User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Study Evaluates Safety of Benzoyl Peroxide Products for Acne
according to results from an analysis that used gas chromatography–mass spectrometry and other methods.
The analysis, which was published in the Journal of Investigative Dermatology and expands on a similar study released more than 6 months ago, also found that encapsulated BPO products break down into benzene at room temperature but that refrigerating them may mitigate this effect.
“Our research provides the first experimental evidence that cold storage can help reduce the rate of benzoyl peroxide breakdown into benzene,” said one of the study authors, Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Connecticut. “Therefore, cold storage throughout the entire supply chain — from manufacturing to patient use — is a reasonable and proportional measure at this time for those continuing to use benzoyl peroxide medicine.” One acne product, the newer prescription triple-combination therapy (adapalene-clindamycin-BPO) “already has a cold shipping process in place; the patient just needs to continue that at home,” he noted.
For the study — which was funded by an independent lab, Valisure — researchers led by Valisure CEO and founder David Light, used gas chromatography-mass spectrometry to detect benzene levels in 111 BPO drug products from major US retailers and selected ion flow tube mass-spectrometry to quantify the release of benzene in real time. Benzene levels ranged from 0.16 ppm to 35.30 ppm, and 38 of the products (34%) had levels above the FDA limit of 2 ppm for drug products. “The results of the products sampled in this study suggest that formulation is likely the strongest contributor to benzene concentrations in BPO drug products that are commercially available, since the magnitude of benzene detected correlates most closely with specific brands or product types within certain brands,” the study authors wrote.
When the researchers tested the stability of a prescription encapsulated BPO drug product at cold (2 °C) and elevated temperature (50 °C), no apparent benzene formation was observed at 2 °C, whereas high levels of benzene formed at 50 °C, “suggesting that encapsulation technology may not stabilize BPO drug products, but cold storage may greatly reduce benzene formation,” they wrote.
In another component of the study, researchers exposed a BP drug product to a UVA/UVB lamp for 2 hours and found detectable benzene through evaporation and substantial benzene formation when exposed to UV light at levels below peak sunlight. The experiment “strongly justifies the package label warnings to avoid sun exposure when using BPO drug products,” the authors wrote. “Further evaluation to determine the influence of sun exposure on BPO drug product degradation and benzene formation is warranted.”
In an interview, John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, Massachusetts characterized the findings as “an important issue that we should take seriously.” However, “we also must not overreact.”
BPO is a foundational acne treatment without any clear alternative, he said, pointing out that no evidence currently exists “to support that routine use of benzoyl peroxide–containing products for acne is associated with a meaningful risk of benzene in the blood or an increased risk of cancer.”
And although it is prudent to minimize benzene exposure as much as possible, Barbieri continued, “it is not clear that these levels are a clinically meaningful incremental risk in the setting of an acne cream or wash. There is minimal cutaneous absorption of benzene, and it is uncertain how much benzene aerosolizes with routine use, particularly for washes which are not left on the skin.”
Bunick said that the combined data from this and the study published in March 2024 affected which BPO products he recommends for patients with acne. “I am using exclusively the triple combination therapy (adapalene-clindamycin-benzoyl peroxide) because I know it has the necessary cold supply chain in place to protect the product’s stability. I further encourage patients to place all their benzoyl peroxide–containing products in the refrigerator at home to reduce benzene formation and exposure.”
Bunick reported having served as an investigator and/or a consultant/speaker for many pharmaceutical companies, including as a consultant for Ortho-Dermatologics; but none related to this study. Barbieri reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to results from an analysis that used gas chromatography–mass spectrometry and other methods.
The analysis, which was published in the Journal of Investigative Dermatology and expands on a similar study released more than 6 months ago, also found that encapsulated BPO products break down into benzene at room temperature but that refrigerating them may mitigate this effect.
“Our research provides the first experimental evidence that cold storage can help reduce the rate of benzoyl peroxide breakdown into benzene,” said one of the study authors, Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Connecticut. “Therefore, cold storage throughout the entire supply chain — from manufacturing to patient use — is a reasonable and proportional measure at this time for those continuing to use benzoyl peroxide medicine.” One acne product, the newer prescription triple-combination therapy (adapalene-clindamycin-BPO) “already has a cold shipping process in place; the patient just needs to continue that at home,” he noted.
For the study — which was funded by an independent lab, Valisure — researchers led by Valisure CEO and founder David Light, used gas chromatography-mass spectrometry to detect benzene levels in 111 BPO drug products from major US retailers and selected ion flow tube mass-spectrometry to quantify the release of benzene in real time. Benzene levels ranged from 0.16 ppm to 35.30 ppm, and 38 of the products (34%) had levels above the FDA limit of 2 ppm for drug products. “The results of the products sampled in this study suggest that formulation is likely the strongest contributor to benzene concentrations in BPO drug products that are commercially available, since the magnitude of benzene detected correlates most closely with specific brands or product types within certain brands,” the study authors wrote.
When the researchers tested the stability of a prescription encapsulated BPO drug product at cold (2 °C) and elevated temperature (50 °C), no apparent benzene formation was observed at 2 °C, whereas high levels of benzene formed at 50 °C, “suggesting that encapsulation technology may not stabilize BPO drug products, but cold storage may greatly reduce benzene formation,” they wrote.
In another component of the study, researchers exposed a BP drug product to a UVA/UVB lamp for 2 hours and found detectable benzene through evaporation and substantial benzene formation when exposed to UV light at levels below peak sunlight. The experiment “strongly justifies the package label warnings to avoid sun exposure when using BPO drug products,” the authors wrote. “Further evaluation to determine the influence of sun exposure on BPO drug product degradation and benzene formation is warranted.”
In an interview, John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, Massachusetts characterized the findings as “an important issue that we should take seriously.” However, “we also must not overreact.”
BPO is a foundational acne treatment without any clear alternative, he said, pointing out that no evidence currently exists “to support that routine use of benzoyl peroxide–containing products for acne is associated with a meaningful risk of benzene in the blood or an increased risk of cancer.”
And although it is prudent to minimize benzene exposure as much as possible, Barbieri continued, “it is not clear that these levels are a clinically meaningful incremental risk in the setting of an acne cream or wash. There is minimal cutaneous absorption of benzene, and it is uncertain how much benzene aerosolizes with routine use, particularly for washes which are not left on the skin.”
Bunick said that the combined data from this and the study published in March 2024 affected which BPO products he recommends for patients with acne. “I am using exclusively the triple combination therapy (adapalene-clindamycin-benzoyl peroxide) because I know it has the necessary cold supply chain in place to protect the product’s stability. I further encourage patients to place all their benzoyl peroxide–containing products in the refrigerator at home to reduce benzene formation and exposure.”
Bunick reported having served as an investigator and/or a consultant/speaker for many pharmaceutical companies, including as a consultant for Ortho-Dermatologics; but none related to this study. Barbieri reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to results from an analysis that used gas chromatography–mass spectrometry and other methods.
The analysis, which was published in the Journal of Investigative Dermatology and expands on a similar study released more than 6 months ago, also found that encapsulated BPO products break down into benzene at room temperature but that refrigerating them may mitigate this effect.
“Our research provides the first experimental evidence that cold storage can help reduce the rate of benzoyl peroxide breakdown into benzene,” said one of the study authors, Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Connecticut. “Therefore, cold storage throughout the entire supply chain — from manufacturing to patient use — is a reasonable and proportional measure at this time for those continuing to use benzoyl peroxide medicine.” One acne product, the newer prescription triple-combination therapy (adapalene-clindamycin-BPO) “already has a cold shipping process in place; the patient just needs to continue that at home,” he noted.
For the study — which was funded by an independent lab, Valisure — researchers led by Valisure CEO and founder David Light, used gas chromatography-mass spectrometry to detect benzene levels in 111 BPO drug products from major US retailers and selected ion flow tube mass-spectrometry to quantify the release of benzene in real time. Benzene levels ranged from 0.16 ppm to 35.30 ppm, and 38 of the products (34%) had levels above the FDA limit of 2 ppm for drug products. “The results of the products sampled in this study suggest that formulation is likely the strongest contributor to benzene concentrations in BPO drug products that are commercially available, since the magnitude of benzene detected correlates most closely with specific brands or product types within certain brands,” the study authors wrote.
When the researchers tested the stability of a prescription encapsulated BPO drug product at cold (2 °C) and elevated temperature (50 °C), no apparent benzene formation was observed at 2 °C, whereas high levels of benzene formed at 50 °C, “suggesting that encapsulation technology may not stabilize BPO drug products, but cold storage may greatly reduce benzene formation,” they wrote.
In another component of the study, researchers exposed a BP drug product to a UVA/UVB lamp for 2 hours and found detectable benzene through evaporation and substantial benzene formation when exposed to UV light at levels below peak sunlight. The experiment “strongly justifies the package label warnings to avoid sun exposure when using BPO drug products,” the authors wrote. “Further evaluation to determine the influence of sun exposure on BPO drug product degradation and benzene formation is warranted.”
In an interview, John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, Massachusetts characterized the findings as “an important issue that we should take seriously.” However, “we also must not overreact.”
BPO is a foundational acne treatment without any clear alternative, he said, pointing out that no evidence currently exists “to support that routine use of benzoyl peroxide–containing products for acne is associated with a meaningful risk of benzene in the blood or an increased risk of cancer.”
And although it is prudent to minimize benzene exposure as much as possible, Barbieri continued, “it is not clear that these levels are a clinically meaningful incremental risk in the setting of an acne cream or wash. There is minimal cutaneous absorption of benzene, and it is uncertain how much benzene aerosolizes with routine use, particularly for washes which are not left on the skin.”
Bunick said that the combined data from this and the study published in March 2024 affected which BPO products he recommends for patients with acne. “I am using exclusively the triple combination therapy (adapalene-clindamycin-benzoyl peroxide) because I know it has the necessary cold supply chain in place to protect the product’s stability. I further encourage patients to place all their benzoyl peroxide–containing products in the refrigerator at home to reduce benzene formation and exposure.”
Bunick reported having served as an investigator and/or a consultant/speaker for many pharmaceutical companies, including as a consultant for Ortho-Dermatologics; but none related to this study. Barbieri reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Utilization, Cost, and Prescription Trends of Antipsychotics Prescribed by Dermatologists for Medicare Patients
To the Editor:
Patients with primary psychiatric disorders with dermatologic manifestations often seek treatment from dermatologists instead of psychiatrists.1 For example, patients with delusions of parasitosis may lack insight into the underlying etiology of their disease and instead fixate on establishing an organic cause for their symptoms. As a result, it is an increasingly common practice for dermatologists to diagnose and treat psychiatric conditions.1 The goal of this study was to evaluate trends for the top 5 antipsychotics most frequently prescribed by dermatologists in the Medicare Part D database.
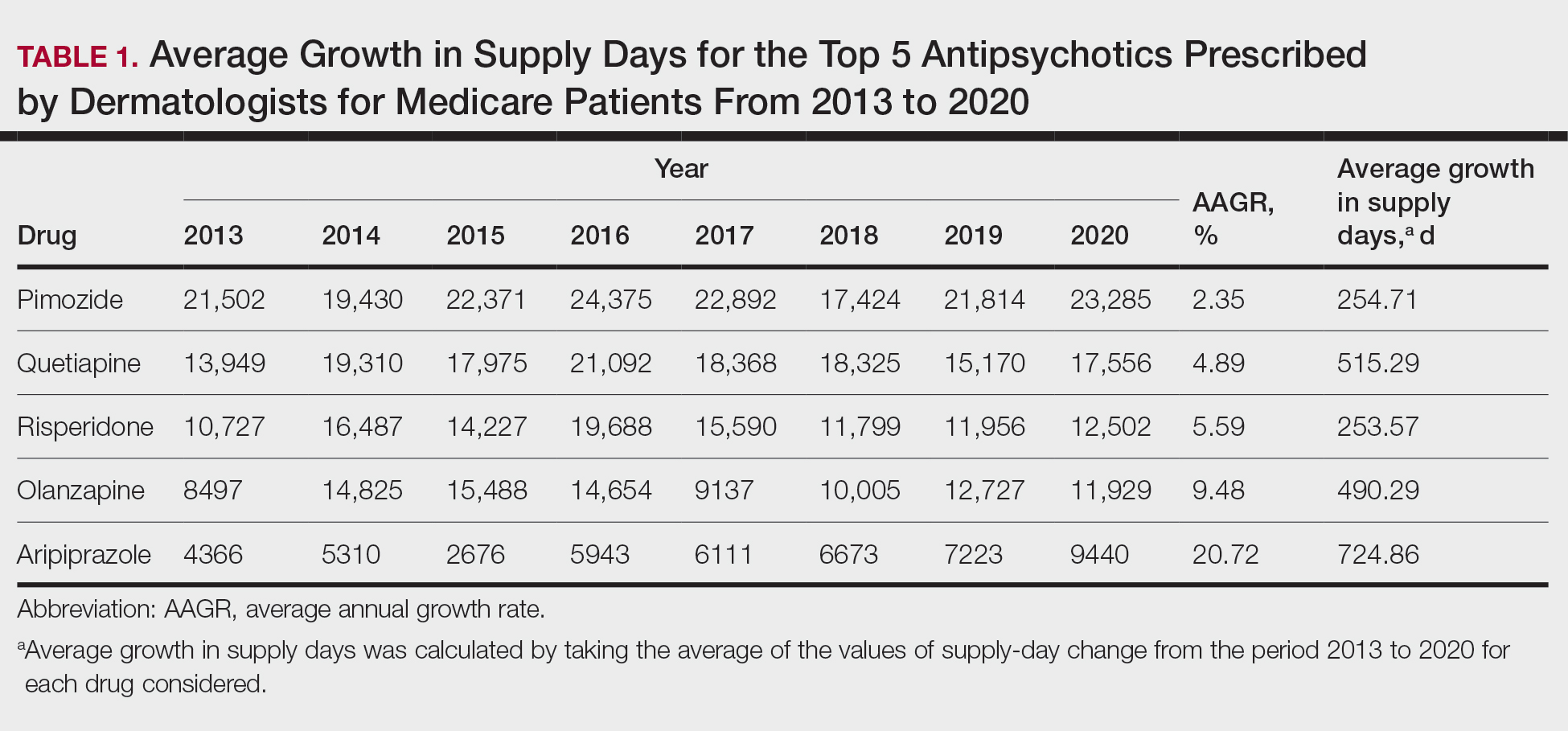
In this retrospective analysis, we consulted the Medicare Provider Utilization and Payment Data for January 2013 through December 2020, which is provided to the public by the Centers for Medicare & Medicaid Services.2 Only prescribing data from dermatologists were included in this study by using the built-in filter on the website to select “dermatology” as the prescriber type. All other provider types were excluded. We chose the top 5 most prescribed antipsychotics based on the number of supply days reported. Supply days—defined by Medicare as the number of days’ worth of medication that is prescribed—were used as a metric for utilization; therefore, each drug’s total supply days prescribed by dermatologists were calculated using this combined filter of drug name and total supply days using the database.
To analyze utilization over time, the annual average growth rate (AAGR) was calculated by determining the growth rate in total supply days annually from 2013 to 2020 and then averaging those rates to determine the overall AAGR. For greater clinical relevance, we calculated the average growth in supply days for the entire study period by determining the difference in the number of supply days for each year and then averaging these values. This was done to consider overall trends across dermatology rather than individual dermatologist prescribing patterns.
