User login
Bump in the Road
On October 14, 2015, the US Food and Drug Administration declined to approve tofacitinib citrate, an oral rheumatoid arthritis drug, for the treatment of moderate to severe chronic plaque psoriasis. The FDA communicated its decision to the manufacturer in the form of a complete response letter, which typically outlines concerns and conditions that must be addressed in order to gain FDA approval following initial review of an application.
A recent press release indicated that the manufacturer is committed to pursuing approval of the product based on the strength of the clinical data for its treatment of psoriasis. The FDA generally does not disclose the contents of its complete response letters, but the manufacturer reported it has been asked to provide additional safety analyses of the drug for psoriasis and that it will work closely with the agency to gain the additional approval for treatment of patients with chronic plaque psoriasis.
What’s the Issue?
With the increasing number of psoriasis drugs on the market and in the pipeline, the risk-benefit profile of all drugs needs to be evaluated very carefully. Therefore, safety is the focal issue in all new drug development. Hopefully these issues with the FDA approval of tofacitinib citrate will be worked out so that we may have another oral option for our psoriasis patients. How will this development influence your approach to new therapies?
On October 14, 2015, the US Food and Drug Administration declined to approve tofacitinib citrate, an oral rheumatoid arthritis drug, for the treatment of moderate to severe chronic plaque psoriasis. The FDA communicated its decision to the manufacturer in the form of a complete response letter, which typically outlines concerns and conditions that must be addressed in order to gain FDA approval following initial review of an application.
A recent press release indicated that the manufacturer is committed to pursuing approval of the product based on the strength of the clinical data for its treatment of psoriasis. The FDA generally does not disclose the contents of its complete response letters, but the manufacturer reported it has been asked to provide additional safety analyses of the drug for psoriasis and that it will work closely with the agency to gain the additional approval for treatment of patients with chronic plaque psoriasis.
What’s the Issue?
With the increasing number of psoriasis drugs on the market and in the pipeline, the risk-benefit profile of all drugs needs to be evaluated very carefully. Therefore, safety is the focal issue in all new drug development. Hopefully these issues with the FDA approval of tofacitinib citrate will be worked out so that we may have another oral option for our psoriasis patients. How will this development influence your approach to new therapies?
On October 14, 2015, the US Food and Drug Administration declined to approve tofacitinib citrate, an oral rheumatoid arthritis drug, for the treatment of moderate to severe chronic plaque psoriasis. The FDA communicated its decision to the manufacturer in the form of a complete response letter, which typically outlines concerns and conditions that must be addressed in order to gain FDA approval following initial review of an application.
A recent press release indicated that the manufacturer is committed to pursuing approval of the product based on the strength of the clinical data for its treatment of psoriasis. The FDA generally does not disclose the contents of its complete response letters, but the manufacturer reported it has been asked to provide additional safety analyses of the drug for psoriasis and that it will work closely with the agency to gain the additional approval for treatment of patients with chronic plaque psoriasis.
What’s the Issue?
With the increasing number of psoriasis drugs on the market and in the pipeline, the risk-benefit profile of all drugs needs to be evaluated very carefully. Therefore, safety is the focal issue in all new drug development. Hopefully these issues with the FDA approval of tofacitinib citrate will be worked out so that we may have another oral option for our psoriasis patients. How will this development influence your approach to new therapies?
Infectious disease in elderly a significant burden for EDs
More U.S. adults over the age of 65 visited an emergency department because of an infectious disease than for myocardial infarction and congestive heart failure combined, according to a new study.
Based on a nationwide ED sample, adults over 65 visited the ED just over 3.1 million times in 2012 due to infectious diseases (ID), more than three times the estimated amount for myocardial infarction and congestive heart failure. The most common diagnoses were lower respiratory infections (26.2%), urinary tract infections (25.3%), and septicemia (18.9%).
Of the 3.1 million cases brought to the ED, nearly 1.8 million cases were hospitalized, with septicemia the most common cause for hospitalization, accounting for 32.2% of ID-related hospitalizations, followed by lower respiratory infections. Septicemia was also the most common cause of mortality, accounting for 74.7% of the nearly 124,000 deaths.
“These observations underscore the importance of integrated strategies aimed at reducing ID-related morbidity and health care use of elderly adults as a national priority for research, health policy, and community action,” Dr. Tadahiro Goto of the University of Fukui (Japan) Hospital and his associates concluded.
Find the full study in the Journal of the American Geriatrics Society (2015 Dec 23. doi: 101111/jgs.13836).
More U.S. adults over the age of 65 visited an emergency department because of an infectious disease than for myocardial infarction and congestive heart failure combined, according to a new study.
Based on a nationwide ED sample, adults over 65 visited the ED just over 3.1 million times in 2012 due to infectious diseases (ID), more than three times the estimated amount for myocardial infarction and congestive heart failure. The most common diagnoses were lower respiratory infections (26.2%), urinary tract infections (25.3%), and septicemia (18.9%).
Of the 3.1 million cases brought to the ED, nearly 1.8 million cases were hospitalized, with septicemia the most common cause for hospitalization, accounting for 32.2% of ID-related hospitalizations, followed by lower respiratory infections. Septicemia was also the most common cause of mortality, accounting for 74.7% of the nearly 124,000 deaths.
“These observations underscore the importance of integrated strategies aimed at reducing ID-related morbidity and health care use of elderly adults as a national priority for research, health policy, and community action,” Dr. Tadahiro Goto of the University of Fukui (Japan) Hospital and his associates concluded.
Find the full study in the Journal of the American Geriatrics Society (2015 Dec 23. doi: 101111/jgs.13836).
More U.S. adults over the age of 65 visited an emergency department because of an infectious disease than for myocardial infarction and congestive heart failure combined, according to a new study.
Based on a nationwide ED sample, adults over 65 visited the ED just over 3.1 million times in 2012 due to infectious diseases (ID), more than three times the estimated amount for myocardial infarction and congestive heart failure. The most common diagnoses were lower respiratory infections (26.2%), urinary tract infections (25.3%), and septicemia (18.9%).
Of the 3.1 million cases brought to the ED, nearly 1.8 million cases were hospitalized, with septicemia the most common cause for hospitalization, accounting for 32.2% of ID-related hospitalizations, followed by lower respiratory infections. Septicemia was also the most common cause of mortality, accounting for 74.7% of the nearly 124,000 deaths.
“These observations underscore the importance of integrated strategies aimed at reducing ID-related morbidity and health care use of elderly adults as a national priority for research, health policy, and community action,” Dr. Tadahiro Goto of the University of Fukui (Japan) Hospital and his associates concluded.
Find the full study in the Journal of the American Geriatrics Society (2015 Dec 23. doi: 101111/jgs.13836).
FROM JOURNAL OF THE AMERICAN GERIATRICS SOCIETY
Consider pyoderma gangrenosum for nonhealing wounds
LAS VEGAS – Though pyoderma gangrenosum and other neutrophilic skin disorders are rare, clinicians should include them in their differential, especially for nonhealing surgical wounds or skin “infections.”
Since these painful areas of ulceration need corticosteroid treatment, not antibiotics, for resolution, accurate diagnosis is critical for healing, Dr. J. Mark Jackson said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In a review of pyoderma gangrenosum (PG) and its cousins at the meeting, Dr. Jackson noted that the etiology of PG is unknown, but disordered neutrophilic chemotaxis is thought to be a factor. The many different manifestations of this disease are now collectively called the “neutrophilic dermatoses,” he said.
“Pyoderma gangrenosum is a very important diagnosis to consider in the differential diagnosis for nonhealing ulcerations, as suspicion and early recognition of this debilitating condition can prevent long-term sequelae such as pain, scarring, and long-term immunosuppressive medications,” said Dr. Jackson of the department of dermatology at the University of Louisville (Ky.).
The diagnosis should be suspected in the setting of a painful cutaneous ulcer with necrolysis. The border is typically irregular, violaceous, and undermined, he said, adding that this classic undermined border is caused by the sheets of neutrophils that characterize the disease.
Noting that half of patients with PG have underlying associated conditions such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and hematologic malignancies, Dr. Jackson emphasized that systemic disease associated with PG should heighten suspicion. “Histopathologic findings may be consistent with but not diagnostic of PG,” and can include a sterile dermal neutrophilia, with or without mixed inflammation and a lymphocytic vasculitis.
“Where you biopsy is important,” he continued, emphasizing that the biopsy must capture the margin of ulceration, where the sheets of neutrophils characteristic of PG will be seen on pathology.
Therapy consists of corticosteroids, with or without an immunosuppressive agent, and cessation of treatments that may continue to provoke pathergy.
Other diseases should also be considered in the differential diagnosis, including dangerous infectious causes, such as atypical mycobacteria, deep fungal infections, and staphylococcal and streptococcal infections. Squamous cell carcinoma, lymphoma, and leukemia may also present with similar lesions, as may metastatic Crohn’s disease, Dr. Jackson said. Several vasculitic and vasculopathic inflammatory conditions can also have similar appearances, including Wegener’s granulomatosis and vasoocclusive disorders such as peripheral vascular disease and cryoglobulinemia.
Classically, PG presents as painful ulcerated areas, most often on the lower extremities, that have a typical undermined border, caused by the sheets of neutrophils that characterize PG, he pointed out. PG may be mistaken for venous stasis ulcers, pressure ulcers, and cellulitis, but it doesn’t improve with antibiotics and mechanical manipulation from exfoliative dressings – and debridement may worsen the condition.
For susceptible individuals, surgery may provoke a pathergic response and trigger PG at the site of the surgical wound, and dogged attempts at conventional wound care may cause continued pathergy and begin a vicious cycle, Dr. Jackson said.
Peristomal pyoderma gangrenosum is a disease subcategory that may be seen in patients whose inflammatory bowel disease has been surgically treated and who have a stoma. Patients will have ulcerating lesions around their stoma site that are often misdiagnosed and treated as infections. Some wound care therapies, such as debridement, may continue to provoke the pathergic response and worsen peristomal PG, he said.
Though associated disease is seen in up to 50% of individuals with PG, there’s no predictable timeline linking the development of PG with the course of the associated disorder. In classic PG, usually occurring on the legs, autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis or another inflammatory arthritis, and paraproteinemia may be seen. Atypical PG, occurring more commonly on the upper extremities and face, is associated with myelogenous leukemia and preleukemic states, Dr. Jackson said.
Pyoderma gangrenosum lesions improve with corticosteroid administration. Depending on disease severity and location, topical, intralesional, or systemic steroids may be used.
Adjunctive treatments for PG and other neutrophilic dermatoses can include antibiotics with anti-inflammatory properties, such as minocycline or doxycycline, dapsone, and metronidazole. Immunosuppressives such as cyclosporine, azathioprine, and mycophenolate mofetil may also help speed resolution. In some cases, skin grafts may be necessary.
PG patients with Crohn’s disease or rheumatoid arthritis who are prescribed tumor necrosis factor–alpha (TNF-alpha) inhibitors for their systemic disease may also see improvement in PG lesions, Dr. Jackson said.
Other rare categories of neutrophilic dermatoses include Sweet’s syndrome, an acute febrile neutrophilic dermatosis, and neutrophilic dermatosis of the dorsum of the hand.
Neutrophilic invasion can also occur in other organs. “These extracutaneous lesions are also ‘sterile’ neutrophilic abscesses, which are often misdiagnosed as infections,” Dr. Jackson said. The most common site of extracutaneous neutrophilic infiltration is the lungs, though any organ system may be affected.
Dr. Jackson disclosed that he has received research support, honoraria, consulting fees, and other support from Abbvie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
The Skin Disease Education Foundation and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Though pyoderma gangrenosum and other neutrophilic skin disorders are rare, clinicians should include them in their differential, especially for nonhealing surgical wounds or skin “infections.”
Since these painful areas of ulceration need corticosteroid treatment, not antibiotics, for resolution, accurate diagnosis is critical for healing, Dr. J. Mark Jackson said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In a review of pyoderma gangrenosum (PG) and its cousins at the meeting, Dr. Jackson noted that the etiology of PG is unknown, but disordered neutrophilic chemotaxis is thought to be a factor. The many different manifestations of this disease are now collectively called the “neutrophilic dermatoses,” he said.
“Pyoderma gangrenosum is a very important diagnosis to consider in the differential diagnosis for nonhealing ulcerations, as suspicion and early recognition of this debilitating condition can prevent long-term sequelae such as pain, scarring, and long-term immunosuppressive medications,” said Dr. Jackson of the department of dermatology at the University of Louisville (Ky.).
The diagnosis should be suspected in the setting of a painful cutaneous ulcer with necrolysis. The border is typically irregular, violaceous, and undermined, he said, adding that this classic undermined border is caused by the sheets of neutrophils that characterize the disease.
Noting that half of patients with PG have underlying associated conditions such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and hematologic malignancies, Dr. Jackson emphasized that systemic disease associated with PG should heighten suspicion. “Histopathologic findings may be consistent with but not diagnostic of PG,” and can include a sterile dermal neutrophilia, with or without mixed inflammation and a lymphocytic vasculitis.
“Where you biopsy is important,” he continued, emphasizing that the biopsy must capture the margin of ulceration, where the sheets of neutrophils characteristic of PG will be seen on pathology.
Therapy consists of corticosteroids, with or without an immunosuppressive agent, and cessation of treatments that may continue to provoke pathergy.
Other diseases should also be considered in the differential diagnosis, including dangerous infectious causes, such as atypical mycobacteria, deep fungal infections, and staphylococcal and streptococcal infections. Squamous cell carcinoma, lymphoma, and leukemia may also present with similar lesions, as may metastatic Crohn’s disease, Dr. Jackson said. Several vasculitic and vasculopathic inflammatory conditions can also have similar appearances, including Wegener’s granulomatosis and vasoocclusive disorders such as peripheral vascular disease and cryoglobulinemia.
