User login
Central Centrifugal Cicatricial Alopecia in Males: Analysis of Time to Diagnosis and Disease Severity
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
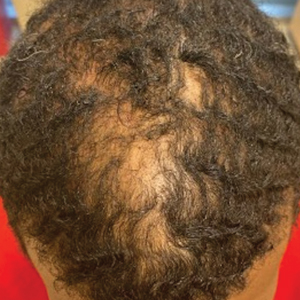
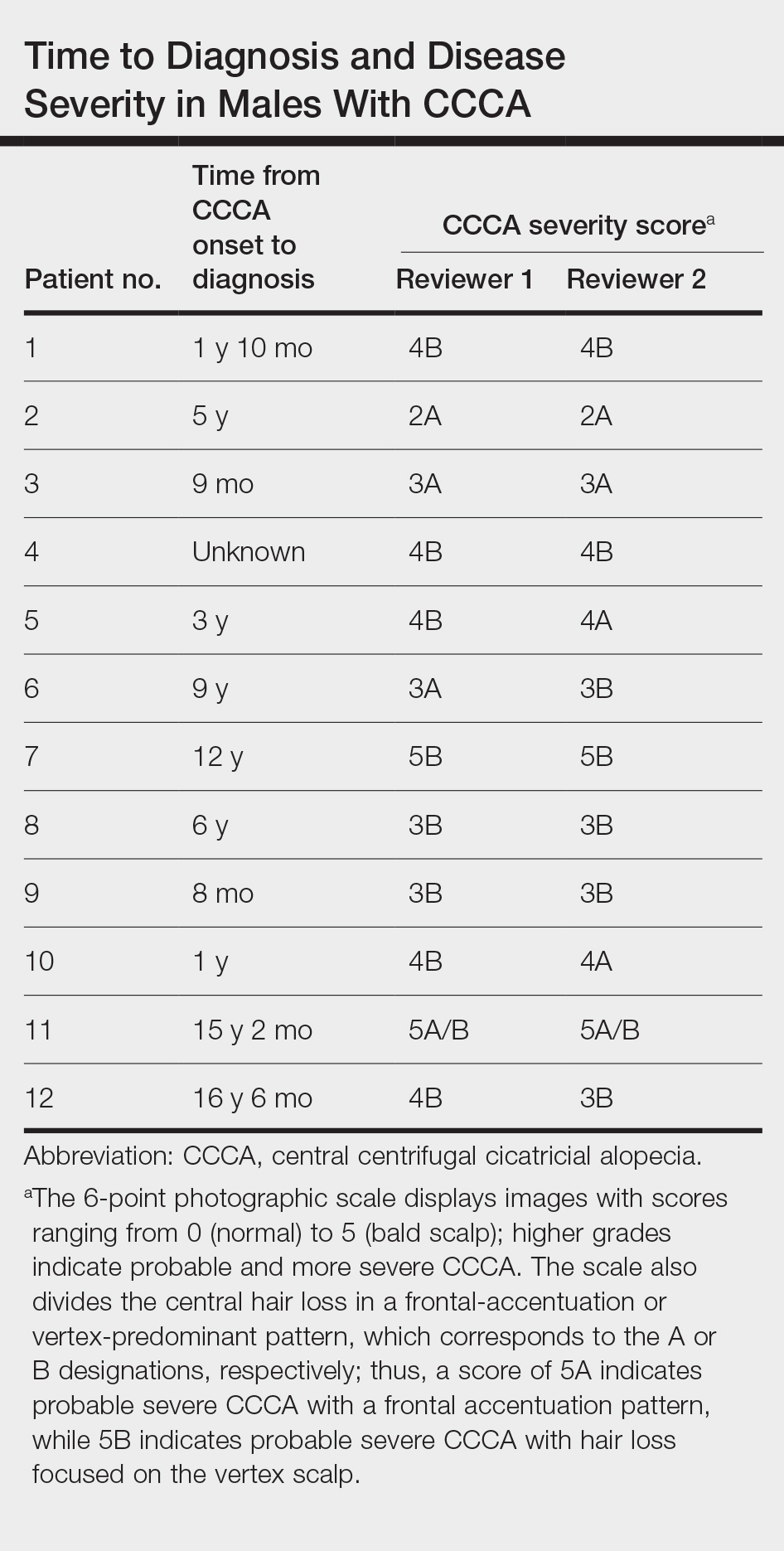
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.
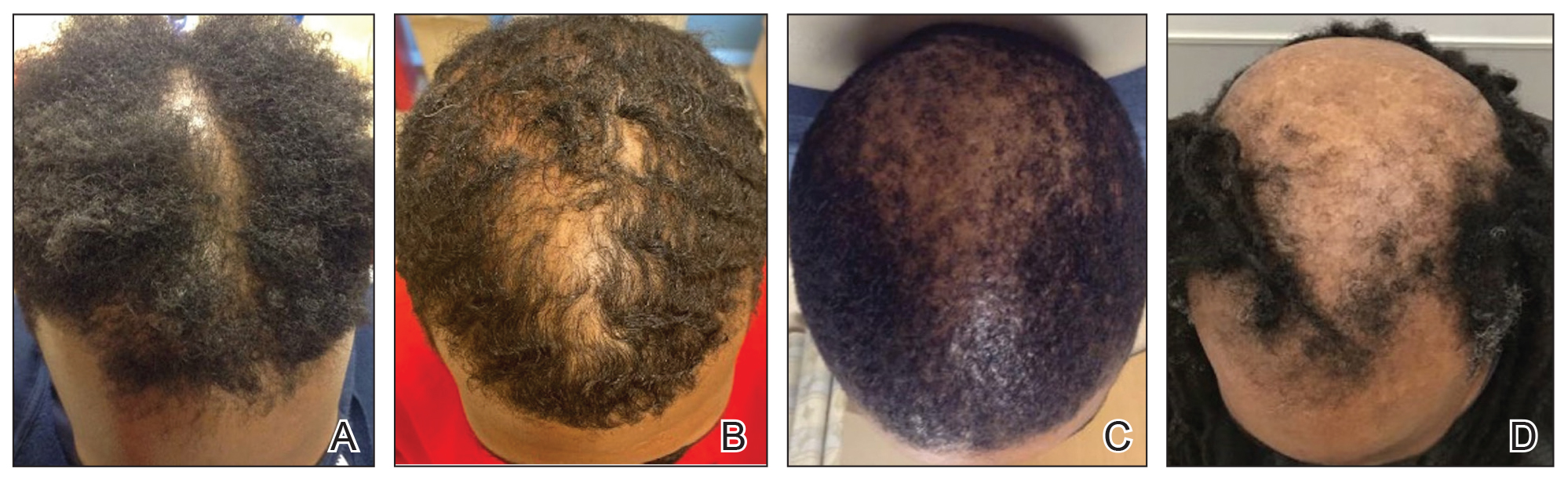
Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.


Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.


Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
Practice Points
- Most males with central centrifugal cicatricial alopecia (CCCA) experience considerable diagnostic delays and typically present to dermatology with late-stage disease.
- Dermatologists should consider CCCA in the differential diagnosis for adult Black males with alopecia.
- More research is needed to explore advanced CCCA in males, including factors limiting timely diagnosis and the impact on quality of life in this population.
Listen to earn your patients’ trust
Recently, I had an interesting conversation while getting my hair cut. It gave me a great deal of insight into some of the problems we have right now with how medical information is shared and some of the disconnect our patients may feel.
The young woman who was cutting my hair asked me what I did for an occupation. I said that I was a physician. She said, “Can I please ask you an important question?” She asked me what my thoughts were about the COVID vaccine. She prefaced it with “I am so confused on whether I should get the vaccine. I have seen a number of TikTok videos that talk about nano particles in the COVID vaccine that can be very dangerous.”
I discussed with her how the COVID vaccine actually works and shared with her the remarkable success of the vaccine. I asked her what side effects she was worried about from the vaccine and what her fears were. She said that she had heard that a lot of people had died from the vaccine. I told her that severe reactions from the vaccine were very uncommon.
She then made a very telling comment: “I wish I could talk to a doctor about my concerns. I have been going to the same health center for the last 5 years and every time I go I see a different person.” She added, “I rarely have more than 5-10 minutes with the person that I am seeing and I rarely get the opportunity to ask questions.”
She thanked me for the information and said that she would be getting the COVID vaccine in the future. She said it is so hard to know where to get information now and the very different things that she heard confused her. She told me that she thought her generation got most of its information from short sound bites or TikTok and Instagram videos.
Why did she trust me? I still think that the medical profession is respected. We are all pressured to do more with less time. Conversations where we can listen and then respond go a long way. We can always listen and learn what information people need and will appreciate. I was also struck by how alone this person felt in our health care system. She did not have a relationship with any one person whom she could trust and reach out to with questions. Relationships with our patients go a long way to establishing trust.
Pearl
It takes time to listen to and answer our patients’ questions. We need to do that to fight the waves of misinformation our patients face.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
Recently, I had an interesting conversation while getting my hair cut. It gave me a great deal of insight into some of the problems we have right now with how medical information is shared and some of the disconnect our patients may feel.
The young woman who was cutting my hair asked me what I did for an occupation. I said that I was a physician. She said, “Can I please ask you an important question?” She asked me what my thoughts were about the COVID vaccine. She prefaced it with “I am so confused on whether I should get the vaccine. I have seen a number of TikTok videos that talk about nano particles in the COVID vaccine that can be very dangerous.”
I discussed with her how the COVID vaccine actually works and shared with her the remarkable success of the vaccine. I asked her what side effects she was worried about from the vaccine and what her fears were. She said that she had heard that a lot of people had died from the vaccine. I told her that severe reactions from the vaccine were very uncommon.
She then made a very telling comment: “I wish I could talk to a doctor about my concerns. I have been going to the same health center for the last 5 years and every time I go I see a different person.” She added, “I rarely have more than 5-10 minutes with the person that I am seeing and I rarely get the opportunity to ask questions.”
She thanked me for the information and said that she would be getting the COVID vaccine in the future. She said it is so hard to know where to get information now and the very different things that she heard confused her. She told me that she thought her generation got most of its information from short sound bites or TikTok and Instagram videos.
Why did she trust me? I still think that the medical profession is respected. We are all pressured to do more with less time. Conversations where we can listen and then respond go a long way. We can always listen and learn what information people need and will appreciate. I was also struck by how alone this person felt in our health care system. She did not have a relationship with any one person whom she could trust and reach out to with questions. Relationships with our patients go a long way to establishing trust.
Pearl
It takes time to listen to and answer our patients’ questions. We need to do that to fight the waves of misinformation our patients face.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
Recently, I had an interesting conversation while getting my hair cut. It gave me a great deal of insight into some of the problems we have right now with how medical information is shared and some of the disconnect our patients may feel.
The young woman who was cutting my hair asked me what I did for an occupation. I said that I was a physician. She said, “Can I please ask you an important question?” She asked me what my thoughts were about the COVID vaccine. She prefaced it with “I am so confused on whether I should get the vaccine. I have seen a number of TikTok videos that talk about nano particles in the COVID vaccine that can be very dangerous.”
I discussed with her how the COVID vaccine actually works and shared with her the remarkable success of the vaccine. I asked her what side effects she was worried about from the vaccine and what her fears were. She said that she had heard that a lot of people had died from the vaccine. I told her that severe reactions from the vaccine were very uncommon.
She then made a very telling comment: “I wish I could talk to a doctor about my concerns. I have been going to the same health center for the last 5 years and every time I go I see a different person.” She added, “I rarely have more than 5-10 minutes with the person that I am seeing and I rarely get the opportunity to ask questions.”
She thanked me for the information and said that she would be getting the COVID vaccine in the future. She said it is so hard to know where to get information now and the very different things that she heard confused her. She told me that she thought her generation got most of its information from short sound bites or TikTok and Instagram videos.
Why did she trust me? I still think that the medical profession is respected. We are all pressured to do more with less time. Conversations where we can listen and then respond go a long way. We can always listen and learn what information people need and will appreciate. I was also struck by how alone this person felt in our health care system. She did not have a relationship with any one person whom she could trust and reach out to with questions. Relationships with our patients go a long way to establishing trust.
Pearl
It takes time to listen to and answer our patients’ questions. We need to do that to fight the waves of misinformation our patients face.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
The Tyranny of Beta-Blockers
Beta-blockers are excellent drugs. They’re cheap and effective; feature prominently in hypertension guidelines; and remain a sine qua non for coronary artery disease, myocardial infarction, and heart failure treatment. They’ve been around forever, and we know they work. Good luck finding an adult medicine patient who isn’t on one.
Beta-blockers act by slowing resting heart rate (and blunting the heart rate response to exercise. The latter is a pernicious cause of activity intolerance that often goes unchecked. Even when the adverse effects of beta-blockers are appreciated, providers are loath to alter dosing, much less stop the drug. After all, beta-blockers are an integral part of guideline-directed medical therapy (GDMT), and GDMT saves lives.
Balancing Heart Rate and Stroke Volume Effects
To augment cardiac output and optimize oxygen uptake (VO2) during exercise, we need the heart rate response. In fact, the heart rate response contributes more to cardiac output than augmenting stroke volume (SV) and more to VO2 than the increase in arteriovenous (AV) oxygen difference. An inability to increase the heart rate commensurate with physiologic work is called chronotropic incompetence (CI). That’s what beta-blockers do ─ they cause CI.
Physiology dictates that CI will cause activity intolerance. That said, it’s hard to quantify the impact from beta-blockers at the individual patient level. Data suggest the heart rate effect is profound. A study in patients without heart failure found that 22% of participants on beta-blockers had CI, and the investigators used a conservative CI definition (≤ 62% of heart rate reserve used). A recent report published in JAMA Cardiology found that stopping beta-blockers in patients with heart failure allowed for an extra 30 beats/min at max exercise.
Wasserman and Whipp’s textbook, the last word on all things exercise, presents a sample subject who undergoes two separate cardiopulmonary exercise tests (CPETs). Before the first, he’s given a placebo, and before the second, he gets an intravenous beta-blocker. He’s a 23-year-old otherwise healthy male — the perfect test case for isolating beta-blocker impact without confounding by comorbid diseases, other medications, or deconditioning. His max heart rate dropped by 30 beats/min after the beta-blocker, identical to what we saw in the JAMA Cardiology study (with the heart rate increasing by 30 beats/min following withdrawal). Case closed. Stop the beta-blockers on your patients so they can meet their exercise goals and get healthy!
Such pithy enthusiasm discounts physiology’s complexities. When blunting our patient’s heart rate response with beta-blockers, we also increase diastolic filling time, which increases SV. For the 23-year-old in Wasserman and Whipp’s physiology textbook, the beta-blocker increased O2 pulse (the product of SV and AV difference). Presumably, this is mediated by the increased SV. There was a net reduction in VO2 peak, but it was nominal, suggesting that the drop in heart rate was largely offset by the increase in O2 pulse. For the patients in the JAMA Cardiology study, the entire group had a small increase in VO2 peak with beta-blocker withdrawal, but the effect differed by left ventricular function. Across different studies, the beta-blocker effect on heart rate is consistent but the change in overall exercise capacity is not.
Patient Variability in Beta-Blocker Response
In addition to left ventricular function, there are other factors likely to drive variability at the patient level. We’ve treated the response to beta-blockers as a class effect — an obvious oversimplification. The impact on exercise and the heart will vary by dose and drug (eg, atenolol vs metoprolol vs carvedilol, and so on). Beta-blockers can also affect the lungs, and we’re still debating how cautious to be in the presence of asthma or chronic obstructive pulmonary disease.
In a world of infinite time, resources, and expertise, we’d CPET everyone before and after beta-blocker use. Our current reality requires the unthinkable: We’ll have to talk to each other and our patients. For example, heart failure guidelines recommend titrating drugs to match the dose from trials that proved efficacy. These doses are quite high. Simple discussion with the cardiologist and the patient may allow for an adjustment back down with careful monitoring and close attention to activity tolerance. With any luck, you’ll preserve the benefits from GDMT while optimizing your patient›s ability to meet their exercise goals.
