User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
A Military Nurse Saves a Life After a Brutal Rollover Crash
Emergencies happen anywhere and anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
A week earlier I’d had a heart surgery and was heading out for a post-op appointment when I saw it: I had a flat tire. It didn’t make sense. The tire was brand new, and there was no puncture. But it was flat.
I swapped out the flat for the spare and went off base to a tire shop. While I was there, my surgeon’s office called and rescheduled my appointment for a couple of hours later. That was lucky because by the time the tire was fixed, I had just enough time to get there.
The hospital is right near I-35 in San Antonio, Texas. I got off the freeway and onto the access road and paused to turn into the parking lot. That’s when I heard an enormous crash.
I saw a big poof of white smoke, and a car barreled off the freeway and came rolling down the embankment.
When the car hit the access road, I saw a woman ejected through the windshield. She bounced and landed in the road about 25 feet in front of me.
I put my car in park, grabbed my face mask and gloves, and started running toward her. But another vehicle — a truck towing a trailer — came from behind to drive around me. The driver didn’t realize what had happened and couldn’t stop in time…
The trailer ran over her.
I didn’t know if anyone could’ve survived that, but I went to her. I saw several other bystanders, but they were frozen in shock. I was praying, dear God, if she’s alive, let me do whatever I need to do to save her life.
It was a horrible scene. This poor lady was in a bloody heap in the middle of the road. Her right arm was twisted up under her neck so tightly, she was choking herself. So, the first thing I did was straighten her arm out to protect her airway.
I started yelling at people, “Call 9-1-1! Run to the hospital! Let them know there’s an accident out here, and I need help!”
The woman had a pulse, but it was super rapid. On first glance, she clearly had multiple fractures and a bad head bleed. With the sheer number of times she’d been injured, I didn’t know what was going on internally, but it was bad. She was gargling on her own blood and spitting it up. She was drowning.
A couple of technicians from the hospital came and brought me a tiny emergency kit. It had a blood pressure cuff and an oral airway. All the vital signs indicated the lady was going into shock. She’d lost a lot of blood on the pavement.
I was able to get the oral airway in. A few minutes later, a fire chief showed up. By now, the traffic had backed up so badly, the emergency vehicles couldn’t get in. But he managed to get there another way and gave me a cervical collar (C collar) and an Ambu bag.
I was hyper-focused on what I could do at that moment and what I needed to do next. Her stats were going down, but she still had a pulse. If she lost the pulse or went into a lethal rhythm, I’d have to start cardiopulmonary resuscitation (CPR). I asked the other people, but nobody else knew CPR, so I wouldn’t have help.
I could tell the lady had a pelvic fracture, and we needed to stabilize her. I directed people how to hold her neck safely and log-roll her flat on the ground. I also needed to put pressure on the back of her head because of all the bleeding. I got people to give me their clothes and tried to do that as I was bagging her.
The windows of her vehicle had all been blown out. I asked somebody to go find her purse with her ID. Then I noticed something …
My heart jumped into my stomach.
A car seat. There was an empty child’s car seat in the back of the car.
I started yelling at everyone, “Look for a baby! Go up and down the embankment and across the road. There might have been a baby in the car!”
But there wasn’t. Thank God. She hadn’t been driving with her child.
At that point, a paramedic came running from behind all the traffic. We did life support together until the ambulance finally arrived.
Emergency medical services got an intravenous line in and used medical anti-shock trousers. Thankfully, I already had the C collar on, and we’d been bagging her, so they could load her very quickly.
I got rid of my bloody gloves. I told a police officer I would come back. And then I went to my doctor’s appointment.
The window at my doctor’s office faced the access road, so the people there had seen all the traffic. They asked me what happened, and I said, “It was me. I saw it happen. I tried to help.” I was a little frazzled.
When I got back to the scene, the police and the fire chief kept thanking me for stopping. Why wouldn’t I stop? It was astounding to realize that they imagined somebody wouldn’t stop in a situation like this.
They told me the lady was alive. She was in the intensive care unit in critical condition, but she had survived. At that moment, I had this overwhelming feeling: God had put me in this exact place at the exact time to save her life.
Looking back, I think about how God ordered my steps. Without the mysterious flat tire, I would’ve gone to the hospital earlier. If my appointment hadn’t been rescheduled, I wouldn’t have been on the access road. All those events brought me there.
Several months later, the woman’s family contacted me and asked if we could meet. I found out more about her injuries. She’d had multiple skull fractures, facial fractures, and a broken jaw. Her upper arm was broken in three places. Her clavicle was broken. She had internal bleeding, a pelvic fracture, and a broken leg. She was 28 years old.
She’d had multiple surgeries, spent 2 months in the ICU, and another 3 months in intensive rehab. But she survived. It was incredible.
We all met up at a McDonald’s. First, her little son — who was the baby I thought might have been in the car — ran up to me and said, “Thank you for saving my mommy’s life.”
Then I turned, and there she was — a beautiful lady looking at me with awe and crying, saying, “It’s me.”
She obviously had gone through a transformation from all the injuries and the medications. She had a little bit of a speech delay, but mentally, she was there. She could walk.
She said, “You’re my angel. God put you there to save my life.” Her family all came up and hugged me. It was so beautiful.
She told me about the accident. She’d been speeding that day, zigzagging through lanes to get around the traffic. And she didn’t have her seatbelt on. She’d driven onto the shoulder to try to pass everyone, but it started narrowing. She clipped somebody’s bumper, went into a tailspin, and collided with a second vehicle, which caused her to flip over and down the embankment.
“God’s given me a new lease on life,” she said, “a fresh start. I will forever wear my seatbelt. And I’m going to do whatever I can to give back to other people because I don’t even feel like I deserve this.”
I just cried.
I’ve been a nurse for 29 years, first on the civilian side and later in the military. I’ve led codes and responded to trauma in a hospital setting or a deployed environment. I was well prepared to do what I did. But doing it under such stress with adrenaline bombarding me ... I’m amazed. I just think God’s hand was on me.
At that time, I was personally going through some things. After my heart surgery, I was in an emotional place where I didn’t feel loved or valued. But when I had that realization — when I knew that I was meant to be there to save her life, I also got the very clear message that I was valued and loved so much.
I know I have a very strong purpose. That day changed my life.
US Air Force Lt. Col. Anne Staley is the officer in charge of the Military Training Network, a division of the Defense Health Agency Education and Training Directorate in San Antonio, Texas.
A version of this article appeared on Medscape.com.
Emergencies happen anywhere and anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
A week earlier I’d had a heart surgery and was heading out for a post-op appointment when I saw it: I had a flat tire. It didn’t make sense. The tire was brand new, and there was no puncture. But it was flat.
I swapped out the flat for the spare and went off base to a tire shop. While I was there, my surgeon’s office called and rescheduled my appointment for a couple of hours later. That was lucky because by the time the tire was fixed, I had just enough time to get there.
The hospital is right near I-35 in San Antonio, Texas. I got off the freeway and onto the access road and paused to turn into the parking lot. That’s when I heard an enormous crash.
I saw a big poof of white smoke, and a car barreled off the freeway and came rolling down the embankment.
When the car hit the access road, I saw a woman ejected through the windshield. She bounced and landed in the road about 25 feet in front of me.
I put my car in park, grabbed my face mask and gloves, and started running toward her. But another vehicle — a truck towing a trailer — came from behind to drive around me. The driver didn’t realize what had happened and couldn’t stop in time…
The trailer ran over her.
I didn’t know if anyone could’ve survived that, but I went to her. I saw several other bystanders, but they were frozen in shock. I was praying, dear God, if she’s alive, let me do whatever I need to do to save her life.
It was a horrible scene. This poor lady was in a bloody heap in the middle of the road. Her right arm was twisted up under her neck so tightly, she was choking herself. So, the first thing I did was straighten her arm out to protect her airway.
I started yelling at people, “Call 9-1-1! Run to the hospital! Let them know there’s an accident out here, and I need help!”
The woman had a pulse, but it was super rapid. On first glance, she clearly had multiple fractures and a bad head bleed. With the sheer number of times she’d been injured, I didn’t know what was going on internally, but it was bad. She was gargling on her own blood and spitting it up. She was drowning.
A couple of technicians from the hospital came and brought me a tiny emergency kit. It had a blood pressure cuff and an oral airway. All the vital signs indicated the lady was going into shock. She’d lost a lot of blood on the pavement.
I was able to get the oral airway in. A few minutes later, a fire chief showed up. By now, the traffic had backed up so badly, the emergency vehicles couldn’t get in. But he managed to get there another way and gave me a cervical collar (C collar) and an Ambu bag.
I was hyper-focused on what I could do at that moment and what I needed to do next. Her stats were going down, but she still had a pulse. If she lost the pulse or went into a lethal rhythm, I’d have to start cardiopulmonary resuscitation (CPR). I asked the other people, but nobody else knew CPR, so I wouldn’t have help.
I could tell the lady had a pelvic fracture, and we needed to stabilize her. I directed people how to hold her neck safely and log-roll her flat on the ground. I also needed to put pressure on the back of her head because of all the bleeding. I got people to give me their clothes and tried to do that as I was bagging her.
The windows of her vehicle had all been blown out. I asked somebody to go find her purse with her ID. Then I noticed something …
My heart jumped into my stomach.
A car seat. There was an empty child’s car seat in the back of the car.
I started yelling at everyone, “Look for a baby! Go up and down the embankment and across the road. There might have been a baby in the car!”
But there wasn’t. Thank God. She hadn’t been driving with her child.
At that point, a paramedic came running from behind all the traffic. We did life support together until the ambulance finally arrived.
Emergency medical services got an intravenous line in and used medical anti-shock trousers. Thankfully, I already had the C collar on, and we’d been bagging her, so they could load her very quickly.
I got rid of my bloody gloves. I told a police officer I would come back. And then I went to my doctor’s appointment.
The window at my doctor’s office faced the access road, so the people there had seen all the traffic. They asked me what happened, and I said, “It was me. I saw it happen. I tried to help.” I was a little frazzled.
When I got back to the scene, the police and the fire chief kept thanking me for stopping. Why wouldn’t I stop? It was astounding to realize that they imagined somebody wouldn’t stop in a situation like this.