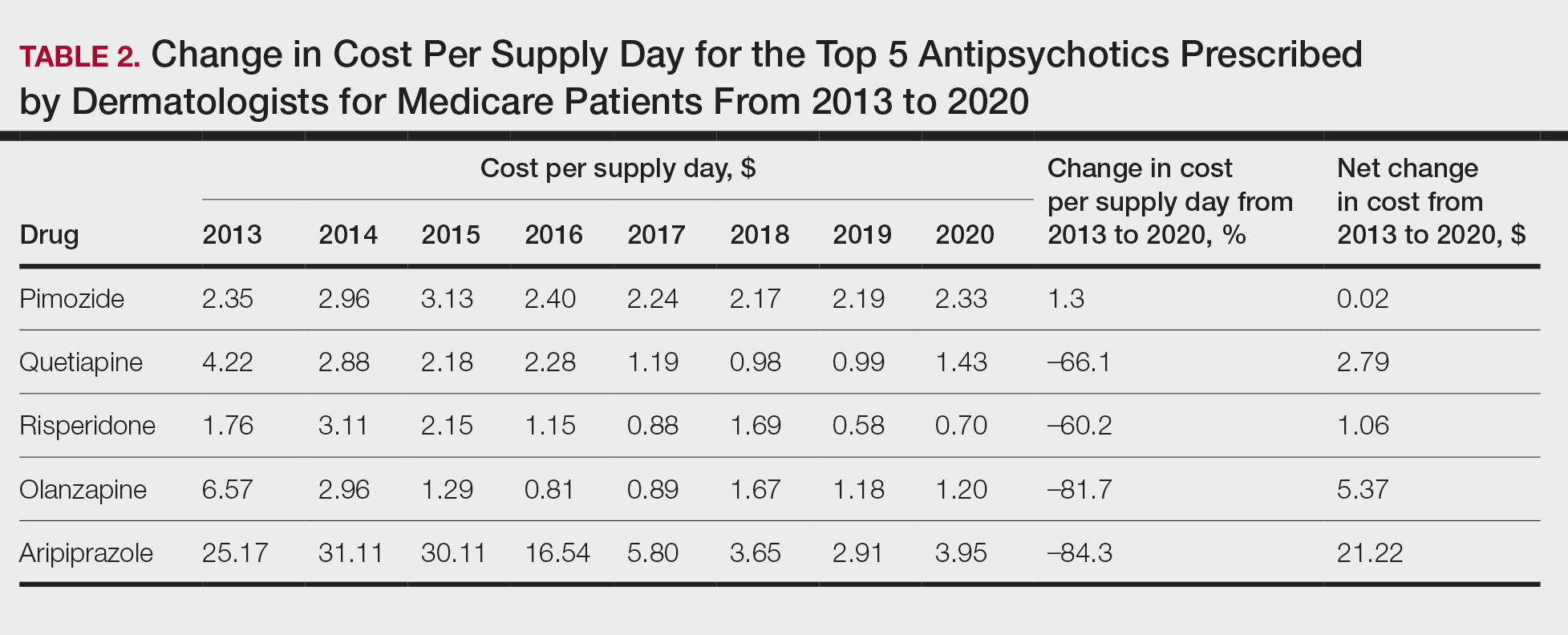
Based on our analysis, the antipsychotics most frequently prescribed by dermatologists for Medicare patients from January 2013 to December 2020 were pimozide, quetiapine, risperidone, olanzapine, and aripiprazole. The AAGR for each drug was 2.35%, 4.89%, 5.59%, 9.48%, and 20.72%, respectively, which is consistent with increased utilization over the study period for all 5 drugs (Table 1). The change in cost per supply day for the same period was 1.3%, –66.1%, –60.2%, –81.7%, and –84.3%, respectively. The net difference in cost per supply day over this entire period was $0.02, –$2.79, –$1.06, –$5.37, and –$21.22, respectively (Table 2).
There were several limitations to our study. Our analysis was limited to the Medicare population. Uninsured patients and those with Medicare Advantage or private health insurance plans were not included. In the Medicare database, only prescribers who prescribed a medication 10 times or more were recorded; therefore, some prescribers were not captured.
Although there was an increase in the dermatologic use of all 5 drugs in this study, perhaps the most marked growth was exhibited by aripiprazole, which had an AAGR of 20.72% (Table 1). Affordability may have been a factor, as the most marked reduction in price per supply day was noted for aripiprazole during the study period. Pimozide, which traditionally has been the first-line therapy for delusions of parasitosis, is the only first-generation antipsychotic drug among the 5 most frequently prescribed antipsychotics.3 Interestingly, pimozide had the lowest AAGR compared with the 4 second-generation antipsychotics. This finding also is corroborated by the average growth in supply days. While pimozide is a first-generation antipsychotic and had the lowest AAGR, pimozide still was the most prescribed antipsychotic in this study. Considering the average growth in Medicare beneficiaries during the study period was 2.70% per year,2 the AAGR of the 4 other drugs excluding pimozide shows that this growth was larger than what can be attributed to an increase in population size.
The most common conditions for which dermatologists prescribe antipsychotics are primary delusional infestation disorders as well as a range of self-inflicted dermatologic manifestations of dermatitis artefacta.4 Particularly, dermatologist-prescribed antipsychotics are first-line for these conditions in which perception of a persistent disease state is present.4 Importantly, dermatologists must differentiate between other dermatology-related psychiatric conditions such as trichotillomania and body dysmorphic disorder, which tend to respond better to selective serotonin reuptake inhibitors.4 Our data suggest that dermatologists are increasing their utilization of second-generation antipsychotics at a higher rate than first-generation antipsychotics, likely due to the lower risk of extrapyramidal symptoms. Patients are more willing to initiate a trial of psychiatric medication when it is prescribed by a dermatologist vs a psychiatrist due to lack of perceived stigma, which can lead to greater treatment compliance rates.5 As mentioned previously, as part of the differential, dermatologists also can effectively prescribe medications such as selective serotonin reuptake inhibitors for symptoms including anxiety, trichotillomania, body dysmorphic disorder, or secondary psychiatric disorders as a result of the burden of skin disease.5
In many cases, a dermatologist may be the first and only specialist to evaluate patients with conditions that overlap within the jurisdiction of dermatology and psychiatry. It is imperative that dermatologists feel comfortable treating this vulnerable patient population. As demonstrated by Medicare prescription data, the increasing utilization of antipsychotics in our specialty demands that dermatologists possess an adequate working knowledge of psychopharmacology, which may be accomplished during residency training through several directives, including focused didactic sessions, elective rotations in psychiatry, increased exposure to psychocutaneous lectures at national conferences, and finally through the establishment of joint dermatology-psychiatry clinics with interdepartmental collaboration.
- Weber MB, Recuero JK, Almeida CS. Use of psychiatric drugs in dermatology. An Bras Dermatol. 2020;95:133-143. doi:10.1016/j.abd.2019.12.002
- Centers for Medicare & Medicaid Services. Medicare provider utilization and payment data: part D prescriber. Updated September 10, 2024. Accessed October 7, 2024. https://www.cms.gov/data -research/statistics-trends-and-reports/medicare-provider-utilization-payment-data/part-d-prescriber
- Bolognia J, Schaffe JV, Lorenzo C. Dermatology. In: Duncan KO, Koo JYM, eds. Psychocutaneous Diseases. Elsevier; 2017:128-136.
- Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi:10.1016/j.clindermatol.2018.08.006
- Jafferany M, Stamu-O’Brien C, Mkhoyan R, et al. Psychotropic drugs in dermatology: a dermatologist’s approach and choice of medications. Dermatol Ther. 2020;33:E13385. doi:10.1111/dth.13385
To the Editor:
Patients with primary psychiatric disorders with dermatologic manifestations often seek treatment from dermatologists instead of psychiatrists.1 For example, patients with delusions of parasitosis may lack insight into the underlying etiology of their disease and instead fixate on establishing an organic cause for their symptoms. As a result, it is an increasingly common practice for dermatologists to diagnose and treat psychiatric conditions.1 The goal of this study was to evaluate trends for the top 5 antipsychotics most frequently prescribed by dermatologists in the Medicare Part D database.
In this retrospective analysis, we consulted the Medicare Provider Utilization and Payment Data for January 2013 through December 2020, which is provided to the public by the Centers for Medicare & Medicaid Services.2 Only prescribing data from dermatologists were included in this study by using the built-in filter on the website to select “dermatology” as the prescriber type. All other provider types were excluded. We chose the top 5 most prescribed antipsychotics based on the number of supply days reported. Supply days—defined by Medicare as the number of days’ worth of medication that is prescribed—were used as a metric for utilization; therefore, each drug’s total supply days prescribed by dermatologists were calculated using this combined filter of drug name and total supply days using the database.
To analyze utilization over time, the annual average growth rate (AAGR) was calculated by determining the growth rate in total supply days annually from 2013 to 2020 and then averaging those rates to determine the overall AAGR. For greater clinical relevance, we calculated the average growth in supply days for the entire study period by determining the difference in the number of supply days for each year and then averaging these values. This was done to consider overall trends across dermatology rather than individual dermatologist prescribing patterns.
Based on our analysis, the antipsychotics most frequently prescribed by dermatologists for Medicare patients from January 2013 to December 2020 were pimozide, quetiapine, risperidone, olanzapine, and aripiprazole. The AAGR for each drug was 2.35%, 4.89%, 5.59%, 9.48%, and 20.72%, respectively, which is consistent with increased utilization over the study period for all 5 drugs (Table 1). The change in cost per supply day for the same period was 1.3%, –66.1%, –60.2%, –81.7%, and –84.3%, respectively. The net difference in cost per supply day over this entire period was $0.02, –$2.79, –$1.06, –$5.37, and –$21.22, respectively (Table 2).


There were several limitations to our study. Our analysis was limited to the Medicare population. Uninsured patients and those with Medicare Advantage or private health insurance plans were not included. In the Medicare database, only prescribers who prescribed a medication 10 times or more were recorded; therefore, some prescribers were not captured.
Although there was an increase in the dermatologic use of all 5 drugs in this study, perhaps the most marked growth was exhibited by aripiprazole, which had an AAGR of 20.72% (Table 1). Affordability may have been a factor, as the most marked reduction in price per supply day was noted for aripiprazole during the study period. Pimozide, which traditionally has been the first-line therapy for delusions of parasitosis, is the only first-generation antipsychotic drug among the 5 most frequently prescribed antipsychotics.3 Interestingly, pimozide had the lowest AAGR compared with the 4 second-generation antipsychotics. This finding also is corroborated by the average growth in supply days. While pimozide is a first-generation antipsychotic and had the lowest AAGR, pimozide still was the most prescribed antipsychotic in this study. Considering the average growth in Medicare beneficiaries during the study period was 2.70% per year,2 the AAGR of the 4 other drugs excluding pimozide shows that this growth was larger than what can be attributed to an increase in population size.
The most common conditions for which dermatologists prescribe antipsychotics are primary delusional infestation disorders as well as a range of self-inflicted dermatologic manifestations of dermatitis artefacta.4 Particularly, dermatologist-prescribed antipsychotics are first-line for these conditions in which perception of a persistent disease state is present.4 Importantly, dermatologists must differentiate between other dermatology-related psychiatric conditions such as trichotillomania and body dysmorphic disorder, which tend to respond better to selective serotonin reuptake inhibitors.4 Our data suggest that dermatologists are increasing their utilization of second-generation antipsychotics at a higher rate than first-generation antipsychotics, likely due to the lower risk of extrapyramidal symptoms. Patients are more willing to initiate a trial of psychiatric medication when it is prescribed by a dermatologist vs a psychiatrist due to lack of perceived stigma, which can lead to greater treatment compliance rates.5 As mentioned previously, as part of the differential, dermatologists also can effectively prescribe medications such as selective serotonin reuptake inhibitors for symptoms including anxiety, trichotillomania, body dysmorphic disorder, or secondary psychiatric disorders as a result of the burden of skin disease.5
In many cases, a dermatologist may be the first and only specialist to evaluate patients with conditions that overlap within the jurisdiction of dermatology and psychiatry. It is imperative that dermatologists feel comfortable treating this vulnerable patient population. As demonstrated by Medicare prescription data, the increasing utilization of antipsychotics in our specialty demands that dermatologists possess an adequate working knowledge of psychopharmacology, which may be accomplished during residency training through several directives, including focused didactic sessions, elective rotations in psychiatry, increased exposure to psychocutaneous lectures at national conferences, and finally through the establishment of joint dermatology-psychiatry clinics with interdepartmental collaboration.
To the Editor:
Patients with primary psychiatric disorders with dermatologic manifestations often seek treatment from dermatologists instead of psychiatrists.1 For example, patients with delusions of parasitosis may lack insight into the underlying etiology of their disease and instead fixate on establishing an organic cause for their symptoms. As a result, it is an increasingly common practice for dermatologists to diagnose and treat psychiatric conditions.1 The goal of this study was to evaluate trends for the top 5 antipsychotics most frequently prescribed by dermatologists in the Medicare Part D database.
In this retrospective analysis, we consulted the Medicare Provider Utilization and Payment Data for January 2013 through December 2020, which is provided to the public by the Centers for Medicare & Medicaid Services.2 Only prescribing data from dermatologists were included in this study by using the built-in filter on the website to select “dermatology” as the prescriber type. All other provider types were excluded. We chose the top 5 most prescribed antipsychotics based on the number of supply days reported. Supply days—defined by Medicare as the number of days’ worth of medication that is prescribed—were used as a metric for utilization; therefore, each drug’s total supply days prescribed by dermatologists were calculated using this combined filter of drug name and total supply days using the database.
To analyze utilization over time, the annual average growth rate (AAGR) was calculated by determining the growth rate in total supply days annually from 2013 to 2020 and then averaging those rates to determine the overall AAGR. For greater clinical relevance, we calculated the average growth in supply days for the entire study period by determining the difference in the number of supply days for each year and then averaging these values. This was done to consider overall trends across dermatology rather than individual dermatologist prescribing patterns.
Based on our analysis, the antipsychotics most frequently prescribed by dermatologists for Medicare patients from January 2013 to December 2020 were pimozide, quetiapine, risperidone, olanzapine, and aripiprazole. The AAGR for each drug was 2.35%, 4.89%, 5.59%, 9.48%, and 20.72%, respectively, which is consistent with increased utilization over the study period for all 5 drugs (Table 1). The change in cost per supply day for the same period was 1.3%, –66.1%, –60.2%, –81.7%, and –84.3%, respectively. The net difference in cost per supply day over this entire period was $0.02, –$2.79, –$1.06, –$5.37, and –$21.22, respectively (Table 2).


There were several limitations to our study. Our analysis was limited to the Medicare population. Uninsured patients and those with Medicare Advantage or private health insurance plans were not included. In the Medicare database, only prescribers who prescribed a medication 10 times or more were recorded; therefore, some prescribers were not captured.
Although there was an increase in the dermatologic use of all 5 drugs in this study, perhaps the most marked growth was exhibited by aripiprazole, which had an AAGR of 20.72% (Table 1). Affordability may have been a factor, as the most marked reduction in price per supply day was noted for aripiprazole during the study period. Pimozide, which traditionally has been the first-line therapy for delusions of parasitosis, is the only first-generation antipsychotic drug among the 5 most frequently prescribed antipsychotics.3 Interestingly, pimozide had the lowest AAGR compared with the 4 second-generation antipsychotics. This finding also is corroborated by the average growth in supply days. While pimozide is a first-generation antipsychotic and had the lowest AAGR, pimozide still was the most prescribed antipsychotic in this study. Considering the average growth in Medicare beneficiaries during the study period was 2.70% per year,2 the AAGR of the 4 other drugs excluding pimozide shows that this growth was larger than what can be attributed to an increase in population size.
