User login
New tech promises better blood oxygen readings on dark skin
A recent study adds weight to earlier findings that their device works.
“It is a new, first-in-class technology,” said Sanjay Gokhale, MD, the bioengineer who is leading this research at the University of Texas at Arlington. “The team conducted extensive preclinical work and carried out phase 1 studies in human volunteers, demonstrating sensitivity and accuracy.”
It’s one of several projects underway to update pulse oximetry, a technology based on research in lighter-skinned people that has not changed much in 50 years.
The pulse oximeter, or “pulse ox,” measures the saturation of oxygen in your hemoglobin (a protein in red blood cells). But it tends to overestimate the oxygen saturation in patients with darker skin by about 2%-3%. That may not sound like a lot, but it’s enough to delay major treatment for respiratory issues like COVID-19.
“Falsely elevated readings from commercial oximeters have delayed treatment of Black COVID-19 patients for hours in some cases,” said Divya Chander, MD, PhD, an anesthesiologist in Oakland, Calif., and chair of neuroscience at The Singularity Group. (Dr. Chander was not involved in the UT Arlington research.)
Early research happening separately at Brown University and Tufts University aims to redesign the pulse oximeter to get accurate readings in patients of all skin tones. University of California, San Diego, researchers are looking into a method that measures blood oxygen using sound in combination with light. Other solutions try to correct for skin tone with algorithms.
The device from UT Arlington uses an algorithm too, but its main innovation is that it replaces red light with green light.
Red light, green light
Traditional oximetry devices, which typically clip on to the patient’s fingertip, use LEDs to beam light through the skin at two wavelengths: one in the red part of the spectrum, the other in the infrared. The light transmits from one side of the clip to the other, passing through arterial blood as it pulses.
The device calculates a patient’s oxygenation based on how much light of each wavelength is absorbed by hemoglobin in the blood. Oxygenated hemoglobin absorbs the light differently than deoxygenated hemoglobin, so oxygenation can be represented as a percentage; 100% means all hemoglobin is completely oxygenated. But the melanin in skin can interfere with the absorption of light and affect the results.
The green light strategy measures not absorption but reflectance – how much of the light bounces back. As with traditional oximetry, the green-light method uses two wavelengths. Each is a different shade of green, and the two forms of hemoglobin reflect them differently.
Using an algorithm developed by the researchers, the device can capture readings in patients of all skin tones, the researchers say. And because it works on the wrist rather than a finger, the device also eliminates issues with cold fingers and dark nail polish – both known to reduce accuracy in traditional oximetry.
In the latest experiments, the researchers tested the technology on synthetic skin samples with varying amounts of melanin, Dr. Gokhale said. The device picked up changes in blood oxygen saturation even in samples with high melanin levels.
In a study published last year, the technology was tested in 16 people against an invasive handheld blood analyzer and a noninvasive commercial pulse oximeter, and found to be comparable to the invasive method.
A drawback
The green light approach could be “game changing,” Dr. Chander said. But there is a drawback.
Since green light doesn’t penetrate as deeply, this approach measures blood oxygen saturation in capillary beds (small blood vessels very close to the skin surface). By contrast, traditional oximetry measures oxygen saturation in an artery as it pulses – thus the name pulse oximetry.
Valuable information can be obtained from an arterial pulse.
Changes in arterial pulse, known as the waveforms, “can tell us about a patient’s hydration status [for instance],” Dr. Chander said. “In a mechanically ventilated patient, this variation with a patient’s respiratory cycle can give us feedback about how responsive the patient will be to fluid resuscitation if their blood pressure is too low.”
Given such considerations, the green light method may be useful as an adjunct, not a full replacement, to a standard pulse ox, Dr. Chander noted.
A version of this article appeared on WebMD.com.
A recent study adds weight to earlier findings that their device works.
“It is a new, first-in-class technology,” said Sanjay Gokhale, MD, the bioengineer who is leading this research at the University of Texas at Arlington. “The team conducted extensive preclinical work and carried out phase 1 studies in human volunteers, demonstrating sensitivity and accuracy.”
It’s one of several projects underway to update pulse oximetry, a technology based on research in lighter-skinned people that has not changed much in 50 years.
The pulse oximeter, or “pulse ox,” measures the saturation of oxygen in your hemoglobin (a protein in red blood cells). But it tends to overestimate the oxygen saturation in patients with darker skin by about 2%-3%. That may not sound like a lot, but it’s enough to delay major treatment for respiratory issues like COVID-19.
“Falsely elevated readings from commercial oximeters have delayed treatment of Black COVID-19 patients for hours in some cases,” said Divya Chander, MD, PhD, an anesthesiologist in Oakland, Calif., and chair of neuroscience at The Singularity Group. (Dr. Chander was not involved in the UT Arlington research.)
Early research happening separately at Brown University and Tufts University aims to redesign the pulse oximeter to get accurate readings in patients of all skin tones. University of California, San Diego, researchers are looking into a method that measures blood oxygen using sound in combination with light. Other solutions try to correct for skin tone with algorithms.
The device from UT Arlington uses an algorithm too, but its main innovation is that it replaces red light with green light.
Red light, green light
Traditional oximetry devices, which typically clip on to the patient’s fingertip, use LEDs to beam light through the skin at two wavelengths: one in the red part of the spectrum, the other in the infrared. The light transmits from one side of the clip to the other, passing through arterial blood as it pulses.
The device calculates a patient’s oxygenation based on how much light of each wavelength is absorbed by hemoglobin in the blood. Oxygenated hemoglobin absorbs the light differently than deoxygenated hemoglobin, so oxygenation can be represented as a percentage; 100% means all hemoglobin is completely oxygenated. But the melanin in skin can interfere with the absorption of light and affect the results.
The green light strategy measures not absorption but reflectance – how much of the light bounces back. As with traditional oximetry, the green-light method uses two wavelengths. Each is a different shade of green, and the two forms of hemoglobin reflect them differently.
Using an algorithm developed by the researchers, the device can capture readings in patients of all skin tones, the researchers say. And because it works on the wrist rather than a finger, the device also eliminates issues with cold fingers and dark nail polish – both known to reduce accuracy in traditional oximetry.
In the latest experiments, the researchers tested the technology on synthetic skin samples with varying amounts of melanin, Dr. Gokhale said. The device picked up changes in blood oxygen saturation even in samples with high melanin levels.
In a study published last year, the technology was tested in 16 people against an invasive handheld blood analyzer and a noninvasive commercial pulse oximeter, and found to be comparable to the invasive method.
A drawback
The green light approach could be “game changing,” Dr. Chander said. But there is a drawback.
Since green light doesn’t penetrate as deeply, this approach measures blood oxygen saturation in capillary beds (small blood vessels very close to the skin surface). By contrast, traditional oximetry measures oxygen saturation in an artery as it pulses – thus the name pulse oximetry.
Valuable information can be obtained from an arterial pulse.
Changes in arterial pulse, known as the waveforms, “can tell us about a patient’s hydration status [for instance],” Dr. Chander said. “In a mechanically ventilated patient, this variation with a patient’s respiratory cycle can give us feedback about how responsive the patient will be to fluid resuscitation if their blood pressure is too low.”
Given such considerations, the green light method may be useful as an adjunct, not a full replacement, to a standard pulse ox, Dr. Chander noted.
A version of this article appeared on WebMD.com.
A recent study adds weight to earlier findings that their device works.
“It is a new, first-in-class technology,” said Sanjay Gokhale, MD, the bioengineer who is leading this research at the University of Texas at Arlington. “The team conducted extensive preclinical work and carried out phase 1 studies in human volunteers, demonstrating sensitivity and accuracy.”
It’s one of several projects underway to update pulse oximetry, a technology based on research in lighter-skinned people that has not changed much in 50 years.
The pulse oximeter, or “pulse ox,” measures the saturation of oxygen in your hemoglobin (a protein in red blood cells). But it tends to overestimate the oxygen saturation in patients with darker skin by about 2%-3%. That may not sound like a lot, but it’s enough to delay major treatment for respiratory issues like COVID-19.
“Falsely elevated readings from commercial oximeters have delayed treatment of Black COVID-19 patients for hours in some cases,” said Divya Chander, MD, PhD, an anesthesiologist in Oakland, Calif., and chair of neuroscience at The Singularity Group. (Dr. Chander was not involved in the UT Arlington research.)
Early research happening separately at Brown University and Tufts University aims to redesign the pulse oximeter to get accurate readings in patients of all skin tones. University of California, San Diego, researchers are looking into a method that measures blood oxygen using sound in combination with light. Other solutions try to correct for skin tone with algorithms.
The device from UT Arlington uses an algorithm too, but its main innovation is that it replaces red light with green light.
Red light, green light
Traditional oximetry devices, which typically clip on to the patient’s fingertip, use LEDs to beam light through the skin at two wavelengths: one in the red part of the spectrum, the other in the infrared. The light transmits from one side of the clip to the other, passing through arterial blood as it pulses.
The device calculates a patient’s oxygenation based on how much light of each wavelength is absorbed by hemoglobin in the blood. Oxygenated hemoglobin absorbs the light differently than deoxygenated hemoglobin, so oxygenation can be represented as a percentage; 100% means all hemoglobin is completely oxygenated. But the melanin in skin can interfere with the absorption of light and affect the results.
The green light strategy measures not absorption but reflectance – how much of the light bounces back. As with traditional oximetry, the green-light method uses two wavelengths. Each is a different shade of green, and the two forms of hemoglobin reflect them differently.
Using an algorithm developed by the researchers, the device can capture readings in patients of all skin tones, the researchers say. And because it works on the wrist rather than a finger, the device also eliminates issues with cold fingers and dark nail polish – both known to reduce accuracy in traditional oximetry.
In the latest experiments, the researchers tested the technology on synthetic skin samples with varying amounts of melanin, Dr. Gokhale said. The device picked up changes in blood oxygen saturation even in samples with high melanin levels.
In a study published last year, the technology was tested in 16 people against an invasive handheld blood analyzer and a noninvasive commercial pulse oximeter, and found to be comparable to the invasive method.
A drawback
The green light approach could be “game changing,” Dr. Chander said. But there is a drawback.
Since green light doesn’t penetrate as deeply, this approach measures blood oxygen saturation in capillary beds (small blood vessels very close to the skin surface). By contrast, traditional oximetry measures oxygen saturation in an artery as it pulses – thus the name pulse oximetry.
Valuable information can be obtained from an arterial pulse.
Changes in arterial pulse, known as the waveforms, “can tell us about a patient’s hydration status [for instance],” Dr. Chander said. “In a mechanically ventilated patient, this variation with a patient’s respiratory cycle can give us feedback about how responsive the patient will be to fluid resuscitation if their blood pressure is too low.”
Given such considerations, the green light method may be useful as an adjunct, not a full replacement, to a standard pulse ox, Dr. Chander noted.
A version of this article appeared on WebMD.com.
Long COVID disability court battles just ‘tip of iceberg’
At least 30 lawsuits have been filed seeking legal resolution of disability insurance claims, according to searches of court records. In addition, the Social Security Administration said it has received about 52,000 disability claims tied to SARS-CoV-2 infections, which represents 1% of all applications.
But legal experts say those cases may not reflect the total number of cases that have gone to court. They note many claims are initially dismissed and are not appealed by claimants.
“With this system, they deny two-thirds of initial applications, then people who appeal get denied almost 90% of the time, and then they can appeal before a judge,” said Kevin LaPorte, a Social Security disability attorney at LaPorte Law Firm in Oakland, Calif. “What happens next doesn’t have a lot of precedent because long COVID is a mass disabling event, and we haven’t seen that many of these cases get all the way through the legal system yet.”
As a result, the exact number of long COVID disability claims and the number of these cases going to court isn’t clear, he said.
“It can take a year or more for cases to get to court, and even longer to reach resolution,” Mr. LaPorte added. “I suspect the few cases we’ve heard about at this point are going to be the tip of the iceberg.”
The process is convoluted and can drag on for months with multiple denials and appeals along the way. Many disabled workers find their only recourse is to take insurers to court.
Long COVID patients typically apply for disability benefits through private insurance or Social Security. But the process can drag on for months, so many find their only recourse is to take insurers to court, according to legal experts.
But even in the courts, many encounter delays and hurdles to resolution.
In one of the first federal lawsuits involving long COVID disability benefits, William Abrams, a trial and appellate attorney and active marathon runner, sued Unum Life Insurance seeking long-term disability income. Symptoms included extreme fatigue, brain fog, decreased attention and concentration, and nearly daily fevers, causing him to stop working in April 2020.
His diagnosis wasn’t definitive. Three doctors said he had long COVID, and four said he had chronic fatigue syndrome. Unum cited this inconsistency as a rationale for rejecting his claim. But the court sided with Mr. Abrams, granting him disability income. The court concluded: “Unum may be correct that [the plaintiff] has not been correctly diagnosed. But that does not mean he is not sick. If [the plaintiff’s] complaints, and [the doctor’s] assessments, are to be believed, [the plaintiff] cannot focus for more than a few minutes at a time, making it impossible for [the plaintiff] to perform the varied and complex tasks his job requires.”
Unum said in an emailed statement that the company doesn’t comment on specific claims as a matter of policy, adding that its total payouts for disability claims from March 2020 to February 2022 were 35% higher than prepandemic levels. “In general, disability and leave claims connected to COVID-19 have been primarily short-term events with the majority of claimants recovering prior to completing the normal qualification period for long-term disability insurance,” Unum said.
Mr. Abrams prevailed in part because he had detailed documentation of the numerous impairments that eventually required him to stop work, said Michelle Roberts of Roberts Disability Law in Oakland, Calif.
He submitted videos of himself taking his temperature to prove he had almost daily fevers, according to court records. He underwent neuropsychological testing, which found learning deficiencies and memory deficits.
Mr. Abrams also submitted statements from a colleague who worked with him on a complex technology patent case involving radiofrequency identification. Before he got COVID, Mr. Abrams “had the analytical ability, legal acumen, and mental energy to attack that learning curve and get up to speed very rapidly,” according to court records.
“The court focused on credulity.” Ms. Roberts said. “There was all this work to be done to show this person was high functioning and ran marathons and worked in an intense, high-pressure occupation but then couldn’t do anything after long COVID.”
Documentation was also crucial in another early federal long COVID disability lawsuit that was filed in 2022 on behalf of Wendy Haut, an educational software sales representative in California who turned to the courts seeking disability income through her company’s employee benefits plan.
Several of Ms. Haut’s doctors documented a detailed list of long COVID symptoms, including “profound fatigue and extreme cognitive difficulties,” that they said prevented her from working as a sales representative or doing any other type of job. A settlement agreement in June 2022 required Reliance Standard Life Insurance to pay Ms. Haut long-term disability benefits, including previously unpaid benefits, according to a report by the advocacy group Pandemic Patients.
Representatives of Reliance Standard didn’t respond to a request for comment.
The growing number of workers being sidelined by long COVID makes more claims and more court cases likely. Right now, an estimated 16 million working-age Americans aged 18-65 years have long COVID, and as many as 4 million of them can’t work, according to a July 2023 Census Bureau report.
Uncertainty about the volume of claims in the pipeline is part of what’s driving some insurers to fight long COVID claims, Ms. Roberts said. Another factor is the lack of clarity around how many years people with long COVID may be out of work, particularly if they’re in their 30s or 40s and might be seeking disability income until they reach retirement age.
“Doctors are not always saying that this person will be permanently disabled,” Ms. Roberts said. “If this person doesn’t get better and they’re disabled until retirement age, this could be a payout in the high six or seven figures if a person is very young and was a very high earner.”
Insurance companies routinely deny claims that can’t be backed up with objective measures, such as specific lab test results or clear findings from a physical exam. But there are steps that can increase the odds of a successful claim for long COVID disability benefits, according to New York–based law firm Hiller.
For starters, patients can document COVID test results, and if testing wasn’t conducted, patients can detail the specific symptoms that led to this diagnosis, Hiller advises. Then patients can keep a daily symptom log at home that run lists all of the specific symptoms that occur at different times during the day and night to help establish a pattern of disability. These logs should provide specific details about every job duty patients have and exactly how specific symptoms of long COVID interfere with these duties.
Even though objective testing is hard to come by for long COVID, people should undergo all the tests they can that may help document the frequency or severity of specific symptoms that make it impossible to carry on with business as usual at work, Hiller advises. This may include neuropsychological testing to document brain fog, a cardiopulmonary exercise test to demonstrate chronic fatigue and the inability to exercise, or a tilt table test to measure dizziness.
Seeking a doctor’s diagnosis can be key to collecting disability payments, in or out of court.
All of this puts a lot of pressure on doctors and patients to build strong cases, said Jonathan Whiteson, MD, codirector of the NYU Langone Health post-COVID care program in New York. “Many physicians are not familiar with the disability benefit paperwork, and so this is a challenge for the doctors to know how to complete and to build the time into their highly scheduled days to take the time needed to complete.
“It’s also challenging because most of the disability benefit forms are ‘generic’ and do not ask specific questions about COVID disability,” Dr. Whiteson added. “It can be like trying to drive a square peg into a round hole.”
