User login
ASCO announces its own COVID-19 and cancer registry
Data will not be commercialized, unlike CancerLinQ
The American Society of Clinical Oncology (ASCO) has launched a registry to collect data on cancer patients with COVID-19 and is asking oncology practices across the United States to share information about their patients with the infection for educational purposes.
The new registry joins at least two other cancer and COVID-19 patient registries already underway in the U.S.
In a statement, ASCO President Howard “Skip” Burris III, MD said there is a need to know “how the virus is impacting our patients, their cancer treatment, and outcomes to inform current cancer care” and future care.
The web-based registry, known as the American Society of Clinical Oncology (ASCO) Survey on COVID-19 in Oncology Registry, is open to all U.S. oncology practices. Participating practices will receive an unspecified “nominal” payment for their data entry efforts.
The registry patient information will be stored on ASCO’s “Big Data” platform, known as CancerLinQ, but is being held apart from that pool of data. The registry information will not be available for commercial purposes, ASCO spokesperson Rachel Martin recently told Medscape Medical News.
Separately, CancerLinQ, which is a wholly owned subsidiary of ASCO, will continue to collect data from its participant oncology practices (as usual), including COVID-19 information.
CancerLinQ has been criticized by ethicists for allowing partner companies to sell access to its data (after stripping off patient identifiers), but without asking for patients’ permission, as reported last year by Medscape Medical News.
Eleven practices, including academic enterprises, have so far expressed interested in participating in the ASCO COVID-19 Registry.
Participating practices are requested to send in details about cancer patients with a confirmed COVID-19 diagnosis. As well as a baseline data capture form, they will need to provide details of subsequent status, treatment, and outcomes. Some patient-identifying data, including zip code, date of birth, gender, race, ethnicity, type of cancer, and comorbidities, will be collected for the purposes of analysis.
ASCO hopes to learn about characteristics of patients with cancer most impacted by COVID-19; estimates of disease severity; treatment modifications or delays; implementation of telemedicine in the cancer treatment setting; and clinical outcomes related to both COVID-19 and cancer.
ASCO says it will deliver periodic reports to the cancer community and the broader public on these and other “key learnings.” It also says that the registry is designed to capture point-in-time data as well as longitudinal data on how the virus will impact care and outcomes into 2021.
ASCO is not alone in its data collection efforts.
The COVID-19 and Cancer Consortium is already collecting information from more than 50 cancer centers and organizations on COVID-19 in patients with cancer. The American Society of Hematology (ASH) Research Collaborative COVID-19 Registry for Hematologic Malignancy is doing the same but with a focus on hematologic malignancies.
This article first appeared on Medscape.com.
Data will not be commercialized, unlike CancerLinQ
Data will not be commercialized, unlike CancerLinQ
The American Society of Clinical Oncology (ASCO) has launched a registry to collect data on cancer patients with COVID-19 and is asking oncology practices across the United States to share information about their patients with the infection for educational purposes.
The new registry joins at least two other cancer and COVID-19 patient registries already underway in the U.S.
In a statement, ASCO President Howard “Skip” Burris III, MD said there is a need to know “how the virus is impacting our patients, their cancer treatment, and outcomes to inform current cancer care” and future care.
The web-based registry, known as the American Society of Clinical Oncology (ASCO) Survey on COVID-19 in Oncology Registry, is open to all U.S. oncology practices. Participating practices will receive an unspecified “nominal” payment for their data entry efforts.
The registry patient information will be stored on ASCO’s “Big Data” platform, known as CancerLinQ, but is being held apart from that pool of data. The registry information will not be available for commercial purposes, ASCO spokesperson Rachel Martin recently told Medscape Medical News.
Separately, CancerLinQ, which is a wholly owned subsidiary of ASCO, will continue to collect data from its participant oncology practices (as usual), including COVID-19 information.
CancerLinQ has been criticized by ethicists for allowing partner companies to sell access to its data (after stripping off patient identifiers), but without asking for patients’ permission, as reported last year by Medscape Medical News.
Eleven practices, including academic enterprises, have so far expressed interested in participating in the ASCO COVID-19 Registry.
Participating practices are requested to send in details about cancer patients with a confirmed COVID-19 diagnosis. As well as a baseline data capture form, they will need to provide details of subsequent status, treatment, and outcomes. Some patient-identifying data, including zip code, date of birth, gender, race, ethnicity, type of cancer, and comorbidities, will be collected for the purposes of analysis.
ASCO hopes to learn about characteristics of patients with cancer most impacted by COVID-19; estimates of disease severity; treatment modifications or delays; implementation of telemedicine in the cancer treatment setting; and clinical outcomes related to both COVID-19 and cancer.
ASCO says it will deliver periodic reports to the cancer community and the broader public on these and other “key learnings.” It also says that the registry is designed to capture point-in-time data as well as longitudinal data on how the virus will impact care and outcomes into 2021.
ASCO is not alone in its data collection efforts.
The COVID-19 and Cancer Consortium is already collecting information from more than 50 cancer centers and organizations on COVID-19 in patients with cancer. The American Society of Hematology (ASH) Research Collaborative COVID-19 Registry for Hematologic Malignancy is doing the same but with a focus on hematologic malignancies.
This article first appeared on Medscape.com.
The American Society of Clinical Oncology (ASCO) has launched a registry to collect data on cancer patients with COVID-19 and is asking oncology practices across the United States to share information about their patients with the infection for educational purposes.
The new registry joins at least two other cancer and COVID-19 patient registries already underway in the U.S.
In a statement, ASCO President Howard “Skip” Burris III, MD said there is a need to know “how the virus is impacting our patients, their cancer treatment, and outcomes to inform current cancer care” and future care.
The web-based registry, known as the American Society of Clinical Oncology (ASCO) Survey on COVID-19 in Oncology Registry, is open to all U.S. oncology practices. Participating practices will receive an unspecified “nominal” payment for their data entry efforts.
The registry patient information will be stored on ASCO’s “Big Data” platform, known as CancerLinQ, but is being held apart from that pool of data. The registry information will not be available for commercial purposes, ASCO spokesperson Rachel Martin recently told Medscape Medical News.
Separately, CancerLinQ, which is a wholly owned subsidiary of ASCO, will continue to collect data from its participant oncology practices (as usual), including COVID-19 information.
CancerLinQ has been criticized by ethicists for allowing partner companies to sell access to its data (after stripping off patient identifiers), but without asking for patients’ permission, as reported last year by Medscape Medical News.
Eleven practices, including academic enterprises, have so far expressed interested in participating in the ASCO COVID-19 Registry.
Participating practices are requested to send in details about cancer patients with a confirmed COVID-19 diagnosis. As well as a baseline data capture form, they will need to provide details of subsequent status, treatment, and outcomes. Some patient-identifying data, including zip code, date of birth, gender, race, ethnicity, type of cancer, and comorbidities, will be collected for the purposes of analysis.
ASCO hopes to learn about characteristics of patients with cancer most impacted by COVID-19; estimates of disease severity; treatment modifications or delays; implementation of telemedicine in the cancer treatment setting; and clinical outcomes related to both COVID-19 and cancer.
