User login
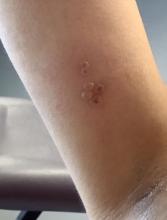
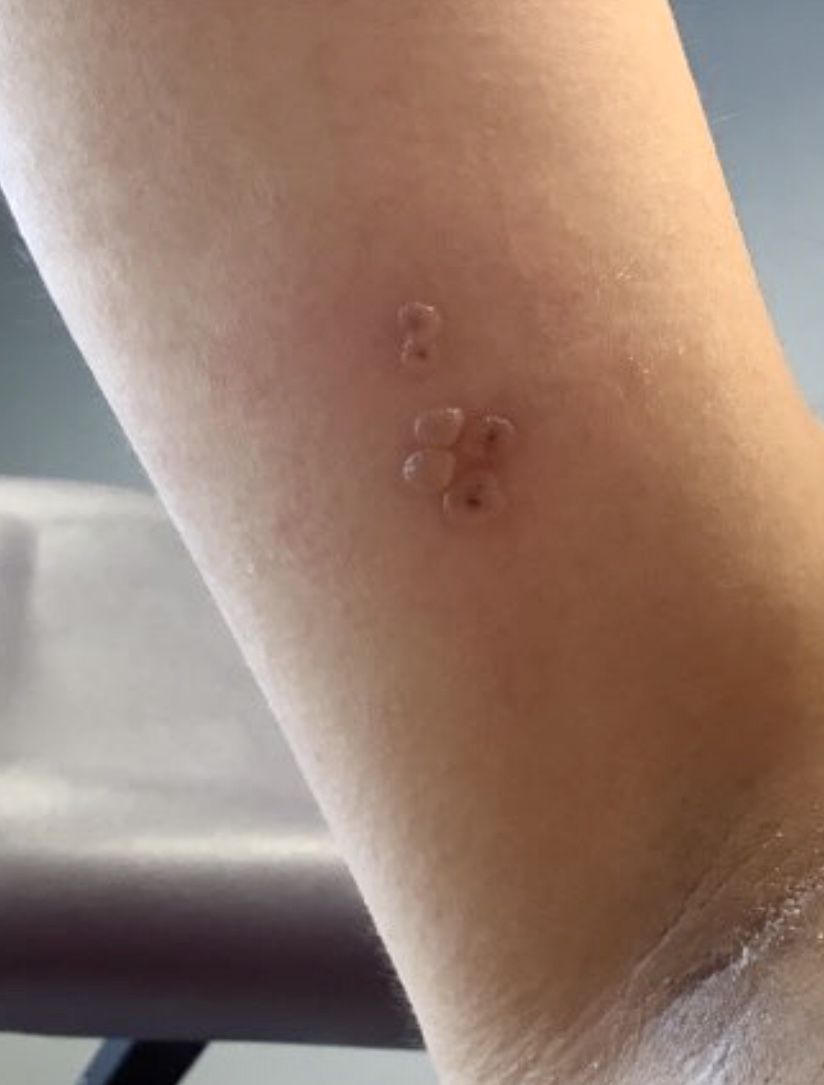
A healthy 36-year-old female presented with 4 days of itchy lesions on the right upper extremity
Additionally, Orthopox DNA by PCR and Monkeypox (mpox) virus DNA by PCR were detected. Herpes simplex virus and bacterial viral cultures were negative. Valacyclovir was started at the time of presentation and the patient’s lesions resolved without sequelae.
Mpox is a zoonotic double-stranded DNA virus that is part of the Orthopoxvirus family, including the West African and Central African variants. This disease presents similarly to smallpox, so most mpox research was conducted around the time smallpox was eradicated. It was not until 1970, when the disease was isolated from a patient with suspected smallpox in the Democratic Republic of the Congo (DRC), that human mpox was considered a distinct disease. An epidemic outbreak in the United States occurred in 2003 related to infected prairie dogs, and travel-related outbreaks have been more recently reported up until May 2022, in which mpox was reported in nonendemic areas including North America, Europe, and Australia. Most cases in this outbreak occurred in men who have sex with men (MSM), but this is not always the case, and mpox is not necessarily considered a sexually transmitted infection. Mpox presents similarly to smallpox and VZV, so using laboratory tests is important in diagnosing and tracking this disease.
Although it is not easily transmitted, the disease can spread through bodily secretions both directly and indirectly. Mpox typically begins with a prodrome that includes fever, headache, myalgia, and fatigue. This is followed by lymphadenopathy that precedes and coincides with rash development. The lymph nodes are firm, tender, may be painful, and are a defining factor in presentation that differs from smallpox and varicella. The rash typically starts on the face, then presents on the body in a centrifugal distribution. However, cases related to sexual transmission present with anogenital lesions. The lesions are characterized by a progression from maculopapular to vesiculopustular, and can vary widely in quantity.
Notably, individuals are contagious from the onset of the prodrome until the lesions have scabbed over and fallen off. The eruptive nature of the later lesions poses a threat of secondary infection, and is often accompanied by a second febrile period that signifies deterioration of the patient’s condition. Other signs of secondary infection are variable and include pulmonary symptoms, vomiting, diarrhea, ocular infections, and in rare cases, encephalitis. These sequelae are more common in unvaccinated and immunocompromised individuals. Long-term complications of mpox include pitted scarring from cutaneous lesions with children being more susceptible to severe disease. The mortality rate for the disease is very low. (As of May 10, 2023, there have been 30,395 mpox cases reported in the United States, and 42 deaths, according to the Centers for Disease Control and Prevention.)
There are a variety of diagnostic tests that can aid in mpox identification, but they are most strongly supported when combined with clinical and epidemiological data. The best, least invasive method includes collection of lesion exudate or crust on a swab, and viral DNA is best preserved by keeping the specimen in a cool, dry, and dark environment. PCR is considered the standard, and electron microscopy and immunohistochemistry are valid tests, but all modalities require sophisticated technicians with the proper laboratory equipment. This is limiting because many cases present in underserved areas that lack the facilities for proper, real-time analysis. Antigen and antibody-based tests can be used, but cross-reactivity of other orthopoxviridae limits confirmation of mpox infection. Vaccination status, history and location must be considered.
Vaccination is the chief form of prevention for mpox, although it is not considered entirely protective. Smallpox vaccination provides protection, but widespread administration of the vaccine is no longer practiced, and an estimated 70% of the global population is no longer vaccinated. Vaccination is recommended for anyone at risk of exposure, but as this is a live, attenuated vaccine, the immune status of the patient is important to keep in mind. Tecovirimat and other antiviral medications including cidofovir and brincidofovir may be considered in severe cases.
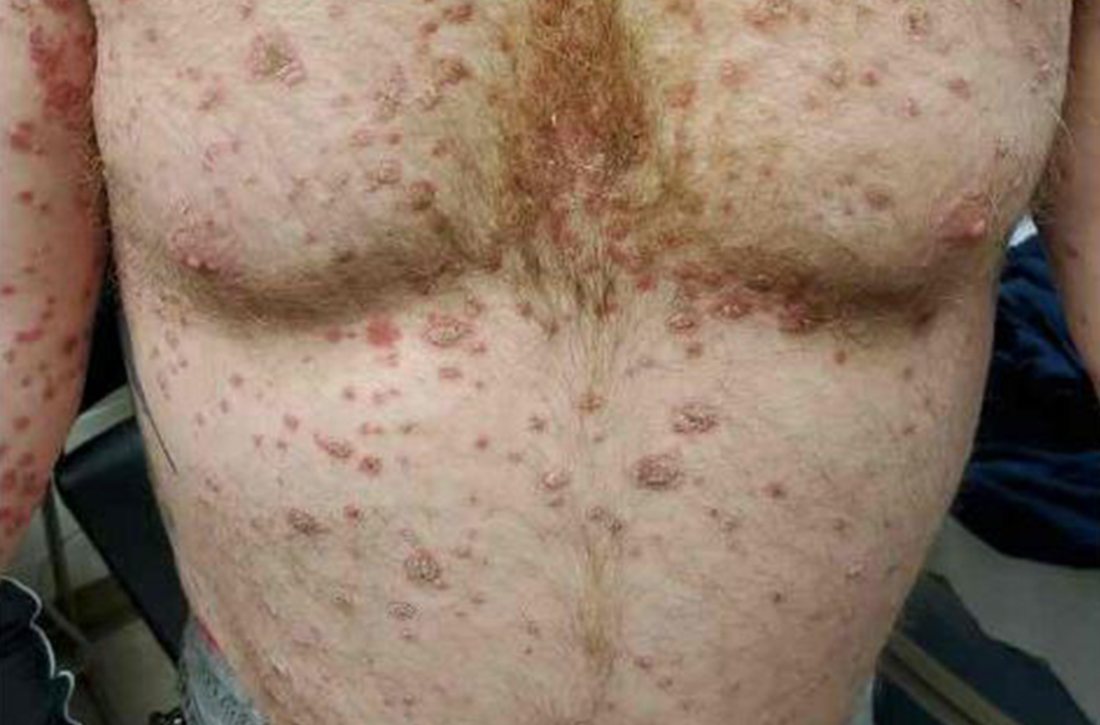
This case is unique as our patient, who had no known risk factors for mpox, presented with mpox and VZV, simultaneously. Although clinical presentation and epidemiological patterns between these diseases differ, there have been a limited number of cases of coinfection reported in the literature, mainly in the DRC where mpox is endemic. Diagnosis must be made by separate laboratory tests and there are differences in presentation between independent and coinfection for these viruses. Notably, patients with mpox/VZV coinfection may be less likely to present with lesions on the face, thorax, arms, palms, and soles than those with only mpox but experience a higher lesion burden than those afflicted by only VZV. Coinfection may be related to reactivation of dormant VZV, or increased susceptibility to secondary infection when infected with one virus.
This case and photo were submitted by Lucas Shapiro, BS, of the Dr. Kiran C. Patel College of Osteopathic Medicine at Nova Southeastern University, Fort Lauderdale, Fla., and Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Macneil A et al. Clin Infect Dis. 2009 Jan 1;48(1):e6-8.
2. Di Gennaro F et al. Microorganisms. 2022 Aug 12;10(8):1633.
3. Hughes CM et al. Am J Trop Med Hyg. 2020 Dec 7;104(2):604-11.
Additionally, Orthopox DNA by PCR and Monkeypox (mpox) virus DNA by PCR were detected. Herpes simplex virus and bacterial viral cultures were negative. Valacyclovir was started at the time of presentation and the patient’s lesions resolved without sequelae.
Mpox is a zoonotic double-stranded DNA virus that is part of the Orthopoxvirus family, including the West African and Central African variants. This disease presents similarly to smallpox, so most mpox research was conducted around the time smallpox was eradicated. It was not until 1970, when the disease was isolated from a patient with suspected smallpox in the Democratic Republic of the Congo (DRC), that human mpox was considered a distinct disease. An epidemic outbreak in the United States occurred in 2003 related to infected prairie dogs, and travel-related outbreaks have been more recently reported up until May 2022, in which mpox was reported in nonendemic areas including North America, Europe, and Australia. Most cases in this outbreak occurred in men who have sex with men (MSM), but this is not always the case, and mpox is not necessarily considered a sexually transmitted infection. Mpox presents similarly to smallpox and VZV, so using laboratory tests is important in diagnosing and tracking this disease.
Although it is not easily transmitted, the disease can spread through bodily secretions both directly and indirectly. Mpox typically begins with a prodrome that includes fever, headache, myalgia, and fatigue. This is followed by lymphadenopathy that precedes and coincides with rash development. The lymph nodes are firm, tender, may be painful, and are a defining factor in presentation that differs from smallpox and varicella. The rash typically starts on the face, then presents on the body in a centrifugal distribution. However, cases related to sexual transmission present with anogenital lesions. The lesions are characterized by a progression from maculopapular to vesiculopustular, and can vary widely in quantity.
Notably, individuals are contagious from the onset of the prodrome until the lesions have scabbed over and fallen off. The eruptive nature of the later lesions poses a threat of secondary infection, and is often accompanied by a second febrile period that signifies deterioration of the patient’s condition. Other signs of secondary infection are variable and include pulmonary symptoms, vomiting, diarrhea, ocular infections, and in rare cases, encephalitis. These sequelae are more common in unvaccinated and immunocompromised individuals. Long-term complications of mpox include pitted scarring from cutaneous lesions with children being more susceptible to severe disease. The mortality rate for the disease is very low. (As of May 10, 2023, there have been 30,395 mpox cases reported in the United States, and 42 deaths, according to the Centers for Disease Control and Prevention.)
There are a variety of diagnostic tests that can aid in mpox identification, but they are most strongly supported when combined with clinical and epidemiological data. The best, least invasive method includes collection of lesion exudate or crust on a swab, and viral DNA is best preserved by keeping the specimen in a cool, dry, and dark environment. PCR is considered the standard, and electron microscopy and immunohistochemistry are valid tests, but all modalities require sophisticated technicians with the proper laboratory equipment. This is limiting because many cases present in underserved areas that lack the facilities for proper, real-time analysis. Antigen and antibody-based tests can be used, but cross-reactivity of other orthopoxviridae limits confirmation of mpox infection. Vaccination status, history and location must be considered.
Vaccination is the chief form of prevention for mpox, although it is not considered entirely protective. Smallpox vaccination provides protection, but widespread administration of the vaccine is no longer practiced, and an estimated 70% of the global population is no longer vaccinated. Vaccination is recommended for anyone at risk of exposure, but as this is a live, attenuated vaccine, the immune status of the patient is important to keep in mind. Tecovirimat and other antiviral medications including cidofovir and brincidofovir may be considered in severe cases.
This case is unique as our patient, who had no known risk factors for mpox, presented with mpox and VZV, simultaneously. Although clinical presentation and epidemiological patterns between these diseases differ, there have been a limited number of cases of coinfection reported in the literature, mainly in the DRC where mpox is endemic. Diagnosis must be made by separate laboratory tests and there are differences in presentation between independent and coinfection for these viruses. Notably, patients with mpox/VZV coinfection may be less likely to present with lesions on the face, thorax, arms, palms, and soles than those with only mpox but experience a higher lesion burden than those afflicted by only VZV. Coinfection may be related to reactivation of dormant VZV, or increased susceptibility to secondary infection when infected with one virus.
This case and photo were submitted by Lucas Shapiro, BS, of the Dr. Kiran C. Patel College of Osteopathic Medicine at Nova Southeastern University, Fort Lauderdale, Fla., and Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Macneil A et al. Clin Infect Dis. 2009 Jan 1;48(1):e6-8.
2. Di Gennaro F et al. Microorganisms. 2022 Aug 12;10(8):1633.
3. Hughes CM et al. Am J Trop Med Hyg. 2020 Dec 7;104(2):604-11.
Additionally, Orthopox DNA by PCR and Monkeypox (mpox) virus DNA by PCR were detected. Herpes simplex virus and bacterial viral cultures were negative. Valacyclovir was started at the time of presentation and the patient’s lesions resolved without sequelae.
Mpox is a zoonotic double-stranded DNA virus that is part of the Orthopoxvirus family, including the West African and Central African variants. This disease presents similarly to smallpox, so most mpox research was conducted around the time smallpox was eradicated. It was not until 1970, when the disease was isolated from a patient with suspected smallpox in the Democratic Republic of the Congo (DRC), that human mpox was considered a distinct disease. An epidemic outbreak in the United States occurred in 2003 related to infected prairie dogs, and travel-related outbreaks have been more recently reported up until May 2022, in which mpox was reported in nonendemic areas including North America, Europe, and Australia. Most cases in this outbreak occurred in men who have sex with men (MSM), but this is not always the case, and mpox is not necessarily considered a sexually transmitted infection. Mpox presents similarly to smallpox and VZV, so using laboratory tests is important in diagnosing and tracking this disease.
