User login
Tucatinib-Trastuzumab Benefit ‘Remarkable’ in HER2-positive mCRC
Only about 3% to 5% of patients with metastatic colorectal cancer have tumors that are positive for human epidermal growth factor receptor 2 (HER2), and until recently there was no treatment approved by the US Food and Drug Administration (FDA) for this subset of patients.
That all changed, in January of 2023. At that time, the FDA granted accelerated approval to tucatinib (Tukysa) in combination with trastuzumab for RAS wild-type HER2-positive unresectable or metastatic colorectal cancer that has progressed following fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
The combination was the first FDA-approved treatment for this patient population.
The only other FDA-approved therapy for metastatic HER2-positive CRC is the antibody-drug conjugate trastuzumab deruxtecan (Enhertu). That drug received accelerated approval from the FDA for metastatic HER2-positive CRC for which no other suitable therapeutic option exists, on April 5, 2024. This FDA action represented an expansion of the drug’s earlier approvals for treating several cancer types, including certain patients with unresectable or metastatic HER2-positive breast cancer and adults with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma who had received a prior trastuzumab-based regimen.
Drug Combo’s Use With Capecitabine in Breast Cancer
Tucatinib is a potent oral tyrosine kinase inhibitor (TKI) that has been shown to be highly selective for HER2. Prior to approval of the colorectal cancer indication, tucatinib had received FDA approval (in April 2020) in combination with trastuzumab and capecitabine for the treatment of patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who had received one or more prior anti-HER2-based regimens in the metastatic setting.
In these patients the combination was associated with significant improvements in both progression-free survival (PFS) and overall survival compared with trastuzumab and capecitabine.
Approval for the colorectal cancer indication was based on results of the phase 2 MOUNTAINEER trial, which were published in The Lancet Oncology.
Real-World Setting
Clinical experience with the combination in a real-world setting is still limited due to the relatively uncommon RAS wild-type HER2-positive CRC subtype, so most of what’s known about the efficacy and safety of tucatinib plus trastuzumab comes from clinical trials. But oncologists interviewed for this article emphasized that the tucatinib-trastuzumab combination nonetheless represents a major breakthrough.
“The population of RAS wild-type HER2-positive is small in colorectal cancer, but the benefit of this treatment is really remarkable. With this combination therapy there was a 38% response rate, and there was a very respectable duration of response. So the population is small, but the benefit of the treatment is by no means small,” said Afsaneh Barzi, MD, PhD, a medical oncologist specializing in gastrointestinal cancers at City of Hope in Duarte, California.
Another medical oncologist interviewed for this piece, who treats patients with HER2-positive metastatic CRC, said that the performance of tucatinib in the real-world setting is in keeping with the efficacy seen in clinical trials.
“There is a group of patients who have a very good response to HER2 [targeted] therapy. Often these are patients who have higher degrees of HER2 amplification, and they do not have concomitant other mutations that activate the pathway, such as RAS mutations,” said Kanwal PS Raghav, MD, MBBS, from the University of Texas MD Anderson Cancer Center in Houston.
Why It Works
In an interview, MOUNTAINEER coinvestigator Tanios S. Bekaii-Saab, MD, from the Mayo Clinic Comprehensive Cancer Center in Phoenix, Arizona, explained why dual HER2 blockade with tucatinib and trastuzumab is an important breakthrough for this population.
“HER2 as a target was already well established in breast cancer and in gastric cancer. In colon cancer we had signals [of anti-HER2 efficacy] but these signals were primarily with dual targeted therapy,” he said.
“What’s unique about tucatinib versus neratinib [Nerlynx], lapatinib [Tykerb] and some of the others is that this is a highly selective tyrosine kinase inhibitor, meaning it is potent just against HER2 and has limited activity against other receptors, classically EGFR, which also goes by the name of HER1,” said MOUNTAINEER trial chair John H. Strickler, MD, of Duke Cancer Center in Durham, North Carolina.
“The reason why that’s valuable is that when you inhibit other receptors like HER1 or EGFR, you can cause significant skin rash and other symptoms that can limit tolerability, which limits your ability to give the full dose. With tucatinib you can more completely inhibit HER2 with fewer side effects,” Dr. Strickler said in an interview.
Dr. Raghav noted that the primary adverse events of therapy with tucatinib have been diarrhea and fatigue, and other common side effects include abdominal pain, fever, nausea, rash, and infusion reactions.
Barriers to Treatment
Dr. Barzi pointed out that in the day-to-day practice setting there are two potential barriers to treatment with tucatinib and trastuzumab for patients with HER2-positive colorectal cancer, hurdles that they would not encounter if they were enrolled in clinical trials.
The first barrier is the requirement for HER2 testing, either through immunohistochemistry or fluorescence in situ hybridization.
“The adoption of HER2 testing in colorectal cancer lags behind other molecular testing, such as testing for KRAS or BRAF, so the provider needs to be aware that HER2 positivity is a possibility,” she said.
The second and more difficult-to-surmount barrier is imposed by the healthcare system. Although the combination is included in National Comprehensive Cancer Network guidelines and, therefore, should not be subject to restrictions or denials by insurers, “the challenge is that this is an oral and IV drug combination,” Dr. Barzi said.
While patients in real-world settings receive intravenous drugs such as trastuzumab in treatment centers, the oral drug component, tucatinib, is dispensed by pharmacies, and patients are often required to shell out high copays for such agents.
Dr. Barzi cited as an example the case of one of her patients who was receiving an oral agent — not tucatinib — for treatment of a different type of colorectal cancer.
“He has very good insurance, and after insurance his out-of-pocket cost on a monthly basis to obtain the drug is $275,” she said.
What’s Next
In colorectal cancer the combination of tucatinib and trastuzumab is approved only in the metastatic setting, but it is also being explored as a first-line therapy in combination with the mFOLFOX6 regimen (5-Fluorouracil, leucovorin, and oxaliplatin) in the MOUNTAINEER-03 trial, which is currently recruiting.
MOUNTAINEER was sponsored by Seagen and Merck. Dr. Strickler reported support from Seagen for the Lancet Oncology manuscript; institutional grants, consulting fees, and travel support from Seagen, and similar relationships with other companies. Dr. Bekaii-Saab reported institutional research and consulting fees from various companies, including Merck, personal consulting fees from various companies, and independent monitoring board/scientific advisory board activities. Dr. Raghav disclosed honoraria and an advisory/consulting role for Seagen and others. Dr. Barzi reported no relevant conflicts of interest.
Only about 3% to 5% of patients with metastatic colorectal cancer have tumors that are positive for human epidermal growth factor receptor 2 (HER2), and until recently there was no treatment approved by the US Food and Drug Administration (FDA) for this subset of patients.
That all changed, in January of 2023. At that time, the FDA granted accelerated approval to tucatinib (Tukysa) in combination with trastuzumab for RAS wild-type HER2-positive unresectable or metastatic colorectal cancer that has progressed following fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
The combination was the first FDA-approved treatment for this patient population.
The only other FDA-approved therapy for metastatic HER2-positive CRC is the antibody-drug conjugate trastuzumab deruxtecan (Enhertu). That drug received accelerated approval from the FDA for metastatic HER2-positive CRC for which no other suitable therapeutic option exists, on April 5, 2024. This FDA action represented an expansion of the drug’s earlier approvals for treating several cancer types, including certain patients with unresectable or metastatic HER2-positive breast cancer and adults with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma who had received a prior trastuzumab-based regimen.
Drug Combo’s Use With Capecitabine in Breast Cancer
Tucatinib is a potent oral tyrosine kinase inhibitor (TKI) that has been shown to be highly selective for HER2. Prior to approval of the colorectal cancer indication, tucatinib had received FDA approval (in April 2020) in combination with trastuzumab and capecitabine for the treatment of patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who had received one or more prior anti-HER2-based regimens in the metastatic setting.
In these patients the combination was associated with significant improvements in both progression-free survival (PFS) and overall survival compared with trastuzumab and capecitabine.
Approval for the colorectal cancer indication was based on results of the phase 2 MOUNTAINEER trial, which were published in The Lancet Oncology.
Real-World Setting
Clinical experience with the combination in a real-world setting is still limited due to the relatively uncommon RAS wild-type HER2-positive CRC subtype, so most of what’s known about the efficacy and safety of tucatinib plus trastuzumab comes from clinical trials. But oncologists interviewed for this article emphasized that the tucatinib-trastuzumab combination nonetheless represents a major breakthrough.
“The population of RAS wild-type HER2-positive is small in colorectal cancer, but the benefit of this treatment is really remarkable. With this combination therapy there was a 38% response rate, and there was a very respectable duration of response. So the population is small, but the benefit of the treatment is by no means small,” said Afsaneh Barzi, MD, PhD, a medical oncologist specializing in gastrointestinal cancers at City of Hope in Duarte, California.
Another medical oncologist interviewed for this piece, who treats patients with HER2-positive metastatic CRC, said that the performance of tucatinib in the real-world setting is in keeping with the efficacy seen in clinical trials.
“There is a group of patients who have a very good response to HER2 [targeted] therapy. Often these are patients who have higher degrees of HER2 amplification, and they do not have concomitant other mutations that activate the pathway, such as RAS mutations,” said Kanwal PS Raghav, MD, MBBS, from the University of Texas MD Anderson Cancer Center in Houston.
Why It Works
In an interview, MOUNTAINEER coinvestigator Tanios S. Bekaii-Saab, MD, from the Mayo Clinic Comprehensive Cancer Center in Phoenix, Arizona, explained why dual HER2 blockade with tucatinib and trastuzumab is an important breakthrough for this population.
“HER2 as a target was already well established in breast cancer and in gastric cancer. In colon cancer we had signals [of anti-HER2 efficacy] but these signals were primarily with dual targeted therapy,” he said.
“What’s unique about tucatinib versus neratinib [Nerlynx], lapatinib [Tykerb] and some of the others is that this is a highly selective tyrosine kinase inhibitor, meaning it is potent just against HER2 and has limited activity against other receptors, classically EGFR, which also goes by the name of HER1,” said MOUNTAINEER trial chair John H. Strickler, MD, of Duke Cancer Center in Durham, North Carolina.
“The reason why that’s valuable is that when you inhibit other receptors like HER1 or EGFR, you can cause significant skin rash and other symptoms that can limit tolerability, which limits your ability to give the full dose. With tucatinib you can more completely inhibit HER2 with fewer side effects,” Dr. Strickler said in an interview.
Dr. Raghav noted that the primary adverse events of therapy with tucatinib have been diarrhea and fatigue, and other common side effects include abdominal pain, fever, nausea, rash, and infusion reactions.
Barriers to Treatment
Dr. Barzi pointed out that in the day-to-day practice setting there are two potential barriers to treatment with tucatinib and trastuzumab for patients with HER2-positive colorectal cancer, hurdles that they would not encounter if they were enrolled in clinical trials.
The first barrier is the requirement for HER2 testing, either through immunohistochemistry or fluorescence in situ hybridization.
“The adoption of HER2 testing in colorectal cancer lags behind other molecular testing, such as testing for KRAS or BRAF, so the provider needs to be aware that HER2 positivity is a possibility,” she said.
The second and more difficult-to-surmount barrier is imposed by the healthcare system. Although the combination is included in National Comprehensive Cancer Network guidelines and, therefore, should not be subject to restrictions or denials by insurers, “the challenge is that this is an oral and IV drug combination,” Dr. Barzi said.
While patients in real-world settings receive intravenous drugs such as trastuzumab in treatment centers, the oral drug component, tucatinib, is dispensed by pharmacies, and patients are often required to shell out high copays for such agents.
Dr. Barzi cited as an example the case of one of her patients who was receiving an oral agent — not tucatinib — for treatment of a different type of colorectal cancer.
“He has very good insurance, and after insurance his out-of-pocket cost on a monthly basis to obtain the drug is $275,” she said.
What’s Next
In colorectal cancer the combination of tucatinib and trastuzumab is approved only in the metastatic setting, but it is also being explored as a first-line therapy in combination with the mFOLFOX6 regimen (5-Fluorouracil, leucovorin, and oxaliplatin) in the MOUNTAINEER-03 trial, which is currently recruiting.
MOUNTAINEER was sponsored by Seagen and Merck. Dr. Strickler reported support from Seagen for the Lancet Oncology manuscript; institutional grants, consulting fees, and travel support from Seagen, and similar relationships with other companies. Dr. Bekaii-Saab reported institutional research and consulting fees from various companies, including Merck, personal consulting fees from various companies, and independent monitoring board/scientific advisory board activities. Dr. Raghav disclosed honoraria and an advisory/consulting role for Seagen and others. Dr. Barzi reported no relevant conflicts of interest.
Only about 3% to 5% of patients with metastatic colorectal cancer have tumors that are positive for human epidermal growth factor receptor 2 (HER2), and until recently there was no treatment approved by the US Food and Drug Administration (FDA) for this subset of patients.
That all changed, in January of 2023. At that time, the FDA granted accelerated approval to tucatinib (Tukysa) in combination with trastuzumab for RAS wild-type HER2-positive unresectable or metastatic colorectal cancer that has progressed following fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
The combination was the first FDA-approved treatment for this patient population.
The only other FDA-approved therapy for metastatic HER2-positive CRC is the antibody-drug conjugate trastuzumab deruxtecan (Enhertu). That drug received accelerated approval from the FDA for metastatic HER2-positive CRC for which no other suitable therapeutic option exists, on April 5, 2024. This FDA action represented an expansion of the drug’s earlier approvals for treating several cancer types, including certain patients with unresectable or metastatic HER2-positive breast cancer and adults with locally advanced or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma who had received a prior trastuzumab-based regimen.
Drug Combo’s Use With Capecitabine in Breast Cancer
Tucatinib is a potent oral tyrosine kinase inhibitor (TKI) that has been shown to be highly selective for HER2. Prior to approval of the colorectal cancer indication, tucatinib had received FDA approval (in April 2020) in combination with trastuzumab and capecitabine for the treatment of patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who had received one or more prior anti-HER2-based regimens in the metastatic setting.
In these patients the combination was associated with significant improvements in both progression-free survival (PFS) and overall survival compared with trastuzumab and capecitabine.
Approval for the colorectal cancer indication was based on results of the phase 2 MOUNTAINEER trial, which were published in The Lancet Oncology.
Real-World Setting
Clinical experience with the combination in a real-world setting is still limited due to the relatively uncommon RAS wild-type HER2-positive CRC subtype, so most of what’s known about the efficacy and safety of tucatinib plus trastuzumab comes from clinical trials. But oncologists interviewed for this article emphasized that the tucatinib-trastuzumab combination nonetheless represents a major breakthrough.
“The population of RAS wild-type HER2-positive is small in colorectal cancer, but the benefit of this treatment is really remarkable. With this combination therapy there was a 38% response rate, and there was a very respectable duration of response. So the population is small, but the benefit of the treatment is by no means small,” said Afsaneh Barzi, MD, PhD, a medical oncologist specializing in gastrointestinal cancers at City of Hope in Duarte, California.
Another medical oncologist interviewed for this piece, who treats patients with HER2-positive metastatic CRC, said that the performance of tucatinib in the real-world setting is in keeping with the efficacy seen in clinical trials.
“There is a group of patients who have a very good response to HER2 [targeted] therapy. Often these are patients who have higher degrees of HER2 amplification, and they do not have concomitant other mutations that activate the pathway, such as RAS mutations,” said Kanwal PS Raghav, MD, MBBS, from the University of Texas MD Anderson Cancer Center in Houston.
Why It Works
In an interview, MOUNTAINEER coinvestigator Tanios S. Bekaii-Saab, MD, from the Mayo Clinic Comprehensive Cancer Center in Phoenix, Arizona, explained why dual HER2 blockade with tucatinib and trastuzumab is an important breakthrough for this population.
“HER2 as a target was already well established in breast cancer and in gastric cancer. In colon cancer we had signals [of anti-HER2 efficacy] but these signals were primarily with dual targeted therapy,” he said.
“What’s unique about tucatinib versus neratinib [Nerlynx], lapatinib [Tykerb] and some of the others is that this is a highly selective tyrosine kinase inhibitor, meaning it is potent just against HER2 and has limited activity against other receptors, classically EGFR, which also goes by the name of HER1,” said MOUNTAINEER trial chair John H. Strickler, MD, of Duke Cancer Center in Durham, North Carolina.
“The reason why that’s valuable is that when you inhibit other receptors like HER1 or EGFR, you can cause significant skin rash and other symptoms that can limit tolerability, which limits your ability to give the full dose. With tucatinib you can more completely inhibit HER2 with fewer side effects,” Dr. Strickler said in an interview.
Dr. Raghav noted that the primary adverse events of therapy with tucatinib have been diarrhea and fatigue, and other common side effects include abdominal pain, fever, nausea, rash, and infusion reactions.
Barriers to Treatment
Dr. Barzi pointed out that in the day-to-day practice setting there are two potential barriers to treatment with tucatinib and trastuzumab for patients with HER2-positive colorectal cancer, hurdles that they would not encounter if they were enrolled in clinical trials.
The first barrier is the requirement for HER2 testing, either through immunohistochemistry or fluorescence in situ hybridization.
“The adoption of HER2 testing in colorectal cancer lags behind other molecular testing, such as testing for KRAS or BRAF, so the provider needs to be aware that HER2 positivity is a possibility,” she said.
The second and more difficult-to-surmount barrier is imposed by the healthcare system. Although the combination is included in National Comprehensive Cancer Network guidelines and, therefore, should not be subject to restrictions or denials by insurers, “the challenge is that this is an oral and IV drug combination,” Dr. Barzi said.
While patients in real-world settings receive intravenous drugs such as trastuzumab in treatment centers, the oral drug component, tucatinib, is dispensed by pharmacies, and patients are often required to shell out high copays for such agents.
