User login
Flu now riding on COVID-19’s coattails
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
Are CRMO and SAPHO syndrome one and the same?
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Chronic recurrent multifocal osteomyelitis (CRMO) in children and SAPHO syndrome in adults may well be a single clinical syndrome.
That contention, recently put forth by Austrian investigators, resonates with Anne M. Stevens, MD, PhD, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
“Is CRMO just for kids? No,” she asserted at the 2020 Rheumatology Winter Clinical Symposium.
First off, she noted that the nomenclature is shifting: The more familiar acronym CRMO is giving way to CNO (chronic nonbacterial osteomyelitis) in light of evidence that roughly 30% of patients with CRMO start out with a single characteristic bone lesion, with the disease turning multifocal in the subsequent 4 years in the great majority of cases.
SAPHO syndrome – an acronym for synovitis, acne, pustulosis, hyperostosis, and osteitis – a formerly obscure disease entity first described in 1987 in France, has suddenly become a trendy research topic, with three small studies presented at the 2019 annual meeting of the American College of Rheumatology.
CNO is a pediatric autoinflammatory bone disease characterized by sterile bone lesions, most often on the clavicle, spine, mandible, and lower extremities. It is marked by prominent focal bone and/or joint pain, worse at night, with or without swelling. With no agreed-upon diagnostic criteria or biomarkers, CNO is a diagnosis of exclusion. Two-thirds of the time the condition is initially misdiagnosed as bacterial osteomyelitis or a malignant tumor.
Austrian investigators at the University of Graz recently conducted a retrospective comparison of 24 pediatric patients diagnosed with CNO and 10 adults with SAPHO syndrome. The median age at diagnosis of CNO was 12.3 years versus 32.5 years for SAPHO syndrome. The two groups shared compelling similarities in mean number of bone lesions, prevalence of skin involvement, and other aspects of initial clinical presentation, as well as laboratory and histopathologic findings on bone biopsy.
There were, however, several notable clinical differences in this small dataset: CNO bone lesions affected mainly the lower extremities, clavicle, spine, and mandible, while SAPHO syndrome more commonly involved the sternum (50% vs. 8%) and vertebrae (50% vs. 21%). Also, the most frequent cutaneous manifestation was palmoplantar pustulosis in adults with SAPHO syndrome, while severe acne predominated in children with CNO. In both children and adults, the skin lesions most often arose after the bone symptoms, making early diagnosis a challenge.
Another similarity: Although there have been no randomized treatment trials in either CNO or SAPHO syndrome, case series suggest the same treatments are effective for both, with NSAIDs as first line, followed by nonbiologic disease-modifying antirheumatic drugs, tumor necrosis factor (TNF) inhibitors, or bisphosphonates.
CNO diagnosis, treatment, and follow-up
Various investigators have pegged the sensitivity of physical examination for diagnosis of CNO at 31%, radiographs at a lowly 13%, and bone scintigraphy at 74%, all in comparison with MRI.
“Our go-to now is MRI with STIR [short tau inversion recovery],” according to Dr. Stevens. “There’s no contrast – so no IV – no radiation, and it’s fast, 20 minutes for a whole body MRI in a little kid, 45 minutes in a big one.”
Insurers are reluctant to pay for serial whole-body MRIs for patient follow-up, so it’s often necessary to order a series of images covering different body parts.
Her University of Washington colleague Dan Zhao, MD, PhD, is developing infrared thermal imaging as an inexpensive, convenient alternative to MRI which could theoretically be done at home. In a pilot study in 30 children with CNO and 31 controls, inflamed leg segments showed significantly higher temperatures. Larger studies are planned.
Dr. Stevens advised leaning towards a diagnosis of CNO with avoidance of bone biopsy in a patient with multifocal osteomyelitis at the typical sites, a normal CBC, the typical extraosseous manifestations, and normal or only mildly elevated erythrocyte sedimentation rate and C-reactive protein in an otherwise well-appearing child. In contrast, strongly consider a bone biopsy to rule out malignancy or infection if the child has unexplained highly elevated C-reactive protein and erythrocyte sedimentation rate, cytopenia, high fever, excessive pain, lymphadenopathy, hepatosplenomegaly, or suspicious imaging findings.
German rheumatologists have developed a clinical score for diagnosis of CNO. A normal blood cell count gets 13 points; symmetric bone lesions 10; lesions with marginal sclerosis 10; a normal body temperature 9; two or more radiologically proven lesions 7; a C-reactive protein of 1 mg/dL or greater 6; and vertebral, clavicular, or sternal lesions 8. A score of 39 points or more out of a possible 63 had a 97% positive predictive value for CNO in a retrospective study of 224 children with CNO, proven bacterial osteomyelitis, or malignant bone tumors. A score of 28 points or less had a 97% negative predictive value for CNO. An indeterminate score of 29-38 warrants close monitoring.
The scoring system hasn’t been validated, but most pediatric rheumatologists agree that it’s useful, according to Dr. Stevens.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) is in the process of developing standardized diagnostic and classification criteria and treatment plans for CNO. Dr. Zhao was first author of a CARRA consensus treatment plan for CNO refractory to NSAID monotherapy. The plan for the first 12 months includes three options: methotrexate or sulfasalazine, TNF inhibitors with or without methotrexate, and bisphosphonates.
“The main point of this is you try a medicine and then wait 3 months. If they’re not responding then, switch medicines or add another drug. Monitor every 3 months based upon pain,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
EXPERT ANALYSIS FROM RWCS 2020
Preventable diseases could gain a foothold because of COVID-19
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
COVID-19 in China: Children have less severe disease, but are vulnerable
Clinical manifestations of COVID-19 infection among children in mainland China generally have been less severe than those among adults, but children of all ages – and infants in particular – are vulnerable to infection, according to a review of 2,143 cases.
Further, infection patterns in the nationwide series of all pediatric patients reported to the Chinese Center for Disease Control and Prevention from Jan. 16 to Feb. 8, 2020, provide strong evidence of human-to-human transmission, Yuanyuan Dong, MPH, a research assistant at Shanghai Children’s Medical Center, Shanghai Jiao Tong University, China, and colleagues reported in Pediatrics.
Of the 2,143 patients included in the review, 57% were boys and the median age was 7 years; 34% had laboratory-confirmed infection and 67% had suspected infection. More than 90% had asymptomatic, mild, or moderate disease (4%, 51%, and 39%, respectively), and 46% were from Hubei Province, where the first cases were reported, the investigators found.
The median time from illness onset to diagnosis was 2 days, and there was a trend of rapid increase of disease at the early stage of the epidemic – with rapid spread from Hubei Province to surrounding provinces – followed by a gradual and steady decrease, they noted.
“The total number of pediatric patients increased remarkably between mid-January and early February, peaked around February 1, and then declined since early February 2020,” they wrote. The proportion of severe and critical cases was 11% for infants under 1 year of age, compared with 7% for those aged 1-5 years; 4% for those aged 6-10 years; 4% for those 11-15 years; and 3% for those 16 years and older.
As of Feb. 8, 2020, only one child in this group of study patients died and most cases of COVID-19 symptoms were mild. There were many fewer severe and critical cases among the children (6%), compared with those reported in adult patients in other studies (19%). “It suggests that, compared with adult patients, clinical manifestations of children’s COVID-19 may be less severe,” the investigators suggested.
“As most of these children were likely to expose themselves to family members and/or other children with COVID-19, it clearly indicates person-to-person transmission ” of novel coronavirus 2019, they said, adding that similar evidence of such transmission also has been reported from studies of adult patients.
The reasons for reduced severity in children versus adults remain unclear, but may be related to both exposure and host factors, Ms. Dong and associates said. “Children were usually well cared for at home and might have relatively [fewer] opportunities to expose themselves to pathogens and/or sick patients.”
The findings demonstrate a pediatric distribution that varied across time and space, with most cases concentrated in the Hubei province and surrounding areas. No significant gender-related difference in infection rates was observed, and although the median patient age was 7 years, the range was 1 day to 18 years, suggesting that “all ages at childhood were susceptible” to the virus, they added.
The declining number of cases over time further suggests that disease control measures implemented by the government were effective, and that cases will “continue to decline, and finally stop in the near future unless sustained human-to-human transmissions occur,” Ms. Dong and associates concluded.
In an accompanying editorial, Andrea T. Cruz, MD, of Baylor College of Medicine, Houston, and Steven L. Zeichner, MD, PhD, of the University of Virginia, Charlottesville, said the findings regarding reduced severity among children versus adults with novel coronavirus 2019 infection are consistent with data on non-COVID-19 coronavirus.
They pointed out that Ms. Dong and associates did find that 13% of virologically-confirmed cases had asymptomatic infection, “a rate that almost certainly understates the true rate of asymptomatic infection, since many asymptomatic children are unlikely to be tested.”
