User login
Bifidobacteria supplementation regulates newborn immune system
Supplementing breastfed infants with bifidobacteria promotes development of a well-regulated immune system, theoretically reducing risk of immune-mediated conditions like allergies and asthma, according to investigators.
These findings support the importance of early gut colonization with beneficial microbes, an event that may affect the immune system throughout life, reported lead author Bethany M. Henrick, PhD, director of immunology and diagnostics at Evolve Biosystems, Davis, Calif., and adjunct assistant professor at the University of Nebraska, Lincoln, and colleagues.
“Dysbiosis of the infant gut microbiome is common in modern societies and a likely contributing factor to the increased incidences of immune-mediated disorders,” the investigators wrote in Cell. “Therefore, there is great interest in identifying microbial factors that can support healthier immune system imprinting and hopefully prevent cases of allergy, autoimmunity, and possibly other conditions involving the immune system.”
Prevailing theory suggests that the rising incidence of neonatal intestinal dysbiosis – which is typical in developed countries – may be caused by a variety of factors, including cesarean sections; modern hygiene practices; antibiotics, antiseptics, and other medications; diets high in fat and sugar; and infant formula.
According to Dr. Henrick and colleagues, a healthy gut microbiome plays the greatest role in immunological development during the first 3 months post partum; specifically, a lack of bifidobacteria during this time has been linked with increased risks of autoimmunity and enteric inflammation, although underlying immune mechanisms remain unclear.
Bifidobacteria also exemplify the symbiotic relationship between mothers, babies, and beneficial microbes. The investigators pointed out that breast milk contains human milk oligosaccharides (HMOs), which humans cannot digest, but are an excellent source of energy for bifidobacteria and other beneficial microbes, giving them a “selective nutritional advantage.”
Bifidobacteria should therefore be common residents within the infant gut, but this is often not now the case, leading Dr. Henrick and colleagues to zero in on the microbe, in hopes of determining the exactly how beneficial bacteria shape immune development.
It is only recently that the necessary knowledge and techniques to perform studies like this one have become available, the investigators wrote, noting a better understanding of cell-regulatory relationships, advances in immune profiling at the systems level, and new technology that allows for profiling small-volume samples from infants.
The present study involved a series of observational experiments and a small interventional trial.
First, the investigators conducted a wide array of blood- and fecal-based longitudinal analyses from 208 infants in Sweden to characterize immune cell expansion and microbiome colonization of the gut, with a focus on bifidobacteria.
Their results showed that infants lacking bifidobacteria, and HMO-utilization genes (which are expressed by bifidobacteria and other beneficial microbes), had higher levels of systemic inflammation, including increased T helper 2 (Th2) and Th17 responses.
“Infants not colonized by Bifidobacteriaceae or in cases where these microbes fail to expand during the first months of life there is evidence of systemic and intestinal inflammation, increased frequencies of activated immune cells, and reduced levels of regulatory cells indicative of systemic immune dysregulation,” the investigators wrote.
The interventional part of the study involved 60 breastfed infants in California. Twenty-nine of the newborns were given 1.8 x 1010 colony-forming units (CFUs) of B. longum subsp. infantis EVC001 daily from postnatal day 7 to day 28, while the remaining 31 infants were given no supplementation.
Fecal samples were collected on day 6 and day 60. At day 60, supplemented infants had high levels of HMO-utilization genes, plus significantly greater alpha diversity (P = .0001; Wilcoxon), compared with controls. Infants receiving EVC001 also had less inflammatory fecal cytokines, suggesting that microbes expressing HMO-utilization genes cause a shift away from proinflammatory Th2 and Th17 responses, and toward Th1.
“It is not the simple presence of bifidobacteria that is responsible for the immune effects but the metabolic partnership between the bacteria and HMOs,” the investigators noted.
According to principal investigator Petter Brodin, MD, PhD, professor of pediatric immunology at Karolinska Institutet, Solna, Sweden, the findings deserve further investigation.
“Our data indicate that substitution with beneficial microbes efficiently metabolizing HMOs could open a way to prevent cases of immune-mediated diseases, but larger, randomized trials aimed at this will be required to determine this potential,” Dr. Brodin said in an interview.
Carolynn Dude, MD, PhD, assistant professor in the division of maternal-fetal medicine at Emory University, Atlanta, agreed that more work is needed.
“While this study provides some insight into the mechanisms that may set up a newborn for poor health outcomes later in life, the data is still very limited, and more long-term follow-up on these infants is needed before recommending any sort of bacterial supplementation to a newborn,” Dr. Dude said in an interview.
Dr. Brodin and colleagues are planning an array of related studies, including larger clinical trials; further investigations into mechanisms of action; comparisons between the present cohort and infants in Kenya, where immune-mediated diseases are rare; and evaluations of vaccine responses and infectious disease susceptibility.
The study was supported by the European Research Council, the Swedish Research Council, the Marianne & Marcus Wallenberg Foundation, and others. The investigators disclosed relationships with Cytodelics, Scailyte, Kancera, and others. Dr. Dude reported no relevant conflicts of interest.
Supplementing breastfed infants with bifidobacteria promotes development of a well-regulated immune system, theoretically reducing risk of immune-mediated conditions like allergies and asthma, according to investigators.
These findings support the importance of early gut colonization with beneficial microbes, an event that may affect the immune system throughout life, reported lead author Bethany M. Henrick, PhD, director of immunology and diagnostics at Evolve Biosystems, Davis, Calif., and adjunct assistant professor at the University of Nebraska, Lincoln, and colleagues.
“Dysbiosis of the infant gut microbiome is common in modern societies and a likely contributing factor to the increased incidences of immune-mediated disorders,” the investigators wrote in Cell. “Therefore, there is great interest in identifying microbial factors that can support healthier immune system imprinting and hopefully prevent cases of allergy, autoimmunity, and possibly other conditions involving the immune system.”
Prevailing theory suggests that the rising incidence of neonatal intestinal dysbiosis – which is typical in developed countries – may be caused by a variety of factors, including cesarean sections; modern hygiene practices; antibiotics, antiseptics, and other medications; diets high in fat and sugar; and infant formula.
According to Dr. Henrick and colleagues, a healthy gut microbiome plays the greatest role in immunological development during the first 3 months post partum; specifically, a lack of bifidobacteria during this time has been linked with increased risks of autoimmunity and enteric inflammation, although underlying immune mechanisms remain unclear.
Bifidobacteria also exemplify the symbiotic relationship between mothers, babies, and beneficial microbes. The investigators pointed out that breast milk contains human milk oligosaccharides (HMOs), which humans cannot digest, but are an excellent source of energy for bifidobacteria and other beneficial microbes, giving them a “selective nutritional advantage.”
Bifidobacteria should therefore be common residents within the infant gut, but this is often not now the case, leading Dr. Henrick and colleagues to zero in on the microbe, in hopes of determining the exactly how beneficial bacteria shape immune development.
It is only recently that the necessary knowledge and techniques to perform studies like this one have become available, the investigators wrote, noting a better understanding of cell-regulatory relationships, advances in immune profiling at the systems level, and new technology that allows for profiling small-volume samples from infants.
The present study involved a series of observational experiments and a small interventional trial.
First, the investigators conducted a wide array of blood- and fecal-based longitudinal analyses from 208 infants in Sweden to characterize immune cell expansion and microbiome colonization of the gut, with a focus on bifidobacteria.
Their results showed that infants lacking bifidobacteria, and HMO-utilization genes (which are expressed by bifidobacteria and other beneficial microbes), had higher levels of systemic inflammation, including increased T helper 2 (Th2) and Th17 responses.
“Infants not colonized by Bifidobacteriaceae or in cases where these microbes fail to expand during the first months of life there is evidence of systemic and intestinal inflammation, increased frequencies of activated immune cells, and reduced levels of regulatory cells indicative of systemic immune dysregulation,” the investigators wrote.
The interventional part of the study involved 60 breastfed infants in California. Twenty-nine of the newborns were given 1.8 x 1010 colony-forming units (CFUs) of B. longum subsp. infantis EVC001 daily from postnatal day 7 to day 28, while the remaining 31 infants were given no supplementation.
Fecal samples were collected on day 6 and day 60. At day 60, supplemented infants had high levels of HMO-utilization genes, plus significantly greater alpha diversity (P = .0001; Wilcoxon), compared with controls. Infants receiving EVC001 also had less inflammatory fecal cytokines, suggesting that microbes expressing HMO-utilization genes cause a shift away from proinflammatory Th2 and Th17 responses, and toward Th1.
“It is not the simple presence of bifidobacteria that is responsible for the immune effects but the metabolic partnership between the bacteria and HMOs,” the investigators noted.
According to principal investigator Petter Brodin, MD, PhD, professor of pediatric immunology at Karolinska Institutet, Solna, Sweden, the findings deserve further investigation.
“Our data indicate that substitution with beneficial microbes efficiently metabolizing HMOs could open a way to prevent cases of immune-mediated diseases, but larger, randomized trials aimed at this will be required to determine this potential,” Dr. Brodin said in an interview.
Carolynn Dude, MD, PhD, assistant professor in the division of maternal-fetal medicine at Emory University, Atlanta, agreed that more work is needed.
“While this study provides some insight into the mechanisms that may set up a newborn for poor health outcomes later in life, the data is still very limited, and more long-term follow-up on these infants is needed before recommending any sort of bacterial supplementation to a newborn,” Dr. Dude said in an interview.
Dr. Brodin and colleagues are planning an array of related studies, including larger clinical trials; further investigations into mechanisms of action; comparisons between the present cohort and infants in Kenya, where immune-mediated diseases are rare; and evaluations of vaccine responses and infectious disease susceptibility.
The study was supported by the European Research Council, the Swedish Research Council, the Marianne & Marcus Wallenberg Foundation, and others. The investigators disclosed relationships with Cytodelics, Scailyte, Kancera, and others. Dr. Dude reported no relevant conflicts of interest.
Supplementing breastfed infants with bifidobacteria promotes development of a well-regulated immune system, theoretically reducing risk of immune-mediated conditions like allergies and asthma, according to investigators.
These findings support the importance of early gut colonization with beneficial microbes, an event that may affect the immune system throughout life, reported lead author Bethany M. Henrick, PhD, director of immunology and diagnostics at Evolve Biosystems, Davis, Calif., and adjunct assistant professor at the University of Nebraska, Lincoln, and colleagues.
“Dysbiosis of the infant gut microbiome is common in modern societies and a likely contributing factor to the increased incidences of immune-mediated disorders,” the investigators wrote in Cell. “Therefore, there is great interest in identifying microbial factors that can support healthier immune system imprinting and hopefully prevent cases of allergy, autoimmunity, and possibly other conditions involving the immune system.”
Prevailing theory suggests that the rising incidence of neonatal intestinal dysbiosis – which is typical in developed countries – may be caused by a variety of factors, including cesarean sections; modern hygiene practices; antibiotics, antiseptics, and other medications; diets high in fat and sugar; and infant formula.
According to Dr. Henrick and colleagues, a healthy gut microbiome plays the greatest role in immunological development during the first 3 months post partum; specifically, a lack of bifidobacteria during this time has been linked with increased risks of autoimmunity and enteric inflammation, although underlying immune mechanisms remain unclear.
Bifidobacteria also exemplify the symbiotic relationship between mothers, babies, and beneficial microbes. The investigators pointed out that breast milk contains human milk oligosaccharides (HMOs), which humans cannot digest, but are an excellent source of energy for bifidobacteria and other beneficial microbes, giving them a “selective nutritional advantage.”
Bifidobacteria should therefore be common residents within the infant gut, but this is often not now the case, leading Dr. Henrick and colleagues to zero in on the microbe, in hopes of determining the exactly how beneficial bacteria shape immune development.
It is only recently that the necessary knowledge and techniques to perform studies like this one have become available, the investigators wrote, noting a better understanding of cell-regulatory relationships, advances in immune profiling at the systems level, and new technology that allows for profiling small-volume samples from infants.
The present study involved a series of observational experiments and a small interventional trial.
First, the investigators conducted a wide array of blood- and fecal-based longitudinal analyses from 208 infants in Sweden to characterize immune cell expansion and microbiome colonization of the gut, with a focus on bifidobacteria.
Their results showed that infants lacking bifidobacteria, and HMO-utilization genes (which are expressed by bifidobacteria and other beneficial microbes), had higher levels of systemic inflammation, including increased T helper 2 (Th2) and Th17 responses.
“Infants not colonized by Bifidobacteriaceae or in cases where these microbes fail to expand during the first months of life there is evidence of systemic and intestinal inflammation, increased frequencies of activated immune cells, and reduced levels of regulatory cells indicative of systemic immune dysregulation,” the investigators wrote.
The interventional part of the study involved 60 breastfed infants in California. Twenty-nine of the newborns were given 1.8 x 1010 colony-forming units (CFUs) of B. longum subsp. infantis EVC001 daily from postnatal day 7 to day 28, while the remaining 31 infants were given no supplementation.
