User login
Team identifies gaps in anticoagulant use

Photo courtesy of NIGMS
Many patients with atrial fibrillation (AF) who are at the highest risk of stroke are not receiving the recommended oral anticoagulant therapy, according to a new study.
Researchers did find that AF patients who had a higher risk of stroke according to CHADS2 score or CHA2DS2-VASc score were more likely to receive an oral anticoagulant.
But the prevalence of oral anticoagulant use did not exceed 50%, even among high-risk patients.
Jonathan C. Hsu, MD, of the University of California, San Diego, and his colleagues reported these findings in JAMA Cardiology.
The researchers said it has not been clear if the prescription of oral anticoagulants increases as the risk of stroke increases in AF patients.
To gain some insight, the team studied 429,417 outpatients with AF enrolled in the American College of Cardiology National Cardiovascular Data Registry’s PINNACLE Registry between January 2008 and December 2012.
The researchers calculated the CHADS2 score and the CHA2DS2-VASc score for all patients and examined the association between increased stroke risk score and prescription of an oral anticoagulant.
In the entire cohort, 44.9% of patients were prescribed an oral anticoagulant, 25.9% aspirin only, 5.5% aspirin plus a thienopyridine, and 23.8% no antithrombotic therapy.
The researchers found that each 1-point increase in risk score was associated with increased odds of oral anticoagulant prescription when compared with aspirin-only prescription.
When using CHADS2 score, the adjusted odds ratio was 1.158 (95% CI, 1.144-1.172, P<0.001). When using CHA2DS2-VASc score, the adjusted odds ratio was 1.163 (95% CI, 1.157-1.169, P<0.001).
Still, the researchers said they observed a plateau in oral anticoagulant prescription.
The prevalence of oral anticoagulant prescription did not exceed 50%, even in patients with a CHADS2 score exceeding 3 or a CHA2DS2-VASc score exceeding 4.
Dr Hsu and his colleagues said these findings draw attention to important gaps in the appropriate treatment of patients with AF at the highest risk of stroke and highlight opportunities to understand the reasons behind these gaps. ![]()

Photo courtesy of NIGMS
Many patients with atrial fibrillation (AF) who are at the highest risk of stroke are not receiving the recommended oral anticoagulant therapy, according to a new study.
Researchers did find that AF patients who had a higher risk of stroke according to CHADS2 score or CHA2DS2-VASc score were more likely to receive an oral anticoagulant.
But the prevalence of oral anticoagulant use did not exceed 50%, even among high-risk patients.
Jonathan C. Hsu, MD, of the University of California, San Diego, and his colleagues reported these findings in JAMA Cardiology.
The researchers said it has not been clear if the prescription of oral anticoagulants increases as the risk of stroke increases in AF patients.
To gain some insight, the team studied 429,417 outpatients with AF enrolled in the American College of Cardiology National Cardiovascular Data Registry’s PINNACLE Registry between January 2008 and December 2012.
The researchers calculated the CHADS2 score and the CHA2DS2-VASc score for all patients and examined the association between increased stroke risk score and prescription of an oral anticoagulant.
In the entire cohort, 44.9% of patients were prescribed an oral anticoagulant, 25.9% aspirin only, 5.5% aspirin plus a thienopyridine, and 23.8% no antithrombotic therapy.
The researchers found that each 1-point increase in risk score was associated with increased odds of oral anticoagulant prescription when compared with aspirin-only prescription.
When using CHADS2 score, the adjusted odds ratio was 1.158 (95% CI, 1.144-1.172, P<0.001). When using CHA2DS2-VASc score, the adjusted odds ratio was 1.163 (95% CI, 1.157-1.169, P<0.001).
Still, the researchers said they observed a plateau in oral anticoagulant prescription.
The prevalence of oral anticoagulant prescription did not exceed 50%, even in patients with a CHADS2 score exceeding 3 or a CHA2DS2-VASc score exceeding 4.
Dr Hsu and his colleagues said these findings draw attention to important gaps in the appropriate treatment of patients with AF at the highest risk of stroke and highlight opportunities to understand the reasons behind these gaps. ![]()

Photo courtesy of NIGMS
Many patients with atrial fibrillation (AF) who are at the highest risk of stroke are not receiving the recommended oral anticoagulant therapy, according to a new study.
Researchers did find that AF patients who had a higher risk of stroke according to CHADS2 score or CHA2DS2-VASc score were more likely to receive an oral anticoagulant.
But the prevalence of oral anticoagulant use did not exceed 50%, even among high-risk patients.
Jonathan C. Hsu, MD, of the University of California, San Diego, and his colleagues reported these findings in JAMA Cardiology.
The researchers said it has not been clear if the prescription of oral anticoagulants increases as the risk of stroke increases in AF patients.
To gain some insight, the team studied 429,417 outpatients with AF enrolled in the American College of Cardiology National Cardiovascular Data Registry’s PINNACLE Registry between January 2008 and December 2012.
The researchers calculated the CHADS2 score and the CHA2DS2-VASc score for all patients and examined the association between increased stroke risk score and prescription of an oral anticoagulant.
In the entire cohort, 44.9% of patients were prescribed an oral anticoagulant, 25.9% aspirin only, 5.5% aspirin plus a thienopyridine, and 23.8% no antithrombotic therapy.
The researchers found that each 1-point increase in risk score was associated with increased odds of oral anticoagulant prescription when compared with aspirin-only prescription.
When using CHADS2 score, the adjusted odds ratio was 1.158 (95% CI, 1.144-1.172, P<0.001). When using CHA2DS2-VASc score, the adjusted odds ratio was 1.163 (95% CI, 1.157-1.169, P<0.001).
Still, the researchers said they observed a plateau in oral anticoagulant prescription.
The prevalence of oral anticoagulant prescription did not exceed 50%, even in patients with a CHADS2 score exceeding 3 or a CHA2DS2-VASc score exceeding 4.
Dr Hsu and his colleagues said these findings draw attention to important gaps in the appropriate treatment of patients with AF at the highest risk of stroke and highlight opportunities to understand the reasons behind these gaps. ![]()
Aesthetic Dermatology: Eyelash extensions
The obsession with longer, fuller, darker eyelashes has become a mainstay in our culture – initially with the ever growing options of mascaras and glue on eyelashes, and now with options that are longer lasting, including eyelash extensions (semipermanent eyelashes) and topical eyelash growth enhancers (such as bimatoprost).
Eyelash extensions are not the same as glue-on strip or individual lashes bought at the drug store or makeup counter that last 1-2 days. These are silk, mink, or poly nylon synthetic lashes that typically last for approximately four weeks, with refills often required at 2-4 week intervals as the natural eyelash sheds. They are adhered to the person’s natural eyelash via an adhesive bonding process that can take 1-2 hours for initial application. Generally, a single lash is applied to each natural lash.
When applied properly, neither the extension eyelash nor the glue should touch the eyelid. The bond is designed to last until the lashes naturally fall out, although the extensions may fall out faster if one uses oil-based eye makeup remover or rubs the eyes regularly, as oil weakens the bond between the glue and the lash. Eyelash extensions are waterproof and give the appearance of having mascara on without wearing it. In the United States, eyelash extension services can range from $100 to $500 for the initial application, with decreased cost for refills. Lash extensions are waterproof and popular for special occasions and vacations, and even more so now for every day.
Potential adverse effects of eyelash extensions include ocular hyperemia, keratoconjunctivitis, allergic blepharitis, and allergic contact dermatitis in the patient. Keratoconjunctivitis is thought to be due to formaldehyde contained in some of the glues used for application.1 Eyelash extensions have also been associated with occupational allergic contact dermatitis, allergic rhinitis, and occupational asthma in the practitioner applying the eyelash extensions, particularly with the cyanoacrylate-based glues.2,3
In a national survey of eyelash extensions and their health-related problems in Japan, 10% (205) of the respondents had experience with eyelash extensions. Of those women, 27% (55) experienced problems that included ocular hyperemia, pain, and itchy swollen eyelids.4 Conjunctival erosion from the eyelid fixing tape used during application and subconjunctival hemorrhage from compression during removal of the extensions has been also reported.1 Hair breakage and even traction alopecia may occur, especially in patients who accidentally or intentionally pull the extensions off.
If permanent eyelash damage occurs, eyelash transplantation may be required to replace the eyelash, as eyelash growth medications such as bimatoprost may not be effective if the follicle is missing or severely damaged. Eyelash transplants often grow long enough where they require trimming, especially if donor sites are taken from the scalp.5
Eyelash extensions offer a nice alternative to daily use of mascara, temporary glue-on eyelashes, and daily application of topical eyelash growth products. As this procedure has increased in number, the dermatologist may be consulted for recommendations and treatment of any potential adverse events associated with it.
References
1. Cornea. 2012 Feb;31(2):121-5.
2. Contact Dermatitis. 2012 Nov;67(5):307-8.
3. Occup Med (Lond). 2013 Jun;63(4):294-7.
4. Nihon Eiseigaku Zasshi. 2013;68(3):168-74.
5. Plast Reconstr Surg Glob Open. 2015 Apr 7;3(3):e324.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
The obsession with longer, fuller, darker eyelashes has become a mainstay in our culture – initially with the ever growing options of mascaras and glue on eyelashes, and now with options that are longer lasting, including eyelash extensions (semipermanent eyelashes) and topical eyelash growth enhancers (such as bimatoprost).
Eyelash extensions are not the same as glue-on strip or individual lashes bought at the drug store or makeup counter that last 1-2 days. These are silk, mink, or poly nylon synthetic lashes that typically last for approximately four weeks, with refills often required at 2-4 week intervals as the natural eyelash sheds. They are adhered to the person’s natural eyelash via an adhesive bonding process that can take 1-2 hours for initial application. Generally, a single lash is applied to each natural lash.
When applied properly, neither the extension eyelash nor the glue should touch the eyelid. The bond is designed to last until the lashes naturally fall out, although the extensions may fall out faster if one uses oil-based eye makeup remover or rubs the eyes regularly, as oil weakens the bond between the glue and the lash. Eyelash extensions are waterproof and give the appearance of having mascara on without wearing it. In the United States, eyelash extension services can range from $100 to $500 for the initial application, with decreased cost for refills. Lash extensions are waterproof and popular for special occasions and vacations, and even more so now for every day.
Potential adverse effects of eyelash extensions include ocular hyperemia, keratoconjunctivitis, allergic blepharitis, and allergic contact dermatitis in the patient. Keratoconjunctivitis is thought to be due to formaldehyde contained in some of the glues used for application.1 Eyelash extensions have also been associated with occupational allergic contact dermatitis, allergic rhinitis, and occupational asthma in the practitioner applying the eyelash extensions, particularly with the cyanoacrylate-based glues.2,3
In a national survey of eyelash extensions and their health-related problems in Japan, 10% (205) of the respondents had experience with eyelash extensions. Of those women, 27% (55) experienced problems that included ocular hyperemia, pain, and itchy swollen eyelids.4 Conjunctival erosion from the eyelid fixing tape used during application and subconjunctival hemorrhage from compression during removal of the extensions has been also reported.1 Hair breakage and even traction alopecia may occur, especially in patients who accidentally or intentionally pull the extensions off.
If permanent eyelash damage occurs, eyelash transplantation may be required to replace the eyelash, as eyelash growth medications such as bimatoprost may not be effective if the follicle is missing or severely damaged. Eyelash transplants often grow long enough where they require trimming, especially if donor sites are taken from the scalp.5
Eyelash extensions offer a nice alternative to daily use of mascara, temporary glue-on eyelashes, and daily application of topical eyelash growth products. As this procedure has increased in number, the dermatologist may be consulted for recommendations and treatment of any potential adverse events associated with it.
References
1. Cornea. 2012 Feb;31(2):121-5.
2. Contact Dermatitis. 2012 Nov;67(5):307-8.
3. Occup Med (Lond). 2013 Jun;63(4):294-7.
4. Nihon Eiseigaku Zasshi. 2013;68(3):168-74.
5. Plast Reconstr Surg Glob Open. 2015 Apr 7;3(3):e324.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
The obsession with longer, fuller, darker eyelashes has become a mainstay in our culture – initially with the ever growing options of mascaras and glue on eyelashes, and now with options that are longer lasting, including eyelash extensions (semipermanent eyelashes) and topical eyelash growth enhancers (such as bimatoprost).
Eyelash extensions are not the same as glue-on strip or individual lashes bought at the drug store or makeup counter that last 1-2 days. These are silk, mink, or poly nylon synthetic lashes that typically last for approximately four weeks, with refills often required at 2-4 week intervals as the natural eyelash sheds. They are adhered to the person’s natural eyelash via an adhesive bonding process that can take 1-2 hours for initial application. Generally, a single lash is applied to each natural lash.
When applied properly, neither the extension eyelash nor the glue should touch the eyelid. The bond is designed to last until the lashes naturally fall out, although the extensions may fall out faster if one uses oil-based eye makeup remover or rubs the eyes regularly, as oil weakens the bond between the glue and the lash. Eyelash extensions are waterproof and give the appearance of having mascara on without wearing it. In the United States, eyelash extension services can range from $100 to $500 for the initial application, with decreased cost for refills. Lash extensions are waterproof and popular for special occasions and vacations, and even more so now for every day.
Potential adverse effects of eyelash extensions include ocular hyperemia, keratoconjunctivitis, allergic blepharitis, and allergic contact dermatitis in the patient. Keratoconjunctivitis is thought to be due to formaldehyde contained in some of the glues used for application.1 Eyelash extensions have also been associated with occupational allergic contact dermatitis, allergic rhinitis, and occupational asthma in the practitioner applying the eyelash extensions, particularly with the cyanoacrylate-based glues.2,3
In a national survey of eyelash extensions and their health-related problems in Japan, 10% (205) of the respondents had experience with eyelash extensions. Of those women, 27% (55) experienced problems that included ocular hyperemia, pain, and itchy swollen eyelids.4 Conjunctival erosion from the eyelid fixing tape used during application and subconjunctival hemorrhage from compression during removal of the extensions has been also reported.1 Hair breakage and even traction alopecia may occur, especially in patients who accidentally or intentionally pull the extensions off.
If permanent eyelash damage occurs, eyelash transplantation may be required to replace the eyelash, as eyelash growth medications such as bimatoprost may not be effective if the follicle is missing or severely damaged. Eyelash transplants often grow long enough where they require trimming, especially if donor sites are taken from the scalp.5
Eyelash extensions offer a nice alternative to daily use of mascara, temporary glue-on eyelashes, and daily application of topical eyelash growth products. As this procedure has increased in number, the dermatologist may be consulted for recommendations and treatment of any potential adverse events associated with it.
References
1. Cornea. 2012 Feb;31(2):121-5.
2. Contact Dermatitis. 2012 Nov;67(5):307-8.
3. Occup Med (Lond). 2013 Jun;63(4):294-7.
4. Nihon Eiseigaku Zasshi. 2013;68(3):168-74.
5. Plast Reconstr Surg Glob Open. 2015 Apr 7;3(3):e324.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Wesley.
CV health may prevent cognitive decline
The closer that older adults come to meeting the American Heart Association’s “ideal” targets for seven factors that determine cardiovascular health, the lower their risk for cognitive decline, according to a report published online March 16 in Journal of the American Heart Association.
A secondary analysis of data from a prospective population-based cohort study of stroke risk demonstrated that better alignment with the AHA’s “Life’s Simple 7” cardiovascular health metrics correlated with less decline in mental processing speed, and, to a lesser extent, in executive function and episodic memory. “The results of this study suggest that achievement of the AHA’s ideal cardiovascular health metrics may have benefits for brain health, in addition to preventing strokes and myocardial infarctions ... underscoring the importance of public health initiatives aimed to better control these seven factors,” said Hannah Gardener, Sc.D., of the department of neurology, University of Miami, and her associates.
The AHA recently defined ideal target levels for seven modifiable cardiovascular (CV) risk factors: smoking status, body mass index, physical activity level, diet, blood pressure, total cholesterol level, and fasting glucose level. Meeting or closely approaching these ideals has already been linked to a decreased risk of stroke and MI. To examine a possible association with brain health, Dr. Gardener and her colleagues assessed these seven metrics in an ethnically diverse cohort of 722 participants aged 50 years and older in the Northern Manhattan Study who underwent serial comprehensive neuropsychological testing including brain MRI.