Of the symptomatic children, “5% had dyspnea or hypoxemia (a substantially lower percentage than what has been reported for adults) and 0.6% progressed to acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction”; this also is at a lower rate than seen in adults, they said.
Very young children –infants or children in preschool – were more likely to have severe clinical manifestations than children who were older.
Thus, it appears that certain subpopulations of children are at increased risk for more significant COVID-19 illness: “younger age, underlying pulmonary pathology, and immunocompromising conditions,” Dr. Cruz and Dr. Zeichner suggested.
The two editorialists said the findings suggest children “may play a major role in community-based viral transmission.” Evidence suggests that children may have more upper respiratory tract involvement and that fecal shedding may occur for several weeks after diagnosis; this raises concerns about fecal-oral transmission, particularly for infants and children, and about viral replication in the gastrointestinal tract, they said. This has substantial implications for community spread in day care centers, schools, and in the home.
A great deal has been learned about COVID-19 in a short time, but there still is much to learn about the effect of the virus on children, the impact of children on viral spread, and about possible vertical transmission, they said.
“Widespread availability of testing will allow for us to more accurately describe the spectrum of illness and may result in adjustment of the apparent morbidity and mortality rate as fewer ill individuals are diagnosed,” Dr. Cruz and Dr. Zeichner wrote, adding that “rigorously gauging the impact of COVID-19 on children will be important to accurately model the pandemic and to ensure that appropriate resources are allocated to children requiring care.”
They noted that understanding differences in children versus adults with COVID-19 “can yield important insights into disease pathogenesis, informing management and the development of therapeutics.”
This study was partially supported by the Science and Technology Commission of Shanghai Municipality. The authors reported having no disclosures. Dr. Cruz and Dr. Zeichner are associate editors for Pediatrics. Dr. Cruz reported having no disclosures. Dr. Zeichner is an inventor of new technologies for the rapid production of vaccines, for which the University of Virginia has filed patent applications.
SOURCE: Dong Y et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0702; Cruz A and Zeichner S. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0834.
Clinical manifestations of COVID-19 infection among children in mainland China generally have been less severe than those among adults, but children of all ages – and infants in particular – are vulnerable to infection, according to a review of 2,143 cases.
Further, infection patterns in the nationwide series of all pediatric patients reported to the Chinese Center for Disease Control and Prevention from Jan. 16 to Feb. 8, 2020, provide strong evidence of human-to-human transmission, Yuanyuan Dong, MPH, a research assistant at Shanghai Children’s Medical Center, Shanghai Jiao Tong University, China, and colleagues reported in Pediatrics.
Of the 2,143 patients included in the review, 57% were boys and the median age was 7 years; 34% had laboratory-confirmed infection and 67% had suspected infection. More than 90% had asymptomatic, mild, or moderate disease (4%, 51%, and 39%, respectively), and 46% were from Hubei Province, where the first cases were reported, the investigators found.
The median time from illness onset to diagnosis was 2 days, and there was a trend of rapid increase of disease at the early stage of the epidemic – with rapid spread from Hubei Province to surrounding provinces – followed by a gradual and steady decrease, they noted.
“The total number of pediatric patients increased remarkably between mid-January and early February, peaked around February 1, and then declined since early February 2020,” they wrote. The proportion of severe and critical cases was 11% for infants under 1 year of age, compared with 7% for those aged 1-5 years; 4% for those aged 6-10 years; 4% for those 11-15 years; and 3% for those 16 years and older.
As of Feb. 8, 2020, only one child in this group of study patients died and most cases of COVID-19 symptoms were mild. There were many fewer severe and critical cases among the children (6%), compared with those reported in adult patients in other studies (19%). “It suggests that, compared with adult patients, clinical manifestations of children’s COVID-19 may be less severe,” the investigators suggested.
“As most of these children were likely to expose themselves to family members and/or other children with COVID-19, it clearly indicates person-to-person transmission ” of novel coronavirus 2019, they said, adding that similar evidence of such transmission also has been reported from studies of adult patients.
The reasons for reduced severity in children versus adults remain unclear, but may be related to both exposure and host factors, Ms. Dong and associates said. “Children were usually well cared for at home and might have relatively [fewer] opportunities to expose themselves to pathogens and/or sick patients.”
The findings demonstrate a pediatric distribution that varied across time and space, with most cases concentrated in the Hubei province and surrounding areas. No significant gender-related difference in infection rates was observed, and although the median patient age was 7 years, the range was 1 day to 18 years, suggesting that “all ages at childhood were susceptible” to the virus, they added.
The declining number of cases over time further suggests that disease control measures implemented by the government were effective, and that cases will “continue to decline, and finally stop in the near future unless sustained human-to-human transmissions occur,” Ms. Dong and associates concluded.
In an accompanying editorial, Andrea T. Cruz, MD, of Baylor College of Medicine, Houston, and Steven L. Zeichner, MD, PhD, of the University of Virginia, Charlottesville, said the findings regarding reduced severity among children versus adults with novel coronavirus 2019 infection are consistent with data on non-COVID-19 coronavirus.
They pointed out that Ms. Dong and associates did find that 13% of virologically-confirmed cases had asymptomatic infection, “a rate that almost certainly understates the true rate of asymptomatic infection, since many asymptomatic children are unlikely to be tested.”
Of the symptomatic children, “5% had dyspnea or hypoxemia (a substantially lower percentage than what has been reported for adults) and 0.6% progressed to acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction”; this also is at a lower rate than seen in adults, they said.
Very young children –infants or children in preschool – were more likely to have severe clinical manifestations than children who were older.
Thus, it appears that certain subpopulations of children are at increased risk for more significant COVID-19 illness: “younger age, underlying pulmonary pathology, and immunocompromising conditions,” Dr. Cruz and Dr. Zeichner suggested.
The two editorialists said the findings suggest children “may play a major role in community-based viral transmission.” Evidence suggests that children may have more upper respiratory tract involvement and that fecal shedding may occur for several weeks after diagnosis; this raises concerns about fecal-oral transmission, particularly for infants and children, and about viral replication in the gastrointestinal tract, they said. This has substantial implications for community spread in day care centers, schools, and in the home.
A great deal has been learned about COVID-19 in a short time, but there still is much to learn about the effect of the virus on children, the impact of children on viral spread, and about possible vertical transmission, they said.
“Widespread availability of testing will allow for us to more accurately describe the spectrum of illness and may result in adjustment of the apparent morbidity and mortality rate as fewer ill individuals are diagnosed,” Dr. Cruz and Dr. Zeichner wrote, adding that “rigorously gauging the impact of COVID-19 on children will be important to accurately model the pandemic and to ensure that appropriate resources are allocated to children requiring care.”
They noted that understanding differences in children versus adults with COVID-19 “can yield important insights into disease pathogenesis, informing management and the development of therapeutics.”
This study was partially supported by the Science and Technology Commission of Shanghai Municipality. The authors reported having no disclosures. Dr. Cruz and Dr. Zeichner are associate editors for Pediatrics. Dr. Cruz reported having no disclosures. Dr. Zeichner is an inventor of new technologies for the rapid production of vaccines, for which the University of Virginia has filed patent applications.
SOURCE: Dong Y et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0702; Cruz A and Zeichner S. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0834.
Clinical manifestations of COVID-19 infection among children in mainland China generally have been less severe than those among adults, but children of all ages – and infants in particular – are vulnerable to infection, according to a review of 2,143 cases.
Further, infection patterns in the nationwide series of all pediatric patients reported to the Chinese Center for Disease Control and Prevention from Jan. 16 to Feb. 8, 2020, provide strong evidence of human-to-human transmission, Yuanyuan Dong, MPH, a research assistant at Shanghai Children’s Medical Center, Shanghai Jiao Tong University, China, and colleagues reported in Pediatrics.
Of the 2,143 patients included in the review, 57% were boys and the median age was 7 years; 34% had laboratory-confirmed infection and 67% had suspected infection. More than 90% had asymptomatic, mild, or moderate disease (4%, 51%, and 39%, respectively), and 46% were from Hubei Province, where the first cases were reported, the investigators found.
The median time from illness onset to diagnosis was 2 days, and there was a trend of rapid increase of disease at the early stage of the epidemic – with rapid spread from Hubei Province to surrounding provinces – followed by a gradual and steady decrease, they noted.
“The total number of pediatric patients increased remarkably between mid-January and early February, peaked around February 1, and then declined since early February 2020,” they wrote. The proportion of severe and critical cases was 11% for infants under 1 year of age, compared with 7% for those aged 1-5 years; 4% for those aged 6-10 years; 4% for those 11-15 years; and 3% for those 16 years and older.
As of Feb. 8, 2020, only one child in this group of study patients died and most cases of COVID-19 symptoms were mild. There were many fewer severe and critical cases among the children (6%), compared with those reported in adult patients in other studies (19%). “It suggests that, compared with adult patients, clinical manifestations of children’s COVID-19 may be less severe,” the investigators suggested.