The most common conditions for which dermatologists prescribe antipsychotics are primary delusional infestation disorders as well as a range of self-inflicted dermatologic manifestations of dermatitis artefacta.4 Particularly, dermatologist-prescribed antipsychotics are first-line for these conditions in which perception of a persistent disease state is present.4 Importantly, dermatologists must differentiate between other dermatology-related psychiatric conditions such as trichotillomania and body dysmorphic disorder, which tend to respond better to selective serotonin reuptake inhibitors.4 Our data suggest that dermatologists are increasing their utilization of second-generation antipsychotics at a higher rate than first-generation antipsychotics, likely due to the lower risk of extrapyramidal symptoms. Patients are more willing to initiate a trial of psychiatric medication when it is prescribed by a dermatologist vs a psychiatrist due to lack of perceived stigma, which can lead to greater treatment compliance rates.5 As mentioned previously, as part of the differential, dermatologists also can effectively prescribe medications such as selective serotonin reuptake inhibitors for symptoms including anxiety, trichotillomania, body dysmorphic disorder, or secondary psychiatric disorders as a result of the burden of skin disease.5
In many cases, a dermatologist may be the first and only specialist to evaluate patients with conditions that overlap within the jurisdiction of dermatology and psychiatry. It is imperative that dermatologists feel comfortable treating this vulnerable patient population. As demonstrated by Medicare prescription data, the increasing utilization of antipsychotics in our specialty demands that dermatologists possess an adequate working knowledge of psychopharmacology, which may be accomplished during residency training through several directives, including focused didactic sessions, elective rotations in psychiatry, increased exposure to psychocutaneous lectures at national conferences, and finally through the establishment of joint dermatology-psychiatry clinics with interdepartmental collaboration.
- Weber MB, Recuero JK, Almeida CS. Use of psychiatric drugs in dermatology. An Bras Dermatol. 2020;95:133-143. doi:10.1016/j.abd.2019.12.002
- Centers for Medicare & Medicaid Services. Medicare provider utilization and payment data: part D prescriber. Updated September 10, 2024. Accessed October 7, 2024. https://www.cms.gov/data -research/statistics-trends-and-reports/medicare-provider-utilization-payment-data/part-d-prescriber
- Bolognia J, Schaffe JV, Lorenzo C. Dermatology. In: Duncan KO, Koo JYM, eds. Psychocutaneous Diseases. Elsevier; 2017:128-136.
- Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi:10.1016/j.clindermatol.2018.08.006
- Jafferany M, Stamu-O’Brien C, Mkhoyan R, et al. Psychotropic drugs in dermatology: a dermatologist’s approach and choice of medications. Dermatol Ther. 2020;33:E13385. doi:10.1111/dth.13385
- Weber MB, Recuero JK, Almeida CS. Use of psychiatric drugs in dermatology. An Bras Dermatol. 2020;95:133-143. doi:10.1016/j.abd.2019.12.002
- Centers for Medicare & Medicaid Services. Medicare provider utilization and payment data: part D prescriber. Updated September 10, 2024. Accessed October 7, 2024. https://www.cms.gov/data -research/statistics-trends-and-reports/medicare-provider-utilization-payment-data/part-d-prescriber
- Bolognia J, Schaffe JV, Lorenzo C. Dermatology. In: Duncan KO, Koo JYM, eds. Psychocutaneous Diseases. Elsevier; 2017:128-136.
- Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi:10.1016/j.clindermatol.2018.08.006
- Jafferany M, Stamu-O’Brien C, Mkhoyan R, et al. Psychotropic drugs in dermatology: a dermatologist’s approach and choice of medications. Dermatol Ther. 2020;33:E13385. doi:10.1111/dth.13385
Practice Points
- Dermatologists are frontline medical providers who can be useful in screening for primary psychiatric disorders in patients with dermatologic manifestations.
- Second-generation antipsychotics are effective for treating many psychiatric disorders.
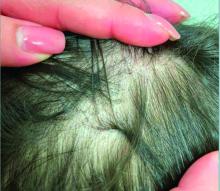
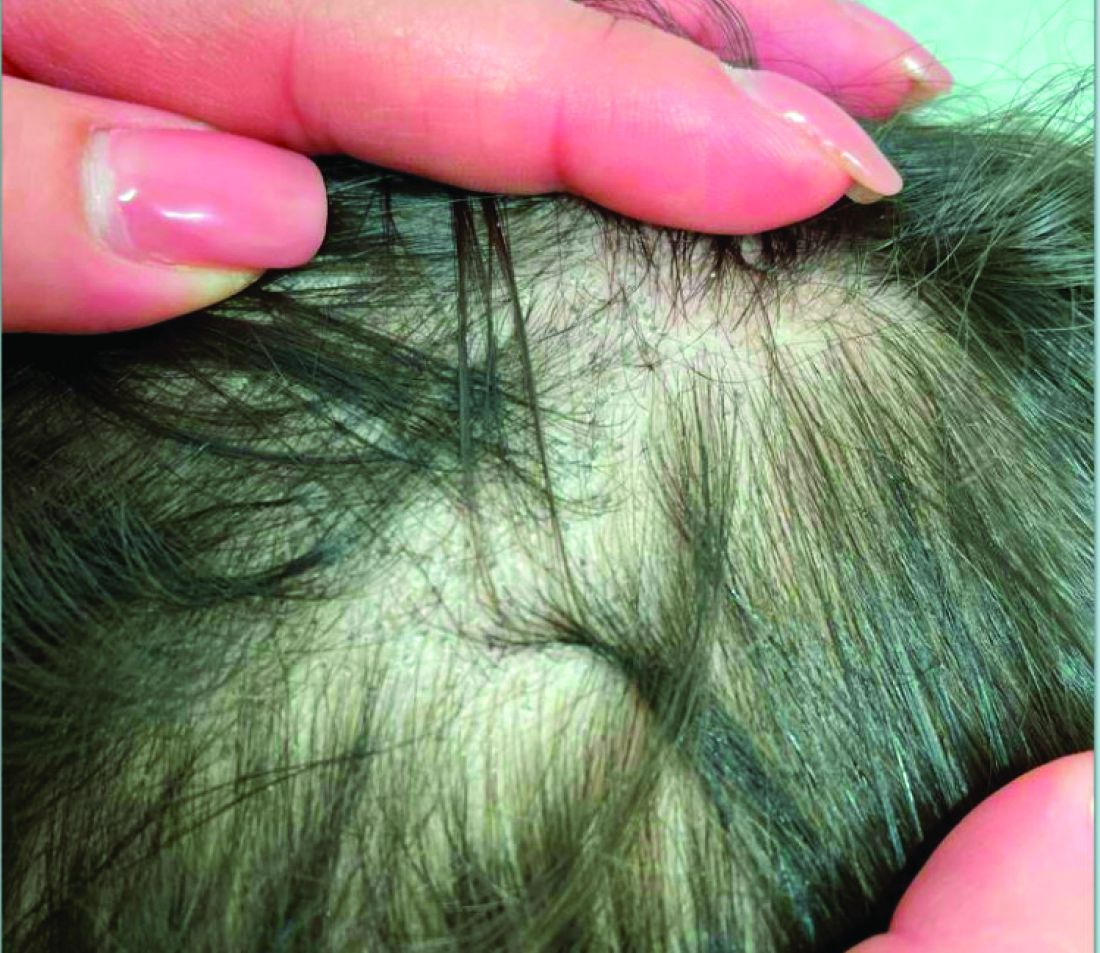
A 7-Year-Old Boy Presents With Dark Spots on His Scalp and Areas of Poor Hair Growth
Given the trichoscopic findings, scrapings from the scaly areas were taken and revealed hyphae, confirming the diagnosis of tinea capitis. A fungal culture identified Trichophyton tonsurans as the causative organism.
Tinea capitis is the most common dermatophyte infection in children. Risk factors include participation in close-contact sports like wrestling or jiu-jitsu, attendance at daycare for younger children, African American hair care practices, pet ownership (particularly cats and rodents), and living in overcrowded conditions.
Diagnosis of tinea capitis requires a thorough clinical history to identify potential risk factors. On physical examination, patchy hair loss with associated scaling should raise suspicion for tinea capitis. Inflammatory signs, such as pustules and swelling, may suggest the presence of a kerion, further supporting the diagnosis. Although some practitioners use Wood’s lamp to help with diagnosis, its utility is limited. It detects fluorescence in Microsporum species (exothrix infections) but not in Trichophyton species (endothrix infections).
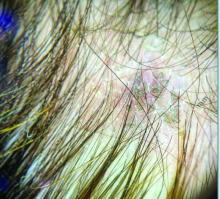
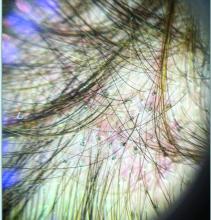
Trichoscopy can be a valuable tool when inflammation is minimal, and only hair loss and scaling are observed. Trichoscopic findings suggestive of tinea capitis include comma hairs, corkscrew hairs (as seen in this patient), Morse code-like hairs, zigzag hairs, bent hairs, block hairs, and i-hairs. Other common, though not characteristic, findings include broken hairs, black dots, perifollicular scaling, and diffuse scaling.
KOH (potassium hydroxide) analysis is another useful method for detecting fungal elements, though it does not identify the specific fungus and may not be available in all clinical settings. Mycologic culture remains the gold standard for diagnosing tinea capitis, though results can take 3-4 weeks. Newer diagnostic techniques, such as PCR analysis and MALDI-TOF/MS, offer more rapid identification of the causative organism.
The differential diagnosis includes:
- Seborrheic dermatitis, which presents with greasy, yellowish scales and itching, with trichoscopy showing twisted, coiled hairs and yellowish scaling.
- Psoriasis, which can mimic tinea capitis but presents with well-demarcated red plaques and silvery-white scales. Trichoscopy shows red dots and uniform scaling.
- Alopecia areata, which causes patchy hair loss without inflammation or scaling, with trichoscopic findings of exclamation mark hairs, black dots, and yellow dots.
- Trichotillomania, a hair-pulling disorder, which results in irregular patches of hair loss. Trichoscopy shows broken hairs of varying lengths, V-sign hairs, and flame-shaped residues at follicular openings.
Treatment of tinea capitis requires systemic antifungals and topical agents to prevent fungal spore spread. Several treatment guidelines are available from different institutions. Griseofulvin (FDA-approved for patients > 2 years of age) has been widely used, particularly for Microsporum canis infections. However, due to limited availability in many countries, terbinafine (FDA-approved for patients > 4 years of age) is now commonly used as first-line therapy, especially for Trichophyton species. Treatment typically lasts 4-6 weeks, and post-treatment cultures may be recommended to confirm mycologic cure.
Concerns about drug resistance have emerged, particularly for terbinafine-resistant dermatophytes linked to mutations in the squalene epoxidase enzyme. Resistance may be driven by limited antifungal availability and poor adherence to prolonged treatment regimens. While fluconazole and itraconazole are used off-label, growing evidence supports their effectiveness, although one large trial showed suboptimal cure rates with fluconazole.
Though systemic antifungals are generally safe, hepatotoxicity remains a concern, especially in patients with hepatic conditions or other comorbidities. Lab monitoring is advised for patients on prolonged or multiple therapies, or for those with coexisting conditions. The decision to conduct lab monitoring should be discussed with parents, balancing the very low risk of hepatotoxicity in healthy children against their comfort level.
An alternative to systemic therapy is photodynamic therapy (PDT), which has been reported as successful in treating tinea capitis infections, particularly in cases of T. mentagrophytes and M. canis. However, large-scale trials are needed to confirm PDT’s efficacy and safety.
In conclusion, children presenting with hair loss, scaling, and associated dark spots on the scalp should be evaluated for fungal infection. While trichoscopy can aid in diagnosis, fungal culture remains the gold standard for confirmation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Rudnicka L et al. Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases. Dermatol Clin. 2013 Oct;31(4):695-708, x. doi: 10.1016/j.det.2013.06.007.
Gupta AK et al. An update on tinea capitis in children. Pediatr Dermatol. 2024 Aug 7. doi: 10.1111/pde.15708.
Anna Waskiel-Burnat et al. Trichoscopy of tinea capitis: A systematic review. Dermatol Ther (Heidelb). 2020 Feb;10(1):43-52. doi: 10.1007/s13555-019-00350-1.
Given the trichoscopic findings, scrapings from the scaly areas were taken and revealed hyphae, confirming the diagnosis of tinea capitis. A fungal culture identified Trichophyton tonsurans as the causative organism.
Tinea capitis is the most common dermatophyte infection in children. Risk factors include participation in close-contact sports like wrestling or jiu-jitsu, attendance at daycare for younger children, African American hair care practices, pet ownership (particularly cats and rodents), and living in overcrowded conditions.
Diagnosis of tinea capitis requires a thorough clinical history to identify potential risk factors. On physical examination, patchy hair loss with associated scaling should raise suspicion for tinea capitis. Inflammatory signs, such as pustules and swelling, may suggest the presence of a kerion, further supporting the diagnosis. Although some practitioners use Wood’s lamp to help with diagnosis, its utility is limited. It detects fluorescence in Microsporum species (exothrix infections) but not in Trichophyton species (endothrix infections).
Trichoscopy can be a valuable tool when inflammation is minimal, and only hair loss and scaling are observed. Trichoscopic findings suggestive of tinea capitis include comma hairs, corkscrew hairs (as seen in this patient), Morse code-like hairs, zigzag hairs, bent hairs, block hairs, and i-hairs. Other common, though not characteristic, findings include broken hairs, black dots, perifollicular scaling, and diffuse scaling.
KOH (potassium hydroxide) analysis is another useful method for detecting fungal elements, though it does not identify the specific fungus and may not be available in all clinical settings. Mycologic culture remains the gold standard for diagnosing tinea capitis, though results can take 3-4 weeks. Newer diagnostic techniques, such as PCR analysis and MALDI-TOF/MS, offer more rapid identification of the causative organism.
The differential diagnosis includes:
- Seborrheic dermatitis, which presents with greasy, yellowish scales and itching, with trichoscopy showing twisted, coiled hairs and yellowish scaling.
- Psoriasis, which can mimic tinea capitis but presents with well-demarcated red plaques and silvery-white scales. Trichoscopy shows red dots and uniform scaling.
- Alopecia areata, which causes patchy hair loss without inflammation or scaling, with trichoscopic findings of exclamation mark hairs, black dots, and yellow dots.
- Trichotillomania, a hair-pulling disorder, which results in irregular patches of hair loss. Trichoscopy shows broken hairs of varying lengths, V-sign hairs, and flame-shaped residues at follicular openings.
Treatment of tinea capitis requires systemic antifungals and topical agents to prevent fungal spore spread. Several treatment guidelines are available from different institutions. Griseofulvin (FDA-approved for patients > 2 years of age) has been widely used, particularly for Microsporum canis infections. However, due to limited availability in many countries, terbinafine (FDA-approved for patients > 4 years of age) is now commonly used as first-line therapy, especially for Trichophyton species. Treatment typically lasts 4-6 weeks, and post-treatment cultures may be recommended to confirm mycologic cure.
Concerns about drug resistance have emerged, particularly for terbinafine-resistant dermatophytes linked to mutations in the squalene epoxidase enzyme. Resistance may be driven by limited antifungal availability and poor adherence to prolonged treatment regimens. While fluconazole and itraconazole are used off-label, growing evidence supports their effectiveness, although one large trial showed suboptimal cure rates with fluconazole.
Though systemic antifungals are generally safe, hepatotoxicity remains a concern, especially in patients with hepatic conditions or other comorbidities. Lab monitoring is advised for patients on prolonged or multiple therapies, or for those with coexisting conditions. The decision to conduct lab monitoring should be discussed with parents, balancing the very low risk of hepatotoxicity in healthy children against their comfort level.
An alternative to systemic therapy is photodynamic therapy (PDT), which has been reported as successful in treating tinea capitis infections, particularly in cases of T. mentagrophytes and M. canis. However, large-scale trials are needed to confirm PDT’s efficacy and safety.
In conclusion, children presenting with hair loss, scaling, and associated dark spots on the scalp should be evaluated for fungal infection. While trichoscopy can aid in diagnosis, fungal culture remains the gold standard for confirmation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Rudnicka L et al. Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases. Dermatol Clin. 2013 Oct;31(4):695-708, x. doi: 10.1016/j.det.2013.06.007.
Gupta AK et al. An update on tinea capitis in children. Pediatr Dermatol. 2024 Aug 7. doi: 10.1111/pde.15708.
Anna Waskiel-Burnat et al. Trichoscopy of tinea capitis: A systematic review. Dermatol Ther (Heidelb). 2020 Feb;10(1):43-52. doi: 10.1007/s13555-019-00350-1.
