User login
How Do Autonomic Symptoms Affect Patients With Early Parkinson’s Disease?
PORTLAND, OR—Autonomic symptoms are present in a majority of patients with early Parkinson's disease, and symptoms correlate with markers of disease severity and impaired quality of life, according to research presented at the Fourth World Parkinson Congress.
Although autonomic dysfunction is common in the later stages of Parkinson's disease, less is known about autonomic symptom presence and severity in early stages of Parkinson's disease. Naveed Malek, MD, Department of Neurology, Institute of Neurological Sciences, Queen Elizabeth University Hospital, in Glasgow, and colleagues conducted a study to explore the prevalence, range, and severity of autonomic symptoms in a cohort of patients who had been diagnosed with Parkinson's disease in the preceding 3.5 years.
The researchers analyzed detailed patient-reported symptoms of autonomic dysfunction that were assessed in the multicenter United Kingdom Tracking Parkinson's study using the Scale for Outcomes in Parkinson's Disease for Autonomic Symptoms (SCOPA-AUT).
The researchers assessed the relationship between baseline SCOPA-AUT score and baseline motor, nonmotor, and quality of life scores. The researchers adjusted for sex, age, and disease duration differences across groups.
A total of 1,738 patients (65.1% male, mean age 67.6) were included in the main analysis. Patients had a mean disease duration of 1.3 years and mean Movement Disorder Society Unified Parkinson's Disease Rating Scale motor score of 22.5. Hoehn and Yahr was stage 1 or 1.5 in 855 cases (49.2%), stage 2 or 2.5 in 783 cases (45.1%), and stage 3 or higher in 100 cases (5.8%).
Autonomic severity by SCOPA-AUT score increased significantly across the motor severity stages, from 10.7 for Hoehn and Yahr stages 1 and 1.5, to 12.7 for Hoehn and Yahr stages 2 and 2.5, and to 13.5 for Hoehn and Yahr stages 3 and higher. Urinary, bowel, and sexual dysfunctions were the most commonly reported autonomic symptoms. The results were between those previously reported in controls and in patients with Parkinson's disease at a mean duration of 10 years. Positive associations were present between autonomic severity and depression, sleep disturbance, and postural instability motor subtype.
"Our study highlights the extent to which we may expect to see autonomic features in early Parkinson's disease, and the relationship between autonomic features and diagnostic consideration, which is important in the balanced diagnostic judgment approach emphasized in recent consensus guidelines," Dr. Malek concluded.
—Jake Remaly
PORTLAND, OR—Autonomic symptoms are present in a majority of patients with early Parkinson's disease, and symptoms correlate with markers of disease severity and impaired quality of life, according to research presented at the Fourth World Parkinson Congress.
Although autonomic dysfunction is common in the later stages of Parkinson's disease, less is known about autonomic symptom presence and severity in early stages of Parkinson's disease. Naveed Malek, MD, Department of Neurology, Institute of Neurological Sciences, Queen Elizabeth University Hospital, in Glasgow, and colleagues conducted a study to explore the prevalence, range, and severity of autonomic symptoms in a cohort of patients who had been diagnosed with Parkinson's disease in the preceding 3.5 years.
The researchers analyzed detailed patient-reported symptoms of autonomic dysfunction that were assessed in the multicenter United Kingdom Tracking Parkinson's study using the Scale for Outcomes in Parkinson's Disease for Autonomic Symptoms (SCOPA-AUT).
The researchers assessed the relationship between baseline SCOPA-AUT score and baseline motor, nonmotor, and quality of life scores. The researchers adjusted for sex, age, and disease duration differences across groups.
A total of 1,738 patients (65.1% male, mean age 67.6) were included in the main analysis. Patients had a mean disease duration of 1.3 years and mean Movement Disorder Society Unified Parkinson's Disease Rating Scale motor score of 22.5. Hoehn and Yahr was stage 1 or 1.5 in 855 cases (49.2%), stage 2 or 2.5 in 783 cases (45.1%), and stage 3 or higher in 100 cases (5.8%).
Autonomic severity by SCOPA-AUT score increased significantly across the motor severity stages, from 10.7 for Hoehn and Yahr stages 1 and 1.5, to 12.7 for Hoehn and Yahr stages 2 and 2.5, and to 13.5 for Hoehn and Yahr stages 3 and higher. Urinary, bowel, and sexual dysfunctions were the most commonly reported autonomic symptoms. The results were between those previously reported in controls and in patients with Parkinson's disease at a mean duration of 10 years. Positive associations were present between autonomic severity and depression, sleep disturbance, and postural instability motor subtype.
"Our study highlights the extent to which we may expect to see autonomic features in early Parkinson's disease, and the relationship between autonomic features and diagnostic consideration, which is important in the balanced diagnostic judgment approach emphasized in recent consensus guidelines," Dr. Malek concluded.
—Jake Remaly
PORTLAND, OR—Autonomic symptoms are present in a majority of patients with early Parkinson's disease, and symptoms correlate with markers of disease severity and impaired quality of life, according to research presented at the Fourth World Parkinson Congress.
Although autonomic dysfunction is common in the later stages of Parkinson's disease, less is known about autonomic symptom presence and severity in early stages of Parkinson's disease. Naveed Malek, MD, Department of Neurology, Institute of Neurological Sciences, Queen Elizabeth University Hospital, in Glasgow, and colleagues conducted a study to explore the prevalence, range, and severity of autonomic symptoms in a cohort of patients who had been diagnosed with Parkinson's disease in the preceding 3.5 years.
The researchers analyzed detailed patient-reported symptoms of autonomic dysfunction that were assessed in the multicenter United Kingdom Tracking Parkinson's study using the Scale for Outcomes in Parkinson's Disease for Autonomic Symptoms (SCOPA-AUT).
The researchers assessed the relationship between baseline SCOPA-AUT score and baseline motor, nonmotor, and quality of life scores. The researchers adjusted for sex, age, and disease duration differences across groups.
A total of 1,738 patients (65.1% male, mean age 67.6) were included in the main analysis. Patients had a mean disease duration of 1.3 years and mean Movement Disorder Society Unified Parkinson's Disease Rating Scale motor score of 22.5. Hoehn and Yahr was stage 1 or 1.5 in 855 cases (49.2%), stage 2 or 2.5 in 783 cases (45.1%), and stage 3 or higher in 100 cases (5.8%).
Autonomic severity by SCOPA-AUT score increased significantly across the motor severity stages, from 10.7 for Hoehn and Yahr stages 1 and 1.5, to 12.7 for Hoehn and Yahr stages 2 and 2.5, and to 13.5 for Hoehn and Yahr stages 3 and higher. Urinary, bowel, and sexual dysfunctions were the most commonly reported autonomic symptoms. The results were between those previously reported in controls and in patients with Parkinson's disease at a mean duration of 10 years. Positive associations were present between autonomic severity and depression, sleep disturbance, and postural instability motor subtype.
"Our study highlights the extent to which we may expect to see autonomic features in early Parkinson's disease, and the relationship between autonomic features and diagnostic consideration, which is important in the balanced diagnostic judgment approach emphasized in recent consensus guidelines," Dr. Malek concluded.
—Jake Remaly
Common Genetic Variants Associated With Cognitive Impairment in Parkinson’s Disease
PORTLAND, OR—A large-scale analysis has identified several new putative susceptibility genes that may affect cognitive impairment in Parkinson's disease, researchers reported at the Fourth World Parkinson Congress.
Cognitive impairment is a common and disabling nonmotor feature of Parkinson's disease. Identifying genetic variants that influence cognitive deficits could clarify the pathophysiology of cognitive impairment, help identify potential therapies, and be useful when considering patient prognosis, said Ignacio F. Mata, PhD, Research Biologist at the Veterans Affairs Puget Sound Health Care System and Acting Assistant Professor of Neurology at the University of Washington in Seattle. Rate of cognitive decline in Parkinson's disease varies. "We are trying to understand why," Dr. Mata said.
Dr. Mata and colleagues conducted a genome-wide exploratory analysis of genetic risk factors for cognitive impairment in a multisite cohort. The investigators genotyped 1,105 patients from the Parkinson's Disease Cognitive Genetics Consortium for 249,336 variants using the NeuroX array, which includes variants that have been linked to neurologic diseases.
Participants completed tests of learning and memory (Hopkins Verbal Learning Test-Revised), working memory and executive function (Letter-Number Sequencing and Trail Making Test Parts A and B), language processing (semantic and phonemic verbal fluency), visuospatial abilities (Benton Judgment of Line Orientation), and global cognitive function (Montreal Cognitive Assessment).
The researchers used linear regression to test for associations between common variants and cognitive performance, with adjustment for important covariates (eg, age, sex, disease duration, and years of education). They analyzed rare variants using the optimal unified sequence kernel association test.
Participants' mean age was 68.8, 67.8% were male, and 17.5% had dementia. Mean disease duration was 8.4 years. Participants had 15.5 years of education on average.
The researchers identified 17 common genetic variants associated with cognitive performance. Three variants were associated with total recall, and two variants were associated with delayed recall on the revised Hopkins Verbal Learning Test. Five variants were associated with performance on the Trail Making Test, and seven variants were associated with performance on the Benton Judgment of Line Orientation test.
The researchers aim to perform a similar analysis using longitudinal data. They also plan to see if these findings can be replicated in an independent cohort.
—Jake Remaly
PORTLAND, OR—A large-scale analysis has identified several new putative susceptibility genes that may affect cognitive impairment in Parkinson's disease, researchers reported at the Fourth World Parkinson Congress.
Cognitive impairment is a common and disabling nonmotor feature of Parkinson's disease. Identifying genetic variants that influence cognitive deficits could clarify the pathophysiology of cognitive impairment, help identify potential therapies, and be useful when considering patient prognosis, said Ignacio F. Mata, PhD, Research Biologist at the Veterans Affairs Puget Sound Health Care System and Acting Assistant Professor of Neurology at the University of Washington in Seattle. Rate of cognitive decline in Parkinson's disease varies. "We are trying to understand why," Dr. Mata said.
Dr. Mata and colleagues conducted a genome-wide exploratory analysis of genetic risk factors for cognitive impairment in a multisite cohort. The investigators genotyped 1,105 patients from the Parkinson's Disease Cognitive Genetics Consortium for 249,336 variants using the NeuroX array, which includes variants that have been linked to neurologic diseases.
Participants completed tests of learning and memory (Hopkins Verbal Learning Test-Revised), working memory and executive function (Letter-Number Sequencing and Trail Making Test Parts A and B), language processing (semantic and phonemic verbal fluency), visuospatial abilities (Benton Judgment of Line Orientation), and global cognitive function (Montreal Cognitive Assessment).
The researchers used linear regression to test for associations between common variants and cognitive performance, with adjustment for important covariates (eg, age, sex, disease duration, and years of education). They analyzed rare variants using the optimal unified sequence kernel association test.
Participants' mean age was 68.8, 67.8% were male, and 17.5% had dementia. Mean disease duration was 8.4 years. Participants had 15.5 years of education on average.
The researchers identified 17 common genetic variants associated with cognitive performance. Three variants were associated with total recall, and two variants were associated with delayed recall on the revised Hopkins Verbal Learning Test. Five variants were associated with performance on the Trail Making Test, and seven variants were associated with performance on the Benton Judgment of Line Orientation test.
The researchers aim to perform a similar analysis using longitudinal data. They also plan to see if these findings can be replicated in an independent cohort.
—Jake Remaly
PORTLAND, OR—A large-scale analysis has identified several new putative susceptibility genes that may affect cognitive impairment in Parkinson's disease, researchers reported at the Fourth World Parkinson Congress.
Cognitive impairment is a common and disabling nonmotor feature of Parkinson's disease. Identifying genetic variants that influence cognitive deficits could clarify the pathophysiology of cognitive impairment, help identify potential therapies, and be useful when considering patient prognosis, said Ignacio F. Mata, PhD, Research Biologist at the Veterans Affairs Puget Sound Health Care System and Acting Assistant Professor of Neurology at the University of Washington in Seattle. Rate of cognitive decline in Parkinson's disease varies. "We are trying to understand why," Dr. Mata said.
Dr. Mata and colleagues conducted a genome-wide exploratory analysis of genetic risk factors for cognitive impairment in a multisite cohort. The investigators genotyped 1,105 patients from the Parkinson's Disease Cognitive Genetics Consortium for 249,336 variants using the NeuroX array, which includes variants that have been linked to neurologic diseases.
Participants completed tests of learning and memory (Hopkins Verbal Learning Test-Revised), working memory and executive function (Letter-Number Sequencing and Trail Making Test Parts A and B), language processing (semantic and phonemic verbal fluency), visuospatial abilities (Benton Judgment of Line Orientation), and global cognitive function (Montreal Cognitive Assessment).
The researchers used linear regression to test for associations between common variants and cognitive performance, with adjustment for important covariates (eg, age, sex, disease duration, and years of education). They analyzed rare variants using the optimal unified sequence kernel association test.
Participants' mean age was 68.8, 67.8% were male, and 17.5% had dementia. Mean disease duration was 8.4 years. Participants had 15.5 years of education on average.
The researchers identified 17 common genetic variants associated with cognitive performance. Three variants were associated with total recall, and two variants were associated with delayed recall on the revised Hopkins Verbal Learning Test. Five variants were associated with performance on the Trail Making Test, and seven variants were associated with performance on the Benton Judgment of Line Orientation test.
The researchers aim to perform a similar analysis using longitudinal data. They also plan to see if these findings can be replicated in an independent cohort.
—Jake Remaly
New research on health-related behaviors of sexual minority youth
The Centers for Disease Control and Prevention released results from the first nationally representative study on health risk behaviors of gay, lesbian, and bisexual (GLB) high school students in August 2016.
These data were collected through the Youth Risk Behavior Survey (YRBS) questionnaire. The YRBS questionnaire was developed in 1990 as a way to monitor health-related behaviors that contribute to the leading causes of mortality and morbidity in youth and young adults. Areas covered by the survey include behaviors related to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, dietary behaviors, and physical activity. Data are collected every 2 years through national, state, territorial, tribal government, and local school-based surveys of representative samples of 9th-12th grade students.
For the study, sexual minority youth were defined as those who identified as GLB; those who reported sexual contact with members of the same sex only; and those who reported sexual contact with members of both sexes. It is important to note that the YRBS is a school-based survey and does not include youth who do not attend school, for example, homeless and runaway youth.