“As most of these children were likely to expose themselves to family members and/or other children with COVID-19, it clearly indicates person-to-person transmission ” of novel coronavirus 2019, they said, adding that similar evidence of such transmission also has been reported from studies of adult patients.
The reasons for reduced severity in children versus adults remain unclear, but may be related to both exposure and host factors, Ms. Dong and associates said. “Children were usually well cared for at home and might have relatively [fewer] opportunities to expose themselves to pathogens and/or sick patients.”
The findings demonstrate a pediatric distribution that varied across time and space, with most cases concentrated in the Hubei province and surrounding areas. No significant gender-related difference in infection rates was observed, and although the median patient age was 7 years, the range was 1 day to 18 years, suggesting that “all ages at childhood were susceptible” to the virus, they added.
The declining number of cases over time further suggests that disease control measures implemented by the government were effective, and that cases will “continue to decline, and finally stop in the near future unless sustained human-to-human transmissions occur,” Ms. Dong and associates concluded.
In an accompanying editorial, Andrea T. Cruz, MD, of Baylor College of Medicine, Houston, and Steven L. Zeichner, MD, PhD, of the University of Virginia, Charlottesville, said the findings regarding reduced severity among children versus adults with novel coronavirus 2019 infection are consistent with data on non-COVID-19 coronavirus.
They pointed out that Ms. Dong and associates did find that 13% of virologically-confirmed cases had asymptomatic infection, “a rate that almost certainly understates the true rate of asymptomatic infection, since many asymptomatic children are unlikely to be tested.”
Of the symptomatic children, “5% had dyspnea or hypoxemia (a substantially lower percentage than what has been reported for adults) and 0.6% progressed to acute respiratory distress syndrome (ARDS) or multiorgan system dysfunction”; this also is at a lower rate than seen in adults, they said.
Very young children –infants or children in preschool – were more likely to have severe clinical manifestations than children who were older.
Thus, it appears that certain subpopulations of children are at increased risk for more significant COVID-19 illness: “younger age, underlying pulmonary pathology, and immunocompromising conditions,” Dr. Cruz and Dr. Zeichner suggested.
The two editorialists said the findings suggest children “may play a major role in community-based viral transmission.” Evidence suggests that children may have more upper respiratory tract involvement and that fecal shedding may occur for several weeks after diagnosis; this raises concerns about fecal-oral transmission, particularly for infants and children, and about viral replication in the gastrointestinal tract, they said. This has substantial implications for community spread in day care centers, schools, and in the home.
A great deal has been learned about COVID-19 in a short time, but there still is much to learn about the effect of the virus on children, the impact of children on viral spread, and about possible vertical transmission, they said.
“Widespread availability of testing will allow for us to more accurately describe the spectrum of illness and may result in adjustment of the apparent morbidity and mortality rate as fewer ill individuals are diagnosed,” Dr. Cruz and Dr. Zeichner wrote, adding that “rigorously gauging the impact of COVID-19 on children will be important to accurately model the pandemic and to ensure that appropriate resources are allocated to children requiring care.”
They noted that understanding differences in children versus adults with COVID-19 “can yield important insights into disease pathogenesis, informing management and the development of therapeutics.”
This study was partially supported by the Science and Technology Commission of Shanghai Municipality. The authors reported having no disclosures. Dr. Cruz and Dr. Zeichner are associate editors for Pediatrics. Dr. Cruz reported having no disclosures. Dr. Zeichner is an inventor of new technologies for the rapid production of vaccines, for which the University of Virginia has filed patent applications.
SOURCE: Dong Y et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0702; Cruz A and Zeichner S. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2020-0834.
FROM PEDIATRICS
COVID-19 in pregnant women and the impact on newborns
Clinical question: How does infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pregnant mothers affect their newborns?
Background: A novel coronavirus, now named SARS-CoV-2 by the World Health Organization (previously referred to as 2019-nCoV), is currently causing a worldwide pandemic. It is believed to have originated in Hubei province, China, but is now rapidly spreading in other countries. Although its effects are most severe in the elderly, SARS-CoV-2 has been infecting younger patients, including pregnant women. The effect of COVID-19, the disease caused by SARS-CoV-2, in pregnant women on their newborn children, is unknown, as is the nature of perinatal transmission of SARS-CoV-2.
Study design: Retrospective analysis.
Setting: Five hospitals in Hubei province, China.
Synopsis: Researchers retrospectively analyzed the clinical features and outcomes of 10 neonates (including two twins) born to nine mothers with confirmed SARS-CoV-2 infection in five hospitals in Hubei province, China, during Jan. 20–Feb. 5, 2020. The mothers were, on average, 30 years of age, but their prior state of health was not described. SARS-CoV-2 infection was confirmed in eight mothers by SARS-CoV-2 nucleic acid testing (NAT). The twins’ mother was diagnosed with COVID-19 based on chest CT scan showing viral interstitial pneumonia with other causes of fever and lung infection being “excluded,” despite a negative SARS-CoV-2 NAT test.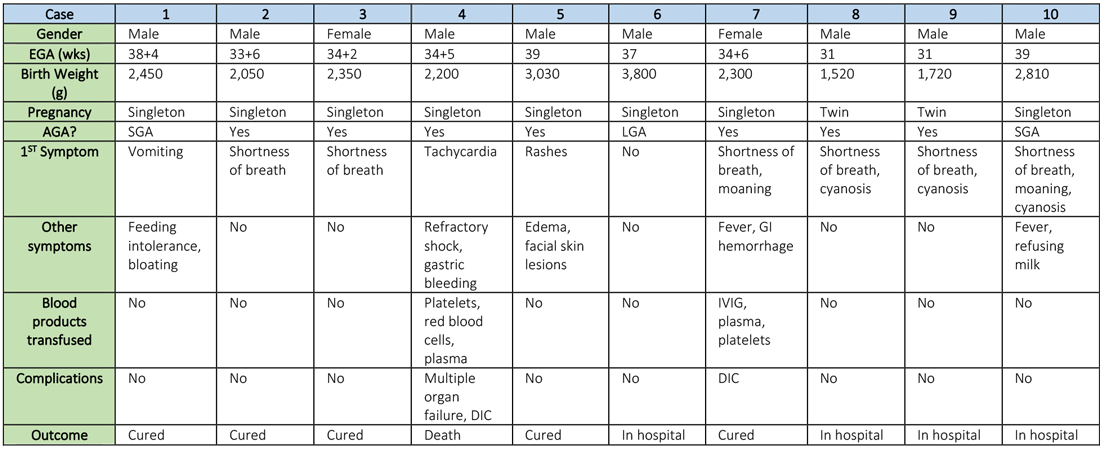
Symptoms occurred in the following:
- Before delivery in four mothers, three of whom were treated with oseltamivir (Tamiflu) after delivery.
- On the day of delivery in two mothers, one of whom was treated with oseltamivir and nebulized inhaled interferon after delivery.
- After delivery in three mothers.
Seven mothers delivered by cesarean section and two by vaginal delivery. Prenatal complications included intrauterine distress in six mothers, premature rupture of membranes in three (5-7 hours before onset of true labor), abnormal amniotic fluid in two, “abnormal” umbilical cord in two, and placenta previa in one.
The neonates born to these mothers included two females and eight males; four were full-term and six were premature (degree of prematurity not described). Symptoms first observed in these newborns included shortness of breath (six), fevers (two), tachycardia (one), and vomiting, feeding intolerance, “bloating,” refusing milk, and “gastric bleeding.” Chest radiographs were abnormal in seven newborns, including evidence of “infection” (four), neonatal respiratory distress syndrome (two), and pneumothorax (one). Two cases were described in detail:
- A neonate delivered at 34+5/7 weeks gestational age, was admitted due to shortness of breath and “moaning.” Eight days later, the neonate developed refractory shock, multiple organ failure, disseminated intravascular coagulation requiring transfusions of platelets, red blood cells, and plasma. He died on the ninth day.
- A neonate delivered at 34+6 weeks gestational age and was admitted 25 minutes after delivery due to shortness of breath and “moaning.” He required 2 days of noninvasive support/oxygen therapy and was observed to later develop “oxygen fluctuations” and thrombocytopenia at 3 days of life. The neonate was treated with “respiratory support,” intravenous immunoglobulin, transfusions of platelets and plasma, hydrocortisone (5 mg/kg per day for 6 days), low-dose heparin (2 units/kg per hr for 6 days), and low molecular weight heparin (2 units/kg per hr for 6 days). He was described to be “cured” 15 days later.
All nine neonates underwent pharyngeal swabs for SARS-CoV-2 NAT, and all were negative.