ASCO says it will deliver periodic reports to the cancer community and the broader public on these and other “key learnings.” It also says that the registry is designed to capture point-in-time data as well as longitudinal data on how the virus will impact care and outcomes into 2021.
ASCO is not alone in its data collection efforts.
The COVID-19 and Cancer Consortium is already collecting information from more than 50 cancer centers and organizations on COVID-19 in patients with cancer. The American Society of Hematology (ASH) Research Collaborative COVID-19 Registry for Hematologic Malignancy is doing the same but with a focus on hematologic malignancies.
This article first appeared on Medscape.com.
Cancer prevalence among COVID-19 patients may be higher than previously reported
An early report pegged the prevalence of cancer among COVID-19 patients at 1%, but authors of a recent meta-analysis found an overall prevalence of 2% and up to 3% depending on the subset of data they reviewed.
However, those findings are limited by the retrospective nature of the studies published to date, according to the authors of the meta-analysis, led by Aakash Desai, MBBS, of the University of Connecticut, Farmington.
Nevertheless, the results do confirm that cancer patients and survivors are an important at-risk population for COVID-19, according to Dr. Desai and colleagues.
“We hope that additional data from China and Italy will provide information on the characteristics of patients with cancer at risk, types of cancer that confer higher risk, and systemic regimens that may increase COVID-19 infection complications,” the authors wrote in JCO Global Oncology.
More than 15 million individuals with cancer and many more cancer survivors are at increased risk of COVID-19 because of compromised immune systems, according to the authors.
Exactly how many individuals with cancer are among the COVID-19 cases remains unclear, though a previous report suggested the prevalence of cancer was 1% (95% confidence interval, 0.61%-1.65%) among COVID-19 patients in China (Lancet Oncol. 2020 Mar;21[3]:335-7). This “seems to be higher” than the 0.29% prevalence of cancer in the overall Chinese population, the investigators noted at the time.
That study revealed 18 cancer patients among 1,590 COVID-19 cases, though it was “hypothesis generating,” according to Dr. Desai and colleagues, who rolled that data into their meta-analysis of 11 reports including 3,661 COVID-19 cases.
Overall, Dr. Desai and colleagues found the pooled prevalence of cancer was 2.0% (95% CI, 2.0%-3.0%) in that population. In a subgroup analysis of five studies with sample sizes of less than 100 COVID-19 patients, the researchers found a “slightly higher” prevalence of 3.0% (95% CI, 1.0%-6.0%).
However, even that data wasn’t robust enough for Dr. Desai and colleagues to make any pronouncements on cancer prevalence. “Overall, current evidence on the association between cancer and COVID-19 remains inconclusive,” they wrote.
Though inconclusive, the findings raise questions about whether treatments or interventions might need to be postponed in certain patients, whether cancer patients and survivors need stronger personal protection, and how to deal with potential delays in cancer clinical trials, according to Dr. Desai and colleagues.
“As the evidence continues to rise, we must strive to answer the unanswered clinical questions,” the authors wrote.
Dr. Desai and colleagues reported no potential conflicts of interest related to the study.
SOURCE: Desai A et al. JCO Glob Oncol. 2020 Apr 6. doi: 10.1200/GO.20.00097.
An early report pegged the prevalence of cancer among COVID-19 patients at 1%, but authors of a recent meta-analysis found an overall prevalence of 2% and up to 3% depending on the subset of data they reviewed.
However, those findings are limited by the retrospective nature of the studies published to date, according to the authors of the meta-analysis, led by Aakash Desai, MBBS, of the University of Connecticut, Farmington.
Nevertheless, the results do confirm that cancer patients and survivors are an important at-risk population for COVID-19, according to Dr. Desai and colleagues.
“We hope that additional data from China and Italy will provide information on the characteristics of patients with cancer at risk, types of cancer that confer higher risk, and systemic regimens that may increase COVID-19 infection complications,” the authors wrote in JCO Global Oncology.
More than 15 million individuals with cancer and many more cancer survivors are at increased risk of COVID-19 because of compromised immune systems, according to the authors.
Exactly how many individuals with cancer are among the COVID-19 cases remains unclear, though a previous report suggested the prevalence of cancer was 1% (95% confidence interval, 0.61%-1.65%) among COVID-19 patients in China (Lancet Oncol. 2020 Mar;21[3]:335-7). This “seems to be higher” than the 0.29% prevalence of cancer in the overall Chinese population, the investigators noted at the time.
That study revealed 18 cancer patients among 1,590 COVID-19 cases, though it was “hypothesis generating,” according to Dr. Desai and colleagues, who rolled that data into their meta-analysis of 11 reports including 3,661 COVID-19 cases.
Overall, Dr. Desai and colleagues found the pooled prevalence of cancer was 2.0% (95% CI, 2.0%-3.0%) in that population. In a subgroup analysis of five studies with sample sizes of less than 100 COVID-19 patients, the researchers found a “slightly higher” prevalence of 3.0% (95% CI, 1.0%-6.0%).
However, even that data wasn’t robust enough for Dr. Desai and colleagues to make any pronouncements on cancer prevalence. “Overall, current evidence on the association between cancer and COVID-19 remains inconclusive,” they wrote.
Though inconclusive, the findings raise questions about whether treatments or interventions might need to be postponed in certain patients, whether cancer patients and survivors need stronger personal protection, and how to deal with potential delays in cancer clinical trials, according to Dr. Desai and colleagues.
“As the evidence continues to rise, we must strive to answer the unanswered clinical questions,” the authors wrote.
Dr. Desai and colleagues reported no potential conflicts of interest related to the study.
SOURCE: Desai A et al. JCO Glob Oncol. 2020 Apr 6. doi: 10.1200/GO.20.00097.
An early report pegged the prevalence of cancer among COVID-19 patients at 1%, but authors of a recent meta-analysis found an overall prevalence of 2% and up to 3% depending on the subset of data they reviewed.
However, those findings are limited by the retrospective nature of the studies published to date, according to the authors of the meta-analysis, led by Aakash Desai, MBBS, of the University of Connecticut, Farmington.
Nevertheless, the results do confirm that cancer patients and survivors are an important at-risk population for COVID-19, according to Dr. Desai and colleagues.
“We hope that additional data from China and Italy will provide information on the characteristics of patients with cancer at risk, types of cancer that confer higher risk, and systemic regimens that may increase COVID-19 infection complications,” the authors wrote in JCO Global Oncology.
More than 15 million individuals with cancer and many more cancer survivors are at increased risk of COVID-19 because of compromised immune systems, according to the authors.
Exactly how many individuals with cancer are among the COVID-19 cases remains unclear, though a previous report suggested the prevalence of cancer was 1% (95% confidence interval, 0.61%-1.65%) among COVID-19 patients in China (Lancet Oncol. 2020 Mar;21[3]:335-7). This “seems to be higher” than the 0.29% prevalence of cancer in the overall Chinese population, the investigators noted at the time.
That study revealed 18 cancer patients among 1,590 COVID-19 cases, though it was “hypothesis generating,” according to Dr. Desai and colleagues, who rolled that data into their meta-analysis of 11 reports including 3,661 COVID-19 cases.
