User login
PACAP38, a Migraine Attack Inducer, Suggests New Treatment Target
OJAI, CA—Infusion of the neuropeptide pituitary adenylate cyclase-activating polypeptide-38 (PACAP38) induces headache and vasodilatation in healthy subjects and migraineurs. Among migraineurs, PACAP38 infusion also may induce migraine-like attacks that are associated with sustained dilatation of extracranial arteries and elevated plasma PACAP38 before attack onset. Taken together, research indicates that PACAP38 is involved in migraine pathophysiology and may have implications for migraine therapy, according to a presentation at the 11th Annual Headache Cooperative of the Pacific Winter Conference.
Investigators at the University of Copenhagen’s Danish Headache Center, including Jes Olesen, MD, Professor of Neurology, have studied PACAP38. Dr. Olesen compared their work on PACAP38 to their prior studies of calcitonin gene-related peptide (CGRP), which contributed to the development of a new class of migraine drugs known as selective CGRP antagonists.
“What got the industry around to producing and testing a drug were our studies with the human model where we infused CGRP and placebo in a crossover study and demonstrated that CGRP infusion can cause a migraine attack,” Dr. Olesen said. Although it makes sense that blocking a peptide that induces migraine may lead to effective treatment, “it is not invariably so,” he said. “With CGRP, it is wonderful that it actually proved to be right, and so now we have the drugs against CGRP and its receptors.”
Similar Peptides With Different Effects
PACAP38 is structurally and functionally related to vasoactive intestinal polypeptide (VIP), but their effects on headache differ. Rahmann et al in 2008 studied 12 patients with migraine without aura who received infusions of VIP. None of the subjects reported a migraine attack after VIP infusion. The few instances of headache were mild. The investigators concluded that VIP does not trigger migraine attacks in migraineurs.
Similar studies of PACAP38, however, found that infusion of PACAP38 does induce headache and migraine-like attacks. Schytz et al in 2009 reported that PACAP38 infusion caused headache in all healthy subjects (n = 12) and in 11 of 12 patients with migraine, seven of whom experienced migraine-like attacks. None of the participants had headache after receiving placebo. Half of the migraineurs reported that onset of migraine-like attacks occurred several hours (mean, six hours) after the start of the PACAP38 infusions. “It’s a long-lasting effect, and there is a tendency for most of the headaches to come pretty late,” Dr. Olesen said.
With such observations in mind, Amin et al in 2014 undertook a head-to-head comparison of PACAP38 and VIP in a double-blind crossover study of female patients with migraine without aura. Patients were randomly allocated to IV infusion of PACAP38 or VIP over 20 minutes. Patients then received the other infusion at least one week later. Of the 22 patients who completed the study (mean age, 24), 16 patients (73%) reported migraine-like attacks after PACAP38 infusion, whereas four patients (18%) reported migraine-like attacks after VIP infusion. Three of the four patients who reported migraine-like attacks after VIP infusion also reported migraine-like attacks after PACAP38 infusion. Some of the migraine attacks could have been spontaneous and unrelated to the infusions, Dr. Olesen noted. Nevertheless, “it is clear that highly significantly more patients got a migraine attack with PACAP than with VIP,” Dr. Olesen said.
Is the PAC1 Receptor Key?
Further insights into PACAP38 have emerged from animal studies, one of which involves the potentially important role of the PAC1 receptor in migraine. PACAP38 works on the three most prominent receptors in the cerebral vasculature: PAC1, VPAC1, and VPAC2. VIP, by contrast, works on VPAC1 and VPAC2, but not on PAC1. The two compounds have the same affinity to VPAC1 and VPAC2. “So, isn’t it almost obvious that since PACAP causes a migraine attack and VIP does not, it must be via the PAC1 receptor?” Dr. Olesen asked. “Well, we think so, but how can we be sure? That is the logic of the data until somebody comes up with something else.”
A study led by Dr. Olesen’s colleague and wife, Inger Jansen-Olesen, DMSc, suggests another difference between VIP and PACAP38 that may be relevant to migraine. Dr. Jansen-Olesen and colleagues compared the effect of PACAP38 and VIP on CGRP release in the trigeminal nucleus caudalis in rats. Increasing doses of PACAP38 increased release of CGRP, whereas increasing doses of VIP did not. “VIP does not liberate CGRP. That is at least one difference between PACAP and VIP that may be relevant,” Dr. Olesen said.
Whether the PAC1 receptor is responsible for migraine induction is the “million-dollar question,” Dr. Olesen said. Several companies, based on their experience with CGRP, have the ability to develop human antibodies against PACAP38 or any of its three receptors relatively quickly, he said. “We would put our money on an antibody that blocks the PAC1 receptor because you like a drug to be as specific as possible,” said Dr. Olesen. “We do not want to block three receptors if we only have to block one receptor.”The same philosophy applies to blocking the PACAP38 molecule itself. “You … take away the signals to all three kinds of receptors, so it is likely that you would have more side effects,” Dr. Olesen said. On the other hand, whether migraine induction involves the PAC1 receptor or other mechanisms remains unclear. “I think [studies] will show in the not-too-distant future whether antibodies against the PAC1 receptor will be effective in migraine,” said Dr. Olesen.
—Fred Balzac
Suggested Reading
Amin FM, Hougaard A, Magon S, et al. Change in brain network connectivity during PACAP38-induced migraine attacks: a resting-state functional MRI study. Neurology. 2016;86(2):180-187.
Amin FM, Hougaard A, Schytz HW, et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain. 2014;137(Pt 3):779-794.
Guo S, Vollesen AL, Hansen RD, et al. Part I: Pituitary adenylate cyclase-activating polypeptide-38 induced migraine-like attacks in patients with and without familial aggregation of migraine. Cephalalgia. 2017;37(2):125-135.
Guo S, Vollesen AL, Hansen YB, et al. Part II: Biochemical changes after pituitary adenylate cyclase-activating polypeptide-38 infusion in migraine patients. Cephalalgia. 2017;37(2):136-147.
Guo S, Vollesen AL, Olesen J, Ashina M. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain. 2016;157(12):2773-2781.
Jansen-Olesen I, Baun M, Amrutkar DV, et al. PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor. Neuropeptides. 2014;48(2):53-64.
Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28(3):226-236.
Schytz HW, Birk S, Wienecke T, et al. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132(Pt 1):16-25.
OJAI, CA—Infusion of the neuropeptide pituitary adenylate cyclase-activating polypeptide-38 (PACAP38) induces headache and vasodilatation in healthy subjects and migraineurs. Among migraineurs, PACAP38 infusion also may induce migraine-like attacks that are associated with sustained dilatation of extracranial arteries and elevated plasma PACAP38 before attack onset. Taken together, research indicates that PACAP38 is involved in migraine pathophysiology and may have implications for migraine therapy, according to a presentation at the 11th Annual Headache Cooperative of the Pacific Winter Conference.
Investigators at the University of Copenhagen’s Danish Headache Center, including Jes Olesen, MD, Professor of Neurology, have studied PACAP38. Dr. Olesen compared their work on PACAP38 to their prior studies of calcitonin gene-related peptide (CGRP), which contributed to the development of a new class of migraine drugs known as selective CGRP antagonists.
“What got the industry around to producing and testing a drug were our studies with the human model where we infused CGRP and placebo in a crossover study and demonstrated that CGRP infusion can cause a migraine attack,” Dr. Olesen said. Although it makes sense that blocking a peptide that induces migraine may lead to effective treatment, “it is not invariably so,” he said. “With CGRP, it is wonderful that it actually proved to be right, and so now we have the drugs against CGRP and its receptors.”
Similar Peptides With Different Effects
PACAP38 is structurally and functionally related to vasoactive intestinal polypeptide (VIP), but their effects on headache differ. Rahmann et al in 2008 studied 12 patients with migraine without aura who received infusions of VIP. None of the subjects reported a migraine attack after VIP infusion. The few instances of headache were mild. The investigators concluded that VIP does not trigger migraine attacks in migraineurs.
Similar studies of PACAP38, however, found that infusion of PACAP38 does induce headache and migraine-like attacks. Schytz et al in 2009 reported that PACAP38 infusion caused headache in all healthy subjects (n = 12) and in 11 of 12 patients with migraine, seven of whom experienced migraine-like attacks. None of the participants had headache after receiving placebo. Half of the migraineurs reported that onset of migraine-like attacks occurred several hours (mean, six hours) after the start of the PACAP38 infusions. “It’s a long-lasting effect, and there is a tendency for most of the headaches to come pretty late,” Dr. Olesen said.
With such observations in mind, Amin et al in 2014 undertook a head-to-head comparison of PACAP38 and VIP in a double-blind crossover study of female patients with migraine without aura. Patients were randomly allocated to IV infusion of PACAP38 or VIP over 20 minutes. Patients then received the other infusion at least one week later. Of the 22 patients who completed the study (mean age, 24), 16 patients (73%) reported migraine-like attacks after PACAP38 infusion, whereas four patients (18%) reported migraine-like attacks after VIP infusion. Three of the four patients who reported migraine-like attacks after VIP infusion also reported migraine-like attacks after PACAP38 infusion. Some of the migraine attacks could have been spontaneous and unrelated to the infusions, Dr. Olesen noted. Nevertheless, “it is clear that highly significantly more patients got a migraine attack with PACAP than with VIP,” Dr. Olesen said.
Is the PAC1 Receptor Key?
Further insights into PACAP38 have emerged from animal studies, one of which involves the potentially important role of the PAC1 receptor in migraine. PACAP38 works on the three most prominent receptors in the cerebral vasculature: PAC1, VPAC1, and VPAC2. VIP, by contrast, works on VPAC1 and VPAC2, but not on PAC1. The two compounds have the same affinity to VPAC1 and VPAC2. “So, isn’t it almost obvious that since PACAP causes a migraine attack and VIP does not, it must be via the PAC1 receptor?” Dr. Olesen asked. “Well, we think so, but how can we be sure? That is the logic of the data until somebody comes up with something else.”
A study led by Dr. Olesen’s colleague and wife, Inger Jansen-Olesen, DMSc, suggests another difference between VIP and PACAP38 that may be relevant to migraine. Dr. Jansen-Olesen and colleagues compared the effect of PACAP38 and VIP on CGRP release in the trigeminal nucleus caudalis in rats. Increasing doses of PACAP38 increased release of CGRP, whereas increasing doses of VIP did not. “VIP does not liberate CGRP. That is at least one difference between PACAP and VIP that may be relevant,” Dr. Olesen said.
Whether the PAC1 receptor is responsible for migraine induction is the “million-dollar question,” Dr. Olesen said. Several companies, based on their experience with CGRP, have the ability to develop human antibodies against PACAP38 or any of its three receptors relatively quickly, he said. “We would put our money on an antibody that blocks the PAC1 receptor because you like a drug to be as specific as possible,” said Dr. Olesen. “We do not want to block three receptors if we only have to block one receptor.”The same philosophy applies to blocking the PACAP38 molecule itself. “You … take away the signals to all three kinds of receptors, so it is likely that you would have more side effects,” Dr. Olesen said. On the other hand, whether migraine induction involves the PAC1 receptor or other mechanisms remains unclear. “I think [studies] will show in the not-too-distant future whether antibodies against the PAC1 receptor will be effective in migraine,” said Dr. Olesen.
—Fred Balzac
Suggested Reading
Amin FM, Hougaard A, Magon S, et al. Change in brain network connectivity during PACAP38-induced migraine attacks: a resting-state functional MRI study. Neurology. 2016;86(2):180-187.
Amin FM, Hougaard A, Schytz HW, et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain. 2014;137(Pt 3):779-794.
Guo S, Vollesen AL, Hansen RD, et al. Part I: Pituitary adenylate cyclase-activating polypeptide-38 induced migraine-like attacks in patients with and without familial aggregation of migraine. Cephalalgia. 2017;37(2):125-135.
Guo S, Vollesen AL, Hansen YB, et al. Part II: Biochemical changes after pituitary adenylate cyclase-activating polypeptide-38 infusion in migraine patients. Cephalalgia. 2017;37(2):136-147.
Guo S, Vollesen AL, Olesen J, Ashina M. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain. 2016;157(12):2773-2781.
Jansen-Olesen I, Baun M, Amrutkar DV, et al. PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor. Neuropeptides. 2014;48(2):53-64.
Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28(3):226-236.
Schytz HW, Birk S, Wienecke T, et al. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132(Pt 1):16-25.
OJAI, CA—Infusion of the neuropeptide pituitary adenylate cyclase-activating polypeptide-38 (PACAP38) induces headache and vasodilatation in healthy subjects and migraineurs. Among migraineurs, PACAP38 infusion also may induce migraine-like attacks that are associated with sustained dilatation of extracranial arteries and elevated plasma PACAP38 before attack onset. Taken together, research indicates that PACAP38 is involved in migraine pathophysiology and may have implications for migraine therapy, according to a presentation at the 11th Annual Headache Cooperative of the Pacific Winter Conference.
Investigators at the University of Copenhagen’s Danish Headache Center, including Jes Olesen, MD, Professor of Neurology, have studied PACAP38. Dr. Olesen compared their work on PACAP38 to their prior studies of calcitonin gene-related peptide (CGRP), which contributed to the development of a new class of migraine drugs known as selective CGRP antagonists.
“What got the industry around to producing and testing a drug were our studies with the human model where we infused CGRP and placebo in a crossover study and demonstrated that CGRP infusion can cause a migraine attack,” Dr. Olesen said. Although it makes sense that blocking a peptide that induces migraine may lead to effective treatment, “it is not invariably so,” he said. “With CGRP, it is wonderful that it actually proved to be right, and so now we have the drugs against CGRP and its receptors.”
Similar Peptides With Different Effects
PACAP38 is structurally and functionally related to vasoactive intestinal polypeptide (VIP), but their effects on headache differ. Rahmann et al in 2008 studied 12 patients with migraine without aura who received infusions of VIP. None of the subjects reported a migraine attack after VIP infusion. The few instances of headache were mild. The investigators concluded that VIP does not trigger migraine attacks in migraineurs.
Similar studies of PACAP38, however, found that infusion of PACAP38 does induce headache and migraine-like attacks. Schytz et al in 2009 reported that PACAP38 infusion caused headache in all healthy subjects (n = 12) and in 11 of 12 patients with migraine, seven of whom experienced migraine-like attacks. None of the participants had headache after receiving placebo. Half of the migraineurs reported that onset of migraine-like attacks occurred several hours (mean, six hours) after the start of the PACAP38 infusions. “It’s a long-lasting effect, and there is a tendency for most of the headaches to come pretty late,” Dr. Olesen said.
With such observations in mind, Amin et al in 2014 undertook a head-to-head comparison of PACAP38 and VIP in a double-blind crossover study of female patients with migraine without aura. Patients were randomly allocated to IV infusion of PACAP38 or VIP over 20 minutes. Patients then received the other infusion at least one week later. Of the 22 patients who completed the study (mean age, 24), 16 patients (73%) reported migraine-like attacks after PACAP38 infusion, whereas four patients (18%) reported migraine-like attacks after VIP infusion. Three of the four patients who reported migraine-like attacks after VIP infusion also reported migraine-like attacks after PACAP38 infusion. Some of the migraine attacks could have been spontaneous and unrelated to the infusions, Dr. Olesen noted. Nevertheless, “it is clear that highly significantly more patients got a migraine attack with PACAP than with VIP,” Dr. Olesen said.
Is the PAC1 Receptor Key?
Further insights into PACAP38 have emerged from animal studies, one of which involves the potentially important role of the PAC1 receptor in migraine. PACAP38 works on the three most prominent receptors in the cerebral vasculature: PAC1, VPAC1, and VPAC2. VIP, by contrast, works on VPAC1 and VPAC2, but not on PAC1. The two compounds have the same affinity to VPAC1 and VPAC2. “So, isn’t it almost obvious that since PACAP causes a migraine attack and VIP does not, it must be via the PAC1 receptor?” Dr. Olesen asked. “Well, we think so, but how can we be sure? That is the logic of the data until somebody comes up with something else.”
