User login
Learning Needs of Physician Assistants
Physician assistants (PA) have rapidly become an integral component in the United States health care delivery system, including in the field of Hospital Medicine, the fastest growing medical field in the United States.1, 2 Since its induction in 1997, hospitalist providers in North America have increased by 30‐fold.3 Correlating with this, the number of PAs practicing in the field of hospital medicine has also increased greatly in recent years. According to the American Academy of Physician Assistants (AAPA) census reports, Hospital Medicine first appeared as one of the specialty choices in the 2006 census (response rate, 33% of all individuals eligible to practice as PAs) when it was selected as the primary specialty by 239 PAs (1.1% of respondents). In the 2008 report (response rate, 35%), the number grew to 421 (1.7%) PAs.2
PA training programs emphasize primary care and offer limited exposure to inpatient medicine. After PA students complete their first 12 months of training in didactic coursework that teach the basic sciences, they typically spend the next year on clinical rotations, largely rooted in outpatient care.2, 4 Upon graduation, PAs do not have to pursue postgraduate training before beginning to practice in their preferred specialty areas. Thus, a majority of PAs going into specialty areas are trained on the job. This is not an exception in the field of hospital medicine.
In recent years, despite an increase in the number of PAs in Hospital Medicine, some medical centers have chosen to phase out the use of midlevel hospitalist providers (including PAs) with the purposeful decision to not hire new midlevel providers.5 The rationale for this strategy is that there is thought to be a steep learning curve that requires much time to overcome before these providers feel comfortable across the breadth of clinical cases. Before they become experienced and confident in caring for a highly complex heterogeneous patient population, they cannot operate autonomously and are not a cost‐effective alternative to physicians. The complexities associated with practicing in this field were clarified in 2006 when the Society of Hospital Medicine identified 51 core competencies in hospital medicine.3, 6 Some hospitalist programs are willing to provide their PAs with on‐the‐job training, but many programs do not have the educational expertise or the resources to make this happen. Structured and focused postgraduate training in hospital medicine seems like a reasonable solution to prepare newly graduating PAs that are interested in pursuing hospitalist careers, but such opportunities are very limited.7
To date, there is no available information about the learning needs of PAs working in hospital medicine settings. We hypothesized that understanding the learning needs of PA hospitalists would inform the development of more effective and efficient training programs. We studied PAs with experience working in hospital medicine to (1) identify self‐perceived gaps in their skills and knowledge upon starting their hospitalist careers and (2) understand their views about optimal training for careers in hospital medicine.
METHODS
Study Design
We conducted a cross‐sectional survey of a convenience sample of self‐identified PAs working in adult Hospital Medicine. The survey was distributed using an electronic survey program.
Participants
The subjects for the survey were identified through the Facebook group PAs in Hospital Medicine, which had 133 members as of July 2010. This source was selected because it was the most comprehensive list of self‐identified hospitalist PAs. Additionally, the group allowed us to send individualized invitations to complete the survey along with subsequent reminder messages to nonresponders. Subjects were eligible to participate if they were PAs with experience working in hospital medicine settings taking care of adult internal medicine inpatients.
Survey Instrument
The survey instrument was developed based on the Core Competencies in Hospital Medicine with the goal of identifying PA hospitalists' knowledge and skill gaps that were present when they started their hospitalist career.
In one section, respondents were asked about content areas among the Core Competencies in Hospital Medicine that they believed would have enhanced their effectiveness in practicing hospital medicine had they had additional training before starting their work as hospitalists. Response options ranged from Strongly Agree to Strongly Disagree. Because there were content areas that seemed more relevant to physicians, through rigorous discussions, our study team (including a hospitalist physician, senior hospitalist PA, two curriculum development experts, one medical education research expert, and an experienced hospital medicine research assistant) selected topics that were felt to be particularly germane to PA hospitalists. The relevance of this content to PA hospitalists was confirmed through pilot testing of the instrument. Another series of questions asked the PAs about their views on formal postgraduate training programs. The subjects were also queried about the frequency with which they performed various procedures (using the following scale: Never, Rarely [1‐2/year], Regularly [1‐2/month], Often [1‐2/week]) and whether they felt it was necessary for PAs to have procedure skills listed as part of the Core Competencies in Hospital Medicine (using the following scale: Not necessary, Preferable, Essential). Finally, the survey included a question about the PAs' preferred learning methods by asking the degree of helpfulness on various approaches (using the following scale: Not at all, Little, Some, A lot, Tremendously). Demographic information was also collected. The instrument was pilot‐tested for clarity on the 9 PA hospitalists who were affiliated with our hospitalist service, and the instrument was iteratively revised based on their feedback.
Data Collection and Analysis
Between September and December 2010, the survey invitations were sent as Facebook messages to the 133 members of the Facebook group PAs in Hospital Medicine. Sixteen members could not be contacted because their account setup did not allow us to send messages, and 14 were excluded because they were non‐PA members. In order to maximize participation, up to 4 reminder messages were sent to the 103 targeted PAs. The survey results were analyzed using Stata 11. Descriptive statistics were used to characterize the responses.
This study protocol was approved by the institution's review board.
RESULTS
Sixty‐nine PAs responded (response rate, 67%). Table 1 provides demographic characteristics of the respondents. The majority of respondents were 2635 years old and had worked as hospitalists for a mean of 4.3 years.
| Characteristics* | Value |
|---|---|
| |
| Age, years, n (%) | |
| <26 | 1 (2) |
| 2630 | 16 (29) |
| 3135 | 14 (25) |
| 3640 | 10 (18) |
| 4145 | 5 (9) |
| >45 | 10 (18) |
| Women, n (%) | 35 (63) |
| Year of graduation from PA school, mode (SD) | 2002 (7) |
| No. of years working/worked as hospitalist, mean (SD) | 4.3 (3.4) |
| Completed any postgraduate training program, n (%) | 0 (0) |
| Hospitalist was the first PA job, n (%) | 30 (49) |
| Salary, US$, n (%) | |
| 50,00170,000 | 1 (2) |
| 70,00190,000 | 32 (57) |
| >90,000 | 23 (41) |
| Location of hospital, n (%) | |
| Urban | 35 (57) |
| Suburban | 21 (34) |
| Rural | 5 (8) |
| Hospital characteristics, n (%) | |
| Academic medical center | 25 (41) |
| Community teaching hospital | 20 (33) |
| Community nonteaching hospital | 16 (26) |
| Responsibilities in addition to taking care of inpatients on medicine floor, n (%) | |
| Care for patients in ICU | 22 (35) |
| Perform inpatient consultations | 31 (50) |
| See outpatients | 11 (18) |
Clinical Conditions
Table 2 shows the respondents' experience with 19 core competency clinical conditions before beginning their careers as hospitalist PAs. They reported having most experience in managing diabetes and urinary tract infections, and least experience in managing healthcare associated pneumonias and sepsis syndrome.
| Clinical Condition | Mean (SD)* |
|---|---|
| |
| Urinary tract infection | 4.5 (0.8) |
| Diabetes mellitus | 4.5 (0.8) |
| Asthma | 4.4 (0.9) |
| Community‐acquired pneumonia | 4.3 (0.9) |
| Chronic obstructive pulmonary disease | 4.3 (1.0) |
| Cellulitis | 4.2 (0.9) |
| Congestive heart failure | 4.1 (1.0) |
| Cardiac arrhythmia | 3.9 (1.1) |
| Delirium and dementia | 3.8 (1.1) |
| Acute coronary syndrome | 3.8 (1.2) |
| Acute renal failure | 3.8 (1.1) |
| Gastrointestinal bleed | 3.7 (1.1) |
| Venous thromboembolism | 3.7 (1.2) |
| Pain management | 3.7 (1.2) |
| Perioperative medicine | 3.6 (1.4) |
| Stroke | 3.5 (1.2) |
| Alcohol and drug withdrawal | 3.4 (1.1) |
| Sepsis syndrome | 3.3 (1.1) |
| Hospital‐acquired pneumonia | 3.2 (1.1) |
Procedures
Most PA hospitalists (67%) perform electrocardiograms and chest X‐ray interpretations regularly (more than 1‐2/ week). However, nearly all PA hospitalists never or rarely (less than 1‐2/year) perform any invasive procedures, including arthrocentesis (98%), lumbar puncture (100%), paracentesis (91%), thoracentesis (98%), central line placement (91%), peripherally inserted central catheter placement (91%), and peripheral intravenous insertion (91%). Despite the infrequency of execution, more than 50% of respondents explained that it is either preferable or essential to be able to perform these procedures.
Content Knowledge
The PA hospitalists indicated which content areas may have allowed them to be more successful had they learned the material before starting their hospitalist career (Table 3). The top 4 topics that PA hospitalists believed would have helped them most to care for inpatients were palliative care (85% agreed or strongly agreed), nutrition for hospitalized patients (84%), performing consultations in the hospital (64%), and prevention of health careassociated infection (61%).
| Health Care System Topics | PAs Who Agreed or Strongly Agreed, n (%) |
|---|---|
| Palliative care | 47 (85) |
| Nutrition for hospitalized patients | 46 (84) |
| Performing consultations in hospital | 35 (64) |
| Prevention of health careassociated infections | 34 (62) |
| Diagnostic decision‐making processes | 32 (58) |
| Patient handoff and transitions of care | 31 (56) |
| Evidence‐based medicine | 28 (51) |
| Communication with patients and families | 27 (49) |
| Drug safety and drug interactions | 27 (49) |
| Team approach and multidisciplinary care | 26 (48) |
| Patient safety and quality improvement processes | 25 (45) |
| Care of elderly patients | 24 (44) |
| Medical ethics | 22 (40) |
| Patient education | 20 (36) |
| Care of uninsured or underinsured patients | 18 (33) |
Professional Growth as Hospitalist Providers
PAs judged working with physician preceptors (mean SD, 4.5 0.6) and discussing patients with consultants (mean SD, 4.3 0.8) to be most helpful for their professional growth, whereas receiving feedback/audits about their performance (mean SD, 3.5 1), attending conferences/lectures (mean SD, 3.6 0.7), and reading journals/textbooks (mean SD, 3.6 0.8) were rated as being less useful. Respondents believed that the mean number of months required for new hospitalist PAs to become fully competent team members was 11 months ( 8.6 SD). Forty‐three percent of respondents shared the perspective that some clinical experience in an inpatient setting was an essential prerequisite for entry into a hospitalist position. Although more than half (58%) felt that completion of postgraduate training program in hospital medicine was not necessary as a prerequisite, almost all (91%) explained that they would have been interested in such a program even if it meant having a lower stipend than a hospitalist PA salary during the first year on the job (Table 4).
| Interest in Training | n (%) |
|---|---|
| Interested and willing to pay tuition | 1 (2) |
| Interested even if there was no stipend, as long as I didn't have to pay any additional tuition | 3 (5) |
| Interested ONLY if a stipend of at least 25% of a hospitalist PA salary was offered | 4 (7) |
| Interested ONLY if a stipend of at least 50% of a hospitalist PA salary was offered | 21 (38) |
| Interested ONLY if a stipend of at least 75% of a hospitalist PA salary was offered | 21 (38) |
| Interested ONLY if 100% of a hospitalist PA salary was offered | 4 (7) |
| Not interested under any circumstances | 1 (2) |
DISCUSSION
Our survey addresses a wide range of topics related to PA hospitalists' learning needs including their experience with the Core Competencies in Hospital Medicine and their views on the benefits of PA training following graduation. Although self‐efficacy is not assessed, our study revealed that PAs who are choosing hospitalist careers have limited prior clinical experience treating many medical conditions that are managed in inpatient settings, such as sepsis syndrome. This inexperience with commonly seen clinical conditions, such as sepsis, wherein following guidelines can both reduce costs and improve outcomes, is problematic. More experience and training with such conditions would almost certainly reduce variability, improve skills, and augment confidence. These observed variations in experience in caring for conditions that often prompt admission to the hospital among PAs starting their hospitalist careers emphasizes the need to be learner‐centered when training PAs, so as to provide tailored guidance and oversight.
Only a few other empiric research articles have focused on PA hospitalists. One article described a postgraduate training program for PAs in hospital medicine that was launched in 2008. The curriculum was developed based on the Core Competencies in Hospital Medicine, and the authors explained that after 12 months of training, their first graduate functioned at the level of a PA with 4 years of experience under her belt.7 Several articles describe experiences using midlevel providers (including PAs) in general surgery, primary care medicine, cardiology, emergency medicine, critical care, pediatrics, and hospital medicine settings.5, 820 Many of these articles reported favorable results showing that using midlevel providers was either superior or just as effective in terms of cost and quality measures to physician‐only models. Many of these papers alluded to the ways in which PAs have enabled graduate medical education training programs to comply with residents' duty‐hour restrictions. A recent analysis that compared outcomes related to inpatient care provided by a hospitalist‐PA model versus a traditional resident‐based model revealed a slightly longer length of stay on the PA team but similar charges, readmission rates, and mortality.19 Yet another paper revealed that patients admitted to a residents' service, compared with the nonteaching hospitalist service that uses PAs and nurse practitioners, were different, having higher comorbidity burdens and higher acuity diagnoses.20 The authors suggested that this variance might be explained by the difference in their training, abilities, and goals of the groups. There was no research article that sought to capture the perspectives of practicing hospitalist PAs.
Our study revealed that although half of respondents became hospitalists immediately after graduating from PA school, a majority agreed that additional clinical training in inpatient settings would have been welcomed and helpful. This study's results reveal that although there is a fair amount of perceived interest in postgraduate training programs in hospital medicine, there are very few training opportunities for PAs in hospital medicine.7, 21 The American Academy of Physician Assistants, the Society of Hospital Medicine, and the American Academy of Nurse Practitioners cosponsor Adult Hospital Medicine Boot Camp for PAs and nurse practitioners annually to facilitate knowledge acquisition, but this course is truly an orientation rather than a comprehensive training program.22 Our findings suggest that more rigorous and thorough training in hospital medicine would be valued and appreciated by PA hospitalists.
Several limitations of this study should be considered. First, our survey respondents may not represent the entire spectrum of practicing PA hospitalists. However, the demographic data of 421 PAs who indicated their specialty as hospital medicine in the 2008 National Physician Assistants Census Report were not dissimilar from our informants; 65% were women, and their mean number of years in hospital medicine was 3.9 years.2 Second, our study sample was small. It was difficult to identify a national sample of hospitalist PAs, and we had to resort to a creative use of social media to find a national sample. Third, the study relied exclusively on self‐report, and since we asked about their perceived learning needs when they started working as hospitalists, recall bias cannot be excluded. However, the questions addressing attitudes and beliefs can only be ascertained from the informants themselves. That said, the input from hospitalist physicians about training needs for the PAs who they are supervising would have strengthened the reliability of the data, but this was not possible given the sampling strategy that we elected to use. Finally, our survey instrument was developed based on the Core Competencies in Hospital Medicine, which is a blueprint to develop standardized curricula for teaching hospital medicine in medical school, postgraduate training programs (ie, residency, fellowship), and continuing medical education programs. It is not clear whether the same competencies should be expected of PA hospitalists who may have different job descriptions from physician hospitalists.
In conclusion, we present the first national data on self‐perceived learning needs of PAs working in hospital medicine settings. This study collates the perceptions of PAs working in hospital medicine and highlights the fact that training in PA school does not adequately prepare them to care for hospitalized patients. Hospitalist groups may use this study's findings to coach and instruct newly hired or inexperienced hospitalist PAs, particularly until postgraduate training opportunities become more prevalent. PA schools may consider the results of this study for modifying their curricula in hopes of emphasizing the clinical content that may be most relevant for a proportion of their graduates.
Acknowledgements
The authors would like to thank Drs. David Kern and Belinda Chen at Johns Hopkins Bayview Medical Center for their assistance in developing the survey instrument.
Financial support: This study was supported by the Linda Brandt Research Award program of the Association of Postgraduate PA Programs. Dr. Wright is a Miller‐Coulson Family Scholar and was supported through the Johns Hopkins Center for Innovative Medicine.
Disclosures: Dr. Torok and Ms. Lackner received a Linda Brandt Research Award from the Association of Postgraduate PA Programs for support of this study. Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine.
- United States Department of Labor, Bureau of Labor Statistics. Available at: http://www.bls.gov. Accessed February 16,2011.
- American Academy of Physician Assistants. Available at: http://www.aapa.org. Accessed April 20,2011.
- Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org. Accessed January 24,2011.
- Accreditation Review Commission on Education for the Physician Assistants Accreditation Standards. Available at: http://www.arc‐pa.org/acc_standards. Accessed February 16,2011.
- ,.Non‐physician providers in hospital medicine: not so fast.J Hosp Med.2010;5(2):103–106.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,,.A hospitalist postgraduate training program for physician assistants.J Hosp Med.2010;5:94–98.
- ,,,.How do surgical residents and non‐physician practitioners play together in the sandbox?Curr Surg.2006;63:155–164.
- ,.Physician assistant influence on surgery residents.Arch Surg.2003;138:971–976.
- ,,, et al.Non‐physician practitioners' overall enhancement to a surgical resident's experience.J Surg Educ.2008;65:50–53.
- ,,,,.Use of midlevel practitioners to achieve labor cost savings in the primary care practice of an MCO.Health Serv Res.2004;39:607–626.
- ,,,,.The economic benefit for family/general medicine practices employing physician assistants.Am J Manag Care.2002;8:613–620.
- ,,.Financial and organizational factors affecting the employment of nurse practitioners and physician assistants in medical group practices.J Ambul Care Manage.2003;26:209–216.
- ,,,,,.A nonresident cardiovascular inpatient service improves residents' experiences in an academic medical center: a new model to meet the challenges of the new millennium.Acad Med.2004;79:426–431.
- ,,.Nurse practitioners and physician assistants in the intensive care unit: an evidence‐based review.Crit Care Med.2008;36:2888–2897.
- ,.A systematic review of the impact of nurse practitioners on cost, quality of care, satisfaction and wait times in the emergency department.CJEM.2007;9:286–295.
- ,,,.Physician assistants as physician extenders in the pediatric intensive care unit setting—a 5‐year experience.Pediatr Crit Care Med.2005;6:14–19.
- ,,,.A process for reducing workload and enhancing residents' education at an academic medical center.Acad Med.2001;76:798–805.
- ,,, et al.A comparison of outcomes of general medical inpatient care provided by a hospitalist‐physician assistant model vs a traditional resident‐based model.J Hosp Med.2011;6:112–130.
- ,,, et al.The effect of nonteaching services on the distribution of inpatient cases for internal medicine residents.Acad Med.2009;84:220–225.
- Association of Postgraduate PA Programs. Available at: http://appap.org/Home/tabid/38/Default.aspx. Accessed February 16,2011.
- Adult Hospital Medicine Boot Camp for PAs and NPs. Available at: http://www.aapa.org/component/content/article/23—general‐/673‐adult‐hospital‐medicine‐boot‐camp‐for‐pas‐and‐nps. Accessed February 16,2011.
Physician assistants (PA) have rapidly become an integral component in the United States health care delivery system, including in the field of Hospital Medicine, the fastest growing medical field in the United States.1, 2 Since its induction in 1997, hospitalist providers in North America have increased by 30‐fold.3 Correlating with this, the number of PAs practicing in the field of hospital medicine has also increased greatly in recent years. According to the American Academy of Physician Assistants (AAPA) census reports, Hospital Medicine first appeared as one of the specialty choices in the 2006 census (response rate, 33% of all individuals eligible to practice as PAs) when it was selected as the primary specialty by 239 PAs (1.1% of respondents). In the 2008 report (response rate, 35%), the number grew to 421 (1.7%) PAs.2
PA training programs emphasize primary care and offer limited exposure to inpatient medicine. After PA students complete their first 12 months of training in didactic coursework that teach the basic sciences, they typically spend the next year on clinical rotations, largely rooted in outpatient care.2, 4 Upon graduation, PAs do not have to pursue postgraduate training before beginning to practice in their preferred specialty areas. Thus, a majority of PAs going into specialty areas are trained on the job. This is not an exception in the field of hospital medicine.
In recent years, despite an increase in the number of PAs in Hospital Medicine, some medical centers have chosen to phase out the use of midlevel hospitalist providers (including PAs) with the purposeful decision to not hire new midlevel providers.5 The rationale for this strategy is that there is thought to be a steep learning curve that requires much time to overcome before these providers feel comfortable across the breadth of clinical cases. Before they become experienced and confident in caring for a highly complex heterogeneous patient population, they cannot operate autonomously and are not a cost‐effective alternative to physicians. The complexities associated with practicing in this field were clarified in 2006 when the Society of Hospital Medicine identified 51 core competencies in hospital medicine.3, 6 Some hospitalist programs are willing to provide their PAs with on‐the‐job training, but many programs do not have the educational expertise or the resources to make this happen. Structured and focused postgraduate training in hospital medicine seems like a reasonable solution to prepare newly graduating PAs that are interested in pursuing hospitalist careers, but such opportunities are very limited.7
To date, there is no available information about the learning needs of PAs working in hospital medicine settings. We hypothesized that understanding the learning needs of PA hospitalists would inform the development of more effective and efficient training programs. We studied PAs with experience working in hospital medicine to (1) identify self‐perceived gaps in their skills and knowledge upon starting their hospitalist careers and (2) understand their views about optimal training for careers in hospital medicine.
METHODS
Study Design
We conducted a cross‐sectional survey of a convenience sample of self‐identified PAs working in adult Hospital Medicine. The survey was distributed using an electronic survey program.
Participants
The subjects for the survey were identified through the Facebook group PAs in Hospital Medicine, which had 133 members as of July 2010. This source was selected because it was the most comprehensive list of self‐identified hospitalist PAs. Additionally, the group allowed us to send individualized invitations to complete the survey along with subsequent reminder messages to nonresponders. Subjects were eligible to participate if they were PAs with experience working in hospital medicine settings taking care of adult internal medicine inpatients.
Survey Instrument
The survey instrument was developed based on the Core Competencies in Hospital Medicine with the goal of identifying PA hospitalists' knowledge and skill gaps that were present when they started their hospitalist career.
