User login
Pooled Analysis Clarifies VNS Efficacy in Cluster Headache
BOSTON—Noninvasive vagal nerve stimulation (VNS) is well tolerated and effectively aborts episodic cluster headache attacks, according to a pooled analysis described at the 59th Annual Scientific Meeting of the American Headache Society. The treatment does not effectively abort attacks in chronic cluster headache, however.
A Pooled Analysis of Two Trials
He and his colleagues studied ElectroCore's gammaCore noninvasive VNS device as an acute treatment of cluster headache in the double-blind, randomized, controlled ACT1 and ACT2 trials. Each trial included more than 100 patients with episodic or chronic cluster headache. The two studies took place at 20 centers in the United States and nine centers in the United Kingdom and Europe.
Treatment was delivered on the patient's right side in ACT1, and on the side ipsilateral to the attack in ACT2. In ACT1, patients were allowed three stimulations, or a total of six minutes of treatment per attack. In ACT2, patients were permitted as many as six stimulations, representing 12 minutes of treatment per attack. In ACT1, the primary end point was response rate (ie, the proportion of patients who achieved pain relief at 15 minutes). The primary end point in ACT2 was pain freedom at 15 minutes, and a secondary end point was the 50% responder rate (ie, mild or no pain) at 15 minutes.
Each study was underpowered for an analysis of results according to headache frequency, so the investigators performed a pooled analysis of the two studies. This examination included a fixed-effects meta-analysis and a first-order interaction test between the treatment group and the cluster headache subgroup to determine whether the magnitude of treatment effect varied significantly by cluster headache subtype.
In all, 253 patients were included in the pooled analysis, 131 of whom had episodic cluster headache, and 122 of whom had chronic cluster headache. Patients' mean age was 46.6. Approximately 21% of patients were female, 2.4% were Asian, and 4.7% were black.
Efficacy in Episodic, But Not Chronic Cluster Headache
Among patients with episodic cluster headache, the rate of pain freedom for all treated attacks at 15 minutes was significantly higher with VNS than with sham (15% vs 6% in ACT1, 35% vs 7% in ACT2, and 24% vs 7% in the pooled analysis). Among patients with chronic cluster headache, the researchers did not find significant differences in this end point between treatment groups (5% vs 14% in ACT1, 7% vs 9% in ACT2, and 7% vs 11% in the pooled analysis).
For patients with episodic cluster headache, the 50% responder rate was 34% in ACT1, 64% in ACT2, and 42% in the pooled analysis. For patients with chronic cluster headache, the responder rates were 13.6% in ACT1, 29.4% in ACT2, and 23.2% in the pooled analysis. For the proportion of attacks that became pain free, the interaction test was significant for ACT1 and the pooled analysis, but it was not significant for ACT2. For the 50% responder end point, the interaction test was significant for ACT1, ACT2, and the pooled analysis.
In April 2017, the FDA cleared the gammaCore device for the acute treatment of pain associated with episodic cluster headache in adults. The device is now available in the US, said Mr. Liebler.
“We are going to continue to examine the mechanism of action [of noninvasive VNS] and the reasons for possible [treatment] failure in chronic cluster headache,” said Mr. Liebler. These investigations could provide further clinical insight and clarify the pathogenesis of cluster headache, he added.
—Erik Greb
Suggested Reading
Silberstein SD, Mechtler LL, Kudrow DB, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56(8):1317-1332.
BOSTON—Noninvasive vagal nerve stimulation (VNS) is well tolerated and effectively aborts episodic cluster headache attacks, according to a pooled analysis described at the 59th Annual Scientific Meeting of the American Headache Society. The treatment does not effectively abort attacks in chronic cluster headache, however.
A Pooled Analysis of Two Trials
He and his colleagues studied ElectroCore's gammaCore noninvasive VNS device as an acute treatment of cluster headache in the double-blind, randomized, controlled ACT1 and ACT2 trials. Each trial included more than 100 patients with episodic or chronic cluster headache. The two studies took place at 20 centers in the United States and nine centers in the United Kingdom and Europe.
Treatment was delivered on the patient's right side in ACT1, and on the side ipsilateral to the attack in ACT2. In ACT1, patients were allowed three stimulations, or a total of six minutes of treatment per attack. In ACT2, patients were permitted as many as six stimulations, representing 12 minutes of treatment per attack. In ACT1, the primary end point was response rate (ie, the proportion of patients who achieved pain relief at 15 minutes). The primary end point in ACT2 was pain freedom at 15 minutes, and a secondary end point was the 50% responder rate (ie, mild or no pain) at 15 minutes.
Each study was underpowered for an analysis of results according to headache frequency, so the investigators performed a pooled analysis of the two studies. This examination included a fixed-effects meta-analysis and a first-order interaction test between the treatment group and the cluster headache subgroup to determine whether the magnitude of treatment effect varied significantly by cluster headache subtype.
In all, 253 patients were included in the pooled analysis, 131 of whom had episodic cluster headache, and 122 of whom had chronic cluster headache. Patients' mean age was 46.6. Approximately 21% of patients were female, 2.4% were Asian, and 4.7% were black.
Efficacy in Episodic, But Not Chronic Cluster Headache
Among patients with episodic cluster headache, the rate of pain freedom for all treated attacks at 15 minutes was significantly higher with VNS than with sham (15% vs 6% in ACT1, 35% vs 7% in ACT2, and 24% vs 7% in the pooled analysis). Among patients with chronic cluster headache, the researchers did not find significant differences in this end point between treatment groups (5% vs 14% in ACT1, 7% vs 9% in ACT2, and 7% vs 11% in the pooled analysis).
For patients with episodic cluster headache, the 50% responder rate was 34% in ACT1, 64% in ACT2, and 42% in the pooled analysis. For patients with chronic cluster headache, the responder rates were 13.6% in ACT1, 29.4% in ACT2, and 23.2% in the pooled analysis. For the proportion of attacks that became pain free, the interaction test was significant for ACT1 and the pooled analysis, but it was not significant for ACT2. For the 50% responder end point, the interaction test was significant for ACT1, ACT2, and the pooled analysis.
In April 2017, the FDA cleared the gammaCore device for the acute treatment of pain associated with episodic cluster headache in adults. The device is now available in the US, said Mr. Liebler.
“We are going to continue to examine the mechanism of action [of noninvasive VNS] and the reasons for possible [treatment] failure in chronic cluster headache,” said Mr. Liebler. These investigations could provide further clinical insight and clarify the pathogenesis of cluster headache, he added.
—Erik Greb
Suggested Reading
Silberstein SD, Mechtler LL, Kudrow DB, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56(8):1317-1332.
BOSTON—Noninvasive vagal nerve stimulation (VNS) is well tolerated and effectively aborts episodic cluster headache attacks, according to a pooled analysis described at the 59th Annual Scientific Meeting of the American Headache Society. The treatment does not effectively abort attacks in chronic cluster headache, however.
A Pooled Analysis of Two Trials
He and his colleagues studied ElectroCore's gammaCore noninvasive VNS device as an acute treatment of cluster headache in the double-blind, randomized, controlled ACT1 and ACT2 trials. Each trial included more than 100 patients with episodic or chronic cluster headache. The two studies took place at 20 centers in the United States and nine centers in the United Kingdom and Europe.
Treatment was delivered on the patient's right side in ACT1, and on the side ipsilateral to the attack in ACT2. In ACT1, patients were allowed three stimulations, or a total of six minutes of treatment per attack. In ACT2, patients were permitted as many as six stimulations, representing 12 minutes of treatment per attack. In ACT1, the primary end point was response rate (ie, the proportion of patients who achieved pain relief at 15 minutes). The primary end point in ACT2 was pain freedom at 15 minutes, and a secondary end point was the 50% responder rate (ie, mild or no pain) at 15 minutes.
Each study was underpowered for an analysis of results according to headache frequency, so the investigators performed a pooled analysis of the two studies. This examination included a fixed-effects meta-analysis and a first-order interaction test between the treatment group and the cluster headache subgroup to determine whether the magnitude of treatment effect varied significantly by cluster headache subtype.
In all, 253 patients were included in the pooled analysis, 131 of whom had episodic cluster headache, and 122 of whom had chronic cluster headache. Patients' mean age was 46.6. Approximately 21% of patients were female, 2.4% were Asian, and 4.7% were black.
Efficacy in Episodic, But Not Chronic Cluster Headache
Among patients with episodic cluster headache, the rate of pain freedom for all treated attacks at 15 minutes was significantly higher with VNS than with sham (15% vs 6% in ACT1, 35% vs 7% in ACT2, and 24% vs 7% in the pooled analysis). Among patients with chronic cluster headache, the researchers did not find significant differences in this end point between treatment groups (5% vs 14% in ACT1, 7% vs 9% in ACT2, and 7% vs 11% in the pooled analysis).
For patients with episodic cluster headache, the 50% responder rate was 34% in ACT1, 64% in ACT2, and 42% in the pooled analysis. For patients with chronic cluster headache, the responder rates were 13.6% in ACT1, 29.4% in ACT2, and 23.2% in the pooled analysis. For the proportion of attacks that became pain free, the interaction test was significant for ACT1 and the pooled analysis, but it was not significant for ACT2. For the 50% responder end point, the interaction test was significant for ACT1, ACT2, and the pooled analysis.
In April 2017, the FDA cleared the gammaCore device for the acute treatment of pain associated with episodic cluster headache in adults. The device is now available in the US, said Mr. Liebler.
“We are going to continue to examine the mechanism of action [of noninvasive VNS] and the reasons for possible [treatment] failure in chronic cluster headache,” said Mr. Liebler. These investigations could provide further clinical insight and clarify the pathogenesis of cluster headache, he added.
—Erik Greb
Suggested Reading
Silberstein SD, Mechtler LL, Kudrow DB, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56(8):1317-1332.
Studies backing certain FDA approvals found lacking
, two studies showed.
Between 2009 and 2013, the FDA granted accelerated approval for 22 drugs with 24 indications, which were supported by 30 preapproval studies with a median of 132 subjects. Only 12 of those studies (40%) were randomized, and only 6 (20%) were double blind. Eight (27%) included fewer than 100 subjects, and 20 (67%) included fewer than 200, reported Huseyin Naci, PhD, of the London School of Economics and Political Science, and his colleagues.
Further, at a minimum of 3 years after the approval, only half of the 38 confirmatory studies required by the FDA were completed, and, ultimately, only 25 of the 48 (66%) examined clinical efficacy, only 7 (18%) evaluated longer follow-up, and only 6 (16%) focused on safety, the investigators reported (JAMA. 2017 Aug 15;318[7]:626-36).
The proportion of studies that were randomized was slightly, but not significantly greater in the postapproval vs. preapproval period (56% vs. 40%), and only one was double blind. For 10 of 24 indications (42%), postapproval study requirements were completed and demonstrated efficacy based on surrogate measures, the investigators said.
Of the 14 remaining indications (58%) for which FDA study requirements had not yet been met, 2 (8%) had at least one confirmatory study that failed to demonstrate clinical benefit (without apparent action on the part of the FDA to rescind approval or impose additional requirements), 2 (8%) had a least one confirmatory study that was terminated, and 3 (13%) had at least one confirmatory study that was delayed by more than a year. The required studies for the remaining indications were progressing as planned, but for eight indications, clinical benefit had not yet been confirmed at 5 or more years after approval.
Similar concerns were seen in a review of clinical studies used to support high-risk medical device modification approvals. Such devices often undergo numerous modifications that receive FDA approval through one of six premarket approval (PMA) supplement pathways, and a total of 83 studies that supported 78 panel-track supplements (one of the 6 pathways and the only one that always required clinical data) approved between April 19, 2006, and Oct. 9, 2015, were identified. Nearly all (98%) of those 78 modifications were supported by just one study; only 45% of those studies were randomized clinical trials, and only 30% were blinded, reported Sarah Y. Zheng, MD, of the University of California, San Francisco, and her colleagues (JAMA. 2017 Aug 15;318[7]:619-25).
The median number of patients in the studies was 185, and the median follow-up was 180 days. Further, of 150 primary endpoints in the studies, 121 (81%) were surrogate endpoints, 57 (38%) were compared with controls, and 6 (11%) of those involved retrospective rather than active controls.
Age and sex were not reported for all enrolled patients in 40% and 30% of the studies, respectively, and in the case of one device modification study, 91% of enrolled patients were not included in the primary analysis.
“Given the extensive modification of many PMA supplement devices and the median preapproval follow-up of 6 months, obtaining additional data via [postapproval studies] is critical. However, the FDA required [postapproval studies] for the minority (37%) of the panel-track supplements,” the investigators noted, adding that only 13% of initiated postapproval studies were completed between 3 and 5 years after FDA approval, and that no warning letters, penalties, or fines were administered for noncompliance.
“These findings suggest that the quality of studies and data evaluated to support approval by the FDA of modifications of high-risk devices should be improved,” they concluded.
Dr. Zheng and her colleagues reported having no conflicts of interest. Dr. Naci reported having no conflicts of interest. One coauthor, Aaron S. Kesselheim, MD, reported receiving unrelated grants from the FDA Office of Generic Drugs and Division of Health Communication.
The findings by Naci and colleagues and Zheng and colleagues raise concerns about whether the current regulatory system is too permissive in not requiring traditional randomized controlled trials for postmarketing evaluation of drugs that receive accelerated approval, and for high-risk medical device supplemental design modifications, Robert Califf, MD, wrote in an editorial.
However, randomization and blinding are not always feasible, and “despite the concerns raised by these two articles … it is important to remember that decisions about postmarket requirements and monitoring of these studies are overseen by full-time FDA employees with no financial conflicts,” he said, adding that “this underscores the importance of a talented workforce at the FDA with the variety of skills needed to assimilate information about manufacturing, quality systems, clinical outcomes, and the well-being and preferences of patients.”
A sweeping overhaul of the overall system is also needed, and is underway, he said, noting that substantial progress in balancing safety with access to effective therapies will come from systemic changes in the ecosystem rather than from imposing more severe demands on individual products (JAMA. 2017 Aug 15;318[7]:614-6).
Indeed, it is time to seriously consider how increasingly robust data and analytic capabilities and more efficient prospective research systems can be used to address the concerns raised in these articles, he said, adding that “as technological improvements and … connected networks of health systems make it feasible to conduct high-quality, low-cost RCTs [randomized, controlled trials] and to continuously monitor product performance, the impediments to progress are mostly those built into the culture of medicine and health care.”
Dr. Califf is with Duke Health and Duke University, Durham, N.C. He was the Commissioner of Food and Drugs, Food and Drug Administration, from February 2016 to January 2017. He currently receives consulting payments from Merck and is employed as a scientific adviser by Verily Life Sciences (Alphabet).
The findings by Naci and colleagues and Zheng and colleagues raise concerns about whether the current regulatory system is too permissive in not requiring traditional randomized controlled trials for postmarketing evaluation of drugs that receive accelerated approval, and for high-risk medical device supplemental design modifications, Robert Califf, MD, wrote in an editorial.
However, randomization and blinding are not always feasible, and “despite the concerns raised by these two articles … it is important to remember that decisions about postmarket requirements and monitoring of these studies are overseen by full-time FDA employees with no financial conflicts,” he said, adding that “this underscores the importance of a talented workforce at the FDA with the variety of skills needed to assimilate information about manufacturing, quality systems, clinical outcomes, and the well-being and preferences of patients.”
A sweeping overhaul of the overall system is also needed, and is underway, he said, noting that substantial progress in balancing safety with access to effective therapies will come from systemic changes in the ecosystem rather than from imposing more severe demands on individual products (JAMA. 2017 Aug 15;318[7]:614-6).
Indeed, it is time to seriously consider how increasingly robust data and analytic capabilities and more efficient prospective research systems can be used to address the concerns raised in these articles, he said, adding that “as technological improvements and … connected networks of health systems make it feasible to conduct high-quality, low-cost RCTs [randomized, controlled trials] and to continuously monitor product performance, the impediments to progress are mostly those built into the culture of medicine and health care.”
Dr. Califf is with Duke Health and Duke University, Durham, N.C. He was the Commissioner of Food and Drugs, Food and Drug Administration, from February 2016 to January 2017. He currently receives consulting payments from Merck and is employed as a scientific adviser by Verily Life Sciences (Alphabet).
The findings by Naci and colleagues and Zheng and colleagues raise concerns about whether the current regulatory system is too permissive in not requiring traditional randomized controlled trials for postmarketing evaluation of drugs that receive accelerated approval, and for high-risk medical device supplemental design modifications, Robert Califf, MD, wrote in an editorial.
However, randomization and blinding are not always feasible, and “despite the concerns raised by these two articles … it is important to remember that decisions about postmarket requirements and monitoring of these studies are overseen by full-time FDA employees with no financial conflicts,” he said, adding that “this underscores the importance of a talented workforce at the FDA with the variety of skills needed to assimilate information about manufacturing, quality systems, clinical outcomes, and the well-being and preferences of patients.”
A sweeping overhaul of the overall system is also needed, and is underway, he said, noting that substantial progress in balancing safety with access to effective therapies will come from systemic changes in the ecosystem rather than from imposing more severe demands on individual products (JAMA. 2017 Aug 15;318[7]:614-6).
Indeed, it is time to seriously consider how increasingly robust data and analytic capabilities and more efficient prospective research systems can be used to address the concerns raised in these articles, he said, adding that “as technological improvements and … connected networks of health systems make it feasible to conduct high-quality, low-cost RCTs [randomized, controlled trials] and to continuously monitor product performance, the impediments to progress are mostly those built into the culture of medicine and health care.”
Dr. Califf is with Duke Health and Duke University, Durham, N.C. He was the Commissioner of Food and Drugs, Food and Drug Administration, from February 2016 to January 2017. He currently receives consulting payments from Merck and is employed as a scientific adviser by Verily Life Sciences (Alphabet).
, two studies showed.
Between 2009 and 2013, the FDA granted accelerated approval for 22 drugs with 24 indications, which were supported by 30 preapproval studies with a median of 132 subjects. Only 12 of those studies (40%) were randomized, and only 6 (20%) were double blind. Eight (27%) included fewer than 100 subjects, and 20 (67%) included fewer than 200, reported Huseyin Naci, PhD, of the London School of Economics and Political Science, and his colleagues.
Further, at a minimum of 3 years after the approval, only half of the 38 confirmatory studies required by the FDA were completed, and, ultimately, only 25 of the 48 (66%) examined clinical efficacy, only 7 (18%) evaluated longer follow-up, and only 6 (16%) focused on safety, the investigators reported (JAMA. 2017 Aug 15;318[7]:626-36).
The proportion of studies that were randomized was slightly, but not significantly greater in the postapproval vs. preapproval period (56% vs. 40%), and only one was double blind. For 10 of 24 indications (42%), postapproval study requirements were completed and demonstrated efficacy based on surrogate measures, the investigators said.
Of the 14 remaining indications (58%) for which FDA study requirements had not yet been met, 2 (8%) had at least one confirmatory study that failed to demonstrate clinical benefit (without apparent action on the part of the FDA to rescind approval or impose additional requirements), 2 (8%) had a least one confirmatory study that was terminated, and 3 (13%) had at least one confirmatory study that was delayed by more than a year. The required studies for the remaining indications were progressing as planned, but for eight indications, clinical benefit had not yet been confirmed at 5 or more years after approval.
Similar concerns were seen in a review of clinical studies used to support high-risk medical device modification approvals. Such devices often undergo numerous modifications that receive FDA approval through one of six premarket approval (PMA) supplement pathways, and a total of 83 studies that supported 78 panel-track supplements (one of the 6 pathways and the only one that always required clinical data) approved between April 19, 2006, and Oct. 9, 2015, were identified. Nearly all (98%) of those 78 modifications were supported by just one study; only 45% of those studies were randomized clinical trials, and only 30% were blinded, reported Sarah Y. Zheng, MD, of the University of California, San Francisco, and her colleagues (JAMA. 2017 Aug 15;318[7]:619-25).
The median number of patients in the studies was 185, and the median follow-up was 180 days. Further, of 150 primary endpoints in the studies, 121 (81%) were surrogate endpoints, 57 (38%) were compared with controls, and 6 (11%) of those involved retrospective rather than active controls.
Age and sex were not reported for all enrolled patients in 40% and 30% of the studies, respectively, and in the case of one device modification study, 91% of enrolled patients were not included in the primary analysis.
“Given the extensive modification of many PMA supplement devices and the median preapproval follow-up of 6 months, obtaining additional data via [postapproval studies] is critical. However, the FDA required [postapproval studies] for the minority (37%) of the panel-track supplements,” the investigators noted, adding that only 13% of initiated postapproval studies were completed between 3 and 5 years after FDA approval, and that no warning letters, penalties, or fines were administered for noncompliance.
“These findings suggest that the quality of studies and data evaluated to support approval by the FDA of modifications of high-risk devices should be improved,” they concluded.
Dr. Zheng and her colleagues reported having no conflicts of interest. Dr. Naci reported having no conflicts of interest. One coauthor, Aaron S. Kesselheim, MD, reported receiving unrelated grants from the FDA Office of Generic Drugs and Division of Health Communication.
, two studies showed.
Between 2009 and 2013, the FDA granted accelerated approval for 22 drugs with 24 indications, which were supported by 30 preapproval studies with a median of 132 subjects. Only 12 of those studies (40%) were randomized, and only 6 (20%) were double blind. Eight (27%) included fewer than 100 subjects, and 20 (67%) included fewer than 200, reported Huseyin Naci, PhD, of the London School of Economics and Political Science, and his colleagues.
Further, at a minimum of 3 years after the approval, only half of the 38 confirmatory studies required by the FDA were completed, and, ultimately, only 25 of the 48 (66%) examined clinical efficacy, only 7 (18%) evaluated longer follow-up, and only 6 (16%) focused on safety, the investigators reported (JAMA. 2017 Aug 15;318[7]:626-36).
The proportion of studies that were randomized was slightly, but not significantly greater in the postapproval vs. preapproval period (56% vs. 40%), and only one was double blind. For 10 of 24 indications (42%), postapproval study requirements were completed and demonstrated efficacy based on surrogate measures, the investigators said.