Bottom line: Although data are currently very limited, neonates born to mothers with COVID-19 appear to be at risk for adverse outcomes, including fetal distress, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. There was no evidence of vertical transmission in this study.
Citation: Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020 Feb;9(1):51-60.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
Clinical question: How does infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pregnant mothers affect their newborns?
Background: A novel coronavirus, now named SARS-CoV-2 by the World Health Organization (previously referred to as 2019-nCoV), is currently causing a worldwide pandemic. It is believed to have originated in Hubei province, China, but is now rapidly spreading in other countries. Although its effects are most severe in the elderly, SARS-CoV-2 has been infecting younger patients, including pregnant women. The effect of COVID-19, the disease caused by SARS-CoV-2, in pregnant women on their newborn children, is unknown, as is the nature of perinatal transmission of SARS-CoV-2.
Study design: Retrospective analysis.
Setting: Five hospitals in Hubei province, China.
Synopsis: Researchers retrospectively analyzed the clinical features and outcomes of 10 neonates (including two twins) born to nine mothers with confirmed SARS-CoV-2 infection in five hospitals in Hubei province, China, during Jan. 20–Feb. 5, 2020. The mothers were, on average, 30 years of age, but their prior state of health was not described. SARS-CoV-2 infection was confirmed in eight mothers by SARS-CoV-2 nucleic acid testing (NAT). The twins’ mother was diagnosed with COVID-19 based on chest CT scan showing viral interstitial pneumonia with other causes of fever and lung infection being “excluded,” despite a negative SARS-CoV-2 NAT test.
Symptoms occurred in the following:
- Before delivery in four mothers, three of whom were treated with oseltamivir (Tamiflu) after delivery.
- On the day of delivery in two mothers, one of whom was treated with oseltamivir and nebulized inhaled interferon after delivery.
- After delivery in three mothers.
Seven mothers delivered by cesarean section and two by vaginal delivery. Prenatal complications included intrauterine distress in six mothers, premature rupture of membranes in three (5-7 hours before onset of true labor), abnormal amniotic fluid in two, “abnormal” umbilical cord in two, and placenta previa in one.
The neonates born to these mothers included two females and eight males; four were full-term and six were premature (degree of prematurity not described). Symptoms first observed in these newborns included shortness of breath (six), fevers (two), tachycardia (one), and vomiting, feeding intolerance, “bloating,” refusing milk, and “gastric bleeding.” Chest radiographs were abnormal in seven newborns, including evidence of “infection” (four), neonatal respiratory distress syndrome (two), and pneumothorax (one). Two cases were described in detail:
- A neonate delivered at 34+5/7 weeks gestational age, was admitted due to shortness of breath and “moaning.” Eight days later, the neonate developed refractory shock, multiple organ failure, disseminated intravascular coagulation requiring transfusions of platelets, red blood cells, and plasma. He died on the ninth day.
- A neonate delivered at 34+6 weeks gestational age and was admitted 25 minutes after delivery due to shortness of breath and “moaning.” He required 2 days of noninvasive support/oxygen therapy and was observed to later develop “oxygen fluctuations” and thrombocytopenia at 3 days of life. The neonate was treated with “respiratory support,” intravenous immunoglobulin, transfusions of platelets and plasma, hydrocortisone (5 mg/kg per day for 6 days), low-dose heparin (2 units/kg per hr for 6 days), and low molecular weight heparin (2 units/kg per hr for 6 days). He was described to be “cured” 15 days later.
All nine neonates underwent pharyngeal swabs for SARS-CoV-2 NAT, and all were negative.
Bottom line: Although data are currently very limited, neonates born to mothers with COVID-19 appear to be at risk for adverse outcomes, including fetal distress, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. There was no evidence of vertical transmission in this study.
Citation: Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020 Feb;9(1):51-60.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
Clinical question: How does infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pregnant mothers affect their newborns?
Background: A novel coronavirus, now named SARS-CoV-2 by the World Health Organization (previously referred to as 2019-nCoV), is currently causing a worldwide pandemic. It is believed to have originated in Hubei province, China, but is now rapidly spreading in other countries. Although its effects are most severe in the elderly, SARS-CoV-2 has been infecting younger patients, including pregnant women. The effect of COVID-19, the disease caused by SARS-CoV-2, in pregnant women on their newborn children, is unknown, as is the nature of perinatal transmission of SARS-CoV-2.
Study design: Retrospective analysis.
Setting: Five hospitals in Hubei province, China.
Synopsis: Researchers retrospectively analyzed the clinical features and outcomes of 10 neonates (including two twins) born to nine mothers with confirmed SARS-CoV-2 infection in five hospitals in Hubei province, China, during Jan. 20–Feb. 5, 2020. The mothers were, on average, 30 years of age, but their prior state of health was not described. SARS-CoV-2 infection was confirmed in eight mothers by SARS-CoV-2 nucleic acid testing (NAT). The twins’ mother was diagnosed with COVID-19 based on chest CT scan showing viral interstitial pneumonia with other causes of fever and lung infection being “excluded,” despite a negative SARS-CoV-2 NAT test.
Symptoms occurred in the following:
- Before delivery in four mothers, three of whom were treated with oseltamivir (Tamiflu) after delivery.
- On the day of delivery in two mothers, one of whom was treated with oseltamivir and nebulized inhaled interferon after delivery.
- After delivery in three mothers.
Seven mothers delivered by cesarean section and two by vaginal delivery. Prenatal complications included intrauterine distress in six mothers, premature rupture of membranes in three (5-7 hours before onset of true labor), abnormal amniotic fluid in two, “abnormal” umbilical cord in two, and placenta previa in one.
The neonates born to these mothers included two females and eight males; four were full-term and six were premature (degree of prematurity not described). Symptoms first observed in these newborns included shortness of breath (six), fevers (two), tachycardia (one), and vomiting, feeding intolerance, “bloating,” refusing milk, and “gastric bleeding.” Chest radiographs were abnormal in seven newborns, including evidence of “infection” (four), neonatal respiratory distress syndrome (two), and pneumothorax (one). Two cases were described in detail:
- A neonate delivered at 34+5/7 weeks gestational age, was admitted due to shortness of breath and “moaning.” Eight days later, the neonate developed refractory shock, multiple organ failure, disseminated intravascular coagulation requiring transfusions of platelets, red blood cells, and plasma. He died on the ninth day.
- A neonate delivered at 34+6 weeks gestational age and was admitted 25 minutes after delivery due to shortness of breath and “moaning.” He required 2 days of noninvasive support/oxygen therapy and was observed to later develop “oxygen fluctuations” and thrombocytopenia at 3 days of life. The neonate was treated with “respiratory support,” intravenous immunoglobulin, transfusions of platelets and plasma, hydrocortisone (5 mg/kg per day for 6 days), low-dose heparin (2 units/kg per hr for 6 days), and low molecular weight heparin (2 units/kg per hr for 6 days). He was described to be “cured” 15 days later.
All nine neonates underwent pharyngeal swabs for SARS-CoV-2 NAT, and all were negative.
Bottom line: Although data are currently very limited, neonates born to mothers with COVID-19 appear to be at risk for adverse outcomes, including fetal distress, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. There was no evidence of vertical transmission in this study.
Citation: Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020 Feb;9(1):51-60.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
COVID-19 guidance for children’s health care providers
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
CNS cancer outcomes worse for black and Hispanic children
according to a study published in Scientific Reports.
While prior studies have shown the effects of racial/ethnic and socioeconomic risk factors on treatment outcomes in adult cancer populations, less is known about how these factors impact children with CNS cancers, explained study author Robert Fineberg, MD, of St. Anthony North Health Campus in Westminster, Colo., and colleagues.
The authors conducted their study to examine the effects of demographic and socioeconomic factors on survival in pediatric CNS cancers. Using data from the Surveillance, Epidemiology, and End Results database, the researchers identified 1,881 patients with CNS tumors, including both spinal and cranial neoplasms.
Data collection encompassed patient characteristics, socioeconomic parameters, tumor characteristics, treatment, and year of diagnosis. The primary outcomes were overall survival and disease stage at diagnosis.
Most patients were white (78.15%) and non-Hispanic (72.09%). The most common brain tumors were gliomas (n = 788), ependymomas (n = 418), and medulloblastomas (n = 393).
On multivariable analysis, the researchers found that black and Hispanic patients had worse survival, compared with white patients (hazard ratio, 1.39; P = .0014) and non-Hispanic patients (HR, 1.36; P = .0002).
After adjustment for socioeconomic parameters and treatment, the hazard ratios for both Hispanic (HR, 1.29; P = .0051) and black patients (HR, 1.29; P = .0206) slightly declined, but the differences remained significant.
On stratified analysis, poorer survival rates were observed for black and Hispanic patients with both metastatic and localized disease at diagnosis, compared with white non-Hispanic patients. However, after adjustment for mediating factors, the difference did not remain significant for black patients (P = .1026).