Exploring and identifying disparities in health behaviors that affect sexual minorities can help us as providers to better target screenings for these health behaviors at the individual level. At the population level, it is important to continue to explore why these differences exist and to continue to develop interventions that help address these differences, while educating families and communities about how to support all of their youth. It is important to note that the majority of sexual minority youth live healthy live; however, this study shows that sexual minority youth do have a higher prevalence of certain health risk behaviors, likely leading to the health disparities we see in this population. Select findings of this study are summarized in the accompanying table.
Continued study is needed to understand the health disparities that occur in sexual minority populations. In October, the National Institutes of Health designated sexual and gender minorities as a specific health disparity population for NIH research. This term encompasses lesbian, gay, bisexual, and transgender individuals as well as any individuals whose sexual identity or gender identity does not align with traditional norms. This hopefully will lead to a growing body of evidence to help all of us learn about the spectrum of sexual and gender identity and better help sexual and gender minority youth reach their full potential.
For more information about the YRBS and the report on health related behaviors in sexual minority youth visit this link:
Selected questionnaire results
Sexual identity
• 88.8% of students identified as heterosexual.
• 6.0% identified as bisexual.
• 3.2% were not sure.
• 2.0% identified as gay or lesbian.
Sexual behaviors
• 48% had had sexual contact with the opposite sex only.
• 4.6% had sexual contact with both sexes.
• 1.7% had had sexual contact with the same sex only.
• 45.7% had no sexual contact.
Mental health
Percent of students who reported making a suicide plan in the 12 months preceding the survey:
• 11.9% of heterosexual students.
• 27.9% of students not sure of sexual identity.
• 38.2% of gay, lesbian, bisexual (GLB) students.
Percent of students who attempted suicide in the 12 months preceding the survey:
• 6.4% of heterosexual students.
• 13.7% of students not sure of sexual identity.
• 29.4% of GLB students.
Sexual Behaviors
First sex before the age of 13:
• 3.4% of heterosexual students.
• 8.8% of students not sure of their sexual identity.
• 7.3% of GLB students.
Drank alcohol or used drugs before last sex:
• 20.0% of heterosexual students.
• 44.5% of students not sure of their sexual identity.
• 22.4% of GLB students.
Tested for HIV:
• 9.3% of heterosexual students.
• 12.8% of students not sure of their sexual identity.
• 18.2% of GLB students.
Substance use
Currently smoking cigarettes daily:
• 1.9% of heterosexual students.
• 7.0% of students not sure of their sexual identity.
• 4.0% of GLB students.
Current alcohol use:
• 32.1% of heterosexual students.
• 34.6% of students not sure of their sexual identity.
• 40.5% of GLB students.
Current marijuana use:
• 20.7% of heterosexual students.
• 26.0% of students not sure of their sexual identity.
• 32.0% of GLB students.
Used hallucinogenic drugs (such as LSD, acid, PCP, angel dust, mescaline, or mushrooms):
• 5.5% of heterosexual students.
• 15.7% of students not sure of their sexual identity.
• 11.5% of GLB students.
Ever used heroin:
• 1.3% of heterosexual students.
• 9.3% of students not sure of their sexual identity.
• 6.0% of GLB students.
Ever took prescription drugs without a doctor’s prescription:
15.5% of heterosexual students.
24.3% of students not sure of their sexual identity.
27.5% of GLB students.
Physical Activity
Did not participate in at least 60 minutes of physical activity on at least 1 day in past week:
• 12.6% of heterosexual students.
• 27.0% of students not sure of their sexual identity.
• 25.7% of GLB students.
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
The Centers for Disease Control and Prevention released results from the first nationally representative study on health risk behaviors of gay, lesbian, and bisexual (GLB) high school students in August 2016.
These data were collected through the Youth Risk Behavior Survey (YRBS) questionnaire. The YRBS questionnaire was developed in 1990 as a way to monitor health-related behaviors that contribute to the leading causes of mortality and morbidity in youth and young adults. Areas covered by the survey include behaviors related to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, dietary behaviors, and physical activity. Data are collected every 2 years through national, state, territorial, tribal government, and local school-based surveys of representative samples of 9th-12th grade students.
For the study, sexual minority youth were defined as those who identified as GLB; those who reported sexual contact with members of the same sex only; and those who reported sexual contact with members of both sexes. It is important to note that the YRBS is a school-based survey and does not include youth who do not attend school, for example, homeless and runaway youth.
Exploring and identifying disparities in health behaviors that affect sexual minorities can help us as providers to better target screenings for these health behaviors at the individual level. At the population level, it is important to continue to explore why these differences exist and to continue to develop interventions that help address these differences, while educating families and communities about how to support all of their youth. It is important to note that the majority of sexual minority youth live healthy live; however, this study shows that sexual minority youth do have a higher prevalence of certain health risk behaviors, likely leading to the health disparities we see in this population. Select findings of this study are summarized in the accompanying table.
Continued study is needed to understand the health disparities that occur in sexual minority populations. In October, the National Institutes of Health designated sexual and gender minorities as a specific health disparity population for NIH research. This term encompasses lesbian, gay, bisexual, and transgender individuals as well as any individuals whose sexual identity or gender identity does not align with traditional norms. This hopefully will lead to a growing body of evidence to help all of us learn about the spectrum of sexual and gender identity and better help sexual and gender minority youth reach their full potential.
For more information about the YRBS and the report on health related behaviors in sexual minority youth visit this link:
Selected questionnaire results
Sexual identity
• 88.8% of students identified as heterosexual.
• 6.0% identified as bisexual.
• 3.2% were not sure.
• 2.0% identified as gay or lesbian.
Sexual behaviors
• 48% had had sexual contact with the opposite sex only.
• 4.6% had sexual contact with both sexes.
• 1.7% had had sexual contact with the same sex only.
• 45.7% had no sexual contact.
Mental health
Percent of students who reported making a suicide plan in the 12 months preceding the survey:
• 11.9% of heterosexual students.
• 27.9% of students not sure of sexual identity.
• 38.2% of gay, lesbian, bisexual (GLB) students.
Percent of students who attempted suicide in the 12 months preceding the survey:
• 6.4% of heterosexual students.
• 13.7% of students not sure of sexual identity.
• 29.4% of GLB students.
Sexual Behaviors
First sex before the age of 13:
• 3.4% of heterosexual students.
• 8.8% of students not sure of their sexual identity.
• 7.3% of GLB students.
Drank alcohol or used drugs before last sex:
• 20.0% of heterosexual students.
• 44.5% of students not sure of their sexual identity.
• 22.4% of GLB students.
Tested for HIV:
• 9.3% of heterosexual students.
• 12.8% of students not sure of their sexual identity.
• 18.2% of GLB students.
Substance use
Currently smoking cigarettes daily:
• 1.9% of heterosexual students.
• 7.0% of students not sure of their sexual identity.
• 4.0% of GLB students.
Current alcohol use:
• 32.1% of heterosexual students.
• 34.6% of students not sure of their sexual identity.
• 40.5% of GLB students.
Current marijuana use:
• 20.7% of heterosexual students.
• 26.0% of students not sure of their sexual identity.
• 32.0% of GLB students.
Used hallucinogenic drugs (such as LSD, acid, PCP, angel dust, mescaline, or mushrooms):
• 5.5% of heterosexual students.
• 15.7% of students not sure of their sexual identity.
• 11.5% of GLB students.
Ever used heroin:
• 1.3% of heterosexual students.
• 9.3% of students not sure of their sexual identity.
• 6.0% of GLB students.
Ever took prescription drugs without a doctor’s prescription:
15.5% of heterosexual students.
24.3% of students not sure of their sexual identity.
27.5% of GLB students.
Physical Activity
Did not participate in at least 60 minutes of physical activity on at least 1 day in past week:
• 12.6% of heterosexual students.
• 27.0% of students not sure of their sexual identity.
• 25.7% of GLB students.
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
The Centers for Disease Control and Prevention released results from the first nationally representative study on health risk behaviors of gay, lesbian, and bisexual (GLB) high school students in August 2016.
These data were collected through the Youth Risk Behavior Survey (YRBS) questionnaire. The YRBS questionnaire was developed in 1990 as a way to monitor health-related behaviors that contribute to the leading causes of mortality and morbidity in youth and young adults. Areas covered by the survey include behaviors related to unintentional injuries and violence, tobacco use, alcohol and other drug use, sexual behaviors, dietary behaviors, and physical activity. Data are collected every 2 years through national, state, territorial, tribal government, and local school-based surveys of representative samples of 9th-12th grade students.
For the study, sexual minority youth were defined as those who identified as GLB; those who reported sexual contact with members of the same sex only; and those who reported sexual contact with members of both sexes. It is important to note that the YRBS is a school-based survey and does not include youth who do not attend school, for example, homeless and runaway youth.
Exploring and identifying disparities in health behaviors that affect sexual minorities can help us as providers to better target screenings for these health behaviors at the individual level. At the population level, it is important to continue to explore why these differences exist and to continue to develop interventions that help address these differences, while educating families and communities about how to support all of their youth. It is important to note that the majority of sexual minority youth live healthy live; however, this study shows that sexual minority youth do have a higher prevalence of certain health risk behaviors, likely leading to the health disparities we see in this population. Select findings of this study are summarized in the accompanying table.
Continued study is needed to understand the health disparities that occur in sexual minority populations. In October, the National Institutes of Health designated sexual and gender minorities as a specific health disparity population for NIH research. This term encompasses lesbian, gay, bisexual, and transgender individuals as well as any individuals whose sexual identity or gender identity does not align with traditional norms. This hopefully will lead to a growing body of evidence to help all of us learn about the spectrum of sexual and gender identity and better help sexual and gender minority youth reach their full potential.
For more information about the YRBS and the report on health related behaviors in sexual minority youth visit this link:
Selected questionnaire results
Sexual identity
• 88.8% of students identified as heterosexual.
• 6.0% identified as bisexual.
• 3.2% were not sure.
• 2.0% identified as gay or lesbian.
Sexual behaviors
• 48% had had sexual contact with the opposite sex only.
• 4.6% had sexual contact with both sexes.
• 1.7% had had sexual contact with the same sex only.
• 45.7% had no sexual contact.
Mental health
Percent of students who reported making a suicide plan in the 12 months preceding the survey:
• 11.9% of heterosexual students.
• 27.9% of students not sure of sexual identity.
• 38.2% of gay, lesbian, bisexual (GLB) students.
Percent of students who attempted suicide in the 12 months preceding the survey:
• 6.4% of heterosexual students.
• 13.7% of students not sure of sexual identity.
• 29.4% of GLB students.
Sexual Behaviors
First sex before the age of 13:
• 3.4% of heterosexual students.
• 8.8% of students not sure of their sexual identity.
• 7.3% of GLB students.
Drank alcohol or used drugs before last sex:
• 20.0% of heterosexual students.
• 44.5% of students not sure of their sexual identity.
• 22.4% of GLB students.
Tested for HIV:
• 9.3% of heterosexual students.
• 12.8% of students not sure of their sexual identity.
• 18.2% of GLB students.
Substance use
Currently smoking cigarettes daily:
• 1.9% of heterosexual students.
• 7.0% of students not sure of their sexual identity.
• 4.0% of GLB students.
Current alcohol use:
• 32.1% of heterosexual students.
• 34.6% of students not sure of their sexual identity.
• 40.5% of GLB students.
Current marijuana use:
• 20.7% of heterosexual students.
• 26.0% of students not sure of their sexual identity.
• 32.0% of GLB students.
Used hallucinogenic drugs (such as LSD, acid, PCP, angel dust, mescaline, or mushrooms):
• 5.5% of heterosexual students.
• 15.7% of students not sure of their sexual identity.
• 11.5% of GLB students.
Ever used heroin:
• 1.3% of heterosexual students.
• 9.3% of students not sure of their sexual identity.
• 6.0% of GLB students.
Ever took prescription drugs without a doctor’s prescription:
15.5% of heterosexual students.
24.3% of students not sure of their sexual identity.
27.5% of GLB students.
Physical Activity
Did not participate in at least 60 minutes of physical activity on at least 1 day in past week:
• 12.6% of heterosexual students.
• 27.0% of students not sure of their sexual identity.
• 25.7% of GLB students.
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at the Ohio State University, both in Columbus.
Metabolic syndrome predicts cardiovascular events in HBV infection
Metabolic syndrome was associated with a fourfold rise in cardiovascular events among patients with chronic hepatitis B virus infection, according to a prospective cohort study published in Hepatology.
Over a median of 7.3 years of follow-up, 8% of patients with metabolic syndrome developed ischemic or hemorrhagic stroke, acute coronary syndrome, or congestive heart failure, or underwent revascularization, compared with 2% of patients without metabolic syndrome (P less than .0001), reported Jenny Yeuk-Ki Cheng, MD, and her associates at The Chinese University of Hong Kong.
But it was liver stiffness measure (LSM), not metabolic syndrome, that predicted hepatic events (hazard ratio, 1.6; 95% confidence interval, 1.0 to 2.5) and death (HR, 1.9; 95% CI, 1.1 to 3.2), the investigators reported (Hepatology. 2016 Sep 29. doi: 10.1002/hep.28778).
Their study included 1,466 patients with chronic HBV infection who averaged 46 years of age, with an average baseline LSM of 8.4 kPa (standard deviation, 6.3 kPa). A total of 188 patients (12.8%) had metabolic syndrome at baseline, defined as at least three of the following five factors: central obesity, hypertriglyceridemia, low high-density lipoprotein cholesterol, hypertension, and type 2 diabetes mellitus or fasting hyperglycemia.
In all, 44 patients (3.0%) developed cardiovascular events during follow-up, while 93 (6.3%) developed cirrhotic events and hepatocellular carcinoma, and 70 (4.8%) died. Patients whose LSM exceeded 8.0 kPa at baseline had a significantly higher cumulative risk of hepatic events over the next 8 years than did patients with lower baseline LSM (12.3% versus 3%; P less than .001).
But high LSM “had no impact on cardiovascular events,” just as metabolic syndrome did not increase the risk of hepatic events or death, the investigators said. The study, the first to evaluate this group of clinical correlates in HBV-infected patients, highlights the need to monitor their metabolic risk factors over time, they concluded.
The researchers reported having no funding sources. Dr. Cheng had no relevant financial disclosures, although other coauthors reported multiple relationships with pharmaceutical and device firms.
Metabolic syndrome was associated with a fourfold rise in cardiovascular events among patients with chronic hepatitis B virus infection, according to a prospective cohort study published in Hepatology.
