User login
Current monkeypox outbreak marked by unconventional spread, clinical features
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
FDA cautions against using OTC products to remove skin spots, moles
Those moles, skin tags, and liver spots should stay on your skin until you see a doctor, according to a new alert from the U.S. Food and Drug Administration. The alert warns against the use of over-the-counter products for removing moles, seborrheic keratoses (wart-like growths that are often brown), or skin tags, emphasizing that none are approved by the FDA for at-home use.
Dermatologists and the FDA say these products may lead to scarring and disfigurement.
Risks include “skin injuries, infection requiring antibiotics, scarring, and delayed skin cancer diagnosis and treatment,” according to the alert, which adds that the agency has received reports of people “who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers. “
These products come in the form of gels, liquids, sticks, or ointments and commonly contain ingredients like salicylic acid, which are cytotoxic, or cell-killing. These chemicals are what make the products potentially dangerous, as each contains unregulated, and likely very high, amounts of these corrosive agents. Even products marketed as natural or organic have these same issues, said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, who notes that bloodroot is another ingredient found in these products.
Dr. Friedman explained that using these products without the supervision of a health care provider can create a chemical burn in the skin, leading to scarring. He’s treated patients for open wounds and infected ulcers caused by these products. “Over my career, I’ve seen many cases of patients coming in with self-inflicted harm due to using these quote, unquote, safe and natural products to remove benign, or even worse, potentially malignant neoplasms,” he told this news organization.
Another concern is that these spots on the skin are often the only sign of a serious issue – cancer. Early signs of melanoma, a type of skin cancer, include large, misshapen, or rapidly changing moles. Dr. Friedman said that if a patient uses one of these products on what is actually a cancerous mole, they will likely only remove the surface, and in turn, destroy the only sign of cancer – effectively killing the canary in the coal mine.
There’s a good chance that the root of the mole has been left intact under the skin surface, and as a result, the cancer has the potential to spread unnoticed. “If people aren’t going to a dermatologist to be properly diagnosed and properly managed, they’re going to cause more harm by thinking that they’ve taken care of a problem,” he said.
If you are concerned about any type of spot on your skin, a visit to the dermatologist will prove much simpler and safer for treating it than doing so at home. In the office, Dr. Friedman said, providers can use a range of highly studied techniques to remove skin lesions with minimal pain and scarring. From freezing, burning, snipping, or a quick moment under a scalpel, you’ll be healed in no time.
Anyone who has experienced an adverse event with one of these products and health care professionals should report cases to the FDA’s MedWatch Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Those moles, skin tags, and liver spots should stay on your skin until you see a doctor, according to a new alert from the U.S. Food and Drug Administration. The alert warns against the use of over-the-counter products for removing moles, seborrheic keratoses (wart-like growths that are often brown), or skin tags, emphasizing that none are approved by the FDA for at-home use.
Dermatologists and the FDA say these products may lead to scarring and disfigurement.
Risks include “skin injuries, infection requiring antibiotics, scarring, and delayed skin cancer diagnosis and treatment,” according to the alert, which adds that the agency has received reports of people “who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers. “
These products come in the form of gels, liquids, sticks, or ointments and commonly contain ingredients like salicylic acid, which are cytotoxic, or cell-killing. These chemicals are what make the products potentially dangerous, as each contains unregulated, and likely very high, amounts of these corrosive agents. Even products marketed as natural or organic have these same issues, said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, who notes that bloodroot is another ingredient found in these products.
Dr. Friedman explained that using these products without the supervision of a health care provider can create a chemical burn in the skin, leading to scarring. He’s treated patients for open wounds and infected ulcers caused by these products. “Over my career, I’ve seen many cases of patients coming in with self-inflicted harm due to using these quote, unquote, safe and natural products to remove benign, or even worse, potentially malignant neoplasms,” he told this news organization.
Another concern is that these spots on the skin are often the only sign of a serious issue – cancer. Early signs of melanoma, a type of skin cancer, include large, misshapen, or rapidly changing moles. Dr. Friedman said that if a patient uses one of these products on what is actually a cancerous mole, they will likely only remove the surface, and in turn, destroy the only sign of cancer – effectively killing the canary in the coal mine.
There’s a good chance that the root of the mole has been left intact under the skin surface, and as a result, the cancer has the potential to spread unnoticed. “If people aren’t going to a dermatologist to be properly diagnosed and properly managed, they’re going to cause more harm by thinking that they’ve taken care of a problem,” he said.
If you are concerned about any type of spot on your skin, a visit to the dermatologist will prove much simpler and safer for treating it than doing so at home. In the office, Dr. Friedman said, providers can use a range of highly studied techniques to remove skin lesions with minimal pain and scarring. From freezing, burning, snipping, or a quick moment under a scalpel, you’ll be healed in no time.
Anyone who has experienced an adverse event with one of these products and health care professionals should report cases to the FDA’s MedWatch Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Those moles, skin tags, and liver spots should stay on your skin until you see a doctor, according to a new alert from the U.S. Food and Drug Administration. The alert warns against the use of over-the-counter products for removing moles, seborrheic keratoses (wart-like growths that are often brown), or skin tags, emphasizing that none are approved by the FDA for at-home use.
Dermatologists and the FDA say these products may lead to scarring and disfigurement.
Risks include “skin injuries, infection requiring antibiotics, scarring, and delayed skin cancer diagnosis and treatment,” according to the alert, which adds that the agency has received reports of people “who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers. “
These products come in the form of gels, liquids, sticks, or ointments and commonly contain ingredients like salicylic acid, which are cytotoxic, or cell-killing. These chemicals are what make the products potentially dangerous, as each contains unregulated, and likely very high, amounts of these corrosive agents. Even products marketed as natural or organic have these same issues, said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, who notes that bloodroot is another ingredient found in these products.
Dr. Friedman explained that using these products without the supervision of a health care provider can create a chemical burn in the skin, leading to scarring. He’s treated patients for open wounds and infected ulcers caused by these products. “Over my career, I’ve seen many cases of patients coming in with self-inflicted harm due to using these quote, unquote, safe and natural products to remove benign, or even worse, potentially malignant neoplasms,” he told this news organization.
Another concern is that these spots on the skin are often the only sign of a serious issue – cancer. Early signs of melanoma, a type of skin cancer, include large, misshapen, or rapidly changing moles. Dr. Friedman said that if a patient uses one of these products on what is actually a cancerous mole, they will likely only remove the surface, and in turn, destroy the only sign of cancer – effectively killing the canary in the coal mine.
There’s a good chance that the root of the mole has been left intact under the skin surface, and as a result, the cancer has the potential to spread unnoticed. “If people aren’t going to a dermatologist to be properly diagnosed and properly managed, they’re going to cause more harm by thinking that they’ve taken care of a problem,” he said.
If you are concerned about any type of spot on your skin, a visit to the dermatologist will prove much simpler and safer for treating it than doing so at home. In the office, Dr. Friedman said, providers can use a range of highly studied techniques to remove skin lesions with minimal pain and scarring. From freezing, burning, snipping, or a quick moment under a scalpel, you’ll be healed in no time.
Anyone who has experienced an adverse event with one of these products and health care professionals should report cases to the FDA’s MedWatch Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Atopic dermatitis: Options abound, and more are coming
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Therapeutic patient education can help with adherence to treatment
, Andreas Wollenberg, MD, said at the Revolutionizing Atopic Dermatitis symposium.
A major goal of patient education is increasing medication adherence, noted Dr. Wollenberg, professor in the department of dermatology and allergy at Ludwig Maximilian University of Munich. Quoting former U.S. Surgeon General C. Everett Koop, MD, he said, “drugs don’t work in patients who don’t take them.”
While this is a simple message, it is important, Dr. Wollenberg said, noting that there can be a gap between a physician’s well-intentioned message and how it is interpreted by the patient. “Our messages may not be heard, not understood, not accepted, and even if they are put into place, how long will they last?” he asked. “We need to find a way [to] place sticky messages in the brains of our patients who are sitting and interacting with us.”
One way to improve treatment adherence is through patient education, such as using a written action plan or graphics; simplifying treatment regimens; minimizing treatment costs; setting up reminder programs, early follow-up visits, and short-term treatment goals; and minimizing nocebo effects. “This is more than providing just leaflets to patients. It is a complete program. It is a holistic approach. It should be structured and should be interdisciplinary, and it should contain a psychological component,” Dr. Wollenberg said.
Therapeutic patient education is recommended at baseline for children and adults with moderate to severe AD in the 2020 European Task Force on Atopic Dermatitis (ETFAD) and European Academy of Dermatology and Venereology (EADV) position paper on the diagnosis and treatment of AD in adults and children, alongside other interventions, such as emollients, bath oils, and avoidance of clinically relevant allergens, noted Dr. Wollenberg, the first author . “Therapeutic patient education is an extremely helpful tool to address patient beliefs and questions regarding disease and treatment,” he and his coauthors wrote in the paper.
When considering a therapeutic patient education program for AD, content is key, but just as important is consideration of legal and cultural conditions in the local area, Dr. Wollenberg explained. Every country will need some degree of standardization of content, he noted. Clinicians interested in adopting a patient education program need to consider who will pay for it – patients, foundations, or insurance companies – as well as the time commitment needed.
Dr. Wollenberg said that his team uses an evidence-based education program for AD in Germany that works across patients with different personalities, with a multidisciplinary team that includes a dermatologist, a specialist nurse, a nutrition expert, and a psychologist. “Sometimes we replace the specialized nurse with the dermatology resident because, in Germany, it’s difficult to find any type of specialized nurse,” although this is not an issue in many other countries, he said.
The model for children involves six 90-minute sessions, which cover topics that include emollients and basic care, food allergies and diet, medical treatment, and psychology of itch. The program for adults involves six 2-hour sessions, which cover topics that include psychology, skin care/nutrition, and medical treatment.
While this education program improves adherence in patients with AD, he acknowledged it is time consuming, and may not work for people who live far away from a clinic or who have other time commitments, making an alternative format necessary.
In terms of improving patient adherence to a doctor’s recommendations regarding chronic skin disease, “we cannot change our patients, we cannot change the disease, but we can strongly influence the treatment that we choose and how we interact as physicians with our patients,” said Dr. Wollenberg.