Overall, Dr. Desai and colleagues found the pooled prevalence of cancer was 2.0% (95% CI, 2.0%-3.0%) in that population. In a subgroup analysis of five studies with sample sizes of less than 100 COVID-19 patients, the researchers found a “slightly higher” prevalence of 3.0% (95% CI, 1.0%-6.0%).
However, even that data wasn’t robust enough for Dr. Desai and colleagues to make any pronouncements on cancer prevalence. “Overall, current evidence on the association between cancer and COVID-19 remains inconclusive,” they wrote.
Though inconclusive, the findings raise questions about whether treatments or interventions might need to be postponed in certain patients, whether cancer patients and survivors need stronger personal protection, and how to deal with potential delays in cancer clinical trials, according to Dr. Desai and colleagues.
“As the evidence continues to rise, we must strive to answer the unanswered clinical questions,” the authors wrote.
Dr. Desai and colleagues reported no potential conflicts of interest related to the study.
SOURCE: Desai A et al. JCO Glob Oncol. 2020 Apr 6. doi: 10.1200/GO.20.00097.
FROM JCO GLOBAL ONCOLOGY
Home-based chemo skyrockets at one U.S. center
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
Major organization opposes concept
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
Cutaneous Metastases From Esophageal Adenocarcinoma on the Scalp
To the Editor:
A 59-year-old man presented with a lesion on the right frontal scalp of 4 months’ duration and a lesion on the left frontal scalp of 1 month’s duration. Both lesions were tender, bleeding, nonhealing, and growing in size. The patient reported no improvement with the use of triple antibiotic ointment. He denied any associated symptoms or trauma to the affected areas. He had a history of stage IV esophageal adenocarcinoma that initially had been surgically removed 6 years prior but metastasized to the lungs and bone. The patient subsequently underwent treatment with FOLFOX (folinic acid, fluorouracil, oxaliplatin), trastuzumab, and radiation therapy.
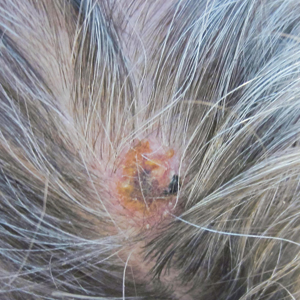
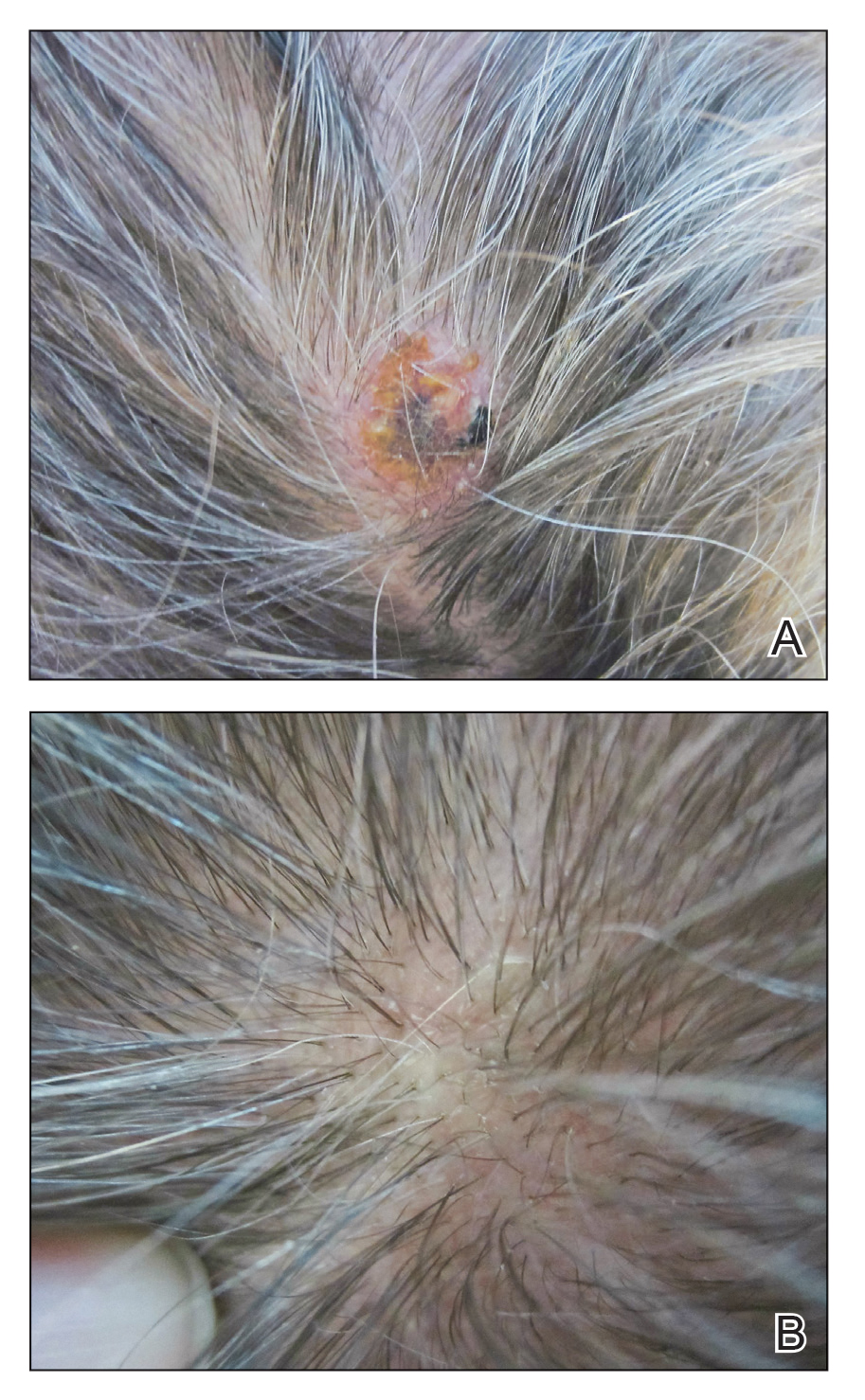
Physical examination revealed a hyperkeratotic pink nodule with a central erosion and crust on the right frontal scalp measuring 1.5×2 cm in diameter (Figure 1A). The left frontal scalp lesion was a smooth pearly papule measuring 5×5 mm in diameter (Figure 1B). The differential diagnosis included basal cell carcinoma, squamous cell carcinoma, and cutaneous metastases from esophageal adenocarcinoma. Shave biopsies were taken of both scalp lesions.