They told me the lady was alive. She was in the intensive care unit in critical condition, but she had survived. At that moment, I had this overwhelming feeling: God had put me in this exact place at the exact time to save her life.
Looking back, I think about how God ordered my steps. Without the mysterious flat tire, I would’ve gone to the hospital earlier. If my appointment hadn’t been rescheduled, I wouldn’t have been on the access road. All those events brought me there.
Several months later, the woman’s family contacted me and asked if we could meet. I found out more about her injuries. She’d had multiple skull fractures, facial fractures, and a broken jaw. Her upper arm was broken in three places. Her clavicle was broken. She had internal bleeding, a pelvic fracture, and a broken leg. She was 28 years old.
She’d had multiple surgeries, spent 2 months in the ICU, and another 3 months in intensive rehab. But she survived. It was incredible.
We all met up at a McDonald’s. First, her little son — who was the baby I thought might have been in the car — ran up to me and said, “Thank you for saving my mommy’s life.”
Then I turned, and there she was — a beautiful lady looking at me with awe and crying, saying, “It’s me.”
She obviously had gone through a transformation from all the injuries and the medications. She had a little bit of a speech delay, but mentally, she was there. She could walk.
She said, “You’re my angel. God put you there to save my life.” Her family all came up and hugged me. It was so beautiful.
She told me about the accident. She’d been speeding that day, zigzagging through lanes to get around the traffic. And she didn’t have her seatbelt on. She’d driven onto the shoulder to try to pass everyone, but it started narrowing. She clipped somebody’s bumper, went into a tailspin, and collided with a second vehicle, which caused her to flip over and down the embankment.
“God’s given me a new lease on life,” she said, “a fresh start. I will forever wear my seatbelt. And I’m going to do whatever I can to give back to other people because I don’t even feel like I deserve this.”
I just cried.
I’ve been a nurse for 29 years, first on the civilian side and later in the military. I’ve led codes and responded to trauma in a hospital setting or a deployed environment. I was well prepared to do what I did. But doing it under such stress with adrenaline bombarding me ... I’m amazed. I just think God’s hand was on me.
At that time, I was personally going through some things. After my heart surgery, I was in an emotional place where I didn’t feel loved or valued. But when I had that realization — when I knew that I was meant to be there to save her life, I also got the very clear message that I was valued and loved so much.
I know I have a very strong purpose. That day changed my life.
US Air Force Lt. Col. Anne Staley is the officer in charge of the Military Training Network, a division of the Defense Health Agency Education and Training Directorate in San Antonio, Texas.
A version of this article appeared on Medscape.com.
Emergencies happen anywhere and anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
A week earlier I’d had a heart surgery and was heading out for a post-op appointment when I saw it: I had a flat tire. It didn’t make sense. The tire was brand new, and there was no puncture. But it was flat.
I swapped out the flat for the spare and went off base to a tire shop. While I was there, my surgeon’s office called and rescheduled my appointment for a couple of hours later. That was lucky because by the time the tire was fixed, I had just enough time to get there.
The hospital is right near I-35 in San Antonio, Texas. I got off the freeway and onto the access road and paused to turn into the parking lot. That’s when I heard an enormous crash.
I saw a big poof of white smoke, and a car barreled off the freeway and came rolling down the embankment.
When the car hit the access road, I saw a woman ejected through the windshield. She bounced and landed in the road about 25 feet in front of me.
I put my car in park, grabbed my face mask and gloves, and started running toward her. But another vehicle — a truck towing a trailer — came from behind to drive around me. The driver didn’t realize what had happened and couldn’t stop in time…
The trailer ran over her.
I didn’t know if anyone could’ve survived that, but I went to her. I saw several other bystanders, but they were frozen in shock. I was praying, dear God, if she’s alive, let me do whatever I need to do to save her life.
It was a horrible scene. This poor lady was in a bloody heap in the middle of the road. Her right arm was twisted up under her neck so tightly, she was choking herself. So, the first thing I did was straighten her arm out to protect her airway.
I started yelling at people, “Call 9-1-1! Run to the hospital! Let them know there’s an accident out here, and I need help!”
The woman had a pulse, but it was super rapid. On first glance, she clearly had multiple fractures and a bad head bleed. With the sheer number of times she’d been injured, I didn’t know what was going on internally, but it was bad. She was gargling on her own blood and spitting it up. She was drowning.
A couple of technicians from the hospital came and brought me a tiny emergency kit. It had a blood pressure cuff and an oral airway. All the vital signs indicated the lady was going into shock. She’d lost a lot of blood on the pavement.
I was able to get the oral airway in. A few minutes later, a fire chief showed up. By now, the traffic had backed up so badly, the emergency vehicles couldn’t get in. But he managed to get there another way and gave me a cervical collar (C collar) and an Ambu bag.
I was hyper-focused on what I could do at that moment and what I needed to do next. Her stats were going down, but she still had a pulse. If she lost the pulse or went into a lethal rhythm, I’d have to start cardiopulmonary resuscitation (CPR). I asked the other people, but nobody else knew CPR, so I wouldn’t have help.
I could tell the lady had a pelvic fracture, and we needed to stabilize her. I directed people how to hold her neck safely and log-roll her flat on the ground. I also needed to put pressure on the back of her head because of all the bleeding. I got people to give me their clothes and tried to do that as I was bagging her.
The windows of her vehicle had all been blown out. I asked somebody to go find her purse with her ID. Then I noticed something …
My heart jumped into my stomach.
A car seat. There was an empty child’s car seat in the back of the car.
I started yelling at everyone, “Look for a baby! Go up and down the embankment and across the road. There might have been a baby in the car!”
But there wasn’t. Thank God. She hadn’t been driving with her child.
At that point, a paramedic came running from behind all the traffic. We did life support together until the ambulance finally arrived.
Emergency medical services got an intravenous line in and used medical anti-shock trousers. Thankfully, I already had the C collar on, and we’d been bagging her, so they could load her very quickly.
I got rid of my bloody gloves. I told a police officer I would come back. And then I went to my doctor’s appointment.
The window at my doctor’s office faced the access road, so the people there had seen all the traffic. They asked me what happened, and I said, “It was me. I saw it happen. I tried to help.” I was a little frazzled.
When I got back to the scene, the police and the fire chief kept thanking me for stopping. Why wouldn’t I stop? It was astounding to realize that they imagined somebody wouldn’t stop in a situation like this.
They told me the lady was alive. She was in the intensive care unit in critical condition, but she had survived. At that moment, I had this overwhelming feeling: God had put me in this exact place at the exact time to save her life.
Looking back, I think about how God ordered my steps. Without the mysterious flat tire, I would’ve gone to the hospital earlier. If my appointment hadn’t been rescheduled, I wouldn’t have been on the access road. All those events brought me there.
Several months later, the woman’s family contacted me and asked if we could meet. I found out more about her injuries. She’d had multiple skull fractures, facial fractures, and a broken jaw. Her upper arm was broken in three places. Her clavicle was broken. She had internal bleeding, a pelvic fracture, and a broken leg. She was 28 years old.
She’d had multiple surgeries, spent 2 months in the ICU, and another 3 months in intensive rehab. But she survived. It was incredible.
We all met up at a McDonald’s. First, her little son — who was the baby I thought might have been in the car — ran up to me and said, “Thank you for saving my mommy’s life.”
Then I turned, and there she was — a beautiful lady looking at me with awe and crying, saying, “It’s me.”
She obviously had gone through a transformation from all the injuries and the medications. She had a little bit of a speech delay, but mentally, she was there. She could walk.
She said, “You’re my angel. God put you there to save my life.” Her family all came up and hugged me. It was so beautiful.
She told me about the accident. She’d been speeding that day, zigzagging through lanes to get around the traffic. And she didn’t have her seatbelt on. She’d driven onto the shoulder to try to pass everyone, but it started narrowing. She clipped somebody’s bumper, went into a tailspin, and collided with a second vehicle, which caused her to flip over and down the embankment.
“God’s given me a new lease on life,” she said, “a fresh start. I will forever wear my seatbelt. And I’m going to do whatever I can to give back to other people because I don’t even feel like I deserve this.”
I just cried.
I’ve been a nurse for 29 years, first on the civilian side and later in the military. I’ve led codes and responded to trauma in a hospital setting or a deployed environment. I was well prepared to do what I did. But doing it under such stress with adrenaline bombarding me ... I’m amazed. I just think God’s hand was on me.
At that time, I was personally going through some things. After my heart surgery, I was in an emotional place where I didn’t feel loved or valued. But when I had that realization — when I knew that I was meant to be there to save her life, I also got the very clear message that I was valued and loved so much.
I know I have a very strong purpose. That day changed my life.
US Air Force Lt. Col. Anne Staley is the officer in charge of the Military Training Network, a division of the Defense Health Agency Education and Training Directorate in San Antonio, Texas.
A version of this article appeared on Medscape.com.