“Our findings on extent of disease at diagnosis demonstrated that neither black race nor Hispanic ethnicity increased the chance of metastatic disease at presentation when controlling for mediating variables,” the authors wrote. “These data suggest that racial and ethnic disparities appear to be partially explained by postdiagnosis mediating factors that may fall in the pathway between race/ethnicity and poorer survival.”
The researchers acknowledged that a key limitation of this study was the exclusion of insurance status because of incomplete access for some patients. As a result, potential associations between insurance and survival or extent of disease could not be determined.
“To better understand underlying causes that contribute to the disparity of outcomes in pediatric brain tumors, patient-level data should be utilized in future studies to investigate both biological factors and pre/postdiagnosis treatment gaps in the care of children diagnosed with CNS tumors in the hopes of improving outcomes,” the authors wrote.
In the meantime, collaboration among physicians could help improve outcomes for these patients, according to study author Adam Green, MD, of the University of Colorado at Denver in Aurora.
“[Clinicians] should establish good working relationships with pediatric oncology and neuro-oncology physicians in their community, and they should ask questions early of those teams when they have patients they’re concerned about,” Dr. Green said. “They can [ensure] that patients of minority race/ethnicity, nonprivate health insurance, and lower socioeconomic status have easy and timely access to appointments.”
This research was supported, in part, by grant funding from the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fineberg R et al. Scientific Reports. 2020 Mar 12. doi: 10.1038/s41598-020-61237-2.
according to a study published in Scientific Reports.
While prior studies have shown the effects of racial/ethnic and socioeconomic risk factors on treatment outcomes in adult cancer populations, less is known about how these factors impact children with CNS cancers, explained study author Robert Fineberg, MD, of St. Anthony North Health Campus in Westminster, Colo., and colleagues.
The authors conducted their study to examine the effects of demographic and socioeconomic factors on survival in pediatric CNS cancers. Using data from the Surveillance, Epidemiology, and End Results database, the researchers identified 1,881 patients with CNS tumors, including both spinal and cranial neoplasms.
Data collection encompassed patient characteristics, socioeconomic parameters, tumor characteristics, treatment, and year of diagnosis. The primary outcomes were overall survival and disease stage at diagnosis.
Most patients were white (78.15%) and non-Hispanic (72.09%). The most common brain tumors were gliomas (n = 788), ependymomas (n = 418), and medulloblastomas (n = 393).
On multivariable analysis, the researchers found that black and Hispanic patients had worse survival, compared with white patients (hazard ratio, 1.39; P = .0014) and non-Hispanic patients (HR, 1.36; P = .0002).
After adjustment for socioeconomic parameters and treatment, the hazard ratios for both Hispanic (HR, 1.29; P = .0051) and black patients (HR, 1.29; P = .0206) slightly declined, but the differences remained significant.
On stratified analysis, poorer survival rates were observed for black and Hispanic patients with both metastatic and localized disease at diagnosis, compared with white non-Hispanic patients. However, after adjustment for mediating factors, the difference did not remain significant for black patients (P = .1026).
“Our findings on extent of disease at diagnosis demonstrated that neither black race nor Hispanic ethnicity increased the chance of metastatic disease at presentation when controlling for mediating variables,” the authors wrote. “These data suggest that racial and ethnic disparities appear to be partially explained by postdiagnosis mediating factors that may fall in the pathway between race/ethnicity and poorer survival.”
The researchers acknowledged that a key limitation of this study was the exclusion of insurance status because of incomplete access for some patients. As a result, potential associations between insurance and survival or extent of disease could not be determined.
“To better understand underlying causes that contribute to the disparity of outcomes in pediatric brain tumors, patient-level data should be utilized in future studies to investigate both biological factors and pre/postdiagnosis treatment gaps in the care of children diagnosed with CNS tumors in the hopes of improving outcomes,” the authors wrote.
In the meantime, collaboration among physicians could help improve outcomes for these patients, according to study author Adam Green, MD, of the University of Colorado at Denver in Aurora.
“[Clinicians] should establish good working relationships with pediatric oncology and neuro-oncology physicians in their community, and they should ask questions early of those teams when they have patients they’re concerned about,” Dr. Green said. “They can [ensure] that patients of minority race/ethnicity, nonprivate health insurance, and lower socioeconomic status have easy and timely access to appointments.”
This research was supported, in part, by grant funding from the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fineberg R et al. Scientific Reports. 2020 Mar 12. doi: 10.1038/s41598-020-61237-2.
according to a study published in Scientific Reports.
While prior studies have shown the effects of racial/ethnic and socioeconomic risk factors on treatment outcomes in adult cancer populations, less is known about how these factors impact children with CNS cancers, explained study author Robert Fineberg, MD, of St. Anthony North Health Campus in Westminster, Colo., and colleagues.
The authors conducted their study to examine the effects of demographic and socioeconomic factors on survival in pediatric CNS cancers. Using data from the Surveillance, Epidemiology, and End Results database, the researchers identified 1,881 patients with CNS tumors, including both spinal and cranial neoplasms.
Data collection encompassed patient characteristics, socioeconomic parameters, tumor characteristics, treatment, and year of diagnosis. The primary outcomes were overall survival and disease stage at diagnosis.
Most patients were white (78.15%) and non-Hispanic (72.09%). The most common brain tumors were gliomas (n = 788), ependymomas (n = 418), and medulloblastomas (n = 393).
On multivariable analysis, the researchers found that black and Hispanic patients had worse survival, compared with white patients (hazard ratio, 1.39; P = .0014) and non-Hispanic patients (HR, 1.36; P = .0002).
After adjustment for socioeconomic parameters and treatment, the hazard ratios for both Hispanic (HR, 1.29; P = .0051) and black patients (HR, 1.29; P = .0206) slightly declined, but the differences remained significant.
On stratified analysis, poorer survival rates were observed for black and Hispanic patients with both metastatic and localized disease at diagnosis, compared with white non-Hispanic patients. However, after adjustment for mediating factors, the difference did not remain significant for black patients (P = .1026).
“Our findings on extent of disease at diagnosis demonstrated that neither black race nor Hispanic ethnicity increased the chance of metastatic disease at presentation when controlling for mediating variables,” the authors wrote. “These data suggest that racial and ethnic disparities appear to be partially explained by postdiagnosis mediating factors that may fall in the pathway between race/ethnicity and poorer survival.”
The researchers acknowledged that a key limitation of this study was the exclusion of insurance status because of incomplete access for some patients. As a result, potential associations between insurance and survival or extent of disease could not be determined.
“To better understand underlying causes that contribute to the disparity of outcomes in pediatric brain tumors, patient-level data should be utilized in future studies to investigate both biological factors and pre/postdiagnosis treatment gaps in the care of children diagnosed with CNS tumors in the hopes of improving outcomes,” the authors wrote.
In the meantime, collaboration among physicians could help improve outcomes for these patients, according to study author Adam Green, MD, of the University of Colorado at Denver in Aurora.
“[Clinicians] should establish good working relationships with pediatric oncology and neuro-oncology physicians in their community, and they should ask questions early of those teams when they have patients they’re concerned about,” Dr. Green said. “They can [ensure] that patients of minority race/ethnicity, nonprivate health insurance, and lower socioeconomic status have easy and timely access to appointments.”
This research was supported, in part, by grant funding from the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fineberg R et al. Scientific Reports. 2020 Mar 12. doi: 10.1038/s41598-020-61237-2.
FROM SCIENTIFIC REPORTS
COVID-19 in pediatric patients: What the hospitalist needs to know
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Dupilumab approval sought for AD under age 12
LAHAINA, HAWAII – Reassuring evidence of the long-term effectiveness and safety of dupilumab in adolescents with moderate to severe atopic dermatitis comes from a phase 3 open-label extension study of the first teenagers in the world to have received the monoclonal antibody, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dupilumab (Dupixent), a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults, received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis (AD) on the strength of a pivotal 251-patient, phase 3 randomized trial of 16 weeks’ duration (JAMA Dermatol. 2019 Nov 6. doi: 10.1001/jamadermatol.2019.3336). But since AD is a chronic disease, it was important to learn how dupilumab performs well beyond the 16-week mark in adolescents, observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
In addition to highlighting some of the emerging fine points of dupilumab therapy in adolescents, Dr. Eichenfield discussed the clinical implications of a potential further expanded indication for treatment of 6- to 12-year-olds, an event he considers likely in the coming months. He also described early data from an ongoing dupilumab clinical trials program in the 2- to 5-year-olds.
Long-term dupilumab in teens
Dr. Eichenfield was a coauthor of the recently published phase 3 international long-term extension study. The 40 participants experienced a mean 85% decrease from baseline at 52 weeks in EASI (Eczema Area and Severity Index) scores on 2 mg/kg per week dosing and an 84% reduction on 4 mg/kg per week dosing. This represented a substantial further improvement from week 2, when the EASI reductions were 34% and 51%, respectively.