In one section, respondents were asked about content areas among the Core Competencies in Hospital Medicine that they believed would have enhanced their effectiveness in practicing hospital medicine had they had additional training before starting their work as hospitalists. Response options ranged from Strongly Agree to Strongly Disagree. Because there were content areas that seemed more relevant to physicians, through rigorous discussions, our study team (including a hospitalist physician, senior hospitalist PA, two curriculum development experts, one medical education research expert, and an experienced hospital medicine research assistant) selected topics that were felt to be particularly germane to PA hospitalists. The relevance of this content to PA hospitalists was confirmed through pilot testing of the instrument. Another series of questions asked the PAs about their views on formal postgraduate training programs. The subjects were also queried about the frequency with which they performed various procedures (using the following scale: Never, Rarely [1‐2/year], Regularly [1‐2/month], Often [1‐2/week]) and whether they felt it was necessary for PAs to have procedure skills listed as part of the Core Competencies in Hospital Medicine (using the following scale: Not necessary, Preferable, Essential). Finally, the survey included a question about the PAs' preferred learning methods by asking the degree of helpfulness on various approaches (using the following scale: Not at all, Little, Some, A lot, Tremendously). Demographic information was also collected. The instrument was pilot‐tested for clarity on the 9 PA hospitalists who were affiliated with our hospitalist service, and the instrument was iteratively revised based on their feedback.
Data Collection and Analysis
Between September and December 2010, the survey invitations were sent as Facebook messages to the 133 members of the Facebook group PAs in Hospital Medicine. Sixteen members could not be contacted because their account setup did not allow us to send messages, and 14 were excluded because they were non‐PA members. In order to maximize participation, up to 4 reminder messages were sent to the 103 targeted PAs. The survey results were analyzed using Stata 11. Descriptive statistics were used to characterize the responses.
This study protocol was approved by the institution's review board.
RESULTS
Sixty‐nine PAs responded (response rate, 67%). Table 1 provides demographic characteristics of the respondents. The majority of respondents were 2635 years old and had worked as hospitalists for a mean of 4.3 years.
| Characteristics* | Value |
|---|---|
| |
| Age, years, n (%) | |
| <26 | 1 (2) |
| 2630 | 16 (29) |
| 3135 | 14 (25) |
| 3640 | 10 (18) |
| 4145 | 5 (9) |
| >45 | 10 (18) |
| Women, n (%) | 35 (63) |
| Year of graduation from PA school, mode (SD) | 2002 (7) |
| No. of years working/worked as hospitalist, mean (SD) | 4.3 (3.4) |
| Completed any postgraduate training program, n (%) | 0 (0) |
| Hospitalist was the first PA job, n (%) | 30 (49) |
| Salary, US$, n (%) | |
| 50,00170,000 | 1 (2) |
| 70,00190,000 | 32 (57) |
| >90,000 | 23 (41) |
| Location of hospital, n (%) | |
| Urban | 35 (57) |
| Suburban | 21 (34) |
| Rural | 5 (8) |
| Hospital characteristics, n (%) | |
| Academic medical center | 25 (41) |
| Community teaching hospital | 20 (33) |
| Community nonteaching hospital | 16 (26) |
| Responsibilities in addition to taking care of inpatients on medicine floor, n (%) | |
| Care for patients in ICU | 22 (35) |
| Perform inpatient consultations | 31 (50) |
| See outpatients | 11 (18) |
Clinical Conditions
Table 2 shows the respondents' experience with 19 core competency clinical conditions before beginning their careers as hospitalist PAs. They reported having most experience in managing diabetes and urinary tract infections, and least experience in managing healthcare associated pneumonias and sepsis syndrome.
| Clinical Condition | Mean (SD)* |
|---|---|
| |
| Urinary tract infection | 4.5 (0.8) |
| Diabetes mellitus | 4.5 (0.8) |
| Asthma | 4.4 (0.9) |
| Community‐acquired pneumonia | 4.3 (0.9) |
| Chronic obstructive pulmonary disease | 4.3 (1.0) |
| Cellulitis | 4.2 (0.9) |
| Congestive heart failure | 4.1 (1.0) |
| Cardiac arrhythmia | 3.9 (1.1) |
| Delirium and dementia | 3.8 (1.1) |
| Acute coronary syndrome | 3.8 (1.2) |
| Acute renal failure | 3.8 (1.1) |
| Gastrointestinal bleed | 3.7 (1.1) |
| Venous thromboembolism | 3.7 (1.2) |
| Pain management | 3.7 (1.2) |
| Perioperative medicine | 3.6 (1.4) |
| Stroke | 3.5 (1.2) |
| Alcohol and drug withdrawal | 3.4 (1.1) |
| Sepsis syndrome | 3.3 (1.1) |
| Hospital‐acquired pneumonia | 3.2 (1.1) |
Procedures
Most PA hospitalists (67%) perform electrocardiograms and chest X‐ray interpretations regularly (more than 1‐2/ week). However, nearly all PA hospitalists never or rarely (less than 1‐2/year) perform any invasive procedures, including arthrocentesis (98%), lumbar puncture (100%), paracentesis (91%), thoracentesis (98%), central line placement (91%), peripherally inserted central catheter placement (91%), and peripheral intravenous insertion (91%). Despite the infrequency of execution, more than 50% of respondents explained that it is either preferable or essential to be able to perform these procedures.
Content Knowledge
The PA hospitalists indicated which content areas may have allowed them to be more successful had they learned the material before starting their hospitalist career (Table 3). The top 4 topics that PA hospitalists believed would have helped them most to care for inpatients were palliative care (85% agreed or strongly agreed), nutrition for hospitalized patients (84%), performing consultations in the hospital (64%), and prevention of health careassociated infection (61%).
| Health Care System Topics | PAs Who Agreed or Strongly Agreed, n (%) |
|---|---|
| Palliative care | 47 (85) |
| Nutrition for hospitalized patients | 46 (84) |
| Performing consultations in hospital | 35 (64) |
| Prevention of health careassociated infections | 34 (62) |
| Diagnostic decision‐making processes | 32 (58) |
| Patient handoff and transitions of care | 31 (56) |
| Evidence‐based medicine | 28 (51) |
| Communication with patients and families | 27 (49) |
| Drug safety and drug interactions | 27 (49) |
| Team approach and multidisciplinary care | 26 (48) |
| Patient safety and quality improvement processes | 25 (45) |
| Care of elderly patients | 24 (44) |
| Medical ethics | 22 (40) |
| Patient education | 20 (36) |
| Care of uninsured or underinsured patients | 18 (33) |
Professional Growth as Hospitalist Providers
PAs judged working with physician preceptors (mean SD, 4.5 0.6) and discussing patients with consultants (mean SD, 4.3 0.8) to be most helpful for their professional growth, whereas receiving feedback/audits about their performance (mean SD, 3.5 1), attending conferences/lectures (mean SD, 3.6 0.7), and reading journals/textbooks (mean SD, 3.6 0.8) were rated as being less useful. Respondents believed that the mean number of months required for new hospitalist PAs to become fully competent team members was 11 months ( 8.6 SD). Forty‐three percent of respondents shared the perspective that some clinical experience in an inpatient setting was an essential prerequisite for entry into a hospitalist position. Although more than half (58%) felt that completion of postgraduate training program in hospital medicine was not necessary as a prerequisite, almost all (91%) explained that they would have been interested in such a program even if it meant having a lower stipend than a hospitalist PA salary during the first year on the job (Table 4).
| Interest in Training | n (%) |
|---|---|
| Interested and willing to pay tuition | 1 (2) |
| Interested even if there was no stipend, as long as I didn't have to pay any additional tuition | 3 (5) |
| Interested ONLY if a stipend of at least 25% of a hospitalist PA salary was offered | 4 (7) |
| Interested ONLY if a stipend of at least 50% of a hospitalist PA salary was offered | 21 (38) |
| Interested ONLY if a stipend of at least 75% of a hospitalist PA salary was offered | 21 (38) |
| Interested ONLY if 100% of a hospitalist PA salary was offered | 4 (7) |
| Not interested under any circumstances | 1 (2) |
DISCUSSION
Our survey addresses a wide range of topics related to PA hospitalists' learning needs including their experience with the Core Competencies in Hospital Medicine and their views on the benefits of PA training following graduation. Although self‐efficacy is not assessed, our study revealed that PAs who are choosing hospitalist careers have limited prior clinical experience treating many medical conditions that are managed in inpatient settings, such as sepsis syndrome. This inexperience with commonly seen clinical conditions, such as sepsis, wherein following guidelines can both reduce costs and improve outcomes, is problematic. More experience and training with such conditions would almost certainly reduce variability, improve skills, and augment confidence. These observed variations in experience in caring for conditions that often prompt admission to the hospital among PAs starting their hospitalist careers emphasizes the need to be learner‐centered when training PAs, so as to provide tailored guidance and oversight.
Only a few other empiric research articles have focused on PA hospitalists. One article described a postgraduate training program for PAs in hospital medicine that was launched in 2008. The curriculum was developed based on the Core Competencies in Hospital Medicine, and the authors explained that after 12 months of training, their first graduate functioned at the level of a PA with 4 years of experience under her belt.7 Several articles describe experiences using midlevel providers (including PAs) in general surgery, primary care medicine, cardiology, emergency medicine, critical care, pediatrics, and hospital medicine settings.5, 820 Many of these articles reported favorable results showing that using midlevel providers was either superior or just as effective in terms of cost and quality measures to physician‐only models. Many of these papers alluded to the ways in which PAs have enabled graduate medical education training programs to comply with residents' duty‐hour restrictions. A recent analysis that compared outcomes related to inpatient care provided by a hospitalist‐PA model versus a traditional resident‐based model revealed a slightly longer length of stay on the PA team but similar charges, readmission rates, and mortality.19 Yet another paper revealed that patients admitted to a residents' service, compared with the nonteaching hospitalist service that uses PAs and nurse practitioners, were different, having higher comorbidity burdens and higher acuity diagnoses.20 The authors suggested that this variance might be explained by the difference in their training, abilities, and goals of the groups. There was no research article that sought to capture the perspectives of practicing hospitalist PAs.
Our study revealed that although half of respondents became hospitalists immediately after graduating from PA school, a majority agreed that additional clinical training in inpatient settings would have been welcomed and helpful. This study's results reveal that although there is a fair amount of perceived interest in postgraduate training programs in hospital medicine, there are very few training opportunities for PAs in hospital medicine.7, 21 The American Academy of Physician Assistants, the Society of Hospital Medicine, and the American Academy of Nurse Practitioners cosponsor Adult Hospital Medicine Boot Camp for PAs and nurse practitioners annually to facilitate knowledge acquisition, but this course is truly an orientation rather than a comprehensive training program.22 Our findings suggest that more rigorous and thorough training in hospital medicine would be valued and appreciated by PA hospitalists.
Several limitations of this study should be considered. First, our survey respondents may not represent the entire spectrum of practicing PA hospitalists. However, the demographic data of 421 PAs who indicated their specialty as hospital medicine in the 2008 National Physician Assistants Census Report were not dissimilar from our informants; 65% were women, and their mean number of years in hospital medicine was 3.9 years.2 Second, our study sample was small. It was difficult to identify a national sample of hospitalist PAs, and we had to resort to a creative use of social media to find a national sample. Third, the study relied exclusively on self‐report, and since we asked about their perceived learning needs when they started working as hospitalists, recall bias cannot be excluded. However, the questions addressing attitudes and beliefs can only be ascertained from the informants themselves. That said, the input from hospitalist physicians about training needs for the PAs who they are supervising would have strengthened the reliability of the data, but this was not possible given the sampling strategy that we elected to use. Finally, our survey instrument was developed based on the Core Competencies in Hospital Medicine, which is a blueprint to develop standardized curricula for teaching hospital medicine in medical school, postgraduate training programs (ie, residency, fellowship), and continuing medical education programs. It is not clear whether the same competencies should be expected of PA hospitalists who may have different job descriptions from physician hospitalists.
In conclusion, we present the first national data on self‐perceived learning needs of PAs working in hospital medicine settings. This study collates the perceptions of PAs working in hospital medicine and highlights the fact that training in PA school does not adequately prepare them to care for hospitalized patients. Hospitalist groups may use this study's findings to coach and instruct newly hired or inexperienced hospitalist PAs, particularly until postgraduate training opportunities become more prevalent. PA schools may consider the results of this study for modifying their curricula in hopes of emphasizing the clinical content that may be most relevant for a proportion of their graduates.
Acknowledgements
The authors would like to thank Drs. David Kern and Belinda Chen at Johns Hopkins Bayview Medical Center for their assistance in developing the survey instrument.
Financial support: This study was supported by the Linda Brandt Research Award program of the Association of Postgraduate PA Programs. Dr. Wright is a Miller‐Coulson Family Scholar and was supported through the Johns Hopkins Center for Innovative Medicine.
Disclosures: Dr. Torok and Ms. Lackner received a Linda Brandt Research Award from the Association of Postgraduate PA Programs for support of this study. Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine.
Physician assistants (PA) have rapidly become an integral component in the United States health care delivery system, including in the field of Hospital Medicine, the fastest growing medical field in the United States.1, 2 Since its induction in 1997, hospitalist providers in North America have increased by 30‐fold.3 Correlating with this, the number of PAs practicing in the field of hospital medicine has also increased greatly in recent years. According to the American Academy of Physician Assistants (AAPA) census reports, Hospital Medicine first appeared as one of the specialty choices in the 2006 census (response rate, 33% of all individuals eligible to practice as PAs) when it was selected as the primary specialty by 239 PAs (1.1% of respondents). In the 2008 report (response rate, 35%), the number grew to 421 (1.7%) PAs.2
PA training programs emphasize primary care and offer limited exposure to inpatient medicine. After PA students complete their first 12 months of training in didactic coursework that teach the basic sciences, they typically spend the next year on clinical rotations, largely rooted in outpatient care.2, 4 Upon graduation, PAs do not have to pursue postgraduate training before beginning to practice in their preferred specialty areas. Thus, a majority of PAs going into specialty areas are trained on the job. This is not an exception in the field of hospital medicine.
In recent years, despite an increase in the number of PAs in Hospital Medicine, some medical centers have chosen to phase out the use of midlevel hospitalist providers (including PAs) with the purposeful decision to not hire new midlevel providers.5 The rationale for this strategy is that there is thought to be a steep learning curve that requires much time to overcome before these providers feel comfortable across the breadth of clinical cases. Before they become experienced and confident in caring for a highly complex heterogeneous patient population, they cannot operate autonomously and are not a cost‐effective alternative to physicians. The complexities associated with practicing in this field were clarified in 2006 when the Society of Hospital Medicine identified 51 core competencies in hospital medicine.3, 6 Some hospitalist programs are willing to provide their PAs with on‐the‐job training, but many programs do not have the educational expertise or the resources to make this happen. Structured and focused postgraduate training in hospital medicine seems like a reasonable solution to prepare newly graduating PAs that are interested in pursuing hospitalist careers, but such opportunities are very limited.7
To date, there is no available information about the learning needs of PAs working in hospital medicine settings. We hypothesized that understanding the learning needs of PA hospitalists would inform the development of more effective and efficient training programs. We studied PAs with experience working in hospital medicine to (1) identify self‐perceived gaps in their skills and knowledge upon starting their hospitalist careers and (2) understand their views about optimal training for careers in hospital medicine.
METHODS
Study Design
We conducted a cross‐sectional survey of a convenience sample of self‐identified PAs working in adult Hospital Medicine. The survey was distributed using an electronic survey program.
Participants
The subjects for the survey were identified through the Facebook group PAs in Hospital Medicine, which had 133 members as of July 2010. This source was selected because it was the most comprehensive list of self‐identified hospitalist PAs. Additionally, the group allowed us to send individualized invitations to complete the survey along with subsequent reminder messages to nonresponders. Subjects were eligible to participate if they were PAs with experience working in hospital medicine settings taking care of adult internal medicine inpatients.
Survey Instrument
The survey instrument was developed based on the Core Competencies in Hospital Medicine with the goal of identifying PA hospitalists' knowledge and skill gaps that were present when they started their hospitalist career.
In one section, respondents were asked about content areas among the Core Competencies in Hospital Medicine that they believed would have enhanced their effectiveness in practicing hospital medicine had they had additional training before starting their work as hospitalists. Response options ranged from Strongly Agree to Strongly Disagree. Because there were content areas that seemed more relevant to physicians, through rigorous discussions, our study team (including a hospitalist physician, senior hospitalist PA, two curriculum development experts, one medical education research expert, and an experienced hospital medicine research assistant) selected topics that were felt to be particularly germane to PA hospitalists. The relevance of this content to PA hospitalists was confirmed through pilot testing of the instrument. Another series of questions asked the PAs about their views on formal postgraduate training programs. The subjects were also queried about the frequency with which they performed various procedures (using the following scale: Never, Rarely [1‐2/year], Regularly [1‐2/month], Often [1‐2/week]) and whether they felt it was necessary for PAs to have procedure skills listed as part of the Core Competencies in Hospital Medicine (using the following scale: Not necessary, Preferable, Essential). Finally, the survey included a question about the PAs' preferred learning methods by asking the degree of helpfulness on various approaches (using the following scale: Not at all, Little, Some, A lot, Tremendously). Demographic information was also collected. The instrument was pilot‐tested for clarity on the 9 PA hospitalists who were affiliated with our hospitalist service, and the instrument was iteratively revised based on their feedback.
Data Collection and Analysis
Between September and December 2010, the survey invitations were sent as Facebook messages to the 133 members of the Facebook group PAs in Hospital Medicine. Sixteen members could not be contacted because their account setup did not allow us to send messages, and 14 were excluded because they were non‐PA members. In order to maximize participation, up to 4 reminder messages were sent to the 103 targeted PAs. The survey results were analyzed using Stata 11. Descriptive statistics were used to characterize the responses.
This study protocol was approved by the institution's review board.
RESULTS
Sixty‐nine PAs responded (response rate, 67%). Table 1 provides demographic characteristics of the respondents. The majority of respondents were 2635 years old and had worked as hospitalists for a mean of 4.3 years.
| Characteristics* | Value |
|---|---|
| |
| Age, years, n (%) | |
| <26 | 1 (2) |
| 2630 | 16 (29) |
| 3135 | 14 (25) |
| 3640 | 10 (18) |
| 4145 | 5 (9) |
| >45 | 10 (18) |
| Women, n (%) | 35 (63) |
| Year of graduation from PA school, mode (SD) | 2002 (7) |
| No. of years working/worked as hospitalist, mean (SD) | 4.3 (3.4) |
| Completed any postgraduate training program, n (%) | 0 (0) |
| Hospitalist was the first PA job, n (%) | 30 (49) |
| Salary, US$, n (%) | |
| 50,00170,000 | 1 (2) |
| 70,00190,000 | 32 (57) |
| >90,000 | 23 (41) |
| Location of hospital, n (%) | |
| Urban | 35 (57) |
| Suburban | 21 (34) |
| Rural | 5 (8) |
| Hospital characteristics, n (%) | |
| Academic medical center | 25 (41) |
| Community teaching hospital | 20 (33) |
| Community nonteaching hospital | 16 (26) |
| Responsibilities in addition to taking care of inpatients on medicine floor, n (%) | |
| Care for patients in ICU | 22 (35) |
| Perform inpatient consultations | 31 (50) |
| See outpatients | 11 (18) |
Clinical Conditions
Table 2 shows the respondents' experience with 19 core competency clinical conditions before beginning their careers as hospitalist PAs. They reported having most experience in managing diabetes and urinary tract infections, and least experience in managing healthcare associated pneumonias and sepsis syndrome.
| Clinical Condition | Mean (SD)* |
|---|---|
| |
| Urinary tract infection | 4.5 (0.8) |
| Diabetes mellitus | 4.5 (0.8) |
| Asthma | 4.4 (0.9) |
| Community‐acquired pneumonia | 4.3 (0.9) |
| Chronic obstructive pulmonary disease | 4.3 (1.0) |
| Cellulitis | 4.2 (0.9) |
| Congestive heart failure | 4.1 (1.0) |
| Cardiac arrhythmia | 3.9 (1.1) |
| Delirium and dementia | 3.8 (1.1) |
| Acute coronary syndrome | 3.8 (1.2) |
| Acute renal failure | 3.8 (1.1) |
| Gastrointestinal bleed | 3.7 (1.1) |
| Venous thromboembolism | 3.7 (1.2) |
| Pain management | 3.7 (1.2) |
| Perioperative medicine | 3.6 (1.4) |
| Stroke | 3.5 (1.2) |
| Alcohol and drug withdrawal | 3.4 (1.1) |
| Sepsis syndrome | 3.3 (1.1) |
| Hospital‐acquired pneumonia | 3.2 (1.1) |
Procedures
Most PA hospitalists (67%) perform electrocardiograms and chest X‐ray interpretations regularly (more than 1‐2/ week). However, nearly all PA hospitalists never or rarely (less than 1‐2/year) perform any invasive procedures, including arthrocentesis (98%), lumbar puncture (100%), paracentesis (91%), thoracentesis (98%), central line placement (91%), peripherally inserted central catheter placement (91%), and peripheral intravenous insertion (91%). Despite the infrequency of execution, more than 50% of respondents explained that it is either preferable or essential to be able to perform these procedures.
Content Knowledge
The PA hospitalists indicated which content areas may have allowed them to be more successful had they learned the material before starting their hospitalist career (Table 3). The top 4 topics that PA hospitalists believed would have helped them most to care for inpatients were palliative care (85% agreed or strongly agreed), nutrition for hospitalized patients (84%), performing consultations in the hospital (64%), and prevention of health careassociated infection (61%).
| Health Care System Topics | PAs Who Agreed or Strongly Agreed, n (%) |
|---|---|
| Palliative care | 47 (85) |
| Nutrition for hospitalized patients | 46 (84) |
| Performing consultations in hospital | 35 (64) |
| Prevention of health careassociated infections | 34 (62) |
| Diagnostic decision‐making processes | 32 (58) |
| Patient handoff and transitions of care | 31 (56) |
| Evidence‐based medicine | 28 (51) |
| Communication with patients and families | 27 (49) |
| Drug safety and drug interactions | 27 (49) |
| Team approach and multidisciplinary care | 26 (48) |
| Patient safety and quality improvement processes | 25 (45) |
| Care of elderly patients | 24 (44) |
| Medical ethics | 22 (40) |
| Patient education | 20 (36) |
| Care of uninsured or underinsured patients | 18 (33) |
Professional Growth as Hospitalist Providers
PAs judged working with physician preceptors (mean SD, 4.5 0.6) and discussing patients with consultants (mean SD, 4.3 0.8) to be most helpful for their professional growth, whereas receiving feedback/audits about their performance (mean SD, 3.5 1), attending conferences/lectures (mean SD, 3.6 0.7), and reading journals/textbooks (mean SD, 3.6 0.8) were rated as being less useful. Respondents believed that the mean number of months required for new hospitalist PAs to become fully competent team members was 11 months ( 8.6 SD). Forty‐three percent of respondents shared the perspective that some clinical experience in an inpatient setting was an essential prerequisite for entry into a hospitalist position. Although more than half (58%) felt that completion of postgraduate training program in hospital medicine was not necessary as a prerequisite, almost all (91%) explained that they would have been interested in such a program even if it meant having a lower stipend than a hospitalist PA salary during the first year on the job (Table 4).
| Interest in Training | n (%) |
|---|---|
| Interested and willing to pay tuition | 1 (2) |
| Interested even if there was no stipend, as long as I didn't have to pay any additional tuition | 3 (5) |
| Interested ONLY if a stipend of at least 25% of a hospitalist PA salary was offered | 4 (7) |
| Interested ONLY if a stipend of at least 50% of a hospitalist PA salary was offered | 21 (38) |
| Interested ONLY if a stipend of at least 75% of a hospitalist PA salary was offered | 21 (38) |
| Interested ONLY if 100% of a hospitalist PA salary was offered | 4 (7) |
| Not interested under any circumstances | 1 (2) |
DISCUSSION
Our survey addresses a wide range of topics related to PA hospitalists' learning needs including their experience with the Core Competencies in Hospital Medicine and their views on the benefits of PA training following graduation. Although self‐efficacy is not assessed, our study revealed that PAs who are choosing hospitalist careers have limited prior clinical experience treating many medical conditions that are managed in inpatient settings, such as sepsis syndrome. This inexperience with commonly seen clinical conditions, such as sepsis, wherein following guidelines can both reduce costs and improve outcomes, is problematic. More experience and training with such conditions would almost certainly reduce variability, improve skills, and augment confidence. These observed variations in experience in caring for conditions that often prompt admission to the hospital among PAs starting their hospitalist careers emphasizes the need to be learner‐centered when training PAs, so as to provide tailored guidance and oversight.
