User login
Concomitant methotrexate has no effect on ustekinumab immunogenicity in PsA
Key clinical point: Concomitant methotrexate had no effect on ustekimumab immunogenicity in patients with psoriatic arthritis (PsA), with the formation of antidrug antibody (ADA) not being associated with reductions in ustekinumab safety or efficacy.
Major finding: The prevalence of ADA at week 52 was not significantly different between the ustekinumab+methotrexate and ustekinumab+placebo groups, with disease activity, treatment response, dropout rates, effect of pretreatment with methotrexate, and safety outcomes not being significantly different in patients with vs without ADA (all P > .05).
Study details: This post hoc analysis of the MUST trial included 112 patients with active PsA who were naive to ustekimumab and were randomly assigned to receive ustekinumab with concomitant methotrexate or placebo.
Disclosures: This study was funded by Innovative Medicines Initiative 2 Joint Undertaking, which received support from the European Union’s Horizon 2020 Research and Innovation Program and others. F Behrens, M Koehm, and H Burkhardt declared receiving research grants from Janssen Cilag. The other authors reported no conflicts of interest.
Source: Poor SM et al. The role of antidrug antibodies in ustekinumab therapy and the impact of methotrexate. Rheumatology (Oxford). 2023 (Apr 20). Doi: 10.1093/rheumatology/kead177
Key clinical point: Concomitant methotrexate had no effect on ustekimumab immunogenicity in patients with psoriatic arthritis (PsA), with the formation of antidrug antibody (ADA) not being associated with reductions in ustekinumab safety or efficacy.
Major finding: The prevalence of ADA at week 52 was not significantly different between the ustekinumab+methotrexate and ustekinumab+placebo groups, with disease activity, treatment response, dropout rates, effect of pretreatment with methotrexate, and safety outcomes not being significantly different in patients with vs without ADA (all P > .05).
Study details: This post hoc analysis of the MUST trial included 112 patients with active PsA who were naive to ustekimumab and were randomly assigned to receive ustekinumab with concomitant methotrexate or placebo.
Disclosures: This study was funded by Innovative Medicines Initiative 2 Joint Undertaking, which received support from the European Union’s Horizon 2020 Research and Innovation Program and others. F Behrens, M Koehm, and H Burkhardt declared receiving research grants from Janssen Cilag. The other authors reported no conflicts of interest.
Source: Poor SM et al. The role of antidrug antibodies in ustekinumab therapy and the impact of methotrexate. Rheumatology (Oxford). 2023 (Apr 20). Doi: 10.1093/rheumatology/kead177
Key clinical point: Concomitant methotrexate had no effect on ustekimumab immunogenicity in patients with psoriatic arthritis (PsA), with the formation of antidrug antibody (ADA) not being associated with reductions in ustekinumab safety or efficacy.
Major finding: The prevalence of ADA at week 52 was not significantly different between the ustekinumab+methotrexate and ustekinumab+placebo groups, with disease activity, treatment response, dropout rates, effect of pretreatment with methotrexate, and safety outcomes not being significantly different in patients with vs without ADA (all P > .05).
Study details: This post hoc analysis of the MUST trial included 112 patients with active PsA who were naive to ustekimumab and were randomly assigned to receive ustekinumab with concomitant methotrexate or placebo.
Disclosures: This study was funded by Innovative Medicines Initiative 2 Joint Undertaking, which received support from the European Union’s Horizon 2020 Research and Innovation Program and others. F Behrens, M Koehm, and H Burkhardt declared receiving research grants from Janssen Cilag. The other authors reported no conflicts of interest.
Source: Poor SM et al. The role of antidrug antibodies in ustekinumab therapy and the impact of methotrexate. Rheumatology (Oxford). 2023 (Apr 20). Doi: 10.1093/rheumatology/kead177
Entheseal fibrocartilage abnormalities: A potential imaging biomarker of PsA
Key clinical point: The entheseal fibrocartilage (EF) thickness assessed during power Doppler ultrasound evaluation was significantly different among patients with psoriatic arthritis (PsA) and control individuals and can be explored as an imaging biomarker for PsA.
Major finding: The median EF thickness was significantly greater in patients with PsA and athletes vs control individuals (0.035 and 0.036 vs 0.030 cm, respectively; P = .05 and P = .008, respectively), with the intra- and inter-reader reliability of the evaluation of EF thickness being excellent (intraclass correlation coefficient [ICC] 0.91) and good (ICC 0.80; both P < .001), respectively.
Study details: This cross-sectional study included patients with PsA (n = 30), athletes (n = 40), and control individuals (n = 20) who underwent power Doppler ultrasound evaluation during bilateral Achilles tendon insertions.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Perrotta FM et al. Ultrasonographic evaluation of entheseal fibrocartilage in patients with psoriatic arthritis, athletes and healthy controls: A comparison study. Diagnostics (Basel). 2023;13(8):1446 (Apr 17). Doi: 10.3390/diagnostics13081446
Key clinical point: The entheseal fibrocartilage (EF) thickness assessed during power Doppler ultrasound evaluation was significantly different among patients with psoriatic arthritis (PsA) and control individuals and can be explored as an imaging biomarker for PsA.
Major finding: The median EF thickness was significantly greater in patients with PsA and athletes vs control individuals (0.035 and 0.036 vs 0.030 cm, respectively; P = .05 and P = .008, respectively), with the intra- and inter-reader reliability of the evaluation of EF thickness being excellent (intraclass correlation coefficient [ICC] 0.91) and good (ICC 0.80; both P < .001), respectively.
Study details: This cross-sectional study included patients with PsA (n = 30), athletes (n = 40), and control individuals (n = 20) who underwent power Doppler ultrasound evaluation during bilateral Achilles tendon insertions.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Perrotta FM et al. Ultrasonographic evaluation of entheseal fibrocartilage in patients with psoriatic arthritis, athletes and healthy controls: A comparison study. Diagnostics (Basel). 2023;13(8):1446 (Apr 17). Doi: 10.3390/diagnostics13081446
Key clinical point: The entheseal fibrocartilage (EF) thickness assessed during power Doppler ultrasound evaluation was significantly different among patients with psoriatic arthritis (PsA) and control individuals and can be explored as an imaging biomarker for PsA.