Given the trichoscopic findings, scrapings from the scaly areas were taken and revealed hyphae, confirming the diagnosis of tinea capitis. A fungal culture identified Trichophyton tonsurans as the causative organism.
Tinea capitis is the most common dermatophyte infection in children. Risk factors include participation in close-contact sports like wrestling or jiu-jitsu, attendance at daycare for younger children, African American hair care practices, pet ownership (particularly cats and rodents), and living in overcrowded conditions.
Diagnosis of tinea capitis requires a thorough clinical history to identify potential risk factors. On physical examination, patchy hair loss with associated scaling should raise suspicion for tinea capitis. Inflammatory signs, such as pustules and swelling, may suggest the presence of a kerion, further supporting the diagnosis. Although some practitioners use Wood’s lamp to help with diagnosis, its utility is limited. It detects fluorescence in Microsporum species (exothrix infections) but not in Trichophyton species (endothrix infections).
Trichoscopy can be a valuable tool when inflammation is minimal, and only hair loss and scaling are observed. Trichoscopic findings suggestive of tinea capitis include comma hairs, corkscrew hairs (as seen in this patient), Morse code-like hairs, zigzag hairs, bent hairs, block hairs, and i-hairs. Other common, though not characteristic, findings include broken hairs, black dots, perifollicular scaling, and diffuse scaling.
KOH (potassium hydroxide) analysis is another useful method for detecting fungal elements, though it does not identify the specific fungus and may not be available in all clinical settings. Mycologic culture remains the gold standard for diagnosing tinea capitis, though results can take 3-4 weeks. Newer diagnostic techniques, such as PCR analysis and MALDI-TOF/MS, offer more rapid identification of the causative organism.
The differential diagnosis includes:
- Seborrheic dermatitis, which presents with greasy, yellowish scales and itching, with trichoscopy showing twisted, coiled hairs and yellowish scaling.
- Psoriasis, which can mimic tinea capitis but presents with well-demarcated red plaques and silvery-white scales. Trichoscopy shows red dots and uniform scaling.
- Alopecia areata, which causes patchy hair loss without inflammation or scaling, with trichoscopic findings of exclamation mark hairs, black dots, and yellow dots.
- Trichotillomania, a hair-pulling disorder, which results in irregular patches of hair loss. Trichoscopy shows broken hairs of varying lengths, V-sign hairs, and flame-shaped residues at follicular openings.
Treatment of tinea capitis requires systemic antifungals and topical agents to prevent fungal spore spread. Several treatment guidelines are available from different institutions. Griseofulvin (FDA-approved for patients > 2 years of age) has been widely used, particularly for Microsporum canis infections. However, due to limited availability in many countries, terbinafine (FDA-approved for patients > 4 years of age) is now commonly used as first-line therapy, especially for Trichophyton species. Treatment typically lasts 4-6 weeks, and post-treatment cultures may be recommended to confirm mycologic cure.
Concerns about drug resistance have emerged, particularly for terbinafine-resistant dermatophytes linked to mutations in the squalene epoxidase enzyme. Resistance may be driven by limited antifungal availability and poor adherence to prolonged treatment regimens. While fluconazole and itraconazole are used off-label, growing evidence supports their effectiveness, although one large trial showed suboptimal cure rates with fluconazole.
Though systemic antifungals are generally safe, hepatotoxicity remains a concern, especially in patients with hepatic conditions or other comorbidities. Lab monitoring is advised for patients on prolonged or multiple therapies, or for those with coexisting conditions. The decision to conduct lab monitoring should be discussed with parents, balancing the very low risk of hepatotoxicity in healthy children against their comfort level.
An alternative to systemic therapy is photodynamic therapy (PDT), which has been reported as successful in treating tinea capitis infections, particularly in cases of T. mentagrophytes and M. canis. However, large-scale trials are needed to confirm PDT’s efficacy and safety.
In conclusion, children presenting with hair loss, scaling, and associated dark spots on the scalp should be evaluated for fungal infection. While trichoscopy can aid in diagnosis, fungal culture remains the gold standard for confirmation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Rudnicka L et al. Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases. Dermatol Clin. 2013 Oct;31(4):695-708, x. doi: 10.1016/j.det.2013.06.007.
Gupta AK et al. An update on tinea capitis in children. Pediatr Dermatol. 2024 Aug 7. doi: 10.1111/pde.15708.
Anna Waskiel-Burnat et al. Trichoscopy of tinea capitis: A systematic review. Dermatol Ther (Heidelb). 2020 Feb;10(1):43-52. doi: 10.1007/s13555-019-00350-1.
Pulsed Dye Laser a “Go-To Device” Option for Acne Treatment When Access to 1726-nm Lasers Is Limited
CARLSBAD, CALIF. — Lasers and energy-based treatments alone or in combination with medical therapy may improve outcomes for patients with moderate to severe acne, according to Arielle Kauvar, MD.
At the Controversies and Conversations in Laser and Cosmetic Surgery annual symposium, Kauvar, director of New York Laser & Skin Care, New York City, highlighted several reasons why using lasers for acne is beneficial. “First, we know that topical therapy alone is often ineffective, and antibiotic treatment does not address the cause of acne and can alter the skin and gut microbiome,” she said. “Isotretinoin is highly effective, but there’s an increasing reluctance to use it. Lasers and energy devices are effective in treating acne and may also treat the post-inflammatory hyperpigmentation and scarring associated with it.”
The pathogenesis of acne is multifactorial, she continued, including a disruption of sebaceous gland activity, with overproduction and alteration of sebum and abnormal follicular keratinization. Acne also causes an imbalance of the skin microbiome, local inflammation, and activation of both innate and adaptive immunity.
“Many studies point to the fact that inflammation and immune system activation may actually be the primary event” of acne formation, said Kauvar, who is also a clinical professor of dermatology at New York University, New York City. “This persistent immune activation is also associated with scarring,” she noted. “So, are we off the mark in terms of trying to kill sebaceous glands? Should we be concentrating on anti-inflammatory approaches?”
AviClear became the first 1726-nm laser cleared by the US Food and Drug Administration (FDA) for the treatment of mild to severe acne vulgaris in 2022, followed a few months later with the FDA clearance of another 1726-nm laser, the Accure Acne Laser System in November 2022. These lasers cause selective photothermolysis of sebaceous glands, but according to Kauvar, “access to these devices is somewhat limited at this time.”
What is available includes her go-to device, the pulsed dye laser (PDL), which has been widely studied and shown in a systematic review and meta-analysis of studies to be effective for acne. The PDL “targets dermal blood vessels facilitating inflammation, upregulates TGF-beta, and inhibits CD4+ T cell-mediated inflammation,” she said. “It can also treat PIH [post-inflammatory hyperpigmentation] and may be helpful in scar prevention.”
In an abstract presented at The American Society for Laser Medicine and Surgery (ASLMS) 2024 annual meeting, Kauvar and colleagues conducted a real-world study of PDL therapy in 15 adult women with recalcitrant acne who were maintained on their medical treatment regimen. Their mean age was 27 years, and they had skin types II-IV; they underwent four monthly PDL treatments with follow-up at 1 and 3 months. At each visit, the researchers took digital photographs and counted inflammatory acne lesions, non-inflammatory acne lesions, and post-inflammatory pigment alteration (PIPA) lesions.
The main outcomes of interest were the investigator global assessment (IGA) scores at the 1- and 3-month follow-up visits. Kauvar and colleagues observed a significant improvement in IGA scores at the 1- and 3-month follow-up visits (P < .05), with an average decrease of 1.8 and 1.6 points in the acne severity scale, respectively, from a baseline score of 3.4. By the 3-month follow-up visits, counts of inflammatory and non-inflammatory lesions decreased significantly (P < .05), and 61% of study participants showed a decrease in the PIPA count. No adverse events occurred.
Kauvar disclosed that she has conducted research for Candela, Lumenis, and Sofwave, and is an adviser to Acclaro.
A version of this article first appeared on Medscape.com.
CARLSBAD, CALIF. — Lasers and energy-based treatments alone or in combination with medical therapy may improve outcomes for patients with moderate to severe acne, according to Arielle Kauvar, MD.
At the Controversies and Conversations in Laser and Cosmetic Surgery annual symposium, Kauvar, director of New York Laser & Skin Care, New York City, highlighted several reasons why using lasers for acne is beneficial. “First, we know that topical therapy alone is often ineffective, and antibiotic treatment does not address the cause of acne and can alter the skin and gut microbiome,” she said. “Isotretinoin is highly effective, but there’s an increasing reluctance to use it. Lasers and energy devices are effective in treating acne and may also treat the post-inflammatory hyperpigmentation and scarring associated with it.”
The pathogenesis of acne is multifactorial, she continued, including a disruption of sebaceous gland activity, with overproduction and alteration of sebum and abnormal follicular keratinization. Acne also causes an imbalance of the skin microbiome, local inflammation, and activation of both innate and adaptive immunity.
“Many studies point to the fact that inflammation and immune system activation may actually be the primary event” of acne formation, said Kauvar, who is also a clinical professor of dermatology at New York University, New York City. “This persistent immune activation is also associated with scarring,” she noted. “So, are we off the mark in terms of trying to kill sebaceous glands? Should we be concentrating on anti-inflammatory approaches?”
AviClear became the first 1726-nm laser cleared by the US Food and Drug Administration (FDA) for the treatment of mild to severe acne vulgaris in 2022, followed a few months later with the FDA clearance of another 1726-nm laser, the Accure Acne Laser System in November 2022. These lasers cause selective photothermolysis of sebaceous glands, but according to Kauvar, “access to these devices is somewhat limited at this time.”
What is available includes her go-to device, the pulsed dye laser (PDL), which has been widely studied and shown in a systematic review and meta-analysis of studies to be effective for acne. The PDL “targets dermal blood vessels facilitating inflammation, upregulates TGF-beta, and inhibits CD4+ T cell-mediated inflammation,” she said. “It can also treat PIH [post-inflammatory hyperpigmentation] and may be helpful in scar prevention.”
In an abstract presented at The American Society for Laser Medicine and Surgery (ASLMS) 2024 annual meeting, Kauvar and colleagues conducted a real-world study of PDL therapy in 15 adult women with recalcitrant acne who were maintained on their medical treatment regimen. Their mean age was 27 years, and they had skin types II-IV; they underwent four monthly PDL treatments with follow-up at 1 and 3 months. At each visit, the researchers took digital photographs and counted inflammatory acne lesions, non-inflammatory acne lesions, and post-inflammatory pigment alteration (PIPA) lesions.
The main outcomes of interest were the investigator global assessment (IGA) scores at the 1- and 3-month follow-up visits. Kauvar and colleagues observed a significant improvement in IGA scores at the 1- and 3-month follow-up visits (P < .05), with an average decrease of 1.8 and 1.6 points in the acne severity scale, respectively, from a baseline score of 3.4. By the 3-month follow-up visits, counts of inflammatory and non-inflammatory lesions decreased significantly (P < .05), and 61% of study participants showed a decrease in the PIPA count. No adverse events occurred.
Kauvar disclosed that she has conducted research for Candela, Lumenis, and Sofwave, and is an adviser to Acclaro.
A version of this article first appeared on Medscape.com.
CARLSBAD, CALIF. — Lasers and energy-based treatments alone or in combination with medical therapy may improve outcomes for patients with moderate to severe acne, according to Arielle Kauvar, MD.
At the Controversies and Conversations in Laser and Cosmetic Surgery annual symposium, Kauvar, director of New York Laser & Skin Care, New York City, highlighted several reasons why using lasers for acne is beneficial. “First, we know that topical therapy alone is often ineffective, and antibiotic treatment does not address the cause of acne and can alter the skin and gut microbiome,” she said. “Isotretinoin is highly effective, but there’s an increasing reluctance to use it. Lasers and energy devices are effective in treating acne and may also treat the post-inflammatory hyperpigmentation and scarring associated with it.”
The pathogenesis of acne is multifactorial, she continued, including a disruption of sebaceous gland activity, with overproduction and alteration of sebum and abnormal follicular keratinization. Acne also causes an imbalance of the skin microbiome, local inflammation, and activation of both innate and adaptive immunity.
“Many studies point to the fact that inflammation and immune system activation may actually be the primary event” of acne formation, said Kauvar, who is also a clinical professor of dermatology at New York University, New York City. “This persistent immune activation is also associated with scarring,” she noted. “So, are we off the mark in terms of trying to kill sebaceous glands? Should we be concentrating on anti-inflammatory approaches?”
AviClear became the first 1726-nm laser cleared by the US Food and Drug Administration (FDA) for the treatment of mild to severe acne vulgaris in 2022, followed a few months later with the FDA clearance of another 1726-nm laser, the Accure Acne Laser System in November 2022. These lasers cause selective photothermolysis of sebaceous glands, but according to Kauvar, “access to these devices is somewhat limited at this time.”
What is available includes her go-to device, the pulsed dye laser (PDL), which has been widely studied and shown in a systematic review and meta-analysis of studies to be effective for acne. The PDL “targets dermal blood vessels facilitating inflammation, upregulates TGF-beta, and inhibits CD4+ T cell-mediated inflammation,” she said. “It can also treat PIH [post-inflammatory hyperpigmentation] and may be helpful in scar prevention.”
In an abstract presented at The American Society for Laser Medicine and Surgery (ASLMS) 2024 annual meeting, Kauvar and colleagues conducted a real-world study of PDL therapy in 15 adult women with recalcitrant acne who were maintained on their medical treatment regimen. Their mean age was 27 years, and they had skin types II-IV; they underwent four monthly PDL treatments with follow-up at 1 and 3 months. At each visit, the researchers took digital photographs and counted inflammatory acne lesions, non-inflammatory acne lesions, and post-inflammatory pigment alteration (PIPA) lesions.
The main outcomes of interest were the investigator global assessment (IGA) scores at the 1- and 3-month follow-up visits. Kauvar and colleagues observed a significant improvement in IGA scores at the 1- and 3-month follow-up visits (P < .05), with an average decrease of 1.8 and 1.6 points in the acne severity scale, respectively, from a baseline score of 3.4. By the 3-month follow-up visits, counts of inflammatory and non-inflammatory lesions decreased significantly (P < .05), and 61% of study participants showed a decrease in the PIPA count. No adverse events occurred.
Kauvar disclosed that she has conducted research for Candela, Lumenis, and Sofwave, and is an adviser to Acclaro.
A version of this article first appeared on Medscape.com.
A Hard Look at Toxic Workplace Culture in Medicine
While Kellie Lease Stecher, MD, was working as an ob.gyn. in Minneapolis, Minnesota, a patient confided in her a sexual assault allegation about one of Stecher’s male colleagues. Stecher shared the allegation with her supervisor, who told Stecher not to file a report and chose not to address the issue with the patient. Stecher weighed how to do the right thing: Should she speak up? What were the ethical and legal implications of speaking up vs staying silent?
After seeking advice from her mentors, Stecher felt it was her moral and legal duty to report the allegation to the Minnesota Medical Board. Once she did, her supervisor chastised her repeatedly for reporting the allegation. Stecher soon found herself in a hostile work environment where she was regularly singled out and silenced by her supervisor and colleagues.
“I got to a point where I felt like I couldn’t say anything at any meetings without somehow being targeted after the meeting. There was an individual who was even allowed to fat-shame me with no consequences,” Stecher said. “[Being bullied at work is] a struggle because you have no voice, you have no opportunities, and there’s someone who is intentionally making your life uncomfortable.”
Stecher’s experience is not unusual. Mistreatment is a common issue among healthcare workers, ranging from rudeness to bullying and harassment and permeating every level and specialty of the medical profession. A 2019 research review estimated that 26.3% of healthcare workers had experienced bullying and found bullying in healthcare to be associated with mental health problems such as burnout and depression, physical health problems such as insomnia and headaches, and physicians taking more sick leave.
