User login
MD-IQ only
Factor XI inhibitors: The promise of a truly safe anticoagulant?
The quest to find an anticoagulant that can prevent strokes, cardiovascular events, and venous thrombosis without significantly increasing risk of bleeding is something of a holy grail in cardiovascular medicine. Could the latest focus of interest in this field – the factor XI inhibitors – be the long–sought-after answer?
Topline results from the largest study so far of a factor XI inhibitor – released on Sep. 18 – are indeed very encouraging. The phase 2 AZALEA-TIMI 71 study was stopped early because of an “overwhelming” reduction in major and clinically relevant nonmajor bleeding shown with the factor XI inhibitor abelacimab (Anthos), compared with apixaban for patients with atrial fibrillation (AFib).
Very few other data from this study have yet been released. Full results are due to be presented at the scientific sessions of the American Heart Association in November. Researchers in the field are optimistic that this new class of drugs may allow millions more patients who are at risk of thrombotic events but are concerned about bleeding risk to be treated, with a consequent reduction in strokes and possibly cardiovascular events as well.
Why factor XI?
In natural physiology, there are two ongoing processes: hemostasis – a set of actions that cause bleeding to stop after an injury – and thrombosis – a pathologic clotting process in which thrombus is formed and causes a stroke, MI, or deep venous thrombosis (DVT).
In patients prone to pathologic clotting, such as those with AFib, the balance of these two processes has shifted toward thrombosis, so anticoagulants are used to reduce the thrombotic risks. For many years, the only available oral anticoagulant was warfarin, a vitamin K antagonist that was very effective at preventing strokes but that comes with a high risk for bleeding, including intracranial hemorrhage (ICH) and fatal bleeding.
The introduction of the direct-acting anticoagulants (DOACs) a few years ago was a step forward in that these drugs have been shown to be as effective as warfarin but are associated with a lower risk of bleeding, particularly of ICH and fatal bleeding. But they still cause bleeding, and concerns over that risk of bleeding prevent millions of patients from taking these drugs and receiving protection against stroke.
John Alexander, MD, professor of medicine at Duke University Medical Center, Durham, N.C., a researcher active in this area, notes that “while the DOACs cause less bleeding than warfarin, they still cause two or three times more bleeding than placebo, and there is a huge, unmet need for safer anticoagulants that don’t cause as much bleeding. We are hopeful that factor XI inhibitors might be those anticoagulants.”
The lead investigator the AZALEA study, Christian Ruff, MD, professor of medicine at Brigham and Women’s Hospital, Boston, explained why it is thought that factor XI inhibitors may be different.
“There’s a lot of different clotting factors, and most of them converge in a central pathway. The problem, therefore, with anticoagulants used to date that block one of these factors is that they prevent clotting but also cause bleeding.
“It has been discovered that factor XI has a really unique position in the cascade of how our body forms clots in that it seems to be important in clot formation, but it doesn’t seem to play a major role in our ability to heal and repair blood vessels.”
Another doctor involved in the field, Manesh Patel, MD, chief of cardiology at Duke University Medical Center, added, “We think that factor XI inhibitors may prevent the pathologic formation of thrombosis while allowing formation of thrombus for natural hemostasis to prevent bleeding. That is why they are so promising.”
This correlates with epidemiologic data suggesting that patients with a genetic factor XI deficiency have low rates of stroke and MI but don’t appear to bleed spontaneously, Dr. Patel notes.
Candidates in development
The pharmaceutical industry is on the case with several factor XI inhibitors now in clinical development. At present, three main candidates lead the field. These are abelacimab (Anthos), a monoclonal antibody given by subcutaneous injection once a month; and two small molecules, milvexian (BMS/Janssen) and asundexian (Bayer), which are both given orally.
Phase 3 trials of these three factor XI inhibitors have recently started for a variety of thrombotic indications, including the prevention of stroke in patients with AFib, prevention of recurrent stroke in patients with ischemic stroke, and prevention of future cardiovascular events in patients with acute coronary syndrome (ACS).
Dr. Alexander, who has been involved in clinical trials of both milvexian and asundexian, commented: “We have pretty good data from a number of phase 2 trials now that these factor XI inhibitors at the doses used in these studies cause a lot less bleeding than therapeutic doses of DOACs and low-molecular-weight heparins.”
He pointed out that, in addition to the AZALEA trial with abelacimab, the phase 2 PACIFIC program of studies has shown less bleeding with asundexian than with apixaban in patients with AFib and a similar amount of bleeding as placebo in ACS/stroke patients on top of antiplatelet therapy. Milvexian has also shown similar results in the AXIOMATIC program of studies.
Dr. Ruff noted that the biggest need for new anticoagulants in general is in the AFib population. “Atrial fibrillation is one of the most common medical conditions in the world. Approximately one in every three people will develop AFib in their lifetime, and it is associated with more than a fivefold increased risk of stroke. But up to half of patients with AFib currently do not take anticoagulants because of concerns about bleeding risks, so these patients are being left unprotected from stroke risk.”
Dr. Ruff pointed out that the AZALEA study was the largest and longest study of a factor XI inhibitor to date; 1,287 patients were followed for a median of 2 years.
“This was the first trial of long-term administration of factor XI inhibitor against a full-dose DOAC, and it was stopped because of an overwhelming reduction in a major bleeding with abelacimab, compared with rivaroxaban,” he noted. “That is very encouraging. It looks like our quest to develop a safe anticoagulant with much lower rates of bleeding, compared with standard of care, seems to have been borne out. I think the field is very excited that we may finally have something that protects patients from thrombosis whilst being much safer than current agents.”
While all this sounds very promising, for these drugs to be successful, in addition to reducing bleeding risk, they will also have to be effective at preventing strokes and other thrombotic events.
“While we are pretty sure that factor XI inhibitors will cause less bleeding than current anticoagulants, what is unknown still is how effective they will be at preventing pathologic blood clots,” Dr. Alexander points out.
“We have some data from studies of these drugs in DVT prophylaxis after orthopedic surgery which suggest that they are effective in preventing blood clots in that scenario. But we don’t know yet about whether they can prevent pathologic blood clots that occur in AFib patients or in poststroke or post-ACS patients. Phase 3 studies are now underway with these three leading drug candidates which will answer some of these questions.”
Dr. Patel agrees that the efficacy data in the phase 3 trials will be key to the success of these drugs. “That is a very important part of the puzzle that is still missing,” he says.
Dr. Ruff notes that the AZALEA study will provide some data on efficacy. “But we already know that in the orthopedic surgery trials there was a 70%-80% reduction in VTE with abelacimab (at the 150-mg dose going forward) vs. prophylactic doses of low-molecular-weight heparin. And we know from the DOACs that the doses preventing clots on the venous side also translated into preventing strokes on the [AFib] side. So that is very encouraging,” Dr. Ruff adds.
Potential indications
The three leading factor XI inhibitors have slightly different phase 3 development programs.
Dr. Ruff notes that not every agent is being investigated in phase 3 trials for all the potential indications, but all three are going for the AFib indication. “This is by far the biggest population, the biggest market, and the biggest clinical need for these agents,” he says.
While the milvexian and asundexian trials are using an active comparator – pitting the factor XI inhibitors against apixaban in AFib patients – the Anthos LILAC trial is taking a slightly different approach and is comparing abelacimab with placebo in patients with AFib who are not currently taking an anticoagulant because of concerns about bleeding risk.
Janssen/BMS is conducting two other phase 3 trials of milvexian in their LIBREXIA phase 3 program. Those trials involve poststroke patients and ACS patients. Bayer is also involved in a poststroke trial of asundexian as part of its OCEANIC phase 3 program.
Dr. Ruff points out that anticoagulants currently do not have a large role in the poststroke or post-ACS population. “But the hope is that, if factor XI inhibitors are so safe, then there will be more enthusiasm about using an anticoagulant on top of antiplatelet therapy, which is the cornerstone of therapy in atherosclerotic cardiovascular disease.”
In addition to its phase 3 LILAC study in patients with AFib, Anthos is conducting two major phase 3 trials with abelacimab for the treatment of cancer-associated venous thromboembolism.
Dr. Ruff notes that the indication of postsurgery or general prevention of VTE is not being pursued at present.
“The orthopedic surgery studies were done mainly for dose finding and proof of principle reasons,” he explains. “In orthopedic surgery the window for anticoagulation is quite short – a few weeks or months. And for the prevention of recurrent VTE in general in the community, those people are at a relatively low risk of bleeding, so there may not be much advantage of the factor XI inhibitors, whereas AFib patients and those with stroke or ACS are usually older and have a much higher bleeding risk. I think this is where the advantages of an anticoagulant with a lower bleeding risk are most needed.”
Dr. Alexander points out that to date anticoagulants have shown more efficacy in venous clotting, which appears to be more dependent on coagulation factors and less dependent on platelets. “Atrial fibrillation is a mix between venous and arterial clotting, but it has more similarities to venous, so I think AFib is a place where new anticoagulants such as the factor XI inhibitors are more likely to have success,” he suggests.
“So far, anticoagulants have had a less clear long-term role in the poststroke and post-ACS populations, so these indications may be a more difficult goal,” he added.
The phase 3 studies are just starting and will take a few years before results are known.
Differences between the agents
The three factor XI inhibitors also have some differences. Dr. Ruff points out that most important will be the safety and efficacy of the drugs in phase 3 trials.
“Early data suggest that the various agents being developed may not have equal inhibition of factor XI. The monoclonal antibody abelacimab may produce a higher degree of inhibition than the small molecules. But we don’t know if that matters or not – whether we need to achieve a certain threshold to prevent stroke. The efficacy and safety data from the phase 3 trials are what will primarily guide use.”
There are also differences in formulations and dosage. Abelacimab is administered by subcutaneous injection once a month and has a long duration of activity, whereas the small molecules are taken orally and their duration of action is much shorter.
Dr. Ruff notes: “If these drugs cause bleeding, having a long-acting drug like abelacimab could be a disadvantage because we wouldn’t be able to stop it. But if they are very safe with regard to bleeding, then having the drug hang around for a long time is not necessarily a disadvantage, and it may improve compliance. These older patients often miss doses, and with a shorter-acting drug, that will mean they will be unprotected from stroke risk for a period of time, so there is a trade-off here.”
Dr. Ruff says that the AZALEA phase 2 study will provide some data on patients being managed around procedures. “The hope is that these drugs are so safe that they will not have to be stopped for procedures. And then the compliance issue of a once-a-month dosing would be an advantage.”
Dr. Patel says he believes there is a place for different formations. “Some patients may prefer a once-monthly injection; others will prefer a daily tablet. It may come down to patient preference, but a lot will depend on the study results with the different agents,” he commented.
What effect could these drugs have?
If these drugs do show efficacy in these phase 3 trials, what difference will they make to clinical practice? The potential appears to be very large.
“If these drugs are as effective at preventing strokes as DOACs, they will be a huge breakthrough, and there is good reason to think they would replace the DOACs,” Dr. Alexander says. “It would be a really big deal to have an anticoagulant that causes almost no bleeding and could prevent clots as well as the DOACs. This would enable a lot more patients to receive protection against stroke.”
Dr. Alexander believes the surgery studies are hopeful. “They show that the factor XI inhibitors are doing something to prevent blood clots. The big question is whether they are as effective as what we already have for the prevention of stroke and if not, what is the trade-off with bleeding?”
He points out that, even if the factor XI inhibitors are not as effective as DOACs but are found to be much safer, they might still have a potential clinical role, especially for those patients who currently do not take an anticoagulant because of concerns regarding bleeding.
But Dr. Patel points out that there is always the issue of costs with new drugs. “New drugs are always expensive. The DOACS are just about to become generic, and there will inevitably be concerns about access to an expensive new therapy.”
Dr. Alexander adds: “Yes, costs could be an issue, but a safer drug will definitely help to get more patients treated and in preventing more strokes, which would be a great thing.”
Dr. Patel has received grants from and acts as an adviser to Bayer (asundexian) and Janssen (milvexian). Dr. Alexander receives research funding from Bayer. Dr. Ruff receives research funding from Anthos for abelacimab trials, is on an AFib executive committee for BMS/Janssen, and has been on an advisory board for Bayer.
A version of this article first appeared on Medscape.com.
The quest to find an anticoagulant that can prevent strokes, cardiovascular events, and venous thrombosis without significantly increasing risk of bleeding is something of a holy grail in cardiovascular medicine. Could the latest focus of interest in this field – the factor XI inhibitors – be the long–sought-after answer?
Topline results from the largest study so far of a factor XI inhibitor – released on Sep. 18 – are indeed very encouraging. The phase 2 AZALEA-TIMI 71 study was stopped early because of an “overwhelming” reduction in major and clinically relevant nonmajor bleeding shown with the factor XI inhibitor abelacimab (Anthos), compared with apixaban for patients with atrial fibrillation (AFib).
Very few other data from this study have yet been released. Full results are due to be presented at the scientific sessions of the American Heart Association in November. Researchers in the field are optimistic that this new class of drugs may allow millions more patients who are at risk of thrombotic events but are concerned about bleeding risk to be treated, with a consequent reduction in strokes and possibly cardiovascular events as well.
Why factor XI?
In natural physiology, there are two ongoing processes: hemostasis – a set of actions that cause bleeding to stop after an injury – and thrombosis – a pathologic clotting process in which thrombus is formed and causes a stroke, MI, or deep venous thrombosis (DVT).
In patients prone to pathologic clotting, such as those with AFib, the balance of these two processes has shifted toward thrombosis, so anticoagulants are used to reduce the thrombotic risks. For many years, the only available oral anticoagulant was warfarin, a vitamin K antagonist that was very effective at preventing strokes but that comes with a high risk for bleeding, including intracranial hemorrhage (ICH) and fatal bleeding.
The introduction of the direct-acting anticoagulants (DOACs) a few years ago was a step forward in that these drugs have been shown to be as effective as warfarin but are associated with a lower risk of bleeding, particularly of ICH and fatal bleeding. But they still cause bleeding, and concerns over that risk of bleeding prevent millions of patients from taking these drugs and receiving protection against stroke.
John Alexander, MD, professor of medicine at Duke University Medical Center, Durham, N.C., a researcher active in this area, notes that “while the DOACs cause less bleeding than warfarin, they still cause two or three times more bleeding than placebo, and there is a huge, unmet need for safer anticoagulants that don’t cause as much bleeding. We are hopeful that factor XI inhibitors might be those anticoagulants.”
The lead investigator the AZALEA study, Christian Ruff, MD, professor of medicine at Brigham and Women’s Hospital, Boston, explained why it is thought that factor XI inhibitors may be different.
“There’s a lot of different clotting factors, and most of them converge in a central pathway. The problem, therefore, with anticoagulants used to date that block one of these factors is that they prevent clotting but also cause bleeding.
“It has been discovered that factor XI has a really unique position in the cascade of how our body forms clots in that it seems to be important in clot formation, but it doesn’t seem to play a major role in our ability to heal and repair blood vessels.”
Another doctor involved in the field, Manesh Patel, MD, chief of cardiology at Duke University Medical Center, added, “We think that factor XI inhibitors may prevent the pathologic formation of thrombosis while allowing formation of thrombus for natural hemostasis to prevent bleeding. That is why they are so promising.”
