User login
Biosimilars and patients: Discussions should address safety, cost, and anxiety about change
Rheumatologist Marcus Snow, MD, is comfortable with prescribing biosimilars as a first-line, first-time biologic, and discussing them with patients.
“If a biosimilar is on the market, it has gone through rigorous study proving its effectiveness and equivalence to a bio-originator,” said Dr. Snow, a rheumatologist with the University of Nebraska Medical Center, Omaha, and chair of the American College of Rheumatology’s Committee on Rheumatologic Care.
The formulary makes a big difference in the conversation about options, he said. “The formularies dictate what we can prescribe. It may not be appropriate, but it is reality. The cost of biologics for a patient without insurance coverage makes it impossible to afford.”
He will often tell patients that he’ll fight any changes or formulary restrictions he does not agree with. “However, when I see patients in follow-up, even if there is no known change on the horizon, I may bring up biosimilars when we have a moment to chat about them to familiarize them with what may happen in the future.”
The need for patient education on biosimilars presents a barrier to realizing their potential to save money and expand choice, noted Cardinal Health in its 2023 biosimilars report. Of 103 rheumatologists who responded to a Cardinal Health survey, 85% agreed that patient education was important. But those conversations can take an uncomfortable turn if the patient pushes back against taking a biosimilar owing to cost or safety concerns.
It’s not uncommon for a patient to express some anxiety about biosimilars, especially if they’re doing well on a current treatment plan. Most patients do not want any changes that may lead to worsening disease control, Dr. Snow said.
Patients and physicians alike often don’t understand the mechanics of biosimilars. “There’s a lot of misinformation about this,” said Sameer Awsare, MD, an associate executive director for The Permanente Medical Group in Campbell, Calif. Patients should know that a biosimilar will be as clinically efficacious as the medicine they’ve been on, with the same safety profiles, said Dr. Awsare, who works with Kaiser Permanente’s pharmacy partners on biosimilars.
Insurance often drives the conversation
The global anti-inflammatory biologics market is anticipated to reach $150 billion by 2027, according to a recent CVS report. As of March 2023, the Food and Drug Administration had approved 40 biosimilars to 11 different reference products. There are 28 on the U.S. market and 100 more in development. Projected to save more than $180 billion over the next 5 years, they are anticipated to expand choice and drive competition.
Rheumatologists, dermatologists, and gastroenterologists are frequent prescribers, although their choices for immune-mediated inflammatory diseases are limited to tumor necrosis factor inhibitors (infliximab [Remicade] originator and adalimumab [Humira] originator) and anti-CD20 agents, such as rituximab (Rituxan) originator.
Benefit design or formulary usually dictates what medicine a patient receives. “Because of significantly higher out-of-pocket cost or formulary positioning, patients may end up with a generic or a biosimilar instead of a brand-name medicine or branded biologic,” said Robert Popovian, PharmD, MS, chief science policy officer of the Global Healthy Living Foundation.
Insurers rarely offer both Remicade and biosimilar infliximab, allowing the doctor to choose, said Miguel Regueiro, MD, chair of the Cleveland Clinic’s Digestive Disease & Surgery Institute, who prescribes infliximab biosimilars. Most often, the payer will choose the lower-cost biosimilar. “I am fine with the biosimilar, either as a new start or a switch from the reference product.”
However, the patient might feel differently. They can form an attachment to the reference medication if it has prevented severe illness. “They do not want to change, as they feel they are going on a ‘new’ medication that will not work as well,” Dr. Regueiro said.
This is where the education comes in: to reassure patients that a biosimilar will work just as well as the reference product. “For patients who have done well for years on a biologic, more time needs to be spent reassuring them and answering questions,” compared with a patient just starting on a biosimilar, he advised.
But not all physicians are quick to prescribe biosimilars.
Especially with psoriasis, which has so many strong options for reference drugs, a switch may be hard to justify, said dermatologist Stephanie K. Fabbro, MD, assistant professor at Northeast Ohio Medical University, Rootstown. “If I have a preference, I would rather switch a patient to a drug from a different class without a biosimilar option to reduce the possibility of pushback.”
Dr. Fabbro, part of the core faculty in the Riverside Methodist Hospital Dermatology Residency Program in Columbus, will share data from clinical trials and postmarket surveillance with patients to support her decision.
Conversations about cost
Patients may also push back if they don’t save money when switching to a biosimilar. “This dilemma raises the question of who is profiting when a biosimilar is dispensed,” Dr. Popovian said. Insurers and pharmacy benefit managers (PBMs) that take additional concessions from biopharmaceutical manufacturers in the form of rebates and fees will often pocket this money as profit instead of passing savings back to the patient to help reduce their out-of-pocket requirement, he added.
If an originator biologic and a biosimilar are available, “as a pharmacist, I will choose the medicine that will incur the lowest out-of-pocket cost for the patient,” Dr. Popovian said.
Discussing cost – and who dictates which biosimilar is on the formulary – is an important conversation to have with patients, said Vivek Kaul, MD, Segal-Watson Professor of Medicine at the University of Rochester (N.Y.) Medical Center.
Providing equivalent clinical efficacy while saving costs is the economic reality of biosimilars, Dr. Kaul said. Third-party payers regularly evaluate how to provide the same quality of care while saving money. Physicians and patients alike “must be mindful that as time goes on, if the science on biosimilars stays robust, if the adoption is more widespread and the cost-saving proposition turns out to be true, more formularies will be attracted to replacing the reference product with the biosimilar counterpart.”
Providers and patients can weigh the options if a formulary suddenly switches to a biosimilar, Dr. Kaul continued. “You can accept the novel product on the formulary or may have to face out-of-pocket expenses as a patient.” If providers and patients have concerns about the biosimilar, they can always appeal if there’s solid scientific evidence that supports reverting back to the reference product.
“If you think the biosimilar is equally efficacious, comes at a lower cost, and is right for the patient, then the providers should tell the patient that,” he added.
Some studies have questioned whether the biosimilars will save money, compared with the reference drug, Dr. Fabbro noted. Medicare, for example, may pay only for a certain percentage of an approved biosimilar, saddling the patient with a monthly copay costing thousands of dollars. “It is unclear whether biosimilar manufacturers will have the same level of patient support programs as the reference drug companies.”
For that reason, physicians should also inform patients about the robust patient assistance and copay assistance programs many reference drug manufacturers offer, she said.
Biosimilars 101: Familiarizing patients
Safety and ease of use are other common concerns about biosimilars. Patients may ask if the application is different, or why it’s advantageous to switch to a biosimilar, Dr. Awsare said.
Sometimes the syringe or injector for a biosimilar might look different from that of the originator drug, he said.
Anecdotally, Dr. Fabbro has heard stories of patients having injection reactions that they did not experience with the reference drug or having a disease flare-up after starting a biosimilar.
As is the case with reference products, in their conversations with patients, clinicians should address the adverse event profile of biosimilars, offering data points from published studies and clinical guidelines that support the use of these products. “There should be an emphasis on patient education around efficacy and any side effects, and how the profile of the reference product compares with a proposed biosimilar,” Dr. Kaul suggested.
When Dr. Snow discusses biosimilars and generics, “I make sure to share this in an understandable way based on the patient’s scientific background, or lack thereof,” he said. If there is enough time, he also discusses how European- and U.S.-sourced biologics are slightly different.
Pharmacists should tell patients to expect the same clinical outcomes from a biosimilar, Dr. Popovian said. However, if they have any reduction in efficacy or potential safety concerns, they should communicate with their physician or pharmacist immediately.
In Dr. Regueiro’s practice, a pharmacist specializing in inflammatory bowel disease often has a one-on-one meeting with patients to educate and answer questions. “Additionally, we provide them the Crohn’s and Colitis Foundation web link on biosimilars,” said Dr. Regueiro.
A village approach to education
When biosimilars first came out, there were no formal education materials, Dr. Awsare said. Kaiser Permanente decided to create its own educational materials, not just for patients but also to help educate its primary care doctors; the rheumatologists, dermatologists, and gastroenterologists using the biosimilars; the nurses infusing patients; and the pharmacists preparing the biosimilars.
The health system also has a different approach to choosing medication. Instead of having an insurance company or PBM decide what’s in the formulary, clinicians work with the pharmacists at Kaiser to look at clinical evidence and decide which biosimilar to use. Most of its plans also provide lower copays to patients when they use the biosimilar.
This was the approach for Humira biosimilars, Dr. Awsare said. Eight will be on the market in 2023. “Our rheumatologists, dermatologists, and gastroenterologists looked at the data from Europe, looked at some real-world evidence, and then said: ‘We think this one’s going to be the best one for our patients.’ ”
Having clinicians choose the biosimilar instead of a health plan makes it a lot easier to have conversations with patients, he said. “Once we’ve moved that market share to that particular biosimilar, we give our physicians the time to have those discussions.”
Clinical pharmacists also provide educational support, offering guidance on issues such as side effects, as patients transition to the biosimilar. “We like to use the word ‘transition’ because it’s essentially the same biologic. So, you’re not actually switching,” Dr. Awsare said.
No consensus on interchangeability
Whether the conversation on interchangeability will affect patient conversations with physicians depends on who you ask.
If a biosimilar has an interchangeability designation, it means that the pharmacist can substitute it without the intervention of the clinician who prescribed the reference product. It does not relate to the quality, safety, or effectiveness of biosimilars or interchangeable biosimilar products, Dr. Popovian said.
The United States is the only country that has this designation. Even though it’s not identical to the originator drug, a biosimilar has the same clinical efficacy and safety profile. “So clinically, interchangeability is meaningless,” Dr. Awsare said.
In its report on biosimilars in the autoimmune category, CVS acknowledged that interchangeability was important but would not be a significant factor in driving adoption of biosimilars. However, in a Cardinal Health survey of 72 gastroenterologists, 38% cited the interchangeability of biosimilars as a top concern for adalimumab biosimilars, along with transitioning patients from Humira to a biosimilar (44%).
“Patient education regarding biosimilar safety, efficacy, and interchangeability appears paramount to the acceptance of these products, particularly for patients who are switched from a reference product,” Dr. Kaul noted in the Cardinal Health report.
Wherever supported by data, Dr. Kaul recommends incorporating biosimilar use and interchangeability into best practice guidelines going forward. “That will go a long way in disseminating the latest information on this topic and position this paradigm for increased adoption among providers.”