The Medscape Physician Workplace Culture Report 2024 found similarly bleak results:
- 38% said workplace culture is declining.
- 70% don’t see a big commitment from employers for positive culture.
- 48% said staff isn’t committed to positive culture.
The irony, of course, is that most physicians enter the field to care for people. As individuals go from medical school to residency and on with the rest of their careers, they often experience a rude awakening.
It’s Everywhere
Noticing the prevalence of workplace bullying in the medical field, endocrinologist Farah Khan, MD, at UW Medicine in Seattle, Washington, decided to conduct a survey on the issue.
Khan collected 122 responses from colleagues, friends, and acquaintances in the field. When asked if they had ever been bullied in medicine, 68% of respondents said yes. But here’s the fascinating part: She tried to pinpoint one particular area or source of toxicity in the progression of a physician’s career — and couldn’t because it existed at all levels.
More than one third of respondents said their worst bullying experiences occurred in residency, while 30% said mistreatment was worst in medical school, and 24% indicated their worst experience had occurred once they became an attending.
The litany of experiences included being belittled, excluded, yelled at, criticized, shamed, unfairly blamed, threatened, sexually harassed, subjected to bigotry and slurs, and humiliated.
“What surprised me the most was how widespread this problem is and the many different layers of healthcare it permeates through, from operating room staff to medical students to hospital HR to residents and attendings,” Khan said of her findings.
Who Cares for the Caregivers?
When hematologist Mikkael Sekeres, MD, was in medical school, he seriously considered a career as a surgeon. Following success in his surgical rotations, he scrubbed in with a cardiothoracic surgeon who was well known for both his status as a surgeon and his fiery temper. Sekeres witnessed the surgeon yelling at whoever was nearby: Medical students, fellows, residents, operating room nurses.
“At the end of that experience, any passing thoughts I had of going into cardiothoracic surgery were gone,” Sekeres said. “Some of the people I met in surgery were truly wonderful. Some were unhappy people.”
He has clear ideas why. Mental health struggles that are all too common among physicians can be caused or exacerbated by mistreatment and can also lead a physician to mistreat others.
“People bully when they themselves are hurting,” Sekeres said. “It begs the question, why are people hurting? What’s driving them to be bullies? I think part of the reason is that they’re working really hard and they’re tired, and nobody’s caring for them. It’s hard to care for others when you feel as if you’re hurting more than they are.”
Gail Gazelle, MD, experienced something like this. In her case, the pressure to please and to be a perfect professional and mother affected how she interacted with those around her. While working as a hospice medical director and an academician and clinician at Harvard Medical School, Boston, Massachusetts, she found herself feeling exhausted and burnt out but simultaneously guilty for not doing enough at work or at home.
Guess what happened? She became irritable, lashing out at her son and not putting her best foot forward with coworkers or patients.
After trying traditional therapy and self-help through books and podcasts, Gazelle found her solution in life coaching. “I realized just how harsh I was being on myself and found ways to reverse that pattern,” she said. “I learned ways of regulating myself emotionally that I definitely didn’t learn in my training.”
Today, Gazelle works as a life coach herself, guiding physicians through common challenges of the profession — particularly bullying, which she sees often. She remembers one client, an oncologist, who was being targeted by a nurse practitioner she was training. The nurse practitioner began talking back to the oncologist, as well as gossiping and bad-mouthing her to the nurses in the practice. The nurses then began excluding the oncologist from their cafeteria table at lunchtime, which felt blatant in such a small practice.
A core component of Gazelle’s coaching strategy was helping the client reclaim her self-esteem by focusing on her strengths. She instructed the client to write down what went well that day each night rather than lying in bed ruminating. Such self-care strategies can not only help bullied physicians but also prevent some of the challenges that might cause a physician to bully or lash out at another in the first place.
Such strategies, along with the recent influx of wellness programs available in healthcare facilities, can help physicians cope with the mental health impacts of bullying and the job in general. But even life coaches like Gazelle acknowledge that they are often band-aids on the system’s deeper wounds. Bullying in healthcare is not an individual issue; at its core, it’s an institutional one.
Negative Hierarchies in Healthcare
When Stecher’s contract expired, she was fired by the supervisor who had been bullying her. Stecher has since filed a lawsuit, claiming sexual discrimination, defamation, and wrongful termination.
The medical field has a long history of hierarchy, and while this rigidity has softened over time, negative hierarchical dynamics are often perpetuated by leaders. Phenomena like cronyism and cliques and behaviors like petty gossip, lunchroom exclusion (which in the worst cases can mimic high school dynamics), and targeting can be at play in the healthcare workplace.
The classic examples, Stecher said, can usually be spotted: “If you threaten the status quo or offer different ideas, you are seen as a threat. Cronyism ... strict hierarchies ... people who elevate individuals in their social arena into leadership positions. Physicians don’t get the leadership training that they really need; they are often just dumped into roles with no previous experience because they’re someone’s golfing buddy.”
The question is how to get workplace culture momentum moving in a positive direction. When Gazelle’s clients are hesitant to voice concerns, she emphasizes doing so can and should benefit leadership, as well as patients and the wider healthcare system.
“The win-win is that you have a healthy culture of respect and dignity and civility rather than the opposite,” she said. “The leader will actually have more staff retention, which everybody’s concerned about, given the shortage of healthcare workers.”
And that’s a key incentive that may not be discussed as much: Talent drain from toxicity. The Medscape Workplace Culture Report asked about culture as it applies to physicians looking to join up. Notably, 93% of doctors say culture is important when mulling a job offer, 70% said culture is equal to money, and 18% ranked it as more important than money, and 46% say a positive atmosphere is the top priority.
Ultimately, it comes down to who is willing to step in and stand up. Respondents to Khan’s survey counted anonymous reporting systems, more supportive administration teams, and zero-tolerance policies as potential remedies. Gazelle, Sekeres, and Stecher all emphasize the need for zero-tolerance policies for bullying and mistreatment.
“We can’t afford to have things going on like this that just destroy the fabric of the healthcare endeavor,” Gazelle said. “They come out sideways eventually. They come out in terms of poor patient care because there are greater errors. There’s a lack of respect for patients. There’s anger and irritability and so much spillover. We have to have zero-tolerance policies from the top down.”
A version of this article appeared on Medscape.com.
While Kellie Lease Stecher, MD, was working as an ob.gyn. in Minneapolis, Minnesota, a patient confided in her a sexual assault allegation about one of Stecher’s male colleagues. Stecher shared the allegation with her supervisor, who told Stecher not to file a report and chose not to address the issue with the patient. Stecher weighed how to do the right thing: Should she speak up? What were the ethical and legal implications of speaking up vs staying silent?
After seeking advice from her mentors, Stecher felt it was her moral and legal duty to report the allegation to the Minnesota Medical Board. Once she did, her supervisor chastised her repeatedly for reporting the allegation. Stecher soon found herself in a hostile work environment where she was regularly singled out and silenced by her supervisor and colleagues.
“I got to a point where I felt like I couldn’t say anything at any meetings without somehow being targeted after the meeting. There was an individual who was even allowed to fat-shame me with no consequences,” Stecher said. “[Being bullied at work is] a struggle because you have no voice, you have no opportunities, and there’s someone who is intentionally making your life uncomfortable.”
Stecher’s experience is not unusual. Mistreatment is a common issue among healthcare workers, ranging from rudeness to bullying and harassment and permeating every level and specialty of the medical profession. A 2019 research review estimated that 26.3% of healthcare workers had experienced bullying and found bullying in healthcare to be associated with mental health problems such as burnout and depression, physical health problems such as insomnia and headaches, and physicians taking more sick leave.
The Medscape Physician Workplace Culture Report 2024 found similarly bleak results:
- 38% said workplace culture is declining.
- 70% don’t see a big commitment from employers for positive culture.
- 48% said staff isn’t committed to positive culture.
The irony, of course, is that most physicians enter the field to care for people. As individuals go from medical school to residency and on with the rest of their careers, they often experience a rude awakening.
It’s Everywhere
Noticing the prevalence of workplace bullying in the medical field, endocrinologist Farah Khan, MD, at UW Medicine in Seattle, Washington, decided to conduct a survey on the issue.
Khan collected 122 responses from colleagues, friends, and acquaintances in the field. When asked if they had ever been bullied in medicine, 68% of respondents said yes. But here’s the fascinating part: She tried to pinpoint one particular area or source of toxicity in the progression of a physician’s career — and couldn’t because it existed at all levels.
More than one third of respondents said their worst bullying experiences occurred in residency, while 30% said mistreatment was worst in medical school, and 24% indicated their worst experience had occurred once they became an attending.
The litany of experiences included being belittled, excluded, yelled at, criticized, shamed, unfairly blamed, threatened, sexually harassed, subjected to bigotry and slurs, and humiliated.
“What surprised me the most was how widespread this problem is and the many different layers of healthcare it permeates through, from operating room staff to medical students to hospital HR to residents and attendings,” Khan said of her findings.
Who Cares for the Caregivers?
When hematologist Mikkael Sekeres, MD, was in medical school, he seriously considered a career as a surgeon. Following success in his surgical rotations, he scrubbed in with a cardiothoracic surgeon who was well known for both his status as a surgeon and his fiery temper. Sekeres witnessed the surgeon yelling at whoever was nearby: Medical students, fellows, residents, operating room nurses.
“At the end of that experience, any passing thoughts I had of going into cardiothoracic surgery were gone,” Sekeres said. “Some of the people I met in surgery were truly wonderful. Some were unhappy people.”
He has clear ideas why. Mental health struggles that are all too common among physicians can be caused or exacerbated by mistreatment and can also lead a physician to mistreat others.
“People bully when they themselves are hurting,” Sekeres said. “It begs the question, why are people hurting? What’s driving them to be bullies? I think part of the reason is that they’re working really hard and they’re tired, and nobody’s caring for them. It’s hard to care for others when you feel as if you’re hurting more than they are.”
Gail Gazelle, MD, experienced something like this. In her case, the pressure to please and to be a perfect professional and mother affected how she interacted with those around her. While working as a hospice medical director and an academician and clinician at Harvard Medical School, Boston, Massachusetts, she found herself feeling exhausted and burnt out but simultaneously guilty for not doing enough at work or at home.
Guess what happened? She became irritable, lashing out at her son and not putting her best foot forward with coworkers or patients.
After trying traditional therapy and self-help through books and podcasts, Gazelle found her solution in life coaching. “I realized just how harsh I was being on myself and found ways to reverse that pattern,” she said. “I learned ways of regulating myself emotionally that I definitely didn’t learn in my training.”
Today, Gazelle works as a life coach herself, guiding physicians through common challenges of the profession — particularly bullying, which she sees often. She remembers one client, an oncologist, who was being targeted by a nurse practitioner she was training. The nurse practitioner began talking back to the oncologist, as well as gossiping and bad-mouthing her to the nurses in the practice. The nurses then began excluding the oncologist from their cafeteria table at lunchtime, which felt blatant in such a small practice.
A core component of Gazelle’s coaching strategy was helping the client reclaim her self-esteem by focusing on her strengths. She instructed the client to write down what went well that day each night rather than lying in bed ruminating. Such self-care strategies can not only help bullied physicians but also prevent some of the challenges that might cause a physician to bully or lash out at another in the first place.
Such strategies, along with the recent influx of wellness programs available in healthcare facilities, can help physicians cope with the mental health impacts of bullying and the job in general. But even life coaches like Gazelle acknowledge that they are often band-aids on the system’s deeper wounds. Bullying in healthcare is not an individual issue; at its core, it’s an institutional one.
Negative Hierarchies in Healthcare
When Stecher’s contract expired, she was fired by the supervisor who had been bullying her. Stecher has since filed a lawsuit, claiming sexual discrimination, defamation, and wrongful termination.
The medical field has a long history of hierarchy, and while this rigidity has softened over time, negative hierarchical dynamics are often perpetuated by leaders. Phenomena like cronyism and cliques and behaviors like petty gossip, lunchroom exclusion (which in the worst cases can mimic high school dynamics), and targeting can be at play in the healthcare workplace.
The classic examples, Stecher said, can usually be spotted: “If you threaten the status quo or offer different ideas, you are seen as a threat. Cronyism ... strict hierarchies ... people who elevate individuals in their social arena into leadership positions. Physicians don’t get the leadership training that they really need; they are often just dumped into roles with no previous experience because they’re someone’s golfing buddy.”
The question is how to get workplace culture momentum moving in a positive direction. When Gazelle’s clients are hesitant to voice concerns, she emphasizes doing so can and should benefit leadership, as well as patients and the wider healthcare system.
“The win-win is that you have a healthy culture of respect and dignity and civility rather than the opposite,” she said. “The leader will actually have more staff retention, which everybody’s concerned about, given the shortage of healthcare workers.”
And that’s a key incentive that may not be discussed as much: Talent drain from toxicity. The Medscape Workplace Culture Report asked about culture as it applies to physicians looking to join up. Notably, 93% of doctors say culture is important when mulling a job offer, 70% said culture is equal to money, and 18% ranked it as more important than money, and 46% say a positive atmosphere is the top priority.
Ultimately, it comes down to who is willing to step in and stand up. Respondents to Khan’s survey counted anonymous reporting systems, more supportive administration teams, and zero-tolerance policies as potential remedies. Gazelle, Sekeres, and Stecher all emphasize the need for zero-tolerance policies for bullying and mistreatment.
“We can’t afford to have things going on like this that just destroy the fabric of the healthcare endeavor,” Gazelle said. “They come out sideways eventually. They come out in terms of poor patient care because there are greater errors. There’s a lack of respect for patients. There’s anger and irritability and so much spillover. We have to have zero-tolerance policies from the top down.”
A version of this article appeared on Medscape.com.
While Kellie Lease Stecher, MD, was working as an ob.gyn. in Minneapolis, Minnesota, a patient confided in her a sexual assault allegation about one of Stecher’s male colleagues. Stecher shared the allegation with her supervisor, who told Stecher not to file a report and chose not to address the issue with the patient. Stecher weighed how to do the right thing: Should she speak up? What were the ethical and legal implications of speaking up vs staying silent?
After seeking advice from her mentors, Stecher felt it was her moral and legal duty to report the allegation to the Minnesota Medical Board. Once she did, her supervisor chastised her repeatedly for reporting the allegation. Stecher soon found herself in a hostile work environment where she was regularly singled out and silenced by her supervisor and colleagues.
“I got to a point where I felt like I couldn’t say anything at any meetings without somehow being targeted after the meeting. There was an individual who was even allowed to fat-shame me with no consequences,” Stecher said. “[Being bullied at work is] a struggle because you have no voice, you have no opportunities, and there’s someone who is intentionally making your life uncomfortable.”
Stecher’s experience is not unusual. Mistreatment is a common issue among healthcare workers, ranging from rudeness to bullying and harassment and permeating every level and specialty of the medical profession. A 2019 research review estimated that 26.3% of healthcare workers had experienced bullying and found bullying in healthcare to be associated with mental health problems such as burnout and depression, physical health problems such as insomnia and headaches, and physicians taking more sick leave.
The Medscape Physician Workplace Culture Report 2024 found similarly bleak results:
- 38% said workplace culture is declining.
- 70% don’t see a big commitment from employers for positive culture.
- 48% said staff isn’t committed to positive culture.
The irony, of course, is that most physicians enter the field to care for people. As individuals go from medical school to residency and on with the rest of their careers, they often experience a rude awakening.
It’s Everywhere
Noticing the prevalence of workplace bullying in the medical field, endocrinologist Farah Khan, MD, at UW Medicine in Seattle, Washington, decided to conduct a survey on the issue.
Khan collected 122 responses from colleagues, friends, and acquaintances in the field. When asked if they had ever been bullied in medicine, 68% of respondents said yes. But here’s the fascinating part: She tried to pinpoint one particular area or source of toxicity in the progression of a physician’s career — and couldn’t because it existed at all levels.
More than one third of respondents said their worst bullying experiences occurred in residency, while 30% said mistreatment was worst in medical school, and 24% indicated their worst experience had occurred once they became an attending.