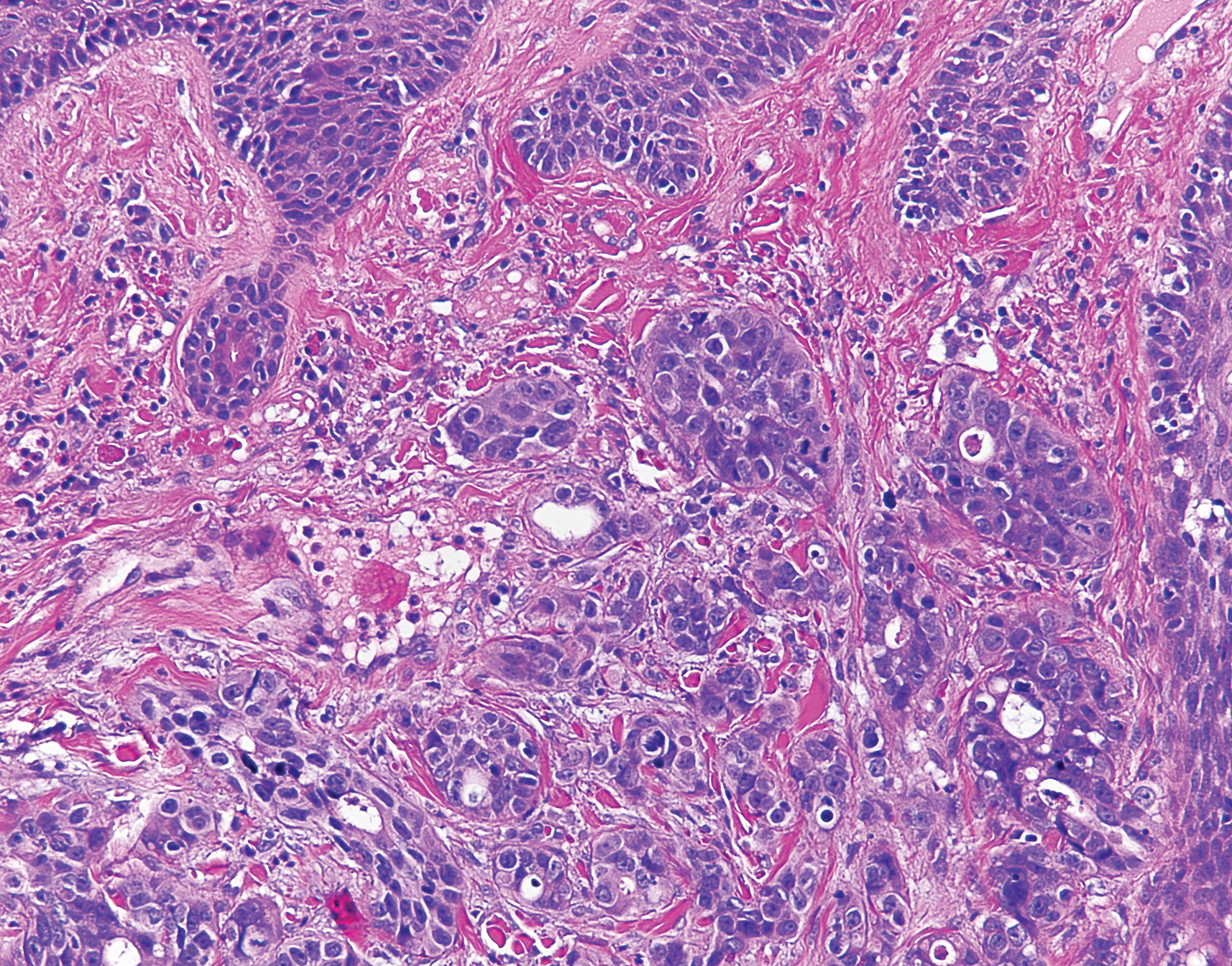
Histologic examination of both scalp lesions demonstrated a dermal gland-forming neoplasm with an infiltrative distribution that was comprised of irregular cribriform glands containing cellular debris (Figure 2). The cells of interest were enlarged and contained pleomorphic crowded nuclei that formed aberrant mitotic division figures. Both biopsies were positive for cytokeratin 7 and negative for cytokeratin 20 and CDX2. The final diagnosis for both scalp lesions was poorly differentiated adenocarcinoma, which was most suggestive of cutaneous metastases of the patient’s known esophageal adenocarcinoma. Given further metastasis, the patient was ultimately switched to ramucirumab and paclitaxel per oncology.
Esophageal carcinoma is the eighth most common cause of death related to cancer worldwide. Adenocarcinoma is the most prevalent histologic type of esophageal carcinoma, with an incidence as high as 5.69 per 100,000 individuals in the United States.1 Internal malignancies that lead to cutaneous metastases are not uncommon; however, the literature is limited on cutaneous scalp metastases from esophageal cancer. Cutaneous metastases secondary to internal malignancies present in less than 10% of overall cases; tend to derive from the breasts, lungs, and large bowel; and usually present in the sixth to seventh decades of life.2 Further, roughly 1% of all skin metastases originate from the esophagus.3 When there are cutaneous metastases to the scalp, they often arise from breast carcinomas and renal cell carcinomas.4,5 Rarely does esophageal cancer spread to the scalp.2,6-9 When cutaneous metastases originate from the esophagus, multiple cancers such as squamous cell carcinomas, mucoepidermoid carcinomas, small cell carcinomas, and adenocarcinomas can be the etiology of origin.10 Metastases originating from esophageal carcinomas frequently are diagnosed in the abdominal lymph nodes (45%), liver (35%), lungs (20%), cervical/supraclavicular lymph nodes (18%), bones (9%), adrenals (5%), peritoneum (2%), brain (2%), stomach (1%), pancreas (1%), pleura (1%), skin/body wall (1%), pericardium (1%), and spleen (1%).3 Additionally, multiple cutaneous scalp metastases from esophageal adenocarcinoma have been reported,7,9 as were seen in our case.
The clinical appearance of cutaneous scalp metastases has been described as inflammatory papules, indurated plaques, or nodules,2 which is consistent with our case, though the spectrum of presentation is admittedly broad. Histopathology of lesions characteristically shows prominent intraluminal necrotic cellular debris, which is common for adenocarcinomas of the gastrointestinal tract.7 However, utilizing immunohistochemical stains to detect specific antigens within tumor cells allows for better specificity of the tumor origin. More specifically, cytokeratin 7 and cytokeratin 20 are stained in esophageal metaplasia, such as Barrett esophagus, rather than in intestinal metaplasia inside the stomach.2,11 Therefore, discerning the location of the adenocarcinoma proves fruitful when using cytokeratin 7 and cytokeratin 20. Although CDX2 is an additional marker that can be used for gastrointestinal adenocarcinomas with decent sensitivity and specificity, it can still be expressed in mucinous ovarian carcinomas and urinary bladder adenocarcinomas.12 In our patient, the strong reactivity of cytokeratin 7 in addition to the characteristic morphology in both presenting biopsies was sufficient to make the diagnosis of cutaneous metastasis of esophageal adenocarcinoma to the scalp.
Our case highlights multiple cutaneous metastases of the scalp from a primary esophageal adenocarcinoma. Although cutaneous scalp metastasis of esophageal adenocarcinoma is rare, it is essential to provide a full-body skin examination, including the scalp, in patients with a history of esophageal cancer and to biopsy any suspicious nodules or plaques. The 1-year survival rate after diagnosis of esophageal carcinoma is less than 50%, and the 5-year survival rate is less than 10%.13 Identifying cutaneous metastasis of an esophageal adenocarcinoma can either change the staging of the cancer (if it was the first distant metastasis noted) or indicate an insufficient response to treatment in a patient with known metastatic disease, prompting a potential change in treatment.7
This case illustrates a rare site of metastasis of a fairly common cancer and highlights the histopathology and accompanying immunohistochemical stains that can be useful in diagnosis as well as the spectrum of its clinical presentation.
