User login
Joint effort: CBD not just innocent bystander in weed
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.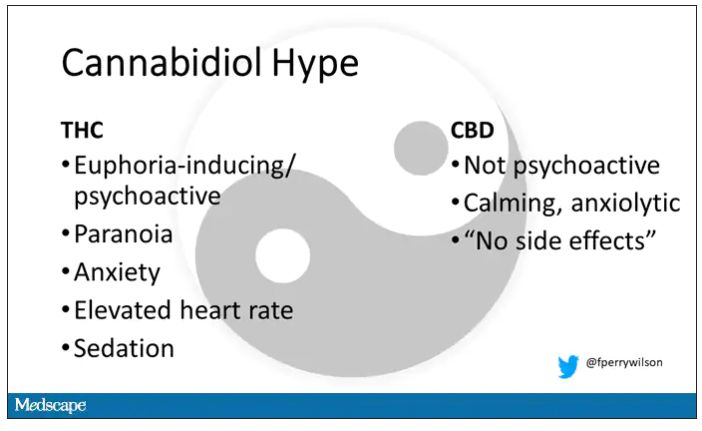
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.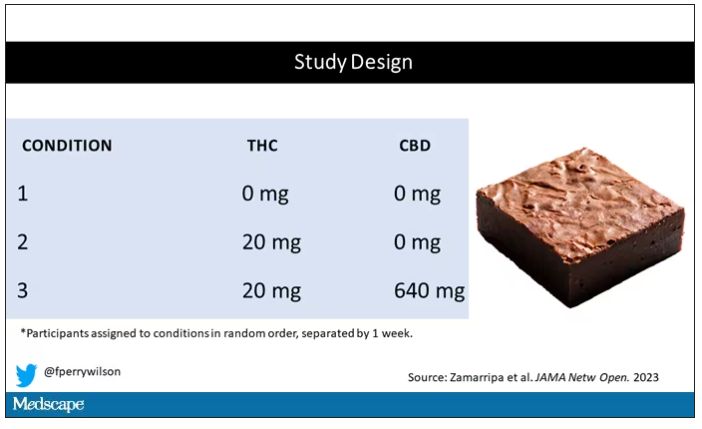
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.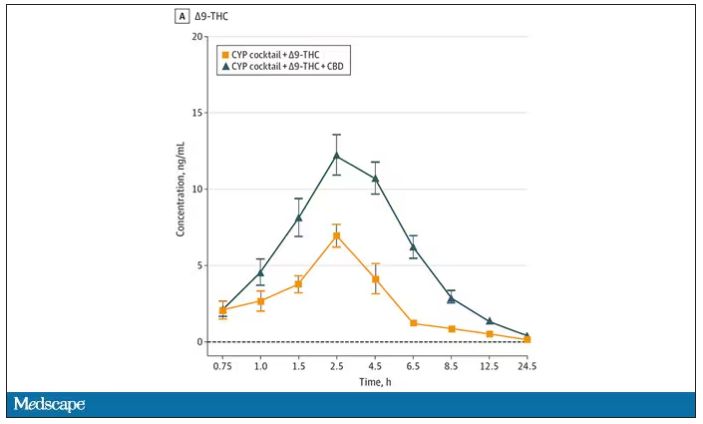
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.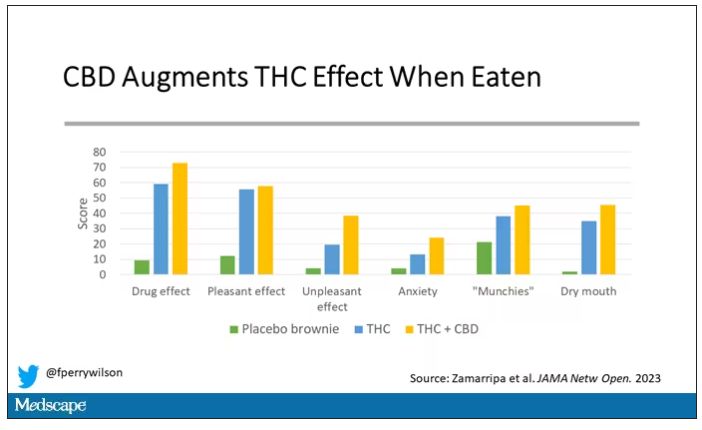
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.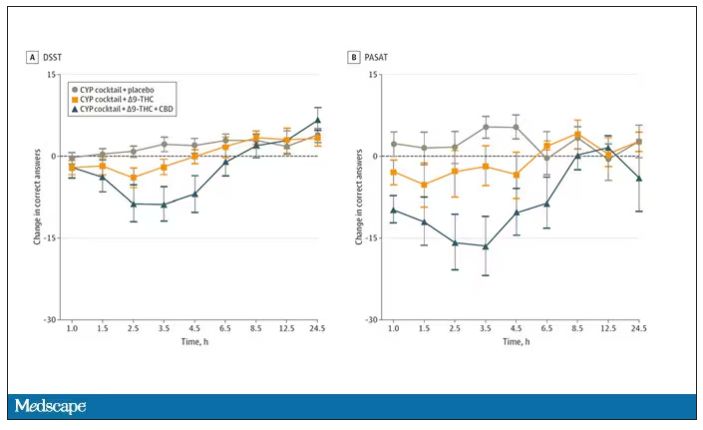
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.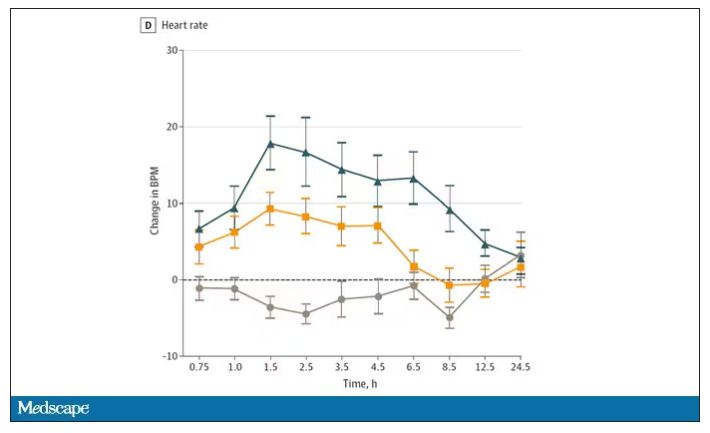
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
UnitedHealthcare tried to deny coverage to a chronically ill patient. He fought back, exposing the insurer’s inner workings.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
STROKE AF at 3 years: High AFib rate after atherosclerotic stroke
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
FROM ISC 2023
Must-read acute care medicine articles from 2022
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Muscle-Related Adverse Events Associated With PCSK9 Inhibitors in a Veteran Population
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
Evaluation of the Appropriateness of Aspirin Therapy in a Veteran Population
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
A doctor intervenes in a fiery car crash
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
USPSTF backs screening for hypertensive disorders of pregnancy
The U.S. Preventive Services Task Force (USPSTF) recommends that clinicians screen for hypertensive disorders of pregnancy, which can cause serious and fatal complications, according to a new draft statement.