A study led by Dr. Olesen’s colleague and wife, Inger Jansen-Olesen, DMSc, suggests another difference between VIP and PACAP38 that may be relevant to migraine. Dr. Jansen-Olesen and colleagues compared the effect of PACAP38 and VIP on CGRP release in the trigeminal nucleus caudalis in rats. Increasing doses of PACAP38 increased release of CGRP, whereas increasing doses of VIP did not. “VIP does not liberate CGRP. That is at least one difference between PACAP and VIP that may be relevant,” Dr. Olesen said.
Whether the PAC1 receptor is responsible for migraine induction is the “million-dollar question,” Dr. Olesen said. Several companies, based on their experience with CGRP, have the ability to develop human antibodies against PACAP38 or any of its three receptors relatively quickly, he said. “We would put our money on an antibody that blocks the PAC1 receptor because you like a drug to be as specific as possible,” said Dr. Olesen. “We do not want to block three receptors if we only have to block one receptor.”The same philosophy applies to blocking the PACAP38 molecule itself. “You … take away the signals to all three kinds of receptors, so it is likely that you would have more side effects,” Dr. Olesen said. On the other hand, whether migraine induction involves the PAC1 receptor or other mechanisms remains unclear. “I think [studies] will show in the not-too-distant future whether antibodies against the PAC1 receptor will be effective in migraine,” said Dr. Olesen.
—Fred Balzac
Suggested Reading
Amin FM, Hougaard A, Magon S, et al. Change in brain network connectivity during PACAP38-induced migraine attacks: a resting-state functional MRI study. Neurology. 2016;86(2):180-187.
Amin FM, Hougaard A, Schytz HW, et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain. 2014;137(Pt 3):779-794.
Guo S, Vollesen AL, Hansen RD, et al. Part I: Pituitary adenylate cyclase-activating polypeptide-38 induced migraine-like attacks in patients with and without familial aggregation of migraine. Cephalalgia. 2017;37(2):125-135.
Guo S, Vollesen AL, Hansen YB, et al. Part II: Biochemical changes after pituitary adenylate cyclase-activating polypeptide-38 infusion in migraine patients. Cephalalgia. 2017;37(2):136-147.
Guo S, Vollesen AL, Olesen J, Ashina M. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain. 2016;157(12):2773-2781.
Jansen-Olesen I, Baun M, Amrutkar DV, et al. PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor. Neuropeptides. 2014;48(2):53-64.
Rahmann A, Wienecke T, Hansen JM, et al. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28(3):226-236.
Schytz HW, Birk S, Wienecke T, et al. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132(Pt 1):16-25.
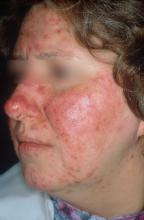
National Rosacea Society designates April as “Rosacea Awareness Month”
April has been designated “Rosacea Awareness Month” by the National Rosacea Society (NRS), with the aim of educating the public about the disease.
“During April and throughout the year, people who suspect they may have rosacea can contact the NRS for more information,” according to a press release issued April 2 by the NRS.
The press release refers to the new standard diagnostic guidelines for rosacea, developed by the National Rosacea Society Expert Committee, which were released online in 2017 (J Am Acad Dermatol. 2018 Jan;78[1]:148-55).
More information for patients is available on the NRS website, at www.rosacea.org, via email at [email protected], or by calling 888-NO-BLUSH (662-5874).
April has been designated “Rosacea Awareness Month” by the National Rosacea Society (NRS), with the aim of educating the public about the disease.
“During April and throughout the year, people who suspect they may have rosacea can contact the NRS for more information,” according to a press release issued April 2 by the NRS.
The press release refers to the new standard diagnostic guidelines for rosacea, developed by the National Rosacea Society Expert Committee, which were released online in 2017 (J Am Acad Dermatol. 2018 Jan;78[1]:148-55).
More information for patients is available on the NRS website, at www.rosacea.org, via email at [email protected], or by calling 888-NO-BLUSH (662-5874).
April has been designated “Rosacea Awareness Month” by the National Rosacea Society (NRS), with the aim of educating the public about the disease.
“During April and throughout the year, people who suspect they may have rosacea can contact the NRS for more information,” according to a press release issued April 2 by the NRS.
The press release refers to the new standard diagnostic guidelines for rosacea, developed by the National Rosacea Society Expert Committee, which were released online in 2017 (J Am Acad Dermatol. 2018 Jan;78[1]:148-55).
More information for patients is available on the NRS website, at www.rosacea.org, via email at [email protected], or by calling 888-NO-BLUSH (662-5874).
Complementary and Integrative Health Therapies for Opioid Overuse
The US has seen a rise in the number of prescriptions of opioids to treat chronic pain; however, the rise has been associated with increased rates of addiction and deaths related to opioid abuse and heroin use. Chronic pain is associated with the use of prescription opioids in veterans, which sometimes complicates the concurrent treatment of mental health disorders.1-3 Also, opioid use issues, including suicide, have affected veterans at higher numbers than it has in the nonveteran population.4,5
Unfortunately, the prevailing Western medical model with its focus on treating disease has not proven to be adequate in solving the problem. Hence, the Department of Veterans Affairs (VA) is in the process of a paradigm shift to a whole person model that prioritizes health and well-being, as defined by the individual, while proactively addressing risk factors before illness develops.
The new model includes an emphasis on complementary and integrative health (CIH) therapies to promote optimal health, healing, and well-being.6 Yoga, massage, acupuncture, meditation, and guided imagery are some examples of VA-approved CIH therapies favored by veterans and their health care providers (HCPs) to treat and/or divert the subject’s attention from physical pain or mental anguish.7,8
In response to opioid overuse, Congress passed the Comprehensive Addiction and Recovery Act of 2016 (CARA).9 Title IX of CARA mandates the VA to work with the Department of Defense (DoD) to limit the amount of time a patient is prescribed an opioid.
Replacing opioids with other ways to control chronic pain may be helpful in addressing the real distress experienced by persons with these diagnoses. Hence, the CARA suggests augmenting opioid therapy with other pain management therapies and modalities, including CIH. Instead of focusing on the treatment of a specific illness after it develops, CIH therapies aim to promote wellness in the whole person. However, good intentions are not enough. Due to existing institutional culture and prioritization of resources, the adoption of CIH therapies across the VA has been inconsistent.10
The CARA furnishes the VA with an opportunity to serve as a leader in the innovative use of CIH therapies. Previous research conducted by the VA has shown that veterans and their HCPs would like increased availability of CIH through the VA.7,10,11 Research also suggests CIH for specific conditions in veterans, such as posttraumatic stress disorder or postoperative pain.12,13 For its part, the VA has declared the provision of personalized, proactive, patient-driven health care for veterans as its top strategic priority.
To achieve the organizational transformation associated with providing this type of care, the VA established the Office of Patient Centered Care and Cultural Transformation (OPCC&CT), which created the Integrative Health Coordinating Center (IHCC).6 The main functions of the IHCC are to identify and remove barriers to providing CIH within VA and to serve as a resource for practice and education for veterans as well as HCPs.
Several VA facilities already have demonstrated what can be done with the support and encouragement of the OPCC&CT plus an enormous amount of dedicated effort from local HCPs and highly supportive service chiefs. Examples include the Perry Point VA Residential Wellness Center in Maryland and the Integrative Medicine and Wellness Center in the Central Arkansas VA Healthcare System in Little Rock. Perry Point has a focus on veterans with substance abuse diagnoses and uses multiple therapies, including acupuncture, yoga, guided meditation, osteopathic manipulation, music, and creative arts. The Little Rock center focuses on skills building, self-care, and accountability with modalities such as yoga, acupuncture, mindfulness, and chiropractic.
The CARA mandates the continuance and expansion of similar pilot projects that assess the feasibility and advisability of CIH programs to complement the provision of pain management and related health care services, including mental health care services to veterans. Thus, the VA Secretary was directed to select at least 15 geographically diverse locations for the pilot projects. The VA has committed to conducting 18 full-scale demonstration projects in 2018—1 project in each VISN (Veterans Integrated Service Network). Section 933 of the CARA, prioritizes medical centers where the “prescription rate of opioids conflicts with or is otherwise inconsistent with the standards of appropriate and safe care.”9
Several issues must be addressed to make the provision of CIH in the VA a success.14 They include but are not limited to the following:
- Clarification that CIH services for veterans are included in the Medical Benefits Package, which requires that care meets generally accepted standards of medical practice.
- Vetting of CIH therapies to determine which ones should be recommended for inclusion in the Medical Benefits Package. Factors to consider include clinical evidence, community standards, practice guidelines, licensing and credentialing requirements, potential for harm, and veteran demand.
- Changes to VA business processes to provide the infrastructure for CIH delivery.
- Competition with existing VA programs for resources.
- Education of HCPs and administrators about CIH through the development of CIH instruction manuals, curriculum, and faculty.
Although the VA faces the daunting task of reducing opioid use while continuing to treat chronic physical and mental pain, CIH therapies seem to offer a viable adjunctive therapy. It will be incumbent on the VA to explore through ongoing research all that CIH therapies may have to offer; veterans deserve no less. If the VA can demonstrate the effectiveness of CIH in treating the challenges faced by veterans, the results will serve as a useful example for treating chronic pain in the nonveteran population as well.
1. Lovejoy TI, Dobscha SK, Turk DC, Weimer MB, Morasco BJ. Correlates of prescription opioid therapy in veterans with chronic pain and history of substance use disorder. J Rehabil Res Dev. 2016;53(1):25-36.
2. Deyo RA, Smith DH, Johnson ES, et al. Opioids for back pain patients: primary care prescribing patterns and use of service. J Am Board Fam Med. 2011;24(6):717-727.
3. Hawkins EJ, Malte CA, Grossbard JR, Saxon AJ. Prevalence and trends of concurrent opioid analgesic and benzodiazepine use among Veterans Affairs patients with post-traumatic stress disorder, 2003-2011. Pain Med. 2015;16(10):1943-1954.
4. Jonas WB, Schoomaker EB. Pain and opioids in the military: we must do better. JAMA Intern Med. 2014;174(8):1402-1403.
5. Ilgen MA, Bohnert ASB, Ganoczy D, Bair MJ, McCarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157(5):1079-1084.
6. Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12) (suppl 5):S5-S8.
7. Fletcher CE, Mitchinson AR, Trumble EL, Hinshaw DB, Dusek JA. Perceptions of other integrative health therapies by veterans with pain who are receiving massage. J Rehabil Res Dev. 2016;53(1):117-126.
8. US Department of Veterans Affairs, Office of Patient Centered Care & Cultural Transformation. IHCC approved CIH. https://vaww.infoshare.va.gov/sites/OPCC/SitePages/IHCC-Approved-CIH.aspx . Published August 8, 2017. Accessed March 26, 2018. [Nonpublic document.]
9. 114th US Congress. Comprehensive Addiction and Recovery Act of 2016 . Public Law 114-198. July 22, 2016 130 STAT.695.
10. Fletcher CE, Mitchinson AR, Trumble EL, Hinshaw DB, Dusek JA. Providers’ and administrators’ perceptions of complementary and integrative health practices across the Veterans Health Administration. J Altern Complement Med. 2017;23(1):26-34.
11. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population studies. Med Care. 2014;52(12)(suppl 5):S83-S90.
12. Bormann JE, Oman D, Walter KH, Johnson BD. Mindful attention increases and mediates psychological outcomes following mantram repetition practice in veterans with posttraumatic stress disorder. Med Care. 2014;52(12)(suppl 5):S13-S18.
13. Mitchinson AR, Kim HM, Rosenberg JM, et al. Acute postoperative pain management using massage as an adjuvant therapy: a randomized trial. Arch Surg. 2007;142(12):1158-1167.
14. US Department of Veterans Affairs, Veterans Health Administration. Complementary and integrative health – expanding research, education, delivery and integration of complementary and integrative health services into the health care services provided to veterans. https://vaww.infoshare.va.gov/sites/OPCC/SiteAssets/SitePages/IHCC-home/2017%20CIH%20Plan_CARA%20932.pdf . Published March 2017. Accessed March 23, 2018. [Nonpublic document.]
The US has seen a rise in the number of prescriptions of opioids to treat chronic pain; however, the rise has been associated with increased rates of addiction and deaths related to opioid abuse and heroin use. Chronic pain is associated with the use of prescription opioids in veterans, which sometimes complicates the concurrent treatment of mental health disorders.1-3 Also, opioid use issues, including suicide, have affected veterans at higher numbers than it has in the nonveteran population.4,5
Unfortunately, the prevailing Western medical model with its focus on treating disease has not proven to be adequate in solving the problem. Hence, the Department of Veterans Affairs (VA) is in the process of a paradigm shift to a whole person model that prioritizes health and well-being, as defined by the individual, while proactively addressing risk factors before illness develops.
The new model includes an emphasis on complementary and integrative health (CIH) therapies to promote optimal health, healing, and well-being.6 Yoga, massage, acupuncture, meditation, and guided imagery are some examples of VA-approved CIH therapies favored by veterans and their health care providers (HCPs) to treat and/or divert the subject’s attention from physical pain or mental anguish.7,8
In response to opioid overuse, Congress passed the Comprehensive Addiction and Recovery Act of 2016 (CARA).9 Title IX of CARA mandates the VA to work with the Department of Defense (DoD) to limit the amount of time a patient is prescribed an opioid.
Replacing opioids with other ways to control chronic pain may be helpful in addressing the real distress experienced by persons with these diagnoses. Hence, the CARA suggests augmenting opioid therapy with other pain management therapies and modalities, including CIH. Instead of focusing on the treatment of a specific illness after it develops, CIH therapies aim to promote wellness in the whole person. However, good intentions are not enough. Due to existing institutional culture and prioritization of resources, the adoption of CIH therapies across the VA has been inconsistent.10
The CARA furnishes the VA with an opportunity to serve as a leader in the innovative use of CIH therapies. Previous research conducted by the VA has shown that veterans and their HCPs would like increased availability of CIH through the VA.7,10,11 Research also suggests CIH for specific conditions in veterans, such as posttraumatic stress disorder or postoperative pain.12,13 For its part, the VA has declared the provision of personalized, proactive, patient-driven health care for veterans as its top strategic priority.
To achieve the organizational transformation associated with providing this type of care, the VA established the Office of Patient Centered Care and Cultural Transformation (OPCC&CT), which created the Integrative Health Coordinating Center (IHCC).6 The main functions of the IHCC are to identify and remove barriers to providing CIH within VA and to serve as a resource for practice and education for veterans as well as HCPs.
Several VA facilities already have demonstrated what can be done with the support and encouragement of the OPCC&CT plus an enormous amount of dedicated effort from local HCPs and highly supportive service chiefs. Examples include the Perry Point VA Residential Wellness Center in Maryland and the Integrative Medicine and Wellness Center in the Central Arkansas VA Healthcare System in Little Rock. Perry Point has a focus on veterans with substance abuse diagnoses and uses multiple therapies, including acupuncture, yoga, guided meditation, osteopathic manipulation, music, and creative arts. The Little Rock center focuses on skills building, self-care, and accountability with modalities such as yoga, acupuncture, mindfulness, and chiropractic.
The CARA mandates the continuance and expansion of similar pilot projects that assess the feasibility and advisability of CIH programs to complement the provision of pain management and related health care services, including mental health care services to veterans. Thus, the VA Secretary was directed to select at least 15 geographically diverse locations for the pilot projects. The VA has committed to conducting 18 full-scale demonstration projects in 2018—1 project in each VISN (Veterans Integrated Service Network). Section 933 of the CARA, prioritizes medical centers where the “prescription rate of opioids conflicts with or is otherwise inconsistent with the standards of appropriate and safe care.”9
Several issues must be addressed to make the provision of CIH in the VA a success.14 They include but are not limited to the following:
- Clarification that CIH services for veterans are included in the Medical Benefits Package, which requires that care meets generally accepted standards of medical practice.