Some physicians like Dr. Snow aren’t that concerned with interchangeability. This hasn’t affected conversations with patients, he said. Multiple studies demonstrating the lack of antibody formation with multiple switches from different biosimilar drugs has eased his concern about multiple switches causing problems.
“Initially, there was a gap in demonstrating the long-term effect of multiple switches on antibody production and drug effectiveness. That gap has started to close as more data from Europe’s experience with biosimilars becomes available,” Dr. Snow said.
Resources for physicians, patients
The federal government has taken steps to advance biosimilars education and adoption. In 2021, President Biden signed the Advancing Education on Biosimilars Act into law, which directs the FDA to develop or improve continuing education programs that address prescribing of biosimilars and biological products.
The FDA provides educational materials on its website, including a comprehensive curriculum toolkit. The Accreditation Council for Medical Affairs has also created an online 40-hour curriculum for health care professionals called the Board-Certified Biologics and Biosimilars Specialist Program.
Dr. Fabbro recommended patients use the FDA page Biosimilar Basics for Patients to educate themselves on biosimilars. The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars, is another free resource for patients.
“While much has changed, the continued need for multistakeholder education, awareness, and dedicated research remains even more important as we expand into newer therapeutic areas and classes,” wrote the authors of the Cardinal Health report.
Help patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars.
Dr. Regueiro is on advisory boards and consults for AbbVie, Janssen, UCB, Takeda, Pfizer, Bristol-Myers Squibb, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET PharmaSolutions, Trellis, and Boehringer Ingelheim. Dr. Fabbro is a principal investigator for Castle Biosciences, on the speakers bureau for Valchlor, and on the advisory boards of Janssen and Bristol-Myers Squibb. Dr. Popovian, Dr. Snow, Dr. Awsare, and Dr. Kaul had no disclosures.
A version of this article originally appeared on Medscape.com.
Rheumatologist Marcus Snow, MD, is comfortable with prescribing biosimilars as a first-line, first-time biologic, and discussing them with patients.
“If a biosimilar is on the market, it has gone through rigorous study proving its effectiveness and equivalence to a bio-originator,” said Dr. Snow, a rheumatologist with the University of Nebraska Medical Center, Omaha, and chair of the American College of Rheumatology’s Committee on Rheumatologic Care.
The formulary makes a big difference in the conversation about options, he said. “The formularies dictate what we can prescribe. It may not be appropriate, but it is reality. The cost of biologics for a patient without insurance coverage makes it impossible to afford.”
He will often tell patients that he’ll fight any changes or formulary restrictions he does not agree with. “However, when I see patients in follow-up, even if there is no known change on the horizon, I may bring up biosimilars when we have a moment to chat about them to familiarize them with what may happen in the future.”
The need for patient education on biosimilars presents a barrier to realizing their potential to save money and expand choice, noted Cardinal Health in its 2023 biosimilars report. Of 103 rheumatologists who responded to a Cardinal Health survey, 85% agreed that patient education was important. But those conversations can take an uncomfortable turn if the patient pushes back against taking a biosimilar owing to cost or safety concerns.
It’s not uncommon for a patient to express some anxiety about biosimilars, especially if they’re doing well on a current treatment plan. Most patients do not want any changes that may lead to worsening disease control, Dr. Snow said.
Patients and physicians alike often don’t understand the mechanics of biosimilars. “There’s a lot of misinformation about this,” said Sameer Awsare, MD, an associate executive director for The Permanente Medical Group in Campbell, Calif. Patients should know that a biosimilar will be as clinically efficacious as the medicine they’ve been on, with the same safety profiles, said Dr. Awsare, who works with Kaiser Permanente’s pharmacy partners on biosimilars.
Insurance often drives the conversation
The global anti-inflammatory biologics market is anticipated to reach $150 billion by 2027, according to a recent CVS report. As of March 2023, the Food and Drug Administration had approved 40 biosimilars to 11 different reference products. There are 28 on the U.S. market and 100 more in development. Projected to save more than $180 billion over the next 5 years, they are anticipated to expand choice and drive competition.
Rheumatologists, dermatologists, and gastroenterologists are frequent prescribers, although their choices for immune-mediated inflammatory diseases are limited to tumor necrosis factor inhibitors (infliximab [Remicade] originator and adalimumab [Humira] originator) and anti-CD20 agents, such as rituximab (Rituxan) originator.
Benefit design or formulary usually dictates what medicine a patient receives. “Because of significantly higher out-of-pocket cost or formulary positioning, patients may end up with a generic or a biosimilar instead of a brand-name medicine or branded biologic,” said Robert Popovian, PharmD, MS, chief science policy officer of the Global Healthy Living Foundation.
Insurers rarely offer both Remicade and biosimilar infliximab, allowing the doctor to choose, said Miguel Regueiro, MD, chair of the Cleveland Clinic’s Digestive Disease & Surgery Institute, who prescribes infliximab biosimilars. Most often, the payer will choose the lower-cost biosimilar. “I am fine with the biosimilar, either as a new start or a switch from the reference product.”
However, the patient might feel differently. They can form an attachment to the reference medication if it has prevented severe illness. “They do not want to change, as they feel they are going on a ‘new’ medication that will not work as well,” Dr. Regueiro said.
This is where the education comes in: to reassure patients that a biosimilar will work just as well as the reference product. “For patients who have done well for years on a biologic, more time needs to be spent reassuring them and answering questions,” compared with a patient just starting on a biosimilar, he advised.
But not all physicians are quick to prescribe biosimilars.
Especially with psoriasis, which has so many strong options for reference drugs, a switch may be hard to justify, said dermatologist Stephanie K. Fabbro, MD, assistant professor at Northeast Ohio Medical University, Rootstown. “If I have a preference, I would rather switch a patient to a drug from a different class without a biosimilar option to reduce the possibility of pushback.”
Dr. Fabbro, part of the core faculty in the Riverside Methodist Hospital Dermatology Residency Program in Columbus, will share data from clinical trials and postmarket surveillance with patients to support her decision.
Conversations about cost
Patients may also push back if they don’t save money when switching to a biosimilar. “This dilemma raises the question of who is profiting when a biosimilar is dispensed,” Dr. Popovian said. Insurers and pharmacy benefit managers (PBMs) that take additional concessions from biopharmaceutical manufacturers in the form of rebates and fees will often pocket this money as profit instead of passing savings back to the patient to help reduce their out-of-pocket requirement, he added.
If an originator biologic and a biosimilar are available, “as a pharmacist, I will choose the medicine that will incur the lowest out-of-pocket cost for the patient,” Dr. Popovian said.
Discussing cost – and who dictates which biosimilar is on the formulary – is an important conversation to have with patients, said Vivek Kaul, MD, Segal-Watson Professor of Medicine at the University of Rochester (N.Y.) Medical Center.
Providing equivalent clinical efficacy while saving costs is the economic reality of biosimilars, Dr. Kaul said. Third-party payers regularly evaluate how to provide the same quality of care while saving money. Physicians and patients alike “must be mindful that as time goes on, if the science on biosimilars stays robust, if the adoption is more widespread and the cost-saving proposition turns out to be true, more formularies will be attracted to replacing the reference product with the biosimilar counterpart.”
Providers and patients can weigh the options if a formulary suddenly switches to a biosimilar, Dr. Kaul continued. “You can accept the novel product on the formulary or may have to face out-of-pocket expenses as a patient.” If providers and patients have concerns about the biosimilar, they can always appeal if there’s solid scientific evidence that supports reverting back to the reference product.
“If you think the biosimilar is equally efficacious, comes at a lower cost, and is right for the patient, then the providers should tell the patient that,” he added.
Some studies have questioned whether the biosimilars will save money, compared with the reference drug, Dr. Fabbro noted. Medicare, for example, may pay only for a certain percentage of an approved biosimilar, saddling the patient with a monthly copay costing thousands of dollars. “It is unclear whether biosimilar manufacturers will have the same level of patient support programs as the reference drug companies.”
For that reason, physicians should also inform patients about the robust patient assistance and copay assistance programs many reference drug manufacturers offer, she said.
Biosimilars 101: Familiarizing patients
Safety and ease of use are other common concerns about biosimilars. Patients may ask if the application is different, or why it’s advantageous to switch to a biosimilar, Dr. Awsare said.
Sometimes the syringe or injector for a biosimilar might look different from that of the originator drug, he said.
Anecdotally, Dr. Fabbro has heard stories of patients having injection reactions that they did not experience with the reference drug or having a disease flare-up after starting a biosimilar.
As is the case with reference products, in their conversations with patients, clinicians should address the adverse event profile of biosimilars, offering data points from published studies and clinical guidelines that support the use of these products. “There should be an emphasis on patient education around efficacy and any side effects, and how the profile of the reference product compares with a proposed biosimilar,” Dr. Kaul suggested.
When Dr. Snow discusses biosimilars and generics, “I make sure to share this in an understandable way based on the patient’s scientific background, or lack thereof,” he said. If there is enough time, he also discusses how European- and U.S.-sourced biologics are slightly different.
Pharmacists should tell patients to expect the same clinical outcomes from a biosimilar, Dr. Popovian said. However, if they have any reduction in efficacy or potential safety concerns, they should communicate with their physician or pharmacist immediately.
In Dr. Regueiro’s practice, a pharmacist specializing in inflammatory bowel disease often has a one-on-one meeting with patients to educate and answer questions. “Additionally, we provide them the Crohn’s and Colitis Foundation web link on biosimilars,” said Dr. Regueiro.
A village approach to education
When biosimilars first came out, there were no formal education materials, Dr. Awsare said. Kaiser Permanente decided to create its own educational materials, not just for patients but also to help educate its primary care doctors; the rheumatologists, dermatologists, and gastroenterologists using the biosimilars; the nurses infusing patients; and the pharmacists preparing the biosimilars.
The health system also has a different approach to choosing medication. Instead of having an insurance company or PBM decide what’s in the formulary, clinicians work with the pharmacists at Kaiser to look at clinical evidence and decide which biosimilar to use. Most of its plans also provide lower copays to patients when they use the biosimilar.