This correlates with epidemiologic data suggesting that patients with a genetic factor XI deficiency have low rates of stroke and MI but don’t appear to bleed spontaneously, Dr. Patel notes.
Candidates in development
The pharmaceutical industry is on the case with several factor XI inhibitors now in clinical development. At present, three main candidates lead the field. These are abelacimab (Anthos), a monoclonal antibody given by subcutaneous injection once a month; and two small molecules, milvexian (BMS/Janssen) and asundexian (Bayer), which are both given orally.
Phase 3 trials of these three factor XI inhibitors have recently started for a variety of thrombotic indications, including the prevention of stroke in patients with AFib, prevention of recurrent stroke in patients with ischemic stroke, and prevention of future cardiovascular events in patients with acute coronary syndrome (ACS).
Dr. Alexander, who has been involved in clinical trials of both milvexian and asundexian, commented: “We have pretty good data from a number of phase 2 trials now that these factor XI inhibitors at the doses used in these studies cause a lot less bleeding than therapeutic doses of DOACs and low-molecular-weight heparins.”
He pointed out that, in addition to the AZALEA trial with abelacimab, the phase 2 PACIFIC program of studies has shown less bleeding with asundexian than with apixaban in patients with AFib and a similar amount of bleeding as placebo in ACS/stroke patients on top of antiplatelet therapy. Milvexian has also shown similar results in the AXIOMATIC program of studies.
Dr. Ruff noted that the biggest need for new anticoagulants in general is in the AFib population. “Atrial fibrillation is one of the most common medical conditions in the world. Approximately one in every three people will develop AFib in their lifetime, and it is associated with more than a fivefold increased risk of stroke. But up to half of patients with AFib currently do not take anticoagulants because of concerns about bleeding risks, so these patients are being left unprotected from stroke risk.”
Dr. Ruff pointed out that the AZALEA study was the largest and longest study of a factor XI inhibitor to date; 1,287 patients were followed for a median of 2 years.
“This was the first trial of long-term administration of factor XI inhibitor against a full-dose DOAC, and it was stopped because of an overwhelming reduction in a major bleeding with abelacimab, compared with rivaroxaban,” he noted. “That is very encouraging. It looks like our quest to develop a safe anticoagulant with much lower rates of bleeding, compared with standard of care, seems to have been borne out. I think the field is very excited that we may finally have something that protects patients from thrombosis whilst being much safer than current agents.”
While all this sounds very promising, for these drugs to be successful, in addition to reducing bleeding risk, they will also have to be effective at preventing strokes and other thrombotic events.
“While we are pretty sure that factor XI inhibitors will cause less bleeding than current anticoagulants, what is unknown still is how effective they will be at preventing pathologic blood clots,” Dr. Alexander points out.
“We have some data from studies of these drugs in DVT prophylaxis after orthopedic surgery which suggest that they are effective in preventing blood clots in that scenario. But we don’t know yet about whether they can prevent pathologic blood clots that occur in AFib patients or in poststroke or post-ACS patients. Phase 3 studies are now underway with these three leading drug candidates which will answer some of these questions.”
Dr. Patel agrees that the efficacy data in the phase 3 trials will be key to the success of these drugs. “That is a very important part of the puzzle that is still missing,” he says.
Dr. Ruff notes that the AZALEA study will provide some data on efficacy. “But we already know that in the orthopedic surgery trials there was a 70%-80% reduction in VTE with abelacimab (at the 150-mg dose going forward) vs. prophylactic doses of low-molecular-weight heparin. And we know from the DOACs that the doses preventing clots on the venous side also translated into preventing strokes on the [AFib] side. So that is very encouraging,” Dr. Ruff adds.
Potential indications
The three leading factor XI inhibitors have slightly different phase 3 development programs.
Dr. Ruff notes that not every agent is being investigated in phase 3 trials for all the potential indications, but all three are going for the AFib indication. “This is by far the biggest population, the biggest market, and the biggest clinical need for these agents,” he says.
While the milvexian and asundexian trials are using an active comparator – pitting the factor XI inhibitors against apixaban in AFib patients – the Anthos LILAC trial is taking a slightly different approach and is comparing abelacimab with placebo in patients with AFib who are not currently taking an anticoagulant because of concerns about bleeding risk.
Janssen/BMS is conducting two other phase 3 trials of milvexian in their LIBREXIA phase 3 program. Those trials involve poststroke patients and ACS patients. Bayer is also involved in a poststroke trial of asundexian as part of its OCEANIC phase 3 program.
Dr. Ruff points out that anticoagulants currently do not have a large role in the poststroke or post-ACS population. “But the hope is that, if factor XI inhibitors are so safe, then there will be more enthusiasm about using an anticoagulant on top of antiplatelet therapy, which is the cornerstone of therapy in atherosclerotic cardiovascular disease.”
In addition to its phase 3 LILAC study in patients with AFib, Anthos is conducting two major phase 3 trials with abelacimab for the treatment of cancer-associated venous thromboembolism.
Dr. Ruff notes that the indication of postsurgery or general prevention of VTE is not being pursued at present.
“The orthopedic surgery studies were done mainly for dose finding and proof of principle reasons,” he explains. “In orthopedic surgery the window for anticoagulation is quite short – a few weeks or months. And for the prevention of recurrent VTE in general in the community, those people are at a relatively low risk of bleeding, so there may not be much advantage of the factor XI inhibitors, whereas AFib patients and those with stroke or ACS are usually older and have a much higher bleeding risk. I think this is where the advantages of an anticoagulant with a lower bleeding risk are most needed.”
Dr. Alexander points out that to date anticoagulants have shown more efficacy in venous clotting, which appears to be more dependent on coagulation factors and less dependent on platelets. “Atrial fibrillation is a mix between venous and arterial clotting, but it has more similarities to venous, so I think AFib is a place where new anticoagulants such as the factor XI inhibitors are more likely to have success,” he suggests.
“So far, anticoagulants have had a less clear long-term role in the poststroke and post-ACS populations, so these indications may be a more difficult goal,” he added.
The phase 3 studies are just starting and will take a few years before results are known.
Differences between the agents
The three factor XI inhibitors also have some differences. Dr. Ruff points out that most important will be the safety and efficacy of the drugs in phase 3 trials.
“Early data suggest that the various agents being developed may not have equal inhibition of factor XI. The monoclonal antibody abelacimab may produce a higher degree of inhibition than the small molecules. But we don’t know if that matters or not – whether we need to achieve a certain threshold to prevent stroke. The efficacy and safety data from the phase 3 trials are what will primarily guide use.”
There are also differences in formulations and dosage. Abelacimab is administered by subcutaneous injection once a month and has a long duration of activity, whereas the small molecules are taken orally and their duration of action is much shorter.
Dr. Ruff notes: “If these drugs cause bleeding, having a long-acting drug like abelacimab could be a disadvantage because we wouldn’t be able to stop it. But if they are very safe with regard to bleeding, then having the drug hang around for a long time is not necessarily a disadvantage, and it may improve compliance. These older patients often miss doses, and with a shorter-acting drug, that will mean they will be unprotected from stroke risk for a period of time, so there is a trade-off here.”
Dr. Ruff says that the AZALEA phase 2 study will provide some data on patients being managed around procedures. “The hope is that these drugs are so safe that they will not have to be stopped for procedures. And then the compliance issue of a once-a-month dosing would be an advantage.”
Dr. Patel says he believes there is a place for different formations. “Some patients may prefer a once-monthly injection; others will prefer a daily tablet. It may come down to patient preference, but a lot will depend on the study results with the different agents,” he commented.
What effect could these drugs have?
If these drugs do show efficacy in these phase 3 trials, what difference will they make to clinical practice? The potential appears to be very large.
“If these drugs are as effective at preventing strokes as DOACs, they will be a huge breakthrough, and there is good reason to think they would replace the DOACs,” Dr. Alexander says. “It would be a really big deal to have an anticoagulant that causes almost no bleeding and could prevent clots as well as the DOACs. This would enable a lot more patients to receive protection against stroke.”
Dr. Alexander believes the surgery studies are hopeful. “They show that the factor XI inhibitors are doing something to prevent blood clots. The big question is whether they are as effective as what we already have for the prevention of stroke and if not, what is the trade-off with bleeding?”
He points out that, even if the factor XI inhibitors are not as effective as DOACs but are found to be much safer, they might still have a potential clinical role, especially for those patients who currently do not take an anticoagulant because of concerns regarding bleeding.
But Dr. Patel points out that there is always the issue of costs with new drugs. “New drugs are always expensive. The DOACS are just about to become generic, and there will inevitably be concerns about access to an expensive new therapy.”
Dr. Alexander adds: “Yes, costs could be an issue, but a safer drug will definitely help to get more patients treated and in preventing more strokes, which would be a great thing.”
Dr. Patel has received grants from and acts as an adviser to Bayer (asundexian) and Janssen (milvexian). Dr. Alexander receives research funding from Bayer. Dr. Ruff receives research funding from Anthos for abelacimab trials, is on an AFib executive committee for BMS/Janssen, and has been on an advisory board for Bayer.
A version of this article first appeared on Medscape.com.
The quest to find an anticoagulant that can prevent strokes, cardiovascular events, and venous thrombosis without significantly increasing risk of bleeding is something of a holy grail in cardiovascular medicine. Could the latest focus of interest in this field – the factor XI inhibitors – be the long–sought-after answer?
Topline results from the largest study so far of a factor XI inhibitor – released on Sep. 18 – are indeed very encouraging. The phase 2 AZALEA-TIMI 71 study was stopped early because of an “overwhelming” reduction in major and clinically relevant nonmajor bleeding shown with the factor XI inhibitor abelacimab (Anthos), compared with apixaban for patients with atrial fibrillation (AFib).
Very few other data from this study have yet been released. Full results are due to be presented at the scientific sessions of the American Heart Association in November. Researchers in the field are optimistic that this new class of drugs may allow millions more patients who are at risk of thrombotic events but are concerned about bleeding risk to be treated, with a consequent reduction in strokes and possibly cardiovascular events as well.
Why factor XI?
In natural physiology, there are two ongoing processes: hemostasis – a set of actions that cause bleeding to stop after an injury – and thrombosis – a pathologic clotting process in which thrombus is formed and causes a stroke, MI, or deep venous thrombosis (DVT).
In patients prone to pathologic clotting, such as those with AFib, the balance of these two processes has shifted toward thrombosis, so anticoagulants are used to reduce the thrombotic risks. For many years, the only available oral anticoagulant was warfarin, a vitamin K antagonist that was very effective at preventing strokes but that comes with a high risk for bleeding, including intracranial hemorrhage (ICH) and fatal bleeding.
The introduction of the direct-acting anticoagulants (DOACs) a few years ago was a step forward in that these drugs have been shown to be as effective as warfarin but are associated with a lower risk of bleeding, particularly of ICH and fatal bleeding. But they still cause bleeding, and concerns over that risk of bleeding prevent millions of patients from taking these drugs and receiving protection against stroke.
John Alexander, MD, professor of medicine at Duke University Medical Center, Durham, N.C., a researcher active in this area, notes that “while the DOACs cause less bleeding than warfarin, they still cause two or three times more bleeding than placebo, and there is a huge, unmet need for safer anticoagulants that don’t cause as much bleeding. We are hopeful that factor XI inhibitors might be those anticoagulants.”
The lead investigator the AZALEA study, Christian Ruff, MD, professor of medicine at Brigham and Women’s Hospital, Boston, explained why it is thought that factor XI inhibitors may be different.
“There’s a lot of different clotting factors, and most of them converge in a central pathway. The problem, therefore, with anticoagulants used to date that block one of these factors is that they prevent clotting but also cause bleeding.
“It has been discovered that factor XI has a really unique position in the cascade of how our body forms clots in that it seems to be important in clot formation, but it doesn’t seem to play a major role in our ability to heal and repair blood vessels.”
Another doctor involved in the field, Manesh Patel, MD, chief of cardiology at Duke University Medical Center, added, “We think that factor XI inhibitors may prevent the pathologic formation of thrombosis while allowing formation of thrombus for natural hemostasis to prevent bleeding. That is why they are so promising.”
This correlates with epidemiologic data suggesting that patients with a genetic factor XI deficiency have low rates of stroke and MI but don’t appear to bleed spontaneously, Dr. Patel notes.
Candidates in development
The pharmaceutical industry is on the case with several factor XI inhibitors now in clinical development. At present, three main candidates lead the field. These are abelacimab (Anthos), a monoclonal antibody given by subcutaneous injection once a month; and two small molecules, milvexian (BMS/Janssen) and asundexian (Bayer), which are both given orally.
Phase 3 trials of these three factor XI inhibitors have recently started for a variety of thrombotic indications, including the prevention of stroke in patients with AFib, prevention of recurrent stroke in patients with ischemic stroke, and prevention of future cardiovascular events in patients with acute coronary syndrome (ACS).
Dr. Alexander, who has been involved in clinical trials of both milvexian and asundexian, commented: “We have pretty good data from a number of phase 2 trials now that these factor XI inhibitors at the doses used in these studies cause a lot less bleeding than therapeutic doses of DOACs and low-molecular-weight heparins.”
He pointed out that, in addition to the AZALEA trial with abelacimab, the phase 2 PACIFIC program of studies has shown less bleeding with asundexian than with apixaban in patients with AFib and a similar amount of bleeding as placebo in ACS/stroke patients on top of antiplatelet therapy. Milvexian has also shown similar results in the AXIOMATIC program of studies.
Dr. Ruff noted that the biggest need for new anticoagulants in general is in the AFib population. “Atrial fibrillation is one of the most common medical conditions in the world. Approximately one in every three people will develop AFib in their lifetime, and it is associated with more than a fivefold increased risk of stroke. But up to half of patients with AFib currently do not take anticoagulants because of concerns about bleeding risks, so these patients are being left unprotected from stroke risk.”
Dr. Ruff pointed out that the AZALEA study was the largest and longest study of a factor XI inhibitor to date; 1,287 patients were followed for a median of 2 years.
“This was the first trial of long-term administration of factor XI inhibitor against a full-dose DOAC, and it was stopped because of an overwhelming reduction in a major bleeding with abelacimab, compared with rivaroxaban,” he noted. “That is very encouraging. It looks like our quest to develop a safe anticoagulant with much lower rates of bleeding, compared with standard of care, seems to have been borne out. I think the field is very excited that we may finally have something that protects patients from thrombosis whilst being much safer than current agents.”
While all this sounds very promising, for these drugs to be successful, in addition to reducing bleeding risk, they will also have to be effective at preventing strokes and other thrombotic events.
“While we are pretty sure that factor XI inhibitors will cause less bleeding than current anticoagulants, what is unknown still is how effective they will be at preventing pathologic blood clots,” Dr. Alexander points out.
“We have some data from studies of these drugs in DVT prophylaxis after orthopedic surgery which suggest that they are effective in preventing blood clots in that scenario. But we don’t know yet about whether they can prevent pathologic blood clots that occur in AFib patients or in poststroke or post-ACS patients. Phase 3 studies are now underway with these three leading drug candidates which will answer some of these questions.”