Dr. Holley, professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center, Washington, disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article appeared on Medscape.com.
Beta-blockers are excellent drugs. They’re cheap and effective; feature prominently in hypertension guidelines; and remain a sine qua non for coronary artery disease, myocardial infarction, and heart failure treatment. They’ve been around forever, and we know they work. Good luck finding an adult medicine patient who isn’t on one.
Beta-blockers act by slowing resting heart rate (and blunting the heart rate response to exercise. The latter is a pernicious cause of activity intolerance that often goes unchecked. Even when the adverse effects of beta-blockers are appreciated, providers are loath to alter dosing, much less stop the drug. After all, beta-blockers are an integral part of guideline-directed medical therapy (GDMT), and GDMT saves lives.
Balancing Heart Rate and Stroke Volume Effects
To augment cardiac output and optimize oxygen uptake (VO2) during exercise, we need the heart rate response. In fact, the heart rate response contributes more to cardiac output than augmenting stroke volume (SV) and more to VO2 than the increase in arteriovenous (AV) oxygen difference. An inability to increase the heart rate commensurate with physiologic work is called chronotropic incompetence (CI). That’s what beta-blockers do ─ they cause CI.
Physiology dictates that CI will cause activity intolerance. That said, it’s hard to quantify the impact from beta-blockers at the individual patient level. Data suggest the heart rate effect is profound. A study in patients without heart failure found that 22% of participants on beta-blockers had CI, and the investigators used a conservative CI definition (≤ 62% of heart rate reserve used). A recent report published in JAMA Cardiology found that stopping beta-blockers in patients with heart failure allowed for an extra 30 beats/min at max exercise.
Wasserman and Whipp’s textbook, the last word on all things exercise, presents a sample subject who undergoes two separate cardiopulmonary exercise tests (CPETs). Before the first, he’s given a placebo, and before the second, he gets an intravenous beta-blocker. He’s a 23-year-old otherwise healthy male — the perfect test case for isolating beta-blocker impact without confounding by comorbid diseases, other medications, or deconditioning. His max heart rate dropped by 30 beats/min after the beta-blocker, identical to what we saw in the JAMA Cardiology study (with the heart rate increasing by 30 beats/min following withdrawal). Case closed. Stop the beta-blockers on your patients so they can meet their exercise goals and get healthy!
Such pithy enthusiasm discounts physiology’s complexities. When blunting our patient’s heart rate response with beta-blockers, we also increase diastolic filling time, which increases SV. For the 23-year-old in Wasserman and Whipp’s physiology textbook, the beta-blocker increased O2 pulse (the product of SV and AV difference). Presumably, this is mediated by the increased SV. There was a net reduction in VO2 peak, but it was nominal, suggesting that the drop in heart rate was largely offset by the increase in O2 pulse. For the patients in the JAMA Cardiology study, the entire group had a small increase in VO2 peak with beta-blocker withdrawal, but the effect differed by left ventricular function. Across different studies, the beta-blocker effect on heart rate is consistent but the change in overall exercise capacity is not.
Patient Variability in Beta-Blocker Response
In addition to left ventricular function, there are other factors likely to drive variability at the patient level. We’ve treated the response to beta-blockers as a class effect — an obvious oversimplification. The impact on exercise and the heart will vary by dose and drug (eg, atenolol vs metoprolol vs carvedilol, and so on). Beta-blockers can also affect the lungs, and we’re still debating how cautious to be in the presence of asthma or chronic obstructive pulmonary disease.
In a world of infinite time, resources, and expertise, we’d CPET everyone before and after beta-blocker use. Our current reality requires the unthinkable: We’ll have to talk to each other and our patients. For example, heart failure guidelines recommend titrating drugs to match the dose from trials that proved efficacy. These doses are quite high. Simple discussion with the cardiologist and the patient may allow for an adjustment back down with careful monitoring and close attention to activity tolerance. With any luck, you’ll preserve the benefits from GDMT while optimizing your patient›s ability to meet their exercise goals.
Dr. Holley, professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center, Washington, disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article appeared on Medscape.com.
Beta-blockers are excellent drugs. They’re cheap and effective; feature prominently in hypertension guidelines; and remain a sine qua non for coronary artery disease, myocardial infarction, and heart failure treatment. They’ve been around forever, and we know they work. Good luck finding an adult medicine patient who isn’t on one.
Beta-blockers act by slowing resting heart rate (and blunting the heart rate response to exercise. The latter is a pernicious cause of activity intolerance that often goes unchecked. Even when the adverse effects of beta-blockers are appreciated, providers are loath to alter dosing, much less stop the drug. After all, beta-blockers are an integral part of guideline-directed medical therapy (GDMT), and GDMT saves lives.
Balancing Heart Rate and Stroke Volume Effects
To augment cardiac output and optimize oxygen uptake (VO2) during exercise, we need the heart rate response. In fact, the heart rate response contributes more to cardiac output than augmenting stroke volume (SV) and more to VO2 than the increase in arteriovenous (AV) oxygen difference. An inability to increase the heart rate commensurate with physiologic work is called chronotropic incompetence (CI). That’s what beta-blockers do ─ they cause CI.
Physiology dictates that CI will cause activity intolerance. That said, it’s hard to quantify the impact from beta-blockers at the individual patient level. Data suggest the heart rate effect is profound. A study in patients without heart failure found that 22% of participants on beta-blockers had CI, and the investigators used a conservative CI definition (≤ 62% of heart rate reserve used). A recent report published in JAMA Cardiology found that stopping beta-blockers in patients with heart failure allowed for an extra 30 beats/min at max exercise.
Wasserman and Whipp’s textbook, the last word on all things exercise, presents a sample subject who undergoes two separate cardiopulmonary exercise tests (CPETs). Before the first, he’s given a placebo, and before the second, he gets an intravenous beta-blocker. He’s a 23-year-old otherwise healthy male — the perfect test case for isolating beta-blocker impact without confounding by comorbid diseases, other medications, or deconditioning. His max heart rate dropped by 30 beats/min after the beta-blocker, identical to what we saw in the JAMA Cardiology study (with the heart rate increasing by 30 beats/min following withdrawal). Case closed. Stop the beta-blockers on your patients so they can meet their exercise goals and get healthy!
Such pithy enthusiasm discounts physiology’s complexities. When blunting our patient’s heart rate response with beta-blockers, we also increase diastolic filling time, which increases SV. For the 23-year-old in Wasserman and Whipp’s physiology textbook, the beta-blocker increased O2 pulse (the product of SV and AV difference). Presumably, this is mediated by the increased SV. There was a net reduction in VO2 peak, but it was nominal, suggesting that the drop in heart rate was largely offset by the increase in O2 pulse. For the patients in the JAMA Cardiology study, the entire group had a small increase in VO2 peak with beta-blocker withdrawal, but the effect differed by left ventricular function. Across different studies, the beta-blocker effect on heart rate is consistent but the change in overall exercise capacity is not.
Patient Variability in Beta-Blocker Response
In addition to left ventricular function, there are other factors likely to drive variability at the patient level. We’ve treated the response to beta-blockers as a class effect — an obvious oversimplification. The impact on exercise and the heart will vary by dose and drug (eg, atenolol vs metoprolol vs carvedilol, and so on). Beta-blockers can also affect the lungs, and we’re still debating how cautious to be in the presence of asthma or chronic obstructive pulmonary disease.
In a world of infinite time, resources, and expertise, we’d CPET everyone before and after beta-blocker use. Our current reality requires the unthinkable: We’ll have to talk to each other and our patients. For example, heart failure guidelines recommend titrating drugs to match the dose from trials that proved efficacy. These doses are quite high. Simple discussion with the cardiologist and the patient may allow for an adjustment back down with careful monitoring and close attention to activity tolerance. With any luck, you’ll preserve the benefits from GDMT while optimizing your patient›s ability to meet their exercise goals.
Dr. Holley, professor in the department of medicine, Uniformed Services University, Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center, Washington, disclosed ties with Metapharm, CHEST College, and WebMD.
A version of this article appeared on Medscape.com.
The Impact of the Recent Supreme Court Ruling on the Dermatology Recruitment Pipeline
The ruling by the Supreme Court of the United States (SCOTUS) in 20231,2 on the use of race-based criteria in college admissions was met with a range of reactions across the country. Given the implications of this decision on the future makeup of higher education, the downstream effects on medical school admissions, and the possible further impact on graduate medical education programs, we sought to explore the potential impact of the landmark decision from the perspective of dermatology residency program directors and offer insights on this pivotal judgment.
Background on the SCOTUS Ruling
In June 2023, SCOTUS issued its formal decision on 2 court cases brought by the organization Students for Fair Admissions (SFFA) against the University of North Carolina at Chapel Hill1 and Harvard University (Cambridge, Massachusetts)2 that addressed college admissions practices dealing with the use of race as a selection criterion in the application process. The cases alleged that these universities had overly emphasized race in the admissions process and thus were in violation of the Civil Rights Act of 1964 as well as the 14th Amendment.1,2
The SCOTUS justices voted 6 to 3 in favor of the argument presented by the SFFA, determining that the use of race in the college admissions process essentially constituted a form of racial discrimination. The ruling was in contrast to a prior decision in 2003 that centered on law school admissions at the University of Michigan (Ann Arbor, Michigan) in which SCOTUS previously had determined that race could be used as one factor amongst other criteria in the higher education selection process.3 In the 2023 decision siding with SFFA, SCOTUS did acknowledge that it was still acceptable for selection processes to consider “an applicant’s discussion of how race affected his or her life, be it through discrimination, inspiration, or otherwise.”2
Effect on Undergraduate Admissions
Prior to the 2023 ruling, several states had already passed independent laws against the use of affirmative action or race-based selection criteria in the admissions process at public colleges and universities.4 As a result, these institutions would already be conforming to the principles set forth in the SCOTUS ruling and major changes to their undergraduate admissions policies would not be expected; however, a considerable number of colleges and universities—particularly those considered highly selective with applicant acceptance rates that are well below the national average—reported the use of race as a factor in their admissions processes in standardized reporting surveys.5 For these institutions, it is no longer considered acceptable (based on the SCOTUS decision) to use race as a singular factor in admissions or to implement race-conscious decision-making—in which individuals are considered differently based solely on their race—as part of the undergraduate selection process.
In light of these rulings, many institutions have explicitly committed to upholding principles of diversity in their recruitment processes, acknowledging the multifaceted nature of diversity beyond strictly racial terms—including but not limited to socioeconomic diversity, religious diversity, or gender diversity—which is in compliance with the interpretation ruling by the US Department of Education and the US Department of Justice.6 Additionally, select institutions have taken approaches to explicitly include questions on ways in which applicants have overcome obstacles or challenges, allowing an opportunity for individuals who have had such experiences related to race an opportunity to incorporate these elements into their applications. Finally, some institutions have taken a more limited approach, eliminating ways in which race is explicitly addressed in the application and focusing on race-neutral elements of the application in their approach to selection.7
Because the first college admission cycle since the 2023 SCOTUS ruling is still underway, we have yet to witness the full impact of this decision on the current undergraduate admissions landscape.
Effect on Medical School Admissions and Rotations
Although SCOTUS specifically examined the undergraduate admissions process, the ruling on race-conscious admissions also had a profound impact on graduate school admissions including medical school admission processes.1,2,8,9 This is because the language of the majority opinion refers to “university programs” in its ruling, which also has been broadly interpreted to include graduate school programs. As with undergraduate admissions, it has been interpreted by national medical education organizations and institutions that medical schools also cannot consider an applicant’s race or ethnicity as a specific factor in the admissions process.1,2,8,9
Lived individual experiences, including essays that speak to an applicant’s lived experiences and career aspirations related to race, still can be taken into account. In particular, holistic review still can be utilized to evaluate medical school candidates and may play a more integral role in the medical school admissions process now than in the past.8,10,11 After the ruling, Justice Sonia Sotomayor noted that “today’s decision leaves intact holistic college admissions and recruitment efforts that seek to enroll diverse classes without using racial classifications.”1
The ruling asserted that universities may define their mission as they see fit. As a result, the ruling did not affect medical school missions or strategic plans, including those that may aim to diversify the health care workforce.8,10,11 The ruling also did not affect the ability to utilize pathway programs to encourage a career in medicine or recruitment relationships with diverse undergraduate or community-based organizations. Student interest groups also can be involved in the relationship-building or recruitment activities for medical schools.8,10,11 Guidance from the US Department of Education and US Department of Justice noted that institutions may consider race in identifying prospective applicants through recruitment and outreach, “provided that their outreach and recruitment programs do not provide targeted groups of prospective students preference in the admissions process, and provided that all students—whether part of a specifically targeted group or not—enjoy the same opportunity to apply and compete for admission.”12
In regard to pathways programs, slots cannot be reserved and preference cannot be given to applicants who participated in these programs if race was a factor in selecting participants.8 Similarly, medical school away electives related to diversity cannot be reserved for those of a specific race or ethnicity; however, these electives can utilize commitment to stated aims and missions of the rotation, such as a commitment to diversity within medicine, as a basis to selecting candidates.8
The ruling did not address how race or ethnicity is factored into financial aid or scholarship determination. There has been concern in higher education that the legal framework utilized in the SCOTUS decision could affect financial aid and scholarship decisions; therefore, many institutions are proceeding with caution in their approach.8
Effect on Residency Selection
Because the SCOTUS ruling references colleges and universities, not health care employers, it should not affect the residency selection process; however, there is variability in how health care institutions are interpreting the impact of the ruling on residency selection, with some taking a more prescriptive and cautious view on the matter. Additionally, with that said, residency selection is considered an employment practice covered by Title VII of the Civil Rights Act of 1964,13 which already prohibits the consideration of race in hiring decisions.7 Under Title VII, it is unlawful for employers to discriminate against someone because of race, color, religion, sex, or national origin, and it is “unlawful to use policies or practices that seem neutral but have the effect of discriminating against people because of their race, color, religion, sex … or national origin.” Title VII also states that employers cannot “make employment decisions based on stereotypes or assumptions about a person’s abilities, traits, or performance because of their race, color, religion, sex … or national origin.”13
Importantly, Title VII does not imply that employers need to abandon their diversity, equity, or inclusion initiatives, and it does not imply that employers must revoke their mission to improve diversity in the workforce. Title VII does not state that racial information cannot be available. It would be permissible to use racial data to assess recruitment trends, identify inequities, and create programs to eliminate barriers and decrease bias14; for example, if a program identified that, based on their current review system, students who are underrepresented in medicine were disproportionately screened out of the applicant pool or interview group, they may wish to revisit their review process to identify and eliminate possible biases. Programs also may wish to adopt educational programs for reviewers (eg, implicit bias training) or educational content on the potential for bias in commonly used review criteria, such as the US Medical Licensing Examination, clerkship grades, and the Medical Student Performance Evaluation.15 Reviewers can and should consider applications in an individualized and holistic manner in which experiences, traits, skills, and academic metrics are assessed together for compatibility with the values and mission of the training program.16
Future Directions for Dermatology
Beyond the SCOTUS ruling, there have been other shifts in the dermatology residency application process that have affected candidate review. Dermatology programs recently have adopted the use of preference signaling in residency applications. Preliminary data from the Association of American Medical Colleges for the 2024 application cycle indicated that of the 81 programs analyzed, there was a nearly 0% chance of an applicant receiving an interview invitation from a program that they did not signal. The median signal-to-interview conversion rate for the 81 dermatology programs analyzed was 55% for gold signals and 15% for silver signals.17 It can be inferred from these data that programs are using preference signaling as important criteria for consideration of interview invitation. Programs may choose to focus most of their attention on the applicant pool who has signaled them. Because the number and type of signals available is equal among all applicants, we hope that this provides an equitable way for all applicants to garner holistic review from programs that interested them. In addition, there has been a 30% decrease in average applications submitted per dermatology applicant.18 With a substantial decline in applications to dermatology, we hope that reviewers are able to spend more time devoted to comprehensive holistic review.