Classically, PG presents as painful ulcerated areas, most often on the lower extremities, that have a typical undermined border, caused by the sheets of neutrophils that characterize PG, he pointed out. PG may be mistaken for venous stasis ulcers, pressure ulcers, and cellulitis, but it doesn’t improve with antibiotics and mechanical manipulation from exfoliative dressings – and debridement may worsen the condition.
For susceptible individuals, surgery may provoke a pathergic response and trigger PG at the site of the surgical wound, and dogged attempts at conventional wound care may cause continued pathergy and begin a vicious cycle, Dr. Jackson said.
Peristomal pyoderma gangrenosum is a disease subcategory that may be seen in patients whose inflammatory bowel disease has been surgically treated and who have a stoma. Patients will have ulcerating lesions around their stoma site that are often misdiagnosed and treated as infections. Some wound care therapies, such as debridement, may continue to provoke the pathergic response and worsen peristomal PG, he said.
Though associated disease is seen in up to 50% of individuals with PG, there’s no predictable timeline linking the development of PG with the course of the associated disorder. In classic PG, usually occurring on the legs, autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis or another inflammatory arthritis, and paraproteinemia may be seen. Atypical PG, occurring more commonly on the upper extremities and face, is associated with myelogenous leukemia and preleukemic states, Dr. Jackson said.
Pyoderma gangrenosum lesions improve with corticosteroid administration. Depending on disease severity and location, topical, intralesional, or systemic steroids may be used.
Adjunctive treatments for PG and other neutrophilic dermatoses can include antibiotics with anti-inflammatory properties, such as minocycline or doxycycline, dapsone, and metronidazole. Immunosuppressives such as cyclosporine, azathioprine, and mycophenolate mofetil may also help speed resolution. In some cases, skin grafts may be necessary.
PG patients with Crohn’s disease or rheumatoid arthritis who are prescribed tumor necrosis factor–alpha (TNF-alpha) inhibitors for their systemic disease may also see improvement in PG lesions, Dr. Jackson said.
Other rare categories of neutrophilic dermatoses include Sweet’s syndrome, an acute febrile neutrophilic dermatosis, and neutrophilic dermatosis of the dorsum of the hand.
Neutrophilic invasion can also occur in other organs. “These extracutaneous lesions are also ‘sterile’ neutrophilic abscesses, which are often misdiagnosed as infections,” Dr. Jackson said. The most common site of extracutaneous neutrophilic infiltration is the lungs, though any organ system may be affected.
Dr. Jackson disclosed that he has received research support, honoraria, consulting fees, and other support from Abbvie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
The Skin Disease Education Foundation and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Though pyoderma gangrenosum and other neutrophilic skin disorders are rare, clinicians should include them in their differential, especially for nonhealing surgical wounds or skin “infections.”
Since these painful areas of ulceration need corticosteroid treatment, not antibiotics, for resolution, accurate diagnosis is critical for healing, Dr. J. Mark Jackson said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In a review of pyoderma gangrenosum (PG) and its cousins at the meeting, Dr. Jackson noted that the etiology of PG is unknown, but disordered neutrophilic chemotaxis is thought to be a factor. The many different manifestations of this disease are now collectively called the “neutrophilic dermatoses,” he said.
“Pyoderma gangrenosum is a very important diagnosis to consider in the differential diagnosis for nonhealing ulcerations, as suspicion and early recognition of this debilitating condition can prevent long-term sequelae such as pain, scarring, and long-term immunosuppressive medications,” said Dr. Jackson of the department of dermatology at the University of Louisville (Ky.).
The diagnosis should be suspected in the setting of a painful cutaneous ulcer with necrolysis. The border is typically irregular, violaceous, and undermined, he said, adding that this classic undermined border is caused by the sheets of neutrophils that characterize the disease.
Noting that half of patients with PG have underlying associated conditions such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and hematologic malignancies, Dr. Jackson emphasized that systemic disease associated with PG should heighten suspicion. “Histopathologic findings may be consistent with but not diagnostic of PG,” and can include a sterile dermal neutrophilia, with or without mixed inflammation and a lymphocytic vasculitis.
“Where you biopsy is important,” he continued, emphasizing that the biopsy must capture the margin of ulceration, where the sheets of neutrophils characteristic of PG will be seen on pathology.
Therapy consists of corticosteroids, with or without an immunosuppressive agent, and cessation of treatments that may continue to provoke pathergy.
Other diseases should also be considered in the differential diagnosis, including dangerous infectious causes, such as atypical mycobacteria, deep fungal infections, and staphylococcal and streptococcal infections. Squamous cell carcinoma, lymphoma, and leukemia may also present with similar lesions, as may metastatic Crohn’s disease, Dr. Jackson said. Several vasculitic and vasculopathic inflammatory conditions can also have similar appearances, including Wegener’s granulomatosis and vasoocclusive disorders such as peripheral vascular disease and cryoglobulinemia.
Classically, PG presents as painful ulcerated areas, most often on the lower extremities, that have a typical undermined border, caused by the sheets of neutrophils that characterize PG, he pointed out. PG may be mistaken for venous stasis ulcers, pressure ulcers, and cellulitis, but it doesn’t improve with antibiotics and mechanical manipulation from exfoliative dressings – and debridement may worsen the condition.
For susceptible individuals, surgery may provoke a pathergic response and trigger PG at the site of the surgical wound, and dogged attempts at conventional wound care may cause continued pathergy and begin a vicious cycle, Dr. Jackson said.
Peristomal pyoderma gangrenosum is a disease subcategory that may be seen in patients whose inflammatory bowel disease has been surgically treated and who have a stoma. Patients will have ulcerating lesions around their stoma site that are often misdiagnosed and treated as infections. Some wound care therapies, such as debridement, may continue to provoke the pathergic response and worsen peristomal PG, he said.
Though associated disease is seen in up to 50% of individuals with PG, there’s no predictable timeline linking the development of PG with the course of the associated disorder. In classic PG, usually occurring on the legs, autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis or another inflammatory arthritis, and paraproteinemia may be seen. Atypical PG, occurring more commonly on the upper extremities and face, is associated with myelogenous leukemia and preleukemic states, Dr. Jackson said.
Pyoderma gangrenosum lesions improve with corticosteroid administration. Depending on disease severity and location, topical, intralesional, or systemic steroids may be used.
Adjunctive treatments for PG and other neutrophilic dermatoses can include antibiotics with anti-inflammatory properties, such as minocycline or doxycycline, dapsone, and metronidazole. Immunosuppressives such as cyclosporine, azathioprine, and mycophenolate mofetil may also help speed resolution. In some cases, skin grafts may be necessary.
PG patients with Crohn’s disease or rheumatoid arthritis who are prescribed tumor necrosis factor–alpha (TNF-alpha) inhibitors for their systemic disease may also see improvement in PG lesions, Dr. Jackson said.
Other rare categories of neutrophilic dermatoses include Sweet’s syndrome, an acute febrile neutrophilic dermatosis, and neutrophilic dermatosis of the dorsum of the hand.
Neutrophilic invasion can also occur in other organs. “These extracutaneous lesions are also ‘sterile’ neutrophilic abscesses, which are often misdiagnosed as infections,” Dr. Jackson said. The most common site of extracutaneous neutrophilic infiltration is the lungs, though any organ system may be affected.
Dr. Jackson disclosed that he has received research support, honoraria, consulting fees, and other support from Abbvie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
The Skin Disease Education Foundation and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
LAIV, IIV almost equally effective against influenza
When vaccinating children against influenza, inactivated and live attenuated influenza vaccines show little significant difference in effectiveness against nearly all strains of the virus, according to a new study in Pediatrics.
However, the study – which examined the effectiveness of IIV and LAIV across four consecutive influenza seasons between 2010 and 2014 – cautions that the 2013-2014 season’s A/(H1N1)pdm09 showed an uncharacteristically large gap in effectiveness favoring IIV, a discrepancy likely due to a problem with a vaccine component in LAIV. (Pediatrics. 2016;137(2):e20153279)
“We found that lower LAIV effectiveness in 2013-2014 was specific to the A/(H1N1)pdm09 vaccine component and was consistent with a previously unexamined effect during the 2010-2011 influenza season,” Jessie R. Chung of the influenza division at the Centers for Disease Control and Prevention and associates wrote, adding that the impetus for the study was the lack of available data “from observational studies after the 2009 pandemic on relative effectiveness of LAIV and IIV in children and adolescents.”
Ms. Chung* and coinvestigators enrolled children aged 2-17 years from clinics and hospitals in Michigan, New York, Pennsylvania, Tennessee, Texas, Washington, and Wisconsin during the 2010-2011, 2011-2012, 2012-2013, and 2013-2014 influenza seasons. Children brought in with symptoms of acute respiratory illness – cough, fever, or feverishness – had nasal and throat swabs collected to test for presence and type of influenza.
In total, 7,718 subjects were evaluated across the four influenza seasons, but after excluding subjects for various reasons – unknown vaccine type, indeterminate vaccine status, and inconclusive reverse transcription polymerase chain reaction results, among others – 6,819 subjects were included for vaccine effectiveness analysis, of which 2,703 were ultimately matched age appropriately and placed into IIV and LAIV cohorts for comparison. The IIV cohort consisted of 2,066 individuals (76.4%), while the LAIV cohort had 637 (23.6%).
During the 2010-2011 season, 66 of the 477 IIV subjects contracted influenza, versus 21 of 116 who received LAIV (14% vs. 18%, respectively). In the 2011-2012 season, 51 of the 499 IIV subjects (10%) contracted influenza, compared with 12 of the 152 LAIV subjects (8%). In the 2012-2013 season, 198 of the 622 IIV subjects (32%) contracted influenza, versus 61 of the 205 LAIV subjects (30%). But, in the 2013-2014 season, 36 of the 468 IIV subjects (8%) contracted influenza, versus 34 of the 164 LAIV subjects (21%).
After adjustment for age and season, the odds ratio for the 2013-2014 season was significantly higher than those of the other seasons across the entire age spectrum of 2-17 years: 2.88, compared with 1.49 (2010-2011), 0.67 (2011-2012), and 0.92 (2012-2013).
When comparing influenza type/subtype, adjusted odds ratio was 5.53 for those with A/(H1N1)pdm09 in the 2010-2011 season, compared with 2.65 for those with the same in the 2013-2014 season. Those with A/H3N2 did not show as significant a difference across seasons (2010-2013), nor did those with influenza type B (2010-2011, 2012-2013).
“We found no statistically significant difference in LAIV effectiveness compared with IIV against medically attended, laboratory-confirmed influenza illness due to A/H3N2 or B viruses,” Ms. Chung and colleagues concluded. “We found significantly higher odds of influenza A/(H1N1)pdm09 among participants vaccinated with LAIV, compared with IIV, [but] reasons for lower effectiveness of LAIV against the A/(H1N1)pdm09 virus, compared with IIV are not fully understood.”
The investigators added that “the finding appears to be specific to the A/(H1N1)pdm09 vaccine component; we did not detect any statistically significant differences in effectiveness for the other components.” Three previous randomized controlled trials indicated that trivalent LAIV was just as effective, if not more so, than IIV, making the findings of this study surprising and “unexpected,” the authors noted.
This study was supported by the CDC through cooperative agreements with a variety of universities and foundations, and funded by the National Institutes of Health. Ms. Chung and associates reported no relevant financial disclosures.
*A previous version of this story misstated Jessie Chung’s academic title. Ms. Chung holds a Master’s in public health.
Influenza vaccination has been recommended for everyone for the past few years. Acceptance of this recommendation has been variable, and vaccine failures do not help the cause of convincing our patients to accept vaccination. In the paper by Chung et al. from the CDC and other coinvestigators who are prominent in influenza research, we learn that the live attenuated intranasally administered flu vaccine was significantly inferior to the killed injection administered flu vaccine for one of the type A flu strains. As a consequence, more kids vaccinated with the live attenuated vaccine got the flu. So parents who claim “the flu shot does not work” were partially correct more often since the 2009 flu season, if their child got the intranasal flu vaccine. However, neither the intranasal nor the injectable flu vaccine have an exceptionally high efficacy because the calculations by the authors for the study described and by citation of prior studies we are reminded that vaccine efficacy varies by strain and yearly by the season between 45% and 71%. We need to have better flu vaccines.

|
Dr. Michael E. Pichichero |
At Legacy Pediatrics, where I am in part-time private practice, we have seen increasing requests for the intranasal flu vaccine each year because parents and kids who can voice their wishes don’t want the shot. Our nurses like it, too, because the crying, wailing, and fighting to hold the kid down is avoided. There had been some reports before 2009 that the intranasal flu vaccine was more effective than the shot. But those of us who have been around long enough practicing medicine have learned about the pendulum of data and opinion sometimes swings back and forth. The article by Chung et al. reminds us once again of this reality.
Dr. Michael E. Pichichero is at the University of Rochester (N.Y.) Medical Center. He has received investigator-initiated grants from Sanofi Pasteur to study novel pneumococcal protein vaccines over the past 3 years and currently but has received no funding from Sanofi regarding injectable influenza vaccine. He also has conducted research with study coauthor Dr. John J. Treanor that was supported by MedImmune, who makes the intranasal flu vaccine.
Influenza vaccination has been recommended for everyone for the past few years. Acceptance of this recommendation has been variable, and vaccine failures do not help the cause of convincing our patients to accept vaccination. In the paper by Chung et al. from the CDC and other coinvestigators who are prominent in influenza research, we learn that the live attenuated intranasally administered flu vaccine was significantly inferior to the killed injection administered flu vaccine for one of the type A flu strains. As a consequence, more kids vaccinated with the live attenuated vaccine got the flu. So parents who claim “the flu shot does not work” were partially correct more often since the 2009 flu season, if their child got the intranasal flu vaccine. However, neither the intranasal nor the injectable flu vaccine have an exceptionally high efficacy because the calculations by the authors for the study described and by citation of prior studies we are reminded that vaccine efficacy varies by strain and yearly by the season between 45% and 71%. We need to have better flu vaccines.