Fecal samples were collected on day 6 and day 60. At day 60, supplemented infants had high levels of HMO-utilization genes, plus significantly greater alpha diversity (P = .0001; Wilcoxon), compared with controls. Infants receiving EVC001 also had less inflammatory fecal cytokines, suggesting that microbes expressing HMO-utilization genes cause a shift away from proinflammatory Th2 and Th17 responses, and toward Th1.
“It is not the simple presence of bifidobacteria that is responsible for the immune effects but the metabolic partnership between the bacteria and HMOs,” the investigators noted.
According to principal investigator Petter Brodin, MD, PhD, professor of pediatric immunology at Karolinska Institutet, Solna, Sweden, the findings deserve further investigation.
“Our data indicate that substitution with beneficial microbes efficiently metabolizing HMOs could open a way to prevent cases of immune-mediated diseases, but larger, randomized trials aimed at this will be required to determine this potential,” Dr. Brodin said in an interview.
Carolynn Dude, MD, PhD, assistant professor in the division of maternal-fetal medicine at Emory University, Atlanta, agreed that more work is needed.
“While this study provides some insight into the mechanisms that may set up a newborn for poor health outcomes later in life, the data is still very limited, and more long-term follow-up on these infants is needed before recommending any sort of bacterial supplementation to a newborn,” Dr. Dude said in an interview.
Dr. Brodin and colleagues are planning an array of related studies, including larger clinical trials; further investigations into mechanisms of action; comparisons between the present cohort and infants in Kenya, where immune-mediated diseases are rare; and evaluations of vaccine responses and infectious disease susceptibility.
The study was supported by the European Research Council, the Swedish Research Council, the Marianne & Marcus Wallenberg Foundation, and others. The investigators disclosed relationships with Cytodelics, Scailyte, Kancera, and others. Dr. Dude reported no relevant conflicts of interest.
FROM CELL
AI-based software demonstrates accuracy in diagnosis of autism
A software program based on artificial intelligence (AI) is effective for distinguishing young children with autism spectrum disorder (ASD) from those with other conditions, according to results of a pivotal trial presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
The AI-based software, which will be submitted to regulatory approval as a device, employs an algorithm that assembles inputs from a caregiver questionnaire, a video, and a clinician questionnaire, according to Sharief Taraman, MD, a pediatric neurologist at CHOC, a pediatric health care system in Orange County, Calif.
Although the device could be employed in a variety of settings, it is envisioned for use by primary care physicians. This will circumvent the need for specialist evaluation except in challenging cases. Currently, nearly all children with ASD are diagnosed in specialty care, according to data cited by Dr. Taraman.
“The lack of diagnostic tools for ASD in primary care settings contributes to an average delay of 3 years between first parental concern and diagnosis and to long wait lists for specialty evaluation,” he reported at the virtual meeting, presented by MedscapeLive.
When used with clinical judgment and criteria from the American Psychiatric Association’s 5th edition of the Diagnostic and Statistical Manual (DSM-5), the data from the trial suggest the diagnostic tool in the hands of primary care physicians “could efficiently and accurately assess ASD in children 18 to 72 months old,” said Dr. Taraman, also an associate clinical professor of pediatrics at the University of California, Irvine.*
The AI-assisted software was evaluated in 425 children at 14 sites in 6 states. The study population was reflective of U.S. demographics. Although only 36% of the children were female, this is consistent with ASD prevalence. Only 60% of the subjects were White. Nearly 30% were Black or Latinx and other populations, such as those of Asian heritage, were represented.
Children between the ages of 18 and 72 months were eligible if both a caregiver and a health care professional were concerned that the child had ASD. About the same time that a caregiver completed a 20-item questionnaire and the primary care physician completed a 15-item questionnaire on a mobile device, the caregiver uploaded two videos of 1-2 minutes in length.
This information, along with a 33-item questionnaire completed by an analyst of the submitted videos, was then processed by the software algorithm. It provided a patient status of positive or negative for ASD, or it concluded that the status was indeterminate.
“To reduce the risk of false classifications, the indeterminate status was included as a safety feature,” Dr. Taraman explained. However, Dr. Taraman considers an indeterminate designation potentially actionable. Rather than a negative result, this status suggests a complex neurodevelopmental disorder and indicates the need for further evaluation.
The reference standard diagnosis, completed in all participants in this study, was a specialist evaluation completed independently by two experts. The presence or absence of ASD was confirmed if the experts agreed. If they did not, a third specialist made the final determination.
In comparison to the specialist determinations, all were correctly classified except for one child, in which the software was determined to have made a false-negative diagnosis. A diagnosis of ASD was reached in 29% of the study participants.
For those with a determinate designation, the sensitivity was 98.4% and the specificity was 78.9%. This translated into positive predictive and negative predictive values of 80.8% and 98.3%, respectively.
Of those identified as indeterminate by the AI-assisted algorithm, 91% were ultimately considered by specialist evaluation to have complex issues. In this group, ASD was part of the complex clinical picture in 20%. The others had non-ASD neurodevelopmental conditions, according to Dr. Taraman.
When the accuracy was evaluated across ages, ethnicity, and factors such as parent education or family income, the tool performed consistently, Dr. Taraman reported. This is important, he said, because the presence or absence of ASD is misdiagnosed in many underserved populations.
The focus on developing a methodology specific for use in primary care was based on evidence that the delay in the diagnosis of ASD is attributable to long wait times for specialty evaluations.
“There will never be enough specialists. There is a need for a way to streamline the diagnosis of ASD,” Dr. Taraman maintained. This is helpful not only to parents concerned about their children, he said, but also there are data to suggest that early intervention improves outcomes.
A specialist in ASD, Paul Carbone, MD, medical director of the child development program at the University of Utah, Salt Lake City, agreed. He said early diagnosis and intervention should be a goal.
“Reducing the age of ASD diagnosis is a priority because early entry into autism-specific interventions is a strong predictor of optimal developmental outcomes for children,” Dr. Carbone said.
Although he is not familiar with this experimental AI-assisted diagnostic program, he has published on the feasibility of ASD diagnosis at the primary care level. In his study, Dr. Carbone examined the Modified Checklist for Autism in Toddlers (M-CHAT) as one of several methodologies that might be considered.
Diagnosis of ASD “can be achieved through systematic processes within primary care that facilitate universal development surveillance and autism screening followed by prompt and timely diagnostic evaluations of at-risk children,” Dr. Carbone said.
MedscapeLive and this news organization are owned by the same parent company. Dr. Taraman reported a financial relationship with Cognoa, the company that is developing the ASD software for clinical use. Dr. Carbone reported that he has no conflicts of interest.
*Updated, 7/7/21
A software program based on artificial intelligence (AI) is effective for distinguishing young children with autism spectrum disorder (ASD) from those with other conditions, according to results of a pivotal trial presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
The AI-based software, which will be submitted to regulatory approval as a device, employs an algorithm that assembles inputs from a caregiver questionnaire, a video, and a clinician questionnaire, according to Sharief Taraman, MD, a pediatric neurologist at CHOC, a pediatric health care system in Orange County, Calif.
Although the device could be employed in a variety of settings, it is envisioned for use by primary care physicians. This will circumvent the need for specialist evaluation except in challenging cases. Currently, nearly all children with ASD are diagnosed in specialty care, according to data cited by Dr. Taraman.
“The lack of diagnostic tools for ASD in primary care settings contributes to an average delay of 3 years between first parental concern and diagnosis and to long wait lists for specialty evaluation,” he reported at the virtual meeting, presented by MedscapeLive.
When used with clinical judgment and criteria from the American Psychiatric Association’s 5th edition of the Diagnostic and Statistical Manual (DSM-5), the data from the trial suggest the diagnostic tool in the hands of primary care physicians “could efficiently and accurately assess ASD in children 18 to 72 months old,” said Dr. Taraman, also an associate clinical professor of pediatrics at the University of California, Irvine.*
The AI-assisted software was evaluated in 425 children at 14 sites in 6 states. The study population was reflective of U.S. demographics. Although only 36% of the children were female, this is consistent with ASD prevalence. Only 60% of the subjects were White. Nearly 30% were Black or Latinx and other populations, such as those of Asian heritage, were represented.
Children between the ages of 18 and 72 months were eligible if both a caregiver and a health care professional were concerned that the child had ASD. About the same time that a caregiver completed a 20-item questionnaire and the primary care physician completed a 15-item questionnaire on a mobile device, the caregiver uploaded two videos of 1-2 minutes in length.
This information, along with a 33-item questionnaire completed by an analyst of the submitted videos, was then processed by the software algorithm. It provided a patient status of positive or negative for ASD, or it concluded that the status was indeterminate.
“To reduce the risk of false classifications, the indeterminate status was included as a safety feature,” Dr. Taraman explained. However, Dr. Taraman considers an indeterminate designation potentially actionable. Rather than a negative result, this status suggests a complex neurodevelopmental disorder and indicates the need for further evaluation.
The reference standard diagnosis, completed in all participants in this study, was a specialist evaluation completed independently by two experts. The presence or absence of ASD was confirmed if the experts agreed. If they did not, a third specialist made the final determination.
In comparison to the specialist determinations, all were correctly classified except for one child, in which the software was determined to have made a false-negative diagnosis. A diagnosis of ASD was reached in 29% of the study participants.
For those with a determinate designation, the sensitivity was 98.4% and the specificity was 78.9%. This translated into positive predictive and negative predictive values of 80.8% and 98.3%, respectively.
Of those identified as indeterminate by the AI-assisted algorithm, 91% were ultimately considered by specialist evaluation to have complex issues. In this group, ASD was part of the complex clinical picture in 20%. The others had non-ASD neurodevelopmental conditions, according to Dr. Taraman.
When the accuracy was evaluated across ages, ethnicity, and factors such as parent education or family income, the tool performed consistently, Dr. Taraman reported. This is important, he said, because the presence or absence of ASD is misdiagnosed in many underserved populations.
The focus on developing a methodology specific for use in primary care was based on evidence that the delay in the diagnosis of ASD is attributable to long wait times for specialty evaluations.
“There will never be enough specialists. There is a need for a way to streamline the diagnosis of ASD,” Dr. Taraman maintained. This is helpful not only to parents concerned about their children, he said, but also there are data to suggest that early intervention improves outcomes.
A specialist in ASD, Paul Carbone, MD, medical director of the child development program at the University of Utah, Salt Lake City, agreed. He said early diagnosis and intervention should be a goal.
“Reducing the age of ASD diagnosis is a priority because early entry into autism-specific interventions is a strong predictor of optimal developmental outcomes for children,” Dr. Carbone said.
Although he is not familiar with this experimental AI-assisted diagnostic program, he has published on the feasibility of ASD diagnosis at the primary care level. In his study, Dr. Carbone examined the Modified Checklist for Autism in Toddlers (M-CHAT) as one of several methodologies that might be considered.
Diagnosis of ASD “can be achieved through systematic processes within primary care that facilitate universal development surveillance and autism screening followed by prompt and timely diagnostic evaluations of at-risk children,” Dr. Carbone said.
MedscapeLive and this news organization are owned by the same parent company. Dr. Taraman reported a financial relationship with Cognoa, the company that is developing the ASD software for clinical use. Dr. Carbone reported that he has no conflicts of interest.
*Updated, 7/7/21
A software program based on artificial intelligence (AI) is effective for distinguishing young children with autism spectrum disorder (ASD) from those with other conditions, according to results of a pivotal trial presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
The AI-based software, which will be submitted to regulatory approval as a device, employs an algorithm that assembles inputs from a caregiver questionnaire, a video, and a clinician questionnaire, according to Sharief Taraman, MD, a pediatric neurologist at CHOC, a pediatric health care system in Orange County, Calif.
Although the device could be employed in a variety of settings, it is envisioned for use by primary care physicians. This will circumvent the need for specialist evaluation except in challenging cases. Currently, nearly all children with ASD are diagnosed in specialty care, according to data cited by Dr. Taraman.
“The lack of diagnostic tools for ASD in primary care settings contributes to an average delay of 3 years between first parental concern and diagnosis and to long wait lists for specialty evaluation,” he reported at the virtual meeting, presented by MedscapeLive.
When used with clinical judgment and criteria from the American Psychiatric Association’s 5th edition of the Diagnostic and Statistical Manual (DSM-5), the data from the trial suggest the diagnostic tool in the hands of primary care physicians “could efficiently and accurately assess ASD in children 18 to 72 months old,” said Dr. Taraman, also an associate clinical professor of pediatrics at the University of California, Irvine.*
The AI-assisted software was evaluated in 425 children at 14 sites in 6 states. The study population was reflective of U.S. demographics. Although only 36% of the children were female, this is consistent with ASD prevalence. Only 60% of the subjects were White. Nearly 30% were Black or Latinx and other populations, such as those of Asian heritage, were represented.
Children between the ages of 18 and 72 months were eligible if both a caregiver and a health care professional were concerned that the child had ASD. About the same time that a caregiver completed a 20-item questionnaire and the primary care physician completed a 15-item questionnaire on a mobile device, the caregiver uploaded two videos of 1-2 minutes in length.
This information, along with a 33-item questionnaire completed by an analyst of the submitted videos, was then processed by the software algorithm. It provided a patient status of positive or negative for ASD, or it concluded that the status was indeterminate.
