User login
Real-world data show SGLT2 inhibitors for diabetes triple DKA risk
according to a new large database analysis.
The findings, which include data on the use of three different SGLT2 inhibitors in Canada and the United Kingdom and suggest a class effect, were published online July 27 in Annals of Internal Medicine by Antonios Douros, MD, PhD, of McGill University and the Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, and colleagues.
“Our results provide robust evidence that SGLT2 inhibitors are associated with an increased risk for DKA. Of note, increased risks were observed in all molecule-specific analyses, with canagliflozin [Invokana, Janssen] showing the highest effect estimate,” they noted.
And because the beneficial effects of SGLT2 inhibitors in the prevention of cardiovascular and renal disease will probably increase their uptake in the coming years, “Physicians should be aware of DKA as a potential adverse effect,” Dr. Douros and colleagues wrote.
Analysis “generally confirms what has already been published”
Asked for comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, said that the study “generally confirms what has already been published” on the topic. He noted that overall “the risk of SGLT2 inhibitor–induced ketoacidosis is quite low in type 2 diabetes, perhaps on the order of 1 episode per 1000 patient-years.”
However, Dr. Taylor cautioned: “Published evidence suggests that the risk of DKA is increased if patients are unable to eat,” such as when hospitalized patients are not permitted to eat.
“In that setting, it is probably prudent to discontinue an SGLT2 inhibitor. Also, it may be prudent not to prescribe SGLT2 inhibitors to patients with a history of DKA,” he added.
Dr. Taylor also advised: “Although not necessarily supported by this publication, I think that caution should be exercised in prescribing SGLT2 inhibitors to insulin-dependent type 2 diabetes patients. ... Some late-stage type 2 diabetes patients may have severe insulin deficiency, and their physiology may resemble that of a type 1 diabetes patient.”
Dr. Taylor has previously advised against using SGLT2 inhibitors altogether in patients with type 1 diabetes.
Increased DKA risk seen across all SGLT2 inhibitors
The study involved electronic health care databases from seven Canadian provinces and the United Kingdom, from which 208,757 new users of SGLT2 inhibitors were propensity-matched 1:1 to new dipeptidyl peptidase-4 (DPP-4) inhibitor users.
Of those taking an SGLT2 inhibitor, 42.3% took canagliflozin, 30.7% dapagliflozin (Farxiga/Forxiga, AstraZeneca), and 27.0% empagliflozin (Jardiance, Boehringer Ingelheim).
Over a mean 0.9-year follow-up, 521 patients were hospitalized with DKA, for an overall incidence rate of 1.41 per 1,000 person-years.
The rate with SGLT2 inhibitors, 2.03 per 1,000 person-years, was nearly three times that seen with DPP-4 inhibitors, at 0.75 per 1,000 person-years, a significant difference (hazard ratio, 2.85).
By individual SGLT2 inhibitor, the hazard ratios compared with DPP-4 inhibitors were 1.86 for dapagliflozin, 2.52 for empagliflozin, and 3.58 for canagliflozin, all statistically significant. Stratification by age, sex, and incident versus prevalent user did not change the association between SGLT2 inhibitors and DKA.
Asked about the higher rate for canagliflozin, Dr. Taylor commented: “It is hard to know whether there are real and reproducible differences in the risks of DKA among the various SGLT2 inhibitors. The differences are not huge and the populations are not well matched.”
But, he noted, “If canagliflozin triggers more glucosuria, it is not surprising that it would also induce more ketosis and possibly ketoacidosis.”
He also noted that the threefold relative increase in DKA with canagliflozin versus comparators is consistent with Janssen’s data, published in 2015.
“It is, of course, reassuring that both [randomized clinical trials] and epidemiology produce similar estimates of the risk of drug-induced adverse events. Interestingly, the incidence of DKA is approximately threefold higher in the Canadian [data] as compared to Janssen’s clinical trials.”
Dr. Taylor also pointed out that, in the Janssen studies, the risk of canagliflozin-induced DKA appeared to be higher among patients with anti-islet antibodies, which suggests that some may have actually had autoimmune (type 1) diabetes. “So the overall risk of SGLT2 inhibitor-induced DKA may depend at least in part on the mix of patients.”
In the current study, individuals who never used insulin had a greater relative increase in risk of DKA with SGLT2 inhibitors, compared with DPP-4 inhibitors, than did those who did use insulin (hazard ratios, 3.96 vs. 2.24, both compared with DPP-4 inhibitors). However, just among those taking SGLT2 inhibitors, the absolute risk for DKA was higher for those with prior insulin use (3.52 vs. 1.43 per 1,000 person-years).
The results of sensitivity analyses were consistent with those of the primary analysis.
The study was funded by the Canadian Institutes of Health Research and supported by ICES. Dr. Douros has reported receiving a salary support award from Fonds de recherche du Quebec – sante. Dr. Taylor was previously employed at Bristol-Myers Squibb. He is currently a consultant for Ionis Pharmaceuticals and has reported receiving research support provided to the University of Maryland School of Medicine by Regeneron.
A version of this article originally appeared on Medscape.com.
according to a new large database analysis.
The findings, which include data on the use of three different SGLT2 inhibitors in Canada and the United Kingdom and suggest a class effect, were published online July 27 in Annals of Internal Medicine by Antonios Douros, MD, PhD, of McGill University and the Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, and colleagues.
“Our results provide robust evidence that SGLT2 inhibitors are associated with an increased risk for DKA. Of note, increased risks were observed in all molecule-specific analyses, with canagliflozin [Invokana, Janssen] showing the highest effect estimate,” they noted.
And because the beneficial effects of SGLT2 inhibitors in the prevention of cardiovascular and renal disease will probably increase their uptake in the coming years, “Physicians should be aware of DKA as a potential adverse effect,” Dr. Douros and colleagues wrote.
Analysis “generally confirms what has already been published”
Asked for comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, said that the study “generally confirms what has already been published” on the topic. He noted that overall “the risk of SGLT2 inhibitor–induced ketoacidosis is quite low in type 2 diabetes, perhaps on the order of 1 episode per 1000 patient-years.”
However, Dr. Taylor cautioned: “Published evidence suggests that the risk of DKA is increased if patients are unable to eat,” such as when hospitalized patients are not permitted to eat.
“In that setting, it is probably prudent to discontinue an SGLT2 inhibitor. Also, it may be prudent not to prescribe SGLT2 inhibitors to patients with a history of DKA,” he added.
Dr. Taylor also advised: “Although not necessarily supported by this publication, I think that caution should be exercised in prescribing SGLT2 inhibitors to insulin-dependent type 2 diabetes patients. ... Some late-stage type 2 diabetes patients may have severe insulin deficiency, and their physiology may resemble that of a type 1 diabetes patient.”
Dr. Taylor has previously advised against using SGLT2 inhibitors altogether in patients with type 1 diabetes.
Increased DKA risk seen across all SGLT2 inhibitors
The study involved electronic health care databases from seven Canadian provinces and the United Kingdom, from which 208,757 new users of SGLT2 inhibitors were propensity-matched 1:1 to new dipeptidyl peptidase-4 (DPP-4) inhibitor users.
Of those taking an SGLT2 inhibitor, 42.3% took canagliflozin, 30.7% dapagliflozin (Farxiga/Forxiga, AstraZeneca), and 27.0% empagliflozin (Jardiance, Boehringer Ingelheim).
Over a mean 0.9-year follow-up, 521 patients were hospitalized with DKA, for an overall incidence rate of 1.41 per 1,000 person-years.
The rate with SGLT2 inhibitors, 2.03 per 1,000 person-years, was nearly three times that seen with DPP-4 inhibitors, at 0.75 per 1,000 person-years, a significant difference (hazard ratio, 2.85).
By individual SGLT2 inhibitor, the hazard ratios compared with DPP-4 inhibitors were 1.86 for dapagliflozin, 2.52 for empagliflozin, and 3.58 for canagliflozin, all statistically significant. Stratification by age, sex, and incident versus prevalent user did not change the association between SGLT2 inhibitors and DKA.
Asked about the higher rate for canagliflozin, Dr. Taylor commented: “It is hard to know whether there are real and reproducible differences in the risks of DKA among the various SGLT2 inhibitors. The differences are not huge and the populations are not well matched.”
But, he noted, “If canagliflozin triggers more glucosuria, it is not surprising that it would also induce more ketosis and possibly ketoacidosis.”
He also noted that the threefold relative increase in DKA with canagliflozin versus comparators is consistent with Janssen’s data, published in 2015.
“It is, of course, reassuring that both [randomized clinical trials] and epidemiology produce similar estimates of the risk of drug-induced adverse events. Interestingly, the incidence of DKA is approximately threefold higher in the Canadian [data] as compared to Janssen’s clinical trials.”
Dr. Taylor also pointed out that, in the Janssen studies, the risk of canagliflozin-induced DKA appeared to be higher among patients with anti-islet antibodies, which suggests that some may have actually had autoimmune (type 1) diabetes. “So the overall risk of SGLT2 inhibitor-induced DKA may depend at least in part on the mix of patients.”
In the current study, individuals who never used insulin had a greater relative increase in risk of DKA with SGLT2 inhibitors, compared with DPP-4 inhibitors, than did those who did use insulin (hazard ratios, 3.96 vs. 2.24, both compared with DPP-4 inhibitors). However, just among those taking SGLT2 inhibitors, the absolute risk for DKA was higher for those with prior insulin use (3.52 vs. 1.43 per 1,000 person-years).
The results of sensitivity analyses were consistent with those of the primary analysis.
The study was funded by the Canadian Institutes of Health Research and supported by ICES. Dr. Douros has reported receiving a salary support award from Fonds de recherche du Quebec – sante. Dr. Taylor was previously employed at Bristol-Myers Squibb. He is currently a consultant for Ionis Pharmaceuticals and has reported receiving research support provided to the University of Maryland School of Medicine by Regeneron.
A version of this article originally appeared on Medscape.com.
according to a new large database analysis.
The findings, which include data on the use of three different SGLT2 inhibitors in Canada and the United Kingdom and suggest a class effect, were published online July 27 in Annals of Internal Medicine by Antonios Douros, MD, PhD, of McGill University and the Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, and colleagues.
“Our results provide robust evidence that SGLT2 inhibitors are associated with an increased risk for DKA. Of note, increased risks were observed in all molecule-specific analyses, with canagliflozin [Invokana, Janssen] showing the highest effect estimate,” they noted.
And because the beneficial effects of SGLT2 inhibitors in the prevention of cardiovascular and renal disease will probably increase their uptake in the coming years, “Physicians should be aware of DKA as a potential adverse effect,” Dr. Douros and colleagues wrote.
Analysis “generally confirms what has already been published”
Asked for comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, said that the study “generally confirms what has already been published” on the topic. He noted that overall “the risk of SGLT2 inhibitor–induced ketoacidosis is quite low in type 2 diabetes, perhaps on the order of 1 episode per 1000 patient-years.”
However, Dr. Taylor cautioned: “Published evidence suggests that the risk of DKA is increased if patients are unable to eat,” such as when hospitalized patients are not permitted to eat.
“In that setting, it is probably prudent to discontinue an SGLT2 inhibitor. Also, it may be prudent not to prescribe SGLT2 inhibitors to patients with a history of DKA,” he added.
Dr. Taylor also advised: “Although not necessarily supported by this publication, I think that caution should be exercised in prescribing SGLT2 inhibitors to insulin-dependent type 2 diabetes patients. ... Some late-stage type 2 diabetes patients may have severe insulin deficiency, and their physiology may resemble that of a type 1 diabetes patient.”
Dr. Taylor has previously advised against using SGLT2 inhibitors altogether in patients with type 1 diabetes.
Increased DKA risk seen across all SGLT2 inhibitors
The study involved electronic health care databases from seven Canadian provinces and the United Kingdom, from which 208,757 new users of SGLT2 inhibitors were propensity-matched 1:1 to new dipeptidyl peptidase-4 (DPP-4) inhibitor users.
Of those taking an SGLT2 inhibitor, 42.3% took canagliflozin, 30.7% dapagliflozin (Farxiga/Forxiga, AstraZeneca), and 27.0% empagliflozin (Jardiance, Boehringer Ingelheim).
Over a mean 0.9-year follow-up, 521 patients were hospitalized with DKA, for an overall incidence rate of 1.41 per 1,000 person-years.
The rate with SGLT2 inhibitors, 2.03 per 1,000 person-years, was nearly three times that seen with DPP-4 inhibitors, at 0.75 per 1,000 person-years, a significant difference (hazard ratio, 2.85).
By individual SGLT2 inhibitor, the hazard ratios compared with DPP-4 inhibitors were 1.86 for dapagliflozin, 2.52 for empagliflozin, and 3.58 for canagliflozin, all statistically significant. Stratification by age, sex, and incident versus prevalent user did not change the association between SGLT2 inhibitors and DKA.
Asked about the higher rate for canagliflozin, Dr. Taylor commented: “It is hard to know whether there are real and reproducible differences in the risks of DKA among the various SGLT2 inhibitors. The differences are not huge and the populations are not well matched.”
But, he noted, “If canagliflozin triggers more glucosuria, it is not surprising that it would also induce more ketosis and possibly ketoacidosis.”
He also noted that the threefold relative increase in DKA with canagliflozin versus comparators is consistent with Janssen’s data, published in 2015.
“It is, of course, reassuring that both [randomized clinical trials] and epidemiology produce similar estimates of the risk of drug-induced adverse events. Interestingly, the incidence of DKA is approximately threefold higher in the Canadian [data] as compared to Janssen’s clinical trials.”
Dr. Taylor also pointed out that, in the Janssen studies, the risk of canagliflozin-induced DKA appeared to be higher among patients with anti-islet antibodies, which suggests that some may have actually had autoimmune (type 1) diabetes. “So the overall risk of SGLT2 inhibitor-induced DKA may depend at least in part on the mix of patients.”
In the current study, individuals who never used insulin had a greater relative increase in risk of DKA with SGLT2 inhibitors, compared with DPP-4 inhibitors, than did those who did use insulin (hazard ratios, 3.96 vs. 2.24, both compared with DPP-4 inhibitors). However, just among those taking SGLT2 inhibitors, the absolute risk for DKA was higher for those with prior insulin use (3.52 vs. 1.43 per 1,000 person-years).
The results of sensitivity analyses were consistent with those of the primary analysis.
The study was funded by the Canadian Institutes of Health Research and supported by ICES. Dr. Douros has reported receiving a salary support award from Fonds de recherche du Quebec – sante. Dr. Taylor was previously employed at Bristol-Myers Squibb. He is currently a consultant for Ionis Pharmaceuticals and has reported receiving research support provided to the University of Maryland School of Medicine by Regeneron.
A version of this article originally appeared on Medscape.com.
The fix is in: AIM bundles to combat maternal morbidity and mortality
“Anytime you have a maternal death, it sticks with you for life,” said Elliott Main, MD, a maternal fetal medicine specialist at Stanford (Calif.) University and one of the nation’s leaders in combating maternal mortality.
Dr. Main has had two maternal deaths in his career, years ago. One woman had a fatal stroke because of severe hypertension, and another died of cardiac complications. “We tried to do everything we possibly could, but you scrounge your memory for years and years [afterward]. To have a young healthy person go into labor and delivery and not come out is a tragedy at all levels. It charged me to not ever want to see that happen again,” he said.
Today, Dr. Main is the medical director of the California Maternal Quality Care Collaborative (CMQCC), a wide-ranging group of clinicians, state officials, hospitals, and others who have come together to address the issue. About 30 states have similar perinatal quality collaboratives (PQCs), and other states are forming them.
They work in collaboration with maternal mortality review committees (MMRCs), state-level groups that review maternal deaths, identify problems to address, and make recommendations to the quality collaboratives on how to prevent maternal deaths.
About 600-800 women die in the United States each year due to pregnancy-related complications, which ranks the United States behind other industrialized nations. Leading causes include hemorrhage and hemorrhagic strokes secondary to hypertension. It’s estimated that the majority of maternal deaths could be prevented with proper care.
To that end, states are enacting safety bundles from the Alliance for Innovation on Maternal Health (AIM), which was established by the American College of Obstetricians and Gynecologist several years ago. There are bundles that address obstetric hypertension, hemorrhage, mental health, venous thromboembolism, opioid use, racial disparities, and other problems. They were developed by experts in the field and published in multiple journals. California and other states have issued toolkits on how to implement them based on local circumstances.
Dr. Main said.
AIM bundle implementation is “what’s happening in New Mexico and a lot of states, mostly through the efforts of state level quality care collaboratives. Some [states] are further ahead than others,” said Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, and president of the New Mexico PQC.
“Most states now have a [MMRC] that collects maternal mortality and near-miss data. Those data are used by the action arm,” which is the PQC. “If the review committee says” opioid use disorder is a significant contributor “like in our state, the collaborative rolls out the opioid use disorder bundle,” she said.
Beginning next January, the Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations, will require that accredited hospitals enact key elements of the AIM bundles for both obstetric hemorrhage and severe hypertension. “Everyone’s [now] motivated to get on that bandwagon,” Dr. Espey said.
“The bundles are here to stay,” and the Joint Commission requirements are “a really important step for sustainability and basic implementation. We really want to get them adopted everywhere,” said Dr. Main, who is also the national implementation director for the AIM initiative.
“The key thing is to work on implementing the hemorrhage and hypertension bundles in your hospital. I would suggest contacting [your] state” PQC, he said.
The California model
California, which has been working to reduce maternal mortality and morbidity since the mid 2000s, has produced among the strongest evidence to date that the efforts make a difference.
By 2013, the state had halved its maternal mortality rate to a 3-year average of 7 deaths per 100,000 live births, which is comparable with the average Western Europe rate of 7.2 deaths. Nationwide, the rate was about 17.4 deaths per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention
The reasons are multifactorial, but “we think” the quality improvement efforts have been “an important contributor,” Dr. Main said.
Improvements especially for Black women
Among the success stories has been California’s implementation of the AIM obstetric hemorrhage bundle about 5 years ago. Among other steps, the 17 evidence-based recommendations included early recognition, immediate access to oxytocin and other medications, immediate access to a hemorrhage cart with instructions for intrauterine balloons and compression stitches, the establishment of a hemorrhage response protocol and team, and regular unit-based drills with debriefing sessions afterward.
Mentoring teams consisting of a physician and nurse with maternal quality improvement experience were created to help hospitals come on board, with each team working with five to eight hospitals. Efforts included monthly telephone calls and face-to-face meetings, and providers were held accountable for progress. Hospitals shared data and tips on implementation, under the aegis of the CMQCC.
When the baseline period of 2011-2014 to the postintervention period of October 2015 to December 2016 were compared, the rate of severe maternal morbidity from hemorrhage fell from 22.1% to 18.5% across 99 hospitals and 73,476 women.
The benefit among Black women exceeded that among White women, with a 9% absolute rate reduction versus 2.1%. “If you adjusted for risk factors, [we found] you could eliminate [racial differences] completely,” which is something that hadn’t been shown before. “This is a really big deal,” Dr. Main said, because the risk of maternal morbidity and mortality is three to four times higher among Black women, compared with White women.
Dr. Main and his team found that the biggest clinical risk factor that accounted for racial differences was a higher rate of cesarean deliveries among Black women, followed by higher rates of anemia at hospital admission. “If you have a C-section when you are anemic, you are going to have a transfusion,” he explained.
More recently, there’s been a push in California to reduce the rate of primary cesarean deliveries by enacting the associated AIM bundle with use of the same approach as with the hemorrhage bundle. Dr. Main and his team recently reported a rate reduction from 29.3% to 25% without compromising birth outcomes.
However, “some changes are easier than others. Hemorrhage was an easy one to change because it didn’t deal with physician autonomy as much, and you saw more immediate results” with fewer hemorrhages. Reducing cesarean delivery rates is “a bigger lift” because “it’s really changing the culture of labor and delivery. It involves more group pressure and more reinforcing, but we were able to do that,” he said.
Problems in the Show Me State
“We’ve patterned a lot” of what’s being done in New Mexico “after California,” Dr. Espey said.
The AIM hemorrhage bundle, for instance, is being rolled out to New Mexico hospitals, with the help of virtual meetings and mentoring programs, plus outreach to the Navajo and others reservations because, as with Black women, rates of maternal morbidity and mortality are higher among Native American women.
It’s been tougher going, however, in states such as Missouri, which recently ranked 44th in the country for maternal mortality.
“We started a little bit late, and we are a little bit behind,” said ob.gyn. Karen L. Florio, DO, at the University of Missouri–Kansas City and also a leader of the state MMRC and member of its PQC.
The main problem is money. California’s efforts are funded by the Centers for Disease Control and Prevention, the state health department, and hospitals, among others.
But Missouri is “not as well funded as California for our mortality review board, and our [PQC] is mostly not funded. If we could get that funding, we would have more resources to implement these AIM bundles,” she said.
In addition to the issue, Missouri didn’t expand Medicaid under the Accountable Care Act – something that’s been linked to reduced maternal morbidity and mortality – and there are entire rural areas with no maternity care. Plus after generations of mistreatment, “our African American population has a valid distrust of the medical system that contributes to maternal mortality,” she said.
Obesity-related heart disease is also prevalent in Missouri, even among young people. “I cannot tell you how many women I have had who have had a heart attack at the age of 30 and who have had stents placed,” Dr. Florio said.
Dr. Florio and her colleagues are currently using teleconferences and other means to roll out the AIM hypertension bundle but can do so only selectively. “We don’t have the resources to reach every single rural hospital all over the state,” she said; they are working to address the funding issues.
For rural hospitals, implementation is “daunting”
Meanwhile, rural hospitals have been a particular concern in South Dakota, said Kimberlee McKay, MD, an ob.gyn. who is the clinical vice president of the ob.gyn. service line at Avera Health, a hospital system based in Sioux Falls, S.D.
She’s been overseeing Avera’s implementation of the hypertension, hemorrhage, and venous thromboembolism bundles. “What’s hard is that” the AIM protocols come “out of academic centers. Implementation of complex algorithms is daunting” for hospitals that only do a couple hundred deliveries a year, she said.
For small hospitals, the approach she’s found that works is to first assess what they can offer, and then have them “do what’s reasonable” for their resources. The second part is making sure high-risk women get to a regional center – with an adequate blood supply, in the case of hemorrhage, for instance – for complications. Dr. McKay and colleagues are working on a system by which regional centers can monitor smaller hospitals for potential maternity problems, and contact them proactively before they emerge.
They’ve also made access to hemorrhage and hypertension drugs easier on labor and delivery units with the help of close-by dedicated medicine boxes, and standardized protocols and order sets across Avera. “We try to make the right thing the easy thing to do,” Dr. McKay said.
Dr. Espey is an editorial adviser for Ob.Gyn. News. The physicians have no relevant financial disclosures.
“Anytime you have a maternal death, it sticks with you for life,” said Elliott Main, MD, a maternal fetal medicine specialist at Stanford (Calif.) University and one of the nation’s leaders in combating maternal mortality.
Dr. Main has had two maternal deaths in his career, years ago. One woman had a fatal stroke because of severe hypertension, and another died of cardiac complications. “We tried to do everything we possibly could, but you scrounge your memory for years and years [afterward]. To have a young healthy person go into labor and delivery and not come out is a tragedy at all levels. It charged me to not ever want to see that happen again,” he said.
Today, Dr. Main is the medical director of the California Maternal Quality Care Collaborative (CMQCC), a wide-ranging group of clinicians, state officials, hospitals, and others who have come together to address the issue. About 30 states have similar perinatal quality collaboratives (PQCs), and other states are forming them.
They work in collaboration with maternal mortality review committees (MMRCs), state-level groups that review maternal deaths, identify problems to address, and make recommendations to the quality collaboratives on how to prevent maternal deaths.
About 600-800 women die in the United States each year due to pregnancy-related complications, which ranks the United States behind other industrialized nations. Leading causes include hemorrhage and hemorrhagic strokes secondary to hypertension. It’s estimated that the majority of maternal deaths could be prevented with proper care.
To that end, states are enacting safety bundles from the Alliance for Innovation on Maternal Health (AIM), which was established by the American College of Obstetricians and Gynecologist several years ago. There are bundles that address obstetric hypertension, hemorrhage, mental health, venous thromboembolism, opioid use, racial disparities, and other problems. They were developed by experts in the field and published in multiple journals. California and other states have issued toolkits on how to implement them based on local circumstances.
Dr. Main said.
AIM bundle implementation is “what’s happening in New Mexico and a lot of states, mostly through the efforts of state level quality care collaboratives. Some [states] are further ahead than others,” said Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, and president of the New Mexico PQC.
“Most states now have a [MMRC] that collects maternal mortality and near-miss data. Those data are used by the action arm,” which is the PQC. “If the review committee says” opioid use disorder is a significant contributor “like in our state, the collaborative rolls out the opioid use disorder bundle,” she said.
Beginning next January, the Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations, will require that accredited hospitals enact key elements of the AIM bundles for both obstetric hemorrhage and severe hypertension. “Everyone’s [now] motivated to get on that bandwagon,” Dr. Espey said.
“The bundles are here to stay,” and the Joint Commission requirements are “a really important step for sustainability and basic implementation. We really want to get them adopted everywhere,” said Dr. Main, who is also the national implementation director for the AIM initiative.
“The key thing is to work on implementing the hemorrhage and hypertension bundles in your hospital. I would suggest contacting [your] state” PQC, he said.
The California model
California, which has been working to reduce maternal mortality and morbidity since the mid 2000s, has produced among the strongest evidence to date that the efforts make a difference.
By 2013, the state had halved its maternal mortality rate to a 3-year average of 7 deaths per 100,000 live births, which is comparable with the average Western Europe rate of 7.2 deaths. Nationwide, the rate was about 17.4 deaths per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention
The reasons are multifactorial, but “we think” the quality improvement efforts have been “an important contributor,” Dr. Main said.
Improvements especially for Black women
Among the success stories has been California’s implementation of the AIM obstetric hemorrhage bundle about 5 years ago. Among other steps, the 17 evidence-based recommendations included early recognition, immediate access to oxytocin and other medications, immediate access to a hemorrhage cart with instructions for intrauterine balloons and compression stitches, the establishment of a hemorrhage response protocol and team, and regular unit-based drills with debriefing sessions afterward.
Mentoring teams consisting of a physician and nurse with maternal quality improvement experience were created to help hospitals come on board, with each team working with five to eight hospitals. Efforts included monthly telephone calls and face-to-face meetings, and providers were held accountable for progress. Hospitals shared data and tips on implementation, under the aegis of the CMQCC.
When the baseline period of 2011-2014 to the postintervention period of October 2015 to December 2016 were compared, the rate of severe maternal morbidity from hemorrhage fell from 22.1% to 18.5% across 99 hospitals and 73,476 women.
The benefit among Black women exceeded that among White women, with a 9% absolute rate reduction versus 2.1%. “If you adjusted for risk factors, [we found] you could eliminate [racial differences] completely,” which is something that hadn’t been shown before. “This is a really big deal,” Dr. Main said, because the risk of maternal morbidity and mortality is three to four times higher among Black women, compared with White women.
Dr. Main and his team found that the biggest clinical risk factor that accounted for racial differences was a higher rate of cesarean deliveries among Black women, followed by higher rates of anemia at hospital admission. “If you have a C-section when you are anemic, you are going to have a transfusion,” he explained.
More recently, there’s been a push in California to reduce the rate of primary cesarean deliveries by enacting the associated AIM bundle with use of the same approach as with the hemorrhage bundle. Dr. Main and his team recently reported a rate reduction from 29.3% to 25% without compromising birth outcomes.
However, “some changes are easier than others. Hemorrhage was an easy one to change because it didn’t deal with physician autonomy as much, and you saw more immediate results” with fewer hemorrhages. Reducing cesarean delivery rates is “a bigger lift” because “it’s really changing the culture of labor and delivery. It involves more group pressure and more reinforcing, but we were able to do that,” he said.
Problems in the Show Me State
“We’ve patterned a lot” of what’s being done in New Mexico “after California,” Dr. Espey said.
The AIM hemorrhage bundle, for instance, is being rolled out to New Mexico hospitals, with the help of virtual meetings and mentoring programs, plus outreach to the Navajo and others reservations because, as with Black women, rates of maternal morbidity and mortality are higher among Native American women.
It’s been tougher going, however, in states such as Missouri, which recently ranked 44th in the country for maternal mortality.
“We started a little bit late, and we are a little bit behind,” said ob.gyn. Karen L. Florio, DO, at the University of Missouri–Kansas City and also a leader of the state MMRC and member of its PQC.
The main problem is money. California’s efforts are funded by the Centers for Disease Control and Prevention, the state health department, and hospitals, among others.
But Missouri is “not as well funded as California for our mortality review board, and our [PQC] is mostly not funded. If we could get that funding, we would have more resources to implement these AIM bundles,” she said.
In addition to the issue, Missouri didn’t expand Medicaid under the Accountable Care Act – something that’s been linked to reduced maternal morbidity and mortality – and there are entire rural areas with no maternity care. Plus after generations of mistreatment, “our African American population has a valid distrust of the medical system that contributes to maternal mortality,” she said.
Obesity-related heart disease is also prevalent in Missouri, even among young people. “I cannot tell you how many women I have had who have had a heart attack at the age of 30 and who have had stents placed,” Dr. Florio said.
Dr. Florio and her colleagues are currently using teleconferences and other means to roll out the AIM hypertension bundle but can do so only selectively. “We don’t have the resources to reach every single rural hospital all over the state,” she said; they are working to address the funding issues.
For rural hospitals, implementation is “daunting”
Meanwhile, rural hospitals have been a particular concern in South Dakota, said Kimberlee McKay, MD, an ob.gyn. who is the clinical vice president of the ob.gyn. service line at Avera Health, a hospital system based in Sioux Falls, S.D.
She’s been overseeing Avera’s implementation of the hypertension, hemorrhage, and venous thromboembolism bundles. “What’s hard is that” the AIM protocols come “out of academic centers. Implementation of complex algorithms is daunting” for hospitals that only do a couple hundred deliveries a year, she said.
For small hospitals, the approach she’s found that works is to first assess what they can offer, and then have them “do what’s reasonable” for their resources. The second part is making sure high-risk women get to a regional center – with an adequate blood supply, in the case of hemorrhage, for instance – for complications. Dr. McKay and colleagues are working on a system by which regional centers can monitor smaller hospitals for potential maternity problems, and contact them proactively before they emerge.
They’ve also made access to hemorrhage and hypertension drugs easier on labor and delivery units with the help of close-by dedicated medicine boxes, and standardized protocols and order sets across Avera. “We try to make the right thing the easy thing to do,” Dr. McKay said.
Dr. Espey is an editorial adviser for Ob.Gyn. News. The physicians have no relevant financial disclosures.
“Anytime you have a maternal death, it sticks with you for life,” said Elliott Main, MD, a maternal fetal medicine specialist at Stanford (Calif.) University and one of the nation’s leaders in combating maternal mortality.
Dr. Main has had two maternal deaths in his career, years ago. One woman had a fatal stroke because of severe hypertension, and another died of cardiac complications. “We tried to do everything we possibly could, but you scrounge your memory for years and years [afterward]. To have a young healthy person go into labor and delivery and not come out is a tragedy at all levels. It charged me to not ever want to see that happen again,” he said.
Today, Dr. Main is the medical director of the California Maternal Quality Care Collaborative (CMQCC), a wide-ranging group of clinicians, state officials, hospitals, and others who have come together to address the issue. About 30 states have similar perinatal quality collaboratives (PQCs), and other states are forming them.
They work in collaboration with maternal mortality review committees (MMRCs), state-level groups that review maternal deaths, identify problems to address, and make recommendations to the quality collaboratives on how to prevent maternal deaths.
About 600-800 women die in the United States each year due to pregnancy-related complications, which ranks the United States behind other industrialized nations. Leading causes include hemorrhage and hemorrhagic strokes secondary to hypertension. It’s estimated that the majority of maternal deaths could be prevented with proper care.
To that end, states are enacting safety bundles from the Alliance for Innovation on Maternal Health (AIM), which was established by the American College of Obstetricians and Gynecologist several years ago. There are bundles that address obstetric hypertension, hemorrhage, mental health, venous thromboembolism, opioid use, racial disparities, and other problems. They were developed by experts in the field and published in multiple journals. California and other states have issued toolkits on how to implement them based on local circumstances.
Dr. Main said.
AIM bundle implementation is “what’s happening in New Mexico and a lot of states, mostly through the efforts of state level quality care collaboratives. Some [states] are further ahead than others,” said Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, and president of the New Mexico PQC.
“Most states now have a [MMRC] that collects maternal mortality and near-miss data. Those data are used by the action arm,” which is the PQC. “If the review committee says” opioid use disorder is a significant contributor “like in our state, the collaborative rolls out the opioid use disorder bundle,” she said.
Beginning next January, the Joint Commission, formerly known as the Joint Commission on Accreditation of Healthcare Organizations, will require that accredited hospitals enact key elements of the AIM bundles for both obstetric hemorrhage and severe hypertension. “Everyone’s [now] motivated to get on that bandwagon,” Dr. Espey said.
“The bundles are here to stay,” and the Joint Commission requirements are “a really important step for sustainability and basic implementation. We really want to get them adopted everywhere,” said Dr. Main, who is also the national implementation director for the AIM initiative.
“The key thing is to work on implementing the hemorrhage and hypertension bundles in your hospital. I would suggest contacting [your] state” PQC, he said.
The California model
California, which has been working to reduce maternal mortality and morbidity since the mid 2000s, has produced among the strongest evidence to date that the efforts make a difference.
By 2013, the state had halved its maternal mortality rate to a 3-year average of 7 deaths per 100,000 live births, which is comparable with the average Western Europe rate of 7.2 deaths. Nationwide, the rate was about 17.4 deaths per 100,000 live births in 2018, according to the Centers for Disease Control and Prevention
The reasons are multifactorial, but “we think” the quality improvement efforts have been “an important contributor,” Dr. Main said.
Improvements especially for Black women
Among the success stories has been California’s implementation of the AIM obstetric hemorrhage bundle about 5 years ago. Among other steps, the 17 evidence-based recommendations included early recognition, immediate access to oxytocin and other medications, immediate access to a hemorrhage cart with instructions for intrauterine balloons and compression stitches, the establishment of a hemorrhage response protocol and team, and regular unit-based drills with debriefing sessions afterward.
Mentoring teams consisting of a physician and nurse with maternal quality improvement experience were created to help hospitals come on board, with each team working with five to eight hospitals. Efforts included monthly telephone calls and face-to-face meetings, and providers were held accountable for progress. Hospitals shared data and tips on implementation, under the aegis of the CMQCC.
When the baseline period of 2011-2014 to the postintervention period of October 2015 to December 2016 were compared, the rate of severe maternal morbidity from hemorrhage fell from 22.1% to 18.5% across 99 hospitals and 73,476 women.
The benefit among Black women exceeded that among White women, with a 9% absolute rate reduction versus 2.1%. “If you adjusted for risk factors, [we found] you could eliminate [racial differences] completely,” which is something that hadn’t been shown before. “This is a really big deal,” Dr. Main said, because the risk of maternal morbidity and mortality is three to four times higher among Black women, compared with White women.
Dr. Main and his team found that the biggest clinical risk factor that accounted for racial differences was a higher rate of cesarean deliveries among Black women, followed by higher rates of anemia at hospital admission. “If you have a C-section when you are anemic, you are going to have a transfusion,” he explained.
More recently, there’s been a push in California to reduce the rate of primary cesarean deliveries by enacting the associated AIM bundle with use of the same approach as with the hemorrhage bundle. Dr. Main and his team recently reported a rate reduction from 29.3% to 25% without compromising birth outcomes.
However, “some changes are easier than others. Hemorrhage was an easy one to change because it didn’t deal with physician autonomy as much, and you saw more immediate results” with fewer hemorrhages. Reducing cesarean delivery rates is “a bigger lift” because “it’s really changing the culture of labor and delivery. It involves more group pressure and more reinforcing, but we were able to do that,” he said.
Problems in the Show Me State
“We’ve patterned a lot” of what’s being done in New Mexico “after California,” Dr. Espey said.
The AIM hemorrhage bundle, for instance, is being rolled out to New Mexico hospitals, with the help of virtual meetings and mentoring programs, plus outreach to the Navajo and others reservations because, as with Black women, rates of maternal morbidity and mortality are higher among Native American women.
It’s been tougher going, however, in states such as Missouri, which recently ranked 44th in the country for maternal mortality.
“We started a little bit late, and we are a little bit behind,” said ob.gyn. Karen L. Florio, DO, at the University of Missouri–Kansas City and also a leader of the state MMRC and member of its PQC.
The main problem is money. California’s efforts are funded by the Centers for Disease Control and Prevention, the state health department, and hospitals, among others.
But Missouri is “not as well funded as California for our mortality review board, and our [PQC] is mostly not funded. If we could get that funding, we would have more resources to implement these AIM bundles,” she said.
In addition to the issue, Missouri didn’t expand Medicaid under the Accountable Care Act – something that’s been linked to reduced maternal morbidity and mortality – and there are entire rural areas with no maternity care. Plus after generations of mistreatment, “our African American population has a valid distrust of the medical system that contributes to maternal mortality,” she said.
Obesity-related heart disease is also prevalent in Missouri, even among young people. “I cannot tell you how many women I have had who have had a heart attack at the age of 30 and who have had stents placed,” Dr. Florio said.
Dr. Florio and her colleagues are currently using teleconferences and other means to roll out the AIM hypertension bundle but can do so only selectively. “We don’t have the resources to reach every single rural hospital all over the state,” she said; they are working to address the funding issues.
For rural hospitals, implementation is “daunting”
Meanwhile, rural hospitals have been a particular concern in South Dakota, said Kimberlee McKay, MD, an ob.gyn. who is the clinical vice president of the ob.gyn. service line at Avera Health, a hospital system based in Sioux Falls, S.D.
She’s been overseeing Avera’s implementation of the hypertension, hemorrhage, and venous thromboembolism bundles. “What’s hard is that” the AIM protocols come “out of academic centers. Implementation of complex algorithms is daunting” for hospitals that only do a couple hundred deliveries a year, she said.
For small hospitals, the approach she’s found that works is to first assess what they can offer, and then have them “do what’s reasonable” for their resources. The second part is making sure high-risk women get to a regional center – with an adequate blood supply, in the case of hemorrhage, for instance – for complications. Dr. McKay and colleagues are working on a system by which regional centers can monitor smaller hospitals for potential maternity problems, and contact them proactively before they emerge.
They’ve also made access to hemorrhage and hypertension drugs easier on labor and delivery units with the help of close-by dedicated medicine boxes, and standardized protocols and order sets across Avera. “We try to make the right thing the easy thing to do,” Dr. McKay said.
Dr. Espey is an editorial adviser for Ob.Gyn. News. The physicians have no relevant financial disclosures.
How to set up your hyperhidrosis patients for treatment success
When children and adolescents first present to George Hightower, MD, PhD, with suspected primary hyperhidrosis, he tries to gauge their level of impairment and distress.
“I ask my patients directly: ‘Does this get in the way of doing things you enjoy?’ ” Dr. Hightower said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. If they say yes, he then asks, “‘What are those things that it gets in the way of?’ Also, so that I can develop a rapport with them, I ask, ‘Is it causing you to view yourself negatively?’ I also ask them how they anticipate treatment is going to change that.”
Dr. Hightower, of the departments of dermatology and pediatrics, University of California, San Diego, and a pediatric dermatologist at Rady Children’s Hospital, defined focal primary hyperhidrosis as focal, visible, excessive sweating for at least 6 months without an apparent cause, plus at least two of the following characteristics: bilateral and relatively symmetric, sweating that impairs daily activities, onset before age 25, at least one episode per week, family history of idiopathic hyperhidrosis, and focal sweating that stops during sleep.
“Based on their prominence in the popular media, armpits relative to body surface area play an oversized role in our patients’ perception of well-being,” he said. “Most of all, patients’ concerns regarding their armpits include one more of the following symptoms: smelly, sweaty, red, and itchy or painful.”
Topical antiperspirants are the preferred initial treatment. “They’re widely available, inexpensive, and well-tolerated therapies,” Dr. Hightower said. Most commercially available antiperspirants contain low-dose aluminum or other metal that keeps the sweat gland ducts from opening.
“Most patients referred to me have failed to improve with over-the-counter antiperspirants or aluminum chloride 20%,” he said. “We start by reviewing the appropriate use of aluminum chloride 20%. If they’re using it appropriately and fail to achieve adequate control, I open the discussion to use glycopyrronium tosylate cloth 2.4%, applied daily. This can be cost prohibitive or not covered by insurance.” Other options include glycopyrrolate 1-6 mg daily and microwave-based procedural intervention.
In a post hoc analysis, researchers examined the efficacy and safety findings by age from two phase three randomized, controlled trials of glycopyrronium tosylate in pediatric primary axillary hyperhidrosis (Pediatr Dermatol. 2019 Jan-Feb;36[1]:89-99). It was well tolerated in the 19 patients aged 9-16 years. “No patients discontinued from the study in this age group [because of] symptomatology,” said Dr. Hightower, who was not involved with the study. “The concerns related to this medication are related to anticholinergic effects such as blurry vision and dry mouth, but overall, randomized clinical trial data support the benefit of this medication in helping patients improve the symptoms of hyperhidrosis.”
In an earlier study, researchers retrospectively studied children with hyperhidrosis who were treated with a mean dosage of 2 mg glycopyrronium tosylate daily (J Am Acad Dermatol 2012 Nov;67[5]:918-23). The average age of patients was 15 years. Most (90%) experienced some improvement and 71% of those who responded saw major improvement. This occurred within hours of administration and disappeared within a day of discontinuation. The two most common side effects were dry mouth (26%) and dry eyes (10%). More worrisome side effects were associated with higher dosing, including blurring of vision (3%) and sensation of palpitations (3%).
When patients return for their first follow-up appointment after starting a treatment plan, Dr. Hightower revisits their level of impairment and distress with hyperhidrosis. “I ask, ‘Remember that activity that you were doing before that this was getting in the way of? Are you doing that more? Do you feel like you can do that in a way that you weren’t able to do before, whether it’s playing an instrument or spending time with friends?’ ”
He also sets expectations with patients and their families with comments such as, “If this treatment does not work for you after 2 months, the next option I would consider is ...” and, “for most people there is no cure, but treatment is helpful.” He also emphasizes the importance of follow-up care, so they “come back to assess the next steps.”
Dr. Hightower reported having no financial disclosures.
When children and adolescents first present to George Hightower, MD, PhD, with suspected primary hyperhidrosis, he tries to gauge their level of impairment and distress.
“I ask my patients directly: ‘Does this get in the way of doing things you enjoy?’ ” Dr. Hightower said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. If they say yes, he then asks, “‘What are those things that it gets in the way of?’ Also, so that I can develop a rapport with them, I ask, ‘Is it causing you to view yourself negatively?’ I also ask them how they anticipate treatment is going to change that.”
Dr. Hightower, of the departments of dermatology and pediatrics, University of California, San Diego, and a pediatric dermatologist at Rady Children’s Hospital, defined focal primary hyperhidrosis as focal, visible, excessive sweating for at least 6 months without an apparent cause, plus at least two of the following characteristics: bilateral and relatively symmetric, sweating that impairs daily activities, onset before age 25, at least one episode per week, family history of idiopathic hyperhidrosis, and focal sweating that stops during sleep.
“Based on their prominence in the popular media, armpits relative to body surface area play an oversized role in our patients’ perception of well-being,” he said. “Most of all, patients’ concerns regarding their armpits include one more of the following symptoms: smelly, sweaty, red, and itchy or painful.”
Topical antiperspirants are the preferred initial treatment. “They’re widely available, inexpensive, and well-tolerated therapies,” Dr. Hightower said. Most commercially available antiperspirants contain low-dose aluminum or other metal that keeps the sweat gland ducts from opening.
“Most patients referred to me have failed to improve with over-the-counter antiperspirants or aluminum chloride 20%,” he said. “We start by reviewing the appropriate use of aluminum chloride 20%. If they’re using it appropriately and fail to achieve adequate control, I open the discussion to use glycopyrronium tosylate cloth 2.4%, applied daily. This can be cost prohibitive or not covered by insurance.” Other options include glycopyrrolate 1-6 mg daily and microwave-based procedural intervention.
In a post hoc analysis, researchers examined the efficacy and safety findings by age from two phase three randomized, controlled trials of glycopyrronium tosylate in pediatric primary axillary hyperhidrosis (Pediatr Dermatol. 2019 Jan-Feb;36[1]:89-99). It was well tolerated in the 19 patients aged 9-16 years. “No patients discontinued from the study in this age group [because of] symptomatology,” said Dr. Hightower, who was not involved with the study. “The concerns related to this medication are related to anticholinergic effects such as blurry vision and dry mouth, but overall, randomized clinical trial data support the benefit of this medication in helping patients improve the symptoms of hyperhidrosis.”
In an earlier study, researchers retrospectively studied children with hyperhidrosis who were treated with a mean dosage of 2 mg glycopyrronium tosylate daily (J Am Acad Dermatol 2012 Nov;67[5]:918-23). The average age of patients was 15 years. Most (90%) experienced some improvement and 71% of those who responded saw major improvement. This occurred within hours of administration and disappeared within a day of discontinuation. The two most common side effects were dry mouth (26%) and dry eyes (10%). More worrisome side effects were associated with higher dosing, including blurring of vision (3%) and sensation of palpitations (3%).
When patients return for their first follow-up appointment after starting a treatment plan, Dr. Hightower revisits their level of impairment and distress with hyperhidrosis. “I ask, ‘Remember that activity that you were doing before that this was getting in the way of? Are you doing that more? Do you feel like you can do that in a way that you weren’t able to do before, whether it’s playing an instrument or spending time with friends?’ ”
He also sets expectations with patients and their families with comments such as, “If this treatment does not work for you after 2 months, the next option I would consider is ...” and, “for most people there is no cure, but treatment is helpful.” He also emphasizes the importance of follow-up care, so they “come back to assess the next steps.”
Dr. Hightower reported having no financial disclosures.
When children and adolescents first present to George Hightower, MD, PhD, with suspected primary hyperhidrosis, he tries to gauge their level of impairment and distress.
“I ask my patients directly: ‘Does this get in the way of doing things you enjoy?’ ” Dr. Hightower said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. If they say yes, he then asks, “‘What are those things that it gets in the way of?’ Also, so that I can develop a rapport with them, I ask, ‘Is it causing you to view yourself negatively?’ I also ask them how they anticipate treatment is going to change that.”
Dr. Hightower, of the departments of dermatology and pediatrics, University of California, San Diego, and a pediatric dermatologist at Rady Children’s Hospital, defined focal primary hyperhidrosis as focal, visible, excessive sweating for at least 6 months without an apparent cause, plus at least two of the following characteristics: bilateral and relatively symmetric, sweating that impairs daily activities, onset before age 25, at least one episode per week, family history of idiopathic hyperhidrosis, and focal sweating that stops during sleep.
“Based on their prominence in the popular media, armpits relative to body surface area play an oversized role in our patients’ perception of well-being,” he said. “Most of all, patients’ concerns regarding their armpits include one more of the following symptoms: smelly, sweaty, red, and itchy or painful.”
Topical antiperspirants are the preferred initial treatment. “They’re widely available, inexpensive, and well-tolerated therapies,” Dr. Hightower said. Most commercially available antiperspirants contain low-dose aluminum or other metal that keeps the sweat gland ducts from opening.
“Most patients referred to me have failed to improve with over-the-counter antiperspirants or aluminum chloride 20%,” he said. “We start by reviewing the appropriate use of aluminum chloride 20%. If they’re using it appropriately and fail to achieve adequate control, I open the discussion to use glycopyrronium tosylate cloth 2.4%, applied daily. This can be cost prohibitive or not covered by insurance.” Other options include glycopyrrolate 1-6 mg daily and microwave-based procedural intervention.
In a post hoc analysis, researchers examined the efficacy and safety findings by age from two phase three randomized, controlled trials of glycopyrronium tosylate in pediatric primary axillary hyperhidrosis (Pediatr Dermatol. 2019 Jan-Feb;36[1]:89-99). It was well tolerated in the 19 patients aged 9-16 years. “No patients discontinued from the study in this age group [because of] symptomatology,” said Dr. Hightower, who was not involved with the study. “The concerns related to this medication are related to anticholinergic effects such as blurry vision and dry mouth, but overall, randomized clinical trial data support the benefit of this medication in helping patients improve the symptoms of hyperhidrosis.”
In an earlier study, researchers retrospectively studied children with hyperhidrosis who were treated with a mean dosage of 2 mg glycopyrronium tosylate daily (J Am Acad Dermatol 2012 Nov;67[5]:918-23). The average age of patients was 15 years. Most (90%) experienced some improvement and 71% of those who responded saw major improvement. This occurred within hours of administration and disappeared within a day of discontinuation. The two most common side effects were dry mouth (26%) and dry eyes (10%). More worrisome side effects were associated with higher dosing, including blurring of vision (3%) and sensation of palpitations (3%).
When patients return for their first follow-up appointment after starting a treatment plan, Dr. Hightower revisits their level of impairment and distress with hyperhidrosis. “I ask, ‘Remember that activity that you were doing before that this was getting in the way of? Are you doing that more? Do you feel like you can do that in a way that you weren’t able to do before, whether it’s playing an instrument or spending time with friends?’ ”
He also sets expectations with patients and their families with comments such as, “If this treatment does not work for you after 2 months, the next option I would consider is ...” and, “for most people there is no cure, but treatment is helpful.” He also emphasizes the importance of follow-up care, so they “come back to assess the next steps.”
Dr. Hightower reported having no financial disclosures.
FROM PEDIATRIC DERMATOLOGY 2020
NSAID continuation linked to less knee OA pain
in a randomized trial.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score was 6.7 out of a possible total of 20 for patients who continued meloxicam for 4 weeks versus 7.8 in those who stopped and switched to a placebo. The estimated mean difference in pain score was 1.4 (P = .92 for noninferiority), which is below the threshold of 2.1 that is considered to be the minimum clinically important difference.
Furthermore, patients who had switched to placebo and then subsequently participated in a telephone-based cognitive behavior therapy (CBT) program for another 10 weeks had higher pain levels compared with those who continued meloxicam. WOMAC scores were 12.1 and 11.8, respectively with a mean difference of 0.8 (P = .28 for noninferiority).
“Among patients with knee osteoarthritis, placebo and CBT (after placebo) are inferior to meloxicam,” Liana Fraenkel, MD, MPH, of Yale University, New Haven, Conn., and coinvestigators concluded in their article, published in JAMA Internal Medicine.
They observed that the WOMAC pain score differences between the two groups were small, however, and that there were no statistically significant differences in participants’ global impression of change or function after 14 weeks.
“Although the overall results of the trial are negative, they provide clinicians with data to support shared decision-making and reassure patients willing to taper NSAIDs and consider self-management approaches such as CBT,” Dr. Fraenkel and coauthors suggested.
The Stopping NSAIDs for Arthritis Pain trial had ultimately included 364 participants, 86% of whom were men, recruited from four veterans affairs health care systems. All had been taking NSAIDs for knee OA pain for at least 3 months and had participated in a 2-week run-in period where the NSAID they had been taking was switched to meloxicam, 15 mg once daily.
The aim of the trial had been to see if discontinuing NSAIDs and starting a CBT program would be noninferior to continuing NSAIDs in patients with knee OA.
The trial does not provide robust information on the use of CBT, David Walsh, a rheumatologist and director of the Pain Centre Versus Arthritis at the University of Nottingham, England, said in an interview.
“It can’t tell you about efficacy of CBT,” Dr. Walsh said as the CBT part of the study was not randomized, was not controlled, and was unblinded. ”It would be a different task to design a CBT trial aiming to help people to stop taking tablets,” he added.
Dr. Fraenkel and coinvestigators had reported that, at week 14, the adjusted mean difference in WOMAC pain score between the placebo (followed by CBT) and meloxicam groups was 0.8 (P = .28 for noninferiority).
“What the trial’s really doing is seeing whether people who’ve been on long-term nonsteroidals, can they just stop them without getting any worse? The conclusion for that is actually they are more likely to get worse than not if you just stop the nonsteroidals,” Dr. Walsh said.
“The withdrawal trial protocol is an important one. You can’t run a prospective trial for years to see whether something works for years. It is just not feasible. So actually, the protocol they’ve got of switching to placebo, or continuing with a nonsteroidal, is probably the best way of working out if an anti-inflammatory still has a pharmacological effect after actually being on it for X years,” Dr. Walsh said.
Dr. Walsh, who was not involved in the trial, observed that while the difference in pain scores between the groups was small, the deterioration in scores might be important for individual patients. Some may do worse, although granted that there may be some that might do better, he said.
“It is suggesting to me that nonsteroidals are still working in people who are on long-term treatment. It is not a very big pharmacological effect, but we already know from the RCTs of anti-inflammatory tablets, that they can be beneficial,” Dr. Walsh noted.
He also pointed out that patients’ pain had been improved after being switched from their current NSAID to meloxicam – the overall WOMAC pain score at recruitment was 9.6 and was 5.6 after the 2-week meloxicam run-in phase.
“Now, whether that’s because they’ve been switched to meloxicam, or whether it’s because they’re in a trial,” is an important question, Dr. Walsh suggested, adding that “it looks as though it’s more likely to be because they’re in a trial, because improvement was maintained during the following 4 weeks on placebo.”
Another point he made was that there was a higher percentage of patients in the placebo group that started taking other types of painkillers, just under half (46%) used acetaminophen versus a quarter (26%) of those who continued using meloxicam.
It is an interesting trial, “trying to tackle some really difficult questions and I think that there are really important implications from it that we can build on, but is it actually going to change the lives of patients at the moment? Not massively,” Dr. Walsh said, ”but it’s another step in the right direction.”
Dr. Fraenkel disclosed receiving research funding from the VA Office of Research and Development, the sponsor of the trial.
SOURCE: Fraenkel L et al. JAMA Intern Med. 2020 Jul 20. doi:10.1001/jamainternmed.2020.2821.
in a randomized trial.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score was 6.7 out of a possible total of 20 for patients who continued meloxicam for 4 weeks versus 7.8 in those who stopped and switched to a placebo. The estimated mean difference in pain score was 1.4 (P = .92 for noninferiority), which is below the threshold of 2.1 that is considered to be the minimum clinically important difference.
Furthermore, patients who had switched to placebo and then subsequently participated in a telephone-based cognitive behavior therapy (CBT) program for another 10 weeks had higher pain levels compared with those who continued meloxicam. WOMAC scores were 12.1 and 11.8, respectively with a mean difference of 0.8 (P = .28 for noninferiority).
“Among patients with knee osteoarthritis, placebo and CBT (after placebo) are inferior to meloxicam,” Liana Fraenkel, MD, MPH, of Yale University, New Haven, Conn., and coinvestigators concluded in their article, published in JAMA Internal Medicine.
They observed that the WOMAC pain score differences between the two groups were small, however, and that there were no statistically significant differences in participants’ global impression of change or function after 14 weeks.
“Although the overall results of the trial are negative, they provide clinicians with data to support shared decision-making and reassure patients willing to taper NSAIDs and consider self-management approaches such as CBT,” Dr. Fraenkel and coauthors suggested.
The Stopping NSAIDs for Arthritis Pain trial had ultimately included 364 participants, 86% of whom were men, recruited from four veterans affairs health care systems. All had been taking NSAIDs for knee OA pain for at least 3 months and had participated in a 2-week run-in period where the NSAID they had been taking was switched to meloxicam, 15 mg once daily.
The aim of the trial had been to see if discontinuing NSAIDs and starting a CBT program would be noninferior to continuing NSAIDs in patients with knee OA.
The trial does not provide robust information on the use of CBT, David Walsh, a rheumatologist and director of the Pain Centre Versus Arthritis at the University of Nottingham, England, said in an interview.
“It can’t tell you about efficacy of CBT,” Dr. Walsh said as the CBT part of the study was not randomized, was not controlled, and was unblinded. ”It would be a different task to design a CBT trial aiming to help people to stop taking tablets,” he added.
Dr. Fraenkel and coinvestigators had reported that, at week 14, the adjusted mean difference in WOMAC pain score between the placebo (followed by CBT) and meloxicam groups was 0.8 (P = .28 for noninferiority).
“What the trial’s really doing is seeing whether people who’ve been on long-term nonsteroidals, can they just stop them without getting any worse? The conclusion for that is actually they are more likely to get worse than not if you just stop the nonsteroidals,” Dr. Walsh said.
“The withdrawal trial protocol is an important one. You can’t run a prospective trial for years to see whether something works for years. It is just not feasible. So actually, the protocol they’ve got of switching to placebo, or continuing with a nonsteroidal, is probably the best way of working out if an anti-inflammatory still has a pharmacological effect after actually being on it for X years,” Dr. Walsh said.
Dr. Walsh, who was not involved in the trial, observed that while the difference in pain scores between the groups was small, the deterioration in scores might be important for individual patients. Some may do worse, although granted that there may be some that might do better, he said.
“It is suggesting to me that nonsteroidals are still working in people who are on long-term treatment. It is not a very big pharmacological effect, but we already know from the RCTs of anti-inflammatory tablets, that they can be beneficial,” Dr. Walsh noted.
He also pointed out that patients’ pain had been improved after being switched from their current NSAID to meloxicam – the overall WOMAC pain score at recruitment was 9.6 and was 5.6 after the 2-week meloxicam run-in phase.
“Now, whether that’s because they’ve been switched to meloxicam, or whether it’s because they’re in a trial,” is an important question, Dr. Walsh suggested, adding that “it looks as though it’s more likely to be because they’re in a trial, because improvement was maintained during the following 4 weeks on placebo.”
Another point he made was that there was a higher percentage of patients in the placebo group that started taking other types of painkillers, just under half (46%) used acetaminophen versus a quarter (26%) of those who continued using meloxicam.
It is an interesting trial, “trying to tackle some really difficult questions and I think that there are really important implications from it that we can build on, but is it actually going to change the lives of patients at the moment? Not massively,” Dr. Walsh said, ”but it’s another step in the right direction.”
Dr. Fraenkel disclosed receiving research funding from the VA Office of Research and Development, the sponsor of the trial.
SOURCE: Fraenkel L et al. JAMA Intern Med. 2020 Jul 20. doi:10.1001/jamainternmed.2020.2821.
in a randomized trial.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score was 6.7 out of a possible total of 20 for patients who continued meloxicam for 4 weeks versus 7.8 in those who stopped and switched to a placebo. The estimated mean difference in pain score was 1.4 (P = .92 for noninferiority), which is below the threshold of 2.1 that is considered to be the minimum clinically important difference.
Furthermore, patients who had switched to placebo and then subsequently participated in a telephone-based cognitive behavior therapy (CBT) program for another 10 weeks had higher pain levels compared with those who continued meloxicam. WOMAC scores were 12.1 and 11.8, respectively with a mean difference of 0.8 (P = .28 for noninferiority).
“Among patients with knee osteoarthritis, placebo and CBT (after placebo) are inferior to meloxicam,” Liana Fraenkel, MD, MPH, of Yale University, New Haven, Conn., and coinvestigators concluded in their article, published in JAMA Internal Medicine.
They observed that the WOMAC pain score differences between the two groups were small, however, and that there were no statistically significant differences in participants’ global impression of change or function after 14 weeks.
“Although the overall results of the trial are negative, they provide clinicians with data to support shared decision-making and reassure patients willing to taper NSAIDs and consider self-management approaches such as CBT,” Dr. Fraenkel and coauthors suggested.
The Stopping NSAIDs for Arthritis Pain trial had ultimately included 364 participants, 86% of whom were men, recruited from four veterans affairs health care systems. All had been taking NSAIDs for knee OA pain for at least 3 months and had participated in a 2-week run-in period where the NSAID they had been taking was switched to meloxicam, 15 mg once daily.
The aim of the trial had been to see if discontinuing NSAIDs and starting a CBT program would be noninferior to continuing NSAIDs in patients with knee OA.
The trial does not provide robust information on the use of CBT, David Walsh, a rheumatologist and director of the Pain Centre Versus Arthritis at the University of Nottingham, England, said in an interview.
“It can’t tell you about efficacy of CBT,” Dr. Walsh said as the CBT part of the study was not randomized, was not controlled, and was unblinded. ”It would be a different task to design a CBT trial aiming to help people to stop taking tablets,” he added.
Dr. Fraenkel and coinvestigators had reported that, at week 14, the adjusted mean difference in WOMAC pain score between the placebo (followed by CBT) and meloxicam groups was 0.8 (P = .28 for noninferiority).
“What the trial’s really doing is seeing whether people who’ve been on long-term nonsteroidals, can they just stop them without getting any worse? The conclusion for that is actually they are more likely to get worse than not if you just stop the nonsteroidals,” Dr. Walsh said.
“The withdrawal trial protocol is an important one. You can’t run a prospective trial for years to see whether something works for years. It is just not feasible. So actually, the protocol they’ve got of switching to placebo, or continuing with a nonsteroidal, is probably the best way of working out if an anti-inflammatory still has a pharmacological effect after actually being on it for X years,” Dr. Walsh said.
Dr. Walsh, who was not involved in the trial, observed that while the difference in pain scores between the groups was small, the deterioration in scores might be important for individual patients. Some may do worse, although granted that there may be some that might do better, he said.
“It is suggesting to me that nonsteroidals are still working in people who are on long-term treatment. It is not a very big pharmacological effect, but we already know from the RCTs of anti-inflammatory tablets, that they can be beneficial,” Dr. Walsh noted.
He also pointed out that patients’ pain had been improved after being switched from their current NSAID to meloxicam – the overall WOMAC pain score at recruitment was 9.6 and was 5.6 after the 2-week meloxicam run-in phase.
“Now, whether that’s because they’ve been switched to meloxicam, or whether it’s because they’re in a trial,” is an important question, Dr. Walsh suggested, adding that “it looks as though it’s more likely to be because they’re in a trial, because improvement was maintained during the following 4 weeks on placebo.”
Another point he made was that there was a higher percentage of patients in the placebo group that started taking other types of painkillers, just under half (46%) used acetaminophen versus a quarter (26%) of those who continued using meloxicam.
It is an interesting trial, “trying to tackle some really difficult questions and I think that there are really important implications from it that we can build on, but is it actually going to change the lives of patients at the moment? Not massively,” Dr. Walsh said, ”but it’s another step in the right direction.”
Dr. Fraenkel disclosed receiving research funding from the VA Office of Research and Development, the sponsor of the trial.
SOURCE: Fraenkel L et al. JAMA Intern Med. 2020 Jul 20. doi:10.1001/jamainternmed.2020.2821.
FROM JAMA INTERNAL MEDICINE
Tendyne transcatheter mitral valve shows sustained benefits at 2 years
Two-year outcomes following transcatheter mitral valve replacement with the Tendyne prosthesis showed durable control of mitral regurgitation, sustained improvement in quality of life and functional capacity, and a marked drop-off in the rate of hospitalization for heart failure, David W.M. Muller, MBBS, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The outcomes can be considered very acceptable considering the advanced age and underlying comorbidities. The data show that, at 2 years, we still have excellent control of the MR [mitral regurgitation], with more than 93% of patients having no MR at all, and there were no patients with 1+ or greater MR,” said Dr. Muller, director of cardiac catheterization laboratories at St. Vincent’s Hospital in Sydney.
He presented for the first time the 2-year results for the first 100 patients enrolled in the long-term Tendyne Expanded Clinical Study. The 1-year outcomes were reported previously (J Am Coll Cardiol. 2019 Mar 26;73[11]:1250-60).
The transcatheter Tendyne mitral valve system received European marketing approval in early 2020 for commercial use in patients with severe symptomatic MR who aren’t candidates for open surgery or transcatheter mitral repair using the MitraClip device. The Tendyne remains investigational in the United States, where a large phase 3 randomized head-to-head trial of the Tendyne device and the FDA-approved MitraClip is ongoing.
At enrollment, the first 100 patients in the long-term prospective Tendyne study averaged 75 years of age, 66% were New York Heart Association functional class III or IV, 89% had secondary or mixed etiology MR, and 39% had been hospitalized for heart failure during the preceding 6 months. Ninety-two percent of participants had 4+ MR before implantation. The group’s average Society of Thoracic Surgeons Predicted Risk of Mortality score was 7.8%.
The all-cause mortality rate was 27% at 1 year and 39% at 2 years, with 87% of deaths being attributable to cardiovascular causes. At 2 years, 82% of survivors were NYHA class I or II. Their Kansas City Cardiomyopathy Questionnaire score improved by 19.1 points from an average of 49 at baseline. There was no evidence of structural valve dysfunction. Their heart failure hospitalization rate improved from 1.3 events per patient per year to 0.6 per patient-year at 1 year and 0.51 at 2 years.
Discussant Francesco Maisano, MD, was favorably impressed by the acute outcomes of transcatheter mitral valve replacement with the Tendyne device.
“There were very few procedural or in-hospital complications. Success was obtained in almost every patient. There was no procedural mortality. The 30-day mortality of 6.0% was reasonable in patients with a baseline STS score of 7.8%; that’s pretty good, I would say,” observed Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
However, he voiced concerns about the 35% incidence of major bleeding and 5% rate of disabling stroke at 2 years, which he deemed “pretty high.”
“This underlies the open issue of anticoagulation following these kinds of procedures. This obviously requires further work in the next years,” he said.
Dr. Muller reported receiving research grants from and serving as a consultant to Abbott, which markets the MitraClip and is developing the Tendyne system. He also serves as a consultant to Edwards Lifesciences and Medtronic. Dr. Maisano also reported serving as a consultant to several medical device companies.
Two-year outcomes following transcatheter mitral valve replacement with the Tendyne prosthesis showed durable control of mitral regurgitation, sustained improvement in quality of life and functional capacity, and a marked drop-off in the rate of hospitalization for heart failure, David W.M. Muller, MBBS, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The outcomes can be considered very acceptable considering the advanced age and underlying comorbidities. The data show that, at 2 years, we still have excellent control of the MR [mitral regurgitation], with more than 93% of patients having no MR at all, and there were no patients with 1+ or greater MR,” said Dr. Muller, director of cardiac catheterization laboratories at St. Vincent’s Hospital in Sydney.
He presented for the first time the 2-year results for the first 100 patients enrolled in the long-term Tendyne Expanded Clinical Study. The 1-year outcomes were reported previously (J Am Coll Cardiol. 2019 Mar 26;73[11]:1250-60).
The transcatheter Tendyne mitral valve system received European marketing approval in early 2020 for commercial use in patients with severe symptomatic MR who aren’t candidates for open surgery or transcatheter mitral repair using the MitraClip device. The Tendyne remains investigational in the United States, where a large phase 3 randomized head-to-head trial of the Tendyne device and the FDA-approved MitraClip is ongoing.
At enrollment, the first 100 patients in the long-term prospective Tendyne study averaged 75 years of age, 66% were New York Heart Association functional class III or IV, 89% had secondary or mixed etiology MR, and 39% had been hospitalized for heart failure during the preceding 6 months. Ninety-two percent of participants had 4+ MR before implantation. The group’s average Society of Thoracic Surgeons Predicted Risk of Mortality score was 7.8%.
The all-cause mortality rate was 27% at 1 year and 39% at 2 years, with 87% of deaths being attributable to cardiovascular causes. At 2 years, 82% of survivors were NYHA class I or II. Their Kansas City Cardiomyopathy Questionnaire score improved by 19.1 points from an average of 49 at baseline. There was no evidence of structural valve dysfunction. Their heart failure hospitalization rate improved from 1.3 events per patient per year to 0.6 per patient-year at 1 year and 0.51 at 2 years.
Discussant Francesco Maisano, MD, was favorably impressed by the acute outcomes of transcatheter mitral valve replacement with the Tendyne device.
“There were very few procedural or in-hospital complications. Success was obtained in almost every patient. There was no procedural mortality. The 30-day mortality of 6.0% was reasonable in patients with a baseline STS score of 7.8%; that’s pretty good, I would say,” observed Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
However, he voiced concerns about the 35% incidence of major bleeding and 5% rate of disabling stroke at 2 years, which he deemed “pretty high.”
“This underlies the open issue of anticoagulation following these kinds of procedures. This obviously requires further work in the next years,” he said.
Dr. Muller reported receiving research grants from and serving as a consultant to Abbott, which markets the MitraClip and is developing the Tendyne system. He also serves as a consultant to Edwards Lifesciences and Medtronic. Dr. Maisano also reported serving as a consultant to several medical device companies.
Two-year outcomes following transcatheter mitral valve replacement with the Tendyne prosthesis showed durable control of mitral regurgitation, sustained improvement in quality of life and functional capacity, and a marked drop-off in the rate of hospitalization for heart failure, David W.M. Muller, MBBS, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The outcomes can be considered very acceptable considering the advanced age and underlying comorbidities. The data show that, at 2 years, we still have excellent control of the MR [mitral regurgitation], with more than 93% of patients having no MR at all, and there were no patients with 1+ or greater MR,” said Dr. Muller, director of cardiac catheterization laboratories at St. Vincent’s Hospital in Sydney.
He presented for the first time the 2-year results for the first 100 patients enrolled in the long-term Tendyne Expanded Clinical Study. The 1-year outcomes were reported previously (J Am Coll Cardiol. 2019 Mar 26;73[11]:1250-60).
The transcatheter Tendyne mitral valve system received European marketing approval in early 2020 for commercial use in patients with severe symptomatic MR who aren’t candidates for open surgery or transcatheter mitral repair using the MitraClip device. The Tendyne remains investigational in the United States, where a large phase 3 randomized head-to-head trial of the Tendyne device and the FDA-approved MitraClip is ongoing.
At enrollment, the first 100 patients in the long-term prospective Tendyne study averaged 75 years of age, 66% were New York Heart Association functional class III or IV, 89% had secondary or mixed etiology MR, and 39% had been hospitalized for heart failure during the preceding 6 months. Ninety-two percent of participants had 4+ MR before implantation. The group’s average Society of Thoracic Surgeons Predicted Risk of Mortality score was 7.8%.
The all-cause mortality rate was 27% at 1 year and 39% at 2 years, with 87% of deaths being attributable to cardiovascular causes. At 2 years, 82% of survivors were NYHA class I or II. Their Kansas City Cardiomyopathy Questionnaire score improved by 19.1 points from an average of 49 at baseline. There was no evidence of structural valve dysfunction. Their heart failure hospitalization rate improved from 1.3 events per patient per year to 0.6 per patient-year at 1 year and 0.51 at 2 years.
Discussant Francesco Maisano, MD, was favorably impressed by the acute outcomes of transcatheter mitral valve replacement with the Tendyne device.
“There were very few procedural or in-hospital complications. Success was obtained in almost every patient. There was no procedural mortality. The 30-day mortality of 6.0% was reasonable in patients with a baseline STS score of 7.8%; that’s pretty good, I would say,” observed Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
However, he voiced concerns about the 35% incidence of major bleeding and 5% rate of disabling stroke at 2 years, which he deemed “pretty high.”
“This underlies the open issue of anticoagulation following these kinds of procedures. This obviously requires further work in the next years,” he said.
Dr. Muller reported receiving research grants from and serving as a consultant to Abbott, which markets the MitraClip and is developing the Tendyne system. He also serves as a consultant to Edwards Lifesciences and Medtronic. Dr. Maisano also reported serving as a consultant to several medical device companies.
FROM EUROPCR 2020
The Role of Process Improvements in Reducing Heart Failure Readmissions
From the Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Objective: To review selected process-of-care interventions that can be applied both during the hospitalization and during the transitional care period to help address the persistent challenge of heart failure readmissions.
Methods: Review of the literature.
Results: Process-of-care interventions that can be implemented to reduce readmissions of heart failure patients include: accurately identifying heart failure patients; providing disease education; titrating guideline-directed medical therapy; ensuring discharge readiness; arranging close discharge follow-up; identifying and addressing social barriers; following up by telephone; using home health; and addressing comorbidities. Importantly, the heart failure hospitalization is an opportunity to set up outpatient success, and setting up feedback loops can aid in post-discharge monitoring.
Conclusion: We encourage teams to consider local capabilities when selecting processes to improve; begin by improving something small to build capacity and team morale, and continually iterate and reexamine processes, as health care systems are continually evolving.
Keywords: heart failure; process improvement; quality improvement; readmission; rehospitalization; transitional care.
The growing population of patients affected by heart failure continues to challenge health systems. The increasing prevalence is paralleled by the rising costs of managing heart failure, which are projected to grow from $30.7 billion in 2012 to $69.8 billion in 2030.1 A significant portion of these costs relate to readmission after an index heart failure hospitalization. The statistics are staggering: for patients hospitalized with heart failure, approximately 15% to 20% are readmitted within 30 days.2,3 Though recent temporal trends suggest a modest reduction in readmission rates, there is a concerning correlation with increasing mortality,3 and a recognition that readmission rate decreases may relate to subtle changes in coding-based risk adjustment.4 Despite these concerns, efforts to reduce readmissions after heart failure hospitalization command significant attention.
Process improvement methodologies may be helpful in reducing hospital readmissions. Various approaches have been employed, and results have been mixed. An analysis of 70 participating hospitals in the American Heart Association’s Get With the Guidelines initiative found that, while overall readmission rates declined by 1.0% over 3 years, only 1 hospital achieved a 20% reduction in readmission rates.5
It is notably difficult to reduce readmissions after heart failure hospitalization. One challenge is that patients with heart failure often have multiple comorbidities, and approximately 50% to 60% of 30-day readmissions after heart failure hospitalization arise from noncardiac causes.1 Another challenge is that a significant fraction of readmissions in general—perhaps 75%—may not be avoidable.6
Recent excellent systematic reviews and meta-analyses provide comprehensive overviews of process improvement strategies that can be used to reduce readmissions after heart failure hospitalizations.7-9 Yet despite this extensive knowledge, few reports discuss the process of actually implementing these changes: the process of process improvement. Here, we seek to not only highlight some of the most promising potential interventions to reduce heart failure readmissions, but also to discuss a process improvement framework to help engender success, using our experience as a case study. We schematize process improvement efforts as having several distinct phases (Figure 1): processes delivered during the hospitalization and prior to discharge; feedback loops set up to maintain clinical stability at home; and the postdischarge clinic visit as an opportunity to further stabilize the patient and advance the plan of care. The discussion of these interventions follows this organization.
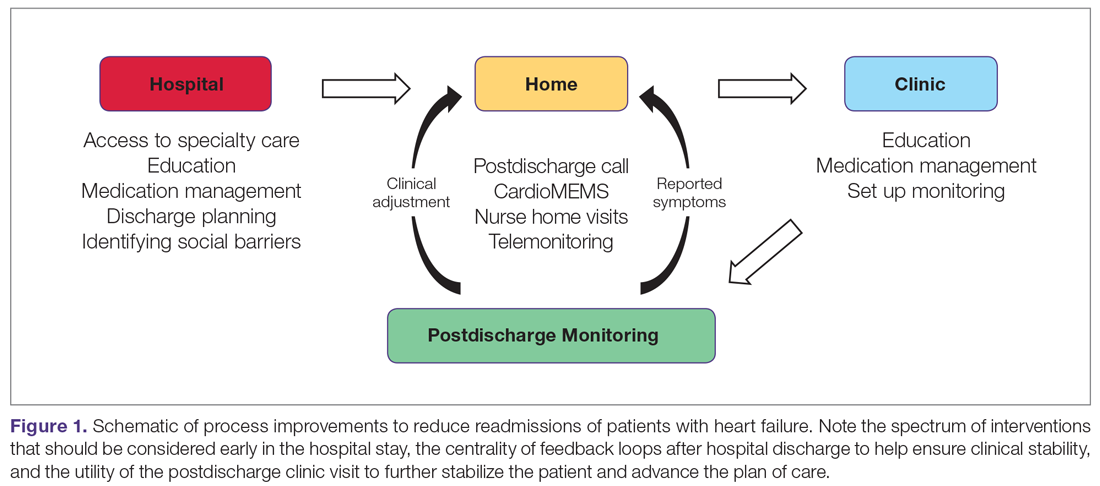
During Hospitalization
The heart failure hospitalization can be used as an opportunity to set up outpatient success, with several goals to target during the index admission. One goal is identifying the root causes of the heart failure syndrome and correcting those root causes, if possible. For example, patients in whom the heart failure syndrome is secondary to valvular heart disease may benefit from transcatheter aortic valve replacement.10 Another clinical goal is decongesting the patient, which is associated with lower readmission rates.11,12 These goals focus on the medical aspects of heart failure care. However, beyond these medical aspects, a patient must be equipped to successfully manage the disease at home.
To support medical and nonmedical interventions for hospitalized heart failure patients, a critical first step is identifying patients with heart failure. This accomplishes at least 2 objectives. First, early identification allows early initiation of interventions, such as heart failure education and social work evaluation. Early initiation of these interventions allows sufficient time during the hospitalization to make meaningful progress on these fronts. Second, early identification allows an opportunity for the delivery of cardiology specialty care, which may help with identifying and correcting root causes of the heart failure syndrome. Such access to cardiology has been shown to improve inpatient mortality and readmission rates.13
In smaller hospitals, identification of patients with heart failure can be as simple as reviewing overnight admissions. More advanced strategies, such as screeners based on brain natriuretic peptide (BNP) levels and administration of intravenous diuretics, can be employed.14,15 In the near future, deep learning-based natural language processing will be applied to mine full-text data in the electronic health record to identify heart failure hospitalizations.16
In the hospital, patients can also receive education about heart failure disease management. This education is a cornerstone of reducing heart failure readmissions. A recent systematic review of nurse education interventions demonstrated reductions in readmissions, hospitalizations, and costs.17 However, the efficacy of heart failure education hinges on many other variables. For patients to adhere to water restriction and daily weights, for example, there must also be patient understanding, compliance, and accessibility to providers to recommend how to strike the fluid balance. Education is therefore necessary, but not sufficient, for setting up outpatient success.
The hospitalization also represents an important time to start or uptitrate guideline-directed medical therapy (GDMT) for heart failure. Doing so takes advantage of an important opportunity to reduce the risk of readmission and even reverse the disease process.18 Uptitration of GDMT in patients with heart failure with reduced ejection fraction is associated with a decreased risk of mortality, while discontinuation is associated with an increased risk of mortality.19 However, recent registry data indicate that intensity of GDMT is just as likely to be decreased as increased during the hospitalization.20 Nevertheless, predischarge initiation of medications may be associated with higher attained doses in follow-up.21
Preparing for Discharge
Preparing a patient for discharge after a heart failure hospitalization involves stabilizing the medical condition as well as ensuring that the patient and caregivers have the medication, equipment, and self-care resources at home necessary to manage the condition. Several frameworks have been put forth to help care teams analyze a patient’s readiness for discharge. One is the B-PREPARED score,22 a validated instrument to discriminate among patients with regard to their readiness to discharge from the hospital. This instrument highlights the importance of several key factors that should be addressed during the discharge process, including counseling and written instructions about medications and their side effects; information about equipment needs and community resources; and information on activity levels and restrictions. Nurse education and discharge coordination can improve patients’ perception of discharge readiness,23 although whether this discharge readiness translates into improved readmission rates appears to depend on the specific follow-up intervention design.9
Prior to discharge, it is important to arrange postdischarge follow-up appointments, as emphasized by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 The use of nurse navigators can help with planning follow-up appointments. For example, the ACC Patient Navigator Program was applied in a single-center study of 120 patients randomized to the program versus usual care.25 This study found a significant increase in patient education and follow-up appointments compared to usual care, and a numerical decrease in hospital readmissions, although the finding was not statistically significant.25
A third critical component of preparing for discharge is identifying and addressing social barriers to care. In a study of patients stratified by household income, patients in the lowest income quartile had a higher readmission rate than patients in the highest income quartile.26 Poverty also correlates with heart failure mortality.27 Social factors play an important role in many aspects of patients’ ability to manage their health, including self-care, medication adherence, and ability to follow-up. Identifying these social factors prior to discharge is the first step to addressing them. While few studies specifically address the role of social workers in the management of heart failure care, the general medical literature suggests that social workers embedded in transitional care teams can augment readmission reduction efforts.28
After Discharge
Patients recently discharged from the hospital who have not yet attended their postdischarge appointment are in an incredibly vulnerable phase of care. Patients who are discharged from the hospital may not yet be connected with outpatient care. During this initial transitional care period, feedback loops involving patient communication back to the clinic, and clinic communication back to the patient, are critical to helping patients remain stable. For example, consider monitoring weights daily after hospital discharge. A patient at home can report increasing weights to a provider, who can then recommend an increased dose of diuretic. The patient can complete the feedback loop by taking the extra medication and monitoring the return of weight back to normal.
While daily weight monitoring is a simple process improvement that relies on the principle of establishing feedback loops, many other strategies exist. One commonly employed tool is the postdischarge telephone follow-up call, which is often coupled with other interventions in a comprehensive care bundle.8 During the telephone call, several process-of-care defects can be corrected, including missing medications or missing information on appointment times.
Beyond the telephone, newer technologies show promise for helping develop feedback loops for patients at home. One such technology is telemonitoring, whereby physiologic information such as weight, heart rate, and blood pressure is collected and sent back to a monitoring center. While the principle holds promise, several studies have not demonstrated significantly different outcomes as compared to usual care.13,29 Another promising technology is the CardioMEMS device (Abbott, Inc., Atlanta, GA), which can remotely transmit the pulmonary artery pressure, a physiologic signal which correlates with volume overload. There is now strong evidence supporting the efficacy of pulmonary artery pressure–guided heart failure management.30,31
Finally, home visits can be an efficient way to communicate symptoms, enable clinical assessment, and provide recommendations. One program that implemented home visits, 24-hour nurses available by call, and telephone follow-up showed a statistically significant reduction in readmissions.32 Furthermore, a meta-analysis of randomized controlled trials comparing home health to usual care showed decreased readmissions and mortality.33 The efficacy may be in strengthening the feedback loop—home care improves compliance with weight monitoring, fluid restriction, and medications.34 These studies provide a strong rationale for the benefits of home health in stabilizing heart failure patients postdischarge. Indeed, nurse home visits were 1 of the 2 process interventions in a Cochrane review of randomized controlled trials that were shown to statistically significantly decrease readmissions and mortality.9 These data underscore the importance of feedback loops for helping ensure patients are clinically stable.
Postdischarge Follow-Up Clinic Visit
The first clinic appointment postdischarge is an important check-in to help advance patient care. Several key tasks can be achieved during the postdischarge visit. First, the patient can be clinically stabilized by adjusting diuretic therapy. If the patient is clinically stable, GDMT can be uptitrated. Second, education around symptoms, medications, diet, and exercise can be reinforced. Finally, clinicians can help connect patients to other members of the multidisciplinary care team, including specialist care, home health, or cardiac rehabilitation.
Achieving 7-day follow-up visits after discharge has been a point of emphasis in national guidelines.24 The ACC promotes a “See You in 7” challenge, advising that all patients discharged with a diagnosis of heart failure have a follow-up appointment within 7 days. Yet based on the latest available data, arrival rates to the postdischarge clinic are dismal, hovering around 30%.35 In a multicenter observational study of hospitals participating in the “See You in 7” collaborative, hospitals were able to increase their 7-day follow-up appointment rates by 2% to 3%, and also noted an absolute decrease in readmission rates by 1% to 2%.36 We have demonstrated, using a mathematical approach called queuing theory, that discharge appointment wait times and clinic access can be significantly improved by providing a modest capacity buffer to clinic availability.37 Those interested in applying this model to their own clinical practice may do so with a free online calculator at http://hfresearch.org.
Another important aspect of postdischarge follow-up is appropriate management of the comorbidity burden, which, as noted, is often significant in patients hospitalized with heart failure.38 For instance, in recent cohorts of hospitalized heart failure patients, the incidence of hypertension was 78%, coronary artery disease was more than 50%, atrial fibrillation was more than 40%, and diabetes was nearly 40%.39 Given this burden of comorbidity, it is not surprising that only 35% of readmissions after an index heart failure hospitalization are for recurrent heart failure.40 Coordinating care among primary care physicians and relevant subspecialists is thus essential. Phone calls and secure electronic messages are very helpful in achieving this. There is increasing interest in more nimble care models, such as the patient-centered specialty practice41 or the dyspnea clinic, to help bring coordinated resources to the patient.42
Process of Process Improvement: Our Experiences
The previous sections outline a series of potential process improvements clinical teams and health systems can implement to impact heart failure readmissions. A plan on paper, however, does not equal a plan in actuality. How does one go about implementing these changes? We offer our local experience starting a heart failure transitional care program as a case study, then draw lessons learned as a set of practical tips for local teams to employ. What we hope to highlight is that there is a large difference between a completed process for transitional care of heart failure patients, and the process of developing that process itself. The former is the hardware, the latter is the software. The latter does not typically get highlighted, but it is absolutely critical to unlocking the capabilities of a team and the institution.
In 2015, Northwestern Memorial Hospital adopted a novel payment arrangement from the Center for Medicare and Medicaid Services for Medicare patients being discharged from the hospital with heart failure. Known as Bundled Payments for Care Improvement,43 this bundled payment model incentivized Northwestern Memorial Hospital charge, principally by reducing hospital readmissions and by collaborating with skilled nursing facilities to control length of stay.
We approached this problem by drawing on the available literature,44,45 and by first creating a schematic of our high-level approach, which comprised 3 major elements (Figure 2): identification of hospitalized heart failure patients, delivery of a care bundle to hospitalized heart failure patients in hospital, and coordinating postdischarge care, centered on a telephone call and a postdischarge visit.
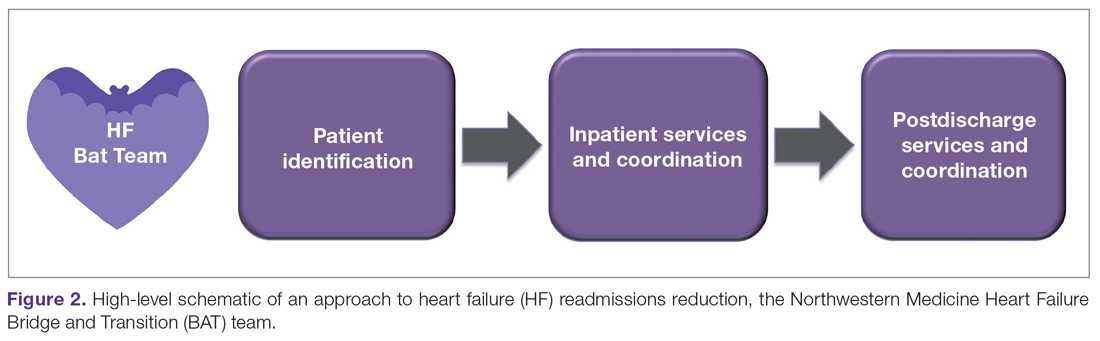
We then proceeded by building out, in stepwise fashion, each component of our value chain, using Agile techniques as a guiding principle.46 Agile, a productivity and process improvement mindset with roots in software development, emphasizes tackling 1 problem at a time, building out new features sequentially and completely, recognizing that the end user does not derive value from a program until new functionality is available for use. Rather than wholesale monolithic change, Agile emphasizes rapid iteration, prototyping, and discarding innovations not found to be helpful. The notion is to stand up new, incremental features rapidly, with each incremental improvement delivering value and helping to accelerate overall change.
Our experience building a robust way to identify heart failure cases is a good example of Agile process improvement in practice. At our hospital, identification of patients with heart failure was a challenge because more than half of heart failure patients are admitted to noncardiology floors. We developed a simple electronic health record query to detect heart failure patients, relying on parameters such as administration of intravenous diuretic or levels of BNP exceeding 100 ng/dL. We deployed this query, finding very high sensitivity for detection of heart failure patients.14 Patients found to have heart failure were then populated into a list in the electronic health record, which made patients’ heart failure status visible to all members of the health care team. Using this list, we were able to automate several processes necessary for heart failure care. For example, the list made it possible for cardiologists to know if there was a patient who perhaps needed cardiology consultation. Nurse navigators could know which patients needed heart failure education without having to be actively consulted by the admitting team. The same nurse navigators could then know upon discharge which patients needed a follow-up telephone call at 48 hours.
This list of heart failure patients was the end product, which was built through prototyping and iteration. For example, with our initial BNP cutoff of 300 ng/dL, we recognized we were missing several cases, and lowered the cutoff for the screener to 100 ng/dL. When we were satisfied this process was working well, we moved on to the next problem to tackle, avoiding trying to work on too many things at once. By doing so, we were able to focus our process improvement resources on 1 problem at a time, building up a suite of interventions. For our hospital, we settled on a bundle of interventions, captured by the mnemonic HEART:
Heart doctor sees patient in the hospital
Education about heart failure in the hospital
After-visit summary with 7-day appointment printed
Reach out to the patient by telephone within 72 hours
Treat the patient in clinic by the 7-day visit
Conclusion
We would like to emphasize that the elements of our heart failure readmissions interventions were not all put in place at once. This was an iterative process that proceeded in a stepwise fashion, with each step improving the care of our patients. We learned a number of lessons from our experience. First, we would advise that teams not try to do everything. One program simply cannot implement all possible readmission reduction interventions, and certainly not all at once. Trade-offs should be made, and interventions more likely to succeed in the local environment should be prioritized. In addition, interventions that do not fit and do not create synergy with the local practice environment should not be pursued.
Second, we would advise teams to start small, tackling a known problem in heart failure transitions of care first. This initial intuition is often right. An example might be improving 7-day appointments upon discharge. Starting with a problem that can be tackled builds process improvement muscle and improves team morale. Third, we would advise teams to consistently iterate on designs, tweaking and improving performance. Complex organizations always evolve; processes that work 1 year may fail the next because another element of the organization may have changed.
Finally, the framework presented in Figure 1 may be helpful in guiding how to structure interventions. Considering interventions to be delivered in the hospital, interventions to be delivered in the clinic, and how to set up feedback loops to support patients as outpatients help develop a comprehensive heart failure readmissions reduction program.
Corresponding author: R. Kannan Mutharasan, MD, Northwestern University Feinberg School of Medicine, 676 North Saint Clair St., Arkes Pavilion, Suite 7-038, Chicago, IL 60611;[email protected].
Financial disclosures: None.
1. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379-385.
2. Kwok CS, Seferovic PM, Van Spall HG, et al. Early unplanned readmissions after admission to hospital with heart failure. Am J Cardiol. 2019;124:736-745.
3. Fonarow GC, Konstam MA, Yancy CW. The hospital readmission reduction program is associated with fewer readmissions, more deaths: time to reconsider. J Am Coll Cardiol. 2017;70:1931-1934.
4. Ody C, Msall L, Dafny LS, et al. Decreases in readmissions credited to medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38:36-43.
5. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9.
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218.
7. Albert NM. A systematic review of transitional-care strategies to reduce rehospitalization in patients with heart failure. Heart Lung. 2016;45:100-113.
8. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1:CD002752.
9. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427-1443.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
11. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8:741-748.
12. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835-843.
13. Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16(1):195.
14. Ahmad FS, Wehbe RM, Kansal P, et al. Targeting the correct population when designing transitional care programs for medicare patients hospitalized with heart failure. JAMA Cardiol. 2017;2:1274-1275.
15. Blecker S, Sontag D, Horwitz LI, et al. Early identification of patients with acute decompensated heart failure. J Card Fail. 2018;24:357-362.
16. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234-1240.
17. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018;101:363-374.
18. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966-2011.
19. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38:406-416.
20. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365-2383.
21. Gattis WA, O’Connor CM, Gallup DS, et al;, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534-1541.
22. Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: Psychometric properties. J Hosp Med. 2008;3:446-454.
23. Van Spall HGC, Lee SF, Xie F, et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019;321:753-761.
24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
25. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30-day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259-266.
26. Patil S, Shah M, Patel B, et al. Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. Mayo Clin Proc. 2019;94:1939-1950.
27. Ahmad K, Chen EW, Nazir U, et al. Regional variation in the association of poverty and heart failure mortality in the 3135 counties of the united states. J Am Heart Assoc. 2019;8:e012422.
28. Bellon JE, Bilderback A, Ahuja-Yende NS, et al. University of Pittsburgh medical center home transitions multidisciplinary care coordination reduces readmissions for older adults. J Am Geriatr Soc. 2019;67:156-163.
29. Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326-1330.
30. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135:1509-1517.
31. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666.
32. Drozda JP, Smith DA, Freiman PC, et al. Heart failure readmission reduction. Am J Med Qual. 2017;32:134-140.
33. Malik AH, Malik SS, Aronow WS; MAGIC (Meta-analysis And oriGinal Investigation in Cardiology) investigators. Effect of home-based follow-up intervention on readmissions and mortality in heart failure patients: a meta-analysis. Future Cardiol. 2019;15:377-386.
34. Strano A, Briggs A, Powell N, et al. Home healthcare visits following hospital discharge: does the timing of visits affect 30-day hospital readmission rates for heart failure patients? Home Healthc Now. 2019;37:152-157.
35. DeVore AD, Cox M, Eapen ZJ, et al. Temporal trends and variation in early scheduled follow-up after a hospitalization for heart failure: findings from get with the guidelines-heart failure. Circ Heart Fail. 2016;9.
36. Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765-773.
37. Mutharasan RK, Ahmad FS, Gurvich I, et al. Buffer or suffer: redesigning heart failure postdischarge clinic using queuing theory. Circ Cardiovasc Qual Outcomes. 2018;11:e004351.
38. Ziaeian B, Hernandez AF, DeVore AD, et al. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1-10.
39. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351-366.
40. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
41. Ward L, Powell RE, Scharf ML, et al. Patient-centered specialty practice: defining the role of specialists in value-based health care. Chest. 2017;151:930-935.
42. Ryan JJ, Waxman AB. The dyspnea clinic. Circulation. 2018;137:1994-1996.
43. Oseran AS, Howard SE, Blumenthal DM. Factors associated with participation in cardiac episode payments included in medicare’s bundled payments for care improvement initiative. JAMA Cardiol. 2018;3:761-766.
44. Takeda A, Taylor SJC, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752.
45. Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384-409.
46. Manifesto for Agile Software Development. http://agilemanifesto.org/ Accessed March 6, 2020.
From the Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Objective: To review selected process-of-care interventions that can be applied both during the hospitalization and during the transitional care period to help address the persistent challenge of heart failure readmissions.
Methods: Review of the literature.
Results: Process-of-care interventions that can be implemented to reduce readmissions of heart failure patients include: accurately identifying heart failure patients; providing disease education; titrating guideline-directed medical therapy; ensuring discharge readiness; arranging close discharge follow-up; identifying and addressing social barriers; following up by telephone; using home health; and addressing comorbidities. Importantly, the heart failure hospitalization is an opportunity to set up outpatient success, and setting up feedback loops can aid in post-discharge monitoring.
Conclusion: We encourage teams to consider local capabilities when selecting processes to improve; begin by improving something small to build capacity and team morale, and continually iterate and reexamine processes, as health care systems are continually evolving.
Keywords: heart failure; process improvement; quality improvement; readmission; rehospitalization; transitional care.
The growing population of patients affected by heart failure continues to challenge health systems. The increasing prevalence is paralleled by the rising costs of managing heart failure, which are projected to grow from $30.7 billion in 2012 to $69.8 billion in 2030.1 A significant portion of these costs relate to readmission after an index heart failure hospitalization. The statistics are staggering: for patients hospitalized with heart failure, approximately 15% to 20% are readmitted within 30 days.2,3 Though recent temporal trends suggest a modest reduction in readmission rates, there is a concerning correlation with increasing mortality,3 and a recognition that readmission rate decreases may relate to subtle changes in coding-based risk adjustment.4 Despite these concerns, efforts to reduce readmissions after heart failure hospitalization command significant attention.
Process improvement methodologies may be helpful in reducing hospital readmissions. Various approaches have been employed, and results have been mixed. An analysis of 70 participating hospitals in the American Heart Association’s Get With the Guidelines initiative found that, while overall readmission rates declined by 1.0% over 3 years, only 1 hospital achieved a 20% reduction in readmission rates.5
It is notably difficult to reduce readmissions after heart failure hospitalization. One challenge is that patients with heart failure often have multiple comorbidities, and approximately 50% to 60% of 30-day readmissions after heart failure hospitalization arise from noncardiac causes.1 Another challenge is that a significant fraction of readmissions in general—perhaps 75%—may not be avoidable.6
Recent excellent systematic reviews and meta-analyses provide comprehensive overviews of process improvement strategies that can be used to reduce readmissions after heart failure hospitalizations.7-9 Yet despite this extensive knowledge, few reports discuss the process of actually implementing these changes: the process of process improvement. Here, we seek to not only highlight some of the most promising potential interventions to reduce heart failure readmissions, but also to discuss a process improvement framework to help engender success, using our experience as a case study. We schematize process improvement efforts as having several distinct phases (Figure 1): processes delivered during the hospitalization and prior to discharge; feedback loops set up to maintain clinical stability at home; and the postdischarge clinic visit as an opportunity to further stabilize the patient and advance the plan of care. The discussion of these interventions follows this organization.

