User login
Black Children With Vitiligo at Increased Risk for Psychiatric Disorders: Study
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Black children with vitiligo are significantly more likely to be diagnosed with psychiatric disorders, including depression, suicidal ideation, and disruptive behavior disorders, than matched controls who did not have vitiligo, according to a case-control study.
METHODOLOGY:
- Researchers conducted a retrospective, single-center, case-control study at Texas Children’s Hospital in Houston on 327 Black children with vitiligo and 981 matched controls without vitiligo.
- The average age of participants was 11.7 years, and 62% were girls.
- The study outcome was the prevalence of psychiatric conditions and rates of treatment (pharmacotherapy and/or psychotherapy) initiation for those conditions.
TAKEAWAY:
- Black children with vitiligo were more likely to be diagnosed with depression (odds ratio [OR], 3.63; P < .001), suicidal ideation (OR, 2.88; P = .005), disruptive behavior disorders (OR, 7.68; P < .001), eating disorders (OR, 15.22; P = .013), generalized anxiety disorder (OR, 2.61; P < .001), and substance abuse (OR, 2.67; P = .011).
- The likelihood of having a psychiatric comorbidity was not significantly different between children with segmental vitiligo and those with generalized vitiligo or between girls and boys.
- Among the patients with vitiligo and psychiatric comorbidities, treatment initiation rates were higher for depression (76.5%), disruptive behavior disorders (82.1%), and eating disorders (100%).
- Treatment initiation rates were lower in patients with vitiligo diagnosed with generalized anxiety disorder (55.3%) and substance abuse (61.5%). Treatment was not initiated in 14% patients with suicidal ideation.
IN PRACTICE:
“Pediatric dermatologists have an important role in screening for psychiatric comorbidities, and implementation of appropriate screening tools while treating vitiligo is likely to have a bidirectional positive impact,” the authors wrote, adding: “By better understanding psychiatric comorbidities of African American children with vitiligo, dermatologists can be more aware of pediatric mental health needs and provide appropriate referrals.”
SOURCE:
This study was led by Emily Strouphauer, BSA, Baylor College of Medicine, Houston, and was published online in JAAD International.
LIMITATIONS:
The study limitations were the retrospective design, small sample size, and heterogeneity in the control group.
DISCLOSURES:
The study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Parents’ Technology Use May Shape Adolescents’ Mental Health
, according to a new study based in Canada.
In fact, this parental “technoference” is associated with higher levels of inattention and hyperactivity symptoms later in the child’s development, the researchers found.
“We hear a lot about children’s and adolescents’ screen time in the media, but we forget that parents are also on their screens a lot. In fact, past research shows that when parents are with their children, they spend 1 in 3 minutes on a screen,” said lead author Audrey-Ann Deneault, PhD, assistant professor of social psychology at the University of Montreal, Montreal, Quebec, Canada.
“We’ve all experienced moments when we’re on the phone and not hearing someone call us or don’t notice something happening right before our eyes,” she said. “We think that’s why it’s important to look at technoference. When parents use screens, they are more likely to miss when their child needs them.”
The study was published online in JAMA Network Open.
Analyzing Parental Technoference
As part of the All Our Families study, Dr. Deneault and colleagues analyzed a cohort of mothers and 1303 emerging adolescents between ages 9 and 11 years in Calgary, with the aim of understanding long-term associations between perceived parental interruptions (or technoference) and their children’s mental health.
Women were recruited during pregnancy between May 2008 and December 2010. For this study, the adolescents were assessed three times — at ages 9 years (in 2020), 10 years (in 2021), and 11 years (in 2021 and 2022). The mothers gave consent for their children to participate, and the children gave assent as well.
During the assessments, the adolescents completed questionnaires about their perceptions of parental technoference and their mental health symptoms, such as anxiety, depression, inattention, and hyperactivity. The study focused on the magnitude of effect sizes rather than statistical significance.
Overall, higher levels of anxiety symptoms at ages 9 and 10 years were prospectively associated with higher levels of perceived parental technoference at ages 10 and 11 years. The effect size was small.
In addition, higher levels of perceived parental technoference at ages 9 and 10 years were prospectively associated with higher levels of hyperactivity at ages 10 and 11 years and higher levels of inattention at age 11 years. There were no significant differences by gender.
“Technoference and youth mental health interact in complex ways. We found that when emerging adolescents have higher rates of anxiety, this can prompt parents to engage in more technoference,” Dr. Deneault said. “This latter bit highlights that parents may be struggling when their youths have mental health difficulties.”
Considering Healthy Changes
The findings call for a multitiered approach, Dr. Deneault said, in which adolescents and parents receive support related to mental health concerns, technology use, and healthy parent-child interactions.
“The key takeaway is that parents’ screen time matters and should begin to be a part of the conversation when we think about child and adolescent mental health,” she said.
Future research should look at the direction of associations between adolescent mental health and parental technoference, as well as underlying mechanisms, specific activities linked to technoference, and different age groups and stages of development, the study authors wrote.
“As a society, we need to understand how parents’ use of technology can interfere or not with youths’ mental health,” said Nicole Letourneau, PhD, a research professor of pediatrics, psychiatry, and community health sciences focused on parent and child health at the University of Calgary, Calgary, Alberta, Canada.
Dr. Letourneau, who wasn’t involved in this study, has researched the effects of parental technoference on parent-child relationships and child health and developmental outcomes. She and her colleagues found that parents recognized changes in their child’s behavior.
“Parental support is important for healthy development, and if parents are distracted by their devices, they can miss important but subtle cues that youth are using to signal their needs,” she said. “Given the troubling rise in youth mental health problems, we need to understand potential contributors so we can offer ways to reduce risks and promote youth mental health.”
Communication with parents should be considered as well. For instance, healthcare providers can address the positive and negative aspects of technology use.
“There is enough research out now that we should be more concerned than we currently are about how parents’ own technology habits might influence child and teen well-being. Yet, taking an overall negative lens to parent technology and smartphone habits may not prove very fruitful,” said Brandon McDaniel, PhD, a senior research scientist at the Parkview Mirro Center for Research & Innovation in Fort Wayne, Indiana.
Dr. McDaniel, who also wasn’t involved with this study, has researched technoference and associations with child behavior problems, as well as parents’ desires to change phone use. He noted that parents may use their devices for positive reasons, such as finding support from others, regulating their own emotions, and escaping from stress, so they can be more emotionally available for their children soon after using their phone.
“Many parents already feel an immense amount of guilt surrounding smartphone use in the presence of their child,” he said. “I suggest that practitioners address parent technology use in ways that validate parents in their positive uses of technology while helping them identify areas of their tech habits that may be counterproductive for their own or their child’s health and mental health.”
The All Our Families study was supported by an Alberta Innovates–Health Solutions Interdisciplinary Team Grant and the Alberta Children’s Hospital Foundation. The current analysis received funding from the Canadian Institutes of Health Research, a Children and Screens: Institute of Digital Media and Child Development COVID-19 grant, an Alberta Innovates grant, and a Banting Postdoctoral Fellowship. Dr. Deneault, Dr. Letourneau, and Dr. McDaniel reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study based in Canada.
In fact, this parental “technoference” is associated with higher levels of inattention and hyperactivity symptoms later in the child’s development, the researchers found.
“We hear a lot about children’s and adolescents’ screen time in the media, but we forget that parents are also on their screens a lot. In fact, past research shows that when parents are with their children, they spend 1 in 3 minutes on a screen,” said lead author Audrey-Ann Deneault, PhD, assistant professor of social psychology at the University of Montreal, Montreal, Quebec, Canada.
“We’ve all experienced moments when we’re on the phone and not hearing someone call us or don’t notice something happening right before our eyes,” she said. “We think that’s why it’s important to look at technoference. When parents use screens, they are more likely to miss when their child needs them.”
The study was published online in JAMA Network Open.
Analyzing Parental Technoference
As part of the All Our Families study, Dr. Deneault and colleagues analyzed a cohort of mothers and 1303 emerging adolescents between ages 9 and 11 years in Calgary, with the aim of understanding long-term associations between perceived parental interruptions (or technoference) and their children’s mental health.
Women were recruited during pregnancy between May 2008 and December 2010. For this study, the adolescents were assessed three times — at ages 9 years (in 2020), 10 years (in 2021), and 11 years (in 2021 and 2022). The mothers gave consent for their children to participate, and the children gave assent as well.
During the assessments, the adolescents completed questionnaires about their perceptions of parental technoference and their mental health symptoms, such as anxiety, depression, inattention, and hyperactivity. The study focused on the magnitude of effect sizes rather than statistical significance.
Overall, higher levels of anxiety symptoms at ages 9 and 10 years were prospectively associated with higher levels of perceived parental technoference at ages 10 and 11 years. The effect size was small.
In addition, higher levels of perceived parental technoference at ages 9 and 10 years were prospectively associated with higher levels of hyperactivity at ages 10 and 11 years and higher levels of inattention at age 11 years. There were no significant differences by gender.
“Technoference and youth mental health interact in complex ways. We found that when emerging adolescents have higher rates of anxiety, this can prompt parents to engage in more technoference,” Dr. Deneault said. “This latter bit highlights that parents may be struggling when their youths have mental health difficulties.”
Considering Healthy Changes
The findings call for a multitiered approach, Dr. Deneault said, in which adolescents and parents receive support related to mental health concerns, technology use, and healthy parent-child interactions.
“The key takeaway is that parents’ screen time matters and should begin to be a part of the conversation when we think about child and adolescent mental health,” she said.
Future research should look at the direction of associations between adolescent mental health and parental technoference, as well as underlying mechanisms, specific activities linked to technoference, and different age groups and stages of development, the study authors wrote.
“As a society, we need to understand how parents’ use of technology can interfere or not with youths’ mental health,” said Nicole Letourneau, PhD, a research professor of pediatrics, psychiatry, and community health sciences focused on parent and child health at the University of Calgary, Calgary, Alberta, Canada.
Dr. Letourneau, who wasn’t involved in this study, has researched the effects of parental technoference on parent-child relationships and child health and developmental outcomes. She and her colleagues found that parents recognized changes in their child’s behavior.
“Parental support is important for healthy development, and if parents are distracted by their devices, they can miss important but subtle cues that youth are using to signal their needs,” she said. “Given the troubling rise in youth mental health problems, we need to understand potential contributors so we can offer ways to reduce risks and promote youth mental health.”
Communication with parents should be considered as well. For instance, healthcare providers can address the positive and negative aspects of technology use.
“There is enough research out now that we should be more concerned than we currently are about how parents’ own technology habits might influence child and teen well-being. Yet, taking an overall negative lens to parent technology and smartphone habits may not prove very fruitful,” said Brandon McDaniel, PhD, a senior research scientist at the Parkview Mirro Center for Research & Innovation in Fort Wayne, Indiana.
Dr. McDaniel, who also wasn’t involved with this study, has researched technoference and associations with child behavior problems, as well as parents’ desires to change phone use. He noted that parents may use their devices for positive reasons, such as finding support from others, regulating their own emotions, and escaping from stress, so they can be more emotionally available for their children soon after using their phone.
“Many parents already feel an immense amount of guilt surrounding smartphone use in the presence of their child,” he said. “I suggest that practitioners address parent technology use in ways that validate parents in their positive uses of technology while helping them identify areas of their tech habits that may be counterproductive for their own or their child’s health and mental health.”
The All Our Families study was supported by an Alberta Innovates–Health Solutions Interdisciplinary Team Grant and the Alberta Children’s Hospital Foundation. The current analysis received funding from the Canadian Institutes of Health Research, a Children and Screens: Institute of Digital Media and Child Development COVID-19 grant, an Alberta Innovates grant, and a Banting Postdoctoral Fellowship. Dr. Deneault, Dr. Letourneau, and Dr. McDaniel reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study based in Canada.
In fact, this parental “technoference” is associated with higher levels of inattention and hyperactivity symptoms later in the child’s development, the researchers found.
“We hear a lot about children’s and adolescents’ screen time in the media, but we forget that parents are also on their screens a lot. In fact, past research shows that when parents are with their children, they spend 1 in 3 minutes on a screen,” said lead author Audrey-Ann Deneault, PhD, assistant professor of social psychology at the University of Montreal, Montreal, Quebec, Canada.
“We’ve all experienced moments when we’re on the phone and not hearing someone call us or don’t notice something happening right before our eyes,” she said. “We think that’s why it’s important to look at technoference. When parents use screens, they are more likely to miss when their child needs them.”
