User login
Disparities of Cutaneous Malignancies in the US Military
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
Occupational sun exposure is a well-known risk factor for the development of melanoma and nonmelanoma skin cancer (NMSC). In addition to sun exposure, US military personnel may face other risk factors such as lack of access to adequate sun protection, work in equatorial latitudes, and increased exposure to carcinogens. In one study, fewer than 30% of surveyed soldiers reported regular sunscreen use during deployment and reported the face, neck, and upper extremities were unprotected at least 70% of the time.1 Skin cancer risk factors that are more common in military service members include inadequate sunscreen access, insufficient sun protection, harsh weather conditions, more immediate safety concerns than sun protection, and male gender. A higher incidence of melanoma and NMSC has been correlated with the more common demographics of US veterans such as male sex, older age, and White race.2
Although not uncommon in both civilian and military populations, we present the case of a military service member who developed skin cancer at an early age potentially due to occupational sun exposure. We also provide a review of the literature to examine the risk factors and incidence of melanoma and NMSC in US military personnel and veterans and provide recommendations for skin cancer prevention, screening, and intervention in the military population.
Case Report
A 37-year-old White active-duty male service member in the US Navy (USN) presented with a nonhealing lesion on the nose of 2 years’ duration that had been gradually growing and bleeding for several weeks. He participated in several sea deployments while onboard a naval destroyer over his 10-year military career. He did not routinely use sunscreen during his deployments. His personal and family medical history lacked risk factors for skin cancer other than his skin tone and frequent sun exposure.
Physical examination revealed a 1-cm ulcerated plaque with rolled borders and prominent telangiectases on the mid nasal dorsum. A shave biopsy was performed to confirm the diagnosis of nodular basal cell carcinoma (BCC). The patient underwent Mohs micrographic surgery, which required repair with an advancement flap. He currently continues his active-duty service and is preparing for his next overseas deployment.
Literature Review
We conducted a review of PubMed articles indexed for MEDLINE using the search terms skin cancer, melanoma, nonmelanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, Merkel cell carcinoma, dermatofibrosarcoma protuberans, or sebaceous carcinoma along with military, Army, Navy, Air Force, or veterans. Studies from January 1984 to April 2020 were included in our qualitative review. All articles were reviewed, and those that did not examine skin cancer and the military population in the United States were excluded. Relevant data, such as results of skin cancer incidence or risk factors or insights about developing skin cancer in this affected population, were extracted from the selected publications.
Several studies showed overall increased age-adjusted incidence rates of melanoma and NMSC among military service personnel compared to age-matched controls in the general population.2 A survey of draft-age men during World War II found a slightly higher percentage of respondents with history of melanoma compared to the control group (83% [74/89] vs 76% [49/65]). Of those who had a history of melanoma, 34% (30/89) served in the tropics compared to 6% (4/65) in the control group.3 A tumor registry review found the age-adjusted melanoma incidence rates per 100,000 person-years for White individuals in the military vs the general population was 33.6 vs 27.5 among those aged 45 to 49 years, 49.8 vs 32.2 among those aged 50 to 54 years, and 178.5 vs 39.2 among those aged 55 to 59 years.4 Among published literature reviews, members of the US Air Force (USAF) had the highest rates of melanoma compared to other military branches, with an incidence rate of 7.6 vs 6.3 among USAF males vs Army males and 9.0 vs 5.5 among USAF females vs Army females.4 These findings were further supported by another study showing a higher incidence rate of melanoma in USAF members compared to Army personnel (17.8 vs 9.5) and a 62% greater melanoma incidence in active-duty military personnel compared to the general population when adjusted for age, race, sex, and year of diagnosis.5 Additionally, a meta-analysis reported a standardized incidence ratio of 1.4 (95% CI, 1.1-1.9) for malignant melanoma and 1.8 (95% CI, 1.3-2.8) for NMSC among military pilots compared to the general population.6 It is important to note that these data are limited to published peer-reviewed studies within PubMed and may not reflect the true skin cancer incidence.
More comprehensive studies are needed to compare NMSC incidence rates in nonpilot military populations compared to the general population. From 2005 to 2014, the average annual NMSC incidence rate in the USAF was 64.4 per 100,000 person-years, with the highest rate at 97.4 per 100,000 person-years in 2007.7 However, this study did not directly compare military service members to the general population. Service in tropical environments among World War II veterans was associated with an increased risk for NMSC. Sixty-six percent of patients with BCC (n=197) and 68% with squamous cell carcinoma (SCC)(n=41) were stationed in the Pacific, despite the number and demographics of soldiers deployed to the Pacific and Europe being approximately equal.8 During a 6-month period in 2008, a Combat Dermatology Clinic in Iraq showed 5% (n=129) of visits were for treatment of actinic keratoses (AKs), while 8% of visits (n=205) were related to skin cancer, including BCC, SCC, mycosis fungoides, and melanoma.9 Overall, these studies confirm a higher rate of melanoma in military service members vs the general population and indicate USAF members may be at the greatest risk for developing melanoma and NMSC among the service branches. Further studies are needed to elucidate why this might be the case and should concentrate on demographics, service locations, uniform wear and personal protective equipment standards, and use of sun-protective measures across each service branch.
Our search yielded no aggregate studies to determine if there is an increased rate of other types of skin cancer in military service members such as Merkel cell carcinoma, dermatofibrosarcoma protuberans, and microcystic adnexal carcinoma (MAC). Gerall et al10 described a case of MAC in a 43-year-old USAF U-2 pilot with a 15-year history of a slow-growing soft-tissue nodule on the cheek. The patient’s young age differed from the typical age of MAC occurrence (ie, 60–70 years), which led to the possibility that his profession contributed to the development of MAC and the relatively young age of onset.10
Etiology of Disease
The results of our literature review indicated that skin cancers are more prevalent among active-duty military personnel and veterans than in the general population; they also suggest that frequent sun exposure and lack of sun protection may be key etiologic factors. In 2015, only 23% of veterans (n=49) reported receiving skin cancer awareness education from the US Military.1 Among soldiers returning from Iraq and Afghanistan (n=212), only 13% reported routine sunscreen use, and
Exposure to UV radiation at higher altitudes (with corresponding higher UV energy) and altered sleep-wake cycles (with resulting altered immune defenses) may contribute to higher rates of melanoma and NMSC among USAF pilots.11 During a 57-minute flight at 30,000-ft altitude, a pilot is exposed to a UVA dose equivalent to 20 minutes inside a tanning booth.12 Although UVB transmission through plastic and glass windshields was reported to be less than 1%, UVA transmission ranged from 0.4% to 53.5%. The UVA dose for a pilot flying a light aircraft in Las Vegas, Nevada, was reported to be 127 μW/cm2 at ground level vs 242 μW/cm2 at a 30,000-ft altitude.12 Therefore, cosmic radiation exposure for military pilots is higher than for commercial pilots, as they fly at higher altitudes. U-2 pilots are exposed to 20 times the cosmic radiation dose at sea level and 10 times the exposure of commercial pilots.10
It currently is unknown why service in the USAF would increase skin cancer risk compared to service in other branches; however, there are some differences between military branches that require further research, including ethnic demographics, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure for each branch of service. Environmental exposures may differ based on the military branch gear requirements; for example, when on the flight line or flight deck, USN aircrews are required to wear cranials (helmets), eyewear (visor or goggles), and long-sleeved shirts. When at sea, USN flight crews wear gloves, headgear, goggles, pants, and long-sleeved shirts to identify their duty onboard. All of these measures offer good sun protection and are carried over to the land-based flight lines in the USN and Marine Corps. Neither the Army nor the USAF commonly utilize these practices. Conversely, the USAF does not allow flight line workers including fuelers, maintainers, and aircrew to wear coveralls due to the risk of being blown off, becoming foreign object debris, and being sucked into jet engines. However, in-flight protective gear such as goggles, gloves, and coveralls are worn.12 Perhaps the USAF may attract, recruit, or commission people with inherently more risk for skin cancer (eg, White individuals). How racial and ethnic factors may affect skin cancer incidence in military branches is an area for future research efforts.
Recommendations
Given the considerable increase in risk factors, efforts are needed to reduce the disparity in skin cancer rates between US military personnel and their civilian counterparts through appropriate prevention, screening, and intervention programs.
Prevention—In wartime settings as well as in training and other peacetime activities, active-duty military members cannot avoid harmful midday sun exposure. Additionally, application and reapplication of sunscreen can be challenging. Sunscreen, broad-spectrum lip balm, and wide-brimmed “boonie” hats can be ordered by supply personnel.13 We recommend that a standard sunscreen supply be available to all active-duty military service members. The long-sleeved, tightly woven fabric of military uniforms also can provide protection from the sun but can be difficult to tolerate for extended periods of time in warm climates. Breathable, lightweight, sun-protective clothing is commercially available and could be incorporated into military uniforms.
All service members should be educated about skin cancer risks while addressing common myths and inaccuracies. Fifty percent (n=50) of surveyed veterans thought discussions of skin cancer prevention and safety during basic training could help prevent skin cancer in service members.14 Suggestions from respondents included education about sun exposure consequences, use of graphic images of skin cancer in teaching, providing protective clothing and sunscreen to active-duty military service members, and discussion about sun protection with physicians during annual physicals. When veterans with a history of skin cancer were surveyed about their personal risk for skin cancer, most believed they were at little risk (average perceived risk response score, 2.2 out of 5 [1=no risk; 5=high risk]).14 The majority explained that they did not seek sun protection after warnings of skin cancer risk because they did not think skin cancer would happen to them,14 though the incidence of NMSC in the United States at the time of these surveys was estimated to be 3.5 million per year.14,15 Another study found that only 13% of veterans knew the back is the most common site of melanoma in men.1 The Army Public Health Center has informational fact sheets available online or in dermatologists’ offices that detail correct sunscreen application techniques and how to reduce sun exposure.16,17 However, military service members reported that they prefer physicians to communicate with them directly about skin cancer risks vs reading brochures in physician offices or gaining information from television, radio, military training, or the Internet (4.4 out 5 rating for communication methods of risks associated with skin cancer [1=ineffective; 5=very effective]).14 However, only 27% of nondermatologist physicians counseled or screened their patients on skin cancer or sunscreen yearly, 49% even less frequently, with 24% never counseling or screening at all. Because not all service members may be able to regularly see a dermatologist, efforts should be focused on increasing primary care physician awareness on counseling and screening.18
Early Detection—Military service members should be educated on how to perform skin self-examinations to alert their providers earlier to concerning lesions. The American Academy of Dermatology publishes infographics regarding the ABCDEs of melanoma and how to perform skin self-examinations.19,20 Although the US Preventive Services Task Force concluded there was insufficient evidence to recommend skin self-examination for all adults, the increased risk that military service members and veterans have requires further studies to examine the utility of self-screening in this population.20 Given the evidence of a higher incidence of melanoma in military service members vs the general population after 45 years of age,4 we recommend starting yearly in-person screenings performed by primary care physicians or dermatologists at this age. Ensuring every service member has routine in-office skin examinations can be difficult given the limited number of active-duty military dermatologists. Civilian dermatologists also could be helpful in this respect.
Teleconsultation, teledermoscopy, or store-and-forward imaging services for concerning lesions could be utilized when in-person consultations with a dermatologist are not feasible or cannot be performed in a timely manner. From 2004 to 2012, 40% of 10,817 teleconsultations were dermatology consultations from deployed or remote environments.21 Teleconsultation can be performed via email through the global military teleconsultation portal.22 These methods can lead to earlier detection of skin cancer rather than delaying evaluation for an in-person consultation.23
Intervention—High-risk patients who have been diagnosed with NMSC or many AKs should consider oral, procedural, or topical chemoprevention to reduce the risk for additional skin cancers as both primary and secondary prevention. In a double-blind, randomized, controlled trial of 386 individuals with a history of 2 or more NMSCs, participants were randomly assigned to receive either 500 mg of nicotinamide twice daily or placebo for 12 months. Compared to the placebo group, the nicotinamide group had a 23% lower rate of new NMSCs and an 11% lower rate of new AKs at 12 months.24 The use of acitretin also has been studied in transplant recipients for the chemoprevention of NMSC. In a double-blind, randomized, controlled trial of renal transplant recipients with more than 10 AKs randomized to receive either 30 mg/d of acitretin or placebo for 6 months, 11% of the acitretin group reported a new NMSC compared to 47% in the placebo group.25 An open-label study of 27 renal transplant recipients treated with methyl-esterified aminolevulinic acid–photodynamic therapy and red light demonstrated an increased mean time to occurrence of an AK, SCC, BCC, keratoacanthoma, or wart from 6.8 months in untreated areas compared to 9.6 months in treated areas.25 In active-duty locations where access to red and blue light sources is unavailable, the use of daylight photodynamic therapy can be considered, as it does not require any special equipment. Topical treatments such as 5-fluorouracil and imiquimod can be used for treatment and chemoprevention of NMSC. In a follow-up study from the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial, patients who applied 5-fluorouracil cream 5% twice daily to the face and ears for 4 weeks had a 75% risk reduction in developing SCC requiring surgery compared to the control group for the first year after treatment.26,27
Final Thoughts
Focusing on the efforts we propose can help the US Military expand their prevention, screening, and intervention programs for skin cancer in service members. Further research can then be performed to determine which programs have the greatest impact on rates of skin cancer among military and veteran personnel. Given these higher incidences and risk of exposure for skin cancer among service members, the various services may consider mandating sunscreen use as part of the uniform to prevent skin cancer. To maximize effectiveness, these efforts to prevent the development of skin cancer among military and veteran personnel should be adopted nationally.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Powers JG, Patel NA, Powers EM, et al. Skin cancer risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192.