“Therapeutic patient education is virtually free of side effects, but evidence based. Have a look [at] it and adapt it to your own practice,” he added.
Dr. Wollenberg is a consultant, speaker and receives fees from numerous pharmaceutical companies.
, Andreas Wollenberg, MD, said at the Revolutionizing Atopic Dermatitis symposium.
A major goal of patient education is increasing medication adherence, noted Dr. Wollenberg, professor in the department of dermatology and allergy at Ludwig Maximilian University of Munich. Quoting former U.S. Surgeon General C. Everett Koop, MD, he said, “drugs don’t work in patients who don’t take them.”
While this is a simple message, it is important, Dr. Wollenberg said, noting that there can be a gap between a physician’s well-intentioned message and how it is interpreted by the patient. “Our messages may not be heard, not understood, not accepted, and even if they are put into place, how long will they last?” he asked. “We need to find a way [to] place sticky messages in the brains of our patients who are sitting and interacting with us.”
One way to improve treatment adherence is through patient education, such as using a written action plan or graphics; simplifying treatment regimens; minimizing treatment costs; setting up reminder programs, early follow-up visits, and short-term treatment goals; and minimizing nocebo effects. “This is more than providing just leaflets to patients. It is a complete program. It is a holistic approach. It should be structured and should be interdisciplinary, and it should contain a psychological component,” Dr. Wollenberg said.
Therapeutic patient education is recommended at baseline for children and adults with moderate to severe AD in the 2020 European Task Force on Atopic Dermatitis (ETFAD) and European Academy of Dermatology and Venereology (EADV) position paper on the diagnosis and treatment of AD in adults and children, alongside other interventions, such as emollients, bath oils, and avoidance of clinically relevant allergens, noted Dr. Wollenberg, the first author . “Therapeutic patient education is an extremely helpful tool to address patient beliefs and questions regarding disease and treatment,” he and his coauthors wrote in the paper.
When considering a therapeutic patient education program for AD, content is key, but just as important is consideration of legal and cultural conditions in the local area, Dr. Wollenberg explained. Every country will need some degree of standardization of content, he noted. Clinicians interested in adopting a patient education program need to consider who will pay for it – patients, foundations, or insurance companies – as well as the time commitment needed.
Dr. Wollenberg said that his team uses an evidence-based education program for AD in Germany that works across patients with different personalities, with a multidisciplinary team that includes a dermatologist, a specialist nurse, a nutrition expert, and a psychologist. “Sometimes we replace the specialized nurse with the dermatology resident because, in Germany, it’s difficult to find any type of specialized nurse,” although this is not an issue in many other countries, he said.
The model for children involves six 90-minute sessions, which cover topics that include emollients and basic care, food allergies and diet, medical treatment, and psychology of itch. The program for adults involves six 2-hour sessions, which cover topics that include psychology, skin care/nutrition, and medical treatment.
While this education program improves adherence in patients with AD, he acknowledged it is time consuming, and may not work for people who live far away from a clinic or who have other time commitments, making an alternative format necessary.
In terms of improving patient adherence to a doctor’s recommendations regarding chronic skin disease, “we cannot change our patients, we cannot change the disease, but we can strongly influence the treatment that we choose and how we interact as physicians with our patients,” said Dr. Wollenberg.
“Therapeutic patient education is virtually free of side effects, but evidence based. Have a look [at] it and adapt it to your own practice,” he added.
Dr. Wollenberg is a consultant, speaker and receives fees from numerous pharmaceutical companies.
, Andreas Wollenberg, MD, said at the Revolutionizing Atopic Dermatitis symposium.
A major goal of patient education is increasing medication adherence, noted Dr. Wollenberg, professor in the department of dermatology and allergy at Ludwig Maximilian University of Munich. Quoting former U.S. Surgeon General C. Everett Koop, MD, he said, “drugs don’t work in patients who don’t take them.”
While this is a simple message, it is important, Dr. Wollenberg said, noting that there can be a gap between a physician’s well-intentioned message and how it is interpreted by the patient. “Our messages may not be heard, not understood, not accepted, and even if they are put into place, how long will they last?” he asked. “We need to find a way [to] place sticky messages in the brains of our patients who are sitting and interacting with us.”
One way to improve treatment adherence is through patient education, such as using a written action plan or graphics; simplifying treatment regimens; minimizing treatment costs; setting up reminder programs, early follow-up visits, and short-term treatment goals; and minimizing nocebo effects. “This is more than providing just leaflets to patients. It is a complete program. It is a holistic approach. It should be structured and should be interdisciplinary, and it should contain a psychological component,” Dr. Wollenberg said.
Therapeutic patient education is recommended at baseline for children and adults with moderate to severe AD in the 2020 European Task Force on Atopic Dermatitis (ETFAD) and European Academy of Dermatology and Venereology (EADV) position paper on the diagnosis and treatment of AD in adults and children, alongside other interventions, such as emollients, bath oils, and avoidance of clinically relevant allergens, noted Dr. Wollenberg, the first author . “Therapeutic patient education is an extremely helpful tool to address patient beliefs and questions regarding disease and treatment,” he and his coauthors wrote in the paper.
When considering a therapeutic patient education program for AD, content is key, but just as important is consideration of legal and cultural conditions in the local area, Dr. Wollenberg explained. Every country will need some degree of standardization of content, he noted. Clinicians interested in adopting a patient education program need to consider who will pay for it – patients, foundations, or insurance companies – as well as the time commitment needed.
Dr. Wollenberg said that his team uses an evidence-based education program for AD in Germany that works across patients with different personalities, with a multidisciplinary team that includes a dermatologist, a specialist nurse, a nutrition expert, and a psychologist. “Sometimes we replace the specialized nurse with the dermatology resident because, in Germany, it’s difficult to find any type of specialized nurse,” although this is not an issue in many other countries, he said.
The model for children involves six 90-minute sessions, which cover topics that include emollients and basic care, food allergies and diet, medical treatment, and psychology of itch. The program for adults involves six 2-hour sessions, which cover topics that include psychology, skin care/nutrition, and medical treatment.
While this education program improves adherence in patients with AD, he acknowledged it is time consuming, and may not work for people who live far away from a clinic or who have other time commitments, making an alternative format necessary.
In terms of improving patient adherence to a doctor’s recommendations regarding chronic skin disease, “we cannot change our patients, we cannot change the disease, but we can strongly influence the treatment that we choose and how we interact as physicians with our patients,” said Dr. Wollenberg.
“Therapeutic patient education is virtually free of side effects, but evidence based. Have a look [at] it and adapt it to your own practice,” he added.
Dr. Wollenberg is a consultant, speaker and receives fees from numerous pharmaceutical companies.
FROM RAD 2022
Infant with mottled skin
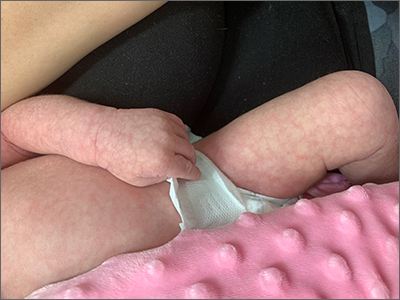
The net-like, violaceous pattern on the infant’s skin was characteristic of livedo reticularis.
Livedo reticularis is thought to arise from a change in underlying cutaneous blood flow.1 The appearance of this condition reflects the configuration of underlying cutaneous vasculature. Arterioles oriented perpendicularly to the skin can surface from a network where blood flows from arteriole to capillary to venule. The reticular appearance is a result of increased visibility of the venous plexus.1
Common culprits of this manifestation are impaired arteriolar perfusion and venous congestion, which may be caused by vasospasm, arterial thrombosis, or hyperviscosity.1 If diagnostic biopsies are needed, they should be taken from the pale central areas.
Livedo reticularis can be categorized into groups to help delineate the patient-specific pathogenesis: idiopathic, primary, secondary (due to underlying disease; see below), and physiologic.1 Physiologic livedo reticularis, which this patient had, is known as cutis marmorata (CM); it occurs in response to cold temperatures. It may be more common, or visible, in individuals with lighter skin types and in preterm infants. The condition typically affects the lower extremities but may also occur on the trunk and upper extremities.1
Physiologic livedo reticularis usually resolves with warming of the extremities. Secondary livedo reticularis usually manifests in older patients and is due to serious conditions such as malignancies, antiphospholipid syndrome, and Sneddon syndrome. For these patients, coagulopathy and malignancy evaluation targeting the underlying cause of the livedo reticularis is warranted. Interestingly, livedo reticularis may also be associated with COVID-19, but this association has not been reported frequently in children.2
The infant in this case experienced rapid resolution with warming. The family was educated about the CM form of livedo reticularis and instructed to keep her warm. No other treatment or evaluation was indicated.
Photo courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of Christy Nwankwo, BA, University of Missouri-Kansas City School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Gibbs MB, English JC 3rd, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019. doi: 10.1016/j.jaad.2004.11.051
2. Lavery MJ, Bouvier CA, Thompson B. Cutaneous manifestations of COVID-19 in children (and adults): a virus that does not discriminate. Clin Dermatol. 2021;39:323-328. doi: 10.1016/j.clindermatol.2020.10.020

The net-like, violaceous pattern on the infant’s skin was characteristic of livedo reticularis.
Livedo reticularis is thought to arise from a change in underlying cutaneous blood flow.1 The appearance of this condition reflects the configuration of underlying cutaneous vasculature. Arterioles oriented perpendicularly to the skin can surface from a network where blood flows from arteriole to capillary to venule. The reticular appearance is a result of increased visibility of the venous plexus.1
Common culprits of this manifestation are impaired arteriolar perfusion and venous congestion, which may be caused by vasospasm, arterial thrombosis, or hyperviscosity.1 If diagnostic biopsies are needed, they should be taken from the pale central areas.
Livedo reticularis can be categorized into groups to help delineate the patient-specific pathogenesis: idiopathic, primary, secondary (due to underlying disease; see below), and physiologic.1 Physiologic livedo reticularis, which this patient had, is known as cutis marmorata (CM); it occurs in response to cold temperatures. It may be more common, or visible, in individuals with lighter skin types and in preterm infants. The condition typically affects the lower extremities but may also occur on the trunk and upper extremities.1
Physiologic livedo reticularis usually resolves with warming of the extremities. Secondary livedo reticularis usually manifests in older patients and is due to serious conditions such as malignancies, antiphospholipid syndrome, and Sneddon syndrome. For these patients, coagulopathy and malignancy evaluation targeting the underlying cause of the livedo reticularis is warranted. Interestingly, livedo reticularis may also be associated with COVID-19, but this association has not been reported frequently in children.2
The infant in this case experienced rapid resolution with warming. The family was educated about the CM form of livedo reticularis and instructed to keep her warm. No other treatment or evaluation was indicated.