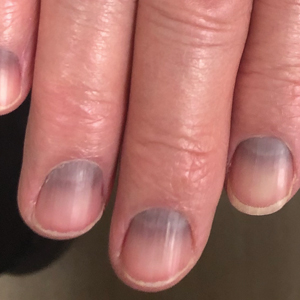
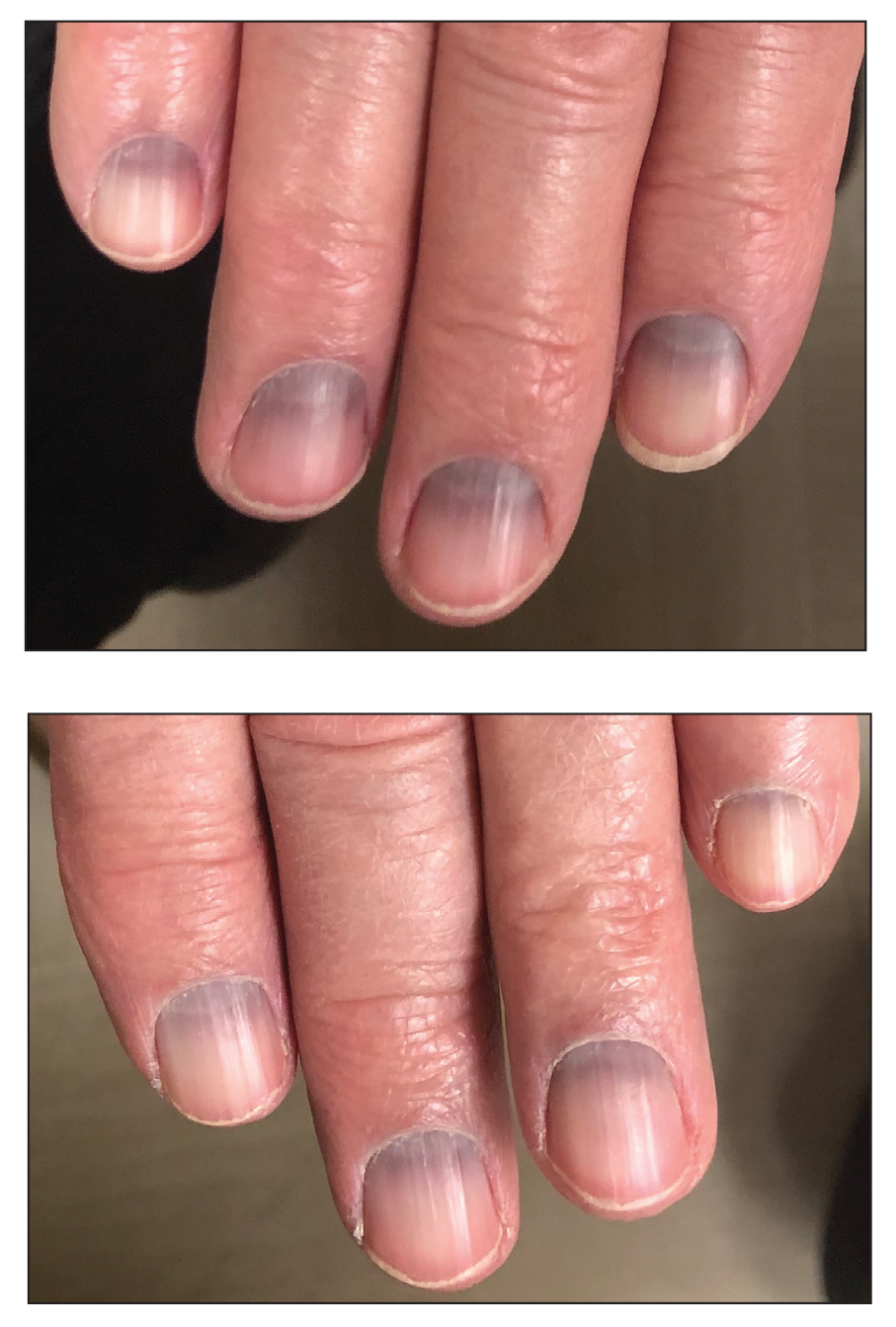
Blue to Slate Gray Discoloration of the Proximal Fingernails
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
An 88-year-old man presented with asymptomatic and unchanging discoloration of the proximal fingernails of both hands of 50 years’ duration. Physical examination revealed blue to slate gray, subungual pigmentary changes of the fingernails of both hands sparing the nail bed distal to the lunulae. There was no overlying plate dystrophy, toenail involvement, or additional mucocutaneous abnormalities. His medical history was notable for heart failure, obstructive sleep apnea, and type 2 diabetes mellitus. He had no history of hepatic, ophthalmologic, or neurologic dysfunction.
Deaths Linked to Substance Use, CVD on the Rise
TOPLINE:
, with the most pronounced rise among women, American Indians, younger people, rural residents, and users of cannabis and psychostimulants, results of new research suggest.
METHODOLOGY:
- From the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) database and using International Classification of Diseases (ICD) codes, researchers collected data on deaths within the United States where both SU and CVD (SU+CVD) were a contributing or an underlying cause and gathered information on location of death (medical facility, home, hospice, nursing home/long-term care facility), demographics (sex, race/ethnicity, age), and region (urban-rural, state).
- Researchers determined crude and age-adjusted mortality rates (AAMRs) per 100,000 population, identified trends in AAMR using annual percent change (APC) and calculated the weighted average of APCs (AAPCs).
- Between 1999 and 2019, there were 636,572 deaths related to CVD+SU, 75.6% of which were among men and 70.6% among non-Hispanic White individuals, with 65% related to alcohol, and where location of death was available, 47.7% occurred in medical facilities.
TAKEAWAY:
- The overall SU+CVD-related AAMR from 1999 to 2019 was 14.3 (95% CI, 14.3-14.3) per 100,000 individuals, with the rate being higher in men (22.5) than in women (6.8) and highest in American Indians or Alaska Natives (37.7) compared with other races/ethnicities.
- Rural areas had higher SU+CVD-related AAMR (15.2; 95% CI, 15.1-15.3) than urban areas, with the District of Columbia having the highest AAMR geographically (25.4), individuals aged 55-69 years having the highest rate agewise (25.1), and alcohol accounting for the highest rate (9.09) among substance types.
- Temporal trends show that the overall SU+CVD-related AAMR increased from 9.9 in 1999 to 21.4 in 2019, a rate that started accelerating in 2012, with an AAPC of 4.0% (95% CI, 3.7-4.3); increases were across all ethnicities and age groups and were particularly pronounced among women (4.8%; 95% CI, 4.5-5.1).
- Cannabis had the highest AAPC of all substances (12.7%), but stimulants had an APC of 21.4 (95% CI, 20.0-22.8) from 2009 to 2019, a period during which stimulants were the fastest-growing substance abuse category.
IN PRACTICE:
These new results identify high-risk groups, which “is crucial for prioritizing preventive measures aiming to reduce substance use and cardiovascular disease-related mortality in these populations,” the researchers wrote.
SOURCE:
Abdul Mannan Khan Minhas, MD, Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, and Jakrin Kewcharoen, MD, Division of Cardiology, Loma Linda University Medical Center, Loma Linda, California, were co-first authors of the study, which was published online in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
TOPLINE:
, with the most pronounced rise among women, American Indians, younger people, rural residents, and users of cannabis and psychostimulants, results of new research suggest.
METHODOLOGY:
- From the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) database and using International Classification of Diseases (ICD) codes, researchers collected data on deaths within the United States where both SU and CVD (SU+CVD) were a contributing or an underlying cause and gathered information on location of death (medical facility, home, hospice, nursing home/long-term care facility), demographics (sex, race/ethnicity, age), and region (urban-rural, state).
- Researchers determined crude and age-adjusted mortality rates (AAMRs) per 100,000 population, identified trends in AAMR using annual percent change (APC) and calculated the weighted average of APCs (AAPCs).
- Between 1999 and 2019, there were 636,572 deaths related to CVD+SU, 75.6% of which were among men and 70.6% among non-Hispanic White individuals, with 65% related to alcohol, and where location of death was available, 47.7% occurred in medical facilities.
TAKEAWAY:
- The overall SU+CVD-related AAMR from 1999 to 2019 was 14.3 (95% CI, 14.3-14.3) per 100,000 individuals, with the rate being higher in men (22.5) than in women (6.8) and highest in American Indians or Alaska Natives (37.7) compared with other races/ethnicities.
- Rural areas had higher SU+CVD-related AAMR (15.2; 95% CI, 15.1-15.3) than urban areas, with the District of Columbia having the highest AAMR geographically (25.4), individuals aged 55-69 years having the highest rate agewise (25.1), and alcohol accounting for the highest rate (9.09) among substance types.
- Temporal trends show that the overall SU+CVD-related AAMR increased from 9.9 in 1999 to 21.4 in 2019, a rate that started accelerating in 2012, with an AAPC of 4.0% (95% CI, 3.7-4.3); increases were across all ethnicities and age groups and were particularly pronounced among women (4.8%; 95% CI, 4.5-5.1).
- Cannabis had the highest AAPC of all substances (12.7%), but stimulants had an APC of 21.4 (95% CI, 20.0-22.8) from 2009 to 2019, a period during which stimulants were the fastest-growing substance abuse category.
IN PRACTICE:
These new results identify high-risk groups, which “is crucial for prioritizing preventive measures aiming to reduce substance use and cardiovascular disease-related mortality in these populations,” the researchers wrote.
SOURCE:
Abdul Mannan Khan Minhas, MD, Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, and Jakrin Kewcharoen, MD, Division of Cardiology, Loma Linda University Medical Center, Loma Linda, California, were co-first authors of the study, which was published online in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
TOPLINE:
, with the most pronounced rise among women, American Indians, younger people, rural residents, and users of cannabis and psychostimulants, results of new research suggest.
METHODOLOGY:
- From the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) database and using International Classification of Diseases (ICD) codes, researchers collected data on deaths within the United States where both SU and CVD (SU+CVD) were a contributing or an underlying cause and gathered information on location of death (medical facility, home, hospice, nursing home/long-term care facility), demographics (sex, race/ethnicity, age), and region (urban-rural, state).
- Researchers determined crude and age-adjusted mortality rates (AAMRs) per 100,000 population, identified trends in AAMR using annual percent change (APC) and calculated the weighted average of APCs (AAPCs).
- Between 1999 and 2019, there were 636,572 deaths related to CVD+SU, 75.6% of which were among men and 70.6% among non-Hispanic White individuals, with 65% related to alcohol, and where location of death was available, 47.7% occurred in medical facilities.
TAKEAWAY:
- The overall SU+CVD-related AAMR from 1999 to 2019 was 14.3 (95% CI, 14.3-14.3) per 100,000 individuals, with the rate being higher in men (22.5) than in women (6.8) and highest in American Indians or Alaska Natives (37.7) compared with other races/ethnicities.
- Rural areas had higher SU+CVD-related AAMR (15.2; 95% CI, 15.1-15.3) than urban areas, with the District of Columbia having the highest AAMR geographically (25.4), individuals aged 55-69 years having the highest rate agewise (25.1), and alcohol accounting for the highest rate (9.09) among substance types.