Dr. Barzi cited as an example the case of one of her patients who was receiving an oral agent — not tucatinib — for treatment of a different type of colorectal cancer.
“He has very good insurance, and after insurance his out-of-pocket cost on a monthly basis to obtain the drug is $275,” she said.
What’s Next
In colorectal cancer the combination of tucatinib and trastuzumab is approved only in the metastatic setting, but it is also being explored as a first-line therapy in combination with the mFOLFOX6 regimen (5-Fluorouracil, leucovorin, and oxaliplatin) in the MOUNTAINEER-03 trial, which is currently recruiting.
MOUNTAINEER was sponsored by Seagen and Merck. Dr. Strickler reported support from Seagen for the Lancet Oncology manuscript; institutional grants, consulting fees, and travel support from Seagen, and similar relationships with other companies. Dr. Bekaii-Saab reported institutional research and consulting fees from various companies, including Merck, personal consulting fees from various companies, and independent monitoring board/scientific advisory board activities. Dr. Raghav disclosed honoraria and an advisory/consulting role for Seagen and others. Dr. Barzi reported no relevant conflicts of interest.
Online, Self-Help Program May Curb Binge Eating
An online program aimed at helping those with binge-eating disorder (BED), based on completing cognitive-behavioral therapy (CBT) modules, showed positive results in a randomized, controlled trial. The findings were published in JAMA Network Open.
In the study, led by Luise Pruessner, MS, with the Department of Psychology at Heidelberg University in Germany, 154 patients (96% female; average age 35.9) who met the criteria for BED were randomized 1-to-1 to the intervention or control group.
12-Week CBT Program with 6 Modules
The intervention group had access to a 12-week CBT online program with a core curriculum of six mandatory modules of texts and videos, focused on self-monitoring of binge eating, psychoeducation, and regulating emotion. Each could be accessed only after the previous module was completed. Participants also chose six specialization areas to personalize the experience. Email reminders were sent to participants who delayed starting the program to boost initial and continuing engagement.
The control group had no access to the program and participants were told they were on a 12-week waiting list for it. They could explore other treatments during that time, an option that mimics real-world experiences. The design choice also helped navigate the ethics of withholding a potentially effective treatment.
Significant Improvement in Outcomes
The intervention group had a significant reduction in binge-eating episodes, the primary outcome, compared with the control group. In the intervention group, the average number of episodes decreased from 14.79 at baseline to 6.07 (95% confidence interval, −11.31 to −6.72; P < .001). The reduction surpassed the clinically meaningful threshold of 3.97 episodes. The control group, as expected, had no significant reductions in episodes.
The intervention group also showed improvement in outcomes including well-being, self-esteem, and emotional regulation and reductions in clinical impairment, depression, and anxiety. “However, there were no meaningful between-group differences regarding changes in work capacity,” the authors noted.
In an invited commentary, Andrea Graham, PhD, with the Center for Behavioral Intervention Technologies at the Feinberg School of Medicine, Northwestern University, Chicago, noted that BED “is a prevalent, serious, and impairing psychiatric illness.”
The study authors pointed out that BED is one of the most prevalent eating disorders, affecting “1.0% to 2.8% of the population over their lifetimes.”
Dr. Graham notes that while there are evidence-based, face-to-face psychological treatments, many patients have considerable barriers to accessing those services.
Digital Intervention Advantages
“Digital interventions, such as the one evaluated by Pruessner and colleagues, have the potential to curb the mental health crisis by reaching large numbers of people in need” in the moments they need help most, she wrote.
She added that with BED, eating decisions and signals for dysregulated eating occur frequently throughout the day, highlighting the need for on-demand and immediate access to self-help, like the solution Ms. Pruessner and colleagues describe.
“The importance of Pruessner and colleagues’ findings is strengthened because their digital intervention did not rely on human support for delivery,” she wrote. Relying on human intervention poses financial challenges for achieving scale.
“Therefore, self-help interventions that achieve clinically significant improvements in outcomes present an important opportunity for closing the treatment gap for binge eating. Given its effectiveness, the critical next step is to learn where and how to implement this intervention to broadly reach individuals in need,” Dr. Graham wrote.
Primary care clinicians don’t typically intervene in eating disorders and a self-help intervention might help address that gap, she added.
“However, a first step would require increasing screening for eating disorders in primary care,” Dr. Graham pointed out.
The authors report no relevant financial relationships. Dr. Graham reports grants from the National Institute of Mental Health, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and the Agency for Healthcare Research and Quality. She reports receiving a grant from the NIDDK-funded Chicago Center for Diabetes Translation Research, Dean’s Office of the Biological Sciences Division of the University of Chicago and Feinberg School of Medicine at Northwestern University; and being an adviser to Alavida Health.
An online program aimed at helping those with binge-eating disorder (BED), based on completing cognitive-behavioral therapy (CBT) modules, showed positive results in a randomized, controlled trial. The findings were published in JAMA Network Open.
In the study, led by Luise Pruessner, MS, with the Department of Psychology at Heidelberg University in Germany, 154 patients (96% female; average age 35.9) who met the criteria for BED were randomized 1-to-1 to the intervention or control group.
12-Week CBT Program with 6 Modules
The intervention group had access to a 12-week CBT online program with a core curriculum of six mandatory modules of texts and videos, focused on self-monitoring of binge eating, psychoeducation, and regulating emotion. Each could be accessed only after the previous module was completed. Participants also chose six specialization areas to personalize the experience. Email reminders were sent to participants who delayed starting the program to boost initial and continuing engagement.
The control group had no access to the program and participants were told they were on a 12-week waiting list for it. They could explore other treatments during that time, an option that mimics real-world experiences. The design choice also helped navigate the ethics of withholding a potentially effective treatment.
Significant Improvement in Outcomes
The intervention group had a significant reduction in binge-eating episodes, the primary outcome, compared with the control group. In the intervention group, the average number of episodes decreased from 14.79 at baseline to 6.07 (95% confidence interval, −11.31 to −6.72; P < .001). The reduction surpassed the clinically meaningful threshold of 3.97 episodes. The control group, as expected, had no significant reductions in episodes.
The intervention group also showed improvement in outcomes including well-being, self-esteem, and emotional regulation and reductions in clinical impairment, depression, and anxiety. “However, there were no meaningful between-group differences regarding changes in work capacity,” the authors noted.
In an invited commentary, Andrea Graham, PhD, with the Center for Behavioral Intervention Technologies at the Feinberg School of Medicine, Northwestern University, Chicago, noted that BED “is a prevalent, serious, and impairing psychiatric illness.”
The study authors pointed out that BED is one of the most prevalent eating disorders, affecting “1.0% to 2.8% of the population over their lifetimes.”
Dr. Graham notes that while there are evidence-based, face-to-face psychological treatments, many patients have considerable barriers to accessing those services.
Digital Intervention Advantages
“Digital interventions, such as the one evaluated by Pruessner and colleagues, have the potential to curb the mental health crisis by reaching large numbers of people in need” in the moments they need help most, she wrote.
She added that with BED, eating decisions and signals for dysregulated eating occur frequently throughout the day, highlighting the need for on-demand and immediate access to self-help, like the solution Ms. Pruessner and colleagues describe.
“The importance of Pruessner and colleagues’ findings is strengthened because their digital intervention did not rely on human support for delivery,” she wrote. Relying on human intervention poses financial challenges for achieving scale.
“Therefore, self-help interventions that achieve clinically significant improvements in outcomes present an important opportunity for closing the treatment gap for binge eating. Given its effectiveness, the critical next step is to learn where and how to implement this intervention to broadly reach individuals in need,” Dr. Graham wrote.
Primary care clinicians don’t typically intervene in eating disorders and a self-help intervention might help address that gap, she added.
“However, a first step would require increasing screening for eating disorders in primary care,” Dr. Graham pointed out.
The authors report no relevant financial relationships. Dr. Graham reports grants from the National Institute of Mental Health, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and the Agency for Healthcare Research and Quality. She reports receiving a grant from the NIDDK-funded Chicago Center for Diabetes Translation Research, Dean’s Office of the Biological Sciences Division of the University of Chicago and Feinberg School of Medicine at Northwestern University; and being an adviser to Alavida Health.
An online program aimed at helping those with binge-eating disorder (BED), based on completing cognitive-behavioral therapy (CBT) modules, showed positive results in a randomized, controlled trial. The findings were published in JAMA Network Open.
In the study, led by Luise Pruessner, MS, with the Department of Psychology at Heidelberg University in Germany, 154 patients (96% female; average age 35.9) who met the criteria for BED were randomized 1-to-1 to the intervention or control group.
12-Week CBT Program with 6 Modules
The intervention group had access to a 12-week CBT online program with a core curriculum of six mandatory modules of texts and videos, focused on self-monitoring of binge eating, psychoeducation, and regulating emotion. Each could be accessed only after the previous module was completed. Participants also chose six specialization areas to personalize the experience. Email reminders were sent to participants who delayed starting the program to boost initial and continuing engagement.
The control group had no access to the program and participants were told they were on a 12-week waiting list for it. They could explore other treatments during that time, an option that mimics real-world experiences. The design choice also helped navigate the ethics of withholding a potentially effective treatment.
Significant Improvement in Outcomes
The intervention group had a significant reduction in binge-eating episodes, the primary outcome, compared with the control group. In the intervention group, the average number of episodes decreased from 14.79 at baseline to 6.07 (95% confidence interval, −11.31 to −6.72; P < .001). The reduction surpassed the clinically meaningful threshold of 3.97 episodes. The control group, as expected, had no significant reductions in episodes.
The intervention group also showed improvement in outcomes including well-being, self-esteem, and emotional regulation and reductions in clinical impairment, depression, and anxiety. “However, there were no meaningful between-group differences regarding changes in work capacity,” the authors noted.
In an invited commentary, Andrea Graham, PhD, with the Center for Behavioral Intervention Technologies at the Feinberg School of Medicine, Northwestern University, Chicago, noted that BED “is a prevalent, serious, and impairing psychiatric illness.”
The study authors pointed out that BED is one of the most prevalent eating disorders, affecting “1.0% to 2.8% of the population over their lifetimes.”
Dr. Graham notes that while there are evidence-based, face-to-face psychological treatments, many patients have considerable barriers to accessing those services.
Digital Intervention Advantages
“Digital interventions, such as the one evaluated by Pruessner and colleagues, have the potential to curb the mental health crisis by reaching large numbers of people in need” in the moments they need help most, she wrote.
She added that with BED, eating decisions and signals for dysregulated eating occur frequently throughout the day, highlighting the need for on-demand and immediate access to self-help, like the solution Ms. Pruessner and colleagues describe.
“The importance of Pruessner and colleagues’ findings is strengthened because their digital intervention did not rely on human support for delivery,” she wrote. Relying on human intervention poses financial challenges for achieving scale.
“Therefore, self-help interventions that achieve clinically significant improvements in outcomes present an important opportunity for closing the treatment gap for binge eating. Given its effectiveness, the critical next step is to learn where and how to implement this intervention to broadly reach individuals in need,” Dr. Graham wrote.
Primary care clinicians don’t typically intervene in eating disorders and a self-help intervention might help address that gap, she added.
“However, a first step would require increasing screening for eating disorders in primary care,” Dr. Graham pointed out.
The authors report no relevant financial relationships. Dr. Graham reports grants from the National Institute of Mental Health, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and the Agency for Healthcare Research and Quality. She reports receiving a grant from the NIDDK-funded Chicago Center for Diabetes Translation Research, Dean’s Office of the Biological Sciences Division of the University of Chicago and Feinberg School of Medicine at Northwestern University; and being an adviser to Alavida Health.
FROM JAMA NETWORK OPEN
Aquagenic Wrinkling Among Skin-Related Signs of Cystic Fibrosis
TOPLINE:
METHODOLOGY:
- Patients with CF, caused by a mutation in the CF Transmembrane Conductance Regulator (CFTR) gene, can develop diverse dermatologic manifestations.
- Researchers reviewed the literature and provided their own clinical experience regarding dermatologic manifestations of CF.
- They also reviewed the cutaneous side effects of CFTR modulators and antibiotics used to treat CF.
TAKEAWAY:
- Aquagenic wrinkling of the palm is common in individuals with CF, affecting up to 80% of patients (and 25% of CF gene carriers), and can be an early manifestation of CF. Treatments include topical medications (such as aluminum chloride, corticosteroids, and salicylic acid), botulinum toxin injections, and recently, CFTR-modulating treatments.
- CF nutrient deficiency dermatitis, often in a diaper distribution, usually appears in infancy and, before newborn screening was available, was sometimes the first sign of CF in some cases. It usually resolves with an adequate diet, pancreatic enzymes, and/or nutritional supplements. Zinc and essential fatty acid deficiencies can lead to acrodermatitis enteropathica–like symptoms and psoriasiform rashes, respectively.
- CF is also associated with vascular disorders, including cutaneous and, rarely, systemic vasculitis. Treatment includes topical and oral steroids and immune-modulating therapies.
- CFTR modulators, now the most common and highly effective treatment for CF, are associated with several skin reactions, which can be managed with treatments that include topical steroids and oral antihistamines. Frequent antibiotic treatment can also trigger skin reactions.
IN PRACTICE:
“Recognition and familiarity with dermatologic clinical manifestations of CF are important for multidisciplinary care” for patients with CF, the authors wrote, adding that “dermatology providers may play a significant role in the diagnosis and management of CF cutaneous comorbidities.”
SOURCE:
Aaron D. Smith, BS, from the University of Virginia (UVA) School of Medicine, Charlottesville, and coauthors were from the departments of dermatology and pulmonology/critical care medicine at UVA. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The authors did not make a comment about the limitations of their review.
DISCLOSURES:
No funding was received for the review. The authors had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Patients with CF, caused by a mutation in the CF Transmembrane Conductance Regulator (CFTR) gene, can develop diverse dermatologic manifestations.
- Researchers reviewed the literature and provided their own clinical experience regarding dermatologic manifestations of CF.
- They also reviewed the cutaneous side effects of CFTR modulators and antibiotics used to treat CF.
TAKEAWAY:
- Aquagenic wrinkling of the palm is common in individuals with CF, affecting up to 80% of patients (and 25% of CF gene carriers), and can be an early manifestation of CF. Treatments include topical medications (such as aluminum chloride, corticosteroids, and salicylic acid), botulinum toxin injections, and recently, CFTR-modulating treatments.
- CF nutrient deficiency dermatitis, often in a diaper distribution, usually appears in infancy and, before newborn screening was available, was sometimes the first sign of CF in some cases. It usually resolves with an adequate diet, pancreatic enzymes, and/or nutritional supplements. Zinc and essential fatty acid deficiencies can lead to acrodermatitis enteropathica–like symptoms and psoriasiform rashes, respectively.
- CF is also associated with vascular disorders, including cutaneous and, rarely, systemic vasculitis. Treatment includes topical and oral steroids and immune-modulating therapies.
- CFTR modulators, now the most common and highly effective treatment for CF, are associated with several skin reactions, which can be managed with treatments that include topical steroids and oral antihistamines. Frequent antibiotic treatment can also trigger skin reactions.
IN PRACTICE:
“Recognition and familiarity with dermatologic clinical manifestations of CF are important for multidisciplinary care” for patients with CF, the authors wrote, adding that “dermatology providers may play a significant role in the diagnosis and management of CF cutaneous comorbidities.”
SOURCE:
Aaron D. Smith, BS, from the University of Virginia (UVA) School of Medicine, Charlottesville, and coauthors were from the departments of dermatology and pulmonology/critical care medicine at UVA. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The authors did not make a comment about the limitations of their review.
DISCLOSURES:
No funding was received for the review. The authors had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Patients with CF, caused by a mutation in the CF Transmembrane Conductance Regulator (CFTR) gene, can develop diverse dermatologic manifestations.
- Researchers reviewed the literature and provided their own clinical experience regarding dermatologic manifestations of CF.
- They also reviewed the cutaneous side effects of CFTR modulators and antibiotics used to treat CF.
TAKEAWAY:
- Aquagenic wrinkling of the palm is common in individuals with CF, affecting up to 80% of patients (and 25% of CF gene carriers), and can be an early manifestation of CF. Treatments include topical medications (such as aluminum chloride, corticosteroids, and salicylic acid), botulinum toxin injections, and recently, CFTR-modulating treatments.
- CF nutrient deficiency dermatitis, often in a diaper distribution, usually appears in infancy and, before newborn screening was available, was sometimes the first sign of CF in some cases. It usually resolves with an adequate diet, pancreatic enzymes, and/or nutritional supplements. Zinc and essential fatty acid deficiencies can lead to acrodermatitis enteropathica–like symptoms and psoriasiform rashes, respectively.
- CF is also associated with vascular disorders, including cutaneous and, rarely, systemic vasculitis. Treatment includes topical and oral steroids and immune-modulating therapies.
- CFTR modulators, now the most common and highly effective treatment for CF, are associated with several skin reactions, which can be managed with treatments that include topical steroids and oral antihistamines. Frequent antibiotic treatment can also trigger skin reactions.
IN PRACTICE:
“Recognition and familiarity with dermatologic clinical manifestations of CF are important for multidisciplinary care” for patients with CF, the authors wrote, adding that “dermatology providers may play a significant role in the diagnosis and management of CF cutaneous comorbidities.”
SOURCE:
Aaron D. Smith, BS, from the University of Virginia (UVA) School of Medicine, Charlottesville, and coauthors were from the departments of dermatology and pulmonology/critical care medicine at UVA. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The authors did not make a comment about the limitations of their review.
DISCLOSURES:
No funding was received for the review. The authors had no disclosures.
A version of this article first appeared on Medscape.com.
When Medicine Isn’t the Last Stop
A distant friend and I were recently chatting by email. After years of trying, she’s become a successful author, and decided to leave medicine to focus on the new career.
