User login
Screening for diabetes at normal BMIs could cut racial disparities
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Longer use of proton pump inhibitors tied to diabetes risk
Long-term use of a proton-pump inhibitor (PPI) was associated with an increased risk of being diagnosed with type 2 diabetes in a large, population-based case-control study in Italy.
The risk of diabetes increased from 19% to 56% as treatment duration increased from 8 weeks to more than 2 years, and prolonged treatment was associated with an even higher risk of diabetes in the youngest patients (age 40-65) and those with the most comorbidities.
The results suggest that “physicians should therefore avoid unnecessary prescription of this class of drugs, particularly for long-term use,” say Stefano Ciardullo, MD, University of Milano-Bicocca, Italy, and colleagues, in their article recently published online in the Journal of Clinical Endocrinology & Metabolism.
“Nonetheless, epidemiologic evidence on the topic remains conflicting,” they acknowledge, adding that “future studies are still needed to validate our findings.”
If the results are confirmed, these “may have important implications for both public health and clinical practice, given the high number of patients being treated with PPIs and the influence of diabetes on morbidity and mortality related to its possible micro- and macrovascular complications,” Dr. Ciardullo and colleagues conclude.
Not enough data to support a change in practice
The current findings align with a recent analysis of three prospective cohort studies of U.S. health care workers that showed a progressively increased risk of diabetes with longer treatment with PPIs, David A. Leiman, MD, MSHP, who was not involved with the current study, told this news organization in an email. “But the effect size remains relatively small and may be explained by residual or unmeasured confounding,” he cautioned.
“Ultimately, there do not seem to be enough data to support a change in clinical practice from this study alone, and, as a result, clinicians should continue to inform patients of the best available evidence regarding the benefits and risks of PPIs,” said Dr. Leiman, assistant professor of medicine, Division of Gastroenterology, Duke University Medical Center, Durham, N.C.
“Recent best practice advice from the American Gastroenterological Association does not recommend screening for insulin resistance among PPI users [and recommends that the decision to discontinue PPIs] should be based solely on the lack of an indication for PPI use, and not because of concern for PPI-associated adverse events,” he noted.
“Clinicians should be prepared to discuss the described risks associated with PPIs,” said Dr. Leiman, but they should “also feel comfortable affirming their safety profile and substantial efficacy in managing symptoms and preventing complications when prescribed for the appropriate indication.”
First-choice therapy for acid-related disorders
PPIs have become first-choice therapy for patients with acid-related disorders such as gastroesophageal reflux disease, Barrett esophagus, and peptic ulcer, and to prevent gastrointestinal bleeding while on nonsteroidal anti-inflammatory drugs (NSAIDs), Dr. Ciardullo and colleagues explain.
However, several studies have identified potential fractures, hypomagnesemia, gastric carcinoids, chronic kidney disease, dementia, and Clostridium difficile diarrhea with prolonged use of PPIs, and these agents can cause changes in the gut microbiome that may play a role in diabetes and other metabolic diseases.
To investigate a potential association between PPIs and type 2 diabetes, the researchers analyzed data from 777,420 patients age 40 and older who were newly treated with PPIs between 2010 and 2015 in Lombardy, Italy.
Of these, 50,540 patients were diagnosed with type 2 diabetes during follow-up until 2020 (a mean follow-up of 6.2 years and a diabetes incidence of 10.6 cases per 1,000 person-years).
The researchers matched 50,535 patients diagnosed with diabetes during follow-up with 50,535 control patients who had the same age, sex, and clinical status.
Patients were a mean age of 66 years and half were men. The most prescribed PPIs were pantoprazole and omeprazole, and the patients diagnosed with diabetes were more likely to use antihypertensives and lipid-lowering drugs.
Compared with patients who received PPIs for less than 8 weeks, those who received PPIs for 8 weeks to 6 months had a 19% increased risk of being diagnosed with diabetes during follow-up (odds ratio, 1.19; 95% confidence interval, 1.15-1.24), after adjusting for age, clinical profile, comorbidities, medical therapy, and PPI type.
Patients who received PPIs for 6 months to 2 years had a 43% increased risk of the outcome (OR, 1.43; 95% CI, 1.38-1.49), and those who received PPIs for more than 2 years had a 56% increased risk of the outcome (OR, 1.56; 95% CI, 1.49-1.64).
The researchers acknowledge limitations including that the study was not a randomized controlled trial, and it lacked information about over-the-counter medications and unmeasured confounders such as body mass index or family history of diabetes that may have affected the outcomes.
Dr. Leiman added that patients may have had prediabetes or undiagnosed diabetes and symptoms such as heartburn or dyspepsia arising from complications of insulin resistance, for which PPIs might have been prescribed.
The study was funded by a grant from the Italian Ministry of Education, University and Research. Dr. Ciardullo and Dr. Leiman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term use of a proton-pump inhibitor (PPI) was associated with an increased risk of being diagnosed with type 2 diabetes in a large, population-based case-control study in Italy.
The risk of diabetes increased from 19% to 56% as treatment duration increased from 8 weeks to more than 2 years, and prolonged treatment was associated with an even higher risk of diabetes in the youngest patients (age 40-65) and those with the most comorbidities.
The results suggest that “physicians should therefore avoid unnecessary prescription of this class of drugs, particularly for long-term use,” say Stefano Ciardullo, MD, University of Milano-Bicocca, Italy, and colleagues, in their article recently published online in the Journal of Clinical Endocrinology & Metabolism.
“Nonetheless, epidemiologic evidence on the topic remains conflicting,” they acknowledge, adding that “future studies are still needed to validate our findings.”
If the results are confirmed, these “may have important implications for both public health and clinical practice, given the high number of patients being treated with PPIs and the influence of diabetes on morbidity and mortality related to its possible micro- and macrovascular complications,” Dr. Ciardullo and colleagues conclude.
Not enough data to support a change in practice
The current findings align with a recent analysis of three prospective cohort studies of U.S. health care workers that showed a progressively increased risk of diabetes with longer treatment with PPIs, David A. Leiman, MD, MSHP, who was not involved with the current study, told this news organization in an email. “But the effect size remains relatively small and may be explained by residual or unmeasured confounding,” he cautioned.
“Ultimately, there do not seem to be enough data to support a change in clinical practice from this study alone, and, as a result, clinicians should continue to inform patients of the best available evidence regarding the benefits and risks of PPIs,” said Dr. Leiman, assistant professor of medicine, Division of Gastroenterology, Duke University Medical Center, Durham, N.C.
“Recent best practice advice from the American Gastroenterological Association does not recommend screening for insulin resistance among PPI users [and recommends that the decision to discontinue PPIs] should be based solely on the lack of an indication for PPI use, and not because of concern for PPI-associated adverse events,” he noted.
“Clinicians should be prepared to discuss the described risks associated with PPIs,” said Dr. Leiman, but they should “also feel comfortable affirming their safety profile and substantial efficacy in managing symptoms and preventing complications when prescribed for the appropriate indication.”
First-choice therapy for acid-related disorders
PPIs have become first-choice therapy for patients with acid-related disorders such as gastroesophageal reflux disease, Barrett esophagus, and peptic ulcer, and to prevent gastrointestinal bleeding while on nonsteroidal anti-inflammatory drugs (NSAIDs), Dr. Ciardullo and colleagues explain.
However, several studies have identified potential fractures, hypomagnesemia, gastric carcinoids, chronic kidney disease, dementia, and Clostridium difficile diarrhea with prolonged use of PPIs, and these agents can cause changes in the gut microbiome that may play a role in diabetes and other metabolic diseases.
To investigate a potential association between PPIs and type 2 diabetes, the researchers analyzed data from 777,420 patients age 40 and older who were newly treated with PPIs between 2010 and 2015 in Lombardy, Italy.
Of these, 50,540 patients were diagnosed with type 2 diabetes during follow-up until 2020 (a mean follow-up of 6.2 years and a diabetes incidence of 10.6 cases per 1,000 person-years).
The researchers matched 50,535 patients diagnosed with diabetes during follow-up with 50,535 control patients who had the same age, sex, and clinical status.
Patients were a mean age of 66 years and half were men. The most prescribed PPIs were pantoprazole and omeprazole, and the patients diagnosed with diabetes were more likely to use antihypertensives and lipid-lowering drugs.
Compared with patients who received PPIs for less than 8 weeks, those who received PPIs for 8 weeks to 6 months had a 19% increased risk of being diagnosed with diabetes during follow-up (odds ratio, 1.19; 95% confidence interval, 1.15-1.24), after adjusting for age, clinical profile, comorbidities, medical therapy, and PPI type.
Patients who received PPIs for 6 months to 2 years had a 43% increased risk of the outcome (OR, 1.43; 95% CI, 1.38-1.49), and those who received PPIs for more than 2 years had a 56% increased risk of the outcome (OR, 1.56; 95% CI, 1.49-1.64).
The researchers acknowledge limitations including that the study was not a randomized controlled trial, and it lacked information about over-the-counter medications and unmeasured confounders such as body mass index or family history of diabetes that may have affected the outcomes.
Dr. Leiman added that patients may have had prediabetes or undiagnosed diabetes and symptoms such as heartburn or dyspepsia arising from complications of insulin resistance, for which PPIs might have been prescribed.
The study was funded by a grant from the Italian Ministry of Education, University and Research. Dr. Ciardullo and Dr. Leiman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term use of a proton-pump inhibitor (PPI) was associated with an increased risk of being diagnosed with type 2 diabetes in a large, population-based case-control study in Italy.
The risk of diabetes increased from 19% to 56% as treatment duration increased from 8 weeks to more than 2 years, and prolonged treatment was associated with an even higher risk of diabetes in the youngest patients (age 40-65) and those with the most comorbidities.