“To reduce the risk of false classifications, the indeterminate status was included as a safety feature,” Dr. Taraman explained. However, Dr. Taraman considers an indeterminate designation potentially actionable. Rather than a negative result, this status suggests a complex neurodevelopmental disorder and indicates the need for further evaluation.
The reference standard diagnosis, completed in all participants in this study, was a specialist evaluation completed independently by two experts. The presence or absence of ASD was confirmed if the experts agreed. If they did not, a third specialist made the final determination.
In comparison to the specialist determinations, all were correctly classified except for one child, in which the software was determined to have made a false-negative diagnosis. A diagnosis of ASD was reached in 29% of the study participants.
For those with a determinate designation, the sensitivity was 98.4% and the specificity was 78.9%. This translated into positive predictive and negative predictive values of 80.8% and 98.3%, respectively.
Of those identified as indeterminate by the AI-assisted algorithm, 91% were ultimately considered by specialist evaluation to have complex issues. In this group, ASD was part of the complex clinical picture in 20%. The others had non-ASD neurodevelopmental conditions, according to Dr. Taraman.
When the accuracy was evaluated across ages, ethnicity, and factors such as parent education or family income, the tool performed consistently, Dr. Taraman reported. This is important, he said, because the presence or absence of ASD is misdiagnosed in many underserved populations.
The focus on developing a methodology specific for use in primary care was based on evidence that the delay in the diagnosis of ASD is attributable to long wait times for specialty evaluations.
“There will never be enough specialists. There is a need for a way to streamline the diagnosis of ASD,” Dr. Taraman maintained. This is helpful not only to parents concerned about their children, he said, but also there are data to suggest that early intervention improves outcomes.
A specialist in ASD, Paul Carbone, MD, medical director of the child development program at the University of Utah, Salt Lake City, agreed. He said early diagnosis and intervention should be a goal.
“Reducing the age of ASD diagnosis is a priority because early entry into autism-specific interventions is a strong predictor of optimal developmental outcomes for children,” Dr. Carbone said.
Although he is not familiar with this experimental AI-assisted diagnostic program, he has published on the feasibility of ASD diagnosis at the primary care level. In his study, Dr. Carbone examined the Modified Checklist for Autism in Toddlers (M-CHAT) as one of several methodologies that might be considered.
Diagnosis of ASD “can be achieved through systematic processes within primary care that facilitate universal development surveillance and autism screening followed by prompt and timely diagnostic evaluations of at-risk children,” Dr. Carbone said.
MedscapeLive and this news organization are owned by the same parent company. Dr. Taraman reported a financial relationship with Cognoa, the company that is developing the ASD software for clinical use. Dr. Carbone reported that he has no conflicts of interest.
*Updated, 7/7/21
FROM CP/AACP PSYCHIATRY UPDATE
Polypharmacy remains common for autism spectrum patients
Approximately one-third of individuals with autism spectrum disorder (ASD) are prescribed multiple medications to manage comorbidities and symptoms, according to data from a retrospective cohort study of more than 26,000 patients.
“Clinicians caring for patients with ASD are tasked with the challenges of managing the primary disease, as well as co-occurring medical conditions, and coordinating with educational and social service professionals to provide holistic care,” wrote Aliya G. Feroe of Harvard Medical School, Boston, and colleagues.
The medication classes used to treat individuals with ASD include ADHD medications, antipsychotics, antidepressants, mood stabilizers, benzodiazepines, anxiolytics, and hypnotics, but the prescription rates of these medications in ASD patients have not been examined in large studies, the researchers said.
In a study published in JAMA Pediatrics, the researchers identified 26,722 individuals with ASD using a United States health care database from Jan. 1, 2014, to Dec. 31, 2019. Data included records of inpatient and outpatient claims, and records of prescriptions filled through commercial pharmacies. Individuals received at least 1 of 24 of the most common medication groups for ASD or comorbidities. The average age of the study participants was 14 years, and 78% were male. Diagnostic codes for ASD were based on the International Classification of Diseases, Ninth Revision, and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision.
Over the 6-year study period, approximately one-third of the participants were taking three or more medications at once, ranging from 28.6% to 31.5%. In any 1 year, approximately 41% of children were prescribed a single medication, 17% received two prescriptions, 7.9% received four, and 3.4% received five. Medication changes occurred more frequently within classes than between classes, and reasons for these changes may include patient preference, adverse effects, and cost, the researchers noted.
The overall number of children prescribed particular drugs remained consistent, the researchers noted. “For example, the total number of individuals prescribed methylphenidate shifted from 832 in 2014 to 850 in 2015, 899 in 2016, 863 in 2017, and 838 in 2018,” they wrote.
In 15 of the 24 medication groups included in the study, at least 15% of the individuals had unspecified anxiety disorder, anxiety neurosis, or major depressive disorder; in 11 of the medication groups, at least 15% had some form of ADHD. ADHD prevalence in patients taking stimulants varied based on ADHD type, the researchers said.
The most common comorbidities in patients taking antipsychotics were combined type ADHD (11.6%-17.8%) and anxiety disorder (13.1%-30.1%). The study findings suggest that many clinicians are incorporating medications into ASD management, the researchers said.
“Although there is no medical treatment for the core deficits of social communication and repetitive behavioral patterns in ASD, the American Academy of Pediatrics recommends that clinicians consider medications in the management of common comorbid conditions, including seizures, ADHD, anxiety disorders, mood disorders, and disruptive behavior disorders,” they said.
The findings were limited by several factors including the potential for inconsistent reporting of diagnoses and pharmacy claims, the researchers noted. Other limitations included a lack of direct clinical assessment to validate diagnoses and the absence of validated diagnostic instruments to screen for comorbidities, they added.
“Our findings suggest that clinicians may be increasingly using integrated approaches to treating patients with ASD and co-occurring conditions, and further work is necessary to determine the relative effects of pharmacotherapy vs. behavioral interventions on outcomes in patients with ASD,” the researchers concluded.
Many reasons for multiple medications
“The researchers put in a lot of effort to provide data on a large scale,” Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“The findings illustrate the reality that autistic children are prescribed a lot of medications for a lot of reasons, some of which are not entirely clear,” Dr. Lessin said. The study also reflects the chronic lack of behavioral health services for children, he noted. Many children with ASD are referred for services they are unable to access, he said. “As a result, they see doctors who can only prescribe medications to try to control behavior or symptoms for which the cause is unclear,” and which could be ASD or other comorbidities, he emphasized.
The large sample size strengthens the study findings, but some of the challenges include the use of claims data, which do not indicate how diagnoses were made, said Dr. Lessin. An additional limitation is the fact that many medications for children with autism are used off label, so the specific reason for their use may be unknown, he said.
The take-home message for clinicians is that children with ASD are getting a lot of medications, and pediatricians are not usually responsible for multiple medications,” Dr. Lessin said. Ultimately, the study is “a plea for more research,” to tease out details of what medications are indicated and helpful, he said.
The study received no outside funding. The researchers and Dr. Lessin had no financial conflicts to disclose. Dr. Lessin serves on the Pediatric News editorial advisory board.
Approximately one-third of individuals with autism spectrum disorder (ASD) are prescribed multiple medications to manage comorbidities and symptoms, according to data from a retrospective cohort study of more than 26,000 patients.
“Clinicians caring for patients with ASD are tasked with the challenges of managing the primary disease, as well as co-occurring medical conditions, and coordinating with educational and social service professionals to provide holistic care,” wrote Aliya G. Feroe of Harvard Medical School, Boston, and colleagues.
The medication classes used to treat individuals with ASD include ADHD medications, antipsychotics, antidepressants, mood stabilizers, benzodiazepines, anxiolytics, and hypnotics, but the prescription rates of these medications in ASD patients have not been examined in large studies, the researchers said.
In a study published in JAMA Pediatrics, the researchers identified 26,722 individuals with ASD using a United States health care database from Jan. 1, 2014, to Dec. 31, 2019. Data included records of inpatient and outpatient claims, and records of prescriptions filled through commercial pharmacies. Individuals received at least 1 of 24 of the most common medication groups for ASD or comorbidities. The average age of the study participants was 14 years, and 78% were male. Diagnostic codes for ASD were based on the International Classification of Diseases, Ninth Revision, and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision.
Over the 6-year study period, approximately one-third of the participants were taking three or more medications at once, ranging from 28.6% to 31.5%. In any 1 year, approximately 41% of children were prescribed a single medication, 17% received two prescriptions, 7.9% received four, and 3.4% received five. Medication changes occurred more frequently within classes than between classes, and reasons for these changes may include patient preference, adverse effects, and cost, the researchers noted.
The overall number of children prescribed particular drugs remained consistent, the researchers noted. “For example, the total number of individuals prescribed methylphenidate shifted from 832 in 2014 to 850 in 2015, 899 in 2016, 863 in 2017, and 838 in 2018,” they wrote.
In 15 of the 24 medication groups included in the study, at least 15% of the individuals had unspecified anxiety disorder, anxiety neurosis, or major depressive disorder; in 11 of the medication groups, at least 15% had some form of ADHD. ADHD prevalence in patients taking stimulants varied based on ADHD type, the researchers said.
The most common comorbidities in patients taking antipsychotics were combined type ADHD (11.6%-17.8%) and anxiety disorder (13.1%-30.1%). The study findings suggest that many clinicians are incorporating medications into ASD management, the researchers said.
“Although there is no medical treatment for the core deficits of social communication and repetitive behavioral patterns in ASD, the American Academy of Pediatrics recommends that clinicians consider medications in the management of common comorbid conditions, including seizures, ADHD, anxiety disorders, mood disorders, and disruptive behavior disorders,” they said.
The findings were limited by several factors including the potential for inconsistent reporting of diagnoses and pharmacy claims, the researchers noted. Other limitations included a lack of direct clinical assessment to validate diagnoses and the absence of validated diagnostic instruments to screen for comorbidities, they added.
“Our findings suggest that clinicians may be increasingly using integrated approaches to treating patients with ASD and co-occurring conditions, and further work is necessary to determine the relative effects of pharmacotherapy vs. behavioral interventions on outcomes in patients with ASD,” the researchers concluded.
Many reasons for multiple medications
“The researchers put in a lot of effort to provide data on a large scale,” Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“The findings illustrate the reality that autistic children are prescribed a lot of medications for a lot of reasons, some of which are not entirely clear,” Dr. Lessin said. The study also reflects the chronic lack of behavioral health services for children, he noted. Many children with ASD are referred for services they are unable to access, he said. “As a result, they see doctors who can only prescribe medications to try to control behavior or symptoms for which the cause is unclear,” and which could be ASD or other comorbidities, he emphasized.
The large sample size strengthens the study findings, but some of the challenges include the use of claims data, which do not indicate how diagnoses were made, said Dr. Lessin. An additional limitation is the fact that many medications for children with autism are used off label, so the specific reason for their use may be unknown, he said.
The take-home message for clinicians is that children with ASD are getting a lot of medications, and pediatricians are not usually responsible for multiple medications,” Dr. Lessin said. Ultimately, the study is “a plea for more research,” to tease out details of what medications are indicated and helpful, he said.
The study received no outside funding. The researchers and Dr. Lessin had no financial conflicts to disclose. Dr. Lessin serves on the Pediatric News editorial advisory board.
Approximately one-third of individuals with autism spectrum disorder (ASD) are prescribed multiple medications to manage comorbidities and symptoms, according to data from a retrospective cohort study of more than 26,000 patients.
“Clinicians caring for patients with ASD are tasked with the challenges of managing the primary disease, as well as co-occurring medical conditions, and coordinating with educational and social service professionals to provide holistic care,” wrote Aliya G. Feroe of Harvard Medical School, Boston, and colleagues.
The medication classes used to treat individuals with ASD include ADHD medications, antipsychotics, antidepressants, mood stabilizers, benzodiazepines, anxiolytics, and hypnotics, but the prescription rates of these medications in ASD patients have not been examined in large studies, the researchers said.
In a study published in JAMA Pediatrics, the researchers identified 26,722 individuals with ASD using a United States health care database from Jan. 1, 2014, to Dec. 31, 2019. Data included records of inpatient and outpatient claims, and records of prescriptions filled through commercial pharmacies. Individuals received at least 1 of 24 of the most common medication groups for ASD or comorbidities. The average age of the study participants was 14 years, and 78% were male. Diagnostic codes for ASD were based on the International Classification of Diseases, Ninth Revision, and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision.
Over the 6-year study period, approximately one-third of the participants were taking three or more medications at once, ranging from 28.6% to 31.5%. In any 1 year, approximately 41% of children were prescribed a single medication, 17% received two prescriptions, 7.9% received four, and 3.4% received five. Medication changes occurred more frequently within classes than between classes, and reasons for these changes may include patient preference, adverse effects, and cost, the researchers noted.
The overall number of children prescribed particular drugs remained consistent, the researchers noted. “For example, the total number of individuals prescribed methylphenidate shifted from 832 in 2014 to 850 in 2015, 899 in 2016, 863 in 2017, and 838 in 2018,” they wrote.
