User login
Survey: Many Mohs surgeons are struggling on the job
.
In a measurement of well-being, 40% of members of the American College of Mohs
Surgery (ACMS) who responded to the survey – and 52% of women – scored at a level considered “at-risk” for adverse outcomes, such as poor quality of life.
“I didn’t think the numbers were going to be that high,” said study author Kemi O. Awe, MD, PhD, a dermatology resident at the University of Alabama at Birmingham, especially in light of Mohs surgery’s reputation as being an especially desirable field in dermatology. She presented the findings at the annual meeting of the ACMS.
Dr. Awe, who hopes to become a Mohs surgeon herself, said in an interview that she launched the study in part to understand how colleagues are faring. “Dermatology is known as a specialty that has a good lifestyle and less stress, but the rate of burnout is actually going up.”
For the study, Dr. Awe and colleagues sent a survey to ACMS members between October and December 2020. The 91 respondents had an average age of 46, and 58% were male. Most practiced in academic facilities (56%), while the rest worked in private practice (39%) or multispecialty (4%) practices. Almost all (89%) were married or in partnerships.
The survey calculated scores on the expanded Physician Well Being Index, a validated tool for measuring physician distress. Forty percent of 68 respondents to this part of the survey got a score of 3 or higher, which the study describes as “a threshold for respondents who are ‘at-risk’ of adverse outcomes such as poor quality of life, depression, and a high level of fatigue.”
Women were more likely to be considered at risk (52%) than men (28%). “This isn’t different than what’s already out there: Female physicians are more likely to be burned out compared to men,” Dr. Awe said.
Compared with their male counterparts, female Mohs surgeons were more likely to say that time at work, malpractice concerns, insurance reimbursement, and compensation structure negatively affected their well-being (P ≤ .05).
It’s unclear whether there’s a well-being gender gap among dermatologists overall, however. Dr. Awe highlighted a 2019 survey of 108 dermatologists that found no significant difference in overall burnout between men and women – about 42% of both genders reported symptoms. But the survey did find that “dermatologists with children living at home had significantly higher levels of burnout,” with a P value of .03.
Dr. Awe said the findings offer insight into what to look out for when pursuing a career as a Mohs surgeon. “There’s potentially excess stress about being a Mohs surgeon,” she said, although the field also has a reputation as being fulfilling and rewarding.
In an interview, Stanford (Calif.) University dermatologist Zakia Rahman, MD, praised the study and said it “certainly provides a framework to address professional fulfillment amongst Mohs surgeons.”
It was especially surprising, she said, that female surgeons didn’t rate their compensation structure as positively as did their male colleagues. “It is possible that there is still a significant amount of gender-based difference in compensation between male and female Mohs surgeons. This is an area that can be further explored.”
Moving forward, she said, “our professional dermatology societies must examine the increase in burnout within our specialty. Further funding and research in this area is needed.”
For now, dermatologists can focus on strategies that can reduce burnout in the field, Sailesh Konda, MD, a Mohs surgeon at the Univeristy of Florida, Gainesville, said in an interview. Dr. Konda highlighted a report published in 2020 that, he said, "recommended focusing on incremental changes that help restore autonomy and control over work, connecting with colleagues within dermatology and the broader medical community, developing self-awareness and recognition of a perfectionist mindset, and restoring meaning and joy to patient care.”*
No funding is reported for the study. Dr. Awe, Dr. Rahman, and Dr. Konda have no relevant disclosures.
*This story was updated on June 2 for clarity.
.
In a measurement of well-being, 40% of members of the American College of Mohs
Surgery (ACMS) who responded to the survey – and 52% of women – scored at a level considered “at-risk” for adverse outcomes, such as poor quality of life.
“I didn’t think the numbers were going to be that high,” said study author Kemi O. Awe, MD, PhD, a dermatology resident at the University of Alabama at Birmingham, especially in light of Mohs surgery’s reputation as being an especially desirable field in dermatology. She presented the findings at the annual meeting of the ACMS.
Dr. Awe, who hopes to become a Mohs surgeon herself, said in an interview that she launched the study in part to understand how colleagues are faring. “Dermatology is known as a specialty that has a good lifestyle and less stress, but the rate of burnout is actually going up.”
For the study, Dr. Awe and colleagues sent a survey to ACMS members between October and December 2020. The 91 respondents had an average age of 46, and 58% were male. Most practiced in academic facilities (56%), while the rest worked in private practice (39%) or multispecialty (4%) practices. Almost all (89%) were married or in partnerships.
The survey calculated scores on the expanded Physician Well Being Index, a validated tool for measuring physician distress. Forty percent of 68 respondents to this part of the survey got a score of 3 or higher, which the study describes as “a threshold for respondents who are ‘at-risk’ of adverse outcomes such as poor quality of life, depression, and a high level of fatigue.”
Women were more likely to be considered at risk (52%) than men (28%). “This isn’t different than what’s already out there: Female physicians are more likely to be burned out compared to men,” Dr. Awe said.
Compared with their male counterparts, female Mohs surgeons were more likely to say that time at work, malpractice concerns, insurance reimbursement, and compensation structure negatively affected their well-being (P ≤ .05).
It’s unclear whether there’s a well-being gender gap among dermatologists overall, however. Dr. Awe highlighted a 2019 survey of 108 dermatologists that found no significant difference in overall burnout between men and women – about 42% of both genders reported symptoms. But the survey did find that “dermatologists with children living at home had significantly higher levels of burnout,” with a P value of .03.
Dr. Awe said the findings offer insight into what to look out for when pursuing a career as a Mohs surgeon. “There’s potentially excess stress about being a Mohs surgeon,” she said, although the field also has a reputation as being fulfilling and rewarding.
In an interview, Stanford (Calif.) University dermatologist Zakia Rahman, MD, praised the study and said it “certainly provides a framework to address professional fulfillment amongst Mohs surgeons.”
It was especially surprising, she said, that female surgeons didn’t rate their compensation structure as positively as did their male colleagues. “It is possible that there is still a significant amount of gender-based difference in compensation between male and female Mohs surgeons. This is an area that can be further explored.”
Moving forward, she said, “our professional dermatology societies must examine the increase in burnout within our specialty. Further funding and research in this area is needed.”
For now, dermatologists can focus on strategies that can reduce burnout in the field, Sailesh Konda, MD, a Mohs surgeon at the Univeristy of Florida, Gainesville, said in an interview. Dr. Konda highlighted a report published in 2020 that, he said, "recommended focusing on incremental changes that help restore autonomy and control over work, connecting with colleagues within dermatology and the broader medical community, developing self-awareness and recognition of a perfectionist mindset, and restoring meaning and joy to patient care.”*
No funding is reported for the study. Dr. Awe, Dr. Rahman, and Dr. Konda have no relevant disclosures.
*This story was updated on June 2 for clarity.
.
In a measurement of well-being, 40% of members of the American College of Mohs
Surgery (ACMS) who responded to the survey – and 52% of women – scored at a level considered “at-risk” for adverse outcomes, such as poor quality of life.
“I didn’t think the numbers were going to be that high,” said study author Kemi O. Awe, MD, PhD, a dermatology resident at the University of Alabama at Birmingham, especially in light of Mohs surgery’s reputation as being an especially desirable field in dermatology. She presented the findings at the annual meeting of the ACMS.
Dr. Awe, who hopes to become a Mohs surgeon herself, said in an interview that she launched the study in part to understand how colleagues are faring. “Dermatology is known as a specialty that has a good lifestyle and less stress, but the rate of burnout is actually going up.”
For the study, Dr. Awe and colleagues sent a survey to ACMS members between October and December 2020. The 91 respondents had an average age of 46, and 58% were male. Most practiced in academic facilities (56%), while the rest worked in private practice (39%) or multispecialty (4%) practices. Almost all (89%) were married or in partnerships.
The survey calculated scores on the expanded Physician Well Being Index, a validated tool for measuring physician distress. Forty percent of 68 respondents to this part of the survey got a score of 3 or higher, which the study describes as “a threshold for respondents who are ‘at-risk’ of adverse outcomes such as poor quality of life, depression, and a high level of fatigue.”
Women were more likely to be considered at risk (52%) than men (28%). “This isn’t different than what’s already out there: Female physicians are more likely to be burned out compared to men,” Dr. Awe said.
Compared with their male counterparts, female Mohs surgeons were more likely to say that time at work, malpractice concerns, insurance reimbursement, and compensation structure negatively affected their well-being (P ≤ .05).
It’s unclear whether there’s a well-being gender gap among dermatologists overall, however. Dr. Awe highlighted a 2019 survey of 108 dermatologists that found no significant difference in overall burnout between men and women – about 42% of both genders reported symptoms. But the survey did find that “dermatologists with children living at home had significantly higher levels of burnout,” with a P value of .03.
Dr. Awe said the findings offer insight into what to look out for when pursuing a career as a Mohs surgeon. “There’s potentially excess stress about being a Mohs surgeon,” she said, although the field also has a reputation as being fulfilling and rewarding.
In an interview, Stanford (Calif.) University dermatologist Zakia Rahman, MD, praised the study and said it “certainly provides a framework to address professional fulfillment amongst Mohs surgeons.”
It was especially surprising, she said, that female surgeons didn’t rate their compensation structure as positively as did their male colleagues. “It is possible that there is still a significant amount of gender-based difference in compensation between male and female Mohs surgeons. This is an area that can be further explored.”
Moving forward, she said, “our professional dermatology societies must examine the increase in burnout within our specialty. Further funding and research in this area is needed.”
For now, dermatologists can focus on strategies that can reduce burnout in the field, Sailesh Konda, MD, a Mohs surgeon at the Univeristy of Florida, Gainesville, said in an interview. Dr. Konda highlighted a report published in 2020 that, he said, "recommended focusing on incremental changes that help restore autonomy and control over work, connecting with colleagues within dermatology and the broader medical community, developing self-awareness and recognition of a perfectionist mindset, and restoring meaning and joy to patient care.”*
No funding is reported for the study. Dr. Awe, Dr. Rahman, and Dr. Konda have no relevant disclosures.
*This story was updated on June 2 for clarity.
FROM THE ACMS ANNUAL MEETING
Making sense of LAMA discharges
Converge 2021 session
LAMA’s DRAMA: Left AMA – Documentation and Rules of AMA
Presenter
Venkatrao Medarametla, MD, SFHM
Session summary
Most hospitalists equate LAMA (left against medical advice) patients with noncompliance and stop at that. During the recent SHM Converge conference session on LAMA, Dr. Venkatrao Medarametla, medical director for hospital medicine at Baystate Medical Center, Springfield, Mass., delved into the etiology and pathophysiology of LAMA discharges.
According to Dr. Medarametla, LAMA accounts for 1.4% of all discharges amounting to more than 500,000 discharges per year nationwide. LAMA discharges are at high risk for readmissions (20%-40% higher), have longer length of stay on readmission, higher morbidity and mortality (10% higher), and result in higher costs of care (56% higher).
The reasons for LAMA discharges could be broadly divided into patient and provider factors. Patient factors include refusal to wait for administrative delays, extenuating domestic and social concerns, conflicts with care providers, disagreement with providers’ judgment of health status, mistrust of the health system, substance dependence with inadequate treatment for withdrawal, patient’s perception of respect, stereotyping or stigma, and even ambiance and diet at the hospital.
Provider factors include conflict with the patient, concerns of legal and ethical responsibilities, formally distancing from nonstandard plan, and deflecting blame for worse outcomes.
Faced with a LAMA discharge, the important role of a hospitalist is to assess capacity. Help may be sought from other specialists such as psychiatrists and geriatricians. Some of the best practices also include a clear discussion of risks of outpatient treatment, exploration of safe alternative care plans, patient-centered care, shared decision-making (e.g., needle exchange), and harm reduction.
Dr. Medarametla advised hospitalists not to rely on the AMA forms the patients are asked to sign for liability protection. The forms may not stand up to legal scrutiny. Excellent documentation regarding the details of discussions with the patient, and determination of capacity encompassing the patients’ understanding, reasoning, and insight should be made. Hospitalists can also assess the barriers and mitigate them. Appropriate outpatient and alternative treatment plans should be explored. Postdischarge care and follow ups also should be facilitated.
According to Dr. Medarametla, another myth about AMA discharge is that insurance will not pay for it. About 57% of a survey sample of attendings and residents believed the same, and 66% heard other providers telling patients that insurance would not cover the AMA discharges. In a multicentric study of 526 patients, payment was refused only in 4.1% of AMA cases, mostly for administrative reasons.
Another prevalent myth is that patients who leave AMA will lose their right to follow up. Prescriptions also could be given to LAMA patients provided hospitalists adhere to detailed and relevant documentation. Overall, the session was very interesting and informative.
Key takeaways
- There are patient and provider factors leading to LAMA.
- Patients signing an AMA form does not provide legal protection for providers, but a stream-lined discharge process and a detailed documentation are likely to.
- There is no evidence that insurance companies will not pay for LAMA discharges.
- LAMA patients could be given prescriptions and follow up as long as they are well documented.
References
Schaefer G et al. Financial responsibility of hospitalized patients who left against medical advice: Medical urban legend? J Gen Intern Med. 2012 Jul;27(7):825-30. doi: 10.1007/s11606-012-1984-x.
Wigder H et al. Insurance companies refusing payment for patients who leave the emergency department against medical advice is a myth. Ann Emerg Med. 2010 Apr;55(4):393. doi: 10.1016/j.annemergmed.2009.11.024.
Dr. Kumar is a hospitalist in Port Huron, Mich. He is a member of the editorial advisory board for the Hospitalist.
Converge 2021 session
LAMA’s DRAMA: Left AMA – Documentation and Rules of AMA
Presenter
Venkatrao Medarametla, MD, SFHM
Session summary
Most hospitalists equate LAMA (left against medical advice) patients with noncompliance and stop at that. During the recent SHM Converge conference session on LAMA, Dr. Venkatrao Medarametla, medical director for hospital medicine at Baystate Medical Center, Springfield, Mass., delved into the etiology and pathophysiology of LAMA discharges.
According to Dr. Medarametla, LAMA accounts for 1.4% of all discharges amounting to more than 500,000 discharges per year nationwide. LAMA discharges are at high risk for readmissions (20%-40% higher), have longer length of stay on readmission, higher morbidity and mortality (10% higher), and result in higher costs of care (56% higher).
The reasons for LAMA discharges could be broadly divided into patient and provider factors. Patient factors include refusal to wait for administrative delays, extenuating domestic and social concerns, conflicts with care providers, disagreement with providers’ judgment of health status, mistrust of the health system, substance dependence with inadequate treatment for withdrawal, patient’s perception of respect, stereotyping or stigma, and even ambiance and diet at the hospital.
Provider factors include conflict with the patient, concerns of legal and ethical responsibilities, formally distancing from nonstandard plan, and deflecting blame for worse outcomes.
Faced with a LAMA discharge, the important role of a hospitalist is to assess capacity. Help may be sought from other specialists such as psychiatrists and geriatricians. Some of the best practices also include a clear discussion of risks of outpatient treatment, exploration of safe alternative care plans, patient-centered care, shared decision-making (e.g., needle exchange), and harm reduction.
Dr. Medarametla advised hospitalists not to rely on the AMA forms the patients are asked to sign for liability protection. The forms may not stand up to legal scrutiny. Excellent documentation regarding the details of discussions with the patient, and determination of capacity encompassing the patients’ understanding, reasoning, and insight should be made. Hospitalists can also assess the barriers and mitigate them. Appropriate outpatient and alternative treatment plans should be explored. Postdischarge care and follow ups also should be facilitated.