The results suggest that “physicians should therefore avoid unnecessary prescription of this class of drugs, particularly for long-term use,” say Stefano Ciardullo, MD, University of Milano-Bicocca, Italy, and colleagues, in their article recently published online in the Journal of Clinical Endocrinology & Metabolism.
“Nonetheless, epidemiologic evidence on the topic remains conflicting,” they acknowledge, adding that “future studies are still needed to validate our findings.”
If the results are confirmed, these “may have important implications for both public health and clinical practice, given the high number of patients being treated with PPIs and the influence of diabetes on morbidity and mortality related to its possible micro- and macrovascular complications,” Dr. Ciardullo and colleagues conclude.
Not enough data to support a change in practice
The current findings align with a recent analysis of three prospective cohort studies of U.S. health care workers that showed a progressively increased risk of diabetes with longer treatment with PPIs, David A. Leiman, MD, MSHP, who was not involved with the current study, told this news organization in an email. “But the effect size remains relatively small and may be explained by residual or unmeasured confounding,” he cautioned.
“Ultimately, there do not seem to be enough data to support a change in clinical practice from this study alone, and, as a result, clinicians should continue to inform patients of the best available evidence regarding the benefits and risks of PPIs,” said Dr. Leiman, assistant professor of medicine, Division of Gastroenterology, Duke University Medical Center, Durham, N.C.
“Recent best practice advice from the American Gastroenterological Association does not recommend screening for insulin resistance among PPI users [and recommends that the decision to discontinue PPIs] should be based solely on the lack of an indication for PPI use, and not because of concern for PPI-associated adverse events,” he noted.
“Clinicians should be prepared to discuss the described risks associated with PPIs,” said Dr. Leiman, but they should “also feel comfortable affirming their safety profile and substantial efficacy in managing symptoms and preventing complications when prescribed for the appropriate indication.”
First-choice therapy for acid-related disorders
PPIs have become first-choice therapy for patients with acid-related disorders such as gastroesophageal reflux disease, Barrett esophagus, and peptic ulcer, and to prevent gastrointestinal bleeding while on nonsteroidal anti-inflammatory drugs (NSAIDs), Dr. Ciardullo and colleagues explain.
However, several studies have identified potential fractures, hypomagnesemia, gastric carcinoids, chronic kidney disease, dementia, and Clostridium difficile diarrhea with prolonged use of PPIs, and these agents can cause changes in the gut microbiome that may play a role in diabetes and other metabolic diseases.
To investigate a potential association between PPIs and type 2 diabetes, the researchers analyzed data from 777,420 patients age 40 and older who were newly treated with PPIs between 2010 and 2015 in Lombardy, Italy.
Of these, 50,540 patients were diagnosed with type 2 diabetes during follow-up until 2020 (a mean follow-up of 6.2 years and a diabetes incidence of 10.6 cases per 1,000 person-years).
The researchers matched 50,535 patients diagnosed with diabetes during follow-up with 50,535 control patients who had the same age, sex, and clinical status.
Patients were a mean age of 66 years and half were men. The most prescribed PPIs were pantoprazole and omeprazole, and the patients diagnosed with diabetes were more likely to use antihypertensives and lipid-lowering drugs.
Compared with patients who received PPIs for less than 8 weeks, those who received PPIs for 8 weeks to 6 months had a 19% increased risk of being diagnosed with diabetes during follow-up (odds ratio, 1.19; 95% confidence interval, 1.15-1.24), after adjusting for age, clinical profile, comorbidities, medical therapy, and PPI type.
Patients who received PPIs for 6 months to 2 years had a 43% increased risk of the outcome (OR, 1.43; 95% CI, 1.38-1.49), and those who received PPIs for more than 2 years had a 56% increased risk of the outcome (OR, 1.56; 95% CI, 1.49-1.64).
The researchers acknowledge limitations including that the study was not a randomized controlled trial, and it lacked information about over-the-counter medications and unmeasured confounders such as body mass index or family history of diabetes that may have affected the outcomes.
Dr. Leiman added that patients may have had prediabetes or undiagnosed diabetes and symptoms such as heartburn or dyspepsia arising from complications of insulin resistance, for which PPIs might have been prescribed.
The study was funded by a grant from the Italian Ministry of Education, University and Research. Dr. Ciardullo and Dr. Leiman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Vegan diet helps shed pounds but doesn’t dint diabetes
on average, new research indicates.
No effect was seen on blood pressure, triglycerides, or high-density lipoprotein cholesterol. HbA1c was reduced by a mean of –0.18 percentage points (P = .002), and there was a small reduction in total cholesterol and low-density lipoprotein cholesterol, on average, across all the studies examined in this meta-analysis.
The work, which compared a number of trials looking at vegan diets versus “normal” eating or other kinds of weight loss diets, “indicates with reasonable certainty that adhering to a vegan diet for at least 12 weeks may result in clinically meaningful weight loss [and] can be used in the management of overweight and type 2 diabetes,” said Anne-Ditte Termannsen, PhD, who reported the findings during a press conference at the European Congress on Obesity 2022, where the work was also presented as a poster.
A vegan diet most likely led to weight loss because it is “associated with a reduced calorie intake due to a lower content of fat and higher content of dietary fiber,” added Dr. Termannsen of the Steno Diabetes Center Copenhagen.
Asked to comment, Janet Cade, PhD, who leads the Nutritional Epidemiology Group at the University of Leeds (England) said the results are likely attributable to fewer calories in the vegan diet, compared with the “control” diets. “Of course, a vegan diet can be healthier in a range of ways, such as higher fruit and vegetables, more fiber and antioxidants; however, the same would be true of a vegetarian diet,” she noted.
And she warned that longer-term data are needed on health outcomes associated with vegan diets, noting, “there have been links to poorer bone health and osteoporosis in people consuming a vegan diet.”
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England) told the UK Science Media Centre: “The authors conducted a systematic review of intervention studies and found that, compared with no dietary interventions, vegan diets showed the strongest association with body-weight reduction.”
However, “When comparing vegan diets with other dietary interventions – such as the Mediterranean diet – the association was much weaker,” he noted.
Vegan, habitual, or a range of weight-loss diets
Dr. Termannsen and colleagues set out to look at the effect of a plant-based diet on cardiometabolic risk factors in people with overweight or type 2 diabetes. They searched the literature for randomized controlled trials with adult participants with overweight (body mass index ≥ 25 kg/m2), prediabetes, or type 2 diabetes.
Participants followed a vegan diet that lasted at least 12 weeks; habitual diets without any changes or energy restriction; a Mediterranean diet; a host of different “diabetes” diets; a low-fat diet; or portion-controlled diets.
“The vegan diets were nearly all low-fat vegan diets but vary substantially regarding the protein, fat, carbohydrate content. All but one study was ad libitum fat, and there were no energy restrictions,” Dr. Termannsen said.
Control diets were more varied. “Some continued their habitual diet, and about half were energy restricted and the others were not,” she acknowledged.
Outcomes comprised body weight, BMI, HbA1c, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, which were assessed across studies.
A total of 11 trials were included in the meta-analysis, and studies were a mean duration of 19 weeks. A total of 796 participants were included.
Compared with control diets, those on vegan diets lost on average –4.1 kg (–9 lb) (P < .001), with a range of –5.9 kg to –2.4 kg.
BMI dropped by –1.38 kg/m2 (P < .001). Total cholesterol dropped by –0.30 mmol/L (–11.6 mg/dL; P = .007) and LDL cholesterol by –0.24 mmol/L (–9.28 mg/dL; P = .005).
Further analyses found even greater reductions in body weight and BMI when vegan diets were compared with continuing a normal diet without dietary changes, on average, at –7.4 kg (–16.3 lb) (P < .001) and –2.78 kg/m2 (P < .001) respectively.
When compared with other intervention diets, however, body weight dropped by –2.7 kg (–6 lb; P < .001) and BMI by –0.87 kg/m2 (P < .001).
Commenting on limitations of studies compared to the real world, Dr. Termannsen said: “Some studies reported high adherence to their diet, usually due to a high level of support, suggesting that providing continued face-to-face contact with participants may partly explain the adherence differences.”
“This also questions the long-term feasibility of the diet and the applicability of this as long-term care,” she added.
Following a vegan diet requires good planning to ensure adequate nutrition and avoid any deficiencies, she urged. “We need to remember that the menu plans in the studies were created by dietitians.”
A version of this article first appeared on Medscape.com.
on average, new research indicates.
No effect was seen on blood pressure, triglycerides, or high-density lipoprotein cholesterol. HbA1c was reduced by a mean of –0.18 percentage points (P = .002), and there was a small reduction in total cholesterol and low-density lipoprotein cholesterol, on average, across all the studies examined in this meta-analysis.
The work, which compared a number of trials looking at vegan diets versus “normal” eating or other kinds of weight loss diets, “indicates with reasonable certainty that adhering to a vegan diet for at least 12 weeks may result in clinically meaningful weight loss [and] can be used in the management of overweight and type 2 diabetes,” said Anne-Ditte Termannsen, PhD, who reported the findings during a press conference at the European Congress on Obesity 2022, where the work was also presented as a poster.
A vegan diet most likely led to weight loss because it is “associated with a reduced calorie intake due to a lower content of fat and higher content of dietary fiber,” added Dr. Termannsen of the Steno Diabetes Center Copenhagen.
Asked to comment, Janet Cade, PhD, who leads the Nutritional Epidemiology Group at the University of Leeds (England) said the results are likely attributable to fewer calories in the vegan diet, compared with the “control” diets. “Of course, a vegan diet can be healthier in a range of ways, such as higher fruit and vegetables, more fiber and antioxidants; however, the same would be true of a vegetarian diet,” she noted.
And she warned that longer-term data are needed on health outcomes associated with vegan diets, noting, “there have been links to poorer bone health and osteoporosis in people consuming a vegan diet.”
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England) told the UK Science Media Centre: “The authors conducted a systematic review of intervention studies and found that, compared with no dietary interventions, vegan diets showed the strongest association with body-weight reduction.”