Photo courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of Christy Nwankwo, BA, University of Missouri-Kansas City School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The net-like, violaceous pattern on the infant’s skin was characteristic of livedo reticularis.
Livedo reticularis is thought to arise from a change in underlying cutaneous blood flow.1 The appearance of this condition reflects the configuration of underlying cutaneous vasculature. Arterioles oriented perpendicularly to the skin can surface from a network where blood flows from arteriole to capillary to venule. The reticular appearance is a result of increased visibility of the venous plexus.1
Common culprits of this manifestation are impaired arteriolar perfusion and venous congestion, which may be caused by vasospasm, arterial thrombosis, or hyperviscosity.1 If diagnostic biopsies are needed, they should be taken from the pale central areas.
Livedo reticularis can be categorized into groups to help delineate the patient-specific pathogenesis: idiopathic, primary, secondary (due to underlying disease; see below), and physiologic.1 Physiologic livedo reticularis, which this patient had, is known as cutis marmorata (CM); it occurs in response to cold temperatures. It may be more common, or visible, in individuals with lighter skin types and in preterm infants. The condition typically affects the lower extremities but may also occur on the trunk and upper extremities.1
Physiologic livedo reticularis usually resolves with warming of the extremities. Secondary livedo reticularis usually manifests in older patients and is due to serious conditions such as malignancies, antiphospholipid syndrome, and Sneddon syndrome. For these patients, coagulopathy and malignancy evaluation targeting the underlying cause of the livedo reticularis is warranted. Interestingly, livedo reticularis may also be associated with COVID-19, but this association has not been reported frequently in children.2
The infant in this case experienced rapid resolution with warming. The family was educated about the CM form of livedo reticularis and instructed to keep her warm. No other treatment or evaluation was indicated.
Photo courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of Christy Nwankwo, BA, University of Missouri-Kansas City School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Gibbs MB, English JC 3rd, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019. doi: 10.1016/j.jaad.2004.11.051
2. Lavery MJ, Bouvier CA, Thompson B. Cutaneous manifestations of COVID-19 in children (and adults): a virus that does not discriminate. Clin Dermatol. 2021;39:323-328. doi: 10.1016/j.clindermatol.2020.10.020
1. Gibbs MB, English JC 3rd, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019. doi: 10.1016/j.jaad.2004.11.051
2. Lavery MJ, Bouvier CA, Thompson B. Cutaneous manifestations of COVID-19 in children (and adults): a virus that does not discriminate. Clin Dermatol. 2021;39:323-328. doi: 10.1016/j.clindermatol.2020.10.020
‘Genetic’ height linked to peripheral neuropathy and certain skin and bone infections
, according to a study published in PLOS Genetics.
Prior studies have investigated height as a risk factor for chronic diseases, such as a higher risk for atrial fibrillation and a reduced risk of cardiovascular disease. It’s been consistently difficult, however, to eliminate the confounding influences of diet, socioeconomics, lifestyle behaviors, and other environmental factors that may interfere with a person’s reaching their expected height based on their genes.
This study, however, was able to better parse those differences by using Mendelian randomization within the comprehensive clinical and genetic dataset of a national health care system biobank. Mendelian randomization uses “genetic instruments for exposures of interest under the assumption that genotype is less susceptible to confounding than measured exposures,” the authors explained. The findings confirmed previously suspected associations between height and a range of cardiovascular and metabolic conditions as well as revealing new associations with several other conditions.
Prior associations confirmed, new associations uncovered
The results confirmed that being tall is linked to a higher risk of atrial fibrillation and varicose veins, and a lower risk of coronary heart disease, high blood pressure, and high cholesterol. The study also uncovered new associations between greater height and a higher risk of peripheral neuropathy, which is caused by damage to nerves on the extremities, as well as skin and bone infections, such as leg and foot ulcers.
The meta-analysis “identified five additional traits associated with genetically-predicted height,” wrote Sridharan Raghavan, MD, assistant professor of medicine at the University of Colorado Anschutz Medical Campus, and colleagues. “Two were genitourinary conditions – erectile dysfunction and urinary retention – that can be associated with neuropathy, and a third was a phecode for nonspecific skin disorders that may be related to skin infections – consistent with the race/ethnicity stratified results.”
Removing potential confounders
F. Perry Wilson, MD, associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study, said the findings were not particularly surprising overall, but it’s striking that the researchers had ”such a large cohort with such detailed electronic health records allowing for the comparison of genetic height with a variety of clinical outcomes.” He also noted the study’s strength in using Mendelian randomization so that the exposure is the predicted genetic height instead of a person’s measured height.
“This is key, since lots of things affect actual height – nutrition is an important one that could certainly be linked to disease as well,” Dr. Wilson said. ”By using genetic height, the authors remove these potential confounders. Since genetic height is “assigned” at birth (or conception), there is little opportunity for confounding. Of course, it is possible that some of the gene variants used to predict genetic height actually do something else, such as make you seek out less nutritious meals, but by and large this is how these types of studies need to be done.”
Height may impact over 100 clinical traits
The study relied on data from the U.S. Veteran Affairs Million Veteran Program with 222,300 non-Hispanic White and 58,151 non-Hispanic Black participants. The researchers first estimated the likelihood of participants’ genetic height based on 3,290 genetic variants determined to affect genetic height in a recent European-ancestry genome-wide meta-analysis. Then they compared these estimates with participants’ actual height in the VA medical record, adjusting for age, sex, and other genetic characteristics.
In doing so, the researchers found 345 clinical traits that were associated with the actual measured height in White participants plus another 17 clinical trials linked to actual measured height in Black participants. An overall 127 of these clinical traits were significantly associated with White participants’ genetically predicted height, and two of them were significantly associated with Black participants’ genetically predicted height.
In analyzing all these data together, the researchers were largely able to separate out those associations between genetically predicted height and certain health conditions from those associations between health conditions and a person’s actual measured height. They also determined that including body mass index as a covariate had little impact on the results. The researchers conducted the appropriate statistical correction to ensure the use of so many variables did not result in spurious statistical significance in some associations.
“Using genetic methods applied to the VA Million Veteran Program, we found evidence that adult height may impact over 100 clinical traits, including several conditions associated with poor outcomes and quality of life – peripheral neuropathy, lower extremity ulcers, and chronic venous insufficiency. We conclude that height may be an unrecognized nonmodifiable risk factor for several common conditions in adults.”
Height linked with health conditions
Genetically predicted height predicted a reduced risk of hyperlipidemia and hypertension independent of coronary heart disease, the analysis revealed. Genetically predicted height was also linked to an approximately 51% increased risk of atrial fibrillation in participants without coronary heart disease but, paradoxically, only a 39% increased risk in those with coronary heart disease, despite coronary heart disease being a risk factor for atrial fibrillation. Genetically predicted height was also associated with a greater risk of varicose veins in the legs and deep vein thrombosis.
Another novel association uncovered by the analysis was between women’s genetically predicted height and both asthma and nonspecific peripheral nerve disorders. “Whether these associations reflect differences by sex in disease pathophysiology related to height may warrant exploration in a sample with better balance between men and women,” the authors wrote. “In sum, our results suggest that an individual’s height may warrant consideration as a nonmodifiable predictor for several common conditions, particularly those affecting peripheral/distal extremities that are most physically impacted by tall stature.”
A substantial limitation of the study was its homogeneity of participants, who were 92% male with an average height of 176 cm and an average BMI of 30.1. The Black participants tended to be younger, with an average age of 58 compared with 64 years in the White participants, but the groups were otherwise similar in height and weight.* The database included data from Hispanic participants, but the researchers excluded these data because of the small sample size.
The smaller dataset for Black participants was a limitation as well as the fact that the genome-wide association study the researchers relied on came from a European population, which may not be as accurate in people with other ancestry, Dr. Wilson said. The bigger limitation, however, is what the findings’ clinical relevance is.
What does it all mean?
“Genetic height is in your genes – there is nothing to be done about it – so it is more of academic interest than clinical interest,” Dr. Wilson said. It’s not even clear whether incorporating a person’s height – actual or genetically predicted, if it could be easily determined for each person – into risk calculators. ”To know whether it would be beneficial to use height (or genetic height) as a risk factor, you’d need to examine each condition of interest, adjusting for all known risk factors, to see if height improved the prediction,” Dr. Wilson said. “I suspect for most conditions, the well-known risk factors would swamp height. For example, high genetic height might truly increase risk for neuropathy. But diabetes might increase the risk so much more that height is not particularly relevant.”
On the other hand, the fact that height in general has any potential influence at all on disease risk may inspire physicians to consider other risk factors in especially tall individuals.
”Physicians may find it interesting that we have some confirmation that height does increase the risk of certain conditions,” Dr. Wilson said. “While this is unlikely to dramatically change practice, they may be a bit more diligent in looking for other relevant risk factors for the diseases found in this study in their very tall patients.”
The research was funded by the U.S. Department of Veteran Affairs, the Boettcher Foundation’s Webb-Waring Biomedical Research Program, the National Institutes of Health, and a Linda Pechenik Montague Investigator award. One study coauthor is a full-time employee of Novartis Institutes of Biomedical Research. The other authors and Dr. Wilson had no disclosures.
*Correction, 6/29/22: An earlier version of this article misstated the average age of Black participants.
, according to a study published in PLOS Genetics.
Prior studies have investigated height as a risk factor for chronic diseases, such as a higher risk for atrial fibrillation and a reduced risk of cardiovascular disease. It’s been consistently difficult, however, to eliminate the confounding influences of diet, socioeconomics, lifestyle behaviors, and other environmental factors that may interfere with a person’s reaching their expected height based on their genes.