Major finding: The median EF thickness was significantly greater in patients with PsA and athletes vs control individuals (0.035 and 0.036 vs 0.030 cm, respectively; P = .05 and P = .008, respectively), with the intra- and inter-reader reliability of the evaluation of EF thickness being excellent (intraclass correlation coefficient [ICC] 0.91) and good (ICC 0.80; both P < .001), respectively.
Study details: This cross-sectional study included patients with PsA (n = 30), athletes (n = 40), and control individuals (n = 20) who underwent power Doppler ultrasound evaluation during bilateral Achilles tendon insertions.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Perrotta FM et al. Ultrasonographic evaluation of entheseal fibrocartilage in patients with psoriatic arthritis, athletes and healthy controls: A comparison study. Diagnostics (Basel). 2023;13(8):1446 (Apr 17). Doi: 10.3390/diagnostics13081446
Upadacitinib safe and effective in PsA patients with axial involvement
Key clinical point: Compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms with a consistent safety profile in patients with psoriatic arthritis (PsA).
Major finding: The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib vs placebo in both SELECT-PsA 1 (−3.12 vs −1.70; P < .0001) and SELECT PsA 2 (−2.06 vs −0.21; P < .0001) trials. Treatment-emergent adverse events were generally similar among the sub-groups.
Study details: This post hoc analysis included patients with active PsA (n = 1,281 and n = 423, respectively) from the SELECT-PsA 1 and SELECT-PsA 2 trials who were randomly assigned to receive either 15 mg upadacitinib, placebo, or adalimumab and were categorized as those with or without axial involvement.
Disclosures: This study was funded by AbbVie. Five authors declared being employees or stockholders of AbbVie, and some authors reported ties with various sources, including AbbVie.
Source: Baraliakos X et al. Efficacy and safety of upadacitinib in patients with active psoriatic arthritis and axial involvement: Results from two phase 3 studies. Arthritis Res Ther. 2023;25:56 (Apr 10). Doi: 10.1186/s13075-023-03027-5
Key clinical point: Compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms with a consistent safety profile in patients with psoriatic arthritis (PsA).
Major finding: The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib vs placebo in both SELECT-PsA 1 (−3.12 vs −1.70; P < .0001) and SELECT PsA 2 (−2.06 vs −0.21; P < .0001) trials. Treatment-emergent adverse events were generally similar among the sub-groups.
Study details: This post hoc analysis included patients with active PsA (n = 1,281 and n = 423, respectively) from the SELECT-PsA 1 and SELECT-PsA 2 trials who were randomly assigned to receive either 15 mg upadacitinib, placebo, or adalimumab and were categorized as those with or without axial involvement.
Disclosures: This study was funded by AbbVie. Five authors declared being employees or stockholders of AbbVie, and some authors reported ties with various sources, including AbbVie.
Source: Baraliakos X et al. Efficacy and safety of upadacitinib in patients with active psoriatic arthritis and axial involvement: Results from two phase 3 studies. Arthritis Res Ther. 2023;25:56 (Apr 10). Doi: 10.1186/s13075-023-03027-5
Key clinical point: Compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms with a consistent safety profile in patients with psoriatic arthritis (PsA).
Major finding: The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib vs placebo in both SELECT-PsA 1 (−3.12 vs −1.70; P < .0001) and SELECT PsA 2 (−2.06 vs −0.21; P < .0001) trials. Treatment-emergent adverse events were generally similar among the sub-groups.
Study details: This post hoc analysis included patients with active PsA (n = 1,281 and n = 423, respectively) from the SELECT-PsA 1 and SELECT-PsA 2 trials who were randomly assigned to receive either 15 mg upadacitinib, placebo, or adalimumab and were categorized as those with or without axial involvement.
Disclosures: This study was funded by AbbVie. Five authors declared being employees or stockholders of AbbVie, and some authors reported ties with various sources, including AbbVie.
Source: Baraliakos X et al. Efficacy and safety of upadacitinib in patients with active psoriatic arthritis and axial involvement: Results from two phase 3 studies. Arthritis Res Ther. 2023;25:56 (Apr 10). Doi: 10.1186/s13075-023-03027-5
The antimicrobial peptide that even Pharma can love
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
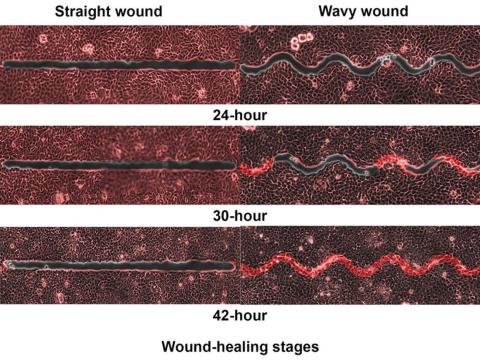
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Sonographic enthesitis associated with sonographic synovitis and tenosynovitis in PsA
Key clinical point: Sonographic enthesitis showed strong association with sonographic synovitis and tenosynovitis in patients with psoriatic arthritis (PsA), suggesting the clinical significance of sonographic enthesitis as a potential marker for inflammation in other musculoskeletal domains.
Major finding: Sonographic enthesitis was significantly associated with sonographic synovitis (β 0.19; P = .004) and sonographic tenosynovitis (β 0.1; P = .003) and showed strong correlation with patient-reported outcomes, such as Health Assessment Questionnaire (P = .003), morning stiffness (P = .002), and others.