However, “When comparing vegan diets with other dietary interventions – such as the Mediterranean diet – the association was much weaker,” he noted.
Vegan, habitual, or a range of weight-loss diets
Dr. Termannsen and colleagues set out to look at the effect of a plant-based diet on cardiometabolic risk factors in people with overweight or type 2 diabetes. They searched the literature for randomized controlled trials with adult participants with overweight (body mass index ≥ 25 kg/m2), prediabetes, or type 2 diabetes.
Participants followed a vegan diet that lasted at least 12 weeks; habitual diets without any changes or energy restriction; a Mediterranean diet; a host of different “diabetes” diets; a low-fat diet; or portion-controlled diets.
“The vegan diets were nearly all low-fat vegan diets but vary substantially regarding the protein, fat, carbohydrate content. All but one study was ad libitum fat, and there were no energy restrictions,” Dr. Termannsen said.
Control diets were more varied. “Some continued their habitual diet, and about half were energy restricted and the others were not,” she acknowledged.
Outcomes comprised body weight, BMI, HbA1c, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, which were assessed across studies.
A total of 11 trials were included in the meta-analysis, and studies were a mean duration of 19 weeks. A total of 796 participants were included.
Compared with control diets, those on vegan diets lost on average –4.1 kg (–9 lb) (P < .001), with a range of –5.9 kg to –2.4 kg.
BMI dropped by –1.38 kg/m2 (P < .001). Total cholesterol dropped by –0.30 mmol/L (–11.6 mg/dL; P = .007) and LDL cholesterol by –0.24 mmol/L (–9.28 mg/dL; P = .005).
Further analyses found even greater reductions in body weight and BMI when vegan diets were compared with continuing a normal diet without dietary changes, on average, at –7.4 kg (–16.3 lb) (P < .001) and –2.78 kg/m2 (P < .001) respectively.
When compared with other intervention diets, however, body weight dropped by –2.7 kg (–6 lb; P < .001) and BMI by –0.87 kg/m2 (P < .001).
Commenting on limitations of studies compared to the real world, Dr. Termannsen said: “Some studies reported high adherence to their diet, usually due to a high level of support, suggesting that providing continued face-to-face contact with participants may partly explain the adherence differences.”
“This also questions the long-term feasibility of the diet and the applicability of this as long-term care,” she added.
Following a vegan diet requires good planning to ensure adequate nutrition and avoid any deficiencies, she urged. “We need to remember that the menu plans in the studies were created by dietitians.”
A version of this article first appeared on Medscape.com.
on average, new research indicates.
No effect was seen on blood pressure, triglycerides, or high-density lipoprotein cholesterol. HbA1c was reduced by a mean of –0.18 percentage points (P = .002), and there was a small reduction in total cholesterol and low-density lipoprotein cholesterol, on average, across all the studies examined in this meta-analysis.
The work, which compared a number of trials looking at vegan diets versus “normal” eating or other kinds of weight loss diets, “indicates with reasonable certainty that adhering to a vegan diet for at least 12 weeks may result in clinically meaningful weight loss [and] can be used in the management of overweight and type 2 diabetes,” said Anne-Ditte Termannsen, PhD, who reported the findings during a press conference at the European Congress on Obesity 2022, where the work was also presented as a poster.
A vegan diet most likely led to weight loss because it is “associated with a reduced calorie intake due to a lower content of fat and higher content of dietary fiber,” added Dr. Termannsen of the Steno Diabetes Center Copenhagen.
Asked to comment, Janet Cade, PhD, who leads the Nutritional Epidemiology Group at the University of Leeds (England) said the results are likely attributable to fewer calories in the vegan diet, compared with the “control” diets. “Of course, a vegan diet can be healthier in a range of ways, such as higher fruit and vegetables, more fiber and antioxidants; however, the same would be true of a vegetarian diet,” she noted.
And she warned that longer-term data are needed on health outcomes associated with vegan diets, noting, “there have been links to poorer bone health and osteoporosis in people consuming a vegan diet.”
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England) told the UK Science Media Centre: “The authors conducted a systematic review of intervention studies and found that, compared with no dietary interventions, vegan diets showed the strongest association with body-weight reduction.”
However, “When comparing vegan diets with other dietary interventions – such as the Mediterranean diet – the association was much weaker,” he noted.
Vegan, habitual, or a range of weight-loss diets
Dr. Termannsen and colleagues set out to look at the effect of a plant-based diet on cardiometabolic risk factors in people with overweight or type 2 diabetes. They searched the literature for randomized controlled trials with adult participants with overweight (body mass index ≥ 25 kg/m2), prediabetes, or type 2 diabetes.
Participants followed a vegan diet that lasted at least 12 weeks; habitual diets without any changes or energy restriction; a Mediterranean diet; a host of different “diabetes” diets; a low-fat diet; or portion-controlled diets.
“The vegan diets were nearly all low-fat vegan diets but vary substantially regarding the protein, fat, carbohydrate content. All but one study was ad libitum fat, and there were no energy restrictions,” Dr. Termannsen said.
Control diets were more varied. “Some continued their habitual diet, and about half were energy restricted and the others were not,” she acknowledged.
Outcomes comprised body weight, BMI, HbA1c, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, which were assessed across studies.
A total of 11 trials were included in the meta-analysis, and studies were a mean duration of 19 weeks. A total of 796 participants were included.
Compared with control diets, those on vegan diets lost on average –4.1 kg (–9 lb) (P < .001), with a range of –5.9 kg to –2.4 kg.
BMI dropped by –1.38 kg/m2 (P < .001). Total cholesterol dropped by –0.30 mmol/L (–11.6 mg/dL; P = .007) and LDL cholesterol by –0.24 mmol/L (–9.28 mg/dL; P = .005).
Further analyses found even greater reductions in body weight and BMI when vegan diets were compared with continuing a normal diet without dietary changes, on average, at –7.4 kg (–16.3 lb) (P < .001) and –2.78 kg/m2 (P < .001) respectively.
When compared with other intervention diets, however, body weight dropped by –2.7 kg (–6 lb; P < .001) and BMI by –0.87 kg/m2 (P < .001).
Commenting on limitations of studies compared to the real world, Dr. Termannsen said: “Some studies reported high adherence to their diet, usually due to a high level of support, suggesting that providing continued face-to-face contact with participants may partly explain the adherence differences.”
“This also questions the long-term feasibility of the diet and the applicability of this as long-term care,” she added.
Following a vegan diet requires good planning to ensure adequate nutrition and avoid any deficiencies, she urged. “We need to remember that the menu plans in the studies were created by dietitians.”
A version of this article first appeared on Medscape.com.
FROM ECO 2022
Impact of the COVID-19 Pandemic on Characteristics of Cutaneous Tumors Treated by Mohs Micrographic Surgery
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
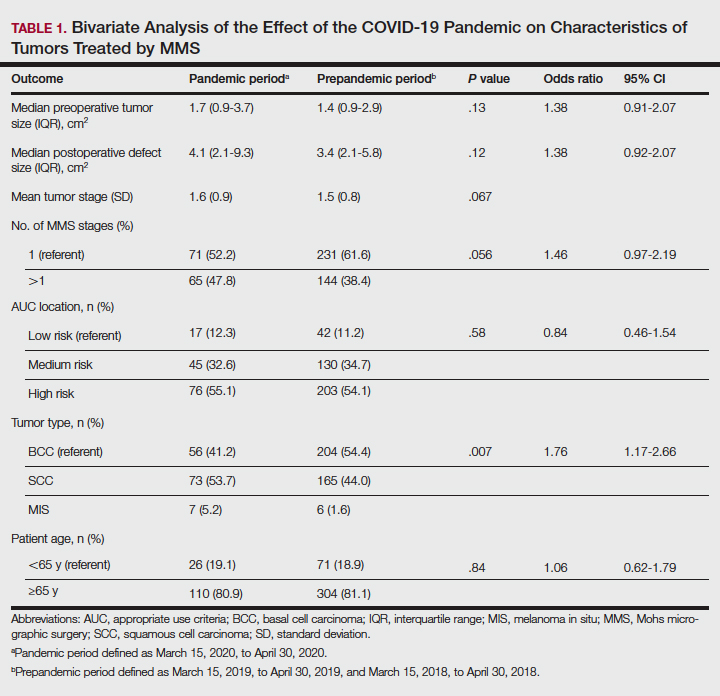
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
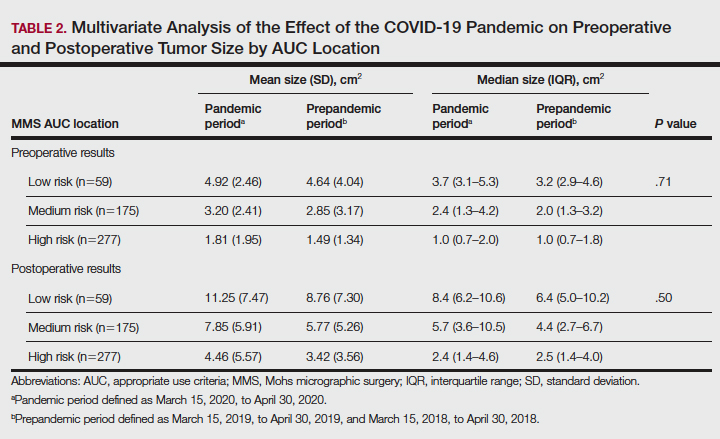
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.

Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).

Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
Practice Points
- Mohs surgeons should follow best practice guidelines dictated by our governing professional societies in selecting skin cancers for treatment by Mohs micrographic surgery (MMS) during the COVID-19 pandemic and beyond.
- The COVID-19 pandemic has impacted the characteristics of skin cancers treated by MMS, largely driven by new guidelines.