This was the approach for Humira biosimilars, Dr. Awsare said. Eight will be on the market in 2023. “Our rheumatologists, dermatologists, and gastroenterologists looked at the data from Europe, looked at some real-world evidence, and then said: ‘We think this one’s going to be the best one for our patients.’ ”
Having clinicians choose the biosimilar instead of a health plan makes it a lot easier to have conversations with patients, he said. “Once we’ve moved that market share to that particular biosimilar, we give our physicians the time to have those discussions.”
Clinical pharmacists also provide educational support, offering guidance on issues such as side effects, as patients transition to the biosimilar. “We like to use the word ‘transition’ because it’s essentially the same biologic. So, you’re not actually switching,” Dr. Awsare said.
No consensus on interchangeability
Whether the conversation on interchangeability will affect patient conversations with physicians depends on who you ask.
If a biosimilar has an interchangeability designation, it means that the pharmacist can substitute it without the intervention of the clinician who prescribed the reference product. It does not relate to the quality, safety, or effectiveness of biosimilars or interchangeable biosimilar products, Dr. Popovian said.
The United States is the only country that has this designation. Even though it’s not identical to the originator drug, a biosimilar has the same clinical efficacy and safety profile. “So clinically, interchangeability is meaningless,” Dr. Awsare said.
In its report on biosimilars in the autoimmune category, CVS acknowledged that interchangeability was important but would not be a significant factor in driving adoption of biosimilars. However, in a Cardinal Health survey of 72 gastroenterologists, 38% cited the interchangeability of biosimilars as a top concern for adalimumab biosimilars, along with transitioning patients from Humira to a biosimilar (44%).
“Patient education regarding biosimilar safety, efficacy, and interchangeability appears paramount to the acceptance of these products, particularly for patients who are switched from a reference product,” Dr. Kaul noted in the Cardinal Health report.
Wherever supported by data, Dr. Kaul recommends incorporating biosimilar use and interchangeability into best practice guidelines going forward. “That will go a long way in disseminating the latest information on this topic and position this paradigm for increased adoption among providers.”
Some physicians like Dr. Snow aren’t that concerned with interchangeability. This hasn’t affected conversations with patients, he said. Multiple studies demonstrating the lack of antibody formation with multiple switches from different biosimilar drugs has eased his concern about multiple switches causing problems.
“Initially, there was a gap in demonstrating the long-term effect of multiple switches on antibody production and drug effectiveness. That gap has started to close as more data from Europe’s experience with biosimilars becomes available,” Dr. Snow said.
Resources for physicians, patients
The federal government has taken steps to advance biosimilars education and adoption. In 2021, President Biden signed the Advancing Education on Biosimilars Act into law, which directs the FDA to develop or improve continuing education programs that address prescribing of biosimilars and biological products.
The FDA provides educational materials on its website, including a comprehensive curriculum toolkit. The Accreditation Council for Medical Affairs has also created an online 40-hour curriculum for health care professionals called the Board-Certified Biologics and Biosimilars Specialist Program.
Dr. Fabbro recommended patients use the FDA page Biosimilar Basics for Patients to educate themselves on biosimilars. The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars, is another free resource for patients.
“While much has changed, the continued need for multistakeholder education, awareness, and dedicated research remains even more important as we expand into newer therapeutic areas and classes,” wrote the authors of the Cardinal Health report.
Help patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars.
Dr. Regueiro is on advisory boards and consults for AbbVie, Janssen, UCB, Takeda, Pfizer, Bristol-Myers Squibb, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET PharmaSolutions, Trellis, and Boehringer Ingelheim. Dr. Fabbro is a principal investigator for Castle Biosciences, on the speakers bureau for Valchlor, and on the advisory boards of Janssen and Bristol-Myers Squibb. Dr. Popovian, Dr. Snow, Dr. Awsare, and Dr. Kaul had no disclosures.
A version of this article originally appeared on Medscape.com.
Rheumatologist Marcus Snow, MD, is comfortable with prescribing biosimilars as a first-line, first-time biologic, and discussing them with patients.
“If a biosimilar is on the market, it has gone through rigorous study proving its effectiveness and equivalence to a bio-originator,” said Dr. Snow, a rheumatologist with the University of Nebraska Medical Center, Omaha, and chair of the American College of Rheumatology’s Committee on Rheumatologic Care.
The formulary makes a big difference in the conversation about options, he said. “The formularies dictate what we can prescribe. It may not be appropriate, but it is reality. The cost of biologics for a patient without insurance coverage makes it impossible to afford.”
He will often tell patients that he’ll fight any changes or formulary restrictions he does not agree with. “However, when I see patients in follow-up, even if there is no known change on the horizon, I may bring up biosimilars when we have a moment to chat about them to familiarize them with what may happen in the future.”
The need for patient education on biosimilars presents a barrier to realizing their potential to save money and expand choice, noted Cardinal Health in its 2023 biosimilars report. Of 103 rheumatologists who responded to a Cardinal Health survey, 85% agreed that patient education was important. But those conversations can take an uncomfortable turn if the patient pushes back against taking a biosimilar owing to cost or safety concerns.
It’s not uncommon for a patient to express some anxiety about biosimilars, especially if they’re doing well on a current treatment plan. Most patients do not want any changes that may lead to worsening disease control, Dr. Snow said.
Patients and physicians alike often don’t understand the mechanics of biosimilars. “There’s a lot of misinformation about this,” said Sameer Awsare, MD, an associate executive director for The Permanente Medical Group in Campbell, Calif. Patients should know that a biosimilar will be as clinically efficacious as the medicine they’ve been on, with the same safety profiles, said Dr. Awsare, who works with Kaiser Permanente’s pharmacy partners on biosimilars.
Insurance often drives the conversation
The global anti-inflammatory biologics market is anticipated to reach $150 billion by 2027, according to a recent CVS report. As of March 2023, the Food and Drug Administration had approved 40 biosimilars to 11 different reference products. There are 28 on the U.S. market and 100 more in development. Projected to save more than $180 billion over the next 5 years, they are anticipated to expand choice and drive competition.
Rheumatologists, dermatologists, and gastroenterologists are frequent prescribers, although their choices for immune-mediated inflammatory diseases are limited to tumor necrosis factor inhibitors (infliximab [Remicade] originator and adalimumab [Humira] originator) and anti-CD20 agents, such as rituximab (Rituxan) originator.
Benefit design or formulary usually dictates what medicine a patient receives. “Because of significantly higher out-of-pocket cost or formulary positioning, patients may end up with a generic or a biosimilar instead of a brand-name medicine or branded biologic,” said Robert Popovian, PharmD, MS, chief science policy officer of the Global Healthy Living Foundation.
Insurers rarely offer both Remicade and biosimilar infliximab, allowing the doctor to choose, said Miguel Regueiro, MD, chair of the Cleveland Clinic’s Digestive Disease & Surgery Institute, who prescribes infliximab biosimilars. Most often, the payer will choose the lower-cost biosimilar. “I am fine with the biosimilar, either as a new start or a switch from the reference product.”
However, the patient might feel differently. They can form an attachment to the reference medication if it has prevented severe illness. “They do not want to change, as they feel they are going on a ‘new’ medication that will not work as well,” Dr. Regueiro said.
This is where the education comes in: to reassure patients that a biosimilar will work just as well as the reference product. “For patients who have done well for years on a biologic, more time needs to be spent reassuring them and answering questions,” compared with a patient just starting on a biosimilar, he advised.
But not all physicians are quick to prescribe biosimilars.
Especially with psoriasis, which has so many strong options for reference drugs, a switch may be hard to justify, said dermatologist Stephanie K. Fabbro, MD, assistant professor at Northeast Ohio Medical University, Rootstown. “If I have a preference, I would rather switch a patient to a drug from a different class without a biosimilar option to reduce the possibility of pushback.”
Dr. Fabbro, part of the core faculty in the Riverside Methodist Hospital Dermatology Residency Program in Columbus, will share data from clinical trials and postmarket surveillance with patients to support her decision.
Conversations about cost
Patients may also push back if they don’t save money when switching to a biosimilar. “This dilemma raises the question of who is profiting when a biosimilar is dispensed,” Dr. Popovian said. Insurers and pharmacy benefit managers (PBMs) that take additional concessions from biopharmaceutical manufacturers in the form of rebates and fees will often pocket this money as profit instead of passing savings back to the patient to help reduce their out-of-pocket requirement, he added.
If an originator biologic and a biosimilar are available, “as a pharmacist, I will choose the medicine that will incur the lowest out-of-pocket cost for the patient,” Dr. Popovian said.
Discussing cost – and who dictates which biosimilar is on the formulary – is an important conversation to have with patients, said Vivek Kaul, MD, Segal-Watson Professor of Medicine at the University of Rochester (N.Y.) Medical Center.
Providing equivalent clinical efficacy while saving costs is the economic reality of biosimilars, Dr. Kaul said. Third-party payers regularly evaluate how to provide the same quality of care while saving money. Physicians and patients alike “must be mindful that as time goes on, if the science on biosimilars stays robust, if the adoption is more widespread and the cost-saving proposition turns out to be true, more formularies will be attracted to replacing the reference product with the biosimilar counterpart.”
Providers and patients can weigh the options if a formulary suddenly switches to a biosimilar, Dr. Kaul continued. “You can accept the novel product on the formulary or may have to face out-of-pocket expenses as a patient.” If providers and patients have concerns about the biosimilar, they can always appeal if there’s solid scientific evidence that supports reverting back to the reference product.
“If you think the biosimilar is equally efficacious, comes at a lower cost, and is right for the patient, then the providers should tell the patient that,” he added.
Some studies have questioned whether the biosimilars will save money, compared with the reference drug, Dr. Fabbro noted. Medicare, for example, may pay only for a certain percentage of an approved biosimilar, saddling the patient with a monthly copay costing thousands of dollars. “It is unclear whether biosimilar manufacturers will have the same level of patient support programs as the reference drug companies.”
For that reason, physicians should also inform patients about the robust patient assistance and copay assistance programs many reference drug manufacturers offer, she said.
Biosimilars 101: Familiarizing patients
Safety and ease of use are other common concerns about biosimilars. Patients may ask if the application is different, or why it’s advantageous to switch to a biosimilar, Dr. Awsare said.
Sometimes the syringe or injector for a biosimilar might look different from that of the originator drug, he said.