Over a median of 7.3 years of follow-up, 8% of patients with metabolic syndrome developed ischemic or hemorrhagic stroke, acute coronary syndrome, or congestive heart failure, or underwent revascularization, compared with 2% of patients without metabolic syndrome (P less than .0001), reported Jenny Yeuk-Ki Cheng, MD, and her associates at The Chinese University of Hong Kong.
But it was liver stiffness measure (LSM), not metabolic syndrome, that predicted hepatic events (hazard ratio, 1.6; 95% confidence interval, 1.0 to 2.5) and death (HR, 1.9; 95% CI, 1.1 to 3.2), the investigators reported (Hepatology. 2016 Sep 29. doi: 10.1002/hep.28778).
Their study included 1,466 patients with chronic HBV infection who averaged 46 years of age, with an average baseline LSM of 8.4 kPa (standard deviation, 6.3 kPa). A total of 188 patients (12.8%) had metabolic syndrome at baseline, defined as at least three of the following five factors: central obesity, hypertriglyceridemia, low high-density lipoprotein cholesterol, hypertension, and type 2 diabetes mellitus or fasting hyperglycemia.
In all, 44 patients (3.0%) developed cardiovascular events during follow-up, while 93 (6.3%) developed cirrhotic events and hepatocellular carcinoma, and 70 (4.8%) died. Patients whose LSM exceeded 8.0 kPa at baseline had a significantly higher cumulative risk of hepatic events over the next 8 years than did patients with lower baseline LSM (12.3% versus 3%; P less than .001).
But high LSM “had no impact on cardiovascular events,” just as metabolic syndrome did not increase the risk of hepatic events or death, the investigators said. The study, the first to evaluate this group of clinical correlates in HBV-infected patients, highlights the need to monitor their metabolic risk factors over time, they concluded.
The researchers reported having no funding sources. Dr. Cheng had no relevant financial disclosures, although other coauthors reported multiple relationships with pharmaceutical and device firms.
Metabolic syndrome was associated with a fourfold rise in cardiovascular events among patients with chronic hepatitis B virus infection, according to a prospective cohort study published in Hepatology.
Over a median of 7.3 years of follow-up, 8% of patients with metabolic syndrome developed ischemic or hemorrhagic stroke, acute coronary syndrome, or congestive heart failure, or underwent revascularization, compared with 2% of patients without metabolic syndrome (P less than .0001), reported Jenny Yeuk-Ki Cheng, MD, and her associates at The Chinese University of Hong Kong.
But it was liver stiffness measure (LSM), not metabolic syndrome, that predicted hepatic events (hazard ratio, 1.6; 95% confidence interval, 1.0 to 2.5) and death (HR, 1.9; 95% CI, 1.1 to 3.2), the investigators reported (Hepatology. 2016 Sep 29. doi: 10.1002/hep.28778).
Their study included 1,466 patients with chronic HBV infection who averaged 46 years of age, with an average baseline LSM of 8.4 kPa (standard deviation, 6.3 kPa). A total of 188 patients (12.8%) had metabolic syndrome at baseline, defined as at least three of the following five factors: central obesity, hypertriglyceridemia, low high-density lipoprotein cholesterol, hypertension, and type 2 diabetes mellitus or fasting hyperglycemia.
In all, 44 patients (3.0%) developed cardiovascular events during follow-up, while 93 (6.3%) developed cirrhotic events and hepatocellular carcinoma, and 70 (4.8%) died. Patients whose LSM exceeded 8.0 kPa at baseline had a significantly higher cumulative risk of hepatic events over the next 8 years than did patients with lower baseline LSM (12.3% versus 3%; P less than .001).
But high LSM “had no impact on cardiovascular events,” just as metabolic syndrome did not increase the risk of hepatic events or death, the investigators said. The study, the first to evaluate this group of clinical correlates in HBV-infected patients, highlights the need to monitor their metabolic risk factors over time, they concluded.
The researchers reported having no funding sources. Dr. Cheng had no relevant financial disclosures, although other coauthors reported multiple relationships with pharmaceutical and device firms.
Key clinical point: Metabolic syndrome significantly increases the risk of cardiovascular events in patients with chronic hepatitis B virus infection.
Major finding: In all, 8% of patients with metabolic syndrome had a cardiovascular event, compared with 2% of patients without metabolic syndrome (P less than .0001).
Data source: A single-center prospective cohort study of 1,466 patients with chronic hepatitis B virus infection.
Disclosures: The researchers reported having no funding sources. Dr. Cheng had no relevant financial disclosures, although other coauthors reported multiple relationships with pharmaceutical and device firms.
Fatal measles complication occurs more often than realized
NEW ORLEANS – A fatal complication of measles known as subacute sclerosing panencephalitis (SSPE) can develop years after measles infection and appears to occur much more often than published reports suggest, according to a review of cases in California from 1998 to 2015.
The findings underscore the vital importance of herd immunity by vaccination, Kristen Wendorf, MD, reported at an annual scientific meeting on infectious diseases.
The incidence of postmeasles SSPE was previously thought be about 1 in 100,000, according to an IDWeek press release.
“There is no cure for SSPE, and the only way to prevent it is to vaccinate everyone against measles,” the release stated.
The cases in the current study were among children with a clinically compatible illness, and either measles IgG antibody detected in cerebrospinal fluid, a characteristic pattern on electroencephalography, typical histologic findings on brain biopsy, or medical record documentation of SSPE-related complications. They were identified based on death certificates, reports from the Centers for Diseases Control and Prevention, or through investigations for undiagnosed neurologic disease. Twelve of the 17 affected children had a clinical history of a febrile rash illness compatible with measles, and all 12 of those experienced illness before age 15 months and before measles vaccination.
Most (67%) were living in the United States when they had measles, Dr. Wendorf said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The median age at diagnosis of SSPE was 12 years, although the range was 3-35 years, and the mean latency period was 9.5 years. In many cases, long-standing cognitive or motor problems were experienced prior to diagnosis, she noted.
The findings suggest that SSPE is more common than previously recognized in unvaccinated children with measles during infancy, Dr. Wendorf said.
Protection of infants younger than 12-15 months of age – before the time when measles vaccine is routinely administered – and in those who can’t be vaccinated because of immune system disorders requires avoidance of travel to endemic areas. Parents also may consider early vaccination prior to such travel.
Further, clinicians should be aware of the risk of SSPE in patients with symptoms suggestive of the disease. This is true even among older patients in whom no specific history of measles infection is known, she said.
In the press release, coauthor James D. Cherry, MD, professor of pediatrics at the University of California, Los Angeles, further stressed the importance of protecting unvaccinated infants.
“Parents of infants who have not yet been vaccinated should avoid putting their children at risk. For example, they should postpone trips overseas – including to Europe – where measles is endemic and epidemic until after their baby has been vaccinated with two doses,” he said. “It’s just not worth the risk.”
The authors reported having no disclosures.
NEW ORLEANS – A fatal complication of measles known as subacute sclerosing panencephalitis (SSPE) can develop years after measles infection and appears to occur much more often than published reports suggest, according to a review of cases in California from 1998 to 2015.
The findings underscore the vital importance of herd immunity by vaccination, Kristen Wendorf, MD, reported at an annual scientific meeting on infectious diseases.
The incidence of postmeasles SSPE was previously thought be about 1 in 100,000, according to an IDWeek press release.
“There is no cure for SSPE, and the only way to prevent it is to vaccinate everyone against measles,” the release stated.
The cases in the current study were among children with a clinically compatible illness, and either measles IgG antibody detected in cerebrospinal fluid, a characteristic pattern on electroencephalography, typical histologic findings on brain biopsy, or medical record documentation of SSPE-related complications. They were identified based on death certificates, reports from the Centers for Diseases Control and Prevention, or through investigations for undiagnosed neurologic disease. Twelve of the 17 affected children had a clinical history of a febrile rash illness compatible with measles, and all 12 of those experienced illness before age 15 months and before measles vaccination.
Most (67%) were living in the United States when they had measles, Dr. Wendorf said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The median age at diagnosis of SSPE was 12 years, although the range was 3-35 years, and the mean latency period was 9.5 years. In many cases, long-standing cognitive or motor problems were experienced prior to diagnosis, she noted.
The findings suggest that SSPE is more common than previously recognized in unvaccinated children with measles during infancy, Dr. Wendorf said.
Protection of infants younger than 12-15 months of age – before the time when measles vaccine is routinely administered – and in those who can’t be vaccinated because of immune system disorders requires avoidance of travel to endemic areas. Parents also may consider early vaccination prior to such travel.
Further, clinicians should be aware of the risk of SSPE in patients with symptoms suggestive of the disease. This is true even among older patients in whom no specific history of measles infection is known, she said.
In the press release, coauthor James D. Cherry, MD, professor of pediatrics at the University of California, Los Angeles, further stressed the importance of protecting unvaccinated infants.
“Parents of infants who have not yet been vaccinated should avoid putting their children at risk. For example, they should postpone trips overseas – including to Europe – where measles is endemic and epidemic until after their baby has been vaccinated with two doses,” he said. “It’s just not worth the risk.”
The authors reported having no disclosures.
NEW ORLEANS – A fatal complication of measles known as subacute sclerosing panencephalitis (SSPE) can develop years after measles infection and appears to occur much more often than published reports suggest, according to a review of cases in California from 1998 to 2015.
The findings underscore the vital importance of herd immunity by vaccination, Kristen Wendorf, MD, reported at an annual scientific meeting on infectious diseases.
The incidence of postmeasles SSPE was previously thought be about 1 in 100,000, according to an IDWeek press release.
“There is no cure for SSPE, and the only way to prevent it is to vaccinate everyone against measles,” the release stated.
The cases in the current study were among children with a clinically compatible illness, and either measles IgG antibody detected in cerebrospinal fluid, a characteristic pattern on electroencephalography, typical histologic findings on brain biopsy, or medical record documentation of SSPE-related complications. They were identified based on death certificates, reports from the Centers for Diseases Control and Prevention, or through investigations for undiagnosed neurologic disease. Twelve of the 17 affected children had a clinical history of a febrile rash illness compatible with measles, and all 12 of those experienced illness before age 15 months and before measles vaccination.
Most (67%) were living in the United States when they had measles, Dr. Wendorf said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The median age at diagnosis of SSPE was 12 years, although the range was 3-35 years, and the mean latency period was 9.5 years. In many cases, long-standing cognitive or motor problems were experienced prior to diagnosis, she noted.
The findings suggest that SSPE is more common than previously recognized in unvaccinated children with measles during infancy, Dr. Wendorf said.
Protection of infants younger than 12-15 months of age – before the time when measles vaccine is routinely administered – and in those who can’t be vaccinated because of immune system disorders requires avoidance of travel to endemic areas. Parents also may consider early vaccination prior to such travel.
Further, clinicians should be aware of the risk of SSPE in patients with symptoms suggestive of the disease. This is true even among older patients in whom no specific history of measles infection is known, she said.
In the press release, coauthor James D. Cherry, MD, professor of pediatrics at the University of California, Los Angeles, further stressed the importance of protecting unvaccinated infants.
“Parents of infants who have not yet been vaccinated should avoid putting their children at risk. For example, they should postpone trips overseas – including to Europe – where measles is endemic and epidemic until after their baby has been vaccinated with two doses,” he said. “It’s just not worth the risk.”
The authors reported having no disclosures.
AT IDWEEK 2016
Key clinical point:
Major finding: The incidence of SSPE among measles cases was 1 in 1,367 children under age 5 years and 1 in 609 children under age 12 months at the time of measles disease.
Data source: A review of records and 17 cases of SSPE.
Disclosures: The authors reported having no disclosures.
Idiopathic Livedo Racemosa Presenting With Splenomegaly and Diffuse Lymphadenopathy
Sneddon syndrome (SS) was first described in 1965 in patients with persistent livedo racemosa and neurological events.1 Because the other manifestations of SS are nonspecific (eg, hypertension, cardiac valvulopathy, arterial and venous occlusion), the diagnosis often is delayed. Many patients who experience prodromal neurologic symptoms such as headaches, depression, anxiety, dizziness, and neuropathy often present to a physician prior to developing ischemic brain manifestations2 but seldom receive the correct diagnosis. Onset of cerebral occlusive events typically occurs in patients younger than 45 years and may present as a transient ischemic attack, stroke, or intracranial hemorrhage.3 The disease is more prevalent in females than males (2:1 ratio). The exact pathogenesis of SS is still unknown, and although it has been thought of as a separate entity from systemic lupus erythematosus and other antiphospholipid disorders, it has been postulated that an immunological dysfunction damages vessel walls leading to thrombosis.
Cutaneous findings associated with SS involve small- to medium-sized dermal-subdermal arteries. Histopathology in some patients demonstrates proliferation of the endothelium and fibrin deposits with subsequent obliteration of involved arteries.4 In many patients including our patient, histopathologic examination of involved skin fails to show specific abnormalities.1 Zelger et al5 reported the sequence of histopathologic skin events in a series of antiphospholipid-negative SS patients. The authors reported that only small arteries at the dermis-subcutis junction were involved and a progression of endothelial dysfunction was observed. The authors believed there were several nonspecific stages prior to fibrin occlusion of involved arteries.5 Stage I involved loosening of endothelial cells with nonspecific perivascular lymphocytic infiltration with perivascular inflammation and lymphocytic infiltration representing the prime mover of the disease.5,6 This stage is thought to be short lived, thus the reason why it has gone undetected for many years in SS patients. Stages II to IV progress through fibrin deposition and occlusion.5 Histological features of stages I to II have not been reported because of late diagnosis of SS. Stage I patients typically present with an average duration of symptoms of 6 months with few neurologic symptoms, the most common being paresthesia of the legs.5
Case Report
A 37-year-old woman with epigastric tenderness on the left side and splenomegaly seen on computed tomography was referred by a hematologist for evaluation of a reticular rash on the left side of the flank of 9 months’ duration with a presumed diagnosis of focal melanoderma. Her medical history was remarkable for a congenital ventricular septal defect and coarctation of the aorta, as well as endometriosis, myalgia, and joint stiffness that had all developed over the last year. Her medical history also was remarkable for nephrolithiasis, irritable bowel syndrome, and chronic sinusitis, as well as psychiatric depression and anxiety disorders. She recently had been diagnosed with moderate hypertension and had experienced difficulty getting pregnant for the last several years with 3 consecutive miscarriages in the first trimester. Neurologic symptoms included neuropathy involving the feet, intermittent paresthesia of the legs, and a history of chronic migraine headaches for several months.