This study, however, was able to better parse those differences by using Mendelian randomization within the comprehensive clinical and genetic dataset of a national health care system biobank. Mendelian randomization uses “genetic instruments for exposures of interest under the assumption that genotype is less susceptible to confounding than measured exposures,” the authors explained. The findings confirmed previously suspected associations between height and a range of cardiovascular and metabolic conditions as well as revealing new associations with several other conditions.
Prior associations confirmed, new associations uncovered
The results confirmed that being tall is linked to a higher risk of atrial fibrillation and varicose veins, and a lower risk of coronary heart disease, high blood pressure, and high cholesterol. The study also uncovered new associations between greater height and a higher risk of peripheral neuropathy, which is caused by damage to nerves on the extremities, as well as skin and bone infections, such as leg and foot ulcers.
The meta-analysis “identified five additional traits associated with genetically-predicted height,” wrote Sridharan Raghavan, MD, assistant professor of medicine at the University of Colorado Anschutz Medical Campus, and colleagues. “Two were genitourinary conditions – erectile dysfunction and urinary retention – that can be associated with neuropathy, and a third was a phecode for nonspecific skin disorders that may be related to skin infections – consistent with the race/ethnicity stratified results.”
Removing potential confounders
F. Perry Wilson, MD, associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study, said the findings were not particularly surprising overall, but it’s striking that the researchers had ”such a large cohort with such detailed electronic health records allowing for the comparison of genetic height with a variety of clinical outcomes.” He also noted the study’s strength in using Mendelian randomization so that the exposure is the predicted genetic height instead of a person’s measured height.
“This is key, since lots of things affect actual height – nutrition is an important one that could certainly be linked to disease as well,” Dr. Wilson said. ”By using genetic height, the authors remove these potential confounders. Since genetic height is “assigned” at birth (or conception), there is little opportunity for confounding. Of course, it is possible that some of the gene variants used to predict genetic height actually do something else, such as make you seek out less nutritious meals, but by and large this is how these types of studies need to be done.”
Height may impact over 100 clinical traits
The study relied on data from the U.S. Veteran Affairs Million Veteran Program with 222,300 non-Hispanic White and 58,151 non-Hispanic Black participants. The researchers first estimated the likelihood of participants’ genetic height based on 3,290 genetic variants determined to affect genetic height in a recent European-ancestry genome-wide meta-analysis. Then they compared these estimates with participants’ actual height in the VA medical record, adjusting for age, sex, and other genetic characteristics.
In doing so, the researchers found 345 clinical traits that were associated with the actual measured height in White participants plus another 17 clinical trials linked to actual measured height in Black participants. An overall 127 of these clinical traits were significantly associated with White participants’ genetically predicted height, and two of them were significantly associated with Black participants’ genetically predicted height.
In analyzing all these data together, the researchers were largely able to separate out those associations between genetically predicted height and certain health conditions from those associations between health conditions and a person’s actual measured height. They also determined that including body mass index as a covariate had little impact on the results. The researchers conducted the appropriate statistical correction to ensure the use of so many variables did not result in spurious statistical significance in some associations.
“Using genetic methods applied to the VA Million Veteran Program, we found evidence that adult height may impact over 100 clinical traits, including several conditions associated with poor outcomes and quality of life – peripheral neuropathy, lower extremity ulcers, and chronic venous insufficiency. We conclude that height may be an unrecognized nonmodifiable risk factor for several common conditions in adults.”
Height linked with health conditions
Genetically predicted height predicted a reduced risk of hyperlipidemia and hypertension independent of coronary heart disease, the analysis revealed. Genetically predicted height was also linked to an approximately 51% increased risk of atrial fibrillation in participants without coronary heart disease but, paradoxically, only a 39% increased risk in those with coronary heart disease, despite coronary heart disease being a risk factor for atrial fibrillation. Genetically predicted height was also associated with a greater risk of varicose veins in the legs and deep vein thrombosis.
Another novel association uncovered by the analysis was between women’s genetically predicted height and both asthma and nonspecific peripheral nerve disorders. “Whether these associations reflect differences by sex in disease pathophysiology related to height may warrant exploration in a sample with better balance between men and women,” the authors wrote. “In sum, our results suggest that an individual’s height may warrant consideration as a nonmodifiable predictor for several common conditions, particularly those affecting peripheral/distal extremities that are most physically impacted by tall stature.”
A substantial limitation of the study was its homogeneity of participants, who were 92% male with an average height of 176 cm and an average BMI of 30.1. The Black participants tended to be younger, with an average age of 58 compared with 64 years in the White participants, but the groups were otherwise similar in height and weight.* The database included data from Hispanic participants, but the researchers excluded these data because of the small sample size.
The smaller dataset for Black participants was a limitation as well as the fact that the genome-wide association study the researchers relied on came from a European population, which may not be as accurate in people with other ancestry, Dr. Wilson said. The bigger limitation, however, is what the findings’ clinical relevance is.
What does it all mean?
“Genetic height is in your genes – there is nothing to be done about it – so it is more of academic interest than clinical interest,” Dr. Wilson said. It’s not even clear whether incorporating a person’s height – actual or genetically predicted, if it could be easily determined for each person – into risk calculators. ”To know whether it would be beneficial to use height (or genetic height) as a risk factor, you’d need to examine each condition of interest, adjusting for all known risk factors, to see if height improved the prediction,” Dr. Wilson said. “I suspect for most conditions, the well-known risk factors would swamp height. For example, high genetic height might truly increase risk for neuropathy. But diabetes might increase the risk so much more that height is not particularly relevant.”
On the other hand, the fact that height in general has any potential influence at all on disease risk may inspire physicians to consider other risk factors in especially tall individuals.
”Physicians may find it interesting that we have some confirmation that height does increase the risk of certain conditions,” Dr. Wilson said. “While this is unlikely to dramatically change practice, they may be a bit more diligent in looking for other relevant risk factors for the diseases found in this study in their very tall patients.”
The research was funded by the U.S. Department of Veteran Affairs, the Boettcher Foundation’s Webb-Waring Biomedical Research Program, the National Institutes of Health, and a Linda Pechenik Montague Investigator award. One study coauthor is a full-time employee of Novartis Institutes of Biomedical Research. The other authors and Dr. Wilson had no disclosures.
*Correction, 6/29/22: An earlier version of this article misstated the average age of Black participants.
, according to a study published in PLOS Genetics.
Prior studies have investigated height as a risk factor for chronic diseases, such as a higher risk for atrial fibrillation and a reduced risk of cardiovascular disease. It’s been consistently difficult, however, to eliminate the confounding influences of diet, socioeconomics, lifestyle behaviors, and other environmental factors that may interfere with a person’s reaching their expected height based on their genes.
This study, however, was able to better parse those differences by using Mendelian randomization within the comprehensive clinical and genetic dataset of a national health care system biobank. Mendelian randomization uses “genetic instruments for exposures of interest under the assumption that genotype is less susceptible to confounding than measured exposures,” the authors explained. The findings confirmed previously suspected associations between height and a range of cardiovascular and metabolic conditions as well as revealing new associations with several other conditions.
Prior associations confirmed, new associations uncovered
The results confirmed that being tall is linked to a higher risk of atrial fibrillation and varicose veins, and a lower risk of coronary heart disease, high blood pressure, and high cholesterol. The study also uncovered new associations between greater height and a higher risk of peripheral neuropathy, which is caused by damage to nerves on the extremities, as well as skin and bone infections, such as leg and foot ulcers.
The meta-analysis “identified five additional traits associated with genetically-predicted height,” wrote Sridharan Raghavan, MD, assistant professor of medicine at the University of Colorado Anschutz Medical Campus, and colleagues. “Two were genitourinary conditions – erectile dysfunction and urinary retention – that can be associated with neuropathy, and a third was a phecode for nonspecific skin disorders that may be related to skin infections – consistent with the race/ethnicity stratified results.”
Removing potential confounders
F. Perry Wilson, MD, associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study, said the findings were not particularly surprising overall, but it’s striking that the researchers had ”such a large cohort with such detailed electronic health records allowing for the comparison of genetic height with a variety of clinical outcomes.” He also noted the study’s strength in using Mendelian randomization so that the exposure is the predicted genetic height instead of a person’s measured height.
“This is key, since lots of things affect actual height – nutrition is an important one that could certainly be linked to disease as well,” Dr. Wilson said. ”By using genetic height, the authors remove these potential confounders. Since genetic height is “assigned” at birth (or conception), there is little opportunity for confounding. Of course, it is possible that some of the gene variants used to predict genetic height actually do something else, such as make you seek out less nutritious meals, but by and large this is how these types of studies need to be done.”
Height may impact over 100 clinical traits
The study relied on data from the U.S. Veteran Affairs Million Veteran Program with 222,300 non-Hispanic White and 58,151 non-Hispanic Black participants. The researchers first estimated the likelihood of participants’ genetic height based on 3,290 genetic variants determined to affect genetic height in a recent European-ancestry genome-wide meta-analysis. Then they compared these estimates with participants’ actual height in the VA medical record, adjusting for age, sex, and other genetic characteristics.
In doing so, the researchers found 345 clinical traits that were associated with the actual measured height in White participants plus another 17 clinical trials linked to actual measured height in Black participants. An overall 127 of these clinical traits were significantly associated with White participants’ genetically predicted height, and two of them were significantly associated with Black participants’ genetically predicted height.
In analyzing all these data together, the researchers were largely able to separate out those associations between genetically predicted height and certain health conditions from those associations between health conditions and a person’s actual measured height. They also determined that including body mass index as a covariate had little impact on the results. The researchers conducted the appropriate statistical correction to ensure the use of so many variables did not result in spurious statistical significance in some associations.
“Using genetic methods applied to the VA Million Veteran Program, we found evidence that adult height may impact over 100 clinical traits, including several conditions associated with poor outcomes and quality of life – peripheral neuropathy, lower extremity ulcers, and chronic venous insufficiency. We conclude that height may be an unrecognized nonmodifiable risk factor for several common conditions in adults.”
Height linked with health conditions
Genetically predicted height predicted a reduced risk of hyperlipidemia and hypertension independent of coronary heart disease, the analysis revealed. Genetically predicted height was also linked to an approximately 51% increased risk of atrial fibrillation in participants without coronary heart disease but, paradoxically, only a 39% increased risk in those with coronary heart disease, despite coronary heart disease being a risk factor for atrial fibrillation. Genetically predicted height was also associated with a greater risk of varicose veins in the legs and deep vein thrombosis.