According to Dr. Medarametla, another myth about AMA discharge is that insurance will not pay for it. About 57% of a survey sample of attendings and residents believed the same, and 66% heard other providers telling patients that insurance would not cover the AMA discharges. In a multicentric study of 526 patients, payment was refused only in 4.1% of AMA cases, mostly for administrative reasons.
Another prevalent myth is that patients who leave AMA will lose their right to follow up. Prescriptions also could be given to LAMA patients provided hospitalists adhere to detailed and relevant documentation. Overall, the session was very interesting and informative.
Key takeaways
- There are patient and provider factors leading to LAMA.
- Patients signing an AMA form does not provide legal protection for providers, but a stream-lined discharge process and a detailed documentation are likely to.
- There is no evidence that insurance companies will not pay for LAMA discharges.
- LAMA patients could be given prescriptions and follow up as long as they are well documented.
References
Schaefer G et al. Financial responsibility of hospitalized patients who left against medical advice: Medical urban legend? J Gen Intern Med. 2012 Jul;27(7):825-30. doi: 10.1007/s11606-012-1984-x.
Wigder H et al. Insurance companies refusing payment for patients who leave the emergency department against medical advice is a myth. Ann Emerg Med. 2010 Apr;55(4):393. doi: 10.1016/j.annemergmed.2009.11.024.
Dr. Kumar is a hospitalist in Port Huron, Mich. He is a member of the editorial advisory board for the Hospitalist.
Converge 2021 session
LAMA’s DRAMA: Left AMA – Documentation and Rules of AMA
Presenter
Venkatrao Medarametla, MD, SFHM
Session summary
Most hospitalists equate LAMA (left against medical advice) patients with noncompliance and stop at that. During the recent SHM Converge conference session on LAMA, Dr. Venkatrao Medarametla, medical director for hospital medicine at Baystate Medical Center, Springfield, Mass., delved into the etiology and pathophysiology of LAMA discharges.
According to Dr. Medarametla, LAMA accounts for 1.4% of all discharges amounting to more than 500,000 discharges per year nationwide. LAMA discharges are at high risk for readmissions (20%-40% higher), have longer length of stay on readmission, higher morbidity and mortality (10% higher), and result in higher costs of care (56% higher).
The reasons for LAMA discharges could be broadly divided into patient and provider factors. Patient factors include refusal to wait for administrative delays, extenuating domestic and social concerns, conflicts with care providers, disagreement with providers’ judgment of health status, mistrust of the health system, substance dependence with inadequate treatment for withdrawal, patient’s perception of respect, stereotyping or stigma, and even ambiance and diet at the hospital.
Provider factors include conflict with the patient, concerns of legal and ethical responsibilities, formally distancing from nonstandard plan, and deflecting blame for worse outcomes.
Faced with a LAMA discharge, the important role of a hospitalist is to assess capacity. Help may be sought from other specialists such as psychiatrists and geriatricians. Some of the best practices also include a clear discussion of risks of outpatient treatment, exploration of safe alternative care plans, patient-centered care, shared decision-making (e.g., needle exchange), and harm reduction.
Dr. Medarametla advised hospitalists not to rely on the AMA forms the patients are asked to sign for liability protection. The forms may not stand up to legal scrutiny. Excellent documentation regarding the details of discussions with the patient, and determination of capacity encompassing the patients’ understanding, reasoning, and insight should be made. Hospitalists can also assess the barriers and mitigate them. Appropriate outpatient and alternative treatment plans should be explored. Postdischarge care and follow ups also should be facilitated.
According to Dr. Medarametla, another myth about AMA discharge is that insurance will not pay for it. About 57% of a survey sample of attendings and residents believed the same, and 66% heard other providers telling patients that insurance would not cover the AMA discharges. In a multicentric study of 526 patients, payment was refused only in 4.1% of AMA cases, mostly for administrative reasons.
Another prevalent myth is that patients who leave AMA will lose their right to follow up. Prescriptions also could be given to LAMA patients provided hospitalists adhere to detailed and relevant documentation. Overall, the session was very interesting and informative.
Key takeaways
- There are patient and provider factors leading to LAMA.
- Patients signing an AMA form does not provide legal protection for providers, but a stream-lined discharge process and a detailed documentation are likely to.
- There is no evidence that insurance companies will not pay for LAMA discharges.
- LAMA patients could be given prescriptions and follow up as long as they are well documented.
References
Schaefer G et al. Financial responsibility of hospitalized patients who left against medical advice: Medical urban legend? J Gen Intern Med. 2012 Jul;27(7):825-30. doi: 10.1007/s11606-012-1984-x.
Wigder H et al. Insurance companies refusing payment for patients who leave the emergency department against medical advice is a myth. Ann Emerg Med. 2010 Apr;55(4):393. doi: 10.1016/j.annemergmed.2009.11.024.
Dr. Kumar is a hospitalist in Port Huron, Mich. He is a member of the editorial advisory board for the Hospitalist.
FROM SHM CONVERGE 2021
Sustained long-term benefit of gene therapy for SMA
, new long-term follow-up data show. At a median of 5.2 years since receiving the approved therapeutic dose, onasemnogene abeparvovec provided “sustained, durable efficacy, with all patients alive and without the need for permanent ventilation,” reported Jerry Mendell, MD, with the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus, Ohio, and colleagues.
The study was published online May 17 in JAMA Neurology.
Single infusion
SMA is a rare genetic disease that can lead to paralysis, breathing difficulty, and death. The disorder is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which encodes the SMN protein critical for maintenance and function of motor neurons.
In 2019, Zolgensma was approved in the United States for children with SMA and younger than 2 years of age.
Zolgensma is an adeno-associated virus vector-based gene therapy that addresses the genetic root cause of SMA by replacing the defective or missing SMN1 gene to halt disease progression. A single, one-time intravenous infusion results in expression of the SMN protein motor neurons, which improves chances of survival, as well as muscle movement and function.
In the phase 1 START study, 15 infants with SMA type 1 were treated with either a low or therapeutic dose of Zolgensma at Nationwide Children’s Hospital between 2014 and 2017.
The START long-term follow-up study (START LTFU) is an ongoing, observational study assessing safety and durability of response over 15 years in 13 of the infants; three infants received the low dose and 10 received the approved high dose.
Prior to baseline, four patients (40%) in the therapeutic dose cohort required noninvasive ventilatory support, and six (60%) did not require regular ventilatory support, which did not change in long-term follow-up.
All 10 patients who received the therapeutic dose remained alive and without the need for permanent ventilation up to 6.2 years after dosing, Dr. Mendell and colleagues report.
These patients also maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen (Spinraza, Biogen).
Serious adverse events occurred in eight patients (62%), none of which resulted in study discontinuation or death. The most common serious adverse events were related to the underlying SMA disease process and included acute respiratory failure (31%), pneumonia (31%), dehydration (23%), respiratory distress (15%), and bronchiolitis (15%).
Importantly, the investigators noted, no new safety signals or “adverse events of special interest” emerged during follow-up, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
The investigators acknowledged that this follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen in several patients. “However, given that the two patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec,” Dr. Mendell and colleagues said.
The study was supported by Novartis Gene Therapies. Dr. Mendell and several co-investigators have disclosed financial relationships with the company.
A version of this article first appeared on Medscape.com.
, new long-term follow-up data show. At a median of 5.2 years since receiving the approved therapeutic dose, onasemnogene abeparvovec provided “sustained, durable efficacy, with all patients alive and without the need for permanent ventilation,” reported Jerry Mendell, MD, with the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus, Ohio, and colleagues.
The study was published online May 17 in JAMA Neurology.
Single infusion
SMA is a rare genetic disease that can lead to paralysis, breathing difficulty, and death. The disorder is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which encodes the SMN protein critical for maintenance and function of motor neurons.
In 2019, Zolgensma was approved in the United States for children with SMA and younger than 2 years of age.
Zolgensma is an adeno-associated virus vector-based gene therapy that addresses the genetic root cause of SMA by replacing the defective or missing SMN1 gene to halt disease progression. A single, one-time intravenous infusion results in expression of the SMN protein motor neurons, which improves chances of survival, as well as muscle movement and function.
In the phase 1 START study, 15 infants with SMA type 1 were treated with either a low or therapeutic dose of Zolgensma at Nationwide Children’s Hospital between 2014 and 2017.
The START long-term follow-up study (START LTFU) is an ongoing, observational study assessing safety and durability of response over 15 years in 13 of the infants; three infants received the low dose and 10 received the approved high dose.
Prior to baseline, four patients (40%) in the therapeutic dose cohort required noninvasive ventilatory support, and six (60%) did not require regular ventilatory support, which did not change in long-term follow-up.
All 10 patients who received the therapeutic dose remained alive and without the need for permanent ventilation up to 6.2 years after dosing, Dr. Mendell and colleagues report.
These patients also maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen (Spinraza, Biogen).
Serious adverse events occurred in eight patients (62%), none of which resulted in study discontinuation or death. The most common serious adverse events were related to the underlying SMA disease process and included acute respiratory failure (31%), pneumonia (31%), dehydration (23%), respiratory distress (15%), and bronchiolitis (15%).
Importantly, the investigators noted, no new safety signals or “adverse events of special interest” emerged during follow-up, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
The investigators acknowledged that this follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen in several patients. “However, given that the two patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec,” Dr. Mendell and colleagues said.
The study was supported by Novartis Gene Therapies. Dr. Mendell and several co-investigators have disclosed financial relationships with the company.
A version of this article first appeared on Medscape.com.
, new long-term follow-up data show. At a median of 5.2 years since receiving the approved therapeutic dose, onasemnogene abeparvovec provided “sustained, durable efficacy, with all patients alive and without the need for permanent ventilation,” reported Jerry Mendell, MD, with the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus, Ohio, and colleagues.
The study was published online May 17 in JAMA Neurology.
Single infusion
SMA is a rare genetic disease that can lead to paralysis, breathing difficulty, and death. The disorder is caused by a mutation in the survival motor neuron 1 (SMN1) gene, which encodes the SMN protein critical for maintenance and function of motor neurons.
In 2019, Zolgensma was approved in the United States for children with SMA and younger than 2 years of age.
Zolgensma is an adeno-associated virus vector-based gene therapy that addresses the genetic root cause of SMA by replacing the defective or missing SMN1 gene to halt disease progression. A single, one-time intravenous infusion results in expression of the SMN protein motor neurons, which improves chances of survival, as well as muscle movement and function.
In the phase 1 START study, 15 infants with SMA type 1 were treated with either a low or therapeutic dose of Zolgensma at Nationwide Children’s Hospital between 2014 and 2017.
The START long-term follow-up study (START LTFU) is an ongoing, observational study assessing safety and durability of response over 15 years in 13 of the infants; three infants received the low dose and 10 received the approved high dose.
Prior to baseline, four patients (40%) in the therapeutic dose cohort required noninvasive ventilatory support, and six (60%) did not require regular ventilatory support, which did not change in long-term follow-up.
All 10 patients who received the therapeutic dose remained alive and without the need for permanent ventilation up to 6.2 years after dosing, Dr. Mendell and colleagues report.
These patients also maintained previously acquired motor milestones. Two patients attained the new milestone of “standing with assistance” without the use of nusinersen (Spinraza, Biogen).
Serious adverse events occurred in eight patients (62%), none of which resulted in study discontinuation or death. The most common serious adverse events were related to the underlying SMA disease process and included acute respiratory failure (31%), pneumonia (31%), dehydration (23%), respiratory distress (15%), and bronchiolitis (15%).
Importantly, the investigators noted, no new safety signals or “adverse events of special interest” emerged during follow-up, including liver function enzyme elevations, new incidences of malignancy or hematologic disorders, and new incidences or exacerbations of existing neurologic or autoimmune disorders.
The investigators acknowledged that this follow-up study is limited by the small sample size of the patient population and confounded by treatment with nusinersen in several patients. “However, given that the two patients who acquired the new motor milestone of standing with assistance did not receive nusinersen at any time, this benefit can be attributed solely to onasemnogene abeparvovec,” Dr. Mendell and colleagues said.
The study was supported by Novartis Gene Therapies. Dr. Mendell and several co-investigators have disclosed financial relationships with the company.
A version of this article first appeared on Medscape.com.
No-cancel culture: How telehealth is making it easier to keep that therapy session
When the COVID-19 pandemic forced behavioral health providers to stop seeing patients in person and instead hold therapy sessions remotely, the switch produced an unintended, positive consequence: Fewer patients skipped appointments.
That had long been a problem in mental health care. Some outpatient programs previously had no-show rates as high as 60%, according to several studies.
Only 9% of psychiatrists reported that all patients kept their appointments before the pandemic, according to an American Psychiatric Association report. Once providers switched to telepsychiatry, that number increased to 32%.
Not only that, but providers and patients say teletherapy has largely been an effective lifeline for people struggling with anxiety, depression, and other psychological issues during an extraordinarily difficult time, even though it created a new set of challenges.
Many providers say they plan to continue offering teletherapy after the pandemic. Some states are making permanent the temporary pandemic rules that allow providers to be reimbursed at the same rates as for in-person visits, which is welcome news to practitioners who take patients’ insurance.
“We are in a mental health crisis right now, so more people are struggling and may be more open to accessing services,” said psychologist Allison Dempsey, PhD, associate professor at University of Colorado at Denver, Aurora. “It’s much easier to connect from your living room.”
The problem for patients who didn’t show up was often as simple as a canceled ride, said Jody Long, a clinical social worker who studied the 60% rate of no-shows or late cancellations at the University of Tennessee Health Science Center psychiatric clinic in Memphis.
But sometimes it was the health problem itself. Mr. Long remembers seeing a first-time patient drive around the parking lot and then exit. The patient later called and told Mr. Long, “I just could not get out of the car; please forgive me and reschedule me.”
Mr. Long, now an assistant professor at Jacksonville (Ala.) State University, said that incident changed his perspective. “I realized when you’re having panic attacks or anxiety attacks or suffering from major depressive disorder, it’s hard,” he said. “It’s like you have built up these walls for protection and then all of a sudden you’re having to let these walls down.”
Absences strain providers whose bosses set billing and productivity expectations and those in private practice who lose billable hours, said Dr. Dempsey, who directs a program to provide mental health care for families of babies with serious medical complications. Psychotherapists often overbooked patients with the expectation that some would not show up.
Now Dr. Dempsey and colleagues no longer need to overbook. When patients don’t show up, staffers can sometimes contact a patient right away and hold the session. Other times, they can reschedule them for later that day or a different day.
And telepsychiatry performs as well as, if not better than, face-to-face delivery of mental health services, according to a World Journal of Psychiatry review of 452 studies.
Virtual visits can also save patients money, because they might not need to travel, take time off work, or pay for child care, said Jay Shore, MD, MPH, chairperson of the American Psychiatric Association’s telepsychiatry committee and a psychiatrist at the University of Colorado.
Dr. Shore started examining the potential of video conferencing to reach rural patients in the late ’90s and concluded that patients and providers can virtually build rapport, which he said is fundamental for effective therapy and medicine management.
But before the pandemic, almost 64% of psychiatrists had never used telehealth, according to the psychiatric association. Amid widespread skepticism, providers then had to do “10 years of implementations in 10 days,” said Dr. Shore, who has consulted with Dr. Dempsey and other providers.
Dr. Dempsey and colleagues faced a steep learning curve. She said she recently held a video therapy session with a mother who “seemed very out of it” before disappearing from the screen while her baby was crying.
She wondered if the patient’s exit was related to the stress of new motherhood or “something more concerning,” like addiction. She thinks she might have better understood the woman’s condition had they been in the same room. The patient called Dr. Dempsey’s team that night and told them she had relapsed into drug use and been taken to the emergency room. The mental health providers directed her to a treatment program, Dr. Dempsey said.
