities
It’s well recognized that 5%-10% of patients with axSpA have concurrent inflammatory bowel disease. But how about irritable bowel syndrome (IBS)?
A study of 186 Swedish patients with axSpA in the population-based SPARTAKUS registry, none with inflammatory bowel disease, concluded that 30% of them met ROME III diagnostic criteria for IBS, compared with 16% of healthy controls. Of note, the axSpA patients with comorbid IBS had significantly worse axSpA disease outcomes, compared with those without IBS as measured by pain, fatigue, and quality-of-life scores, as well as significantly greater disease activity on the Bath Ankylosing Spondylitis Disease Activity Index.
New-onset inflammatory back pain occurred in a hefty 24% of 513 Saudi patients placed on isotretinoin for acne. About 42% of those with inflammatory back pain displayed evidence of sacroiliitis on MRI. Moreover, 52% of patients with MRI-proven sacroiliitis fulfilled ASAS criteria for axSpA. In this longitudinal study, the MRI abnormalities and back pain symptoms completely resolved after isotretinoin discontinuation, but it took a long time: up to 9 months.
“When you see these people with inflammatory back pain on isotretinoin, I think it’s important that before you saddle them with a diagnosis that they have axSpA – a diagnosis that will go with them forever – give them time off drug, because this can look like the real thing. It’s something to think about as these pretty young kids come in to see you with back pain: Always ask about their medication history because it could be important,” Dr. Ruderman said.
A successful biologic remission induction-and-maintenance strategy
The phase 3b, multicenter C-OPTIMISE study sought to determine the best strategy for avoiding axSpA flares once sustained clinical remission has been achieved with a TNF inhibitor, in this case certolizumab pegol (Cimzia). The first part of the trial involved 736 patients with early axSpA, including 329 with nonradiographic disease. During the 48-week open-label induction period, 43.9% of patients achieved sustained clinical remission at the approved dose of 200 mg every 2 weeks, with similar success rates in radiographic and nonradiographic axSpA.
Those in sustained remission were then randomized double blind to one of three groups: an additional 48 weeks of certolizumab pegol at the full maintenance dose of 200 mg every 2 weeks, reduced maintenance dosing at 200 mg every 4 weeks, or placebo. During this period, 83.7% of the group who continued on full-dose certolizumab remained flare free, as did 79% of those on the reduced maintenance dose. In contrast, only 20.2% of patients in whom the biologic was completely withdrawn remained flare free. The investigators concluded that certolizumab dose reduction is a winning strategy for maintenance of clinical remission, as it reduces costs and limits long-term exposure to immunosuppressive therapy while maintaining clinical benefits.
All biologics aren’t equal when it comes to anterior uveitis risk
An analysis of Swedish Rheumatology Quality Register data presented at the 2020 EULAR meeting concluded that, among 3,568 patients started on one of four biologics for treatment of spondyloarthritis, the incidence of anterior uveitis was 2.9 per 100 patient-years in those on infliximab (Remicade), 4.0 per 100 patient-years with adalimumab (Humira), 6.8 per 100 patient-years with secukinumab (Cosentyx), and 7.5 per 100 patient-years with etanercept (Enbrel).
“This is important information for us in the clinic. The big question has been, do we see a reduced risk of anterior uveitis with secukinumab, an interleukin-17 inhibitor,” observed RWCS director Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy in the division of rheumatology, allergy, and immunology at the University of California, San Diego.
“When I’ve switched people with uveitis to secukinumab or ixekizumab [Taltz], I do see it come back. So I think it’s important to have these data out there,” Dr. Ogdie said.
Certolizumab pegol markedly reduced the incidence of anterior uveitis flares in patients with radiographic and nonradiographic axSpA and a history of recurrent uveitis flares in the ongoing phase 4 C-VIEW study. In the 48 weeks prior to going on certolizumab pegol, the 89 participants included in this analysis had an acute anterior uveitis incidence of 1.5 episodes per patient; during their first 48 weeks on the TNF inhibitor, the rate plunged to 0.2 episodes, representing an 87% reduction.