Anecdotally, Dr. Fabbro has heard stories of patients having injection reactions that they did not experience with the reference drug or having a disease flare-up after starting a biosimilar.
As is the case with reference products, in their conversations with patients, clinicians should address the adverse event profile of biosimilars, offering data points from published studies and clinical guidelines that support the use of these products. “There should be an emphasis on patient education around efficacy and any side effects, and how the profile of the reference product compares with a proposed biosimilar,” Dr. Kaul suggested.
When Dr. Snow discusses biosimilars and generics, “I make sure to share this in an understandable way based on the patient’s scientific background, or lack thereof,” he said. If there is enough time, he also discusses how European- and U.S.-sourced biologics are slightly different.
Pharmacists should tell patients to expect the same clinical outcomes from a biosimilar, Dr. Popovian said. However, if they have any reduction in efficacy or potential safety concerns, they should communicate with their physician or pharmacist immediately.
In Dr. Regueiro’s practice, a pharmacist specializing in inflammatory bowel disease often has a one-on-one meeting with patients to educate and answer questions. “Additionally, we provide them the Crohn’s and Colitis Foundation web link on biosimilars,” said Dr. Regueiro.
A village approach to education
When biosimilars first came out, there were no formal education materials, Dr. Awsare said. Kaiser Permanente decided to create its own educational materials, not just for patients but also to help educate its primary care doctors; the rheumatologists, dermatologists, and gastroenterologists using the biosimilars; the nurses infusing patients; and the pharmacists preparing the biosimilars.
The health system also has a different approach to choosing medication. Instead of having an insurance company or PBM decide what’s in the formulary, clinicians work with the pharmacists at Kaiser to look at clinical evidence and decide which biosimilar to use. Most of its plans also provide lower copays to patients when they use the biosimilar.
This was the approach for Humira biosimilars, Dr. Awsare said. Eight will be on the market in 2023. “Our rheumatologists, dermatologists, and gastroenterologists looked at the data from Europe, looked at some real-world evidence, and then said: ‘We think this one’s going to be the best one for our patients.’ ”
Having clinicians choose the biosimilar instead of a health plan makes it a lot easier to have conversations with patients, he said. “Once we’ve moved that market share to that particular biosimilar, we give our physicians the time to have those discussions.”
Clinical pharmacists also provide educational support, offering guidance on issues such as side effects, as patients transition to the biosimilar. “We like to use the word ‘transition’ because it’s essentially the same biologic. So, you’re not actually switching,” Dr. Awsare said.
No consensus on interchangeability
Whether the conversation on interchangeability will affect patient conversations with physicians depends on who you ask.
If a biosimilar has an interchangeability designation, it means that the pharmacist can substitute it without the intervention of the clinician who prescribed the reference product. It does not relate to the quality, safety, or effectiveness of biosimilars or interchangeable biosimilar products, Dr. Popovian said.
The United States is the only country that has this designation. Even though it’s not identical to the originator drug, a biosimilar has the same clinical efficacy and safety profile. “So clinically, interchangeability is meaningless,” Dr. Awsare said.
In its report on biosimilars in the autoimmune category, CVS acknowledged that interchangeability was important but would not be a significant factor in driving adoption of biosimilars. However, in a Cardinal Health survey of 72 gastroenterologists, 38% cited the interchangeability of biosimilars as a top concern for adalimumab biosimilars, along with transitioning patients from Humira to a biosimilar (44%).
“Patient education regarding biosimilar safety, efficacy, and interchangeability appears paramount to the acceptance of these products, particularly for patients who are switched from a reference product,” Dr. Kaul noted in the Cardinal Health report.
Wherever supported by data, Dr. Kaul recommends incorporating biosimilar use and interchangeability into best practice guidelines going forward. “That will go a long way in disseminating the latest information on this topic and position this paradigm for increased adoption among providers.”
Some physicians like Dr. Snow aren’t that concerned with interchangeability. This hasn’t affected conversations with patients, he said. Multiple studies demonstrating the lack of antibody formation with multiple switches from different biosimilar drugs has eased his concern about multiple switches causing problems.
“Initially, there was a gap in demonstrating the long-term effect of multiple switches on antibody production and drug effectiveness. That gap has started to close as more data from Europe’s experience with biosimilars becomes available,” Dr. Snow said.
Resources for physicians, patients
The federal government has taken steps to advance biosimilars education and adoption. In 2021, President Biden signed the Advancing Education on Biosimilars Act into law, which directs the FDA to develop or improve continuing education programs that address prescribing of biosimilars and biological products.
The FDA provides educational materials on its website, including a comprehensive curriculum toolkit. The Accreditation Council for Medical Affairs has also created an online 40-hour curriculum for health care professionals called the Board-Certified Biologics and Biosimilars Specialist Program.
Dr. Fabbro recommended patients use the FDA page Biosimilar Basics for Patients to educate themselves on biosimilars. The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars, is another free resource for patients.
“While much has changed, the continued need for multistakeholder education, awareness, and dedicated research remains even more important as we expand into newer therapeutic areas and classes,” wrote the authors of the Cardinal Health report.
Help patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars.
Dr. Regueiro is on advisory boards and consults for AbbVie, Janssen, UCB, Takeda, Pfizer, Bristol-Myers Squibb, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET PharmaSolutions, Trellis, and Boehringer Ingelheim. Dr. Fabbro is a principal investigator for Castle Biosciences, on the speakers bureau for Valchlor, and on the advisory boards of Janssen and Bristol-Myers Squibb. Dr. Popovian, Dr. Snow, Dr. Awsare, and Dr. Kaul had no disclosures.
A version of this article originally appeared on Medscape.com.
FDA approves new formulation of Hyrimoz adalimumab biosimilar
The Food and Drug Administration has approved a citrate-free, 100 mg/mL formulation of the biosimilar adalimumab-adaz (Hyrimoz), according to a statement from manufacturer Sandoz.
Hyrimoz, a tumor necrosis factor (TNF) blocker that is biosimilar to its reference product Humira, was approved by the FDA in 2018 at a concentration of 50 mg/mL for rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The high-concentration formula is indicated for these same conditions.
Sandoz said that it intends to launch the citrate-free formulation in the United States on July 1. It will be one of up to nine other adalimumab biosimilars that are expected to launch in July. On January 31, Amjevita (adalimumab-atto) became the first adalimumab biosimilar to launch in the United States.
The current label for Hyrimoz contains a black box warning emphasizing certain risks, notably the increased risk for serious infections, such as tuberculosis or sepsis, and an increased risk of malignancy, particularly lymphomas.
Adverse effects associated with Hyrimoz with an incidence greater than 10% include upper respiratory infections and sinusitis, injection-site reactions, headache, and rash.
The approval for the high-concentration formulation was based on data from a phase 1 pharmacokinetics bridging study that compared Hyrimoz 50 mg/mL and citrate-free Hyrimoz 100 mg/mL.
“This study met all of the primary objectives, demonstrating comparable pharmacokinetics and showing similar safety and immunogenicity of the Hyrimoz 50 mg/mL and Hyrimoz [100 mg/mL],” according to Sandoz, a division of Novartis.
The approval for Hyrimoz 50 mg/mL in 2018 was based on preclinical and clinical research comparing Hyrimoz and Humira. In a phase 3 trial published in the British Journal of Dermatology, which included adults with clinically stable but active moderate to severe chronic plaque psoriasis, Hyrimoz and Humira showed a similar percentage of patients met the primary endpoint of a 75% reduction or more in Psoriasis Area and Severity Index (PASI 75) score at 16 weeks, compared with baseline (66.8% and 65%, respectively).
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved a citrate-free, 100 mg/mL formulation of the biosimilar adalimumab-adaz (Hyrimoz), according to a statement from manufacturer Sandoz.
Hyrimoz, a tumor necrosis factor (TNF) blocker that is biosimilar to its reference product Humira, was approved by the FDA in 2018 at a concentration of 50 mg/mL for rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The high-concentration formula is indicated for these same conditions.
Sandoz said that it intends to launch the citrate-free formulation in the United States on July 1. It will be one of up to nine other adalimumab biosimilars that are expected to launch in July. On January 31, Amjevita (adalimumab-atto) became the first adalimumab biosimilar to launch in the United States.
The current label for Hyrimoz contains a black box warning emphasizing certain risks, notably the increased risk for serious infections, such as tuberculosis or sepsis, and an increased risk of malignancy, particularly lymphomas.
Adverse effects associated with Hyrimoz with an incidence greater than 10% include upper respiratory infections and sinusitis, injection-site reactions, headache, and rash.
The approval for the high-concentration formulation was based on data from a phase 1 pharmacokinetics bridging study that compared Hyrimoz 50 mg/mL and citrate-free Hyrimoz 100 mg/mL.
“This study met all of the primary objectives, demonstrating comparable pharmacokinetics and showing similar safety and immunogenicity of the Hyrimoz 50 mg/mL and Hyrimoz [100 mg/mL],” according to Sandoz, a division of Novartis.
The approval for Hyrimoz 50 mg/mL in 2018 was based on preclinical and clinical research comparing Hyrimoz and Humira. In a phase 3 trial published in the British Journal of Dermatology, which included adults with clinically stable but active moderate to severe chronic plaque psoriasis, Hyrimoz and Humira showed a similar percentage of patients met the primary endpoint of a 75% reduction or more in Psoriasis Area and Severity Index (PASI 75) score at 16 weeks, compared with baseline (66.8% and 65%, respectively).
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved a citrate-free, 100 mg/mL formulation of the biosimilar adalimumab-adaz (Hyrimoz), according to a statement from manufacturer Sandoz.
Hyrimoz, a tumor necrosis factor (TNF) blocker that is biosimilar to its reference product Humira, was approved by the FDA in 2018 at a concentration of 50 mg/mL for rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The high-concentration formula is indicated for these same conditions.
Sandoz said that it intends to launch the citrate-free formulation in the United States on July 1. It will be one of up to nine other adalimumab biosimilars that are expected to launch in July. On January 31, Amjevita (adalimumab-atto) became the first adalimumab biosimilar to launch in the United States.
The current label for Hyrimoz contains a black box warning emphasizing certain risks, notably the increased risk for serious infections, such as tuberculosis or sepsis, and an increased risk of malignancy, particularly lymphomas.