- Vetting of CIH therapies to determine which ones should be recommended for inclusion in the Medical Benefits Package. Factors to consider include clinical evidence, community standards, practice guidelines, licensing and credentialing requirements, potential for harm, and veteran demand.
- Changes to VA business processes to provide the infrastructure for CIH delivery.
- Competition with existing VA programs for resources.
- Education of HCPs and administrators about CIH through the development of CIH instruction manuals, curriculum, and faculty.
Although the VA faces the daunting task of reducing opioid use while continuing to treat chronic physical and mental pain, CIH therapies seem to offer a viable adjunctive therapy. It will be incumbent on the VA to explore through ongoing research all that CIH therapies may have to offer; veterans deserve no less. If the VA can demonstrate the effectiveness of CIH in treating the challenges faced by veterans, the results will serve as a useful example for treating chronic pain in the nonveteran population as well.
The US has seen a rise in the number of prescriptions of opioids to treat chronic pain; however, the rise has been associated with increased rates of addiction and deaths related to opioid abuse and heroin use. Chronic pain is associated with the use of prescription opioids in veterans, which sometimes complicates the concurrent treatment of mental health disorders.1-3 Also, opioid use issues, including suicide, have affected veterans at higher numbers than it has in the nonveteran population.4,5
Unfortunately, the prevailing Western medical model with its focus on treating disease has not proven to be adequate in solving the problem. Hence, the Department of Veterans Affairs (VA) is in the process of a paradigm shift to a whole person model that prioritizes health and well-being, as defined by the individual, while proactively addressing risk factors before illness develops.
The new model includes an emphasis on complementary and integrative health (CIH) therapies to promote optimal health, healing, and well-being.6 Yoga, massage, acupuncture, meditation, and guided imagery are some examples of VA-approved CIH therapies favored by veterans and their health care providers (HCPs) to treat and/or divert the subject’s attention from physical pain or mental anguish.7,8
In response to opioid overuse, Congress passed the Comprehensive Addiction and Recovery Act of 2016 (CARA).9 Title IX of CARA mandates the VA to work with the Department of Defense (DoD) to limit the amount of time a patient is prescribed an opioid.
Replacing opioids with other ways to control chronic pain may be helpful in addressing the real distress experienced by persons with these diagnoses. Hence, the CARA suggests augmenting opioid therapy with other pain management therapies and modalities, including CIH. Instead of focusing on the treatment of a specific illness after it develops, CIH therapies aim to promote wellness in the whole person. However, good intentions are not enough. Due to existing institutional culture and prioritization of resources, the adoption of CIH therapies across the VA has been inconsistent.10
The CARA furnishes the VA with an opportunity to serve as a leader in the innovative use of CIH therapies. Previous research conducted by the VA has shown that veterans and their HCPs would like increased availability of CIH through the VA.7,10,11 Research also suggests CIH for specific conditions in veterans, such as posttraumatic stress disorder or postoperative pain.12,13 For its part, the VA has declared the provision of personalized, proactive, patient-driven health care for veterans as its top strategic priority.
To achieve the organizational transformation associated with providing this type of care, the VA established the Office of Patient Centered Care and Cultural Transformation (OPCC&CT), which created the Integrative Health Coordinating Center (IHCC).6 The main functions of the IHCC are to identify and remove barriers to providing CIH within VA and to serve as a resource for practice and education for veterans as well as HCPs.
Several VA facilities already have demonstrated what can be done with the support and encouragement of the OPCC&CT plus an enormous amount of dedicated effort from local HCPs and highly supportive service chiefs. Examples include the Perry Point VA Residential Wellness Center in Maryland and the Integrative Medicine and Wellness Center in the Central Arkansas VA Healthcare System in Little Rock. Perry Point has a focus on veterans with substance abuse diagnoses and uses multiple therapies, including acupuncture, yoga, guided meditation, osteopathic manipulation, music, and creative arts. The Little Rock center focuses on skills building, self-care, and accountability with modalities such as yoga, acupuncture, mindfulness, and chiropractic.
The CARA mandates the continuance and expansion of similar pilot projects that assess the feasibility and advisability of CIH programs to complement the provision of pain management and related health care services, including mental health care services to veterans. Thus, the VA Secretary was directed to select at least 15 geographically diverse locations for the pilot projects. The VA has committed to conducting 18 full-scale demonstration projects in 2018—1 project in each VISN (Veterans Integrated Service Network). Section 933 of the CARA, prioritizes medical centers where the “prescription rate of opioids conflicts with or is otherwise inconsistent with the standards of appropriate and safe care.”9
Several issues must be addressed to make the provision of CIH in the VA a success.14 They include but are not limited to the following:
- Clarification that CIH services for veterans are included in the Medical Benefits Package, which requires that care meets generally accepted standards of medical practice.
- Vetting of CIH therapies to determine which ones should be recommended for inclusion in the Medical Benefits Package. Factors to consider include clinical evidence, community standards, practice guidelines, licensing and credentialing requirements, potential for harm, and veteran demand.
- Changes to VA business processes to provide the infrastructure for CIH delivery.
- Competition with existing VA programs for resources.
- Education of HCPs and administrators about CIH through the development of CIH instruction manuals, curriculum, and faculty.
Although the VA faces the daunting task of reducing opioid use while continuing to treat chronic physical and mental pain, CIH therapies seem to offer a viable adjunctive therapy. It will be incumbent on the VA to explore through ongoing research all that CIH therapies may have to offer; veterans deserve no less. If the VA can demonstrate the effectiveness of CIH in treating the challenges faced by veterans, the results will serve as a useful example for treating chronic pain in the nonveteran population as well.
1. Lovejoy TI, Dobscha SK, Turk DC, Weimer MB, Morasco BJ. Correlates of prescription opioid therapy in veterans with chronic pain and history of substance use disorder. J Rehabil Res Dev. 2016;53(1):25-36.
2. Deyo RA, Smith DH, Johnson ES, et al. Opioids for back pain patients: primary care prescribing patterns and use of service. J Am Board Fam Med. 2011;24(6):717-727.
3. Hawkins EJ, Malte CA, Grossbard JR, Saxon AJ. Prevalence and trends of concurrent opioid analgesic and benzodiazepine use among Veterans Affairs patients with post-traumatic stress disorder, 2003-2011. Pain Med. 2015;16(10):1943-1954.
4. Jonas WB, Schoomaker EB. Pain and opioids in the military: we must do better. JAMA Intern Med. 2014;174(8):1402-1403.
5. Ilgen MA, Bohnert ASB, Ganoczy D, Bair MJ, McCarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157(5):1079-1084.
6. Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12) (suppl 5):S5-S8.
7. Fletcher CE, Mitchinson AR, Trumble EL, Hinshaw DB, Dusek JA. Perceptions of other integrative health therapies by veterans with pain who are receiving massage. J Rehabil Res Dev. 2016;53(1):117-126.
8. US Department of Veterans Affairs, Office of Patient Centered Care & Cultural Transformation. IHCC approved CIH. https://vaww.infoshare.va.gov/sites/OPCC/SitePages/IHCC-Approved-CIH.aspx . Published August 8, 2017. Accessed March 26, 2018. [Nonpublic document.]
9. 114th US Congress. Comprehensive Addiction and Recovery Act of 2016 . Public Law 114-198. July 22, 2016 130 STAT.695.
10. Fletcher CE, Mitchinson AR, Trumble EL, Hinshaw DB, Dusek JA. Providers’ and administrators’ perceptions of complementary and integrative health practices across the Veterans Health Administration. J Altern Complement Med. 2017;23(1):26-34.
11. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population studies. Med Care. 2014;52(12)(suppl 5):S83-S90.
12. Bormann JE, Oman D, Walter KH, Johnson BD. Mindful attention increases and mediates psychological outcomes following mantram repetition practice in veterans with posttraumatic stress disorder. Med Care. 2014;52(12)(suppl 5):S13-S18.
13. Mitchinson AR, Kim HM, Rosenberg JM, et al. Acute postoperative pain management using massage as an adjuvant therapy: a randomized trial. Arch Surg. 2007;142(12):1158-1167.
14. US Department of Veterans Affairs, Veterans Health Administration. Complementary and integrative health – expanding research, education, delivery and integration of complementary and integrative health services into the health care services provided to veterans. https://vaww.infoshare.va.gov/sites/OPCC/SiteAssets/SitePages/IHCC-home/2017%20CIH%20Plan_CARA%20932.pdf . Published March 2017. Accessed March 23, 2018. [Nonpublic document.]
1. Lovejoy TI, Dobscha SK, Turk DC, Weimer MB, Morasco BJ. Correlates of prescription opioid therapy in veterans with chronic pain and history of substance use disorder. J Rehabil Res Dev. 2016;53(1):25-36.
2. Deyo RA, Smith DH, Johnson ES, et al. Opioids for back pain patients: primary care prescribing patterns and use of service. J Am Board Fam Med. 2011;24(6):717-727.
3. Hawkins EJ, Malte CA, Grossbard JR, Saxon AJ. Prevalence and trends of concurrent opioid analgesic and benzodiazepine use among Veterans Affairs patients with post-traumatic stress disorder, 2003-2011. Pain Med. 2015;16(10):1943-1954.
4. Jonas WB, Schoomaker EB. Pain and opioids in the military: we must do better. JAMA Intern Med. 2014;174(8):1402-1403.
5. Ilgen MA, Bohnert ASB, Ganoczy D, Bair MJ, McCarthy JF, Blow FC. Opioid dose and risk of suicide. Pain. 2016;157(5):1079-1084.
6. Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12) (suppl 5):S5-S8.
7. Fletcher CE, Mitchinson AR, Trumble EL, Hinshaw DB, Dusek JA. Perceptions of other integrative health therapies by veterans with pain who are receiving massage. J Rehabil Res Dev. 2016;53(1):117-126.
8. US Department of Veterans Affairs, Office of Patient Centered Care & Cultural Transformation. IHCC approved CIH. https://vaww.infoshare.va.gov/sites/OPCC/SitePages/IHCC-Approved-CIH.aspx . Published August 8, 2017. Accessed March 26, 2018. [Nonpublic document.]
9. 114th US Congress. Comprehensive Addiction and Recovery Act of 2016 . Public Law 114-198. July 22, 2016 130 STAT.695.
10. Fletcher CE, Mitchinson AR, Trumble EL, Hinshaw DB, Dusek JA. Providers’ and administrators’ perceptions of complementary and integrative health practices across the Veterans Health Administration. J Altern Complement Med. 2017;23(1):26-34.
11. Davis MT, Mulvaney-Day N, Larson MJ, Hoover R, Mauch D. Complementary and alternative medicine among veterans and military personnel: a synthesis of population studies. Med Care. 2014;52(12)(suppl 5):S83-S90.
12. Bormann JE, Oman D, Walter KH, Johnson BD. Mindful attention increases and mediates psychological outcomes following mantram repetition practice in veterans with posttraumatic stress disorder. Med Care. 2014;52(12)(suppl 5):S13-S18.
13. Mitchinson AR, Kim HM, Rosenberg JM, et al. Acute postoperative pain management using massage as an adjuvant therapy: a randomized trial. Arch Surg. 2007;142(12):1158-1167.
14. US Department of Veterans Affairs, Veterans Health Administration. Complementary and integrative health – expanding research, education, delivery and integration of complementary and integrative health services into the health care services provided to veterans. https://vaww.infoshare.va.gov/sites/OPCC/SiteAssets/SitePages/IHCC-home/2017%20CIH%20Plan_CARA%20932.pdf . Published March 2017. Accessed March 23, 2018. [Nonpublic document.]
MDedge Daily News: How gastric bypass helps fight diabetes
New efforts aim to get addiction specialists on the job. Preconception hypertension poses pregnancy risks. And don’t leave transgender patients out of breast cancer surveillance.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
New efforts aim to get addiction specialists on the job. Preconception hypertension poses pregnancy risks. And don’t leave transgender patients out of breast cancer surveillance.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
New efforts aim to get addiction specialists on the job. Preconception hypertension poses pregnancy risks. And don’t leave transgender patients out of breast cancer surveillance.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Understanding, Assessing, and Conceptualizing Suicide Risk Among Veterans With PTSD
Increased risk of suicide among veterans with posttraumatic stress disorder (PTSD) is well established. Posttraumatic stress disorder and related consequences are associated with higher rates of suicidal ideation and suicidal self-directed violence (S-SDV).1 Based on a systematic review, several explanations for this relationship have been hypothesized.1 Particular emphasis has been placed on trauma type (eg, premilitary childhood abuse, combat exposure), frequency of trauma exposure (ie, a single traumatic episode vs multiple traumatic experiences), specific PTSD symptoms (eg, avoidance, sleep disturbance, alteration in mood and cognitions, risky behaviors), and other psychosocial consequences associated with PTSD (eg, low social support, psychiatric comorbidity, substance use). However, there is limited understanding regarding how to conceptualize and assess risk for suicide when treating veterans who have PTSD.
PTSD and the Interpersonal-Psychological Theory of Suicide
Although PTSD is associated with risk for S-SDV among veterans, a diagnosis-specific approach to conceptualizing risk of suicide (ie, an explanation specific to PTSD) might not be enough because most individuals with a psychiatric diagnosis do not engage in S-SDV.2 Rather, theories that are able to conceptualize suicide risk across many different psychiatric diagnoses are likely to improve mental health providers’ ability to understand risk of suicide. Although many theories attempt to understand suicide risk, the Interpersonal-Psychological Theory of Suicide (IPTS) has robust empirical support.3
The IPTS proposes that suicidal ideation is driven by perceptions of stable and unchanging thwarted belongingness (TB), defined as an unmet psychological need to socially belong, and perceived burdensomeness (PB), defined as the perception that one is a burden on others.4 However, PB and TB are not considered sufficient for S-SDV to occur unless an individual also has acquired the capability for suicide. Capability for suicide is thought to happen when an individual loses the fear of dying by suicide and develops tolerance to physical pain, which is proposed to occur through habituation or repeated exposure to painful stimuli.3
Several studies have examined the IPTS in a number of clinical populations, including veterans and active-duty service members; yet limited research has applied the IPTS to veterans with PTSD.3 However, a recent article proposed that a number of PTSD-related factors increase risk of suicide through the lens of the IPTS.5 In particular, repeated exposure to painful and provocative events—especially those characterized by violence and aggression—might increase acquired capability for suicide by causing habituation to physical pain and discomfort and reducing fear of injury and death. This concept is especially concerning because of the frequent occurrence of both military- (eg, combat, military sexual trauma) and nonmilitary-related (eg, childhood abuse, intimate partner violence) stressful and traumatic events among veterans, especially individuals with PTSD.
Moreover, the acquired capability for suicide correlates highly with anxious, intrusive, and hyperarousal symptoms of PTSD.5-7 Over time, these PTSD symptoms are thought to increase habituation to the physically painful and frightening aspects of S-SDV, resulting in increased pain tolerance and fearlessness about death.3
In addition, PTSD-related cognitive-affective states (ie, thoughts and emotions), such as guilt, shame, and self-deprecation, might drive beliefs of PB and TB.5,8 Repeated exposure to such trauma-related thoughts and emotions could further reinforce beliefs of self-hate or inadequacy (PB).2 Trauma-related beliefs that the world or others are unsafe also might reduce the likelihood of seeking social support, thereby increasing TB.2 The PTSD symptoms of avoidance and self-blame also are likely to reinforce beliefs of PB and TB.2
Assessing Suicide Risk in the Context of PTSD
The IPTS framework is one that can be used by mental health providers to conceptualize risk of suicide across populations and psychiatric diagnoses, including veterans with PTSD. However, integrating additional risk assessment and management techniques is essential to guide appropriate risk stratification and treatment.
