User login
Fundoplication works best for true PPI-refractory heartburn
WASHINGTON – Less than a quarter of patients with heartburn that appears refractory to proton pump inhibitor treatment truly have reflux-related, drug-refractory heartburn with a high symptom–related probability, but patients who fall into this select subgroup often have significant symptom relief from surgical fundoplication, based on results from a randomized, multicenter, Department of Veterans Affairs study with 78 patients.
Although laparoscopic Nissen fundoplication relieved the heartburn symptoms of just two-thirds of patients who met the study’s definition of having true proton pump inhibitor (PPI)–refractory heartburn, this level of efficacy far exceeded the impact of drug therapy with baclofen or desipramine, which was little better than placebo, Stuart J. Spechler, MD, said at the annual Digestive Disease Week®.
“Fundoplication fell out of favor because of the success of PPI treatment, and because of complications from the surgery, but what our results show is that there is a subgroup of patients who can benefit from fundoplication. The challenge is identifying them,” said Dr. Spechler, a gastroenterologist and professor of medicine at the University of Texas, Dallas. “If you go through a careful work-up you will find the patients who have true PPI-refractory acid reflux and heartburn, and in the end we don’t have good medical treatments for these patients,” leaving fundoplication as their best hope for symptom relief.
The study he ran included 366 patients seen at about 30 VA Medical Centers across the United States who had been referred to his center because of presumed PPI-refractory heartburn. The careful work-up that Dr. Spechler and his associates ran included a closely supervised, 2-week trial of a standardized PPI regimen with omeprazole, careful symptom scoring on this treatment with a reflux-specific, health-related quality of life questionnaire, endoscopic esophageal manometry, and esophageal pH monitoring while on omeprazole.
This process placed patients into several distinct subgroups: About 19% dropped out of the study during this assessment, and another 15% left the study because of their intolerance of various stages of the work-up. Nearly 12% of patients wound up being responsive to the PPI regimen, about 6% had organic disorders not related to gastroesophageal reflux disease, and 27% had functional heartburn with a normal level of acid reflux, which left 78 patients (21%) who demonstrated true reflux-related, PPI-refractory heartburn symptoms.
The researchers then randomized this 78-patient subgroup into three treatment arms, with one group of 27 underwent fundoplication surgery. A group of 25 underwent active medical therapy with 20 mg omeprazole b.i.d. plus baclofen, which was started at 5 mg t.i.d. and increased to 20 mg t.i.d. In baclofen-intolerant or nonresponding patients, this treatment was followed up with desipramine, increasing from a starting dosage of 25 mg/day to 100 mg/day. A third group of 26 control patients received active omeprazole at the same dosage but placebo in place of the baclofen and desipramine. These three subgroups showed no statistically significant differences at baseline for all demographic and clinical parameters recorded.
The study’s primary endpoint was the percentage of patients in each treatment arm who had a “successful” outcome, defined as at least a 50% improvement in their gastroesophageal reflux health-related quality of life score (J Gastrointest Surg. 1998 Mar-Apr;2[2]:141-5) after 1 year on treatment, which occurred in 67% of the fundoplication patients, 28% in the active medical arm, and 12% in the control arm. The fundoplication-treated patients had a significantly higher rate of a successful outcome, compared with patients in each of the other two treatment groups, while the success rates among patients in the active medical group and the control group did not differ significantly, Dr. Spechler said.
Dr. Spechler had no disclosures to report.
WASHINGTON – Less than a quarter of patients with heartburn that appears refractory to proton pump inhibitor treatment truly have reflux-related, drug-refractory heartburn with a high symptom–related probability, but patients who fall into this select subgroup often have significant symptom relief from surgical fundoplication, based on results from a randomized, multicenter, Department of Veterans Affairs study with 78 patients.
Although laparoscopic Nissen fundoplication relieved the heartburn symptoms of just two-thirds of patients who met the study’s definition of having true proton pump inhibitor (PPI)–refractory heartburn, this level of efficacy far exceeded the impact of drug therapy with baclofen or desipramine, which was little better than placebo, Stuart J. Spechler, MD, said at the annual Digestive Disease Week®.
“Fundoplication fell out of favor because of the success of PPI treatment, and because of complications from the surgery, but what our results show is that there is a subgroup of patients who can benefit from fundoplication. The challenge is identifying them,” said Dr. Spechler, a gastroenterologist and professor of medicine at the University of Texas, Dallas. “If you go through a careful work-up you will find the patients who have true PPI-refractory acid reflux and heartburn, and in the end we don’t have good medical treatments for these patients,” leaving fundoplication as their best hope for symptom relief.
The study he ran included 366 patients seen at about 30 VA Medical Centers across the United States who had been referred to his center because of presumed PPI-refractory heartburn. The careful work-up that Dr. Spechler and his associates ran included a closely supervised, 2-week trial of a standardized PPI regimen with omeprazole, careful symptom scoring on this treatment with a reflux-specific, health-related quality of life questionnaire, endoscopic esophageal manometry, and esophageal pH monitoring while on omeprazole.
This process placed patients into several distinct subgroups: About 19% dropped out of the study during this assessment, and another 15% left the study because of their intolerance of various stages of the work-up. Nearly 12% of patients wound up being responsive to the PPI regimen, about 6% had organic disorders not related to gastroesophageal reflux disease, and 27% had functional heartburn with a normal level of acid reflux, which left 78 patients (21%) who demonstrated true reflux-related, PPI-refractory heartburn symptoms.
The researchers then randomized this 78-patient subgroup into three treatment arms, with one group of 27 underwent fundoplication surgery. A group of 25 underwent active medical therapy with 20 mg omeprazole b.i.d. plus baclofen, which was started at 5 mg t.i.d. and increased to 20 mg t.i.d. In baclofen-intolerant or nonresponding patients, this treatment was followed up with desipramine, increasing from a starting dosage of 25 mg/day to 100 mg/day. A third group of 26 control patients received active omeprazole at the same dosage but placebo in place of the baclofen and desipramine. These three subgroups showed no statistically significant differences at baseline for all demographic and clinical parameters recorded.
The study’s primary endpoint was the percentage of patients in each treatment arm who had a “successful” outcome, defined as at least a 50% improvement in their gastroesophageal reflux health-related quality of life score (J Gastrointest Surg. 1998 Mar-Apr;2[2]:141-5) after 1 year on treatment, which occurred in 67% of the fundoplication patients, 28% in the active medical arm, and 12% in the control arm. The fundoplication-treated patients had a significantly higher rate of a successful outcome, compared with patients in each of the other two treatment groups, while the success rates among patients in the active medical group and the control group did not differ significantly, Dr. Spechler said.
Dr. Spechler had no disclosures to report.
WASHINGTON – Less than a quarter of patients with heartburn that appears refractory to proton pump inhibitor treatment truly have reflux-related, drug-refractory heartburn with a high symptom–related probability, but patients who fall into this select subgroup often have significant symptom relief from surgical fundoplication, based on results from a randomized, multicenter, Department of Veterans Affairs study with 78 patients.
Although laparoscopic Nissen fundoplication relieved the heartburn symptoms of just two-thirds of patients who met the study’s definition of having true proton pump inhibitor (PPI)–refractory heartburn, this level of efficacy far exceeded the impact of drug therapy with baclofen or desipramine, which was little better than placebo, Stuart J. Spechler, MD, said at the annual Digestive Disease Week®.
“Fundoplication fell out of favor because of the success of PPI treatment, and because of complications from the surgery, but what our results show is that there is a subgroup of patients who can benefit from fundoplication. The challenge is identifying them,” said Dr. Spechler, a gastroenterologist and professor of medicine at the University of Texas, Dallas. “If you go through a careful work-up you will find the patients who have true PPI-refractory acid reflux and heartburn, and in the end we don’t have good medical treatments for these patients,” leaving fundoplication as their best hope for symptom relief.
The study he ran included 366 patients seen at about 30 VA Medical Centers across the United States who had been referred to his center because of presumed PPI-refractory heartburn. The careful work-up that Dr. Spechler and his associates ran included a closely supervised, 2-week trial of a standardized PPI regimen with omeprazole, careful symptom scoring on this treatment with a reflux-specific, health-related quality of life questionnaire, endoscopic esophageal manometry, and esophageal pH monitoring while on omeprazole.
This process placed patients into several distinct subgroups: About 19% dropped out of the study during this assessment, and another 15% left the study because of their intolerance of various stages of the work-up. Nearly 12% of patients wound up being responsive to the PPI regimen, about 6% had organic disorders not related to gastroesophageal reflux disease, and 27% had functional heartburn with a normal level of acid reflux, which left 78 patients (21%) who demonstrated true reflux-related, PPI-refractory heartburn symptoms.
The researchers then randomized this 78-patient subgroup into three treatment arms, with one group of 27 underwent fundoplication surgery. A group of 25 underwent active medical therapy with 20 mg omeprazole b.i.d. plus baclofen, which was started at 5 mg t.i.d. and increased to 20 mg t.i.d. In baclofen-intolerant or nonresponding patients, this treatment was followed up with desipramine, increasing from a starting dosage of 25 mg/day to 100 mg/day. A third group of 26 control patients received active omeprazole at the same dosage but placebo in place of the baclofen and desipramine. These three subgroups showed no statistically significant differences at baseline for all demographic and clinical parameters recorded.