The study was published online in JAMA Network Open.
Analyzing Parental Technoference
As part of the All Our Families study, Dr. Deneault and colleagues analyzed a cohort of mothers and 1303 emerging adolescents between ages 9 and 11 years in Calgary, with the aim of understanding long-term associations between perceived parental interruptions (or technoference) and their children’s mental health.
Women were recruited during pregnancy between May 2008 and December 2010. For this study, the adolescents were assessed three times — at ages 9 years (in 2020), 10 years (in 2021), and 11 years (in 2021 and 2022). The mothers gave consent for their children to participate, and the children gave assent as well.
During the assessments, the adolescents completed questionnaires about their perceptions of parental technoference and their mental health symptoms, such as anxiety, depression, inattention, and hyperactivity. The study focused on the magnitude of effect sizes rather than statistical significance.
Overall, higher levels of anxiety symptoms at ages 9 and 10 years were prospectively associated with higher levels of perceived parental technoference at ages 10 and 11 years. The effect size was small.
In addition, higher levels of perceived parental technoference at ages 9 and 10 years were prospectively associated with higher levels of hyperactivity at ages 10 and 11 years and higher levels of inattention at age 11 years. There were no significant differences by gender.
“Technoference and youth mental health interact in complex ways. We found that when emerging adolescents have higher rates of anxiety, this can prompt parents to engage in more technoference,” Dr. Deneault said. “This latter bit highlights that parents may be struggling when their youths have mental health difficulties.”
Considering Healthy Changes
The findings call for a multitiered approach, Dr. Deneault said, in which adolescents and parents receive support related to mental health concerns, technology use, and healthy parent-child interactions.
“The key takeaway is that parents’ screen time matters and should begin to be a part of the conversation when we think about child and adolescent mental health,” she said.
Future research should look at the direction of associations between adolescent mental health and parental technoference, as well as underlying mechanisms, specific activities linked to technoference, and different age groups and stages of development, the study authors wrote.
“As a society, we need to understand how parents’ use of technology can interfere or not with youths’ mental health,” said Nicole Letourneau, PhD, a research professor of pediatrics, psychiatry, and community health sciences focused on parent and child health at the University of Calgary, Calgary, Alberta, Canada.
Dr. Letourneau, who wasn’t involved in this study, has researched the effects of parental technoference on parent-child relationships and child health and developmental outcomes. She and her colleagues found that parents recognized changes in their child’s behavior.
“Parental support is important for healthy development, and if parents are distracted by their devices, they can miss important but subtle cues that youth are using to signal their needs,” she said. “Given the troubling rise in youth mental health problems, we need to understand potential contributors so we can offer ways to reduce risks and promote youth mental health.”
Communication with parents should be considered as well. For instance, healthcare providers can address the positive and negative aspects of technology use.
“There is enough research out now that we should be more concerned than we currently are about how parents’ own technology habits might influence child and teen well-being. Yet, taking an overall negative lens to parent technology and smartphone habits may not prove very fruitful,” said Brandon McDaniel, PhD, a senior research scientist at the Parkview Mirro Center for Research & Innovation in Fort Wayne, Indiana.
Dr. McDaniel, who also wasn’t involved with this study, has researched technoference and associations with child behavior problems, as well as parents’ desires to change phone use. He noted that parents may use their devices for positive reasons, such as finding support from others, regulating their own emotions, and escaping from stress, so they can be more emotionally available for their children soon after using their phone.
“Many parents already feel an immense amount of guilt surrounding smartphone use in the presence of their child,” he said. “I suggest that practitioners address parent technology use in ways that validate parents in their positive uses of technology while helping them identify areas of their tech habits that may be counterproductive for their own or their child’s health and mental health.”
The All Our Families study was supported by an Alberta Innovates–Health Solutions Interdisciplinary Team Grant and the Alberta Children’s Hospital Foundation. The current analysis received funding from the Canadian Institutes of Health Research, a Children and Screens: Institute of Digital Media and Child Development COVID-19 grant, an Alberta Innovates grant, and a Banting Postdoctoral Fellowship. Dr. Deneault, Dr. Letourneau, and Dr. McDaniel reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
How Intermittent Fasting Could Transform Adolescent Obesity
TOPLINE:
METHODOLOGY:
- Researchers conducted a 52-week randomized clinical trial at two pediatric centers in Australia that involved 141 adolescents aged 13-17 years with obesity and at least one associated complication.
- Participants were divided into two groups: IER and CER, with three phases: Very low-energy diet (weeks 0-4), intensive intervention (weeks 5-16), and continued intervention/maintenance (weeks 17-52).
- Interventions included a very low-energy diet of 3350 kJ/d (800 kcal/d) for the first 4 weeks, followed by either IER intervention (2500-2950 kJ [600-700 kcal 3 days/wk]) or a daily CER intervention (6000-8000 kJ/d based on age; 1430-1670 kcal/d for teens aged 13-14 years and 1670-1900 kcal/d for teens aged 15-17 years).
- Participants were provided with multivitamins and met with dietitians regularly, with additional support via telephone, text message, or email.
TAKEAWAY:
- Teens in both the IER and CER groups showed a 0.28 reduction in BMI z-scores at 52 weeks with no significant differences between the two.
- The researchers observed no differences in body composition or cardiometabolic outcomes between the IER and CER groups.
- The occurrence of insulin resistance was reduced in both groups at week 16, but this effect was maintained only in the CER group at week 52.
- The study found no significant differences in the occurrence of dyslipidemia or impaired hepatic function between the IER and CER groups.
IN PRACTICE:
“These findings suggest that for adolescents with obesity-associated complications, IER can be incorporated into a behavioral weight management program, providing an option in addition to CER and offering participants more choice,” the authors of the study wrote.
SOURCE:
The study was led by Natalie B. Lister, PhD, of the University of Sydney in Australia and was published online in JAMA Pediatrics.
LIMITATIONS:
The COVID-19 pandemic and subsequent lockdowns limited the sample size. Some dietitian visits were conducted via telehealth.
DISCLOSURES:
Dr. Lister received grants from the National Health and Medical Research Council of Australia. A coauthor, Louise A. Baur, MBBS, PhD, received speakers’ fees from Novo Nordisk and served as a member of the Eli Lilly Advisory Committee.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a 52-week randomized clinical trial at two pediatric centers in Australia that involved 141 adolescents aged 13-17 years with obesity and at least one associated complication.
- Participants were divided into two groups: IER and CER, with three phases: Very low-energy diet (weeks 0-4), intensive intervention (weeks 5-16), and continued intervention/maintenance (weeks 17-52).
- Interventions included a very low-energy diet of 3350 kJ/d (800 kcal/d) for the first 4 weeks, followed by either IER intervention (2500-2950 kJ [600-700 kcal 3 days/wk]) or a daily CER intervention (6000-8000 kJ/d based on age; 1430-1670 kcal/d for teens aged 13-14 years and 1670-1900 kcal/d for teens aged 15-17 years).
- Participants were provided with multivitamins and met with dietitians regularly, with additional support via telephone, text message, or email.
TAKEAWAY:
- Teens in both the IER and CER groups showed a 0.28 reduction in BMI z-scores at 52 weeks with no significant differences between the two.
- The researchers observed no differences in body composition or cardiometabolic outcomes between the IER and CER groups.
- The occurrence of insulin resistance was reduced in both groups at week 16, but this effect was maintained only in the CER group at week 52.
- The study found no significant differences in the occurrence of dyslipidemia or impaired hepatic function between the IER and CER groups.
IN PRACTICE:
“These findings suggest that for adolescents with obesity-associated complications, IER can be incorporated into a behavioral weight management program, providing an option in addition to CER and offering participants more choice,” the authors of the study wrote.
SOURCE:
The study was led by Natalie B. Lister, PhD, of the University of Sydney in Australia and was published online in JAMA Pediatrics.
LIMITATIONS:
The COVID-19 pandemic and subsequent lockdowns limited the sample size. Some dietitian visits were conducted via telehealth.
DISCLOSURES:
Dr. Lister received grants from the National Health and Medical Research Council of Australia. A coauthor, Louise A. Baur, MBBS, PhD, received speakers’ fees from Novo Nordisk and served as a member of the Eli Lilly Advisory Committee.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a 52-week randomized clinical trial at two pediatric centers in Australia that involved 141 adolescents aged 13-17 years with obesity and at least one associated complication.
- Participants were divided into two groups: IER and CER, with three phases: Very low-energy diet (weeks 0-4), intensive intervention (weeks 5-16), and continued intervention/maintenance (weeks 17-52).
- Interventions included a very low-energy diet of 3350 kJ/d (800 kcal/d) for the first 4 weeks, followed by either IER intervention (2500-2950 kJ [600-700 kcal 3 days/wk]) or a daily CER intervention (6000-8000 kJ/d based on age; 1430-1670 kcal/d for teens aged 13-14 years and 1670-1900 kcal/d for teens aged 15-17 years).
- Participants were provided with multivitamins and met with dietitians regularly, with additional support via telephone, text message, or email.
TAKEAWAY:
- Teens in both the IER and CER groups showed a 0.28 reduction in BMI z-scores at 52 weeks with no significant differences between the two.
- The researchers observed no differences in body composition or cardiometabolic outcomes between the IER and CER groups.
- The occurrence of insulin resistance was reduced in both groups at week 16, but this effect was maintained only in the CER group at week 52.
- The study found no significant differences in the occurrence of dyslipidemia or impaired hepatic function between the IER and CER groups.
IN PRACTICE:
“These findings suggest that for adolescents with obesity-associated complications, IER can be incorporated into a behavioral weight management program, providing an option in addition to CER and offering participants more choice,” the authors of the study wrote.
SOURCE:
The study was led by Natalie B. Lister, PhD, of the University of Sydney in Australia and was published online in JAMA Pediatrics.
LIMITATIONS:
The COVID-19 pandemic and subsequent lockdowns limited the sample size. Some dietitian visits were conducted via telehealth.
DISCLOSURES:
Dr. Lister received grants from the National Health and Medical Research Council of Australia. A coauthor, Louise A. Baur, MBBS, PhD, received speakers’ fees from Novo Nordisk and served as a member of the Eli Lilly Advisory Committee.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
COVID-19 Booster Vaccine Shortens Menstrual Cycles in Teens
TOPLINE:
The vaccine did not appear to be associated with shifts in menstrual flow, pain, or other symptoms.
METHODOLOGY:
- Reports of menstrual cycle changes following the COVID-19 vaccination began to emerge in early 2021, raising concerns about the impact of the vaccine on menstrual health.
- Researchers conducted a prospective study including 65 adolescent girls (mean age, 17.3 years), of whom 47 had received an initial series of COVID-19 vaccination at least 6 months prior to receiving a booster dose (booster group), and 18 had not received the booster vaccine (control group), two of whom had never received any COVID-19 vaccine, four who had received an initial vaccine but not a booster, and 12 who had received an initial vaccine and booster but more than 6 months prior to the study.
- Menstrual cycle length was measured for three cycles prior to and four cycles after vaccination in the booster group and for seven cycles in the control group.
- Menstrual flow, pain, and stress were measured at baseline and monthly for 3 months post vaccination.
TAKEAWAY:
- Participants in the booster group experienced shorter cycles by an average of 5.35 days after receiving the COVID-19 booster vaccine (P = .03), particularly during the second cycle. In contrast, those in the control group did not experience any changes in the menstrual cycle length.
- Receiving the booster dose in the follicular phase was associated with significantly shorter menstrual cycles, compared with pre-booster cycles (P = .0157).
- Menstrual flow, pain, and other symptoms remained unaffected after the COVID-19 booster vaccination.
- Higher stress levels at baseline were also associated with a shorter length of the menstrual cycle (P = .03) in both groups, regardless of the booster vaccination status.
IN PRACTICE:
“These data are potentially important for counseling parents regarding potential vaccine refusal in the future for their teen daughters,” the authors wrote.
SOURCE:
This study was led by Laura A. Payne, PhD, from McLean Hospital in Boston, and was published online in the Journal of Adolescent Health.
LIMITATIONS:
The sample size for the booster and control groups was relatively small and homogeneous. The study did not include the height, weight, birth control use, or other chronic conditions of the participants, which may have influenced the functioning of the menstrual cycle. The control group included a majority of teens who had previously received a vaccine and even a booster, which could have affected results.