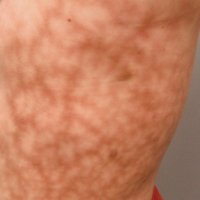
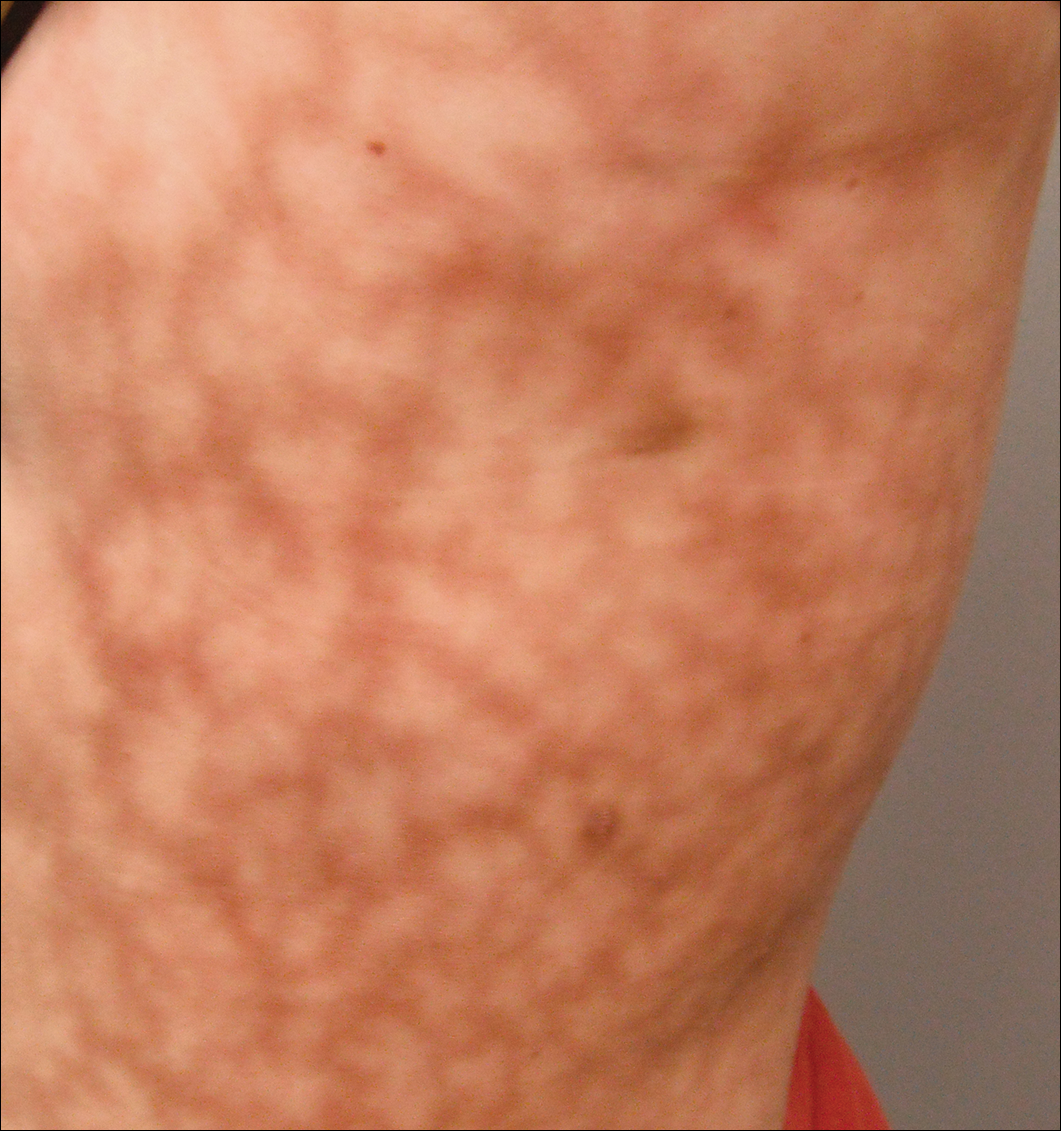
Dermatologic examination revealed a slightly overweight woman with a 25×30-cm dusky, erythematous, irregular, netlike pattern on the left side of the upper and lower trunk (Figure 1). Extensive livedo racemosa was not altered by changes in temperature and had been unchanged for more than 9 months. There were no signs of pruritus or ulcerations, and areas of livedo racemosa were slightly tender to palpation.
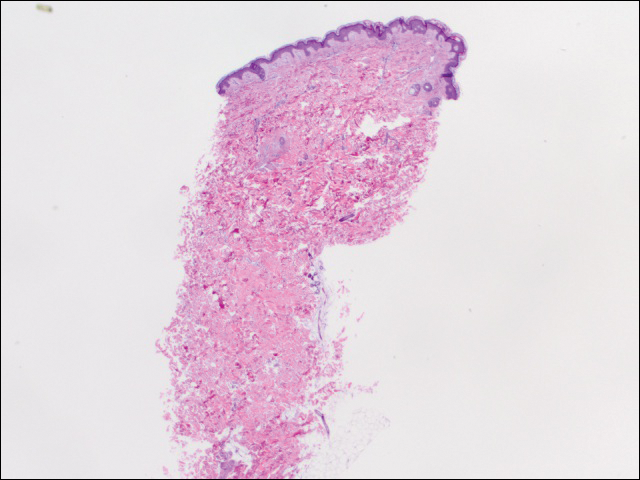
We performed 2 sets of three 4-mm biopsies. The first set targeted areas within the violaceous pattern, while the second set targeted areas of normal tissue between the mottled areas. All 6 specimens demonstrated superficial perivascular lymphocytic infiltrate with no evidence of vasculitis or connective tissue disease. The vessels showed no microthrombi or surrounding fibrosis. No eosinophils were identified within the epidermis. There was no evidence of increased dermal mucin. Both the superficial and deep vascular plexuses were unremarkable and showed no evidence of damage to the walls (Figure 2).
To rule out other possible causes of livedo racemosa, complete blood cell count, comprehensive metabolic panel, coagulation profile, lipase test, urinalysis, serologic testing, and immunologic workup were performed. Lipase was within reference range. The complete blood cell count revealed mild anemia, while the rest of the values were within reference range. An immunologic workup included Sjögren syndrome antigen A, Sjögren syndrome antigen B, anticardiolipin antibodies, and antinuclear antibody, which were all negative. Family history was remarkable for first-degree relatives with systemic lupus erythematosus and Crohn disease.
Computed tomography revealed enlargement of the spleen, as well as periaortic, portacaval, and porta hepatis lymphadenopathy. Based on the laboratory findings and clinical presentation as well as the patient’s medical history, the diagnosis of exclusion was idiopathic livedo racemosa with unknown progression to full-blown SS. The patient did not meet the current diagnostic criteria for SS, and her immunologic studies failed to confirm any present antibodies, but involvement of the reticuloendothelial system pointed to production of antibodies that were not yet detectable on laboratory testing.
Comment
More than 50 years after the first case of SS was diagnosed, better laboratory workup is available and more information is known about the pathophysiology. Sneddon syndrome is a rare disorder, affecting only approximately 4 patients per million each year worldwide. Seronegative antiphospholipid antibody syndrome (SNAPS) describes patients with clinical presentations of antiphospholipid syndrome (APS) without detectable serological markers.7 Antiphospholipid-negative SS, which was seen in our patient, would be categorized under SNAPS. A PubMed search of articles indexed for MEDLINE using the terms livedo racemosa, Sneddon syndrome, and SNAPS and splenomegaly revealed there currently are no known cases of SNAPS that have been reported with splenomegaly and lymphadenopathy. Our patient presented with the following clinical features of SS: livedo racemosa, history of miscarriage, psychiatric disturbances, and hypertension. Surprisingly, biopsies from affected skin did not show any fibrin deposition or microthrombi but did reveal perivascular lymphocytic infiltrations. Magnetic resonance imaging did not show any pathological lesions or vascular changes.
Sneddon syndrome and APS share a common pathway to occlusive arteriolopathy for which 4 stages have been described by Zelger et al.5 Stage I involves a nonspecific Langerhans cell infiltrate with polymorphonuclear leukocytes. The tunica media and elastic lamina usually are unaltered at this early stage, while the surrounding connective tissue may appear edematous.5 This early stage of histopathology has not been evaluated in SS patients, primarily because of delay of diagnosis. Late stages III and IV will show fibrin deposition and shrinkage of affected vessels.7
A PubMed search using the terms Sneddon syndrome, lymphadenopathy and livedo racemosa, and Sneddon syndrome and lymphadenopathy revealed that splenomegaly and lymphadenopathy have not been reported in patients with SS. In patients with antiphospholipid-negative SS, one can assume that antibodies to other phospholipids not tested must exist because of striking similarities between APS and antiphospholipid-negative SS.8 Although our patient did not test positive for any of these antibodies, she did present with lymphadenopathy and splenic enlargement, leading us to believe that involvement of the reticuloendothelial system may be a feature of SS that has not been previously reported. Further studies are required to name specific antigens responsible for clinical manifestations in SS.
Currently, no single diagnostic test for SS exists, thus delaying both diagnosis and initiation of treatment. Histopathologic examination may be helpful, but in many cases it is nonspecific, as are serologic markers. Neuroradiological confirmation of involvement usually is the confirmatory feature in many patients with late-stage diagnosis.2 A diagnostic schematic for SS, which was first described by Daoud et al,2 illustrates classification of symptoms and aids in diagnosis. A working diagnosis of idiopathic livedo racemosa is made after ruling out other causes of SS in a patient with nonspecific biopsy findings and negative magnetic resonance imaging results with prodromal symptoms. The prognosis for such patients progressing to full SS is unknown with or without management using anticoagulant therapy.
Conclusion
Early diagnosis of livedo racemosa and SS is essential, as prevention of cerebrovascular accidents, myocardial infarction, and other thromboembolic diseases can be minimized by attacking risk factors such as smoking, taking oral contraceptive pills, becoming pregnant,9 and by initiating either antiplatelet or anticoagulation treatments. These treatments have been shown to delay the development of neurovascular damage and early-onset dementia. We present this case to demonstrate the variability of early-presenting symptoms in idiopathic livedo racemosa. Recognizing some of the early manifestations can lead to early diagnosis and initiation of treatment.
- Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Daoud MS, Wilmoth GJ, Su WP, et al. Sneddon syndrome. Semin Dermatol. 1995;14:166-172.
- Besnier R, Francès C, Ankri A, et al. Factor V Leiden mutation in Sneddon syndrome. Lupus. 2003;12:406-408.
- K aragülle AT, Karadağ D, Erden A, et al. Sneddon’s syndrome: MR imaging findings. Eur Radiol. 2002;12:144-146.
- Zelg er B, Sepp N, Schmid KW, et al. Life-history of cutaneous vascular-lesions in Sneddon’s syndrome. Hum Pathol. 1992;23:668-675.
- Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329-1332.
- Duva l A, Darnige L, Glowacki F, et al. Livedo, dementia, thrombocytopenia, and endotheliitis without antiphospholipid antibodies: seronegative antiphospholipid-like syndrome. J Am Acad Dermatol. 2009;61:1076-1078.
- Kala shnikova LA, Nasonov EL, Kushekbaeva AE, et al. Anticardiolipin antibodies in Sneddon’s syndrome. Neurology. 1990;40:464-467.
- Wohl rab J, Fischer M, Wolter M, et al. Diagnostic impact and sensitivity of skin biopsies in Sneddon’s syndrome. a report of 15 cases. Br J Dermatol. 2001;145:285-288.
Sneddon syndrome (SS) was first described in 1965 in patients with persistent livedo racemosa and neurological events.1 Because the other manifestations of SS are nonspecific (eg, hypertension, cardiac valvulopathy, arterial and venous occlusion), the diagnosis often is delayed. Many patients who experience prodromal neurologic symptoms such as headaches, depression, anxiety, dizziness, and neuropathy often present to a physician prior to developing ischemic brain manifestations2 but seldom receive the correct diagnosis. Onset of cerebral occlusive events typically occurs in patients younger than 45 years and may present as a transient ischemic attack, stroke, or intracranial hemorrhage.3 The disease is more prevalent in females than males (2:1 ratio). The exact pathogenesis of SS is still unknown, and although it has been thought of as a separate entity from systemic lupus erythematosus and other antiphospholipid disorders, it has been postulated that an immunological dysfunction damages vessel walls leading to thrombosis.
Cutaneous findings associated with SS involve small- to medium-sized dermal-subdermal arteries. Histopathology in some patients demonstrates proliferation of the endothelium and fibrin deposits with subsequent obliteration of involved arteries.4 In many patients including our patient, histopathologic examination of involved skin fails to show specific abnormalities.1 Zelger et al5 reported the sequence of histopathologic skin events in a series of antiphospholipid-negative SS patients. The authors reported that only small arteries at the dermis-subcutis junction were involved and a progression of endothelial dysfunction was observed. The authors believed there were several nonspecific stages prior to fibrin occlusion of involved arteries.5 Stage I involved loosening of endothelial cells with nonspecific perivascular lymphocytic infiltration with perivascular inflammation and lymphocytic infiltration representing the prime mover of the disease.5,6 This stage is thought to be short lived, thus the reason why it has gone undetected for many years in SS patients. Stages II to IV progress through fibrin deposition and occlusion.5 Histological features of stages I to II have not been reported because of late diagnosis of SS. Stage I patients typically present with an average duration of symptoms of 6 months with few neurologic symptoms, the most common being paresthesia of the legs.5
Case Report
A 37-year-old woman with epigastric tenderness on the left side and splenomegaly seen on computed tomography was referred by a hematologist for evaluation of a reticular rash on the left side of the flank of 9 months’ duration with a presumed diagnosis of focal melanoderma. Her medical history was remarkable for a congenital ventricular septal defect and coarctation of the aorta, as well as endometriosis, myalgia, and joint stiffness that had all developed over the last year. Her medical history also was remarkable for nephrolithiasis, irritable bowel syndrome, and chronic sinusitis, as well as psychiatric depression and anxiety disorders. She recently had been diagnosed with moderate hypertension and had experienced difficulty getting pregnant for the last several years with 3 consecutive miscarriages in the first trimester. Neurologic symptoms included neuropathy involving the feet, intermittent paresthesia of the legs, and a history of chronic migraine headaches for several months.
Dermatologic examination revealed a slightly overweight woman with a 25×30-cm dusky, erythematous, irregular, netlike pattern on the left side of the upper and lower trunk (Figure 1). Extensive livedo racemosa was not altered by changes in temperature and had been unchanged for more than 9 months. There were no signs of pruritus or ulcerations, and areas of livedo racemosa were slightly tender to palpation.