Study details: This study prospectively recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses points along with clinical evaluation.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Balulu G et al. The association between sonographic enthesitis with sonographic synovitis and tenosynovitis in psoriatic arthritis patients. Rheumatology (Oxford). 2023 (May 11). Doi: 10.1093/rheumatology/kead202
Key clinical point: Sonographic enthesitis showed strong association with sonographic synovitis and tenosynovitis in patients with psoriatic arthritis (PsA), suggesting the clinical significance of sonographic enthesitis as a potential marker for inflammation in other musculoskeletal domains.
Major finding: Sonographic enthesitis was significantly associated with sonographic synovitis (β 0.19; P = .004) and sonographic tenosynovitis (β 0.1; P = .003) and showed strong correlation with patient-reported outcomes, such as Health Assessment Questionnaire (P = .003), morning stiffness (P = .002), and others.
Study details: This study prospectively recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses points along with clinical evaluation.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Balulu G et al. The association between sonographic enthesitis with sonographic synovitis and tenosynovitis in psoriatic arthritis patients. Rheumatology (Oxford). 2023 (May 11). Doi: 10.1093/rheumatology/kead202
Key clinical point: Sonographic enthesitis showed strong association with sonographic synovitis and tenosynovitis in patients with psoriatic arthritis (PsA), suggesting the clinical significance of sonographic enthesitis as a potential marker for inflammation in other musculoskeletal domains.
Major finding: Sonographic enthesitis was significantly associated with sonographic synovitis (β 0.19; P = .004) and sonographic tenosynovitis (β 0.1; P = .003) and showed strong correlation with patient-reported outcomes, such as Health Assessment Questionnaire (P = .003), morning stiffness (P = .002), and others.
Study details: This study prospectively recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses points along with clinical evaluation.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Balulu G et al. The association between sonographic enthesitis with sonographic synovitis and tenosynovitis in psoriatic arthritis patients. Rheumatology (Oxford). 2023 (May 11). Doi: 10.1093/rheumatology/kead202
Enthesitis resolution similar with secukinumab and adalimumab in PsA
Key clinical point: Patients with psoriatic arthritis (PsA) achieved enthesitis resolution over 52 weeks with secukinumab treatment, which was comparable to that with adalimumab treatment.
Major finding: At week 52, secukinumab vs adalimumab led to a similar proportion of patients achieving enthesitis resolution (53.2% vs 51.4%) along with site-specific resolution of lateral epicondyle enthesitis (84.6% vs 87.1%) and showing relapse after first resolution (21.0% vs 15.6%), as assessed by the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC). Moreover, secukinumab vs adalimumab had a comparable response time to SPARCC enthesitis resolution (113 vs 88 days).
Study details: This post hoc analysis of the EXCEED study included 853 patients with PsA who received either secukinumab (300 mg) or adalimumab (40 mg) over 52 weeks.
Disclosures: This study was supported by Novartis Pharmaceuticals Corporation, USA. C Gaillez and B Parikh declared being current or former employees of Novartis Pharma or Novartis Pharmaceuticals Corporation, and several authors reported ties with various sources, including Novartis.
Source: Kaeley GS et al. Enthesitis in patients with psoriatic arthritis treated with secukinumab or adalimumab: A post hoc analysis of the EXCEED study. Rheumatology (Oxford). 2023 (Apr 25). Doi: 10.1093/rheumatology/kead181
Key clinical point: Patients with psoriatic arthritis (PsA) achieved enthesitis resolution over 52 weeks with secukinumab treatment, which was comparable to that with adalimumab treatment.
Major finding: At week 52, secukinumab vs adalimumab led to a similar proportion of patients achieving enthesitis resolution (53.2% vs 51.4%) along with site-specific resolution of lateral epicondyle enthesitis (84.6% vs 87.1%) and showing relapse after first resolution (21.0% vs 15.6%), as assessed by the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC). Moreover, secukinumab vs adalimumab had a comparable response time to SPARCC enthesitis resolution (113 vs 88 days).
Study details: This post hoc analysis of the EXCEED study included 853 patients with PsA who received either secukinumab (300 mg) or adalimumab (40 mg) over 52 weeks.
Disclosures: This study was supported by Novartis Pharmaceuticals Corporation, USA. C Gaillez and B Parikh declared being current or former employees of Novartis Pharma or Novartis Pharmaceuticals Corporation, and several authors reported ties with various sources, including Novartis.
Source: Kaeley GS et al. Enthesitis in patients with psoriatic arthritis treated with secukinumab or adalimumab: A post hoc analysis of the EXCEED study. Rheumatology (Oxford). 2023 (Apr 25). Doi: 10.1093/rheumatology/kead181
Key clinical point: Patients with psoriatic arthritis (PsA) achieved enthesitis resolution over 52 weeks with secukinumab treatment, which was comparable to that with adalimumab treatment.
Major finding: At week 52, secukinumab vs adalimumab led to a similar proportion of patients achieving enthesitis resolution (53.2% vs 51.4%) along with site-specific resolution of lateral epicondyle enthesitis (84.6% vs 87.1%) and showing relapse after first resolution (21.0% vs 15.6%), as assessed by the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC). Moreover, secukinumab vs adalimumab had a comparable response time to SPARCC enthesitis resolution (113 vs 88 days).
Study details: This post hoc analysis of the EXCEED study included 853 patients with PsA who received either secukinumab (300 mg) or adalimumab (40 mg) over 52 weeks.
Disclosures: This study was supported by Novartis Pharmaceuticals Corporation, USA. C Gaillez and B Parikh declared being current or former employees of Novartis Pharma or Novartis Pharmaceuticals Corporation, and several authors reported ties with various sources, including Novartis.
Source: Kaeley GS et al. Enthesitis in patients with psoriatic arthritis treated with secukinumab or adalimumab: A post hoc analysis of the EXCEED study. Rheumatology (Oxford). 2023 (Apr 25). Doi: 10.1093/rheumatology/kead181
Secukinumab improves treatment outcomes and inhibits structural damage in PsA
Key clinical point: The achievement of stringent treatment goals with secukinumab led to clinically meaningful benefits in physical function in patients with psoriatic arthritis (PsA), with secukinumab showing a protective effect on radiographic progression.