DISCLOSURES:
This study was supported by grants from the Eunice Kennedy Shriver National Institute for Child Health and Human Development. Some authors received consulting fees, travel reimbursements, honoraria, research funding, and royalties from Bayer Healthcare, Mahana Therapeutics, Gates, and Merck, among others.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The vaccine did not appear to be associated with shifts in menstrual flow, pain, or other symptoms.
METHODOLOGY:
- Reports of menstrual cycle changes following the COVID-19 vaccination began to emerge in early 2021, raising concerns about the impact of the vaccine on menstrual health.
- Researchers conducted a prospective study including 65 adolescent girls (mean age, 17.3 years), of whom 47 had received an initial series of COVID-19 vaccination at least 6 months prior to receiving a booster dose (booster group), and 18 had not received the booster vaccine (control group), two of whom had never received any COVID-19 vaccine, four who had received an initial vaccine but not a booster, and 12 who had received an initial vaccine and booster but more than 6 months prior to the study.
- Menstrual cycle length was measured for three cycles prior to and four cycles after vaccination in the booster group and for seven cycles in the control group.
- Menstrual flow, pain, and stress were measured at baseline and monthly for 3 months post vaccination.
TAKEAWAY:
- Participants in the booster group experienced shorter cycles by an average of 5.35 days after receiving the COVID-19 booster vaccine (P = .03), particularly during the second cycle. In contrast, those in the control group did not experience any changes in the menstrual cycle length.
- Receiving the booster dose in the follicular phase was associated with significantly shorter menstrual cycles, compared with pre-booster cycles (P = .0157).
- Menstrual flow, pain, and other symptoms remained unaffected after the COVID-19 booster vaccination.
- Higher stress levels at baseline were also associated with a shorter length of the menstrual cycle (P = .03) in both groups, regardless of the booster vaccination status.
IN PRACTICE:
“These data are potentially important for counseling parents regarding potential vaccine refusal in the future for their teen daughters,” the authors wrote.
SOURCE:
This study was led by Laura A. Payne, PhD, from McLean Hospital in Boston, and was published online in the Journal of Adolescent Health.
LIMITATIONS:
The sample size for the booster and control groups was relatively small and homogeneous. The study did not include the height, weight, birth control use, or other chronic conditions of the participants, which may have influenced the functioning of the menstrual cycle. The control group included a majority of teens who had previously received a vaccine and even a booster, which could have affected results.
DISCLOSURES:
This study was supported by grants from the Eunice Kennedy Shriver National Institute for Child Health and Human Development. Some authors received consulting fees, travel reimbursements, honoraria, research funding, and royalties from Bayer Healthcare, Mahana Therapeutics, Gates, and Merck, among others.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The vaccine did not appear to be associated with shifts in menstrual flow, pain, or other symptoms.
METHODOLOGY:
- Reports of menstrual cycle changes following the COVID-19 vaccination began to emerge in early 2021, raising concerns about the impact of the vaccine on menstrual health.
- Researchers conducted a prospective study including 65 adolescent girls (mean age, 17.3 years), of whom 47 had received an initial series of COVID-19 vaccination at least 6 months prior to receiving a booster dose (booster group), and 18 had not received the booster vaccine (control group), two of whom had never received any COVID-19 vaccine, four who had received an initial vaccine but not a booster, and 12 who had received an initial vaccine and booster but more than 6 months prior to the study.
- Menstrual cycle length was measured for three cycles prior to and four cycles after vaccination in the booster group and for seven cycles in the control group.
- Menstrual flow, pain, and stress were measured at baseline and monthly for 3 months post vaccination.
TAKEAWAY:
- Participants in the booster group experienced shorter cycles by an average of 5.35 days after receiving the COVID-19 booster vaccine (P = .03), particularly during the second cycle. In contrast, those in the control group did not experience any changes in the menstrual cycle length.
- Receiving the booster dose in the follicular phase was associated with significantly shorter menstrual cycles, compared with pre-booster cycles (P = .0157).
- Menstrual flow, pain, and other symptoms remained unaffected after the COVID-19 booster vaccination.
- Higher stress levels at baseline were also associated with a shorter length of the menstrual cycle (P = .03) in both groups, regardless of the booster vaccination status.
IN PRACTICE:
“These data are potentially important for counseling parents regarding potential vaccine refusal in the future for their teen daughters,” the authors wrote.
SOURCE:
This study was led by Laura A. Payne, PhD, from McLean Hospital in Boston, and was published online in the Journal of Adolescent Health.
LIMITATIONS:
The sample size for the booster and control groups was relatively small and homogeneous. The study did not include the height, weight, birth control use, or other chronic conditions of the participants, which may have influenced the functioning of the menstrual cycle. The control group included a majority of teens who had previously received a vaccine and even a booster, which could have affected results.
DISCLOSURES:
This study was supported by grants from the Eunice Kennedy Shriver National Institute for Child Health and Human Development. Some authors received consulting fees, travel reimbursements, honoraria, research funding, and royalties from Bayer Healthcare, Mahana Therapeutics, Gates, and Merck, among others.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Identifying Child Abuse Through Oral Health: What Every Clinician Should Know
TOPLINE:
Researchers detail best practices for pediatricians in evaluating dental indications of child abuse and how to work with other physicians to detect and report these incidents.
METHODOLOGY:
- Approximately 323,000 children in the United States were identified as having experienced physical abuse in 2006, the most recent year evaluated, according to the Fourth National Incidence Study of Child Abuse and Neglect.
- One in seven children in the United States are abused or neglected each year; craniofacial, head, face, and neck injuries occur in more than half of child abuse cases.
- Children with orofacial and torso bruising who are younger than age 4 years are at risk for future, more serious abuse.
- Child trafficking survivors are twice as likely to have dental issues due to poor nutrition and inadequate care.
TAKEAWAY:
- In cases of possible oral sexual abuse, physicians should test for sexually transmitted infections and document incidents to support forensic investigations.
- Pediatricians should consult with forensic pediatric dentists or child abuse specialists for assistance in evaluating bite marks or any other indications of abuse.
- If a parent fails to seek treatment for a child’s oral or dental disease after detection, pediatricians should report the case to child protective services regarding concerns of dental neglect.
- Because trafficked children may receive medical or dental care while in captivity, physicians should use screening tools to identify children at risk of trafficking, regardless of gender.
- Physicians should be mindful of having a bias against reporting because of sharing a similar background to the parents or other caregivers of a child who is suspected of experiencing abuse.
IN PRACTICE:
“Pediatric dentists and oral and maxillofacial surgeons, whose advanced education programs include a mandated child abuse curriculum, can provide valuable information and assistance to other health care providers about oral and dental aspects of child abuse and neglect,” the study authors wrote.
SOURCE:
The study was led by Anupama Rao Tate, DMD, MPH, of the American Academy of Pediatrics, and was published online in Pediatrics.
LIMITATIONS:
No limitations were reported.
DISCLOSURES:
Susan A. Fischer-Owens reported financial connections with Colgate. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Researchers detail best practices for pediatricians in evaluating dental indications of child abuse and how to work with other physicians to detect and report these incidents.
METHODOLOGY:
- Approximately 323,000 children in the United States were identified as having experienced physical abuse in 2006, the most recent year evaluated, according to the Fourth National Incidence Study of Child Abuse and Neglect.
- One in seven children in the United States are abused or neglected each year; craniofacial, head, face, and neck injuries occur in more than half of child abuse cases.
- Children with orofacial and torso bruising who are younger than age 4 years are at risk for future, more serious abuse.
- Child trafficking survivors are twice as likely to have dental issues due to poor nutrition and inadequate care.
TAKEAWAY:
- In cases of possible oral sexual abuse, physicians should test for sexually transmitted infections and document incidents to support forensic investigations.
- Pediatricians should consult with forensic pediatric dentists or child abuse specialists for assistance in evaluating bite marks or any other indications of abuse.
- If a parent fails to seek treatment for a child’s oral or dental disease after detection, pediatricians should report the case to child protective services regarding concerns of dental neglect.
- Because trafficked children may receive medical or dental care while in captivity, physicians should use screening tools to identify children at risk of trafficking, regardless of gender.
- Physicians should be mindful of having a bias against reporting because of sharing a similar background to the parents or other caregivers of a child who is suspected of experiencing abuse.
IN PRACTICE:
“Pediatric dentists and oral and maxillofacial surgeons, whose advanced education programs include a mandated child abuse curriculum, can provide valuable information and assistance to other health care providers about oral and dental aspects of child abuse and neglect,” the study authors wrote.
SOURCE:
The study was led by Anupama Rao Tate, DMD, MPH, of the American Academy of Pediatrics, and was published online in Pediatrics.
LIMITATIONS:
No limitations were reported.
DISCLOSURES:
Susan A. Fischer-Owens reported financial connections with Colgate. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Researchers detail best practices for pediatricians in evaluating dental indications of child abuse and how to work with other physicians to detect and report these incidents.
METHODOLOGY:
- Approximately 323,000 children in the United States were identified as having experienced physical abuse in 2006, the most recent year evaluated, according to the Fourth National Incidence Study of Child Abuse and Neglect.
- One in seven children in the United States are abused or neglected each year; craniofacial, head, face, and neck injuries occur in more than half of child abuse cases.
- Children with orofacial and torso bruising who are younger than age 4 years are at risk for future, more serious abuse.
- Child trafficking survivors are twice as likely to have dental issues due to poor nutrition and inadequate care.
TAKEAWAY:
- In cases of possible oral sexual abuse, physicians should test for sexually transmitted infections and document incidents to support forensic investigations.
- Pediatricians should consult with forensic pediatric dentists or child abuse specialists for assistance in evaluating bite marks or any other indications of abuse.
- If a parent fails to seek treatment for a child’s oral or dental disease after detection, pediatricians should report the case to child protective services regarding concerns of dental neglect.
- Because trafficked children may receive medical or dental care while in captivity, physicians should use screening tools to identify children at risk of trafficking, regardless of gender.
- Physicians should be mindful of having a bias against reporting because of sharing a similar background to the parents or other caregivers of a child who is suspected of experiencing abuse.
IN PRACTICE:
“Pediatric dentists and oral and maxillofacial surgeons, whose advanced education programs include a mandated child abuse curriculum, can provide valuable information and assistance to other health care providers about oral and dental aspects of child abuse and neglect,” the study authors wrote.
SOURCE:
The study was led by Anupama Rao Tate, DMD, MPH, of the American Academy of Pediatrics, and was published online in Pediatrics.
LIMITATIONS:
No limitations were reported.
DISCLOSURES:
Susan A. Fischer-Owens reported financial connections with Colgate. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Timing of iPLEDGE Updates Unclear
iPLEDGE, the Food and Drug Administration (FDA)–required Risk Evaluation and Mitigation Strategy (REMS) program launched in 2010, aims to manage the risks for the teratogenic acne drug isotretinoin and prevent fetal exposure. But it’s been dogged by issues and controversy, causing difficulties for patients and prescribers.
Late in 2023, there seemed to be a reason for optimism that improvements were coming. On November 30, 2023, the FDA informed isotretinoin manufacturers — known as the Isotretinoin Products Manufacturing Group (IPMG) — that they had 6 months to make five changes to the existing iPLEDGE REMS, addressing the controversies and potentially reducing glitches in the program and minimizing the burden of the program on patients, prescribers, and pharmacies — while maintaining safe use of the drug — and to submit their proposal by May 30, 2024.
The timeline for when an improved program might be in place remains unclear.
An FDA spokesperson, without confirming that the submission was submitted on time, recently said the review timeline once such a submission is received is generally 6 months.
‘Radio Silence’
No official FDA announcement has been made about the timeline, nor has information been forthcoming from the IPMG, and the silence has been frustrating for John S. Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, both in Boston, Massachusetts. He chairs the American Academy of Dermatology Association’s IPLEDGE Work Group, which works with both the FDA and IPMG.
He began writing about issues with iPLEDGE about 4 years ago, when he and colleagues suggested, among other changes, simplifying the iPLEDGE contraception requirements in a paper published in the Journal of the American Academy of Dermatology.
In an interview, Dr. Barbieri expressed frustration about the lack of information on the status of the iPLEDGE changes. “We’ve been given no timeline [beyond the FDA’s May 30 deadline for the IPMG to respond] of what might happen when. We’ve asked what was submitted. No one will share it with us or tell us anything about it. It’s just radio silence.”
Dr. Barbieri is also frustrated at the lack of response from IPMG. Despite repeated requests to the group to include the dermatologists in the discussions, IPMG has repeatedly declined the help, he said.
