User login
Subclinical hypothyroidism: Let the evidence be your guide
Subclinical hypothyroidism (SCH) is a biochemical state in which the thyroid-stimulating hormone (TSH) is elevated while the free thyroxine (T4) level is normal. Overt hypothyroidism is not diagnosed until the free T4 level is decreased, regardless of the degree of TSH elevation.
The overall prevalence of SCH in iodine-rich areas is 4% to 10%, with a risk for progression to overt hypothyroidism of between 2% and 6% annually.1 The prevalence of SCH varies depending on the TSH reference range used.1 The normal reference range for TSH varies depending on the laboratory and/or the reference population surveyed, with the range likely widening with increasing age.
SCH is most common among women, the elderly, and White individuals.2 The discovery of SCH is often incidental, given that usually it is detected by laboratory findings alone without associated symptoms of overt hypothyroidism.3
The not-so-significant role of symptoms in subclinical hypothyroidism
Symptoms associated with overt hypothyroidism include constipation, dry skin, fatigue, slow thinking, poor memory, muscle cramps, weakness, and cold intolerance. In SCH, these symptoms are inconsistent, with around 1 in 3 patients having no symptoms
One study reported that roughly 18% of euthyroid individuals, 22% of SCH patients, and 26% of those with overt hypothyroidism reported 4 or more symptoms classically thought to be related to hypothyroidism.4 A large Danish cohort study found that hypothyroid symptoms were no more common in patients with SCH than in euthyroid individuals in the general population.5 These studies question the validity of attributing symptoms to SCH.
Adverse health associations
Observational data suggest that SCH is associated with an increased risk for dyslipidemia, coronary heart disease, heart failure, and cardiovascular mortality, particularly in those with TSH levels ≥ 10 mIU/L.6,7 Such associations were not found for most adults with TSH levels between 5 and 10 mIU/L.8 There are also potential associations of SCH with obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.9,10 Despite thyroid studies being commonly ordered as part of a mental health evaluation, SCH has not been statistically associated with depressive symptoms.11,12
Caveats with laboratory testing
There are several issues to consider when performing a laboratory assessment of thyroid function. TSH levels fluctuate considerably during the day, as TSH secretion has a circadian rhythm. TSH values are 50% higher at night and in the early morning than during the rest of the day.13 TSH values also may rise in response to current illness or stress. Due to this biologic variability, repeat testing to confirm TSH levels is recommended if an initial test result is abnormal.14
Continue to: An exact reference range...
An exact reference range for TSH is not widely agreed upon—although most laboratories regard 4.0 to 5.0 mIU/L as the high-end cutoff for normal. Also, “normal” TSH levels appear to differ by age. Accordingly, some experts have recommended an age-based reference range for TSH levels,15 although this is not implemented widely by laboratories. A TSH level of 6.0 mIU/L (or even higher) may be more appropriate for adults older than 65 years.1
Biotin supplementation has been shown to cause spurious thyroid testing results (TSH, T3, T4) depending on the type of assay used. Therefore, supplements containing biotin should be withheld for several days before assessing thyroid function.16Patients with SCH are often categorized as having TSH levels between 4.5 and 10 mIU/L (around 90% of patients) or levels ≥ 10 mIU/L.8,17 If followed for 5 years, approximately 60% of patients with SCH and TSH levels between 4 and 10 mIU/L will normalize without intervention.18 Normalization is less common in patients with a TSH level greater than 10 mIU/L.18
The risk for progression to overt hypothyroidism also appears to be higher for those with certain risk factors. These include higher baseline TSH levels, presence of thyroid peroxidase antibodies (TPOAbs), or history of neck irradiation or radioactive iodine uptake.1 Other risk factors for eventual thyroid dysfunction include female sex, older age, goiter, and high iodine intake.13
Evidence for treatment varies
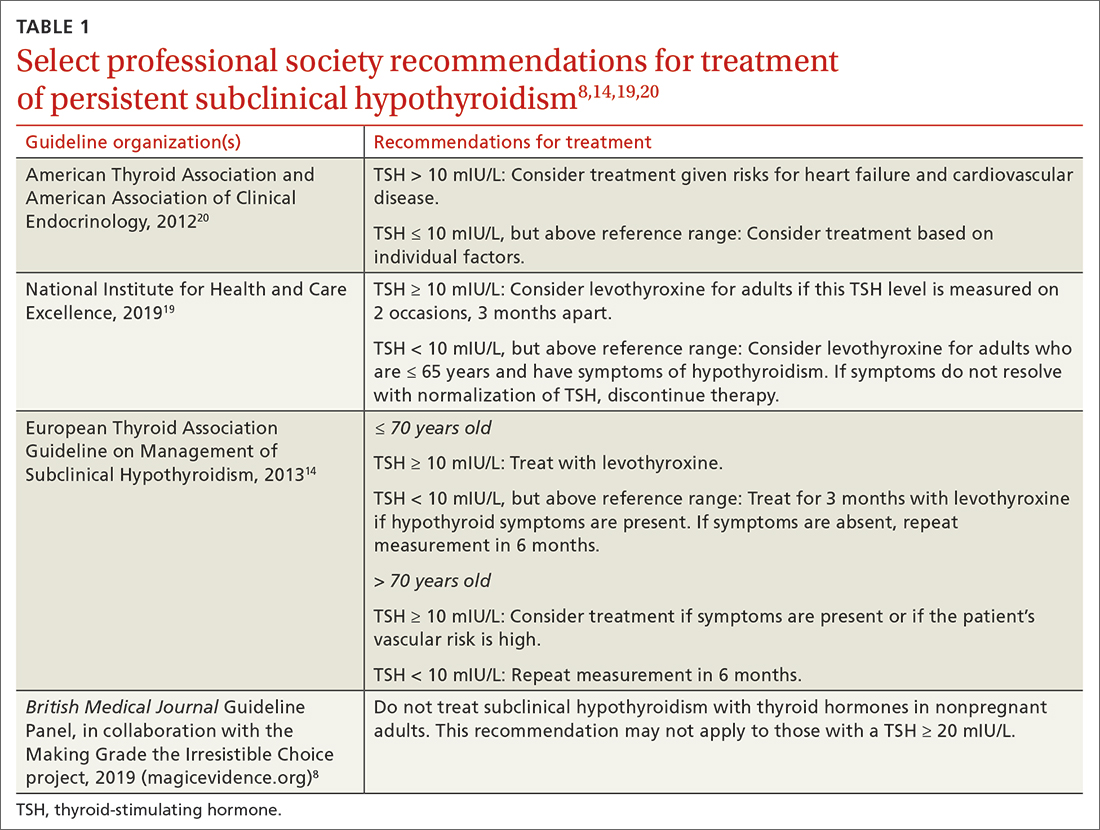
Guidelines for the treatment of SCH (TABLE 18,14,19,20) are founded on the condition’s risk for progression to overt hypothyroidism and its association with health consequences such as cardiovascular disease. Guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA), and those of the United Kingdom–based National Institute for Health and Care Excellence (NICE), prioritize treatment for individuals with a TSH level > 10 mIU/La and for those with
There are few large RCTs of treatment outcomes for SCH. A 2017 RCT (the Thyroid Hormone Replacement for Untreated Older Adults with Subclinical Hypothyroidism, or TRUST, trial) of 737 adults older than 65 years with SCH evaluated the ability of levothyroxine to normalize TSH values compared with placebo. At 1 year, there was no difference in hypothyroid symptoms or tiredness scale scores with levothyroxine treatment compared with placebo.21 This finding was consistent even in the subgroup with a higher baseline symptom burden.22
Continue to: Two small RCTs evaluated...
Two small RCTs evaluated treatment of SCH with depressive symptoms and cognitive function, neither finding benefit compared with placebo.12,23 A 2018 systematic review and meta-analysis of 21 studies and 2192 adults did not show a benefit to quality of life or thyroid-specific symptoms in those treated for SCH compared with controls.24
RCT support also is lacking for a reduction in cardiovascular mortality following treatment for SCH. A large population-level retrospective cohort from Denmark showed no difference in cardiovascular mortality or myocardial infarction in those treated for SCH compared with controls.25 Pooled results from 2 RCTs (for patients older than 65 years, and those older than 80 years) showed no change in risk for cardiovascular outcomes in older adults treated for SCH.26 Older adults treated for SCH in the TRUST trial showed no improvements in systolic or diastolic function on echocardiography.27 Two trials showed no difference in carotid intima-media thickness with treatment of SCH compared with placebo.28,29
While most of the RCT data come from older adults, a retrospective cohort study in the United Kingdom of younger (ages 40-70 years; n = 3093) and older (age > 70 years; n = 1642) patients showed a reduction in cardiovascular mortality among treated patients who were younger (hazard ratio [HR] = 0.61; 4.2% vs. 6.6%) but not those who were older (HR = 0.99; 12.7% vs. 10.7%).30 There is also evidence that thyroid size in those with goiter can be reduced with treatment of SCH.31
A measured approach to treating subclinical hypothyroidism
Consider several factors when deciding whether to treat SCH. For instance, RCT data suggest a lack of treatment benefit in relieving depression, improving cognition, or reducing general hypothyroid symptoms. Treatment of SCH in older adults does not appear to improve cardiovascular outcomes. The question of whether long-term treatment of SCH in younger patients reduces cardiovascular morbidity or mortality lacks answers from RCTs. Before diagnosing SCH or starting treatment, always confirm SCH with repeat testing in 2 to 3 months, as a high percentage of those with untreated SCH will have normal thyroid function on repeat testing.
In the event you and your patient elect to treat SCH, guidelines and trials generally support a low initial daily dose of 25 to 50 mcg of levothyroxine (T4), followed with dose changes every 4 to 8 weeks and a goal of normalizing TSH to within the lower half of the reference range (0.4-2.5 mIU/L).14 This is generally similar to published treatment goals for primary hypothyroidism and is based on studies suggesting the lower half of the reference range is normal for young, healthy, euthyroid individuals.32 Though full replacement doses (1.6-1.8 mcg/kg of ideal body weight) can be started for those who are elderly or who have ischemic heart disease or angina, this approach should be avoided in favor of low-dose initial therapy.33 Thyroid supplements are best absorbed when taken apart from food, calcium, or iron supplements. The ATA suggests taking thyroid medication 60 minutes before breakfast or at bedtime (3 or more hours after the evening meal).33
Continue to: Screening guidelines differ
Screening guidelines differ
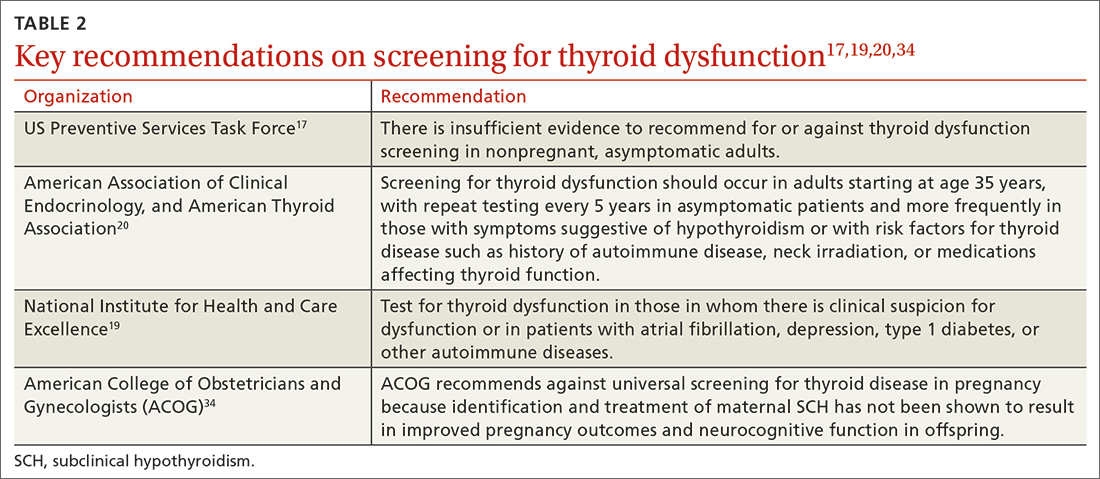
Lacking population-level screening data from RCTs, most organizations do not recommend screening for thyroid dysfunction or they note insufficient evidence to make a screening recommendation (TABLE 217,19,20,34). In their most recent recommendation statement on the subject in 2015, the US Preventive Services Task Force (USPSTF) concluded the current evidence was insufficient to recommend for or against thyroid dysfunction screening in nonpregnant, asymptomatic adults.17 This differs from the ATA and the American Association of Clinical Endocrinology (AACE; formerly known as the American Association of Clinical Endocrinologists), which both recommend targeted screening for thyroid dysfunction based on symptoms or risk factors.20
What about subclinical hypothyroidism in pregnancy?
Overt hypothyroidism is associated with adverse events during pregnancy and with subsequent neurodevelopmental complications in children, although the effects of SCH during pregnancy remain less certain. Concerns have been raised over the potential association of SCH with pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death.35 Historically, the prevalence of SCH during pregnancy has ranged from 2% to 2.5%, but using lower trimester-based TSH reference ranges, the prevalence of SCH in pregnancy may be as high as 15%.35
Guided by a large RCT that failed to find benefit (pregnancy outcomes, neurodevelopmental outcomes in children) following treatment of SCH in pregnancy,36 the American College of Obstetricians and Gynecologists (ACOG) recommends against routine screening for thyroid disease in pregnancy.34 The ATA notes insufficient evidence to rec-ommend universal screening for thyroid dysfunction in pregnancy but recommends targeted screening of those with risk factors.37 Data are conflicting on the benefit of treating known or recently detected SCH on pregnancy outcomes including pregnancy loss.35,38 As such, the American Society of Reproductive Medicine and the ATA both generally recommend treatment of SCH in pregnant patients, particularly when the TSH is ≥ 4.0 mIU/L and TPOAbs are present.37,39
a The ATA, ETA, and NICE have slightly different recommendations when a TSH level = 10 mIU/L. ETA and NICE recommend prioritizing treatment for individuals with this level, while ATA recommends treatment when individual factors are also considered.
ACKNOWLEDGEMENT
The authors thank Family Medicine Medical Librarian Gwen Wilson, MLS, AHIP, for her assistance with literature searches.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8:260. doi: 10.1186/s13643-019-1181-7
2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. doi: 10.1016/S0140-6736(11)60276-6
3. Bauer BS, Azcoaga-Lorenzo A, Agrawal U, et al. Management strategies for patients with subclinical hypothyroidism: a protocol for an umbrella review. Syst Rev. 2021;10:290. doi: 10.1186/s13643-021-01842-y
4. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
5. Carlé A, Karmisholt JS, Knudsen N, et al. Does subclinical hypothyroidism add any symptoms? Evidence from a Danish population-based study. Am J Med. 2021;134:1115-1126.e1. doi: 10.1016/j.amjmed.2021.03.009
6. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040-1049. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. doi: 10.1001/jama.2010.1361
8. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006
9. Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. doi: 10.1016/j.jhep.2012.02.027
10. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. doi: 10.1016/j.cgh.2017.08.014
11. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab. 2018;103:1827-1833. doi: 10.1210/jc.2017-01247
12. Jorde R, Waterloo K, Storhaug H, et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145-53. doi: 10.1210/jc.2005-1775
13. Azim S, Nasr C. Subclinical hypothyroidism: when to treat. Cleve Clin J Med. 2019;86:101-110. doi: 10.3949/ccjm.86a.17053
14. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
15. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322:1961-1962. doi: 10.1001/jama.2019.14728
16. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. 2017;318:1150-1160.
17. LeFevre ML, USPSTF. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
18. Meyerovitch J, Rotman-Pikielni P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533-1538. doi: 10.1001/archinte.167.14.1533
19. NICE. Thyroid Disease: assessment and management (NICE guideline NG145). 2019. Accessed March 14, 2023. www.nice.org.uk/guidance/ng145/resources/thyroid-disease-assessment-and-management-pdf-66141781496773
20. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534-2544. doi: 10.1056/NEJMoa1603825
22. de Montmollin M, Feller M, Beglinger S, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Intern Med. 2020;172:709-716. doi: 10.7326/M19-3193
23. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95:3623-3632. doi: 10.1210/jc.2009-2571
24. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
25. Andersen MN, Schjerning Olsen A-M, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10:e0129793. doi: 10.1371/journal.pone.0129793
26. Zijlstra LE, Jukema JW, Westendorp RG, et al. Levothyroxine treatment and cardiovascular outcomes in older people with subclinical hypothyroidism: pooled individual results of two randomised controlled trials. Front Endocrinol (Lausanne). 2021;12:674841. doi: 10.3389/fendo.2021.674841
27. Gencer B, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020;133:848-856.e5. doi: 10.1016/j.amjmed.2020.01.018
28. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988-2997. doi: 10.1210/jc.2018-00279
29. Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643-659. doi: 10.5551/jat.39917
30. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. doi: 10.1001/archinternmed.2012.1159
31. Romaldini JH, Biancalana MM, Figueiredo DI, et al. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6:183-188. doi: 10.1089/thy.1996.6.183
32. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131. doi: 10.1210/er.2006-0043
33. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
34. ACOG. Thyroid disease in pregnancy: ACOG practice bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
35. Maraka S, Ospina NM, O’Keeffe ET, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590. doi: 10.1089/thy.2015.0418
36. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825. doi: 10.1056/NEJMoa1606205
37. Alexander EK, Pearce EN, Brent FA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. doi: 10.1089/thy.2016.0457
38. Dong AC, Morgan J, Kane M, et al. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113:587-600.e1. doi: 10.1016/j.fertnstert.2019.11.003
39. Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545-553. doi: 10.1016/j.fertnstert.2015.05.028
Subclinical hypothyroidism (SCH) is a biochemical state in which the thyroid-stimulating hormone (TSH) is elevated while the free thyroxine (T4) level is normal. Overt hypothyroidism is not diagnosed until the free T4 level is decreased, regardless of the degree of TSH elevation.
The overall prevalence of SCH in iodine-rich areas is 4% to 10%, with a risk for progression to overt hypothyroidism of between 2% and 6% annually.1 The prevalence of SCH varies depending on the TSH reference range used.1 The normal reference range for TSH varies depending on the laboratory and/or the reference population surveyed, with the range likely widening with increasing age.
SCH is most common among women, the elderly, and White individuals.2 The discovery of SCH is often incidental, given that usually it is detected by laboratory findings alone without associated symptoms of overt hypothyroidism.3
The not-so-significant role of symptoms in subclinical hypothyroidism
Symptoms associated with overt hypothyroidism include constipation, dry skin, fatigue, slow thinking, poor memory, muscle cramps, weakness, and cold intolerance. In SCH, these symptoms are inconsistent, with around 1 in 3 patients having no symptoms
One study reported that roughly 18% of euthyroid individuals, 22% of SCH patients, and 26% of those with overt hypothyroidism reported 4 or more symptoms classically thought to be related to hypothyroidism.4 A large Danish cohort study found that hypothyroid symptoms were no more common in patients with SCH than in euthyroid individuals in the general population.5 These studies question the validity of attributing symptoms to SCH.
Adverse health associations
Observational data suggest that SCH is associated with an increased risk for dyslipidemia, coronary heart disease, heart failure, and cardiovascular mortality, particularly in those with TSH levels ≥ 10 mIU/L.6,7 Such associations were not found for most adults with TSH levels between 5 and 10 mIU/L.8 There are also potential associations of SCH with obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.9,10 Despite thyroid studies being commonly ordered as part of a mental health evaluation, SCH has not been statistically associated with depressive symptoms.11,12
Caveats with laboratory testing
There are several issues to consider when performing a laboratory assessment of thyroid function. TSH levels fluctuate considerably during the day, as TSH secretion has a circadian rhythm. TSH values are 50% higher at night and in the early morning than during the rest of the day.13 TSH values also may rise in response to current illness or stress. Due to this biologic variability, repeat testing to confirm TSH levels is recommended if an initial test result is abnormal.14
Continue to: An exact reference range...