Major finding: Overall, over 2 years, a relatively small percentage of patients receiving secukinumab achieved sustained remission (REM) according to very low disease activity (LDA; 19%-24%) or Disease Activity index for PsA (DAPSA) REM (30%-36%), with those achieving DAPSA LDA+REM or DAPSA REM showing numerically greater improvement in physical function. A higher proportion of secukinumab-treated patients were non-structural progressors at 2 years irrespective of achieving sustained LDA/REM.
Study details: This was a retrospective analysis from the FUTURE 5 study including 996 patients with active PsA who were randomly assigned to receive secukinumab or placebo.
Disclosures: This study was funded by Novartis Pharma AG. Two authors declared being employees of or owning stocks/shares in Novartis, and several authors reported ties with various sources, including Novartis.
Source: Coates LC et al. Secukinumab improves physical function and quality of life and inhibits structural damage in patients with PsA with sustained remission or low disease activity: Results from the 2-year phase 3 FUTURE 5 study. RMD Open. 2023;9(2):e002939 (Apr 24). Doi: 10.1136/rmdopen-2022-002939
Key clinical point: The achievement of stringent treatment goals with secukinumab led to clinically meaningful benefits in physical function in patients with psoriatic arthritis (PsA), with secukinumab showing a protective effect on radiographic progression.
Major finding: Overall, over 2 years, a relatively small percentage of patients receiving secukinumab achieved sustained remission (REM) according to very low disease activity (LDA; 19%-24%) or Disease Activity index for PsA (DAPSA) REM (30%-36%), with those achieving DAPSA LDA+REM or DAPSA REM showing numerically greater improvement in physical function. A higher proportion of secukinumab-treated patients were non-structural progressors at 2 years irrespective of achieving sustained LDA/REM.
Study details: This was a retrospective analysis from the FUTURE 5 study including 996 patients with active PsA who were randomly assigned to receive secukinumab or placebo.
Disclosures: This study was funded by Novartis Pharma AG. Two authors declared being employees of or owning stocks/shares in Novartis, and several authors reported ties with various sources, including Novartis.
Source: Coates LC et al. Secukinumab improves physical function and quality of life and inhibits structural damage in patients with PsA with sustained remission or low disease activity: Results from the 2-year phase 3 FUTURE 5 study. RMD Open. 2023;9(2):e002939 (Apr 24). Doi: 10.1136/rmdopen-2022-002939
Key clinical point: The achievement of stringent treatment goals with secukinumab led to clinically meaningful benefits in physical function in patients with psoriatic arthritis (PsA), with secukinumab showing a protective effect on radiographic progression.
Major finding: Overall, over 2 years, a relatively small percentage of patients receiving secukinumab achieved sustained remission (REM) according to very low disease activity (LDA; 19%-24%) or Disease Activity index for PsA (DAPSA) REM (30%-36%), with those achieving DAPSA LDA+REM or DAPSA REM showing numerically greater improvement in physical function. A higher proportion of secukinumab-treated patients were non-structural progressors at 2 years irrespective of achieving sustained LDA/REM.
Study details: This was a retrospective analysis from the FUTURE 5 study including 996 patients with active PsA who were randomly assigned to receive secukinumab or placebo.
Disclosures: This study was funded by Novartis Pharma AG. Two authors declared being employees of or owning stocks/shares in Novartis, and several authors reported ties with various sources, including Novartis.
Source: Coates LC et al. Secukinumab improves physical function and quality of life and inhibits structural damage in patients with PsA with sustained remission or low disease activity: Results from the 2-year phase 3 FUTURE 5 study. RMD Open. 2023;9(2):e002939 (Apr 24). Doi: 10.1136/rmdopen-2022-002939
PARP inhibitors and breast cancer: Questions remain about wider use
oncologists explained at the European Society for Medical Oncology Breast Cancer annual congress.
For now, the drugs are only approved in high-risk germline BRCA mutation (gBRCAmut) early breast cancer, oncologist Kevin Punie, MD, of Saint Augustine Hospital in Wilrijk, Belgium, said during a session at the meeting. Combining the drugs with chemotherapy “has not yet demonstrated significant benefits, and this is irrespective whether platinum was part of the chemotherapy backbone.”
PARP is a kind of enzyme that repairs damaged DNA in cells, especially cancerous ones. PARP inhibitors block the enzyme, potentially leading more cancer cells to die, the Dana-Farber Cancer Institute states.
In a separate presentation during the same session, oncologist Andrew Tutt, MBChB, PhD, noted that a study he led – a phase 3, double-blinded, randomized 2021 trial – found that patients with BRCA1- or BRCA2-mutated breast cancer who took the PARP inhibitor olarapib (Lynparza) versus placebo had improved outcomes on several measures, including 3-year invasive disease-free survival (85.9% vs. 77.1%, P < .001). However, the study noted that “olaparib had limited effects on global patient-reported quality of life.”
Dr. Tutt, of the Institute of Cancer Research, London, and Kings College London, said 57% of patients who took olarapib suffered nausea versus 24% of those who took placebo, and fatigue and anemia were also more common in the olarapib group. Anemia can be severe and lead to transfusions in some cases.
As Dr. Punie explained, there are many reasons to consider combining PARP inhibitors with other treatments such as chemotherapy, immunotherapy, and radiotherapy. The combinations may have synergetic effects, and they could have potential in both the neoadjuvant and adjuvant settings.
The combination of the PARP inhibitor olaparib and endocrine therapy is now approved by the European Medicines Agency for the adjuvant treatment of certain patients with germline BRCA1/2 mutations who have HER2-negative, high-risk early breast cancer, Dr. Punie noted.
The 2021 study led by Dr. Tutt reported that treatment or safety differences were found in those who received both olaparib and endocrine therapy versus those who only received olarapib.