During Hospitalization
The heart failure hospitalization can be used as an opportunity to set up outpatient success, with several goals to target during the index admission. One goal is identifying the root causes of the heart failure syndrome and correcting those root causes, if possible. For example, patients in whom the heart failure syndrome is secondary to valvular heart disease may benefit from transcatheter aortic valve replacement.10 Another clinical goal is decongesting the patient, which is associated with lower readmission rates.11,12 These goals focus on the medical aspects of heart failure care. However, beyond these medical aspects, a patient must be equipped to successfully manage the disease at home.
To support medical and nonmedical interventions for hospitalized heart failure patients, a critical first step is identifying patients with heart failure. This accomplishes at least 2 objectives. First, early identification allows early initiation of interventions, such as heart failure education and social work evaluation. Early initiation of these interventions allows sufficient time during the hospitalization to make meaningful progress on these fronts. Second, early identification allows an opportunity for the delivery of cardiology specialty care, which may help with identifying and correcting root causes of the heart failure syndrome. Such access to cardiology has been shown to improve inpatient mortality and readmission rates.13
In smaller hospitals, identification of patients with heart failure can be as simple as reviewing overnight admissions. More advanced strategies, such as screeners based on brain natriuretic peptide (BNP) levels and administration of intravenous diuretics, can be employed.14,15 In the near future, deep learning-based natural language processing will be applied to mine full-text data in the electronic health record to identify heart failure hospitalizations.16
In the hospital, patients can also receive education about heart failure disease management. This education is a cornerstone of reducing heart failure readmissions. A recent systematic review of nurse education interventions demonstrated reductions in readmissions, hospitalizations, and costs.17 However, the efficacy of heart failure education hinges on many other variables. For patients to adhere to water restriction and daily weights, for example, there must also be patient understanding, compliance, and accessibility to providers to recommend how to strike the fluid balance. Education is therefore necessary, but not sufficient, for setting up outpatient success.
The hospitalization also represents an important time to start or uptitrate guideline-directed medical therapy (GDMT) for heart failure. Doing so takes advantage of an important opportunity to reduce the risk of readmission and even reverse the disease process.18 Uptitration of GDMT in patients with heart failure with reduced ejection fraction is associated with a decreased risk of mortality, while discontinuation is associated with an increased risk of mortality.19 However, recent registry data indicate that intensity of GDMT is just as likely to be decreased as increased during the hospitalization.20 Nevertheless, predischarge initiation of medications may be associated with higher attained doses in follow-up.21
Preparing for Discharge
Preparing a patient for discharge after a heart failure hospitalization involves stabilizing the medical condition as well as ensuring that the patient and caregivers have the medication, equipment, and self-care resources at home necessary to manage the condition. Several frameworks have been put forth to help care teams analyze a patient’s readiness for discharge. One is the B-PREPARED score,22 a validated instrument to discriminate among patients with regard to their readiness to discharge from the hospital. This instrument highlights the importance of several key factors that should be addressed during the discharge process, including counseling and written instructions about medications and their side effects; information about equipment needs and community resources; and information on activity levels and restrictions. Nurse education and discharge coordination can improve patients’ perception of discharge readiness,23 although whether this discharge readiness translates into improved readmission rates appears to depend on the specific follow-up intervention design.9
Prior to discharge, it is important to arrange postdischarge follow-up appointments, as emphasized by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 The use of nurse navigators can help with planning follow-up appointments. For example, the ACC Patient Navigator Program was applied in a single-center study of 120 patients randomized to the program versus usual care.25 This study found a significant increase in patient education and follow-up appointments compared to usual care, and a numerical decrease in hospital readmissions, although the finding was not statistically significant.25
A third critical component of preparing for discharge is identifying and addressing social barriers to care. In a study of patients stratified by household income, patients in the lowest income quartile had a higher readmission rate than patients in the highest income quartile.26 Poverty also correlates with heart failure mortality.27 Social factors play an important role in many aspects of patients’ ability to manage their health, including self-care, medication adherence, and ability to follow-up. Identifying these social factors prior to discharge is the first step to addressing them. While few studies specifically address the role of social workers in the management of heart failure care, the general medical literature suggests that social workers embedded in transitional care teams can augment readmission reduction efforts.28
After Discharge
Patients recently discharged from the hospital who have not yet attended their postdischarge appointment are in an incredibly vulnerable phase of care. Patients who are discharged from the hospital may not yet be connected with outpatient care. During this initial transitional care period, feedback loops involving patient communication back to the clinic, and clinic communication back to the patient, are critical to helping patients remain stable. For example, consider monitoring weights daily after hospital discharge. A patient at home can report increasing weights to a provider, who can then recommend an increased dose of diuretic. The patient can complete the feedback loop by taking the extra medication and monitoring the return of weight back to normal.
While daily weight monitoring is a simple process improvement that relies on the principle of establishing feedback loops, many other strategies exist. One commonly employed tool is the postdischarge telephone follow-up call, which is often coupled with other interventions in a comprehensive care bundle.8 During the telephone call, several process-of-care defects can be corrected, including missing medications or missing information on appointment times.
Beyond the telephone, newer technologies show promise for helping develop feedback loops for patients at home. One such technology is telemonitoring, whereby physiologic information such as weight, heart rate, and blood pressure is collected and sent back to a monitoring center. While the principle holds promise, several studies have not demonstrated significantly different outcomes as compared to usual care.13,29 Another promising technology is the CardioMEMS device (Abbott, Inc., Atlanta, GA), which can remotely transmit the pulmonary artery pressure, a physiologic signal which correlates with volume overload. There is now strong evidence supporting the efficacy of pulmonary artery pressure–guided heart failure management.30,31
Finally, home visits can be an efficient way to communicate symptoms, enable clinical assessment, and provide recommendations. One program that implemented home visits, 24-hour nurses available by call, and telephone follow-up showed a statistically significant reduction in readmissions.32 Furthermore, a meta-analysis of randomized controlled trials comparing home health to usual care showed decreased readmissions and mortality.33 The efficacy may be in strengthening the feedback loop—home care improves compliance with weight monitoring, fluid restriction, and medications.34 These studies provide a strong rationale for the benefits of home health in stabilizing heart failure patients postdischarge. Indeed, nurse home visits were 1 of the 2 process interventions in a Cochrane review of randomized controlled trials that were shown to statistically significantly decrease readmissions and mortality.9 These data underscore the importance of feedback loops for helping ensure patients are clinically stable.
Postdischarge Follow-Up Clinic Visit
The first clinic appointment postdischarge is an important check-in to help advance patient care. Several key tasks can be achieved during the postdischarge visit. First, the patient can be clinically stabilized by adjusting diuretic therapy. If the patient is clinically stable, GDMT can be uptitrated. Second, education around symptoms, medications, diet, and exercise can be reinforced. Finally, clinicians can help connect patients to other members of the multidisciplinary care team, including specialist care, home health, or cardiac rehabilitation.
Achieving 7-day follow-up visits after discharge has been a point of emphasis in national guidelines.24 The ACC promotes a “See You in 7” challenge, advising that all patients discharged with a diagnosis of heart failure have a follow-up appointment within 7 days. Yet based on the latest available data, arrival rates to the postdischarge clinic are dismal, hovering around 30%.35 In a multicenter observational study of hospitals participating in the “See You in 7” collaborative, hospitals were able to increase their 7-day follow-up appointment rates by 2% to 3%, and also noted an absolute decrease in readmission rates by 1% to 2%.36 We have demonstrated, using a mathematical approach called queuing theory, that discharge appointment wait times and clinic access can be significantly improved by providing a modest capacity buffer to clinic availability.37 Those interested in applying this model to their own clinical practice may do so with a free online calculator at http://hfresearch.org.
Another important aspect of postdischarge follow-up is appropriate management of the comorbidity burden, which, as noted, is often significant in patients hospitalized with heart failure.38 For instance, in recent cohorts of hospitalized heart failure patients, the incidence of hypertension was 78%, coronary artery disease was more than 50%, atrial fibrillation was more than 40%, and diabetes was nearly 40%.39 Given this burden of comorbidity, it is not surprising that only 35% of readmissions after an index heart failure hospitalization are for recurrent heart failure.40 Coordinating care among primary care physicians and relevant subspecialists is thus essential. Phone calls and secure electronic messages are very helpful in achieving this. There is increasing interest in more nimble care models, such as the patient-centered specialty practice41 or the dyspnea clinic, to help bring coordinated resources to the patient.42
Process of Process Improvement: Our Experiences
The previous sections outline a series of potential process improvements clinical teams and health systems can implement to impact heart failure readmissions. A plan on paper, however, does not equal a plan in actuality. How does one go about implementing these changes? We offer our local experience starting a heart failure transitional care program as a case study, then draw lessons learned as a set of practical tips for local teams to employ. What we hope to highlight is that there is a large difference between a completed process for transitional care of heart failure patients, and the process of developing that process itself. The former is the hardware, the latter is the software. The latter does not typically get highlighted, but it is absolutely critical to unlocking the capabilities of a team and the institution.
In 2015, Northwestern Memorial Hospital adopted a novel payment arrangement from the Center for Medicare and Medicaid Services for Medicare patients being discharged from the hospital with heart failure. Known as Bundled Payments for Care Improvement,43 this bundled payment model incentivized Northwestern Memorial Hospital charge, principally by reducing hospital readmissions and by collaborating with skilled nursing facilities to control length of stay.
We approached this problem by drawing on the available literature,44,45 and by first creating a schematic of our high-level approach, which comprised 3 major elements (Figure 2): identification of hospitalized heart failure patients, delivery of a care bundle to hospitalized heart failure patients in hospital, and coordinating postdischarge care, centered on a telephone call and a postdischarge visit.