Although it is not easily transmitted, the disease can spread through bodily secretions both directly and indirectly. Mpox typically begins with a prodrome that includes fever, headache, myalgia, and fatigue. This is followed by lymphadenopathy that precedes and coincides with rash development. The lymph nodes are firm, tender, may be painful, and are a defining factor in presentation that differs from smallpox and varicella. The rash typically starts on the face, then presents on the body in a centrifugal distribution. However, cases related to sexual transmission present with anogenital lesions. The lesions are characterized by a progression from maculopapular to vesiculopustular, and can vary widely in quantity.
Notably, individuals are contagious from the onset of the prodrome until the lesions have scabbed over and fallen off. The eruptive nature of the later lesions poses a threat of secondary infection, and is often accompanied by a second febrile period that signifies deterioration of the patient’s condition. Other signs of secondary infection are variable and include pulmonary symptoms, vomiting, diarrhea, ocular infections, and in rare cases, encephalitis. These sequelae are more common in unvaccinated and immunocompromised individuals. Long-term complications of mpox include pitted scarring from cutaneous lesions with children being more susceptible to severe disease. The mortality rate for the disease is very low. (As of May 10, 2023, there have been 30,395 mpox cases reported in the United States, and 42 deaths, according to the Centers for Disease Control and Prevention.)
There are a variety of diagnostic tests that can aid in mpox identification, but they are most strongly supported when combined with clinical and epidemiological data. The best, least invasive method includes collection of lesion exudate or crust on a swab, and viral DNA is best preserved by keeping the specimen in a cool, dry, and dark environment. PCR is considered the standard, and electron microscopy and immunohistochemistry are valid tests, but all modalities require sophisticated technicians with the proper laboratory equipment. This is limiting because many cases present in underserved areas that lack the facilities for proper, real-time analysis. Antigen and antibody-based tests can be used, but cross-reactivity of other orthopoxviridae limits confirmation of mpox infection. Vaccination status, history and location must be considered.
Vaccination is the chief form of prevention for mpox, although it is not considered entirely protective. Smallpox vaccination provides protection, but widespread administration of the vaccine is no longer practiced, and an estimated 70% of the global population is no longer vaccinated. Vaccination is recommended for anyone at risk of exposure, but as this is a live, attenuated vaccine, the immune status of the patient is important to keep in mind. Tecovirimat and other antiviral medications including cidofovir and brincidofovir may be considered in severe cases.
This case is unique as our patient, who had no known risk factors for mpox, presented with mpox and VZV, simultaneously. Although clinical presentation and epidemiological patterns between these diseases differ, there have been a limited number of cases of coinfection reported in the literature, mainly in the DRC where mpox is endemic. Diagnosis must be made by separate laboratory tests and there are differences in presentation between independent and coinfection for these viruses. Notably, patients with mpox/VZV coinfection may be less likely to present with lesions on the face, thorax, arms, palms, and soles than those with only mpox but experience a higher lesion burden than those afflicted by only VZV. Coinfection may be related to reactivation of dormant VZV, or increased susceptibility to secondary infection when infected with one virus.
This case and photo were submitted by Lucas Shapiro, BS, of the Dr. Kiran C. Patel College of Osteopathic Medicine at Nova Southeastern University, Fort Lauderdale, Fla., and Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Macneil A et al. Clin Infect Dis. 2009 Jan 1;48(1):e6-8.
2. Di Gennaro F et al. Microorganisms. 2022 Aug 12;10(8):1633.
3. Hughes CM et al. Am J Trop Med Hyg. 2020 Dec 7;104(2):604-11.
CGM completes picture of A1c in type 2 diabetes
in a post hoc analysis of the SWITCH PRO clinical trial.
TIR was inversely related to A1c, with the strongest correlation following treatment intensification.
However, “there was a wide scatter of data, indicating that TIR (and other metrics) provides information about glycemic control that cannot be discerned from A1c alone, and which at least complements it,” Ronald M. Goldenberg, MD, from LMC Diabetes & Endocrinology in Thornhill, Ont., and colleagues write in their article published in Diabetes Therapy.
Other work has shown that more than a third of people with type 2 diabetes are not achieving the internationally recommended A1c target of < 7% to 8.5%, they note.
When used with A1c, CGM data – such as TIR, time below range (TBR), and time above range (TAR) – “provide a more complete picture of glucose levels throughout the day and night,” they write.
“This may help empower people with diabetes to better manage their condition, giving them practical insights into the factors driving daily fluctuations in glucose levels, such as diet, exercise, insulin dosage, and insulin timing,” they add. “These metrics may also be used to inform treatment decisions by health care professionals.”
“Ultimately,” the researchers conclude, “it is hoped that the use of these new metrics should lead to an improved quality of glycemic control and, in turn, to a reduction in the number of diabetes-related complications.”
‘Important study’
Invited to comment, Celeste C. Thomas, MD, who was not involved with the research, said: “This study is important because it is consistent with previous analyses that found a correlation between TIR and A1c.”
But, “I was surprised by the scatter plots which identified participants with TIR of 70% that also had A1c > 9%,” she added. “This highlights the importance of using multiple glycemic metrics to understand an individual’s risk for diabetes complications and to be aware of the limitations of the metrics.”
Dr. Thomas, from the University of Chicago, also noted that CGM is used in endocrinology clinics and increasingly in primary care clinics, “often to determine glycemic patterns to optimize therapeutic management but also to review TIR and, importantly, time below range to reduce the incidence of hypoglycemia.”
And people with type 2 diabetes are using CGM, Dr. Thomas noted, to understand their individual responses to medications, food choices, sleep quality and duration, exercise, and other day-to-day variables that affect glucose levels. “In my clinical practice, the information provided by personal CGM is empowering,” she said.
Effective April 4, 2023, Medicare “allows for the coverage of CGM in patients [with type 2 diabetes] treated with one injection of insulin daily and those not taking insulin but with a history of hypoglycemia,” Dr. Thomas noted, whereas “previously, patients needed to be prescribed at least three injections of insulin daily. Other insurers will hopefully soon follow.”
“I foresee CGM and TIR being widely used in clinical practice for people living with type 2 diabetes,” she said, “especially those who have ever had an A1c over 8%, those with a history of hypoglycemia, and those treated with medications that are known to cause hypoglycemia.”
How does TIR compare with A1c?
Dr. Goldenberg and colleagues set out to better understand how the emerging TIR metric compares with the traditional A1c value.
They performed a post-hoc analysis of data from the phase 4 SWITCH PRO study of basal insulin–treated patients with type 2 diabetes with at least one risk factor for hypoglycemia.
The patients were treated with insulin degludec or glargine 100 during a 16-week titration and 2-week maintenance phase, and then crossed over to the other treatment for the same time periods.
Glycemic control was evaluated using a blinded professional CGM (Abbott Freestyle Libro Pro). The primary outcome was TIR, which was defined as the percentage of time spent in the blood glucose range of 70-180 mg/dL.
There were 419 participants in the full analysis. Patients were a mean age of 63 and 48% were men. They had a mean body mass index of 32 kg/m2 and had diabetes for a mean of 15 years.
There was a moderate inverse linear correlation between TIR and A1c at baseline, which became stronger following treatment intensification during the maintenance periods in the full cohort, and in a subgroup of patients with median A1c ≥ 7.5% (212 patients).
This correlation between TIR and A1c was poorer in the subgroup of patients with baseline median A1c < 7.5% (307 patients).
The data were widely scattered, “supporting the premise that A1c and TIR can be relatively crude surrogates of each other when it comes to individual patients,” Dr. Goldenberg and colleagues note.
Where individual patients have both low A1c and low TIR values, this might indicate frequent episodes of hypoglycemia.
A few individual patients had TIR > 70% but A1c approaching 9%. These patients may have different red blood cell physiology whereby A1c does not reflect average glycemic values, the researchers suggest.
The study was sponsored by Novo Nordisk and several authors are Novo Nordisk employees. The complete author disclosures are listed with the article. Dr. Thomas has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a post hoc analysis of the SWITCH PRO clinical trial.
TIR was inversely related to A1c, with the strongest correlation following treatment intensification.
However, “there was a wide scatter of data, indicating that TIR (and other metrics) provides information about glycemic control that cannot be discerned from A1c alone, and which at least complements it,” Ronald M. Goldenberg, MD, from LMC Diabetes & Endocrinology in Thornhill, Ont., and colleagues write in their article published in Diabetes Therapy.
Other work has shown that more than a third of people with type 2 diabetes are not achieving the internationally recommended A1c target of < 7% to 8.5%, they note.
When used with A1c, CGM data – such as TIR, time below range (TBR), and time above range (TAR) – “provide a more complete picture of glucose levels throughout the day and night,” they write.
“This may help empower people with diabetes to better manage their condition, giving them practical insights into the factors driving daily fluctuations in glucose levels, such as diet, exercise, insulin dosage, and insulin timing,” they add. “These metrics may also be used to inform treatment decisions by health care professionals.”
“Ultimately,” the researchers conclude, “it is hoped that the use of these new metrics should lead to an improved quality of glycemic control and, in turn, to a reduction in the number of diabetes-related complications.”
‘Important study’
Invited to comment, Celeste C. Thomas, MD, who was not involved with the research, said: “This study is important because it is consistent with previous analyses that found a correlation between TIR and A1c.”
But, “I was surprised by the scatter plots which identified participants with TIR of 70% that also had A1c > 9%,” she added. “This highlights the importance of using multiple glycemic metrics to understand an individual’s risk for diabetes complications and to be aware of the limitations of the metrics.”
Dr. Thomas, from the University of Chicago, also noted that CGM is used in endocrinology clinics and increasingly in primary care clinics, “often to determine glycemic patterns to optimize therapeutic management but also to review TIR and, importantly, time below range to reduce the incidence of hypoglycemia.”
And people with type 2 diabetes are using CGM, Dr. Thomas noted, to understand their individual responses to medications, food choices, sleep quality and duration, exercise, and other day-to-day variables that affect glucose levels. “In my clinical practice, the information provided by personal CGM is empowering,” she said.
Effective April 4, 2023, Medicare “allows for the coverage of CGM in patients [with type 2 diabetes] treated with one injection of insulin daily and those not taking insulin but with a history of hypoglycemia,” Dr. Thomas noted, whereas “previously, patients needed to be prescribed at least three injections of insulin daily. Other insurers will hopefully soon follow.”
“I foresee CGM and TIR being widely used in clinical practice for people living with type 2 diabetes,” she said, “especially those who have ever had an A1c over 8%, those with a history of hypoglycemia, and those treated with medications that are known to cause hypoglycemia.”
How does TIR compare with A1c?
Dr. Goldenberg and colleagues set out to better understand how the emerging TIR metric compares with the traditional A1c value.
They performed a post-hoc analysis of data from the phase 4 SWITCH PRO study of basal insulin–treated patients with type 2 diabetes with at least one risk factor for hypoglycemia.
The patients were treated with insulin degludec or glargine 100 during a 16-week titration and 2-week maintenance phase, and then crossed over to the other treatment for the same time periods.
Glycemic control was evaluated using a blinded professional CGM (Abbott Freestyle Libro Pro). The primary outcome was TIR, which was defined as the percentage of time spent in the blood glucose range of 70-180 mg/dL.
There were 419 participants in the full analysis. Patients were a mean age of 63 and 48% were men. They had a mean body mass index of 32 kg/m2 and had diabetes for a mean of 15 years.
There was a moderate inverse linear correlation between TIR and A1c at baseline, which became stronger following treatment intensification during the maintenance periods in the full cohort, and in a subgroup of patients with median A1c ≥ 7.5% (212 patients).
This correlation between TIR and A1c was poorer in the subgroup of patients with baseline median A1c < 7.5% (307 patients).
The data were widely scattered, “supporting the premise that A1c and TIR can be relatively crude surrogates of each other when it comes to individual patients,” Dr. Goldenberg and colleagues note.
Where individual patients have both low A1c and low TIR values, this might indicate frequent episodes of hypoglycemia.
A few individual patients had TIR > 70% but A1c approaching 9%. These patients may have different red blood cell physiology whereby A1c does not reflect average glycemic values, the researchers suggest.
The study was sponsored by Novo Nordisk and several authors are Novo Nordisk employees. The complete author disclosures are listed with the article. Dr. Thomas has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a post hoc analysis of the SWITCH PRO clinical trial.
TIR was inversely related to A1c, with the strongest correlation following treatment intensification.
However, “there was a wide scatter of data, indicating that TIR (and other metrics) provides information about glycemic control that cannot be discerned from A1c alone, and which at least complements it,” Ronald M. Goldenberg, MD, from LMC Diabetes & Endocrinology in Thornhill, Ont., and colleagues write in their article published in Diabetes Therapy.
Other work has shown that more than a third of people with type 2 diabetes are not achieving the internationally recommended A1c target of < 7% to 8.5%, they note.
When used with A1c, CGM data – such as TIR, time below range (TBR), and time above range (TAR) – “provide a more complete picture of glucose levels throughout the day and night,” they write.
“This may help empower people with diabetes to better manage their condition, giving them practical insights into the factors driving daily fluctuations in glucose levels, such as diet, exercise, insulin dosage, and insulin timing,” they add. “These metrics may also be used to inform treatment decisions by health care professionals.”
“Ultimately,” the researchers conclude, “it is hoped that the use of these new metrics should lead to an improved quality of glycemic control and, in turn, to a reduction in the number of diabetes-related complications.”
‘Important study’
Invited to comment, Celeste C. Thomas, MD, who was not involved with the research, said: “This study is important because it is consistent with previous analyses that found a correlation between TIR and A1c.”
But, “I was surprised by the scatter plots which identified participants with TIR of 70% that also had A1c > 9%,” she added. “This highlights the importance of using multiple glycemic metrics to understand an individual’s risk for diabetes complications and to be aware of the limitations of the metrics.”
Dr. Thomas, from the University of Chicago, also noted that CGM is used in endocrinology clinics and increasingly in primary care clinics, “often to determine glycemic patterns to optimize therapeutic management but also to review TIR and, importantly, time below range to reduce the incidence of hypoglycemia.”
And people with type 2 diabetes are using CGM, Dr. Thomas noted, to understand their individual responses to medications, food choices, sleep quality and duration, exercise, and other day-to-day variables that affect glucose levels. “In my clinical practice, the information provided by personal CGM is empowering,” she said.
Effective April 4, 2023, Medicare “allows for the coverage of CGM in patients [with type 2 diabetes] treated with one injection of insulin daily and those not taking insulin but with a history of hypoglycemia,” Dr. Thomas noted, whereas “previously, patients needed to be prescribed at least three injections of insulin daily. Other insurers will hopefully soon follow.”
“I foresee CGM and TIR being widely used in clinical practice for people living with type 2 diabetes,” she said, “especially those who have ever had an A1c over 8%, those with a history of hypoglycemia, and those treated with medications that are known to cause hypoglycemia.”
How does TIR compare with A1c?
Dr. Goldenberg and colleagues set out to better understand how the emerging TIR metric compares with the traditional A1c value.
They performed a post-hoc analysis of data from the phase 4 SWITCH PRO study of basal insulin–treated patients with type 2 diabetes with at least one risk factor for hypoglycemia.
The patients were treated with insulin degludec or glargine 100 during a 16-week titration and 2-week maintenance phase, and then crossed over to the other treatment for the same time periods.
Glycemic control was evaluated using a blinded professional CGM (Abbott Freestyle Libro Pro). The primary outcome was TIR, which was defined as the percentage of time spent in the blood glucose range of 70-180 mg/dL.