The study’s primary endpoint was the percentage of patients in each treatment arm who had a “successful” outcome, defined as at least a 50% improvement in their gastroesophageal reflux health-related quality of life score (J Gastrointest Surg. 1998 Mar-Apr;2[2]:141-5) after 1 year on treatment, which occurred in 67% of the fundoplication patients, 28% in the active medical arm, and 12% in the control arm. The fundoplication-treated patients had a significantly higher rate of a successful outcome, compared with patients in each of the other two treatment groups, while the success rates among patients in the active medical group and the control group did not differ significantly, Dr. Spechler said.
Dr. Spechler had no disclosures to report.
REPORTING FROM DDW 2018
Key clinical point: Fundoplication produces the best outcomes in patients with true proton pump inhibitor–refractory heartburn.
Major finding: Two-thirds of patients treated with fundoplication had successful outcomes, compared with 28% in medical controls and 12% in placebo controls.
Study details: A multicenter, randomized study with 78 patients.
Disclosures: Dr. Spechler had no disclosures to report.
Summer colds
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
New Guidelines for Nonmelanoma Skin Cancer: What You Need to Know
British good practice paper offers MCL diagnosis pearls
Immunohistochemical panels used in the diagnosis of mantle cell lymphoma should include cyclin D1 and SOX11 immunostaining, according to a good practice paper from the British Society of Haematology.
Pamela McKay, MD, of the Beatson West of Scotland Cancer Centre, Glasgow, and her colleagues provided based on a review of literature from 1980 to 2017. The good practice paper aims to offer best practice advice based on consensus where the evidence is limited. Specifically, the paper incorporates new information on molecular pathology and the use of positron emission tomography/computed tomography (PET/CT) scanning in staging of disease.
The top recommendations related to MCL diagnosis include performing lymph node excision or adequate core biopsy for diagnosis of nodal MCL. For non-nodal presentation, a tissue biopsy or peripheral blood can be used. Additionally, immunohistochemical panels should include cyclin D1 and SOX11 immunostaining.
In cases of atypical morphology, aberrant immunophenotype, equivocal cyclin D1 positivity, or unusual clinical presentation, the authors recommended fluorescence in situ hybridization (FISH) to demonstrate the presence of the t(11;14) translocation. They also recommended recording the Ki67 Proliferation Index at baseline, with an index of greater than 30% being indicative of a poorer outcome.
In terms of staging disease, Dr. McKay and her associates recommended that patients undergo staging with CT of the neck, chest, abdomen, and pelvis. They recommended against routine use of fluorodeoxyglucose PET for MCL staging, but said it could be considered if radical radiotherapy is being proposed for early-stage disease.
For cases with suspicion of central nervous system involvement, lumbar puncture with cytospin and immunophenotyping is recommended.
They recommended that all MCL patients have either their simplified or combined MCL international prognostic index score recorded at baseline.
All the authors made a declaration of interest to the British Society of Haematology and task force chairs, which may be viewed on request.
SOURCE: McKay P et al. Br J Haematol. 2018 Jun 8. doi: 10.1111/bjh.15281.
Immunohistochemical panels used in the diagnosis of mantle cell lymphoma should include cyclin D1 and SOX11 immunostaining, according to a good practice paper from the British Society of Haematology.
Pamela McKay, MD, of the Beatson West of Scotland Cancer Centre, Glasgow, and her colleagues provided based on a review of literature from 1980 to 2017. The good practice paper aims to offer best practice advice based on consensus where the evidence is limited. Specifically, the paper incorporates new information on molecular pathology and the use of positron emission tomography/computed tomography (PET/CT) scanning in staging of disease.
The top recommendations related to MCL diagnosis include performing lymph node excision or adequate core biopsy for diagnosis of nodal MCL. For non-nodal presentation, a tissue biopsy or peripheral blood can be used. Additionally, immunohistochemical panels should include cyclin D1 and SOX11 immunostaining.
In cases of atypical morphology, aberrant immunophenotype, equivocal cyclin D1 positivity, or unusual clinical presentation, the authors recommended fluorescence in situ hybridization (FISH) to demonstrate the presence of the t(11;14) translocation. They also recommended recording the Ki67 Proliferation Index at baseline, with an index of greater than 30% being indicative of a poorer outcome.
In terms of staging disease, Dr. McKay and her associates recommended that patients undergo staging with CT of the neck, chest, abdomen, and pelvis. They recommended against routine use of fluorodeoxyglucose PET for MCL staging, but said it could be considered if radical radiotherapy is being proposed for early-stage disease.
For cases with suspicion of central nervous system involvement, lumbar puncture with cytospin and immunophenotyping is recommended.
They recommended that all MCL patients have either their simplified or combined MCL international prognostic index score recorded at baseline.
All the authors made a declaration of interest to the British Society of Haematology and task force chairs, which may be viewed on request.
SOURCE: McKay P et al. Br J Haematol. 2018 Jun 8. doi: 10.1111/bjh.15281.
Immunohistochemical panels used in the diagnosis of mantle cell lymphoma should include cyclin D1 and SOX11 immunostaining, according to a good practice paper from the British Society of Haematology.
Pamela McKay, MD, of the Beatson West of Scotland Cancer Centre, Glasgow, and her colleagues provided based on a review of literature from 1980 to 2017. The good practice paper aims to offer best practice advice based on consensus where the evidence is limited. Specifically, the paper incorporates new information on molecular pathology and the use of positron emission tomography/computed tomography (PET/CT) scanning in staging of disease.
The top recommendations related to MCL diagnosis include performing lymph node excision or adequate core biopsy for diagnosis of nodal MCL. For non-nodal presentation, a tissue biopsy or peripheral blood can be used. Additionally, immunohistochemical panels should include cyclin D1 and SOX11 immunostaining.
In cases of atypical morphology, aberrant immunophenotype, equivocal cyclin D1 positivity, or unusual clinical presentation, the authors recommended fluorescence in situ hybridization (FISH) to demonstrate the presence of the t(11;14) translocation. They also recommended recording the Ki67 Proliferation Index at baseline, with an index of greater than 30% being indicative of a poorer outcome.
In terms of staging disease, Dr. McKay and her associates recommended that patients undergo staging with CT of the neck, chest, abdomen, and pelvis. They recommended against routine use of fluorodeoxyglucose PET for MCL staging, but said it could be considered if radical radiotherapy is being proposed for early-stage disease.
For cases with suspicion of central nervous system involvement, lumbar puncture with cytospin and immunophenotyping is recommended.
They recommended that all MCL patients have either their simplified or combined MCL international prognostic index score recorded at baseline.
All the authors made a declaration of interest to the British Society of Haematology and task force chairs, which may be viewed on request.
SOURCE: McKay P et al. Br J Haematol. 2018 Jun 8. doi: 10.1111/bjh.15281.
FROM THE BRITISH JOURNAL OF HAEMATOLOGY
FDA database reveals many rheumatic and musculoskeletal adverse events on immunotherapies
AMSTERDAM – Mining of the Food and Drug Administration adverse events database revealed a more substantial risk of rheumatic and musculoskeletal events on checkpoint inhibitor therapy than has been previously reported, according to Xerxes N. Pundole, PhD, an instructor in the research faculty at the University of Texas MD Anderson Cancer Center, Houston.
In a video interview, Dr. Pundole summarized data he presented at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
So far, according to Dr. Pundole, there have been a relatively limited number of reports in the medical literature of inflammatory rheumatic or musculoskeletal events from checkpoint inhibitors. However, other inflammatory conditions, such as colitis and pneumonitis, are known to occur commonly with these agents. The FDA adverse event database provided an opportunity to evaluate how often rheumatic and musculoskeletal events are reported in the real world.
In this interview, Dr. Pundole explained that rheumatic and musculoskeletal events do occur at higher rates than would be expected in patients not treated with a checkpoint inhibitor. With data from more than 30,000 unique patients, the relative risks of some of these adverse events, such as polymyositis, were more than doubled, although the event rates were not evenly distributed.
Specifically, rheumatic and musculoskeletal adverse events were far less common with the cytotoxic T-lymphocyte antigen 4 checkpoint inhibitor ipilimumab (Yervoy) relative to programmed cell death protein 1 inhibitors, particularly nivolumab (Opdivo).
In another notable finding, a demographic stratification of the FDA database found elderly men to be overrepresented among patients developing adverse events related to musculoskeletal inflammation.
Overall, his data do support a relationship between checkpoint inhibitors and a greater risk of rheumatic and musculoskeletal adverse events than has been previously reported, but he noted that these data provide no specific guidance for those who already have RA or another inflammatory condition.
“Can you identify these adverse events early on to keep the patients on immune checkpoint inhibitor therapy and not have to stop their cancer treatment? That’s a question,” Dr. Pundole said. However, he suggested that the FDA data support clinician awareness of the problem and the studies that will establish strategies for preserving the benefit-to-risk ratio of checkpoint inhibitors in patients who are at greater risk of adverse events relative to immune function because of a preexisting inflammatory condition.