So far, Dr. Punie said, “we not yet have enough clinical evidence to say that there’s really synergy between PNP inhibitors and other anticancer therapies.” According to the National Institutes of Health, medical synergy “describes the interaction of two or more drugs when their combined effect is greater than the sum of the effects seen when each drug is given alone.”
In regard to chemotherapy, it makes sense that PARP inhibitors would be helpful in combination, Dr. Punie said. DNA damage to cancer cells accumulates during chemotherapy, he said, and they’re more depending on PARP for repair.
Study results so far have been mixed. A 2022 study, for example, found that adding the experimental PARP inhibitor veliparib to the chemotherapy regimen carboplatin-paclitaxel didn’t improve outcomes, he said. A similar study examining the addition of olaparib to carboplatin-paclitaxel is ongoing.
As for combining radiotherapy and PARP inhibitors, Dr. Punie said that preclinical findings are promising, and research is underway. There’s also ongoing research into combining PARP inhibitors with immunotherapy.
Off-label use of olaparib with immunotherapy or sequential treatment may be appropriate in the setting of adjuvant gBRCAmut triple-negative breast cancer with residual disease, he said.
During his presentation, Dr. Tutt called for researchers to investigate the use of PARP inhibitors in the de-escalation of treatment in lower-risk gBRCAmut disease.
“Clearly, some patients require chemotherapy, and we know patients respond very well to neoadjuvant chemotherapy if they have a BRCA mutation, but we don’t yet know who we can de-escalate in,” he said.
He also highlighted the need to reduce anemia in patients on PARP inhibitors, “particularly if we’re moving into lower-risk populations or possibly considering prevention trials.
“The study of PARP inhibitor resistance ... is now urgent, so that we can address it,” he said.
Dr. Punie disclosed financial relationships with AstraZeneca, Eli Lilly, Exact Sciences, Focus Patient, Medscape, MSD, Mundi Pharma, Need, Novartis, Pierre Fabre, Pfizer, F. Hoffmann–La Roche, Sanofi, Seagen, and PharmaMar. Dr. Tutt disclosed financial relationships with Artios, Gilead, MD Anderson, Merck KGaA, Pfizer, Vertex, AstraZeneca, EM Partners, Medscape Education, CRUK, Inbiomotion, Myriad Genetics, and Breast Cancer Now.
oncologists explained at the European Society for Medical Oncology Breast Cancer annual congress.
For now, the drugs are only approved in high-risk germline BRCA mutation (gBRCAmut) early breast cancer, oncologist Kevin Punie, MD, of Saint Augustine Hospital in Wilrijk, Belgium, said during a session at the meeting. Combining the drugs with chemotherapy “has not yet demonstrated significant benefits, and this is irrespective whether platinum was part of the chemotherapy backbone.”
PARP is a kind of enzyme that repairs damaged DNA in cells, especially cancerous ones. PARP inhibitors block the enzyme, potentially leading more cancer cells to die, the Dana-Farber Cancer Institute states.
In a separate presentation during the same session, oncologist Andrew Tutt, MBChB, PhD, noted that a study he led – a phase 3, double-blinded, randomized 2021 trial – found that patients with BRCA1- or BRCA2-mutated breast cancer who took the PARP inhibitor olarapib (Lynparza) versus placebo had improved outcomes on several measures, including 3-year invasive disease-free survival (85.9% vs. 77.1%, P < .001). However, the study noted that “olaparib had limited effects on global patient-reported quality of life.”
Dr. Tutt, of the Institute of Cancer Research, London, and Kings College London, said 57% of patients who took olarapib suffered nausea versus 24% of those who took placebo, and fatigue and anemia were also more common in the olarapib group. Anemia can be severe and lead to transfusions in some cases.
As Dr. Punie explained, there are many reasons to consider combining PARP inhibitors with other treatments such as chemotherapy, immunotherapy, and radiotherapy. The combinations may have synergetic effects, and they could have potential in both the neoadjuvant and adjuvant settings.
The combination of the PARP inhibitor olaparib and endocrine therapy is now approved by the European Medicines Agency for the adjuvant treatment of certain patients with germline BRCA1/2 mutations who have HER2-negative, high-risk early breast cancer, Dr. Punie noted.
The 2021 study led by Dr. Tutt reported that treatment or safety differences were found in those who received both olaparib and endocrine therapy versus those who only received olarapib.
So far, Dr. Punie said, “we not yet have enough clinical evidence to say that there’s really synergy between PNP inhibitors and other anticancer therapies.” According to the National Institutes of Health, medical synergy “describes the interaction of two or more drugs when their combined effect is greater than the sum of the effects seen when each drug is given alone.”
In regard to chemotherapy, it makes sense that PARP inhibitors would be helpful in combination, Dr. Punie said. DNA damage to cancer cells accumulates during chemotherapy, he said, and they’re more depending on PARP for repair.
Study results so far have been mixed. A 2022 study, for example, found that adding the experimental PARP inhibitor veliparib to the chemotherapy regimen carboplatin-paclitaxel didn’t improve outcomes, he said. A similar study examining the addition of olaparib to carboplatin-paclitaxel is ongoing.
As for combining radiotherapy and PARP inhibitors, Dr. Punie said that preclinical findings are promising, and research is underway. There’s also ongoing research into combining PARP inhibitors with immunotherapy.
Off-label use of olaparib with immunotherapy or sequential treatment may be appropriate in the setting of adjuvant gBRCAmut triple-negative breast cancer with residual disease, he said.
During his presentation, Dr. Tutt called for researchers to investigate the use of PARP inhibitors in the de-escalation of treatment in lower-risk gBRCAmut disease.
“Clearly, some patients require chemotherapy, and we know patients respond very well to neoadjuvant chemotherapy if they have a BRCA mutation, but we don’t yet know who we can de-escalate in,” he said.
He also highlighted the need to reduce anemia in patients on PARP inhibitors, “particularly if we’re moving into lower-risk populations or possibly considering prevention trials.