Although signals are equitable for applicants, their distribution among programs may not be; for example, in a given year, a program might find that all their gold signals came from non–underrepresented in medicine students. We encourage programs to carefully review applicant data to ensure their recruitment process is not inadvertently discriminatory and is in alignment with their goals and mission.
- Students for Fair Admissions, Inc. v University of North Carolina, 567 F. Supp. 3d 580 (M.D.N.C. 2021).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306 (2003).
- Saul S. 9 states have banned affirmative action. here’s what that looks like. The New York Times. October 31, 2022. https://www.nytimes.com/2022/10/31/us/politics/affirmative-action-ban-states.html
- Desilver D. Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions. Pew Research Center. July 14, 2023. Accessed May 29, 2024. https://www.pewresearch.org/short-reads/2023/07/14/private-selective-colleges-are-most-likely-to-use-race-ethnicity-as-a-factor-in-admissions-decisions/
- US Department of Education. Justice and education departments release resources to advance diversity and opportunity in higher education. August 14, 2023. Accessed May 17, 2024. https://www.ed.gov/news/press-releases/advance-diversity-and-opportunity-higher-education-justice-and-education-departments-release-resources-advance-diversity-and-opportunity-higher-education
- Amponsah MN, Hamid RD. Harvard overhauls college application in wake of affirmative action decision. The Harvard Crimson. August 3, 2023. Accessed May 17, 2024. https://www.thecrimson.com/article/2023/8/3/harvard-admission-essay-change/
- Association of American Medical Colleges. Frequently asked questions: what does the Harvard and UNC decision mean for medical education? August 24, 2023. Accessed May 17, 2024. https://www.aamc.org/media/68771/download?attachment%3Fattachment
- American Medical Association. Affirmative action ends: how Supreme Court ruling impacts medical schools & the health care workforce. July 7, 2023. Accessed May 17, 2024. https://www.ama-assn.org/medical-students/medical-school-life/affirmative-action-ends-how-supreme-court-ruling-impacts
- Association of American Medical Colleges. How can medical schools boost racial diversity in the wake of the recent Supreme Court ruling? July 27, 2023. Accessed May 17, 2024. https://www.aamc.org/news/how-can-medical-schools-boost-racial-diversity-wake-recent-supreme-court-ruling
- Association of American Medical Colleges. Diversity in medical school admissions. Updated March 18, 2024. Accessed May 17, 2024. https://www.aamc.org/about-us/mission-areas/medical-education/diversity-medical-school-admissions
- United States Department of Justice. Questions and answers regarding the Supreme Court’s decision in Students For Fair Admissions, Inc. v. Harvard College and University of North Carolina. August 14, 2023. Accessed May 29, 2024. https://www.justice.gov/d9/2023-08/post-sffa_resource_faq_final_508.pdf
- US Department of Justice. Title VII of the Civil Rights Act of 1964. Accessed May 17, 2024. https://www.justice.gov/crt/laws-we-enforce
- Zheng L. How to effectively—and legally—use racial data for DEI. Harvard Business Review. July 24, 2023. Accessed May 17, 2024. https://hbr.org/2023/07/how-to-effectively-and-legally-use-racial-data-for-dei
- Crites K, Johnson J, Scott N, et al. Increasing diversity in residency training programs. Cureus. 2022;14:E25962. doi:10.7759/cureus.25962
- Association of American Medical Colleges. Holistic principles in resident selection: an introduction. Accessed May 17, 2024. https://www.aamc.org/media/44586/download?attachment
- Association of American Medical Colleges. Exploring the relationship between program signaling & interview invitations across specialties 2024 ERAS® preliminary analysis. December 29, 2023. Accessed May 17, 2024. https://www.aamc.org/media/74811/download?attachment
- Association of American Medical Colleges. Preliminary program signaling data and their impact on residency selection. October 24, 2023. Accessed May 17, 2024. https://www.aamc.org/services/eras-institutions/program-signaling-data#:~:text=Preliminary%20Program%20Signaling%20Data%20and%20Their%20Impact%20on%20Residency%20Selection,-Oct.&text=Program%20signals%20are%20a%20mechanism,whom%20to%20invite%20for%20interview
The ruling by the Supreme Court of the United States (SCOTUS) in 20231,2 on the use of race-based criteria in college admissions was met with a range of reactions across the country. Given the implications of this decision on the future makeup of higher education, the downstream effects on medical school admissions, and the possible further impact on graduate medical education programs, we sought to explore the potential impact of the landmark decision from the perspective of dermatology residency program directors and offer insights on this pivotal judgment.
Background on the SCOTUS Ruling
In June 2023, SCOTUS issued its formal decision on 2 court cases brought by the organization Students for Fair Admissions (SFFA) against the University of North Carolina at Chapel Hill1 and Harvard University (Cambridge, Massachusetts)2 that addressed college admissions practices dealing with the use of race as a selection criterion in the application process. The cases alleged that these universities had overly emphasized race in the admissions process and thus were in violation of the Civil Rights Act of 1964 as well as the 14th Amendment.1,2
The SCOTUS justices voted 6 to 3 in favor of the argument presented by the SFFA, determining that the use of race in the college admissions process essentially constituted a form of racial discrimination. The ruling was in contrast to a prior decision in 2003 that centered on law school admissions at the University of Michigan (Ann Arbor, Michigan) in which SCOTUS previously had determined that race could be used as one factor amongst other criteria in the higher education selection process.3 In the 2023 decision siding with SFFA, SCOTUS did acknowledge that it was still acceptable for selection processes to consider “an applicant’s discussion of how race affected his or her life, be it through discrimination, inspiration, or otherwise.”2
Effect on Undergraduate Admissions
Prior to the 2023 ruling, several states had already passed independent laws against the use of affirmative action or race-based selection criteria in the admissions process at public colleges and universities.4 As a result, these institutions would already be conforming to the principles set forth in the SCOTUS ruling and major changes to their undergraduate admissions policies would not be expected; however, a considerable number of colleges and universities—particularly those considered highly selective with applicant acceptance rates that are well below the national average—reported the use of race as a factor in their admissions processes in standardized reporting surveys.5 For these institutions, it is no longer considered acceptable (based on the SCOTUS decision) to use race as a singular factor in admissions or to implement race-conscious decision-making—in which individuals are considered differently based solely on their race—as part of the undergraduate selection process.
In light of these rulings, many institutions have explicitly committed to upholding principles of diversity in their recruitment processes, acknowledging the multifaceted nature of diversity beyond strictly racial terms—including but not limited to socioeconomic diversity, religious diversity, or gender diversity—which is in compliance with the interpretation ruling by the US Department of Education and the US Department of Justice.6 Additionally, select institutions have taken approaches to explicitly include questions on ways in which applicants have overcome obstacles or challenges, allowing an opportunity for individuals who have had such experiences related to race an opportunity to incorporate these elements into their applications. Finally, some institutions have taken a more limited approach, eliminating ways in which race is explicitly addressed in the application and focusing on race-neutral elements of the application in their approach to selection.7
Because the first college admission cycle since the 2023 SCOTUS ruling is still underway, we have yet to witness the full impact of this decision on the current undergraduate admissions landscape.
Effect on Medical School Admissions and Rotations
Although SCOTUS specifically examined the undergraduate admissions process, the ruling on race-conscious admissions also had a profound impact on graduate school admissions including medical school admission processes.1,2,8,9 This is because the language of the majority opinion refers to “university programs” in its ruling, which also has been broadly interpreted to include graduate school programs. As with undergraduate admissions, it has been interpreted by national medical education organizations and institutions that medical schools also cannot consider an applicant’s race or ethnicity as a specific factor in the admissions process.1,2,8,9
Lived individual experiences, including essays that speak to an applicant’s lived experiences and career aspirations related to race, still can be taken into account. In particular, holistic review still can be utilized to evaluate medical school candidates and may play a more integral role in the medical school admissions process now than in the past.8,10,11 After the ruling, Justice Sonia Sotomayor noted that “today’s decision leaves intact holistic college admissions and recruitment efforts that seek to enroll diverse classes without using racial classifications.”1
The ruling asserted that universities may define their mission as they see fit. As a result, the ruling did not affect medical school missions or strategic plans, including those that may aim to diversify the health care workforce.8,10,11 The ruling also did not affect the ability to utilize pathway programs to encourage a career in medicine or recruitment relationships with diverse undergraduate or community-based organizations. Student interest groups also can be involved in the relationship-building or recruitment activities for medical schools.8,10,11 Guidance from the US Department of Education and US Department of Justice noted that institutions may consider race in identifying prospective applicants through recruitment and outreach, “provided that their outreach and recruitment programs do not provide targeted groups of prospective students preference in the admissions process, and provided that all students—whether part of a specifically targeted group or not—enjoy the same opportunity to apply and compete for admission.”12
In regard to pathways programs, slots cannot be reserved and preference cannot be given to applicants who participated in these programs if race was a factor in selecting participants.8 Similarly, medical school away electives related to diversity cannot be reserved for those of a specific race or ethnicity; however, these electives can utilize commitment to stated aims and missions of the rotation, such as a commitment to diversity within medicine, as a basis to selecting candidates.8
The ruling did not address how race or ethnicity is factored into financial aid or scholarship determination. There has been concern in higher education that the legal framework utilized in the SCOTUS decision could affect financial aid and scholarship decisions; therefore, many institutions are proceeding with caution in their approach.8
Effect on Residency Selection
Because the SCOTUS ruling references colleges and universities, not health care employers, it should not affect the residency selection process; however, there is variability in how health care institutions are interpreting the impact of the ruling on residency selection, with some taking a more prescriptive and cautious view on the matter. Additionally, with that said, residency selection is considered an employment practice covered by Title VII of the Civil Rights Act of 1964,13 which already prohibits the consideration of race in hiring decisions.7 Under Title VII, it is unlawful for employers to discriminate against someone because of race, color, religion, sex, or national origin, and it is “unlawful to use policies or practices that seem neutral but have the effect of discriminating against people because of their race, color, religion, sex … or national origin.” Title VII also states that employers cannot “make employment decisions based on stereotypes or assumptions about a person’s abilities, traits, or performance because of their race, color, religion, sex … or national origin.”13
Importantly, Title VII does not imply that employers need to abandon their diversity, equity, or inclusion initiatives, and it does not imply that employers must revoke their mission to improve diversity in the workforce. Title VII does not state that racial information cannot be available. It would be permissible to use racial data to assess recruitment trends, identify inequities, and create programs to eliminate barriers and decrease bias14; for example, if a program identified that, based on their current review system, students who are underrepresented in medicine were disproportionately screened out of the applicant pool or interview group, they may wish to revisit their review process to identify and eliminate possible biases. Programs also may wish to adopt educational programs for reviewers (eg, implicit bias training) or educational content on the potential for bias in commonly used review criteria, such as the US Medical Licensing Examination, clerkship grades, and the Medical Student Performance Evaluation.15 Reviewers can and should consider applications in an individualized and holistic manner in which experiences, traits, skills, and academic metrics are assessed together for compatibility with the values and mission of the training program.16
Future Directions for Dermatology
Beyond the SCOTUS ruling, there have been other shifts in the dermatology residency application process that have affected candidate review. Dermatology programs recently have adopted the use of preference signaling in residency applications. Preliminary data from the Association of American Medical Colleges for the 2024 application cycle indicated that of the 81 programs analyzed, there was a nearly 0% chance of an applicant receiving an interview invitation from a program that they did not signal. The median signal-to-interview conversion rate for the 81 dermatology programs analyzed was 55% for gold signals and 15% for silver signals.17 It can be inferred from these data that programs are using preference signaling as important criteria for consideration of interview invitation. Programs may choose to focus most of their attention on the applicant pool who has signaled them. Because the number and type of signals available is equal among all applicants, we hope that this provides an equitable way for all applicants to garner holistic review from programs that interested them. In addition, there has been a 30% decrease in average applications submitted per dermatology applicant.18 With a substantial decline in applications to dermatology, we hope that reviewers are able to spend more time devoted to comprehensive holistic review.
Although signals are equitable for applicants, their distribution among programs may not be; for example, in a given year, a program might find that all their gold signals came from non–underrepresented in medicine students. We encourage programs to carefully review applicant data to ensure their recruitment process is not inadvertently discriminatory and is in alignment with their goals and mission.
The ruling by the Supreme Court of the United States (SCOTUS) in 20231,2 on the use of race-based criteria in college admissions was met with a range of reactions across the country. Given the implications of this decision on the future makeup of higher education, the downstream effects on medical school admissions, and the possible further impact on graduate medical education programs, we sought to explore the potential impact of the landmark decision from the perspective of dermatology residency program directors and offer insights on this pivotal judgment.
Background on the SCOTUS Ruling
In June 2023, SCOTUS issued its formal decision on 2 court cases brought by the organization Students for Fair Admissions (SFFA) against the University of North Carolina at Chapel Hill1 and Harvard University (Cambridge, Massachusetts)2 that addressed college admissions practices dealing with the use of race as a selection criterion in the application process. The cases alleged that these universities had overly emphasized race in the admissions process and thus were in violation of the Civil Rights Act of 1964 as well as the 14th Amendment.1,2
The SCOTUS justices voted 6 to 3 in favor of the argument presented by the SFFA, determining that the use of race in the college admissions process essentially constituted a form of racial discrimination. The ruling was in contrast to a prior decision in 2003 that centered on law school admissions at the University of Michigan (Ann Arbor, Michigan) in which SCOTUS previously had determined that race could be used as one factor amongst other criteria in the higher education selection process.3 In the 2023 decision siding with SFFA, SCOTUS did acknowledge that it was still acceptable for selection processes to consider “an applicant’s discussion of how race affected his or her life, be it through discrimination, inspiration, or otherwise.”2
Effect on Undergraduate Admissions
Prior to the 2023 ruling, several states had already passed independent laws against the use of affirmative action or race-based selection criteria in the admissions process at public colleges and universities.4 As a result, these institutions would already be conforming to the principles set forth in the SCOTUS ruling and major changes to their undergraduate admissions policies would not be expected; however, a considerable number of colleges and universities—particularly those considered highly selective with applicant acceptance rates that are well below the national average—reported the use of race as a factor in their admissions processes in standardized reporting surveys.5 For these institutions, it is no longer considered acceptable (based on the SCOTUS decision) to use race as a singular factor in admissions or to implement race-conscious decision-making—in which individuals are considered differently based solely on their race—as part of the undergraduate selection process.
In light of these rulings, many institutions have explicitly committed to upholding principles of diversity in their recruitment processes, acknowledging the multifaceted nature of diversity beyond strictly racial terms—including but not limited to socioeconomic diversity, religious diversity, or gender diversity—which is in compliance with the interpretation ruling by the US Department of Education and the US Department of Justice.6 Additionally, select institutions have taken approaches to explicitly include questions on ways in which applicants have overcome obstacles or challenges, allowing an opportunity for individuals who have had such experiences related to race an opportunity to incorporate these elements into their applications. Finally, some institutions have taken a more limited approach, eliminating ways in which race is explicitly addressed in the application and focusing on race-neutral elements of the application in their approach to selection.7
Because the first college admission cycle since the 2023 SCOTUS ruling is still underway, we have yet to witness the full impact of this decision on the current undergraduate admissions landscape.