Of the total cohort, 3% had zero ideal factors, 15% had one factor, 33% had two factors, 30% had three factors, 14% had four factors, 14% had five factors, 1% had six factors, and none had all seven factors.
“An increasing number of ideal cardiovascular health factors was positively associated with processing speed,” and the association was particularly strong for three of the factors: ideal body mass index, lack of smoking, and ideal fasting glucose level. This association persisted when the data were adjusted to account for MRI markers of subclinical vascular damage, such as abnormalities in white matter volume, brain atrophy, and previous infarctions. A similar but less strong association was seen between an increasing number of ideal cardiovascular health factors and performance on measures of episodic memory and executive function.
These seven CV factors also were associated with less decline over time in these three areas of cognitive function. In contrast, the CV factors showed no association with measures of semantic memory, the investigators said (J Am Heart Assoc. 2016 Mar 16).
The associations remained unchanged in sensitivity analyses that controlled for the presence and severity of depression.
“The results of our study add to a growing body of literature suggesting the effects of smoking and blood glucose levels on cognitive health in particular,” and support the role of vascular damage and metabolic processes in the etiology of cognitive aging and dementia, they added.
The closer that older adults come to meeting the American Heart Association’s “ideal” targets for seven factors that determine cardiovascular health, the lower their risk for cognitive decline, according to a report published online March 16 in Journal of the American Heart Association.
A secondary analysis of data from a prospective population-based cohort study of stroke risk demonstrated that better alignment with the AHA’s “Life’s Simple 7” cardiovascular health metrics correlated with less decline in mental processing speed, and, to a lesser extent, in executive function and episodic memory. “The results of this study suggest that achievement of the AHA’s ideal cardiovascular health metrics may have benefits for brain health, in addition to preventing strokes and myocardial infarctions ... underscoring the importance of public health initiatives aimed to better control these seven factors,” said Hannah Gardener, Sc.D., of the department of neurology, University of Miami, and her associates.
The AHA recently defined ideal target levels for seven modifiable cardiovascular (CV) risk factors: smoking status, body mass index, physical activity level, diet, blood pressure, total cholesterol level, and fasting glucose level. Meeting or closely approaching these ideals has already been linked to a decreased risk of stroke and MI. To examine a possible association with brain health, Dr. Gardener and her colleagues assessed these seven metrics in an ethnically diverse cohort of 722 participants aged 50 years and older in the Northern Manhattan Study who underwent serial comprehensive neuropsychological testing including brain MRI.
Of the total cohort, 3% had zero ideal factors, 15% had one factor, 33% had two factors, 30% had three factors, 14% had four factors, 14% had five factors, 1% had six factors, and none had all seven factors.
“An increasing number of ideal cardiovascular health factors was positively associated with processing speed,” and the association was particularly strong for three of the factors: ideal body mass index, lack of smoking, and ideal fasting glucose level. This association persisted when the data were adjusted to account for MRI markers of subclinical vascular damage, such as abnormalities in white matter volume, brain atrophy, and previous infarctions. A similar but less strong association was seen between an increasing number of ideal cardiovascular health factors and performance on measures of episodic memory and executive function.
These seven CV factors also were associated with less decline over time in these three areas of cognitive function. In contrast, the CV factors showed no association with measures of semantic memory, the investigators said (J Am Heart Assoc. 2016 Mar 16).
The associations remained unchanged in sensitivity analyses that controlled for the presence and severity of depression.
“The results of our study add to a growing body of literature suggesting the effects of smoking and blood glucose levels on cognitive health in particular,” and support the role of vascular damage and metabolic processes in the etiology of cognitive aging and dementia, they added.
The closer that older adults come to meeting the American Heart Association’s “ideal” targets for seven factors that determine cardiovascular health, the lower their risk for cognitive decline, according to a report published online March 16 in Journal of the American Heart Association.
A secondary analysis of data from a prospective population-based cohort study of stroke risk demonstrated that better alignment with the AHA’s “Life’s Simple 7” cardiovascular health metrics correlated with less decline in mental processing speed, and, to a lesser extent, in executive function and episodic memory. “The results of this study suggest that achievement of the AHA’s ideal cardiovascular health metrics may have benefits for brain health, in addition to preventing strokes and myocardial infarctions ... underscoring the importance of public health initiatives aimed to better control these seven factors,” said Hannah Gardener, Sc.D., of the department of neurology, University of Miami, and her associates.
The AHA recently defined ideal target levels for seven modifiable cardiovascular (CV) risk factors: smoking status, body mass index, physical activity level, diet, blood pressure, total cholesterol level, and fasting glucose level. Meeting or closely approaching these ideals has already been linked to a decreased risk of stroke and MI. To examine a possible association with brain health, Dr. Gardener and her colleagues assessed these seven metrics in an ethnically diverse cohort of 722 participants aged 50 years and older in the Northern Manhattan Study who underwent serial comprehensive neuropsychological testing including brain MRI.
Of the total cohort, 3% had zero ideal factors, 15% had one factor, 33% had two factors, 30% had three factors, 14% had four factors, 14% had five factors, 1% had six factors, and none had all seven factors.
“An increasing number of ideal cardiovascular health factors was positively associated with processing speed,” and the association was particularly strong for three of the factors: ideal body mass index, lack of smoking, and ideal fasting glucose level. This association persisted when the data were adjusted to account for MRI markers of subclinical vascular damage, such as abnormalities in white matter volume, brain atrophy, and previous infarctions. A similar but less strong association was seen between an increasing number of ideal cardiovascular health factors and performance on measures of episodic memory and executive function.
These seven CV factors also were associated with less decline over time in these three areas of cognitive function. In contrast, the CV factors showed no association with measures of semantic memory, the investigators said (J Am Heart Assoc. 2016 Mar 16).
The associations remained unchanged in sensitivity analyses that controlled for the presence and severity of depression.
“The results of our study add to a growing body of literature suggesting the effects of smoking and blood glucose levels on cognitive health in particular,” and support the role of vascular damage and metabolic processes in the etiology of cognitive aging and dementia, they added.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point: The closer adults come to meeting “ideal” American Heart Association targets for seven factors related to cardiovascular health, the lower their risk for cognitive decline.
Major finding: An increasing number of the seven ideal cardiovascular health factors was positively associated with mental processing speed.
Data source: A secondary analysis of data from the Northern Manhattan Study, a prospective population-based cohort study of stroke risk, involving 722 people aged 50 years and older at baseline in 1993-2001.
Disclosures: This study was funded by the Evelyn F. McKnight Brain Institute and the National Institutes of Health. Dr. Gardener and her associates reported having no relevant financial disclosures.
Receiving the Flu Vaccine While at the Hospital Does Not Increase Adverse Effects
NEW YORK (Reuters Health) - Receiving the seasonal flu vaccine while in the hospital does not increase surgical patients' health care utilization or their likelihood of being evaluated for infection after discharge, according to a new retrospective cohort study.
The Advisory Committee on Immunization Practices recommends that hospitalized patients who are eligible for the flu vaccine receive it before discharge, but rates of vaccination remain low in surgical patients, Dr. Sara Tartof of Kaiser Permanente Southern California in Pasadena and her colleagues note in their report, published online March 14 in the Annals of Internal Medicine.
This could be due to surgeons' concerns that adverse effects of influenza vaccine such as myalgia or fever could be attributed to surgical complications, or could complicate post-surgical care, they add.
"When we searched in the literature, we really just couldn't find any data that really speak to this question," Dr. Tartof told Reuters Health in a telephone interview.
She and her colleagues looked at Kaiser Permanente Southern California patients aged six months or older who had inpatient surgery between September 2010 and March 2013. Of the 42,777 surgeries in their analysis, 6,420 included seasonal flu vaccination during hospitalization.
The researchers found no differences between the vaccinated and unvaccinated groups in the risk of inpatient visits,emergency department visits, post-discharge fever, or clinical evaluation for infection. There was a marginal increase in the risk of outpatient visits (relative risk 1.05, p=0.032).
"We feel that the benefits of vaccination outweigh this risk," Dr. Tartof said. "For high-risk patients, this is a health care contact, this is an opportunity to vaccinate, and we don't want to miss those."
Many patients in the study who were vaccinated against the flu received the shot when they were discharged, the researcher noted. "This may be a more comfortable time for patients and for their clinicians to vaccinate," she said.
Dr. Tartof and her colleagues are now planning to repeat the study in a larger population of nonsurgical inpatients, including children.
The Centers for Disease Control and Prevention funded this research. Five coauthors reported disclosures.
NEW YORK (Reuters Health) - Receiving the seasonal flu vaccine while in the hospital does not increase surgical patients' health care utilization or their likelihood of being evaluated for infection after discharge, according to a new retrospective cohort study.
The Advisory Committee on Immunization Practices recommends that hospitalized patients who are eligible for the flu vaccine receive it before discharge, but rates of vaccination remain low in surgical patients, Dr. Sara Tartof of Kaiser Permanente Southern California in Pasadena and her colleagues note in their report, published online March 14 in the Annals of Internal Medicine.
This could be due to surgeons' concerns that adverse effects of influenza vaccine such as myalgia or fever could be attributed to surgical complications, or could complicate post-surgical care, they add.
"When we searched in the literature, we really just couldn't find any data that really speak to this question," Dr. Tartof told Reuters Health in a telephone interview.
She and her colleagues looked at Kaiser Permanente Southern California patients aged six months or older who had inpatient surgery between September 2010 and March 2013. Of the 42,777 surgeries in their analysis, 6,420 included seasonal flu vaccination during hospitalization.
The researchers found no differences between the vaccinated and unvaccinated groups in the risk of inpatient visits,emergency department visits, post-discharge fever, or clinical evaluation for infection. There was a marginal increase in the risk of outpatient visits (relative risk 1.05, p=0.032).
"We feel that the benefits of vaccination outweigh this risk," Dr. Tartof said. "For high-risk patients, this is a health care contact, this is an opportunity to vaccinate, and we don't want to miss those."
Many patients in the study who were vaccinated against the flu received the shot when they were discharged, the researcher noted. "This may be a more comfortable time for patients and for their clinicians to vaccinate," she said.
Dr. Tartof and her colleagues are now planning to repeat the study in a larger population of nonsurgical inpatients, including children.
The Centers for Disease Control and Prevention funded this research. Five coauthors reported disclosures.
NEW YORK (Reuters Health) - Receiving the seasonal flu vaccine while in the hospital does not increase surgical patients' health care utilization or their likelihood of being evaluated for infection after discharge, according to a new retrospective cohort study.
The Advisory Committee on Immunization Practices recommends that hospitalized patients who are eligible for the flu vaccine receive it before discharge, but rates of vaccination remain low in surgical patients, Dr. Sara Tartof of Kaiser Permanente Southern California in Pasadena and her colleagues note in their report, published online March 14 in the Annals of Internal Medicine.
This could be due to surgeons' concerns that adverse effects of influenza vaccine such as myalgia or fever could be attributed to surgical complications, or could complicate post-surgical care, they add.
"When we searched in the literature, we really just couldn't find any data that really speak to this question," Dr. Tartof told Reuters Health in a telephone interview.
She and her colleagues looked at Kaiser Permanente Southern California patients aged six months or older who had inpatient surgery between September 2010 and March 2013. Of the 42,777 surgeries in their analysis, 6,420 included seasonal flu vaccination during hospitalization.
The researchers found no differences between the vaccinated and unvaccinated groups in the risk of inpatient visits,emergency department visits, post-discharge fever, or clinical evaluation for infection. There was a marginal increase in the risk of outpatient visits (relative risk 1.05, p=0.032).
"We feel that the benefits of vaccination outweigh this risk," Dr. Tartof said. "For high-risk patients, this is a health care contact, this is an opportunity to vaccinate, and we don't want to miss those."
Many patients in the study who were vaccinated against the flu received the shot when they were discharged, the researcher noted. "This may be a more comfortable time for patients and for their clinicians to vaccinate," she said.
Dr. Tartof and her colleagues are now planning to repeat the study in a larger population of nonsurgical inpatients, including children.
The Centers for Disease Control and Prevention funded this research. Five coauthors reported disclosures.
Nicotinamide Prevents Actinic Keratoses, Basal Cell Carcinomas, and Squamous Cell Carcinomas

Chen et al (N Engl J Med. 2015;373:1618-1626) performed a multicenter, phase 3, double-blind, randomized, placebo-controlled trial. Results demonstrated that nicotinamide effectively decreased the rates of new nonmelanoma skin cancers (NMSCs) and actinic keratoses (AKs) in high-risk patients who had at least 2 histologically confirmed NMSCs in the last 5 years. In comparison to participants who received placebo, individuals who received nicotinamide 500 mg twice daily during the 12-month study (branded with a predictive acronym ONTRAC [oral nicotinamide to reduce actinic cancer]) had reduced rates of AKs of up to 20%, basal cell carcinomas of 20%, squamous cell carcinomas of 30%, and NMSCs of 23%. However, the effect of nicotinamide on NMSCs was not maintained at evaluation 6 months after discontinuation; the number of basal cell carcinomas was similar, and the number of squamous cell carcinomas was greater in participants who received nicotinamide in comparison to individuals who received placebo.
What’s the issue?
The risk for skin cancer is increased by UV radiation that damages DNA, suppresses cutaneous immunity, and inhibits DNA repair by depleting cellular adenosine triphosphate. Nicotinamide, an amide form of vitamin B3, has been demonstrated to not only reduce UV radiation–induced immunosuppression but also to prevent UV radiation–induced adenosine triphosphate depletion and glycolic blockade. Nicotinamide, which is classified as a food additive, also has neuroprotective and antioxidant functions and reduces pigmentation, wrinkles, and sebum production. Although oral nicotinamide has been demonstrated to reduce NMSCs and AKs, topical application has been shown to improve many skin conditions such as acne, atopic dermatitis, isoniazid-induced pellagra, and rosacea.
In contrast to nicotinic acid (niacin), nicotinamide is not associated with headaches, hypotension, flushing, itching, or vasodilatation. At high oral doses, side effects of nicotinamide that have been hypothesized or observed in animals, humans, or both have included the development of Parkinson disease, insulin sensitivity and diabetes mellitus, and liver toxicity. Although there are no reports in humans of growth retardation, teratogenicity, or oncogenicity, Rolfe (J Cosmet Dermatol. 2014;13:324-328) discussed that fetal blood levels of nicotinamide are greater than corresponding maternal blood levels because it is able to cross the placenta. However, according to Chen et al, no clinically significant between-group differences were found with respect to the number or types of adverse events that occurred in the placebo participants and the individuals who received 1000 mg daily of nicotinamide. Chen et al implied that there were additional benefits in the recipients of nicotinamide with regards to cognitive function and transepidermal water loss.
Perhaps all patients with a history of AKs, basal cell carcinomas, or squamous cell carcinomas should receive lifelong nicotinamide. Also, it might be reasonable to consider that all individuals older than 18 years who are not pregnant or breastfeeding with increased sun exposure but no history of AKs or NMSC add nicotinamide to their daily diets as a proactive measure for chemoprevention. Would you suggest nicotinamide to your patients?

Chen et al (N Engl J Med. 2015;373:1618-1626) performed a multicenter, phase 3, double-blind, randomized, placebo-controlled trial. Results demonstrated that nicotinamide effectively decreased the rates of new nonmelanoma skin cancers (NMSCs) and actinic keratoses (AKs) in high-risk patients who had at least 2 histologically confirmed NMSCs in the last 5 years. In comparison to participants who received placebo, individuals who received nicotinamide 500 mg twice daily during the 12-month study (branded with a predictive acronym ONTRAC [oral nicotinamide to reduce actinic cancer]) had reduced rates of AKs of up to 20%, basal cell carcinomas of 20%, squamous cell carcinomas of 30%, and NMSCs of 23%. However, the effect of nicotinamide on NMSCs was not maintained at evaluation 6 months after discontinuation; the number of basal cell carcinomas was similar, and the number of squamous cell carcinomas was greater in participants who received nicotinamide in comparison to individuals who received placebo.
What’s the issue?
The risk for skin cancer is increased by UV radiation that damages DNA, suppresses cutaneous immunity, and inhibits DNA repair by depleting cellular adenosine triphosphate. Nicotinamide, an amide form of vitamin B3, has been demonstrated to not only reduce UV radiation–induced immunosuppression but also to prevent UV radiation–induced adenosine triphosphate depletion and glycolic blockade. Nicotinamide, which is classified as a food additive, also has neuroprotective and antioxidant functions and reduces pigmentation, wrinkles, and sebum production. Although oral nicotinamide has been demonstrated to reduce NMSCs and AKs, topical application has been shown to improve many skin conditions such as acne, atopic dermatitis, isoniazid-induced pellagra, and rosacea.