All pregnant people should have their blood pressure measured at each prenatal visit to identify and prevent serious health problems. The grade B recommendation expands on the task force’s 2017 recommendation on screening for preeclampsia to include all hypertensive disorders of pregnancy.
“Hypertensive disorders of pregnancy are some of the leading causes of serious complications and death for pregnant people,” Esa Davis, MD, a USPSTF member and associate professor of medicine and clinical and translational science at the University of Pittsburgh School of Medicine, told this news organization.
In the U.S., the rate of hypertensive disorders of pregnancy has increased in recent decades, jumping from about 500 cases per 10,000 deliveries in the early 1990s to more than 1,000 cases per 10,000 deliveries in the mid-2010s.
“The U.S. Preventive Services Task Force wants to help save the lives of pregnant people and their babies by ensuring that clinicians have the most up-to-date guidance on how to find these conditions early,” she said.
The draft recommendation statement was published online .
Screening recommendation
Hypertensive disorders of pregnancy, including gestational hypertension, preeclampsia, eclampsia, and chronic hypertension with and without superimposed preeclampsia, are marked by elevated blood pressure during pregnancy.
The disorders can lead to complications for the pregnant person, such as stroke, retinal detachment, organ damage or failure, and seizures, as well as for the baby, including restricted growth, low birth weight, and stillbirth. Many complications can lead to early induction of labor, cesarean delivery, and preterm birth.