An exact reference range for TSH is not widely agreed upon—although most laboratories regard 4.0 to 5.0 mIU/L as the high-end cutoff for normal. Also, “normal” TSH levels appear to differ by age. Accordingly, some experts have recommended an age-based reference range for TSH levels,15 although this is not implemented widely by laboratories. A TSH level of 6.0 mIU/L (or even higher) may be more appropriate for adults older than 65 years.1
Biotin supplementation has been shown to cause spurious thyroid testing results (TSH, T3, T4) depending on the type of assay used. Therefore, supplements containing biotin should be withheld for several days before assessing thyroid function.16Patients with SCH are often categorized as having TSH levels between 4.5 and 10 mIU/L (around 90% of patients) or levels ≥ 10 mIU/L.8,17 If followed for 5 years, approximately 60% of patients with SCH and TSH levels between 4 and 10 mIU/L will normalize without intervention.18 Normalization is less common in patients with a TSH level greater than 10 mIU/L.18
The risk for progression to overt hypothyroidism also appears to be higher for those with certain risk factors. These include higher baseline TSH levels, presence of thyroid peroxidase antibodies (TPOAbs), or history of neck irradiation or radioactive iodine uptake.1 Other risk factors for eventual thyroid dysfunction include female sex, older age, goiter, and high iodine intake.13
Evidence for treatment varies
Guidelines for the treatment of SCH (TABLE 18,14,19,20) are founded on the condition’s risk for progression to overt hypothyroidism and its association with health consequences such as cardiovascular disease. Guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA), and those of the United Kingdom–based National Institute for Health and Care Excellence (NICE), prioritize treatment for individuals with a TSH level > 10 mIU/La and for those with

There are few large RCTs of treatment outcomes for SCH. A 2017 RCT (the Thyroid Hormone Replacement for Untreated Older Adults with Subclinical Hypothyroidism, or TRUST, trial) of 737 adults older than 65 years with SCH evaluated the ability of levothyroxine to normalize TSH values compared with placebo. At 1 year, there was no difference in hypothyroid symptoms or tiredness scale scores with levothyroxine treatment compared with placebo.21 This finding was consistent even in the subgroup with a higher baseline symptom burden.22
Continue to: Two small RCTs evaluated...
Two small RCTs evaluated treatment of SCH with depressive symptoms and cognitive function, neither finding benefit compared with placebo.12,23 A 2018 systematic review and meta-analysis of 21 studies and 2192 adults did not show a benefit to quality of life or thyroid-specific symptoms in those treated for SCH compared with controls.24
RCT support also is lacking for a reduction in cardiovascular mortality following treatment for SCH. A large population-level retrospective cohort from Denmark showed no difference in cardiovascular mortality or myocardial infarction in those treated for SCH compared with controls.25 Pooled results from 2 RCTs (for patients older than 65 years, and those older than 80 years) showed no change in risk for cardiovascular outcomes in older adults treated for SCH.26 Older adults treated for SCH in the TRUST trial showed no improvements in systolic or diastolic function on echocardiography.27 Two trials showed no difference in carotid intima-media thickness with treatment of SCH compared with placebo.28,29
While most of the RCT data come from older adults, a retrospective cohort study in the United Kingdom of younger (ages 40-70 years; n = 3093) and older (age > 70 years; n = 1642) patients showed a reduction in cardiovascular mortality among treated patients who were younger (hazard ratio [HR] = 0.61; 4.2% vs. 6.6%) but not those who were older (HR = 0.99; 12.7% vs. 10.7%).30 There is also evidence that thyroid size in those with goiter can be reduced with treatment of SCH.31
A measured approach to treating subclinical hypothyroidism
Consider several factors when deciding whether to treat SCH. For instance, RCT data suggest a lack of treatment benefit in relieving depression, improving cognition, or reducing general hypothyroid symptoms. Treatment of SCH in older adults does not appear to improve cardiovascular outcomes. The question of whether long-term treatment of SCH in younger patients reduces cardiovascular morbidity or mortality lacks answers from RCTs. Before diagnosing SCH or starting treatment, always confirm SCH with repeat testing in 2 to 3 months, as a high percentage of those with untreated SCH will have normal thyroid function on repeat testing.
In the event you and your patient elect to treat SCH, guidelines and trials generally support a low initial daily dose of 25 to 50 mcg of levothyroxine (T4), followed with dose changes every 4 to 8 weeks and a goal of normalizing TSH to within the lower half of the reference range (0.4-2.5 mIU/L).14 This is generally similar to published treatment goals for primary hypothyroidism and is based on studies suggesting the lower half of the reference range is normal for young, healthy, euthyroid individuals.32 Though full replacement doses (1.6-1.8 mcg/kg of ideal body weight) can be started for those who are elderly or who have ischemic heart disease or angina, this approach should be avoided in favor of low-dose initial therapy.33 Thyroid supplements are best absorbed when taken apart from food, calcium, or iron supplements. The ATA suggests taking thyroid medication 60 minutes before breakfast or at bedtime (3 or more hours after the evening meal).33
Continue to: Screening guidelines differ
Screening guidelines differ
Lacking population-level screening data from RCTs, most organizations do not recommend screening for thyroid dysfunction or they note insufficient evidence to make a screening recommendation (TABLE 217,19,20,34). In their most recent recommendation statement on the subject in 2015, the US Preventive Services Task Force (USPSTF) concluded the current evidence was insufficient to recommend for or against thyroid dysfunction screening in nonpregnant, asymptomatic adults.17 This differs from the ATA and the American Association of Clinical Endocrinology (AACE; formerly known as the American Association of Clinical Endocrinologists), which both recommend targeted screening for thyroid dysfunction based on symptoms or risk factors.20

What about subclinical hypothyroidism in pregnancy?
Overt hypothyroidism is associated with adverse events during pregnancy and with subsequent neurodevelopmental complications in children, although the effects of SCH during pregnancy remain less certain. Concerns have been raised over the potential association of SCH with pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death.35 Historically, the prevalence of SCH during pregnancy has ranged from 2% to 2.5%, but using lower trimester-based TSH reference ranges, the prevalence of SCH in pregnancy may be as high as 15%.35
Guided by a large RCT that failed to find benefit (pregnancy outcomes, neurodevelopmental outcomes in children) following treatment of SCH in pregnancy,36 the American College of Obstetricians and Gynecologists (ACOG) recommends against routine screening for thyroid disease in pregnancy.34 The ATA notes insufficient evidence to rec-ommend universal screening for thyroid dysfunction in pregnancy but recommends targeted screening of those with risk factors.37 Data are conflicting on the benefit of treating known or recently detected SCH on pregnancy outcomes including pregnancy loss.35,38 As such, the American Society of Reproductive Medicine and the ATA both generally recommend treatment of SCH in pregnant patients, particularly when the TSH is ≥ 4.0 mIU/L and TPOAbs are present.37,39
a The ATA, ETA, and NICE have slightly different recommendations when a TSH level = 10 mIU/L. ETA and NICE recommend prioritizing treatment for individuals with this level, while ATA recommends treatment when individual factors are also considered.
ACKNOWLEDGEMENT
The authors thank Family Medicine Medical Librarian Gwen Wilson, MLS, AHIP, for her assistance with literature searches.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
Subclinical hypothyroidism (SCH) is a biochemical state in which the thyroid-stimulating hormone (TSH) is elevated while the free thyroxine (T4) level is normal. Overt hypothyroidism is not diagnosed until the free T4 level is decreased, regardless of the degree of TSH elevation.
The overall prevalence of SCH in iodine-rich areas is 4% to 10%, with a risk for progression to overt hypothyroidism of between 2% and 6% annually.1 The prevalence of SCH varies depending on the TSH reference range used.1 The normal reference range for TSH varies depending on the laboratory and/or the reference population surveyed, with the range likely widening with increasing age.
SCH is most common among women, the elderly, and White individuals.2 The discovery of SCH is often incidental, given that usually it is detected by laboratory findings alone without associated symptoms of overt hypothyroidism.3
The not-so-significant role of symptoms in subclinical hypothyroidism
Symptoms associated with overt hypothyroidism include constipation, dry skin, fatigue, slow thinking, poor memory, muscle cramps, weakness, and cold intolerance. In SCH, these symptoms are inconsistent, with around 1 in 3 patients having no symptoms
One study reported that roughly 18% of euthyroid individuals, 22% of SCH patients, and 26% of those with overt hypothyroidism reported 4 or more symptoms classically thought to be related to hypothyroidism.4 A large Danish cohort study found that hypothyroid symptoms were no more common in patients with SCH than in euthyroid individuals in the general population.5 These studies question the validity of attributing symptoms to SCH.
Adverse health associations
Observational data suggest that SCH is associated with an increased risk for dyslipidemia, coronary heart disease, heart failure, and cardiovascular mortality, particularly in those with TSH levels ≥ 10 mIU/L.6,7 Such associations were not found for most adults with TSH levels between 5 and 10 mIU/L.8 There are also potential associations of SCH with obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.9,10 Despite thyroid studies being commonly ordered as part of a mental health evaluation, SCH has not been statistically associated with depressive symptoms.11,12
Caveats with laboratory testing
There are several issues to consider when performing a laboratory assessment of thyroid function. TSH levels fluctuate considerably during the day, as TSH secretion has a circadian rhythm. TSH values are 50% higher at night and in the early morning than during the rest of the day.13 TSH values also may rise in response to current illness or stress. Due to this biologic variability, repeat testing to confirm TSH levels is recommended if an initial test result is abnormal.14
Continue to: An exact reference range...
An exact reference range for TSH is not widely agreed upon—although most laboratories regard 4.0 to 5.0 mIU/L as the high-end cutoff for normal. Also, “normal” TSH levels appear to differ by age. Accordingly, some experts have recommended an age-based reference range for TSH levels,15 although this is not implemented widely by laboratories. A TSH level of 6.0 mIU/L (or even higher) may be more appropriate for adults older than 65 years.1
Biotin supplementation has been shown to cause spurious thyroid testing results (TSH, T3, T4) depending on the type of assay used. Therefore, supplements containing biotin should be withheld for several days before assessing thyroid function.16Patients with SCH are often categorized as having TSH levels between 4.5 and 10 mIU/L (around 90% of patients) or levels ≥ 10 mIU/L.8,17 If followed for 5 years, approximately 60% of patients with SCH and TSH levels between 4 and 10 mIU/L will normalize without intervention.18 Normalization is less common in patients with a TSH level greater than 10 mIU/L.18
The risk for progression to overt hypothyroidism also appears to be higher for those with certain risk factors. These include higher baseline TSH levels, presence of thyroid peroxidase antibodies (TPOAbs), or history of neck irradiation or radioactive iodine uptake.1 Other risk factors for eventual thyroid dysfunction include female sex, older age, goiter, and high iodine intake.13
Evidence for treatment varies
Guidelines for the treatment of SCH (TABLE 18,14,19,20) are founded on the condition’s risk for progression to overt hypothyroidism and its association with health consequences such as cardiovascular disease. Guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA), and those of the United Kingdom–based National Institute for Health and Care Excellence (NICE), prioritize treatment for individuals with a TSH level > 10 mIU/La and for those with

There are few large RCTs of treatment outcomes for SCH. A 2017 RCT (the Thyroid Hormone Replacement for Untreated Older Adults with Subclinical Hypothyroidism, or TRUST, trial) of 737 adults older than 65 years with SCH evaluated the ability of levothyroxine to normalize TSH values compared with placebo. At 1 year, there was no difference in hypothyroid symptoms or tiredness scale scores with levothyroxine treatment compared with placebo.21 This finding was consistent even in the subgroup with a higher baseline symptom burden.22
Continue to: Two small RCTs evaluated...
Two small RCTs evaluated treatment of SCH with depressive symptoms and cognitive function, neither finding benefit compared with placebo.12,23 A 2018 systematic review and meta-analysis of 21 studies and 2192 adults did not show a benefit to quality of life or thyroid-specific symptoms in those treated for SCH compared with controls.24
RCT support also is lacking for a reduction in cardiovascular mortality following treatment for SCH. A large population-level retrospective cohort from Denmark showed no difference in cardiovascular mortality or myocardial infarction in those treated for SCH compared with controls.25 Pooled results from 2 RCTs (for patients older than 65 years, and those older than 80 years) showed no change in risk for cardiovascular outcomes in older adults treated for SCH.26 Older adults treated for SCH in the TRUST trial showed no improvements in systolic or diastolic function on echocardiography.27 Two trials showed no difference in carotid intima-media thickness with treatment of SCH compared with placebo.28,29
While most of the RCT data come from older adults, a retrospective cohort study in the United Kingdom of younger (ages 40-70 years; n = 3093) and older (age > 70 years; n = 1642) patients showed a reduction in cardiovascular mortality among treated patients who were younger (hazard ratio [HR] = 0.61; 4.2% vs. 6.6%) but not those who were older (HR = 0.99; 12.7% vs. 10.7%).30 There is also evidence that thyroid size in those with goiter can be reduced with treatment of SCH.31
A measured approach to treating subclinical hypothyroidism
Consider several factors when deciding whether to treat SCH. For instance, RCT data suggest a lack of treatment benefit in relieving depression, improving cognition, or reducing general hypothyroid symptoms. Treatment of SCH in older adults does not appear to improve cardiovascular outcomes. The question of whether long-term treatment of SCH in younger patients reduces cardiovascular morbidity or mortality lacks answers from RCTs. Before diagnosing SCH or starting treatment, always confirm SCH with repeat testing in 2 to 3 months, as a high percentage of those with untreated SCH will have normal thyroid function on repeat testing.
In the event you and your patient elect to treat SCH, guidelines and trials generally support a low initial daily dose of 25 to 50 mcg of levothyroxine (T4), followed with dose changes every 4 to 8 weeks and a goal of normalizing TSH to within the lower half of the reference range (0.4-2.5 mIU/L).14 This is generally similar to published treatment goals for primary hypothyroidism and is based on studies suggesting the lower half of the reference range is normal for young, healthy, euthyroid individuals.32 Though full replacement doses (1.6-1.8 mcg/kg of ideal body weight) can be started for those who are elderly or who have ischemic heart disease or angina, this approach should be avoided in favor of low-dose initial therapy.33 Thyroid supplements are best absorbed when taken apart from food, calcium, or iron supplements. The ATA suggests taking thyroid medication 60 minutes before breakfast or at bedtime (3 or more hours after the evening meal).33
Continue to: Screening guidelines differ
Screening guidelines differ
Lacking population-level screening data from RCTs, most organizations do not recommend screening for thyroid dysfunction or they note insufficient evidence to make a screening recommendation (TABLE 217,19,20,34). In their most recent recommendation statement on the subject in 2015, the US Preventive Services Task Force (USPSTF) concluded the current evidence was insufficient to recommend for or against thyroid dysfunction screening in nonpregnant, asymptomatic adults.17 This differs from the ATA and the American Association of Clinical Endocrinology (AACE; formerly known as the American Association of Clinical Endocrinologists), which both recommend targeted screening for thyroid dysfunction based on symptoms or risk factors.20

What about subclinical hypothyroidism in pregnancy?
Overt hypothyroidism is associated with adverse events during pregnancy and with subsequent neurodevelopmental complications in children, although the effects of SCH during pregnancy remain less certain. Concerns have been raised over the potential association of SCH with pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death.35 Historically, the prevalence of SCH during pregnancy has ranged from 2% to 2.5%, but using lower trimester-based TSH reference ranges, the prevalence of SCH in pregnancy may be as high as 15%.35
Guided by a large RCT that failed to find benefit (pregnancy outcomes, neurodevelopmental outcomes in children) following treatment of SCH in pregnancy,36 the American College of Obstetricians and Gynecologists (ACOG) recommends against routine screening for thyroid disease in pregnancy.34 The ATA notes insufficient evidence to rec-ommend universal screening for thyroid dysfunction in pregnancy but recommends targeted screening of those with risk factors.37 Data are conflicting on the benefit of treating known or recently detected SCH on pregnancy outcomes including pregnancy loss.35,38 As such, the American Society of Reproductive Medicine and the ATA both generally recommend treatment of SCH in pregnant patients, particularly when the TSH is ≥ 4.0 mIU/L and TPOAbs are present.37,39
a The ATA, ETA, and NICE have slightly different recommendations when a TSH level = 10 mIU/L. ETA and NICE recommend prioritizing treatment for individuals with this level, while ATA recommends treatment when individual factors are also considered.
ACKNOWLEDGEMENT
The authors thank Family Medicine Medical Librarian Gwen Wilson, MLS, AHIP, for her assistance with literature searches.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8:260. doi: 10.1186/s13643-019-1181-7
2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. doi: 10.1016/S0140-6736(11)60276-6
3. Bauer BS, Azcoaga-Lorenzo A, Agrawal U, et al. Management strategies for patients with subclinical hypothyroidism: a protocol for an umbrella review. Syst Rev. 2021;10:290. doi: 10.1186/s13643-021-01842-y
4. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
5. Carlé A, Karmisholt JS, Knudsen N, et al. Does subclinical hypothyroidism add any symptoms? Evidence from a Danish population-based study. Am J Med. 2021;134:1115-1126.e1. doi: 10.1016/j.amjmed.2021.03.009
6. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040-1049. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. doi: 10.1001/jama.2010.1361
8. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006
9. Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. doi: 10.1016/j.jhep.2012.02.027
10. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. doi: 10.1016/j.cgh.2017.08.014
11. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab. 2018;103:1827-1833. doi: 10.1210/jc.2017-01247
12. Jorde R, Waterloo K, Storhaug H, et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145-53. doi: 10.1210/jc.2005-1775
13. Azim S, Nasr C. Subclinical hypothyroidism: when to treat. Cleve Clin J Med. 2019;86:101-110. doi: 10.3949/ccjm.86a.17053
14. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
15. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322:1961-1962. doi: 10.1001/jama.2019.14728
16. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. 2017;318:1150-1160.