Of the 14 remaining indications (58%) for which FDA study requirements had not yet been met, 2 (8%) had at least one confirmatory study that failed to demonstrate clinical benefit (without apparent action on the part of the FDA to rescind approval or impose additional requirements), 2 (8%) had a least one confirmatory study that was terminated, and 3 (13%) had at least one confirmatory study that was delayed by more than a year. The required studies for the remaining indications were progressing as planned, but for eight indications, clinical benefit had not yet been confirmed at 5 or more years after approval.
Similar concerns were seen in a review of clinical studies used to support high-risk medical device modification approvals. Such devices often undergo numerous modifications that receive FDA approval through one of six premarket approval (PMA) supplement pathways, and a total of 83 studies that supported 78 panel-track supplements (one of the 6 pathways and the only one that always required clinical data) approved between April 19, 2006, and Oct. 9, 2015, were identified. Nearly all (98%) of those 78 modifications were supported by just one study; only 45% of those studies were randomized clinical trials, and only 30% were blinded, reported Sarah Y. Zheng, MD, of the University of California, San Francisco, and her colleagues (JAMA. 2017 Aug 15;318[7]:619-25).
The median number of patients in the studies was 185, and the median follow-up was 180 days. Further, of 150 primary endpoints in the studies, 121 (81%) were surrogate endpoints, 57 (38%) were compared with controls, and 6 (11%) of those involved retrospective rather than active controls.
Age and sex were not reported for all enrolled patients in 40% and 30% of the studies, respectively, and in the case of one device modification study, 91% of enrolled patients were not included in the primary analysis.
“Given the extensive modification of many PMA supplement devices and the median preapproval follow-up of 6 months, obtaining additional data via [postapproval studies] is critical. However, the FDA required [postapproval studies] for the minority (37%) of the panel-track supplements,” the investigators noted, adding that only 13% of initiated postapproval studies were completed between 3 and 5 years after FDA approval, and that no warning letters, penalties, or fines were administered for noncompliance.
“These findings suggest that the quality of studies and data evaluated to support approval by the FDA of modifications of high-risk devices should be improved,” they concluded.
Dr. Zheng and her colleagues reported having no conflicts of interest. Dr. Naci reported having no conflicts of interest. One coauthor, Aaron S. Kesselheim, MD, reported receiving unrelated grants from the FDA Office of Generic Drugs and Division of Health Communication.
FROM JAMA
Key clinical point:
Major finding: For 8 of 24 drug indications, clinical benefit had not yet been confirmed at 5 or more years after approval, and only 13% of initiated postapproval studies were completed between 3 and 5 years after FDA device supplement approvals.
Data source: A review of FDA documents regarding 22 drugs with 24 indications, and a descriptive study of 83 clinical studies supporting 78 panel-track supplements.
Disclosures: Dr. Zheng and her colleagues reported having no conflicts of interest. Dr. Naci reported having no conflicts of interest. One coauthor, Aaron S. Kesselheim, MD, reported receiving unrelated grants from the FDA Office of Generic Drugs and Division of Health Communication.
Cannabidiol Changes Serum Levels of Antiepileptic Drugs
The pharmaceutical formulation of cannabidiol (CBD) is associated with significant changes in serum levels of clobazam, rufinamide, topiramate, zonisamide, and eslicarbazepine, according to research published online ahead of print August 6 in Epilepsia. Furthermore, patients taking CBD and valproate may have abnormal liver function test results.
“A perception exists that since CBD is plant-based, it is natural and safe; and while this may be mostly true, our study shows that CBD, just like other antiepileptic drugs [AEDs], has interactions with other seizure drugs that patients and providers need to be aware of,” said Tyler Gaston, MD, Assistant Professor of Neurology at the University of Alabama at Birmingham.
Patients in a Compassionate-Use Study
Few data about CBD’s interactions with AEDs are available. To gain more information, Dr. Gaston and colleagues examined 42 children and 39 adults in the University of Alabama’s open-label compassionate-use study of CBD as an add-on therapy for treatment-resistant epilepsy. At baseline, the investigators obtained participants’ serum AED levels. Participants initiated treatment with 5 mg/kg/day of CBD along with their other AEDs. Every two weeks, participants underwent a physical examination and laboratory testing. The investigators obtained serum AED levels and increased the dose of CBD by 5 mg/kg/day to a maximum of 50 mg/kg/day. AED doses were adjusted if a clinical symptom or laboratory result was related to a potential interaction.
Dr. Gaston and colleagues used a mixed linear model to determine whether the plasma levels of each AED changed significantly with increasing CBD dose.
Many Serum Levels Remained in Therapeutic Range
The mean age of pediatric participants was 10, and 20 of the children were female. The mean age of adults was 29, and 20 of the adult participants were female. The mean number of concomitant AEDs at enrollment for all participants was three.
The investigators noted increases in serum levels of topiramate, rufinamide, and N-desmethylclobazam (ie, the active metabolite of clobazam) and a decrease in serum levels of clobazam with increasing CBD dose. They also observed increases in serum levels of zonisamide and eslicarbazepine with increasing CBD dose in adults. All mean level changes were within the accepted therapeutic range, except for those in clobazam and N-desmethylclobazam. Sedation was more frequent with higher N-desmethylclobazam levels in adults. Levels of aspartate aminotransferase and alanine aminotransferase were significantly higher in participants taking concomitant valproate.
One of the study’s limitations was that the sample sizes of patients taking each AED were small, which may have masked potential interactions with CBD, according to the researchers. In addition, the naturalistic study design resulted in significant noise in the data for which the researchers could not completely account in their analyses. Nevertheless, “this study emphasizes the importance of monitoring serum AED levels and liver function tests during treatment with CBD,” said Dr. Gaston.
—Erik Greb
Suggested Reading
Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017 Aug 6 [Epub ahead of print].
The pharmaceutical formulation of cannabidiol (CBD) is associated with significant changes in serum levels of clobazam, rufinamide, topiramate, zonisamide, and eslicarbazepine, according to research published online ahead of print August 6 in Epilepsia. Furthermore, patients taking CBD and valproate may have abnormal liver function test results.
“A perception exists that since CBD is plant-based, it is natural and safe; and while this may be mostly true, our study shows that CBD, just like other antiepileptic drugs [AEDs], has interactions with other seizure drugs that patients and providers need to be aware of,” said Tyler Gaston, MD, Assistant Professor of Neurology at the University of Alabama at Birmingham.
Patients in a Compassionate-Use Study
Few data about CBD’s interactions with AEDs are available. To gain more information, Dr. Gaston and colleagues examined 42 children and 39 adults in the University of Alabama’s open-label compassionate-use study of CBD as an add-on therapy for treatment-resistant epilepsy. At baseline, the investigators obtained participants’ serum AED levels. Participants initiated treatment with 5 mg/kg/day of CBD along with their other AEDs. Every two weeks, participants underwent a physical examination and laboratory testing. The investigators obtained serum AED levels and increased the dose of CBD by 5 mg/kg/day to a maximum of 50 mg/kg/day. AED doses were adjusted if a clinical symptom or laboratory result was related to a potential interaction.
Dr. Gaston and colleagues used a mixed linear model to determine whether the plasma levels of each AED changed significantly with increasing CBD dose.
Many Serum Levels Remained in Therapeutic Range
The mean age of pediatric participants was 10, and 20 of the children were female. The mean age of adults was 29, and 20 of the adult participants were female. The mean number of concomitant AEDs at enrollment for all participants was three.
The investigators noted increases in serum levels of topiramate, rufinamide, and N-desmethylclobazam (ie, the active metabolite of clobazam) and a decrease in serum levels of clobazam with increasing CBD dose. They also observed increases in serum levels of zonisamide and eslicarbazepine with increasing CBD dose in adults. All mean level changes were within the accepted therapeutic range, except for those in clobazam and N-desmethylclobazam. Sedation was more frequent with higher N-desmethylclobazam levels in adults. Levels of aspartate aminotransferase and alanine aminotransferase were significantly higher in participants taking concomitant valproate.
One of the study’s limitations was that the sample sizes of patients taking each AED were small, which may have masked potential interactions with CBD, according to the researchers. In addition, the naturalistic study design resulted in significant noise in the data for which the researchers could not completely account in their analyses. Nevertheless, “this study emphasizes the importance of monitoring serum AED levels and liver function tests during treatment with CBD,” said Dr. Gaston.
—Erik Greb
Suggested Reading
Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017 Aug 6 [Epub ahead of print].
The pharmaceutical formulation of cannabidiol (CBD) is associated with significant changes in serum levels of clobazam, rufinamide, topiramate, zonisamide, and eslicarbazepine, according to research published online ahead of print August 6 in Epilepsia. Furthermore, patients taking CBD and valproate may have abnormal liver function test results.
“A perception exists that since CBD is plant-based, it is natural and safe; and while this may be mostly true, our study shows that CBD, just like other antiepileptic drugs [AEDs], has interactions with other seizure drugs that patients and providers need to be aware of,” said Tyler Gaston, MD, Assistant Professor of Neurology at the University of Alabama at Birmingham.
Patients in a Compassionate-Use Study
Few data about CBD’s interactions with AEDs are available. To gain more information, Dr. Gaston and colleagues examined 42 children and 39 adults in the University of Alabama’s open-label compassionate-use study of CBD as an add-on therapy for treatment-resistant epilepsy. At baseline, the investigators obtained participants’ serum AED levels. Participants initiated treatment with 5 mg/kg/day of CBD along with their other AEDs. Every two weeks, participants underwent a physical examination and laboratory testing. The investigators obtained serum AED levels and increased the dose of CBD by 5 mg/kg/day to a maximum of 50 mg/kg/day. AED doses were adjusted if a clinical symptom or laboratory result was related to a potential interaction.
Dr. Gaston and colleagues used a mixed linear model to determine whether the plasma levels of each AED changed significantly with increasing CBD dose.
Many Serum Levels Remained in Therapeutic Range
The mean age of pediatric participants was 10, and 20 of the children were female. The mean age of adults was 29, and 20 of the adult participants were female. The mean number of concomitant AEDs at enrollment for all participants was three.
The investigators noted increases in serum levels of topiramate, rufinamide, and N-desmethylclobazam (ie, the active metabolite of clobazam) and a decrease in serum levels of clobazam with increasing CBD dose. They also observed increases in serum levels of zonisamide and eslicarbazepine with increasing CBD dose in adults. All mean level changes were within the accepted therapeutic range, except for those in clobazam and N-desmethylclobazam. Sedation was more frequent with higher N-desmethylclobazam levels in adults. Levels of aspartate aminotransferase and alanine aminotransferase were significantly higher in participants taking concomitant valproate.
One of the study’s limitations was that the sample sizes of patients taking each AED were small, which may have masked potential interactions with CBD, according to the researchers. In addition, the naturalistic study design resulted in significant noise in the data for which the researchers could not completely account in their analyses. Nevertheless, “this study emphasizes the importance of monitoring serum AED levels and liver function tests during treatment with CBD,” said Dr. Gaston.
—Erik Greb
Suggested Reading
Gaston TE, Bebin EM, Cutter GR, et al. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017 Aug 6 [Epub ahead of print].
Headache Trajectories Differ Five Years After TBI
BOSTON—Over five years after traumatic brain injury (TBI), a relatively large (24% to 30%) group of individuals experience chronic headache pain and a significant functional impact of headache, according to a pattern analysis of headache pain and impact following moderate to severe TBI. These results were presented at the 59th Annual Scientific Meeting of the American Headache Society. “Identification of members within these groups may be important to assist with an appropriate intensity of treatment to improve satisfaction with life and employment opportunity after TBI,” said Sylvia Lucas, MD, PhD, Clinical Professor of Neurology and Neurological Surgery at the University of Washington in Seattle. “The identification of trajectory membership may also be useful in evaluation of appropriate subjects for inclusion in studies of headache treatment after TBI.”
Headache is the most common symptom following TBI of any severity. Dr. Lucas and her research colleagues have previously reported high rates of headache in a prospective cohort of patients who experienced moderate to severe TBI. These patients have been followed for five years. New or worse headache occurred at a rate of 37% at three months, 33% at six months, 34% at 12 months, and 35% at 60 months post injury. The present study examined whether certain patterns or trajectories of headache occur over five years after TBI and whether demographic or injury characteristics are related to these trajectories. Trajectory type was also examined in relation to satisfaction with life and employment status five years after injury.
Data on 316 individuals were evaluated at five years. These patients were initially enrolled during inpatient rehabilitation after moderate to severe TBI. Enrollment was performed in person in the hospital. Structured telephone interviews were conducted at three, six, 12, and 60 months after TBI. At each time point, individuals who reported headache in the previous three months were asked to rate their headache pain on a 0 to 10 scale (0 = no pain, 10 = worst pain) and to complete the Headache Impact Test (HIT-6). Discrete mixture modeling was used to estimate trajectory groups based on pain rating and on headache impact scores.
Four trajectories were found for headache pain over five years: minimal pain over time (25% of individuals), worsening pain over time (37%), improving pain over time (7%), and chronic pain over time (30%). A chronic pain trajectory was more common in females, those incurring TBI by violence, those who were unemployed prior to injury, those with a headache history prior to injury, and those with mental health problems. Those with a chronic pain trajectory had significantly lower satisfaction with life five years after injury, compared with other trajectory groups.
A chronic impact trajectory was more common in females and in those who incurred TBI by violence, had a prior history of headache, or were unemployed prior to injury. At five years post TBI, the chronic impact group was significantly more likely to be unemployed and less satisfied with life, compared with individuals in the minimal or worsening impact trajectory groups. Those employed prior to injury were more frequently in the worsening group for pain and impact, compared with those not employed prior to injury. No relationship was found for other demographic and injury data, including age, posttraumatic amnesia, or substance abuse prior to injury.
BOSTON—Over five years after traumatic brain injury (TBI), a relatively large (24% to 30%) group of individuals experience chronic headache pain and a significant functional impact of headache, according to a pattern analysis of headache pain and impact following moderate to severe TBI. These results were presented at the 59th Annual Scientific Meeting of the American Headache Society. “Identification of members within these groups may be important to assist with an appropriate intensity of treatment to improve satisfaction with life and employment opportunity after TBI,” said Sylvia Lucas, MD, PhD, Clinical Professor of Neurology and Neurological Surgery at the University of Washington in Seattle. “The identification of trajectory membership may also be useful in evaluation of appropriate subjects for inclusion in studies of headache treatment after TBI.”
Headache is the most common symptom following TBI of any severity. Dr. Lucas and her research colleagues have previously reported high rates of headache in a prospective cohort of patients who experienced moderate to severe TBI. These patients have been followed for five years. New or worse headache occurred at a rate of 37% at three months, 33% at six months, 34% at 12 months, and 35% at 60 months post injury. The present study examined whether certain patterns or trajectories of headache occur over five years after TBI and whether demographic or injury characteristics are related to these trajectories. Trajectory type was also examined in relation to satisfaction with life and employment status five years after injury.
Data on 316 individuals were evaluated at five years. These patients were initially enrolled during inpatient rehabilitation after moderate to severe TBI. Enrollment was performed in person in the hospital. Structured telephone interviews were conducted at three, six, 12, and 60 months after TBI. At each time point, individuals who reported headache in the previous three months were asked to rate their headache pain on a 0 to 10 scale (0 = no pain, 10 = worst pain) and to complete the Headache Impact Test (HIT-6). Discrete mixture modeling was used to estimate trajectory groups based on pain rating and on headache impact scores.
Four trajectories were found for headache pain over five years: minimal pain over time (25% of individuals), worsening pain over time (37%), improving pain over time (7%), and chronic pain over time (30%). A chronic pain trajectory was more common in females, those incurring TBI by violence, those who were unemployed prior to injury, those with a headache history prior to injury, and those with mental health problems. Those with a chronic pain trajectory had significantly lower satisfaction with life five years after injury, compared with other trajectory groups.
A chronic impact trajectory was more common in females and in those who incurred TBI by violence, had a prior history of headache, or were unemployed prior to injury. At five years post TBI, the chronic impact group was significantly more likely to be unemployed and less satisfied with life, compared with individuals in the minimal or worsening impact trajectory groups. Those employed prior to injury were more frequently in the worsening group for pain and impact, compared with those not employed prior to injury. No relationship was found for other demographic and injury data, including age, posttraumatic amnesia, or substance abuse prior to injury.
BOSTON—Over five years after traumatic brain injury (TBI), a relatively large (24% to 30%) group of individuals experience chronic headache pain and a significant functional impact of headache, according to a pattern analysis of headache pain and impact following moderate to severe TBI. These results were presented at the 59th Annual Scientific Meeting of the American Headache Society. “Identification of members within these groups may be important to assist with an appropriate intensity of treatment to improve satisfaction with life and employment opportunity after TBI,” said Sylvia Lucas, MD, PhD, Clinical Professor of Neurology and Neurological Surgery at the University of Washington in Seattle. “The identification of trajectory membership may also be useful in evaluation of appropriate subjects for inclusion in studies of headache treatment after TBI.”
Headache is the most common symptom following TBI of any severity. Dr. Lucas and her research colleagues have previously reported high rates of headache in a prospective cohort of patients who experienced moderate to severe TBI. These patients have been followed for five years. New or worse headache occurred at a rate of 37% at three months, 33% at six months, 34% at 12 months, and 35% at 60 months post injury. The present study examined whether certain patterns or trajectories of headache occur over five years after TBI and whether demographic or injury characteristics are related to these trajectories. Trajectory type was also examined in relation to satisfaction with life and employment status five years after injury.
Data on 316 individuals were evaluated at five years. These patients were initially enrolled during inpatient rehabilitation after moderate to severe TBI. Enrollment was performed in person in the hospital. Structured telephone interviews were conducted at three, six, 12, and 60 months after TBI. At each time point, individuals who reported headache in the previous three months were asked to rate their headache pain on a 0 to 10 scale (0 = no pain, 10 = worst pain) and to complete the Headache Impact Test (HIT-6). Discrete mixture modeling was used to estimate trajectory groups based on pain rating and on headache impact scores.
Four trajectories were found for headache pain over five years: minimal pain over time (25% of individuals), worsening pain over time (37%), improving pain over time (7%), and chronic pain over time (30%). A chronic pain trajectory was more common in females, those incurring TBI by violence, those who were unemployed prior to injury, those with a headache history prior to injury, and those with mental health problems. Those with a chronic pain trajectory had significantly lower satisfaction with life five years after injury, compared with other trajectory groups.
A chronic impact trajectory was more common in females and in those who incurred TBI by violence, had a prior history of headache, or were unemployed prior to injury. At five years post TBI, the chronic impact group was significantly more likely to be unemployed and less satisfied with life, compared with individuals in the minimal or worsening impact trajectory groups. Those employed prior to injury were more frequently in the worsening group for pain and impact, compared with those not employed prior to injury. No relationship was found for other demographic and injury data, including age, posttraumatic amnesia, or substance abuse prior to injury.
Model Predicts Outcomes After AED Withdrawal
An evidence-based model allows neurologists to predict individual patient outcomes following the withdrawal of antiepileptic drugs (AEDs), according to research published in the July issue of Epilepsia. The model indicates a patient’s risk of relapse and chance of long-term seizure freedom. The model therefore might help physicians and patients make individualized choices about treatment, said the authors.
Patients with epilepsy who have achieved seizure freedom may want to discontinue their AEDs to avoid their associated side effects. Discontinuation raises the risk of seizure recurrence, however. Previous prognostic meta-analyses have been unable to calculate individual outcome predictors’ effect sizes because of the heterogeneous methods and reporting in the literature.
Analyzing Individual Participant Data
To overcome this limitation, Herm J. Lamberink, MD, a doctoral student at University Medical Center Utrecht in the Netherlands, and colleagues conducted a meta-analysis using individual participant data from previous studies. They reviewed PubMed and Embase for articles that reported on patients with epilepsy who were seizure-free and had started withdrawal of AEDs. Eligible articles contained information regarding seizure recurrences during and after withdrawal. The investigators selected 25 candidate predictors based on a systematic review of the predictors of seizure recurrence after AED withdrawal.
Dr. Lamberink and colleagues identified 45 studies that included 7,082 patients in all. The meta-analysis included 10 studies with 1,769 patients. The populations included selected and nonselected children and adults. Median follow-up time was 5.3 years. In all, 812 patients (46%) had seizure relapse, which was a higher rate than the average reported in the literature. Approximately 9% of participants for whom data were available had seizures in their last year of follow-up, which suggests that they had not regained lasting seizure control.
Model Had Stable Performance
Epilepsy duration before remission, seizure-free interval before AED withdrawal, age at onset of epilepsy, history of febrile seizures, number of seizures before remission, absence of a self-limiting epilepsy syndrome, developmental delay, and epileptiform abnormality on EEG before withdrawal were independent predictors of seizure recurrence. Epilepsy duration before remission, seizure-free interval before AED withdrawal, number of AEDs before withdrawal, female sex, family history of epilepsy, number of seizures before remission, focal seizures, and epileptiform abnormality on EEG before withdrawal were independent predictors of seizures in the last year of follow-up.
The adjusted concordance statistics for the model were 0.65 for predicting seizure recurrence and 0.71 for predicting long-term seizure freedom. Internal–external cross validation indicated that the model had good and stable performance in all cohorts.
One limitation of the study is that the population included only participants who attempted to withdraw AEDs. In addition, too few cases of epileptic encephalopathy and juvenile myoclonic epilepsy were included in the population to determine whether these disorders predict outcomes after AED withdrawal.
—Erik Greb
Suggested Reading
Lamberink HJ, Otte WM, Geerts AT, et al. Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. Lancet Neurol. 2017;16(7):523-531.
An evidence-based model allows neurologists to predict individual patient outcomes following the withdrawal of antiepileptic drugs (AEDs), according to research published in the July issue of Epilepsia. The model indicates a patient’s risk of relapse and chance of long-term seizure freedom. The model therefore might help physicians and patients make individualized choices about treatment, said the authors.