“The study of PARP inhibitor resistance ... is now urgent, so that we can address it,” he said.
Dr. Punie disclosed financial relationships with AstraZeneca, Eli Lilly, Exact Sciences, Focus Patient, Medscape, MSD, Mundi Pharma, Need, Novartis, Pierre Fabre, Pfizer, F. Hoffmann–La Roche, Sanofi, Seagen, and PharmaMar. Dr. Tutt disclosed financial relationships with Artios, Gilead, MD Anderson, Merck KGaA, Pfizer, Vertex, AstraZeneca, EM Partners, Medscape Education, CRUK, Inbiomotion, Myriad Genetics, and Breast Cancer Now.
oncologists explained at the European Society for Medical Oncology Breast Cancer annual congress.
For now, the drugs are only approved in high-risk germline BRCA mutation (gBRCAmut) early breast cancer, oncologist Kevin Punie, MD, of Saint Augustine Hospital in Wilrijk, Belgium, said during a session at the meeting. Combining the drugs with chemotherapy “has not yet demonstrated significant benefits, and this is irrespective whether platinum was part of the chemotherapy backbone.”
PARP is a kind of enzyme that repairs damaged DNA in cells, especially cancerous ones. PARP inhibitors block the enzyme, potentially leading more cancer cells to die, the Dana-Farber Cancer Institute states.
In a separate presentation during the same session, oncologist Andrew Tutt, MBChB, PhD, noted that a study he led – a phase 3, double-blinded, randomized 2021 trial – found that patients with BRCA1- or BRCA2-mutated breast cancer who took the PARP inhibitor olarapib (Lynparza) versus placebo had improved outcomes on several measures, including 3-year invasive disease-free survival (85.9% vs. 77.1%, P < .001). However, the study noted that “olaparib had limited effects on global patient-reported quality of life.”
Dr. Tutt, of the Institute of Cancer Research, London, and Kings College London, said 57% of patients who took olarapib suffered nausea versus 24% of those who took placebo, and fatigue and anemia were also more common in the olarapib group. Anemia can be severe and lead to transfusions in some cases.
As Dr. Punie explained, there are many reasons to consider combining PARP inhibitors with other treatments such as chemotherapy, immunotherapy, and radiotherapy. The combinations may have synergetic effects, and they could have potential in both the neoadjuvant and adjuvant settings.
The combination of the PARP inhibitor olaparib and endocrine therapy is now approved by the European Medicines Agency for the adjuvant treatment of certain patients with germline BRCA1/2 mutations who have HER2-negative, high-risk early breast cancer, Dr. Punie noted.
The 2021 study led by Dr. Tutt reported that treatment or safety differences were found in those who received both olaparib and endocrine therapy versus those who only received olarapib.
So far, Dr. Punie said, “we not yet have enough clinical evidence to say that there’s really synergy between PNP inhibitors and other anticancer therapies.” According to the National Institutes of Health, medical synergy “describes the interaction of two or more drugs when their combined effect is greater than the sum of the effects seen when each drug is given alone.”
In regard to chemotherapy, it makes sense that PARP inhibitors would be helpful in combination, Dr. Punie said. DNA damage to cancer cells accumulates during chemotherapy, he said, and they’re more depending on PARP for repair.
Study results so far have been mixed. A 2022 study, for example, found that adding the experimental PARP inhibitor veliparib to the chemotherapy regimen carboplatin-paclitaxel didn’t improve outcomes, he said. A similar study examining the addition of olaparib to carboplatin-paclitaxel is ongoing.
As for combining radiotherapy and PARP inhibitors, Dr. Punie said that preclinical findings are promising, and research is underway. There’s also ongoing research into combining PARP inhibitors with immunotherapy.
Off-label use of olaparib with immunotherapy or sequential treatment may be appropriate in the setting of adjuvant gBRCAmut triple-negative breast cancer with residual disease, he said.
During his presentation, Dr. Tutt called for researchers to investigate the use of PARP inhibitors in the de-escalation of treatment in lower-risk gBRCAmut disease.
“Clearly, some patients require chemotherapy, and we know patients respond very well to neoadjuvant chemotherapy if they have a BRCA mutation, but we don’t yet know who we can de-escalate in,” he said.
He also highlighted the need to reduce anemia in patients on PARP inhibitors, “particularly if we’re moving into lower-risk populations or possibly considering prevention trials.
“The study of PARP inhibitor resistance ... is now urgent, so that we can address it,” he said.
Dr. Punie disclosed financial relationships with AstraZeneca, Eli Lilly, Exact Sciences, Focus Patient, Medscape, MSD, Mundi Pharma, Need, Novartis, Pierre Fabre, Pfizer, F. Hoffmann–La Roche, Sanofi, Seagen, and PharmaMar. Dr. Tutt disclosed financial relationships with Artios, Gilead, MD Anderson, Merck KGaA, Pfizer, Vertex, AstraZeneca, EM Partners, Medscape Education, CRUK, Inbiomotion, Myriad Genetics, and Breast Cancer Now.
FROM ESMO BREAST CANCER 2023
Highlights in Multiple Sclerosis From AAN 2023
Conversion to multiple sclerosis (MS) and a novel drug to prevent it are among the MS highlights from the 2023 American Academy of Neurology (AAN) Annual Meeting, as reported by Dr Fred Lublin of the Icahn School of Medicine in New York.
Dr Lublin starts with three studies examining radiologic isolated syndrome (RIS), the first of which looked at MRI and spinal fluid factors associated with conversion to MS. The study indicated that less stringent criteria for RIS may have to be considered.
The second study, again using data obtained via MRI, showed that the presence of pragmatic rim lesions may be an indicator of more severe disease. The third study was a clinical trial of teriflunomide. This suggested that the drug reduced conversion rates to MS by almost two thirds compared with placebo.
Dr Lublin then turns to an investigation of the impact of assisted reproductive technologies on relapse risk in women with MS. Reassuringly, women who continued with disease-modifying therapy during reproductive assistance were less likely to relapse.