Adverse effects associated with Hyrimoz with an incidence greater than 10% include upper respiratory infections and sinusitis, injection-site reactions, headache, and rash.
The approval for the high-concentration formulation was based on data from a phase 1 pharmacokinetics bridging study that compared Hyrimoz 50 mg/mL and citrate-free Hyrimoz 100 mg/mL.
“This study met all of the primary objectives, demonstrating comparable pharmacokinetics and showing similar safety and immunogenicity of the Hyrimoz 50 mg/mL and Hyrimoz [100 mg/mL],” according to Sandoz, a division of Novartis.
The approval for Hyrimoz 50 mg/mL in 2018 was based on preclinical and clinical research comparing Hyrimoz and Humira. In a phase 3 trial published in the British Journal of Dermatology, which included adults with clinically stable but active moderate to severe chronic plaque psoriasis, Hyrimoz and Humira showed a similar percentage of patients met the primary endpoint of a 75% reduction or more in Psoriasis Area and Severity Index (PASI 75) score at 16 weeks, compared with baseline (66.8% and 65%, respectively).
A version of this article originally appeared on Medscape.com.
New data forecast more oral PDE4 inhibitors for psoriasis
NEW ORLEANS – according to results of a phase 2 clinical trial presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
The phase 2b data, which are prompting a phase 3 trial, suggest that the drug, called orismilast, “is a potential new addition to the psoriasis armamentarium,” reported Lars E. French, MD, professor and chair, department of dermatology, Ludwig Maximilian University of Munich (Germany).
At the same session, findings from another study supported off-label use of oral roflumilast (Daliresp and generic), a PDE4 inhibitor approved for severe chronic obstructive pulmonary disease (COPD). The only PDE4 inhibitors with an indication for psoriasis are roflumilast, approved as a cream (Zoryve), and apremilast (Otezla), approved as an oral therapy.
Phase 2 study of orismilast
In the orismilast trial, Dr. French attributed the efficacy observed to the potency of orismilast on the B and D subtypes of PDE4 associated with inflammation. One clue is that these specific subtypes are overly expressed in the skin of patients with either psoriasis or atopic dermatitis.
“When compared to apremilast, orismilast is at least two to fivefold more potent on all PDE4 isoforms and up to 39 times more potent on some of the PDE4 B and D isoforms,” said Dr. French, referring to preclinical findings in human whole blood and blood cells and in a mouse model of chronic inflammation.
The efficacy of orismilast in an immediate-release oral formulation was previously demonstrated in a recently published phase 2a trial, but the newest study tested a modified-release formulation of orismilast to test its potential to improve tolerability.
In the study, 202 adult patients with moderate to severe psoriasis (Psoriasis Area Severity Index [PASI] score ≥ 12) were randomly assigned to one of three doses of orismilast or to placebo. Each of the three doses – 20 mg, 30 mg, or 40 mg – were administered twice daily. The primary endpoint was change in PASI score at 16 weeks. Secondary endpoints included PASI 75 responses (signifying 75% clearance) and safety.
Relative to placebo, which was associated with a PASI improvement of 17%, all three of the tested orismilast doses were superior in a dose-dependent manner. The rates of response were 53%, 61%, and 64% for the 20-mg, 30-mg, and 40-mg twice-daily doses, respectively.
The PASI improvements were rapid, Dr. French said. At 4 weeks, PASI scores climbed from baseline by nearly 40% for those on all orismilast doses, which was more than double the improvement in the placebo group.
In the intention-to-treat analysis with missing data counted as nonresponders, the proportion of patients reaching PASI-75 scores at 16 weeks were 39%, 49%, 45%, and 17%, in the 20-mg, 30-mg, 40-mg, and placebo groups, respectively. The proportion of patients experiencing complete or near-complete skin clearance defined by a PASI 90 were 24%, 22%, 28%, and 8%, respectively.
The side-effect profile was consistent with other PDE4 inhibitors. The most common adverse events included gastrointestinal complaints, such as diarrhea and nausea, as well as headache and dizziness. But the majority of these events were of low grade, and they were largely confined to the first 4 weeks of treatment, which is a pattern reported with other PDE4 inhibitors in psoriasis and other chronic inflammatory diseases, such as COPD, according to Dr. French.
“There were no discontinuations for a treatment-related adverse event in the arms receiving either the 20-mg or the 30-mg doses,” Dr. French reported. There were only two serious adverse events, and neither were considered by trial investigators to be related to orismilast.
Based on the limited therapeutic gain but greater risk for adverse events on the 40-mg twice-daily dose, “the question is now whether to move forward with the 20-mg or the 30-mg dose,” said Dr. French, who said planning of a phase 3 trial is underway.
Phase 2 study of roflumilast
However, this was not the only set of data on an oral PDE4 inhibitor presented as a late-breaker at the AAD meeting. For clinicians looking for a more immediate and less expensive alternative to apremilast, another study indicated that off-label use of oral roflumilast is an option.
In an investigator-initiated, multicenter, double-blind, placebo-controlled trial conducted in Denmark, the rate of response to oral roflumilast at 24 weeks, including the clear or almost clear response, was on the same general order of magnitude as that seen in the orismilast study, reported Alexander Egeberg, MD, PhD, professor of dermatology, University of Copenhagen.
“At 24 weeks, 21.7% had achieved a PASI 90, and 8.7% achieved a PASI 100,” Dr. Egeberg said.
Oral roflumilast has been available for the treatment of COPD for more than 10 years and is now available in a generic formulation. This study was conducted independent of any pharmaceutical company involvement, and the high rate of response and low risk of adverse events suggests that patients can benefit from a PDE4 inhibitor in a very low-cost form.
“Generic oral roflumilast is cheaper than a Starbucks coffee,” Dr. Egeberg said.
In this trial, 46 patients were randomly assigned to placebo or to the COPD-approved roflumilast dose of 500 mcg once daily. The primary endpoint was change in PASI scores from baseline to week 12, which Dr. Egeberg pointed out is a shorter time frame than the 16 weeks more typical of psoriasis treatment studies.
At week 12, the median improvement in PASI was 34.8% in the roflumilast group versus 0% in the placebo group. Patients were then followed for an additional 12 weeks, but those randomized to placebo were switched to the active treatment. By week 24, the switch patients had largely caught up to those initiated on roflumilast for median PASI improvement (39.1% vs. 43.5%).
Similar to orismilast, roflumilast “was generally well tolerated,” Dr. Egeberg said. The adverse events were consistent with those associated with PDE4 inhibitors in previous trials, whether in psoriasis or COPD. There was only one serious adverse event, and it was not considered treatment related. Discontinuations for adverse events “were very low.”
In a population with a relatively high rate of smoking, Dr. Egeberg further reported, lung function was improved, a remark initially interpreted as a joke by some attending the presentation. However, Dr. Egeberg confirmed that lung function was monitored, and objective improvements were recorded.
By Danish law, the investigators were required to inform the manufacturers of roflumilast. Despite the results of this study, he is not aware of any plans to seek an indication for roflumilast in psoriasis, but he noted that the drug is readily available at a low price.
For those willing to offer this therapy off label, “you can start using it tomorrow if you’d like,” he said.
Dr. French reports financial relationships with Almirall, Amgen, Biotest, Galderma, Janssen Cilag, Leo Pharma, Pincell, Regeneron, UCB, and UNION Therapeutics, which provided funding for this trial. Dr. Egeberg reports financial relationships with Eli Lilly, Galderma, Janssen-Cilag, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – according to results of a phase 2 clinical trial presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
The phase 2b data, which are prompting a phase 3 trial, suggest that the drug, called orismilast, “is a potential new addition to the psoriasis armamentarium,” reported Lars E. French, MD, professor and chair, department of dermatology, Ludwig Maximilian University of Munich (Germany).
At the same session, findings from another study supported off-label use of oral roflumilast (Daliresp and generic), a PDE4 inhibitor approved for severe chronic obstructive pulmonary disease (COPD). The only PDE4 inhibitors with an indication for psoriasis are roflumilast, approved as a cream (Zoryve), and apremilast (Otezla), approved as an oral therapy.
Phase 2 study of orismilast
In the orismilast trial, Dr. French attributed the efficacy observed to the potency of orismilast on the B and D subtypes of PDE4 associated with inflammation. One clue is that these specific subtypes are overly expressed in the skin of patients with either psoriasis or atopic dermatitis.
“When compared to apremilast, orismilast is at least two to fivefold more potent on all PDE4 isoforms and up to 39 times more potent on some of the PDE4 B and D isoforms,” said Dr. French, referring to preclinical findings in human whole blood and blood cells and in a mouse model of chronic inflammation.
The efficacy of orismilast in an immediate-release oral formulation was previously demonstrated in a recently published phase 2a trial, but the newest study tested a modified-release formulation of orismilast to test its potential to improve tolerability.
In the study, 202 adult patients with moderate to severe psoriasis (Psoriasis Area Severity Index [PASI] score ≥ 12) were randomly assigned to one of three doses of orismilast or to placebo. Each of the three doses – 20 mg, 30 mg, or 40 mg – were administered twice daily. The primary endpoint was change in PASI score at 16 weeks. Secondary endpoints included PASI 75 responses (signifying 75% clearance) and safety.
Relative to placebo, which was associated with a PASI improvement of 17%, all three of the tested orismilast doses were superior in a dose-dependent manner. The rates of response were 53%, 61%, and 64% for the 20-mg, 30-mg, and 40-mg twice-daily doses, respectively.
The PASI improvements were rapid, Dr. French said. At 4 weeks, PASI scores climbed from baseline by nearly 40% for those on all orismilast doses, which was more than double the improvement in the placebo group.
In the intention-to-treat analysis with missing data counted as nonresponders, the proportion of patients reaching PASI-75 scores at 16 weeks were 39%, 49%, 45%, and 17%, in the 20-mg, 30-mg, 40-mg, and placebo groups, respectively. The proportion of patients experiencing complete or near-complete skin clearance defined by a PASI 90 were 24%, 22%, 28%, and 8%, respectively.
The side-effect profile was consistent with other PDE4 inhibitors. The most common adverse events included gastrointestinal complaints, such as diarrhea and nausea, as well as headache and dizziness. But the majority of these events were of low grade, and they were largely confined to the first 4 weeks of treatment, which is a pattern reported with other PDE4 inhibitors in psoriasis and other chronic inflammatory diseases, such as COPD, according to Dr. French.