IPMG appears to have no dedicated website. No response had been received to an email sent to an address attributed to the group asking if it would share the submission to the FDA.
Currently, isotretinoin, originally marketed as Accutane, is marketed under such brand names as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
Asked for specific information on the proposed changes, an FDA spokesperson said in an August 19 email that “the submission to the FDA from the isotretinoin manufacturers will be a major modification, and the review timeline is generally 6 months. Once approved, the isotretinoin manufacturers will need additional time to implement the changes.”
The spokesperson declined to provide additional information on the status of the IPMG proposal, to share the proposal itself, or to estimate the implementation period.
Reason for Hope?
In response to the comment that the review generally takes 6 months, Dr. Barbieri said it doesn’t give him much hope, adding that “any delay of implementing these reforms is a missed opportunity to improve the care of patients with acne.” He is also hopeful that the FDA will invite some public comment during the review period “so that stakeholders can share their feedback about the proposal to help guide FDA decision-making and ensure effective implementation.”
From Meeting to Mandate
The FDA order for the changes followed a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee in March 2023 about the program requirements. It included feedback from patients and dermatologists and recommendations for changes, with a goal of reducing the burden of the program on patients, pharmacies, and prescribers without compromising patient safety.
The Five Requested Changes
In the November 30 letter, the FDA requested the following from the IPMG:
- Remove the requirement that pregnancy tests be performed in a specially certified lab (such as a Clinical Laboratory Improvement Amendments lab). This would enable the tests to be done in a clinic setting rather than sending patients to a separate lab.
- Allow prescribers the option of letting patients use home pregnancy tests during and after treatment, with steps in place to minimize falsification.
- Remove the waiting period requirement, known as the “19-day lockout,” for patients if they don’t obtain the isotretinoin from the pharmacy within the first 7-day prescription window. Before initiation of isotretinoin, a repeat confirmatory test must be done in a medical setting without any required waiting period.
- Revise the pregnancy registry requirement, removing the objective to document the outcome and associated collection of data for each pregnancy.
- Revise the requirement for prescribers to document patient counseling for those who can’t become pregnant from monthly counseling to counseling at enrollment only. Before each prescription is dispensed, the authorization must verify patient enrollment and prescriber certification. (In December 2021, a new, gender-neutral approach, approved by the FDA, was launched. It places potential patients into two risk categories — those who can become pregnant and those who cannot. Previously, there were three such categories: Females of reproductive potential, females not of reproductive potential, and males.)
Perspective on the Requested Changes
Of the requested changes, “really the most important is eliminating the request for monthly counseling for patients who cannot become pregnant,” Dr. Barbieri said. Because of that requirement, all patients need to have monthly visits with a dermatologist to get the medication refills, “and that creates a logistical barrier,” plus reducing time available for dermatologists to care for other patients with other dermatologic issues.
As for missing the 7-day prescription window, Dr. Barbieri said, in his experience, “it’s almost never the patient’s fault; it’s almost always an insurance problem.”
Dr. Barbieri reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
iPLEDGE, the Food and Drug Administration (FDA)–required Risk Evaluation and Mitigation Strategy (REMS) program launched in 2010, aims to manage the risks for the teratogenic acne drug isotretinoin and prevent fetal exposure. But it’s been dogged by issues and controversy, causing difficulties for patients and prescribers.
Late in 2023, there seemed to be a reason for optimism that improvements were coming. On November 30, 2023, the FDA informed isotretinoin manufacturers — known as the Isotretinoin Products Manufacturing Group (IPMG) — that they had 6 months to make five changes to the existing iPLEDGE REMS, addressing the controversies and potentially reducing glitches in the program and minimizing the burden of the program on patients, prescribers, and pharmacies — while maintaining safe use of the drug — and to submit their proposal by May 30, 2024.
The timeline for when an improved program might be in place remains unclear.
An FDA spokesperson, without confirming that the submission was submitted on time, recently said the review timeline once such a submission is received is generally 6 months.
‘Radio Silence’
No official FDA announcement has been made about the timeline, nor has information been forthcoming from the IPMG, and the silence has been frustrating for John S. Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, both in Boston, Massachusetts. He chairs the American Academy of Dermatology Association’s IPLEDGE Work Group, which works with both the FDA and IPMG.
He began writing about issues with iPLEDGE about 4 years ago, when he and colleagues suggested, among other changes, simplifying the iPLEDGE contraception requirements in a paper published in the Journal of the American Academy of Dermatology.
In an interview, Dr. Barbieri expressed frustration about the lack of information on the status of the iPLEDGE changes. “We’ve been given no timeline [beyond the FDA’s May 30 deadline for the IPMG to respond] of what might happen when. We’ve asked what was submitted. No one will share it with us or tell us anything about it. It’s just radio silence.”
Dr. Barbieri is also frustrated at the lack of response from IPMG. Despite repeated requests to the group to include the dermatologists in the discussions, IPMG has repeatedly declined the help, he said.
IPMG appears to have no dedicated website. No response had been received to an email sent to an address attributed to the group asking if it would share the submission to the FDA.
Currently, isotretinoin, originally marketed as Accutane, is marketed under such brand names as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
Asked for specific information on the proposed changes, an FDA spokesperson said in an August 19 email that “the submission to the FDA from the isotretinoin manufacturers will be a major modification, and the review timeline is generally 6 months. Once approved, the isotretinoin manufacturers will need additional time to implement the changes.”
The spokesperson declined to provide additional information on the status of the IPMG proposal, to share the proposal itself, or to estimate the implementation period.
Reason for Hope?
In response to the comment that the review generally takes 6 months, Dr. Barbieri said it doesn’t give him much hope, adding that “any delay of implementing these reforms is a missed opportunity to improve the care of patients with acne.” He is also hopeful that the FDA will invite some public comment during the review period “so that stakeholders can share their feedback about the proposal to help guide FDA decision-making and ensure effective implementation.”
From Meeting to Mandate
The FDA order for the changes followed a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee in March 2023 about the program requirements. It included feedback from patients and dermatologists and recommendations for changes, with a goal of reducing the burden of the program on patients, pharmacies, and prescribers without compromising patient safety.
The Five Requested Changes
In the November 30 letter, the FDA requested the following from the IPMG:
- Remove the requirement that pregnancy tests be performed in a specially certified lab (such as a Clinical Laboratory Improvement Amendments lab). This would enable the tests to be done in a clinic setting rather than sending patients to a separate lab.
- Allow prescribers the option of letting patients use home pregnancy tests during and after treatment, with steps in place to minimize falsification.
- Remove the waiting period requirement, known as the “19-day lockout,” for patients if they don’t obtain the isotretinoin from the pharmacy within the first 7-day prescription window. Before initiation of isotretinoin, a repeat confirmatory test must be done in a medical setting without any required waiting period.
- Revise the pregnancy registry requirement, removing the objective to document the outcome and associated collection of data for each pregnancy.
- Revise the requirement for prescribers to document patient counseling for those who can’t become pregnant from monthly counseling to counseling at enrollment only. Before each prescription is dispensed, the authorization must verify patient enrollment and prescriber certification. (In December 2021, a new, gender-neutral approach, approved by the FDA, was launched. It places potential patients into two risk categories — those who can become pregnant and those who cannot. Previously, there were three such categories: Females of reproductive potential, females not of reproductive potential, and males.)
Perspective on the Requested Changes
Of the requested changes, “really the most important is eliminating the request for monthly counseling for patients who cannot become pregnant,” Dr. Barbieri said. Because of that requirement, all patients need to have monthly visits with a dermatologist to get the medication refills, “and that creates a logistical barrier,” plus reducing time available for dermatologists to care for other patients with other dermatologic issues.
As for missing the 7-day prescription window, Dr. Barbieri said, in his experience, “it’s almost never the patient’s fault; it’s almost always an insurance problem.”
Dr. Barbieri reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
iPLEDGE, the Food and Drug Administration (FDA)–required Risk Evaluation and Mitigation Strategy (REMS) program launched in 2010, aims to manage the risks for the teratogenic acne drug isotretinoin and prevent fetal exposure. But it’s been dogged by issues and controversy, causing difficulties for patients and prescribers.
Late in 2023, there seemed to be a reason for optimism that improvements were coming. On November 30, 2023, the FDA informed isotretinoin manufacturers — known as the Isotretinoin Products Manufacturing Group (IPMG) — that they had 6 months to make five changes to the existing iPLEDGE REMS, addressing the controversies and potentially reducing glitches in the program and minimizing the burden of the program on patients, prescribers, and pharmacies — while maintaining safe use of the drug — and to submit their proposal by May 30, 2024.
The timeline for when an improved program might be in place remains unclear.
An FDA spokesperson, without confirming that the submission was submitted on time, recently said the review timeline once such a submission is received is generally 6 months.
‘Radio Silence’
No official FDA announcement has been made about the timeline, nor has information been forthcoming from the IPMG, and the silence has been frustrating for John S. Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, both in Boston, Massachusetts. He chairs the American Academy of Dermatology Association’s IPLEDGE Work Group, which works with both the FDA and IPMG.
He began writing about issues with iPLEDGE about 4 years ago, when he and colleagues suggested, among other changes, simplifying the iPLEDGE contraception requirements in a paper published in the Journal of the American Academy of Dermatology.
In an interview, Dr. Barbieri expressed frustration about the lack of information on the status of the iPLEDGE changes. “We’ve been given no timeline [beyond the FDA’s May 30 deadline for the IPMG to respond] of what might happen when. We’ve asked what was submitted. No one will share it with us or tell us anything about it. It’s just radio silence.”
Dr. Barbieri is also frustrated at the lack of response from IPMG. Despite repeated requests to the group to include the dermatologists in the discussions, IPMG has repeatedly declined the help, he said.
IPMG appears to have no dedicated website. No response had been received to an email sent to an address attributed to the group asking if it would share the submission to the FDA.
Currently, isotretinoin, originally marketed as Accutane, is marketed under such brand names as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
Asked for specific information on the proposed changes, an FDA spokesperson said in an August 19 email that “the submission to the FDA from the isotretinoin manufacturers will be a major modification, and the review timeline is generally 6 months. Once approved, the isotretinoin manufacturers will need additional time to implement the changes.”
The spokesperson declined to provide additional information on the status of the IPMG proposal, to share the proposal itself, or to estimate the implementation period.
Reason for Hope?
In response to the comment that the review generally takes 6 months, Dr. Barbieri said it doesn’t give him much hope, adding that “any delay of implementing these reforms is a missed opportunity to improve the care of patients with acne.” He is also hopeful that the FDA will invite some public comment during the review period “so that stakeholders can share their feedback about the proposal to help guide FDA decision-making and ensure effective implementation.”
From Meeting to Mandate
The FDA order for the changes followed a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and the Dermatologic and Ophthalmic Drugs Advisory Committee in March 2023 about the program requirements. It included feedback from patients and dermatologists and recommendations for changes, with a goal of reducing the burden of the program on patients, pharmacies, and prescribers without compromising patient safety.
The Five Requested Changes
In the November 30 letter, the FDA requested the following from the IPMG:
- Remove the requirement that pregnancy tests be performed in a specially certified lab (such as a Clinical Laboratory Improvement Amendments lab). This would enable the tests to be done in a clinic setting rather than sending patients to a separate lab.
- Allow prescribers the option of letting patients use home pregnancy tests during and after treatment, with steps in place to minimize falsification.
- Remove the waiting period requirement, known as the “19-day lockout,” for patients if they don’t obtain the isotretinoin from the pharmacy within the first 7-day prescription window. Before initiation of isotretinoin, a repeat confirmatory test must be done in a medical setting without any required waiting period.
- Revise the pregnancy registry requirement, removing the objective to document the outcome and associated collection of data for each pregnancy.
- Revise the requirement for prescribers to document patient counseling for those who can’t become pregnant from monthly counseling to counseling at enrollment only. Before each prescription is dispensed, the authorization must verify patient enrollment and prescriber certification. (In December 2021, a new, gender-neutral approach, approved by the FDA, was launched. It places potential patients into two risk categories — those who can become pregnant and those who cannot. Previously, there were three such categories: Females of reproductive potential, females not of reproductive potential, and males.)
Perspective on the Requested Changes
Of the requested changes, “really the most important is eliminating the request for monthly counseling for patients who cannot become pregnant,” Dr. Barbieri said. Because of that requirement, all patients need to have monthly visits with a dermatologist to get the medication refills, “and that creates a logistical barrier,” plus reducing time available for dermatologists to care for other patients with other dermatologic issues.