|
Dr. Michael E. Pichichero |
At Legacy Pediatrics, where I am in part-time private practice, we have seen increasing requests for the intranasal flu vaccine each year because parents and kids who can voice their wishes don’t want the shot. Our nurses like it, too, because the crying, wailing, and fighting to hold the kid down is avoided. There had been some reports before 2009 that the intranasal flu vaccine was more effective than the shot. But those of us who have been around long enough practicing medicine have learned about the pendulum of data and opinion sometimes swings back and forth. The article by Chung et al. reminds us once again of this reality.
Dr. Michael E. Pichichero is at the University of Rochester (N.Y.) Medical Center. He has received investigator-initiated grants from Sanofi Pasteur to study novel pneumococcal protein vaccines over the past 3 years and currently but has received no funding from Sanofi regarding injectable influenza vaccine. He also has conducted research with study coauthor Dr. John J. Treanor that was supported by MedImmune, who makes the intranasal flu vaccine.
Influenza vaccination has been recommended for everyone for the past few years. Acceptance of this recommendation has been variable, and vaccine failures do not help the cause of convincing our patients to accept vaccination. In the paper by Chung et al. from the CDC and other coinvestigators who are prominent in influenza research, we learn that the live attenuated intranasally administered flu vaccine was significantly inferior to the killed injection administered flu vaccine for one of the type A flu strains. As a consequence, more kids vaccinated with the live attenuated vaccine got the flu. So parents who claim “the flu shot does not work” were partially correct more often since the 2009 flu season, if their child got the intranasal flu vaccine. However, neither the intranasal nor the injectable flu vaccine have an exceptionally high efficacy because the calculations by the authors for the study described and by citation of prior studies we are reminded that vaccine efficacy varies by strain and yearly by the season between 45% and 71%. We need to have better flu vaccines.

|
Dr. Michael E. Pichichero |
At Legacy Pediatrics, where I am in part-time private practice, we have seen increasing requests for the intranasal flu vaccine each year because parents and kids who can voice their wishes don’t want the shot. Our nurses like it, too, because the crying, wailing, and fighting to hold the kid down is avoided. There had been some reports before 2009 that the intranasal flu vaccine was more effective than the shot. But those of us who have been around long enough practicing medicine have learned about the pendulum of data and opinion sometimes swings back and forth. The article by Chung et al. reminds us once again of this reality.
Dr. Michael E. Pichichero is at the University of Rochester (N.Y.) Medical Center. He has received investigator-initiated grants from Sanofi Pasteur to study novel pneumococcal protein vaccines over the past 3 years and currently but has received no funding from Sanofi regarding injectable influenza vaccine. He also has conducted research with study coauthor Dr. John J. Treanor that was supported by MedImmune, who makes the intranasal flu vaccine.
When vaccinating children against influenza, inactivated and live attenuated influenza vaccines show little significant difference in effectiveness against nearly all strains of the virus, according to a new study in Pediatrics.
However, the study – which examined the effectiveness of IIV and LAIV across four consecutive influenza seasons between 2010 and 2014 – cautions that the 2013-2014 season’s A/(H1N1)pdm09 showed an uncharacteristically large gap in effectiveness favoring IIV, a discrepancy likely due to a problem with a vaccine component in LAIV. (Pediatrics. 2016;137(2):e20153279)
“We found that lower LAIV effectiveness in 2013-2014 was specific to the A/(H1N1)pdm09 vaccine component and was consistent with a previously unexamined effect during the 2010-2011 influenza season,” Jessie R. Chung of the influenza division at the Centers for Disease Control and Prevention and associates wrote, adding that the impetus for the study was the lack of available data “from observational studies after the 2009 pandemic on relative effectiveness of LAIV and IIV in children and adolescents.”
Ms. Chung* and coinvestigators enrolled children aged 2-17 years from clinics and hospitals in Michigan, New York, Pennsylvania, Tennessee, Texas, Washington, and Wisconsin during the 2010-2011, 2011-2012, 2012-2013, and 2013-2014 influenza seasons. Children brought in with symptoms of acute respiratory illness – cough, fever, or feverishness – had nasal and throat swabs collected to test for presence and type of influenza.
In total, 7,718 subjects were evaluated across the four influenza seasons, but after excluding subjects for various reasons – unknown vaccine type, indeterminate vaccine status, and inconclusive reverse transcription polymerase chain reaction results, among others – 6,819 subjects were included for vaccine effectiveness analysis, of which 2,703 were ultimately matched age appropriately and placed into IIV and LAIV cohorts for comparison. The IIV cohort consisted of 2,066 individuals (76.4%), while the LAIV cohort had 637 (23.6%).
During the 2010-2011 season, 66 of the 477 IIV subjects contracted influenza, versus 21 of 116 who received LAIV (14% vs. 18%, respectively). In the 2011-2012 season, 51 of the 499 IIV subjects (10%) contracted influenza, compared with 12 of the 152 LAIV subjects (8%). In the 2012-2013 season, 198 of the 622 IIV subjects (32%) contracted influenza, versus 61 of the 205 LAIV subjects (30%). But, in the 2013-2014 season, 36 of the 468 IIV subjects (8%) contracted influenza, versus 34 of the 164 LAIV subjects (21%).
After adjustment for age and season, the odds ratio for the 2013-2014 season was significantly higher than those of the other seasons across the entire age spectrum of 2-17 years: 2.88, compared with 1.49 (2010-2011), 0.67 (2011-2012), and 0.92 (2012-2013).
When comparing influenza type/subtype, adjusted odds ratio was 5.53 for those with A/(H1N1)pdm09 in the 2010-2011 season, compared with 2.65 for those with the same in the 2013-2014 season. Those with A/H3N2 did not show as significant a difference across seasons (2010-2013), nor did those with influenza type B (2010-2011, 2012-2013).
“We found no statistically significant difference in LAIV effectiveness compared with IIV against medically attended, laboratory-confirmed influenza illness due to A/H3N2 or B viruses,” Ms. Chung and colleagues concluded. “We found significantly higher odds of influenza A/(H1N1)pdm09 among participants vaccinated with LAIV, compared with IIV, [but] reasons for lower effectiveness of LAIV against the A/(H1N1)pdm09 virus, compared with IIV are not fully understood.”
The investigators added that “the finding appears to be specific to the A/(H1N1)pdm09 vaccine component; we did not detect any statistically significant differences in effectiveness for the other components.” Three previous randomized controlled trials indicated that trivalent LAIV was just as effective, if not more so, than IIV, making the findings of this study surprising and “unexpected,” the authors noted.
This study was supported by the CDC through cooperative agreements with a variety of universities and foundations, and funded by the National Institutes of Health. Ms. Chung and associates reported no relevant financial disclosures.
*A previous version of this story misstated Jessie Chung’s academic title. Ms. Chung holds a Master’s in public health.
When vaccinating children against influenza, inactivated and live attenuated influenza vaccines show little significant difference in effectiveness against nearly all strains of the virus, according to a new study in Pediatrics.
However, the study – which examined the effectiveness of IIV and LAIV across four consecutive influenza seasons between 2010 and 2014 – cautions that the 2013-2014 season’s A/(H1N1)pdm09 showed an uncharacteristically large gap in effectiveness favoring IIV, a discrepancy likely due to a problem with a vaccine component in LAIV. (Pediatrics. 2016;137(2):e20153279)
“We found that lower LAIV effectiveness in 2013-2014 was specific to the A/(H1N1)pdm09 vaccine component and was consistent with a previously unexamined effect during the 2010-2011 influenza season,” Jessie R. Chung of the influenza division at the Centers for Disease Control and Prevention and associates wrote, adding that the impetus for the study was the lack of available data “from observational studies after the 2009 pandemic on relative effectiveness of LAIV and IIV in children and adolescents.”
Ms. Chung* and coinvestigators enrolled children aged 2-17 years from clinics and hospitals in Michigan, New York, Pennsylvania, Tennessee, Texas, Washington, and Wisconsin during the 2010-2011, 2011-2012, 2012-2013, and 2013-2014 influenza seasons. Children brought in with symptoms of acute respiratory illness – cough, fever, or feverishness – had nasal and throat swabs collected to test for presence and type of influenza.
In total, 7,718 subjects were evaluated across the four influenza seasons, but after excluding subjects for various reasons – unknown vaccine type, indeterminate vaccine status, and inconclusive reverse transcription polymerase chain reaction results, among others – 6,819 subjects were included for vaccine effectiveness analysis, of which 2,703 were ultimately matched age appropriately and placed into IIV and LAIV cohorts for comparison. The IIV cohort consisted of 2,066 individuals (76.4%), while the LAIV cohort had 637 (23.6%).
During the 2010-2011 season, 66 of the 477 IIV subjects contracted influenza, versus 21 of 116 who received LAIV (14% vs. 18%, respectively). In the 2011-2012 season, 51 of the 499 IIV subjects (10%) contracted influenza, compared with 12 of the 152 LAIV subjects (8%). In the 2012-2013 season, 198 of the 622 IIV subjects (32%) contracted influenza, versus 61 of the 205 LAIV subjects (30%). But, in the 2013-2014 season, 36 of the 468 IIV subjects (8%) contracted influenza, versus 34 of the 164 LAIV subjects (21%).
After adjustment for age and season, the odds ratio for the 2013-2014 season was significantly higher than those of the other seasons across the entire age spectrum of 2-17 years: 2.88, compared with 1.49 (2010-2011), 0.67 (2011-2012), and 0.92 (2012-2013).
When comparing influenza type/subtype, adjusted odds ratio was 5.53 for those with A/(H1N1)pdm09 in the 2010-2011 season, compared with 2.65 for those with the same in the 2013-2014 season. Those with A/H3N2 did not show as significant a difference across seasons (2010-2013), nor did those with influenza type B (2010-2011, 2012-2013).
“We found no statistically significant difference in LAIV effectiveness compared with IIV against medically attended, laboratory-confirmed influenza illness due to A/H3N2 or B viruses,” Ms. Chung and colleagues concluded. “We found significantly higher odds of influenza A/(H1N1)pdm09 among participants vaccinated with LAIV, compared with IIV, [but] reasons for lower effectiveness of LAIV against the A/(H1N1)pdm09 virus, compared with IIV are not fully understood.”
The investigators added that “the finding appears to be specific to the A/(H1N1)pdm09 vaccine component; we did not detect any statistically significant differences in effectiveness for the other components.” Three previous randomized controlled trials indicated that trivalent LAIV was just as effective, if not more so, than IIV, making the findings of this study surprising and “unexpected,” the authors noted.
This study was supported by the CDC through cooperative agreements with a variety of universities and foundations, and funded by the National Institutes of Health. Ms. Chung and associates reported no relevant financial disclosures.
*A previous version of this story misstated Jessie Chung’s academic title. Ms. Chung holds a Master’s in public health.
FROM PEDIATRICS
Key clinical point: Inactivated influenza vaccine was significantly more effective against at least one strain of influenza than live attenuated influenza vaccine in 2013-2014.
Major finding: While no significant differences were seen in influenza rates between the IIV and LAIV cohorts for three consecutive seasons (2010-2013), the A/(H1N1)pdm09 strain of 2013-2014 affected subjects with LAIV at a significantly higher rate than did those with IIV.
Data source: Prospective cohort study of 2,703 children aged 2-17 years vaccinated between 2010 and 2014 with either IIV or LAIV.
Disclosures: This study was supported by the CDC through cooperative agreements with a variety of universities and foundations, and funded by the National Institutes of Health. Dr. Chung and his associates reported no relevant financial disclosures.
Novel agent for adult GH can be administered once weekly
Use of a novel reversible albumin-binding human growth hormone (GH) derivative administered subcutaneously once weekly for 4 weeks was safe and effective in adults with growth hormone deficiency, according to a phase I, randomized, open-label trial.
Results from a recent clinical trial of the agent, known as NNC0195-0092 and being developed by Norvo Nordisk, indicated the feasibility of a once-weekly dosing regimen in healthy men (J Clin Endocrinol Metab. 2014;99:E1819-29). The purpose of the current study was to report the first data obtained from a multiple-dose trial of NNC0195-0092 conducted in men and women at three hospitals in Denmark and one in Sweden.
“GH is currently administered as daily subcutaneous injections; however, a long-acting GH formulation that decreases injection frequency may improve treatment adherence and reduce the inconvenience associated with daily injections,” researchers led by Dr. Michael Højby Rasmussen wrote in the article published online Jan. 4 in the Journal of Clinical Endocrinology and Metabolism (2016. doi: 10.1210/jc.2015-1991). They went on to note that the plasma half-life of therapeutic peptides such as GH can be extended through binding to serum albumin, which “has a high affinity and binding capacity for fatty acids, and acylation of fatty acids to therapeutic proteins has been used to facilitate binding of these molecules to circulating albumin. In NNC0195-0092, fatty acids with noncovalent albumin-binding properties have been attached by acylation.”
Dr. Rasmussen of Novo Nordisk, Denmark, and his associates reported results from 25 men and nine women with a mean age of 53 years who were assigned into four cohorts of eight subjects and randomized to receive once-weekly NNC0195-0092 for 4 weeks in doses that ranged from 0.02 to 0.12 mg/kg, or daily injections of Norditropin NordiFlex for 4 weeks with a dose replicating the pretrial dose of somatropin. They found that the number of adverse events was similar at the 0.02, 0.04, and 0.08 mg/kg doses of NNC0195-0092, compared with the daily injections of Norditropin NordiFlex, while the number of adverse events was greatest at the 0.12 mg/kg dose of NNC0195-0092.
“No clinically significant safety and tolerability signals causally related to NNC0195-0092 were identified, nor were any immunogenicity concerns revealed,” the investigators concluded. “The IGF-I profiles were consistent with a once-weekly treatment profile of NNC0195-0092 at a starting dose of 0.02-0.04 mg/kg/wk.”
The trial was supported by Novo Nordisk. Dr. Rasmussen disclosed that he is an employee of the company.
Use of a novel reversible albumin-binding human growth hormone (GH) derivative administered subcutaneously once weekly for 4 weeks was safe and effective in adults with growth hormone deficiency, according to a phase I, randomized, open-label trial.