“There were no discontinuations for a treatment-related adverse event in the arms receiving either the 20-mg or the 30-mg doses,” Dr. French reported. There were only two serious adverse events, and neither were considered by trial investigators to be related to orismilast.
Based on the limited therapeutic gain but greater risk for adverse events on the 40-mg twice-daily dose, “the question is now whether to move forward with the 20-mg or the 30-mg dose,” said Dr. French, who said planning of a phase 3 trial is underway.
Phase 2 study of roflumilast
However, this was not the only set of data on an oral PDE4 inhibitor presented as a late-breaker at the AAD meeting. For clinicians looking for a more immediate and less expensive alternative to apremilast, another study indicated that off-label use of oral roflumilast is an option.
In an investigator-initiated, multicenter, double-blind, placebo-controlled trial conducted in Denmark, the rate of response to oral roflumilast at 24 weeks, including the clear or almost clear response, was on the same general order of magnitude as that seen in the orismilast study, reported Alexander Egeberg, MD, PhD, professor of dermatology, University of Copenhagen.
“At 24 weeks, 21.7% had achieved a PASI 90, and 8.7% achieved a PASI 100,” Dr. Egeberg said.
Oral roflumilast has been available for the treatment of COPD for more than 10 years and is now available in a generic formulation. This study was conducted independent of any pharmaceutical company involvement, and the high rate of response and low risk of adverse events suggests that patients can benefit from a PDE4 inhibitor in a very low-cost form.
“Generic oral roflumilast is cheaper than a Starbucks coffee,” Dr. Egeberg said.
In this trial, 46 patients were randomly assigned to placebo or to the COPD-approved roflumilast dose of 500 mcg once daily. The primary endpoint was change in PASI scores from baseline to week 12, which Dr. Egeberg pointed out is a shorter time frame than the 16 weeks more typical of psoriasis treatment studies.
At week 12, the median improvement in PASI was 34.8% in the roflumilast group versus 0% in the placebo group. Patients were then followed for an additional 12 weeks, but those randomized to placebo were switched to the active treatment. By week 24, the switch patients had largely caught up to those initiated on roflumilast for median PASI improvement (39.1% vs. 43.5%).
Similar to orismilast, roflumilast “was generally well tolerated,” Dr. Egeberg said. The adverse events were consistent with those associated with PDE4 inhibitors in previous trials, whether in psoriasis or COPD. There was only one serious adverse event, and it was not considered treatment related. Discontinuations for adverse events “were very low.”
In a population with a relatively high rate of smoking, Dr. Egeberg further reported, lung function was improved, a remark initially interpreted as a joke by some attending the presentation. However, Dr. Egeberg confirmed that lung function was monitored, and objective improvements were recorded.
By Danish law, the investigators were required to inform the manufacturers of roflumilast. Despite the results of this study, he is not aware of any plans to seek an indication for roflumilast in psoriasis, but he noted that the drug is readily available at a low price.
For those willing to offer this therapy off label, “you can start using it tomorrow if you’d like,” he said.
Dr. French reports financial relationships with Almirall, Amgen, Biotest, Galderma, Janssen Cilag, Leo Pharma, Pincell, Regeneron, UCB, and UNION Therapeutics, which provided funding for this trial. Dr. Egeberg reports financial relationships with Eli Lilly, Galderma, Janssen-Cilag, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – according to results of a phase 2 clinical trial presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
The phase 2b data, which are prompting a phase 3 trial, suggest that the drug, called orismilast, “is a potential new addition to the psoriasis armamentarium,” reported Lars E. French, MD, professor and chair, department of dermatology, Ludwig Maximilian University of Munich (Germany).
At the same session, findings from another study supported off-label use of oral roflumilast (Daliresp and generic), a PDE4 inhibitor approved for severe chronic obstructive pulmonary disease (COPD). The only PDE4 inhibitors with an indication for psoriasis are roflumilast, approved as a cream (Zoryve), and apremilast (Otezla), approved as an oral therapy.
Phase 2 study of orismilast
In the orismilast trial, Dr. French attributed the efficacy observed to the potency of orismilast on the B and D subtypes of PDE4 associated with inflammation. One clue is that these specific subtypes are overly expressed in the skin of patients with either psoriasis or atopic dermatitis.
“When compared to apremilast, orismilast is at least two to fivefold more potent on all PDE4 isoforms and up to 39 times more potent on some of the PDE4 B and D isoforms,” said Dr. French, referring to preclinical findings in human whole blood and blood cells and in a mouse model of chronic inflammation.
The efficacy of orismilast in an immediate-release oral formulation was previously demonstrated in a recently published phase 2a trial, but the newest study tested a modified-release formulation of orismilast to test its potential to improve tolerability.
In the study, 202 adult patients with moderate to severe psoriasis (Psoriasis Area Severity Index [PASI] score ≥ 12) were randomly assigned to one of three doses of orismilast or to placebo. Each of the three doses – 20 mg, 30 mg, or 40 mg – were administered twice daily. The primary endpoint was change in PASI score at 16 weeks. Secondary endpoints included PASI 75 responses (signifying 75% clearance) and safety.
Relative to placebo, which was associated with a PASI improvement of 17%, all three of the tested orismilast doses were superior in a dose-dependent manner. The rates of response were 53%, 61%, and 64% for the 20-mg, 30-mg, and 40-mg twice-daily doses, respectively.
The PASI improvements were rapid, Dr. French said. At 4 weeks, PASI scores climbed from baseline by nearly 40% for those on all orismilast doses, which was more than double the improvement in the placebo group.
In the intention-to-treat analysis with missing data counted as nonresponders, the proportion of patients reaching PASI-75 scores at 16 weeks were 39%, 49%, 45%, and 17%, in the 20-mg, 30-mg, 40-mg, and placebo groups, respectively. The proportion of patients experiencing complete or near-complete skin clearance defined by a PASI 90 were 24%, 22%, 28%, and 8%, respectively.
The side-effect profile was consistent with other PDE4 inhibitors. The most common adverse events included gastrointestinal complaints, such as diarrhea and nausea, as well as headache and dizziness. But the majority of these events were of low grade, and they were largely confined to the first 4 weeks of treatment, which is a pattern reported with other PDE4 inhibitors in psoriasis and other chronic inflammatory diseases, such as COPD, according to Dr. French.
“There were no discontinuations for a treatment-related adverse event in the arms receiving either the 20-mg or the 30-mg doses,” Dr. French reported. There were only two serious adverse events, and neither were considered by trial investigators to be related to orismilast.
Based on the limited therapeutic gain but greater risk for adverse events on the 40-mg twice-daily dose, “the question is now whether to move forward with the 20-mg or the 30-mg dose,” said Dr. French, who said planning of a phase 3 trial is underway.
Phase 2 study of roflumilast
However, this was not the only set of data on an oral PDE4 inhibitor presented as a late-breaker at the AAD meeting. For clinicians looking for a more immediate and less expensive alternative to apremilast, another study indicated that off-label use of oral roflumilast is an option.
In an investigator-initiated, multicenter, double-blind, placebo-controlled trial conducted in Denmark, the rate of response to oral roflumilast at 24 weeks, including the clear or almost clear response, was on the same general order of magnitude as that seen in the orismilast study, reported Alexander Egeberg, MD, PhD, professor of dermatology, University of Copenhagen.
“At 24 weeks, 21.7% had achieved a PASI 90, and 8.7% achieved a PASI 100,” Dr. Egeberg said.
Oral roflumilast has been available for the treatment of COPD for more than 10 years and is now available in a generic formulation. This study was conducted independent of any pharmaceutical company involvement, and the high rate of response and low risk of adverse events suggests that patients can benefit from a PDE4 inhibitor in a very low-cost form.
“Generic oral roflumilast is cheaper than a Starbucks coffee,” Dr. Egeberg said.
In this trial, 46 patients were randomly assigned to placebo or to the COPD-approved roflumilast dose of 500 mcg once daily. The primary endpoint was change in PASI scores from baseline to week 12, which Dr. Egeberg pointed out is a shorter time frame than the 16 weeks more typical of psoriasis treatment studies.
At week 12, the median improvement in PASI was 34.8% in the roflumilast group versus 0% in the placebo group. Patients were then followed for an additional 12 weeks, but those randomized to placebo were switched to the active treatment. By week 24, the switch patients had largely caught up to those initiated on roflumilast for median PASI improvement (39.1% vs. 43.5%).
Similar to orismilast, roflumilast “was generally well tolerated,” Dr. Egeberg said. The adverse events were consistent with those associated with PDE4 inhibitors in previous trials, whether in psoriasis or COPD. There was only one serious adverse event, and it was not considered treatment related. Discontinuations for adverse events “were very low.”
In a population with a relatively high rate of smoking, Dr. Egeberg further reported, lung function was improved, a remark initially interpreted as a joke by some attending the presentation. However, Dr. Egeberg confirmed that lung function was monitored, and objective improvements were recorded.
By Danish law, the investigators were required to inform the manufacturers of roflumilast. Despite the results of this study, he is not aware of any plans to seek an indication for roflumilast in psoriasis, but he noted that the drug is readily available at a low price.
For those willing to offer this therapy off label, “you can start using it tomorrow if you’d like,” he said.
Dr. French reports financial relationships with Almirall, Amgen, Biotest, Galderma, Janssen Cilag, Leo Pharma, Pincell, Regeneron, UCB, and UNION Therapeutics, which provided funding for this trial. Dr. Egeberg reports financial relationships with Eli Lilly, Galderma, Janssen-Cilag, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
AT AAD 2023
JAK inhibitor safety warnings drawn from rheumatologic data may be misleading in dermatology
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
NEW ORLEANS – , even though the basis for all the risks is a rheumatoid arthritis study, according to a critical review at the annual meeting of the American Academy of Dermatology.
Given the fact that the postmarketing RA study was specifically enriched with high-risk patients by requiring an age at enrollment of at least 50 years and the presence of at least one cardiovascular risk factor, the extrapolation of these risks to dermatologic indications is “not necessarily data-driven,” said Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
The recently approved deucravacitinib is the only JAK inhibitor that has so far been exempt from these warnings. Instead, based on the ORAL Surveillance study, published in the New England Journal of Medicine, the Food and Drug Administration requires a boxed warning in nearly identical language for all the other JAK inhibitors. Relative to tofacitinib, the JAK inhibitor tested in ORAL Surveillance, many of these drugs differ by JAK selectivity and other characteristics that are likely relevant to risk of adverse events, Dr. King said. The same language has even been applied to topical ruxolitinib cream.