As for missing the 7-day prescription window, Dr. Barbieri said, in his experience, “it’s almost never the patient’s fault; it’s almost always an insurance problem.”
Dr. Barbieri reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Cancer Treatment 101: A Primer for Non-Oncologists
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
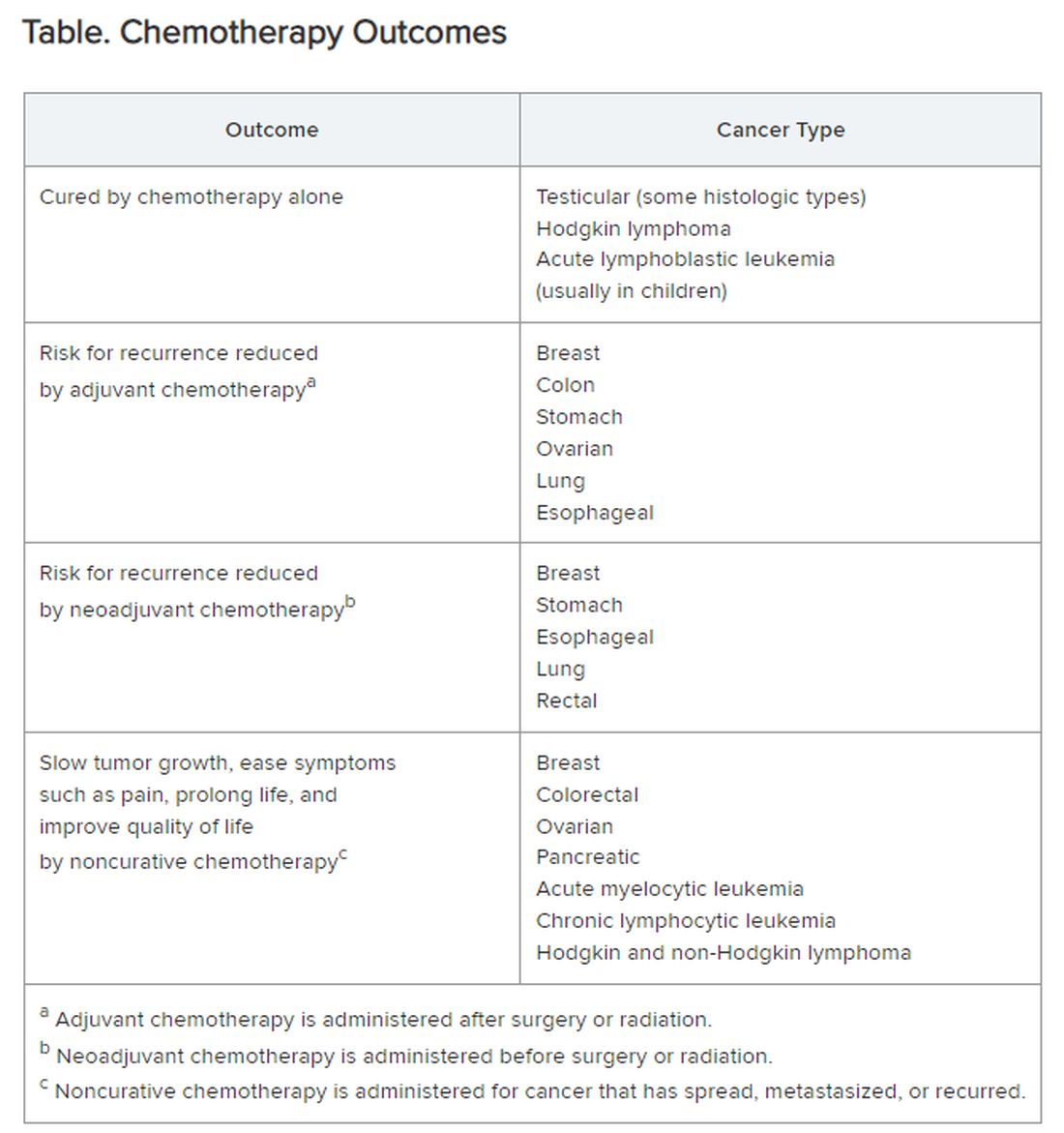
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The remaining 700,000 or so often proceed to chemotherapy either immediately or upon cancer recurrence, spread, or newly recognized metastases. “Cures” after that point are rare.
I’m speaking in generalities, understanding that each cancer and each patient is unique.
Chemotherapy
Chemotherapy alone can cure a small number of cancer types. When added to radiation or surgery, chemotherapy can help to cure a wider range of cancer types. As an add-on, chemotherapy can extend the length and quality of life for many patients with cancer. Since chemotherapy is by definition “toxic,” it can also shorten the duration or harm the quality of life and provide false hope. The Table summarizes what chemotherapy can and cannot achieve in selected cancer types.
Careful, compassionate communication between patient and physician is key. Goals and expectations must be clearly understood.
Organized chemotherapeutic efforts are further categorized as first line, second line, and third line.
First-line treatment. The initial round of recommended chemotherapy for a specific cancer. It is typically considered the most effective treatment for that type and stage of cancer on the basis of current research and clinical trials.
Second-line treatment. This is the treatment used if the first-line chemotherapy doesn’t work as desired. Reasons to switch to second-line chemo include:
- Lack of response (the tumor failed to shrink).
- Progression (the cancer may have grown or spread further).
- Adverse side effects were too severe to continue.
The drugs used in second-line chemo will typically be different from those used in first line, sometimes because cancer cells can develop resistance to chemotherapy drugs over time. Moreover, the goal of second-line chemo may differ from that of first-line therapy. Rather than chiefly aiming for a cure, second-line treatment might focus on slowing cancer growth, managing symptoms, or improving quality of life. Unfortunately, not every type of cancer has a readily available second-line option.
Third-line treatment. Third-line options come into play when both the initial course of chemo (first line) and the subsequent treatment (second line) have failed to achieve remission or control the cancer’s spread. Owing to the progressive nature of advanced cancers, patients might not be eligible or healthy enough for third-line therapy. Depending on cancer type, the patient’s general health, and response to previous treatments, third-line options could include:
- New or different chemotherapy drugs compared with prior lines.
- Surgery to debulk the tumor.
- Radiation for symptom control.
- Targeted therapy: drugs designed to target specific vulnerabilities in cancer cells.
- Immunotherapy: agents that help the body’s immune system fight cancer cells.
- Clinical trials testing new or investigational treatments, which may be applicable at any time, depending on the questions being addressed.
The goals of third-line therapy may shift from aiming for a cure to managing symptoms, improving quality of life, and potentially slowing cancer growth. The decision to pursue third-line therapy involves careful consideration by the doctor and patient, weighing the potential benefits and risks of treatment considering the individual’s overall health and specific situation.
It’s important to have realistic expectations about the potential outcomes of third-line therapy. Although remission may be unlikely, third-line therapy can still play a role in managing the disease.
Navigating advanced cancer treatment is very complex. The patient and physician must together consider detailed explanations and clarifications to set expectations and make informed decisions about care.
Interventions to Consider Earlier
In traditional clinical oncology practice, other interventions are possible, but these may not be offered until treatment has reached the third line:
- Molecular testing.
- Palliation.
- Clinical trials.
- Innovative testing to guide targeted therapy by ascertaining which agents are most likely (or not likely at all) to be effective.
I would argue that the patient’s interests are better served by considering and offering these other interventions much earlier, even before starting first-line chemotherapy.
Molecular testing. The best time for molecular testing of a new malignant tumor is typically at the time of diagnosis. Here’s why:
- Molecular testing helps identify specific genetic mutations in the cancer cells. This information can be crucial for selecting targeted therapies that are most effective against those specific mutations. Early detection allows for the most treatment options. For example, for non–small cell lung cancer, early is best because treatment and outcomes may well be changed by test results.
- Knowing the tumor’s molecular makeup can help determine whether a patient qualifies for clinical trials of new drugs designed for specific mutations.
- Some molecular markers can offer information about the tumor’s aggressiveness and potential for metastasis so that prognosis can be informed.
Molecular testing can be a valuable tool throughout a cancer patient’s journey. With genetically diverse tumors, the initial biopsy might not capture the full picture. Molecular testing of circulating tumor DNA can be used to monitor a patient’s response to treatment and detect potential mutations that might arise during treatment resistance. Retesting after metastasis can provide additional information that can aid in treatment decisions.
Palliative care. The ideal time to discuss palliative care with a patient with cancer is early in the diagnosis and treatment process. Palliative care is not the same as hospice care; it isn’t just about end-of-life. Palliative care focuses on improving a patient’s quality of life throughout cancer treatment. Palliative care specialists can address a wide range of symptoms a patient might experience from cancer or its treatment, including pain, fatigue, nausea, and anxiety.
Early discussions allow for a more comprehensive care plan. Open communication about all treatment options, including palliative care, empowers patients to make informed decisions about their care goals and preferences.
Specific situations where discussing palliative care might be appropriate are:
- Soon after a cancer diagnosis.
- If the patient experiences significant side effects from cancer treatment.
- When considering different treatment options, palliative care can complement those treatments.
- In advanced stages of cancer, to focus on comfort and quality of life.
Clinical trials. Participation in a clinical trial to explore new or investigational treatments should always be considered.
In theory, clinical trials should be an option at any time in the patient’s course. But the organized clinical trial experience may not be available or appropriate. Then, the individual becomes a de facto “clinical trial with an n of 1.” Read this brief open-access blog post at Cancer Commons to learn more about that circumstance.
Innovative testing. The best choice of chemotherapeutic or targeted therapies is often unclear. The clinician is likely to follow published guidelines, often from the National Comprehensive Cancer Network.
These are evidence based and driven by consensus of experts. But guideline-recommended therapy is not always effective, and weeks or months can pass before this ineffectiveness becomes apparent. Thus, many researchers and companies are seeking methods of testing each patient’s specific cancer to determine in advance, or very quickly, whether a particular drug is likely to be effective.
Read more about these leading innovations:
SAGE Oncotest: Entering the Next Generation of Tailored Cancer Treatment
Alibrex: A New Blood Test to Reveal Whether a Cancer Treatment is Working
PARIS Test Uses Lab-Grown Mini-Tumors to Find a Patient’s Best Treatment
Using Live Cells from Patients to Find the Right Cancer Drug
Other innovative therapies under investigation could even be agnostic to cancer type:
Treating Pancreatic Cancer: Could Metabolism — Not Genomics — Be the Key?
High-Energy Blue Light Powers a Promising New Treatment to Destroy Cancer Cells
All-Clear Follow-Up: Hydrogen Peroxide Appears to Treat Oral and Skin Lesions
Cancer is a tough nut to crack. Many people and organizations are trying very hard. So much is being learned. Some approaches will be effective. We can all hope.
Dr. Lundberg, editor in chief, Cancer Commons, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Necrotic Papules in a Pediatric Patient
The Diagnosis: Pityriasis Lichenoides et Varioliformis Acuta
Sectioned punch biopsies were performed on the patient’s right arm. Histopathology showed acanthosis and parakeratosis in the epidermis, with vacuolar degeneration and dyskeratosis in the basal layer. Dermal changes included extravasated red blood cells in the papillary dermis as well as perivascular lymphocytic infiltrates in both the papillary and reticular dermis (Figure). Direct immunofluorescence of a perilesional biopsy using anti–human IgG, IgM, IgA, C3, and fibrin conjugates showed no findings of immune deposition. Biopsy results were consistent with pityriasis lichenoides et varioliformis acuta (PLEVA), and the patient was treated with a 5-day course of oral azithromycin, triamcinolone ointment 0.1% twice daily, and phototherapy with narrowband UVB 3 times weekly. Rapid improvement was noted at 2-month follow-up.
Pityriasis lichenoides et varioliformis acuta is a form of pityriasis lichenoides, a group of inflammatory dermatoses that are characterized clinically by successive crops of morphologically diverse lesions. Epidemiologic studies have shown a slight male predominance. It primarily affects children and young adults, with peak ages of 8 and 32 years in pediatric and adult populations, respectively.1
The pathogenesis of PLEVA remains unclear. An abnormal immune response to Toxoplasma, Epstein-Barr virus, HIV, and other pathogens has been suggested based on serologic evidence of concurrent disease activity with the onset of lesions as well as cutaneous improvement in some patients after treatment of the infection.1 A T-cell lymphoproliferative etiology also has been considered based on histopathologic similarities between PLEVA and lymphomatoid papulosis (LyP) as well as findings of clonality in T-cell receptor gene rearrangement in many patients.1,2 Some clinicians consider LyP and PLEVA as separate entities on one disease spectrum.