- Temporal trends show that the overall SU+CVD-related AAMR increased from 9.9 in 1999 to 21.4 in 2019, a rate that started accelerating in 2012, with an AAPC of 4.0% (95% CI, 3.7-4.3); increases were across all ethnicities and age groups and were particularly pronounced among women (4.8%; 95% CI, 4.5-5.1).
- Cannabis had the highest AAPC of all substances (12.7%), but stimulants had an APC of 21.4 (95% CI, 20.0-22.8) from 2009 to 2019, a period during which stimulants were the fastest-growing substance abuse category.
IN PRACTICE:
These new results identify high-risk groups, which “is crucial for prioritizing preventive measures aiming to reduce substance use and cardiovascular disease-related mortality in these populations,” the researchers wrote.
SOURCE:
Abdul Mannan Khan Minhas, MD, Department of Medicine, University of Mississippi Medical Center, Jackson, Mississippi, and Jakrin Kewcharoen, MD, Division of Cardiology, Loma Linda University Medical Center, Loma Linda, California, were co-first authors of the study, which was published online in the Journal of the American Heart Association.
A version of this article first appeared on Medscape.com.
Corticosteroid Injections Don’t Move Blood Sugar for Most
TOPLINE:
Intra-articular corticosteroid (IACS) injections pose a minimal risk of accelerating diabetes for most people, despite temporarily elevating blood glucose levels, according to a study published in Clinical Diabetes.
METHODOLOGY:
- Almost half of Americans with diabetes have arthritis, so glycemic control is a concern for many receiving IACS injections.
- IACS injections are known to cause short-term hyperglycemia, but their long-term effects on glycemic control are not well studied.
- For the retrospective cohort study, researchers at Mayo Clinic in Rochester, Minnesota, used electronic health records from 1169 adults who had received an IACS injection in one large joint between 2012 and 2018.
- They analyzed data on A1C levels for study participants from 18 months before and after the injections.
- Researchers assessed if participants had a greater-than-expected (defined as an increase of more than 0.5% above expected) concentration of A1C after the injection, and examined rates of diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome in the 30 days following an injection.
TAKEAWAY:
- Nearly 16% of people experienced a greater-than-expected A1C level after receiving an injection.
- A1C levels rose by an average of 1.2% in the greater-than-expected group, but decreased by an average of 0.2% in the average group.
- One patient had an episode of severe hyperglycemia that was linked to the injection.
- A baseline level of A1C above 8% was the only factor associated with a greater-than-expected increase in the marker after an IACS injection.
IN PRACTICE:
“Although most patients do not experience an increase in A1C after IACS, clinicians should counsel patients with suboptimally controlled diabetes about risks of further hyperglycemia after IACS administration,” the researchers wrote.
SOURCE:
The study was led by Terin T. Sytsma, MD, of Mayo Clinic in Rochester, Minnesota.
LIMITATIONS:
The study was retrospective and could not establish causation. In addition, the population was of residents from one county in Minnesota, and was not racially or ethnically diverse. Details about the injection, such as location and total dose, were not available. The study also did not include a control group.
DISCLOSURES:
The study was funded by Mayo Clinic and the National Center for Advancing Translational Sciences. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Intra-articular corticosteroid (IACS) injections pose a minimal risk of accelerating diabetes for most people, despite temporarily elevating blood glucose levels, according to a study published in Clinical Diabetes.
METHODOLOGY:
- Almost half of Americans with diabetes have arthritis, so glycemic control is a concern for many receiving IACS injections.
- IACS injections are known to cause short-term hyperglycemia, but their long-term effects on glycemic control are not well studied.
- For the retrospective cohort study, researchers at Mayo Clinic in Rochester, Minnesota, used electronic health records from 1169 adults who had received an IACS injection in one large joint between 2012 and 2018.
- They analyzed data on A1C levels for study participants from 18 months before and after the injections.
- Researchers assessed if participants had a greater-than-expected (defined as an increase of more than 0.5% above expected) concentration of A1C after the injection, and examined rates of diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome in the 30 days following an injection.
TAKEAWAY:
- Nearly 16% of people experienced a greater-than-expected A1C level after receiving an injection.
- A1C levels rose by an average of 1.2% in the greater-than-expected group, but decreased by an average of 0.2% in the average group.
- One patient had an episode of severe hyperglycemia that was linked to the injection.
- A baseline level of A1C above 8% was the only factor associated with a greater-than-expected increase in the marker after an IACS injection.
IN PRACTICE:
“Although most patients do not experience an increase in A1C after IACS, clinicians should counsel patients with suboptimally controlled diabetes about risks of further hyperglycemia after IACS administration,” the researchers wrote.
SOURCE:
The study was led by Terin T. Sytsma, MD, of Mayo Clinic in Rochester, Minnesota.
LIMITATIONS:
The study was retrospective and could not establish causation. In addition, the population was of residents from one county in Minnesota, and was not racially or ethnically diverse. Details about the injection, such as location and total dose, were not available. The study also did not include a control group.
DISCLOSURES:
The study was funded by Mayo Clinic and the National Center for Advancing Translational Sciences. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Intra-articular corticosteroid (IACS) injections pose a minimal risk of accelerating diabetes for most people, despite temporarily elevating blood glucose levels, according to a study published in Clinical Diabetes.
METHODOLOGY:
- Almost half of Americans with diabetes have arthritis, so glycemic control is a concern for many receiving IACS injections.
- IACS injections are known to cause short-term hyperglycemia, but their long-term effects on glycemic control are not well studied.
- For the retrospective cohort study, researchers at Mayo Clinic in Rochester, Minnesota, used electronic health records from 1169 adults who had received an IACS injection in one large joint between 2012 and 2018.
- They analyzed data on A1C levels for study participants from 18 months before and after the injections.
- Researchers assessed if participants had a greater-than-expected (defined as an increase of more than 0.5% above expected) concentration of A1C after the injection, and examined rates of diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome in the 30 days following an injection.
TAKEAWAY:
- Nearly 16% of people experienced a greater-than-expected A1C level after receiving an injection.
- A1C levels rose by an average of 1.2% in the greater-than-expected group, but decreased by an average of 0.2% in the average group.
- One patient had an episode of severe hyperglycemia that was linked to the injection.
- A baseline level of A1C above 8% was the only factor associated with a greater-than-expected increase in the marker after an IACS injection.
IN PRACTICE:
“Although most patients do not experience an increase in A1C after IACS, clinicians should counsel patients with suboptimally controlled diabetes about risks of further hyperglycemia after IACS administration,” the researchers wrote.
SOURCE:
The study was led by Terin T. Sytsma, MD, of Mayo Clinic in Rochester, Minnesota.
LIMITATIONS:
The study was retrospective and could not establish causation. In addition, the population was of residents from one county in Minnesota, and was not racially or ethnically diverse. Details about the injection, such as location and total dose, were not available. The study also did not include a control group.
DISCLOSURES:
The study was funded by Mayo Clinic and the National Center for Advancing Translational Sciences. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
High Rate of Rehospitalization After First Ischemic Stroke
TOPLINE:
Among patients hospitalized with a first ischemic stroke, 80% were rehospitalized, primarily because of subsequent primary cardiovascular and cerebrovascular diagnoses.
METHODOLOGY:
- To gather information on post-stroke hospital admission, investigators followed 1412 participants (mean age, 72.4 years; 52.1% women, 35.3% Black individuals) from the Atherosclerosis Risk in Communities (ARIC) study who were living in Maryland, Minnesota, North Carolina, and Mississippi.
- Participants were recruited between 1987 and 1989 when they were 45-64 years old and were followed on an annual and then semiannual basis from the index discharge until discharge after their second hospitalization, death, or end of the study in December 2019.
- Specific diagnoses for each hospitalization were based on hospital records, discharge diagnoses, and annual and semiannual phone interviews.
TAKEAWAY:
- During the study period, 1143 hospitalizations occurred over 41,849 person-months.
- 81% of participants were hospitalized over a maximum of 26.6 years of follow-up. Primary cardiovascular and cerebrovascular diagnoses were reported for half of readmissions.
- Over the follow-up period, compared with cardioembolic stroke, readmission risk was lower for thrombotic/lacunar stroke (adjusted hazard ratio [aHR], 0.82; 95% CI, 0.71-0.95) and hemorrhagic stroke (aHR, 0.74; 95% CI, 0.58-0.93). However, when adjusting for atrial fibrillation and competing risk for death, there were no significant differences between stroke subtypes.
- Compared with cardioembolic stroke, thrombotic/lacunar stroke was associated with lower readmission risk within 1 month (aHR, 0.66; 95% CI, 0.46-0.93) and from 1 month to 1 year (aHR, 0.78; 95% CI, 0.62-0.97), and hemorrhagic stroke was associated with lower risk from 1 month to 1 year (aHR, 0.60; 95% CI, 0.41-0.87).
IN PRACTICE:
“These results suggest that prevention strategies focused on cardiovascular and cerebrovascular health warrant further investigation, especially within the first year after incident stroke and perhaps particularly among individuals with an incident cardioembolic stroke,” the authors wrote.
SOURCE:
Kelly Sloane, MD, of the University of Pennsylvania Perelman School of Medicine in Philadelphia, led the study along with colleagues at the National Institute of Neurological Disorders and Stroke, Johns Hopkins University in Baltimore, and the University of North Carolina, Chapel Hill. The article was published online on January 5 in Neurology.
LIMITATIONS:
The ARIC study classification of stroke subtype grouped embolic strokes of undetermined source as thrombotic strokes, and investigators were unable to distinguish between the groups. In addition, there was no way to measure stroke severity, which could have played a role in readmission risk.
DISCLOSURES:
The study was funded by the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institutes of Health.
A version of this article appeared on Medscape.com.
TOPLINE:
Among patients hospitalized with a first ischemic stroke, 80% were rehospitalized, primarily because of subsequent primary cardiovascular and cerebrovascular diagnoses.
METHODOLOGY:
- To gather information on post-stroke hospital admission, investigators followed 1412 participants (mean age, 72.4 years; 52.1% women, 35.3% Black individuals) from the Atherosclerosis Risk in Communities (ARIC) study who were living in Maryland, Minnesota, North Carolina, and Mississippi.