The litany of experiences included being belittled, excluded, yelled at, criticized, shamed, unfairly blamed, threatened, sexually harassed, subjected to bigotry and slurs, and humiliated.
“What surprised me the most was how widespread this problem is and the many different layers of healthcare it permeates through, from operating room staff to medical students to hospital HR to residents and attendings,” Khan said of her findings.
Who Cares for the Caregivers?
When hematologist Mikkael Sekeres, MD, was in medical school, he seriously considered a career as a surgeon. Following success in his surgical rotations, he scrubbed in with a cardiothoracic surgeon who was well known for both his status as a surgeon and his fiery temper. Sekeres witnessed the surgeon yelling at whoever was nearby: Medical students, fellows, residents, operating room nurses.
“At the end of that experience, any passing thoughts I had of going into cardiothoracic surgery were gone,” Sekeres said. “Some of the people I met in surgery were truly wonderful. Some were unhappy people.”
He has clear ideas why. Mental health struggles that are all too common among physicians can be caused or exacerbated by mistreatment and can also lead a physician to mistreat others.
“People bully when they themselves are hurting,” Sekeres said. “It begs the question, why are people hurting? What’s driving them to be bullies? I think part of the reason is that they’re working really hard and they’re tired, and nobody’s caring for them. It’s hard to care for others when you feel as if you’re hurting more than they are.”
Gail Gazelle, MD, experienced something like this. In her case, the pressure to please and to be a perfect professional and mother affected how she interacted with those around her. While working as a hospice medical director and an academician and clinician at Harvard Medical School, Boston, Massachusetts, she found herself feeling exhausted and burnt out but simultaneously guilty for not doing enough at work or at home.
Guess what happened? She became irritable, lashing out at her son and not putting her best foot forward with coworkers or patients.
After trying traditional therapy and self-help through books and podcasts, Gazelle found her solution in life coaching. “I realized just how harsh I was being on myself and found ways to reverse that pattern,” she said. “I learned ways of regulating myself emotionally that I definitely didn’t learn in my training.”
Today, Gazelle works as a life coach herself, guiding physicians through common challenges of the profession — particularly bullying, which she sees often. She remembers one client, an oncologist, who was being targeted by a nurse practitioner she was training. The nurse practitioner began talking back to the oncologist, as well as gossiping and bad-mouthing her to the nurses in the practice. The nurses then began excluding the oncologist from their cafeteria table at lunchtime, which felt blatant in such a small practice.
A core component of Gazelle’s coaching strategy was helping the client reclaim her self-esteem by focusing on her strengths. She instructed the client to write down what went well that day each night rather than lying in bed ruminating. Such self-care strategies can not only help bullied physicians but also prevent some of the challenges that might cause a physician to bully or lash out at another in the first place.
Such strategies, along with the recent influx of wellness programs available in healthcare facilities, can help physicians cope with the mental health impacts of bullying and the job in general. But even life coaches like Gazelle acknowledge that they are often band-aids on the system’s deeper wounds. Bullying in healthcare is not an individual issue; at its core, it’s an institutional one.
Negative Hierarchies in Healthcare
When Stecher’s contract expired, she was fired by the supervisor who had been bullying her. Stecher has since filed a lawsuit, claiming sexual discrimination, defamation, and wrongful termination.
The medical field has a long history of hierarchy, and while this rigidity has softened over time, negative hierarchical dynamics are often perpetuated by leaders. Phenomena like cronyism and cliques and behaviors like petty gossip, lunchroom exclusion (which in the worst cases can mimic high school dynamics), and targeting can be at play in the healthcare workplace.
The classic examples, Stecher said, can usually be spotted: “If you threaten the status quo or offer different ideas, you are seen as a threat. Cronyism ... strict hierarchies ... people who elevate individuals in their social arena into leadership positions. Physicians don’t get the leadership training that they really need; they are often just dumped into roles with no previous experience because they’re someone’s golfing buddy.”
The question is how to get workplace culture momentum moving in a positive direction. When Gazelle’s clients are hesitant to voice concerns, she emphasizes doing so can and should benefit leadership, as well as patients and the wider healthcare system.
“The win-win is that you have a healthy culture of respect and dignity and civility rather than the opposite,” she said. “The leader will actually have more staff retention, which everybody’s concerned about, given the shortage of healthcare workers.”
And that’s a key incentive that may not be discussed as much: Talent drain from toxicity. The Medscape Workplace Culture Report asked about culture as it applies to physicians looking to join up. Notably, 93% of doctors say culture is important when mulling a job offer, 70% said culture is equal to money, and 18% ranked it as more important than money, and 46% say a positive atmosphere is the top priority.
Ultimately, it comes down to who is willing to step in and stand up. Respondents to Khan’s survey counted anonymous reporting systems, more supportive administration teams, and zero-tolerance policies as potential remedies. Gazelle, Sekeres, and Stecher all emphasize the need for zero-tolerance policies for bullying and mistreatment.
“We can’t afford to have things going on like this that just destroy the fabric of the healthcare endeavor,” Gazelle said. “They come out sideways eventually. They come out in terms of poor patient care because there are greater errors. There’s a lack of respect for patients. There’s anger and irritability and so much spillover. We have to have zero-tolerance policies from the top down.”
A version of this article appeared on Medscape.com.
NY Nurse Practitioners Sue State Over Pay Equity, Alleged Gender Inequality
A
The New York State Civil Service Commission understates the job function of NPs, overstates their dependence on physicians, and inadequately pays them for their work, according to the complaint filed in the US District Court for the Northern District of New York.
The nurses claim the mistreatment is a consequence of the fact that “at least 80% of the state’s employed NPs are women.”
Michael H. Sussman, a Goshen, New York–based attorney for the nurses, said in an interview that New York NPs are increasingly being used essentially as doctors at state-run facilities, including prisons, yet the state has failed to adequately pay them.
The lawsuit comes after a decade-long attempt by NPs to attain equitable pay and the ability to advance their civil service careers, he said.
“New York state has not addressed the heart of the issue, which is that the classification of this position is much lower than other positions in the state which are not so female-dominated and which engage in very similar activities,” Sussman said.
The lawsuit claims that “the work of NPs is complex, equaling that of a medical specialist, psychiatrist, or clinical physician.”
A spokesman for the New York State Civil Service Commission declined comment, saying the department does not comment on pending litigation.
Novel Gender Discrimination Argument
Gender discrimination is a relatively new argument avenue in the larger equal work, equal pay debate, said Joanne Spetz, PhD, director of the Institute for Health Policy Studies at the University of California, San Francisco.
“This is the first time I’ve heard of [such] a case being really gender discrimination focused,” she said in an interview. “On one level, I think it’s groundbreaking as a legal approach, but it’s also limited because it’s focused on public, state employees.”
Spetz noted that New York has significantly expanded NPs’ scope of practice, enacting in 2022 legislation that granted NPs full practice authority. The law means NPs can evaluate, order, diagnose, manage treatments, and prescribe medications for patients without physician supervision.
“They are in a role where they are stepping back and saying, ‘Wait, why are [we] not receiving equal pay for equal work?’ ” Spetz said. “It’s a totally fair area for debate, especially because they are now authorized to do essentially equal work with a high degree of autonomy.”
Debate Over Pay Grade
The nurses’ complaint centers on the New York State Civil Service Commission’s classification for NPs, which hasn’t changed since 2006. NPs are classified at grade 24, and they have no possibility of internal advancement associated with their title, according to the legal complaint filed on September 17.
To comply with a state legislative directive, the commission in 2018 conducted a study of the NP classification but recommended against reclassification or implementing a career ladder. The study noted the subordinate role of NPs to physicians and the substantial difference between physician classification (entry at grade 34) and that of NPs, psychologists (grade 25), and pharmacists (grade 25).
The study concluded that higher classified positions have higher levels of educational attainment and licensure requirements and no supervision or collaboration requirements, according to the complaint.
At the time, groups such as the Nurse Practitioner Association and the Public Employees Federation (PEF) criticized the findings, but the commission stuck to its classification.
Following the NP Modernization Act that allowed NPs to practice independently, PEF sought an increase for NPs to grade 28 with a progression to grade 34 depending on experience.
“But to this date, despite altering the starting salaries of NPs, defendants have failed and refused to alter the compensation offered to the substantial majority of NPs, and each plaintiff remains cabined in a grade 24 with a discriminatorily low salary when compared with males in other job classifications doing highly similar functions,” the lawsuit contended.
Six plaintiffs are named in the lawsuit, all of whom are women and work for state agencies. Plaintiff Rachel Burns, for instance, works as a psychiatric mental health NP in West Seneca and is responsible for performing psychiatric evaluations for patients, diagnosis, prescribing medication, ordering labs, and determining risks. The evaluations are identical for a psychiatrist and require her to complete the same forms, according to the suit.
Another plaintiff, Amber Hawthorne Lashway, works at a correctional facility in Altona, where for many years she was the sole medical provider, according to the lawsuit. Lashway’s duties, which include diagnoses and treatment of inmates’ medical conditions, mirror those performed by clinical physicians, the suit stated.
The plaintiffs are requesting the court accept jurisdiction of the matter and certify the class they seek to represent. They are also demanding prospective pay equity and compensatory damages for the distress caused by “the long-standing discriminatory” treatment by the state.
The Civil Service Commission and state of New York have not yet responded to the complaint. Their responses are due on November 12.
Attorney: Case Impact Limited
Benjamin McMichael, PhD, JD, said the New York case is not surprising as more states across the country are granting nurses more practice autonomy. The current landscape tends to favor the nurses, he said, with about half of states now allowing NPs full practice authority.
“I think the [New York] NPs are correct that they are underpaid,” said McMichael, an associate professor of law and director of the Interdisciplinary Legal Studies Initiative at The University of Alabama in Tuscaloosa. “With that said, the nature of the case does not clearly lend itself to national change.”
The fact that the NP plaintiffs are employed by the state means they are using a specific set of laws to advance their cause, he said. Other NPs in other employment situations may not have access to the same laws.
A version of this article first appeared on Medscape.com.
A
The New York State Civil Service Commission understates the job function of NPs, overstates their dependence on physicians, and inadequately pays them for their work, according to the complaint filed in the US District Court for the Northern District of New York.
The nurses claim the mistreatment is a consequence of the fact that “at least 80% of the state’s employed NPs are women.”
Michael H. Sussman, a Goshen, New York–based attorney for the nurses, said in an interview that New York NPs are increasingly being used essentially as doctors at state-run facilities, including prisons, yet the state has failed to adequately pay them.
The lawsuit comes after a decade-long attempt by NPs to attain equitable pay and the ability to advance their civil service careers, he said.
“New York state has not addressed the heart of the issue, which is that the classification of this position is much lower than other positions in the state which are not so female-dominated and which engage in very similar activities,” Sussman said.
The lawsuit claims that “the work of NPs is complex, equaling that of a medical specialist, psychiatrist, or clinical physician.”
A spokesman for the New York State Civil Service Commission declined comment, saying the department does not comment on pending litigation.
Novel Gender Discrimination Argument
Gender discrimination is a relatively new argument avenue in the larger equal work, equal pay debate, said Joanne Spetz, PhD, director of the Institute for Health Policy Studies at the University of California, San Francisco.
“This is the first time I’ve heard of [such] a case being really gender discrimination focused,” she said in an interview. “On one level, I think it’s groundbreaking as a legal approach, but it’s also limited because it’s focused on public, state employees.”
Spetz noted that New York has significantly expanded NPs’ scope of practice, enacting in 2022 legislation that granted NPs full practice authority. The law means NPs can evaluate, order, diagnose, manage treatments, and prescribe medications for patients without physician supervision.
“They are in a role where they are stepping back and saying, ‘Wait, why are [we] not receiving equal pay for equal work?’ ” Spetz said. “It’s a totally fair area for debate, especially because they are now authorized to do essentially equal work with a high degree of autonomy.”
Debate Over Pay Grade
The nurses’ complaint centers on the New York State Civil Service Commission’s classification for NPs, which hasn’t changed since 2006. NPs are classified at grade 24, and they have no possibility of internal advancement associated with their title, according to the legal complaint filed on September 17.
To comply with a state legislative directive, the commission in 2018 conducted a study of the NP classification but recommended against reclassification or implementing a career ladder. The study noted the subordinate role of NPs to physicians and the substantial difference between physician classification (entry at grade 34) and that of NPs, psychologists (grade 25), and pharmacists (grade 25).
The study concluded that higher classified positions have higher levels of educational attainment and licensure requirements and no supervision or collaboration requirements, according to the complaint.
At the time, groups such as the Nurse Practitioner Association and the Public Employees Federation (PEF) criticized the findings, but the commission stuck to its classification.
Following the NP Modernization Act that allowed NPs to practice independently, PEF sought an increase for NPs to grade 28 with a progression to grade 34 depending on experience.
“But to this date, despite altering the starting salaries of NPs, defendants have failed and refused to alter the compensation offered to the substantial majority of NPs, and each plaintiff remains cabined in a grade 24 with a discriminatorily low salary when compared with males in other job classifications doing highly similar functions,” the lawsuit contended.
Six plaintiffs are named in the lawsuit, all of whom are women and work for state agencies. Plaintiff Rachel Burns, for instance, works as a psychiatric mental health NP in West Seneca and is responsible for performing psychiatric evaluations for patients, diagnosis, prescribing medication, ordering labs, and determining risks. The evaluations are identical for a psychiatrist and require her to complete the same forms, according to the suit.
Another plaintiff, Amber Hawthorne Lashway, works at a correctional facility in Altona, where for many years she was the sole medical provider, according to the lawsuit. Lashway’s duties, which include diagnoses and treatment of inmates’ medical conditions, mirror those performed by clinical physicians, the suit stated.
The plaintiffs are requesting the court accept jurisdiction of the matter and certify the class they seek to represent. They are also demanding prospective pay equity and compensatory damages for the distress caused by “the long-standing discriminatory” treatment by the state.
The Civil Service Commission and state of New York have not yet responded to the complaint. Their responses are due on November 12.
Attorney: Case Impact Limited
Benjamin McMichael, PhD, JD, said the New York case is not surprising as more states across the country are granting nurses more practice autonomy. The current landscape tends to favor the nurses, he said, with about half of states now allowing NPs full practice authority.
“I think the [New York] NPs are correct that they are underpaid,” said McMichael, an associate professor of law and director of the Interdisciplinary Legal Studies Initiative at The University of Alabama in Tuscaloosa. “With that said, the nature of the case does not clearly lend itself to national change.”
The fact that the NP plaintiffs are employed by the state means they are using a specific set of laws to advance their cause, he said. Other NPs in other employment situations may not have access to the same laws.
A version of this article first appeared on Medscape.com.
A
The New York State Civil Service Commission understates the job function of NPs, overstates their dependence on physicians, and inadequately pays them for their work, according to the complaint filed in the US District Court for the Northern District of New York.
The nurses claim the mistreatment is a consequence of the fact that “at least 80% of the state’s employed NPs are women.”
Michael H. Sussman, a Goshen, New York–based attorney for the nurses, said in an interview that New York NPs are increasingly being used essentially as doctors at state-run facilities, including prisons, yet the state has failed to adequately pay them.
The lawsuit comes after a decade-long attempt by NPs to attain equitable pay and the ability to advance their civil service careers, he said.
“New York state has not addressed the heart of the issue, which is that the classification of this position is much lower than other positions in the state which are not so female-dominated and which engage in very similar activities,” Sussman said.
The lawsuit claims that “the work of NPs is complex, equaling that of a medical specialist, psychiatrist, or clinical physician.”
A spokesman for the New York State Civil Service Commission declined comment, saying the department does not comment on pending litigation.
Novel Gender Discrimination Argument
Gender discrimination is a relatively new argument avenue in the larger equal work, equal pay debate, said Joanne Spetz, PhD, director of the Institute for Health Policy Studies at the University of California, San Francisco.