Basis of boxed warnings
In ORAL Surveillance, about 4,300 high-risk patients with RA were randomized to one of two doses of tofacitinib (5 mg or 10 mg) twice daily or a tumor necrosis factor (TNF) inhibitor. All patients in the trial were taking methotrexate, and almost 60% were taking concomitant corticosteroids. The average body mass index of the study population was about 30 kg/m2.
After a median 4 years of follow-up (about 5,000 patient-years), the incidence of many of the adverse events tracked in the study were higher in the tofacitinib groups, including serious infections, MACE, thromboembolic events, and cancer. Dr. King did not challenge the importance of these data, but he questioned whether they are reasonably extrapolated to dermatologic indications, particularly as many of those treated are younger than those common to an RA population.
In fact, despite a study enriched for a higher risk of many events tracked, most adverse events were only slightly elevated, Dr. King pointed out. For example, the incidence of MACE over the 4 years of follow-up was 3.4% among those taking any dose of tofacitinib versus 2.5% of those randomized to TNF inhibitor. Rates of cancer were 4.2% versus 2.9%, respectively. There were also absolute increases in the number of serious infections and thromboembolic events for tofacitinib relative to TNF inhibitor.
Dr. King acknowledged that the numbers in ORAL Surveillance associated tofacitinib with a higher risk of serious events than TNF inhibitor in patients with RA, but he believes that “JAK inhibitor safety is almost certainly not the same in dermatology as it is in rheumatology patients.”
Evidence of difference in dermatology
There is some evidence to back this up. Dr. King cited a recently published study in RMD Open that evaluated the safety profile of the JAK inhibitor upadacitinib in nearly 7,000 patients over 15,000 patient-years of follow-up. Drug safety data were evaluated with up to 5.5 years of follow-up from 12 clinical trials of the four diseases for which upadacitinib is now indicated. Three were rheumatologic (RA, psoriatic arthritis, and ankylosing spondylitis), and the fourth was atopic dermatitis (AD). Fourteen outcomes, including numerous types of infection, MACE, hepatic complications, and malignancy, were compared with methotrexate and the TNF inhibitor adalimumab.
For the RA diseases, upadacitinib was associated with a greater risk than comparators for several outcomes, including serious infections. But in AD, there was a smaller increased risk of adverse outcomes for the JAK inhibitor relative to comparators.
When evaluated by risk of adverse events across indications, for MACE, the exposure-adjusted event rates for upadacitinib were less than 0.1 in patients treated for AD over the observation period versus 0.3 and 0.4 for RA and psoriatic arthritis, respectively. Similarly, for venous thromboembolism, the rates for upadacitinib were again less than 0.1 in patients with AD versus 0.4 and 0.2 in RA and psoriatic arthritis, respectively.
Referring back to the postmarketing study, Dr. King emphasized that it is essential to consider how the boxed warning for JAK inhibitors was generated before applying them to dermatologic indications.
“Is a 30-year-old patient with a dermatologic disorder possibly at the same risk as the patients in the study from which we got the boxed warning? The answer is simply no,” he said.
Like the tofacitinib data in the ORAL Surveillance study, the upadacitinib clinical trial data are not necessarily relevant to other JAK inhibitors. In fact, Dr. King pointed out that the safety profiles of the available JAK inhibitors are not identical, an observation that is consistent with differences in JAK inhibitor selectivity that has implications for off-target events.
Dr. King does not dismiss the potential risks outlined in the current regulatory cautions about the use of JAK inhibitors, but he believes that dermatologists should be cognizant of “where the black box warning comes from.”
“We need to think carefully about the risk-to-benefit ratio in older patients or patients with risk factors, such as obesity and diabetes,” he said. But the safety profile of JAK inhibitors “is almost certainly better” than the profile suggested in boxed warnings applied to JAK inhibitors for dermatologic indications, he advised.
Risk-benefit considerations in dermatology
This position was supported by numerous other experts when asked for their perspectives. “I fully agree,” said Emma Guttman-Yassky, MD, PhD, system chair of dermatology and immunology, Icahn School of Medicine, Mount Sinai, New York.
Like Dr. King, Dr. Guttman-Yassky did not dismiss the potential risks of JAK inhibitors when treating dermatologic diseases.
“While JAK inhibitors need monitoring as advised, adopting a boxed warning from an RA study for patients who are older [is problematic],” she commented. A study with the nonselective tofacitinib in this population “cannot be compared to more selective inhibitors in a much younger population, such as those treated [for] alopecia areata or atopic dermatitis.”
George Z. Han, MD, PhD, an associate professor of dermatology, Zucker School of Medicine, Hofstra, Northwell Medical Center, New Hyde Park, New York, also agreed but added some caveats.
“The comments about the ORAL Surveillance study are salient,” he said in an interview. “This kind of data should not directly be extrapolated to other patient types or to other medications.” However, one of Dr. Han’s most important caveats involves long-term use.
“JAK inhibitors are still relatively narrow-therapeutic-window drugs that in a dose-dependent fashion could lead to negative effects, including thromboembolic events, abnormalities in red blood cells, white blood cells, platelets, and lipids,” he said. While doses used in dermatology “are generally below the level of any major concern,” Dr. Han cautioned that “we lack definitive data” on long-term use, and this is important for understanding “any potential small risk of rare events, such as malignancy or thromboembolism.”
Saakshi Khattri, MD, a colleague of Dr. Guttman-Yassky at Mount Sinai, said the risks of JAK inhibitors should not be underestimated, but she also agreed that risk “needs to be delivered in the right context.” Dr. Khattri, who is board certified in both dermatology and rheumatology, noted the safety profiles of available JAK inhibitors differ and that extrapolating safety from an RA study to dermatologic indications does not make sense. “Different diseases, different age groups,” she said.
Dr. King has reported financial relationships with more than 15 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Han reports financial relationships with Amgen, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, PellePharm, Pfizer, and UCB. Dr. Khattri has reported financial relationships with AbbVie, Arcutis, Bristol-Myers Squibb, Janssen, Leo, Lilly, Novartis, Pfizer, and UCB.
A version of this article originally appeared on Medscape.com.
AT AAD 2023
A 9-year-old male presents with multiple thick scaly plaques on scalp, ears, and trunk
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
A 9-year-old male is seen in the clinic with a 1-year history of multiple thick scaly plaques on scalp, ears, and trunk. He has been treated with hydrocortisone 1% ointment with no change in the lesions. He had upper respiratory tract symptoms 3 weeks prior to the visit.
Examination reveals erythematous, well-demarcated plaques of the anterior scalp with thick overlying micaceous scale with some extension onto the forehead and temples. Additionally, erythematous scaly patches on the ear, axilla, and umbilicus were noted. There was no palmar or plantar involvement. He denied joint swelling, stiffness, or pain in the morning.
Biologics show signs of delaying arthritis in psoriasis patients
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
FROM LANCET RHEUMATOLOGY
Dermatologic Implications of Sleep Deprivation in the US Military
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Practice Points
- Sleep deprivation may have negative effects on skin function and worsen dermatologic conditions.
- Proposed mechanisms of action for these negative effects include dysregulation of the hypothalamic-pituitary-adrenal axis, impairment of cutaneous barrier function, and alteration of cutaneous immune function.
- Members of the US Military are at an increased risk for sleep deprivation, especially during training and overseas deployments.
Isolated nail psoriasis may bring arthritis into play
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.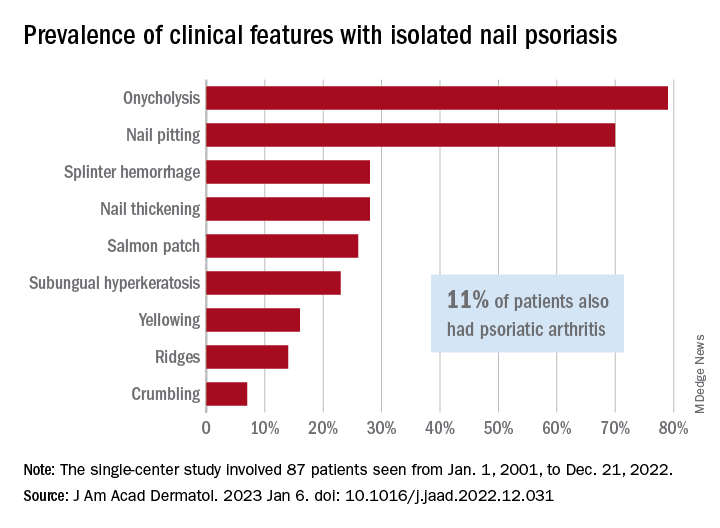
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Treating nail psoriasis: Intralesional injections and biologics
HONOLULU – combined with systemic therapy.
One might think of intralesional injections “as a torture method from the medieval days,” she said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!, but intramatricial corticosteroid injections have been performed for many years as a treatment for nail psoriasis, typically with triamcinolone acetonide.
According to Dr. Armstrong, professor of dermatology and associate dean of clinical research at the University of Southern California, Los Angeles, nail matrix psoriasis can present as pitting, leukonychia, red macules in the lunula, crumbling, or trachyonychia. Nail bed psoriasis can present as splinter hemorrhages and onycholysis, hyperkeratosis and splinter hemorrhages, salmon patch or oil spot dyschromia, or onycholysis and salmon patch dyschromia.
In a German cross-sectional study of patients with psoriasis, nails were one of the body sites that have the greatest impact on quality of life – especially those in younger age groups.
While topical treatments are generally considered first for limited disease involving special areas such as the nails, systemic therapy is warranted in patients with moderate-to-severe involvement of specific sites or in those refractory to topical therapy, Dr. Armstrong said.