Only a few other empiric research articles have focused on PA hospitalists. One article described a postgraduate training program for PAs in hospital medicine that was launched in 2008. The curriculum was developed based on the Core Competencies in Hospital Medicine, and the authors explained that after 12 months of training, their first graduate functioned at the level of a PA with 4 years of experience under her belt.7 Several articles describe experiences using midlevel providers (including PAs) in general surgery, primary care medicine, cardiology, emergency medicine, critical care, pediatrics, and hospital medicine settings.5, 820 Many of these articles reported favorable results showing that using midlevel providers was either superior or just as effective in terms of cost and quality measures to physician‐only models. Many of these papers alluded to the ways in which PAs have enabled graduate medical education training programs to comply with residents' duty‐hour restrictions. A recent analysis that compared outcomes related to inpatient care provided by a hospitalist‐PA model versus a traditional resident‐based model revealed a slightly longer length of stay on the PA team but similar charges, readmission rates, and mortality.19 Yet another paper revealed that patients admitted to a residents' service, compared with the nonteaching hospitalist service that uses PAs and nurse practitioners, were different, having higher comorbidity burdens and higher acuity diagnoses.20 The authors suggested that this variance might be explained by the difference in their training, abilities, and goals of the groups. There was no research article that sought to capture the perspectives of practicing hospitalist PAs.
Our study revealed that although half of respondents became hospitalists immediately after graduating from PA school, a majority agreed that additional clinical training in inpatient settings would have been welcomed and helpful. This study's results reveal that although there is a fair amount of perceived interest in postgraduate training programs in hospital medicine, there are very few training opportunities for PAs in hospital medicine.7, 21 The American Academy of Physician Assistants, the Society of Hospital Medicine, and the American Academy of Nurse Practitioners cosponsor Adult Hospital Medicine Boot Camp for PAs and nurse practitioners annually to facilitate knowledge acquisition, but this course is truly an orientation rather than a comprehensive training program.22 Our findings suggest that more rigorous and thorough training in hospital medicine would be valued and appreciated by PA hospitalists.
Several limitations of this study should be considered. First, our survey respondents may not represent the entire spectrum of practicing PA hospitalists. However, the demographic data of 421 PAs who indicated their specialty as hospital medicine in the 2008 National Physician Assistants Census Report were not dissimilar from our informants; 65% were women, and their mean number of years in hospital medicine was 3.9 years.2 Second, our study sample was small. It was difficult to identify a national sample of hospitalist PAs, and we had to resort to a creative use of social media to find a national sample. Third, the study relied exclusively on self‐report, and since we asked about their perceived learning needs when they started working as hospitalists, recall bias cannot be excluded. However, the questions addressing attitudes and beliefs can only be ascertained from the informants themselves. That said, the input from hospitalist physicians about training needs for the PAs who they are supervising would have strengthened the reliability of the data, but this was not possible given the sampling strategy that we elected to use. Finally, our survey instrument was developed based on the Core Competencies in Hospital Medicine, which is a blueprint to develop standardized curricula for teaching hospital medicine in medical school, postgraduate training programs (ie, residency, fellowship), and continuing medical education programs. It is not clear whether the same competencies should be expected of PA hospitalists who may have different job descriptions from physician hospitalists.
In conclusion, we present the first national data on self‐perceived learning needs of PAs working in hospital medicine settings. This study collates the perceptions of PAs working in hospital medicine and highlights the fact that training in PA school does not adequately prepare them to care for hospitalized patients. Hospitalist groups may use this study's findings to coach and instruct newly hired or inexperienced hospitalist PAs, particularly until postgraduate training opportunities become more prevalent. PA schools may consider the results of this study for modifying their curricula in hopes of emphasizing the clinical content that may be most relevant for a proportion of their graduates.
Acknowledgements
The authors would like to thank Drs. David Kern and Belinda Chen at Johns Hopkins Bayview Medical Center for their assistance in developing the survey instrument.
Financial support: This study was supported by the Linda Brandt Research Award program of the Association of Postgraduate PA Programs. Dr. Wright is a Miller‐Coulson Family Scholar and was supported through the Johns Hopkins Center for Innovative Medicine.
Disclosures: Dr. Torok and Ms. Lackner received a Linda Brandt Research Award from the Association of Postgraduate PA Programs for support of this study. Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine.
- United States Department of Labor, Bureau of Labor Statistics. Available at: http://www.bls.gov. Accessed February 16,2011.
- American Academy of Physician Assistants. Available at: http://www.aapa.org. Accessed April 20,2011.
- Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org. Accessed January 24,2011.
- Accreditation Review Commission on Education for the Physician Assistants Accreditation Standards. Available at: http://www.arc‐pa.org/acc_standards. Accessed February 16,2011.
- ,.Non‐physician providers in hospital medicine: not so fast.J Hosp Med.2010;5(2):103–106.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,,.A hospitalist postgraduate training program for physician assistants.J Hosp Med.2010;5:94–98.
- ,,,.How do surgical residents and non‐physician practitioners play together in the sandbox?Curr Surg.2006;63:155–164.
- ,.Physician assistant influence on surgery residents.Arch Surg.2003;138:971–976.
- ,,, et al.Non‐physician practitioners' overall enhancement to a surgical resident's experience.J Surg Educ.2008;65:50–53.
- ,,,,.Use of midlevel practitioners to achieve labor cost savings in the primary care practice of an MCO.Health Serv Res.2004;39:607–626.
- ,,,,.The economic benefit for family/general medicine practices employing physician assistants.Am J Manag Care.2002;8:613–620.
- ,,.Financial and organizational factors affecting the employment of nurse practitioners and physician assistants in medical group practices.J Ambul Care Manage.2003;26:209–216.
- ,,,,,.A nonresident cardiovascular inpatient service improves residents' experiences in an academic medical center: a new model to meet the challenges of the new millennium.Acad Med.2004;79:426–431.
- ,,.Nurse practitioners and physician assistants in the intensive care unit: an evidence‐based review.Crit Care Med.2008;36:2888–2897.
- ,.A systematic review of the impact of nurse practitioners on cost, quality of care, satisfaction and wait times in the emergency department.CJEM.2007;9:286–295.
- ,,,.Physician assistants as physician extenders in the pediatric intensive care unit setting—a 5‐year experience.Pediatr Crit Care Med.2005;6:14–19.
- ,,,.A process for reducing workload and enhancing residents' education at an academic medical center.Acad Med.2001;76:798–805.
- ,,, et al.A comparison of outcomes of general medical inpatient care provided by a hospitalist‐physician assistant model vs a traditional resident‐based model.J Hosp Med.2011;6:112–130.
- ,,, et al.The effect of nonteaching services on the distribution of inpatient cases for internal medicine residents.Acad Med.2009;84:220–225.
- Association of Postgraduate PA Programs. Available at: http://appap.org/Home/tabid/38/Default.aspx. Accessed February 16,2011.
- Adult Hospital Medicine Boot Camp for PAs and NPs. Available at: http://www.aapa.org/component/content/article/23—general‐/673‐adult‐hospital‐medicine‐boot‐camp‐for‐pas‐and‐nps. Accessed February 16,2011.
- United States Department of Labor, Bureau of Labor Statistics. Available at: http://www.bls.gov. Accessed February 16,2011.
- American Academy of Physician Assistants. Available at: http://www.aapa.org. Accessed April 20,2011.
- Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org. Accessed January 24,2011.
- Accreditation Review Commission on Education for the Physician Assistants Accreditation Standards. Available at: http://www.arc‐pa.org/acc_standards. Accessed February 16,2011.
- ,.Non‐physician providers in hospital medicine: not so fast.J Hosp Med.2010;5(2):103–106.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,,.A hospitalist postgraduate training program for physician assistants.J Hosp Med.2010;5:94–98.
- ,,,.How do surgical residents and non‐physician practitioners play together in the sandbox?Curr Surg.2006;63:155–164.
- ,.Physician assistant influence on surgery residents.Arch Surg.2003;138:971–976.
- ,,, et al.Non‐physician practitioners' overall enhancement to a surgical resident's experience.J Surg Educ.2008;65:50–53.
- ,,,,.Use of midlevel practitioners to achieve labor cost savings in the primary care practice of an MCO.Health Serv Res.2004;39:607–626.
- ,,,,.The economic benefit for family/general medicine practices employing physician assistants.Am J Manag Care.2002;8:613–620.
- ,,.Financial and organizational factors affecting the employment of nurse practitioners and physician assistants in medical group practices.J Ambul Care Manage.2003;26:209–216.
- ,,,,,.A nonresident cardiovascular inpatient service improves residents' experiences in an academic medical center: a new model to meet the challenges of the new millennium.Acad Med.2004;79:426–431.
- ,,.Nurse practitioners and physician assistants in the intensive care unit: an evidence‐based review.Crit Care Med.2008;36:2888–2897.
- ,.A systematic review of the impact of nurse practitioners on cost, quality of care, satisfaction and wait times in the emergency department.CJEM.2007;9:286–295.
- ,,,.Physician assistants as physician extenders in the pediatric intensive care unit setting—a 5‐year experience.Pediatr Crit Care Med.2005;6:14–19.
- ,,,.A process for reducing workload and enhancing residents' education at an academic medical center.Acad Med.2001;76:798–805.
- ,,, et al.A comparison of outcomes of general medical inpatient care provided by a hospitalist‐physician assistant model vs a traditional resident‐based model.J Hosp Med.2011;6:112–130.
- ,,, et al.The effect of nonteaching services on the distribution of inpatient cases for internal medicine residents.Acad Med.2009;84:220–225.
- Association of Postgraduate PA Programs. Available at: http://appap.org/Home/tabid/38/Default.aspx. Accessed February 16,2011.
- Adult Hospital Medicine Boot Camp for PAs and NPs. Available at: http://www.aapa.org/component/content/article/23—general‐/673‐adult‐hospital‐medicine‐boot‐camp‐for‐pas‐and‐nps. Accessed February 16,2011.
Copyright © 2011 Society of Hospital Medicine
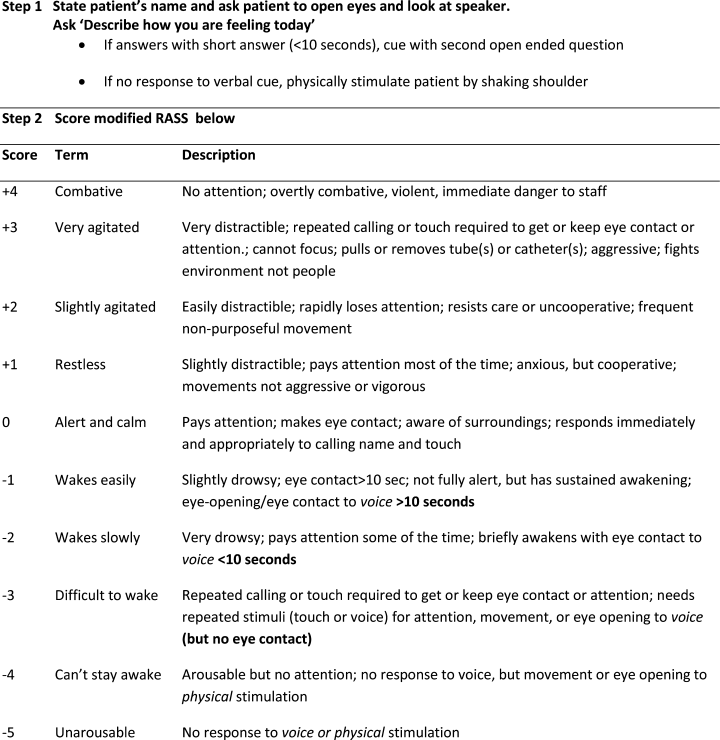
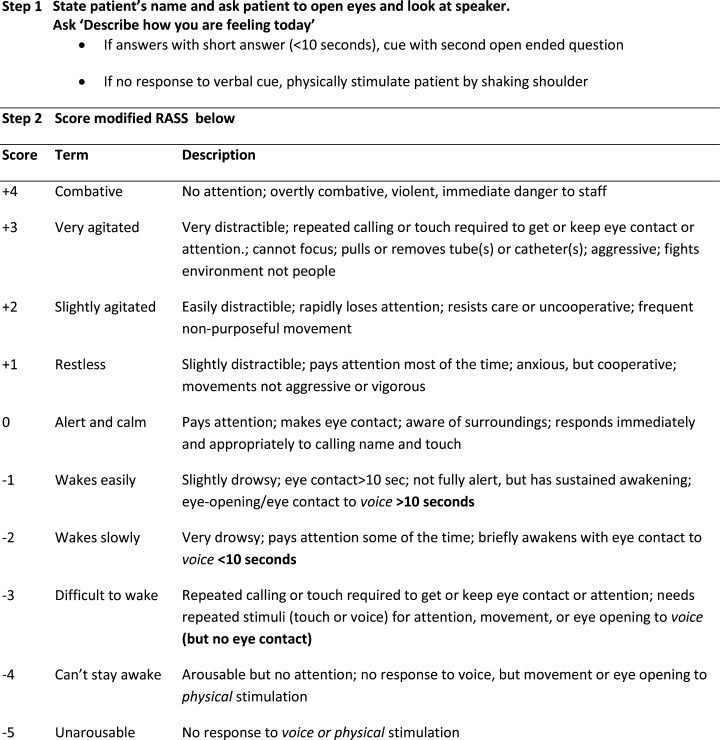
Modified RASS for Identifying Delirium
Vital signs constitute a fundamental component of the physical examination and serve key diagnostic and monitoring purposes. The brain is as vital to life as the cardiovascular, respiratory, and immune/thermoregulatory systems, yet currently no vital sign exists that would allow rapid, reliable, and easily reproducible assessment of cognition.1 As a result, acute mental status changes frequently go undetected and untreated.24 Delirium is defined as an acute change in attention with fluctuations in cognition, thought, and/or consciousness throughout the course of the day.5 Because delirium in older patients is common and is associated with increased morbidity, mortality, functional decline, and costs,69 development and validation of a rapid, objective screening assessment could be used by nursing staff to identify patients at high risk for delirium.
Current recommendations for inpatient delirium monitoring usually involve daily cognitive screening with a standardized screening instrument.6 Because this process is often time‐consuming (8‐12 minutes), most patients do not undergo routine screening. To facilitate clinical implementation, we focused on developing a brief (30‐second) inpatient screening measure of a feature of mental status that could be administered serially. The purpose of this study was to (1) develop a brief screening tool for a core feature of mental status and (2) validate this screening tool for delirium in an older inpatient population.
METHODS
Consensus Panel
In June 2009, the Veterans Administration sponsored an interdisciplinary conference that solicited input on identifying the most targetable components of delirium and discussing potential clinical instruments. Following this, a consensus panel of 8 representatives from medicine, geriatrics, nursing, psychiatry, and psychology used a modified Delphi method to target characteristic features of delirium and identify instruments that could best capture mental status change. While inattention was agreed upon as the core cognitive feature of delirium, the group came to consensus that capturing the acute onset and fluctuating nature of delirium was better suited as a vital sign. To meet these criteria, the group modified the Richmond Agitation Sedation Scale (RASS).10
The RASS is an observational instrument that has been validated in the intesive care unit setting for objectively determining level of sedation. It has been shown to be highly reliable and associated with delirium.11 The RASS is a quick, objective scale of consciousness with a scoring system that captures both hyperactive and hypoactive levels of consciousness. A disadvantage of using the RASS includes its limited attention assessment. The Consensus panel modified the RASS to improve its assessment of attention, using a brief open‐ended question that was asked before scoring (Figure 1).
Participants
For this prospective validation study, we recruited 95 medical patients 65 years of age who had been admitted to a VA hospital. The study was approved by the institutional review board, and participants provided written informed consent. Patients were excluded if they refused (n = 64), anticipated leaving the hospital within 1 day (n = 42), or had vision or cognition impairments that would prevent their ability to complete informed consent forms and cognitive screening tools (n = 19). Five participants were discharged between enrollment and expert assessment.
Mental Status Assessments
After enrollment, 3 study staff members visited each participant independently. First, the trained research assistant obtained informed consent and demographic, cognitive, and functional assessments. The mini‐mental state examination was then administered to provide a baseline measure of cognitive function at the time of admission.12 A nurse‐interviewer later administered the modified RASS (mRASS) separately. Finally, a delirium expert performed an independent comprehensive mental status interview including assessments of attention, executive function, memory, and mood. Delirium was diagnosed by the delirium expert according to DSM‐IV criteria.5 Each investigator was blinded to the others' ratings. After the initial assessments, each participant was visited daily throughout the hospitalization by an mRASS assessor and, independently, by the delirium expert.
To determine inter‐rater reliability, 60 participants were evaluated with the mRASS by the trained research assistant and the nurse‐interviewer simultaneously. The mRASS was scored independently and the assessors were blinded to each others' ratings.
Statistics
The paired mRASS‐delirium assessments were analyzed in 3 ways: (1) as single‐day independent assessments; (2) longitudinally as a change from baseline including prevalent delirium; and (3) longitudinally as a change from baseline, excluding prevalent delirium cases. We examined 1‐point and 2‐point changes on the mRASS from baseline, which allowed determination of the most appropriate cut‐point for clinical use. Sensitivity, specificity, and likelihood ratios were calculated. The C‐statistic was calculated using absolute mRASS score for the single‐time assessments, and as a difference between minimum and maximum mRASS for the longitudinal analyses.
RESULTS
Characteristics of the study population are presented in Table 1. Because this was a VA population, the vast majority (94%) of participants were men, with a mean age of 81 years (range, 66‐96 years), and 89% were white. This population had a high Charlson Comorbidity Index (mean SD, 4.0 2.4), which was reflected in functional assessments, with 37% reporting difficulty with activities of daily living and 58% reporting difficulty with instrumental activities of daily living. Despite the age and comorbidity, delirium prevalence was 11% (n = 10) and incidence was 14% (n = 13). Interrater reliability of the mRASS yielded 98% agreement with a weighted kappa of 0.48 (P 0.001).
| Characteristics | Values |
|---|---|
| |
| Age, years, mean (SD) | 81.0 (7.3) |
| Gender, male, no. (%) | 89 (94) |
| Race, white, no. (%) | 85 (89) |
| Charlson Comorbidity Index, mean (SD) | 4.0 (2.4) |
| BMI, kg/m2, mean (SD) | 27.2 (6.3) |
| Mini‐mental state examination, mean (SD) | 24.4 (4.1) |
| AUDIT, mean (SD) | 2.4 (2.9) |
| Tobacco use, pack‐years, no. (%) | 54 (56) |
| Current | 8 (8) |
| Never | 16 (17) |
| Prior | 68 (72) |
| Functional impairment, no. (%) | |
| Difficulty with 1 ADL | 35 (37) |
| Difficulty with 1 IADL | 55 (58) |
| Length of hospital stay | |
| Mean (SD), days | 6.3 (5.4) |
| Median, days | 5 |
| mRASS per patient, mean (SD) | 3.8 (3.3) |
When the mRASS was analyzed as a single‐day independent assessment, any abnormal score (ie, a score 0) had a sensitivity of 64% and a specificity of 93% for delirium relative to the expert evaluation (Table 2). With an abnormal mRASS as 2 or 2, the sensitivity fell to 34%, while the specificity increased to 99.6%.
| Criteria | mRASS | Sensitivity* (95% CI) | Specificity* (95% CI) | LR+ | LR |
|---|---|---|---|---|---|
| |||||
| Single‐day independent assessments | |||||
| Any abnormal | 63.9% (51.976.0) | 93.2% (90.396.4) | 9.4 | 0.4 | |
| RASS 2 or 2 | 34.4% (22.546.3) | 99.6% (98.8100) | 86 | 0.7 | |
| Longitudinal assessments | |||||
| Any delirium | Any change | 73.9% (56.091.9) | 91.7% (85.398.1) | 8.9 | 0.3 |
| Change in 2 points | 21.7% | 100% | 0.8 | ||
| Incident delirium | Any change | 84.6% (65.0100.0) | 91.7% (85.398.1) | 10.2 | 0.2 |
| Change in 2 points | 23.1% | 100% | 0.8 | ||
When the mRASS was used longitudinally to detect change in delirium during the hospital stay among all participants, it had a sensitivity of 74% and specificity of 92% for any change. Increasing the stringency of the criteria by looking at a change of 2 mRASS points decreased the sensitivity (22%) and increased the specificity (100%).
To capture the potential of the mRASS administered on a longitudinal basis as a diagnostic aid, the prevalent cases of delirium were excluded. In this analysis, any change in the mRASS had a sensitivity of 85% and a specificity of 92% for incident delirium. With more stringent criteria of a change of 2 points, the sensitivity was 23% and the specificity was 100%.