Effect on Medical School Admissions and Rotations
Although SCOTUS specifically examined the undergraduate admissions process, the ruling on race-conscious admissions also had a profound impact on graduate school admissions including medical school admission processes.1,2,8,9 This is because the language of the majority opinion refers to “university programs” in its ruling, which also has been broadly interpreted to include graduate school programs. As with undergraduate admissions, it has been interpreted by national medical education organizations and institutions that medical schools also cannot consider an applicant’s race or ethnicity as a specific factor in the admissions process.1,2,8,9
Lived individual experiences, including essays that speak to an applicant’s lived experiences and career aspirations related to race, still can be taken into account. In particular, holistic review still can be utilized to evaluate medical school candidates and may play a more integral role in the medical school admissions process now than in the past.8,10,11 After the ruling, Justice Sonia Sotomayor noted that “today’s decision leaves intact holistic college admissions and recruitment efforts that seek to enroll diverse classes without using racial classifications.”1
The ruling asserted that universities may define their mission as they see fit. As a result, the ruling did not affect medical school missions or strategic plans, including those that may aim to diversify the health care workforce.8,10,11 The ruling also did not affect the ability to utilize pathway programs to encourage a career in medicine or recruitment relationships with diverse undergraduate or community-based organizations. Student interest groups also can be involved in the relationship-building or recruitment activities for medical schools.8,10,11 Guidance from the US Department of Education and US Department of Justice noted that institutions may consider race in identifying prospective applicants through recruitment and outreach, “provided that their outreach and recruitment programs do not provide targeted groups of prospective students preference in the admissions process, and provided that all students—whether part of a specifically targeted group or not—enjoy the same opportunity to apply and compete for admission.”12
In regard to pathways programs, slots cannot be reserved and preference cannot be given to applicants who participated in these programs if race was a factor in selecting participants.8 Similarly, medical school away electives related to diversity cannot be reserved for those of a specific race or ethnicity; however, these electives can utilize commitment to stated aims and missions of the rotation, such as a commitment to diversity within medicine, as a basis to selecting candidates.8
The ruling did not address how race or ethnicity is factored into financial aid or scholarship determination. There has been concern in higher education that the legal framework utilized in the SCOTUS decision could affect financial aid and scholarship decisions; therefore, many institutions are proceeding with caution in their approach.8
Effect on Residency Selection
Because the SCOTUS ruling references colleges and universities, not health care employers, it should not affect the residency selection process; however, there is variability in how health care institutions are interpreting the impact of the ruling on residency selection, with some taking a more prescriptive and cautious view on the matter. Additionally, with that said, residency selection is considered an employment practice covered by Title VII of the Civil Rights Act of 1964,13 which already prohibits the consideration of race in hiring decisions.7 Under Title VII, it is unlawful for employers to discriminate against someone because of race, color, religion, sex, or national origin, and it is “unlawful to use policies or practices that seem neutral but have the effect of discriminating against people because of their race, color, religion, sex … or national origin.” Title VII also states that employers cannot “make employment decisions based on stereotypes or assumptions about a person’s abilities, traits, or performance because of their race, color, religion, sex … or national origin.”13
Importantly, Title VII does not imply that employers need to abandon their diversity, equity, or inclusion initiatives, and it does not imply that employers must revoke their mission to improve diversity in the workforce. Title VII does not state that racial information cannot be available. It would be permissible to use racial data to assess recruitment trends, identify inequities, and create programs to eliminate barriers and decrease bias14; for example, if a program identified that, based on their current review system, students who are underrepresented in medicine were disproportionately screened out of the applicant pool or interview group, they may wish to revisit their review process to identify and eliminate possible biases. Programs also may wish to adopt educational programs for reviewers (eg, implicit bias training) or educational content on the potential for bias in commonly used review criteria, such as the US Medical Licensing Examination, clerkship grades, and the Medical Student Performance Evaluation.15 Reviewers can and should consider applications in an individualized and holistic manner in which experiences, traits, skills, and academic metrics are assessed together for compatibility with the values and mission of the training program.16
Future Directions for Dermatology
Beyond the SCOTUS ruling, there have been other shifts in the dermatology residency application process that have affected candidate review. Dermatology programs recently have adopted the use of preference signaling in residency applications. Preliminary data from the Association of American Medical Colleges for the 2024 application cycle indicated that of the 81 programs analyzed, there was a nearly 0% chance of an applicant receiving an interview invitation from a program that they did not signal. The median signal-to-interview conversion rate for the 81 dermatology programs analyzed was 55% for gold signals and 15% for silver signals.17 It can be inferred from these data that programs are using preference signaling as important criteria for consideration of interview invitation. Programs may choose to focus most of their attention on the applicant pool who has signaled them. Because the number and type of signals available is equal among all applicants, we hope that this provides an equitable way for all applicants to garner holistic review from programs that interested them. In addition, there has been a 30% decrease in average applications submitted per dermatology applicant.18 With a substantial decline in applications to dermatology, we hope that reviewers are able to spend more time devoted to comprehensive holistic review.
Although signals are equitable for applicants, their distribution among programs may not be; for example, in a given year, a program might find that all their gold signals came from non–underrepresented in medicine students. We encourage programs to carefully review applicant data to ensure their recruitment process is not inadvertently discriminatory and is in alignment with their goals and mission.
- Students for Fair Admissions, Inc. v University of North Carolina, 567 F. Supp. 3d 580 (M.D.N.C. 2021).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306 (2003).
- Saul S. 9 states have banned affirmative action. here’s what that looks like. The New York Times. October 31, 2022. https://www.nytimes.com/2022/10/31/us/politics/affirmative-action-ban-states.html
- Desilver D. Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions. Pew Research Center. July 14, 2023. Accessed May 29, 2024. https://www.pewresearch.org/short-reads/2023/07/14/private-selective-colleges-are-most-likely-to-use-race-ethnicity-as-a-factor-in-admissions-decisions/
- US Department of Education. Justice and education departments release resources to advance diversity and opportunity in higher education. August 14, 2023. Accessed May 17, 2024. https://www.ed.gov/news/press-releases/advance-diversity-and-opportunity-higher-education-justice-and-education-departments-release-resources-advance-diversity-and-opportunity-higher-education
- Amponsah MN, Hamid RD. Harvard overhauls college application in wake of affirmative action decision. The Harvard Crimson. August 3, 2023. Accessed May 17, 2024. https://www.thecrimson.com/article/2023/8/3/harvard-admission-essay-change/
- Association of American Medical Colleges. Frequently asked questions: what does the Harvard and UNC decision mean for medical education? August 24, 2023. Accessed May 17, 2024. https://www.aamc.org/media/68771/download?attachment%3Fattachment
- American Medical Association. Affirmative action ends: how Supreme Court ruling impacts medical schools & the health care workforce. July 7, 2023. Accessed May 17, 2024. https://www.ama-assn.org/medical-students/medical-school-life/affirmative-action-ends-how-supreme-court-ruling-impacts
- Association of American Medical Colleges. How can medical schools boost racial diversity in the wake of the recent Supreme Court ruling? July 27, 2023. Accessed May 17, 2024. https://www.aamc.org/news/how-can-medical-schools-boost-racial-diversity-wake-recent-supreme-court-ruling
- Association of American Medical Colleges. Diversity in medical school admissions. Updated March 18, 2024. Accessed May 17, 2024. https://www.aamc.org/about-us/mission-areas/medical-education/diversity-medical-school-admissions
- United States Department of Justice. Questions and answers regarding the Supreme Court’s decision in Students For Fair Admissions, Inc. v. Harvard College and University of North Carolina. August 14, 2023. Accessed May 29, 2024. https://www.justice.gov/d9/2023-08/post-sffa_resource_faq_final_508.pdf
- US Department of Justice. Title VII of the Civil Rights Act of 1964. Accessed May 17, 2024. https://www.justice.gov/crt/laws-we-enforce
- Zheng L. How to effectively—and legally—use racial data for DEI. Harvard Business Review. July 24, 2023. Accessed May 17, 2024. https://hbr.org/2023/07/how-to-effectively-and-legally-use-racial-data-for-dei
- Crites K, Johnson J, Scott N, et al. Increasing diversity in residency training programs. Cureus. 2022;14:E25962. doi:10.7759/cureus.25962
- Association of American Medical Colleges. Holistic principles in resident selection: an introduction. Accessed May 17, 2024. https://www.aamc.org/media/44586/download?attachment
- Association of American Medical Colleges. Exploring the relationship between program signaling & interview invitations across specialties 2024 ERAS® preliminary analysis. December 29, 2023. Accessed May 17, 2024. https://www.aamc.org/media/74811/download?attachment
- Association of American Medical Colleges. Preliminary program signaling data and their impact on residency selection. October 24, 2023. Accessed May 17, 2024. https://www.aamc.org/services/eras-institutions/program-signaling-data#:~:text=Preliminary%20Program%20Signaling%20Data%20and%20Their%20Impact%20on%20Residency%20Selection,-Oct.&text=Program%20signals%20are%20a%20mechanism,whom%20to%20invite%20for%20interview
- Students for Fair Admissions, Inc. v University of North Carolina, 567 F. Supp. 3d 580 (M.D.N.C. 2021).
- Students for Fair Admissions, Inc. v President and Fellows of Harvard College, 600 US ___ (2023).
- Grutter v Bollinger, 539 US 306 (2003).
- Saul S. 9 states have banned affirmative action. here’s what that looks like. The New York Times. October 31, 2022. https://www.nytimes.com/2022/10/31/us/politics/affirmative-action-ban-states.html
- Desilver D. Private, selective colleges are most likely to use race, ethnicity as a factor in admissions decisions. Pew Research Center. July 14, 2023. Accessed May 29, 2024. https://www.pewresearch.org/short-reads/2023/07/14/private-selective-colleges-are-most-likely-to-use-race-ethnicity-as-a-factor-in-admissions-decisions/
- US Department of Education. Justice and education departments release resources to advance diversity and opportunity in higher education. August 14, 2023. Accessed May 17, 2024. https://www.ed.gov/news/press-releases/advance-diversity-and-opportunity-higher-education-justice-and-education-departments-release-resources-advance-diversity-and-opportunity-higher-education
- Amponsah MN, Hamid RD. Harvard overhauls college application in wake of affirmative action decision. The Harvard Crimson. August 3, 2023. Accessed May 17, 2024. https://www.thecrimson.com/article/2023/8/3/harvard-admission-essay-change/
- Association of American Medical Colleges. Frequently asked questions: what does the Harvard and UNC decision mean for medical education? August 24, 2023. Accessed May 17, 2024. https://www.aamc.org/media/68771/download?attachment%3Fattachment
- American Medical Association. Affirmative action ends: how Supreme Court ruling impacts medical schools & the health care workforce. July 7, 2023. Accessed May 17, 2024. https://www.ama-assn.org/medical-students/medical-school-life/affirmative-action-ends-how-supreme-court-ruling-impacts
- Association of American Medical Colleges. How can medical schools boost racial diversity in the wake of the recent Supreme Court ruling? July 27, 2023. Accessed May 17, 2024. https://www.aamc.org/news/how-can-medical-schools-boost-racial-diversity-wake-recent-supreme-court-ruling
- Association of American Medical Colleges. Diversity in medical school admissions. Updated March 18, 2024. Accessed May 17, 2024. https://www.aamc.org/about-us/mission-areas/medical-education/diversity-medical-school-admissions
- United States Department of Justice. Questions and answers regarding the Supreme Court’s decision in Students For Fair Admissions, Inc. v. Harvard College and University of North Carolina. August 14, 2023. Accessed May 29, 2024. https://www.justice.gov/d9/2023-08/post-sffa_resource_faq_final_508.pdf
- US Department of Justice. Title VII of the Civil Rights Act of 1964. Accessed May 17, 2024. https://www.justice.gov/crt/laws-we-enforce
- Zheng L. How to effectively—and legally—use racial data for DEI. Harvard Business Review. July 24, 2023. Accessed May 17, 2024. https://hbr.org/2023/07/how-to-effectively-and-legally-use-racial-data-for-dei
- Crites K, Johnson J, Scott N, et al. Increasing diversity in residency training programs. Cureus. 2022;14:E25962. doi:10.7759/cureus.25962
- Association of American Medical Colleges. Holistic principles in resident selection: an introduction. Accessed May 17, 2024. https://www.aamc.org/media/44586/download?attachment
- Association of American Medical Colleges. Exploring the relationship between program signaling & interview invitations across specialties 2024 ERAS® preliminary analysis. December 29, 2023. Accessed May 17, 2024. https://www.aamc.org/media/74811/download?attachment
- Association of American Medical Colleges. Preliminary program signaling data and their impact on residency selection. October 24, 2023. Accessed May 17, 2024. https://www.aamc.org/services/eras-institutions/program-signaling-data#:~:text=Preliminary%20Program%20Signaling%20Data%20and%20Their%20Impact%20on%20Residency%20Selection,-Oct.&text=Program%20signals%20are%20a%20mechanism,whom%20to%20invite%20for%20interview
Practice Points
- The 2023 ruling by the Supreme Court of the United States on the use of race-based criteria in college admissions may have implications for the selection of individuals into the dermatology workforce.
- We highlight the impacts of these decisions at the college, medical school, and dermatology residency levels and provide context for future directions in the selection processes for practicing dermatologists.
Predicting and Understanding Vaccine Response Determinants
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
In this column, I recently discussed the impact of the microbiome on childhood vaccine responses. My group has been expanding our research on the topic of childhood vaccine response and its relationship to infection proneness. Therefore, I want to share new research findings.
Immune responsiveness to vaccines varies among children, leaving some susceptible to infections. We also have evidence that the immune deficiencies that contribute to poor vaccine responsiveness also manifest in children as respiratory infection proneness.
Predicting Vaccine Response in the Neonatal Period
The first 100 days of life is an amazing transition time in early life. During that time, the immune system is highly influenced by environmental factors that generate epigenetic changes affecting vaccine responsiveness. Some publications have used the term “window of opportunity,” because it is thought that interventions to change a negative trajectory to a positive one for vaccine responsiveness have a better potential to be effective. Predicting which children will be poorly responsive to vaccines would be desirable, so those children could be specifically identified for intervention. Doing so in the neonatal age time frame using easy-to-obtain clinical samples would be a bonus.
In our most recent study, we sought to identify cytokine biosignatures in the neonatal period, measured in convenient nasopharyngeal secretions, that predict vaccine responses, measured as antibody levels to various vaccines at 1 year of life. Secondly, we assessed the effect of antibiotic exposures on vaccine responses in the study cohort. Third, we tested for induction of CD4+ T-cell vaccine-specific immune memory at infant age 1 year. Fourth, we studied antigen presenting cells (APCs) at rest and in response to an adjuvant called R848, known to stimulate toll-like receptor (TLR) 7/8 agonist, to assess its effects on the immune cells of low vaccine responder children, compared with other children.1
The study population consisted of 101 infants recruited from two primary care pediatric practices in/near Rochester, New York. Children lived in suburban and rural environments. Enrollment and sampling occurred during 2017-2020. All participants received regularly scheduled childhood vaccinations according to the recommendations by US Centers for Disease Control. Nasopharyngeal swabs were used to collect nasal secretions. Antibody titers against six antigens were measured at approximately 1 year of age from all 72 available blood samples. The protective threshold of the corresponding vaccine antigen divided each vaccine-induced antibody level and the ratio considered a normalized titer. The normalized antibody titers were used to define vaccine responsiveness groups as Low Vaccine Responder (bottom 25th percentile of vaccine responders, n = 18 children), as Normal Vaccine Responder (25-75th percentile of vaccine responders, n = 36 children) and as High Vaccine Responder (top 25th percentile of vaccine responders, n = 18 children).
We found that specific nasal cytokine levels measured at newborn age 1 week old, 2 weeks old, and 3 weeks old were predictive of the vaccine response groupings measured at child age 1 year old, following their primary series of vaccinations. The P values varied between less than .05 to .001.
Five newborns had antibiotic exposure at/near the time of birth; 4 [80%] of the 5 were Low Vaccine Responders vs 1 [2%] of 60 Normal+High Vaccine Responder children, P = .006. Also, the cumulative days of antibiotic exposure up to 1 year was highly associated with low vaccine responders, compared with Normal+High Vaccine Responder children (P = 2 x 10-16).
We found that Low Vaccine Responder infants had reduced vaccine-specific T-helper memory cells producing INFg and IL-2 (Th1 cytokines) and IL-4 (Th2 cytokines), compared with Normal+High Vaccine Responder children. In the absence of sufficient numbers of antigen-specific memory CD4+ T-cells, a child would become unprotected from the target infection that the vaccines were intended to prevent after the antibody levels wane.