Dr. Patel agrees that the efficacy data in the phase 3 trials will be key to the success of these drugs. “That is a very important part of the puzzle that is still missing,” he says.
Dr. Ruff notes that the AZALEA study will provide some data on efficacy. “But we already know that in the orthopedic surgery trials there was a 70%-80% reduction in VTE with abelacimab (at the 150-mg dose going forward) vs. prophylactic doses of low-molecular-weight heparin. And we know from the DOACs that the doses preventing clots on the venous side also translated into preventing strokes on the [AFib] side. So that is very encouraging,” Dr. Ruff adds.
Potential indications
The three leading factor XI inhibitors have slightly different phase 3 development programs.
Dr. Ruff notes that not every agent is being investigated in phase 3 trials for all the potential indications, but all three are going for the AFib indication. “This is by far the biggest population, the biggest market, and the biggest clinical need for these agents,” he says.
While the milvexian and asundexian trials are using an active comparator – pitting the factor XI inhibitors against apixaban in AFib patients – the Anthos LILAC trial is taking a slightly different approach and is comparing abelacimab with placebo in patients with AFib who are not currently taking an anticoagulant because of concerns about bleeding risk.
Janssen/BMS is conducting two other phase 3 trials of milvexian in their LIBREXIA phase 3 program. Those trials involve poststroke patients and ACS patients. Bayer is also involved in a poststroke trial of asundexian as part of its OCEANIC phase 3 program.
Dr. Ruff points out that anticoagulants currently do not have a large role in the poststroke or post-ACS population. “But the hope is that, if factor XI inhibitors are so safe, then there will be more enthusiasm about using an anticoagulant on top of antiplatelet therapy, which is the cornerstone of therapy in atherosclerotic cardiovascular disease.”
In addition to its phase 3 LILAC study in patients with AFib, Anthos is conducting two major phase 3 trials with abelacimab for the treatment of cancer-associated venous thromboembolism.
Dr. Ruff notes that the indication of postsurgery or general prevention of VTE is not being pursued at present.
“The orthopedic surgery studies were done mainly for dose finding and proof of principle reasons,” he explains. “In orthopedic surgery the window for anticoagulation is quite short – a few weeks or months. And for the prevention of recurrent VTE in general in the community, those people are at a relatively low risk of bleeding, so there may not be much advantage of the factor XI inhibitors, whereas AFib patients and those with stroke or ACS are usually older and have a much higher bleeding risk. I think this is where the advantages of an anticoagulant with a lower bleeding risk are most needed.”
Dr. Alexander points out that to date anticoagulants have shown more efficacy in venous clotting, which appears to be more dependent on coagulation factors and less dependent on platelets. “Atrial fibrillation is a mix between venous and arterial clotting, but it has more similarities to venous, so I think AFib is a place where new anticoagulants such as the factor XI inhibitors are more likely to have success,” he suggests.
“So far, anticoagulants have had a less clear long-term role in the poststroke and post-ACS populations, so these indications may be a more difficult goal,” he added.
The phase 3 studies are just starting and will take a few years before results are known.
Differences between the agents
The three factor XI inhibitors also have some differences. Dr. Ruff points out that most important will be the safety and efficacy of the drugs in phase 3 trials.
“Early data suggest that the various agents being developed may not have equal inhibition of factor XI. The monoclonal antibody abelacimab may produce a higher degree of inhibition than the small molecules. But we don’t know if that matters or not – whether we need to achieve a certain threshold to prevent stroke. The efficacy and safety data from the phase 3 trials are what will primarily guide use.”
There are also differences in formulations and dosage. Abelacimab is administered by subcutaneous injection once a month and has a long duration of activity, whereas the small molecules are taken orally and their duration of action is much shorter.
Dr. Ruff notes: “If these drugs cause bleeding, having a long-acting drug like abelacimab could be a disadvantage because we wouldn’t be able to stop it. But if they are very safe with regard to bleeding, then having the drug hang around for a long time is not necessarily a disadvantage, and it may improve compliance. These older patients often miss doses, and with a shorter-acting drug, that will mean they will be unprotected from stroke risk for a period of time, so there is a trade-off here.”
Dr. Ruff says that the AZALEA phase 2 study will provide some data on patients being managed around procedures. “The hope is that these drugs are so safe that they will not have to be stopped for procedures. And then the compliance issue of a once-a-month dosing would be an advantage.”
Dr. Patel says he believes there is a place for different formations. “Some patients may prefer a once-monthly injection; others will prefer a daily tablet. It may come down to patient preference, but a lot will depend on the study results with the different agents,” he commented.
What effect could these drugs have?
If these drugs do show efficacy in these phase 3 trials, what difference will they make to clinical practice? The potential appears to be very large.
“If these drugs are as effective at preventing strokes as DOACs, they will be a huge breakthrough, and there is good reason to think they would replace the DOACs,” Dr. Alexander says. “It would be a really big deal to have an anticoagulant that causes almost no bleeding and could prevent clots as well as the DOACs. This would enable a lot more patients to receive protection against stroke.”
Dr. Alexander believes the surgery studies are hopeful. “They show that the factor XI inhibitors are doing something to prevent blood clots. The big question is whether they are as effective as what we already have for the prevention of stroke and if not, what is the trade-off with bleeding?”
He points out that, even if the factor XI inhibitors are not as effective as DOACs but are found to be much safer, they might still have a potential clinical role, especially for those patients who currently do not take an anticoagulant because of concerns regarding bleeding.
But Dr. Patel points out that there is always the issue of costs with new drugs. “New drugs are always expensive. The DOACS are just about to become generic, and there will inevitably be concerns about access to an expensive new therapy.”
Dr. Alexander adds: “Yes, costs could be an issue, but a safer drug will definitely help to get more patients treated and in preventing more strokes, which would be a great thing.”
Dr. Patel has received grants from and acts as an adviser to Bayer (asundexian) and Janssen (milvexian). Dr. Alexander receives research funding from Bayer. Dr. Ruff receives research funding from Anthos for abelacimab trials, is on an AFib executive committee for BMS/Janssen, and has been on an advisory board for Bayer.
A version of this article first appeared on Medscape.com.
Steady VKA therapy beats switch to NOAC in frail AFib patients: FRAIL-AF
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
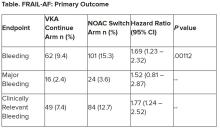
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
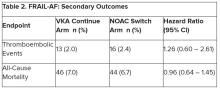
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Switching frail patients with atrial fibrillation (AFib) from anticoagulation therapy with vitamin K antagonists (VKAs) to a novel oral anticoagulant (NOAC) resulted in more bleeding without any reduction in thromboembolic complications or all-cause mortality, randomized trial results show.
The study, FRAIL-AF, is the first randomized NOAC trial to exclusively include frail older patients, said lead author Linda P.T. Joosten, MD, Julius Center for Health Sciences and Primary Care in Utrecht, the Netherlands, and these unexpected findings provide evidence that goes beyond what is currently available.
“Data from the FRAIL-AF trial showed that switching from a VKA to a NOAC should not be considered without a clear indication in frail older patients with AF[ib], as switching to a NOAC leads to 69% more bleeding,” she concluded, without any benefit on secondary clinical endpoints, including thromboembolic events and all-cause mortality.
“The results turned out different than we expected,” Dr. Joosten said. “The hypothesis of this superiority trial was that switching from VKA therapy to a NOAC would result in less bleeding. However, we observed the opposite. After the interim analysis, the data and safety monitoring board advised to stop inclusion because switching from a VKA to a NOAC was clearly contraindicated with a hazard ratio of 1.69 and a highly significant P value of .001.”
Results of FRAIL-AF were presented at the annual congress of the European Society of Cardiology and published online in the journal Circulation.
Session moderator Renate B. Schnabel, MD, interventional cardiologist with University Heart & Vascular Center Hamburg (Germany), congratulated the researchers on these “astonishing” data.
“The thing I want to emphasize here is that, in the absence of randomized controlled trial data, we should be very cautious in extrapolating data from the landmark trials to populations not enrolled in those, and to rely on observational data only,” Dr. Schnabel told Dr. Joosten. “We need randomized controlled trials that sometimes give astonishing results.”
Frailty a clinical syndrome
Frailty is “a lot more than just aging, multiple comorbidities and polypharmacy,” Dr. Joosten explained. “It’s really a clinical syndrome, with people with a high biological vulnerability, dependency on significant others, and a reduced capacity to resist stressors, all leading to a reduced homeostatic reserve.”
Frailty is common in the community, with a prevalence of about 12%, she noted, “and even more important, AF[ib] in frail older people is very common, with a prevalence of 18%. And “without any doubt, we have to adequately anticoagulate frail AF[ib] patients, as they have a high stroke risk, with an incidence of 12.4% per year,” Dr. Joosten noted, compared with 3.9% per year among nonfrail AFib patients.
NOACs are preferred over VKAs in nonfrail AFib patients, after four major trials, RE-LY with dabigatran, ROCKET-AF with rivaroxaban, ARISTOTLE with apixaban, and ENGAGE-AF with edoxaban, showed that NOAC treatment resulted in less major bleeding while stroke risk was comparable with treatment with warfarin, she noted.
The 2023 European Heart Rhythm Association consensus document on management of arrhythmias in frailty syndrome concludes that the advantages of NOACs relative to VKAs are “likely consistent” in frail and nonfrail AFib patients, but the level of evidence is low.
So it’s unknown if NOACs are preferred over VKAs in frail AFib patients, “and it’s even more questionable whether patients on VKAs should switch to NOAC therapy,” Dr. Joosten said.
This new trial aimed to answer the question of whether switching frail AFib patients currently managed on a VKA to a NOAC would reduce bleeding. FRAIL-AF was a pragmatic, multicenter, open-label, randomized, controlled superiority trial.
Older AFib patients were deemed frail if they were aged 75 years or older and had a score of 3 or more on the validated Groningen Frailty Indicator (GFI). Patients with a glomerular filtration rate of less than 30 mL/min per 1.73 m2 or with valvular AFib were excluded.
Eligible patients were then assigned randomly to switch from their international normalized ratio (INR)–guided VKA treatment with either 1 mg acenocoumarol or 3 mg phenprocoumon, to a NOAC, or to continue VKA treatment. They were followed for 12 months for the primary outcome – major bleeding or clinically relevant nonmajor bleeding complication, whichever came first – accounting for death as a competing risk.
A total of 1,330 patients were randomly assigned between January 2018 and June 2022. Their mean age was 83 years, and they had a median GFI of 4. After randomization, 6 patients in the switch-to-NOAC arm, and 1 in the continue-VKA arm were found to have exclusion criteria, so in the end, 662 patients were switched from a VKA to NOAC, while 661 continued on VKA therapy. The choice of NOAC was made by the treating physician.
Major bleeding was defined as a fatal bleeding; bleeding in a critical area or organ; bleeding leading to transfusion; and/or bleeding leading to a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more. Nonmajor bleeding was bleeding not considered major but requiring face-to-face consultation, hospitalization or increased level of care, or medical intervention.
After a prespecified futility analysis planned after 163 primary outcome events, the trial was halted when it was seen that there were 101 primary outcome events in the switch arm compared to 62 in the continue arm, Dr. Joosten said. The difference appeared to be driven by clinically relevant nonmajor bleeding.
Secondary outcomes of thromboembolic events and all-cause mortality were similar between the groups.
Completely different patients
Discussant at the meeting for the presentation was Isabelle C. Van Gelder, MD, University Medical Centre Groningen (the Netherlands). She said the results are important and relevant because it “provides data on an important gap of knowledge in our AF[ib] guidelines, and a note for all the cardiologists – this study was not done in the hospital. This trial was done in general practitioner practices, so that’s important to consider.”
Comparing FRAIL-AF patients with those of the four previous NOAC trials, “you see that enormous difference in age,” with an average age of 83 years versus 70-73 years in those trials. “These are completely different patients than have been included previously,” she said.
That GFI score of 4 or more includes patients on four or more different types of medication, as well as memory complaints, an inability to walk around the house, and problems with vision or hearing.
The finding of a 69% increase in bleeding with NOACs in FRAIL-AF was “completely unexpected, and I think that we as cardiologists and as NOAC believers did not expect it at all, but it is as clear as it is.” The curves don’t diverge immediately, but rather after 3 months or thereafter, “so it has nothing to do with the switching process. So why did it occur?”
The Netherlands has dedicated thrombosis services that might improve time in therapeutic range for VKA patients, but there is no real difference in TTRs in FRAIL-AF versus the other NOAC trials, Dr. Van Gelder noted.
The most likely suspect in her view is frailty itself, in particular the tendency for patients to be on a high number of medications. A previous study showed, for example, that polypharmacy could be used as a proxy for the effect of frailty on bleeding risk; patients on 10 or more medications had a higher risk for bleeding on treatment with rivaroxaban versus those on 4 or fewer medications.
“Therefore, in my view, why was there such a high risk of bleeding? It’s because these are other patients than we are normally used to treat, we as cardiologists,” although general practitioners see these patients all the time. “It’s all about frailty.”
NOACs are still relatively new drugs, with possible unknown interactions, she added. Because of their frailty and polypharmacy, these patients may benefit from INR control, Dr. Van Gelder speculated. “Therefore, I agree with them that we should be careful; if such old, frail patients survive on VKA, do not change medications and do not switch!”
The study was supported by the Dutch government with additional and unrestricted educational grants from Boehringer Ingelheim, BMS-Pfizer, Bayer, and Daiichi Sankyo. Dr. Joosten reported no relevant financial relationships. Dr. Van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
Medicare announces 10 drugs targeted for price cuts in 2026
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
American Geriatrics Society 2023 updated Beers Criteria highlights
Every 4 years, an interprofessional panel of experts from the American Geriatrics Society provides updated guidelines on safe prescribing of medications in older adults, known as the Beers Criteria. A 2023 update was released in May 2023 after panel review of more 1,500 clinical trials and research studies published since the last update.
Anticoagulants
Notable changes to the 2023 guidelines include updated recommendations for anticoagulation. Warfarin should be avoided as initial therapy for venous thromboembolism or nonvalvular atrial fibrillation unless there are contraindications to direct oral anticoagulants (DOACs) or other substantial barriers to use.
Rivaroxaban should also be avoided, and dabigatran used with caution in favor of apixaban, which is felt to have a better safety profile in older adults. Rivaroxaban may be considered if once daily dosing is deemed to be more clinically appropriate. Financial barriers regarding drug coverage and formulary options were acknowledged as a significant barrier to equitable access to preferred direct oral anticoagulants in older adults.
Diabetes medication
Regarding diabetes management, short-acting sulfonylureas should be avoided in addition to long-acting sulfonylureas, because of the increased risk of hypoglycemia, and cardiovascular and all-cause mortality in older adults. Sodium-glucose cotransporter 2 inhibitors as an entire class are recommended to be used with caution, as older adults are at higher risk of euglycemic ketoacidosis and urogenital infections, particularly in women in the first month of initiating treatment.
Like DOACs, the panel acknowledged that financial considerations may lead to limited options for oral diabetic treatment. In circumstances where a sulfonylurea is used, short-acting forms are preferred over long acting to reduce the risk of prolonged hypoglycemia.