SOURCE: Pundole XN et al. Ann Rheum Dis. 2018;77(Suppl 2):147-148. Abstract OP0197.
AMSTERDAM – Mining of the Food and Drug Administration adverse events database revealed a more substantial risk of rheumatic and musculoskeletal events on checkpoint inhibitor therapy than has been previously reported, according to Xerxes N. Pundole, PhD, an instructor in the research faculty at the University of Texas MD Anderson Cancer Center, Houston.
In a video interview, Dr. Pundole summarized data he presented at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
So far, according to Dr. Pundole, there have been a relatively limited number of reports in the medical literature of inflammatory rheumatic or musculoskeletal events from checkpoint inhibitors. However, other inflammatory conditions, such as colitis and pneumonitis, are known to occur commonly with these agents. The FDA adverse event database provided an opportunity to evaluate how often rheumatic and musculoskeletal events are reported in the real world.
In this interview, Dr. Pundole explained that rheumatic and musculoskeletal events do occur at higher rates than would be expected in patients not treated with a checkpoint inhibitor. With data from more than 30,000 unique patients, the relative risks of some of these adverse events, such as polymyositis, were more than doubled, although the event rates were not evenly distributed.
Specifically, rheumatic and musculoskeletal adverse events were far less common with the cytotoxic T-lymphocyte antigen 4 checkpoint inhibitor ipilimumab (Yervoy) relative to programmed cell death protein 1 inhibitors, particularly nivolumab (Opdivo).
In another notable finding, a demographic stratification of the FDA database found elderly men to be overrepresented among patients developing adverse events related to musculoskeletal inflammation.
Overall, his data do support a relationship between checkpoint inhibitors and a greater risk of rheumatic and musculoskeletal adverse events than has been previously reported, but he noted that these data provide no specific guidance for those who already have RA or another inflammatory condition.
“Can you identify these adverse events early on to keep the patients on immune checkpoint inhibitor therapy and not have to stop their cancer treatment? That’s a question,” Dr. Pundole said. However, he suggested that the FDA data support clinician awareness of the problem and the studies that will establish strategies for preserving the benefit-to-risk ratio of checkpoint inhibitors in patients who are at greater risk of adverse events relative to immune function because of a preexisting inflammatory condition.
SOURCE: Pundole XN et al. Ann Rheum Dis. 2018;77(Suppl 2):147-148. Abstract OP0197.
AMSTERDAM – Mining of the Food and Drug Administration adverse events database revealed a more substantial risk of rheumatic and musculoskeletal events on checkpoint inhibitor therapy than has been previously reported, according to Xerxes N. Pundole, PhD, an instructor in the research faculty at the University of Texas MD Anderson Cancer Center, Houston.
In a video interview, Dr. Pundole summarized data he presented at the European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
So far, according to Dr. Pundole, there have been a relatively limited number of reports in the medical literature of inflammatory rheumatic or musculoskeletal events from checkpoint inhibitors. However, other inflammatory conditions, such as colitis and pneumonitis, are known to occur commonly with these agents. The FDA adverse event database provided an opportunity to evaluate how often rheumatic and musculoskeletal events are reported in the real world.
In this interview, Dr. Pundole explained that rheumatic and musculoskeletal events do occur at higher rates than would be expected in patients not treated with a checkpoint inhibitor. With data from more than 30,000 unique patients, the relative risks of some of these adverse events, such as polymyositis, were more than doubled, although the event rates were not evenly distributed.
Specifically, rheumatic and musculoskeletal adverse events were far less common with the cytotoxic T-lymphocyte antigen 4 checkpoint inhibitor ipilimumab (Yervoy) relative to programmed cell death protein 1 inhibitors, particularly nivolumab (Opdivo).
In another notable finding, a demographic stratification of the FDA database found elderly men to be overrepresented among patients developing adverse events related to musculoskeletal inflammation.
Overall, his data do support a relationship between checkpoint inhibitors and a greater risk of rheumatic and musculoskeletal adverse events than has been previously reported, but he noted that these data provide no specific guidance for those who already have RA or another inflammatory condition.
“Can you identify these adverse events early on to keep the patients on immune checkpoint inhibitor therapy and not have to stop their cancer treatment? That’s a question,” Dr. Pundole said. However, he suggested that the FDA data support clinician awareness of the problem and the studies that will establish strategies for preserving the benefit-to-risk ratio of checkpoint inhibitors in patients who are at greater risk of adverse events relative to immune function because of a preexisting inflammatory condition.
SOURCE: Pundole XN et al. Ann Rheum Dis. 2018;77(Suppl 2):147-148. Abstract OP0197.
REPORTING FROM THE EULAR 2018 CONGRESS
Generic versions of Suboxone approved for opioid dependence
The Food and Drug Administration has approved the first generic versions of Suboxone (buprenorphine and naloxone) sublingual film for the medication-assisted treatment of opioid dependence.
Medication-assisted treatment (MAT) combines approved medication, such as methadone, buprenorphine, or naltrexone, with counseling and other behavioral therapies to treat opioid use disorders. Suboxone adherence reduces opioid withdrawal symptoms, the desire to use opioid, and the pleasurable effects of opioid use. Patients who receive MAT reduce their risk of death from all causes by about 50%.
“The FDA is taking new steps to advance the development of improved treatments for opioid use disorder and to make sure these medicines are accessible to the patients who need them. That includes promoting the development of better drugs and also facilitating market entry of generic versions of approved drugs to help ensure broader access,” FDA Commissioner Scott Gottlieb, MD, said in the June 14 press release.
.
The agency said data from the Substance Abuse and Mental Health Services Administration show that patients who receive MAT as an intervention for opioid use reduce their risk of death from all causes by 50%.
Common adverse events associated with Suboxone include oral hypoesthesia, glossodynia, oral mucosal erythema, headache, nausea, vomiting, hyperhidrosis, constipation, signs and symptoms of withdrawal, insomnia, pain, and peripheral edema.
Find the full press release on the FDA website.
The Food and Drug Administration has approved the first generic versions of Suboxone (buprenorphine and naloxone) sublingual film for the medication-assisted treatment of opioid dependence.
Medication-assisted treatment (MAT) combines approved medication, such as methadone, buprenorphine, or naltrexone, with counseling and other behavioral therapies to treat opioid use disorders. Suboxone adherence reduces opioid withdrawal symptoms, the desire to use opioid, and the pleasurable effects of opioid use. Patients who receive MAT reduce their risk of death from all causes by about 50%.
“The FDA is taking new steps to advance the development of improved treatments for opioid use disorder and to make sure these medicines are accessible to the patients who need them. That includes promoting the development of better drugs and also facilitating market entry of generic versions of approved drugs to help ensure broader access,” FDA Commissioner Scott Gottlieb, MD, said in the June 14 press release.
.
The agency said data from the Substance Abuse and Mental Health Services Administration show that patients who receive MAT as an intervention for opioid use reduce their risk of death from all causes by 50%.
Common adverse events associated with Suboxone include oral hypoesthesia, glossodynia, oral mucosal erythema, headache, nausea, vomiting, hyperhidrosis, constipation, signs and symptoms of withdrawal, insomnia, pain, and peripheral edema.
Find the full press release on the FDA website.
The Food and Drug Administration has approved the first generic versions of Suboxone (buprenorphine and naloxone) sublingual film for the medication-assisted treatment of opioid dependence.
Medication-assisted treatment (MAT) combines approved medication, such as methadone, buprenorphine, or naltrexone, with counseling and other behavioral therapies to treat opioid use disorders. Suboxone adherence reduces opioid withdrawal symptoms, the desire to use opioid, and the pleasurable effects of opioid use. Patients who receive MAT reduce their risk of death from all causes by about 50%.
“The FDA is taking new steps to advance the development of improved treatments for opioid use disorder and to make sure these medicines are accessible to the patients who need them. That includes promoting the development of better drugs and also facilitating market entry of generic versions of approved drugs to help ensure broader access,” FDA Commissioner Scott Gottlieb, MD, said in the June 14 press release.
.
The agency said data from the Substance Abuse and Mental Health Services Administration show that patients who receive MAT as an intervention for opioid use reduce their risk of death from all causes by 50%.
Common adverse events associated with Suboxone include oral hypoesthesia, glossodynia, oral mucosal erythema, headache, nausea, vomiting, hyperhidrosis, constipation, signs and symptoms of withdrawal, insomnia, pain, and peripheral edema.
Find the full press release on the FDA website.
A patient portal for the inpatient experience
Hospitalists, nurses most impacted.
Hospitalists see patients at their most fragile – and, as a result, they have a unique opportunity to affect their health going forward.
“These moments can transform the way patients see their health and their behaviors, and any opportunity to position patients as empowered to influence their experience is one that can not be squandered,” said Timothy Huerta, PhD, MS, lead author on a study of patient portals and tablets during inpatient care.1 “In that context, hospitals have the opportunity to set expectations for engagement that can be influenced by technology. Patient portals, positioned within the inpatient setting, offer a platform to engage, empower, and educate.”
