User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
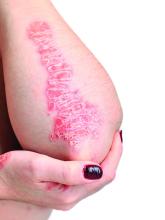
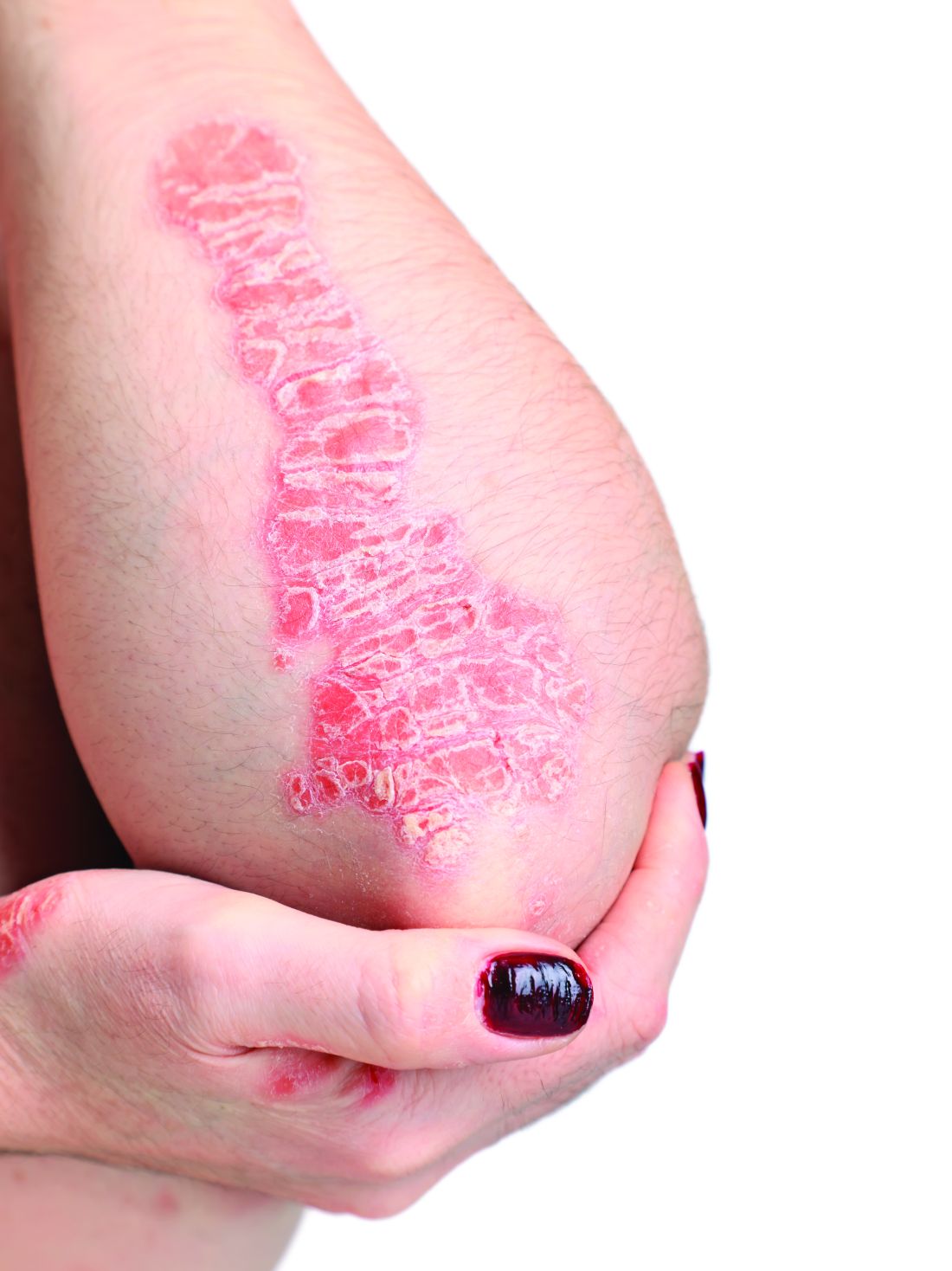
Elevated PCSK9 levels associated with psoriasis suggest new treatment target
A Mendelian randomization study employing data from nearly 300,000 individuals has linked elevated levels of the PCSK9 enzyme with an increased risk of psoriasis, suggesting it might be targetable as an intervention.
. Conversely, psoriasis risk did not appear to be affected when LDL-C was reduced by other pathways of lipid control.
This study “suggests that PCSK9 inhibition is causally associated with reduced risk of psoriasis,” reported a team of investigators led by Sizheng Steven Zhao, MD, PhD, of the division of musculoskeletal and dermatological sciences, University of Manchester (England). “Existing PCSK9 inhibitors hold potential as therapeutic targets for prevention, and possibly treatment, of psoriasis, although further clinical studies are needed,” they concluded.
In an interview, Dr. Zhao also noted that it will be interesting to look at psoriasis susceptibility in post hoc analyses of large randomized controlled trials of PCSK9 inhibitors for cardiovascular disease.
“Genetically proxied” inhibition of HMG-CoA reductase, which is targeted by statins, and NPC1L1 which is targeted by ezetimibe, “were not associated with psoriasis risk,” the investigators reported in the study, published in JAMA Dermatology.
Abnormal lipid metabolism is sufficiently common among people with psoriasis that screening in patients with moderate to severe disease is recommended in 2019 psoriasis guidelines from the American Academy of Dermatology and the National Psoriasis Foundation. However, the link between these diseases is unclear. This study was launched to explore genetically proxied relationships between psoriasis and LDL-C reductions as well as specific treatments for elevated LDL-C.
Mendelian randomizations were applied to deidentified data from two sources, a UK biobank and FinnGen, a Finnish-based project for identifying genotype-to-phenotype correlations. Genetic proxies for these variables were established on the basis of genomewide association studies on large population samples.
Ultimately, 34 genetic variants were selected to proxy for lipid lowering by PCSK9, 19 were selected to proxy for HMG-CoA reductase, and 9 for NPC1L1. In the Mendelian analyses performed on the two sources, genetically proxied PCSK9 inhibition was associated with about a 30% reduction in the odds ratio of psoriasis (OR, 0.69; P = .003). There were no robust associations with proxies for reductions in either HMG-CoA reductase or NPC1L1.
In sensitivity analyses, there was no evidence of bias from pleiotropy or genetic confounding, according to Dr. Zhao and his coauthors, who noted that the relationship between reductions in PCSK9 and reduced risk of psoriasis appeared to be independent of change in circulating LDL-C.
Given the prior evidence implicating the PCSK9 enzyme in psoriasis risk, “this is an exciting study that really highlights the importance of studying and targeting lipid metabolism in psoriasis for a few reasons,” according to Michael S. Garshick, MD, a researcher, cardiologist, and director of the cardio-rheumatology program, New York University Langone Health.
An investigator who has participated in several studies evaluating the relationship between cardiovascular risk and psoriasis, Dr. Garshick said there is increasing interest in PCSK9 as a biomarker or even a mediator of inflammation independent of blood lipid levels.
“In psoriasis regarding PCSK9, we and others have shown PCSK9 is elevated in psoriatic lesion skin, and studies are starting to investigate the unique lipidomic profile in psoriasis,” Dr. Garshick said in an interview. The study he led that showed elevated PCSK9 levels in psoriatic skin was published in 2021 in the Journal of Investigative Dermatology.
While the Mendelian randomization provides only “an inference” that PCSK9 plays a role in mediating risk of psoriasis, Dr. Zhao and coauthors cited numerous studies linking elevated PCSK9 to psoriasis pathophysiology. This not only includes the elevated PCSK9 expression in psoriatic plaques as shown by Dr. Garshick and others but several sets of experimental evidence linking PCSK9 to inflammatory pathways, including upregulation of interleukin-17 and stimulation of macrophage activation.
While Dr. Zhao and coauthors suggested that clinical trials are now needed to test the potential of PCSK9 inhibitors to modify the risk of psoriasis, Dr. Garshick indicated that there are numerous variables to unravel in the relationship between elevated lipids, PCSK9, and psoriasis.
“In our own studies, we did see a statistical correlation between circulating PCSK9 and psoriasis severity,” Dr. Garshick said. But he added, “I think we are just beginning to understand the functions of circulating (extrahepatic) PCSK9 independent of lipid metabolism.”
While he is intrigued by the evidence that PCSK9 is linked to systemic inflammation, he pointed out that several medications used to treat dyslipidemias, such as statins, are associated with an anti-inflammatory effect.
This study “further emphasizes the need to conduct clinical trials treating dyslipidemia in psoriasis, including the targeting of PCSK9, whether it is with statins with lipid lowering and potential pleiotropic anti-inflammatory properties or PCSK9 inhibition,” he said. If positive, “both would be exciting.“
From a cardiologist’s point of view, there is an upside for including patients with psoriasis in lipid-lowering trials even if the effect on psoriasis is modest. Either way, “you still get the lipid-lowering benefit, which is important for reducing atherosclerotic cardiovascular disease,” Dr. Garshick said.
Dr. Zhao reported financial relationships with UCB, although UCB did not provide funding for this study. One author reported grants from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre during the study, grants from Bristol Myers Squibb, Galapagos, and Pfizer, and personal fees from Chugai Roche outside the submitted work. No other disclosures were reported. The study was supported by grants from Versus Arthritis and the NIHR Manchester Biomedical Research Centre. Dr. Garshick reported financial relationships with AbbVie and Horizon Therapeutics.
A Mendelian randomization study employing data from nearly 300,000 individuals has linked elevated levels of the PCSK9 enzyme with an increased risk of psoriasis, suggesting it might be targetable as an intervention.
. Conversely, psoriasis risk did not appear to be affected when LDL-C was reduced by other pathways of lipid control.
This study “suggests that PCSK9 inhibition is causally associated with reduced risk of psoriasis,” reported a team of investigators led by Sizheng Steven Zhao, MD, PhD, of the division of musculoskeletal and dermatological sciences, University of Manchester (England). “Existing PCSK9 inhibitors hold potential as therapeutic targets for prevention, and possibly treatment, of psoriasis, although further clinical studies are needed,” they concluded.
In an interview, Dr. Zhao also noted that it will be interesting to look at psoriasis susceptibility in post hoc analyses of large randomized controlled trials of PCSK9 inhibitors for cardiovascular disease.
“Genetically proxied” inhibition of HMG-CoA reductase, which is targeted by statins, and NPC1L1 which is targeted by ezetimibe, “were not associated with psoriasis risk,” the investigators reported in the study, published in JAMA Dermatology.