There were 419 participants in the full analysis. Patients were a mean age of 63 and 48% were men. They had a mean body mass index of 32 kg/m2 and had diabetes for a mean of 15 years.
There was a moderate inverse linear correlation between TIR and A1c at baseline, which became stronger following treatment intensification during the maintenance periods in the full cohort, and in a subgroup of patients with median A1c ≥ 7.5% (212 patients).
This correlation between TIR and A1c was poorer in the subgroup of patients with baseline median A1c < 7.5% (307 patients).
The data were widely scattered, “supporting the premise that A1c and TIR can be relatively crude surrogates of each other when it comes to individual patients,” Dr. Goldenberg and colleagues note.
Where individual patients have both low A1c and low TIR values, this might indicate frequent episodes of hypoglycemia.
A few individual patients had TIR > 70% but A1c approaching 9%. These patients may have different red blood cell physiology whereby A1c does not reflect average glycemic values, the researchers suggest.
The study was sponsored by Novo Nordisk and several authors are Novo Nordisk employees. The complete author disclosures are listed with the article. Dr. Thomas has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETES THERAPY
Key red flags for early-onset colorectal cancer
As the number of cases of early-onset colorectal cancer (CRC) diagnosed before age 50 continues to rise, early detection has become increasingly important.
The signs and symptoms are abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia.
Two symptoms in particular – rectal bleeding and iron-deficiency anemia – point to the need for timely endoscopy and follow-up, the researchers say.
“Colorectal cancer is not simply a disease affecting older people; we want younger adults to be aware of and act on these potentially very telling signs and symptoms – particularly because people under 50 are considered to be at low risk, and they don’t receive routine colorectal cancer screening,” senior investigator Yin Cao, ScD, with Washington University School of Medicine, St. Louis, said in a news release.
“It’s also crucial to spread awareness among primary care doctors, gastroenterologists, and emergency medicine doctors,” Dr. Cao added. “To date, many early-onset colorectal cancers are detected in emergency rooms, and there often are significant diagnostic delays with this cancer.”
The study was published online in the Journal of the National Cancer Institute.
Although previous research has identified rectal bleeding, iron-deficiency anemia, and rectal/abdominal pain as symptoms of early-onset CRC, most studies “have aggregated symptoms till the time of diagnosis,” which limits their use for early detection, the authors explain.
In the current study, the researchers analyzed data from more than 5,000 cases of early-onset CRC and from more than 22,000 control patients using the IBM MarketScan commercial database.
Dr. Cao and colleagues found that between 3 months and 2 years before diagnosis, abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia each indicated an increased risk for early-onset CRC.
Among patients with early-onset CRC, 19.3% presented with one or more of the four red flags between 3 months and 2 years prior to the index date; 15.6% had one symptom, and 3.7% had two or more.
After multivariable adjustment, having one symptom almost doubled the risk for early-onset CRC (odds ratio, 1.94); having two symptoms increased risk by more than threefold (OR, 3.59); and having three or more boosted the risk by more than 6.5-fold (OR, 6.52).
Abdominal pain was associated with a 34% higher risk of early-onset CRC (11.6% among case patients vs. 7.7% among controls; OR, 1.34).
Although not as common, rectal bleeding was associated with the highest odds for early-onset CRC (7.2% case patients vs. 1.3% controls; OR, 5.13).
The other predictive signs and symptoms included diarrhea (2.8% case patients vs. 1.4% controls; OR, 1.43) and iron-deficiency anemia (2.3% case patients vs. 0.9% controls; OR, 2.07).
No differences were observed by gender for each sign or symptom.
Among patients with a red-flag symptom who presented between 3 months and 2 years before diagnosis, for those with early-onset CRC, the median diagnostic interval was 8.7 months.
The researchers suggest that clinicians prioritize prompt diagnostic workups for patients younger than 50 who present with rectal bleeding and/or iron-deficiency anemia and that they also keep abdominal pain and diarrhea in mind as early symptoms.
Dr. Cao noted that since most early-onset CRC cases “have been and will continue to be diagnosed after symptom presentation, it is crucial to recognize these red-flag signs and symptoms promptly and conduct a diagnostic workup as soon as possible.
“By doing so, we can diagnose the disease earlier, which in turn can reduce the need for more aggressive treatment and improve patients’ quality of life and survival rates,” said Dr. Cao.
The study was supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article originally appeared on Medscape.com.
As the number of cases of early-onset colorectal cancer (CRC) diagnosed before age 50 continues to rise, early detection has become increasingly important.
The signs and symptoms are abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia.
Two symptoms in particular – rectal bleeding and iron-deficiency anemia – point to the need for timely endoscopy and follow-up, the researchers say.
“Colorectal cancer is not simply a disease affecting older people; we want younger adults to be aware of and act on these potentially very telling signs and symptoms – particularly because people under 50 are considered to be at low risk, and they don’t receive routine colorectal cancer screening,” senior investigator Yin Cao, ScD, with Washington University School of Medicine, St. Louis, said in a news release.
“It’s also crucial to spread awareness among primary care doctors, gastroenterologists, and emergency medicine doctors,” Dr. Cao added. “To date, many early-onset colorectal cancers are detected in emergency rooms, and there often are significant diagnostic delays with this cancer.”
The study was published online in the Journal of the National Cancer Institute.
Although previous research has identified rectal bleeding, iron-deficiency anemia, and rectal/abdominal pain as symptoms of early-onset CRC, most studies “have aggregated symptoms till the time of diagnosis,” which limits their use for early detection, the authors explain.
In the current study, the researchers analyzed data from more than 5,000 cases of early-onset CRC and from more than 22,000 control patients using the IBM MarketScan commercial database.
Dr. Cao and colleagues found that between 3 months and 2 years before diagnosis, abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia each indicated an increased risk for early-onset CRC.
Among patients with early-onset CRC, 19.3% presented with one or more of the four red flags between 3 months and 2 years prior to the index date; 15.6% had one symptom, and 3.7% had two or more.
After multivariable adjustment, having one symptom almost doubled the risk for early-onset CRC (odds ratio, 1.94); having two symptoms increased risk by more than threefold (OR, 3.59); and having three or more boosted the risk by more than 6.5-fold (OR, 6.52).
Abdominal pain was associated with a 34% higher risk of early-onset CRC (11.6% among case patients vs. 7.7% among controls; OR, 1.34).
Although not as common, rectal bleeding was associated with the highest odds for early-onset CRC (7.2% case patients vs. 1.3% controls; OR, 5.13).
The other predictive signs and symptoms included diarrhea (2.8% case patients vs. 1.4% controls; OR, 1.43) and iron-deficiency anemia (2.3% case patients vs. 0.9% controls; OR, 2.07).
No differences were observed by gender for each sign or symptom.
Among patients with a red-flag symptom who presented between 3 months and 2 years before diagnosis, for those with early-onset CRC, the median diagnostic interval was 8.7 months.
The researchers suggest that clinicians prioritize prompt diagnostic workups for patients younger than 50 who present with rectal bleeding and/or iron-deficiency anemia and that they also keep abdominal pain and diarrhea in mind as early symptoms.
Dr. Cao noted that since most early-onset CRC cases “have been and will continue to be diagnosed after symptom presentation, it is crucial to recognize these red-flag signs and symptoms promptly and conduct a diagnostic workup as soon as possible.
“By doing so, we can diagnose the disease earlier, which in turn can reduce the need for more aggressive treatment and improve patients’ quality of life and survival rates,” said Dr. Cao.
The study was supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article originally appeared on Medscape.com.
As the number of cases of early-onset colorectal cancer (CRC) diagnosed before age 50 continues to rise, early detection has become increasingly important.
The signs and symptoms are abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia.
Two symptoms in particular – rectal bleeding and iron-deficiency anemia – point to the need for timely endoscopy and follow-up, the researchers say.
“Colorectal cancer is not simply a disease affecting older people; we want younger adults to be aware of and act on these potentially very telling signs and symptoms – particularly because people under 50 are considered to be at low risk, and they don’t receive routine colorectal cancer screening,” senior investigator Yin Cao, ScD, with Washington University School of Medicine, St. Louis, said in a news release.
“It’s also crucial to spread awareness among primary care doctors, gastroenterologists, and emergency medicine doctors,” Dr. Cao added. “To date, many early-onset colorectal cancers are detected in emergency rooms, and there often are significant diagnostic delays with this cancer.”
The study was published online in the Journal of the National Cancer Institute.
Although previous research has identified rectal bleeding, iron-deficiency anemia, and rectal/abdominal pain as symptoms of early-onset CRC, most studies “have aggregated symptoms till the time of diagnosis,” which limits their use for early detection, the authors explain.
In the current study, the researchers analyzed data from more than 5,000 cases of early-onset CRC and from more than 22,000 control patients using the IBM MarketScan commercial database.
Dr. Cao and colleagues found that between 3 months and 2 years before diagnosis, abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia each indicated an increased risk for early-onset CRC.
Among patients with early-onset CRC, 19.3% presented with one or more of the four red flags between 3 months and 2 years prior to the index date; 15.6% had one symptom, and 3.7% had two or more.
After multivariable adjustment, having one symptom almost doubled the risk for early-onset CRC (odds ratio, 1.94); having two symptoms increased risk by more than threefold (OR, 3.59); and having three or more boosted the risk by more than 6.5-fold (OR, 6.52).
Abdominal pain was associated with a 34% higher risk of early-onset CRC (11.6% among case patients vs. 7.7% among controls; OR, 1.34).
Although not as common, rectal bleeding was associated with the highest odds for early-onset CRC (7.2% case patients vs. 1.3% controls; OR, 5.13).
The other predictive signs and symptoms included diarrhea (2.8% case patients vs. 1.4% controls; OR, 1.43) and iron-deficiency anemia (2.3% case patients vs. 0.9% controls; OR, 2.07).
No differences were observed by gender for each sign or symptom.
Among patients with a red-flag symptom who presented between 3 months and 2 years before diagnosis, for those with early-onset CRC, the median diagnostic interval was 8.7 months.
The researchers suggest that clinicians prioritize prompt diagnostic workups for patients younger than 50 who present with rectal bleeding and/or iron-deficiency anemia and that they also keep abdominal pain and diarrhea in mind as early symptoms.
Dr. Cao noted that since most early-onset CRC cases “have been and will continue to be diagnosed after symptom presentation, it is crucial to recognize these red-flag signs and symptoms promptly and conduct a diagnostic workup as soon as possible.
“By doing so, we can diagnose the disease earlier, which in turn can reduce the need for more aggressive treatment and improve patients’ quality of life and survival rates,” said Dr. Cao.
The study was supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article originally appeared on Medscape.com.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
ChatGPT delivers credible answers to colonoscopy queries
, new research suggests.
“This study shows that a conversational AI program can generate credible medical information in response to common patient questions,” say the investigators, led by Tsung-Chun Lee, MD, division of gastroenterology and hepatology, Taipei Medical University Shuang Ho Hospital, New Taipei City, Taiwan.
“With dedicated domain training, there is meaningful potential to optimize clinical communication to patients undergoing colonoscopy,” they add.
The study was published online in Gastroenterology.
ChatGPT, developed by OpenAI, is a natural language processing tool that allows users to have personalized conversations with an artificial intelligence (AI) bot capable of providing a detailed response to any question posed.
For their first-of-its-kind study, Dr. Lee and colleagues assessed the quality of ChatGPT-generated answers to eight common patient questions about colonoscopy, including what a colonoscopy entails, why it’s performed, how to prepare for it, potential complications, what to expect after the procedure, and what happens with a positive/negative result.
They retrieved the questions from the websites of three randomly selected top hospitals for gastroenterology and gastrointestinal surgery and had ChatGPT (Jan. 30, 2023, version) answer the questions twice.
Using plagiarism detection software, they found that text similarity was extremely low between ChatGPT answers and those on hospital websites (0%-16%). Text similarity ranged from 28% to 77% between the two ChatGPT answers for the same question, except on the question of what to do after a positive colonoscopy result, which had 0% text similarity.
To objectively gauge the quality of the ChatGPT answers, four gastroenterologists (two senior gastroenterologists and two fellows) rated 36 pairs of common questions and answers on a seven-point Likert scale according to ease of understanding, scientific adequacy, and satisfaction with the answer.
The gastroenterologists rated the ChatGPT answers highly and similarly to non-AI answers for all three quality indicators, with some AI scores even higher than non-AI scores.
Interestingly, they could correctly identify AI-generated answers only 48% of the time. Three raters had an accuracy of less than 50%, whereas one (a fellow) was 81% accurate.
The researchers note that publications about ChatGPT in PubMed grew 10-fold from Feb. 3 to April 14, 2023, with topics such as board examinations authorship, editorial policies, medical education, and clinical decision support.
Although in their early days, ChatGPT and other AI bots may represent a “transformative innovation” in how medical information is created by physicians and consumed by patients, they say.
It could also be a time-saver for health care professionals.
“AI-generated medical information, with appropriate provider oversight, accreditation, and periodic surveillance, could improve efficiency of care and free providers for more cognitively intensive patient communications,” they add.
However, several challenges remain, such as the lack of clinical evidence in constructing AI-generated answers.
In addition, AI-generated answers were written at significantly higher reading levels than were answers on hospital websites, which could be a barrier for some patients.
The study received no specific funding. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This study shows that a conversational AI program can generate credible medical information in response to common patient questions,” say the investigators, led by Tsung-Chun Lee, MD, division of gastroenterology and hepatology, Taipei Medical University Shuang Ho Hospital, New Taipei City, Taiwan.
“With dedicated domain training, there is meaningful potential to optimize clinical communication to patients undergoing colonoscopy,” they add.
The study was published online in Gastroenterology.
ChatGPT, developed by OpenAI, is a natural language processing tool that allows users to have personalized conversations with an artificial intelligence (AI) bot capable of providing a detailed response to any question posed.
For their first-of-its-kind study, Dr. Lee and colleagues assessed the quality of ChatGPT-generated answers to eight common patient questions about colonoscopy, including what a colonoscopy entails, why it’s performed, how to prepare for it, potential complications, what to expect after the procedure, and what happens with a positive/negative result.
They retrieved the questions from the websites of three randomly selected top hospitals for gastroenterology and gastrointestinal surgery and had ChatGPT (Jan. 30, 2023, version) answer the questions twice.
Using plagiarism detection software, they found that text similarity was extremely low between ChatGPT answers and those on hospital websites (0%-16%). Text similarity ranged from 28% to 77% between the two ChatGPT answers for the same question, except on the question of what to do after a positive colonoscopy result, which had 0% text similarity.
To objectively gauge the quality of the ChatGPT answers, four gastroenterologists (two senior gastroenterologists and two fellows) rated 36 pairs of common questions and answers on a seven-point Likert scale according to ease of understanding, scientific adequacy, and satisfaction with the answer.
The gastroenterologists rated the ChatGPT answers highly and similarly to non-AI answers for all three quality indicators, with some AI scores even higher than non-AI scores.
Interestingly, they could correctly identify AI-generated answers only 48% of the time. Three raters had an accuracy of less than 50%, whereas one (a fellow) was 81% accurate.
The researchers note that publications about ChatGPT in PubMed grew 10-fold from Feb. 3 to April 14, 2023, with topics such as board examinations authorship, editorial policies, medical education, and clinical decision support.
Although in their early days, ChatGPT and other AI bots may represent a “transformative innovation” in how medical information is created by physicians and consumed by patients, they say.
It could also be a time-saver for health care professionals.