Still, when it comes to long COVID, completing disability paperwork is increasingly becoming part of standard care, along with managing medication, rehabilitation therapies, and lifestyle changes to navigate daily life with this illness, Dr. Whiteson noted.
Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the Post-COVID-19 Recovery Clinic at the University of Texas Health Science Center, San Antonio, agreed with this assessment.
“I have done letter upon letter of appeal to disability insurance companies,” she said.
Some doctors, however, are reluctant to step up in such cases, in part because no standard diagnostic guidelines exist for long COVID and because it can be frustrating.
“This is the work that is not paid and causes burnout in physicians,” Dr. Verduzco-Gutierrez said. “The paperwork, the fighting with insurance companies, the resubmission of forms for disability all to get what your patient needs – and then it gets denied.
“We will keep doing this because our patients need this disability income in order to live their lives and to afford what they need for recovery,” said Dr. Verduzco-Gutierrez. “But at some point something has to change because this isn’t sustainable.”
A version of this article appeared on Medscape.com.
At least 30 lawsuits have been filed seeking legal resolution of disability insurance claims, according to searches of court records. In addition, the Social Security Administration said it has received about 52,000 disability claims tied to SARS-CoV-2 infections, which represents 1% of all applications.
But legal experts say those cases may not reflect the total number of cases that have gone to court. They note many claims are initially dismissed and are not appealed by claimants.
“With this system, they deny two-thirds of initial applications, then people who appeal get denied almost 90% of the time, and then they can appeal before a judge,” said Kevin LaPorte, a Social Security disability attorney at LaPorte Law Firm in Oakland, Calif. “What happens next doesn’t have a lot of precedent because long COVID is a mass disabling event, and we haven’t seen that many of these cases get all the way through the legal system yet.”
As a result, the exact number of long COVID disability claims and the number of these cases going to court isn’t clear, he said.
“It can take a year or more for cases to get to court, and even longer to reach resolution,” Mr. LaPorte added. “I suspect the few cases we’ve heard about at this point are going to be the tip of the iceberg.”
The process is convoluted and can drag on for months with multiple denials and appeals along the way. Many disabled workers find their only recourse is to take insurers to court.
Long COVID patients typically apply for disability benefits through private insurance or Social Security. But the process can drag on for months, so many find their only recourse is to take insurers to court, according to legal experts.
But even in the courts, many encounter delays and hurdles to resolution.
In one of the first federal lawsuits involving long COVID disability benefits, William Abrams, a trial and appellate attorney and active marathon runner, sued Unum Life Insurance seeking long-term disability income. Symptoms included extreme fatigue, brain fog, decreased attention and concentration, and nearly daily fevers, causing him to stop working in April 2020.
His diagnosis wasn’t definitive. Three doctors said he had long COVID, and four said he had chronic fatigue syndrome. Unum cited this inconsistency as a rationale for rejecting his claim. But the court sided with Mr. Abrams, granting him disability income. The court concluded: “Unum may be correct that [the plaintiff] has not been correctly diagnosed. But that does not mean he is not sick. If [the plaintiff’s] complaints, and [the doctor’s] assessments, are to be believed, [the plaintiff] cannot focus for more than a few minutes at a time, making it impossible for [the plaintiff] to perform the varied and complex tasks his job requires.”
Unum said in an emailed statement that the company doesn’t comment on specific claims as a matter of policy, adding that its total payouts for disability claims from March 2020 to February 2022 were 35% higher than prepandemic levels. “In general, disability and leave claims connected to COVID-19 have been primarily short-term events with the majority of claimants recovering prior to completing the normal qualification period for long-term disability insurance,” Unum said.
Mr. Abrams prevailed in part because he had detailed documentation of the numerous impairments that eventually required him to stop work, said Michelle Roberts of Roberts Disability Law in Oakland, Calif.
He submitted videos of himself taking his temperature to prove he had almost daily fevers, according to court records. He underwent neuropsychological testing, which found learning deficiencies and memory deficits.
Mr. Abrams also submitted statements from a colleague who worked with him on a complex technology patent case involving radiofrequency identification. Before he got COVID, Mr. Abrams “had the analytical ability, legal acumen, and mental energy to attack that learning curve and get up to speed very rapidly,” according to court records.
“The court focused on credulity.” Ms. Roberts said. “There was all this work to be done to show this person was high functioning and ran marathons and worked in an intense, high-pressure occupation but then couldn’t do anything after long COVID.”
Documentation was also crucial in another early federal long COVID disability lawsuit that was filed in 2022 on behalf of Wendy Haut, an educational software sales representative in California who turned to the courts seeking disability income through her company’s employee benefits plan.
Several of Ms. Haut’s doctors documented a detailed list of long COVID symptoms, including “profound fatigue and extreme cognitive difficulties,” that they said prevented her from working as a sales representative or doing any other type of job. A settlement agreement in June 2022 required Reliance Standard Life Insurance to pay Ms. Haut long-term disability benefits, including previously unpaid benefits, according to a report by the advocacy group Pandemic Patients.
Representatives of Reliance Standard didn’t respond to a request for comment.
The growing number of workers being sidelined by long COVID makes more claims and more court cases likely. Right now, an estimated 16 million working-age Americans aged 18-65 years have long COVID, and as many as 4 million of them can’t work, according to a July 2023 Census Bureau report.
Uncertainty about the volume of claims in the pipeline is part of what’s driving some insurers to fight long COVID claims, Ms. Roberts said. Another factor is the lack of clarity around how many years people with long COVID may be out of work, particularly if they’re in their 30s or 40s and might be seeking disability income until they reach retirement age.
“Doctors are not always saying that this person will be permanently disabled,” Ms. Roberts said. “If this person doesn’t get better and they’re disabled until retirement age, this could be a payout in the high six or seven figures if a person is very young and was a very high earner.”
Insurance companies routinely deny claims that can’t be backed up with objective measures, such as specific lab test results or clear findings from a physical exam. But there are steps that can increase the odds of a successful claim for long COVID disability benefits, according to New York–based law firm Hiller.
For starters, patients can document COVID test results, and if testing wasn’t conducted, patients can detail the specific symptoms that led to this diagnosis, Hiller advises. Then patients can keep a daily symptom log at home that run lists all of the specific symptoms that occur at different times during the day and night to help establish a pattern of disability. These logs should provide specific details about every job duty patients have and exactly how specific symptoms of long COVID interfere with these duties.
Even though objective testing is hard to come by for long COVID, people should undergo all the tests they can that may help document the frequency or severity of specific symptoms that make it impossible to carry on with business as usual at work, Hiller advises. This may include neuropsychological testing to document brain fog, a cardiopulmonary exercise test to demonstrate chronic fatigue and the inability to exercise, or a tilt table test to measure dizziness.
Seeking a doctor’s diagnosis can be key to collecting disability payments, in or out of court.
All of this puts a lot of pressure on doctors and patients to build strong cases, said Jonathan Whiteson, MD, codirector of the NYU Langone Health post-COVID care program in New York. “Many physicians are not familiar with the disability benefit paperwork, and so this is a challenge for the doctors to know how to complete and to build the time into their highly scheduled days to take the time needed to complete.
“It’s also challenging because most of the disability benefit forms are ‘generic’ and do not ask specific questions about COVID disability,” Dr. Whiteson added. “It can be like trying to drive a square peg into a round hole.”
Still, when it comes to long COVID, completing disability paperwork is increasingly becoming part of standard care, along with managing medication, rehabilitation therapies, and lifestyle changes to navigate daily life with this illness, Dr. Whiteson noted.
Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the Post-COVID-19 Recovery Clinic at the University of Texas Health Science Center, San Antonio, agreed with this assessment.
“I have done letter upon letter of appeal to disability insurance companies,” she said.
Some doctors, however, are reluctant to step up in such cases, in part because no standard diagnostic guidelines exist for long COVID and because it can be frustrating.
“This is the work that is not paid and causes burnout in physicians,” Dr. Verduzco-Gutierrez said. “The paperwork, the fighting with insurance companies, the resubmission of forms for disability all to get what your patient needs – and then it gets denied.
“We will keep doing this because our patients need this disability income in order to live their lives and to afford what they need for recovery,” said Dr. Verduzco-Gutierrez. “But at some point something has to change because this isn’t sustainable.”
A version of this article appeared on Medscape.com.
At least 30 lawsuits have been filed seeking legal resolution of disability insurance claims, according to searches of court records. In addition, the Social Security Administration said it has received about 52,000 disability claims tied to SARS-CoV-2 infections, which represents 1% of all applications.
But legal experts say those cases may not reflect the total number of cases that have gone to court. They note many claims are initially dismissed and are not appealed by claimants.
“With this system, they deny two-thirds of initial applications, then people who appeal get denied almost 90% of the time, and then they can appeal before a judge,” said Kevin LaPorte, a Social Security disability attorney at LaPorte Law Firm in Oakland, Calif. “What happens next doesn’t have a lot of precedent because long COVID is a mass disabling event, and we haven’t seen that many of these cases get all the way through the legal system yet.”
As a result, the exact number of long COVID disability claims and the number of these cases going to court isn’t clear, he said.
“It can take a year or more for cases to get to court, and even longer to reach resolution,” Mr. LaPorte added. “I suspect the few cases we’ve heard about at this point are going to be the tip of the iceberg.”
The process is convoluted and can drag on for months with multiple denials and appeals along the way. Many disabled workers find their only recourse is to take insurers to court.
Long COVID patients typically apply for disability benefits through private insurance or Social Security. But the process can drag on for months, so many find their only recourse is to take insurers to court, according to legal experts.
But even in the courts, many encounter delays and hurdles to resolution.
In one of the first federal lawsuits involving long COVID disability benefits, William Abrams, a trial and appellate attorney and active marathon runner, sued Unum Life Insurance seeking long-term disability income. Symptoms included extreme fatigue, brain fog, decreased attention and concentration, and nearly daily fevers, causing him to stop working in April 2020.
His diagnosis wasn’t definitive. Three doctors said he had long COVID, and four said he had chronic fatigue syndrome. Unum cited this inconsistency as a rationale for rejecting his claim. But the court sided with Mr. Abrams, granting him disability income. The court concluded: “Unum may be correct that [the plaintiff] has not been correctly diagnosed. But that does not mean he is not sick. If [the plaintiff’s] complaints, and [the doctor’s] assessments, are to be believed, [the plaintiff] cannot focus for more than a few minutes at a time, making it impossible for [the plaintiff] to perform the varied and complex tasks his job requires.”
Unum said in an emailed statement that the company doesn’t comment on specific claims as a matter of policy, adding that its total payouts for disability claims from March 2020 to February 2022 were 35% higher than prepandemic levels. “In general, disability and leave claims connected to COVID-19 have been primarily short-term events with the majority of claimants recovering prior to completing the normal qualification period for long-term disability insurance,” Unum said.
Mr. Abrams prevailed in part because he had detailed documentation of the numerous impairments that eventually required him to stop work, said Michelle Roberts of Roberts Disability Law in Oakland, Calif.
He submitted videos of himself taking his temperature to prove he had almost daily fevers, according to court records. He underwent neuropsychological testing, which found learning deficiencies and memory deficits.
Mr. Abrams also submitted statements from a colleague who worked with him on a complex technology patent case involving radiofrequency identification. Before he got COVID, Mr. Abrams “had the analytical ability, legal acumen, and mental energy to attack that learning curve and get up to speed very rapidly,” according to court records.
“The court focused on credulity.” Ms. Roberts said. “There was all this work to be done to show this person was high functioning and ran marathons and worked in an intense, high-pressure occupation but then couldn’t do anything after long COVID.”
Documentation was also crucial in another early federal long COVID disability lawsuit that was filed in 2022 on behalf of Wendy Haut, an educational software sales representative in California who turned to the courts seeking disability income through her company’s employee benefits plan.
Several of Ms. Haut’s doctors documented a detailed list of long COVID symptoms, including “profound fatigue and extreme cognitive difficulties,” that they said prevented her from working as a sales representative or doing any other type of job. A settlement agreement in June 2022 required Reliance Standard Life Insurance to pay Ms. Haut long-term disability benefits, including previously unpaid benefits, according to a report by the advocacy group Pandemic Patients.
Representatives of Reliance Standard didn’t respond to a request for comment.
The growing number of workers being sidelined by long COVID makes more claims and more court cases likely. Right now, an estimated 16 million working-age Americans aged 18-65 years have long COVID, and as many as 4 million of them can’t work, according to a July 2023 Census Bureau report.
Uncertainty about the volume of claims in the pipeline is part of what’s driving some insurers to fight long COVID claims, Ms. Roberts said. Another factor is the lack of clarity around how many years people with long COVID may be out of work, particularly if they’re in their 30s or 40s and might be seeking disability income until they reach retirement age.
“Doctors are not always saying that this person will be permanently disabled,” Ms. Roberts said. “If this person doesn’t get better and they’re disabled until retirement age, this could be a payout in the high six or seven figures if a person is very young and was a very high earner.”
Insurance companies routinely deny claims that can’t be backed up with objective measures, such as specific lab test results or clear findings from a physical exam. But there are steps that can increase the odds of a successful claim for long COVID disability benefits, according to New York–based law firm Hiller.
For starters, patients can document COVID test results, and if testing wasn’t conducted, patients can detail the specific symptoms that led to this diagnosis, Hiller advises. Then patients can keep a daily symptom log at home that run lists all of the specific symptoms that occur at different times during the day and night to help establish a pattern of disability. These logs should provide specific details about every job duty patients have and exactly how specific symptoms of long COVID interfere with these duties.
Even though objective testing is hard to come by for long COVID, people should undergo all the tests they can that may help document the frequency or severity of specific symptoms that make it impossible to carry on with business as usual at work, Hiller advises. This may include neuropsychological testing to document brain fog, a cardiopulmonary exercise test to demonstrate chronic fatigue and the inability to exercise, or a tilt table test to measure dizziness.
Seeking a doctor’s diagnosis can be key to collecting disability payments, in or out of court.
All of this puts a lot of pressure on doctors and patients to build strong cases, said Jonathan Whiteson, MD, codirector of the NYU Langone Health post-COVID care program in New York. “Many physicians are not familiar with the disability benefit paperwork, and so this is a challenge for the doctors to know how to complete and to build the time into their highly scheduled days to take the time needed to complete.
“It’s also challenging because most of the disability benefit forms are ‘generic’ and do not ask specific questions about COVID disability,” Dr. Whiteson added. “It can be like trying to drive a square peg into a round hole.”
Still, when it comes to long COVID, completing disability paperwork is increasingly becoming part of standard care, along with managing medication, rehabilitation therapies, and lifestyle changes to navigate daily life with this illness, Dr. Whiteson noted.
Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the Post-COVID-19 Recovery Clinic at the University of Texas Health Science Center, San Antonio, agreed with this assessment.
“I have done letter upon letter of appeal to disability insurance companies,” she said.
Some doctors, however, are reluctant to step up in such cases, in part because no standard diagnostic guidelines exist for long COVID and because it can be frustrating.
“This is the work that is not paid and causes burnout in physicians,” Dr. Verduzco-Gutierrez said. “The paperwork, the fighting with insurance companies, the resubmission of forms for disability all to get what your patient needs – and then it gets denied.
“We will keep doing this because our patients need this disability income in order to live their lives and to afford what they need for recovery,” said Dr. Verduzco-Gutierrez. “But at some point something has to change because this isn’t sustainable.”
A version of this article appeared on Medscape.com.
How soybean oil could lead to gut inflammation
A popular ingredient in the American diet has been linked to ulcerative colitis. The ingredient is soybean oil, which is very common in processed foods. In fact, U.S. per capita consumption of soybean oil increased more than 1,000-fold during the 20th century.
In a study from the University of California, Riverside, and UC Davis, published in Gut Microbes, mice fed a diet high in soybean oil were more at risk of developing colitis.
The likely culprit? Linoleic acid, an omega-6 fatty acid that composes up to 60% of soybean oil.
Small amounts of linoleic acid help maintain the body’s water balance. But Americans derive as much as 10% of their daily energy from linoleic acid, when they need only 1%-2%, the researchers say.
The findings build on earlier research linking a high-linoleic acid diet with inflammatory bowel disease, or IBD, in humans. (Previous research in mice has also linked high consumption of the oil with obesity and diabetes in the rodents.)
For the new study, the researchers wanted to drill down into how linoleic acid affects the gut.
How linoleic acid may promote inflammation
In mice, the soybean oil diet upset the ratio of omega-3 to omega-6 fatty acids in the gut. This led to a decrease in endocannabinoids, lipid-based molecules that help block inflammation.
Enzymes that metabolize fatty acids are “shared between two pathways,” said study coauthor Frances Sladek, PhD, professor of cell biology at UC Riverside. “If you swamp the system with linoleic acid, you’ll have less enzymes available to metabolize omega-3s into good endocannabinoids.”