We found that Low Vaccine Responder antigen-presenting cells are different from those in normal vaccine responders and they can be distinguished when at rest and when stimulated by a specific adjuvant — R848. Our previous findings suggested that Low Vaccine Responder children have a prolonged neonatal-like immune profile (PNIP).2 Therefore, stimulating the immune system of a Low Vaccine Responder could shift their cellular immune responses to behave like cells of Normal+High Vaccine Responder children.
In summary, we identified cytokine biosignatures measured in nasopharyngeal secretions in the neonatal period that predicted vaccine response groups measured as antibody levels at 1 year of life. We showed that reduced vaccine responsiveness was associated with antibiotic exposure at/near birth and with cumulative exposure during the first year of life. We found that Low Vaccine Responder children at 1 year old have fewer vaccine-specific memory CD4+ Th1 and Th2-cells and that antigen-presenting cells at rest and in response to R848 antigen stimulation differ, compared with Normal+High Vaccine Responder children.
Future work by our group will focus on exploring early-life risk factors that influence differences in vaccine responsiveness and interventions that might shift a child’s responsiveness from low to normal or high.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (New York) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. Variability of Vaccine Responsiveness in Young Children. J Infect Dis. 2023 Nov 22:jiad524. doi: 10.1093/infdis/jiad524.
2. Pichichero ME et al. Functional Immune Cell Differences Associated with Low Vaccine Responses in Infants. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
A 7-year-old female presents with persistent pimples on the nose and cheeks for approximately 1 year
Diagnosis
During the visit, skin scrapings were performed, revealing several Demodex mites, confirming the diagnosis of demodicosis.
Demodex folliculorum and Demodex brevis are the two common species implicated. The life cycle of Demodex spp. occurs in the sebaceous glands, leading to mechanical and chemical irritation of the skin.
Various immune responses are also triggered, such as a keratinocyte response via Toll-like receptor 2. Patients usually present with non-specific symptoms such as skin erythema, irritation, peeling, and dryness on the cheeks, eyelids, and paranasal areas. Patients may develop a maculopapular or rosacea-like rash.
Diagnosis is often made through microscopic examination of a skin sample in KOH solution. In rare occasions, a skin surface standardization biopsy method may be used, which determines the density of mites per 1 cm2. Dermoscopy may identify spiky white structures. Molecular methods such as PCR can be used but are not standard.
The differential diagnosis may include acne, rosacea, folliculitis, and Candida infection. Demodicosis can be differentiated by history and further studies including dermoscopy.
Acne vulgaris is an inflammatory disease of the skin’s pilosebaceous unit, primarily involving the face and trunk. It can present with comedones, papules, pustules, and nodules. Secondary signs suggestive of acne vulgaris include scars, erythema, and hyperpigmentation. All forms of acne share a common pathogenesis resulting in the formation of microcomedones, precursors for all clinical acne lesions. In this patient, the absence of microcomedones and the presence of primary inflammatory papules localized to the nose and cheeks suggested an alternative diagnosis.
Rosacea was also considered in the differential diagnosis. Rosacea is an inflammatory dermatosis characterized by erythema, telangiectasia, recurrent flushing, and inflammatory lesions including papulopustules and swelling, primarily affecting the face. The pathogenesis of rosacea is not fully understood but is suggested to involve immune-mediated responses. Vascular dysregulation and reactive oxygen species damage keratinocytes, fibroblasts, and endothelial cells. A higher incidence of rosacea in those with a family history and UV exposure is a known trigger. Demodex folliculorum and Helicobacter pylori are also implicated. Occasionally, Demodex infestation and rosacea may co-occur, and treatment with topical metronidazole can be helpful.
Folliculitis is an infection and inflammation of the hair follicles, forming pustules or erythematous papules over hair-covered skin. It is commonly caused by bacterial infection but can also be due to fungi, viruses, and noninfectious causes such as eosinophilic folliculitis. Diagnosis is clinical, based on physical exam and history, such as recent increased sweating or scratching. KOH prep can be used for Malassezia folliculitis and skin biopsy for eosinophilic folliculitis. Treatment targets the underlying cause. Most bacterial folliculitis cases resolve without treatment, but topical antibiotics may be used. Fungal folliculitis requires oral antifungals, and herpes simplex folliculitis can be treated with antiviral medications.
Cutaneous candidiasis is an infection of the skin by various Candida species, commonly C. albicans. Superficial infections of the skin and mucous membranes, such as intertrigo, are common types. Risk factors include immunosuppression, endocrine disorders, or compromised blood flow. Increased humidity, occlusion, broken skin barriers, and altered skin microbial flora contribute to Candida infection. Diagnosis is clinical but can be confirmed by KOH prep, microscopy, and culture. Treatment involves anti-inflammatory, antibacterial, and antifungal medications. Topical clotrimazole, nystatin, and miconazole are commonly used. Recurrence is prevented by keeping the affected area dry with barrier creams.
Therapeutic goals include arresting mite reproduction, elimination, and preventing recurrent infestations. Treatment may last several months, and the choice of drug depends on patient factors. There have been no standardized treatment studies or long-term effectiveness analyses. Antibiotics such as tetracycline, metronidazole, doxycycline, and ivermectin may be used to prevent proliferation. Permethrin, benzyl benzoate, crotamiton, lindane, and sulfur have also been used. Metronidazole is a common treatment for demodicosis, as was used in our patient for several weeks until the lesions cleared. Systemic metronidazole therapy may be indicated for reducing Demodex spp. density. Severe cases, particularly in immunocompromised individuals, may require oral ivermectin. Appropriate hygiene is important for prevention, such as washing the face with non-oily cleansers and laundering linens regularly.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Mr. Lee is a medical student at the University of California San Diego.
Suggested Reading
Chudzicka-Strugała I et al. Demodicosis in different age groups and alternative treatment options—A review. J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649.
Eichenfield DZ et al. Management of acne vulgaris: A review. JAMA. 2021 Nov 23;326(20):2055-2067. doi: 10.1001/jama.2021.17633.
Sharma A et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022 May;21(5):1895-1904. doi: 10.1111/jocd.14816.
Taudorf EH et al. Cutaneous candidiasis — an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782.
Winters RD, Mitchell M. Folliculitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547754/
Diagnosis
During the visit, skin scrapings were performed, revealing several Demodex mites, confirming the diagnosis of demodicosis.
Demodex folliculorum and Demodex brevis are the two common species implicated. The life cycle of Demodex spp. occurs in the sebaceous glands, leading to mechanical and chemical irritation of the skin.
Various immune responses are also triggered, such as a keratinocyte response via Toll-like receptor 2. Patients usually present with non-specific symptoms such as skin erythema, irritation, peeling, and dryness on the cheeks, eyelids, and paranasal areas. Patients may develop a maculopapular or rosacea-like rash.
Diagnosis is often made through microscopic examination of a skin sample in KOH solution. In rare occasions, a skin surface standardization biopsy method may be used, which determines the density of mites per 1 cm2. Dermoscopy may identify spiky white structures. Molecular methods such as PCR can be used but are not standard.
The differential diagnosis may include acne, rosacea, folliculitis, and Candida infection. Demodicosis can be differentiated by history and further studies including dermoscopy.
Acne vulgaris is an inflammatory disease of the skin’s pilosebaceous unit, primarily involving the face and trunk. It can present with comedones, papules, pustules, and nodules. Secondary signs suggestive of acne vulgaris include scars, erythema, and hyperpigmentation. All forms of acne share a common pathogenesis resulting in the formation of microcomedones, precursors for all clinical acne lesions. In this patient, the absence of microcomedones and the presence of primary inflammatory papules localized to the nose and cheeks suggested an alternative diagnosis.
Rosacea was also considered in the differential diagnosis. Rosacea is an inflammatory dermatosis characterized by erythema, telangiectasia, recurrent flushing, and inflammatory lesions including papulopustules and swelling, primarily affecting the face. The pathogenesis of rosacea is not fully understood but is suggested to involve immune-mediated responses. Vascular dysregulation and reactive oxygen species damage keratinocytes, fibroblasts, and endothelial cells. A higher incidence of rosacea in those with a family history and UV exposure is a known trigger. Demodex folliculorum and Helicobacter pylori are also implicated. Occasionally, Demodex infestation and rosacea may co-occur, and treatment with topical metronidazole can be helpful.
Folliculitis is an infection and inflammation of the hair follicles, forming pustules or erythematous papules over hair-covered skin. It is commonly caused by bacterial infection but can also be due to fungi, viruses, and noninfectious causes such as eosinophilic folliculitis. Diagnosis is clinical, based on physical exam and history, such as recent increased sweating or scratching. KOH prep can be used for Malassezia folliculitis and skin biopsy for eosinophilic folliculitis. Treatment targets the underlying cause. Most bacterial folliculitis cases resolve without treatment, but topical antibiotics may be used. Fungal folliculitis requires oral antifungals, and herpes simplex folliculitis can be treated with antiviral medications.
Cutaneous candidiasis is an infection of the skin by various Candida species, commonly C. albicans. Superficial infections of the skin and mucous membranes, such as intertrigo, are common types. Risk factors include immunosuppression, endocrine disorders, or compromised blood flow. Increased humidity, occlusion, broken skin barriers, and altered skin microbial flora contribute to Candida infection. Diagnosis is clinical but can be confirmed by KOH prep, microscopy, and culture. Treatment involves anti-inflammatory, antibacterial, and antifungal medications. Topical clotrimazole, nystatin, and miconazole are commonly used. Recurrence is prevented by keeping the affected area dry with barrier creams.
Therapeutic goals include arresting mite reproduction, elimination, and preventing recurrent infestations. Treatment may last several months, and the choice of drug depends on patient factors. There have been no standardized treatment studies or long-term effectiveness analyses. Antibiotics such as tetracycline, metronidazole, doxycycline, and ivermectin may be used to prevent proliferation. Permethrin, benzyl benzoate, crotamiton, lindane, and sulfur have also been used. Metronidazole is a common treatment for demodicosis, as was used in our patient for several weeks until the lesions cleared. Systemic metronidazole therapy may be indicated for reducing Demodex spp. density. Severe cases, particularly in immunocompromised individuals, may require oral ivermectin. Appropriate hygiene is important for prevention, such as washing the face with non-oily cleansers and laundering linens regularly.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Mr. Lee is a medical student at the University of California San Diego.
Suggested Reading
Chudzicka-Strugała I et al. Demodicosis in different age groups and alternative treatment options—A review. J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649.
Eichenfield DZ et al. Management of acne vulgaris: A review. JAMA. 2021 Nov 23;326(20):2055-2067. doi: 10.1001/jama.2021.17633.
Sharma A et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022 May;21(5):1895-1904. doi: 10.1111/jocd.14816.
Taudorf EH et al. Cutaneous candidiasis — an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782.
Winters RD, Mitchell M. Folliculitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547754/
Diagnosis
During the visit, skin scrapings were performed, revealing several Demodex mites, confirming the diagnosis of demodicosis.
Demodex folliculorum and Demodex brevis are the two common species implicated. The life cycle of Demodex spp. occurs in the sebaceous glands, leading to mechanical and chemical irritation of the skin.
Various immune responses are also triggered, such as a keratinocyte response via Toll-like receptor 2. Patients usually present with non-specific symptoms such as skin erythema, irritation, peeling, and dryness on the cheeks, eyelids, and paranasal areas. Patients may develop a maculopapular or rosacea-like rash.
Diagnosis is often made through microscopic examination of a skin sample in KOH solution. In rare occasions, a skin surface standardization biopsy method may be used, which determines the density of mites per 1 cm2. Dermoscopy may identify spiky white structures. Molecular methods such as PCR can be used but are not standard.
The differential diagnosis may include acne, rosacea, folliculitis, and Candida infection. Demodicosis can be differentiated by history and further studies including dermoscopy.
Acne vulgaris is an inflammatory disease of the skin’s pilosebaceous unit, primarily involving the face and trunk. It can present with comedones, papules, pustules, and nodules. Secondary signs suggestive of acne vulgaris include scars, erythema, and hyperpigmentation. All forms of acne share a common pathogenesis resulting in the formation of microcomedones, precursors for all clinical acne lesions. In this patient, the absence of microcomedones and the presence of primary inflammatory papules localized to the nose and cheeks suggested an alternative diagnosis.
Rosacea was also considered in the differential diagnosis. Rosacea is an inflammatory dermatosis characterized by erythema, telangiectasia, recurrent flushing, and inflammatory lesions including papulopustules and swelling, primarily affecting the face. The pathogenesis of rosacea is not fully understood but is suggested to involve immune-mediated responses. Vascular dysregulation and reactive oxygen species damage keratinocytes, fibroblasts, and endothelial cells. A higher incidence of rosacea in those with a family history and UV exposure is a known trigger. Demodex folliculorum and Helicobacter pylori are also implicated. Occasionally, Demodex infestation and rosacea may co-occur, and treatment with topical metronidazole can be helpful.
Folliculitis is an infection and inflammation of the hair follicles, forming pustules or erythematous papules over hair-covered skin. It is commonly caused by bacterial infection but can also be due to fungi, viruses, and noninfectious causes such as eosinophilic folliculitis. Diagnosis is clinical, based on physical exam and history, such as recent increased sweating or scratching. KOH prep can be used for Malassezia folliculitis and skin biopsy for eosinophilic folliculitis. Treatment targets the underlying cause. Most bacterial folliculitis cases resolve without treatment, but topical antibiotics may be used. Fungal folliculitis requires oral antifungals, and herpes simplex folliculitis can be treated with antiviral medications.
Cutaneous candidiasis is an infection of the skin by various Candida species, commonly C. albicans. Superficial infections of the skin and mucous membranes, such as intertrigo, are common types. Risk factors include immunosuppression, endocrine disorders, or compromised blood flow. Increased humidity, occlusion, broken skin barriers, and altered skin microbial flora contribute to Candida infection. Diagnosis is clinical but can be confirmed by KOH prep, microscopy, and culture. Treatment involves anti-inflammatory, antibacterial, and antifungal medications. Topical clotrimazole, nystatin, and miconazole are commonly used. Recurrence is prevented by keeping the affected area dry with barrier creams.
Therapeutic goals include arresting mite reproduction, elimination, and preventing recurrent infestations. Treatment may last several months, and the choice of drug depends on patient factors. There have been no standardized treatment studies or long-term effectiveness analyses. Antibiotics such as tetracycline, metronidazole, doxycycline, and ivermectin may be used to prevent proliferation. Permethrin, benzyl benzoate, crotamiton, lindane, and sulfur have also been used. Metronidazole is a common treatment for demodicosis, as was used in our patient for several weeks until the lesions cleared. Systemic metronidazole therapy may be indicated for reducing Demodex spp. density. Severe cases, particularly in immunocompromised individuals, may require oral ivermectin. Appropriate hygiene is important for prevention, such as washing the face with non-oily cleansers and laundering linens regularly.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Mr. Lee is a medical student at the University of California San Diego.
Suggested Reading
Chudzicka-Strugała I et al. Demodicosis in different age groups and alternative treatment options—A review. J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649.
Eichenfield DZ et al. Management of acne vulgaris: A review. JAMA. 2021 Nov 23;326(20):2055-2067. doi: 10.1001/jama.2021.17633.
Sharma A et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022 May;21(5):1895-1904. doi: 10.1111/jocd.14816.
Taudorf EH et al. Cutaneous candidiasis — an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782.
Winters RD, Mitchell M. Folliculitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547754/
A 7-year-old female presents with persistent pimples on the nose and cheeks for approximately 1 year. She had been treated with several topical antibiotics and acne washes without resolution of the lesions. There were no signs of early puberty, and the child had no history of medical conditions. Her mother has a history of rosacea. Physical examination revealed erythematous papules on the nose and cheeks bilaterally.
Early-Life Excess Weight Tied to Subsequent Stroke Risk
, new research suggested.
An analysis of more than five decades of health data on 10,000 adults revealed that close to 5% experienced a stroke during the follow-up period, with the risk for ischemic stroke being more than twice as high in women who had obesity as teens or young adults. The risk was even higher for hemorrhagic stroke in both men and women with a history of obesity in youth.