After commissioning a systematic evidence review, the USPSTF provided a grade B recommendation for clinicians to offer or provide screening for hypertensive disorders of pregnancy. The recommendation concludes with “moderate certainty” that screening with blood pressure measurements has “substantial net benefit.”
The task force notes that it is “essential” for all pregnant women and pregnant people of all genders to be screened and that those who screen positive receive evidence-based management of their condition.
Risk factors include a history of eclampsia or preeclampsia, a family history of preeclampsia, a previous adverse pregnancy outcome, having gestational diabetes or chronic hypertension, being pregnant with more than one baby, having a first pregnancy, having a high body mass index prior to pregnancy, and being 35 years of age or older.
In addition, Black, American Indian, and Alaska Native people face higher risks and are more likely both to have and to die from a hypertensive disorder of pregnancy. In particular, Black people experience higher rates of maternal and infant morbidity and perinatal mortality than other racial and ethnic groups, and hypertensive disorders of pregnancy account for a larger proportion of these outcomes.
Although measuring blood pressure throughout pregnancy is an important first step, it’s not enough to improve inequities in health outcomes, the task force notes. Identifying hypertensive disorders of pregnancy requires adequate prenatal follow-up visits, surveillance, and evidence-based care, which can be a barrier for some pregnant people.
Follow-up visits with health care providers such as nurses, nurse midwives, pediatricians, and lactation consultants could help, as well as screening and monitoring during the postpartum period. Other approaches include telehealth, connections to community resources during the perinatal period, collaborative care provided in medical homes, and multilevel interventions to address underlying health inequities that increase health risks during pregnancy.
“Since screening is not enough to address the health disparities experienced by Black, American Indian, and Alaska Native people, health care professionals should also do what they can to help address these inequities,” Dr. Davis said. “For example, the task force identified a few promising approaches, including using standardized clinical bundles of best practices for disease management to help ensure that all pregnant persons receive appropriate, equitable care.”
Additional considerations
The USPSTF looked at the evidence on additional methods of screening but continued to find that measuring blood pressure at each prenatal visit is the best approach. Other evaluations, such as testing for proteinuria when preeclampsia is suspected, have low accuracy for detecting proteinuria in pregnancy.
Although there is no currently available treatment for preeclampsia except delivery, management strategies for diagnosed hypertensive disorders of pregnancy include close fetal and maternal monitoring, antihypertension medications, and magnesium sulfate for seizure prophylaxis when indicated.
Previously, the USPSTF also recommended that pregnant Black people be considered for treatment with low-dose aspirin to prevent preeclampsia, with aspirin use recommended for those with at least one additional moderate risk factor. Clinicians should also be aware of the complications of poor health outcomes among populations who face higher risks.
The USPSTF noted several gaps for future research, including the best approaches for blood pressure monitoring during pregnancy and the postpartum period, how to address health inequities through multilevel interventions, how to increase access to care through telehealth services, and how to mitigate cardiovascular complications later in life in patients diagnosed with hypertensive disorders of pregnancy.
“Continued research is needed in these promising areas,” Dr. Davis said. “We hope all clinicians will join us in helping ensure that all parents and babies have access to the care they need to be as healthy as possible.”
The draft recommendation statement and draft evidence review were posted for public comment on the USPSTF website. Comments can be submitted until March 6.
No relevant financial relationships have been disclosed.
A version of this article originally appeared on Medscape.com.
The U.S. Preventive Services Task Force (USPSTF) recommends that clinicians screen for hypertensive disorders of pregnancy, which can cause serious and fatal complications, according to a new draft statement.
All pregnant people should have their blood pressure measured at each prenatal visit to identify and prevent serious health problems. The grade B recommendation expands on the task force’s 2017 recommendation on screening for preeclampsia to include all hypertensive disorders of pregnancy.
“Hypertensive disorders of pregnancy are some of the leading causes of serious complications and death for pregnant people,” Esa Davis, MD, a USPSTF member and associate professor of medicine and clinical and translational science at the University of Pittsburgh School of Medicine, told this news organization.
In the U.S., the rate of hypertensive disorders of pregnancy has increased in recent decades, jumping from about 500 cases per 10,000 deliveries in the early 1990s to more than 1,000 cases per 10,000 deliveries in the mid-2010s.