- Changing characteristics of skin cancers treated by MMS are of clinical significance, potentially affecting the extent of reconstructive surgery, cost, operating time, and future tumor characteristics.
Three symptoms suggest higher risk for self-injury in cancer
, according to a Canadian study.
In a population-based, case-control study, each of these symptoms was associated with an increase of at least 60% in the risk for NFSI in the following 180 days, the investigators report.
“Clinicians should know that self-injury is a real problem after a cancer diagnosis,” lead investigator Julie Hallet, MD, an associate scientist at Sunnybrook Health Sciences Centre in Toronto, told this news organization.
Self-injury “does not necessarily represent an attempted suicide,” she added. “While our data do not allow us to know what the intent was, we know from other work that the repercussions of distress in patients with cancer are much broader than suicide. Self-injury can be a means to cope with psychological difficulties for some patients, without intent for suicide.”
The study was published online in JAMA Oncology.
Nine common symptoms
The study included adults who were diagnosed with cancer between Jan. 1, 2007, and March 31, 2019, and had completed the Edmonton Symptom Assessment System (ESAS) evaluation within 36 months of their index cancer diagnosis. ESAS evaluates nine common cancer-associated symptoms, including pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath, on a patient-reported scale of 0 (absence of symptom) to 10 (worst possible symptom).
The analysis included 406 patients who had visited an emergency department for an NFSI within 180 days of their ESAS evaluation, as well as 1,624 matched control patients with cancer who did not have an NFSI. Case patients and control patients were matched according to age at cancer diagnosis, sex, prior self-injury within 5 years of being diagnosed with cancer, and cancer type. Nonmatched covariates included psychiatric illness and therapy received before NFSI, comorbidity burden, material deprivation, and cancer stage.
Toward tailored intervention
A higher proportion of case patients than control patients reported moderate to severe scores for all nine ESAS symptoms. In an adjusted analysis, moderate to severe anxiety (odds ratio, 1.61), depression (OR, 1.66), and shortness of breath (OR, 1.65) were independently associated with higher odds of subsequent NFSI. Each 10-point increase in total ESAS score also was associated with increased risk (OR, 1.51).
“These findings are important to enhance the use of screening ESAS scores to better support patients,” say the authors. “Scores from ESAS assessments can be used to identify patients at higher risk of NFSI, indicating higher level of distress, and help direct tailored assessment and intervention.”
In prior work, Dr. Hallet’s group showed that NFSI occurs in 3 of every 1,000 patients with cancer. NFSI is more frequent among younger patients and those with a history of prior mental illness. “Identifying patients at risk in clinical practice requires you to inquire about a patient’s prior history, identify high symptom scores and ask about them, and trigger intervention pathways when risk is identified,” said Dr. Hallet.
“For example, a young patient with head and neck cancer and a prior history of mental illness who reports high scores for anxiety and drowsiness would be at high risk of self-injury,” she added. Such a patient should be referred to psycho-oncology, psychiatry, or social work. “To facilitate this, we are working on prognostic scores that can be integrated in clinical practice, such as an electronic medical record, to flag patients at risk,” said Dr. Hallet. “Future work will also need to identify the optimal care pathways for at-risk patients.”
Self-injury vs. suicidality
Commenting on the study for this news organization, Madeline Li, MD, PhD, a psychiatrist and clinician-scientist at Toronto’s Princess Margaret Cancer Centre, said that the findings are “underwhelming” because they tell us what is already known – that “NFSI is associated with distress, and cancer is a stressor.” It would have been more interesting to ask how to distinguish patients at risk for suicide from those at risk for self-harm without suicide, she added.
“The way these authors formulated NFSI included both self-harm intent and suicidal intent,” she explained. The researchers compared patients who were at risk for these two types of events with patients without NFSI. “When we see self-harm without suicidal intent in the emergency room, it’s mostly people making cries for help,” said Dr. Li. “These are people who cut their wrists or take small overdoses on purpose without the intent to die. It would have been more interesting to see if there are different risk factors for people who are just going to self-harm vs. those who are actually going to attempt suicide.”
The study’s identification of risk factors for NSFI is important because “it does tell us that when there’s anxiety, depression, and shortness of breath, we should pay attention to these patients and do something about it,” said Dr. Li. Still, research in cancer psychiatry needs to shift its focus from identifying and addressing existing risk factors to preventing them from developing, she added.
“We need to move earlier and provide emotional and mental health support to cancer patients to prevent them from becoming suicidal, rather than intervening when somebody already is,” Dr. Li concluded.
The study was funded by the Hanna Research Award from the division of surgical oncology at the Odette Cancer Centre–Sunnybrook Health Sciences Centre and by a Sunnybrook Health Sciences Centre Alternate Funding Plan Innovation grant. It was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Hallet has received personal fees from Ipsen Biopharmaceuticals Canada and AAA outside the submitted work. Dr. Li reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a Canadian study.
In a population-based, case-control study, each of these symptoms was associated with an increase of at least 60% in the risk for NFSI in the following 180 days, the investigators report.
“Clinicians should know that self-injury is a real problem after a cancer diagnosis,” lead investigator Julie Hallet, MD, an associate scientist at Sunnybrook Health Sciences Centre in Toronto, told this news organization.
Self-injury “does not necessarily represent an attempted suicide,” she added. “While our data do not allow us to know what the intent was, we know from other work that the repercussions of distress in patients with cancer are much broader than suicide. Self-injury can be a means to cope with psychological difficulties for some patients, without intent for suicide.”
The study was published online in JAMA Oncology.
Nine common symptoms
The study included adults who were diagnosed with cancer between Jan. 1, 2007, and March 31, 2019, and had completed the Edmonton Symptom Assessment System (ESAS) evaluation within 36 months of their index cancer diagnosis. ESAS evaluates nine common cancer-associated symptoms, including pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath, on a patient-reported scale of 0 (absence of symptom) to 10 (worst possible symptom).
The analysis included 406 patients who had visited an emergency department for an NFSI within 180 days of their ESAS evaluation, as well as 1,624 matched control patients with cancer who did not have an NFSI. Case patients and control patients were matched according to age at cancer diagnosis, sex, prior self-injury within 5 years of being diagnosed with cancer, and cancer type. Nonmatched covariates included psychiatric illness and therapy received before NFSI, comorbidity burden, material deprivation, and cancer stage.
Toward tailored intervention
A higher proportion of case patients than control patients reported moderate to severe scores for all nine ESAS symptoms. In an adjusted analysis, moderate to severe anxiety (odds ratio, 1.61), depression (OR, 1.66), and shortness of breath (OR, 1.65) were independently associated with higher odds of subsequent NFSI. Each 10-point increase in total ESAS score also was associated with increased risk (OR, 1.51).
“These findings are important to enhance the use of screening ESAS scores to better support patients,” say the authors. “Scores from ESAS assessments can be used to identify patients at higher risk of NFSI, indicating higher level of distress, and help direct tailored assessment and intervention.”
In prior work, Dr. Hallet’s group showed that NFSI occurs in 3 of every 1,000 patients with cancer. NFSI is more frequent among younger patients and those with a history of prior mental illness. “Identifying patients at risk in clinical practice requires you to inquire about a patient’s prior history, identify high symptom scores and ask about them, and trigger intervention pathways when risk is identified,” said Dr. Hallet.
“For example, a young patient with head and neck cancer and a prior history of mental illness who reports high scores for anxiety and drowsiness would be at high risk of self-injury,” she added. Such a patient should be referred to psycho-oncology, psychiatry, or social work. “To facilitate this, we are working on prognostic scores that can be integrated in clinical practice, such as an electronic medical record, to flag patients at risk,” said Dr. Hallet. “Future work will also need to identify the optimal care pathways for at-risk patients.”
Self-injury vs. suicidality
Commenting on the study for this news organization, Madeline Li, MD, PhD, a psychiatrist and clinician-scientist at Toronto’s Princess Margaret Cancer Centre, said that the findings are “underwhelming” because they tell us what is already known – that “NFSI is associated with distress, and cancer is a stressor.” It would have been more interesting to ask how to distinguish patients at risk for suicide from those at risk for self-harm without suicide, she added.
“The way these authors formulated NFSI included both self-harm intent and suicidal intent,” she explained. The researchers compared patients who were at risk for these two types of events with patients without NFSI. “When we see self-harm without suicidal intent in the emergency room, it’s mostly people making cries for help,” said Dr. Li. “These are people who cut their wrists or take small overdoses on purpose without the intent to die. It would have been more interesting to see if there are different risk factors for people who are just going to self-harm vs. those who are actually going to attempt suicide.”
The study’s identification of risk factors for NSFI is important because “it does tell us that when there’s anxiety, depression, and shortness of breath, we should pay attention to these patients and do something about it,” said Dr. Li. Still, research in cancer psychiatry needs to shift its focus from identifying and addressing existing risk factors to preventing them from developing, she added.
“We need to move earlier and provide emotional and mental health support to cancer patients to prevent them from becoming suicidal, rather than intervening when somebody already is,” Dr. Li concluded.
The study was funded by the Hanna Research Award from the division of surgical oncology at the Odette Cancer Centre–Sunnybrook Health Sciences Centre and by a Sunnybrook Health Sciences Centre Alternate Funding Plan Innovation grant. It was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Hallet has received personal fees from Ipsen Biopharmaceuticals Canada and AAA outside the submitted work. Dr. Li reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a Canadian study.
In a population-based, case-control study, each of these symptoms was associated with an increase of at least 60% in the risk for NFSI in the following 180 days, the investigators report.
“Clinicians should know that self-injury is a real problem after a cancer diagnosis,” lead investigator Julie Hallet, MD, an associate scientist at Sunnybrook Health Sciences Centre in Toronto, told this news organization.
Self-injury “does not necessarily represent an attempted suicide,” she added. “While our data do not allow us to know what the intent was, we know from other work that the repercussions of distress in patients with cancer are much broader than suicide. Self-injury can be a means to cope with psychological difficulties for some patients, without intent for suicide.”