In 15 of the 24 medication groups included in the study, at least 15% of the individuals had unspecified anxiety disorder, anxiety neurosis, or major depressive disorder; in 11 of the medication groups, at least 15% had some form of ADHD. ADHD prevalence in patients taking stimulants varied based on ADHD type, the researchers said.
The most common comorbidities in patients taking antipsychotics were combined type ADHD (11.6%-17.8%) and anxiety disorder (13.1%-30.1%). The study findings suggest that many clinicians are incorporating medications into ASD management, the researchers said.
“Although there is no medical treatment for the core deficits of social communication and repetitive behavioral patterns in ASD, the American Academy of Pediatrics recommends that clinicians consider medications in the management of common comorbid conditions, including seizures, ADHD, anxiety disorders, mood disorders, and disruptive behavior disorders,” they said.
The findings were limited by several factors including the potential for inconsistent reporting of diagnoses and pharmacy claims, the researchers noted. Other limitations included a lack of direct clinical assessment to validate diagnoses and the absence of validated diagnostic instruments to screen for comorbidities, they added.
“Our findings suggest that clinicians may be increasingly using integrated approaches to treating patients with ASD and co-occurring conditions, and further work is necessary to determine the relative effects of pharmacotherapy vs. behavioral interventions on outcomes in patients with ASD,” the researchers concluded.
Many reasons for multiple medications
“The researchers put in a lot of effort to provide data on a large scale,” Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“The findings illustrate the reality that autistic children are prescribed a lot of medications for a lot of reasons, some of which are not entirely clear,” Dr. Lessin said. The study also reflects the chronic lack of behavioral health services for children, he noted. Many children with ASD are referred for services they are unable to access, he said. “As a result, they see doctors who can only prescribe medications to try to control behavior or symptoms for which the cause is unclear,” and which could be ASD or other comorbidities, he emphasized.
The large sample size strengthens the study findings, but some of the challenges include the use of claims data, which do not indicate how diagnoses were made, said Dr. Lessin. An additional limitation is the fact that many medications for children with autism are used off label, so the specific reason for their use may be unknown, he said.
The take-home message for clinicians is that children with ASD are getting a lot of medications, and pediatricians are not usually responsible for multiple medications,” Dr. Lessin said. Ultimately, the study is “a plea for more research,” to tease out details of what medications are indicated and helpful, he said.
The study received no outside funding. The researchers and Dr. Lessin had no financial conflicts to disclose. Dr. Lessin serves on the Pediatric News editorial advisory board.
FROM JAMA PEDIATRICS
Children and COVID: Vaccination trends beginning to diverge
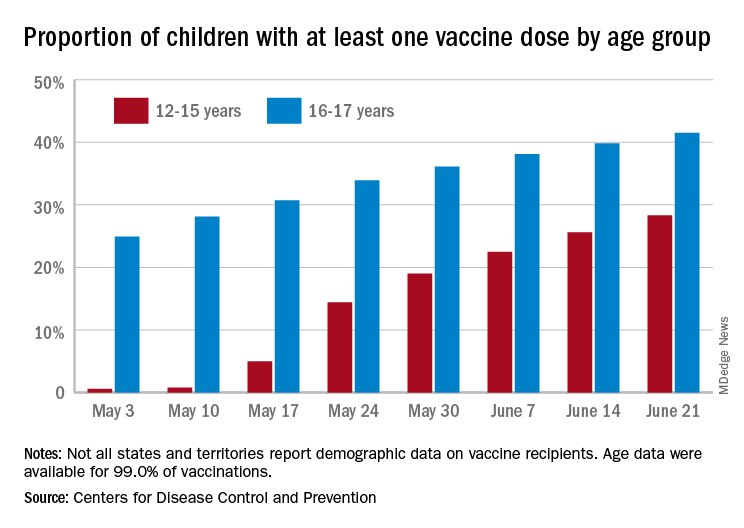
As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.
AAP updates guidance for return to sports and physical activities
As pandemic restrictions ease and young athletes once again take to fields, courts, tracks, and rinks, doctors are sharing ways to help them get back to sports safely.
That means taking steps to prevent COVID-19.
It also means trying to avoid sports-related injuries, which may be more likely if young athletes didn’t move around so much during the pandemic.
For adolescents who are eligible, getting a COVID-19 vaccine may be the most important thing they can do, according to the American Academy of Pediatrics.
“The AAP encourages all people who are eligible to receive the COVID-19 vaccine as soon as it is available,” the organization wrote in updated guidance on returning to sports and physical activity.
“I don’t think it can be overemphasized how important these vaccines are, both for the individual and at the community level,” says Aaron L. Baggish, MD, an associate professor of medicine at Harvard Medical School, Boston, and director of the Cardiovascular Performance Program at Massachusetts General Hospital in Boston.
Dr. Baggish, team cardiologist for the New England Patriots, the Boston Bruins, the New England Revolution, U.S. Men’s and Women’s Soccer, and U.S. Rowing, as well as medical director for the Boston Marathon, has studied the effects of COVID-19 on the heart in college athletes and written return-to-play recommendations for athletes of high school age and older.
“Millions of people have received these vaccines from age 12 up,” Dr. Baggish says. “The efficacy continues to look very durable and near complete, and the risk associated with vaccination is incredibly low, to the point where the risk-benefit ratio across the age spectrum, whether you’re athletic or not, strongly favors getting vaccinated. There is really no reason to hold off at this point.”
While outdoor activities are lower-risk for spreading COVID-19 and many people have been vaccinated, masks still should be worn in certain settings, the AAP notes.
“Indoor spaces that are crowded are still high-risk for COVID-19 transmission. And we recognize that not everyone in these settings may be vaccinated,” says Susannah Briskin, MD, lead author of the AAP guidance.
“So for indoor sporting events with spectators, in locker rooms or other small spaces such as a training room, and during shared car rides or school transportation to and from events, individuals should continue to mask,” adds Dr. Briskin, a pediatrician in the Division of Sports Medicine and fellowship director for the Primary Care Sports Medicine program at University Hospitals Rainbow Babies & Children’s Hospital.
For outdoor sports, athletes who are not fully vaccinated should be encouraged to wear masks on the sidelines and during group training and competition when they are within 3 feet of others for sustained amounts of time, according to the AAP.
Get back into exercise gradually
In general, athletes who have not been active for more than a month should resume exercise gradually, Dr. Briskin says. Starting at 25% of normal volume and increasing slowly over time – with 10% increases each week – is one rule of thumb.
“Those who have taken a prolonged break from sports are at a higher risk of injury when they return,” she notes. “Families should also be aware of an increased risk for heat-related illness if they are not acclimated.”
Caitlyn Mooney, MD, a team doctor for the University of Texas, San Antonio, has heard reports of doctors seeing more injuries like stress fractures. Some cases may relate to people going from “months of doing nothing to all of a sudden going back to sports,” says Dr. Mooney, who is also a clinical assistant professor of pediatrics and orthopedics at UT Health San Antonio.
“The coaches, the parents, and the athletes themselves really need to keep in mind that it’s not like a regular season,” Dr. Mooney says. She suggests gradually ramping up activity and paying attention to any pain. “That’s a good indicator that maybe you’re going too fast,” she adds.
Athletes should be mindful of other symptoms too when restarting exercise, especially after illness.
It is “very important that any athlete with recent COVID-19 monitor for new symptoms when they return to exercise,” says Jonathan Drezner, MD, a professor of family medicine at the University of Washington, Seattle. “A little fatigue from detraining may be expected, but exertional chest pain deserves more evaluation.”
Dr. Drezner – editor-in-chief of the British Journal of Sports Medicine and team doctor for the Seattle Seahawks – along with Dr. Baggish and colleagues, found a low prevalence of cardiac involvement in a study of more than 3,000 college athletes with prior SARS-CoV-2 infection.
“Any athlete, despite their initial symptom course, who has cardiopulmonary symptoms on return to exercise, particularly chest pain, should see their physician for a comprehensive cardiac evaluation,” Dr. Drezner says. “Cardiac MRI should be reserved for athletes with abnormal testing or when clinical suspicion of myocardial involvement is high.”
If an athlete had COVID-19 with moderate symptoms (such as fever, chills, or a flu-like syndrome) or cardiopulmonary symptoms (such as chest pain or shortness of breath), cardiac testing should be considered, he notes.
These symptoms “were associated with a higher prevalence of cardiac involvement,” Dr. Drezner said in an email. “Testing may include an ECG, echocardiogram (ultrasound), and troponin (blood test).”
For kids who test positive for SARS-CoV-2 but do not have symptoms, or their symptoms last less than 4 days, a phone call or telemedicine visit with their doctor may be enough to clear them to play, says Dr. Briskin, who’s also an assistant professor of pediatrics at Case Western Reserve University, Cleveland.
“This will allow the physician an opportunity to screen for any concerning cardiac signs or symptoms, update the patient’s electronic medical record with the recent COVID-19 infection, and provide appropriate guidance back to exercise,” she adds.
Dr. Baggish, Dr. Briskin, Dr. Mooney, and Dr. Drezner had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
As pandemic restrictions ease and young athletes once again take to fields, courts, tracks, and rinks, doctors are sharing ways to help them get back to sports safely.
That means taking steps to prevent COVID-19.
It also means trying to avoid sports-related injuries, which may be more likely if young athletes didn’t move around so much during the pandemic.
For adolescents who are eligible, getting a COVID-19 vaccine may be the most important thing they can do, according to the American Academy of Pediatrics.
“The AAP encourages all people who are eligible to receive the COVID-19 vaccine as soon as it is available,” the organization wrote in updated guidance on returning to sports and physical activity.
“I don’t think it can be overemphasized how important these vaccines are, both for the individual and at the community level,” says Aaron L. Baggish, MD, an associate professor of medicine at Harvard Medical School, Boston, and director of the Cardiovascular Performance Program at Massachusetts General Hospital in Boston.
Dr. Baggish, team cardiologist for the New England Patriots, the Boston Bruins, the New England Revolution, U.S. Men’s and Women’s Soccer, and U.S. Rowing, as well as medical director for the Boston Marathon, has studied the effects of COVID-19 on the heart in college athletes and written return-to-play recommendations for athletes of high school age and older.
“Millions of people have received these vaccines from age 12 up,” Dr. Baggish says. “The efficacy continues to look very durable and near complete, and the risk associated with vaccination is incredibly low, to the point where the risk-benefit ratio across the age spectrum, whether you’re athletic or not, strongly favors getting vaccinated. There is really no reason to hold off at this point.”
While outdoor activities are lower-risk for spreading COVID-19 and many people have been vaccinated, masks still should be worn in certain settings, the AAP notes.
“Indoor spaces that are crowded are still high-risk for COVID-19 transmission. And we recognize that not everyone in these settings may be vaccinated,” says Susannah Briskin, MD, lead author of the AAP guidance.
“So for indoor sporting events with spectators, in locker rooms or other small spaces such as a training room, and during shared car rides or school transportation to and from events, individuals should continue to mask,” adds Dr. Briskin, a pediatrician in the Division of Sports Medicine and fellowship director for the Primary Care Sports Medicine program at University Hospitals Rainbow Babies & Children’s Hospital.
For outdoor sports, athletes who are not fully vaccinated should be encouraged to wear masks on the sidelines and during group training and competition when they are within 3 feet of others for sustained amounts of time, according to the AAP.
Get back into exercise gradually
In general, athletes who have not been active for more than a month should resume exercise gradually, Dr. Briskin says. Starting at 25% of normal volume and increasing slowly over time – with 10% increases each week – is one rule of thumb.
“Those who have taken a prolonged break from sports are at a higher risk of injury when they return,” she notes. “Families should also be aware of an increased risk for heat-related illness if they are not acclimated.”
Caitlyn Mooney, MD, a team doctor for the University of Texas, San Antonio, has heard reports of doctors seeing more injuries like stress fractures. Some cases may relate to people going from “months of doing nothing to all of a sudden going back to sports,” says Dr. Mooney, who is also a clinical assistant professor of pediatrics and orthopedics at UT Health San Antonio.
“The coaches, the parents, and the athletes themselves really need to keep in mind that it’s not like a regular season,” Dr. Mooney says. She suggests gradually ramping up activity and paying attention to any pain. “That’s a good indicator that maybe you’re going too fast,” she adds.
Athletes should be mindful of other symptoms too when restarting exercise, especially after illness.
It is “very important that any athlete with recent COVID-19 monitor for new symptoms when they return to exercise,” says Jonathan Drezner, MD, a professor of family medicine at the University of Washington, Seattle. “A little fatigue from detraining may be expected, but exertional chest pain deserves more evaluation.”
Dr. Drezner – editor-in-chief of the British Journal of Sports Medicine and team doctor for the Seattle Seahawks – along with Dr. Baggish and colleagues, found a low prevalence of cardiac involvement in a study of more than 3,000 college athletes with prior SARS-CoV-2 infection.