The endocannabinoid system has been linked to “visceral pain” in the gut, said Punyanganie de Silva, MD, MPH, an assistant professor at Brigham & Women’s Hospital, Boston, who was not involved in the study. But the relationship between the endocannabinoid system and inflammation has yet to be fully explored.
“This is one of the first papers that has looked at the association between linoleic acid and the endocannabinoid system,” Dr. de Silva said.
Changes in the gut microbiome
The gut microbiome of the mice also showed increased amounts of adherent invasive E. coli, a type of bacteria that grows by using linoleic acid as a carbon source. A “very close relative” of this bacteria has been linked to IBD in humans, Dr. Sladek said.
Using a method known as metabolomics, the researchers studied 3,000 metabolites in the intestinal cells of both the mice and the bacteria. Endocannabinoids decreased in both.
“We were actually quite surprised. I didn’t realize that bacteria made endocannabinoids,” Dr. Sladek said.
Helpful bacteria, such as the probiotic lactobacillus species, died off. The mice also had increased levels of oxylipins, which are correlated with obesity in mice and colitis in humans.
A high–linoleic acid diet could mean a leaky gut
Linoleic acid binds to a protein known as HNF-4 alpha. Disrupting the expression of this protein can weaken the intestinal barrier, letting toxins flow into the body – more commonly known as leaky gut. Mice on the soybean oil diet had decreased levels of the protein and more porous intestinal barriers, raising the risk for inflammation and colitis. “The HNF-4 alpha protein is conserved from mouse to human, so whatever’s happening to it in the context of the mouse gut, there’s a very high chance that a similar effect could be seen in humans as well,” said study coauthor Poonamjot Deol, PhD, an assistant professional researcher at UC Riverside.
Still, Dr. de Silva urges “some caution when interpreting these results,” given that “this is still experimental and needs to be reproduced in clinical studies as humans have a far more varied microbiome and more variable environmental exposures than these very controlled mouse model studies.”
Dr. de Silva says cooking with olive oil can “help increase omega-3 to omega-6 ratios” and advises eating a varied diet that includes omega-3 fats, such as flaxseed and walnuts, and minimal amounts of processed foods and saturated fats.
Looking ahead, endocannabinoids are being explored as “a potential therapy for treating IBD symptoms,” said Dr. Deol. She hopes to delve further into how linoleic acid affects the endocannabinoid system.
A version of this article first appeared on WebMD.com.
A popular ingredient in the American diet has been linked to ulcerative colitis. The ingredient is soybean oil, which is very common in processed foods. In fact, U.S. per capita consumption of soybean oil increased more than 1,000-fold during the 20th century.
In a study from the University of California, Riverside, and UC Davis, published in Gut Microbes, mice fed a diet high in soybean oil were more at risk of developing colitis.
The likely culprit? Linoleic acid, an omega-6 fatty acid that composes up to 60% of soybean oil.
Small amounts of linoleic acid help maintain the body’s water balance. But Americans derive as much as 10% of their daily energy from linoleic acid, when they need only 1%-2%, the researchers say.
The findings build on earlier research linking a high-linoleic acid diet with inflammatory bowel disease, or IBD, in humans. (Previous research in mice has also linked high consumption of the oil with obesity and diabetes in the rodents.)
For the new study, the researchers wanted to drill down into how linoleic acid affects the gut.
How linoleic acid may promote inflammation
In mice, the soybean oil diet upset the ratio of omega-3 to omega-6 fatty acids in the gut. This led to a decrease in endocannabinoids, lipid-based molecules that help block inflammation.
Enzymes that metabolize fatty acids are “shared between two pathways,” said study coauthor Frances Sladek, PhD, professor of cell biology at UC Riverside. “If you swamp the system with linoleic acid, you’ll have less enzymes available to metabolize omega-3s into good endocannabinoids.”
The endocannabinoid system has been linked to “visceral pain” in the gut, said Punyanganie de Silva, MD, MPH, an assistant professor at Brigham & Women’s Hospital, Boston, who was not involved in the study. But the relationship between the endocannabinoid system and inflammation has yet to be fully explored.
“This is one of the first papers that has looked at the association between linoleic acid and the endocannabinoid system,” Dr. de Silva said.
Changes in the gut microbiome
The gut microbiome of the mice also showed increased amounts of adherent invasive E. coli, a type of bacteria that grows by using linoleic acid as a carbon source. A “very close relative” of this bacteria has been linked to IBD in humans, Dr. Sladek said.
Using a method known as metabolomics, the researchers studied 3,000 metabolites in the intestinal cells of both the mice and the bacteria. Endocannabinoids decreased in both.
“We were actually quite surprised. I didn’t realize that bacteria made endocannabinoids,” Dr. Sladek said.
Helpful bacteria, such as the probiotic lactobacillus species, died off. The mice also had increased levels of oxylipins, which are correlated with obesity in mice and colitis in humans.
A high–linoleic acid diet could mean a leaky gut
Linoleic acid binds to a protein known as HNF-4 alpha. Disrupting the expression of this protein can weaken the intestinal barrier, letting toxins flow into the body – more commonly known as leaky gut. Mice on the soybean oil diet had decreased levels of the protein and more porous intestinal barriers, raising the risk for inflammation and colitis. “The HNF-4 alpha protein is conserved from mouse to human, so whatever’s happening to it in the context of the mouse gut, there’s a very high chance that a similar effect could be seen in humans as well,” said study coauthor Poonamjot Deol, PhD, an assistant professional researcher at UC Riverside.
Still, Dr. de Silva urges “some caution when interpreting these results,” given that “this is still experimental and needs to be reproduced in clinical studies as humans have a far more varied microbiome and more variable environmental exposures than these very controlled mouse model studies.”
Dr. de Silva says cooking with olive oil can “help increase omega-3 to omega-6 ratios” and advises eating a varied diet that includes omega-3 fats, such as flaxseed and walnuts, and minimal amounts of processed foods and saturated fats.
Looking ahead, endocannabinoids are being explored as “a potential therapy for treating IBD symptoms,” said Dr. Deol. She hopes to delve further into how linoleic acid affects the endocannabinoid system.
A version of this article first appeared on WebMD.com.
A popular ingredient in the American diet has been linked to ulcerative colitis. The ingredient is soybean oil, which is very common in processed foods. In fact, U.S. per capita consumption of soybean oil increased more than 1,000-fold during the 20th century.
In a study from the University of California, Riverside, and UC Davis, published in Gut Microbes, mice fed a diet high in soybean oil were more at risk of developing colitis.
The likely culprit? Linoleic acid, an omega-6 fatty acid that composes up to 60% of soybean oil.
Small amounts of linoleic acid help maintain the body’s water balance. But Americans derive as much as 10% of their daily energy from linoleic acid, when they need only 1%-2%, the researchers say.
The findings build on earlier research linking a high-linoleic acid diet with inflammatory bowel disease, or IBD, in humans. (Previous research in mice has also linked high consumption of the oil with obesity and diabetes in the rodents.)
For the new study, the researchers wanted to drill down into how linoleic acid affects the gut.
How linoleic acid may promote inflammation
In mice, the soybean oil diet upset the ratio of omega-3 to omega-6 fatty acids in the gut. This led to a decrease in endocannabinoids, lipid-based molecules that help block inflammation.
Enzymes that metabolize fatty acids are “shared between two pathways,” said study coauthor Frances Sladek, PhD, professor of cell biology at UC Riverside. “If you swamp the system with linoleic acid, you’ll have less enzymes available to metabolize omega-3s into good endocannabinoids.”
The endocannabinoid system has been linked to “visceral pain” in the gut, said Punyanganie de Silva, MD, MPH, an assistant professor at Brigham & Women’s Hospital, Boston, who was not involved in the study. But the relationship between the endocannabinoid system and inflammation has yet to be fully explored.
“This is one of the first papers that has looked at the association between linoleic acid and the endocannabinoid system,” Dr. de Silva said.
Changes in the gut microbiome
The gut microbiome of the mice also showed increased amounts of adherent invasive E. coli, a type of bacteria that grows by using linoleic acid as a carbon source. A “very close relative” of this bacteria has been linked to IBD in humans, Dr. Sladek said.
Using a method known as metabolomics, the researchers studied 3,000 metabolites in the intestinal cells of both the mice and the bacteria. Endocannabinoids decreased in both.
“We were actually quite surprised. I didn’t realize that bacteria made endocannabinoids,” Dr. Sladek said.
Helpful bacteria, such as the probiotic lactobacillus species, died off. The mice also had increased levels of oxylipins, which are correlated with obesity in mice and colitis in humans.
A high–linoleic acid diet could mean a leaky gut
Linoleic acid binds to a protein known as HNF-4 alpha. Disrupting the expression of this protein can weaken the intestinal barrier, letting toxins flow into the body – more commonly known as leaky gut. Mice on the soybean oil diet had decreased levels of the protein and more porous intestinal barriers, raising the risk for inflammation and colitis. “The HNF-4 alpha protein is conserved from mouse to human, so whatever’s happening to it in the context of the mouse gut, there’s a very high chance that a similar effect could be seen in humans as well,” said study coauthor Poonamjot Deol, PhD, an assistant professional researcher at UC Riverside.
Still, Dr. de Silva urges “some caution when interpreting these results,” given that “this is still experimental and needs to be reproduced in clinical studies as humans have a far more varied microbiome and more variable environmental exposures than these very controlled mouse model studies.”
Dr. de Silva says cooking with olive oil can “help increase omega-3 to omega-6 ratios” and advises eating a varied diet that includes omega-3 fats, such as flaxseed and walnuts, and minimal amounts of processed foods and saturated fats.
Looking ahead, endocannabinoids are being explored as “a potential therapy for treating IBD symptoms,” said Dr. Deol. She hopes to delve further into how linoleic acid affects the endocannabinoid system.
A version of this article first appeared on WebMD.com.
FROM GUT MICROBES
Offering HPV vaccine at age 9 linked to greater series completion
BALTIMORE – , according to a retrospective cohort study of commercially insured youth presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The research was published ahead of print in Human Vaccines and Immunotherapeutics.
Changing attitudes
“These findings are novel because they emphasize starting at age 9, and that is different than prior studies that emphasize bundling of these vaccines,” Kevin Ault, MD, professor and chair of the department of obstetrics and gynecology at Western Michigan University Homer Stryker MD School of Medicine and a former member of the CDC’s Advisory Committee on Immunization Practices, said in an interview.
Dr. Ault was not involved in the study but noted that these findings support the AAP’s recommendation to start the HPV vaccine series at age 9. The Centers for Disease Control and Prevention currently recommends giving the first dose of the HPV vaccine at ages 11-12, at the same time as the Tdap and meningitis vaccines. This recommendation to “bundle” the HPV vaccine with the Tdap and meningitis vaccines aims to facilitate provider-family discussion about the HPV vaccine, ideally reducing parent hesitancy and concerns about the vaccines. Multiple studies have shown improved HPV vaccine uptake when providers offer the HPV vaccine at the same time as the Tdap and meningococcal vaccines.
However, shifts in parents’ attitudes have occurred toward the HPV vaccine since those studies on bundling: Concerns about sexual activity have receded while concerns about safety remain high. The American Academy of Pediatrics and the American Cancer Society both advise starting the HPV vaccine series at age 9, based on evidence showing that more children complete the series when they get the first shot before age 11 compared to getting it at 11 or 12.
“The bundling was really to vaccinate people by the age of 13, thinking that onset of sexual activity was after that,” study author Sidika Kajtezovic, MD, a resident at Boston Medical Center and Boston University Obstetrics and Gynecology, said in an interview. But Dr. Kajtezovic said she delivers babies for 13-year-old patients. “Kids are having sex sooner or sooner.” It’s also clear that using the bundling strategy is not making up the entire gap right now: Ninety percent of children are getting the meningococcal vaccine while only 49% are getting the HPV vaccine, Dr. Kajtezovic pointed out. “There’s a disconnect happening there, even with the bundling,” she said.
Debundling vaccines
Dr. Kajtezovic and her colleagues used a national database of employee-sponsored health insurance to analyze the records of 100,857 children who were continuously enrolled in a plan from age 9 in 2015 to age 13 in 2019. They calculated the odds of children completing the HPV vaccine series based on whether they started the series before, at the same time as, or after the Tdap vaccination.
Youth who received the HPV vaccine before their Tdap vaccine had 38% greater odds of completing the series – getting both doses – than did those who received the HPV vaccine at the same time as the Tdap vaccine. Meanwhile, in line with prior evidence, those who got the first HPV dose after their Tdap were less likely – 68% lower odds – to complete the two- or three-dose (if starting above age 14) series.
The researchers identified several other factors that were linked to completing the HPV vaccine series. Females had greater odds than did males of completing the series, as did those living in urban, rather than rural, areas. Other factors associated with completing the series included living in the Northeast United States and receiving primary care from a pediatrician rather than a family medicine physician.
Timing is important
“I am encouraged by the findings of this study,” Dr. Ault said in an interview. “However, I would have liked the authors to expand the age range a bit higher. There are data that continuing to discuss the HPV vaccine with parents and teens will increase uptake into the later teen years.”
One challenge is that research shows attendance at primary care visits declines in older adolescence. Since there is no second Tdap or meningitis shot, families need to return for the second HPV vaccine dose after those shots, though they could get the second dose at the same time as other two vaccines if they receive the first dose before age 11. There’s also evidence suggesting that providers find conversations about the HPV vaccine easier when sexual activity is not the focus.
“I often feel that, before a child reaches adolescence, they’re almost, in a way, not sexualized yet, so talking about cancer prevention for an 8- or 9-year-old sometimes sounds a little different to patients versus protecting your 12-year-old, who’s starting to go through adolescence and developing breasts” and other signs of puberty, Dr. Kajtezovic said. Keeping the focus of HPV vaccine discussions on cancer prevention also allows providers to point out the protection against anal cancer, vulvar cancer, vaginal cancer, and head and neck cancer. “They are horrible, and even if they’re treatable, they’re often very hard to treat at an advanced stage,” Dr. Kajtezovic said. “The surgery required is so life disabling and disfiguring.”
The HPV Roundtable advises continuing bundling at practices having success with it but encourages practices to consider earlier vaccination if their uptake is lagging. Quality improvement initiatives, such as earlier electronic medical record prompts and multi-level interventions in pediatric practices, have shown substantial increases in HPV vaccine uptake at 9 and 10 years old. One survey in 2021 found that one in five primary care providers already routinely recommend the HPV vaccine at ages 9-10, and nearly half of others would consider doing so.
“My hope is in the next few years, when [the CDC] refreshes their vaccine recommendations, that they will either unbundle it or move the bar a few years earlier so that you can initiate it to encourage earlier initiation,” Dr. Kajtezovic said.
Dr. Ault had no other disclosures besides prior service on ACIP. Dr. Kajtezovic had no disclosures.
BALTIMORE – , according to a retrospective cohort study of commercially insured youth presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The research was published ahead of print in Human Vaccines and Immunotherapeutics.
Changing attitudes
“These findings are novel because they emphasize starting at age 9, and that is different than prior studies that emphasize bundling of these vaccines,” Kevin Ault, MD, professor and chair of the department of obstetrics and gynecology at Western Michigan University Homer Stryker MD School of Medicine and a former member of the CDC’s Advisory Committee on Immunization Practices, said in an interview.
Dr. Ault was not involved in the study but noted that these findings support the AAP’s recommendation to start the HPV vaccine series at age 9. The Centers for Disease Control and Prevention currently recommends giving the first dose of the HPV vaccine at ages 11-12, at the same time as the Tdap and meningitis vaccines. This recommendation to “bundle” the HPV vaccine with the Tdap and meningitis vaccines aims to facilitate provider-family discussion about the HPV vaccine, ideally reducing parent hesitancy and concerns about the vaccines. Multiple studies have shown improved HPV vaccine uptake when providers offer the HPV vaccine at the same time as the Tdap and meningococcal vaccines.
However, shifts in parents’ attitudes have occurred toward the HPV vaccine since those studies on bundling: Concerns about sexual activity have receded while concerns about safety remain high. The American Academy of Pediatrics and the American Cancer Society both advise starting the HPV vaccine series at age 9, based on evidence showing that more children complete the series when they get the first shot before age 11 compared to getting it at 11 or 12.
“The bundling was really to vaccinate people by the age of 13, thinking that onset of sexual activity was after that,” study author Sidika Kajtezovic, MD, a resident at Boston Medical Center and Boston University Obstetrics and Gynecology, said in an interview. But Dr. Kajtezovic said she delivers babies for 13-year-old patients. “Kids are having sex sooner or sooner.” It’s also clear that using the bundling strategy is not making up the entire gap right now: Ninety percent of children are getting the meningococcal vaccine while only 49% are getting the HPV vaccine, Dr. Kajtezovic pointed out. “There’s a disconnect happening there, even with the bundling,” she said.
Debundling vaccines
Dr. Kajtezovic and her colleagues used a national database of employee-sponsored health insurance to analyze the records of 100,857 children who were continuously enrolled in a plan from age 9 in 2015 to age 13 in 2019. They calculated the odds of children completing the HPV vaccine series based on whether they started the series before, at the same time as, or after the Tdap vaccination.