- Brown J, Kopf AW, Rigel DS, et al. Malignant melanoma in World War II veterans. Int J Dermatol. 1984;23:661-663.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the U.S. Military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Ramani ML, Bennett RG. High prevalence of skin cancer in World War II servicemen stationed in the Pacific theater. J Am Acad Dermatol. 1993;28:733-737.
- Henning JS, Firoz, BF. Combat dermatology: the prevalence of skin disease in a deployed dermatology clinic in Iraq. J Drugs Dermatol. 2010;9:210-214.
- Gerall CD, Sippel MR, Yracheta JL, et al. Microcystic adnexal carcinoma: a rare, commonly misdiagnosed malignancy. Mil Med. 2019;184:948-950.
- Wilkison B, Wong E. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- Proctor SP, Heaton KJ, Smith KW, et al. The Occupational JP8 Neuroepidemiology Study (OJENES): repeated workday exposure and central nervous system functioning among US Air Force personnel. Neurotoxicology. 2011;32:799-808.
- Soldiers protect themselves from skin cancer. US Army website. Published February 28, 2019. Accessed August 21, 2022. https://www.army.mil/article/17601/soldiers_protect_themselves_from_skin_cancer
- Fisher V, Lee D, McGrath J, et al. Veterans speak up: current warnings on skin cancer miss the target, suggestions for improvement. Mil Med. 2015;180:892-897.
- Rogers HW, Weinstick MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Sun safety. Army Public Health Center website. Updated June 6, 2019. Accessed August 21, 2022. https://phc.amedd.army.mil/topics/discond/hipss/Pages/Sun-Safety.aspx
- Outdoor ultraviolet radiation hazards and protection. Army Public Health Center website. Accessed August 21, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/OutdoorUltravioletRadiationHazardsandProtection_FS_24-017-1115.pdf
- Saraiya M, Frank E, Elon L, et al. Personal and clinical skin cancer prevention practices of US women physicians. Arch Dermatol. 2000;136:633-642.
- What to look for: ABCDEs of melanoma. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/at-risk/abcdes
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology website. Accessed August 21, 2022. https://www.aad.org/public/diseases/skin-cancer/find/check-skin
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the US military in a deployed setting. Mil Med. 2014;179:1347-1353.
- Bartling SJ, Rivard SC, Meyerle JH. Melanoma in an active duty marine. Mil Med. 2017;182:2034-2039.
- Day WG, Shirvastava V, Roman JW. Synchronous teledermoscopy in military treatment facilities. Mil Med. 2020;185:1334-1337.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- Wulf HC, Pavel S, Stender I, et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol. 2006;86:25-28.
- Weinstock MA, Thwin SS, Siegel JA, et al; Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCC) Group. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
Practice Points
- Skin cancer is more prevalent among military personnel and veterans, especially those in the US Air Force. Frequent and/or prolonged sun exposure and lack of sun protection may be key factors.
- Future research should compare the prevalence of skin cancer in nonpilot military populations to the general US population; explore racial and ethnic differences by military branch and their influence on skin cancers; analyze each branch’s sun-protective measures, uniform wear and personal protective equipment standards, duty assignment locations, and the hours the military members are asked to work outside with direct sunlight exposure; and explore the effects of appropriate military skin cancer intervention and screening programs.
Association of BRAF V600E Status of Incident Melanoma and Risk for a Second Primary Malignancy: A Population-Based Study
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results
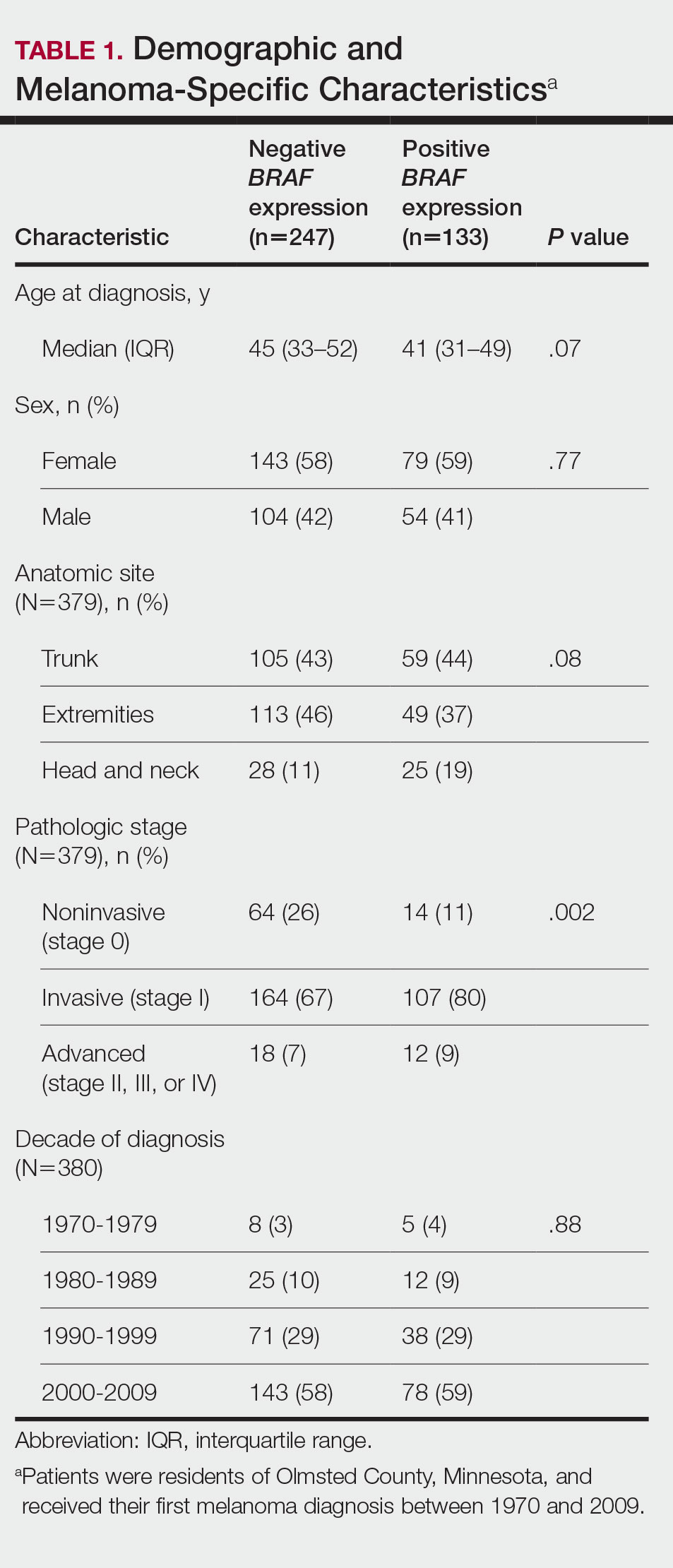
Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
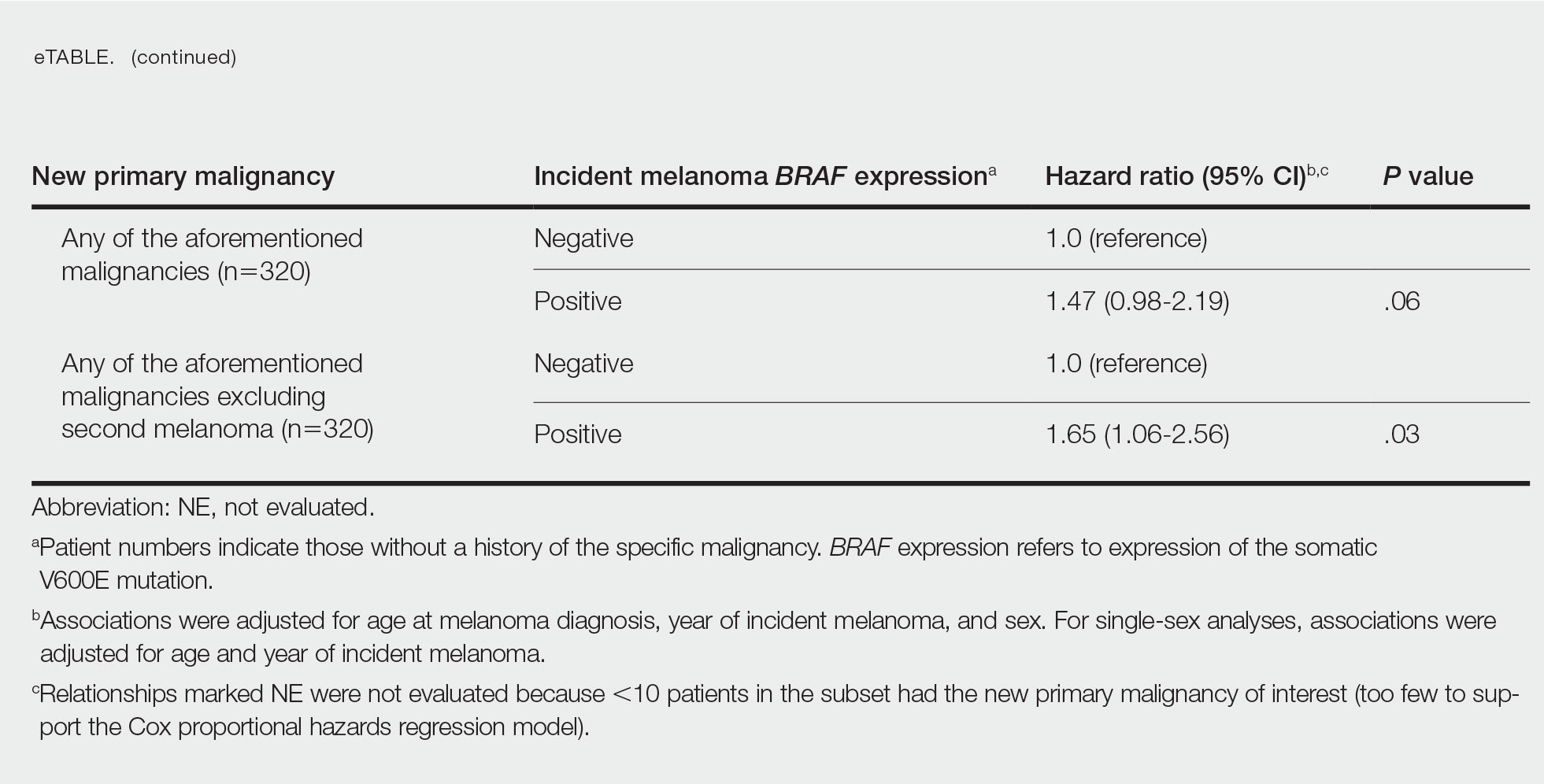
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.