DISCUSSION
In this study, we developed a modified RASS (mRASS) for serial mental status assessment. Whereas a single measurement of the mRASS had modest sensitivity and good specificity for delirium, longitudinal measurement increased the sensitivity with no loss in specificity. Importantly, the 30 seconds required for the mRASS could be incorporated into daily workflow and provides an objective measure of consciousness. As such, we believe the mRASS can potentially serve as a longitudinal measure of consciousnessmuch like a vital sign for mental status.
Altered consciousness is a clinical and diagnostic feature of delirium,5, 13 and fluctuation in mental status is a diagnostic feature of delirium. As such, a screening instrument able to quantify the level of consciousness longitudinally and allow comparison to prior and subsequent determinations has face validity as a delirium screening instrument.
The mRASS has other features that make it appropriate for serial measurement in a manner similar to a vital sign. First, it objectively described consciousness on a scale, which is an improvement relative to many of the subjective descriptions clinically used. Consistent with other studies of the RASS,10, 11 the mRASS has good interrater reliability, allowing a common language to be used to describe level of consciousness across health care settings that can become the basis for a systematic and standardized monitor of cognitive change, improving continuity of care and communication between providers. It can be further used to objectively establish a patient's baseline and monitor change longitudinally.
The current study is limited by the lack of diversity and small size of the study population, which limits external validity (generalizability). Additional studies evaluating the utility of the mRASS by a variety of health care team members in a larger, more ethnically/racially diverse and heterogeneous population should be completed before we can determine if it can perform as a mental status vital sign, and if it is associated with better patient outcomes. Additionally, this study selected patients who were physically and cognitively capable of enrolling and excluded patients with severe cognitive and sensory impairment who were unable to provide consent to participate. Thus, some of the sickest, frailest, and most cognitively impaired patients were excluded. Unfortunately, this study therefore excluded a population significantly more vulnerable to the development of delirium.
Because a change in mental status (such as delirium) is common, morbid, and costly, a brief tool that can reliably and effectively assess mental status is needed. The mRASS used in this study provided an objective measurement of consciousness, a key component of mental status, and was demonstrated to reliably screen for presence or absence of delirium when administered longitudinally. Further study in diverse populations with administration by a variety of health care team members is needed to determine whether the mRASS can accurately serve as a mental status vital sign. If adopted widely, the mRASS could be used alongside the traditional vital signs to establish patient baselines, monitor change, improve provider communication, and potentially improve patient outcomes.
Acknowledgements
The authors are indebted to all of the veterans who willingly participated in this project. The VA Delirium Working Group Consensus Panel Consisted of Kenneth Boockvar, Joseph Flaherty, Sharon Gordon, Barbara Kamholz, James Rudolph, Marianne Shaughnessy, Kenneth Shay, and Joan Stein.
The authors maintained independence in the development, execution, and reporting of this study.
This article was presented in abstract form at the American Geriatrics Society Annual Meeting, May 12, 2011.
Funding: Jennifer G. Chester was funded by an Einstein Research Fellowship. James L. Rudolph is supported by a VA Rehabilitation Research Career Development Award. Additional support was provided by the American Federation for Aging Research, the Boston MSTAR, and National Institutes of Health grants AG 026781‐05 and AG 038027. James L. Rudolph and Mary Beth Harrington and the VA Delirium Working Group Consensus Panel are VA employees. The authors have no additional disclosures to report.
- ,.Vital signs in older patients: age‐related changes.J Am Med Dir Assoc.2011;12:337–343.
- ,,.Acute confusional states (delirium) in the hospitalized elderly.Annu Rev Gerontol Geriatr.1986;6:1–26.
- ,,,,.Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients.J Am Geriatr Soc.1991;39:760–765.
- ,,,,.Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings.Arch Intern Med.2001;161:2467–2473.
- Diagnostic and Statistical Manual of Mental Disorders.4th ed.Washington, DC:American Psychiatric Association;1994.
- .Delirium in older persons.N Engl J Med.2006;354:1157–1165.
- ,,,,.One‐year health care costs associated with delirium in the elderly population.Arch Intern Med.2008;168:27–32.
- ,,,,.Delirium predicts 12‐month mortality.Arch Intern Med.2002;162:457–463.
- ,,, et al.Delirium: an independent predictor of functional decline after cardiac surgery.J Am Geriatr Soc.2010;58:643–649.
- ,,, et al.The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients.Am J Respir Crit Care Med.2002;166:1338–1344.
- ,,, et al.Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS).JAMA.2003;289:2983–2991.
- ,,.“Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12:189–198.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
Vital signs constitute a fundamental component of the physical examination and serve key diagnostic and monitoring purposes. The brain is as vital to life as the cardiovascular, respiratory, and immune/thermoregulatory systems, yet currently no vital sign exists that would allow rapid, reliable, and easily reproducible assessment of cognition.1 As a result, acute mental status changes frequently go undetected and untreated.24 Delirium is defined as an acute change in attention with fluctuations in cognition, thought, and/or consciousness throughout the course of the day.5 Because delirium in older patients is common and is associated with increased morbidity, mortality, functional decline, and costs,69 development and validation of a rapid, objective screening assessment could be used by nursing staff to identify patients at high risk for delirium.
Current recommendations for inpatient delirium monitoring usually involve daily cognitive screening with a standardized screening instrument.6 Because this process is often time‐consuming (8‐12 minutes), most patients do not undergo routine screening. To facilitate clinical implementation, we focused on developing a brief (30‐second) inpatient screening measure of a feature of mental status that could be administered serially. The purpose of this study was to (1) develop a brief screening tool for a core feature of mental status and (2) validate this screening tool for delirium in an older inpatient population.
METHODS
Consensus Panel
In June 2009, the Veterans Administration sponsored an interdisciplinary conference that solicited input on identifying the most targetable components of delirium and discussing potential clinical instruments. Following this, a consensus panel of 8 representatives from medicine, geriatrics, nursing, psychiatry, and psychology used a modified Delphi method to target characteristic features of delirium and identify instruments that could best capture mental status change. While inattention was agreed upon as the core cognitive feature of delirium, the group came to consensus that capturing the acute onset and fluctuating nature of delirium was better suited as a vital sign. To meet these criteria, the group modified the Richmond Agitation Sedation Scale (RASS).10
The RASS is an observational instrument that has been validated in the intesive care unit setting for objectively determining level of sedation. It has been shown to be highly reliable and associated with delirium.11 The RASS is a quick, objective scale of consciousness with a scoring system that captures both hyperactive and hypoactive levels of consciousness. A disadvantage of using the RASS includes its limited attention assessment. The Consensus panel modified the RASS to improve its assessment of attention, using a brief open‐ended question that was asked before scoring (Figure 1).

Participants
For this prospective validation study, we recruited 95 medical patients 65 years of age who had been admitted to a VA hospital. The study was approved by the institutional review board, and participants provided written informed consent. Patients were excluded if they refused (n = 64), anticipated leaving the hospital within 1 day (n = 42), or had vision or cognition impairments that would prevent their ability to complete informed consent forms and cognitive screening tools (n = 19). Five participants were discharged between enrollment and expert assessment.
Mental Status Assessments
After enrollment, 3 study staff members visited each participant independently. First, the trained research assistant obtained informed consent and demographic, cognitive, and functional assessments. The mini‐mental state examination was then administered to provide a baseline measure of cognitive function at the time of admission.12 A nurse‐interviewer later administered the modified RASS (mRASS) separately. Finally, a delirium expert performed an independent comprehensive mental status interview including assessments of attention, executive function, memory, and mood. Delirium was diagnosed by the delirium expert according to DSM‐IV criteria.5 Each investigator was blinded to the others' ratings. After the initial assessments, each participant was visited daily throughout the hospitalization by an mRASS assessor and, independently, by the delirium expert.
To determine inter‐rater reliability, 60 participants were evaluated with the mRASS by the trained research assistant and the nurse‐interviewer simultaneously. The mRASS was scored independently and the assessors were blinded to each others' ratings.
Statistics
The paired mRASS‐delirium assessments were analyzed in 3 ways: (1) as single‐day independent assessments; (2) longitudinally as a change from baseline including prevalent delirium; and (3) longitudinally as a change from baseline, excluding prevalent delirium cases. We examined 1‐point and 2‐point changes on the mRASS from baseline, which allowed determination of the most appropriate cut‐point for clinical use. Sensitivity, specificity, and likelihood ratios were calculated. The C‐statistic was calculated using absolute mRASS score for the single‐time assessments, and as a difference between minimum and maximum mRASS for the longitudinal analyses.
RESULTS
Characteristics of the study population are presented in Table 1. Because this was a VA population, the vast majority (94%) of participants were men, with a mean age of 81 years (range, 66‐96 years), and 89% were white. This population had a high Charlson Comorbidity Index (mean SD, 4.0 2.4), which was reflected in functional assessments, with 37% reporting difficulty with activities of daily living and 58% reporting difficulty with instrumental activities of daily living. Despite the age and comorbidity, delirium prevalence was 11% (n = 10) and incidence was 14% (n = 13). Interrater reliability of the mRASS yielded 98% agreement with a weighted kappa of 0.48 (P 0.001).
| Characteristics | Values |
|---|---|
| |
| Age, years, mean (SD) | 81.0 (7.3) |
| Gender, male, no. (%) | 89 (94) |
| Race, white, no. (%) | 85 (89) |
| Charlson Comorbidity Index, mean (SD) | 4.0 (2.4) |
| BMI, kg/m2, mean (SD) | 27.2 (6.3) |
| Mini‐mental state examination, mean (SD) | 24.4 (4.1) |
| AUDIT, mean (SD) | 2.4 (2.9) |
| Tobacco use, pack‐years, no. (%) | 54 (56) |
| Current | 8 (8) |
| Never | 16 (17) |
| Prior | 68 (72) |
| Functional impairment, no. (%) | |
| Difficulty with 1 ADL | 35 (37) |
| Difficulty with 1 IADL | 55 (58) |
| Length of hospital stay | |
| Mean (SD), days | 6.3 (5.4) |
| Median, days | 5 |
| mRASS per patient, mean (SD) | 3.8 (3.3) |
When the mRASS was analyzed as a single‐day independent assessment, any abnormal score (ie, a score 0) had a sensitivity of 64% and a specificity of 93% for delirium relative to the expert evaluation (Table 2). With an abnormal mRASS as 2 or 2, the sensitivity fell to 34%, while the specificity increased to 99.6%.
| Criteria | mRASS | Sensitivity* (95% CI) | Specificity* (95% CI) | LR+ | LR |
|---|---|---|---|---|---|
| |||||
| Single‐day independent assessments | |||||
| Any abnormal | 63.9% (51.976.0) | 93.2% (90.396.4) | 9.4 | 0.4 | |
| RASS 2 or 2 | 34.4% (22.546.3) | 99.6% (98.8100) | 86 | 0.7 | |
| Longitudinal assessments | |||||
| Any delirium | Any change | 73.9% (56.091.9) | 91.7% (85.398.1) | 8.9 | 0.3 |
| Change in 2 points | 21.7% | 100% | 0.8 | ||
| Incident delirium | Any change | 84.6% (65.0100.0) | 91.7% (85.398.1) | 10.2 | 0.2 |
| Change in 2 points | 23.1% | 100% | 0.8 | ||
When the mRASS was used longitudinally to detect change in delirium during the hospital stay among all participants, it had a sensitivity of 74% and specificity of 92% for any change. Increasing the stringency of the criteria by looking at a change of 2 mRASS points decreased the sensitivity (22%) and increased the specificity (100%).
To capture the potential of the mRASS administered on a longitudinal basis as a diagnostic aid, the prevalent cases of delirium were excluded. In this analysis, any change in the mRASS had a sensitivity of 85% and a specificity of 92% for incident delirium. With more stringent criteria of a change of 2 points, the sensitivity was 23% and the specificity was 100%.
DISCUSSION
In this study, we developed a modified RASS (mRASS) for serial mental status assessment. Whereas a single measurement of the mRASS had modest sensitivity and good specificity for delirium, longitudinal measurement increased the sensitivity with no loss in specificity. Importantly, the 30 seconds required for the mRASS could be incorporated into daily workflow and provides an objective measure of consciousness. As such, we believe the mRASS can potentially serve as a longitudinal measure of consciousnessmuch like a vital sign for mental status.
Altered consciousness is a clinical and diagnostic feature of delirium,5, 13 and fluctuation in mental status is a diagnostic feature of delirium. As such, a screening instrument able to quantify the level of consciousness longitudinally and allow comparison to prior and subsequent determinations has face validity as a delirium screening instrument.
The mRASS has other features that make it appropriate for serial measurement in a manner similar to a vital sign. First, it objectively described consciousness on a scale, which is an improvement relative to many of the subjective descriptions clinically used. Consistent with other studies of the RASS,10, 11 the mRASS has good interrater reliability, allowing a common language to be used to describe level of consciousness across health care settings that can become the basis for a systematic and standardized monitor of cognitive change, improving continuity of care and communication between providers. It can be further used to objectively establish a patient's baseline and monitor change longitudinally.
The current study is limited by the lack of diversity and small size of the study population, which limits external validity (generalizability). Additional studies evaluating the utility of the mRASS by a variety of health care team members in a larger, more ethnically/racially diverse and heterogeneous population should be completed before we can determine if it can perform as a mental status vital sign, and if it is associated with better patient outcomes. Additionally, this study selected patients who were physically and cognitively capable of enrolling and excluded patients with severe cognitive and sensory impairment who were unable to provide consent to participate. Thus, some of the sickest, frailest, and most cognitively impaired patients were excluded. Unfortunately, this study therefore excluded a population significantly more vulnerable to the development of delirium.
Because a change in mental status (such as delirium) is common, morbid, and costly, a brief tool that can reliably and effectively assess mental status is needed. The mRASS used in this study provided an objective measurement of consciousness, a key component of mental status, and was demonstrated to reliably screen for presence or absence of delirium when administered longitudinally. Further study in diverse populations with administration by a variety of health care team members is needed to determine whether the mRASS can accurately serve as a mental status vital sign. If adopted widely, the mRASS could be used alongside the traditional vital signs to establish patient baselines, monitor change, improve provider communication, and potentially improve patient outcomes.
Acknowledgements
The authors are indebted to all of the veterans who willingly participated in this project. The VA Delirium Working Group Consensus Panel Consisted of Kenneth Boockvar, Joseph Flaherty, Sharon Gordon, Barbara Kamholz, James Rudolph, Marianne Shaughnessy, Kenneth Shay, and Joan Stein.
The authors maintained independence in the development, execution, and reporting of this study.
This article was presented in abstract form at the American Geriatrics Society Annual Meeting, May 12, 2011.
Funding: Jennifer G. Chester was funded by an Einstein Research Fellowship. James L. Rudolph is supported by a VA Rehabilitation Research Career Development Award. Additional support was provided by the American Federation for Aging Research, the Boston MSTAR, and National Institutes of Health grants AG 026781‐05 and AG 038027. James L. Rudolph and Mary Beth Harrington and the VA Delirium Working Group Consensus Panel are VA employees. The authors have no additional disclosures to report.
Vital signs constitute a fundamental component of the physical examination and serve key diagnostic and monitoring purposes. The brain is as vital to life as the cardiovascular, respiratory, and immune/thermoregulatory systems, yet currently no vital sign exists that would allow rapid, reliable, and easily reproducible assessment of cognition.1 As a result, acute mental status changes frequently go undetected and untreated.24 Delirium is defined as an acute change in attention with fluctuations in cognition, thought, and/or consciousness throughout the course of the day.5 Because delirium in older patients is common and is associated with increased morbidity, mortality, functional decline, and costs,69 development and validation of a rapid, objective screening assessment could be used by nursing staff to identify patients at high risk for delirium.
Current recommendations for inpatient delirium monitoring usually involve daily cognitive screening with a standardized screening instrument.6 Because this process is often time‐consuming (8‐12 minutes), most patients do not undergo routine screening. To facilitate clinical implementation, we focused on developing a brief (30‐second) inpatient screening measure of a feature of mental status that could be administered serially. The purpose of this study was to (1) develop a brief screening tool for a core feature of mental status and (2) validate this screening tool for delirium in an older inpatient population.
METHODS
Consensus Panel
In June 2009, the Veterans Administration sponsored an interdisciplinary conference that solicited input on identifying the most targetable components of delirium and discussing potential clinical instruments. Following this, a consensus panel of 8 representatives from medicine, geriatrics, nursing, psychiatry, and psychology used a modified Delphi method to target characteristic features of delirium and identify instruments that could best capture mental status change. While inattention was agreed upon as the core cognitive feature of delirium, the group came to consensus that capturing the acute onset and fluctuating nature of delirium was better suited as a vital sign. To meet these criteria, the group modified the Richmond Agitation Sedation Scale (RASS).10
The RASS is an observational instrument that has been validated in the intesive care unit setting for objectively determining level of sedation. It has been shown to be highly reliable and associated with delirium.11 The RASS is a quick, objective scale of consciousness with a scoring system that captures both hyperactive and hypoactive levels of consciousness. A disadvantage of using the RASS includes its limited attention assessment. The Consensus panel modified the RASS to improve its assessment of attention, using a brief open‐ended question that was asked before scoring (Figure 1).

Participants
For this prospective validation study, we recruited 95 medical patients 65 years of age who had been admitted to a VA hospital. The study was approved by the institutional review board, and participants provided written informed consent. Patients were excluded if they refused (n = 64), anticipated leaving the hospital within 1 day (n = 42), or had vision or cognition impairments that would prevent their ability to complete informed consent forms and cognitive screening tools (n = 19). Five participants were discharged between enrollment and expert assessment.
Mental Status Assessments
After enrollment, 3 study staff members visited each participant independently. First, the trained research assistant obtained informed consent and demographic, cognitive, and functional assessments. The mini‐mental state examination was then administered to provide a baseline measure of cognitive function at the time of admission.12 A nurse‐interviewer later administered the modified RASS (mRASS) separately. Finally, a delirium expert performed an independent comprehensive mental status interview including assessments of attention, executive function, memory, and mood. Delirium was diagnosed by the delirium expert according to DSM‐IV criteria.5 Each investigator was blinded to the others' ratings. After the initial assessments, each participant was visited daily throughout the hospitalization by an mRASS assessor and, independently, by the delirium expert.
To determine inter‐rater reliability, 60 participants were evaluated with the mRASS by the trained research assistant and the nurse‐interviewer simultaneously. The mRASS was scored independently and the assessors were blinded to each others' ratings.
Statistics
The paired mRASS‐delirium assessments were analyzed in 3 ways: (1) as single‐day independent assessments; (2) longitudinally as a change from baseline including prevalent delirium; and (3) longitudinally as a change from baseline, excluding prevalent delirium cases. We examined 1‐point and 2‐point changes on the mRASS from baseline, which allowed determination of the most appropriate cut‐point for clinical use. Sensitivity, specificity, and likelihood ratios were calculated. The C‐statistic was calculated using absolute mRASS score for the single‐time assessments, and as a difference between minimum and maximum mRASS for the longitudinal analyses.
RESULTS
Characteristics of the study population are presented in Table 1. Because this was a VA population, the vast majority (94%) of participants were men, with a mean age of 81 years (range, 66‐96 years), and 89% were white. This population had a high Charlson Comorbidity Index (mean SD, 4.0 2.4), which was reflected in functional assessments, with 37% reporting difficulty with activities of daily living and 58% reporting difficulty with instrumental activities of daily living. Despite the age and comorbidity, delirium prevalence was 11% (n = 10) and incidence was 14% (n = 13). Interrater reliability of the mRASS yielded 98% agreement with a weighted kappa of 0.48 (P 0.001).
| Characteristics | Values |
|---|---|
| |
| Age, years, mean (SD) | 81.0 (7.3) |
| Gender, male, no. (%) | 89 (94) |
| Race, white, no. (%) | 85 (89) |
| Charlson Comorbidity Index, mean (SD) | 4.0 (2.4) |
| BMI, kg/m2, mean (SD) | 27.2 (6.3) |
| Mini‐mental state examination, mean (SD) | 24.4 (4.1) |
| AUDIT, mean (SD) | 2.4 (2.9) |
| Tobacco use, pack‐years, no. (%) | 54 (56) |
| Current | 8 (8) |
| Never | 16 (17) |
| Prior | 68 (72) |
| Functional impairment, no. (%) | |
| Difficulty with 1 ADL | 35 (37) |
| Difficulty with 1 IADL | 55 (58) |
| Length of hospital stay | |
| Mean (SD), days | 6.3 (5.4) |
| Median, days | 5 |
| mRASS per patient, mean (SD) | 3.8 (3.3) |
When the mRASS was analyzed as a single‐day independent assessment, any abnormal score (ie, a score 0) had a sensitivity of 64% and a specificity of 93% for delirium relative to the expert evaluation (Table 2). With an abnormal mRASS as 2 or 2, the sensitivity fell to 34%, while the specificity increased to 99.6%.
| Criteria | mRASS | Sensitivity* (95% CI) | Specificity* (95% CI) | LR+ | LR |
|---|---|---|---|---|---|
| |||||
| Single‐day independent assessments | |||||
| Any abnormal | 63.9% (51.976.0) | 93.2% (90.396.4) | 9.4 | 0.4 | |
| RASS 2 or 2 | 34.4% (22.546.3) | 99.6% (98.8100) | 86 | 0.7 | |
| Longitudinal assessments | |||||
| Any delirium | Any change | 73.9% (56.091.9) | 91.7% (85.398.1) | 8.9 | 0.3 |
| Change in 2 points | 21.7% | 100% | 0.8 | ||
| Incident delirium | Any change | 84.6% (65.0100.0) | 91.7% (85.398.1) | 10.2 | 0.2 |
| Change in 2 points | 23.1% | 100% | 0.8 | ||
When the mRASS was used longitudinally to detect change in delirium during the hospital stay among all participants, it had a sensitivity of 74% and specificity of 92% for any change. Increasing the stringency of the criteria by looking at a change of 2 mRASS points decreased the sensitivity (22%) and increased the specificity (100%).
To capture the potential of the mRASS administered on a longitudinal basis as a diagnostic aid, the prevalent cases of delirium were excluded. In this analysis, any change in the mRASS had a sensitivity of 85% and a specificity of 92% for incident delirium. With more stringent criteria of a change of 2 points, the sensitivity was 23% and the specificity was 100%.
DISCUSSION
In this study, we developed a modified RASS (mRASS) for serial mental status assessment. Whereas a single measurement of the mRASS had modest sensitivity and good specificity for delirium, longitudinal measurement increased the sensitivity with no loss in specificity. Importantly, the 30 seconds required for the mRASS could be incorporated into daily workflow and provides an objective measure of consciousness. As such, we believe the mRASS can potentially serve as a longitudinal measure of consciousnessmuch like a vital sign for mental status.