17. LeFevre ML, USPSTF. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
18. Meyerovitch J, Rotman-Pikielni P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533-1538. doi: 10.1001/archinte.167.14.1533
19. NICE. Thyroid Disease: assessment and management (NICE guideline NG145). 2019. Accessed March 14, 2023. www.nice.org.uk/guidance/ng145/resources/thyroid-disease-assessment-and-management-pdf-66141781496773
20. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534-2544. doi: 10.1056/NEJMoa1603825
22. de Montmollin M, Feller M, Beglinger S, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Intern Med. 2020;172:709-716. doi: 10.7326/M19-3193
23. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95:3623-3632. doi: 10.1210/jc.2009-2571
24. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
25. Andersen MN, Schjerning Olsen A-M, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10:e0129793. doi: 10.1371/journal.pone.0129793
26. Zijlstra LE, Jukema JW, Westendorp RG, et al. Levothyroxine treatment and cardiovascular outcomes in older people with subclinical hypothyroidism: pooled individual results of two randomised controlled trials. Front Endocrinol (Lausanne). 2021;12:674841. doi: 10.3389/fendo.2021.674841
27. Gencer B, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020;133:848-856.e5. doi: 10.1016/j.amjmed.2020.01.018
28. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988-2997. doi: 10.1210/jc.2018-00279
29. Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643-659. doi: 10.5551/jat.39917
30. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. doi: 10.1001/archinternmed.2012.1159
31. Romaldini JH, Biancalana MM, Figueiredo DI, et al. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6:183-188. doi: 10.1089/thy.1996.6.183
32. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131. doi: 10.1210/er.2006-0043
33. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
34. ACOG. Thyroid disease in pregnancy: ACOG practice bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
35. Maraka S, Ospina NM, O’Keeffe ET, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590. doi: 10.1089/thy.2015.0418
36. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825. doi: 10.1056/NEJMoa1606205
37. Alexander EK, Pearce EN, Brent FA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. doi: 10.1089/thy.2016.0457
38. Dong AC, Morgan J, Kane M, et al. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113:587-600.e1. doi: 10.1016/j.fertnstert.2019.11.003
39. Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545-553. doi: 10.1016/j.fertnstert.2015.05.028
1. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8:260. doi: 10.1186/s13643-019-1181-7
2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. doi: 10.1016/S0140-6736(11)60276-6
3. Bauer BS, Azcoaga-Lorenzo A, Agrawal U, et al. Management strategies for patients with subclinical hypothyroidism: a protocol for an umbrella review. Syst Rev. 2021;10:290. doi: 10.1186/s13643-021-01842-y
4. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
5. Carlé A, Karmisholt JS, Knudsen N, et al. Does subclinical hypothyroidism add any symptoms? Evidence from a Danish population-based study. Am J Med. 2021;134:1115-1126.e1. doi: 10.1016/j.amjmed.2021.03.009
6. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040-1049. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. doi: 10.1001/jama.2010.1361
8. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006
9. Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. doi: 10.1016/j.jhep.2012.02.027
10. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. doi: 10.1016/j.cgh.2017.08.014
11. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab. 2018;103:1827-1833. doi: 10.1210/jc.2017-01247
12. Jorde R, Waterloo K, Storhaug H, et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145-53. doi: 10.1210/jc.2005-1775
13. Azim S, Nasr C. Subclinical hypothyroidism: when to treat. Cleve Clin J Med. 2019;86:101-110. doi: 10.3949/ccjm.86a.17053
14. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
15. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322:1961-1962. doi: 10.1001/jama.2019.14728
16. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. 2017;318:1150-1160.
17. LeFevre ML, USPSTF. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
18. Meyerovitch J, Rotman-Pikielni P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533-1538. doi: 10.1001/archinte.167.14.1533
19. NICE. Thyroid Disease: assessment and management (NICE guideline NG145). 2019. Accessed March 14, 2023. www.nice.org.uk/guidance/ng145/resources/thyroid-disease-assessment-and-management-pdf-66141781496773
20. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534-2544. doi: 10.1056/NEJMoa1603825
22. de Montmollin M, Feller M, Beglinger S, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Intern Med. 2020;172:709-716. doi: 10.7326/M19-3193
23. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95:3623-3632. doi: 10.1210/jc.2009-2571
24. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
25. Andersen MN, Schjerning Olsen A-M, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10:e0129793. doi: 10.1371/journal.pone.0129793
26. Zijlstra LE, Jukema JW, Westendorp RG, et al. Levothyroxine treatment and cardiovascular outcomes in older people with subclinical hypothyroidism: pooled individual results of two randomised controlled trials. Front Endocrinol (Lausanne). 2021;12:674841. doi: 10.3389/fendo.2021.674841
27. Gencer B, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020;133:848-856.e5. doi: 10.1016/j.amjmed.2020.01.018
28. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988-2997. doi: 10.1210/jc.2018-00279
29. Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643-659. doi: 10.5551/jat.39917
30. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. doi: 10.1001/archinternmed.2012.1159
31. Romaldini JH, Biancalana MM, Figueiredo DI, et al. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6:183-188. doi: 10.1089/thy.1996.6.183
32. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131. doi: 10.1210/er.2006-0043
33. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
34. ACOG. Thyroid disease in pregnancy: ACOG practice bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
35. Maraka S, Ospina NM, O’Keeffe ET, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590. doi: 10.1089/thy.2015.0418
36. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825. doi: 10.1056/NEJMoa1606205
37. Alexander EK, Pearce EN, Brent FA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. doi: 10.1089/thy.2016.0457
38. Dong AC, Morgan J, Kane M, et al. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113:587-600.e1. doi: 10.1016/j.fertnstert.2019.11.003
39. Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545-553. doi: 10.1016/j.fertnstert.2015.05.028
PRACTICE RECOMMENDATIONS
› Do not routinely screen for subclinical or overt hypothyroidism in asymptomatic nonpregnant adults. B
› Consider treatment of known or screening-detected subclinical hypothyroidism (SCH) in patients who are pregnant or trying to conceive. C
› Consider treating SCH in younger adults whose thyroidstimulating hormone level is ≥ 10 mIU/L. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Physician wellness: Managing stress and preventing burnout
Meet Dr. A and Dr. M
Dr. A is a 50-year-old family physician who provides prenatal care in a busy practice. She sees patients in eight 4-hour clinic sessions per week and is on inpatient call 1 week out of every 2 months. Dr. A has become disillusioned with her practice. She typically works until 7
Dr. M is a single, 32-year-old family physician working at an academic medical center. Dr. M is unhappy in his job, is trying to grow his practice, and views himself as having little impact or autonomy. He finds himself lost while navigating the electronic health record (EHR) and struggles to be efficient in the clinic. Dr. M has multiple administrative responsibilities that require him to work evenings and weekends. Debt from medical school loans also motivates him to moonlight several weekends per month. Over the past few months, Dr. M has become frustrated and discouraged, making his depression more difficult to manage. He feels drained by the time he arrives home, where he lives alone. He has stopped exercising, socializing with friends, and dating. Dr. M often wonders if he is in the wrong profession.
Defining burnout, stress, and wellness
Dr. A and Dr. M are experiencing symptoms of burnout, common to physicians and other health care professionals. Recent studies showed an increase in burnout during the COVID-19 pandemic.1,2 In a survey using the Maslach Burnout Inventory (MBI), approximately 44% of physicians reported at least one symptom of burnout.3 After adjusting for age, gender, relationship status, and hours worked per week, physicians were found to be at greater risk for burnout than nonphysician workers.3 The latest Medscape physician burnout survey found an increase in burnout among US physicians from 42% in 2021 to 47% in 2022 during the COVID-19 pandemic.1 Rates of burnout were even higher among family physicians and other frontline (eg, emergency, infectious disease, and critical care) physicians.1
Burnout has 3 key dimensions: (1) overwhelming exhaustion; (2) feelings of cynicism and detachment from the job; and (3) a sense of ineffectiveness and lack of accomplishment.4 The MBI is considered the standard tool for research in the field of burnout and has been repeatedly assessed for reliability and validity.4 The original MBI includes such items as: “I feel emotionally drained from my work,” “I feel like I’m working too hard on my job,” and “I worry that this job is hardening me emotionally.”5
According to the World Health Organization, burnout is an occupational phenomenon associated with chronic work-related stress that is not successfully managed.6 This definition emphasizes work stress as the cause of burnout, thus highlighting the importance of addressing the work environment.7 Physician burnout can affect physician health and wellness and the quality of patient care.8-13 Because of the cost of burnout to individuals and the health care system, it is important to understand stressors that can lead to physician burnout.
Stress has been described as “physical, mental, or emotional strain or tension … when a person perceives that demands exceed the personal and social resources the individual is able to mobilize.”14 Work-related sources of stress affecting practicing physicians include long workdays, multiple bureaucratic tasks, lack of autonomy/control, and complex patients.1,15
The COVID-19 pandemic is a stressor that increased physicians’ exposure to patient suffering and deaths and physicians’ vulnerability to disease at work.16 Physicians taking care of patients with COVID-19 risk infection and the possibility of infecting others.Online health records are another source of stress for many physicians.17,18 Access to online health records on personal devices can blur the line between work and home. For each hour of direct patient contact, a physician spends an additional 2 hours interacting with an EHR.19 Among family physicians and other primary care physicians, increased EHR interaction outside clinic hours has been associated with decreased workplace satisfaction and increased rates of burnout.11,19,20 Time spent on non-patient-facing clinical tasks, such as peer-to-peer reviews and billing queries, contributes more to burnout than clinic time alone.17
Continue to: These and other organizational factors...
These and other organizational factors contribute to the stress experienced by physicians. Many describe themselves as feeling consumed by their work. At the beginning of the COVID-19 pandemic, physicians (and the rest of the health care team) had to quickly learn how to conduct virtual office visits. Clerical responsibilities increased as patients relied more on patient portals and telephone calls to receive care.
Who is predisposed to burnout? Although burnout is a work-related syndrome, studies have shown an increase in burnout associated with individual (ie, personal) factors. For example, female physicians have been shown to have higher rates of burnout compared with male physicians.1,3 The stress of balancing the demands of the profession can begin during medical school and residency, with younger physicians having nearly twice the risk for stress-related symptoms when compared with older colleagues.15,20-23 Having a child younger than 21 years old, and other personal factors related to balancing family and life demands, increases the likelihood of burnout.11,21,22
Physicians with certain personality types and predispositions are at increased risk for burnout.23-25 For example, neuroticism on the Big Five Personality Inventory (one of the most well-known of the psychology inventories) is associated with an increased risk for burnout. Neuroticism may manifest as sadness or related emotional dysregulation (eg, irritability, anxiety).26 Other traits measured by the Big Five Personality Inventory include extraversion, agreeableness, conscientiousness, and openness to experience.26
A history of depression is also associated with an increased risk for burnout.27 Although depression and burnout are separate conditions, a 2016 study found significant overlap between the two.27 Physicians in this study who were depressed were more likely to experience burnout symptoms (87.5%); however, only 26.2% of physicians experiencing burnout were diagnosed as having depression.27 Rates of depression are higher among physicians when compared with nonphysicians, yet physicians are less likely to seek help due to fear of stigma and potential licensing concerns.28,29 Because of this, when physicians experience depressive symptoms, they may respond by working harder rather than seeking professional counseling or emotional support. They might believe that “asking for help is a sign of weakness,” thus sacrificing their wellness.
Wellness encompasses a sense of thriving characterized by thoughts and feelings of contentment, joy, and fulfillment—and the absence of severe distress.30 Wellness is a multifaceted condition that includes physical, psychological, and social aspects of an individual’s personal and professional life. Individuals experience a sense of wellness when they nurture their physical selves, minds, and relationships. People experience a sense of wellness when they balance their schedules, eat well, and maintain physical activity. Making time to enjoy family and friends also contributes to wellness.
Continue to: The culture of medicine often rewards...
The culture of medicine often rewards physician attitudes and behaviors that detract from wellness.31 Physicians internalize the culture of medicine that promotes perfectionism and downplays personal vulnerability.32 Physicians are reluctant to protect and preserve their wellness, believing self-sacrifice makes them good doctors. Physicians may spend countless hours counseling patients on the importance of wellness, but then work when ill or neglect their personal health needs and self-care—potentially decreasing their resilience and increasing the risk for burnout.31
Two paths to managing stress and preventing burnout
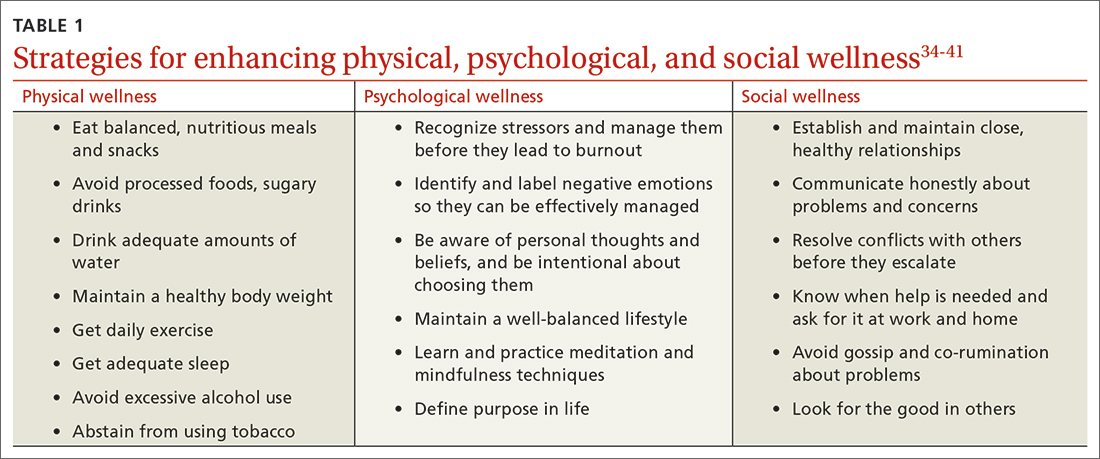
Patel and colleagues distinguish between 2 burnout intervention categories: (1) those that focus on individual physicians and (2) those that focus on the organizational environment.33 We find these distinctions useful and offer strategies for enhancing individual physician wellness (TABLE 134-41). Similar to West and colleagues,11 we offer strategies for addressing organizational sources of stress (TABLE 242-48). The following text describes these burnout intervention categories, emphasizing increasing self-care and changes that enable physicians to adapt effectively.

The recommendations outlined in this article are based on published stress and burnout literature, as well as the experiences of the authors. However, the number of randomized controlled studies of interventions aimed at reducing physician stress and burnout is limited. In addition, strategies proposed to reduce burnout in other professions may not address the unique stressors physicians encounter. Hence, our recommendations are limited. We have included interventions that seem optimal for individual physicians and the organizations that employ them.
Individual strategies target physical, psychological, and social wellness
Physician wellness strategies are divided into 3 categories: physical, psychological, and social wellness. Most strategies to improve physical wellness are widely known, evidence based, and recommended to patients by physicians.34-36 For example, most physicians advise their patients to eat healthy balanced meals, avoid unhealthy foods and beverages, maintain a healthy body weight, get daily exercise and adequate sleep, avoid excessive alcohol use, and abstain from tobacco use. However, discrepancies between physicians’ advice to patients and their own behaviors are common. Simply stated, physicians are well advised to follow their own advice regarding physical self-care.
CBT and mindfulness are key to psychological wellness. Recommendations for enhancing psychological wellness are primarily derived from cognitive behavioral therapy (CBT) and mindfulness principles and practices.37,38 CBT has been called the “gold standard” of psychotherapy, based on the breadth of research demonstrating that “no other form of psychotherapy has been shown to be systematically superior to CBT.”39
Continue to: CBT is based on the premise...
CBT is based on the premise that individuals’ thoughts and beliefs largely determine how they feel (emotions) and act (behaviors). Certain thoughts lead to positive feelings and effective behaviors, while others lead to negative feelings and less effective behaviors. For example, when a physician has self-critical or helpless thoughts (eg, “I’m just no good at managing my life”), they are more likely to feel unhappy and abandon problem-solving. In contrast, when a physician has self-affirming or hopeful thoughts (eg, “This is difficult, but I have the personal resources to succeed”), they are more likely to feel confident and act to solve problems.
Physicians vacillate between these thoughts and beliefs, and their emotions and behaviors follow accordingly. When hyper-focused on “the hassles of medicine,” physicians feel defeated, depressed, and anxious about their work. In contrast, when physicians recognize and challenge problematic thoughts and focus on what they love about medicine, they feel good and interact with patients and coworkers in positive and self-reinforcing ways.
Mindfulness can help reduce psychological stress and increase personal fulfillment. Mindfulness is characterized as being in the present moment, fully accepting “what is,” and having a sense of gratitude and compassion for self and others.40 In practice, mindfulness involves being intentional.
Dahl and colleagues41 describe a framework for human flourishing that includes 4 core dimensions of well-being (awareness, insight, connection, and purpose) that are all closely linked to mindful, intentional living. Based on their work, it is apparent that those who maintain a “heightened and flexible attentiveness” to their thoughts and feelings are likely to benefit by experiencing “improved mental health and psychological well-being.”41
However, the utility of CBT and mindfulness practices depends on receptivity to psychological interventions. Individuals who are not receptive may be hesitant to use these practices or likely will not benefit from them. Given these limitations of behavioral interventions, it would be helpful if more attention were paid to preventing and managing physician stress and burnout, especially through research focused on organizational changes.
Continue to: Supportive relationships are powerful
Supportive relationships are powerful. Finally, to enhance social wellness, it would be difficult to overstate the potential benefits of positive, supportive, close relationships.42 However, the demands of a career in medicine, starting in medical school, have the potential for inhibiting (rather than enhancing) close relationships.
Placing value on relationships with friends and family members is essential. As Dr. M began experiencing burnout, he felt increasingly lonely, yet he isolated himself from those who cared about him. Dr. A felt lonely at home, even though she was surrounded by family. Physicians are often reluctant to initiate vulnerable communication with others, believing “no one wants to hear about my problems.” However, by realizing the need for help and asking friends and family for emotional support, physicians can improve their wellness. Fostering supportive relationships can help provide the resilience needed to address organizational stressors.
Tackling organizational challenges
Long hours and pressure to see large numbers of patients (production demands) are a challenge across practice settings. Limiting work hours has been effective in improving the well-being of physician trainees but has had an inconsistent effect on burnout.43,44
Organizations can offer flexible scheduling, and physicians considering limiting work hours may switch to part-time status or shift work. However, decreasing work hours may have the unintended consequence of increased stress as some physicians feel pressure to do more in less time.45 Therefore, it’s important to set clear boundaries around work time and when and where work tasks are completed (eg, home vs office).
How we use technology matters. Given technology’s ever-increasing role in medicine, organizations must identify and use the most efficient, effective technology for managing clerical processes. When physicians participate in these decisions and share their experiences, technology is likely to be more user-friendly and impose less stress.46
Continue to: If technology contributes to stress...
If technology contributes to stress by being too complex or impractical, it’s important to identify individuals in the workplace (eg, IT support or “super-users”) to help address these challenges. Organizations can implement multidisciplinary teams to address EHR challenges and decrease physician stress and burnout by training support staff to assist with clerical duties, allowing physicians to focus on patient care.47,48 Such organizational-directed interventions will be most successful when physicians are included in the decision-making process.47
Take on leadership roles to influence change. Leadership may be formal (involving a title and authority) or informal (leading by example). Health care organizations that are committed to the well-being of physicians will make the effort to improve the systems in which physicians work. Physicians working in organizations that are reluctant to change have several choices: implement individual strategies, take on leadership roles to influence change, or reconsider their fit for the organization. Physicians in solo practice might consider joining others in solo practices to share systems (call, phone triage, technical resources, etc) to implement some of these interventions.
Dr. A and Dr. M implement new wellness strategies
Dr. A and Dr. M have recently committed to addressing stressors in their lives and improving their wellness. Dr. A has become more assertive at work, highlighting her need for additional resources to function effectively. In response, her practice has hired scribes to assist in documenting visits. This success has inspired Dr. A to pay attention to her lifestyle choices. Gradually, she has begun to exercise and engage in healthy eating.
Dr. M has begun to utilize resources at his medical center to improve his EHR efficiency and patient flow. He has taken steps to address his financial concerns, developing a budget and spending judiciously. He practices mindfulness and ensures that he gets at least 7 hours of sleep per night, improving his mental and physical health. By doing so, he has more energy to connect with friends, exercise, and date.