“AI-generated medical information, with appropriate provider oversight, accreditation, and periodic surveillance, could improve efficiency of care and free providers for more cognitively intensive patient communications,” they add.
However, several challenges remain, such as the lack of clinical evidence in constructing AI-generated answers.
In addition, AI-generated answers were written at significantly higher reading levels than were answers on hospital websites, which could be a barrier for some patients.
The study received no specific funding. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This study shows that a conversational AI program can generate credible medical information in response to common patient questions,” say the investigators, led by Tsung-Chun Lee, MD, division of gastroenterology and hepatology, Taipei Medical University Shuang Ho Hospital, New Taipei City, Taiwan.
“With dedicated domain training, there is meaningful potential to optimize clinical communication to patients undergoing colonoscopy,” they add.
The study was published online in Gastroenterology.
ChatGPT, developed by OpenAI, is a natural language processing tool that allows users to have personalized conversations with an artificial intelligence (AI) bot capable of providing a detailed response to any question posed.
For their first-of-its-kind study, Dr. Lee and colleagues assessed the quality of ChatGPT-generated answers to eight common patient questions about colonoscopy, including what a colonoscopy entails, why it’s performed, how to prepare for it, potential complications, what to expect after the procedure, and what happens with a positive/negative result.
They retrieved the questions from the websites of three randomly selected top hospitals for gastroenterology and gastrointestinal surgery and had ChatGPT (Jan. 30, 2023, version) answer the questions twice.
Using plagiarism detection software, they found that text similarity was extremely low between ChatGPT answers and those on hospital websites (0%-16%). Text similarity ranged from 28% to 77% between the two ChatGPT answers for the same question, except on the question of what to do after a positive colonoscopy result, which had 0% text similarity.
To objectively gauge the quality of the ChatGPT answers, four gastroenterologists (two senior gastroenterologists and two fellows) rated 36 pairs of common questions and answers on a seven-point Likert scale according to ease of understanding, scientific adequacy, and satisfaction with the answer.
The gastroenterologists rated the ChatGPT answers highly and similarly to non-AI answers for all three quality indicators, with some AI scores even higher than non-AI scores.
Interestingly, they could correctly identify AI-generated answers only 48% of the time. Three raters had an accuracy of less than 50%, whereas one (a fellow) was 81% accurate.
The researchers note that publications about ChatGPT in PubMed grew 10-fold from Feb. 3 to April 14, 2023, with topics such as board examinations authorship, editorial policies, medical education, and clinical decision support.
Although in their early days, ChatGPT and other AI bots may represent a “transformative innovation” in how medical information is created by physicians and consumed by patients, they say.
It could also be a time-saver for health care professionals.
“AI-generated medical information, with appropriate provider oversight, accreditation, and periodic surveillance, could improve efficiency of care and free providers for more cognitively intensive patient communications,” they add.
However, several challenges remain, such as the lack of clinical evidence in constructing AI-generated answers.
In addition, AI-generated answers were written at significantly higher reading levels than were answers on hospital websites, which could be a barrier for some patients.
The study received no specific funding. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
T-DXd for HER2-low BC: Analysis confirms adverse effects
Interstitial lung disease (ILD) also remains a concern, and it’s not clear if retreatment after resolution is warranted.
In general, however, “T-DXd demonstrates a manageable safety profile consistent with prior reports. Results from this safety analysis continued to support its use as a new standard of care in patients with HER2-low metastatic breast cancer,” said report lead author Hope Rugo, MD, of the University of California, San Francisco, during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
T-DXd, an antibody-drug conjugate, received FDA approval in August 2022 for patients with HER2-low disease. The drug has been touted as “practice changing” and a “new standard of care.”
However, physicians have noted that the benefits of the drug come at the cost of significant adverse effects, including some that can cause hospitalization. There’s special concern about high-grade interstitial lung disease/pneumonitis, and an FDA boxed warning cautions clinicians about this possible side effect.
For the new analysis, researchers presented additional safety data from the industry-funded DESTINY-Breast04 trial, whose results was published in July 2022 in the New England Journal of Medicine. That study randomized 373 patients to T-DXd and 184 to physician’s choice of treatment. It found that, “among all patients, the median progression-free survival was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice group (hazard ratio for disease progression or death, 0.50; P < .001), and overall survival was 23.4 months and 16.8 months, respectively (hazard ratio for death, 0.64; P = .001).”
Exposure-adjusted incidence rates for any-grade treatment-emergent adverse events were lower for T-DXd versus physician’s choice of treatment (1.30 vs. 2.66). However, nausea and vomiting events were more than twice as common in patients who took T-DXd versus the physician’s choice (79.5% vs. 35.5%).
A total of 50.9% of patients in the T-DXd arm received antiemetic prophylaxis versus 37.2% in the physician’s choice arm. A single patient discontinued T-DXd treatment because of vomiting, and a single patient discontinued treatment because of nausea. No patients in the physician choice group discontinued treatment because of nausea or vomiting.
Neutropenia and febrile neutropenia were less frequent in the T-DXd arm versus physician’s choice (12.9% vs. 18.0% and 0.3% vs. 2.9%, respectively.)
ILD occurred in 45 patients (12.1%) of those in the T-DXd arm versus 1 (0.6%) in the physician choice arm. Ten patients of the patients in the T-DXd arm had not recovered by the data cutoff point.
Six patients with ILD were retreated following resolution; one discontinued because of an adverse event, two discontinued because of progressive disease, and three remained on the drug. “Given that there was only a small number of patients who were retreated with T-DXd, it’s difficult to make clinically meaningful conclusions on the effect of retreatment following grade IDL events that have resolved,” Dr. Rugo said.
In the big picture, “ILD pneumonitis remains an important identified risk and an adverse event of interest associated with T-DXd,” Dr. Rugo said. “It’s important that we adhere to management guidelines and updated toxicity management guidelines.”
In a discussion, Dr. Rugo said she prescribes three antiemetic drugs to help patients tolerate T-DXd therapy: “It makes a big difference. Anecdotally, it really has improved management of nausea. Start more and back down [as symptoms fade].”
Gustavo Werutsky, MD, PhD, of Moinhos de Vento Hospital, Porto Alegre, Brazil, the discussant for the presentation, also emphasized the importance of prevention and said he prescribes two or three prophylactic drugs. “In the beginning, we didn’t know these events were so important. A big part of the message is that patients from the beginning need to have a good prophylaxis for the nausea and vomiting.”
The researchers also presented a related report at the conference, an analysis of patient-reported outcomes from DESTINY-Breast02, a randomized phase 3 study of T-DXd (n = 406) versus physician’s choice of treatment (n = 202) in patients with HER2-positive metastatic breast cancer who were resistant/refractory to trastuzumab emtansine.
The analysis, led by Tanja Fehm, MD, of University Hospital Düsseldorf (Germany), found that the median time to definitive deterioration was longer with T-DXd versus the other arm per the EORTC QLQ-C30 global health status/quality of life score (14.1 vs. 5.9 months; HR, 0.56; 95% confidence interval, 0.44-0.71).
The studies were funded by Daiichi Sankyo and AstraZeneca, which make T-DXd. Dr. Hugo discloses relationships with Puma, NAPO, Blueprint, Scorpion Therapeutics, Merck, AstraZeneca, Gilead, Astellas, Daiichi Sankyo, F. Hoffmann–La Roche/Genentech, GlaxoSmithKline, Lilly, Novartis, OBI, Pfizer, Pionyr, Sermonix, Taiho Oncology, and Veru. Multiple other authors of both studies have various disclosures.
Interstitial lung disease (ILD) also remains a concern, and it’s not clear if retreatment after resolution is warranted.
In general, however, “T-DXd demonstrates a manageable safety profile consistent with prior reports. Results from this safety analysis continued to support its use as a new standard of care in patients with HER2-low metastatic breast cancer,” said report lead author Hope Rugo, MD, of the University of California, San Francisco, during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
T-DXd, an antibody-drug conjugate, received FDA approval in August 2022 for patients with HER2-low disease. The drug has been touted as “practice changing” and a “new standard of care.”
However, physicians have noted that the benefits of the drug come at the cost of significant adverse effects, including some that can cause hospitalization. There’s special concern about high-grade interstitial lung disease/pneumonitis, and an FDA boxed warning cautions clinicians about this possible side effect.
For the new analysis, researchers presented additional safety data from the industry-funded DESTINY-Breast04 trial, whose results was published in July 2022 in the New England Journal of Medicine. That study randomized 373 patients to T-DXd and 184 to physician’s choice of treatment. It found that, “among all patients, the median progression-free survival was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice group (hazard ratio for disease progression or death, 0.50; P < .001), and overall survival was 23.4 months and 16.8 months, respectively (hazard ratio for death, 0.64; P = .001).”
Exposure-adjusted incidence rates for any-grade treatment-emergent adverse events were lower for T-DXd versus physician’s choice of treatment (1.30 vs. 2.66). However, nausea and vomiting events were more than twice as common in patients who took T-DXd versus the physician’s choice (79.5% vs. 35.5%).
A total of 50.9% of patients in the T-DXd arm received antiemetic prophylaxis versus 37.2% in the physician’s choice arm. A single patient discontinued T-DXd treatment because of vomiting, and a single patient discontinued treatment because of nausea. No patients in the physician choice group discontinued treatment because of nausea or vomiting.
Neutropenia and febrile neutropenia were less frequent in the T-DXd arm versus physician’s choice (12.9% vs. 18.0% and 0.3% vs. 2.9%, respectively.)
ILD occurred in 45 patients (12.1%) of those in the T-DXd arm versus 1 (0.6%) in the physician choice arm. Ten patients of the patients in the T-DXd arm had not recovered by the data cutoff point.
Six patients with ILD were retreated following resolution; one discontinued because of an adverse event, two discontinued because of progressive disease, and three remained on the drug. “Given that there was only a small number of patients who were retreated with T-DXd, it’s difficult to make clinically meaningful conclusions on the effect of retreatment following grade IDL events that have resolved,” Dr. Rugo said.
In the big picture, “ILD pneumonitis remains an important identified risk and an adverse event of interest associated with T-DXd,” Dr. Rugo said. “It’s important that we adhere to management guidelines and updated toxicity management guidelines.”
In a discussion, Dr. Rugo said she prescribes three antiemetic drugs to help patients tolerate T-DXd therapy: “It makes a big difference. Anecdotally, it really has improved management of nausea. Start more and back down [as symptoms fade].”
Gustavo Werutsky, MD, PhD, of Moinhos de Vento Hospital, Porto Alegre, Brazil, the discussant for the presentation, also emphasized the importance of prevention and said he prescribes two or three prophylactic drugs. “In the beginning, we didn’t know these events were so important. A big part of the message is that patients from the beginning need to have a good prophylaxis for the nausea and vomiting.”
The researchers also presented a related report at the conference, an analysis of patient-reported outcomes from DESTINY-Breast02, a randomized phase 3 study of T-DXd (n = 406) versus physician’s choice of treatment (n = 202) in patients with HER2-positive metastatic breast cancer who were resistant/refractory to trastuzumab emtansine.
The analysis, led by Tanja Fehm, MD, of University Hospital Düsseldorf (Germany), found that the median time to definitive deterioration was longer with T-DXd versus the other arm per the EORTC QLQ-C30 global health status/quality of life score (14.1 vs. 5.9 months; HR, 0.56; 95% confidence interval, 0.44-0.71).
The studies were funded by Daiichi Sankyo and AstraZeneca, which make T-DXd. Dr. Hugo discloses relationships with Puma, NAPO, Blueprint, Scorpion Therapeutics, Merck, AstraZeneca, Gilead, Astellas, Daiichi Sankyo, F. Hoffmann–La Roche/Genentech, GlaxoSmithKline, Lilly, Novartis, OBI, Pfizer, Pionyr, Sermonix, Taiho Oncology, and Veru. Multiple other authors of both studies have various disclosures.
Interstitial lung disease (ILD) also remains a concern, and it’s not clear if retreatment after resolution is warranted.
In general, however, “T-DXd demonstrates a manageable safety profile consistent with prior reports. Results from this safety analysis continued to support its use as a new standard of care in patients with HER2-low metastatic breast cancer,” said report lead author Hope Rugo, MD, of the University of California, San Francisco, during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
T-DXd, an antibody-drug conjugate, received FDA approval in August 2022 for patients with HER2-low disease. The drug has been touted as “practice changing” and a “new standard of care.”
However, physicians have noted that the benefits of the drug come at the cost of significant adverse effects, including some that can cause hospitalization. There’s special concern about high-grade interstitial lung disease/pneumonitis, and an FDA boxed warning cautions clinicians about this possible side effect.
For the new analysis, researchers presented additional safety data from the industry-funded DESTINY-Breast04 trial, whose results was published in July 2022 in the New England Journal of Medicine. That study randomized 373 patients to T-DXd and 184 to physician’s choice of treatment. It found that, “among all patients, the median progression-free survival was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice group (hazard ratio for disease progression or death, 0.50; P < .001), and overall survival was 23.4 months and 16.8 months, respectively (hazard ratio for death, 0.64; P = .001).”
Exposure-adjusted incidence rates for any-grade treatment-emergent adverse events were lower for T-DXd versus physician’s choice of treatment (1.30 vs. 2.66). However, nausea and vomiting events were more than twice as common in patients who took T-DXd versus the physician’s choice (79.5% vs. 35.5%).
A total of 50.9% of patients in the T-DXd arm received antiemetic prophylaxis versus 37.2% in the physician’s choice arm. A single patient discontinued T-DXd treatment because of vomiting, and a single patient discontinued treatment because of nausea. No patients in the physician choice group discontinued treatment because of nausea or vomiting.
Neutropenia and febrile neutropenia were less frequent in the T-DXd arm versus physician’s choice (12.9% vs. 18.0% and 0.3% vs. 2.9%, respectively.)
ILD occurred in 45 patients (12.1%) of those in the T-DXd arm versus 1 (0.6%) in the physician choice arm. Ten patients of the patients in the T-DXd arm had not recovered by the data cutoff point.
Six patients with ILD were retreated following resolution; one discontinued because of an adverse event, two discontinued because of progressive disease, and three remained on the drug. “Given that there was only a small number of patients who were retreated with T-DXd, it’s difficult to make clinically meaningful conclusions on the effect of retreatment following grade IDL events that have resolved,” Dr. Rugo said.
In the big picture, “ILD pneumonitis remains an important identified risk and an adverse event of interest associated with T-DXd,” Dr. Rugo said. “It’s important that we adhere to management guidelines and updated toxicity management guidelines.”
In a discussion, Dr. Rugo said she prescribes three antiemetic drugs to help patients tolerate T-DXd therapy: “It makes a big difference. Anecdotally, it really has improved management of nausea. Start more and back down [as symptoms fade].”
Gustavo Werutsky, MD, PhD, of Moinhos de Vento Hospital, Porto Alegre, Brazil, the discussant for the presentation, also emphasized the importance of prevention and said he prescribes two or three prophylactic drugs. “In the beginning, we didn’t know these events were so important. A big part of the message is that patients from the beginning need to have a good prophylaxis for the nausea and vomiting.”
The researchers also presented a related report at the conference, an analysis of patient-reported outcomes from DESTINY-Breast02, a randomized phase 3 study of T-DXd (n = 406) versus physician’s choice of treatment (n = 202) in patients with HER2-positive metastatic breast cancer who were resistant/refractory to trastuzumab emtansine.
The analysis, led by Tanja Fehm, MD, of University Hospital Düsseldorf (Germany), found that the median time to definitive deterioration was longer with T-DXd versus the other arm per the EORTC QLQ-C30 global health status/quality of life score (14.1 vs. 5.9 months; HR, 0.56; 95% confidence interval, 0.44-0.71).