In contrast to nicotinic acid (niacin), nicotinamide is not associated with headaches, hypotension, flushing, itching, or vasodilatation. At high oral doses, side effects of nicotinamide that have been hypothesized or observed in animals, humans, or both have included the development of Parkinson disease, insulin sensitivity and diabetes mellitus, and liver toxicity. Although there are no reports in humans of growth retardation, teratogenicity, or oncogenicity, Rolfe (J Cosmet Dermatol. 2014;13:324-328) discussed that fetal blood levels of nicotinamide are greater than corresponding maternal blood levels because it is able to cross the placenta. However, according to Chen et al, no clinically significant between-group differences were found with respect to the number or types of adverse events that occurred in the placebo participants and the individuals who received 1000 mg daily of nicotinamide. Chen et al implied that there were additional benefits in the recipients of nicotinamide with regards to cognitive function and transepidermal water loss.
Perhaps all patients with a history of AKs, basal cell carcinomas, or squamous cell carcinomas should receive lifelong nicotinamide. Also, it might be reasonable to consider that all individuals older than 18 years who are not pregnant or breastfeeding with increased sun exposure but no history of AKs or NMSC add nicotinamide to their daily diets as a proactive measure for chemoprevention. Would you suggest nicotinamide to your patients?

Chen et al (N Engl J Med. 2015;373:1618-1626) performed a multicenter, phase 3, double-blind, randomized, placebo-controlled trial. Results demonstrated that nicotinamide effectively decreased the rates of new nonmelanoma skin cancers (NMSCs) and actinic keratoses (AKs) in high-risk patients who had at least 2 histologically confirmed NMSCs in the last 5 years. In comparison to participants who received placebo, individuals who received nicotinamide 500 mg twice daily during the 12-month study (branded with a predictive acronym ONTRAC [oral nicotinamide to reduce actinic cancer]) had reduced rates of AKs of up to 20%, basal cell carcinomas of 20%, squamous cell carcinomas of 30%, and NMSCs of 23%. However, the effect of nicotinamide on NMSCs was not maintained at evaluation 6 months after discontinuation; the number of basal cell carcinomas was similar, and the number of squamous cell carcinomas was greater in participants who received nicotinamide in comparison to individuals who received placebo.
What’s the issue?
The risk for skin cancer is increased by UV radiation that damages DNA, suppresses cutaneous immunity, and inhibits DNA repair by depleting cellular adenosine triphosphate. Nicotinamide, an amide form of vitamin B3, has been demonstrated to not only reduce UV radiation–induced immunosuppression but also to prevent UV radiation–induced adenosine triphosphate depletion and glycolic blockade. Nicotinamide, which is classified as a food additive, also has neuroprotective and antioxidant functions and reduces pigmentation, wrinkles, and sebum production. Although oral nicotinamide has been demonstrated to reduce NMSCs and AKs, topical application has been shown to improve many skin conditions such as acne, atopic dermatitis, isoniazid-induced pellagra, and rosacea.
In contrast to nicotinic acid (niacin), nicotinamide is not associated with headaches, hypotension, flushing, itching, or vasodilatation. At high oral doses, side effects of nicotinamide that have been hypothesized or observed in animals, humans, or both have included the development of Parkinson disease, insulin sensitivity and diabetes mellitus, and liver toxicity. Although there are no reports in humans of growth retardation, teratogenicity, or oncogenicity, Rolfe (J Cosmet Dermatol. 2014;13:324-328) discussed that fetal blood levels of nicotinamide are greater than corresponding maternal blood levels because it is able to cross the placenta. However, according to Chen et al, no clinically significant between-group differences were found with respect to the number or types of adverse events that occurred in the placebo participants and the individuals who received 1000 mg daily of nicotinamide. Chen et al implied that there were additional benefits in the recipients of nicotinamide with regards to cognitive function and transepidermal water loss.
Perhaps all patients with a history of AKs, basal cell carcinomas, or squamous cell carcinomas should receive lifelong nicotinamide. Also, it might be reasonable to consider that all individuals older than 18 years who are not pregnant or breastfeeding with increased sun exposure but no history of AKs or NMSC add nicotinamide to their daily diets as a proactive measure for chemoprevention. Would you suggest nicotinamide to your patients?
RPS15 mutations prevalent in aggressive chronic lymphocytic leukemia
Mutations in the RPS15 gene occurred in 8 of 41 patients with relapsing chronic lymphocytic leukemia (CLL), and the mutations were present before treatment in 7 of the 8, a possible indication that the aberrations are early genetic events in aggressive CLL pathobiology.
RPS15 mutations may lead to defective p53 stability and increased degradation, representing a potential novel mechanism in CLL pathobiology. The findings suggest “RPS15-mutant cases should be treated with alternative regimens that act independently of the p53 pathway,” wrote Dr. Viktor Ljungström of the department of immunology, genetics, and pathology, Uppsala (Sweden) University, and colleagues (Blood 2016 Feb 25. doi: 10.1182/blood-2015-10-674572).
In their study, the researchers performed whole exome sequencing of 110 samples collected before and after treatment from 41 patients with aggressive CLL that relapsed after a median of 2 years; 7 patients had mutations in RPS15 before treatment, and 8 had RPS15 mutations after treatment. The findings suggest that standard therapy with fludarabine, cyclophosphamide, and rituximab was not intrinsically mutagenic.
High frequencies of mutations were linked to poor outcome in both pretreated and relapse samples. These mutations included NOTCH1, TP53, ATM, SF3B1, MGA, and BIRC3. At least one mutation was seen before treatment in 26 of the 41 patients, and that rate rose to 33 of 41 patients at relapse. Two or more mutations were noted before treatment in 12 of 41 patients, and that rose to 15 of 41 at relapse.
In response to their findings, the researchers next performed targeted resequencing of the RPS15 hot spot (exon 4) in an extended series of 790 patients with CLL, intentionally enriched with 605 cases with adverse prognostic profiles. They found an additional 36 mutations in RPS15 (36/605, 6%). In contrast, none of the 185 patients with more favorable prognostic, IGHV-mutated CLL carried RPS15 mutations. RPS15-mutant patients without concomitant TP53 aberrations had an overall survival similar to other aggressive CLL subgroups, but none of the patients with both mutations survived at 10 years, compared with 59% of patients with wild-type RPS15 and wild-type TP53, “pointing to a dismal prognosis for RPS15-mutated CLL,” they wrote.
They also analyzed 30 cases with Richter syndrome (CLL transformed into diffuse large B-cell lymphoma), and only a single case was found to carry an RPS15 mutation, and the mutation was also observed in the preceding CLL phase. This finding indicates that RPS15 mutation probably does not underlie the transformation of CLL to Richter syndrome, according to the researchers.
Dr. Ljungström and coauthors reported having no relevant financial disclosures.
In support of the authors’ hypothesis that RPS15 mutations may be an early-acquired driver in high-risk disease, the variant allele frequency in eight serially analyzed cases remained static, with only one case gaining a mutation in RPS15, whereas the variable allele frequency increased at relapse for other well-characterized mutations in ATM, BIRC3, NFKBIE, and TP53.
Pilot experiments demonstrated specific interactions between TP53 and RPS15, and p53 stability was reduced in the presence of mutant RPS15.
The findings should prompt further investigation to determine if the consequences of RPS15 mutations depend on its interaction with TP53, or if the mutations found in other ribosomal proteins indicate a different mechanism related to the 40S subunit.
Given that RPS15 is not included in common academic or commercial sequencing panels, the presence of RPS15 mutations in other diseases may be underestimated as well.
More generally, are there other cancers with subgroups enriched for other benign-appearing genes?
Dr. James Blachly is with Wexner Medical Center, the Ohio State University, Columbus. These remarks were part of an editorial accompanying a report in Blood (2016 Feb 25. doi: 10.1182/blood-2015-10-674572).
In support of the authors’ hypothesis that RPS15 mutations may be an early-acquired driver in high-risk disease, the variant allele frequency in eight serially analyzed cases remained static, with only one case gaining a mutation in RPS15, whereas the variable allele frequency increased at relapse for other well-characterized mutations in ATM, BIRC3, NFKBIE, and TP53.
Pilot experiments demonstrated specific interactions between TP53 and RPS15, and p53 stability was reduced in the presence of mutant RPS15.
The findings should prompt further investigation to determine if the consequences of RPS15 mutations depend on its interaction with TP53, or if the mutations found in other ribosomal proteins indicate a different mechanism related to the 40S subunit.
Given that RPS15 is not included in common academic or commercial sequencing panels, the presence of RPS15 mutations in other diseases may be underestimated as well.
More generally, are there other cancers with subgroups enriched for other benign-appearing genes?
Dr. James Blachly is with Wexner Medical Center, the Ohio State University, Columbus. These remarks were part of an editorial accompanying a report in Blood (2016 Feb 25. doi: 10.1182/blood-2015-10-674572).
In support of the authors’ hypothesis that RPS15 mutations may be an early-acquired driver in high-risk disease, the variant allele frequency in eight serially analyzed cases remained static, with only one case gaining a mutation in RPS15, whereas the variable allele frequency increased at relapse for other well-characterized mutations in ATM, BIRC3, NFKBIE, and TP53.
Pilot experiments demonstrated specific interactions between TP53 and RPS15, and p53 stability was reduced in the presence of mutant RPS15.
The findings should prompt further investigation to determine if the consequences of RPS15 mutations depend on its interaction with TP53, or if the mutations found in other ribosomal proteins indicate a different mechanism related to the 40S subunit.
Given that RPS15 is not included in common academic or commercial sequencing panels, the presence of RPS15 mutations in other diseases may be underestimated as well.
More generally, are there other cancers with subgroups enriched for other benign-appearing genes?
Dr. James Blachly is with Wexner Medical Center, the Ohio State University, Columbus. These remarks were part of an editorial accompanying a report in Blood (2016 Feb 25. doi: 10.1182/blood-2015-10-674572).
Mutations in the RPS15 gene occurred in 8 of 41 patients with relapsing chronic lymphocytic leukemia (CLL), and the mutations were present before treatment in 7 of the 8, a possible indication that the aberrations are early genetic events in aggressive CLL pathobiology.
RPS15 mutations may lead to defective p53 stability and increased degradation, representing a potential novel mechanism in CLL pathobiology. The findings suggest “RPS15-mutant cases should be treated with alternative regimens that act independently of the p53 pathway,” wrote Dr. Viktor Ljungström of the department of immunology, genetics, and pathology, Uppsala (Sweden) University, and colleagues (Blood 2016 Feb 25. doi: 10.1182/blood-2015-10-674572).
In their study, the researchers performed whole exome sequencing of 110 samples collected before and after treatment from 41 patients with aggressive CLL that relapsed after a median of 2 years; 7 patients had mutations in RPS15 before treatment, and 8 had RPS15 mutations after treatment. The findings suggest that standard therapy with fludarabine, cyclophosphamide, and rituximab was not intrinsically mutagenic.
High frequencies of mutations were linked to poor outcome in both pretreated and relapse samples. These mutations included NOTCH1, TP53, ATM, SF3B1, MGA, and BIRC3. At least one mutation was seen before treatment in 26 of the 41 patients, and that rate rose to 33 of 41 patients at relapse. Two or more mutations were noted before treatment in 12 of 41 patients, and that rose to 15 of 41 at relapse.
In response to their findings, the researchers next performed targeted resequencing of the RPS15 hot spot (exon 4) in an extended series of 790 patients with CLL, intentionally enriched with 605 cases with adverse prognostic profiles. They found an additional 36 mutations in RPS15 (36/605, 6%). In contrast, none of the 185 patients with more favorable prognostic, IGHV-mutated CLL carried RPS15 mutations. RPS15-mutant patients without concomitant TP53 aberrations had an overall survival similar to other aggressive CLL subgroups, but none of the patients with both mutations survived at 10 years, compared with 59% of patients with wild-type RPS15 and wild-type TP53, “pointing to a dismal prognosis for RPS15-mutated CLL,” they wrote.
They also analyzed 30 cases with Richter syndrome (CLL transformed into diffuse large B-cell lymphoma), and only a single case was found to carry an RPS15 mutation, and the mutation was also observed in the preceding CLL phase. This finding indicates that RPS15 mutation probably does not underlie the transformation of CLL to Richter syndrome, according to the researchers.
Dr. Ljungström and coauthors reported having no relevant financial disclosures.
Mutations in the RPS15 gene occurred in 8 of 41 patients with relapsing chronic lymphocytic leukemia (CLL), and the mutations were present before treatment in 7 of the 8, a possible indication that the aberrations are early genetic events in aggressive CLL pathobiology.
RPS15 mutations may lead to defective p53 stability and increased degradation, representing a potential novel mechanism in CLL pathobiology. The findings suggest “RPS15-mutant cases should be treated with alternative regimens that act independently of the p53 pathway,” wrote Dr. Viktor Ljungström of the department of immunology, genetics, and pathology, Uppsala (Sweden) University, and colleagues (Blood 2016 Feb 25. doi: 10.1182/blood-2015-10-674572).
In their study, the researchers performed whole exome sequencing of 110 samples collected before and after treatment from 41 patients with aggressive CLL that relapsed after a median of 2 years; 7 patients had mutations in RPS15 before treatment, and 8 had RPS15 mutations after treatment. The findings suggest that standard therapy with fludarabine, cyclophosphamide, and rituximab was not intrinsically mutagenic.
High frequencies of mutations were linked to poor outcome in both pretreated and relapse samples. These mutations included NOTCH1, TP53, ATM, SF3B1, MGA, and BIRC3. At least one mutation was seen before treatment in 26 of the 41 patients, and that rate rose to 33 of 41 patients at relapse. Two or more mutations were noted before treatment in 12 of 41 patients, and that rose to 15 of 41 at relapse.
In response to their findings, the researchers next performed targeted resequencing of the RPS15 hot spot (exon 4) in an extended series of 790 patients with CLL, intentionally enriched with 605 cases with adverse prognostic profiles. They found an additional 36 mutations in RPS15 (36/605, 6%). In contrast, none of the 185 patients with more favorable prognostic, IGHV-mutated CLL carried RPS15 mutations. RPS15-mutant patients without concomitant TP53 aberrations had an overall survival similar to other aggressive CLL subgroups, but none of the patients with both mutations survived at 10 years, compared with 59% of patients with wild-type RPS15 and wild-type TP53, “pointing to a dismal prognosis for RPS15-mutated CLL,” they wrote.
They also analyzed 30 cases with Richter syndrome (CLL transformed into diffuse large B-cell lymphoma), and only a single case was found to carry an RPS15 mutation, and the mutation was also observed in the preceding CLL phase. This finding indicates that RPS15 mutation probably does not underlie the transformation of CLL to Richter syndrome, according to the researchers.
Dr. Ljungström and coauthors reported having no relevant financial disclosures.
FROM BLOOD
Key clinical point: Aberrations in the RPS15 gene before therapy may be an indicator of aggressive pathobiology in chronic lymphocytic leukemia.
Major finding: Mutations in the RPS15 gene occurred in 8 of 41 patients with relapsing CLL, and the mutations were present before treatment in 7 of the 8.
Data sources: Whole exome sequencing of 110 samples collected before and after fludarabine, cyclophosphamide, and rituximab therapy from 41 patients with relapsed CLL.
Disclosures: Dr. Ljungström and coauthors reported having no relevant financial disclosures.
Experts Suggest Ways to Deal with Challenges Surrounding Care of Psychiatric Patients
In 1955, there was one psychiatric bed for every 300 Americans. By 2005, following the widespread shuttering or downsizing of psychiatric hospitals in the 1990s, that number had shrunk to one bed for every 3,000 Americans.1
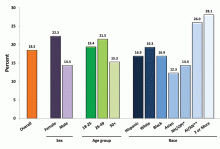
In 2008, an estimated 39.8 million Americans age 18 or older had mental illness, which represents 17.7% of U.S. adults.2 In 2013, this number rose to an estimated 43.8 million, or 18.5% of U.S. adults (see Figure 1).3
“It’s like we have returned to the early 19th century, when mentally ill persons were held in prisons or temporarily kept in hospital settings,” says Ricardo Bianco, PsyD, program director of the Master of Arts in counseling and health psychology at William James College in Newton, Mass. “The problem is that the healthcare system did not catch up to absorb the mentally ill population.