Results from a recent clinical trial of the agent, known as NNC0195-0092 and being developed by Norvo Nordisk, indicated the feasibility of a once-weekly dosing regimen in healthy men (J Clin Endocrinol Metab. 2014;99:E1819-29). The purpose of the current study was to report the first data obtained from a multiple-dose trial of NNC0195-0092 conducted in men and women at three hospitals in Denmark and one in Sweden.
“GH is currently administered as daily subcutaneous injections; however, a long-acting GH formulation that decreases injection frequency may improve treatment adherence and reduce the inconvenience associated with daily injections,” researchers led by Dr. Michael Højby Rasmussen wrote in the article published online Jan. 4 in the Journal of Clinical Endocrinology and Metabolism (2016. doi: 10.1210/jc.2015-1991). They went on to note that the plasma half-life of therapeutic peptides such as GH can be extended through binding to serum albumin, which “has a high affinity and binding capacity for fatty acids, and acylation of fatty acids to therapeutic proteins has been used to facilitate binding of these molecules to circulating albumin. In NNC0195-0092, fatty acids with noncovalent albumin-binding properties have been attached by acylation.”
Dr. Rasmussen of Novo Nordisk, Denmark, and his associates reported results from 25 men and nine women with a mean age of 53 years who were assigned into four cohorts of eight subjects and randomized to receive once-weekly NNC0195-0092 for 4 weeks in doses that ranged from 0.02 to 0.12 mg/kg, or daily injections of Norditropin NordiFlex for 4 weeks with a dose replicating the pretrial dose of somatropin. They found that the number of adverse events was similar at the 0.02, 0.04, and 0.08 mg/kg doses of NNC0195-0092, compared with the daily injections of Norditropin NordiFlex, while the number of adverse events was greatest at the 0.12 mg/kg dose of NNC0195-0092.
“No clinically significant safety and tolerability signals causally related to NNC0195-0092 were identified, nor were any immunogenicity concerns revealed,” the investigators concluded. “The IGF-I profiles were consistent with a once-weekly treatment profile of NNC0195-0092 at a starting dose of 0.02-0.04 mg/kg/wk.”
The trial was supported by Novo Nordisk. Dr. Rasmussen disclosed that he is an employee of the company.
Use of a novel reversible albumin-binding human growth hormone (GH) derivative administered subcutaneously once weekly for 4 weeks was safe and effective in adults with growth hormone deficiency, according to a phase I, randomized, open-label trial.
Results from a recent clinical trial of the agent, known as NNC0195-0092 and being developed by Norvo Nordisk, indicated the feasibility of a once-weekly dosing regimen in healthy men (J Clin Endocrinol Metab. 2014;99:E1819-29). The purpose of the current study was to report the first data obtained from a multiple-dose trial of NNC0195-0092 conducted in men and women at three hospitals in Denmark and one in Sweden.
“GH is currently administered as daily subcutaneous injections; however, a long-acting GH formulation that decreases injection frequency may improve treatment adherence and reduce the inconvenience associated with daily injections,” researchers led by Dr. Michael Højby Rasmussen wrote in the article published online Jan. 4 in the Journal of Clinical Endocrinology and Metabolism (2016. doi: 10.1210/jc.2015-1991). They went on to note that the plasma half-life of therapeutic peptides such as GH can be extended through binding to serum albumin, which “has a high affinity and binding capacity for fatty acids, and acylation of fatty acids to therapeutic proteins has been used to facilitate binding of these molecules to circulating albumin. In NNC0195-0092, fatty acids with noncovalent albumin-binding properties have been attached by acylation.”
Dr. Rasmussen of Novo Nordisk, Denmark, and his associates reported results from 25 men and nine women with a mean age of 53 years who were assigned into four cohorts of eight subjects and randomized to receive once-weekly NNC0195-0092 for 4 weeks in doses that ranged from 0.02 to 0.12 mg/kg, or daily injections of Norditropin NordiFlex for 4 weeks with a dose replicating the pretrial dose of somatropin. They found that the number of adverse events was similar at the 0.02, 0.04, and 0.08 mg/kg doses of NNC0195-0092, compared with the daily injections of Norditropin NordiFlex, while the number of adverse events was greatest at the 0.12 mg/kg dose of NNC0195-0092.
“No clinically significant safety and tolerability signals causally related to NNC0195-0092 were identified, nor were any immunogenicity concerns revealed,” the investigators concluded. “The IGF-I profiles were consistent with a once-weekly treatment profile of NNC0195-0092 at a starting dose of 0.02-0.04 mg/kg/wk.”
The trial was supported by Novo Nordisk. Dr. Rasmussen disclosed that he is an employee of the company.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Key clinical point: Four once-weekly doses of NNC0195-0092 administered to patients with adult growth hormone deficiency were well tolerated.
Major finding: The number of adverse events was similar at the 0.02, 0.04, and 0.08 mg/kg doses of NNC0195-0092, compared with the daily injections of Norditropin NordiFlex, while the number of adverse events was greatest at the 0.12 mg/kg dose of NNC0195-0092.
Data source: A phase I, open-label, randomized study that set out to evaluate the safety and tolerability of multiple once-weekly doses of NNC0195-0092, compared with daily GH in 34 patients with adult growth hormone deficiency.
Disclosures: The trial was supported by Novo Nordisk. Dr. Rasmussen disclosed that he is an employee of the company.
Reconstructive Shelf Arthroplasty as a Salvage Procedure for Complex Fifth Tarsometatarsal Joint Complex Injuries: A Case Review and Discussion
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
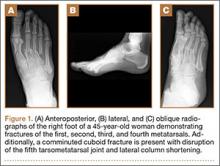
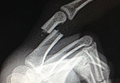
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
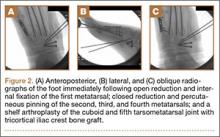
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
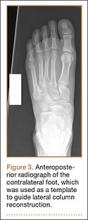
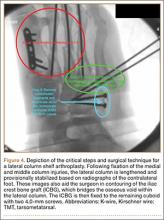
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
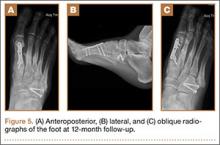
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
Royal jelly
Used for centuries by humans for its health-promoting qualities, royal jelly is a yellowish, viscous secretion from the hypopharyngeal and mandibular glands of worker bees that nourishes bee larvae of all kinds (i.e., drones, workers, queens) after which it becomes the exclusive nourishment for queens throughout their development.1-3 A wide range of biologic activity has been attributed to royal jelly, including antitumor, antibacterial, anti-inflammatory, antioxidant, collagen production-promoting, immunomodulatory, and wound healing.3-8 Royal jelly is used in cosmetics, health tonics (particularly in Asia), dietary supplements, and beverages.2,9
Produced from pollen, royal jelly contains water, proteins (82%-90% of which are known as the major royal jelly proteins, with five primary members), lipids – including its primary unsaturated fatty acid, 10-hydroxy-2-decenoic acid (10-HDA) – sugars, carbohydrates, free amino acids, vitamins, and minerals.4,7,10 Many of the benefits to human health linked to royal jelly can be partly attributed to the activity of its lipids, particularly 10-HDA, which render the royal jelly emulsion highly acidic and impart antimicrobial properties.10 These and other constituents of royal jelly operate in ways that are thought to yield broad protection against skin aging and cancer development, modulation of the immune system, induction of neurogenesis, and alleviation of menopausal symptoms.1 This column will focus on recent studies pertaining to the topical use of royal jelly.
Wound healing
In 2008, Abdelatif et al. conducted a pilot study to determine the safety and effectiveness of a then-new ointment combining royal jelly and panthenol (Pedyphar) in 60 patients with limb-threatening diabetic foot infections. After 9 weeks of treatment and through 6 months of follow-up, 96% of subjects with full-thickness skin ulcers (Wagner grades 1 and 2) or deep tissue infection and suspected osteomyelitis (grade 3) responded well, with all grade 1 and 2 ulcers healing and 92% of grade 3 ulcers healing. All patients with gangrenous lesions (grades 4 and 5) healed after surgical excision, debridement, and conservative treatment with the royal jelly/panthenol product. The researchers called for more double-blind, randomized controlled studies to confirm their promising findings of the safety and efficacy of the royal jelly/panthenol combination.11
Two years later, Kim et al. treated freshly scratched normal human dermal fibroblasts with different concentrations of royal jelly (0.1 mg/mL, 1.0 mg/mL, or 5 mg/mL) for up to 48 hours. Fibroblast migration was found to have peaked at 24 hours after wound induction, with royal jelly significantly and dose-dependently accelerating the migration at the 8-hour mark. Royal jelly also influenced several fibroblast lipids involved in the wound healing process, with a decrease in cholesterol level and an increase in sphinganines.12
A small study with eight subjects was done in 2011 by Siavash et al. to evaluate the efficacy of topically applied royal jelly for diabetic foot ulcers. Seven of the eight ulcers treated healed, with a mean healing time of 41 days. The eighth ulcer improved, diminishing significantly in size. The researchers concluded that a royal jelly dressing is an effective alternative for treatment of diabetic foot ulcers.13 However, the same team conducted a double-blind, placebo-controlled clinical trial of topical royal jelly on diabetic foot ulcers in 25 patients (6 females, 19 males) and found no significant differences between 5% sterile topical royal jelly or placebo.6
Collagen production
A decade ago, Koya-Miyata et al. showed that royal jelly promotes collagen synthesis by skin fibroblasts in the presence of ascorbic acid-2-O-alpha-glucoside. They also showed that its primary fatty acid constituent, 10-HDA, facilitates the collagen production by fibroblasts treated with ascorbic acid-2-O-alpha-glucoside through activation of transforming growth factor-beta 1 production.5
Photoprotection
Park et al. measured the 10-HDA content of royal jelly in 2011 and studied its effects on UVB-induced skin photoaging in normal human dermal fibroblasts. The introduction of royal jelly (0.211% 10-HDA) promoted the production of procollagen type I and transforming growth factor (TGF)-beta-1 without affecting matrix metalloproteinase (MMP)-1 levels. The investigators concluded that the impact of royal jelly on collagen production positioned the bee product as a potential photoprotectant against UVB-induced photoaging.14 The next year, Park et al. observed that the production of type I collagen in the dorsal skin of ovariectomized Sprague-Dawley rats was enhanced by the dietary supplementation of 1% royal jelly extract. Although MMP-1 levels were unaffected, the investigators speculated that the effects on collagen synthesis alone were sufficient for royal jelly to provide anti-aging activity.4
In 2013, Zheng et al. found that 10-HDA significantly protected fibroblasts from UVA-induced cytotoxicity, reactive oxygen species, and cellular senescence. They also noted that 10-HDA inhibited the UVA-generated expression of MMP-1 and -3, and stimulated collagen production. Treatment with 10-HDA also reduced the activation of the c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways. The researchers concluded that this royal jelly fatty acid appears to be a promising agent for the prevention and treatment of cutaneous photoaging.8
Skin whitening
In 2011, Han et al. reported that royal jelly dose-dependently inhibited melanin biosynthesis in the B16F1 mouse melanocyte cell line by reducing tyrosinase activity. Royal jelly also lowered mRNA levels of tyrosinase. The investigators concluded that royal jelly may be a viable option in the skin-lightening arsenal.3
Safety
There are some reports of contact dermatitis from the use of topical royal jelly.15 Far more significant, while rare, adverse reactions have been linked to oral use of royal jelly, including acute asthma, anaphylaxis, and even death.2,16,17
Conclusion
Royal jelly is one of several bee products found to have beneficial health effects in humans. Various dermatologic applications of royal jelly have been employed in recent decades. More research is necessary, though, to determine just how useful this bee product may be for a range of cutaneous conditions.
References
1. J Med Food. 2013;16(2):96-102.
2. Biosci Biotechnol Biochem. 2013;77(4):789-95.
3. Am J Chin Med. 2011;39(6):1253-60.
4. J Med Food. 2012;15(6):568-75.
5. Biosci Biotechnol Biochem. 2004 Apr;68(4):767-73.
6. Int Wound J. 2015;12(2):137-42.
7. J Food Sci. 2008 Nov;73(9):R117-24.
8. J Eur Acad Dermatol. Venereol. 2013;27(10):1269-77.
9. Pharmacogn Mag. 2013;9(33):9-13.
11. J Wound Care. 2008;17(3):108-10.
12. Nutr Res Pract. 2010;4(5):362-8.
13. J Res Med Sci. 2011;16(7):904-9.
14. J Med Food. 2011;14(9):899-906.
15. Contact Dermatitis. 1983;9(6):452-5.
16. Trop Biomed. 2008;25(3):243-51.
17. J Dermatol. 2011;38(11):1079-81.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Used for centuries by humans for its health-promoting qualities, royal jelly is a yellowish, viscous secretion from the hypopharyngeal and mandibular glands of worker bees that nourishes bee larvae of all kinds (i.e., drones, workers, queens) after which it becomes the exclusive nourishment for queens throughout their development.1-3 A wide range of biologic activity has been attributed to royal jelly, including antitumor, antibacterial, anti-inflammatory, antioxidant, collagen production-promoting, immunomodulatory, and wound healing.3-8 Royal jelly is used in cosmetics, health tonics (particularly in Asia), dietary supplements, and beverages.2,9
Produced from pollen, royal jelly contains water, proteins (82%-90% of which are known as the major royal jelly proteins, with five primary members), lipids – including its primary unsaturated fatty acid, 10-hydroxy-2-decenoic acid (10-HDA) – sugars, carbohydrates, free amino acids, vitamins, and minerals.4,7,10 Many of the benefits to human health linked to royal jelly can be partly attributed to the activity of its lipids, particularly 10-HDA, which render the royal jelly emulsion highly acidic and impart antimicrobial properties.10 These and other constituents of royal jelly operate in ways that are thought to yield broad protection against skin aging and cancer development, modulation of the immune system, induction of neurogenesis, and alleviation of menopausal symptoms.1 This column will focus on recent studies pertaining to the topical use of royal jelly.