“We spent a lot of time reviewing what happened with that case and thinking about what we need to do differently,” Dr. Dempsey said.
Providers now routinely ask for the name of someone to call if they lose a connection and can no longer reach the patient.
In another session, Dr. Dempsey noticed that a patient seemed guarded and saw her partner hovering in the background. She said she worried about the possibility of domestic violence or “some other form of controlling behavior.”
In such cases, Dr. Dempsey called after the appointments or sent the patients secure messages to their online health portal. She asked if they felt safe and suggested they talk in person.
Such inability to maintain privacy remains a concern.
In a Walmart parking lot recently, psychologist Kristy Keefe, PsyD, of Western Illinois University, Macomb, heard a patient talking with her therapist from her car. Dr. Keefe said she wondered if the patient “had no other safe place to go to.”
To avoid that scenario, Dr. Keefe does 30-minute consultations with patients before their first telehealth appointment. She asks if they have space to talk where no one can overhear them and makes sure they have sufficient internet access and know how to use video conferencing.
To ensure that she, too, was prepared, Dr. Keefe upgraded her WiFi router, purchased two white-noise machines to drown out her conversations, and placed a stop sign on her door during appointments so her 5-year-old son knew she was seeing patients.
Dr. Keefe concluded that audio alone sometimes works better than video, which often lags. Over the phone, she and her psychology students “got really sensitive to tone fluctuations” in a patient’s voice and were better able to “pick up the emotion” than with video conferencing.
With those telehealth visits, her 20% no-show rate evaporated.
Kate Barnes, a 29-year-old middle school teacher in Fayetteville, Ark., who struggles with anxiety and depression, also has found visits easier by phone than by Zoom, because she doesn’t feel like a spotlight is on her.
“I can focus more on what I want to say,” she said.
In one of Dr. Keefe’s video sessions, though, a patient reached out, touched the camera and started to cry as she said how appreciative she was that someone was there, Dr. Keefe recalled.
“I am so very thankful that they had something in this terrible time of loss and trauma and isolation,” said Dr. Keefe.
Demand for mental health services will likely continue even after the lifting of all COVID restrictions. according to data from the U.S. Census Bureau and the National Health Interview Survey.
“That is not going to go away with snapping our fingers,” Dr. Dempsey said.
After the pandemic, Dr. Shore said, providers should review data from the past year and determine when virtual care or in-person care is more effective. He also said the health care industry needs to work to bridge the digital divide that exists because of lack of access to devices and broadband internet.
Even though Ms. Barnes said she did not see teletherapy as less effective than in-person therapy, she would like to return to seeing her therapist in person.
“When you are in person with someone, you can pick up on their body language better,” she said. “It’s a lot harder over a video call to do that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When the COVID-19 pandemic forced behavioral health providers to stop seeing patients in person and instead hold therapy sessions remotely, the switch produced an unintended, positive consequence: Fewer patients skipped appointments.
That had long been a problem in mental health care. Some outpatient programs previously had no-show rates as high as 60%, according to several studies.
Only 9% of psychiatrists reported that all patients kept their appointments before the pandemic, according to an American Psychiatric Association report. Once providers switched to telepsychiatry, that number increased to 32%.
Not only that, but providers and patients say teletherapy has largely been an effective lifeline for people struggling with anxiety, depression, and other psychological issues during an extraordinarily difficult time, even though it created a new set of challenges.
Many providers say they plan to continue offering teletherapy after the pandemic. Some states are making permanent the temporary pandemic rules that allow providers to be reimbursed at the same rates as for in-person visits, which is welcome news to practitioners who take patients’ insurance.
“We are in a mental health crisis right now, so more people are struggling and may be more open to accessing services,” said psychologist Allison Dempsey, PhD, associate professor at University of Colorado at Denver, Aurora. “It’s much easier to connect from your living room.”
The problem for patients who didn’t show up was often as simple as a canceled ride, said Jody Long, a clinical social worker who studied the 60% rate of no-shows or late cancellations at the University of Tennessee Health Science Center psychiatric clinic in Memphis.
But sometimes it was the health problem itself. Mr. Long remembers seeing a first-time patient drive around the parking lot and then exit. The patient later called and told Mr. Long, “I just could not get out of the car; please forgive me and reschedule me.”
Mr. Long, now an assistant professor at Jacksonville (Ala.) State University, said that incident changed his perspective. “I realized when you’re having panic attacks or anxiety attacks or suffering from major depressive disorder, it’s hard,” he said. “It’s like you have built up these walls for protection and then all of a sudden you’re having to let these walls down.”
Absences strain providers whose bosses set billing and productivity expectations and those in private practice who lose billable hours, said Dr. Dempsey, who directs a program to provide mental health care for families of babies with serious medical complications. Psychotherapists often overbooked patients with the expectation that some would not show up.
Now Dr. Dempsey and colleagues no longer need to overbook. When patients don’t show up, staffers can sometimes contact a patient right away and hold the session. Other times, they can reschedule them for later that day or a different day.
And telepsychiatry performs as well as, if not better than, face-to-face delivery of mental health services, according to a World Journal of Psychiatry review of 452 studies.
Virtual visits can also save patients money, because they might not need to travel, take time off work, or pay for child care, said Jay Shore, MD, MPH, chairperson of the American Psychiatric Association’s telepsychiatry committee and a psychiatrist at the University of Colorado.
Dr. Shore started examining the potential of video conferencing to reach rural patients in the late ’90s and concluded that patients and providers can virtually build rapport, which he said is fundamental for effective therapy and medicine management.
But before the pandemic, almost 64% of psychiatrists had never used telehealth, according to the psychiatric association. Amid widespread skepticism, providers then had to do “10 years of implementations in 10 days,” said Dr. Shore, who has consulted with Dr. Dempsey and other providers.
Dr. Dempsey and colleagues faced a steep learning curve. She said she recently held a video therapy session with a mother who “seemed very out of it” before disappearing from the screen while her baby was crying.
She wondered if the patient’s exit was related to the stress of new motherhood or “something more concerning,” like addiction. She thinks she might have better understood the woman’s condition had they been in the same room. The patient called Dr. Dempsey’s team that night and told them she had relapsed into drug use and been taken to the emergency room. The mental health providers directed her to a treatment program, Dr. Dempsey said.
“We spent a lot of time reviewing what happened with that case and thinking about what we need to do differently,” Dr. Dempsey said.
Providers now routinely ask for the name of someone to call if they lose a connection and can no longer reach the patient.
In another session, Dr. Dempsey noticed that a patient seemed guarded and saw her partner hovering in the background. She said she worried about the possibility of domestic violence or “some other form of controlling behavior.”
In such cases, Dr. Dempsey called after the appointments or sent the patients secure messages to their online health portal. She asked if they felt safe and suggested they talk in person.
Such inability to maintain privacy remains a concern.
In a Walmart parking lot recently, psychologist Kristy Keefe, PsyD, of Western Illinois University, Macomb, heard a patient talking with her therapist from her car. Dr. Keefe said she wondered if the patient “had no other safe place to go to.”
To avoid that scenario, Dr. Keefe does 30-minute consultations with patients before their first telehealth appointment. She asks if they have space to talk where no one can overhear them and makes sure they have sufficient internet access and know how to use video conferencing.
To ensure that she, too, was prepared, Dr. Keefe upgraded her WiFi router, purchased two white-noise machines to drown out her conversations, and placed a stop sign on her door during appointments so her 5-year-old son knew she was seeing patients.
Dr. Keefe concluded that audio alone sometimes works better than video, which often lags. Over the phone, she and her psychology students “got really sensitive to tone fluctuations” in a patient’s voice and were better able to “pick up the emotion” than with video conferencing.
With those telehealth visits, her 20% no-show rate evaporated.
Kate Barnes, a 29-year-old middle school teacher in Fayetteville, Ark., who struggles with anxiety and depression, also has found visits easier by phone than by Zoom, because she doesn’t feel like a spotlight is on her.
“I can focus more on what I want to say,” she said.
In one of Dr. Keefe’s video sessions, though, a patient reached out, touched the camera and started to cry as she said how appreciative she was that someone was there, Dr. Keefe recalled.
“I am so very thankful that they had something in this terrible time of loss and trauma and isolation,” said Dr. Keefe.
Demand for mental health services will likely continue even after the lifting of all COVID restrictions. according to data from the U.S. Census Bureau and the National Health Interview Survey.
“That is not going to go away with snapping our fingers,” Dr. Dempsey said.
After the pandemic, Dr. Shore said, providers should review data from the past year and determine when virtual care or in-person care is more effective. He also said the health care industry needs to work to bridge the digital divide that exists because of lack of access to devices and broadband internet.
Even though Ms. Barnes said she did not see teletherapy as less effective than in-person therapy, she would like to return to seeing her therapist in person.
“When you are in person with someone, you can pick up on their body language better,” she said. “It’s a lot harder over a video call to do that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When the COVID-19 pandemic forced behavioral health providers to stop seeing patients in person and instead hold therapy sessions remotely, the switch produced an unintended, positive consequence: Fewer patients skipped appointments.
That had long been a problem in mental health care. Some outpatient programs previously had no-show rates as high as 60%, according to several studies.
Only 9% of psychiatrists reported that all patients kept their appointments before the pandemic, according to an American Psychiatric Association report. Once providers switched to telepsychiatry, that number increased to 32%.
Not only that, but providers and patients say teletherapy has largely been an effective lifeline for people struggling with anxiety, depression, and other psychological issues during an extraordinarily difficult time, even though it created a new set of challenges.
Many providers say they plan to continue offering teletherapy after the pandemic. Some states are making permanent the temporary pandemic rules that allow providers to be reimbursed at the same rates as for in-person visits, which is welcome news to practitioners who take patients’ insurance.
“We are in a mental health crisis right now, so more people are struggling and may be more open to accessing services,” said psychologist Allison Dempsey, PhD, associate professor at University of Colorado at Denver, Aurora. “It’s much easier to connect from your living room.”
The problem for patients who didn’t show up was often as simple as a canceled ride, said Jody Long, a clinical social worker who studied the 60% rate of no-shows or late cancellations at the University of Tennessee Health Science Center psychiatric clinic in Memphis.
But sometimes it was the health problem itself. Mr. Long remembers seeing a first-time patient drive around the parking lot and then exit. The patient later called and told Mr. Long, “I just could not get out of the car; please forgive me and reschedule me.”
Mr. Long, now an assistant professor at Jacksonville (Ala.) State University, said that incident changed his perspective. “I realized when you’re having panic attacks or anxiety attacks or suffering from major depressive disorder, it’s hard,” he said. “It’s like you have built up these walls for protection and then all of a sudden you’re having to let these walls down.”
Absences strain providers whose bosses set billing and productivity expectations and those in private practice who lose billable hours, said Dr. Dempsey, who directs a program to provide mental health care for families of babies with serious medical complications. Psychotherapists often overbooked patients with the expectation that some would not show up.
Now Dr. Dempsey and colleagues no longer need to overbook. When patients don’t show up, staffers can sometimes contact a patient right away and hold the session. Other times, they can reschedule them for later that day or a different day.
And telepsychiatry performs as well as, if not better than, face-to-face delivery of mental health services, according to a World Journal of Psychiatry review of 452 studies.
Virtual visits can also save patients money, because they might not need to travel, take time off work, or pay for child care, said Jay Shore, MD, MPH, chairperson of the American Psychiatric Association’s telepsychiatry committee and a psychiatrist at the University of Colorado.
Dr. Shore started examining the potential of video conferencing to reach rural patients in the late ’90s and concluded that patients and providers can virtually build rapport, which he said is fundamental for effective therapy and medicine management.
But before the pandemic, almost 64% of psychiatrists had never used telehealth, according to the psychiatric association. Amid widespread skepticism, providers then had to do “10 years of implementations in 10 days,” said Dr. Shore, who has consulted with Dr. Dempsey and other providers.
Dr. Dempsey and colleagues faced a steep learning curve. She said she recently held a video therapy session with a mother who “seemed very out of it” before disappearing from the screen while her baby was crying.
She wondered if the patient’s exit was related to the stress of new motherhood or “something more concerning,” like addiction. She thinks she might have better understood the woman’s condition had they been in the same room. The patient called Dr. Dempsey’s team that night and told them she had relapsed into drug use and been taken to the emergency room. The mental health providers directed her to a treatment program, Dr. Dempsey said.
“We spent a lot of time reviewing what happened with that case and thinking about what we need to do differently,” Dr. Dempsey said.
Providers now routinely ask for the name of someone to call if they lose a connection and can no longer reach the patient.
In another session, Dr. Dempsey noticed that a patient seemed guarded and saw her partner hovering in the background. She said she worried about the possibility of domestic violence or “some other form of controlling behavior.”
In such cases, Dr. Dempsey called after the appointments or sent the patients secure messages to their online health portal. She asked if they felt safe and suggested they talk in person.
Such inability to maintain privacy remains a concern.
In a Walmart parking lot recently, psychologist Kristy Keefe, PsyD, of Western Illinois University, Macomb, heard a patient talking with her therapist from her car. Dr. Keefe said she wondered if the patient “had no other safe place to go to.”
To avoid that scenario, Dr. Keefe does 30-minute consultations with patients before their first telehealth appointment. She asks if they have space to talk where no one can overhear them and makes sure they have sufficient internet access and know how to use video conferencing.
To ensure that she, too, was prepared, Dr. Keefe upgraded her WiFi router, purchased two white-noise machines to drown out her conversations, and placed a stop sign on her door during appointments so her 5-year-old son knew she was seeing patients.
Dr. Keefe concluded that audio alone sometimes works better than video, which often lags. Over the phone, she and her psychology students “got really sensitive to tone fluctuations” in a patient’s voice and were better able to “pick up the emotion” than with video conferencing.
With those telehealth visits, her 20% no-show rate evaporated.
Kate Barnes, a 29-year-old middle school teacher in Fayetteville, Ark., who struggles with anxiety and depression, also has found visits easier by phone than by Zoom, because she doesn’t feel like a spotlight is on her.
“I can focus more on what I want to say,” she said.
In one of Dr. Keefe’s video sessions, though, a patient reached out, touched the camera and started to cry as she said how appreciative she was that someone was there, Dr. Keefe recalled.
“I am so very thankful that they had something in this terrible time of loss and trauma and isolation,” said Dr. Keefe.
Demand for mental health services will likely continue even after the lifting of all COVID restrictions. according to data from the U.S. Census Bureau and the National Health Interview Survey.
“That is not going to go away with snapping our fingers,” Dr. Dempsey said.
After the pandemic, Dr. Shore said, providers should review data from the past year and determine when virtual care or in-person care is more effective. He also said the health care industry needs to work to bridge the digital divide that exists because of lack of access to devices and broadband internet.
Even though Ms. Barnes said she did not see teletherapy as less effective than in-person therapy, she would like to return to seeing her therapist in person.
“When you are in person with someone, you can pick up on their body language better,” she said. “It’s a lot harder over a video call to do that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Gene therapy is bad business, and hugging chickens is just … bad
Look ma, I’m writing with no hands
Imagine being able to type every thought you had without using your hands, the words just magically appearing on the screen as fast as you can think of writing them down. Well, with the help of a new brain-computer interface (BCI), you can.
In a recent paper published in Nature, a team of researchers described how they developed a whole new way of communicating that blows previous BCIs, which used a method of pointing and clicking on letters, out of the water as far as accuracy and speed are concerned.
Developed for individuals with medical conditions or other disabilities that prevent them from communicating verbally or manually, the technology involves placing tiny sensors on the brain in the areas that control hand and arm movements. All the individual has to do is think of the process of writing and the system does the rest.