Another novel association uncovered by the analysis was between women’s genetically predicted height and both asthma and nonspecific peripheral nerve disorders. “Whether these associations reflect differences by sex in disease pathophysiology related to height may warrant exploration in a sample with better balance between men and women,” the authors wrote. “In sum, our results suggest that an individual’s height may warrant consideration as a nonmodifiable predictor for several common conditions, particularly those affecting peripheral/distal extremities that are most physically impacted by tall stature.”
A substantial limitation of the study was its homogeneity of participants, who were 92% male with an average height of 176 cm and an average BMI of 30.1. The Black participants tended to be younger, with an average age of 58 compared with 64 years in the White participants, but the groups were otherwise similar in height and weight.* The database included data from Hispanic participants, but the researchers excluded these data because of the small sample size.
The smaller dataset for Black participants was a limitation as well as the fact that the genome-wide association study the researchers relied on came from a European population, which may not be as accurate in people with other ancestry, Dr. Wilson said. The bigger limitation, however, is what the findings’ clinical relevance is.
What does it all mean?
“Genetic height is in your genes – there is nothing to be done about it – so it is more of academic interest than clinical interest,” Dr. Wilson said. It’s not even clear whether incorporating a person’s height – actual or genetically predicted, if it could be easily determined for each person – into risk calculators. ”To know whether it would be beneficial to use height (or genetic height) as a risk factor, you’d need to examine each condition of interest, adjusting for all known risk factors, to see if height improved the prediction,” Dr. Wilson said. “I suspect for most conditions, the well-known risk factors would swamp height. For example, high genetic height might truly increase risk for neuropathy. But diabetes might increase the risk so much more that height is not particularly relevant.”
On the other hand, the fact that height in general has any potential influence at all on disease risk may inspire physicians to consider other risk factors in especially tall individuals.
”Physicians may find it interesting that we have some confirmation that height does increase the risk of certain conditions,” Dr. Wilson said. “While this is unlikely to dramatically change practice, they may be a bit more diligent in looking for other relevant risk factors for the diseases found in this study in their very tall patients.”
The research was funded by the U.S. Department of Veteran Affairs, the Boettcher Foundation’s Webb-Waring Biomedical Research Program, the National Institutes of Health, and a Linda Pechenik Montague Investigator award. One study coauthor is a full-time employee of Novartis Institutes of Biomedical Research. The other authors and Dr. Wilson had no disclosures.
*Correction, 6/29/22: An earlier version of this article misstated the average age of Black participants.
FROM PLOS GENETICS
Biologics, Women, and Pregnancy: What’s Known?
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
FDA approves dupilumab for children with eczema aged 6 months to 5 years
The whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The approval, announced on June 7, 2022, makes dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, the first biologic available in the United States to treat uncontrolled moderate to severe atopic dermatitis in this age group. In this age group, it is administered subcutaneously every 4 weeks. Dupilumab remains the only biologic treatment approved for patients aged 6 years and older for this indication.
Approval was based on data from a 16-week pivotal phase 3 trial that evaluated the efficacy and safety of dupilumab added to standard of care topical corticosteroids (TCS) in children aged 6 months to 5 years with uncontrolled moderate to severe atopic dermatitis. The trial’s principal investigator, Amy S. Paller, MD, professor and chair of dermatology at Northwestern University, Chicago, and colleagues, found that, at 16 weeks, 28% of patients who were treated with dupilumab, added to low-potency TCS, met the primary endpoint of clear or almost clear skin, compared with 4% of those who received low-potency TCS alone (P < .0001).
In addition, patients who received the combined treatment experienced a 70% average improvement in disease severity from baseline, compared with a 20% improvement among those in the TCS-only group (P < .0001). They also experienced a 49% improvement in itch, compared with a 2% improvement among their counterparts in the TCS-only group (P < .0001).
Outside of the United States, the study’s coprimary endpoint was achievement of 75% or greater improvement in overall disease severity. More than half of the patients who received combined treatment (53%) met this endpoint, compared with 11% in the TCS-only group (P < .0001), according to the company.
Safety results were generally consistent with the safety profile of dupilumab in atopic dermatitis for patients aged 6 years and older. The most common adverse events that were more commonly observed with dupilumab included conjunctivitis (5% vs 0% in the placebo group) and herpes viral infections (6% vs. 5% in the placebo group). Among those on dupilumab, ages 6 months to 5 years, hand,foot, and mouth disease was reported in 5% and skin papilloma were reported in 2%, but these cases did not lead to discontinuation of treatment, according to the company release.
A version of this article first appeared on Medscape.com.
The whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The approval, announced on June 7, 2022, makes dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, the first biologic available in the United States to treat uncontrolled moderate to severe atopic dermatitis in this age group. In this age group, it is administered subcutaneously every 4 weeks. Dupilumab remains the only biologic treatment approved for patients aged 6 years and older for this indication.
Approval was based on data from a 16-week pivotal phase 3 trial that evaluated the efficacy and safety of dupilumab added to standard of care topical corticosteroids (TCS) in children aged 6 months to 5 years with uncontrolled moderate to severe atopic dermatitis. The trial’s principal investigator, Amy S. Paller, MD, professor and chair of dermatology at Northwestern University, Chicago, and colleagues, found that, at 16 weeks, 28% of patients who were treated with dupilumab, added to low-potency TCS, met the primary endpoint of clear or almost clear skin, compared with 4% of those who received low-potency TCS alone (P < .0001).
In addition, patients who received the combined treatment experienced a 70% average improvement in disease severity from baseline, compared with a 20% improvement among those in the TCS-only group (P < .0001). They also experienced a 49% improvement in itch, compared with a 2% improvement among their counterparts in the TCS-only group (P < .0001).
Outside of the United States, the study’s coprimary endpoint was achievement of 75% or greater improvement in overall disease severity. More than half of the patients who received combined treatment (53%) met this endpoint, compared with 11% in the TCS-only group (P < .0001), according to the company.
Safety results were generally consistent with the safety profile of dupilumab in atopic dermatitis for patients aged 6 years and older. The most common adverse events that were more commonly observed with dupilumab included conjunctivitis (5% vs 0% in the placebo group) and herpes viral infections (6% vs. 5% in the placebo group). Among those on dupilumab, ages 6 months to 5 years, hand,foot, and mouth disease was reported in 5% and skin papilloma were reported in 2%, but these cases did not lead to discontinuation of treatment, according to the company release.
A version of this article first appeared on Medscape.com.
The whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The approval, announced on June 7, 2022, makes dupilumab (Dupixent), an interleukin-4 receptor alpha antagonist, the first biologic available in the United States to treat uncontrolled moderate to severe atopic dermatitis in this age group. In this age group, it is administered subcutaneously every 4 weeks. Dupilumab remains the only biologic treatment approved for patients aged 6 years and older for this indication.
Approval was based on data from a 16-week pivotal phase 3 trial that evaluated the efficacy and safety of dupilumab added to standard of care topical corticosteroids (TCS) in children aged 6 months to 5 years with uncontrolled moderate to severe atopic dermatitis. The trial’s principal investigator, Amy S. Paller, MD, professor and chair of dermatology at Northwestern University, Chicago, and colleagues, found that, at 16 weeks, 28% of patients who were treated with dupilumab, added to low-potency TCS, met the primary endpoint of clear or almost clear skin, compared with 4% of those who received low-potency TCS alone (P < .0001).
In addition, patients who received the combined treatment experienced a 70% average improvement in disease severity from baseline, compared with a 20% improvement among those in the TCS-only group (P < .0001). They also experienced a 49% improvement in itch, compared with a 2% improvement among their counterparts in the TCS-only group (P < .0001).
Outside of the United States, the study’s coprimary endpoint was achievement of 75% or greater improvement in overall disease severity. More than half of the patients who received combined treatment (53%) met this endpoint, compared with 11% in the TCS-only group (P < .0001), according to the company.
Safety results were generally consistent with the safety profile of dupilumab in atopic dermatitis for patients aged 6 years and older. The most common adverse events that were more commonly observed with dupilumab included conjunctivitis (5% vs 0% in the placebo group) and herpes viral infections (6% vs. 5% in the placebo group). Among those on dupilumab, ages 6 months to 5 years, hand,foot, and mouth disease was reported in 5% and skin papilloma were reported in 2%, but these cases did not lead to discontinuation of treatment, according to the company release.
A version of this article first appeared on Medscape.com.
Is benzophenone safe in skin care? Part 2: Environmental effects
Although it has been . DiNardo and Downs point out that BP-3 has been linked to contact and photocontact allergies in humans and implicated as a potential endocrine disruptor. They add that it can yield deleterious by-products when reacting with chlorine in swimming pools and wastewater treatment plants and can cause additional side effects in humans who ingest fish.1 This column will focus on recent studies, mainly on the role of benzophenones in sunscreen agents that pose considerable risks to waterways and marine life, with concomitant effects on the food chain.