Aspirin for primary prevention
Alongside the U.S. Preventive Services Task Force guideline update in 2022 regarding aspirin for primary prevention of cardiovascular disease and stroke, the Beer’s Criteria recommend against initiation of aspirin for primary prevention in older adults. Ticagrelor and prasugrel should be used with caution because of the increased risk of major bleeding in older adults over the age of 75, compared with clopidogrel. If prasugrel is used, a lower dose of 5 mg is recommended, in line with guidelines by the American College of Cardiology and American Heart Association.
Pain medication
For pain management, the Beer’s Criteria updated recommendations to avoid NSAIDs, particularly when used in combination with steroids or anticoagulants. The panel highlights that even short-term use of NSAIDs is high risk when used in combination with steroids or anticoagulants. If no other alternatives are possible, patients should be placed on a proton pump inhibitor or misoprostol while taking NSAIDs.
Baclofen should be avoided in older adults with renal insufficiency (estimated glomerular filtration rate < 60 mL/min per 1.73 m2) because of the increased risk of encephalopathy, and when used, should be given at the lowest effective dose with close monitoring for mental status changes.
Androgen and estrogen replacement therapy
For androgen replacement therapy, the panel notes that testosterone supplementation should be avoided because of cardiovascular risks unless there is confirmed hypogonadism. The panel revised their recommendation on the basis of emerging data that a history of prostate cancer is not an absolute contraindication for exogenous testosterone. A risk versus benefit discussion about exogenous testosterone should be had with a medical oncologist or urologist in those with a history of prostate cancer.
Regarding estrogen, systemic formulations should not be initiated in women over the age of 60 because of increased risk of cardiovascular events, venous thromboembolism, and dementia. In women with a history of breast cancer, vaginal estrogens are generally felt to be safe to use at low doses, such as less than 25 mcg twice weekly.
Dr. Wang is a geriatrician and general internist at Harborview Medical Center, Seattle.
Every 4 years, an interprofessional panel of experts from the American Geriatrics Society provides updated guidelines on safe prescribing of medications in older adults, known as the Beers Criteria. A 2023 update was released in May 2023 after panel review of more 1,500 clinical trials and research studies published since the last update.
Anticoagulants
Notable changes to the 2023 guidelines include updated recommendations for anticoagulation. Warfarin should be avoided as initial therapy for venous thromboembolism or nonvalvular atrial fibrillation unless there are contraindications to direct oral anticoagulants (DOACs) or other substantial barriers to use.
Rivaroxaban should also be avoided, and dabigatran used with caution in favor of apixaban, which is felt to have a better safety profile in older adults. Rivaroxaban may be considered if once daily dosing is deemed to be more clinically appropriate. Financial barriers regarding drug coverage and formulary options were acknowledged as a significant barrier to equitable access to preferred direct oral anticoagulants in older adults.
Diabetes medication
Regarding diabetes management, short-acting sulfonylureas should be avoided in addition to long-acting sulfonylureas, because of the increased risk of hypoglycemia, and cardiovascular and all-cause mortality in older adults. Sodium-glucose cotransporter 2 inhibitors as an entire class are recommended to be used with caution, as older adults are at higher risk of euglycemic ketoacidosis and urogenital infections, particularly in women in the first month of initiating treatment.
Like DOACs, the panel acknowledged that financial considerations may lead to limited options for oral diabetic treatment. In circumstances where a sulfonylurea is used, short-acting forms are preferred over long acting to reduce the risk of prolonged hypoglycemia.
Aspirin for primary prevention
Alongside the U.S. Preventive Services Task Force guideline update in 2022 regarding aspirin for primary prevention of cardiovascular disease and stroke, the Beer’s Criteria recommend against initiation of aspirin for primary prevention in older adults. Ticagrelor and prasugrel should be used with caution because of the increased risk of major bleeding in older adults over the age of 75, compared with clopidogrel. If prasugrel is used, a lower dose of 5 mg is recommended, in line with guidelines by the American College of Cardiology and American Heart Association.
Pain medication
For pain management, the Beer’s Criteria updated recommendations to avoid NSAIDs, particularly when used in combination with steroids or anticoagulants. The panel highlights that even short-term use of NSAIDs is high risk when used in combination with steroids or anticoagulants. If no other alternatives are possible, patients should be placed on a proton pump inhibitor or misoprostol while taking NSAIDs.
Baclofen should be avoided in older adults with renal insufficiency (estimated glomerular filtration rate < 60 mL/min per 1.73 m2) because of the increased risk of encephalopathy, and when used, should be given at the lowest effective dose with close monitoring for mental status changes.
Androgen and estrogen replacement therapy
For androgen replacement therapy, the panel notes that testosterone supplementation should be avoided because of cardiovascular risks unless there is confirmed hypogonadism. The panel revised their recommendation on the basis of emerging data that a history of prostate cancer is not an absolute contraindication for exogenous testosterone. A risk versus benefit discussion about exogenous testosterone should be had with a medical oncologist or urologist in those with a history of prostate cancer.
Regarding estrogen, systemic formulations should not be initiated in women over the age of 60 because of increased risk of cardiovascular events, venous thromboembolism, and dementia. In women with a history of breast cancer, vaginal estrogens are generally felt to be safe to use at low doses, such as less than 25 mcg twice weekly.
Dr. Wang is a geriatrician and general internist at Harborview Medical Center, Seattle.
Every 4 years, an interprofessional panel of experts from the American Geriatrics Society provides updated guidelines on safe prescribing of medications in older adults, known as the Beers Criteria. A 2023 update was released in May 2023 after panel review of more 1,500 clinical trials and research studies published since the last update.
Anticoagulants
Notable changes to the 2023 guidelines include updated recommendations for anticoagulation. Warfarin should be avoided as initial therapy for venous thromboembolism or nonvalvular atrial fibrillation unless there are contraindications to direct oral anticoagulants (DOACs) or other substantial barriers to use.
Rivaroxaban should also be avoided, and dabigatran used with caution in favor of apixaban, which is felt to have a better safety profile in older adults. Rivaroxaban may be considered if once daily dosing is deemed to be more clinically appropriate. Financial barriers regarding drug coverage and formulary options were acknowledged as a significant barrier to equitable access to preferred direct oral anticoagulants in older adults.
Diabetes medication
Regarding diabetes management, short-acting sulfonylureas should be avoided in addition to long-acting sulfonylureas, because of the increased risk of hypoglycemia, and cardiovascular and all-cause mortality in older adults. Sodium-glucose cotransporter 2 inhibitors as an entire class are recommended to be used with caution, as older adults are at higher risk of euglycemic ketoacidosis and urogenital infections, particularly in women in the first month of initiating treatment.
Like DOACs, the panel acknowledged that financial considerations may lead to limited options for oral diabetic treatment. In circumstances where a sulfonylurea is used, short-acting forms are preferred over long acting to reduce the risk of prolonged hypoglycemia.
Aspirin for primary prevention
Alongside the U.S. Preventive Services Task Force guideline update in 2022 regarding aspirin for primary prevention of cardiovascular disease and stroke, the Beer’s Criteria recommend against initiation of aspirin for primary prevention in older adults. Ticagrelor and prasugrel should be used with caution because of the increased risk of major bleeding in older adults over the age of 75, compared with clopidogrel. If prasugrel is used, a lower dose of 5 mg is recommended, in line with guidelines by the American College of Cardiology and American Heart Association.
Pain medication
For pain management, the Beer’s Criteria updated recommendations to avoid NSAIDs, particularly when used in combination with steroids or anticoagulants. The panel highlights that even short-term use of NSAIDs is high risk when used in combination with steroids or anticoagulants. If no other alternatives are possible, patients should be placed on a proton pump inhibitor or misoprostol while taking NSAIDs.
Baclofen should be avoided in older adults with renal insufficiency (estimated glomerular filtration rate < 60 mL/min per 1.73 m2) because of the increased risk of encephalopathy, and when used, should be given at the lowest effective dose with close monitoring for mental status changes.
Androgen and estrogen replacement therapy
For androgen replacement therapy, the panel notes that testosterone supplementation should be avoided because of cardiovascular risks unless there is confirmed hypogonadism. The panel revised their recommendation on the basis of emerging data that a history of prostate cancer is not an absolute contraindication for exogenous testosterone. A risk versus benefit discussion about exogenous testosterone should be had with a medical oncologist or urologist in those with a history of prostate cancer.
Regarding estrogen, systemic formulations should not be initiated in women over the age of 60 because of increased risk of cardiovascular events, venous thromboembolism, and dementia. In women with a history of breast cancer, vaginal estrogens are generally felt to be safe to use at low doses, such as less than 25 mcg twice weekly.
Dr. Wang is a geriatrician and general internist at Harborview Medical Center, Seattle.
Pulmonary embolism confers higher mortality long term
Topline
Long-term mortality rates among individuals who have had a pulmonary embolism are significantly higher than rates in the general population.
Methodology
Researchers investigated long-term outcomes of patients with pulmonary embolism in a single-center registry.
They followed 896 patients for up to 14 years.
Data were from consecutive cases treated between May 2005 and December 2017.
Takeaway
The total follow-up time was 3,908 patient-years (median, 3.1 years).
One-year and five-year mortality rates were 19.7% (95% confidence interval, 17.2%-22.4%) and 37.1% (95% CI, 33.6%-40.5%), respectively, for patients with pulmonary embolism.
The most frequent causes of death were cancer (28.5%), pulmonary embolism (19.4%), infections (13.9%), and cardiovascular events (11.6%).
Late mortality (>30 days) was more frequent than in the general population for patients with cancer (5-year standardized mortality ratio, 2.77; 95% CI, 2.41-3.16) and for patients without cancer (1.80; 95% CI, 1.50-2.14), compared with expected rates.
In practice
stated Johannes Eckelt, Clinic of Cardiology and Pneumology, University Medical Center Göttingen (Germany).
Source
“Long-term Mortality in Pulmonary Embolism: Results in a Single-Center Registry,” by Mr. Eckelt and colleagues was published in Research and Practice in Thrombosis and Haemostasis.
Limitations
Owing to the single-center study design, selection bias cannot be excluded, limiting the generalizability of the study findings, the authors stated.
Disclosures
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Topline
Long-term mortality rates among individuals who have had a pulmonary embolism are significantly higher than rates in the general population.
Methodology
Researchers investigated long-term outcomes of patients with pulmonary embolism in a single-center registry.
They followed 896 patients for up to 14 years.
Data were from consecutive cases treated between May 2005 and December 2017.
Takeaway
The total follow-up time was 3,908 patient-years (median, 3.1 years).
One-year and five-year mortality rates were 19.7% (95% confidence interval, 17.2%-22.4%) and 37.1% (95% CI, 33.6%-40.5%), respectively, for patients with pulmonary embolism.
The most frequent causes of death were cancer (28.5%), pulmonary embolism (19.4%), infections (13.9%), and cardiovascular events (11.6%).
Late mortality (>30 days) was more frequent than in the general population for patients with cancer (5-year standardized mortality ratio, 2.77; 95% CI, 2.41-3.16) and for patients without cancer (1.80; 95% CI, 1.50-2.14), compared with expected rates.
In practice
stated Johannes Eckelt, Clinic of Cardiology and Pneumology, University Medical Center Göttingen (Germany).
Source
“Long-term Mortality in Pulmonary Embolism: Results in a Single-Center Registry,” by Mr. Eckelt and colleagues was published in Research and Practice in Thrombosis and Haemostasis.
Limitations
Owing to the single-center study design, selection bias cannot be excluded, limiting the generalizability of the study findings, the authors stated.
Disclosures
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Topline
Long-term mortality rates among individuals who have had a pulmonary embolism are significantly higher than rates in the general population.
Methodology
Researchers investigated long-term outcomes of patients with pulmonary embolism in a single-center registry.
They followed 896 patients for up to 14 years.
Data were from consecutive cases treated between May 2005 and December 2017.
Takeaway
The total follow-up time was 3,908 patient-years (median, 3.1 years).
One-year and five-year mortality rates were 19.7% (95% confidence interval, 17.2%-22.4%) and 37.1% (95% CI, 33.6%-40.5%), respectively, for patients with pulmonary embolism.
The most frequent causes of death were cancer (28.5%), pulmonary embolism (19.4%), infections (13.9%), and cardiovascular events (11.6%).
Late mortality (>30 days) was more frequent than in the general population for patients with cancer (5-year standardized mortality ratio, 2.77; 95% CI, 2.41-3.16) and for patients without cancer (1.80; 95% CI, 1.50-2.14), compared with expected rates.
In practice
stated Johannes Eckelt, Clinic of Cardiology and Pneumology, University Medical Center Göttingen (Germany).
Source
“Long-term Mortality in Pulmonary Embolism: Results in a Single-Center Registry,” by Mr. Eckelt and colleagues was published in Research and Practice in Thrombosis and Haemostasis.
Limitations
Owing to the single-center study design, selection bias cannot be excluded, limiting the generalizability of the study findings, the authors stated.
Disclosures
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Indefinite anticoagulation likely not cost effective after unprovoked VTE
Continuing anticoagulation indefinitely in patients with a first unprovoked venous thromboembolism (VTE) may have benefits for certain patients but is unlikely to be cost effective, say authors of a new study.
Continued anticoagulation for such patients “has little chance of improving life expectancy but might provide a mortality benefit in certain subgroups including patients with an initial PE (pulmonary embolism) or those at a very low risk for major bleeding,” wrote the authors, led by Faizan Khan, PhD, with the O’Brien Institute for Public Health, University of Calgary (Alta.).
Therefore, shared decision-making between patients with unprovoked VTE and physicians that includes discussion of preferences and values and use of validated prediction tools is important.
The authors noted that some patients might value avoiding morbidities of recurrent VTE the most and want to have lifelong anticoagulation. Some might be more fearful of major bleeding than VTE repercussions or don’t want the inconveniences of taking anticoagulants for a lifetime.
The findings were published in Annals of Internal Medicine.
Current guidelines recommend indefinite anticoagulation
Clinical practice guidelines now recommend indefinite anticoagulation for a first unprovoked VTE.
The authors did a modeling study in a hypothetical cohort of 1,000 patients aged 55 years with a first unprovoked VTE who had completed 3-6 months of initial anticoagulation. The study found indefinite anticoagulation, compared with discontinuing anticoagulation, on average, resulted in 368 fewer recurrent VTE events and 14 fewer fatal PE events.
At the same time, indefinite coagulation in the hypothetical group induced an additional 114 major bleeding events, 30 intracerebral hemorrhages, and 11 fatal bleeding events over 40 years.
As for cost effectiveness, from the perspective of Canada’s health care system, continuing anticoagulation indefinitely, on average, increased costs by $16,014 Canadian dollars per person ($12,140 USD) without improving quality-adjusted life-years (incremental difference, 0.075 per person; 95% uncertainty interval, –0.192 to 0.017).
The authors noted that cost is a prime consideration as the estimated annual health care costs of VTE and its complications is $600 Canadian dollars ($7 billion–$10 billion USD).
High probability of small benefit
The authors spelled out the small benefit in patients with an initial PE.
According to the study, indefinite anticoagulation would result in an 80% probability of a marginal added clinical benefit (average increase of 57 days of perfect health over a lifetime) in patients with an initial PE (but with only a 24% chance of being cost effective).