“The U.S. Preventive Services Task Force wants to help save the lives of pregnant people and their babies by ensuring that clinicians have the most up-to-date guidance on how to find these conditions early,” she said.
The draft recommendation statement was published online .
Screening recommendation
Hypertensive disorders of pregnancy, including gestational hypertension, preeclampsia, eclampsia, and chronic hypertension with and without superimposed preeclampsia, are marked by elevated blood pressure during pregnancy.
The disorders can lead to complications for the pregnant person, such as stroke, retinal detachment, organ damage or failure, and seizures, as well as for the baby, including restricted growth, low birth weight, and stillbirth. Many complications can lead to early induction of labor, cesarean delivery, and preterm birth.
After commissioning a systematic evidence review, the USPSTF provided a grade B recommendation for clinicians to offer or provide screening for hypertensive disorders of pregnancy. The recommendation concludes with “moderate certainty” that screening with blood pressure measurements has “substantial net benefit.”
The task force notes that it is “essential” for all pregnant women and pregnant people of all genders to be screened and that those who screen positive receive evidence-based management of their condition.
Risk factors include a history of eclampsia or preeclampsia, a family history of preeclampsia, a previous adverse pregnancy outcome, having gestational diabetes or chronic hypertension, being pregnant with more than one baby, having a first pregnancy, having a high body mass index prior to pregnancy, and being 35 years of age or older.
In addition, Black, American Indian, and Alaska Native people face higher risks and are more likely both to have and to die from a hypertensive disorder of pregnancy. In particular, Black people experience higher rates of maternal and infant morbidity and perinatal mortality than other racial and ethnic groups, and hypertensive disorders of pregnancy account for a larger proportion of these outcomes.
Although measuring blood pressure throughout pregnancy is an important first step, it’s not enough to improve inequities in health outcomes, the task force notes. Identifying hypertensive disorders of pregnancy requires adequate prenatal follow-up visits, surveillance, and evidence-based care, which can be a barrier for some pregnant people.
Follow-up visits with health care providers such as nurses, nurse midwives, pediatricians, and lactation consultants could help, as well as screening and monitoring during the postpartum period. Other approaches include telehealth, connections to community resources during the perinatal period, collaborative care provided in medical homes, and multilevel interventions to address underlying health inequities that increase health risks during pregnancy.
“Since screening is not enough to address the health disparities experienced by Black, American Indian, and Alaska Native people, health care professionals should also do what they can to help address these inequities,” Dr. Davis said. “For example, the task force identified a few promising approaches, including using standardized clinical bundles of best practices for disease management to help ensure that all pregnant persons receive appropriate, equitable care.”
Additional considerations
The USPSTF looked at the evidence on additional methods of screening but continued to find that measuring blood pressure at each prenatal visit is the best approach. Other evaluations, such as testing for proteinuria when preeclampsia is suspected, have low accuracy for detecting proteinuria in pregnancy.
Although there is no currently available treatment for preeclampsia except delivery, management strategies for diagnosed hypertensive disorders of pregnancy include close fetal and maternal monitoring, antihypertension medications, and magnesium sulfate for seizure prophylaxis when indicated.
Previously, the USPSTF also recommended that pregnant Black people be considered for treatment with low-dose aspirin to prevent preeclampsia, with aspirin use recommended for those with at least one additional moderate risk factor. Clinicians should also be aware of the complications of poor health outcomes among populations who face higher risks.
The USPSTF noted several gaps for future research, including the best approaches for blood pressure monitoring during pregnancy and the postpartum period, how to address health inequities through multilevel interventions, how to increase access to care through telehealth services, and how to mitigate cardiovascular complications later in life in patients diagnosed with hypertensive disorders of pregnancy.
“Continued research is needed in these promising areas,” Dr. Davis said. “We hope all clinicians will join us in helping ensure that all parents and babies have access to the care they need to be as healthy as possible.”
The draft recommendation statement and draft evidence review were posted for public comment on the USPSTF website. Comments can be submitted until March 6.
No relevant financial relationships have been disclosed.
A version of this article originally appeared on Medscape.com.
The U.S. Preventive Services Task Force (USPSTF) recommends that clinicians screen for hypertensive disorders of pregnancy, which can cause serious and fatal complications, according to a new draft statement.