“This is the first time I’ve heard of [such] a case being really gender discrimination focused,” she said in an interview. “On one level, I think it’s groundbreaking as a legal approach, but it’s also limited because it’s focused on public, state employees.”
Spetz noted that New York has significantly expanded NPs’ scope of practice, enacting in 2022 legislation that granted NPs full practice authority. The law means NPs can evaluate, order, diagnose, manage treatments, and prescribe medications for patients without physician supervision.
“They are in a role where they are stepping back and saying, ‘Wait, why are [we] not receiving equal pay for equal work?’ ” Spetz said. “It’s a totally fair area for debate, especially because they are now authorized to do essentially equal work with a high degree of autonomy.”
Debate Over Pay Grade
The nurses’ complaint centers on the New York State Civil Service Commission’s classification for NPs, which hasn’t changed since 2006. NPs are classified at grade 24, and they have no possibility of internal advancement associated with their title, according to the legal complaint filed on September 17.
To comply with a state legislative directive, the commission in 2018 conducted a study of the NP classification but recommended against reclassification or implementing a career ladder. The study noted the subordinate role of NPs to physicians and the substantial difference between physician classification (entry at grade 34) and that of NPs, psychologists (grade 25), and pharmacists (grade 25).
The study concluded that higher classified positions have higher levels of educational attainment and licensure requirements and no supervision or collaboration requirements, according to the complaint.
At the time, groups such as the Nurse Practitioner Association and the Public Employees Federation (PEF) criticized the findings, but the commission stuck to its classification.
Following the NP Modernization Act that allowed NPs to practice independently, PEF sought an increase for NPs to grade 28 with a progression to grade 34 depending on experience.
“But to this date, despite altering the starting salaries of NPs, defendants have failed and refused to alter the compensation offered to the substantial majority of NPs, and each plaintiff remains cabined in a grade 24 with a discriminatorily low salary when compared with males in other job classifications doing highly similar functions,” the lawsuit contended.
Six plaintiffs are named in the lawsuit, all of whom are women and work for state agencies. Plaintiff Rachel Burns, for instance, works as a psychiatric mental health NP in West Seneca and is responsible for performing psychiatric evaluations for patients, diagnosis, prescribing medication, ordering labs, and determining risks. The evaluations are identical for a psychiatrist and require her to complete the same forms, according to the suit.
Another plaintiff, Amber Hawthorne Lashway, works at a correctional facility in Altona, where for many years she was the sole medical provider, according to the lawsuit. Lashway’s duties, which include diagnoses and treatment of inmates’ medical conditions, mirror those performed by clinical physicians, the suit stated.
The plaintiffs are requesting the court accept jurisdiction of the matter and certify the class they seek to represent. They are also demanding prospective pay equity and compensatory damages for the distress caused by “the long-standing discriminatory” treatment by the state.
The Civil Service Commission and state of New York have not yet responded to the complaint. Their responses are due on November 12.
Attorney: Case Impact Limited
Benjamin McMichael, PhD, JD, said the New York case is not surprising as more states across the country are granting nurses more practice autonomy. The current landscape tends to favor the nurses, he said, with about half of states now allowing NPs full practice authority.
“I think the [New York] NPs are correct that they are underpaid,” said McMichael, an associate professor of law and director of the Interdisciplinary Legal Studies Initiative at The University of Alabama in Tuscaloosa. “With that said, the nature of the case does not clearly lend itself to national change.”
The fact that the NP plaintiffs are employed by the state means they are using a specific set of laws to advance their cause, he said. Other NPs in other employment situations may not have access to the same laws.
A version of this article first appeared on Medscape.com.
Beyond Scope Creep: Why Physicians and PAs Should Come Together for Patients
Over the past few years, many states have attempted to address the ongoing shortage of healthcare workers by introducing new bills to increase the scope of practice for nurse practitioners (NPs) and physician assistants (PAs). The goal of each bill was to improve access to care, particularly for patients who may live in areas where it’s difficult to find a doctor.
In response, the American Medical Association (AMA) launched a targeted campaign to fight “scope creep.” Their goal was to gain the momentum necessary to block proposed legislation to modify or expand the practice authority of nonphysicians, including PAs. A spokesperson for the organization told this news organization that the AMA “greatly values and respects the contributions of PAs as important members of the healthcare team” but emphasized that they do not have the same “skill set or breadth of experience of physicians.”
As such, the AMA argued that expanded practice authority would not only dismantle physician-led care teams but also ultimately lead to higher costs and lower-quality patient care.
The AMA has since launched a large-scale advocacy effort to fight practice expansion legislation — and has a specific page on its website to highlight those efforts. In addition, they have authored model legislation, talking points for AMA members, and a widely read article in AMA News to help them in what they call a “fight for physicians.”
These resources have also been disseminated to the greater healthcare stakeholder community.
Marilyn Suri, PA-C, chief operating officer and senior executive for Advanced Practice Professional Affairs at Vincenzo Novara MDPA and Associates, a critical care pulmonary medicine practice in Miami, Florida, said she found the AMA’s campaign to be “very misleading.”
“PAs are created in the image of physicians to help manage the physician shortage. We are trained very rigorously — to diagnose illness, develop treatment plans, and prescribe medications,” she said. “We’re not trying to expand our scope. We are trying to eliminate or lessen barriers that prevent patients from getting access to care.”
Suri is not alone. Last summer, the American Academy of Physician Associates (AAPA) requested a meeting with the AMA to find ways for the two organizations to collaborate to improve care delivery — as well as find common ground to address issues regarding patient access to care. When the AMA did not respond, the AAPA sent a second letter in September 2024, reiterating their request for a meeting.
That correspondence also included a letter, signed by more than 8000 PAs from across the country, calling for an end to what the AAPA refers to as “damaging rhetoric,” as well as data from a recent survey of PAs regarding the fallout of AMA’s scope creep messaging.
Those survey results highlighted that the vast majority of PAs surveyed feel that the AMA is doing more than just attacking proposed legislation: They believe the association is negatively influencing patients’ understanding of PA qualifications, ultimately affecting their ability to provide care.
“The campaign is unintentionally harming patients by suggesting we are doing more than what we are trained to do,” said Elisa Hock, PA-C, a behavioral health PA in Texas. “And when you work in a place with limited resources, medically speaking — including limited access to providers — this kind of campaign is really detrimental to helping patients.”
Lisa M. Gables, CEO of the AAPA, said the organization is “deeply disappointed” in the AMA’s lack of response to their letters thus far — but remains committed to working with the organization to bring forward new solutions to address healthcare’s most pressing challenges.
“AAPA remains committed to pushing for modernization of practice laws to ensure all providers can practice medicine to the fullest extent of their training, education, and experience,” she said. “That is what patients deserve and want.”
Hock agreed. She told this news organization that the public is not always aware of what PAs can offer in terms of patient care. That said, she believes newer generations of physicians understand the value of PAs and the many skills they bring to the table.
“I’ve been doing this for 17 years, and it’s been an uphill battle, at times, to educate the public about what PAs can and can’t do,” she explained. “To throw more mud in the mix that will confuse patients more about what we do doesn’t help. Healthcare works best with a team-based approach. And that team has been and always will be led by the physician. We are aware of our role and our limitations. But we also know what we can offer patients, especially in areas like El Paso, where there is a real shortage of providers.”
With a growing aging population — and the physician shortage expected to increase in the coming decade — Suri hopes that the AMA will accept AAPA’s invitation to meet — because no one wins with this kind of healthcare infighting. In fact, she said patients will suffer because of it. She hopes that future discussions and collaborations can show providers and patients what team-based healthcare can offer.
“I think it’s important for those in healthcare to be aware that none of us work alone. Even physicians collaborate with other subspecialties, as well as nurses and other healthcare professionals,” said Suri.
A version of this article appeared on Medscape.com.
Over the past few years, many states have attempted to address the ongoing shortage of healthcare workers by introducing new bills to increase the scope of practice for nurse practitioners (NPs) and physician assistants (PAs). The goal of each bill was to improve access to care, particularly for patients who may live in areas where it’s difficult to find a doctor.
In response, the American Medical Association (AMA) launched a targeted campaign to fight “scope creep.” Their goal was to gain the momentum necessary to block proposed legislation to modify or expand the practice authority of nonphysicians, including PAs. A spokesperson for the organization told this news organization that the AMA “greatly values and respects the contributions of PAs as important members of the healthcare team” but emphasized that they do not have the same “skill set or breadth of experience of physicians.”
As such, the AMA argued that expanded practice authority would not only dismantle physician-led care teams but also ultimately lead to higher costs and lower-quality patient care.
The AMA has since launched a large-scale advocacy effort to fight practice expansion legislation — and has a specific page on its website to highlight those efforts. In addition, they have authored model legislation, talking points for AMA members, and a widely read article in AMA News to help them in what they call a “fight for physicians.”
These resources have also been disseminated to the greater healthcare stakeholder community.
Marilyn Suri, PA-C, chief operating officer and senior executive for Advanced Practice Professional Affairs at Vincenzo Novara MDPA and Associates, a critical care pulmonary medicine practice in Miami, Florida, said she found the AMA’s campaign to be “very misleading.”
“PAs are created in the image of physicians to help manage the physician shortage. We are trained very rigorously — to diagnose illness, develop treatment plans, and prescribe medications,” she said. “We’re not trying to expand our scope. We are trying to eliminate or lessen barriers that prevent patients from getting access to care.”
Suri is not alone. Last summer, the American Academy of Physician Associates (AAPA) requested a meeting with the AMA to find ways for the two organizations to collaborate to improve care delivery — as well as find common ground to address issues regarding patient access to care. When the AMA did not respond, the AAPA sent a second letter in September 2024, reiterating their request for a meeting.
That correspondence also included a letter, signed by more than 8000 PAs from across the country, calling for an end to what the AAPA refers to as “damaging rhetoric,” as well as data from a recent survey of PAs regarding the fallout of AMA’s scope creep messaging.
Those survey results highlighted that the vast majority of PAs surveyed feel that the AMA is doing more than just attacking proposed legislation: They believe the association is negatively influencing patients’ understanding of PA qualifications, ultimately affecting their ability to provide care.
“The campaign is unintentionally harming patients by suggesting we are doing more than what we are trained to do,” said Elisa Hock, PA-C, a behavioral health PA in Texas. “And when you work in a place with limited resources, medically speaking — including limited access to providers — this kind of campaign is really detrimental to helping patients.”
Lisa M. Gables, CEO of the AAPA, said the organization is “deeply disappointed” in the AMA’s lack of response to their letters thus far — but remains committed to working with the organization to bring forward new solutions to address healthcare’s most pressing challenges.
“AAPA remains committed to pushing for modernization of practice laws to ensure all providers can practice medicine to the fullest extent of their training, education, and experience,” she said. “That is what patients deserve and want.”
Hock agreed. She told this news organization that the public is not always aware of what PAs can offer in terms of patient care. That said, she believes newer generations of physicians understand the value of PAs and the many skills they bring to the table.
“I’ve been doing this for 17 years, and it’s been an uphill battle, at times, to educate the public about what PAs can and can’t do,” she explained. “To throw more mud in the mix that will confuse patients more about what we do doesn’t help. Healthcare works best with a team-based approach. And that team has been and always will be led by the physician. We are aware of our role and our limitations. But we also know what we can offer patients, especially in areas like El Paso, where there is a real shortage of providers.”
With a growing aging population — and the physician shortage expected to increase in the coming decade — Suri hopes that the AMA will accept AAPA’s invitation to meet — because no one wins with this kind of healthcare infighting. In fact, she said patients will suffer because of it. She hopes that future discussions and collaborations can show providers and patients what team-based healthcare can offer.
“I think it’s important for those in healthcare to be aware that none of us work alone. Even physicians collaborate with other subspecialties, as well as nurses and other healthcare professionals,” said Suri.
A version of this article appeared on Medscape.com.
Over the past few years, many states have attempted to address the ongoing shortage of healthcare workers by introducing new bills to increase the scope of practice for nurse practitioners (NPs) and physician assistants (PAs). The goal of each bill was to improve access to care, particularly for patients who may live in areas where it’s difficult to find a doctor.
In response, the American Medical Association (AMA) launched a targeted campaign to fight “scope creep.” Their goal was to gain the momentum necessary to block proposed legislation to modify or expand the practice authority of nonphysicians, including PAs. A spokesperson for the organization told this news organization that the AMA “greatly values and respects the contributions of PAs as important members of the healthcare team” but emphasized that they do not have the same “skill set or breadth of experience of physicians.”
As such, the AMA argued that expanded practice authority would not only dismantle physician-led care teams but also ultimately lead to higher costs and lower-quality patient care.
The AMA has since launched a large-scale advocacy effort to fight practice expansion legislation — and has a specific page on its website to highlight those efforts. In addition, they have authored model legislation, talking points for AMA members, and a widely read article in AMA News to help them in what they call a “fight for physicians.”
These resources have also been disseminated to the greater healthcare stakeholder community.
Marilyn Suri, PA-C, chief operating officer and senior executive for Advanced Practice Professional Affairs at Vincenzo Novara MDPA and Associates, a critical care pulmonary medicine practice in Miami, Florida, said she found the AMA’s campaign to be “very misleading.”
“PAs are created in the image of physicians to help manage the physician shortage. We are trained very rigorously — to diagnose illness, develop treatment plans, and prescribe medications,” she said. “We’re not trying to expand our scope. We are trying to eliminate or lessen barriers that prevent patients from getting access to care.”
Suri is not alone. Last summer, the American Academy of Physician Associates (AAPA) requested a meeting with the AMA to find ways for the two organizations to collaborate to improve care delivery — as well as find common ground to address issues regarding patient access to care. When the AMA did not respond, the AAPA sent a second letter in September 2024, reiterating their request for a meeting.
That correspondence also included a letter, signed by more than 8000 PAs from across the country, calling for an end to what the AAPA refers to as “damaging rhetoric,” as well as data from a recent survey of PAs regarding the fallout of AMA’s scope creep messaging.
Those survey results highlighted that the vast majority of PAs surveyed feel that the AMA is doing more than just attacking proposed legislation: They believe the association is negatively influencing patients’ understanding of PA qualifications, ultimately affecting their ability to provide care.
“The campaign is unintentionally harming patients by suggesting we are doing more than what we are trained to do,” said Elisa Hock, PA-C, a behavioral health PA in Texas. “And when you work in a place with limited resources, medically speaking — including limited access to providers — this kind of campaign is really detrimental to helping patients.”
Lisa M. Gables, CEO of the AAPA, said the organization is “deeply disappointed” in the AMA’s lack of response to their letters thus far — but remains committed to working with the organization to bring forward new solutions to address healthcare’s most pressing challenges.
“AAPA remains committed to pushing for modernization of practice laws to ensure all providers can practice medicine to the fullest extent of their training, education, and experience,” she said. “That is what patients deserve and want.”
Hock agreed. She told this news organization that the public is not always aware of what PAs can offer in terms of patient care. That said, she believes newer generations of physicians understand the value of PAs and the many skills they bring to the table.
“I’ve been doing this for 17 years, and it’s been an uphill battle, at times, to educate the public about what PAs can and can’t do,” she explained. “To throw more mud in the mix that will confuse patients more about what we do doesn’t help. Healthcare works best with a team-based approach. And that team has been and always will be led by the physician. We are aware of our role and our limitations. But we also know what we can offer patients, especially in areas like El Paso, where there is a real shortage of providers.”
With a growing aging population — and the physician shortage expected to increase in the coming decade — Suri hopes that the AMA will accept AAPA’s invitation to meet — because no one wins with this kind of healthcare infighting. In fact, she said patients will suffer because of it. She hopes that future discussions and collaborations can show providers and patients what team-based healthcare can offer.
“I think it’s important for those in healthcare to be aware that none of us work alone. Even physicians collaborate with other subspecialties, as well as nurses and other healthcare professionals,” said Suri.
A version of this article appeared on Medscape.com.
Lawsuit Targets Publishers: Is Peer Review Flawed?