The mean trough serum dupilumab concentrations over the course of the year were markedly lower in the 2 mg/kg group: 74 mg/L, as compared to 161 mg/L with dosing at 4 mg/kg per week (Br J Dermatol. 2020 Jan;182[1]:85-96).
“It’ll be interesting to see how this works out over time,” the dermatologist commented. “The issue of dose by weight becomes important as we start to treat younger patients because the pharmacokinetics are very different at 4 and 2 mg/kg, and it may have an impact on efficacy.”
The extension study also established the safety and effectiveness of utilizing dupilumab in combination with standard topical corticosteroid therapy, which wasn’t allowed in the pivotal 16-week trial.
Some have commented that dupilumab may be less effective in adolescents than in adults. They point to the 24% rate of an Investigator Global Assessment (IGA) of 0 or 1 – that is, clear or almost clear – at week 16 in the pivotal adolescent trial, a substantially lower rate than in the adult trials. However, Dr. Eichenfield noted that the adolescent study population was heavily skewed to the severe end of the disease spectrum, the placebo response rate was very low, and the absolute placebo-subtracted benefit turned out to be quite similar to what was seen in the adult trials. Moreover, he added, in a post hoc analysis of the pivotal trial data which utilized a different measure of clinically meaningful response – a composite of either a 50% reduction in EASI score, a 3-point or greater improvement on a 10-point pruritus scale, or at least a 6-point improvement from baseline on the Children’s Dermatology Quality Life Index – that outcome was achieved by 74% of adolescents who didn’t achieve clear or almost clear.
What’s next for dupilumab in pediatric AD
Approval of dupilumab in children under aged 12 years is eagerly awaited, Dr. Eichenfield said. The Food and Drug Administration is now analyzing as-yet unreleased data from completed clinical trials of dupilumab in 6- to 12-year-olds with moderate to severe AD with an eye toward a possible further expanded indication. The side effect profile appears to be the same as in 12- to 18-year-olds.
“I assume it will be approved,” Dr. Eichenfield said. “We don’t know what’s going to happen in 6- to 12-year-olds in terms of the ultimate dosing recommendations that will be put out, but be aware that the pharmacokinetics vary by weight over time.”
Early data in children aged 2-5 years with severe AD from the phase 2, open-label, single ascending dose Liberty AD PRESCHOOL study showed that weight-based dosing in that age group made a big difference in terms of pharmacokinetics. In terms of efficacy, the mean reduction in EASI scores 4 weeks after a single dose of dupilumab was 27% with 3 mg/kg and 49% with 6 mg/kg.
Avoidance of live vaccines while on dupilumab becomes more of a consideration in the under-12 population. The second dose of varicella is supposed to be administered at 4 to 6 years of age, as is the second dose of MMR. The nasal influenza vaccine is a live virus vaccine, as is the yellow fever vaccine.
“We don’t know if live vaccines are dangerous for someone on dupilumab, it’s just that it’s listed that you shouldn’t use them and they haven’t been studied,” Dr. Eichenfield observed.
He reported receiving research grants from or serving as a consultant to several dozen pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the long-term effectiveness and safety of dupilumab in adolescents with moderate to severe atopic dermatitis comes from a phase 3 open-label extension study of the first teenagers in the world to have received the monoclonal antibody, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dupilumab (Dupixent), a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults, received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis (AD) on the strength of a pivotal 251-patient, phase 3 randomized trial of 16 weeks’ duration (JAMA Dermatol. 2019 Nov 6. doi: 10.1001/jamadermatol.2019.3336). But since AD is a chronic disease, it was important to learn how dupilumab performs well beyond the 16-week mark in adolescents, observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
In addition to highlighting some of the emerging fine points of dupilumab therapy in adolescents, Dr. Eichenfield discussed the clinical implications of a potential further expanded indication for treatment of 6- to 12-year-olds, an event he considers likely in the coming months. He also described early data from an ongoing dupilumab clinical trials program in the 2- to 5-year-olds.
Long-term dupilumab in teens
Dr. Eichenfield was a coauthor of the recently published phase 3 international long-term extension study. The 40 participants experienced a mean 85% decrease from baseline at 52 weeks in EASI (Eczema Area and Severity Index) scores on 2 mg/kg per week dosing and an 84% reduction on 4 mg/kg per week dosing. This represented a substantial further improvement from week 2, when the EASI reductions were 34% and 51%, respectively.
The mean trough serum dupilumab concentrations over the course of the year were markedly lower in the 2 mg/kg group: 74 mg/L, as compared to 161 mg/L with dosing at 4 mg/kg per week (Br J Dermatol. 2020 Jan;182[1]:85-96).
“It’ll be interesting to see how this works out over time,” the dermatologist commented. “The issue of dose by weight becomes important as we start to treat younger patients because the pharmacokinetics are very different at 4 and 2 mg/kg, and it may have an impact on efficacy.”
The extension study also established the safety and effectiveness of utilizing dupilumab in combination with standard topical corticosteroid therapy, which wasn’t allowed in the pivotal 16-week trial.
Some have commented that dupilumab may be less effective in adolescents than in adults. They point to the 24% rate of an Investigator Global Assessment (IGA) of 0 or 1 – that is, clear or almost clear – at week 16 in the pivotal adolescent trial, a substantially lower rate than in the adult trials. However, Dr. Eichenfield noted that the adolescent study population was heavily skewed to the severe end of the disease spectrum, the placebo response rate was very low, and the absolute placebo-subtracted benefit turned out to be quite similar to what was seen in the adult trials. Moreover, he added, in a post hoc analysis of the pivotal trial data which utilized a different measure of clinically meaningful response – a composite of either a 50% reduction in EASI score, a 3-point or greater improvement on a 10-point pruritus scale, or at least a 6-point improvement from baseline on the Children’s Dermatology Quality Life Index – that outcome was achieved by 74% of adolescents who didn’t achieve clear or almost clear.
What’s next for dupilumab in pediatric AD
Approval of dupilumab in children under aged 12 years is eagerly awaited, Dr. Eichenfield said. The Food and Drug Administration is now analyzing as-yet unreleased data from completed clinical trials of dupilumab in 6- to 12-year-olds with moderate to severe AD with an eye toward a possible further expanded indication. The side effect profile appears to be the same as in 12- to 18-year-olds.
“I assume it will be approved,” Dr. Eichenfield said. “We don’t know what’s going to happen in 6- to 12-year-olds in terms of the ultimate dosing recommendations that will be put out, but be aware that the pharmacokinetics vary by weight over time.”
Early data in children aged 2-5 years with severe AD from the phase 2, open-label, single ascending dose Liberty AD PRESCHOOL study showed that weight-based dosing in that age group made a big difference in terms of pharmacokinetics. In terms of efficacy, the mean reduction in EASI scores 4 weeks after a single dose of dupilumab was 27% with 3 mg/kg and 49% with 6 mg/kg.
Avoidance of live vaccines while on dupilumab becomes more of a consideration in the under-12 population. The second dose of varicella is supposed to be administered at 4 to 6 years of age, as is the second dose of MMR. The nasal influenza vaccine is a live virus vaccine, as is the yellow fever vaccine.
“We don’t know if live vaccines are dangerous for someone on dupilumab, it’s just that it’s listed that you shouldn’t use them and they haven’t been studied,” Dr. Eichenfield observed.
He reported receiving research grants from or serving as a consultant to several dozen pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Reassuring evidence of the long-term effectiveness and safety of dupilumab in adolescents with moderate to severe atopic dermatitis comes from a phase 3 open-label extension study of the first teenagers in the world to have received the monoclonal antibody, Lawrence F. Eichenfield, MD, reported at the SDEF Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dupilumab (Dupixent), a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults, received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis (AD) on the strength of a pivotal 251-patient, phase 3 randomized trial of 16 weeks’ duration (JAMA Dermatol. 2019 Nov 6. doi: 10.1001/jamadermatol.2019.3336). But since AD is a chronic disease, it was important to learn how dupilumab performs well beyond the 16-week mark in adolescents, observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital.
In addition to highlighting some of the emerging fine points of dupilumab therapy in adolescents, Dr. Eichenfield discussed the clinical implications of a potential further expanded indication for treatment of 6- to 12-year-olds, an event he considers likely in the coming months. He also described early data from an ongoing dupilumab clinical trials program in the 2- to 5-year-olds.
Long-term dupilumab in teens
Dr. Eichenfield was a coauthor of the recently published phase 3 international long-term extension study. The 40 participants experienced a mean 85% decrease from baseline at 52 weeks in EASI (Eczema Area and Severity Index) scores on 2 mg/kg per week dosing and an 84% reduction on 4 mg/kg per week dosing. This represented a substantial further improvement from week 2, when the EASI reductions were 34% and 51%, respectively.