- Melhado R, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404.
- Park JM, Kim DS, Oh SH, et al. A case of esophageal adenocarcinoma metastasized to the scalp [published online May 31, 2009]. Ann Dermatol. 2009;21:164-167.
- Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120.
- Dobson C, Tagor V, Myint A, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635-636.
- Riter H, Ghobrial I. Renal cell carcinoma with acrometastasis and scalp metastasis. Mayo Clin Proc. 2004;79:76.
- Roh EK, Nord R, Jukic DM. Scalp metastasis from esophageal adenocarcinoma. Cutis. 2006;77:106.
- Doumit G, Abouhassan W, Piliang M, et al. Scalp metastasis from esophageal adenocarcinoma: comparative histopathology dictates surgical approach. Ann Plast Surg. 2011;71:60-62.
- Roy AD, Sherparpa M, Prasad PR, et al. Scalp metastasis of gastro-esophageal junction adenocarcinoma: a rare occurrence. 2014;8:159-160.
- Stein R, Spencer J. Painful cutaneous metastases from esophageal carcinoma. Cutis. 2002;70:230.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182.
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Smith KJ, Williams J, Skelton H. Metastatic adenocarcinoma of the esophagus to the skin: new patterns of tumor recurrence and alternate treatments for palliation. J Cutan Pathol. 2001;28:425-431.
To the Editor:
A 59-year-old man presented with a lesion on the right frontal scalp of 4 months’ duration and a lesion on the left frontal scalp of 1 month’s duration. Both lesions were tender, bleeding, nonhealing, and growing in size. The patient reported no improvement with the use of triple antibiotic ointment. He denied any associated symptoms or trauma to the affected areas. He had a history of stage IV esophageal adenocarcinoma that initially had been surgically removed 6 years prior but metastasized to the lungs and bone. The patient subsequently underwent treatment with FOLFOX (folinic acid, fluorouracil, oxaliplatin), trastuzumab, and radiation therapy.
Physical examination revealed a hyperkeratotic pink nodule with a central erosion and crust on the right frontal scalp measuring 1.5×2 cm in diameter (Figure 1A). The left frontal scalp lesion was a smooth pearly papule measuring 5×5 mm in diameter (Figure 1B). The differential diagnosis included basal cell carcinoma, squamous cell carcinoma, and cutaneous metastases from esophageal adenocarcinoma. Shave biopsies were taken of both scalp lesions.

Histologic examination of both scalp lesions demonstrated a dermal gland-forming neoplasm with an infiltrative distribution that was comprised of irregular cribriform glands containing cellular debris (Figure 2). The cells of interest were enlarged and contained pleomorphic crowded nuclei that formed aberrant mitotic division figures. Both biopsies were positive for cytokeratin 7 and negative for cytokeratin 20 and CDX2. The final diagnosis for both scalp lesions was poorly differentiated adenocarcinoma, which was most suggestive of cutaneous metastases of the patient’s known esophageal adenocarcinoma. Given further metastasis, the patient was ultimately switched to ramucirumab and paclitaxel per oncology.

Esophageal carcinoma is the eighth most common cause of death related to cancer worldwide. Adenocarcinoma is the most prevalent histologic type of esophageal carcinoma, with an incidence as high as 5.69 per 100,000 individuals in the United States.1 Internal malignancies that lead to cutaneous metastases are not uncommon; however, the literature is limited on cutaneous scalp metastases from esophageal cancer. Cutaneous metastases secondary to internal malignancies present in less than 10% of overall cases; tend to derive from the breasts, lungs, and large bowel; and usually present in the sixth to seventh decades of life.2 Further, roughly 1% of all skin metastases originate from the esophagus.3 When there are cutaneous metastases to the scalp, they often arise from breast carcinomas and renal cell carcinomas.4,5 Rarely does esophageal cancer spread to the scalp.2,6-9 When cutaneous metastases originate from the esophagus, multiple cancers such as squamous cell carcinomas, mucoepidermoid carcinomas, small cell carcinomas, and adenocarcinomas can be the etiology of origin.10 Metastases originating from esophageal carcinomas frequently are diagnosed in the abdominal lymph nodes (45%), liver (35%), lungs (20%), cervical/supraclavicular lymph nodes (18%), bones (9%), adrenals (5%), peritoneum (2%), brain (2%), stomach (1%), pancreas (1%), pleura (1%), skin/body wall (1%), pericardium (1%), and spleen (1%).3 Additionally, multiple cutaneous scalp metastases from esophageal adenocarcinoma have been reported,7,9 as were seen in our case.
The clinical appearance of cutaneous scalp metastases has been described as inflammatory papules, indurated plaques, or nodules,2 which is consistent with our case, though the spectrum of presentation is admittedly broad. Histopathology of lesions characteristically shows prominent intraluminal necrotic cellular debris, which is common for adenocarcinomas of the gastrointestinal tract.7 However, utilizing immunohistochemical stains to detect specific antigens within tumor cells allows for better specificity of the tumor origin. More specifically, cytokeratin 7 and cytokeratin 20 are stained in esophageal metaplasia, such as Barrett esophagus, rather than in intestinal metaplasia inside the stomach.2,11 Therefore, discerning the location of the adenocarcinoma proves fruitful when using cytokeratin 7 and cytokeratin 20. Although CDX2 is an additional marker that can be used for gastrointestinal adenocarcinomas with decent sensitivity and specificity, it can still be expressed in mucinous ovarian carcinomas and urinary bladder adenocarcinomas.12 In our patient, the strong reactivity of cytokeratin 7 in addition to the characteristic morphology in both presenting biopsies was sufficient to make the diagnosis of cutaneous metastasis of esophageal adenocarcinoma to the scalp.
Our case highlights multiple cutaneous metastases of the scalp from a primary esophageal adenocarcinoma. Although cutaneous scalp metastasis of esophageal adenocarcinoma is rare, it is essential to provide a full-body skin examination, including the scalp, in patients with a history of esophageal cancer and to biopsy any suspicious nodules or plaques. The 1-year survival rate after diagnosis of esophageal carcinoma is less than 50%, and the 5-year survival rate is less than 10%.13 Identifying cutaneous metastasis of an esophageal adenocarcinoma can either change the staging of the cancer (if it was the first distant metastasis noted) or indicate an insufficient response to treatment in a patient with known metastatic disease, prompting a potential change in treatment.7
This case illustrates a rare site of metastasis of a fairly common cancer and highlights the histopathology and accompanying immunohistochemical stains that can be useful in diagnosis as well as the spectrum of its clinical presentation.