“As a result, hospital staffs are inadequately trained, there is insufficient funding for these patients, and there are not enough human resource personnel to manage them. Consequently, hospitalists are overwhelmed with cases that should be primarily treated by psychologists, psychiatrists, and social workers.”
According to David M. Grace, MD, SFHM, hospitalist and senior medical officer at the Schumacher Group in Lafayette, La., two groups of psychiatric patients present to the acute-care hospital environment: those who are there for a primary psychiatric problem and those who have a medical problem and a psychiatric comorbidity. The first group of patients presents distinct challenges. U.S. hospitals lack two-thirds of the minimum number of beds needed to care for this population. The second group is problematic because psychiatric issues often cloud the medical issues of a patient, increasing both diagnostic uncertainty and resource utilization.
Challenges Abound
Psychiatric patients present a number of problems for hospitalists. First, it is difficult to decipher what comprises a psychiatric issue and what does not because “many psychiatric conditions manifest as physical symptoms and they often require significant resource consumption to diagnose,” Dr. Grace says. Secondly, some patients present with a severe primary psychiatric problem in which they are homicidal, suicidal, or gravely disabled.
In addition, psychiatric patients tend to have a greater incidence of noncompliance with imaging, laboratory work, medication, and general medical care, says Daniel Sussman, MD, a hospitalist at IPC Healthcare, Inc., based in North Hollywood, Calif. He also serves as interim chairman in the department of psychiatry at St. John’s Episcopal Hospital in Far Rockaway, N.Y.
Clinically, potential interactions between psychiatric medications and medically related prescription drugs are always a concern, notes Dr. Sussman, who says more than 70% of patients admitted to St. John’s Episcopal Hospital have a major psychiatric illness in addition to their medical problem. Psychiatric medications, which patients may have tolerated well when they were stable, may be too sedating when patients are ill. Side effects and adverse reactions of psychotropic medications must also be considered when diagnosing and treating medical illnesses. Metabolic syndrome is more commonly seen and is a factor in the development and subsequent treatment of other illnesses.
Another challenge stems from the fact that patients with substantial psychiatric comorbidities can have significant and rapid mood and behavioral changes as well as sudden, volatile, and aggressive outbursts—both verbal and physical.
“Staff members who interact with the patient are at risk if an outburst occurs,” says Emily Fingado, MD, FAAP, a pediatric hospitalist and clinical assistant professor of pediatrics at Nemours/Alfred I. duPont Hospital for Children (Nemours/AIDHC) of the Sidney Kimmel Medical College at Thomas Jefferson University in Wilmington, Del.
Such situations can become scary, particularly if someone with psychiatric expertise is not available to intervene. This can be very frustrating for hospitalists who want to provide high-level care but may lack the training needed to be successful with such patients. This can ultimately lead to burnout, says Sarah Rivelli, MD, medical director of psychiatry clinical services at Duke University Hospital and Duke University Medical Center in Durham, N.C.
Another challenge is that although there are protocols in place designed to follow specific steps for patients with physical illnesses or disorders, that’s not the case for psychiatric illness. “Many hospital facilities are not designed, or have yet to implement, protocols to attend to mentally ill individuals,” Dr. Bianco says.
Because of the unpredictability of patients and lack of practice protocols, mental illness can introduce a wild card into the standard treatment process. A more individualized approach with these patients is needed, but with increasing focus on length of stay and operational efficiency, medical and nursing staffs are pressured to do things quickly and to do more with less. It can be very time-consuming for a nurse to have to explain to paranoid patients why they should take their medication or for a phlebotomist to try to calm patients in order to obtain blood. When patients refuse needed tests, or only provide a limited history, the hospitalist ends up working with incomplete information, which makes choosing and monitoring the treatment approach problematic.
A Look at Best Practices
In light of challenges, some best practices have been identified for handling psychiatric patients. As previously mentioned, most hospital staffs have limited formal training in interacting with psychiatric patients. In fact, the American Board of Internal Medicine only devotes 4% of the certification exam to psychiatry.4
Ideally, staff members who care for psychiatric patients will have specialized or additional training in managing patients with psychiatric conditions or comorbidities. Nemours/AIDHC has a committee assigned to evaluate psychiatric patients’ care and help manage them when a behavioral emergency occurs, Dr. Fingado says. This team, which has been trained on de-escalation, restraint techniques and policies, as well as medications to use in these situations, intervenes when patients have an aggressive event that places patients, visitors, or staff at risk. The team includes nursing staff, the hospitalist on-call, and security personnel and involves the social work, psychology, and psychiatry departments.
Training focused on treating patients with psychiatric conditions should include how to recognize substance abuse and treat substance withdrawal because mental illness and substance abuse often track together, Dr. Sussman notes. At St. John’s Episcopal Hospital, patients with chronic mental illness are not the ones who typically become aggressive or violent. Rather, this is more often the case among patients with substance abuse either in states of acute intoxication or withdrawal.
Recently, Dr. Sussman has seen a significant increase in patients who abuse K2, or spice, a synthetic form of cannabis. Side effects of using K2 include rapid heart rate, anxiety, hallucinations, and paranoia to the point of delusional thinking. These side effects can frequently wax and wane for days after the drug is used, and they can be associated with significant psychomotor agitation and assaultive behavior.
When patients abuse flake, another synthetic drug that has been reported in the Southeast, they tend to become very paranoid and violent. “These patients can be extremely unpredictable and aggressive,” he says. “Patients with dementia can be impulsive and aggressive during care, and caution is needed, but it’s not a directed violence like that seen in patients who are agitated secondary to substance abuse.”
Dr. Bianco advises having a predetermined triage system or a scale that can assess and measure patients’ level of psychological distress, which can ensure timely and appropriate evaluation and treatment of psychiatric patients, as well as toxicology screens and mental health protocols, which can aid in diagnosis.
“Technology is an important tool in facilitating integration, including identifying and screening patients, tracking patient progress, encouraging adherence to clinical protocols, facilitating communication between providers, and evaluating the impact of integrated programs,” Dr. Bianco says. Academic hospitals struggle less with this problem, he adds, because they tend to be more adequately funded in all areas of operations, including the field of mental health.
IPC Healthcare hospitalist James N. Horst, DO, a psychiatrist who manages mental health patients in a long-term care and nursing home facility, says he has found standardized general screening tools to be useful. The Hamilton Depression Scale, Beck Anxiety Inventory, and CAGE exam for chemical dependency can be easily administered and scored in any facility setting, he says. These tests include self-administered questions to which the patient answers yes or no. Laboratory work is a secondary tool in psychiatry since few mental illness disorders are based on medical comorbidities.
Dr. Sussman looks to the past, when psychiatrists were part of medical teams rounding in hospitals, for a solution. “An integrated model provides an approach where patient care is less compartmentalized,” he says. “In this model, clinicians are responsible for making sure their patient is treated, not simply focusing on their individual area of expertise. This involves working more closely with an integrated care management team.”
Ideally, this will occur at every level of care: outpatient, inpatient, and emergency department (ED). New York State is attempting to redesign the Medicaid system in this fashion, with the goal of improving overall care and reducing reliance on inpatient treatment to provide that care. This is an enormous initiative, costing more than $8 billion. If successful, it will result in a more patient-centered care system that treats the whole individual, not just the illness, and will positively impact patients’ overall health.
For now, St. John’s Episcopal Hospital has an active psychiatric consultation liaison service that is staffed by both in-house residents and attending physicians who are there 24-7 to help with psychiatric patients.
A ‘Utopia Management’ Perspective
In a dream world, patients with significant psychiatric problems or comorbidities would have coordinated, multidisciplinary care from admission to discharge, Dr. Fingado adds. Ideally, hospitals would have dedicated rooms or areas in the ED that are safe for patients and staff. Psychiatric patients who require observation or admission to a non-psychiatric hospital would be placed in rooms or units dedicated for psychiatric patients, again providing safety for patients and staff, Dr. Fingado surmises.
In addition, all staff members would have training in behavioral health management, including instruction on de-escalation, restraint techniques, and medication use for patients. Ideally, units would be staffed by specially trained aides, nurses, and healthcare providers (i.e., physician assistants, nurse practitioners, physicians), as well as psychologists and psychiatrists, Dr. Fingado says. This type of management would require buy-in from a multitude of groups, including healthcare administrators, nursing and provider staff, as well as health insurance companies. A reallocation or increase in funds would be needed to help build and staff these types of management models and locations, she adds.
In a perfect world, all hospitalized patients would be adequately screened for mental health issues and have their issues appropriately addressed by well-qualified professionals in real time, Dr. Grace says. Telemedicine services have great potential in helping to meet that goal, he says, and more relaxed regulatory guidelines around telemedicine could help make such physician-patient interactions less difficult. Many, if not most, hospitals currently have limited or no access to qualified mental health professionals, a conundrum based on supply, reimbursement, and need.
“Telemedicine, which is already having great success in neurology and intensive care unit medicine, is a great fit for this space,” Dr. Grace says. “Widespread access to a tele-psychiatrist would bring significant tangible benefits to patients, hospitals, hospital staff, and the population at large, who ultimately pay for healthcare in the nation.”
Dr. Horst says he believes everyone who treats psychiatric patients should have education in psychiatric medicine education. One way to achieve this would be to mandate continuing medical education coursework in mental health disorders.
The Reality of a Utopia
Traditionally, our healthcare system has been designed to react to illness, meaning that physicians treat illnesses when individuals become sick.
“But as science and technology now better understand the etiology of most illnesses, we are more equipped to design more preventative interventions rather than wait for individuals to become sick,” Dr. Bianco says. “Prevention interventions require an initial investment that the healthcare system is not necessarily willing to invest in at this time and a shift in the way it charges for services. If the healthcare system is unwilling to go that route, and we know we can prevent many illnesses by shifting the focus of treatment, consequently, human suffering is augmented and quality of life jeopardized.”
More recently, the general population and providers have acknowledged that healing takes place more effectively when it is applied in more integrated approaches (i.e., the utilization of the bio-psycho-social-spiritual model), Dr. Bianco adds. This greater appreciation is demonstrated by different research studies applied to different populations (both the general public and different providers). Despite this, the system (i.e., training) does not support a full integration of interventions.
“The system continues to operate under the traditional medical model that is fragmented and hyper-specialized,” Dr. Bianco says. “Science has demonstrated that the mind and the body work in more complex ways, requiring a more holistic approach to treatment. Although all segments among providers now understand and accept that, the system they dwell in does not support the daily challenges of treatment.
“Treatment continues to be fragmented as it is the medical model. At this point, at a minimum, a hospital should have a psychiatric department composed of individuals who are adequately trained (e.g., health psychology, behavioral medicine) to absorb a portion of individuals who primarily present with mental health issues.” TH
Karen Appold is a freelance medical writer in Pennsylvania.
References
- Torrey EF, Kennard AD, Eslinger D, Lamb R, Pavle J. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. Treatment Advocacy Center website. Available at: http://www.treatmentadvocacycenter.org/storage/documents/final_jails_v_hospitals_study.pdf. May 2010. Accessed August 18, 2015.
- Results from the 2013 national survey on drug use and health: mental health detailed tables. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services website. Available at: http://www.samhsa.gov/data/sites/default/files/2013MHDetTabs/NSDUH-MHDetTabs2013.pdf. Accessed August 19, 2015.
- Any mental illness among adults. National Institute of Mental Health website. Available at: http://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-adults.shtml. Accessed August 19, 2015.
- Internal medicine certification examination blueprint. American Board of Internal Medicine website. Available at: https://www.abim.org/pdf/blueprint/im_cert.pdf. January 2015. Accessed August 19, 2015.
In 1955, there was one psychiatric bed for every 300 Americans. By 2005, following the widespread shuttering or downsizing of psychiatric hospitals in the 1990s, that number had shrunk to one bed for every 3,000 Americans.1
In 2008, an estimated 39.8 million Americans age 18 or older had mental illness, which represents 17.7% of U.S. adults.2 In 2013, this number rose to an estimated 43.8 million, or 18.5% of U.S. adults (see Figure 1).3
“It’s like we have returned to the early 19th century, when mentally ill persons were held in prisons or temporarily kept in hospital settings,” says Ricardo Bianco, PsyD, program director of the Master of Arts in counseling and health psychology at William James College in Newton, Mass. “The problem is that the healthcare system did not catch up to absorb the mentally ill population.
“As a result, hospital staffs are inadequately trained, there is insufficient funding for these patients, and there are not enough human resource personnel to manage them. Consequently, hospitalists are overwhelmed with cases that should be primarily treated by psychologists, psychiatrists, and social workers.”
According to David M. Grace, MD, SFHM, hospitalist and senior medical officer at the Schumacher Group in Lafayette, La., two groups of psychiatric patients present to the acute-care hospital environment: those who are there for a primary psychiatric problem and those who have a medical problem and a psychiatric comorbidity. The first group of patients presents distinct challenges. U.S. hospitals lack two-thirds of the minimum number of beds needed to care for this population. The second group is problematic because psychiatric issues often cloud the medical issues of a patient, increasing both diagnostic uncertainty and resource utilization.
Challenges Abound
Psychiatric patients present a number of problems for hospitalists. First, it is difficult to decipher what comprises a psychiatric issue and what does not because “many psychiatric conditions manifest as physical symptoms and they often require significant resource consumption to diagnose,” Dr. Grace says. Secondly, some patients present with a severe primary psychiatric problem in which they are homicidal, suicidal, or gravely disabled.
In addition, psychiatric patients tend to have a greater incidence of noncompliance with imaging, laboratory work, medication, and general medical care, says Daniel Sussman, MD, a hospitalist at IPC Healthcare, Inc., based in North Hollywood, Calif. He also serves as interim chairman in the department of psychiatry at St. John’s Episcopal Hospital in Far Rockaway, N.Y.
Clinically, potential interactions between psychiatric medications and medically related prescription drugs are always a concern, notes Dr. Sussman, who says more than 70% of patients admitted to St. John’s Episcopal Hospital have a major psychiatric illness in addition to their medical problem. Psychiatric medications, which patients may have tolerated well when they were stable, may be too sedating when patients are ill. Side effects and adverse reactions of psychotropic medications must also be considered when diagnosing and treating medical illnesses. Metabolic syndrome is more commonly seen and is a factor in the development and subsequent treatment of other illnesses.
Another challenge stems from the fact that patients with substantial psychiatric comorbidities can have significant and rapid mood and behavioral changes as well as sudden, volatile, and aggressive outbursts—both verbal and physical.
“Staff members who interact with the patient are at risk if an outburst occurs,” says Emily Fingado, MD, FAAP, a pediatric hospitalist and clinical assistant professor of pediatrics at Nemours/Alfred I. duPont Hospital for Children (Nemours/AIDHC) of the Sidney Kimmel Medical College at Thomas Jefferson University in Wilmington, Del.
Such situations can become scary, particularly if someone with psychiatric expertise is not available to intervene. This can be very frustrating for hospitalists who want to provide high-level care but may lack the training needed to be successful with such patients. This can ultimately lead to burnout, says Sarah Rivelli, MD, medical director of psychiatry clinical services at Duke University Hospital and Duke University Medical Center in Durham, N.C.
Another challenge is that although there are protocols in place designed to follow specific steps for patients with physical illnesses or disorders, that’s not the case for psychiatric illness. “Many hospital facilities are not designed, or have yet to implement, protocols to attend to mentally ill individuals,” Dr. Bianco says.
Because of the unpredictability of patients and lack of practice protocols, mental illness can introduce a wild card into the standard treatment process. A more individualized approach with these patients is needed, but with increasing focus on length of stay and operational efficiency, medical and nursing staffs are pressured to do things quickly and to do more with less. It can be very time-consuming for a nurse to have to explain to paranoid patients why they should take their medication or for a phlebotomist to try to calm patients in order to obtain blood. When patients refuse needed tests, or only provide a limited history, the hospitalist ends up working with incomplete information, which makes choosing and monitoring the treatment approach problematic.