CORRESPONDENCE
Margaret L. Smith, MD, MPH, MHSA, KUMC, Family Medicine and Community Health, 3901 Rainbow Boulevard – Mailstop 4010, Kansas City, KS 66160; [email protected]
1. Kane L. Physician burnout & depression report: stress, anxiety, and anger. Medscape. January 21, 2022. Accessed February 23, 2023. www.medscape.com/slideshow/2022-lifestyle-burnout-6014664
2. Lockwood L, Patel N, Bukelis I. 45.5 Physician burnout and the COVID-19 pandemic: the silent epidemic. J Am Acad Child Adolesc Psychiatry. 2021;60:S242. doi: 10.1016/j.jaac.2021.09.354
3. Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clin Proc. 2019;94:1681-1694. doi: 10.1016/j.mayocp.2018.10.023
4. Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15:103-111. doi: 10.1002/wps.20311
5. Maslach C, Jackson SE. The measurement of experienced burnout. J Organ Behav. 1981;2:99-113. doi: 10.1002/job.4030020205
6. World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed February 23, 2023. www.who.int/news/item/28-05-2019-burn-out-an-occupational-phenomenon-international-classification-of-diseases
7. Berg S. WHO adds burnout to ICD-11. What it means for physicians. American Medical Association. July 23, 2019. Accessed February 23, 2023. www.ama-assn.org/practice-management/physician-health/who-adds-burnout-icd-11-what-it-means-physicians
8. Brown SD, Goske MJ, Johnson CM. Beyond substance abuse: stress, burnout, and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009;6:479-485. doi: 10.1016/j.jacr.2008.11.029
9. Williams ES, Rathert C, Buttigieg SC. The personal and professional consequences of physician burnout: a systematic review of the literature. Med Care Res Rev. 2020;77:371-386. doi: 10.1177/ 1077558719856787
10. Yates SW. Physician Stress and Burnout. Am J Med. 2020;133:160-164. doi: 10.1016/j.amjmed.2019.08.034
11. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529. doi: 10.1111/joim.12752
12. Firth-Cozens J, Greenhalgh J. Doctors’ perceptions of the links between stress and lowered clinical care. Soc Sci Med. 1997;44:1017-1022. doi: 10.1016/s0277-9536(96)00227-4
13. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: a systematic review. BMJ Open. 2017;7:e015141. doi: 10.1136/bmjopen-2016-015141
14. American Institute of Stress. What is stress? April 29, 2022. Accessed February 23, 2023. www.stress.org/daily-life
15. Regehr C, Glancy D, Pitts A, et al. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202:353-359. doi: 10.1097/NMD. 0000000000000130
16. Fitzpatrick K, Patterson R, Morley K, et al. Physician wellness during a pandemic. West J Emerg Med. 2020;21:83-87. doi: 10.5811/westjem.2020.7.48472
17. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848. doi: 10.1016/j.mayocp.2016.05.007
18. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15:419-426. doi: 10.1370/afm.2121
19. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760. doi: 10.7326/M16-0961
20. Robertson SL, Robinson MD, Reid A. Electronic health record effects on work-life balance and burnout within the I3 Population Collaborative. J Grad Med Educ. 2017;9:479-484. doi: 10.4300/JGME-D-16-00123.1
21. Fares J, Al Tabosh H, Saadeddin Z, et al. Stress, burnout and coping strategies in preclinical medical students. N Am J Med Sci. 2016;8:75-81. doi: 10.4103/1947-2714.177299
22. Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sci (Basel). 2018; 8:98. doi: 10.3390/bs8110098
23. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114:513-519. doi: 10.1016/s0002-9343(03)00117-7
24. Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
25. Brown PA, Slater M, Lofters A. Personality and burnout among primary care physicians: an international study. Psychol Res Behav Manag. 2019;12:169-177. doi: 10.2147/PRBM.S195633.
26. John OP, Donahue EM, Kentle RL. The Big Five Inventory – Versions 4A and 54. Institute of Personality and Social Research, University of California; 1991.
27. Wurm W, Vogel K, Holl A, et al. Depression-burnout overlap in physicians. PLoS One. 2016;11:e0149913. doi: 10.1371/journal.pone.0149913
28. Mehta SS, Edwards ML. Suffering in silence: Mental health stigma and physicians’ licensing fears. Am J Psychiatry Resid J. 2018;13:2-4.
29. Adam AR, Golu FT. Prevalence of depression among physicians: A comprehensive meta-analysis. Ro Med J. 2021;68:327-337. doi: 10.37897/RMJ.2021.3.1
30. Brady KJS, Trockel MT, Khan CT, et al. What do we mean by physician wellness? A systematic review of its definition and measurement. Acad Psychiatry. 2018;42:94-108. doi: 10.1007/s40596-017-0781-6
31. Shanafelt TD, Schein E, Minor LB, et al. Healing the professional culture of medicine. Mayo Clin Proc. 2019;94:1556-1566. doi: 10.1016/j.mayocp.2019.03.026
32. Horan S, Flaxman PE, Stride CB. The perfect recovery? Interactive influence of perfectionism and spillover work tasks on changes in exhaustion and mood around a vacation. J Occup Health Psychol. 2021;26:86-107. doi: 10.1037/ocp0000208
33. Patel RS, Sekhri S, Bhimanadham NN, et al. A review on strategies to manage physician burnout. Cureus. 2019;11:e4805. doi: 10.7759/cureus.4805
34. US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. US Department of Health and Human Services; 2018.
35. Kim ES, Chen Y, Nakamura JS, et al. Sense of purpose in life and subsequent physical, behavioral, and psychosocial health: an outcome-wide approach. Am J Health Promot. 2022;36:137-147. doi: 10.1177/08901171211038545
36. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
37. Fordham B, Sugavanam T, Edwards K, et al. The evidence for cognitive behavioural therapy in any condition, population or context: a meta-review of systematic reviews and panoramic meta-analysis. Psychol Med. 2021;51:21-29. doi: 10.1017/S0033291720005292
38. Goldberg SB, Tucker RP, Greene PA, et al. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin Psychol Rev. 2018;59:52-60. doi: 10.1016/j.cpr.2017.10.011
39. David D, Cristea I, Hofmann SG. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front Psychiatry. 2018;9:4. doi: 10.3389/fpsyt.2018.00004
40. Fendel JC, Bürkle JJ, Göritz AS. Mindfulness-based interventions to reduce burnout and stress in physicians: a systematic review and meta-analysis. Acad Med. 2021;96:751-764. doi: 10.1097/ACM.0000000000003936
41. Dahl CJ, Wilson-Mendenhall CD, Davidson RJ. The plasticity of well-being: a training-based framework for the cultivation of human flourishing. Proc Natl Acad Sci USA. 2020;117:32197-32206. doi: 10.1073/pnas.2014859117
42. Holt-Lunstad J. Why social relationships are important for physical health: a systems approach to understanding and modifying risk and protection. Annu Rev Psychol. 2018;69:437-458. doi: 10.1146/annurev-psych-122216-011902
43. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018; 378:1494-1508. doi: 10.1056/NEJMoa1800965
44. Shea JA, Bellini LM, Dinges DF, et al. Impact of protected sleep period for internal medicine interns on overnight call on depression, burnout, and empathy. J Grad Med Educ. 2014;6:256-263. doi: 10.4300/JGME-D-13-00241.1
45. Morrow G, Burford B, Carter M, et al. Have restricted working hours reduced junior doctors’ experience of fatigue? A focus group and telephone interview study. BMJ Open. 2014;4:e004222. doi: 10.1136/bmjopen-2013-004222
46. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92:129-146. doi: 10.1016/j.mayocp.2016.10.004
47. Sequeira L, Almilaji K, Strudwick G, et al. EHR “SWAT” teams: a physician engagement initiative to improve Electronic Health Record (EHR) experiences and mitigate possible causes of EHR-related burnout. JAMA Open. 2021;4:1-7. doi: 10.1093/jamiaopen/ooab018
48. Smith PC, Lyon C, English AF, et al. Practice transformation under the University of Colorado’s primary care redesign model. Ann Fam Med. 2019;17:S24-S32. doi: 10.1370/afm.2424
Meet Dr. A and Dr. M
Dr. A is a 50-year-old family physician who provides prenatal care in a busy practice. She sees patients in eight 4-hour clinic sessions per week and is on inpatient call 1 week out of every 2 months. Dr. A has become disillusioned with her practice. She typically works until 7
Dr. M is a single, 32-year-old family physician working at an academic medical center. Dr. M is unhappy in his job, is trying to grow his practice, and views himself as having little impact or autonomy. He finds himself lost while navigating the electronic health record (EHR) and struggles to be efficient in the clinic. Dr. M has multiple administrative responsibilities that require him to work evenings and weekends. Debt from medical school loans also motivates him to moonlight several weekends per month. Over the past few months, Dr. M has become frustrated and discouraged, making his depression more difficult to manage. He feels drained by the time he arrives home, where he lives alone. He has stopped exercising, socializing with friends, and dating. Dr. M often wonders if he is in the wrong profession.
Defining burnout, stress, and wellness
Dr. A and Dr. M are experiencing symptoms of burnout, common to physicians and other health care professionals. Recent studies showed an increase in burnout during the COVID-19 pandemic.1,2 In a survey using the Maslach Burnout Inventory (MBI), approximately 44% of physicians reported at least one symptom of burnout.3 After adjusting for age, gender, relationship status, and hours worked per week, physicians were found to be at greater risk for burnout than nonphysician workers.3 The latest Medscape physician burnout survey found an increase in burnout among US physicians from 42% in 2021 to 47% in 2022 during the COVID-19 pandemic.1 Rates of burnout were even higher among family physicians and other frontline (eg, emergency, infectious disease, and critical care) physicians.1
Burnout has 3 key dimensions: (1) overwhelming exhaustion; (2) feelings of cynicism and detachment from the job; and (3) a sense of ineffectiveness and lack of accomplishment.4 The MBI is considered the standard tool for research in the field of burnout and has been repeatedly assessed for reliability and validity.4 The original MBI includes such items as: “I feel emotionally drained from my work,” “I feel like I’m working too hard on my job,” and “I worry that this job is hardening me emotionally.”5
According to the World Health Organization, burnout is an occupational phenomenon associated with chronic work-related stress that is not successfully managed.6 This definition emphasizes work stress as the cause of burnout, thus highlighting the importance of addressing the work environment.7 Physician burnout can affect physician health and wellness and the quality of patient care.8-13 Because of the cost of burnout to individuals and the health care system, it is important to understand stressors that can lead to physician burnout.
Stress has been described as “physical, mental, or emotional strain or tension … when a person perceives that demands exceed the personal and social resources the individual is able to mobilize.”14 Work-related sources of stress affecting practicing physicians include long workdays, multiple bureaucratic tasks, lack of autonomy/control, and complex patients.1,15
The COVID-19 pandemic is a stressor that increased physicians’ exposure to patient suffering and deaths and physicians’ vulnerability to disease at work.16 Physicians taking care of patients with COVID-19 risk infection and the possibility of infecting others.Online health records are another source of stress for many physicians.17,18 Access to online health records on personal devices can blur the line between work and home. For each hour of direct patient contact, a physician spends an additional 2 hours interacting with an EHR.19 Among family physicians and other primary care physicians, increased EHR interaction outside clinic hours has been associated with decreased workplace satisfaction and increased rates of burnout.11,19,20 Time spent on non-patient-facing clinical tasks, such as peer-to-peer reviews and billing queries, contributes more to burnout than clinic time alone.17
Continue to: These and other organizational factors...
These and other organizational factors contribute to the stress experienced by physicians. Many describe themselves as feeling consumed by their work. At the beginning of the COVID-19 pandemic, physicians (and the rest of the health care team) had to quickly learn how to conduct virtual office visits. Clerical responsibilities increased as patients relied more on patient portals and telephone calls to receive care.
Who is predisposed to burnout? Although burnout is a work-related syndrome, studies have shown an increase in burnout associated with individual (ie, personal) factors. For example, female physicians have been shown to have higher rates of burnout compared with male physicians.1,3 The stress of balancing the demands of the profession can begin during medical school and residency, with younger physicians having nearly twice the risk for stress-related symptoms when compared with older colleagues.15,20-23 Having a child younger than 21 years old, and other personal factors related to balancing family and life demands, increases the likelihood of burnout.11,21,22
Physicians with certain personality types and predispositions are at increased risk for burnout.23-25 For example, neuroticism on the Big Five Personality Inventory (one of the most well-known of the psychology inventories) is associated with an increased risk for burnout. Neuroticism may manifest as sadness or related emotional dysregulation (eg, irritability, anxiety).26 Other traits measured by the Big Five Personality Inventory include extraversion, agreeableness, conscientiousness, and openness to experience.26
A history of depression is also associated with an increased risk for burnout.27 Although depression and burnout are separate conditions, a 2016 study found significant overlap between the two.27 Physicians in this study who were depressed were more likely to experience burnout symptoms (87.5%); however, only 26.2% of physicians experiencing burnout were diagnosed as having depression.27 Rates of depression are higher among physicians when compared with nonphysicians, yet physicians are less likely to seek help due to fear of stigma and potential licensing concerns.28,29 Because of this, when physicians experience depressive symptoms, they may respond by working harder rather than seeking professional counseling or emotional support. They might believe that “asking for help is a sign of weakness,” thus sacrificing their wellness.
Wellness encompasses a sense of thriving characterized by thoughts and feelings of contentment, joy, and fulfillment—and the absence of severe distress.30 Wellness is a multifaceted condition that includes physical, psychological, and social aspects of an individual’s personal and professional life. Individuals experience a sense of wellness when they nurture their physical selves, minds, and relationships. People experience a sense of wellness when they balance their schedules, eat well, and maintain physical activity. Making time to enjoy family and friends also contributes to wellness.
Continue to: The culture of medicine often rewards...
The culture of medicine often rewards physician attitudes and behaviors that detract from wellness.31 Physicians internalize the culture of medicine that promotes perfectionism and downplays personal vulnerability.32 Physicians are reluctant to protect and preserve their wellness, believing self-sacrifice makes them good doctors. Physicians may spend countless hours counseling patients on the importance of wellness, but then work when ill or neglect their personal health needs and self-care—potentially decreasing their resilience and increasing the risk for burnout.31

Two paths to managing stress and preventing burnout
Patel and colleagues distinguish between 2 burnout intervention categories: (1) those that focus on individual physicians and (2) those that focus on the organizational environment.33 We find these distinctions useful and offer strategies for enhancing individual physician wellness (TABLE 134-41). Similar to West and colleagues,11 we offer strategies for addressing organizational sources of stress (TABLE 242-48). The following text describes these burnout intervention categories, emphasizing increasing self-care and changes that enable physicians to adapt effectively.

The recommendations outlined in this article are based on published stress and burnout literature, as well as the experiences of the authors. However, the number of randomized controlled studies of interventions aimed at reducing physician stress and burnout is limited. In addition, strategies proposed to reduce burnout in other professions may not address the unique stressors physicians encounter. Hence, our recommendations are limited. We have included interventions that seem optimal for individual physicians and the organizations that employ them.
Individual strategies target physical, psychological, and social wellness
Physician wellness strategies are divided into 3 categories: physical, psychological, and social wellness. Most strategies to improve physical wellness are widely known, evidence based, and recommended to patients by physicians.34-36 For example, most physicians advise their patients to eat healthy balanced meals, avoid unhealthy foods and beverages, maintain a healthy body weight, get daily exercise and adequate sleep, avoid excessive alcohol use, and abstain from tobacco use. However, discrepancies between physicians’ advice to patients and their own behaviors are common. Simply stated, physicians are well advised to follow their own advice regarding physical self-care.
CBT and mindfulness are key to psychological wellness. Recommendations for enhancing psychological wellness are primarily derived from cognitive behavioral therapy (CBT) and mindfulness principles and practices.37,38 CBT has been called the “gold standard” of psychotherapy, based on the breadth of research demonstrating that “no other form of psychotherapy has been shown to be systematically superior to CBT.”39
Continue to: CBT is based on the premise...
CBT is based on the premise that individuals’ thoughts and beliefs largely determine how they feel (emotions) and act (behaviors). Certain thoughts lead to positive feelings and effective behaviors, while others lead to negative feelings and less effective behaviors. For example, when a physician has self-critical or helpless thoughts (eg, “I’m just no good at managing my life”), they are more likely to feel unhappy and abandon problem-solving. In contrast, when a physician has self-affirming or hopeful thoughts (eg, “This is difficult, but I have the personal resources to succeed”), they are more likely to feel confident and act to solve problems.
Physicians vacillate between these thoughts and beliefs, and their emotions and behaviors follow accordingly. When hyper-focused on “the hassles of medicine,” physicians feel defeated, depressed, and anxious about their work. In contrast, when physicians recognize and challenge problematic thoughts and focus on what they love about medicine, they feel good and interact with patients and coworkers in positive and self-reinforcing ways.
Mindfulness can help reduce psychological stress and increase personal fulfillment. Mindfulness is characterized as being in the present moment, fully accepting “what is,” and having a sense of gratitude and compassion for self and others.40 In practice, mindfulness involves being intentional.
Dahl and colleagues41 describe a framework for human flourishing that includes 4 core dimensions of well-being (awareness, insight, connection, and purpose) that are all closely linked to mindful, intentional living. Based on their work, it is apparent that those who maintain a “heightened and flexible attentiveness” to their thoughts and feelings are likely to benefit by experiencing “improved mental health and psychological well-being.”41
However, the utility of CBT and mindfulness practices depends on receptivity to psychological interventions. Individuals who are not receptive may be hesitant to use these practices or likely will not benefit from them. Given these limitations of behavioral interventions, it would be helpful if more attention were paid to preventing and managing physician stress and burnout, especially through research focused on organizational changes.
Continue to: Supportive relationships are powerful
Supportive relationships are powerful. Finally, to enhance social wellness, it would be difficult to overstate the potential benefits of positive, supportive, close relationships.42 However, the demands of a career in medicine, starting in medical school, have the potential for inhibiting (rather than enhancing) close relationships.
Placing value on relationships with friends and family members is essential. As Dr. M began experiencing burnout, he felt increasingly lonely, yet he isolated himself from those who cared about him. Dr. A felt lonely at home, even though she was surrounded by family. Physicians are often reluctant to initiate vulnerable communication with others, believing “no one wants to hear about my problems.” However, by realizing the need for help and asking friends and family for emotional support, physicians can improve their wellness. Fostering supportive relationships can help provide the resilience needed to address organizational stressors.
Tackling organizational challenges
Long hours and pressure to see large numbers of patients (production demands) are a challenge across practice settings. Limiting work hours has been effective in improving the well-being of physician trainees but has had an inconsistent effect on burnout.43,44
Organizations can offer flexible scheduling, and physicians considering limiting work hours may switch to part-time status or shift work. However, decreasing work hours may have the unintended consequence of increased stress as some physicians feel pressure to do more in less time.45 Therefore, it’s important to set clear boundaries around work time and when and where work tasks are completed (eg, home vs office).
How we use technology matters. Given technology’s ever-increasing role in medicine, organizations must identify and use the most efficient, effective technology for managing clerical processes. When physicians participate in these decisions and share their experiences, technology is likely to be more user-friendly and impose less stress.46
Continue to: If technology contributes to stress...
If technology contributes to stress by being too complex or impractical, it’s important to identify individuals in the workplace (eg, IT support or “super-users”) to help address these challenges. Organizations can implement multidisciplinary teams to address EHR challenges and decrease physician stress and burnout by training support staff to assist with clerical duties, allowing physicians to focus on patient care.47,48 Such organizational-directed interventions will be most successful when physicians are included in the decision-making process.47
Take on leadership roles to influence change. Leadership may be formal (involving a title and authority) or informal (leading by example). Health care organizations that are committed to the well-being of physicians will make the effort to improve the systems in which physicians work. Physicians working in organizations that are reluctant to change have several choices: implement individual strategies, take on leadership roles to influence change, or reconsider their fit for the organization. Physicians in solo practice might consider joining others in solo practices to share systems (call, phone triage, technical resources, etc) to implement some of these interventions.