His experience – at the first and largest academic medical center to provide this technology across the entire hospital system – offers the first insight into the demands that such a process shift requires, he said. The researchers ran a 90-day pilot program giving tablets to 179 patients; subsequently, the health system committed to providing tablets for accessing inpatient portals in all seven of its hospitals. “Adopting this technology is not easy, and we continue to explore how we can use it more effectively. Our hope is that our experience can make the journey of others easier.”
Providing the technology is a necessary but insufficient component of implementation, he added. “This is not like the movie ‘Field of Dreams’ – if you build it they will come. It requires leaders to see the value proposition, champions throughout the organization to make a reality where the technology matters to the provision of care, and clinicians to see the tool as a means to a greater good.”
In hospitals, nursing staff and hospitalists are likely to be most impacted by the addition of these tools. “They will require choices – for example who will respond on what timeline to patient communication when using these tools – which requires collaboration across the institution.”
Reference
1. Huerta T, McAlearney AS, Rizer MK. “Introducing a Patient Portal and Electronic Tablets to Inpatient Care.” Ann Intern Med. 2017;167(11):816-7.
Hospitalists, nurses most impacted.
Hospitalists, nurses most impacted.
Hospitalists see patients at their most fragile – and, as a result, they have a unique opportunity to affect their health going forward.
“These moments can transform the way patients see their health and their behaviors, and any opportunity to position patients as empowered to influence their experience is one that can not be squandered,” said Timothy Huerta, PhD, MS, lead author on a study of patient portals and tablets during inpatient care.1 “In that context, hospitals have the opportunity to set expectations for engagement that can be influenced by technology. Patient portals, positioned within the inpatient setting, offer a platform to engage, empower, and educate.”
His experience – at the first and largest academic medical center to provide this technology across the entire hospital system – offers the first insight into the demands that such a process shift requires, he said. The researchers ran a 90-day pilot program giving tablets to 179 patients; subsequently, the health system committed to providing tablets for accessing inpatient portals in all seven of its hospitals. “Adopting this technology is not easy, and we continue to explore how we can use it more effectively. Our hope is that our experience can make the journey of others easier.”
Providing the technology is a necessary but insufficient component of implementation, he added. “This is not like the movie ‘Field of Dreams’ – if you build it they will come. It requires leaders to see the value proposition, champions throughout the organization to make a reality where the technology matters to the provision of care, and clinicians to see the tool as a means to a greater good.”
In hospitals, nursing staff and hospitalists are likely to be most impacted by the addition of these tools. “They will require choices – for example who will respond on what timeline to patient communication when using these tools – which requires collaboration across the institution.”
Reference
1. Huerta T, McAlearney AS, Rizer MK. “Introducing a Patient Portal and Electronic Tablets to Inpatient Care.” Ann Intern Med. 2017;167(11):816-7.
Hospitalists see patients at their most fragile – and, as a result, they have a unique opportunity to affect their health going forward.
“These moments can transform the way patients see their health and their behaviors, and any opportunity to position patients as empowered to influence their experience is one that can not be squandered,” said Timothy Huerta, PhD, MS, lead author on a study of patient portals and tablets during inpatient care.1 “In that context, hospitals have the opportunity to set expectations for engagement that can be influenced by technology. Patient portals, positioned within the inpatient setting, offer a platform to engage, empower, and educate.”
His experience – at the first and largest academic medical center to provide this technology across the entire hospital system – offers the first insight into the demands that such a process shift requires, he said. The researchers ran a 90-day pilot program giving tablets to 179 patients; subsequently, the health system committed to providing tablets for accessing inpatient portals in all seven of its hospitals. “Adopting this technology is not easy, and we continue to explore how we can use it more effectively. Our hope is that our experience can make the journey of others easier.”
Providing the technology is a necessary but insufficient component of implementation, he added. “This is not like the movie ‘Field of Dreams’ – if you build it they will come. It requires leaders to see the value proposition, champions throughout the organization to make a reality where the technology matters to the provision of care, and clinicians to see the tool as a means to a greater good.”
In hospitals, nursing staff and hospitalists are likely to be most impacted by the addition of these tools. “They will require choices – for example who will respond on what timeline to patient communication when using these tools – which requires collaboration across the institution.”
Reference
1. Huerta T, McAlearney AS, Rizer MK. “Introducing a Patient Portal and Electronic Tablets to Inpatient Care.” Ann Intern Med. 2017;167(11):816-7.
Novel blood test that predicts gestational age, fetal development, could improve prenatal care
Researchers have identified cell-free RNA transcripts obtained from a noninvasive blood test during pregnancy that can predict risk of preterm birth in addition to predicting gestational age with an accuracy similar to ultrasound, which may soon pave the way for a low-cost alternative to ultrasound for prenatal care in developing areas, according to recent results from two pilot studies.
“Our results are thus generally comparable to ultrasound measurements, can be performed throughout pregnancy, and do not require a priori physiological knowledge such as the woman’s last menstrual period,” Stephen Quake, PhD, of Stanford (Calif.) University, and his colleagues wrote in Science.
Dr. Quake and his colleagues recruited 31 women from Denmark who provided weekly blood samples (521 samples) during pregnancy up until they delivered full-term. After analyzing the cell-free RNA (cfRNA) genes, researchers found cfRNA placenta, fetal, and immune genes were highly correlated with one another. They created a random forest model based on nine cfRNA genes (CGA, CAPN6, CGB, ALPP, CSHL1, PLAC4, PSG7, PAPPA, and LGALS14) that corresponded with the placenta. They estimated that those nine genes would predict gestational age and tested the model using 306 samples from 21 women in a training cohort and validated the test using 215 samples from 10 women in a validation cohort. The blood test predicted gestational age within 14 days of delivery in 32% of cases at the second trimester (T2), 23% at the third trimester (3T), and 45% at T2 and T3, compared with a 48% with ultrasound.
In a second pilot study, Dr. Quake and his colleagues created a polymerase chain reaction panel for 38 genes identified from sequencing RNA from patients in Pennsylvania, Alabama, and Denmark, with full-term and preterm deliveries up to 2 months before labor to determine “cfRNA transcripts that might be able to discriminate a spontaneous preterm delivery from a full-term delivery.” The top seven cfRNA transcripts (CLCN3, DAPP1, PPBP, MAP3K7CL, MOB1B, RAB27B, and RGS18), when grouped in “unique combinations” of three genes, predicted 75% of preterm samples and misclassified 1 of 26 samples (4%) from Denmark and Pennsylvania; in a validated cohort of Alabama patients, the test predicted 4 of 5 preterm samples (80%) and misclassified 3 of 18 full-term samples (17%).
“These cfRNA [polymerase chain reaction]–based tests have two advantages over alternatives: broader applicability and lower cost,” Dr. Quake and his colleagues wrote. “They can be applied across the globe as a complement to or substitute for ultrasound, which can be expensive and inaccurate during the second and third trimesters.”
The authors noted a larger sample size and blinded testing on a broader patient population is needed before clinics can apply this blood test in a diagnostic or screening tool for widespread use.
Dr. Quake and three other authors have a patent application submitted by the Chan Zuckerberg Biohub relating to “noninvasive estimates of gestational age, delivery, and preterm birth.” The other authors have no relevant financial disclosures.
SOURCE: Ngo TTM et al. Science. 2018 Jun 7. doi: 10.1126/science.aar3819.
Researchers have identified cell-free RNA transcripts obtained from a noninvasive blood test during pregnancy that can predict risk of preterm birth in addition to predicting gestational age with an accuracy similar to ultrasound, which may soon pave the way for a low-cost alternative to ultrasound for prenatal care in developing areas, according to recent results from two pilot studies.
“Our results are thus generally comparable to ultrasound measurements, can be performed throughout pregnancy, and do not require a priori physiological knowledge such as the woman’s last menstrual period,” Stephen Quake, PhD, of Stanford (Calif.) University, and his colleagues wrote in Science.
Dr. Quake and his colleagues recruited 31 women from Denmark who provided weekly blood samples (521 samples) during pregnancy up until they delivered full-term. After analyzing the cell-free RNA (cfRNA) genes, researchers found cfRNA placenta, fetal, and immune genes were highly correlated with one another. They created a random forest model based on nine cfRNA genes (CGA, CAPN6, CGB, ALPP, CSHL1, PLAC4, PSG7, PAPPA, and LGALS14) that corresponded with the placenta. They estimated that those nine genes would predict gestational age and tested the model using 306 samples from 21 women in a training cohort and validated the test using 215 samples from 10 women in a validation cohort. The blood test predicted gestational age within 14 days of delivery in 32% of cases at the second trimester (T2), 23% at the third trimester (3T), and 45% at T2 and T3, compared with a 48% with ultrasound.
In a second pilot study, Dr. Quake and his colleagues created a polymerase chain reaction panel for 38 genes identified from sequencing RNA from patients in Pennsylvania, Alabama, and Denmark, with full-term and preterm deliveries up to 2 months before labor to determine “cfRNA transcripts that might be able to discriminate a spontaneous preterm delivery from a full-term delivery.” The top seven cfRNA transcripts (CLCN3, DAPP1, PPBP, MAP3K7CL, MOB1B, RAB27B, and RGS18), when grouped in “unique combinations” of three genes, predicted 75% of preterm samples and misclassified 1 of 26 samples (4%) from Denmark and Pennsylvania; in a validated cohort of Alabama patients, the test predicted 4 of 5 preterm samples (80%) and misclassified 3 of 18 full-term samples (17%).