We then proceeded by building out, in stepwise fashion, each component of our value chain, using Agile techniques as a guiding principle.46 Agile, a productivity and process improvement mindset with roots in software development, emphasizes tackling 1 problem at a time, building out new features sequentially and completely, recognizing that the end user does not derive value from a program until new functionality is available for use. Rather than wholesale monolithic change, Agile emphasizes rapid iteration, prototyping, and discarding innovations not found to be helpful. The notion is to stand up new, incremental features rapidly, with each incremental improvement delivering value and helping to accelerate overall change.
Our experience building a robust way to identify heart failure cases is a good example of Agile process improvement in practice. At our hospital, identification of patients with heart failure was a challenge because more than half of heart failure patients are admitted to noncardiology floors. We developed a simple electronic health record query to detect heart failure patients, relying on parameters such as administration of intravenous diuretic or levels of BNP exceeding 100 ng/dL. We deployed this query, finding very high sensitivity for detection of heart failure patients.14 Patients found to have heart failure were then populated into a list in the electronic health record, which made patients’ heart failure status visible to all members of the health care team. Using this list, we were able to automate several processes necessary for heart failure care. For example, the list made it possible for cardiologists to know if there was a patient who perhaps needed cardiology consultation. Nurse navigators could know which patients needed heart failure education without having to be actively consulted by the admitting team. The same nurse navigators could then know upon discharge which patients needed a follow-up telephone call at 48 hours.
This list of heart failure patients was the end product, which was built through prototyping and iteration. For example, with our initial BNP cutoff of 300 ng/dL, we recognized we were missing several cases, and lowered the cutoff for the screener to 100 ng/dL. When we were satisfied this process was working well, we moved on to the next problem to tackle, avoiding trying to work on too many things at once. By doing so, we were able to focus our process improvement resources on 1 problem at a time, building up a suite of interventions. For our hospital, we settled on a bundle of interventions, captured by the mnemonic HEART:
Heart doctor sees patient in the hospital
Education about heart failure in the hospital
After-visit summary with 7-day appointment printed
Reach out to the patient by telephone within 72 hours
Treat the patient in clinic by the 7-day visit
Conclusion
We would like to emphasize that the elements of our heart failure readmissions interventions were not all put in place at once. This was an iterative process that proceeded in a stepwise fashion, with each step improving the care of our patients. We learned a number of lessons from our experience. First, we would advise that teams not try to do everything. One program simply cannot implement all possible readmission reduction interventions, and certainly not all at once. Trade-offs should be made, and interventions more likely to succeed in the local environment should be prioritized. In addition, interventions that do not fit and do not create synergy with the local practice environment should not be pursued.
Second, we would advise teams to start small, tackling a known problem in heart failure transitions of care first. This initial intuition is often right. An example might be improving 7-day appointments upon discharge. Starting with a problem that can be tackled builds process improvement muscle and improves team morale. Third, we would advise teams to consistently iterate on designs, tweaking and improving performance. Complex organizations always evolve; processes that work 1 year may fail the next because another element of the organization may have changed.
Finally, the framework presented in Figure 1 may be helpful in guiding how to structure interventions. Considering interventions to be delivered in the hospital, interventions to be delivered in the clinic, and how to set up feedback loops to support patients as outpatients help develop a comprehensive heart failure readmissions reduction program.
Corresponding author: R. Kannan Mutharasan, MD, Northwestern University Feinberg School of Medicine, 676 North Saint Clair St., Arkes Pavilion, Suite 7-038, Chicago, IL 60611;[email protected].
Financial disclosures: None.
From the Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL.
Objective: To review selected process-of-care interventions that can be applied both during the hospitalization and during the transitional care period to help address the persistent challenge of heart failure readmissions.
Methods: Review of the literature.
Results: Process-of-care interventions that can be implemented to reduce readmissions of heart failure patients include: accurately identifying heart failure patients; providing disease education; titrating guideline-directed medical therapy; ensuring discharge readiness; arranging close discharge follow-up; identifying and addressing social barriers; following up by telephone; using home health; and addressing comorbidities. Importantly, the heart failure hospitalization is an opportunity to set up outpatient success, and setting up feedback loops can aid in post-discharge monitoring.
Conclusion: We encourage teams to consider local capabilities when selecting processes to improve; begin by improving something small to build capacity and team morale, and continually iterate and reexamine processes, as health care systems are continually evolving.
Keywords: heart failure; process improvement; quality improvement; readmission; rehospitalization; transitional care.
The growing population of patients affected by heart failure continues to challenge health systems. The increasing prevalence is paralleled by the rising costs of managing heart failure, which are projected to grow from $30.7 billion in 2012 to $69.8 billion in 2030.1 A significant portion of these costs relate to readmission after an index heart failure hospitalization. The statistics are staggering: for patients hospitalized with heart failure, approximately 15% to 20% are readmitted within 30 days.2,3 Though recent temporal trends suggest a modest reduction in readmission rates, there is a concerning correlation with increasing mortality,3 and a recognition that readmission rate decreases may relate to subtle changes in coding-based risk adjustment.4 Despite these concerns, efforts to reduce readmissions after heart failure hospitalization command significant attention.
Process improvement methodologies may be helpful in reducing hospital readmissions. Various approaches have been employed, and results have been mixed. An analysis of 70 participating hospitals in the American Heart Association’s Get With the Guidelines initiative found that, while overall readmission rates declined by 1.0% over 3 years, only 1 hospital achieved a 20% reduction in readmission rates.5
It is notably difficult to reduce readmissions after heart failure hospitalization. One challenge is that patients with heart failure often have multiple comorbidities, and approximately 50% to 60% of 30-day readmissions after heart failure hospitalization arise from noncardiac causes.1 Another challenge is that a significant fraction of readmissions in general—perhaps 75%—may not be avoidable.6
Recent excellent systematic reviews and meta-analyses provide comprehensive overviews of process improvement strategies that can be used to reduce readmissions after heart failure hospitalizations.7-9 Yet despite this extensive knowledge, few reports discuss the process of actually implementing these changes: the process of process improvement. Here, we seek to not only highlight some of the most promising potential interventions to reduce heart failure readmissions, but also to discuss a process improvement framework to help engender success, using our experience as a case study. We schematize process improvement efforts as having several distinct phases (Figure 1): processes delivered during the hospitalization and prior to discharge; feedback loops set up to maintain clinical stability at home; and the postdischarge clinic visit as an opportunity to further stabilize the patient and advance the plan of care. The discussion of these interventions follows this organization.