One such method of suicide risk assessment and management is therapeutic risk management (TRM).9 Therapeutic risk management involves a stratification process by which temporal aspects (ie, acute and chronic) and severity (ie, low, moderate, and high) of suicide risk are assessed using a combination of clinical interview and psychometrically sound self-report measures, such as the Beck Scale for Suicide Ideation, Beck Hopelessness Scale, and Reasons for Living Inventory. Appropriate clinical interventions that correspond to acute and chronic suicide risk stratification are then implemented (eg, safety planning, lethal means counseling, increasing frequency of care, hospitalization if warranted).
Therapeutic risk management emphasizes the necessity of assessing current and past suicidal ideation, intent, plan, and access to means. Moreover, additional considerations might be indicated when assessing and conceptualizing suicide risk among veterans with PTSD. Assessing lifetime trauma history, including traumas that occurred before, during, and after military service, is important for understanding whether traumatic experiences influence acute and chronic risks of suicide. As previously described, careful attention to stressful and traumatic experiences with violent and aggressive characteristics is recommended because research suggests that these experiences are associated with increased capability for suicide.5 Awareness of the diversity of traumatic experiences and the importance of contextual factors surrounding such experiences also are essential. For example, the nature of violence and proximity to violence (eg, directly involved in a firefight vs hearing a mortar explosion in the distance) are key components of military-related combat trauma that might differentially influence risk of suicide.10
Similarly, although military sexual trauma can include repeated threatening sexual harassment or sexual assault, research suggests that military sexual assault is particularly important for understanding suicidal ideation, and experiences of military sexual harassment are less important.11 Therefore, a careful and nuanced understanding of how contextual aspects of a veteran’s trauma history might relate to his or her chronic and acute risk of suicide is critical.
Also important is considering the individual and institutional reactions to trauma. For example, veterans whose behaviors during traumatic experiences violated their values and moral code (ie, moral injury) might be at increased risk for S-SDV. Similarly, veterans who believe that the military institution did not adequately protect them from or support them in the aftermath of traumatic experience(s) (ie, institutional betrayal) might be at higher risk of suicide.
During a clinical interview, mental health providers should pay attention to beliefs and behaviors the veteran is reporting. For example, endorsement of perceptions of low social support (eg, “no one likes me”) or self-esteem (eg, “I’m just not as good as I used to be”) might be indicative of TB or PB, respectively. Additionally, providers should be aware of current or lifetime exposure to painful stimuli (eg, nonsuicidal self-injury, such as cutting or burning, previous suicide attempts) because these exposures might increase the veteran’s acquired capability of future S-SDV.
Although unstructured clinical interviews are a common suicide risk assessment approach, TRM proposes that using a thorough clinical interview along with valid self-report measures could further illuminate a patient’s risk of suicide.9 Implementing brief measures allows mental health providers to quickly assess several risk factors and decrease the likelihood of missing important aspects of suicide risk assessment. Providers can use a number of measures to inform their suicide risk assessment, including augmenting a clinical interview of suicide risk with a valid self-report measure of recent suicidal ideation (eg, Beck Scale for Suicide Ideation, which assesses the severity of suicidal ideation in the past week).
Additionally for veterans with PTSD, mental health providers can include measures of PTSD symptoms (eg, PTSD checklist in the Diagnostic and Statistical Manual of Mental Disorders–5) and common PTSD comorbidities (eg, Beck Depression Inventory-II for depressive symptoms) that might contribute to current risk of suicide. Based on previous research, providers also might consider adding measures of trauma-related beliefs (eg, Posttraumatic Cognitions Inventory) and emotions, such as guilt (eg, Trauma-Related Guilt Inventory).5
These measures could aid in identifying modifiable risk factors of suicide among veterans with PTSD, such as the extent to which certain beliefs or emotions relate to an individual’s risk of suicide. In addition to asking about characteristics of traumatic events during the clinical interview, measures of moral injury (eg, Moral Injury Events Scale) and institutional betrayal (eg, Institutional Betrayal Questionnaire) might further inform understanding of contextual aspects of trauma that could help explain an individual’s risk of suicide.
Finally, interpersonal measures also could be helpful. For example, because avoidance and social isolation are risk factors for suicidal ideation among veterans with PTSD, measures of perceived interpersonal functioning (eg, Interpersonal Needs Questionnaire) might add further data to assist in suicide risk conceptualization. Although the selection of specific measures likely varies based on the specific needs of an individual patient, these are examples of measures that can be used with veterans with PTSD to inform suicide risk assessment and conceptualization.
By combining data from various measures across multiple domains with a thorough clinical interview, mental health providers can use a TRM approach to understand and conceptualize suicide risk among veterans with PTSD. This approach can facilitate mental health providers’ ability to provide optimal care and guide intervention(s) for veterans with PTSD. One brief intervention that has been used with veterans is safety planning. During safety planning, the provider assists the veteran in identifying warning signs, internal and external coping strategies, and individuals the veteran can reach out to for help (eg, friends and family, providers, Veterans Crisis Line), in addition to collaboratively brainstorming ways the veteran can make his or her environment safer (eg, reducing access to lethal means, identifying reminders of their reasons for living).
Specific to veterans with PTSD, symptoms such as avoidance, hyperarousal, social isolation, and beliefs that others and the world are unsafe might affect safety planning. Such symptoms could hinder identification and use of coping strategies while deterring openness to reach out to others for help. A collaborative method can be used to identify alternate means of coping that take into account PTSD-related avoidance and hyperarousal (eg, rather than going to a crowded store or isolating at home, taking a walk in a quiet park with few people). Similarly, because substance use and risky behaviors are common among veterans with PTSD and might further increase risk of suicide, exploring healthy (eg, exercise) vs unhealthy (eg, substance use; unprotected sex) coping strategies could be helpful.
Further, based on their lived experience, veterans with PTSD could experience difficulty identifying a support system or be reluctant to reach out to others during acute crisis. This might be particularly daunting in the presence of PB and TB. In these situations, it is important to validate the veteran’s difficulty with reaching out while simultaneously encouraging the veteran to examine the accuracy of such beliefs and/or helping the veteran develop skills to overcome these obstacles.
The mental health provider also can work with the individual to ensure that the veteran understands that if he or she does engage emergency resources (eg, Veterans Crisis Line), information likely will be held confidential. Providers can tell their patients that breaks in confidentiality are rare and occur only in circumstances in which it is necessary to protect the veteran. In doing so, the provider facilitates the veteran’s understanding of the role of crisis resources and clarifies any misconceptions the veteran might have (eg, calling the crisis line will always result in hospitalization or police presence).
Conclusion
Several PTSD-related factors might increase PB, TB, and the acquired capability for suicide among veterans with PTSD. Because suicide risk assessment and management can be time sensitive and anxiety provoking, mental health providers can use a TRM approach to increase their confidence in instituting optimal care and mitigating risk by having a structured, therapeutic assessment process that gathers appropriate suicide- and PTSD-related data to assist in developing suicide risk-related treatment. However, more research is needed to determine the most useful self-report measures and effective interventions when working with veterans with PTSD at risk of suicide.
1. Pompili M, Sher L, Serafini G, et al. Posttraumatic stress disorder and suicide risk among veterans: a literature review
2. Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE, eds. Reducing Suicide: A National Imperative. Washington, DC: The National Academies Press; 2002.
3. Chu C, Buchman-Schmitt JM, Stanley IH, et al. The interpersonal psychological theory of suicide: a systematic review and meta-analysis of a decade of cross-national research. Psychol Bull. 2017;143(12):1313-1345.
4. Van Orden KA, Witte TK, Cukrowicz KC, et al. The interpersonal theory of suicide. Psychol Rev. 2010;117(2):575-600.
5. Bryan CJ, Grove JL, Kimbrel NA. Theory-driven models of self-directed violence among individuals with PTSD. Curr Opin Psychol. 2017;14:12-17.
6. Bryan CJ, Anestis M. Reexperiencing symptoms and the interpersonal-psychological theory of suicidal behavior among deployed service members evaluated for traumatic brain injury. J Clin Psychol. 2011;67(9):856-865.
7. Zuromski KL, Davis MT, Witte TK, Weathers F, Blevins C. PTSD symptom clusters are differentially associated with components of the acquired capability for suicide. Suicide Life Threat Behav. 2014;44(6):682-697.
8. Davis MT, Witte TK, Weathers FW, Blevins CA. The role of posttraumatic stress disorder symptom clusters in the prediction of passive suicidal ideation. Psychol Trauma. 2014;6(suppl 1):S82-S91.
9. Wortzel HS, Matarazzo B, Homaifar B. A model for therapeutic risk management of the suicidal patient. J Psychiatr Pract. 2013;19(4):323-326.
10. Bryan CJ, Cukrowicz KC. Associations between types of combat violence and the acquired capability for suicide. Suicide Life Threat Behav. 2011;41(2):126-136.
11. Monteith LL, Menefee DS, Forster JE, Bahraini NH. A closer examination of sexual trauma during deployment: not all sexual traumas are associated with suicidal ideation. Suicide Life Threat Behav. 2016;46(1):46-54.
Increased risk of suicide among veterans with posttraumatic stress disorder (PTSD) is well established. Posttraumatic stress disorder and related consequences are associated with higher rates of suicidal ideation and suicidal self-directed violence (S-SDV).1 Based on a systematic review, several explanations for this relationship have been hypothesized.1 Particular emphasis has been placed on trauma type (eg, premilitary childhood abuse, combat exposure), frequency of trauma exposure (ie, a single traumatic episode vs multiple traumatic experiences), specific PTSD symptoms (eg, avoidance, sleep disturbance, alteration in mood and cognitions, risky behaviors), and other psychosocial consequences associated with PTSD (eg, low social support, psychiatric comorbidity, substance use). However, there is limited understanding regarding how to conceptualize and assess risk for suicide when treating veterans who have PTSD.
PTSD and the Interpersonal-Psychological Theory of Suicide
Although PTSD is associated with risk for S-SDV among veterans, a diagnosis-specific approach to conceptualizing risk of suicide (ie, an explanation specific to PTSD) might not be enough because most individuals with a psychiatric diagnosis do not engage in S-SDV.2 Rather, theories that are able to conceptualize suicide risk across many different psychiatric diagnoses are likely to improve mental health providers’ ability to understand risk of suicide. Although many theories attempt to understand suicide risk, the Interpersonal-Psychological Theory of Suicide (IPTS) has robust empirical support.3
The IPTS proposes that suicidal ideation is driven by perceptions of stable and unchanging thwarted belongingness (TB), defined as an unmet psychological need to socially belong, and perceived burdensomeness (PB), defined as the perception that one is a burden on others.4 However, PB and TB are not considered sufficient for S-SDV to occur unless an individual also has acquired the capability for suicide. Capability for suicide is thought to happen when an individual loses the fear of dying by suicide and develops tolerance to physical pain, which is proposed to occur through habituation or repeated exposure to painful stimuli.3
Several studies have examined the IPTS in a number of clinical populations, including veterans and active-duty service members; yet limited research has applied the IPTS to veterans with PTSD.3 However, a recent article proposed that a number of PTSD-related factors increase risk of suicide through the lens of the IPTS.5 In particular, repeated exposure to painful and provocative events—especially those characterized by violence and aggression—might increase acquired capability for suicide by causing habituation to physical pain and discomfort and reducing fear of injury and death. This concept is especially concerning because of the frequent occurrence of both military- (eg, combat, military sexual trauma) and nonmilitary-related (eg, childhood abuse, intimate partner violence) stressful and traumatic events among veterans, especially individuals with PTSD.
Moreover, the acquired capability for suicide correlates highly with anxious, intrusive, and hyperarousal symptoms of PTSD.5-7 Over time, these PTSD symptoms are thought to increase habituation to the physically painful and frightening aspects of S-SDV, resulting in increased pain tolerance and fearlessness about death.3
In addition, PTSD-related cognitive-affective states (ie, thoughts and emotions), such as guilt, shame, and self-deprecation, might drive beliefs of PB and TB.5,8 Repeated exposure to such trauma-related thoughts and emotions could further reinforce beliefs of self-hate or inadequacy (PB).2 Trauma-related beliefs that the world or others are unsafe also might reduce the likelihood of seeking social support, thereby increasing TB.2 The PTSD symptoms of avoidance and self-blame also are likely to reinforce beliefs of PB and TB.2
Assessing Suicide Risk in the Context of PTSD
The IPTS framework is one that can be used by mental health providers to conceptualize risk of suicide across populations and psychiatric diagnoses, including veterans with PTSD. However, integrating additional risk assessment and management techniques is essential to guide appropriate risk stratification and treatment.
One such method of suicide risk assessment and management is therapeutic risk management (TRM).9 Therapeutic risk management involves a stratification process by which temporal aspects (ie, acute and chronic) and severity (ie, low, moderate, and high) of suicide risk are assessed using a combination of clinical interview and psychometrically sound self-report measures, such as the Beck Scale for Suicide Ideation, Beck Hopelessness Scale, and Reasons for Living Inventory. Appropriate clinical interventions that correspond to acute and chronic suicide risk stratification are then implemented (eg, safety planning, lethal means counseling, increasing frequency of care, hospitalization if warranted).
Therapeutic risk management emphasizes the necessity of assessing current and past suicidal ideation, intent, plan, and access to means. Moreover, additional considerations might be indicated when assessing and conceptualizing suicide risk among veterans with PTSD. Assessing lifetime trauma history, including traumas that occurred before, during, and after military service, is important for understanding whether traumatic experiences influence acute and chronic risks of suicide. As previously described, careful attention to stressful and traumatic experiences with violent and aggressive characteristics is recommended because research suggests that these experiences are associated with increased capability for suicide.5 Awareness of the diversity of traumatic experiences and the importance of contextual factors surrounding such experiences also are essential. For example, the nature of violence and proximity to violence (eg, directly involved in a firefight vs hearing a mortar explosion in the distance) are key components of military-related combat trauma that might differentially influence risk of suicide.10
Similarly, although military sexual trauma can include repeated threatening sexual harassment or sexual assault, research suggests that military sexual assault is particularly important for understanding suicidal ideation, and experiences of military sexual harassment are less important.11 Therefore, a careful and nuanced understanding of how contextual aspects of a veteran’s trauma history might relate to his or her chronic and acute risk of suicide is critical.
Also important is considering the individual and institutional reactions to trauma. For example, veterans whose behaviors during traumatic experiences violated their values and moral code (ie, moral injury) might be at increased risk for S-SDV. Similarly, veterans who believe that the military institution did not adequately protect them from or support them in the aftermath of traumatic experience(s) (ie, institutional betrayal) might be at higher risk of suicide.
During a clinical interview, mental health providers should pay attention to beliefs and behaviors the veteran is reporting. For example, endorsement of perceptions of low social support (eg, “no one likes me”) or self-esteem (eg, “I’m just not as good as I used to be”) might be indicative of TB or PB, respectively. Additionally, providers should be aware of current or lifetime exposure to painful stimuli (eg, nonsuicidal self-injury, such as cutting or burning, previous suicide attempts) because these exposures might increase the veteran’s acquired capability of future S-SDV.
Although unstructured clinical interviews are a common suicide risk assessment approach, TRM proposes that using a thorough clinical interview along with valid self-report measures could further illuminate a patient’s risk of suicide.9 Implementing brief measures allows mental health providers to quickly assess several risk factors and decrease the likelihood of missing important aspects of suicide risk assessment. Providers can use a number of measures to inform their suicide risk assessment, including augmenting a clinical interview of suicide risk with a valid self-report measure of recent suicidal ideation (eg, Beck Scale for Suicide Ideation, which assesses the severity of suicidal ideation in the past week).