A Look at Best Practices
In light of challenges, some best practices have been identified for handling psychiatric patients. As previously mentioned, most hospital staffs have limited formal training in interacting with psychiatric patients. In fact, the American Board of Internal Medicine only devotes 4% of the certification exam to psychiatry.4
Ideally, staff members who care for psychiatric patients will have specialized or additional training in managing patients with psychiatric conditions or comorbidities. Nemours/AIDHC has a committee assigned to evaluate psychiatric patients’ care and help manage them when a behavioral emergency occurs, Dr. Fingado says. This team, which has been trained on de-escalation, restraint techniques and policies, as well as medications to use in these situations, intervenes when patients have an aggressive event that places patients, visitors, or staff at risk. The team includes nursing staff, the hospitalist on-call, and security personnel and involves the social work, psychology, and psychiatry departments.
Training focused on treating patients with psychiatric conditions should include how to recognize substance abuse and treat substance withdrawal because mental illness and substance abuse often track together, Dr. Sussman notes. At St. John’s Episcopal Hospital, patients with chronic mental illness are not the ones who typically become aggressive or violent. Rather, this is more often the case among patients with substance abuse either in states of acute intoxication or withdrawal.
Recently, Dr. Sussman has seen a significant increase in patients who abuse K2, or spice, a synthetic form of cannabis. Side effects of using K2 include rapid heart rate, anxiety, hallucinations, and paranoia to the point of delusional thinking. These side effects can frequently wax and wane for days after the drug is used, and they can be associated with significant psychomotor agitation and assaultive behavior.
When patients abuse flake, another synthetic drug that has been reported in the Southeast, they tend to become very paranoid and violent. “These patients can be extremely unpredictable and aggressive,” he says. “Patients with dementia can be impulsive and aggressive during care, and caution is needed, but it’s not a directed violence like that seen in patients who are agitated secondary to substance abuse.”
Dr. Bianco advises having a predetermined triage system or a scale that can assess and measure patients’ level of psychological distress, which can ensure timely and appropriate evaluation and treatment of psychiatric patients, as well as toxicology screens and mental health protocols, which can aid in diagnosis.
“Technology is an important tool in facilitating integration, including identifying and screening patients, tracking patient progress, encouraging adherence to clinical protocols, facilitating communication between providers, and evaluating the impact of integrated programs,” Dr. Bianco says. Academic hospitals struggle less with this problem, he adds, because they tend to be more adequately funded in all areas of operations, including the field of mental health.
IPC Healthcare hospitalist James N. Horst, DO, a psychiatrist who manages mental health patients in a long-term care and nursing home facility, says he has found standardized general screening tools to be useful. The Hamilton Depression Scale, Beck Anxiety Inventory, and CAGE exam for chemical dependency can be easily administered and scored in any facility setting, he says. These tests include self-administered questions to which the patient answers yes or no. Laboratory work is a secondary tool in psychiatry since few mental illness disorders are based on medical comorbidities.
Dr. Sussman looks to the past, when psychiatrists were part of medical teams rounding in hospitals, for a solution. “An integrated model provides an approach where patient care is less compartmentalized,” he says. “In this model, clinicians are responsible for making sure their patient is treated, not simply focusing on their individual area of expertise. This involves working more closely with an integrated care management team.”
Ideally, this will occur at every level of care: outpatient, inpatient, and emergency department (ED). New York State is attempting to redesign the Medicaid system in this fashion, with the goal of improving overall care and reducing reliance on inpatient treatment to provide that care. This is an enormous initiative, costing more than $8 billion. If successful, it will result in a more patient-centered care system that treats the whole individual, not just the illness, and will positively impact patients’ overall health.
For now, St. John’s Episcopal Hospital has an active psychiatric consultation liaison service that is staffed by both in-house residents and attending physicians who are there 24-7 to help with psychiatric patients.
A ‘Utopia Management’ Perspective
In a dream world, patients with significant psychiatric problems or comorbidities would have coordinated, multidisciplinary care from admission to discharge, Dr. Fingado adds. Ideally, hospitals would have dedicated rooms or areas in the ED that are safe for patients and staff. Psychiatric patients who require observation or admission to a non-psychiatric hospital would be placed in rooms or units dedicated for psychiatric patients, again providing safety for patients and staff, Dr. Fingado surmises.
In addition, all staff members would have training in behavioral health management, including instruction on de-escalation, restraint techniques, and medication use for patients. Ideally, units would be staffed by specially trained aides, nurses, and healthcare providers (i.e., physician assistants, nurse practitioners, physicians), as well as psychologists and psychiatrists, Dr. Fingado says. This type of management would require buy-in from a multitude of groups, including healthcare administrators, nursing and provider staff, as well as health insurance companies. A reallocation or increase in funds would be needed to help build and staff these types of management models and locations, she adds.
In a perfect world, all hospitalized patients would be adequately screened for mental health issues and have their issues appropriately addressed by well-qualified professionals in real time, Dr. Grace says. Telemedicine services have great potential in helping to meet that goal, he says, and more relaxed regulatory guidelines around telemedicine could help make such physician-patient interactions less difficult. Many, if not most, hospitals currently have limited or no access to qualified mental health professionals, a conundrum based on supply, reimbursement, and need.
“Telemedicine, which is already having great success in neurology and intensive care unit medicine, is a great fit for this space,” Dr. Grace says. “Widespread access to a tele-psychiatrist would bring significant tangible benefits to patients, hospitals, hospital staff, and the population at large, who ultimately pay for healthcare in the nation.”
Dr. Horst says he believes everyone who treats psychiatric patients should have education in psychiatric medicine education. One way to achieve this would be to mandate continuing medical education coursework in mental health disorders.
The Reality of a Utopia
Traditionally, our healthcare system has been designed to react to illness, meaning that physicians treat illnesses when individuals become sick.
“But as science and technology now better understand the etiology of most illnesses, we are more equipped to design more preventative interventions rather than wait for individuals to become sick,” Dr. Bianco says. “Prevention interventions require an initial investment that the healthcare system is not necessarily willing to invest in at this time and a shift in the way it charges for services. If the healthcare system is unwilling to go that route, and we know we can prevent many illnesses by shifting the focus of treatment, consequently, human suffering is augmented and quality of life jeopardized.”
More recently, the general population and providers have acknowledged that healing takes place more effectively when it is applied in more integrated approaches (i.e., the utilization of the bio-psycho-social-spiritual model), Dr. Bianco adds. This greater appreciation is demonstrated by different research studies applied to different populations (both the general public and different providers). Despite this, the system (i.e., training) does not support a full integration of interventions.
“The system continues to operate under the traditional medical model that is fragmented and hyper-specialized,” Dr. Bianco says. “Science has demonstrated that the mind and the body work in more complex ways, requiring a more holistic approach to treatment. Although all segments among providers now understand and accept that, the system they dwell in does not support the daily challenges of treatment.
“Treatment continues to be fragmented as it is the medical model. At this point, at a minimum, a hospital should have a psychiatric department composed of individuals who are adequately trained (e.g., health psychology, behavioral medicine) to absorb a portion of individuals who primarily present with mental health issues.” TH
Karen Appold is a freelance medical writer in Pennsylvania.
References
- Torrey EF, Kennard AD, Eslinger D, Lamb R, Pavle J. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. Treatment Advocacy Center website. Available at: http://www.treatmentadvocacycenter.org/storage/documents/final_jails_v_hospitals_study.pdf. May 2010. Accessed August 18, 2015.
- Results from the 2013 national survey on drug use and health: mental health detailed tables. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services website. Available at: http://www.samhsa.gov/data/sites/default/files/2013MHDetTabs/NSDUH-MHDetTabs2013.pdf. Accessed August 19, 2015.
- Any mental illness among adults. National Institute of Mental Health website. Available at: http://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-adults.shtml. Accessed August 19, 2015.
- Internal medicine certification examination blueprint. American Board of Internal Medicine website. Available at: https://www.abim.org/pdf/blueprint/im_cert.pdf. January 2015. Accessed August 19, 2015.
In 1955, there was one psychiatric bed for every 300 Americans. By 2005, following the widespread shuttering or downsizing of psychiatric hospitals in the 1990s, that number had shrunk to one bed for every 3,000 Americans.1
In 2008, an estimated 39.8 million Americans age 18 or older had mental illness, which represents 17.7% of U.S. adults.2 In 2013, this number rose to an estimated 43.8 million, or 18.5% of U.S. adults (see Figure 1).3
“It’s like we have returned to the early 19th century, when mentally ill persons were held in prisons or temporarily kept in hospital settings,” says Ricardo Bianco, PsyD, program director of the Master of Arts in counseling and health psychology at William James College in Newton, Mass. “The problem is that the healthcare system did not catch up to absorb the mentally ill population.
“As a result, hospital staffs are inadequately trained, there is insufficient funding for these patients, and there are not enough human resource personnel to manage them. Consequently, hospitalists are overwhelmed with cases that should be primarily treated by psychologists, psychiatrists, and social workers.”
According to David M. Grace, MD, SFHM, hospitalist and senior medical officer at the Schumacher Group in Lafayette, La., two groups of psychiatric patients present to the acute-care hospital environment: those who are there for a primary psychiatric problem and those who have a medical problem and a psychiatric comorbidity. The first group of patients presents distinct challenges. U.S. hospitals lack two-thirds of the minimum number of beds needed to care for this population. The second group is problematic because psychiatric issues often cloud the medical issues of a patient, increasing both diagnostic uncertainty and resource utilization.
Challenges Abound
Psychiatric patients present a number of problems for hospitalists. First, it is difficult to decipher what comprises a psychiatric issue and what does not because “many psychiatric conditions manifest as physical symptoms and they often require significant resource consumption to diagnose,” Dr. Grace says. Secondly, some patients present with a severe primary psychiatric problem in which they are homicidal, suicidal, or gravely disabled.
In addition, psychiatric patients tend to have a greater incidence of noncompliance with imaging, laboratory work, medication, and general medical care, says Daniel Sussman, MD, a hospitalist at IPC Healthcare, Inc., based in North Hollywood, Calif. He also serves as interim chairman in the department of psychiatry at St. John’s Episcopal Hospital in Far Rockaway, N.Y.
Clinically, potential interactions between psychiatric medications and medically related prescription drugs are always a concern, notes Dr. Sussman, who says more than 70% of patients admitted to St. John’s Episcopal Hospital have a major psychiatric illness in addition to their medical problem. Psychiatric medications, which patients may have tolerated well when they were stable, may be too sedating when patients are ill. Side effects and adverse reactions of psychotropic medications must also be considered when diagnosing and treating medical illnesses. Metabolic syndrome is more commonly seen and is a factor in the development and subsequent treatment of other illnesses.
Another challenge stems from the fact that patients with substantial psychiatric comorbidities can have significant and rapid mood and behavioral changes as well as sudden, volatile, and aggressive outbursts—both verbal and physical.
“Staff members who interact with the patient are at risk if an outburst occurs,” says Emily Fingado, MD, FAAP, a pediatric hospitalist and clinical assistant professor of pediatrics at Nemours/Alfred I. duPont Hospital for Children (Nemours/AIDHC) of the Sidney Kimmel Medical College at Thomas Jefferson University in Wilmington, Del.
Such situations can become scary, particularly if someone with psychiatric expertise is not available to intervene. This can be very frustrating for hospitalists who want to provide high-level care but may lack the training needed to be successful with such patients. This can ultimately lead to burnout, says Sarah Rivelli, MD, medical director of psychiatry clinical services at Duke University Hospital and Duke University Medical Center in Durham, N.C.
Another challenge is that although there are protocols in place designed to follow specific steps for patients with physical illnesses or disorders, that’s not the case for psychiatric illness. “Many hospital facilities are not designed, or have yet to implement, protocols to attend to mentally ill individuals,” Dr. Bianco says.
Because of the unpredictability of patients and lack of practice protocols, mental illness can introduce a wild card into the standard treatment process. A more individualized approach with these patients is needed, but with increasing focus on length of stay and operational efficiency, medical and nursing staffs are pressured to do things quickly and to do more with less. It can be very time-consuming for a nurse to have to explain to paranoid patients why they should take their medication or for a phlebotomist to try to calm patients in order to obtain blood. When patients refuse needed tests, or only provide a limited history, the hospitalist ends up working with incomplete information, which makes choosing and monitoring the treatment approach problematic.
A Look at Best Practices
In light of challenges, some best practices have been identified for handling psychiatric patients. As previously mentioned, most hospital staffs have limited formal training in interacting with psychiatric patients. In fact, the American Board of Internal Medicine only devotes 4% of the certification exam to psychiatry.4
Ideally, staff members who care for psychiatric patients will have specialized or additional training in managing patients with psychiatric conditions or comorbidities. Nemours/AIDHC has a committee assigned to evaluate psychiatric patients’ care and help manage them when a behavioral emergency occurs, Dr. Fingado says. This team, which has been trained on de-escalation, restraint techniques and policies, as well as medications to use in these situations, intervenes when patients have an aggressive event that places patients, visitors, or staff at risk. The team includes nursing staff, the hospitalist on-call, and security personnel and involves the social work, psychology, and psychiatry departments.
Training focused on treating patients with psychiatric conditions should include how to recognize substance abuse and treat substance withdrawal because mental illness and substance abuse often track together, Dr. Sussman notes. At St. John’s Episcopal Hospital, patients with chronic mental illness are not the ones who typically become aggressive or violent. Rather, this is more often the case among patients with substance abuse either in states of acute intoxication or withdrawal.
Recently, Dr. Sussman has seen a significant increase in patients who abuse K2, or spice, a synthetic form of cannabis. Side effects of using K2 include rapid heart rate, anxiety, hallucinations, and paranoia to the point of delusional thinking. These side effects can frequently wax and wane for days after the drug is used, and they can be associated with significant psychomotor agitation and assaultive behavior.
When patients abuse flake, another synthetic drug that has been reported in the Southeast, they tend to become very paranoid and violent. “These patients can be extremely unpredictable and aggressive,” he says. “Patients with dementia can be impulsive and aggressive during care, and caution is needed, but it’s not a directed violence like that seen in patients who are agitated secondary to substance abuse.”
Dr. Bianco advises having a predetermined triage system or a scale that can assess and measure patients’ level of psychological distress, which can ensure timely and appropriate evaluation and treatment of psychiatric patients, as well as toxicology screens and mental health protocols, which can aid in diagnosis.
“Technology is an important tool in facilitating integration, including identifying and screening patients, tracking patient progress, encouraging adherence to clinical protocols, facilitating communication between providers, and evaluating the impact of integrated programs,” Dr. Bianco says. Academic hospitals struggle less with this problem, he adds, because they tend to be more adequately funded in all areas of operations, including the field of mental health.
IPC Healthcare hospitalist James N. Horst, DO, a psychiatrist who manages mental health patients in a long-term care and nursing home facility, says he has found standardized general screening tools to be useful. The Hamilton Depression Scale, Beck Anxiety Inventory, and CAGE exam for chemical dependency can be easily administered and scored in any facility setting, he says. These tests include self-administered questions to which the patient answers yes or no. Laboratory work is a secondary tool in psychiatry since few mental illness disorders are based on medical comorbidities.
Dr. Sussman looks to the past, when psychiatrists were part of medical teams rounding in hospitals, for a solution. “An integrated model provides an approach where patient care is less compartmentalized,” he says. “In this model, clinicians are responsible for making sure their patient is treated, not simply focusing on their individual area of expertise. This involves working more closely with an integrated care management team.”
Ideally, this will occur at every level of care: outpatient, inpatient, and emergency department (ED). New York State is attempting to redesign the Medicaid system in this fashion, with the goal of improving overall care and reducing reliance on inpatient treatment to provide that care. This is an enormous initiative, costing more than $8 billion. If successful, it will result in a more patient-centered care system that treats the whole individual, not just the illness, and will positively impact patients’ overall health.
For now, St. John’s Episcopal Hospital has an active psychiatric consultation liaison service that is staffed by both in-house residents and attending physicians who are there 24-7 to help with psychiatric patients.
A ‘Utopia Management’ Perspective
In a dream world, patients with significant psychiatric problems or comorbidities would have coordinated, multidisciplinary care from admission to discharge, Dr. Fingado adds. Ideally, hospitals would have dedicated rooms or areas in the ED that are safe for patients and staff. Psychiatric patients who require observation or admission to a non-psychiatric hospital would be placed in rooms or units dedicated for psychiatric patients, again providing safety for patients and staff, Dr. Fingado surmises.