Abnormal lipid metabolism is sufficiently common among people with psoriasis that screening in patients with moderate to severe disease is recommended in 2019 psoriasis guidelines from the American Academy of Dermatology and the National Psoriasis Foundation. However, the link between these diseases is unclear. This study was launched to explore genetically proxied relationships between psoriasis and LDL-C reductions as well as specific treatments for elevated LDL-C.
Mendelian randomizations were applied to deidentified data from two sources, a UK biobank and FinnGen, a Finnish-based project for identifying genotype-to-phenotype correlations. Genetic proxies for these variables were established on the basis of genomewide association studies on large population samples.
Ultimately, 34 genetic variants were selected to proxy for lipid lowering by PCSK9, 19 were selected to proxy for HMG-CoA reductase, and 9 for NPC1L1. In the Mendelian analyses performed on the two sources, genetically proxied PCSK9 inhibition was associated with about a 30% reduction in the odds ratio of psoriasis (OR, 0.69; P = .003). There were no robust associations with proxies for reductions in either HMG-CoA reductase or NPC1L1.
In sensitivity analyses, there was no evidence of bias from pleiotropy or genetic confounding, according to Dr. Zhao and his coauthors, who noted that the relationship between reductions in PCSK9 and reduced risk of psoriasis appeared to be independent of change in circulating LDL-C.
Given the prior evidence implicating the PCSK9 enzyme in psoriasis risk, “this is an exciting study that really highlights the importance of studying and targeting lipid metabolism in psoriasis for a few reasons,” according to Michael S. Garshick, MD, a researcher, cardiologist, and director of the cardio-rheumatology program, New York University Langone Health.
An investigator who has participated in several studies evaluating the relationship between cardiovascular risk and psoriasis, Dr. Garshick said there is increasing interest in PCSK9 as a biomarker or even a mediator of inflammation independent of blood lipid levels.
“In psoriasis regarding PCSK9, we and others have shown PCSK9 is elevated in psoriatic lesion skin, and studies are starting to investigate the unique lipidomic profile in psoriasis,” Dr. Garshick said in an interview. The study he led that showed elevated PCSK9 levels in psoriatic skin was published in 2021 in the Journal of Investigative Dermatology.
While the Mendelian randomization provides only “an inference” that PCSK9 plays a role in mediating risk of psoriasis, Dr. Zhao and coauthors cited numerous studies linking elevated PCSK9 to psoriasis pathophysiology. This not only includes the elevated PCSK9 expression in psoriatic plaques as shown by Dr. Garshick and others but several sets of experimental evidence linking PCSK9 to inflammatory pathways, including upregulation of interleukin-17 and stimulation of macrophage activation.
While Dr. Zhao and coauthors suggested that clinical trials are now needed to test the potential of PCSK9 inhibitors to modify the risk of psoriasis, Dr. Garshick indicated that there are numerous variables to unravel in the relationship between elevated lipids, PCSK9, and psoriasis.
“In our own studies, we did see a statistical correlation between circulating PCSK9 and psoriasis severity,” Dr. Garshick said. But he added, “I think we are just beginning to understand the functions of circulating (extrahepatic) PCSK9 independent of lipid metabolism.”
While he is intrigued by the evidence that PCSK9 is linked to systemic inflammation, he pointed out that several medications used to treat dyslipidemias, such as statins, are associated with an anti-inflammatory effect.
This study “further emphasizes the need to conduct clinical trials treating dyslipidemia in psoriasis, including the targeting of PCSK9, whether it is with statins with lipid lowering and potential pleiotropic anti-inflammatory properties or PCSK9 inhibition,” he said. If positive, “both would be exciting.“
From a cardiologist’s point of view, there is an upside for including patients with psoriasis in lipid-lowering trials even if the effect on psoriasis is modest. Either way, “you still get the lipid-lowering benefit, which is important for reducing atherosclerotic cardiovascular disease,” Dr. Garshick said.
Dr. Zhao reported financial relationships with UCB, although UCB did not provide funding for this study. One author reported grants from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre during the study, grants from Bristol Myers Squibb, Galapagos, and Pfizer, and personal fees from Chugai Roche outside the submitted work. No other disclosures were reported. The study was supported by grants from Versus Arthritis and the NIHR Manchester Biomedical Research Centre. Dr. Garshick reported financial relationships with AbbVie and Horizon Therapeutics.
A Mendelian randomization study employing data from nearly 300,000 individuals has linked elevated levels of the PCSK9 enzyme with an increased risk of psoriasis, suggesting it might be targetable as an intervention.
. Conversely, psoriasis risk did not appear to be affected when LDL-C was reduced by other pathways of lipid control.
This study “suggests that PCSK9 inhibition is causally associated with reduced risk of psoriasis,” reported a team of investigators led by Sizheng Steven Zhao, MD, PhD, of the division of musculoskeletal and dermatological sciences, University of Manchester (England). “Existing PCSK9 inhibitors hold potential as therapeutic targets for prevention, and possibly treatment, of psoriasis, although further clinical studies are needed,” they concluded.
In an interview, Dr. Zhao also noted that it will be interesting to look at psoriasis susceptibility in post hoc analyses of large randomized controlled trials of PCSK9 inhibitors for cardiovascular disease.
“Genetically proxied” inhibition of HMG-CoA reductase, which is targeted by statins, and NPC1L1 which is targeted by ezetimibe, “were not associated with psoriasis risk,” the investigators reported in the study, published in JAMA Dermatology.
Abnormal lipid metabolism is sufficiently common among people with psoriasis that screening in patients with moderate to severe disease is recommended in 2019 psoriasis guidelines from the American Academy of Dermatology and the National Psoriasis Foundation. However, the link between these diseases is unclear. This study was launched to explore genetically proxied relationships between psoriasis and LDL-C reductions as well as specific treatments for elevated LDL-C.
Mendelian randomizations were applied to deidentified data from two sources, a UK biobank and FinnGen, a Finnish-based project for identifying genotype-to-phenotype correlations. Genetic proxies for these variables were established on the basis of genomewide association studies on large population samples.
Ultimately, 34 genetic variants were selected to proxy for lipid lowering by PCSK9, 19 were selected to proxy for HMG-CoA reductase, and 9 for NPC1L1. In the Mendelian analyses performed on the two sources, genetically proxied PCSK9 inhibition was associated with about a 30% reduction in the odds ratio of psoriasis (OR, 0.69; P = .003). There were no robust associations with proxies for reductions in either HMG-CoA reductase or NPC1L1.
In sensitivity analyses, there was no evidence of bias from pleiotropy or genetic confounding, according to Dr. Zhao and his coauthors, who noted that the relationship between reductions in PCSK9 and reduced risk of psoriasis appeared to be independent of change in circulating LDL-C.
Given the prior evidence implicating the PCSK9 enzyme in psoriasis risk, “this is an exciting study that really highlights the importance of studying and targeting lipid metabolism in psoriasis for a few reasons,” according to Michael S. Garshick, MD, a researcher, cardiologist, and director of the cardio-rheumatology program, New York University Langone Health.
An investigator who has participated in several studies evaluating the relationship between cardiovascular risk and psoriasis, Dr. Garshick said there is increasing interest in PCSK9 as a biomarker or even a mediator of inflammation independent of blood lipid levels.
“In psoriasis regarding PCSK9, we and others have shown PCSK9 is elevated in psoriatic lesion skin, and studies are starting to investigate the unique lipidomic profile in psoriasis,” Dr. Garshick said in an interview. The study he led that showed elevated PCSK9 levels in psoriatic skin was published in 2021 in the Journal of Investigative Dermatology.
While the Mendelian randomization provides only “an inference” that PCSK9 plays a role in mediating risk of psoriasis, Dr. Zhao and coauthors cited numerous studies linking elevated PCSK9 to psoriasis pathophysiology. This not only includes the elevated PCSK9 expression in psoriatic plaques as shown by Dr. Garshick and others but several sets of experimental evidence linking PCSK9 to inflammatory pathways, including upregulation of interleukin-17 and stimulation of macrophage activation.
While Dr. Zhao and coauthors suggested that clinical trials are now needed to test the potential of PCSK9 inhibitors to modify the risk of psoriasis, Dr. Garshick indicated that there are numerous variables to unravel in the relationship between elevated lipids, PCSK9, and psoriasis.
“In our own studies, we did see a statistical correlation between circulating PCSK9 and psoriasis severity,” Dr. Garshick said. But he added, “I think we are just beginning to understand the functions of circulating (extrahepatic) PCSK9 independent of lipid metabolism.”
While he is intrigued by the evidence that PCSK9 is linked to systemic inflammation, he pointed out that several medications used to treat dyslipidemias, such as statins, are associated with an anti-inflammatory effect.
This study “further emphasizes the need to conduct clinical trials treating dyslipidemia in psoriasis, including the targeting of PCSK9, whether it is with statins with lipid lowering and potential pleiotropic anti-inflammatory properties or PCSK9 inhibition,” he said. If positive, “both would be exciting.“
From a cardiologist’s point of view, there is an upside for including patients with psoriasis in lipid-lowering trials even if the effect on psoriasis is modest. Either way, “you still get the lipid-lowering benefit, which is important for reducing atherosclerotic cardiovascular disease,” Dr. Garshick said.
Dr. Zhao reported financial relationships with UCB, although UCB did not provide funding for this study. One author reported grants from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre during the study, grants from Bristol Myers Squibb, Galapagos, and Pfizer, and personal fees from Chugai Roche outside the submitted work. No other disclosures were reported. The study was supported by grants from Versus Arthritis and the NIHR Manchester Biomedical Research Centre. Dr. Garshick reported financial relationships with AbbVie and Horizon Therapeutics.
FROM JAMA DERMATOLOGY
Dermatologists address cultural competence and unconscious biases in the specialty
ORLANDO – When he was applying for residency, Omar N. Qutub, MD, eagerly arrived at his first interview of the day. But he was quickly thrown off his game.
The interviewer, he said, spent a surprising amount of time asking about his ethnicity and his last name. “I think I spent about 3-5 minutes in the first interview talking about my last name,” said Dr. Qutub, who practices in Portland, Ore., during a session titled “unconscious bias and microaggressions in dermatology” at the ODAC Dermatology, Aesthetic and Surgical Conference. “I really would have rather talked about my research interests.” The interaction threw him off for the rest of the interview process, he said.
The experience is an example of how the field has a ways to go in acquiring cultural competence and in overcoming unconscious biases, said Dr. Qutub. In 2020, a review in Clinics in Dermatology referred to a report that dermatology was the second-least diverse medical specialty, only behind orthopedic surgery, because of its low numbers of residents and faculty from groups underrepresented in medicine.
“We really need to put cultural competency at the forefront in order to do better for our patients,” he said.
Adam Friedman, MD, professor and chair of dermatology and director of the residency program at George Washington University, Washington, who also spoke during the session, said that the process of diversifying the field has to go deeper than the resident interviewing process. “If we just focus on trying to increase the diversity of our applicant pool for residents, it’s too late.”