Wound healing
In 2008, Abdelatif et al. conducted a pilot study to determine the safety and effectiveness of a then-new ointment combining royal jelly and panthenol (Pedyphar) in 60 patients with limb-threatening diabetic foot infections. After 9 weeks of treatment and through 6 months of follow-up, 96% of subjects with full-thickness skin ulcers (Wagner grades 1 and 2) or deep tissue infection and suspected osteomyelitis (grade 3) responded well, with all grade 1 and 2 ulcers healing and 92% of grade 3 ulcers healing. All patients with gangrenous lesions (grades 4 and 5) healed after surgical excision, debridement, and conservative treatment with the royal jelly/panthenol product. The researchers called for more double-blind, randomized controlled studies to confirm their promising findings of the safety and efficacy of the royal jelly/panthenol combination.11
Two years later, Kim et al. treated freshly scratched normal human dermal fibroblasts with different concentrations of royal jelly (0.1 mg/mL, 1.0 mg/mL, or 5 mg/mL) for up to 48 hours. Fibroblast migration was found to have peaked at 24 hours after wound induction, with royal jelly significantly and dose-dependently accelerating the migration at the 8-hour mark. Royal jelly also influenced several fibroblast lipids involved in the wound healing process, with a decrease in cholesterol level and an increase in sphinganines.12
A small study with eight subjects was done in 2011 by Siavash et al. to evaluate the efficacy of topically applied royal jelly for diabetic foot ulcers. Seven of the eight ulcers treated healed, with a mean healing time of 41 days. The eighth ulcer improved, diminishing significantly in size. The researchers concluded that a royal jelly dressing is an effective alternative for treatment of diabetic foot ulcers.13 However, the same team conducted a double-blind, placebo-controlled clinical trial of topical royal jelly on diabetic foot ulcers in 25 patients (6 females, 19 males) and found no significant differences between 5% sterile topical royal jelly or placebo.6
Collagen production
A decade ago, Koya-Miyata et al. showed that royal jelly promotes collagen synthesis by skin fibroblasts in the presence of ascorbic acid-2-O-alpha-glucoside. They also showed that its primary fatty acid constituent, 10-HDA, facilitates the collagen production by fibroblasts treated with ascorbic acid-2-O-alpha-glucoside through activation of transforming growth factor-beta 1 production.5
Photoprotection
Park et al. measured the 10-HDA content of royal jelly in 2011 and studied its effects on UVB-induced skin photoaging in normal human dermal fibroblasts. The introduction of royal jelly (0.211% 10-HDA) promoted the production of procollagen type I and transforming growth factor (TGF)-beta-1 without affecting matrix metalloproteinase (MMP)-1 levels. The investigators concluded that the impact of royal jelly on collagen production positioned the bee product as a potential photoprotectant against UVB-induced photoaging.14 The next year, Park et al. observed that the production of type I collagen in the dorsal skin of ovariectomized Sprague-Dawley rats was enhanced by the dietary supplementation of 1% royal jelly extract. Although MMP-1 levels were unaffected, the investigators speculated that the effects on collagen synthesis alone were sufficient for royal jelly to provide anti-aging activity.4
In 2013, Zheng et al. found that 10-HDA significantly protected fibroblasts from UVA-induced cytotoxicity, reactive oxygen species, and cellular senescence. They also noted that 10-HDA inhibited the UVA-generated expression of MMP-1 and -3, and stimulated collagen production. Treatment with 10-HDA also reduced the activation of the c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways. The researchers concluded that this royal jelly fatty acid appears to be a promising agent for the prevention and treatment of cutaneous photoaging.8
Skin whitening
In 2011, Han et al. reported that royal jelly dose-dependently inhibited melanin biosynthesis in the B16F1 mouse melanocyte cell line by reducing tyrosinase activity. Royal jelly also lowered mRNA levels of tyrosinase. The investigators concluded that royal jelly may be a viable option in the skin-lightening arsenal.3
Safety
There are some reports of contact dermatitis from the use of topical royal jelly.15 Far more significant, while rare, adverse reactions have been linked to oral use of royal jelly, including acute asthma, anaphylaxis, and even death.2,16,17
Conclusion
Royal jelly is one of several bee products found to have beneficial health effects in humans. Various dermatologic applications of royal jelly have been employed in recent decades. More research is necessary, though, to determine just how useful this bee product may be for a range of cutaneous conditions.
References
1. J Med Food. 2013;16(2):96-102.
2. Biosci Biotechnol Biochem. 2013;77(4):789-95.
3. Am J Chin Med. 2011;39(6):1253-60.
4. J Med Food. 2012;15(6):568-75.
5. Biosci Biotechnol Biochem. 2004 Apr;68(4):767-73.
6. Int Wound J. 2015;12(2):137-42.
7. J Food Sci. 2008 Nov;73(9):R117-24.
8. J Eur Acad Dermatol. Venereol. 2013;27(10):1269-77.
9. Pharmacogn Mag. 2013;9(33):9-13.
11. J Wound Care. 2008;17(3):108-10.
12. Nutr Res Pract. 2010;4(5):362-8.
13. J Res Med Sci. 2011;16(7):904-9.
14. J Med Food. 2011;14(9):899-906.
15. Contact Dermatitis. 1983;9(6):452-5.
16. Trop Biomed. 2008;25(3):243-51.
17. J Dermatol. 2011;38(11):1079-81.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Used for centuries by humans for its health-promoting qualities, royal jelly is a yellowish, viscous secretion from the hypopharyngeal and mandibular glands of worker bees that nourishes bee larvae of all kinds (i.e., drones, workers, queens) after which it becomes the exclusive nourishment for queens throughout their development.1-3 A wide range of biologic activity has been attributed to royal jelly, including antitumor, antibacterial, anti-inflammatory, antioxidant, collagen production-promoting, immunomodulatory, and wound healing.3-8 Royal jelly is used in cosmetics, health tonics (particularly in Asia), dietary supplements, and beverages.2,9
Produced from pollen, royal jelly contains water, proteins (82%-90% of which are known as the major royal jelly proteins, with five primary members), lipids – including its primary unsaturated fatty acid, 10-hydroxy-2-decenoic acid (10-HDA) – sugars, carbohydrates, free amino acids, vitamins, and minerals.4,7,10 Many of the benefits to human health linked to royal jelly can be partly attributed to the activity of its lipids, particularly 10-HDA, which render the royal jelly emulsion highly acidic and impart antimicrobial properties.10 These and other constituents of royal jelly operate in ways that are thought to yield broad protection against skin aging and cancer development, modulation of the immune system, induction of neurogenesis, and alleviation of menopausal symptoms.1 This column will focus on recent studies pertaining to the topical use of royal jelly.
Wound healing
In 2008, Abdelatif et al. conducted a pilot study to determine the safety and effectiveness of a then-new ointment combining royal jelly and panthenol (Pedyphar) in 60 patients with limb-threatening diabetic foot infections. After 9 weeks of treatment and through 6 months of follow-up, 96% of subjects with full-thickness skin ulcers (Wagner grades 1 and 2) or deep tissue infection and suspected osteomyelitis (grade 3) responded well, with all grade 1 and 2 ulcers healing and 92% of grade 3 ulcers healing. All patients with gangrenous lesions (grades 4 and 5) healed after surgical excision, debridement, and conservative treatment with the royal jelly/panthenol product. The researchers called for more double-blind, randomized controlled studies to confirm their promising findings of the safety and efficacy of the royal jelly/panthenol combination.11
Two years later, Kim et al. treated freshly scratched normal human dermal fibroblasts with different concentrations of royal jelly (0.1 mg/mL, 1.0 mg/mL, or 5 mg/mL) for up to 48 hours. Fibroblast migration was found to have peaked at 24 hours after wound induction, with royal jelly significantly and dose-dependently accelerating the migration at the 8-hour mark. Royal jelly also influenced several fibroblast lipids involved in the wound healing process, with a decrease in cholesterol level and an increase in sphinganines.12
A small study with eight subjects was done in 2011 by Siavash et al. to evaluate the efficacy of topically applied royal jelly for diabetic foot ulcers. Seven of the eight ulcers treated healed, with a mean healing time of 41 days. The eighth ulcer improved, diminishing significantly in size. The researchers concluded that a royal jelly dressing is an effective alternative for treatment of diabetic foot ulcers.13 However, the same team conducted a double-blind, placebo-controlled clinical trial of topical royal jelly on diabetic foot ulcers in 25 patients (6 females, 19 males) and found no significant differences between 5% sterile topical royal jelly or placebo.6
Collagen production
A decade ago, Koya-Miyata et al. showed that royal jelly promotes collagen synthesis by skin fibroblasts in the presence of ascorbic acid-2-O-alpha-glucoside. They also showed that its primary fatty acid constituent, 10-HDA, facilitates the collagen production by fibroblasts treated with ascorbic acid-2-O-alpha-glucoside through activation of transforming growth factor-beta 1 production.5
Photoprotection
Park et al. measured the 10-HDA content of royal jelly in 2011 and studied its effects on UVB-induced skin photoaging in normal human dermal fibroblasts. The introduction of royal jelly (0.211% 10-HDA) promoted the production of procollagen type I and transforming growth factor (TGF)-beta-1 without affecting matrix metalloproteinase (MMP)-1 levels. The investigators concluded that the impact of royal jelly on collagen production positioned the bee product as a potential photoprotectant against UVB-induced photoaging.14 The next year, Park et al. observed that the production of type I collagen in the dorsal skin of ovariectomized Sprague-Dawley rats was enhanced by the dietary supplementation of 1% royal jelly extract. Although MMP-1 levels were unaffected, the investigators speculated that the effects on collagen synthesis alone were sufficient for royal jelly to provide anti-aging activity.4
In 2013, Zheng et al. found that 10-HDA significantly protected fibroblasts from UVA-induced cytotoxicity, reactive oxygen species, and cellular senescence. They also noted that 10-HDA inhibited the UVA-generated expression of MMP-1 and -3, and stimulated collagen production. Treatment with 10-HDA also reduced the activation of the c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) pathways. The researchers concluded that this royal jelly fatty acid appears to be a promising agent for the prevention and treatment of cutaneous photoaging.8
Skin whitening
In 2011, Han et al. reported that royal jelly dose-dependently inhibited melanin biosynthesis in the B16F1 mouse melanocyte cell line by reducing tyrosinase activity. Royal jelly also lowered mRNA levels of tyrosinase. The investigators concluded that royal jelly may be a viable option in the skin-lightening arsenal.3
Safety
There are some reports of contact dermatitis from the use of topical royal jelly.15 Far more significant, while rare, adverse reactions have been linked to oral use of royal jelly, including acute asthma, anaphylaxis, and even death.2,16,17
Conclusion
Royal jelly is one of several bee products found to have beneficial health effects in humans. Various dermatologic applications of royal jelly have been employed in recent decades. More research is necessary, though, to determine just how useful this bee product may be for a range of cutaneous conditions.
References
1. J Med Food. 2013;16(2):96-102.
2. Biosci Biotechnol Biochem. 2013;77(4):789-95.
3. Am J Chin Med. 2011;39(6):1253-60.
4. J Med Food. 2012;15(6):568-75.
5. Biosci Biotechnol Biochem. 2004 Apr;68(4):767-73.
6. Int Wound J. 2015;12(2):137-42.
7. J Food Sci. 2008 Nov;73(9):R117-24.
8. J Eur Acad Dermatol. Venereol. 2013;27(10):1269-77.
9. Pharmacogn Mag. 2013;9(33):9-13.
11. J Wound Care. 2008;17(3):108-10.
12. Nutr Res Pract. 2010;4(5):362-8.
13. J Res Med Sci. 2011;16(7):904-9.
14. J Med Food. 2011;14(9):899-906.
15. Contact Dermatitis. 1983;9(6):452-5.
16. Trop Biomed. 2008;25(3):243-51.
17. J Dermatol. 2011;38(11):1079-81.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Definitive Fixation of Hand and Wrist Fractures in the Emergency Department
A mentor—now in his 60s—related his experiences as a resident. On call as a second-year resident, he would often be alone at a busy trauma center with no backup. When a case came in, he would quickly read about it in the library, then manage it in the emergency department (ED) if possible, or, if necessary, take the patient to the operating room (OR).
In the era of improved patient care, increased supervision, and decreased autonomy, this is not the reality anymore.1 In theory, more reliable patient care is the result; however, the pendulum may have swung too far.
There are a number of injuries that are amenable to definitive fixation in the ED, but not as limited an array of injuries as we have perhaps grown accustomed to. Hand injuries are among the most common orthopedic injuries seen in the ED, with fractures of the metacarpals and phalanges constituting nearly one-half of all hand injuries.2 The authors recently attended an excellent instructional course lecture on “The Lost and Found Art of Percutaneous Pinning in the Hand and Wrist” at the annual conference of the American Academy of Orthopaedic Surgeons.3 The presenters itemized a comprehensive list of fractures and simple dislocations of the hand, which could be simply, safely, effectively, and definitively managed through percutaneous pinning techniques. A significant number of unstable fractures of the phalanges and metacarpals can be treated in the ED under mini–C-arm fluoroscopy without an admission and trip to the OR.3,4 Most phalangeal and metacarpal fractures are nondisplaced or minimally displaced and stable, and can often be handled with a combination of closed reduction, buddy-taping, and splinting.5 The indications for percutaneous versus internal fixation depend on a number of factors, including bone quality, degree of comminution, quality of the soft-tissue envelope, articular involvement, acuity of presentation, and goals for motion.6,7
Many simple injury patterns involving unstable fractures or dislocations may be definitively managed in the ED with percutaneous pinning (eg, injuries that are unstable with closed reduction alone but that do not necessitate soft-tissue dissection). These include but are not limited to bony mallet injuries, unstable transverse or oblique fractures or fracture-dislocations of the phalanges and metacarpals, carpometacarpal fracture- dislocations, and underlying fractures that need protection of nail-bed repairs, soft-tissue flaps, or extensor tendon injuries (Figures 1, 2).7,8 The techniques for specific fracture types are beyond the scope of this article but are readily available.5,6
There are certain situations that undoubtedly warrant surgery in the OR, such as neurovascular injury necessitating microvascular repair, flexor tendon laceration, severely comminuted or segmental fractures, irreducible dislocations, and fractures with severe soft-tissue injury or contamination not amenable to primary irrigation, débridement, and closure at bedside.4,7,8
You might ask, “Why would one treat an operative injury in the ED and not formally in the OR?,” and we submit that there are a number of reasons.