Patients with epilepsy who have achieved seizure freedom may want to discontinue their AEDs to avoid their associated side effects. Discontinuation raises the risk of seizure recurrence, however. Previous prognostic meta-analyses have been unable to calculate individual outcome predictors’ effect sizes because of the heterogeneous methods and reporting in the literature.
Analyzing Individual Participant Data
To overcome this limitation, Herm J. Lamberink, MD, a doctoral student at University Medical Center Utrecht in the Netherlands, and colleagues conducted a meta-analysis using individual participant data from previous studies. They reviewed PubMed and Embase for articles that reported on patients with epilepsy who were seizure-free and had started withdrawal of AEDs. Eligible articles contained information regarding seizure recurrences during and after withdrawal. The investigators selected 25 candidate predictors based on a systematic review of the predictors of seizure recurrence after AED withdrawal.
Dr. Lamberink and colleagues identified 45 studies that included 7,082 patients in all. The meta-analysis included 10 studies with 1,769 patients. The populations included selected and nonselected children and adults. Median follow-up time was 5.3 years. In all, 812 patients (46%) had seizure relapse, which was a higher rate than the average reported in the literature. Approximately 9% of participants for whom data were available had seizures in their last year of follow-up, which suggests that they had not regained lasting seizure control.
Model Had Stable Performance
Epilepsy duration before remission, seizure-free interval before AED withdrawal, age at onset of epilepsy, history of febrile seizures, number of seizures before remission, absence of a self-limiting epilepsy syndrome, developmental delay, and epileptiform abnormality on EEG before withdrawal were independent predictors of seizure recurrence. Epilepsy duration before remission, seizure-free interval before AED withdrawal, number of AEDs before withdrawal, female sex, family history of epilepsy, number of seizures before remission, focal seizures, and epileptiform abnormality on EEG before withdrawal were independent predictors of seizures in the last year of follow-up.
The adjusted concordance statistics for the model were 0.65 for predicting seizure recurrence and 0.71 for predicting long-term seizure freedom. Internal–external cross validation indicated that the model had good and stable performance in all cohorts.
One limitation of the study is that the population included only participants who attempted to withdraw AEDs. In addition, too few cases of epileptic encephalopathy and juvenile myoclonic epilepsy were included in the population to determine whether these disorders predict outcomes after AED withdrawal.
—Erik Greb
Suggested Reading
Lamberink HJ, Otte WM, Geerts AT, et al. Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. Lancet Neurol. 2017;16(7):523-531.
An evidence-based model allows neurologists to predict individual patient outcomes following the withdrawal of antiepileptic drugs (AEDs), according to research published in the July issue of Epilepsia. The model indicates a patient’s risk of relapse and chance of long-term seizure freedom. The model therefore might help physicians and patients make individualized choices about treatment, said the authors.
Patients with epilepsy who have achieved seizure freedom may want to discontinue their AEDs to avoid their associated side effects. Discontinuation raises the risk of seizure recurrence, however. Previous prognostic meta-analyses have been unable to calculate individual outcome predictors’ effect sizes because of the heterogeneous methods and reporting in the literature.
Analyzing Individual Participant Data
To overcome this limitation, Herm J. Lamberink, MD, a doctoral student at University Medical Center Utrecht in the Netherlands, and colleagues conducted a meta-analysis using individual participant data from previous studies. They reviewed PubMed and Embase for articles that reported on patients with epilepsy who were seizure-free and had started withdrawal of AEDs. Eligible articles contained information regarding seizure recurrences during and after withdrawal. The investigators selected 25 candidate predictors based on a systematic review of the predictors of seizure recurrence after AED withdrawal.
Dr. Lamberink and colleagues identified 45 studies that included 7,082 patients in all. The meta-analysis included 10 studies with 1,769 patients. The populations included selected and nonselected children and adults. Median follow-up time was 5.3 years. In all, 812 patients (46%) had seizure relapse, which was a higher rate than the average reported in the literature. Approximately 9% of participants for whom data were available had seizures in their last year of follow-up, which suggests that they had not regained lasting seizure control.
Model Had Stable Performance
Epilepsy duration before remission, seizure-free interval before AED withdrawal, age at onset of epilepsy, history of febrile seizures, number of seizures before remission, absence of a self-limiting epilepsy syndrome, developmental delay, and epileptiform abnormality on EEG before withdrawal were independent predictors of seizure recurrence. Epilepsy duration before remission, seizure-free interval before AED withdrawal, number of AEDs before withdrawal, female sex, family history of epilepsy, number of seizures before remission, focal seizures, and epileptiform abnormality on EEG before withdrawal were independent predictors of seizures in the last year of follow-up.
The adjusted concordance statistics for the model were 0.65 for predicting seizure recurrence and 0.71 for predicting long-term seizure freedom. Internal–external cross validation indicated that the model had good and stable performance in all cohorts.
One limitation of the study is that the population included only participants who attempted to withdraw AEDs. In addition, too few cases of epileptic encephalopathy and juvenile myoclonic epilepsy were included in the population to determine whether these disorders predict outcomes after AED withdrawal.
—Erik Greb
Suggested Reading
Lamberink HJ, Otte WM, Geerts AT, et al. Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. Lancet Neurol. 2017;16(7):523-531.
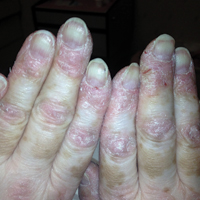
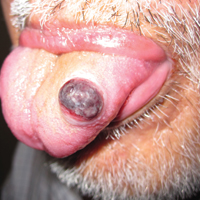
Sunny With a Chance of Skin Damage
A 56-year-old woman has several lesions she is worried might be cancerous. Added to that, there have been changes to her facial skin over the past several years that are increasingly obvious to her friends and family and therefore concerning to the patient.
She has an extensive history of nonmelanoma skin cancers, including basal cell carcinomas and squamous cell carcinomas, which were removed from her trunk in the distant past. She has lived in the southwestern United States all her life and has been smoking cigarettes since age 14.
EXAMINATION
The patient’s skin is quite fair, with abundant evidence of sun damage. She looks considerably older than her stated age.
Fortunately, no skin cancers are found on examination, but many closed and open comedones can be seen on both of her cheeks, stippled on rough, weathered skin. Solar elastosis manifests in this area as diffuse white thickening—what some might call “chicken skin.”

What is the diagnosis?
DISCUSSION
Favre-Racouchot syndrome (FRS) is a fairly common result of chronic overexposure to UV sources; it is especially prevalent among men who smoke. For reasons not fully understood, the changes associated with FRS tend to be relegated to the bilateral malar cheeks, roughly even with the eyes. Apart from the patient being female, this case is quite typical.
Chronic overexposure to UV light is known to result in dermatologic changes such as solar elastosis and the aforementioned whitish plaques—which, on microscopic exam, are simply basophilic degeneration of the dermis. This degeneration can be seen all over the face, but it is particularly evident on the forehead and cheeks; the concentration of comedones on the cheeks is unique to FRS.
Treatment options include lasers and peels, which involve considerable expenditure of time and money. While the comedones can be extracted, they are likely to recur unless more invasive methods are used.
TAKE-HOME LEARNING POINTS
- Favre-Racouchot syndrome (FRS) is seen primarily in men with chronic overexposure to sunlight—particularly those who smoke.
- FRS is characterized by localized collections of open and closed comedones superimposed on thickened, white “chicken skin” (solar elastosis).
- These changes typically occur on the bilateral cheeks of patients in the later decades of life, though they have been seen on patients in their 20s.
- Treatment is possible by means of laser resurfacing and/or chemical peels.
A 56-year-old woman has several lesions she is worried might be cancerous. Added to that, there have been changes to her facial skin over the past several years that are increasingly obvious to her friends and family and therefore concerning to the patient.
She has an extensive history of nonmelanoma skin cancers, including basal cell carcinomas and squamous cell carcinomas, which were removed from her trunk in the distant past. She has lived in the southwestern United States all her life and has been smoking cigarettes since age 14.
EXAMINATION
The patient’s skin is quite fair, with abundant evidence of sun damage. She looks considerably older than her stated age.
Fortunately, no skin cancers are found on examination, but many closed and open comedones can be seen on both of her cheeks, stippled on rough, weathered skin. Solar elastosis manifests in this area as diffuse white thickening—what some might call “chicken skin.”

What is the diagnosis?
DISCUSSION
Favre-Racouchot syndrome (FRS) is a fairly common result of chronic overexposure to UV sources; it is especially prevalent among men who smoke. For reasons not fully understood, the changes associated with FRS tend to be relegated to the bilateral malar cheeks, roughly even with the eyes. Apart from the patient being female, this case is quite typical.
Chronic overexposure to UV light is known to result in dermatologic changes such as solar elastosis and the aforementioned whitish plaques—which, on microscopic exam, are simply basophilic degeneration of the dermis. This degeneration can be seen all over the face, but it is particularly evident on the forehead and cheeks; the concentration of comedones on the cheeks is unique to FRS.
Treatment options include lasers and peels, which involve considerable expenditure of time and money. While the comedones can be extracted, they are likely to recur unless more invasive methods are used.
TAKE-HOME LEARNING POINTS
- Favre-Racouchot syndrome (FRS) is seen primarily in men with chronic overexposure to sunlight—particularly those who smoke.
- FRS is characterized by localized collections of open and closed comedones superimposed on thickened, white “chicken skin” (solar elastosis).
- These changes typically occur on the bilateral cheeks of patients in the later decades of life, though they have been seen on patients in their 20s.
- Treatment is possible by means of laser resurfacing and/or chemical peels.
A 56-year-old woman has several lesions she is worried might be cancerous. Added to that, there have been changes to her facial skin over the past several years that are increasingly obvious to her friends and family and therefore concerning to the patient.
She has an extensive history of nonmelanoma skin cancers, including basal cell carcinomas and squamous cell carcinomas, which were removed from her trunk in the distant past. She has lived in the southwestern United States all her life and has been smoking cigarettes since age 14.
EXAMINATION
The patient’s skin is quite fair, with abundant evidence of sun damage. She looks considerably older than her stated age.
Fortunately, no skin cancers are found on examination, but many closed and open comedones can be seen on both of her cheeks, stippled on rough, weathered skin. Solar elastosis manifests in this area as diffuse white thickening—what some might call “chicken skin.”

What is the diagnosis?
DISCUSSION
Favre-Racouchot syndrome (FRS) is a fairly common result of chronic overexposure to UV sources; it is especially prevalent among men who smoke. For reasons not fully understood, the changes associated with FRS tend to be relegated to the bilateral malar cheeks, roughly even with the eyes. Apart from the patient being female, this case is quite typical.
Chronic overexposure to UV light is known to result in dermatologic changes such as solar elastosis and the aforementioned whitish plaques—which, on microscopic exam, are simply basophilic degeneration of the dermis. This degeneration can be seen all over the face, but it is particularly evident on the forehead and cheeks; the concentration of comedones on the cheeks is unique to FRS.
Treatment options include lasers and peels, which involve considerable expenditure of time and money. While the comedones can be extracted, they are likely to recur unless more invasive methods are used.
TAKE-HOME LEARNING POINTS
- Favre-Racouchot syndrome (FRS) is seen primarily in men with chronic overexposure to sunlight—particularly those who smoke.
- FRS is characterized by localized collections of open and closed comedones superimposed on thickened, white “chicken skin” (solar elastosis).
- These changes typically occur on the bilateral cheeks of patients in the later decades of life, though they have been seen on patients in their 20s.
- Treatment is possible by means of laser resurfacing and/or chemical peels.
Lessons on using cannabinoids for pediatric epilepsy
DENVER – holds useful lessons for physicians in states where legal marijuana is a far more recent development, Amy R. Brooks-Kayal, MD, said at the annual meeting of the Teratology Society.
Medical marijuana has been legal in Colorado for nearly 20 years. But the drug’s potential role in treating intractable pediatric epilepsy started getting a lot more attention in 2013 when a CNN report by Sanjay Gupta, MD, chronicled a child’s remarkable turnaround in response to medical marijuana. The story triggered a migration to the state by what has been termed “marijuana refugees”: desperate families with children who had the most severe, complex, treatment-refractory seizure disorders, said Dr. Brooks-Kayal, professor of pediatrics and neurology and chief of pediatric neurology at the University of Colorado at Denver, Aurora.
The situation, fortunately, has improved. There is now phase 3 randomized, double-blind, placebo-controlled clinical trial evidence of efficacy for an investigational proprietary cannabidiol oral solution known as Epidiolex for children and young adults with Dravet syndrome and drug-resistant seizures, as well as documentation of multiple adverse effects (N Engl J Med. 2017 May 25;376[21]:2011-20).
Dr. Brooks-Kayal, a past president of the American Epilepsy Society, said she believes this medication is potentially approvable by the Food and Drug Administration.
“In the world of new seizure medications, what is usually required by the FDA is a 50% reduction in seizures, which this agent gets close to reaching. But it does have a higher adverse event rate than many of our medications. However, this is a tough crowd. These are very, very difficult-to-treat children. So I think any addition to our armamentarium for these kids is going to be beneficial,” she said. “Unfortunately, though, it’s not going to be the panacea that I think some of our families are looking for.”
Based upon the Colorado experience, Dr. Brooks-Kayal offered the following suggestions for colleagues around the country as they begin fielding questions from families about medical marijuana for pediatric epilepsy:
- Provide families with the current data, discuss what’s known and still unknown, and encourage families to disclose the use of cannabinoids so the child can be monitored.
- Have the family keep a seizure diary. Get a baseline EEG and another at about 12 weeks. Do routine laboratory monitoring every 4 weeks, including liver function tests. “We think CBDs [cannabinoids] have the potential to worsen liver function,” she said.
- Stress the importance of leaving other seizure medications unchanged. “When this first started, the medical marijuana providers were recommending patients stop their other medications. The providers don’t do that anymore, fortunately,” Dr. Brooks-Kayal said. “Every week we were putting a child in a medically induced coma because they had status epilepticus, and it was the only way to stop their seizures. They started using marijuana products, they were sure it was going to be the cure, they stopped all their other medications, and they developed status epilepticus.”
- Establish policies with the hospital administration and pharmacy about how to handle marijuana products when a child is in the hospital. The Children’s Hospital Colorado pharmacy cannot store or dispense marijuana products because of federal regulations. And again, it’s unsafe to stop seizure medications abruptly, including marijuana products. Informed consent procedures need to be developed for when patients on cannabinoids are hospitalized.
- Encourage families to participate in one of the six Food and Drug Administration–approved double-blind, placebo-controlled trials of Epidiolex for Dravet syndrome, Lennox-Gastaut syndrome, tuberous sclerosis complex, and infantile spasms sponsored by GW Pharmaceuticals.
Breaking down the evidence
Here’s what’s known and what is still unknown about the safety and efficacy of cannabinoids for the treatment of refractory pediatric epilepsy, according to Dr. Brooks-Kayal.
The knowns
Cannabinoids show activity against seizures in animal models. Moreover, initial clinical data suggest they may decrease seizures in some children with refractory epilepsy. This evidence includes a retrospective study from Children’s Hospital Colorado reliant upon parental reports of improvement (Epilepsy Behav. 2015 Apr;45:49-52), an Israeli retrospective study (Seizure. 2016 Feb;35:41-4), a positive open-label trial of an investigational oral oil-based solution of a pharmaceutical-grade cannabidiol known as Epidiolex (Lancet Neurol. 2016 Mar;15[3]:270-8), and evidence from a Food and Drug Administration–authorized phase 3, randomized clinical trial of Epidiolex (N Engl J Med. 2017 May 25;376[21]:2011-20).
The incidence of short-term adverse events associated with cannabinoids is substantial. The rate seems to be higher with Epidiolex than with many other medical marijuana products, although the potency is greater, too. These include somnolence, fatigue, and convulsions.
In addition, gastrointestinal side effects are common with Epidiolex. “Some are probably due to the oil base; some [are] probably due to the cannabidiol itself,” said Dr. Brooks-Kayal.
The unknowns
What types of seizures does it work for? This is under study in a series of FDA-authorized phase 3 randomized trials.
What is the placebo-subtracted response rate to cannabidiol? In the randomized trial published in the New England Journal of Medicine, the median monthly frequency of seizures decreased from 12.4 to 5.9 with cannabidiol, compared with a reduction from 14.9 to 14.1 with placebo. This needs confirmation in additional trials.
What’s the optimal dose? The randomized trial tested just one dose – 20 mg/kg per day.
What are the drug interactions and their possible impact on cannabidiol efficacy? Outcomes appear to be better in patients on concomitant clobazam (Onfi), perhaps because of the significantly higher blood levels of clobazam’s major metabolite in children on cannabidiol.
Long-term effects
The jury is still out on the long-term adverse effects. “These medical marijuana products are being given by families to 2- and 3-month-olds. It will be years before we know about potential long-term cognitive and behavioral effects,” Dr. Brooks-Kayal said.
Dr. Brooks-Kayal reported having no financial conflicts of interest regarding her presentation.
DENVER – holds useful lessons for physicians in states where legal marijuana is a far more recent development, Amy R. Brooks-Kayal, MD, said at the annual meeting of the Teratology Society.
Medical marijuana has been legal in Colorado for nearly 20 years. But the drug’s potential role in treating intractable pediatric epilepsy started getting a lot more attention in 2013 when a CNN report by Sanjay Gupta, MD, chronicled a child’s remarkable turnaround in response to medical marijuana. The story triggered a migration to the state by what has been termed “marijuana refugees”: desperate families with children who had the most severe, complex, treatment-refractory seizure disorders, said Dr. Brooks-Kayal, professor of pediatrics and neurology and chief of pediatric neurology at the University of Colorado at Denver, Aurora.
The situation, fortunately, has improved. There is now phase 3 randomized, double-blind, placebo-controlled clinical trial evidence of efficacy for an investigational proprietary cannabidiol oral solution known as Epidiolex for children and young adults with Dravet syndrome and drug-resistant seizures, as well as documentation of multiple adverse effects (N Engl J Med. 2017 May 25;376[21]:2011-20).
Dr. Brooks-Kayal, a past president of the American Epilepsy Society, said she believes this medication is potentially approvable by the Food and Drug Administration.
“In the world of new seizure medications, what is usually required by the FDA is a 50% reduction in seizures, which this agent gets close to reaching. But it does have a higher adverse event rate than many of our medications. However, this is a tough crowd. These are very, very difficult-to-treat children. So I think any addition to our armamentarium for these kids is going to be beneficial,” she said. “Unfortunately, though, it’s not going to be the panacea that I think some of our families are looking for.”
Based upon the Colorado experience, Dr. Brooks-Kayal offered the following suggestions for colleagues around the country as they begin fielding questions from families about medical marijuana for pediatric epilepsy:
- Provide families with the current data, discuss what’s known and still unknown, and encourage families to disclose the use of cannabinoids so the child can be monitored.
- Have the family keep a seizure diary. Get a baseline EEG and another at about 12 weeks. Do routine laboratory monitoring every 4 weeks, including liver function tests. “We think CBDs [cannabinoids] have the potential to worsen liver function,” she said.
- Stress the importance of leaving other seizure medications unchanged. “When this first started, the medical marijuana providers were recommending patients stop their other medications. The providers don’t do that anymore, fortunately,” Dr. Brooks-Kayal said. “Every week we were putting a child in a medically induced coma because they had status epilepticus, and it was the only way to stop their seizures. They started using marijuana products, they were sure it was going to be the cure, they stopped all their other medications, and they developed status epilepticus.”
- Establish policies with the hospital administration and pharmacy about how to handle marijuana products when a child is in the hospital. The Children’s Hospital Colorado pharmacy cannot store or dispense marijuana products because of federal regulations. And again, it’s unsafe to stop seizure medications abruptly, including marijuana products. Informed consent procedures need to be developed for when patients on cannabinoids are hospitalized.
- Encourage families to participate in one of the six Food and Drug Administration–approved double-blind, placebo-controlled trials of Epidiolex for Dravet syndrome, Lennox-Gastaut syndrome, tuberous sclerosis complex, and infantile spasms sponsored by GW Pharmaceuticals.
Breaking down the evidence
Here’s what’s known and what is still unknown about the safety and efficacy of cannabinoids for the treatment of refractory pediatric epilepsy, according to Dr. Brooks-Kayal.
The knowns
Cannabinoids show activity against seizures in animal models. Moreover, initial clinical data suggest they may decrease seizures in some children with refractory epilepsy. This evidence includes a retrospective study from Children’s Hospital Colorado reliant upon parental reports of improvement (Epilepsy Behav. 2015 Apr;45:49-52), an Israeli retrospective study (Seizure. 2016 Feb;35:41-4), a positive open-label trial of an investigational oral oil-based solution of a pharmaceutical-grade cannabidiol known as Epidiolex (Lancet Neurol. 2016 Mar;15[3]:270-8), and evidence from a Food and Drug Administration–authorized phase 3, randomized clinical trial of Epidiolex (N Engl J Med. 2017 May 25;376[21]:2011-20).
The incidence of short-term adverse events associated with cannabinoids is substantial. The rate seems to be higher with Epidiolex than with many other medical marijuana products, although the potency is greater, too. These include somnolence, fatigue, and convulsions.
In addition, gastrointestinal side effects are common with Epidiolex. “Some are probably due to the oil base; some [are] probably due to the cannabidiol itself,” said Dr. Brooks-Kayal.
The unknowns
What types of seizures does it work for? This is under study in a series of FDA-authorized phase 3 randomized trials.
What is the placebo-subtracted response rate to cannabidiol? In the randomized trial published in the New England Journal of Medicine, the median monthly frequency of seizures decreased from 12.4 to 5.9 with cannabidiol, compared with a reduction from 14.9 to 14.1 with placebo. This needs confirmation in additional trials.
What’s the optimal dose? The randomized trial tested just one dose – 20 mg/kg per day.
What are the drug interactions and their possible impact on cannabidiol efficacy? Outcomes appear to be better in patients on concomitant clobazam (Onfi), perhaps because of the significantly higher blood levels of clobazam’s major metabolite in children on cannabidiol.