Additionally for veterans with PTSD, mental health providers can include measures of PTSD symptoms (eg, PTSD checklist in the Diagnostic and Statistical Manual of Mental Disorders–5) and common PTSD comorbidities (eg, Beck Depression Inventory-II for depressive symptoms) that might contribute to current risk of suicide. Based on previous research, providers also might consider adding measures of trauma-related beliefs (eg, Posttraumatic Cognitions Inventory) and emotions, such as guilt (eg, Trauma-Related Guilt Inventory).5
These measures could aid in identifying modifiable risk factors of suicide among veterans with PTSD, such as the extent to which certain beliefs or emotions relate to an individual’s risk of suicide. In addition to asking about characteristics of traumatic events during the clinical interview, measures of moral injury (eg, Moral Injury Events Scale) and institutional betrayal (eg, Institutional Betrayal Questionnaire) might further inform understanding of contextual aspects of trauma that could help explain an individual’s risk of suicide.
Finally, interpersonal measures also could be helpful. For example, because avoidance and social isolation are risk factors for suicidal ideation among veterans with PTSD, measures of perceived interpersonal functioning (eg, Interpersonal Needs Questionnaire) might add further data to assist in suicide risk conceptualization. Although the selection of specific measures likely varies based on the specific needs of an individual patient, these are examples of measures that can be used with veterans with PTSD to inform suicide risk assessment and conceptualization.
By combining data from various measures across multiple domains with a thorough clinical interview, mental health providers can use a TRM approach to understand and conceptualize suicide risk among veterans with PTSD. This approach can facilitate mental health providers’ ability to provide optimal care and guide intervention(s) for veterans with PTSD. One brief intervention that has been used with veterans is safety planning. During safety planning, the provider assists the veteran in identifying warning signs, internal and external coping strategies, and individuals the veteran can reach out to for help (eg, friends and family, providers, Veterans Crisis Line), in addition to collaboratively brainstorming ways the veteran can make his or her environment safer (eg, reducing access to lethal means, identifying reminders of their reasons for living).
Specific to veterans with PTSD, symptoms such as avoidance, hyperarousal, social isolation, and beliefs that others and the world are unsafe might affect safety planning. Such symptoms could hinder identification and use of coping strategies while deterring openness to reach out to others for help. A collaborative method can be used to identify alternate means of coping that take into account PTSD-related avoidance and hyperarousal (eg, rather than going to a crowded store or isolating at home, taking a walk in a quiet park with few people). Similarly, because substance use and risky behaviors are common among veterans with PTSD and might further increase risk of suicide, exploring healthy (eg, exercise) vs unhealthy (eg, substance use; unprotected sex) coping strategies could be helpful.
Further, based on their lived experience, veterans with PTSD could experience difficulty identifying a support system or be reluctant to reach out to others during acute crisis. This might be particularly daunting in the presence of PB and TB. In these situations, it is important to validate the veteran’s difficulty with reaching out while simultaneously encouraging the veteran to examine the accuracy of such beliefs and/or helping the veteran develop skills to overcome these obstacles.
The mental health provider also can work with the individual to ensure that the veteran understands that if he or she does engage emergency resources (eg, Veterans Crisis Line), information likely will be held confidential. Providers can tell their patients that breaks in confidentiality are rare and occur only in circumstances in which it is necessary to protect the veteran. In doing so, the provider facilitates the veteran’s understanding of the role of crisis resources and clarifies any misconceptions the veteran might have (eg, calling the crisis line will always result in hospitalization or police presence).
Conclusion
Several PTSD-related factors might increase PB, TB, and the acquired capability for suicide among veterans with PTSD. Because suicide risk assessment and management can be time sensitive and anxiety provoking, mental health providers can use a TRM approach to increase their confidence in instituting optimal care and mitigating risk by having a structured, therapeutic assessment process that gathers appropriate suicide- and PTSD-related data to assist in developing suicide risk-related treatment. However, more research is needed to determine the most useful self-report measures and effective interventions when working with veterans with PTSD at risk of suicide.
Increased risk of suicide among veterans with posttraumatic stress disorder (PTSD) is well established. Posttraumatic stress disorder and related consequences are associated with higher rates of suicidal ideation and suicidal self-directed violence (S-SDV).1 Based on a systematic review, several explanations for this relationship have been hypothesized.1 Particular emphasis has been placed on trauma type (eg, premilitary childhood abuse, combat exposure), frequency of trauma exposure (ie, a single traumatic episode vs multiple traumatic experiences), specific PTSD symptoms (eg, avoidance, sleep disturbance, alteration in mood and cognitions, risky behaviors), and other psychosocial consequences associated with PTSD (eg, low social support, psychiatric comorbidity, substance use). However, there is limited understanding regarding how to conceptualize and assess risk for suicide when treating veterans who have PTSD.
PTSD and the Interpersonal-Psychological Theory of Suicide
Although PTSD is associated with risk for S-SDV among veterans, a diagnosis-specific approach to conceptualizing risk of suicide (ie, an explanation specific to PTSD) might not be enough because most individuals with a psychiatric diagnosis do not engage in S-SDV.2 Rather, theories that are able to conceptualize suicide risk across many different psychiatric diagnoses are likely to improve mental health providers’ ability to understand risk of suicide. Although many theories attempt to understand suicide risk, the Interpersonal-Psychological Theory of Suicide (IPTS) has robust empirical support.3
The IPTS proposes that suicidal ideation is driven by perceptions of stable and unchanging thwarted belongingness (TB), defined as an unmet psychological need to socially belong, and perceived burdensomeness (PB), defined as the perception that one is a burden on others.4 However, PB and TB are not considered sufficient for S-SDV to occur unless an individual also has acquired the capability for suicide. Capability for suicide is thought to happen when an individual loses the fear of dying by suicide and develops tolerance to physical pain, which is proposed to occur through habituation or repeated exposure to painful stimuli.3
Several studies have examined the IPTS in a number of clinical populations, including veterans and active-duty service members; yet limited research has applied the IPTS to veterans with PTSD.3 However, a recent article proposed that a number of PTSD-related factors increase risk of suicide through the lens of the IPTS.5 In particular, repeated exposure to painful and provocative events—especially those characterized by violence and aggression—might increase acquired capability for suicide by causing habituation to physical pain and discomfort and reducing fear of injury and death. This concept is especially concerning because of the frequent occurrence of both military- (eg, combat, military sexual trauma) and nonmilitary-related (eg, childhood abuse, intimate partner violence) stressful and traumatic events among veterans, especially individuals with PTSD.
Moreover, the acquired capability for suicide correlates highly with anxious, intrusive, and hyperarousal symptoms of PTSD.5-7 Over time, these PTSD symptoms are thought to increase habituation to the physically painful and frightening aspects of S-SDV, resulting in increased pain tolerance and fearlessness about death.3
In addition, PTSD-related cognitive-affective states (ie, thoughts and emotions), such as guilt, shame, and self-deprecation, might drive beliefs of PB and TB.5,8 Repeated exposure to such trauma-related thoughts and emotions could further reinforce beliefs of self-hate or inadequacy (PB).2 Trauma-related beliefs that the world or others are unsafe also might reduce the likelihood of seeking social support, thereby increasing TB.2 The PTSD symptoms of avoidance and self-blame also are likely to reinforce beliefs of PB and TB.2
Assessing Suicide Risk in the Context of PTSD
The IPTS framework is one that can be used by mental health providers to conceptualize risk of suicide across populations and psychiatric diagnoses, including veterans with PTSD. However, integrating additional risk assessment and management techniques is essential to guide appropriate risk stratification and treatment.
One such method of suicide risk assessment and management is therapeutic risk management (TRM).9 Therapeutic risk management involves a stratification process by which temporal aspects (ie, acute and chronic) and severity (ie, low, moderate, and high) of suicide risk are assessed using a combination of clinical interview and psychometrically sound self-report measures, such as the Beck Scale for Suicide Ideation, Beck Hopelessness Scale, and Reasons for Living Inventory. Appropriate clinical interventions that correspond to acute and chronic suicide risk stratification are then implemented (eg, safety planning, lethal means counseling, increasing frequency of care, hospitalization if warranted).
Therapeutic risk management emphasizes the necessity of assessing current and past suicidal ideation, intent, plan, and access to means. Moreover, additional considerations might be indicated when assessing and conceptualizing suicide risk among veterans with PTSD. Assessing lifetime trauma history, including traumas that occurred before, during, and after military service, is important for understanding whether traumatic experiences influence acute and chronic risks of suicide. As previously described, careful attention to stressful and traumatic experiences with violent and aggressive characteristics is recommended because research suggests that these experiences are associated with increased capability for suicide.5 Awareness of the diversity of traumatic experiences and the importance of contextual factors surrounding such experiences also are essential. For example, the nature of violence and proximity to violence (eg, directly involved in a firefight vs hearing a mortar explosion in the distance) are key components of military-related combat trauma that might differentially influence risk of suicide.10
Similarly, although military sexual trauma can include repeated threatening sexual harassment or sexual assault, research suggests that military sexual assault is particularly important for understanding suicidal ideation, and experiences of military sexual harassment are less important.11 Therefore, a careful and nuanced understanding of how contextual aspects of a veteran’s trauma history might relate to his or her chronic and acute risk of suicide is critical.
Also important is considering the individual and institutional reactions to trauma. For example, veterans whose behaviors during traumatic experiences violated their values and moral code (ie, moral injury) might be at increased risk for S-SDV. Similarly, veterans who believe that the military institution did not adequately protect them from or support them in the aftermath of traumatic experience(s) (ie, institutional betrayal) might be at higher risk of suicide.
During a clinical interview, mental health providers should pay attention to beliefs and behaviors the veteran is reporting. For example, endorsement of perceptions of low social support (eg, “no one likes me”) or self-esteem (eg, “I’m just not as good as I used to be”) might be indicative of TB or PB, respectively. Additionally, providers should be aware of current or lifetime exposure to painful stimuli (eg, nonsuicidal self-injury, such as cutting or burning, previous suicide attempts) because these exposures might increase the veteran’s acquired capability of future S-SDV.
Although unstructured clinical interviews are a common suicide risk assessment approach, TRM proposes that using a thorough clinical interview along with valid self-report measures could further illuminate a patient’s risk of suicide.9 Implementing brief measures allows mental health providers to quickly assess several risk factors and decrease the likelihood of missing important aspects of suicide risk assessment. Providers can use a number of measures to inform their suicide risk assessment, including augmenting a clinical interview of suicide risk with a valid self-report measure of recent suicidal ideation (eg, Beck Scale for Suicide Ideation, which assesses the severity of suicidal ideation in the past week).
Additionally for veterans with PTSD, mental health providers can include measures of PTSD symptoms (eg, PTSD checklist in the Diagnostic and Statistical Manual of Mental Disorders–5) and common PTSD comorbidities (eg, Beck Depression Inventory-II for depressive symptoms) that might contribute to current risk of suicide. Based on previous research, providers also might consider adding measures of trauma-related beliefs (eg, Posttraumatic Cognitions Inventory) and emotions, such as guilt (eg, Trauma-Related Guilt Inventory).5
These measures could aid in identifying modifiable risk factors of suicide among veterans with PTSD, such as the extent to which certain beliefs or emotions relate to an individual’s risk of suicide. In addition to asking about characteristics of traumatic events during the clinical interview, measures of moral injury (eg, Moral Injury Events Scale) and institutional betrayal (eg, Institutional Betrayal Questionnaire) might further inform understanding of contextual aspects of trauma that could help explain an individual’s risk of suicide.
Finally, interpersonal measures also could be helpful. For example, because avoidance and social isolation are risk factors for suicidal ideation among veterans with PTSD, measures of perceived interpersonal functioning (eg, Interpersonal Needs Questionnaire) might add further data to assist in suicide risk conceptualization. Although the selection of specific measures likely varies based on the specific needs of an individual patient, these are examples of measures that can be used with veterans with PTSD to inform suicide risk assessment and conceptualization.
By combining data from various measures across multiple domains with a thorough clinical interview, mental health providers can use a TRM approach to understand and conceptualize suicide risk among veterans with PTSD. This approach can facilitate mental health providers’ ability to provide optimal care and guide intervention(s) for veterans with PTSD. One brief intervention that has been used with veterans is safety planning. During safety planning, the provider assists the veteran in identifying warning signs, internal and external coping strategies, and individuals the veteran can reach out to for help (eg, friends and family, providers, Veterans Crisis Line), in addition to collaboratively brainstorming ways the veteran can make his or her environment safer (eg, reducing access to lethal means, identifying reminders of their reasons for living).
Specific to veterans with PTSD, symptoms such as avoidance, hyperarousal, social isolation, and beliefs that others and the world are unsafe might affect safety planning. Such symptoms could hinder identification and use of coping strategies while deterring openness to reach out to others for help. A collaborative method can be used to identify alternate means of coping that take into account PTSD-related avoidance and hyperarousal (eg, rather than going to a crowded store or isolating at home, taking a walk in a quiet park with few people). Similarly, because substance use and risky behaviors are common among veterans with PTSD and might further increase risk of suicide, exploring healthy (eg, exercise) vs unhealthy (eg, substance use; unprotected sex) coping strategies could be helpful.
Further, based on their lived experience, veterans with PTSD could experience difficulty identifying a support system or be reluctant to reach out to others during acute crisis. This might be particularly daunting in the presence of PB and TB. In these situations, it is important to validate the veteran’s difficulty with reaching out while simultaneously encouraging the veteran to examine the accuracy of such beliefs and/or helping the veteran develop skills to overcome these obstacles.
The mental health provider also can work with the individual to ensure that the veteran understands that if he or she does engage emergency resources (eg, Veterans Crisis Line), information likely will be held confidential. Providers can tell their patients that breaks in confidentiality are rare and occur only in circumstances in which it is necessary to protect the veteran. In doing so, the provider facilitates the veteran’s understanding of the role of crisis resources and clarifies any misconceptions the veteran might have (eg, calling the crisis line will always result in hospitalization or police presence).
Conclusion
Several PTSD-related factors might increase PB, TB, and the acquired capability for suicide among veterans with PTSD. Because suicide risk assessment and management can be time sensitive and anxiety provoking, mental health providers can use a TRM approach to increase their confidence in instituting optimal care and mitigating risk by having a structured, therapeutic assessment process that gathers appropriate suicide- and PTSD-related data to assist in developing suicide risk-related treatment. However, more research is needed to determine the most useful self-report measures and effective interventions when working with veterans with PTSD at risk of suicide.
1. Pompili M, Sher L, Serafini G, et al. Posttraumatic stress disorder and suicide risk among veterans: a literature review
2. Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE, eds. Reducing Suicide: A National Imperative. Washington, DC: The National Academies Press; 2002.
3. Chu C, Buchman-Schmitt JM, Stanley IH, et al. The interpersonal psychological theory of suicide: a systematic review and meta-analysis of a decade of cross-national research. Psychol Bull. 2017;143(12):1313-1345.
4. Van Orden KA, Witte TK, Cukrowicz KC, et al. The interpersonal theory of suicide. Psychol Rev. 2010;117(2):575-600.
5. Bryan CJ, Grove JL, Kimbrel NA. Theory-driven models of self-directed violence among individuals with PTSD. Curr Opin Psychol. 2017;14:12-17.
6. Bryan CJ, Anestis M. Reexperiencing symptoms and the interpersonal-psychological theory of suicidal behavior among deployed service members evaluated for traumatic brain injury. J Clin Psychol. 2011;67(9):856-865.
7. Zuromski KL, Davis MT, Witte TK, Weathers F, Blevins C. PTSD symptom clusters are differentially associated with components of the acquired capability for suicide. Suicide Life Threat Behav. 2014;44(6):682-697.
8. Davis MT, Witte TK, Weathers FW, Blevins CA. The role of posttraumatic stress disorder symptom clusters in the prediction of passive suicidal ideation. Psychol Trauma. 2014;6(suppl 1):S82-S91.
9. Wortzel HS, Matarazzo B, Homaifar B. A model for therapeutic risk management of the suicidal patient. J Psychiatr Pract. 2013;19(4):323-326.
10. Bryan CJ, Cukrowicz KC. Associations between types of combat violence and the acquired capability for suicide. Suicide Life Threat Behav. 2011;41(2):126-136.