Dr. A and Dr. M implement new wellness strategies
Dr. A and Dr. M have recently committed to addressing stressors in their lives and improving their wellness. Dr. A has become more assertive at work, highlighting her need for additional resources to function effectively. In response, her practice has hired scribes to assist in documenting visits. This success has inspired Dr. A to pay attention to her lifestyle choices. Gradually, she has begun to exercise and engage in healthy eating.
Dr. M has begun to utilize resources at his medical center to improve his EHR efficiency and patient flow. He has taken steps to address his financial concerns, developing a budget and spending judiciously. He practices mindfulness and ensures that he gets at least 7 hours of sleep per night, improving his mental and physical health. By doing so, he has more energy to connect with friends, exercise, and date.
CORRESPONDENCE
Margaret L. Smith, MD, MPH, MHSA, KUMC, Family Medicine and Community Health, 3901 Rainbow Boulevard – Mailstop 4010, Kansas City, KS 66160; [email protected]
Meet Dr. A and Dr. M
Dr. A is a 50-year-old family physician who provides prenatal care in a busy practice. She sees patients in eight 4-hour clinic sessions per week and is on inpatient call 1 week out of every 2 months. Dr. A has become disillusioned with her practice. She typically works until 7
Dr. M is a single, 32-year-old family physician working at an academic medical center. Dr. M is unhappy in his job, is trying to grow his practice, and views himself as having little impact or autonomy. He finds himself lost while navigating the electronic health record (EHR) and struggles to be efficient in the clinic. Dr. M has multiple administrative responsibilities that require him to work evenings and weekends. Debt from medical school loans also motivates him to moonlight several weekends per month. Over the past few months, Dr. M has become frustrated and discouraged, making his depression more difficult to manage. He feels drained by the time he arrives home, where he lives alone. He has stopped exercising, socializing with friends, and dating. Dr. M often wonders if he is in the wrong profession.
Defining burnout, stress, and wellness
Dr. A and Dr. M are experiencing symptoms of burnout, common to physicians and other health care professionals. Recent studies showed an increase in burnout during the COVID-19 pandemic.1,2 In a survey using the Maslach Burnout Inventory (MBI), approximately 44% of physicians reported at least one symptom of burnout.3 After adjusting for age, gender, relationship status, and hours worked per week, physicians were found to be at greater risk for burnout than nonphysician workers.3 The latest Medscape physician burnout survey found an increase in burnout among US physicians from 42% in 2021 to 47% in 2022 during the COVID-19 pandemic.1 Rates of burnout were even higher among family physicians and other frontline (eg, emergency, infectious disease, and critical care) physicians.1
Burnout has 3 key dimensions: (1) overwhelming exhaustion; (2) feelings of cynicism and detachment from the job; and (3) a sense of ineffectiveness and lack of accomplishment.4 The MBI is considered the standard tool for research in the field of burnout and has been repeatedly assessed for reliability and validity.4 The original MBI includes such items as: “I feel emotionally drained from my work,” “I feel like I’m working too hard on my job,” and “I worry that this job is hardening me emotionally.”5
According to the World Health Organization, burnout is an occupational phenomenon associated with chronic work-related stress that is not successfully managed.6 This definition emphasizes work stress as the cause of burnout, thus highlighting the importance of addressing the work environment.7 Physician burnout can affect physician health and wellness and the quality of patient care.8-13 Because of the cost of burnout to individuals and the health care system, it is important to understand stressors that can lead to physician burnout.
Stress has been described as “physical, mental, or emotional strain or tension … when a person perceives that demands exceed the personal and social resources the individual is able to mobilize.”14 Work-related sources of stress affecting practicing physicians include long workdays, multiple bureaucratic tasks, lack of autonomy/control, and complex patients.1,15
The COVID-19 pandemic is a stressor that increased physicians’ exposure to patient suffering and deaths and physicians’ vulnerability to disease at work.16 Physicians taking care of patients with COVID-19 risk infection and the possibility of infecting others.Online health records are another source of stress for many physicians.17,18 Access to online health records on personal devices can blur the line between work and home. For each hour of direct patient contact, a physician spends an additional 2 hours interacting with an EHR.19 Among family physicians and other primary care physicians, increased EHR interaction outside clinic hours has been associated with decreased workplace satisfaction and increased rates of burnout.11,19,20 Time spent on non-patient-facing clinical tasks, such as peer-to-peer reviews and billing queries, contributes more to burnout than clinic time alone.17
Continue to: These and other organizational factors...
These and other organizational factors contribute to the stress experienced by physicians. Many describe themselves as feeling consumed by their work. At the beginning of the COVID-19 pandemic, physicians (and the rest of the health care team) had to quickly learn how to conduct virtual office visits. Clerical responsibilities increased as patients relied more on patient portals and telephone calls to receive care.
Who is predisposed to burnout? Although burnout is a work-related syndrome, studies have shown an increase in burnout associated with individual (ie, personal) factors. For example, female physicians have been shown to have higher rates of burnout compared with male physicians.1,3 The stress of balancing the demands of the profession can begin during medical school and residency, with younger physicians having nearly twice the risk for stress-related symptoms when compared with older colleagues.15,20-23 Having a child younger than 21 years old, and other personal factors related to balancing family and life demands, increases the likelihood of burnout.11,21,22
Physicians with certain personality types and predispositions are at increased risk for burnout.23-25 For example, neuroticism on the Big Five Personality Inventory (one of the most well-known of the psychology inventories) is associated with an increased risk for burnout. Neuroticism may manifest as sadness or related emotional dysregulation (eg, irritability, anxiety).26 Other traits measured by the Big Five Personality Inventory include extraversion, agreeableness, conscientiousness, and openness to experience.26
A history of depression is also associated with an increased risk for burnout.27 Although depression and burnout are separate conditions, a 2016 study found significant overlap between the two.27 Physicians in this study who were depressed were more likely to experience burnout symptoms (87.5%); however, only 26.2% of physicians experiencing burnout were diagnosed as having depression.27 Rates of depression are higher among physicians when compared with nonphysicians, yet physicians are less likely to seek help due to fear of stigma and potential licensing concerns.28,29 Because of this, when physicians experience depressive symptoms, they may respond by working harder rather than seeking professional counseling or emotional support. They might believe that “asking for help is a sign of weakness,” thus sacrificing their wellness.
Wellness encompasses a sense of thriving characterized by thoughts and feelings of contentment, joy, and fulfillment—and the absence of severe distress.30 Wellness is a multifaceted condition that includes physical, psychological, and social aspects of an individual’s personal and professional life. Individuals experience a sense of wellness when they nurture their physical selves, minds, and relationships. People experience a sense of wellness when they balance their schedules, eat well, and maintain physical activity. Making time to enjoy family and friends also contributes to wellness.
Continue to: The culture of medicine often rewards...
The culture of medicine often rewards physician attitudes and behaviors that detract from wellness.31 Physicians internalize the culture of medicine that promotes perfectionism and downplays personal vulnerability.32 Physicians are reluctant to protect and preserve their wellness, believing self-sacrifice makes them good doctors. Physicians may spend countless hours counseling patients on the importance of wellness, but then work when ill or neglect their personal health needs and self-care—potentially decreasing their resilience and increasing the risk for burnout.31

Two paths to managing stress and preventing burnout
Patel and colleagues distinguish between 2 burnout intervention categories: (1) those that focus on individual physicians and (2) those that focus on the organizational environment.33 We find these distinctions useful and offer strategies for enhancing individual physician wellness (TABLE 134-41). Similar to West and colleagues,11 we offer strategies for addressing organizational sources of stress (TABLE 242-48). The following text describes these burnout intervention categories, emphasizing increasing self-care and changes that enable physicians to adapt effectively.

The recommendations outlined in this article are based on published stress and burnout literature, as well as the experiences of the authors. However, the number of randomized controlled studies of interventions aimed at reducing physician stress and burnout is limited. In addition, strategies proposed to reduce burnout in other professions may not address the unique stressors physicians encounter. Hence, our recommendations are limited. We have included interventions that seem optimal for individual physicians and the organizations that employ them.
Individual strategies target physical, psychological, and social wellness
Physician wellness strategies are divided into 3 categories: physical, psychological, and social wellness. Most strategies to improve physical wellness are widely known, evidence based, and recommended to patients by physicians.34-36 For example, most physicians advise their patients to eat healthy balanced meals, avoid unhealthy foods and beverages, maintain a healthy body weight, get daily exercise and adequate sleep, avoid excessive alcohol use, and abstain from tobacco use. However, discrepancies between physicians’ advice to patients and their own behaviors are common. Simply stated, physicians are well advised to follow their own advice regarding physical self-care.
CBT and mindfulness are key to psychological wellness. Recommendations for enhancing psychological wellness are primarily derived from cognitive behavioral therapy (CBT) and mindfulness principles and practices.37,38 CBT has been called the “gold standard” of psychotherapy, based on the breadth of research demonstrating that “no other form of psychotherapy has been shown to be systematically superior to CBT.”39
Continue to: CBT is based on the premise...
CBT is based on the premise that individuals’ thoughts and beliefs largely determine how they feel (emotions) and act (behaviors). Certain thoughts lead to positive feelings and effective behaviors, while others lead to negative feelings and less effective behaviors. For example, when a physician has self-critical or helpless thoughts (eg, “I’m just no good at managing my life”), they are more likely to feel unhappy and abandon problem-solving. In contrast, when a physician has self-affirming or hopeful thoughts (eg, “This is difficult, but I have the personal resources to succeed”), they are more likely to feel confident and act to solve problems.
Physicians vacillate between these thoughts and beliefs, and their emotions and behaviors follow accordingly. When hyper-focused on “the hassles of medicine,” physicians feel defeated, depressed, and anxious about their work. In contrast, when physicians recognize and challenge problematic thoughts and focus on what they love about medicine, they feel good and interact with patients and coworkers in positive and self-reinforcing ways.
Mindfulness can help reduce psychological stress and increase personal fulfillment. Mindfulness is characterized as being in the present moment, fully accepting “what is,” and having a sense of gratitude and compassion for self and others.40 In practice, mindfulness involves being intentional.
Dahl and colleagues41 describe a framework for human flourishing that includes 4 core dimensions of well-being (awareness, insight, connection, and purpose) that are all closely linked to mindful, intentional living. Based on their work, it is apparent that those who maintain a “heightened and flexible attentiveness” to their thoughts and feelings are likely to benefit by experiencing “improved mental health and psychological well-being.”41
However, the utility of CBT and mindfulness practices depends on receptivity to psychological interventions. Individuals who are not receptive may be hesitant to use these practices or likely will not benefit from them. Given these limitations of behavioral interventions, it would be helpful if more attention were paid to preventing and managing physician stress and burnout, especially through research focused on organizational changes.
Continue to: Supportive relationships are powerful
Supportive relationships are powerful. Finally, to enhance social wellness, it would be difficult to overstate the potential benefits of positive, supportive, close relationships.42 However, the demands of a career in medicine, starting in medical school, have the potential for inhibiting (rather than enhancing) close relationships.
Placing value on relationships with friends and family members is essential. As Dr. M began experiencing burnout, he felt increasingly lonely, yet he isolated himself from those who cared about him. Dr. A felt lonely at home, even though she was surrounded by family. Physicians are often reluctant to initiate vulnerable communication with others, believing “no one wants to hear about my problems.” However, by realizing the need for help and asking friends and family for emotional support, physicians can improve their wellness. Fostering supportive relationships can help provide the resilience needed to address organizational stressors.
Tackling organizational challenges
Long hours and pressure to see large numbers of patients (production demands) are a challenge across practice settings. Limiting work hours has been effective in improving the well-being of physician trainees but has had an inconsistent effect on burnout.43,44
Organizations can offer flexible scheduling, and physicians considering limiting work hours may switch to part-time status or shift work. However, decreasing work hours may have the unintended consequence of increased stress as some physicians feel pressure to do more in less time.45 Therefore, it’s important to set clear boundaries around work time and when and where work tasks are completed (eg, home vs office).
How we use technology matters. Given technology’s ever-increasing role in medicine, organizations must identify and use the most efficient, effective technology for managing clerical processes. When physicians participate in these decisions and share their experiences, technology is likely to be more user-friendly and impose less stress.46
Continue to: If technology contributes to stress...
If technology contributes to stress by being too complex or impractical, it’s important to identify individuals in the workplace (eg, IT support or “super-users”) to help address these challenges. Organizations can implement multidisciplinary teams to address EHR challenges and decrease physician stress and burnout by training support staff to assist with clerical duties, allowing physicians to focus on patient care.47,48 Such organizational-directed interventions will be most successful when physicians are included in the decision-making process.47
Take on leadership roles to influence change. Leadership may be formal (involving a title and authority) or informal (leading by example). Health care organizations that are committed to the well-being of physicians will make the effort to improve the systems in which physicians work. Physicians working in organizations that are reluctant to change have several choices: implement individual strategies, take on leadership roles to influence change, or reconsider their fit for the organization. Physicians in solo practice might consider joining others in solo practices to share systems (call, phone triage, technical resources, etc) to implement some of these interventions.
Dr. A and Dr. M implement new wellness strategies
Dr. A and Dr. M have recently committed to addressing stressors in their lives and improving their wellness. Dr. A has become more assertive at work, highlighting her need for additional resources to function effectively. In response, her practice has hired scribes to assist in documenting visits. This success has inspired Dr. A to pay attention to her lifestyle choices. Gradually, she has begun to exercise and engage in healthy eating.
Dr. M has begun to utilize resources at his medical center to improve his EHR efficiency and patient flow. He has taken steps to address his financial concerns, developing a budget and spending judiciously. He practices mindfulness and ensures that he gets at least 7 hours of sleep per night, improving his mental and physical health. By doing so, he has more energy to connect with friends, exercise, and date.
CORRESPONDENCE
Margaret L. Smith, MD, MPH, MHSA, KUMC, Family Medicine and Community Health, 3901 Rainbow Boulevard – Mailstop 4010, Kansas City, KS 66160; [email protected]
1. Kane L. Physician burnout & depression report: stress, anxiety, and anger. Medscape. January 21, 2022. Accessed February 23, 2023. www.medscape.com/slideshow/2022-lifestyle-burnout-6014664
2. Lockwood L, Patel N, Bukelis I. 45.5 Physician burnout and the COVID-19 pandemic: the silent epidemic. J Am Acad Child Adolesc Psychiatry. 2021;60:S242. doi: 10.1016/j.jaac.2021.09.354
3. Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clin Proc. 2019;94:1681-1694. doi: 10.1016/j.mayocp.2018.10.023
4. Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15:103-111. doi: 10.1002/wps.20311
5. Maslach C, Jackson SE. The measurement of experienced burnout. J Organ Behav. 1981;2:99-113. doi: 10.1002/job.4030020205
6. World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed February 23, 2023. www.who.int/news/item/28-05-2019-burn-out-an-occupational-phenomenon-international-classification-of-diseases
7. Berg S. WHO adds burnout to ICD-11. What it means for physicians. American Medical Association. July 23, 2019. Accessed February 23, 2023. www.ama-assn.org/practice-management/physician-health/who-adds-burnout-icd-11-what-it-means-physicians
8. Brown SD, Goske MJ, Johnson CM. Beyond substance abuse: stress, burnout, and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009;6:479-485. doi: 10.1016/j.jacr.2008.11.029
9. Williams ES, Rathert C, Buttigieg SC. The personal and professional consequences of physician burnout: a systematic review of the literature. Med Care Res Rev. 2020;77:371-386. doi: 10.1177/ 1077558719856787
10. Yates SW. Physician Stress and Burnout. Am J Med. 2020;133:160-164. doi: 10.1016/j.amjmed.2019.08.034
11. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529. doi: 10.1111/joim.12752
12. Firth-Cozens J, Greenhalgh J. Doctors’ perceptions of the links between stress and lowered clinical care. Soc Sci Med. 1997;44:1017-1022. doi: 10.1016/s0277-9536(96)00227-4
13. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: a systematic review. BMJ Open. 2017;7:e015141. doi: 10.1136/bmjopen-2016-015141
14. American Institute of Stress. What is stress? April 29, 2022. Accessed February 23, 2023. www.stress.org/daily-life
15. Regehr C, Glancy D, Pitts A, et al. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202:353-359. doi: 10.1097/NMD. 0000000000000130
16. Fitzpatrick K, Patterson R, Morley K, et al. Physician wellness during a pandemic. West J Emerg Med. 2020;21:83-87. doi: 10.5811/westjem.2020.7.48472
17. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848. doi: 10.1016/j.mayocp.2016.05.007
18. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15:419-426. doi: 10.1370/afm.2121
19. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760. doi: 10.7326/M16-0961
20. Robertson SL, Robinson MD, Reid A. Electronic health record effects on work-life balance and burnout within the I3 Population Collaborative. J Grad Med Educ. 2017;9:479-484. doi: 10.4300/JGME-D-16-00123.1
21. Fares J, Al Tabosh H, Saadeddin Z, et al. Stress, burnout and coping strategies in preclinical medical students. N Am J Med Sci. 2016;8:75-81. doi: 10.4103/1947-2714.177299
22. Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sci (Basel). 2018; 8:98. doi: 10.3390/bs8110098
23. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114:513-519. doi: 10.1016/s0002-9343(03)00117-7
24. Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
25. Brown PA, Slater M, Lofters A. Personality and burnout among primary care physicians: an international study. Psychol Res Behav Manag. 2019;12:169-177. doi: 10.2147/PRBM.S195633.
26. John OP, Donahue EM, Kentle RL. The Big Five Inventory – Versions 4A and 54. Institute of Personality and Social Research, University of California; 1991.
27. Wurm W, Vogel K, Holl A, et al. Depression-burnout overlap in physicians. PLoS One. 2016;11:e0149913. doi: 10.1371/journal.pone.0149913
28. Mehta SS, Edwards ML. Suffering in silence: Mental health stigma and physicians’ licensing fears. Am J Psychiatry Resid J. 2018;13:2-4.