“This high probability of an additional clinical benefit is plausible due to the higher proportion of recurrent VTE events presenting as PE (approximately 70% of episodes) in patients initially presenting with PE, in turn, resulting in a two- to threefold higher case-fatality rate of recurrent VTE in this patient subgroup.”
Tools to estimate bleeding risk imprecise
Scott Woller, MD, an internal medicine specialist and chair of medicine at Intermountain Medical Center, Murray, Utah, said in an interview that these results should help physicians’ discuss with their patients about duration of anticoagulation after the treatment phase.
He noted that the authors suggest that a low estimated annual risk for major bleeding should be assumed (< 0.67%) to make the choice for indefinite anticoagulation.
“This is a sticky wicket,” he said, “as tools to estimate bleeding risk among VTE patients are presently imprecise. For these reasons PCPs should take into account patient risk estimates – and the limitations that exist surrounding how we calculate these estimates – in addition to their values and preferences. This is really key in electing duration of anticoagulation.”
A limitation of the study is that the model assumed that risks for recurrent VTE and major bleeding in clinical trials at 1 year remained constant during extended anticoagulation.
Dr. Woller said about that limitation: “One might argue that this is unlikely; age is a risk factor for major bleeding and therefore risks may be underestimated. However, in the ‘real world’ those that are perceived at lowest risk and demonstrate good tolerance to anticoagulation might likely preferentially continue anticoagulants and therefore risks may be overestimated.”
One coauthor reported being a clinical investigator for trials sponsored by Pfizer and Bristol-Myers Squibb and receiving honoraria from Pfizer, Sanofi and Aspen Pharma. The other authors disclosed no other relevant financial relationships. Dr. Woller is cochair of the CHEST guidelines on the treatment of venous thromboembolic disease.
Continuing anticoagulation indefinitely in patients with a first unprovoked venous thromboembolism (VTE) may have benefits for certain patients but is unlikely to be cost effective, say authors of a new study.
Continued anticoagulation for such patients “has little chance of improving life expectancy but might provide a mortality benefit in certain subgroups including patients with an initial PE (pulmonary embolism) or those at a very low risk for major bleeding,” wrote the authors, led by Faizan Khan, PhD, with the O’Brien Institute for Public Health, University of Calgary (Alta.).
Therefore, shared decision-making between patients with unprovoked VTE and physicians that includes discussion of preferences and values and use of validated prediction tools is important.
The authors noted that some patients might value avoiding morbidities of recurrent VTE the most and want to have lifelong anticoagulation. Some might be more fearful of major bleeding than VTE repercussions or don’t want the inconveniences of taking anticoagulants for a lifetime.
The findings were published in Annals of Internal Medicine.
Current guidelines recommend indefinite anticoagulation
Clinical practice guidelines now recommend indefinite anticoagulation for a first unprovoked VTE.
The authors did a modeling study in a hypothetical cohort of 1,000 patients aged 55 years with a first unprovoked VTE who had completed 3-6 months of initial anticoagulation. The study found indefinite anticoagulation, compared with discontinuing anticoagulation, on average, resulted in 368 fewer recurrent VTE events and 14 fewer fatal PE events.
At the same time, indefinite coagulation in the hypothetical group induced an additional 114 major bleeding events, 30 intracerebral hemorrhages, and 11 fatal bleeding events over 40 years.
As for cost effectiveness, from the perspective of Canada’s health care system, continuing anticoagulation indefinitely, on average, increased costs by $16,014 Canadian dollars per person ($12,140 USD) without improving quality-adjusted life-years (incremental difference, 0.075 per person; 95% uncertainty interval, –0.192 to 0.017).
The authors noted that cost is a prime consideration as the estimated annual health care costs of VTE and its complications is $600 Canadian dollars ($7 billion–$10 billion USD).
High probability of small benefit
The authors spelled out the small benefit in patients with an initial PE.
According to the study, indefinite anticoagulation would result in an 80% probability of a marginal added clinical benefit (average increase of 57 days of perfect health over a lifetime) in patients with an initial PE (but with only a 24% chance of being cost effective).
“This high probability of an additional clinical benefit is plausible due to the higher proportion of recurrent VTE events presenting as PE (approximately 70% of episodes) in patients initially presenting with PE, in turn, resulting in a two- to threefold higher case-fatality rate of recurrent VTE in this patient subgroup.”
Tools to estimate bleeding risk imprecise
Scott Woller, MD, an internal medicine specialist and chair of medicine at Intermountain Medical Center, Murray, Utah, said in an interview that these results should help physicians’ discuss with their patients about duration of anticoagulation after the treatment phase.
He noted that the authors suggest that a low estimated annual risk for major bleeding should be assumed (< 0.67%) to make the choice for indefinite anticoagulation.
“This is a sticky wicket,” he said, “as tools to estimate bleeding risk among VTE patients are presently imprecise. For these reasons PCPs should take into account patient risk estimates – and the limitations that exist surrounding how we calculate these estimates – in addition to their values and preferences. This is really key in electing duration of anticoagulation.”
A limitation of the study is that the model assumed that risks for recurrent VTE and major bleeding in clinical trials at 1 year remained constant during extended anticoagulation.
Dr. Woller said about that limitation: “One might argue that this is unlikely; age is a risk factor for major bleeding and therefore risks may be underestimated. However, in the ‘real world’ those that are perceived at lowest risk and demonstrate good tolerance to anticoagulation might likely preferentially continue anticoagulants and therefore risks may be overestimated.”
One coauthor reported being a clinical investigator for trials sponsored by Pfizer and Bristol-Myers Squibb and receiving honoraria from Pfizer, Sanofi and Aspen Pharma. The other authors disclosed no other relevant financial relationships. Dr. Woller is cochair of the CHEST guidelines on the treatment of venous thromboembolic disease.
Continuing anticoagulation indefinitely in patients with a first unprovoked venous thromboembolism (VTE) may have benefits for certain patients but is unlikely to be cost effective, say authors of a new study.
Continued anticoagulation for such patients “has little chance of improving life expectancy but might provide a mortality benefit in certain subgroups including patients with an initial PE (pulmonary embolism) or those at a very low risk for major bleeding,” wrote the authors, led by Faizan Khan, PhD, with the O’Brien Institute for Public Health, University of Calgary (Alta.).
Therefore, shared decision-making between patients with unprovoked VTE and physicians that includes discussion of preferences and values and use of validated prediction tools is important.
The authors noted that some patients might value avoiding morbidities of recurrent VTE the most and want to have lifelong anticoagulation. Some might be more fearful of major bleeding than VTE repercussions or don’t want the inconveniences of taking anticoagulants for a lifetime.
The findings were published in Annals of Internal Medicine.
Current guidelines recommend indefinite anticoagulation
Clinical practice guidelines now recommend indefinite anticoagulation for a first unprovoked VTE.
The authors did a modeling study in a hypothetical cohort of 1,000 patients aged 55 years with a first unprovoked VTE who had completed 3-6 months of initial anticoagulation. The study found indefinite anticoagulation, compared with discontinuing anticoagulation, on average, resulted in 368 fewer recurrent VTE events and 14 fewer fatal PE events.
At the same time, indefinite coagulation in the hypothetical group induced an additional 114 major bleeding events, 30 intracerebral hemorrhages, and 11 fatal bleeding events over 40 years.
As for cost effectiveness, from the perspective of Canada’s health care system, continuing anticoagulation indefinitely, on average, increased costs by $16,014 Canadian dollars per person ($12,140 USD) without improving quality-adjusted life-years (incremental difference, 0.075 per person; 95% uncertainty interval, –0.192 to 0.017).
The authors noted that cost is a prime consideration as the estimated annual health care costs of VTE and its complications is $600 Canadian dollars ($7 billion–$10 billion USD).
High probability of small benefit
The authors spelled out the small benefit in patients with an initial PE.
According to the study, indefinite anticoagulation would result in an 80% probability of a marginal added clinical benefit (average increase of 57 days of perfect health over a lifetime) in patients with an initial PE (but with only a 24% chance of being cost effective).
“This high probability of an additional clinical benefit is plausible due to the higher proportion of recurrent VTE events presenting as PE (approximately 70% of episodes) in patients initially presenting with PE, in turn, resulting in a two- to threefold higher case-fatality rate of recurrent VTE in this patient subgroup.”
Tools to estimate bleeding risk imprecise
Scott Woller, MD, an internal medicine specialist and chair of medicine at Intermountain Medical Center, Murray, Utah, said in an interview that these results should help physicians’ discuss with their patients about duration of anticoagulation after the treatment phase.
He noted that the authors suggest that a low estimated annual risk for major bleeding should be assumed (< 0.67%) to make the choice for indefinite anticoagulation.
“This is a sticky wicket,” he said, “as tools to estimate bleeding risk among VTE patients are presently imprecise. For these reasons PCPs should take into account patient risk estimates – and the limitations that exist surrounding how we calculate these estimates – in addition to their values and preferences. This is really key in electing duration of anticoagulation.”
A limitation of the study is that the model assumed that risks for recurrent VTE and major bleeding in clinical trials at 1 year remained constant during extended anticoagulation.
Dr. Woller said about that limitation: “One might argue that this is unlikely; age is a risk factor for major bleeding and therefore risks may be underestimated. However, in the ‘real world’ those that are perceived at lowest risk and demonstrate good tolerance to anticoagulation might likely preferentially continue anticoagulants and therefore risks may be overestimated.”
One coauthor reported being a clinical investigator for trials sponsored by Pfizer and Bristol-Myers Squibb and receiving honoraria from Pfizer, Sanofi and Aspen Pharma. The other authors disclosed no other relevant financial relationships. Dr. Woller is cochair of the CHEST guidelines on the treatment of venous thromboembolic disease.
FROM ANNALS OF INTERNAL MEDICINE
Rehabilitation improves walk test results for post–pulmonary embolism patients with persistent dyspnea
In patients with persistent dyspnea following a pulmonary embolism, rehabilitation should be considered as a treatment option, according to findings from a randomized, controlled trial comparing usual care to a twice-weekly, 8-week physical exercise program.
The prevalence of persistent dyspnea, functional limitations, and reduced quality of life (QoL) after pulmonary embolism (PE) ranges from 30% to 50% in published studies. While the underlying mechanisms remain unclear and are likely multifactorial, Øyvind Jervan, MD, and colleagues reported, research suggests that deconditioning and psychological factors contribute substantially to post-PE impairment. Optimal management remains unknown.
The investigators randomized adult patients 1:1 from two hospitals (Osfold Hospital and Akershus University Hospital) with PE identified via computed tomography pulmonary angiography 6-72 months prior to study inclusion to either a supervised outpatient exercise program or usual care. The once- or twice-weekly home-based program was tailored to each participant and included a 90-minute educational session on the cardiopulmonary system, diagnosis and treatment of PE and its possible long-term effects, the benefits of exercise and physical activity, and the management of breathlessness. Also during the intervention period, participants were given a simple home-based exercise program to be performed once or twice weekly. Differences between groups in the Incremental Shuttle Walk Test (ISWT), a standardized walking test that assesses exercise capacity, was the primary endpoint. Secondary endpoints included an endurance walk test (ESWT) and measures of symptoms and QoL.
Among 211 participants (median age 57 years; 56% men), the median time from diagnosis to inclusion was 10.3 months. Median baseline walking distance on the ISWT was 695 m with 21% achieving the 1,020-m maximum distance. At follow-up, a between-group difference of 53.0 m favored the rehabilitation group (89 evaluable subjects; 87 in usual care) (P = .0035). While subgroup analysis revealed a greater difference for those with shorter time from diagnosis (6-12 months vs. 12.1-72 months), the between-group differences were nonsignificant. Also, no ISWT differences between the intervention and control group were found for those with higher pulmonary embolism severity and dyspnea scores. The walk endurance test revealed no between-group differences.
Scores at follow-up on the Pulmonary Embolism-QoL questionnaire favored the rehabilitation group (mean difference –4%; P = .041), but there were no differences in generic QoL, dyspnea scores, or the ESWT.
“The present study adds to the growing evidence of the benefits of rehabilitation after PE,” the researchers stated. Although several recent studies have shown rehabilitation after PE results that were promising, the authors pointed out that most of these studies have been small or have lacked a control group, with great variations between them with respect to time, mode, and duration of intervention. In addition, the current study is the largest one addressing the effect of rehabilitation after PE to demonstrate in subjects with persistent dyspnea a positive effect on exercise capacity and QoL.
The researchers also commented that the small detected mean difference of 53 m in walking distance was lower than has been considered a worthwhile improvement by some, and its clinical relevance can be debated. Other studies, however, have used mean group differences of 40-62 m as clinically meaningful. The authors underscored also that the ISWT data were subject to a considerable ceiling effect which may underestimate the effect size.
Addressing study limitations, the researchers added that: “The rehabilitation program in the present study consisted mainly of exercise training. It is unknown whether the addition of occupational therapy, psychology, or dietary therapy would provide additional benefits for the participants. Most participants had mild symptoms, which may have limited the potential benefits of our rehabilitation program.”
The project was funded by Østfold Hospital Trust. Dr. Jervan reported no relevant conflicts of interest.
In patients with persistent dyspnea following a pulmonary embolism, rehabilitation should be considered as a treatment option, according to findings from a randomized, controlled trial comparing usual care to a twice-weekly, 8-week physical exercise program.
The prevalence of persistent dyspnea, functional limitations, and reduced quality of life (QoL) after pulmonary embolism (PE) ranges from 30% to 50% in published studies. While the underlying mechanisms remain unclear and are likely multifactorial, Øyvind Jervan, MD, and colleagues reported, research suggests that deconditioning and psychological factors contribute substantially to post-PE impairment. Optimal management remains unknown.
The investigators randomized adult patients 1:1 from two hospitals (Osfold Hospital and Akershus University Hospital) with PE identified via computed tomography pulmonary angiography 6-72 months prior to study inclusion to either a supervised outpatient exercise program or usual care. The once- or twice-weekly home-based program was tailored to each participant and included a 90-minute educational session on the cardiopulmonary system, diagnosis and treatment of PE and its possible long-term effects, the benefits of exercise and physical activity, and the management of breathlessness. Also during the intervention period, participants were given a simple home-based exercise program to be performed once or twice weekly. Differences between groups in the Incremental Shuttle Walk Test (ISWT), a standardized walking test that assesses exercise capacity, was the primary endpoint. Secondary endpoints included an endurance walk test (ESWT) and measures of symptoms and QoL.
Among 211 participants (median age 57 years; 56% men), the median time from diagnosis to inclusion was 10.3 months. Median baseline walking distance on the ISWT was 695 m with 21% achieving the 1,020-m maximum distance. At follow-up, a between-group difference of 53.0 m favored the rehabilitation group (89 evaluable subjects; 87 in usual care) (P = .0035). While subgroup analysis revealed a greater difference for those with shorter time from diagnosis (6-12 months vs. 12.1-72 months), the between-group differences were nonsignificant. Also, no ISWT differences between the intervention and control group were found for those with higher pulmonary embolism severity and dyspnea scores. The walk endurance test revealed no between-group differences.