Environmental effects of BPs and legislative responses
Various UV filters, including BP-3, octinoxate, octocrylene, and ethylhexyl salicylate, are thought to pose considerable peril to the marine environment.2,3 In particular, BP-3 has been demonstrated to provoke coral reef bleaching in vitro, leading to ossification and deforming DNA in the larval stage.3,4
According to a 2018 report, BP-3 is believed to be present in approximately two thirds of organic sunscreens used in the United States.3 In addition, several studies have revealed that detectable levels of organic sunscreen ingredients, including BP-3, have been identified in coastal waters around the globe, including Hawaii and the U.S. Virgin Islands.4-8
A surfeit of tourists has been blamed in part, given that an estimated 25% of applied sunscreen is eliminated within 20 minutes of entering the water and thought to release about 4,000-6,000 tons/year into the surrounding coral reefs.9,10 In Hawaii in particular, sewage contamination of the waterways has resulted from wastewater treatment facilities ill-equipped to filter out organic substances such as BP-3 and octinoxate.10,11 In light of such circumstances, the use of sunscreens containing BP-3 and octinoxate have been restricted in Hawaii, particularly in proximity to beaches, since Jan. 1, 2021, because of their apparent environmental impact.10
The exposure of coral to these compounds is believed to result in bleaching because of impaired membrane integrity and photosynthetic pigment loss in the zooxanthellae that coral releases.9,10 Coral and the algae zooxanthellae have a symbiotic relationship, Siller et al. explain, with the coral delivering protection and components essential for photosynthesis and the algae ultimately serving as nutrients for the coral.10 Stress endured by coral is believed to cause algae to detach, rendering coral more vulnerable to disease and less viable overall.10
In 2016, Downs et al. showed that four out of five sampled locations had detectable levels of BP-3 (100 pp trillion) with a fifth tested site measured at 19.2 pp billion.4
In 2019, Sirois acknowledges the problem of coral bleaching around the world but speculates that banning sunscreen ingredients for this purpose will delude people that such a measure will reverse the decline of coral and may lead to the unintended consequence of lower use of sunscreens. Sirois adds that a more comprehensive investigation of the multiple causes of coral reef bleaching is warranted, as are deeper examinations of studies using higher concentrations of sunscreen ingredients in artificial conditions.12
In the same year, Raffa et al. discussed the impending ban in Hawaii of the two sunscreen ingredients (BP-3 and octinoxate) to help preserve coral reefs. In so doing, they detailed the natural and human-induced harm to coral reefs, including pollution, fishing practices, overall impact of global climate change, and alterations in ocean temperature and chemistry. The implication is that sunscreen ingredients, which help prevent sun damage in users, are not the only causes of harm to coral reefs. Nevertheless, they point out that concentration estimates and mechanism studies buttress the argument that sunscreen ingredients contribute to coral bleaching. Still, the ban in Hawaii is thought to be a trend. Opponents of the ban are concerned that human skin cancers will rise in such circumstances. Alternative chemical sunscreens are being investigated, and physical sunscreens have emerged as the go-to recommendation.13
Notably, oxybenzone has been virtually replaced in the European Union with other UV filters with broad-spectrum action, but the majority of such filters have not yet been approved for use in the United States by the Food and Drug Administration.3
Food chain implications
BP-3 and other UV filters have been investigated for their effects on fish and mammals. Schneider and Lim illustrate that BP-3 is among the frequently used organic UV filters (along with 4-methylbenzylidene camphor, octocrylene, and octinoxate [ethylhexyl methoxycinnamate]) found in most water sources in the world, as well as multiple fish species.2 Cod liver in Norway, for instance, was found to contain octocrylene in 80% of cod, with BP-3 identified in 50% of the sample. BP-3 and octinoxate were also found in white fish.2,14 In laboratory studies, BP-3 in particular has been found in high concentrations in rainbow trout and Japanese rice fish (medaka), causing reduced egg production and hatchlings in females and increased vitellogenin protein production in males, suggesting potential feminization.2,15
Schneider and Lim note that standard wastewater treatment approaches cannot address this issue and the presence of such contaminants in fish can pose dangerous ramifications in the food chain. They assert that, despite relatively low concentrations in the fish, bioaccumulation and biomagnification present the potential for chemicals accumulating over time and becoming more deleterious as such ingredients travel up the food chain. As higher-chain organisms absorb higher concentrations of the chemicals not broken down in the lower-chain organisms, though, there have not yet been reports of adverse effects of biomagnification in humans.2
BP-3 has been found by Brausch and Rand to have bioaccumulated in fish at higher levels than the ambient water, however.1,2,16 Schneider and Lim present these issues as relevant to the sun protection discussion, while advocating for dermatologists to continue to counsel wise sun-protective behaviors.2
Conclusion
While calls for additional research are necessary and encouraging, I think human, and likely environmental, health would be better protected by the use of inorganic sunscreens in general and near or in coastal waterways. In light of legislative actions, in particular, it is important for dermatologists to intervene to ensure that patients do not engage in riskier behaviors in the sun in areas facing imminent organic sunscreen bans.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. DiNardo JC and Downs CA. J Cosmet Dermatol. 2018 Feb;17(1):15-9.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Downs CA et al. Arch Environ Contam Toxicol 2016 Feb;70(2):265-88.
5. Sánchez Rodríguez A et al. Chemosphere. 2015 Jul;131:85-90.
6. Tovar-Sánchez A et al. PLoS One. 2013 Jun 5;8(6):e65451.
7. Danovaro R and Corinaldesi C. Microb Ecol. 2003 Feb;45(2):109-18.
8. Daughton CG and Ternes TA. Environ Health Perspect. 1999 Dec;107 Suppl 6:907-38.
9. Danovaro R et al. Environ Health Perspect. 2008 Apr;116(4):441-7.
10. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
11. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
12. Sirois J. Sci Total Environ. 2019 Jul 15;674:211-2.
13. Raffa RB et al. J Clin Pharm Ther. 2019 Feb;44(1):134-9.
14. Langford KH et al. Environ Int. 2015 Jul;80:1-7.
15. Coronado M et al. Aquat Toxicol. 2008 Nov 21;90(3):182-7.
16. Brausch JM and Rand GM. Chemosphere. 2011 Mar;82(11):1518-32.
Although it has been . DiNardo and Downs point out that BP-3 has been linked to contact and photocontact allergies in humans and implicated as a potential endocrine disruptor. They add that it can yield deleterious by-products when reacting with chlorine in swimming pools and wastewater treatment plants and can cause additional side effects in humans who ingest fish.1 This column will focus on recent studies, mainly on the role of benzophenones in sunscreen agents that pose considerable risks to waterways and marine life, with concomitant effects on the food chain.
Environmental effects of BPs and legislative responses
Various UV filters, including BP-3, octinoxate, octocrylene, and ethylhexyl salicylate, are thought to pose considerable peril to the marine environment.2,3 In particular, BP-3 has been demonstrated to provoke coral reef bleaching in vitro, leading to ossification and deforming DNA in the larval stage.3,4
According to a 2018 report, BP-3 is believed to be present in approximately two thirds of organic sunscreens used in the United States.3 In addition, several studies have revealed that detectable levels of organic sunscreen ingredients, including BP-3, have been identified in coastal waters around the globe, including Hawaii and the U.S. Virgin Islands.4-8
A surfeit of tourists has been blamed in part, given that an estimated 25% of applied sunscreen is eliminated within 20 minutes of entering the water and thought to release about 4,000-6,000 tons/year into the surrounding coral reefs.9,10 In Hawaii in particular, sewage contamination of the waterways has resulted from wastewater treatment facilities ill-equipped to filter out organic substances such as BP-3 and octinoxate.10,11 In light of such circumstances, the use of sunscreens containing BP-3 and octinoxate have been restricted in Hawaii, particularly in proximity to beaches, since Jan. 1, 2021, because of their apparent environmental impact.10
The exposure of coral to these compounds is believed to result in bleaching because of impaired membrane integrity and photosynthetic pigment loss in the zooxanthellae that coral releases.9,10 Coral and the algae zooxanthellae have a symbiotic relationship, Siller et al. explain, with the coral delivering protection and components essential for photosynthesis and the algae ultimately serving as nutrients for the coral.10 Stress endured by coral is believed to cause algae to detach, rendering coral more vulnerable to disease and less viable overall.10
In 2016, Downs et al. showed that four out of five sampled locations had detectable levels of BP-3 (100 pp trillion) with a fifth tested site measured at 19.2 pp billion.4
In 2019, Sirois acknowledges the problem of coral bleaching around the world but speculates that banning sunscreen ingredients for this purpose will delude people that such a measure will reverse the decline of coral and may lead to the unintended consequence of lower use of sunscreens. Sirois adds that a more comprehensive investigation of the multiple causes of coral reef bleaching is warranted, as are deeper examinations of studies using higher concentrations of sunscreen ingredients in artificial conditions.12
In the same year, Raffa et al. discussed the impending ban in Hawaii of the two sunscreen ingredients (BP-3 and octinoxate) to help preserve coral reefs. In so doing, they detailed the natural and human-induced harm to coral reefs, including pollution, fishing practices, overall impact of global climate change, and alterations in ocean temperature and chemistry. The implication is that sunscreen ingredients, which help prevent sun damage in users, are not the only causes of harm to coral reefs. Nevertheless, they point out that concentration estimates and mechanism studies buttress the argument that sunscreen ingredients contribute to coral bleaching. Still, the ban in Hawaii is thought to be a trend. Opponents of the ban are concerned that human skin cancers will rise in such circumstances. Alternative chemical sunscreens are being investigated, and physical sunscreens have emerged as the go-to recommendation.13
Notably, oxybenzone has been virtually replaced in the European Union with other UV filters with broad-spectrum action, but the majority of such filters have not yet been approved for use in the United States by the Food and Drug Administration.3
Food chain implications
BP-3 and other UV filters have been investigated for their effects on fish and mammals. Schneider and Lim illustrate that BP-3 is among the frequently used organic UV filters (along with 4-methylbenzylidene camphor, octocrylene, and octinoxate [ethylhexyl methoxycinnamate]) found in most water sources in the world, as well as multiple fish species.2 Cod liver in Norway, for instance, was found to contain octocrylene in 80% of cod, with BP-3 identified in 50% of the sample. BP-3 and octinoxate were also found in white fish.2,14 In laboratory studies, BP-3 in particular has been found in high concentrations in rainbow trout and Japanese rice fish (medaka), causing reduced egg production and hatchlings in females and increased vitellogenin protein production in males, suggesting potential feminization.2,15
Schneider and Lim note that standard wastewater treatment approaches cannot address this issue and the presence of such contaminants in fish can pose dangerous ramifications in the food chain. They assert that, despite relatively low concentrations in the fish, bioaccumulation and biomagnification present the potential for chemicals accumulating over time and becoming more deleterious as such ingredients travel up the food chain. As higher-chain organisms absorb higher concentrations of the chemicals not broken down in the lower-chain organisms, though, there have not yet been reports of adverse effects of biomagnification in humans.2
BP-3 has been found by Brausch and Rand to have bioaccumulated in fish at higher levels than the ambient water, however.1,2,16 Schneider and Lim present these issues as relevant to the sun protection discussion, while advocating for dermatologists to continue to counsel wise sun-protective behaviors.2
Conclusion
While calls for additional research are necessary and encouraging, I think human, and likely environmental, health would be better protected by the use of inorganic sunscreens in general and near or in coastal waterways. In light of legislative actions, in particular, it is important for dermatologists to intervene to ensure that patients do not engage in riskier behaviors in the sun in areas facing imminent organic sunscreen bans.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. DiNardo JC and Downs CA. J Cosmet Dermatol. 2018 Feb;17(1):15-9.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Downs CA et al. Arch Environ Contam Toxicol 2016 Feb;70(2):265-88.