Even better, with continual use, the program’s algorithm comes to recognize the patterns of each letter, speeding up the number of words written. The previous record held for a BCI was about 40 characters per minute, but this new program enables users to type 90 characters per minute.
Think of how many emails you could reply to with just a thought. Or the LOTMEs we could write … or think? … Or think about writing?
Chicken noodle salmonella
Chickens and ducks sure are cute, especially babies, but humans should be extra careful around these animals for risk of salmonella. This isn’t a new thing to loyal readers of Livin’ on the MDedge.
As more people keep such creatures at home – Emily Shoop of Penn State University told the N.Y. Times that raising poultry was “the fastest-growing animal-related hobby in the United States” – the ducks and chickens are being treated more like house pets, which is sweet but not safe.
In the latest outbreak, more than 160 people, mostly children under 5 years old, have fallen ill from salmonella poisoning and more than 30 have been hospitalized across 43 states, and the Centers for Disease Control and Prevention suspects the numbers could be higher because many did not get tested and recovered on their own.
People should refrain from kissing these animals and should wash their hands for at least 20 seconds after handling them, their products, or their manure. If they do happen to kiss and cuddle these animals, they should wash their face and brush their teeth.
It’s not that ducks and chickens are dirty creatures, but they naturally carry bacteria. Some can get salmonella from contaminated food, or even contract it from their mothers before birth.
We can’t speak for everyone, but we would find it hard to connect with an animal that’s going to end up on our dinner plate.
This kidney research rocks!
When kids pick teams on the playground, someone is going to get their feelings hurt by being chosen last. There’s no way around it. Someone has to be last.
It’s the same way with research teams. When scientists are trying to cure diseases or pioneer new surgical techniques, they get a team together. And who always gets picked last? That’s right, the geologist, because who needs a geologist when you’re studying brain-computer interfaces?
Turns out, though, that there was a research team that needed a geologist: The one studying kidney stones.
Illinois geology professor Bruce Fouke explains: “The process of kidney stone formation is part of the natural process of the stone formation seen throughout nature. We are bringing together geology, biology, and medicine to map the entire process of kidney stone formation, step by step.”
In its latest work, the team found that kidney stones develop as tiny bits of mineral called microspherules, which can then come together to form larger crystals if they are not flushed out of the kidney tissue. Some eventually become large enough to cause excruciating pain.
Their transdisciplinary approach, known as GeoBioMed, has produced a device the team calls the GeoBioCell, which is “a microfluidic cartridge designed to mimic the intricate internal structures of the kidney,” they said.
Great stuff, no doubt, but we’re thinking the geologists haven’t quite gotten over the whole last-picked-for-the-team business, or maybe they’re just really into Batman. They’ve named the GeoBioCell after themselves, and he had the Batmobile and the Bat-tweezers. Also the Bat-funnel. And the Bat-scilloscope.
Gene therapy: What is it good for? Absolutely nothing!
Gene therapy has the potential to permanently cure all sorts of terrible diseases, and one would assume that this would be something we all could agree on. Yes, no more cancer or diabetes or anything like that, no sane person could possibly be against this, right?
Oh, you poor naive fool.
To be fair, the report written by Goldman Sachs does lay out many potential applications for gene therapy, and all the markets it can expand into. But then the writers ask the question that they’re not supposed to say out loud: Is curing patients a sustainable business model?
They go on to say that, while it would obviously be of enormous benefit to patients and society to give a one-shot cure rather than forcing a long, drawn-out series of treatments, current therapies for chronic disease represent a major source of money that would be cut off if a permanent treatment were found. They specifically mentioned hepatitis C, which has achieved a cure rate of over 90% in the past few years. In 2015, Gilead – the maker of these treatments – brought in sales of over $12 billion from its hepatitis C cure, but the report estimated that in 2021 they would bring in only $4 billion.
The authors of the report suggested that developers focus on “large markets,” such as hemophilia; diseases with high incidence like spinal muscular atrophy; and on diseases such as the various inherited retinal disorders, where there’s plenty of room to constantly bring out new and exciting treatments without sabotaging the all-important money flow.
While we can accept that Goldman Sachs may be technically correct in their assertion that curing disease is bad for business, that’s about as far as our sympathy goes, unless the big biotech companies of the world would like a sad song played on the world’s smallest violin.
Look ma, I’m writing with no hands
Imagine being able to type every thought you had without using your hands, the words just magically appearing on the screen as fast as you can think of writing them down. Well, with the help of a new brain-computer interface (BCI), you can.
In a recent paper published in Nature, a team of researchers described how they developed a whole new way of communicating that blows previous BCIs, which used a method of pointing and clicking on letters, out of the water as far as accuracy and speed are concerned.
Developed for individuals with medical conditions or other disabilities that prevent them from communicating verbally or manually, the technology involves placing tiny sensors on the brain in the areas that control hand and arm movements. All the individual has to do is think of the process of writing and the system does the rest.
Even better, with continual use, the program’s algorithm comes to recognize the patterns of each letter, speeding up the number of words written. The previous record held for a BCI was about 40 characters per minute, but this new program enables users to type 90 characters per minute.
Think of how many emails you could reply to with just a thought. Or the LOTMEs we could write … or think? … Or think about writing?
Chicken noodle salmonella
Chickens and ducks sure are cute, especially babies, but humans should be extra careful around these animals for risk of salmonella. This isn’t a new thing to loyal readers of Livin’ on the MDedge.
As more people keep such creatures at home – Emily Shoop of Penn State University told the N.Y. Times that raising poultry was “the fastest-growing animal-related hobby in the United States” – the ducks and chickens are being treated more like house pets, which is sweet but not safe.
In the latest outbreak, more than 160 people, mostly children under 5 years old, have fallen ill from salmonella poisoning and more than 30 have been hospitalized across 43 states, and the Centers for Disease Control and Prevention suspects the numbers could be higher because many did not get tested and recovered on their own.
People should refrain from kissing these animals and should wash their hands for at least 20 seconds after handling them, their products, or their manure. If they do happen to kiss and cuddle these animals, they should wash their face and brush their teeth.
It’s not that ducks and chickens are dirty creatures, but they naturally carry bacteria. Some can get salmonella from contaminated food, or even contract it from their mothers before birth.
We can’t speak for everyone, but we would find it hard to connect with an animal that’s going to end up on our dinner plate.
This kidney research rocks!
When kids pick teams on the playground, someone is going to get their feelings hurt by being chosen last. There’s no way around it. Someone has to be last.
It’s the same way with research teams. When scientists are trying to cure diseases or pioneer new surgical techniques, they get a team together. And who always gets picked last? That’s right, the geologist, because who needs a geologist when you’re studying brain-computer interfaces?
Turns out, though, that there was a research team that needed a geologist: The one studying kidney stones.
Illinois geology professor Bruce Fouke explains: “The process of kidney stone formation is part of the natural process of the stone formation seen throughout nature. We are bringing together geology, biology, and medicine to map the entire process of kidney stone formation, step by step.”
In its latest work, the team found that kidney stones develop as tiny bits of mineral called microspherules, which can then come together to form larger crystals if they are not flushed out of the kidney tissue. Some eventually become large enough to cause excruciating pain.
Their transdisciplinary approach, known as GeoBioMed, has produced a device the team calls the GeoBioCell, which is “a microfluidic cartridge designed to mimic the intricate internal structures of the kidney,” they said.
Great stuff, no doubt, but we’re thinking the geologists haven’t quite gotten over the whole last-picked-for-the-team business, or maybe they’re just really into Batman. They’ve named the GeoBioCell after themselves, and he had the Batmobile and the Bat-tweezers. Also the Bat-funnel. And the Bat-scilloscope.
Gene therapy: What is it good for? Absolutely nothing!
Gene therapy has the potential to permanently cure all sorts of terrible diseases, and one would assume that this would be something we all could agree on. Yes, no more cancer or diabetes or anything like that, no sane person could possibly be against this, right?
Oh, you poor naive fool.
To be fair, the report written by Goldman Sachs does lay out many potential applications for gene therapy, and all the markets it can expand into. But then the writers ask the question that they’re not supposed to say out loud: Is curing patients a sustainable business model?
They go on to say that, while it would obviously be of enormous benefit to patients and society to give a one-shot cure rather than forcing a long, drawn-out series of treatments, current therapies for chronic disease represent a major source of money that would be cut off if a permanent treatment were found. They specifically mentioned hepatitis C, which has achieved a cure rate of over 90% in the past few years. In 2015, Gilead – the maker of these treatments – brought in sales of over $12 billion from its hepatitis C cure, but the report estimated that in 2021 they would bring in only $4 billion.
The authors of the report suggested that developers focus on “large markets,” such as hemophilia; diseases with high incidence like spinal muscular atrophy; and on diseases such as the various inherited retinal disorders, where there’s plenty of room to constantly bring out new and exciting treatments without sabotaging the all-important money flow.
While we can accept that Goldman Sachs may be technically correct in their assertion that curing disease is bad for business, that’s about as far as our sympathy goes, unless the big biotech companies of the world would like a sad song played on the world’s smallest violin.
Look ma, I’m writing with no hands
Imagine being able to type every thought you had without using your hands, the words just magically appearing on the screen as fast as you can think of writing them down. Well, with the help of a new brain-computer interface (BCI), you can.
In a recent paper published in Nature, a team of researchers described how they developed a whole new way of communicating that blows previous BCIs, which used a method of pointing and clicking on letters, out of the water as far as accuracy and speed are concerned.
Developed for individuals with medical conditions or other disabilities that prevent them from communicating verbally or manually, the technology involves placing tiny sensors on the brain in the areas that control hand and arm movements. All the individual has to do is think of the process of writing and the system does the rest.
Even better, with continual use, the program’s algorithm comes to recognize the patterns of each letter, speeding up the number of words written. The previous record held for a BCI was about 40 characters per minute, but this new program enables users to type 90 characters per minute.
Think of how many emails you could reply to with just a thought. Or the LOTMEs we could write … or think? … Or think about writing?
Chicken noodle salmonella
Chickens and ducks sure are cute, especially babies, but humans should be extra careful around these animals for risk of salmonella. This isn’t a new thing to loyal readers of Livin’ on the MDedge.
As more people keep such creatures at home – Emily Shoop of Penn State University told the N.Y. Times that raising poultry was “the fastest-growing animal-related hobby in the United States” – the ducks and chickens are being treated more like house pets, which is sweet but not safe.
In the latest outbreak, more than 160 people, mostly children under 5 years old, have fallen ill from salmonella poisoning and more than 30 have been hospitalized across 43 states, and the Centers for Disease Control and Prevention suspects the numbers could be higher because many did not get tested and recovered on their own.
People should refrain from kissing these animals and should wash their hands for at least 20 seconds after handling them, their products, or their manure. If they do happen to kiss and cuddle these animals, they should wash their face and brush their teeth.
It’s not that ducks and chickens are dirty creatures, but they naturally carry bacteria. Some can get salmonella from contaminated food, or even contract it from their mothers before birth.
We can’t speak for everyone, but we would find it hard to connect with an animal that’s going to end up on our dinner plate.
This kidney research rocks!
When kids pick teams on the playground, someone is going to get their feelings hurt by being chosen last. There’s no way around it. Someone has to be last.
It’s the same way with research teams. When scientists are trying to cure diseases or pioneer new surgical techniques, they get a team together. And who always gets picked last? That’s right, the geologist, because who needs a geologist when you’re studying brain-computer interfaces?
Turns out, though, that there was a research team that needed a geologist: The one studying kidney stones.
Illinois geology professor Bruce Fouke explains: “The process of kidney stone formation is part of the natural process of the stone formation seen throughout nature. We are bringing together geology, biology, and medicine to map the entire process of kidney stone formation, step by step.”
In its latest work, the team found that kidney stones develop as tiny bits of mineral called microspherules, which can then come together to form larger crystals if they are not flushed out of the kidney tissue. Some eventually become large enough to cause excruciating pain.
Their transdisciplinary approach, known as GeoBioMed, has produced a device the team calls the GeoBioCell, which is “a microfluidic cartridge designed to mimic the intricate internal structures of the kidney,” they said.
Great stuff, no doubt, but we’re thinking the geologists haven’t quite gotten over the whole last-picked-for-the-team business, or maybe they’re just really into Batman. They’ve named the GeoBioCell after themselves, and he had the Batmobile and the Bat-tweezers. Also the Bat-funnel. And the Bat-scilloscope.
Gene therapy: What is it good for? Absolutely nothing!
Gene therapy has the potential to permanently cure all sorts of terrible diseases, and one would assume that this would be something we all could agree on. Yes, no more cancer or diabetes or anything like that, no sane person could possibly be against this, right?
Oh, you poor naive fool.
To be fair, the report written by Goldman Sachs does lay out many potential applications for gene therapy, and all the markets it can expand into. But then the writers ask the question that they’re not supposed to say out loud: Is curing patients a sustainable business model?
They go on to say that, while it would obviously be of enormous benefit to patients and society to give a one-shot cure rather than forcing a long, drawn-out series of treatments, current therapies for chronic disease represent a major source of money that would be cut off if a permanent treatment were found. They specifically mentioned hepatitis C, which has achieved a cure rate of over 90% in the past few years. In 2015, Gilead – the maker of these treatments – brought in sales of over $12 billion from its hepatitis C cure, but the report estimated that in 2021 they would bring in only $4 billion.
The authors of the report suggested that developers focus on “large markets,” such as hemophilia; diseases with high incidence like spinal muscular atrophy; and on diseases such as the various inherited retinal disorders, where there’s plenty of room to constantly bring out new and exciting treatments without sabotaging the all-important money flow.
While we can accept that Goldman Sachs may be technically correct in their assertion that curing disease is bad for business, that’s about as far as our sympathy goes, unless the big biotech companies of the world would like a sad song played on the world’s smallest violin.
COVID’s big impact made a fairly small dent in GI earnings
Despite the shutdowns and plummeting patient volumes early in the COVID-19 pandemic, gastroenterologists’ earnings were just 3.1% lower in 2020 than in 2019, according to new survey results from Medscape.
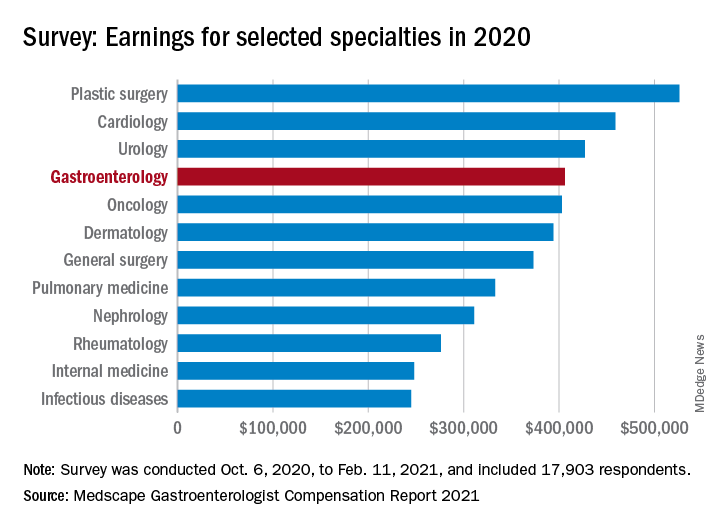
That drop, from $419,000 to $406,000, was larger, however, than the average for all specialists, which slipped just 0.6% in 2020.
“Most gastroenterologists who saw a drop in income cited COVID-19–related issues, such as job loss, fewer hours, and fewer patients,” Keith L. Martin wrote in the 2021 Medscape Gastroenterologist Compensation Report.
Specialties with larger declines than gastroenterology included dermatology (–4%), pediatrics (–5%), and otolaryngology (–9%). Conversely, plastic surgeons saw the largest increases last year, with their average compensation rising 10% over 2019. Oncologists (+7%) and cardiologists (+5%) also did well, the Medscape data show.