“These cfRNA [polymerase chain reaction]–based tests have two advantages over alternatives: broader applicability and lower cost,” Dr. Quake and his colleagues wrote. “They can be applied across the globe as a complement to or substitute for ultrasound, which can be expensive and inaccurate during the second and third trimesters.”
The authors noted a larger sample size and blinded testing on a broader patient population is needed before clinics can apply this blood test in a diagnostic or screening tool for widespread use.
Dr. Quake and three other authors have a patent application submitted by the Chan Zuckerberg Biohub relating to “noninvasive estimates of gestational age, delivery, and preterm birth.” The other authors have no relevant financial disclosures.
SOURCE: Ngo TTM et al. Science. 2018 Jun 7. doi: 10.1126/science.aar3819.
Researchers have identified cell-free RNA transcripts obtained from a noninvasive blood test during pregnancy that can predict risk of preterm birth in addition to predicting gestational age with an accuracy similar to ultrasound, which may soon pave the way for a low-cost alternative to ultrasound for prenatal care in developing areas, according to recent results from two pilot studies.
“Our results are thus generally comparable to ultrasound measurements, can be performed throughout pregnancy, and do not require a priori physiological knowledge such as the woman’s last menstrual period,” Stephen Quake, PhD, of Stanford (Calif.) University, and his colleagues wrote in Science.
Dr. Quake and his colleagues recruited 31 women from Denmark who provided weekly blood samples (521 samples) during pregnancy up until they delivered full-term. After analyzing the cell-free RNA (cfRNA) genes, researchers found cfRNA placenta, fetal, and immune genes were highly correlated with one another. They created a random forest model based on nine cfRNA genes (CGA, CAPN6, CGB, ALPP, CSHL1, PLAC4, PSG7, PAPPA, and LGALS14) that corresponded with the placenta. They estimated that those nine genes would predict gestational age and tested the model using 306 samples from 21 women in a training cohort and validated the test using 215 samples from 10 women in a validation cohort. The blood test predicted gestational age within 14 days of delivery in 32% of cases at the second trimester (T2), 23% at the third trimester (3T), and 45% at T2 and T3, compared with a 48% with ultrasound.
In a second pilot study, Dr. Quake and his colleagues created a polymerase chain reaction panel for 38 genes identified from sequencing RNA from patients in Pennsylvania, Alabama, and Denmark, with full-term and preterm deliveries up to 2 months before labor to determine “cfRNA transcripts that might be able to discriminate a spontaneous preterm delivery from a full-term delivery.” The top seven cfRNA transcripts (CLCN3, DAPP1, PPBP, MAP3K7CL, MOB1B, RAB27B, and RGS18), when grouped in “unique combinations” of three genes, predicted 75% of preterm samples and misclassified 1 of 26 samples (4%) from Denmark and Pennsylvania; in a validated cohort of Alabama patients, the test predicted 4 of 5 preterm samples (80%) and misclassified 3 of 18 full-term samples (17%).
“These cfRNA [polymerase chain reaction]–based tests have two advantages over alternatives: broader applicability and lower cost,” Dr. Quake and his colleagues wrote. “They can be applied across the globe as a complement to or substitute for ultrasound, which can be expensive and inaccurate during the second and third trimesters.”
The authors noted a larger sample size and blinded testing on a broader patient population is needed before clinics can apply this blood test in a diagnostic or screening tool for widespread use.
Dr. Quake and three other authors have a patent application submitted by the Chan Zuckerberg Biohub relating to “noninvasive estimates of gestational age, delivery, and preterm birth.” The other authors have no relevant financial disclosures.
SOURCE: Ngo TTM et al. Science. 2018 Jun 7. doi: 10.1126/science.aar3819.
FROM SCIENCE
Key clinical point: Cell-free RNA transcripts identified from a single blood sample can reliably predict gestational age similar to ultrasound and can identify risk of preterm birth.
Major finding: Nine cell-free RNA transcripts predicted gestational age at an accuracy similar to ultrasound, while seven cell-free RNA transcripts predicted an increased risk of preterm birth until 2 months prior to delivery.
Study details: A pilot study of 31 pregnant women and a related pilot study of 38 women with full-term or preterm deliveries.
Disclosures: Dr. Quake and three other authors have a patent application submitted by the Chan Zuckerberg Biohub relating to “noninvasive estimates of gestational age, delivery, and preterm birth.” The other authors have no relevant financial disclosures.
Source: Ngo TTM et al. Science. 2018 Jun 7. doi: 10.1126/science.aar3819.
LLDAS shows potential as routine lupus treatment target
AMSTERDAM – The Lupus Low Disease Activity State measure of treatment response offers clinicians an attainable target for patients with systemic lupus erythematosus that correlates with a substantially reduced rate of organ damage, based on a retrospective assessment of data collected from more than 2,000 lupus patients at a single U.S. center.
The analysis showed that when patients with systemic lupus erythematosus (SLE) met the Lupus Low Disease Activity State (LLDAS) criteria at least half the time while on treatment, their overall rate of organ damage was reduced by 52%, compared with patients who never achieved LLDAS, Michelle A. Petri, MD, said at the European Congress of Rheumatology.

“LLDAS is something that anyone can use in practice,” and has the advantage of including a low steroid dose – no more than 7.5 mg prednisolone/day or an equivalent steroid – as one of its criteria, “a major bad actor” for SLE patients, Dr. Petri said in an interview. LLDAS “is absolutely ready for routine use,” although until now few clinicians have used it to monitor SLE patients, she noted.
“The LLDAS can be a useful target,” commented Ian N. Bruce, MD, professor of rheumatology at the University of Manchester (England), adding that the steroid dosage an SLE patient receives “is an important parameter to measure when assessing an SLE patient.
“It’s not far from being ready for routine use, but I’d like to see more evidence” that it’s a meaningful measure of an SLE patient’s disease status, he said in an interview.
To examine the clinical relevance of the LLDAS criteria, a five-point assessment for SLE first introduced in a 2016 report (Ann Rheum Dis. 2016 Sept;75[9]:1615-21), Dr. Petri and her associates applied it retrospectively to their records for 2,026 SLE patients in a Johns Hopkins registry. Clinicians at Johns Hopkins routinely assessed their SLE patients every 3 months and followed the patients for a median of about 10 years, and so had data from more than 81,000 patient encounters. The researchers used the longitudinal follow-up records to calculate an area under the curve for each patient that tracked their LLDAS state over time. This showed a clear dose-response relationship: The more time an SLE patient spent in LLDAS, the less organ damage they had. Patients who remained in LLDAS at least 75% of the time had a 60% reduction in cumulative organ damage, compared with patients who never achieved LLDAS, Dr. Petri said. The analysis also showed that LLDAS was substantially easier for patients to achieve than the Definitions of Remission in SLE (Ann Rheum Dis. 2017 March;76[3]:554-61). The Johns Hopkins cohort met the LLDAS definition about three times more often than they met the Definitions of Remission in SLE criteria, Dr. Petri said.
The new analysis also showed that LLDAS was especially effective in correlating with statistically significant reductions in future strokes, MI, and end-stage renal disease, though it did not significantly correlate with subsequent reductions in the incidence of cognitive impairment, deep vein thrombosis, malignancy, pulmonary fibrosis, pulmonary hypertension, or cataract development. But the strong correlation of time in LLDAS and the future rate of stroke, MI, or end-stage renal disease was very meaningful because those are the most important types of damage associated with SLE, Dr. Petri said. “LLDAS is a good treatment target as a surrogate” for future risk of SLE complications.
The study had no commercial funding, and Dr. Petri had no disclosures to report. Dr. Bruce has been a consultant to and speaker for GlaxoSmithKline, MedImmune, Pfizer, Roche, and UCB, and he has received research support from Genzyme, GlaxoSmithKline, Human Genome Sciences, Roche, and UCB.
SOURCE: Petri MA et al. Ann Rheum Dis. 2018;77(Suppl 2):111. Abstract OP0122.
AMSTERDAM – The Lupus Low Disease Activity State measure of treatment response offers clinicians an attainable target for patients with systemic lupus erythematosus that correlates with a substantially reduced rate of organ damage, based on a retrospective assessment of data collected from more than 2,000 lupus patients at a single U.S. center.
The analysis showed that when patients with systemic lupus erythematosus (SLE) met the Lupus Low Disease Activity State (LLDAS) criteria at least half the time while on treatment, their overall rate of organ damage was reduced by 52%, compared with patients who never achieved LLDAS, Michelle A. Petri, MD, said at the European Congress of Rheumatology.

“LLDAS is something that anyone can use in practice,” and has the advantage of including a low steroid dose – no more than 7.5 mg prednisolone/day or an equivalent steroid – as one of its criteria, “a major bad actor” for SLE patients, Dr. Petri said in an interview. LLDAS “is absolutely ready for routine use,” although until now few clinicians have used it to monitor SLE patients, she noted.
“The LLDAS can be a useful target,” commented Ian N. Bruce, MD, professor of rheumatology at the University of Manchester (England), adding that the steroid dosage an SLE patient receives “is an important parameter to measure when assessing an SLE patient.