The studies were funded by Daiichi Sankyo and AstraZeneca, which make T-DXd. Dr. Hugo discloses relationships with Puma, NAPO, Blueprint, Scorpion Therapeutics, Merck, AstraZeneca, Gilead, Astellas, Daiichi Sankyo, F. Hoffmann–La Roche/Genentech, GlaxoSmithKline, Lilly, Novartis, OBI, Pfizer, Pionyr, Sermonix, Taiho Oncology, and Veru. Multiple other authors of both studies have various disclosures.
FROM ESMO BREAST CANCER 2023
At-home monitoring device can predict Crohn’s disease flares
CHICAGO – , including an inability to signal a change in disease activity without laboratory testing or before symptoms arise.
A new device developed at Massachusetts Institute of Technology could change all that.
Using data collected via a passive at-home monitoring device (Emerald sensor, Emerald Innovations Inc.), researchers found that increases in breathing rate, more awakenings at night, and slower walking speed accurately predicted that a person’s Crohn’s disease activity was about to flare, according to a study presented May 7 at Digestive Disease Week® (DDW) 2023.
In some cases, the prediction of a flare came up to 25 days sooner than via traditional measures.
“In order to provide optimal care, providers need to monitor patients closely with regard to accurate active disease.” said Joshua Korzenik, MD, a gastroenterologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School in Boston. “The problem is the clinical symptoms are not accurate.”
Tracking flares with technology
Traditionally, measuring flares in Crohn’s disease activity depends on imaging, colonoscopy, and/or laboratory measures of calprotectin or other biomarkers. These approaches can be costly, can involve delays, and can carry risks, Dr. Korzenik said.
“They are also a single snapshot in time,” he added.
To determine how well a noninvasive device could perform, investigators enrolled 120 people with 105 continuing in the study long enough to be evaluable; 44 people whose Crohn’s disease was in remission, 35 with active Crohn’s disease, as well as 26 healthy controls. Among those with Crohn’s disease, 83% were on biologic therapy.
The groups were matched for age and gender, with a mean age of 47 years and mean disease duration of 13 years.
People with certain medical conditions were excluded, as was anyone who owned a large dog that sometimes slept in bed with them because that might throw off the readings.
The participants put the device – which resembles a closed laptop or a large Wi-Fi router – in their homes and were monitored for a mean 306 days. Participants wore an ankle bracelet the first 2 weeks of the study so the device could learn to distinguish them from others in the home.
The device sent out radio waves with frequencies like Wi-Fi for researchers to measure the factors that may be associated with flares, such as sleep quality and cycles, breathing rate, and gait speed.
Traditional clinical measures based on blood and stool samples, along with patient-reported outcome surveys, were taken to compare with the accuracy of the device.
Data from the device were collected and transmitted securely to a cloud database without any interaction from the participant. Data included information on more than 25,000 nights of sleep, 200,000 hours of breathing signals, and 400,000 measurements of walking speed.
Sleep quality and cycles were straightforward, as was breathing rate. But gait speed was a little more complicated to measure. To illustrate, Dr. Korzenik showed the layout of an example apartment with data on how someone moved around. To distill the data, the researchers focused on one path in the home, relatively straight and not obstructed by furniture, and limited the measurements to a certain amount of time. People who spent more time at home during the COVID-19 pandemic did not skew the results, according to Dr. Korzenik, who added that it wasn’t total time walking around but a snippet in time.
A variety of sleep, breathing, and mobility metrics extracted by the device were integrated to assess disease activity. Investigators noted that during flares, sleep quality decreased, and more nocturnal awakenings occurred. They also found that gait speed slowed, and respiratory rate increased with flares.
When the investigators looked at sleep as a function of disease activity in the patient-reported surveys, they found a significant difference between people in remission and those with active disease. For example, people with active disease had a greater number of awakenings at night (P = .0016), less REM sleep at night (P = .0000), and less time in deep sleep (P = .000) compared with those in remission.
The technology “can identify flares with a predictive value that approaches fecal calprotectin,” Dr. Korzenik said.
Machine learning was used to look at severity of disease vs. fecal calprotectin values and “showed the data could be used as a marker of disease,” he added.
Use of a remote monitor, the comparison of validated vs. conventional data, and the large dataset were among the strengths of the study. The single-center design and exclusion of people with some comorbidities are potential limitations.
Further studies are warranted to confirm these findings and guide optimal care of people with Crohn’s disease, the investigators noted.
Earlier detection, earlier intervention
“The study is really important,” said session comoderator Raymond K. Cross Jr., MD, professor of medicine and director of the IBD program at the University of Maryland, Baltimore.
Monitoring devices like this “could be very useful,” Dr. Cross said. “It is not invasive, unless you consider a device in your house invasive. But, to me, I don’t think a box in my bedroom would be unnerving to me.”
Dr. Cross shared a couple of caveats. “The one devil in the details is always going to be cost,” he said. Also, it’s unclear who will read and interpret all the data generated by the device among “providers who are already overwhelmed with the volume of information.”
Moving forward, a device like this could offer multiple uses, Dr. Cross noted. If the device can detect relapses earlier, physicians could intervene sooner, he said. Also, the device could potentially flag people who are not taking their medications as recommended, or it could be used as a guide to optimize treatment response.
Whether data from the device could indicate when it’s appropriate to reduce the frequency or dose of medication or even when to withdraw therapy would be “really aspirational,” Dr. Cross added.
The study was funded by The Leona M. and Harry B. Helmsley Charitable Trust. Dr. Korzenik and Dr. Cross report no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
A version of this article first appeared on Medscape.com.
CHICAGO – , including an inability to signal a change in disease activity without laboratory testing or before symptoms arise.
A new device developed at Massachusetts Institute of Technology could change all that.
Using data collected via a passive at-home monitoring device (Emerald sensor, Emerald Innovations Inc.), researchers found that increases in breathing rate, more awakenings at night, and slower walking speed accurately predicted that a person’s Crohn’s disease activity was about to flare, according to a study presented May 7 at Digestive Disease Week® (DDW) 2023.
In some cases, the prediction of a flare came up to 25 days sooner than via traditional measures.
“In order to provide optimal care, providers need to monitor patients closely with regard to accurate active disease.” said Joshua Korzenik, MD, a gastroenterologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School in Boston. “The problem is the clinical symptoms are not accurate.”
Tracking flares with technology
Traditionally, measuring flares in Crohn’s disease activity depends on imaging, colonoscopy, and/or laboratory measures of calprotectin or other biomarkers. These approaches can be costly, can involve delays, and can carry risks, Dr. Korzenik said.
“They are also a single snapshot in time,” he added.
To determine how well a noninvasive device could perform, investigators enrolled 120 people with 105 continuing in the study long enough to be evaluable; 44 people whose Crohn’s disease was in remission, 35 with active Crohn’s disease, as well as 26 healthy controls. Among those with Crohn’s disease, 83% were on biologic therapy.
The groups were matched for age and gender, with a mean age of 47 years and mean disease duration of 13 years.
People with certain medical conditions were excluded, as was anyone who owned a large dog that sometimes slept in bed with them because that might throw off the readings.
The participants put the device – which resembles a closed laptop or a large Wi-Fi router – in their homes and were monitored for a mean 306 days. Participants wore an ankle bracelet the first 2 weeks of the study so the device could learn to distinguish them from others in the home.
The device sent out radio waves with frequencies like Wi-Fi for researchers to measure the factors that may be associated with flares, such as sleep quality and cycles, breathing rate, and gait speed.
Traditional clinical measures based on blood and stool samples, along with patient-reported outcome surveys, were taken to compare with the accuracy of the device.
Data from the device were collected and transmitted securely to a cloud database without any interaction from the participant. Data included information on more than 25,000 nights of sleep, 200,000 hours of breathing signals, and 400,000 measurements of walking speed.
Sleep quality and cycles were straightforward, as was breathing rate. But gait speed was a little more complicated to measure. To illustrate, Dr. Korzenik showed the layout of an example apartment with data on how someone moved around. To distill the data, the researchers focused on one path in the home, relatively straight and not obstructed by furniture, and limited the measurements to a certain amount of time. People who spent more time at home during the COVID-19 pandemic did not skew the results, according to Dr. Korzenik, who added that it wasn’t total time walking around but a snippet in time.
A variety of sleep, breathing, and mobility metrics extracted by the device were integrated to assess disease activity. Investigators noted that during flares, sleep quality decreased, and more nocturnal awakenings occurred. They also found that gait speed slowed, and respiratory rate increased with flares.
When the investigators looked at sleep as a function of disease activity in the patient-reported surveys, they found a significant difference between people in remission and those with active disease. For example, people with active disease had a greater number of awakenings at night (P = .0016), less REM sleep at night (P = .0000), and less time in deep sleep (P = .000) compared with those in remission.
The technology “can identify flares with a predictive value that approaches fecal calprotectin,” Dr. Korzenik said.
Machine learning was used to look at severity of disease vs. fecal calprotectin values and “showed the data could be used as a marker of disease,” he added.
Use of a remote monitor, the comparison of validated vs. conventional data, and the large dataset were among the strengths of the study. The single-center design and exclusion of people with some comorbidities are potential limitations.
Further studies are warranted to confirm these findings and guide optimal care of people with Crohn’s disease, the investigators noted.
Earlier detection, earlier intervention
“The study is really important,” said session comoderator Raymond K. Cross Jr., MD, professor of medicine and director of the IBD program at the University of Maryland, Baltimore.
Monitoring devices like this “could be very useful,” Dr. Cross said. “It is not invasive, unless you consider a device in your house invasive. But, to me, I don’t think a box in my bedroom would be unnerving to me.”
Dr. Cross shared a couple of caveats. “The one devil in the details is always going to be cost,” he said. Also, it’s unclear who will read and interpret all the data generated by the device among “providers who are already overwhelmed with the volume of information.”
Moving forward, a device like this could offer multiple uses, Dr. Cross noted. If the device can detect relapses earlier, physicians could intervene sooner, he said. Also, the device could potentially flag people who are not taking their medications as recommended, or it could be used as a guide to optimize treatment response.
Whether data from the device could indicate when it’s appropriate to reduce the frequency or dose of medication or even when to withdraw therapy would be “really aspirational,” Dr. Cross added.
The study was funded by The Leona M. and Harry B. Helmsley Charitable Trust. Dr. Korzenik and Dr. Cross report no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
A version of this article first appeared on Medscape.com.
CHICAGO – , including an inability to signal a change in disease activity without laboratory testing or before symptoms arise.
A new device developed at Massachusetts Institute of Technology could change all that.
Using data collected via a passive at-home monitoring device (Emerald sensor, Emerald Innovations Inc.), researchers found that increases in breathing rate, more awakenings at night, and slower walking speed accurately predicted that a person’s Crohn’s disease activity was about to flare, according to a study presented May 7 at Digestive Disease Week® (DDW) 2023.
In some cases, the prediction of a flare came up to 25 days sooner than via traditional measures.
“In order to provide optimal care, providers need to monitor patients closely with regard to accurate active disease.” said Joshua Korzenik, MD, a gastroenterologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School in Boston. “The problem is the clinical symptoms are not accurate.”
Tracking flares with technology
Traditionally, measuring flares in Crohn’s disease activity depends on imaging, colonoscopy, and/or laboratory measures of calprotectin or other biomarkers. These approaches can be costly, can involve delays, and can carry risks, Dr. Korzenik said.
“They are also a single snapshot in time,” he added.
To determine how well a noninvasive device could perform, investigators enrolled 120 people with 105 continuing in the study long enough to be evaluable; 44 people whose Crohn’s disease was in remission, 35 with active Crohn’s disease, as well as 26 healthy controls. Among those with Crohn’s disease, 83% were on biologic therapy.
The groups were matched for age and gender, with a mean age of 47 years and mean disease duration of 13 years.
People with certain medical conditions were excluded, as was anyone who owned a large dog that sometimes slept in bed with them because that might throw off the readings.
The participants put the device – which resembles a closed laptop or a large Wi-Fi router – in their homes and were monitored for a mean 306 days. Participants wore an ankle bracelet the first 2 weeks of the study so the device could learn to distinguish them from others in the home.
The device sent out radio waves with frequencies like Wi-Fi for researchers to measure the factors that may be associated with flares, such as sleep quality and cycles, breathing rate, and gait speed.
Traditional clinical measures based on blood and stool samples, along with patient-reported outcome surveys, were taken to compare with the accuracy of the device.
Data from the device were collected and transmitted securely to a cloud database without any interaction from the participant. Data included information on more than 25,000 nights of sleep, 200,000 hours of breathing signals, and 400,000 measurements of walking speed.
Sleep quality and cycles were straightforward, as was breathing rate. But gait speed was a little more complicated to measure. To illustrate, Dr. Korzenik showed the layout of an example apartment with data on how someone moved around. To distill the data, the researchers focused on one path in the home, relatively straight and not obstructed by furniture, and limited the measurements to a certain amount of time. People who spent more time at home during the COVID-19 pandemic did not skew the results, according to Dr. Korzenik, who added that it wasn’t total time walking around but a snippet in time.
A variety of sleep, breathing, and mobility metrics extracted by the device were integrated to assess disease activity. Investigators noted that during flares, sleep quality decreased, and more nocturnal awakenings occurred. They also found that gait speed slowed, and respiratory rate increased with flares.
When the investigators looked at sleep as a function of disease activity in the patient-reported surveys, they found a significant difference between people in remission and those with active disease. For example, people with active disease had a greater number of awakenings at night (P = .0016), less REM sleep at night (P = .0000), and less time in deep sleep (P = .000) compared with those in remission.
The technology “can identify flares with a predictive value that approaches fecal calprotectin,” Dr. Korzenik said.
Machine learning was used to look at severity of disease vs. fecal calprotectin values and “showed the data could be used as a marker of disease,” he added.
Use of a remote monitor, the comparison of validated vs. conventional data, and the large dataset were among the strengths of the study. The single-center design and exclusion of people with some comorbidities are potential limitations.
Further studies are warranted to confirm these findings and guide optimal care of people with Crohn’s disease, the investigators noted.
Earlier detection, earlier intervention
“The study is really important,” said session comoderator Raymond K. Cross Jr., MD, professor of medicine and director of the IBD program at the University of Maryland, Baltimore.
Monitoring devices like this “could be very useful,” Dr. Cross said. “It is not invasive, unless you consider a device in your house invasive. But, to me, I don’t think a box in my bedroom would be unnerving to me.”
Dr. Cross shared a couple of caveats. “The one devil in the details is always going to be cost,” he said. Also, it’s unclear who will read and interpret all the data generated by the device among “providers who are already overwhelmed with the volume of information.”
Moving forward, a device like this could offer multiple uses, Dr. Cross noted. If the device can detect relapses earlier, physicians could intervene sooner, he said. Also, the device could potentially flag people who are not taking their medications as recommended, or it could be used as a guide to optimize treatment response.
Whether data from the device could indicate when it’s appropriate to reduce the frequency or dose of medication or even when to withdraw therapy would be “really aspirational,” Dr. Cross added.
The study was funded by The Leona M. and Harry B. Helmsley Charitable Trust. Dr. Korzenik and Dr. Cross report no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
A version of this article first appeared on Medscape.com.
AT DDW 2023
Fewer discontinuations with infliximab vs. vedolizumab for UC maintenance therapy
CHICAGO – an updated meta-analysis of randomized clinical trials reveals.
At 1 year, 2% of people taking the anti–tumor necrosis factor (TNF) agent infliximab discontinued for lack of efficacy, compared with 24% of patients taking vedolizumab (Entyvio), an integrin receptor antagonist.