Youth who received the HPV vaccine before their Tdap vaccine had 38% greater odds of completing the series – getting both doses – than did those who received the HPV vaccine at the same time as the Tdap vaccine. Meanwhile, in line with prior evidence, those who got the first HPV dose after their Tdap were less likely – 68% lower odds – to complete the two- or three-dose (if starting above age 14) series.
The researchers identified several other factors that were linked to completing the HPV vaccine series. Females had greater odds than did males of completing the series, as did those living in urban, rather than rural, areas. Other factors associated with completing the series included living in the Northeast United States and receiving primary care from a pediatrician rather than a family medicine physician.
Timing is important
“I am encouraged by the findings of this study,” Dr. Ault said in an interview. “However, I would have liked the authors to expand the age range a bit higher. There are data that continuing to discuss the HPV vaccine with parents and teens will increase uptake into the later teen years.”
One challenge is that research shows attendance at primary care visits declines in older adolescence. Since there is no second Tdap or meningitis shot, families need to return for the second HPV vaccine dose after those shots, though they could get the second dose at the same time as other two vaccines if they receive the first dose before age 11. There’s also evidence suggesting that providers find conversations about the HPV vaccine easier when sexual activity is not the focus.
“I often feel that, before a child reaches adolescence, they’re almost, in a way, not sexualized yet, so talking about cancer prevention for an 8- or 9-year-old sometimes sounds a little different to patients versus protecting your 12-year-old, who’s starting to go through adolescence and developing breasts” and other signs of puberty, Dr. Kajtezovic said. Keeping the focus of HPV vaccine discussions on cancer prevention also allows providers to point out the protection against anal cancer, vulvar cancer, vaginal cancer, and head and neck cancer. “They are horrible, and even if they’re treatable, they’re often very hard to treat at an advanced stage,” Dr. Kajtezovic said. “The surgery required is so life disabling and disfiguring.”
The HPV Roundtable advises continuing bundling at practices having success with it but encourages practices to consider earlier vaccination if their uptake is lagging. Quality improvement initiatives, such as earlier electronic medical record prompts and multi-level interventions in pediatric practices, have shown substantial increases in HPV vaccine uptake at 9 and 10 years old. One survey in 2021 found that one in five primary care providers already routinely recommend the HPV vaccine at ages 9-10, and nearly half of others would consider doing so.
“My hope is in the next few years, when [the CDC] refreshes their vaccine recommendations, that they will either unbundle it or move the bar a few years earlier so that you can initiate it to encourage earlier initiation,” Dr. Kajtezovic said.
Dr. Ault had no other disclosures besides prior service on ACIP. Dr. Kajtezovic had no disclosures.
BALTIMORE – , according to a retrospective cohort study of commercially insured youth presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The research was published ahead of print in Human Vaccines and Immunotherapeutics.
Changing attitudes
“These findings are novel because they emphasize starting at age 9, and that is different than prior studies that emphasize bundling of these vaccines,” Kevin Ault, MD, professor and chair of the department of obstetrics and gynecology at Western Michigan University Homer Stryker MD School of Medicine and a former member of the CDC’s Advisory Committee on Immunization Practices, said in an interview.
Dr. Ault was not involved in the study but noted that these findings support the AAP’s recommendation to start the HPV vaccine series at age 9. The Centers for Disease Control and Prevention currently recommends giving the first dose of the HPV vaccine at ages 11-12, at the same time as the Tdap and meningitis vaccines. This recommendation to “bundle” the HPV vaccine with the Tdap and meningitis vaccines aims to facilitate provider-family discussion about the HPV vaccine, ideally reducing parent hesitancy and concerns about the vaccines. Multiple studies have shown improved HPV vaccine uptake when providers offer the HPV vaccine at the same time as the Tdap and meningococcal vaccines.
However, shifts in parents’ attitudes have occurred toward the HPV vaccine since those studies on bundling: Concerns about sexual activity have receded while concerns about safety remain high. The American Academy of Pediatrics and the American Cancer Society both advise starting the HPV vaccine series at age 9, based on evidence showing that more children complete the series when they get the first shot before age 11 compared to getting it at 11 or 12.
“The bundling was really to vaccinate people by the age of 13, thinking that onset of sexual activity was after that,” study author Sidika Kajtezovic, MD, a resident at Boston Medical Center and Boston University Obstetrics and Gynecology, said in an interview. But Dr. Kajtezovic said she delivers babies for 13-year-old patients. “Kids are having sex sooner or sooner.” It’s also clear that using the bundling strategy is not making up the entire gap right now: Ninety percent of children are getting the meningococcal vaccine while only 49% are getting the HPV vaccine, Dr. Kajtezovic pointed out. “There’s a disconnect happening there, even with the bundling,” she said.
Debundling vaccines
Dr. Kajtezovic and her colleagues used a national database of employee-sponsored health insurance to analyze the records of 100,857 children who were continuously enrolled in a plan from age 9 in 2015 to age 13 in 2019. They calculated the odds of children completing the HPV vaccine series based on whether they started the series before, at the same time as, or after the Tdap vaccination.
Youth who received the HPV vaccine before their Tdap vaccine had 38% greater odds of completing the series – getting both doses – than did those who received the HPV vaccine at the same time as the Tdap vaccine. Meanwhile, in line with prior evidence, those who got the first HPV dose after their Tdap were less likely – 68% lower odds – to complete the two- or three-dose (if starting above age 14) series.
The researchers identified several other factors that were linked to completing the HPV vaccine series. Females had greater odds than did males of completing the series, as did those living in urban, rather than rural, areas. Other factors associated with completing the series included living in the Northeast United States and receiving primary care from a pediatrician rather than a family medicine physician.
Timing is important
“I am encouraged by the findings of this study,” Dr. Ault said in an interview. “However, I would have liked the authors to expand the age range a bit higher. There are data that continuing to discuss the HPV vaccine with parents and teens will increase uptake into the later teen years.”
One challenge is that research shows attendance at primary care visits declines in older adolescence. Since there is no second Tdap or meningitis shot, families need to return for the second HPV vaccine dose after those shots, though they could get the second dose at the same time as other two vaccines if they receive the first dose before age 11. There’s also evidence suggesting that providers find conversations about the HPV vaccine easier when sexual activity is not the focus.
“I often feel that, before a child reaches adolescence, they’re almost, in a way, not sexualized yet, so talking about cancer prevention for an 8- or 9-year-old sometimes sounds a little different to patients versus protecting your 12-year-old, who’s starting to go through adolescence and developing breasts” and other signs of puberty, Dr. Kajtezovic said. Keeping the focus of HPV vaccine discussions on cancer prevention also allows providers to point out the protection against anal cancer, vulvar cancer, vaginal cancer, and head and neck cancer. “They are horrible, and even if they’re treatable, they’re often very hard to treat at an advanced stage,” Dr. Kajtezovic said. “The surgery required is so life disabling and disfiguring.”
The HPV Roundtable advises continuing bundling at practices having success with it but encourages practices to consider earlier vaccination if their uptake is lagging. Quality improvement initiatives, such as earlier electronic medical record prompts and multi-level interventions in pediatric practices, have shown substantial increases in HPV vaccine uptake at 9 and 10 years old. One survey in 2021 found that one in five primary care providers already routinely recommend the HPV vaccine at ages 9-10, and nearly half of others would consider doing so.
“My hope is in the next few years, when [the CDC] refreshes their vaccine recommendations, that they will either unbundle it or move the bar a few years earlier so that you can initiate it to encourage earlier initiation,” Dr. Kajtezovic said.
Dr. Ault had no other disclosures besides prior service on ACIP. Dr. Kajtezovic had no disclosures.
AT ACOG 2023
Affixing a Scalp Dressing With Hairpins
Practice Gap
Wound dressings protect the skin and prevent contamination. The hair often makes it difficult to affix a dressing after a minor scalp trauma or local surgery on the head. Traditional approaches for fastening a dressing on the head include bandage winding or adhesive tape, but these methods often affect aesthetics or cause discomfort—bandage winding can make it inconvenient for the patient to move their head, and adhesive tape can cause pain by pulling the hair during removal.
To better position a scalp dressing, tie-over dressings, braid dressings, and paper clips have been used as fixators.1-3 These methods have benefits and disadvantages.
Tie-over Dressing—The dressing is clasped with long sutures that were reserved during wound closure. This method is sturdy, can slightly compress the wound, and is applicable to any part of the scalp. However, it requires more sutures, and more careful wound care may be required due to the edge of the dressing being close to the wound.
Braid Dressing—Tape, a rubber band, or braided hair is used to bind the gauze pad. This dressing is simple and inexpensive. However, it is limited to patients with long hair; even then, it often is difficult to anchor the dressing by braiding hair. Moreover, removal of the rubber band and tape can cause discomfort or pain.
Paper Clip—This is a simple scalp dressing fixator. However, due to the short and circular structure of the clip, it is not conducive to affixing a gauze dressing for patients with short hair, and it often hooks the gauze and hair, making it inconvenient for the physician and a source of discomfort for the patient when the paper clip is being removed.
The Technique
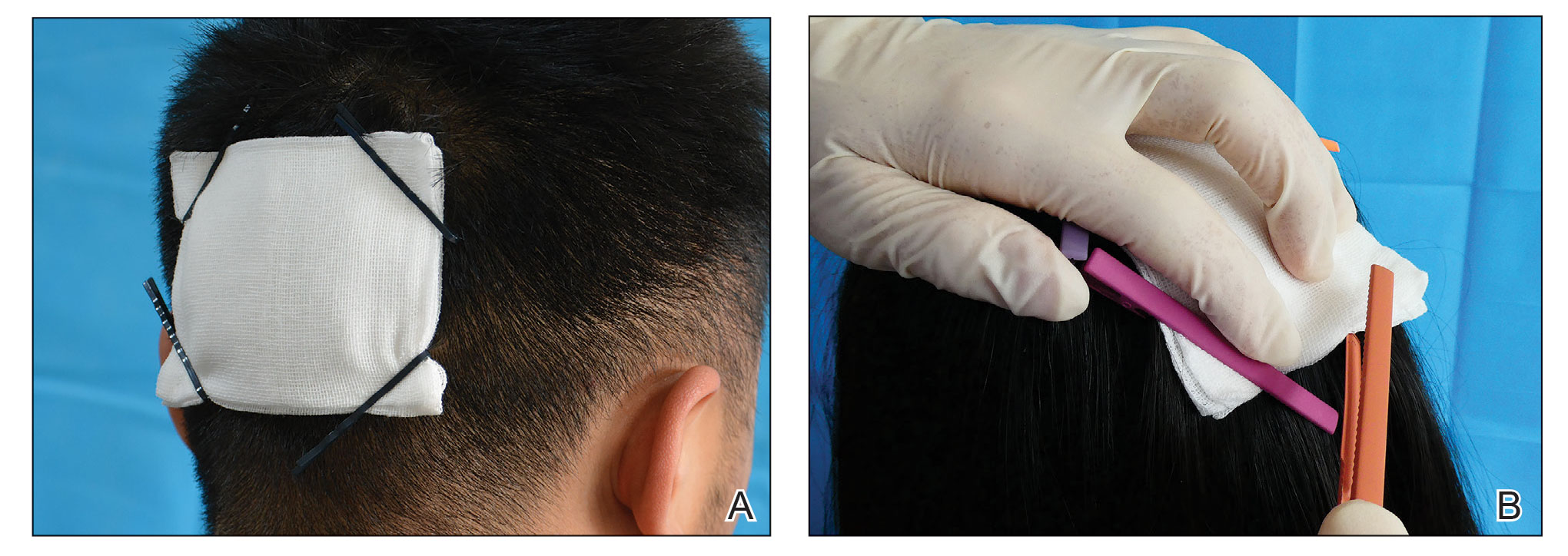
To address shortcomings of traditional methods, we encourage the use of hairpins to affix a dressing after a scalp wound is sutured. Two steps are required:
- Position the gauze to cover the wound and press the gauze down with your hand.
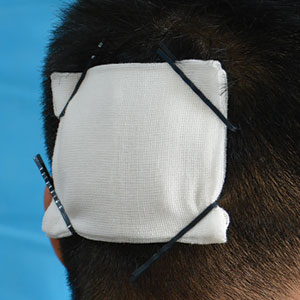
- Clamp the 4 corners of the dressing and adjacent hair with hairpins (Figure, A).
Practical Implications
Hairpins are common for fixing hairstyles and decorating hair. They are inexpensive, easy to obtain, simple in structure, convenient to use without additional discomfort, and easy to remove (Figure, B). Because most hairpins have a powerful clamping force, they can affix dressings in short hair (Figure, A). All medical staff can use hairpins to anchor the scalp dressing. Even a patient’s family members can carry out simple dressing replacement and wound cleaning using this method. Patients also have many options for hairpin styles, which is especially useful in easing the apprehension of surgery in pediatric patients.
- Ginzburg A, Mutalik S. Another method of tie-over dressing for surgical wounds of hair-bearing areas. Dermatol Surg. 1999;25:893-894. doi:10.1046/j.1524-4725.1999.99155.x
- Yanaka K, Nose T. Braid dressing for hair-bearing scalp wound. Neurocrit Care. 2004;1:217-218. doi:10.1385/NCC:1:2:217
- Bu W, Zhang Q, Fang F, et al. Fixation of head dressing gauzes with paper clips is similar to and better than using tape. J Am Acad Dermatol. 2019;81:E95-E96. doi:10.1016/j.jaad.2018.10.046
Practice Gap
Wound dressings protect the skin and prevent contamination. The hair often makes it difficult to affix a dressing after a minor scalp trauma or local surgery on the head. Traditional approaches for fastening a dressing on the head include bandage winding or adhesive tape, but these methods often affect aesthetics or cause discomfort—bandage winding can make it inconvenient for the patient to move their head, and adhesive tape can cause pain by pulling the hair during removal.
To better position a scalp dressing, tie-over dressings, braid dressings, and paper clips have been used as fixators.1-3 These methods have benefits and disadvantages.
Tie-over Dressing—The dressing is clasped with long sutures that were reserved during wound closure. This method is sturdy, can slightly compress the wound, and is applicable to any part of the scalp. However, it requires more sutures, and more careful wound care may be required due to the edge of the dressing being close to the wound.
Braid Dressing—Tape, a rubber band, or braided hair is used to bind the gauze pad. This dressing is simple and inexpensive. However, it is limited to patients with long hair; even then, it often is difficult to anchor the dressing by braiding hair. Moreover, removal of the rubber band and tape can cause discomfort or pain.
Paper Clip—This is a simple scalp dressing fixator. However, due to the short and circular structure of the clip, it is not conducive to affixing a gauze dressing for patients with short hair, and it often hooks the gauze and hair, making it inconvenient for the physician and a source of discomfort for the patient when the paper clip is being removed.
The Technique
To address shortcomings of traditional methods, we encourage the use of hairpins to affix a dressing after a scalp wound is sutured. Two steps are required:
- Position the gauze to cover the wound and press the gauze down with your hand.
- Clamp the 4 corners of the dressing and adjacent hair with hairpins (Figure, A).

Practical Implications
Hairpins are common for fixing hairstyles and decorating hair. They are inexpensive, easy to obtain, simple in structure, convenient to use without additional discomfort, and easy to remove (Figure, B). Because most hairpins have a powerful clamping force, they can affix dressings in short hair (Figure, A). All medical staff can use hairpins to anchor the scalp dressing. Even a patient’s family members can carry out simple dressing replacement and wound cleaning using this method. Patients also have many options for hairpin styles, which is especially useful in easing the apprehension of surgery in pediatric patients.
Practice Gap
Wound dressings protect the skin and prevent contamination. The hair often makes it difficult to affix a dressing after a minor scalp trauma or local surgery on the head. Traditional approaches for fastening a dressing on the head include bandage winding or adhesive tape, but these methods often affect aesthetics or cause discomfort—bandage winding can make it inconvenient for the patient to move their head, and adhesive tape can cause pain by pulling the hair during removal.
To better position a scalp dressing, tie-over dressings, braid dressings, and paper clips have been used as fixators.1-3 These methods have benefits and disadvantages.
Tie-over Dressing—The dressing is clasped with long sutures that were reserved during wound closure. This method is sturdy, can slightly compress the wound, and is applicable to any part of the scalp. However, it requires more sutures, and more careful wound care may be required due to the edge of the dressing being close to the wound.
Braid Dressing—Tape, a rubber band, or braided hair is used to bind the gauze pad. This dressing is simple and inexpensive. However, it is limited to patients with long hair; even then, it often is difficult to anchor the dressing by braiding hair. Moreover, removal of the rubber band and tape can cause discomfort or pain.
Paper Clip—This is a simple scalp dressing fixator. However, due to the short and circular structure of the clip, it is not conducive to affixing a gauze dressing for patients with short hair, and it often hooks the gauze and hair, making it inconvenient for the physician and a source of discomfort for the patient when the paper clip is being removed.