We performed 2 sets of three 4-mm biopsies. The first set targeted areas within the violaceous pattern, while the second set targeted areas of normal tissue between the mottled areas. All 6 specimens demonstrated superficial perivascular lymphocytic infiltrate with no evidence of vasculitis or connective tissue disease. The vessels showed no microthrombi or surrounding fibrosis. No eosinophils were identified within the epidermis. There was no evidence of increased dermal mucin. Both the superficial and deep vascular plexuses were unremarkable and showed no evidence of damage to the walls (Figure 2).

To rule out other possible causes of livedo racemosa, complete blood cell count, comprehensive metabolic panel, coagulation profile, lipase test, urinalysis, serologic testing, and immunologic workup were performed. Lipase was within reference range. The complete blood cell count revealed mild anemia, while the rest of the values were within reference range. An immunologic workup included Sjögren syndrome antigen A, Sjögren syndrome antigen B, anticardiolipin antibodies, and antinuclear antibody, which were all negative. Family history was remarkable for first-degree relatives with systemic lupus erythematosus and Crohn disease.
Computed tomography revealed enlargement of the spleen, as well as periaortic, portacaval, and porta hepatis lymphadenopathy. Based on the laboratory findings and clinical presentation as well as the patient’s medical history, the diagnosis of exclusion was idiopathic livedo racemosa with unknown progression to full-blown SS. The patient did not meet the current diagnostic criteria for SS, and her immunologic studies failed to confirm any present antibodies, but involvement of the reticuloendothelial system pointed to production of antibodies that were not yet detectable on laboratory testing.
Comment
More than 50 years after the first case of SS was diagnosed, better laboratory workup is available and more information is known about the pathophysiology. Sneddon syndrome is a rare disorder, affecting only approximately 4 patients per million each year worldwide. Seronegative antiphospholipid antibody syndrome (SNAPS) describes patients with clinical presentations of antiphospholipid syndrome (APS) without detectable serological markers.7 Antiphospholipid-negative SS, which was seen in our patient, would be categorized under SNAPS. A PubMed search of articles indexed for MEDLINE using the terms livedo racemosa, Sneddon syndrome, and SNAPS and splenomegaly revealed there currently are no known cases of SNAPS that have been reported with splenomegaly and lymphadenopathy. Our patient presented with the following clinical features of SS: livedo racemosa, history of miscarriage, psychiatric disturbances, and hypertension. Surprisingly, biopsies from affected skin did not show any fibrin deposition or microthrombi but did reveal perivascular lymphocytic infiltrations. Magnetic resonance imaging did not show any pathological lesions or vascular changes.
Sneddon syndrome and APS share a common pathway to occlusive arteriolopathy for which 4 stages have been described by Zelger et al.5 Stage I involves a nonspecific Langerhans cell infiltrate with polymorphonuclear leukocytes. The tunica media and elastic lamina usually are unaltered at this early stage, while the surrounding connective tissue may appear edematous.5 This early stage of histopathology has not been evaluated in SS patients, primarily because of delay of diagnosis. Late stages III and IV will show fibrin deposition and shrinkage of affected vessels.7
A PubMed search using the terms Sneddon syndrome, lymphadenopathy and livedo racemosa, and Sneddon syndrome and lymphadenopathy revealed that splenomegaly and lymphadenopathy have not been reported in patients with SS. In patients with antiphospholipid-negative SS, one can assume that antibodies to other phospholipids not tested must exist because of striking similarities between APS and antiphospholipid-negative SS.8 Although our patient did not test positive for any of these antibodies, she did present with lymphadenopathy and splenic enlargement, leading us to believe that involvement of the reticuloendothelial system may be a feature of SS that has not been previously reported. Further studies are required to name specific antigens responsible for clinical manifestations in SS.
Currently, no single diagnostic test for SS exists, thus delaying both diagnosis and initiation of treatment. Histopathologic examination may be helpful, but in many cases it is nonspecific, as are serologic markers. Neuroradiological confirmation of involvement usually is the confirmatory feature in many patients with late-stage diagnosis.2 A diagnostic schematic for SS, which was first described by Daoud et al,2 illustrates classification of symptoms and aids in diagnosis. A working diagnosis of idiopathic livedo racemosa is made after ruling out other causes of SS in a patient with nonspecific biopsy findings and negative magnetic resonance imaging results with prodromal symptoms. The prognosis for such patients progressing to full SS is unknown with or without management using anticoagulant therapy.
Conclusion
Early diagnosis of livedo racemosa and SS is essential, as prevention of cerebrovascular accidents, myocardial infarction, and other thromboembolic diseases can be minimized by attacking risk factors such as smoking, taking oral contraceptive pills, becoming pregnant,9 and by initiating either antiplatelet or anticoagulation treatments. These treatments have been shown to delay the development of neurovascular damage and early-onset dementia. We present this case to demonstrate the variability of early-presenting symptoms in idiopathic livedo racemosa. Recognizing some of the early manifestations can lead to early diagnosis and initiation of treatment.
Sneddon syndrome (SS) was first described in 1965 in patients with persistent livedo racemosa and neurological events.1 Because the other manifestations of SS are nonspecific (eg, hypertension, cardiac valvulopathy, arterial and venous occlusion), the diagnosis often is delayed. Many patients who experience prodromal neurologic symptoms such as headaches, depression, anxiety, dizziness, and neuropathy often present to a physician prior to developing ischemic brain manifestations2 but seldom receive the correct diagnosis. Onset of cerebral occlusive events typically occurs in patients younger than 45 years and may present as a transient ischemic attack, stroke, or intracranial hemorrhage.3 The disease is more prevalent in females than males (2:1 ratio). The exact pathogenesis of SS is still unknown, and although it has been thought of as a separate entity from systemic lupus erythematosus and other antiphospholipid disorders, it has been postulated that an immunological dysfunction damages vessel walls leading to thrombosis.
Cutaneous findings associated with SS involve small- to medium-sized dermal-subdermal arteries. Histopathology in some patients demonstrates proliferation of the endothelium and fibrin deposits with subsequent obliteration of involved arteries.4 In many patients including our patient, histopathologic examination of involved skin fails to show specific abnormalities.1 Zelger et al5 reported the sequence of histopathologic skin events in a series of antiphospholipid-negative SS patients. The authors reported that only small arteries at the dermis-subcutis junction were involved and a progression of endothelial dysfunction was observed. The authors believed there were several nonspecific stages prior to fibrin occlusion of involved arteries.5 Stage I involved loosening of endothelial cells with nonspecific perivascular lymphocytic infiltration with perivascular inflammation and lymphocytic infiltration representing the prime mover of the disease.5,6 This stage is thought to be short lived, thus the reason why it has gone undetected for many years in SS patients. Stages II to IV progress through fibrin deposition and occlusion.5 Histological features of stages I to II have not been reported because of late diagnosis of SS. Stage I patients typically present with an average duration of symptoms of 6 months with few neurologic symptoms, the most common being paresthesia of the legs.5
Case Report
A 37-year-old woman with epigastric tenderness on the left side and splenomegaly seen on computed tomography was referred by a hematologist for evaluation of a reticular rash on the left side of the flank of 9 months’ duration with a presumed diagnosis of focal melanoderma. Her medical history was remarkable for a congenital ventricular septal defect and coarctation of the aorta, as well as endometriosis, myalgia, and joint stiffness that had all developed over the last year. Her medical history also was remarkable for nephrolithiasis, irritable bowel syndrome, and chronic sinusitis, as well as psychiatric depression and anxiety disorders. She recently had been diagnosed with moderate hypertension and had experienced difficulty getting pregnant for the last several years with 3 consecutive miscarriages in the first trimester. Neurologic symptoms included neuropathy involving the feet, intermittent paresthesia of the legs, and a history of chronic migraine headaches for several months.
Dermatologic examination revealed a slightly overweight woman with a 25×30-cm dusky, erythematous, irregular, netlike pattern on the left side of the upper and lower trunk (Figure 1). Extensive livedo racemosa was not altered by changes in temperature and had been unchanged for more than 9 months. There were no signs of pruritus or ulcerations, and areas of livedo racemosa were slightly tender to palpation.

We performed 2 sets of three 4-mm biopsies. The first set targeted areas within the violaceous pattern, while the second set targeted areas of normal tissue between the mottled areas. All 6 specimens demonstrated superficial perivascular lymphocytic infiltrate with no evidence of vasculitis or connective tissue disease. The vessels showed no microthrombi or surrounding fibrosis. No eosinophils were identified within the epidermis. There was no evidence of increased dermal mucin. Both the superficial and deep vascular plexuses were unremarkable and showed no evidence of damage to the walls (Figure 2).

To rule out other possible causes of livedo racemosa, complete blood cell count, comprehensive metabolic panel, coagulation profile, lipase test, urinalysis, serologic testing, and immunologic workup were performed. Lipase was within reference range. The complete blood cell count revealed mild anemia, while the rest of the values were within reference range. An immunologic workup included Sjögren syndrome antigen A, Sjögren syndrome antigen B, anticardiolipin antibodies, and antinuclear antibody, which were all negative. Family history was remarkable for first-degree relatives with systemic lupus erythematosus and Crohn disease.
Computed tomography revealed enlargement of the spleen, as well as periaortic, portacaval, and porta hepatis lymphadenopathy. Based on the laboratory findings and clinical presentation as well as the patient’s medical history, the diagnosis of exclusion was idiopathic livedo racemosa with unknown progression to full-blown SS. The patient did not meet the current diagnostic criteria for SS, and her immunologic studies failed to confirm any present antibodies, but involvement of the reticuloendothelial system pointed to production of antibodies that were not yet detectable on laboratory testing.
Comment
More than 50 years after the first case of SS was diagnosed, better laboratory workup is available and more information is known about the pathophysiology. Sneddon syndrome is a rare disorder, affecting only approximately 4 patients per million each year worldwide. Seronegative antiphospholipid antibody syndrome (SNAPS) describes patients with clinical presentations of antiphospholipid syndrome (APS) without detectable serological markers.7 Antiphospholipid-negative SS, which was seen in our patient, would be categorized under SNAPS. A PubMed search of articles indexed for MEDLINE using the terms livedo racemosa, Sneddon syndrome, and SNAPS and splenomegaly revealed there currently are no known cases of SNAPS that have been reported with splenomegaly and lymphadenopathy. Our patient presented with the following clinical features of SS: livedo racemosa, history of miscarriage, psychiatric disturbances, and hypertension. Surprisingly, biopsies from affected skin did not show any fibrin deposition or microthrombi but did reveal perivascular lymphocytic infiltrations. Magnetic resonance imaging did not show any pathological lesions or vascular changes.
Sneddon syndrome and APS share a common pathway to occlusive arteriolopathy for which 4 stages have been described by Zelger et al.5 Stage I involves a nonspecific Langerhans cell infiltrate with polymorphonuclear leukocytes. The tunica media and elastic lamina usually are unaltered at this early stage, while the surrounding connective tissue may appear edematous.5 This early stage of histopathology has not been evaluated in SS patients, primarily because of delay of diagnosis. Late stages III and IV will show fibrin deposition and shrinkage of affected vessels.7
A PubMed search using the terms Sneddon syndrome, lymphadenopathy and livedo racemosa, and Sneddon syndrome and lymphadenopathy revealed that splenomegaly and lymphadenopathy have not been reported in patients with SS. In patients with antiphospholipid-negative SS, one can assume that antibodies to other phospholipids not tested must exist because of striking similarities between APS and antiphospholipid-negative SS.8 Although our patient did not test positive for any of these antibodies, she did present with lymphadenopathy and splenic enlargement, leading us to believe that involvement of the reticuloendothelial system may be a feature of SS that has not been previously reported. Further studies are required to name specific antigens responsible for clinical manifestations in SS.
Currently, no single diagnostic test for SS exists, thus delaying both diagnosis and initiation of treatment. Histopathologic examination may be helpful, but in many cases it is nonspecific, as are serologic markers. Neuroradiological confirmation of involvement usually is the confirmatory feature in many patients with late-stage diagnosis.2 A diagnostic schematic for SS, which was first described by Daoud et al,2 illustrates classification of symptoms and aids in diagnosis. A working diagnosis of idiopathic livedo racemosa is made after ruling out other causes of SS in a patient with nonspecific biopsy findings and negative magnetic resonance imaging results with prodromal symptoms. The prognosis for such patients progressing to full SS is unknown with or without management using anticoagulant therapy.
Conclusion
Early diagnosis of livedo racemosa and SS is essential, as prevention of cerebrovascular accidents, myocardial infarction, and other thromboembolic diseases can be minimized by attacking risk factors such as smoking, taking oral contraceptive pills, becoming pregnant,9 and by initiating either antiplatelet or anticoagulation treatments. These treatments have been shown to delay the development of neurovascular damage and early-onset dementia. We present this case to demonstrate the variability of early-presenting symptoms in idiopathic livedo racemosa. Recognizing some of the early manifestations can lead to early diagnosis and initiation of treatment.
- Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Daoud MS, Wilmoth GJ, Su WP, et al. Sneddon syndrome. Semin Dermatol. 1995;14:166-172.
- Besnier R, Francès C, Ankri A, et al. Factor V Leiden mutation in Sneddon syndrome. Lupus. 2003;12:406-408.
- K aragülle AT, Karadağ D, Erden A, et al. Sneddon’s syndrome: MR imaging findings. Eur Radiol. 2002;12:144-146.
- Zelg er B, Sepp N, Schmid KW, et al. Life-history of cutaneous vascular-lesions in Sneddon’s syndrome. Hum Pathol. 1992;23:668-675.
- Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329-1332.
- Duva l A, Darnige L, Glowacki F, et al. Livedo, dementia, thrombocytopenia, and endotheliitis without antiphospholipid antibodies: seronegative antiphospholipid-like syndrome. J Am Acad Dermatol. 2009;61:1076-1078.
- Kala shnikova LA, Nasonov EL, Kushekbaeva AE, et al. Anticardiolipin antibodies in Sneddon’s syndrome. Neurology. 1990;40:464-467.
- Wohl rab J, Fischer M, Wolter M, et al. Diagnostic impact and sensitivity of skin biopsies in Sneddon’s syndrome. a report of 15 cases. Br J Dermatol. 2001;145:285-288.
- Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Daoud MS, Wilmoth GJ, Su WP, et al. Sneddon syndrome. Semin Dermatol. 1995;14:166-172.