For the physicians who did encounter financial hardships, relief came in several forms.
Many turned to “the federal Paycheck Protection Program to help keep themselves afloat; some were able to renegotiate their lease contracts; a large percentage reduced their staff, which reduced their expenses; and those in capitated plans were still getting paid even though they weren’t seeing as many patients,” Michael Belkin, JD, divisional vice president at Merritt Hawkins and Associates in Dallas, said in an interview.
One complication on the road to recovery was the furloughs experienced by some gastroenterologists, but work hours for the specialty have largely recovered. The prepandemic average was 53 hours per week, compared with 52 hours for this year’s gastroenterologist respondents, who represented about 2% of the 17,903 Medscape member physicians who completed the survey.
Among the gastroenterologists surveyed who did experience negative financial or practice-related effects from the pandemic, about one-third estimated that it would take 2-3 years for their income to return to the pre-COVID level, and 14% believe that it will never return to those levels. It is worth noting, however, that 45% of physicians overall reported no such harms last year.
Despite the drop in their incomes last year, more gastroenterologists said that they felt fairly compensated in 2020 than indicated as such in 2019 (55% vs. 52%). This year’s higher figure, though, is on the low end of the scale: of the 29 specialties included, only 4 were lower, and 19 were higher. Five others were the same, according to Medscape’s findings.
In other matters covered by the survey, gastroenterologists found themselves closer to the top. When asked if they would choose medicine again, 81% said yes. Only 8 of the 29 specialties were higher; 93% of gastroenterologists said they would choose gastroenterology again. Only four specialties were higher.
The survey was conducted from Oct. 6, 2020, to Feb. 11, 2021, and had a sampling error of ±0.73%. The salary figures were calculated using data for full-time physicians only.
A version of this article first appeared on Medscape.com.
Despite the shutdowns and plummeting patient volumes early in the COVID-19 pandemic, gastroenterologists’ earnings were just 3.1% lower in 2020 than in 2019, according to new survey results from Medscape.

That drop, from $419,000 to $406,000, was larger, however, than the average for all specialists, which slipped just 0.6% in 2020.
“Most gastroenterologists who saw a drop in income cited COVID-19–related issues, such as job loss, fewer hours, and fewer patients,” Keith L. Martin wrote in the 2021 Medscape Gastroenterologist Compensation Report.
Specialties with larger declines than gastroenterology included dermatology (–4%), pediatrics (–5%), and otolaryngology (–9%). Conversely, plastic surgeons saw the largest increases last year, with their average compensation rising 10% over 2019. Oncologists (+7%) and cardiologists (+5%) also did well, the Medscape data show.
For the physicians who did encounter financial hardships, relief came in several forms.
Many turned to “the federal Paycheck Protection Program to help keep themselves afloat; some were able to renegotiate their lease contracts; a large percentage reduced their staff, which reduced their expenses; and those in capitated plans were still getting paid even though they weren’t seeing as many patients,” Michael Belkin, JD, divisional vice president at Merritt Hawkins and Associates in Dallas, said in an interview.
One complication on the road to recovery was the furloughs experienced by some gastroenterologists, but work hours for the specialty have largely recovered. The prepandemic average was 53 hours per week, compared with 52 hours for this year’s gastroenterologist respondents, who represented about 2% of the 17,903 Medscape member physicians who completed the survey.
Among the gastroenterologists surveyed who did experience negative financial or practice-related effects from the pandemic, about one-third estimated that it would take 2-3 years for their income to return to the pre-COVID level, and 14% believe that it will never return to those levels. It is worth noting, however, that 45% of physicians overall reported no such harms last year.
Despite the drop in their incomes last year, more gastroenterologists said that they felt fairly compensated in 2020 than indicated as such in 2019 (55% vs. 52%). This year’s higher figure, though, is on the low end of the scale: of the 29 specialties included, only 4 were lower, and 19 were higher. Five others were the same, according to Medscape’s findings.
In other matters covered by the survey, gastroenterologists found themselves closer to the top. When asked if they would choose medicine again, 81% said yes. Only 8 of the 29 specialties were higher; 93% of gastroenterologists said they would choose gastroenterology again. Only four specialties were higher.
The survey was conducted from Oct. 6, 2020, to Feb. 11, 2021, and had a sampling error of ±0.73%. The salary figures were calculated using data for full-time physicians only.
A version of this article first appeared on Medscape.com.
Despite the shutdowns and plummeting patient volumes early in the COVID-19 pandemic, gastroenterologists’ earnings were just 3.1% lower in 2020 than in 2019, according to new survey results from Medscape.

That drop, from $419,000 to $406,000, was larger, however, than the average for all specialists, which slipped just 0.6% in 2020.
“Most gastroenterologists who saw a drop in income cited COVID-19–related issues, such as job loss, fewer hours, and fewer patients,” Keith L. Martin wrote in the 2021 Medscape Gastroenterologist Compensation Report.
Specialties with larger declines than gastroenterology included dermatology (–4%), pediatrics (–5%), and otolaryngology (–9%). Conversely, plastic surgeons saw the largest increases last year, with their average compensation rising 10% over 2019. Oncologists (+7%) and cardiologists (+5%) also did well, the Medscape data show.
For the physicians who did encounter financial hardships, relief came in several forms.
Many turned to “the federal Paycheck Protection Program to help keep themselves afloat; some were able to renegotiate their lease contracts; a large percentage reduced their staff, which reduced their expenses; and those in capitated plans were still getting paid even though they weren’t seeing as many patients,” Michael Belkin, JD, divisional vice president at Merritt Hawkins and Associates in Dallas, said in an interview.
One complication on the road to recovery was the furloughs experienced by some gastroenterologists, but work hours for the specialty have largely recovered. The prepandemic average was 53 hours per week, compared with 52 hours for this year’s gastroenterologist respondents, who represented about 2% of the 17,903 Medscape member physicians who completed the survey.
Among the gastroenterologists surveyed who did experience negative financial or practice-related effects from the pandemic, about one-third estimated that it would take 2-3 years for their income to return to the pre-COVID level, and 14% believe that it will never return to those levels. It is worth noting, however, that 45% of physicians overall reported no such harms last year.
Despite the drop in their incomes last year, more gastroenterologists said that they felt fairly compensated in 2020 than indicated as such in 2019 (55% vs. 52%). This year’s higher figure, though, is on the low end of the scale: of the 29 specialties included, only 4 were lower, and 19 were higher. Five others were the same, according to Medscape’s findings.
In other matters covered by the survey, gastroenterologists found themselves closer to the top. When asked if they would choose medicine again, 81% said yes. Only 8 of the 29 specialties were higher; 93% of gastroenterologists said they would choose gastroenterology again. Only four specialties were higher.
The survey was conducted from Oct. 6, 2020, to Feb. 11, 2021, and had a sampling error of ±0.73%. The salary figures were calculated using data for full-time physicians only.
A version of this article first appeared on Medscape.com.
‘Overbasalization’ common in type 2 diabetes management
that impedes achievement of optimal glycemic control, new research suggests.
Such ‘overbasalization,’ defined as a hemoglobin A1c of greater than 8% despite use of more than 0.5 units/kg per day of basal insulin, was identified in about 40% of patients seen in a Florida primary care clinic during 2015-2018. The findings were published in the April 2021 issue of Clinical Diabetes by Kevin Cowart, PharmD, a diabetes care and education specialist at the University of South Florida, Tampa, and colleagues.
The literature suggests that once people with type 2 diabetes start basal insulin, the chance that they’ll achieve a given hemoglobin A1c target, i.e., less than 7%, diminishes significantly if that goal isn’t achieved within the first year of starting insulin, Dr. Cowart said in an interview.
“Our analysis suggests that overbasalization plays a role in patients with type 2 diabetes on basal insulin not achieving optimal glycemic control. Basal insulin is not designed to address postprandial hyperglycemia. I think there’s a clear need to address hesitancy in therapeutic progression beyond basal insulin. A lot of factors underlie the delays, with therapeutic inertia being one of them. It’s complex,” he said.
Overbasalization seen in large proportion of patients
The study comprised 655 adults diagnosed with type 2 diabetes for at least a year who received a prescription for a basal insulin (glargine U-100, glargine U-300, detemir, degludec U-100, degludec U-200, regular U-500, or NPH insulin).
The patients had a mean hemoglobin A1c of 8.4% and a mean basal insulin dose 0.4 units/kg per day. The prevalence of overbasalization was 38.1% for those with hemoglobin A1c above 8%, 42.7% for those with A1c of 9% or above, and 42% with A1c of 10% or greater.
Patient characteristics independently associated with overbasalization were age 35-54 years (odds ratio 1.89), age 65-80 years (0.44), A1c 9% or greater (13.97), and A1c 10% or greater (6.04). Having a prescription for insulin glargine U-100 was associated with a lower overbasalization risk (0.62). In multivariate analysis, only an A1c of 9% or greater remained significant.
Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic, Rochester, Minn., said in an interview that she sees [overbasalization] frequently in patients who are referred to her. “It’s kind of that wall that patients with type 2 diabetes hit because their A1c is high but their fasting blood sugars are normal. Sometimes it’s assumed that there’s a discrepancy, because people don’t always think about postprandial hyperglycemia.”
She also noted that there has been a push in recent years to simplify regimens, particularly in older patients.
“We really want to avoid rapid-acting insulin in older patients because we’re afraid of hypoglycemia, so we start them on basal and keep the noninsulins like metformin and sulfonylureas around. Initially those control the postprandial blood sugar but over time they’re no longer enough.”
Options exist for addressing postmeal blood sugar highs while minimizing lows
While in the past adding premeal insulin was the only option, today there are alternatives for addressing postmeal hyperglycemia, at least in the short term.
Dr. Cowart advised that the first step is to have patients self-monitor their blood glucose and titrate their basal insulin to address fasting hyperglycemia first. Once that appropriate dose is reached, if the patient’s hemoglobin A1c is still above target, the next step is to evaluate the need for postmeal control.
For patients who are at high cardiovascular risk, the next step might involve adding a sodium-glucose cotransporter 2 inhibitor (SGLT2i) or a glucagon-like peptide 1 receptor agonist (GLP-1RA) instead of premeal insulin. But for patients in whom overbasalization is the main concern, a GLP-1RA might be the better choice since it will have a greater impact on postprandial glucose levels, while an SGLT2i will have more effect on fasting blood sugar, he said.
Another option is to use a fixed-dose combination of basal insulin and a glucagon-like peptide 1 receptor agonist (GLP-1RA), provided there aren’t cost or formulary barriers. “We want to use the right combination of drugs and not use too much of one to lead to hypoglycemia,” Dr. Cowart said.
Dr. McCoy doesn’t use fixed-dose combinations because they don’t allow as much flexibility in dosing. To correct overbasalization, she also recommends adding either a GLP-1RA or SGLT2i instead of premeal insulin. However, she cautions, “you still have to monitor those patients because after a few years it still won’t be enough and you’ll have to add mealtime insulin.”
If cost or lack of coverage prevents a patient’s use of SLGT2i/GLP-1RAs, Dr. McCoy said that adding just one premeal injection of rapid-acting insulin before the largest meal of the day is one option. Another is to use twice-daily NPH insulin instead of analog basal insulin, since that does offer some postprandial coverage.
Dr. Cowart said his approach in cost barrier situations is to try to use patient assistance programs and to look into the patient’s formulary to see if there is step therapy or tier considerations, and maybe have a discussion with the insurance company. “We often have to navigate that, and it does take a significant amount of time and could potentially delay patients getting the right therapy when it’s warranted. That is an area where there is a particular role for pharmacists in helping to overcome that and get patients on the right drugs,” he explained.
Problem may be even more common; testing is key
Dr. McCoy said that the A1c cutoff of 8% used to define overbasalization in the study probably resulted in an underestimation of the problem, since many patients are experiencing nighttime hypoglycemia from the basal insulin. The lows bring down their A1c level, but they’re still experiencing postmeal highs.
“I think they’re missing a lot of people, to be honest. I see a lot of patients with A1cs that aren’t that bad, say 7.5%, and their fasting blood sugars are okay, but if you were to put a [continuous glucose monitor] on those patients, invariably there’s hypoglycemia at night that no one knew about.”
Of course, for insurance reasons, most people with type 2 diabetes don’t currently have access to continuous glucose monitors. And often those who are not taking multiple daily injections are limited to one fingerstick test strip a day.
Dr. McCoy says that if hypoglycemia is a concern she will write a prior authorization justifying more test strips.
“I state explicitly in my notes why I recommend frequent monitoring. If they’re on a sulfonylurea, they should be able to check more frequently because they can have hypoglycemia. Same thing with basal insulin.”
Dr. McCoy advises that patients test their blood sugar 2 hours after the largest meal on one day, and at other times on different days. “Blood glucose after a meal shouldn’t be more than 200 [mg/dL]. If it is, that’s not a failure of basal insulin. It’s doing its job. You just need a different agent.”
Dr. Cowart has no disclosures. Dr. McCoy receives funding from the National Institutes of Health.
that impedes achievement of optimal glycemic control, new research suggests.
Such ‘overbasalization,’ defined as a hemoglobin A1c of greater than 8% despite use of more than 0.5 units/kg per day of basal insulin, was identified in about 40% of patients seen in a Florida primary care clinic during 2015-2018. The findings were published in the April 2021 issue of Clinical Diabetes by Kevin Cowart, PharmD, a diabetes care and education specialist at the University of South Florida, Tampa, and colleagues.
The literature suggests that once people with type 2 diabetes start basal insulin, the chance that they’ll achieve a given hemoglobin A1c target, i.e., less than 7%, diminishes significantly if that goal isn’t achieved within the first year of starting insulin, Dr. Cowart said in an interview.
“Our analysis suggests that overbasalization plays a role in patients with type 2 diabetes on basal insulin not achieving optimal glycemic control. Basal insulin is not designed to address postprandial hyperglycemia. I think there’s a clear need to address hesitancy in therapeutic progression beyond basal insulin. A lot of factors underlie the delays, with therapeutic inertia being one of them. It’s complex,” he said.
Overbasalization seen in large proportion of patients
The study comprised 655 adults diagnosed with type 2 diabetes for at least a year who received a prescription for a basal insulin (glargine U-100, glargine U-300, detemir, degludec U-100, degludec U-200, regular U-500, or NPH insulin).
The patients had a mean hemoglobin A1c of 8.4% and a mean basal insulin dose 0.4 units/kg per day. The prevalence of overbasalization was 38.1% for those with hemoglobin A1c above 8%, 42.7% for those with A1c of 9% or above, and 42% with A1c of 10% or greater.
Patient characteristics independently associated with overbasalization were age 35-54 years (odds ratio 1.89), age 65-80 years (0.44), A1c 9% or greater (13.97), and A1c 10% or greater (6.04). Having a prescription for insulin glargine U-100 was associated with a lower overbasalization risk (0.62). In multivariate analysis, only an A1c of 9% or greater remained significant.
Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic, Rochester, Minn., said in an interview that she sees [overbasalization] frequently in patients who are referred to her. “It’s kind of that wall that patients with type 2 diabetes hit because their A1c is high but their fasting blood sugars are normal. Sometimes it’s assumed that there’s a discrepancy, because people don’t always think about postprandial hyperglycemia.”
She also noted that there has been a push in recent years to simplify regimens, particularly in older patients.
“We really want to avoid rapid-acting insulin in older patients because we’re afraid of hypoglycemia, so we start them on basal and keep the noninsulins like metformin and sulfonylureas around. Initially those control the postprandial blood sugar but over time they’re no longer enough.”