“It’s not far from being ready for routine use, but I’d like to see more evidence” that it’s a meaningful measure of an SLE patient’s disease status, he said in an interview.
To examine the clinical relevance of the LLDAS criteria, a five-point assessment for SLE first introduced in a 2016 report (Ann Rheum Dis. 2016 Sept;75[9]:1615-21), Dr. Petri and her associates applied it retrospectively to their records for 2,026 SLE patients in a Johns Hopkins registry. Clinicians at Johns Hopkins routinely assessed their SLE patients every 3 months and followed the patients for a median of about 10 years, and so had data from more than 81,000 patient encounters. The researchers used the longitudinal follow-up records to calculate an area under the curve for each patient that tracked their LLDAS state over time. This showed a clear dose-response relationship: The more time an SLE patient spent in LLDAS, the less organ damage they had. Patients who remained in LLDAS at least 75% of the time had a 60% reduction in cumulative organ damage, compared with patients who never achieved LLDAS, Dr. Petri said. The analysis also showed that LLDAS was substantially easier for patients to achieve than the Definitions of Remission in SLE (Ann Rheum Dis. 2017 March;76[3]:554-61). The Johns Hopkins cohort met the LLDAS definition about three times more often than they met the Definitions of Remission in SLE criteria, Dr. Petri said.
The new analysis also showed that LLDAS was especially effective in correlating with statistically significant reductions in future strokes, MI, and end-stage renal disease, though it did not significantly correlate with subsequent reductions in the incidence of cognitive impairment, deep vein thrombosis, malignancy, pulmonary fibrosis, pulmonary hypertension, or cataract development. But the strong correlation of time in LLDAS and the future rate of stroke, MI, or end-stage renal disease was very meaningful because those are the most important types of damage associated with SLE, Dr. Petri said. “LLDAS is a good treatment target as a surrogate” for future risk of SLE complications.
The study had no commercial funding, and Dr. Petri had no disclosures to report. Dr. Bruce has been a consultant to and speaker for GlaxoSmithKline, MedImmune, Pfizer, Roche, and UCB, and he has received research support from Genzyme, GlaxoSmithKline, Human Genome Sciences, Roche, and UCB.
SOURCE: Petri MA et al. Ann Rheum Dis. 2018;77(Suppl 2):111. Abstract OP0122.
AMSTERDAM – The Lupus Low Disease Activity State measure of treatment response offers clinicians an attainable target for patients with systemic lupus erythematosus that correlates with a substantially reduced rate of organ damage, based on a retrospective assessment of data collected from more than 2,000 lupus patients at a single U.S. center.
The analysis showed that when patients with systemic lupus erythematosus (SLE) met the Lupus Low Disease Activity State (LLDAS) criteria at least half the time while on treatment, their overall rate of organ damage was reduced by 52%, compared with patients who never achieved LLDAS, Michelle A. Petri, MD, said at the European Congress of Rheumatology.

“LLDAS is something that anyone can use in practice,” and has the advantage of including a low steroid dose – no more than 7.5 mg prednisolone/day or an equivalent steroid – as one of its criteria, “a major bad actor” for SLE patients, Dr. Petri said in an interview. LLDAS “is absolutely ready for routine use,” although until now few clinicians have used it to monitor SLE patients, she noted.
“The LLDAS can be a useful target,” commented Ian N. Bruce, MD, professor of rheumatology at the University of Manchester (England), adding that the steroid dosage an SLE patient receives “is an important parameter to measure when assessing an SLE patient.
“It’s not far from being ready for routine use, but I’d like to see more evidence” that it’s a meaningful measure of an SLE patient’s disease status, he said in an interview.
To examine the clinical relevance of the LLDAS criteria, a five-point assessment for SLE first introduced in a 2016 report (Ann Rheum Dis. 2016 Sept;75[9]:1615-21), Dr. Petri and her associates applied it retrospectively to their records for 2,026 SLE patients in a Johns Hopkins registry. Clinicians at Johns Hopkins routinely assessed their SLE patients every 3 months and followed the patients for a median of about 10 years, and so had data from more than 81,000 patient encounters. The researchers used the longitudinal follow-up records to calculate an area under the curve for each patient that tracked their LLDAS state over time. This showed a clear dose-response relationship: The more time an SLE patient spent in LLDAS, the less organ damage they had. Patients who remained in LLDAS at least 75% of the time had a 60% reduction in cumulative organ damage, compared with patients who never achieved LLDAS, Dr. Petri said. The analysis also showed that LLDAS was substantially easier for patients to achieve than the Definitions of Remission in SLE (Ann Rheum Dis. 2017 March;76[3]:554-61). The Johns Hopkins cohort met the LLDAS definition about three times more often than they met the Definitions of Remission in SLE criteria, Dr. Petri said.
The new analysis also showed that LLDAS was especially effective in correlating with statistically significant reductions in future strokes, MI, and end-stage renal disease, though it did not significantly correlate with subsequent reductions in the incidence of cognitive impairment, deep vein thrombosis, malignancy, pulmonary fibrosis, pulmonary hypertension, or cataract development. But the strong correlation of time in LLDAS and the future rate of stroke, MI, or end-stage renal disease was very meaningful because those are the most important types of damage associated with SLE, Dr. Petri said. “LLDAS is a good treatment target as a surrogate” for future risk of SLE complications.
The study had no commercial funding, and Dr. Petri had no disclosures to report. Dr. Bruce has been a consultant to and speaker for GlaxoSmithKline, MedImmune, Pfizer, Roche, and UCB, and he has received research support from Genzyme, GlaxoSmithKline, Human Genome Sciences, Roche, and UCB.
SOURCE: Petri MA et al. Ann Rheum Dis. 2018;77(Suppl 2):111. Abstract OP0122.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: The Lupus Low Disease Activity State is a good treatment target for systemic lupus erythematosus patients.
Major finding: Patients who achieved LLDAS at least half the time had 52% less organ damage than patients who never achieved LLDAS.
Study details: A review of case records from 2,026 SLE patients followed regularly at one U.S. center.
Disclosures: The study had no commercial funding, and Dr. Petri had no disclosures to report. Dr. Bruce has been a consultant to and speaker for GlaxoSmithKline, MedImmune, Pfizer, Roche, and UCB, and he has received research support from Genzyme, GlaxoSmithKline, Human Genome Sciences, Roche, and UCB.
Source: Petri MA et al. Ann Rheum Dis. 2018;77(Suppl 2):111. Abstract OP0122.
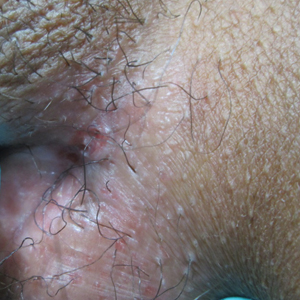
Painful Nonhealing Vulvar and Perianal Erosions
The Diagnosis: Cutaneous Crohn Disease
A punch biopsy of the vulvar skin revealed epidermal hyperplasia with moderate spongiosis and exocytosis of lymphocytes and neutrophils in the epidermis. A brisk mixed inflammatory infiltrate of epithelioid histiocytes, multinucleate foreign body-type giant cells, lymphocytes, plasma cells, neutrophils, and eosinophils in a granulomatous pattern also were present in the dermis (Figure). Periodic acid-Schiff and acid-fast bacillus stains were negative. Given the history of Crohn disease (CD) and the characteristic dermal noncaseating granulomas on histology, the patient was diagnosed with cutaneous CD.