The Technique
To address shortcomings of traditional methods, we encourage the use of hairpins to affix a dressing after a scalp wound is sutured. Two steps are required:
- Position the gauze to cover the wound and press the gauze down with your hand.
- Clamp the 4 corners of the dressing and adjacent hair with hairpins (Figure, A).

Practical Implications
Hairpins are common for fixing hairstyles and decorating hair. They are inexpensive, easy to obtain, simple in structure, convenient to use without additional discomfort, and easy to remove (Figure, B). Because most hairpins have a powerful clamping force, they can affix dressings in short hair (Figure, A). All medical staff can use hairpins to anchor the scalp dressing. Even a patient’s family members can carry out simple dressing replacement and wound cleaning using this method. Patients also have many options for hairpin styles, which is especially useful in easing the apprehension of surgery in pediatric patients.
- Ginzburg A, Mutalik S. Another method of tie-over dressing for surgical wounds of hair-bearing areas. Dermatol Surg. 1999;25:893-894. doi:10.1046/j.1524-4725.1999.99155.x
- Yanaka K, Nose T. Braid dressing for hair-bearing scalp wound. Neurocrit Care. 2004;1:217-218. doi:10.1385/NCC:1:2:217
- Bu W, Zhang Q, Fang F, et al. Fixation of head dressing gauzes with paper clips is similar to and better than using tape. J Am Acad Dermatol. 2019;81:E95-E96. doi:10.1016/j.jaad.2018.10.046
- Ginzburg A, Mutalik S. Another method of tie-over dressing for surgical wounds of hair-bearing areas. Dermatol Surg. 1999;25:893-894. doi:10.1046/j.1524-4725.1999.99155.x
- Yanaka K, Nose T. Braid dressing for hair-bearing scalp wound. Neurocrit Care. 2004;1:217-218. doi:10.1385/NCC:1:2:217
- Bu W, Zhang Q, Fang F, et al. Fixation of head dressing gauzes with paper clips is similar to and better than using tape. J Am Acad Dermatol. 2019;81:E95-E96. doi:10.1016/j.jaad.2018.10.046
Cancer Screening for Dermatomyositis: A Survey of Indirect Costs, Burden, and Patient Willingness to Pay
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18
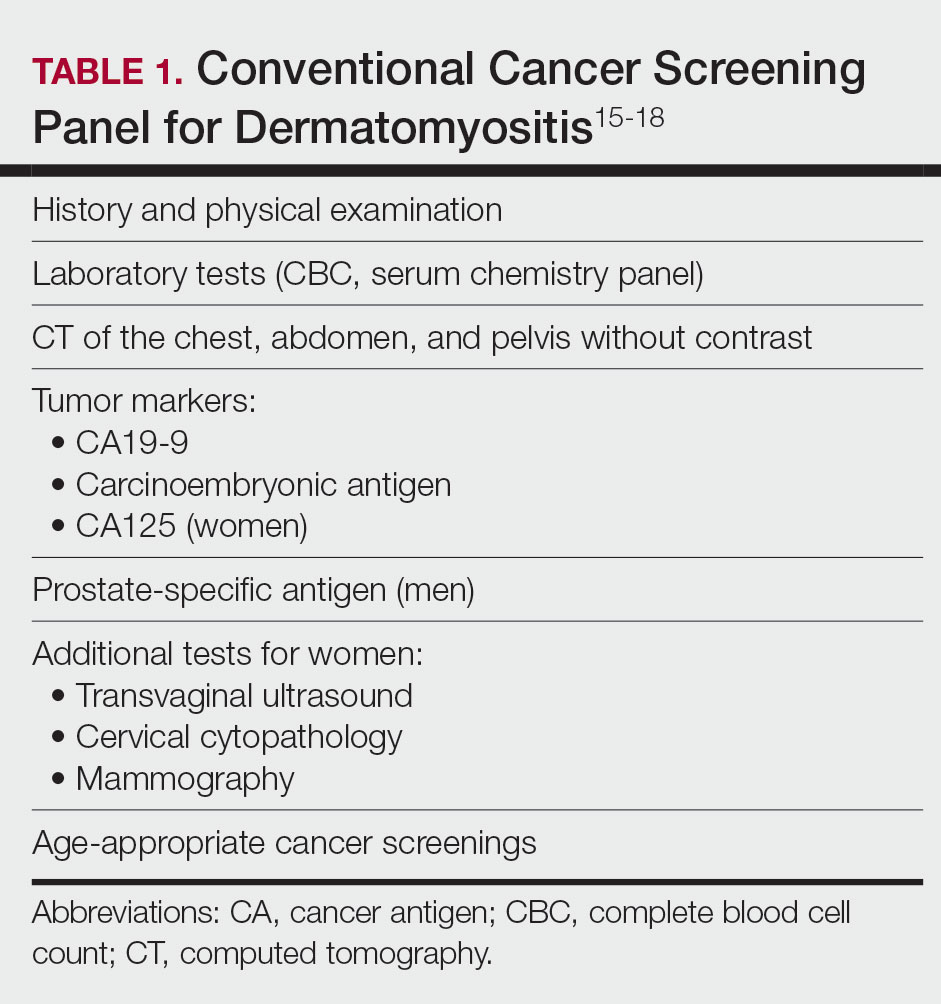
The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).
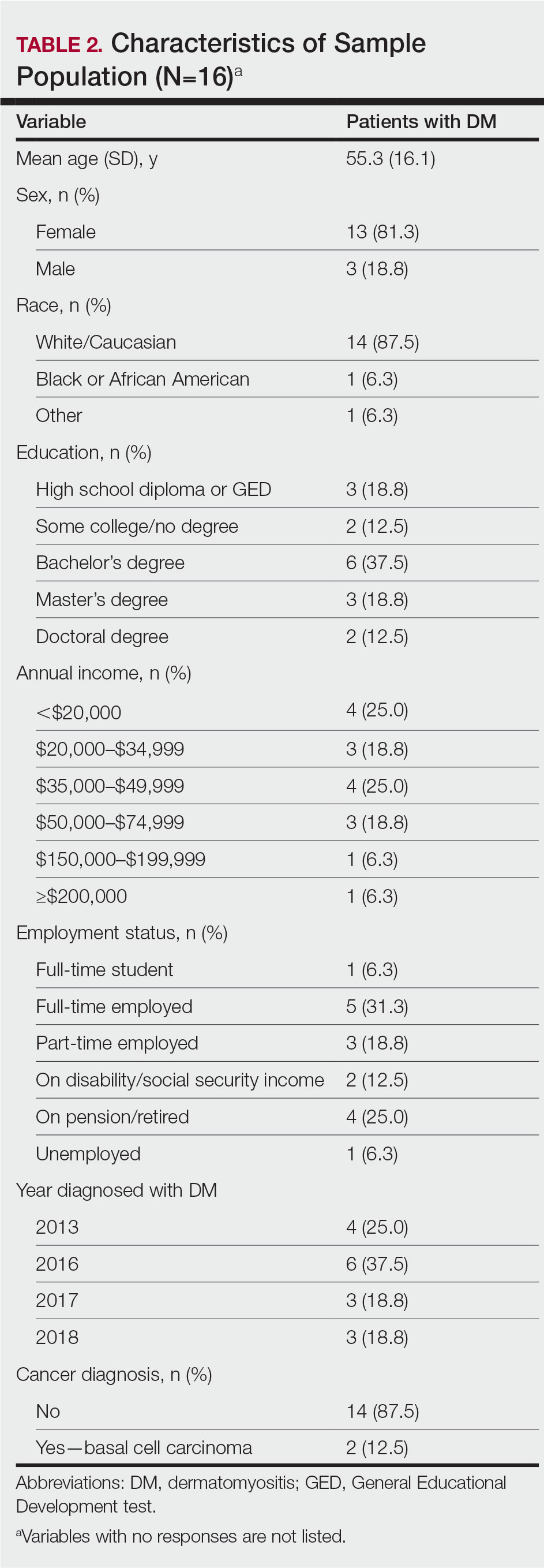
Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.
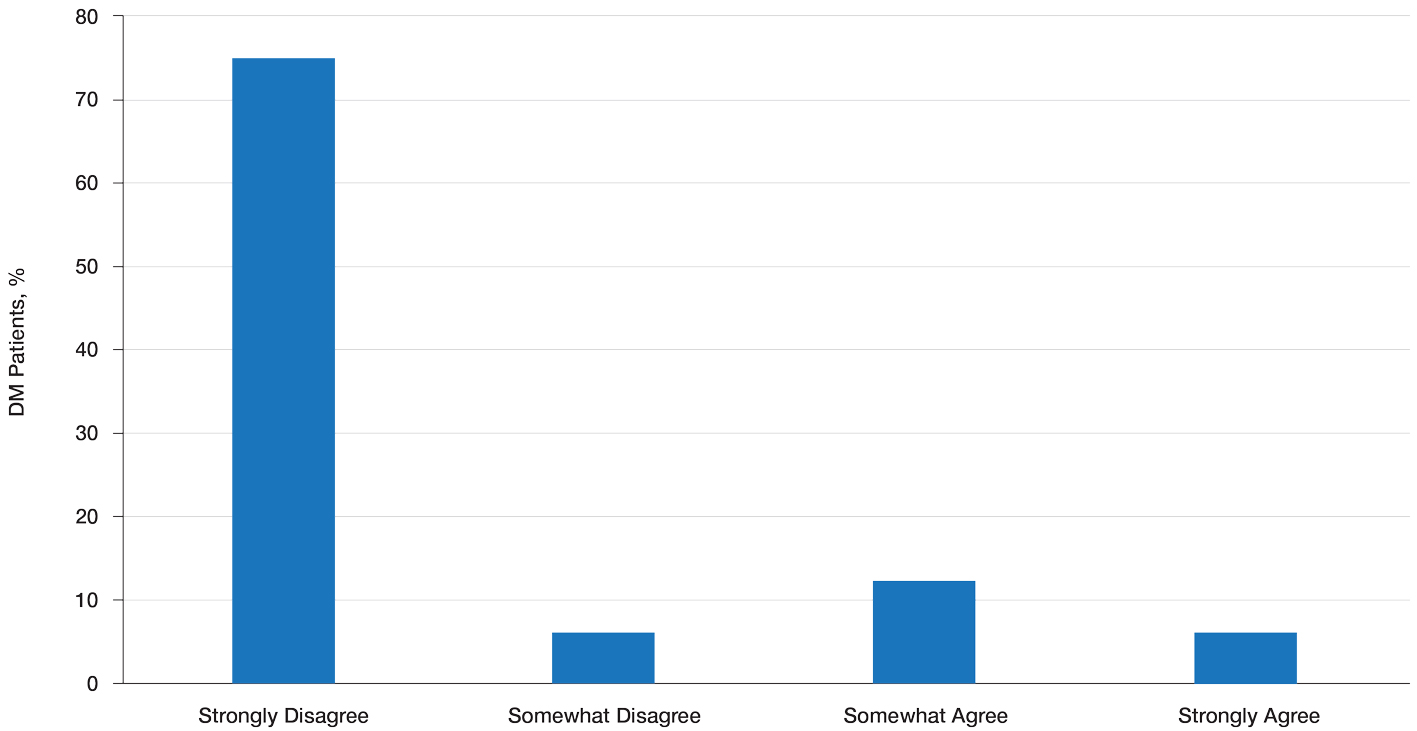
Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.
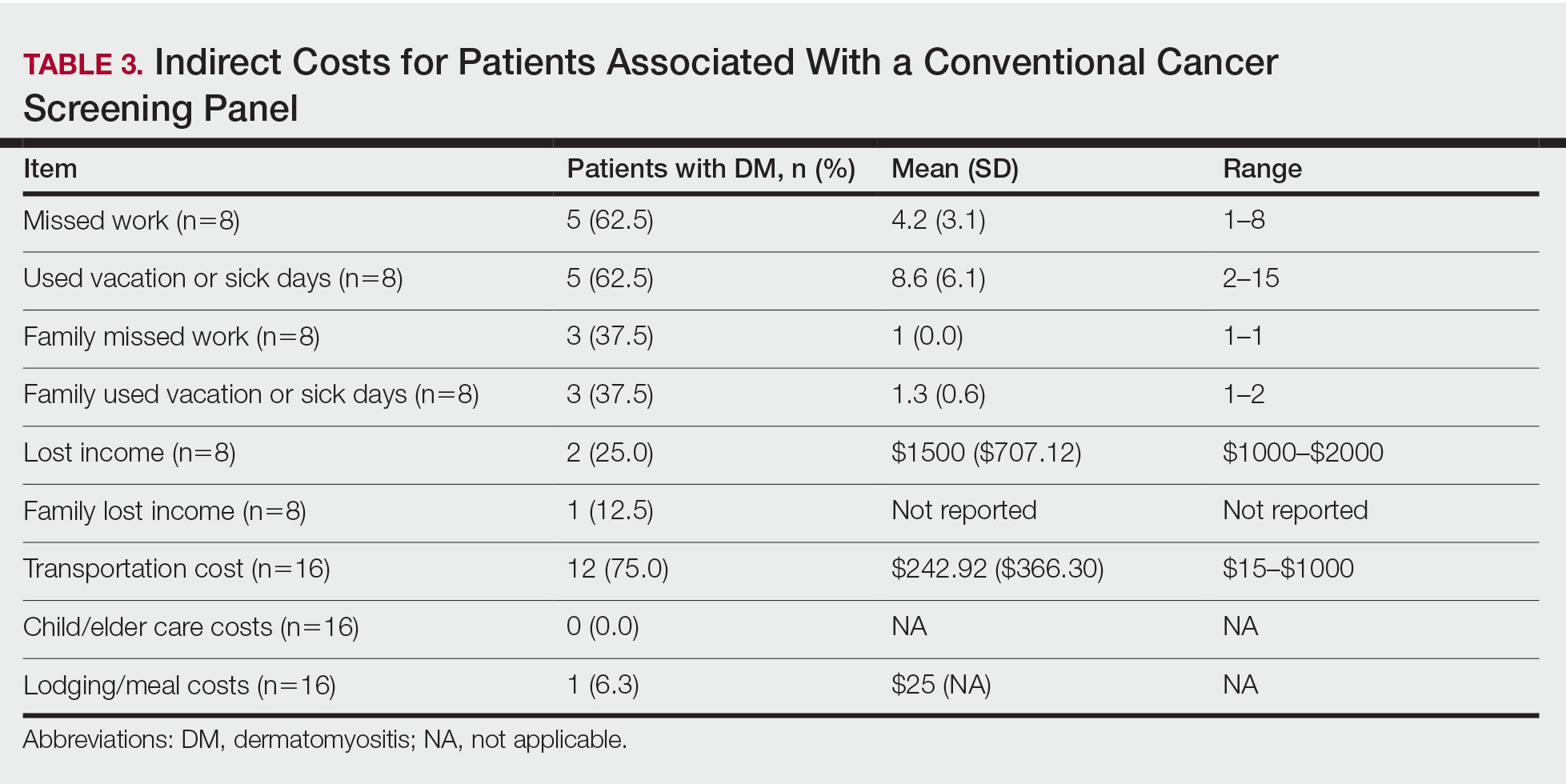
Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17
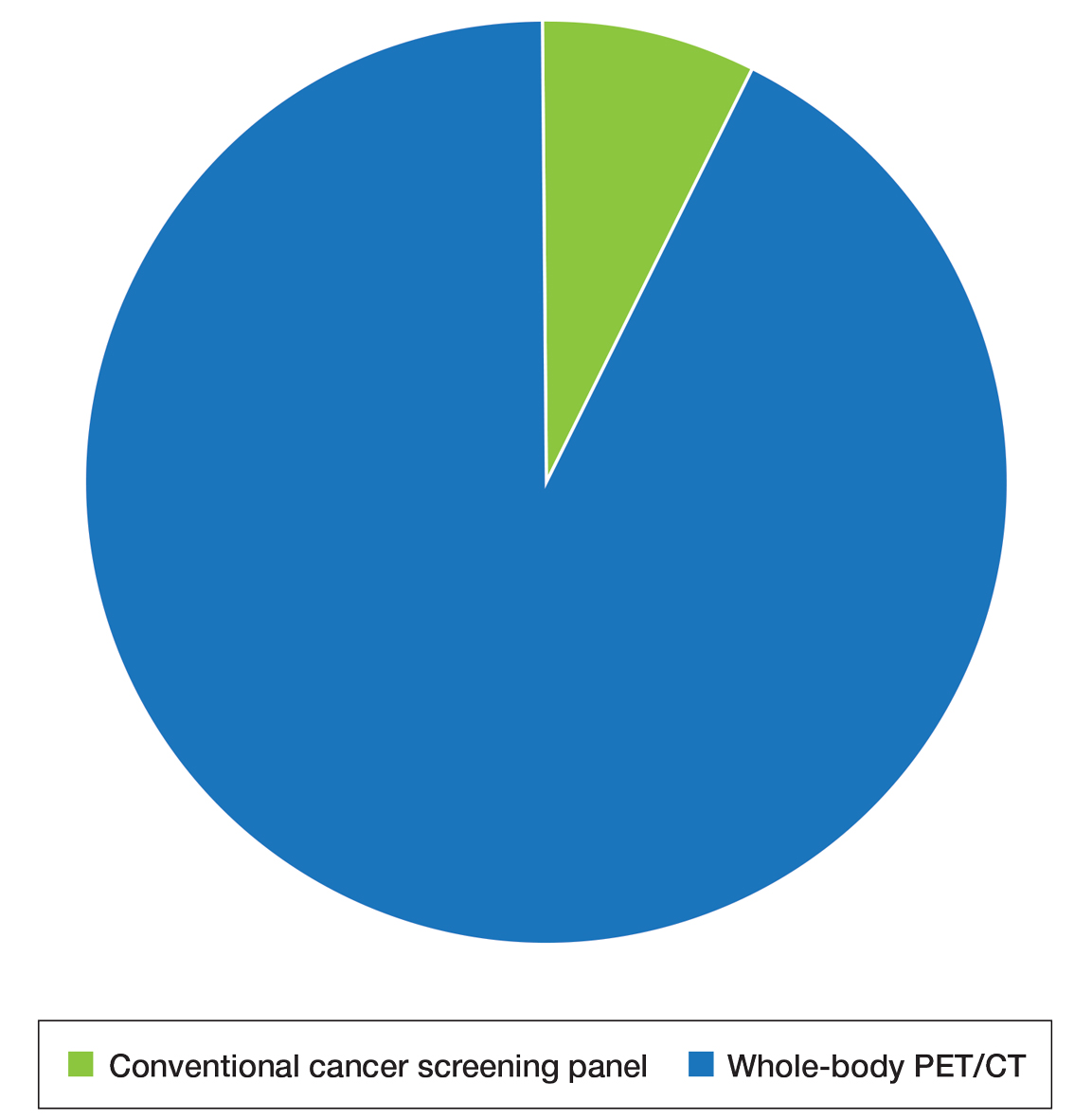
The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)
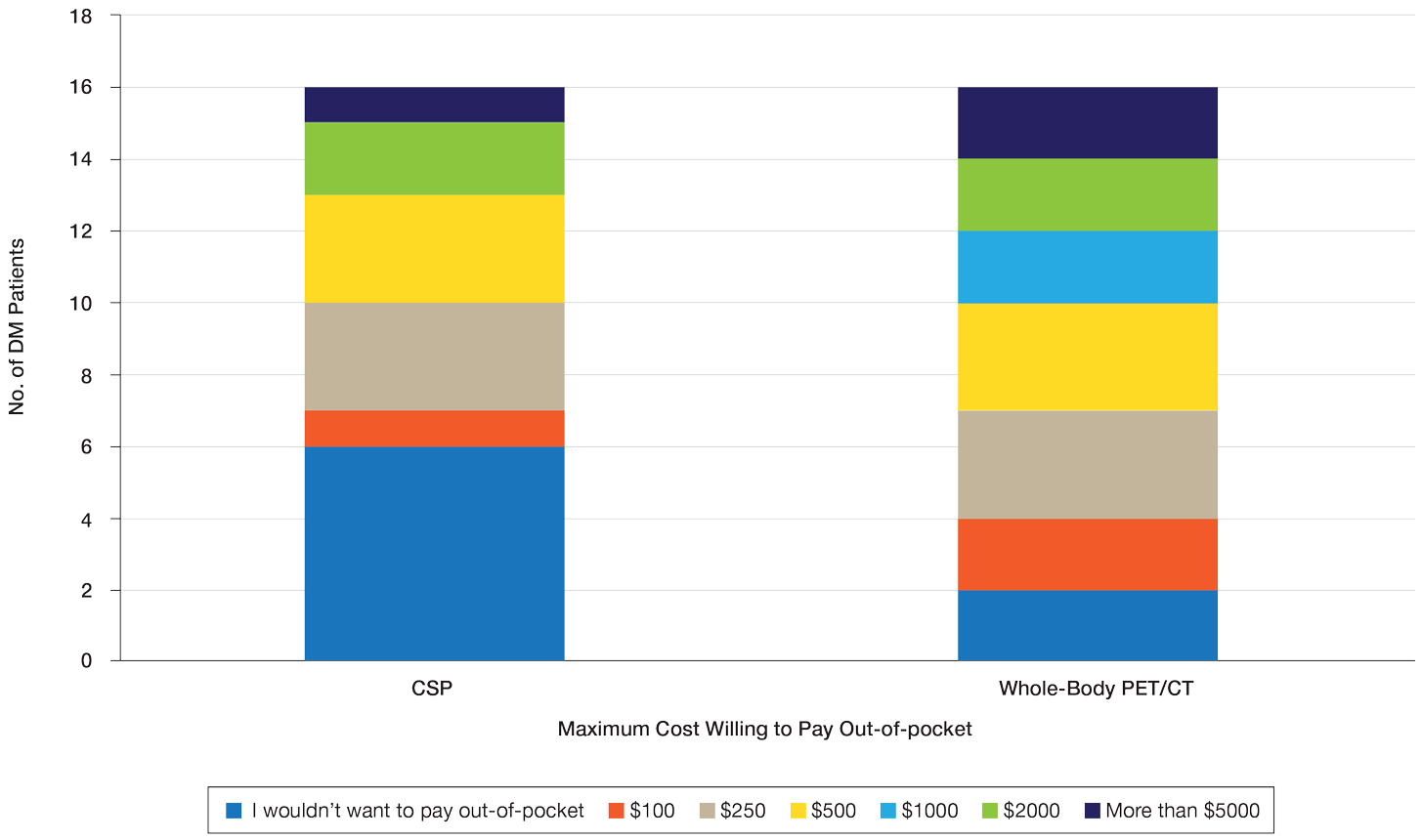
“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Practice Points
- Dermatomyositis (DM) is associated with an increased risk for malignancy. Patient perspective needs to be considered in developing cancer screening guidelines for patients with DM, particularly given the similar efficacy of available screening modalities.
- Current modalities for cancer screening in DM include whole-body positron emission tomography/computed tomography (PET/CT) and a conventional cancer screening panel (CSP), which includes a battery of tests typically requiring multiple visits. Patients may find the simplicity of PET/CT more preferrable than the more complex CSP.
- Indirect costs of cancer screening include missed work, travel and childcare expenses, and lost wages. Conventional cancer screening has greater indirect costs than PET/CT.
Brachioradial Pruritus: An Etiologic Review and Treatment Summary
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).
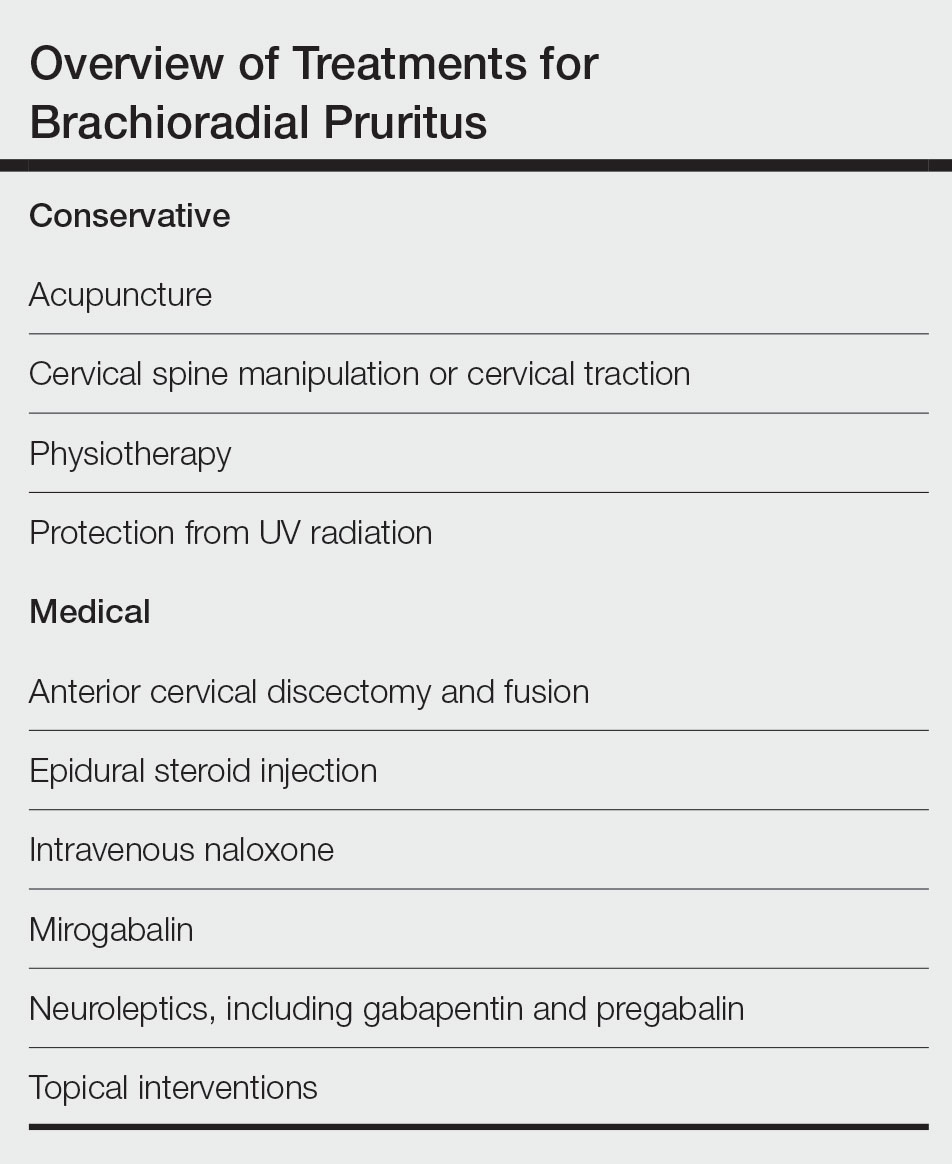
Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months. To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).

Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months. To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).

Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months. To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
Practice Points
- The etiology of brachioradial pruritus (BRP) has been associated with cervical spine pathology and/or UV radiation exposure.
- Treatment options for BRP range from conservative to invasive, and clinicians should consider the evidence for all options to decide what is best for each patient.
Violaceous Plaque on the Metacarpophalangeal Joints
The Diagnosis: Mycobacterial Infection