During Hospitalization
The heart failure hospitalization can be used as an opportunity to set up outpatient success, with several goals to target during the index admission. One goal is identifying the root causes of the heart failure syndrome and correcting those root causes, if possible. For example, patients in whom the heart failure syndrome is secondary to valvular heart disease may benefit from transcatheter aortic valve replacement.10 Another clinical goal is decongesting the patient, which is associated with lower readmission rates.11,12 These goals focus on the medical aspects of heart failure care. However, beyond these medical aspects, a patient must be equipped to successfully manage the disease at home.
To support medical and nonmedical interventions for hospitalized heart failure patients, a critical first step is identifying patients with heart failure. This accomplishes at least 2 objectives. First, early identification allows early initiation of interventions, such as heart failure education and social work evaluation. Early initiation of these interventions allows sufficient time during the hospitalization to make meaningful progress on these fronts. Second, early identification allows an opportunity for the delivery of cardiology specialty care, which may help with identifying and correcting root causes of the heart failure syndrome. Such access to cardiology has been shown to improve inpatient mortality and readmission rates.13
In smaller hospitals, identification of patients with heart failure can be as simple as reviewing overnight admissions. More advanced strategies, such as screeners based on brain natriuretic peptide (BNP) levels and administration of intravenous diuretics, can be employed.14,15 In the near future, deep learning-based natural language processing will be applied to mine full-text data in the electronic health record to identify heart failure hospitalizations.16
In the hospital, patients can also receive education about heart failure disease management. This education is a cornerstone of reducing heart failure readmissions. A recent systematic review of nurse education interventions demonstrated reductions in readmissions, hospitalizations, and costs.17 However, the efficacy of heart failure education hinges on many other variables. For patients to adhere to water restriction and daily weights, for example, there must also be patient understanding, compliance, and accessibility to providers to recommend how to strike the fluid balance. Education is therefore necessary, but not sufficient, for setting up outpatient success.
The hospitalization also represents an important time to start or uptitrate guideline-directed medical therapy (GDMT) for heart failure. Doing so takes advantage of an important opportunity to reduce the risk of readmission and even reverse the disease process.18 Uptitration of GDMT in patients with heart failure with reduced ejection fraction is associated with a decreased risk of mortality, while discontinuation is associated with an increased risk of mortality.19 However, recent registry data indicate that intensity of GDMT is just as likely to be decreased as increased during the hospitalization.20 Nevertheless, predischarge initiation of medications may be associated with higher attained doses in follow-up.21
Preparing for Discharge
Preparing a patient for discharge after a heart failure hospitalization involves stabilizing the medical condition as well as ensuring that the patient and caregivers have the medication, equipment, and self-care resources at home necessary to manage the condition. Several frameworks have been put forth to help care teams analyze a patient’s readiness for discharge. One is the B-PREPARED score,22 a validated instrument to discriminate among patients with regard to their readiness to discharge from the hospital. This instrument highlights the importance of several key factors that should be addressed during the discharge process, including counseling and written instructions about medications and their side effects; information about equipment needs and community resources; and information on activity levels and restrictions. Nurse education and discharge coordination can improve patients’ perception of discharge readiness,23 although whether this discharge readiness translates into improved readmission rates appears to depend on the specific follow-up intervention design.9
Prior to discharge, it is important to arrange postdischarge follow-up appointments, as emphasized by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.24 The use of nurse navigators can help with planning follow-up appointments. For example, the ACC Patient Navigator Program was applied in a single-center study of 120 patients randomized to the program versus usual care.25 This study found a significant increase in patient education and follow-up appointments compared to usual care, and a numerical decrease in hospital readmissions, although the finding was not statistically significant.25
A third critical component of preparing for discharge is identifying and addressing social barriers to care. In a study of patients stratified by household income, patients in the lowest income quartile had a higher readmission rate than patients in the highest income quartile.26 Poverty also correlates with heart failure mortality.27 Social factors play an important role in many aspects of patients’ ability to manage their health, including self-care, medication adherence, and ability to follow-up. Identifying these social factors prior to discharge is the first step to addressing them. While few studies specifically address the role of social workers in the management of heart failure care, the general medical literature suggests that social workers embedded in transitional care teams can augment readmission reduction efforts.28
After Discharge
Patients recently discharged from the hospital who have not yet attended their postdischarge appointment are in an incredibly vulnerable phase of care. Patients who are discharged from the hospital may not yet be connected with outpatient care. During this initial transitional care period, feedback loops involving patient communication back to the clinic, and clinic communication back to the patient, are critical to helping patients remain stable. For example, consider monitoring weights daily after hospital discharge. A patient at home can report increasing weights to a provider, who can then recommend an increased dose of diuretic. The patient can complete the feedback loop by taking the extra medication and monitoring the return of weight back to normal.
While daily weight monitoring is a simple process improvement that relies on the principle of establishing feedback loops, many other strategies exist. One commonly employed tool is the postdischarge telephone follow-up call, which is often coupled with other interventions in a comprehensive care bundle.8 During the telephone call, several process-of-care defects can be corrected, including missing medications or missing information on appointment times.
Beyond the telephone, newer technologies show promise for helping develop feedback loops for patients at home. One such technology is telemonitoring, whereby physiologic information such as weight, heart rate, and blood pressure is collected and sent back to a monitoring center. While the principle holds promise, several studies have not demonstrated significantly different outcomes as compared to usual care.13,29 Another promising technology is the CardioMEMS device (Abbott, Inc., Atlanta, GA), which can remotely transmit the pulmonary artery pressure, a physiologic signal which correlates with volume overload. There is now strong evidence supporting the efficacy of pulmonary artery pressure–guided heart failure management.30,31
Finally, home visits can be an efficient way to communicate symptoms, enable clinical assessment, and provide recommendations. One program that implemented home visits, 24-hour nurses available by call, and telephone follow-up showed a statistically significant reduction in readmissions.32 Furthermore, a meta-analysis of randomized controlled trials comparing home health to usual care showed decreased readmissions and mortality.33 The efficacy may be in strengthening the feedback loop—home care improves compliance with weight monitoring, fluid restriction, and medications.34 These studies provide a strong rationale for the benefits of home health in stabilizing heart failure patients postdischarge. Indeed, nurse home visits were 1 of the 2 process interventions in a Cochrane review of randomized controlled trials that were shown to statistically significantly decrease readmissions and mortality.9 These data underscore the importance of feedback loops for helping ensure patients are clinically stable.
Postdischarge Follow-Up Clinic Visit
The first clinic appointment postdischarge is an important check-in to help advance patient care. Several key tasks can be achieved during the postdischarge visit. First, the patient can be clinically stabilized by adjusting diuretic therapy. If the patient is clinically stable, GDMT can be uptitrated. Second, education around symptoms, medications, diet, and exercise can be reinforced. Finally, clinicians can help connect patients to other members of the multidisciplinary care team, including specialist care, home health, or cardiac rehabilitation.
Achieving 7-day follow-up visits after discharge has been a point of emphasis in national guidelines.24 The ACC promotes a “See You in 7” challenge, advising that all patients discharged with a diagnosis of heart failure have a follow-up appointment within 7 days. Yet based on the latest available data, arrival rates to the postdischarge clinic are dismal, hovering around 30%.35 In a multicenter observational study of hospitals participating in the “See You in 7” collaborative, hospitals were able to increase their 7-day follow-up appointment rates by 2% to 3%, and also noted an absolute decrease in readmission rates by 1% to 2%.36 We have demonstrated, using a mathematical approach called queuing theory, that discharge appointment wait times and clinic access can be significantly improved by providing a modest capacity buffer to clinic availability.37 Those interested in applying this model to their own clinical practice may do so with a free online calculator at http://hfresearch.org.
Another important aspect of postdischarge follow-up is appropriate management of the comorbidity burden, which, as noted, is often significant in patients hospitalized with heart failure.38 For instance, in recent cohorts of hospitalized heart failure patients, the incidence of hypertension was 78%, coronary artery disease was more than 50%, atrial fibrillation was more than 40%, and diabetes was nearly 40%.39 Given this burden of comorbidity, it is not surprising that only 35% of readmissions after an index heart failure hospitalization are for recurrent heart failure.40 Coordinating care among primary care physicians and relevant subspecialists is thus essential. Phone calls and secure electronic messages are very helpful in achieving this. There is increasing interest in more nimble care models, such as the patient-centered specialty practice41 or the dyspnea clinic, to help bring coordinated resources to the patient.42
Process of Process Improvement: Our Experiences
The previous sections outline a series of potential process improvements clinical teams and health systems can implement to impact heart failure readmissions. A plan on paper, however, does not equal a plan in actuality. How does one go about implementing these changes? We offer our local experience starting a heart failure transitional care program as a case study, then draw lessons learned as a set of practical tips for local teams to employ. What we hope to highlight is that there is a large difference between a completed process for transitional care of heart failure patients, and the process of developing that process itself. The former is the hardware, the latter is the software. The latter does not typically get highlighted, but it is absolutely critical to unlocking the capabilities of a team and the institution.
In 2015, Northwestern Memorial Hospital adopted a novel payment arrangement from the Center for Medicare and Medicaid Services for Medicare patients being discharged from the hospital with heart failure. Known as Bundled Payments for Care Improvement,43 this bundled payment model incentivized Northwestern Memorial Hospital charge, principally by reducing hospital readmissions and by collaborating with skilled nursing facilities to control length of stay.
We approached this problem by drawing on the available literature,44,45 and by first creating a schematic of our high-level approach, which comprised 3 major elements (Figure 2): identification of hospitalized heart failure patients, delivery of a care bundle to hospitalized heart failure patients in hospital, and coordinating postdischarge care, centered on a telephone call and a postdischarge visit.