- Participants were recruited between 1987 and 1989 when they were 45-64 years old and were followed on an annual and then semiannual basis from the index discharge until discharge after their second hospitalization, death, or end of the study in December 2019.
- Specific diagnoses for each hospitalization were based on hospital records, discharge diagnoses, and annual and semiannual phone interviews.
TAKEAWAY:
- During the study period, 1143 hospitalizations occurred over 41,849 person-months.
- 81% of participants were hospitalized over a maximum of 26.6 years of follow-up. Primary cardiovascular and cerebrovascular diagnoses were reported for half of readmissions.
- Over the follow-up period, compared with cardioembolic stroke, readmission risk was lower for thrombotic/lacunar stroke (adjusted hazard ratio [aHR], 0.82; 95% CI, 0.71-0.95) and hemorrhagic stroke (aHR, 0.74; 95% CI, 0.58-0.93). However, when adjusting for atrial fibrillation and competing risk for death, there were no significant differences between stroke subtypes.
- Compared with cardioembolic stroke, thrombotic/lacunar stroke was associated with lower readmission risk within 1 month (aHR, 0.66; 95% CI, 0.46-0.93) and from 1 month to 1 year (aHR, 0.78; 95% CI, 0.62-0.97), and hemorrhagic stroke was associated with lower risk from 1 month to 1 year (aHR, 0.60; 95% CI, 0.41-0.87).
IN PRACTICE:
“These results suggest that prevention strategies focused on cardiovascular and cerebrovascular health warrant further investigation, especially within the first year after incident stroke and perhaps particularly among individuals with an incident cardioembolic stroke,” the authors wrote.
SOURCE:
Kelly Sloane, MD, of the University of Pennsylvania Perelman School of Medicine in Philadelphia, led the study along with colleagues at the National Institute of Neurological Disorders and Stroke, Johns Hopkins University in Baltimore, and the University of North Carolina, Chapel Hill. The article was published online on January 5 in Neurology.
LIMITATIONS:
The ARIC study classification of stroke subtype grouped embolic strokes of undetermined source as thrombotic strokes, and investigators were unable to distinguish between the groups. In addition, there was no way to measure stroke severity, which could have played a role in readmission risk.
DISCLOSURES:
The study was funded by the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institutes of Health.
A version of this article appeared on Medscape.com.
TOPLINE:
Among patients hospitalized with a first ischemic stroke, 80% were rehospitalized, primarily because of subsequent primary cardiovascular and cerebrovascular diagnoses.
METHODOLOGY:
- To gather information on post-stroke hospital admission, investigators followed 1412 participants (mean age, 72.4 years; 52.1% women, 35.3% Black individuals) from the Atherosclerosis Risk in Communities (ARIC) study who were living in Maryland, Minnesota, North Carolina, and Mississippi.
- Participants were recruited between 1987 and 1989 when they were 45-64 years old and were followed on an annual and then semiannual basis from the index discharge until discharge after their second hospitalization, death, or end of the study in December 2019.
- Specific diagnoses for each hospitalization were based on hospital records, discharge diagnoses, and annual and semiannual phone interviews.
TAKEAWAY:
- During the study period, 1143 hospitalizations occurred over 41,849 person-months.
- 81% of participants were hospitalized over a maximum of 26.6 years of follow-up. Primary cardiovascular and cerebrovascular diagnoses were reported for half of readmissions.
- Over the follow-up period, compared with cardioembolic stroke, readmission risk was lower for thrombotic/lacunar stroke (adjusted hazard ratio [aHR], 0.82; 95% CI, 0.71-0.95) and hemorrhagic stroke (aHR, 0.74; 95% CI, 0.58-0.93). However, when adjusting for atrial fibrillation and competing risk for death, there were no significant differences between stroke subtypes.
- Compared with cardioembolic stroke, thrombotic/lacunar stroke was associated with lower readmission risk within 1 month (aHR, 0.66; 95% CI, 0.46-0.93) and from 1 month to 1 year (aHR, 0.78; 95% CI, 0.62-0.97), and hemorrhagic stroke was associated with lower risk from 1 month to 1 year (aHR, 0.60; 95% CI, 0.41-0.87).
IN PRACTICE:
“These results suggest that prevention strategies focused on cardiovascular and cerebrovascular health warrant further investigation, especially within the first year after incident stroke and perhaps particularly among individuals with an incident cardioembolic stroke,” the authors wrote.
SOURCE:
Kelly Sloane, MD, of the University of Pennsylvania Perelman School of Medicine in Philadelphia, led the study along with colleagues at the National Institute of Neurological Disorders and Stroke, Johns Hopkins University in Baltimore, and the University of North Carolina, Chapel Hill. The article was published online on January 5 in Neurology.
LIMITATIONS:
The ARIC study classification of stroke subtype grouped embolic strokes of undetermined source as thrombotic strokes, and investigators were unable to distinguish between the groups. In addition, there was no way to measure stroke severity, which could have played a role in readmission risk.
DISCLOSURES:
The study was funded by the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institutes of Health.
A version of this article appeared on Medscape.com.
SGLT2 Inhibitors Protective Against Retinopathy in T2D
TOPLINE:
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are associated with a lower risk for sight-threatening retinopathy than other second-line glucose-lowering medications in patients with type 2 diabetes (T2D).
METHODOLOGY:
- Researchers conducted a nationwide cohort study including 3,544,383 patients with newly diagnosed T2D.
- During the 5-year study period, 159,965 patients were treated with SGLT2 inhibitors, 304,383 received dipeptidyl peptidase-4 (DPP-4) inhibitors, 108,420 took pioglitazone, and 189,618 received sulfonylurea.
- The propensity score matching found 65,930 pairs of patients treated with SGLT2 inhibitors vs DPP-4 inhibitors, 93,760 pairs treated with SGLT2 inhibitors vs pioglitazone, and 42,121 pairs treated with SGLT2 inhibitors vs sulfonylurea.
- The main outcome was sight-threatening retinopathy in patients with at least two outpatient visits or one hospitalization or anti-vascular endothelial growth factor injections.
TAKEAWAY:
- SGLT2 inhibitors reduced sight-threatening retinopathy risk by 43% vs DPP-4 inhibitors (adjusted hazard ratio [aHR], 0.57), 38% vs sulfonylurea (aHR, 0.62), and 25% vs pioglitazone (aHR, 0.75; P < .001 for all).
- Similarly, the cumulative incidence of sight-threatening retinopathy was significantly lower with SGLT2 inhibitors vs DPP-4i, pioglitazone, or sulfonylurea (P < .001 for all).
- All three SGLT2 inhibitor treatments, namely, empagliflozin, dapagliflozin, and canagliflozin, were more effective than DPP-4 inhibitors, pioglitazone, or sulfonylurea in reducing the risk for sight-threatening retinopathy.
IN PRACTICE:
“SGLT2i treatments were as safe and effective in slowing the progression of diabetic retinopathy as in lowering the risk for diabetic nephropathy in patients with T2D,” the authors wrote.
SOURCE:
This study was led by Fu-Shun Yen, MD, a private practitioner from Taiwan, and was published online on December 20, 2023, in JAMA Network Open.
LIMITATIONS:
There were insufficient data regarding the participants’ alcohol use, physical activity, smoking status, and family history, which may have had an impact on the results.
The study mainly involved individuals of Taiwanese ethnicity.
DISCLOSURES:
This study was supported partly by the Taiwan Ministry of Health and Welfare Clinical Trial Center, the MOST Clinical Trial Consortium for Stroke, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are associated with a lower risk for sight-threatening retinopathy than other second-line glucose-lowering medications in patients with type 2 diabetes (T2D).
METHODOLOGY:
- Researchers conducted a nationwide cohort study including 3,544,383 patients with newly diagnosed T2D.
- During the 5-year study period, 159,965 patients were treated with SGLT2 inhibitors, 304,383 received dipeptidyl peptidase-4 (DPP-4) inhibitors, 108,420 took pioglitazone, and 189,618 received sulfonylurea.
- The propensity score matching found 65,930 pairs of patients treated with SGLT2 inhibitors vs DPP-4 inhibitors, 93,760 pairs treated with SGLT2 inhibitors vs pioglitazone, and 42,121 pairs treated with SGLT2 inhibitors vs sulfonylurea.
- The main outcome was sight-threatening retinopathy in patients with at least two outpatient visits or one hospitalization or anti-vascular endothelial growth factor injections.
TAKEAWAY:
- SGLT2 inhibitors reduced sight-threatening retinopathy risk by 43% vs DPP-4 inhibitors (adjusted hazard ratio [aHR], 0.57), 38% vs sulfonylurea (aHR, 0.62), and 25% vs pioglitazone (aHR, 0.75; P < .001 for all).
- Similarly, the cumulative incidence of sight-threatening retinopathy was significantly lower with SGLT2 inhibitors vs DPP-4i, pioglitazone, or sulfonylurea (P < .001 for all).
- All three SGLT2 inhibitor treatments, namely, empagliflozin, dapagliflozin, and canagliflozin, were more effective than DPP-4 inhibitors, pioglitazone, or sulfonylurea in reducing the risk for sight-threatening retinopathy.
IN PRACTICE:
“SGLT2i treatments were as safe and effective in slowing the progression of diabetic retinopathy as in lowering the risk for diabetic nephropathy in patients with T2D,” the authors wrote.
SOURCE:
This study was led by Fu-Shun Yen, MD, a private practitioner from Taiwan, and was published online on December 20, 2023, in JAMA Network Open.
LIMITATIONS:
There were insufficient data regarding the participants’ alcohol use, physical activity, smoking status, and family history, which may have had an impact on the results.
The study mainly involved individuals of Taiwanese ethnicity.
DISCLOSURES:
This study was supported partly by the Taiwan Ministry of Health and Welfare Clinical Trial Center, the MOST Clinical Trial Consortium for Stroke, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are associated with a lower risk for sight-threatening retinopathy than other second-line glucose-lowering medications in patients with type 2 diabetes (T2D).
METHODOLOGY:
- Researchers conducted a nationwide cohort study including 3,544,383 patients with newly diagnosed T2D.