Altered consciousness is a clinical and diagnostic feature of delirium,5, 13 and fluctuation in mental status is a diagnostic feature of delirium. As such, a screening instrument able to quantify the level of consciousness longitudinally and allow comparison to prior and subsequent determinations has face validity as a delirium screening instrument.
The mRASS has other features that make it appropriate for serial measurement in a manner similar to a vital sign. First, it objectively described consciousness on a scale, which is an improvement relative to many of the subjective descriptions clinically used. Consistent with other studies of the RASS,10, 11 the mRASS has good interrater reliability, allowing a common language to be used to describe level of consciousness across health care settings that can become the basis for a systematic and standardized monitor of cognitive change, improving continuity of care and communication between providers. It can be further used to objectively establish a patient's baseline and monitor change longitudinally.
The current study is limited by the lack of diversity and small size of the study population, which limits external validity (generalizability). Additional studies evaluating the utility of the mRASS by a variety of health care team members in a larger, more ethnically/racially diverse and heterogeneous population should be completed before we can determine if it can perform as a mental status vital sign, and if it is associated with better patient outcomes. Additionally, this study selected patients who were physically and cognitively capable of enrolling and excluded patients with severe cognitive and sensory impairment who were unable to provide consent to participate. Thus, some of the sickest, frailest, and most cognitively impaired patients were excluded. Unfortunately, this study therefore excluded a population significantly more vulnerable to the development of delirium.
Because a change in mental status (such as delirium) is common, morbid, and costly, a brief tool that can reliably and effectively assess mental status is needed. The mRASS used in this study provided an objective measurement of consciousness, a key component of mental status, and was demonstrated to reliably screen for presence or absence of delirium when administered longitudinally. Further study in diverse populations with administration by a variety of health care team members is needed to determine whether the mRASS can accurately serve as a mental status vital sign. If adopted widely, the mRASS could be used alongside the traditional vital signs to establish patient baselines, monitor change, improve provider communication, and potentially improve patient outcomes.
Acknowledgements
The authors are indebted to all of the veterans who willingly participated in this project. The VA Delirium Working Group Consensus Panel Consisted of Kenneth Boockvar, Joseph Flaherty, Sharon Gordon, Barbara Kamholz, James Rudolph, Marianne Shaughnessy, Kenneth Shay, and Joan Stein.
The authors maintained independence in the development, execution, and reporting of this study.
This article was presented in abstract form at the American Geriatrics Society Annual Meeting, May 12, 2011.
Funding: Jennifer G. Chester was funded by an Einstein Research Fellowship. James L. Rudolph is supported by a VA Rehabilitation Research Career Development Award. Additional support was provided by the American Federation for Aging Research, the Boston MSTAR, and National Institutes of Health grants AG 026781‐05 and AG 038027. James L. Rudolph and Mary Beth Harrington and the VA Delirium Working Group Consensus Panel are VA employees. The authors have no additional disclosures to report.
- ,.Vital signs in older patients: age‐related changes.J Am Med Dir Assoc.2011;12:337–343.
- ,,.Acute confusional states (delirium) in the hospitalized elderly.Annu Rev Gerontol Geriatr.1986;6:1–26.
- ,,,,.Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients.J Am Geriatr Soc.1991;39:760–765.
- ,,,,.Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings.Arch Intern Med.2001;161:2467–2473.
- Diagnostic and Statistical Manual of Mental Disorders.4th ed.Washington, DC:American Psychiatric Association;1994.
- .Delirium in older persons.N Engl J Med.2006;354:1157–1165.
- ,,,,.One‐year health care costs associated with delirium in the elderly population.Arch Intern Med.2008;168:27–32.
- ,,,,.Delirium predicts 12‐month mortality.Arch Intern Med.2002;162:457–463.
- ,,, et al.Delirium: an independent predictor of functional decline after cardiac surgery.J Am Geriatr Soc.2010;58:643–649.
- ,,, et al.The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients.Am J Respir Crit Care Med.2002;166:1338–1344.
- ,,, et al.Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS).JAMA.2003;289:2983–2991.
- ,,.“Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12:189–198.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
- ,.Vital signs in older patients: age‐related changes.J Am Med Dir Assoc.2011;12:337–343.
- ,,.Acute confusional states (delirium) in the hospitalized elderly.Annu Rev Gerontol Geriatr.1986;6:1–26.
- ,,,,.Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients.J Am Geriatr Soc.1991;39:760–765.
- ,,,,.Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings.Arch Intern Med.2001;161:2467–2473.
- Diagnostic and Statistical Manual of Mental Disorders.4th ed.Washington, DC:American Psychiatric Association;1994.
- .Delirium in older persons.N Engl J Med.2006;354:1157–1165.
- ,,,,.One‐year health care costs associated with delirium in the elderly population.Arch Intern Med.2008;168:27–32.
- ,,,,.Delirium predicts 12‐month mortality.Arch Intern Med.2002;162:457–463.
- ,,, et al.Delirium: an independent predictor of functional decline after cardiac surgery.J Am Geriatr Soc.2010;58:643–649.
- ,,, et al.The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients.Am J Respir Crit Care Med.2002;166:1338–1344.
- ,,, et al.Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation‐Sedation Scale (RASS).JAMA.2003;289:2983–2991.
- ,,.“Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12:189–198.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
New ACO Rules Could Improve Enrollment
The recently published Centers for Medicare & Medicaid Services (CMS) rules on accountable-care organizations (ACOs) are an improvement over the draft rules from this spring, but whether they spur wider formation of the new care model remains to be seen, a leading hospitalist says.
"They did some smart things," says Ron Greeno, MD, MHM, chief medical officer of Brentwood, Tenn.-based Cogent HMG and chair of SHM's Public Policy Committee. "I don't know yet that I have a great feel for how many people are going to apply—certainly more than before."
Released in March, the draft rules prompted more than 1,000 formal comments, spurring changes in several areas, including a significant reduction in the number of quality measures that CMS would monitor (33 from 65), and the elimination of assigning patients to ACOs retrospectively.
The revisions have been made to the Pioneer ACO Model, which offers higher payments to providers and organizations that already have experience with shared savings contracts, and a related program, the Medicare Shared Savings Program, which requires no previous experience.
Overall, the changes "increase incentives and eliminate downside risk," Dr. Greeno says, adding that he would like even more incentives to encourage more providers and organizations to participate. A recent white paper from healthcare research group Leavitt Partners of Salt Lake City identified 165 ACOs nationwide. Dr. Greeno believes that with more opportunity for providers and health systems to share in potential savings, that number can grow quickly.
In a comment letter posted to its website, SHM suggested "that limiting the incentive will also limit the results. ACOs that continually innovate to achieve progressive savings should have ongoing incentives to do so.”
"It's like [CMS] wants this to work, but they're almost scared people will make money doing this," he adds. "If you think this is important, make it worthwhile ... let organizations find the level of risk they're comfortable taking. And reward them accordingly if they're successful."
The recently published Centers for Medicare & Medicaid Services (CMS) rules on accountable-care organizations (ACOs) are an improvement over the draft rules from this spring, but whether they spur wider formation of the new care model remains to be seen, a leading hospitalist says.
"They did some smart things," says Ron Greeno, MD, MHM, chief medical officer of Brentwood, Tenn.-based Cogent HMG and chair of SHM's Public Policy Committee. "I don't know yet that I have a great feel for how many people are going to apply—certainly more than before."
Released in March, the draft rules prompted more than 1,000 formal comments, spurring changes in several areas, including a significant reduction in the number of quality measures that CMS would monitor (33 from 65), and the elimination of assigning patients to ACOs retrospectively.
The revisions have been made to the Pioneer ACO Model, which offers higher payments to providers and organizations that already have experience with shared savings contracts, and a related program, the Medicare Shared Savings Program, which requires no previous experience.
Overall, the changes "increase incentives and eliminate downside risk," Dr. Greeno says, adding that he would like even more incentives to encourage more providers and organizations to participate. A recent white paper from healthcare research group Leavitt Partners of Salt Lake City identified 165 ACOs nationwide. Dr. Greeno believes that with more opportunity for providers and health systems to share in potential savings, that number can grow quickly.
In a comment letter posted to its website, SHM suggested "that limiting the incentive will also limit the results. ACOs that continually innovate to achieve progressive savings should have ongoing incentives to do so.”
"It's like [CMS] wants this to work, but they're almost scared people will make money doing this," he adds. "If you think this is important, make it worthwhile ... let organizations find the level of risk they're comfortable taking. And reward them accordingly if they're successful."
The recently published Centers for Medicare & Medicaid Services (CMS) rules on accountable-care organizations (ACOs) are an improvement over the draft rules from this spring, but whether they spur wider formation of the new care model remains to be seen, a leading hospitalist says.
"They did some smart things," says Ron Greeno, MD, MHM, chief medical officer of Brentwood, Tenn.-based Cogent HMG and chair of SHM's Public Policy Committee. "I don't know yet that I have a great feel for how many people are going to apply—certainly more than before."
Released in March, the draft rules prompted more than 1,000 formal comments, spurring changes in several areas, including a significant reduction in the number of quality measures that CMS would monitor (33 from 65), and the elimination of assigning patients to ACOs retrospectively.
The revisions have been made to the Pioneer ACO Model, which offers higher payments to providers and organizations that already have experience with shared savings contracts, and a related program, the Medicare Shared Savings Program, which requires no previous experience.
Overall, the changes "increase incentives and eliminate downside risk," Dr. Greeno says, adding that he would like even more incentives to encourage more providers and organizations to participate. A recent white paper from healthcare research group Leavitt Partners of Salt Lake City identified 165 ACOs nationwide. Dr. Greeno believes that with more opportunity for providers and health systems to share in potential savings, that number can grow quickly.
In a comment letter posted to its website, SHM suggested "that limiting the incentive will also limit the results. ACOs that continually innovate to achieve progressive savings should have ongoing incentives to do so.”
"It's like [CMS] wants this to work, but they're almost scared people will make money doing this," he adds. "If you think this is important, make it worthwhile ... let organizations find the level of risk they're comfortable taking. And reward them accordingly if they're successful."
Hospitalist Leads Project to Improve Antimicrobial Prescribing
Hospitalists are at the center of many proposed interventions to improve antimicrobial prescribing practices, half of which are estimated to be unnecessary or inappropriate, with serious cost and safety consequences, says Scott Flanders, MD, FACP, SFHM, professor of medicine and director of the hospitalist program at the University of Michigan Health System in Ann Arbor.
Dr. Flanders, past president of SHM, is on the faculty of a new Institute for Healthcare Improvement (IHI) initiative kicked off in Boston in late October to test the feasibility of those interventions in hospitals of varying models.
The CDC's national campaign Get Smart for Healthcare recommends formal antimicrobial stewardship programs in hospitals to ensure that patients routinely receive the right antibiotic in the right dose at the right time for the right duration. The CDC website cites a number of studies showing the positive effects of such stewardship on antimicrobial use, antimicrobial resistance, the incidence of Clostridium difficile infections, cost, and other endpoints.
The CDC has engaged IHI to define and pilot-test the feasibility of expert-recommended interventions and approaches at eight hospitals through June 2012. The testing could lead to modifications in approaches, perhaps a second round of testing, and an IHI collaborative, says Diane Jacobsen, IHI project manager.
Eventually, Dr. Flanders adds, SHM might offer its own toolkit of resources for hospitals and hospitalists, and mentored implementation along the lines of its other major quality initiatives.
"The biggest thing for hospitalists is awareness of the problem, and then a commitment to appropriate, evidence-based selection and de-escalation of antibiotics," Jacobsen adds.
Hospitalists are at the center of many proposed interventions to improve antimicrobial prescribing practices, half of which are estimated to be unnecessary or inappropriate, with serious cost and safety consequences, says Scott Flanders, MD, FACP, SFHM, professor of medicine and director of the hospitalist program at the University of Michigan Health System in Ann Arbor.
Dr. Flanders, past president of SHM, is on the faculty of a new Institute for Healthcare Improvement (IHI) initiative kicked off in Boston in late October to test the feasibility of those interventions in hospitals of varying models.
The CDC's national campaign Get Smart for Healthcare recommends formal antimicrobial stewardship programs in hospitals to ensure that patients routinely receive the right antibiotic in the right dose at the right time for the right duration. The CDC website cites a number of studies showing the positive effects of such stewardship on antimicrobial use, antimicrobial resistance, the incidence of Clostridium difficile infections, cost, and other endpoints.
The CDC has engaged IHI to define and pilot-test the feasibility of expert-recommended interventions and approaches at eight hospitals through June 2012. The testing could lead to modifications in approaches, perhaps a second round of testing, and an IHI collaborative, says Diane Jacobsen, IHI project manager.
Eventually, Dr. Flanders adds, SHM might offer its own toolkit of resources for hospitals and hospitalists, and mentored implementation along the lines of its other major quality initiatives.
"The biggest thing for hospitalists is awareness of the problem, and then a commitment to appropriate, evidence-based selection and de-escalation of antibiotics," Jacobsen adds.
Hospitalists are at the center of many proposed interventions to improve antimicrobial prescribing practices, half of which are estimated to be unnecessary or inappropriate, with serious cost and safety consequences, says Scott Flanders, MD, FACP, SFHM, professor of medicine and director of the hospitalist program at the University of Michigan Health System in Ann Arbor.
Dr. Flanders, past president of SHM, is on the faculty of a new Institute for Healthcare Improvement (IHI) initiative kicked off in Boston in late October to test the feasibility of those interventions in hospitals of varying models.
The CDC's national campaign Get Smart for Healthcare recommends formal antimicrobial stewardship programs in hospitals to ensure that patients routinely receive the right antibiotic in the right dose at the right time for the right duration. The CDC website cites a number of studies showing the positive effects of such stewardship on antimicrobial use, antimicrobial resistance, the incidence of Clostridium difficile infections, cost, and other endpoints.
The CDC has engaged IHI to define and pilot-test the feasibility of expert-recommended interventions and approaches at eight hospitals through June 2012. The testing could lead to modifications in approaches, perhaps a second round of testing, and an IHI collaborative, says Diane Jacobsen, IHI project manager.
Eventually, Dr. Flanders adds, SHM might offer its own toolkit of resources for hospitals and hospitalists, and mentored implementation along the lines of its other major quality initiatives.
"The biggest thing for hospitalists is awareness of the problem, and then a commitment to appropriate, evidence-based selection and de-escalation of antibiotics," Jacobsen adds.
CPAP Improves Metabolic Syndrome in Apnea Patients
Continuous positive airway pressure therapy improved several components of the metabolic syndrome along with obstructive sleep apnea in patients who had both disorders, according to a report in the Dec. 15 issue of the New England Journal of Medicine.
In most cases, only one component of the metabolic syndrome improved significantly after CPAP, but that improvement was significant enough to "reverse" the syndrome, said Dr. Surendra K. Sharma of All India Institute of Medical Sciences, New Delhi, and his associates.
No particular component stood out as being the most responsive to CPAP; statistically significant improvements were seen in systolic BP, diastolic BP, total cholesterol, non-HDL cholesterol, LDL cholesterol, triglycerides, glycated hemoglobin, weight, and visceral and subcutaneous fat. "These results suggest a significant clinical benefit that will lead to a reduction in cardiovascular risk," they noted.
To examine the effect of CPAP on components of the metabolic syndrome, the researchers recruited 86 patients aged 30-65 years from the sleep laboratory at the institute who had obstructive sleep apnea that was moderate or worse in severity. All the subjects reported excessive daytime somnolence.
A total of 75 study subjects (87%) had the metabolic syndrome, and the remainder had some of the components of the metabolic syndrome.
These patients were randomly assigned to undergo either CPAP or sham CPAP for 3 months, followed by a washout period of 1 month. They then crossed over to receive the other intervention for 3 months.
The sham CPAP was not discernible to the study subjects or the investigators.
The metabolic syndrome resolved in 14 (20%) of the study subjects after CPAP. This was due to decreased blood pressure in five; decreased fasting blood glucose in two; decreased triglycerides in two; increased HDL cholesterol in three; improved triglycerides plus HDL cholesterol in one; and improved triglycerides, HDL cholesterol, and fasting blood glucose in one, Dr. Sharma and his colleagues said. Symptoms of the syndrome developed in three patients who did not have metabolic syndrome at the start of the study.
Overall, CPAP was associated with a mean decrease in systolic BP of 3.9 mm Hg, a mean decrease in diastolic BP of 2.5 mm Hg, a mean decrease in total cholesterol of 13.3 mg/dL, and a mean decrease in triglycerides of 18.7 mg/dL.
CT scans revealed a significant decrease in both visceral and subcutaneous fat, which was accompanied by a decrease in BMI, with CPAP therapy. "These findings could be secondary to a decrease in daytime somnolence and a consequent increase in physical activity after CPAP use at night."
In addition, "we speculate that CPAP has a favorable effect on leptin levels, which have been shown to be elevated in patients with obstructive sleep apnea and to normalize with CPAP therapy," the investigators said (N. Engl. J. Med. 2011;365:2277-86).
In a subgroup analysis involving only the 51 subjects who were most compliant with CPAP, with a mean use of at least 5 hours every night, the improvements in components of the metabolic syndrome were even greater. In particular, systolic BP decreased by 5.6 mm Hg and diastolic BP decreased by 3.3 mm Hg.
This subgroup of patients also showed significant improvement in carotid intima-media thickness, "suggesting a potential role for CPAP therapy in reversing endothelial damage due to obstructive sleep apnea and the metabolic syndrome," Dr. Sharma and his associates said.
Two patients could not tolerate CPAP and one could not tolerate sham CPAP within the first month of treatment, and they withdrew from the study. "Other adverse events reported included skin irritation (in 51% of all patients), nasal bridge discomfort (in 44%), nasal congestion (in 28%), headache (in 26%), and mask leaks (in 30%)."
This study was funded by Pfizer. All investigators reported having no financial conflicts of interest. The investigators received technical support from ResMed Corp. in designing a sham CPAP machine.
Continuous positive airway pressure therapy improved several components of the metabolic syndrome along with obstructive sleep apnea in patients who had both disorders, according to a report in the Dec. 15 issue of the New England Journal of Medicine.
In most cases, only one component of the metabolic syndrome improved significantly after CPAP, but that improvement was significant enough to "reverse" the syndrome, said Dr. Surendra K. Sharma of All India Institute of Medical Sciences, New Delhi, and his associates.
No particular component stood out as being the most responsive to CPAP; statistically significant improvements were seen in systolic BP, diastolic BP, total cholesterol, non-HDL cholesterol, LDL cholesterol, triglycerides, glycated hemoglobin, weight, and visceral and subcutaneous fat. "These results suggest a significant clinical benefit that will lead to a reduction in cardiovascular risk," they noted.
To examine the effect of CPAP on components of the metabolic syndrome, the researchers recruited 86 patients aged 30-65 years from the sleep laboratory at the institute who had obstructive sleep apnea that was moderate or worse in severity. All the subjects reported excessive daytime somnolence.
A total of 75 study subjects (87%) had the metabolic syndrome, and the remainder had some of the components of the metabolic syndrome.
These patients were randomly assigned to undergo either CPAP or sham CPAP for 3 months, followed by a washout period of 1 month. They then crossed over to receive the other intervention for 3 months.
The sham CPAP was not discernible to the study subjects or the investigators.
The metabolic syndrome resolved in 14 (20%) of the study subjects after CPAP. This was due to decreased blood pressure in five; decreased fasting blood glucose in two; decreased triglycerides in two; increased HDL cholesterol in three; improved triglycerides plus HDL cholesterol in one; and improved triglycerides, HDL cholesterol, and fasting blood glucose in one, Dr. Sharma and his colleagues said. Symptoms of the syndrome developed in three patients who did not have metabolic syndrome at the start of the study.
Overall, CPAP was associated with a mean decrease in systolic BP of 3.9 mm Hg, a mean decrease in diastolic BP of 2.5 mm Hg, a mean decrease in total cholesterol of 13.3 mg/dL, and a mean decrease in triglycerides of 18.7 mg/dL.
CT scans revealed a significant decrease in both visceral and subcutaneous fat, which was accompanied by a decrease in BMI, with CPAP therapy. "These findings could be secondary to a decrease in daytime somnolence and a consequent increase in physical activity after CPAP use at night."
In addition, "we speculate that CPAP has a favorable effect on leptin levels, which have been shown to be elevated in patients with obstructive sleep apnea and to normalize with CPAP therapy," the investigators said (N. Engl. J. Med. 2011;365:2277-86).
In a subgroup analysis involving only the 51 subjects who were most compliant with CPAP, with a mean use of at least 5 hours every night, the improvements in components of the metabolic syndrome were even greater. In particular, systolic BP decreased by 5.6 mm Hg and diastolic BP decreased by 3.3 mm Hg.
This subgroup of patients also showed significant improvement in carotid intima-media thickness, "suggesting a potential role for CPAP therapy in reversing endothelial damage due to obstructive sleep apnea and the metabolic syndrome," Dr. Sharma and his associates said.
Two patients could not tolerate CPAP and one could not tolerate sham CPAP within the first month of treatment, and they withdrew from the study. "Other adverse events reported included skin irritation (in 51% of all patients), nasal bridge discomfort (in 44%), nasal congestion (in 28%), headache (in 26%), and mask leaks (in 30%)."
This study was funded by Pfizer. All investigators reported having no financial conflicts of interest. The investigators received technical support from ResMed Corp. in designing a sham CPAP machine.
Continuous positive airway pressure therapy improved several components of the metabolic syndrome along with obstructive sleep apnea in patients who had both disorders, according to a report in the Dec. 15 issue of the New England Journal of Medicine.
In most cases, only one component of the metabolic syndrome improved significantly after CPAP, but that improvement was significant enough to "reverse" the syndrome, said Dr. Surendra K. Sharma of All India Institute of Medical Sciences, New Delhi, and his associates.
No particular component stood out as being the most responsive to CPAP; statistically significant improvements were seen in systolic BP, diastolic BP, total cholesterol, non-HDL cholesterol, LDL cholesterol, triglycerides, glycated hemoglobin, weight, and visceral and subcutaneous fat. "These results suggest a significant clinical benefit that will lead to a reduction in cardiovascular risk," they noted.
To examine the effect of CPAP on components of the metabolic syndrome, the researchers recruited 86 patients aged 30-65 years from the sleep laboratory at the institute who had obstructive sleep apnea that was moderate or worse in severity. All the subjects reported excessive daytime somnolence.
A total of 75 study subjects (87%) had the metabolic syndrome, and the remainder had some of the components of the metabolic syndrome.