Long-term effects
The jury is still out on the long-term adverse effects. “These medical marijuana products are being given by families to 2- and 3-month-olds. It will be years before we know about potential long-term cognitive and behavioral effects,” Dr. Brooks-Kayal said.
Dr. Brooks-Kayal reported having no financial conflicts of interest regarding her presentation.
DENVER – holds useful lessons for physicians in states where legal marijuana is a far more recent development, Amy R. Brooks-Kayal, MD, said at the annual meeting of the Teratology Society.
Medical marijuana has been legal in Colorado for nearly 20 years. But the drug’s potential role in treating intractable pediatric epilepsy started getting a lot more attention in 2013 when a CNN report by Sanjay Gupta, MD, chronicled a child’s remarkable turnaround in response to medical marijuana. The story triggered a migration to the state by what has been termed “marijuana refugees”: desperate families with children who had the most severe, complex, treatment-refractory seizure disorders, said Dr. Brooks-Kayal, professor of pediatrics and neurology and chief of pediatric neurology at the University of Colorado at Denver, Aurora.
The situation, fortunately, has improved. There is now phase 3 randomized, double-blind, placebo-controlled clinical trial evidence of efficacy for an investigational proprietary cannabidiol oral solution known as Epidiolex for children and young adults with Dravet syndrome and drug-resistant seizures, as well as documentation of multiple adverse effects (N Engl J Med. 2017 May 25;376[21]:2011-20).
Dr. Brooks-Kayal, a past president of the American Epilepsy Society, said she believes this medication is potentially approvable by the Food and Drug Administration.
“In the world of new seizure medications, what is usually required by the FDA is a 50% reduction in seizures, which this agent gets close to reaching. But it does have a higher adverse event rate than many of our medications. However, this is a tough crowd. These are very, very difficult-to-treat children. So I think any addition to our armamentarium for these kids is going to be beneficial,” she said. “Unfortunately, though, it’s not going to be the panacea that I think some of our families are looking for.”
Based upon the Colorado experience, Dr. Brooks-Kayal offered the following suggestions for colleagues around the country as they begin fielding questions from families about medical marijuana for pediatric epilepsy:
- Provide families with the current data, discuss what’s known and still unknown, and encourage families to disclose the use of cannabinoids so the child can be monitored.
- Have the family keep a seizure diary. Get a baseline EEG and another at about 12 weeks. Do routine laboratory monitoring every 4 weeks, including liver function tests. “We think CBDs [cannabinoids] have the potential to worsen liver function,” she said.
- Stress the importance of leaving other seizure medications unchanged. “When this first started, the medical marijuana providers were recommending patients stop their other medications. The providers don’t do that anymore, fortunately,” Dr. Brooks-Kayal said. “Every week we were putting a child in a medically induced coma because they had status epilepticus, and it was the only way to stop their seizures. They started using marijuana products, they were sure it was going to be the cure, they stopped all their other medications, and they developed status epilepticus.”
- Establish policies with the hospital administration and pharmacy about how to handle marijuana products when a child is in the hospital. The Children’s Hospital Colorado pharmacy cannot store or dispense marijuana products because of federal regulations. And again, it’s unsafe to stop seizure medications abruptly, including marijuana products. Informed consent procedures need to be developed for when patients on cannabinoids are hospitalized.
- Encourage families to participate in one of the six Food and Drug Administration–approved double-blind, placebo-controlled trials of Epidiolex for Dravet syndrome, Lennox-Gastaut syndrome, tuberous sclerosis complex, and infantile spasms sponsored by GW Pharmaceuticals.
Breaking down the evidence
Here’s what’s known and what is still unknown about the safety and efficacy of cannabinoids for the treatment of refractory pediatric epilepsy, according to Dr. Brooks-Kayal.
The knowns
Cannabinoids show activity against seizures in animal models. Moreover, initial clinical data suggest they may decrease seizures in some children with refractory epilepsy. This evidence includes a retrospective study from Children’s Hospital Colorado reliant upon parental reports of improvement (Epilepsy Behav. 2015 Apr;45:49-52), an Israeli retrospective study (Seizure. 2016 Feb;35:41-4), a positive open-label trial of an investigational oral oil-based solution of a pharmaceutical-grade cannabidiol known as Epidiolex (Lancet Neurol. 2016 Mar;15[3]:270-8), and evidence from a Food and Drug Administration–authorized phase 3, randomized clinical trial of Epidiolex (N Engl J Med. 2017 May 25;376[21]:2011-20).
The incidence of short-term adverse events associated with cannabinoids is substantial. The rate seems to be higher with Epidiolex than with many other medical marijuana products, although the potency is greater, too. These include somnolence, fatigue, and convulsions.
In addition, gastrointestinal side effects are common with Epidiolex. “Some are probably due to the oil base; some [are] probably due to the cannabidiol itself,” said Dr. Brooks-Kayal.
The unknowns
What types of seizures does it work for? This is under study in a series of FDA-authorized phase 3 randomized trials.
What is the placebo-subtracted response rate to cannabidiol? In the randomized trial published in the New England Journal of Medicine, the median monthly frequency of seizures decreased from 12.4 to 5.9 with cannabidiol, compared with a reduction from 14.9 to 14.1 with placebo. This needs confirmation in additional trials.
What’s the optimal dose? The randomized trial tested just one dose – 20 mg/kg per day.
What are the drug interactions and their possible impact on cannabidiol efficacy? Outcomes appear to be better in patients on concomitant clobazam (Onfi), perhaps because of the significantly higher blood levels of clobazam’s major metabolite in children on cannabidiol.
Long-term effects
The jury is still out on the long-term adverse effects. “These medical marijuana products are being given by families to 2- and 3-month-olds. It will be years before we know about potential long-term cognitive and behavioral effects,” Dr. Brooks-Kayal said.
Dr. Brooks-Kayal reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM TERATOLOGY SOCIETY 2017
Amyopathic Dermatomyositis With Plantar Keratoderma Responding to Methotrexate Therapy
Case Report
A 54-year-old woman presented with a painful pruritic rash on the hands and feet of 7 years’ duration. She reported intermittent joint pain but denied muscle weakness. Physical examination revealed fissured fingertips and heavy scaling of the palms and lateral fingers (Figure 1). Violaceous scaly papules were seen on the distal and proximal interphalangeal joints (Figure 2). A severe plantar keratoderma also was noted (Figure 3). Pink scaly plaques were present on the bilateral elbows and postauricular skin. Diffuse mat telangiectases covered the malar skin. Extensive poikilodermatous skin changes covered approximately 20% of the total body surface area. Salt-and-pepper patches and papules were noted over the bilateral thighs. She reported an uncertain history of recent radiographs of one or both hands, which showed no joint degeneration characteristic of psoriatic arthritis. She previously had been given a diagnosis of psoriasis by an outside dermatologist but was not responding to topical therapy.

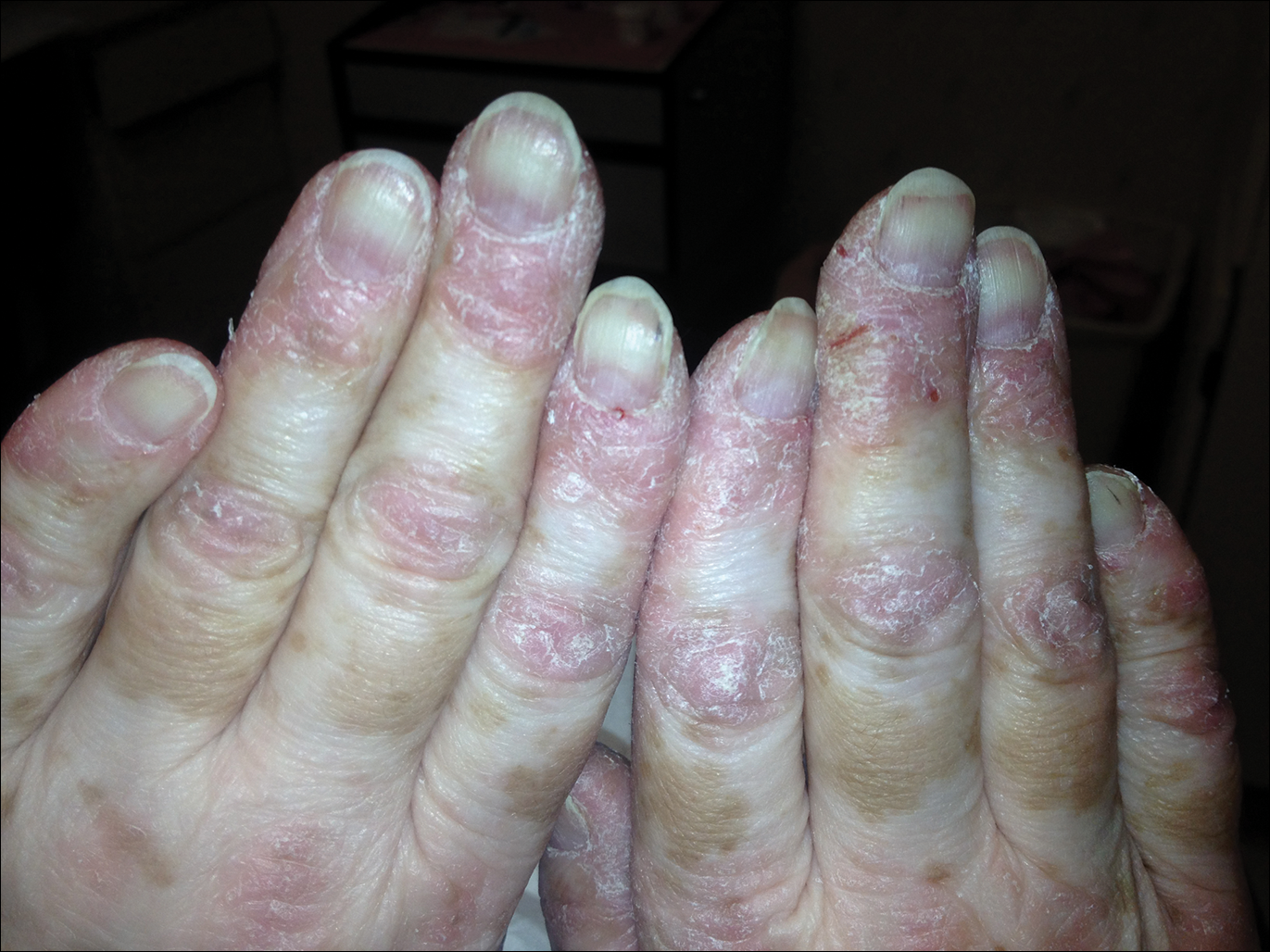
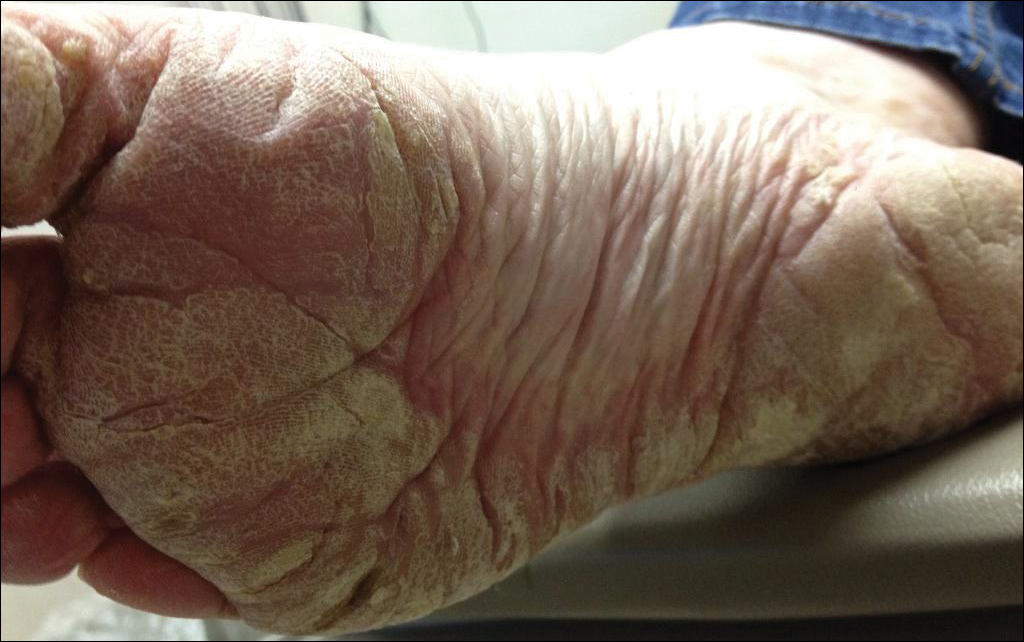
Several skin biopsies showed histologic evidence of dermatomyositis (DM)(Figure 4). Prominent basement thickening also was seen on periodic acid–Schiff staining (not shown). Laboratory workup showed negative antinuclear antibodies and anti–Jo-1, anti-Ku, and anti-Mi2 antibodies. Muscle enzymes including creatinine kinase and aldolase were within reference range. Pelvic ultrasonography and mammography were negative. Pulmonary function tests were unremarkable. High-resolution chest computed tomography (CT) was ordered because of a history of chronic cough; however, no evidence of malignancy or interstitial lung disease was seen. The patient was diagnosed with amyopathic dermatomyositis (ADM). Rheumatology was consulted and initiated oral hydroxychloroquine therapy. After 3 months, the patient’s cutaneous disease did not respond and she reported having headaches associated with this medication; therefore, methotrexate was started. Within 2 months of treatment, full resolution of the plantar keratoderma (Figure 5) and clearance of the scaling/fissuring of the hands as well as the psoriatic-appearing plaques on the elbows was noted.
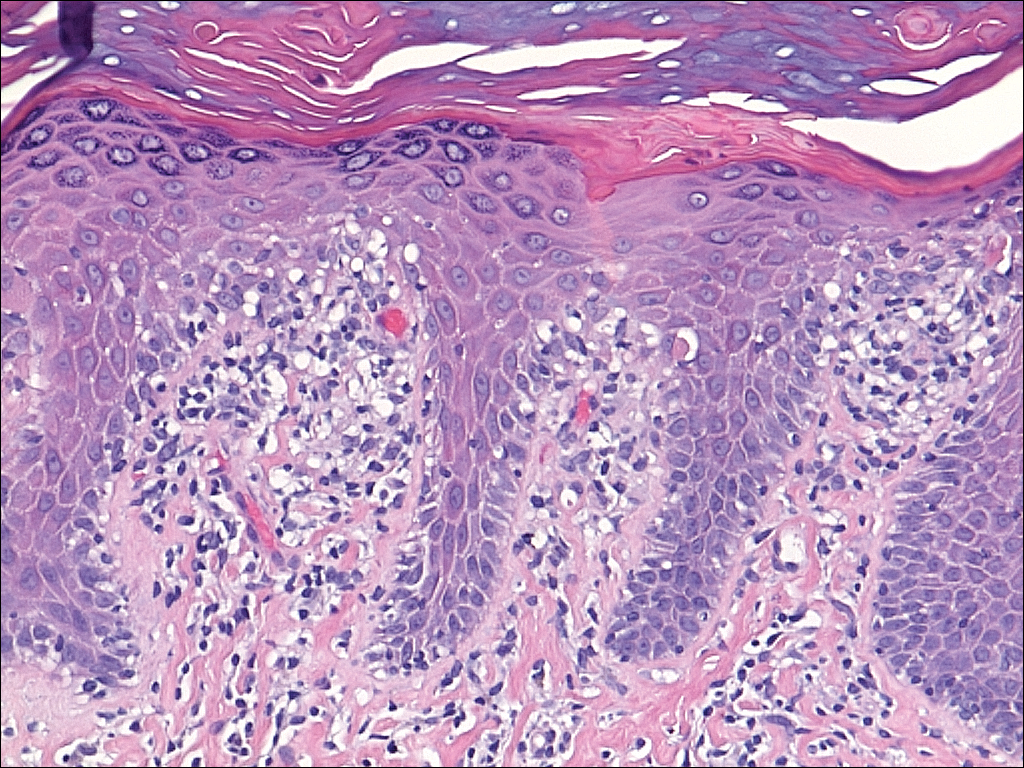
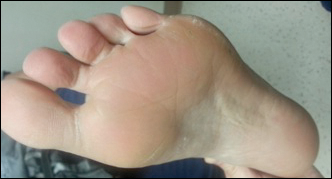
Comment
Amyopathic DM is a subset of DM that accounts for 10% to 20% of DM cases.1,2 Sontheimer’s3 diagnostic criteria for ADM require histopathologic confirmation of the hallmark skin findings of classic DM and lack of muscle weakness or muscle enzyme (creatine kinase/aldolase) elevation for at least 2 years.
Similar to classic DM, ADM typically presents in the fifth decade of life and has a female predilection.1,4 The term hypomyopathic DM is used to describe patients who exhibit classic skin findings and evidence of muscle involvement on magnetic resonance imaging, electromyography, biopsy, or serum enzymes but have no clinical evidence of muscle weakness for at least 6 months. Together, hypomyopathic DM and ADM are referred to as clinically ADM (CADM). Patients who have met the criteria for hypomyopathic DM or ADM may later develop frank myopathy, progressing to a diagnosis of CADM, which may occur in as many as 10% to 13% of cases of CADM.1,2 Clinical evidence of muscle weakness typically is heralded by elevation of creatine kinase and aldolase; therefore, patients with ADM should have muscle enzymes periodically checked.
Cutaneous findings of ADM are the same as the hallmark skin findings in CADM.3 Poikiloderma appears as thin telangiectatic skin in a background of mottled hyperpigmentation and hypopigmentation. It represents chronic inflammation and often occurs in sun-exposed areas. Poikiloderma located on the posterior neck and shoulders is known as the shawl sign and on the lateral thighs as the holster sign.5 The term mechanic’s hands is used to describe the clinical finding of palmar erythema with scaling and fissuring of the fingertips.6 Scalp findings include erythematous, atrophic, scaly plaques resembling psoriasis and nonscarring alopecia.7 Gottron papules are nearly pathognomonic for DM. These violaceous papules often are pruritic and found over the finger joints, in contrast to the hand rash of lupus erythematosus that involves the skin between finger joints.8 Psoriatic-appearing plaques overlying the elbows and knees are known as Gottron sign and can contribute to misdiagnosis as psoriasis.8 The classic heliotrope rash presents as a violaceous hue in the periorbital area and may be associated with periorbital edema.9 Calcinosis cutis is common in CADM but rarely is reported in ADM.10 Nail findings include periungual hyperemia, cuticular overgrowth, and nail bed changes due to avascular areas and dilated capillaries. The cutaneous histopathologic findings in ADM are the same as with CADM: a smudged dermoepidermal interface, vacuolar alterations of the basal layer, and dermal mucin deposits.
Palmoplantar keratoderma rarely is reported as a cutaneous finding in DM. The finding of keratoderma has mainly been reported in association with Wong-type DM, a rare subtype of DM with features of pityriasis rubra pilaris.11-13 Palmoplantar keratoderma also has been reported in a case of an ADM-like hydroxyurea-induced eruption14 and as an early presenting feature in one patient with CADM and one with juvenile DM.15,16
The autoantibody profile in patients with ADM varies from that of CADM and can be helpful in both diagnosis and prognosis. Similar to CADM, the majority of patients with ADM have positive antinuclear antibodies.2,17 Anti–Jo-1 (an anti–aminoacyl-transfer RNA synthetase) antibody frequently is found in CADM but rarely in ADM.2 Anti–Jo-1 is predictive of interstitial lung disease (ILD) in CADM. Positive anti–Jo-1 in combination with Raynaud phenomenon and mechanic’s hands is referred to as antisynthetase syndrome in patients with CADM.18,19 An antibody uniquely linked with CADM is the anti–CADM-140/MDA5 antibody and can be a marker of rapidly progressing ILD in these patients.20 Anti–Mi-2 is another myositis-specific antibody not commonly found in ADM but is present in 15% to 30% of DM cases.2,21 In CADM, the anti–Mi-2 antibody is associated with the shawl sign, ragged cuticles, and carpal tunnel syndrome and has a favorable prognosis.17,21 Myositis-associated autoantibodies (eg, anti-Ku) are found in patients with symptoms overlapping both DM and scleroderma or other connective tissue diseases.22 More recently described, the anti-p155/140 antibody is highly specific (up to 89%) for occult malignancy in DM.23
Lung disease is an important association in ADM. When it develops, it may be more aggressive compared to lung disease associated with CADM.24-26 In a systematic review of 197 cases of ADM by Gerami et al,2 10% of patients had ILD, and it was fatal in 42% of cases. Most cases of ILD associated with CADM were diagnosed as interstitial pneumonitis or diffuse alveolar disease; bronchiolitis obliterans organizing pneumonia and basilar fibrosis also were recorded.2 Anti–Jo-1 antibodies often accompany lung disease in CADM but are not typically found in lung disease associated with ADM. The anti–CADM-140/MDA5 antibody is associated with an increased risk for rapidly progressing ILD in patients with CADM.20 Recommended baseline screening for lung disease in DM includes chest radiography, pulmonary function tests with diffusion capacity,8 and in some instances high-resolution chest CT.27 Follow-up visits should include screening for symptoms of ILD such as cough, shortness of breath, or dyspnea. Treatment of myopathy-associated ILD is systemic steroids combined with various immunosuppressants including cyclophosphamide, azathioprine, mycophenolate mofetil, cyclosporine, tacrolimus, and intravenous immunoglobulin.28,29
The risk of malignancy in ADM is thought to be similar to the rate of 20% to 25% found in CADM.1,30-32 The most commonly reported malignancies associated with ADM are nasopharyngeal, breast, lung, ovarian, colorectal, pancreatic, and stomach cancers and lymphoma/leukemia.2,33 Patients with ADM should be screened for malignancy at diagnosis, then yearly for 3 years.8,31,33 In addition to history, physical examination, and age/sex-appropriate screening, a complete blood cell count, chemistry panel, urinalysis, stool guaiac, CA 125, CA 19-9, chest radiograph, and abdominal ultrasound should be performed. For women, mammography and pelvic ultrasonography should be completed.31 Some experts also recommend a full-body CT scan. Because Asian patients have a higher risk for nasopharyngeal carcinoma, referral to an ear, nose, and throat surgeon for direct visualization also can be considered.33 The risk of cancer in patients with DM compared to the general population is increased for at least the first 5 years after diagnosis, but most associated cancers are found within the first 3 years.34
Several therapies have been found useful in ADM. Because lesions often are photoexacerbated, sun protection is essential. Antimalarials such as hydroxychloroquine are considered first-line therapy. Clinicians must be aware of 2 possible hydroxychloroquine side effects that can uniquely confuse the clinical picture in ADM. The first is a rash, most often morbilliform and pruritic, that occurs in DM more frequently than in other diseases.35 The second is a myopathy found in as many as 6.7% of patients using antimalarials for rheumatic disease,36 which can clinically mimic the progression of ADM to CADM.37 Two small retrospective case series found that methotrexate was beneficial in ADM.38,39 Methotrexate also has been reported as an efficacious treatment of ILD in patients with connective tissue diseases.40,41 Intravenous immunoglobulin and other immunosuppressants are additional agents to be considered.42
In summary, ADM is an important subset of DM and is more likely to present to dermatology practices than to other specialists. Amyopathic DM shares cutaneous findings with DM, and both overlap and differ with respect to other key disease characteristics including autoantibody profile, associated lung disease, and malignancy risk. Palmoplantar keratoderma is a rarely reported skin finding in DM. We report a case of ADM with the unique finding of severe plantar keratoderma. The fact that our patient’s keratoderma and other skin findings resolved concomitantly during methotrexate therapy leads us to believe that the keratoderma was a unique skin manifestation of the ADM itself.