Secukinumab ‘not the obvious choice’ after inadequate response to a TNF inhibitor
While it might seem logical to turn to an IL-17 inhibitor in patients with an inadequate response to one or more TNF inhibitors, two recently published studies suggest that starting secukinumab is not more effective than trying yet another TNF inhibitor.
A retrospective analysis of Swiss registry data on next-step therapy in 390 axSpA patients who had withdrawn from one or more TNF inhibitors concluded that efficacy at 1 year in the 106 who switched to secukinumab wasn’t significantly different than in the 284 who moved on to another TNF inhibitor.
Similarly, an analysis of 10,583 courses of biologic therapy in 8,050 axSpA patients in five Nordic registries concluded that secukinumab and adalimumab as second-line therapy in patients with inadequate response to an initial TNF inhibitor performed similarly through 1 year of follow-up. However, in patients who’d previously failed to respond to two or three different biologic agents, adalimumab proved superior to the interleukin-17 inhibitor.
“These are two studies that don’t support the intuitive notion of trying a drug with a different mechanism of action when a patient has an inadequate response to a TNF inhibitor. It’s not clear that’s going to make a difference. It doesn’t mean secukinumab can’t work, but it means secukinumab is not the obvious choice,” Dr. Ruderman commented.
All three speakers reported financial relationships with numerous pharmaceutical companies.
FROM RWCS 2021
Pediatric COVID-19: Data to guide practice
With the daily stream of new information, it is difficult to keep up with data on how the coronavirus epidemic affects children and school attendance, as well as how pediatricians can advise parents. The following is a summary of recently published information about birth and infant outcomes, and symptoms seen in infants and children, along with a review of recent information on transmission in schools.
COVID-19 in newborns
In November 2020, the Centers for Disease Control and Prevention published data from 16 jurisdictions detailing pregnancy and infant outcomes of more than 5,000 women with SARS-CoV-2 infection. The data were collected from March to October 2020. More than 80% of the women found to be positive for SARS-CoV-2 were identified during their third trimester. The surveillance found that 12.9% of infants born to infected mothers were born preterm, compared with an expected rate in the population of approximately 10%, suggesting that third-trimester infection may be associated with an increase in premature birth. Among 610 infants born to infected mothers and tested for SARS-CoV-2 during their nursery stay, 2.6% were positive. The infant positivity rate was as high as 4.3% among infants who were born to women with a documented SARS-CoV-2 infection within 2 weeks of the delivery date. No newborn infections were found among the infants whose mothers’ infection occurred more than 14 days before delivery. Current CDC and American Academy of Pediatrics recommendations are to test infants born to mothers with suspected or confirmed SARS-CoV-2 infection.
Data on clinical characteristics of a series of hospitalized infants in Montreal was published in December 2020. The study identified infants 0-12 months old who were diagnosed or treated at a single Montreal hospital from February until May 2020. In all, 25 (2.0%) of 1,165 infants were confirmed to have SARS-CoV-2, and approximately 8 of those were hospitalized; 85% had gastrointestinal symptoms and 81% had a fever. Upper respiratory tract symptoms were present in 59%, and none of the hospitalized infants required supplemental oxygen. The data overall support the idea that infants are generally only mildly symptomatic when infected, and respiratory symptoms do not appear to be the most prevalent finding.
COVID-19 in children
The lack of prominent respiratory symptoms among children with SARS-CoV-2 infection symptoms was echoed in another study that evaluated more than 2,400 children in Alberta, Canada. Among the 1,987 children who tested positive for SARS-CoV-2, one-third (35.9%) were asymptomatic. Some symptoms were not helpful in differentiating children who tested positive vs. those who tested negative. The frequency of muscle or joint pain, myalgia, malaise, and respiratory symptoms such as nasal congestion, difficulty breathing, and sore throat was indistinguishable between the SARS-CoV-2–infected and –noninfected children. However, anosmia was much more prevalent (7.7%) among those who tested positive for SARS-CoV-2, compared with 1.1% of those who were negative. Headache was present in 15.7% of those who were positive vs. 6.3% of those who were negative. Fever was slightly more prevalent, at 25.5% among the positive patients and 15% of the negative patients.