These patients were randomly assigned to undergo either CPAP or sham CPAP for 3 months, followed by a washout period of 1 month. They then crossed over to receive the other intervention for 3 months.
The sham CPAP was not discernible to the study subjects or the investigators.
The metabolic syndrome resolved in 14 (20%) of the study subjects after CPAP. This was due to decreased blood pressure in five; decreased fasting blood glucose in two; decreased triglycerides in two; increased HDL cholesterol in three; improved triglycerides plus HDL cholesterol in one; and improved triglycerides, HDL cholesterol, and fasting blood glucose in one, Dr. Sharma and his colleagues said. Symptoms of the syndrome developed in three patients who did not have metabolic syndrome at the start of the study.
Overall, CPAP was associated with a mean decrease in systolic BP of 3.9 mm Hg, a mean decrease in diastolic BP of 2.5 mm Hg, a mean decrease in total cholesterol of 13.3 mg/dL, and a mean decrease in triglycerides of 18.7 mg/dL.
CT scans revealed a significant decrease in both visceral and subcutaneous fat, which was accompanied by a decrease in BMI, with CPAP therapy. "These findings could be secondary to a decrease in daytime somnolence and a consequent increase in physical activity after CPAP use at night."
In addition, "we speculate that CPAP has a favorable effect on leptin levels, which have been shown to be elevated in patients with obstructive sleep apnea and to normalize with CPAP therapy," the investigators said (N. Engl. J. Med. 2011;365:2277-86).
In a subgroup analysis involving only the 51 subjects who were most compliant with CPAP, with a mean use of at least 5 hours every night, the improvements in components of the metabolic syndrome were even greater. In particular, systolic BP decreased by 5.6 mm Hg and diastolic BP decreased by 3.3 mm Hg.
This subgroup of patients also showed significant improvement in carotid intima-media thickness, "suggesting a potential role for CPAP therapy in reversing endothelial damage due to obstructive sleep apnea and the metabolic syndrome," Dr. Sharma and his associates said.
Two patients could not tolerate CPAP and one could not tolerate sham CPAP within the first month of treatment, and they withdrew from the study. "Other adverse events reported included skin irritation (in 51% of all patients), nasal bridge discomfort (in 44%), nasal congestion (in 28%), headache (in 26%), and mask leaks (in 30%)."
This study was funded by Pfizer. All investigators reported having no financial conflicts of interest. The investigators received technical support from ResMed Corp. in designing a sham CPAP machine.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: The metabolic syndrome resolved in 11 of 86 patients after CPAP therapy, compared with 1 of those same patients after sham therapy. The treatment also significantly improved systolic and diastolic BP; total, LDL, and non-HDL cholesterol; triglycerides; glycated hemoglobin; weight; and visceral and subcutaneous fat.
Data Source: A double-blind, randomized trial involving 86 patients with moderate to severe obstructive sleep apnea and components of the metabolic syndrome who received 3 months of real and 3 months of sham CPAP therapy.
Disclosures: This study was funded by Pfizer. All investigators reported having no financial conflicts of interest. The investigators received technical support from ResMed Corp. in designing a sham CPAP machine.
Most Lymphomatoid Papulosis Has Benign Course
LAS VEGAS – Although certain characteristics of lymphomatoid papulosis appear be associated with progression to lymphoma, the majority of patients will have a benign course of disease.
The recurrent papulonodular skin eruption lymphomatoid papulosis (LyP) can be a confusing dermatologic entity because it appears malignant histologically, but it usually follows a clinically benign and indolent course (Arch. Dermatol. 1968;97:23-30). And even the 10%-20% of patients who do progress to lymphoma tend to have less aggressive disease, said Dr. Lawrence E. Gibson, professor of dermatology at the Mayo Clinic in Rochester, Minn. The reasons for this disconnect are not yet known.
"Patients with LyP look like lymphoma under the microscope but have clinically indolent cutaneous disease. ... It is an example of a very important clinical-pathological relationship that really needs to be made to help us understand how to treat patients better," he said.
"The bottom line is most of our patients who have LyP do not go on to have aggressive lymphomas."
To identify which LyP patients are more likely to progress to lymphoma, Dr. Gibson, Dr. Rokea A. el-Azhary, Dr. Aieska de Souza, and their associates conducted a retrospective analysis of 123 patients seen at the Mayo Clinic between 1991 and 2008. The patients were followed for a mean of 4 years (range, 2 months to 14 years). The 65 males and 58 females had a mean age of 47 years (range, 1-83 years), and a mean of 14 lesions (range, 1-100). Most (88%) of the lesions were papules, with a reported mean duration of 5.5 weeks. Pruritis was present in 38% and scar formation in 58%, the researchers reported (J. Am. Acad. Dermatol. 2011 [doi:10.1016/j.jaad.2011.07.012]).
Hematologic malignancies were present in 17 patients (14%). Of those, 10 were cutaneous lymphomas – 8 mycosis fungoides (MF) and 2 anaplastic large-cell lymphomas (ALCL). Hodgkin lymphoma was present in three patients (including the two with ALCL), multiple myeloma or monoclonal gammopathy in three, and myelodysplastic syndrome in one.
"The bottom line is most of our patients who have LyP do not go on to have aggressive lymphomas. They certainly don’t have cytotoxic lymphomas. And if they have lymphoma, they usually have MF. I think that’s somewhat reassuring to us. And for the most part, most of the patients don’t have anything. They have a normal life," Dr. Gibson said at the seminar sponsored by Skin Disease Education Foundation (SDEF).
Of 97 LyP patients for whom original biopsy slides were available, the majority (69) had World Health Organization/European Organization for Research and Treatment of Cancer histologic classification type A, including 35 with immunophenotypic subtype CD8 and 34 with subtype CD4. Another 13 patients had type B lesions (8 CD4, 5 CD8), and 6 had type C, all of which were CD4. The other 9 patients had more than one histologic type (A, B, or C), and/or more than one immunophenotypic subtype (CD4 or CD8). They were designated mixed type.
Clinically, there were no distinguishing features among the subtypes. This finding contrasts with some previous reports that the CD8 subtype might predispose to worse disease outcome (Am. J. Pathol. 1999;155:483-92).
"Our findings indicated that the LyP subtype CD8 does not signify more aggressive disease, a poor prognosis, or an association with malignancy," Dr. Gibson and his colleagues wrote.
Hematologic malignancies were present in 5 of the 9 mixed-type patients (55.5%), compared with 4 of the 34 with A/CD4 (12%), 4 of the 35 A/CD8 (11.5%), 1 of the 8 B/CD4 patients (12.5%), and 1 of the 5 B/CD8 patients (20%). (Two of the 17 malignancies were excluded from analysis because original slides were not available.) The odds ratio for malignancy for the patients with mixed-type LyP versus all other types was a statistically significant 4.33 (P = .03).
In a molecular genetics substudy of 84 LyP lesions from 76 patients, 42 (50%) were positive for clonal T-cell receptor gene rearrangement (TCRGR), 34 (40%) were negative, and 8 (10%) showed equivocal results or had insufficient DNA for analysis. Among the LyP patients who had a hematologic malignancy, 9 of 11 (82%) had positive TCRGR, compared with 30 of 68 (44%) LyP patients without malignancy. That association was also significant, with an odds ratio of 5.7 (P = .02), noted the investigators.
"A positive T-cell receptor gene rearrangement or having more than one type of LyP may have a higher risk of progression to lymphoma, but the evidence is not hard and fast. ... The take-home message is most of these patients do just fine," Dr. Gibson said.
Dr. Gibson stated that he had no relevant financial disclosures or conflicts of interest. SDEF and this news organization are owned by Elsevier.
LAS VEGAS – Although certain characteristics of lymphomatoid papulosis appear be associated with progression to lymphoma, the majority of patients will have a benign course of disease.
The recurrent papulonodular skin eruption lymphomatoid papulosis (LyP) can be a confusing dermatologic entity because it appears malignant histologically, but it usually follows a clinically benign and indolent course (Arch. Dermatol. 1968;97:23-30). And even the 10%-20% of patients who do progress to lymphoma tend to have less aggressive disease, said Dr. Lawrence E. Gibson, professor of dermatology at the Mayo Clinic in Rochester, Minn. The reasons for this disconnect are not yet known.
"Patients with LyP look like lymphoma under the microscope but have clinically indolent cutaneous disease. ... It is an example of a very important clinical-pathological relationship that really needs to be made to help us understand how to treat patients better," he said.
"The bottom line is most of our patients who have LyP do not go on to have aggressive lymphomas."
To identify which LyP patients are more likely to progress to lymphoma, Dr. Gibson, Dr. Rokea A. el-Azhary, Dr. Aieska de Souza, and their associates conducted a retrospective analysis of 123 patients seen at the Mayo Clinic between 1991 and 2008. The patients were followed for a mean of 4 years (range, 2 months to 14 years). The 65 males and 58 females had a mean age of 47 years (range, 1-83 years), and a mean of 14 lesions (range, 1-100). Most (88%) of the lesions were papules, with a reported mean duration of 5.5 weeks. Pruritis was present in 38% and scar formation in 58%, the researchers reported (J. Am. Acad. Dermatol. 2011 [doi:10.1016/j.jaad.2011.07.012]).
Hematologic malignancies were present in 17 patients (14%). Of those, 10 were cutaneous lymphomas – 8 mycosis fungoides (MF) and 2 anaplastic large-cell lymphomas (ALCL). Hodgkin lymphoma was present in three patients (including the two with ALCL), multiple myeloma or monoclonal gammopathy in three, and myelodysplastic syndrome in one.
"The bottom line is most of our patients who have LyP do not go on to have aggressive lymphomas. They certainly don’t have cytotoxic lymphomas. And if they have lymphoma, they usually have MF. I think that’s somewhat reassuring to us. And for the most part, most of the patients don’t have anything. They have a normal life," Dr. Gibson said at the seminar sponsored by Skin Disease Education Foundation (SDEF).
Of 97 LyP patients for whom original biopsy slides were available, the majority (69) had World Health Organization/European Organization for Research and Treatment of Cancer histologic classification type A, including 35 with immunophenotypic subtype CD8 and 34 with subtype CD4. Another 13 patients had type B lesions (8 CD4, 5 CD8), and 6 had type C, all of which were CD4. The other 9 patients had more than one histologic type (A, B, or C), and/or more than one immunophenotypic subtype (CD4 or CD8). They were designated mixed type.
Clinically, there were no distinguishing features among the subtypes. This finding contrasts with some previous reports that the CD8 subtype might predispose to worse disease outcome (Am. J. Pathol. 1999;155:483-92).
"Our findings indicated that the LyP subtype CD8 does not signify more aggressive disease, a poor prognosis, or an association with malignancy," Dr. Gibson and his colleagues wrote.
Hematologic malignancies were present in 5 of the 9 mixed-type patients (55.5%), compared with 4 of the 34 with A/CD4 (12%), 4 of the 35 A/CD8 (11.5%), 1 of the 8 B/CD4 patients (12.5%), and 1 of the 5 B/CD8 patients (20%). (Two of the 17 malignancies were excluded from analysis because original slides were not available.) The odds ratio for malignancy for the patients with mixed-type LyP versus all other types was a statistically significant 4.33 (P = .03).
In a molecular genetics substudy of 84 LyP lesions from 76 patients, 42 (50%) were positive for clonal T-cell receptor gene rearrangement (TCRGR), 34 (40%) were negative, and 8 (10%) showed equivocal results or had insufficient DNA for analysis. Among the LyP patients who had a hematologic malignancy, 9 of 11 (82%) had positive TCRGR, compared with 30 of 68 (44%) LyP patients without malignancy. That association was also significant, with an odds ratio of 5.7 (P = .02), noted the investigators.
"A positive T-cell receptor gene rearrangement or having more than one type of LyP may have a higher risk of progression to lymphoma, but the evidence is not hard and fast. ... The take-home message is most of these patients do just fine," Dr. Gibson said.
Dr. Gibson stated that he had no relevant financial disclosures or conflicts of interest. SDEF and this news organization are owned by Elsevier.
LAS VEGAS – Although certain characteristics of lymphomatoid papulosis appear be associated with progression to lymphoma, the majority of patients will have a benign course of disease.
The recurrent papulonodular skin eruption lymphomatoid papulosis (LyP) can be a confusing dermatologic entity because it appears malignant histologically, but it usually follows a clinically benign and indolent course (Arch. Dermatol. 1968;97:23-30). And even the 10%-20% of patients who do progress to lymphoma tend to have less aggressive disease, said Dr. Lawrence E. Gibson, professor of dermatology at the Mayo Clinic in Rochester, Minn. The reasons for this disconnect are not yet known.
"Patients with LyP look like lymphoma under the microscope but have clinically indolent cutaneous disease. ... It is an example of a very important clinical-pathological relationship that really needs to be made to help us understand how to treat patients better," he said.
"The bottom line is most of our patients who have LyP do not go on to have aggressive lymphomas."
To identify which LyP patients are more likely to progress to lymphoma, Dr. Gibson, Dr. Rokea A. el-Azhary, Dr. Aieska de Souza, and their associates conducted a retrospective analysis of 123 patients seen at the Mayo Clinic between 1991 and 2008. The patients were followed for a mean of 4 years (range, 2 months to 14 years). The 65 males and 58 females had a mean age of 47 years (range, 1-83 years), and a mean of 14 lesions (range, 1-100). Most (88%) of the lesions were papules, with a reported mean duration of 5.5 weeks. Pruritis was present in 38% and scar formation in 58%, the researchers reported (J. Am. Acad. Dermatol. 2011 [doi:10.1016/j.jaad.2011.07.012]).
Hematologic malignancies were present in 17 patients (14%). Of those, 10 were cutaneous lymphomas – 8 mycosis fungoides (MF) and 2 anaplastic large-cell lymphomas (ALCL). Hodgkin lymphoma was present in three patients (including the two with ALCL), multiple myeloma or monoclonal gammopathy in three, and myelodysplastic syndrome in one.
"The bottom line is most of our patients who have LyP do not go on to have aggressive lymphomas. They certainly don’t have cytotoxic lymphomas. And if they have lymphoma, they usually have MF. I think that’s somewhat reassuring to us. And for the most part, most of the patients don’t have anything. They have a normal life," Dr. Gibson said at the seminar sponsored by Skin Disease Education Foundation (SDEF).
Of 97 LyP patients for whom original biopsy slides were available, the majority (69) had World Health Organization/European Organization for Research and Treatment of Cancer histologic classification type A, including 35 with immunophenotypic subtype CD8 and 34 with subtype CD4. Another 13 patients had type B lesions (8 CD4, 5 CD8), and 6 had type C, all of which were CD4. The other 9 patients had more than one histologic type (A, B, or C), and/or more than one immunophenotypic subtype (CD4 or CD8). They were designated mixed type.
Clinically, there were no distinguishing features among the subtypes. This finding contrasts with some previous reports that the CD8 subtype might predispose to worse disease outcome (Am. J. Pathol. 1999;155:483-92).
"Our findings indicated that the LyP subtype CD8 does not signify more aggressive disease, a poor prognosis, or an association with malignancy," Dr. Gibson and his colleagues wrote.
Hematologic malignancies were present in 5 of the 9 mixed-type patients (55.5%), compared with 4 of the 34 with A/CD4 (12%), 4 of the 35 A/CD8 (11.5%), 1 of the 8 B/CD4 patients (12.5%), and 1 of the 5 B/CD8 patients (20%). (Two of the 17 malignancies were excluded from analysis because original slides were not available.) The odds ratio for malignancy for the patients with mixed-type LyP versus all other types was a statistically significant 4.33 (P = .03).
In a molecular genetics substudy of 84 LyP lesions from 76 patients, 42 (50%) were positive for clonal T-cell receptor gene rearrangement (TCRGR), 34 (40%) were negative, and 8 (10%) showed equivocal results or had insufficient DNA for analysis. Among the LyP patients who had a hematologic malignancy, 9 of 11 (82%) had positive TCRGR, compared with 30 of 68 (44%) LyP patients without malignancy. That association was also significant, with an odds ratio of 5.7 (P = .02), noted the investigators.
"A positive T-cell receptor gene rearrangement or having more than one type of LyP may have a higher risk of progression to lymphoma, but the evidence is not hard and fast. ... The take-home message is most of these patients do just fine," Dr. Gibson said.
Dr. Gibson stated that he had no relevant financial disclosures or conflicts of interest. SDEF and this news organization are owned by Elsevier.
FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
Major Finding: Hematologic malignancies were present in 17 patients (14%).
Data Source: Retrospective analysis of 123 LyP patients seen at the Mayo Clinic between 1991 and 2008.
Disclosures: Dr. Gibson has no relevant financial disclosures or conflicts of interest. SDEF and this news organization are owned by Elsevier.
Obinutuzumab Equals Rituximab in Relapsed Indolent NHL
SAN DIEGO – Induction therapy with obinutuzumab, a bioengineered CD20 inhibitor, induced a slightly higher overall response rate in induction therapy than did rituximab in patients with relapsed, CD20-positive indolent B-cell non-Hodgkin’s lymphoma, investigators reported at the annual meeting of the American Society of Hematology.
According to independent radiology reviewers who were blinded to treatment type, the overall response rate – a composite of complete responses (CR), CR-unconfirmed (CRu), and partial responses – was 44.6% at the end of induction among 74 patients with follicular lymphoma that was treated with obinutuzumab (Genentech’s GA101), compared with 26.7% of patients on rituximab (Rituxan) (P = .01), said Dr. Laurie H. Sehn, a medical oncologist at the University of British Columbia, Vancouver.
However, the overall response rate (ORR) by trial investigator ratings – the primary end point – showed a trend that was not significant, at 44.6% vs. 33.3% (P = .08). There is currently no difference in progression-free survival, said Dr. Sehn, on behalf of her colleagues in the phase II GAUSS trial, touted at the first head-to-head comparison of the two anti-CD20 antibodies.
Patients with other indolent lymphoma histologies were included in the trial, but Dr. Sehn reported full data on patients with follicular lymphomas only.
Obinutuzumab is considered to be a type II monoclonal antibody, shown in preclinical testing to have better ability than does rituximab to cause cell death and to invoke a cellular immune response, with lower complement-dependent cytotoxicity, Dr, Sehn said at a briefing on Dec. 11 in advance of presentation of the results on Dec. 12, 2011.
"As a single drug, rituximab really has had one of the most significant impacts in outcome in B-cell lymphomas in probably the last several decades, so there’s a real motivation to take it one step further and possibly develop a new anti-CD20 antibody that might work better than rituximab or one that may continue to work when rituximab stops working, so as to further improve outcomes," Dr, Sehn said.
A lymphoma specialist who was not involved in the GAUSS trial commented that despite rituximab’s significant benefits, new treatments still are needed, but whether obinutuzumab or another pretender is heir to rituximab’s throne is still unclear.
"We’ve been waiting for a better rituximab for some period of time. It has certainly been the major advance in the therapy of lymphomas in the last 10 to 12 years. The question is, can we do better? Genentech has made an attempt with obinutuzumab to improve the outcome for these patients who are still rituximab-sensitive," commented Dr. Ephraim P. Hochberg of the division of hematology/oncology at the Massachusetts General Hospital Cancer Center in Boston.
"The results are not overwhelming: there’s certainly a trend toward better progression-free survival, there’s a trend toward a slightly improved response rate, and a trend toward a slightly improved complete response rate, but this doesn’t look to be the home run that we might have hoped for with this patient population," he said.
The GAUSS trial is an ongoing, open-label, phase II study. Patients with relapsed CD20+ NHL who had a prior response lasting at least 6 months to a rituximab-containing regimen were randomly assigned to receive either rituximab 375 mg/m2 IV weekly for 4 weeks or obinutuzumab 1 g IV weekly for 4 weeks. At the end of the induction phase, patients were assessed and those without disease progression continued with maintenance therapy on the same drug and dose every 2 months for up to 2 years.
In addition to the primary end point results shown above, they found that the best overall response rates by investigator determination occurred in 66.2% of patients on obinutuzumab (35.1% CR/Cru, and 31.1% partial response), and 64% among those on rituximab (18.7% CR/Cru, and 45.3% PR; P = .39). By independent review, the best ORR was 60.8% with obinutuzumab (27% CR/Cru, 33.8% PR), and 46.7% with rituximab (20% and 26.7%; P = .04).*
There were no differences in progression-survival with 39.2% of patients on obinutuzumab having an event at a median time to event of 17.3 months, compared with 34.7% of patients on rituximab with a median time to event of 17.4 months.
In the obinutuzumab arm, 93% of patients had at least one adverse event vs. 81% in the rituximab arm. There were no adverse events leading to death within 28 months of the last dose of obinutuzumab compared with 2 patients on rituximab. Adverse events leading to withdrawal occurred in 8% and 10%, respectively. The proportion of patients having at least one adverse event in each group was identical, at 14%.
Phase III trials with obinutuzumab are underway.
The study was supported by Roche. Dr. Sehn and three coauthors disclosed consulting and receiving honoraria and/or research funding from Roche/Genentech. Two coauthors are Roche employees. Dr. Hochberg has received consulting fees or research support from the company.
*Correction, 12/15/2011: The best overall response rate by independent review was incorrectly stated for rituximab. This version has been updated.
SAN DIEGO – Induction therapy with obinutuzumab, a bioengineered CD20 inhibitor, induced a slightly higher overall response rate in induction therapy than did rituximab in patients with relapsed, CD20-positive indolent B-cell non-Hodgkin’s lymphoma, investigators reported at the annual meeting of the American Society of Hematology.
According to independent radiology reviewers who were blinded to treatment type, the overall response rate – a composite of complete responses (CR), CR-unconfirmed (CRu), and partial responses – was 44.6% at the end of induction among 74 patients with follicular lymphoma that was treated with obinutuzumab (Genentech’s GA101), compared with 26.7% of patients on rituximab (Rituxan) (P = .01), said Dr. Laurie H. Sehn, a medical oncologist at the University of British Columbia, Vancouver.
However, the overall response rate (ORR) by trial investigator ratings – the primary end point – showed a trend that was not significant, at 44.6% vs. 33.3% (P = .08). There is currently no difference in progression-free survival, said Dr. Sehn, on behalf of her colleagues in the phase II GAUSS trial, touted at the first head-to-head comparison of the two anti-CD20 antibodies.
Patients with other indolent lymphoma histologies were included in the trial, but Dr. Sehn reported full data on patients with follicular lymphomas only.