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26-30.
- Gerami P, Schope JM, McDonald L, et al. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597-613.
- Sontheimer RD. Cutaneous features of classic dermatomyositis and amyopathic dermatomyositis. Curr Opin Rheumatol. 1999;11:475-482.
- Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23-27.
- Marvi U, Chung L, Fiorentino DF. Clinical presentation and evaluation of dermatomyositis. Indian J Dermatol. 2012;57:375-381.
- Stahl NI, Klippel JH, Decker JL. A cutaneous lesion associated with myositis. Ann Intern Med. 1979;91:577-579.
- Kasteler JS, Callen JP. Scalp involvement in dermatomyositis. often overlooked or misdiagnosed. JAMA. 1994;272:1939-1941.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Russo T, Piccolo V, Ruocco E, et al. The heliotrope sign of dermatomyositis: the correct meaning of the term heliotrope. Arch Dermatol. 2012;148:1178.
- Peñate Y, Guillermo N, Melwani P, et al. Calcinosis cutis associated with amyopathic dermatomyositis: response to intravenous immunoglobulin. J Am Acad Dermatol. 2009;60:1076-1077.
- Requena L, Grilli R, Soriano L, et al. Dermatomyositis with a pityriasis rubra pilaris-like eruption: a little-known distinctive cutaneous manifestation of dermatomyositis. Br J Dermatol. 1997;136:768-771.
- Lupton JR, Figueroa P, Berberian BJ, et al. An unusual presentation of dermatomyositis: the type Wong variant revisited. J Am Acad Dermatol. 2000;43(5 part 2):908-912.
- Caporali R, Cavagna L, Bellosta M, et al. Inflammatory myopathy in a patient with cutaneous findings of pityriasis rubra pilaris: a case of Wong’s dermatomyositis. Clin Rheumatol. 2004;23:63-65.
- Nofal A, El-Din ES. Hydroxyurea-induced dermatomyositis: true amyopathic dermatomyositis or dermatomyositis-like eruption? Int J Dermatol. 2012;51:535-541.
- See Y, Rooney M, Woo P. Palmar plantar hyperkeratosis—a previously undescribed skin manifestation of juvenile dermatomyositis. Br J Rheumatol. 1997;36(8):917-919.
- Chang LY, Yang LJ, Wu YJJ. Keratoderma plantaris and mechanic’s hands as the initial presentation in a case of dermatomyositis. Dermatol Sinica. 2002;20:329-334.
- Love L, Leff R, Fraser D, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore). 1991;70:360-374.
- Marguerie C, Bunn CC, Beynon HL, et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med. 1990;77:1019-1038.
- Marie I, Hatron PY, Hachulla E, et al. Pulmonary involvement in polymyositis and in dermatomyositis. J Rheumatol. 1998;25:1336-1343.
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571-1576.
- Dimachkie MM. Idiopathic inflammatory myopathies. J Neuroimmunol. 2011;231:32-42.
- Betteridge ZE, Gunawardena H, McHugh NJ. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther. 2011;13:209.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, et al. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol. 2010;22:627-632.
- Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis, and amyopathic dermatomyositis. Rheumatology (Oxford). 2005;44:1282-1286.
- Ye S, Chen XX, Lu XY, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol. 2007;26:1647-1654.
- Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341-1347.
- Fathi M, Dastmalchi, M, Rasmussen E, et al. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63:297-301.
- Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med. 2010;31:501-512.
- Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med. 2013;107:890-896.
- Klein RQ, Teal V, Taylor L, et al. Number, characteristics, and classification of patients with dermatomyositis seen by dermatology and rheumatology departments at a large tertiary medical center. J Am Acad Dermatol. 2007;57:937-943.
- Sontheimer RD. Clinically amyopathic dermatomyositis: what can we now tell our patients? Arch Dermatol. 2010;146:76-80.
- Azuma K, Yamada H, Ohkubo M, et al. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod Rheumatol. 2011;21:178-183.
- Femia AN, Vleugels RA, Callen JP. Cutaneous dermatomyositis: an updated review of treatment options and internal associations. Am J Clin Dermatol. 2013;14:291-313.
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095.
- Pelle MT, Callen JP. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol. 2002;138:1231-1233.
- Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385-390.
- Zieglschmid-Adams ME, Pandya AG, Cohen SB, et al. Treatment of dermatomyositis with methotrexate. J Am Acad Dermatol. 1995;32(5, pt 1):754-757.
- Foulke G, Baccon J, Marks JG, et al. Antimalarial myopathy in amyopathic dermatomyositis. Arch Dermatol. 2012;148:1100-1101.
- Kasteler JS, Callen JP. Low-dose methotrexate administered weekly is an effective corticosteroid-sparing agent for the treatment of the cutaneous manifestations of dermatomyositis. J Am Acad Dermatol. 1997;36:67-71.
- Scott DG, Bacon PA. Response to methotrexate in fibrosing alveolitis associated with connective tissue disease. Thorax. 1980;35:725-731.
- Fink SD, Kremer JM. Successful treatment of interstitial lung disease in systemic lupus erythematosus with methotrexate. J Rheumatol. 1995;22:967-969.
- Ernste FC, Reed AM. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88:83-105.
Case Report
A 54-year-old woman presented with a painful pruritic rash on the hands and feet of 7 years’ duration. She reported intermittent joint pain but denied muscle weakness. Physical examination revealed fissured fingertips and heavy scaling of the palms and lateral fingers (Figure 1). Violaceous scaly papules were seen on the distal and proximal interphalangeal joints (Figure 2). A severe plantar keratoderma also was noted (Figure 3). Pink scaly plaques were present on the bilateral elbows and postauricular skin. Diffuse mat telangiectases covered the malar skin. Extensive poikilodermatous skin changes covered approximately 20% of the total body surface area. Salt-and-pepper patches and papules were noted over the bilateral thighs. She reported an uncertain history of recent radiographs of one or both hands, which showed no joint degeneration characteristic of psoriatic arthritis. She previously had been given a diagnosis of psoriasis by an outside dermatologist but was not responding to topical therapy.



Several skin biopsies showed histologic evidence of dermatomyositis (DM)(Figure 4). Prominent basement thickening also was seen on periodic acid–Schiff staining (not shown). Laboratory workup showed negative antinuclear antibodies and anti–Jo-1, anti-Ku, and anti-Mi2 antibodies. Muscle enzymes including creatinine kinase and aldolase were within reference range. Pelvic ultrasonography and mammography were negative. Pulmonary function tests were unremarkable. High-resolution chest computed tomography (CT) was ordered because of a history of chronic cough; however, no evidence of malignancy or interstitial lung disease was seen. The patient was diagnosed with amyopathic dermatomyositis (ADM). Rheumatology was consulted and initiated oral hydroxychloroquine therapy. After 3 months, the patient’s cutaneous disease did not respond and she reported having headaches associated with this medication; therefore, methotrexate was started. Within 2 months of treatment, full resolution of the plantar keratoderma (Figure 5) and clearance of the scaling/fissuring of the hands as well as the psoriatic-appearing plaques on the elbows was noted.


Comment
Amyopathic DM is a subset of DM that accounts for 10% to 20% of DM cases.1,2 Sontheimer’s3 diagnostic criteria for ADM require histopathologic confirmation of the hallmark skin findings of classic DM and lack of muscle weakness or muscle enzyme (creatine kinase/aldolase) elevation for at least 2 years.
Similar to classic DM, ADM typically presents in the fifth decade of life and has a female predilection.1,4 The term hypomyopathic DM is used to describe patients who exhibit classic skin findings and evidence of muscle involvement on magnetic resonance imaging, electromyography, biopsy, or serum enzymes but have no clinical evidence of muscle weakness for at least 6 months. Together, hypomyopathic DM and ADM are referred to as clinically ADM (CADM). Patients who have met the criteria for hypomyopathic DM or ADM may later develop frank myopathy, progressing to a diagnosis of CADM, which may occur in as many as 10% to 13% of cases of CADM.1,2 Clinical evidence of muscle weakness typically is heralded by elevation of creatine kinase and aldolase; therefore, patients with ADM should have muscle enzymes periodically checked.
Cutaneous findings of ADM are the same as the hallmark skin findings in CADM.3 Poikiloderma appears as thin telangiectatic skin in a background of mottled hyperpigmentation and hypopigmentation. It represents chronic inflammation and often occurs in sun-exposed areas. Poikiloderma located on the posterior neck and shoulders is known as the shawl sign and on the lateral thighs as the holster sign.5 The term mechanic’s hands is used to describe the clinical finding of palmar erythema with scaling and fissuring of the fingertips.6 Scalp findings include erythematous, atrophic, scaly plaques resembling psoriasis and nonscarring alopecia.7 Gottron papules are nearly pathognomonic for DM. These violaceous papules often are pruritic and found over the finger joints, in contrast to the hand rash of lupus erythematosus that involves the skin between finger joints.8 Psoriatic-appearing plaques overlying the elbows and knees are known as Gottron sign and can contribute to misdiagnosis as psoriasis.8 The classic heliotrope rash presents as a violaceous hue in the periorbital area and may be associated with periorbital edema.9 Calcinosis cutis is common in CADM but rarely is reported in ADM.10 Nail findings include periungual hyperemia, cuticular overgrowth, and nail bed changes due to avascular areas and dilated capillaries. The cutaneous histopathologic findings in ADM are the same as with CADM: a smudged dermoepidermal interface, vacuolar alterations of the basal layer, and dermal mucin deposits.
Palmoplantar keratoderma rarely is reported as a cutaneous finding in DM. The finding of keratoderma has mainly been reported in association with Wong-type DM, a rare subtype of DM with features of pityriasis rubra pilaris.11-13 Palmoplantar keratoderma also has been reported in a case of an ADM-like hydroxyurea-induced eruption14 and as an early presenting feature in one patient with CADM and one with juvenile DM.15,16
The autoantibody profile in patients with ADM varies from that of CADM and can be helpful in both diagnosis and prognosis. Similar to CADM, the majority of patients with ADM have positive antinuclear antibodies.2,17 Anti–Jo-1 (an anti–aminoacyl-transfer RNA synthetase) antibody frequently is found in CADM but rarely in ADM.2 Anti–Jo-1 is predictive of interstitial lung disease (ILD) in CADM. Positive anti–Jo-1 in combination with Raynaud phenomenon and mechanic’s hands is referred to as antisynthetase syndrome in patients with CADM.18,19 An antibody uniquely linked with CADM is the anti–CADM-140/MDA5 antibody and can be a marker of rapidly progressing ILD in these patients.20 Anti–Mi-2 is another myositis-specific antibody not commonly found in ADM but is present in 15% to 30% of DM cases.2,21 In CADM, the anti–Mi-2 antibody is associated with the shawl sign, ragged cuticles, and carpal tunnel syndrome and has a favorable prognosis.17,21 Myositis-associated autoantibodies (eg, anti-Ku) are found in patients with symptoms overlapping both DM and scleroderma or other connective tissue diseases.22 More recently described, the anti-p155/140 antibody is highly specific (up to 89%) for occult malignancy in DM.23
Lung disease is an important association in ADM. When it develops, it may be more aggressive compared to lung disease associated with CADM.24-26 In a systematic review of 197 cases of ADM by Gerami et al,2 10% of patients had ILD, and it was fatal in 42% of cases. Most cases of ILD associated with CADM were diagnosed as interstitial pneumonitis or diffuse alveolar disease; bronchiolitis obliterans organizing pneumonia and basilar fibrosis also were recorded.2 Anti–Jo-1 antibodies often accompany lung disease in CADM but are not typically found in lung disease associated with ADM. The anti–CADM-140/MDA5 antibody is associated with an increased risk for rapidly progressing ILD in patients with CADM.20 Recommended baseline screening for lung disease in DM includes chest radiography, pulmonary function tests with diffusion capacity,8 and in some instances high-resolution chest CT.27 Follow-up visits should include screening for symptoms of ILD such as cough, shortness of breath, or dyspnea. Treatment of myopathy-associated ILD is systemic steroids combined with various immunosuppressants including cyclophosphamide, azathioprine, mycophenolate mofetil, cyclosporine, tacrolimus, and intravenous immunoglobulin.28,29
The risk of malignancy in ADM is thought to be similar to the rate of 20% to 25% found in CADM.1,30-32 The most commonly reported malignancies associated with ADM are nasopharyngeal, breast, lung, ovarian, colorectal, pancreatic, and stomach cancers and lymphoma/leukemia.2,33 Patients with ADM should be screened for malignancy at diagnosis, then yearly for 3 years.8,31,33 In addition to history, physical examination, and age/sex-appropriate screening, a complete blood cell count, chemistry panel, urinalysis, stool guaiac, CA 125, CA 19-9, chest radiograph, and abdominal ultrasound should be performed. For women, mammography and pelvic ultrasonography should be completed.31 Some experts also recommend a full-body CT scan. Because Asian patients have a higher risk for nasopharyngeal carcinoma, referral to an ear, nose, and throat surgeon for direct visualization also can be considered.33 The risk of cancer in patients with DM compared to the general population is increased for at least the first 5 years after diagnosis, but most associated cancers are found within the first 3 years.34
Several therapies have been found useful in ADM. Because lesions often are photoexacerbated, sun protection is essential. Antimalarials such as hydroxychloroquine are considered first-line therapy. Clinicians must be aware of 2 possible hydroxychloroquine side effects that can uniquely confuse the clinical picture in ADM. The first is a rash, most often morbilliform and pruritic, that occurs in DM more frequently than in other diseases.35 The second is a myopathy found in as many as 6.7% of patients using antimalarials for rheumatic disease,36 which can clinically mimic the progression of ADM to CADM.37 Two small retrospective case series found that methotrexate was beneficial in ADM.38,39 Methotrexate also has been reported as an efficacious treatment of ILD in patients with connective tissue diseases.40,41 Intravenous immunoglobulin and other immunosuppressants are additional agents to be considered.42
In summary, ADM is an important subset of DM and is more likely to present to dermatology practices than to other specialists. Amyopathic DM shares cutaneous findings with DM, and both overlap and differ with respect to other key disease characteristics including autoantibody profile, associated lung disease, and malignancy risk. Palmoplantar keratoderma is a rarely reported skin finding in DM. We report a case of ADM with the unique finding of severe plantar keratoderma. The fact that our patient’s keratoderma and other skin findings resolved concomitantly during methotrexate therapy leads us to believe that the keratoderma was a unique skin manifestation of the ADM itself.
Case Report
A 54-year-old woman presented with a painful pruritic rash on the hands and feet of 7 years’ duration. She reported intermittent joint pain but denied muscle weakness. Physical examination revealed fissured fingertips and heavy scaling of the palms and lateral fingers (Figure 1). Violaceous scaly papules were seen on the distal and proximal interphalangeal joints (Figure 2). A severe plantar keratoderma also was noted (Figure 3). Pink scaly plaques were present on the bilateral elbows and postauricular skin. Diffuse mat telangiectases covered the malar skin. Extensive poikilodermatous skin changes covered approximately 20% of the total body surface area. Salt-and-pepper patches and papules were noted over the bilateral thighs. She reported an uncertain history of recent radiographs of one or both hands, which showed no joint degeneration characteristic of psoriatic arthritis. She previously had been given a diagnosis of psoriasis by an outside dermatologist but was not responding to topical therapy.



Several skin biopsies showed histologic evidence of dermatomyositis (DM)(Figure 4). Prominent basement thickening also was seen on periodic acid–Schiff staining (not shown). Laboratory workup showed negative antinuclear antibodies and anti–Jo-1, anti-Ku, and anti-Mi2 antibodies. Muscle enzymes including creatinine kinase and aldolase were within reference range. Pelvic ultrasonography and mammography were negative. Pulmonary function tests were unremarkable. High-resolution chest computed tomography (CT) was ordered because of a history of chronic cough; however, no evidence of malignancy or interstitial lung disease was seen. The patient was diagnosed with amyopathic dermatomyositis (ADM). Rheumatology was consulted and initiated oral hydroxychloroquine therapy. After 3 months, the patient’s cutaneous disease did not respond and she reported having headaches associated with this medication; therefore, methotrexate was started. Within 2 months of treatment, full resolution of the plantar keratoderma (Figure 5) and clearance of the scaling/fissuring of the hands as well as the psoriatic-appearing plaques on the elbows was noted.


Comment
Amyopathic DM is a subset of DM that accounts for 10% to 20% of DM cases.1,2 Sontheimer’s3 diagnostic criteria for ADM require histopathologic confirmation of the hallmark skin findings of classic DM and lack of muscle weakness or muscle enzyme (creatine kinase/aldolase) elevation for at least 2 years.
Similar to classic DM, ADM typically presents in the fifth decade of life and has a female predilection.1,4 The term hypomyopathic DM is used to describe patients who exhibit classic skin findings and evidence of muscle involvement on magnetic resonance imaging, electromyography, biopsy, or serum enzymes but have no clinical evidence of muscle weakness for at least 6 months. Together, hypomyopathic DM and ADM are referred to as clinically ADM (CADM). Patients who have met the criteria for hypomyopathic DM or ADM may later develop frank myopathy, progressing to a diagnosis of CADM, which may occur in as many as 10% to 13% of cases of CADM.1,2 Clinical evidence of muscle weakness typically is heralded by elevation of creatine kinase and aldolase; therefore, patients with ADM should have muscle enzymes periodically checked.
Cutaneous findings of ADM are the same as the hallmark skin findings in CADM.3 Poikiloderma appears as thin telangiectatic skin in a background of mottled hyperpigmentation and hypopigmentation. It represents chronic inflammation and often occurs in sun-exposed areas. Poikiloderma located on the posterior neck and shoulders is known as the shawl sign and on the lateral thighs as the holster sign.5 The term mechanic’s hands is used to describe the clinical finding of palmar erythema with scaling and fissuring of the fingertips.6 Scalp findings include erythematous, atrophic, scaly plaques resembling psoriasis and nonscarring alopecia.7 Gottron papules are nearly pathognomonic for DM. These violaceous papules often are pruritic and found over the finger joints, in contrast to the hand rash of lupus erythematosus that involves the skin between finger joints.8 Psoriatic-appearing plaques overlying the elbows and knees are known as Gottron sign and can contribute to misdiagnosis as psoriasis.8 The classic heliotrope rash presents as a violaceous hue in the periorbital area and may be associated with periorbital edema.9 Calcinosis cutis is common in CADM but rarely is reported in ADM.10 Nail findings include periungual hyperemia, cuticular overgrowth, and nail bed changes due to avascular areas and dilated capillaries. The cutaneous histopathologic findings in ADM are the same as with CADM: a smudged dermoepidermal interface, vacuolar alterations of the basal layer, and dermal mucin deposits.