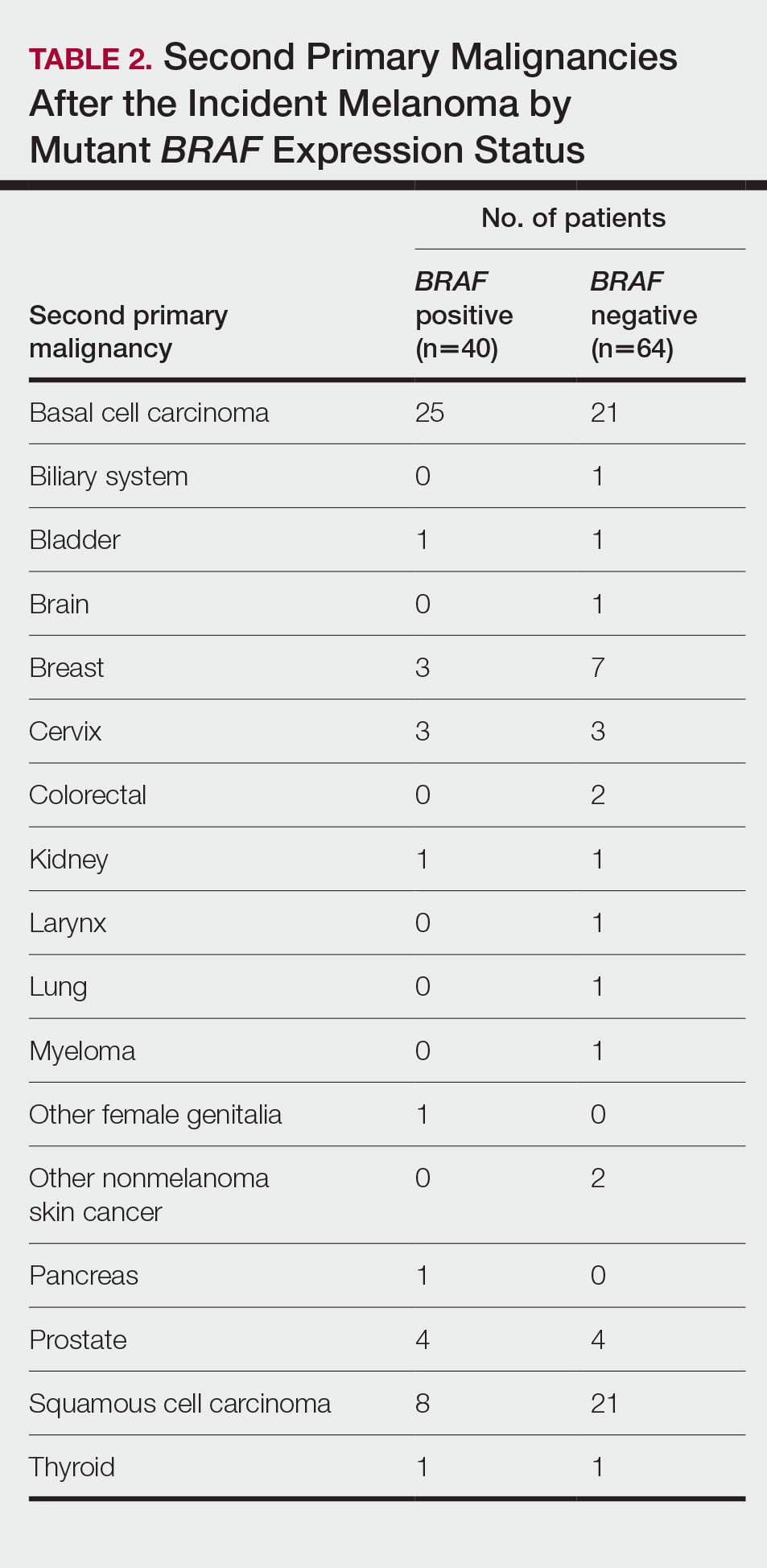
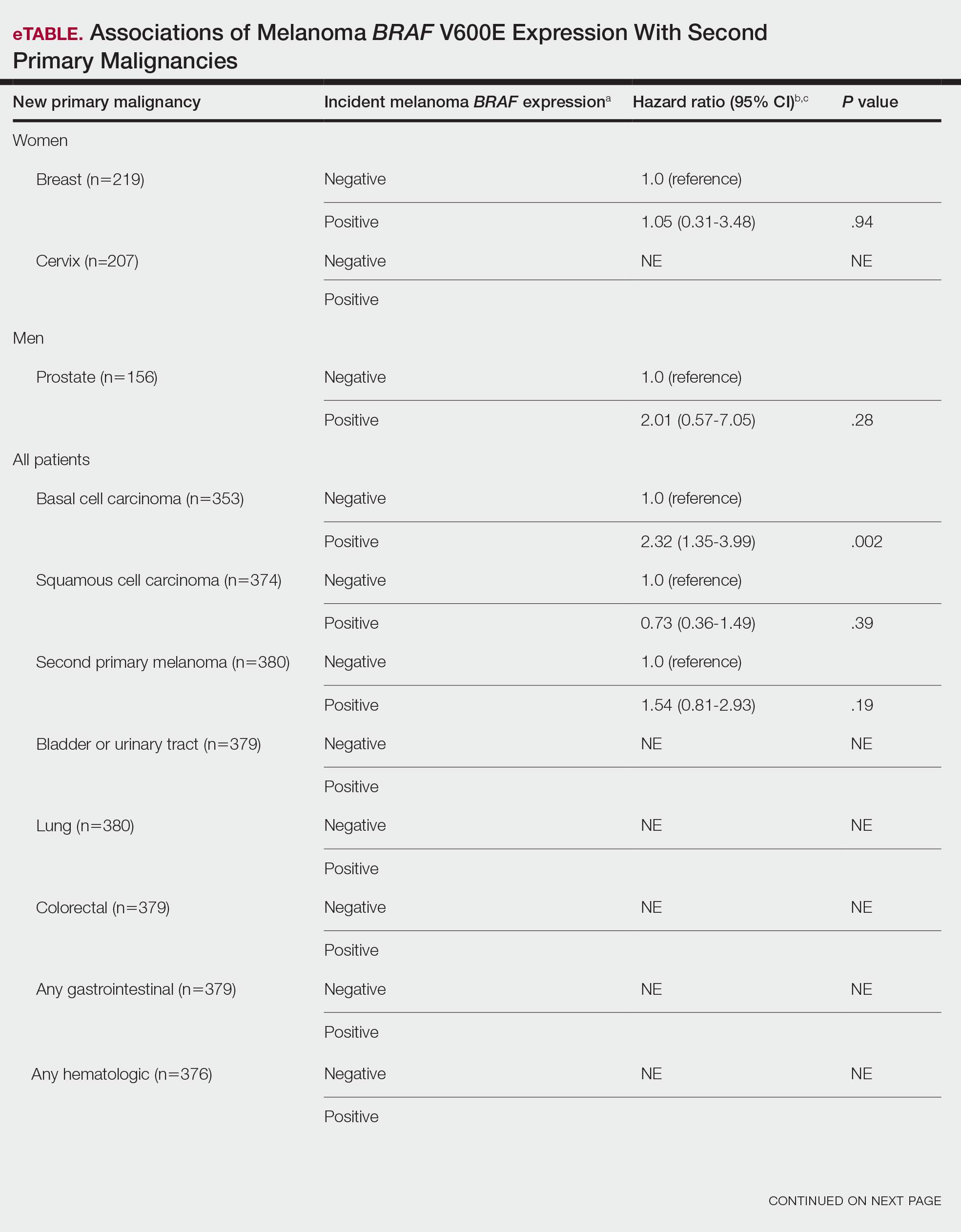
BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.
Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.
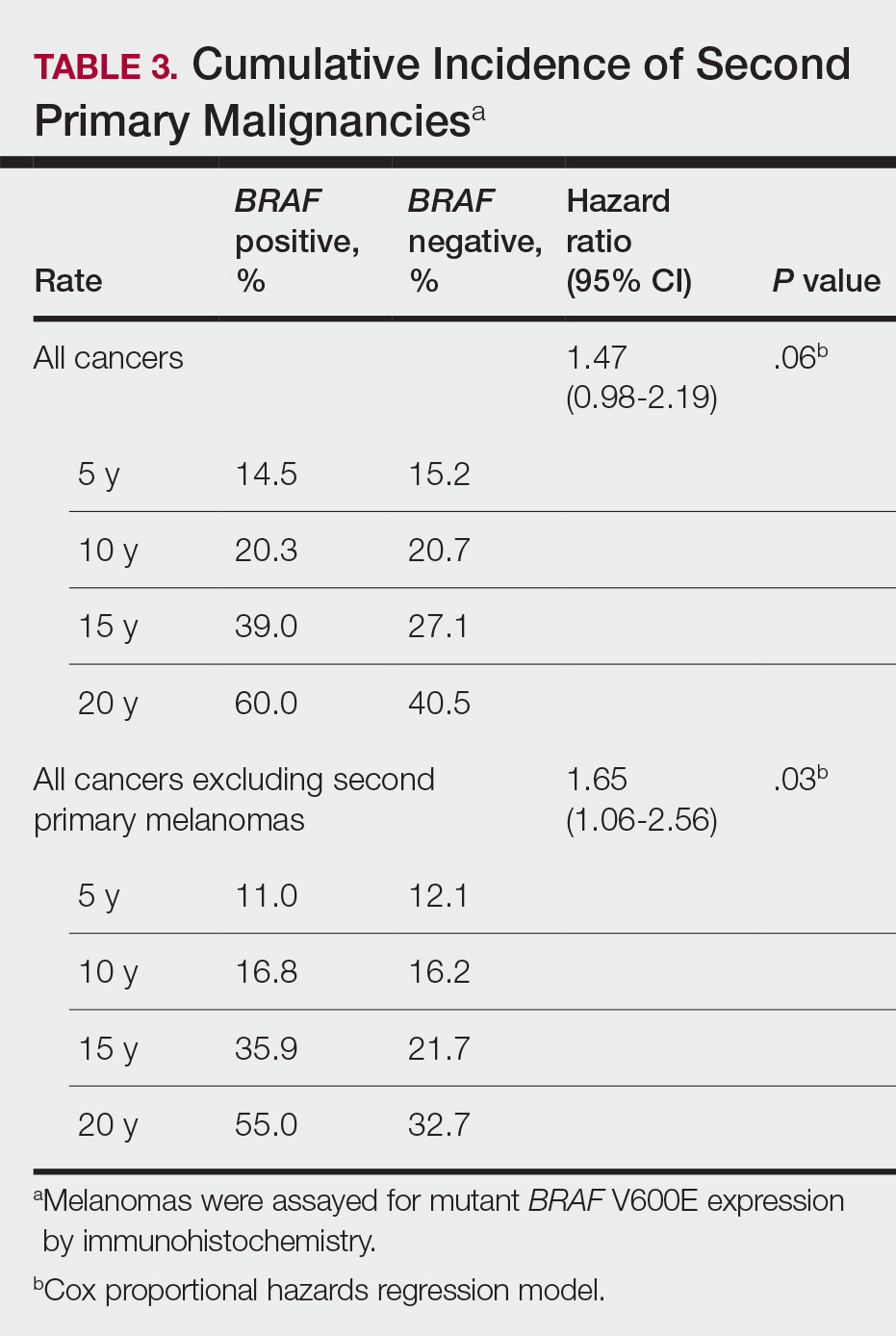
The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
The incidence of cutaneous melanoma in the United States has increased in the last 30 years, with the American Cancer Society estimating that 99,780 new melanomas will be diagnosed and 7650 melanoma-related deaths will occur in 2022.1 Patients with melanoma have an increased risk for developing a second primary melanoma or other malignancy, such as salivary gland, small intestine, breast, prostate, renal, or thyroid cancer, but most commonly nonmelanoma skin cancer.2,3 The incidence rate of melanoma among residents of Olmsted County, Minnesota, from 1970 through 2009 has already been described for various age groups4-7; however, the incidence of a second primary malignancy, including melanoma, within these incident cohorts remains unknown.
Mutations in the BRAF oncogene occur in approximately 50% of melanomas.8,9
Although the BRAF mutation event in melanoma is sporadic and should not necessarily affect the development of an unrelated malignancy, we hypothesized that the exposures that may have predisposed a particular individual to a BRAF-mutated melanoma also may have a higher chance of predisposing that individual to the development of another primary malignancy. In this population-based study, we aimed to determine whether the specific melanoma feature of mutant BRAF V600E expression was associated with the development of a second primary malignancy.
Methods
This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center (both in Rochester, Minnesota). The reporting of this study is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology statement.15
Patient Selection and BRAF Assessment—The Rochester Epidemiology Project (REP) links comprehensive health care records for virtually all residents of Olmsted County, Minnesota, across different medical providers. The REP provides an index of diagnostic and therapeutic procedures, tracks timelines and outcomes of individuals and their medical conditions, and is ideal for population-based studies.
We obtained a list of all residents of Olmsted County aged 18 to 60 years who had a melanoma diagnosed according to the International Classification of Diseases, Ninth Revision, from January 1, 1970, through December 30, 2009; these cohorts have been analyzed previously.4-7 Of the 638 individuals identified, 380 had a melanoma tissue block on file at Mayo Clinic with enough tumor present in available tissue blocks for BRAF assessment. All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) to confirm the diagnosis of melanoma. Tissue blocks were recut, and formalin-fixed, paraffin-embedded tissue sections were stained for BRAF V600E (Spring Bioscience Corporation). BRAF-stained specimens and the associated hematoxylin and eosin−stained slides were reviewed. Melanocyte cytoplasmic staining for BRAF was graded as negative if no staining was evident. BRAF was graded as positive if focal or partial staining was observed (<50% of tumor or low BRAF expression) or if diffuse staining was evident (>50% of tumor or high BRAF expression).