Obinutuzumab is considered to be a type II monoclonal antibody, shown in preclinical testing to have better ability than does rituximab to cause cell death and to invoke a cellular immune response, with lower complement-dependent cytotoxicity, Dr, Sehn said at a briefing on Dec. 11 in advance of presentation of the results on Dec. 12, 2011.
"As a single drug, rituximab really has had one of the most significant impacts in outcome in B-cell lymphomas in probably the last several decades, so there’s a real motivation to take it one step further and possibly develop a new anti-CD20 antibody that might work better than rituximab or one that may continue to work when rituximab stops working, so as to further improve outcomes," Dr, Sehn said.
A lymphoma specialist who was not involved in the GAUSS trial commented that despite rituximab’s significant benefits, new treatments still are needed, but whether obinutuzumab or another pretender is heir to rituximab’s throne is still unclear.
"We’ve been waiting for a better rituximab for some period of time. It has certainly been the major advance in the therapy of lymphomas in the last 10 to 12 years. The question is, can we do better? Genentech has made an attempt with obinutuzumab to improve the outcome for these patients who are still rituximab-sensitive," commented Dr. Ephraim P. Hochberg of the division of hematology/oncology at the Massachusetts General Hospital Cancer Center in Boston.
"The results are not overwhelming: there’s certainly a trend toward better progression-free survival, there’s a trend toward a slightly improved response rate, and a trend toward a slightly improved complete response rate, but this doesn’t look to be the home run that we might have hoped for with this patient population," he said.
The GAUSS trial is an ongoing, open-label, phase II study. Patients with relapsed CD20+ NHL who had a prior response lasting at least 6 months to a rituximab-containing regimen were randomly assigned to receive either rituximab 375 mg/m2 IV weekly for 4 weeks or obinutuzumab 1 g IV weekly for 4 weeks. At the end of the induction phase, patients were assessed and those without disease progression continued with maintenance therapy on the same drug and dose every 2 months for up to 2 years.
In addition to the primary end point results shown above, they found that the best overall response rates by investigator determination occurred in 66.2% of patients on obinutuzumab (35.1% CR/Cru, and 31.1% partial response), and 64% among those on rituximab (18.7% CR/Cru, and 45.3% PR; P = .39). By independent review, the best ORR was 60.8% with obinutuzumab (27% CR/Cru, 33.8% PR), and 46.7% with rituximab (20% and 26.7%; P = .04).*
There were no differences in progression-survival with 39.2% of patients on obinutuzumab having an event at a median time to event of 17.3 months, compared with 34.7% of patients on rituximab with a median time to event of 17.4 months.
In the obinutuzumab arm, 93% of patients had at least one adverse event vs. 81% in the rituximab arm. There were no adverse events leading to death within 28 months of the last dose of obinutuzumab compared with 2 patients on rituximab. Adverse events leading to withdrawal occurred in 8% and 10%, respectively. The proportion of patients having at least one adverse event in each group was identical, at 14%.
Phase III trials with obinutuzumab are underway.
The study was supported by Roche. Dr. Sehn and three coauthors disclosed consulting and receiving honoraria and/or research funding from Roche/Genentech. Two coauthors are Roche employees. Dr. Hochberg has received consulting fees or research support from the company.
*Correction, 12/15/2011: The best overall response rate by independent review was incorrectly stated for rituximab. This version has been updated.
SAN DIEGO – Induction therapy with obinutuzumab, a bioengineered CD20 inhibitor, induced a slightly higher overall response rate in induction therapy than did rituximab in patients with relapsed, CD20-positive indolent B-cell non-Hodgkin’s lymphoma, investigators reported at the annual meeting of the American Society of Hematology.
According to independent radiology reviewers who were blinded to treatment type, the overall response rate – a composite of complete responses (CR), CR-unconfirmed (CRu), and partial responses – was 44.6% at the end of induction among 74 patients with follicular lymphoma that was treated with obinutuzumab (Genentech’s GA101), compared with 26.7% of patients on rituximab (Rituxan) (P = .01), said Dr. Laurie H. Sehn, a medical oncologist at the University of British Columbia, Vancouver.
However, the overall response rate (ORR) by trial investigator ratings – the primary end point – showed a trend that was not significant, at 44.6% vs. 33.3% (P = .08). There is currently no difference in progression-free survival, said Dr. Sehn, on behalf of her colleagues in the phase II GAUSS trial, touted at the first head-to-head comparison of the two anti-CD20 antibodies.
Patients with other indolent lymphoma histologies were included in the trial, but Dr. Sehn reported full data on patients with follicular lymphomas only.
Obinutuzumab is considered to be a type II monoclonal antibody, shown in preclinical testing to have better ability than does rituximab to cause cell death and to invoke a cellular immune response, with lower complement-dependent cytotoxicity, Dr, Sehn said at a briefing on Dec. 11 in advance of presentation of the results on Dec. 12, 2011.
"As a single drug, rituximab really has had one of the most significant impacts in outcome in B-cell lymphomas in probably the last several decades, so there’s a real motivation to take it one step further and possibly develop a new anti-CD20 antibody that might work better than rituximab or one that may continue to work when rituximab stops working, so as to further improve outcomes," Dr, Sehn said.
A lymphoma specialist who was not involved in the GAUSS trial commented that despite rituximab’s significant benefits, new treatments still are needed, but whether obinutuzumab or another pretender is heir to rituximab’s throne is still unclear.
"We’ve been waiting for a better rituximab for some period of time. It has certainly been the major advance in the therapy of lymphomas in the last 10 to 12 years. The question is, can we do better? Genentech has made an attempt with obinutuzumab to improve the outcome for these patients who are still rituximab-sensitive," commented Dr. Ephraim P. Hochberg of the division of hematology/oncology at the Massachusetts General Hospital Cancer Center in Boston.
"The results are not overwhelming: there’s certainly a trend toward better progression-free survival, there’s a trend toward a slightly improved response rate, and a trend toward a slightly improved complete response rate, but this doesn’t look to be the home run that we might have hoped for with this patient population," he said.
The GAUSS trial is an ongoing, open-label, phase II study. Patients with relapsed CD20+ NHL who had a prior response lasting at least 6 months to a rituximab-containing regimen were randomly assigned to receive either rituximab 375 mg/m2 IV weekly for 4 weeks or obinutuzumab 1 g IV weekly for 4 weeks. At the end of the induction phase, patients were assessed and those without disease progression continued with maintenance therapy on the same drug and dose every 2 months for up to 2 years.
In addition to the primary end point results shown above, they found that the best overall response rates by investigator determination occurred in 66.2% of patients on obinutuzumab (35.1% CR/Cru, and 31.1% partial response), and 64% among those on rituximab (18.7% CR/Cru, and 45.3% PR; P = .39). By independent review, the best ORR was 60.8% with obinutuzumab (27% CR/Cru, 33.8% PR), and 46.7% with rituximab (20% and 26.7%; P = .04).*
There were no differences in progression-survival with 39.2% of patients on obinutuzumab having an event at a median time to event of 17.3 months, compared with 34.7% of patients on rituximab with a median time to event of 17.4 months.
In the obinutuzumab arm, 93% of patients had at least one adverse event vs. 81% in the rituximab arm. There were no adverse events leading to death within 28 months of the last dose of obinutuzumab compared with 2 patients on rituximab. Adverse events leading to withdrawal occurred in 8% and 10%, respectively. The proportion of patients having at least one adverse event in each group was identical, at 14%.
Phase III trials with obinutuzumab are underway.
The study was supported by Roche. Dr. Sehn and three coauthors disclosed consulting and receiving honoraria and/or research funding from Roche/Genentech. Two coauthors are Roche employees. Dr. Hochberg has received consulting fees or research support from the company.
*Correction, 12/15/2011: The best overall response rate by independent review was incorrectly stated for rituximab. This version has been updated.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: The overall investigator-rated response rate among patients with relapsed CD20+ follicular lymphoma assigned to obinutuzumab was 44.6%, compared with 33.3% for those assigned to rituximab (P = .08).
Data Source: A randomized, open label phase II trial with independent, investigator-blinded radiologic review.
Disclosures: The study was supported by Roche. Dr. Sehn and three coauthors disclosed consulting and receiving honoraria and/or research funding from Roche/Genentech. Two coauthors are Roche employees. Dr. Hochberg has received consulting fees or research support from the company.
Chemotherapy Alone Bests Radiation for Nonbulky Hodgkin's Lymphoma
SAN DIEGO – In the long run, standard chemotherapy alone is more effective than radiation in keeping patients with limited-stage nonbulky Hodgkin’s lymphoma alive, according to updated results from an intergroup trial in 405 patients.
At 12 years, 94% of patients receiving ABVD – doxorubicin (Adriamycin), bleomycin (Blenoxane), vinblastine (Velbe), and dacarbazine – chemotherapy were alive, compared with 87% receiving subtotal nodal radiation with or without chemotherapy (hazard ratio for death, 0.50; P = .04).
Although 5-year data showed that patients treated with radiation experienced greater disease control, the survival advantage with chemotherapy resulted from a lower rate of death from other causes, Dr. Ralph M. Meyer said at the annual meeting of the American Society of Hematology.
A total of 12 patients in the ABVD arm died: six due to Hodgkin’s, four to a second cancer, and two due to cardiac causes. In contrast, 10 of the 24 deaths in the radiation arm were due to a second cancer. There were two deaths due to cardiac events, four to Hodgkin’s, three fatal infections, and five other deaths.
Dr. Meyer acknowledged that interpretation of the results is bound to be controversial because subtotal nodal radiation is no longer current practice as it is considered excessive. Patients today with low-risk stage IA or IIA nonbulky disease typically receive two cycles of ABVD with 20 Gy of involved-field radiation therapy. Although modern technology has reduced radiation exposure, radiation therapy would still include the coronary artery, heart, and substantial areas of the subdiaphragmatic, he said at a press briefing.
What is clear from the National Cancer Institute of Canada Clinical Trials Group and Eastern Cooperative Oncology Group study is that measures of disease control, like freedom from progressive disease, are not accurate surrogates for long-term overall survival in patients with stage I-II nonbulky Hodgkin’s lymphoma, said Dr. Meyer, director of the National Cancer Institute of Canada Clinical Trials Group.
"New proxies that predict for risks of late-treatment effects are needed," he said.
Trials are testing the use of PET scanning during therapy to identify patients who may receive chemotherapy alone. It is hypothesized that if PET scans are negative and patients are in remission after two cycles of chemotherapy, the cure rate will be high with further chemotherapy alone, whereas radiation therapy or some other form of chemotherapy should be considered if there is residual PET activity.
How the use of PET will alter current treatment management will be another contentious issue since results from ongoing trials are not expected for 2-3 years. Moreover, the trials are limited because they are using disease control and progression-free survival as end points, which the current trial has shown are not good proxies for overall survival, Dr. Meyer said.
"Thus it will cause issues and interpretation as to how to proceed with these results," he said.
Dr. Meyer and his associates randomly assigned 405 patients with previously untreated stage IA or IIA nonbulky Hodgkin’s lymphoma to receive ABVD chemotherapy alone or subtotal nodal radiation at a dose of 35 Gy in 20 daily fractions. Patients in the radiation group with a favorable risk profile received radiation only, while those with an unfavorable risk received two cycles of ABVD followed by radiation therapy. Median follow-up was 11.3 years.
At 12 years, freedom from disease progression was 92% with radiation vs. 87% with ABVD chemotherapy (HR, 1.91; P = .05,), Dr. Meyer said. Event-free survival was similar at 80% with radiation therapy and 85% with ABVD (HR, 0.88; P = .60).
The results were simultaneously published in the New England Journal of Medicine (N. Engl. J. Med. 2011 [doi:10.1056/NEJMoa1111961]).
Dr. Meyer reported honoraria from Lilly and Celgene.
chemotherapy for lymphoma, ABVD lymphoma, nodal radiation, nonbulky Hodgkin's,
SAN DIEGO – In the long run, standard chemotherapy alone is more effective than radiation in keeping patients with limited-stage nonbulky Hodgkin’s lymphoma alive, according to updated results from an intergroup trial in 405 patients.
At 12 years, 94% of patients receiving ABVD – doxorubicin (Adriamycin), bleomycin (Blenoxane), vinblastine (Velbe), and dacarbazine – chemotherapy were alive, compared with 87% receiving subtotal nodal radiation with or without chemotherapy (hazard ratio for death, 0.50; P = .04).
Although 5-year data showed that patients treated with radiation experienced greater disease control, the survival advantage with chemotherapy resulted from a lower rate of death from other causes, Dr. Ralph M. Meyer said at the annual meeting of the American Society of Hematology.
A total of 12 patients in the ABVD arm died: six due to Hodgkin’s, four to a second cancer, and two due to cardiac causes. In contrast, 10 of the 24 deaths in the radiation arm were due to a second cancer. There were two deaths due to cardiac events, four to Hodgkin’s, three fatal infections, and five other deaths.
Dr. Meyer acknowledged that interpretation of the results is bound to be controversial because subtotal nodal radiation is no longer current practice as it is considered excessive. Patients today with low-risk stage IA or IIA nonbulky disease typically receive two cycles of ABVD with 20 Gy of involved-field radiation therapy. Although modern technology has reduced radiation exposure, radiation therapy would still include the coronary artery, heart, and substantial areas of the subdiaphragmatic, he said at a press briefing.
What is clear from the National Cancer Institute of Canada Clinical Trials Group and Eastern Cooperative Oncology Group study is that measures of disease control, like freedom from progressive disease, are not accurate surrogates for long-term overall survival in patients with stage I-II nonbulky Hodgkin’s lymphoma, said Dr. Meyer, director of the National Cancer Institute of Canada Clinical Trials Group.
"New proxies that predict for risks of late-treatment effects are needed," he said.
Trials are testing the use of PET scanning during therapy to identify patients who may receive chemotherapy alone. It is hypothesized that if PET scans are negative and patients are in remission after two cycles of chemotherapy, the cure rate will be high with further chemotherapy alone, whereas radiation therapy or some other form of chemotherapy should be considered if there is residual PET activity.
How the use of PET will alter current treatment management will be another contentious issue since results from ongoing trials are not expected for 2-3 years. Moreover, the trials are limited because they are using disease control and progression-free survival as end points, which the current trial has shown are not good proxies for overall survival, Dr. Meyer said.
"Thus it will cause issues and interpretation as to how to proceed with these results," he said.
Dr. Meyer and his associates randomly assigned 405 patients with previously untreated stage IA or IIA nonbulky Hodgkin’s lymphoma to receive ABVD chemotherapy alone or subtotal nodal radiation at a dose of 35 Gy in 20 daily fractions. Patients in the radiation group with a favorable risk profile received radiation only, while those with an unfavorable risk received two cycles of ABVD followed by radiation therapy. Median follow-up was 11.3 years.
At 12 years, freedom from disease progression was 92% with radiation vs. 87% with ABVD chemotherapy (HR, 1.91; P = .05,), Dr. Meyer said. Event-free survival was similar at 80% with radiation therapy and 85% with ABVD (HR, 0.88; P = .60).
The results were simultaneously published in the New England Journal of Medicine (N. Engl. J. Med. 2011 [doi:10.1056/NEJMoa1111961]).
Dr. Meyer reported honoraria from Lilly and Celgene.
SAN DIEGO – In the long run, standard chemotherapy alone is more effective than radiation in keeping patients with limited-stage nonbulky Hodgkin’s lymphoma alive, according to updated results from an intergroup trial in 405 patients.
At 12 years, 94% of patients receiving ABVD – doxorubicin (Adriamycin), bleomycin (Blenoxane), vinblastine (Velbe), and dacarbazine – chemotherapy were alive, compared with 87% receiving subtotal nodal radiation with or without chemotherapy (hazard ratio for death, 0.50; P = .04).
Although 5-year data showed that patients treated with radiation experienced greater disease control, the survival advantage with chemotherapy resulted from a lower rate of death from other causes, Dr. Ralph M. Meyer said at the annual meeting of the American Society of Hematology.
A total of 12 patients in the ABVD arm died: six due to Hodgkin’s, four to a second cancer, and two due to cardiac causes. In contrast, 10 of the 24 deaths in the radiation arm were due to a second cancer. There were two deaths due to cardiac events, four to Hodgkin’s, three fatal infections, and five other deaths.
Dr. Meyer acknowledged that interpretation of the results is bound to be controversial because subtotal nodal radiation is no longer current practice as it is considered excessive. Patients today with low-risk stage IA or IIA nonbulky disease typically receive two cycles of ABVD with 20 Gy of involved-field radiation therapy. Although modern technology has reduced radiation exposure, radiation therapy would still include the coronary artery, heart, and substantial areas of the subdiaphragmatic, he said at a press briefing.
What is clear from the National Cancer Institute of Canada Clinical Trials Group and Eastern Cooperative Oncology Group study is that measures of disease control, like freedom from progressive disease, are not accurate surrogates for long-term overall survival in patients with stage I-II nonbulky Hodgkin’s lymphoma, said Dr. Meyer, director of the National Cancer Institute of Canada Clinical Trials Group.
"New proxies that predict for risks of late-treatment effects are needed," he said.
Trials are testing the use of PET scanning during therapy to identify patients who may receive chemotherapy alone. It is hypothesized that if PET scans are negative and patients are in remission after two cycles of chemotherapy, the cure rate will be high with further chemotherapy alone, whereas radiation therapy or some other form of chemotherapy should be considered if there is residual PET activity.
How the use of PET will alter current treatment management will be another contentious issue since results from ongoing trials are not expected for 2-3 years. Moreover, the trials are limited because they are using disease control and progression-free survival as end points, which the current trial has shown are not good proxies for overall survival, Dr. Meyer said.
"Thus it will cause issues and interpretation as to how to proceed with these results," he said.
Dr. Meyer and his associates randomly assigned 405 patients with previously untreated stage IA or IIA nonbulky Hodgkin’s lymphoma to receive ABVD chemotherapy alone or subtotal nodal radiation at a dose of 35 Gy in 20 daily fractions. Patients in the radiation group with a favorable risk profile received radiation only, while those with an unfavorable risk received two cycles of ABVD followed by radiation therapy. Median follow-up was 11.3 years.
At 12 years, freedom from disease progression was 92% with radiation vs. 87% with ABVD chemotherapy (HR, 1.91; P = .05,), Dr. Meyer said. Event-free survival was similar at 80% with radiation therapy and 85% with ABVD (HR, 0.88; P = .60).
The results were simultaneously published in the New England Journal of Medicine (N. Engl. J. Med. 2011 [doi:10.1056/NEJMoa1111961]).
Dr. Meyer reported honoraria from Lilly and Celgene.
chemotherapy for lymphoma, ABVD lymphoma, nodal radiation, nonbulky Hodgkin's,
chemotherapy for lymphoma, ABVD lymphoma, nodal radiation, nonbulky Hodgkin's,
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: At 12 years, the overall survival rate was 94% with chemotherapy alone and 87% with subtotal nodal radiation.
Data Source: Prospective, randomized trial in 405 patients with untreated nonbulky Hodgkin’s lymphoma.
Disclosures: Dr. Meyers reported honoraria from Lilly and Celgene.
Observation Suffices After Rituximab in Low-Burden Follicular Lymphoma
SAN DIEGO – Maintain with more rituximab or re-treat? For patients with low tumor burden follicular lymphomas, the answer seems to be that it doesn’t much matter, said investigators at the annual meeting of the American Society of Hematology.
Following 4 weeks of induction with rituximab monotherapy, time to treatment failure (TTTF), the primary end point, was virtually identical between patients randomized to 12 weeks of rituximab (Rituxan) maintenance (3.9 years) or rituximab re-treatment at progression (3.6 years, P = .80) in the E4402 RESORT (Rituximab Extended Schedule or Re-Treatment Trial) study.
One year after randomization, there were no significant differences in patient quality of life or stress burden between the treatment strategies, said Dr. Brad S. Kahl of the University of Wisconsin, Madison, on behalf of coinvestigators a briefing in advance of his presentation of the results at a late-breaking abstracts session on Dec. 13.
For the end point of time to first cytotoxic therapy, however, maintenance was significantly better, with 95% of patients not on chemotherapy at 3 years, compared with 86% for the re-treatment strategy (hazard ratio, 2.5; P = .027).
"Both strategies performed extremely well," Dr. Kahl said.
The re-treatment strategy was less costly: Patients in the maintenance arm received a mean of 15.8 total doses of rituximab, compared with a mean 4.5 doses in the re-treatment arm, Dr. Kahl noted. "To get this very small improvement in time to chemotherapy took roughly 4 times more drug in the maintenance arm," he said,
"Given that there was no difference in time to treatment failure, and given the excellent results with re-treatment or time to first chemotherapy, given a slightly better toxicity profile [with re-treatment], given a lack of a quality-of-life benefit for maintenance, and given the resource utilization strategy, the re-treatment strategy would be our recommended strategy when you\'re administering single-agent rituximab in this patient population," he said.
Maintenance does, however, provide a progression-free survival advantage for patients with high tumor burden follicular lymphoma following induction with a combination immunochemotherapy regimen, as shown in the PRIMA trial, in which 2-years of rituximab maintenance was associated with significantly better progression-free survival compared with observation alone (Lancet 2011;377:42-51 [doi:10.1016/S0140-6736(10)62175-7]).
But some patients with low tumor burden may still be anxious about "doing nothing," and for them maintenance is still an appropriate option, said Dr. Ephraim P. Hochberg from the division of hematology/oncology at the Massachusetts General Hospital Cancer Center in Boston.
"In my practice, the patients who I think are going to be psychologically intolerant of relapse, or for whom relapse is a devastating personal event, those are patients for whom there is a benefit to maintenance rituximab that\'s not measured in this sort of analysis," he said in an interview. Dr. Hochberg was not involved in RESORT.
The RESORT trial enrolled 545 patients with non-Hodgkin’s lymphoma, 384 of whom (71%) had a follicular histology; patients with nonfollicular histologies are being analyzed separately. Among the 384 patients, 274 had a response to induction rituximab, and were then randomized to either re-treatment at progression (134) or to maintenance with rituximab 375 mg/m2 every 12 weeks. Each strategy was continued until treatment failure.
Time to treatment failure was defined as progression within 6 months of the last rituximab infusion, no response to re-treatment, initiation of alternative therapy, or inability to complete the protocol therapy. Patients were evaluated every 3 months and had restaging CT scans every 6 months,
Grade 3 or 4 hematologic toxicities were seen in less than 5% of patients, and there were two on-study deaths, one in each treatment arm. Both strategies were "extremely well tolerated with minimal toxicities," Dr. Kahl said. There were more toxicities leading to a failure event in the maintenance arm, however.
"Our analysis so far shows that there is no quality-of-life benefit for the maintenance strategy relative to retreatment," he said.