11. Monteith LL, Menefee DS, Forster JE, Bahraini NH. A closer examination of sexual trauma during deployment: not all sexual traumas are associated with suicidal ideation. Suicide Life Threat Behav. 2016;46(1):46-54.
1. Pompili M, Sher L, Serafini G, et al. Posttraumatic stress disorder and suicide risk among veterans: a literature review
2. Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE, eds. Reducing Suicide: A National Imperative. Washington, DC: The National Academies Press; 2002.
3. Chu C, Buchman-Schmitt JM, Stanley IH, et al. The interpersonal psychological theory of suicide: a systematic review and meta-analysis of a decade of cross-national research. Psychol Bull. 2017;143(12):1313-1345.
4. Van Orden KA, Witte TK, Cukrowicz KC, et al. The interpersonal theory of suicide. Psychol Rev. 2010;117(2):575-600.
5. Bryan CJ, Grove JL, Kimbrel NA. Theory-driven models of self-directed violence among individuals with PTSD. Curr Opin Psychol. 2017;14:12-17.
6. Bryan CJ, Anestis M. Reexperiencing symptoms and the interpersonal-psychological theory of suicidal behavior among deployed service members evaluated for traumatic brain injury. J Clin Psychol. 2011;67(9):856-865.
7. Zuromski KL, Davis MT, Witte TK, Weathers F, Blevins C. PTSD symptom clusters are differentially associated with components of the acquired capability for suicide. Suicide Life Threat Behav. 2014;44(6):682-697.
8. Davis MT, Witte TK, Weathers FW, Blevins CA. The role of posttraumatic stress disorder symptom clusters in the prediction of passive suicidal ideation. Psychol Trauma. 2014;6(suppl 1):S82-S91.
9. Wortzel HS, Matarazzo B, Homaifar B. A model for therapeutic risk management of the suicidal patient. J Psychiatr Pract. 2013;19(4):323-326.
10. Bryan CJ, Cukrowicz KC. Associations between types of combat violence and the acquired capability for suicide. Suicide Life Threat Behav. 2011;41(2):126-136.
11. Monteith LL, Menefee DS, Forster JE, Bahraini NH. A closer examination of sexual trauma during deployment: not all sexual traumas are associated with suicidal ideation. Suicide Life Threat Behav. 2016;46(1):46-54.
Team uses CRISPR to turn on fetal hemoglobin
Researchers have used CRISPR-Cas9 gene editing to reproduce naturally occurring mutations that boost the production of fetal hemoglobin.
The mutations are associated with hereditary persistence of fetal hemoglobin (HPFH), and the researchers believe that introducing these mutations into erythroid cells could be a safe way to treat β-hemoglobinopathies such as sickle cell disease (SCD) and β-thalassemia.
This research was published in Nature Genetics.
“Our new approach can be seen as a forerunner to ‘organic gene therapy’ for a range of common inherited blood disorders, including β-thalassemia and sickle cell anemia,” said study author Merlin Crossley, DPhil, of the University of New South Wales in Sydney, Australia.
“It is organic because no new DNA is introduced into the cells; rather, we engineer in naturally occurring, benign mutations that are known to be beneficial to people with these conditions. It should prove to be a safe and effective therapy, although more research would be needed to scale the processes up into effective treatments.”
Dr Crossley and his colleagues noted that reactivating fetal hemoglobin production has long been a therapeutic goal for SCD and β-thalassemia.
“The fetal hemoglobin gene is naturally silenced after birth,” Dr Crossley explained. “For 50 years, researchers have been competing furiously to find out how it is switched off, so it can be turned back on. Our study, which is the culmination of many years of work, solves that mystery.”
“We have found that 2 genes, called BCL11A and ZBTB7A, switch off the fetal hemoglobin gene by binding directly to it. And the beneficial mutations work by disrupting the 2 sites where these 2 genes bind.”
The “beneficial mutations,” which cause some forms of HPFH, are point mutations in the γ-globin gene promoter at -115 and -200 bp upstream of the transcription start site.
Dr Crossley and his colleagues found that BCL11A31 and ZBTB7A23—2 well-established fetal globin repressors—bind these regions of the γ-globin gene proximal promoter, and mutations at –115 and –200 bp disrupt the binding.
The researchers used CRISPR-Cas9 to introduce the HPFH-associated mutations into erythroid cells and observed both disruption of repressor binding and increased γ-globin gene expression.
“This landmark finding not only contributes to our appreciation of how these globin genes are regulated,” Dr Crossley said. “It means we can now shift our focus to developing therapies for these genetic diseases using CRISPR to target precise changes in the genome.”
Researchers have used CRISPR-Cas9 gene editing to reproduce naturally occurring mutations that boost the production of fetal hemoglobin.
The mutations are associated with hereditary persistence of fetal hemoglobin (HPFH), and the researchers believe that introducing these mutations into erythroid cells could be a safe way to treat β-hemoglobinopathies such as sickle cell disease (SCD) and β-thalassemia.
This research was published in Nature Genetics.
“Our new approach can be seen as a forerunner to ‘organic gene therapy’ for a range of common inherited blood disorders, including β-thalassemia and sickle cell anemia,” said study author Merlin Crossley, DPhil, of the University of New South Wales in Sydney, Australia.
“It is organic because no new DNA is introduced into the cells; rather, we engineer in naturally occurring, benign mutations that are known to be beneficial to people with these conditions. It should prove to be a safe and effective therapy, although more research would be needed to scale the processes up into effective treatments.”
Dr Crossley and his colleagues noted that reactivating fetal hemoglobin production has long been a therapeutic goal for SCD and β-thalassemia.
“The fetal hemoglobin gene is naturally silenced after birth,” Dr Crossley explained. “For 50 years, researchers have been competing furiously to find out how it is switched off, so it can be turned back on. Our study, which is the culmination of many years of work, solves that mystery.”
“We have found that 2 genes, called BCL11A and ZBTB7A, switch off the fetal hemoglobin gene by binding directly to it. And the beneficial mutations work by disrupting the 2 sites where these 2 genes bind.”
The “beneficial mutations,” which cause some forms of HPFH, are point mutations in the γ-globin gene promoter at -115 and -200 bp upstream of the transcription start site.
Dr Crossley and his colleagues found that BCL11A31 and ZBTB7A23—2 well-established fetal globin repressors—bind these regions of the γ-globin gene proximal promoter, and mutations at –115 and –200 bp disrupt the binding.
The researchers used CRISPR-Cas9 to introduce the HPFH-associated mutations into erythroid cells and observed both disruption of repressor binding and increased γ-globin gene expression.
“This landmark finding not only contributes to our appreciation of how these globin genes are regulated,” Dr Crossley said. “It means we can now shift our focus to developing therapies for these genetic diseases using CRISPR to target precise changes in the genome.”
Researchers have used CRISPR-Cas9 gene editing to reproduce naturally occurring mutations that boost the production of fetal hemoglobin.
The mutations are associated with hereditary persistence of fetal hemoglobin (HPFH), and the researchers believe that introducing these mutations into erythroid cells could be a safe way to treat β-hemoglobinopathies such as sickle cell disease (SCD) and β-thalassemia.
This research was published in Nature Genetics.
“Our new approach can be seen as a forerunner to ‘organic gene therapy’ for a range of common inherited blood disorders, including β-thalassemia and sickle cell anemia,” said study author Merlin Crossley, DPhil, of the University of New South Wales in Sydney, Australia.
“It is organic because no new DNA is introduced into the cells; rather, we engineer in naturally occurring, benign mutations that are known to be beneficial to people with these conditions. It should prove to be a safe and effective therapy, although more research would be needed to scale the processes up into effective treatments.”
Dr Crossley and his colleagues noted that reactivating fetal hemoglobin production has long been a therapeutic goal for SCD and β-thalassemia.
“The fetal hemoglobin gene is naturally silenced after birth,” Dr Crossley explained. “For 50 years, researchers have been competing furiously to find out how it is switched off, so it can be turned back on. Our study, which is the culmination of many years of work, solves that mystery.”
“We have found that 2 genes, called BCL11A and ZBTB7A, switch off the fetal hemoglobin gene by binding directly to it. And the beneficial mutations work by disrupting the 2 sites where these 2 genes bind.”
The “beneficial mutations,” which cause some forms of HPFH, are point mutations in the γ-globin gene promoter at -115 and -200 bp upstream of the transcription start site.
Dr Crossley and his colleagues found that BCL11A31 and ZBTB7A23—2 well-established fetal globin repressors—bind these regions of the γ-globin gene proximal promoter, and mutations at –115 and –200 bp disrupt the binding.
The researchers used CRISPR-Cas9 to introduce the HPFH-associated mutations into erythroid cells and observed both disruption of repressor binding and increased γ-globin gene expression.
“This landmark finding not only contributes to our appreciation of how these globin genes are regulated,” Dr Crossley said. “It means we can now shift our focus to developing therapies for these genetic diseases using CRISPR to target precise changes in the genome.”
FDA approves blinatumomab to treat MRD+ BCP-ALL
The US Food and Drug Administration (FDA) has expanded the approved indication for blinatumomab (Blincyto®).
The drug is now approved to treat adults and children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission (CR) with minimal residual disease (MRD) greater than or equal to 0.1%.
Blinatumomab received accelerated approval for this indication because the drug has not yet shown a clinical benefit in these patients.
The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on surrogate or intermediate endpoints—in this case, MRD response rate and hematologic relapse-free survival (RFS)—that are reasonably likely to predict clinical benefit.
Continued approval of blinatumomab for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
“This is the first FDA-approved treatment for patients with MRD-positive ALL,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
“Because patients who have MRD are more likely to relapse, having a treatment option that eliminates even very low amounts of residual leukemia cells may help keep the cancer in remission longer. We look forward to furthering our understanding about the reduction in MRD after treatment with Blincyto. Studies are being conducted to assess how Blincyto affects long-term survival outcomes in patients with MRD.”
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
In 2014, the FDA granted blinatumomab accelerated approval to treat adults with Philadelphia chromosome-negative (Ph-) relapsed/refractory BCP-ALL.
In 2016, the FDA granted the therapy accelerated approval for pediatric patients with Ph- relapsed/refractory BCP-ALL.
Last year, the FDA granted blinatumomab full approval for pediatric and adult patients with Ph- or Ph+ relapsed/refractory BCP-ALL.
The FDA-approved prescribing information for blinatumomab includes a boxed warning for cytokine release syndrome and neurologic toxicities. Blinatumomab is also under a risk evaluation and mitigation strategy program in the US.
BLAST study
The new accelerated approval for blinatumomab was supported by results from the phase 2 BLAST study, which were published in Blood in January.
The study enrolled adults with MRD-positive BCP-ALL in complete hematologic remission after 3 or more cycles of intensive chemotherapy.
Patients received continuous intravenous infusions of blinatumomab at 15 μg/m2/day for 4 weeks, followed by 2 weeks off. They received up to 4 cycles of treatment and could undergo hematopoietic stem cell transplant (HSCT) at any time after the first cycle.
In all, there were 116 patients who received at least 1 infusion of blinatumomab. Seventy-six patients went on to HSCT while in continuous CR after cycle 1 (n=27), 2 (n=36), or 3/4 (n=13).
The study’s primary endpoint was the rate of complete MRD response within the first treatment cycle, and 78% of evaluable patients (88/113) achieved this endpoint.
A key secondary endpoint was RFS at 18 months. There were 110 patients evaluable for this endpoint. They all had Ph- BCP-ALL and <5% blasts at baseline.
The estimated RFS at 18 months was 54%, and the median RFS was 18.9 months. The median RFS was 24.6 months for patients treated in first CR and 11.0 months for patients treated in a later CR (P=0.004).
Another key endpoint was overall survival (OS). The median OS was 36.5 months, both for the 110 patients in the RFS analysis and for the entire study population.
In a landmark analysis, complete MRD responders had longer OS than MRD nonresponders—38.9 months and 12.5 months, respectively (P=0.002). And complete MRD responders had longer RFS than nonresponders—23.6 months and 5.7 months, respectively (P=0.002).
All 116 patients who started cycle 1 had at least 1 adverse event (AE). The rate of grade 3 AEs was 33%, and the rate of grade 4 AEs was 27%. These AEs were considered treatment-related in 29% (grade 3) and 22% (grade 4) of patients.
Four (3%) patients developed cytokine release syndrome—2 with grade 1 and 2 with grade 3. All of these events occurred during cycle 1.
Fifty-three percent of patients (n=61) had neurologic events. In most cases (97%, n=59), these events resolved.
There were 2 fatal AEs during the treatment period, both in cycle 1. One of these events—atypical pneumonitis with H1N1 influenza—was considered treatment-related. The other event—subdural hemorrhage—was considered unrelated to treatment.
There were 4 fatal AEs reported after blinatumomab treatment. Two of these deaths—due to multifocal CNS lesions and graft-versus-host disease—occurred in HSCT recipients. The other 2 deaths—due to disease progression and multi-organ failure—occurred in nontransplanted patients after relapse.
The US Food and Drug Administration (FDA) has expanded the approved indication for blinatumomab (Blincyto®).
The drug is now approved to treat adults and children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission (CR) with minimal residual disease (MRD) greater than or equal to 0.1%.
Blinatumomab received accelerated approval for this indication because the drug has not yet shown a clinical benefit in these patients.
The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on surrogate or intermediate endpoints—in this case, MRD response rate and hematologic relapse-free survival (RFS)—that are reasonably likely to predict clinical benefit.
Continued approval of blinatumomab for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
“This is the first FDA-approved treatment for patients with MRD-positive ALL,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
“Because patients who have MRD are more likely to relapse, having a treatment option that eliminates even very low amounts of residual leukemia cells may help keep the cancer in remission longer. We look forward to furthering our understanding about the reduction in MRD after treatment with Blincyto. Studies are being conducted to assess how Blincyto affects long-term survival outcomes in patients with MRD.”
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
In 2014, the FDA granted blinatumomab accelerated approval to treat adults with Philadelphia chromosome-negative (Ph-) relapsed/refractory BCP-ALL.
In 2016, the FDA granted the therapy accelerated approval for pediatric patients with Ph- relapsed/refractory BCP-ALL.
Last year, the FDA granted blinatumomab full approval for pediatric and adult patients with Ph- or Ph+ relapsed/refractory BCP-ALL.
The FDA-approved prescribing information for blinatumomab includes a boxed warning for cytokine release syndrome and neurologic toxicities. Blinatumomab is also under a risk evaluation and mitigation strategy program in the US.
BLAST study
The new accelerated approval for blinatumomab was supported by results from the phase 2 BLAST study, which were published in Blood in January.
The study enrolled adults with MRD-positive BCP-ALL in complete hematologic remission after 3 or more cycles of intensive chemotherapy.
Patients received continuous intravenous infusions of blinatumomab at 15 μg/m2/day for 4 weeks, followed by 2 weeks off. They received up to 4 cycles of treatment and could undergo hematopoietic stem cell transplant (HSCT) at any time after the first cycle.
In all, there were 116 patients who received at least 1 infusion of blinatumomab. Seventy-six patients went on to HSCT while in continuous CR after cycle 1 (n=27), 2 (n=36), or 3/4 (n=13).
The study’s primary endpoint was the rate of complete MRD response within the first treatment cycle, and 78% of evaluable patients (88/113) achieved this endpoint.
A key secondary endpoint was RFS at 18 months. There were 110 patients evaluable for this endpoint. They all had Ph- BCP-ALL and <5% blasts at baseline.
The estimated RFS at 18 months was 54%, and the median RFS was 18.9 months. The median RFS was 24.6 months for patients treated in first CR and 11.0 months for patients treated in a later CR (P=0.004).
Another key endpoint was overall survival (OS). The median OS was 36.5 months, both for the 110 patients in the RFS analysis and for the entire study population.
In a landmark analysis, complete MRD responders had longer OS than MRD nonresponders—38.9 months and 12.5 months, respectively (P=0.002). And complete MRD responders had longer RFS than nonresponders—23.6 months and 5.7 months, respectively (P=0.002).