Using resources of the REP, we confirmed patients’ residency status in Olmsted County at the time of diagnosis of the incident melanoma. Patients who denied access to their medical records for research purposes were excluded. We used the complete record of each patient to confirm the date of diagnosis of the incident melanoma. Baseline characteristics of patients and their incident melanomas (eg, anatomic site and pathologic stage according to the American Joint Committee on Cancer classification) were obtained. When only the Clark level was included in the dermatopathology report, the corresponding Breslow thickness was extrapolated from the Clark level,18 and the pathologic stage according to the American Joint Committee on Cancer classification (7th edition) was determined.
For our study, specific diagnostic codes—International Classification of Diseases, Ninth and Tenth Revisions; Hospital International Classification of Diseases Adaptation19; and Berkson16—were applied across individual records to identify all second primary malignancies using the resources of the REP. The diagnosis date, morphology, and anatomic location of second primary malignancies were confirmed from examination of the clinical records.
Statistical Analysis—Baseline characteristics were compared by BRAF V600E expression using Wilcoxon rank sum and χ2 tests. The rate of developing a second primary malignancy at 5, 10, 15, and 20 years after the incident malignant melanoma was estimated with the Kaplan-Meier method. The duration of follow-up was calculated from the incident melanoma date to the second primary malignancy date or the last follow-up date. Patients with a history of the malignancy of interest, except skin cancers, before the incident melanoma date were excluded because it was not possible to distinguish between recurrence of a prior malignancy and a second primary malignancy. Associations of BRAF V600E expression with the development of a second primary malignancy were evaluated with Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs; all associations were adjusted for potential confounders such as age at the incident melanoma, year of the incident melanoma, and sex.
Results

Cumulative Incidence of Second Primary Melanoma—Of 133 patients with positive BRAF V600E expression, we identified 14 (10.5%), 1 (0.8%), and 1 (0.8%) who had 1, 2, and 4 subsequent melanomas, respectively. Of the 247 patients with negative BRAF V600E expression, we identified 15 (6%), 4 (1.6%), 2 (0.8%), and 1 (0.4%) patients who had 1, 2, 3, and 4 subsequent melanomas, respectively; BRAF V600E expression was not associated with the number of subsequent melanomas (P=.37; Wilcoxon rank sum test). The cumulative incidences of developing a second primary melanoma (n=38 among the 380 patients studied) at 5, 10, 15, and 20 years after the incident melanoma were 5.3%, 7.6%, 8.1%, and 14.6%, respectively.
Cumulative Incidence of All Second Primary Malignancies—Of the 380 patients studied, 60 (16%) had at least 1 malignancy diagnosed before the incident melanoma. Of the remaining 320 patients, 104 later had at least 1 malignancy develop, including a second primary melanoma, at a median (IQR) of 8.0 (2.7–16.2) years after the incident melanoma; the 104 patients with at least 1 subsequent malignancy included 40 with BRAF-positive and 64 with BRAF-negative melanomas. The cumulative incidences of developing at least 1 malignancy of any kind at 5, 10, 15, and 20 years after the incident melanoma were 15.0%, 20.5%, 31.2%, and 47.0%, respectively. Table 2 shows the number of patients with at least 1 second primary malignancy after the incident melanoma stratified by BRAF status.

BRAF V600E Expression and Association With Second Primary Malignancy—The eTable shows the associations of mutant BRAF V600E expression status with the development of a new primary malignancy. Malignancies affecting fewer than 10 patients were excluded from the analysis because there were too few events to support the Cox model. Positive BRAF V600E expression was associated with subsequent development of BCCs (HR, 2.32; 95% CI, 1.35-3.99; P=.002) and the development of all combined second primary malignancies excluding melanoma (HR, 1.65; 95% CI, 1.06-2.56; P=.03). However, BRAF V600E status was no longer a significant factor when all second primary malignancies, including second melanomas, were considered (P=.06). Table 3 shows the 5-, 10-, 15-, and 20-year cumulative incidences of all second primary malignancies according to mutant BRAF status.


Comment
Association of BRAF V600E Expression With Second Primary Malignancies—BRAF V600E expression of an incident melanoma was associated with the development of all combined second primary malignancies excluding melanoma; however, this association was not statistically significant when second primary melanomas were included. A possible explanation is that individuals with more than 1 primary melanoma possess additional genetic risk—CDKN2A or CDKN4 gene mutations or MC1R variation—that outweighed the effect of BRAF expression in the statistical analysis.

The 5- and 10-year cumulative incidences of all second primary malignancies excluding second primary melanoma were similar between BRAF-positive and BRAF-negative melanoma, but the 15- and 20-year cumulative incidences were greater for the BRAF-positive cohort. This could reflect the association of BRAF expression with BCCs and the increased likelihood of their occurrence with cumulative sun exposure and advancing age. BRAF expression was associated with the development of BCCs, but the reason for this association was unclear. BRAF-mutated melanoma occurs more frequently on sun-protected sites,20 whereas sporadic BCC generally occurs on sun-exposed sites. However, BRAF-mutated melanoma is associated with high levels of ambient UV exposure early in life, particularly birth through 20 years of age,21 and we speculate that such early UV exposure influences the later development of BCCs.
Development of BRAF-Mutated Cancers—It currently is not understood why the same somatic mutation can cause different types of cancer. A recent translational research study showed that in mice models, precursor cells of the pancreas and bile duct responded differently when exposed to PIK3CA and KRAS oncogenes, and tumorigenesis is influenced by specific cooperating genetic events in the tissue microenvironment. Future research investigating these molecular interactions may lead to better understanding of cancer pathogenesis and direct the design of new targeted therapies.22,23
Regarding environmental influences on the development of BRAF-mutated cancers, we found 1 population-based study that identified an association between high iodine content of drinking water and the prevalence of T1799A BRAF papillary thyroid carcinoma in 5 regions in China.24 Another study identified an increased risk for colorectal cancer and nonmelanoma skin cancer in the first-degree relatives of index patients with BRAF V600E colorectal cancer.25 Two studies by institutions in China and Sweden reported the frequency of BRAF mutations in cohorts of patients with melanoma.26,27
Additional studies investigating a possible association between BRAF-mutated melanoma and other cancers with larger numbers of participants than in our study may become more feasible in the future with increased routine genetic testing of biopsied cancers.
Study Limitations—Limitations of this retrospective epidemiologic study include the possibility of ascertainment bias during data collection. We did not account for known risk factors for cancer (eg, excessive sun exposure, smoking).
The main clinical implications from this study are that we do not have enough evidence to recommend BRAF testing for all incident melanomas, and BRAF-mutated melanomas cannot be associated with increased risk for developing other forms of cancer, with the possible exception of BCCs
Conclusion
Physicians should be aware of the risk for a second primary malignancy after an incident melanoma, and we emphasize the importance of long-term cancer surveillance.
Acknowledgment—We thank Ms. Jayne H. Feind (Rochester, Minnesota) for assistance with study coordination.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 12, 2022. Accessed August 15, 2022.https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- American Cancer Society. Second Cancers After Melanoma Skin Cancer. Accessed August 19, 2022. https://www.cancer.org/cancer/melanoma-skin-cancer/after-treatment/second-cancers.html
- Spanogle JP, Clarke CA, Aroner S, et al. Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol. 2010;62:757-767.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Lowe GC, Brewer JD, Peters MS, et al. Incidence of melanoma in the pediatric population: a population-based study in Olmsted County, Minnesota. Pediatr Derm. 2015;32:618-620.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma [editorial]. J Transl Med. 2012;10:85.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954.
- Miller AJ, Mihm MC Jr. Melanoma. N Engl J Med. 2006;355:51-65.
- Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305-2315.
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245-262.
- Moreau S, Saiag P, Aegerter P, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314-4321.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- St. Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614-1624.
- National Cancer Institute. Staging: melanoma of the skin, vulva, penis and scrotum staging. Accessed August 15, 2022. https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html
- Pakhomov SV, Buntrock JD, Chute CG. Automating the assignment of diagnosis codes to patient encounters using example-based and machine learning techniques. J Am Med Inform Assoc. 2006;13:516-525.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- German Cancer Research Center. Why identical mutations cause different types of cancer. July 19, 2021. Accessed August 15, 2022. https://www.dkfz.de/en/presse/pressemitteilungen/2021/dkfz-pm-21-41-Why-identical-mutations-cause-different-types-of-cancer.php
- Falcomatà C, Bärthel S, Ulrich A, et al. Genetic screens identify a context-specific PI3K/p27Kip1 node driving extrahepatic biliary cancer. Cancer Discov. 2021;11:3158-3177.
- Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab. 2009;94:1612-1617.
- Wish TA, Hyde AJ, Parfrey PS, et al. Increased cancer predisposition in family members of colorectal cancer patients harboring the p.V600E BRAF mutation: a population-based study. Cancer Epidemiol Biomarkers Prev. 2010;19:1831-1839.
- Zebary A, Omholt K, Vassilaki I, et al. KIT, NRAS, BRAF and PTEN mutations in a sample of Swedish patients with acral lentiginous melanoma. J Dermatol Sci. 2013;72:284-289.
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94-100.
- Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma. Br J Dermatol. 2013;169:320-328.
Practice Points
- Dermatologists should be aware of the long-term risk of second primary malignancies after an incident melanoma.
- BRAF mutations occur in melanomas and several other cancers. Our study found that melanoma BRAF V600E expression is associated with an increased risk for basal cell carcinomas.
Hydroquinone, found in skin-lightening agents worldwide, linked with increased skin cancer risk
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
an analysis of records from a large research database suggests.
In the study, hydroquinone use was associated with an approximately threefold increase for skin cancer risk, coauthor Brittany Miles, a fourth-year medical student at the University of Texas Medical Branch at Galveston’s John Sealy School of Medicine, told this news organization. “The magnitude of the risk was surprising. Increased risk should be disclosed to patients considering hydroquinone treatment.”
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
Hydroquinone (multiple brand names), a tyrosinase inhibitor used worldwide for skin lightening because of its inhibition of melanin production, was once considered “generally safe and effective” by the Food and Drug Administration, the authors wrote.
The compound’s use in over-the-counter products in the United States has been restricted based on suspicion of carcinogenicity, but few human studies have been conducted. In April, the FDA issued warning letters to 12 companies that sold hydroquinone in concentrations not generally recognized as safe and effective, because of other concerns including rashes, facial swelling, and ochronosis (skin discoloration).
Ms. Miles and her coauthor, Michael Wilkerson, MD, professor and chair of the department of dermatology at UTMB, analyzed data from TriNetX, the medical research database of anonymized medical record information from 61 million patients in 57 large health care organizations, almost all of them in the United States.
The researchers created two cohorts of patients aged 15 years and older with no prior diagnosis of skin cancer: one group had been treated with hydroquinone (medication code 5509 in the TriNetX system), and the other had not been exposed to the drug. Using ICD-10 codes for melanoma, nonmelanoma skin cancer, and all skin cancers, they investigated which groups of people were likely to develop these cancers.
They found that hydroquinone exposure was linked with a significant increase in melanoma (relative risk, 3.0; 95% confidence interval, 1.704-5.281; P < .0001), nonmelanoma skin cancers (RR, 3.6; 95%; CI, 2.815-4.561; P < .0001), and all reported skin cancers combined (relative risk, 3.4; 95% CI, 2.731-4.268; P < .0001)
While “the source of the data and the number of patients in the study are significant strengths,” Ms. Miles said, “the inability to determine how long and how consistently the patients used hydroquinone is likely the biggest weakness.”
Skin lightening is big business and more research is needed
“The U.S. market for skin-lightening agents was approximately 330 million dollars in 2021, and 330,000 prescriptions containing hydroquinone were dispensed in 2019,” Ms. Miles said.
Valencia D. Thomas, MD, professor in the department of dermatology of the University of Texas MD Anderson Cancer Center, Houston, said in an email that over-the-counter skin-lightening products containing low-concentration hydroquinone are in widespread use and are commonly used in populations of color.
“Hydroquinone preparations in higher concentrations are unfortunately also available in the United States,” added Dr. Thomas, who was not involved in the study and referred to the FDA warning letter issued in April.
Only one hydroquinone-containing medication – Tri-Luma at 4% concentration, used to treat melasma – is currently FDA-approved, she said.
The data in the study do not show an increased risk for skin cancer with hydroquinone exposure, but do show “an increased risk of cancer in the TriNetX medication code 5509 hydroquinone exposure group, which does not prove causation,” Dr. Thomas commented.
“Because ‘hydroquinone exposure’ is not defined, it is unclear how TriNetX identified the hydroquinone exposure cohort,” she noted. “Does ‘exposure’ count prescriptions written and potentially not used, the use of hydroquinone products of high concentration not approved by the FDA, or the use of over-the-counter hydroquinone products?