- Besnier R, Francès C, Ankri A, et al. Factor V Leiden mutation in Sneddon syndrome. Lupus. 2003;12:406-408.
- K aragülle AT, Karadağ D, Erden A, et al. Sneddon’s syndrome: MR imaging findings. Eur Radiol. 2002;12:144-146.
- Zelg er B, Sepp N, Schmid KW, et al. Life-history of cutaneous vascular-lesions in Sneddon’s syndrome. Hum Pathol. 1992;23:668-675.
- Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329-1332.
- Duva l A, Darnige L, Glowacki F, et al. Livedo, dementia, thrombocytopenia, and endotheliitis without antiphospholipid antibodies: seronegative antiphospholipid-like syndrome. J Am Acad Dermatol. 2009;61:1076-1078.
- Kala shnikova LA, Nasonov EL, Kushekbaeva AE, et al. Anticardiolipin antibodies in Sneddon’s syndrome. Neurology. 1990;40:464-467.
- Wohl rab J, Fischer M, Wolter M, et al. Diagnostic impact and sensitivity of skin biopsies in Sneddon’s syndrome. a report of 15 cases. Br J Dermatol. 2001;145:285-288.
Practice Points
- The classic physical diagnostic finding of Sneddon syndrome (SS) is livedo racemosa.
- Early identification and treatment of SS can prevent serious morbidity due to stroke, myocardial infarction, and other thrombotic events.
- Preventive care in SS should include antiplatelet therapy or anticoagulants and smoking cessation along with avoidance of birth control pills.
Letters to the Editor: Risk-reducing surgery for BRCA mutation carriers

“SHOULD RISK-REDUCING GYNECOLOGIC SURGERY FOR BRCA MUTATION CARRIERS INCLUDE HYSTERECTOMY?”
ANDREW M. KAUNITZ, MD (WEB EXCLUSIVE, AUGUST 28, 2016)
Hysterectomy warranted?
I am wondering if Dr. Kaunitz really is recommending performing 270 hysterectomies to prevent one endometrial cancer? Is this justified given the risks from the hysterectomy itself, the economics of the disease, or any significant reductions in endometrial cancer mortality?
David O. Holtz, MD
Paoli, Pennsylvania
Dr. Kaunitz responds
I appreciate Dr. Holtz’s interest in my commentary on the role of hysterectomy as part of risk-reducing surgery in BRCA mutation carriers. Women who are mutation carriers are at increased risk for serous or serous-like endometrial cancers. Further, hysterectomy offers specific advantages for young mutation carriers for whom menopausal hormone therapy is often indicated after risk-reducing salpingo-oophorectomy. Accordingly, I would indeed encourage such women to consider hysterectomy as part of risk-reducing gynecologic surgery if such surgery can be accomplished via minimally invasive techniques.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

“SHOULD RISK-REDUCING GYNECOLOGIC SURGERY FOR BRCA MUTATION CARRIERS INCLUDE HYSTERECTOMY?”
ANDREW M. KAUNITZ, MD (WEB EXCLUSIVE, AUGUST 28, 2016)
Hysterectomy warranted?
I am wondering if Dr. Kaunitz really is recommending performing 270 hysterectomies to prevent one endometrial cancer? Is this justified given the risks from the hysterectomy itself, the economics of the disease, or any significant reductions in endometrial cancer mortality?
David O. Holtz, MD
Paoli, Pennsylvania
Dr. Kaunitz responds
I appreciate Dr. Holtz’s interest in my commentary on the role of hysterectomy as part of risk-reducing surgery in BRCA mutation carriers. Women who are mutation carriers are at increased risk for serous or serous-like endometrial cancers. Further, hysterectomy offers specific advantages for young mutation carriers for whom menopausal hormone therapy is often indicated after risk-reducing salpingo-oophorectomy. Accordingly, I would indeed encourage such women to consider hysterectomy as part of risk-reducing gynecologic surgery if such surgery can be accomplished via minimally invasive techniques.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

“SHOULD RISK-REDUCING GYNECOLOGIC SURGERY FOR BRCA MUTATION CARRIERS INCLUDE HYSTERECTOMY?”
ANDREW M. KAUNITZ, MD (WEB EXCLUSIVE, AUGUST 28, 2016)
Hysterectomy warranted?
I am wondering if Dr. Kaunitz really is recommending performing 270 hysterectomies to prevent one endometrial cancer? Is this justified given the risks from the hysterectomy itself, the economics of the disease, or any significant reductions in endometrial cancer mortality?
David O. Holtz, MD
Paoli, Pennsylvania
Dr. Kaunitz responds
I appreciate Dr. Holtz’s interest in my commentary on the role of hysterectomy as part of risk-reducing surgery in BRCA mutation carriers. Women who are mutation carriers are at increased risk for serous or serous-like endometrial cancers. Further, hysterectomy offers specific advantages for young mutation carriers for whom menopausal hormone therapy is often indicated after risk-reducing salpingo-oophorectomy. Accordingly, I would indeed encourage such women to consider hysterectomy as part of risk-reducing gynecologic surgery if such surgery can be accomplished via minimally invasive techniques.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
VIDEO: Biologic therapy for multidrug-resistant HIV offers new hope
NEW ORLEANS – Ibalizumab, the first monoclonal antibody to reach a phase III trial for multidrug resistant HIV therapy, has demonstrated efficacy, safety, and a novel mechanism of action, offering promise to many patients with few remaining options.
“The drug has now shown very significant antiretroviral activity, with 83% of patients demonstrating a half log decrease after 7 days, and a mean and median decrease of 1.1 log,” Jacob Lalezari, MD, lead author of the study and medical director of Quest Clinical Research in San Francisco, said at IDWeek 2016, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “The big message here is the novel mechanism of action provides new treatment to patients with limited options, really offering hope to those left behind by an otherwise amazing evolution of HIV treatment.”
Dr. Lalezari said there is currently no evidence of cross-resistance with existing antiretrovirals. “I think we got lucky with that,” he noted. “For the primary care HIV doc, who is increasingly overwhelmed by drug-drug interactions, the good news is ibalizumab does not have obvious drug-drug interactions with antiretrovirals or other [HIV] drugs in other classes.”
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, announced the findings with Dr. Lalezari at an IDWeek 2016 press conference. “This study is very important, because although we have terrific therapies for initial and second line treatment, we’ve really come to need treatment for the core group of patients who have developed resistance to all the drugs in the armamentarium,” he said.
Multidrug resistance emerges in less than 5% of people with HIV, affecting up to about 10,000 people in the United States. “It’s not a huge population, but it’s the most vulnerable and in need,” Dr. Lalezari said.
The 40 treatment-experienced patients in the study had their viral load and CD4+ counts measured at day 0. At day 7, they received a 2,000 mg IV loading dose of ibalizumab. At day 14, the response to ibalizumab monotherapy was measured and participants began an optimized background regimen with at least one other agent to which HIV showed sensitivity. Unfortunately, for about 50% of the patients, there was no such agent remaining, so researchers added BMS-663068, an investigational oral attachment inhibitor.
The cohort was 85% men, 45% nonwhite, and had a mean duration of HIV infection of 21 years. At study entry, patients’ mean viral load was approximately 100,000 copies/mL and mean CD4+ T-cell count was 160/mcL. However, half of the patients had T-cell counts below 100, and one third had counts below 10, “meaning they are at the very edge of sustainability,” Dr. Lalezari said.
Efficacy and safety
“The story is pretty simple – the drug worked,” Dr. Lalezari said. In addition to the 83% who met the primary endpoint of a half-life log decrease in HIV-1 RNA, 60% had a full log decrease or more at day 14. The mean and median HIV-1 RNA decrease for the entire cohort was 1.1 log10, a significant difference, compared with the day 0 to 7 control period (P less than .0001).
Putting the findings in perspective, Dr. Lalezari said, “So 1 log is not the most potent drug we see for HIV, but it’s pretty good. And in the setting of multidrug-resistant virus, it’s very good.” Although the agent is not potent enough for monotherapy, he said it is a strong candidate for combination therapy. The goal is to “give somebody a chance, potentially their last chance, to get control of the virus and prevent the progression of disease, and importantly, prevent them from spreading it to somebody else.”
“It’s a bit of a mystery” why 7 of the 40 patients did not meet the primary endpoint, Dr. Lalezari added. None of the factors the investigators compared between responders and nonresponders were significantly different.
Ibalizumab appears safe with no discontinuations and no treatment-related serious adverse events, Dr. Lalezari said. “We have not seen anything that strikes me as concerning at all in terms of safety, and it’s in a patient population that is quite ill.”
When asked during the press conference to address cost concerns, an issue in other specialties when biologics are introduced, Dr. Lalezari responded, “The one comment I will make is that whatever this drug is, it’s not a ‘me too drug.’ It’s unique and offers a unique mechanism of action. For those patients, the patients we saw whose T cells were under 10, whose health was failing and they were getting ready to die, their viral loads got suppressed and now they’re living, so it brings great value.”
Ibalizumab’s antiretroviral activity stems from blocking post-attachment conformational changes that are required to enable the HIV virus to bind with its co-receptors and ultimately gain entry into the target T cell. “Importantly the drug is away from the binding site for MHC class II molecules, and therefore not thought to cause T-cell depletion or be immunosuppressive,” Dr. Lalezari said.
In terms of the bigger picture, unlike most HIV drugs taken daily, ibalizumab is the first of the long-acting antiretrovirals, he noted. “I do think a paradigm shift is looming for at least some patients for whom long-acting therapy might be advantageous. There is also a movement toward IM and hopefully subcutaneous therapy as well, which would be very advantageous for patient self-administration.”
Although Dr. Lalezari and Dr. Kuritzkes presented the 14-day findings, the study is ongoing and participants continue to receive 800 mg ibalizumab intravenously every two weeks.
TaiMed Biologics, the sponsor of the study, has an expanded access program. Dr. Lalezari said, “So if you [have] a patient who is in trouble, in need of rescue therapy, there is an option for you now to consider.”
Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Ibalizumab, the first monoclonal antibody to reach a phase III trial for multidrug resistant HIV therapy, has demonstrated efficacy, safety, and a novel mechanism of action, offering promise to many patients with few remaining options.
“The drug has now shown very significant antiretroviral activity, with 83% of patients demonstrating a half log decrease after 7 days, and a mean and median decrease of 1.1 log,” Jacob Lalezari, MD, lead author of the study and medical director of Quest Clinical Research in San Francisco, said at IDWeek 2016, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “The big message here is the novel mechanism of action provides new treatment to patients with limited options, really offering hope to those left behind by an otherwise amazing evolution of HIV treatment.”
Dr. Lalezari said there is currently no evidence of cross-resistance with existing antiretrovirals. “I think we got lucky with that,” he noted. “For the primary care HIV doc, who is increasingly overwhelmed by drug-drug interactions, the good news is ibalizumab does not have obvious drug-drug interactions with antiretrovirals or other [HIV] drugs in other classes.”
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, announced the findings with Dr. Lalezari at an IDWeek 2016 press conference. “This study is very important, because although we have terrific therapies for initial and second line treatment, we’ve really come to need treatment for the core group of patients who have developed resistance to all the drugs in the armamentarium,” he said.
Multidrug resistance emerges in less than 5% of people with HIV, affecting up to about 10,000 people in the United States. “It’s not a huge population, but it’s the most vulnerable and in need,” Dr. Lalezari said.
The 40 treatment-experienced patients in the study had their viral load and CD4+ counts measured at day 0. At day 7, they received a 2,000 mg IV loading dose of ibalizumab. At day 14, the response to ibalizumab monotherapy was measured and participants began an optimized background regimen with at least one other agent to which HIV showed sensitivity. Unfortunately, for about 50% of the patients, there was no such agent remaining, so researchers added BMS-663068, an investigational oral attachment inhibitor.
The cohort was 85% men, 45% nonwhite, and had a mean duration of HIV infection of 21 years. At study entry, patients’ mean viral load was approximately 100,000 copies/mL and mean CD4+ T-cell count was 160/mcL. However, half of the patients had T-cell counts below 100, and one third had counts below 10, “meaning they are at the very edge of sustainability,” Dr. Lalezari said.
Efficacy and safety
“The story is pretty simple – the drug worked,” Dr. Lalezari said. In addition to the 83% who met the primary endpoint of a half-life log decrease in HIV-1 RNA, 60% had a full log decrease or more at day 14. The mean and median HIV-1 RNA decrease for the entire cohort was 1.1 log10, a significant difference, compared with the day 0 to 7 control period (P less than .0001).
Putting the findings in perspective, Dr. Lalezari said, “So 1 log is not the most potent drug we see for HIV, but it’s pretty good. And in the setting of multidrug-resistant virus, it’s very good.” Although the agent is not potent enough for monotherapy, he said it is a strong candidate for combination therapy. The goal is to “give somebody a chance, potentially their last chance, to get control of the virus and prevent the progression of disease, and importantly, prevent them from spreading it to somebody else.”
“It’s a bit of a mystery” why 7 of the 40 patients did not meet the primary endpoint, Dr. Lalezari added. None of the factors the investigators compared between responders and nonresponders were significantly different.
Ibalizumab appears safe with no discontinuations and no treatment-related serious adverse events, Dr. Lalezari said. “We have not seen anything that strikes me as concerning at all in terms of safety, and it’s in a patient population that is quite ill.”
When asked during the press conference to address cost concerns, an issue in other specialties when biologics are introduced, Dr. Lalezari responded, “The one comment I will make is that whatever this drug is, it’s not a ‘me too drug.’ It’s unique and offers a unique mechanism of action. For those patients, the patients we saw whose T cells were under 10, whose health was failing and they were getting ready to die, their viral loads got suppressed and now they’re living, so it brings great value.”
Ibalizumab’s antiretroviral activity stems from blocking post-attachment conformational changes that are required to enable the HIV virus to bind with its co-receptors and ultimately gain entry into the target T cell. “Importantly the drug is away from the binding site for MHC class II molecules, and therefore not thought to cause T-cell depletion or be immunosuppressive,” Dr. Lalezari said.
In terms of the bigger picture, unlike most HIV drugs taken daily, ibalizumab is the first of the long-acting antiretrovirals, he noted. “I do think a paradigm shift is looming for at least some patients for whom long-acting therapy might be advantageous. There is also a movement toward IM and hopefully subcutaneous therapy as well, which would be very advantageous for patient self-administration.”
Although Dr. Lalezari and Dr. Kuritzkes presented the 14-day findings, the study is ongoing and participants continue to receive 800 mg ibalizumab intravenously every two weeks.