The E4402 (RESORT) trial was sponsored by the Eastern Cooperative Oncology Group and the National Cancer Institute. Dr. Kahl disclosed being a consultant to Genentech and Roche, comarketer of rituximab. Two of his coauthors also are consultants to the company, and one is an employee. Dr. Hochberg has received consulting fees or research support from the company.
SAN DIEGO – Maintain with more rituximab or re-treat? For patients with low tumor burden follicular lymphomas, the answer seems to be that it doesn’t much matter, said investigators at the annual meeting of the American Society of Hematology.
Following 4 weeks of induction with rituximab monotherapy, time to treatment failure (TTTF), the primary end point, was virtually identical between patients randomized to 12 weeks of rituximab (Rituxan) maintenance (3.9 years) or rituximab re-treatment at progression (3.6 years, P = .80) in the E4402 RESORT (Rituximab Extended Schedule or Re-Treatment Trial) study.
One year after randomization, there were no significant differences in patient quality of life or stress burden between the treatment strategies, said Dr. Brad S. Kahl of the University of Wisconsin, Madison, on behalf of coinvestigators a briefing in advance of his presentation of the results at a late-breaking abstracts session on Dec. 13.
For the end point of time to first cytotoxic therapy, however, maintenance was significantly better, with 95% of patients not on chemotherapy at 3 years, compared with 86% for the re-treatment strategy (hazard ratio, 2.5; P = .027).
"Both strategies performed extremely well," Dr. Kahl said.
The re-treatment strategy was less costly: Patients in the maintenance arm received a mean of 15.8 total doses of rituximab, compared with a mean 4.5 doses in the re-treatment arm, Dr. Kahl noted. "To get this very small improvement in time to chemotherapy took roughly 4 times more drug in the maintenance arm," he said,
"Given that there was no difference in time to treatment failure, and given the excellent results with re-treatment or time to first chemotherapy, given a slightly better toxicity profile [with re-treatment], given a lack of a quality-of-life benefit for maintenance, and given the resource utilization strategy, the re-treatment strategy would be our recommended strategy when you\'re administering single-agent rituximab in this patient population," he said.
Maintenance does, however, provide a progression-free survival advantage for patients with high tumor burden follicular lymphoma following induction with a combination immunochemotherapy regimen, as shown in the PRIMA trial, in which 2-years of rituximab maintenance was associated with significantly better progression-free survival compared with observation alone (Lancet 2011;377:42-51 [doi:10.1016/S0140-6736(10)62175-7]).
But some patients with low tumor burden may still be anxious about "doing nothing," and for them maintenance is still an appropriate option, said Dr. Ephraim P. Hochberg from the division of hematology/oncology at the Massachusetts General Hospital Cancer Center in Boston.
"In my practice, the patients who I think are going to be psychologically intolerant of relapse, or for whom relapse is a devastating personal event, those are patients for whom there is a benefit to maintenance rituximab that\'s not measured in this sort of analysis," he said in an interview. Dr. Hochberg was not involved in RESORT.
The RESORT trial enrolled 545 patients with non-Hodgkin’s lymphoma, 384 of whom (71%) had a follicular histology; patients with nonfollicular histologies are being analyzed separately. Among the 384 patients, 274 had a response to induction rituximab, and were then randomized to either re-treatment at progression (134) or to maintenance with rituximab 375 mg/m2 every 12 weeks. Each strategy was continued until treatment failure.
Time to treatment failure was defined as progression within 6 months of the last rituximab infusion, no response to re-treatment, initiation of alternative therapy, or inability to complete the protocol therapy. Patients were evaluated every 3 months and had restaging CT scans every 6 months,
Grade 3 or 4 hematologic toxicities were seen in less than 5% of patients, and there were two on-study deaths, one in each treatment arm. Both strategies were "extremely well tolerated with minimal toxicities," Dr. Kahl said. There were more toxicities leading to a failure event in the maintenance arm, however.
"Our analysis so far shows that there is no quality-of-life benefit for the maintenance strategy relative to retreatment," he said.
The E4402 (RESORT) trial was sponsored by the Eastern Cooperative Oncology Group and the National Cancer Institute. Dr. Kahl disclosed being a consultant to Genentech and Roche, comarketer of rituximab. Two of his coauthors also are consultants to the company, and one is an employee. Dr. Hochberg has received consulting fees or research support from the company.
SAN DIEGO – Maintain with more rituximab or re-treat? For patients with low tumor burden follicular lymphomas, the answer seems to be that it doesn’t much matter, said investigators at the annual meeting of the American Society of Hematology.
Following 4 weeks of induction with rituximab monotherapy, time to treatment failure (TTTF), the primary end point, was virtually identical between patients randomized to 12 weeks of rituximab (Rituxan) maintenance (3.9 years) or rituximab re-treatment at progression (3.6 years, P = .80) in the E4402 RESORT (Rituximab Extended Schedule or Re-Treatment Trial) study.
One year after randomization, there were no significant differences in patient quality of life or stress burden between the treatment strategies, said Dr. Brad S. Kahl of the University of Wisconsin, Madison, on behalf of coinvestigators a briefing in advance of his presentation of the results at a late-breaking abstracts session on Dec. 13.
For the end point of time to first cytotoxic therapy, however, maintenance was significantly better, with 95% of patients not on chemotherapy at 3 years, compared with 86% for the re-treatment strategy (hazard ratio, 2.5; P = .027).
"Both strategies performed extremely well," Dr. Kahl said.
The re-treatment strategy was less costly: Patients in the maintenance arm received a mean of 15.8 total doses of rituximab, compared with a mean 4.5 doses in the re-treatment arm, Dr. Kahl noted. "To get this very small improvement in time to chemotherapy took roughly 4 times more drug in the maintenance arm," he said,
"Given that there was no difference in time to treatment failure, and given the excellent results with re-treatment or time to first chemotherapy, given a slightly better toxicity profile [with re-treatment], given a lack of a quality-of-life benefit for maintenance, and given the resource utilization strategy, the re-treatment strategy would be our recommended strategy when you\'re administering single-agent rituximab in this patient population," he said.
Maintenance does, however, provide a progression-free survival advantage for patients with high tumor burden follicular lymphoma following induction with a combination immunochemotherapy regimen, as shown in the PRIMA trial, in which 2-years of rituximab maintenance was associated with significantly better progression-free survival compared with observation alone (Lancet 2011;377:42-51 [doi:10.1016/S0140-6736(10)62175-7]).
But some patients with low tumor burden may still be anxious about "doing nothing," and for them maintenance is still an appropriate option, said Dr. Ephraim P. Hochberg from the division of hematology/oncology at the Massachusetts General Hospital Cancer Center in Boston.
"In my practice, the patients who I think are going to be psychologically intolerant of relapse, or for whom relapse is a devastating personal event, those are patients for whom there is a benefit to maintenance rituximab that\'s not measured in this sort of analysis," he said in an interview. Dr. Hochberg was not involved in RESORT.
The RESORT trial enrolled 545 patients with non-Hodgkin’s lymphoma, 384 of whom (71%) had a follicular histology; patients with nonfollicular histologies are being analyzed separately. Among the 384 patients, 274 had a response to induction rituximab, and were then randomized to either re-treatment at progression (134) or to maintenance with rituximab 375 mg/m2 every 12 weeks. Each strategy was continued until treatment failure.
Time to treatment failure was defined as progression within 6 months of the last rituximab infusion, no response to re-treatment, initiation of alternative therapy, or inability to complete the protocol therapy. Patients were evaluated every 3 months and had restaging CT scans every 6 months,
Grade 3 or 4 hematologic toxicities were seen in less than 5% of patients, and there were two on-study deaths, one in each treatment arm. Both strategies were "extremely well tolerated with minimal toxicities," Dr. Kahl said. There were more toxicities leading to a failure event in the maintenance arm, however.
"Our analysis so far shows that there is no quality-of-life benefit for the maintenance strategy relative to retreatment," he said.
The E4402 (RESORT) trial was sponsored by the Eastern Cooperative Oncology Group and the National Cancer Institute. Dr. Kahl disclosed being a consultant to Genentech and Roche, comarketer of rituximab. Two of his coauthors also are consultants to the company, and one is an employee. Dr. Hochberg has received consulting fees or research support from the company.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: Following 4 weeks of induction with rituximab monotherapy, time to treatment failure was not significantly different between patients randomized to either 12 weeks rituximab maintenance (3.9 years) or rituximab retreatment at progression (3.6 years, P = .80).
Data Source: Randomized comparison trial.
Disclosures: The E4402 (RESORT) trial was sponsored by the Eastern Cooperative Oncology Group and the National Cancer Institute. Dr. Kahl disclosed being a consultant to Genentech and Roche, comarketer of Rituximab. Two of his coauthors also are consultants to the company, and one is an employee. Dr. Hochberg has received consulting fees or research support from the company.
BTK Inhibitor Draws High Response Rate in CLL
SAN DIEGO – A novel targeted agent was associated with high objective response rates at 10 months in patients with relapsed/refractory, heavily pretreated chronic lymphocytic leukemia/small lymphocytic lymphoma, investigators reported at the annual meeting of the American Society of Hematology.
At 10.2 months’ median follow-up, objective responses (combined complete and partial responses) were seen in 70% of 27 CLL/SLL patients assigned to a 420-mg daily dose of PCI-32765, an inhibitor of Bruton’s tyrosine kinase (BTK). Objective responses were seen at 6.5 months’ follow-up in 44% of 34 patients on an 840 mg daily dose, reported Dr. Susan O’Brien, professor in the department of leukemia at the University of Texas MD Anderson Cancer Center in Houston.
The researchers had previously reported a 48% objective response rate at 6.2 months median follow-up.
Among patients who did not have a complete or partial response, a nodal partial response was noted in 19% of those in the 420-mg cohort and in 35% of those in the 840-mg group. A nodal partial response was defined as a reduction of greater than 50% in aggregate lymph node size, but with residual lymphocytosis.
Progression-free survival at 6 months is 92% in the 27 patients in the 420-mg cohort, and 90% in the 34 patients in the 840-mg group.
Although it’s still too early to tell which dose will be more effective, the evidence to date suggests that the 420-mg dose completely inhibits activity of the targeted kinase. Thus, the 840-mg dose may not be necessary, Dr. O'Brien said.
Further, PC-32765 does not cause myelosuppression, a trait noted with imatinib (Gleevec) and other tyrosine kinase inhibitors that are effective in other leukemia subtypes.
"This is a big deal in CLL because all of the treatments that we have are pretty much chemo-based or antibody-based treatments. The biggest complication in treating CLL with pretty much every therapy we have is myelosuppression and infection, and of course these people are [already] immune suppressed. To have an agent that’s not myelosuppressive and this effective is very exciting," she said in a briefing prior to her presentation of the results at a session on Tuesday, Dec.13.
A leukemia specialist who was not involved in the study said that the results are particularly impressive given the nature of the patient population.
Patients also tolerated the drug well. The incidence of serious adverse events potentially related to PCI-32765 was 10%. The most common adverse event was diarrhea, which was generally mild, easily controlled, and self-limited, Dr. O’Brien said.
PCI-32765 binds selectively and irreversibly to BTK, an essential element of the B-cell antigen receptor signaling pathway, thereby blocking receptor signaling, inducing cell death via apoptosis, and inhibiting cellular migration and adhesion of malignant B cells.
"To have an agent that’s not myelo-suppressive and this effective is very exciting."
The investigators enrolled both treatment-naive patients with CLL and those who had relapsed/refractory disease following at least two prior therapies, including fludarabine. The patients were treated with PCI-32765 administered daily for 28-day cycles until disease progression. Treatment-naive patients and 27 patients with relapsed/refractory disease were assigned to the 420-mg dose; 34 patients with relapsed/refractory disease were assigned to the 840-mg dose.
In all, 72% of patients had one or more poor-risk molecular features. Of this group, 31% had the 17p deletion, 33% had the 11q deletion, and 57% had IgVH un-mutated.
Two patients dropped out of the trial because of adverse events (dose group not specified), and six patients (two in the 420-mg group, four in the 840-mg group) required a dose reduction.
The most frequently reported grade 1 or 2 adverse events were diarrhea, fatigue, nausea, and ecchymosis. Serious adverse events were reported in 38% of patients. Serious events potentially related to the drug occurred in 10% of all patients.
The investigators noted that, in a majority of patients "a characteristic pattern of response, with a transient phase of lymphocytosis typically peaking within the first 2 months of therapy, followed by resolution over time."
At last follow-up, 22 of 27 patients on the 420-mg dose and 28 of 34 on the 840-mg dose were still on therapy. Phase-III trials are planned.
The trial was funded by Pharmacyclics. Dr. O’Brien disclosed serving on the board of directors or advisory committee, and has received research funding from the company. All of her coauthors disclosed either receiving research funding or consulting fees from the company, or being employees and receiving equity ownership in Phamacyclics.
"There are several remarkable things about this report. Considering the kinds of patients that were enrolled, particularly the fact that 72% of the patients had at least one poor-risk molecular feature, and in addition that patients had between three and five prior treatments, the objective responses seen here are remarkable for CLL," said Dr. Eyal C. Attar.
Dr. Attar is with the division of hematology/oncology at the Massachusetts General Hospital Cancer Center in Boston.
"There are several remarkable things about this report. Considering the kinds of patients that were enrolled, particularly the fact that 72% of the patients had at least one poor-risk molecular feature, and in addition that patients had between three and five prior treatments, the objective responses seen here are remarkable for CLL," said Dr. Eyal C. Attar.
Dr. Attar is with the division of hematology/oncology at the Massachusetts General Hospital Cancer Center in Boston.
"There are several remarkable things about this report. Considering the kinds of patients that were enrolled, particularly the fact that 72% of the patients had at least one poor-risk molecular feature, and in addition that patients had between three and five prior treatments, the objective responses seen here are remarkable for CLL," said Dr. Eyal C. Attar.
Dr. Attar is with the division of hematology/oncology at the Massachusetts General Hospital Cancer Center in Boston.
SAN DIEGO – A novel targeted agent was associated with high objective response rates at 10 months in patients with relapsed/refractory, heavily pretreated chronic lymphocytic leukemia/small lymphocytic lymphoma, investigators reported at the annual meeting of the American Society of Hematology.
At 10.2 months’ median follow-up, objective responses (combined complete and partial responses) were seen in 70% of 27 CLL/SLL patients assigned to a 420-mg daily dose of PCI-32765, an inhibitor of Bruton’s tyrosine kinase (BTK). Objective responses were seen at 6.5 months’ follow-up in 44% of 34 patients on an 840 mg daily dose, reported Dr. Susan O’Brien, professor in the department of leukemia at the University of Texas MD Anderson Cancer Center in Houston.
The researchers had previously reported a 48% objective response rate at 6.2 months median follow-up.
Among patients who did not have a complete or partial response, a nodal partial response was noted in 19% of those in the 420-mg cohort and in 35% of those in the 840-mg group. A nodal partial response was defined as a reduction of greater than 50% in aggregate lymph node size, but with residual lymphocytosis.
Progression-free survival at 6 months is 92% in the 27 patients in the 420-mg cohort, and 90% in the 34 patients in the 840-mg group.
Although it’s still too early to tell which dose will be more effective, the evidence to date suggests that the 420-mg dose completely inhibits activity of the targeted kinase. Thus, the 840-mg dose may not be necessary, Dr. O'Brien said.
Further, PC-32765 does not cause myelosuppression, a trait noted with imatinib (Gleevec) and other tyrosine kinase inhibitors that are effective in other leukemia subtypes.
"This is a big deal in CLL because all of the treatments that we have are pretty much chemo-based or antibody-based treatments. The biggest complication in treating CLL with pretty much every therapy we have is myelosuppression and infection, and of course these people are [already] immune suppressed. To have an agent that’s not myelosuppressive and this effective is very exciting," she said in a briefing prior to her presentation of the results at a session on Tuesday, Dec.13.
A leukemia specialist who was not involved in the study said that the results are particularly impressive given the nature of the patient population.
Patients also tolerated the drug well. The incidence of serious adverse events potentially related to PCI-32765 was 10%. The most common adverse event was diarrhea, which was generally mild, easily controlled, and self-limited, Dr. O’Brien said.
PCI-32765 binds selectively and irreversibly to BTK, an essential element of the B-cell antigen receptor signaling pathway, thereby blocking receptor signaling, inducing cell death via apoptosis, and inhibiting cellular migration and adhesion of malignant B cells.
"To have an agent that’s not myelo-suppressive and this effective is very exciting."
The investigators enrolled both treatment-naive patients with CLL and those who had relapsed/refractory disease following at least two prior therapies, including fludarabine. The patients were treated with PCI-32765 administered daily for 28-day cycles until disease progression. Treatment-naive patients and 27 patients with relapsed/refractory disease were assigned to the 420-mg dose; 34 patients with relapsed/refractory disease were assigned to the 840-mg dose.
In all, 72% of patients had one or more poor-risk molecular features. Of this group, 31% had the 17p deletion, 33% had the 11q deletion, and 57% had IgVH un-mutated.
Two patients dropped out of the trial because of adverse events (dose group not specified), and six patients (two in the 420-mg group, four in the 840-mg group) required a dose reduction.
The most frequently reported grade 1 or 2 adverse events were diarrhea, fatigue, nausea, and ecchymosis. Serious adverse events were reported in 38% of patients. Serious events potentially related to the drug occurred in 10% of all patients.
The investigators noted that, in a majority of patients "a characteristic pattern of response, with a transient phase of lymphocytosis typically peaking within the first 2 months of therapy, followed by resolution over time."
At last follow-up, 22 of 27 patients on the 420-mg dose and 28 of 34 on the 840-mg dose were still on therapy. Phase-III trials are planned.
The trial was funded by Pharmacyclics. Dr. O’Brien disclosed serving on the board of directors or advisory committee, and has received research funding from the company. All of her coauthors disclosed either receiving research funding or consulting fees from the company, or being employees and receiving equity ownership in Phamacyclics.
SAN DIEGO – A novel targeted agent was associated with high objective response rates at 10 months in patients with relapsed/refractory, heavily pretreated chronic lymphocytic leukemia/small lymphocytic lymphoma, investigators reported at the annual meeting of the American Society of Hematology.
At 10.2 months’ median follow-up, objective responses (combined complete and partial responses) were seen in 70% of 27 CLL/SLL patients assigned to a 420-mg daily dose of PCI-32765, an inhibitor of Bruton’s tyrosine kinase (BTK). Objective responses were seen at 6.5 months’ follow-up in 44% of 34 patients on an 840 mg daily dose, reported Dr. Susan O’Brien, professor in the department of leukemia at the University of Texas MD Anderson Cancer Center in Houston.
The researchers had previously reported a 48% objective response rate at 6.2 months median follow-up.
Among patients who did not have a complete or partial response, a nodal partial response was noted in 19% of those in the 420-mg cohort and in 35% of those in the 840-mg group. A nodal partial response was defined as a reduction of greater than 50% in aggregate lymph node size, but with residual lymphocytosis.
Progression-free survival at 6 months is 92% in the 27 patients in the 420-mg cohort, and 90% in the 34 patients in the 840-mg group.
Although it’s still too early to tell which dose will be more effective, the evidence to date suggests that the 420-mg dose completely inhibits activity of the targeted kinase. Thus, the 840-mg dose may not be necessary, Dr. O'Brien said.
Further, PC-32765 does not cause myelosuppression, a trait noted with imatinib (Gleevec) and other tyrosine kinase inhibitors that are effective in other leukemia subtypes.
"This is a big deal in CLL because all of the treatments that we have are pretty much chemo-based or antibody-based treatments. The biggest complication in treating CLL with pretty much every therapy we have is myelosuppression and infection, and of course these people are [already] immune suppressed. To have an agent that’s not myelosuppressive and this effective is very exciting," she said in a briefing prior to her presentation of the results at a session on Tuesday, Dec.13.
A leukemia specialist who was not involved in the study said that the results are particularly impressive given the nature of the patient population.
Patients also tolerated the drug well. The incidence of serious adverse events potentially related to PCI-32765 was 10%. The most common adverse event was diarrhea, which was generally mild, easily controlled, and self-limited, Dr. O’Brien said.
PCI-32765 binds selectively and irreversibly to BTK, an essential element of the B-cell antigen receptor signaling pathway, thereby blocking receptor signaling, inducing cell death via apoptosis, and inhibiting cellular migration and adhesion of malignant B cells.
"To have an agent that’s not myelo-suppressive and this effective is very exciting."
The investigators enrolled both treatment-naive patients with CLL and those who had relapsed/refractory disease following at least two prior therapies, including fludarabine. The patients were treated with PCI-32765 administered daily for 28-day cycles until disease progression. Treatment-naive patients and 27 patients with relapsed/refractory disease were assigned to the 420-mg dose; 34 patients with relapsed/refractory disease were assigned to the 840-mg dose.
In all, 72% of patients had one or more poor-risk molecular features. Of this group, 31% had the 17p deletion, 33% had the 11q deletion, and 57% had IgVH un-mutated.
Two patients dropped out of the trial because of adverse events (dose group not specified), and six patients (two in the 420-mg group, four in the 840-mg group) required a dose reduction.
The most frequently reported grade 1 or 2 adverse events were diarrhea, fatigue, nausea, and ecchymosis. Serious adverse events were reported in 38% of patients. Serious events potentially related to the drug occurred in 10% of all patients.
The investigators noted that, in a majority of patients "a characteristic pattern of response, with a transient phase of lymphocytosis typically peaking within the first 2 months of therapy, followed by resolution over time."
At last follow-up, 22 of 27 patients on the 420-mg dose and 28 of 34 on the 840-mg dose were still on therapy. Phase-III trials are planned.
The trial was funded by Pharmacyclics. Dr. O’Brien disclosed serving on the board of directors or advisory committee, and has received research funding from the company. All of her coauthors disclosed either receiving research funding or consulting fees from the company, or being employees and receiving equity ownership in Phamacyclics.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: At 10.2 months’ median follow-up, 70% of 27 patients with chronic lymphocytic leukemia/small lymphocytic lymphoma assigned to a 420-mg oral daily dose of PCI-32765 had an objective response (combined complete and partial responses), as did 44% of 34 patients on an 840-mg dose.
Data Source: A follow-up of the multicenter phase Ib/II study PCYC-1102.
Disclosures: The trial was funded by Pharmacyclics. Dr. O’Brien disclosed serving on the board of directors or advisory committee and has received research funding from the company. All of her coauthors disclosed either receiving research funding or consulting fees from the company, or being employees and receiving equity ownership in it.