In addition, all staff members would have training in behavioral health management, including instruction on de-escalation, restraint techniques, and medication use for patients. Ideally, units would be staffed by specially trained aides, nurses, and healthcare providers (i.e., physician assistants, nurse practitioners, physicians), as well as psychologists and psychiatrists, Dr. Fingado says. This type of management would require buy-in from a multitude of groups, including healthcare administrators, nursing and provider staff, as well as health insurance companies. A reallocation or increase in funds would be needed to help build and staff these types of management models and locations, she adds.
In a perfect world, all hospitalized patients would be adequately screened for mental health issues and have their issues appropriately addressed by well-qualified professionals in real time, Dr. Grace says. Telemedicine services have great potential in helping to meet that goal, he says, and more relaxed regulatory guidelines around telemedicine could help make such physician-patient interactions less difficult. Many, if not most, hospitals currently have limited or no access to qualified mental health professionals, a conundrum based on supply, reimbursement, and need.
“Telemedicine, which is already having great success in neurology and intensive care unit medicine, is a great fit for this space,” Dr. Grace says. “Widespread access to a tele-psychiatrist would bring significant tangible benefits to patients, hospitals, hospital staff, and the population at large, who ultimately pay for healthcare in the nation.”
Dr. Horst says he believes everyone who treats psychiatric patients should have education in psychiatric medicine education. One way to achieve this would be to mandate continuing medical education coursework in mental health disorders.
The Reality of a Utopia
Traditionally, our healthcare system has been designed to react to illness, meaning that physicians treat illnesses when individuals become sick.
“But as science and technology now better understand the etiology of most illnesses, we are more equipped to design more preventative interventions rather than wait for individuals to become sick,” Dr. Bianco says. “Prevention interventions require an initial investment that the healthcare system is not necessarily willing to invest in at this time and a shift in the way it charges for services. If the healthcare system is unwilling to go that route, and we know we can prevent many illnesses by shifting the focus of treatment, consequently, human suffering is augmented and quality of life jeopardized.”
More recently, the general population and providers have acknowledged that healing takes place more effectively when it is applied in more integrated approaches (i.e., the utilization of the bio-psycho-social-spiritual model), Dr. Bianco adds. This greater appreciation is demonstrated by different research studies applied to different populations (both the general public and different providers). Despite this, the system (i.e., training) does not support a full integration of interventions.
“The system continues to operate under the traditional medical model that is fragmented and hyper-specialized,” Dr. Bianco says. “Science has demonstrated that the mind and the body work in more complex ways, requiring a more holistic approach to treatment. Although all segments among providers now understand and accept that, the system they dwell in does not support the daily challenges of treatment.
“Treatment continues to be fragmented as it is the medical model. At this point, at a minimum, a hospital should have a psychiatric department composed of individuals who are adequately trained (e.g., health psychology, behavioral medicine) to absorb a portion of individuals who primarily present with mental health issues.” TH
Karen Appold is a freelance medical writer in Pennsylvania.
References
- Torrey EF, Kennard AD, Eslinger D, Lamb R, Pavle J. More mentally ill persons are in jails and prisons than hospitals: a survey of the states. Treatment Advocacy Center website. Available at: http://www.treatmentadvocacycenter.org/storage/documents/final_jails_v_hospitals_study.pdf. May 2010. Accessed August 18, 2015.
- Results from the 2013 national survey on drug use and health: mental health detailed tables. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services website. Available at: http://www.samhsa.gov/data/sites/default/files/2013MHDetTabs/NSDUH-MHDetTabs2013.pdf. Accessed August 19, 2015.
- Any mental illness among adults. National Institute of Mental Health website. Available at: http://www.nimh.nih.gov/health/statistics/prevalence/any-mental-illness-ami-among-adults.shtml. Accessed August 19, 2015.
- Internal medicine certification examination blueprint. American Board of Internal Medicine website. Available at: https://www.abim.org/pdf/blueprint/im_cert.pdf. January 2015. Accessed August 19, 2015.
Should a Patient Who Requests Alcohol Detoxification Be Admitted or Treated as Outpatient?
Case
A 42-year-old man with a history of posttraumatic stress disorder (PTSD), hypertension, and alcohol use disorder (AUD) presents to the ED requesting alcohol detoxification. He has had six admissions in the last six months for alcohol detoxification. Two years ago, the patient had a documented alcohol withdrawal seizure. His last drink was eight hours ago, and he currently drinks a liter of vodka a day. On exam, his pulse rate is 126 bpm, and his blood pressure is 162/91 mm Hg. He appears anxious and has bilateral hand tremors. His serum ethanol level is 388.6 mg/dL.
Overview
DSM-5 integrated alcohol abuse and alcohol dependence that were previously classified in DSM-IV into AUDs with mild, moderate, and severe subclassifications. AUDs are the most serious substance abuse problem in the U.S. In the general population, the lifetime prevalence of alcohol abuse is 17.8% and of alcohol dependence is 12.5%.1–3 One study estimates that 24% of adult patients brought to the ED by ambulance suffer from alcoholism, and approximately 10% to 32% of hospitalized medical patients have an AUD.4–8 Patients who stop drinking will develop alcohol withdrawal as early as six hours after their last drink (see Figure 1). The majority of patients at risk of alcohol withdrawal syndrome (AWS) will develop only minor uncomplicated symptoms, but up to 20% will develop symptoms associated with complicated AWS, including withdrawal seizures and delirium tremens (DT).9 It is not entirely clear why some individuals suffer from more severe withdrawal symptoms than others, but genetic predisposition may play a role.10
DT is a syndrome characterized by agitation, disorientation, hallucinations, and autonomic instability (tachycardia, hypertension, hyperthermia, and diaphoresis) in the setting of acute reduction or abstinence from alcohol and is associated with a mortality rate as high as 20%.11 Complicated AWS is associated with increased in-hospital morbidity and mortality, longer lengths of stay, inflated costs of care, increased burden and frustration of nursing and medical staff, and worse cognitive functioning.9 In 80% of cases, the symptoms of uncomplicated alcohol withdrawal do not require aggressive medical intervention and usually disappear within two to seven days of the last drink.12 Physicians making triage decisions for patients who present to the ED in need of detoxification face a difficult dilemma concerning inpatient versus outpatient treatment.
Review of the Data
The literature on both inpatient and outpatient management and treatment of AWS is well-described. Currently, there are no guidelines or consensus on whether to admit patients with alcohol abuse syndromes to the hospital when the request for detoxification is made. Admission should be considered for all patients experiencing alcohol withdrawal who present to the ED.13 Patients with mild AWS may be discharged if they do not require admission for an additional medical condition, but patients experiencing moderate to severe withdrawal require admission for monitoring and treatment. Many physicians use a simple assessment of past history of DT and pulse rate, which may be easily evaluated in clinical settings, to readily identify patients who are at high risk of developing DT during an alcohol dependence period.14
Since 1978, the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) has been consistently used for both monitoring patients with alcohol withdrawal and for making an initial assessment. CIWA-Ar was developed as a revised scale and is frequently used to monitor the severity of ongoing alcohol withdrawal and the response to treatment for the clinical care of patients in alcohol withdrawal (see Figure 2). CIWA-Ar was not developed to identify patients at risk for AWS but is frequently used to determine if patients require admission to the hospital for detoxification.15 Patients with CIWA-Ar scores > 15 require inpatient detoxification. Patients with scores between 8 and 15 should be admitted if they have a history of prior seizures or DT but could otherwise be considered for outpatient detoxification. Patients with scores < 8, which are considered mild alcohol withdrawal, can likely be safely treated as outpatients unless they have a history of DT or alcohol withdrawal seizures.16 Because symptoms of severe alcohol withdrawal are often not present for more than six hours after the patient’s last drink, or often longer, CIWA-Ar is limited and does not identify patients who are otherwise at high risk for complicated withdrawal. A protocol was developed incorporating the patient’s history of alcohol withdrawal seizure, DT, and the CIWA to evaluate the outcome of outpatient versus inpatient detoxification.16
The most promising tool to screen patients for AWS was developed recently by researchers at Stanford University in Stanford, Calif., using an extensive systematic literature search to identify evidence-based clinical factors associated with the development of AWS.15 The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) was subsequently constructed from 10 items correlating with complicated AWS (see Figure 3). When using a PAWSS score cutoff of ≥ 4, the predictive value of identifying a patient who is at risk for complicated withdrawal is significantly increased to 93.1%. This tool has only been used in medically ill patients but could be extrapolated for use in patients who present to an acute-care setting requesting inpatient detoxification.
Patients presenting to the ED with alcohol withdrawal seizures have been shown to have an associated 35% risk of progression to DT when found to have a low platelet count, low blood pyridoxine, and a high blood level of homocysteine. In another retrospective cohort study in Hepatology, three clinical features were identified to be associated with an increased risk for DT: alcohol dependence, a prior history of DT, and a higher pulse rate at admission (> 100 bpm).14
Instructions for the assessment of the patient who requests detoxification are as follows:
- A patient whose last drink of alcohol was more than five days ago and who shows no signs of withdrawal is unlikely to develop significant withdrawal symptoms and does not require inpatient detoxification.
- Other medical and psychiatric conditions should be evaluated for admission including alcohol use disorder complications.
- Calculate CIWA-Ar score:
Scores < 8 may not need detoxification; consider calculating PAWSS score.
Scores of 8 to 15 without symptoms of DT or seizures can be treated as an outpatient detoxification if no contraindication.
Scores of ≥ 15 should be admitted to the hospital.
- Calculate PAWSS score:
Scores ≥ 4 suggest high risk for moderate to severe complicated AWS, and admission should be considered.
Scores < 4 suggest lower risk for complicated AWS, and outpatient treatment should be considered if patients do not have a medical or surgical diagnosis requiring admission.
Back to the Case
At the time of his presentation, the patient was beginning to show signs of early withdrawal symptoms, including tremor and tachycardia, despite having an elevated blood alcohol level. This patient had a PAWSS score of 6, placing him at increased risk of complicated AWS, and a CIWA-Ar score of 13. He was subsequently admitted to the hospital, and symptom-triggered therapy for treatment of his alcohol withdrawal was used. The patient’s CIWA-Ar score peaked at 21 some 24 hours after his last drink. The patient otherwise had an uncomplicated four-day hospital course due to persistent nausea.
Bottom Line
Hospitalists unsure of which patients should be admitted for alcohol detoxification can use the PAWSS tool and an initial CIWA-Ar score to help determine a patient’s risk for developing complicated AWS. TH
Dr. Velasquez and Dr. Kornsawad are assistant professors and hospitalists at the University of Texas Health Science Center at San Antonio. Dr. Velasquez also serves as assistant professor and hospitalist at the South Texas Veterans Health Care System serving the San Antonio area.
References
- Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorder and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807-816.
- Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333(16):1058-1065.
- Hasin SD, Stinson SF, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842.
- Whiteman PJ, Hoffman RS, Goldfrank LR. Alcoholism in the emergency department: an epidemiologic study. Acad Emerg Med. 2000;7(1):14-20.
- Nielson SD, Storgarrd H, Moesgarrd F, Gluud C. Prevalence of alcohol problems among adult somatic in-patients of a Copenhagen hospital. Alcohol Alcohol. 1994;29(5):583-590.
- Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004;164(7):749-756.
- Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40(6):515-519.
- Doering-Silveira J, Fidalgo TM, Nascimento CL, et al. Assessing alcohol dependence in hospitalized patients. Int J Environ Res Public Health. 2014;11(6):5783-5791.
- Maldonado JR, Sher Y, Das S, et al. Prospective validation study of the prediction of alcohol withdrawal severity scale (PAWSS) in medically ill inpatients: a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50(5):509-518.
- Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
- Turner RC, Lichstein PR, Pedan Jr JG, Busher JT, Waivers LE. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492-501.
- Stehman CR, Mycyk MB. A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med. 2013;31(4):734-742.
- Lee JH, Jang MK, Lee JY, et al. Clinical predictors for delirium tremens in alcohol dependence. J Gastroenterol Hepatol. 2005;20(12):1833-1837.
- Maldonado JR, Sher Y, Ashouri JF, et al. The “prediction of alcohol withdrawal severity scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48(4):375-390.
- Stephens JR, Liles AE, Dancel R, Gilchrist M, Kirsch J, DeWalt DA. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J Gen Intern Med. 2014;29(4):587-593.
Case
A 42-year-old man with a history of posttraumatic stress disorder (PTSD), hypertension, and alcohol use disorder (AUD) presents to the ED requesting alcohol detoxification. He has had six admissions in the last six months for alcohol detoxification. Two years ago, the patient had a documented alcohol withdrawal seizure. His last drink was eight hours ago, and he currently drinks a liter of vodka a day. On exam, his pulse rate is 126 bpm, and his blood pressure is 162/91 mm Hg. He appears anxious and has bilateral hand tremors. His serum ethanol level is 388.6 mg/dL.
Overview
DSM-5 integrated alcohol abuse and alcohol dependence that were previously classified in DSM-IV into AUDs with mild, moderate, and severe subclassifications. AUDs are the most serious substance abuse problem in the U.S. In the general population, the lifetime prevalence of alcohol abuse is 17.8% and of alcohol dependence is 12.5%.1–3 One study estimates that 24% of adult patients brought to the ED by ambulance suffer from alcoholism, and approximately 10% to 32% of hospitalized medical patients have an AUD.4–8 Patients who stop drinking will develop alcohol withdrawal as early as six hours after their last drink (see Figure 1). The majority of patients at risk of alcohol withdrawal syndrome (AWS) will develop only minor uncomplicated symptoms, but up to 20% will develop symptoms associated with complicated AWS, including withdrawal seizures and delirium tremens (DT).9 It is not entirely clear why some individuals suffer from more severe withdrawal symptoms than others, but genetic predisposition may play a role.10
DT is a syndrome characterized by agitation, disorientation, hallucinations, and autonomic instability (tachycardia, hypertension, hyperthermia, and diaphoresis) in the setting of acute reduction or abstinence from alcohol and is associated with a mortality rate as high as 20%.11 Complicated AWS is associated with increased in-hospital morbidity and mortality, longer lengths of stay, inflated costs of care, increased burden and frustration of nursing and medical staff, and worse cognitive functioning.9 In 80% of cases, the symptoms of uncomplicated alcohol withdrawal do not require aggressive medical intervention and usually disappear within two to seven days of the last drink.12 Physicians making triage decisions for patients who present to the ED in need of detoxification face a difficult dilemma concerning inpatient versus outpatient treatment.
Review of the Data
The literature on both inpatient and outpatient management and treatment of AWS is well-described. Currently, there are no guidelines or consensus on whether to admit patients with alcohol abuse syndromes to the hospital when the request for detoxification is made. Admission should be considered for all patients experiencing alcohol withdrawal who present to the ED.13 Patients with mild AWS may be discharged if they do not require admission for an additional medical condition, but patients experiencing moderate to severe withdrawal require admission for monitoring and treatment. Many physicians use a simple assessment of past history of DT and pulse rate, which may be easily evaluated in clinical settings, to readily identify patients who are at high risk of developing DT during an alcohol dependence period.14
Since 1978, the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) has been consistently used for both monitoring patients with alcohol withdrawal and for making an initial assessment. CIWA-Ar was developed as a revised scale and is frequently used to monitor the severity of ongoing alcohol withdrawal and the response to treatment for the clinical care of patients in alcohol withdrawal (see Figure 2). CIWA-Ar was not developed to identify patients at risk for AWS but is frequently used to determine if patients require admission to the hospital for detoxification.15 Patients with CIWA-Ar scores > 15 require inpatient detoxification. Patients with scores between 8 and 15 should be admitted if they have a history of prior seizures or DT but could otherwise be considered for outpatient detoxification. Patients with scores < 8, which are considered mild alcohol withdrawal, can likely be safely treated as outpatients unless they have a history of DT or alcohol withdrawal seizures.16 Because symptoms of severe alcohol withdrawal are often not present for more than six hours after the patient’s last drink, or often longer, CIWA-Ar is limited and does not identify patients who are otherwise at high risk for complicated withdrawal. A protocol was developed incorporating the patient’s history of alcohol withdrawal seizure, DT, and the CIWA to evaluate the outcome of outpatient versus inpatient detoxification.16
The most promising tool to screen patients for AWS was developed recently by researchers at Stanford University in Stanford, Calif., using an extensive systematic literature search to identify evidence-based clinical factors associated with the development of AWS.15 The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) was subsequently constructed from 10 items correlating with complicated AWS (see Figure 3). When using a PAWSS score cutoff of ≥ 4, the predictive value of identifying a patient who is at risk for complicated withdrawal is significantly increased to 93.1%. This tool has only been used in medically ill patients but could be extrapolated for use in patients who present to an acute-care setting requesting inpatient detoxification.