She’s excited about this, as it’s really what she’s always dreamed of doing, but at the same time feels guilty about it. Leaving medicine for a new career isn’t quite the same as quitting your job as a waitress or insurance salesman. You’ve put a lot of time, and effort, and money, into becoming an attending physician.
I also once dreamed of being a successful writer (amongst other things) but have no complaints about where I landed. I like what I do. Besides, I don’t have her kind of imagination.
It’s a valid point, though. Becoming a doc in practice takes a minimum of 4 years of college and 4 years of medical school. Then you tack on a residency of 3 years (internal medicine) to 7 years (neurosurgery). On top of that many add another 1-2 years for fellowship training. So you’re talking a bare minimum of at least 11 years, ranging up to 17 years.
Then you think of how much money was spent on college and medical school — tuition, living expenses, loan interest, not to mention the emotional toll of the training.
You also have to think that somewhere in there you got a chance to become a doctor while someone else didn’t.
So, I can see why she feels guilty, but she shouldn’t. She’s paid back all her loans, so no one else is left carrying the financial bag. The argument about denying someone else a spot can be kind of flimsy when you don’t know how that person might have turned out (the medical school dropout rate is 15%-18%).
Life is unpredictable. We often don’t really know what we want until we get there, and those journeys are rarely a straight line. That doesn’t mean those years were a waste, they’re just part of the trip — stepping stones to get you to the right place and realize who you really are. They also make these things possible — the experiences add to the background, and give you time and support to make the change.
She joins a group of other physicians who found their calling elsewhere, such as Graham Chapman or Michael Crichton. A nonmedical example is the renowned British astrophysicist, Sir Brian May.
I have no plans to leave medicine for another career. This fall will be 35 years since I started at Creighton Medical School, and I have no regrets. But if others have found something they enjoy more and are successful at, they have nothing to feel guilty about.
Good luck, friend.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
A distant friend and I were recently chatting by email. After years of trying, she’s become a successful author, and decided to leave medicine to focus on the new career.
She’s excited about this, as it’s really what she’s always dreamed of doing, but at the same time feels guilty about it. Leaving medicine for a new career isn’t quite the same as quitting your job as a waitress or insurance salesman. You’ve put a lot of time, and effort, and money, into becoming an attending physician.
I also once dreamed of being a successful writer (amongst other things) but have no complaints about where I landed. I like what I do. Besides, I don’t have her kind of imagination.
It’s a valid point, though. Becoming a doc in practice takes a minimum of 4 years of college and 4 years of medical school. Then you tack on a residency of 3 years (internal medicine) to 7 years (neurosurgery). On top of that many add another 1-2 years for fellowship training. So you’re talking a bare minimum of at least 11 years, ranging up to 17 years.
Then you think of how much money was spent on college and medical school — tuition, living expenses, loan interest, not to mention the emotional toll of the training.
You also have to think that somewhere in there you got a chance to become a doctor while someone else didn’t.
So, I can see why she feels guilty, but she shouldn’t. She’s paid back all her loans, so no one else is left carrying the financial bag. The argument about denying someone else a spot can be kind of flimsy when you don’t know how that person might have turned out (the medical school dropout rate is 15%-18%).
Life is unpredictable. We often don’t really know what we want until we get there, and those journeys are rarely a straight line. That doesn’t mean those years were a waste, they’re just part of the trip — stepping stones to get you to the right place and realize who you really are. They also make these things possible — the experiences add to the background, and give you time and support to make the change.
She joins a group of other physicians who found their calling elsewhere, such as Graham Chapman or Michael Crichton. A nonmedical example is the renowned British astrophysicist, Sir Brian May.
I have no plans to leave medicine for another career. This fall will be 35 years since I started at Creighton Medical School, and I have no regrets. But if others have found something they enjoy more and are successful at, they have nothing to feel guilty about.
Good luck, friend.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
A distant friend and I were recently chatting by email. After years of trying, she’s become a successful author, and decided to leave medicine to focus on the new career.
She’s excited about this, as it’s really what she’s always dreamed of doing, but at the same time feels guilty about it. Leaving medicine for a new career isn’t quite the same as quitting your job as a waitress or insurance salesman. You’ve put a lot of time, and effort, and money, into becoming an attending physician.
I also once dreamed of being a successful writer (amongst other things) but have no complaints about where I landed. I like what I do. Besides, I don’t have her kind of imagination.
It’s a valid point, though. Becoming a doc in practice takes a minimum of 4 years of college and 4 years of medical school. Then you tack on a residency of 3 years (internal medicine) to 7 years (neurosurgery). On top of that many add another 1-2 years for fellowship training. So you’re talking a bare minimum of at least 11 years, ranging up to 17 years.
Then you think of how much money was spent on college and medical school — tuition, living expenses, loan interest, not to mention the emotional toll of the training.
You also have to think that somewhere in there you got a chance to become a doctor while someone else didn’t.
So, I can see why she feels guilty, but she shouldn’t. She’s paid back all her loans, so no one else is left carrying the financial bag. The argument about denying someone else a spot can be kind of flimsy when you don’t know how that person might have turned out (the medical school dropout rate is 15%-18%).
Life is unpredictable. We often don’t really know what we want until we get there, and those journeys are rarely a straight line. That doesn’t mean those years were a waste, they’re just part of the trip — stepping stones to get you to the right place and realize who you really are. They also make these things possible — the experiences add to the background, and give you time and support to make the change.
She joins a group of other physicians who found their calling elsewhere, such as Graham Chapman or Michael Crichton. A nonmedical example is the renowned British astrophysicist, Sir Brian May.
I have no plans to leave medicine for another career. This fall will be 35 years since I started at Creighton Medical School, and I have no regrets. But if others have found something they enjoy more and are successful at, they have nothing to feel guilty about.
Good luck, friend.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
PCP Compensation, Part 4
I have already shared with you that healthcare systems value panel size and productivity when they are considering primary care physician compensation. Your employers also know that the market won’t bear a substantial price increase for the procedure-poor practice style typical of primary care. You know that the relative value unit (RVU) system for calculating complexity of service is time consuming and discourages the inclusion of customer-friendly short visits that could allow an efficient provider to see more patients. Unfortunately, there is little hope that RVUs will become more PCP-friendly in the near future.
However, before leaving the topic of value and moving on to a consideration of quality, I can’t resist sharing some thoughts about efficiency and time management.
First, it must be said that the inexpert development and the clumsy rollout of electronic medical records (EMRs) have struck the biggest blow to the compensation potential and mental health of even the most efficient PCPs. Until that chasm is filled, there will be little progress in improving the efficiency and, consequently, the fair compensation of PCPs.
However, there is a myth that there is a direct correlation between the time spent with the patient and the quality of care. Eighty-five percent of PCPs report they would like to spend more time to get to know their patients. On the other hand, in my experience, really getting to know a patient is a process best done over multiple visits — some long, many of them short. It is unrealistic and inefficient to gain an in-depth understanding of the patient in a single visit.
Yes, one often hears a patient complain “they only spent 5 minutes with me.” While the patient may be technically correct, I contend that the provider’s manner has a major influence on the patient’s perception of the time spent in the exam room.
Was the provider reasonably prompt? In other words did they value my time? Did they appear rushed? Were they aware of my relevant history and prepared to deal with the current situation? In other words, did they do their homework? Did they engage me visually and seem to know what they were talking about? But, most importantly, did they exude sympathy and seem to care? Was I treated in the same manner that they would like to have been treated? If the answer is YES to those questions, then likely the patient could care less about the time spent.
It may seem counterintuitive to some of you, but there is a simple strategy that a provider can employ that will give them more time with the patient and at the same time allow them to claim to the boss that they are lowering the overhead costs. Management consultants often lean heavily on delegation as a more efficient use of resources. However, when the provider takes the patient’s vital signs and gives the injections, this multitasking provides an excellent hands-on opportunity to take the history and get to know the patient better. And, by giving the immunizations the provider is making the clearest statement possible that these vaccines are so important that they administer them personally.
You may have been wondering why I haven’t included the quality of PCP care in a discussion of compensation. It is because I don’t believe anyone has figured out how to do it in a manner that makes sense and is fair. PCPs don’t do procedures on which their success rate can be measured. A PCP’s patient panel almost by definition is going to be a mix of ages with a broad variety of complaints. Do they see enough diabetics to use their panel’s hemoglobin A1cs as a metric, or enough asthmatics to use emergency department visits as a quality-of-care measurement? In pediatrics, the closest we can come to a valid measure may be the provider’s vaccine acceptance rate.
But, then how does one factor in the general health of the community? If I open a practice in an underserved community, can you measure the quality of my care based on how quickly I can improve the metrics when I have no control over the poverty and educational system?
Since we aren’t surgeons, outcomes can’t be used to judge our quality. I’m afraid the only way we can assure quality is to demand evidence of our efforts to keep abreast of the current knowledge in our field and hope that at some level CME credits accumulated translate to the care we provide. A recent study has demonstrated an association between board certification exam board scores and newly trained internists and the care they provide. The patients of the physicians with the top scores had a lower risk of being readmitted to the hospital and were less likely to die in the first seven days of hospitalization.
We now may have come full circle. The fact is that, like it or not, our value to the folks that pay us lies in the number of patients we can bring into the system. To keep our overhead down, we will always be encouraged to see as many patients as we can, or at least be efficient. Even if there were a way to quantify the quality of our care using outcome metrics, the patients will continue to select their providers based on availability, and the professional and consumer-friendly behavior of those providers. The patients’ perception of how good we are at making them feel better may be our strongest argument for better compensation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I have already shared with you that healthcare systems value panel size and productivity when they are considering primary care physician compensation. Your employers also know that the market won’t bear a substantial price increase for the procedure-poor practice style typical of primary care. You know that the relative value unit (RVU) system for calculating complexity of service is time consuming and discourages the inclusion of customer-friendly short visits that could allow an efficient provider to see more patients. Unfortunately, there is little hope that RVUs will become more PCP-friendly in the near future.
However, before leaving the topic of value and moving on to a consideration of quality, I can’t resist sharing some thoughts about efficiency and time management.
First, it must be said that the inexpert development and the clumsy rollout of electronic medical records (EMRs) have struck the biggest blow to the compensation potential and mental health of even the most efficient PCPs. Until that chasm is filled, there will be little progress in improving the efficiency and, consequently, the fair compensation of PCPs.
However, there is a myth that there is a direct correlation between the time spent with the patient and the quality of care. Eighty-five percent of PCPs report they would like to spend more time to get to know their patients. On the other hand, in my experience, really getting to know a patient is a process best done over multiple visits — some long, many of them short. It is unrealistic and inefficient to gain an in-depth understanding of the patient in a single visit.
Yes, one often hears a patient complain “they only spent 5 minutes with me.” While the patient may be technically correct, I contend that the provider’s manner has a major influence on the patient’s perception of the time spent in the exam room.
Was the provider reasonably prompt? In other words did they value my time? Did they appear rushed? Were they aware of my relevant history and prepared to deal with the current situation? In other words, did they do their homework? Did they engage me visually and seem to know what they were talking about? But, most importantly, did they exude sympathy and seem to care? Was I treated in the same manner that they would like to have been treated? If the answer is YES to those questions, then likely the patient could care less about the time spent.
It may seem counterintuitive to some of you, but there is a simple strategy that a provider can employ that will give them more time with the patient and at the same time allow them to claim to the boss that they are lowering the overhead costs. Management consultants often lean heavily on delegation as a more efficient use of resources. However, when the provider takes the patient’s vital signs and gives the injections, this multitasking provides an excellent hands-on opportunity to take the history and get to know the patient better. And, by giving the immunizations the provider is making the clearest statement possible that these vaccines are so important that they administer them personally.
You may have been wondering why I haven’t included the quality of PCP care in a discussion of compensation. It is because I don’t believe anyone has figured out how to do it in a manner that makes sense and is fair. PCPs don’t do procedures on which their success rate can be measured. A PCP’s patient panel almost by definition is going to be a mix of ages with a broad variety of complaints. Do they see enough diabetics to use their panel’s hemoglobin A1cs as a metric, or enough asthmatics to use emergency department visits as a quality-of-care measurement? In pediatrics, the closest we can come to a valid measure may be the provider’s vaccine acceptance rate.
But, then how does one factor in the general health of the community? If I open a practice in an underserved community, can you measure the quality of my care based on how quickly I can improve the metrics when I have no control over the poverty and educational system?
Since we aren’t surgeons, outcomes can’t be used to judge our quality. I’m afraid the only way we can assure quality is to demand evidence of our efforts to keep abreast of the current knowledge in our field and hope that at some level CME credits accumulated translate to the care we provide. A recent study has demonstrated an association between board certification exam board scores and newly trained internists and the care they provide. The patients of the physicians with the top scores had a lower risk of being readmitted to the hospital and were less likely to die in the first seven days of hospitalization.
We now may have come full circle. The fact is that, like it or not, our value to the folks that pay us lies in the number of patients we can bring into the system. To keep our overhead down, we will always be encouraged to see as many patients as we can, or at least be efficient. Even if there were a way to quantify the quality of our care using outcome metrics, the patients will continue to select their providers based on availability, and the professional and consumer-friendly behavior of those providers. The patients’ perception of how good we are at making them feel better may be our strongest argument for better compensation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I have already shared with you that healthcare systems value panel size and productivity when they are considering primary care physician compensation. Your employers also know that the market won’t bear a substantial price increase for the procedure-poor practice style typical of primary care. You know that the relative value unit (RVU) system for calculating complexity of service is time consuming and discourages the inclusion of customer-friendly short visits that could allow an efficient provider to see more patients. Unfortunately, there is little hope that RVUs will become more PCP-friendly in the near future.
However, before leaving the topic of value and moving on to a consideration of quality, I can’t resist sharing some thoughts about efficiency and time management.
First, it must be said that the inexpert development and the clumsy rollout of electronic medical records (EMRs) have struck the biggest blow to the compensation potential and mental health of even the most efficient PCPs. Until that chasm is filled, there will be little progress in improving the efficiency and, consequently, the fair compensation of PCPs.
However, there is a myth that there is a direct correlation between the time spent with the patient and the quality of care. Eighty-five percent of PCPs report they would like to spend more time to get to know their patients. On the other hand, in my experience, really getting to know a patient is a process best done over multiple visits — some long, many of them short. It is unrealistic and inefficient to gain an in-depth understanding of the patient in a single visit.
Yes, one often hears a patient complain “they only spent 5 minutes with me.” While the patient may be technically correct, I contend that the provider’s manner has a major influence on the patient’s perception of the time spent in the exam room.
Was the provider reasonably prompt? In other words did they value my time? Did they appear rushed? Were they aware of my relevant history and prepared to deal with the current situation? In other words, did they do their homework? Did they engage me visually and seem to know what they were talking about? But, most importantly, did they exude sympathy and seem to care? Was I treated in the same manner that they would like to have been treated? If the answer is YES to those questions, then likely the patient could care less about the time spent.
It may seem counterintuitive to some of you, but there is a simple strategy that a provider can employ that will give them more time with the patient and at the same time allow them to claim to the boss that they are lowering the overhead costs. Management consultants often lean heavily on delegation as a more efficient use of resources. However, when the provider takes the patient’s vital signs and gives the injections, this multitasking provides an excellent hands-on opportunity to take the history and get to know the patient better. And, by giving the immunizations the provider is making the clearest statement possible that these vaccines are so important that they administer them personally.
You may have been wondering why I haven’t included the quality of PCP care in a discussion of compensation. It is because I don’t believe anyone has figured out how to do it in a manner that makes sense and is fair. PCPs don’t do procedures on which their success rate can be measured. A PCP’s patient panel almost by definition is going to be a mix of ages with a broad variety of complaints. Do they see enough diabetics to use their panel’s hemoglobin A1cs as a metric, or enough asthmatics to use emergency department visits as a quality-of-care measurement? In pediatrics, the closest we can come to a valid measure may be the provider’s vaccine acceptance rate.
But, then how does one factor in the general health of the community? If I open a practice in an underserved community, can you measure the quality of my care based on how quickly I can improve the metrics when I have no control over the poverty and educational system?
Since we aren’t surgeons, outcomes can’t be used to judge our quality. I’m afraid the only way we can assure quality is to demand evidence of our efforts to keep abreast of the current knowledge in our field and hope that at some level CME credits accumulated translate to the care we provide. A recent study has demonstrated an association between board certification exam board scores and newly trained internists and the care they provide. The patients of the physicians with the top scores had a lower risk of being readmitted to the hospital and were less likely to die in the first seven days of hospitalization.