Scores at follow-up on the Pulmonary Embolism-QoL questionnaire favored the rehabilitation group (mean difference –4%; P = .041), but there were no differences in generic QoL, dyspnea scores, or the ESWT.
“The present study adds to the growing evidence of the benefits of rehabilitation after PE,” the researchers stated. Although several recent studies have shown rehabilitation after PE results that were promising, the authors pointed out that most of these studies have been small or have lacked a control group, with great variations between them with respect to time, mode, and duration of intervention. In addition, the current study is the largest one addressing the effect of rehabilitation after PE to demonstrate in subjects with persistent dyspnea a positive effect on exercise capacity and QoL.
The researchers also commented that the small detected mean difference of 53 m in walking distance was lower than has been considered a worthwhile improvement by some, and its clinical relevance can be debated. Other studies, however, have used mean group differences of 40-62 m as clinically meaningful. The authors underscored also that the ISWT data were subject to a considerable ceiling effect which may underestimate the effect size.
Addressing study limitations, the researchers added that: “The rehabilitation program in the present study consisted mainly of exercise training. It is unknown whether the addition of occupational therapy, psychology, or dietary therapy would provide additional benefits for the participants. Most participants had mild symptoms, which may have limited the potential benefits of our rehabilitation program.”
The project was funded by Østfold Hospital Trust. Dr. Jervan reported no relevant conflicts of interest.
In patients with persistent dyspnea following a pulmonary embolism, rehabilitation should be considered as a treatment option, according to findings from a randomized, controlled trial comparing usual care to a twice-weekly, 8-week physical exercise program.
The prevalence of persistent dyspnea, functional limitations, and reduced quality of life (QoL) after pulmonary embolism (PE) ranges from 30% to 50% in published studies. While the underlying mechanisms remain unclear and are likely multifactorial, Øyvind Jervan, MD, and colleagues reported, research suggests that deconditioning and psychological factors contribute substantially to post-PE impairment. Optimal management remains unknown.
The investigators randomized adult patients 1:1 from two hospitals (Osfold Hospital and Akershus University Hospital) with PE identified via computed tomography pulmonary angiography 6-72 months prior to study inclusion to either a supervised outpatient exercise program or usual care. The once- or twice-weekly home-based program was tailored to each participant and included a 90-minute educational session on the cardiopulmonary system, diagnosis and treatment of PE and its possible long-term effects, the benefits of exercise and physical activity, and the management of breathlessness. Also during the intervention period, participants were given a simple home-based exercise program to be performed once or twice weekly. Differences between groups in the Incremental Shuttle Walk Test (ISWT), a standardized walking test that assesses exercise capacity, was the primary endpoint. Secondary endpoints included an endurance walk test (ESWT) and measures of symptoms and QoL.
Among 211 participants (median age 57 years; 56% men), the median time from diagnosis to inclusion was 10.3 months. Median baseline walking distance on the ISWT was 695 m with 21% achieving the 1,020-m maximum distance. At follow-up, a between-group difference of 53.0 m favored the rehabilitation group (89 evaluable subjects; 87 in usual care) (P = .0035). While subgroup analysis revealed a greater difference for those with shorter time from diagnosis (6-12 months vs. 12.1-72 months), the between-group differences were nonsignificant. Also, no ISWT differences between the intervention and control group were found for those with higher pulmonary embolism severity and dyspnea scores. The walk endurance test revealed no between-group differences.
Scores at follow-up on the Pulmonary Embolism-QoL questionnaire favored the rehabilitation group (mean difference –4%; P = .041), but there were no differences in generic QoL, dyspnea scores, or the ESWT.
“The present study adds to the growing evidence of the benefits of rehabilitation after PE,” the researchers stated. Although several recent studies have shown rehabilitation after PE results that were promising, the authors pointed out that most of these studies have been small or have lacked a control group, with great variations between them with respect to time, mode, and duration of intervention. In addition, the current study is the largest one addressing the effect of rehabilitation after PE to demonstrate in subjects with persistent dyspnea a positive effect on exercise capacity and QoL.
The researchers also commented that the small detected mean difference of 53 m in walking distance was lower than has been considered a worthwhile improvement by some, and its clinical relevance can be debated. Other studies, however, have used mean group differences of 40-62 m as clinically meaningful. The authors underscored also that the ISWT data were subject to a considerable ceiling effect which may underestimate the effect size.
Addressing study limitations, the researchers added that: “The rehabilitation program in the present study consisted mainly of exercise training. It is unknown whether the addition of occupational therapy, psychology, or dietary therapy would provide additional benefits for the participants. Most participants had mild symptoms, which may have limited the potential benefits of our rehabilitation program.”
The project was funded by Østfold Hospital Trust. Dr. Jervan reported no relevant conflicts of interest.
FROM THE JOURNAL CHEST
Circulatory support for RV failure caused by pulmonary embolism
A new review article highlights approaches for mechanical circulatory support in patients with high-risk acute pulmonary embolism (PE).
Pulmonary embolism with hemodynamic significance is widely underdiagnosed, and the mortality rate can be as high as 30%, but new therapeutic developments offer promise. “Over the past few years, a renewed interest in mechanical circulatory support (MCS; both percutaneous and surgical) for acute RVF has emerged, increasing viable treatment options for high-risk acute PE,” wrote the authors of the review, which was published online in Interventional Cardiology Clinics.
Poor outcomes are often driven by RVF, which is tricky to diagnose and manage, and it stems from a sudden increase in pulmonary vascular resistance (PVR) following PE. “The mechanism for increased PVR in acute PE is multifactorial, including direct blood flow impedance, local hypoxia-induced vasoconstriction, and platelet/thrombin-induced release of vasoactive peptides. The cascade of events that then leads to RVF includes decreased RV stoke volume, increased RV wall tension, and RV dilation,” the authors wrote.
The authors noted that diuretics help to correct changes to RV geometry and can improve left ventricle filling, which improves hemodynamics. Diuretics can be used in patients who are hypotensive and volume overloaded, but vasopressors should be employed to support blood pressure.
When using mechanical ventilation, strategies such as low tidal volumes, minimization of positive end expiratory pressure, and prevention of hypoxemia and acidemia should be employed to prevent an increase of pulmonary vascular resistance, which can worsen RV failure.
Pulmonary vasodilators aren’t recommended for acute PE, but inhaled pulmonary vasodilators may be considered in hemodynamically unstable patients.
Surgically implanted right ventricle assistance device are generally not used for acute RV failure in high-risk PE, unless the patient has not improved after medical management.
Percutaneous devices
Percutaneous mechanical circulatory support devices can be used for patients experiencing refractory shock. The review highlighted three such devices, including the Impella RP, tandem-heart right ventricular assist devices (TH-RVAD) or Protek Duo, and venoarterial extracorporeal membrane oxygenation (VA-ECMO), but they are not without limitations. “Challenges to using these devices in patients with acute PE include clot dislodgement, vascular complications, infections, device migration, and fracture of individual elements,” the authors wrote.
The Impella RP is easy to deploy and bypasses the RV, but it can’t provide blood oxygenation and may cause bleeding or hemolysis. TH-RVAD oxygenates the blood and bypasses the RV, but suffers from a large sheath size. VA-ECMO oxygenates the blood but may cause bleeding.
There are important differences among the mechanical support devices, according to Jonathan Ludmir, MD, who was asked to comment. “In reality, if someone has a large pulmonary embolism burden, to put in the Impella RP or the Protek Duo would be a little bit risky, because you’d be sometimes putting the device right where the clot is. At least what we do in our institution, when someone is in extremis despite using [intravenous] medications like vasopressors or inotropes, VA-ECMO is kind of the go to. This is both the quickest and probably most effective way to support the patient. I say the quickest because this is a procedure you can do at the bedside.”
Benefits of PERT
One message that the review only briefly mentions, but Dr. Ludmir believes is key, is employing a pulmonary embolism response team. “That’s been looked at extensively, and it’s a really key part of any decision-making. If someone presents to the emergency room or someone inside the hospital has an acute pulmonary embolism, you have a team of people that can respond and help assess the next step. Typically, that involves a cardiologist or an interventional cardiologist, a hematologist, vascular surgeon, often a cardiac surgeon, so it’s a whole slew of people. Based on the patient assessment they can quickly decide, can this patient just be okay with a blood thinner like heparin? Does this patient need something more aggressive, like a thrombectomy? Or is this a serious case where you involve the shock team or the ECMO team, and you have to stabilize the patient on mechanical circulatory support, so you can accomplish what you need to do to get rid of the pulmonary embolism,” said Dr. Ludmir, who is an assistant professor of medicine at Corrigan Minehan Heart Center at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“Every case is individualized, hence the importance of having a team of a variety of different backgrounds and thoughts to approach it. And I think that’s kind of like the key takeaway. Yes, you have to be familiar with all the therapies, but at the end of the day, not every patient is going to fit into the algorithm for how you approach pulmonary embolism,” said Dr. Ludmir.
Dr. Ludmir has no relevant conflicts of interest.
A new review article highlights approaches for mechanical circulatory support in patients with high-risk acute pulmonary embolism (PE).
Pulmonary embolism with hemodynamic significance is widely underdiagnosed, and the mortality rate can be as high as 30%, but new therapeutic developments offer promise. “Over the past few years, a renewed interest in mechanical circulatory support (MCS; both percutaneous and surgical) for acute RVF has emerged, increasing viable treatment options for high-risk acute PE,” wrote the authors of the review, which was published online in Interventional Cardiology Clinics.
Poor outcomes are often driven by RVF, which is tricky to diagnose and manage, and it stems from a sudden increase in pulmonary vascular resistance (PVR) following PE. “The mechanism for increased PVR in acute PE is multifactorial, including direct blood flow impedance, local hypoxia-induced vasoconstriction, and platelet/thrombin-induced release of vasoactive peptides. The cascade of events that then leads to RVF includes decreased RV stoke volume, increased RV wall tension, and RV dilation,” the authors wrote.
The authors noted that diuretics help to correct changes to RV geometry and can improve left ventricle filling, which improves hemodynamics. Diuretics can be used in patients who are hypotensive and volume overloaded, but vasopressors should be employed to support blood pressure.
When using mechanical ventilation, strategies such as low tidal volumes, minimization of positive end expiratory pressure, and prevention of hypoxemia and acidemia should be employed to prevent an increase of pulmonary vascular resistance, which can worsen RV failure.
Pulmonary vasodilators aren’t recommended for acute PE, but inhaled pulmonary vasodilators may be considered in hemodynamically unstable patients.
Surgically implanted right ventricle assistance device are generally not used for acute RV failure in high-risk PE, unless the patient has not improved after medical management.
Percutaneous devices
Percutaneous mechanical circulatory support devices can be used for patients experiencing refractory shock. The review highlighted three such devices, including the Impella RP, tandem-heart right ventricular assist devices (TH-RVAD) or Protek Duo, and venoarterial extracorporeal membrane oxygenation (VA-ECMO), but they are not without limitations. “Challenges to using these devices in patients with acute PE include clot dislodgement, vascular complications, infections, device migration, and fracture of individual elements,” the authors wrote.
The Impella RP is easy to deploy and bypasses the RV, but it can’t provide blood oxygenation and may cause bleeding or hemolysis. TH-RVAD oxygenates the blood and bypasses the RV, but suffers from a large sheath size. VA-ECMO oxygenates the blood but may cause bleeding.
There are important differences among the mechanical support devices, according to Jonathan Ludmir, MD, who was asked to comment. “In reality, if someone has a large pulmonary embolism burden, to put in the Impella RP or the Protek Duo would be a little bit risky, because you’d be sometimes putting the device right where the clot is. At least what we do in our institution, when someone is in extremis despite using [intravenous] medications like vasopressors or inotropes, VA-ECMO is kind of the go to. This is both the quickest and probably most effective way to support the patient. I say the quickest because this is a procedure you can do at the bedside.”
Benefits of PERT
One message that the review only briefly mentions, but Dr. Ludmir believes is key, is employing a pulmonary embolism response team. “That’s been looked at extensively, and it’s a really key part of any decision-making. If someone presents to the emergency room or someone inside the hospital has an acute pulmonary embolism, you have a team of people that can respond and help assess the next step. Typically, that involves a cardiologist or an interventional cardiologist, a hematologist, vascular surgeon, often a cardiac surgeon, so it’s a whole slew of people. Based on the patient assessment they can quickly decide, can this patient just be okay with a blood thinner like heparin? Does this patient need something more aggressive, like a thrombectomy? Or is this a serious case where you involve the shock team or the ECMO team, and you have to stabilize the patient on mechanical circulatory support, so you can accomplish what you need to do to get rid of the pulmonary embolism,” said Dr. Ludmir, who is an assistant professor of medicine at Corrigan Minehan Heart Center at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“Every case is individualized, hence the importance of having a team of a variety of different backgrounds and thoughts to approach it. And I think that’s kind of like the key takeaway. Yes, you have to be familiar with all the therapies, but at the end of the day, not every patient is going to fit into the algorithm for how you approach pulmonary embolism,” said Dr. Ludmir.
Dr. Ludmir has no relevant conflicts of interest.
A new review article highlights approaches for mechanical circulatory support in patients with high-risk acute pulmonary embolism (PE).
Pulmonary embolism with hemodynamic significance is widely underdiagnosed, and the mortality rate can be as high as 30%, but new therapeutic developments offer promise. “Over the past few years, a renewed interest in mechanical circulatory support (MCS; both percutaneous and surgical) for acute RVF has emerged, increasing viable treatment options for high-risk acute PE,” wrote the authors of the review, which was published online in Interventional Cardiology Clinics.
Poor outcomes are often driven by RVF, which is tricky to diagnose and manage, and it stems from a sudden increase in pulmonary vascular resistance (PVR) following PE. “The mechanism for increased PVR in acute PE is multifactorial, including direct blood flow impedance, local hypoxia-induced vasoconstriction, and platelet/thrombin-induced release of vasoactive peptides. The cascade of events that then leads to RVF includes decreased RV stoke volume, increased RV wall tension, and RV dilation,” the authors wrote.
The authors noted that diuretics help to correct changes to RV geometry and can improve left ventricle filling, which improves hemodynamics. Diuretics can be used in patients who are hypotensive and volume overloaded, but vasopressors should be employed to support blood pressure.
When using mechanical ventilation, strategies such as low tidal volumes, minimization of positive end expiratory pressure, and prevention of hypoxemia and acidemia should be employed to prevent an increase of pulmonary vascular resistance, which can worsen RV failure.
Pulmonary vasodilators aren’t recommended for acute PE, but inhaled pulmonary vasodilators may be considered in hemodynamically unstable patients.
Surgically implanted right ventricle assistance device are generally not used for acute RV failure in high-risk PE, unless the patient has not improved after medical management.
Percutaneous devices
Percutaneous mechanical circulatory support devices can be used for patients experiencing refractory shock. The review highlighted three such devices, including the Impella RP, tandem-heart right ventricular assist devices (TH-RVAD) or Protek Duo, and venoarterial extracorporeal membrane oxygenation (VA-ECMO), but they are not without limitations. “Challenges to using these devices in patients with acute PE include clot dislodgement, vascular complications, infections, device migration, and fracture of individual elements,” the authors wrote.