“The strength of this study is its size,” Dr. Thomas acknowledged. “This study is a wonderful starting point to further investigate the ‘hydroquinone exposure’ cohort to determine if hydroquinone is a driver of cancer, or if hydroquinone is itself a confounder.”
These results highlight the need to examine the social determinants of health that may explain increased risk for cancer, including race, geography, and poverty, she added.
“Given the global consumption of hydroquinone, multinational collaboration investigating hydroquinone and cancer data will likely be needed to provide insight into this continuing question,” Dr. Thomas advised.
Christiane Querfeld, MD, PhD, associate professor of dermatology and dermatopathology at City of Hope in Duarte, Calif., agreed that the occurrence of skin cancer following use of hydroquinone is largely understudied.
“The findings have a huge impact on how we counsel and monitor future patients,” Dr. Querfeld, who also was not involved in the study, said in an email. “There may be a trade-off at the start of treatment: Get rid of melasma but develop a skin cancer or melanoma with potentially severe outcomes.
“It remains to be seen if there is a higher incidence of skin cancer following use of hydroquinone or other voluntary bleaching and depigmentation remedies in ethnic groups such as African American or Hispanic patient populations, who have historically been at low risk of developing skin cancer,” she added. “It also remains to be seen if increased risk is due to direct effects or to indirect effects on already-photodamaged skin.
“These data are critical, and I am sure this will open further investigations to study effects in more detail,” Dr. Querfeld said.
The study authors, Dr. Thomas, and Dr. Querfeld reported no relevant financial relationships. The study did not receive external funding.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Consider the ‘long game’ in tumor management following Mohs surgery
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
PORTLAND, ORE. – In his nearly 2 decades of dermatology practice, Keith L. Duffy, MD, has seen his share of cases where Mohs surgery was misused or misappropriated.
, Salt Lake City, said at the annual meeting of the Pacific Dermatologic Association. “I want to protect our specialty. I see patients who have dozens of skin cancers. I want to emphasize the long game of management in those patients. You have to think about the tumors in terms of decades.”
In 2012, an ad hoc task force from the American Academy of Dermatology (AAD), the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery developed appropriate use criteria (AUC) for 270 scenarios for which Mohs micrographic surgery (MMS) is frequently considered. The task force used a 9-point scale to rate each indication, as follows:
- A score of 7 to 9: The use of MMS is appropriate for the specific indication and is generally considered acceptable.
- A score of 4 to 6: The use of MMS is uncertain for the specific indication, although its use may be appropriate and acceptable.
- A score of 1 to 3: The use of MMS is inappropriate for the specific indication and is generally not considered acceptable.
These ratings were translated into a free Mohs Surgery Appropriate Use Criteria App developed by the AAD.
Subsequently, Dr. Duffy and colleagues retrospectively examined the University of Utah’s adherence to the Mohs AUC over the course of 3 months. Their analysis, published in 2015, included 1,026 nonmelanoma skin cancers in 724 patients. Of the 1,026 cancers, 350 (34.1%) were treated with MMS. Of these, 339 (96.9%) were deemed appropriate based on the AUC guidelines, 4 (1.1%) were deemed uncertain, and 7 (2%) were deemed inappropriate.
There were also 611 skin cancers that were not treated with Mohs but met criteria for treatment with Mohs. “Most of these were AUC 7 tumors,” Dr. Duffy said. “When I see an AUC 7 tumor, I give high consideration for certain anatomic locations, especially the lower leg, scalp, eyelid, genitalia, ear, hands, and feet. I also think about the patient’s age, the number of skin cancers, and histological characteristics. Consider the long game in management and remember that skin cancer patients can make a near infinite amount of skin cancers, so be conservative when excising skin cancers to preserve precious skin.”
In his opinion, full thickness wounds requiring sutures should be avoided on the scalp and lower leg, if possible. “Most carcinomas in these locations are superficial and not aggressive in immunocompetent patients,” said Dr. Duffy, who said he has had one patient in 12 years who was not a transplant patient who had a metastatic squamous cell carcinoma on the lower leg. “Postop complications can be totally avoided. I don’t worry about these patients bleeding or [about] dehiscence. They can go back and play golf the next day, so you save valuable skin where the real estate is precious. This underscores a practice pearl: Incorporate the Mohs AUC and consideration of anatomic location when considering the most appropriate treatment of skin cancers.”
He also advises dermatologists to consider the histopathologic characteristics of the tumor when treating skin cancers to reduce complications and save tissue, so that patients can resume their lifestyle. “When you read the pathology report, really think about what the dermatopathologist saw under the microscope,” said Dr. Duffy, who is an investigator at the University of Utah’s Huntsman Cancer Institute. He said that he is able to review the slides for 90% of his own cases before surgery. “I’m lucky that way, but if you have any questions, your dermatopathologist should be on speed dial.”
Ultimately, he concluded, proper selection of a treatment modality for a specific tumor and patient rules the day. “Tumors should be thought about in the context of the patient and not as a single or isolated cancer,” he said.
Dr. Duffy reported having no relevant disclosures.
AT PDA 2022
Second opinions on melanocytic lesions swayed when first opinion is known
, diminishing the value and accuracy of an independent analysis.
In a novel effort to determine whether previous interpretations sway second opinions, 149 dermatopathologists were asked to read melanocytic skin biopsy specimens without access to the initial pathology report. A year or more later they read them again but now with access to the initial reading.
The study showed that the participants, independent of many variables, such as years of experience or frequency with which they offered second options, were more likely to upgrade or downgrade the severity of the specimens in accordance with the initial report even if their original reading was correct.
If the goal of a second dermatopathologist opinion is to obtain an independent diagnostic opinion, the message from this study is that they “should be blinded to first opinions,” according to the authors of this study, led by Joann G. Elmore, MD, professor of medicine, University of California, Los Angeles. The study was published online in JAMA Dermatology.
Two-phase study has 1-year washout
The study was conducted in two phases. In phase 1, a nationally representative sample of volunteer dermatopathologists performed 878 interpretations. In phase 2, conducted after a washout period of 12 months or more, the dermatopathologists read a random subset of the same cases evaluated in phase 1, but this time, unlike the first, they were first exposed to prior pathology reports.
Ultimately, “the dermatologists provided more than 5,000 interpretations of study cases, which was a big contribution of time,” Dr. Elmore said in an interview. Grateful for their critical contribution, she speculated that they were driven by the importance of the question being asked.
When categorized by the Melanocytic Pathology Assessment Tool (MPAT), which rates specimens from benign (class 1) to pT1b invasive melanoma (class 4), the influence of the prior report went in both directions, so that the likelihood of upgrading or downgrading went in accordance with the grading in the original dermatopathology report.
As a result, the risk of a less severe interpretation on the second relative to the first reading was 38% greater if the initial dermatopathology report had a lower grade (relative risk, 1.38; 95% confidence interval [CI], 1.19-1.59). The risk of upgrading the second report if the initial pathology report had a higher grade was increased by more than 50% (RR, 1.52; 95% CI, 1.34-1.73).
The greater likelihood of upgrading than downgrading is “understandable,” Dr. Elmore said. “I think this is consistent with the concern about missing something,” she explained.
According to Dr. Elmore, one of the greatest concerns regarding the bias imposed by the original pathology report is that the switch of opinions often went from one that was accurate to one that was inaccurate.
If the phase 1 diagnosis was accurate but upgraded in the phase 2 diagnosis, the risk of inaccuracy was almost doubled (RR, 1.96; 95% CI, 1.31-2.93). If the phase 1 report was inaccurate, the relative risk of changing the phase 2 diagnosis was still high but lower than if it was accurate (RR, 1.46; 95% CI, 1.27-1.68).
“That is, even when the phase 1 diagnoses agreed with the consensus reference diagnosis, they were swayed away from the correct diagnosis in phase 2 [when the initial pathology report characterized the specimen as higher grade],” Dr. Elmore reported.
Conversely, the risk of downgrading was about the same whether the phase 1 evaluation was accurate (RR, 1.37; 95% CI, 1.14-1.64) or inaccurate (RR 1.32; 95% CI, 1.07-1.64).
Downward and upward shifts in severity from an accurate diagnosis are concerning because of the likelihood they will lead to overtreatment or undertreatment. The problem, according to data from this study, is that dermatologists making a second opinion cannot judge their own susceptibility to being swayed by the original report.
Pathologists might be unaware of bias
At baseline, the participants were asked whether they thought they were influenced by the first interpretation when providing a second opinion. Although 69% acknowledged that they might be “somewhat influenced,” 31% maintained that they do not take initial reports into consideration. When the two groups were compared, the risk of downgrading was nearly identical. The risk of upgrading was lower in those claiming to disregard initial reports (RR, 1.29) relative to those who said they were “somewhat influenced” by a previous diagnosis (RR, 1.64), but the difference was not significant.
The actual risk of bias incurred by prior pathology reports might be greater than that captured in this study for several reasons, according to the investigators. They pointed out that all participants were experienced and board-certified and might therefore be expected to be more confident in their interpretations than an unselected group of dermatopathologists. In addition, participants might have been more careful in their interpretations knowing they were participating in a study.
“There are a lot of data to support the value of second opinions [in dermatopathology and other areas], but we need to consider the process of how they are being obtained,” Dr. Elmore said. “There needs to be a greater emphasis on providing an independent analysis.”
More than 60% of the dermatologists participating in this study reported that they agreed or strongly agreed with the premise that they prefer to have the original dermatopathology report when they offer a second opinion. Dr. Elmore said that the desire of those offering a second opinion to have as much information in front of them as possible is understandable, but the bias imposed by the original report weakens the value of the second opinion.
Blind reading of pathology reports needed
“These data suggest that seeing the original report sways opinions and that includes swaying opinions away from an accurate reading,” Dr. Elmore said. She thinks that for dermatopathologists to render a valuable and independent second opinion, the specimens should be examined “at least initially” without access to the first report.
The results of this study were not surprising to Vishal Anil Patel, MD, director of the Cutaneous Oncology Program, George Washington University Cancer Center, Washington. He made the point that physicians “are human first and foremost and not perfect machines.” As a result, he suggested bias and error are inevitable.
Although strategies to avoid bias are likely to offer some protection against inaccuracy, he said that diagnostic support tools such as artificial intelligence might be the right direction for improving inter- and intra-rater reliability.
Ruifeng Guo, MD, PhD, a consultant in the division of anatomic pathology at the Mayo Clinic, Rochester, Minn., agreed with the basic premise of the study, but he cautioned that restricting access to the initial pathology report might not always be the right approach.
It is true that “dermatopathologists providing a second opinion in diagnosing cutaneous melanoma are mostly unaware of the risk of bias if they read the initial pathology report,” said Dr. Guo, but restricting access comes with risks.
“There are also times critical information may be contained in the initial pathology report that needs to be considered when providing a second opinion consultation,” he noted. Ultimately, the decision to read or not read the initial report should be decided “on an individual basis.”
The study was funded by grants from the National Cancer Institute. Dr. Elmore, Dr. Patel, and Dr. Guo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, diminishing the value and accuracy of an independent analysis.
In a novel effort to determine whether previous interpretations sway second opinions, 149 dermatopathologists were asked to read melanocytic skin biopsy specimens without access to the initial pathology report. A year or more later they read them again but now with access to the initial reading.
The study showed that the participants, independent of many variables, such as years of experience or frequency with which they offered second options, were more likely to upgrade or downgrade the severity of the specimens in accordance with the initial report even if their original reading was correct.
If the goal of a second dermatopathologist opinion is to obtain an independent diagnostic opinion, the message from this study is that they “should be blinded to first opinions,” according to the authors of this study, led by Joann G. Elmore, MD, professor of medicine, University of California, Los Angeles. The study was published online in JAMA Dermatology.
Two-phase study has 1-year washout
The study was conducted in two phases. In phase 1, a nationally representative sample of volunteer dermatopathologists performed 878 interpretations. In phase 2, conducted after a washout period of 12 months or more, the dermatopathologists read a random subset of the same cases evaluated in phase 1, but this time, unlike the first, they were first exposed to prior pathology reports.
Ultimately, “the dermatologists provided more than 5,000 interpretations of study cases, which was a big contribution of time,” Dr. Elmore said in an interview. Grateful for their critical contribution, she speculated that they were driven by the importance of the question being asked.
When categorized by the Melanocytic Pathology Assessment Tool (MPAT), which rates specimens from benign (class 1) to pT1b invasive melanoma (class 4), the influence of the prior report went in both directions, so that the likelihood of upgrading or downgrading went in accordance with the grading in the original dermatopathology report.
As a result, the risk of a less severe interpretation on the second relative to the first reading was 38% greater if the initial dermatopathology report had a lower grade (relative risk, 1.38; 95% confidence interval [CI], 1.19-1.59). The risk of upgrading the second report if the initial pathology report had a higher grade was increased by more than 50% (RR, 1.52; 95% CI, 1.34-1.73).