We now may have come full circle. The fact is that, like it or not, our value to the folks that pay us lies in the number of patients we can bring into the system. To keep our overhead down, we will always be encouraged to see as many patients as we can, or at least be efficient. Even if there were a way to quantify the quality of our care using outcome metrics, the patients will continue to select their providers based on availability, and the professional and consumer-friendly behavior of those providers. The patients’ perception of how good we are at making them feel better may be our strongest argument for better compensation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Why Cardiac Biomarkers Don’t Help Predict Heart Disease
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.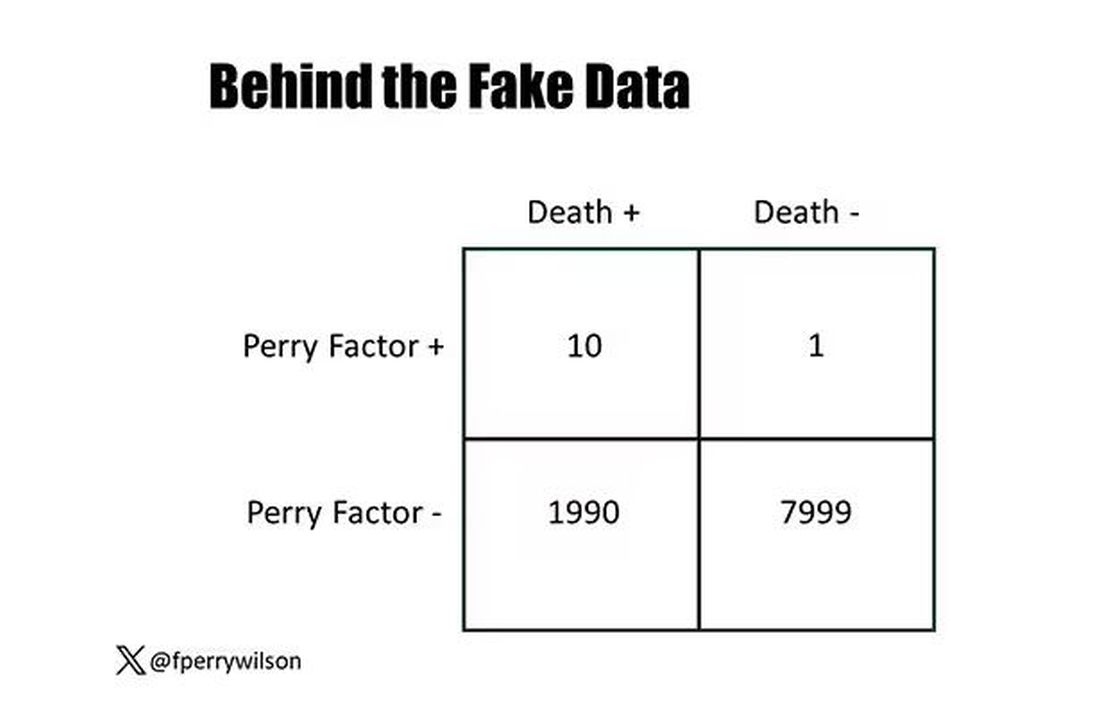
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.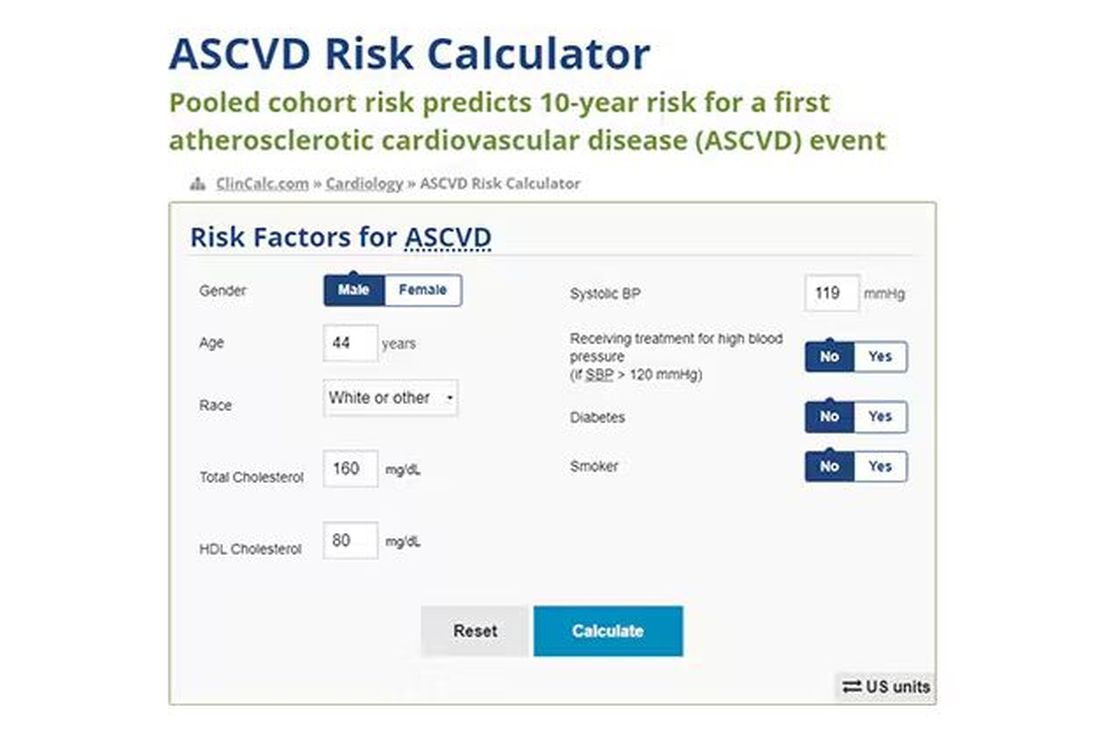
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.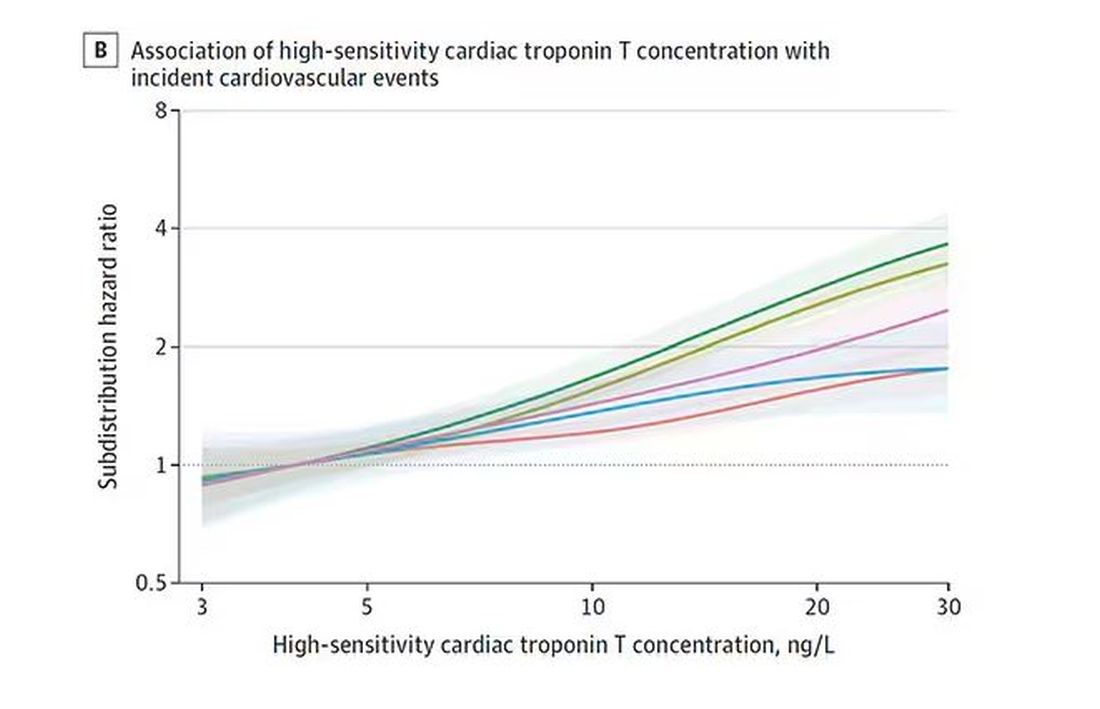
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.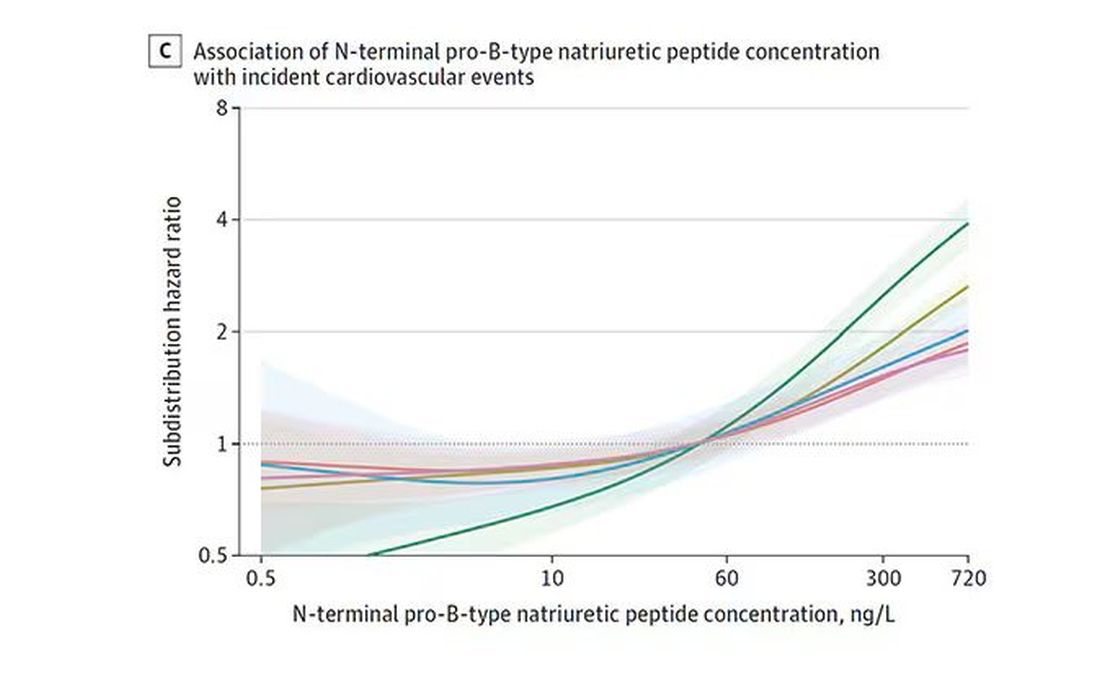
And CRP does a similar thing, with levels above 1.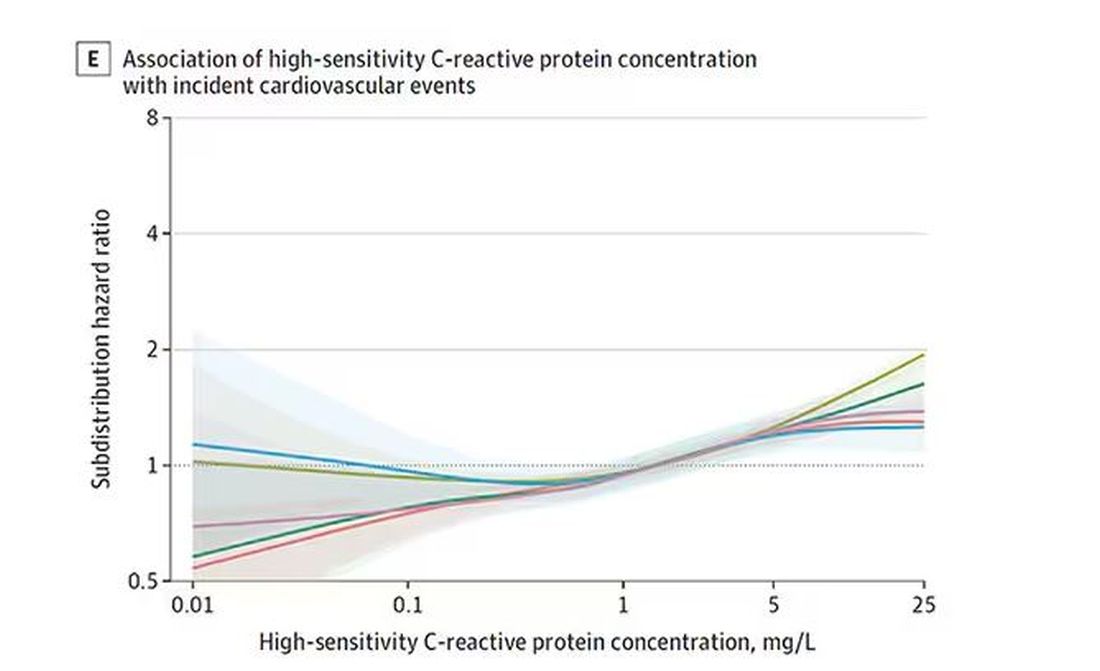
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.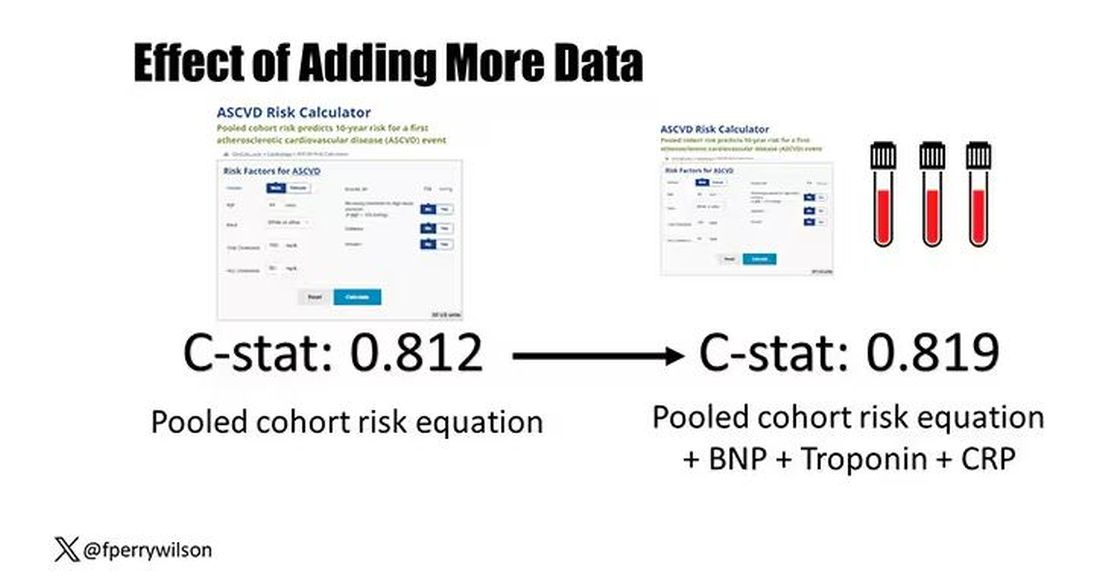
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s the counterintuitive stuff in epidemiology that always really interests me. One intuition many of us have is that if a risk factor is significantly associated with an outcome, knowledge of that risk factor would help to predict that outcome. Makes sense. Feels right.
But it’s not right. Not always.
Here’s a fake example to illustrate my point. Let’s say we have 10,000 individuals who we follow for 10 years and 2000 of them die. (It’s been a rough decade.) At baseline, I measured a novel biomarker, the Perry Factor, in everyone. To keep it simple, the Perry Factor has only two values: 0 or 1.
I then do a standard associational analysis and find that individuals who are positive for the Perry Factor have a 40-fold higher odds of death than those who are negative for it. I am beginning to reconsider ascribing my good name to this biomarker. This is a highly statistically significant result — a P value <.001.
Clearly, knowledge of the Perry Factor should help me predict who will die in the cohort. I evaluate predictive power using a metric called the area under the receiver operating characteristic curve (AUC, referred to as the C-statistic in time-to-event studies). It tells you, given two people — one who dies and one who doesn’t — how frequently you “pick” the right person, given the knowledge of their Perry Factor.
A C-statistic of 0.5, or 50%, would mean the Perry Factor gives you no better results than a coin flip; it’s chance. A C-statistic of 1 is perfect prediction. So, what will the C-statistic be, given the incredibly strong association of the Perry Factor with outcomes? 0.9? 0.95?
0.5024. Almost useless.

Let’s figure out why strength of association and usefulness for prediction are not always the same thing.
I constructed my fake Perry Factor dataset quite carefully to illustrate this point. Let me show you what happened. What you see here is a breakdown of the patients in my fake study. You can see that just 11 of them were Perry Factor positive, but 10 of those 11 ended up dying.
That’s quite unlikely by chance alone. It really does appear that if you have Perry Factor, your risk for death is much higher. But the reason that Perry Factor is a bad predictor is because it is so rare in the population. Sure, you can use it to correctly predict the outcome of 10 of the 11 people who have it, but the vast majority of people don’t have Perry Factor. It’s useless to distinguish who will die vs who will live in that population.
Why have I spent so much time trying to reverse our intuition that strength of association and strength of predictive power must be related? Because it helps to explain this paper, “Prognostic Value of Cardiovascular Biomarkers in the Population,” appearing in JAMA, which is a very nice piece of work trying to help us better predict cardiovascular disease.
I don’t need to tell you that cardiovascular disease is the number-one killer in this country and most of the world. I don’t need to tell you that we have really good preventive therapies and lifestyle interventions that can reduce the risk. But it would be nice to know in whom, specifically, we should use those interventions.
Cardiovascular risk scores, to date, are pretty simple. The most common one in use in the United States, the pooled cohort risk equation, has nine variables, two of which require a cholesterol panel and one a blood pressure test. It’s easy and it’s pretty accurate.
Using the score from the pooled cohort risk calculator, you get a C-statistic as high as 0.82 when applied to Black women, a low of 0.71 when applied to Black men. Non-Black individuals are in the middle. Not bad. But, clearly, not perfect.
And aren’t we in the era of big data, the era of personalized medicine? We have dozens, maybe hundreds, of quantifiable biomarkers that are associated with subsequent heart disease. Surely, by adding these biomarkers into the risk equation, we can improve prediction. Right?
The JAMA study includes 164,054 patients pooled from 28 cohort studies from 12 countries. All the studies measured various key biomarkers at baseline and followed their participants for cardiovascular events like heart attack, stroke, coronary revascularization, and so on.
The biomarkers in question are really the big guns in this space: troponin, a marker of stress on the heart muscle; NT-proBNP, a marker of stretch on the heart muscle; and C-reactive protein, a marker of inflammation. In every case, higher levels of these markers at baseline were associated with a higher risk for cardiovascular disease in the future.
Troponin T, shown here, has a basically linear risk with subsequent cardiovascular disease.
BNP seems to demonstrate more of a threshold effect, where levels above 60 start to associate with problems.