“Any athlete, despite their initial symptom course, who has cardiopulmonary symptoms on return to exercise, particularly chest pain, should see their physician for a comprehensive cardiac evaluation,” Dr. Drezner says. “Cardiac MRI should be reserved for athletes with abnormal testing or when clinical suspicion of myocardial involvement is high.”
If an athlete had COVID-19 with moderate symptoms (such as fever, chills, or a flu-like syndrome) or cardiopulmonary symptoms (such as chest pain or shortness of breath), cardiac testing should be considered, he notes.
These symptoms “were associated with a higher prevalence of cardiac involvement,” Dr. Drezner said in an email. “Testing may include an ECG, echocardiogram (ultrasound), and troponin (blood test).”
For kids who test positive for SARS-CoV-2 but do not have symptoms, or their symptoms last less than 4 days, a phone call or telemedicine visit with their doctor may be enough to clear them to play, says Dr. Briskin, who’s also an assistant professor of pediatrics at Case Western Reserve University, Cleveland.
“This will allow the physician an opportunity to screen for any concerning cardiac signs or symptoms, update the patient’s electronic medical record with the recent COVID-19 infection, and provide appropriate guidance back to exercise,” she adds.
Dr. Baggish, Dr. Briskin, Dr. Mooney, and Dr. Drezner had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
As pandemic restrictions ease and young athletes once again take to fields, courts, tracks, and rinks, doctors are sharing ways to help them get back to sports safely.
That means taking steps to prevent COVID-19.
It also means trying to avoid sports-related injuries, which may be more likely if young athletes didn’t move around so much during the pandemic.
For adolescents who are eligible, getting a COVID-19 vaccine may be the most important thing they can do, according to the American Academy of Pediatrics.
“The AAP encourages all people who are eligible to receive the COVID-19 vaccine as soon as it is available,” the organization wrote in updated guidance on returning to sports and physical activity.
“I don’t think it can be overemphasized how important these vaccines are, both for the individual and at the community level,” says Aaron L. Baggish, MD, an associate professor of medicine at Harvard Medical School, Boston, and director of the Cardiovascular Performance Program at Massachusetts General Hospital in Boston.
Dr. Baggish, team cardiologist for the New England Patriots, the Boston Bruins, the New England Revolution, U.S. Men’s and Women’s Soccer, and U.S. Rowing, as well as medical director for the Boston Marathon, has studied the effects of COVID-19 on the heart in college athletes and written return-to-play recommendations for athletes of high school age and older.
“Millions of people have received these vaccines from age 12 up,” Dr. Baggish says. “The efficacy continues to look very durable and near complete, and the risk associated with vaccination is incredibly low, to the point where the risk-benefit ratio across the age spectrum, whether you’re athletic or not, strongly favors getting vaccinated. There is really no reason to hold off at this point.”
While outdoor activities are lower-risk for spreading COVID-19 and many people have been vaccinated, masks still should be worn in certain settings, the AAP notes.
“Indoor spaces that are crowded are still high-risk for COVID-19 transmission. And we recognize that not everyone in these settings may be vaccinated,” says Susannah Briskin, MD, lead author of the AAP guidance.
“So for indoor sporting events with spectators, in locker rooms or other small spaces such as a training room, and during shared car rides or school transportation to and from events, individuals should continue to mask,” adds Dr. Briskin, a pediatrician in the Division of Sports Medicine and fellowship director for the Primary Care Sports Medicine program at University Hospitals Rainbow Babies & Children’s Hospital.
For outdoor sports, athletes who are not fully vaccinated should be encouraged to wear masks on the sidelines and during group training and competition when they are within 3 feet of others for sustained amounts of time, according to the AAP.
Get back into exercise gradually
In general, athletes who have not been active for more than a month should resume exercise gradually, Dr. Briskin says. Starting at 25% of normal volume and increasing slowly over time – with 10% increases each week – is one rule of thumb.
“Those who have taken a prolonged break from sports are at a higher risk of injury when they return,” she notes. “Families should also be aware of an increased risk for heat-related illness if they are not acclimated.”
Caitlyn Mooney, MD, a team doctor for the University of Texas, San Antonio, has heard reports of doctors seeing more injuries like stress fractures. Some cases may relate to people going from “months of doing nothing to all of a sudden going back to sports,” says Dr. Mooney, who is also a clinical assistant professor of pediatrics and orthopedics at UT Health San Antonio.
“The coaches, the parents, and the athletes themselves really need to keep in mind that it’s not like a regular season,” Dr. Mooney says. She suggests gradually ramping up activity and paying attention to any pain. “That’s a good indicator that maybe you’re going too fast,” she adds.
Athletes should be mindful of other symptoms too when restarting exercise, especially after illness.
It is “very important that any athlete with recent COVID-19 monitor for new symptoms when they return to exercise,” says Jonathan Drezner, MD, a professor of family medicine at the University of Washington, Seattle. “A little fatigue from detraining may be expected, but exertional chest pain deserves more evaluation.”
Dr. Drezner – editor-in-chief of the British Journal of Sports Medicine and team doctor for the Seattle Seahawks – along with Dr. Baggish and colleagues, found a low prevalence of cardiac involvement in a study of more than 3,000 college athletes with prior SARS-CoV-2 infection.
“Any athlete, despite their initial symptom course, who has cardiopulmonary symptoms on return to exercise, particularly chest pain, should see their physician for a comprehensive cardiac evaluation,” Dr. Drezner says. “Cardiac MRI should be reserved for athletes with abnormal testing or when clinical suspicion of myocardial involvement is high.”
If an athlete had COVID-19 with moderate symptoms (such as fever, chills, or a flu-like syndrome) or cardiopulmonary symptoms (such as chest pain or shortness of breath), cardiac testing should be considered, he notes.
These symptoms “were associated with a higher prevalence of cardiac involvement,” Dr. Drezner said in an email. “Testing may include an ECG, echocardiogram (ultrasound), and troponin (blood test).”
For kids who test positive for SARS-CoV-2 but do not have symptoms, or their symptoms last less than 4 days, a phone call or telemedicine visit with their doctor may be enough to clear them to play, says Dr. Briskin, who’s also an assistant professor of pediatrics at Case Western Reserve University, Cleveland.
“This will allow the physician an opportunity to screen for any concerning cardiac signs or symptoms, update the patient’s electronic medical record with the recent COVID-19 infection, and provide appropriate guidance back to exercise,” she adds.
Dr. Baggish, Dr. Briskin, Dr. Mooney, and Dr. Drezner had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FDA approves OTC antihistamine nasal spray
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
5-year-old boy • calf pain • fever • cough & rhinitis • Dx?
THE CASE
A 5-year-old previously healthy white boy presented to clinic with bilateral calf pain and refusal to bear weight since awakening that morning. Associated symptoms included a 3-day history of generalized fatigue, subjective fevers, cough, congestion, and rhinitis. The night prior to presentation, he showed no symptoms of gait abnormalities, muscle pain, or weakness. There was no history of similar symptoms, trauma, overexertion, foreign travel, or family history of musculoskeletal disease. He was fully immunized, except for the annual influenza vaccine. He was not taking any medications. This case occurred before the onset of the COVID-19 pandemic.
Objective findings included fever of 101 °F, refusal to bear weight, and symmetrical bilateral tenderness to palpation of the gastrocnemius-soleus complex. Pain was elicited with passive dorsiflexion. There was no erythema, edema, or sensory deficits, and the distal leg compartments were soft. There was normal range of motion of the hips, knees, and ankles. Dorsalis pedis pulses were 2+, and patella reflexes were 2/4 bilaterally.
Lab results included a white blood cell count of 2500/μL (normal range, 4500 to 11,000/μL);absolute neutrophil count, 900/μL (1500 to 8000/μL); platelet count, 131,000/μL (150,000 to 450,000/μL); creatine kinase level, 869 IU/L (22 to 198 U/L); and aspartate aminotransferase level, 116 U/L (8 to 33 U/L). A rapid influenza swab was positive for influenza B. Plain films of the bilateral hips and lower extremities were unremarkable. C-reactive protein (CRP) level, urinalysis, and renal function tests were within normal limits. Creatine kinase (CK) level peaked (1935 U/L; normal range, 22 to 198 U/L) within the first 24 hours of presentation and then trended down.
The Diagnosis
The patient’s sudden onset of symmetrical bilateral calf pain in the setting of an upper respiratory tract infection was extremely suspicious for benign acute childhood myositis (BACM). Lab work and radiologic evaluation were performed to rule out more ominous causes of refusal to bear weight. The suspicion of BACM was further validated by influenza B serology, an elevated CK, and a normal CRP.
Discussion
BACM was first described by Lundberg in 1957.1 The overall incidence and prevalence are unclear.2 A viral prodrome involving rhinorrhea, low-grade fever, sore throat, cough, and malaise typically precedes bilateral calf pain by 3 days.2-4 Myositis symptoms typically last for 4 days.3 While several infectious etiologies have been linked to this condition, influenza B has the greatest association.5,6
❚ Patient population. BACM occurs predominately in school-aged children (6-8 years old) and has a male-to-female ratio of 2:1.3,5,6 In a retrospective study of 219 children, BACM was strongly associated with male gender and ages 6 to 9 years.3 In another retrospective study of 54 children,80% of patients were male, and the mean age was 7.3 years.5
❚ Key symptoms and differential. The distinguishing feature of BACM is bilateral symmetric gastrocnemius-soleus tenderness.2,4 Additionally, the lack of neurologic symptoms is an important differentiator, as long as refusal to bear weight is not mistaken for weakness.6 These features help to distinguish BACM from other items in the differential, including trauma, Guillain-Barre syndrome, osteomyelitis, malignancy, deep vein thrombosis, and inherited musculoskeletal disorders.2
Continue to: Labratory evaluation...
❚ Laboratory evaluation will often show mild neutropenia, thrombocytopenia, and mild elevation in CK.7,8 CRP is typically normal.4,7,9 In a retrospective study of 28 admissions for BACM from 2001 to 2012, common findings included leukopenia (35%), neutropenia (25%), and thrombocytopenia (21%). The median CK value was 4181 U/L.4 In another analysis of BACM cases, mean CK was 1872 U/L.5
❚ Biopsy is unnecessary; however, calf muscle samples from 11 of 12 children with suspected BACM due to influenza B infection were consistent with patchy necrosis without significant myositis.10
❚ Complications. Rhabdomyolysis, although rare, has been reported with BACM. In 1 analysis, 10 of 316 patients with influenza-associated myositis developed rhabdomyolysis; 8 experienced renal failure. Rhabdomyolysis was 4 times more likely to occur in girls, and 86% of cases were associated with influenza A.6 Common manifestations of rhabdomyolysis associated with influenza include diffuse myopathy, gross hematuria, and myoglobinuria.6
❚ Treatment is mainly supportive.4,8,9 Antivirals typically are not indicated, as the bilateral calf pain manifests during the recovery phase of the illness.4,9,11 BACM is self-limited and should resolve within 3 days of myositis manifestation.2 Patients should follow up in 2 to 3 weeks to verify symptom resolution.2
If muscle pain, swelling, and tenderness worsen, further work-up is indicated. In more severe cases, including those involving renal failure, intensive care management and even dialysis may be necessary.4,6
❚ Our patient was hospitalized due to fever in the setting of neutropenia. Ultimately, he was treated with acetaminophen and intravenous fluids for mild dehydration and elevated CK levels. He was discharged home after 3 days, at which time he had complete resolution of pain and was able to resume normal activities.
The Takeaway
Benign acute childhood myositis is a self-limited disorder with an excellent prognosis. It has a typical presentation and therefore should be a clinical diagnosis; however, investigative studies may be warranted to rule out more ominous causes. Reassurance to family that the condition should self-resolve in a few days is important. Close follow-up should be scheduled to ensure resolution of symptoms.
CORRESPONDENCE
Nicholas A. Rathjen, DO, William Beaumont Army Medical Center, Department of Soldier and Family Care, 11335 SSG Sims Street, Fort Bliss, TX 79918; nicholas.a.rathjen@gmail. com
- Lundberg A. Myalgia cruris epidemica. Acta Paediatr. 1957;46:18-31. doi: 10.1111/j.1651-2227.1957.tb08627.x
- Magee H, Goldman RD. Viral myositis in children. Can Fam Physician. 2017;63:365-368.