We then proceeded by building out, in stepwise fashion, each component of our value chain, using Agile techniques as a guiding principle.46 Agile, a productivity and process improvement mindset with roots in software development, emphasizes tackling 1 problem at a time, building out new features sequentially and completely, recognizing that the end user does not derive value from a program until new functionality is available for use. Rather than wholesale monolithic change, Agile emphasizes rapid iteration, prototyping, and discarding innovations not found to be helpful. The notion is to stand up new, incremental features rapidly, with each incremental improvement delivering value and helping to accelerate overall change.
Our experience building a robust way to identify heart failure cases is a good example of Agile process improvement in practice. At our hospital, identification of patients with heart failure was a challenge because more than half of heart failure patients are admitted to noncardiology floors. We developed a simple electronic health record query to detect heart failure patients, relying on parameters such as administration of intravenous diuretic or levels of BNP exceeding 100 ng/dL. We deployed this query, finding very high sensitivity for detection of heart failure patients.14 Patients found to have heart failure were then populated into a list in the electronic health record, which made patients’ heart failure status visible to all members of the health care team. Using this list, we were able to automate several processes necessary for heart failure care. For example, the list made it possible for cardiologists to know if there was a patient who perhaps needed cardiology consultation. Nurse navigators could know which patients needed heart failure education without having to be actively consulted by the admitting team. The same nurse navigators could then know upon discharge which patients needed a follow-up telephone call at 48 hours.
This list of heart failure patients was the end product, which was built through prototyping and iteration. For example, with our initial BNP cutoff of 300 ng/dL, we recognized we were missing several cases, and lowered the cutoff for the screener to 100 ng/dL. When we were satisfied this process was working well, we moved on to the next problem to tackle, avoiding trying to work on too many things at once. By doing so, we were able to focus our process improvement resources on 1 problem at a time, building up a suite of interventions. For our hospital, we settled on a bundle of interventions, captured by the mnemonic HEART:
Heart doctor sees patient in the hospital
Education about heart failure in the hospital
After-visit summary with 7-day appointment printed
Reach out to the patient by telephone within 72 hours
Treat the patient in clinic by the 7-day visit
Conclusion
We would like to emphasize that the elements of our heart failure readmissions interventions were not all put in place at once. This was an iterative process that proceeded in a stepwise fashion, with each step improving the care of our patients. We learned a number of lessons from our experience. First, we would advise that teams not try to do everything. One program simply cannot implement all possible readmission reduction interventions, and certainly not all at once. Trade-offs should be made, and interventions more likely to succeed in the local environment should be prioritized. In addition, interventions that do not fit and do not create synergy with the local practice environment should not be pursued.
Second, we would advise teams to start small, tackling a known problem in heart failure transitions of care first. This initial intuition is often right. An example might be improving 7-day appointments upon discharge. Starting with a problem that can be tackled builds process improvement muscle and improves team morale. Third, we would advise teams to consistently iterate on designs, tweaking and improving performance. Complex organizations always evolve; processes that work 1 year may fail the next because another element of the organization may have changed.
Finally, the framework presented in Figure 1 may be helpful in guiding how to structure interventions. Considering interventions to be delivered in the hospital, interventions to be delivered in the clinic, and how to set up feedback loops to support patients as outpatients help develop a comprehensive heart failure readmissions reduction program.
Corresponding author: R. Kannan Mutharasan, MD, Northwestern University Feinberg School of Medicine, 676 North Saint Clair St., Arkes Pavilion, Suite 7-038, Chicago, IL 60611;[email protected].
Financial disclosures: None.
1. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379-385.
2. Kwok CS, Seferovic PM, Van Spall HG, et al. Early unplanned readmissions after admission to hospital with heart failure. Am J Cardiol. 2019;124:736-745.
3. Fonarow GC, Konstam MA, Yancy CW. The hospital readmission reduction program is associated with fewer readmissions, more deaths: time to reconsider. J Am Coll Cardiol. 2017;70:1931-1934.
4. Ody C, Msall L, Dafny LS, et al. Decreases in readmissions credited to medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38:36-43.
5. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9.
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218.
7. Albert NM. A systematic review of transitional-care strategies to reduce rehospitalization in patients with heart failure. Heart Lung. 2016;45:100-113.
8. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1:CD002752.
9. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427-1443.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
11. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8:741-748.
12. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835-843.
13. Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16(1):195.
14. Ahmad FS, Wehbe RM, Kansal P, et al. Targeting the correct population when designing transitional care programs for medicare patients hospitalized with heart failure. JAMA Cardiol. 2017;2:1274-1275.
15. Blecker S, Sontag D, Horwitz LI, et al. Early identification of patients with acute decompensated heart failure. J Card Fail. 2018;24:357-362.
16. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234-1240.
17. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018;101:363-374.
18. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966-2011.
19. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38:406-416.
20. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365-2383.
21. Gattis WA, O’Connor CM, Gallup DS, et al;, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534-1541.
22. Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: Psychometric properties. J Hosp Med. 2008;3:446-454.
23. Van Spall HGC, Lee SF, Xie F, et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019;321:753-761.
24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
25. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30-day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259-266.
26. Patil S, Shah M, Patel B, et al. Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. Mayo Clin Proc. 2019;94:1939-1950.
27. Ahmad K, Chen EW, Nazir U, et al. Regional variation in the association of poverty and heart failure mortality in the 3135 counties of the united states. J Am Heart Assoc. 2019;8:e012422.
28. Bellon JE, Bilderback A, Ahuja-Yende NS, et al. University of Pittsburgh medical center home transitions multidisciplinary care coordination reduces readmissions for older adults. J Am Geriatr Soc. 2019;67:156-163.
29. Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326-1330.
30. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135:1509-1517.
31. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666.
32. Drozda JP, Smith DA, Freiman PC, et al. Heart failure readmission reduction. Am J Med Qual. 2017;32:134-140.
33. Malik AH, Malik SS, Aronow WS; MAGIC (Meta-analysis And oriGinal Investigation in Cardiology) investigators. Effect of home-based follow-up intervention on readmissions and mortality in heart failure patients: a meta-analysis. Future Cardiol. 2019;15:377-386.
34. Strano A, Briggs A, Powell N, et al. Home healthcare visits following hospital discharge: does the timing of visits affect 30-day hospital readmission rates for heart failure patients? Home Healthc Now. 2019;37:152-157.
35. DeVore AD, Cox M, Eapen ZJ, et al. Temporal trends and variation in early scheduled follow-up after a hospitalization for heart failure: findings from get with the guidelines-heart failure. Circ Heart Fail. 2016;9.
36. Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765-773.
37. Mutharasan RK, Ahmad FS, Gurvich I, et al. Buffer or suffer: redesigning heart failure postdischarge clinic using queuing theory. Circ Cardiovasc Qual Outcomes. 2018;11:e004351.
38. Ziaeian B, Hernandez AF, DeVore AD, et al. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1-10.
39. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351-366.
40. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
41. Ward L, Powell RE, Scharf ML, et al. Patient-centered specialty practice: defining the role of specialists in value-based health care. Chest. 2017;151:930-935.
42. Ryan JJ, Waxman AB. The dyspnea clinic. Circulation. 2018;137:1994-1996.
43. Oseran AS, Howard SE, Blumenthal DM. Factors associated with participation in cardiac episode payments included in medicare’s bundled payments for care improvement initiative. JAMA Cardiol. 2018;3:761-766.
44. Takeda A, Taylor SJC, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752.
45. Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384-409.
46. Manifesto for Agile Software Development. http://agilemanifesto.org/ Accessed March 6, 2020.
1. Ziaeian B, Fonarow GC. The prevention of hospital readmissions in heart failure. Prog Cardiovasc Dis. 2016;58:379-385.
2. Kwok CS, Seferovic PM, Van Spall HG, et al. Early unplanned readmissions after admission to hospital with heart failure. Am J Cardiol. 2019;124:736-745.
3. Fonarow GC, Konstam MA, Yancy CW. The hospital readmission reduction program is associated with fewer readmissions, more deaths: time to reconsider. J Am Coll Cardiol. 2017;70:1931-1934.
4. Ody C, Msall L, Dafny LS, et al. Decreases in readmissions credited to medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38:36-43.
5. Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the Get With The Guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9.
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. 2012;18(6):1211-1218.
7. Albert NM. A systematic review of transitional-care strategies to reduce rehospitalization in patients with heart failure. Heart Lung. 2016;45:100-113.
8. Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1:CD002752.
9. Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427-1443.
10. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
11. Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8:741-748.
12. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835-843.
13. Driscoll A, Meagher S, Kennedy R, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. 2016;16(1):195.
14. Ahmad FS, Wehbe RM, Kansal P, et al. Targeting the correct population when designing transitional care programs for medicare patients hospitalized with heart failure. JAMA Cardiol. 2017;2:1274-1275.
15. Blecker S, Sontag D, Horwitz LI, et al. Early identification of patients with acute decompensated heart failure. J Card Fail. 2018;24:357-362.
16. Lee J, Yoon W, Kim S, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. 2020;36:1234-1240.
17. Rice H, Say R, Betihavas V. The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns. 2018;101:363-374.
18. Hollenberg SM, Warner Stevenson L, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966-2011.
19. Tran RH, Aldemerdash A, Chang P, et al. Guideline-directed medical therapy and survival following hospitalization in patients with heart failure. Pharmacotherapy. 2018;38:406-416.
20. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365-2383.
21. Gattis WA, O’Connor CM, Gallup DS, et al;, IMPACT-HF Investigators and Coordinators. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534-1541.
22. Graumlich JF, Novotny NL, Aldag JC. Brief scale measuring patient preparedness for hospital discharge to home: Psychometric properties. J Hosp Med. 2008;3:446-454.
23. Van Spall HGC, Lee SF, Xie F, et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT-HF Randomized Clinical Trial. JAMA. 2019;321:753-761.
24. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-327.
25. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30-day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259-266.
26. Patil S, Shah M, Patel B, et al. Readmissions among patients admitted with acute decompensated heart failure based on income quartiles. Mayo Clin Proc. 2019;94:1939-1950.
27. Ahmad K, Chen EW, Nazir U, et al. Regional variation in the association of poverty and heart failure mortality in the 3135 counties of the united states. J Am Heart Assoc. 2019;8:e012422.
28. Bellon JE, Bilderback A, Ahuja-Yende NS, et al. University of Pittsburgh medical center home transitions multidisciplinary care coordination reduces readmissions for older adults. J Am Geriatr Soc. 2019;67:156-163.
29. Rosen D, McCall JD, Primack BA. Telehealth protocol to prevent readmission among high-risk patients with congestive heart failure. Am J Med. 2017;130:1326-1330.
30. Heywood JT, Jermyn R, Shavelle D, et al. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS sensor. Circulation. 2017;135:1509-1517.
31. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666.
32. Drozda JP, Smith DA, Freiman PC, et al. Heart failure readmission reduction. Am J Med Qual. 2017;32:134-140.
33. Malik AH, Malik SS, Aronow WS; MAGIC (Meta-analysis And oriGinal Investigation in Cardiology) investigators. Effect of home-based follow-up intervention on readmissions and mortality in heart failure patients: a meta-analysis. Future Cardiol. 2019;15:377-386.
34. Strano A, Briggs A, Powell N, et al. Home healthcare visits following hospital discharge: does the timing of visits affect 30-day hospital readmission rates for heart failure patients? Home Healthc Now. 2019;37:152-157.
35. DeVore AD, Cox M, Eapen ZJ, et al. Temporal trends and variation in early scheduled follow-up after a hospitalization for heart failure: findings from get with the guidelines-heart failure. Circ Heart Fail. 2016;9.
36. Baker H, Oliver-McNeil S, Deng L, Hummel SL. Regional hospital collaboration and outcomes in medicare heart failure patients: see you in 7. JACC Heart Fail. 2015;3:765-773.
37. Mutharasan RK, Ahmad FS, Gurvich I, et al. Buffer or suffer: redesigning heart failure postdischarge clinic using queuing theory. Circ Cardiovasc Qual Outcomes. 2018;11:e004351.
38. Ziaeian B, Hernandez AF, DeVore AD, et al. Long-term outcomes for heart failure patients with and without diabetes: From the Get With The Guidelines-Heart Failure Registry. Am Heart J. 2019;211:1-10.
39. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF Registry. J Am Coll Cardiol. 2018;72:351-366.
40. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
41. Ward L, Powell RE, Scharf ML, et al. Patient-centered specialty practice: defining the role of specialists in value-based health care. Chest. 2017;151:930-935.
42. Ryan JJ, Waxman AB. The dyspnea clinic. Circulation. 2018;137:1994-1996.
43. Oseran AS, Howard SE, Blumenthal DM. Factors associated with participation in cardiac episode payments included in medicare’s bundled payments for care improvement initiative. JAMA Cardiol. 2018;3:761-766.
44. Takeda A, Taylor SJC, Taylor RS, et al. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;(9):CD002752.
45. Albert NM, Barnason S, Deswal A, et al. Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384-409.
46. Manifesto for Agile Software Development. http://agilemanifesto.org/ Accessed March 6, 2020.
Pandemic-related stress causing health issues in many Americans
Over the last 2 months, more than half of Americans have experienced some sort of adverse effect caused by stress related to the COVID-19 pandemic, according to a survey from the Kaiser Family Foundation (KFF).
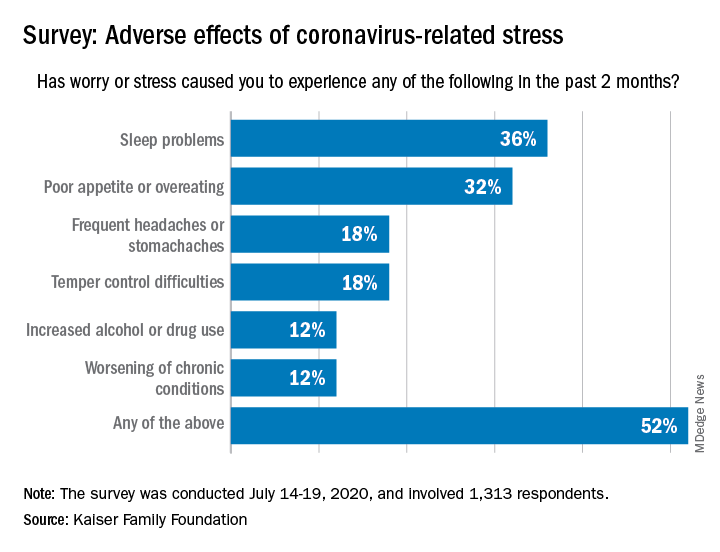
More than a third (36%) of the 1,313 respondents said they either had difficulty sleeping, falling asleep, or sleeping too much, KFF said in its latest Health Tracking Poll, conducted July 14-19, 2020. That was followed by poor appetite or overeating, which was mentioned by 32% of those surveyed.
Other adverse effects included frequent headaches or stomachaches (18%), temper-control issues (18%), increased drug or alcohol use (12%), and worsening of chronic conditions such as diabetes or hypertension (12%). Altogether, 52% of Americans have had at least one of these issues in the past 2 months, Liz Hamel and associates at KFF reported.
breaking down to 26% reporting a major impact and 28% reporting a minor impact (figures have been rounded), they said.
“As life with the coronavirus pandemic wears on, Americans increasingly say it is taking a negative toll on their mental health,” the investigators wrote. Earlier polls showed that pandemic-related stress was having an impact on mental health for 39% of respondents in May, compared with 45% in early April and 32% in March.
In the July poll, Black adults were much more likely to report a negative mental health impact (68%) than were Hispanics or Whites, who were both at 51%. Age was also a factor: The youngest group of respondents (ages 18-29 years) had the highest negative-impact rate (62%), and the oldest group (65 years and older) had the lowest (47%), they said.
When it came to reporting the adverse effects of stress or worry, however, the situation was somewhat different. Hispanics had the highest rate of such effects at 63%, while Blacks had a rate of 57% and 47% of Whites reported issues with sleep, eating, temper, and other problems, Ms. Hamel and associates reported.
Over the last 2 months, more than half of Americans have experienced some sort of adverse effect caused by stress related to the COVID-19 pandemic, according to a survey from the Kaiser Family Foundation (KFF).