Nada Elbuluk, MD, associate professor of dermatology at the University of Southern California, Los Angeles, pointed to USC’s Derm RISES initiative, a service program that aims to reach inner-city students through education in the sciences, starting from kindergarten to 12th grade. The program also includes premed undergraduate and medical students, “with the goal of increasing exposure to the sciences, medicine, and dermatology,” according to the USC website. “It’s crucial to begin the process early to get a high yield of students who reach medical school and eventually dermatology, she said, because of the inevitable attrition at each level of the education process.
“It’s incredibly rewarding,” added Dr. Elbuluk, who is also director of the dermatology diversity and inclusion program at USC. “And we get these thank-you letters back from students who [say], ‘I didn’t know I could be a doctor.’ ”
In another presentation, Kavita Mariwalla, MD, who practices in West Islip, N.Y., provided tips on boosting cultural competence during aesthetic consults.
One was to “know your fillers,” she said, noting that fillers have different effects on different skin tones, because of differences in fibroblast content, and fat cells will interact with fillers in different ways across skin tones.
Another is to “understand the shortfall of facial canons,” the idea that you can divide a face into sections that can be viewed and enhanced discretely. This concept was based on a White European model and has to be expanded when considering other ethnicities, Dr. Mariwalla said.
Overgeneralizing categories is another pitfall, she said. “Asian” is a term that covers countries from India to Japan, but within that category are a multitude of notions and nuances about aesthetics, and dermatologists have to be sensitive to all of them.
When meeting with a patient, Dr. Mariwalla said, asking the typical “Where are you from?” is not a helpful question. Instead, she suggested asking: “What is your cultural background? Can you tell me more about what your expectations are?”
“I ask for pictures,” she said. “I want to know what they looked like as a kid. I want to know what their family looks like. And I always hand patients a mirror. Patients will say to me: ‘I want to do what you think.’ It’s not about what I think, because what I see, and what you see in your magnifying mirror, are totally different things.”
After the session ended, a member of the audience, Sharon Stokes, MD, a dermatologist in the Orlando area, provided her view of the presentations, noting that it was an important discussion.
“I think it’s past time in medicine for cultural diversity training and awareness for physicians to understand their patients better and getting to know them – and how to even approach the patient and not to offensively and microaggressively approach the patient,” she said.
Dr. Elbuluk reported relevant relationships with Avita, Incyte, Beiersdorf, and other companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis and other companies. Dr. Mariwalla reported relevant financial relationships with Abbvie, Sanofi, Regeneron and other companies. Dr. Qutub reported no relevant financial relationships. Dr. Qutub is the ODAC director of equity, diversity, and inclusion.
ORLANDO – When he was applying for residency, Omar N. Qutub, MD, eagerly arrived at his first interview of the day. But he was quickly thrown off his game.
The interviewer, he said, spent a surprising amount of time asking about his ethnicity and his last name. “I think I spent about 3-5 minutes in the first interview talking about my last name,” said Dr. Qutub, who practices in Portland, Ore., during a session titled “unconscious bias and microaggressions in dermatology” at the ODAC Dermatology, Aesthetic and Surgical Conference. “I really would have rather talked about my research interests.” The interaction threw him off for the rest of the interview process, he said.
The experience is an example of how the field has a ways to go in acquiring cultural competence and in overcoming unconscious biases, said Dr. Qutub. In 2020, a review in Clinics in Dermatology referred to a report that dermatology was the second-least diverse medical specialty, only behind orthopedic surgery, because of its low numbers of residents and faculty from groups underrepresented in medicine.
“We really need to put cultural competency at the forefront in order to do better for our patients,” he said.
Adam Friedman, MD, professor and chair of dermatology and director of the residency program at George Washington University, Washington, who also spoke during the session, said that the process of diversifying the field has to go deeper than the resident interviewing process. “If we just focus on trying to increase the diversity of our applicant pool for residents, it’s too late.”
Nada Elbuluk, MD, associate professor of dermatology at the University of Southern California, Los Angeles, pointed to USC’s Derm RISES initiative, a service program that aims to reach inner-city students through education in the sciences, starting from kindergarten to 12th grade. The program also includes premed undergraduate and medical students, “with the goal of increasing exposure to the sciences, medicine, and dermatology,” according to the USC website. “It’s crucial to begin the process early to get a high yield of students who reach medical school and eventually dermatology, she said, because of the inevitable attrition at each level of the education process.
“It’s incredibly rewarding,” added Dr. Elbuluk, who is also director of the dermatology diversity and inclusion program at USC. “And we get these thank-you letters back from students who [say], ‘I didn’t know I could be a doctor.’ ”
In another presentation, Kavita Mariwalla, MD, who practices in West Islip, N.Y., provided tips on boosting cultural competence during aesthetic consults.
One was to “know your fillers,” she said, noting that fillers have different effects on different skin tones, because of differences in fibroblast content, and fat cells will interact with fillers in different ways across skin tones.
Another is to “understand the shortfall of facial canons,” the idea that you can divide a face into sections that can be viewed and enhanced discretely. This concept was based on a White European model and has to be expanded when considering other ethnicities, Dr. Mariwalla said.
Overgeneralizing categories is another pitfall, she said. “Asian” is a term that covers countries from India to Japan, but within that category are a multitude of notions and nuances about aesthetics, and dermatologists have to be sensitive to all of them.
When meeting with a patient, Dr. Mariwalla said, asking the typical “Where are you from?” is not a helpful question. Instead, she suggested asking: “What is your cultural background? Can you tell me more about what your expectations are?”
“I ask for pictures,” she said. “I want to know what they looked like as a kid. I want to know what their family looks like. And I always hand patients a mirror. Patients will say to me: ‘I want to do what you think.’ It’s not about what I think, because what I see, and what you see in your magnifying mirror, are totally different things.”
After the session ended, a member of the audience, Sharon Stokes, MD, a dermatologist in the Orlando area, provided her view of the presentations, noting that it was an important discussion.
“I think it’s past time in medicine for cultural diversity training and awareness for physicians to understand their patients better and getting to know them – and how to even approach the patient and not to offensively and microaggressively approach the patient,” she said.
Dr. Elbuluk reported relevant relationships with Avita, Incyte, Beiersdorf, and other companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis and other companies. Dr. Mariwalla reported relevant financial relationships with Abbvie, Sanofi, Regeneron and other companies. Dr. Qutub reported no relevant financial relationships. Dr. Qutub is the ODAC director of equity, diversity, and inclusion.
ORLANDO – When he was applying for residency, Omar N. Qutub, MD, eagerly arrived at his first interview of the day. But he was quickly thrown off his game.
The interviewer, he said, spent a surprising amount of time asking about his ethnicity and his last name. “I think I spent about 3-5 minutes in the first interview talking about my last name,” said Dr. Qutub, who practices in Portland, Ore., during a session titled “unconscious bias and microaggressions in dermatology” at the ODAC Dermatology, Aesthetic and Surgical Conference. “I really would have rather talked about my research interests.” The interaction threw him off for the rest of the interview process, he said.
The experience is an example of how the field has a ways to go in acquiring cultural competence and in overcoming unconscious biases, said Dr. Qutub. In 2020, a review in Clinics in Dermatology referred to a report that dermatology was the second-least diverse medical specialty, only behind orthopedic surgery, because of its low numbers of residents and faculty from groups underrepresented in medicine.
“We really need to put cultural competency at the forefront in order to do better for our patients,” he said.
Adam Friedman, MD, professor and chair of dermatology and director of the residency program at George Washington University, Washington, who also spoke during the session, said that the process of diversifying the field has to go deeper than the resident interviewing process. “If we just focus on trying to increase the diversity of our applicant pool for residents, it’s too late.”
Nada Elbuluk, MD, associate professor of dermatology at the University of Southern California, Los Angeles, pointed to USC’s Derm RISES initiative, a service program that aims to reach inner-city students through education in the sciences, starting from kindergarten to 12th grade. The program also includes premed undergraduate and medical students, “with the goal of increasing exposure to the sciences, medicine, and dermatology,” according to the USC website. “It’s crucial to begin the process early to get a high yield of students who reach medical school and eventually dermatology, she said, because of the inevitable attrition at each level of the education process.
“It’s incredibly rewarding,” added Dr. Elbuluk, who is also director of the dermatology diversity and inclusion program at USC. “And we get these thank-you letters back from students who [say], ‘I didn’t know I could be a doctor.’ ”
In another presentation, Kavita Mariwalla, MD, who practices in West Islip, N.Y., provided tips on boosting cultural competence during aesthetic consults.
One was to “know your fillers,” she said, noting that fillers have different effects on different skin tones, because of differences in fibroblast content, and fat cells will interact with fillers in different ways across skin tones.
Another is to “understand the shortfall of facial canons,” the idea that you can divide a face into sections that can be viewed and enhanced discretely. This concept was based on a White European model and has to be expanded when considering other ethnicities, Dr. Mariwalla said.
Overgeneralizing categories is another pitfall, she said. “Asian” is a term that covers countries from India to Japan, but within that category are a multitude of notions and nuances about aesthetics, and dermatologists have to be sensitive to all of them.
When meeting with a patient, Dr. Mariwalla said, asking the typical “Where are you from?” is not a helpful question. Instead, she suggested asking: “What is your cultural background? Can you tell me more about what your expectations are?”
“I ask for pictures,” she said. “I want to know what they looked like as a kid. I want to know what their family looks like. And I always hand patients a mirror. Patients will say to me: ‘I want to do what you think.’ It’s not about what I think, because what I see, and what you see in your magnifying mirror, are totally different things.”
After the session ended, a member of the audience, Sharon Stokes, MD, a dermatologist in the Orlando area, provided her view of the presentations, noting that it was an important discussion.
“I think it’s past time in medicine for cultural diversity training and awareness for physicians to understand their patients better and getting to know them – and how to even approach the patient and not to offensively and microaggressively approach the patient,” she said.
Dr. Elbuluk reported relevant relationships with Avita, Incyte, Beiersdorf, and other companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis and other companies. Dr. Mariwalla reported relevant financial relationships with Abbvie, Sanofi, Regeneron and other companies. Dr. Qutub reported no relevant financial relationships. Dr. Qutub is the ODAC director of equity, diversity, and inclusion.
AT ODAC 2023
Dermatopathologist reflects on the early history of melanoma
SAN DIEGO – Evidence of melanoma in the ancient past is rare, but according to James W. Patterson, MD, .
“Radiocarbon dating indicated that these mummies were 2,400 years old,” Dr. Patterson, professor emeritus of pathology and dermatology at the University of Virginia, Charlottesville, said at the annual Cutaneous Malignancy Update.