- Mall S, Buchholz U, Tibussek D, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30:e142-e146. doi: 10.1097/INF.0b013e318217e356
- Santos JA, Albuquerque C, Lito D, et al. Benign acute childhood myositis: an alarming condition with an excellent prognosis! Am J Emerg Med. 2014;32:1418-1419. doi: 10.1016/j.ajem.2014.08.022
- Rosenberg T, Heitner S, Scolnik D, et al. Outcome of benign acute childhood myositis: the experience of 2 large tertiary care pediatric hospitals. Pediatr Emerg Care. 2018;34:400-402. doi: 10.1097/PEC.0000000000000830
- Agyeman P, Duppenthaler A, Heininger U, et al. Influenza-associated myositis in children. Infection. 2004;32:199-203. doi: 10.1007/s15010-004-4003-2
- Mackay MT, Kornberg AJ, Shield LK, et al. Benign acute childhood myositis: laboratory and clinical features. Neurology. 1999;53:2127-2131. doi: 10.1212/wnl.53.9.2127
- Neocleous C, Spanou C, Mpampalis E, et al. Unnecessary diagnostic investigations in benign acute childhood myositis: a case series report. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012023
- Felipe Cavagnaro SM, Alejandra Aird G, Ingrid Harwardt R, et al. Benign acute childhood myositis: clinical series and literature review. Rev Chil Pediatr. 2017;88:268-274. doi: 10.1016/j.rchipe.2016.07.002
- Bove KE, Hilton PK, Partin J, et al. Morphology of acute myopathy associated with influenza B infection. Pediatric Pathology. 1983;1:51-66. https://doi.org/10.3109/15513818309048284
- Koliou M, Hadjiloizou S, Ourani S, et al. A case of benign acute childhood myositis associated with influenza A (HINI) virus infection. Clin Microbiol Infect. 2010;16:193-195. doi: 10.1111/j.1469-0691.2009.03064.x
THE CASE
A 5-year-old previously healthy white boy presented to clinic with bilateral calf pain and refusal to bear weight since awakening that morning. Associated symptoms included a 3-day history of generalized fatigue, subjective fevers, cough, congestion, and rhinitis. The night prior to presentation, he showed no symptoms of gait abnormalities, muscle pain, or weakness. There was no history of similar symptoms, trauma, overexertion, foreign travel, or family history of musculoskeletal disease. He was fully immunized, except for the annual influenza vaccine. He was not taking any medications. This case occurred before the onset of the COVID-19 pandemic.
Objective findings included fever of 101 °F, refusal to bear weight, and symmetrical bilateral tenderness to palpation of the gastrocnemius-soleus complex. Pain was elicited with passive dorsiflexion. There was no erythema, edema, or sensory deficits, and the distal leg compartments were soft. There was normal range of motion of the hips, knees, and ankles. Dorsalis pedis pulses were 2+, and patella reflexes were 2/4 bilaterally.
Lab results included a white blood cell count of 2500/μL (normal range, 4500 to 11,000/μL);absolute neutrophil count, 900/μL (1500 to 8000/μL); platelet count, 131,000/μL (150,000 to 450,000/μL); creatine kinase level, 869 IU/L (22 to 198 U/L); and aspartate aminotransferase level, 116 U/L (8 to 33 U/L). A rapid influenza swab was positive for influenza B. Plain films of the bilateral hips and lower extremities were unremarkable. C-reactive protein (CRP) level, urinalysis, and renal function tests were within normal limits. Creatine kinase (CK) level peaked (1935 U/L; normal range, 22 to 198 U/L) within the first 24 hours of presentation and then trended down.
The Diagnosis
The patient’s sudden onset of symmetrical bilateral calf pain in the setting of an upper respiratory tract infection was extremely suspicious for benign acute childhood myositis (BACM). Lab work and radiologic evaluation were performed to rule out more ominous causes of refusal to bear weight. The suspicion of BACM was further validated by influenza B serology, an elevated CK, and a normal CRP.
Discussion
BACM was first described by Lundberg in 1957.1 The overall incidence and prevalence are unclear.2 A viral prodrome involving rhinorrhea, low-grade fever, sore throat, cough, and malaise typically precedes bilateral calf pain by 3 days.2-4 Myositis symptoms typically last for 4 days.3 While several infectious etiologies have been linked to this condition, influenza B has the greatest association.5,6
❚ Patient population. BACM occurs predominately in school-aged children (6-8 years old) and has a male-to-female ratio of 2:1.3,5,6 In a retrospective study of 219 children, BACM was strongly associated with male gender and ages 6 to 9 years.3 In another retrospective study of 54 children,80% of patients were male, and the mean age was 7.3 years.5
❚ Key symptoms and differential. The distinguishing feature of BACM is bilateral symmetric gastrocnemius-soleus tenderness.2,4 Additionally, the lack of neurologic symptoms is an important differentiator, as long as refusal to bear weight is not mistaken for weakness.6 These features help to distinguish BACM from other items in the differential, including trauma, Guillain-Barre syndrome, osteomyelitis, malignancy, deep vein thrombosis, and inherited musculoskeletal disorders.2
Continue to: Labratory evaluation...
❚ Laboratory evaluation will often show mild neutropenia, thrombocytopenia, and mild elevation in CK.7,8 CRP is typically normal.4,7,9 In a retrospective study of 28 admissions for BACM from 2001 to 2012, common findings included leukopenia (35%), neutropenia (25%), and thrombocytopenia (21%). The median CK value was 4181 U/L.4 In another analysis of BACM cases, mean CK was 1872 U/L.5
❚ Biopsy is unnecessary; however, calf muscle samples from 11 of 12 children with suspected BACM due to influenza B infection were consistent with patchy necrosis without significant myositis.10
❚ Complications. Rhabdomyolysis, although rare, has been reported with BACM. In 1 analysis, 10 of 316 patients with influenza-associated myositis developed rhabdomyolysis; 8 experienced renal failure. Rhabdomyolysis was 4 times more likely to occur in girls, and 86% of cases were associated with influenza A.6 Common manifestations of rhabdomyolysis associated with influenza include diffuse myopathy, gross hematuria, and myoglobinuria.6
❚ Treatment is mainly supportive.4,8,9 Antivirals typically are not indicated, as the bilateral calf pain manifests during the recovery phase of the illness.4,9,11 BACM is self-limited and should resolve within 3 days of myositis manifestation.2 Patients should follow up in 2 to 3 weeks to verify symptom resolution.2
If muscle pain, swelling, and tenderness worsen, further work-up is indicated. In more severe cases, including those involving renal failure, intensive care management and even dialysis may be necessary.4,6
❚ Our patient was hospitalized due to fever in the setting of neutropenia. Ultimately, he was treated with acetaminophen and intravenous fluids for mild dehydration and elevated CK levels. He was discharged home after 3 days, at which time he had complete resolution of pain and was able to resume normal activities.
The Takeaway
Benign acute childhood myositis is a self-limited disorder with an excellent prognosis. It has a typical presentation and therefore should be a clinical diagnosis; however, investigative studies may be warranted to rule out more ominous causes. Reassurance to family that the condition should self-resolve in a few days is important. Close follow-up should be scheduled to ensure resolution of symptoms.
CORRESPONDENCE
Nicholas A. Rathjen, DO, William Beaumont Army Medical Center, Department of Soldier and Family Care, 11335 SSG Sims Street, Fort Bliss, TX 79918; nicholas.a.rathjen@gmail. com
THE CASE
A 5-year-old previously healthy white boy presented to clinic with bilateral calf pain and refusal to bear weight since awakening that morning. Associated symptoms included a 3-day history of generalized fatigue, subjective fevers, cough, congestion, and rhinitis. The night prior to presentation, he showed no symptoms of gait abnormalities, muscle pain, or weakness. There was no history of similar symptoms, trauma, overexertion, foreign travel, or family history of musculoskeletal disease. He was fully immunized, except for the annual influenza vaccine. He was not taking any medications. This case occurred before the onset of the COVID-19 pandemic.
Objective findings included fever of 101 °F, refusal to bear weight, and symmetrical bilateral tenderness to palpation of the gastrocnemius-soleus complex. Pain was elicited with passive dorsiflexion. There was no erythema, edema, or sensory deficits, and the distal leg compartments were soft. There was normal range of motion of the hips, knees, and ankles. Dorsalis pedis pulses were 2+, and patella reflexes were 2/4 bilaterally.
Lab results included a white blood cell count of 2500/μL (normal range, 4500 to 11,000/μL);absolute neutrophil count, 900/μL (1500 to 8000/μL); platelet count, 131,000/μL (150,000 to 450,000/μL); creatine kinase level, 869 IU/L (22 to 198 U/L); and aspartate aminotransferase level, 116 U/L (8 to 33 U/L). A rapid influenza swab was positive for influenza B. Plain films of the bilateral hips and lower extremities were unremarkable. C-reactive protein (CRP) level, urinalysis, and renal function tests were within normal limits. Creatine kinase (CK) level peaked (1935 U/L; normal range, 22 to 198 U/L) within the first 24 hours of presentation and then trended down.
The Diagnosis
The patient’s sudden onset of symmetrical bilateral calf pain in the setting of an upper respiratory tract infection was extremely suspicious for benign acute childhood myositis (BACM). Lab work and radiologic evaluation were performed to rule out more ominous causes of refusal to bear weight. The suspicion of BACM was further validated by influenza B serology, an elevated CK, and a normal CRP.
Discussion
BACM was first described by Lundberg in 1957.1 The overall incidence and prevalence are unclear.2 A viral prodrome involving rhinorrhea, low-grade fever, sore throat, cough, and malaise typically precedes bilateral calf pain by 3 days.2-4 Myositis symptoms typically last for 4 days.3 While several infectious etiologies have been linked to this condition, influenza B has the greatest association.5,6
❚ Patient population. BACM occurs predominately in school-aged children (6-8 years old) and has a male-to-female ratio of 2:1.3,5,6 In a retrospective study of 219 children, BACM was strongly associated with male gender and ages 6 to 9 years.3 In another retrospective study of 54 children,80% of patients were male, and the mean age was 7.3 years.5
❚ Key symptoms and differential. The distinguishing feature of BACM is bilateral symmetric gastrocnemius-soleus tenderness.2,4 Additionally, the lack of neurologic symptoms is an important differentiator, as long as refusal to bear weight is not mistaken for weakness.6 These features help to distinguish BACM from other items in the differential, including trauma, Guillain-Barre syndrome, osteomyelitis, malignancy, deep vein thrombosis, and inherited musculoskeletal disorders.2
Continue to: Labratory evaluation...
❚ Laboratory evaluation will often show mild neutropenia, thrombocytopenia, and mild elevation in CK.7,8 CRP is typically normal.4,7,9 In a retrospective study of 28 admissions for BACM from 2001 to 2012, common findings included leukopenia (35%), neutropenia (25%), and thrombocytopenia (21%). The median CK value was 4181 U/L.4 In another analysis of BACM cases, mean CK was 1872 U/L.5
❚ Biopsy is unnecessary; however, calf muscle samples from 11 of 12 children with suspected BACM due to influenza B infection were consistent with patchy necrosis without significant myositis.10
❚ Complications. Rhabdomyolysis, although rare, has been reported with BACM. In 1 analysis, 10 of 316 patients with influenza-associated myositis developed rhabdomyolysis; 8 experienced renal failure. Rhabdomyolysis was 4 times more likely to occur in girls, and 86% of cases were associated with influenza A.6 Common manifestations of rhabdomyolysis associated with influenza include diffuse myopathy, gross hematuria, and myoglobinuria.6
❚ Treatment is mainly supportive.4,8,9 Antivirals typically are not indicated, as the bilateral calf pain manifests during the recovery phase of the illness.4,9,11 BACM is self-limited and should resolve within 3 days of myositis manifestation.2 Patients should follow up in 2 to 3 weeks to verify symptom resolution.2
If muscle pain, swelling, and tenderness worsen, further work-up is indicated. In more severe cases, including those involving renal failure, intensive care management and even dialysis may be necessary.4,6
❚ Our patient was hospitalized due to fever in the setting of neutropenia. Ultimately, he was treated with acetaminophen and intravenous fluids for mild dehydration and elevated CK levels. He was discharged home after 3 days, at which time he had complete resolution of pain and was able to resume normal activities.
The Takeaway
Benign acute childhood myositis is a self-limited disorder with an excellent prognosis. It has a typical presentation and therefore should be a clinical diagnosis; however, investigative studies may be warranted to rule out more ominous causes. Reassurance to family that the condition should self-resolve in a few days is important. Close follow-up should be scheduled to ensure resolution of symptoms.
CORRESPONDENCE
Nicholas A. Rathjen, DO, William Beaumont Army Medical Center, Department of Soldier and Family Care, 11335 SSG Sims Street, Fort Bliss, TX 79918; nicholas.a.rathjen@gmail. com
- Lundberg A. Myalgia cruris epidemica. Acta Paediatr. 1957;46:18-31. doi: 10.1111/j.1651-2227.1957.tb08627.x
- Magee H, Goldman RD. Viral myositis in children. Can Fam Physician. 2017;63:365-368.