More than a third (36%) of the 1,313 respondents said they either had difficulty sleeping, falling asleep, or sleeping too much, KFF said in its latest Health Tracking Poll, conducted July 14-19, 2020. That was followed by poor appetite or overeating, which was mentioned by 32% of those surveyed.
Other adverse effects included frequent headaches or stomachaches (18%), temper-control issues (18%), increased drug or alcohol use (12%), and worsening of chronic conditions such as diabetes or hypertension (12%). Altogether, 52% of Americans have had at least one of these issues in the past 2 months, Liz Hamel and associates at KFF reported.
breaking down to 26% reporting a major impact and 28% reporting a minor impact (figures have been rounded), they said.
“As life with the coronavirus pandemic wears on, Americans increasingly say it is taking a negative toll on their mental health,” the investigators wrote. Earlier polls showed that pandemic-related stress was having an impact on mental health for 39% of respondents in May, compared with 45% in early April and 32% in March.
In the July poll, Black adults were much more likely to report a negative mental health impact (68%) than were Hispanics or Whites, who were both at 51%. Age was also a factor: The youngest group of respondents (ages 18-29 years) had the highest negative-impact rate (62%), and the oldest group (65 years and older) had the lowest (47%), they said.
When it came to reporting the adverse effects of stress or worry, however, the situation was somewhat different. Hispanics had the highest rate of such effects at 63%, while Blacks had a rate of 57% and 47% of Whites reported issues with sleep, eating, temper, and other problems, Ms. Hamel and associates reported.
Over the last 2 months, more than half of Americans have experienced some sort of adverse effect caused by stress related to the COVID-19 pandemic, according to a survey from the Kaiser Family Foundation (KFF).

More than a third (36%) of the 1,313 respondents said they either had difficulty sleeping, falling asleep, or sleeping too much, KFF said in its latest Health Tracking Poll, conducted July 14-19, 2020. That was followed by poor appetite or overeating, which was mentioned by 32% of those surveyed.
Other adverse effects included frequent headaches or stomachaches (18%), temper-control issues (18%), increased drug or alcohol use (12%), and worsening of chronic conditions such as diabetes or hypertension (12%). Altogether, 52% of Americans have had at least one of these issues in the past 2 months, Liz Hamel and associates at KFF reported.
breaking down to 26% reporting a major impact and 28% reporting a minor impact (figures have been rounded), they said.
“As life with the coronavirus pandemic wears on, Americans increasingly say it is taking a negative toll on their mental health,” the investigators wrote. Earlier polls showed that pandemic-related stress was having an impact on mental health for 39% of respondents in May, compared with 45% in early April and 32% in March.
In the July poll, Black adults were much more likely to report a negative mental health impact (68%) than were Hispanics or Whites, who were both at 51%. Age was also a factor: The youngest group of respondents (ages 18-29 years) had the highest negative-impact rate (62%), and the oldest group (65 years and older) had the lowest (47%), they said.
When it came to reporting the adverse effects of stress or worry, however, the situation was somewhat different. Hispanics had the highest rate of such effects at 63%, while Blacks had a rate of 57% and 47% of Whites reported issues with sleep, eating, temper, and other problems, Ms. Hamel and associates reported.
AHA statement addresses genetic testing for CVD
A new scientific statement from the American Heart Association recommends that genetic testing for inherited cardiovascular disease should be reserved for four specific types of heart diseases – cardiomyopathies, thoracic aortic aneurysms and dissections, arrhythmias, and familial hypercholesterolemia – and should enlist skilled geneticists and genetic counselors in the care team.
The guidance comes in a scientific statement published online in the journal Circulation: Genomic and Precision Medicine.
Kiran Musunuru, MD, PhD, MPH, ML, chair of the writing group for the scientific statement, described in an interview the rationale for publishing the statement at this time. “There was no prior single statement that summarized best practices for the whole gamut of inherited cardiovascular diseases in adults, only statements for individual diseases,” he said in an interview. “With genetic testing seeing explosive growth in the past few years, both in the clinical setting and with direct-to-consumer testing, we felt that cardiovascular practitioners would benefit from having a single document to serve as a general resource on genetic testing.”
The statement describes two types of patients who would be suitable for genetic testing for cardiovascular disease (CVD), Dr. Musunuru noted: “Patients who have been diagnosed with or are strongly suspected to have a cardiovascular disease that is often inherited and family members of patients who have been diagnosed with an inherited cardiovascular disease and found by genetic testing to have a mutation that is felt to be the cause of the disease.”
The statement also spells out two crucial elements for genetic testing: thorough disease-specific phenotyping – that is, using genetic information to identify the individual’s disease characteristics and a comprehensive family history that spans at least three generations. Testing should only proceed after patients has had genetic counseling and made a shared decision with their doctors.
“Genetic counseling is absolutely essential both before genetic testing to educate patients on what genetic testing entails and what potential results to expect, as well as the risks of testing; and after genetic testing, to review the results of the genetic testing and explain the potential consequences for the patient’s health and the health of family members, including children,” Dr. Musunuru said.
The process should involve board-certified geneticists or at least cardiovascular specialists well-versed in genetics and genetic counselors, the statement noted. The latter are “critical” in the care team, Dr. Musunuru said.
After the decision is made to do genetic testing, the next step is to decide the scope of the testing. That can range from targeted sequencing of a single gene or a few genes linked to the disease to large gene panels; the latter “may not increase the likelihood of clinically actionable results in adult patients,” Dr. Musunuru and colleagues wrote.
But genetic testing is no guarantee to identify a cause or confirm a diagnosis of CVD, the statement noted. “The yield for any genetic testing for any inherited cardiovascular disease remains <100%, usually much less than 100%,” the writing committee stated.
Dr. Musunuru explained that the results can sometimes be inconclusive. “In many cases, genetic testing reveals a mutation that is uninterpretable, what we call a variant of uncertain significance,” he said. “It is not clear whether the mutation increases the risk of disease or is entirely benign, which makes it very challenging to counsel patients as to whether anything should be done about the mutation.”
Even in a diagnosed patient the test results can be uncertain. “This makes it challenging to explain why the patient has the disease and whether any of the family members are at risk,” Dr. Musunuru said.
According to the statement, providers should encourage patients with a confirmed or likely pathogenic variant for CVD to share that information with “all of their at-risk relative,” the statement noted, suggesting “family letters” given to patients are a way to navigate HIPAA’s privacy limits.
The statement was written on behalf of the American Heart Association’s Council on Genomic and Precision Medicine; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology.
Dr. Musunuru and writing group members have no relevant financial relationships to disclose.
SOURCE: Musunuru K et al. Circ Genom Precis Med. 2020 Jul 23. doi: 10.1161/HCG.0000000000000067.
A new scientific statement from the American Heart Association recommends that genetic testing for inherited cardiovascular disease should be reserved for four specific types of heart diseases – cardiomyopathies, thoracic aortic aneurysms and dissections, arrhythmias, and familial hypercholesterolemia – and should enlist skilled geneticists and genetic counselors in the care team.
The guidance comes in a scientific statement published online in the journal Circulation: Genomic and Precision Medicine.
Kiran Musunuru, MD, PhD, MPH, ML, chair of the writing group for the scientific statement, described in an interview the rationale for publishing the statement at this time. “There was no prior single statement that summarized best practices for the whole gamut of inherited cardiovascular diseases in adults, only statements for individual diseases,” he said in an interview. “With genetic testing seeing explosive growth in the past few years, both in the clinical setting and with direct-to-consumer testing, we felt that cardiovascular practitioners would benefit from having a single document to serve as a general resource on genetic testing.”
The statement describes two types of patients who would be suitable for genetic testing for cardiovascular disease (CVD), Dr. Musunuru noted: “Patients who have been diagnosed with or are strongly suspected to have a cardiovascular disease that is often inherited and family members of patients who have been diagnosed with an inherited cardiovascular disease and found by genetic testing to have a mutation that is felt to be the cause of the disease.”
The statement also spells out two crucial elements for genetic testing: thorough disease-specific phenotyping – that is, using genetic information to identify the individual’s disease characteristics and a comprehensive family history that spans at least three generations. Testing should only proceed after patients has had genetic counseling and made a shared decision with their doctors.
“Genetic counseling is absolutely essential both before genetic testing to educate patients on what genetic testing entails and what potential results to expect, as well as the risks of testing; and after genetic testing, to review the results of the genetic testing and explain the potential consequences for the patient’s health and the health of family members, including children,” Dr. Musunuru said.
The process should involve board-certified geneticists or at least cardiovascular specialists well-versed in genetics and genetic counselors, the statement noted. The latter are “critical” in the care team, Dr. Musunuru said.
After the decision is made to do genetic testing, the next step is to decide the scope of the testing. That can range from targeted sequencing of a single gene or a few genes linked to the disease to large gene panels; the latter “may not increase the likelihood of clinically actionable results in adult patients,” Dr. Musunuru and colleagues wrote.
But genetic testing is no guarantee to identify a cause or confirm a diagnosis of CVD, the statement noted. “The yield for any genetic testing for any inherited cardiovascular disease remains <100%, usually much less than 100%,” the writing committee stated.
Dr. Musunuru explained that the results can sometimes be inconclusive. “In many cases, genetic testing reveals a mutation that is uninterpretable, what we call a variant of uncertain significance,” he said. “It is not clear whether the mutation increases the risk of disease or is entirely benign, which makes it very challenging to counsel patients as to whether anything should be done about the mutation.”
Even in a diagnosed patient the test results can be uncertain. “This makes it challenging to explain why the patient has the disease and whether any of the family members are at risk,” Dr. Musunuru said.
According to the statement, providers should encourage patients with a confirmed or likely pathogenic variant for CVD to share that information with “all of their at-risk relative,” the statement noted, suggesting “family letters” given to patients are a way to navigate HIPAA’s privacy limits.
The statement was written on behalf of the American Heart Association’s Council on Genomic and Precision Medicine; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology.
Dr. Musunuru and writing group members have no relevant financial relationships to disclose.
SOURCE: Musunuru K et al. Circ Genom Precis Med. 2020 Jul 23. doi: 10.1161/HCG.0000000000000067.
A new scientific statement from the American Heart Association recommends that genetic testing for inherited cardiovascular disease should be reserved for four specific types of heart diseases – cardiomyopathies, thoracic aortic aneurysms and dissections, arrhythmias, and familial hypercholesterolemia – and should enlist skilled geneticists and genetic counselors in the care team.
The guidance comes in a scientific statement published online in the journal Circulation: Genomic and Precision Medicine.
Kiran Musunuru, MD, PhD, MPH, ML, chair of the writing group for the scientific statement, described in an interview the rationale for publishing the statement at this time. “There was no prior single statement that summarized best practices for the whole gamut of inherited cardiovascular diseases in adults, only statements for individual diseases,” he said in an interview. “With genetic testing seeing explosive growth in the past few years, both in the clinical setting and with direct-to-consumer testing, we felt that cardiovascular practitioners would benefit from having a single document to serve as a general resource on genetic testing.”
The statement describes two types of patients who would be suitable for genetic testing for cardiovascular disease (CVD), Dr. Musunuru noted: “Patients who have been diagnosed with or are strongly suspected to have a cardiovascular disease that is often inherited and family members of patients who have been diagnosed with an inherited cardiovascular disease and found by genetic testing to have a mutation that is felt to be the cause of the disease.”
The statement also spells out two crucial elements for genetic testing: thorough disease-specific phenotyping – that is, using genetic information to identify the individual’s disease characteristics and a comprehensive family history that spans at least three generations. Testing should only proceed after patients has had genetic counseling and made a shared decision with their doctors.
“Genetic counseling is absolutely essential both before genetic testing to educate patients on what genetic testing entails and what potential results to expect, as well as the risks of testing; and after genetic testing, to review the results of the genetic testing and explain the potential consequences for the patient’s health and the health of family members, including children,” Dr. Musunuru said.
The process should involve board-certified geneticists or at least cardiovascular specialists well-versed in genetics and genetic counselors, the statement noted. The latter are “critical” in the care team, Dr. Musunuru said.
After the decision is made to do genetic testing, the next step is to decide the scope of the testing. That can range from targeted sequencing of a single gene or a few genes linked to the disease to large gene panels; the latter “may not increase the likelihood of clinically actionable results in adult patients,” Dr. Musunuru and colleagues wrote.
But genetic testing is no guarantee to identify a cause or confirm a diagnosis of CVD, the statement noted. “The yield for any genetic testing for any inherited cardiovascular disease remains <100%, usually much less than 100%,” the writing committee stated.
Dr. Musunuru explained that the results can sometimes be inconclusive. “In many cases, genetic testing reveals a mutation that is uninterpretable, what we call a variant of uncertain significance,” he said. “It is not clear whether the mutation increases the risk of disease or is entirely benign, which makes it very challenging to counsel patients as to whether anything should be done about the mutation.”
Even in a diagnosed patient the test results can be uncertain. “This makes it challenging to explain why the patient has the disease and whether any of the family members are at risk,” Dr. Musunuru said.
According to the statement, providers should encourage patients with a confirmed or likely pathogenic variant for CVD to share that information with “all of their at-risk relative,” the statement noted, suggesting “family letters” given to patients are a way to navigate HIPAA’s privacy limits.
The statement was written on behalf of the American Heart Association’s Council on Genomic and Precision Medicine; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology.
Dr. Musunuru and writing group members have no relevant financial relationships to disclose.
SOURCE: Musunuru K et al. Circ Genom Precis Med. 2020 Jul 23. doi: 10.1161/HCG.0000000000000067.
FROM CIRCULATION: GENOMIC AND PRECISION MEDICINE
A Multidisciplinary Ambulation Protocol to Reduce Postoperative Venous Thromboembolism After Colorectal Surgery
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.
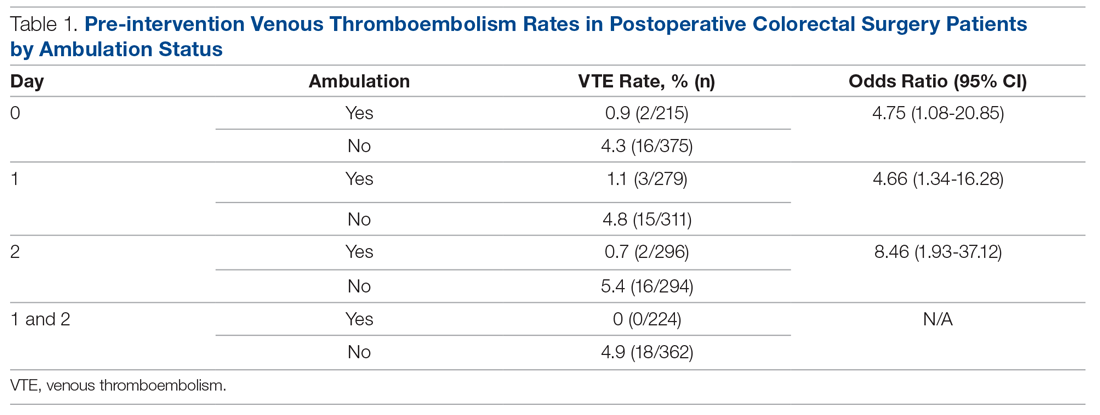
Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).
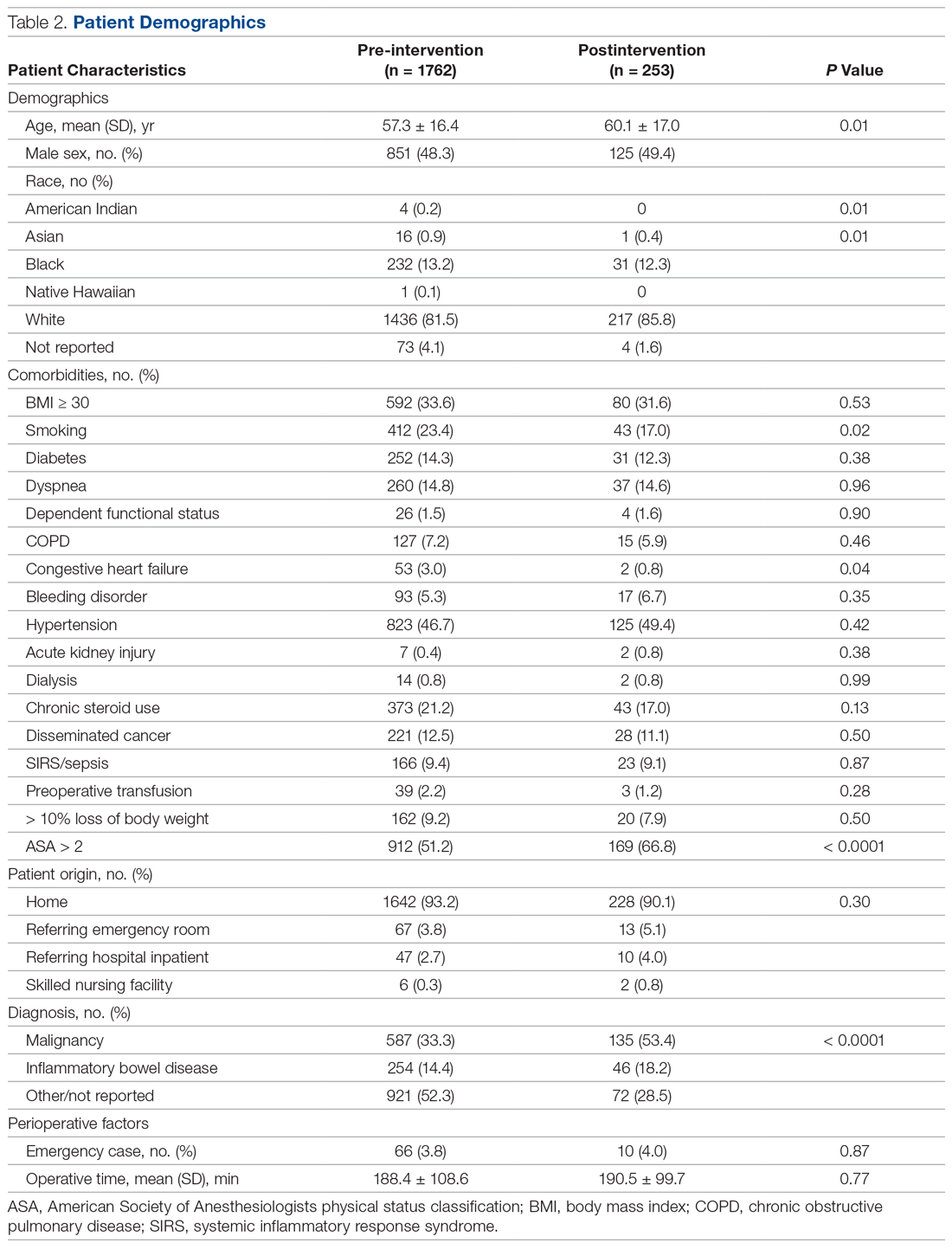
The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.
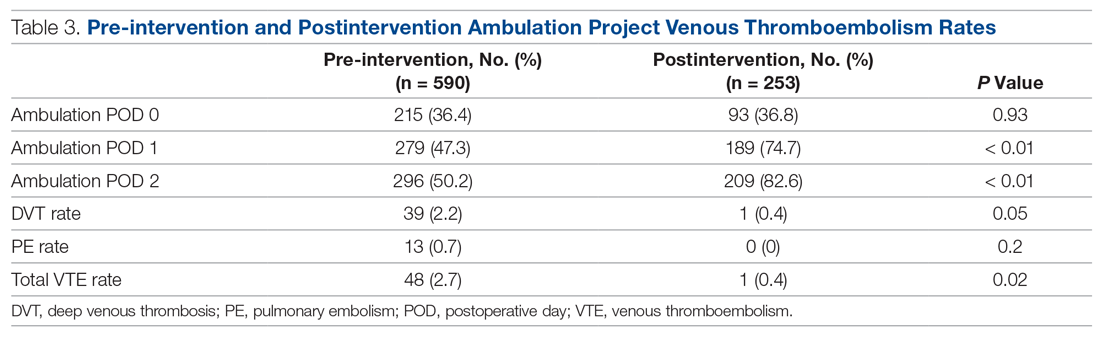
Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.

Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).

The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.

Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.

Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).

The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.

Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
A Curriculum for Training Medical Faculty to Teach Mental Health Care—and Their Responses to the Learning
From Michigan State University, East Lansing, MI.
Abstract
- Objective: We previously reported that training medical faculty to teach mental health care to residents was effective. We here describe the faculty’s training curriculum and their responses to learning and teaching mental health care, a unique focus in the educational literature.
- Design: Qualitative researchers assessed the experiences of medical faculty trainees in learning and teaching mental health care.
- Setting: Internal medicine residency training program at Michigan State University.
- Participants: One early career medicine faculty learner and another faculty learner at mid-career, 4 faculty trainers, and 2 qualitative researchers.
- Measurements: Typed qualitative research reports were evaluated by the authors from 4 time periods: (1) following didactic and interviewing training; (2) following training in a mental health clinic; (3) following training to teach residents mental health care; and (4) 8 months after training.
- Results: Faculty expressed anxiety and low confidence at each of 3 levels of training, but progressively developed confidence and satisfaction during training at each level. They rated didactic experiences as least valuable, seeing these experiences as lacking practical application. Experiential training in interviewing and mental health care were positively viewed, as was the benefit from mentoring. Teaching mental health skills to residents was initially difficult, but faculty became comfortable with experience, which solidified the faculty’s confidence in their own skills.
- Conclusion: A new curriculum for training medical faculty to teach mental health care was demonstrated to be acceptable to the faculty, based on findings from multiple focus groups.
Keywords: psychiatry; primary care mental health; medical education; curriculum; formative evaluation.
We previously trained general medicine faculty intensively in 3 evidence-based models essential for mental health care.1-4 They, in turn, trained medical residents in the models over all 3 years of residency training.5 The results of this quasi-experimental trial demonstrated highly significant learning by residents on all 3 models.6 To address the mental health care crisis caused by the severe shortage of psychiatrists in the United States,7-14 we propose this train-the-trainer intervention as a model for widescale training of medical faculty in mental health care, thus enabling them to then train their own residents and students indefinitely.6
This brief report details the faculty training curriculum in mental health care and its teaching, along with the responses of medical faculty to the training; no similar training experiences have been reported in the medical or psychiatric literature. While the residency training curriculum has been published,5 the faculty training curriculum has not. Additionally, faculty responses to the training are important because they can provide key information about what did and did not work. Even though demonstrated to be effective for teaching mental health care to residents,6 the training must also be acceptable to its new teachers.15
Methods
Design, Setting, and Participants
This descriptive study was conducted by 2 experienced qualitative researchers in the setting of a 5-year quantitative study of residents’ learning of mental health care.5,6 They interviewed 2 general medicine faculty undergoing training in mental health care on 4 occasions: 3 times during training and once following training. Learners were taught by 4 faculty trainers (2 general medicine, 2 psychiatry). The setting was the internal medicine residency program at Michigan State University. The project was approved by the local Institutional Review Board.
Faculty Training Intervention
The 2 training faculty evaluated in this study were taught in a predominantly experiential way.5 Learning objectives were behaviorally defined (see Table 1, which also presents the teaching methods). Teaching occurred in 3 segments over 15 months, with a 10% weekly commitment to training supported by a research grant.
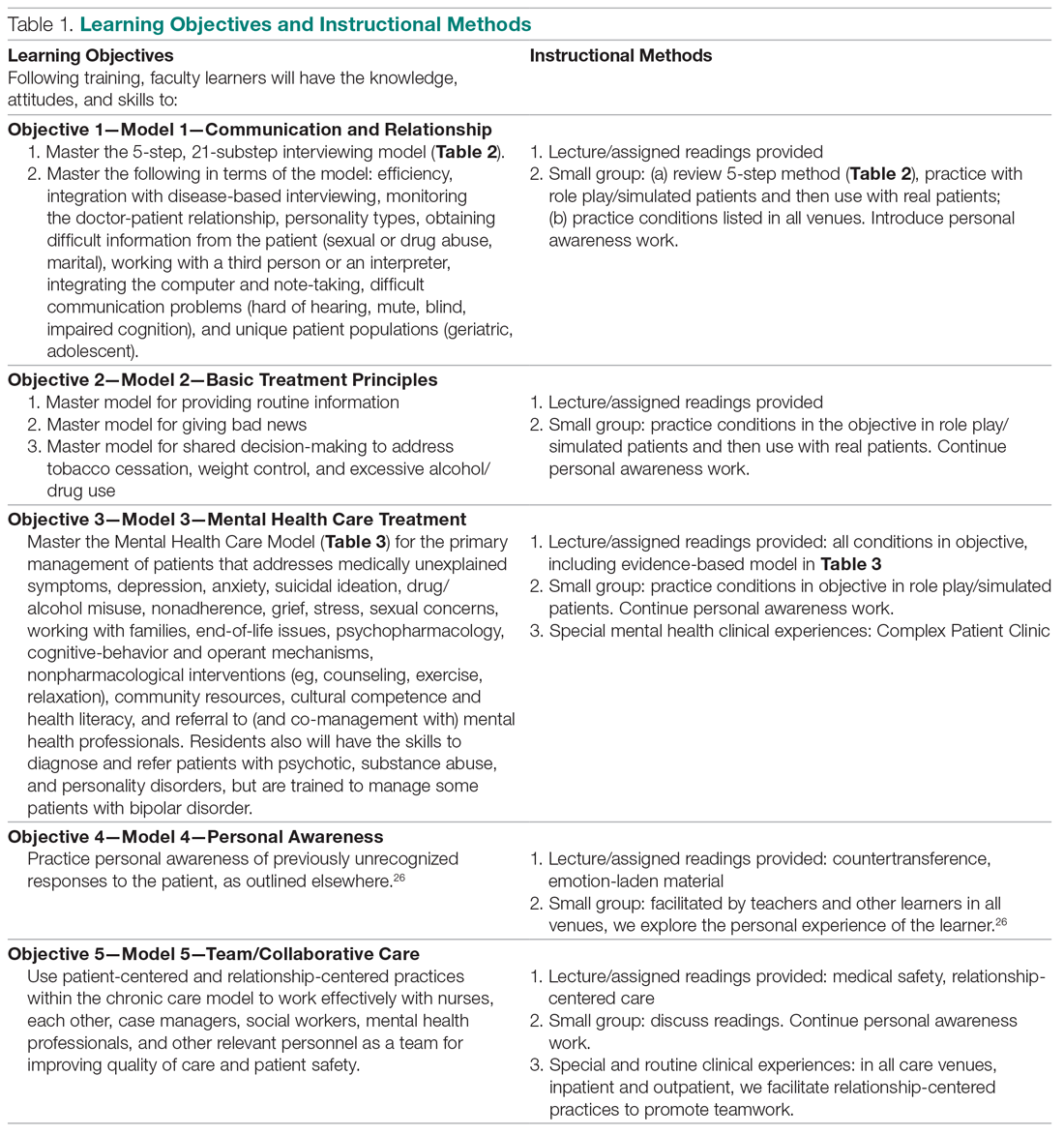
First 6 Months. For 1 half-day (4 hours) every week, teaching sessions were divided into 2 parts:
1. Experiential learning of the objectives, particularly patient-centered interviewing (Table 2)16 and mental health care models (Table 3).3,17 This initially involved role playing and was followed by using the models with hospital and clinic patients, sometimes directly observed, other times evaluated via audiotaped recordings.