Mycobacterium marinum is a waterborne nontuberculous mycobacterium prevailing in salt water, brackish water, and still or streaming fresh water that infects fish and amphibians worldwide.1,2 Although first described in 1926 as the organism responsible for the demise of fish in an aquarium in Philadelphia, Pennsylvania, it was not until 1954 that the organism was linked to the cause of infection in humans after it was identified in 80 individuals who had utilized the same swimming pool.1 Due to its ability to secondarily contaminate aquariums, swimming pools, and rivers, this species can give rise to infection in humans, likely though an impaired skin barrier or points of trauma. It commonly is known as swimming pool or fish tank granuloma.3,4
Infection by M marinum commonly presents with lesions on the upper extremities, particularly the hands, that appear approximately 2 to 3 weeks following exposure to the organism.2 Lesions are categorized as superficial (type 1), granulomatous (type 2), or deep (type 3).1 Superficial lesions usually are solitary and painless; may exhibit purulent secretions; and consist of papulonodular, verrucose, or ulcerated granulomatous inflammation.1 These lesions may spread in a sporotrichoidlike pattern or in a linear fashion along lymphatic channels, similar to sporotrichosis. Granulomatous lesions present as solitary or numerous granulomas that typically are swollen, tender, and purulent. Deep lesions are the rarest form and primarily are seen in immunocompromised patients, particularly transplant recipients. Infection can lead to arthritis, tenosynovitis, or osteomyelitis.1
Mycobacterium marinum infection is diagnosed via tissue biopsy for concomitant histopathologic examination and culture from a nonulcerated area close to the lesion.1,2 If cultures do not grow, polymerase chain reaction (PCR) or PCR restriction fragment length polymorphism analysis can be conducted. These techniques can exclude other potential diagnoses; however, PCR is unable to provide information on antibiotic susceptibility.1 Biopsy of lesions reveals a nonspecific inflammatory type of reaction within the dermis consisting of lymphocytes, polymorphonuclear cells, and histiocytes.1,4 Additionally, a granulomatous inflammatory infiltrate resembling tuberculoid granuloma, sarcoidlike granuloma, or rheumatoidlike nodules also may be observed.1 With staining, the acid-fast organisms can be viewed within histiocytes, sometimes demonstrating transverse bands.4
The preferred treatment of M marinum infection is antibiotic therapy.2 It generally is not recommended to obtain in vitro drug sensitivity testing, as mutational resistance to the commonly utilized drugs is minimal. Microbiologic investigation may be warranted in cases of treatment failure or persistently positive cultures over a period of several months.1,2 Due to its rarity, no clinical trials exist to guide optimal management of M marinum infection, according to a search of ClinicalTrials.gov. Nonetheless, anecdotal evidence of prior cases can direct the selection of antibiotics. Mycobacterium marinum appears to respond to certain tetracyclines, including minocycline followed by doxycycline. Other options include clarithromycin, clarithromycin in combination with rifampin, rifampin in combination with ethambutol, trimethoprimsulfamethoxazole, and ciprofloxacin.1,2 Surgical debridement or excision may be indicated, especially in an infection involving deep structures, though recurrences have been reported in some individuals following surgery.2,4 Nonspecific treatment such as hyperthermic or liquid nitrogen local treatment have been used experimentally with positive outcomes; however, experience with this treatment modality is limited.2
Sarcoidosis is an immune-mediated systemic disorder that most commonly affects the lungs and skin. Histopathology shows sarcoidal granulomas with features similar to M marinum infection. The clinical presentation often is described as red-brown macules or papules affecting the face, rarely with overlying scale or ulceration.5 Majocchi granuloma is a dermatophyte fungal infection involving the hair follicles. Although application of topical steroids can worsen the involvement, it commonly displays perifollicular pustules,6 which were not seen in our patient. Granuloma annulare is a benign granulomatous disorder that will spontaneously resolve, typically within 2 years of onset. It presents as an annular or arcuate red-brown papule or plaque without overlying scale or ulceration,7 unlike the lesion seen in our patient. Cutaneous lymphoma is a malignant lymphoproliferative disease most commonly affecting middle-aged White men. The presentation is variable and may include an ulcerated plaque8; the lack of systemic symptoms and notable progression over several years in our patient made this a less likely diagnosis.