- During the 5-year study period, 159,965 patients were treated with SGLT2 inhibitors, 304,383 received dipeptidyl peptidase-4 (DPP-4) inhibitors, 108,420 took pioglitazone, and 189,618 received sulfonylurea.
- The propensity score matching found 65,930 pairs of patients treated with SGLT2 inhibitors vs DPP-4 inhibitors, 93,760 pairs treated with SGLT2 inhibitors vs pioglitazone, and 42,121 pairs treated with SGLT2 inhibitors vs sulfonylurea.
- The main outcome was sight-threatening retinopathy in patients with at least two outpatient visits or one hospitalization or anti-vascular endothelial growth factor injections.
TAKEAWAY:
- SGLT2 inhibitors reduced sight-threatening retinopathy risk by 43% vs DPP-4 inhibitors (adjusted hazard ratio [aHR], 0.57), 38% vs sulfonylurea (aHR, 0.62), and 25% vs pioglitazone (aHR, 0.75; P < .001 for all).
- Similarly, the cumulative incidence of sight-threatening retinopathy was significantly lower with SGLT2 inhibitors vs DPP-4i, pioglitazone, or sulfonylurea (P < .001 for all).
- All three SGLT2 inhibitor treatments, namely, empagliflozin, dapagliflozin, and canagliflozin, were more effective than DPP-4 inhibitors, pioglitazone, or sulfonylurea in reducing the risk for sight-threatening retinopathy.
IN PRACTICE:
“SGLT2i treatments were as safe and effective in slowing the progression of diabetic retinopathy as in lowering the risk for diabetic nephropathy in patients with T2D,” the authors wrote.
SOURCE:
This study was led by Fu-Shun Yen, MD, a private practitioner from Taiwan, and was published online on December 20, 2023, in JAMA Network Open.
LIMITATIONS:
There were insufficient data regarding the participants’ alcohol use, physical activity, smoking status, and family history, which may have had an impact on the results.
The study mainly involved individuals of Taiwanese ethnicity.
DISCLOSURES:
This study was supported partly by the Taiwan Ministry of Health and Welfare Clinical Trial Center, the MOST Clinical Trial Consortium for Stroke, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Hair Creams: Do You Know the Health Risks?
In late December 2023, Brazil’s National Health Surveillance Agency (ANVISA) suspended the commercialization of approximately 1200 hair creams because of reports of eye irritation and temporary blindness.
A similar measure encompassing all hair creams sold in the country had already been announced by the agency in March. However, after a few weeks, ANVISA issued a resolution with rules for the products’ commercialization, allowing them back on the shelves.
With the new resolution, the sale of products that do not comply with the standards has once again been suspended. The reason is that reports of adverse events have reemerged. These events include temporary vision loss, headaches, and burning, tearing, itching, redness, and swelling of the eyes. According to reports, these adverse effects occurred mainly in people who used the specific products before swimming in the sea or in pools, or even going out in the rain.
The banned products contain 20% or more ethoxylated alcohols in their formulations. , potentially causing allergies and burns to the eyes and skin. They also have a high pulmonary and neurological toxicity. All these substances are eye irritants and can cause chemical keratitis. In extreme cases, corneal ulcers may develop, leading to vision loss.
The Brazilian Council of Ophthalmology also issued a warning on these products. It emphasized that, in addition to the sales prohibition, consumers should check the labels of hair creams to make sure that these toxic substances are not present in the product formulation.
The ANVISA website contains a list of creams that are considered safe and have not had their commercialization suspended, along with links to adverse event notifications reported by healthcare professionals or consumers.
For consumers who have recently used hair creams, the agency advises careful hair washing, including tilting the head backward to prevent the product from coming into contact with the eye area. If there is accidental eye contact, the eyes should be washed with plenty of water.
If there are any undesired effects after using these products, users should immediately seek the nearest healthcare service. Treatment should be individualized, possibly including ocular occlusion and the use of eye drops containing antibiotics or corticosteroids, among other medications.
Not every patient has easy access to an ophthalmologist in an emergency, so it is crucial for general practitioners to be prepared for initial care. In this regard, one of the most important measures is eye washing with copious amounts of clean water or saline solution for 5-10 minutes.
Eye itching is a frequent manifestation of using hair creams, and scratching the area may worsen the condition. Ocular occlusion can protect the cornea until an evaluation can be performed by a specialist.
Although we prefer our patients to stay away from these creams, it is also important to disseminate this information and advise them to read labels and use safe cosmetics.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
In late December 2023, Brazil’s National Health Surveillance Agency (ANVISA) suspended the commercialization of approximately 1200 hair creams because of reports of eye irritation and temporary blindness.
A similar measure encompassing all hair creams sold in the country had already been announced by the agency in March. However, after a few weeks, ANVISA issued a resolution with rules for the products’ commercialization, allowing them back on the shelves.
With the new resolution, the sale of products that do not comply with the standards has once again been suspended. The reason is that reports of adverse events have reemerged. These events include temporary vision loss, headaches, and burning, tearing, itching, redness, and swelling of the eyes. According to reports, these adverse effects occurred mainly in people who used the specific products before swimming in the sea or in pools, or even going out in the rain.
The banned products contain 20% or more ethoxylated alcohols in their formulations. , potentially causing allergies and burns to the eyes and skin. They also have a high pulmonary and neurological toxicity. All these substances are eye irritants and can cause chemical keratitis. In extreme cases, corneal ulcers may develop, leading to vision loss.
The Brazilian Council of Ophthalmology also issued a warning on these products. It emphasized that, in addition to the sales prohibition, consumers should check the labels of hair creams to make sure that these toxic substances are not present in the product formulation.
The ANVISA website contains a list of creams that are considered safe and have not had their commercialization suspended, along with links to adverse event notifications reported by healthcare professionals or consumers.
For consumers who have recently used hair creams, the agency advises careful hair washing, including tilting the head backward to prevent the product from coming into contact with the eye area. If there is accidental eye contact, the eyes should be washed with plenty of water.
If there are any undesired effects after using these products, users should immediately seek the nearest healthcare service. Treatment should be individualized, possibly including ocular occlusion and the use of eye drops containing antibiotics or corticosteroids, among other medications.
Not every patient has easy access to an ophthalmologist in an emergency, so it is crucial for general practitioners to be prepared for initial care. In this regard, one of the most important measures is eye washing with copious amounts of clean water or saline solution for 5-10 minutes.
Eye itching is a frequent manifestation of using hair creams, and scratching the area may worsen the condition. Ocular occlusion can protect the cornea until an evaluation can be performed by a specialist.
Although we prefer our patients to stay away from these creams, it is also important to disseminate this information and advise them to read labels and use safe cosmetics.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
In late December 2023, Brazil’s National Health Surveillance Agency (ANVISA) suspended the commercialization of approximately 1200 hair creams because of reports of eye irritation and temporary blindness.
A similar measure encompassing all hair creams sold in the country had already been announced by the agency in March. However, after a few weeks, ANVISA issued a resolution with rules for the products’ commercialization, allowing them back on the shelves.
With the new resolution, the sale of products that do not comply with the standards has once again been suspended. The reason is that reports of adverse events have reemerged. These events include temporary vision loss, headaches, and burning, tearing, itching, redness, and swelling of the eyes. According to reports, these adverse effects occurred mainly in people who used the specific products before swimming in the sea or in pools, or even going out in the rain.
The banned products contain 20% or more ethoxylated alcohols in their formulations. , potentially causing allergies and burns to the eyes and skin. They also have a high pulmonary and neurological toxicity. All these substances are eye irritants and can cause chemical keratitis. In extreme cases, corneal ulcers may develop, leading to vision loss.
The Brazilian Council of Ophthalmology also issued a warning on these products. It emphasized that, in addition to the sales prohibition, consumers should check the labels of hair creams to make sure that these toxic substances are not present in the product formulation.
The ANVISA website contains a list of creams that are considered safe and have not had their commercialization suspended, along with links to adverse event notifications reported by healthcare professionals or consumers.
For consumers who have recently used hair creams, the agency advises careful hair washing, including tilting the head backward to prevent the product from coming into contact with the eye area. If there is accidental eye contact, the eyes should be washed with plenty of water.
If there are any undesired effects after using these products, users should immediately seek the nearest healthcare service. Treatment should be individualized, possibly including ocular occlusion and the use of eye drops containing antibiotics or corticosteroids, among other medications.
Not every patient has easy access to an ophthalmologist in an emergency, so it is crucial for general practitioners to be prepared for initial care. In this regard, one of the most important measures is eye washing with copious amounts of clean water or saline solution for 5-10 minutes.
Eye itching is a frequent manifestation of using hair creams, and scratching the area may worsen the condition. Ocular occlusion can protect the cornea until an evaluation can be performed by a specialist.
Although we prefer our patients to stay away from these creams, it is also important to disseminate this information and advise them to read labels and use safe cosmetics.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
High Salt Intake Linked to Increased Risk for Kidney Disease
People who habitually add salt to their meals at the table may unknowingly be risking their kidneys, according to a study utilizing UK Biobank data. Chronic salt additions are associated with an elevated risk of developing chronic kidney disease (CKD), as revealed by researchers led by Rui Tang, a doctoral candidate in epidemiology at Tulane University in New Orleans, Louisiana. The study was published in JAMA Network Open.
Large Study Sample
In a population-based cohort study comprising over 460,000 UK Biobank participants aged 37-73 years, the researchers explored the association between adding table salt to food and increased CKD risk.
Participants indicated how often they added salt to their meals: Never or rarely, sometimes, often, or always. The follow-up period exceeded a decade, and median duration was 11.8 years. During this time, approximately 22,000 new CKD cases were documented. Data analysis revealed a significantly higher CKD risk among those who frequently added salt.
The extent of risk elevation varied with the frequency of salt additions. Even occasional salters had a 7% higher risk than those who never or rarely added salt. For frequent salters, the risk increased by 12%, and for those who always added salt, it rose to 29%. These results were adjusted for age and gender.
Worse Overall Health
The research group noted that individuals who frequently added salt were generally less healthy, adopting an unhealthier lifestyle and having lower socioeconomic status. They exhibited higher body mass index (BMI), were more likely to smoke, had diabetes or cardiovascular diseases, and had reduced estimated glomerular filtration rate (eGFR) at the beginning of the study. Moreover, their Townsend Deprivation Index, indicating material deprivation, was higher.