- Mall S, Buchholz U, Tibussek D, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30:e142-e146. doi: 10.1097/INF.0b013e318217e356
- Santos JA, Albuquerque C, Lito D, et al. Benign acute childhood myositis: an alarming condition with an excellent prognosis! Am J Emerg Med. 2014;32:1418-1419. doi: 10.1016/j.ajem.2014.08.022
- Rosenberg T, Heitner S, Scolnik D, et al. Outcome of benign acute childhood myositis: the experience of 2 large tertiary care pediatric hospitals. Pediatr Emerg Care. 2018;34:400-402. doi: 10.1097/PEC.0000000000000830
- Agyeman P, Duppenthaler A, Heininger U, et al. Influenza-associated myositis in children. Infection. 2004;32:199-203. doi: 10.1007/s15010-004-4003-2
- Mackay MT, Kornberg AJ, Shield LK, et al. Benign acute childhood myositis: laboratory and clinical features. Neurology. 1999;53:2127-2131. doi: 10.1212/wnl.53.9.2127
- Neocleous C, Spanou C, Mpampalis E, et al. Unnecessary diagnostic investigations in benign acute childhood myositis: a case series report. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012023
- Felipe Cavagnaro SM, Alejandra Aird G, Ingrid Harwardt R, et al. Benign acute childhood myositis: clinical series and literature review. Rev Chil Pediatr. 2017;88:268-274. doi: 10.1016/j.rchipe.2016.07.002
- Bove KE, Hilton PK, Partin J, et al. Morphology of acute myopathy associated with influenza B infection. Pediatric Pathology. 1983;1:51-66. https://doi.org/10.3109/15513818309048284
- Koliou M, Hadjiloizou S, Ourani S, et al. A case of benign acute childhood myositis associated with influenza A (HINI) virus infection. Clin Microbiol Infect. 2010;16:193-195. doi: 10.1111/j.1469-0691.2009.03064.x
- Lundberg A. Myalgia cruris epidemica. Acta Paediatr. 1957;46:18-31. doi: 10.1111/j.1651-2227.1957.tb08627.x
- Magee H, Goldman RD. Viral myositis in children. Can Fam Physician. 2017;63:365-368.
- Mall S, Buchholz U, Tibussek D, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30:e142-e146. doi: 10.1097/INF.0b013e318217e356
- Santos JA, Albuquerque C, Lito D, et al. Benign acute childhood myositis: an alarming condition with an excellent prognosis! Am J Emerg Med. 2014;32:1418-1419. doi: 10.1016/j.ajem.2014.08.022
- Rosenberg T, Heitner S, Scolnik D, et al. Outcome of benign acute childhood myositis: the experience of 2 large tertiary care pediatric hospitals. Pediatr Emerg Care. 2018;34:400-402. doi: 10.1097/PEC.0000000000000830
- Agyeman P, Duppenthaler A, Heininger U, et al. Influenza-associated myositis in children. Infection. 2004;32:199-203. doi: 10.1007/s15010-004-4003-2
- Mackay MT, Kornberg AJ, Shield LK, et al. Benign acute childhood myositis: laboratory and clinical features. Neurology. 1999;53:2127-2131. doi: 10.1212/wnl.53.9.2127
- Neocleous C, Spanou C, Mpampalis E, et al. Unnecessary diagnostic investigations in benign acute childhood myositis: a case series report. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012023
- Felipe Cavagnaro SM, Alejandra Aird G, Ingrid Harwardt R, et al. Benign acute childhood myositis: clinical series and literature review. Rev Chil Pediatr. 2017;88:268-274. doi: 10.1016/j.rchipe.2016.07.002
- Bove KE, Hilton PK, Partin J, et al. Morphology of acute myopathy associated with influenza B infection. Pediatric Pathology. 1983;1:51-66. https://doi.org/10.3109/15513818309048284
- Koliou M, Hadjiloizou S, Ourani S, et al. A case of benign acute childhood myositis associated with influenza A (HINI) virus infection. Clin Microbiol Infect. 2010;16:193-195. doi: 10.1111/j.1469-0691.2009.03064.x
To screen or not to screen children for hypertension?
In this issue of JFP, Smith et al recommend following guidelines from the American Academy of Pediatrics to annually screen children for hypertension (see page 220). This recommendation appears to be at odds with the recent US Preventive Services Task Force (USPSTF) statement that concluded there is insufficient evidence for screening children and adolescents for hypertension. But an “I” recommendation from the USPSTF is not the same as a “D” recommendation. “D” means don’t do it, because the evidence indicates that the harms outweigh the benefits. “I” means we don’t have enough evidence to weigh the harms and benefits, so it is up to you and your patients to decide what to do.
So whose recommendations should we follow?
Our decision should be based on a thorough understanding of the evidence, and that evidence is well summarized in the recent USPSTF report.1 The reviewers found no studies that evaluated the benefits and harms of screening children and adolescents for hypertension and no studies evaluating disease outcomes from treating hypertension in these patients.
There is, however, an association between elevated blood pressure in childhood and outcomes such as left ventricular hypertrophy and carotid intimal thickness.2 Some physicians contend that these “disease-oriented outcomes” are sufficient reason to identify and treat hypertension in children and adolescents.3 The USPSTF, however, requires a higher level of evidence that includes patient-oriented outcomes, such as a lower risk of congestive heart failure, renal failure, or death, before recommending treatment. Physicians and patients have to choose what level of evidence is sufficient to take action.
Dr. Smith comments: “As noted in their report, the USPSTF acknowledges that observational studies indicate an association between hypertension in childhood and hypertension in adulthood, but there have been no randomized trials to determine if treating hypertension in children and adolescents reduces risk of cardiovascular events. Although it is a cohort study, not a randomized trial, the ongoing i3C Consortium Outcomes Study4 may provide better information to guide decision-making for children and adolescents with elevated blood pressure.”
What we can all agree on is that, when hypertension is identified in a child or adolescent, it is important to determine if there is a treatable cause of elevated blood pressure such as coarctation of the aorta or renal disease. It is also important to address risk factors for elevated blood pressure and cardiovascular disease, such as obesity, poor dietary habits, and smoking. The treatment is lifestyle modification with diet, exercise, and smoking cessation.
- USPSTF: High blood pressure in children and adolescents: screening. Accessed June 2, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
- Yang L, Magnussen CG, Yang L, et al. Elevated blood pressure in childhood or adolescence and cardiovascular outcomes in adulthood: a systematic review. Hypertension. 2020;75:948–955. doi: 10.1161/hypertensionaha.119.14168
- Falkner B, Lurbe E. The USPSTF call to inaction on blood pressure screening in children and adolescents. Pediatr Nephrol. 2021;36:1327-1329. doi: 10.1007/s00467-021-04926-y
- Sinaiko AR, Jacobs DR Jr, Woo JG, et al. The International Childhood Cardiovascular Cohort (i3C) consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: Design and recruitment. Contemp Clin Trials. 2018;69:55-64. doi: 10.1016/j.cct.2018.04.009
In this issue of JFP, Smith et al recommend following guidelines from the American Academy of Pediatrics to annually screen children for hypertension (see page 220). This recommendation appears to be at odds with the recent US Preventive Services Task Force (USPSTF) statement that concluded there is insufficient evidence for screening children and adolescents for hypertension. But an “I” recommendation from the USPSTF is not the same as a “D” recommendation. “D” means don’t do it, because the evidence indicates that the harms outweigh the benefits. “I” means we don’t have enough evidence to weigh the harms and benefits, so it is up to you and your patients to decide what to do.
So whose recommendations should we follow?
Our decision should be based on a thorough understanding of the evidence, and that evidence is well summarized in the recent USPSTF report.1 The reviewers found no studies that evaluated the benefits and harms of screening children and adolescents for hypertension and no studies evaluating disease outcomes from treating hypertension in these patients.
There is, however, an association between elevated blood pressure in childhood and outcomes such as left ventricular hypertrophy and carotid intimal thickness.2 Some physicians contend that these “disease-oriented outcomes” are sufficient reason to identify and treat hypertension in children and adolescents.3 The USPSTF, however, requires a higher level of evidence that includes patient-oriented outcomes, such as a lower risk of congestive heart failure, renal failure, or death, before recommending treatment. Physicians and patients have to choose what level of evidence is sufficient to take action.
Dr. Smith comments: “As noted in their report, the USPSTF acknowledges that observational studies indicate an association between hypertension in childhood and hypertension in adulthood, but there have been no randomized trials to determine if treating hypertension in children and adolescents reduces risk of cardiovascular events. Although it is a cohort study, not a randomized trial, the ongoing i3C Consortium Outcomes Study4 may provide better information to guide decision-making for children and adolescents with elevated blood pressure.”
What we can all agree on is that, when hypertension is identified in a child or adolescent, it is important to determine if there is a treatable cause of elevated blood pressure such as coarctation of the aorta or renal disease. It is also important to address risk factors for elevated blood pressure and cardiovascular disease, such as obesity, poor dietary habits, and smoking. The treatment is lifestyle modification with diet, exercise, and smoking cessation.
In this issue of JFP, Smith et al recommend following guidelines from the American Academy of Pediatrics to annually screen children for hypertension (see page 220). This recommendation appears to be at odds with the recent US Preventive Services Task Force (USPSTF) statement that concluded there is insufficient evidence for screening children and adolescents for hypertension. But an “I” recommendation from the USPSTF is not the same as a “D” recommendation. “D” means don’t do it, because the evidence indicates that the harms outweigh the benefits. “I” means we don’t have enough evidence to weigh the harms and benefits, so it is up to you and your patients to decide what to do.
So whose recommendations should we follow?
Our decision should be based on a thorough understanding of the evidence, and that evidence is well summarized in the recent USPSTF report.1 The reviewers found no studies that evaluated the benefits and harms of screening children and adolescents for hypertension and no studies evaluating disease outcomes from treating hypertension in these patients.
There is, however, an association between elevated blood pressure in childhood and outcomes such as left ventricular hypertrophy and carotid intimal thickness.2 Some physicians contend that these “disease-oriented outcomes” are sufficient reason to identify and treat hypertension in children and adolescents.3 The USPSTF, however, requires a higher level of evidence that includes patient-oriented outcomes, such as a lower risk of congestive heart failure, renal failure, or death, before recommending treatment. Physicians and patients have to choose what level of evidence is sufficient to take action.
Dr. Smith comments: “As noted in their report, the USPSTF acknowledges that observational studies indicate an association between hypertension in childhood and hypertension in adulthood, but there have been no randomized trials to determine if treating hypertension in children and adolescents reduces risk of cardiovascular events. Although it is a cohort study, not a randomized trial, the ongoing i3C Consortium Outcomes Study4 may provide better information to guide decision-making for children and adolescents with elevated blood pressure.”
What we can all agree on is that, when hypertension is identified in a child or adolescent, it is important to determine if there is a treatable cause of elevated blood pressure such as coarctation of the aorta or renal disease. It is also important to address risk factors for elevated blood pressure and cardiovascular disease, such as obesity, poor dietary habits, and smoking. The treatment is lifestyle modification with diet, exercise, and smoking cessation.
- USPSTF: High blood pressure in children and adolescents: screening. Accessed June 2, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
- Yang L, Magnussen CG, Yang L, et al. Elevated blood pressure in childhood or adolescence and cardiovascular outcomes in adulthood: a systematic review. Hypertension. 2020;75:948–955. doi: 10.1161/hypertensionaha.119.14168
- Falkner B, Lurbe E. The USPSTF call to inaction on blood pressure screening in children and adolescents. Pediatr Nephrol. 2021;36:1327-1329. doi: 10.1007/s00467-021-04926-y
- Sinaiko AR, Jacobs DR Jr, Woo JG, et al. The International Childhood Cardiovascular Cohort (i3C) consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: Design and recruitment. Contemp Clin Trials. 2018;69:55-64. doi: 10.1016/j.cct.2018.04.009
- USPSTF: High blood pressure in children and adolescents: screening. Accessed June 2, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
- Yang L, Magnussen CG, Yang L, et al. Elevated blood pressure in childhood or adolescence and cardiovascular outcomes in adulthood: a systematic review. Hypertension. 2020;75:948–955. doi: 10.1161/hypertensionaha.119.14168
- Falkner B, Lurbe E. The USPSTF call to inaction on blood pressure screening in children and adolescents. Pediatr Nephrol. 2021;36:1327-1329. doi: 10.1007/s00467-021-04926-y
- Sinaiko AR, Jacobs DR Jr, Woo JG, et al. The International Childhood Cardiovascular Cohort (i3C) consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: Design and recruitment. Contemp Clin Trials. 2018;69:55-64. doi: 10.1016/j.cct.2018.04.009
Why getting a COVID-19 vaccine to children could take time
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Testing COVID-19 vaccines in young children is going to be tricky. Deciding how to approve them and who should get them may be even more difficult.
So far, the vaccines available to Americans ages 12 and up have sailed through the U.S. Food and Drug Administration’s regulatory checks, taking advantage of an accelerated clearance process called an Emergency Use Authorization (EUA).
EUAs set a lower bar for effectiveness, saying the vaccines may be safe and effective based on just a few months of data.
But with COVID cases plummeting in the United States and children historically seeing far less serious disease than adults, a panel of expert advisors to the FDA was asked to deliberate on Thursday whether the agency could consider vaccines for this age group under the same standard.
Stated another way: Is COVID an emergency for kids?
There’s another wrinkle in the mix, too – heart inflammation, which appears to be a very rare emerging adverse event tied to vaccination. It seems to happen more often in teens and young adults. To date, cases of myocarditis and pericarditis appear to be happening in 16 to 30 people for every 1 million doses given.
But if it is conclusively linked to the shots, some wonder whether it might tip the balance between benefits and risks for kids.
That left some of the experts who sit on the FDA’s advisory committee for vaccines and related biological products urging the FDA to take its time and more thoroughly study the shots before they’re given to millions of children.
Vaccine studies different in children?