2. Lecture and reading series, which occurred in 2 parts: (a) For the first 3 months, a biopsychosocial and patient-centered medicine seminar was guided by readings from a patient-centered interviewing textbook and 4 articles.3,16,18-20 These readings were supplemented by a large collection of material on our website that was utilized in a learner-centered fashion, depending on learners’ interests (these are available from the authors, along with a detailed outline we followed for each teaching session). (b) For the last 3 months, a psychiatry lecture series addressed the material needed for primary care mental health. The lectures were guided by a psychiatry textbook (the schedule and content of presentations is available from the authors).21
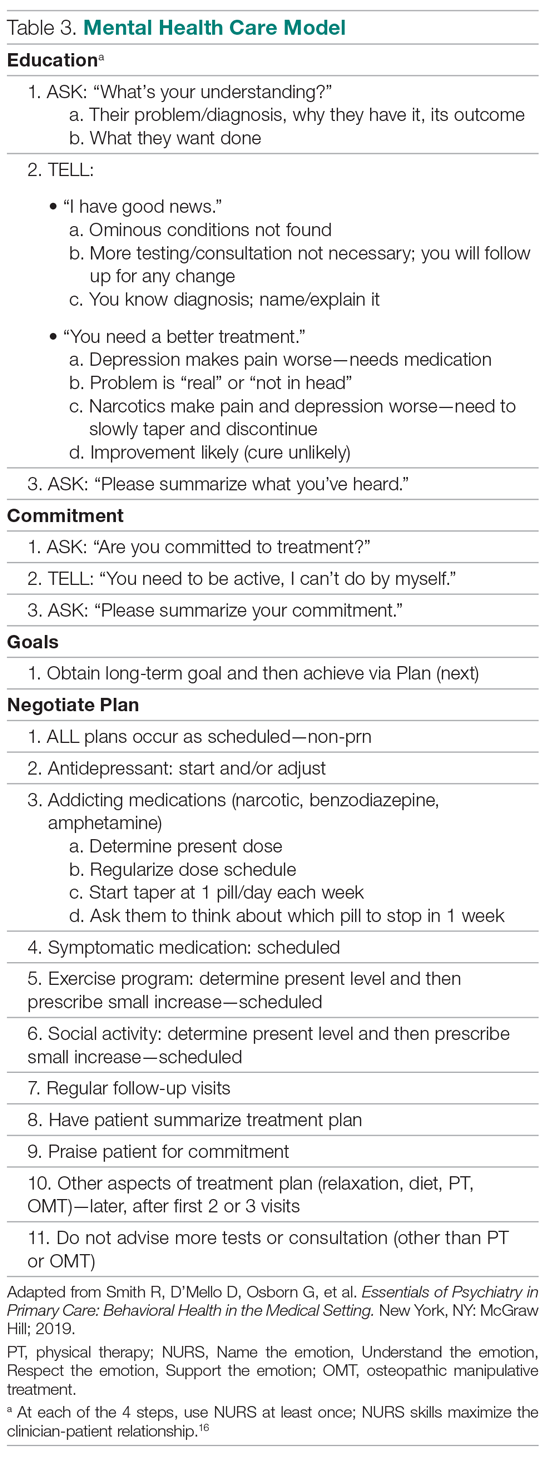
Beginning in the first 6 months, faculty also participated as co-teachers with their trainers in a long-standing psychosocial rotation, a 1-month full-time rotation for PGY-1 residents that occurred twice yearly during training. This initially helped them learn the models, and they later received experience in how to teach the models.
Middle 4 Months. During this period, faculty learners were supervised by trainers as they transitioned to learn mental health care in a Complex Patient Clinic (CPC). Training was guided by a syllabus now contained in a textbook.17 The CPC is a unique mental health care clinic located in the clinic area where faculty and residents observe other patients. Rooms resemble other exam rooms, except they have a computer attached to an audio-video camera that delivers the physician-patient interaction live to another room, where faculty observe it via a software program (Vidyo, Hackensack, NJ)22,23; no recordings are made of the live interactions. The details of patient recruitment and the CPC are described elsewhere.22 CPC patients had an average of 2.3 DSM-V diagnoses and 3.3 major medical diagnoses. Faculty trainees evaluated 2 or 3 patients each day.
Final 5 Months. Supervision continued for faculty learners as they taught mental health care to postgraduate year (PGY) 2 and 3 residents in the CPC. Residents had between 6 and 8 sessions in each of their last 2 years of training; 2 residents were assigned for each half-day CPC session and each evaluated 2 or 3 patients under faculty-learner supervision.
Data Collection
The qualitative interviewers were independent of the study. The research team members did not see the transcripts until preparing this report in conjunction with the interviewers. Data were collected from faculty at 4 points: following the initial 6 months of training in the models; following training in mental health care in the CPC; following supervision of faculty training of residents; and 8 months following completion of training, during which time they independently taught residents.
Data were collected in a systematic way over 1 hour, beginning and continuing open-endedly for about 30 minutes and concluding with closed-ended inquiry to pin down details and to ask any pre-planned questions that had not been answered. The protocol that guided focus group interviews is available from the authors.
Audio recordings were made from each group, and a 500- to 1000-word report was written by the interviewers, which served as the basis of the present descriptive evaluation. The authors independently analyzed the data at each collection point and then came to the consensus that follows.
Results
Lectures/Didactic Training
The training sessions involved 2 parts: lectures and didactic material around interviewing, general system theory, and psychiatry diagnoses; and skills practice in interviewing and the mental health care models. The trainers and faculty met weekly for 4 hours, and the first 2 hours of these sessions were spent reviewing the background of what would become the mainstay of the teaching, the models for interviewing and mental health care (Table 2 and Table 3). These readings differed in content and style from the typical clinical readings that physicians use, and they required considerable outside time and preparation, beyond that anticipated by the trainees. Digging into these theoretical concepts was described as interesting and “refreshing,” but the trainees at first found the readings disconnected from their clinical work. Faculty trainees later recognized the importance of understanding the models as they prepared for their roles as teachers. All told, however, the trainees believed there was too much didactic material.
Receiving education on diagnosis and management of common psychiatric disorders from academic psychiatrists was appreciated, but the trainees also expressed the greatest frustrations about this part of the curriculum. They felt that the level of these sessions was not always appropriately gauged—ranging from too simplistic, as in medical school, to too detailed, especially around neurochemical and neurobiological mechanisms. Although they appreciated learning about advanced psychiatric illness and treatments (eg, electroconvulsive treatment, especially), they did not believe the information was necessary in primary care. Trainees were experienced primary care providers and were more interested in case-based education that could highlight the types of patients seen in their office every day. One trainee indicated that these sessions were lacking “the patient voice.” Abstract discussion of diagnoses and treatments made it challenging to apply this new knowledge to the trainees’ practices. Trainees also suggested trying to integrate this section of the training with the interviewing skills training to better highlight that interplay. The trainees believed that their understanding and familiarity with the diagnosis and management of mental disorders occurred primarily in later CPC training. The trainees recommended that all didactic material be reduced by half or more in future teaching.
Skills Practice
The patient-centered interviewing skills practice, which occurred in the second 2-hour period during the first 6 months, was lauded by the faculty trainees. It was considered the “most immediately relevant component” of this period of training. Because the trainees were experienced physicians when they began this project, they felt this part of training made the “…material more accessible to myself, more germane to what I do day in and day out.” The insight of modifying the interviewing techniques to connect with different patient personality types was particularly helpful. One trainee described an “aha moment” of “getting patients to open up in a way I had not been able to do before.” As time went on, the trainees felt empowered to adapt “the interviewing script” modestly to fit their already developed “rhythm and style with their patients.”
Wellness/Mentoring
The 2 trainees were at different stages of their careers, 1 early-career faculty and 1 mid-career faculty. This academic diversity within the small training group provided varied perspectives not only on the concepts presented and discussed, but also on a more personal level. In an otherwise hectic academic medicine environment, this group had a weekly chance to stop, “check in” with each other, and truly connect on a personal level. To be asked “about your week and actually mean it and want to hear the answer” is an unusual opportunity, one noted. It also offered time and support for purposeful self-reflection, which “often brought some emotions to the surface…at different times.” These connections were perhaps one of the most valuable parts of the experience. With burnout among physicians rampant,24 establishing these networks is invaluable. In addition to introspection and personal connections, there was a strong element of mentoring during these weekly meetings. The opportunity to meet in a small group with senior faculty was highly valued by the trainees.
Mental Health Care: Complex Patient Clinic
The faculty were eager, but very apprehensive, in beginning the second segment of training, where work shifted from lectures and practicing skills to mental health care training in the CPC. The trainees expressed anxiety about several areas. These included additional clinical workload, patient referral/selection, and transition of patient care back to the primary care provider. Of note, they did not particularly express worries about the care they would be providing, because a psychiatrist would be available to them on site. In reflection, after spending 4 months in the clinic, trainees noted “how important observing live interviews for evaluation/feedback was to their learning.” The CPC provided “learning in the moment on specific patients [which] was without question the most powerful teaching tool.” The support of the training faculty who were present at each clinic was invaluable. Whereas the earlier didactics given by psychiatrists were received by trainees with lukewarm enthusiasm, the point-of-care, case-by-case learning and feedback truly advanced the trainees’ knowledge, as well as skills, and improved their confidence in providing mental health care.
One of the tenets of the mental health care models is collaborative care.25 Recognizing this critical component of patient care, the CPC experience integrated a clinical social worker. The faculty noted the critical role she played in the patient care experience. They described her as “fabulous and awesome.” Her grasp of the health care system and community resources (particularly for an underserved population) was indispensable. Additionally, she was able to serve as a steady contact to follow patients through multiple visits and improve their feelings of continuity.
Teaching: Psychosocial Rotation
The first psychosocial teaching occurred after the interviewing skills and didactic experiences in the first 6 months. The trainees expressed great doubt about tackling this initial teaching experience. From residents challenging the need for interviewing and other aspects of “touchy-feely” teaching, to patients expressing raw emotions, the trainees lacked confidence in their ability to handle these moments. At this early stage of their training, one trainee said, “I feel like I am becoming a better interrogator, but I haven’t learned the skills to be a better healer yet.” Over time, this concern disappeared. As training evolved, the trainees began to thrive in their role as educator. At the final focus group, it was noted that “teaching has enhanced [my] confidence in the framework and in turn has made it easier to teach.”
Teaching: Complex Patient Clinic
This powerful teaching tool to train residents was the centerpiece of training. The faculty trainees had some hesitation about their role as teacher before it began. The faculty trainees were at different stages of their careers, and their confidence in their own teaching skills was not uniform. Importantly, the initial structure of the CPC, which included psychiatrists and senior faculty supervision, provided strong and continued support for the faculty trainees. Later work in the CPC as teacher, rather than trainee, further bolstered the faculty’s confidence in the treatment models. As confidence with their own skills grew, faculty noted that it became “easier to teach” as well. Faculty also recognized the unique opportunity that the CPC provided in directly observing a resident’s patient interaction. This allows them to “monitor progress, provide specific feedback, and address issues.” The time spent debriefing after each patient encounter was noted to be particularly important. When they became too busy to adequately provide this debriefing, changes to the schedule were made to accommodate it (follow-up visits were lengthened from 30 to 60 minutes). In addition to giving an opportunity to provide feedback, this extra time available for residents to interact with a patient—to utilize and practice the interviewing skills, for example—was quite valuable, independent of actual mental health care training. Finally, the faculty were able to create a “relaxed and comfortable” space in the CPC. Indeed, the faculty felt comfortable sharing some of their struggles and reflections on caring for a mental health patient population, and residents were able, in turn, to engage in some self-reflection and debriefing as well.
Discussion
Faculty trainees demonstrated a striking evolution as they progressed through this curriculum. At each of the 3 stages of training, they endorsed a broad range of feelings, from anxiety and uncertainty initially, to confidence and growth and appreciation later. They felt satisfied with having participated in the project and are engaged in exploring next steps.
Of note, these faculty members had some exposure to the skills models prior to starting the program because the residency program has integrated patient-centered interviewing into its program for many years. The faculty were supportive of the models prior to engaging in the curriculum, and they volunteered to participate. Similarly, the residents were familiar with the expectations as they went through the psychosocial rotation and the CPC. It is conceivable that the interviewing and mental health material may not be received as easily at an institution where the culture has had less exposure to such teaching.
While describing a faculty curriculum for mental health training is unique5 and the primary intent of this paper, we wanted to present its formative evaluation even though only 2 faculty trainees were involved. Simply put, the grant for this project supported only 2 trainees, and no more were required. Nevertheless, we propose that this only reported experience of medical faculty with mental health training is an important addition to the literature in mental health education. It will be a critical guide for others who choose the new direction of training medical faculty to teach mental health care.
As the research team looks to foster dissemination of the curriculum, it continues to be streamlined to highlight the components most useful and germane to learners. The early didactic readings on subjects such as general system theory were less engaging. (In later training of new medical faculty learners, the focus on theory and other didactics was reduced.) In contrast, the trainees clearly valued the interviewing skills experience (both learning and teaching). While the mental health curriculum and the CPC were associated with much greater anxiety in the trainees, with practical, respectful, and supervised teaching, they became confident and satisfied—as well as effective.6 Future teachers will benefit from slowly and understandingly addressing trainees’ personal issues, particularly during the initial phases of training.26 It appeared to us to be the key factor enabling the faculty to successfully learn and teach mental health care. Once they overcame their personal reactions to mental health material, they learned mental health skills just as they learn the more familiar physical disease material.
Conclusion
In a new direction in medical education, a curriculum for training medical faculty to teach mental health care is presented. Not only did prior research demonstrate that the faculty effectively trained residents, but we also demonstrated here that the training was acceptable to and valued by faculty. With mental health often an alien dimension of medicine, acceptability is especially important when we recommend disseminating the curriculum as a way to offset the national mental health care crisis.
Corresponding author: Robert C. Smith, 788 Service Road, B314 Clinical Center, East Lansing, MI 48824; [email protected].
Financial disclosures: None.
Funding support: The authors are grateful for the generous support from the Health Resources and Services Administration (D58HP23259).
1. Smith R, Gardiner J, Luo Z, et al. Primary care physicians treat somatization. J Gen Int Med. 2009;24:829-832.
2. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms—a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
3. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
4. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
5. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns. 2014;94:33-42.
6. Smith R, Laird-Fick H, Dwamena F, et al. Teaching residents mental health care. Patient Educ Couns. 2018;101:2145-2155.
7. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood). 2009;28:w490-501.
8. US Department of Health and Human Services: Healthy People 2020: The Road Ahead. Washington, DC: US Governmant Printing Office; 2011.
9. US Department of Health and Human Services. Facing Addiction in America—The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Dept of Health and Human Services; 2016.
10. US Department of Health and Human Services. Mental Health and Mental Disorders. Washington, DC: US Government Printing Office; 2000.
11. Hogan MF. The President’s New Freedom Commission: recommendations to transform mental health care in America. Psychiatr Serv. 2003;54:1467-1474.
12. Morrisey J, Thomas K, Ellis A, et al. Development of a New Method for Designation of Mental Health Professional Shortage Areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
13. US Department of Health and Human Services. Mental Health: a Report of the Surgeon General. Rockville, MD: Dept. of Health and Human Services; 1999.
14. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
15. Kern DE, Thomas PA, Hughes MT. Curriculum Development for Medical Education: A Six-Step Approach. Baltimore, MD: The Johns Hopkins University Press; 2009.
16. Fortin 6th AH, Dwamena F, Frankel R, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 4th ed. New York, NY: McGraw-Hill; 2018.
17. Smith R, D’Mello D, Osborn G, et al. Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting. New York, NY: McGraw Hill; 2019 .
18. Smith R, Fortin AH 6th, Dwamena F, et al. An evidence-based patient-centered method makes the biopsychosocial model scientific. Patient Educ Couns. 2013;90:265-270.
19. Smith R, Dwamena F, Grover M, et al. Behaviorally-defined patient-centered communication—a narrative review of the literature. J Gen Intern Med. 2010;26:185-191.
20. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
21. Schneider RK, Levenson JL. Psychiatry Essentials for Primary Care. Philadelphia, PA: American College of Physicians; 2008.
22. Dwamena F, Laird-Fick H, Freilich L, et al. Behavioral health problems in medical patients. J Clin Outcomes Manage. 2014;21:497-505.
23. Vidyo (Hackensack, NJ). http://www.vidyo.com/products/use/. 2014.
24. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:195-205.
25. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55:109-122.
26. Smith RC, Dwamena FC, Fortin AH 6th. Teaching personal awareness. J Gen Intern Med. 2005;20:201-207.
From Michigan State University, East Lansing, MI.
Abstract
- Objective: We previously reported that training medical faculty to teach mental health care to residents was effective. We here describe the faculty’s training curriculum and their responses to learning and teaching mental health care, a unique focus in the educational literature.
- Design: Qualitative researchers assessed the experiences of medical faculty trainees in learning and teaching mental health care.
- Setting: Internal medicine residency training program at Michigan State University.
- Participants: One early career medicine faculty learner and another faculty learner at mid-career, 4 faculty trainers, and 2 qualitative researchers.
- Measurements: Typed qualitative research reports were evaluated by the authors from 4 time periods: (1) following didactic and interviewing training; (2) following training in a mental health clinic; (3) following training to teach residents mental health care; and (4) 8 months after training.
- Results: Faculty expressed anxiety and low confidence at each of 3 levels of training, but progressively developed confidence and satisfaction during training at each level. They rated didactic experiences as least valuable, seeing these experiences as lacking practical application. Experiential training in interviewing and mental health care were positively viewed, as was the benefit from mentoring. Teaching mental health skills to residents was initially difficult, but faculty became comfortable with experience, which solidified the faculty’s confidence in their own skills.
- Conclusion: A new curriculum for training medical faculty to teach mental health care was demonstrated to be acceptable to the faculty, based on findings from multiple focus groups.
Keywords: psychiatry; primary care mental health; medical education; curriculum; formative evaluation.
We previously trained general medicine faculty intensively in 3 evidence-based models essential for mental health care.1-4 They, in turn, trained medical residents in the models over all 3 years of residency training.5 The results of this quasi-experimental trial demonstrated highly significant learning by residents on all 3 models.6 To address the mental health care crisis caused by the severe shortage of psychiatrists in the United States,7-14 we propose this train-the-trainer intervention as a model for widescale training of medical faculty in mental health care, thus enabling them to then train their own residents and students indefinitely.6
This brief report details the faculty training curriculum in mental health care and its teaching, along with the responses of medical faculty to the training; no similar training experiences have been reported in the medical or psychiatric literature. While the residency training curriculum has been published,5 the faculty training curriculum has not. Additionally, faculty responses to the training are important because they can provide key information about what did and did not work. Even though demonstrated to be effective for teaching mental health care to residents,6 the training must also be acceptable to its new teachers.15
Methods
Design, Setting, and Participants
This descriptive study was conducted by 2 experienced qualitative researchers in the setting of a 5-year quantitative study of residents’ learning of mental health care.5,6 They interviewed 2 general medicine faculty undergoing training in mental health care on 4 occasions: 3 times during training and once following training. Learners were taught by 4 faculty trainers (2 general medicine, 2 psychiatry). The setting was the internal medicine residency program at Michigan State University. The project was approved by the local Institutional Review Board.
Faculty Training Intervention
The 2 training faculty evaluated in this study were taught in a predominantly experiential way.5 Learning objectives were behaviorally defined (see Table 1, which also presents the teaching methods). Teaching occurred in 3 segments over 15 months, with a 10% weekly commitment to training supported by a research grant.

First 6 Months. For 1 half-day (4 hours) every week, teaching sessions were divided into 2 parts:
1. Experiential learning of the objectives, particularly patient-centered interviewing (Table 2)16 and mental health care models (Table 3).3,17 This initially involved role playing and was followed by using the models with hospital and clinic patients, sometimes directly observed, other times evaluated via audiotaped recordings.

2. Lecture and reading series, which occurred in 2 parts: (a) For the first 3 months, a biopsychosocial and patient-centered medicine seminar was guided by readings from a patient-centered interviewing textbook and 4 articles.3,16,18-20 These readings were supplemented by a large collection of material on our website that was utilized in a learner-centered fashion, depending on learners’ interests (these are available from the authors, along with a detailed outline we followed for each teaching session). (b) For the last 3 months, a psychiatry lecture series addressed the material needed for primary care mental health. The lectures were guided by a psychiatry textbook (the schedule and content of presentations is available from the authors).21