The authors calculated likelihood ratios for individual symptoms and found that almost all individual symptoms had likelihood ratios of 1:1.8 for testing positive. However, nausea and vomiting had a likelihood ratio of 5.5, and for anosmia it was 7.3. The combination of symptoms of nausea, nausea and vomiting, and headache produced a likelihood ratio of nearly 66. The authors suggest that these data on ambulatory children indicate that, in general, respiratory symptoms are not helpful for distinguishing patients who are likely to be positive, although the symptoms of nausea, headache, and both along with fever can be highly predictive. The authors propose that it may be more helpful for schools to focus on identifying children with combinations of these high-yield symptoms for potential testing and exclusion from school rather than on random or isolated respiratory symptoms.
COVID-19 in schools
Transmission risk in different settings is certainly something parents quiz pediatricians about, so data released in January and February 2021 may help provide some context. A CDC report on the experience of 17 schools in Wisconsin from August to November 2020 is illuminating. In that study, the SARS-CoV-2 case rate in students, school teachers, and staff members was 63% of the rate in the general public at the time, suggesting that the mitigation strategies used by the schools were effective. In addition, among the students who contracted SARS-CoV-2, only 5% of cases were attributable to school exposure. No cases of SARS-CoV-2 among faculty or staff were linked to school exposure.
Indeed, data released on Feb. 2, 2021, demonstrate that younger adults are the largest source of sustaining the epidemic. On the basis of data from August to October 2020, the opening of schools does not appear to be associated with population-level changes in SARS-CoV-2–attributable deaths. For October 2020, the authors estimate that 2.7% of infections were from children 0-9 years old, 7.1% from those ages 10-19 years, but 34% from those 20-34 years old and 38% from those 35-49 years old, by far the largest two groups contributing to spread. It should be noted that ages 20-49 years are the peak working years for adults, but the source of the data did not allow the authors to conclude whether infections were work related or social activity related. Their data do suggest that prioritizing vaccination of younger working-age adults may put more of a dent in the pandemic spread than vaccinating older individuals.
In a similar vein, a systematic review and meta-analysis of recent studies looked at household transmission of SARS-CoV-2 and demonstrated an attack rate within households of 16.6%. Of note, secondary household attack rates were only 0.7% from asymptomatic cases and 18% from symptomatic cases, with spouses and adult household contacts having higher secondary attack rates than children in the household.
COVID-19 in student athletes
A recent MMWR report described a SARS-CoV-2 outbreak associated with a series of wrestling tournaments in Florida, held in December and January 2021. While everyone would like children to be able to participate in sports, such events potentially violate several of the precepts for preventing spread: Avoid close contact and don’t mix contacts from different schools. Moreover, the events occurred during some of the highest incident case rates in the counties where the tournaments took place.
On Dec. 4, 2020, the AAP released updated guidance for athletic activities and recommended cloth face coverings for student athletes during training, in competition, while traveling, and even while waiting on the sidelines and not actively playing. Notable exceptions to the recommendation were competitive cheerleading, gymnastics, wrestling, and water sports, where the risk for entanglement from face coverings was too high or was not practical.
Taken as a whole, the evolving data continue to show that school mitigation practices can be effective in reducing the risk for SARS-CoV-2 infection. In addition, SARS-CoV-2 rates among schoolchildren more closely mirror community rates and are probably more influenced by what happens outside the schools than inside the schools.
A version of this article first appeared on Medscape.com.
With the daily stream of new information, it is difficult to keep up with data on how the coronavirus epidemic affects children and school attendance, as well as how pediatricians can advise parents. The following is a summary of recently published information about birth and infant outcomes, and symptoms seen in infants and children, along with a review of recent information on transmission in schools.