First, and most important, with increasing health care costs and decreasing reimbursements, physicians are faced with providing safe but economical care. Percutaneous Kirschner wire (K-wire) fixation is dramatically more cost-effective when performed in the ED than in the OR. The cost of a procedure performed in either setting is similarly dependent on a variety of factors, generally including complexity of the patient or procedure, costs of supplies and pharmacologic agents, fixed versus variable overhead costs, and the professional fees of providers and ancillary personnel.9,10
While the patient is not charged per hour in the ED, it is estimated that ORs in the United States cost, on average, $62 per minute, ranging from as low as $22 to as high as $133 per minute.9 Additionally, the number of personnel involved in running an OR exceeds those for a similar procedure performed in the ED, considering (at a minimum) the orthopedic surgeon, anesthesiologist, scrub and radiology technicians, and nursing personnel required before, during, and after an operation.
While analgesia and procedural sedation can be performed similarly in either setting, it is our experience that patients are managed much more often in the ED with local anesthesia under direct care of only the orthopedic provider, whereas intravenous sedation and general anesthesia are far more commonly implemented in the OR. There are exceptions for pediatric patients or those who are unable to tolerate the procedure under only local anesthesia. Local anesthesia or even intravenous conscious sedation entails less risk as well as lower associated drug costs.11
The difference in risk is especially true for sicker patients undergoing minimally invasive procedures.11 Although administration of adequate procedural analgesia grows increasingly difficult the more proximal the injury, the hand and the fingers are easily and reliably anesthetized with well-placed wrist or digital blocks, with infrequent complications.12 Application of a lidocaine/bupivacaine mixture provides up to 6 to 8 hours of analgesia. A small tourniquet alternative, such as the finger of a sterile glove or phlebotomy tourniquet, applied to the base of the finger or the wrist additionally provides a relatively bloodless field and effectively acts as a Bier block.
Percutaneous pins are much more forgiving than rigid internal fixation. If the initial placement of a pin is unsatisfactory, the pin can be reinserted at little cost.12 Conversely, it may not be possible to reposition a misplaced screw or screw with inadequate purchase and still maintain adequate fixation. While percutaneous pin fixation is not as rigid as screw fixation, the degree of stability provided is adequate for the small forces affecting the hand in most cases. Accordingly, there is a very low incidence of fibrous union or nonunion.13,14 With an increasing appreciation of soft-tissue handling over the past few decades, another significant advantage of K-wire fixation is the obviation of soft-tissue dissection, preserving the biology to maximize healing and minimize adverse sequelae.12 Percutaneous fixation has been shown to achieve functional outcomes comparable to open reduction with internal fixation of operative phalangeal and metacarpal fractures, without soft-tissue disruption, scarring, or implant irritation, and with minimal risk of infection.3,13,15,16 Ultimate range of motion after percutaneous fixation is comparable, if not superior, to that of internal fixation, despite the initial advantage of rigid internal fixation secondary to decreased scarring and lack of indwelling hardware.16,17
While the risk of infection, perhaps the primary concern with percutaneous fixation, has been cited as high as 7%, osteomyelitis is exceedingly rare (<0.5%).3,13,14 Furthermore, pins are often left in place for 3 to 6 weeks, and infection has been found to occur most often at a mean of 10 weeks.7,13 Infection can also be mitigated by intelligent pin placement, relief of residual tension, and splint immobilization.4,15 Pin loosening has similarly been reported in up to 4% of cases in large retrospective studies, occurring at an average of 8 weeks, by which time most pins would have been extricated.13 Other complications related to impaling adjacent neurovascular or tendinous structures have also been cited but are rare.13 A 12-month prospective study of 75 patients specifically evaluating the outcomes after closed reduction with percutaneous fixation of unstable hand fractures in the ED reported only 6 complications at final follow-up.4 Complications were all minor, with no cases of nonunion, delayed union, malunion, pin-tract infection, pyarthrosis, or cellulitis, even in the setting of open fractures. Three patients required revision in the OR for pin migration, initial malreduction, and bone loss in the setting of comminution, respectively. The authors credited their low complication rate to supplementary immobilization.
In conclusion, many unstable simple fractures and dislocations of the hand and wrist can be safely and effectively treated in the ED. While it may seem daunting for a junior resident who is unfamiliar with percutaneous techniques, the authors advocate learning from a more senior mentor. The only additional training required is an understanding of how to apply this skill set in a different setting.
1. Levine WN, Spang RC 3rd. ACGME duty hour requirements: perceptions and impact on resident training and patient care. J Am Acad Orthop Surg. 2014;22(9):535-544.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Catalano LW 3rd, Glickel SZ, Strauch RJ, Barron AO. The lost and found art of percutaneous pinning in the hand and wrist. Instructional Course Lectures. Annual Meeting of the American Academy of Orthopaedic Surgeons; March 24, 2015; Las Vegas, NV.
4. Starker I, Eaton RG. Kirschner wire placement in the emergency room. Is there a risk? J Hand Surg Br. 1995;20(4):535-538.
5. Meals C, Meals R. Hand fractures: a review of current treatment strategies. J Hand Surg Am. 2013;38(5):1021-1031.
6. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595.
7. Klein DM, Belsole RJ. Percutaneous treatment of carpal, metacarpal, and phalangeal injuries. Clin Orthop Relat Res. 2000;(375):116-125.
8. Bernstein ML, Chung KC. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048.
9. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
10. Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
11. Bodenham AR, Howell SJ. General anesthesia vs local anaesthesia: an ongoing story. Br J Anaesth. 2009;103(6):785-789.
12. Stern PJ. Management of fractures of the hand over the last 25 years. J Hand Surg Am. 2000;25(5):817-823.
13. Botte MJ, Davis JL, Rose BA, et al. Complications of smooth pin fixation of fractures and dislocations in the hand and wrist. Clin Orthop Relat Res. 1992;(276):194-201.
14. Wray RC Jr, Glunk R. Treatment of delayed union, nonunion, and malunion of the phalanges of the hand. Ann Plast Surg. 1989;22(1):14-18.
15. Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am. 2011;36(4):610-616.
16. Stem PJ, Wieser MJ, Reilly DG. Complications of plate fixation in the hand skeleton. Clin Orthop Relat Res. 1987;(214):59-65.
17. Page SM, Stern PJ. Complications and range of motion following plate fixation of metacarpal and phalangeal fractures. J Hand Surg Am. 1998;23(5):827-832.
A mentor—now in his 60s—related his experiences as a resident. On call as a second-year resident, he would often be alone at a busy trauma center with no backup. When a case came in, he would quickly read about it in the library, then manage it in the emergency department (ED) if possible, or, if necessary, take the patient to the operating room (OR).
In the era of improved patient care, increased supervision, and decreased autonomy, this is not the reality anymore.1 In theory, more reliable patient care is the result; however, the pendulum may have swung too far.
There are a number of injuries that are amenable to definitive fixation in the ED, but not as limited an array of injuries as we have perhaps grown accustomed to. Hand injuries are among the most common orthopedic injuries seen in the ED, with fractures of the metacarpals and phalanges constituting nearly one-half of all hand injuries.2 The authors recently attended an excellent instructional course lecture on “The Lost and Found Art of Percutaneous Pinning in the Hand and Wrist” at the annual conference of the American Academy of Orthopaedic Surgeons.3 The presenters itemized a comprehensive list of fractures and simple dislocations of the hand, which could be simply, safely, effectively, and definitively managed through percutaneous pinning techniques. A significant number of unstable fractures of the phalanges and metacarpals can be treated in the ED under mini–C-arm fluoroscopy without an admission and trip to the OR.3,4 Most phalangeal and metacarpal fractures are nondisplaced or minimally displaced and stable, and can often be handled with a combination of closed reduction, buddy-taping, and splinting.5 The indications for percutaneous versus internal fixation depend on a number of factors, including bone quality, degree of comminution, quality of the soft-tissue envelope, articular involvement, acuity of presentation, and goals for motion.6,7
Many simple injury patterns involving unstable fractures or dislocations may be definitively managed in the ED with percutaneous pinning (eg, injuries that are unstable with closed reduction alone but that do not necessitate soft-tissue dissection). These include but are not limited to bony mallet injuries, unstable transverse or oblique fractures or fracture-dislocations of the phalanges and metacarpals, carpometacarpal fracture- dislocations, and underlying fractures that need protection of nail-bed repairs, soft-tissue flaps, or extensor tendon injuries (Figures 1, 2).7,8 The techniques for specific fracture types are beyond the scope of this article but are readily available.5,6
There are certain situations that undoubtedly warrant surgery in the OR, such as neurovascular injury necessitating microvascular repair, flexor tendon laceration, severely comminuted or segmental fractures, irreducible dislocations, and fractures with severe soft-tissue injury or contamination not amenable to primary irrigation, débridement, and closure at bedside.4,7,8
You might ask, “Why would one treat an operative injury in the ED and not formally in the OR?,” and we submit that there are a number of reasons.
First, and most important, with increasing health care costs and decreasing reimbursements, physicians are faced with providing safe but economical care. Percutaneous Kirschner wire (K-wire) fixation is dramatically more cost-effective when performed in the ED than in the OR. The cost of a procedure performed in either setting is similarly dependent on a variety of factors, generally including complexity of the patient or procedure, costs of supplies and pharmacologic agents, fixed versus variable overhead costs, and the professional fees of providers and ancillary personnel.9,10
While the patient is not charged per hour in the ED, it is estimated that ORs in the United States cost, on average, $62 per minute, ranging from as low as $22 to as high as $133 per minute.9 Additionally, the number of personnel involved in running an OR exceeds those for a similar procedure performed in the ED, considering (at a minimum) the orthopedic surgeon, anesthesiologist, scrub and radiology technicians, and nursing personnel required before, during, and after an operation.
While analgesia and procedural sedation can be performed similarly in either setting, it is our experience that patients are managed much more often in the ED with local anesthesia under direct care of only the orthopedic provider, whereas intravenous sedation and general anesthesia are far more commonly implemented in the OR. There are exceptions for pediatric patients or those who are unable to tolerate the procedure under only local anesthesia. Local anesthesia or even intravenous conscious sedation entails less risk as well as lower associated drug costs.11
The difference in risk is especially true for sicker patients undergoing minimally invasive procedures.11 Although administration of adequate procedural analgesia grows increasingly difficult the more proximal the injury, the hand and the fingers are easily and reliably anesthetized with well-placed wrist or digital blocks, with infrequent complications.12 Application of a lidocaine/bupivacaine mixture provides up to 6 to 8 hours of analgesia. A small tourniquet alternative, such as the finger of a sterile glove or phlebotomy tourniquet, applied to the base of the finger or the wrist additionally provides a relatively bloodless field and effectively acts as a Bier block.
Percutaneous pins are much more forgiving than rigid internal fixation. If the initial placement of a pin is unsatisfactory, the pin can be reinserted at little cost.12 Conversely, it may not be possible to reposition a misplaced screw or screw with inadequate purchase and still maintain adequate fixation. While percutaneous pin fixation is not as rigid as screw fixation, the degree of stability provided is adequate for the small forces affecting the hand in most cases. Accordingly, there is a very low incidence of fibrous union or nonunion.13,14 With an increasing appreciation of soft-tissue handling over the past few decades, another significant advantage of K-wire fixation is the obviation of soft-tissue dissection, preserving the biology to maximize healing and minimize adverse sequelae.12 Percutaneous fixation has been shown to achieve functional outcomes comparable to open reduction with internal fixation of operative phalangeal and metacarpal fractures, without soft-tissue disruption, scarring, or implant irritation, and with minimal risk of infection.3,13,15,16 Ultimate range of motion after percutaneous fixation is comparable, if not superior, to that of internal fixation, despite the initial advantage of rigid internal fixation secondary to decreased scarring and lack of indwelling hardware.16,17
While the risk of infection, perhaps the primary concern with percutaneous fixation, has been cited as high as 7%, osteomyelitis is exceedingly rare (<0.5%).3,13,14 Furthermore, pins are often left in place for 3 to 6 weeks, and infection has been found to occur most often at a mean of 10 weeks.7,13 Infection can also be mitigated by intelligent pin placement, relief of residual tension, and splint immobilization.4,15 Pin loosening has similarly been reported in up to 4% of cases in large retrospective studies, occurring at an average of 8 weeks, by which time most pins would have been extricated.13 Other complications related to impaling adjacent neurovascular or tendinous structures have also been cited but are rare.13 A 12-month prospective study of 75 patients specifically evaluating the outcomes after closed reduction with percutaneous fixation of unstable hand fractures in the ED reported only 6 complications at final follow-up.4 Complications were all minor, with no cases of nonunion, delayed union, malunion, pin-tract infection, pyarthrosis, or cellulitis, even in the setting of open fractures. Three patients required revision in the OR for pin migration, initial malreduction, and bone loss in the setting of comminution, respectively. The authors credited their low complication rate to supplementary immobilization.
In conclusion, many unstable simple fractures and dislocations of the hand and wrist can be safely and effectively treated in the ED. While it may seem daunting for a junior resident who is unfamiliar with percutaneous techniques, the authors advocate learning from a more senior mentor. The only additional training required is an understanding of how to apply this skill set in a different setting.
A mentor—now in his 60s—related his experiences as a resident. On call as a second-year resident, he would often be alone at a busy trauma center with no backup. When a case came in, he would quickly read about it in the library, then manage it in the emergency department (ED) if possible, or, if necessary, take the patient to the operating room (OR).