All 116 patients who started cycle 1 had at least 1 adverse event (AE). The rate of grade 3 AEs was 33%, and the rate of grade 4 AEs was 27%. These AEs were considered treatment-related in 29% (grade 3) and 22% (grade 4) of patients.
Four (3%) patients developed cytokine release syndrome—2 with grade 1 and 2 with grade 3. All of these events occurred during cycle 1.
Fifty-three percent of patients (n=61) had neurologic events. In most cases (97%, n=59), these events resolved.
There were 2 fatal AEs during the treatment period, both in cycle 1. One of these events—atypical pneumonitis with H1N1 influenza—was considered treatment-related. The other event—subdural hemorrhage—was considered unrelated to treatment.
There were 4 fatal AEs reported after blinatumomab treatment. Two of these deaths—due to multifocal CNS lesions and graft-versus-host disease—occurred in HSCT recipients. The other 2 deaths—due to disease progression and multi-organ failure—occurred in nontransplanted patients after relapse.
The US Food and Drug Administration (FDA) has expanded the approved indication for blinatumomab (Blincyto®).
The drug is now approved to treat adults and children with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) in first or second complete remission (CR) with minimal residual disease (MRD) greater than or equal to 0.1%.
Blinatumomab received accelerated approval for this indication because the drug has not yet shown a clinical benefit in these patients.
The FDA’s accelerated approval program allows conditional approval of a drug that fills an unmet medical need for a serious condition.
Accelerated approval is based on surrogate or intermediate endpoints—in this case, MRD response rate and hematologic relapse-free survival (RFS)—that are reasonably likely to predict clinical benefit.
Continued approval of blinatumomab for the aforementioned indication may be contingent upon verification of clinical benefit in confirmatory trials.
“This is the first FDA-approved treatment for patients with MRD-positive ALL,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
“Because patients who have MRD are more likely to relapse, having a treatment option that eliminates even very low amounts of residual leukemia cells may help keep the cancer in remission longer. We look forward to furthering our understanding about the reduction in MRD after treatment with Blincyto. Studies are being conducted to assess how Blincyto affects long-term survival outcomes in patients with MRD.”
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
In 2014, the FDA granted blinatumomab accelerated approval to treat adults with Philadelphia chromosome-negative (Ph-) relapsed/refractory BCP-ALL.
In 2016, the FDA granted the therapy accelerated approval for pediatric patients with Ph- relapsed/refractory BCP-ALL.
Last year, the FDA granted blinatumomab full approval for pediatric and adult patients with Ph- or Ph+ relapsed/refractory BCP-ALL.
The FDA-approved prescribing information for blinatumomab includes a boxed warning for cytokine release syndrome and neurologic toxicities. Blinatumomab is also under a risk evaluation and mitigation strategy program in the US.
BLAST study
The new accelerated approval for blinatumomab was supported by results from the phase 2 BLAST study, which were published in Blood in January.
The study enrolled adults with MRD-positive BCP-ALL in complete hematologic remission after 3 or more cycles of intensive chemotherapy.
Patients received continuous intravenous infusions of blinatumomab at 15 μg/m2/day for 4 weeks, followed by 2 weeks off. They received up to 4 cycles of treatment and could undergo hematopoietic stem cell transplant (HSCT) at any time after the first cycle.
In all, there were 116 patients who received at least 1 infusion of blinatumomab. Seventy-six patients went on to HSCT while in continuous CR after cycle 1 (n=27), 2 (n=36), or 3/4 (n=13).
The study’s primary endpoint was the rate of complete MRD response within the first treatment cycle, and 78% of evaluable patients (88/113) achieved this endpoint.
A key secondary endpoint was RFS at 18 months. There were 110 patients evaluable for this endpoint. They all had Ph- BCP-ALL and <5% blasts at baseline.
The estimated RFS at 18 months was 54%, and the median RFS was 18.9 months. The median RFS was 24.6 months for patients treated in first CR and 11.0 months for patients treated in a later CR (P=0.004).
Another key endpoint was overall survival (OS). The median OS was 36.5 months, both for the 110 patients in the RFS analysis and for the entire study population.
In a landmark analysis, complete MRD responders had longer OS than MRD nonresponders—38.9 months and 12.5 months, respectively (P=0.002). And complete MRD responders had longer RFS than nonresponders—23.6 months and 5.7 months, respectively (P=0.002).
All 116 patients who started cycle 1 had at least 1 adverse event (AE). The rate of grade 3 AEs was 33%, and the rate of grade 4 AEs was 27%. These AEs were considered treatment-related in 29% (grade 3) and 22% (grade 4) of patients.
Four (3%) patients developed cytokine release syndrome—2 with grade 1 and 2 with grade 3. All of these events occurred during cycle 1.
Fifty-three percent of patients (n=61) had neurologic events. In most cases (97%, n=59), these events resolved.
There were 2 fatal AEs during the treatment period, both in cycle 1. One of these events—atypical pneumonitis with H1N1 influenza—was considered treatment-related. The other event—subdural hemorrhage—was considered unrelated to treatment.
There were 4 fatal AEs reported after blinatumomab treatment. Two of these deaths—due to multifocal CNS lesions and graft-versus-host disease—occurred in HSCT recipients. The other 2 deaths—due to disease progression and multi-organ failure—occurred in nontransplanted patients after relapse.
Drug receives orphan designation for AML
The US Food and Drug Administration (FDA) has granted orphan designation to MAX-40279 for the treatment of acute myeloid leukemia (AML).
MAX-40279 is a multi-target kinase inhibitor being developed by MaxiNovel Pharmaceuticals, Inc.
The drug mainly targets FMS-related tyrosine kinase 3 (FLT3) and fibroblast growth factor receptor (FGFR).
MAX-40279 demonstrated “potent” inhibition of FLT3 and FGFR in preclinical testing, according to MaxiNovel Pharmaceuticals, Inc.
The company is currently testing MAX-40279 in a phase 1 trial of patients with AML (NCT03412292).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan designation to MAX-40279 for the treatment of acute myeloid leukemia (AML).
MAX-40279 is a multi-target kinase inhibitor being developed by MaxiNovel Pharmaceuticals, Inc.
The drug mainly targets FMS-related tyrosine kinase 3 (FLT3) and fibroblast growth factor receptor (FGFR).
MAX-40279 demonstrated “potent” inhibition of FLT3 and FGFR in preclinical testing, according to MaxiNovel Pharmaceuticals, Inc.
The company is currently testing MAX-40279 in a phase 1 trial of patients with AML (NCT03412292).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan designation to MAX-40279 for the treatment of acute myeloid leukemia (AML).
MAX-40279 is a multi-target kinase inhibitor being developed by MaxiNovel Pharmaceuticals, Inc.
The drug mainly targets FMS-related tyrosine kinase 3 (FLT3) and fibroblast growth factor receptor (FGFR).
MAX-40279 demonstrated “potent” inhibition of FLT3 and FGFR in preclinical testing, according to MaxiNovel Pharmaceuticals, Inc.
The company is currently testing MAX-40279 in a phase 1 trial of patients with AML (NCT03412292).
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
Cleaning Out Your Emotional Junk Drawer
It’s no secret that health care providers are at high risk for burnout. In my time as a provider, a patient, and a supportive companion to family members, I have witnessed too many of what I call “walking dead” providers—those who barely function in their jobs, leave the profession reluctantly, or count the days until retirement.
One key to avoiding burnout is self-care. I know, you’ve heard this before. But knowing something and acting on it are entirely different.
In my case, it was my employer who broke down my self-care barrier. Through the hospital I work for, I received repeated invitations to participate in free workshops. The first email read: “Healing loss workshops provided for interested staff.” The workshops were based on the Kübler-Ross model (otherwise known as the five stages of grief). First introduced by Swiss psychiatrist Elisabeth Kübler-Ross in 1969, the model postulates a series of emotional reactions to loss.
At the time, I thought grief and loss were only about someone dying. I didn’t know that it could encompass other forms of loss, such as relationships, jobs, physical mobility, major rejection, childhood dreams, or children. So, even though around this time I was in the middle of a divorce, ending a 20-year relationship, breaking up a family, and leaving a house I had lived in for two decades (talk about grief and loss!), I deleted several of these messages before I decided to consider the invitation.
Even when I did, my reason for filling out an application was based on the fact that the workshop, food, and lodgings were free—and I would receive continuing education credit! My mindset was focused on what I could gain tangibly rather than emotionally. I was surprised when I was accepted as a participant—and unaware of how much this experience would change both my personal and professional lives.
I arrived for the two-and-a-half-day seminar with no expectations. I knew that the workshop was touted as providing a safe, comfortable, and confidential environment in which facilitators and staff would provide education on and tools for healing. It was emphasized that this was not a form of medical therapy and that participants could choose to discontinue the workshop at any time. The goal was for participants to learn how to resolve inner issues they have built up and carried around with them.
It is difficult to explain in words the internal change that took place within me during my first workshop. The group participation helped me to recognize that everyone carries a mask. Behind that mask, every individual—strangers, colleagues, patients, even family—has his or her own story and journey. Witnessing others sharing their personal pain and grief in a confidential, nonjudgmental environment made me more compassionate.
Continue to: But I also learned more about myself ...
But I also learned more about myself than I expected to, including that I need to continually take care of myself. Otherwise, I will carry baggage with me wherever I am. And the contents of that baggage came as a surprise to me. Yes, I was dealing with loss in my immediate life—divorce, moving, etc—but I became aware that my earlier life experiences were impacting my current behaviors and relationships.
It was difficult for me to conceive that my loving parents, who had given me food and shelter, had neglected my emotional needs. I love my parents, but the resentments I uncovered during that first workshop startled me. I realized that, while they did not overtly teach me prejudice, their actions caused me to cultivate a general distrust of others. They would often say, “Don’t tell anyone.” I now understand that this cast others as untrustworthy and suspicious.
The memory that encapsulated this best for me was of one summer, when I returned home from traveling abroad and was unable to find my old toys (which were always in a large cardboard box in our garage). My parents didn’t have any explanation for where they had gone. I continued to search the garage for years, literally, not understanding how the toys had disappeared. It was only many years later, when the subject of the toys came up in a conversation with my mother, that she replied, nonchalantly, “Oh, your father threw them out.” It’s no wonder I had issues with trust!
I often hear people say, “I just want to forget about what happened to me,” “I don’t want to think about my past,” or “I buried all that old stuff.” But if I learned one thing from these workshops, it’s that life experiences do not stay buried. I carry the effects of my experiences around with me without being conscious of it. The workshop taught me how to acknowledge my grief and loss and work on becoming more emotionally available. I learned that it is OK to be angry about my parents’ behavior and to express my emotions about it, while still loving my parents very much. Acknowledging these suppressed emotions and the effects they had on my life has helped me to grow and move forward.
By the time the
Continue to: Some of the changes I've made ...
Some of the changes I’ve made as a result might seem subtle to an outsider, but they have made a huge difference to me internally—and that seems to reflect onto the people surrounding me.
The biggest complaints I hear from hospitalized patients are that “nobody tells me anything” or “they come in with one leg out the door.” So now I ask for permission to enter the patient’s room. I introduce myself, then ask if it’s OK to sit down with them. This seemingly small action makes such an impact. I began to notice that, in response, patients were thanking me for coming to see them, for listening, and for spending time with them.
The best example I have is a hospitalized patient who had undergone major surgery. She was upset about her care and getting frustrated with staff. I had seen her once before, and when she looked up and saw me at the door of her room, she said, “Oh, let her in! I know she’s really ‘here’ with me.” What a confirmation that this simple change of mindset on my part is helping to make a difference in the care I deliver to my patients!
I realize many of you might be as skeptical as I was when I read the first workshop invitation. You may be thinking, “This won’t help me.” I understand your doubts—I shared them. But that first workshop was so inspiring that I felt compelled to share my experience with others. I believe in the benefits so completely that I pursued training to become a voluntary facilitator. It is indescribable the results I witness in participants. I can only encourage you to give these types of workshops a chance. (If you are uncomfortable about doing a workshop with coworkers in attendance, or just want to do some traveling, workshops are available in many states and different countries.) A simple search for “internalization/externalization workshops” could change your life as much as it did mine!
It’s no secret that health care providers are at high risk for burnout. In my time as a provider, a patient, and a supportive companion to family members, I have witnessed too many of what I call “walking dead” providers—those who barely function in their jobs, leave the profession reluctantly, or count the days until retirement.
One key to avoiding burnout is self-care. I know, you’ve heard this before. But knowing something and acting on it are entirely different.
In my case, it was my employer who broke down my self-care barrier. Through the hospital I work for, I received repeated invitations to participate in free workshops. The first email read: “Healing loss workshops provided for interested staff.” The workshops were based on the Kübler-Ross model (otherwise known as the five stages of grief). First introduced by Swiss psychiatrist Elisabeth Kübler-Ross in 1969, the model postulates a series of emotional reactions to loss.
At the time, I thought grief and loss were only about someone dying. I didn’t know that it could encompass other forms of loss, such as relationships, jobs, physical mobility, major rejection, childhood dreams, or children. So, even though around this time I was in the middle of a divorce, ending a 20-year relationship, breaking up a family, and leaving a house I had lived in for two decades (talk about grief and loss!), I deleted several of these messages before I decided to consider the invitation.
Even when I did, my reason for filling out an application was based on the fact that the workshop, food, and lodgings were free—and I would receive continuing education credit! My mindset was focused on what I could gain tangibly rather than emotionally. I was surprised when I was accepted as a participant—and unaware of how much this experience would change both my personal and professional lives.
I arrived for the two-and-a-half-day seminar with no expectations. I knew that the workshop was touted as providing a safe, comfortable, and confidential environment in which facilitators and staff would provide education on and tools for healing. It was emphasized that this was not a form of medical therapy and that participants could choose to discontinue the workshop at any time. The goal was for participants to learn how to resolve inner issues they have built up and carried around with them.
It is difficult to explain in words the internal change that took place within me during my first workshop. The group participation helped me to recognize that everyone carries a mask. Behind that mask, every individual—strangers, colleagues, patients, even family—has his or her own story and journey. Witnessing others sharing their personal pain and grief in a confidential, nonjudgmental environment made me more compassionate.
Continue to: But I also learned more about myself ...
But I also learned more about myself than I expected to, including that I need to continually take care of myself. Otherwise, I will carry baggage with me wherever I am. And the contents of that baggage came as a surprise to me. Yes, I was dealing with loss in my immediate life—divorce, moving, etc—but I became aware that my earlier life experiences were impacting my current behaviors and relationships.
It was difficult for me to conceive that my loving parents, who had given me food and shelter, had neglected my emotional needs. I love my parents, but the resentments I uncovered during that first workshop startled me. I realized that, while they did not overtly teach me prejudice, their actions caused me to cultivate a general distrust of others. They would often say, “Don’t tell anyone.” I now understand that this cast others as untrustworthy and suspicious.
The memory that encapsulated this best for me was of one summer, when I returned home from traveling abroad and was unable to find my old toys (which were always in a large cardboard box in our garage). My parents didn’t have any explanation for where they had gone. I continued to search the garage for years, literally, not understanding how the toys had disappeared. It was only many years later, when the subject of the toys came up in a conversation with my mother, that she replied, nonchalantly, “Oh, your father threw them out.” It’s no wonder I had issues with trust!
I often hear people say, “I just want to forget about what happened to me,” “I don’t want to think about my past,” or “I buried all that old stuff.” But if I learned one thing from these workshops, it’s that life experiences do not stay buried. I carry the effects of my experiences around with me without being conscious of it. The workshop taught me how to acknowledge my grief and loss and work on becoming more emotionally available. I learned that it is OK to be angry about my parents’ behavior and to express my emotions about it, while still loving my parents very much. Acknowledging these suppressed emotions and the effects they had on my life has helped me to grow and move forward.
By the time the
Continue to: Some of the changes I've made ...