John Hunter, a famous British surgeon who lived from 1728 to 1793, had the first known reported encounter with melanoma in 1787. “He thought it was a form of cancerous fungus,” said Dr. Patterson, a former president of the American Board of Dermatology. “That tumor was preserved in the Hunterian Museum of the Royal College of Surgeons in London, and in 1968 it was reexamined and turned out to be melanoma.”
René Laënnec, the French physician who invented the stethoscope in 1816, is believed to be the first person to lecture on melanoma while a medical student in 1804. The lecture was published about a year later. He originated the term “melanose” (becoming black), a French word derived from the Greek language, to describe metastatic melanoma and reported metastasis to the lungs. During the early part of his career, Dr. Laënnec had studied dissection in the laboratory of the French anatomist and military surgeon Guillaume Dupuytren, best known for his description of Dupuytren’s contracture. Dr. Dupuytren took exception to Dr. Laënnec’s publication about melanoma and called foul.
“As sometimes happens these days, there was some rivalry between these two outstanding physicians of their time,” Dr. Patterson said at the meeting, hosted by Scripps MD Anderson Cancer Center. “Dupuytren was unhappy that Laënnec took credit for this because he claimed credit for originally describing melanoma. He claimed that Laënnec stole the idea from his lectures. I’m not sure that issue was ever resolved.”
In 1820, William Norris, a general practitioner from Stourbridge, England, published the first English language report of melanoma in the Edinburgh Medical and Surgical Journal. “The report was titled ‘A case of fungoid disease,’ so it appears that melanoma was often regarded as a fungal infection back then,” Dr. Patterson said. In the report, Dr. Norris described the tumor in a 59-year-old man as “nearly half the size of a hen’s egg, of a deep brown color, of a firm and fleshy feel, [and] ulcerated on its surface.” Dr. Norris authored a later work titled “Eight cases of melanosis, with pathological and therapeutical remarks on that disease.”
In 1840, a full 2 decades following the first published report from Dr. Norris, the British surgeon Samuel Cooper published a book titled “First Lines of Theory and Practice of Surgery,” in which he described patients with advanced stage melanoma as untreatable and postulated that the only chance for survival was early removal of the tumor.
Dr. Patterson reported having no relevant disclosures.
SAN DIEGO – Evidence of melanoma in the ancient past is rare, but according to James W. Patterson, MD, .
“Radiocarbon dating indicated that these mummies were 2,400 years old,” Dr. Patterson, professor emeritus of pathology and dermatology at the University of Virginia, Charlottesville, said at the annual Cutaneous Malignancy Update.
John Hunter, a famous British surgeon who lived from 1728 to 1793, had the first known reported encounter with melanoma in 1787. “He thought it was a form of cancerous fungus,” said Dr. Patterson, a former president of the American Board of Dermatology. “That tumor was preserved in the Hunterian Museum of the Royal College of Surgeons in London, and in 1968 it was reexamined and turned out to be melanoma.”
René Laënnec, the French physician who invented the stethoscope in 1816, is believed to be the first person to lecture on melanoma while a medical student in 1804. The lecture was published about a year later. He originated the term “melanose” (becoming black), a French word derived from the Greek language, to describe metastatic melanoma and reported metastasis to the lungs. During the early part of his career, Dr. Laënnec had studied dissection in the laboratory of the French anatomist and military surgeon Guillaume Dupuytren, best known for his description of Dupuytren’s contracture. Dr. Dupuytren took exception to Dr. Laënnec’s publication about melanoma and called foul.
“As sometimes happens these days, there was some rivalry between these two outstanding physicians of their time,” Dr. Patterson said at the meeting, hosted by Scripps MD Anderson Cancer Center. “Dupuytren was unhappy that Laënnec took credit for this because he claimed credit for originally describing melanoma. He claimed that Laënnec stole the idea from his lectures. I’m not sure that issue was ever resolved.”
In 1820, William Norris, a general practitioner from Stourbridge, England, published the first English language report of melanoma in the Edinburgh Medical and Surgical Journal. “The report was titled ‘A case of fungoid disease,’ so it appears that melanoma was often regarded as a fungal infection back then,” Dr. Patterson said. In the report, Dr. Norris described the tumor in a 59-year-old man as “nearly half the size of a hen’s egg, of a deep brown color, of a firm and fleshy feel, [and] ulcerated on its surface.” Dr. Norris authored a later work titled “Eight cases of melanosis, with pathological and therapeutical remarks on that disease.”
In 1840, a full 2 decades following the first published report from Dr. Norris, the British surgeon Samuel Cooper published a book titled “First Lines of Theory and Practice of Surgery,” in which he described patients with advanced stage melanoma as untreatable and postulated that the only chance for survival was early removal of the tumor.
Dr. Patterson reported having no relevant disclosures.
SAN DIEGO – Evidence of melanoma in the ancient past is rare, but according to James W. Patterson, MD, .
“Radiocarbon dating indicated that these mummies were 2,400 years old,” Dr. Patterson, professor emeritus of pathology and dermatology at the University of Virginia, Charlottesville, said at the annual Cutaneous Malignancy Update.
John Hunter, a famous British surgeon who lived from 1728 to 1793, had the first known reported encounter with melanoma in 1787. “He thought it was a form of cancerous fungus,” said Dr. Patterson, a former president of the American Board of Dermatology. “That tumor was preserved in the Hunterian Museum of the Royal College of Surgeons in London, and in 1968 it was reexamined and turned out to be melanoma.”
René Laënnec, the French physician who invented the stethoscope in 1816, is believed to be the first person to lecture on melanoma while a medical student in 1804. The lecture was published about a year later. He originated the term “melanose” (becoming black), a French word derived from the Greek language, to describe metastatic melanoma and reported metastasis to the lungs. During the early part of his career, Dr. Laënnec had studied dissection in the laboratory of the French anatomist and military surgeon Guillaume Dupuytren, best known for his description of Dupuytren’s contracture. Dr. Dupuytren took exception to Dr. Laënnec’s publication about melanoma and called foul.
“As sometimes happens these days, there was some rivalry between these two outstanding physicians of their time,” Dr. Patterson said at the meeting, hosted by Scripps MD Anderson Cancer Center. “Dupuytren was unhappy that Laënnec took credit for this because he claimed credit for originally describing melanoma. He claimed that Laënnec stole the idea from his lectures. I’m not sure that issue was ever resolved.”
In 1820, William Norris, a general practitioner from Stourbridge, England, published the first English language report of melanoma in the Edinburgh Medical and Surgical Journal. “The report was titled ‘A case of fungoid disease,’ so it appears that melanoma was often regarded as a fungal infection back then,” Dr. Patterson said. In the report, Dr. Norris described the tumor in a 59-year-old man as “nearly half the size of a hen’s egg, of a deep brown color, of a firm and fleshy feel, [and] ulcerated on its surface.” Dr. Norris authored a later work titled “Eight cases of melanosis, with pathological and therapeutical remarks on that disease.”
In 1840, a full 2 decades following the first published report from Dr. Norris, the British surgeon Samuel Cooper published a book titled “First Lines of Theory and Practice of Surgery,” in which he described patients with advanced stage melanoma as untreatable and postulated that the only chance for survival was early removal of the tumor.
Dr. Patterson reported having no relevant disclosures.
AT MELANOMA 2023
Commentary: A New Drug, and Pediatric Concerns, February 2023
I love registries! With large numbers of participants, registries can be very helpful to identify rare side effects and to assess the efficacy and safety of medications in populations that may not be fully represented in clinical trials. I also love dupilumab; it was revolutionary in the management of patients with AD.
Vittrup and colleagues have created a registry of 347 participants treated with dupilumab. This does not yet have the large number of participants needed to identify new issues that wouldn't have been detected in clinical trials, but the study is informative about real-life use. The dramatic improvement in the Eczema Area and Severity Index (EASI) score is consistent with the high efficacy of dupilumab. The high rate of treatment persistence is also consistent with dupilumab being a very effective and safe treatment (because if the drug wasn't working well or was causing a severe problem, patients would probably stop the treatment). Though the study reported persistent head and neck involvement, the residual involvement may be quite minimal.
The EASI-75 and Investigator Global Assessment response rates reported in dupilumab trials underestimate the value of this drug. With a 2-year persistence rate of nearly 90%, it's clear that dupilumab is making a huge difference in the lives of patients with AD.
Fatigue is a fascinating issue in AD. We might wonder if all the inflammation in patients with AD would directly cause fatigue. Almost certainly all the itching in AD adversely affects sleep and would cause tremendous fatigue. It surprised me that most of the children in the study by Rangel and colleagues were reported as having no or mild fatigue; severe fatigue was very uncommon. It leaves me wondering whether the assessments of fatigue fully capture what's happening. Also, since the fatigue score was reported by the parents, I (as the parent of a child with AD) am wondering whether the parents were projecting, with the score more reflective of the parents' fatigue than with that of the child; alternatively, perhaps the child's hyperactivity leaves parents thinking there is no fatigue when there actually is (and possibly even causing the perceived hyperactivity).
The lack of a control group without AD is another major limitation in our ability to interpret the study findings. Is fatigue more common or less common in children with AD than in children without AD? I cannot tell from these findings. Does fatigue warrant, as the authors suggest, more attention in clinical practice? I don't know. If we are already treating our patients based on patients' global impressions of how they are doing — combined, of course, with our observations of their objective disease severity — I'm not sure how asking about fatigue would change anything, even if future studies were to definitively show that AD is associated with fatigue.
I hate new drugs (well, maybe not hate, but I worry about unknown long-term risks). Clinical trials that help a drug get approved can tell us a lot about a drug's efficacy, but these studies are generally limited in what they tell us about a drug's safety. Clinical trials are generally not powered enough (not enough participants and not followed for long enough) to be informative about rare risks. I love long-term studies of new drugs in large numbers of people because those studies can be very reassuring about the risks of medications. Studying nearly 10,000 patients for 5 years is quite reassuring, confirming my impression that dupilumab has a remarkable, excellent safety profile (Owji et al). Blocking interleukin 4 and interleukin 13 seems to be very specific to AD. Finding no association to cancer is what I would have expected; being able to share this information with patients is likely to be reassuring to them.
Oh, lord help me, another study that claims we should change our disease management because they've identified an increased risk for something. When you compare 70,000 patients with 270,000 controls, you have huge power to detect statistically significant associations of no clinical consequence. Let's assume for the moment that the detected association the authors found between AD and juvenile idiopathic arthritis (JIA) is real. The odds ratio is 2; the odds ratio for smoking causing cancer is on the order of 100.
In this study, over 99% of individuals in both AD and control groups did not have JIA. The difference between rates of JIA in patients with AD compared with controls was 0.3%! The authors conclude "it is important to inquire actively about symptoms not directly linked to the patients' skin disease"; based on the findings of this study, I would conclude that we don't need to worry about JIA in patients with AD even if there is a (marginally) higher prevalence of JIA in this group.