All pregnant people should have their blood pressure measured at each prenatal visit to identify and prevent serious health problems. The grade B recommendation expands on the task force’s 2017 recommendation on screening for preeclampsia to include all hypertensive disorders of pregnancy.
“Hypertensive disorders of pregnancy are some of the leading causes of serious complications and death for pregnant people,” Esa Davis, MD, a USPSTF member and associate professor of medicine and clinical and translational science at the University of Pittsburgh School of Medicine, told this news organization.
In the U.S., the rate of hypertensive disorders of pregnancy has increased in recent decades, jumping from about 500 cases per 10,000 deliveries in the early 1990s to more than 1,000 cases per 10,000 deliveries in the mid-2010s.
“The U.S. Preventive Services Task Force wants to help save the lives of pregnant people and their babies by ensuring that clinicians have the most up-to-date guidance on how to find these conditions early,” she said.
The draft recommendation statement was published online .
Screening recommendation
Hypertensive disorders of pregnancy, including gestational hypertension, preeclampsia, eclampsia, and chronic hypertension with and without superimposed preeclampsia, are marked by elevated blood pressure during pregnancy.
The disorders can lead to complications for the pregnant person, such as stroke, retinal detachment, organ damage or failure, and seizures, as well as for the baby, including restricted growth, low birth weight, and stillbirth. Many complications can lead to early induction of labor, cesarean delivery, and preterm birth.
After commissioning a systematic evidence review, the USPSTF provided a grade B recommendation for clinicians to offer or provide screening for hypertensive disorders of pregnancy. The recommendation concludes with “moderate certainty” that screening with blood pressure measurements has “substantial net benefit.”
The task force notes that it is “essential” for all pregnant women and pregnant people of all genders to be screened and that those who screen positive receive evidence-based management of their condition.
Risk factors include a history of eclampsia or preeclampsia, a family history of preeclampsia, a previous adverse pregnancy outcome, having gestational diabetes or chronic hypertension, being pregnant with more than one baby, having a first pregnancy, having a high body mass index prior to pregnancy, and being 35 years of age or older.
In addition, Black, American Indian, and Alaska Native people face higher risks and are more likely both to have and to die from a hypertensive disorder of pregnancy. In particular, Black people experience higher rates of maternal and infant morbidity and perinatal mortality than other racial and ethnic groups, and hypertensive disorders of pregnancy account for a larger proportion of these outcomes.
Although measuring blood pressure throughout pregnancy is an important first step, it’s not enough to improve inequities in health outcomes, the task force notes. Identifying hypertensive disorders of pregnancy requires adequate prenatal follow-up visits, surveillance, and evidence-based care, which can be a barrier for some pregnant people.
Follow-up visits with health care providers such as nurses, nurse midwives, pediatricians, and lactation consultants could help, as well as screening and monitoring during the postpartum period. Other approaches include telehealth, connections to community resources during the perinatal period, collaborative care provided in medical homes, and multilevel interventions to address underlying health inequities that increase health risks during pregnancy.
“Since screening is not enough to address the health disparities experienced by Black, American Indian, and Alaska Native people, health care professionals should also do what they can to help address these inequities,” Dr. Davis said. “For example, the task force identified a few promising approaches, including using standardized clinical bundles of best practices for disease management to help ensure that all pregnant persons receive appropriate, equitable care.”
Additional considerations
The USPSTF looked at the evidence on additional methods of screening but continued to find that measuring blood pressure at each prenatal visit is the best approach. Other evaluations, such as testing for proteinuria when preeclampsia is suspected, have low accuracy for detecting proteinuria in pregnancy.
Although there is no currently available treatment for preeclampsia except delivery, management strategies for diagnosed hypertensive disorders of pregnancy include close fetal and maternal monitoring, antihypertension medications, and magnesium sulfate for seizure prophylaxis when indicated.
Previously, the USPSTF also recommended that pregnant Black people be considered for treatment with low-dose aspirin to prevent preeclampsia, with aspirin use recommended for those with at least one additional moderate risk factor. Clinicians should also be aware of the complications of poor health outcomes among populations who face higher risks.
The USPSTF noted several gaps for future research, including the best approaches for blood pressure monitoring during pregnancy and the postpartum period, how to address health inequities through multilevel interventions, how to increase access to care through telehealth services, and how to mitigate cardiovascular complications later in life in patients diagnosed with hypertensive disorders of pregnancy.