- Karim S, Devani A, Brassard A. Dermacase. can you identify this condition? Mycobacterium marinum infection. Can Fam Physician. 2013;59:53-54.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006; 25:609-613. doi:10.1007/s10096-006-0201-4
- Gray SF, Smith RS, Reynolds NJ, et al. Fish tank granuloma. BMJ. 1990;300:1069-1070. doi:10.1136/bmj.300.6731.1069
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma: a study of 290 cases. Arch Dermatol. 1963;88:158-162. doi:10.1001/archderm.1963.01590200046008
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives [published online May 22, 2018]. Infect Drug Resist. 2018;11:751-760. doi:10.2147/IDR.S145027
- Joshi TP, Duvic M. Granuloma annulare: an updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol. 2022;23:37-50. doi:10.1007/s40257-021-00636-1
- Charli-Joseph YV, Gatica-Torres M, Pincus LB. Approach to cutaneous lymphoid infiltrates: when to consider lymphoma? Indian J Dermatol. 2016;61:351-374. doi:10.4103/0019-5154.185698
The Diagnosis: Mycobacterial Infection
Mycobacterium marinum is a waterborne nontuberculous mycobacterium prevailing in salt water, brackish water, and still or streaming fresh water that infects fish and amphibians worldwide.1,2 Although first described in 1926 as the organism responsible for the demise of fish in an aquarium in Philadelphia, Pennsylvania, it was not until 1954 that the organism was linked to the cause of infection in humans after it was identified in 80 individuals who had utilized the same swimming pool.1 Due to its ability to secondarily contaminate aquariums, swimming pools, and rivers, this species can give rise to infection in humans, likely though an impaired skin barrier or points of trauma. It commonly is known as swimming pool or fish tank granuloma.3,4
Infection by M marinum commonly presents with lesions on the upper extremities, particularly the hands, that appear approximately 2 to 3 weeks following exposure to the organism.2 Lesions are categorized as superficial (type 1), granulomatous (type 2), or deep (type 3).1 Superficial lesions usually are solitary and painless; may exhibit purulent secretions; and consist of papulonodular, verrucose, or ulcerated granulomatous inflammation.1 These lesions may spread in a sporotrichoidlike pattern or in a linear fashion along lymphatic channels, similar to sporotrichosis. Granulomatous lesions present as solitary or numerous granulomas that typically are swollen, tender, and purulent. Deep lesions are the rarest form and primarily are seen in immunocompromised patients, particularly transplant recipients. Infection can lead to arthritis, tenosynovitis, or osteomyelitis.1
Mycobacterium marinum infection is diagnosed via tissue biopsy for concomitant histopathologic examination and culture from a nonulcerated area close to the lesion.1,2 If cultures do not grow, polymerase chain reaction (PCR) or PCR restriction fragment length polymorphism analysis can be conducted. These techniques can exclude other potential diagnoses; however, PCR is unable to provide information on antibiotic susceptibility.1 Biopsy of lesions reveals a nonspecific inflammatory type of reaction within the dermis consisting of lymphocytes, polymorphonuclear cells, and histiocytes.1,4 Additionally, a granulomatous inflammatory infiltrate resembling tuberculoid granuloma, sarcoidlike granuloma, or rheumatoidlike nodules also may be observed.1 With staining, the acid-fast organisms can be viewed within histiocytes, sometimes demonstrating transverse bands.4
The preferred treatment of M marinum infection is antibiotic therapy.2 It generally is not recommended to obtain in vitro drug sensitivity testing, as mutational resistance to the commonly utilized drugs is minimal. Microbiologic investigation may be warranted in cases of treatment failure or persistently positive cultures over a period of several months.1,2 Due to its rarity, no clinical trials exist to guide optimal management of M marinum infection, according to a search of ClinicalTrials.gov. Nonetheless, anecdotal evidence of prior cases can direct the selection of antibiotics. Mycobacterium marinum appears to respond to certain tetracyclines, including minocycline followed by doxycycline. Other options include clarithromycin, clarithromycin in combination with rifampin, rifampin in combination with ethambutol, trimethoprimsulfamethoxazole, and ciprofloxacin.1,2 Surgical debridement or excision may be indicated, especially in an infection involving deep structures, though recurrences have been reported in some individuals following surgery.2,4 Nonspecific treatment such as hyperthermic or liquid nitrogen local treatment have been used experimentally with positive outcomes; however, experience with this treatment modality is limited.2
Sarcoidosis is an immune-mediated systemic disorder that most commonly affects the lungs and skin. Histopathology shows sarcoidal granulomas with features similar to M marinum infection. The clinical presentation often is described as red-brown macules or papules affecting the face, rarely with overlying scale or ulceration.5 Majocchi granuloma is a dermatophyte fungal infection involving the hair follicles. Although application of topical steroids can worsen the involvement, it commonly displays perifollicular pustules,6 which were not seen in our patient. Granuloma annulare is a benign granulomatous disorder that will spontaneously resolve, typically within 2 years of onset. It presents as an annular or arcuate red-brown papule or plaque without overlying scale or ulceration,7 unlike the lesion seen in our patient. Cutaneous lymphoma is a malignant lymphoproliferative disease most commonly affecting middle-aged White men. The presentation is variable and may include an ulcerated plaque8; the lack of systemic symptoms and notable progression over several years in our patient made this a less likely diagnosis.
The Diagnosis: Mycobacterial Infection
Mycobacterium marinum is a waterborne nontuberculous mycobacterium prevailing in salt water, brackish water, and still or streaming fresh water that infects fish and amphibians worldwide.1,2 Although first described in 1926 as the organism responsible for the demise of fish in an aquarium in Philadelphia, Pennsylvania, it was not until 1954 that the organism was linked to the cause of infection in humans after it was identified in 80 individuals who had utilized the same swimming pool.1 Due to its ability to secondarily contaminate aquariums, swimming pools, and rivers, this species can give rise to infection in humans, likely though an impaired skin barrier or points of trauma. It commonly is known as swimming pool or fish tank granuloma.3,4
Infection by M marinum commonly presents with lesions on the upper extremities, particularly the hands, that appear approximately 2 to 3 weeks following exposure to the organism.2 Lesions are categorized as superficial (type 1), granulomatous (type 2), or deep (type 3).1 Superficial lesions usually are solitary and painless; may exhibit purulent secretions; and consist of papulonodular, verrucose, or ulcerated granulomatous inflammation.1 These lesions may spread in a sporotrichoidlike pattern or in a linear fashion along lymphatic channels, similar to sporotrichosis. Granulomatous lesions present as solitary or numerous granulomas that typically are swollen, tender, and purulent. Deep lesions are the rarest form and primarily are seen in immunocompromised patients, particularly transplant recipients. Infection can lead to arthritis, tenosynovitis, or osteomyelitis.1
Mycobacterium marinum infection is diagnosed via tissue biopsy for concomitant histopathologic examination and culture from a nonulcerated area close to the lesion.1,2 If cultures do not grow, polymerase chain reaction (PCR) or PCR restriction fragment length polymorphism analysis can be conducted. These techniques can exclude other potential diagnoses; however, PCR is unable to provide information on antibiotic susceptibility.1 Biopsy of lesions reveals a nonspecific inflammatory type of reaction within the dermis consisting of lymphocytes, polymorphonuclear cells, and histiocytes.1,4 Additionally, a granulomatous inflammatory infiltrate resembling tuberculoid granuloma, sarcoidlike granuloma, or rheumatoidlike nodules also may be observed.1 With staining, the acid-fast organisms can be viewed within histiocytes, sometimes demonstrating transverse bands.4
The preferred treatment of M marinum infection is antibiotic therapy.2 It generally is not recommended to obtain in vitro drug sensitivity testing, as mutational resistance to the commonly utilized drugs is minimal. Microbiologic investigation may be warranted in cases of treatment failure or persistently positive cultures over a period of several months.1,2 Due to its rarity, no clinical trials exist to guide optimal management of M marinum infection, according to a search of ClinicalTrials.gov. Nonetheless, anecdotal evidence of prior cases can direct the selection of antibiotics. Mycobacterium marinum appears to respond to certain tetracyclines, including minocycline followed by doxycycline. Other options include clarithromycin, clarithromycin in combination with rifampin, rifampin in combination with ethambutol, trimethoprimsulfamethoxazole, and ciprofloxacin.1,2 Surgical debridement or excision may be indicated, especially in an infection involving deep structures, though recurrences have been reported in some individuals following surgery.2,4 Nonspecific treatment such as hyperthermic or liquid nitrogen local treatment have been used experimentally with positive outcomes; however, experience with this treatment modality is limited.2
Sarcoidosis is an immune-mediated systemic disorder that most commonly affects the lungs and skin. Histopathology shows sarcoidal granulomas with features similar to M marinum infection. The clinical presentation often is described as red-brown macules or papules affecting the face, rarely with overlying scale or ulceration.5 Majocchi granuloma is a dermatophyte fungal infection involving the hair follicles. Although application of topical steroids can worsen the involvement, it commonly displays perifollicular pustules,6 which were not seen in our patient. Granuloma annulare is a benign granulomatous disorder that will spontaneously resolve, typically within 2 years of onset. It presents as an annular or arcuate red-brown papule or plaque without overlying scale or ulceration,7 unlike the lesion seen in our patient. Cutaneous lymphoma is a malignant lymphoproliferative disease most commonly affecting middle-aged White men. The presentation is variable and may include an ulcerated plaque8; the lack of systemic symptoms and notable progression over several years in our patient made this a less likely diagnosis.
- Karim S, Devani A, Brassard A. Dermacase. can you identify this condition? Mycobacterium marinum infection. Can Fam Physician. 2013;59:53-54.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006; 25:609-613. doi:10.1007/s10096-006-0201-4
- Gray SF, Smith RS, Reynolds NJ, et al. Fish tank granuloma. BMJ. 1990;300:1069-1070. doi:10.1136/bmj.300.6731.1069
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma: a study of 290 cases. Arch Dermatol. 1963;88:158-162. doi:10.1001/archderm.1963.01590200046008
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives [published online May 22, 2018]. Infect Drug Resist. 2018;11:751-760. doi:10.2147/IDR.S145027
- Joshi TP, Duvic M. Granuloma annulare: an updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol. 2022;23:37-50. doi:10.1007/s40257-021-00636-1
- Charli-Joseph YV, Gatica-Torres M, Pincus LB. Approach to cutaneous lymphoid infiltrates: when to consider lymphoma? Indian J Dermatol. 2016;61:351-374. doi:10.4103/0019-5154.185698
- Karim S, Devani A, Brassard A. Dermacase. can you identify this condition? Mycobacterium marinum infection. Can Fam Physician. 2013;59:53-54.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006; 25:609-613. doi:10.1007/s10096-006-0201-4
- Gray SF, Smith RS, Reynolds NJ, et al. Fish tank granuloma. BMJ. 1990;300:1069-1070. doi:10.1136/bmj.300.6731.1069
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma: a study of 290 cases. Arch Dermatol. 1963;88:158-162. doi:10.1001/archderm.1963.01590200046008
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives [published online May 22, 2018]. Infect Drug Resist. 2018;11:751-760. doi:10.2147/IDR.S145027
- Joshi TP, Duvic M. Granuloma annulare: an updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol. 2022;23:37-50. doi:10.1007/s40257-021-00636-1
- Charli-Joseph YV, Gatica-Torres M, Pincus LB. Approach to cutaneous lymphoid infiltrates: when to consider lymphoma? Indian J Dermatol. 2016;61:351-374. doi:10.4103/0019-5154.185698
A 24-year-old man presented with a slowly growing, asymptomatic lesion on the left dorsal fourth and fifth metacarpophalangeal joints of 5 years’ duration that was recalcitrant to potent topical corticosteroids. Physical examination revealed an L-shaped, violaceous, firm plaque with focal areas of serous crust. There was no regional lymphadenopathy or lymphangitic spread. The patient had no history of recent travel, and he reported no associated pain or signs of systemic infection.
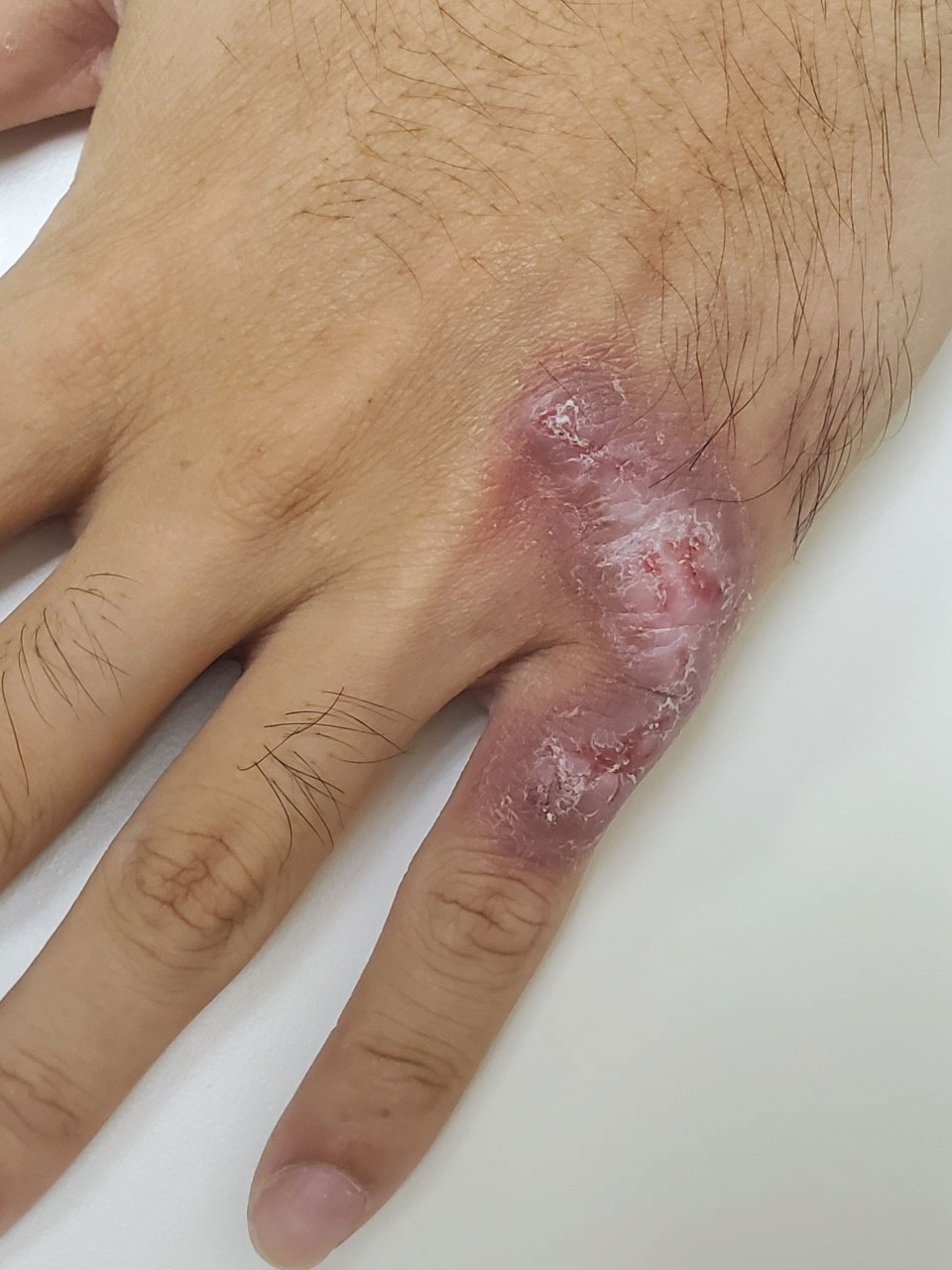
Depression at any stage of life tied to increased dementia risk
Adults with depression have more than double the risk of developing dementia and the risk persists regardless of when in life depression is diagnosed, a large population-based study shows.
That the association between depression and dementia persisted even among individuals first diagnosed with depression in early or mid-life provides “strong evidence that depression is not only an early symptom of dementia, but also that depression increases dementia risk,” study investigator Holly Elser, MD, PhD, epidemiologist and resident physician, University of Pennsylvania, Philadelphia, told this news organization.
The study was published online in JAMA Neurology.
Double the risk
Several prior studies that have examined the relationship between depression and dementia over the life course have consistently shown depression later in life is associated with subsequent dementia.
“Late-life depression is generally thought to be an early symptom of dementia or a reaction to subclinical cognitive decline,” said Dr. Elser.
The investigators wanted to examine whether the association between depression and dementia persists even when depression is diagnosed earlier in life, which may suggest it increases the risk of dementia.
“To my knowledge, ours is the largest study on this topic to date, leveraging routinely and prospectively collected data from more than 1.4 million Danish citizens followed from 1977 to 2018,” Dr. Elser noted.
The cohort included 246,499 individuals diagnosed with depression and 1,190,302 individuals without depression.
In both groups, the median age was 50 years and 65% were women. Roughly two-thirds (68%) of those diagnosed with depression were diagnosed before age 60 years.
In Cox proportional hazards regression models, the overall hazard of dementia was more than doubled in those diagnosed with depression (hazard ratio [HR] 2.41). The risk of dementia with depression was more pronounced for men (HR, 2.98) than in women (HR, 2.21).
This association persisted even when the time elapsed from depression diagnosis was between 20 and 39 years (HR, 1.79) and whether depression was diagnosed in early life (18-44 years: HR, 3.08), mid-life (45-59 years: HR, 2.95), or late life (≥ 60 years: HR, 2.31).
It remains unclear whether effective treatment of depression modifies the risk of dementia, as the current study explored the role of antidepressants in a “very limited fashion,” Dr. Elser said.
Specifically, the researchers considered whether an individual was treated with an antidepressant within 6 months of the initial depression diagnosis and found no evidence of a difference in dementia risk between the treated and untreated groups.
“Research that explores implications of the timing and duration of treatment with antidepressants for dementia, treatment with cognitive behavioral therapy, and is able to evaluate the effectiveness of those treatments will be extremely important,” Dr. Elser said.
‘An assault on the brain’
Reached for comment, John Showalter, MD, chief product officer at Linus Health, said one of the most “intriguing” findings of the study is that a depression diagnosis earlier in adulthood conferred a greater risk of developing vascular dementia (HR, 3.28) than did dementia due to Alzheimer’s disease (HR, 1.73).
“The difference in risk for subtypes of dementia is a meaningful addition to our understanding of depression’s connection to dementia,” said Dr. Showalter, who was not involved in the study.
Also weighing in, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the findings from this “far-reaching investigation leave little room for doubt – depression unleashes a devastating storm within the brain, wreaking havoc on the lives of those ensnared by its grip.
“This massive, multi-decade, and high-data quality registry study adds another brick to the growing edifice of evidence attesting to the profound connection between psychiatric health and the very essence of brain health,” said Dr. Lakhan, who was not involved in the study.
“In a resounding declaration, this research underscores that psychiatric health should be perceived as an integral component of overall health – a paradigm shift that challenges long-standing misconceptions and stigmas surrounding mental disorders. Depression, once marginalized, now claims its rightful place on the pedestal of health concerns that must be addressed with unwavering resolve,” said Dr. Lakhan.
He noted that depression is “not just a mental battle, it’s a profound assault on the very fabric of the brain, leaving lives in turmoil and hearts in search of hope. No longer shrouded in silence, depression demands society’s attention.”
The study had no specific funding. Dr. Elser, Dr. Showalter, and Dr. Lakhan have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Adults with depression have more than double the risk of developing dementia and the risk persists regardless of when in life depression is diagnosed, a large population-based study shows.