The safety profile of each agent is also important.
“We know that vedolizumab has less safety risks than an anti-TNF agent, but we also have the gut feeling that the anti-TNF agents are more efficacious,” lead author Marc Ferrante, MD, said in an interview.
“Of course, I don’t think we can really say that vedolizumab is the safest and infliximab is not safe, but there is some difference,” added Dr. Ferrante, a professor in the department of gastroenterology and hepatology, University Hospitals Leuven (Belgium).
The study was presented as a poster at the annual Digestive Disease Week (DDW).
The researchers conducted a pooled analysis of six randomized controlled trials from the past 10 years. They analyzed the NOR-SWITCH IV Q8W, the NCT02883452 SC Q2W, and LIBERTY-UC SC Q2W studies for infliximab, and the VISIBLE 1 SC Q2W, GEMINI 1 IV Q4W, and VARSITY IV Q8W trials for vedolizumab.
Their work expands on a meta-analysis by Dr. Ferrante and colleagues presented at DDW 2022. They added the 1-year results from the phase 3 LIBERTY-UC study to increase the number of participants taking infliximab or an infliximab biosimilar.
“Luckily, the results are very similar,” Dr. Ferrante said, and noted that they support previous findings that discontinuation of infliximab was lower than that of vedolizumab.
Most of the patients in the infliximab group were taking an infliximab biosimilar, whereas the vedolizumab group received the originator. Dr. Ferrante noted that the economic considerations involved in deciding between a biosimilar and an originator were not part of the research but that “there will be a difference in costs.”
Same mechanism, different route
The novel finding from the study includes the subcutaneous form of infliximab, which is not yet available in the United States, noted Joshua M. Steinberg, MD, director of inflammatory bowel disease at Gastroenterology of the Rockies, Denver, when asked to comment on the study. Currently, intravenously administered infliximab and vedolizumab are available in the United States.
A better comparator in the future would be looking at subcutaneous forms of both agents, especially “with the impending launch of subcutaneous vedolizumab in the United States,” said Dr. Steinberg, who is also a clinical instructor of medicine at the University of Colorado at Denver, Aurora.
He added that it’s reassuring overall that with a newer mode of administration but same mechanism of action, it is still feasible and durable for at least 1 year.
“The general consensus is that in terms of our biologics, vedolizumab is the safest because of its targeted mechanism of action. But sometimes the ‘safest choice’ isn’t the best choice,” Dr. Steinberg said. “I think in the right patient, the most effective treatment is going to be the one that works the best, and that’s not going to be universal.”
The study was sponsored by Celltrion, which makes an infliximab biosimilar. Dr. Ferrante receives honoraria as a consultant and speaker for Celltrion. Dr. Steinberg reported no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
CHICAGO – an updated meta-analysis of randomized clinical trials reveals.
At 1 year, 2% of people taking the anti–tumor necrosis factor (TNF) agent infliximab discontinued for lack of efficacy, compared with 24% of patients taking vedolizumab (Entyvio), an integrin receptor antagonist.
The safety profile of each agent is also important.
“We know that vedolizumab has less safety risks than an anti-TNF agent, but we also have the gut feeling that the anti-TNF agents are more efficacious,” lead author Marc Ferrante, MD, said in an interview.
“Of course, I don’t think we can really say that vedolizumab is the safest and infliximab is not safe, but there is some difference,” added Dr. Ferrante, a professor in the department of gastroenterology and hepatology, University Hospitals Leuven (Belgium).
The study was presented as a poster at the annual Digestive Disease Week (DDW).
The researchers conducted a pooled analysis of six randomized controlled trials from the past 10 years. They analyzed the NOR-SWITCH IV Q8W, the NCT02883452 SC Q2W, and LIBERTY-UC SC Q2W studies for infliximab, and the VISIBLE 1 SC Q2W, GEMINI 1 IV Q4W, and VARSITY IV Q8W trials for vedolizumab.
Their work expands on a meta-analysis by Dr. Ferrante and colleagues presented at DDW 2022. They added the 1-year results from the phase 3 LIBERTY-UC study to increase the number of participants taking infliximab or an infliximab biosimilar.
“Luckily, the results are very similar,” Dr. Ferrante said, and noted that they support previous findings that discontinuation of infliximab was lower than that of vedolizumab.
Most of the patients in the infliximab group were taking an infliximab biosimilar, whereas the vedolizumab group received the originator. Dr. Ferrante noted that the economic considerations involved in deciding between a biosimilar and an originator were not part of the research but that “there will be a difference in costs.”
Same mechanism, different route
The novel finding from the study includes the subcutaneous form of infliximab, which is not yet available in the United States, noted Joshua M. Steinberg, MD, director of inflammatory bowel disease at Gastroenterology of the Rockies, Denver, when asked to comment on the study. Currently, intravenously administered infliximab and vedolizumab are available in the United States.
A better comparator in the future would be looking at subcutaneous forms of both agents, especially “with the impending launch of subcutaneous vedolizumab in the United States,” said Dr. Steinberg, who is also a clinical instructor of medicine at the University of Colorado at Denver, Aurora.
He added that it’s reassuring overall that with a newer mode of administration but same mechanism of action, it is still feasible and durable for at least 1 year.
“The general consensus is that in terms of our biologics, vedolizumab is the safest because of its targeted mechanism of action. But sometimes the ‘safest choice’ isn’t the best choice,” Dr. Steinberg said. “I think in the right patient, the most effective treatment is going to be the one that works the best, and that’s not going to be universal.”
The study was sponsored by Celltrion, which makes an infliximab biosimilar. Dr. Ferrante receives honoraria as a consultant and speaker for Celltrion. Dr. Steinberg reported no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
CHICAGO – an updated meta-analysis of randomized clinical trials reveals.
At 1 year, 2% of people taking the anti–tumor necrosis factor (TNF) agent infliximab discontinued for lack of efficacy, compared with 24% of patients taking vedolizumab (Entyvio), an integrin receptor antagonist.
The safety profile of each agent is also important.
“We know that vedolizumab has less safety risks than an anti-TNF agent, but we also have the gut feeling that the anti-TNF agents are more efficacious,” lead author Marc Ferrante, MD, said in an interview.
“Of course, I don’t think we can really say that vedolizumab is the safest and infliximab is not safe, but there is some difference,” added Dr. Ferrante, a professor in the department of gastroenterology and hepatology, University Hospitals Leuven (Belgium).
The study was presented as a poster at the annual Digestive Disease Week (DDW).
The researchers conducted a pooled analysis of six randomized controlled trials from the past 10 years. They analyzed the NOR-SWITCH IV Q8W, the NCT02883452 SC Q2W, and LIBERTY-UC SC Q2W studies for infliximab, and the VISIBLE 1 SC Q2W, GEMINI 1 IV Q4W, and VARSITY IV Q8W trials for vedolizumab.
Their work expands on a meta-analysis by Dr. Ferrante and colleagues presented at DDW 2022. They added the 1-year results from the phase 3 LIBERTY-UC study to increase the number of participants taking infliximab or an infliximab biosimilar.
“Luckily, the results are very similar,” Dr. Ferrante said, and noted that they support previous findings that discontinuation of infliximab was lower than that of vedolizumab.
Most of the patients in the infliximab group were taking an infliximab biosimilar, whereas the vedolizumab group received the originator. Dr. Ferrante noted that the economic considerations involved in deciding between a biosimilar and an originator were not part of the research but that “there will be a difference in costs.”
Same mechanism, different route
The novel finding from the study includes the subcutaneous form of infliximab, which is not yet available in the United States, noted Joshua M. Steinberg, MD, director of inflammatory bowel disease at Gastroenterology of the Rockies, Denver, when asked to comment on the study. Currently, intravenously administered infliximab and vedolizumab are available in the United States.
A better comparator in the future would be looking at subcutaneous forms of both agents, especially “with the impending launch of subcutaneous vedolizumab in the United States,” said Dr. Steinberg, who is also a clinical instructor of medicine at the University of Colorado at Denver, Aurora.
He added that it’s reassuring overall that with a newer mode of administration but same mechanism of action, it is still feasible and durable for at least 1 year.
“The general consensus is that in terms of our biologics, vedolizumab is the safest because of its targeted mechanism of action. But sometimes the ‘safest choice’ isn’t the best choice,” Dr. Steinberg said. “I think in the right patient, the most effective treatment is going to be the one that works the best, and that’s not going to be universal.”
The study was sponsored by Celltrion, which makes an infliximab biosimilar. Dr. Ferrante receives honoraria as a consultant and speaker for Celltrion. Dr. Steinberg reported no relevant financial relationships.
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and The Society for Surgery of the Alimentary Tract.
A version of this article first appeared on Medscape.com.
AT DDW 2023
Severe rash after COVID-19 vaccination
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.
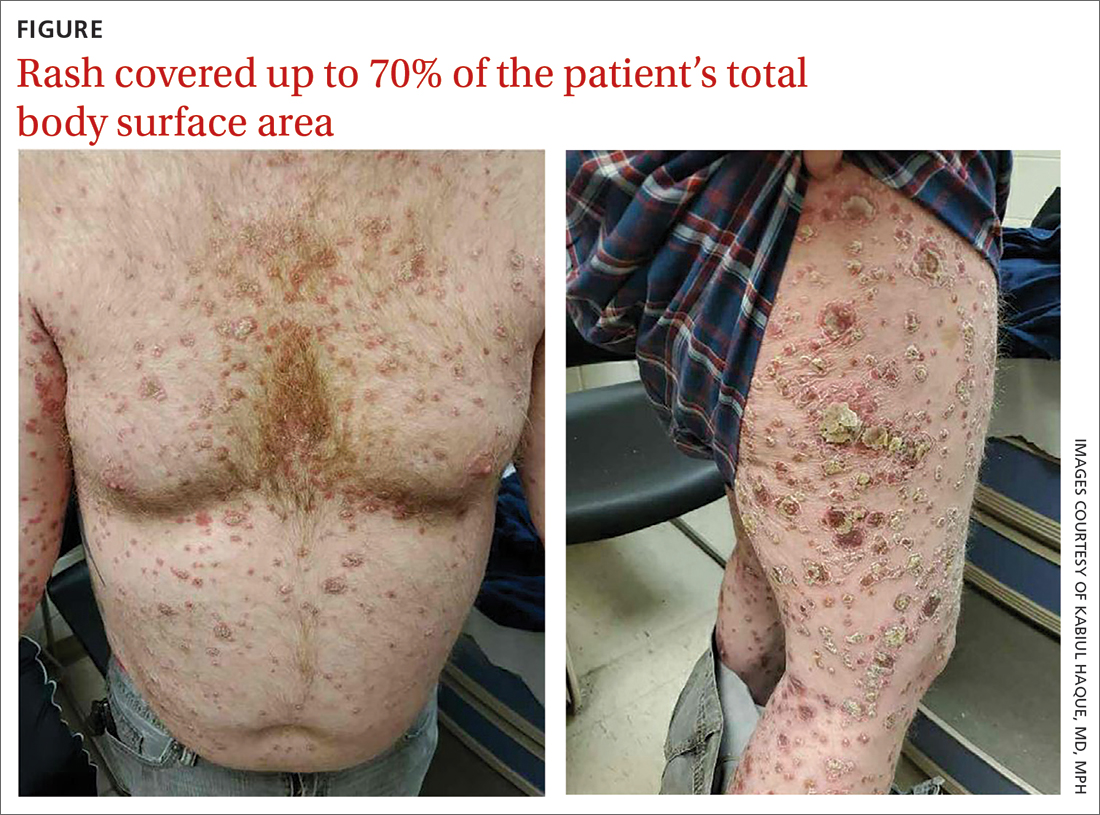
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
What BP target is appropriate for pregnant patients with mild chronic hypertension?
ILLUSTRATIVE CASE
A 32-year-old primigravida at 10 weeks’ gestation presents for an initial prenatal visit. Medical history includes hypertension that is currently well controlled on labetalol 200 mg twice daily. The patient’s blood pressure (BP) at today’s visit is 125/80 mm Hg. Should labetalol be discontinued?
Chronic hypertension in pregnancy is hypertension that predates the pregnancy or with onset prior to 20 weeks’ gestation. Diagnostic criteria include systolic BP > 140 mm Hg or diastolic BP > 90 mm Hg, use of antihypertensive medications prior to pregnancy, or pregnancy-related hypertension persisting > 12 weeks postpartum.2,3 Chronic hypertension affects 0.9% to 5% of pregnancies and is associated with increased risk for complications, such as superimposed preeclampsia, small-for-gestational-age infant, preterm birth, cesarean delivery, and neonatal intensive care unit admission.4 Superimposed preeclampsia occurs in about 17% to 25% of pregnancies affected by chronic hypertension, compared with 3% to 5% of the general population.3
Historically, a higher treatment threshold of 160/110 mm Hg was preferred to avoid theoretical complications of low placental perfusion.2 Practically, this often meant discontinuing antihypertensives at the onset of prenatal care if BP was well controlled. A few small trials previously demonstrated that tight BP goals reduced the risk for severe hypertension, but they did not show an improvement in pregnancy outcomes.5-7 This larger RCT evaluated whether treatment of mild chronic hypertension in pregnancy at lower BP thresholds is associated with improved pregnancy outcomes without negative impact on fetal growth.
STUDY SUMMARY
Active BP treatment yielded better pregnancy outcomes
In a US multicenter, open-label RCT, 2419 pregnant patients with chronic hypertension and singleton fetuses at gestational age < 23 weeks were randomized to receive either active pharmacologic treatment with a BP goal of 140/90 mm Hg or standard treatment, in which BP medication was withheld unless BP reached 160/105 mm Hg (severe hypertension). If medication was initiated in the standard-treatment group, the goal was also 140/90 mm Hg. Exclusion criteria included severe hypertension or suspected intrauterine growth restriction at randomization, known secondary hypertension, certain high-risk comorbidities (eg, cardiac or renal disease), or a major fetal anomaly.
First-line medications were labetalol or extended-release nifedipine in the majority of patients in the active-treatment group and in standard-treatment patients who developed severe hypertension. Patients were followed until 6 weeks after delivery. Intention-to-treat analyses were performed. The primary outcome was a composite of fetal or neonatal death before 28 days of life, superimposed preeclampsia with severe features up to 2 weeks postpartum, placental abruption leading to delivery, and medically indicated preterm birth before 35 weeks’ gestation. Safety outcomes included birthweight < 10th and < 5th percentile for gestational age.
Primary outcome events occurred in 30.2% of the active-treatment group compared with 37% of the standard-treatment group (adjusted risk ratio [aRR] = 0.82; 95% CI, 0.74-0.92; number needed to treat [NNT] = 15). Preeclampsia with severe features (23.3% vs 29.1%; aRR = 0.80; 95% CI, 0.70-0.92) and medically indicated preterm birth before 35 weeks (12.2% vs 16.7%; aRR = 0.73; 95% CI, 0.6-0.89) occurred less often in the active-treatment group compared with the standard-treatment group. There were no differences in rates of placental abruption, fetal or neonatal death, or small-for-gestational-age infants.
WHAT’S NEW
Target BP of < 140/90 mm Hg reduced risk
This trial provides high-quality evidence that initiating or maintaining treatment at a nonsevere BP threshold (< 140/90 mm Hg) in pregnant patients with mild chronic hypertension reduces maternal and neonatal risk without increasing the risk for small-for-gestational-age infants. The American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine have issued statements recommending a change in practice based on this trial.8,9
Continue to: CAVEATS
CAVEATS
Patient characteristics and medication choices were limited
This trial does not identify a BP goal for patients who are at highest risk for complications of hypertension or who already have been given a diagnosis of a growth-restricted fetus, as those patients were excluded.