“Continued research is needed in these promising areas,” Dr. Davis said. “We hope all clinicians will join us in helping ensure that all parents and babies have access to the care they need to be as healthy as possible.”
The draft recommendation statement and draft evidence review were posted for public comment on the USPSTF website. Comments can be submitted until March 6.
No relevant financial relationships have been disclosed.
A version of this article originally appeared on Medscape.com.
Cardiac monitoring company settles DOJ false claims allegations
Beyond Reps (dba IronRod Health and Cardiac Monitoring Services) has agreed to pay $673,200 to resolve allegations that it submitted false claims to federal health care programs relating to remote cardiac monitoring services.
The U.S. Department of Justice alleges that between Jan. 1, 2018, and April 30, 2021, IronRod, with headquarters in Phoenix, used technicians who lacked required credentials to conduct remote cardiac monitoring readings.
The government further alleges that between June 1, 2018, and Aug. 20, 2018, the company misrepresented that it performed services in New York state in order to get higher reimbursements from Medicare for remote cardiac monitoring services.
“Providers that seek payment from federal health programs are required to follow laws meant to protect beneficiaries, as well as to protect the integrity of those programs,” U.S. Attorney Trini E. Ross said in a statement.
“Our office is committed to pursuing cases against any provider that cuts corners or seeks to obtain payments for which they are not entitled,” Ms. Ross said.
A request to Beyond Reps for comment was not returned.
The civil settlement resolves claims brought under the qui tam (whistleblower) provisions of the False Claims Act by Coleen DeGroat.
Under those provisions, a private party can file an action on behalf of the United States and receive a portion of any recovery. Ms. DeGroat will receive a share of the settlement.
A version of this article first appeared on Medscape.com.
Beyond Reps (dba IronRod Health and Cardiac Monitoring Services) has agreed to pay $673,200 to resolve allegations that it submitted false claims to federal health care programs relating to remote cardiac monitoring services.
The U.S. Department of Justice alleges that between Jan. 1, 2018, and April 30, 2021, IronRod, with headquarters in Phoenix, used technicians who lacked required credentials to conduct remote cardiac monitoring readings.
The government further alleges that between June 1, 2018, and Aug. 20, 2018, the company misrepresented that it performed services in New York state in order to get higher reimbursements from Medicare for remote cardiac monitoring services.
“Providers that seek payment from federal health programs are required to follow laws meant to protect beneficiaries, as well as to protect the integrity of those programs,” U.S. Attorney Trini E. Ross said in a statement.
“Our office is committed to pursuing cases against any provider that cuts corners or seeks to obtain payments for which they are not entitled,” Ms. Ross said.
A request to Beyond Reps for comment was not returned.
The civil settlement resolves claims brought under the qui tam (whistleblower) provisions of the False Claims Act by Coleen DeGroat.
Under those provisions, a private party can file an action on behalf of the United States and receive a portion of any recovery. Ms. DeGroat will receive a share of the settlement.
A version of this article first appeared on Medscape.com.
Beyond Reps (dba IronRod Health and Cardiac Monitoring Services) has agreed to pay $673,200 to resolve allegations that it submitted false claims to federal health care programs relating to remote cardiac monitoring services.
The U.S. Department of Justice alleges that between Jan. 1, 2018, and April 30, 2021, IronRod, with headquarters in Phoenix, used technicians who lacked required credentials to conduct remote cardiac monitoring readings.
The government further alleges that between June 1, 2018, and Aug. 20, 2018, the company misrepresented that it performed services in New York state in order to get higher reimbursements from Medicare for remote cardiac monitoring services.
“Providers that seek payment from federal health programs are required to follow laws meant to protect beneficiaries, as well as to protect the integrity of those programs,” U.S. Attorney Trini E. Ross said in a statement.
“Our office is committed to pursuing cases against any provider that cuts corners or seeks to obtain payments for which they are not entitled,” Ms. Ross said.
A request to Beyond Reps for comment was not returned.
The civil settlement resolves claims brought under the qui tam (whistleblower) provisions of the False Claims Act by Coleen DeGroat.
Under those provisions, a private party can file an action on behalf of the United States and receive a portion of any recovery. Ms. DeGroat will receive a share of the settlement.
A version of this article first appeared on Medscape.com.
Three wild technologies about to change health care
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.