I love registries! With large numbers of participants, registries can be very helpful to identify rare side effects and to assess the efficacy and safety of medications in populations that may not be fully represented in clinical trials. I also love dupilumab; it was revolutionary in the management of patients with AD.
Vittrup and colleagues have created a registry of 347 participants treated with dupilumab. This does not yet have the large number of participants needed to identify new issues that wouldn't have been detected in clinical trials, but the study is informative about real-life use. The dramatic improvement in the Eczema Area and Severity Index (EASI) score is consistent with the high efficacy of dupilumab. The high rate of treatment persistence is also consistent with dupilumab being a very effective and safe treatment (because if the drug wasn't working well or was causing a severe problem, patients would probably stop the treatment). Though the study reported persistent head and neck involvement, the residual involvement may be quite minimal.
The EASI-75 and Investigator Global Assessment response rates reported in dupilumab trials underestimate the value of this drug. With a 2-year persistence rate of nearly 90%, it's clear that dupilumab is making a huge difference in the lives of patients with AD.
Fatigue is a fascinating issue in AD. We might wonder if all the inflammation in patients with AD would directly cause fatigue. Almost certainly all the itching in AD adversely affects sleep and would cause tremendous fatigue. It surprised me that most of the children in the study by Rangel and colleagues were reported as having no or mild fatigue; severe fatigue was very uncommon. It leaves me wondering whether the assessments of fatigue fully capture what's happening. Also, since the fatigue score was reported by the parents, I (as the parent of a child with AD) am wondering whether the parents were projecting, with the score more reflective of the parents' fatigue than with that of the child; alternatively, perhaps the child's hyperactivity leaves parents thinking there is no fatigue when there actually is (and possibly even causing the perceived hyperactivity).
The lack of a control group without AD is another major limitation in our ability to interpret the study findings. Is fatigue more common or less common in children with AD than in children without AD? I cannot tell from these findings. Does fatigue warrant, as the authors suggest, more attention in clinical practice? I don't know. If we are already treating our patients based on patients' global impressions of how they are doing — combined, of course, with our observations of their objective disease severity — I'm not sure how asking about fatigue would change anything, even if future studies were to definitively show that AD is associated with fatigue.
I hate new drugs (well, maybe not hate, but I worry about unknown long-term risks). Clinical trials that help a drug get approved can tell us a lot about a drug's efficacy, but these studies are generally limited in what they tell us about a drug's safety. Clinical trials are generally not powered enough (not enough participants and not followed for long enough) to be informative about rare risks. I love long-term studies of new drugs in large numbers of people because those studies can be very reassuring about the risks of medications. Studying nearly 10,000 patients for 5 years is quite reassuring, confirming my impression that dupilumab has a remarkable, excellent safety profile (Owji et al). Blocking interleukin 4 and interleukin 13 seems to be very specific to AD. Finding no association to cancer is what I would have expected; being able to share this information with patients is likely to be reassuring to them.
Oh, lord help me, another study that claims we should change our disease management because they've identified an increased risk for something. When you compare 70,000 patients with 270,000 controls, you have huge power to detect statistically significant associations of no clinical consequence. Let's assume for the moment that the detected association the authors found between AD and juvenile idiopathic arthritis (JIA) is real. The odds ratio is 2; the odds ratio for smoking causing cancer is on the order of 100.
In this study, over 99% of individuals in both AD and control groups did not have JIA. The difference between rates of JIA in patients with AD compared with controls was 0.3%! The authors conclude "it is important to inquire actively about symptoms not directly linked to the patients' skin disease"; based on the findings of this study, I would conclude that we don't need to worry about JIA in patients with AD even if there is a (marginally) higher prevalence of JIA in this group.
I love registries! With large numbers of participants, registries can be very helpful to identify rare side effects and to assess the efficacy and safety of medications in populations that may not be fully represented in clinical trials. I also love dupilumab; it was revolutionary in the management of patients with AD.
Vittrup and colleagues have created a registry of 347 participants treated with dupilumab. This does not yet have the large number of participants needed to identify new issues that wouldn't have been detected in clinical trials, but the study is informative about real-life use. The dramatic improvement in the Eczema Area and Severity Index (EASI) score is consistent with the high efficacy of dupilumab. The high rate of treatment persistence is also consistent with dupilumab being a very effective and safe treatment (because if the drug wasn't working well or was causing a severe problem, patients would probably stop the treatment). Though the study reported persistent head and neck involvement, the residual involvement may be quite minimal.
The EASI-75 and Investigator Global Assessment response rates reported in dupilumab trials underestimate the value of this drug. With a 2-year persistence rate of nearly 90%, it's clear that dupilumab is making a huge difference in the lives of patients with AD.
Fatigue is a fascinating issue in AD. We might wonder if all the inflammation in patients with AD would directly cause fatigue. Almost certainly all the itching in AD adversely affects sleep and would cause tremendous fatigue. It surprised me that most of the children in the study by Rangel and colleagues were reported as having no or mild fatigue; severe fatigue was very uncommon. It leaves me wondering whether the assessments of fatigue fully capture what's happening. Also, since the fatigue score was reported by the parents, I (as the parent of a child with AD) am wondering whether the parents were projecting, with the score more reflective of the parents' fatigue than with that of the child; alternatively, perhaps the child's hyperactivity leaves parents thinking there is no fatigue when there actually is (and possibly even causing the perceived hyperactivity).
The lack of a control group without AD is another major limitation in our ability to interpret the study findings. Is fatigue more common or less common in children with AD than in children without AD? I cannot tell from these findings. Does fatigue warrant, as the authors suggest, more attention in clinical practice? I don't know. If we are already treating our patients based on patients' global impressions of how they are doing — combined, of course, with our observations of their objective disease severity — I'm not sure how asking about fatigue would change anything, even if future studies were to definitively show that AD is associated with fatigue.
I hate new drugs (well, maybe not hate, but I worry about unknown long-term risks). Clinical trials that help a drug get approved can tell us a lot about a drug's efficacy, but these studies are generally limited in what they tell us about a drug's safety. Clinical trials are generally not powered enough (not enough participants and not followed for long enough) to be informative about rare risks. I love long-term studies of new drugs in large numbers of people because those studies can be very reassuring about the risks of medications. Studying nearly 10,000 patients for 5 years is quite reassuring, confirming my impression that dupilumab has a remarkable, excellent safety profile (Owji et al). Blocking interleukin 4 and interleukin 13 seems to be very specific to AD. Finding no association to cancer is what I would have expected; being able to share this information with patients is likely to be reassuring to them.
Oh, lord help me, another study that claims we should change our disease management because they've identified an increased risk for something. When you compare 70,000 patients with 270,000 controls, you have huge power to detect statistically significant associations of no clinical consequence. Let's assume for the moment that the detected association the authors found between AD and juvenile idiopathic arthritis (JIA) is real. The odds ratio is 2; the odds ratio for smoking causing cancer is on the order of 100.
In this study, over 99% of individuals in both AD and control groups did not have JIA. The difference between rates of JIA in patients with AD compared with controls was 0.3%! The authors conclude "it is important to inquire actively about symptoms not directly linked to the patients' skin disease"; based on the findings of this study, I would conclude that we don't need to worry about JIA in patients with AD even if there is a (marginally) higher prevalence of JIA in this group.
Fluorescence-optical imaging may detect preclinical PsA
Fluorescence-optical imaging (FOI) identified early signs of psoriatic arthritis, based on data from 2 years of follow-up of a cohort of 389 adults at 14 rheumatology centers.
Approximately 25% of individuals with psoriasis go on to develop psoriatic arthritis (PsA), but there are no validated biomarkers to identify patients at risk for progression to PsA, Michaela Koehm, MD, of Goethe University, Frankfurt am Main, Germany, and colleagues wrote in RMD Open.
FOI is a technique that allows assessment of changes in microvascularization and subdermal skin inflammation, and because individuals with psoriasis who develop PsA have shown changes in blood vessel formation in the early stages of disease, the researchers sought to determine if FOI could be used to predict early PsA.
The researchers conducted a multicenter, two-part observational cohort study. The two parts, known as XCITING and XTEND, included 389 adults aged 18-75 years with plaque psoriasis deemed at increased risk for PsA. The patients were seen at rheumatology sites in Germany between Jan. 28, 2014, and March 16, 2017. The XTEND study included clinic visits 18-24 months after the XCITING study.
Participants underwent a complete clinical examination, with musculoskeletal ultrasound (MSUS) and FOI on both hands at a single visit. Those with positive FOI findings not seen with clinical exam or MSUS underwent MRI within 7 days. Patients with positive FOI but negative findings on clinical exam, MSUS, and MRI were followed for 2 years in the XTEND study.
The primary outcome was the ability of FOI to detect musculoskeletal inflammation, compared with clinical examination and MSUS.
Overall, 50% of the patients were diagnosed with PsA. A total of 116 (30%) had positive FOI findings; complete MRI data were available for 108 of these patients, including 68 negative MRIs and 40 positive MRIs.
In the XTEND study, another 12% of patients who were positive on FOI but not on MRI also developed PsA by the end of the 2-year follow-up. In comparison, the researchers noted that “literature data on yearly incidence rates [of PsA] in different national cohorts indicate an incidence rate of approximately 4.3% per year.”
A total of 149 of the 196 patients with PsA confirmed by either clinical exam or MSUS were also positive on FOI, yielding a sensitivity of 76.0%. The specificity of FOI was 39.5%.
The sensitive visualization of musculoskeletal inflammation possible with FOI “may exceed its ability to detect clinically manifest PsA at high sensitivity or specificity, but early visualization is arguably of greater value as other imaging methods are currently available for detection of later stages of PsA,” the researchers wrote in their discussion. “A technique allowing early identification of PsA may be especially valuable for nonrheumatologists, including dermatologists and general practitioners, and help expedite more efficient referral to specialists.”
The findings were limited by several factors, including the nonrandomized design and small subgroup numbers, the researchers noted. Other limitations include the presence of alternative conditions such as osteoarthritis that might have complicated the imaging; the focus only on the hands; and potential variation in FOI assessment related to technical standards such as temperature and positioning.
However, the results support FOI as a safe and effective method of detecting early signs of joint inflammation that could predict increased risk for PsA in psoriasis patients, the researchers said.
The researchers added that more work is needed to evaluate FOI in clinical practice, but FOI has the potential to identify vascularization changes earlier than other imaging modalities and in advance of clinical symptoms.
“Accordingly, FOI may have the potential to improve patient outcomes in PsA by reducing the time to initiation of early treatment,” they concluded.