“Our findings suggest that being overweight may have long-term health effects, even if the excess weight is temporary,” lead author Ursula Mikkola, BM, an investigator in the Research Unit of Population Health at the University of Oulu, Oulu, Finland, said in a news release.
“Health care professionals should pay attention to overweight and obesity in young people and work with them to develop healthier eating patterns and physical activity — however, conversations with teens and young adults about weight should be approached in a nonjudgmental and nonstigmatizing manner,” she added.
The study was published online in Stroke.
Gender Differences
Childhood obesity has been associated with a heightened risk for cerebrovascular disease later in life, but most studies have focused on body mass index (BMI) at a single time point without considering its fluctuations throughout life, the investigators noted.
For the study, investigators used data from the Northern Finland Birth Cohort 1966, a prospective, general population-based birth cohort that followed 10,491 individuals (5185 women) until 2020 or the first stroke, death, or moving abroad, whichever came first.
Mean (SD) follow-up for each participant was 39 years from age 14 onward and 23 years from age 31 onward. The analysis was conducted between 1980 and 2020.
BMI data were collected from participants at the age of 14 and 31 years. Age 14 covariates included smoking, parental socioeconomic status, and age at menarche (for girls). Age 31 covariates included smoking and participants’ educational level.
During the follow-up period, 4.7% of participants experienced stroke. Of these events, 31% were ischemic strokes and 40% were transient ischemic attacks. The remainder were hemorrhagic or other cerebrovascular events.
Using normal weight as a reference, researchers found that the risk for ischemic stroke was over twice as high for women who had been overweight at ages 14 (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.44-4.31) and 31 (HR, 2.13; 95% CI, 1.14-3.97) years. The risk was also considerably higher for women who had obesity at ages 14 (HR, 1.87; 95% CI, 0.76-4.58) and 31 (HR, 2.67; 95% CI, 1.26-5.65) years.
The risk for hemorrhagic stroke was even higher, both among women (HR, 3.49; 95% CI, 1.13-10.7) and men (HR, 5.75; 95% CI, 1.43-23.1) who had obesity at age 31.
No similar associations were found among men, and the findings were independent of earlier or later BMI.
The risk for any cerebrovascular disease related to overweight at age 14 was twice as high among girls vs boys (HR, 2.09; 95% CI, 1.06-4.15), and the risk for ischemic stroke related to obesity at age 31 was nearly seven times higher among women vs men (HR, 6.96; 95% CI, 1.36-35.7).
“Stroke at a young age is rare, so the difference of just a few strokes could have an outsized impact on the risk estimates,” the study authors said. “Also, BMI relies solely on a person’s height and weight; therefore, a high BMI may be a misleading way to define obesity, especially in muscular people who may carry little fat even while weighing more.”
Caveats
In an accompanying editorial, Larry Goldstein, MD, chair of the Department of Neurology, University of Kentucky, Lexington, Kentucky, and codirector of the Kentucky Neuroscience Institute, said the study “provides additional evidence of an association between overweight/obesity and stroke in young adults.”
However, Dr. Goldstein added that “while it is tempting to assume that reductions in overweight/obesity in younger populations would translate to lower stroke rates in young adults, this remains to be proven.”
Moreover, it is “always important to acknowledge that associations found in observational studies may not reflect causality.”
This study was supported by Orion Research Foundation, Päivikki and Sakari Sohlberg Foundation, and Paulo Foundation. Dr. Mikkola reported no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Goldstein reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggested.
An analysis of more than five decades of health data on 10,000 adults revealed that close to 5% experienced a stroke during the follow-up period, with the risk for ischemic stroke being more than twice as high in women who had obesity as teens or young adults. The risk was even higher for hemorrhagic stroke in both men and women with a history of obesity in youth.
“Our findings suggest that being overweight may have long-term health effects, even if the excess weight is temporary,” lead author Ursula Mikkola, BM, an investigator in the Research Unit of Population Health at the University of Oulu, Oulu, Finland, said in a news release.
“Health care professionals should pay attention to overweight and obesity in young people and work with them to develop healthier eating patterns and physical activity — however, conversations with teens and young adults about weight should be approached in a nonjudgmental and nonstigmatizing manner,” she added.
The study was published online in Stroke.
Gender Differences
Childhood obesity has been associated with a heightened risk for cerebrovascular disease later in life, but most studies have focused on body mass index (BMI) at a single time point without considering its fluctuations throughout life, the investigators noted.
For the study, investigators used data from the Northern Finland Birth Cohort 1966, a prospective, general population-based birth cohort that followed 10,491 individuals (5185 women) until 2020 or the first stroke, death, or moving abroad, whichever came first.
Mean (SD) follow-up for each participant was 39 years from age 14 onward and 23 years from age 31 onward. The analysis was conducted between 1980 and 2020.
BMI data were collected from participants at the age of 14 and 31 years. Age 14 covariates included smoking, parental socioeconomic status, and age at menarche (for girls). Age 31 covariates included smoking and participants’ educational level.
During the follow-up period, 4.7% of participants experienced stroke. Of these events, 31% were ischemic strokes and 40% were transient ischemic attacks. The remainder were hemorrhagic or other cerebrovascular events.
Using normal weight as a reference, researchers found that the risk for ischemic stroke was over twice as high for women who had been overweight at ages 14 (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.44-4.31) and 31 (HR, 2.13; 95% CI, 1.14-3.97) years. The risk was also considerably higher for women who had obesity at ages 14 (HR, 1.87; 95% CI, 0.76-4.58) and 31 (HR, 2.67; 95% CI, 1.26-5.65) years.
The risk for hemorrhagic stroke was even higher, both among women (HR, 3.49; 95% CI, 1.13-10.7) and men (HR, 5.75; 95% CI, 1.43-23.1) who had obesity at age 31.
No similar associations were found among men, and the findings were independent of earlier or later BMI.
The risk for any cerebrovascular disease related to overweight at age 14 was twice as high among girls vs boys (HR, 2.09; 95% CI, 1.06-4.15), and the risk for ischemic stroke related to obesity at age 31 was nearly seven times higher among women vs men (HR, 6.96; 95% CI, 1.36-35.7).
“Stroke at a young age is rare, so the difference of just a few strokes could have an outsized impact on the risk estimates,” the study authors said. “Also, BMI relies solely on a person’s height and weight; therefore, a high BMI may be a misleading way to define obesity, especially in muscular people who may carry little fat even while weighing more.”
Caveats
In an accompanying editorial, Larry Goldstein, MD, chair of the Department of Neurology, University of Kentucky, Lexington, Kentucky, and codirector of the Kentucky Neuroscience Institute, said the study “provides additional evidence of an association between overweight/obesity and stroke in young adults.”
However, Dr. Goldstein added that “while it is tempting to assume that reductions in overweight/obesity in younger populations would translate to lower stroke rates in young adults, this remains to be proven.”
Moreover, it is “always important to acknowledge that associations found in observational studies may not reflect causality.”
This study was supported by Orion Research Foundation, Päivikki and Sakari Sohlberg Foundation, and Paulo Foundation. Dr. Mikkola reported no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Goldstein reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggested.
An analysis of more than five decades of health data on 10,000 adults revealed that close to 5% experienced a stroke during the follow-up period, with the risk for ischemic stroke being more than twice as high in women who had obesity as teens or young adults. The risk was even higher for hemorrhagic stroke in both men and women with a history of obesity in youth.
“Our findings suggest that being overweight may have long-term health effects, even if the excess weight is temporary,” lead author Ursula Mikkola, BM, an investigator in the Research Unit of Population Health at the University of Oulu, Oulu, Finland, said in a news release.
“Health care professionals should pay attention to overweight and obesity in young people and work with them to develop healthier eating patterns and physical activity — however, conversations with teens and young adults about weight should be approached in a nonjudgmental and nonstigmatizing manner,” she added.
The study was published online in Stroke.
Gender Differences
Childhood obesity has been associated with a heightened risk for cerebrovascular disease later in life, but most studies have focused on body mass index (BMI) at a single time point without considering its fluctuations throughout life, the investigators noted.
For the study, investigators used data from the Northern Finland Birth Cohort 1966, a prospective, general population-based birth cohort that followed 10,491 individuals (5185 women) until 2020 or the first stroke, death, or moving abroad, whichever came first.
Mean (SD) follow-up for each participant was 39 years from age 14 onward and 23 years from age 31 onward. The analysis was conducted between 1980 and 2020.
BMI data were collected from participants at the age of 14 and 31 years. Age 14 covariates included smoking, parental socioeconomic status, and age at menarche (for girls). Age 31 covariates included smoking and participants’ educational level.
During the follow-up period, 4.7% of participants experienced stroke. Of these events, 31% were ischemic strokes and 40% were transient ischemic attacks. The remainder were hemorrhagic or other cerebrovascular events.
Using normal weight as a reference, researchers found that the risk for ischemic stroke was over twice as high for women who had been overweight at ages 14 (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.44-4.31) and 31 (HR, 2.13; 95% CI, 1.14-3.97) years. The risk was also considerably higher for women who had obesity at ages 14 (HR, 1.87; 95% CI, 0.76-4.58) and 31 (HR, 2.67; 95% CI, 1.26-5.65) years.
The risk for hemorrhagic stroke was even higher, both among women (HR, 3.49; 95% CI, 1.13-10.7) and men (HR, 5.75; 95% CI, 1.43-23.1) who had obesity at age 31.
No similar associations were found among men, and the findings were independent of earlier or later BMI.
The risk for any cerebrovascular disease related to overweight at age 14 was twice as high among girls vs boys (HR, 2.09; 95% CI, 1.06-4.15), and the risk for ischemic stroke related to obesity at age 31 was nearly seven times higher among women vs men (HR, 6.96; 95% CI, 1.36-35.7).
“Stroke at a young age is rare, so the difference of just a few strokes could have an outsized impact on the risk estimates,” the study authors said. “Also, BMI relies solely on a person’s height and weight; therefore, a high BMI may be a misleading way to define obesity, especially in muscular people who may carry little fat even while weighing more.”
Caveats
In an accompanying editorial, Larry Goldstein, MD, chair of the Department of Neurology, University of Kentucky, Lexington, Kentucky, and codirector of the Kentucky Neuroscience Institute, said the study “provides additional evidence of an association between overweight/obesity and stroke in young adults.”
However, Dr. Goldstein added that “while it is tempting to assume that reductions in overweight/obesity in younger populations would translate to lower stroke rates in young adults, this remains to be proven.”
Moreover, it is “always important to acknowledge that associations found in observational studies may not reflect causality.”
This study was supported by Orion Research Foundation, Päivikki and Sakari Sohlberg Foundation, and Paulo Foundation. Dr. Mikkola reported no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Goldstein reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sharp Rise in US Pediatric ADHD Diagnoses
TOPLINE:
METHODOLOGY:
- Researchers used 2022 data from the National Survey of Children’s Health to estimate the prevalence of ever-diagnosed and current ADHD among US children between the ages of 3 and 18 years.
- They also estimated, among children with current ADHD, the severity of the condition and the presence of current co-occurring disorders and the receipt of medication and behavioral treatments.
- The researchers calculated overall weighted estimates as well as estimates for specific demographic and clinical subgroups (n = 45,169).
TAKEAWAY:
- The number of children who had ever received an ADHD diagnosis increased from 6.1 million in 2016 to 7.1 million in 2022, and the number with current ADHD increased from 5.4 million to 6.5 million.
- Of those with current ADHD in 2022, 58.1% had moderate or severe ADHD, and 77.9% had at least one co-occurring disorder.
- A total of 53.6% had received ADHD medication, 44.4% had received behavioral treatment in the past year, and 30.1% had received no ADHD-specific treatment.
- A similar percentage of children with ADHD were receiving behavioral treatment in 2022 as in 2016 (44.4% vs 46.7%, respectively), but treatment with ADHD medication was lower in 2022 than in 2016 (53.6% vs 62.0%, respectively).
IN PRACTICE:
The estimates “can be used by clinicians to understand current ADHD diagnosis and treatment utilization patterns to inform clinical practice, such as accounting for the frequency and management of co-occurring conditions and considering the notable percentage of children with ADHD not currently receiving ADHD treatment,” and can be used by policymakers, practitioners, and others “to plan for the needs of children with ADHD, such as by ensuring access to care and services for ADHD,” investigators wrote.
SOURCE:
Melissa L. Danielson, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, led the study, which was published online in the Journal of Clinical Child & Adolescent Psychology.
LIMITATIONS:
Indicators reported in the analysis were on the basis of the parent report, which may be limited by recall and reporting decisions and were not validated against medical records or clinical judgment. Moreover, details about the types of treatment were not included.
DISCLOSURES:
The work was authorized as part of the contributor’s official duties as an employee of the US Government, and therefore is a work of the US Government. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers used 2022 data from the National Survey of Children’s Health to estimate the prevalence of ever-diagnosed and current ADHD among US children between the ages of 3 and 18 years.
- They also estimated, among children with current ADHD, the severity of the condition and the presence of current co-occurring disorders and the receipt of medication and behavioral treatments.
- The researchers calculated overall weighted estimates as well as estimates for specific demographic and clinical subgroups (n = 45,169).
TAKEAWAY:
- The number of children who had ever received an ADHD diagnosis increased from 6.1 million in 2016 to 7.1 million in 2022, and the number with current ADHD increased from 5.4 million to 6.5 million.
- Of those with current ADHD in 2022, 58.1% had moderate or severe ADHD, and 77.9% had at least one co-occurring disorder.
- A total of 53.6% had received ADHD medication, 44.4% had received behavioral treatment in the past year, and 30.1% had received no ADHD-specific treatment.
- A similar percentage of children with ADHD were receiving behavioral treatment in 2022 as in 2016 (44.4% vs 46.7%, respectively), but treatment with ADHD medication was lower in 2022 than in 2016 (53.6% vs 62.0%, respectively).
IN PRACTICE:
The estimates “can be used by clinicians to understand current ADHD diagnosis and treatment utilization patterns to inform clinical practice, such as accounting for the frequency and management of co-occurring conditions and considering the notable percentage of children with ADHD not currently receiving ADHD treatment,” and can be used by policymakers, practitioners, and others “to plan for the needs of children with ADHD, such as by ensuring access to care and services for ADHD,” investigators wrote.
SOURCE:
Melissa L. Danielson, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, led the study, which was published online in the Journal of Clinical Child & Adolescent Psychology.
LIMITATIONS:
Indicators reported in the analysis were on the basis of the parent report, which may be limited by recall and reporting decisions and were not validated against medical records or clinical judgment. Moreover, details about the types of treatment were not included.
DISCLOSURES:
The work was authorized as part of the contributor’s official duties as an employee of the US Government, and therefore is a work of the US Government. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers used 2022 data from the National Survey of Children’s Health to estimate the prevalence of ever-diagnosed and current ADHD among US children between the ages of 3 and 18 years.
- They also estimated, among children with current ADHD, the severity of the condition and the presence of current co-occurring disorders and the receipt of medication and behavioral treatments.
- The researchers calculated overall weighted estimates as well as estimates for specific demographic and clinical subgroups (n = 45,169).
TAKEAWAY:
- The number of children who had ever received an ADHD diagnosis increased from 6.1 million in 2016 to 7.1 million in 2022, and the number with current ADHD increased from 5.4 million to 6.5 million.
- Of those with current ADHD in 2022, 58.1% had moderate or severe ADHD, and 77.9% had at least one co-occurring disorder.
- A total of 53.6% had received ADHD medication, 44.4% had received behavioral treatment in the past year, and 30.1% had received no ADHD-specific treatment.
- A similar percentage of children with ADHD were receiving behavioral treatment in 2022 as in 2016 (44.4% vs 46.7%, respectively), but treatment with ADHD medication was lower in 2022 than in 2016 (53.6% vs 62.0%, respectively).