The Impella RP is easy to deploy and bypasses the RV, but it can’t provide blood oxygenation and may cause bleeding or hemolysis. TH-RVAD oxygenates the blood and bypasses the RV, but suffers from a large sheath size. VA-ECMO oxygenates the blood but may cause bleeding.
There are important differences among the mechanical support devices, according to Jonathan Ludmir, MD, who was asked to comment. “In reality, if someone has a large pulmonary embolism burden, to put in the Impella RP or the Protek Duo would be a little bit risky, because you’d be sometimes putting the device right where the clot is. At least what we do in our institution, when someone is in extremis despite using [intravenous] medications like vasopressors or inotropes, VA-ECMO is kind of the go to. This is both the quickest and probably most effective way to support the patient. I say the quickest because this is a procedure you can do at the bedside.”
Benefits of PERT
One message that the review only briefly mentions, but Dr. Ludmir believes is key, is employing a pulmonary embolism response team. “That’s been looked at extensively, and it’s a really key part of any decision-making. If someone presents to the emergency room or someone inside the hospital has an acute pulmonary embolism, you have a team of people that can respond and help assess the next step. Typically, that involves a cardiologist or an interventional cardiologist, a hematologist, vascular surgeon, often a cardiac surgeon, so it’s a whole slew of people. Based on the patient assessment they can quickly decide, can this patient just be okay with a blood thinner like heparin? Does this patient need something more aggressive, like a thrombectomy? Or is this a serious case where you involve the shock team or the ECMO team, and you have to stabilize the patient on mechanical circulatory support, so you can accomplish what you need to do to get rid of the pulmonary embolism,” said Dr. Ludmir, who is an assistant professor of medicine at Corrigan Minehan Heart Center at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“Every case is individualized, hence the importance of having a team of a variety of different backgrounds and thoughts to approach it. And I think that’s kind of like the key takeaway. Yes, you have to be familiar with all the therapies, but at the end of the day, not every patient is going to fit into the algorithm for how you approach pulmonary embolism,” said Dr. Ludmir.
Dr. Ludmir has no relevant conflicts of interest.
FROM INTERVENTIONAL CARDIOLOGY CLINICS
Dabigatran recalled over potential carcinogen
The nationwide recall, to the consumer level, is because of the detection of the nitrosamine impurity, N-nitroso-dabigatran, which may increase the risk of cancer with prolonged exposure to levels higher than acceptable.
To date, Ascend Laboratories has not received any reports of adverse events related to this recall.
The recalled product was distributed nationwide to wholesalers, distributors, and retailers in the United States from June 2022 to October 2022.
Complete details of the recalled product, including national drug code, lot numbers, expiration dates, and configuration/counts, are provided in a company announcement that was posted on the Food and Drug Administration website.
The company is advising patients who have any dabigatran that has been recalled to continue taking their medication and to contact their physician for advice regarding an alternative treatment.
Wholesalers/distributors and pharmacies with an existing inventory of the affected lots should stop use and distribution and quarantine the product immediately. Wholesalers and distributors should also recall the distributed product.
Questions regarding this recall can call Ascend Laboratories at 877.272.7901 (24 hours, 7 days a week).
Problems with this product should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article originally appeared on Medscape.com.
The nationwide recall, to the consumer level, is because of the detection of the nitrosamine impurity, N-nitroso-dabigatran, which may increase the risk of cancer with prolonged exposure to levels higher than acceptable.
To date, Ascend Laboratories has not received any reports of adverse events related to this recall.
The recalled product was distributed nationwide to wholesalers, distributors, and retailers in the United States from June 2022 to October 2022.
Complete details of the recalled product, including national drug code, lot numbers, expiration dates, and configuration/counts, are provided in a company announcement that was posted on the Food and Drug Administration website.
The company is advising patients who have any dabigatran that has been recalled to continue taking their medication and to contact their physician for advice regarding an alternative treatment.
Wholesalers/distributors and pharmacies with an existing inventory of the affected lots should stop use and distribution and quarantine the product immediately. Wholesalers and distributors should also recall the distributed product.
Questions regarding this recall can call Ascend Laboratories at 877.272.7901 (24 hours, 7 days a week).
Problems with this product should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article originally appeared on Medscape.com.
The nationwide recall, to the consumer level, is because of the detection of the nitrosamine impurity, N-nitroso-dabigatran, which may increase the risk of cancer with prolonged exposure to levels higher than acceptable.
To date, Ascend Laboratories has not received any reports of adverse events related to this recall.
The recalled product was distributed nationwide to wholesalers, distributors, and retailers in the United States from June 2022 to October 2022.
Complete details of the recalled product, including national drug code, lot numbers, expiration dates, and configuration/counts, are provided in a company announcement that was posted on the Food and Drug Administration website.
The company is advising patients who have any dabigatran that has been recalled to continue taking their medication and to contact their physician for advice regarding an alternative treatment.
Wholesalers/distributors and pharmacies with an existing inventory of the affected lots should stop use and distribution and quarantine the product immediately. Wholesalers and distributors should also recall the distributed product.
Questions regarding this recall can call Ascend Laboratories at 877.272.7901 (24 hours, 7 days a week).
Problems with this product should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article originally appeared on Medscape.com.
From PERT to AI, high-risk PE care evolves
In 2012, a small group of specialists, consisting of a critical care pulmonologist, cardiologist, cardiac surgeon, and vascular specialist, at Massachusetts General Hospital, Boston, met to Monday morning quarterback an acute pulmonary embolism case that didn’t go as well as they’d hoped. They came up with a concept known as the pulmonary embolism response team – PERT for short – an idea that soon took hold in other centers and served as the vanguard to other innovative approaches to managing critical care patients with PE, which is the third-leading cause of cardiovascular death in the United States (Intern Emerg Med. 2023. doi: 10.1007/s11739-022-03180-w).
Three years later the PERT Consortium came together, which today has 102 members, according to the organization’s website (www.pertconsortium.org), and members in South America, Europe, Asia, and Australia. Since then, and apps to expedite diagnosis and treatment. The PERT Consortium, meanwhile, is in the process of creating the PE Centers of Excellence program to certify centers that meet certain requirements.
“Part of the reason we recognized that a discussion across specialties was important was because there weren’t the large clinical trials that could tell us exactly what to do for any given case,” said Christopher Kabrhel, MD, MPH, director of the Center for Vascular Emergencies at Mass General and a professor at Harvard Medical School in Boston, who assembled that formative meeting. “Without a clear basis in data, it was really important to have all the different specialists weigh in and give their perspective and talk about what was the best approach for the patient’s care.”
Filling data gaps
Some of those data gaps persist today, Dr. Kabrhel said. “It’s precisely that lack of head-to-head data that existed in 2012, and to a great extent still exists today, that led us to create this system.” The American Heart Association just this January issued a scientific statement on surgical management and mechanical circulatory support in high-risk PE (Circulation. 2023;147:e628-47).
But the intervening research has been uneven. The Pulmonary Embolism Thrombolysis (PEITHO) trial in 2014 evaluated systemic thrombolysis and anticoagulation alone (N Engl J Med. 2014;370:1402-11), but head-to-head studies of catheter-directed thrombolysis (CDT), which was just emerging in 2012, and either systemic thrombolysis or anticoagulation have been lacking, Dr. Kabrhel said. The Hi-PEITHO trial in high-risk PE patients is evaluating ultrasound-guided CDT plus anticoagulation vs. anticoagulation alone (Am Heart J. 2022:251:43-54), but it isn’t complete.
“The therapeutic landscape for PE is evolving incredibly rapidly,” he said. “When we first started PERT we were just starting to see CDT. Since then, we’ve seen several new thrombolytic catheters come onto the market, but there’s also been a proliferation of suction embolectomy catheters and we’ve seen a potentially larger role for surgery and the use of ECMO [extracorporeal membrane oxygenation] or cardiac bypass to bridge patients to definitive therapy. With the rapid evolution and the seemingly daily addition of new therapeutic options, I think the need for PERT is only increasing.”
A recent study out of the University of Michigan reported that the PERT there led to a decrease in the use of advanced therapies given to acute PE patients without reducing mortality or extending hospital stays (Thromb Res. 2023;221:73-8). A study in Spain reported that patients with high-risk and intermediate high-risk PE who had PERT-coordinated care had half the 12-month mortality rate of non-PERT counterparts, 9% vs. 22.2% (P = .02) (Med Clin [Barc]. 2023;S0025-7753(23)00017-9). And a 2021 study at University Hospitals in Cleveland reported that PERT-managed PE patients had a 60% lower rate of adverse outcomes at 90 days than non–PERT-managed patients (J Invasive Cardiol. 2021;33:E173-E180).
Nelish Ardeshna, MD, MA, the lead author of the Michigan study, said the PERT there was formed in 2017. Besides the multispecialty team that can be summoned to a teleconference on short notice, the protocol includes having at least one noninvasive specialist, such as a cardiologist or hospitalist, and one interventionalist, such as a radiologist, always on call. The PERT gets activated through the paging system after a hospital or emergency department physician identifies a suspected or established high-risk PE.
“High-risk PE patients can present in all settings, including the emergency department, ICU, surgical floor, or medical floor,” said Dr. Ardeshna, an internal medicine resident. “Management for these patients is equally varied from anticoagulation to systemic thrombolytics. Not all providers may be familiar with current guidelines to select the optimal therapy for high-risk pulmonary embolism patients. PERT aims to bridge that gap by providing a multidisciplinary discussion with PE specialists that can help identify the correct therapeutic options for optimal outcomes.”
At Cleveland Clinic, where the PERT has been in place since 2012, the PERT can consist of six to eight different specialties and involve up to 15 providers on a conference call, said Leben Tefera, MD, a vascular specialist and head of the PERT team there.
“Each patient will come in and have certain comorbidities,” Dr. Tefera said. “The unfortunate thing about a majority of the PEs that we see, in particular ones [in patients] that are very sick and require inpatient treatment, is that they don’t really fit into a box; you can’t come up with one kind of generic care routine or care path that treats the majority of patients with PE.”
Evolving to follow-up care
As the PERT protocol led to better inpatient outcomes, the teams became more aware that discharged PE patients were struggling with mental health and other quality-of-life issues – symptoms that have been understudied, according to a protocol Dr. Tefera coauthored for a prospective observational study of psychological distress symptoms in PE survivors. By contrast, the protocol noted, these symptoms have been studied extensively in myocardial infarction and stroke patients (Res Pract Thromb Hemost. 2023. doi: 10.1016/j.rpth.2023.10045). Other studies have found that 35%-50% of patients reported mental health symptoms 3 months after PE (Chest. 2021;159:2428-38; Qual Life Res. 2019;28:2111-24).
“A lot of physicians have known it for quite some time, but it wasn’t really until the last couple of years that physicians started saying psychological stress is something that we need to quantify and that we need to actually treat, that we actually need to address,” Dr. Tefera said. That led Dr. Tefera and his Cleveland Clinic PERT colleagues to set up a follow-up clinic for PE patients.
At their follow-up visits, patients complete validated questionnaires about anxiety, depression, fear of recurrence, PE-specific quality of life, and posttraumatic stress disorder. “If they flag as positive, we give them a referral to an in-house psychologist,” he said. “One thing I can report is that patients absolutely, positively love this, because it’s something that they are all experiencing that a lot of physicians just aren’t addressing.”
Artificial intelligence emerges
At the University of Pittsburgh Medical Center, the PERT has started evaluating artificial intelligence to aid in PE diagnosis. Belinda Rivera-Lebron, MD, director of the acute and chronic embolism program at Pitt, explained that the AI protocol hasn’t been adopted yet, but the concept is to have a platform that’s compatible with the hospital system’s electronic medical record.
She described how AI would work once the PERT is activated. “Once the patient goes through the CT scanner, within 60 seconds of that scan being completed, the scan gets uploaded into the cloud and the app or the platform is able to tell you whether there is PE present or absent, and whether there is right ventricle dilation on that scan. This is even before you probably even think about opening up the computer to look at the scan, and even before radiology opens up the scan to read,” she said. “It’s so fast.”
The idea is to send the scans rapidly to the PERT. “It will send you a text, a notification on your phone that will tell you Mr. Smith is PE positive,” Dr. Rivera-Lebron said. “Then you open it and you are able to scroll through the CT scan in your phone. So, it’s really remarkable.”
Clinical trials worth watching
Meanwhile, a number of clinical trials have started to enroll patients, or will soon, that Dr. Rivera-Lebron said are worth paying attention to.
PEITHO-3 is a randomized, placebo-controlled trial with long-term follow-up comparing the efficacy of a reduced-dose alteplase regimen or standard heparin anticoagulation in patients with intermediate to high-risk PE (Thromb Haemost. 2022;122:867-66).
PEERLESS is a prospective randomized trial comparing mechanical thrombectomy and CDT (ClinicalTrials.gov identifier NCT05111613).
PE-Thrombus Removal with Catheter-directed Therapy (PE-TRACT) is an open-label Phase 3 trial comparing anticoagulation and CDT that’s not yet recruiting (ClinicalTrials.gov identifier NCT05591118).
FlowTriever for Acute Massive Pulmonary Embolism (FLAME) is a prospective cohort study evaluating a clot-retrieving device in high-risk PE patients (ClinicalTrials.gov identifier NCT04795167).
When completed and published, these trials could provide PERTs more evidence for their decision-making.
Dr. Ardeshna and Dr. Tefera have no relevant relationships to disclose. Dr. Rivera-Lebron disclosed relationships with INARI Catheter and Johnson & Johnson. Dr. Kabrhel disclosed relationships with Bristol Myers Squibb and Pfizer.
In 2012, a small group of specialists, consisting of a critical care pulmonologist, cardiologist, cardiac surgeon, and vascular specialist, at Massachusetts General Hospital, Boston, met to Monday morning quarterback an acute pulmonary embolism case that didn’t go as well as they’d hoped. They came up with a concept known as the pulmonary embolism response team – PERT for short – an idea that soon took hold in other centers and served as the vanguard to other innovative approaches to managing critical care patients with PE, which is the third-leading cause of cardiovascular death in the United States (Intern Emerg Med. 2023. doi: 10.1007/s11739-022-03180-w).
Three years later the PERT Consortium came together, which today has 102 members, according to the organization’s website (www.pertconsortium.org), and members in South America, Europe, Asia, and Australia. Since then, and apps to expedite diagnosis and treatment. The PERT Consortium, meanwhile, is in the process of creating the PE Centers of Excellence program to certify centers that meet certain requirements.
“Part of the reason we recognized that a discussion across specialties was important was because there weren’t the large clinical trials that could tell us exactly what to do for any given case,” said Christopher Kabrhel, MD, MPH, director of the Center for Vascular Emergencies at Mass General and a professor at Harvard Medical School in Boston, who assembled that formative meeting. “Without a clear basis in data, it was really important to have all the different specialists weigh in and give their perspective and talk about what was the best approach for the patient’s care.”
Filling data gaps
Some of those data gaps persist today, Dr. Kabrhel said. “It’s precisely that lack of head-to-head data that existed in 2012, and to a great extent still exists today, that led us to create this system.” The American Heart Association just this January issued a scientific statement on surgical management and mechanical circulatory support in high-risk PE (Circulation. 2023;147:e628-47).