Palmoplantar keratoderma rarely is reported as a cutaneous finding in DM. The finding of keratoderma has mainly been reported in association with Wong-type DM, a rare subtype of DM with features of pityriasis rubra pilaris.11-13 Palmoplantar keratoderma also has been reported in a case of an ADM-like hydroxyurea-induced eruption14 and as an early presenting feature in one patient with CADM and one with juvenile DM.15,16
The autoantibody profile in patients with ADM varies from that of CADM and can be helpful in both diagnosis and prognosis. Similar to CADM, the majority of patients with ADM have positive antinuclear antibodies.2,17 Anti–Jo-1 (an anti–aminoacyl-transfer RNA synthetase) antibody frequently is found in CADM but rarely in ADM.2 Anti–Jo-1 is predictive of interstitial lung disease (ILD) in CADM. Positive anti–Jo-1 in combination with Raynaud phenomenon and mechanic’s hands is referred to as antisynthetase syndrome in patients with CADM.18,19 An antibody uniquely linked with CADM is the anti–CADM-140/MDA5 antibody and can be a marker of rapidly progressing ILD in these patients.20 Anti–Mi-2 is another myositis-specific antibody not commonly found in ADM but is present in 15% to 30% of DM cases.2,21 In CADM, the anti–Mi-2 antibody is associated with the shawl sign, ragged cuticles, and carpal tunnel syndrome and has a favorable prognosis.17,21 Myositis-associated autoantibodies (eg, anti-Ku) are found in patients with symptoms overlapping both DM and scleroderma or other connective tissue diseases.22 More recently described, the anti-p155/140 antibody is highly specific (up to 89%) for occult malignancy in DM.23
Lung disease is an important association in ADM. When it develops, it may be more aggressive compared to lung disease associated with CADM.24-26 In a systematic review of 197 cases of ADM by Gerami et al,2 10% of patients had ILD, and it was fatal in 42% of cases. Most cases of ILD associated with CADM were diagnosed as interstitial pneumonitis or diffuse alveolar disease; bronchiolitis obliterans organizing pneumonia and basilar fibrosis also were recorded.2 Anti–Jo-1 antibodies often accompany lung disease in CADM but are not typically found in lung disease associated with ADM. The anti–CADM-140/MDA5 antibody is associated with an increased risk for rapidly progressing ILD in patients with CADM.20 Recommended baseline screening for lung disease in DM includes chest radiography, pulmonary function tests with diffusion capacity,8 and in some instances high-resolution chest CT.27 Follow-up visits should include screening for symptoms of ILD such as cough, shortness of breath, or dyspnea. Treatment of myopathy-associated ILD is systemic steroids combined with various immunosuppressants including cyclophosphamide, azathioprine, mycophenolate mofetil, cyclosporine, tacrolimus, and intravenous immunoglobulin.28,29
The risk of malignancy in ADM is thought to be similar to the rate of 20% to 25% found in CADM.1,30-32 The most commonly reported malignancies associated with ADM are nasopharyngeal, breast, lung, ovarian, colorectal, pancreatic, and stomach cancers and lymphoma/leukemia.2,33 Patients with ADM should be screened for malignancy at diagnosis, then yearly for 3 years.8,31,33 In addition to history, physical examination, and age/sex-appropriate screening, a complete blood cell count, chemistry panel, urinalysis, stool guaiac, CA 125, CA 19-9, chest radiograph, and abdominal ultrasound should be performed. For women, mammography and pelvic ultrasonography should be completed.31 Some experts also recommend a full-body CT scan. Because Asian patients have a higher risk for nasopharyngeal carcinoma, referral to an ear, nose, and throat surgeon for direct visualization also can be considered.33 The risk of cancer in patients with DM compared to the general population is increased for at least the first 5 years after diagnosis, but most associated cancers are found within the first 3 years.34
Several therapies have been found useful in ADM. Because lesions often are photoexacerbated, sun protection is essential. Antimalarials such as hydroxychloroquine are considered first-line therapy. Clinicians must be aware of 2 possible hydroxychloroquine side effects that can uniquely confuse the clinical picture in ADM. The first is a rash, most often morbilliform and pruritic, that occurs in DM more frequently than in other diseases.35 The second is a myopathy found in as many as 6.7% of patients using antimalarials for rheumatic disease,36 which can clinically mimic the progression of ADM to CADM.37 Two small retrospective case series found that methotrexate was beneficial in ADM.38,39 Methotrexate also has been reported as an efficacious treatment of ILD in patients with connective tissue diseases.40,41 Intravenous immunoglobulin and other immunosuppressants are additional agents to be considered.42
In summary, ADM is an important subset of DM and is more likely to present to dermatology practices than to other specialists. Amyopathic DM shares cutaneous findings with DM, and both overlap and differ with respect to other key disease characteristics including autoantibody profile, associated lung disease, and malignancy risk. Palmoplantar keratoderma is a rarely reported skin finding in DM. We report a case of ADM with the unique finding of severe plantar keratoderma. The fact that our patient’s keratoderma and other skin findings resolved concomitantly during methotrexate therapy leads us to believe that the keratoderma was a unique skin manifestation of the ADM itself.
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26-30.
- Gerami P, Schope JM, McDonald L, et al. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597-613.
- Sontheimer RD. Cutaneous features of classic dermatomyositis and amyopathic dermatomyositis. Curr Opin Rheumatol. 1999;11:475-482.
- Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23-27.
- Marvi U, Chung L, Fiorentino DF. Clinical presentation and evaluation of dermatomyositis. Indian J Dermatol. 2012;57:375-381.
- Stahl NI, Klippel JH, Decker JL. A cutaneous lesion associated with myositis. Ann Intern Med. 1979;91:577-579.
- Kasteler JS, Callen JP. Scalp involvement in dermatomyositis. often overlooked or misdiagnosed. JAMA. 1994;272:1939-1941.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Russo T, Piccolo V, Ruocco E, et al. The heliotrope sign of dermatomyositis: the correct meaning of the term heliotrope. Arch Dermatol. 2012;148:1178.
- Peñate Y, Guillermo N, Melwani P, et al. Calcinosis cutis associated with amyopathic dermatomyositis: response to intravenous immunoglobulin. J Am Acad Dermatol. 2009;60:1076-1077.
- Requena L, Grilli R, Soriano L, et al. Dermatomyositis with a pityriasis rubra pilaris-like eruption: a little-known distinctive cutaneous manifestation of dermatomyositis. Br J Dermatol. 1997;136:768-771.
- Lupton JR, Figueroa P, Berberian BJ, et al. An unusual presentation of dermatomyositis: the type Wong variant revisited. J Am Acad Dermatol. 2000;43(5 part 2):908-912.
- Caporali R, Cavagna L, Bellosta M, et al. Inflammatory myopathy in a patient with cutaneous findings of pityriasis rubra pilaris: a case of Wong’s dermatomyositis. Clin Rheumatol. 2004;23:63-65.
- Nofal A, El-Din ES. Hydroxyurea-induced dermatomyositis: true amyopathic dermatomyositis or dermatomyositis-like eruption? Int J Dermatol. 2012;51:535-541.
- See Y, Rooney M, Woo P. Palmar plantar hyperkeratosis—a previously undescribed skin manifestation of juvenile dermatomyositis. Br J Rheumatol. 1997;36(8):917-919.
- Chang LY, Yang LJ, Wu YJJ. Keratoderma plantaris and mechanic’s hands as the initial presentation in a case of dermatomyositis. Dermatol Sinica. 2002;20:329-334.
- Love L, Leff R, Fraser D, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore). 1991;70:360-374.
- Marguerie C, Bunn CC, Beynon HL, et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med. 1990;77:1019-1038.
- Marie I, Hatron PY, Hachulla E, et al. Pulmonary involvement in polymyositis and in dermatomyositis. J Rheumatol. 1998;25:1336-1343.
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571-1576.
- Dimachkie MM. Idiopathic inflammatory myopathies. J Neuroimmunol. 2011;231:32-42.
- Betteridge ZE, Gunawardena H, McHugh NJ. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther. 2011;13:209.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, et al. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol. 2010;22:627-632.
- Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis, and amyopathic dermatomyositis. Rheumatology (Oxford). 2005;44:1282-1286.
- Ye S, Chen XX, Lu XY, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol. 2007;26:1647-1654.
- Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341-1347.
- Fathi M, Dastmalchi, M, Rasmussen E, et al. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63:297-301.
- Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med. 2010;31:501-512.
- Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med. 2013;107:890-896.
- Klein RQ, Teal V, Taylor L, et al. Number, characteristics, and classification of patients with dermatomyositis seen by dermatology and rheumatology departments at a large tertiary medical center. J Am Acad Dermatol. 2007;57:937-943.
- Sontheimer RD. Clinically amyopathic dermatomyositis: what can we now tell our patients? Arch Dermatol. 2010;146:76-80.
- Azuma K, Yamada H, Ohkubo M, et al. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod Rheumatol. 2011;21:178-183.
- Femia AN, Vleugels RA, Callen JP. Cutaneous dermatomyositis: an updated review of treatment options and internal associations. Am J Clin Dermatol. 2013;14:291-313.
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095.
- Pelle MT, Callen JP. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol. 2002;138:1231-1233.
- Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385-390.
- Zieglschmid-Adams ME, Pandya AG, Cohen SB, et al. Treatment of dermatomyositis with methotrexate. J Am Acad Dermatol. 1995;32(5, pt 1):754-757.
- Foulke G, Baccon J, Marks JG, et al. Antimalarial myopathy in amyopathic dermatomyositis. Arch Dermatol. 2012;148:1100-1101.
- Kasteler JS, Callen JP. Low-dose methotrexate administered weekly is an effective corticosteroid-sparing agent for the treatment of the cutaneous manifestations of dermatomyositis. J Am Acad Dermatol. 1997;36:67-71.
- Scott DG, Bacon PA. Response to methotrexate in fibrosing alveolitis associated with connective tissue disease. Thorax. 1980;35:725-731.
- Fink SD, Kremer JM. Successful treatment of interstitial lung disease in systemic lupus erythematosus with methotrexate. J Rheumatol. 1995;22:967-969.
- Ernste FC, Reed AM. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88:83-105.
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26-30.
- Gerami P, Schope JM, McDonald L, et al. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597-613.
- Sontheimer RD. Cutaneous features of classic dermatomyositis and amyopathic dermatomyositis. Curr Opin Rheumatol. 1999;11:475-482.
- Caproni M, Cardinali C, Parodi A, et al. Amyopathic dermatomyositis: a review by the Italian Group of Immunodermatology. Arch Dermatol. 2002;138:23-27.
- Marvi U, Chung L, Fiorentino DF. Clinical presentation and evaluation of dermatomyositis. Indian J Dermatol. 2012;57:375-381.
- Stahl NI, Klippel JH, Decker JL. A cutaneous lesion associated with myositis. Ann Intern Med. 1979;91:577-579.
- Kasteler JS, Callen JP. Scalp involvement in dermatomyositis. often overlooked or misdiagnosed. JAMA. 1994;272:1939-1941.
- Callen JP. Dermatomyositis. Lancet. 2000;355:53-57.
- Russo T, Piccolo V, Ruocco E, et al. The heliotrope sign of dermatomyositis: the correct meaning of the term heliotrope. Arch Dermatol. 2012;148:1178.
- Peñate Y, Guillermo N, Melwani P, et al. Calcinosis cutis associated with amyopathic dermatomyositis: response to intravenous immunoglobulin. J Am Acad Dermatol. 2009;60:1076-1077.
- Requena L, Grilli R, Soriano L, et al. Dermatomyositis with a pityriasis rubra pilaris-like eruption: a little-known distinctive cutaneous manifestation of dermatomyositis. Br J Dermatol. 1997;136:768-771.
- Lupton JR, Figueroa P, Berberian BJ, et al. An unusual presentation of dermatomyositis: the type Wong variant revisited. J Am Acad Dermatol. 2000;43(5 part 2):908-912.
- Caporali R, Cavagna L, Bellosta M, et al. Inflammatory myopathy in a patient with cutaneous findings of pityriasis rubra pilaris: a case of Wong’s dermatomyositis. Clin Rheumatol. 2004;23:63-65.
- Nofal A, El-Din ES. Hydroxyurea-induced dermatomyositis: true amyopathic dermatomyositis or dermatomyositis-like eruption? Int J Dermatol. 2012;51:535-541.
- See Y, Rooney M, Woo P. Palmar plantar hyperkeratosis—a previously undescribed skin manifestation of juvenile dermatomyositis. Br J Rheumatol. 1997;36(8):917-919.
- Chang LY, Yang LJ, Wu YJJ. Keratoderma plantaris and mechanic’s hands as the initial presentation in a case of dermatomyositis. Dermatol Sinica. 2002;20:329-334.
- Love L, Leff R, Fraser D, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore). 1991;70:360-374.
- Marguerie C, Bunn CC, Beynon HL, et al. Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med. 1990;77:1019-1038.
- Marie I, Hatron PY, Hachulla E, et al. Pulmonary involvement in polymyositis and in dermatomyositis. J Rheumatol. 1998;25:1336-1343.
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571-1576.
- Dimachkie MM. Idiopathic inflammatory myopathies. J Neuroimmunol. 2011;231:32-42.
- Betteridge ZE, Gunawardena H, McHugh NJ. Novel autoantibodies and clinical phenotypes in adult and juvenile myositis. Arthritis Res Ther. 2011;13:209.
- Selva-O’Callaghan A, Trallero-Araguás E, Grau-Junyent JM, et al. Malignancy and myositis: novel autoantibodies and new insights. Curr Opin Rheumatol. 2010;22:627-632.
- Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis, and amyopathic dermatomyositis. Rheumatology (Oxford). 2005;44:1282-1286.
- Ye S, Chen XX, Lu XY, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol. 2007;26:1647-1654.
- Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341-1347.
- Fathi M, Dastmalchi, M, Rasmussen E, et al. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63:297-301.
- Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med. 2010;31:501-512.
- Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med. 2013;107:890-896.
- Klein RQ, Teal V, Taylor L, et al. Number, characteristics, and classification of patients with dermatomyositis seen by dermatology and rheumatology departments at a large tertiary medical center. J Am Acad Dermatol. 2007;57:937-943.
- Sontheimer RD. Clinically amyopathic dermatomyositis: what can we now tell our patients? Arch Dermatol. 2010;146:76-80.
- Azuma K, Yamada H, Ohkubo M, et al. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod Rheumatol. 2011;21:178-183.
- Femia AN, Vleugels RA, Callen JP. Cutaneous dermatomyositis: an updated review of treatment options and internal associations. Am J Clin Dermatol. 2013;14:291-313.
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095.
- Pelle MT, Callen JP. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol. 2002;138:1231-1233.
- Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65:385-390.
- Zieglschmid-Adams ME, Pandya AG, Cohen SB, et al. Treatment of dermatomyositis with methotrexate. J Am Acad Dermatol. 1995;32(5, pt 1):754-757.
- Foulke G, Baccon J, Marks JG, et al. Antimalarial myopathy in amyopathic dermatomyositis. Arch Dermatol. 2012;148:1100-1101.
- Kasteler JS, Callen JP. Low-dose methotrexate administered weekly is an effective corticosteroid-sparing agent for the treatment of the cutaneous manifestations of dermatomyositis. J Am Acad Dermatol. 1997;36:67-71.
- Scott DG, Bacon PA. Response to methotrexate in fibrosing alveolitis associated with connective tissue disease. Thorax. 1980;35:725-731.
- Fink SD, Kremer JM. Successful treatment of interstitial lung disease in systemic lupus erythematosus with methotrexate. J Rheumatol. 1995;22:967-969.
- Ernste FC, Reed AM. Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc. 2013;88:83-105.
Practice Points
- Dermatomyositis (DM) can present without muscular weakness as clinically amyopathic dermatomyositis (CADM).
- Clinically amyopathic dermatomyositis has cutaneous findings that can mimic other diseases including psoriasis.
- Clinically amyopathic dermatomyositis may have similar systemic associations as DM in general, such as an increased risk for malignancies.
- Treatments to consider for CADM should include systemic methotrexate.
Traumatic Ulcerative Granuloma With Stromal Eosinophilia: A Malignant-Appearing Benign Lesion
Traumatic ulcerative granuloma with stromal eosinophilia (TUGSE) is an uncommon, benign, self-limited condition that is restricted to the oral mucosa, most commonly seen in the fifth to seventh decades of life.1-3 The pathogenesis of TUGSE is unknown, but current theory suggests trauma is the instigating factor. The presence of CD30+ mononuclear cells within TUGSE raises the possibility of a CD30+ lymphoproliferative disorder in some cases.4 However, because CD30+ cells are not uncommon in other benign reactive processes, they may simply represent a reactive phenomenon.3
Traumatic ulcerative granuloma with stromal eosinophilia traverses multiple disciplines, including dermatology, oral surgery, dentistry, and pathology, resulting in a diverse nomenclature including traumatic granuloma of the tongue, traumatic eosinophilic granuloma of the oral mucosa, ulcerated granuloma eosinophilicum diutinum, and eosinophilic ulcer of the oral mucosa.1,4-6 It is important to differentiate eosinophilic granuloma of the oral mucosa from the eosinophilic granuloma that is associated with Langerhans cell histiocytosis. Although both may present with oral ulceration, Langerhans cell–associated eosinophilic granuloma typically develops from underlying bone, whereas eosinophilic granuloma of the oral mucosa (TUGSE) is described as nonosseous.7,8 Furthermore, the gingiva is the most common oral site in Langerhans cell–associated eosinophilic granuloma, whereas the tongue is most commonly involved in TUGSE.8 Shapiro and Juhlin9 clearly distinguished TUGSE from Langerhans cell–associated eosinophilic granuloma in 1970. Histologically, the 2 conditions are completely different.
When ulcerative granulomas develop in the pediatric population, usually in children younger than 2 years, it is termed Riga-Fede disease.10 These children were typically breastfeeding, suckling, or teething, suggesting trauma as a triggering event. In 1961, Hjorting-Hansen and Schmidt5 described 3 separate lesions similar to Riga-Fede disease in an adult patient. Subsequently, Riga-Fede disease was grouped under TUGSE.3
Histologically, TUGSE shows an ulcerated epithelium with a polymorphic inflammatory cell infiltrate that has a large predominance of eosinophils. The infiltrate affects the superficial and deep layers of the muscle tissue and penetrates into the salivary glands. Large atypical mononuclear cells with an ovoid and pale-appearing nucleus often are present. These cells may be mitotically active and stain positively for CD30.1,4,11 CD68+ macrophages, T lymphocytes, and factor XIIIa–positive dendritic cells commonly are present.12
Given the presence of large atypical CD30+ cells in many lesions, the possibility of a CD30+ lymphoproliferative disorder has been postulated by some authors. Indeed, lymphomatoid papulosis (LyP) has been documented to involve the oral mucosa.2,4
Case Report
An 81-year-old man presented with a rapidly enlarging, 1.7×1.3-cm, vascular-appearing nodule with a collarette of mucosal epithelium on the left side of the dorsal surface of the tongue of 2 weeks’ duration (Figure 1). He denied any history of trauma, tobacco chewing, weight change, fever, or fatigue; however, he did report a 30 pack-year smoking history. There was no other pertinent medical history to include medications or allergies.

The differential diagnosis included pyogenic granuloma, granular cell tumor, squamous cell carcinoma, other neoplasms (eg, oral lymphoma, salivary gland tumors), and a traumatic blood blister from tongue biting. The patient was referred to the oral maxillofacial surgery department for an excisional biopsy, which showed a solitary ulcerated nodule with associated granulation tissue, thrombus, and fibrinoid debris (Figure 2). A surrounding dense mixed inflammatory cell infiltrate composed of lymphocytes, histiocytes, and numerous eosinophils was noted extending through the submucosal tissue and underlying striated muscle fibers (Figure 3). The adjacent mucosal epithelium appeared normal. CD30 staining showed only rare positive cells. These findings were consistent with TUGSE.


Due to the benign nature of TUGSE, the patient was released with symptomatic care and instructed to return for any new growth. The growth spontaneously resolved over 1 month and no recurrence or new lesions were reported 1 year later.
Comment
Despite encompassing multiple disciplines of medicine, TUGSE has minimal exposure in the dermatologic literature. It is an important clinical and histologic diagnosis that will provide reassurance to the patient when accurately identified and reduce potentially harmful treatments.
Clinical Presentation
Typically, TUGSE presents as a painful solitary nodule with a central ulcer and yellow fibrinous base. The margins of the ulcer typically have an indurated and rolled appearance.1,4 More than 50% of the lesions develop on the tongue, specifically the dorsal or lateral surfaces, but they may present anywhere in the oral mucosa.7 Traumatic ulcerative granuloma with stromal eosinophilia is a fast-growing lesion, typically developing in days to weeks. Although it spontaneously regresses, the lesion may take weeks or months to resolve. In one case, it resolved 1 year later.1 Traumatic ulcerative granuloma with stromal eosinophilia has a bimodal age distribution, generally appearing in the first 2 years of life and later in the fifth through seventh decades. The male-to-female predominance is equal.1,7,11 Reoccurrence is rare, but some reports have shown patients with multiple episodes of TUGSE.13,14
Differential Diagnosis
The clinical differential diagnosis for TUGSE includes squamous cell carcinoma, pyogenic granuloma, lymphoproliferative disorder, traumatic neuroma, Langerhans cell histiocytosis, granulomatous disorders, and oral lymphoma. Inflammatory disorders such as syphilis, Behçet’s disease, herpes, histoplasmosis, Wegener granulomatosis, and others also should be considered.