Clinical studies of the vaccines in teens and adults have thus far relied on some straightforward math. You take two groups of similar people. You give half the vaccine and half a placebo. Then you wait and see which group has more symptomatic infections. To date, the vaccines have dramatically cut the risk of getting severely ill with COVID for every age group tested.
But COVID infections are falling rapidly in the U.S., and that may make it more difficult for researchers to conduct a similar kind of experiment in children.
The FDA is considering different approaches to figure out whether a vaccine would be effective in kids, including something called an “immunobridging trial.”
In bridging trials, researchers don’t look for infections; rather, they look for proven signs that someone has developed immunity, like antibody levels. Those biomarkers are then compared to the immune responses of younger adults who have demonstrated good protection against infection.
The main advantage of bridging studies is speed. It’s possible to get a snapshot of how the immune system responds to a vaccine within weeks of the final dose.
The drawback is that researchers don’t know exactly what to look for to judge how well the shots are generating protection.
That’s made even more difficult because kids’ immune systems are still developing, so it may be tough to draw direct parallels to adults.
“We don’t know what the serologic correlate of immunity is now. We don’t know how much antibody you have to get in order to be protected. We don’t know what the role of T cells will be,” said H. Cody Meissner, MD, chief of the division of pediatric infectious disease at Tufts Medical Center, Boston.
“I have so much sympathy for the FDA because these are enormous problems, and you have to make a decision,” said Dr. Meissner, who is a member of the FDA’s vaccines and related biological products advisory committee.
Speed vaccines to market, or gather more data?
The plummeting rate of infections in the United States also means that it may be more difficult for the FDA to justify allowing a vaccine on the market for emergency use for children under age 12.
In its recent advisory committee meeting, the agency asked the panel whether it should consider COVID vaccines for children under an EUA or a biologics license application (BLA), aka full approval.
A BLA typically means the agency considers a year or two of data on a new product, rather than just 2 months’ worth. Emergency use also allows products on the market under a looser standard – they “may be” safe and effective, instead of has been proven to be safe and effective.
Several committee members said they didn’t feel the United States was still in an emergency with COVID and couldn’t see the FDA allowing a vaccine to be used in kids that wasn’t given the agency’s highest level of scrutiny, particularly with reports of adverse events like myocarditis coming to light.
“I just want to be sure the price we pay for vaccinating millions of children justifies the side effects, and I don’t think we know that yet,” Dr. Meissner said.
Others acknowledged that there was little risk to kids now with infections on the decline but said that picture could change as variants spread, schools reopen, and colder temperatures force people indoors.
The FDA must decide whether to act based on where we are now or where we could be in a few months.
“I think it’s the million-dollar question right now,” said Hannah Kirking, MD, a medical epidemiologist with the Centers for Disease Control and Prevention who presented new and unpublished data on COVID’s impact in children to the FDA’s advisory committee.
She said prospective studies tracking the way COVID moves through a household with weekly testing from New York City and Utah had found that children catch and transmit COVID almost as readily as adults. But they don’t usually get as sick as adults do, so their cases are easy to miss.
She also presented the results of blood tests from samples around the country looking for evidence of past infection. In these seroprevalence studies, about 27% of children under age 17 had antibodies to COVID – the most of any age group. So more than 1 in 4 kids already has some natural immunity.
That means the main benefit of vaccinating children might be the protection of others, while they still bear the risks – however tiny.
Some experts felt that wasn’t enough reason to justify mass distribution of the vaccines to kids, and from a regulatory standpoint, it might not be permissible.
“FDA can only approve a medical product in a population if the benefits outweigh the risks in that population,” said Peter Doshi, PhD, assistant professor of pharmaceutical health services research in the University of Maryland’s school of pharmacy, Baltimore.
“If benefits don’t outweigh risks in children, it can’t be indicated for children. Full stop,” said Dr. Doshi, who is also an editor at the BMJ.
He said there’s another way to give children access to vaccines, through an expanded access or compassionate use program. Because most COVID deaths have been in children with underlying health conditions, Dr. Doshi and others said it might make sense to allow expanded access – which would get vaccines to children at high risk for complications – without turning them loose on millions before they are more thoroughly studied.
“It’s not a particularly attractive option for industry, because there’s no money to be made. Your medicine can’t be commercialized under expanded access. The most you can reap is manufacturing cost, which is not a lot,” he said.
Art Caplan, a professor of bioethics at New York University’s Langone medical center, said the argument for vaccinating children for flu falls along the same lines. The benefit-to-risk ratio is finely balanced in children. The main value of protecting them is to protect others.
“Flu rarely kills young folks. But you’re really trying to protect old folks and that’s the classic example,” he said.
What’s more, he said the idea that children would take on some risk with a vaccine for little personal benefit is oversimplified.
“Yes, you might get vaccinated to prevent harm to others, but those others are providing benefits to you. It’s not a one-way street. I think that’s a little morally distorted,” Mr. Caplan said. “Being able to keep society open benefits kids and adults alike.”
Other committee members felt like it was too early to sound the all-clear on COVID and said the FDA should authorize vaccines for children as quickly as it had for other age groups.
“We are still, I believe, in an emergency situation. I think that when this virus goes into our children, which is what it’s going to do, that will give it an incubator to change,” said Oveta Fuller, PhD, associate professor of microbiology and immunology at the University of Michigan, Ann Arbor.
Fuller said that for the good of the world, Americans needed to vaccinate children to prevent the virus from mutating and creating new and potentially more dangerous variants.
Weighing risk over safety
Beth Thielen, MD, PhD, pediatric infectious disease specialist and virologist at the University of Minnesota, Minneapolis, said she had not followed the committee’s discussions, but about once a month she treats kids who are very sick because of the virus – either because of a COVID infection or because of multisystem inflammatory syndrome (MIS-C), an inflammatory reaction that strikes after infection.
She’s worried about how the virus has already changed. She said the kind of disease she’s seeing in kids now is different than what she saw in the early months of the pandemic.
“In the last couple of months, I’ve actually seen a few cases of severe pulmonary disease, more similar to adult disease in children,” Dr. Thielen said. “I see on the horizon that we could start seeing more significant disease in young people, and then the risks of being unvaccinated go up substantially.”
But she also knows nobody has a crystal ball, and right now, everything seems to be trending in the right direction with COVID. That makes the risk-to-benefit consideration murkier.
“The question in my mind is, what is the risk of side effects from the vaccine?” she said. “I think we really need to know what the safety profile of vaccine looks like in children because we do have a decent understanding now what risk from disease looks like, because it’s small, but we are seeing it.”
Dr. Thielen said she’ll be keeping an eye on the next meeting of the CDC’s Advisory Committee on Immunization Practices for more answers.
A version of this article first appeared on Medscape.com.
Insomnia in children tied to mood and anxiety disorders in adulthood
, new research indicates. However, insomnia symptoms in childhood that remit in the transition to adolescence do not confer increased risk of mood or anxiety disorders later on, the study found.
“As insomnia symptoms may precipitate or maintain internalizing disorders, our findings further reinforce the need for early sleep interventions to prevent future mental health disorders,” said lead investigator Julio Fernandez-Mendoza, PhD, associate professor at Penn State University, Hershey.
He presented his research at Virtual SLEEP 2021, the 35th annual meeting of the Associated Professional Sleep Societies.
Results ‘very clear’
The findings are based on data from the Penn State Child Cohort, a longitudinal, population-based sample of 700 children with a median age of 9 years, including 421 who were followed up 8 years later as adolescents (median age, 16 years) and 502 who were followed up 15 years later as young adults (median age, 24 years).
The data are “very clear that the risk of having internalizing disorders in young adulthood associated with having persistent insomnia symptoms, since childhood through adolescence into young adulthood,” Dr. Fernandez-Mendoza said in his presentation.
A persistent developmental trajectory was associated with a threefold increased risk of adult internalizing disorder (hazard ratio, 3.19).
The risk of having an internalizing disorder in young adulthood associated with newly developing (incident) insomnia symptoms is about twofold higher (HR, 1.94), whereas the risk associated with the waxing and waning pattern of insomnia is 1.5-fold (HR, 1.53) higher and only marginally significant, he reported.
An equally important finding, said Dr. Fernandez-Mendoza, is that those who had remitted insomnia symptoms in the transition to adolescence and throughout young adulthood were not at increased risk of having an internalizing disorder in young adulthood.
“Insomnia symptoms in a persistent manner associated with long-term adverse mental health outcomes, but remission of those insomnia symptoms associated with a good prognosis,” he said.
It’s also important to note, he said, that about 40% of children do not outgrow their insomnia symptoms in the transition to adolescence and are at risk of developing mental health disorders later on during early adulthood.
Reached for comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said: “There is a connection with mood and anxiety disorders with sleep, especially insomnia. This is a good reminder that reviewing someone’s sleep habits should always be a part of assessing someone’s mental health.”
A version of this article first appeared on Medscape.com.
, new research indicates. However, insomnia symptoms in childhood that remit in the transition to adolescence do not confer increased risk of mood or anxiety disorders later on, the study found.
“As insomnia symptoms may precipitate or maintain internalizing disorders, our findings further reinforce the need for early sleep interventions to prevent future mental health disorders,” said lead investigator Julio Fernandez-Mendoza, PhD, associate professor at Penn State University, Hershey.
He presented his research at Virtual SLEEP 2021, the 35th annual meeting of the Associated Professional Sleep Societies.
Results ‘very clear’
The findings are based on data from the Penn State Child Cohort, a longitudinal, population-based sample of 700 children with a median age of 9 years, including 421 who were followed up 8 years later as adolescents (median age, 16 years) and 502 who were followed up 15 years later as young adults (median age, 24 years).
The data are “very clear that the risk of having internalizing disorders in young adulthood associated with having persistent insomnia symptoms, since childhood through adolescence into young adulthood,” Dr. Fernandez-Mendoza said in his presentation.
A persistent developmental trajectory was associated with a threefold increased risk of adult internalizing disorder (hazard ratio, 3.19).
The risk of having an internalizing disorder in young adulthood associated with newly developing (incident) insomnia symptoms is about twofold higher (HR, 1.94), whereas the risk associated with the waxing and waning pattern of insomnia is 1.5-fold (HR, 1.53) higher and only marginally significant, he reported.
An equally important finding, said Dr. Fernandez-Mendoza, is that those who had remitted insomnia symptoms in the transition to adolescence and throughout young adulthood were not at increased risk of having an internalizing disorder in young adulthood.
“Insomnia symptoms in a persistent manner associated with long-term adverse mental health outcomes, but remission of those insomnia symptoms associated with a good prognosis,” he said.
It’s also important to note, he said, that about 40% of children do not outgrow their insomnia symptoms in the transition to adolescence and are at risk of developing mental health disorders later on during early adulthood.
Reached for comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said: “There is a connection with mood and anxiety disorders with sleep, especially insomnia. This is a good reminder that reviewing someone’s sleep habits should always be a part of assessing someone’s mental health.”
A version of this article first appeared on Medscape.com.
, new research indicates. However, insomnia symptoms in childhood that remit in the transition to adolescence do not confer increased risk of mood or anxiety disorders later on, the study found.
“As insomnia symptoms may precipitate or maintain internalizing disorders, our findings further reinforce the need for early sleep interventions to prevent future mental health disorders,” said lead investigator Julio Fernandez-Mendoza, PhD, associate professor at Penn State University, Hershey.
He presented his research at Virtual SLEEP 2021, the 35th annual meeting of the Associated Professional Sleep Societies.
Results ‘very clear’
The findings are based on data from the Penn State Child Cohort, a longitudinal, population-based sample of 700 children with a median age of 9 years, including 421 who were followed up 8 years later as adolescents (median age, 16 years) and 502 who were followed up 15 years later as young adults (median age, 24 years).
The data are “very clear that the risk of having internalizing disorders in young adulthood associated with having persistent insomnia symptoms, since childhood through adolescence into young adulthood,” Dr. Fernandez-Mendoza said in his presentation.
A persistent developmental trajectory was associated with a threefold increased risk of adult internalizing disorder (hazard ratio, 3.19).
The risk of having an internalizing disorder in young adulthood associated with newly developing (incident) insomnia symptoms is about twofold higher (HR, 1.94), whereas the risk associated with the waxing and waning pattern of insomnia is 1.5-fold (HR, 1.53) higher and only marginally significant, he reported.
An equally important finding, said Dr. Fernandez-Mendoza, is that those who had remitted insomnia symptoms in the transition to adolescence and throughout young adulthood were not at increased risk of having an internalizing disorder in young adulthood.
“Insomnia symptoms in a persistent manner associated with long-term adverse mental health outcomes, but remission of those insomnia symptoms associated with a good prognosis,” he said.
It’s also important to note, he said, that about 40% of children do not outgrow their insomnia symptoms in the transition to adolescence and are at risk of developing mental health disorders later on during early adulthood.
Reached for comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said: “There is a connection with mood and anxiety disorders with sleep, especially insomnia. This is a good reminder that reviewing someone’s sleep habits should always be a part of assessing someone’s mental health.”
A version of this article first appeared on Medscape.com.