In the era of improved patient care, increased supervision, and decreased autonomy, this is not the reality anymore.1 In theory, more reliable patient care is the result; however, the pendulum may have swung too far.
There are a number of injuries that are amenable to definitive fixation in the ED, but not as limited an array of injuries as we have perhaps grown accustomed to. Hand injuries are among the most common orthopedic injuries seen in the ED, with fractures of the metacarpals and phalanges constituting nearly one-half of all hand injuries.2 The authors recently attended an excellent instructional course lecture on “The Lost and Found Art of Percutaneous Pinning in the Hand and Wrist” at the annual conference of the American Academy of Orthopaedic Surgeons.3 The presenters itemized a comprehensive list of fractures and simple dislocations of the hand, which could be simply, safely, effectively, and definitively managed through percutaneous pinning techniques. A significant number of unstable fractures of the phalanges and metacarpals can be treated in the ED under mini–C-arm fluoroscopy without an admission and trip to the OR.3,4 Most phalangeal and metacarpal fractures are nondisplaced or minimally displaced and stable, and can often be handled with a combination of closed reduction, buddy-taping, and splinting.5 The indications for percutaneous versus internal fixation depend on a number of factors, including bone quality, degree of comminution, quality of the soft-tissue envelope, articular involvement, acuity of presentation, and goals for motion.6,7
Many simple injury patterns involving unstable fractures or dislocations may be definitively managed in the ED with percutaneous pinning (eg, injuries that are unstable with closed reduction alone but that do not necessitate soft-tissue dissection). These include but are not limited to bony mallet injuries, unstable transverse or oblique fractures or fracture-dislocations of the phalanges and metacarpals, carpometacarpal fracture- dislocations, and underlying fractures that need protection of nail-bed repairs, soft-tissue flaps, or extensor tendon injuries (Figures 1, 2).7,8 The techniques for specific fracture types are beyond the scope of this article but are readily available.5,6
There are certain situations that undoubtedly warrant surgery in the OR, such as neurovascular injury necessitating microvascular repair, flexor tendon laceration, severely comminuted or segmental fractures, irreducible dislocations, and fractures with severe soft-tissue injury or contamination not amenable to primary irrigation, débridement, and closure at bedside.4,7,8
You might ask, “Why would one treat an operative injury in the ED and not formally in the OR?,” and we submit that there are a number of reasons.
First, and most important, with increasing health care costs and decreasing reimbursements, physicians are faced with providing safe but economical care. Percutaneous Kirschner wire (K-wire) fixation is dramatically more cost-effective when performed in the ED than in the OR. The cost of a procedure performed in either setting is similarly dependent on a variety of factors, generally including complexity of the patient or procedure, costs of supplies and pharmacologic agents, fixed versus variable overhead costs, and the professional fees of providers and ancillary personnel.9,10
While the patient is not charged per hour in the ED, it is estimated that ORs in the United States cost, on average, $62 per minute, ranging from as low as $22 to as high as $133 per minute.9 Additionally, the number of personnel involved in running an OR exceeds those for a similar procedure performed in the ED, considering (at a minimum) the orthopedic surgeon, anesthesiologist, scrub and radiology technicians, and nursing personnel required before, during, and after an operation.
While analgesia and procedural sedation can be performed similarly in either setting, it is our experience that patients are managed much more often in the ED with local anesthesia under direct care of only the orthopedic provider, whereas intravenous sedation and general anesthesia are far more commonly implemented in the OR. There are exceptions for pediatric patients or those who are unable to tolerate the procedure under only local anesthesia. Local anesthesia or even intravenous conscious sedation entails less risk as well as lower associated drug costs.11
The difference in risk is especially true for sicker patients undergoing minimally invasive procedures.11 Although administration of adequate procedural analgesia grows increasingly difficult the more proximal the injury, the hand and the fingers are easily and reliably anesthetized with well-placed wrist or digital blocks, with infrequent complications.12 Application of a lidocaine/bupivacaine mixture provides up to 6 to 8 hours of analgesia. A small tourniquet alternative, such as the finger of a sterile glove or phlebotomy tourniquet, applied to the base of the finger or the wrist additionally provides a relatively bloodless field and effectively acts as a Bier block.
Percutaneous pins are much more forgiving than rigid internal fixation. If the initial placement of a pin is unsatisfactory, the pin can be reinserted at little cost.12 Conversely, it may not be possible to reposition a misplaced screw or screw with inadequate purchase and still maintain adequate fixation. While percutaneous pin fixation is not as rigid as screw fixation, the degree of stability provided is adequate for the small forces affecting the hand in most cases. Accordingly, there is a very low incidence of fibrous union or nonunion.13,14 With an increasing appreciation of soft-tissue handling over the past few decades, another significant advantage of K-wire fixation is the obviation of soft-tissue dissection, preserving the biology to maximize healing and minimize adverse sequelae.12 Percutaneous fixation has been shown to achieve functional outcomes comparable to open reduction with internal fixation of operative phalangeal and metacarpal fractures, without soft-tissue disruption, scarring, or implant irritation, and with minimal risk of infection.3,13,15,16 Ultimate range of motion after percutaneous fixation is comparable, if not superior, to that of internal fixation, despite the initial advantage of rigid internal fixation secondary to decreased scarring and lack of indwelling hardware.16,17
While the risk of infection, perhaps the primary concern with percutaneous fixation, has been cited as high as 7%, osteomyelitis is exceedingly rare (<0.5%).3,13,14 Furthermore, pins are often left in place for 3 to 6 weeks, and infection has been found to occur most often at a mean of 10 weeks.7,13 Infection can also be mitigated by intelligent pin placement, relief of residual tension, and splint immobilization.4,15 Pin loosening has similarly been reported in up to 4% of cases in large retrospective studies, occurring at an average of 8 weeks, by which time most pins would have been extricated.13 Other complications related to impaling adjacent neurovascular or tendinous structures have also been cited but are rare.13 A 12-month prospective study of 75 patients specifically evaluating the outcomes after closed reduction with percutaneous fixation of unstable hand fractures in the ED reported only 6 complications at final follow-up.4 Complications were all minor, with no cases of nonunion, delayed union, malunion, pin-tract infection, pyarthrosis, or cellulitis, even in the setting of open fractures. Three patients required revision in the OR for pin migration, initial malreduction, and bone loss in the setting of comminution, respectively. The authors credited their low complication rate to supplementary immobilization.
In conclusion, many unstable simple fractures and dislocations of the hand and wrist can be safely and effectively treated in the ED. While it may seem daunting for a junior resident who is unfamiliar with percutaneous techniques, the authors advocate learning from a more senior mentor. The only additional training required is an understanding of how to apply this skill set in a different setting.
1. Levine WN, Spang RC 3rd. ACGME duty hour requirements: perceptions and impact on resident training and patient care. J Am Acad Orthop Surg. 2014;22(9):535-544.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Catalano LW 3rd, Glickel SZ, Strauch RJ, Barron AO. The lost and found art of percutaneous pinning in the hand and wrist. Instructional Course Lectures. Annual Meeting of the American Academy of Orthopaedic Surgeons; March 24, 2015; Las Vegas, NV.
4. Starker I, Eaton RG. Kirschner wire placement in the emergency room. Is there a risk? J Hand Surg Br. 1995;20(4):535-538.
5. Meals C, Meals R. Hand fractures: a review of current treatment strategies. J Hand Surg Am. 2013;38(5):1021-1031.
6. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595.
7. Klein DM, Belsole RJ. Percutaneous treatment of carpal, metacarpal, and phalangeal injuries. Clin Orthop Relat Res. 2000;(375):116-125.
8. Bernstein ML, Chung KC. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048.
9. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
10. Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
11. Bodenham AR, Howell SJ. General anesthesia vs local anaesthesia: an ongoing story. Br J Anaesth. 2009;103(6):785-789.
12. Stern PJ. Management of fractures of the hand over the last 25 years. J Hand Surg Am. 2000;25(5):817-823.
13. Botte MJ, Davis JL, Rose BA, et al. Complications of smooth pin fixation of fractures and dislocations in the hand and wrist. Clin Orthop Relat Res. 1992;(276):194-201.
14. Wray RC Jr, Glunk R. Treatment of delayed union, nonunion, and malunion of the phalanges of the hand. Ann Plast Surg. 1989;22(1):14-18.
15. Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am. 2011;36(4):610-616.
16. Stem PJ, Wieser MJ, Reilly DG. Complications of plate fixation in the hand skeleton. Clin Orthop Relat Res. 1987;(214):59-65.
17. Page SM, Stern PJ. Complications and range of motion following plate fixation of metacarpal and phalangeal fractures. J Hand Surg Am. 1998;23(5):827-832.
1. Levine WN, Spang RC 3rd. ACGME duty hour requirements: perceptions and impact on resident training and patient care. J Am Acad Orthop Surg. 2014;22(9):535-544.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Catalano LW 3rd, Glickel SZ, Strauch RJ, Barron AO. The lost and found art of percutaneous pinning in the hand and wrist. Instructional Course Lectures. Annual Meeting of the American Academy of Orthopaedic Surgeons; March 24, 2015; Las Vegas, NV.
4. Starker I, Eaton RG. Kirschner wire placement in the emergency room. Is there a risk? J Hand Surg Br. 1995;20(4):535-538.
5. Meals C, Meals R. Hand fractures: a review of current treatment strategies. J Hand Surg Am. 2013;38(5):1021-1031.
6. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595.
7. Klein DM, Belsole RJ. Percutaneous treatment of carpal, metacarpal, and phalangeal injuries. Clin Orthop Relat Res. 2000;(375):116-125.
8. Bernstein ML, Chung KC. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048.
9. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
10. Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
11. Bodenham AR, Howell SJ. General anesthesia vs local anaesthesia: an ongoing story. Br J Anaesth. 2009;103(6):785-789.
12. Stern PJ. Management of fractures of the hand over the last 25 years. J Hand Surg Am. 2000;25(5):817-823.
13. Botte MJ, Davis JL, Rose BA, et al. Complications of smooth pin fixation of fractures and dislocations in the hand and wrist. Clin Orthop Relat Res. 1992;(276):194-201.
14. Wray RC Jr, Glunk R. Treatment of delayed union, nonunion, and malunion of the phalanges of the hand. Ann Plast Surg. 1989;22(1):14-18.
15. Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am. 2011;36(4):610-616.
16. Stem PJ, Wieser MJ, Reilly DG. Complications of plate fixation in the hand skeleton. Clin Orthop Relat Res. 1987;(214):59-65.
17. Page SM, Stern PJ. Complications and range of motion following plate fixation of metacarpal and phalangeal fractures. J Hand Surg Am. 1998;23(5):827-832.
Increased prevalence of pancreatic cysts due to MRI improvements
The apparent increase in the prevalence of incidental pancreatic cysts in recent years may be tied to improvements in MRI scanning technology, results of a study suggest.
Researchers conducted a retrospective analysis of data from 500 patients who underwent an MRI for nonpancreatic indications at a single center between 2005 and 2014; 50 were sampled from each year in chronological order.
A total of 208 patients (41.6%) were found to have an incidental cyst, of which less than a quarter were described in the original MRI report, according to a paper published online in Clinical Gastroenterology and Hepatology.
Analysis showed a very strong association between the type of imaging hardware and software, and the presence of cysts; older hardware found pancreatic cysts in 30.3% of patients and the newest hardware found cysts in 56.3% of patients.
However, MRI field strength was not associated with the frequency of lesion discovery (Clin Gastro Hepatol. 2015 Sep 11. doi: 10.1016/j.cgh.2015.08.038).
Most cysts were relatively small, with a median size of 4 mm. Nearly half of the patients with a cyst only had one described.
Nearly two-thirds of these cysts (62%) had an uncertain diagnosis, but 35% of patients were diagnosed with an intraductal papillary mucinous neoplasm, and one patient showed radiologic evidence of subacute pancreatitis.
When compared to the rest of the cohort, individuals with pancreatic cysts were more likely to be older, have diabetes mellitus, or have a personal history of cancer, particularly nonmelanoma skin cancer and hepatocellular carcinoma.
“Our study demonstrates the relationship between the higher trend of incidental pancreatic cysts observed in the recent years and the improvements in the technical features of MRIs,” wrote Dr. Maria Moris and colleagues from the Mayo Clinic, Jacksonville.
The authors said the real prevalence of pancreatic cystic lesions is estimated to range from 0.2% to 44.7%.
Their finding of a prevalence of 41.6% was higher than that found in similar imaging studies, but the authors suggested some of this may be due to the lack of a size cutoff in their study, as opposed to a 5-mm cutoff used in one earlier study that found a prevalence of 10%.
“Moreover, we believe that we may have even underestimated the real prevalence because of the absence of magnetic resonance cholangiopancreatography sequences (only 19% of the examinations), and the lack of 3-T studies (6% of the MRIs),” they wrote.
The median size of the lesions was lower than those found in previous studies.
“This smaller size was unexpected because, as a result of the exclusion criteria applied, the technical features of the MRIs were not the most specific for [pancreatic cystic lesion] PCL visualization,” the authors wrote, suggesting that this may have been due to the radiologist’s experience in this field.
The study was funded by the Joyce E. Baker Foundation for Research at Mayo Clinic in Jacksonville. One author disclosed grants or travel support from Olympus, Boston Scientific, and Cosmo Pharmaceuticals. There were no other conflicts of interest declared.
AGA Resource
Review the AGA guideline on the management of asymptomatic pancreatic neoplastic cysts at http://www.gastro.org/guidelines/2015/5/29/pancreatic-cysts.
The apparent increase in the prevalence of incidental pancreatic cysts in recent years may be tied to improvements in MRI scanning technology, results of a study suggest.
Researchers conducted a retrospective analysis of data from 500 patients who underwent an MRI for nonpancreatic indications at a single center between 2005 and 2014; 50 were sampled from each year in chronological order.
A total of 208 patients (41.6%) were found to have an incidental cyst, of which less than a quarter were described in the original MRI report, according to a paper published online in Clinical Gastroenterology and Hepatology.