The greater likelihood of upgrading than downgrading is “understandable,” Dr. Elmore said. “I think this is consistent with the concern about missing something,” she explained.
According to Dr. Elmore, one of the greatest concerns regarding the bias imposed by the original pathology report is that the switch of opinions often went from one that was accurate to one that was inaccurate.
If the phase 1 diagnosis was accurate but upgraded in the phase 2 diagnosis, the risk of inaccuracy was almost doubled (RR, 1.96; 95% CI, 1.31-2.93). If the phase 1 report was inaccurate, the relative risk of changing the phase 2 diagnosis was still high but lower than if it was accurate (RR, 1.46; 95% CI, 1.27-1.68).
“That is, even when the phase 1 diagnoses agreed with the consensus reference diagnosis, they were swayed away from the correct diagnosis in phase 2 [when the initial pathology report characterized the specimen as higher grade],” Dr. Elmore reported.
Conversely, the risk of downgrading was about the same whether the phase 1 evaluation was accurate (RR, 1.37; 95% CI, 1.14-1.64) or inaccurate (RR 1.32; 95% CI, 1.07-1.64).
Downward and upward shifts in severity from an accurate diagnosis are concerning because of the likelihood they will lead to overtreatment or undertreatment. The problem, according to data from this study, is that dermatologists making a second opinion cannot judge their own susceptibility to being swayed by the original report.
Pathologists might be unaware of bias
At baseline, the participants were asked whether they thought they were influenced by the first interpretation when providing a second opinion. Although 69% acknowledged that they might be “somewhat influenced,” 31% maintained that they do not take initial reports into consideration. When the two groups were compared, the risk of downgrading was nearly identical. The risk of upgrading was lower in those claiming to disregard initial reports (RR, 1.29) relative to those who said they were “somewhat influenced” by a previous diagnosis (RR, 1.64), but the difference was not significant.
The actual risk of bias incurred by prior pathology reports might be greater than that captured in this study for several reasons, according to the investigators. They pointed out that all participants were experienced and board-certified and might therefore be expected to be more confident in their interpretations than an unselected group of dermatopathologists. In addition, participants might have been more careful in their interpretations knowing they were participating in a study.
“There are a lot of data to support the value of second opinions [in dermatopathology and other areas], but we need to consider the process of how they are being obtained,” Dr. Elmore said. “There needs to be a greater emphasis on providing an independent analysis.”
More than 60% of the dermatologists participating in this study reported that they agreed or strongly agreed with the premise that they prefer to have the original dermatopathology report when they offer a second opinion. Dr. Elmore said that the desire of those offering a second opinion to have as much information in front of them as possible is understandable, but the bias imposed by the original report weakens the value of the second opinion.
Blind reading of pathology reports needed
“These data suggest that seeing the original report sways opinions and that includes swaying opinions away from an accurate reading,” Dr. Elmore said. She thinks that for dermatopathologists to render a valuable and independent second opinion, the specimens should be examined “at least initially” without access to the first report.
The results of this study were not surprising to Vishal Anil Patel, MD, director of the Cutaneous Oncology Program, George Washington University Cancer Center, Washington. He made the point that physicians “are human first and foremost and not perfect machines.” As a result, he suggested bias and error are inevitable.
Although strategies to avoid bias are likely to offer some protection against inaccuracy, he said that diagnostic support tools such as artificial intelligence might be the right direction for improving inter- and intra-rater reliability.
Ruifeng Guo, MD, PhD, a consultant in the division of anatomic pathology at the Mayo Clinic, Rochester, Minn., agreed with the basic premise of the study, but he cautioned that restricting access to the initial pathology report might not always be the right approach.
It is true that “dermatopathologists providing a second opinion in diagnosing cutaneous melanoma are mostly unaware of the risk of bias if they read the initial pathology report,” said Dr. Guo, but restricting access comes with risks.
“There are also times critical information may be contained in the initial pathology report that needs to be considered when providing a second opinion consultation,” he noted. Ultimately, the decision to read or not read the initial report should be decided “on an individual basis.”
The study was funded by grants from the National Cancer Institute. Dr. Elmore, Dr. Patel, and Dr. Guo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, diminishing the value and accuracy of an independent analysis.
In a novel effort to determine whether previous interpretations sway second opinions, 149 dermatopathologists were asked to read melanocytic skin biopsy specimens without access to the initial pathology report. A year or more later they read them again but now with access to the initial reading.
The study showed that the participants, independent of many variables, such as years of experience or frequency with which they offered second options, were more likely to upgrade or downgrade the severity of the specimens in accordance with the initial report even if their original reading was correct.
If the goal of a second dermatopathologist opinion is to obtain an independent diagnostic opinion, the message from this study is that they “should be blinded to first opinions,” according to the authors of this study, led by Joann G. Elmore, MD, professor of medicine, University of California, Los Angeles. The study was published online in JAMA Dermatology.
Two-phase study has 1-year washout
The study was conducted in two phases. In phase 1, a nationally representative sample of volunteer dermatopathologists performed 878 interpretations. In phase 2, conducted after a washout period of 12 months or more, the dermatopathologists read a random subset of the same cases evaluated in phase 1, but this time, unlike the first, they were first exposed to prior pathology reports.
Ultimately, “the dermatologists provided more than 5,000 interpretations of study cases, which was a big contribution of time,” Dr. Elmore said in an interview. Grateful for their critical contribution, she speculated that they were driven by the importance of the question being asked.
When categorized by the Melanocytic Pathology Assessment Tool (MPAT), which rates specimens from benign (class 1) to pT1b invasive melanoma (class 4), the influence of the prior report went in both directions, so that the likelihood of upgrading or downgrading went in accordance with the grading in the original dermatopathology report.
As a result, the risk of a less severe interpretation on the second relative to the first reading was 38% greater if the initial dermatopathology report had a lower grade (relative risk, 1.38; 95% confidence interval [CI], 1.19-1.59). The risk of upgrading the second report if the initial pathology report had a higher grade was increased by more than 50% (RR, 1.52; 95% CI, 1.34-1.73).
The greater likelihood of upgrading than downgrading is “understandable,” Dr. Elmore said. “I think this is consistent with the concern about missing something,” she explained.
According to Dr. Elmore, one of the greatest concerns regarding the bias imposed by the original pathology report is that the switch of opinions often went from one that was accurate to one that was inaccurate.
If the phase 1 diagnosis was accurate but upgraded in the phase 2 diagnosis, the risk of inaccuracy was almost doubled (RR, 1.96; 95% CI, 1.31-2.93). If the phase 1 report was inaccurate, the relative risk of changing the phase 2 diagnosis was still high but lower than if it was accurate (RR, 1.46; 95% CI, 1.27-1.68).
“That is, even when the phase 1 diagnoses agreed with the consensus reference diagnosis, they were swayed away from the correct diagnosis in phase 2 [when the initial pathology report characterized the specimen as higher grade],” Dr. Elmore reported.
Conversely, the risk of downgrading was about the same whether the phase 1 evaluation was accurate (RR, 1.37; 95% CI, 1.14-1.64) or inaccurate (RR 1.32; 95% CI, 1.07-1.64).
Downward and upward shifts in severity from an accurate diagnosis are concerning because of the likelihood they will lead to overtreatment or undertreatment. The problem, according to data from this study, is that dermatologists making a second opinion cannot judge their own susceptibility to being swayed by the original report.
Pathologists might be unaware of bias
At baseline, the participants were asked whether they thought they were influenced by the first interpretation when providing a second opinion. Although 69% acknowledged that they might be “somewhat influenced,” 31% maintained that they do not take initial reports into consideration. When the two groups were compared, the risk of downgrading was nearly identical. The risk of upgrading was lower in those claiming to disregard initial reports (RR, 1.29) relative to those who said they were “somewhat influenced” by a previous diagnosis (RR, 1.64), but the difference was not significant.
The actual risk of bias incurred by prior pathology reports might be greater than that captured in this study for several reasons, according to the investigators. They pointed out that all participants were experienced and board-certified and might therefore be expected to be more confident in their interpretations than an unselected group of dermatopathologists. In addition, participants might have been more careful in their interpretations knowing they were participating in a study.
“There are a lot of data to support the value of second opinions [in dermatopathology and other areas], but we need to consider the process of how they are being obtained,” Dr. Elmore said. “There needs to be a greater emphasis on providing an independent analysis.”
More than 60% of the dermatologists participating in this study reported that they agreed or strongly agreed with the premise that they prefer to have the original dermatopathology report when they offer a second opinion. Dr. Elmore said that the desire of those offering a second opinion to have as much information in front of them as possible is understandable, but the bias imposed by the original report weakens the value of the second opinion.
Blind reading of pathology reports needed
“These data suggest that seeing the original report sways opinions and that includes swaying opinions away from an accurate reading,” Dr. Elmore said. She thinks that for dermatopathologists to render a valuable and independent second opinion, the specimens should be examined “at least initially” without access to the first report.
The results of this study were not surprising to Vishal Anil Patel, MD, director of the Cutaneous Oncology Program, George Washington University Cancer Center, Washington. He made the point that physicians “are human first and foremost and not perfect machines.” As a result, he suggested bias and error are inevitable.
Although strategies to avoid bias are likely to offer some protection against inaccuracy, he said that diagnostic support tools such as artificial intelligence might be the right direction for improving inter- and intra-rater reliability.
Ruifeng Guo, MD, PhD, a consultant in the division of anatomic pathology at the Mayo Clinic, Rochester, Minn., agreed with the basic premise of the study, but he cautioned that restricting access to the initial pathology report might not always be the right approach.
It is true that “dermatopathologists providing a second opinion in diagnosing cutaneous melanoma are mostly unaware of the risk of bias if they read the initial pathology report,” said Dr. Guo, but restricting access comes with risks.
“There are also times critical information may be contained in the initial pathology report that needs to be considered when providing a second opinion consultation,” he noted. Ultimately, the decision to read or not read the initial report should be decided “on an individual basis.”
The study was funded by grants from the National Cancer Institute. Dr. Elmore, Dr. Patel, and Dr. Guo reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Getting cancer research on track again may require a ‘behemoth’ effort
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
In 2016, as vice president, Joe Biden launched the Cancer Moonshot program just 1 year after his son Beau died from glioblastoma multiforme. His objective, he said, was to “cure” cancer, but to get close to that goal, to get cancer research just back up to pre-COVID-19 pandemic levels.
There has been a significant decrease in the launch of new clinical trials for cancer and biologic therapies since 2020. “That can affect every aspect of our research operation. It really affected our capacity to continue to move forward at a fast pace. It will require a behemoth effort to get back to pre-COVID times,” said Tanios S. Bekaii-Saab, MD, leader of the gastrointestinal cancer program at Mayo Clinic in Phoenix.
Congress passed the 21st Century Cures Act in 2016 authorizing $1.8 billion for Cancer Moonshot over 7 years. More recently, the program received $194 million from the $6.9 billion National Cancer Institute budget in FY 2022.
Joseph Alvarnas, MD, a hematologist oncologist and vice president of government affairs at City of Hope, Duarte, Calif., sees the Moonshot budget as a potential shortcoming.
“The priorities are well founded and based on what we would think are the most important things to cover, but, if we’re going to achieve these extraordinarily ambitious goals of halving cancer mortality and serving communities more equitably, it’s going to need more funding positioned at making these things real,” he said.
Moonshot is being positioned as an opportunity to double down on efforts started in 2016, but treating cancer is complex and goes well beyond funding new research.
“We know that we have amazing research and progress around innovations that will drive us toward the goal of reducing the death rate from cancer. But we also know that we have tools that aren’t reaching all parts of the country, so we have a great opportunity to make sure that we’re doing all we can to prevent, detect and treat cancer,” Dr. Carnival said.
Can cancer be cured?
The Biden administration relaunched Moonshot in 2022 with newly defined goals: Cut the rate of cancer-related deaths in half within 25 years; improve the experience of people with cancer, cancer survivors, and their families; and “end cancer as we know it,” President Biden said in a press conference in February.
Cancer is the second leading cause of death in the United States after heart disease, but it may indeed be possible to cut the total number of cancer-related deaths in half over the next 25 years.
“As a hematologist who’s been involved in both research and clinical care, I think it’s important to realize this is actually doable. Between 1990 and 2020 cancer mortality rates decreased by 31%, and in the last American Cancer Society’s annual report, mortality rates dropped by the largest percentages for 2 consecutive years in a row. The question shifts now from ‘Is this possible? to ‘How do we ensure that it’s possible?’ The spirit of Cancer Moonshot 2.0 is identifying the multiple paths to move this effort forward,” Dr. Alvarnas said.
But without a significant infusion of cash for research, it’s doubtful cancer-related deaths will drop by 50% over the next 25 years.