5. Sánchez Rodríguez A et al. Chemosphere. 2015 Jul;131:85-90.
6. Tovar-Sánchez A et al. PLoS One. 2013 Jun 5;8(6):e65451.
7. Danovaro R and Corinaldesi C. Microb Ecol. 2003 Feb;45(2):109-18.
8. Daughton CG and Ternes TA. Environ Health Perspect. 1999 Dec;107 Suppl 6:907-38.
9. Danovaro R et al. Environ Health Perspect. 2008 Apr;116(4):441-7.
10. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
11. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
12. Sirois J. Sci Total Environ. 2019 Jul 15;674:211-2.
13. Raffa RB et al. J Clin Pharm Ther. 2019 Feb;44(1):134-9.
14. Langford KH et al. Environ Int. 2015 Jul;80:1-7.
15. Coronado M et al. Aquat Toxicol. 2008 Nov 21;90(3):182-7.
16. Brausch JM and Rand GM. Chemosphere. 2011 Mar;82(11):1518-32.
Although it has been . DiNardo and Downs point out that BP-3 has been linked to contact and photocontact allergies in humans and implicated as a potential endocrine disruptor. They add that it can yield deleterious by-products when reacting with chlorine in swimming pools and wastewater treatment plants and can cause additional side effects in humans who ingest fish.1 This column will focus on recent studies, mainly on the role of benzophenones in sunscreen agents that pose considerable risks to waterways and marine life, with concomitant effects on the food chain.
Environmental effects of BPs and legislative responses
Various UV filters, including BP-3, octinoxate, octocrylene, and ethylhexyl salicylate, are thought to pose considerable peril to the marine environment.2,3 In particular, BP-3 has been demonstrated to provoke coral reef bleaching in vitro, leading to ossification and deforming DNA in the larval stage.3,4
According to a 2018 report, BP-3 is believed to be present in approximately two thirds of organic sunscreens used in the United States.3 In addition, several studies have revealed that detectable levels of organic sunscreen ingredients, including BP-3, have been identified in coastal waters around the globe, including Hawaii and the U.S. Virgin Islands.4-8
A surfeit of tourists has been blamed in part, given that an estimated 25% of applied sunscreen is eliminated within 20 minutes of entering the water and thought to release about 4,000-6,000 tons/year into the surrounding coral reefs.9,10 In Hawaii in particular, sewage contamination of the waterways has resulted from wastewater treatment facilities ill-equipped to filter out organic substances such as BP-3 and octinoxate.10,11 In light of such circumstances, the use of sunscreens containing BP-3 and octinoxate have been restricted in Hawaii, particularly in proximity to beaches, since Jan. 1, 2021, because of their apparent environmental impact.10
The exposure of coral to these compounds is believed to result in bleaching because of impaired membrane integrity and photosynthetic pigment loss in the zooxanthellae that coral releases.9,10 Coral and the algae zooxanthellae have a symbiotic relationship, Siller et al. explain, with the coral delivering protection and components essential for photosynthesis and the algae ultimately serving as nutrients for the coral.10 Stress endured by coral is believed to cause algae to detach, rendering coral more vulnerable to disease and less viable overall.10
In 2016, Downs et al. showed that four out of five sampled locations had detectable levels of BP-3 (100 pp trillion) with a fifth tested site measured at 19.2 pp billion.4
In 2019, Sirois acknowledges the problem of coral bleaching around the world but speculates that banning sunscreen ingredients for this purpose will delude people that such a measure will reverse the decline of coral and may lead to the unintended consequence of lower use of sunscreens. Sirois adds that a more comprehensive investigation of the multiple causes of coral reef bleaching is warranted, as are deeper examinations of studies using higher concentrations of sunscreen ingredients in artificial conditions.12
In the same year, Raffa et al. discussed the impending ban in Hawaii of the two sunscreen ingredients (BP-3 and octinoxate) to help preserve coral reefs. In so doing, they detailed the natural and human-induced harm to coral reefs, including pollution, fishing practices, overall impact of global climate change, and alterations in ocean temperature and chemistry. The implication is that sunscreen ingredients, which help prevent sun damage in users, are not the only causes of harm to coral reefs. Nevertheless, they point out that concentration estimates and mechanism studies buttress the argument that sunscreen ingredients contribute to coral bleaching. Still, the ban in Hawaii is thought to be a trend. Opponents of the ban are concerned that human skin cancers will rise in such circumstances. Alternative chemical sunscreens are being investigated, and physical sunscreens have emerged as the go-to recommendation.13
Notably, oxybenzone has been virtually replaced in the European Union with other UV filters with broad-spectrum action, but the majority of such filters have not yet been approved for use in the United States by the Food and Drug Administration.3
Food chain implications
BP-3 and other UV filters have been investigated for their effects on fish and mammals. Schneider and Lim illustrate that BP-3 is among the frequently used organic UV filters (along with 4-methylbenzylidene camphor, octocrylene, and octinoxate [ethylhexyl methoxycinnamate]) found in most water sources in the world, as well as multiple fish species.2 Cod liver in Norway, for instance, was found to contain octocrylene in 80% of cod, with BP-3 identified in 50% of the sample. BP-3 and octinoxate were also found in white fish.2,14 In laboratory studies, BP-3 in particular has been found in high concentrations in rainbow trout and Japanese rice fish (medaka), causing reduced egg production and hatchlings in females and increased vitellogenin protein production in males, suggesting potential feminization.2,15
Schneider and Lim note that standard wastewater treatment approaches cannot address this issue and the presence of such contaminants in fish can pose dangerous ramifications in the food chain. They assert that, despite relatively low concentrations in the fish, bioaccumulation and biomagnification present the potential for chemicals accumulating over time and becoming more deleterious as such ingredients travel up the food chain. As higher-chain organisms absorb higher concentrations of the chemicals not broken down in the lower-chain organisms, though, there have not yet been reports of adverse effects of biomagnification in humans.2
BP-3 has been found by Brausch and Rand to have bioaccumulated in fish at higher levels than the ambient water, however.1,2,16 Schneider and Lim present these issues as relevant to the sun protection discussion, while advocating for dermatologists to continue to counsel wise sun-protective behaviors.2
Conclusion
While calls for additional research are necessary and encouraging, I think human, and likely environmental, health would be better protected by the use of inorganic sunscreens in general and near or in coastal waterways. In light of legislative actions, in particular, it is important for dermatologists to intervene to ensure that patients do not engage in riskier behaviors in the sun in areas facing imminent organic sunscreen bans.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. DiNardo JC and Downs CA. J Cosmet Dermatol. 2018 Feb;17(1):15-9.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Downs CA et al. Arch Environ Contam Toxicol 2016 Feb;70(2):265-88.
5. Sánchez Rodríguez A et al. Chemosphere. 2015 Jul;131:85-90.
6. Tovar-Sánchez A et al. PLoS One. 2013 Jun 5;8(6):e65451.
7. Danovaro R and Corinaldesi C. Microb Ecol. 2003 Feb;45(2):109-18.
8. Daughton CG and Ternes TA. Environ Health Perspect. 1999 Dec;107 Suppl 6:907-38.
9. Danovaro R et al. Environ Health Perspect. 2008 Apr;116(4):441-7.
10. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
11. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
12. Sirois J. Sci Total Environ. 2019 Jul 15;674:211-2.
13. Raffa RB et al. J Clin Pharm Ther. 2019 Feb;44(1):134-9.
14. Langford KH et al. Environ Int. 2015 Jul;80:1-7.
15. Coronado M et al. Aquat Toxicol. 2008 Nov 21;90(3):182-7.
16. Brausch JM and Rand GM. Chemosphere. 2011 Mar;82(11):1518-32.
Surgical site infections not increased in immunocompromised patients after Mohs surgery
, suggesting that antibiotic prophylaxis, which is often used for these patients, may not be necessary, according to new research.
The retrospective cohort study found that “immunosuppressed patients had similar infection rates as immunocompetent patients following Mohs micrographic surgery,” first author Tuyet A. Nguyen, MD, of the department of dermatology, Cedars-Sinai Medical Center, Los Angeles, told this news organization.
“Therefore, antibiotic prescribing patterns should not change simply due to immunosuppression. Furthermore, immunosuppressed patients appear to respond well to antibiotics and recover similarly to immunocompetent patients,” she said.
The study was presented at the annual meeting of the American College of Mohs Surgery.
Mohs surgery is increasingly being performed for patients who are immunosuppressed because of the higher incidence of skin cancer in this group of patients and their higher risk of more aggressive skin cancers.
Overall, the rate of surgical site infections following Mohs surgery generally ranges from 0.5% to 2.4%. However, research is lacking on the risk among patients who are immunosuppressed and on how effective the use of prophylactic antibiotics is for these patients.
For the retrospective study, Dr. Nguyen and her colleagues evaluated data on 5,886 patients who underwent Mohs surgery at Cedars-Sinai between October 2014 and August 2021. Among these patients, 741 (12.6%) were immunocompromised.
Causes of immunosuppression in the cohort included the following: immunosuppression after transplant surgery; having HIV, chronic myeloid leukemia, multiple myeloma, or other hematogenous forms of immunosuppression; or immunosuppression related to other conditions, such as chronic inflammatory diseases.
Overall, postprocedural infections occurred in 1.6% (95) of patients, a rate that mirrors that of the general population, Dr. Nguyen noted. No significant differences in surgical site infection rates were observed between immunocompromised patients (2.1%, n = 15) and those who were immunocompetent (1.6%, n = 80; P = .30).
Importantly, among those who were immunocompromised, the rates of infection were not significantly different between those who did receive antibiotics (3.0%, n = 8) and those who did not receive antibiotics (1.5%, n = 7; P = .19).
The lack of a difference in surgical site infection rates among those who did and those who did not receive antibiotics extended to the entire study population (2.0% vs. 1.4%; P = .12).
The study cohort mainly comprised immunosuppressed transplant patients, notably, heart, lung, and kidney transplant patients. However, “even in this population, we did not see a higher rate of infection,” senior author Nima M. Gharavi, MD, PhD, director of dermatologic surgery and Mohs micrographic surgery and associate professor of medicine and pathology and laboratory medicine at Cedars-Sinai Medical Center, said in an interview.