Most patients in the trial who required medications received labetalol or extended-release nifedipine. It is unclear if other medications would produce similar outcomes.
CHALLENGES TO IMPLEMENTATION
Limited challenges anticipated
There should be limited challenges to implementation.
1. Tita AT, Szychowski JM, Boggess K, et al; Chronic Hypertension and Pregnancy (CHAP) Trial Consortium. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50. doi: 10.1097/AOG.0000000000003020
3. Guedes-Martins L. Chronic hypertension and pregnancy. Adv Exp Med Biol. 2017;956:395-407. doi: 10.1007/5584_2016_81
4. Bramham K, Parnell B, Nelson-Piercy C, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301
5. Sibai BM, Mabie WC, Shamsa F, et al. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-967. doi: 10.1016/0002-9378(90)91297-p
6. Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718-722. doi: 10.1111/j.1471-0528.1998.tb10201.x
7. Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407-417. doi: 10.1056/NEJMoa1404595
8. American College of Obstetricians and Gynecologists’ Committee on Clinical Practice Guidelines—Obstetrics. Clinical guidance for the integration of the findings of the Chronic Hypertension and Pregnancy (CHAP) study. Practice Advisory. April 2022. Accessed December 4, 2022. www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2022/04/clinical-guidance-for-the-integration-of-the-findings-of-the-chronic-hypertension-and-pregnancy-chap-study
9. Society for Maternal-Fetal Medicine; Publications Committee. Society for Maternal-Fetal Medicine statement: antihypertensive therapy for mild chronic hypertension in pregnancy—the Chronic Hypertension and Pregnancy trial. Am J Obstet Gynecol. 2022;227:B24-B27. doi: 10.1016/j.ajog.2022.04.011
ILLUSTRATIVE CASE
A 32-year-old primigravida at 10 weeks’ gestation presents for an initial prenatal visit. Medical history includes hypertension that is currently well controlled on labetalol 200 mg twice daily. The patient’s blood pressure (BP) at today’s visit is 125/80 mm Hg. Should labetalol be discontinued?
Chronic hypertension in pregnancy is hypertension that predates the pregnancy or with onset prior to 20 weeks’ gestation. Diagnostic criteria include systolic BP > 140 mm Hg or diastolic BP > 90 mm Hg, use of antihypertensive medications prior to pregnancy, or pregnancy-related hypertension persisting > 12 weeks postpartum.2,3 Chronic hypertension affects 0.9% to 5% of pregnancies and is associated with increased risk for complications, such as superimposed preeclampsia, small-for-gestational-age infant, preterm birth, cesarean delivery, and neonatal intensive care unit admission.4 Superimposed preeclampsia occurs in about 17% to 25% of pregnancies affected by chronic hypertension, compared with 3% to 5% of the general population.3
Historically, a higher treatment threshold of 160/110 mm Hg was preferred to avoid theoretical complications of low placental perfusion.2 Practically, this often meant discontinuing antihypertensives at the onset of prenatal care if BP was well controlled. A few small trials previously demonstrated that tight BP goals reduced the risk for severe hypertension, but they did not show an improvement in pregnancy outcomes.5-7 This larger RCT evaluated whether treatment of mild chronic hypertension in pregnancy at lower BP thresholds is associated with improved pregnancy outcomes without negative impact on fetal growth.
STUDY SUMMARY
Active BP treatment yielded better pregnancy outcomes
In a US multicenter, open-label RCT, 2419 pregnant patients with chronic hypertension and singleton fetuses at gestational age < 23 weeks were randomized to receive either active pharmacologic treatment with a BP goal of 140/90 mm Hg or standard treatment, in which BP medication was withheld unless BP reached 160/105 mm Hg (severe hypertension). If medication was initiated in the standard-treatment group, the goal was also 140/90 mm Hg. Exclusion criteria included severe hypertension or suspected intrauterine growth restriction at randomization, known secondary hypertension, certain high-risk comorbidities (eg, cardiac or renal disease), or a major fetal anomaly.
First-line medications were labetalol or extended-release nifedipine in the majority of patients in the active-treatment group and in standard-treatment patients who developed severe hypertension. Patients were followed until 6 weeks after delivery. Intention-to-treat analyses were performed. The primary outcome was a composite of fetal or neonatal death before 28 days of life, superimposed preeclampsia with severe features up to 2 weeks postpartum, placental abruption leading to delivery, and medically indicated preterm birth before 35 weeks’ gestation. Safety outcomes included birthweight < 10th and < 5th percentile for gestational age.
Primary outcome events occurred in 30.2% of the active-treatment group compared with 37% of the standard-treatment group (adjusted risk ratio [aRR] = 0.82; 95% CI, 0.74-0.92; number needed to treat [NNT] = 15). Preeclampsia with severe features (23.3% vs 29.1%; aRR = 0.80; 95% CI, 0.70-0.92) and medically indicated preterm birth before 35 weeks (12.2% vs 16.7%; aRR = 0.73; 95% CI, 0.6-0.89) occurred less often in the active-treatment group compared with the standard-treatment group. There were no differences in rates of placental abruption, fetal or neonatal death, or small-for-gestational-age infants.
WHAT’S NEW
Target BP of < 140/90 mm Hg reduced risk
This trial provides high-quality evidence that initiating or maintaining treatment at a nonsevere BP threshold (< 140/90 mm Hg) in pregnant patients with mild chronic hypertension reduces maternal and neonatal risk without increasing the risk for small-for-gestational-age infants. The American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine have issued statements recommending a change in practice based on this trial.8,9
Continue to: CAVEATS
CAVEATS
Patient characteristics and medication choices were limited
This trial does not identify a BP goal for patients who are at highest risk for complications of hypertension or who already have been given a diagnosis of a growth-restricted fetus, as those patients were excluded.
Most patients in the trial who required medications received labetalol or extended-release nifedipine. It is unclear if other medications would produce similar outcomes.
CHALLENGES TO IMPLEMENTATION
Limited challenges anticipated
There should be limited challenges to implementation.
ILLUSTRATIVE CASE
A 32-year-old primigravida at 10 weeks’ gestation presents for an initial prenatal visit. Medical history includes hypertension that is currently well controlled on labetalol 200 mg twice daily. The patient’s blood pressure (BP) at today’s visit is 125/80 mm Hg. Should labetalol be discontinued?
Chronic hypertension in pregnancy is hypertension that predates the pregnancy or with onset prior to 20 weeks’ gestation. Diagnostic criteria include systolic BP > 140 mm Hg or diastolic BP > 90 mm Hg, use of antihypertensive medications prior to pregnancy, or pregnancy-related hypertension persisting > 12 weeks postpartum.2,3 Chronic hypertension affects 0.9% to 5% of pregnancies and is associated with increased risk for complications, such as superimposed preeclampsia, small-for-gestational-age infant, preterm birth, cesarean delivery, and neonatal intensive care unit admission.4 Superimposed preeclampsia occurs in about 17% to 25% of pregnancies affected by chronic hypertension, compared with 3% to 5% of the general population.3
Historically, a higher treatment threshold of 160/110 mm Hg was preferred to avoid theoretical complications of low placental perfusion.2 Practically, this often meant discontinuing antihypertensives at the onset of prenatal care if BP was well controlled. A few small trials previously demonstrated that tight BP goals reduced the risk for severe hypertension, but they did not show an improvement in pregnancy outcomes.5-7 This larger RCT evaluated whether treatment of mild chronic hypertension in pregnancy at lower BP thresholds is associated with improved pregnancy outcomes without negative impact on fetal growth.
STUDY SUMMARY
Active BP treatment yielded better pregnancy outcomes
In a US multicenter, open-label RCT, 2419 pregnant patients with chronic hypertension and singleton fetuses at gestational age < 23 weeks were randomized to receive either active pharmacologic treatment with a BP goal of 140/90 mm Hg or standard treatment, in which BP medication was withheld unless BP reached 160/105 mm Hg (severe hypertension). If medication was initiated in the standard-treatment group, the goal was also 140/90 mm Hg. Exclusion criteria included severe hypertension or suspected intrauterine growth restriction at randomization, known secondary hypertension, certain high-risk comorbidities (eg, cardiac or renal disease), or a major fetal anomaly.
First-line medications were labetalol or extended-release nifedipine in the majority of patients in the active-treatment group and in standard-treatment patients who developed severe hypertension. Patients were followed until 6 weeks after delivery. Intention-to-treat analyses were performed. The primary outcome was a composite of fetal or neonatal death before 28 days of life, superimposed preeclampsia with severe features up to 2 weeks postpartum, placental abruption leading to delivery, and medically indicated preterm birth before 35 weeks’ gestation. Safety outcomes included birthweight < 10th and < 5th percentile for gestational age.
Primary outcome events occurred in 30.2% of the active-treatment group compared with 37% of the standard-treatment group (adjusted risk ratio [aRR] = 0.82; 95% CI, 0.74-0.92; number needed to treat [NNT] = 15). Preeclampsia with severe features (23.3% vs 29.1%; aRR = 0.80; 95% CI, 0.70-0.92) and medically indicated preterm birth before 35 weeks (12.2% vs 16.7%; aRR = 0.73; 95% CI, 0.6-0.89) occurred less often in the active-treatment group compared with the standard-treatment group. There were no differences in rates of placental abruption, fetal or neonatal death, or small-for-gestational-age infants.
WHAT’S NEW
Target BP of < 140/90 mm Hg reduced risk
This trial provides high-quality evidence that initiating or maintaining treatment at a nonsevere BP threshold (< 140/90 mm Hg) in pregnant patients with mild chronic hypertension reduces maternal and neonatal risk without increasing the risk for small-for-gestational-age infants. The American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine have issued statements recommending a change in practice based on this trial.8,9
Continue to: CAVEATS
CAVEATS
Patient characteristics and medication choices were limited
This trial does not identify a BP goal for patients who are at highest risk for complications of hypertension or who already have been given a diagnosis of a growth-restricted fetus, as those patients were excluded.
Most patients in the trial who required medications received labetalol or extended-release nifedipine. It is unclear if other medications would produce similar outcomes.
CHALLENGES TO IMPLEMENTATION
Limited challenges anticipated
There should be limited challenges to implementation.
1. Tita AT, Szychowski JM, Boggess K, et al; Chronic Hypertension and Pregnancy (CHAP) Trial Consortium. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50. doi: 10.1097/AOG.0000000000003020
3. Guedes-Martins L. Chronic hypertension and pregnancy. Adv Exp Med Biol. 2017;956:395-407. doi: 10.1007/5584_2016_81
4. Bramham K, Parnell B, Nelson-Piercy C, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301
5. Sibai BM, Mabie WC, Shamsa F, et al. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-967. doi: 10.1016/0002-9378(90)91297-p
6. Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718-722. doi: 10.1111/j.1471-0528.1998.tb10201.x
7. Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407-417. doi: 10.1056/NEJMoa1404595
8. American College of Obstetricians and Gynecologists’ Committee on Clinical Practice Guidelines—Obstetrics. Clinical guidance for the integration of the findings of the Chronic Hypertension and Pregnancy (CHAP) study. Practice Advisory. April 2022. Accessed December 4, 2022. www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2022/04/clinical-guidance-for-the-integration-of-the-findings-of-the-chronic-hypertension-and-pregnancy-chap-study
9. Society for Maternal-Fetal Medicine; Publications Committee. Society for Maternal-Fetal Medicine statement: antihypertensive therapy for mild chronic hypertension in pregnancy—the Chronic Hypertension and Pregnancy trial. Am J Obstet Gynecol. 2022;227:B24-B27. doi: 10.1016/j.ajog.2022.04.011
1. Tita AT, Szychowski JM, Boggess K, et al; Chronic Hypertension and Pregnancy (CHAP) Trial Consortium. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26-e50. doi: 10.1097/AOG.0000000000003020
3. Guedes-Martins L. Chronic hypertension and pregnancy. Adv Exp Med Biol. 2017;956:395-407. doi: 10.1007/5584_2016_81
4. Bramham K, Parnell B, Nelson-Piercy C, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301
5. Sibai BM, Mabie WC, Shamsa F, et al. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol. 1990;162:960-967. doi: 10.1016/0002-9378(90)91297-p
6. Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718-722. doi: 10.1111/j.1471-0528.1998.tb10201.x
7. Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407-417. doi: 10.1056/NEJMoa1404595
8. American College of Obstetricians and Gynecologists’ Committee on Clinical Practice Guidelines—Obstetrics. Clinical guidance for the integration of the findings of the Chronic Hypertension and Pregnancy (CHAP) study. Practice Advisory. April 2022. Accessed December 4, 2022. www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2022/04/clinical-guidance-for-the-integration-of-the-findings-of-the-chronic-hypertension-and-pregnancy-chap-study
9. Society for Maternal-Fetal Medicine; Publications Committee. Society for Maternal-Fetal Medicine statement: antihypertensive therapy for mild chronic hypertension in pregnancy—the Chronic Hypertension and Pregnancy trial. Am J Obstet Gynecol. 2022;227:B24-B27. doi: 10.1016/j.ajog.2022.04.011
PRACTICE CHANGER
Treat mild chronic hypertension during pregnancy to a target of < 140/90 mm Hg to reduce the risk for adverse pregnancy outcomes.
STRENGTH OF RECOMMENDATION
B: Based on a single high-quality randomized controlled trial (RCT).1
Tita AT, Szychowski JM, Boggess K, et al; Chronic Hypertension and Pregnancy (CHAP) Trial Consortium. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295
These USPSTF recommendations should be on your radar
The US Preventive Services Task Force (USPSTF) had a productive year in 2022. In total, the USPSTF
- reviewed and made recommendations on 4 new topics
- re-assessed 19 previous recommendations on 11 topics
- made 24 separate recommendations, including 1 “A,” 3 “B,” 3 “C,” and 5 “D” recommendations and 12 “I” statements (see TABLE 11).

A note about grading. TABLE 22 outlines the USPSTF’s grade definitions and suggestions for practice. The importance of an “A” or “B” recommendation rests historically with the requirement in the Affordable Care Act (ACA) that all USPSTF-recommended services with either of these grades have to be provided by commercial health insurance plans with no co-pay or deductible applied. (The legal challenge in Texas to the ACA’s preventive care provision may change that.)
What’s new?
The USPSTF’s review of 4 new topics exceeds the entity’s output in each of the prior 4 years, when the Task Force was able to add only 1 or 2 topics annually. However, 3 of the 4 new topics in 2022 resulted in an insufficient evidence or “I” statement, which means there was not enough evidence to judge the relative benefits and harms of the intervention.
These 3 included screening for type 2 diabetes in children and adolescents younger than 18 years; screening for obstructive sleep apnea in the general adult population (ages ≥ 18 years); and screening for eating disorders in adolescents and adults. The fourth new topic, screening for anxiety in children and adolescents, resulted in a “B” recommendation and was described in a recent Practice Alert.3
Major revision to 1 prior recommendation
Only 1 of the 19 revisited recommendations resulted in a major revision: the use of daily aspirin for primary prevention of cardiovascular disease (CVD). Note that it does not apply to those who have established CVD, in whom the use of aspirin would be considered tertiary prevention or harm reduction.
In 2016, the USPSTF recommended (with a “B” grade) the use of daily low-dose aspirin for those ages 50 to 59 years who had a 10-year risk for a CVD event > 10%; no increased risk for bleeding; at least a 10-year life expectancy; and a willingness to take aspirin for 10 years. For those ages 60 to 69 years with a 10-year risk for a CVD event > 10%, the recommendation was a “C.” For those younger than 50 and older than 70, an “I” statement was issued.