Finally, he discusses a study showing that women with MS who followed a Mediterranean diet had better cognition scores.
--
Fred Lublin, MD, Saunders Family Professor of Neurology; Director, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY
Fred D. Lublin, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biogen; EMD Serono; Novartis; Teva; Actelion/Janssen; Sanofi/Genzyme; Acorda; Roche/Genentech; Viela Bio/Horizon
Serve(d) as a speaker or a member of a speakers bureau for: Sanofi; Biogen
Received research grant from: Biogen; Novartis; Actelion; Biogen; Sanofi; National Multiple Sclerosis Society; Brainstorm Cell Therapeutics; National Institutes of Health
Conversion to multiple sclerosis (MS) and a novel drug to prevent it are among the MS highlights from the 2023 American Academy of Neurology (AAN) Annual Meeting, as reported by Dr Fred Lublin of the Icahn School of Medicine in New York.
Dr Lublin starts with three studies examining radiologic isolated syndrome (RIS), the first of which looked at MRI and spinal fluid factors associated with conversion to MS. The study indicated that less stringent criteria for RIS may have to be considered.
The second study, again using data obtained via MRI, showed that the presence of pragmatic rim lesions may be an indicator of more severe disease. The third study was a clinical trial of teriflunomide. This suggested that the drug reduced conversion rates to MS by almost two thirds compared with placebo.
Dr Lublin then turns to an investigation of the impact of assisted reproductive technologies on relapse risk in women with MS. Reassuringly, women who continued with disease-modifying therapy during reproductive assistance were less likely to relapse.
Finally, he discusses a study showing that women with MS who followed a Mediterranean diet had better cognition scores.
--
Fred Lublin, MD, Saunders Family Professor of Neurology; Director, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY
Fred D. Lublin, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biogen; EMD Serono; Novartis; Teva; Actelion/Janssen; Sanofi/Genzyme; Acorda; Roche/Genentech; Viela Bio/Horizon
Serve(d) as a speaker or a member of a speakers bureau for: Sanofi; Biogen
Received research grant from: Biogen; Novartis; Actelion; Biogen; Sanofi; National Multiple Sclerosis Society; Brainstorm Cell Therapeutics; National Institutes of Health
Conversion to multiple sclerosis (MS) and a novel drug to prevent it are among the MS highlights from the 2023 American Academy of Neurology (AAN) Annual Meeting, as reported by Dr Fred Lublin of the Icahn School of Medicine in New York.
Dr Lublin starts with three studies examining radiologic isolated syndrome (RIS), the first of which looked at MRI and spinal fluid factors associated with conversion to MS. The study indicated that less stringent criteria for RIS may have to be considered.
The second study, again using data obtained via MRI, showed that the presence of pragmatic rim lesions may be an indicator of more severe disease. The third study was a clinical trial of teriflunomide. This suggested that the drug reduced conversion rates to MS by almost two thirds compared with placebo.
Dr Lublin then turns to an investigation of the impact of assisted reproductive technologies on relapse risk in women with MS. Reassuringly, women who continued with disease-modifying therapy during reproductive assistance were less likely to relapse.
Finally, he discusses a study showing that women with MS who followed a Mediterranean diet had better cognition scores.
--
Fred Lublin, MD, Saunders Family Professor of Neurology; Director, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY
Fred D. Lublin, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biogen; EMD Serono; Novartis; Teva; Actelion/Janssen; Sanofi/Genzyme; Acorda; Roche/Genentech; Viela Bio/Horizon
Serve(d) as a speaker or a member of a speakers bureau for: Sanofi; Biogen
Received research grant from: Biogen; Novartis; Actelion; Biogen; Sanofi; National Multiple Sclerosis Society; Brainstorm Cell Therapeutics; National Institutes of Health

Mailed HPV test kits boost cervical cancer screening
The self-sampling kits, which detect human papillomavirus (HPV), are available only for use in clinical trials, but the researchers hope that eventually these kits will be approved for use by the general public.
The researchers, from the University of North Carolina, explored use of these kits in the My Body, My Test-3 study, which was published online in The Lancet Public Health.
In a commentary published with the study, Runzhi Wang, MD, and Jennell Coleman, MD, MPH, both of Johns Hopkins University, Baltimore, said it “provides the required evidence that ... self-collected samples can be an effective strategy for hard-to-reach populations.”
The study involved 665 women (aged 25-64) in North Carolina who were either uninsured or enrolled in Medicaid or Medicare. The patients had low-income backgrounds and lived in urban areas. More than half self-reported as Black or Hispanic (55%), uninsured (78%) or unemployed (57%). None had a Pap smear in at least 4 years or a high-risk HPV test in the last 6 years.
Two-thirds of the women were mailed an HPV self-collection kit and received assistance with scheduling an in-person screening appointment. The kit included a Viba-Brush device, which is inserted into the vagina like a tampon to collect the sample.
The other third of women, the control group, only received scheduling assistance.
The team found that mailing the self-collection tests along with helping women book in-clinic appointments improved screening rates twofold, compared with just assisting patients to schedule an appointment.
Screening success among those who received the at-home collection kit was 72%, compared with 37% in the control group.
Of those who received the kits, 78% returned them. This is “impressive,” said Dr. Wang and Dr. Coleman, as previous studies have reported return rates of only 8%-20%.
About 23% of eligible women are overdue for cervical cancer screening by at least a year, according to the National Cancer Institute. Jennifer Smith, PhD, MPH, professor of epidemiology at the University of North Carolina at Chapel Hill and an author of the study, believes every woman deserves equal access to cervical screening.
“I think we really need to make efforts to increase cervical cancer screening among women who are overdue for screening by a year or more from the recommended guidelines,” Dr. Smith said. “We’ve proven along with the wide evidence both in the U.S. and globally that self-collection intervention works well and can motivate screening uptake by breaking down barriers for populations that have less access to care.”