29. Adam AR, Golu FT. Prevalence of depression among physicians: A comprehensive meta-analysis. Ro Med J. 2021;68:327-337. doi: 10.37897/RMJ.2021.3.1
30. Brady KJS, Trockel MT, Khan CT, et al. What do we mean by physician wellness? A systematic review of its definition and measurement. Acad Psychiatry. 2018;42:94-108. doi: 10.1007/s40596-017-0781-6
31. Shanafelt TD, Schein E, Minor LB, et al. Healing the professional culture of medicine. Mayo Clin Proc. 2019;94:1556-1566. doi: 10.1016/j.mayocp.2019.03.026
32. Horan S, Flaxman PE, Stride CB. The perfect recovery? Interactive influence of perfectionism and spillover work tasks on changes in exhaustion and mood around a vacation. J Occup Health Psychol. 2021;26:86-107. doi: 10.1037/ocp0000208
33. Patel RS, Sekhri S, Bhimanadham NN, et al. A review on strategies to manage physician burnout. Cureus. 2019;11:e4805. doi: 10.7759/cureus.4805
34. US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. US Department of Health and Human Services; 2018.
35. Kim ES, Chen Y, Nakamura JS, et al. Sense of purpose in life and subsequent physical, behavioral, and psychosocial health: an outcome-wide approach. Am J Health Promot. 2022;36:137-147. doi: 10.1177/08901171211038545
36. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
37. Fordham B, Sugavanam T, Edwards K, et al. The evidence for cognitive behavioural therapy in any condition, population or context: a meta-review of systematic reviews and panoramic meta-analysis. Psychol Med. 2021;51:21-29. doi: 10.1017/S0033291720005292
38. Goldberg SB, Tucker RP, Greene PA, et al. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin Psychol Rev. 2018;59:52-60. doi: 10.1016/j.cpr.2017.10.011
39. David D, Cristea I, Hofmann SG. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front Psychiatry. 2018;9:4. doi: 10.3389/fpsyt.2018.00004
40. Fendel JC, Bürkle JJ, Göritz AS. Mindfulness-based interventions to reduce burnout and stress in physicians: a systematic review and meta-analysis. Acad Med. 2021;96:751-764. doi: 10.1097/ACM.0000000000003936
41. Dahl CJ, Wilson-Mendenhall CD, Davidson RJ. The plasticity of well-being: a training-based framework for the cultivation of human flourishing. Proc Natl Acad Sci USA. 2020;117:32197-32206. doi: 10.1073/pnas.2014859117
42. Holt-Lunstad J. Why social relationships are important for physical health: a systems approach to understanding and modifying risk and protection. Annu Rev Psychol. 2018;69:437-458. doi: 10.1146/annurev-psych-122216-011902
43. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018; 378:1494-1508. doi: 10.1056/NEJMoa1800965
44. Shea JA, Bellini LM, Dinges DF, et al. Impact of protected sleep period for internal medicine interns on overnight call on depression, burnout, and empathy. J Grad Med Educ. 2014;6:256-263. doi: 10.4300/JGME-D-13-00241.1
45. Morrow G, Burford B, Carter M, et al. Have restricted working hours reduced junior doctors’ experience of fatigue? A focus group and telephone interview study. BMJ Open. 2014;4:e004222. doi: 10.1136/bmjopen-2013-004222
46. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92:129-146. doi: 10.1016/j.mayocp.2016.10.004
47. Sequeira L, Almilaji K, Strudwick G, et al. EHR “SWAT” teams: a physician engagement initiative to improve Electronic Health Record (EHR) experiences and mitigate possible causes of EHR-related burnout. JAMA Open. 2021;4:1-7. doi: 10.1093/jamiaopen/ooab018
48. Smith PC, Lyon C, English AF, et al. Practice transformation under the University of Colorado’s primary care redesign model. Ann Fam Med. 2019;17:S24-S32. doi: 10.1370/afm.2424
1. Kane L. Physician burnout & depression report: stress, anxiety, and anger. Medscape. January 21, 2022. Accessed February 23, 2023. www.medscape.com/slideshow/2022-lifestyle-burnout-6014664
2. Lockwood L, Patel N, Bukelis I. 45.5 Physician burnout and the COVID-19 pandemic: the silent epidemic. J Am Acad Child Adolesc Psychiatry. 2021;60:S242. doi: 10.1016/j.jaac.2021.09.354
3. Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clin Proc. 2019;94:1681-1694. doi: 10.1016/j.mayocp.2018.10.023
4. Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15:103-111. doi: 10.1002/wps.20311
5. Maslach C, Jackson SE. The measurement of experienced burnout. J Organ Behav. 1981;2:99-113. doi: 10.1002/job.4030020205
6. World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed February 23, 2023. www.who.int/news/item/28-05-2019-burn-out-an-occupational-phenomenon-international-classification-of-diseases
7. Berg S. WHO adds burnout to ICD-11. What it means for physicians. American Medical Association. July 23, 2019. Accessed February 23, 2023. www.ama-assn.org/practice-management/physician-health/who-adds-burnout-icd-11-what-it-means-physicians
8. Brown SD, Goske MJ, Johnson CM. Beyond substance abuse: stress, burnout, and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009;6:479-485. doi: 10.1016/j.jacr.2008.11.029
9. Williams ES, Rathert C, Buttigieg SC. The personal and professional consequences of physician burnout: a systematic review of the literature. Med Care Res Rev. 2020;77:371-386. doi: 10.1177/ 1077558719856787
10. Yates SW. Physician Stress and Burnout. Am J Med. 2020;133:160-164. doi: 10.1016/j.amjmed.2019.08.034
11. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529. doi: 10.1111/joim.12752
12. Firth-Cozens J, Greenhalgh J. Doctors’ perceptions of the links between stress and lowered clinical care. Soc Sci Med. 1997;44:1017-1022. doi: 10.1016/s0277-9536(96)00227-4
13. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: a systematic review. BMJ Open. 2017;7:e015141. doi: 10.1136/bmjopen-2016-015141
14. American Institute of Stress. What is stress? April 29, 2022. Accessed February 23, 2023. www.stress.org/daily-life
15. Regehr C, Glancy D, Pitts A, et al. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202:353-359. doi: 10.1097/NMD. 0000000000000130
16. Fitzpatrick K, Patterson R, Morley K, et al. Physician wellness during a pandemic. West J Emerg Med. 2020;21:83-87. doi: 10.5811/westjem.2020.7.48472
17. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848. doi: 10.1016/j.mayocp.2016.05.007
18. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15:419-426. doi: 10.1370/afm.2121
19. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760. doi: 10.7326/M16-0961
20. Robertson SL, Robinson MD, Reid A. Electronic health record effects on work-life balance and burnout within the I3 Population Collaborative. J Grad Med Educ. 2017;9:479-484. doi: 10.4300/JGME-D-16-00123.1
21. Fares J, Al Tabosh H, Saadeddin Z, et al. Stress, burnout and coping strategies in preclinical medical students. N Am J Med Sci. 2016;8:75-81. doi: 10.4103/1947-2714.177299
22. Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sci (Basel). 2018; 8:98. doi: 10.3390/bs8110098
23. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114:513-519. doi: 10.1016/s0002-9343(03)00117-7
24. Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
25. Brown PA, Slater M, Lofters A. Personality and burnout among primary care physicians: an international study. Psychol Res Behav Manag. 2019;12:169-177. doi: 10.2147/PRBM.S195633.
26. John OP, Donahue EM, Kentle RL. The Big Five Inventory – Versions 4A and 54. Institute of Personality and Social Research, University of California; 1991.
27. Wurm W, Vogel K, Holl A, et al. Depression-burnout overlap in physicians. PLoS One. 2016;11:e0149913. doi: 10.1371/journal.pone.0149913
28. Mehta SS, Edwards ML. Suffering in silence: Mental health stigma and physicians’ licensing fears. Am J Psychiatry Resid J. 2018;13:2-4.
29. Adam AR, Golu FT. Prevalence of depression among physicians: A comprehensive meta-analysis. Ro Med J. 2021;68:327-337. doi: 10.37897/RMJ.2021.3.1
30. Brady KJS, Trockel MT, Khan CT, et al. What do we mean by physician wellness? A systematic review of its definition and measurement. Acad Psychiatry. 2018;42:94-108. doi: 10.1007/s40596-017-0781-6
31. Shanafelt TD, Schein E, Minor LB, et al. Healing the professional culture of medicine. Mayo Clin Proc. 2019;94:1556-1566. doi: 10.1016/j.mayocp.2019.03.026
32. Horan S, Flaxman PE, Stride CB. The perfect recovery? Interactive influence of perfectionism and spillover work tasks on changes in exhaustion and mood around a vacation. J Occup Health Psychol. 2021;26:86-107. doi: 10.1037/ocp0000208
33. Patel RS, Sekhri S, Bhimanadham NN, et al. A review on strategies to manage physician burnout. Cureus. 2019;11:e4805. doi: 10.7759/cureus.4805
34. US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. US Department of Health and Human Services; 2018.
35. Kim ES, Chen Y, Nakamura JS, et al. Sense of purpose in life and subsequent physical, behavioral, and psychosocial health: an outcome-wide approach. Am J Health Promot. 2022;36:137-147. doi: 10.1177/08901171211038545
36. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
37. Fordham B, Sugavanam T, Edwards K, et al. The evidence for cognitive behavioural therapy in any condition, population or context: a meta-review of systematic reviews and panoramic meta-analysis. Psychol Med. 2021;51:21-29. doi: 10.1017/S0033291720005292
38. Goldberg SB, Tucker RP, Greene PA, et al. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin Psychol Rev. 2018;59:52-60. doi: 10.1016/j.cpr.2017.10.011
39. David D, Cristea I, Hofmann SG. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front Psychiatry. 2018;9:4. doi: 10.3389/fpsyt.2018.00004
40. Fendel JC, Bürkle JJ, Göritz AS. Mindfulness-based interventions to reduce burnout and stress in physicians: a systematic review and meta-analysis. Acad Med. 2021;96:751-764. doi: 10.1097/ACM.0000000000003936
41. Dahl CJ, Wilson-Mendenhall CD, Davidson RJ. The plasticity of well-being: a training-based framework for the cultivation of human flourishing. Proc Natl Acad Sci USA. 2020;117:32197-32206. doi: 10.1073/pnas.2014859117
42. Holt-Lunstad J. Why social relationships are important for physical health: a systems approach to understanding and modifying risk and protection. Annu Rev Psychol. 2018;69:437-458. doi: 10.1146/annurev-psych-122216-011902
43. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018; 378:1494-1508. doi: 10.1056/NEJMoa1800965
44. Shea JA, Bellini LM, Dinges DF, et al. Impact of protected sleep period for internal medicine interns on overnight call on depression, burnout, and empathy. J Grad Med Educ. 2014;6:256-263. doi: 10.4300/JGME-D-13-00241.1
45. Morrow G, Burford B, Carter M, et al. Have restricted working hours reduced junior doctors’ experience of fatigue? A focus group and telephone interview study. BMJ Open. 2014;4:e004222. doi: 10.1136/bmjopen-2013-004222
46. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92:129-146. doi: 10.1016/j.mayocp.2016.10.004
47. Sequeira L, Almilaji K, Strudwick G, et al. EHR “SWAT” teams: a physician engagement initiative to improve Electronic Health Record (EHR) experiences and mitigate possible causes of EHR-related burnout. JAMA Open. 2021;4:1-7. doi: 10.1093/jamiaopen/ooab018
48. Smith PC, Lyon C, English AF, et al. Practice transformation under the University of Colorado’s primary care redesign model. Ann Fam Med. 2019;17:S24-S32. doi: 10.1370/afm.2424
PRACTICE RECOMMENDATIONS
› Serve as a leader and positively influence the systems (ie, organizations, institutions, offices) in which you practice as a way to address organizational stress. C
› Establish and maintain positive, supportive, and close relationships with friends, family, and colleagues to improve social wellness. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Common gut bacteria linked to Parkinson’s disease
, a small study suggests.
Environmental factors as well as genetics are also suspected to play a role in PD etiology, although the exact cause remains unknown.
“Our findings indicate that specific strains of Desulfovibrio bacteria are likely to cause Parkinson’s disease,” study investigator Per Erik Saris, PhD, from the University of Helsinki, Finland, says in a news release.
The study was published online in Frontiers in Cellular and Infection Microbiology.
Screen and treat?
It builds on earlier work by the researchers that showed that Desulfovibrio bacteria were more prevalent and more abundant in quantity in patients with PD, especially patients with more severe disease, than in healthy individuals.
Desulfovibrio is a genus of gram-negative bacteria commonly found in aquatic environments in which levels of organic material are elevated, as well as in waterlogged soils.
In their latest study, Dr. Saris and colleagues looked for Desulfovibrio species in fecal samples from 10 patients with PD and their healthy spouses. Isolated Desulfovibrio strains were fed to a strain of Caenorhabditis elegans roundworms that expressed human alpha-syn fused with yellow fluorescent protein.
They found that worms fed Desulfovibrio bacteria from patients with PD harbored significantly more (P < .001) and larger alpha-syn aggregates (P < .001) than worms fed Desulfovibrio bacteria from healthy individuals or worms fed Escherichia coli strains.
In addition, worms fed Desulfovibrio strains from patients with PD died in significantly higher quantities than worms fed E. coli bacteria (P < .01).
Desulfovibrio strains isolated from patients with PD and strains isolated from healthy individuals appear to have different traits. Comparative genomics studies are needed to identify genetic differences and pathogenic genes from Desulfovibrio strains from patients with PD, the researchers note.
“Taking into account that aggregation of alpha-syn is a hallmark of PD, the ability of Desulfovibrio bacteria to induce alpha-syn aggregation in large numbers and sizes, as demonstrated in the present study, provides further evidence for the pathogenic role of Desulfovibrio bacteria in PD, as previously suggested,” they add.
The findings highlight the potential for screening and targeted removal of harmful Desulfovibrio bacteria, Dr. Saris suggests in the news release.
No clinical implications
In a comment, James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, cautioned that “this research is in a very early stage, uses a nonvertebrate animal model, and the number of participants is small.
“Understanding the role of the gut microbiome in influencing PD is in its infancy. These are important steps to determining what – if any – link may be between gut bacteria and PD,” Dr. Beck said.
“Right now, there are no implications for the screening/treatment of carriers,” Dr. Beck said.
“It seems that a lot of people, whether with PD or not, harbor Desulfovibrio bacteria in their gut. More research is needed to understand what is different between the Desulfovibrio bacteria of people with PD vs. those who do not have PD,” Dr. Beck added.
The study was supported by the Magnus Ehrnrooth Foundation and the Jane and Aatos Erkko Foundation. Dr. Saris and Dr. Beck have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a small study suggests.
Environmental factors as well as genetics are also suspected to play a role in PD etiology, although the exact cause remains unknown.
“Our findings indicate that specific strains of Desulfovibrio bacteria are likely to cause Parkinson’s disease,” study investigator Per Erik Saris, PhD, from the University of Helsinki, Finland, says in a news release.
The study was published online in Frontiers in Cellular and Infection Microbiology.
Screen and treat?
It builds on earlier work by the researchers that showed that Desulfovibrio bacteria were more prevalent and more abundant in quantity in patients with PD, especially patients with more severe disease, than in healthy individuals.
Desulfovibrio is a genus of gram-negative bacteria commonly found in aquatic environments in which levels of organic material are elevated, as well as in waterlogged soils.
In their latest study, Dr. Saris and colleagues looked for Desulfovibrio species in fecal samples from 10 patients with PD and their healthy spouses. Isolated Desulfovibrio strains were fed to a strain of Caenorhabditis elegans roundworms that expressed human alpha-syn fused with yellow fluorescent protein.
They found that worms fed Desulfovibrio bacteria from patients with PD harbored significantly more (P < .001) and larger alpha-syn aggregates (P < .001) than worms fed Desulfovibrio bacteria from healthy individuals or worms fed Escherichia coli strains.
In addition, worms fed Desulfovibrio strains from patients with PD died in significantly higher quantities than worms fed E. coli bacteria (P < .01).
Desulfovibrio strains isolated from patients with PD and strains isolated from healthy individuals appear to have different traits. Comparative genomics studies are needed to identify genetic differences and pathogenic genes from Desulfovibrio strains from patients with PD, the researchers note.
“Taking into account that aggregation of alpha-syn is a hallmark of PD, the ability of Desulfovibrio bacteria to induce alpha-syn aggregation in large numbers and sizes, as demonstrated in the present study, provides further evidence for the pathogenic role of Desulfovibrio bacteria in PD, as previously suggested,” they add.
The findings highlight the potential for screening and targeted removal of harmful Desulfovibrio bacteria, Dr. Saris suggests in the news release.
No clinical implications
In a comment, James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, cautioned that “this research is in a very early stage, uses a nonvertebrate animal model, and the number of participants is small.
“Understanding the role of the gut microbiome in influencing PD is in its infancy. These are important steps to determining what – if any – link may be between gut bacteria and PD,” Dr. Beck said.
“Right now, there are no implications for the screening/treatment of carriers,” Dr. Beck said.
“It seems that a lot of people, whether with PD or not, harbor Desulfovibrio bacteria in their gut. More research is needed to understand what is different between the Desulfovibrio bacteria of people with PD vs. those who do not have PD,” Dr. Beck added.
The study was supported by the Magnus Ehrnrooth Foundation and the Jane and Aatos Erkko Foundation. Dr. Saris and Dr. Beck have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a small study suggests.
Environmental factors as well as genetics are also suspected to play a role in PD etiology, although the exact cause remains unknown.
“Our findings indicate that specific strains of Desulfovibrio bacteria are likely to cause Parkinson’s disease,” study investigator Per Erik Saris, PhD, from the University of Helsinki, Finland, says in a news release.
The study was published online in Frontiers in Cellular and Infection Microbiology.
Screen and treat?
It builds on earlier work by the researchers that showed that Desulfovibrio bacteria were more prevalent and more abundant in quantity in patients with PD, especially patients with more severe disease, than in healthy individuals.
Desulfovibrio is a genus of gram-negative bacteria commonly found in aquatic environments in which levels of organic material are elevated, as well as in waterlogged soils.
In their latest study, Dr. Saris and colleagues looked for Desulfovibrio species in fecal samples from 10 patients with PD and their healthy spouses. Isolated Desulfovibrio strains were fed to a strain of Caenorhabditis elegans roundworms that expressed human alpha-syn fused with yellow fluorescent protein.
They found that worms fed Desulfovibrio bacteria from patients with PD harbored significantly more (P < .001) and larger alpha-syn aggregates (P < .001) than worms fed Desulfovibrio bacteria from healthy individuals or worms fed Escherichia coli strains.
In addition, worms fed Desulfovibrio strains from patients with PD died in significantly higher quantities than worms fed E. coli bacteria (P < .01).
Desulfovibrio strains isolated from patients with PD and strains isolated from healthy individuals appear to have different traits. Comparative genomics studies are needed to identify genetic differences and pathogenic genes from Desulfovibrio strains from patients with PD, the researchers note.
“Taking into account that aggregation of alpha-syn is a hallmark of PD, the ability of Desulfovibrio bacteria to induce alpha-syn aggregation in large numbers and sizes, as demonstrated in the present study, provides further evidence for the pathogenic role of Desulfovibrio bacteria in PD, as previously suggested,” they add.
The findings highlight the potential for screening and targeted removal of harmful Desulfovibrio bacteria, Dr. Saris suggests in the news release.
No clinical implications
In a comment, James Beck, PhD, chief scientific officer at the Parkinson’s Foundation, cautioned that “this research is in a very early stage, uses a nonvertebrate animal model, and the number of participants is small.
“Understanding the role of the gut microbiome in influencing PD is in its infancy. These are important steps to determining what – if any – link may be between gut bacteria and PD,” Dr. Beck said.
“Right now, there are no implications for the screening/treatment of carriers,” Dr. Beck said.
“It seems that a lot of people, whether with PD or not, harbor Desulfovibrio bacteria in their gut. More research is needed to understand what is different between the Desulfovibrio bacteria of people with PD vs. those who do not have PD,” Dr. Beck added.
The study was supported by the Magnus Ehrnrooth Foundation and the Jane and Aatos Erkko Foundation. Dr. Saris and Dr. Beck have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN CELLULAR AND INFECTION MICROBIOLOGY
Noninvasive skin test may aid in Cushing diagnosis
SEATTLE – new research suggests.
Tissue accumulation of AGEs – harmful compounds formed by glycation of macromolecules – has been implicated in aging, diabetes, and cardiovascular disease. Now, in a new single-center prospective study, a group of 208 patients with endogenous hypercortisolism was found to have significantly higher median tissue AGE levels than 103 reference subjects without hypercortisolism.
The findings were presented at the annual meeting of the American Association of Clinical Endocrinology by Rashi Sandooja, MD, an endocrinology fellow at the Mayo Clinic, Rochester, Minn.
“Diagnosis of endogenous hypercortisolism can be quite challenging. Often patients can have nonspecific symptoms with biochemical testing being equivocal. In these situations, new biomarkers of hypercortisolism such as AGE measurement could potentially be useful,” Dr. Sandooja said in an interview.
“After proper validation, it could help clinicians in cases which may not be straightforward and could serve as an additional” instrument in the toolkit to reach a conclusive diagnosis, she added.