Patients presenting to the ED with alcohol withdrawal seizures have been shown to have an associated 35% risk of progression to DT when found to have a low platelet count, low blood pyridoxine, and a high blood level of homocysteine. In another retrospective cohort study in Hepatology, three clinical features were identified to be associated with an increased risk for DT: alcohol dependence, a prior history of DT, and a higher pulse rate at admission (> 100 bpm).14
Instructions for the assessment of the patient who requests detoxification are as follows:
- A patient whose last drink of alcohol was more than five days ago and who shows no signs of withdrawal is unlikely to develop significant withdrawal symptoms and does not require inpatient detoxification.
- Other medical and psychiatric conditions should be evaluated for admission including alcohol use disorder complications.
- Calculate CIWA-Ar score:
Scores < 8 may not need detoxification; consider calculating PAWSS score.
Scores of 8 to 15 without symptoms of DT or seizures can be treated as an outpatient detoxification if no contraindication.
Scores of ≥ 15 should be admitted to the hospital.
- Calculate PAWSS score:
Scores ≥ 4 suggest high risk for moderate to severe complicated AWS, and admission should be considered.
Scores < 4 suggest lower risk for complicated AWS, and outpatient treatment should be considered if patients do not have a medical or surgical diagnosis requiring admission.
Back to the Case
At the time of his presentation, the patient was beginning to show signs of early withdrawal symptoms, including tremor and tachycardia, despite having an elevated blood alcohol level. This patient had a PAWSS score of 6, placing him at increased risk of complicated AWS, and a CIWA-Ar score of 13. He was subsequently admitted to the hospital, and symptom-triggered therapy for treatment of his alcohol withdrawal was used. The patient’s CIWA-Ar score peaked at 21 some 24 hours after his last drink. The patient otherwise had an uncomplicated four-day hospital course due to persistent nausea.
Bottom Line
Hospitalists unsure of which patients should be admitted for alcohol detoxification can use the PAWSS tool and an initial CIWA-Ar score to help determine a patient’s risk for developing complicated AWS. TH
Dr. Velasquez and Dr. Kornsawad are assistant professors and hospitalists at the University of Texas Health Science Center at San Antonio. Dr. Velasquez also serves as assistant professor and hospitalist at the South Texas Veterans Health Care System serving the San Antonio area.
References
- Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorder and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807-816.
- Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333(16):1058-1065.
- Hasin SD, Stinson SF, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842.
- Whiteman PJ, Hoffman RS, Goldfrank LR. Alcoholism in the emergency department: an epidemiologic study. Acad Emerg Med. 2000;7(1):14-20.
- Nielson SD, Storgarrd H, Moesgarrd F, Gluud C. Prevalence of alcohol problems among adult somatic in-patients of a Copenhagen hospital. Alcohol Alcohol. 1994;29(5):583-590.
- Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004;164(7):749-756.
- Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40(6):515-519.
- Doering-Silveira J, Fidalgo TM, Nascimento CL, et al. Assessing alcohol dependence in hospitalized patients. Int J Environ Res Public Health. 2014;11(6):5783-5791.
- Maldonado JR, Sher Y, Das S, et al. Prospective validation study of the prediction of alcohol withdrawal severity scale (PAWSS) in medically ill inpatients: a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50(5):509-518.
- Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
- Turner RC, Lichstein PR, Pedan Jr JG, Busher JT, Waivers LE. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492-501.
- Stehman CR, Mycyk MB. A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med. 2013;31(4):734-742.
- Lee JH, Jang MK, Lee JY, et al. Clinical predictors for delirium tremens in alcohol dependence. J Gastroenterol Hepatol. 2005;20(12):1833-1837.
- Maldonado JR, Sher Y, Ashouri JF, et al. The “prediction of alcohol withdrawal severity scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48(4):375-390.
- Stephens JR, Liles AE, Dancel R, Gilchrist M, Kirsch J, DeWalt DA. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J Gen Intern Med. 2014;29(4):587-593.
Case
A 42-year-old man with a history of posttraumatic stress disorder (PTSD), hypertension, and alcohol use disorder (AUD) presents to the ED requesting alcohol detoxification. He has had six admissions in the last six months for alcohol detoxification. Two years ago, the patient had a documented alcohol withdrawal seizure. His last drink was eight hours ago, and he currently drinks a liter of vodka a day. On exam, his pulse rate is 126 bpm, and his blood pressure is 162/91 mm Hg. He appears anxious and has bilateral hand tremors. His serum ethanol level is 388.6 mg/dL.
Overview
DSM-5 integrated alcohol abuse and alcohol dependence that were previously classified in DSM-IV into AUDs with mild, moderate, and severe subclassifications. AUDs are the most serious substance abuse problem in the U.S. In the general population, the lifetime prevalence of alcohol abuse is 17.8% and of alcohol dependence is 12.5%.1–3 One study estimates that 24% of adult patients brought to the ED by ambulance suffer from alcoholism, and approximately 10% to 32% of hospitalized medical patients have an AUD.4–8 Patients who stop drinking will develop alcohol withdrawal as early as six hours after their last drink (see Figure 1). The majority of patients at risk of alcohol withdrawal syndrome (AWS) will develop only minor uncomplicated symptoms, but up to 20% will develop symptoms associated with complicated AWS, including withdrawal seizures and delirium tremens (DT).9 It is not entirely clear why some individuals suffer from more severe withdrawal symptoms than others, but genetic predisposition may play a role.10
DT is a syndrome characterized by agitation, disorientation, hallucinations, and autonomic instability (tachycardia, hypertension, hyperthermia, and diaphoresis) in the setting of acute reduction or abstinence from alcohol and is associated with a mortality rate as high as 20%.11 Complicated AWS is associated with increased in-hospital morbidity and mortality, longer lengths of stay, inflated costs of care, increased burden and frustration of nursing and medical staff, and worse cognitive functioning.9 In 80% of cases, the symptoms of uncomplicated alcohol withdrawal do not require aggressive medical intervention and usually disappear within two to seven days of the last drink.12 Physicians making triage decisions for patients who present to the ED in need of detoxification face a difficult dilemma concerning inpatient versus outpatient treatment.
Review of the Data
The literature on both inpatient and outpatient management and treatment of AWS is well-described. Currently, there are no guidelines or consensus on whether to admit patients with alcohol abuse syndromes to the hospital when the request for detoxification is made. Admission should be considered for all patients experiencing alcohol withdrawal who present to the ED.13 Patients with mild AWS may be discharged if they do not require admission for an additional medical condition, but patients experiencing moderate to severe withdrawal require admission for monitoring and treatment. Many physicians use a simple assessment of past history of DT and pulse rate, which may be easily evaluated in clinical settings, to readily identify patients who are at high risk of developing DT during an alcohol dependence period.14
Since 1978, the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) has been consistently used for both monitoring patients with alcohol withdrawal and for making an initial assessment. CIWA-Ar was developed as a revised scale and is frequently used to monitor the severity of ongoing alcohol withdrawal and the response to treatment for the clinical care of patients in alcohol withdrawal (see Figure 2). CIWA-Ar was not developed to identify patients at risk for AWS but is frequently used to determine if patients require admission to the hospital for detoxification.15 Patients with CIWA-Ar scores > 15 require inpatient detoxification. Patients with scores between 8 and 15 should be admitted if they have a history of prior seizures or DT but could otherwise be considered for outpatient detoxification. Patients with scores < 8, which are considered mild alcohol withdrawal, can likely be safely treated as outpatients unless they have a history of DT or alcohol withdrawal seizures.16 Because symptoms of severe alcohol withdrawal are often not present for more than six hours after the patient’s last drink, or often longer, CIWA-Ar is limited and does not identify patients who are otherwise at high risk for complicated withdrawal. A protocol was developed incorporating the patient’s history of alcohol withdrawal seizure, DT, and the CIWA to evaluate the outcome of outpatient versus inpatient detoxification.16
The most promising tool to screen patients for AWS was developed recently by researchers at Stanford University in Stanford, Calif., using an extensive systematic literature search to identify evidence-based clinical factors associated with the development of AWS.15 The Prediction of Alcohol Withdrawal Severity Scale (PAWSS) was subsequently constructed from 10 items correlating with complicated AWS (see Figure 3). When using a PAWSS score cutoff of ≥ 4, the predictive value of identifying a patient who is at risk for complicated withdrawal is significantly increased to 93.1%. This tool has only been used in medically ill patients but could be extrapolated for use in patients who present to an acute-care setting requesting inpatient detoxification.
Patients presenting to the ED with alcohol withdrawal seizures have been shown to have an associated 35% risk of progression to DT when found to have a low platelet count, low blood pyridoxine, and a high blood level of homocysteine. In another retrospective cohort study in Hepatology, three clinical features were identified to be associated with an increased risk for DT: alcohol dependence, a prior history of DT, and a higher pulse rate at admission (> 100 bpm).14
Instructions for the assessment of the patient who requests detoxification are as follows:
- A patient whose last drink of alcohol was more than five days ago and who shows no signs of withdrawal is unlikely to develop significant withdrawal symptoms and does not require inpatient detoxification.
- Other medical and psychiatric conditions should be evaluated for admission including alcohol use disorder complications.
- Calculate CIWA-Ar score:
Scores < 8 may not need detoxification; consider calculating PAWSS score.
Scores of 8 to 15 without symptoms of DT or seizures can be treated as an outpatient detoxification if no contraindication.
Scores of ≥ 15 should be admitted to the hospital.
- Calculate PAWSS score:
Scores ≥ 4 suggest high risk for moderate to severe complicated AWS, and admission should be considered.
Scores < 4 suggest lower risk for complicated AWS, and outpatient treatment should be considered if patients do not have a medical or surgical diagnosis requiring admission.
Back to the Case
At the time of his presentation, the patient was beginning to show signs of early withdrawal symptoms, including tremor and tachycardia, despite having an elevated blood alcohol level. This patient had a PAWSS score of 6, placing him at increased risk of complicated AWS, and a CIWA-Ar score of 13. He was subsequently admitted to the hospital, and symptom-triggered therapy for treatment of his alcohol withdrawal was used. The patient’s CIWA-Ar score peaked at 21 some 24 hours after his last drink. The patient otherwise had an uncomplicated four-day hospital course due to persistent nausea.
Bottom Line
Hospitalists unsure of which patients should be admitted for alcohol detoxification can use the PAWSS tool and an initial CIWA-Ar score to help determine a patient’s risk for developing complicated AWS. TH
Dr. Velasquez and Dr. Kornsawad are assistant professors and hospitalists at the University of Texas Health Science Center at San Antonio. Dr. Velasquez also serves as assistant professor and hospitalist at the South Texas Veterans Health Care System serving the San Antonio area.
References
- Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorder and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807-816.
- Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333(16):1058-1065.
- Hasin SD, Stinson SF, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830-842.
- Whiteman PJ, Hoffman RS, Goldfrank LR. Alcoholism in the emergency department: an epidemiologic study. Acad Emerg Med. 2000;7(1):14-20.
- Nielson SD, Storgarrd H, Moesgarrd F, Gluud C. Prevalence of alcohol problems among adult somatic in-patients of a Copenhagen hospital. Alcohol Alcohol. 1994;29(5):583-590.
- Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004;164(7):749-756.
- Dolman JM, Hawkes ND. Combining the audit questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40(6):515-519.
- Doering-Silveira J, Fidalgo TM, Nascimento CL, et al. Assessing alcohol dependence in hospitalized patients. Int J Environ Res Public Health. 2014;11(6):5783-5791.
- Maldonado JR, Sher Y, Das S, et al. Prospective validation study of the prediction of alcohol withdrawal severity scale (PAWSS) in medically ill inpatients: a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50(5):509-518.
- Saitz R, O’Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med Clin North Am. 1997;81(4):881-907.
- Turner RC, Lichstein PR, Pedan Jr JG, Busher JT, Waivers LE. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492-501.
- Stehman CR, Mycyk MB. A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med. 2013;31(4):734-742.
- Lee JH, Jang MK, Lee JY, et al. Clinical predictors for delirium tremens in alcohol dependence. J Gastroenterol Hepatol. 2005;20(12):1833-1837.
- Maldonado JR, Sher Y, Ashouri JF, et al. The “prediction of alcohol withdrawal severity scale” (PAWSS): systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48(4):375-390.
- Stephens JR, Liles AE, Dancel R, Gilchrist M, Kirsch J, DeWalt DA. Who needs inpatient detox? Development and implementation of a hospitalist protocol for the evaluation of patients for alcohol detoxification. J Gen Intern Med. 2014;29(4):587-593.
FDA approves drug for 2 indications in MM

Photo courtesy of
Spectrum Pharmaceuticals
The US Food and Drug Administration (FDA) has approved a new formulation of melphalan for injection (Evomela) for 2 indications.
The drug is now approved for use as a high-dose conditioning treatment prior to autologous hematopoietic stem cell transplant (HSCT) in patients with multiple myeloma (MM) and for the palliative treatment of patients with MM for whom oral therapy is not appropriate.
Evomela is the first product to be FDA-approved for the high-dose conditioning indication in MM. The FDA previously granted the drug orphan designation for this indication.
About Evomela
Evomela is a Captisol-enabled, propylene glycol-free melphalan formulation. This formulation eliminates the need to use a propylene glycol-containing custom diluent, which is required with other intravenous melphalan formulations and has been reported to cause renal and cardiac side effects.
Captisol is a chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
The use of Captisol technology to reformulate melphalan is reported to improve the drug’s stability, extending its use time. The technology allows the admixture solution to be stable for 4 hours at room temperature, in addition to the 1 hour following reconstitution, and for 24 hours at refrigerated temperature (5°C).
This is anticipated to simplify preparation and administration logistics and allow for slower infusion rates and longer administration durations for pre-transplant chemotherapy.
The full prescribing information for Evomela is available at www.evomela.com.
Spectrum Pharmaceuticals gained global development and commercialization rights to Evomela from Ligand Pharmaceuticals Incorporated in March 2013.
Spectrum assumed responsibility for completing the pivotal phase 2 trial of Evomela and was responsible for filing the new drug application. Spectrum filed the application in December 2014, and the FDA accepted it the following March.
Phase 2 study
Evomela was approved by the FDA based on its bioequivalence to the standard melphalan formulation (Alkeran) in a phase 2 study.
Initial results from this trial (phase 2a) were published in Bone Marrow Transplantation in June 2014. Phase 2b results were published in Biology of Blood and Marrow Transplantation in September 2015.
Phase 2b included 61 patients. Fifty-six had newly diagnosed MM, and 5 had relapsed MM following prior HSCT.
The patients received Evomela at 200 mg/m2, given as 2 doses on day -3 and day -2 prior to autologous HSCT (day 0).
All 61 patients achieved myeloablation at a median of 5 days post-HSCT. And all patients had successful neutrophil and platelet engraftment at a median of 12 days and 13 days post-HSCT, respectively.
Efficacy was assessed by clinical response at day 100. According to investigator assessment, the overall response rate was 95%, and the complete response (CR) rate was 31%.
According to independent pathology review, the overall response rate was 100%, and the CR rate was 21%. The lower rate of confirmed CRs in the independent review was due to missing data.
Treatment-related mortality was 0%, and non-hematologic adverse events were mostly grade 1 and 2 in severity. The incidence of grade 3 mucositis and grade 3 stomatitis were 10% and 5%, respectively, with no grade 4 mucositis or stomatitis reported.
Twenty percent of patients experienced treatment-emergent serious adverse events, most of which were grade 3 and consisted of events commonly reported in patients undergoing myeloablative chemotherapy. No new safety signals were identified. ![]()

Photo courtesy of
Spectrum Pharmaceuticals
The US Food and Drug Administration (FDA) has approved a new formulation of melphalan for injection (Evomela) for 2 indications.
The drug is now approved for use as a high-dose conditioning treatment prior to autologous hematopoietic stem cell transplant (HSCT) in patients with multiple myeloma (MM) and for the palliative treatment of patients with MM for whom oral therapy is not appropriate.