The study was supported by Fraunhofer ITMP, a nonprofit organization, and a research grant from Pfizer Germany. Some of the researchers disclosed financial relationships with many pharmaceutical companies, including Pfizer.
Fluorescence-optical imaging (FOI) identified early signs of psoriatic arthritis, based on data from 2 years of follow-up of a cohort of 389 adults at 14 rheumatology centers.
Approximately 25% of individuals with psoriasis go on to develop psoriatic arthritis (PsA), but there are no validated biomarkers to identify patients at risk for progression to PsA, Michaela Koehm, MD, of Goethe University, Frankfurt am Main, Germany, and colleagues wrote in RMD Open.
FOI is a technique that allows assessment of changes in microvascularization and subdermal skin inflammation, and because individuals with psoriasis who develop PsA have shown changes in blood vessel formation in the early stages of disease, the researchers sought to determine if FOI could be used to predict early PsA.
The researchers conducted a multicenter, two-part observational cohort study. The two parts, known as XCITING and XTEND, included 389 adults aged 18-75 years with plaque psoriasis deemed at increased risk for PsA. The patients were seen at rheumatology sites in Germany between Jan. 28, 2014, and March 16, 2017. The XTEND study included clinic visits 18-24 months after the XCITING study.
Participants underwent a complete clinical examination, with musculoskeletal ultrasound (MSUS) and FOI on both hands at a single visit. Those with positive FOI findings not seen with clinical exam or MSUS underwent MRI within 7 days. Patients with positive FOI but negative findings on clinical exam, MSUS, and MRI were followed for 2 years in the XTEND study.
The primary outcome was the ability of FOI to detect musculoskeletal inflammation, compared with clinical examination and MSUS.
Overall, 50% of the patients were diagnosed with PsA. A total of 116 (30%) had positive FOI findings; complete MRI data were available for 108 of these patients, including 68 negative MRIs and 40 positive MRIs.
In the XTEND study, another 12% of patients who were positive on FOI but not on MRI also developed PsA by the end of the 2-year follow-up. In comparison, the researchers noted that “literature data on yearly incidence rates [of PsA] in different national cohorts indicate an incidence rate of approximately 4.3% per year.”
A total of 149 of the 196 patients with PsA confirmed by either clinical exam or MSUS were also positive on FOI, yielding a sensitivity of 76.0%. The specificity of FOI was 39.5%.
The sensitive visualization of musculoskeletal inflammation possible with FOI “may exceed its ability to detect clinically manifest PsA at high sensitivity or specificity, but early visualization is arguably of greater value as other imaging methods are currently available for detection of later stages of PsA,” the researchers wrote in their discussion. “A technique allowing early identification of PsA may be especially valuable for nonrheumatologists, including dermatologists and general practitioners, and help expedite more efficient referral to specialists.”
The findings were limited by several factors, including the nonrandomized design and small subgroup numbers, the researchers noted. Other limitations include the presence of alternative conditions such as osteoarthritis that might have complicated the imaging; the focus only on the hands; and potential variation in FOI assessment related to technical standards such as temperature and positioning.
However, the results support FOI as a safe and effective method of detecting early signs of joint inflammation that could predict increased risk for PsA in psoriasis patients, the researchers said.
The researchers added that more work is needed to evaluate FOI in clinical practice, but FOI has the potential to identify vascularization changes earlier than other imaging modalities and in advance of clinical symptoms.
“Accordingly, FOI may have the potential to improve patient outcomes in PsA by reducing the time to initiation of early treatment,” they concluded.
The study was supported by Fraunhofer ITMP, a nonprofit organization, and a research grant from Pfizer Germany. Some of the researchers disclosed financial relationships with many pharmaceutical companies, including Pfizer.
Fluorescence-optical imaging (FOI) identified early signs of psoriatic arthritis, based on data from 2 years of follow-up of a cohort of 389 adults at 14 rheumatology centers.
Approximately 25% of individuals with psoriasis go on to develop psoriatic arthritis (PsA), but there are no validated biomarkers to identify patients at risk for progression to PsA, Michaela Koehm, MD, of Goethe University, Frankfurt am Main, Germany, and colleagues wrote in RMD Open.
FOI is a technique that allows assessment of changes in microvascularization and subdermal skin inflammation, and because individuals with psoriasis who develop PsA have shown changes in blood vessel formation in the early stages of disease, the researchers sought to determine if FOI could be used to predict early PsA.
The researchers conducted a multicenter, two-part observational cohort study. The two parts, known as XCITING and XTEND, included 389 adults aged 18-75 years with plaque psoriasis deemed at increased risk for PsA. The patients were seen at rheumatology sites in Germany between Jan. 28, 2014, and March 16, 2017. The XTEND study included clinic visits 18-24 months after the XCITING study.
Participants underwent a complete clinical examination, with musculoskeletal ultrasound (MSUS) and FOI on both hands at a single visit. Those with positive FOI findings not seen with clinical exam or MSUS underwent MRI within 7 days. Patients with positive FOI but negative findings on clinical exam, MSUS, and MRI were followed for 2 years in the XTEND study.
The primary outcome was the ability of FOI to detect musculoskeletal inflammation, compared with clinical examination and MSUS.
Overall, 50% of the patients were diagnosed with PsA. A total of 116 (30%) had positive FOI findings; complete MRI data were available for 108 of these patients, including 68 negative MRIs and 40 positive MRIs.
In the XTEND study, another 12% of patients who were positive on FOI but not on MRI also developed PsA by the end of the 2-year follow-up. In comparison, the researchers noted that “literature data on yearly incidence rates [of PsA] in different national cohorts indicate an incidence rate of approximately 4.3% per year.”
A total of 149 of the 196 patients with PsA confirmed by either clinical exam or MSUS were also positive on FOI, yielding a sensitivity of 76.0%. The specificity of FOI was 39.5%.
The sensitive visualization of musculoskeletal inflammation possible with FOI “may exceed its ability to detect clinically manifest PsA at high sensitivity or specificity, but early visualization is arguably of greater value as other imaging methods are currently available for detection of later stages of PsA,” the researchers wrote in their discussion. “A technique allowing early identification of PsA may be especially valuable for nonrheumatologists, including dermatologists and general practitioners, and help expedite more efficient referral to specialists.”
The findings were limited by several factors, including the nonrandomized design and small subgroup numbers, the researchers noted. Other limitations include the presence of alternative conditions such as osteoarthritis that might have complicated the imaging; the focus only on the hands; and potential variation in FOI assessment related to technical standards such as temperature and positioning.
However, the results support FOI as a safe and effective method of detecting early signs of joint inflammation that could predict increased risk for PsA in psoriasis patients, the researchers said.
The researchers added that more work is needed to evaluate FOI in clinical practice, but FOI has the potential to identify vascularization changes earlier than other imaging modalities and in advance of clinical symptoms.
“Accordingly, FOI may have the potential to improve patient outcomes in PsA by reducing the time to initiation of early treatment,” they concluded.
The study was supported by Fraunhofer ITMP, a nonprofit organization, and a research grant from Pfizer Germany. Some of the researchers disclosed financial relationships with many pharmaceutical companies, including Pfizer.
FROM RMD OPEN
Don’t cross the friends line with patients
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
All that moving can make it hard to maintain friendships. Factor in the challenges from the pandemic, and a physician’s life can be lonely. So, when a patient invites you for coffee or a game of pickleball, do you accept? For almost one-third of the physicians who responded to the Medscape Physician Friendships: The Joys and Challenges 2022, the answer might be yes.
About 29% said they develop friendships with patients. However, a lot depends on the circumstances. As one physician in the report said: “I have been a pediatrician for 35 years, and my patients have grown up and become productive adults in our small, rural, isolated area. You can’t help but know almost everyone.”
As the daughter of a cardiologist, Nishi Mehta, MD, a radiologist and founder of the largest physician-only Facebook group in the country, grew up with that small-town-everyone-knows-the-doctor model.
“When I was a kid, I’d go to the mall, and my friends and I would play a game: How long before a patient [of my dad’s] comes up to me?” she said. At the time, Dr. Mehta was embarrassed, but now she marvels that her dad knew his patients so well that they would recognize his daughter in crowded suburban mall.
In other instances, a physician may develop a friendly relationship after a patient leaves their care. For example, Leo Nissola, MD, now a full-time researcher and immunotherapy scientist in San Francisco, has stayed in touch with some of the patients he treated while at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Nissola said it was important to stay connected with the patients he had meaningful relationships with. “It becomes challenging, though, when a former patient asks for medical advice.” At that moment, “you have to be explicitly clear that the relationship has changed.”
A hard line in the sand
The blurring of lines is one reason many doctors refuse to befriend patients, even after they are no longer treating them. The American College of Physicians Ethics Manual advises against treating anyone with whom you have a close relationship, including family and friends.
“Friendships can get in the way of patients being honest with you, which can interfere with medical care,” Dr. Mehta said. “If a patient has a concern related to something they wouldn’t want you to know as friends, it can get awkward. They may elect not to tell you.”
And on the flip side, friendship can provide a view into your private life that you may not welcome in the exam room.
“Let’s say you go out for drinks [with a patient], and you’re up late, but you have surgery the next day,” said Brandi Ring, MD, an ob.gyn. and the associate medical director at the Center for Children and Women in Houston. Now, one of your patients knows you were out until midnight when you had to be in the OR at 5:00 a.m.
Worse still, your relationship could color your decisions about a patient’s care, even unconsciously. It can be hard to maintain objectivity when you have an emotional investment in someone’s well-being.
“We don’t necessarily treat family and friends to the standards of medical care,” said Dr. Ring. “We go above and beyond. We might order more tests and more scans. We don’t always follow the guidelines, especially in critical illness.”
For all these reasons and more, the ACP advises against treating friends.
Put physician before friend
But adhering to those guidelines can lead physicians to make some painful decisions. Cutting yourself off from the possibility of friendship is never easy, and the Medscape report found that physicians tend to have fewer friends than the average American.
“Especially earlier in my practice, when I was a young parent, and I would see a lot of other young parents in the same stage in life, I’d think, ‘In other circumstances, I would be hanging out at the park with this person,’ “ said Kathleen Rowland, MD, a family medicine physician and vice chair of education in the department of family medicine at Rush University, Chicago. “But the hard part is, the doctor-patient relationship always comes first.”
To a certain extent, one’s specialty may determine the feasibility of becoming friends with a patient. While Dr. Mehta has never done so, as a radiologist, she doesn’t usually see patients repeatedly. Likewise, a young gerontologist may have little in common with his octogenarian patients. And an older pediatrician is not in the same life stage as his patients’ sleep-deprived new parents, possibly making them less attractive friends.
However, practicing family medicine is all about long-term physician-patient relationships. Getting to know patients and their families over many years can lead to a certain intimacy. Dr. Rowland said that, while a wonderful part of being a physician is getting that unique trust whereby patients tell you all sorts of things about their lives, she’s never gone down the friendship path.