TaiMed Biologics, the sponsor of the study, has an expanded access program. Dr. Lalezari said, “So if you [have] a patient who is in trouble, in need of rescue therapy, there is an option for you now to consider.”
Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Ibalizumab, the first monoclonal antibody to reach a phase III trial for multidrug resistant HIV therapy, has demonstrated efficacy, safety, and a novel mechanism of action, offering promise to many patients with few remaining options.
“The drug has now shown very significant antiretroviral activity, with 83% of patients demonstrating a half log decrease after 7 days, and a mean and median decrease of 1.1 log,” Jacob Lalezari, MD, lead author of the study and medical director of Quest Clinical Research in San Francisco, said at IDWeek 2016, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “The big message here is the novel mechanism of action provides new treatment to patients with limited options, really offering hope to those left behind by an otherwise amazing evolution of HIV treatment.”
Dr. Lalezari said there is currently no evidence of cross-resistance with existing antiretrovirals. “I think we got lucky with that,” he noted. “For the primary care HIV doc, who is increasingly overwhelmed by drug-drug interactions, the good news is ibalizumab does not have obvious drug-drug interactions with antiretrovirals or other [HIV] drugs in other classes.”
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, announced the findings with Dr. Lalezari at an IDWeek 2016 press conference. “This study is very important, because although we have terrific therapies for initial and second line treatment, we’ve really come to need treatment for the core group of patients who have developed resistance to all the drugs in the armamentarium,” he said.
Multidrug resistance emerges in less than 5% of people with HIV, affecting up to about 10,000 people in the United States. “It’s not a huge population, but it’s the most vulnerable and in need,” Dr. Lalezari said.
The 40 treatment-experienced patients in the study had their viral load and CD4+ counts measured at day 0. At day 7, they received a 2,000 mg IV loading dose of ibalizumab. At day 14, the response to ibalizumab monotherapy was measured and participants began an optimized background regimen with at least one other agent to which HIV showed sensitivity. Unfortunately, for about 50% of the patients, there was no such agent remaining, so researchers added BMS-663068, an investigational oral attachment inhibitor.
The cohort was 85% men, 45% nonwhite, and had a mean duration of HIV infection of 21 years. At study entry, patients’ mean viral load was approximately 100,000 copies/mL and mean CD4+ T-cell count was 160/mcL. However, half of the patients had T-cell counts below 100, and one third had counts below 10, “meaning they are at the very edge of sustainability,” Dr. Lalezari said.
Efficacy and safety
“The story is pretty simple – the drug worked,” Dr. Lalezari said. In addition to the 83% who met the primary endpoint of a half-life log decrease in HIV-1 RNA, 60% had a full log decrease or more at day 14. The mean and median HIV-1 RNA decrease for the entire cohort was 1.1 log10, a significant difference, compared with the day 0 to 7 control period (P less than .0001).
Putting the findings in perspective, Dr. Lalezari said, “So 1 log is not the most potent drug we see for HIV, but it’s pretty good. And in the setting of multidrug-resistant virus, it’s very good.” Although the agent is not potent enough for monotherapy, he said it is a strong candidate for combination therapy. The goal is to “give somebody a chance, potentially their last chance, to get control of the virus and prevent the progression of disease, and importantly, prevent them from spreading it to somebody else.”
“It’s a bit of a mystery” why 7 of the 40 patients did not meet the primary endpoint, Dr. Lalezari added. None of the factors the investigators compared between responders and nonresponders were significantly different.
Ibalizumab appears safe with no discontinuations and no treatment-related serious adverse events, Dr. Lalezari said. “We have not seen anything that strikes me as concerning at all in terms of safety, and it’s in a patient population that is quite ill.”
When asked during the press conference to address cost concerns, an issue in other specialties when biologics are introduced, Dr. Lalezari responded, “The one comment I will make is that whatever this drug is, it’s not a ‘me too drug.’ It’s unique and offers a unique mechanism of action. For those patients, the patients we saw whose T cells were under 10, whose health was failing and they were getting ready to die, their viral loads got suppressed and now they’re living, so it brings great value.”
Ibalizumab’s antiretroviral activity stems from blocking post-attachment conformational changes that are required to enable the HIV virus to bind with its co-receptors and ultimately gain entry into the target T cell. “Importantly the drug is away from the binding site for MHC class II molecules, and therefore not thought to cause T-cell depletion or be immunosuppressive,” Dr. Lalezari said.
In terms of the bigger picture, unlike most HIV drugs taken daily, ibalizumab is the first of the long-acting antiretrovirals, he noted. “I do think a paradigm shift is looming for at least some patients for whom long-acting therapy might be advantageous. There is also a movement toward IM and hopefully subcutaneous therapy as well, which would be very advantageous for patient self-administration.”
Although Dr. Lalezari and Dr. Kuritzkes presented the 14-day findings, the study is ongoing and participants continue to receive 800 mg ibalizumab intravenously every two weeks.
TaiMed Biologics, the sponsor of the study, has an expanded access program. Dr. Lalezari said, “So if you [have] a patient who is in trouble, in need of rescue therapy, there is an option for you now to consider.”
Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT IDWEEK 2016
Key clinical point: Ibalizumab, a new biologic in a phase III trial, significantly reduces viral loads in patients with multidrug-resistant HIV infection.
Major finding: At 14 days, 83% of 40 trial participants experienced at least a one-half log reduction in HIV viral load.
Data source: Single arm, 24-week study of ibalizumab plus optimized background regimen in treatment experienced patients with multidrug-resistant HIV-1.
Disclosures: TaiMed Biologics, the manufacturer of ibalizumab, sponsored the study. Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
Staple line reinforcement linked to increased leak risk in bariatric surgery
Laparoscopic sleeve gastrectomy is safe and effective overall, but staple line reinforcement appears to increase the rate of postsurgical leaks – which were associated with readmissions and, in some cases, reoperations.
A large review of quality improvement data found that staple line reinforcement – an extremely common technique – was associated with a 60% increased risk of leak, compared with closures without staple line reinforcement, Elizabeth R. Berger, MD, and her colleagues reported in the October issue of the Annals of Surgery (2016;264:464-73).
“This study also demonstrates that leaks were significantly more morbid than bleeding with higher readmission and reoperation rates in patients with a leak vs. a bleed,” wrote Dr. Berger of Loyola University, Chicago, and her coauthors. “Therefore, a surgeon should consider the benefits, risks, and costs of each surgical technique in performing a laparoscopic sleeve gastrectomy and selectively utilize those that, in their hands, minimize morbidity while maximizing clinical effectiveness.”
The team examined outcomes in 189,477 laparoscopic sleeve gastrectomies performed by 1,634 surgeons at 720 centers from 2012 to 2014. All of the data were extracted from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program, created in 2012 by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery.
They examined the impact of staple line reinforcement, oversewing the staple line, bougie size, and distance of the staple line from the pylorus on 30-day outcomes, and their effect on weight loss and weight-related comorbidities at 1 year. Outcomes included morbidity, leak rates, and bleeding, which were examined at both the patient and surgeon levels.
Most patients (126,904; 67%) patients had some type of staple line reinforcement (SLR); the rest had only oversewn staple lines (OSL) or no reinforcement. Leaks occurred in 1,703 patients and bleeds in 1,436 patients. Leaks were more serious than bleeds: Patients with a leak were almost 28% more likely to readmitted and 11% more likely to need a reoperation than were patients who had only a bleed.
At the patient level, those with SLR with or without OSL were 20%-46% more likely to experience a leak than were those who had neither. Bleeding rates were about 70% lower in the SLR groups.
Most surgeons in the analysis (80%) used some type of SLR; almost 20% routinely used only OSL, and 30% routinely used only SLR. At the surgeon level, SLR was associated with a 60% increased risk of a postoperative leak, compared with no reinforcement. There was no association between SLR and bleeding risk, however.
Oversewing had an effect on 1-year weight loss. Patients with oversewn staple lines lost an additional 1.3 points on the body mass index (BMI) scale, compared with patients with no type of reinforcement.
“The reason for increased leaks from SLR is relatively unclear,” the authors wrote. “The two layers of material that are placed within the staple line could increase ischemia or decrease the relative staple heights. At the notches, where one staple firing ends and the next one begins, there is sandwiching of the two layers of staples and a combined four layers of SLR. This bulk may predispose to leaks.”
Larger bougie sizes (BS) seemed more beneficial than did smaller ones, in both the surgeon- and patient-level analyses. A BS of at least 38 French was associated with a 28% decreased risk of a leak (odds ratio 0.72) at the patient level and a 10% decreased risk at the surgeon level (OR 0.90). There were no associations with bleeding.
“Our findings support literature that describes narrower BSs leads to increased ischemia secondary to increased intraluminal pressure, causing more leaks,” the authors wrote.
A BS of at least 40 French had a significant impact on weight loss. At 1 year, patients with the larger BS had lost 2.45 points more on the BMI scale than did those with smaller sizes.
This finding is in accord with other studies, including one that found the best weight-loss outcomes associated with a BS of more than 60 French. “Perhaps the sleeve works because of more rapid emptying, which is favored by a relatively larger BS, rather than because of restriction,” they said.
The distance to the pylorus (DP) from the staple line initiation point was divided into four sections: less than 4 cm; 4-5 cm; 5-6 cm; and 6 cm or more.
On a patient level, there was no association between DP and leak rates. There was, however, an association with bleeding. A DP of 4-4.99 cm had the highest rate, 90%, while a DP of 5-5.99 cm had the lowest (71%). DP was also associated with weight loss on this level, with a distance of more than 6 cm being associated with the biggest BMI decrease (3.7 points).
“Our data show significantly increased excess weight loss in a stepwise fashion as the DP increases,” the authors said. “Our data suggest that as DP increased, there was an increased excess weight loss, possibly explained by preserving the ‘antral mill.’ Stapling further from the pylorus perhaps keeps the antrum’s functional component intact and allows food to enter the distal gut more quickly, leading to earlier satiety and increased weight loss.”
Only 114 surgeons (8%) used a DP of less than 4 cm. There were no significant associations with any 30-day outcomes and DP after adjustment.
The authors had no financial disclosures.
Before drawing overarching conclusions and implementing recommendations based on this study, there are several limitations that must be borne in mind when considering data-mining exercises such as this one:
• It should be taken into account that there was significant intraoperative variation in technique and experience among the surgeons that was not captured through the data acquisition.
• Similarly, the true distance between the stapler and the selected bougie is also variable, adding an inherent lack of accuracy of the true real diameter of the completed gastric tube.
• There is a lack of granular information, including the type of SLR or staplers used, thereby also limiting any reliable conclusions that could be drawn.
• There are additional techniques, such as omental buttressing, and the use of clips, sutures, or hemostatic agents that are not reported, yet may have an impact on leak and bleeding rates.
• The reported follow-up rate of 39.4% at 1 year is typically considered to be suboptimal.
• SLR techniques may also include oversewing, and these are also subject to wide variation, including the type of suture material used, and the actual suturing technique that was implemented.
• Only those patients whose bleeding was severe enough to warrant transfusions were included, such that lower level bleeding would have not been represented in this report.
• There were also deficiencies in correlating leaks or bleeding rates with staple height selection, or the experience and learning curve of the surgeon.
Samer Mattar, MD, is a bariatric surgeon and professor of surgery at Oregon Health and Science University, Portland. Dr. Mattar has no disclosures.
Before drawing overarching conclusions and implementing recommendations based on this study, there are several limitations that must be borne in mind when considering data-mining exercises such as this one:
• It should be taken into account that there was significant intraoperative variation in technique and experience among the surgeons that was not captured through the data acquisition.
• Similarly, the true distance between the stapler and the selected bougie is also variable, adding an inherent lack of accuracy of the true real diameter of the completed gastric tube.
• There is a lack of granular information, including the type of SLR or staplers used, thereby also limiting any reliable conclusions that could be drawn.
• There are additional techniques, such as omental buttressing, and the use of clips, sutures, or hemostatic agents that are not reported, yet may have an impact on leak and bleeding rates.
• The reported follow-up rate of 39.4% at 1 year is typically considered to be suboptimal.
• SLR techniques may also include oversewing, and these are also subject to wide variation, including the type of suture material used, and the actual suturing technique that was implemented.
• Only those patients whose bleeding was severe enough to warrant transfusions were included, such that lower level bleeding would have not been represented in this report.
• There were also deficiencies in correlating leaks or bleeding rates with staple height selection, or the experience and learning curve of the surgeon.
Samer Mattar, MD, is a bariatric surgeon and professor of surgery at Oregon Health and Science University, Portland. Dr. Mattar has no disclosures.
Before drawing overarching conclusions and implementing recommendations based on this study, there are several limitations that must be borne in mind when considering data-mining exercises such as this one:
• It should be taken into account that there was significant intraoperative variation in technique and experience among the surgeons that was not captured through the data acquisition.
• Similarly, the true distance between the stapler and the selected bougie is also variable, adding an inherent lack of accuracy of the true real diameter of the completed gastric tube.
• There is a lack of granular information, including the type of SLR or staplers used, thereby also limiting any reliable conclusions that could be drawn.
• There are additional techniques, such as omental buttressing, and the use of clips, sutures, or hemostatic agents that are not reported, yet may have an impact on leak and bleeding rates.
• The reported follow-up rate of 39.4% at 1 year is typically considered to be suboptimal.
• SLR techniques may also include oversewing, and these are also subject to wide variation, including the type of suture material used, and the actual suturing technique that was implemented.
• Only those patients whose bleeding was severe enough to warrant transfusions were included, such that lower level bleeding would have not been represented in this report.
• There were also deficiencies in correlating leaks or bleeding rates with staple height selection, or the experience and learning curve of the surgeon.
Samer Mattar, MD, is a bariatric surgeon and professor of surgery at Oregon Health and Science University, Portland. Dr. Mattar has no disclosures.
Laparoscopic sleeve gastrectomy is safe and effective overall, but staple line reinforcement appears to increase the rate of postsurgical leaks – which were associated with readmissions and, in some cases, reoperations.
A large review of quality improvement data found that staple line reinforcement – an extremely common technique – was associated with a 60% increased risk of leak, compared with closures without staple line reinforcement, Elizabeth R. Berger, MD, and her colleagues reported in the October issue of the Annals of Surgery (2016;264:464-73).
“This study also demonstrates that leaks were significantly more morbid than bleeding with higher readmission and reoperation rates in patients with a leak vs. a bleed,” wrote Dr. Berger of Loyola University, Chicago, and her coauthors. “Therefore, a surgeon should consider the benefits, risks, and costs of each surgical technique in performing a laparoscopic sleeve gastrectomy and selectively utilize those that, in their hands, minimize morbidity while maximizing clinical effectiveness.”