To the Editor:
A 59-year-old man presented with a lesion on the right frontal scalp of 4 months’ duration and a lesion on the left frontal scalp of 1 month’s duration. Both lesions were tender, bleeding, nonhealing, and growing in size. The patient reported no improvement with the use of triple antibiotic ointment. He denied any associated symptoms or trauma to the affected areas. He had a history of stage IV esophageal adenocarcinoma that initially had been surgically removed 6 years prior but metastasized to the lungs and bone. The patient subsequently underwent treatment with FOLFOX (folinic acid, fluorouracil, oxaliplatin), trastuzumab, and radiation therapy.
Physical examination revealed a hyperkeratotic pink nodule with a central erosion and crust on the right frontal scalp measuring 1.5×2 cm in diameter (Figure 1A). The left frontal scalp lesion was a smooth pearly papule measuring 5×5 mm in diameter (Figure 1B). The differential diagnosis included basal cell carcinoma, squamous cell carcinoma, and cutaneous metastases from esophageal adenocarcinoma. Shave biopsies were taken of both scalp lesions.

Histologic examination of both scalp lesions demonstrated a dermal gland-forming neoplasm with an infiltrative distribution that was comprised of irregular cribriform glands containing cellular debris (Figure 2). The cells of interest were enlarged and contained pleomorphic crowded nuclei that formed aberrant mitotic division figures. Both biopsies were positive for cytokeratin 7 and negative for cytokeratin 20 and CDX2. The final diagnosis for both scalp lesions was poorly differentiated adenocarcinoma, which was most suggestive of cutaneous metastases of the patient’s known esophageal adenocarcinoma. Given further metastasis, the patient was ultimately switched to ramucirumab and paclitaxel per oncology.

Esophageal carcinoma is the eighth most common cause of death related to cancer worldwide. Adenocarcinoma is the most prevalent histologic type of esophageal carcinoma, with an incidence as high as 5.69 per 100,000 individuals in the United States.1 Internal malignancies that lead to cutaneous metastases are not uncommon; however, the literature is limited on cutaneous scalp metastases from esophageal cancer. Cutaneous metastases secondary to internal malignancies present in less than 10% of overall cases; tend to derive from the breasts, lungs, and large bowel; and usually present in the sixth to seventh decades of life.2 Further, roughly 1% of all skin metastases originate from the esophagus.3 When there are cutaneous metastases to the scalp, they often arise from breast carcinomas and renal cell carcinomas.4,5 Rarely does esophageal cancer spread to the scalp.2,6-9 When cutaneous metastases originate from the esophagus, multiple cancers such as squamous cell carcinomas, mucoepidermoid carcinomas, small cell carcinomas, and adenocarcinomas can be the etiology of origin.10 Metastases originating from esophageal carcinomas frequently are diagnosed in the abdominal lymph nodes (45%), liver (35%), lungs (20%), cervical/supraclavicular lymph nodes (18%), bones (9%), adrenals (5%), peritoneum (2%), brain (2%), stomach (1%), pancreas (1%), pleura (1%), skin/body wall (1%), pericardium (1%), and spleen (1%).3 Additionally, multiple cutaneous scalp metastases from esophageal adenocarcinoma have been reported,7,9 as were seen in our case.
The clinical appearance of cutaneous scalp metastases has been described as inflammatory papules, indurated plaques, or nodules,2 which is consistent with our case, though the spectrum of presentation is admittedly broad. Histopathology of lesions characteristically shows prominent intraluminal necrotic cellular debris, which is common for adenocarcinomas of the gastrointestinal tract.7 However, utilizing immunohistochemical stains to detect specific antigens within tumor cells allows for better specificity of the tumor origin. More specifically, cytokeratin 7 and cytokeratin 20 are stained in esophageal metaplasia, such as Barrett esophagus, rather than in intestinal metaplasia inside the stomach.2,11 Therefore, discerning the location of the adenocarcinoma proves fruitful when using cytokeratin 7 and cytokeratin 20. Although CDX2 is an additional marker that can be used for gastrointestinal adenocarcinomas with decent sensitivity and specificity, it can still be expressed in mucinous ovarian carcinomas and urinary bladder adenocarcinomas.12 In our patient, the strong reactivity of cytokeratin 7 in addition to the characteristic morphology in both presenting biopsies was sufficient to make the diagnosis of cutaneous metastasis of esophageal adenocarcinoma to the scalp.
Our case highlights multiple cutaneous metastases of the scalp from a primary esophageal adenocarcinoma. Although cutaneous scalp metastasis of esophageal adenocarcinoma is rare, it is essential to provide a full-body skin examination, including the scalp, in patients with a history of esophageal cancer and to biopsy any suspicious nodules or plaques. The 1-year survival rate after diagnosis of esophageal carcinoma is less than 50%, and the 5-year survival rate is less than 10%.13 Identifying cutaneous metastasis of an esophageal adenocarcinoma can either change the staging of the cancer (if it was the first distant metastasis noted) or indicate an insufficient response to treatment in a patient with known metastatic disease, prompting a potential change in treatment.7
This case illustrates a rare site of metastasis of a fairly common cancer and highlights the histopathology and accompanying immunohistochemical stains that can be useful in diagnosis as well as the spectrum of its clinical presentation.
- Melhado R, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404.
- Park JM, Kim DS, Oh SH, et al. A case of esophageal adenocarcinoma metastasized to the scalp [published online May 31, 2009]. Ann Dermatol. 2009;21:164-167.
- Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120.
- Dobson C, Tagor V, Myint A, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635-636.
- Riter H, Ghobrial I. Renal cell carcinoma with acrometastasis and scalp metastasis. Mayo Clin Proc. 2004;79:76.
- Roh EK, Nord R, Jukic DM. Scalp metastasis from esophageal adenocarcinoma. Cutis. 2006;77:106.
- Doumit G, Abouhassan W, Piliang M, et al. Scalp metastasis from esophageal adenocarcinoma: comparative histopathology dictates surgical approach. Ann Plast Surg. 2011;71:60-62.
- Roy AD, Sherparpa M, Prasad PR, et al. Scalp metastasis of gastro-esophageal junction adenocarcinoma: a rare occurrence. 2014;8:159-160.
- Stein R, Spencer J. Painful cutaneous metastases from esophageal carcinoma. Cutis. 2002;70:230.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182.
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Smith KJ, Williams J, Skelton H. Metastatic adenocarcinoma of the esophagus to the skin: new patterns of tumor recurrence and alternate treatments for palliation. J Cutan Pathol. 2001;28:425-431.
- Melhado R, Alderson D, Tucker O. The changing face of esophageal cancer. Cancers (Basel). 2010;2:1379-1404.
- Park JM, Kim DS, Oh SH, et al. A case of esophageal adenocarcinoma metastasized to the scalp [published online May 31, 2009]. Ann Dermatol. 2009;21:164-167.
- Quint LE, Hepburn LM, Francis IR, et al. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120.
- Dobson C, Tagor V, Myint A, et al. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635-636.
- Riter H, Ghobrial I. Renal cell carcinoma with acrometastasis and scalp metastasis. Mayo Clin Proc. 2004;79:76.
- Roh EK, Nord R, Jukic DM. Scalp metastasis from esophageal adenocarcinoma. Cutis. 2006;77:106.