IN PRACTICE:
The estimates “can be used by clinicians to understand current ADHD diagnosis and treatment utilization patterns to inform clinical practice, such as accounting for the frequency and management of co-occurring conditions and considering the notable percentage of children with ADHD not currently receiving ADHD treatment,” and can be used by policymakers, practitioners, and others “to plan for the needs of children with ADHD, such as by ensuring access to care and services for ADHD,” investigators wrote.
SOURCE:
Melissa L. Danielson, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, led the study, which was published online in the Journal of Clinical Child & Adolescent Psychology.
LIMITATIONS:
Indicators reported in the analysis were on the basis of the parent report, which may be limited by recall and reporting decisions and were not validated against medical records or clinical judgment. Moreover, details about the types of treatment were not included.
DISCLOSURES:
The work was authorized as part of the contributor’s official duties as an employee of the US Government, and therefore is a work of the US Government. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
Engineering Mind Helps Investigator Develop New Cancer Therapies
Dr. Kopetz received the AACR-Waun Ki Hong Award in April. The American Association for Cancer Research (AACR) granted Dr. Kopetz this award to recognize his leadership in the development of novel therapies for patients with BRAF-mutated metastatic colon cancer with poor prognoses, according to a statement from the AACR.
Using molecular profiling and patient-derived xenografts, Dr. Kopetz discovered resistance mechanisms and helped develop approaches to overcome such resistant pathways. His clinical studies analyzing vemurafenib, cetuximab, and irinotecan resulted in new additions to National Comprehensive Cancer Network guidelines and led to the FDA approval of encorafenib plus cetuximab for adult patients with metastatic colorectal cancer (CRC) with a BRAF V600E mutation after prior therapy.
In an interview, Dr. Kopetz shared his unique road to research, how his engineering background influences his work, and why his recent award’s namesake holds special significance to him.
What led to your medical career? Growing up, did you always want to be a doctor?
Dr. Kopetz: My interest initially was in engineering. I grew up in Tennessee from a family of engineers and doctors. In college, I completed a degree in biomedical engineering and electrical engineering.
I had the opportunity to spend one summer at the National Institutes of Health, where I did some research on the structure of the HIV integrase enzyme. It was fundamental basic research with some engineering overlay and required spending 4 days a week working in the dark in a laser lab to analyze the structure of this protein.
One day a week, I was at Georgetown in the HIV/AIDS Clinic, where I collected blood samples and saw HIV/AIDS patients. At the end of the summer, I reflected and realized that I really enjoyed that 1 day out of the week, much more than the other 4. I enjoyed working with patients and interacting with people and thought I’d enjoy the more direct way to help patients, so made a pivot into medicine.
Was the rest of your medical training more traditional?
Dr. Kopetz: My path was a little atypical for a physician scientist. I pursued a medical degree at Johns Hopkins, did internal medicine training at Duke, and then came down to MD Anderson Cancer Center [in Houston, Texas] to do a fellowship in medical oncology, and also obtained a PhD in cancer biology, where I explored mechanisms of resistance to colorectal cancer treatment.
While a traditional physician scientist typically obtains a PhD training in the middle of their medical school, I completed my medical training and then went back to get a PhD. It was a different, nontraditional route.
What is your current role, and what is most inspiring about your work?
Dr. Kopetz: I’ve been at MD Anderson now for 20 years in GI medical oncology. I recently stepped into a new role of helping facilitate translational research at the institution and am now Associate VP for translational research.
I’m excited about where we are in cancer research. I think we’re moving into an era where the amount of information that we can get out of patients and the rapidity in which we can move discoveries is much greater than it has ever been.
Our ability to extract information out of patient biopsies, surgical samples, or even minimally invasive techniques to sample the tumors, such as liquid biopsy, has provided tremendous insights into how tumors are evolving and adapting to therapies and [provides us] opportunities for novel interventions. This opens up ways where I think as a field, we can more readily accelerate our understanding of cancer.
The second component is seeing the rapidity in which we’re now able to execute ideas in the drug development space compared to years before. The pace of new drug development has increased and the innovations in the chemistries have opened up new opportunities and new targets that in the past were considered undruggable. For example, the mutated oncogene, KRAS, was once an extremely challenging therapeutic target and considered undruggable. Mutations in the p53 gene, a tumor suppressor gene, were similarly challenging. I think the convergence of these two trends are going to more rapidly accelerate the advances for our patients. I’m optimistic about the future.
Tell us more about the novel therapies for patients with BRAF-mutated metastatic colon cancer for which you were a lead researcher.
Dr. Kopetz: A lot of [my] work goes back over 10 years, where my [research colleagues and I] were targeting the BRAF V600E oncogene in colorectal cancer melanoma and identified that this worked well in melanoma but was relatively inactive in colorectal cancer despite the same drugs and the same mutations. This led to a recognition of optimal combination drugs that really blocked some of the adaptive feedback that we saw in colorectal cancer. This was a key recognition that these tumors, after you block one node of signaling, rapidly adapt and reactivate the signaling through alternate nodes. This finding really resonated with me with my engineering background, thinking about the networks, signaling networks, and the concepts of feedback regulation of complex systems.
The strategy of blocking the primary oncogene and then blocking the feedback mechanisms that the tumors were utilizing was adopted in colorectal cancer through this work. It took us 10 years to get to an FDA approval with this strategy, but it’s really encouraging that we’re now using this strategy and applying it to the new wave of KRAS inhibitors, where the exact same feedback pathway appears to be at play.
Does your engineering background impact your work today?
Dr. Kopetz: Yes, I’ve found that my engineering training has provided me with complementary skills that can significantly contribute to the development of innovative technologies, computational approaches, and interdisciplinary strategies for advancing cancer research.
Today, I do a lot of work understanding and recognizing complex networks of signaling, and it’s the same network theories that we learned and developed in engineering.
These same theories are now being applied to biology. For example, we are very interested in how tumors adapt over the longer term, over multiple lines of therapy, where there is both clonal selection and clonal evolution occurring with our various standard-of-care therapies. Our hope is that application of engineering principles can help uncover new vulnerabilities in cancer that weren’t evident when we were thinking about CRC as a static tumor.
I understand your recently awarded AACR-Waun Ki Hong Award for Outstanding Achievement in Translational and Clinical Cancer Research has special significance to you. Can you explain why that is?
Dr. Kopetz: This holds a special meaning for me, because Dr. Hong provided a lot of guidance [to me] over the years. He was the division head for cancer medicine at MD Anderson for many years and was instrumental in helping advocate [for me] and advance my career as well as the careers of so many others in and outside of the institution. I considered him a key mentor and sponsor. He helped provide me with guidance early in my oncology career, helping me identify high-value projects and critically evaluate research directions to pursue. He also helped me think about how to balance my research portfolio and provided guidance about how to work well within a team.
It’s really humbling to have a reward bearing his name as somebody who I so deeply respected, and I’m so grateful for the impact he had on my life.
Dr. Kopetz received the AACR-Waun Ki Hong Award in April. The American Association for Cancer Research (AACR) granted Dr. Kopetz this award to recognize his leadership in the development of novel therapies for patients with BRAF-mutated metastatic colon cancer with poor prognoses, according to a statement from the AACR.
Using molecular profiling and patient-derived xenografts, Dr. Kopetz discovered resistance mechanisms and helped develop approaches to overcome such resistant pathways. His clinical studies analyzing vemurafenib, cetuximab, and irinotecan resulted in new additions to National Comprehensive Cancer Network guidelines and led to the FDA approval of encorafenib plus cetuximab for adult patients with metastatic colorectal cancer (CRC) with a BRAF V600E mutation after prior therapy.
In an interview, Dr. Kopetz shared his unique road to research, how his engineering background influences his work, and why his recent award’s namesake holds special significance to him.
What led to your medical career? Growing up, did you always want to be a doctor?
Dr. Kopetz: My interest initially was in engineering. I grew up in Tennessee from a family of engineers and doctors. In college, I completed a degree in biomedical engineering and electrical engineering.
I had the opportunity to spend one summer at the National Institutes of Health, where I did some research on the structure of the HIV integrase enzyme. It was fundamental basic research with some engineering overlay and required spending 4 days a week working in the dark in a laser lab to analyze the structure of this protein.
One day a week, I was at Georgetown in the HIV/AIDS Clinic, where I collected blood samples and saw HIV/AIDS patients. At the end of the summer, I reflected and realized that I really enjoyed that 1 day out of the week, much more than the other 4. I enjoyed working with patients and interacting with people and thought I’d enjoy the more direct way to help patients, so made a pivot into medicine.
Was the rest of your medical training more traditional?
Dr. Kopetz: My path was a little atypical for a physician scientist. I pursued a medical degree at Johns Hopkins, did internal medicine training at Duke, and then came down to MD Anderson Cancer Center [in Houston, Texas] to do a fellowship in medical oncology, and also obtained a PhD in cancer biology, where I explored mechanisms of resistance to colorectal cancer treatment.
While a traditional physician scientist typically obtains a PhD training in the middle of their medical school, I completed my medical training and then went back to get a PhD. It was a different, nontraditional route.
What is your current role, and what is most inspiring about your work?
Dr. Kopetz: I’ve been at MD Anderson now for 20 years in GI medical oncology. I recently stepped into a new role of helping facilitate translational research at the institution and am now Associate VP for translational research.
I’m excited about where we are in cancer research. I think we’re moving into an era where the amount of information that we can get out of patients and the rapidity in which we can move discoveries is much greater than it has ever been.
Our ability to extract information out of patient biopsies, surgical samples, or even minimally invasive techniques to sample the tumors, such as liquid biopsy, has provided tremendous insights into how tumors are evolving and adapting to therapies and [provides us] opportunities for novel interventions. This opens up ways where I think as a field, we can more readily accelerate our understanding of cancer.
The second component is seeing the rapidity in which we’re now able to execute ideas in the drug development space compared to years before. The pace of new drug development has increased and the innovations in the chemistries have opened up new opportunities and new targets that in the past were considered undruggable. For example, the mutated oncogene, KRAS, was once an extremely challenging therapeutic target and considered undruggable. Mutations in the p53 gene, a tumor suppressor gene, were similarly challenging. I think the convergence of these two trends are going to more rapidly accelerate the advances for our patients. I’m optimistic about the future.
Tell us more about the novel therapies for patients with BRAF-mutated metastatic colon cancer for which you were a lead researcher.
Dr. Kopetz: A lot of [my] work goes back over 10 years, where my [research colleagues and I] were targeting the BRAF V600E oncogene in colorectal cancer melanoma and identified that this worked well in melanoma but was relatively inactive in colorectal cancer despite the same drugs and the same mutations. This led to a recognition of optimal combination drugs that really blocked some of the adaptive feedback that we saw in colorectal cancer. This was a key recognition that these tumors, after you block one node of signaling, rapidly adapt and reactivate the signaling through alternate nodes. This finding really resonated with me with my engineering background, thinking about the networks, signaling networks, and the concepts of feedback regulation of complex systems.
The strategy of blocking the primary oncogene and then blocking the feedback mechanisms that the tumors were utilizing was adopted in colorectal cancer through this work. It took us 10 years to get to an FDA approval with this strategy, but it’s really encouraging that we’re now using this strategy and applying it to the new wave of KRAS inhibitors, where the exact same feedback pathway appears to be at play.
Does your engineering background impact your work today?
Dr. Kopetz: Yes, I’ve found that my engineering training has provided me with complementary skills that can significantly contribute to the development of innovative technologies, computational approaches, and interdisciplinary strategies for advancing cancer research.
Today, I do a lot of work understanding and recognizing complex networks of signaling, and it’s the same network theories that we learned and developed in engineering.
These same theories are now being applied to biology. For example, we are very interested in how tumors adapt over the longer term, over multiple lines of therapy, where there is both clonal selection and clonal evolution occurring with our various standard-of-care therapies. Our hope is that application of engineering principles can help uncover new vulnerabilities in cancer that weren’t evident when we were thinking about CRC as a static tumor.
I understand your recently awarded AACR-Waun Ki Hong Award for Outstanding Achievement in Translational and Clinical Cancer Research has special significance to you. Can you explain why that is?
Dr. Kopetz: This holds a special meaning for me, because Dr. Hong provided a lot of guidance [to me] over the years. He was the division head for cancer medicine at MD Anderson for many years and was instrumental in helping advocate [for me] and advance my career as well as the careers of so many others in and outside of the institution. I considered him a key mentor and sponsor. He helped provide me with guidance early in my oncology career, helping me identify high-value projects and critically evaluate research directions to pursue. He also helped me think about how to balance my research portfolio and provided guidance about how to work well within a team.
It’s really humbling to have a reward bearing his name as somebody who I so deeply respected, and I’m so grateful for the impact he had on my life.
Dr. Kopetz received the AACR-Waun Ki Hong Award in April. The American Association for Cancer Research (AACR) granted Dr. Kopetz this award to recognize his leadership in the development of novel therapies for patients with BRAF-mutated metastatic colon cancer with poor prognoses, according to a statement from the AACR.
Using molecular profiling and patient-derived xenografts, Dr. Kopetz discovered resistance mechanisms and helped develop approaches to overcome such resistant pathways. His clinical studies analyzing vemurafenib, cetuximab, and irinotecan resulted in new additions to National Comprehensive Cancer Network guidelines and led to the FDA approval of encorafenib plus cetuximab for adult patients with metastatic colorectal cancer (CRC) with a BRAF V600E mutation after prior therapy.
In an interview, Dr. Kopetz shared his unique road to research, how his engineering background influences his work, and why his recent award’s namesake holds special significance to him.
What led to your medical career? Growing up, did you always want to be a doctor?
Dr. Kopetz: My interest initially was in engineering. I grew up in Tennessee from a family of engineers and doctors. In college, I completed a degree in biomedical engineering and electrical engineering.
I had the opportunity to spend one summer at the National Institutes of Health, where I did some research on the structure of the HIV integrase enzyme. It was fundamental basic research with some engineering overlay and required spending 4 days a week working in the dark in a laser lab to analyze the structure of this protein.
One day a week, I was at Georgetown in the HIV/AIDS Clinic, where I collected blood samples and saw HIV/AIDS patients. At the end of the summer, I reflected and realized that I really enjoyed that 1 day out of the week, much more than the other 4. I enjoyed working with patients and interacting with people and thought I’d enjoy the more direct way to help patients, so made a pivot into medicine.
Was the rest of your medical training more traditional?
Dr. Kopetz: My path was a little atypical for a physician scientist. I pursued a medical degree at Johns Hopkins, did internal medicine training at Duke, and then came down to MD Anderson Cancer Center [in Houston, Texas] to do a fellowship in medical oncology, and also obtained a PhD in cancer biology, where I explored mechanisms of resistance to colorectal cancer treatment.
While a traditional physician scientist typically obtains a PhD training in the middle of their medical school, I completed my medical training and then went back to get a PhD. It was a different, nontraditional route.
What is your current role, and what is most inspiring about your work?
Dr. Kopetz: I’ve been at MD Anderson now for 20 years in GI medical oncology. I recently stepped into a new role of helping facilitate translational research at the institution and am now Associate VP for translational research.
I’m excited about where we are in cancer research. I think we’re moving into an era where the amount of information that we can get out of patients and the rapidity in which we can move discoveries is much greater than it has ever been.
Our ability to extract information out of patient biopsies, surgical samples, or even minimally invasive techniques to sample the tumors, such as liquid biopsy, has provided tremendous insights into how tumors are evolving and adapting to therapies and [provides us] opportunities for novel interventions. This opens up ways where I think as a field, we can more readily accelerate our understanding of cancer.
The second component is seeing the rapidity in which we’re now able to execute ideas in the drug development space compared to years before. The pace of new drug development has increased and the innovations in the chemistries have opened up new opportunities and new targets that in the past were considered undruggable. For example, the mutated oncogene, KRAS, was once an extremely challenging therapeutic target and considered undruggable. Mutations in the p53 gene, a tumor suppressor gene, were similarly challenging. I think the convergence of these two trends are going to more rapidly accelerate the advances for our patients. I’m optimistic about the future.