The study was published online in JAMA Oncology.
Nine common symptoms
The study included adults who were diagnosed with cancer between Jan. 1, 2007, and March 31, 2019, and had completed the Edmonton Symptom Assessment System (ESAS) evaluation within 36 months of their index cancer diagnosis. ESAS evaluates nine common cancer-associated symptoms, including pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath, on a patient-reported scale of 0 (absence of symptom) to 10 (worst possible symptom).
The analysis included 406 patients who had visited an emergency department for an NFSI within 180 days of their ESAS evaluation, as well as 1,624 matched control patients with cancer who did not have an NFSI. Case patients and control patients were matched according to age at cancer diagnosis, sex, prior self-injury within 5 years of being diagnosed with cancer, and cancer type. Nonmatched covariates included psychiatric illness and therapy received before NFSI, comorbidity burden, material deprivation, and cancer stage.
Toward tailored intervention
A higher proportion of case patients than control patients reported moderate to severe scores for all nine ESAS symptoms. In an adjusted analysis, moderate to severe anxiety (odds ratio, 1.61), depression (OR, 1.66), and shortness of breath (OR, 1.65) were independently associated with higher odds of subsequent NFSI. Each 10-point increase in total ESAS score also was associated with increased risk (OR, 1.51).
“These findings are important to enhance the use of screening ESAS scores to better support patients,” say the authors. “Scores from ESAS assessments can be used to identify patients at higher risk of NFSI, indicating higher level of distress, and help direct tailored assessment and intervention.”
In prior work, Dr. Hallet’s group showed that NFSI occurs in 3 of every 1,000 patients with cancer. NFSI is more frequent among younger patients and those with a history of prior mental illness. “Identifying patients at risk in clinical practice requires you to inquire about a patient’s prior history, identify high symptom scores and ask about them, and trigger intervention pathways when risk is identified,” said Dr. Hallet.
“For example, a young patient with head and neck cancer and a prior history of mental illness who reports high scores for anxiety and drowsiness would be at high risk of self-injury,” she added. Such a patient should be referred to psycho-oncology, psychiatry, or social work. “To facilitate this, we are working on prognostic scores that can be integrated in clinical practice, such as an electronic medical record, to flag patients at risk,” said Dr. Hallet. “Future work will also need to identify the optimal care pathways for at-risk patients.”
Self-injury vs. suicidality
Commenting on the study for this news organization, Madeline Li, MD, PhD, a psychiatrist and clinician-scientist at Toronto’s Princess Margaret Cancer Centre, said that the findings are “underwhelming” because they tell us what is already known – that “NFSI is associated with distress, and cancer is a stressor.” It would have been more interesting to ask how to distinguish patients at risk for suicide from those at risk for self-harm without suicide, she added.
“The way these authors formulated NFSI included both self-harm intent and suicidal intent,” she explained. The researchers compared patients who were at risk for these two types of events with patients without NFSI. “When we see self-harm without suicidal intent in the emergency room, it’s mostly people making cries for help,” said Dr. Li. “These are people who cut their wrists or take small overdoses on purpose without the intent to die. It would have been more interesting to see if there are different risk factors for people who are just going to self-harm vs. those who are actually going to attempt suicide.”
The study’s identification of risk factors for NSFI is important because “it does tell us that when there’s anxiety, depression, and shortness of breath, we should pay attention to these patients and do something about it,” said Dr. Li. Still, research in cancer psychiatry needs to shift its focus from identifying and addressing existing risk factors to preventing them from developing, she added.
“We need to move earlier and provide emotional and mental health support to cancer patients to prevent them from becoming suicidal, rather than intervening when somebody already is,” Dr. Li concluded.
The study was funded by the Hanna Research Award from the division of surgical oncology at the Odette Cancer Centre–Sunnybrook Health Sciences Centre and by a Sunnybrook Health Sciences Centre Alternate Funding Plan Innovation grant. It was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Hallet has received personal fees from Ipsen Biopharmaceuticals Canada and AAA outside the submitted work. Dr. Li reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Which solid organ transplant recipients face the highest risk of skin cancer?
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
AT AAD 22
Biomarker testing gains momentum in NSCLC
Despite Spain’s lack of a national project or standard protocol for biomarker testing, , according to a Spanish national registry study reported at the 2022 European Lung Cancer Congress.
“In recent years we’ve developed drugs that target biomarkers, so it’s important to identify those biomarkers to guide treatment and have an impact on the survival of our patients,” said lead author Virginia Calvo, MD, a medical oncologist with the Puerta de Hierro Majadahonda University Hospital, Madrid.
“If we don’t know our patients’ biomarkers, we can’t treat them with targeted therapies,” she added, noting that the overall survival of lung cancer patients has increased by 15% in the last 10 years, largely because of better therapies such as targeted drugs for advanced stage disease and immunotherapies.
To assess the status of biomarker testing in Spain, Dr. Calvo and colleagues analyzed data from the country’s Thoracic Tumor Registry on 9,239 patients diagnosed with metastatic NSCLC from 2016 to the present, 7,467 (81%) with nonsquamous tumors and 1,772 (19%) with squamous tumors.
They found that 85% of patients with nonsquamous NSCLC and about 53% of those with squamous cancers had undergone biomarker testing. They discovered that 4,115 (44%) of patients tested positive for EGFR, ALK, KRAS, BRAF, ROS1, or PD-L1.
Dr. Calvo attributes the widespread use of biomarker testing and its significant increase in the last 5 years to the growing knowledge and understanding of the disease.
“We are learning more about NSCLC, and I think in the next few years the number of biomarkers are going to grow,” she said.
The study’s findings also highlight the importance of establishing and maintaining cancer registries, Dr. Calvo said, noting that 182 hospitals across Spain and more than 550 experts participate in the Thoracic Tumors Registry, which includes data on patients from every Spanish territory.
“It’s important to collect information on real-life cancer care so that we know what our real situation is and take steps to improve it,” she said.
She anticipates that treatment for NSCLC patients will become increasingly complex in the future with the growing number of different biomarkers and the proportion of patients who test positive for them. “We may need to establish national strategies to implement next generation sequencing so that we can identify different biomarkers and improve the survival of our patients.”
In a press release, Rolf Stahel, MD, president of the European Thoracic Oncology Platform, said that it would be helpful to look at how frequently molecular testing led to patients receiving appropriate targeted treatment.
In the United States, the National Comprehensive Cancer Network recommends biomarker testing for eligible patients with newly diagnosed stage 4 NSCLC, and it can be considered for patients with squamous histology because 5%-10% of these tumors have targetable mutations. “This is because numerous lines of evidence show that patients with stage 4 NSCLC and a targetable mutation, typically have improved overall survival when treated with a targeted therapy,” wrote the authors of the NCCN recommendations.
“For newly diagnosed stage 4 NSCLC, there is always a tension between the need to start therapy versus waiting for molecular results. This is because if a recommended targeted option is identified, it is the optimal first-line therapy. Targeted therapy cannot be given to everyone. Different biomarkers predict response to different agents. This has been well illustrated and it makes testing critically important for patients with NSCLC,” Dara Aisner, MD, PhD, associate professor of pathology with the University of Colorado at Denver, Aurora, wrote in the NCCN guideline.
The study presented at ELCC was funded by a grant from the European Union’s Horizon 2020 Research and Innovation Program. Dr. Calvo has received fees from Roche, Bristol-Myers Squibb, MSD and AstraZeneca.
Despite Spain’s lack of a national project or standard protocol for biomarker testing, , according to a Spanish national registry study reported at the 2022 European Lung Cancer Congress.
“In recent years we’ve developed drugs that target biomarkers, so it’s important to identify those biomarkers to guide treatment and have an impact on the survival of our patients,” said lead author Virginia Calvo, MD, a medical oncologist with the Puerta de Hierro Majadahonda University Hospital, Madrid.
“If we don’t know our patients’ biomarkers, we can’t treat them with targeted therapies,” she added, noting that the overall survival of lung cancer patients has increased by 15% in the last 10 years, largely because of better therapies such as targeted drugs for advanced stage disease and immunotherapies.
To assess the status of biomarker testing in Spain, Dr. Calvo and colleagues analyzed data from the country’s Thoracic Tumor Registry on 9,239 patients diagnosed with metastatic NSCLC from 2016 to the present, 7,467 (81%) with nonsquamous tumors and 1,772 (19%) with squamous tumors.
They found that 85% of patients with nonsquamous NSCLC and about 53% of those with squamous cancers had undergone biomarker testing. They discovered that 4,115 (44%) of patients tested positive for EGFR, ALK, KRAS, BRAF, ROS1, or PD-L1.
Dr. Calvo attributes the widespread use of biomarker testing and its significant increase in the last 5 years to the growing knowledge and understanding of the disease.
“We are learning more about NSCLC, and I think in the next few years the number of biomarkers are going to grow,” she said.
The study’s findings also highlight the importance of establishing and maintaining cancer registries, Dr. Calvo said, noting that 182 hospitals across Spain and more than 550 experts participate in the Thoracic Tumors Registry, which includes data on patients from every Spanish territory.
“It’s important to collect information on real-life cancer care so that we know what our real situation is and take steps to improve it,” she said.
She anticipates that treatment for NSCLC patients will become increasingly complex in the future with the growing number of different biomarkers and the proportion of patients who test positive for them. “We may need to establish national strategies to implement next generation sequencing so that we can identify different biomarkers and improve the survival of our patients.”
In a press release, Rolf Stahel, MD, president of the European Thoracic Oncology Platform, said that it would be helpful to look at how frequently molecular testing led to patients receiving appropriate targeted treatment.