Beginning in the first 6 months, faculty also participated as co-teachers with their trainers in a long-standing psychosocial rotation, a 1-month full-time rotation for PGY-1 residents that occurred twice yearly during training. This initially helped them learn the models, and they later received experience in how to teach the models.
Middle 4 Months. During this period, faculty learners were supervised by trainers as they transitioned to learn mental health care in a Complex Patient Clinic (CPC). Training was guided by a syllabus now contained in a textbook.17 The CPC is a unique mental health care clinic located in the clinic area where faculty and residents observe other patients. Rooms resemble other exam rooms, except they have a computer attached to an audio-video camera that delivers the physician-patient interaction live to another room, where faculty observe it via a software program (Vidyo, Hackensack, NJ)22,23; no recordings are made of the live interactions. The details of patient recruitment and the CPC are described elsewhere.22 CPC patients had an average of 2.3 DSM-V diagnoses and 3.3 major medical diagnoses. Faculty trainees evaluated 2 or 3 patients each day.
Final 5 Months. Supervision continued for faculty learners as they taught mental health care to postgraduate year (PGY) 2 and 3 residents in the CPC. Residents had between 6 and 8 sessions in each of their last 2 years of training; 2 residents were assigned for each half-day CPC session and each evaluated 2 or 3 patients under faculty-learner supervision.
Data Collection
The qualitative interviewers were independent of the study. The research team members did not see the transcripts until preparing this report in conjunction with the interviewers. Data were collected from faculty at 4 points: following the initial 6 months of training in the models; following training in mental health care in the CPC; following supervision of faculty training of residents; and 8 months following completion of training, during which time they independently taught residents.
Data were collected in a systematic way over 1 hour, beginning and continuing open-endedly for about 30 minutes and concluding with closed-ended inquiry to pin down details and to ask any pre-planned questions that had not been answered. The protocol that guided focus group interviews is available from the authors.
Audio recordings were made from each group, and a 500- to 1000-word report was written by the interviewers, which served as the basis of the present descriptive evaluation. The authors independently analyzed the data at each collection point and then came to the consensus that follows.
Results
Lectures/Didactic Training
The training sessions involved 2 parts: lectures and didactic material around interviewing, general system theory, and psychiatry diagnoses; and skills practice in interviewing and the mental health care models. The trainers and faculty met weekly for 4 hours, and the first 2 hours of these sessions were spent reviewing the background of what would become the mainstay of the teaching, the models for interviewing and mental health care (Table 2 and Table 3). These readings differed in content and style from the typical clinical readings that physicians use, and they required considerable outside time and preparation, beyond that anticipated by the trainees. Digging into these theoretical concepts was described as interesting and “refreshing,” but the trainees at first found the readings disconnected from their clinical work. Faculty trainees later recognized the importance of understanding the models as they prepared for their roles as teachers. All told, however, the trainees believed there was too much didactic material.
Receiving education on diagnosis and management of common psychiatric disorders from academic psychiatrists was appreciated, but the trainees also expressed the greatest frustrations about this part of the curriculum. They felt that the level of these sessions was not always appropriately gauged—ranging from too simplistic, as in medical school, to too detailed, especially around neurochemical and neurobiological mechanisms. Although they appreciated learning about advanced psychiatric illness and treatments (eg, electroconvulsive treatment, especially), they did not believe the information was necessary in primary care. Trainees were experienced primary care providers and were more interested in case-based education that could highlight the types of patients seen in their office every day. One trainee indicated that these sessions were lacking “the patient voice.” Abstract discussion of diagnoses and treatments made it challenging to apply this new knowledge to the trainees’ practices. Trainees also suggested trying to integrate this section of the training with the interviewing skills training to better highlight that interplay. The trainees believed that their understanding and familiarity with the diagnosis and management of mental disorders occurred primarily in later CPC training. The trainees recommended that all didactic material be reduced by half or more in future teaching.
Skills Practice
The patient-centered interviewing skills practice, which occurred in the second 2-hour period during the first 6 months, was lauded by the faculty trainees. It was considered the “most immediately relevant component” of this period of training. Because the trainees were experienced physicians when they began this project, they felt this part of training made the “…material more accessible to myself, more germane to what I do day in and day out.” The insight of modifying the interviewing techniques to connect with different patient personality types was particularly helpful. One trainee described an “aha moment” of “getting patients to open up in a way I had not been able to do before.” As time went on, the trainees felt empowered to adapt “the interviewing script” modestly to fit their already developed “rhythm and style with their patients.”
Wellness/Mentoring
The 2 trainees were at different stages of their careers, 1 early-career faculty and 1 mid-career faculty. This academic diversity within the small training group provided varied perspectives not only on the concepts presented and discussed, but also on a more personal level. In an otherwise hectic academic medicine environment, this group had a weekly chance to stop, “check in” with each other, and truly connect on a personal level. To be asked “about your week and actually mean it and want to hear the answer” is an unusual opportunity, one noted. It also offered time and support for purposeful self-reflection, which “often brought some emotions to the surface…at different times.” These connections were perhaps one of the most valuable parts of the experience. With burnout among physicians rampant,24 establishing these networks is invaluable. In addition to introspection and personal connections, there was a strong element of mentoring during these weekly meetings. The opportunity to meet in a small group with senior faculty was highly valued by the trainees.
Mental Health Care: Complex Patient Clinic
The faculty were eager, but very apprehensive, in beginning the second segment of training, where work shifted from lectures and practicing skills to mental health care training in the CPC. The trainees expressed anxiety about several areas. These included additional clinical workload, patient referral/selection, and transition of patient care back to the primary care provider. Of note, they did not particularly express worries about the care they would be providing, because a psychiatrist would be available to them on site. In reflection, after spending 4 months in the clinic, trainees noted “how important observing live interviews for evaluation/feedback was to their learning.” The CPC provided “learning in the moment on specific patients [which] was without question the most powerful teaching tool.” The support of the training faculty who were present at each clinic was invaluable. Whereas the earlier didactics given by psychiatrists were received by trainees with lukewarm enthusiasm, the point-of-care, case-by-case learning and feedback truly advanced the trainees’ knowledge, as well as skills, and improved their confidence in providing mental health care.
One of the tenets of the mental health care models is collaborative care.25 Recognizing this critical component of patient care, the CPC experience integrated a clinical social worker. The faculty noted the critical role she played in the patient care experience. They described her as “fabulous and awesome.” Her grasp of the health care system and community resources (particularly for an underserved population) was indispensable. Additionally, she was able to serve as a steady contact to follow patients through multiple visits and improve their feelings of continuity.
Teaching: Psychosocial Rotation
The first psychosocial teaching occurred after the interviewing skills and didactic experiences in the first 6 months. The trainees expressed great doubt about tackling this initial teaching experience. From residents challenging the need for interviewing and other aspects of “touchy-feely” teaching, to patients expressing raw emotions, the trainees lacked confidence in their ability to handle these moments. At this early stage of their training, one trainee said, “I feel like I am becoming a better interrogator, but I haven’t learned the skills to be a better healer yet.” Over time, this concern disappeared. As training evolved, the trainees began to thrive in their role as educator. At the final focus group, it was noted that “teaching has enhanced [my] confidence in the framework and in turn has made it easier to teach.”
Teaching: Complex Patient Clinic
This powerful teaching tool to train residents was the centerpiece of training. The faculty trainees had some hesitation about their role as teacher before it began. The faculty trainees were at different stages of their careers, and their confidence in their own teaching skills was not uniform. Importantly, the initial structure of the CPC, which included psychiatrists and senior faculty supervision, provided strong and continued support for the faculty trainees. Later work in the CPC as teacher, rather than trainee, further bolstered the faculty’s confidence in the treatment models. As confidence with their own skills grew, faculty noted that it became “easier to teach” as well. Faculty also recognized the unique opportunity that the CPC provided in directly observing a resident’s patient interaction. This allows them to “monitor progress, provide specific feedback, and address issues.” The time spent debriefing after each patient encounter was noted to be particularly important. When they became too busy to adequately provide this debriefing, changes to the schedule were made to accommodate it (follow-up visits were lengthened from 30 to 60 minutes). In addition to giving an opportunity to provide feedback, this extra time available for residents to interact with a patient—to utilize and practice the interviewing skills, for example—was quite valuable, independent of actual mental health care training. Finally, the faculty were able to create a “relaxed and comfortable” space in the CPC. Indeed, the faculty felt comfortable sharing some of their struggles and reflections on caring for a mental health patient population, and residents were able, in turn, to engage in some self-reflection and debriefing as well.
Discussion
Faculty trainees demonstrated a striking evolution as they progressed through this curriculum. At each of the 3 stages of training, they endorsed a broad range of feelings, from anxiety and uncertainty initially, to confidence and growth and appreciation later. They felt satisfied with having participated in the project and are engaged in exploring next steps.
Of note, these faculty members had some exposure to the skills models prior to starting the program because the residency program has integrated patient-centered interviewing into its program for many years. The faculty were supportive of the models prior to engaging in the curriculum, and they volunteered to participate. Similarly, the residents were familiar with the expectations as they went through the psychosocial rotation and the CPC. It is conceivable that the interviewing and mental health material may not be received as easily at an institution where the culture has had less exposure to such teaching.
While describing a faculty curriculum for mental health training is unique5 and the primary intent of this paper, we wanted to present its formative evaluation even though only 2 faculty trainees were involved. Simply put, the grant for this project supported only 2 trainees, and no more were required. Nevertheless, we propose that this only reported experience of medical faculty with mental health training is an important addition to the literature in mental health education. It will be a critical guide for others who choose the new direction of training medical faculty to teach mental health care.
As the research team looks to foster dissemination of the curriculum, it continues to be streamlined to highlight the components most useful and germane to learners. The early didactic readings on subjects such as general system theory were less engaging. (In later training of new medical faculty learners, the focus on theory and other didactics was reduced.) In contrast, the trainees clearly valued the interviewing skills experience (both learning and teaching). While the mental health curriculum and the CPC were associated with much greater anxiety in the trainees, with practical, respectful, and supervised teaching, they became confident and satisfied—as well as effective.6 Future teachers will benefit from slowly and understandingly addressing trainees’ personal issues, particularly during the initial phases of training.26 It appeared to us to be the key factor enabling the faculty to successfully learn and teach mental health care. Once they overcame their personal reactions to mental health material, they learned mental health skills just as they learn the more familiar physical disease material.
Conclusion
In a new direction in medical education, a curriculum for training medical faculty to teach mental health care is presented. Not only did prior research demonstrate that the faculty effectively trained residents, but we also demonstrated here that the training was acceptable to and valued by faculty. With mental health often an alien dimension of medicine, acceptability is especially important when we recommend disseminating the curriculum as a way to offset the national mental health care crisis.
Corresponding author: Robert C. Smith, 788 Service Road, B314 Clinical Center, East Lansing, MI 48824; [email protected].
Financial disclosures: None.
Funding support: The authors are grateful for the generous support from the Health Resources and Services Administration (D58HP23259).
From Michigan State University, East Lansing, MI.
Abstract
- Objective: We previously reported that training medical faculty to teach mental health care to residents was effective. We here describe the faculty’s training curriculum and their responses to learning and teaching mental health care, a unique focus in the educational literature.
- Design: Qualitative researchers assessed the experiences of medical faculty trainees in learning and teaching mental health care.
- Setting: Internal medicine residency training program at Michigan State University.
- Participants: One early career medicine faculty learner and another faculty learner at mid-career, 4 faculty trainers, and 2 qualitative researchers.
- Measurements: Typed qualitative research reports were evaluated by the authors from 4 time periods: (1) following didactic and interviewing training; (2) following training in a mental health clinic; (3) following training to teach residents mental health care; and (4) 8 months after training.
- Results: Faculty expressed anxiety and low confidence at each of 3 levels of training, but progressively developed confidence and satisfaction during training at each level. They rated didactic experiences as least valuable, seeing these experiences as lacking practical application. Experiential training in interviewing and mental health care were positively viewed, as was the benefit from mentoring. Teaching mental health skills to residents was initially difficult, but faculty became comfortable with experience, which solidified the faculty’s confidence in their own skills.
- Conclusion: A new curriculum for training medical faculty to teach mental health care was demonstrated to be acceptable to the faculty, based on findings from multiple focus groups.
Keywords: psychiatry; primary care mental health; medical education; curriculum; formative evaluation.
We previously trained general medicine faculty intensively in 3 evidence-based models essential for mental health care.1-4 They, in turn, trained medical residents in the models over all 3 years of residency training.5 The results of this quasi-experimental trial demonstrated highly significant learning by residents on all 3 models.6 To address the mental health care crisis caused by the severe shortage of psychiatrists in the United States,7-14 we propose this train-the-trainer intervention as a model for widescale training of medical faculty in mental health care, thus enabling them to then train their own residents and students indefinitely.6
This brief report details the faculty training curriculum in mental health care and its teaching, along with the responses of medical faculty to the training; no similar training experiences have been reported in the medical or psychiatric literature. While the residency training curriculum has been published,5 the faculty training curriculum has not. Additionally, faculty responses to the training are important because they can provide key information about what did and did not work. Even though demonstrated to be effective for teaching mental health care to residents,6 the training must also be acceptable to its new teachers.15
Methods
Design, Setting, and Participants
This descriptive study was conducted by 2 experienced qualitative researchers in the setting of a 5-year quantitative study of residents’ learning of mental health care.5,6 They interviewed 2 general medicine faculty undergoing training in mental health care on 4 occasions: 3 times during training and once following training. Learners were taught by 4 faculty trainers (2 general medicine, 2 psychiatry). The setting was the internal medicine residency program at Michigan State University. The project was approved by the local Institutional Review Board.
Faculty Training Intervention
The 2 training faculty evaluated in this study were taught in a predominantly experiential way.5 Learning objectives were behaviorally defined (see Table 1, which also presents the teaching methods). Teaching occurred in 3 segments over 15 months, with a 10% weekly commitment to training supported by a research grant.

First 6 Months. For 1 half-day (4 hours) every week, teaching sessions were divided into 2 parts:
1. Experiential learning of the objectives, particularly patient-centered interviewing (Table 2)16 and mental health care models (Table 3).3,17 This initially involved role playing and was followed by using the models with hospital and clinic patients, sometimes directly observed, other times evaluated via audiotaped recordings.

2. Lecture and reading series, which occurred in 2 parts: (a) For the first 3 months, a biopsychosocial and patient-centered medicine seminar was guided by readings from a patient-centered interviewing textbook and 4 articles.3,16,18-20 These readings were supplemented by a large collection of material on our website that was utilized in a learner-centered fashion, depending on learners’ interests (these are available from the authors, along with a detailed outline we followed for each teaching session). (b) For the last 3 months, a psychiatry lecture series addressed the material needed for primary care mental health. The lectures were guided by a psychiatry textbook (the schedule and content of presentations is available from the authors).21

Beginning in the first 6 months, faculty also participated as co-teachers with their trainers in a long-standing psychosocial rotation, a 1-month full-time rotation for PGY-1 residents that occurred twice yearly during training. This initially helped them learn the models, and they later received experience in how to teach the models.
Middle 4 Months. During this period, faculty learners were supervised by trainers as they transitioned to learn mental health care in a Complex Patient Clinic (CPC). Training was guided by a syllabus now contained in a textbook.17 The CPC is a unique mental health care clinic located in the clinic area where faculty and residents observe other patients. Rooms resemble other exam rooms, except they have a computer attached to an audio-video camera that delivers the physician-patient interaction live to another room, where faculty observe it via a software program (Vidyo, Hackensack, NJ)22,23; no recordings are made of the live interactions. The details of patient recruitment and the CPC are described elsewhere.22 CPC patients had an average of 2.3 DSM-V diagnoses and 3.3 major medical diagnoses. Faculty trainees evaluated 2 or 3 patients each day.
Final 5 Months. Supervision continued for faculty learners as they taught mental health care to postgraduate year (PGY) 2 and 3 residents in the CPC. Residents had between 6 and 8 sessions in each of their last 2 years of training; 2 residents were assigned for each half-day CPC session and each evaluated 2 or 3 patients under faculty-learner supervision.
Data Collection
The qualitative interviewers were independent of the study. The research team members did not see the transcripts until preparing this report in conjunction with the interviewers. Data were collected from faculty at 4 points: following the initial 6 months of training in the models; following training in mental health care in the CPC; following supervision of faculty training of residents; and 8 months following completion of training, during which time they independently taught residents.
Data were collected in a systematic way over 1 hour, beginning and continuing open-endedly for about 30 minutes and concluding with closed-ended inquiry to pin down details and to ask any pre-planned questions that had not been answered. The protocol that guided focus group interviews is available from the authors.
Audio recordings were made from each group, and a 500- to 1000-word report was written by the interviewers, which served as the basis of the present descriptive evaluation. The authors independently analyzed the data at each collection point and then came to the consensus that follows.
Results
Lectures/Didactic Training
The training sessions involved 2 parts: lectures and didactic material around interviewing, general system theory, and psychiatry diagnoses; and skills practice in interviewing and the mental health care models. The trainers and faculty met weekly for 4 hours, and the first 2 hours of these sessions were spent reviewing the background of what would become the mainstay of the teaching, the models for interviewing and mental health care (Table 2 and Table 3). These readings differed in content and style from the typical clinical readings that physicians use, and they required considerable outside time and preparation, beyond that anticipated by the trainees. Digging into these theoretical concepts was described as interesting and “refreshing,” but the trainees at first found the readings disconnected from their clinical work. Faculty trainees later recognized the importance of understanding the models as they prepared for their roles as teachers. All told, however, the trainees believed there was too much didactic material.
Receiving education on diagnosis and management of common psychiatric disorders from academic psychiatrists was appreciated, but the trainees also expressed the greatest frustrations about this part of the curriculum. They felt that the level of these sessions was not always appropriately gauged—ranging from too simplistic, as in medical school, to too detailed, especially around neurochemical and neurobiological mechanisms. Although they appreciated learning about advanced psychiatric illness and treatments (eg, electroconvulsive treatment, especially), they did not believe the information was necessary in primary care. Trainees were experienced primary care providers and were more interested in case-based education that could highlight the types of patients seen in their office every day. One trainee indicated that these sessions were lacking “the patient voice.” Abstract discussion of diagnoses and treatments made it challenging to apply this new knowledge to the trainees’ practices. Trainees also suggested trying to integrate this section of the training with the interviewing skills training to better highlight that interplay. The trainees believed that their understanding and familiarity with the diagnosis and management of mental disorders occurred primarily in later CPC training. The trainees recommended that all didactic material be reduced by half or more in future teaching.
Skills Practice
The patient-centered interviewing skills practice, which occurred in the second 2-hour period during the first 6 months, was lauded by the faculty trainees. It was considered the “most immediately relevant component” of this period of training. Because the trainees were experienced physicians when they began this project, they felt this part of training made the “…material more accessible to myself, more germane to what I do day in and day out.” The insight of modifying the interviewing techniques to connect with different patient personality types was particularly helpful. One trainee described an “aha moment” of “getting patients to open up in a way I had not been able to do before.” As time went on, the trainees felt empowered to adapt “the interviewing script” modestly to fit their already developed “rhythm and style with their patients.”
Wellness/Mentoring
The 2 trainees were at different stages of their careers, 1 early-career faculty and 1 mid-career faculty. This academic diversity within the small training group provided varied perspectives not only on the concepts presented and discussed, but also on a more personal level. In an otherwise hectic academic medicine environment, this group had a weekly chance to stop, “check in” with each other, and truly connect on a personal level. To be asked “about your week and actually mean it and want to hear the answer” is an unusual opportunity, one noted. It also offered time and support for purposeful self-reflection, which “often brought some emotions to the surface…at different times.” These connections were perhaps one of the most valuable parts of the experience. With burnout among physicians rampant,24 establishing these networks is invaluable. In addition to introspection and personal connections, there was a strong element of mentoring during these weekly meetings. The opportunity to meet in a small group with senior faculty was highly valued by the trainees.
Mental Health Care: Complex Patient Clinic
The faculty were eager, but very apprehensive, in beginning the second segment of training, where work shifted from lectures and practicing skills to mental health care training in the CPC. The trainees expressed anxiety about several areas. These included additional clinical workload, patient referral/selection, and transition of patient care back to the primary care provider. Of note, they did not particularly express worries about the care they would be providing, because a psychiatrist would be available to them on site. In reflection, after spending 4 months in the clinic, trainees noted “how important observing live interviews for evaluation/feedback was to their learning.” The CPC provided “learning in the moment on specific patients [which] was without question the most powerful teaching tool.” The support of the training faculty who were present at each clinic was invaluable. Whereas the earlier didactics given by psychiatrists were received by trainees with lukewarm enthusiasm, the point-of-care, case-by-case learning and feedback truly advanced the trainees’ knowledge, as well as skills, and improved their confidence in providing mental health care.
One of the tenets of the mental health care models is collaborative care.25 Recognizing this critical component of patient care, the CPC experience integrated a clinical social worker. The faculty noted the critical role she played in the patient care experience. They described her as “fabulous and awesome.” Her grasp of the health care system and community resources (particularly for an underserved population) was indispensable. Additionally, she was able to serve as a steady contact to follow patients through multiple visits and improve their feelings of continuity.
Teaching: Psychosocial Rotation
The first psychosocial teaching occurred after the interviewing skills and didactic experiences in the first 6 months. The trainees expressed great doubt about tackling this initial teaching experience. From residents challenging the need for interviewing and other aspects of “touchy-feely” teaching, to patients expressing raw emotions, the trainees lacked confidence in their ability to handle these moments. At this early stage of their training, one trainee said, “I feel like I am becoming a better interrogator, but I haven’t learned the skills to be a better healer yet.” Over time, this concern disappeared. As training evolved, the trainees began to thrive in their role as educator. At the final focus group, it was noted that “teaching has enhanced [my] confidence in the framework and in turn has made it easier to teach.”
Teaching: Complex Patient Clinic
This powerful teaching tool to train residents was the centerpiece of training. The faculty trainees had some hesitation about their role as teacher before it began. The faculty trainees were at different stages of their careers, and their confidence in their own teaching skills was not uniform. Importantly, the initial structure of the CPC, which included psychiatrists and senior faculty supervision, provided strong and continued support for the faculty trainees. Later work in the CPC as teacher, rather than trainee, further bolstered the faculty’s confidence in the treatment models. As confidence with their own skills grew, faculty noted that it became “easier to teach” as well. Faculty also recognized the unique opportunity that the CPC provided in directly observing a resident’s patient interaction. This allows them to “monitor progress, provide specific feedback, and address issues.” The time spent debriefing after each patient encounter was noted to be particularly important. When they became too busy to adequately provide this debriefing, changes to the schedule were made to accommodate it (follow-up visits were lengthened from 30 to 60 minutes). In addition to giving an opportunity to provide feedback, this extra time available for residents to interact with a patient—to utilize and practice the interviewing skills, for example—was quite valuable, independent of actual mental health care training. Finally, the faculty were able to create a “relaxed and comfortable” space in the CPC. Indeed, the faculty felt comfortable sharing some of their struggles and reflections on caring for a mental health patient population, and residents were able, in turn, to engage in some self-reflection and debriefing as well.
Discussion
Faculty trainees demonstrated a striking evolution as they progressed through this curriculum. At each of the 3 stages of training, they endorsed a broad range of feelings, from anxiety and uncertainty initially, to confidence and growth and appreciation later. They felt satisfied with having participated in the project and are engaged in exploring next steps.
Of note, these faculty members had some exposure to the skills models prior to starting the program because the residency program has integrated patient-centered interviewing into its program for many years. The faculty were supportive of the models prior to engaging in the curriculum, and they volunteered to participate. Similarly, the residents were familiar with the expectations as they went through the psychosocial rotation and the CPC. It is conceivable that the interviewing and mental health material may not be received as easily at an institution where the culture has had less exposure to such teaching.
While describing a faculty curriculum for mental health training is unique5 and the primary intent of this paper, we wanted to present its formative evaluation even though only 2 faculty trainees were involved. Simply put, the grant for this project supported only 2 trainees, and no more were required. Nevertheless, we propose that this only reported experience of medical faculty with mental health training is an important addition to the literature in mental health education. It will be a critical guide for others who choose the new direction of training medical faculty to teach mental health care.
As the research team looks to foster dissemination of the curriculum, it continues to be streamlined to highlight the components most useful and germane to learners. The early didactic readings on subjects such as general system theory were less engaging. (In later training of new medical faculty learners, the focus on theory and other didactics was reduced.) In contrast, the trainees clearly valued the interviewing skills experience (both learning and teaching). While the mental health curriculum and the CPC were associated with much greater anxiety in the trainees, with practical, respectful, and supervised teaching, they became confident and satisfied—as well as effective.6 Future teachers will benefit from slowly and understandingly addressing trainees’ personal issues, particularly during the initial phases of training.26 It appeared to us to be the key factor enabling the faculty to successfully learn and teach mental health care. Once they overcame their personal reactions to mental health material, they learned mental health skills just as they learn the more familiar physical disease material.
Conclusion
In a new direction in medical education, a curriculum for training medical faculty to teach mental health care is presented. Not only did prior research demonstrate that the faculty effectively trained residents, but we also demonstrated here that the training was acceptable to and valued by faculty. With mental health often an alien dimension of medicine, acceptability is especially important when we recommend disseminating the curriculum as a way to offset the national mental health care crisis.
Corresponding author: Robert C. Smith, 788 Service Road, B314 Clinical Center, East Lansing, MI 48824; [email protected].
Financial disclosures: None.
Funding support: The authors are grateful for the generous support from the Health Resources and Services Administration (D58HP23259).
1. Smith R, Gardiner J, Luo Z, et al. Primary care physicians treat somatization. J Gen Int Med. 2009;24:829-832.
2. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms—a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
3. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
4. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
5. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns. 2014;94:33-42.
6. Smith R, Laird-Fick H, Dwamena F, et al. Teaching residents mental health care. Patient Educ Couns. 2018;101:2145-2155.
7. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood). 2009;28:w490-501.
8. US Department of Health and Human Services: Healthy People 2020: The Road Ahead. Washington, DC: US Governmant Printing Office; 2011.
9. US Department of Health and Human Services. Facing Addiction in America—The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Dept of Health and Human Services; 2016.
10. US Department of Health and Human Services. Mental Health and Mental Disorders. Washington, DC: US Government Printing Office; 2000.
11. Hogan MF. The President’s New Freedom Commission: recommendations to transform mental health care in America. Psychiatr Serv. 2003;54:1467-1474.
12. Morrisey J, Thomas K, Ellis A, et al. Development of a New Method for Designation of Mental Health Professional Shortage Areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
13. US Department of Health and Human Services. Mental Health: a Report of the Surgeon General. Rockville, MD: Dept. of Health and Human Services; 1999.
14. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
15. Kern DE, Thomas PA, Hughes MT. Curriculum Development for Medical Education: A Six-Step Approach. Baltimore, MD: The Johns Hopkins University Press; 2009.
16. Fortin 6th AH, Dwamena F, Frankel R, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 4th ed. New York, NY: McGraw-Hill; 2018.
17. Smith R, D’Mello D, Osborn G, et al. Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting. New York, NY: McGraw Hill; 2019 .
18. Smith R, Fortin AH 6th, Dwamena F, et al. An evidence-based patient-centered method makes the biopsychosocial model scientific. Patient Educ Couns. 2013;90:265-270.
19. Smith R, Dwamena F, Grover M, et al. Behaviorally-defined patient-centered communication—a narrative review of the literature. J Gen Intern Med. 2010;26:185-191.
20. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
21. Schneider RK, Levenson JL. Psychiatry Essentials for Primary Care. Philadelphia, PA: American College of Physicians; 2008.
22. Dwamena F, Laird-Fick H, Freilich L, et al. Behavioral health problems in medical patients. J Clin Outcomes Manage. 2014;21:497-505.
23. Vidyo (Hackensack, NJ). http://www.vidyo.com/products/use/. 2014.
24. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:195-205.
25. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55:109-122.
26. Smith RC, Dwamena FC, Fortin AH 6th. Teaching personal awareness. J Gen Intern Med. 2005;20:201-207.
1. Smith R, Gardiner J, Luo Z, et al. Primary care physicians treat somatization. J Gen Int Med. 2009;24:829-832.
2. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms—a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
3. Smith RC, Lein C, Collins C, et al. Treating patients with medically unexplained symptoms in primary care. J Gen Intern Med. 2003;18:478-489.
4. Smith RC, Lyles JS, Mettler J, et al. The effectiveness of intensive training for residents in interviewing. A randomized, controlled study. Ann Intern Med. 1998;128:118-126.
5. Smith R, Laird-Fick H, D’Mello D, et al. Addressing mental health issues in primary care: an initial curriculum for medical residents. Patient Educ Couns. 2014;94:33-42.
6. Smith R, Laird-Fick H, Dwamena F, et al. Teaching residents mental health care. Patient Educ Couns. 2018;101:2145-2155.
7. Cunningham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood). 2009;28:w490-501.
8. US Department of Health and Human Services: Healthy People 2020: The Road Ahead. Washington, DC: US Governmant Printing Office; 2011.
9. US Department of Health and Human Services. Facing Addiction in America—The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: US Dept of Health and Human Services; 2016.
10. US Department of Health and Human Services. Mental Health and Mental Disorders. Washington, DC: US Government Printing Office; 2000.
11. Hogan MF. The President’s New Freedom Commission: recommendations to transform mental health care in America. Psychiatr Serv. 2003;54:1467-1474.
12. Morrisey J, Thomas K, Ellis A, et al. Development of a New Method for Designation of Mental Health Professional Shortage Areas. Chapel Hill, NC: University of North Carolina at Chapel Hill; 2007.
13. US Department of Health and Human Services. Mental Health: a Report of the Surgeon General. Rockville, MD: Dept. of Health and Human Services; 1999.
14. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:629-640.
15. Kern DE, Thomas PA, Hughes MT. Curriculum Development for Medical Education: A Six-Step Approach. Baltimore, MD: The Johns Hopkins University Press; 2009.
16. Fortin 6th AH, Dwamena F, Frankel R, et al. Smith’s Patient-Centered Interviewing: An Evidence-Based Method. 4th ed. New York, NY: McGraw-Hill; 2018.
17. Smith R, D’Mello D, Osborn G, et al. Essentials of Psychiatry in Primary Care: Behavioral Health in the Medical Setting. New York, NY: McGraw Hill; 2019 .
18. Smith R, Fortin AH 6th, Dwamena F, et al. An evidence-based patient-centered method makes the biopsychosocial model scientific. Patient Educ Couns. 2013;90:265-270.
19. Smith R, Dwamena F, Grover M, et al. Behaviorally-defined patient-centered communication—a narrative review of the literature. J Gen Intern Med. 2010;26:185-191.
20. Smith RC, Dwamena FC. Classification and diagnosis of patients with medically unexplained symptoms. J Gen Intern Med. 2007;22:685-691.
21. Schneider RK, Levenson JL. Psychiatry Essentials for Primary Care. Philadelphia, PA: American College of Physicians; 2008.
22. Dwamena F, Laird-Fick H, Freilich L, et al. Behavioral health problems in medical patients. J Clin Outcomes Manage. 2014;21:497-505.
23. Vidyo (Hackensack, NJ). http://www.vidyo.com/products/use/. 2014.
24. Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:195-205.
25. Huffman JC, Niazi SK, Rundell JR, et al. Essential articles on collaborative care models for the treatment of psychiatric disorders in medical settings: a publication by the Academy of Psychosomatic Medicine Research and Evidence-Based Practice Committee. Psychosomatics. 2014;55:109-122.
26. Smith RC, Dwamena FC, Fortin AH 6th. Teaching personal awareness. J Gen Intern Med. 2005;20:201-207.