COVID-19 in newborns
In November 2020, the Centers for Disease Control and Prevention published data from 16 jurisdictions detailing pregnancy and infant outcomes of more than 5,000 women with SARS-CoV-2 infection. The data were collected from March to October 2020. More than 80% of the women found to be positive for SARS-CoV-2 were identified during their third trimester. The surveillance found that 12.9% of infants born to infected mothers were born preterm, compared with an expected rate in the population of approximately 10%, suggesting that third-trimester infection may be associated with an increase in premature birth. Among 610 infants born to infected mothers and tested for SARS-CoV-2 during their nursery stay, 2.6% were positive. The infant positivity rate was as high as 4.3% among infants who were born to women with a documented SARS-CoV-2 infection within 2 weeks of the delivery date. No newborn infections were found among the infants whose mothers’ infection occurred more than 14 days before delivery. Current CDC and American Academy of Pediatrics recommendations are to test infants born to mothers with suspected or confirmed SARS-CoV-2 infection.
Data on clinical characteristics of a series of hospitalized infants in Montreal was published in December 2020. The study identified infants 0-12 months old who were diagnosed or treated at a single Montreal hospital from February until May 2020. In all, 25 (2.0%) of 1,165 infants were confirmed to have SARS-CoV-2, and approximately 8 of those were hospitalized; 85% had gastrointestinal symptoms and 81% had a fever. Upper respiratory tract symptoms were present in 59%, and none of the hospitalized infants required supplemental oxygen. The data overall support the idea that infants are generally only mildly symptomatic when infected, and respiratory symptoms do not appear to be the most prevalent finding.
COVID-19 in children
The lack of prominent respiratory symptoms among children with SARS-CoV-2 infection symptoms was echoed in another study that evaluated more than 2,400 children in Alberta, Canada. Among the 1,987 children who tested positive for SARS-CoV-2, one-third (35.9%) were asymptomatic. Some symptoms were not helpful in differentiating children who tested positive vs. those who tested negative. The frequency of muscle or joint pain, myalgia, malaise, and respiratory symptoms such as nasal congestion, difficulty breathing, and sore throat was indistinguishable between the SARS-CoV-2–infected and –noninfected children. However, anosmia was much more prevalent (7.7%) among those who tested positive for SARS-CoV-2, compared with 1.1% of those who were negative. Headache was present in 15.7% of those who were positive vs. 6.3% of those who were negative. Fever was slightly more prevalent, at 25.5% among the positive patients and 15% of the negative patients.
The authors calculated likelihood ratios for individual symptoms and found that almost all individual symptoms had likelihood ratios of 1:1.8 for testing positive. However, nausea and vomiting had a likelihood ratio of 5.5, and for anosmia it was 7.3. The combination of symptoms of nausea, nausea and vomiting, and headache produced a likelihood ratio of nearly 66. The authors suggest that these data on ambulatory children indicate that, in general, respiratory symptoms are not helpful for distinguishing patients who are likely to be positive, although the symptoms of nausea, headache, and both along with fever can be highly predictive. The authors propose that it may be more helpful for schools to focus on identifying children with combinations of these high-yield symptoms for potential testing and exclusion from school rather than on random or isolated respiratory symptoms.
COVID-19 in schools
Transmission risk in different settings is certainly something parents quiz pediatricians about, so data released in January and February 2021 may help provide some context. A CDC report on the experience of 17 schools in Wisconsin from August to November 2020 is illuminating. In that study, the SARS-CoV-2 case rate in students, school teachers, and staff members was 63% of the rate in the general public at the time, suggesting that the mitigation strategies used by the schools were effective. In addition, among the students who contracted SARS-CoV-2, only 5% of cases were attributable to school exposure. No cases of SARS-CoV-2 among faculty or staff were linked to school exposure.
Indeed, data released on Feb. 2, 2021, demonstrate that younger adults are the largest source of sustaining the epidemic. On the basis of data from August to October 2020, the opening of schools does not appear to be associated with population-level changes in SARS-CoV-2–attributable deaths. For October 2020, the authors estimate that 2.7% of infections were from children 0-9 years old, 7.1% from those ages 10-19 years, but 34% from those 20-34 years old and 38% from those 35-49 years old, by far the largest two groups contributing to spread. It should be noted that ages 20-49 years are the peak working years for adults, but the source of the data did not allow the authors to conclude whether infections were work related or social activity related. Their data do suggest that prioritizing vaccination of younger working-age adults may put more of a dent in the pandemic spread than vaccinating older individuals.