Immunohistochemistry
Immunohistochemical analysis of TUGSE lesions recently has revealed the presence of CD30+ cells. These cells are associated with cutaneous lymphoproliferative disorders including LyP, anaplastic large cell lymphoma (ALCL), and borderline CD30+ lesions, among others. Systemic diseases with CD30+ cells include mycosis fungoides, other T-cell lymphomas, and Hodgkin lymphoma.15,16 Once CD30+ cells were recognized, multiple authors began speculating there was a correlation between TUGSE and the CD30+ lymphoproliferative disorders.1,2,13 Anaplastic large cell lymphoma and LyP of the oral mucosa have been reported in several cases.17-20 One report described 2 cases of ulcerated CD30+ T-cell non-Hodgkin lymphoma of the oral mucosa, one of which showed eosinophilic infiltrates and was initially thought to be TUGSE. Based on these overlapping clinical and histologic features, the authors hypothesized there was a correlation between oral ALCL, LyP, and TUGSE.17 In one report, a patient developed multiple TUGSE lesions throughout his life, suggesting a pathologic process similar to LyP. The lesion biopsied showed that 70% of the T cells expressed CD30 (Ki-1) antigen.13
Underlying Causes
In support of an underlying immunologic process that augments the growth of these lesions, 2 separate case reports of TUGSE in the presence of human T-lymphotropic virus 1 (HTLV-1) and Epstein-Barr virus have been documented.2,21 Concurrent presentation of TUGSE and HTLV-1 in one report demonstrated eosinophilia in both the oral lesion and peripheral blood, suggesting an immunologic relationship. Furthermore, the authors postulated that local trauma initiated the development of TUGSE, providing the catalyst for the HTLV-1 carrier to develop peripheral eosinophilia.21
In the second case, a 12-year-old boy developed TUGSE in the presence of Epstein-Barr virus.2 Immunologically, this virus can be reactivated from its latent stage during immunosuppression. Epstein-Barr virus has been implicated in lymphoproliferative diseases of both B- and T-cell origin, including CD30+ ALCL and LyP.22,23 The authors in this report again hypothesized there was a correlation between lymphoproliferative disorders and TUGSE lesions.2,24
Alternatively, TUGSE may simply be a reactive process to trauma or another underlying trigger. It has been speculated that the presence of eosinophils correlates with antigen insertion into the oral mucosa, whereas other ulcers of the oral mucosa are devoid of eosinophils.1 These antigens may include microorganisms, endogenous degradation products, or foreign proteins.7,25 Additionally, the presence of CD30+ lymphocytes is not isolated to lymphoproliferative disorders. CD30+ cells have been documented in arthropod bite reactions, atopic dermatitis, drug reactions, molluscum contagiosum, and scabies, among others.1,26
Healing and Management
The length of healing in TUGSE ulcers has substantial variability, from days to up to 1 year in an isolated case.1,24 Sequential expression of transforming growth factor (TGF) α and TGF-β expressed by tissue eosinophils may be underlying factors associated with a quicker healing response as demonstrated by similar ulcers in hamsters.27 Chronic nonhealing oral ulcers, particularly TUGSE lesions that demonstrated the typical increase in eosinophils in 11 of 12 cases, showed minimal TGF-α or TGF-β expression by eosinophils, perhaps indicating a possible mechanism leading to delayed wound healing in some cases. Interestingly, incisional biopsies often led to rapid wound healing, suggesting that the biopsy itself allowed for a transition back to the regular wound-healing processes.28
Traumatic ulcerative granuloma with stromal eosinophilia spontaneously resolves on its own in most cases; however, because of the concern for malignancy, it has the potential to be overtreated.26 Symptomatic treatment only is the mainstay of therapy. The patient should be instructed to avoid trauma, and referral to a dental professional is indicated when associated with dentures or other periprosthetic devices. Diet should consist of soft foods while avoiding spicy foods. Topical or oral analgesics may be necessary if substantial pain is associated with the lesion.2 Oral prednisolone was used in a patient with concurrent HTLV-1 and TUGSE to treat peripheral eosinophilia.21 The patient’s peripheral eosinophils dropped to 1% in 1 day, and the patient’s oral lesion began to improve at day 3 and disappeared by day 10. Although TUGSE may spontaneously resolve within a 10-day period without steroids, it may be a reasonable treatment to improve healing time in an otherwise healthy individual.21,26 If there is concern for malignancy, the patient should have the lesion biopsied to provide reassurance and for the added benefit of a transition to normal healing response and decreased healing time.28
Clinical Recognition
The clinician should be aware of the possibility of a CD30+ lymphoproliferative disorder, which has been associated with TUGSE in some cases, or may simulate TUGSE both clinically and histologically. Further studies are needed to clarify the relationship between these 2 entities. Whether it is a true relationship, simple coincidence, or simply overlapping clinical and histologic features remains to be determined.
- Hirshberg A, Amariglio N, Akrish S, et al. Traumatic ulcerative granuloma with stromal eosinophilia: reactive lesion of the oral mucosa. Am J Clin Pathol. 2006;126:522-529.
- Abdel-Naser MB, Tsatsou F, Hippe S, et al. Oral eosinophilic ulcer, an Epstein-Barr virus-associated CD30+ lymphoproliferation? [published online April 5, 2011]. Dermatology. 2011;222:113-118.
- Fonseca FP, Benevenuto de Andrade BA, Coletta RD, et al. Clinicopathological and immunohistochemical analysis of 19 cases of oral eosinophilic ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:532-540.
- Alobeid B, Pan LX, Milligan L, et al. Eosinophil-rich CD30+ lymphoproliferative disorder of the oral mucosa. Am J Clin Pathol. 2004;121:43-50.
- Hjorting-Hansen E, Schmidt H. Ulcerated granuloma eosinophilicum diutinum of the tongue. report of a case. Acta Derm Venereol. 1961;41:235-239.
- Velez A, Alamillos FJ, Dean A, et al. Eosinophilic ulcer of the oral mucosa: report of a recurrent case on the tongue. Clin Exp Dermatol. 1997;22:154-156.
- Elzay RP. Traumatic ulcerative granuloma with stromal eosinophilia (Riga-Fede’s disease and traumatic eosinophilic granuloma). Oral Surg Oral Med Oral Pathol. 1983;55:497-506.
- Val-Bernal JF, Gonzalez-Vela MC, Sanchez-Santolino S, et al. Localized eosinophilic (Langerhans’ cell) granuloma of the lower lip. a lesion that may cause diagnostic error. J Cutan Pathol. 2009;36:1109-1113.
- Shapiro L, Juhlin EA. Eosinophilic ulcer of the tongue report of two cases and review of the literature. Dermatologica. 1970;140:242-250.
- Amberg S. Sublingual growth in infants. Am J Med Sci. 1902;126:257-269.
- EI-Mofty SK, Swanson PE, Wick MR, et al. Eosinophilic ulcer of the oral mucosa: report of 38 new cases with immunohistochemical observations. Oral Surg Oral Med Oral Pathol. 1993;75:716-722.
- Regezi JA, Zarbo RJ, Daniels TE, et al. Oral traumatic granuloma: characterization of the cellular infiltrate. Oral Surg Oral Med Oral Pathol. 1993;75:723-727.
- Ficarra G, Prignano F, Romagnoli P. Traumatic eosinophilic granuloma of the oral mucosa: a CD30+ (Ki-1) lymphoproliferative disorder? Oral Oncol. 1997;33:375-379.
- Doyle JL, Geary W, Baden E. Eosinophilic ulcer. J Oral Maxillofac Surg. 1989;47:349-352.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848-858.
- Rosenberg A, Biesma DH, Sie-Go DMDS, et al. Primary extranodal CD30-positive T-cell non-Hodgkin’s lymphoma of the oral mucosa. report of two cases. Int J Oral Maxillofac Surg. 1996;25:57-59.
- Kato N, Tomita Y, Yoshida K, et al. Involvement of the tongue by lymphomatoid papulosis. Am J Dermatopathol. 1998;20:522-526.
- Savarrio L, Gibson J, Dunlop DJ, et al. Spontaneous regression of an anaplastic large cell lymphoma in the oral cavity: first reported case and review of the literature. Oral Oncol. 1999;35:609-613.
- Sciubba J, Said-Al-Naief N, Fantasia J. Critical review of lymphomatoid papulosis of the oral cavity with case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:195-204.
- Yamazaki H, Shirasugi Y, Kajiwara H, et al. Concurrent onset of eosinophilic ulcer of the oral mucosa with peripheral eosinophilia in a human T-cell leukemia virus type I carrier. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:E43-E48.
- Dojcinov SD, Venkataram G, Raffeld M, et al. EBV positive mucocutaneous ulcer—a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405-417.
- Kim YC, Yang WI, Lee MG, et al. Epstein-Barr virus in CD30 anaplastic large cell lymphoma involving the skin and lymphomatoid papulosis in South Korea. Int J Dermatol. 2006;45:1312-1316.
- Pietersma F, Piriou E, van Baarle D. Immune surveillance of EBV-infected B cells and the development of non-Hodgkin lymphomas in immunocompromised patients. Leuk Lymphoma. 2008;49:1028-1041.
- Salisbury CL, Budnick SD, Li S. T cell receptor gene rearrangement and CD 30 immunoreactivity in traumatic ulcerative granuloma with stromal eosinophilia of oral cavity. Am J Clin Pathol. 2009;132:722-727.
- Marszalek A, Neska-Dlugosz I. Traumatic ulcerative granuloma with stromal eosinophilia. a case report and short literature review. Pol J Pathol. 2011;3:172-175.
- Wong DT, Donoff RB, Yang J, et al. Sequential expression of transforming growth factors alpha and beta 1 by eosinophils during cutaneous wound healing in the hamster. Am J Pathol. 1993;143:130-142.
- Elovic AE, Gallagher GT, Kabani S, et al. Lack of TGF-alpha and TGF-beta synthesis by human eosinophils in chronic oral ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:672-681.
Traumatic ulcerative granuloma with stromal eosinophilia (TUGSE) is an uncommon, benign, self-limited condition that is restricted to the oral mucosa, most commonly seen in the fifth to seventh decades of life.1-3 The pathogenesis of TUGSE is unknown, but current theory suggests trauma is the instigating factor. The presence of CD30+ mononuclear cells within TUGSE raises the possibility of a CD30+ lymphoproliferative disorder in some cases.4 However, because CD30+ cells are not uncommon in other benign reactive processes, they may simply represent a reactive phenomenon.3
Traumatic ulcerative granuloma with stromal eosinophilia traverses multiple disciplines, including dermatology, oral surgery, dentistry, and pathology, resulting in a diverse nomenclature including traumatic granuloma of the tongue, traumatic eosinophilic granuloma of the oral mucosa, ulcerated granuloma eosinophilicum diutinum, and eosinophilic ulcer of the oral mucosa.1,4-6 It is important to differentiate eosinophilic granuloma of the oral mucosa from the eosinophilic granuloma that is associated with Langerhans cell histiocytosis. Although both may present with oral ulceration, Langerhans cell–associated eosinophilic granuloma typically develops from underlying bone, whereas eosinophilic granuloma of the oral mucosa (TUGSE) is described as nonosseous.7,8 Furthermore, the gingiva is the most common oral site in Langerhans cell–associated eosinophilic granuloma, whereas the tongue is most commonly involved in TUGSE.8 Shapiro and Juhlin9 clearly distinguished TUGSE from Langerhans cell–associated eosinophilic granuloma in 1970. Histologically, the 2 conditions are completely different.
When ulcerative granulomas develop in the pediatric population, usually in children younger than 2 years, it is termed Riga-Fede disease.10 These children were typically breastfeeding, suckling, or teething, suggesting trauma as a triggering event. In 1961, Hjorting-Hansen and Schmidt5 described 3 separate lesions similar to Riga-Fede disease in an adult patient. Subsequently, Riga-Fede disease was grouped under TUGSE.3
Histologically, TUGSE shows an ulcerated epithelium with a polymorphic inflammatory cell infiltrate that has a large predominance of eosinophils. The infiltrate affects the superficial and deep layers of the muscle tissue and penetrates into the salivary glands. Large atypical mononuclear cells with an ovoid and pale-appearing nucleus often are present. These cells may be mitotically active and stain positively for CD30.1,4,11 CD68+ macrophages, T lymphocytes, and factor XIIIa–positive dendritic cells commonly are present.12
Given the presence of large atypical CD30+ cells in many lesions, the possibility of a CD30+ lymphoproliferative disorder has been postulated by some authors. Indeed, lymphomatoid papulosis (LyP) has been documented to involve the oral mucosa.2,4
Case Report
An 81-year-old man presented with a rapidly enlarging, 1.7×1.3-cm, vascular-appearing nodule with a collarette of mucosal epithelium on the left side of the dorsal surface of the tongue of 2 weeks’ duration (Figure 1). He denied any history of trauma, tobacco chewing, weight change, fever, or fatigue; however, he did report a 30 pack-year smoking history. There was no other pertinent medical history to include medications or allergies.

The differential diagnosis included pyogenic granuloma, granular cell tumor, squamous cell carcinoma, other neoplasms (eg, oral lymphoma, salivary gland tumors), and a traumatic blood blister from tongue biting. The patient was referred to the oral maxillofacial surgery department for an excisional biopsy, which showed a solitary ulcerated nodule with associated granulation tissue, thrombus, and fibrinoid debris (Figure 2). A surrounding dense mixed inflammatory cell infiltrate composed of lymphocytes, histiocytes, and numerous eosinophils was noted extending through the submucosal tissue and underlying striated muscle fibers (Figure 3). The adjacent mucosal epithelium appeared normal. CD30 staining showed only rare positive cells. These findings were consistent with TUGSE.


Due to the benign nature of TUGSE, the patient was released with symptomatic care and instructed to return for any new growth. The growth spontaneously resolved over 1 month and no recurrence or new lesions were reported 1 year later.
Comment
Despite encompassing multiple disciplines of medicine, TUGSE has minimal exposure in the dermatologic literature. It is an important clinical and histologic diagnosis that will provide reassurance to the patient when accurately identified and reduce potentially harmful treatments.
Clinical Presentation
Typically, TUGSE presents as a painful solitary nodule with a central ulcer and yellow fibrinous base. The margins of the ulcer typically have an indurated and rolled appearance.1,4 More than 50% of the lesions develop on the tongue, specifically the dorsal or lateral surfaces, but they may present anywhere in the oral mucosa.7 Traumatic ulcerative granuloma with stromal eosinophilia is a fast-growing lesion, typically developing in days to weeks. Although it spontaneously regresses, the lesion may take weeks or months to resolve. In one case, it resolved 1 year later.1 Traumatic ulcerative granuloma with stromal eosinophilia has a bimodal age distribution, generally appearing in the first 2 years of life and later in the fifth through seventh decades. The male-to-female predominance is equal.1,7,11 Reoccurrence is rare, but some reports have shown patients with multiple episodes of TUGSE.13,14
Differential Diagnosis
The clinical differential diagnosis for TUGSE includes squamous cell carcinoma, pyogenic granuloma, lymphoproliferative disorder, traumatic neuroma, Langerhans cell histiocytosis, granulomatous disorders, and oral lymphoma. Inflammatory disorders such as syphilis, Behçet’s disease, herpes, histoplasmosis, Wegener granulomatosis, and others also should be considered.
Immunohistochemistry
Immunohistochemical analysis of TUGSE lesions recently has revealed the presence of CD30+ cells. These cells are associated with cutaneous lymphoproliferative disorders including LyP, anaplastic large cell lymphoma (ALCL), and borderline CD30+ lesions, among others. Systemic diseases with CD30+ cells include mycosis fungoides, other T-cell lymphomas, and Hodgkin lymphoma.15,16 Once CD30+ cells were recognized, multiple authors began speculating there was a correlation between TUGSE and the CD30+ lymphoproliferative disorders.1,2,13 Anaplastic large cell lymphoma and LyP of the oral mucosa have been reported in several cases.17-20 One report described 2 cases of ulcerated CD30+ T-cell non-Hodgkin lymphoma of the oral mucosa, one of which showed eosinophilic infiltrates and was initially thought to be TUGSE. Based on these overlapping clinical and histologic features, the authors hypothesized there was a correlation between oral ALCL, LyP, and TUGSE.17 In one report, a patient developed multiple TUGSE lesions throughout his life, suggesting a pathologic process similar to LyP. The lesion biopsied showed that 70% of the T cells expressed CD30 (Ki-1) antigen.13
Underlying Causes
In support of an underlying immunologic process that augments the growth of these lesions, 2 separate case reports of TUGSE in the presence of human T-lymphotropic virus 1 (HTLV-1) and Epstein-Barr virus have been documented.2,21 Concurrent presentation of TUGSE and HTLV-1 in one report demonstrated eosinophilia in both the oral lesion and peripheral blood, suggesting an immunologic relationship. Furthermore, the authors postulated that local trauma initiated the development of TUGSE, providing the catalyst for the HTLV-1 carrier to develop peripheral eosinophilia.21
In the second case, a 12-year-old boy developed TUGSE in the presence of Epstein-Barr virus.2 Immunologically, this virus can be reactivated from its latent stage during immunosuppression. Epstein-Barr virus has been implicated in lymphoproliferative diseases of both B- and T-cell origin, including CD30+ ALCL and LyP.22,23 The authors in this report again hypothesized there was a correlation between lymphoproliferative disorders and TUGSE lesions.2,24
Alternatively, TUGSE may simply be a reactive process to trauma or another underlying trigger. It has been speculated that the presence of eosinophils correlates with antigen insertion into the oral mucosa, whereas other ulcers of the oral mucosa are devoid of eosinophils.1 These antigens may include microorganisms, endogenous degradation products, or foreign proteins.7,25 Additionally, the presence of CD30+ lymphocytes is not isolated to lymphoproliferative disorders. CD30+ cells have been documented in arthropod bite reactions, atopic dermatitis, drug reactions, molluscum contagiosum, and scabies, among others.1,26
Healing and Management
The length of healing in TUGSE ulcers has substantial variability, from days to up to 1 year in an isolated case.1,24 Sequential expression of transforming growth factor (TGF) α and TGF-β expressed by tissue eosinophils may be underlying factors associated with a quicker healing response as demonstrated by similar ulcers in hamsters.27 Chronic nonhealing oral ulcers, particularly TUGSE lesions that demonstrated the typical increase in eosinophils in 11 of 12 cases, showed minimal TGF-α or TGF-β expression by eosinophils, perhaps indicating a possible mechanism leading to delayed wound healing in some cases. Interestingly, incisional biopsies often led to rapid wound healing, suggesting that the biopsy itself allowed for a transition back to the regular wound-healing processes.28
Traumatic ulcerative granuloma with stromal eosinophilia spontaneously resolves on its own in most cases; however, because of the concern for malignancy, it has the potential to be overtreated.26 Symptomatic treatment only is the mainstay of therapy. The patient should be instructed to avoid trauma, and referral to a dental professional is indicated when associated with dentures or other periprosthetic devices. Diet should consist of soft foods while avoiding spicy foods. Topical or oral analgesics may be necessary if substantial pain is associated with the lesion.2 Oral prednisolone was used in a patient with concurrent HTLV-1 and TUGSE to treat peripheral eosinophilia.21 The patient’s peripheral eosinophils dropped to 1% in 1 day, and the patient’s oral lesion began to improve at day 3 and disappeared by day 10. Although TUGSE may spontaneously resolve within a 10-day period without steroids, it may be a reasonable treatment to improve healing time in an otherwise healthy individual.21,26 If there is concern for malignancy, the patient should have the lesion biopsied to provide reassurance and for the added benefit of a transition to normal healing response and decreased healing time.28
Clinical Recognition
The clinician should be aware of the possibility of a CD30+ lymphoproliferative disorder, which has been associated with TUGSE in some cases, or may simulate TUGSE both clinically and histologically. Further studies are needed to clarify the relationship between these 2 entities. Whether it is a true relationship, simple coincidence, or simply overlapping clinical and histologic features remains to be determined.
Traumatic ulcerative granuloma with stromal eosinophilia (TUGSE) is an uncommon, benign, self-limited condition that is restricted to the oral mucosa, most commonly seen in the fifth to seventh decades of life.1-3 The pathogenesis of TUGSE is unknown, but current theory suggests trauma is the instigating factor. The presence of CD30+ mononuclear cells within TUGSE raises the possibility of a CD30+ lymphoproliferative disorder in some cases.4 However, because CD30+ cells are not uncommon in other benign reactive processes, they may simply represent a reactive phenomenon.3
Traumatic ulcerative granuloma with stromal eosinophilia traverses multiple disciplines, including dermatology, oral surgery, dentistry, and pathology, resulting in a diverse nomenclature including traumatic granuloma of the tongue, traumatic eosinophilic granuloma of the oral mucosa, ulcerated granuloma eosinophilicum diutinum, and eosinophilic ulcer of the oral mucosa.1,4-6 It is important to differentiate eosinophilic granuloma of the oral mucosa from the eosinophilic granuloma that is associated with Langerhans cell histiocytosis. Although both may present with oral ulceration, Langerhans cell–associated eosinophilic granuloma typically develops from underlying bone, whereas eosinophilic granuloma of the oral mucosa (TUGSE) is described as nonosseous.7,8 Furthermore, the gingiva is the most common oral site in Langerhans cell–associated eosinophilic granuloma, whereas the tongue is most commonly involved in TUGSE.8 Shapiro and Juhlin9 clearly distinguished TUGSE from Langerhans cell–associated eosinophilic granuloma in 1970. Histologically, the 2 conditions are completely different.
When ulcerative granulomas develop in the pediatric population, usually in children younger than 2 years, it is termed Riga-Fede disease.10 These children were typically breastfeeding, suckling, or teething, suggesting trauma as a triggering event. In 1961, Hjorting-Hansen and Schmidt5 described 3 separate lesions similar to Riga-Fede disease in an adult patient. Subsequently, Riga-Fede disease was grouped under TUGSE.3
Histologically, TUGSE shows an ulcerated epithelium with a polymorphic inflammatory cell infiltrate that has a large predominance of eosinophils. The infiltrate affects the superficial and deep layers of the muscle tissue and penetrates into the salivary glands. Large atypical mononuclear cells with an ovoid and pale-appearing nucleus often are present. These cells may be mitotically active and stain positively for CD30.1,4,11 CD68+ macrophages, T lymphocytes, and factor XIIIa–positive dendritic cells commonly are present.12
Given the presence of large atypical CD30+ cells in many lesions, the possibility of a CD30+ lymphoproliferative disorder has been postulated by some authors. Indeed, lymphomatoid papulosis (LyP) has been documented to involve the oral mucosa.2,4
Case Report
An 81-year-old man presented with a rapidly enlarging, 1.7×1.3-cm, vascular-appearing nodule with a collarette of mucosal epithelium on the left side of the dorsal surface of the tongue of 2 weeks’ duration (Figure 1). He denied any history of trauma, tobacco chewing, weight change, fever, or fatigue; however, he did report a 30 pack-year smoking history. There was no other pertinent medical history to include medications or allergies.

The differential diagnosis included pyogenic granuloma, granular cell tumor, squamous cell carcinoma, other neoplasms (eg, oral lymphoma, salivary gland tumors), and a traumatic blood blister from tongue biting. The patient was referred to the oral maxillofacial surgery department for an excisional biopsy, which showed a solitary ulcerated nodule with associated granulation tissue, thrombus, and fibrinoid debris (Figure 2). A surrounding dense mixed inflammatory cell infiltrate composed of lymphocytes, histiocytes, and numerous eosinophils was noted extending through the submucosal tissue and underlying striated muscle fibers (Figure 3). The adjacent mucosal epithelium appeared normal. CD30 staining showed only rare positive cells. These findings were consistent with TUGSE.


Due to the benign nature of TUGSE, the patient was released with symptomatic care and instructed to return for any new growth. The growth spontaneously resolved over 1 month and no recurrence or new lesions were reported 1 year later.
Comment
Despite encompassing multiple disciplines of medicine, TUGSE has minimal exposure in the dermatologic literature. It is an important clinical and histologic diagnosis that will provide reassurance to the patient when accurately identified and reduce potentially harmful treatments.