The peer-review process, which is used by scientific journals to validate legitimate research, is now under legal scrutiny. The US District Court for the Southern District of New York will soon rule on whether scientific publishers have compromised this system for profit. In mid-September, University of California, Los Angeles neuroscientist Lucina Uddin filed a class action lawsuit against six leading academic publishers — Elsevier, Wolters Kluwer, Wiley, Sage Publications, Taylor & Francis, and Springer Nature — accusing them of violating antitrust laws and obstructing academic research.
The lawsuit targets several long-standing practices in scientific publishing, including the lack of compensation for peer reviewers, restrictions that require submitting to only one journal at a time, and bans on sharing manuscripts under review. Uddin’s complaint argues that these practices contribute to inefficiencies in the review process, thus delaying the publication of critical discoveries, which could hinder research, clinical advancements, and the development of new medical treatments.
The suit also noted that these publishers generated $10 billion in revenue in 2023 in peer-reviewed journals. However, the complaint seemingly overlooks the widespread practice of preprint repositories, where many manuscripts are shared while awaiting peer review.
Flawed Reviews
A growing number of studies have highlighted subpar or unethical behaviors among reviewers, who are supposed to adhere to the highest standards of methodological rigor, both in conducting research and reviewing work for journals. One recent study published in Scientometrics in August examined 263 reviews from 37 journals across various disciplines and found alarming patterns of duplication. Many of the reviews contained identical or highly similar language. Some reviewers were found to be suggesting that the authors expand their bibliographies to include the reviewers’ own work, thus inflating their citation counts.
As María Ángeles Oviedo-García from the University of Seville in Spain, pointed out: “The analysis of 263 review reports shows a pattern of vague, repetitive statements — often identical or very similar — along with coercive citations, ultimately resulting in misleading reviews.”
Experts in research integrity and ethics argue that while issues persist, the integrity of scientific research is improving. Increasing research and public disclosure reflect a heightened awareness of problems long overlooked.
Speaking to this news organization, Fanelli, who has been studying scientific misconduct for about 20 years, noted that while his early work left him disillusioned, further research has replaced his cynicism with what he describes as healthy skepticism and a more optimistic outlook. Fanelli also collaborates with the Luxembourg Agency for Research Integrity and the Advisory Committee on Research Ethics and Bioethics at the Italian National Research Council (CNR), where he helped develop the first research integrity guidelines.
Lack of Awareness
A recurring challenge is the difficulty in distinguishing between honest mistakes and intentional misconduct. “This is why greater investment in education is essential,” said Daniel Pizzolato, European Network of Research Ethics Committees, Bonn, Germany, and the Centre for Biomedical Ethics and Law, KU Leuven in Belgium.
While Pizzolato acknowledged that institutions such as the CNR in Italy provide a positive example, awareness of research integrity is generally still lacking across much of Europe, and there are few offices dedicated to promoting research integrity. However, he pointed to promising developments in other countries. “In France and Denmark, researchers are required to be familiar with integrity norms because codes of conduct have legal standing. Some major international funding bodies like the European Molecular Biology Organization are making participation in research integrity courses a condition for receiving grants.”
Pizzolato remains optimistic. “There is a growing willingness to move past this impasse,” he said.
A recent study published in The Journal of Clinical Epidemiology reveals troubling gaps in how retracted biomedical articles are flagged and cited. Led by Caitlin Bakkera, Department of Epidemiology, Maastricht University, Maastricht, the Netherlands, the research sought to determine whether articles retracted because of errors or fraud were properly flagged across various databases.
The results were concerning: Less than 5% of retracted articles had consistent retraction notices across all databases that hosted them, and less than 50% of citations referenced the retraction. None of the 414 retraction notices analyzed met best-practice guidelines for completeness. Bakkera and colleagues warned that these shortcomings threaten the integrity of public health research.
Fanelli’s Perspective
Despite the concerns, Fanelli remains calm. “Science is based on debate and a perspective called organized skepticism, which helps reveal the truth,” he explained. “While there is often excessive skepticism today, the overall quality of clinical trials is improving.
“It’s important to remember that reliable results take time and shouldn’t depend on the outcome of a single study. It’s essential to consider the broader context, the history of the research field, and potential conflicts of interest, both financial and otherwise. Biomedical research requires constant updates,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The peer-review process, which is used by scientific journals to validate legitimate research, is now under legal scrutiny. The US District Court for the Southern District of New York will soon rule on whether scientific publishers have compromised this system for profit. In mid-September, University of California, Los Angeles neuroscientist Lucina Uddin filed a class action lawsuit against six leading academic publishers — Elsevier, Wolters Kluwer, Wiley, Sage Publications, Taylor & Francis, and Springer Nature — accusing them of violating antitrust laws and obstructing academic research.
The lawsuit targets several long-standing practices in scientific publishing, including the lack of compensation for peer reviewers, restrictions that require submitting to only one journal at a time, and bans on sharing manuscripts under review. Uddin’s complaint argues that these practices contribute to inefficiencies in the review process, thus delaying the publication of critical discoveries, which could hinder research, clinical advancements, and the development of new medical treatments.
The suit also noted that these publishers generated $10 billion in revenue in 2023 in peer-reviewed journals. However, the complaint seemingly overlooks the widespread practice of preprint repositories, where many manuscripts are shared while awaiting peer review.
Flawed Reviews
A growing number of studies have highlighted subpar or unethical behaviors among reviewers, who are supposed to adhere to the highest standards of methodological rigor, both in conducting research and reviewing work for journals. One recent study published in Scientometrics in August examined 263 reviews from 37 journals across various disciplines and found alarming patterns of duplication. Many of the reviews contained identical or highly similar language. Some reviewers were found to be suggesting that the authors expand their bibliographies to include the reviewers’ own work, thus inflating their citation counts.
As María Ángeles Oviedo-García from the University of Seville in Spain, pointed out: “The analysis of 263 review reports shows a pattern of vague, repetitive statements — often identical or very similar — along with coercive citations, ultimately resulting in misleading reviews.”
Experts in research integrity and ethics argue that while issues persist, the integrity of scientific research is improving. Increasing research and public disclosure reflect a heightened awareness of problems long overlooked.
Speaking to this news organization, Fanelli, who has been studying scientific misconduct for about 20 years, noted that while his early work left him disillusioned, further research has replaced his cynicism with what he describes as healthy skepticism and a more optimistic outlook. Fanelli also collaborates with the Luxembourg Agency for Research Integrity and the Advisory Committee on Research Ethics and Bioethics at the Italian National Research Council (CNR), where he helped develop the first research integrity guidelines.
Lack of Awareness
A recurring challenge is the difficulty in distinguishing between honest mistakes and intentional misconduct. “This is why greater investment in education is essential,” said Daniel Pizzolato, European Network of Research Ethics Committees, Bonn, Germany, and the Centre for Biomedical Ethics and Law, KU Leuven in Belgium.
While Pizzolato acknowledged that institutions such as the CNR in Italy provide a positive example, awareness of research integrity is generally still lacking across much of Europe, and there are few offices dedicated to promoting research integrity. However, he pointed to promising developments in other countries. “In France and Denmark, researchers are required to be familiar with integrity norms because codes of conduct have legal standing. Some major international funding bodies like the European Molecular Biology Organization are making participation in research integrity courses a condition for receiving grants.”
Pizzolato remains optimistic. “There is a growing willingness to move past this impasse,” he said.
A recent study published in The Journal of Clinical Epidemiology reveals troubling gaps in how retracted biomedical articles are flagged and cited. Led by Caitlin Bakkera, Department of Epidemiology, Maastricht University, Maastricht, the Netherlands, the research sought to determine whether articles retracted because of errors or fraud were properly flagged across various databases.
The results were concerning: Less than 5% of retracted articles had consistent retraction notices across all databases that hosted them, and less than 50% of citations referenced the retraction. None of the 414 retraction notices analyzed met best-practice guidelines for completeness. Bakkera and colleagues warned that these shortcomings threaten the integrity of public health research.
Fanelli’s Perspective
Despite the concerns, Fanelli remains calm. “Science is based on debate and a perspective called organized skepticism, which helps reveal the truth,” he explained. “While there is often excessive skepticism today, the overall quality of clinical trials is improving.
“It’s important to remember that reliable results take time and shouldn’t depend on the outcome of a single study. It’s essential to consider the broader context, the history of the research field, and potential conflicts of interest, both financial and otherwise. Biomedical research requires constant updates,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The peer-review process, which is used by scientific journals to validate legitimate research, is now under legal scrutiny. The US District Court for the Southern District of New York will soon rule on whether scientific publishers have compromised this system for profit. In mid-September, University of California, Los Angeles neuroscientist Lucina Uddin filed a class action lawsuit against six leading academic publishers — Elsevier, Wolters Kluwer, Wiley, Sage Publications, Taylor & Francis, and Springer Nature — accusing them of violating antitrust laws and obstructing academic research.
The lawsuit targets several long-standing practices in scientific publishing, including the lack of compensation for peer reviewers, restrictions that require submitting to only one journal at a time, and bans on sharing manuscripts under review. Uddin’s complaint argues that these practices contribute to inefficiencies in the review process, thus delaying the publication of critical discoveries, which could hinder research, clinical advancements, and the development of new medical treatments.
The suit also noted that these publishers generated $10 billion in revenue in 2023 in peer-reviewed journals. However, the complaint seemingly overlooks the widespread practice of preprint repositories, where many manuscripts are shared while awaiting peer review.
Flawed Reviews
A growing number of studies have highlighted subpar or unethical behaviors among reviewers, who are supposed to adhere to the highest standards of methodological rigor, both in conducting research and reviewing work for journals. One recent study published in Scientometrics in August examined 263 reviews from 37 journals across various disciplines and found alarming patterns of duplication. Many of the reviews contained identical or highly similar language. Some reviewers were found to be suggesting that the authors expand their bibliographies to include the reviewers’ own work, thus inflating their citation counts.
As María Ángeles Oviedo-García from the University of Seville in Spain, pointed out: “The analysis of 263 review reports shows a pattern of vague, repetitive statements — often identical or very similar — along with coercive citations, ultimately resulting in misleading reviews.”
Experts in research integrity and ethics argue that while issues persist, the integrity of scientific research is improving. Increasing research and public disclosure reflect a heightened awareness of problems long overlooked.
Speaking to this news organization, Fanelli, who has been studying scientific misconduct for about 20 years, noted that while his early work left him disillusioned, further research has replaced his cynicism with what he describes as healthy skepticism and a more optimistic outlook. Fanelli also collaborates with the Luxembourg Agency for Research Integrity and the Advisory Committee on Research Ethics and Bioethics at the Italian National Research Council (CNR), where he helped develop the first research integrity guidelines.
Lack of Awareness
A recurring challenge is the difficulty in distinguishing between honest mistakes and intentional misconduct. “This is why greater investment in education is essential,” said Daniel Pizzolato, European Network of Research Ethics Committees, Bonn, Germany, and the Centre for Biomedical Ethics and Law, KU Leuven in Belgium.
While Pizzolato acknowledged that institutions such as the CNR in Italy provide a positive example, awareness of research integrity is generally still lacking across much of Europe, and there are few offices dedicated to promoting research integrity. However, he pointed to promising developments in other countries. “In France and Denmark, researchers are required to be familiar with integrity norms because codes of conduct have legal standing. Some major international funding bodies like the European Molecular Biology Organization are making participation in research integrity courses a condition for receiving grants.”
Pizzolato remains optimistic. “There is a growing willingness to move past this impasse,” he said.
A recent study published in The Journal of Clinical Epidemiology reveals troubling gaps in how retracted biomedical articles are flagged and cited. Led by Caitlin Bakkera, Department of Epidemiology, Maastricht University, Maastricht, the Netherlands, the research sought to determine whether articles retracted because of errors or fraud were properly flagged across various databases.
The results were concerning: Less than 5% of retracted articles had consistent retraction notices across all databases that hosted them, and less than 50% of citations referenced the retraction. None of the 414 retraction notices analyzed met best-practice guidelines for completeness. Bakkera and colleagues warned that these shortcomings threaten the integrity of public health research.
Fanelli’s Perspective
Despite the concerns, Fanelli remains calm. “Science is based on debate and a perspective called organized skepticism, which helps reveal the truth,” he explained. “While there is often excessive skepticism today, the overall quality of clinical trials is improving.
“It’s important to remember that reliable results take time and shouldn’t depend on the outcome of a single study. It’s essential to consider the broader context, the history of the research field, and potential conflicts of interest, both financial and otherwise. Biomedical research requires constant updates,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
How AI Is Revolutionizing Drug Repurposing for Faster, Broader Impact
Summary:
In this segment, the speaker discusses how AI is revolutionizing the drug repurposing process. Previously, drug repurposing was limited by manual research on individual diseases and drugs. With AI, scientists can now analyze a vast array of drugs and diseases simultaneously, generating a ranking system based on the likelihood of success. The Center for Cytokine Storm Treatment and Laboratory, along with the platform Every Cure, uses AI to score 3000 drugs against 18,000 diseases. This platform dramatically reduces the time and resources required for drug repurposing, enabling predictions that can be tested in a fraction of the time.
Key Takeaways:
AI is accelerating the drug repurposing process, offering faster and more comprehensive analysis of possible drug-disease matches.
The AI-based platform assigns a likelihood score to each potential match, streamlining the process for testing and validation.
Our Editors Also Recommend:
AI’s Drug Revolution, Part 1: Faster Trials and Approvals
From AI to Obesity Drugs to Soaring Costs: Medscape Hot Topics in the Medical Profession Report 2024
AI Voice Analysis for Diabetes Screening Shows Promise
To see the full event recording, click here.
A version of this article appeared on Medscape.com.
Summary:
In this segment, the speaker discusses how AI is revolutionizing the drug repurposing process. Previously, drug repurposing was limited by manual research on individual diseases and drugs. With AI, scientists can now analyze a vast array of drugs and diseases simultaneously, generating a ranking system based on the likelihood of success. The Center for Cytokine Storm Treatment and Laboratory, along with the platform Every Cure, uses AI to score 3000 drugs against 18,000 diseases. This platform dramatically reduces the time and resources required for drug repurposing, enabling predictions that can be tested in a fraction of the time.
Key Takeaways:
AI is accelerating the drug repurposing process, offering faster and more comprehensive analysis of possible drug-disease matches.
The AI-based platform assigns a likelihood score to each potential match, streamlining the process for testing and validation.
Our Editors Also Recommend:
AI’s Drug Revolution, Part 1: Faster Trials and Approvals
From AI to Obesity Drugs to Soaring Costs: Medscape Hot Topics in the Medical Profession Report 2024
AI Voice Analysis for Diabetes Screening Shows Promise
To see the full event recording, click here.
A version of this article appeared on Medscape.com.
Summary:
In this segment, the speaker discusses how AI is revolutionizing the drug repurposing process. Previously, drug repurposing was limited by manual research on individual diseases and drugs. With AI, scientists can now analyze a vast array of drugs and diseases simultaneously, generating a ranking system based on the likelihood of success. The Center for Cytokine Storm Treatment and Laboratory, along with the platform Every Cure, uses AI to score 3000 drugs against 18,000 diseases. This platform dramatically reduces the time and resources required for drug repurposing, enabling predictions that can be tested in a fraction of the time.
Key Takeaways:
AI is accelerating the drug repurposing process, offering faster and more comprehensive analysis of possible drug-disease matches.
The AI-based platform assigns a likelihood score to each potential match, streamlining the process for testing and validation.
Our Editors Also Recommend:
AI’s Drug Revolution, Part 1: Faster Trials and Approvals
From AI to Obesity Drugs to Soaring Costs: Medscape Hot Topics in the Medical Profession Report 2024
AI Voice Analysis for Diabetes Screening Shows Promise
To see the full event recording, click here.
A version of this article appeared on Medscape.com.
Mycosis Fungoides: Measured Approach Key to Treatment
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
FROM PDA 2024