Considering these factors, the researchers adjusted the results not only for age and gender but also for ethnicity, Townsend Deprivation Index, eGFR, BMI, smoking status, alcohol consumption, physical activity, elevated cholesterol levels, diabetes, cardiovascular diseases, hypertension, infectious diseases, immune system disorders, and the use of nephrotoxic medications.
Association Persists
Even after accounting for these factors, a significant, albeit attenuated, association between salt additions and CKD risk remained. The risk increased by 2% for occasional salters, 5% for frequent salters, and 6% for those who always added salt.
The research group concluded that adding salt to meals could be associated with an increased risk for CKD in the general population. However, they highlighted several limitations that should be considered when interpreting the study results.
Reducing Salt
Primarily, self-reported frequency of salt addition doesn’t precisely reflect actual salt consumption. While earlier studies validated the accuracy of this variable, the researchers acknowledged the possibility that frequent salt addition may merely be a marker for an unhealthy lifestyle.
Nevertheless, the authors speculated that reducing the frequency of salt additions to meals could contribute to lowering CKD risk in the general population. They suggested validating their results in post hoc analyses or follow-up studies from clinical trials.
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
People who habitually add salt to their meals at the table may unknowingly be risking their kidneys, according to a study utilizing UK Biobank data. Chronic salt additions are associated with an elevated risk of developing chronic kidney disease (CKD), as revealed by researchers led by Rui Tang, a doctoral candidate in epidemiology at Tulane University in New Orleans, Louisiana. The study was published in JAMA Network Open.
Large Study Sample
In a population-based cohort study comprising over 460,000 UK Biobank participants aged 37-73 years, the researchers explored the association between adding table salt to food and increased CKD risk.
Participants indicated how often they added salt to their meals: Never or rarely, sometimes, often, or always. The follow-up period exceeded a decade, and median duration was 11.8 years. During this time, approximately 22,000 new CKD cases were documented. Data analysis revealed a significantly higher CKD risk among those who frequently added salt.
The extent of risk elevation varied with the frequency of salt additions. Even occasional salters had a 7% higher risk than those who never or rarely added salt. For frequent salters, the risk increased by 12%, and for those who always added salt, it rose to 29%. These results were adjusted for age and gender.
Worse Overall Health
The research group noted that individuals who frequently added salt were generally less healthy, adopting an unhealthier lifestyle and having lower socioeconomic status. They exhibited higher body mass index (BMI), were more likely to smoke, had diabetes or cardiovascular diseases, and had reduced estimated glomerular filtration rate (eGFR) at the beginning of the study. Moreover, their Townsend Deprivation Index, indicating material deprivation, was higher.
Considering these factors, the researchers adjusted the results not only for age and gender but also for ethnicity, Townsend Deprivation Index, eGFR, BMI, smoking status, alcohol consumption, physical activity, elevated cholesterol levels, diabetes, cardiovascular diseases, hypertension, infectious diseases, immune system disorders, and the use of nephrotoxic medications.
Association Persists
Even after accounting for these factors, a significant, albeit attenuated, association between salt additions and CKD risk remained. The risk increased by 2% for occasional salters, 5% for frequent salters, and 6% for those who always added salt.
The research group concluded that adding salt to meals could be associated with an increased risk for CKD in the general population. However, they highlighted several limitations that should be considered when interpreting the study results.
Reducing Salt
Primarily, self-reported frequency of salt addition doesn’t precisely reflect actual salt consumption. While earlier studies validated the accuracy of this variable, the researchers acknowledged the possibility that frequent salt addition may merely be a marker for an unhealthy lifestyle.
Nevertheless, the authors speculated that reducing the frequency of salt additions to meals could contribute to lowering CKD risk in the general population. They suggested validating their results in post hoc analyses or follow-up studies from clinical trials.
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
People who habitually add salt to their meals at the table may unknowingly be risking their kidneys, according to a study utilizing UK Biobank data. Chronic salt additions are associated with an elevated risk of developing chronic kidney disease (CKD), as revealed by researchers led by Rui Tang, a doctoral candidate in epidemiology at Tulane University in New Orleans, Louisiana. The study was published in JAMA Network Open.
Large Study Sample
In a population-based cohort study comprising over 460,000 UK Biobank participants aged 37-73 years, the researchers explored the association between adding table salt to food and increased CKD risk.
Participants indicated how often they added salt to their meals: Never or rarely, sometimes, often, or always. The follow-up period exceeded a decade, and median duration was 11.8 years. During this time, approximately 22,000 new CKD cases were documented. Data analysis revealed a significantly higher CKD risk among those who frequently added salt.
The extent of risk elevation varied with the frequency of salt additions. Even occasional salters had a 7% higher risk than those who never or rarely added salt. For frequent salters, the risk increased by 12%, and for those who always added salt, it rose to 29%. These results were adjusted for age and gender.
Worse Overall Health
The research group noted that individuals who frequently added salt were generally less healthy, adopting an unhealthier lifestyle and having lower socioeconomic status. They exhibited higher body mass index (BMI), were more likely to smoke, had diabetes or cardiovascular diseases, and had reduced estimated glomerular filtration rate (eGFR) at the beginning of the study. Moreover, their Townsend Deprivation Index, indicating material deprivation, was higher.
Considering these factors, the researchers adjusted the results not only for age and gender but also for ethnicity, Townsend Deprivation Index, eGFR, BMI, smoking status, alcohol consumption, physical activity, elevated cholesterol levels, diabetes, cardiovascular diseases, hypertension, infectious diseases, immune system disorders, and the use of nephrotoxic medications.
Association Persists
Even after accounting for these factors, a significant, albeit attenuated, association between salt additions and CKD risk remained. The risk increased by 2% for occasional salters, 5% for frequent salters, and 6% for those who always added salt.
The research group concluded that adding salt to meals could be associated with an increased risk for CKD in the general population. However, they highlighted several limitations that should be considered when interpreting the study results.
Reducing Salt
Primarily, self-reported frequency of salt addition doesn’t precisely reflect actual salt consumption. While earlier studies validated the accuracy of this variable, the researchers acknowledged the possibility that frequent salt addition may merely be a marker for an unhealthy lifestyle.
Nevertheless, the authors speculated that reducing the frequency of salt additions to meals could contribute to lowering CKD risk in the general population. They suggested validating their results in post hoc analyses or follow-up studies from clinical trials.
This article was translated from the Medscape German edition. A version of this article appeared on Medscape.com.
No Compelling Evidence of Pancreatic Cancer Risk With GLP-1s
TOPLINE:
New data provide no support for an increased risk for pancreatic cancer with use of a glucagon-like peptide-1 receptor agonist (GLP-1 RA) for up to 7 years, although longer-term data are needed, researchers said.
METHODOLOGY:
- Some studies have raised concern about a possible increased risk for pancreatitis and pancreatic cancer in patients taking a GLP-1 RA.
- Investigators behind this population-based cohort study assessed the association of GLP-1 RA treatment with pancreatic cancer incidence over a median of 7 years in 543,595 adults (mean age, 59.9 years; 51% women) with type 2 diabetes.
- Treatment with basal insulin was used as an active comparator.
- The analyses accounted for major confounding factors and time-related biases and adjusted for treatment with other glucose-lowering medications and a history of pancreatitis.
TAKEAWAY:
- During the study period, 33,377 patients (6.1%) used GLP-1 RAs and 106,849 (19.7%) used basal insulin, with 1665 of all patients diagnosed with pancreatic cancer.
- There was no evidence that GLP-1 RA use increased pancreatic cancer risk compared with basal insulin.
- The estimated hazard ratio (HR) for pancreatic cancer associated with incremental use of one defined daily dose per day of GLP-1 RA compared with basal insulin in years 5-7 was 0.50 (95% CI, 0.15-1.71).
- New-user and prevalent new-user analyses showed HRs from year 5 onward following initiation of a GLP-1 RA vs basal insulin was 0.52 (95% CI, 0.19-1.41) and 0.75 (95% CI, 0.37-1.53), respectively.
IN PRACTICE:
Using several analytical approaches, these findings do not suggest an increase in pancreatic cancer incidence over 7 years following the start of GLP-1 RA treatment, according to the investigation. “However, monitoring for pancreatic cancer risk beyond 7 years following initiation of treatment is still required,” the authors wrote.
SOURCE:
The study, with first author Rachel Dankner, MD, MPH, Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Israel, was published online on January 4, 2024, in JAMA Network Open.
LIMITATIONS:
Data on the exact type of GLP-1 RA were not available. The analyses accounted for history of pancreatitis but not alcohol use or exposure to pesticides/chemicals. Because of the risk for bias due to reverse causation, an emphasis was placed on drug effects several years after their initiation. However, this reduced the number of pancreatic cancer cases available and led to estimated HRs with wider CIs.
DISCLOSURES:
The study received no specific funding. The authors disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
New data provide no support for an increased risk for pancreatic cancer with use of a glucagon-like peptide-1 receptor agonist (GLP-1 RA) for up to 7 years, although longer-term data are needed, researchers said.
METHODOLOGY:
- Some studies have raised concern about a possible increased risk for pancreatitis and pancreatic cancer in patients taking a GLP-1 RA.
- Investigators behind this population-based cohort study assessed the association of GLP-1 RA treatment with pancreatic cancer incidence over a median of 7 years in 543,595 adults (mean age, 59.9 years; 51% women) with type 2 diabetes.
- Treatment with basal insulin was used as an active comparator.
- The analyses accounted for major confounding factors and time-related biases and adjusted for treatment with other glucose-lowering medications and a history of pancreatitis.
TAKEAWAY:
- During the study period, 33,377 patients (6.1%) used GLP-1 RAs and 106,849 (19.7%) used basal insulin, with 1665 of all patients diagnosed with pancreatic cancer.
- There was no evidence that GLP-1 RA use increased pancreatic cancer risk compared with basal insulin.
- The estimated hazard ratio (HR) for pancreatic cancer associated with incremental use of one defined daily dose per day of GLP-1 RA compared with basal insulin in years 5-7 was 0.50 (95% CI, 0.15-1.71).