Eruptions of PLEVA tend to favor the trunk and proximal extremities. Lesions may begin as macules measuring 2 to 3 mm in diameter that quickly evolve into papules with fine scale that remains attached centrally. Ulcerations with hemorrhagic crusts also may be noted as the lesions progress in stage. The rash may persist for weeks to years, and overlapping crops of macules and papules at varying stages of development may be seen in the same patient.1
Histopathologic findings of PLEVA include spongiosis, dyskeratosis, parakeratosis, and focal keratinocyte necrosis within the epidermis, as well as vacuolar degeneration of the basal layer. Lymphocyte and erythrocyte extravasation may extend into the epidermis. Dermal findings may include edema and wedge-shaped perivascular lymphocytic infiltrates extending into the reticular dermis.1

Important differential diagnoses to consider include LyP, mycosis fungoides (MF), pemphigus foliaceus, and varicella. Lymphomatoid papulosis is a benign CD30+ lymphoproliferative disorder that is characterized by an indolent course of recurrent, often self-resolving papules that occur most frequently on the trunk, arms, and legs of older patients. There are several histologic subtypes of LyP, but the most common (type A) may manifest with wedge-shaped perivascular lymphocytic infiltrates in the dermis, similar to PLEVA. T-cell receptor gene rearrangement studies characteristically reveal clonality in LyP, and clonality has been reported in PLEVA. However, LyP demonstrates a higher cytologic grade and lacks the characteristic parakeratotic scale and superficial dermal microhemorrhage of PLEVA.3
Mycosis fungoides is a malignant lymphoproliferative disorder that is characterized by an indolent clinical course of persistent patches, plaques, or tumors of various sizes that often manifest in non–sun-exposed areas of the skin. Early stages of MF are difficult to detect histologically, but biopsies may show atypical lymphocytes with hyperchromatic, irregularly contoured nuclei arranged along the basal layer of the epidermis. Epidermal aggregates of atypical lymphocytes (also known as Pautrier microabscesses) are considered highly specific for MF. T-cell receptor and immunopathologic studies also are important adjuncts in the diagnosis of MF.4
Pemphigus foliaceus is an autoimmune blistering disease caused by antibodies directed against desmoglein 1, which is found in the granular layer of the epidermis. It manifests with a subtle onset of scattered crusted lesions in the seborrheic areas, such as the scalp, face, chest, and upper back. Histopathologic findings of early blisters may include acantholysis and dyskeratosis in the stratum granulosum as well as vacuolization of the granular layer. The blisters may coalesce into superficial bullae containing fibrin and neutrophils. Immunofluorescence studies that demonstrate intraepidermal C3 and IgG deposition are key to the diagnosis of pemphigus.5
Varicella (also known as chickenpox) manifests with crops of vesicles on an erythematous base in a centripetal distribution favoring the trunk and proximal extremities. It often is preceded by prodromal fever, malaise, and myalgia. Histopathologic evaluation of varicella is uncommon but may reveal acantholysis, multinucleation, and nuclear margination of keratinocytes. Viral culture or nucleic acid amplification testing of lesions can be used to verify the diagnosis.6
Most cases of PLEVA resolve without intervention.7 Treatment is directed at speeding recovery, providing symptomatic relief, and limiting permanent sequelae. Topical steroids often are used to alleviate inflammation and pruritus. Systemic antibiotics such as doxycycline, minocycline, and erythromycin have been used for their anti-inflammatory properties. Phototherapy of various wavelengths, including broadband and narrowband UVB as well as psoralen plus UVA, have led to improvements in affected patients. Refractory disease may warrant consideration of therapy with methotrexate, acitretin, dapsone, or cyclosporine.7
There have been rare reports of PLEVA evolving into its potentially lethal variant, febrile ulceronecrotic Mucha-Habermann disease, which is differentiated by the presence of systemic manifestations, including high fever, sore throat, diarrhea, central nervous system symptoms, abdominal pain, interstitial pneumonitis, splenomegaly, arthritis, sepsis, megaloblastic anemia, or conjunctival ulcers. The orogenital mucosa may be affected. Cutaneous lesions may rapidly progress to large, generalized, coalescent ulcers with necrotic crusts and vasculitic features on biopsy.8 Malignant transformation of PLEVA into LyP or MF rarely may occur and warrants continued follow-up of unresolved lesions.9
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-572. doi:10.1016/j.jaad.2005.07.058
- Teklehaimanot F, Gade A, Rubenstein R. Pityriasis lichenoides et varioliformis acuta (PLEVA). In: StatPearls. StatPearls Publishing; 2023.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73. doi:10.1111/jdv.15931
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Lepe K, Yarrarapu SNS, Zito PM. Pemphigus foliaceus. In: StatPearls. StatPearls Publishing; 2023.
- Ayoade F, Kumar S. Varicella zoster (chickenpox). In: StatPearls. StatPearls Publishing; 2023.
- Bellinato F, Maurelli M, Gisondi P, et al. A systematic review of treatments for pityriasis lichenoides. J Eur Acad Dermatol Venereol. 2019;33:2039-2049. doi:10.1111/jdv.15813
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738. doi:10.1111/ijd.13195
- Thomson KF, Whittaker SJ, Russell-Jones R, et al. Childhood cutaneous T-cell lymphoma in association with pityriasis lichenoides chronica. Br J Dermatol. 1999;141:1136-1152. doi:10.1046/j.1365-2133.1999.03232.x
The Diagnosis: Pityriasis Lichenoides et Varioliformis Acuta
Sectioned punch biopsies were performed on the patient’s right arm. Histopathology showed acanthosis and parakeratosis in the epidermis, with vacuolar degeneration and dyskeratosis in the basal layer. Dermal changes included extravasated red blood cells in the papillary dermis as well as perivascular lymphocytic infiltrates in both the papillary and reticular dermis (Figure). Direct immunofluorescence of a perilesional biopsy using anti–human IgG, IgM, IgA, C3, and fibrin conjugates showed no findings of immune deposition. Biopsy results were consistent with pityriasis lichenoides et varioliformis acuta (PLEVA), and the patient was treated with a 5-day course of oral azithromycin, triamcinolone ointment 0.1% twice daily, and phototherapy with narrowband UVB 3 times weekly. Rapid improvement was noted at 2-month follow-up.
Pityriasis lichenoides et varioliformis acuta is a form of pityriasis lichenoides, a group of inflammatory dermatoses that are characterized clinically by successive crops of morphologically diverse lesions. Epidemiologic studies have shown a slight male predominance. It primarily affects children and young adults, with peak ages of 8 and 32 years in pediatric and adult populations, respectively.1
The pathogenesis of PLEVA remains unclear. An abnormal immune response to Toxoplasma, Epstein-Barr virus, HIV, and other pathogens has been suggested based on serologic evidence of concurrent disease activity with the onset of lesions as well as cutaneous improvement in some patients after treatment of the infection.1 A T-cell lymphoproliferative etiology also has been considered based on histopathologic similarities between PLEVA and lymphomatoid papulosis (LyP) as well as findings of clonality in T-cell receptor gene rearrangement in many patients.1,2 Some clinicians consider LyP and PLEVA as separate entities on one disease spectrum.
Eruptions of PLEVA tend to favor the trunk and proximal extremities. Lesions may begin as macules measuring 2 to 3 mm in diameter that quickly evolve into papules with fine scale that remains attached centrally. Ulcerations with hemorrhagic crusts also may be noted as the lesions progress in stage. The rash may persist for weeks to years, and overlapping crops of macules and papules at varying stages of development may be seen in the same patient.1
Histopathologic findings of PLEVA include spongiosis, dyskeratosis, parakeratosis, and focal keratinocyte necrosis within the epidermis, as well as vacuolar degeneration of the basal layer. Lymphocyte and erythrocyte extravasation may extend into the epidermis. Dermal findings may include edema and wedge-shaped perivascular lymphocytic infiltrates extending into the reticular dermis.1

Important differential diagnoses to consider include LyP, mycosis fungoides (MF), pemphigus foliaceus, and varicella. Lymphomatoid papulosis is a benign CD30+ lymphoproliferative disorder that is characterized by an indolent course of recurrent, often self-resolving papules that occur most frequently on the trunk, arms, and legs of older patients. There are several histologic subtypes of LyP, but the most common (type A) may manifest with wedge-shaped perivascular lymphocytic infiltrates in the dermis, similar to PLEVA. T-cell receptor gene rearrangement studies characteristically reveal clonality in LyP, and clonality has been reported in PLEVA. However, LyP demonstrates a higher cytologic grade and lacks the characteristic parakeratotic scale and superficial dermal microhemorrhage of PLEVA.3
Mycosis fungoides is a malignant lymphoproliferative disorder that is characterized by an indolent clinical course of persistent patches, plaques, or tumors of various sizes that often manifest in non–sun-exposed areas of the skin. Early stages of MF are difficult to detect histologically, but biopsies may show atypical lymphocytes with hyperchromatic, irregularly contoured nuclei arranged along the basal layer of the epidermis. Epidermal aggregates of atypical lymphocytes (also known as Pautrier microabscesses) are considered highly specific for MF. T-cell receptor and immunopathologic studies also are important adjuncts in the diagnosis of MF.4
Pemphigus foliaceus is an autoimmune blistering disease caused by antibodies directed against desmoglein 1, which is found in the granular layer of the epidermis. It manifests with a subtle onset of scattered crusted lesions in the seborrheic areas, such as the scalp, face, chest, and upper back. Histopathologic findings of early blisters may include acantholysis and dyskeratosis in the stratum granulosum as well as vacuolization of the granular layer. The blisters may coalesce into superficial bullae containing fibrin and neutrophils. Immunofluorescence studies that demonstrate intraepidermal C3 and IgG deposition are key to the diagnosis of pemphigus.5
Varicella (also known as chickenpox) manifests with crops of vesicles on an erythematous base in a centripetal distribution favoring the trunk and proximal extremities. It often is preceded by prodromal fever, malaise, and myalgia. Histopathologic evaluation of varicella is uncommon but may reveal acantholysis, multinucleation, and nuclear margination of keratinocytes. Viral culture or nucleic acid amplification testing of lesions can be used to verify the diagnosis.6
Most cases of PLEVA resolve without intervention.7 Treatment is directed at speeding recovery, providing symptomatic relief, and limiting permanent sequelae. Topical steroids often are used to alleviate inflammation and pruritus. Systemic antibiotics such as doxycycline, minocycline, and erythromycin have been used for their anti-inflammatory properties. Phototherapy of various wavelengths, including broadband and narrowband UVB as well as psoralen plus UVA, have led to improvements in affected patients. Refractory disease may warrant consideration of therapy with methotrexate, acitretin, dapsone, or cyclosporine.7
There have been rare reports of PLEVA evolving into its potentially lethal variant, febrile ulceronecrotic Mucha-Habermann disease, which is differentiated by the presence of systemic manifestations, including high fever, sore throat, diarrhea, central nervous system symptoms, abdominal pain, interstitial pneumonitis, splenomegaly, arthritis, sepsis, megaloblastic anemia, or conjunctival ulcers. The orogenital mucosa may be affected. Cutaneous lesions may rapidly progress to large, generalized, coalescent ulcers with necrotic crusts and vasculitic features on biopsy.8 Malignant transformation of PLEVA into LyP or MF rarely may occur and warrants continued follow-up of unresolved lesions.9
The Diagnosis: Pityriasis Lichenoides et Varioliformis Acuta
Sectioned punch biopsies were performed on the patient’s right arm. Histopathology showed acanthosis and parakeratosis in the epidermis, with vacuolar degeneration and dyskeratosis in the basal layer. Dermal changes included extravasated red blood cells in the papillary dermis as well as perivascular lymphocytic infiltrates in both the papillary and reticular dermis (Figure). Direct immunofluorescence of a perilesional biopsy using anti–human IgG, IgM, IgA, C3, and fibrin conjugates showed no findings of immune deposition. Biopsy results were consistent with pityriasis lichenoides et varioliformis acuta (PLEVA), and the patient was treated with a 5-day course of oral azithromycin, triamcinolone ointment 0.1% twice daily, and phototherapy with narrowband UVB 3 times weekly. Rapid improvement was noted at 2-month follow-up.