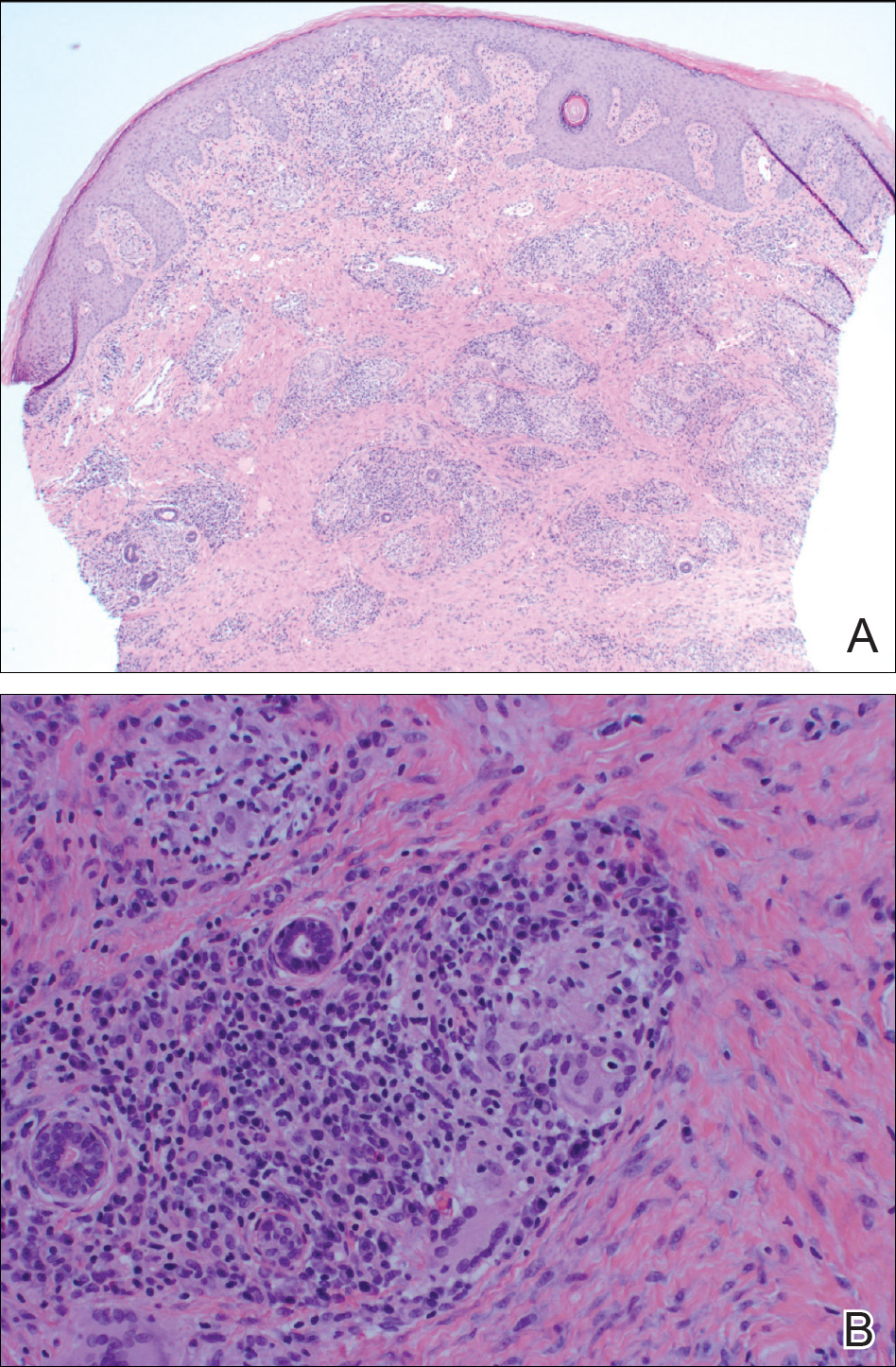
Although the patient was offered a topical corticosteroid, she deferred topical therapy. Given the lack of response to adalimumab, the gastroenterology department switched the patient to a treatment of infliximab 5 mg/kg every 8 weeks. Azathioprine was discontinued and the patient was switched to intramuscular methotrexate 25 mg/mL weekly. Slow reepithelialization of the vulvar and perianal erosions occurred on this regimen.
Although CD has numerous cutaneous features, cutaneous CD, also known as metastatic CD, is the rarest cutaneous manifestation of CD.1 This disease process is characterized by noncaseating granulomatous cutaneous lesions that are not contiguous with the affected gastrointestinal tract.2 The pathogenesis of cutaneous CD is unknown. Young adults tend to be more predisposed to developing cutaneous CD, likely due to the age distribution of CD.3
Cutaneous CD commonly presents in patients with a well-established history of gastrointestinal CD but occasionally can be the presenting sign of CD.1 The most common sites of involvement are the legs, vulva, penis, trunk, face, and intertriginous areas. Cutaneous CD findings can be divided into 2 subgroups: genital and nongenital lesions. Genital findings involve ulceration, erythema, edema, and fissuring of the vulva, labia, clitoris, scrotum, penis, and perineum. Nongenital cutaneous manifestations include ulcers; erythematous papules, plaques, and nodules; abscesslike lesions; and lichenoid papules.4,5 The severity of cutaneous lesions does not correlate to the severity of gastrointestinal disease; however, colon involvement is more common in patients with cutaneous CD.6
Histologically, cutaneous CD presents as noncaseating granulomatous inflammation in the papillary and reticular dermis. These granulomas consist of epithelioid histiocytes and multinucleated giant cells with a lymphocytic infiltrate.5
Given the rarity of cutaneous CD, treatment approach is based on anecdotal evidence from case reports and case series. For a single lesion or localized disease, topical superpotent or intralesional steroids are recommended for initial therapy.3 Oral metronidazole also is an effective treatment and can be combined with topical or intralesional steroids.7 For disseminated disease, systemic corticosteroids have shown efficacy.3 Other reported treatment options include oral corticosteroids, sulfasalazine, azathioprine, 6-mercaptopurine, infliximab, and adalimumab. If monotherapy fails, combination therapy may be needed. Surgical debridement may be attempted if medical therapy fails but is complicated by wound dehiscence and disease recurrence.3
Although genital ulcers can be a presentation of Behçet disease and genital herpes infection, genital nodules and plaques are not typical for these 2 diseases. Also, the patient did not have oral ulcers, which is a common feature of Behçet disease. Genital sarcoidosis is extremely rare, and cutaneous CD was more likely given the patient's medical history. Finally, Jacquet dermatitis is more common in children, and patients with this condition typically have history of fecal and urinary incontinence.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Stingeni L, Neve D, Bassotti G, et al. Cutaneous Crohn's disease successfully treated with adalimumab [published online Sep 15, 2015]. J Eur Acad Dermatol Venerol. 2016;30:E72-E74.
- Kurtzman DJ, Jones T, Fangru L, et al. Metastatic Crohn's disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813.
- Hagen JW, Swoger JM, Grandinetti LM. Cutaneous manifestations of Crohn disease. Dermatol Clin. 2015;33:417-431.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review [published online June 19, 2008]. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease, part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
The Diagnosis: Cutaneous Crohn Disease
A punch biopsy of the vulvar skin revealed epidermal hyperplasia with moderate spongiosis and exocytosis of lymphocytes and neutrophils in the epidermis. A brisk mixed inflammatory infiltrate of epithelioid histiocytes, multinucleate foreign body-type giant cells, lymphocytes, plasma cells, neutrophils, and eosinophils in a granulomatous pattern also were present in the dermis (Figure). Periodic acid-Schiff and acid-fast bacillus stains were negative. Given the history of Crohn disease (CD) and the characteristic dermal noncaseating granulomas on histology, the patient was diagnosed with cutaneous CD.

Although the patient was offered a topical corticosteroid, she deferred topical therapy. Given the lack of response to adalimumab, the gastroenterology department switched the patient to a treatment of infliximab 5 mg/kg every 8 weeks. Azathioprine was discontinued and the patient was switched to intramuscular methotrexate 25 mg/mL weekly. Slow reepithelialization of the vulvar and perianal erosions occurred on this regimen.
Although CD has numerous cutaneous features, cutaneous CD, also known as metastatic CD, is the rarest cutaneous manifestation of CD.1 This disease process is characterized by noncaseating granulomatous cutaneous lesions that are not contiguous with the affected gastrointestinal tract.2 The pathogenesis of cutaneous CD is unknown. Young adults tend to be more predisposed to developing cutaneous CD, likely due to the age distribution of CD.3
Cutaneous CD commonly presents in patients with a well-established history of gastrointestinal CD but occasionally can be the presenting sign of CD.1 The most common sites of involvement are the legs, vulva, penis, trunk, face, and intertriginous areas. Cutaneous CD findings can be divided into 2 subgroups: genital and nongenital lesions. Genital findings involve ulceration, erythema, edema, and fissuring of the vulva, labia, clitoris, scrotum, penis, and perineum. Nongenital cutaneous manifestations include ulcers; erythematous papules, plaques, and nodules; abscesslike lesions; and lichenoid papules.4,5 The severity of cutaneous lesions does not correlate to the severity of gastrointestinal disease; however, colon involvement is more common in patients with cutaneous CD.6
Histologically, cutaneous CD presents as noncaseating granulomatous inflammation in the papillary and reticular dermis. These granulomas consist of epithelioid histiocytes and multinucleated giant cells with a lymphocytic infiltrate.5
Given the rarity of cutaneous CD, treatment approach is based on anecdotal evidence from case reports and case series. For a single lesion or localized disease, topical superpotent or intralesional steroids are recommended for initial therapy.3 Oral metronidazole also is an effective treatment and can be combined with topical or intralesional steroids.7 For disseminated disease, systemic corticosteroids have shown efficacy.3 Other reported treatment options include oral corticosteroids, sulfasalazine, azathioprine, 6-mercaptopurine, infliximab, and adalimumab. If monotherapy fails, combination therapy may be needed. Surgical debridement may be attempted if medical therapy fails but is complicated by wound dehiscence and disease recurrence.3
Although genital ulcers can be a presentation of Behçet disease and genital herpes infection, genital nodules and plaques are not typical for these 2 diseases. Also, the patient did not have oral ulcers, which is a common feature of Behçet disease. Genital sarcoidosis is extremely rare, and cutaneous CD was more likely given the patient's medical history. Finally, Jacquet dermatitis is more common in children, and patients with this condition typically have history of fecal and urinary incontinence.