Evomela is the first product to be FDA-approved for the high-dose conditioning indication in MM. The FDA previously granted the drug orphan designation for this indication.
About Evomela
Evomela is a Captisol-enabled, propylene glycol-free melphalan formulation. This formulation eliminates the need to use a propylene glycol-containing custom diluent, which is required with other intravenous melphalan formulations and has been reported to cause renal and cardiac side effects.
Captisol is a chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
The use of Captisol technology to reformulate melphalan is reported to improve the drug’s stability, extending its use time. The technology allows the admixture solution to be stable for 4 hours at room temperature, in addition to the 1 hour following reconstitution, and for 24 hours at refrigerated temperature (5°C).
This is anticipated to simplify preparation and administration logistics and allow for slower infusion rates and longer administration durations for pre-transplant chemotherapy.
The full prescribing information for Evomela is available at www.evomela.com.
Spectrum Pharmaceuticals gained global development and commercialization rights to Evomela from Ligand Pharmaceuticals Incorporated in March 2013.
Spectrum assumed responsibility for completing the pivotal phase 2 trial of Evomela and was responsible for filing the new drug application. Spectrum filed the application in December 2014, and the FDA accepted it the following March.
Phase 2 study
Evomela was approved by the FDA based on its bioequivalence to the standard melphalan formulation (Alkeran) in a phase 2 study.
Initial results from this trial (phase 2a) were published in Bone Marrow Transplantation in June 2014. Phase 2b results were published in Biology of Blood and Marrow Transplantation in September 2015.
Phase 2b included 61 patients. Fifty-six had newly diagnosed MM, and 5 had relapsed MM following prior HSCT.
The patients received Evomela at 200 mg/m2, given as 2 doses on day -3 and day -2 prior to autologous HSCT (day 0).
All 61 patients achieved myeloablation at a median of 5 days post-HSCT. And all patients had successful neutrophil and platelet engraftment at a median of 12 days and 13 days post-HSCT, respectively.
Efficacy was assessed by clinical response at day 100. According to investigator assessment, the overall response rate was 95%, and the complete response (CR) rate was 31%.
According to independent pathology review, the overall response rate was 100%, and the CR rate was 21%. The lower rate of confirmed CRs in the independent review was due to missing data.
Treatment-related mortality was 0%, and non-hematologic adverse events were mostly grade 1 and 2 in severity. The incidence of grade 3 mucositis and grade 3 stomatitis were 10% and 5%, respectively, with no grade 4 mucositis or stomatitis reported.
Twenty percent of patients experienced treatment-emergent serious adverse events, most of which were grade 3 and consisted of events commonly reported in patients undergoing myeloablative chemotherapy. No new safety signals were identified. ![]()

Photo courtesy of
Spectrum Pharmaceuticals
The US Food and Drug Administration (FDA) has approved a new formulation of melphalan for injection (Evomela) for 2 indications.
The drug is now approved for use as a high-dose conditioning treatment prior to autologous hematopoietic stem cell transplant (HSCT) in patients with multiple myeloma (MM) and for the palliative treatment of patients with MM for whom oral therapy is not appropriate.
Evomela is the first product to be FDA-approved for the high-dose conditioning indication in MM. The FDA previously granted the drug orphan designation for this indication.
About Evomela
Evomela is a Captisol-enabled, propylene glycol-free melphalan formulation. This formulation eliminates the need to use a propylene glycol-containing custom diluent, which is required with other intravenous melphalan formulations and has been reported to cause renal and cardiac side effects.
Captisol is a chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs.
The use of Captisol technology to reformulate melphalan is reported to improve the drug’s stability, extending its use time. The technology allows the admixture solution to be stable for 4 hours at room temperature, in addition to the 1 hour following reconstitution, and for 24 hours at refrigerated temperature (5°C).
This is anticipated to simplify preparation and administration logistics and allow for slower infusion rates and longer administration durations for pre-transplant chemotherapy.
The full prescribing information for Evomela is available at www.evomela.com.
Spectrum Pharmaceuticals gained global development and commercialization rights to Evomela from Ligand Pharmaceuticals Incorporated in March 2013.
Spectrum assumed responsibility for completing the pivotal phase 2 trial of Evomela and was responsible for filing the new drug application. Spectrum filed the application in December 2014, and the FDA accepted it the following March.
Phase 2 study
Evomela was approved by the FDA based on its bioequivalence to the standard melphalan formulation (Alkeran) in a phase 2 study.
Initial results from this trial (phase 2a) were published in Bone Marrow Transplantation in June 2014. Phase 2b results were published in Biology of Blood and Marrow Transplantation in September 2015.
Phase 2b included 61 patients. Fifty-six had newly diagnosed MM, and 5 had relapsed MM following prior HSCT.
The patients received Evomela at 200 mg/m2, given as 2 doses on day -3 and day -2 prior to autologous HSCT (day 0).
All 61 patients achieved myeloablation at a median of 5 days post-HSCT. And all patients had successful neutrophil and platelet engraftment at a median of 12 days and 13 days post-HSCT, respectively.
Efficacy was assessed by clinical response at day 100. According to investigator assessment, the overall response rate was 95%, and the complete response (CR) rate was 31%.
According to independent pathology review, the overall response rate was 100%, and the CR rate was 21%. The lower rate of confirmed CRs in the independent review was due to missing data.
Treatment-related mortality was 0%, and non-hematologic adverse events were mostly grade 1 and 2 in severity. The incidence of grade 3 mucositis and grade 3 stomatitis were 10% and 5%, respectively, with no grade 4 mucositis or stomatitis reported.
Twenty percent of patients experienced treatment-emergent serious adverse events, most of which were grade 3 and consisted of events commonly reported in patients undergoing myeloablative chemotherapy. No new safety signals were identified. ![]()
Combo appears superior to G-CSF for mobilizing HSCs

in the bone marrow
A 2-drug combination can be more effective than granulocyte colony-stimulating factor (G-CSF) for mobilizing hematopoietic stem cells (HSCs), according to preclinical research published in Nature Communications.
The combination consists of the dual α9β1/α4β1 antagonist BOP and the CXCR4 antagonist AMD3100, also known as plerixafor.
In experiments with mice, researchers found that treatment with BOP and AMD3100 directly impacts HSCs so they can be seen in the blood stream within an hour.
And when these HSCs are transplanted into recipient mice, they can replenish the entire hematopoietic system.
“Current treatment requires [a transplant donor] to have growth factor injections for several days leading up to the [harvesting] procedure,” said study author Susie Nilsson, PhD, of Monash University in Clayton, Victoria, Australia.
“Using the [2-drug combination] eliminates the need for this, meaning a procedure that once took days can be reduced to around an hour.”
Combination vs monotherapy
Dr Nilsson and her colleagues found that, when given alone, AMD3100 and BOP produced similar increases in white blood cell (WBC) counts and the proportion of progenitors (LSK cells) in the peripheral blood (PB) of mice.
However, BOP produced a significantly greater increase in the proportion of HSCs (LSKSLAM cells) in the PB. The researchers said this suggests that AMD3100 predominantly mobilizes progenitors, and BOP predominantly mobilizes HSCs.
The team also found that, in combination, BOP and AMD3100 mobilized WBCs, progenitors, and HSCs more effectively than either agent alone.
To determine if these drugs could mobilize HSCs and progenitors with long-term multi-lineage engraftment potential, the researchers performed limiting dilution transplant analysis using BOP, AMD3100, or both drugs.
The team observed superior survival in mice that received 30 ml of PB mobilized using the combination, when compared to either drug alone.
In addition, PB mobilized with the combination resulted in a greater repopulation frequency (1 HSC in 23 ml PB) than PB mobilized with BOP (1 HSC in 327 ml) or AMD3100 (1 HSC in 351 ml).
The researchers said this was a more than 10-fold improvement with the combination, as compared to monotherapy.
Comparisons with G-CSF
Treating mice with a single dose of BOP following 4 days of G-CSF treatment resulted in significant synergistic increases in HSCs (LSKSLAM cells) and progenitors (LSK cells), when compared to G-CSF alone.
BOP and AMD3100 in combination produced equivalent numbers of HSCs and progenitors as G-CSF alone.
And the combination of G-CSF and AMD3100 mobilized significantly more HSCs and progenitors than G-CSF alone.
But the greatest number of HSCs and progenitors were mobilized with the combination of G-CSF, AMD3100, and BOP. This combination produced a significant increase in cells when compared with G-CSF alone or the combination of AMD3100 and BOP.
The researchers also compared the hematopoietic potential of PB mobilized with multiple doses of G-CSF to PB mobilized with a single dose of BOP and AMD3100 in combination.
Although equivalent numbers of HSCs were mobilized, BOP and AMD3100 significantly enhanced short-term and long-term multi-lineage engraftment, when compared to G-CSF.
To determine whether the HSC mobilization observed in these experiments is equivalent in humans, the researchers tested the mobilizing agents in humanized NSG mice.
They found that a single dose of BOP or AMD3100 alone, or multiple doses of G-CSF for 4 days, did not significantly increase human WBCs or human CD34+ stem and progenitor cells.
However, a single dose of BOP and AMD3100 in combination produced a significant increase in both WBCs and stem and progenitor cells.
The researchers said the next step is a phase 1 trial assessing the combination of BOP with G-CSF before they can test the combination of BOP and AMD3100 in humans. ![]()

in the bone marrow
A 2-drug combination can be more effective than granulocyte colony-stimulating factor (G-CSF) for mobilizing hematopoietic stem cells (HSCs), according to preclinical research published in Nature Communications.
The combination consists of the dual α9β1/α4β1 antagonist BOP and the CXCR4 antagonist AMD3100, also known as plerixafor.
In experiments with mice, researchers found that treatment with BOP and AMD3100 directly impacts HSCs so they can be seen in the blood stream within an hour.
And when these HSCs are transplanted into recipient mice, they can replenish the entire hematopoietic system.
“Current treatment requires [a transplant donor] to have growth factor injections for several days leading up to the [harvesting] procedure,” said study author Susie Nilsson, PhD, of Monash University in Clayton, Victoria, Australia.
“Using the [2-drug combination] eliminates the need for this, meaning a procedure that once took days can be reduced to around an hour.”
Combination vs monotherapy
Dr Nilsson and her colleagues found that, when given alone, AMD3100 and BOP produced similar increases in white blood cell (WBC) counts and the proportion of progenitors (LSK cells) in the peripheral blood (PB) of mice.
However, BOP produced a significantly greater increase in the proportion of HSCs (LSKSLAM cells) in the PB. The researchers said this suggests that AMD3100 predominantly mobilizes progenitors, and BOP predominantly mobilizes HSCs.
The team also found that, in combination, BOP and AMD3100 mobilized WBCs, progenitors, and HSCs more effectively than either agent alone.
To determine if these drugs could mobilize HSCs and progenitors with long-term multi-lineage engraftment potential, the researchers performed limiting dilution transplant analysis using BOP, AMD3100, or both drugs.
The team observed superior survival in mice that received 30 ml of PB mobilized using the combination, when compared to either drug alone.
In addition, PB mobilized with the combination resulted in a greater repopulation frequency (1 HSC in 23 ml PB) than PB mobilized with BOP (1 HSC in 327 ml) or AMD3100 (1 HSC in 351 ml).
The researchers said this was a more than 10-fold improvement with the combination, as compared to monotherapy.
Comparisons with G-CSF
Treating mice with a single dose of BOP following 4 days of G-CSF treatment resulted in significant synergistic increases in HSCs (LSKSLAM cells) and progenitors (LSK cells), when compared to G-CSF alone.
BOP and AMD3100 in combination produced equivalent numbers of HSCs and progenitors as G-CSF alone.
And the combination of G-CSF and AMD3100 mobilized significantly more HSCs and progenitors than G-CSF alone.
But the greatest number of HSCs and progenitors were mobilized with the combination of G-CSF, AMD3100, and BOP. This combination produced a significant increase in cells when compared with G-CSF alone or the combination of AMD3100 and BOP.
The researchers also compared the hematopoietic potential of PB mobilized with multiple doses of G-CSF to PB mobilized with a single dose of BOP and AMD3100 in combination.
Although equivalent numbers of HSCs were mobilized, BOP and AMD3100 significantly enhanced short-term and long-term multi-lineage engraftment, when compared to G-CSF.
To determine whether the HSC mobilization observed in these experiments is equivalent in humans, the researchers tested the mobilizing agents in humanized NSG mice.
They found that a single dose of BOP or AMD3100 alone, or multiple doses of G-CSF for 4 days, did not significantly increase human WBCs or human CD34+ stem and progenitor cells.
However, a single dose of BOP and AMD3100 in combination produced a significant increase in both WBCs and stem and progenitor cells.
The researchers said the next step is a phase 1 trial assessing the combination of BOP with G-CSF before they can test the combination of BOP and AMD3100 in humans. ![]()

in the bone marrow
A 2-drug combination can be more effective than granulocyte colony-stimulating factor (G-CSF) for mobilizing hematopoietic stem cells (HSCs), according to preclinical research published in Nature Communications.
The combination consists of the dual α9β1/α4β1 antagonist BOP and the CXCR4 antagonist AMD3100, also known as plerixafor.
In experiments with mice, researchers found that treatment with BOP and AMD3100 directly impacts HSCs so they can be seen in the blood stream within an hour.
And when these HSCs are transplanted into recipient mice, they can replenish the entire hematopoietic system.
“Current treatment requires [a transplant donor] to have growth factor injections for several days leading up to the [harvesting] procedure,” said study author Susie Nilsson, PhD, of Monash University in Clayton, Victoria, Australia.
“Using the [2-drug combination] eliminates the need for this, meaning a procedure that once took days can be reduced to around an hour.”
Combination vs monotherapy
Dr Nilsson and her colleagues found that, when given alone, AMD3100 and BOP produced similar increases in white blood cell (WBC) counts and the proportion of progenitors (LSK cells) in the peripheral blood (PB) of mice.
However, BOP produced a significantly greater increase in the proportion of HSCs (LSKSLAM cells) in the PB. The researchers said this suggests that AMD3100 predominantly mobilizes progenitors, and BOP predominantly mobilizes HSCs.
The team also found that, in combination, BOP and AMD3100 mobilized WBCs, progenitors, and HSCs more effectively than either agent alone.
To determine if these drugs could mobilize HSCs and progenitors with long-term multi-lineage engraftment potential, the researchers performed limiting dilution transplant analysis using BOP, AMD3100, or both drugs.
The team observed superior survival in mice that received 30 ml of PB mobilized using the combination, when compared to either drug alone.
In addition, PB mobilized with the combination resulted in a greater repopulation frequency (1 HSC in 23 ml PB) than PB mobilized with BOP (1 HSC in 327 ml) or AMD3100 (1 HSC in 351 ml).
The researchers said this was a more than 10-fold improvement with the combination, as compared to monotherapy.
Comparisons with G-CSF
Treating mice with a single dose of BOP following 4 days of G-CSF treatment resulted in significant synergistic increases in HSCs (LSKSLAM cells) and progenitors (LSK cells), when compared to G-CSF alone.
BOP and AMD3100 in combination produced equivalent numbers of HSCs and progenitors as G-CSF alone.
And the combination of G-CSF and AMD3100 mobilized significantly more HSCs and progenitors than G-CSF alone.
But the greatest number of HSCs and progenitors were mobilized with the combination of G-CSF, AMD3100, and BOP. This combination produced a significant increase in cells when compared with G-CSF alone or the combination of AMD3100 and BOP.
The researchers also compared the hematopoietic potential of PB mobilized with multiple doses of G-CSF to PB mobilized with a single dose of BOP and AMD3100 in combination.
Although equivalent numbers of HSCs were mobilized, BOP and AMD3100 significantly enhanced short-term and long-term multi-lineage engraftment, when compared to G-CSF.
To determine whether the HSC mobilization observed in these experiments is equivalent in humans, the researchers tested the mobilizing agents in humanized NSG mice.
They found that a single dose of BOP or AMD3100 alone, or multiple doses of G-CSF for 4 days, did not significantly increase human WBCs or human CD34+ stem and progenitor cells.
However, a single dose of BOP and AMD3100 in combination produced a significant increase in both WBCs and stem and progenitor cells.
The researchers said the next step is a phase 1 trial assessing the combination of BOP with G-CSF before they can test the combination of BOP and AMD3100 in humans. ![]()