Pityriasis lichenoides et varioliformis acuta is a form of pityriasis lichenoides, a group of inflammatory dermatoses that are characterized clinically by successive crops of morphologically diverse lesions. Epidemiologic studies have shown a slight male predominance. It primarily affects children and young adults, with peak ages of 8 and 32 years in pediatric and adult populations, respectively.1
The pathogenesis of PLEVA remains unclear. An abnormal immune response to Toxoplasma, Epstein-Barr virus, HIV, and other pathogens has been suggested based on serologic evidence of concurrent disease activity with the onset of lesions as well as cutaneous improvement in some patients after treatment of the infection.1 A T-cell lymphoproliferative etiology also has been considered based on histopathologic similarities between PLEVA and lymphomatoid papulosis (LyP) as well as findings of clonality in T-cell receptor gene rearrangement in many patients.1,2 Some clinicians consider LyP and PLEVA as separate entities on one disease spectrum.
Eruptions of PLEVA tend to favor the trunk and proximal extremities. Lesions may begin as macules measuring 2 to 3 mm in diameter that quickly evolve into papules with fine scale that remains attached centrally. Ulcerations with hemorrhagic crusts also may be noted as the lesions progress in stage. The rash may persist for weeks to years, and overlapping crops of macules and papules at varying stages of development may be seen in the same patient.1
Histopathologic findings of PLEVA include spongiosis, dyskeratosis, parakeratosis, and focal keratinocyte necrosis within the epidermis, as well as vacuolar degeneration of the basal layer. Lymphocyte and erythrocyte extravasation may extend into the epidermis. Dermal findings may include edema and wedge-shaped perivascular lymphocytic infiltrates extending into the reticular dermis.1

Important differential diagnoses to consider include LyP, mycosis fungoides (MF), pemphigus foliaceus, and varicella. Lymphomatoid papulosis is a benign CD30+ lymphoproliferative disorder that is characterized by an indolent course of recurrent, often self-resolving papules that occur most frequently on the trunk, arms, and legs of older patients. There are several histologic subtypes of LyP, but the most common (type A) may manifest with wedge-shaped perivascular lymphocytic infiltrates in the dermis, similar to PLEVA. T-cell receptor gene rearrangement studies characteristically reveal clonality in LyP, and clonality has been reported in PLEVA. However, LyP demonstrates a higher cytologic grade and lacks the characteristic parakeratotic scale and superficial dermal microhemorrhage of PLEVA.3
Mycosis fungoides is a malignant lymphoproliferative disorder that is characterized by an indolent clinical course of persistent patches, plaques, or tumors of various sizes that often manifest in non–sun-exposed areas of the skin. Early stages of MF are difficult to detect histologically, but biopsies may show atypical lymphocytes with hyperchromatic, irregularly contoured nuclei arranged along the basal layer of the epidermis. Epidermal aggregates of atypical lymphocytes (also known as Pautrier microabscesses) are considered highly specific for MF. T-cell receptor and immunopathologic studies also are important adjuncts in the diagnosis of MF.4
Pemphigus foliaceus is an autoimmune blistering disease caused by antibodies directed against desmoglein 1, which is found in the granular layer of the epidermis. It manifests with a subtle onset of scattered crusted lesions in the seborrheic areas, such as the scalp, face, chest, and upper back. Histopathologic findings of early blisters may include acantholysis and dyskeratosis in the stratum granulosum as well as vacuolization of the granular layer. The blisters may coalesce into superficial bullae containing fibrin and neutrophils. Immunofluorescence studies that demonstrate intraepidermal C3 and IgG deposition are key to the diagnosis of pemphigus.5
Varicella (also known as chickenpox) manifests with crops of vesicles on an erythematous base in a centripetal distribution favoring the trunk and proximal extremities. It often is preceded by prodromal fever, malaise, and myalgia. Histopathologic evaluation of varicella is uncommon but may reveal acantholysis, multinucleation, and nuclear margination of keratinocytes. Viral culture or nucleic acid amplification testing of lesions can be used to verify the diagnosis.6
Most cases of PLEVA resolve without intervention.7 Treatment is directed at speeding recovery, providing symptomatic relief, and limiting permanent sequelae. Topical steroids often are used to alleviate inflammation and pruritus. Systemic antibiotics such as doxycycline, minocycline, and erythromycin have been used for their anti-inflammatory properties. Phototherapy of various wavelengths, including broadband and narrowband UVB as well as psoralen plus UVA, have led to improvements in affected patients. Refractory disease may warrant consideration of therapy with methotrexate, acitretin, dapsone, or cyclosporine.7
There have been rare reports of PLEVA evolving into its potentially lethal variant, febrile ulceronecrotic Mucha-Habermann disease, which is differentiated by the presence of systemic manifestations, including high fever, sore throat, diarrhea, central nervous system symptoms, abdominal pain, interstitial pneumonitis, splenomegaly, arthritis, sepsis, megaloblastic anemia, or conjunctival ulcers. The orogenital mucosa may be affected. Cutaneous lesions may rapidly progress to large, generalized, coalescent ulcers with necrotic crusts and vasculitic features on biopsy.8 Malignant transformation of PLEVA into LyP or MF rarely may occur and warrants continued follow-up of unresolved lesions.9
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-572. doi:10.1016/j.jaad.2005.07.058
- Teklehaimanot F, Gade A, Rubenstein R. Pityriasis lichenoides et varioliformis acuta (PLEVA). In: StatPearls. StatPearls Publishing; 2023.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73. doi:10.1111/jdv.15931
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Lepe K, Yarrarapu SNS, Zito PM. Pemphigus foliaceus. In: StatPearls. StatPearls Publishing; 2023.
- Ayoade F, Kumar S. Varicella zoster (chickenpox). In: StatPearls. StatPearls Publishing; 2023.
- Bellinato F, Maurelli M, Gisondi P, et al. A systematic review of treatments for pityriasis lichenoides. J Eur Acad Dermatol Venereol. 2019;33:2039-2049. doi:10.1111/jdv.15813
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738. doi:10.1111/ijd.13195
- Thomson KF, Whittaker SJ, Russell-Jones R, et al. Childhood cutaneous T-cell lymphoma in association with pityriasis lichenoides chronica. Br J Dermatol. 1999;141:1136-1152. doi:10.1046/j.1365-2133.1999.03232.x
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-572. doi:10.1016/j.jaad.2005.07.058
- Teklehaimanot F, Gade A, Rubenstein R. Pityriasis lichenoides et varioliformis acuta (PLEVA). In: StatPearls. StatPearls Publishing; 2023.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73. doi:10.1111/jdv.15931
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Lepe K, Yarrarapu SNS, Zito PM. Pemphigus foliaceus. In: StatPearls. StatPearls Publishing; 2023.
- Ayoade F, Kumar S. Varicella zoster (chickenpox). In: StatPearls. StatPearls Publishing; 2023.
- Bellinato F, Maurelli M, Gisondi P, et al. A systematic review of treatments for pityriasis lichenoides. J Eur Acad Dermatol Venereol. 2019;33:2039-2049. doi:10.1111/jdv.15813
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738. doi:10.1111/ijd.13195
- Thomson KF, Whittaker SJ, Russell-Jones R, et al. Childhood cutaneous T-cell lymphoma in association with pityriasis lichenoides chronica. Br J Dermatol. 1999;141:1136-1152. doi:10.1046/j.1365-2133.1999.03232.x
A 7-year-old boy was referred to the dermatology clinic for evaluation of a diffuse pruritic rash of 3 months’ duration. The rash began as scant erythematous papules on the face, and crops of similar lesions later erupted on the trunk, arms, and legs. He was treated previously by a pediatrician for scabies with topical permethrin followed by 2 doses of oral ivermectin 200 μg/kg without improvement. Physical examination revealed innumerable erythematous macules and papules with centrally adherent scaling distributed on the trunk, arms, and legs, as well as scant necrotic papules with a hemorrhagic crust and a peripheral rim of scale.

When Childhood Cancer Survivors Face Sexual Challenges
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Childhood cancers represent a diverse group of neoplasms, and thanks to advances in treatment, survival rates have improved significantly. Today, more than 80%-85% of children diagnosed with cancer in developed countries survive into adulthood.
This increase in survival has brought new challenges, however. Compared with the general population, childhood cancer survivors (CCS) are at a notably higher risk for early mortality, developing secondary cancers, and experiencing various long-term clinical and psychosocial issues stemming from their disease or its treatment.
Long-term follow-up care for CCS is a complex and evolving field. Despite ongoing efforts to establish global and national guidelines, current evidence indicates that the care and management of these patients remain suboptimal.
The disruptions caused by cancer and its treatment can interfere with normal physiological and psychological development, leading to issues with sexual function. This aspect of health is critical as it influences not just physical well-being but also psychosocial, developmental, and emotional health.
Characteristics and Mechanisms
Sexual functioning encompasses the physiological and psychological aspects of sexual behavior, including desire, arousal, orgasm, sexual pleasure, and overall satisfaction.
As CCS reach adolescence or adulthood, they often face sexual and reproductive issues, particularly as they enter romantic relationships.
Sexual functioning is a complex process that relies on the interaction of various factors, including physiological health, psychosexual development, romantic relationships, body image, and desire.
Despite its importance, the impact of childhood cancer on sexual function is often overlooked, even though cancer and its treatments can have lifelong effects.
Sexual Function in CCS
A recent review aimed to summarize the existing research on sexual function among CCS, highlighting assessment tools, key stages of psychosexual development, common sexual problems, and the prevalence of sexual dysfunction.
The review study included 22 studies published between 2000 and 2022, comprising two qualitative, six cohort, and 14 cross-sectional studies.
Most CCS reached all key stages of psychosexual development at an average age of 29.8 years. Although some milestones were achieved later than is typical, many survivors felt they reached these stages at the appropriate time. Sexual initiation was less common among those who had undergone intensive neurotoxic treatments, such as those diagnosed with brain tumors or leukemia in childhood.
In a cross-sectional study of CCS aged 17-39 years, about one third had never engaged in sexual intercourse, 41.4% reported never experiencing sexual attraction, 44.8% were dissatisfied with their sex lives, and many rarely felt sexually attractive to others. Another study found that common issues among CCS included a lack of interest in sex (30%), difficulty enjoying sex (24%), and difficulty becoming aroused (23%). However, comparing and analyzing these problems was challenging due to the lack of standardized assessment criteria.
The prevalence of sexual dysfunction among CCS ranged from 12.3% to 46.5%. For males, the prevalence ranged from 12.3% to 54.0%, while for females, it ranged from 19.9% to 57.0%.
Factors Influencing Sexual Function
The review identified the following four categories of factors influencing sexual function in CCS: Demographic, treatment-related, psychological, and physiological.
Demographic factors: Gender, age, education level, relationship status, income level, and race all play roles in sexual function.
Female survivors reported more severe sexual dysfunction and poorer sexual health than did male survivors. Age at cancer diagnosis, age at evaluation, and the time since diagnosis were closely linked to sexual experiences. Patients diagnosed with cancer during childhood tended to report better sexual function than those diagnosed during adolescence.
Treatment-related factors: The type of cancer and intensity of treatment, along with surgical history, were significant factors. Surgeries involving the spinal cord or sympathetic nerves, as well as a history of prostate or pelvic surgery, were strongly associated with erectile dysfunction in men. In women, pelvic surgeries and treatments to the pelvic area were commonly linked to sexual dysfunction.
The association between treatment intensity and sexual function was noted across several studies, although the results were not always consistent. For example, testicular radiation above 10 Gy was positively correlated with sexual dysfunction. Women who underwent more intensive treatments were more likely to report issues in multiple areas of sexual function, while men in this group were less likely to have children.
Among female CCS, certain types of cancer, such as germ cell tumors, renal tumors, and leukemia, present a higher risk for sexual dysfunction. Women who had CNS tumors in childhood frequently reported problems like difficulty in sexual arousal, low sexual satisfaction, infrequent sexual activity, and fewer sexual partners, compared with survivors of other cancers. Survivors of acute lymphoblastic leukemia and those who underwent hematopoietic stem cell transplantation (HSCT) also showed varying degrees of impaired sexual function, compared with the general population. The HSCT group showed significant testicular damage, including reduced testicular volumes, low testosterone levels, and low sperm counts.
Psychological factors: These factors, such as emotional distress, play a significant role in sexual dysfunction among CCS. Symptoms like anxiety, nervousness during sexual activity, and depression are commonly reported by those with sexual dysfunction. The connection between body image and sexual function is complex. Many CCS with sexual dysfunction express concern about how others, particularly their partners, perceived their altered body image due to cancer and its treatment.