In a similar vein, a systematic review and meta-analysis of recent studies looked at household transmission of SARS-CoV-2 and demonstrated an attack rate within households of 16.6%. Of note, secondary household attack rates were only 0.7% from asymptomatic cases and 18% from symptomatic cases, with spouses and adult household contacts having higher secondary attack rates than children in the household.
COVID-19 in student athletes
A recent MMWR report described a SARS-CoV-2 outbreak associated with a series of wrestling tournaments in Florida, held in December and January 2021. While everyone would like children to be able to participate in sports, such events potentially violate several of the precepts for preventing spread: Avoid close contact and don’t mix contacts from different schools. Moreover, the events occurred during some of the highest incident case rates in the counties where the tournaments took place.
On Dec. 4, 2020, the AAP released updated guidance for athletic activities and recommended cloth face coverings for student athletes during training, in competition, while traveling, and even while waiting on the sidelines and not actively playing. Notable exceptions to the recommendation were competitive cheerleading, gymnastics, wrestling, and water sports, where the risk for entanglement from face coverings was too high or was not practical.
Taken as a whole, the evolving data continue to show that school mitigation practices can be effective in reducing the risk for SARS-CoV-2 infection. In addition, SARS-CoV-2 rates among schoolchildren more closely mirror community rates and are probably more influenced by what happens outside the schools than inside the schools.
A version of this article first appeared on Medscape.com.
With the daily stream of new information, it is difficult to keep up with data on how the coronavirus epidemic affects children and school attendance, as well as how pediatricians can advise parents. The following is a summary of recently published information about birth and infant outcomes, and symptoms seen in infants and children, along with a review of recent information on transmission in schools.
COVID-19 in newborns
In November 2020, the Centers for Disease Control and Prevention published data from 16 jurisdictions detailing pregnancy and infant outcomes of more than 5,000 women with SARS-CoV-2 infection. The data were collected from March to October 2020. More than 80% of the women found to be positive for SARS-CoV-2 were identified during their third trimester. The surveillance found that 12.9% of infants born to infected mothers were born preterm, compared with an expected rate in the population of approximately 10%, suggesting that third-trimester infection may be associated with an increase in premature birth. Among 610 infants born to infected mothers and tested for SARS-CoV-2 during their nursery stay, 2.6% were positive. The infant positivity rate was as high as 4.3% among infants who were born to women with a documented SARS-CoV-2 infection within 2 weeks of the delivery date. No newborn infections were found among the infants whose mothers’ infection occurred more than 14 days before delivery. Current CDC and American Academy of Pediatrics recommendations are to test infants born to mothers with suspected or confirmed SARS-CoV-2 infection.
Data on clinical characteristics of a series of hospitalized infants in Montreal was published in December 2020. The study identified infants 0-12 months old who were diagnosed or treated at a single Montreal hospital from February until May 2020. In all, 25 (2.0%) of 1,165 infants were confirmed to have SARS-CoV-2, and approximately 8 of those were hospitalized; 85% had gastrointestinal symptoms and 81% had a fever. Upper respiratory tract symptoms were present in 59%, and none of the hospitalized infants required supplemental oxygen. The data overall support the idea that infants are generally only mildly symptomatic when infected, and respiratory symptoms do not appear to be the most prevalent finding.
COVID-19 in children
The lack of prominent respiratory symptoms among children with SARS-CoV-2 infection symptoms was echoed in another study that evaluated more than 2,400 children in Alberta, Canada. Among the 1,987 children who tested positive for SARS-CoV-2, one-third (35.9%) were asymptomatic. Some symptoms were not helpful in differentiating children who tested positive vs. those who tested negative. The frequency of muscle or joint pain, myalgia, malaise, and respiratory symptoms such as nasal congestion, difficulty breathing, and sore throat was indistinguishable between the SARS-CoV-2–infected and –noninfected children. However, anosmia was much more prevalent (7.7%) among those who tested positive for SARS-CoV-2, compared with 1.1% of those who were negative. Headache was present in 15.7% of those who were positive vs. 6.3% of those who were negative. Fever was slightly more prevalent, at 25.5% among the positive patients and 15% of the negative patients.