In the United States, the National Comprehensive Cancer Network recommends biomarker testing for eligible patients with newly diagnosed stage 4 NSCLC, and it can be considered for patients with squamous histology because 5%-10% of these tumors have targetable mutations. “This is because numerous lines of evidence show that patients with stage 4 NSCLC and a targetable mutation, typically have improved overall survival when treated with a targeted therapy,” wrote the authors of the NCCN recommendations.
“For newly diagnosed stage 4 NSCLC, there is always a tension between the need to start therapy versus waiting for molecular results. This is because if a recommended targeted option is identified, it is the optimal first-line therapy. Targeted therapy cannot be given to everyone. Different biomarkers predict response to different agents. This has been well illustrated and it makes testing critically important for patients with NSCLC,” Dara Aisner, MD, PhD, associate professor of pathology with the University of Colorado at Denver, Aurora, wrote in the NCCN guideline.
The study presented at ELCC was funded by a grant from the European Union’s Horizon 2020 Research and Innovation Program. Dr. Calvo has received fees from Roche, Bristol-Myers Squibb, MSD and AstraZeneca.
Despite Spain’s lack of a national project or standard protocol for biomarker testing, , according to a Spanish national registry study reported at the 2022 European Lung Cancer Congress.
“In recent years we’ve developed drugs that target biomarkers, so it’s important to identify those biomarkers to guide treatment and have an impact on the survival of our patients,” said lead author Virginia Calvo, MD, a medical oncologist with the Puerta de Hierro Majadahonda University Hospital, Madrid.
“If we don’t know our patients’ biomarkers, we can’t treat them with targeted therapies,” she added, noting that the overall survival of lung cancer patients has increased by 15% in the last 10 years, largely because of better therapies such as targeted drugs for advanced stage disease and immunotherapies.
To assess the status of biomarker testing in Spain, Dr. Calvo and colleagues analyzed data from the country’s Thoracic Tumor Registry on 9,239 patients diagnosed with metastatic NSCLC from 2016 to the present, 7,467 (81%) with nonsquamous tumors and 1,772 (19%) with squamous tumors.
They found that 85% of patients with nonsquamous NSCLC and about 53% of those with squamous cancers had undergone biomarker testing. They discovered that 4,115 (44%) of patients tested positive for EGFR, ALK, KRAS, BRAF, ROS1, or PD-L1.
Dr. Calvo attributes the widespread use of biomarker testing and its significant increase in the last 5 years to the growing knowledge and understanding of the disease.
“We are learning more about NSCLC, and I think in the next few years the number of biomarkers are going to grow,” she said.
The study’s findings also highlight the importance of establishing and maintaining cancer registries, Dr. Calvo said, noting that 182 hospitals across Spain and more than 550 experts participate in the Thoracic Tumors Registry, which includes data on patients from every Spanish territory.
“It’s important to collect information on real-life cancer care so that we know what our real situation is and take steps to improve it,” she said.
She anticipates that treatment for NSCLC patients will become increasingly complex in the future with the growing number of different biomarkers and the proportion of patients who test positive for them. “We may need to establish national strategies to implement next generation sequencing so that we can identify different biomarkers and improve the survival of our patients.”
In a press release, Rolf Stahel, MD, president of the European Thoracic Oncology Platform, said that it would be helpful to look at how frequently molecular testing led to patients receiving appropriate targeted treatment.
In the United States, the National Comprehensive Cancer Network recommends biomarker testing for eligible patients with newly diagnosed stage 4 NSCLC, and it can be considered for patients with squamous histology because 5%-10% of these tumors have targetable mutations. “This is because numerous lines of evidence show that patients with stage 4 NSCLC and a targetable mutation, typically have improved overall survival when treated with a targeted therapy,” wrote the authors of the NCCN recommendations.
“For newly diagnosed stage 4 NSCLC, there is always a tension between the need to start therapy versus waiting for molecular results. This is because if a recommended targeted option is identified, it is the optimal first-line therapy. Targeted therapy cannot be given to everyone. Different biomarkers predict response to different agents. This has been well illustrated and it makes testing critically important for patients with NSCLC,” Dara Aisner, MD, PhD, associate professor of pathology with the University of Colorado at Denver, Aurora, wrote in the NCCN guideline.
The study presented at ELCC was funded by a grant from the European Union’s Horizon 2020 Research and Innovation Program. Dr. Calvo has received fees from Roche, Bristol-Myers Squibb, MSD and AstraZeneca.
FROM ELCC 2022
Childhood abuse may increase risk of MS in women
, according to the first prospective cohort study of its kind.
More research is needed to uncover underlying mechanisms of action, according to lead author Karine Eid, MD, a PhD candidate at Haukeland University Hospital, Bergen, Norway, and colleagues.
“Trauma and stressful life events have been associated with an increased risk of autoimmune disorders,” the investigators wrote in the Journal Of Neurology, Neurosurgery, & Psychiatry. “Whether adverse events in childhood can have an impact on MS susceptibility is not known.”
The present study recruited participants from the Norwegian Mother, Father and Child cohort, a population consisting of Norwegian women who were pregnant from 1999 to 2008. Of the 77,997 participating women, 14,477 reported emotional, sexual, and/or physical abuse in childhood, while the remaining 63,520 women reported no abuse. After a mean follow-up of 13 years, 300 women were diagnosed with MS, among whom 24% reported a history of childhood abuse, compared with 19% among women who did not develop MS.
To look for associations between childhood abuse and risk of MS, the investigators used a Cox model adjusted for confounders and mediators, including smoking, obesity, adult socioeconomic factors, and childhood social status. The model revealed that emotional abuse increased the risk of MS by 40% (hazard ratio [HR] 1.40; 95% confidence interval [CI], 1.03-1.90), and sexual abuse increased the risk of MS by 65% (HR 1.65; 95% CI, 1.13-2.39).
Although physical abuse alone did not significantly increase risk of MS (HR 1.31; 95% CI, 0.83-2.06), it did contribute to a dose-response relationship when women were exposed to more than one type of childhood abuse. Women exposed to two out of three abuse categories had a 66% increased risk of MS (HR 1.66; 95% CI, 1.04-2.67), whereas women exposed to all three types of abuse had the highest risk of MS, at 93% (HR 1.93; 95% CI, 1.02-3.67).
Dr. Eid and colleagues noted that their findings are supported by previous retrospective research, and discussed possible mechanisms of action.
“The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation,” they wrote. “Childhood abuse can cause dysregulation of the hypothalamic-pituitary-adrenal axis, lead to oxidative stress, and induce a proinflammatory state decades into adulthood. Psychological stress has been shown to disrupt the blood-brain barrier and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.
“The underlying mechanisms behind this association should be investigated further,” they concluded.
Study findings should guide interventions
Commenting on the research, Ruth Ann Marrie, MD, PhD, professor of medicine and community health sciences and director of the multiple sclerosis clinic at Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, said that the present study “has several strengths compared to prior studies – including that it is prospective and the sample size.”
Dr. Marrie, who was not involved in the study, advised clinicians in the field to take note of the findings, as patients with a history of abuse may need unique interventions.
“Providers need to recognize the higher prevalence of childhood maltreatment in people with MS,” Dr. Marrie said in an interview. “These findings dovetail with others that suggest that adverse childhood experiences are associated with increased mental health concerns and pain catastrophizing in people with MS. Affected individuals may benefit from additional psychological supports and trauma-informed care.”
Tiffany Joy Braley, MD, associate professor of neurology, and Carri Polick, RN and PhD candidate at the school of nursing, University of Michigan, Ann Arbor, who published a case report last year highlighting the importance of evaluating stress exposure in MS, suggested that the findings should guide interventions at both a system and patient level.
“Although a cause-and-effect relationship cannot be established by the current study, these and related findings should be considered in the context of system level and policy interventions that address links between environment and health care disparities,” they said in a joint, written comment. “Given recent impetus to provide trauma-informed health care, these data could be particularly informative in neurological conditions which are associated with high mental health comorbidity. Traumatic stress screening practices could lead to referrals for appropriate support services and more personalized health care.”
While several mechanisms have been proposed to explain the link between traumatic stress and MS, more work is needed in this area, they added.
This knowledge gap was acknowledged by Dr. Marrie.
“Our understanding of the etiology of MS remains incomplete,” Dr. Marrie said. “We still need a better understanding of mechanisms by which adverse childhood experiences lead to MS, how they interact with other risk factors for MS (beyond smoking and obesity), and whether there are any interventions that can mitigate the risk of developing MS that is associated with adverse childhood experiences.”
The investigators disclosed relationships with Novartis, Biogen, Merck, and others. Dr. Marrie receives research support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, MS Society of Canada, the Consortium of Multiple Sclerosis Centers, Crohn’s and Colitis Canada, Research Manitoba, and the Arthritis Society; she has no pharmaceutical support. Dr. Braley and Ms. Polick reported no conflicts of interest.
, according to the first prospective cohort study of its kind.
More research is needed to uncover underlying mechanisms of action, according to lead author Karine Eid, MD, a PhD candidate at Haukeland University Hospital, Bergen, Norway, and colleagues.
“Trauma and stressful life events have been associated with an increased risk of autoimmune disorders,” the investigators wrote in the Journal Of Neurology, Neurosurgery, & Psychiatry. “Whether adverse events in childhood can have an impact on MS susceptibility is not known.”
The present study recruited participants from the Norwegian Mother, Father and Child cohort, a population consisting of Norwegian women who were pregnant from 1999 to 2008. Of the 77,997 participating women, 14,477 reported emotional, sexual, and/or physical abuse in childhood, while the remaining 63,520 women reported no abuse. After a mean follow-up of 13 years, 300 women were diagnosed with MS, among whom 24% reported a history of childhood abuse, compared with 19% among women who did not develop MS.
To look for associations between childhood abuse and risk of MS, the investigators used a Cox model adjusted for confounders and mediators, including smoking, obesity, adult socioeconomic factors, and childhood social status. The model revealed that emotional abuse increased the risk of MS by 40% (hazard ratio [HR] 1.40; 95% confidence interval [CI], 1.03-1.90), and sexual abuse increased the risk of MS by 65% (HR 1.65; 95% CI, 1.13-2.39).