- Doumit G, Abouhassan W, Piliang M, et al. Scalp metastasis from esophageal adenocarcinoma: comparative histopathology dictates surgical approach. Ann Plast Surg. 2011;71:60-62.
- Roy AD, Sherparpa M, Prasad PR, et al. Scalp metastasis of gastro-esophageal junction adenocarcinoma: a rare occurrence. 2014;8:159-160.
- Stein R, Spencer J. Painful cutaneous metastases from esophageal carcinoma. Cutis. 2002;70:230.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182.
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294.
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310.
- Smith KJ, Williams J, Skelton H. Metastatic adenocarcinoma of the esophagus to the skin: new patterns of tumor recurrence and alternate treatments for palliation. J Cutan Pathol. 2001;28:425-431.
Practice Points
- In the setting of underlying esophageal adenocarcinoma, metastatic spread to the scalp should be considered in the differential diagnosis for any suspicious scalp lesions.
- Coupling histopathology with immunohistochemical stains may aid in the diagnosis for cutaneous metastasis of esophageal adenocarcinoma.
Conducting cancer trials amid the COVID-19 pandemic
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
NCCN panel: Defer nonurgent skin cancer care during pandemic
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
‘Brutal’ plan to restrict palliative radiation during pandemic
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
Advice from the front lines: How cancer centers can cope with COVID-19
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
No staff COVID-19 diagnoses after plan at Chinese cancer center
Short-term results
No staff members or patients were diagnosed with COVID-19 after “strict protective measures” for screening and managing patients were implemented at the National Cancer Center/Cancer Hospital, Chinese Academy of Sciences, in Beijing, according to a report published online April 1 in JAMA Oncology.
However, the time period for the analysis, which included nearly 3000 patients, was short — only about 3 weeks (February 12 to March 3). Also, Beijing is more than 1100 kilometers from Wuhan, the center of the Chinese outbreak of COVID-19.
The Beijing cancer hospital implemented a multipronged safety plan in February in order to “avoid COVID-19 related nosocomial cross-infection between patients and medical staff,” explain the authors, led by medical oncologist Zhijie Wang, MD.
Notably, “all of the measures taken in China are actively being implemented and used in major oncology centers in the United States,” Robert Carlson, MD, chief executive officer, National Comprehensive Cancer Network (NCCN), told Medscape Medical News.
John Greene, MD, section chief, Infectious Disease and Tropical Medicine, Moffitt Cancer Center, Tampa, Florida, pointed out that the Chinese safety plan, which is full of “good measures,” is being largely used at his center. However, he observed that one tool — doing a temperature check at the hospital front door — is not well supported by most of the literature. “It gives good optics and looks like you are doing the most you possibly can, but scientifically it may not be as effective [as other screening measures],” he said.
The Chinese plan consists of four broad elements
First, the above-mentioned on-site temperature tests are performed at the entrances of the hospital, outpatient clinic, and wards. Contact and travel histories related to the Wuhan epidemic area are also established and recorded.
Second, an outpatient appointment scheduling system allows both online scheduling and on-site registration. Online consultation channels are open daily, featuring instruction on medication taking and cancer-related symptom management. These “substantially reduced the flow of people in the hospital,” write the authors. On-site patients must wear a mask and have their own disinfectant.
Third, for patients with cancer preparing to be admitted to hospital, symptoms associated with COVID-19, such as fever and cough, are recorded. Mandatory blood tests and CT scans of the lungs are performed. COVID-19 virus nucleic acid tests are performed for patients with suspected pneumonia on imaging.
Fourth, some anticancer drugs conventionally administered by infusion have been changed to oral administration, such as etoposide and vinorelbine. For adjuvant or maintenance chemotherapy, the infusion intervals were appropriately prolonged depending on patients’ conditions.
Eight out of 2,900 patients had imaging suspicious for infection
The Chinese authors report that a total of 2,944 patients with cancer were seen for clinic consultation and treatment in the wards (2795 outpatients and 149 inpatients).
Patients with cancer are believed to have a higher probability of severe illness and increased mortality compared with the healthy population once infected with COVID-19, point out the authors.
Under the new “strict screening strategy,” 27 patients showed radiologic manifestations of inflammatory changes or multiple-site exudative pneumonia in the lungs, including eight suspected of having COVID-19 infection. “Fortunately, negative results from nucleic acid testing ultimately excluded COVID-19 infection in all these patients,” the authors report.
However, two of these patients “presented with recovered pneumonia after symptomatic treatment.” Commenting on this finding, Moffitt’s Greene said that may mean these two patients were tested and found to be positive but were early in the infection and not yet shedding the virus, or they were infected after the initial negative result.
Greene said his center has implemented some measures not mentioned in the Chinese plan. For example, the Florida center no longer allows inpatient visitation. Also, one third of staff now work from home, resulting in less social interaction. Social distancing in meetings, the cafeteria, and hallways is being observed “aggressively,” and most meetings are now on Zoom, he said.
Moffitt has not been hard hit with COVID-19 and is at level one preparedness, the lowest rung. The center has performed 60 tests to date, with only one positive for the virus (< 2%), Greene told Medscape Medical News.
Currently, in the larger Tampa Bay community setting, about 12% of tests are positive.
The low percentage found among the Moffitt patients “tells you that a lot of cancer patients have fever and respiratory symptoms due to other viruses and, more importantly, other reasons, whether it’s their immunotherapy or chemotherapy or their cancer,” said Greene.
NCCN’s Carlson said the publication of the Chinese data was a good sign in terms of international science.
“This is a strong example of how the global oncology community rapidly shares information and experience whenever it makes a difference in patient care,” he commented.
The authors, as well as Carlson and Greene, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Short-term results
Short-term results
No staff members or patients were diagnosed with COVID-19 after “strict protective measures” for screening and managing patients were implemented at the National Cancer Center/Cancer Hospital, Chinese Academy of Sciences, in Beijing, according to a report published online April 1 in JAMA Oncology.
However, the time period for the analysis, which included nearly 3000 patients, was short — only about 3 weeks (February 12 to March 3). Also, Beijing is more than 1100 kilometers from Wuhan, the center of the Chinese outbreak of COVID-19.
The Beijing cancer hospital implemented a multipronged safety plan in February in order to “avoid COVID-19 related nosocomial cross-infection between patients and medical staff,” explain the authors, led by medical oncologist Zhijie Wang, MD.
Notably, “all of the measures taken in China are actively being implemented and used in major oncology centers in the United States,” Robert Carlson, MD, chief executive officer, National Comprehensive Cancer Network (NCCN), told Medscape Medical News.
John Greene, MD, section chief, Infectious Disease and Tropical Medicine, Moffitt Cancer Center, Tampa, Florida, pointed out that the Chinese safety plan, which is full of “good measures,” is being largely used at his center. However, he observed that one tool — doing a temperature check at the hospital front door — is not well supported by most of the literature. “It gives good optics and looks like you are doing the most you possibly can, but scientifically it may not be as effective [as other screening measures],” he said.
The Chinese plan consists of four broad elements
First, the above-mentioned on-site temperature tests are performed at the entrances of the hospital, outpatient clinic, and wards. Contact and travel histories related to the Wuhan epidemic area are also established and recorded.