The Diagnosis: Cutaneous Crohn Disease
A punch biopsy of the vulvar skin revealed epidermal hyperplasia with moderate spongiosis and exocytosis of lymphocytes and neutrophils in the epidermis. A brisk mixed inflammatory infiltrate of epithelioid histiocytes, multinucleate foreign body-type giant cells, lymphocytes, plasma cells, neutrophils, and eosinophils in a granulomatous pattern also were present in the dermis (Figure). Periodic acid-Schiff and acid-fast bacillus stains were negative. Given the history of Crohn disease (CD) and the characteristic dermal noncaseating granulomas on histology, the patient was diagnosed with cutaneous CD.

Although the patient was offered a topical corticosteroid, she deferred topical therapy. Given the lack of response to adalimumab, the gastroenterology department switched the patient to a treatment of infliximab 5 mg/kg every 8 weeks. Azathioprine was discontinued and the patient was switched to intramuscular methotrexate 25 mg/mL weekly. Slow reepithelialization of the vulvar and perianal erosions occurred on this regimen.
Although CD has numerous cutaneous features, cutaneous CD, also known as metastatic CD, is the rarest cutaneous manifestation of CD.1 This disease process is characterized by noncaseating granulomatous cutaneous lesions that are not contiguous with the affected gastrointestinal tract.2 The pathogenesis of cutaneous CD is unknown. Young adults tend to be more predisposed to developing cutaneous CD, likely due to the age distribution of CD.3
Cutaneous CD commonly presents in patients with a well-established history of gastrointestinal CD but occasionally can be the presenting sign of CD.1 The most common sites of involvement are the legs, vulva, penis, trunk, face, and intertriginous areas. Cutaneous CD findings can be divided into 2 subgroups: genital and nongenital lesions. Genital findings involve ulceration, erythema, edema, and fissuring of the vulva, labia, clitoris, scrotum, penis, and perineum. Nongenital cutaneous manifestations include ulcers; erythematous papules, plaques, and nodules; abscesslike lesions; and lichenoid papules.4,5 The severity of cutaneous lesions does not correlate to the severity of gastrointestinal disease; however, colon involvement is more common in patients with cutaneous CD.6
Histologically, cutaneous CD presents as noncaseating granulomatous inflammation in the papillary and reticular dermis. These granulomas consist of epithelioid histiocytes and multinucleated giant cells with a lymphocytic infiltrate.5
Given the rarity of cutaneous CD, treatment approach is based on anecdotal evidence from case reports and case series. For a single lesion or localized disease, topical superpotent or intralesional steroids are recommended for initial therapy.3 Oral metronidazole also is an effective treatment and can be combined with topical or intralesional steroids.7 For disseminated disease, systemic corticosteroids have shown efficacy.3 Other reported treatment options include oral corticosteroids, sulfasalazine, azathioprine, 6-mercaptopurine, infliximab, and adalimumab. If monotherapy fails, combination therapy may be needed. Surgical debridement may be attempted if medical therapy fails but is complicated by wound dehiscence and disease recurrence.3
Although genital ulcers can be a presentation of Behçet disease and genital herpes infection, genital nodules and plaques are not typical for these 2 diseases. Also, the patient did not have oral ulcers, which is a common feature of Behçet disease. Genital sarcoidosis is extremely rare, and cutaneous CD was more likely given the patient's medical history. Finally, Jacquet dermatitis is more common in children, and patients with this condition typically have history of fecal and urinary incontinence.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Stingeni L, Neve D, Bassotti G, et al. Cutaneous Crohn's disease successfully treated with adalimumab [published online Sep 15, 2015]. J Eur Acad Dermatol Venerol. 2016;30:E72-E74.
- Kurtzman DJ, Jones T, Fangru L, et al. Metastatic Crohn's disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813.
- Hagen JW, Swoger JM, Grandinetti LM. Cutaneous manifestations of Crohn disease. Dermatol Clin. 2015;33:417-431.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review [published online June 19, 2008]. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease, part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
- Teixeira M, Machado S, Lago P, et al. Cutaneous Crohn's disease. Int J Dermatol. 2006;45:1074-1076.
- Stingeni L, Neve D, Bassotti G, et al. Cutaneous Crohn's disease successfully treated with adalimumab [published online Sep 15, 2015]. J Eur Acad Dermatol Venerol. 2016;30:E72-E74.
- Kurtzman DJ, Jones T, Fangru L, et al. Metastatic Crohn's disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813.
- Hagen JW, Swoger JM, Grandinetti LM. Cutaneous manifestations of Crohn disease. Dermatol Clin. 2015;33:417-431.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review [published online June 19, 2008]. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease, part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33.
- Abide JM. Metastatic Crohn disease: clearance with metronidazole. J Am Acad Dermatol. 2011;64:448-449.
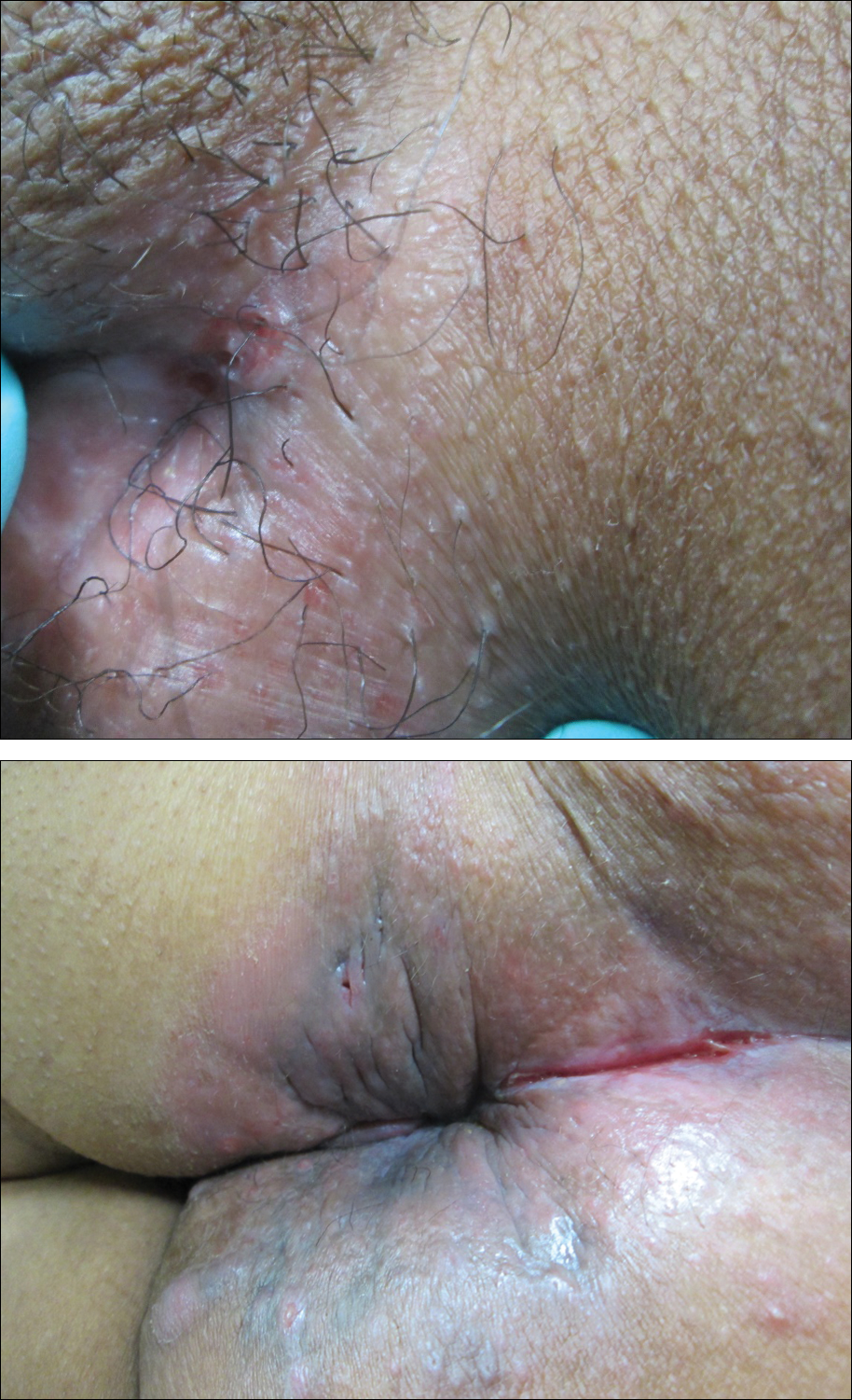
A 38-year-old woman with a history of Crohn disease presented with painful nonhealing vulvar and perianal erosions of 6 months' duration. The erosions developed 4 months after discontinuing adalimumab for a planned surgery. During this time, the patient also had an exacerbation of Crohn colitis and developed an anal fistula. Prior to this break in adalimumab, the patient's Crohn disease was well controlled on adalimumab 40 mg every 2 weeks, azathioprine 100 mg daily, and mesalamine 4.8 g daily. Despite restarting adalimumab and therapy with multiple antibiotics (ie, metronidazole, ciprofloxacin), the erosions persisted. On physical examination erythematous plaques and nodules were present at the vulvar (top) and perianal (bottom) skin. In addition, well-demarcated erosions measuring 20 mm and 80 mm were present on the vulvar and perianal skin, respectively. Human immunodeficiency virus screening and rapid plasma reagin were negative.