Tell us more about the novel therapies for patients with BRAF-mutated metastatic colon cancer for which you were a lead researcher.
Dr. Kopetz: A lot of [my] work goes back over 10 years, where my [research colleagues and I] were targeting the BRAF V600E oncogene in colorectal cancer melanoma and identified that this worked well in melanoma but was relatively inactive in colorectal cancer despite the same drugs and the same mutations. This led to a recognition of optimal combination drugs that really blocked some of the adaptive feedback that we saw in colorectal cancer. This was a key recognition that these tumors, after you block one node of signaling, rapidly adapt and reactivate the signaling through alternate nodes. This finding really resonated with me with my engineering background, thinking about the networks, signaling networks, and the concepts of feedback regulation of complex systems.
The strategy of blocking the primary oncogene and then blocking the feedback mechanisms that the tumors were utilizing was adopted in colorectal cancer through this work. It took us 10 years to get to an FDA approval with this strategy, but it’s really encouraging that we’re now using this strategy and applying it to the new wave of KRAS inhibitors, where the exact same feedback pathway appears to be at play.
Does your engineering background impact your work today?
Dr. Kopetz: Yes, I’ve found that my engineering training has provided me with complementary skills that can significantly contribute to the development of innovative technologies, computational approaches, and interdisciplinary strategies for advancing cancer research.
Today, I do a lot of work understanding and recognizing complex networks of signaling, and it’s the same network theories that we learned and developed in engineering.
These same theories are now being applied to biology. For example, we are very interested in how tumors adapt over the longer term, over multiple lines of therapy, where there is both clonal selection and clonal evolution occurring with our various standard-of-care therapies. Our hope is that application of engineering principles can help uncover new vulnerabilities in cancer that weren’t evident when we were thinking about CRC as a static tumor.
I understand your recently awarded AACR-Waun Ki Hong Award for Outstanding Achievement in Translational and Clinical Cancer Research has special significance to you. Can you explain why that is?
Dr. Kopetz: This holds a special meaning for me, because Dr. Hong provided a lot of guidance [to me] over the years. He was the division head for cancer medicine at MD Anderson for many years and was instrumental in helping advocate [for me] and advance my career as well as the careers of so many others in and outside of the institution. I considered him a key mentor and sponsor. He helped provide me with guidance early in my oncology career, helping me identify high-value projects and critically evaluate research directions to pursue. He also helped me think about how to balance my research portfolio and provided guidance about how to work well within a team.
It’s really humbling to have a reward bearing his name as somebody who I so deeply respected, and I’m so grateful for the impact he had on my life.
Prospective MS Trial Proves Ocrelizumab Efficacy in Under-Represented Populations
NASHVILLE, Tennessee — , according to the results of a 1-year analysis of the CHIMES trial. The study is the first-ever prospective study of an MS disease-modifying therapy (DMT) exclusively performed in under-represented populations, and offers lessons to researchers aiming to design more inclusive clinical trials to bolster participation by under-represented populations.
“The goal was to better understand efficacy of therapy in under-represented populations because we typically have very low numbers of these patients in our clinical trials, although there are multiple studies over the past decades suggesting that there may be poorer outcomes in Black and Hispanic individuals, particularly in the United States, and that there also may be more aggressive disease,” said Mitzi Williams, MD, who presented the study in a poster session at the annual meeting of the Consortium of Multiple Sclerosis Centers.
The team recruited 113 Black participants and 69 Hispanic participants, and, in fact, over-recruited the target number by 25%, and did so 2 months before the launch of the study in July 2020, which just happened to be in the midst of a global pandemic.
After 48 weeks of ocrelizumab treatment, 46.0% of Black participants and 58.0% of Hispanic participants achieved no evidence of disease activity in three components (NEDA-3), while 94.7% and 95.7% were free from relapses, respectively, and 94.7% and 94.2% were free from disease worsening. Serious adverse events occurred in 6.2% and 4.3% of each group, respectively, and there were no new safety signals in either group.
“The good news is that the efficacy and safety was very similar to what we saw in other clinical trials. I don’t think we really expected it to be much different, because when we think about race, it’s a social construct, not a biologic construct. What we do hope to find out is more about some of the interplay of social determinants of health, and how getting on high efficacy treatment can improve and increase productivity and outcomes in the long term,” said Dr. Williams, who is medical director of Joi Life Wellness Group, Smyrna, Georgia.
The researchers succeeded by involving patient advocates and advocacy organizations at the very earliest stages of the trial design. “We were very intentional about looking at things like social determinants of health, childcare, transportation, and things like that to ease some of the burden of participating in the trial, obviously in a legal and compliant way,” said Dr. Williams. The team also ensured complete and accurate translation of patient materials into Spanish.
The study was also a phase 4 trial, which may have simplified recruitment. “So it’s a therapy that’s already approved, which may make people feel more comfortable, but obviously the goal is for our phase 3 trials to make sure that we are recruiting represented populations. We’re taking these learnings and applying them to the broader clinical trial population so that hopefully we won’t have to come back and do phase 4 studies like this,” said Dr. Williams.
She noted that the results of more inclusive studies don’t just benefit underserved populations. “You have groups of people that are suffering and having more disability from a condition, and you need to understand why. When we broaden the population to understand those that are most vulnerable and underserved and [having the worst outcomes], it really helps us to better treat everybody. Because if we can get a hold of those factors that make us do the worst, then we can also better understand the factors that make us do the best,” said Dr. Williams.
Inclusive Recruitment in Clinical Trials
Asked for comment, Ahmed Obeidat, MD, PhD, highlighted the importance of inclusive recruitment. “The study is very important because historically and even in most recent clinical trials, these groups were markedly under-represented and most completed clinical trials derive conclusions based on the study of a nondiverse, White-non-Hispanic predominant population,” said Dr. Obeidat, who is an associate professor at the Medical College of Wisconsin, Milwaukee. He pointed to a systematic review showing that the median percentage of White participants in MS clinical trials was 93% and ranged from 86% to 98%.
“Several factors may contribute to the disparity in clinical trial participation, and solutions must be explored and developed. CHIMES is a first step in this direction where the study itself is designed to address disparity in MS clinical trial participation,” said Dr. Obeidat.
Dr. Obeidat also pointed to the need to consider other forms of diversity in clinical trials, such as older patients and those with advanced disability. “Investigators, coordinators, and other staff should all strive to be as inclusive as possible in clinical trials,” he said.
Dr. Williams has received consulting fees from Alexion, Biogen, Bristol Myers Squibb, EMD Serono, Genentech Inc., Janssen, Novartis, Sanofi, and TG Therapeutics, and serves on speakers bureaus for Biogen, Bristol Myers Squibb, EMD Serono, Janssen, Genentech, and TG Therapeutics. Dr. Ahmed Z. Obeidat has financial relationships with Alexion Pharmaceuticals, Banner Life Sciences, BD Biosciences, Biogen, Biologix Solutions, Bristol Myers Squibb, Celgene, EMD Serono, Genentech, GW Pharmaceuticals, Horizon Therapeutics, Jazz Pharmaceuticals, Novartis, Sandoz, Sanofi Genzyme, TG Therapeutics, and Viela Bio.
NASHVILLE, Tennessee — , according to the results of a 1-year analysis of the CHIMES trial. The study is the first-ever prospective study of an MS disease-modifying therapy (DMT) exclusively performed in under-represented populations, and offers lessons to researchers aiming to design more inclusive clinical trials to bolster participation by under-represented populations.
“The goal was to better understand efficacy of therapy in under-represented populations because we typically have very low numbers of these patients in our clinical trials, although there are multiple studies over the past decades suggesting that there may be poorer outcomes in Black and Hispanic individuals, particularly in the United States, and that there also may be more aggressive disease,” said Mitzi Williams, MD, who presented the study in a poster session at the annual meeting of the Consortium of Multiple Sclerosis Centers.
The team recruited 113 Black participants and 69 Hispanic participants, and, in fact, over-recruited the target number by 25%, and did so 2 months before the launch of the study in July 2020, which just happened to be in the midst of a global pandemic.
After 48 weeks of ocrelizumab treatment, 46.0% of Black participants and 58.0% of Hispanic participants achieved no evidence of disease activity in three components (NEDA-3), while 94.7% and 95.7% were free from relapses, respectively, and 94.7% and 94.2% were free from disease worsening. Serious adverse events occurred in 6.2% and 4.3% of each group, respectively, and there were no new safety signals in either group.
“The good news is that the efficacy and safety was very similar to what we saw in other clinical trials. I don’t think we really expected it to be much different, because when we think about race, it’s a social construct, not a biologic construct. What we do hope to find out is more about some of the interplay of social determinants of health, and how getting on high efficacy treatment can improve and increase productivity and outcomes in the long term,” said Dr. Williams, who is medical director of Joi Life Wellness Group, Smyrna, Georgia.
The researchers succeeded by involving patient advocates and advocacy organizations at the very earliest stages of the trial design. “We were very intentional about looking at things like social determinants of health, childcare, transportation, and things like that to ease some of the burden of participating in the trial, obviously in a legal and compliant way,” said Dr. Williams. The team also ensured complete and accurate translation of patient materials into Spanish.
The study was also a phase 4 trial, which may have simplified recruitment. “So it’s a therapy that’s already approved, which may make people feel more comfortable, but obviously the goal is for our phase 3 trials to make sure that we are recruiting represented populations. We’re taking these learnings and applying them to the broader clinical trial population so that hopefully we won’t have to come back and do phase 4 studies like this,” said Dr. Williams.
She noted that the results of more inclusive studies don’t just benefit underserved populations. “You have groups of people that are suffering and having more disability from a condition, and you need to understand why. When we broaden the population to understand those that are most vulnerable and underserved and [having the worst outcomes], it really helps us to better treat everybody. Because if we can get a hold of those factors that make us do the worst, then we can also better understand the factors that make us do the best,” said Dr. Williams.
Inclusive Recruitment in Clinical Trials
Asked for comment, Ahmed Obeidat, MD, PhD, highlighted the importance of inclusive recruitment. “The study is very important because historically and even in most recent clinical trials, these groups were markedly under-represented and most completed clinical trials derive conclusions based on the study of a nondiverse, White-non-Hispanic predominant population,” said Dr. Obeidat, who is an associate professor at the Medical College of Wisconsin, Milwaukee. He pointed to a systematic review showing that the median percentage of White participants in MS clinical trials was 93% and ranged from 86% to 98%.
“Several factors may contribute to the disparity in clinical trial participation, and solutions must be explored and developed. CHIMES is a first step in this direction where the study itself is designed to address disparity in MS clinical trial participation,” said Dr. Obeidat.
Dr. Obeidat also pointed to the need to consider other forms of diversity in clinical trials, such as older patients and those with advanced disability. “Investigators, coordinators, and other staff should all strive to be as inclusive as possible in clinical trials,” he said.
Dr. Williams has received consulting fees from Alexion, Biogen, Bristol Myers Squibb, EMD Serono, Genentech Inc., Janssen, Novartis, Sanofi, and TG Therapeutics, and serves on speakers bureaus for Biogen, Bristol Myers Squibb, EMD Serono, Janssen, Genentech, and TG Therapeutics. Dr. Ahmed Z. Obeidat has financial relationships with Alexion Pharmaceuticals, Banner Life Sciences, BD Biosciences, Biogen, Biologix Solutions, Bristol Myers Squibb, Celgene, EMD Serono, Genentech, GW Pharmaceuticals, Horizon Therapeutics, Jazz Pharmaceuticals, Novartis, Sandoz, Sanofi Genzyme, TG Therapeutics, and Viela Bio.
NASHVILLE, Tennessee — , according to the results of a 1-year analysis of the CHIMES trial. The study is the first-ever prospective study of an MS disease-modifying therapy (DMT) exclusively performed in under-represented populations, and offers lessons to researchers aiming to design more inclusive clinical trials to bolster participation by under-represented populations.
“The goal was to better understand efficacy of therapy in under-represented populations because we typically have very low numbers of these patients in our clinical trials, although there are multiple studies over the past decades suggesting that there may be poorer outcomes in Black and Hispanic individuals, particularly in the United States, and that there also may be more aggressive disease,” said Mitzi Williams, MD, who presented the study in a poster session at the annual meeting of the Consortium of Multiple Sclerosis Centers.
The team recruited 113 Black participants and 69 Hispanic participants, and, in fact, over-recruited the target number by 25%, and did so 2 months before the launch of the study in July 2020, which just happened to be in the midst of a global pandemic.
After 48 weeks of ocrelizumab treatment, 46.0% of Black participants and 58.0% of Hispanic participants achieved no evidence of disease activity in three components (NEDA-3), while 94.7% and 95.7% were free from relapses, respectively, and 94.7% and 94.2% were free from disease worsening. Serious adverse events occurred in 6.2% and 4.3% of each group, respectively, and there were no new safety signals in either group.
“The good news is that the efficacy and safety was very similar to what we saw in other clinical trials. I don’t think we really expected it to be much different, because when we think about race, it’s a social construct, not a biologic construct. What we do hope to find out is more about some of the interplay of social determinants of health, and how getting on high efficacy treatment can improve and increase productivity and outcomes in the long term,” said Dr. Williams, who is medical director of Joi Life Wellness Group, Smyrna, Georgia.
The researchers succeeded by involving patient advocates and advocacy organizations at the very earliest stages of the trial design. “We were very intentional about looking at things like social determinants of health, childcare, transportation, and things like that to ease some of the burden of participating in the trial, obviously in a legal and compliant way,” said Dr. Williams. The team also ensured complete and accurate translation of patient materials into Spanish.
The study was also a phase 4 trial, which may have simplified recruitment. “So it’s a therapy that’s already approved, which may make people feel more comfortable, but obviously the goal is for our phase 3 trials to make sure that we are recruiting represented populations. We’re taking these learnings and applying them to the broader clinical trial population so that hopefully we won’t have to come back and do phase 4 studies like this,” said Dr. Williams.
She noted that the results of more inclusive studies don’t just benefit underserved populations. “You have groups of people that are suffering and having more disability from a condition, and you need to understand why. When we broaden the population to understand those that are most vulnerable and underserved and [having the worst outcomes], it really helps us to better treat everybody. Because if we can get a hold of those factors that make us do the worst, then we can also better understand the factors that make us do the best,” said Dr. Williams.
Inclusive Recruitment in Clinical Trials
Asked for comment, Ahmed Obeidat, MD, PhD, highlighted the importance of inclusive recruitment. “The study is very important because historically and even in most recent clinical trials, these groups were markedly under-represented and most completed clinical trials derive conclusions based on the study of a nondiverse, White-non-Hispanic predominant population,” said Dr. Obeidat, who is an associate professor at the Medical College of Wisconsin, Milwaukee. He pointed to a systematic review showing that the median percentage of White participants in MS clinical trials was 93% and ranged from 86% to 98%.
“Several factors may contribute to the disparity in clinical trial participation, and solutions must be explored and developed. CHIMES is a first step in this direction where the study itself is designed to address disparity in MS clinical trial participation,” said Dr. Obeidat.
Dr. Obeidat also pointed to the need to consider other forms of diversity in clinical trials, such as older patients and those with advanced disability. “Investigators, coordinators, and other staff should all strive to be as inclusive as possible in clinical trials,” he said.
Dr. Williams has received consulting fees from Alexion, Biogen, Bristol Myers Squibb, EMD Serono, Genentech Inc., Janssen, Novartis, Sanofi, and TG Therapeutics, and serves on speakers bureaus for Biogen, Bristol Myers Squibb, EMD Serono, Janssen, Genentech, and TG Therapeutics. Dr. Ahmed Z. Obeidat has financial relationships with Alexion Pharmaceuticals, Banner Life Sciences, BD Biosciences, Biogen, Biologix Solutions, Bristol Myers Squibb, Celgene, EMD Serono, Genentech, GW Pharmaceuticals, Horizon Therapeutics, Jazz Pharmaceuticals, Novartis, Sandoz, Sanofi Genzyme, TG Therapeutics, and Viela Bio.
FROM CMSC 2024