“There are a lot of big and lofty goals in Cancer Moonshot, and the words ‘ending cancer,’ well those are big words,” Dr. Bekaii-Saab said. “The reality is how do we measure in 25 years the impact of this today? I think it will require significantly more funding over the next few years to achieve the goals set by the Moonshot. Otherwise it will be a 7-year done deal that will accrue a lot of great numbers but won’t make a dent in those goals for the next 25 years. To stop it at some point and not invest more into it, we will probably lose most of the benefit.”
Closing the loop on data sharing
Moonshot has been instrumental in fostering research collaborations by encouraging data sharing among scientists.
“It also brought together a new way for the National Cancer Institute and Department of Energy to drive progress on some of the big data initiatives. The initial Cancer Moonshot infused a sense of urgency and hope into this effort,” said Danielle Carnival, PhD, coordinator of Cancer Moonshot.
Between 2017 and 2022, Cancer Moonshot created more than 70 consortiums or programs, and funded about 240 research projects. Its fundamental goals of improving data sharing and encouraging collaboration are very important, Dr. Bekaii-Saab said.
“Because, historically, what happens with cancer is that researchers compete for resources...and they become very protective of their data. Sharing gets more difficult, collaborations become more onerous, and it becomes counterproductive,” he said.
Dr. Bekaii-Saab highlighted two networks created specifically for data sharing. They include the Human Tumor Atlas for cellular, morphological, and molecular tumor data, and PDXNet, a patient derived xenograft research network.
A shift in funding priorities?
Cancer funding has been stagnant for years. When adjusted for growth, it hasn’t had a significant infusion of funding since at least 2003—at least in relative terms, Dr. Bekaii-Saab said. “This affects a lot of the things we do, including NCI-funded clinical trials. It pushes us to work with the private sector, which is not necessarily a detriment, but it doesn’t advance the academic mission at the same level. So, overall, I wouldn’t call it tragic, but I do think we’re falling behind,” he said.
“I think when we do the process for the budget for FY24 and after we’ve had time to really explore the best ideas and build the foundation for some of these new aspects of the Cancer Moonshot, we hope to have something more concrete going toward these efforts,” Dr. Carnival said.
But in addition to funding, Dr. Alvarnas says, it is equally important to address gaps in care. Not all patients have access to existing cancer treatments.
“The great challenge to us in the 2020s is not only about developing new and more effective technologies, but also in doing a better job of getting existing life-saving treatments into the hands of underserved populations. One of the really positive challenges set forth by the Biden administration is the idea that financing care equity is as important, if not more so, than advancing technologies. If there’s been stagnation, it’s because from a government and resourcing point of view, that priority has been ineffectively supported financially.”
The pandemic stymies cancer research
The pandemic has had a significant impact on cancer research. As in other fields, it disrupted ongoing research, but it may have also contributed to the loss of employees who resigned in what’s been called the “Great Resignation.” “A lot of employees just decided to change jobs in the middle of the pandemic, which led to a cancer research staffing crisis,” Dr. Bekaii-Saab said.
“We all recognized that turning so much of the attention of the entire biomedical research engine and health system to the COVID-19 pandemic would have an impact across cancer research, screenings and care,” Dr. Carnival said. “There is work to do to get us back to whole, but from a research perspective, we’ve seen a reorientation of the trial networks we were using for COVID-19 research, back to their initial purpose. Some of those are cancer and oncology networks, so we’re excited about that and fully believe that we can catch up.”
But then there’s also the impact the pandemic has had on cancer patients who delayed their care at the primary level. This, Dr. Bekaii-Saab fears, will lead to more patients presenting with more advanced disease in years to come. “One of the biggest problems was that a lot of patients delayed their care at the primary level. My biggest concern is that in the years to come we will see a lot more patients presenting with more advanced cancer.”
Experts: EPA should assess risk of sunscreens’ UV filters
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Firm Exophytic Tumor on the Shin
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
A 62-year-old man presented with a firm, exophytic, 2.8×1.5-cm tumor on the left shin of 6 to 7 years’ duration. An excisional biopsy was obtained for histopathologic evaluation.
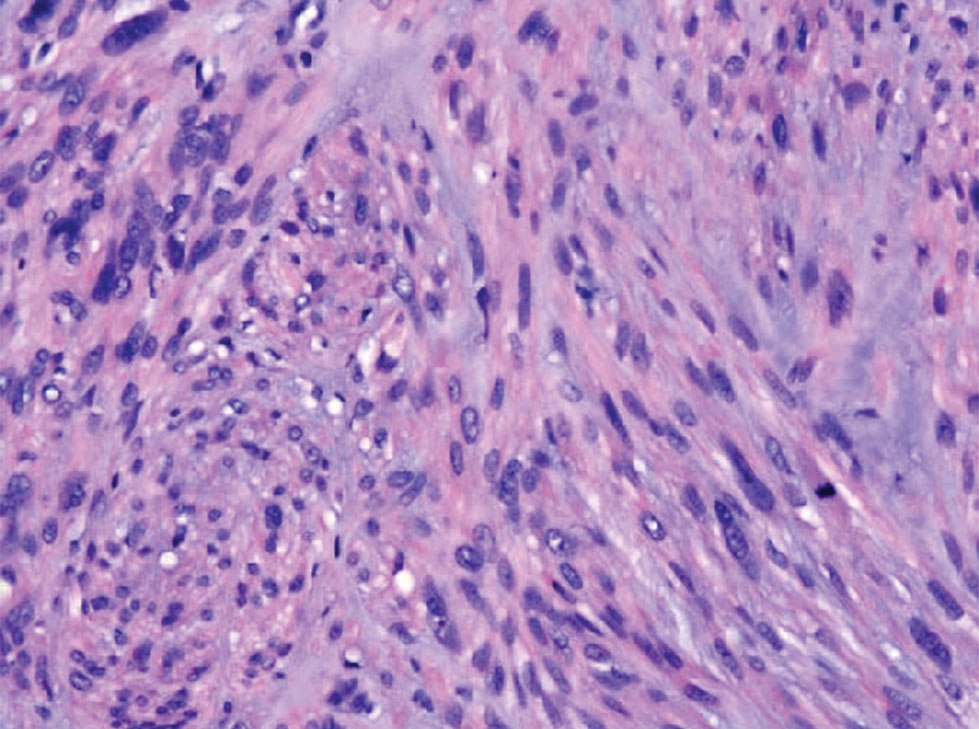
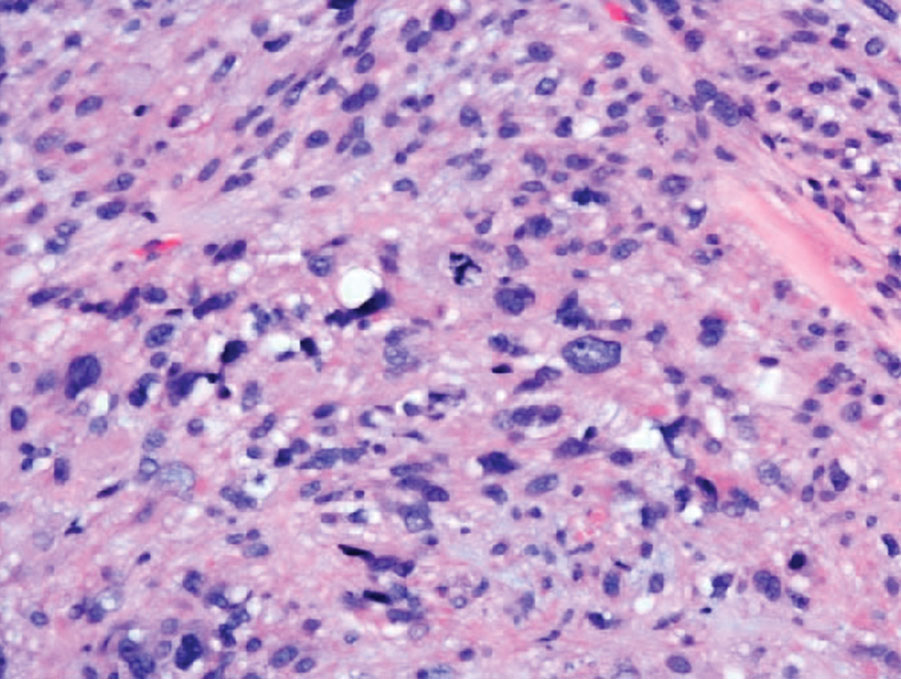
FDA acts against sales of unapproved mole and skin tag products on Amazon, other sites
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
Audit Proof Your Mohs Note
In October 2020, Medicare released an updated guidance to reduce Mohs micrographic surgery (MMS) reimbursement issues,1 which initially was released in 2013. This guidance defines the latest performance and documentation requirements that Medicare requires for MMS. Understanding these requirements and making sure that your Mohs surgical reports have all the needed documentation details are critical because auditors from not only Medicare Administrative Contractors (MACs) but also private insurers and Medicare Advantage plans have adopted these standards and will deny payment for Mohs surgical codes if they are not met. This article provides a review of the updated Medicare requirements to make sure your MMS procedure notes are audit proof.
Notes Must Indicate Mohs Is the Most Appropriate Treatment
I review many of my colleagues’ Mohs notes and can tell you that some of the requirements laid out in the updated guidance typically are already reported by Mohs surgeons in their notes, including the location, number, and size of the lesion or lesions treated and the number of stages performed. However, there are some new requirements that often are not reported by Mohs surgeons that now need to be included. The guidance indicates the following:
The majority of skin cancers can be managed by simple excision or destruction techniques. The medical record of a patient undergoing MMS should clearly show that this procedure was chosen because of the complexity (eg, poorly defined clinical borders, possible deep invasion, prior irradiation), size or location (eg, maximum conservation of tumor-free tissue is important). Medicare will consider reimbursement for MMS for accepted diagnoses and indications, which you must document in the patient’s medical record as being appropriate for MMS and that MMS is the most appropriate choice for the treatment of a particular lesion.1
In my experience, most Mohs notes include some statement that the skin cancer treated is appropriate based on the Mohs appropriate use criteria (AUC) or the AUC score. However, notes should make clear not just that the lesion treated is “appropriate” for MMS but also that it is the most appropriate treatment (eg, why the lesion was not managed by standard excision or destruction technique).
Mohs Surgeon Must Perform the Surgery and Interpret Slides
The updated guidance clearly indicates that MMS may only be performed by a physician who is specifically trained and highly skilled in Mohs techniques and pathologic identification: “Medicare will only reimburse for MMS services when the Mohs surgeon acts as both surgeon and pathologist.”1 Mohs micrographic surgery codes may not be billed if preparation or interpretation of the pathology slides is performed by a physician other than the Mohs surgeon. Operative notes and pathology documentation in the patient’s medical record should clearly show that MMS was performed using an accepted MMS technique in which the physician acts in 2 integrated and distinct capacities—surgeon and pathologist—thereby confirming that the procedure meets the definition of the Current Procedural Terminology code(s).
Furthermore, the Mohs operative report should detail “the number of specimens per stage.”1 I interpret this statement to indicate that the Mohs surgeon should document the number of tissue blocks examined in each stage of Mohs surgery. For example, a statement in the notes such as “the specimen from the first Mohs stage was oriented, mapped, and divided into 4 blocks” should suffice to meet this requirement.
Histologic Description Must Be Included in Mohs Notes
Medicare will require the Mohs surgeon to document “the histology of the specimens taken. That description should include depth of invasion, pathological pattern, cell morphology, and, if present, perineural invasion or presence of scar tissue.”1 Although this histologic description requirement appears daunting, it is common for Mohs surgeons to indicate their pathologic findings on their Mohs map such as “NBCC” next to a red area to indicate “nodular basal cell carcinoma visualized.” A template-based system to translate typical pathologic findings can be employed to rapidly and accurately populate a Mohs note with histologic description such as “NBBC=nodular aggregates of palisaded basaloid epithelial tumor arising from the epidermis forming a palisade with a cleft forming from the adjacent mucinous stroma extending to the mid dermis. Centrally the nuclei become crowded with scattered mitotic figures and necrotic bodies evident.”
Recent Improvement for 1-Stage Mohs Surgeries
The most notable improvement in the
Final Thoughts
Overall, the updated Medicare guidance provides important details in the requirements for performance and documentation of Mohs surgery cases. However, additional critical information will be found in Mohs coverage policies and local coverage determinations (LCDs) from MACs and private insurers.2-4 Each LCD and insurer Mohs payment policy has unique wording and requirements. Coverage of MMS for specific malignant diagnoses, histologic subtypes, locations, and clinical scenarios varies between LCDs; most are based directly on the Mohs AUC, while others have a less specific coverage criteria. To understand the specific documentation and coverage requirements of the MAC for a particular region or private insurer, Mohs surgeons are encouraged to familiarize themselves with the Mohs surgery LCD of their local MAC and coverage policies of their insurers and to ensure their documentation substantiates these requirements. Making sure that your MMS documentation is accurate and complies with Medicare and insurer requirements will keep you out of hot water with auditors and allow reimbursement for this critical skin cancer procedure.