“There’s the assumption I’ll take care of someone for a long period of time, and their partner and their kids, maybe another generation or two,” Dr. Rowland said. “People really do rely on that relationship to contribute to their health.”
Worse, nowadays, when people may be starved for connection, many patients want to feel emotionally close and cared for by their doctor, so it’d be easy to cross the line. While patients deserve a compassionate, caring doctor, the physician is left to walk the line between those boundaries. Dr. Rowland said, “It’s up to the clinician to say: ‘My role is as a doctor. You deserve caring friends, but I have to order your mammogram and your blood counts. My role is different.’ ”
Friendly but not friends
It can be tricky to navigate the boundary between a cordial, warm relationship with a patient and that patient inviting you to their daughter’s wedding.
“People may mistake being pleasant and friendly for being friends,” said Larry Blosser, MD, chief medical officer at Central Ohio Primary Care, Westerville. In his position, he sometimes hears from patients who have misunderstood their relationship with a doctor in the practice. When that happens, he advises the physician to consider the persona they’re presenting to the patient. If you’re overly friendly, there’s the potential for confusion, but you can’t be aloof and cold, he said.
Maintaining that awareness helps to prevent a patient’s offhand invitation to catch a movie or go on a hike. And verbalizing it to your patients can make your relationship clear from the get-go.
“I tell patients we’re a team. I’m the captain, and they’re my MVP. When the match is over, whatever the results, we’re done,” said Karenne Fru, MD, PhD, a fertility specialist at Oma Fertility Atlanta. Making deep connections is essential to her practice, so Dr. Fru structures her patient interactions carefully. “Infertility is such an isolating experience. While you’re with us, we care about what’s going on in your life, your pets, and your mom’s chemo. We need mutual trust for you to be compliant with the care.”
However, that approach won’t work when you see patients regularly, as with family practice or specialties that see the same patients repeatedly throughout the year. In those circumstances, the match is never over but one in which the onus is on the physician to establish a friendly yet professional rapport without letting your self-interest, loneliness, or lack of friends interfere.
“It’s been a very difficult couple of years for a lot of us. Depending on what kind of clinical work we do, some of us took care of healthy people that got very sick or passed away,” Dr. Rowland said. “Having the chance to reconnect with people and reestablish some of that closeness, both physical and emotional, is going to be good for us.”
Just continue conveying warm, trusting compassion for your patients without blurring the friend lines.
A version of this article first appeared on Medscape.com.
How should PRAME be used to evaluate melanocytic lesions?
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
AT MELANOMA 2023
FDA panel backs shift toward one-dose COVID shot
The FDA is looking to give clearer direction to vaccine makers about future development of COVID-19 vaccines. The plan is to narrow down the current complex landscape of options for vaccinations, and thus help increase use of these shots.
COVID remains a serious threat, causing about 4,000 deaths a week recently, according to the Centers for Disease Control and Prevention. The 21 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Jan. 26 voted unanimously “yes” on a single question posed by the FDA:
“Does the committee recommend harmonizing the vaccine strain composition of primary series and booster doses in the U.S. to a single composition, e.g., the composition for all vaccines administered currently would be a bivalent vaccine (Original plus Omicron BA.4/BA.5)?”
In other words, would it be better to have one vaccine potentially combining multiple strains of the virus, instead of multiple vaccines – such as a two-shot primary series then a booster containing different combinations of viral strains.
The FDA will consider the panel’s advice as it outlines new strategies for keeping ahead of the evolving virus.
In explaining their support for the FDA plan, panel members said they hoped that a simpler regime would aid in persuading more people to get COVID vaccines.
Pamela McInnes, DDS, MSc, noted that it’s difficult to explain to many people that the vaccine works to protect them from more severe illness if they contract COVID after getting vaccinated.
“That is a real challenge,” said Dr. McInness, retired deputy director of the National Center for Advancing Translational Sciences at the National Institutes of Health.
“The message that you would have gotten more sick and landed in the hospital resonates with me, but I’m not sure if it resonates with” many people who become infected, she said.
The plan
In the briefing document for the meeting, the FDA outlined a plan for transitioning from the current complex landscape of COVID-19 vaccines to a single vaccine composition for the primary series and booster vaccination.
This would require harmonizing the strain composition of all COVID-19 vaccines; simplifying the immunization schedule for future vaccination campaigns to administer a two-dose series in certain young children and in older adults and persons with compromised immunity, and only one dose in all other individuals; and establishing a process for vaccine strain selection recommendations, similar in many ways to that used for seasonal influenza vaccines, based on prevailing and predicted variants that would take place by June to allow for vaccine production by September.
During the discussion, though, questions arose about the June target date. Given the production schedule for some vaccines, that date might need to shift, said Jerry Weir, PhD, director of the division of viral products at FDA’s Center for Biologics Evaluation and Research.
“We’re all just going to have to maintain flexibility,” Dr. Weir said, adding that there is not yet a “good pattern” established for updating these vaccines.
Increasing vaccination rates
There was broad consensus about the need to boost public support for COVID-19 vaccinations. While about 81% of the U.S. population has had at least one dose of this vaccine, only 15.3% have had an updated bivalent booster dose, according to the CDC.
“Anything that results in better public communication would be extremely valuable,” said committee member Henry H. Bernstein, DO, MHCM, of the Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
But it’s unclear what expectations will be prioritized for the COVID vaccine program, he said.
“Realistically, I don’t think we can have it all – less infection, less transmission, less severe disease, and less long COVID,” Dr. Bernstein said. “And that seems to be a major challenge for public messaging.”
Panelists press for more data
Other committee members also pressed for clearer targets in evaluating the goals for COVID vaccines, and for more robust data.
Like his fellow VRBPAC members, Cody Meissner, MD, of Dartmouth’s Geisel School of Medicine, Hanover, N.H., supported a move toward harmonizing the strains used in different companies’ vaccines. But he added that it wasn’t clear yet how frequently they should be administered.
“We need to see what happens with disease burden,” Dr. Meissner said. “We may or may not need annual vaccination. It’s just awfully early, it seems to me, in this process to answer that question.”
Among those serving on VRBPAC was one of the FDA’s more vocal critics on these points, Paul A. Offit, MD, a vaccine expert from Children’s Hospital of Philadelphia. Dr. Offit, for example, joined former FDA officials in writing a November opinion article for the Washington Post, arguing that the evidence for boosters for healthy younger adults was not strong.
At the Jan. 26 meeting, he supported the drive toward simplification of COVID vaccine schedules, while arguing for more data about how well these products are working.
“This virus is going to be with us for years, if not decades, and there will always be vulnerable groups who are going to be hospitalized and killed by the virus,” Dr. Offit said.
The CDC needs to provide more information about the characteristics of people being hospitalized with COVID infections, including their ages and comorbidities as well as details about their vaccine history, he said. In addition, academic researchers should provide a clearer picture of what immunological predictors are at play in increasing people’s risk from COVID.
“Then and only then can we really best make the decision about who gets vaccinated with what and when,” Dr. Offit said.
VRBPAC member Ofer Levy, MD, PhD, also urged the FDA to press for a collection of more robust and detailed information about the immune response to COVID-19 vaccinations, such as a deeper look at what’s happening with antibodies.
“I hope FDA will continue to reflect on how to best take this information forward, and encourage – or require – sponsors to gather more information in a standardized way across these different arms of the human immune system,” Dr. Levy said. “So we keep learning and keep doing this better.”
In recapping the panel’s suggestions at the end of the meeting, Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research, addressed the requests made during the day’s meeting about better data on how the vaccines work.
“We heard loud and clear that we need to use a data-driven approach to get to the simplest possible scheme that we can for vaccination,” Dr. Marks said. “And it should be as simple as possible but not oversimplified, a little bit like they say about Mozart’s music.”
A version of this article first appeared on WebMD.com.
The FDA is looking to give clearer direction to vaccine makers about future development of COVID-19 vaccines. The plan is to narrow down the current complex landscape of options for vaccinations, and thus help increase use of these shots.
COVID remains a serious threat, causing about 4,000 deaths a week recently, according to the Centers for Disease Control and Prevention. The 21 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Jan. 26 voted unanimously “yes” on a single question posed by the FDA:
“Does the committee recommend harmonizing the vaccine strain composition of primary series and booster doses in the U.S. to a single composition, e.g., the composition for all vaccines administered currently would be a bivalent vaccine (Original plus Omicron BA.4/BA.5)?”
In other words, would it be better to have one vaccine potentially combining multiple strains of the virus, instead of multiple vaccines – such as a two-shot primary series then a booster containing different combinations of viral strains.
The FDA will consider the panel’s advice as it outlines new strategies for keeping ahead of the evolving virus.
In explaining their support for the FDA plan, panel members said they hoped that a simpler regime would aid in persuading more people to get COVID vaccines.
Pamela McInnes, DDS, MSc, noted that it’s difficult to explain to many people that the vaccine works to protect them from more severe illness if they contract COVID after getting vaccinated.
“That is a real challenge,” said Dr. McInness, retired deputy director of the National Center for Advancing Translational Sciences at the National Institutes of Health.
“The message that you would have gotten more sick and landed in the hospital resonates with me, but I’m not sure if it resonates with” many people who become infected, she said.
The plan
In the briefing document for the meeting, the FDA outlined a plan for transitioning from the current complex landscape of COVID-19 vaccines to a single vaccine composition for the primary series and booster vaccination.
This would require harmonizing the strain composition of all COVID-19 vaccines; simplifying the immunization schedule for future vaccination campaigns to administer a two-dose series in certain young children and in older adults and persons with compromised immunity, and only one dose in all other individuals; and establishing a process for vaccine strain selection recommendations, similar in many ways to that used for seasonal influenza vaccines, based on prevailing and predicted variants that would take place by June to allow for vaccine production by September.
During the discussion, though, questions arose about the June target date. Given the production schedule for some vaccines, that date might need to shift, said Jerry Weir, PhD, director of the division of viral products at FDA’s Center for Biologics Evaluation and Research.
“We’re all just going to have to maintain flexibility,” Dr. Weir said, adding that there is not yet a “good pattern” established for updating these vaccines.
Increasing vaccination rates
There was broad consensus about the need to boost public support for COVID-19 vaccinations. While about 81% of the U.S. population has had at least one dose of this vaccine, only 15.3% have had an updated bivalent booster dose, according to the CDC.
“Anything that results in better public communication would be extremely valuable,” said committee member Henry H. Bernstein, DO, MHCM, of the Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
But it’s unclear what expectations will be prioritized for the COVID vaccine program, he said.