Some of the changes I’ve made as a result might seem subtle to an outsider, but they have made a huge difference to me internally—and that seems to reflect onto the people surrounding me.
The biggest complaints I hear from hospitalized patients are that “nobody tells me anything” or “they come in with one leg out the door.” So now I ask for permission to enter the patient’s room. I introduce myself, then ask if it’s OK to sit down with them. This seemingly small action makes such an impact. I began to notice that, in response, patients were thanking me for coming to see them, for listening, and for spending time with them.
The best example I have is a hospitalized patient who had undergone major surgery. She was upset about her care and getting frustrated with staff. I had seen her once before, and when she looked up and saw me at the door of her room, she said, “Oh, let her in! I know she’s really ‘here’ with me.” What a confirmation that this simple change of mindset on my part is helping to make a difference in the care I deliver to my patients!
I realize many of you might be as skeptical as I was when I read the first workshop invitation. You may be thinking, “This won’t help me.” I understand your doubts—I shared them. But that first workshop was so inspiring that I felt compelled to share my experience with others. I believe in the benefits so completely that I pursued training to become a voluntary facilitator. It is indescribable the results I witness in participants. I can only encourage you to give these types of workshops a chance. (If you are uncomfortable about doing a workshop with coworkers in attendance, or just want to do some traveling, workshops are available in many states and different countries.) A simple search for “internalization/externalization workshops” could change your life as much as it did mine!
It’s no secret that health care providers are at high risk for burnout. In my time as a provider, a patient, and a supportive companion to family members, I have witnessed too many of what I call “walking dead” providers—those who barely function in their jobs, leave the profession reluctantly, or count the days until retirement.
One key to avoiding burnout is self-care. I know, you’ve heard this before. But knowing something and acting on it are entirely different.
In my case, it was my employer who broke down my self-care barrier. Through the hospital I work for, I received repeated invitations to participate in free workshops. The first email read: “Healing loss workshops provided for interested staff.” The workshops were based on the Kübler-Ross model (otherwise known as the five stages of grief). First introduced by Swiss psychiatrist Elisabeth Kübler-Ross in 1969, the model postulates a series of emotional reactions to loss.
At the time, I thought grief and loss were only about someone dying. I didn’t know that it could encompass other forms of loss, such as relationships, jobs, physical mobility, major rejection, childhood dreams, or children. So, even though around this time I was in the middle of a divorce, ending a 20-year relationship, breaking up a family, and leaving a house I had lived in for two decades (talk about grief and loss!), I deleted several of these messages before I decided to consider the invitation.
Even when I did, my reason for filling out an application was based on the fact that the workshop, food, and lodgings were free—and I would receive continuing education credit! My mindset was focused on what I could gain tangibly rather than emotionally. I was surprised when I was accepted as a participant—and unaware of how much this experience would change both my personal and professional lives.
I arrived for the two-and-a-half-day seminar with no expectations. I knew that the workshop was touted as providing a safe, comfortable, and confidential environment in which facilitators and staff would provide education on and tools for healing. It was emphasized that this was not a form of medical therapy and that participants could choose to discontinue the workshop at any time. The goal was for participants to learn how to resolve inner issues they have built up and carried around with them.
It is difficult to explain in words the internal change that took place within me during my first workshop. The group participation helped me to recognize that everyone carries a mask. Behind that mask, every individual—strangers, colleagues, patients, even family—has his or her own story and journey. Witnessing others sharing their personal pain and grief in a confidential, nonjudgmental environment made me more compassionate.
Continue to: But I also learned more about myself ...
But I also learned more about myself than I expected to, including that I need to continually take care of myself. Otherwise, I will carry baggage with me wherever I am. And the contents of that baggage came as a surprise to me. Yes, I was dealing with loss in my immediate life—divorce, moving, etc—but I became aware that my earlier life experiences were impacting my current behaviors and relationships.
It was difficult for me to conceive that my loving parents, who had given me food and shelter, had neglected my emotional needs. I love my parents, but the resentments I uncovered during that first workshop startled me. I realized that, while they did not overtly teach me prejudice, their actions caused me to cultivate a general distrust of others. They would often say, “Don’t tell anyone.” I now understand that this cast others as untrustworthy and suspicious.
The memory that encapsulated this best for me was of one summer, when I returned home from traveling abroad and was unable to find my old toys (which were always in a large cardboard box in our garage). My parents didn’t have any explanation for where they had gone. I continued to search the garage for years, literally, not understanding how the toys had disappeared. It was only many years later, when the subject of the toys came up in a conversation with my mother, that she replied, nonchalantly, “Oh, your father threw them out.” It’s no wonder I had issues with trust!
I often hear people say, “I just want to forget about what happened to me,” “I don’t want to think about my past,” or “I buried all that old stuff.” But if I learned one thing from these workshops, it’s that life experiences do not stay buried. I carry the effects of my experiences around with me without being conscious of it. The workshop taught me how to acknowledge my grief and loss and work on becoming more emotionally available. I learned that it is OK to be angry about my parents’ behavior and to express my emotions about it, while still loving my parents very much. Acknowledging these suppressed emotions and the effects they had on my life has helped me to grow and move forward.
By the time the
Continue to: Some of the changes I've made ...
Some of the changes I’ve made as a result might seem subtle to an outsider, but they have made a huge difference to me internally—and that seems to reflect onto the people surrounding me.
The biggest complaints I hear from hospitalized patients are that “nobody tells me anything” or “they come in with one leg out the door.” So now I ask for permission to enter the patient’s room. I introduce myself, then ask if it’s OK to sit down with them. This seemingly small action makes such an impact. I began to notice that, in response, patients were thanking me for coming to see them, for listening, and for spending time with them.
The best example I have is a hospitalized patient who had undergone major surgery. She was upset about her care and getting frustrated with staff. I had seen her once before, and when she looked up and saw me at the door of her room, she said, “Oh, let her in! I know she’s really ‘here’ with me.” What a confirmation that this simple change of mindset on my part is helping to make a difference in the care I deliver to my patients!
I realize many of you might be as skeptical as I was when I read the first workshop invitation. You may be thinking, “This won’t help me.” I understand your doubts—I shared them. But that first workshop was so inspiring that I felt compelled to share my experience with others. I believe in the benefits so completely that I pursued training to become a voluntary facilitator. It is indescribable the results I witness in participants. I can only encourage you to give these types of workshops a chance. (If you are uncomfortable about doing a workshop with coworkers in attendance, or just want to do some traveling, workshops are available in many states and different countries.) A simple search for “internalization/externalization workshops” could change your life as much as it did mine!
ACOG: Ob.gyns. can help protect pregnant women’s workplace rights
Obstetrician-gynecologists have a central role in helping pregnant patients maintain employment by writing appropriate notes to employers and informing patients of their rights, according to a new committee opinion by the American College of Obstetricians and Gynecologists.
The most common employment issues that pregnant women face include pregnancy-related discrimination, work accommodations that allow continued employment, job-protected leave, and wage replacement while on leave, according to the opinion. After delivery, top employment concerns involve lactation, and accommodations and leave for recovery, bonding, and caring for the infant.
“It’s important that ob.gyns. discuss workplace expectations with their working pregnant patient,” Yasser El-Sayed, MD, vice chair of the ACOG’s committee on obstetric practice said in a statement. “Some women may be unaware of attendant risks to pregnancy at their job, while others may be unaware that their ob.gyn. can offer both support and potential solutions or resources, whether in the form of slight modifications or a formal medical note detailing the need for an accommodation to protect the patient’s health.”
To address these issues, ACOG recommends that ob.gyns. reassure patients that working during pregnancy is generally safe. In the case of high-risk or complicated pregnancy, ob.gyns. should inform patients that work accommodations often can allow for continued safe employment. Appropriately drafted notes by physicians to employers are key, the opinion stresses.
“By writing appropriate notes to employers, obstetrician-gynecologists and other obstetric care providers can be instrumental in obtaining accommodations for their patients who are able to continue working,” the authors wrote. “Accommodations that allow a woman to keep working are the most reliable way to guarantee pay, benefits, and job protection.”
Because the way in which medical certification paperwork is written can greatly affect whether employers comply with medical suggestions, physicians should familiarize themselves with the most effective ways to write such notes, according to ACOG. The recent opinion provides an overview of the necessary structure and contents of medical notes to optimize their effects. The opinion highlights the work of Pregnant@Work, an online note-writing resource developed by the University of California’s Hastings Center for WorkLife Law, San Francisco, that assists health providers in writing legally appropriate work accommodations letters.
Also important for ob.gyns. is knowing the relevant state and federal protections for pregnant patients and new mothers who work or take medical leave. In some instances, it may be necessary for women to consult with a legal counselor in cases in which discrimination has occurred, accommodations are denied, or complex legal questions arise, according to the opinion.
The ACOG opinion is timely because more women than ever before are a part of the workforce, said Rebecca Jackson, MD, a member of ACOG’s committee on obstetric practice and a coauthor of the opinion. Dr. Jackson recently participated in a multidisciplinary work group that addressed legal issues around employment accommodations for pregnant women. Dr. Jackson said she approached ACOG about creating formal guidance on the subject after hearing about the many employment challenges pregnant women experience and how the way physician notes are written can greatly influence accommodations.
“As health care providers responsible for caring for women, it’s essential our guidance addresses the key considerations for maintaining women’s health – this often includes work environments, particularly for pregnant and immediately postpartum women who may have specific health care needs requiring adjustments or accommodations,” Dr. Jackson said in an interview. “Obstetric care providers must be equipped with the necessary and relevant guidance to appropriately support their patients or refer them to additional resources, like legal assistance. This guidance is an important component in building that knowledge among women’s health care providers and ensuring more women can access the care and treatment they need when they need it without compromising their careers or income.”
Obstetrician-gynecologists have a central role in helping pregnant patients maintain employment by writing appropriate notes to employers and informing patients of their rights, according to a new committee opinion by the American College of Obstetricians and Gynecologists.
The most common employment issues that pregnant women face include pregnancy-related discrimination, work accommodations that allow continued employment, job-protected leave, and wage replacement while on leave, according to the opinion. After delivery, top employment concerns involve lactation, and accommodations and leave for recovery, bonding, and caring for the infant.
“It’s important that ob.gyns. discuss workplace expectations with their working pregnant patient,” Yasser El-Sayed, MD, vice chair of the ACOG’s committee on obstetric practice said in a statement. “Some women may be unaware of attendant risks to pregnancy at their job, while others may be unaware that their ob.gyn. can offer both support and potential solutions or resources, whether in the form of slight modifications or a formal medical note detailing the need for an accommodation to protect the patient’s health.”
To address these issues, ACOG recommends that ob.gyns. reassure patients that working during pregnancy is generally safe. In the case of high-risk or complicated pregnancy, ob.gyns. should inform patients that work accommodations often can allow for continued safe employment. Appropriately drafted notes by physicians to employers are key, the opinion stresses.
“By writing appropriate notes to employers, obstetrician-gynecologists and other obstetric care providers can be instrumental in obtaining accommodations for their patients who are able to continue working,” the authors wrote. “Accommodations that allow a woman to keep working are the most reliable way to guarantee pay, benefits, and job protection.”
Because the way in which medical certification paperwork is written can greatly affect whether employers comply with medical suggestions, physicians should familiarize themselves with the most effective ways to write such notes, according to ACOG. The recent opinion provides an overview of the necessary structure and contents of medical notes to optimize their effects. The opinion highlights the work of Pregnant@Work, an online note-writing resource developed by the University of California’s Hastings Center for WorkLife Law, San Francisco, that assists health providers in writing legally appropriate work accommodations letters.
Also important for ob.gyns. is knowing the relevant state and federal protections for pregnant patients and new mothers who work or take medical leave. In some instances, it may be necessary for women to consult with a legal counselor in cases in which discrimination has occurred, accommodations are denied, or complex legal questions arise, according to the opinion.
The ACOG opinion is timely because more women than ever before are a part of the workforce, said Rebecca Jackson, MD, a member of ACOG’s committee on obstetric practice and a coauthor of the opinion. Dr. Jackson recently participated in a multidisciplinary work group that addressed legal issues around employment accommodations for pregnant women. Dr. Jackson said she approached ACOG about creating formal guidance on the subject after hearing about the many employment challenges pregnant women experience and how the way physician notes are written can greatly influence accommodations.
“As health care providers responsible for caring for women, it’s essential our guidance addresses the key considerations for maintaining women’s health – this often includes work environments, particularly for pregnant and immediately postpartum women who may have specific health care needs requiring adjustments or accommodations,” Dr. Jackson said in an interview. “Obstetric care providers must be equipped with the necessary and relevant guidance to appropriately support their patients or refer them to additional resources, like legal assistance. This guidance is an important component in building that knowledge among women’s health care providers and ensuring more women can access the care and treatment they need when they need it without compromising their careers or income.”
Obstetrician-gynecologists have a central role in helping pregnant patients maintain employment by writing appropriate notes to employers and informing patients of their rights, according to a new committee opinion by the American College of Obstetricians and Gynecologists.
The most common employment issues that pregnant women face include pregnancy-related discrimination, work accommodations that allow continued employment, job-protected leave, and wage replacement while on leave, according to the opinion. After delivery, top employment concerns involve lactation, and accommodations and leave for recovery, bonding, and caring for the infant.
“It’s important that ob.gyns. discuss workplace expectations with their working pregnant patient,” Yasser El-Sayed, MD, vice chair of the ACOG’s committee on obstetric practice said in a statement. “Some women may be unaware of attendant risks to pregnancy at their job, while others may be unaware that their ob.gyn. can offer both support and potential solutions or resources, whether in the form of slight modifications or a formal medical note detailing the need for an accommodation to protect the patient’s health.”
To address these issues, ACOG recommends that ob.gyns. reassure patients that working during pregnancy is generally safe. In the case of high-risk or complicated pregnancy, ob.gyns. should inform patients that work accommodations often can allow for continued safe employment. Appropriately drafted notes by physicians to employers are key, the opinion stresses.
“By writing appropriate notes to employers, obstetrician-gynecologists and other obstetric care providers can be instrumental in obtaining accommodations for their patients who are able to continue working,” the authors wrote. “Accommodations that allow a woman to keep working are the most reliable way to guarantee pay, benefits, and job protection.”
Because the way in which medical certification paperwork is written can greatly affect whether employers comply with medical suggestions, physicians should familiarize themselves with the most effective ways to write such notes, according to ACOG. The recent opinion provides an overview of the necessary structure and contents of medical notes to optimize their effects. The opinion highlights the work of Pregnant@Work, an online note-writing resource developed by the University of California’s Hastings Center for WorkLife Law, San Francisco, that assists health providers in writing legally appropriate work accommodations letters.
Also important for ob.gyns. is knowing the relevant state and federal protections for pregnant patients and new mothers who work or take medical leave. In some instances, it may be necessary for women to consult with a legal counselor in cases in which discrimination has occurred, accommodations are denied, or complex legal questions arise, according to the opinion.
The ACOG opinion is timely because more women than ever before are a part of the workforce, said Rebecca Jackson, MD, a member of ACOG’s committee on obstetric practice and a coauthor of the opinion. Dr. Jackson recently participated in a multidisciplinary work group that addressed legal issues around employment accommodations for pregnant women. Dr. Jackson said she approached ACOG about creating formal guidance on the subject after hearing about the many employment challenges pregnant women experience and how the way physician notes are written can greatly influence accommodations.
“As health care providers responsible for caring for women, it’s essential our guidance addresses the key considerations for maintaining women’s health – this often includes work environments, particularly for pregnant and immediately postpartum women who may have specific health care needs requiring adjustments or accommodations,” Dr. Jackson said in an interview. “Obstetric care providers must be equipped with the necessary and relevant guidance to appropriately support their patients or refer them to additional resources, like legal assistance. This guidance is an important component in building that knowledge among women’s health care providers and ensuring more women can access the care and treatment they need when they need it without compromising their careers or income.”