- Centers for Disease Control and Prevention. Guidance to reduce Mohs surgery reimbursement issues. MLN Matters. Published October 27, 2020. Accessed July 18, 2022. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1318.pdf
- Mohs micrographic surgery policy, professional. United Healthcare website. Accessed July 12, 2022. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-reimbursement/COMM-Mohs-Micrographic-Surgery-Policy.pdf#:~:text=This%20policy%20describes%20reimbursement%20guidelines%20for%20reporting%20Mohs,CCI%20Editing%20Policy%20and%20the%20Laboratory%20Services%20Policy.
- Clinical UM guideline—Mohs micrographic surgery. Anthem Insurance Companies website. Published October 6, 2021. Accessed July 27, 2022. https://www.anthem.com/dam/medpolicies/abcbs/active/guidelines/gl_pw_d085074.html
- Local coverage determinations. Centers for Medicare and Medicaid Services website. Updated July 12, 2022. Accessed July 12, 2022. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
In October 2020, Medicare released an updated guidance to reduce Mohs micrographic surgery (MMS) reimbursement issues,1 which initially was released in 2013. This guidance defines the latest performance and documentation requirements that Medicare requires for MMS. Understanding these requirements and making sure that your Mohs surgical reports have all the needed documentation details are critical because auditors from not only Medicare Administrative Contractors (MACs) but also private insurers and Medicare Advantage plans have adopted these standards and will deny payment for Mohs surgical codes if they are not met. This article provides a review of the updated Medicare requirements to make sure your MMS procedure notes are audit proof.
Notes Must Indicate Mohs Is the Most Appropriate Treatment
I review many of my colleagues’ Mohs notes and can tell you that some of the requirements laid out in the updated guidance typically are already reported by Mohs surgeons in their notes, including the location, number, and size of the lesion or lesions treated and the number of stages performed. However, there are some new requirements that often are not reported by Mohs surgeons that now need to be included. The guidance indicates the following:
The majority of skin cancers can be managed by simple excision or destruction techniques. The medical record of a patient undergoing MMS should clearly show that this procedure was chosen because of the complexity (eg, poorly defined clinical borders, possible deep invasion, prior irradiation), size or location (eg, maximum conservation of tumor-free tissue is important). Medicare will consider reimbursement for MMS for accepted diagnoses and indications, which you must document in the patient’s medical record as being appropriate for MMS and that MMS is the most appropriate choice for the treatment of a particular lesion.1
In my experience, most Mohs notes include some statement that the skin cancer treated is appropriate based on the Mohs appropriate use criteria (AUC) or the AUC score. However, notes should make clear not just that the lesion treated is “appropriate” for MMS but also that it is the most appropriate treatment (eg, why the lesion was not managed by standard excision or destruction technique).
Mohs Surgeon Must Perform the Surgery and Interpret Slides
The updated guidance clearly indicates that MMS may only be performed by a physician who is specifically trained and highly skilled in Mohs techniques and pathologic identification: “Medicare will only reimburse for MMS services when the Mohs surgeon acts as both surgeon and pathologist.”1 Mohs micrographic surgery codes may not be billed if preparation or interpretation of the pathology slides is performed by a physician other than the Mohs surgeon. Operative notes and pathology documentation in the patient’s medical record should clearly show that MMS was performed using an accepted MMS technique in which the physician acts in 2 integrated and distinct capacities—surgeon and pathologist—thereby confirming that the procedure meets the definition of the Current Procedural Terminology code(s).
Furthermore, the Mohs operative report should detail “the number of specimens per stage.”1 I interpret this statement to indicate that the Mohs surgeon should document the number of tissue blocks examined in each stage of Mohs surgery. For example, a statement in the notes such as “the specimen from the first Mohs stage was oriented, mapped, and divided into 4 blocks” should suffice to meet this requirement.
Histologic Description Must Be Included in Mohs Notes
Medicare will require the Mohs surgeon to document “the histology of the specimens taken. That description should include depth of invasion, pathological pattern, cell morphology, and, if present, perineural invasion or presence of scar tissue.”1 Although this histologic description requirement appears daunting, it is common for Mohs surgeons to indicate their pathologic findings on their Mohs map such as “NBCC” next to a red area to indicate “nodular basal cell carcinoma visualized.” A template-based system to translate typical pathologic findings can be employed to rapidly and accurately populate a Mohs note with histologic description such as “NBBC=nodular aggregates of palisaded basaloid epithelial tumor arising from the epidermis forming a palisade with a cleft forming from the adjacent mucinous stroma extending to the mid dermis. Centrally the nuclei become crowded with scattered mitotic figures and necrotic bodies evident.”
Recent Improvement for 1-Stage Mohs Surgeries
The most notable improvement in the
Final Thoughts
Overall, the updated Medicare guidance provides important details in the requirements for performance and documentation of Mohs surgery cases. However, additional critical information will be found in Mohs coverage policies and local coverage determinations (LCDs) from MACs and private insurers.2-4 Each LCD and insurer Mohs payment policy has unique wording and requirements. Coverage of MMS for specific malignant diagnoses, histologic subtypes, locations, and clinical scenarios varies between LCDs; most are based directly on the Mohs AUC, while others have a less specific coverage criteria. To understand the specific documentation and coverage requirements of the MAC for a particular region or private insurer, Mohs surgeons are encouraged to familiarize themselves with the Mohs surgery LCD of their local MAC and coverage policies of their insurers and to ensure their documentation substantiates these requirements. Making sure that your MMS documentation is accurate and complies with Medicare and insurer requirements will keep you out of hot water with auditors and allow reimbursement for this critical skin cancer procedure.
In October 2020, Medicare released an updated guidance to reduce Mohs micrographic surgery (MMS) reimbursement issues,1 which initially was released in 2013. This guidance defines the latest performance and documentation requirements that Medicare requires for MMS. Understanding these requirements and making sure that your Mohs surgical reports have all the needed documentation details are critical because auditors from not only Medicare Administrative Contractors (MACs) but also private insurers and Medicare Advantage plans have adopted these standards and will deny payment for Mohs surgical codes if they are not met. This article provides a review of the updated Medicare requirements to make sure your MMS procedure notes are audit proof.
Notes Must Indicate Mohs Is the Most Appropriate Treatment
I review many of my colleagues’ Mohs notes and can tell you that some of the requirements laid out in the updated guidance typically are already reported by Mohs surgeons in their notes, including the location, number, and size of the lesion or lesions treated and the number of stages performed. However, there are some new requirements that often are not reported by Mohs surgeons that now need to be included. The guidance indicates the following:
The majority of skin cancers can be managed by simple excision or destruction techniques. The medical record of a patient undergoing MMS should clearly show that this procedure was chosen because of the complexity (eg, poorly defined clinical borders, possible deep invasion, prior irradiation), size or location (eg, maximum conservation of tumor-free tissue is important). Medicare will consider reimbursement for MMS for accepted diagnoses and indications, which you must document in the patient’s medical record as being appropriate for MMS and that MMS is the most appropriate choice for the treatment of a particular lesion.1
In my experience, most Mohs notes include some statement that the skin cancer treated is appropriate based on the Mohs appropriate use criteria (AUC) or the AUC score. However, notes should make clear not just that the lesion treated is “appropriate” for MMS but also that it is the most appropriate treatment (eg, why the lesion was not managed by standard excision or destruction technique).
Mohs Surgeon Must Perform the Surgery and Interpret Slides
The updated guidance clearly indicates that MMS may only be performed by a physician who is specifically trained and highly skilled in Mohs techniques and pathologic identification: “Medicare will only reimburse for MMS services when the Mohs surgeon acts as both surgeon and pathologist.”1 Mohs micrographic surgery codes may not be billed if preparation or interpretation of the pathology slides is performed by a physician other than the Mohs surgeon. Operative notes and pathology documentation in the patient’s medical record should clearly show that MMS was performed using an accepted MMS technique in which the physician acts in 2 integrated and distinct capacities—surgeon and pathologist—thereby confirming that the procedure meets the definition of the Current Procedural Terminology code(s).
Furthermore, the Mohs operative report should detail “the number of specimens per stage.”1 I interpret this statement to indicate that the Mohs surgeon should document the number of tissue blocks examined in each stage of Mohs surgery. For example, a statement in the notes such as “the specimen from the first Mohs stage was oriented, mapped, and divided into 4 blocks” should suffice to meet this requirement.
Histologic Description Must Be Included in Mohs Notes
Medicare will require the Mohs surgeon to document “the histology of the specimens taken. That description should include depth of invasion, pathological pattern, cell morphology, and, if present, perineural invasion or presence of scar tissue.”1 Although this histologic description requirement appears daunting, it is common for Mohs surgeons to indicate their pathologic findings on their Mohs map such as “NBCC” next to a red area to indicate “nodular basal cell carcinoma visualized.” A template-based system to translate typical pathologic findings can be employed to rapidly and accurately populate a Mohs note with histologic description such as “NBBC=nodular aggregates of palisaded basaloid epithelial tumor arising from the epidermis forming a palisade with a cleft forming from the adjacent mucinous stroma extending to the mid dermis. Centrally the nuclei become crowded with scattered mitotic figures and necrotic bodies evident.”
Recent Improvement for 1-Stage Mohs Surgeries
The most notable improvement in the
Final Thoughts
Overall, the updated Medicare guidance provides important details in the requirements for performance and documentation of Mohs surgery cases. However, additional critical information will be found in Mohs coverage policies and local coverage determinations (LCDs) from MACs and private insurers.2-4 Each LCD and insurer Mohs payment policy has unique wording and requirements. Coverage of MMS for specific malignant diagnoses, histologic subtypes, locations, and clinical scenarios varies between LCDs; most are based directly on the Mohs AUC, while others have a less specific coverage criteria. To understand the specific documentation and coverage requirements of the MAC for a particular region or private insurer, Mohs surgeons are encouraged to familiarize themselves with the Mohs surgery LCD of their local MAC and coverage policies of their insurers and to ensure their documentation substantiates these requirements. Making sure that your MMS documentation is accurate and complies with Medicare and insurer requirements will keep you out of hot water with auditors and allow reimbursement for this critical skin cancer procedure.
- Centers for Disease Control and Prevention. Guidance to reduce Mohs surgery reimbursement issues. MLN Matters. Published October 27, 2020. Accessed July 18, 2022. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1318.pdf
- Mohs micrographic surgery policy, professional. United Healthcare website. Accessed July 12, 2022. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-reimbursement/COMM-Mohs-Micrographic-Surgery-Policy.pdf#:~:text=This%20policy%20describes%20reimbursement%20guidelines%20for%20reporting%20Mohs,CCI%20Editing%20Policy%20and%20the%20Laboratory%20Services%20Policy.
- Clinical UM guideline—Mohs micrographic surgery. Anthem Insurance Companies website. Published October 6, 2021. Accessed July 27, 2022. https://www.anthem.com/dam/medpolicies/abcbs/active/guidelines/gl_pw_d085074.html
- Local coverage determinations. Centers for Medicare and Medicaid Services website. Updated July 12, 2022. Accessed July 12, 2022. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
- Centers for Disease Control and Prevention. Guidance to reduce Mohs surgery reimbursement issues. MLN Matters. Published October 27, 2020. Accessed July 18, 2022. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1318.pdf
- Mohs micrographic surgery policy, professional. United Healthcare website. Accessed July 12, 2022. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-reimbursement/COMM-Mohs-Micrographic-Surgery-Policy.pdf#:~:text=This%20policy%20describes%20reimbursement%20guidelines%20for%20reporting%20Mohs,CCI%20Editing%20Policy%20and%20the%20Laboratory%20Services%20Policy.
- Clinical UM guideline—Mohs micrographic surgery. Anthem Insurance Companies website. Published October 6, 2021. Accessed July 27, 2022. https://www.anthem.com/dam/medpolicies/abcbs/active/guidelines/gl_pw_d085074.html
- Local coverage determinations. Centers for Medicare and Medicaid Services website. Updated July 12, 2022. Accessed July 12, 2022. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
Practice Points
- Medicare’s updated guidance for documentation of Mohs micrographic surgery (MMS) includes some new requirements that Mohs surgeons should ensure are implemented in their Mohs records.
- Per Medicare guidance, MMS records should include a justification of why MMS was the most appropriate treatment and a description of the histologic findings from the Mohs slides.
- One major improvement with the updated documentation requirements is that if no tumor is visualized in the first stage of MMS, then no histology description of the tumor is required.