Analysis showed a very strong association between the type of imaging hardware and software, and the presence of cysts; older hardware found pancreatic cysts in 30.3% of patients and the newest hardware found cysts in 56.3% of patients.
However, MRI field strength was not associated with the frequency of lesion discovery (Clin Gastro Hepatol. 2015 Sep 11. doi: 10.1016/j.cgh.2015.08.038).
Most cysts were relatively small, with a median size of 4 mm. Nearly half of the patients with a cyst only had one described.
Nearly two-thirds of these cysts (62%) had an uncertain diagnosis, but 35% of patients were diagnosed with an intraductal papillary mucinous neoplasm, and one patient showed radiologic evidence of subacute pancreatitis.
When compared to the rest of the cohort, individuals with pancreatic cysts were more likely to be older, have diabetes mellitus, or have a personal history of cancer, particularly nonmelanoma skin cancer and hepatocellular carcinoma.
“Our study demonstrates the relationship between the higher trend of incidental pancreatic cysts observed in the recent years and the improvements in the technical features of MRIs,” wrote Dr. Maria Moris and colleagues from the Mayo Clinic, Jacksonville.
The authors said the real prevalence of pancreatic cystic lesions is estimated to range from 0.2% to 44.7%.
Their finding of a prevalence of 41.6% was higher than that found in similar imaging studies, but the authors suggested some of this may be due to the lack of a size cutoff in their study, as opposed to a 5-mm cutoff used in one earlier study that found a prevalence of 10%.
“Moreover, we believe that we may have even underestimated the real prevalence because of the absence of magnetic resonance cholangiopancreatography sequences (only 19% of the examinations), and the lack of 3-T studies (6% of the MRIs),” they wrote.
The median size of the lesions was lower than those found in previous studies.
“This smaller size was unexpected because, as a result of the exclusion criteria applied, the technical features of the MRIs were not the most specific for [pancreatic cystic lesion] PCL visualization,” the authors wrote, suggesting that this may have been due to the radiologist’s experience in this field.
The study was funded by the Joyce E. Baker Foundation for Research at Mayo Clinic in Jacksonville. One author disclosed grants or travel support from Olympus, Boston Scientific, and Cosmo Pharmaceuticals. There were no other conflicts of interest declared.
AGA Resource
Review the AGA guideline on the management of asymptomatic pancreatic neoplastic cysts at http://www.gastro.org/guidelines/2015/5/29/pancreatic-cysts.
The apparent increase in the prevalence of incidental pancreatic cysts in recent years may be tied to improvements in MRI scanning technology, results of a study suggest.
Researchers conducted a retrospective analysis of data from 500 patients who underwent an MRI for nonpancreatic indications at a single center between 2005 and 2014; 50 were sampled from each year in chronological order.
A total of 208 patients (41.6%) were found to have an incidental cyst, of which less than a quarter were described in the original MRI report, according to a paper published online in Clinical Gastroenterology and Hepatology.
Analysis showed a very strong association between the type of imaging hardware and software, and the presence of cysts; older hardware found pancreatic cysts in 30.3% of patients and the newest hardware found cysts in 56.3% of patients.
However, MRI field strength was not associated with the frequency of lesion discovery (Clin Gastro Hepatol. 2015 Sep 11. doi: 10.1016/j.cgh.2015.08.038).
Most cysts were relatively small, with a median size of 4 mm. Nearly half of the patients with a cyst only had one described.
Nearly two-thirds of these cysts (62%) had an uncertain diagnosis, but 35% of patients were diagnosed with an intraductal papillary mucinous neoplasm, and one patient showed radiologic evidence of subacute pancreatitis.
When compared to the rest of the cohort, individuals with pancreatic cysts were more likely to be older, have diabetes mellitus, or have a personal history of cancer, particularly nonmelanoma skin cancer and hepatocellular carcinoma.
“Our study demonstrates the relationship between the higher trend of incidental pancreatic cysts observed in the recent years and the improvements in the technical features of MRIs,” wrote Dr. Maria Moris and colleagues from the Mayo Clinic, Jacksonville.
The authors said the real prevalence of pancreatic cystic lesions is estimated to range from 0.2% to 44.7%.
Their finding of a prevalence of 41.6% was higher than that found in similar imaging studies, but the authors suggested some of this may be due to the lack of a size cutoff in their study, as opposed to a 5-mm cutoff used in one earlier study that found a prevalence of 10%.
“Moreover, we believe that we may have even underestimated the real prevalence because of the absence of magnetic resonance cholangiopancreatography sequences (only 19% of the examinations), and the lack of 3-T studies (6% of the MRIs),” they wrote.
The median size of the lesions was lower than those found in previous studies.
“This smaller size was unexpected because, as a result of the exclusion criteria applied, the technical features of the MRIs were not the most specific for [pancreatic cystic lesion] PCL visualization,” the authors wrote, suggesting that this may have been due to the radiologist’s experience in this field.
The study was funded by the Joyce E. Baker Foundation for Research at Mayo Clinic in Jacksonville. One author disclosed grants or travel support from Olympus, Boston Scientific, and Cosmo Pharmaceuticals. There were no other conflicts of interest declared.
AGA Resource
Review the AGA guideline on the management of asymptomatic pancreatic neoplastic cysts at http://www.gastro.org/guidelines/2015/5/29/pancreatic-cysts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The apparent increase of the prevalence of incidental pancreatic cysts in recent years may be tied to improvements in MRI scanning technology.
Major finding: Older MRI hardware found pancreatic cysts in 30.3% of patients and the newest hardware found cysts in 56.3% of patients.
Data source: A retrospective analysis of data from 500 patients who underwent an MRI for nonpancreatic indications.
Disclosures: The study was funded by the Joyce E. Baker Foundation for Research at Mayo Clinic in Jacksonville, Fla. One author disclosed grants or travel support from Olympus, Boston Scientific, and Cosmo Pharmaceuticals. There were no other conflicts of interest declared.
Does Medication Use Decrease After Hip-Replacement Surgery?
Results of a new study provide information on the trajectories of prescription drug use before and after total hip arthroplasty (THA). The study was published online ahead of print November 14 in Pain.
Researchers merged Norwegian national joint replacement and prescription databases to analyze the medication use of nearly 40,000 patients undergoing THA from 2005 to 2011. The patients’ average age was 68.5 and about three-fourths of patients underwent THA because of primary osteoarthritis.
The investigators analyzed trends in prescription drug use over 2 years: 4 quarters before and 4 quarters after hip-replacement surgery. The study focused on analgesics and hypnotics as well as drugs to treat anxiety and depression.
Overall, about half of patients filled a prescription for an analgesic in the year before surgery. Analgesic use included nonsteroidal anti-inflammatory drugs (NSAIDs) in 38% of patients, opioids in 16%, and other non-opioid analgesics in 12%.
Use of pain medications continued to increase during the last quarter before THA and then increased dramatically in the first quarter after surgery. The sharpest increases were for opioids, which increased to 28% in the last quarter before THA, then to 65% in the first quarter afterward; non-opioid analgesics increased to 21% and then to 60.5%.
The percentage of patients who filled prescriptions for hypnotics also increased from the quarter before to the quarter after surgery—from 14% to 25%. Analysis of the dosage showed a similar pattern.
With continued follow-up after THA, medication use decreased. By 1 year after THA, opioid use had decreased to 14%, NSAID use had decreased to 18%, and non-opioid analgesic use had decreased to 13%. Use of hypnotic drugs also decreased, along with medications to treat anxiety. There was little or no change in the use of antidepressants.
“Patients with chronic pain are frequent users of analgesic and psychotropic drugs and thereby risk adverse drug events,” said Tone Blågestad, a PhD candidate from the Department of Clinical Psychology at the University of Bergen in Norway, and coauthors. They cited special concern about the potential for serious adverse effects of opioids, including drug overdose.
The results suggest that use of pain medications increases in the year before THA, with a further increase immediately afterward, followed by a long-term decrease. That pattern is consistent with previous studies on pain scores in the period before and after THA.
Hypnotic drug use shows a similar trend, suggesting that sleep problems get worse, then improve with long-term pain relief after THA. The lack of change in antidepressant use suggests that depression in patients undergoing hip replacement isn’t necessarily related to hip pain.
“Overall, the present results extend the positive effects of THA to include reduced reliance on medication to alleviate symptoms,” said Ms. Blågestad and colleagues. The finding that hypnotics follow the same prescription trajectory as analgesics highlights the link between pain and sleep. The researchers add, “Our results warrant attention to the increased risk of adverse medication effects occurring with the increased use of both opioids and hypnotics in the recovery phase.”
Suggested Reading
Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2015 Nov 14. [Epub ahead of print].
Results of a new study provide information on the trajectories of prescription drug use before and after total hip arthroplasty (THA). The study was published online ahead of print November 14 in Pain.
Researchers merged Norwegian national joint replacement and prescription databases to analyze the medication use of nearly 40,000 patients undergoing THA from 2005 to 2011. The patients’ average age was 68.5 and about three-fourths of patients underwent THA because of primary osteoarthritis.
The investigators analyzed trends in prescription drug use over 2 years: 4 quarters before and 4 quarters after hip-replacement surgery. The study focused on analgesics and hypnotics as well as drugs to treat anxiety and depression.
Overall, about half of patients filled a prescription for an analgesic in the year before surgery. Analgesic use included nonsteroidal anti-inflammatory drugs (NSAIDs) in 38% of patients, opioids in 16%, and other non-opioid analgesics in 12%.
Use of pain medications continued to increase during the last quarter before THA and then increased dramatically in the first quarter after surgery. The sharpest increases were for opioids, which increased to 28% in the last quarter before THA, then to 65% in the first quarter afterward; non-opioid analgesics increased to 21% and then to 60.5%.
The percentage of patients who filled prescriptions for hypnotics also increased from the quarter before to the quarter after surgery—from 14% to 25%. Analysis of the dosage showed a similar pattern.
With continued follow-up after THA, medication use decreased. By 1 year after THA, opioid use had decreased to 14%, NSAID use had decreased to 18%, and non-opioid analgesic use had decreased to 13%. Use of hypnotic drugs also decreased, along with medications to treat anxiety. There was little or no change in the use of antidepressants.
“Patients with chronic pain are frequent users of analgesic and psychotropic drugs and thereby risk adverse drug events,” said Tone Blågestad, a PhD candidate from the Department of Clinical Psychology at the University of Bergen in Norway, and coauthors. They cited special concern about the potential for serious adverse effects of opioids, including drug overdose.
The results suggest that use of pain medications increases in the year before THA, with a further increase immediately afterward, followed by a long-term decrease. That pattern is consistent with previous studies on pain scores in the period before and after THA.
Hypnotic drug use shows a similar trend, suggesting that sleep problems get worse, then improve with long-term pain relief after THA. The lack of change in antidepressant use suggests that depression in patients undergoing hip replacement isn’t necessarily related to hip pain.
“Overall, the present results extend the positive effects of THA to include reduced reliance on medication to alleviate symptoms,” said Ms. Blågestad and colleagues. The finding that hypnotics follow the same prescription trajectory as analgesics highlights the link between pain and sleep. The researchers add, “Our results warrant attention to the increased risk of adverse medication effects occurring with the increased use of both opioids and hypnotics in the recovery phase.”
Results of a new study provide information on the trajectories of prescription drug use before and after total hip arthroplasty (THA). The study was published online ahead of print November 14 in Pain.
Researchers merged Norwegian national joint replacement and prescription databases to analyze the medication use of nearly 40,000 patients undergoing THA from 2005 to 2011. The patients’ average age was 68.5 and about three-fourths of patients underwent THA because of primary osteoarthritis.
The investigators analyzed trends in prescription drug use over 2 years: 4 quarters before and 4 quarters after hip-replacement surgery. The study focused on analgesics and hypnotics as well as drugs to treat anxiety and depression.
Overall, about half of patients filled a prescription for an analgesic in the year before surgery. Analgesic use included nonsteroidal anti-inflammatory drugs (NSAIDs) in 38% of patients, opioids in 16%, and other non-opioid analgesics in 12%.
Use of pain medications continued to increase during the last quarter before THA and then increased dramatically in the first quarter after surgery. The sharpest increases were for opioids, which increased to 28% in the last quarter before THA, then to 65% in the first quarter afterward; non-opioid analgesics increased to 21% and then to 60.5%.
The percentage of patients who filled prescriptions for hypnotics also increased from the quarter before to the quarter after surgery—from 14% to 25%. Analysis of the dosage showed a similar pattern.
With continued follow-up after THA, medication use decreased. By 1 year after THA, opioid use had decreased to 14%, NSAID use had decreased to 18%, and non-opioid analgesic use had decreased to 13%. Use of hypnotic drugs also decreased, along with medications to treat anxiety. There was little or no change in the use of antidepressants.
“Patients with chronic pain are frequent users of analgesic and psychotropic drugs and thereby risk adverse drug events,” said Tone Blågestad, a PhD candidate from the Department of Clinical Psychology at the University of Bergen in Norway, and coauthors. They cited special concern about the potential for serious adverse effects of opioids, including drug overdose.
The results suggest that use of pain medications increases in the year before THA, with a further increase immediately afterward, followed by a long-term decrease. That pattern is consistent with previous studies on pain scores in the period before and after THA.
Hypnotic drug use shows a similar trend, suggesting that sleep problems get worse, then improve with long-term pain relief after THA. The lack of change in antidepressant use suggests that depression in patients undergoing hip replacement isn’t necessarily related to hip pain.
“Overall, the present results extend the positive effects of THA to include reduced reliance on medication to alleviate symptoms,” said Ms. Blågestad and colleagues. The finding that hypnotics follow the same prescription trajectory as analgesics highlights the link between pain and sleep. The researchers add, “Our results warrant attention to the increased risk of adverse medication effects occurring with the increased use of both opioids and hypnotics in the recovery phase.”
Suggested Reading
Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2015 Nov 14. [Epub ahead of print].
Suggested Reading
Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2015 Nov 14. [Epub ahead of print].