“We’re hoping this research in combination with all of the extensive evidence on the positive performance of HPV self-collection will provide additional information to be considered by the FDA for approval of the kits for primary screening,” Dr. Smith said.
“Government approval of at-home HPV tests would have a huge impact,” said coauthor Noel Brewer, PhD, also of UNC Chapel Hill. “We could better reach those in rural areas where cervical cancer screening is hard to come by.”
Dr. Smith has received research grants, supply donations, and consultancies for Hologic and BD Diagnostics. Dr. Brewer, Dr. Wang, and Dr. Coleman reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
The self-sampling kits, which detect human papillomavirus (HPV), are available only for use in clinical trials, but the researchers hope that eventually these kits will be approved for use by the general public.
The researchers, from the University of North Carolina, explored use of these kits in the My Body, My Test-3 study, which was published online in The Lancet Public Health.
In a commentary published with the study, Runzhi Wang, MD, and Jennell Coleman, MD, MPH, both of Johns Hopkins University, Baltimore, said it “provides the required evidence that ... self-collected samples can be an effective strategy for hard-to-reach populations.”
The study involved 665 women (aged 25-64) in North Carolina who were either uninsured or enrolled in Medicaid or Medicare. The patients had low-income backgrounds and lived in urban areas. More than half self-reported as Black or Hispanic (55%), uninsured (78%) or unemployed (57%). None had a Pap smear in at least 4 years or a high-risk HPV test in the last 6 years.
Two-thirds of the women were mailed an HPV self-collection kit and received assistance with scheduling an in-person screening appointment. The kit included a Viba-Brush device, which is inserted into the vagina like a tampon to collect the sample.
The other third of women, the control group, only received scheduling assistance.
The team found that mailing the self-collection tests along with helping women book in-clinic appointments improved screening rates twofold, compared with just assisting patients to schedule an appointment.
Screening success among those who received the at-home collection kit was 72%, compared with 37% in the control group.
Of those who received the kits, 78% returned them. This is “impressive,” said Dr. Wang and Dr. Coleman, as previous studies have reported return rates of only 8%-20%.
About 23% of eligible women are overdue for cervical cancer screening by at least a year, according to the National Cancer Institute. Jennifer Smith, PhD, MPH, professor of epidemiology at the University of North Carolina at Chapel Hill and an author of the study, believes every woman deserves equal access to cervical screening.
“I think we really need to make efforts to increase cervical cancer screening among women who are overdue for screening by a year or more from the recommended guidelines,” Dr. Smith said. “We’ve proven along with the wide evidence both in the U.S. and globally that self-collection intervention works well and can motivate screening uptake by breaking down barriers for populations that have less access to care.”
“We’re hoping this research in combination with all of the extensive evidence on the positive performance of HPV self-collection will provide additional information to be considered by the FDA for approval of the kits for primary screening,” Dr. Smith said.
“Government approval of at-home HPV tests would have a huge impact,” said coauthor Noel Brewer, PhD, also of UNC Chapel Hill. “We could better reach those in rural areas where cervical cancer screening is hard to come by.”
Dr. Smith has received research grants, supply donations, and consultancies for Hologic and BD Diagnostics. Dr. Brewer, Dr. Wang, and Dr. Coleman reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
The self-sampling kits, which detect human papillomavirus (HPV), are available only for use in clinical trials, but the researchers hope that eventually these kits will be approved for use by the general public.
The researchers, from the University of North Carolina, explored use of these kits in the My Body, My Test-3 study, which was published online in The Lancet Public Health.
In a commentary published with the study, Runzhi Wang, MD, and Jennell Coleman, MD, MPH, both of Johns Hopkins University, Baltimore, said it “provides the required evidence that ... self-collected samples can be an effective strategy for hard-to-reach populations.”
The study involved 665 women (aged 25-64) in North Carolina who were either uninsured or enrolled in Medicaid or Medicare. The patients had low-income backgrounds and lived in urban areas. More than half self-reported as Black or Hispanic (55%), uninsured (78%) or unemployed (57%). None had a Pap smear in at least 4 years or a high-risk HPV test in the last 6 years.
Two-thirds of the women were mailed an HPV self-collection kit and received assistance with scheduling an in-person screening appointment. The kit included a Viba-Brush device, which is inserted into the vagina like a tampon to collect the sample.
The other third of women, the control group, only received scheduling assistance.
The team found that mailing the self-collection tests along with helping women book in-clinic appointments improved screening rates twofold, compared with just assisting patients to schedule an appointment.
Screening success among those who received the at-home collection kit was 72%, compared with 37% in the control group.
Of those who received the kits, 78% returned them. This is “impressive,” said Dr. Wang and Dr. Coleman, as previous studies have reported return rates of only 8%-20%.
About 23% of eligible women are overdue for cervical cancer screening by at least a year, according to the National Cancer Institute. Jennifer Smith, PhD, MPH, professor of epidemiology at the University of North Carolina at Chapel Hill and an author of the study, believes every woman deserves equal access to cervical screening.
“I think we really need to make efforts to increase cervical cancer screening among women who are overdue for screening by a year or more from the recommended guidelines,” Dr. Smith said. “We’ve proven along with the wide evidence both in the U.S. and globally that self-collection intervention works well and can motivate screening uptake by breaking down barriers for populations that have less access to care.”
“We’re hoping this research in combination with all of the extensive evidence on the positive performance of HPV self-collection will provide additional information to be considered by the FDA for approval of the kits for primary screening,” Dr. Smith said.
“Government approval of at-home HPV tests would have a huge impact,” said coauthor Noel Brewer, PhD, also of UNC Chapel Hill. “We could better reach those in rural areas where cervical cancer screening is hard to come by.”
Dr. Smith has received research grants, supply donations, and consultancies for Hologic and BD Diagnostics. Dr. Brewer, Dr. Wang, and Dr. Coleman reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
FROM THE LANCET PUBLIC HEALTH