Options exist for addressing postmeal blood sugar highs while minimizing lows
While in the past adding premeal insulin was the only option, today there are alternatives for addressing postmeal hyperglycemia, at least in the short term.
Dr. Cowart advised that the first step is to have patients self-monitor their blood glucose and titrate their basal insulin to address fasting hyperglycemia first. Once that appropriate dose is reached, if the patient’s hemoglobin A1c is still above target, the next step is to evaluate the need for postmeal control.
For patients who are at high cardiovascular risk, the next step might involve adding a sodium-glucose cotransporter 2 inhibitor (SGLT2i) or a glucagon-like peptide 1 receptor agonist (GLP-1RA) instead of premeal insulin. But for patients in whom overbasalization is the main concern, a GLP-1RA might be the better choice since it will have a greater impact on postprandial glucose levels, while an SGLT2i will have more effect on fasting blood sugar, he said.
Another option is to use a fixed-dose combination of basal insulin and a glucagon-like peptide 1 receptor agonist (GLP-1RA), provided there aren’t cost or formulary barriers. “We want to use the right combination of drugs and not use too much of one to lead to hypoglycemia,” Dr. Cowart said.
Dr. McCoy doesn’t use fixed-dose combinations because they don’t allow as much flexibility in dosing. To correct overbasalization, she also recommends adding either a GLP-1RA or SGLT2i instead of premeal insulin. However, she cautions, “you still have to monitor those patients because after a few years it still won’t be enough and you’ll have to add mealtime insulin.”
If cost or lack of coverage prevents a patient’s use of SLGT2i/GLP-1RAs, Dr. McCoy said that adding just one premeal injection of rapid-acting insulin before the largest meal of the day is one option. Another is to use twice-daily NPH insulin instead of analog basal insulin, since that does offer some postprandial coverage.
Dr. Cowart said his approach in cost barrier situations is to try to use patient assistance programs and to look into the patient’s formulary to see if there is step therapy or tier considerations, and maybe have a discussion with the insurance company. “We often have to navigate that, and it does take a significant amount of time and could potentially delay patients getting the right therapy when it’s warranted. That is an area where there is a particular role for pharmacists in helping to overcome that and get patients on the right drugs,” he explained.
Problem may be even more common; testing is key
Dr. McCoy said that the A1c cutoff of 8% used to define overbasalization in the study probably resulted in an underestimation of the problem, since many patients are experiencing nighttime hypoglycemia from the basal insulin. The lows bring down their A1c level, but they’re still experiencing postmeal highs.
“I think they’re missing a lot of people, to be honest. I see a lot of patients with A1cs that aren’t that bad, say 7.5%, and their fasting blood sugars are okay, but if you were to put a [continuous glucose monitor] on those patients, invariably there’s hypoglycemia at night that no one knew about.”
Of course, for insurance reasons, most people with type 2 diabetes don’t currently have access to continuous glucose monitors. And often those who are not taking multiple daily injections are limited to one fingerstick test strip a day.
Dr. McCoy says that if hypoglycemia is a concern she will write a prior authorization justifying more test strips.
“I state explicitly in my notes why I recommend frequent monitoring. If they’re on a sulfonylurea, they should be able to check more frequently because they can have hypoglycemia. Same thing with basal insulin.”
Dr. McCoy advises that patients test their blood sugar 2 hours after the largest meal on one day, and at other times on different days. “Blood glucose after a meal shouldn’t be more than 200 [mg/dL]. If it is, that’s not a failure of basal insulin. It’s doing its job. You just need a different agent.”
Dr. Cowart has no disclosures. Dr. McCoy receives funding from the National Institutes of Health.
that impedes achievement of optimal glycemic control, new research suggests.
Such ‘overbasalization,’ defined as a hemoglobin A1c of greater than 8% despite use of more than 0.5 units/kg per day of basal insulin, was identified in about 40% of patients seen in a Florida primary care clinic during 2015-2018. The findings were published in the April 2021 issue of Clinical Diabetes by Kevin Cowart, PharmD, a diabetes care and education specialist at the University of South Florida, Tampa, and colleagues.
The literature suggests that once people with type 2 diabetes start basal insulin, the chance that they’ll achieve a given hemoglobin A1c target, i.e., less than 7%, diminishes significantly if that goal isn’t achieved within the first year of starting insulin, Dr. Cowart said in an interview.
“Our analysis suggests that overbasalization plays a role in patients with type 2 diabetes on basal insulin not achieving optimal glycemic control. Basal insulin is not designed to address postprandial hyperglycemia. I think there’s a clear need to address hesitancy in therapeutic progression beyond basal insulin. A lot of factors underlie the delays, with therapeutic inertia being one of them. It’s complex,” he said.
Overbasalization seen in large proportion of patients
The study comprised 655 adults diagnosed with type 2 diabetes for at least a year who received a prescription for a basal insulin (glargine U-100, glargine U-300, detemir, degludec U-100, degludec U-200, regular U-500, or NPH insulin).
The patients had a mean hemoglobin A1c of 8.4% and a mean basal insulin dose 0.4 units/kg per day. The prevalence of overbasalization was 38.1% for those with hemoglobin A1c above 8%, 42.7% for those with A1c of 9% or above, and 42% with A1c of 10% or greater.
Patient characteristics independently associated with overbasalization were age 35-54 years (odds ratio 1.89), age 65-80 years (0.44), A1c 9% or greater (13.97), and A1c 10% or greater (6.04). Having a prescription for insulin glargine U-100 was associated with a lower overbasalization risk (0.62). In multivariate analysis, only an A1c of 9% or greater remained significant.
Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic, Rochester, Minn., said in an interview that she sees [overbasalization] frequently in patients who are referred to her. “It’s kind of that wall that patients with type 2 diabetes hit because their A1c is high but their fasting blood sugars are normal. Sometimes it’s assumed that there’s a discrepancy, because people don’t always think about postprandial hyperglycemia.”
She also noted that there has been a push in recent years to simplify regimens, particularly in older patients.
“We really want to avoid rapid-acting insulin in older patients because we’re afraid of hypoglycemia, so we start them on basal and keep the noninsulins like metformin and sulfonylureas around. Initially those control the postprandial blood sugar but over time they’re no longer enough.”
Options exist for addressing postmeal blood sugar highs while minimizing lows
While in the past adding premeal insulin was the only option, today there are alternatives for addressing postmeal hyperglycemia, at least in the short term.
Dr. Cowart advised that the first step is to have patients self-monitor their blood glucose and titrate their basal insulin to address fasting hyperglycemia first. Once that appropriate dose is reached, if the patient’s hemoglobin A1c is still above target, the next step is to evaluate the need for postmeal control.
For patients who are at high cardiovascular risk, the next step might involve adding a sodium-glucose cotransporter 2 inhibitor (SGLT2i) or a glucagon-like peptide 1 receptor agonist (GLP-1RA) instead of premeal insulin. But for patients in whom overbasalization is the main concern, a GLP-1RA might be the better choice since it will have a greater impact on postprandial glucose levels, while an SGLT2i will have more effect on fasting blood sugar, he said.
Another option is to use a fixed-dose combination of basal insulin and a glucagon-like peptide 1 receptor agonist (GLP-1RA), provided there aren’t cost or formulary barriers. “We want to use the right combination of drugs and not use too much of one to lead to hypoglycemia,” Dr. Cowart said.
Dr. McCoy doesn’t use fixed-dose combinations because they don’t allow as much flexibility in dosing. To correct overbasalization, she also recommends adding either a GLP-1RA or SGLT2i instead of premeal insulin. However, she cautions, “you still have to monitor those patients because after a few years it still won’t be enough and you’ll have to add mealtime insulin.”
If cost or lack of coverage prevents a patient’s use of SLGT2i/GLP-1RAs, Dr. McCoy said that adding just one premeal injection of rapid-acting insulin before the largest meal of the day is one option. Another is to use twice-daily NPH insulin instead of analog basal insulin, since that does offer some postprandial coverage.
Dr. Cowart said his approach in cost barrier situations is to try to use patient assistance programs and to look into the patient’s formulary to see if there is step therapy or tier considerations, and maybe have a discussion with the insurance company. “We often have to navigate that, and it does take a significant amount of time and could potentially delay patients getting the right therapy when it’s warranted. That is an area where there is a particular role for pharmacists in helping to overcome that and get patients on the right drugs,” he explained.
Problem may be even more common; testing is key
Dr. McCoy said that the A1c cutoff of 8% used to define overbasalization in the study probably resulted in an underestimation of the problem, since many patients are experiencing nighttime hypoglycemia from the basal insulin. The lows bring down their A1c level, but they’re still experiencing postmeal highs.
“I think they’re missing a lot of people, to be honest. I see a lot of patients with A1cs that aren’t that bad, say 7.5%, and their fasting blood sugars are okay, but if you were to put a [continuous glucose monitor] on those patients, invariably there’s hypoglycemia at night that no one knew about.”
Of course, for insurance reasons, most people with type 2 diabetes don’t currently have access to continuous glucose monitors. And often those who are not taking multiple daily injections are limited to one fingerstick test strip a day.
Dr. McCoy says that if hypoglycemia is a concern she will write a prior authorization justifying more test strips.
“I state explicitly in my notes why I recommend frequent monitoring. If they’re on a sulfonylurea, they should be able to check more frequently because they can have hypoglycemia. Same thing with basal insulin.”
Dr. McCoy advises that patients test their blood sugar 2 hours after the largest meal on one day, and at other times on different days. “Blood glucose after a meal shouldn’t be more than 200 [mg/dL]. If it is, that’s not a failure of basal insulin. It’s doing its job. You just need a different agent.”
Dr. Cowart has no disclosures. Dr. McCoy receives funding from the National Institutes of Health.
FROM CLINICAL DIABETES
Rivaroxaban cut recurrent limb events in VOYAGER-PAD
After patients with peripheral artery disease undergo lower-extremity revascularization, they are at high risk for major adverse limb events, and new findings from a prespecified analysis of data from the VOYAGER-PAD trial show that treatment with the direct-acting oral anticoagulant rivaroxaban along with aspirin significantly cut the rate of total major adverse limb events in these patients.
These findings confirm the drop in first major adverse limb events linked to rivaroxaban treatment that was VOYAGER-PAD’s primary result, reported just over a year ago.
The new total-event analysis also provides important insight into the huge magnitude of total major adverse limb events that patients with PAD can develop following lower-extremity revascularization (LER).
The 6,564 patients who all received aspirin and were randomized to either rivaroxaban (Xarelto) or placebo had 4,714 total events during a median follow-up of 2.5 years following their revascularization procedure. This included 1,092 first primary events (a composite of acute limb ischemia, major amputation for vascular causes, MI, ischemic stroke, or cardiovascular death), 522 primary events that occurred as second or subsequent events among patients after a first primary event (a nearly 50% increase from first events only), and 3,100 additional vascular events that did not fit into the primary-event category, most often a peripheral revascularization procedure, Rupert M. Bauersachs, MD, said at the annual scientific sessions of the American College of Cardiology.
“We were all astonished by this high event rate,” Dr. Bauersachs said during his report.
The total-event analysis that he reported showed that treatment with rivaroxaban resulted in a significant 14% relative reduction, compared with placebo in the incidence of total primary events, which closely tracks the significant 15% relative reduction in first primary events reported from the VOYAGER-PAD trial in 2020. Treatment with rivaroxaban also significantly linked with a 14% cut in total vascular events, compared with placebo, including the many events not included in the primary endpoint, said Dr. Bauersachs, who until his retirement in May 2021 was director of the Clinic for Vascular Medicine at the Darmstadt (Germany) Clinic. Concurrently with the report, the results appeared online.
“If one focuses only on first events, you miss the totality of disease burden. There is even greater benefit by reducing total events,” Dr. Bauersachs said during a press briefing. Adding rivaroxaban prevented roughly 2.6 first primary events for every 100 patients treated, but it also prevented 4.4 total primary events and 12.5 total vascular events for every 100 treated patients.
An ‘incredibly high’ event rate
“I don’t think any of us imagined the level of morbidity in this population. The event rate is incredibly high,” commented Joshua A. Beckman, MD, professor and director of vascular medicine at Vanderbilt University Medical Center, Nashville, Tenn.
Because treatment with rivaroxaban showed clear efficacy for also preventing subsequent events it should not be considered to have failed in patients who have a vascular event while on rivaroxaban treatment, he added as designated discussant for the report. Treatment with rivaroxaban “should be continued indefinitely,” he concluded.
“It’s quite astonishing to see the magnitude of [total] events in these patients,” commented Sahil A. Parikh, MD, a cardiologist and director of endovascular services at Columbia University Medical Center in New York. “We’ve always known that these are high-risk patients, but exactly how high their risk is was not well understood until these data came to light.”
Dr. Parikh also noted that, despite the clear evidence reported from VOYAGER-PAD more than a year ago proving the efficacy and safety of adding rivaroxaban to aspirin for long-term treatment of patients with PAD following LER, this regimen has not yet become standard U.S. practice.
Rivaroxaban use falls short of the expected level
“This paradigm shift has not seen the level of adoption that we would expect based on the data,” he said. “There have been numerous editorials and discussions of this at every major medical meeting” during the past year, but those expert opinions have not translated into changed practice. “Perhaps the pandemic has muted enthusiasm for adoption of a new therapeutic paradigm,” suggested Dr. Parikh, and “on top of that guidelines have yet to be updated,” although he noted that updated guidelines from the ACC and American Heart Association for PAD that include the types of patients enrolled in VOYAGER-PAD are now under review and should be released by the first half of 2022.
“I think the additional data [reported by Dr. Bauersachs] will encourage us to use rivaroxaban in patients with claudication,” Dr. Parikh said. “Perhaps we should use rivaroxaban and aspirin in a broader swath of patients, but it will take time to convince some constituencies.”
VOYAGER-PAD randomized patients with PAD who underwent successful LER within 10 days prior to enrollment at 542 sites in 34 countries during 2015-2018. In addition to every patient receiving 100 mg aspirin daily and either 2.5 mg rivaroxaban twice daily or placebo once daily, patients who received an intra-arterial device such as a stent could also receive the antiplatelet agent clopidogrel for a planned maximum of 30 days after revascularization at the discretion of their physician, and the trial protocol allowed for extending clopidogrel treatment to as many as 60 days.
In addition to the efficacy outcomes, the safety results showed that adding rivaroxaban to aspirin appeared to increase bleeding episodes, but at rates that generally did not reach significance and that were dwarfed by the efficacy benefit. The study’s primary safety outcome was the incidence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding episodes, which occurred in 2.65% of patients who received rivaroxaban and in 1.87% on those on placebo, a 43% relative increase that fell short of significance (P = .07). The analyses overall indicated that 10,000 similar patients treated for 1 year with rivaroxaban would have 181 fewer primary events, compared with placebo-treated patients at the cost of also having 29 additional TIMI major bleeding events compared with patient on placebo.
Adding clopidogrel adds little except bleeding
Further analysis showed that just over half of enrolled patients also received clopidogrel for a median of 29 days following their LER procedure. This added agent produced no significant added benefit during 3-year follow-up, but did boost bleeding risk, especially in patients who received clopidogrel for more than 30 days. This led the study investigators to suggest that, while rivaroxaban plus aspirin is indicated for long-term treatment, addition of clopidogrel on top of this should be limited to 30 days or fewer to minimize bleeding risk.
“I’m sure there is a bleeding hazard associated with rivaroxaban plus aspirin, but this is attenuated by using dual therapy and not using triple therapy” by also adding clopidogrel, noted Dr. Parikh.