But the intervening research has been uneven. The Pulmonary Embolism Thrombolysis (PEITHO) trial in 2014 evaluated systemic thrombolysis and anticoagulation alone (N Engl J Med. 2014;370:1402-11), but head-to-head studies of catheter-directed thrombolysis (CDT), which was just emerging in 2012, and either systemic thrombolysis or anticoagulation have been lacking, Dr. Kabrhel said. The Hi-PEITHO trial in high-risk PE patients is evaluating ultrasound-guided CDT plus anticoagulation vs. anticoagulation alone (Am Heart J. 2022:251:43-54), but it isn’t complete.
“The therapeutic landscape for PE is evolving incredibly rapidly,” he said. “When we first started PERT we were just starting to see CDT. Since then, we’ve seen several new thrombolytic catheters come onto the market, but there’s also been a proliferation of suction embolectomy catheters and we’ve seen a potentially larger role for surgery and the use of ECMO [extracorporeal membrane oxygenation] or cardiac bypass to bridge patients to definitive therapy. With the rapid evolution and the seemingly daily addition of new therapeutic options, I think the need for PERT is only increasing.”
A recent study out of the University of Michigan reported that the PERT there led to a decrease in the use of advanced therapies given to acute PE patients without reducing mortality or extending hospital stays (Thromb Res. 2023;221:73-8). A study in Spain reported that patients with high-risk and intermediate high-risk PE who had PERT-coordinated care had half the 12-month mortality rate of non-PERT counterparts, 9% vs. 22.2% (P = .02) (Med Clin [Barc]. 2023;S0025-7753(23)00017-9). And a 2021 study at University Hospitals in Cleveland reported that PERT-managed PE patients had a 60% lower rate of adverse outcomes at 90 days than non–PERT-managed patients (J Invasive Cardiol. 2021;33:E173-E180).
Nelish Ardeshna, MD, MA, the lead author of the Michigan study, said the PERT there was formed in 2017. Besides the multispecialty team that can be summoned to a teleconference on short notice, the protocol includes having at least one noninvasive specialist, such as a cardiologist or hospitalist, and one interventionalist, such as a radiologist, always on call. The PERT gets activated through the paging system after a hospital or emergency department physician identifies a suspected or established high-risk PE.
“High-risk PE patients can present in all settings, including the emergency department, ICU, surgical floor, or medical floor,” said Dr. Ardeshna, an internal medicine resident. “Management for these patients is equally varied from anticoagulation to systemic thrombolytics. Not all providers may be familiar with current guidelines to select the optimal therapy for high-risk pulmonary embolism patients. PERT aims to bridge that gap by providing a multidisciplinary discussion with PE specialists that can help identify the correct therapeutic options for optimal outcomes.”
At Cleveland Clinic, where the PERT has been in place since 2012, the PERT can consist of six to eight different specialties and involve up to 15 providers on a conference call, said Leben Tefera, MD, a vascular specialist and head of the PERT team there.
“Each patient will come in and have certain comorbidities,” Dr. Tefera said. “The unfortunate thing about a majority of the PEs that we see, in particular ones [in patients] that are very sick and require inpatient treatment, is that they don’t really fit into a box; you can’t come up with one kind of generic care routine or care path that treats the majority of patients with PE.”
Evolving to follow-up care
As the PERT protocol led to better inpatient outcomes, the teams became more aware that discharged PE patients were struggling with mental health and other quality-of-life issues – symptoms that have been understudied, according to a protocol Dr. Tefera coauthored for a prospective observational study of psychological distress symptoms in PE survivors. By contrast, the protocol noted, these symptoms have been studied extensively in myocardial infarction and stroke patients (Res Pract Thromb Hemost. 2023. doi: 10.1016/j.rpth.2023.10045). Other studies have found that 35%-50% of patients reported mental health symptoms 3 months after PE (Chest. 2021;159:2428-38; Qual Life Res. 2019;28:2111-24).
“A lot of physicians have known it for quite some time, but it wasn’t really until the last couple of years that physicians started saying psychological stress is something that we need to quantify and that we need to actually treat, that we actually need to address,” Dr. Tefera said. That led Dr. Tefera and his Cleveland Clinic PERT colleagues to set up a follow-up clinic for PE patients.
At their follow-up visits, patients complete validated questionnaires about anxiety, depression, fear of recurrence, PE-specific quality of life, and posttraumatic stress disorder. “If they flag as positive, we give them a referral to an in-house psychologist,” he said. “One thing I can report is that patients absolutely, positively love this, because it’s something that they are all experiencing that a lot of physicians just aren’t addressing.”
Artificial intelligence emerges
At the University of Pittsburgh Medical Center, the PERT has started evaluating artificial intelligence to aid in PE diagnosis. Belinda Rivera-Lebron, MD, director of the acute and chronic embolism program at Pitt, explained that the AI protocol hasn’t been adopted yet, but the concept is to have a platform that’s compatible with the hospital system’s electronic medical record.
She described how AI would work once the PERT is activated. “Once the patient goes through the CT scanner, within 60 seconds of that scan being completed, the scan gets uploaded into the cloud and the app or the platform is able to tell you whether there is PE present or absent, and whether there is right ventricle dilation on that scan. This is even before you probably even think about opening up the computer to look at the scan, and even before radiology opens up the scan to read,” she said. “It’s so fast.”
The idea is to send the scans rapidly to the PERT. “It will send you a text, a notification on your phone that will tell you Mr. Smith is PE positive,” Dr. Rivera-Lebron said. “Then you open it and you are able to scroll through the CT scan in your phone. So, it’s really remarkable.”
Clinical trials worth watching
Meanwhile, a number of clinical trials have started to enroll patients, or will soon, that Dr. Rivera-Lebron said are worth paying attention to.
PEITHO-3 is a randomized, placebo-controlled trial with long-term follow-up comparing the efficacy of a reduced-dose alteplase regimen or standard heparin anticoagulation in patients with intermediate to high-risk PE (Thromb Haemost. 2022;122:867-66).
PEERLESS is a prospective randomized trial comparing mechanical thrombectomy and CDT (ClinicalTrials.gov identifier NCT05111613).
PE-Thrombus Removal with Catheter-directed Therapy (PE-TRACT) is an open-label Phase 3 trial comparing anticoagulation and CDT that’s not yet recruiting (ClinicalTrials.gov identifier NCT05591118).
FlowTriever for Acute Massive Pulmonary Embolism (FLAME) is a prospective cohort study evaluating a clot-retrieving device in high-risk PE patients (ClinicalTrials.gov identifier NCT04795167).
When completed and published, these trials could provide PERTs more evidence for their decision-making.
Dr. Ardeshna and Dr. Tefera have no relevant relationships to disclose. Dr. Rivera-Lebron disclosed relationships with INARI Catheter and Johnson & Johnson. Dr. Kabrhel disclosed relationships with Bristol Myers Squibb and Pfizer.
In 2012, a small group of specialists, consisting of a critical care pulmonologist, cardiologist, cardiac surgeon, and vascular specialist, at Massachusetts General Hospital, Boston, met to Monday morning quarterback an acute pulmonary embolism case that didn’t go as well as they’d hoped. They came up with a concept known as the pulmonary embolism response team – PERT for short – an idea that soon took hold in other centers and served as the vanguard to other innovative approaches to managing critical care patients with PE, which is the third-leading cause of cardiovascular death in the United States (Intern Emerg Med. 2023. doi: 10.1007/s11739-022-03180-w).
Three years later the PERT Consortium came together, which today has 102 members, according to the organization’s website (www.pertconsortium.org), and members in South America, Europe, Asia, and Australia. Since then, and apps to expedite diagnosis and treatment. The PERT Consortium, meanwhile, is in the process of creating the PE Centers of Excellence program to certify centers that meet certain requirements.
“Part of the reason we recognized that a discussion across specialties was important was because there weren’t the large clinical trials that could tell us exactly what to do for any given case,” said Christopher Kabrhel, MD, MPH, director of the Center for Vascular Emergencies at Mass General and a professor at Harvard Medical School in Boston, who assembled that formative meeting. “Without a clear basis in data, it was really important to have all the different specialists weigh in and give their perspective and talk about what was the best approach for the patient’s care.”
Filling data gaps
Some of those data gaps persist today, Dr. Kabrhel said. “It’s precisely that lack of head-to-head data that existed in 2012, and to a great extent still exists today, that led us to create this system.” The American Heart Association just this January issued a scientific statement on surgical management and mechanical circulatory support in high-risk PE (Circulation. 2023;147:e628-47).
But the intervening research has been uneven. The Pulmonary Embolism Thrombolysis (PEITHO) trial in 2014 evaluated systemic thrombolysis and anticoagulation alone (N Engl J Med. 2014;370:1402-11), but head-to-head studies of catheter-directed thrombolysis (CDT), which was just emerging in 2012, and either systemic thrombolysis or anticoagulation have been lacking, Dr. Kabrhel said. The Hi-PEITHO trial in high-risk PE patients is evaluating ultrasound-guided CDT plus anticoagulation vs. anticoagulation alone (Am Heart J. 2022:251:43-54), but it isn’t complete.
“The therapeutic landscape for PE is evolving incredibly rapidly,” he said. “When we first started PERT we were just starting to see CDT. Since then, we’ve seen several new thrombolytic catheters come onto the market, but there’s also been a proliferation of suction embolectomy catheters and we’ve seen a potentially larger role for surgery and the use of ECMO [extracorporeal membrane oxygenation] or cardiac bypass to bridge patients to definitive therapy. With the rapid evolution and the seemingly daily addition of new therapeutic options, I think the need for PERT is only increasing.”
A recent study out of the University of Michigan reported that the PERT there led to a decrease in the use of advanced therapies given to acute PE patients without reducing mortality or extending hospital stays (Thromb Res. 2023;221:73-8). A study in Spain reported that patients with high-risk and intermediate high-risk PE who had PERT-coordinated care had half the 12-month mortality rate of non-PERT counterparts, 9% vs. 22.2% (P = .02) (Med Clin [Barc]. 2023;S0025-7753(23)00017-9). And a 2021 study at University Hospitals in Cleveland reported that PERT-managed PE patients had a 60% lower rate of adverse outcomes at 90 days than non–PERT-managed patients (J Invasive Cardiol. 2021;33:E173-E180).
Nelish Ardeshna, MD, MA, the lead author of the Michigan study, said the PERT there was formed in 2017. Besides the multispecialty team that can be summoned to a teleconference on short notice, the protocol includes having at least one noninvasive specialist, such as a cardiologist or hospitalist, and one interventionalist, such as a radiologist, always on call. The PERT gets activated through the paging system after a hospital or emergency department physician identifies a suspected or established high-risk PE.
“High-risk PE patients can present in all settings, including the emergency department, ICU, surgical floor, or medical floor,” said Dr. Ardeshna, an internal medicine resident. “Management for these patients is equally varied from anticoagulation to systemic thrombolytics. Not all providers may be familiar with current guidelines to select the optimal therapy for high-risk pulmonary embolism patients. PERT aims to bridge that gap by providing a multidisciplinary discussion with PE specialists that can help identify the correct therapeutic options for optimal outcomes.”
At Cleveland Clinic, where the PERT has been in place since 2012, the PERT can consist of six to eight different specialties and involve up to 15 providers on a conference call, said Leben Tefera, MD, a vascular specialist and head of the PERT team there.
“Each patient will come in and have certain comorbidities,” Dr. Tefera said. “The unfortunate thing about a majority of the PEs that we see, in particular ones [in patients] that are very sick and require inpatient treatment, is that they don’t really fit into a box; you can’t come up with one kind of generic care routine or care path that treats the majority of patients with PE.”
Evolving to follow-up care
As the PERT protocol led to better inpatient outcomes, the teams became more aware that discharged PE patients were struggling with mental health and other quality-of-life issues – symptoms that have been understudied, according to a protocol Dr. Tefera coauthored for a prospective observational study of psychological distress symptoms in PE survivors. By contrast, the protocol noted, these symptoms have been studied extensively in myocardial infarction and stroke patients (Res Pract Thromb Hemost. 2023. doi: 10.1016/j.rpth.2023.10045). Other studies have found that 35%-50% of patients reported mental health symptoms 3 months after PE (Chest. 2021;159:2428-38; Qual Life Res. 2019;28:2111-24).
“A lot of physicians have known it for quite some time, but it wasn’t really until the last couple of years that physicians started saying psychological stress is something that we need to quantify and that we need to actually treat, that we actually need to address,” Dr. Tefera said. That led Dr. Tefera and his Cleveland Clinic PERT colleagues to set up a follow-up clinic for PE patients.
At their follow-up visits, patients complete validated questionnaires about anxiety, depression, fear of recurrence, PE-specific quality of life, and posttraumatic stress disorder. “If they flag as positive, we give them a referral to an in-house psychologist,” he said. “One thing I can report is that patients absolutely, positively love this, because it’s something that they are all experiencing that a lot of physicians just aren’t addressing.”
Artificial intelligence emerges
At the University of Pittsburgh Medical Center, the PERT has started evaluating artificial intelligence to aid in PE diagnosis. Belinda Rivera-Lebron, MD, director of the acute and chronic embolism program at Pitt, explained that the AI protocol hasn’t been adopted yet, but the concept is to have a platform that’s compatible with the hospital system’s electronic medical record.
She described how AI would work once the PERT is activated. “Once the patient goes through the CT scanner, within 60 seconds of that scan being completed, the scan gets uploaded into the cloud and the app or the platform is able to tell you whether there is PE present or absent, and whether there is right ventricle dilation on that scan. This is even before you probably even think about opening up the computer to look at the scan, and even before radiology opens up the scan to read,” she said. “It’s so fast.”
The idea is to send the scans rapidly to the PERT. “It will send you a text, a notification on your phone that will tell you Mr. Smith is PE positive,” Dr. Rivera-Lebron said. “Then you open it and you are able to scroll through the CT scan in your phone. So, it’s really remarkable.”
Clinical trials worth watching
Meanwhile, a number of clinical trials have started to enroll patients, or will soon, that Dr. Rivera-Lebron said are worth paying attention to.
PEITHO-3 is a randomized, placebo-controlled trial with long-term follow-up comparing the efficacy of a reduced-dose alteplase regimen or standard heparin anticoagulation in patients with intermediate to high-risk PE (Thromb Haemost. 2022;122:867-66).
PEERLESS is a prospective randomized trial comparing mechanical thrombectomy and CDT (ClinicalTrials.gov identifier NCT05111613).
PE-Thrombus Removal with Catheter-directed Therapy (PE-TRACT) is an open-label Phase 3 trial comparing anticoagulation and CDT that’s not yet recruiting (ClinicalTrials.gov identifier NCT05591118).
FlowTriever for Acute Massive Pulmonary Embolism (FLAME) is a prospective cohort study evaluating a clot-retrieving device in high-risk PE patients (ClinicalTrials.gov identifier NCT04795167).
When completed and published, these trials could provide PERTs more evidence for their decision-making.
Dr. Ardeshna and Dr. Tefera have no relevant relationships to disclose. Dr. Rivera-Lebron disclosed relationships with INARI Catheter and Johnson & Johnson. Dr. Kabrhel disclosed relationships with Bristol Myers Squibb and Pfizer.