In 2018, Indian researchers published results from an open-label study of 17 patients, with nail psoriasis, comparing three treatments . Patients were assigned to three groups of 30 nails each and treated with intramatricial injections of triamcinolone acetonide (10 mg/mL), methotrexate (25 mg/mL), and cyclosporine (50 mg/mL), respectively. Each nail was treated with two injections at 6-week intervals and graded at 24 weeks using the Nail Psoriasis Severity Index (NAPSI). In the triamcinolone acetonide and methotrexate groups, 50% of treated nails showed a greater than 75% improvement at 24 weeks, compared with 33% of those in the cyclosporine group. The most side effects occurred in the nails treated with cyclosporine.
When Dr. Armstrong performs intramatricial injections, she uses triamcinolone acetonide at 10 mg/mL. However, she said, “my favorite way of treating severe nail psoriasis is with biologics.”
In an early study of patients with moderate to severe psoriasis treated with the tumor necrosis factor blocker adalimumab 80 mg subcutaneously at week 0, followed by 40 mg subcutaneously every other week from weeks 1 to 15, a post hoc analysis on the effects on nail psoriasis showed a 10-point decrease in the median NAPSI score through week 16 – from 21 to 11 .
In VOYAGE 2, which compared the interleukin-23 blocker guselkumab and adalimumab in patients with moderate to severe psoriasis, the mean percent improvement from baseline in the NAPSI score was similar in patients treated with adalimumab or guselkumab at week 16 (39.6% vs. 46.9%, respectively) and at week 24 (55% vs. 53.7%).
In another study of patients with nail psoriasis, researchers evaluated the efficacy of the IL-17A antagonist secukinumab 150 mg, 300 mg, or placebo at weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter for 2.5 years. At 2.5 years, the mean reduction in NAPSI score was 63.6% in the secukinumab 150 mg group and 73.3% in the secukinumab 300 mg group.
“I do have to tell my patients what to expect, because the nails grow out slowly, but over time we do see this increase in efficacy,” Dr. Armstrong said.
Studies of another IL-17A antagonist, ixekizumab, have yielded positive results as well, she noted. In 2021, Taiwanese researchers published a systematic review and network meta-analysis to evaluate the efficacy of small molecule inhibitors and biologics in treating nail psoriasis. They drew from 39 studies involving 15,673 patients with nail psoriasis and found that the oral Janus kinase inhibitor tofacitinib and ixekizumab had the best efficacy for treating nail psoriasis in 10-16 weeks and 24-26 weeks, respectively.
“They found that overall, the biologics have a good effect on nail psoriasis and that the treatment effects are overall quite similar,” Dr. Armstrong said.
Dr. Armstrong disclosed that she is a consultant or adviser for numerous pharmaceutical companies. She has also received research funding from Bristol-Myers Squibb, Dermavant, Dermira, Leo, Lilly, Pfizer, and UCB Pharma.
HONOLULU – combined with systemic therapy.
One might think of intralesional injections “as a torture method from the medieval days,” she said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!, but intramatricial corticosteroid injections have been performed for many years as a treatment for nail psoriasis, typically with triamcinolone acetonide.
According to Dr. Armstrong, professor of dermatology and associate dean of clinical research at the University of Southern California, Los Angeles, nail matrix psoriasis can present as pitting, leukonychia, red macules in the lunula, crumbling, or trachyonychia. Nail bed psoriasis can present as splinter hemorrhages and onycholysis, hyperkeratosis and splinter hemorrhages, salmon patch or oil spot dyschromia, or onycholysis and salmon patch dyschromia.
In a German cross-sectional study of patients with psoriasis, nails were one of the body sites that have the greatest impact on quality of life – especially those in younger age groups.
While topical treatments are generally considered first for limited disease involving special areas such as the nails, systemic therapy is warranted in patients with moderate-to-severe involvement of specific sites or in those refractory to topical therapy, Dr. Armstrong said.
In 2018, Indian researchers published results from an open-label study of 17 patients, with nail psoriasis, comparing three treatments . Patients were assigned to three groups of 30 nails each and treated with intramatricial injections of triamcinolone acetonide (10 mg/mL), methotrexate (25 mg/mL), and cyclosporine (50 mg/mL), respectively. Each nail was treated with two injections at 6-week intervals and graded at 24 weeks using the Nail Psoriasis Severity Index (NAPSI). In the triamcinolone acetonide and methotrexate groups, 50% of treated nails showed a greater than 75% improvement at 24 weeks, compared with 33% of those in the cyclosporine group. The most side effects occurred in the nails treated with cyclosporine.
When Dr. Armstrong performs intramatricial injections, she uses triamcinolone acetonide at 10 mg/mL. However, she said, “my favorite way of treating severe nail psoriasis is with biologics.”
In an early study of patients with moderate to severe psoriasis treated with the tumor necrosis factor blocker adalimumab 80 mg subcutaneously at week 0, followed by 40 mg subcutaneously every other week from weeks 1 to 15, a post hoc analysis on the effects on nail psoriasis showed a 10-point decrease in the median NAPSI score through week 16 – from 21 to 11 .
In VOYAGE 2, which compared the interleukin-23 blocker guselkumab and adalimumab in patients with moderate to severe psoriasis, the mean percent improvement from baseline in the NAPSI score was similar in patients treated with adalimumab or guselkumab at week 16 (39.6% vs. 46.9%, respectively) and at week 24 (55% vs. 53.7%).
In another study of patients with nail psoriasis, researchers evaluated the efficacy of the IL-17A antagonist secukinumab 150 mg, 300 mg, or placebo at weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter for 2.5 years. At 2.5 years, the mean reduction in NAPSI score was 63.6% in the secukinumab 150 mg group and 73.3% in the secukinumab 300 mg group.
“I do have to tell my patients what to expect, because the nails grow out slowly, but over time we do see this increase in efficacy,” Dr. Armstrong said.
Studies of another IL-17A antagonist, ixekizumab, have yielded positive results as well, she noted. In 2021, Taiwanese researchers published a systematic review and network meta-analysis to evaluate the efficacy of small molecule inhibitors and biologics in treating nail psoriasis. They drew from 39 studies involving 15,673 patients with nail psoriasis and found that the oral Janus kinase inhibitor tofacitinib and ixekizumab had the best efficacy for treating nail psoriasis in 10-16 weeks and 24-26 weeks, respectively.
“They found that overall, the biologics have a good effect on nail psoriasis and that the treatment effects are overall quite similar,” Dr. Armstrong said.
Dr. Armstrong disclosed that she is a consultant or adviser for numerous pharmaceutical companies. She has also received research funding from Bristol-Myers Squibb, Dermavant, Dermira, Leo, Lilly, Pfizer, and UCB Pharma.
HONOLULU – combined with systemic therapy.
One might think of intralesional injections “as a torture method from the medieval days,” she said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!, but intramatricial corticosteroid injections have been performed for many years as a treatment for nail psoriasis, typically with triamcinolone acetonide.
According to Dr. Armstrong, professor of dermatology and associate dean of clinical research at the University of Southern California, Los Angeles, nail matrix psoriasis can present as pitting, leukonychia, red macules in the lunula, crumbling, or trachyonychia. Nail bed psoriasis can present as splinter hemorrhages and onycholysis, hyperkeratosis and splinter hemorrhages, salmon patch or oil spot dyschromia, or onycholysis and salmon patch dyschromia.
In a German cross-sectional study of patients with psoriasis, nails were one of the body sites that have the greatest impact on quality of life – especially those in younger age groups.
While topical treatments are generally considered first for limited disease involving special areas such as the nails, systemic therapy is warranted in patients with moderate-to-severe involvement of specific sites or in those refractory to topical therapy, Dr. Armstrong said.
In 2018, Indian researchers published results from an open-label study of 17 patients, with nail psoriasis, comparing three treatments . Patients were assigned to three groups of 30 nails each and treated with intramatricial injections of triamcinolone acetonide (10 mg/mL), methotrexate (25 mg/mL), and cyclosporine (50 mg/mL), respectively. Each nail was treated with two injections at 6-week intervals and graded at 24 weeks using the Nail Psoriasis Severity Index (NAPSI). In the triamcinolone acetonide and methotrexate groups, 50% of treated nails showed a greater than 75% improvement at 24 weeks, compared with 33% of those in the cyclosporine group. The most side effects occurred in the nails treated with cyclosporine.
When Dr. Armstrong performs intramatricial injections, she uses triamcinolone acetonide at 10 mg/mL. However, she said, “my favorite way of treating severe nail psoriasis is with biologics.”
In an early study of patients with moderate to severe psoriasis treated with the tumor necrosis factor blocker adalimumab 80 mg subcutaneously at week 0, followed by 40 mg subcutaneously every other week from weeks 1 to 15, a post hoc analysis on the effects on nail psoriasis showed a 10-point decrease in the median NAPSI score through week 16 – from 21 to 11 .
In VOYAGE 2, which compared the interleukin-23 blocker guselkumab and adalimumab in patients with moderate to severe psoriasis, the mean percent improvement from baseline in the NAPSI score was similar in patients treated with adalimumab or guselkumab at week 16 (39.6% vs. 46.9%, respectively) and at week 24 (55% vs. 53.7%).
In another study of patients with nail psoriasis, researchers evaluated the efficacy of the IL-17A antagonist secukinumab 150 mg, 300 mg, or placebo at weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter for 2.5 years. At 2.5 years, the mean reduction in NAPSI score was 63.6% in the secukinumab 150 mg group and 73.3% in the secukinumab 300 mg group.
“I do have to tell my patients what to expect, because the nails grow out slowly, but over time we do see this increase in efficacy,” Dr. Armstrong said.
Studies of another IL-17A antagonist, ixekizumab, have yielded positive results as well, she noted. In 2021, Taiwanese researchers published a systematic review and network meta-analysis to evaluate the efficacy of small molecule inhibitors and biologics in treating nail psoriasis. They drew from 39 studies involving 15,673 patients with nail psoriasis and found that the oral Janus kinase inhibitor tofacitinib and ixekizumab had the best efficacy for treating nail psoriasis in 10-16 weeks and 24-26 weeks, respectively.
“They found that overall, the biologics have a good effect on nail psoriasis and that the treatment effects are overall quite similar,” Dr. Armstrong said.
Dr. Armstrong disclosed that she is a consultant or adviser for numerous pharmaceutical companies. She has also received research funding from Bristol-Myers Squibb, Dermavant, Dermira, Leo, Lilly, Pfizer, and UCB Pharma.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
What’s holding back physicians from prescribing biosimilars? Four specialties weigh in
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.