The new VOYAGER-PAD results also showed that the ongoing risk faced by patients with PAD following LER applies globally to their peripheral arteries. Of the 3,034 total peripheral revascularizations performed in the cohort during follow-up, 64% occurred in the index limb and 36% in the contralateral limb. Another striking finding was that the need for ipsilateral repeat revascularization was more common after an index endovascular procedure, 2,329 repeat revascularizations in 4,379 of these patients (53%), compared with 2,185 patients who had surgical revascularization for their index procedure and subsequently 705 of these patients (32%) needed repeat revascularization.
But rivaroxaban treatment appeared to provide little benefit for the much less frequent incidence of first and subsequent events in the coronary and cerebral circulation. During follow-up, the rates of major adverse cardiovascular events – cardiovascular death, nonfatal MI, and nonfatal stroke – were virtually identical in the rivaroxaban and placebo groups.
“This study makes it clear that we are learning about differences in presentation between the vascular beds, and the benefits of specific treatments in each vascular bed,” Dr. Beckman said.
VOYAGER-PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). Dr. Bauersachs has received personal fees from Bayer, as well as from Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer, and has received grant support from Aspen Pharma. Dr. Beckman been a consultant to and received honoraria from Janssen, as well as from Amgen, JanOne, Novartis, and Sanofi, and he has served on a data and safety monitoring board for Bayer. Dr. Parikh has been a consultant to and received honoraria from Janssen, as well as from Abbott, Boston Scientific, Cordis, Medtronic, Penumbra, Philips, and Terumo, he has been a speaker on behalf of Inari, and he has received grant support from Abbott, Shockwave Medical, Surmodics, and TriReme Medical.
After patients with peripheral artery disease undergo lower-extremity revascularization, they are at high risk for major adverse limb events, and new findings from a prespecified analysis of data from the VOYAGER-PAD trial show that treatment with the direct-acting oral anticoagulant rivaroxaban along with aspirin significantly cut the rate of total major adverse limb events in these patients.
These findings confirm the drop in first major adverse limb events linked to rivaroxaban treatment that was VOYAGER-PAD’s primary result, reported just over a year ago.
The new total-event analysis also provides important insight into the huge magnitude of total major adverse limb events that patients with PAD can develop following lower-extremity revascularization (LER).
The 6,564 patients who all received aspirin and were randomized to either rivaroxaban (Xarelto) or placebo had 4,714 total events during a median follow-up of 2.5 years following their revascularization procedure. This included 1,092 first primary events (a composite of acute limb ischemia, major amputation for vascular causes, MI, ischemic stroke, or cardiovascular death), 522 primary events that occurred as second or subsequent events among patients after a first primary event (a nearly 50% increase from first events only), and 3,100 additional vascular events that did not fit into the primary-event category, most often a peripheral revascularization procedure, Rupert M. Bauersachs, MD, said at the annual scientific sessions of the American College of Cardiology.
“We were all astonished by this high event rate,” Dr. Bauersachs said during his report.
The total-event analysis that he reported showed that treatment with rivaroxaban resulted in a significant 14% relative reduction, compared with placebo in the incidence of total primary events, which closely tracks the significant 15% relative reduction in first primary events reported from the VOYAGER-PAD trial in 2020. Treatment with rivaroxaban also significantly linked with a 14% cut in total vascular events, compared with placebo, including the many events not included in the primary endpoint, said Dr. Bauersachs, who until his retirement in May 2021 was director of the Clinic for Vascular Medicine at the Darmstadt (Germany) Clinic. Concurrently with the report, the results appeared online.
“If one focuses only on first events, you miss the totality of disease burden. There is even greater benefit by reducing total events,” Dr. Bauersachs said during a press briefing. Adding rivaroxaban prevented roughly 2.6 first primary events for every 100 patients treated, but it also prevented 4.4 total primary events and 12.5 total vascular events for every 100 treated patients.
An ‘incredibly high’ event rate
“I don’t think any of us imagined the level of morbidity in this population. The event rate is incredibly high,” commented Joshua A. Beckman, MD, professor and director of vascular medicine at Vanderbilt University Medical Center, Nashville, Tenn.
Because treatment with rivaroxaban showed clear efficacy for also preventing subsequent events it should not be considered to have failed in patients who have a vascular event while on rivaroxaban treatment, he added as designated discussant for the report. Treatment with rivaroxaban “should be continued indefinitely,” he concluded.
“It’s quite astonishing to see the magnitude of [total] events in these patients,” commented Sahil A. Parikh, MD, a cardiologist and director of endovascular services at Columbia University Medical Center in New York. “We’ve always known that these are high-risk patients, but exactly how high their risk is was not well understood until these data came to light.”
Dr. Parikh also noted that, despite the clear evidence reported from VOYAGER-PAD more than a year ago proving the efficacy and safety of adding rivaroxaban to aspirin for long-term treatment of patients with PAD following LER, this regimen has not yet become standard U.S. practice.
Rivaroxaban use falls short of the expected level
“This paradigm shift has not seen the level of adoption that we would expect based on the data,” he said. “There have been numerous editorials and discussions of this at every major medical meeting” during the past year, but those expert opinions have not translated into changed practice. “Perhaps the pandemic has muted enthusiasm for adoption of a new therapeutic paradigm,” suggested Dr. Parikh, and “on top of that guidelines have yet to be updated,” although he noted that updated guidelines from the ACC and American Heart Association for PAD that include the types of patients enrolled in VOYAGER-PAD are now under review and should be released by the first half of 2022.
“I think the additional data [reported by Dr. Bauersachs] will encourage us to use rivaroxaban in patients with claudication,” Dr. Parikh said. “Perhaps we should use rivaroxaban and aspirin in a broader swath of patients, but it will take time to convince some constituencies.”
VOYAGER-PAD randomized patients with PAD who underwent successful LER within 10 days prior to enrollment at 542 sites in 34 countries during 2015-2018. In addition to every patient receiving 100 mg aspirin daily and either 2.5 mg rivaroxaban twice daily or placebo once daily, patients who received an intra-arterial device such as a stent could also receive the antiplatelet agent clopidogrel for a planned maximum of 30 days after revascularization at the discretion of their physician, and the trial protocol allowed for extending clopidogrel treatment to as many as 60 days.
In addition to the efficacy outcomes, the safety results showed that adding rivaroxaban to aspirin appeared to increase bleeding episodes, but at rates that generally did not reach significance and that were dwarfed by the efficacy benefit. The study’s primary safety outcome was the incidence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding episodes, which occurred in 2.65% of patients who received rivaroxaban and in 1.87% on those on placebo, a 43% relative increase that fell short of significance (P = .07). The analyses overall indicated that 10,000 similar patients treated for 1 year with rivaroxaban would have 181 fewer primary events, compared with placebo-treated patients at the cost of also having 29 additional TIMI major bleeding events compared with patient on placebo.
Adding clopidogrel adds little except bleeding
Further analysis showed that just over half of enrolled patients also received clopidogrel for a median of 29 days following their LER procedure. This added agent produced no significant added benefit during 3-year follow-up, but did boost bleeding risk, especially in patients who received clopidogrel for more than 30 days. This led the study investigators to suggest that, while rivaroxaban plus aspirin is indicated for long-term treatment, addition of clopidogrel on top of this should be limited to 30 days or fewer to minimize bleeding risk.
“I’m sure there is a bleeding hazard associated with rivaroxaban plus aspirin, but this is attenuated by using dual therapy and not using triple therapy” by also adding clopidogrel, noted Dr. Parikh.
The new VOYAGER-PAD results also showed that the ongoing risk faced by patients with PAD following LER applies globally to their peripheral arteries. Of the 3,034 total peripheral revascularizations performed in the cohort during follow-up, 64% occurred in the index limb and 36% in the contralateral limb. Another striking finding was that the need for ipsilateral repeat revascularization was more common after an index endovascular procedure, 2,329 repeat revascularizations in 4,379 of these patients (53%), compared with 2,185 patients who had surgical revascularization for their index procedure and subsequently 705 of these patients (32%) needed repeat revascularization.
But rivaroxaban treatment appeared to provide little benefit for the much less frequent incidence of first and subsequent events in the coronary and cerebral circulation. During follow-up, the rates of major adverse cardiovascular events – cardiovascular death, nonfatal MI, and nonfatal stroke – were virtually identical in the rivaroxaban and placebo groups.
“This study makes it clear that we are learning about differences in presentation between the vascular beds, and the benefits of specific treatments in each vascular bed,” Dr. Beckman said.
VOYAGER-PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). Dr. Bauersachs has received personal fees from Bayer, as well as from Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer, and has received grant support from Aspen Pharma. Dr. Beckman been a consultant to and received honoraria from Janssen, as well as from Amgen, JanOne, Novartis, and Sanofi, and he has served on a data and safety monitoring board for Bayer. Dr. Parikh has been a consultant to and received honoraria from Janssen, as well as from Abbott, Boston Scientific, Cordis, Medtronic, Penumbra, Philips, and Terumo, he has been a speaker on behalf of Inari, and he has received grant support from Abbott, Shockwave Medical, Surmodics, and TriReme Medical.
After patients with peripheral artery disease undergo lower-extremity revascularization, they are at high risk for major adverse limb events, and new findings from a prespecified analysis of data from the VOYAGER-PAD trial show that treatment with the direct-acting oral anticoagulant rivaroxaban along with aspirin significantly cut the rate of total major adverse limb events in these patients.
These findings confirm the drop in first major adverse limb events linked to rivaroxaban treatment that was VOYAGER-PAD’s primary result, reported just over a year ago.
The new total-event analysis also provides important insight into the huge magnitude of total major adverse limb events that patients with PAD can develop following lower-extremity revascularization (LER).
The 6,564 patients who all received aspirin and were randomized to either rivaroxaban (Xarelto) or placebo had 4,714 total events during a median follow-up of 2.5 years following their revascularization procedure. This included 1,092 first primary events (a composite of acute limb ischemia, major amputation for vascular causes, MI, ischemic stroke, or cardiovascular death), 522 primary events that occurred as second or subsequent events among patients after a first primary event (a nearly 50% increase from first events only), and 3,100 additional vascular events that did not fit into the primary-event category, most often a peripheral revascularization procedure, Rupert M. Bauersachs, MD, said at the annual scientific sessions of the American College of Cardiology.
“We were all astonished by this high event rate,” Dr. Bauersachs said during his report.
The total-event analysis that he reported showed that treatment with rivaroxaban resulted in a significant 14% relative reduction, compared with placebo in the incidence of total primary events, which closely tracks the significant 15% relative reduction in first primary events reported from the VOYAGER-PAD trial in 2020. Treatment with rivaroxaban also significantly linked with a 14% cut in total vascular events, compared with placebo, including the many events not included in the primary endpoint, said Dr. Bauersachs, who until his retirement in May 2021 was director of the Clinic for Vascular Medicine at the Darmstadt (Germany) Clinic. Concurrently with the report, the results appeared online.
“If one focuses only on first events, you miss the totality of disease burden. There is even greater benefit by reducing total events,” Dr. Bauersachs said during a press briefing. Adding rivaroxaban prevented roughly 2.6 first primary events for every 100 patients treated, but it also prevented 4.4 total primary events and 12.5 total vascular events for every 100 treated patients.
An ‘incredibly high’ event rate
“I don’t think any of us imagined the level of morbidity in this population. The event rate is incredibly high,” commented Joshua A. Beckman, MD, professor and director of vascular medicine at Vanderbilt University Medical Center, Nashville, Tenn.
Because treatment with rivaroxaban showed clear efficacy for also preventing subsequent events it should not be considered to have failed in patients who have a vascular event while on rivaroxaban treatment, he added as designated discussant for the report. Treatment with rivaroxaban “should be continued indefinitely,” he concluded.
“It’s quite astonishing to see the magnitude of [total] events in these patients,” commented Sahil A. Parikh, MD, a cardiologist and director of endovascular services at Columbia University Medical Center in New York. “We’ve always known that these are high-risk patients, but exactly how high their risk is was not well understood until these data came to light.”
Dr. Parikh also noted that, despite the clear evidence reported from VOYAGER-PAD more than a year ago proving the efficacy and safety of adding rivaroxaban to aspirin for long-term treatment of patients with PAD following LER, this regimen has not yet become standard U.S. practice.
Rivaroxaban use falls short of the expected level
“This paradigm shift has not seen the level of adoption that we would expect based on the data,” he said. “There have been numerous editorials and discussions of this at every major medical meeting” during the past year, but those expert opinions have not translated into changed practice. “Perhaps the pandemic has muted enthusiasm for adoption of a new therapeutic paradigm,” suggested Dr. Parikh, and “on top of that guidelines have yet to be updated,” although he noted that updated guidelines from the ACC and American Heart Association for PAD that include the types of patients enrolled in VOYAGER-PAD are now under review and should be released by the first half of 2022.
“I think the additional data [reported by Dr. Bauersachs] will encourage us to use rivaroxaban in patients with claudication,” Dr. Parikh said. “Perhaps we should use rivaroxaban and aspirin in a broader swath of patients, but it will take time to convince some constituencies.”
VOYAGER-PAD randomized patients with PAD who underwent successful LER within 10 days prior to enrollment at 542 sites in 34 countries during 2015-2018. In addition to every patient receiving 100 mg aspirin daily and either 2.5 mg rivaroxaban twice daily or placebo once daily, patients who received an intra-arterial device such as a stent could also receive the antiplatelet agent clopidogrel for a planned maximum of 30 days after revascularization at the discretion of their physician, and the trial protocol allowed for extending clopidogrel treatment to as many as 60 days.
In addition to the efficacy outcomes, the safety results showed that adding rivaroxaban to aspirin appeared to increase bleeding episodes, but at rates that generally did not reach significance and that were dwarfed by the efficacy benefit. The study’s primary safety outcome was the incidence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding episodes, which occurred in 2.65% of patients who received rivaroxaban and in 1.87% on those on placebo, a 43% relative increase that fell short of significance (P = .07). The analyses overall indicated that 10,000 similar patients treated for 1 year with rivaroxaban would have 181 fewer primary events, compared with placebo-treated patients at the cost of also having 29 additional TIMI major bleeding events compared with patient on placebo.
Adding clopidogrel adds little except bleeding
Further analysis showed that just over half of enrolled patients also received clopidogrel for a median of 29 days following their LER procedure. This added agent produced no significant added benefit during 3-year follow-up, but did boost bleeding risk, especially in patients who received clopidogrel for more than 30 days. This led the study investigators to suggest that, while rivaroxaban plus aspirin is indicated for long-term treatment, addition of clopidogrel on top of this should be limited to 30 days or fewer to minimize bleeding risk.
“I’m sure there is a bleeding hazard associated with rivaroxaban plus aspirin, but this is attenuated by using dual therapy and not using triple therapy” by also adding clopidogrel, noted Dr. Parikh.
The new VOYAGER-PAD results also showed that the ongoing risk faced by patients with PAD following LER applies globally to their peripheral arteries. Of the 3,034 total peripheral revascularizations performed in the cohort during follow-up, 64% occurred in the index limb and 36% in the contralateral limb. Another striking finding was that the need for ipsilateral repeat revascularization was more common after an index endovascular procedure, 2,329 repeat revascularizations in 4,379 of these patients (53%), compared with 2,185 patients who had surgical revascularization for their index procedure and subsequently 705 of these patients (32%) needed repeat revascularization.
But rivaroxaban treatment appeared to provide little benefit for the much less frequent incidence of first and subsequent events in the coronary and cerebral circulation. During follow-up, the rates of major adverse cardiovascular events – cardiovascular death, nonfatal MI, and nonfatal stroke – were virtually identical in the rivaroxaban and placebo groups.
“This study makes it clear that we are learning about differences in presentation between the vascular beds, and the benefits of specific treatments in each vascular bed,” Dr. Beckman said.