Second, an outpatient appointment scheduling system allows both online scheduling and on-site registration. Online consultation channels are open daily, featuring instruction on medication taking and cancer-related symptom management. These “substantially reduced the flow of people in the hospital,” write the authors. On-site patients must wear a mask and have their own disinfectant.
Third, for patients with cancer preparing to be admitted to hospital, symptoms associated with COVID-19, such as fever and cough, are recorded. Mandatory blood tests and CT scans of the lungs are performed. COVID-19 virus nucleic acid tests are performed for patients with suspected pneumonia on imaging.
Fourth, some anticancer drugs conventionally administered by infusion have been changed to oral administration, such as etoposide and vinorelbine. For adjuvant or maintenance chemotherapy, the infusion intervals were appropriately prolonged depending on patients’ conditions.
Eight out of 2,900 patients had imaging suspicious for infection
The Chinese authors report that a total of 2,944 patients with cancer were seen for clinic consultation and treatment in the wards (2795 outpatients and 149 inpatients).
Patients with cancer are believed to have a higher probability of severe illness and increased mortality compared with the healthy population once infected with COVID-19, point out the authors.
Under the new “strict screening strategy,” 27 patients showed radiologic manifestations of inflammatory changes or multiple-site exudative pneumonia in the lungs, including eight suspected of having COVID-19 infection. “Fortunately, negative results from nucleic acid testing ultimately excluded COVID-19 infection in all these patients,” the authors report.
However, two of these patients “presented with recovered pneumonia after symptomatic treatment.” Commenting on this finding, Moffitt’s Greene said that may mean these two patients were tested and found to be positive but were early in the infection and not yet shedding the virus, or they were infected after the initial negative result.
Greene said his center has implemented some measures not mentioned in the Chinese plan. For example, the Florida center no longer allows inpatient visitation. Also, one third of staff now work from home, resulting in less social interaction. Social distancing in meetings, the cafeteria, and hallways is being observed “aggressively,” and most meetings are now on Zoom, he said.
Moffitt has not been hard hit with COVID-19 and is at level one preparedness, the lowest rung. The center has performed 60 tests to date, with only one positive for the virus (< 2%), Greene told Medscape Medical News.
Currently, in the larger Tampa Bay community setting, about 12% of tests are positive.
The low percentage found among the Moffitt patients “tells you that a lot of cancer patients have fever and respiratory symptoms due to other viruses and, more importantly, other reasons, whether it’s their immunotherapy or chemotherapy or their cancer,” said Greene.
NCCN’s Carlson said the publication of the Chinese data was a good sign in terms of international science.
“This is a strong example of how the global oncology community rapidly shares information and experience whenever it makes a difference in patient care,” he commented.
The authors, as well as Carlson and Greene, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
No staff members or patients were diagnosed with COVID-19 after “strict protective measures” for screening and managing patients were implemented at the National Cancer Center/Cancer Hospital, Chinese Academy of Sciences, in Beijing, according to a report published online April 1 in JAMA Oncology.
However, the time period for the analysis, which included nearly 3000 patients, was short — only about 3 weeks (February 12 to March 3). Also, Beijing is more than 1100 kilometers from Wuhan, the center of the Chinese outbreak of COVID-19.
The Beijing cancer hospital implemented a multipronged safety plan in February in order to “avoid COVID-19 related nosocomial cross-infection between patients and medical staff,” explain the authors, led by medical oncologist Zhijie Wang, MD.
Notably, “all of the measures taken in China are actively being implemented and used in major oncology centers in the United States,” Robert Carlson, MD, chief executive officer, National Comprehensive Cancer Network (NCCN), told Medscape Medical News.
John Greene, MD, section chief, Infectious Disease and Tropical Medicine, Moffitt Cancer Center, Tampa, Florida, pointed out that the Chinese safety plan, which is full of “good measures,” is being largely used at his center. However, he observed that one tool — doing a temperature check at the hospital front door — is not well supported by most of the literature. “It gives good optics and looks like you are doing the most you possibly can, but scientifically it may not be as effective [as other screening measures],” he said.
The Chinese plan consists of four broad elements
First, the above-mentioned on-site temperature tests are performed at the entrances of the hospital, outpatient clinic, and wards. Contact and travel histories related to the Wuhan epidemic area are also established and recorded.
Second, an outpatient appointment scheduling system allows both online scheduling and on-site registration. Online consultation channels are open daily, featuring instruction on medication taking and cancer-related symptom management. These “substantially reduced the flow of people in the hospital,” write the authors. On-site patients must wear a mask and have their own disinfectant.
Third, for patients with cancer preparing to be admitted to hospital, symptoms associated with COVID-19, such as fever and cough, are recorded. Mandatory blood tests and CT scans of the lungs are performed. COVID-19 virus nucleic acid tests are performed for patients with suspected pneumonia on imaging.
Fourth, some anticancer drugs conventionally administered by infusion have been changed to oral administration, such as etoposide and vinorelbine. For adjuvant or maintenance chemotherapy, the infusion intervals were appropriately prolonged depending on patients’ conditions.
Eight out of 2,900 patients had imaging suspicious for infection
The Chinese authors report that a total of 2,944 patients with cancer were seen for clinic consultation and treatment in the wards (2795 outpatients and 149 inpatients).
Patients with cancer are believed to have a higher probability of severe illness and increased mortality compared with the healthy population once infected with COVID-19, point out the authors.
Under the new “strict screening strategy,” 27 patients showed radiologic manifestations of inflammatory changes or multiple-site exudative pneumonia in the lungs, including eight suspected of having COVID-19 infection. “Fortunately, negative results from nucleic acid testing ultimately excluded COVID-19 infection in all these patients,” the authors report.
However, two of these patients “presented with recovered pneumonia after symptomatic treatment.” Commenting on this finding, Moffitt’s Greene said that may mean these two patients were tested and found to be positive but were early in the infection and not yet shedding the virus, or they were infected after the initial negative result.
Greene said his center has implemented some measures not mentioned in the Chinese plan. For example, the Florida center no longer allows inpatient visitation. Also, one third of staff now work from home, resulting in less social interaction. Social distancing in meetings, the cafeteria, and hallways is being observed “aggressively,” and most meetings are now on Zoom, he said.
Moffitt has not been hard hit with COVID-19 and is at level one preparedness, the lowest rung. The center has performed 60 tests to date, with only one positive for the virus (< 2%), Greene told Medscape Medical News.
Currently, in the larger Tampa Bay community setting, about 12% of tests are positive.
The low percentage found among the Moffitt patients “tells you that a lot of cancer patients have fever and respiratory symptoms due to other viruses and, more importantly, other reasons, whether it’s their immunotherapy or chemotherapy or their cancer,” said Greene.
NCCN’s Carlson said the publication of the Chinese data was a good sign in terms of international science.
“This is a strong example of how the global oncology community rapidly shares information and experience whenever it makes a difference in patient care,” he commented.
The authors, as well as Carlson and Greene, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Maintaining cancer care in the face of COVID-19
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.