And CRP does a similar thing, with levels above 1.
All of these findings were statistically significant. If you have higher levels of one or more of these biomarkers, you are more likely to have cardiovascular disease in the future.
Of course, our old friend the pooled cohort risk equation is still here — in the background — requiring just that one blood test and measurement of blood pressure. Let’s talk about predictive power.
The pooled cohort risk equation score, in this study, had a C-statistic of 0.812.
By adding troponin, BNP, and CRP to the equation, the new C-statistic is 0.819. Barely any change.
Now, the authors looked at different types of prediction here. The greatest improvement in the AUC was seen when they tried to predict heart failure within 1 year of measurement; there the AUC improved by 0.04. But the presence of BNP as a biomarker and the short time window of 1 year makes me wonder whether this is really prediction at all or whether they were essentially just diagnosing people with existing heart failure.
Why does this happen? Why do these promising biomarkers, clearly associated with bad outcomes, fail to improve our ability to predict the future? I already gave one example, which has to do with how the markers are distributed in the population. But even more relevant here is that the new markers will only improve prediction insofar as they are not already represented in the old predictive model.
Of course, BNP, for example, wasn’t in the old model. But smoking was. Diabetes was. Blood pressure was. All of that data might actually tell you something about the patient’s BNP through their mutual correlation. And improvement in prediction requires new information.
This is actually why I consider this a really successful study. We need to do studies like this to help us find what those new sources of information might be.
We will never get to a C-statistic of 1. Perfect prediction is the domain of palm readers and astrophysicists. But better prediction is always possible through data. The big question, of course, is which data?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Smart Use of Smartphones
Amid the declarations about the current mental health crisis among youth, it has become increasingly common to link rising rates of anxiety and depression among youth to screen time, and more specifically to hours spent on social media. But in truth, this matter is far from settled. The evidence linking mood and anxiety disorders to social media use is inconsistent. And where the evidence is stronger, causality has not been established. Does screen time precipitate an episode of depression or does a preteen at risk for depression, in the midst of a divorce or burdened by learning problems, use screen time excessively as a solution to these problems? There is also substantial variation across age groups, genders, and other factors that suggests that time spent on smartphone apps may not be the primary factor creating risk. Indeed, there is growing uncertainty about whether the climbing rates of anxiety and mood disorders among youth reflect yet to be identified factors increasing the burden of mental illness or the altered screening and tracking landscape in the United States after COVID and the Affordable Care Act. This uncertainty does not mean that we cannot make recommendations about how to guide patients and their families. Smartphones (and watches, glasses, etc) are here to stay.
Start by asking your patients how much time they spend on screens of all sorts and on social media in particular. Find out if there are rules at school or at home limiting screen time or social media. Are there disagreements about screen time? Are patients frustrated with their parents’ use of screens? What are their favorite apps to use? How much time do they think they spend on them? If they don’t know, point out how they can track it on their phone directly. Is it painful to be separated from their phone? Do they have interests or hobbies that are not screen-based? What would they do if the power or Wi-Fi was out for a week? These questions can be the start of an ongoing project for screen time and social media literacy.
Recognize That Apps Are Designed to Be Addictive
Smartphones are useful tools designed to help people stay connected, manage their bank accounts, keep up with current events, access entertainment, and much more. It is easy to spend more time than one intended on them. The applications developed for smartphones promise, and often deliver, efficiency and ease, including staying connected to friends and families. But social media applications have been developed to make their parent companies profit from ad revenue or selling user data. They are designed to encourage more and more use, and for some may become addictive. Start the literacy course with a clear statement of this fact. Remind teenagers that they are often the target audience for the corporations making money from these apps. They are especially sensitive to the likes and followers that can be the currency of social media. For every minute they spend on the apps, a corporation is profiting. It can be helpful to remind teenagers to bring their healthy skepticism of authority to their use of these corporate products.
Develop Awareness of Their Time, Energy, and Mood
Time is our most precious commodity, and most teenagers are stressed by not having enough of it. Ask your patients about the variety of things they need to do and want to do each day. Do they have enough time to do the things they want beyond their smartphones? Is the time on their smartphones more or less than they want? How do they feel when they finish with different activities? Energized? Engaged? Exhilarated? Drained? Irritable? Sad? Do they feel connected? Lonely? Loved? Left out? Suggest that they pay attention to how they feel after engaging in all kinds of activities (including homework, sports, hobbies, and time with friends), as these are the types of choices they will make throughout their adult lives. Some tasks are simply required (homework), some are relaxing (leaving us feeling calm and even sleepy), and some are recharging (leaving us feeling focused and energized). If an activity consistently leaves them drained and irritable, sad, and lonely or discouraged and insecure, they need to step back and ask themselves why they are making this choice and if that is the choice they want to make. Support their developing self-awareness, activating their sense of agency and independence in making choices that will serve them.
Develop Awareness of Their Sense of Connection to Others
As your patients are paying attention to their mood, focus, interest, and energy, they can also pay attention to these components of their social life. How do they feel with individual friends? With different groups? In different settings? How does this compare with how they feel when engaged with social media? In general, when technology is supporting strong connections with friends, it can enhance their health and well-being. When it helps youth isolated by interests or identity to become connected to supportive youth who are physically far away, it can be a social lifeline. But sometimes, social media exploits youth sensitivity to peer opinions and social comparison to keep their attention without the payoff of deepened or new relationships. Do they know the youth they are chatting with or following? Could they spend 2 hours with them offline? How do they feel after spending 2 hours “with” them online? Once again, the goal is to develop teens’ awareness of the quality of their relationships and of their control over how to manage this.
Acknowledge Their Own Vulnerabilities
Does your patient have attention-deficit/hyperactivity disorder (ADHD)? Are they being treated for depression? An anxiety disorder? An eating disorder? While we cannot say whether excessive use of social media can cause these problems, we know that it can be counterproductive to their treatment. Youth with ADHD have great difficulty switching their cognitive focus away from something rewarding, so are particularly prone to spending excessive time in addictive apps. Those with depression often have low energy and initiative alongside feelings of worthlessness that can make engaging in physical, in-person activities challenging. Those with anxiety disorders are prone to rumination and avoidance. The possibility of escaping into virtual social activities or distractions can be very hard to resist and counter-therapeutic for these youth (and adults). Those with eating disorders are vulnerable to comparing themselves with idealized (airbrushed) images online, which can intensify the body image distortion and competitiveness that are common in eating disorders. While there may be helpful information about diagnoses, treatment, and support, there is also troubling information about self-injury, restrictive eating, and even suicide that can increase the risk for these behaviors in vulnerable youth. You can help your patients cultivate awareness of how to take good care of themselves.
Create Habits That Support Sleep, Exercise, and Relationships
Talk with your patients and their parents about strategies to set habits that will make it easier for them to be smart users of their smartphones. Can they explore new apps or games together? Can they talk together about how each of them relaxes and recharges? Then they can work together on how this tool (and toy) can fit into a healthy life. The task is to prioritize sleep, exercise, and live, in-person social time, so virtual activities don’t take over the time needed for them. This can be as simple as consistent bed and waking times and ensuring that smartphones are not at the dining table or in bedrooms at night. Having dinner together as a family most nights (an especially positive habit), going for walks, runs, or hikes together, or doing activities that everyone enjoys (playing music or board games, making cookies or art, gardening) are beneficial for every family member’s physical and mental health and ensure that screen time is not at the expense of real connection. Invite your patients to tell you how they practice putting their smartphones away, getting their homework done, or making time for activities that matter to them. And find out how they relax and recharge beyond using their smartphones. Healthy habits evolve over a lifetime, and there will surely be new technologies that require new limits in the coming years. Helping your patients to make good choices will serve them well as they enter adulthood and throughout their lives.
Dr. Swick is physician in chief at Ohana Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (California) Peninsula. Dr. Michael S. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Amid the declarations about the current mental health crisis among youth, it has become increasingly common to link rising rates of anxiety and depression among youth to screen time, and more specifically to hours spent on social media. But in truth, this matter is far from settled. The evidence linking mood and anxiety disorders to social media use is inconsistent. And where the evidence is stronger, causality has not been established. Does screen time precipitate an episode of depression or does a preteen at risk for depression, in the midst of a divorce or burdened by learning problems, use screen time excessively as a solution to these problems? There is also substantial variation across age groups, genders, and other factors that suggests that time spent on smartphone apps may not be the primary factor creating risk. Indeed, there is growing uncertainty about whether the climbing rates of anxiety and mood disorders among youth reflect yet to be identified factors increasing the burden of mental illness or the altered screening and tracking landscape in the United States after COVID and the Affordable Care Act. This uncertainty does not mean that we cannot make recommendations about how to guide patients and their families. Smartphones (and watches, glasses, etc) are here to stay.
Start by asking your patients how much time they spend on screens of all sorts and on social media in particular. Find out if there are rules at school or at home limiting screen time or social media. Are there disagreements about screen time? Are patients frustrated with their parents’ use of screens? What are their favorite apps to use? How much time do they think they spend on them? If they don’t know, point out how they can track it on their phone directly. Is it painful to be separated from their phone? Do they have interests or hobbies that are not screen-based? What would they do if the power or Wi-Fi was out for a week? These questions can be the start of an ongoing project for screen time and social media literacy.
Recognize That Apps Are Designed to Be Addictive
Smartphones are useful tools designed to help people stay connected, manage their bank accounts, keep up with current events, access entertainment, and much more. It is easy to spend more time than one intended on them. The applications developed for smartphones promise, and often deliver, efficiency and ease, including staying connected to friends and families. But social media applications have been developed to make their parent companies profit from ad revenue or selling user data. They are designed to encourage more and more use, and for some may become addictive. Start the literacy course with a clear statement of this fact. Remind teenagers that they are often the target audience for the corporations making money from these apps. They are especially sensitive to the likes and followers that can be the currency of social media. For every minute they spend on the apps, a corporation is profiting. It can be helpful to remind teenagers to bring their healthy skepticism of authority to their use of these corporate products.
Develop Awareness of Their Time, Energy, and Mood
Time is our most precious commodity, and most teenagers are stressed by not having enough of it. Ask your patients about the variety of things they need to do and want to do each day. Do they have enough time to do the things they want beyond their smartphones? Is the time on their smartphones more or less than they want? How do they feel when they finish with different activities? Energized? Engaged? Exhilarated? Drained? Irritable? Sad? Do they feel connected? Lonely? Loved? Left out? Suggest that they pay attention to how they feel after engaging in all kinds of activities (including homework, sports, hobbies, and time with friends), as these are the types of choices they will make throughout their adult lives. Some tasks are simply required (homework), some are relaxing (leaving us feeling calm and even sleepy), and some are recharging (leaving us feeling focused and energized). If an activity consistently leaves them drained and irritable, sad, and lonely or discouraged and insecure, they need to step back and ask themselves why they are making this choice and if that is the choice they want to make. Support their developing self-awareness, activating their sense of agency and independence in making choices that will serve them.
Develop Awareness of Their Sense of Connection to Others
As your patients are paying attention to their mood, focus, interest, and energy, they can also pay attention to these components of their social life. How do they feel with individual friends? With different groups? In different settings? How does this compare with how they feel when engaged with social media? In general, when technology is supporting strong connections with friends, it can enhance their health and well-being. When it helps youth isolated by interests or identity to become connected to supportive youth who are physically far away, it can be a social lifeline. But sometimes, social media exploits youth sensitivity to peer opinions and social comparison to keep their attention without the payoff of deepened or new relationships. Do they know the youth they are chatting with or following? Could they spend 2 hours with them offline? How do they feel after spending 2 hours “with” them online? Once again, the goal is to develop teens’ awareness of the quality of their relationships and of their control over how to manage this.
Acknowledge Their Own Vulnerabilities
Does your patient have attention-deficit/hyperactivity disorder (ADHD)? Are they being treated for depression? An anxiety disorder? An eating disorder? While we cannot say whether excessive use of social media can cause these problems, we know that it can be counterproductive to their treatment. Youth with ADHD have great difficulty switching their cognitive focus away from something rewarding, so are particularly prone to spending excessive time in addictive apps. Those with depression often have low energy and initiative alongside feelings of worthlessness that can make engaging in physical, in-person activities challenging. Those with anxiety disorders are prone to rumination and avoidance. The possibility of escaping into virtual social activities or distractions can be very hard to resist and counter-therapeutic for these youth (and adults). Those with eating disorders are vulnerable to comparing themselves with idealized (airbrushed) images online, which can intensify the body image distortion and competitiveness that are common in eating disorders. While there may be helpful information about diagnoses, treatment, and support, there is also troubling information about self-injury, restrictive eating, and even suicide that can increase the risk for these behaviors in vulnerable youth. You can help your patients cultivate awareness of how to take good care of themselves.
Create Habits That Support Sleep, Exercise, and Relationships
Talk with your patients and their parents about strategies to set habits that will make it easier for them to be smart users of their smartphones. Can they explore new apps or games together? Can they talk together about how each of them relaxes and recharges? Then they can work together on how this tool (and toy) can fit into a healthy life. The task is to prioritize sleep, exercise, and live, in-person social time, so virtual activities don’t take over the time needed for them. This can be as simple as consistent bed and waking times and ensuring that smartphones are not at the dining table or in bedrooms at night. Having dinner together as a family most nights (an especially positive habit), going for walks, runs, or hikes together, or doing activities that everyone enjoys (playing music or board games, making cookies or art, gardening) are beneficial for every family member’s physical and mental health and ensure that screen time is not at the expense of real connection. Invite your patients to tell you how they practice putting their smartphones away, getting their homework done, or making time for activities that matter to them. And find out how they relax and recharge beyond using their smartphones. Healthy habits evolve over a lifetime, and there will surely be new technologies that require new limits in the coming years. Helping your patients to make good choices will serve them well as they enter adulthood and throughout their lives.
Dr. Swick is physician in chief at Ohana Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (California) Peninsula. Dr. Michael S. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Amid the declarations about the current mental health crisis among youth, it has become increasingly common to link rising rates of anxiety and depression among youth to screen time, and more specifically to hours spent on social media. But in truth, this matter is far from settled. The evidence linking mood and anxiety disorders to social media use is inconsistent. And where the evidence is stronger, causality has not been established. Does screen time precipitate an episode of depression or does a preteen at risk for depression, in the midst of a divorce or burdened by learning problems, use screen time excessively as a solution to these problems? There is also substantial variation across age groups, genders, and other factors that suggests that time spent on smartphone apps may not be the primary factor creating risk. Indeed, there is growing uncertainty about whether the climbing rates of anxiety and mood disorders among youth reflect yet to be identified factors increasing the burden of mental illness or the altered screening and tracking landscape in the United States after COVID and the Affordable Care Act. This uncertainty does not mean that we cannot make recommendations about how to guide patients and their families. Smartphones (and watches, glasses, etc) are here to stay.
Start by asking your patients how much time they spend on screens of all sorts and on social media in particular. Find out if there are rules at school or at home limiting screen time or social media. Are there disagreements about screen time? Are patients frustrated with their parents’ use of screens? What are their favorite apps to use? How much time do they think they spend on them? If they don’t know, point out how they can track it on their phone directly. Is it painful to be separated from their phone? Do they have interests or hobbies that are not screen-based? What would they do if the power or Wi-Fi was out for a week? These questions can be the start of an ongoing project for screen time and social media literacy.
Recognize That Apps Are Designed to Be Addictive
Smartphones are useful tools designed to help people stay connected, manage their bank accounts, keep up with current events, access entertainment, and much more. It is easy to spend more time than one intended on them. The applications developed for smartphones promise, and often deliver, efficiency and ease, including staying connected to friends and families. But social media applications have been developed to make their parent companies profit from ad revenue or selling user data. They are designed to encourage more and more use, and for some may become addictive. Start the literacy course with a clear statement of this fact. Remind teenagers that they are often the target audience for the corporations making money from these apps. They are especially sensitive to the likes and followers that can be the currency of social media. For every minute they spend on the apps, a corporation is profiting. It can be helpful to remind teenagers to bring their healthy skepticism of authority to their use of these corporate products.
Develop Awareness of Their Time, Energy, and Mood
Time is our most precious commodity, and most teenagers are stressed by not having enough of it. Ask your patients about the variety of things they need to do and want to do each day. Do they have enough time to do the things they want beyond their smartphones? Is the time on their smartphones more or less than they want? How do they feel when they finish with different activities? Energized? Engaged? Exhilarated? Drained? Irritable? Sad? Do they feel connected? Lonely? Loved? Left out? Suggest that they pay attention to how they feel after engaging in all kinds of activities (including homework, sports, hobbies, and time with friends), as these are the types of choices they will make throughout their adult lives. Some tasks are simply required (homework), some are relaxing (leaving us feeling calm and even sleepy), and some are recharging (leaving us feeling focused and energized). If an activity consistently leaves them drained and irritable, sad, and lonely or discouraged and insecure, they need to step back and ask themselves why they are making this choice and if that is the choice they want to make. Support their developing self-awareness, activating their sense of agency and independence in making choices that will serve them.
Develop Awareness of Their Sense of Connection to Others
As your patients are paying attention to their mood, focus, interest, and energy, they can also pay attention to these components of their social life. How do they feel with individual friends? With different groups? In different settings? How does this compare with how they feel when engaged with social media? In general, when technology is supporting strong connections with friends, it can enhance their health and well-being. When it helps youth isolated by interests or identity to become connected to supportive youth who are physically far away, it can be a social lifeline. But sometimes, social media exploits youth sensitivity to peer opinions and social comparison to keep their attention without the payoff of deepened or new relationships. Do they know the youth they are chatting with or following? Could they spend 2 hours with them offline? How do they feel after spending 2 hours “with” them online? Once again, the goal is to develop teens’ awareness of the quality of their relationships and of their control over how to manage this.