The authors calculated likelihood ratios for individual symptoms and found that almost all individual symptoms had likelihood ratios of 1:1.8 for testing positive. However, nausea and vomiting had a likelihood ratio of 5.5, and for anosmia it was 7.3. The combination of symptoms of nausea, nausea and vomiting, and headache produced a likelihood ratio of nearly 66. The authors suggest that these data on ambulatory children indicate that, in general, respiratory symptoms are not helpful for distinguishing patients who are likely to be positive, although the symptoms of nausea, headache, and both along with fever can be highly predictive. The authors propose that it may be more helpful for schools to focus on identifying children with combinations of these high-yield symptoms for potential testing and exclusion from school rather than on random or isolated respiratory symptoms.
COVID-19 in schools
Transmission risk in different settings is certainly something parents quiz pediatricians about, so data released in January and February 2021 may help provide some context. A CDC report on the experience of 17 schools in Wisconsin from August to November 2020 is illuminating. In that study, the SARS-CoV-2 case rate in students, school teachers, and staff members was 63% of the rate in the general public at the time, suggesting that the mitigation strategies used by the schools were effective. In addition, among the students who contracted SARS-CoV-2, only 5% of cases were attributable to school exposure. No cases of SARS-CoV-2 among faculty or staff were linked to school exposure.
Indeed, data released on Feb. 2, 2021, demonstrate that younger adults are the largest source of sustaining the epidemic. On the basis of data from August to October 2020, the opening of schools does not appear to be associated with population-level changes in SARS-CoV-2–attributable deaths. For October 2020, the authors estimate that 2.7% of infections were from children 0-9 years old, 7.1% from those ages 10-19 years, but 34% from those 20-34 years old and 38% from those 35-49 years old, by far the largest two groups contributing to spread. It should be noted that ages 20-49 years are the peak working years for adults, but the source of the data did not allow the authors to conclude whether infections were work related or social activity related. Their data do suggest that prioritizing vaccination of younger working-age adults may put more of a dent in the pandemic spread than vaccinating older individuals.
In a similar vein, a systematic review and meta-analysis of recent studies looked at household transmission of SARS-CoV-2 and demonstrated an attack rate within households of 16.6%. Of note, secondary household attack rates were only 0.7% from asymptomatic cases and 18% from symptomatic cases, with spouses and adult household contacts having higher secondary attack rates than children in the household.
COVID-19 in student athletes
A recent MMWR report described a SARS-CoV-2 outbreak associated with a series of wrestling tournaments in Florida, held in December and January 2021. While everyone would like children to be able to participate in sports, such events potentially violate several of the precepts for preventing spread: Avoid close contact and don’t mix contacts from different schools. Moreover, the events occurred during some of the highest incident case rates in the counties where the tournaments took place.
On Dec. 4, 2020, the AAP released updated guidance for athletic activities and recommended cloth face coverings for student athletes during training, in competition, while traveling, and even while waiting on the sidelines and not actively playing. Notable exceptions to the recommendation were competitive cheerleading, gymnastics, wrestling, and water sports, where the risk for entanglement from face coverings was too high or was not practical.
Taken as a whole, the evolving data continue to show that school mitigation practices can be effective in reducing the risk for SARS-CoV-2 infection. In addition, SARS-CoV-2 rates among schoolchildren more closely mirror community rates and are probably more influenced by what happens outside the schools than inside the schools.
A version of this article first appeared on Medscape.com.
Painful hand and foot plaques
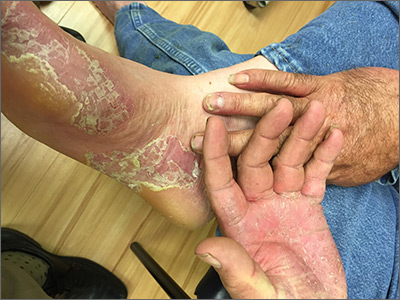
This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.