Clinical Presentation
Typically, TUGSE presents as a painful solitary nodule with a central ulcer and yellow fibrinous base. The margins of the ulcer typically have an indurated and rolled appearance.1,4 More than 50% of the lesions develop on the tongue, specifically the dorsal or lateral surfaces, but they may present anywhere in the oral mucosa.7 Traumatic ulcerative granuloma with stromal eosinophilia is a fast-growing lesion, typically developing in days to weeks. Although it spontaneously regresses, the lesion may take weeks or months to resolve. In one case, it resolved 1 year later.1 Traumatic ulcerative granuloma with stromal eosinophilia has a bimodal age distribution, generally appearing in the first 2 years of life and later in the fifth through seventh decades. The male-to-female predominance is equal.1,7,11 Reoccurrence is rare, but some reports have shown patients with multiple episodes of TUGSE.13,14
Differential Diagnosis
The clinical differential diagnosis for TUGSE includes squamous cell carcinoma, pyogenic granuloma, lymphoproliferative disorder, traumatic neuroma, Langerhans cell histiocytosis, granulomatous disorders, and oral lymphoma. Inflammatory disorders such as syphilis, Behçet’s disease, herpes, histoplasmosis, Wegener granulomatosis, and others also should be considered.
Immunohistochemistry
Immunohistochemical analysis of TUGSE lesions recently has revealed the presence of CD30+ cells. These cells are associated with cutaneous lymphoproliferative disorders including LyP, anaplastic large cell lymphoma (ALCL), and borderline CD30+ lesions, among others. Systemic diseases with CD30+ cells include mycosis fungoides, other T-cell lymphomas, and Hodgkin lymphoma.15,16 Once CD30+ cells were recognized, multiple authors began speculating there was a correlation between TUGSE and the CD30+ lymphoproliferative disorders.1,2,13 Anaplastic large cell lymphoma and LyP of the oral mucosa have been reported in several cases.17-20 One report described 2 cases of ulcerated CD30+ T-cell non-Hodgkin lymphoma of the oral mucosa, one of which showed eosinophilic infiltrates and was initially thought to be TUGSE. Based on these overlapping clinical and histologic features, the authors hypothesized there was a correlation between oral ALCL, LyP, and TUGSE.17 In one report, a patient developed multiple TUGSE lesions throughout his life, suggesting a pathologic process similar to LyP. The lesion biopsied showed that 70% of the T cells expressed CD30 (Ki-1) antigen.13
Underlying Causes
In support of an underlying immunologic process that augments the growth of these lesions, 2 separate case reports of TUGSE in the presence of human T-lymphotropic virus 1 (HTLV-1) and Epstein-Barr virus have been documented.2,21 Concurrent presentation of TUGSE and HTLV-1 in one report demonstrated eosinophilia in both the oral lesion and peripheral blood, suggesting an immunologic relationship. Furthermore, the authors postulated that local trauma initiated the development of TUGSE, providing the catalyst for the HTLV-1 carrier to develop peripheral eosinophilia.21
In the second case, a 12-year-old boy developed TUGSE in the presence of Epstein-Barr virus.2 Immunologically, this virus can be reactivated from its latent stage during immunosuppression. Epstein-Barr virus has been implicated in lymphoproliferative diseases of both B- and T-cell origin, including CD30+ ALCL and LyP.22,23 The authors in this report again hypothesized there was a correlation between lymphoproliferative disorders and TUGSE lesions.2,24
Alternatively, TUGSE may simply be a reactive process to trauma or another underlying trigger. It has been speculated that the presence of eosinophils correlates with antigen insertion into the oral mucosa, whereas other ulcers of the oral mucosa are devoid of eosinophils.1 These antigens may include microorganisms, endogenous degradation products, or foreign proteins.7,25 Additionally, the presence of CD30+ lymphocytes is not isolated to lymphoproliferative disorders. CD30+ cells have been documented in arthropod bite reactions, atopic dermatitis, drug reactions, molluscum contagiosum, and scabies, among others.1,26
Healing and Management
The length of healing in TUGSE ulcers has substantial variability, from days to up to 1 year in an isolated case.1,24 Sequential expression of transforming growth factor (TGF) α and TGF-β expressed by tissue eosinophils may be underlying factors associated with a quicker healing response as demonstrated by similar ulcers in hamsters.27 Chronic nonhealing oral ulcers, particularly TUGSE lesions that demonstrated the typical increase in eosinophils in 11 of 12 cases, showed minimal TGF-α or TGF-β expression by eosinophils, perhaps indicating a possible mechanism leading to delayed wound healing in some cases. Interestingly, incisional biopsies often led to rapid wound healing, suggesting that the biopsy itself allowed for a transition back to the regular wound-healing processes.28
Traumatic ulcerative granuloma with stromal eosinophilia spontaneously resolves on its own in most cases; however, because of the concern for malignancy, it has the potential to be overtreated.26 Symptomatic treatment only is the mainstay of therapy. The patient should be instructed to avoid trauma, and referral to a dental professional is indicated when associated with dentures or other periprosthetic devices. Diet should consist of soft foods while avoiding spicy foods. Topical or oral analgesics may be necessary if substantial pain is associated with the lesion.2 Oral prednisolone was used in a patient with concurrent HTLV-1 and TUGSE to treat peripheral eosinophilia.21 The patient’s peripheral eosinophils dropped to 1% in 1 day, and the patient’s oral lesion began to improve at day 3 and disappeared by day 10. Although TUGSE may spontaneously resolve within a 10-day period without steroids, it may be a reasonable treatment to improve healing time in an otherwise healthy individual.21,26 If there is concern for malignancy, the patient should have the lesion biopsied to provide reassurance and for the added benefit of a transition to normal healing response and decreased healing time.28
Clinical Recognition
The clinician should be aware of the possibility of a CD30+ lymphoproliferative disorder, which has been associated with TUGSE in some cases, or may simulate TUGSE both clinically and histologically. Further studies are needed to clarify the relationship between these 2 entities. Whether it is a true relationship, simple coincidence, or simply overlapping clinical and histologic features remains to be determined.
- Hirshberg A, Amariglio N, Akrish S, et al. Traumatic ulcerative granuloma with stromal eosinophilia: reactive lesion of the oral mucosa. Am J Clin Pathol. 2006;126:522-529.
- Abdel-Naser MB, Tsatsou F, Hippe S, et al. Oral eosinophilic ulcer, an Epstein-Barr virus-associated CD30+ lymphoproliferation? [published online April 5, 2011]. Dermatology. 2011;222:113-118.
- Fonseca FP, Benevenuto de Andrade BA, Coletta RD, et al. Clinicopathological and immunohistochemical analysis of 19 cases of oral eosinophilic ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:532-540.
- Alobeid B, Pan LX, Milligan L, et al. Eosinophil-rich CD30+ lymphoproliferative disorder of the oral mucosa. Am J Clin Pathol. 2004;121:43-50.
- Hjorting-Hansen E, Schmidt H. Ulcerated granuloma eosinophilicum diutinum of the tongue. report of a case. Acta Derm Venereol. 1961;41:235-239.
- Velez A, Alamillos FJ, Dean A, et al. Eosinophilic ulcer of the oral mucosa: report of a recurrent case on the tongue. Clin Exp Dermatol. 1997;22:154-156.
- Elzay RP. Traumatic ulcerative granuloma with stromal eosinophilia (Riga-Fede’s disease and traumatic eosinophilic granuloma). Oral Surg Oral Med Oral Pathol. 1983;55:497-506.
- Val-Bernal JF, Gonzalez-Vela MC, Sanchez-Santolino S, et al. Localized eosinophilic (Langerhans’ cell) granuloma of the lower lip. a lesion that may cause diagnostic error. J Cutan Pathol. 2009;36:1109-1113.
- Shapiro L, Juhlin EA. Eosinophilic ulcer of the tongue report of two cases and review of the literature. Dermatologica. 1970;140:242-250.
- Amberg S. Sublingual growth in infants. Am J Med Sci. 1902;126:257-269.
- EI-Mofty SK, Swanson PE, Wick MR, et al. Eosinophilic ulcer of the oral mucosa: report of 38 new cases with immunohistochemical observations. Oral Surg Oral Med Oral Pathol. 1993;75:716-722.
- Regezi JA, Zarbo RJ, Daniels TE, et al. Oral traumatic granuloma: characterization of the cellular infiltrate. Oral Surg Oral Med Oral Pathol. 1993;75:723-727.
- Ficarra G, Prignano F, Romagnoli P. Traumatic eosinophilic granuloma of the oral mucosa: a CD30+ (Ki-1) lymphoproliferative disorder? Oral Oncol. 1997;33:375-379.
- Doyle JL, Geary W, Baden E. Eosinophilic ulcer. J Oral Maxillofac Surg. 1989;47:349-352.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848-858.
- Rosenberg A, Biesma DH, Sie-Go DMDS, et al. Primary extranodal CD30-positive T-cell non-Hodgkin’s lymphoma of the oral mucosa. report of two cases. Int J Oral Maxillofac Surg. 1996;25:57-59.
- Kato N, Tomita Y, Yoshida K, et al. Involvement of the tongue by lymphomatoid papulosis. Am J Dermatopathol. 1998;20:522-526.
- Savarrio L, Gibson J, Dunlop DJ, et al. Spontaneous regression of an anaplastic large cell lymphoma in the oral cavity: first reported case and review of the literature. Oral Oncol. 1999;35:609-613.
- Sciubba J, Said-Al-Naief N, Fantasia J. Critical review of lymphomatoid papulosis of the oral cavity with case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:195-204.
- Yamazaki H, Shirasugi Y, Kajiwara H, et al. Concurrent onset of eosinophilic ulcer of the oral mucosa with peripheral eosinophilia in a human T-cell leukemia virus type I carrier. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:E43-E48.
- Dojcinov SD, Venkataram G, Raffeld M, et al. EBV positive mucocutaneous ulcer—a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405-417.
- Kim YC, Yang WI, Lee MG, et al. Epstein-Barr virus in CD30 anaplastic large cell lymphoma involving the skin and lymphomatoid papulosis in South Korea. Int J Dermatol. 2006;45:1312-1316.
- Pietersma F, Piriou E, van Baarle D. Immune surveillance of EBV-infected B cells and the development of non-Hodgkin lymphomas in immunocompromised patients. Leuk Lymphoma. 2008;49:1028-1041.
- Salisbury CL, Budnick SD, Li S. T cell receptor gene rearrangement and CD 30 immunoreactivity in traumatic ulcerative granuloma with stromal eosinophilia of oral cavity. Am J Clin Pathol. 2009;132:722-727.
- Marszalek A, Neska-Dlugosz I. Traumatic ulcerative granuloma with stromal eosinophilia. a case report and short literature review. Pol J Pathol. 2011;3:172-175.
- Wong DT, Donoff RB, Yang J, et al. Sequential expression of transforming growth factors alpha and beta 1 by eosinophils during cutaneous wound healing in the hamster. Am J Pathol. 1993;143:130-142.
- Elovic AE, Gallagher GT, Kabani S, et al. Lack of TGF-alpha and TGF-beta synthesis by human eosinophils in chronic oral ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:672-681.
- Hirshberg A, Amariglio N, Akrish S, et al. Traumatic ulcerative granuloma with stromal eosinophilia: reactive lesion of the oral mucosa. Am J Clin Pathol. 2006;126:522-529.
- Abdel-Naser MB, Tsatsou F, Hippe S, et al. Oral eosinophilic ulcer, an Epstein-Barr virus-associated CD30+ lymphoproliferation? [published online April 5, 2011]. Dermatology. 2011;222:113-118.
- Fonseca FP, Benevenuto de Andrade BA, Coletta RD, et al. Clinicopathological and immunohistochemical analysis of 19 cases of oral eosinophilic ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:532-540.
- Alobeid B, Pan LX, Milligan L, et al. Eosinophil-rich CD30+ lymphoproliferative disorder of the oral mucosa. Am J Clin Pathol. 2004;121:43-50.
- Hjorting-Hansen E, Schmidt H. Ulcerated granuloma eosinophilicum diutinum of the tongue. report of a case. Acta Derm Venereol. 1961;41:235-239.
- Velez A, Alamillos FJ, Dean A, et al. Eosinophilic ulcer of the oral mucosa: report of a recurrent case on the tongue. Clin Exp Dermatol. 1997;22:154-156.
- Elzay RP. Traumatic ulcerative granuloma with stromal eosinophilia (Riga-Fede’s disease and traumatic eosinophilic granuloma). Oral Surg Oral Med Oral Pathol. 1983;55:497-506.
- Val-Bernal JF, Gonzalez-Vela MC, Sanchez-Santolino S, et al. Localized eosinophilic (Langerhans’ cell) granuloma of the lower lip. a lesion that may cause diagnostic error. J Cutan Pathol. 2009;36:1109-1113.
- Shapiro L, Juhlin EA. Eosinophilic ulcer of the tongue report of two cases and review of the literature. Dermatologica. 1970;140:242-250.
- Amberg S. Sublingual growth in infants. Am J Med Sci. 1902;126:257-269.
- EI-Mofty SK, Swanson PE, Wick MR, et al. Eosinophilic ulcer of the oral mucosa: report of 38 new cases with immunohistochemical observations. Oral Surg Oral Med Oral Pathol. 1993;75:716-722.
- Regezi JA, Zarbo RJ, Daniels TE, et al. Oral traumatic granuloma: characterization of the cellular infiltrate. Oral Surg Oral Med Oral Pathol. 1993;75:723-727.
- Ficarra G, Prignano F, Romagnoli P. Traumatic eosinophilic granuloma of the oral mucosa: a CD30+ (Ki-1) lymphoproliferative disorder? Oral Oncol. 1997;33:375-379.
- Doyle JL, Geary W, Baden E. Eosinophilic ulcer. J Oral Maxillofac Surg. 1989;47:349-352.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848-858.
- Rosenberg A, Biesma DH, Sie-Go DMDS, et al. Primary extranodal CD30-positive T-cell non-Hodgkin’s lymphoma of the oral mucosa. report of two cases. Int J Oral Maxillofac Surg. 1996;25:57-59.
- Kato N, Tomita Y, Yoshida K, et al. Involvement of the tongue by lymphomatoid papulosis. Am J Dermatopathol. 1998;20:522-526.
- Savarrio L, Gibson J, Dunlop DJ, et al. Spontaneous regression of an anaplastic large cell lymphoma in the oral cavity: first reported case and review of the literature. Oral Oncol. 1999;35:609-613.
- Sciubba J, Said-Al-Naief N, Fantasia J. Critical review of lymphomatoid papulosis of the oral cavity with case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:195-204.
- Yamazaki H, Shirasugi Y, Kajiwara H, et al. Concurrent onset of eosinophilic ulcer of the oral mucosa with peripheral eosinophilia in a human T-cell leukemia virus type I carrier. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:E43-E48.
- Dojcinov SD, Venkataram G, Raffeld M, et al. EBV positive mucocutaneous ulcer—a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405-417.
- Kim YC, Yang WI, Lee MG, et al. Epstein-Barr virus in CD30 anaplastic large cell lymphoma involving the skin and lymphomatoid papulosis in South Korea. Int J Dermatol. 2006;45:1312-1316.
- Pietersma F, Piriou E, van Baarle D. Immune surveillance of EBV-infected B cells and the development of non-Hodgkin lymphomas in immunocompromised patients. Leuk Lymphoma. 2008;49:1028-1041.
- Salisbury CL, Budnick SD, Li S. T cell receptor gene rearrangement and CD 30 immunoreactivity in traumatic ulcerative granuloma with stromal eosinophilia of oral cavity. Am J Clin Pathol. 2009;132:722-727.
- Marszalek A, Neska-Dlugosz I. Traumatic ulcerative granuloma with stromal eosinophilia. a case report and short literature review. Pol J Pathol. 2011;3:172-175.
- Wong DT, Donoff RB, Yang J, et al. Sequential expression of transforming growth factors alpha and beta 1 by eosinophils during cutaneous wound healing in the hamster. Am J Pathol. 1993;143:130-142.
- Elovic AE, Gallagher GT, Kabani S, et al. Lack of TGF-alpha and TGF-beta synthesis by human eosinophils in chronic oral ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:672-681.
Practice Points
- Immunohistochemical staining of traumatic ulcerative granuloma with stromal eosinophilia (TUGSE) may suggest an underlying lymphoproliferative disorder.
- Early recognition of TUGSE, which often is malignant appearing, is key, with watchful waiting as the mainstay therapy.
- Adjunctive therapy for TUGSE includes prednisolone and oral analgesics.
ASCO issues new guideline on stage IV NSCLC
The American Society of Clinical Oncology has updated its clinical practice guideline on systemic therapy for stage IV non–small-cell lung cancer (NSCLC), incorporating recommendations on using checkpoint inhibitors in these patients.
The guideline was published recently on the Journal of Clinical Oncology website (J Clin Oncol. 2017 Aug 14. doi: 10.1200/JCO.2017.74.6065).
Among the recommendations is that, for patients with non–squamous cell carcinoma or squamous cell carcinoma without positive markers such as epidermal growth factor receptor who have high programmed death ligand 1 (PD-L1) expression, first-line treatment should be pembrolizumab alone. For those with low PD-L1 expression, standard chemotherapy should be used.
For second-line treatment in patients who received first-line chemotherapy without prior immune checkpoint treatment, if the NSCLC tumor is positive for PD-L1 expression, single-agent nivolumab, pembrolizumab, or atezolizumab should be used.
The guideline is an update of its 2015 recommendations. An expert panel made the changes after a systematic review of randomized controlled trials from February 2014 to December 2016.
Panelists said that there’s still a lot to learn about the use of checkpoint inhibitors in these patients.
“Cancer immunotherapy allows some patients to live longer with a better quality of life than chemotherapy; however, not all patients respond to this treatment,” panelists wrote. “Many factors remain unknown in the understanding of optimal sequencing of immune checkpoint therapy and other agents previously recommended in ASCO guidelines. Contraindications to receiving immune checkpoint therapy are not yet well defined.”
Several panelists report receiving research funding and/or consulting fees from Merck, Bristol-Meyers Squibb, Peloton Therapeutics, Genentech, and other companies.
The American Society of Clinical Oncology has updated its clinical practice guideline on systemic therapy for stage IV non–small-cell lung cancer (NSCLC), incorporating recommendations on using checkpoint inhibitors in these patients.
The guideline was published recently on the Journal of Clinical Oncology website (J Clin Oncol. 2017 Aug 14. doi: 10.1200/JCO.2017.74.6065).
Among the recommendations is that, for patients with non–squamous cell carcinoma or squamous cell carcinoma without positive markers such as epidermal growth factor receptor who have high programmed death ligand 1 (PD-L1) expression, first-line treatment should be pembrolizumab alone. For those with low PD-L1 expression, standard chemotherapy should be used.
For second-line treatment in patients who received first-line chemotherapy without prior immune checkpoint treatment, if the NSCLC tumor is positive for PD-L1 expression, single-agent nivolumab, pembrolizumab, or atezolizumab should be used.
The guideline is an update of its 2015 recommendations. An expert panel made the changes after a systematic review of randomized controlled trials from February 2014 to December 2016.
Panelists said that there’s still a lot to learn about the use of checkpoint inhibitors in these patients.
“Cancer immunotherapy allows some patients to live longer with a better quality of life than chemotherapy; however, not all patients respond to this treatment,” panelists wrote. “Many factors remain unknown in the understanding of optimal sequencing of immune checkpoint therapy and other agents previously recommended in ASCO guidelines. Contraindications to receiving immune checkpoint therapy are not yet well defined.”
Several panelists report receiving research funding and/or consulting fees from Merck, Bristol-Meyers Squibb, Peloton Therapeutics, Genentech, and other companies.
The American Society of Clinical Oncology has updated its clinical practice guideline on systemic therapy for stage IV non–small-cell lung cancer (NSCLC), incorporating recommendations on using checkpoint inhibitors in these patients.
The guideline was published recently on the Journal of Clinical Oncology website (J Clin Oncol. 2017 Aug 14. doi: 10.1200/JCO.2017.74.6065).
Among the recommendations is that, for patients with non–squamous cell carcinoma or squamous cell carcinoma without positive markers such as epidermal growth factor receptor who have high programmed death ligand 1 (PD-L1) expression, first-line treatment should be pembrolizumab alone. For those with low PD-L1 expression, standard chemotherapy should be used.
For second-line treatment in patients who received first-line chemotherapy without prior immune checkpoint treatment, if the NSCLC tumor is positive for PD-L1 expression, single-agent nivolumab, pembrolizumab, or atezolizumab should be used.
The guideline is an update of its 2015 recommendations. An expert panel made the changes after a systematic review of randomized controlled trials from February 2014 to December 2016.
Panelists said that there’s still a lot to learn about the use of checkpoint inhibitors in these patients.
“Cancer immunotherapy allows some patients to live longer with a better quality of life than chemotherapy; however, not all patients respond to this treatment,” panelists wrote. “Many factors remain unknown in the understanding of optimal sequencing of immune checkpoint therapy and other agents previously recommended in ASCO guidelines. Contraindications to receiving immune checkpoint therapy are not yet well defined.”
Several panelists report receiving research funding and/or consulting fees from Merck, Bristol-Meyers Squibb, Peloton Therapeutics, Genentech, and other companies.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The American Society of Clinical Oncology has issued its latest clinical practice guideline on the systemic treatment of non–small-cell lung cancer, an update of its 2015 guideline.
Major finding: For patients with non–squamous cell carcinoma or squamous cell carcinoma without positive markers such as epidermal growth factor receptor (EGFR), if a patient has high programmed death ligand 1 (PD-L1) expression, first-line treatment should be pembrolizumab alone. For those with low PD-L1 expression, standard chemotherapy should be used.
Data source: A systematic review of randomized controlled trials from February 2014 to December 2016.
Disclosures: Several panelists report receiving research funding and/or consulting fees from Merck, Bristol-Meyers Squibb, Peloton Therapeutics, Genentech, and other companies.