Acknowledge Their Own Vulnerabilities
Does your patient have attention-deficit/hyperactivity disorder (ADHD)? Are they being treated for depression? An anxiety disorder? An eating disorder? While we cannot say whether excessive use of social media can cause these problems, we know that it can be counterproductive to their treatment. Youth with ADHD have great difficulty switching their cognitive focus away from something rewarding, so are particularly prone to spending excessive time in addictive apps. Those with depression often have low energy and initiative alongside feelings of worthlessness that can make engaging in physical, in-person activities challenging. Those with anxiety disorders are prone to rumination and avoidance. The possibility of escaping into virtual social activities or distractions can be very hard to resist and counter-therapeutic for these youth (and adults). Those with eating disorders are vulnerable to comparing themselves with idealized (airbrushed) images online, which can intensify the body image distortion and competitiveness that are common in eating disorders. While there may be helpful information about diagnoses, treatment, and support, there is also troubling information about self-injury, restrictive eating, and even suicide that can increase the risk for these behaviors in vulnerable youth. You can help your patients cultivate awareness of how to take good care of themselves.
Create Habits That Support Sleep, Exercise, and Relationships
Talk with your patients and their parents about strategies to set habits that will make it easier for them to be smart users of their smartphones. Can they explore new apps or games together? Can they talk together about how each of them relaxes and recharges? Then they can work together on how this tool (and toy) can fit into a healthy life. The task is to prioritize sleep, exercise, and live, in-person social time, so virtual activities don’t take over the time needed for them. This can be as simple as consistent bed and waking times and ensuring that smartphones are not at the dining table or in bedrooms at night. Having dinner together as a family most nights (an especially positive habit), going for walks, runs, or hikes together, or doing activities that everyone enjoys (playing music or board games, making cookies or art, gardening) are beneficial for every family member’s physical and mental health and ensure that screen time is not at the expense of real connection. Invite your patients to tell you how they practice putting their smartphones away, getting their homework done, or making time for activities that matter to them. And find out how they relax and recharge beyond using their smartphones. Healthy habits evolve over a lifetime, and there will surely be new technologies that require new limits in the coming years. Helping your patients to make good choices will serve them well as they enter adulthood and throughout their lives.
Dr. Swick is physician in chief at Ohana Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (California) Peninsula. Dr. Michael S. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
What Does Natural Healing of ACL Ruptures Mean for Long-Term Outcomes?
VIENNA — Nearly one third of anterior cruciate ligament (ACL) injuries appear to heal without surgery, according to an analysis of three-dimensional MRI data taken from the NACOX study, presented as a late-breaking poster at the OARSI 2024 World Congress.
At 2 years after injury, three-dimensional MRI showed that 13 of 43 (30%) knees had evidence of normal, continuous ACL fibers. Moreover, a further 14 (33%) knees had a continuous ACL fiber structure following rehabilitation alone. ACL fibers were partly (16%) or completely (21%) ruptured in the remainder of cases.
“If you think of the ACL like a rope, when there is continuity, it means those fibers have rejoined,” study coauthor Stephanie Filbay, PhD, an associate professor at the University of Melbourne in Australia, told this news organization.
“Within that, there’s a few variations of healing that we’re seeing. Some look like they’ve never been injured, while some have rejoined but appear thinner or longer than a normal ACL,” Dr. Filbay said.
She added: “What all this research is showing is that it’s happening at a much higher rate than we thought possible. And in some of the studies, it looks like ACL healing is associated with very favorable outcomes.”
At OARSI 2024, Dr. Filbay presented additional data from her and others’ research on the relationships between ACL healing and long-term functional outcomes and osteoarthritis (OA) incidence in comparisons between patients’ treatment pathways: Early ACL surgery, rehabilitation followed by delayed surgery, or rehabilitation only.
Healing Without Surgery
The idea that the ACL can heal without surgery is relatively recent and perhaps still not widely accepted as a concept, as Dr. Filbay explained during a plenary lecture at the congress.
Dr. Filbay explained that the ideal management of ACL injury depends on the severity of knee injury and whether someone’s knee is stable after trying nonsurgical management. Results of the ACL SNNAP trial, for example, have suggested that surgical reconstruction is superior to a rehabilitation strategy for managing non-acute ACL injuries where there are persistent symptoms of instability.
However, there have been two trials — COMPARE performed in the Netherlands and KANON performed in Sweden — that found that early surgery was no better than a strategy of initial rehabilitation with the option of having a delayed ACL surgery if needed.
What Happens Long Term?
Posttraumatic OA is a well-known long-term consequence of ACL injury. According to a recent meta-analysis, there is a sevenfold increased risk for OA comparing people who have and have not had an ACL injury.
ACL injury also results in OA occurring at an earlier age than in people with OA who have not had an ACL injury. This has been shown to progress at a faster rate and be associated with a longer period of disability, Dr. Filbay said at the congress, sponsored by the Osteoarthritis Research Society International.
But does the ACL really heal? Dr. Filbay thinks that it does and has been involved in several studies that have used MRI to look at how the ACL may do so.
In a recently published paper, Dr. Filbay and colleagues reported the findings from a secondary analysis of the KANON trial and found that nearly one in three (30%) of the participants who had been randomized to optional delayed surgery had MRI evidence of healing at 2 years. But when they excluded people who had delayed surgery, 53% of people managed by rehabilitation alone had evidence of healing.
The evaluation also found that those who had a healed vs non-healed ligament had better results using the Knee Injury and Osteoarthritis Outcome Score (KOOS), and that there were better outcomes at 2 years among those with ACL healing vs those who had early or delayed ACL surgery.
ACL Continuity and Long-Term Outcomes
At OARSI 2024, Dr. Filbay and colleagues reported an even longer-term secondary analysis of the KANON trial on the relationship between ACL healing at 5 years and outcomes at 11 years. The results were first reported in NEJM Evidence.
Dr. Filbay reported that participants with ACL continuity on MRI at 5 years actually had worse patient-reported outcomes 11 years later than those who were managed with early or delayed ACL reconstruction.
“This does not align with previous findings suggesting better 2-year outcomes compared to the surgically managed groups,” Dr. Filbay said.
However, people with ACL continuity following rehabilitation did seem to show numerically similar or fewer signs of radiographic OA at 11 years vs the surgical groups.
Radiographic OA of the tibiofemoral joint (TFJ) or patellofemoral joint (PFJ) at 11 years was observed in a respective 14% and 21% of people with ACL continuity at 5 years (n = 14) and in 22% and 11% of people with ACL discontinuity at 5 years in the rehabilitation alone group.
By comparison, radiographic OA of the TFJ or PFJ at 11 years was seen in a respective 23% and 35% of people who had rehabilitation with delayed surgery (n = 26) and in 18% and 41% of those who had early surgery (n = 49).
These are descriptive results, Dr. Filbay said, because the numbers were too small to do a statistical analysis. Further, larger, longitudinal studies will be needed.
Posttraumatic OA After ACL Surgery
Elsewhere at OARSI 2024, Matthew Harkey, PhD, and colleagues from Michigan State University, East Lansing, Michigan, reported data showing that nearly two thirds of people who undergo surgical reconstruction have symptoms at 6 months that could be indicative of early knee OA.
Knee symptoms indicative of OA declined to 53% at 12 months and 45% at 24 months.
“It’s a bit complex — we can’t outright say arthritis is developing, but there’s a large group of patients whose symptoms linger long after surgery,” Dr. Harkey said in a press release.
“Often, clinicians assume that these postoperative symptoms will naturally improve as patients reengage with their usual activities. However, what we’re seeing suggests these symptoms persist and likely require a targeted approach to manage or improve them,” Dr. Harkey said.
The analysis used data on 3752 individuals aged 14-40 years who were enrolled in the New Zealand ACL Registry and who completed the KOOS at 6, 12, and 24 months after having ACL reconstruction.
Dr. Harkey and team reported that one in three people had persistent early OA symptoms at 2 years, while 23% had no early OA symptoms at any timepoint.
The studies were independently supported. Dr. Filbay and Dr. Harkey had no relevant financial relationships to report.
Dr. Filbay and colleagues have developed a treatment decision aid for individuals who have sustained an ACL injury. This provides information on the different treatment options available and how they compare.
A version of this article appeared on Medscape.com.
VIENNA — Nearly one third of anterior cruciate ligament (ACL) injuries appear to heal without surgery, according to an analysis of three-dimensional MRI data taken from the NACOX study, presented as a late-breaking poster at the OARSI 2024 World Congress.
At 2 years after injury, three-dimensional MRI showed that 13 of 43 (30%) knees had evidence of normal, continuous ACL fibers. Moreover, a further 14 (33%) knees had a continuous ACL fiber structure following rehabilitation alone. ACL fibers were partly (16%) or completely (21%) ruptured in the remainder of cases.
“If you think of the ACL like a rope, when there is continuity, it means those fibers have rejoined,” study coauthor Stephanie Filbay, PhD, an associate professor at the University of Melbourne in Australia, told this news organization.
“Within that, there’s a few variations of healing that we’re seeing. Some look like they’ve never been injured, while some have rejoined but appear thinner or longer than a normal ACL,” Dr. Filbay said.
She added: “What all this research is showing is that it’s happening at a much higher rate than we thought possible. And in some of the studies, it looks like ACL healing is associated with very favorable outcomes.”
At OARSI 2024, Dr. Filbay presented additional data from her and others’ research on the relationships between ACL healing and long-term functional outcomes and osteoarthritis (OA) incidence in comparisons between patients’ treatment pathways: Early ACL surgery, rehabilitation followed by delayed surgery, or rehabilitation only.
Healing Without Surgery
The idea that the ACL can heal without surgery is relatively recent and perhaps still not widely accepted as a concept, as Dr. Filbay explained during a plenary lecture at the congress.
Dr. Filbay explained that the ideal management of ACL injury depends on the severity of knee injury and whether someone’s knee is stable after trying nonsurgical management. Results of the ACL SNNAP trial, for example, have suggested that surgical reconstruction is superior to a rehabilitation strategy for managing non-acute ACL injuries where there are persistent symptoms of instability.
However, there have been two trials — COMPARE performed in the Netherlands and KANON performed in Sweden — that found that early surgery was no better than a strategy of initial rehabilitation with the option of having a delayed ACL surgery if needed.
What Happens Long Term?
Posttraumatic OA is a well-known long-term consequence of ACL injury. According to a recent meta-analysis, there is a sevenfold increased risk for OA comparing people who have and have not had an ACL injury.
ACL injury also results in OA occurring at an earlier age than in people with OA who have not had an ACL injury. This has been shown to progress at a faster rate and be associated with a longer period of disability, Dr. Filbay said at the congress, sponsored by the Osteoarthritis Research Society International.
But does the ACL really heal? Dr. Filbay thinks that it does and has been involved in several studies that have used MRI to look at how the ACL may do so.
In a recently published paper, Dr. Filbay and colleagues reported the findings from a secondary analysis of the KANON trial and found that nearly one in three (30%) of the participants who had been randomized to optional delayed surgery had MRI evidence of healing at 2 years. But when they excluded people who had delayed surgery, 53% of people managed by rehabilitation alone had evidence of healing.
The evaluation also found that those who had a healed vs non-healed ligament had better results using the Knee Injury and Osteoarthritis Outcome Score (KOOS), and that there were better outcomes at 2 years among those with ACL healing vs those who had early or delayed ACL surgery.
ACL Continuity and Long-Term Outcomes
At OARSI 2024, Dr. Filbay and colleagues reported an even longer-term secondary analysis of the KANON trial on the relationship between ACL healing at 5 years and outcomes at 11 years. The results were first reported in NEJM Evidence.
Dr. Filbay reported that participants with ACL continuity on MRI at 5 years actually had worse patient-reported outcomes 11 years later than those who were managed with early or delayed ACL reconstruction.
“This does not align with previous findings suggesting better 2-year outcomes compared to the surgically managed groups,” Dr. Filbay said.
However, people with ACL continuity following rehabilitation did seem to show numerically similar or fewer signs of radiographic OA at 11 years vs the surgical groups.
Radiographic OA of the tibiofemoral joint (TFJ) or patellofemoral joint (PFJ) at 11 years was observed in a respective 14% and 21% of people with ACL continuity at 5 years (n = 14) and in 22% and 11% of people with ACL discontinuity at 5 years in the rehabilitation alone group.
By comparison, radiographic OA of the TFJ or PFJ at 11 years was seen in a respective 23% and 35% of people who had rehabilitation with delayed surgery (n = 26) and in 18% and 41% of those who had early surgery (n = 49).
These are descriptive results, Dr. Filbay said, because the numbers were too small to do a statistical analysis. Further, larger, longitudinal studies will be needed.
Posttraumatic OA After ACL Surgery
Elsewhere at OARSI 2024, Matthew Harkey, PhD, and colleagues from Michigan State University, East Lansing, Michigan, reported data showing that nearly two thirds of people who undergo surgical reconstruction have symptoms at 6 months that could be indicative of early knee OA.
Knee symptoms indicative of OA declined to 53% at 12 months and 45% at 24 months.
“It’s a bit complex — we can’t outright say arthritis is developing, but there’s a large group of patients whose symptoms linger long after surgery,” Dr. Harkey said in a press release.
“Often, clinicians assume that these postoperative symptoms will naturally improve as patients reengage with their usual activities. However, what we’re seeing suggests these symptoms persist and likely require a targeted approach to manage or improve them,” Dr. Harkey said.
The analysis used data on 3752 individuals aged 14-40 years who were enrolled in the New Zealand ACL Registry and who completed the KOOS at 6, 12, and 24 months after having ACL reconstruction.
Dr. Harkey and team reported that one in three people had persistent early OA symptoms at 2 years, while 23% had no early OA symptoms at any timepoint.
The studies were independently supported. Dr. Filbay and Dr. Harkey had no relevant financial relationships to report.
Dr. Filbay and colleagues have developed a treatment decision aid for individuals who have sustained an ACL injury. This provides information on the different treatment options available and how they compare.
A version of this article appeared on Medscape.com.
VIENNA — Nearly one third of anterior cruciate ligament (ACL) injuries appear to heal without surgery, according to an analysis of three-dimensional MRI data taken from the NACOX study, presented as a late-breaking poster at the OARSI 2024 World Congress.
At 2 years after injury, three-dimensional MRI showed that 13 of 43 (30%) knees had evidence of normal, continuous ACL fibers. Moreover, a further 14 (33%) knees had a continuous ACL fiber structure following rehabilitation alone. ACL fibers were partly (16%) or completely (21%) ruptured in the remainder of cases.
“If you think of the ACL like a rope, when there is continuity, it means those fibers have rejoined,” study coauthor Stephanie Filbay, PhD, an associate professor at the University of Melbourne in Australia, told this news organization.
“Within that, there’s a few variations of healing that we’re seeing. Some look like they’ve never been injured, while some have rejoined but appear thinner or longer than a normal ACL,” Dr. Filbay said.
She added: “What all this research is showing is that it’s happening at a much higher rate than we thought possible. And in some of the studies, it looks like ACL healing is associated with very favorable outcomes.”
At OARSI 2024, Dr. Filbay presented additional data from her and others’ research on the relationships between ACL healing and long-term functional outcomes and osteoarthritis (OA) incidence in comparisons between patients’ treatment pathways: Early ACL surgery, rehabilitation followed by delayed surgery, or rehabilitation only.
Healing Without Surgery
The idea that the ACL can heal without surgery is relatively recent and perhaps still not widely accepted as a concept, as Dr. Filbay explained during a plenary lecture at the congress.
Dr. Filbay explained that the ideal management of ACL injury depends on the severity of knee injury and whether someone’s knee is stable after trying nonsurgical management. Results of the ACL SNNAP trial, for example, have suggested that surgical reconstruction is superior to a rehabilitation strategy for managing non-acute ACL injuries where there are persistent symptoms of instability.
However, there have been two trials — COMPARE performed in the Netherlands and KANON performed in Sweden — that found that early surgery was no better than a strategy of initial rehabilitation with the option of having a delayed ACL surgery if needed.
What Happens Long Term?
Posttraumatic OA is a well-known long-term consequence of ACL injury. According to a recent meta-analysis, there is a sevenfold increased risk for OA comparing people who have and have not had an ACL injury.
ACL injury also results in OA occurring at an earlier age than in people with OA who have not had an ACL injury. This has been shown to progress at a faster rate and be associated with a longer period of disability, Dr. Filbay said at the congress, sponsored by the Osteoarthritis Research Society International.
But does the ACL really heal? Dr. Filbay thinks that it does and has been involved in several studies that have used MRI to look at how the ACL may do so.
In a recently published paper, Dr. Filbay and colleagues reported the findings from a secondary analysis of the KANON trial and found that nearly one in three (30%) of the participants who had been randomized to optional delayed surgery had MRI evidence of healing at 2 years. But when they excluded people who had delayed surgery, 53% of people managed by rehabilitation alone had evidence of healing.
The evaluation also found that those who had a healed vs non-healed ligament had better results using the Knee Injury and Osteoarthritis Outcome Score (KOOS), and that there were better outcomes at 2 years among those with ACL healing vs those who had early or delayed ACL surgery.
ACL Continuity and Long-Term Outcomes
At OARSI 2024, Dr. Filbay and colleagues reported an even longer-term secondary analysis of the KANON trial on the relationship between ACL healing at 5 years and outcomes at 11 years. The results were first reported in NEJM Evidence.
Dr. Filbay reported that participants with ACL continuity on MRI at 5 years actually had worse patient-reported outcomes 11 years later than those who were managed with early or delayed ACL reconstruction.
“This does not align with previous findings suggesting better 2-year outcomes compared to the surgically managed groups,” Dr. Filbay said.
However, people with ACL continuity following rehabilitation did seem to show numerically similar or fewer signs of radiographic OA at 11 years vs the surgical groups.