Physiological factors: In male CCS, low serum testosterone levels and low lean muscle mass are linked to an increased risk for sexual dysfunction. Treatments involving alkylating agents or testicular radiation, and surgery or radiotherapy targeting the genitourinary organs or the hypothalamic-pituitary region, can lead to various physiological and endocrine disorders, contributing to sexual dysfunction. Despite these risks, there is a lack of research evaluating sexual function through the lens of the hypothalamic-pituitary-gonadal axis and neuroendocrine pathways.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Do You Have Patients With JAKne — JAK Inhibitor–Associated Acne? Here’s What to Know
Since the first Food and Drug Administration approval of a Janus kinase (JAK) inhibitor in 2011, the number of these medications available — and their treatment indications — have continued to grow. Prescribing physicians are familiar with the benefits and risks for these drugs, including higher risk for cardiac events and malignancy; however, one adverse effect may be overlooked, especially by specialties outside of dermatology: acne. Though less serious than some other side effects, JAK inhibitor–associated acne — JAKne, for short — can be a concern for patients.
“Your physical appearance and how you present yourself to the world is an important part of your self-confidence and living life on your own terms,” said Arash Mostaghimi, MD, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. “I think letting people know about [JAKne] and then addressing it when it occurs should be a normal part of managing these medications.”
What Is JAKne?
JAKne generally looks like other kinds of acne, explained Janelle Nassim, MD, director of laser and cosmetic dermatology at the Indiana University School of Medicine, Indianapolis. “It can affect the same areas that typical acne affects, including the face, chest, back, neck, and upper shoulders.”
Though it appears like typical forms of acne, it is not clear what drives these skin eruptions in patients taking JAK inhibitors.
“We don’t understand the underlying pathophysiology,” Dr. Mostaghimi said. “It looks like acne, but we don’t know if the exact underlying inflammatory process is the same or if it’s different.”
In a 2023 systematic review of clinical studies, Dr. Mostaghimi and colleagues found that patients on any JAK inhibitor were nearly four times more likely to experience acne than patients who received placebo, but risk varied between medications. Patients taking JAK inhibitors for skin conditions had higher risk for acne than those given the medications for other indications. However, Dr. Mostaghimi thinks this finding is the result of selection bias.
Participants may not mention side effects like acne in trials for rheumatologic or gastrointestinal conditions, he said, unlike in trials for skin conditions. “Clinically, I’ve seen it in patients across every indication.”
Patients with a history of acne seem to be more likely to develop this side effect, though formal studies looking into risk factors are lacking. In Dr. Mostaghimi’s own clinical experience, JAKne is also more common in younger patients, but it can happen to anyone. “I’ve seen 70-year-olds develop acne — patients who’ve never had an issue their whole life — when they’re taking a JAK inhibitor.”
This issue also appears to be more common earlier in treatment, he added, and may improve over time as a patient continues with the medication.
How Do You Treat It?
“I think in other specialties, you will often feel awkward addressing skin conditions or pointing out acne,” Dr. Mostaghimi said. The most important steps are being aware of this potential side effect, and if you see it practice, to bring it up.
“Say: I’m noticing there’s some changes in your skin. Some patients on JAK inhibitors develop more acne. Have you noticed this? And if so, is this bothering you?”
Generally, JAKne is mild to moderate, explained Dr. Nassim, and if non-dermatologists are comfortable, they can prescribe a first-line topical regimen for patients. Dr. Mostaghimi recommends prescribing a clindamycin 1% lotion or gel. In addition, patients can use a benzoyl peroxide wash (4% or 10%) combined with a gentle retinoid, such as adapalene. (Both of these treatments are now available over the counter.)
In patients with scalp or hairline involvement, he often prescribes a ketoconazole 2% shampoo, which patients can use to wash their scalp, face, chest, and back in the shower.
If they aren’t responding to these initial treatments, then refer to a dermatologist for further assessment.
“Ultimately, referring to a dermatologist is the best course of action,” Dr. Nassim said. “I have had patients on JAK inhibitors who improved with topical acne treatments, and some that required more aggressive treatment with oral medications.”
Dr. Mostaghimi reported consulting fees from AbbVie, Concert Pharmaceuticals, Pfizer, and 3Derm Systems; research funding from Incyte, Aclaris Therapeutics, Eli Lilly, and Concert Pharmaceuticals; personal fees from Equillium, ASLAN Pharmaceuticals, ACOM, and Boehringer Ingelheim; and advisory board fees from Fig.1 Beauty, Eli Lilly, Pfizer, and Hims & Hers Health. Dr. Nassim had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Since the first Food and Drug Administration approval of a Janus kinase (JAK) inhibitor in 2011, the number of these medications available — and their treatment indications — have continued to grow. Prescribing physicians are familiar with the benefits and risks for these drugs, including higher risk for cardiac events and malignancy; however, one adverse effect may be overlooked, especially by specialties outside of dermatology: acne. Though less serious than some other side effects, JAK inhibitor–associated acne — JAKne, for short — can be a concern for patients.
“Your physical appearance and how you present yourself to the world is an important part of your self-confidence and living life on your own terms,” said Arash Mostaghimi, MD, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. “I think letting people know about [JAKne] and then addressing it when it occurs should be a normal part of managing these medications.”
What Is JAKne?
JAKne generally looks like other kinds of acne, explained Janelle Nassim, MD, director of laser and cosmetic dermatology at the Indiana University School of Medicine, Indianapolis. “It can affect the same areas that typical acne affects, including the face, chest, back, neck, and upper shoulders.”
Though it appears like typical forms of acne, it is not clear what drives these skin eruptions in patients taking JAK inhibitors.
“We don’t understand the underlying pathophysiology,” Dr. Mostaghimi said. “It looks like acne, but we don’t know if the exact underlying inflammatory process is the same or if it’s different.”
In a 2023 systematic review of clinical studies, Dr. Mostaghimi and colleagues found that patients on any JAK inhibitor were nearly four times more likely to experience acne than patients who received placebo, but risk varied between medications. Patients taking JAK inhibitors for skin conditions had higher risk for acne than those given the medications for other indications. However, Dr. Mostaghimi thinks this finding is the result of selection bias.
Participants may not mention side effects like acne in trials for rheumatologic or gastrointestinal conditions, he said, unlike in trials for skin conditions. “Clinically, I’ve seen it in patients across every indication.”
Patients with a history of acne seem to be more likely to develop this side effect, though formal studies looking into risk factors are lacking. In Dr. Mostaghimi’s own clinical experience, JAKne is also more common in younger patients, but it can happen to anyone. “I’ve seen 70-year-olds develop acne — patients who’ve never had an issue their whole life — when they’re taking a JAK inhibitor.”
This issue also appears to be more common earlier in treatment, he added, and may improve over time as a patient continues with the medication.
How Do You Treat It?
“I think in other specialties, you will often feel awkward addressing skin conditions or pointing out acne,” Dr. Mostaghimi said. The most important steps are being aware of this potential side effect, and if you see it practice, to bring it up.
“Say: I’m noticing there’s some changes in your skin. Some patients on JAK inhibitors develop more acne. Have you noticed this? And if so, is this bothering you?”
Generally, JAKne is mild to moderate, explained Dr. Nassim, and if non-dermatologists are comfortable, they can prescribe a first-line topical regimen for patients. Dr. Mostaghimi recommends prescribing a clindamycin 1% lotion or gel. In addition, patients can use a benzoyl peroxide wash (4% or 10%) combined with a gentle retinoid, such as adapalene. (Both of these treatments are now available over the counter.)
In patients with scalp or hairline involvement, he often prescribes a ketoconazole 2% shampoo, which patients can use to wash their scalp, face, chest, and back in the shower.
If they aren’t responding to these initial treatments, then refer to a dermatologist for further assessment.
“Ultimately, referring to a dermatologist is the best course of action,” Dr. Nassim said. “I have had patients on JAK inhibitors who improved with topical acne treatments, and some that required more aggressive treatment with oral medications.”
Dr. Mostaghimi reported consulting fees from AbbVie, Concert Pharmaceuticals, Pfizer, and 3Derm Systems; research funding from Incyte, Aclaris Therapeutics, Eli Lilly, and Concert Pharmaceuticals; personal fees from Equillium, ASLAN Pharmaceuticals, ACOM, and Boehringer Ingelheim; and advisory board fees from Fig.1 Beauty, Eli Lilly, Pfizer, and Hims & Hers Health. Dr. Nassim had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Since the first Food and Drug Administration approval of a Janus kinase (JAK) inhibitor in 2011, the number of these medications available — and their treatment indications — have continued to grow. Prescribing physicians are familiar with the benefits and risks for these drugs, including higher risk for cardiac events and malignancy; however, one adverse effect may be overlooked, especially by specialties outside of dermatology: acne. Though less serious than some other side effects, JAK inhibitor–associated acne — JAKne, for short — can be a concern for patients.
“Your physical appearance and how you present yourself to the world is an important part of your self-confidence and living life on your own terms,” said Arash Mostaghimi, MD, the director of inpatient dermatology at Brigham and Women’s Hospital in Boston, Massachusetts. “I think letting people know about [JAKne] and then addressing it when it occurs should be a normal part of managing these medications.”
What Is JAKne?
JAKne generally looks like other kinds of acne, explained Janelle Nassim, MD, director of laser and cosmetic dermatology at the Indiana University School of Medicine, Indianapolis. “It can affect the same areas that typical acne affects, including the face, chest, back, neck, and upper shoulders.”
Though it appears like typical forms of acne, it is not clear what drives these skin eruptions in patients taking JAK inhibitors.
“We don’t understand the underlying pathophysiology,” Dr. Mostaghimi said. “It looks like acne, but we don’t know if the exact underlying inflammatory process is the same or if it’s different.”
In a 2023 systematic review of clinical studies, Dr. Mostaghimi and colleagues found that patients on any JAK inhibitor were nearly four times more likely to experience acne than patients who received placebo, but risk varied between medications. Patients taking JAK inhibitors for skin conditions had higher risk for acne than those given the medications for other indications. However, Dr. Mostaghimi thinks this finding is the result of selection bias.
Participants may not mention side effects like acne in trials for rheumatologic or gastrointestinal conditions, he said, unlike in trials for skin conditions. “Clinically, I’ve seen it in patients across every indication.”
Patients with a history of acne seem to be more likely to develop this side effect, though formal studies looking into risk factors are lacking. In Dr. Mostaghimi’s own clinical experience, JAKne is also more common in younger patients, but it can happen to anyone. “I’ve seen 70-year-olds develop acne — patients who’ve never had an issue their whole life — when they’re taking a JAK inhibitor.”
This issue also appears to be more common earlier in treatment, he added, and may improve over time as a patient continues with the medication.
How Do You Treat It?
“I think in other specialties, you will often feel awkward addressing skin conditions or pointing out acne,” Dr. Mostaghimi said. The most important steps are being aware of this potential side effect, and if you see it practice, to bring it up.
“Say: I’m noticing there’s some changes in your skin. Some patients on JAK inhibitors develop more acne. Have you noticed this? And if so, is this bothering you?”
Generally, JAKne is mild to moderate, explained Dr. Nassim, and if non-dermatologists are comfortable, they can prescribe a first-line topical regimen for patients. Dr. Mostaghimi recommends prescribing a clindamycin 1% lotion or gel. In addition, patients can use a benzoyl peroxide wash (4% or 10%) combined with a gentle retinoid, such as adapalene. (Both of these treatments are now available over the counter.)
In patients with scalp or hairline involvement, he often prescribes a ketoconazole 2% shampoo, which patients can use to wash their scalp, face, chest, and back in the shower.
If they aren’t responding to these initial treatments, then refer to a dermatologist for further assessment.
“Ultimately, referring to a dermatologist is the best course of action,” Dr. Nassim said. “I have had patients on JAK inhibitors who improved with topical acne treatments, and some that required more aggressive treatment with oral medications.”
Dr. Mostaghimi reported consulting fees from AbbVie, Concert Pharmaceuticals, Pfizer, and 3Derm Systems; research funding from Incyte, Aclaris Therapeutics, Eli Lilly, and Concert Pharmaceuticals; personal fees from Equillium, ASLAN Pharmaceuticals, ACOM, and Boehringer Ingelheim; and advisory board fees from Fig.1 Beauty, Eli Lilly, Pfizer, and Hims & Hers Health. Dr. Nassim had no relevant disclosures.
A version of this article first appeared on Medscape.com.