Asked to comment, session moderator Anupam Kotwal, MD, said in an interview: “I think it’s very exciting data. ... I envision its use in mild autonomous cortisol secretion, where there are not a lot of overt Cushing features but they may have a small adrenal mass. ... It might be used to guide care when there’s not a clear-cut answer.”
However, he cautioned that more validation is needed to determine the correlates of AGEs by different etiologies and magnitudes of cortisol excess.
Moreover, “skin can become thin in hypercortisolism, so is [the reader device] just detecting it more with skin testing? I think a blood test for validation would be a very good next step,” added Dr. Kotwal, who is an assistant professor in the division of diabetes, endocrinology and metabolism at the University of Nebraska, Omaha.
More work will be needed
Future directions for research should include adding a longitudinal arm and looking at the impact on AGE after patients undergo curative surgery and achieve remission, Dr. Sandooja explained.
“It will be interesting to see if AGE levels continue to be persistently high or decrease after patients achieve sustained remission of hypercortisolism. We are also interested in whether AGE measurement at baseline, prior to surgery may be associated with glucocorticoid withdrawal, myopathy, and metabolic outcomes following the surgery.”
Dr. Kotwal observed: “If the answer is clear for Cushing disease, I don’t know what extra information this would give. Maybe they would monitor people more closely afterward. It would be useful to see, but I think the first low-hanging fruit is use it in a way to guide the care of patients where we’re unclear as to whether initial treatment of this [mild autonomous cortisol secretion] is going to improve their outcomes.”
But, he added, “keeping in mind issues of skin ... we don’t want to distract clinicians and patients from using the tried and tested methods of characterizing Cushing syndrome. I’m always hesitant to bring something into practice before there is a little more information on how it can be used.”
Dr. Sandooja and Dr. Kotwal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SEATTLE – new research suggests.
Tissue accumulation of AGEs – harmful compounds formed by glycation of macromolecules – has been implicated in aging, diabetes, and cardiovascular disease. Now, in a new single-center prospective study, a group of 208 patients with endogenous hypercortisolism was found to have significantly higher median tissue AGE levels than 103 reference subjects without hypercortisolism.
The findings were presented at the annual meeting of the American Association of Clinical Endocrinology by Rashi Sandooja, MD, an endocrinology fellow at the Mayo Clinic, Rochester, Minn.
“Diagnosis of endogenous hypercortisolism can be quite challenging. Often patients can have nonspecific symptoms with biochemical testing being equivocal. In these situations, new biomarkers of hypercortisolism such as AGE measurement could potentially be useful,” Dr. Sandooja said in an interview.
“After proper validation, it could help clinicians in cases which may not be straightforward and could serve as an additional” instrument in the toolkit to reach a conclusive diagnosis, she added.
Asked to comment, session moderator Anupam Kotwal, MD, said in an interview: “I think it’s very exciting data. ... I envision its use in mild autonomous cortisol secretion, where there are not a lot of overt Cushing features but they may have a small adrenal mass. ... It might be used to guide care when there’s not a clear-cut answer.”
However, he cautioned that more validation is needed to determine the correlates of AGEs by different etiologies and magnitudes of cortisol excess.
Moreover, “skin can become thin in hypercortisolism, so is [the reader device] just detecting it more with skin testing? I think a blood test for validation would be a very good next step,” added Dr. Kotwal, who is an assistant professor in the division of diabetes, endocrinology and metabolism at the University of Nebraska, Omaha.
More work will be needed
Future directions for research should include adding a longitudinal arm and looking at the impact on AGE after patients undergo curative surgery and achieve remission, Dr. Sandooja explained.
“It will be interesting to see if AGE levels continue to be persistently high or decrease after patients achieve sustained remission of hypercortisolism. We are also interested in whether AGE measurement at baseline, prior to surgery may be associated with glucocorticoid withdrawal, myopathy, and metabolic outcomes following the surgery.”
Dr. Kotwal observed: “If the answer is clear for Cushing disease, I don’t know what extra information this would give. Maybe they would monitor people more closely afterward. It would be useful to see, but I think the first low-hanging fruit is use it in a way to guide the care of patients where we’re unclear as to whether initial treatment of this [mild autonomous cortisol secretion] is going to improve their outcomes.”
But, he added, “keeping in mind issues of skin ... we don’t want to distract clinicians and patients from using the tried and tested methods of characterizing Cushing syndrome. I’m always hesitant to bring something into practice before there is a little more information on how it can be used.”
Dr. Sandooja and Dr. Kotwal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SEATTLE – new research suggests.
Tissue accumulation of AGEs – harmful compounds formed by glycation of macromolecules – has been implicated in aging, diabetes, and cardiovascular disease. Now, in a new single-center prospective study, a group of 208 patients with endogenous hypercortisolism was found to have significantly higher median tissue AGE levels than 103 reference subjects without hypercortisolism.
The findings were presented at the annual meeting of the American Association of Clinical Endocrinology by Rashi Sandooja, MD, an endocrinology fellow at the Mayo Clinic, Rochester, Minn.
“Diagnosis of endogenous hypercortisolism can be quite challenging. Often patients can have nonspecific symptoms with biochemical testing being equivocal. In these situations, new biomarkers of hypercortisolism such as AGE measurement could potentially be useful,” Dr. Sandooja said in an interview.
“After proper validation, it could help clinicians in cases which may not be straightforward and could serve as an additional” instrument in the toolkit to reach a conclusive diagnosis, she added.
Asked to comment, session moderator Anupam Kotwal, MD, said in an interview: “I think it’s very exciting data. ... I envision its use in mild autonomous cortisol secretion, where there are not a lot of overt Cushing features but they may have a small adrenal mass. ... It might be used to guide care when there’s not a clear-cut answer.”
However, he cautioned that more validation is needed to determine the correlates of AGEs by different etiologies and magnitudes of cortisol excess.
Moreover, “skin can become thin in hypercortisolism, so is [the reader device] just detecting it more with skin testing? I think a blood test for validation would be a very good next step,” added Dr. Kotwal, who is an assistant professor in the division of diabetes, endocrinology and metabolism at the University of Nebraska, Omaha.
More work will be needed
Future directions for research should include adding a longitudinal arm and looking at the impact on AGE after patients undergo curative surgery and achieve remission, Dr. Sandooja explained.
“It will be interesting to see if AGE levels continue to be persistently high or decrease after patients achieve sustained remission of hypercortisolism. We are also interested in whether AGE measurement at baseline, prior to surgery may be associated with glucocorticoid withdrawal, myopathy, and metabolic outcomes following the surgery.”
Dr. Kotwal observed: “If the answer is clear for Cushing disease, I don’t know what extra information this would give. Maybe they would monitor people more closely afterward. It would be useful to see, but I think the first low-hanging fruit is use it in a way to guide the care of patients where we’re unclear as to whether initial treatment of this [mild autonomous cortisol secretion] is going to improve their outcomes.”
But, he added, “keeping in mind issues of skin ... we don’t want to distract clinicians and patients from using the tried and tested methods of characterizing Cushing syndrome. I’m always hesitant to bring something into practice before there is a little more information on how it can be used.”
Dr. Sandooja and Dr. Kotwal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AACE 2023
Men underrepresented in clinical trials of laser hair removal, review finds
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
AT ASLMS 2023
A legacy of unfair admissions
This is where people related to successful alumni and/or big donors can get preferential admission, possibly over more qualified people.
All of us likely experienced this from one side or another, though realistically I haven’t thought about it years. My kids went to the same state school I did, but I’m pretty sure I had nothing to do with their being accepted. I never gave the school a single donation, nor did I call anyone there to try and get them in. Not that anyone would have known who I was if I’d tried. I’m just another one of many who went there, preserved only in some filing cabinet of transcripts somewhere.
I’m all for the legacy system ending, though, for one simple reason: It’s not fair.
If someone is qualified, great. They should be admitted on their own merits. But if they’re not, they shouldn’t get into medical school just because one (or both) of their parents went there, or is a VIP, or paid for a new library wing.
The reason I’m writing this is because the recent reporting did bring back a memory.
A long time ago, when I was in college, I hung out with other premed students. We knew we were all competing with each other for the same spots at the state medical school, but also knew that we wouldn’t all get in there. That didn’t make us enemies, it was just the truth. It’s that point in life where ANY medical school admission is all you want.
Pete (not his real name) was a nice guy, but his grades weren’t the best. His MCAT scores lagged behind the rest of us in the clique, and ... he didn’t care.
Pete’s dad had graduated from the state medical school, and was still on staff there. He was now on the teaching staff ... and on the school’s admissions board. To Pete, tests and grades didn’t matter. His admission was assured.
So it was no surprise when he got in ahead of the rest of us with better qualifications. Most of us, including me, did get in somewhere, so we were still happy. We just had to move farther and pay more, but that’s life.
I really didn’t think much about Pete again after that. I was now in medical school, I had a whole new social group, and more importantly I didn’t really have time to think of much beyond when the next exam was.
Then I moved home, and started residency. During my PGY-2 year we had a changing group of medical students assigned to my wards rotation.
And, as you probably guessed, one of them was Pete.
Pete was in his last year of medical school. But we’d both started in the same year, and now I was 2 years ahead of him. I didn’t ask him what happened, but another medical student told me he wasn’t known to be the best student, but the university refused to drop him, and just kept setting him back a class here, a year there.
Maybe they’d have done the same for anyone, but I doubt it.
I never saw Pete again after that. When I looked him up online tonight he’s not listed as being a doctor, and isn’t even in medicine. Granted, a lot of doctors have left medicine, and maybe he did too.
But the more likely reason is that Pete never should have been there in the first place. He got in as a legacy, taking a medical school slot from someone who may have been more capable and driven.
And that just doesn’t seem right to me. It didn’t then and it doesn’t now.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This is where people related to successful alumni and/or big donors can get preferential admission, possibly over more qualified people.
All of us likely experienced this from one side or another, though realistically I haven’t thought about it years. My kids went to the same state school I did, but I’m pretty sure I had nothing to do with their being accepted. I never gave the school a single donation, nor did I call anyone there to try and get them in. Not that anyone would have known who I was if I’d tried. I’m just another one of many who went there, preserved only in some filing cabinet of transcripts somewhere.
I’m all for the legacy system ending, though, for one simple reason: It’s not fair.
If someone is qualified, great. They should be admitted on their own merits. But if they’re not, they shouldn’t get into medical school just because one (or both) of their parents went there, or is a VIP, or paid for a new library wing.
The reason I’m writing this is because the recent reporting did bring back a memory.
A long time ago, when I was in college, I hung out with other premed students. We knew we were all competing with each other for the same spots at the state medical school, but also knew that we wouldn’t all get in there. That didn’t make us enemies, it was just the truth. It’s that point in life where ANY medical school admission is all you want.
Pete (not his real name) was a nice guy, but his grades weren’t the best. His MCAT scores lagged behind the rest of us in the clique, and ... he didn’t care.
Pete’s dad had graduated from the state medical school, and was still on staff there. He was now on the teaching staff ... and on the school’s admissions board. To Pete, tests and grades didn’t matter. His admission was assured.
So it was no surprise when he got in ahead of the rest of us with better qualifications. Most of us, including me, did get in somewhere, so we were still happy. We just had to move farther and pay more, but that’s life.
I really didn’t think much about Pete again after that. I was now in medical school, I had a whole new social group, and more importantly I didn’t really have time to think of much beyond when the next exam was.
Then I moved home, and started residency. During my PGY-2 year we had a changing group of medical students assigned to my wards rotation.
And, as you probably guessed, one of them was Pete.
Pete was in his last year of medical school. But we’d both started in the same year, and now I was 2 years ahead of him. I didn’t ask him what happened, but another medical student told me he wasn’t known to be the best student, but the university refused to drop him, and just kept setting him back a class here, a year there.
Maybe they’d have done the same for anyone, but I doubt it.
I never saw Pete again after that. When I looked him up online tonight he’s not listed as being a doctor, and isn’t even in medicine. Granted, a lot of doctors have left medicine, and maybe he did too.
But the more likely reason is that Pete never should have been there in the first place. He got in as a legacy, taking a medical school slot from someone who may have been more capable and driven.
And that just doesn’t seem right to me. It didn’t then and it doesn’t now.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This is where people related to successful alumni and/or big donors can get preferential admission, possibly over more qualified people.
All of us likely experienced this from one side or another, though realistically I haven’t thought about it years. My kids went to the same state school I did, but I’m pretty sure I had nothing to do with their being accepted. I never gave the school a single donation, nor did I call anyone there to try and get them in. Not that anyone would have known who I was if I’d tried. I’m just another one of many who went there, preserved only in some filing cabinet of transcripts somewhere.
I’m all for the legacy system ending, though, for one simple reason: It’s not fair.
If someone is qualified, great. They should be admitted on their own merits. But if they’re not, they shouldn’t get into medical school just because one (or both) of their parents went there, or is a VIP, or paid for a new library wing.
The reason I’m writing this is because the recent reporting did bring back a memory.
A long time ago, when I was in college, I hung out with other premed students. We knew we were all competing with each other for the same spots at the state medical school, but also knew that we wouldn’t all get in there. That didn’t make us enemies, it was just the truth. It’s that point in life where ANY medical school admission is all you want.
Pete (not his real name) was a nice guy, but his grades weren’t the best. His MCAT scores lagged behind the rest of us in the clique, and ... he didn’t care.
Pete’s dad had graduated from the state medical school, and was still on staff there. He was now on the teaching staff ... and on the school’s admissions board. To Pete, tests and grades didn’t matter. His admission was assured.
So it was no surprise when he got in ahead of the rest of us with better qualifications. Most of us, including me, did get in somewhere, so we were still happy. We just had to move farther and pay more, but that’s life.
I really didn’t think much about Pete again after that. I was now in medical school, I had a whole new social group, and more importantly I didn’t really have time to think of much beyond when the next exam was.
Then I moved home, and started residency. During my PGY-2 year we had a changing group of medical students assigned to my wards rotation.
And, as you probably guessed, one of them was Pete.
Pete was in his last year of medical school. But we’d both started in the same year, and now I was 2 years ahead of him. I didn’t ask him what happened, but another medical student told me he wasn’t known to be the best student, but the university refused to drop him, and just kept setting him back a class here, a year there.
Maybe they’d have done the same for anyone, but I doubt it.
I never saw Pete again after that. When I looked him up online tonight he’s not listed as being a doctor, and isn’t even in medicine. Granted, a lot of doctors have left medicine, and maybe he did too.
But the more likely reason is that Pete never should have been there in the first place. He got in as a legacy, taking a medical school slot from someone who may have been more capable and driven.
And that just doesn’t seem right to me. It didn’t then and it doesn’t now.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
FDA moves to curb misuse of ADHD meds
“The current prescribing information for some prescription stimulants does not provide up-to-date warnings about the harms of misuse and abuse, and particularly that most individuals who misuse prescription stimulants get their drugs from other family members or peers,” the FDA said in a drug safety communication.
Going forward, updated drug labels will clearly state that patients should never share their prescription stimulants with anyone, and the boxed warning will describe the risks of misuse, abuse, addiction, and overdose consistently for all medicines in the class, the FDA said.
The boxed warning will also advise heath care professionals to monitor patients closely for signs and symptoms of misuse, abuse, and addiction.
Patient medication guides will be updated to educate patients and caregivers about these risks.
The FDA encourages prescribers to assess patient risk of misuse, abuse, and addiction before prescribing a stimulant and to counsel patients not to share the medication.
Friends and family
A recent literature review by the FDA found that friends and family members are the most common source of prescription stimulant misuse and abuse (nonmedical use). Estimates of such use range from 56% to 80%.
Misuse/abuse of a patient’s own prescription make up 10%-20% of people who report nonmedical stimulant use.
Less commonly reported sources include drug dealers or strangers (4%-7% of people who report nonmedical use) and the Internet (1%-2%).
The groups at highest risk for misuse/abuse of prescription stimulants are young adults aged 18-25 years, college students, and adolescents and young adults who have been diagnosed with ADHD, the FDA said.
Recent data from the Centers for Disease Control and Prevention show that during the first year of the COVID-19 pandemic, prescriptions for stimulants increased 10% among older children and adults.
A version of this article first appeared on Medscape.com.
“The current prescribing information for some prescription stimulants does not provide up-to-date warnings about the harms of misuse and abuse, and particularly that most individuals who misuse prescription stimulants get their drugs from other family members or peers,” the FDA said in a drug safety communication.
Going forward, updated drug labels will clearly state that patients should never share their prescription stimulants with anyone, and the boxed warning will describe the risks of misuse, abuse, addiction, and overdose consistently for all medicines in the class, the FDA said.
The boxed warning will also advise heath care professionals to monitor patients closely for signs and symptoms of misuse, abuse, and addiction.
Patient medication guides will be updated to educate patients and caregivers about these risks.
The FDA encourages prescribers to assess patient risk of misuse, abuse, and addiction before prescribing a stimulant and to counsel patients not to share the medication.
Friends and family
A recent literature review by the FDA found that friends and family members are the most common source of prescription stimulant misuse and abuse (nonmedical use). Estimates of such use range from 56% to 80%.
Misuse/abuse of a patient’s own prescription make up 10%-20% of people who report nonmedical stimulant use.
Less commonly reported sources include drug dealers or strangers (4%-7% of people who report nonmedical use) and the Internet (1%-2%).
The groups at highest risk for misuse/abuse of prescription stimulants are young adults aged 18-25 years, college students, and adolescents and young adults who have been diagnosed with ADHD, the FDA said.
Recent data from the Centers for Disease Control and Prevention show that during the first year of the COVID-19 pandemic, prescriptions for stimulants increased 10% among older children and adults.
A version of this article first appeared on Medscape.com.
“The current prescribing information for some prescription stimulants does not provide up-to-date warnings about the harms of misuse and abuse, and particularly that most individuals who misuse prescription stimulants get their drugs from other family members or peers,” the FDA said in a drug safety communication.
Going forward, updated drug labels will clearly state that patients should never share their prescription stimulants with anyone, and the boxed warning will describe the risks of misuse, abuse, addiction, and overdose consistently for all medicines in the class, the FDA said.
The boxed warning will also advise heath care professionals to monitor patients closely for signs and symptoms of misuse, abuse, and addiction.
Patient medication guides will be updated to educate patients and caregivers about these risks.
The FDA encourages prescribers to assess patient risk of misuse, abuse, and addiction before prescribing a stimulant and to counsel patients not to share the medication.
Friends and family
A recent literature review by the FDA found that friends and family members are the most common source of prescription stimulant misuse and abuse (nonmedical use). Estimates of such use range from 56% to 80%.
Misuse/abuse of a patient’s own prescription make up 10%-20% of people who report nonmedical stimulant use.
Less commonly reported sources include drug dealers or strangers (4%-7% of people who report nonmedical use) and the Internet (1%-2%).
The groups at highest risk for misuse/abuse of prescription stimulants are young adults aged 18-25 years, college students, and adolescents and young adults who have been diagnosed with ADHD, the FDA said.
Recent data from the Centers for Disease Control and Prevention show that during the first year of the COVID-19 pandemic, prescriptions for stimulants increased 10% among older children and adults.
A version of this article first appeared on Medscape.com.
Statins appear to guard against liver disease progression
The Swedish population-based study found that adults with noncirrhotic CLD who were on statin therapy had a statistically significant 40% lower risk of developing severe liver disease, compared with matched patients who were not on statin therapy.
The statin users were also less apt to progress to cirrhosis or hepatocellular carcinoma (HCC) and to die of liver disease, Rajani Sharma, MD, MSc, division of digestive and liver diseases, Columbia University Irving Medical Center, New York, and colleagues reported.