That the association between depression and dementia persisted even among individuals first diagnosed with depression in early or mid-life provides “strong evidence that depression is not only an early symptom of dementia, but also that depression increases dementia risk,” study investigator Holly Elser, MD, PhD, epidemiologist and resident physician, University of Pennsylvania, Philadelphia, told this news organization.
The study was published online in JAMA Neurology.
Double the risk
Several prior studies that have examined the relationship between depression and dementia over the life course have consistently shown depression later in life is associated with subsequent dementia.
“Late-life depression is generally thought to be an early symptom of dementia or a reaction to subclinical cognitive decline,” said Dr. Elser.
The investigators wanted to examine whether the association between depression and dementia persists even when depression is diagnosed earlier in life, which may suggest it increases the risk of dementia.
“To my knowledge, ours is the largest study on this topic to date, leveraging routinely and prospectively collected data from more than 1.4 million Danish citizens followed from 1977 to 2018,” Dr. Elser noted.
The cohort included 246,499 individuals diagnosed with depression and 1,190,302 individuals without depression.
In both groups, the median age was 50 years and 65% were women. Roughly two-thirds (68%) of those diagnosed with depression were diagnosed before age 60 years.
In Cox proportional hazards regression models, the overall hazard of dementia was more than doubled in those diagnosed with depression (hazard ratio [HR] 2.41). The risk of dementia with depression was more pronounced for men (HR, 2.98) than in women (HR, 2.21).
This association persisted even when the time elapsed from depression diagnosis was between 20 and 39 years (HR, 1.79) and whether depression was diagnosed in early life (18-44 years: HR, 3.08), mid-life (45-59 years: HR, 2.95), or late life (≥ 60 years: HR, 2.31).
It remains unclear whether effective treatment of depression modifies the risk of dementia, as the current study explored the role of antidepressants in a “very limited fashion,” Dr. Elser said.
Specifically, the researchers considered whether an individual was treated with an antidepressant within 6 months of the initial depression diagnosis and found no evidence of a difference in dementia risk between the treated and untreated groups.
“Research that explores implications of the timing and duration of treatment with antidepressants for dementia, treatment with cognitive behavioral therapy, and is able to evaluate the effectiveness of those treatments will be extremely important,” Dr. Elser said.
‘An assault on the brain’
Reached for comment, John Showalter, MD, chief product officer at Linus Health, said one of the most “intriguing” findings of the study is that a depression diagnosis earlier in adulthood conferred a greater risk of developing vascular dementia (HR, 3.28) than did dementia due to Alzheimer’s disease (HR, 1.73).
“The difference in risk for subtypes of dementia is a meaningful addition to our understanding of depression’s connection to dementia,” said Dr. Showalter, who was not involved in the study.
Also weighing in, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the findings from this “far-reaching investigation leave little room for doubt – depression unleashes a devastating storm within the brain, wreaking havoc on the lives of those ensnared by its grip.
“This massive, multi-decade, and high-data quality registry study adds another brick to the growing edifice of evidence attesting to the profound connection between psychiatric health and the very essence of brain health,” said Dr. Lakhan, who was not involved in the study.
“In a resounding declaration, this research underscores that psychiatric health should be perceived as an integral component of overall health – a paradigm shift that challenges long-standing misconceptions and stigmas surrounding mental disorders. Depression, once marginalized, now claims its rightful place on the pedestal of health concerns that must be addressed with unwavering resolve,” said Dr. Lakhan.
He noted that depression is “not just a mental battle, it’s a profound assault on the very fabric of the brain, leaving lives in turmoil and hearts in search of hope. No longer shrouded in silence, depression demands society’s attention.”
The study had no specific funding. Dr. Elser, Dr. Showalter, and Dr. Lakhan have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Adults with depression have more than double the risk of developing dementia and the risk persists regardless of when in life depression is diagnosed, a large population-based study shows.
That the association between depression and dementia persisted even among individuals first diagnosed with depression in early or mid-life provides “strong evidence that depression is not only an early symptom of dementia, but also that depression increases dementia risk,” study investigator Holly Elser, MD, PhD, epidemiologist and resident physician, University of Pennsylvania, Philadelphia, told this news organization.
The study was published online in JAMA Neurology.
Double the risk
Several prior studies that have examined the relationship between depression and dementia over the life course have consistently shown depression later in life is associated with subsequent dementia.
“Late-life depression is generally thought to be an early symptom of dementia or a reaction to subclinical cognitive decline,” said Dr. Elser.
The investigators wanted to examine whether the association between depression and dementia persists even when depression is diagnosed earlier in life, which may suggest it increases the risk of dementia.
“To my knowledge, ours is the largest study on this topic to date, leveraging routinely and prospectively collected data from more than 1.4 million Danish citizens followed from 1977 to 2018,” Dr. Elser noted.
The cohort included 246,499 individuals diagnosed with depression and 1,190,302 individuals without depression.
In both groups, the median age was 50 years and 65% were women. Roughly two-thirds (68%) of those diagnosed with depression were diagnosed before age 60 years.
In Cox proportional hazards regression models, the overall hazard of dementia was more than doubled in those diagnosed with depression (hazard ratio [HR] 2.41). The risk of dementia with depression was more pronounced for men (HR, 2.98) than in women (HR, 2.21).
This association persisted even when the time elapsed from depression diagnosis was between 20 and 39 years (HR, 1.79) and whether depression was diagnosed in early life (18-44 years: HR, 3.08), mid-life (45-59 years: HR, 2.95), or late life (≥ 60 years: HR, 2.31).
It remains unclear whether effective treatment of depression modifies the risk of dementia, as the current study explored the role of antidepressants in a “very limited fashion,” Dr. Elser said.
Specifically, the researchers considered whether an individual was treated with an antidepressant within 6 months of the initial depression diagnosis and found no evidence of a difference in dementia risk between the treated and untreated groups.
“Research that explores implications of the timing and duration of treatment with antidepressants for dementia, treatment with cognitive behavioral therapy, and is able to evaluate the effectiveness of those treatments will be extremely important,” Dr. Elser said.
‘An assault on the brain’
Reached for comment, John Showalter, MD, chief product officer at Linus Health, said one of the most “intriguing” findings of the study is that a depression diagnosis earlier in adulthood conferred a greater risk of developing vascular dementia (HR, 3.28) than did dementia due to Alzheimer’s disease (HR, 1.73).
“The difference in risk for subtypes of dementia is a meaningful addition to our understanding of depression’s connection to dementia,” said Dr. Showalter, who was not involved in the study.
Also weighing in, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the findings from this “far-reaching investigation leave little room for doubt – depression unleashes a devastating storm within the brain, wreaking havoc on the lives of those ensnared by its grip.
“This massive, multi-decade, and high-data quality registry study adds another brick to the growing edifice of evidence attesting to the profound connection between psychiatric health and the very essence of brain health,” said Dr. Lakhan, who was not involved in the study.
“In a resounding declaration, this research underscores that psychiatric health should be perceived as an integral component of overall health – a paradigm shift that challenges long-standing misconceptions and stigmas surrounding mental disorders. Depression, once marginalized, now claims its rightful place on the pedestal of health concerns that must be addressed with unwavering resolve,” said Dr. Lakhan.
He noted that depression is “not just a mental battle, it’s a profound assault on the very fabric of the brain, leaving lives in turmoil and hearts in search of hope. No longer shrouded in silence, depression demands society’s attention.”
The study had no specific funding. Dr. Elser, Dr. Showalter, and Dr. Lakhan have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Palifermin-Associated Cutaneous Papular Rash of the Head and Neck
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.
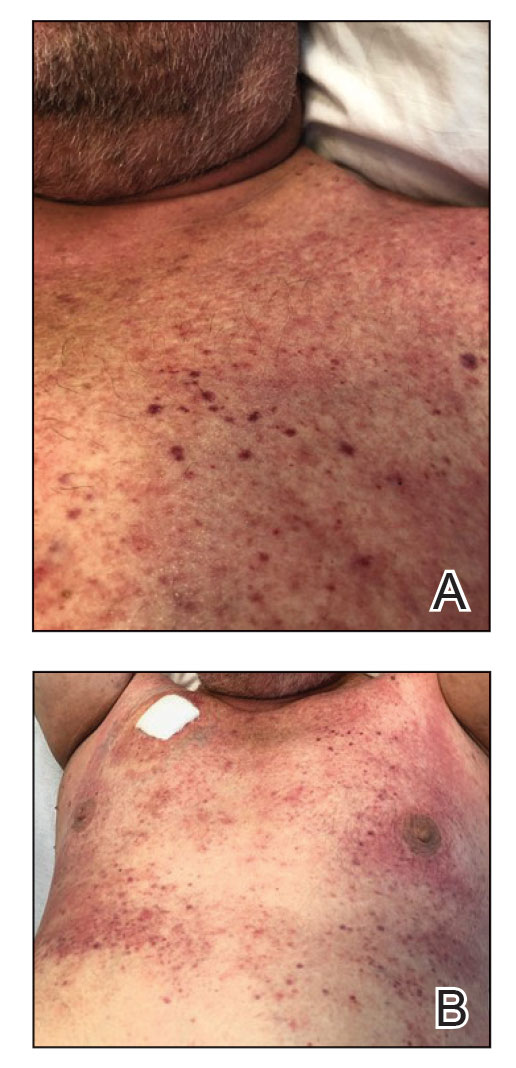
Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
To the Editor:
Palifermin is a recombinant keratinocyte growth factor (KGF) approved by the US Food and Drug Administration to prevent oral mucositis following radiation therapy or chemotherapy. Cutaneous reactions associated with palifermin have been reported.1-5 One case described a distinctive polymorphous eruption in a patient treated with palifermin.6 On histologic analysis, papules demonstrated findings similar to verrucae, with evidence of papillomatosis, hypergranulosis, and hyperorthokeratosis. Given its mechanism of action as a KGF, it was concluded that these findings were likely the direct result of palifermin.6 We report a similar case of a patient who was given palifermin prior to an autologous stem cell transplant. Histopathologic analysis confirmed epidermal dysmaturation and marked hypergranulosis. We present this case to expand the paucity of data on palifermin-associated cutaneous reactions.
A 63-year-old man with a history of psoriasis, eczema, and relapsed diffuse large B-cell lymphoma was admitted to the hospital for routine management of an autologous stem cell transplant with a conditioning regimen involving thiotepa, busulfan, and cyclophosphamide. The patient had completed a 3-day course of palifermin 1 day prior to the current presentation. On admission, he developed a pruritic erythematous rash over the face and axillae. Within 24 hours, the facial rash progressed with appreciable edema, and he reported difficulty opening his eyes. He denied any fever, nausea, vomiting, diarrhea, or increased fatigue. He also denied use of any other medications other than starting a course of prophylactic trimethoprim-sulfamethoxazole 3 times weekly 2 months prior to admission.
Diffuse blanching erythema with a well-demarcated linear border was noted along the lower anterior neck extending to the posterior hairline. There was notable edema but no evidence of pustules or overlying scale. Similar areas of blanchable erythema were present along the axillae and inguinal folds. There also were flesh-colored to pink papules within the axillary vaults and on the back that occasionally coalesced into plaques. There was no involvement of the mucous membranes or acral sites.
A complete blood cell count with differential and a comprehensive metabolic profile largely were unremarkable. A potassium hydroxide preparation of the face and groin was negative for hyphae and Demodex mites. Histopathologic analysis from a punch biopsy of a representative papule from the posterior neck demonstrated epidermal dysmaturation with marked thickening of the granular cell layer with notably large keratohyalin granules (Figure 1).

In the setting of treatment with thiotepa, we recommended supportive care with cool compresses rather than topical medication because he was neutropenic, and we wanted to avoid further immunosuppression or toxicity. By 24 hours after completing the course of palifermin, the patient experienced complete resolution of the rash. At his request, the trial of palifermin was restarted 10 days into conditioning therapy. A similar rash with less facial edema but more prominent involvement of the chest appeared 3 days into the retrial (Figure 2). The medication was discontinued, which resulted in resolution of the rash. Again, the patient remained afebrile without involvement of the mucous membranes. Liver enzyme and creatinine levels remained within reference range.Eosinophilia and the level of atypical lymphocytes could not be assessed because of leukopenia in the setting of recent chemotherapy. The rash self-resolved in 4 days.

Palifermin is a recombinant form of human KGF that is more stable than the endogenous form but retains all vital properties of the protein.5-7 Similar to other growth factors, KGF induces differentiation, proliferation, and migration of cells in vivo.8 However, it uniquely produces a targeted effect on epithelial cells in the skin, oral mucosa, lungs, gastrointestinal tract, and genitourinary system.7-9
Palifermin was approved by the US Food and Drug Administration in 2004 for the prevention and treatment of severe oral mucositis in patients receiving myelotoxic therapy prior to stem cell transplantation.7,9 Severe mucositis occurs in approximately 70% to 80% of patients receiving radiation or chemotherapy-based conditioning treatments.4,7 Compared to placebo, palifermin has been shown to greatly reduce the incidence of Grade 4 oral mucositis, defined as severe enough to prevent alimentation.10
The proliferative effect of palifermin on the oral mucosa is beneficial to patients but likely is the driving force behind its cutaneous adverse effects. A nonspecific rash is the most commonly cited treatment-related adverse event associated with palifermin, occurring in approximately 62% of patients.5,7,9
Our case is a rare report of a palifermin-associated cutaneous reaction. Previous cases have cited the occurrence of palmoplantar erythrodysesthesias, papulopustular eruptions involving the face and chest, and a papular rash involving the dorsal hands and intertriginous areas.1-4 Another report documented a “mild rash” but failed to further characterize the morphology or the body site involved.5
In 2009, King et al6 reported the occurrence of a lichen planus–like eruption involving the intertriginous regions and of white oral plaques in a patient treated with palifermin. Hematoxylin and eosin staining of a representative lesion in that patient demonstrated an appearance similar to that of verrucae, including papillomatosis, hypergranulosis, and hyperorthokeratosis.
King et al6 expanded analysis of the reaction to include immunohistochemical study, using targeted antibody stains for cytokeratin 5/6 and Ki-67 protein. Staining with Ki-67 showed dramatically increased activity within basilar and suprabasilar keratinocytes in a biopsy taken at the height of the reaction. Biopsy specimens obtained when the eruption was clinically resolving—2 days after the first biopsy—showed decreased Ki-67 staining. These findings taken together suggest a direct causal effect of palifermin inducing hyperkeratotic changes appreciated on examination of treated patients.6
We present this case to add to current data regarding palifermin-induced cutaneous changes. Unique to our patient was a strikingly well-demarcated rash confined to the head and neck. Although a photosensitive eruption due to trimethoprim-sulfamethoxazole is conceivable, the fixed time course of the eruption—corresponding to (1) initiation and discontinuation of palifermin and (2) histologic findings—led us to conclude that this self-limited eruption likely was due to palifermin.
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
- Gorcey L, Lewin JM, Trufant J, et al. Papular eruption associated with palifermin. J Am Acad Dermatol. 2014;71:E101-E102. doi:10.1016/j.jaad.2014.04.006
- Grzegorczyk-Jaz´win´ska A, Kozak I, Karakulska-Prystupiuk E, et al. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. case history. Adv Med Sci. 2006;51(suppl 1):66-68.
- Keijzer A, Huijgens PC, van de Loosdrecht AA. Palifermin and palmar–plantar erythrodysesthesia. Br J Haematol. 2007;136:856-857. doi:10.1111/j.1365-2141.2007.06509.x
- Sibelt LAG, Aboosy N, van der Velden WJFM, et al. Palifermin-induced flexural hyperpigmentation: a clinical and histological study of five cases. Br J Dermatol. 2008;159:1200-1203. doi:10.1111/j.1365-2133.2008.08816.x
- Keefe D, Lees J, Horvath N. Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer. 2006;14:580-582. doi:10.1007/s00520-006-0048-3
- King B, Knopp E, Galan A, et al. Palifermin-associated papular eruption. Arch Dermatol. 2009;145:179-182. doi:10.1001/archdermatol.2008.548
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590-2598. doi: 10.1056/NEJMoa040125
- Rubin JS, Bottaro DP, Chedid M, et al. Keratinocyte growth factor. Cell Biol Int. 1995;19:399-411. doi:10.1006/cbir.1995.1085
- McDonnell AM, Lenz KL. Palifermin: role in the prevention of chemotherapy- and radiation-induced mucositis. Ann Pharmacother. 2007;41:86-94. doi:10.1345/aph.1G473
- Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol. 2017;7:89. doi:10.3389/fonc.2017.00089
Practice Points
- Palifermin is a recombinant keratinocyte growth factor that is US Food and Drug Administration approved to prevent oral mucositis in patients undergoing chemotherapy or radiation therapy.
- Histologically, the rash can resemble verrucae with evidence of hypergranulosis, hyperorthokeratosis, and papillomatosis.
- Cutaneous reactions have been reported with use of palifermin and generally are benign and self-limited with removal of the offending agent.