Although physical abuse alone did not significantly increase risk of MS (HR 1.31; 95% CI, 0.83-2.06), it did contribute to a dose-response relationship when women were exposed to more than one type of childhood abuse. Women exposed to two out of three abuse categories had a 66% increased risk of MS (HR 1.66; 95% CI, 1.04-2.67), whereas women exposed to all three types of abuse had the highest risk of MS, at 93% (HR 1.93; 95% CI, 1.02-3.67).
Dr. Eid and colleagues noted that their findings are supported by previous retrospective research, and discussed possible mechanisms of action.
“The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation,” they wrote. “Childhood abuse can cause dysregulation of the hypothalamic-pituitary-adrenal axis, lead to oxidative stress, and induce a proinflammatory state decades into adulthood. Psychological stress has been shown to disrupt the blood-brain barrier and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.
“The underlying mechanisms behind this association should be investigated further,” they concluded.
Study findings should guide interventions
Commenting on the research, Ruth Ann Marrie, MD, PhD, professor of medicine and community health sciences and director of the multiple sclerosis clinic at Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, said that the present study “has several strengths compared to prior studies – including that it is prospective and the sample size.”
Dr. Marrie, who was not involved in the study, advised clinicians in the field to take note of the findings, as patients with a history of abuse may need unique interventions.
“Providers need to recognize the higher prevalence of childhood maltreatment in people with MS,” Dr. Marrie said in an interview. “These findings dovetail with others that suggest that adverse childhood experiences are associated with increased mental health concerns and pain catastrophizing in people with MS. Affected individuals may benefit from additional psychological supports and trauma-informed care.”
Tiffany Joy Braley, MD, associate professor of neurology, and Carri Polick, RN and PhD candidate at the school of nursing, University of Michigan, Ann Arbor, who published a case report last year highlighting the importance of evaluating stress exposure in MS, suggested that the findings should guide interventions at both a system and patient level.
“Although a cause-and-effect relationship cannot be established by the current study, these and related findings should be considered in the context of system level and policy interventions that address links between environment and health care disparities,” they said in a joint, written comment. “Given recent impetus to provide trauma-informed health care, these data could be particularly informative in neurological conditions which are associated with high mental health comorbidity. Traumatic stress screening practices could lead to referrals for appropriate support services and more personalized health care.”
While several mechanisms have been proposed to explain the link between traumatic stress and MS, more work is needed in this area, they added.
This knowledge gap was acknowledged by Dr. Marrie.
“Our understanding of the etiology of MS remains incomplete,” Dr. Marrie said. “We still need a better understanding of mechanisms by which adverse childhood experiences lead to MS, how they interact with other risk factors for MS (beyond smoking and obesity), and whether there are any interventions that can mitigate the risk of developing MS that is associated with adverse childhood experiences.”
The investigators disclosed relationships with Novartis, Biogen, Merck, and others. Dr. Marrie receives research support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, MS Society of Canada, the Consortium of Multiple Sclerosis Centers, Crohn’s and Colitis Canada, Research Manitoba, and the Arthritis Society; she has no pharmaceutical support. Dr. Braley and Ms. Polick reported no conflicts of interest.
, according to the first prospective cohort study of its kind.
More research is needed to uncover underlying mechanisms of action, according to lead author Karine Eid, MD, a PhD candidate at Haukeland University Hospital, Bergen, Norway, and colleagues.
“Trauma and stressful life events have been associated with an increased risk of autoimmune disorders,” the investigators wrote in the Journal Of Neurology, Neurosurgery, & Psychiatry. “Whether adverse events in childhood can have an impact on MS susceptibility is not known.”
The present study recruited participants from the Norwegian Mother, Father and Child cohort, a population consisting of Norwegian women who were pregnant from 1999 to 2008. Of the 77,997 participating women, 14,477 reported emotional, sexual, and/or physical abuse in childhood, while the remaining 63,520 women reported no abuse. After a mean follow-up of 13 years, 300 women were diagnosed with MS, among whom 24% reported a history of childhood abuse, compared with 19% among women who did not develop MS.
To look for associations between childhood abuse and risk of MS, the investigators used a Cox model adjusted for confounders and mediators, including smoking, obesity, adult socioeconomic factors, and childhood social status. The model revealed that emotional abuse increased the risk of MS by 40% (hazard ratio [HR] 1.40; 95% confidence interval [CI], 1.03-1.90), and sexual abuse increased the risk of MS by 65% (HR 1.65; 95% CI, 1.13-2.39).
Although physical abuse alone did not significantly increase risk of MS (HR 1.31; 95% CI, 0.83-2.06), it did contribute to a dose-response relationship when women were exposed to more than one type of childhood abuse. Women exposed to two out of three abuse categories had a 66% increased risk of MS (HR 1.66; 95% CI, 1.04-2.67), whereas women exposed to all three types of abuse had the highest risk of MS, at 93% (HR 1.93; 95% CI, 1.02-3.67).
Dr. Eid and colleagues noted that their findings are supported by previous retrospective research, and discussed possible mechanisms of action.
“The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation,” they wrote. “Childhood abuse can cause dysregulation of the hypothalamic-pituitary-adrenal axis, lead to oxidative stress, and induce a proinflammatory state decades into adulthood. Psychological stress has been shown to disrupt the blood-brain barrier and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.
“The underlying mechanisms behind this association should be investigated further,” they concluded.
Study findings should guide interventions
Commenting on the research, Ruth Ann Marrie, MD, PhD, professor of medicine and community health sciences and director of the multiple sclerosis clinic at Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, said that the present study “has several strengths compared to prior studies – including that it is prospective and the sample size.”
Dr. Marrie, who was not involved in the study, advised clinicians in the field to take note of the findings, as patients with a history of abuse may need unique interventions.
“Providers need to recognize the higher prevalence of childhood maltreatment in people with MS,” Dr. Marrie said in an interview. “These findings dovetail with others that suggest that adverse childhood experiences are associated with increased mental health concerns and pain catastrophizing in people with MS. Affected individuals may benefit from additional psychological supports and trauma-informed care.”
Tiffany Joy Braley, MD, associate professor of neurology, and Carri Polick, RN and PhD candidate at the school of nursing, University of Michigan, Ann Arbor, who published a case report last year highlighting the importance of evaluating stress exposure in MS, suggested that the findings should guide interventions at both a system and patient level.
“Although a cause-and-effect relationship cannot be established by the current study, these and related findings should be considered in the context of system level and policy interventions that address links between environment and health care disparities,” they said in a joint, written comment. “Given recent impetus to provide trauma-informed health care, these data could be particularly informative in neurological conditions which are associated with high mental health comorbidity. Traumatic stress screening practices could lead to referrals for appropriate support services and more personalized health care.”
While several mechanisms have been proposed to explain the link between traumatic stress and MS, more work is needed in this area, they added.
This knowledge gap was acknowledged by Dr. Marrie.
“Our understanding of the etiology of MS remains incomplete,” Dr. Marrie said. “We still need a better understanding of mechanisms by which adverse childhood experiences lead to MS, how they interact with other risk factors for MS (beyond smoking and obesity), and whether there are any interventions that can mitigate the risk of developing MS that is associated with adverse childhood experiences.”
The investigators disclosed relationships with Novartis, Biogen, Merck, and others. Dr. Marrie receives research support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, MS Society of Canada, the Consortium of Multiple Sclerosis Centers, Crohn’s and Colitis Canada, Research Manitoba, and the Arthritis Society; she has no pharmaceutical support. Dr. Braley and Ms. Polick reported no conflicts of interest.
FROM THE JOURNAL OF NEUROLOGY, NEUROSURGERY, & PSYCHIATRY
Cancer diet studies: Veggies get another rave, while red meat’s busted again
Researchers report that high consumption of vegetables – especially lettuce, legumes, and cruciferous varieties – appears to lower the risk of liver cancer/liver disease. A separate team suggests that high consumption of red meat, organ meats, and processed meats boosts the risk of gastric cancer.
The findings of the latter study “reinforce the idea that avoidance of red meat and processed meat is probably good beyond [the prevention of] colorectal cancer,” said corresponding author and epidemiologist Paolo Boffetta, MD, MPH, of Stony Brook University Cancer Center, New York, in an interview. “The possible carcinogenic effect may extend beyond the colon.”
Both studies were released at the annual meeting of the American Association for Cancer Research.
For the red meat study, researchers examined statistics from the Golestan cohort study, which is prospectively tracking 50,045 people aged 40-75 from northeastern Iran. The study focuses on esophageal cancer due to the region’s high rate of the disease.
Red meat consumption is fairly rare in the region, where residents typically prefer chicken, said study lead author Giulia Collatuzzo, MD, a resident physician in occupational medicine at the University of Bologna, Italy, in an interview. On average, participants reported eating 18.4 grams daily of red meat and 72.1 grams daily of white meat.
The researchers tracked study participants for a median 12-year follow-up, during which 369 developed esophageal cancer and 368 developed gastric cancer. Red meat was only linked to more esophageal cancer in women (hazard ratio, 1.13, 95% confidence interval, 1.00-1.18, for each quintile increase in consumption).
Overall red meat consumption (including red meat, organ meat, and processed meat) was linked to higher rates of gastric cancer (HR, 1.08, 95% CI, 1.00-1.17) for each quartile increase in consumption, as was consumption of the red meat subtype alone (HR, 1.09, 95% CI, 1.00-1.18).
According to Dr. Collatuzzo, the findings suggest that those in the highest quartile of overall red meat consumption may have around a 25% increase in risk, compared with the lowest quartile.
Overall, she said, the study findings aren’t surprising. The lack of a connection between red meat consumption and esophageal cancer may be due to the fact that meat only temporarily transits through the esophagus, she said.