“Realistically, I don’t think we can have it all – less infection, less transmission, less severe disease, and less long COVID,” Dr. Bernstein said. “And that seems to be a major challenge for public messaging.”
Panelists press for more data
Other committee members also pressed for clearer targets in evaluating the goals for COVID vaccines, and for more robust data.
Like his fellow VRBPAC members, Cody Meissner, MD, of Dartmouth’s Geisel School of Medicine, Hanover, N.H., supported a move toward harmonizing the strains used in different companies’ vaccines. But he added that it wasn’t clear yet how frequently they should be administered.
“We need to see what happens with disease burden,” Dr. Meissner said. “We may or may not need annual vaccination. It’s just awfully early, it seems to me, in this process to answer that question.”
Among those serving on VRBPAC was one of the FDA’s more vocal critics on these points, Paul A. Offit, MD, a vaccine expert from Children’s Hospital of Philadelphia. Dr. Offit, for example, joined former FDA officials in writing a November opinion article for the Washington Post, arguing that the evidence for boosters for healthy younger adults was not strong.
At the Jan. 26 meeting, he supported the drive toward simplification of COVID vaccine schedules, while arguing for more data about how well these products are working.
“This virus is going to be with us for years, if not decades, and there will always be vulnerable groups who are going to be hospitalized and killed by the virus,” Dr. Offit said.
The CDC needs to provide more information about the characteristics of people being hospitalized with COVID infections, including their ages and comorbidities as well as details about their vaccine history, he said. In addition, academic researchers should provide a clearer picture of what immunological predictors are at play in increasing people’s risk from COVID.
“Then and only then can we really best make the decision about who gets vaccinated with what and when,” Dr. Offit said.
VRBPAC member Ofer Levy, MD, PhD, also urged the FDA to press for a collection of more robust and detailed information about the immune response to COVID-19 vaccinations, such as a deeper look at what’s happening with antibodies.
“I hope FDA will continue to reflect on how to best take this information forward, and encourage – or require – sponsors to gather more information in a standardized way across these different arms of the human immune system,” Dr. Levy said. “So we keep learning and keep doing this better.”
In recapping the panel’s suggestions at the end of the meeting, Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research, addressed the requests made during the day’s meeting about better data on how the vaccines work.
“We heard loud and clear that we need to use a data-driven approach to get to the simplest possible scheme that we can for vaccination,” Dr. Marks said. “And it should be as simple as possible but not oversimplified, a little bit like they say about Mozart’s music.”
A version of this article first appeared on WebMD.com.
The FDA is looking to give clearer direction to vaccine makers about future development of COVID-19 vaccines. The plan is to narrow down the current complex landscape of options for vaccinations, and thus help increase use of these shots.
COVID remains a serious threat, causing about 4,000 deaths a week recently, according to the Centers for Disease Control and Prevention. The 21 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Jan. 26 voted unanimously “yes” on a single question posed by the FDA:
“Does the committee recommend harmonizing the vaccine strain composition of primary series and booster doses in the U.S. to a single composition, e.g., the composition for all vaccines administered currently would be a bivalent vaccine (Original plus Omicron BA.4/BA.5)?”
In other words, would it be better to have one vaccine potentially combining multiple strains of the virus, instead of multiple vaccines – such as a two-shot primary series then a booster containing different combinations of viral strains.
The FDA will consider the panel’s advice as it outlines new strategies for keeping ahead of the evolving virus.
In explaining their support for the FDA plan, panel members said they hoped that a simpler regime would aid in persuading more people to get COVID vaccines.
Pamela McInnes, DDS, MSc, noted that it’s difficult to explain to many people that the vaccine works to protect them from more severe illness if they contract COVID after getting vaccinated.
“That is a real challenge,” said Dr. McInness, retired deputy director of the National Center for Advancing Translational Sciences at the National Institutes of Health.
“The message that you would have gotten more sick and landed in the hospital resonates with me, but I’m not sure if it resonates with” many people who become infected, she said.
The plan
In the briefing document for the meeting, the FDA outlined a plan for transitioning from the current complex landscape of COVID-19 vaccines to a single vaccine composition for the primary series and booster vaccination.
This would require harmonizing the strain composition of all COVID-19 vaccines; simplifying the immunization schedule for future vaccination campaigns to administer a two-dose series in certain young children and in older adults and persons with compromised immunity, and only one dose in all other individuals; and establishing a process for vaccine strain selection recommendations, similar in many ways to that used for seasonal influenza vaccines, based on prevailing and predicted variants that would take place by June to allow for vaccine production by September.
During the discussion, though, questions arose about the June target date. Given the production schedule for some vaccines, that date might need to shift, said Jerry Weir, PhD, director of the division of viral products at FDA’s Center for Biologics Evaluation and Research.
“We’re all just going to have to maintain flexibility,” Dr. Weir said, adding that there is not yet a “good pattern” established for updating these vaccines.
Increasing vaccination rates
There was broad consensus about the need to boost public support for COVID-19 vaccinations. While about 81% of the U.S. population has had at least one dose of this vaccine, only 15.3% have had an updated bivalent booster dose, according to the CDC.
“Anything that results in better public communication would be extremely valuable,” said committee member Henry H. Bernstein, DO, MHCM, of the Zucker School of Medicine at Hofstra/Northwell Health in Hempstead, N.Y.
But it’s unclear what expectations will be prioritized for the COVID vaccine program, he said.
“Realistically, I don’t think we can have it all – less infection, less transmission, less severe disease, and less long COVID,” Dr. Bernstein said. “And that seems to be a major challenge for public messaging.”
Panelists press for more data
Other committee members also pressed for clearer targets in evaluating the goals for COVID vaccines, and for more robust data.
Like his fellow VRBPAC members, Cody Meissner, MD, of Dartmouth’s Geisel School of Medicine, Hanover, N.H., supported a move toward harmonizing the strains used in different companies’ vaccines. But he added that it wasn’t clear yet how frequently they should be administered.
“We need to see what happens with disease burden,” Dr. Meissner said. “We may or may not need annual vaccination. It’s just awfully early, it seems to me, in this process to answer that question.”
Among those serving on VRBPAC was one of the FDA’s more vocal critics on these points, Paul A. Offit, MD, a vaccine expert from Children’s Hospital of Philadelphia. Dr. Offit, for example, joined former FDA officials in writing a November opinion article for the Washington Post, arguing that the evidence for boosters for healthy younger adults was not strong.
At the Jan. 26 meeting, he supported the drive toward simplification of COVID vaccine schedules, while arguing for more data about how well these products are working.
“This virus is going to be with us for years, if not decades, and there will always be vulnerable groups who are going to be hospitalized and killed by the virus,” Dr. Offit said.
The CDC needs to provide more information about the characteristics of people being hospitalized with COVID infections, including their ages and comorbidities as well as details about their vaccine history, he said. In addition, academic researchers should provide a clearer picture of what immunological predictors are at play in increasing people’s risk from COVID.
“Then and only then can we really best make the decision about who gets vaccinated with what and when,” Dr. Offit said.
VRBPAC member Ofer Levy, MD, PhD, also urged the FDA to press for a collection of more robust and detailed information about the immune response to COVID-19 vaccinations, such as a deeper look at what’s happening with antibodies.
“I hope FDA will continue to reflect on how to best take this information forward, and encourage – or require – sponsors to gather more information in a standardized way across these different arms of the human immune system,” Dr. Levy said. “So we keep learning and keep doing this better.”
In recapping the panel’s suggestions at the end of the meeting, Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research, addressed the requests made during the day’s meeting about better data on how the vaccines work.
“We heard loud and clear that we need to use a data-driven approach to get to the simplest possible scheme that we can for vaccination,” Dr. Marks said. “And it should be as simple as possible but not oversimplified, a little bit like they say about Mozart’s music.”
A version of this article first appeared on WebMD.com.
75 years: A look back on the fascinating history of methotrexate and folate antagonists
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
Meta-analysis reveals that most atopic dermatitis therapies are effective against pruritus
Key clinical point: The majority of topical and systemic therapies for atopic dermatitis (AD) effectively reduced pruritus, the most common patient-reported symptom.
Major finding: Topical and systemic treatments led to a mean reduction of 3.32 (99% CI 2.32-4.33) and 3.07 (99% CI 2.58-3.56) points in pruritus score, respectively. Wet-wrap therapy using halometasone (−4.75 points) was the most effective topical treatment, and 30 mg upadacitinib (−4.90 points) was the most effective systemic treatment.
Study details: This study analyzed 22 studies that included patients aged ≥ 10 years with AD who received topical or systemic treatments.
Disclosures: No information on the source of funding was provided. Two authors reported ties with various organizations.
Source: Rodriguez-Le Roy Y et al. Efficacy of topical and systemic treatments for atopic dermatitis on pruritus: A systematic literature review and meta-analysis. Front Med (Lausanne). 2022;9:1079323 (Dec 22). Doi: 10.3389/fmed.2022.1079323
Key clinical point: The majority of topical and systemic therapies for atopic dermatitis (AD) effectively reduced pruritus, the most common patient-reported symptom.
Major finding: Topical and systemic treatments led to a mean reduction of 3.32 (99% CI 2.32-4.33) and 3.07 (99% CI 2.58-3.56) points in pruritus score, respectively. Wet-wrap therapy using halometasone (−4.75 points) was the most effective topical treatment, and 30 mg upadacitinib (−4.90 points) was the most effective systemic treatment.
Study details: This study analyzed 22 studies that included patients aged ≥ 10 years with AD who received topical or systemic treatments.
Disclosures: No information on the source of funding was provided. Two authors reported ties with various organizations.
Source: Rodriguez-Le Roy Y et al. Efficacy of topical and systemic treatments for atopic dermatitis on pruritus: A systematic literature review and meta-analysis. Front Med (Lausanne). 2022;9:1079323 (Dec 22). Doi: 10.3389/fmed.2022.1079323
Key clinical point: The majority of topical and systemic therapies for atopic dermatitis (AD) effectively reduced pruritus, the most common patient-reported symptom.
Major finding: Topical and systemic treatments led to a mean reduction of 3.32 (99% CI 2.32-4.33) and 3.07 (99% CI 2.58-3.56) points in pruritus score, respectively. Wet-wrap therapy using halometasone (−4.75 points) was the most effective topical treatment, and 30 mg upadacitinib (−4.90 points) was the most effective systemic treatment.
Study details: This study analyzed 22 studies that included patients aged ≥ 10 years with AD who received topical or systemic treatments.
Disclosures: No information on the source of funding was provided. Two authors reported ties with various organizations.
Source: Rodriguez-Le Roy Y et al. Efficacy of topical and systemic treatments for atopic dermatitis on pruritus: A systematic literature review and meta-analysis. Front Med (Lausanne). 2022;9:1079323 (Dec 22). Doi: 10.3389/fmed.2022.1079323