The team examined outcomes in 189,477 laparoscopic sleeve gastrectomies performed by 1,634 surgeons at 720 centers from 2012 to 2014. All of the data were extracted from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program, created in 2012 by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery.
They examined the impact of staple line reinforcement, oversewing the staple line, bougie size, and distance of the staple line from the pylorus on 30-day outcomes, and their effect on weight loss and weight-related comorbidities at 1 year. Outcomes included morbidity, leak rates, and bleeding, which were examined at both the patient and surgeon levels.
Most patients (126,904; 67%) patients had some type of staple line reinforcement (SLR); the rest had only oversewn staple lines (OSL) or no reinforcement. Leaks occurred in 1,703 patients and bleeds in 1,436 patients. Leaks were more serious than bleeds: Patients with a leak were almost 28% more likely to readmitted and 11% more likely to need a reoperation than were patients who had only a bleed.
At the patient level, those with SLR with or without OSL were 20%-46% more likely to experience a leak than were those who had neither. Bleeding rates were about 70% lower in the SLR groups.
Most surgeons in the analysis (80%) used some type of SLR; almost 20% routinely used only OSL, and 30% routinely used only SLR. At the surgeon level, SLR was associated with a 60% increased risk of a postoperative leak, compared with no reinforcement. There was no association between SLR and bleeding risk, however.
Oversewing had an effect on 1-year weight loss. Patients with oversewn staple lines lost an additional 1.3 points on the body mass index (BMI) scale, compared with patients with no type of reinforcement.
“The reason for increased leaks from SLR is relatively unclear,” the authors wrote. “The two layers of material that are placed within the staple line could increase ischemia or decrease the relative staple heights. At the notches, where one staple firing ends and the next one begins, there is sandwiching of the two layers of staples and a combined four layers of SLR. This bulk may predispose to leaks.”
Larger bougie sizes (BS) seemed more beneficial than did smaller ones, in both the surgeon- and patient-level analyses. A BS of at least 38 French was associated with a 28% decreased risk of a leak (odds ratio 0.72) at the patient level and a 10% decreased risk at the surgeon level (OR 0.90). There were no associations with bleeding.
“Our findings support literature that describes narrower BSs leads to increased ischemia secondary to increased intraluminal pressure, causing more leaks,” the authors wrote.
A BS of at least 40 French had a significant impact on weight loss. At 1 year, patients with the larger BS had lost 2.45 points more on the BMI scale than did those with smaller sizes.
This finding is in accord with other studies, including one that found the best weight-loss outcomes associated with a BS of more than 60 French. “Perhaps the sleeve works because of more rapid emptying, which is favored by a relatively larger BS, rather than because of restriction,” they said.
The distance to the pylorus (DP) from the staple line initiation point was divided into four sections: less than 4 cm; 4-5 cm; 5-6 cm; and 6 cm or more.
On a patient level, there was no association between DP and leak rates. There was, however, an association with bleeding. A DP of 4-4.99 cm had the highest rate, 90%, while a DP of 5-5.99 cm had the lowest (71%). DP was also associated with weight loss on this level, with a distance of more than 6 cm being associated with the biggest BMI decrease (3.7 points).
“Our data show significantly increased excess weight loss in a stepwise fashion as the DP increases,” the authors said. “Our data suggest that as DP increased, there was an increased excess weight loss, possibly explained by preserving the ‘antral mill.’ Stapling further from the pylorus perhaps keeps the antrum’s functional component intact and allows food to enter the distal gut more quickly, leading to earlier satiety and increased weight loss.”
Only 114 surgeons (8%) used a DP of less than 4 cm. There were no significant associations with any 30-day outcomes and DP after adjustment.
The authors had no financial disclosures.
Laparoscopic sleeve gastrectomy is safe and effective overall, but staple line reinforcement appears to increase the rate of postsurgical leaks – which were associated with readmissions and, in some cases, reoperations.
A large review of quality improvement data found that staple line reinforcement – an extremely common technique – was associated with a 60% increased risk of leak, compared with closures without staple line reinforcement, Elizabeth R. Berger, MD, and her colleagues reported in the October issue of the Annals of Surgery (2016;264:464-73).
“This study also demonstrates that leaks were significantly more morbid than bleeding with higher readmission and reoperation rates in patients with a leak vs. a bleed,” wrote Dr. Berger of Loyola University, Chicago, and her coauthors. “Therefore, a surgeon should consider the benefits, risks, and costs of each surgical technique in performing a laparoscopic sleeve gastrectomy and selectively utilize those that, in their hands, minimize morbidity while maximizing clinical effectiveness.”
The team examined outcomes in 189,477 laparoscopic sleeve gastrectomies performed by 1,634 surgeons at 720 centers from 2012 to 2014. All of the data were extracted from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program, created in 2012 by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery.
They examined the impact of staple line reinforcement, oversewing the staple line, bougie size, and distance of the staple line from the pylorus on 30-day outcomes, and their effect on weight loss and weight-related comorbidities at 1 year. Outcomes included morbidity, leak rates, and bleeding, which were examined at both the patient and surgeon levels.
Most patients (126,904; 67%) patients had some type of staple line reinforcement (SLR); the rest had only oversewn staple lines (OSL) or no reinforcement. Leaks occurred in 1,703 patients and bleeds in 1,436 patients. Leaks were more serious than bleeds: Patients with a leak were almost 28% more likely to readmitted and 11% more likely to need a reoperation than were patients who had only a bleed.
At the patient level, those with SLR with or without OSL were 20%-46% more likely to experience a leak than were those who had neither. Bleeding rates were about 70% lower in the SLR groups.
Most surgeons in the analysis (80%) used some type of SLR; almost 20% routinely used only OSL, and 30% routinely used only SLR. At the surgeon level, SLR was associated with a 60% increased risk of a postoperative leak, compared with no reinforcement. There was no association between SLR and bleeding risk, however.
Oversewing had an effect on 1-year weight loss. Patients with oversewn staple lines lost an additional 1.3 points on the body mass index (BMI) scale, compared with patients with no type of reinforcement.
“The reason for increased leaks from SLR is relatively unclear,” the authors wrote. “The two layers of material that are placed within the staple line could increase ischemia or decrease the relative staple heights. At the notches, where one staple firing ends and the next one begins, there is sandwiching of the two layers of staples and a combined four layers of SLR. This bulk may predispose to leaks.”
Larger bougie sizes (BS) seemed more beneficial than did smaller ones, in both the surgeon- and patient-level analyses. A BS of at least 38 French was associated with a 28% decreased risk of a leak (odds ratio 0.72) at the patient level and a 10% decreased risk at the surgeon level (OR 0.90). There were no associations with bleeding.
“Our findings support literature that describes narrower BSs leads to increased ischemia secondary to increased intraluminal pressure, causing more leaks,” the authors wrote.
A BS of at least 40 French had a significant impact on weight loss. At 1 year, patients with the larger BS had lost 2.45 points more on the BMI scale than did those with smaller sizes.
This finding is in accord with other studies, including one that found the best weight-loss outcomes associated with a BS of more than 60 French. “Perhaps the sleeve works because of more rapid emptying, which is favored by a relatively larger BS, rather than because of restriction,” they said.
The distance to the pylorus (DP) from the staple line initiation point was divided into four sections: less than 4 cm; 4-5 cm; 5-6 cm; and 6 cm or more.
On a patient level, there was no association between DP and leak rates. There was, however, an association with bleeding. A DP of 4-4.99 cm had the highest rate, 90%, while a DP of 5-5.99 cm had the lowest (71%). DP was also associated with weight loss on this level, with a distance of more than 6 cm being associated with the biggest BMI decrease (3.7 points).
“Our data show significantly increased excess weight loss in a stepwise fashion as the DP increases,” the authors said. “Our data suggest that as DP increased, there was an increased excess weight loss, possibly explained by preserving the ‘antral mill.’ Stapling further from the pylorus perhaps keeps the antrum’s functional component intact and allows food to enter the distal gut more quickly, leading to earlier satiety and increased weight loss.”
Only 114 surgeons (8%) used a DP of less than 4 cm. There were no significant associations with any 30-day outcomes and DP after adjustment.
The authors had no financial disclosures.
FROM THE ANNALS OF SURGERY
Key clinical point:
Major finding: Compared to not reinforcing the staple line, doing sow as associated with up to a 60% increase in the risk of a postsurgical leak.
Data source: The database review contained outcomes on 189,477 laparoscopic sleeve gastrectomies.
Disclosures: None of the study authors had any financial disclosures.
Heart failure targets African Americans
ORLANDO – The disparity in U.S. heart failure incidence continued undiminished during 2002-2013, with African Americans maintaining a steady 2.3-fold increased rate of heart failure, compared with whites, based on national levels of heart failure hospitalizations, a reasonable surrogate for incidence rates, Boback Ziaeian, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
The same period also showed a substantial relative improvement in the heart failure hospitalization rates among U.S. Hispanics, compared with whites, so that, by 2013, the ethnic disparity seen in 2002 between Hispanics and whites largely disappeared, reported Dr. Ziaeian, a cardiologist at the University of California, Los Angeles. The data he analyzed also showed that Asian Americans had the lowest heart failure hospitalization rates of any racial or ethnic group throughout the 11-year period, and that the incidence of heart failure fell more sharply in women than in men during the period, based on the hospitalization numbers.
Age-adjusted heart failure hospitalizations among whites dropped by 30%, and among African Americans by a nearly identical 29%. But this maintained a greater than twofold disparity in rates between the two groups. Among whites, the rate per 100,000 fell from 448 to 315; among African Americans, it dropped from 1,048 to 741. In 2013, the rate of heart failure hospitalizations was 2.4-fold higher in African Americans, compared with whites.
Heart failure hospitalizations fell among Hispanics from 650 per 100,000 to 337 per 100,000 in 2013, a 48% drop that brought the rate among Hispanics to nearly the same as among whites. Asian Americans remained the group with the least heart failure throughout the period, falling from 343 hospitalizations per 100,000 in 2002 to 181 per 100,000 in 2013, a 47% drop.
Among women, the age-adjusted rate per 100,000 fell from 486 to 311, a 36% drop, compared with a decrease from 582 to 431 per 100,000 in men, a 26% reduction. Lower incidence in women may reflect better risk factor control during the study period, compared with men, such as a higher rate of quiting smoking and better treatment compliance, Dr. Ziaeian suggested.
[email protected]
On Twitter @mitchelzoler
ORLANDO – The disparity in U.S. heart failure incidence continued undiminished during 2002-2013, with African Americans maintaining a steady 2.3-fold increased rate of heart failure, compared with whites, based on national levels of heart failure hospitalizations, a reasonable surrogate for incidence rates, Boback Ziaeian, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
The same period also showed a substantial relative improvement in the heart failure hospitalization rates among U.S. Hispanics, compared with whites, so that, by 2013, the ethnic disparity seen in 2002 between Hispanics and whites largely disappeared, reported Dr. Ziaeian, a cardiologist at the University of California, Los Angeles. The data he analyzed also showed that Asian Americans had the lowest heart failure hospitalization rates of any racial or ethnic group throughout the 11-year period, and that the incidence of heart failure fell more sharply in women than in men during the period, based on the hospitalization numbers.
Age-adjusted heart failure hospitalizations among whites dropped by 30%, and among African Americans by a nearly identical 29%. But this maintained a greater than twofold disparity in rates between the two groups. Among whites, the rate per 100,000 fell from 448 to 315; among African Americans, it dropped from 1,048 to 741. In 2013, the rate of heart failure hospitalizations was 2.4-fold higher in African Americans, compared with whites.
Heart failure hospitalizations fell among Hispanics from 650 per 100,000 to 337 per 100,000 in 2013, a 48% drop that brought the rate among Hispanics to nearly the same as among whites. Asian Americans remained the group with the least heart failure throughout the period, falling from 343 hospitalizations per 100,000 in 2002 to 181 per 100,000 in 2013, a 47% drop.
Among women, the age-adjusted rate per 100,000 fell from 486 to 311, a 36% drop, compared with a decrease from 582 to 431 per 100,000 in men, a 26% reduction. Lower incidence in women may reflect better risk factor control during the study period, compared with men, such as a higher rate of quiting smoking and better treatment compliance, Dr. Ziaeian suggested.
[email protected]
On Twitter @mitchelzoler
ORLANDO – The disparity in U.S. heart failure incidence continued undiminished during 2002-2013, with African Americans maintaining a steady 2.3-fold increased rate of heart failure, compared with whites, based on national levels of heart failure hospitalizations, a reasonable surrogate for incidence rates, Boback Ziaeian, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
The same period also showed a substantial relative improvement in the heart failure hospitalization rates among U.S. Hispanics, compared with whites, so that, by 2013, the ethnic disparity seen in 2002 between Hispanics and whites largely disappeared, reported Dr. Ziaeian, a cardiologist at the University of California, Los Angeles. The data he analyzed also showed that Asian Americans had the lowest heart failure hospitalization rates of any racial or ethnic group throughout the 11-year period, and that the incidence of heart failure fell more sharply in women than in men during the period, based on the hospitalization numbers.
Age-adjusted heart failure hospitalizations among whites dropped by 30%, and among African Americans by a nearly identical 29%. But this maintained a greater than twofold disparity in rates between the two groups. Among whites, the rate per 100,000 fell from 448 to 315; among African Americans, it dropped from 1,048 to 741. In 2013, the rate of heart failure hospitalizations was 2.4-fold higher in African Americans, compared with whites.
Heart failure hospitalizations fell among Hispanics from 650 per 100,000 to 337 per 100,000 in 2013, a 48% drop that brought the rate among Hispanics to nearly the same as among whites. Asian Americans remained the group with the least heart failure throughout the period, falling from 343 hospitalizations per 100,000 in 2002 to 181 per 100,000 in 2013, a 47% drop.
Among women, the age-adjusted rate per 100,000 fell from 486 to 311, a 36% drop, compared with a decrease from 582 to 431 per 100,000 in men, a 26% reduction. Lower incidence in women may reflect better risk factor control during the study period, compared with men, such as a higher rate of quiting smoking and better treatment compliance, Dr. Ziaeian suggested.
[email protected]
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: In 2013, age-adjusted heart failure hospitalization was 741/100,000 in African Americans and 315/100,000 in whites.
Data source: The National Inpatient Sample and U.S. Census data.
Disclosures: Dr. Ziaeian had no disclosures.