For the liver cancer/liver disease study, researchers examined the medical records of 470,653 subjects in the NIH-AARP Diet and Health Study. They were recruited in 1995-1996 when they were 50-71 years old. Over a median follow-up of 15.5 years, 899 developed liver cancer, and 934 died of chronic liver disease.
The median intakes of vegetables in quintile 5 (highest) and quintile 1 (lowest) were 3.7 cups daily and 1.0 cups daily, respectively, said study lead author Long-Gang Zhao, MS, a graduate student at Harvard University.
After adjusting for possible cofounders, those in the highest quintile of vegetable consumption were a third less likely to develop liver cancer, compared with the lowest quintile (HR, 0.66, 95% CI, 0.53-0.82, P < 0.01). Several types of vegetables appeared to be the strongest cancer fighters: cruciferous (broccoli, cauliflower), lettuce, legumes, and carrots. These kinds of vegetables were also linked to lower rates of chronic liver disease mortality (all P < 0.01), as was total vegetable intake for the top quintile versus the lowest quintile (HR, 0.60, 95% CI, 0.49-0.74, P = < 0.01).
“A one-cup increase (8 oz or 225 g) in vegetable intake was associated with about 20% decreased risk of liver cancer incidence and chronic liver mortality,” Zhao said.
There was no statistically significant link between fruit consumption and liver cancer or chronic liver disease mortality.
The findings provide more insight into diet and liver disease, Zhao said. “Chronic liver disease, which predisposes to liver cancer, is the tenth cause of death worldwide, causing two million deaths each year. It shares some etiological processes with liver cancer. Therefore, examining both chronic liver disease mortality and liver cancer incidence in our study may provide a more general picture for the prevention of liver diseases.”
As for limitations, both studies are based on self-reports about food consumption, which can be unreliable, and the subjects in the fruit/vegetable analysis were mainly of European origin.
The authors of both studies report no relevant disclosures. No funding is reported for either study.
Researchers report that high consumption of vegetables – especially lettuce, legumes, and cruciferous varieties – appears to lower the risk of liver cancer/liver disease. A separate team suggests that high consumption of red meat, organ meats, and processed meats boosts the risk of gastric cancer.
The findings of the latter study “reinforce the idea that avoidance of red meat and processed meat is probably good beyond [the prevention of] colorectal cancer,” said corresponding author and epidemiologist Paolo Boffetta, MD, MPH, of Stony Brook University Cancer Center, New York, in an interview. “The possible carcinogenic effect may extend beyond the colon.”
Both studies were released at the annual meeting of the American Association for Cancer Research.
For the red meat study, researchers examined statistics from the Golestan cohort study, which is prospectively tracking 50,045 people aged 40-75 from northeastern Iran. The study focuses on esophageal cancer due to the region’s high rate of the disease.
Red meat consumption is fairly rare in the region, where residents typically prefer chicken, said study lead author Giulia Collatuzzo, MD, a resident physician in occupational medicine at the University of Bologna, Italy, in an interview. On average, participants reported eating 18.4 grams daily of red meat and 72.1 grams daily of white meat.
The researchers tracked study participants for a median 12-year follow-up, during which 369 developed esophageal cancer and 368 developed gastric cancer. Red meat was only linked to more esophageal cancer in women (hazard ratio, 1.13, 95% confidence interval, 1.00-1.18, for each quintile increase in consumption).
Overall red meat consumption (including red meat, organ meat, and processed meat) was linked to higher rates of gastric cancer (HR, 1.08, 95% CI, 1.00-1.17) for each quartile increase in consumption, as was consumption of the red meat subtype alone (HR, 1.09, 95% CI, 1.00-1.18).
According to Dr. Collatuzzo, the findings suggest that those in the highest quartile of overall red meat consumption may have around a 25% increase in risk, compared with the lowest quartile.
Overall, she said, the study findings aren’t surprising. The lack of a connection between red meat consumption and esophageal cancer may be due to the fact that meat only temporarily transits through the esophagus, she said.
For the liver cancer/liver disease study, researchers examined the medical records of 470,653 subjects in the NIH-AARP Diet and Health Study. They were recruited in 1995-1996 when they were 50-71 years old. Over a median follow-up of 15.5 years, 899 developed liver cancer, and 934 died of chronic liver disease.
The median intakes of vegetables in quintile 5 (highest) and quintile 1 (lowest) were 3.7 cups daily and 1.0 cups daily, respectively, said study lead author Long-Gang Zhao, MS, a graduate student at Harvard University.
After adjusting for possible cofounders, those in the highest quintile of vegetable consumption were a third less likely to develop liver cancer, compared with the lowest quintile (HR, 0.66, 95% CI, 0.53-0.82, P < 0.01). Several types of vegetables appeared to be the strongest cancer fighters: cruciferous (broccoli, cauliflower), lettuce, legumes, and carrots. These kinds of vegetables were also linked to lower rates of chronic liver disease mortality (all P < 0.01), as was total vegetable intake for the top quintile versus the lowest quintile (HR, 0.60, 95% CI, 0.49-0.74, P = < 0.01).
“A one-cup increase (8 oz or 225 g) in vegetable intake was associated with about 20% decreased risk of liver cancer incidence and chronic liver mortality,” Zhao said.
There was no statistically significant link between fruit consumption and liver cancer or chronic liver disease mortality.
The findings provide more insight into diet and liver disease, Zhao said. “Chronic liver disease, which predisposes to liver cancer, is the tenth cause of death worldwide, causing two million deaths each year. It shares some etiological processes with liver cancer. Therefore, examining both chronic liver disease mortality and liver cancer incidence in our study may provide a more general picture for the prevention of liver diseases.”
As for limitations, both studies are based on self-reports about food consumption, which can be unreliable, and the subjects in the fruit/vegetable analysis were mainly of European origin.
The authors of both studies report no relevant disclosures. No funding is reported for either study.
Researchers report that high consumption of vegetables – especially lettuce, legumes, and cruciferous varieties – appears to lower the risk of liver cancer/liver disease. A separate team suggests that high consumption of red meat, organ meats, and processed meats boosts the risk of gastric cancer.
The findings of the latter study “reinforce the idea that avoidance of red meat and processed meat is probably good beyond [the prevention of] colorectal cancer,” said corresponding author and epidemiologist Paolo Boffetta, MD, MPH, of Stony Brook University Cancer Center, New York, in an interview. “The possible carcinogenic effect may extend beyond the colon.”
Both studies were released at the annual meeting of the American Association for Cancer Research.
For the red meat study, researchers examined statistics from the Golestan cohort study, which is prospectively tracking 50,045 people aged 40-75 from northeastern Iran. The study focuses on esophageal cancer due to the region’s high rate of the disease.
Red meat consumption is fairly rare in the region, where residents typically prefer chicken, said study lead author Giulia Collatuzzo, MD, a resident physician in occupational medicine at the University of Bologna, Italy, in an interview. On average, participants reported eating 18.4 grams daily of red meat and 72.1 grams daily of white meat.
The researchers tracked study participants for a median 12-year follow-up, during which 369 developed esophageal cancer and 368 developed gastric cancer. Red meat was only linked to more esophageal cancer in women (hazard ratio, 1.13, 95% confidence interval, 1.00-1.18, for each quintile increase in consumption).
Overall red meat consumption (including red meat, organ meat, and processed meat) was linked to higher rates of gastric cancer (HR, 1.08, 95% CI, 1.00-1.17) for each quartile increase in consumption, as was consumption of the red meat subtype alone (HR, 1.09, 95% CI, 1.00-1.18).
According to Dr. Collatuzzo, the findings suggest that those in the highest quartile of overall red meat consumption may have around a 25% increase in risk, compared with the lowest quartile.
Overall, she said, the study findings aren’t surprising. The lack of a connection between red meat consumption and esophageal cancer may be due to the fact that meat only temporarily transits through the esophagus, she said.
For the liver cancer/liver disease study, researchers examined the medical records of 470,653 subjects in the NIH-AARP Diet and Health Study. They were recruited in 1995-1996 when they were 50-71 years old. Over a median follow-up of 15.5 years, 899 developed liver cancer, and 934 died of chronic liver disease.
The median intakes of vegetables in quintile 5 (highest) and quintile 1 (lowest) were 3.7 cups daily and 1.0 cups daily, respectively, said study lead author Long-Gang Zhao, MS, a graduate student at Harvard University.
After adjusting for possible cofounders, those in the highest quintile of vegetable consumption were a third less likely to develop liver cancer, compared with the lowest quintile (HR, 0.66, 95% CI, 0.53-0.82, P < 0.01). Several types of vegetables appeared to be the strongest cancer fighters: cruciferous (broccoli, cauliflower), lettuce, legumes, and carrots. These kinds of vegetables were also linked to lower rates of chronic liver disease mortality (all P < 0.01), as was total vegetable intake for the top quintile versus the lowest quintile (HR, 0.60, 95% CI, 0.49-0.74, P = < 0.01).
“A one-cup increase (8 oz or 225 g) in vegetable intake was associated with about 20% decreased risk of liver cancer incidence and chronic liver mortality,” Zhao said.
There was no statistically significant link between fruit consumption and liver cancer or chronic liver disease mortality.
The findings provide more insight into diet and liver disease, Zhao said. “Chronic liver disease, which predisposes to liver cancer, is the tenth cause of death worldwide, causing two million deaths each year. It shares some etiological processes with liver cancer. Therefore, examining both chronic liver disease mortality and liver cancer incidence in our study may provide a more general picture for the prevention of liver diseases.”
As for limitations, both studies are based on self-reports about food consumption, which can be unreliable, and the subjects in the fruit/vegetable analysis were mainly of European origin.
The authors of both studies report no relevant disclosures. No funding is reported for either study.
FROM AACR 2022
New York NPs join half of states with full practice authority
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.