Their study was published online in Clinical Gastroenterology and Hepatology.
More than just cholesterol lowering
The study “continues the theme that cholesterol-lowering statins are good for a lot more things than just lowering cholesterol,” William Carey, MD, who wasn’t involved with the study, said in an interview.
The results are “very consistent with other trials that show that people with liver disease on statins do better in many respects than those who are not on statins,” said Dr. Carey, acting head of the hepatology section, department of gastroenterology, hepatology, and nutrition, Cleveland Clinic.
“The effects are not trivial,” Dr. Carey added. “It’s a very significant advantage in terms of fibrosis progression and survival.”
Statins have been shown to inhibit inflammatory pathways, promote endothelial cell function, and reduce hepatic stellate cell activity, suggesting that statins could lessen the progression of liver fibrosis, Dr. Sharma and coauthors wrote.
A few prior studies have looked at the effects of statins in noncirrhotic CLD specifically, but most only included patients with viral hepatitis, and the identification of precirrhotic liver disease was largely based on fibrosis scores or ICD coding, leading to a risk for misclassification and heterogeneity in results, they wrote.
Using histopathology data in a nationwide Swedish cohort, Dr. Sharma and colleagues identified 3862 adults with noncirrhotic CLD who were statin users and a like number of propensity score–matched nonstatin users with noncirrhotic CLD. The adults with CLD included in the study were required to have a liver biopsy showing fibrosis or inflammation between the years 1969 and 2017 and at least one ICD code for CLD.
In both groups, 45% of patients had nonalcoholic fatty liver disease (NAFLD), 22% had alcohol-related liver disease (ALD), 18% had viral hepatitis, and 15% had autoimmune hepatitis (AIH).
The analysis found 234 (6.1%) statin users developed severe liver disease versus 276 (7.1%) nonusers, with incidence rates of 10.5 versus 18.1 per 1,000 person-years, respectively.
Statin use was associated with a statistically significant 40% lower rate of severe liver disease (hazard ratio, 0.60; 95% confidence interval, 0.48-0.74).
This was the case in ALD (HR, 0.30; 95% CI, 0.19-0.49) and NAFLD (HR, 0.68; 95% CI 0.45-1.00), but the results were not statistically significant for individuals with viral hepatitis (HR, 0.76; 95% CI, 0.51-1.14) or AIH (HR, 0.88; 0.48-1.58).
Statin use had a protective association in both prefibrosis and fibrosis stages at diagnosis, the researchers reported.
Statin use was also associated with lower rates of progression to cirrhosis (HR, 0.62; 95% CI, 0.49-0.78), HCC (HR, 0.44; 95% CI, 0.27-0.71) and liver-related death or liver transplant (HR, 0.55; 95% CI, 0.36-0.82).
The authors noted that their “study provides the most robust estimates available thus far.” However, they cautioned that “prospective randomized controlled trials are necessary in order to recommend statin use in clinical practice.”
‘Reassuring and pleasantly surprising’
The study is “very interesting, reassuring, and pleasantly surprising,” Scott L. Friedman, MD, chief of the division of liver diseases and dean for therapeutic discovery at the Icahn School of Medicine at Mount Sinai. New York, said in an interview.
“Statins have been around for a long time, and in earlier days, there was fear of using them because they might induce liver injury. But ample and consistent data exclude the possibility that they are more toxic in patients with liver disease,” said Dr. Friedman, who was not associated with this research.
“What’s interesting and new about this paper is that those studies that have looked at the effects of statins on liver disease have primarily focused on patients who have cirrhosis because there’s some scientific evidence [that] statins can lead to vasodilation and reduce the elevated liver blood flow that occurs in cirrhosis,” he explained.
“Instead, this study, which is quite sizable, includes patients who do not have evidence of cirrhosis based on biopsies. The results suggest that statins have a significant protective effect in these patients,” Dr. Friedman said.
The study was supported by the Karolinska Institute in Stockholm, the Columbia University Irving Medical Center, the Swedish Research Council, the Swedish Cancer Society, and the U.S. National Institutes of Health. Dr. Sharma is a consultant for Takeda and Volv. Other coauthors reported current or past relationships with Bristol-Myers Squibb, Gilead, Salix, and GlaxoSmithKline. Dr. Carey and Dr. Friedman reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Swedish population-based study found that adults with noncirrhotic CLD who were on statin therapy had a statistically significant 40% lower risk of developing severe liver disease, compared with matched patients who were not on statin therapy.
The statin users were also less apt to progress to cirrhosis or hepatocellular carcinoma (HCC) and to die of liver disease, Rajani Sharma, MD, MSc, division of digestive and liver diseases, Columbia University Irving Medical Center, New York, and colleagues reported.
Their study was published online in Clinical Gastroenterology and Hepatology.
More than just cholesterol lowering
The study “continues the theme that cholesterol-lowering statins are good for a lot more things than just lowering cholesterol,” William Carey, MD, who wasn’t involved with the study, said in an interview.
The results are “very consistent with other trials that show that people with liver disease on statins do better in many respects than those who are not on statins,” said Dr. Carey, acting head of the hepatology section, department of gastroenterology, hepatology, and nutrition, Cleveland Clinic.
“The effects are not trivial,” Dr. Carey added. “It’s a very significant advantage in terms of fibrosis progression and survival.”
Statins have been shown to inhibit inflammatory pathways, promote endothelial cell function, and reduce hepatic stellate cell activity, suggesting that statins could lessen the progression of liver fibrosis, Dr. Sharma and coauthors wrote.
A few prior studies have looked at the effects of statins in noncirrhotic CLD specifically, but most only included patients with viral hepatitis, and the identification of precirrhotic liver disease was largely based on fibrosis scores or ICD coding, leading to a risk for misclassification and heterogeneity in results, they wrote.
Using histopathology data in a nationwide Swedish cohort, Dr. Sharma and colleagues identified 3862 adults with noncirrhotic CLD who were statin users and a like number of propensity score–matched nonstatin users with noncirrhotic CLD. The adults with CLD included in the study were required to have a liver biopsy showing fibrosis or inflammation between the years 1969 and 2017 and at least one ICD code for CLD.
In both groups, 45% of patients had nonalcoholic fatty liver disease (NAFLD), 22% had alcohol-related liver disease (ALD), 18% had viral hepatitis, and 15% had autoimmune hepatitis (AIH).
The analysis found 234 (6.1%) statin users developed severe liver disease versus 276 (7.1%) nonusers, with incidence rates of 10.5 versus 18.1 per 1,000 person-years, respectively.
Statin use was associated with a statistically significant 40% lower rate of severe liver disease (hazard ratio, 0.60; 95% confidence interval, 0.48-0.74).
This was the case in ALD (HR, 0.30; 95% CI, 0.19-0.49) and NAFLD (HR, 0.68; 95% CI 0.45-1.00), but the results were not statistically significant for individuals with viral hepatitis (HR, 0.76; 95% CI, 0.51-1.14) or AIH (HR, 0.88; 0.48-1.58).
Statin use had a protective association in both prefibrosis and fibrosis stages at diagnosis, the researchers reported.
Statin use was also associated with lower rates of progression to cirrhosis (HR, 0.62; 95% CI, 0.49-0.78), HCC (HR, 0.44; 95% CI, 0.27-0.71) and liver-related death or liver transplant (HR, 0.55; 95% CI, 0.36-0.82).
The authors noted that their “study provides the most robust estimates available thus far.” However, they cautioned that “prospective randomized controlled trials are necessary in order to recommend statin use in clinical practice.”
‘Reassuring and pleasantly surprising’
The study is “very interesting, reassuring, and pleasantly surprising,” Scott L. Friedman, MD, chief of the division of liver diseases and dean for therapeutic discovery at the Icahn School of Medicine at Mount Sinai. New York, said in an interview.
“Statins have been around for a long time, and in earlier days, there was fear of using them because they might induce liver injury. But ample and consistent data exclude the possibility that they are more toxic in patients with liver disease,” said Dr. Friedman, who was not associated with this research.
“What’s interesting and new about this paper is that those studies that have looked at the effects of statins on liver disease have primarily focused on patients who have cirrhosis because there’s some scientific evidence [that] statins can lead to vasodilation and reduce the elevated liver blood flow that occurs in cirrhosis,” he explained.
“Instead, this study, which is quite sizable, includes patients who do not have evidence of cirrhosis based on biopsies. The results suggest that statins have a significant protective effect in these patients,” Dr. Friedman said.
The study was supported by the Karolinska Institute in Stockholm, the Columbia University Irving Medical Center, the Swedish Research Council, the Swedish Cancer Society, and the U.S. National Institutes of Health. Dr. Sharma is a consultant for Takeda and Volv. Other coauthors reported current or past relationships with Bristol-Myers Squibb, Gilead, Salix, and GlaxoSmithKline. Dr. Carey and Dr. Friedman reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Swedish population-based study found that adults with noncirrhotic CLD who were on statin therapy had a statistically significant 40% lower risk of developing severe liver disease, compared with matched patients who were not on statin therapy.
The statin users were also less apt to progress to cirrhosis or hepatocellular carcinoma (HCC) and to die of liver disease, Rajani Sharma, MD, MSc, division of digestive and liver diseases, Columbia University Irving Medical Center, New York, and colleagues reported.
Their study was published online in Clinical Gastroenterology and Hepatology.
More than just cholesterol lowering
The study “continues the theme that cholesterol-lowering statins are good for a lot more things than just lowering cholesterol,” William Carey, MD, who wasn’t involved with the study, said in an interview.
The results are “very consistent with other trials that show that people with liver disease on statins do better in many respects than those who are not on statins,” said Dr. Carey, acting head of the hepatology section, department of gastroenterology, hepatology, and nutrition, Cleveland Clinic.
“The effects are not trivial,” Dr. Carey added. “It’s a very significant advantage in terms of fibrosis progression and survival.”
Statins have been shown to inhibit inflammatory pathways, promote endothelial cell function, and reduce hepatic stellate cell activity, suggesting that statins could lessen the progression of liver fibrosis, Dr. Sharma and coauthors wrote.
A few prior studies have looked at the effects of statins in noncirrhotic CLD specifically, but most only included patients with viral hepatitis, and the identification of precirrhotic liver disease was largely based on fibrosis scores or ICD coding, leading to a risk for misclassification and heterogeneity in results, they wrote.
Using histopathology data in a nationwide Swedish cohort, Dr. Sharma and colleagues identified 3862 adults with noncirrhotic CLD who were statin users and a like number of propensity score–matched nonstatin users with noncirrhotic CLD. The adults with CLD included in the study were required to have a liver biopsy showing fibrosis or inflammation between the years 1969 and 2017 and at least one ICD code for CLD.
In both groups, 45% of patients had nonalcoholic fatty liver disease (NAFLD), 22% had alcohol-related liver disease (ALD), 18% had viral hepatitis, and 15% had autoimmune hepatitis (AIH).
The analysis found 234 (6.1%) statin users developed severe liver disease versus 276 (7.1%) nonusers, with incidence rates of 10.5 versus 18.1 per 1,000 person-years, respectively.
Statin use was associated with a statistically significant 40% lower rate of severe liver disease (hazard ratio, 0.60; 95% confidence interval, 0.48-0.74).
This was the case in ALD (HR, 0.30; 95% CI, 0.19-0.49) and NAFLD (HR, 0.68; 95% CI 0.45-1.00), but the results were not statistically significant for individuals with viral hepatitis (HR, 0.76; 95% CI, 0.51-1.14) or AIH (HR, 0.88; 0.48-1.58).
Statin use had a protective association in both prefibrosis and fibrosis stages at diagnosis, the researchers reported.
Statin use was also associated with lower rates of progression to cirrhosis (HR, 0.62; 95% CI, 0.49-0.78), HCC (HR, 0.44; 95% CI, 0.27-0.71) and liver-related death or liver transplant (HR, 0.55; 95% CI, 0.36-0.82).
The authors noted that their “study provides the most robust estimates available thus far.” However, they cautioned that “prospective randomized controlled trials are necessary in order to recommend statin use in clinical practice.”
‘Reassuring and pleasantly surprising’
The study is “very interesting, reassuring, and pleasantly surprising,” Scott L. Friedman, MD, chief of the division of liver diseases and dean for therapeutic discovery at the Icahn School of Medicine at Mount Sinai. New York, said in an interview.
“Statins have been around for a long time, and in earlier days, there was fear of using them because they might induce liver injury. But ample and consistent data exclude the possibility that they are more toxic in patients with liver disease,” said Dr. Friedman, who was not associated with this research.
“What’s interesting and new about this paper is that those studies that have looked at the effects of statins on liver disease have primarily focused on patients who have cirrhosis because there’s some scientific evidence [that] statins can lead to vasodilation and reduce the elevated liver blood flow that occurs in cirrhosis,” he explained.
“Instead, this study, which is quite sizable, includes patients who do not have evidence of cirrhosis based on biopsies. The results suggest that statins have a significant protective effect in these patients,” Dr. Friedman said.
The study was supported by the Karolinska Institute in Stockholm, the Columbia University Irving Medical Center, the Swedish Research Council, the Swedish Cancer Society, and the U.S. National Institutes of Health. Dr. Sharma is a consultant for Takeda and Volv. Other coauthors reported current or past relationships with Bristol-Myers Squibb, Gilead, Salix, and GlaxoSmithKline. Dr. Carey and Dr. Friedman reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Facial, hand, and foot dermatitis: Lebrikizumab and dupilumab show efficacy in new studies
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
AT RAD 2023
FDA approves new drug to manage menopausal hot flashes
The Food and Drug Administration has approved the oral medication fezolinetant (Veozah) for the treatment of moderate to severe hot flashes in menopausal women, according to an FDA statement. The approved dose is 45 mg once daily.
Fezolinetant, a neurokinin 3 (NK3) receptor antagonist, is the first drug of its kind to earn FDA approval for the vasomotor symptoms associated with menopause, according to the statement. The drug works by binding to the NK3 receptor, which plays a role in regulating body temperature, and blocking its activity. Fezolinetant is not a hormone, and can be taken by women for whom hormones are contraindicated, such as those with a history of vaginal bleeding, stroke, heart attack, blood clots, or liver disease, the FDA stated.
The approval was based on data from the SKYLIGHT 2 trial, results of which were presented at the annual meeting of the Endocrine Society, reported by this news organization, and published in the Journal of Clinical Endocrinology and Metabolism.
In the two-phase trial, women were randomized to 30 mg or 45 mg of fezolinetant or a placebo. After 12 weeks, women in placebo groups were rerandomized to fezolinetant for a 40-week safety study.
The study population included women aged 40-65 years, with an average minimum of seven moderate-to-severe hot flashes per day. The study included 120 sites in North America and Europe.
At 12 weeks, both placebo and fezolinetant patients experienced reductions in moderate to severe vasomotor symptoms of approximately 60%, as well as a significant decrease in vasomotor symptom severity.
The FDA statement noted that patients should undergo baseline blood work before starting fezolinetant to test for liver infection or damage, and the prescribing information includes a warning for liver injury; blood work should be repeated at 3, 6, and 9 months after starting the medication, according to the FDA and a press release from the manufacturer Astellas.
The most common side effects associated with fezolinetant include abdominal pain, diarrhea, insomnia, back pain, hot flashes, and elevated liver values, according to the FDA statement. The FDA granted Astellas Pharma’s application a Priority Review designation. Astellas has priced the drug at $550 for a 30-day supply, significantly higher than the Institute for Clinical and Economic Review’s previously recommended range of $2,000 to $2,500 per year.
Full prescribing information is available here.
The Food and Drug Administration has approved the oral medication fezolinetant (Veozah) for the treatment of moderate to severe hot flashes in menopausal women, according to an FDA statement. The approved dose is 45 mg once daily.
Fezolinetant, a neurokinin 3 (NK3) receptor antagonist, is the first drug of its kind to earn FDA approval for the vasomotor symptoms associated with menopause, according to the statement. The drug works by binding to the NK3 receptor, which plays a role in regulating body temperature, and blocking its activity. Fezolinetant is not a hormone, and can be taken by women for whom hormones are contraindicated, such as those with a history of vaginal bleeding, stroke, heart attack, blood clots, or liver disease, the FDA stated.
The approval was based on data from the SKYLIGHT 2 trial, results of which were presented at the annual meeting of the Endocrine Society, reported by this news organization, and published in the Journal of Clinical Endocrinology and Metabolism.
In the two-phase trial, women were randomized to 30 mg or 45 mg of fezolinetant or a placebo. After 12 weeks, women in placebo groups were rerandomized to fezolinetant for a 40-week safety study.
The study population included women aged 40-65 years, with an average minimum of seven moderate-to-severe hot flashes per day. The study included 120 sites in North America and Europe.
At 12 weeks, both placebo and fezolinetant patients experienced reductions in moderate to severe vasomotor symptoms of approximately 60%, as well as a significant decrease in vasomotor symptom severity.
The FDA statement noted that patients should undergo baseline blood work before starting fezolinetant to test for liver infection or damage, and the prescribing information includes a warning for liver injury; blood work should be repeated at 3, 6, and 9 months after starting the medication, according to the FDA and a press release from the manufacturer Astellas.
The most common side effects associated with fezolinetant include abdominal pain, diarrhea, insomnia, back pain, hot flashes, and elevated liver values, according to the FDA statement. The FDA granted Astellas Pharma’s application a Priority Review designation. Astellas has priced the drug at $550 for a 30-day supply, significantly higher than the Institute for Clinical and Economic Review’s previously recommended range of $2,000 to $2,500 per year.
Full prescribing information is available here.
The Food and Drug Administration has approved the oral medication fezolinetant (Veozah) for the treatment of moderate to severe hot flashes in menopausal women, according to an FDA statement. The approved dose is 45 mg once daily.
Fezolinetant, a neurokinin 3 (NK3) receptor antagonist, is the first drug of its kind to earn FDA approval for the vasomotor symptoms associated with menopause, according to the statement. The drug works by binding to the NK3 receptor, which plays a role in regulating body temperature, and blocking its activity. Fezolinetant is not a hormone, and can be taken by women for whom hormones are contraindicated, such as those with a history of vaginal bleeding, stroke, heart attack, blood clots, or liver disease, the FDA stated.
The approval was based on data from the SKYLIGHT 2 trial, results of which were presented at the annual meeting of the Endocrine Society, reported by this news organization, and published in the Journal of Clinical Endocrinology and Metabolism.
In the two-phase trial, women were randomized to 30 mg or 45 mg of fezolinetant or a placebo. After 12 weeks, women in placebo groups were rerandomized to fezolinetant for a 40-week safety study.
The study population included women aged 40-65 years, with an average minimum of seven moderate-to-severe hot flashes per day. The study included 120 sites in North America and Europe.
At 12 weeks, both placebo and fezolinetant patients experienced reductions in moderate to severe vasomotor symptoms of approximately 60%, as well as a significant decrease in vasomotor symptom severity.
The FDA statement noted that patients should undergo baseline blood work before starting fezolinetant to test for liver infection or damage, and the prescribing information includes a warning for liver injury; blood work should be repeated at 3, 6, and 9 months after starting the medication, according to the FDA and a press release from the manufacturer Astellas.
The most common side effects associated with fezolinetant include abdominal pain, diarrhea, insomnia, back pain, hot flashes, and elevated liver values, according to the FDA statement. The FDA granted Astellas Pharma’s application a Priority Review designation. Astellas has priced the drug at $550 for a 30-day supply, significantly higher than the Institute for Clinical and Economic Review’s previously recommended range of $2,000 to $2,500 per year.
Full prescribing information is available here.