The mean trough serum dupilumab concentrations over the course of the year were markedly lower in the 2 mg/kg group: 74 mg/L, as compared to 161 mg/L with dosing at 4 mg/kg per week (Br J Dermatol. 2020 Jan;182[1]:85-96).
“It’ll be interesting to see how this works out over time,” the dermatologist commented. “The issue of dose by weight becomes important as we start to treat younger patients because the pharmacokinetics are very different at 4 and 2 mg/kg, and it may have an impact on efficacy.”
The extension study also established the safety and effectiveness of utilizing dupilumab in combination with standard topical corticosteroid therapy, which wasn’t allowed in the pivotal 16-week trial.
Some have commented that dupilumab may be less effective in adolescents than in adults. They point to the 24% rate of an Investigator Global Assessment (IGA) of 0 or 1 – that is, clear or almost clear – at week 16 in the pivotal adolescent trial, a substantially lower rate than in the adult trials. However, Dr. Eichenfield noted that the adolescent study population was heavily skewed to the severe end of the disease spectrum, the placebo response rate was very low, and the absolute placebo-subtracted benefit turned out to be quite similar to what was seen in the adult trials. Moreover, he added, in a post hoc analysis of the pivotal trial data which utilized a different measure of clinically meaningful response – a composite of either a 50% reduction in EASI score, a 3-point or greater improvement on a 10-point pruritus scale, or at least a 6-point improvement from baseline on the Children’s Dermatology Quality Life Index – that outcome was achieved by 74% of adolescents who didn’t achieve clear or almost clear.
What’s next for dupilumab in pediatric AD
Approval of dupilumab in children under aged 12 years is eagerly awaited, Dr. Eichenfield said. The Food and Drug Administration is now analyzing as-yet unreleased data from completed clinical trials of dupilumab in 6- to 12-year-olds with moderate to severe AD with an eye toward a possible further expanded indication. The side effect profile appears to be the same as in 12- to 18-year-olds.
“I assume it will be approved,” Dr. Eichenfield said. “We don’t know what’s going to happen in 6- to 12-year-olds in terms of the ultimate dosing recommendations that will be put out, but be aware that the pharmacokinetics vary by weight over time.”
Early data in children aged 2-5 years with severe AD from the phase 2, open-label, single ascending dose Liberty AD PRESCHOOL study showed that weight-based dosing in that age group made a big difference in terms of pharmacokinetics. In terms of efficacy, the mean reduction in EASI scores 4 weeks after a single dose of dupilumab was 27% with 3 mg/kg and 49% with 6 mg/kg.
Avoidance of live vaccines while on dupilumab becomes more of a consideration in the under-12 population. The second dose of varicella is supposed to be administered at 4 to 6 years of age, as is the second dose of MMR. The nasal influenza vaccine is a live virus vaccine, as is the yellow fever vaccine.
“We don’t know if live vaccines are dangerous for someone on dupilumab, it’s just that it’s listed that you shouldn’t use them and they haven’t been studied,” Dr. Eichenfield observed.
He reported receiving research grants from or serving as a consultant to several dozen pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Childhood CV health tied to reduced risk later in life
Two observational studies link better cardiovascular health (CVH) in childhood and midlife to reduced CV mortality and subclinical atherosclerosis in later life. Though many studies have examined CVH and CV mortality in later life, the two studies, published in JAMA Cardiology, examine longitudinal CVH and could inform lifestyle modification.
Together, the studies lend support to the American Heart Association 2010 Strategic Initiative, which put an emphasis on health promotion in children rather than CV disease prevention, Erica Spatz, MD, of Yale University, New Haven, Conn., wrote in an accompanying editorial.
Dr. Spatz pointed out that CV disease prevention can be a tough sell, especially in younger patients for whom the threat of heart disease is distant. These studies and others like them could capture evolving risk factors through patients’ lives, and connect them to current lifestyle and experiences. Such data could overcome barriers to behavioral change and lead to more personalized interventions, she wrote.
Framingham Offspring Study
One study, led by Vanessa Xanthakis, PhD, of Boston University, examined the relationship between the length of time during midlife spent in ideal CVH and various CV disease and mortality outcomes at the final examination.
The prospective study included 1,445 participants (mean age 60 years, 52% women) from a Framingham Heart Study Offspring investigation based in Massachusetts. The subjects had completed seven examinations. The current study ranged from 1991 to 2015, and encompassed the fifth, sixth, and seventh examinations. Researchers calculated CVH scores based on resting blood pressure, height, weight, total cholesterol level, fasting blood glucose level, smoking status, diet, and physical activity.
At the seventh examination, 39% of participants had poor CVH scores and 54% had intermediate scores. For each 5-year period of intermediate or ideal CVH (compared with poor) measured in previous examinations, during the follow-up period after the seventh examination, there was an associated reduction in risk for adverse outcomes including incident hypertension (hazard ratio, 0.67; 95% confidence interval, 056-0.80), diabetes (HR, 0.73; 95% CI, 0.57-0.93), chronic kidney disease (HR, 0.75; 95% CI, 0.63-0.89), CV disease (HR, 0.73; 95% CI, 0.63-0.85), and all-cause mortality (HR, 0.86; 95% CI, 0.76-0.97).
“Our results indicated that living longer in adulthood with better CVH may be potentially beneficial regardless of age because we did not observe statistically significant effect modification by age of the associations between duration in a given CVH score category and any outcome. Overall, our findings support the importance of promoting healthy behaviors throughout the life course,” the authors wrote.
The study was limited by several factors. Diet and physical activity were self-reported, and about half of participants were excluded after missing an examination, which could introduce bias.
International cohort study
The second study analyzed data from 9,388 individuals in five prospective cohorts in the United States and Finland. During 1973-2015, it tracked participants from childhood through middle age (age 8-55 years), linking CVH measures to subclinical atherosclerosis as measured by carotid intima-media thickness (cIMT) in middle age. Led by Norrina Allen, PhD, of the Northwestern University, Chicago, the researchers measured body mass index, total cholesterol level, blood pressure, glucose level, diet, physical activity, and smoking status during a minimum of three examinations. Based on those data, they classified participants as having ideal, intermediate, or poor CVH.
The researchers grouped the participants into five CVH trajectories: High-late decline, which started with high CVH scores at age 8 and maintained them through early adulthood (16%); high-moderate decline (high early scores, moderate decline; 26%); high-early decline (high early scores, early-life decline; 32%); intermediate-late decline (intermediate initial scores, late decline; 16%); and intermediate-early decline (10%). CVH stratification began early: At age 8, 25% of individuals had intermediate CVH scores.
After adjustment for demographics and baseline smoking, diet, and physical activity, the high-late decline CVH group had the smallest mean cIMT value (0.64 mm; 95 % CI, 0.63-0.65 mm), while the intermediate-early decline group, which had the poorest CVH, had the largest (0.72 mm; 95% CI, 0.69-0.76 mm; P less than .001). The relationship was the same even after adjustment for baseline or proximal CVH scores, showing that the trajectory of CVH scores was driving the measure of subclinical atherosclerosis.
“Although it remains important to provide treatment to individuals with elevated risk factor levels, the most effective way to reduce the burden of future CV disease may be to prevent the development of those CV disease risk factors, an approach termed primordial prevention. There is a large body of literature showing effective interventions that may help individuals maintain ideal CV health. Our findings suggest that these interventions are critical and should be implemented early in life to prevent the loss of CVH and future CV [disease] development,” the authors wrote.
The study’s limitations include the fact that analyzed cohorts were drawn from studies with varying protocols and CVH measurement methods. It is also limited by its observational nature.
The two studies were funded by a range of nonindustry sources.
SOURCES: Allen N et al. JAMA Cardiol. 2020 Mar 11. doi: 10.1001/jamacardio.2020.0140; Corlin N et al. JAMA Cardiol. 2020 Mar 11. doi: 10.1001/jamacardio.2020.0109.
Two observational studies link better cardiovascular health (CVH) in childhood and midlife to reduced CV mortality and subclinical atherosclerosis in later life. Though many studies have examined CVH and CV mortality in later life, the two studies, published in JAMA Cardiology, examine longitudinal CVH and could inform lifestyle modification.
Together, the studies lend support to the American Heart Association 2010 Strategic Initiative, which put an emphasis on health promotion in children rather than CV disease prevention, Erica Spatz, MD, of Yale University, New Haven, Conn., wrote in an accompanying editorial.
Dr. Spatz pointed out that CV disease prevention can be a tough sell, especially in younger patients for whom the threat of heart disease is distant. These studies and others like them could capture evolving risk factors through patients’ lives, and connect them to current lifestyle and experiences. Such data could overcome barriers to behavioral change and lead to more personalized interventions, she wrote.