Radiographic OA of the tibiofemoral joint (TFJ) or patellofemoral joint (PFJ) at 11 years was observed in a respective 14% and 21% of people with ACL continuity at 5 years (n = 14) and in 22% and 11% of people with ACL discontinuity at 5 years in the rehabilitation alone group.
By comparison, radiographic OA of the TFJ or PFJ at 11 years was seen in a respective 23% and 35% of people who had rehabilitation with delayed surgery (n = 26) and in 18% and 41% of those who had early surgery (n = 49).
These are descriptive results, Dr. Filbay said, because the numbers were too small to do a statistical analysis. Further, larger, longitudinal studies will be needed.
Posttraumatic OA After ACL Surgery
Elsewhere at OARSI 2024, Matthew Harkey, PhD, and colleagues from Michigan State University, East Lansing, Michigan, reported data showing that nearly two thirds of people who undergo surgical reconstruction have symptoms at 6 months that could be indicative of early knee OA.
Knee symptoms indicative of OA declined to 53% at 12 months and 45% at 24 months.
“It’s a bit complex — we can’t outright say arthritis is developing, but there’s a large group of patients whose symptoms linger long after surgery,” Dr. Harkey said in a press release.
“Often, clinicians assume that these postoperative symptoms will naturally improve as patients reengage with their usual activities. However, what we’re seeing suggests these symptoms persist and likely require a targeted approach to manage or improve them,” Dr. Harkey said.
The analysis used data on 3752 individuals aged 14-40 years who were enrolled in the New Zealand ACL Registry and who completed the KOOS at 6, 12, and 24 months after having ACL reconstruction.
Dr. Harkey and team reported that one in three people had persistent early OA symptoms at 2 years, while 23% had no early OA symptoms at any timepoint.
The studies were independently supported. Dr. Filbay and Dr. Harkey had no relevant financial relationships to report.
Dr. Filbay and colleagues have developed a treatment decision aid for individuals who have sustained an ACL injury. This provides information on the different treatment options available and how they compare.
A version of this article appeared on Medscape.com.
FROM OARSI 2024
Outside the Guidelines: Prostate Cancer Screening Overused in Older Men
In its most recent guidance, the US Preventive Services Task Force (USPSTF) revised a previous 2012 recommendation against routine screening for prostate cancer to instead endorse individual decision-making for men aged 55 to 69 years (grade C).
In the update guidance, which was published in 2018, the task force still recommended against PSA-based screening for prostate cancer in men 70 years and older (grade D) due to a range of potential risks and harms. Guidelines from the American Urological Association and American Cancer Society have echoed that recommendation, in general agreement that men over the age of 70 or with limited life expectancy show little benefit from the screening.
To take a closer look at how commonly men are being screened for prostate cancer, based not only on their age but their estimated life expectancy, Kevin H. Kensler, ScD, of Weill Cornell Medicine, and colleagues conducted a cross-sectional study using data from the 2020 Behavioral Risk Factor Surveillance System (BRFSS).
“Our findings indicate that many males aged 70 years and older or those with a high risk of death within 10 years undergo prostate cancer screening despite the recommendation against screening in these populations by current guidelines,” the authors wrote in their paper, published in JAMA Network Open. The results underscore that “enhancements to the shared decision-making process are needed to ensure that older males who undergo screening are those who may potentially benefit,” they noted.
For the study, the authors identified 57,397 men aged 60 and older without a history of prostate cancer who reported undergoing a screening PSA test in the prior 2 years.
Using a risk factor system, mortality risk was estimated based on the scales ranging from 5.5 or less to 10.0 or greater, corresponding to the estimated 10-year mortality of less than 30% to 71% or more, respectively.
Of the men, 19.2% were aged 70 to 74 years, 13.0% were aged 75 to 79 years, and 12.3% were aged 80 years or older. The rest were 69 years or younger.
While the estimated 2-year prostate cancer screening rates were 36.3% among those aged 60 to 64 years and 42.8% for those 65 to 69 years, the rates were even higher, at 47.1%, among those aged 70 to 74 years, and similar, at 42.7%, in the 75 to 79 years of age range. Among those aged 80 years and older, 30.4% had been screened.
While the screening frequency was 43.4% among males with the greatest estimated life expectancy, a fair percentage of men, 30.4%, with the lowest life expectancy, indicative of a 71% or greater risk of death within 10 years, received prostate cancer screening.
In fact, among those with lowest life expectancy, the screening rates were greater than 20% in all age groups.
Screening in Older Age: Benefit in Reducing Mortality Low
Autopsy research indicates that, in fact, as many as 50% of men do have prostate cancer at age 80; however, many of those tumors are low-risk and unlikely to affect the health of the men.
If detected early, as is the intention of screening, prostate cancer can take years to advance and the likelihood of receiving any mortality benefit from continued screening in older age is low.
Furthermore, screening in older age can have implications, including a higher risk of complications following a false positive prostate biopsy that may not have been necessary in the first place, the authors explained.
“Given the long natural history of prostate cancer and lead time associated with PSA-based screening, these males [aged 70 and older or with a high risk of death within 10 years] have a low likelihood of receiving any mortality benefit from continued screening,” the authors reported.
“Yet they face the potential harms of overdiagnosis, such as complications after prostate biopsy for a false-positive screening and psychological stress associated with a cancer diagnosis.”
Guideline Confusion, Habit, Among Reasons for Continued Screening
Among key reasons for the continued screening of men well into old age is the fluctuating history of the guidelines, Dr. Kensler said in an interview.
“There has been considerable variation in prostate cancer screening guidelines over time and across organizations that make screening recommendations, and this has inevitably led to some confusion among clinicians,” he explained.
However, the evidence of a lack of benefit over the age of 70 is strong enough that not performing PSA-based screening among men ages 70 or older is a Healthcare Effectiveness Data and Information Set (HEDIS) measure for quality of care, he noted.
Nevertheless, “I think the trends we found in our analysis reflect that it is difficult for patients and providers to stop providing screening once they have already started it,” Dr. Kensler said.
Another motivator may be an inclination by clinicians to err on the side of caution, he added.
“For clinicians, although they may be aware of the guidelines, they may perhaps fear that they will not have offered screening to one of the older individuals who would have benefited from it even though they recognize that most would not,” Dr. Kensler noted.
Too often, however, such screenings “can lead to a cascade of other events that end up harming the patient without extending their lifespan,” he said.
Difficult Discussions
Complicating matters is the task of informing patients that due to their life expectancy, screening is considered to not likely be worthwhile — which may not be an easy discussion.
“For patients, hearing that they are at a stage of life where they may not benefit from screening is an unpleasant message to receive,” Dr. Kensler said.
“Having an in-depth conversation on this topic is also difficult given the many other health topics that clinicians and patients must cover during a visit.”
Ultimately, “these and other factors lead to inertia, where it is easier to stick to the status quo of continuing screening.”
The challenges underscore the need for improvements to the shared decision-making process to make sure that older men who do undergo prostrate screening will benefit, Dr. Kensler argued.
“If the guidelines are going to recommend shared decision-making, we need to provide tools to help patients and clinicians navigate these potentially difficult conversations.
Life Expectancy Uncertainties
Commenting on the research in an interview, Kyle Richards, MD, associate professor with the Department of Urology at the University of Wisconsin School of Medicine and Public Health, in Madison, noted that, “while most urology experts agree that we should not screen for prostate cancer in men with less than 5-10 years life expectancy, the challenge is deciding which patients have a more limited life expectancy.”
Tools and calculators are available to try to calculate life expectancy, “but they can be cumbersome and difficult to incorporate into clinical practice,” he added.
Indeed, the difficulty in accurately estimating life expectancy is also a limitation of the study, he noted.
“The challenge with a study like this is it is very difficult to accurately estimate life expectancy,” he said. “It is easy to pick a cut point (i.e. age 70) but it is very difficult to calculate one’s life expectancy from survey data alone.”
Another limitation is that “screening PSA testing implies that the patient is not having any symptoms, and we do not know from this study if any of these men were getting PSA checks due to some urinary symptoms or other issues,” Dr. Richards added.
“So, while the study does raise some concern about screening PSA in older men, the data source makes it quite difficult to home in on this question.”
When it can be estimated, life expectancy can indeed provide a more useful guide in assessing the options if a patient is found to have prostate cancer, Dr. Richards noted.
“If a patient has a 5- to 10-year life expectancy, and they are diagnosed with a clinically significant prostate cancer, they absolutely may still benefit from treatment,” he said.
“If they have a clinically significant prostate cancer that is unrecognized, it could metastasize and cause symptoms or lead to death, as roughly 30,000 men die from prostate cancer each year in the USA.”
However, “if a patient has a limited life expectancy of less than 5 to 10 years, don’t screen for prostate cancer,” he advised. Proper guidance should furthermore be made loud and clear in guideline recommendations.
“I do think the USPSTF and AUA need to be the primary voices educating primary care and patients regarding prostate cancer screening,” Dr. Richards said.
“We need to be smart about whom to screen, when to screen, and how often to screen. And this message needs to be heard by the primary care providers that perform the screening.”
The study was supported by the Sandra and Edward Meyer Cancer Center and a grant from the National Cancer Institute of the National Institutes of Health.
Dr. Kensler and Dr. Richards had no disclosures to report.
In its most recent guidance, the US Preventive Services Task Force (USPSTF) revised a previous 2012 recommendation against routine screening for prostate cancer to instead endorse individual decision-making for men aged 55 to 69 years (grade C).
In the update guidance, which was published in 2018, the task force still recommended against PSA-based screening for prostate cancer in men 70 years and older (grade D) due to a range of potential risks and harms. Guidelines from the American Urological Association and American Cancer Society have echoed that recommendation, in general agreement that men over the age of 70 or with limited life expectancy show little benefit from the screening.
To take a closer look at how commonly men are being screened for prostate cancer, based not only on their age but their estimated life expectancy, Kevin H. Kensler, ScD, of Weill Cornell Medicine, and colleagues conducted a cross-sectional study using data from the 2020 Behavioral Risk Factor Surveillance System (BRFSS).
“Our findings indicate that many males aged 70 years and older or those with a high risk of death within 10 years undergo prostate cancer screening despite the recommendation against screening in these populations by current guidelines,” the authors wrote in their paper, published in JAMA Network Open. The results underscore that “enhancements to the shared decision-making process are needed to ensure that older males who undergo screening are those who may potentially benefit,” they noted.
For the study, the authors identified 57,397 men aged 60 and older without a history of prostate cancer who reported undergoing a screening PSA test in the prior 2 years.
Using a risk factor system, mortality risk was estimated based on the scales ranging from 5.5 or less to 10.0 or greater, corresponding to the estimated 10-year mortality of less than 30% to 71% or more, respectively.
Of the men, 19.2% were aged 70 to 74 years, 13.0% were aged 75 to 79 years, and 12.3% were aged 80 years or older. The rest were 69 years or younger.
While the estimated 2-year prostate cancer screening rates were 36.3% among those aged 60 to 64 years and 42.8% for those 65 to 69 years, the rates were even higher, at 47.1%, among those aged 70 to 74 years, and similar, at 42.7%, in the 75 to 79 years of age range. Among those aged 80 years and older, 30.4% had been screened.
While the screening frequency was 43.4% among males with the greatest estimated life expectancy, a fair percentage of men, 30.4%, with the lowest life expectancy, indicative of a 71% or greater risk of death within 10 years, received prostate cancer screening.
In fact, among those with lowest life expectancy, the screening rates were greater than 20% in all age groups.
Screening in Older Age: Benefit in Reducing Mortality Low
Autopsy research indicates that, in fact, as many as 50% of men do have prostate cancer at age 80; however, many of those tumors are low-risk and unlikely to affect the health of the men.
If detected early, as is the intention of screening, prostate cancer can take years to advance and the likelihood of receiving any mortality benefit from continued screening in older age is low.
Furthermore, screening in older age can have implications, including a higher risk of complications following a false positive prostate biopsy that may not have been necessary in the first place, the authors explained.
“Given the long natural history of prostate cancer and lead time associated with PSA-based screening, these males [aged 70 and older or with a high risk of death within 10 years] have a low likelihood of receiving any mortality benefit from continued screening,” the authors reported.
“Yet they face the potential harms of overdiagnosis, such as complications after prostate biopsy for a false-positive screening and psychological stress associated with a cancer diagnosis.”
Guideline Confusion, Habit, Among Reasons for Continued Screening
Among key reasons for the continued screening of men well into old age is the fluctuating history of the guidelines, Dr. Kensler said in an interview.
“There has been considerable variation in prostate cancer screening guidelines over time and across organizations that make screening recommendations, and this has inevitably led to some confusion among clinicians,” he explained.
However, the evidence of a lack of benefit over the age of 70 is strong enough that not performing PSA-based screening among men ages 70 or older is a Healthcare Effectiveness Data and Information Set (HEDIS) measure for quality of care, he noted.
Nevertheless, “I think the trends we found in our analysis reflect that it is difficult for patients and providers to stop providing screening once they have already started it,” Dr. Kensler said.
Another motivator may be an inclination by clinicians to err on the side of caution, he added.
“For clinicians, although they may be aware of the guidelines, they may perhaps fear that they will not have offered screening to one of the older individuals who would have benefited from it even though they recognize that most would not,” Dr. Kensler noted.
Too often, however, such screenings “can lead to a cascade of other events that end up harming the patient without extending their lifespan,” he said.
Difficult Discussions
Complicating matters is the task of informing patients that due to their life expectancy, screening is considered to not likely be worthwhile — which may not be an easy discussion.
“For patients, hearing that they are at a stage of life where they may not benefit from screening is an unpleasant message to receive,” Dr. Kensler said.
“Having an in-depth conversation on this topic is also difficult given the many other health topics that clinicians and patients must cover during a visit.”
Ultimately, “these and other factors lead to inertia, where it is easier to stick to the status quo of continuing screening.”
The challenges underscore the need for improvements to the shared decision-making process to make sure that older men who do undergo prostrate screening will benefit, Dr. Kensler argued.
“If the guidelines are going to recommend shared decision-making, we need to provide tools to help patients and clinicians navigate these potentially difficult conversations.
Life Expectancy Uncertainties
Commenting on the research in an interview, Kyle Richards, MD, associate professor with the Department of Urology at the University of Wisconsin School of Medicine and Public Health, in Madison, noted that, “while most urology experts agree that we should not screen for prostate cancer in men with less than 5-10 years life expectancy, the challenge is deciding which patients have a more limited life expectancy.”
Tools and calculators are available to try to calculate life expectancy, “but they can be cumbersome and difficult to incorporate into clinical practice,” he added.
Indeed, the difficulty in accurately estimating life expectancy is also a limitation of the study, he noted.
“The challenge with a study like this is it is very difficult to accurately estimate life expectancy,” he said. “It is easy to pick a cut point (i.e. age 70) but it is very difficult to calculate one’s life expectancy from survey data alone.”
Another limitation is that “screening PSA testing implies that the patient is not having any symptoms, and we do not know from this study if any of these men were getting PSA checks due to some urinary symptoms or other issues,” Dr. Richards added.
“So, while the study does raise some concern about screening PSA in older men, the data source makes it quite difficult to home in on this question.”
When it can be estimated, life expectancy can indeed provide a more useful guide in assessing the options if a patient is found to have prostate cancer, Dr. Richards noted.
“If a patient has a 5- to 10-year life expectancy, and they are diagnosed with a clinically significant prostate cancer, they absolutely may still benefit from treatment,” he said.
“If they have a clinically significant prostate cancer that is unrecognized, it could metastasize and cause symptoms or lead to death, as roughly 30,000 men die from prostate cancer each year in the USA.”
However, “if a patient has a limited life expectancy of less than 5 to 10 years, don’t screen for prostate cancer,” he advised. Proper guidance should furthermore be made loud and clear in guideline recommendations.
“I do think the USPSTF and AUA need to be the primary voices educating primary care and patients regarding prostate cancer screening,” Dr. Richards said.
“We need to be smart about whom to screen, when to screen, and how often to screen. And this message needs to be heard by the primary care providers that perform the screening.”
The study was supported by the Sandra and Edward Meyer Cancer Center and a grant from the National Cancer Institute of the National Institutes of Health.
Dr. Kensler and Dr. Richards had no disclosures to report.
In its most recent guidance, the US Preventive Services Task Force (USPSTF) revised a previous 2012 recommendation against routine screening for prostate cancer to instead endorse individual decision-making for men aged 55 to 69 years (grade C).
In the update guidance, which was published in 2018, the task force still recommended against PSA-based screening for prostate cancer in men 70 years and older (grade D) due to a range of potential risks and harms. Guidelines from the American Urological Association and American Cancer Society have echoed that recommendation, in general agreement that men over the age of 70 or with limited life expectancy show little benefit from the screening.
To take a closer look at how commonly men are being screened for prostate cancer, based not only on their age but their estimated life expectancy, Kevin H. Kensler, ScD, of Weill Cornell Medicine, and colleagues conducted a cross-sectional study using data from the 2020 Behavioral Risk Factor Surveillance System (BRFSS).
“Our findings indicate that many males aged 70 years and older or those with a high risk of death within 10 years undergo prostate cancer screening despite the recommendation against screening in these populations by current guidelines,” the authors wrote in their paper, published in JAMA Network Open. The results underscore that “enhancements to the shared decision-making process are needed to ensure that older males who undergo screening are those who may potentially benefit,” they noted.
For the study, the authors identified 57,397 men aged 60 and older without a history of prostate cancer who reported undergoing a screening PSA test in the prior 2 years.
Using a risk factor system, mortality risk was estimated based on the scales ranging from 5.5 or less to 10.0 or greater, corresponding to the estimated 10-year mortality of less than 30% to 71% or more, respectively.