VOYAGER-PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). Dr. Bauersachs has received personal fees from Bayer, as well as from Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer, and has received grant support from Aspen Pharma. Dr. Beckman been a consultant to and received honoraria from Janssen, as well as from Amgen, JanOne, Novartis, and Sanofi, and he has served on a data and safety monitoring board for Bayer. Dr. Parikh has been a consultant to and received honoraria from Janssen, as well as from Abbott, Boston Scientific, Cordis, Medtronic, Penumbra, Philips, and Terumo, he has been a speaker on behalf of Inari, and he has received grant support from Abbott, Shockwave Medical, Surmodics, and TriReme Medical.
FROM ACC 2021
Surprising percentage of biopsy samples found retained in GI endoscopes
Researchers examining GI endoscopes after colonoscopy and esophagogastroduodenoscopy (EGD) procedures found a “startlingly high” rate of retained biopsy samples in the endoscope accessory channel or cap.
Investigators found 64% of 105 total endoscopies featured retained biopsy samples, including 76% of EGDs and 50% of colonoscopies examined.
“The take-home message would be that retained biopsies are much more common than most endoscopists would think. In our institution, many endoscopists guessed 10%-15%, while the actual number was 64%,” Gregory Toy, MD, said in an interview.
Raising awareness about the high proportion of retained biopsy samples “could help change behavior to make this happen less often,” added Dr. Toy, an internal medicine resident at the University of Utah Health in Salt Lake City.
“Another finding of this study was that there were significantly more retained biopsies found in EGDs compared to colonoscopies,” Dr. Toy said.
Dr. Toy presented the findings at the annual Digestive Disease Week® (DDW).
‘Very surprising’ findings
“The study is very important as it points out a significant rate of tissue retention in the biopsy channel at the conclusion of endoscopic procedures,” session moderator Serge Sorser, MD, said in an interview.
The high rate of tissue retention “is very surprising,” added Dr. Sorser, a gastroenterologist at Ascension Michigan Providence Hospital in Novi, Mich.
“Not only does this mean that not all tissue is submitted for pathologic review, but it also brings to light the need for diligent endoscope processing between procedures,” he said.
Because biopsy specimens during GI endoscopy procedures must pass through the device’s biopsy channel and cap, Dr. Toy and colleagues decided to examine the rate of potentially retained samples.
Endoscopists “have noted anecdotally that retained biopsies can be found in the accessory channel and/or cap,” Dr. Toy said during his presentation at DDW. “However, this has not been formally studied.”
After 55 EGDs and 50 colonoscopies, each a standard outpatient procedure, the researchers removed the cap and the male end where the cap attaches. They brushed these areas for residual tissue. Next, they applied a new suction trap and cleared the channel using water and suction. They then brushed the channel and repeated the water and suction procedure. As a final check, they visually inspected the cleaning brush.
They sent any recovered tissue – designated from either the cap or channel – to pathology for evaluation. “The new pathology reads from these retained biopsies changed or added to the diagnosis in only five of our patients. All of these changes were minor, and patients were already on appropriate treatment,” Dr. Toy said.
Dr. Toy and colleagues found no differences between EGDs and colonoscopies with and without retained biopsy samples according to procedure time, doses of propofol or fentanyl, and age or gender of the patient. Likewise, the number of samples collected did not appear to influence the retention rates.
Of retained samples discovered after 42 EGDs, 71% were in the cap, 35% were in the channel, and 29% were found in both locations. Of the 25 colonoscopies with retained samples, 40% were in the cap, 34% were in the channel, and 24% were found in both places.
“The overall incidence of retained biopsies during standard upper and lower endoscopy is high,” the researchers noted.
Inclusion of multiple endoscopists and a hospital outpatient setting were strengths of the study. Limitations included a single center study with a relatively small sample size.
Dr. Toy and Dr. Sorser have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers examining GI endoscopes after colonoscopy and esophagogastroduodenoscopy (EGD) procedures found a “startlingly high” rate of retained biopsy samples in the endoscope accessory channel or cap.
Investigators found 64% of 105 total endoscopies featured retained biopsy samples, including 76% of EGDs and 50% of colonoscopies examined.
“The take-home message would be that retained biopsies are much more common than most endoscopists would think. In our institution, many endoscopists guessed 10%-15%, while the actual number was 64%,” Gregory Toy, MD, said in an interview.
Raising awareness about the high proportion of retained biopsy samples “could help change behavior to make this happen less often,” added Dr. Toy, an internal medicine resident at the University of Utah Health in Salt Lake City.
“Another finding of this study was that there were significantly more retained biopsies found in EGDs compared to colonoscopies,” Dr. Toy said.
Dr. Toy presented the findings at the annual Digestive Disease Week® (DDW).
‘Very surprising’ findings
“The study is very important as it points out a significant rate of tissue retention in the biopsy channel at the conclusion of endoscopic procedures,” session moderator Serge Sorser, MD, said in an interview.
The high rate of tissue retention “is very surprising,” added Dr. Sorser, a gastroenterologist at Ascension Michigan Providence Hospital in Novi, Mich.
“Not only does this mean that not all tissue is submitted for pathologic review, but it also brings to light the need for diligent endoscope processing between procedures,” he said.
Because biopsy specimens during GI endoscopy procedures must pass through the device’s biopsy channel and cap, Dr. Toy and colleagues decided to examine the rate of potentially retained samples.
Endoscopists “have noted anecdotally that retained biopsies can be found in the accessory channel and/or cap,” Dr. Toy said during his presentation at DDW. “However, this has not been formally studied.”
After 55 EGDs and 50 colonoscopies, each a standard outpatient procedure, the researchers removed the cap and the male end where the cap attaches. They brushed these areas for residual tissue. Next, they applied a new suction trap and cleared the channel using water and suction. They then brushed the channel and repeated the water and suction procedure. As a final check, they visually inspected the cleaning brush.
They sent any recovered tissue – designated from either the cap or channel – to pathology for evaluation. “The new pathology reads from these retained biopsies changed or added to the diagnosis in only five of our patients. All of these changes were minor, and patients were already on appropriate treatment,” Dr. Toy said.
Dr. Toy and colleagues found no differences between EGDs and colonoscopies with and without retained biopsy samples according to procedure time, doses of propofol or fentanyl, and age or gender of the patient. Likewise, the number of samples collected did not appear to influence the retention rates.
Of retained samples discovered after 42 EGDs, 71% were in the cap, 35% were in the channel, and 29% were found in both locations. Of the 25 colonoscopies with retained samples, 40% were in the cap, 34% were in the channel, and 24% were found in both places.
“The overall incidence of retained biopsies during standard upper and lower endoscopy is high,” the researchers noted.
Inclusion of multiple endoscopists and a hospital outpatient setting were strengths of the study. Limitations included a single center study with a relatively small sample size.
Dr. Toy and Dr. Sorser have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers examining GI endoscopes after colonoscopy and esophagogastroduodenoscopy (EGD) procedures found a “startlingly high” rate of retained biopsy samples in the endoscope accessory channel or cap.
Investigators found 64% of 105 total endoscopies featured retained biopsy samples, including 76% of EGDs and 50% of colonoscopies examined.
“The take-home message would be that retained biopsies are much more common than most endoscopists would think. In our institution, many endoscopists guessed 10%-15%, while the actual number was 64%,” Gregory Toy, MD, said in an interview.
Raising awareness about the high proportion of retained biopsy samples “could help change behavior to make this happen less often,” added Dr. Toy, an internal medicine resident at the University of Utah Health in Salt Lake City.
“Another finding of this study was that there were significantly more retained biopsies found in EGDs compared to colonoscopies,” Dr. Toy said.
Dr. Toy presented the findings at the annual Digestive Disease Week® (DDW).
‘Very surprising’ findings
“The study is very important as it points out a significant rate of tissue retention in the biopsy channel at the conclusion of endoscopic procedures,” session moderator Serge Sorser, MD, said in an interview.
The high rate of tissue retention “is very surprising,” added Dr. Sorser, a gastroenterologist at Ascension Michigan Providence Hospital in Novi, Mich.
“Not only does this mean that not all tissue is submitted for pathologic review, but it also brings to light the need for diligent endoscope processing between procedures,” he said.
Because biopsy specimens during GI endoscopy procedures must pass through the device’s biopsy channel and cap, Dr. Toy and colleagues decided to examine the rate of potentially retained samples.
Endoscopists “have noted anecdotally that retained biopsies can be found in the accessory channel and/or cap,” Dr. Toy said during his presentation at DDW. “However, this has not been formally studied.”
After 55 EGDs and 50 colonoscopies, each a standard outpatient procedure, the researchers removed the cap and the male end where the cap attaches. They brushed these areas for residual tissue. Next, they applied a new suction trap and cleared the channel using water and suction. They then brushed the channel and repeated the water and suction procedure. As a final check, they visually inspected the cleaning brush.
They sent any recovered tissue – designated from either the cap or channel – to pathology for evaluation. “The new pathology reads from these retained biopsies changed or added to the diagnosis in only five of our patients. All of these changes were minor, and patients were already on appropriate treatment,” Dr. Toy said.
Dr. Toy and colleagues found no differences between EGDs and colonoscopies with and without retained biopsy samples according to procedure time, doses of propofol or fentanyl, and age or gender of the patient. Likewise, the number of samples collected did not appear to influence the retention rates.
Of retained samples discovered after 42 EGDs, 71% were in the cap, 35% were in the channel, and 29% were found in both locations. Of the 25 colonoscopies with retained samples, 40% were in the cap, 34% were in the channel, and 24% were found in both places.
“The overall incidence of retained biopsies during standard upper and lower endoscopy is high,” the researchers noted.
Inclusion of multiple endoscopists and a hospital outpatient setting were strengths of the study. Limitations included a single center study with a relatively small sample size.
Dr. Toy and Dr. Sorser have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Large vessel stroke linked to AstraZeneca COVID vaccine
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.
The three cases (one of which was fatal) occurred in two women and one man in their 30s or 40s and involved blockages of the carotid and middle cerebral artery. Two of the three patients also had venous thrombosis involving the portal and cerebral venous system. All three also had extremely low platelet counts, confirmed antibodies to platelet factor 4, and raised D-dimer levels, all characteristic of the vaccine-induced immune thrombotic thrombocytopenia (VITT) reaction associated with the AstraZeneca vaccine.
They are described in detail in a letter published online on May 25 in the Journal of Neurology, Neurosurgery & Psychiatry
“These are [the] first detailed reports of arterial stroke believed to be caused by VITT after the AstraZeneca COVID vaccine, although stroke has been mentioned previously in the VITT data,” said senior author David Werring, PhD, FRCP.
“VITT has more commonly presented as CVST [Cerebral venous sinus thrombosis] which is stroke caused by a venous thrombosis; these cases are showing that it can also cause stroke caused by an arterial thrombosis,” explained Dr. Werring, professor of clinical neurology at the Stroke Research Centre, University College London.
“In patients who present with ischemic stroke, especially younger patients, and who have had the AstraZeneca vaccine within the past month, clinicians need to consider VITT as a possible cause, as there is a specific treatment needed for this syndrome,” he said.
Young patients presenting with ischemic stroke after receiving the AstraZeneca vaccine should urgently be evaluated for VITT with laboratory tests, including platelet count, D-dimers, fibrinogen, and anti-PF4 antibodies, the authors wrote, and then managed by a multidisciplinary team, including hematology, neurology, stroke, neurosurgery, and neuroradiology, for rapid access to treatments including intravenous immune globulin, methylprednisolone, plasmapheresis, and nonheparin anticoagulants such as fondaparinux, argatroban, or direct oral anticoagulants.
Dr. Werring noted that these reports do not add anything to the overall risk/benefit of the vaccine, as they are only describing three cases. “While VITT is very serious, the benefit of the vaccine still outweighs its risks,” he said. “Around 40% of patients hospitalized with COVID-19 experience some sort of thrombosis and about 1.5% have an ischemic stroke. Whereas latest figures from the U.K. estimate the incidence of VITT with the AstraZeneca vaccine of 1 in 50,000 to 1 in 100,000.
“Our report doesn’t suggest that VITT is more common than these latest figures estimate, but we are just drawing attention to an alternative presentation,” he added.
Three cases
The first patient in the current case series, a woman in her 30s, experienced an intermittent headache on the right side and around her eyes 6 days after the vaccine. Five days later, she awoke feeling drowsy and with weakness to her left face, arm, and leg.
Imaging revealed a blocked right middle cerebral artery with brain infarction and clots in the right portal vein. She underwent brain surgery to reduce the pressure in her skull, plasma removal and replacement, and received the anticoagulant fondaparinux, but she still unfortunately died.
The second patient, a woman in her late 30s, presented with headache, confusion, weakness in her left arm, and loss of vision on the left side 12 days after having received the vaccine. Imaging showed occlusion of both carotid arteries, as well as pulmonary embolism and a left cerebral venous sinus thrombosis.
Her platelet count increased following plasma removal and replacement and intravenous corticosteroids, and her condition improved after fondaparinux treatment.
The third patient, a man in his early 40s, presented 3 weeks after receiving his vaccination with problems speaking. Imaging showed a clot in the left middle cerebral artery, but there was no evidence of clots in the cerebral venous sinuses. He received a platelet and plasma transfusion, and fondaparinux, and remains stable.
High index of suspicion required
In a linked commentary, Hugh Markus, PhD, FRCP, professor of stroke medicine at the University of Cambridge, United Kingdom, wrote: “This report emphasizes that the immune mediated coagulopathy can also cause arterial thrombosis, including ischemic stroke, although venous thrombosis and especially cerebral venous sinus thrombosis appear more frequent.
“During the current period of COVID vaccination, a high index of suspicion is required to identify thrombotic episodes following vaccination,” he added. “However, it is important to remember that these side effects are rare and much less common than both cerebral venous thrombosis and ischemic stroke associated with COVID-19 infection itself.”
Risk/benefit unaltered
Several experts who commented on these reports for the Science Media Centre all agreed with Dr. Werring and Dr. Markus that these reports do not alter the current risk/benefit estimates with the vaccine.
Ian Douglas, PhD, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, who sits on the U.K.’s Medicines and Healthcare Products Regulatory Agency’s Pharmacovigilance Expert Advisory Group, said: “The picture regarding the rare syndrome of blood clots combined with low platelet counts associated with the AstraZeneca vaccine is becoming clearer. Until now, the cases described have tended to involve clots in veins such as cerebral vein thrombosis. In this series of three case reports, we now have some evidence that the types of blood vessels affected include arteries as well as veins.”
“It’s important to stress that such cases remain very rare, and it’s certainly much rarer in people who have had the AstraZeneca vaccine than it is in people affected by COVID-19 itself,” Dr. Douglas emphasized.
“The description of the cases suggests the patients involved presented with the same kind of symptoms as already described in cases involving cerebral vein thrombosis, and they don’t suggest patients need to be on the alert for anything different,” he added.
“However, the emergence of details like this will help guide health professionals who may be faced with similar cases in future; the sooner such cases are recognized, the more chance they will quickly receive the right kind of treatment, hopefully leading to better outcomes.”
Will Lester, MBChB, PhD, consultant hematologist, University Hospitals Birmingham NHS Foundation Trust, said: “VITT remains a rare complication, and patients with a history of thrombosis, including stroke, should not consider themselves to be at any higher risk of this type of rare thrombosis after vaccination, and COVID infection itself is a significant risk for stroke and other types of thrombosis.”
Many countries have paused use of the AstraZeneca vaccine because of its link to the VITT syndrome or restricted its use to older people as the VITT reaction appears to be slightly more common in younger people. In the United Kingdom, the current recommendation is that individuals under 40 years of age should be offered an alternative to the AstraZeneca vaccine where possible.
A version of this article first appeared on Medscape.com.