Framingham Offspring Study
One study, led by Vanessa Xanthakis, PhD, of Boston University, examined the relationship between the length of time during midlife spent in ideal CVH and various CV disease and mortality outcomes at the final examination.
The prospective study included 1,445 participants (mean age 60 years, 52% women) from a Framingham Heart Study Offspring investigation based in Massachusetts. The subjects had completed seven examinations. The current study ranged from 1991 to 2015, and encompassed the fifth, sixth, and seventh examinations. Researchers calculated CVH scores based on resting blood pressure, height, weight, total cholesterol level, fasting blood glucose level, smoking status, diet, and physical activity.
At the seventh examination, 39% of participants had poor CVH scores and 54% had intermediate scores. For each 5-year period of intermediate or ideal CVH (compared with poor) measured in previous examinations, during the follow-up period after the seventh examination, there was an associated reduction in risk for adverse outcomes including incident hypertension (hazard ratio, 0.67; 95% confidence interval, 056-0.80), diabetes (HR, 0.73; 95% CI, 0.57-0.93), chronic kidney disease (HR, 0.75; 95% CI, 0.63-0.89), CV disease (HR, 0.73; 95% CI, 0.63-0.85), and all-cause mortality (HR, 0.86; 95% CI, 0.76-0.97).
“Our results indicated that living longer in adulthood with better CVH may be potentially beneficial regardless of age because we did not observe statistically significant effect modification by age of the associations between duration in a given CVH score category and any outcome. Overall, our findings support the importance of promoting healthy behaviors throughout the life course,” the authors wrote.
The study was limited by several factors. Diet and physical activity were self-reported, and about half of participants were excluded after missing an examination, which could introduce bias.
International cohort study
The second study analyzed data from 9,388 individuals in five prospective cohorts in the United States and Finland. During 1973-2015, it tracked participants from childhood through middle age (age 8-55 years), linking CVH measures to subclinical atherosclerosis as measured by carotid intima-media thickness (cIMT) in middle age. Led by Norrina Allen, PhD, of the Northwestern University, Chicago, the researchers measured body mass index, total cholesterol level, blood pressure, glucose level, diet, physical activity, and smoking status during a minimum of three examinations. Based on those data, they classified participants as having ideal, intermediate, or poor CVH.
The researchers grouped the participants into five CVH trajectories: High-late decline, which started with high CVH scores at age 8 and maintained them through early adulthood (16%); high-moderate decline (high early scores, moderate decline; 26%); high-early decline (high early scores, early-life decline; 32%); intermediate-late decline (intermediate initial scores, late decline; 16%); and intermediate-early decline (10%). CVH stratification began early: At age 8, 25% of individuals had intermediate CVH scores.
After adjustment for demographics and baseline smoking, diet, and physical activity, the high-late decline CVH group had the smallest mean cIMT value (0.64 mm; 95 % CI, 0.63-0.65 mm), while the intermediate-early decline group, which had the poorest CVH, had the largest (0.72 mm; 95% CI, 0.69-0.76 mm; P less than .001). The relationship was the same even after adjustment for baseline or proximal CVH scores, showing that the trajectory of CVH scores was driving the measure of subclinical atherosclerosis.
“Although it remains important to provide treatment to individuals with elevated risk factor levels, the most effective way to reduce the burden of future CV disease may be to prevent the development of those CV disease risk factors, an approach termed primordial prevention. There is a large body of literature showing effective interventions that may help individuals maintain ideal CV health. Our findings suggest that these interventions are critical and should be implemented early in life to prevent the loss of CVH and future CV [disease] development,” the authors wrote.
The study’s limitations include the fact that analyzed cohorts were drawn from studies with varying protocols and CVH measurement methods. It is also limited by its observational nature.
The two studies were funded by a range of nonindustry sources.
SOURCES: Allen N et al. JAMA Cardiol. 2020 Mar 11. doi: 10.1001/jamacardio.2020.0140; Corlin N et al. JAMA Cardiol. 2020 Mar 11. doi: 10.1001/jamacardio.2020.0109.
Two observational studies link better cardiovascular health (CVH) in childhood and midlife to reduced CV mortality and subclinical atherosclerosis in later life. Though many studies have examined CVH and CV mortality in later life, the two studies, published in JAMA Cardiology, examine longitudinal CVH and could inform lifestyle modification.
Together, the studies lend support to the American Heart Association 2010 Strategic Initiative, which put an emphasis on health promotion in children rather than CV disease prevention, Erica Spatz, MD, of Yale University, New Haven, Conn., wrote in an accompanying editorial.
Dr. Spatz pointed out that CV disease prevention can be a tough sell, especially in younger patients for whom the threat of heart disease is distant. These studies and others like them could capture evolving risk factors through patients’ lives, and connect them to current lifestyle and experiences. Such data could overcome barriers to behavioral change and lead to more personalized interventions, she wrote.
Framingham Offspring Study
One study, led by Vanessa Xanthakis, PhD, of Boston University, examined the relationship between the length of time during midlife spent in ideal CVH and various CV disease and mortality outcomes at the final examination.
The prospective study included 1,445 participants (mean age 60 years, 52% women) from a Framingham Heart Study Offspring investigation based in Massachusetts. The subjects had completed seven examinations. The current study ranged from 1991 to 2015, and encompassed the fifth, sixth, and seventh examinations. Researchers calculated CVH scores based on resting blood pressure, height, weight, total cholesterol level, fasting blood glucose level, smoking status, diet, and physical activity.
At the seventh examination, 39% of participants had poor CVH scores and 54% had intermediate scores. For each 5-year period of intermediate or ideal CVH (compared with poor) measured in previous examinations, during the follow-up period after the seventh examination, there was an associated reduction in risk for adverse outcomes including incident hypertension (hazard ratio, 0.67; 95% confidence interval, 056-0.80), diabetes (HR, 0.73; 95% CI, 0.57-0.93), chronic kidney disease (HR, 0.75; 95% CI, 0.63-0.89), CV disease (HR, 0.73; 95% CI, 0.63-0.85), and all-cause mortality (HR, 0.86; 95% CI, 0.76-0.97).
“Our results indicated that living longer in adulthood with better CVH may be potentially beneficial regardless of age because we did not observe statistically significant effect modification by age of the associations between duration in a given CVH score category and any outcome. Overall, our findings support the importance of promoting healthy behaviors throughout the life course,” the authors wrote.
The study was limited by several factors. Diet and physical activity were self-reported, and about half of participants were excluded after missing an examination, which could introduce bias.
International cohort study
The second study analyzed data from 9,388 individuals in five prospective cohorts in the United States and Finland. During 1973-2015, it tracked participants from childhood through middle age (age 8-55 years), linking CVH measures to subclinical atherosclerosis as measured by carotid intima-media thickness (cIMT) in middle age. Led by Norrina Allen, PhD, of the Northwestern University, Chicago, the researchers measured body mass index, total cholesterol level, blood pressure, glucose level, diet, physical activity, and smoking status during a minimum of three examinations. Based on those data, they classified participants as having ideal, intermediate, or poor CVH.
The researchers grouped the participants into five CVH trajectories: High-late decline, which started with high CVH scores at age 8 and maintained them through early adulthood (16%); high-moderate decline (high early scores, moderate decline; 26%); high-early decline (high early scores, early-life decline; 32%); intermediate-late decline (intermediate initial scores, late decline; 16%); and intermediate-early decline (10%). CVH stratification began early: At age 8, 25% of individuals had intermediate CVH scores.
After adjustment for demographics and baseline smoking, diet, and physical activity, the high-late decline CVH group had the smallest mean cIMT value (0.64 mm; 95 % CI, 0.63-0.65 mm), while the intermediate-early decline group, which had the poorest CVH, had the largest (0.72 mm; 95% CI, 0.69-0.76 mm; P less than .001). The relationship was the same even after adjustment for baseline or proximal CVH scores, showing that the trajectory of CVH scores was driving the measure of subclinical atherosclerosis.
“Although it remains important to provide treatment to individuals with elevated risk factor levels, the most effective way to reduce the burden of future CV disease may be to prevent the development of those CV disease risk factors, an approach termed primordial prevention. There is a large body of literature showing effective interventions that may help individuals maintain ideal CV health. Our findings suggest that these interventions are critical and should be implemented early in life to prevent the loss of CVH and future CV [disease] development,” the authors wrote.
The study’s limitations include the fact that analyzed cohorts were drawn from studies with varying protocols and CVH measurement methods. It is also limited by its observational nature.
The two studies were funded by a range of nonindustry sources.
SOURCES: Allen N et al. JAMA Cardiol. 2020 Mar 11. doi: 10.1001/jamacardio.2020.0140; Corlin N et al. JAMA Cardiol. 2020 Mar 11. doi: 10.1001/jamacardio.2020.0109.
FROM JAMA CARDIOLOGY



