Yet the risk of infection among those patients has been shown to be high and of consequence. Data indicate that infections account for 13%-16% of deaths among kidney and heart transplant patients and up to 21% of deaths among lung transplant patients. The rate of mortality appears to parallel the level of immunosuppression, Dr. Nguyen explained.
Furthermore, up to 25% of patients who undergo heart and lung transplantation develop bacteremia.
In terms of why worse infections or bacteremia surgeries may not occur in association with Mohs, Dr. Nguyen speculated that, as opposed to other surgeries, those involving the skin may benefit from unique defense mechanisms.
“The skin is a complex system in its defense against foreign pathogens and infectious agents,” she explained during her presentation. “There is the physical barrier, the antimicrobial peptides, and an adaptive as well as innate immune response.”
“In immunosuppressed patients, with the decrease in adaptive immunity, it’s possible this loss is less important because the skin has such a robust immune system in general.”
In her presentation, Dr. Nguyen noted that “further studies are necessary to investigate why patients aren’t presenting with greater severity, and we plan to try to investigate whether the unique nature of skin-mediated immunity makes this organ less susceptible to severe or life-threatening infections in patients on immunosuppression.”
Of note, the rate of prophylactic antibiotic prescriptions was no higher for those who were and those who were not immunosuppressed (37.9% vs. 34.1%; P = .14), which Dr. Nguyen said is consistent with recommendations.
“Immunosuppression is not an indication for antibiotic use, and hence, we did not have a higher rate of antibiotics use in this population,” she told this news organization. However, a 2021 ACMS survey found that a high percentage of Mohs surgeons prescribe antibiotics for procedures in which antibiotics are not indicated so as to reduce the risk of infections and that immunosuppression is a common reason for doing so.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggesting that antibiotic prophylaxis, which is often used for these patients, may not be necessary, according to new research.
The retrospective cohort study found that “immunosuppressed patients had similar infection rates as immunocompetent patients following Mohs micrographic surgery,” first author Tuyet A. Nguyen, MD, of the department of dermatology, Cedars-Sinai Medical Center, Los Angeles, told this news organization.
“Therefore, antibiotic prescribing patterns should not change simply due to immunosuppression. Furthermore, immunosuppressed patients appear to respond well to antibiotics and recover similarly to immunocompetent patients,” she said.
The study was presented at the annual meeting of the American College of Mohs Surgery.
Mohs surgery is increasingly being performed for patients who are immunosuppressed because of the higher incidence of skin cancer in this group of patients and their higher risk of more aggressive skin cancers.
Overall, the rate of surgical site infections following Mohs surgery generally ranges from 0.5% to 2.4%. However, research is lacking on the risk among patients who are immunosuppressed and on how effective the use of prophylactic antibiotics is for these patients.
For the retrospective study, Dr. Nguyen and her colleagues evaluated data on 5,886 patients who underwent Mohs surgery at Cedars-Sinai between October 2014 and August 2021. Among these patients, 741 (12.6%) were immunocompromised.
Causes of immunosuppression in the cohort included the following: immunosuppression after transplant surgery; having HIV, chronic myeloid leukemia, multiple myeloma, or other hematogenous forms of immunosuppression; or immunosuppression related to other conditions, such as chronic inflammatory diseases.
Overall, postprocedural infections occurred in 1.6% (95) of patients, a rate that mirrors that of the general population, Dr. Nguyen noted. No significant differences in surgical site infection rates were observed between immunocompromised patients (2.1%, n = 15) and those who were immunocompetent (1.6%, n = 80; P = .30).
Importantly, among those who were immunocompromised, the rates of infection were not significantly different between those who did receive antibiotics (3.0%, n = 8) and those who did not receive antibiotics (1.5%, n = 7; P = .19).
The lack of a difference in surgical site infection rates among those who did and those who did not receive antibiotics extended to the entire study population (2.0% vs. 1.4%; P = .12).
The study cohort mainly comprised immunosuppressed transplant patients, notably, heart, lung, and kidney transplant patients. However, “even in this population, we did not see a higher rate of infection,” senior author Nima M. Gharavi, MD, PhD, director of dermatologic surgery and Mohs micrographic surgery and associate professor of medicine and pathology and laboratory medicine at Cedars-Sinai Medical Center, said in an interview.
Yet the risk of infection among those patients has been shown to be high and of consequence. Data indicate that infections account for 13%-16% of deaths among kidney and heart transplant patients and up to 21% of deaths among lung transplant patients. The rate of mortality appears to parallel the level of immunosuppression, Dr. Nguyen explained.
Furthermore, up to 25% of patients who undergo heart and lung transplantation develop bacteremia.
In terms of why worse infections or bacteremia surgeries may not occur in association with Mohs, Dr. Nguyen speculated that, as opposed to other surgeries, those involving the skin may benefit from unique defense mechanisms.
“The skin is a complex system in its defense against foreign pathogens and infectious agents,” she explained during her presentation. “There is the physical barrier, the antimicrobial peptides, and an adaptive as well as innate immune response.”
“In immunosuppressed patients, with the decrease in adaptive immunity, it’s possible this loss is less important because the skin has such a robust immune system in general.”
In her presentation, Dr. Nguyen noted that “further studies are necessary to investigate why patients aren’t presenting with greater severity, and we plan to try to investigate whether the unique nature of skin-mediated immunity makes this organ less susceptible to severe or life-threatening infections in patients on immunosuppression.”
Of note, the rate of prophylactic antibiotic prescriptions was no higher for those who were and those who were not immunosuppressed (37.9% vs. 34.1%; P = .14), which Dr. Nguyen said is consistent with recommendations.
“Immunosuppression is not an indication for antibiotic use, and hence, we did not have a higher rate of antibiotics use in this population,” she told this news organization. However, a 2021 ACMS survey found that a high percentage of Mohs surgeons prescribe antibiotics for procedures in which antibiotics are not indicated so as to reduce the risk of infections and that immunosuppression is a common reason for doing so.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, suggesting that antibiotic prophylaxis, which is often used for these patients, may not be necessary, according to new research.
The retrospective cohort study found that “immunosuppressed patients had similar infection rates as immunocompetent patients following Mohs micrographic surgery,” first author Tuyet A. Nguyen, MD, of the department of dermatology, Cedars-Sinai Medical Center, Los Angeles, told this news organization.
“Therefore, antibiotic prescribing patterns should not change simply due to immunosuppression. Furthermore, immunosuppressed patients appear to respond well to antibiotics and recover similarly to immunocompetent patients,” she said.
The study was presented at the annual meeting of the American College of Mohs Surgery.
Mohs surgery is increasingly being performed for patients who are immunosuppressed because of the higher incidence of skin cancer in this group of patients and their higher risk of more aggressive skin cancers.
Overall, the rate of surgical site infections following Mohs surgery generally ranges from 0.5% to 2.4%. However, research is lacking on the risk among patients who are immunosuppressed and on how effective the use of prophylactic antibiotics is for these patients.
For the retrospective study, Dr. Nguyen and her colleagues evaluated data on 5,886 patients who underwent Mohs surgery at Cedars-Sinai between October 2014 and August 2021. Among these patients, 741 (12.6%) were immunocompromised.
Causes of immunosuppression in the cohort included the following: immunosuppression after transplant surgery; having HIV, chronic myeloid leukemia, multiple myeloma, or other hematogenous forms of immunosuppression; or immunosuppression related to other conditions, such as chronic inflammatory diseases.
Overall, postprocedural infections occurred in 1.6% (95) of patients, a rate that mirrors that of the general population, Dr. Nguyen noted. No significant differences in surgical site infection rates were observed between immunocompromised patients (2.1%, n = 15) and those who were immunocompetent (1.6%, n = 80; P = .30).
Importantly, among those who were immunocompromised, the rates of infection were not significantly different between those who did receive antibiotics (3.0%, n = 8) and those who did not receive antibiotics (1.5%, n = 7; P = .19).
The lack of a difference in surgical site infection rates among those who did and those who did not receive antibiotics extended to the entire study population (2.0% vs. 1.4%; P = .12).
The study cohort mainly comprised immunosuppressed transplant patients, notably, heart, lung, and kidney transplant patients. However, “even in this population, we did not see a higher rate of infection,” senior author Nima M. Gharavi, MD, PhD, director of dermatologic surgery and Mohs micrographic surgery and associate professor of medicine and pathology and laboratory medicine at Cedars-Sinai Medical Center, said in an interview.
Yet the risk of infection among those patients has been shown to be high and of consequence. Data indicate that infections account for 13%-16% of deaths among kidney and heart transplant patients and up to 21% of deaths among lung transplant patients. The rate of mortality appears to parallel the level of immunosuppression, Dr. Nguyen explained.
Furthermore, up to 25% of patients who undergo heart and lung transplantation develop bacteremia.
In terms of why worse infections or bacteremia surgeries may not occur in association with Mohs, Dr. Nguyen speculated that, as opposed to other surgeries, those involving the skin may benefit from unique defense mechanisms.
“The skin is a complex system in its defense against foreign pathogens and infectious agents,” she explained during her presentation. “There is the physical barrier, the antimicrobial peptides, and an adaptive as well as innate immune response.”
“In immunosuppressed patients, with the decrease in adaptive immunity, it’s possible this loss is less important because the skin has such a robust immune system in general.”
In her presentation, Dr. Nguyen noted that “further studies are necessary to investigate why patients aren’t presenting with greater severity, and we plan to try to investigate whether the unique nature of skin-mediated immunity makes this organ less susceptible to severe or life-threatening infections in patients on immunosuppression.”
Of note, the rate of prophylactic antibiotic prescriptions was no higher for those who were and those who were not immunosuppressed (37.9% vs. 34.1%; P = .14), which Dr. Nguyen said is consistent with recommendations.
“Immunosuppression is not an indication for antibiotic use, and hence, we did not have a higher rate of antibiotics use in this population,” she told this news organization. However, a 2021 ACMS survey found that a high percentage of Mohs surgeons prescribe antibiotics for procedures in which antibiotics are not indicated so as to reduce the risk of infections and that immunosuppression is a common reason for doing so.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACMS ANNUAL MEETING