- New-user and prevalent new-user analyses showed HRs from year 5 onward following initiation of a GLP-1 RA vs basal insulin was 0.52 (95% CI, 0.19-1.41) and 0.75 (95% CI, 0.37-1.53), respectively.
IN PRACTICE:
Using several analytical approaches, these findings do not suggest an increase in pancreatic cancer incidence over 7 years following the start of GLP-1 RA treatment, according to the investigation. “However, monitoring for pancreatic cancer risk beyond 7 years following initiation of treatment is still required,” the authors wrote.
SOURCE:
The study, with first author Rachel Dankner, MD, MPH, Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Israel, was published online on January 4, 2024, in JAMA Network Open.
LIMITATIONS:
Data on the exact type of GLP-1 RA were not available. The analyses accounted for history of pancreatitis but not alcohol use or exposure to pesticides/chemicals. Because of the risk for bias due to reverse causation, an emphasis was placed on drug effects several years after their initiation. However, this reduced the number of pancreatic cancer cases available and led to estimated HRs with wider CIs.
DISCLOSURES:
The study received no specific funding. The authors disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
New data provide no support for an increased risk for pancreatic cancer with use of a glucagon-like peptide-1 receptor agonist (GLP-1 RA) for up to 7 years, although longer-term data are needed, researchers said.
METHODOLOGY:
- Some studies have raised concern about a possible increased risk for pancreatitis and pancreatic cancer in patients taking a GLP-1 RA.
- Investigators behind this population-based cohort study assessed the association of GLP-1 RA treatment with pancreatic cancer incidence over a median of 7 years in 543,595 adults (mean age, 59.9 years; 51% women) with type 2 diabetes.
- Treatment with basal insulin was used as an active comparator.
- The analyses accounted for major confounding factors and time-related biases and adjusted for treatment with other glucose-lowering medications and a history of pancreatitis.
TAKEAWAY:
- During the study period, 33,377 patients (6.1%) used GLP-1 RAs and 106,849 (19.7%) used basal insulin, with 1665 of all patients diagnosed with pancreatic cancer.
- There was no evidence that GLP-1 RA use increased pancreatic cancer risk compared with basal insulin.
- The estimated hazard ratio (HR) for pancreatic cancer associated with incremental use of one defined daily dose per day of GLP-1 RA compared with basal insulin in years 5-7 was 0.50 (95% CI, 0.15-1.71).
- New-user and prevalent new-user analyses showed HRs from year 5 onward following initiation of a GLP-1 RA vs basal insulin was 0.52 (95% CI, 0.19-1.41) and 0.75 (95% CI, 0.37-1.53), respectively.
IN PRACTICE:
Using several analytical approaches, these findings do not suggest an increase in pancreatic cancer incidence over 7 years following the start of GLP-1 RA treatment, according to the investigation. “However, monitoring for pancreatic cancer risk beyond 7 years following initiation of treatment is still required,” the authors wrote.
SOURCE:
The study, with first author Rachel Dankner, MD, MPH, Gertner Institute for Epidemiology and Health Policy Research, Sheba Medical Center, Israel, was published online on January 4, 2024, in JAMA Network Open.
LIMITATIONS:
Data on the exact type of GLP-1 RA were not available. The analyses accounted for history of pancreatitis but not alcohol use or exposure to pesticides/chemicals. Because of the risk for bias due to reverse causation, an emphasis was placed on drug effects several years after their initiation. However, this reduced the number of pancreatic cancer cases available and led to estimated HRs with wider CIs.
DISCLOSURES:
The study received no specific funding. The authors disclosed no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
High and Low Body Mass Indices Promote Respiratory Symptoms
TOPLINE:
Individuals with either high or low body mass index (BMI) showed an increased risk for respiratory symptoms and diseases than those with BMI in the normal range.
METHODOLOGY:
- The researchers reviewed data from the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2012; the study population included 12,719 adults older than 40 years with data on respiratory symptoms; 51% were female, and 53.3% were non-Hispanic White individuals.
- The study population was divided into quartiles based on BMI as follows: 3180 individuals with BMI of 13.2-24.9 kg/m2, 3175 with BMI of 24.9-28.4 kg/m2, 3180 with BMI of 28.4-32.5 kg/m2, and 3184 with BMI of 32.5-82.0 kg/m2.
- The study sought to assess the correlation between BMI and respiratory symptoms (cough, wheezing, and dyspnea), chronic obstructive pulmonary disease (COPD), and asthma in unadjusted and adjusted models based on sex, race, marital status, poverty-income ratio (PIR), education level, and smoking status.
TAKEAWAY:
- In a logistic regression and curve fitting analysis, BMI showed a U-shaped relationship with respiratory symptoms, asthma, and COPD, with increased risk in individuals with high or low BMI than those with BMIs in the middle quartiles.
- In a stratified analysis by race, the risk for cough was significantly higher among non-Hispanic Black individuals than other races (P < .0001), and a higher BMI was associated with an increased risk for COPD in non-Hispanic Black individuals (odds ratio, 1.053; P < .0001).
- The researchers found no significant impact of biological sex on the relationship between BMI and respiratory symptoms, COPD, or asthma.
- The results support previous studies showing that a BMI that is too low can be detrimental to health.
IN PRACTICE:
“These results suggest that the risk of small airway obstruction in underweight individuals deserves more attention and that excessive wasting may also affect the prognosis of patients with COPD,” the researchers wrote.
SOURCE:
The lead author on the study was Yuefeng Sun of Shandong University of Traditional Chinese Medicine, Jinan, China. The study was published online on January 10, 2024, in Scientific Reports.
LIMITATIONS:
The cross-sectional NHANES database prevented conclusions of causality, and potential confounding factors that were not accounted for could have affected the results.
DISCLOSURES:
The study was supported by the Shandong Province Taishan Scholar Project. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals with either high or low body mass index (BMI) showed an increased risk for respiratory symptoms and diseases than those with BMI in the normal range.
METHODOLOGY:
- The researchers reviewed data from the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2012; the study population included 12,719 adults older than 40 years with data on respiratory symptoms; 51% were female, and 53.3% were non-Hispanic White individuals.
- The study population was divided into quartiles based on BMI as follows: 3180 individuals with BMI of 13.2-24.9 kg/m2, 3175 with BMI of 24.9-28.4 kg/m2, 3180 with BMI of 28.4-32.5 kg/m2, and 3184 with BMI of 32.5-82.0 kg/m2.
- The study sought to assess the correlation between BMI and respiratory symptoms (cough, wheezing, and dyspnea), chronic obstructive pulmonary disease (COPD), and asthma in unadjusted and adjusted models based on sex, race, marital status, poverty-income ratio (PIR), education level, and smoking status.
TAKEAWAY:
- In a logistic regression and curve fitting analysis, BMI showed a U-shaped relationship with respiratory symptoms, asthma, and COPD, with increased risk in individuals with high or low BMI than those with BMIs in the middle quartiles.
- In a stratified analysis by race, the risk for cough was significantly higher among non-Hispanic Black individuals than other races (P < .0001), and a higher BMI was associated with an increased risk for COPD in non-Hispanic Black individuals (odds ratio, 1.053; P < .0001).
- The researchers found no significant impact of biological sex on the relationship between BMI and respiratory symptoms, COPD, or asthma.
- The results support previous studies showing that a BMI that is too low can be detrimental to health.
IN PRACTICE:
“These results suggest that the risk of small airway obstruction in underweight individuals deserves more attention and that excessive wasting may also affect the prognosis of patients with COPD,” the researchers wrote.
SOURCE:
The lead author on the study was Yuefeng Sun of Shandong University of Traditional Chinese Medicine, Jinan, China. The study was published online on January 10, 2024, in Scientific Reports.
LIMITATIONS:
The cross-sectional NHANES database prevented conclusions of causality, and potential confounding factors that were not accounted for could have affected the results.
DISCLOSURES:
The study was supported by the Shandong Province Taishan Scholar Project. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals with either high or low body mass index (BMI) showed an increased risk for respiratory symptoms and diseases than those with BMI in the normal range.
METHODOLOGY:
- The researchers reviewed data from the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2012; the study population included 12,719 adults older than 40 years with data on respiratory symptoms; 51% were female, and 53.3% were non-Hispanic White individuals.
- The study population was divided into quartiles based on BMI as follows: 3180 individuals with BMI of 13.2-24.9 kg/m2, 3175 with BMI of 24.9-28.4 kg/m2, 3180 with BMI of 28.4-32.5 kg/m2, and 3184 with BMI of 32.5-82.0 kg/m2.
- The study sought to assess the correlation between BMI and respiratory symptoms (cough, wheezing, and dyspnea), chronic obstructive pulmonary disease (COPD), and asthma in unadjusted and adjusted models based on sex, race, marital status, poverty-income ratio (PIR), education level, and smoking status.
TAKEAWAY:
- In a logistic regression and curve fitting analysis, BMI showed a U-shaped relationship with respiratory symptoms, asthma, and COPD, with increased risk in individuals with high or low BMI than those with BMIs in the middle quartiles.
- In a stratified analysis by race, the risk for cough was significantly higher among non-Hispanic Black individuals than other races (P < .0001), and a higher BMI was associated with an increased risk for COPD in non-Hispanic Black individuals (odds ratio, 1.053; P < .0001).
- The researchers found no significant impact of biological sex on the relationship between BMI and respiratory symptoms, COPD, or asthma.
- The results support previous studies showing that a BMI that is too low can be detrimental to health.
IN PRACTICE:
“These results suggest that the risk of small airway obstruction in underweight individuals deserves more attention and that excessive wasting may also affect the prognosis of patients with COPD,” the researchers wrote.
SOURCE:
The lead author on the study was Yuefeng Sun of Shandong University of Traditional Chinese Medicine, Jinan, China. The study was published online on January 10, 2024, in Scientific Reports.
LIMITATIONS:
The cross-sectional NHANES database prevented conclusions of causality, and potential confounding factors that were not accounted for could have affected the results.
DISCLOSURES:
The study was supported by the Shandong Province Taishan Scholar Project. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.