In 2022, the USPSTF was much less enthusiastic about daily aspirin as a primary preventative.4 The recommendation is now a “C” for those ages 40 to 59 years who have a 10-year CVD risk ≥ 10%. Those most likely to benefit have a 10-year CVD risk > 15%.
Continue to: The recommendation pertains...
The recommendation pertains to the initiation of aspirin, not the continuation or discontinuation for those who have been using aspirin without complications. The USPSTF suggests that the dose of aspirin, if used, should be 81 mg and that it should not be continued past age 75 years. A more detailed discussion of this recommendation and some of its clinical considerations is contained in a recent Practice Alert.5
“D” is for “don’t”(with a few caveats)
Avoiding unnecessary or harmful testing and treatments is just as important as offering preventive services of proven benefit. Those practices listed in TABLE 11 with a “D” recommendation should be avoided in practice.
However, it is worth mentioning that, while postmenopausal hormone replacement therapy should not be prescribed for the prevention of chronic conditions, this does not mean it should not be used to alleviate postmenopausal vasomotor symptoms—albeit for a limited period of time.
Also, it is important to appreciate the difference between screening and diagnostic tests. When the USPSTF recommends for or against screening, they are referring to the practice in asymptomatic people. The recommendation does not pertain to diagnostic testing to confirm or rule out a condition in a person with symptoms suggestive of a condition. Thus, the recommendation against screening adults for chronic obstructive pulmonary disease applies only to those without symptoms.
Be selective with services graded “C” or “I”
The USPSTF recommendations that require the most clinical judgment and are the most difficult to implement are those with a “C.” Few individuals will benefit from these interventions, and those most likely to benefit usually are described in the clinical considerations that accompany the recommendation. These interventions are time consuming and may be subject to insurance co-pays and deductibles. All 3 “C” recommendations made in 2022 (see TABLE 11) pertained to the prevention of CVD, still the leading cause of death in the United States.
Continue it: As "I" statement is not the same...
An “I” statement is not the same as a recommendation against the service—but if the service is offered, both the physician and the patient should understand the uncertainty involved. The services the USPSTF has determined lack sufficient evidence of benefits and/or harms are often recommended by other organizations—and in fact, the use of the “I” statement distinguishes the USPSTF from other clinical guideline groups.
If good evidence does not exist, the USPSTF will not make a recommendation. This is the main reason that, when the USPSTF reevaluates a topic (about every 6 to 7 years), they seldom make significant changes to their previous recommendations. Good evidence tends to survive the test of time.
However, adherence to this standard can cause the USPSTF to lag behind other guideline producers for some commonly used interventions. This delay can be considered a detriment if the intervention eventually proves to be effective, but it is a benefit if the intervention proves to be nonbeneficial or even harmful.
Putting recommendations into best practice
Given the time constraints in primary care practice, the most efficient way of providing high-quality, clinical preventive services is by implementing USPSTF “A” and “B” recommendations, being very selective about who receives an intervention with a “C” recommendation or “I” statement, and avoiding interventions with a “D” recommendation.
BREAKING NEWS
At press time, the USPSTF issued a draft recommendation statement that women begin receiving biennial mammograms starting at age 40 years (through age 74 years). For more, see: www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults#fullrecommendation start
1. USPSTF. Recommendation topics. Accessed April 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Grade definitions. Updated October 2018. Accessed April 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions
3. Campos-Outcalt D. Whom to screen for anxiety and depression: updated USPSTF recommendations. J Fam Pract. 2022;71:423-425. doi: 10.12788/jfp.0519
4. USPSTF. Aspirin use to prevent cardiovascular disease: USPSTF recommendation statement. JAMA. 2022;327:1577-1584. doi: 10.1001/jama.2022.4983
5. Campos-Outcalt D. USPSTF updates recommendations on aspirin and CVD. J Fam Pract. 2022;71:262-264. doi: 10.12788/jfp.0452
The US Preventive Services Task Force (USPSTF) had a productive year in 2022. In total, the USPSTF
- reviewed and made recommendations on 4 new topics
- re-assessed 19 previous recommendations on 11 topics
- made 24 separate recommendations, including 1 “A,” 3 “B,” 3 “C,” and 5 “D” recommendations and 12 “I” statements (see TABLE 11).

A note about grading. TABLE 22 outlines the USPSTF’s grade definitions and suggestions for practice. The importance of an “A” or “B” recommendation rests historically with the requirement in the Affordable Care Act (ACA) that all USPSTF-recommended services with either of these grades have to be provided by commercial health insurance plans with no co-pay or deductible applied. (The legal challenge in Texas to the ACA’s preventive care provision may change that.)

What’s new?
The USPSTF’s review of 4 new topics exceeds the entity’s output in each of the prior 4 years, when the Task Force was able to add only 1 or 2 topics annually. However, 3 of the 4 new topics in 2022 resulted in an insufficient evidence or “I” statement, which means there was not enough evidence to judge the relative benefits and harms of the intervention.
These 3 included screening for type 2 diabetes in children and adolescents younger than 18 years; screening for obstructive sleep apnea in the general adult population (ages ≥ 18 years); and screening for eating disorders in adolescents and adults. The fourth new topic, screening for anxiety in children and adolescents, resulted in a “B” recommendation and was described in a recent Practice Alert.3
Major revision to 1 prior recommendation
Only 1 of the 19 revisited recommendations resulted in a major revision: the use of daily aspirin for primary prevention of cardiovascular disease (CVD). Note that it does not apply to those who have established CVD, in whom the use of aspirin would be considered tertiary prevention or harm reduction.
In 2016, the USPSTF recommended (with a “B” grade) the use of daily low-dose aspirin for those ages 50 to 59 years who had a 10-year risk for a CVD event > 10%; no increased risk for bleeding; at least a 10-year life expectancy; and a willingness to take aspirin for 10 years. For those ages 60 to 69 years with a 10-year risk for a CVD event > 10%, the recommendation was a “C.” For those younger than 50 and older than 70, an “I” statement was issued.
In 2022, the USPSTF was much less enthusiastic about daily aspirin as a primary preventative.4 The recommendation is now a “C” for those ages 40 to 59 years who have a 10-year CVD risk ≥ 10%. Those most likely to benefit have a 10-year CVD risk > 15%.
Continue to: The recommendation pertains...
The recommendation pertains to the initiation of aspirin, not the continuation or discontinuation for those who have been using aspirin without complications. The USPSTF suggests that the dose of aspirin, if used, should be 81 mg and that it should not be continued past age 75 years. A more detailed discussion of this recommendation and some of its clinical considerations is contained in a recent Practice Alert.5
“D” is for “don’t”(with a few caveats)
Avoiding unnecessary or harmful testing and treatments is just as important as offering preventive services of proven benefit. Those practices listed in TABLE 11 with a “D” recommendation should be avoided in practice.
However, it is worth mentioning that, while postmenopausal hormone replacement therapy should not be prescribed for the prevention of chronic conditions, this does not mean it should not be used to alleviate postmenopausal vasomotor symptoms—albeit for a limited period of time.
Also, it is important to appreciate the difference between screening and diagnostic tests. When the USPSTF recommends for or against screening, they are referring to the practice in asymptomatic people. The recommendation does not pertain to diagnostic testing to confirm or rule out a condition in a person with symptoms suggestive of a condition. Thus, the recommendation against screening adults for chronic obstructive pulmonary disease applies only to those without symptoms.
Be selective with services graded “C” or “I”
The USPSTF recommendations that require the most clinical judgment and are the most difficult to implement are those with a “C.” Few individuals will benefit from these interventions, and those most likely to benefit usually are described in the clinical considerations that accompany the recommendation. These interventions are time consuming and may be subject to insurance co-pays and deductibles. All 3 “C” recommendations made in 2022 (see TABLE 11) pertained to the prevention of CVD, still the leading cause of death in the United States.
Continue it: As "I" statement is not the same...
An “I” statement is not the same as a recommendation against the service—but if the service is offered, both the physician and the patient should understand the uncertainty involved. The services the USPSTF has determined lack sufficient evidence of benefits and/or harms are often recommended by other organizations—and in fact, the use of the “I” statement distinguishes the USPSTF from other clinical guideline groups.
If good evidence does not exist, the USPSTF will not make a recommendation. This is the main reason that, when the USPSTF reevaluates a topic (about every 6 to 7 years), they seldom make significant changes to their previous recommendations. Good evidence tends to survive the test of time.
However, adherence to this standard can cause the USPSTF to lag behind other guideline producers for some commonly used interventions. This delay can be considered a detriment if the intervention eventually proves to be effective, but it is a benefit if the intervention proves to be nonbeneficial or even harmful.
Putting recommendations into best practice
Given the time constraints in primary care practice, the most efficient way of providing high-quality, clinical preventive services is by implementing USPSTF “A” and “B” recommendations, being very selective about who receives an intervention with a “C” recommendation or “I” statement, and avoiding interventions with a “D” recommendation.
BREAKING NEWS
At press time, the USPSTF issued a draft recommendation statement that women begin receiving biennial mammograms starting at age 40 years (through age 74 years). For more, see: www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults#fullrecommendation start
The US Preventive Services Task Force (USPSTF) had a productive year in 2022. In total, the USPSTF
- reviewed and made recommendations on 4 new topics
- re-assessed 19 previous recommendations on 11 topics
- made 24 separate recommendations, including 1 “A,” 3 “B,” 3 “C,” and 5 “D” recommendations and 12 “I” statements (see TABLE 11).

A note about grading. TABLE 22 outlines the USPSTF’s grade definitions and suggestions for practice. The importance of an “A” or “B” recommendation rests historically with the requirement in the Affordable Care Act (ACA) that all USPSTF-recommended services with either of these grades have to be provided by commercial health insurance plans with no co-pay or deductible applied. (The legal challenge in Texas to the ACA’s preventive care provision may change that.)

What’s new?
The USPSTF’s review of 4 new topics exceeds the entity’s output in each of the prior 4 years, when the Task Force was able to add only 1 or 2 topics annually. However, 3 of the 4 new topics in 2022 resulted in an insufficient evidence or “I” statement, which means there was not enough evidence to judge the relative benefits and harms of the intervention.
These 3 included screening for type 2 diabetes in children and adolescents younger than 18 years; screening for obstructive sleep apnea in the general adult population (ages ≥ 18 years); and screening for eating disorders in adolescents and adults. The fourth new topic, screening for anxiety in children and adolescents, resulted in a “B” recommendation and was described in a recent Practice Alert.3
Major revision to 1 prior recommendation
Only 1 of the 19 revisited recommendations resulted in a major revision: the use of daily aspirin for primary prevention of cardiovascular disease (CVD). Note that it does not apply to those who have established CVD, in whom the use of aspirin would be considered tertiary prevention or harm reduction.
In 2016, the USPSTF recommended (with a “B” grade) the use of daily low-dose aspirin for those ages 50 to 59 years who had a 10-year risk for a CVD event > 10%; no increased risk for bleeding; at least a 10-year life expectancy; and a willingness to take aspirin for 10 years. For those ages 60 to 69 years with a 10-year risk for a CVD event > 10%, the recommendation was a “C.” For those younger than 50 and older than 70, an “I” statement was issued.
In 2022, the USPSTF was much less enthusiastic about daily aspirin as a primary preventative.4 The recommendation is now a “C” for those ages 40 to 59 years who have a 10-year CVD risk ≥ 10%. Those most likely to benefit have a 10-year CVD risk > 15%.
Continue to: The recommendation pertains...
The recommendation pertains to the initiation of aspirin, not the continuation or discontinuation for those who have been using aspirin without complications. The USPSTF suggests that the dose of aspirin, if used, should be 81 mg and that it should not be continued past age 75 years. A more detailed discussion of this recommendation and some of its clinical considerations is contained in a recent Practice Alert.5
“D” is for “don’t”(with a few caveats)
Avoiding unnecessary or harmful testing and treatments is just as important as offering preventive services of proven benefit. Those practices listed in TABLE 11 with a “D” recommendation should be avoided in practice.
However, it is worth mentioning that, while postmenopausal hormone replacement therapy should not be prescribed for the prevention of chronic conditions, this does not mean it should not be used to alleviate postmenopausal vasomotor symptoms—albeit for a limited period of time.
Also, it is important to appreciate the difference between screening and diagnostic tests. When the USPSTF recommends for or against screening, they are referring to the practice in asymptomatic people. The recommendation does not pertain to diagnostic testing to confirm or rule out a condition in a person with symptoms suggestive of a condition. Thus, the recommendation against screening adults for chronic obstructive pulmonary disease applies only to those without symptoms.
Be selective with services graded “C” or “I”
The USPSTF recommendations that require the most clinical judgment and are the most difficult to implement are those with a “C.” Few individuals will benefit from these interventions, and those most likely to benefit usually are described in the clinical considerations that accompany the recommendation. These interventions are time consuming and may be subject to insurance co-pays and deductibles. All 3 “C” recommendations made in 2022 (see TABLE 11) pertained to the prevention of CVD, still the leading cause of death in the United States.
Continue it: As "I" statement is not the same...
An “I” statement is not the same as a recommendation against the service—but if the service is offered, both the physician and the patient should understand the uncertainty involved. The services the USPSTF has determined lack sufficient evidence of benefits and/or harms are often recommended by other organizations—and in fact, the use of the “I” statement distinguishes the USPSTF from other clinical guideline groups.
If good evidence does not exist, the USPSTF will not make a recommendation. This is the main reason that, when the USPSTF reevaluates a topic (about every 6 to 7 years), they seldom make significant changes to their previous recommendations. Good evidence tends to survive the test of time.
However, adherence to this standard can cause the USPSTF to lag behind other guideline producers for some commonly used interventions. This delay can be considered a detriment if the intervention eventually proves to be effective, but it is a benefit if the intervention proves to be nonbeneficial or even harmful.
Putting recommendations into best practice
Given the time constraints in primary care practice, the most efficient way of providing high-quality, clinical preventive services is by implementing USPSTF “A” and “B” recommendations, being very selective about who receives an intervention with a “C” recommendation or “I” statement, and avoiding interventions with a “D” recommendation.
BREAKING NEWS
At press time, the USPSTF issued a draft recommendation statement that women begin receiving biennial mammograms starting at age 40 years (through age 74 years). For more, see: www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults#fullrecommendation start
1. USPSTF. Recommendation topics. Accessed April 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Grade definitions. Updated October 2018. Accessed April 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions
3. Campos-Outcalt D. Whom to screen for anxiety and depression: updated USPSTF recommendations. J Fam Pract. 2022;71:423-425. doi: 10.12788/jfp.0519
4. USPSTF. Aspirin use to prevent cardiovascular disease: USPSTF recommendation statement. JAMA. 2022;327:1577-1584. doi: 10.1001/jama.2022.4983
5. Campos-Outcalt D. USPSTF updates recommendations on aspirin and CVD. J Fam Pract. 2022;71:262-264. doi: 10.12788/jfp.0452
1. USPSTF. Recommendation topics. Accessed April 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Grade definitions. Updated October 2018. Accessed April 18, 2023. www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions
3. Campos-Outcalt D. Whom to screen for anxiety and depression: updated USPSTF recommendations. J Fam Pract. 2022;71:423-425. doi: 10.12788/jfp.0519
4. USPSTF. Aspirin use to prevent cardiovascular disease: USPSTF recommendation statement. JAMA. 2022;327:1577-1584. doi: 10.1001/jama.2022.4983
5. Campos-Outcalt D. USPSTF updates recommendations on aspirin and CVD. J Fam Pract. 2022;71:262-264. doi: 10.12788/jfp.0452