Of the men, 19.2% were aged 70 to 74 years, 13.0% were aged 75 to 79 years, and 12.3% were aged 80 years or older. The rest were 69 years or younger.
While the estimated 2-year prostate cancer screening rates were 36.3% among those aged 60 to 64 years and 42.8% for those 65 to 69 years, the rates were even higher, at 47.1%, among those aged 70 to 74 years, and similar, at 42.7%, in the 75 to 79 years of age range. Among those aged 80 years and older, 30.4% had been screened.
While the screening frequency was 43.4% among males with the greatest estimated life expectancy, a fair percentage of men, 30.4%, with the lowest life expectancy, indicative of a 71% or greater risk of death within 10 years, received prostate cancer screening.
In fact, among those with lowest life expectancy, the screening rates were greater than 20% in all age groups.
Screening in Older Age: Benefit in Reducing Mortality Low
Autopsy research indicates that, in fact, as many as 50% of men do have prostate cancer at age 80; however, many of those tumors are low-risk and unlikely to affect the health of the men.
If detected early, as is the intention of screening, prostate cancer can take years to advance and the likelihood of receiving any mortality benefit from continued screening in older age is low.
Furthermore, screening in older age can have implications, including a higher risk of complications following a false positive prostate biopsy that may not have been necessary in the first place, the authors explained.
“Given the long natural history of prostate cancer and lead time associated with PSA-based screening, these males [aged 70 and older or with a high risk of death within 10 years] have a low likelihood of receiving any mortality benefit from continued screening,” the authors reported.
“Yet they face the potential harms of overdiagnosis, such as complications after prostate biopsy for a false-positive screening and psychological stress associated with a cancer diagnosis.”
Guideline Confusion, Habit, Among Reasons for Continued Screening
Among key reasons for the continued screening of men well into old age is the fluctuating history of the guidelines, Dr. Kensler said in an interview.
“There has been considerable variation in prostate cancer screening guidelines over time and across organizations that make screening recommendations, and this has inevitably led to some confusion among clinicians,” he explained.
However, the evidence of a lack of benefit over the age of 70 is strong enough that not performing PSA-based screening among men ages 70 or older is a Healthcare Effectiveness Data and Information Set (HEDIS) measure for quality of care, he noted.
Nevertheless, “I think the trends we found in our analysis reflect that it is difficult for patients and providers to stop providing screening once they have already started it,” Dr. Kensler said.
Another motivator may be an inclination by clinicians to err on the side of caution, he added.
“For clinicians, although they may be aware of the guidelines, they may perhaps fear that they will not have offered screening to one of the older individuals who would have benefited from it even though they recognize that most would not,” Dr. Kensler noted.
Too often, however, such screenings “can lead to a cascade of other events that end up harming the patient without extending their lifespan,” he said.
Difficult Discussions
Complicating matters is the task of informing patients that due to their life expectancy, screening is considered to not likely be worthwhile — which may not be an easy discussion.
“For patients, hearing that they are at a stage of life where they may not benefit from screening is an unpleasant message to receive,” Dr. Kensler said.
“Having an in-depth conversation on this topic is also difficult given the many other health topics that clinicians and patients must cover during a visit.”
Ultimately, “these and other factors lead to inertia, where it is easier to stick to the status quo of continuing screening.”
The challenges underscore the need for improvements to the shared decision-making process to make sure that older men who do undergo prostrate screening will benefit, Dr. Kensler argued.
“If the guidelines are going to recommend shared decision-making, we need to provide tools to help patients and clinicians navigate these potentially difficult conversations.
Life Expectancy Uncertainties
Commenting on the research in an interview, Kyle Richards, MD, associate professor with the Department of Urology at the University of Wisconsin School of Medicine and Public Health, in Madison, noted that, “while most urology experts agree that we should not screen for prostate cancer in men with less than 5-10 years life expectancy, the challenge is deciding which patients have a more limited life expectancy.”
Tools and calculators are available to try to calculate life expectancy, “but they can be cumbersome and difficult to incorporate into clinical practice,” he added.
Indeed, the difficulty in accurately estimating life expectancy is also a limitation of the study, he noted.
“The challenge with a study like this is it is very difficult to accurately estimate life expectancy,” he said. “It is easy to pick a cut point (i.e. age 70) but it is very difficult to calculate one’s life expectancy from survey data alone.”
Another limitation is that “screening PSA testing implies that the patient is not having any symptoms, and we do not know from this study if any of these men were getting PSA checks due to some urinary symptoms or other issues,” Dr. Richards added.
“So, while the study does raise some concern about screening PSA in older men, the data source makes it quite difficult to home in on this question.”
When it can be estimated, life expectancy can indeed provide a more useful guide in assessing the options if a patient is found to have prostate cancer, Dr. Richards noted.
“If a patient has a 5- to 10-year life expectancy, and they are diagnosed with a clinically significant prostate cancer, they absolutely may still benefit from treatment,” he said.
“If they have a clinically significant prostate cancer that is unrecognized, it could metastasize and cause symptoms or lead to death, as roughly 30,000 men die from prostate cancer each year in the USA.”
However, “if a patient has a limited life expectancy of less than 5 to 10 years, don’t screen for prostate cancer,” he advised. Proper guidance should furthermore be made loud and clear in guideline recommendations.
“I do think the USPSTF and AUA need to be the primary voices educating primary care and patients regarding prostate cancer screening,” Dr. Richards said.
“We need to be smart about whom to screen, when to screen, and how often to screen. And this message needs to be heard by the primary care providers that perform the screening.”
The study was supported by the Sandra and Edward Meyer Cancer Center and a grant from the National Cancer Institute of the National Institutes of Health.
Dr. Kensler and Dr. Richards had no disclosures to report.
Is Meningitis a Risk Factor for Trigeminal Neuralgia? New Data
In multivariate analysis, the odds of meningitis were threefold higher in patients admitted with trigeminal neuralgia than in matched controls without trigeminal neuralgia.
This is the first nationwide population-based study of the rare, chronic pain disorder to identify the prevalence of trigeminal neuralgia admissions in the United States and risk factors contributing to trigeminal neuralgia development.
“Our results affirm known associations between trigeminal neuralgia and comorbidities like multiple sclerosis, and they also identify meningitis as a novel risk factor for trigeminal neuralgia,” said investigator Megan Tang, BS, a medical student at the Icahn School of Medicine at Mount Sinai, New York City.
The findings were presented at the American Association of Neurological Surgeons (AANS) 2024 annual meeting.
Strong Clinical Risk Factors
Trigeminal neuralgia is a rare pain disorder involving neurovascular compression of the trigeminal nerve. Its etiology and risk factors are poorly understood. Current literature is based on limited datasets and reports inconsistent risk factors across studies.
To better understand the disorder, researchers used International Classification of Diseases (ICD)-9 codes to identify trigeminal neuralgia admissions in the National Inpatient Sample from 2016 to 2019, and then propensity matched them 1:1 to non-trigeminal neuralgia admissions based on demographics, socioeconomic status, and Charlson comorbidity index scores.
Univariate analysis identified 136,345 trigeminal neuralgia admissions or an overall prevalence of 0.096%.
Trigeminal neuralgia admissions had lower morbidity than non-trigeminal neuralgia admissions and a higher prevalence of non-White patients, private insurance, and prolonged length of stay, Ms. Tang said.
Patients admitted for trigeminal neuralgia also had a higher prevalence of several chronic conditions, including hypertension, hyperlipidemia, and osteoarthritis; inflammatory conditions like lupus, meningitis, rheumatoid arthritis, and inflammatory bowel disease; and neurologic conditions including multiple sclerosis, epilepsy, stroke, and neurovascular compression disorders.
In multivariate analysis, investigators identified meningitis as a previously unknown risk factor for trigeminal neuralgia (odds ratio [OR], 3.1; P < .001).
Other strong risk factors were neurovascular compression disorders (OR, 39.82; P < .001) and multiple sclerosis (OR, 12.41; P < .001). Non-White race (Black; OR, 1.09; Hispanic; OR, 1.23; Other; OR, 1.24) and use of Medicaid (OR, 1.07) and other insurance (OR, 1.17) were demographic risk factors for trigeminal neuralgia.
“This finding points us toward future work exploring the potential mechanisms of predictors, most notably inflammatory conditions in trigeminal neuralgia development,” Ms. Tang concluded.
She declined to comment further on the findings, noting the investigators are still finalizing the results and interpretation.
Ask About Meningitis, Fever
Commenting on the findings, Michael D. Staudt, MD, MSc, University Hospitals Cleveland Medical Center, said that many patients who present with classical trigeminal neuralgia will have a blood vessel on MRI that is pressing on the trigeminal nerve.
“Obviously, the nerve is bathed in cerebrospinal fluid. So, if there’s an inflammatory marker, inflammation, or infection that could be injuring the nerve in a way that we don’t yet understand, that could be something that could cause trigeminal neuralgia without having to see a blood vessel,” said Dr. Staudt, who was not involved in the study. “It makes sense, theoretically. Something that’s inflammatory, something that’s irritating, that’s novel.”
Currently, predictive markers include clinical history, response to classical medications such as carbamazepine, and MRI findings, Dr. Staudt noted.
“Someone shows up with symptoms and MRI, and it’s basically do they have a blood vessel or not,” he said. “Treatments are generally within the same categories, but we don’t think it’s the same sort of success rate as seeing a blood vessel.”
Further research is needed, but, in the meantime, Dr. Staudt said, “We can ask patients who show up with facial pain if they’ve ever had meningitis or some sort of fever that preceded their onset of pain.”
The study had no specific funding. Ms. Tang and coauthor Jack Y. Zhang, MS, reported no relevant financial disclosures. Dr. Staudt reported serving as a consultant for Abbott and as a scientific adviser and consultant for Boston Scientific.
A version of this article appeared on Medscape.com.
In multivariate analysis, the odds of meningitis were threefold higher in patients admitted with trigeminal neuralgia than in matched controls without trigeminal neuralgia.
This is the first nationwide population-based study of the rare, chronic pain disorder to identify the prevalence of trigeminal neuralgia admissions in the United States and risk factors contributing to trigeminal neuralgia development.
“Our results affirm known associations between trigeminal neuralgia and comorbidities like multiple sclerosis, and they also identify meningitis as a novel risk factor for trigeminal neuralgia,” said investigator Megan Tang, BS, a medical student at the Icahn School of Medicine at Mount Sinai, New York City.
The findings were presented at the American Association of Neurological Surgeons (AANS) 2024 annual meeting.
Strong Clinical Risk Factors
Trigeminal neuralgia is a rare pain disorder involving neurovascular compression of the trigeminal nerve. Its etiology and risk factors are poorly understood. Current literature is based on limited datasets and reports inconsistent risk factors across studies.
To better understand the disorder, researchers used International Classification of Diseases (ICD)-9 codes to identify trigeminal neuralgia admissions in the National Inpatient Sample from 2016 to 2019, and then propensity matched them 1:1 to non-trigeminal neuralgia admissions based on demographics, socioeconomic status, and Charlson comorbidity index scores.
Univariate analysis identified 136,345 trigeminal neuralgia admissions or an overall prevalence of 0.096%.
Trigeminal neuralgia admissions had lower morbidity than non-trigeminal neuralgia admissions and a higher prevalence of non-White patients, private insurance, and prolonged length of stay, Ms. Tang said.
Patients admitted for trigeminal neuralgia also had a higher prevalence of several chronic conditions, including hypertension, hyperlipidemia, and osteoarthritis; inflammatory conditions like lupus, meningitis, rheumatoid arthritis, and inflammatory bowel disease; and neurologic conditions including multiple sclerosis, epilepsy, stroke, and neurovascular compression disorders.
In multivariate analysis, investigators identified meningitis as a previously unknown risk factor for trigeminal neuralgia (odds ratio [OR], 3.1; P < .001).
Other strong risk factors were neurovascular compression disorders (OR, 39.82; P < .001) and multiple sclerosis (OR, 12.41; P < .001). Non-White race (Black; OR, 1.09; Hispanic; OR, 1.23; Other; OR, 1.24) and use of Medicaid (OR, 1.07) and other insurance (OR, 1.17) were demographic risk factors for trigeminal neuralgia.
“This finding points us toward future work exploring the potential mechanisms of predictors, most notably inflammatory conditions in trigeminal neuralgia development,” Ms. Tang concluded.
She declined to comment further on the findings, noting the investigators are still finalizing the results and interpretation.
Ask About Meningitis, Fever
Commenting on the findings, Michael D. Staudt, MD, MSc, University Hospitals Cleveland Medical Center, said that many patients who present with classical trigeminal neuralgia will have a blood vessel on MRI that is pressing on the trigeminal nerve.
“Obviously, the nerve is bathed in cerebrospinal fluid. So, if there’s an inflammatory marker, inflammation, or infection that could be injuring the nerve in a way that we don’t yet understand, that could be something that could cause trigeminal neuralgia without having to see a blood vessel,” said Dr. Staudt, who was not involved in the study. “It makes sense, theoretically. Something that’s inflammatory, something that’s irritating, that’s novel.”
Currently, predictive markers include clinical history, response to classical medications such as carbamazepine, and MRI findings, Dr. Staudt noted.
“Someone shows up with symptoms and MRI, and it’s basically do they have a blood vessel or not,” he said. “Treatments are generally within the same categories, but we don’t think it’s the same sort of success rate as seeing a blood vessel.”
Further research is needed, but, in the meantime, Dr. Staudt said, “We can ask patients who show up with facial pain if they’ve ever had meningitis or some sort of fever that preceded their onset of pain.”
The study had no specific funding. Ms. Tang and coauthor Jack Y. Zhang, MS, reported no relevant financial disclosures. Dr. Staudt reported serving as a consultant for Abbott and as a scientific adviser and consultant for Boston Scientific.
A version of this article appeared on Medscape.com.
In multivariate analysis, the odds of meningitis were threefold higher in patients admitted with trigeminal neuralgia than in matched controls without trigeminal neuralgia.
This is the first nationwide population-based study of the rare, chronic pain disorder to identify the prevalence of trigeminal neuralgia admissions in the United States and risk factors contributing to trigeminal neuralgia development.
“Our results affirm known associations between trigeminal neuralgia and comorbidities like multiple sclerosis, and they also identify meningitis as a novel risk factor for trigeminal neuralgia,” said investigator Megan Tang, BS, a medical student at the Icahn School of Medicine at Mount Sinai, New York City.
The findings were presented at the American Association of Neurological Surgeons (AANS) 2024 annual meeting.
Strong Clinical Risk Factors
Trigeminal neuralgia is a rare pain disorder involving neurovascular compression of the trigeminal nerve. Its etiology and risk factors are poorly understood. Current literature is based on limited datasets and reports inconsistent risk factors across studies.
To better understand the disorder, researchers used International Classification of Diseases (ICD)-9 codes to identify trigeminal neuralgia admissions in the National Inpatient Sample from 2016 to 2019, and then propensity matched them 1:1 to non-trigeminal neuralgia admissions based on demographics, socioeconomic status, and Charlson comorbidity index scores.
Univariate analysis identified 136,345 trigeminal neuralgia admissions or an overall prevalence of 0.096%.
Trigeminal neuralgia admissions had lower morbidity than non-trigeminal neuralgia admissions and a higher prevalence of non-White patients, private insurance, and prolonged length of stay, Ms. Tang said.
Patients admitted for trigeminal neuralgia also had a higher prevalence of several chronic conditions, including hypertension, hyperlipidemia, and osteoarthritis; inflammatory conditions like lupus, meningitis, rheumatoid arthritis, and inflammatory bowel disease; and neurologic conditions including multiple sclerosis, epilepsy, stroke, and neurovascular compression disorders.
In multivariate analysis, investigators identified meningitis as a previously unknown risk factor for trigeminal neuralgia (odds ratio [OR], 3.1; P < .001).
Other strong risk factors were neurovascular compression disorders (OR, 39.82; P < .001) and multiple sclerosis (OR, 12.41; P < .001). Non-White race (Black; OR, 1.09; Hispanic; OR, 1.23; Other; OR, 1.24) and use of Medicaid (OR, 1.07) and other insurance (OR, 1.17) were demographic risk factors for trigeminal neuralgia.
“This finding points us toward future work exploring the potential mechanisms of predictors, most notably inflammatory conditions in trigeminal neuralgia development,” Ms. Tang concluded.
She declined to comment further on the findings, noting the investigators are still finalizing the results and interpretation.
Ask About Meningitis, Fever
Commenting on the findings, Michael D. Staudt, MD, MSc, University Hospitals Cleveland Medical Center, said that many patients who present with classical trigeminal neuralgia will have a blood vessel on MRI that is pressing on the trigeminal nerve.
“Obviously, the nerve is bathed in cerebrospinal fluid. So, if there’s an inflammatory marker, inflammation, or infection that could be injuring the nerve in a way that we don’t yet understand, that could be something that could cause trigeminal neuralgia without having to see a blood vessel,” said Dr. Staudt, who was not involved in the study. “It makes sense, theoretically. Something that’s inflammatory, something that’s irritating, that’s novel.”
Currently, predictive markers include clinical history, response to classical medications such as carbamazepine, and MRI findings, Dr. Staudt noted.
“Someone shows up with symptoms and MRI, and it’s basically do they have a blood vessel or not,” he said. “Treatments are generally within the same categories, but we don’t think it’s the same sort of success rate as seeing a blood vessel.”
Further research is needed, but, in the meantime, Dr. Staudt said, “We can ask patients who show up with facial pain if they’ve ever had meningitis or some sort of fever that preceded their onset of pain.”
The study had no specific funding. Ms. Tang and coauthor Jack Y. Zhang, MS, reported no relevant financial disclosures. Dr. Staudt reported serving as a consultant for Abbott and as a scientific adviser and consultant for Boston Scientific.
A version of this article appeared on Medscape.com.
FROM AANS 2024








