User login
In head-to-head trial, two biologics differ markedly for control of psoriasis
with other biologics, according to data from two simultaneously published trials, one of which was a head-to-head comparison with ustekinumab.
In the head-to-head trial called BE VIVID, which included a placebo arm, there was a large advantage of bimekizumab over ustekinumab, a biologic that targets IL-12 and IL-23 and is approved for treating psoriasis, for both coprimary endpoints, according to a multinational group of investigators led by Kristian Reich, MD, PhD, professor of dermatology at the University Medical Center, Hamburg-Eppendorf, Germany.
The proportion of patients with skin clearance was not only greater but faster, “with responses observed after one dose,” Dr. Reich and coinvestigators reported.
The data from the BE VIVID trial was published simultaneously with the BE READY trial, which was placebo-controlled but did not include an active comparator.
Evaluated at week 16, the coprimary endpoints in both studies were skin clearance as measured by a Psoriasis Area Severity Index greater than 90% (PASI 90) and Investigators Global Assessment (IGA) score of 0 (clear) or 1 (almost clear).
In BE VIVID, 567 patients were randomized in 11 countries, including the United States. The dose of bimekizumab was 320 mg administered subcutaneously every 4 weeks. In a randomization scheme of 4:2:1, half as many patients (163) were randomized to ustekinumab (Stelara), which was administered in weight-based dosing of 45 mg or 90 mg at enrollment, at 4 weeks, and then every 12 weeks. The placebo arm had 83 patients. All were switched to bimekizumab at 16 weeks.
At week 16, PASI 90 was achieved in 85% of patients randomized to bimekizumab, compared with 50% of patients randomized to ustekinumab (P < .0001). The rate in the placebo group was 5%.
The bimekizumab advantage for an IGA response of 0 or 1 was of similar magnitude, relative to ustekinumab (84% vs. 53%; P < .0001) and placebo (5%). All secondary efficacy endpoints, such as PASI 90 at week 12 (85% vs. 44%) and PASI 100 at week 16 (59% vs. 21%), favored bimekizumab over ustekinumab.
In the BE READY trial, which evaluated the same dose and schedule of bimekizumab, the rates of PASI 90 at week 16 were 91% and 1% (P < .0001) for the experimental arm and placebo, respectively. The proportion of patients with an IGA score of 0 or 1 were 93% and 1% (P < .0001), respectively.
In BE READY, patients who achieved PASI 90 at week 16 were reallocated to receive bimekizumab every 4 weeks, bimekizumab every 8 weeks (also 320 mg), or placebo. Both schedules of bimekizumab maintained responses through week 56, according to the authors, led by Kenneth B. Gordon, MD, professor and chair of dermatology, Medical College of Wisconsin, Milwaukee.
In both trials, safety was evaluated over the first 16 weeks as well as over a subsequent maintenance period, which extended to 52 weeks in BE VIVID and 56 weeks in BE READY. For bimekizumab, oral candidiasis was the most common treatment-related adverse event. In BE VIVID, this adverse event was reported in 9% of bimekizumab patients, compared with 0% of either the ustekinumab or placebo groups, up to week 16. Out to week 52, the rates were 15% in the bimekizumab group and 1% in the ustekinumab group.
In the BE READY trial, the rates of oral candidiasis were 6% and 0% for bimekizumab and placebo, respectively, through week 16. Over the maintenance periods, the rates were 9% and 11% for the every-8-week and every-4-week doses, respectively.
Discontinuation for adverse events was not higher on bimekizumab than placebo in either trial, nor was the proportion of serious treatment-emergent adverse events.
Nevertheless, the potential for adverse events was a key part of the discussion regarding the future role of bimekizumab, if approved, in an editorial that accompanied the publication of these studies.
“Bimekizumab might be our most effective biologic for psoriasis yet,” coauthors, William W. Huang, MD, PhD, associate professor of dermatology, and Steven R. Feldman, MD, PhD, professor of dermatology, both at Wake Forest University, Winston-Salem, NC, wrote in the editorial. “If the goal of psoriasis treatment is complete clearance, bimekizumab seems like a good option from an efficacy perspective.”
However, they noted that other IL-17 blockers, like secukinumab (Cosentyx) and brodalumab (Siliq), have been associated with risks, including the development of inflammatory bowel disease. In addition to the oral candidiasis seen in the BE VIVID and BE READY trials, they cautioned that other issues might arise with longer follow-up and greater numbers of patients exposed to this therapy.
In an interview, Dr. Feldman said adequately informed patients might be willing to accept these risks for the potential of greater efficacy, but he emphasized the need for appropriate warnings and education.
“We have a lot of very good treatments that offer patients an excellent chance of an excellent outcome – treatments that have been around and in use in large numbers of people for years,” Dr. Feldman said. “Unless the doctor and patient felt strongly about the need to use this new, perhaps more potent option, I would be personally inclined to use treatment with well-established safety profiles first.”
The senior author of the BE VIVID trial, Mark Lebwohl, MD, dean for clinical therapeutics and professor of dermatology, at the Icahn School of Medicine at Mount Sinai, New York, disagreed. He acknowledged that other agents targeting IL-17 have been associated with IBD, but risk of IBD is already elevated in patients with psoriasis and the risk appears to be lower with bimekizumab relative to prior agents in this class.
“Bimekizumab has now been studied in thousands of patients over several years. We can say with support from a sizable amount of data that IBD is very uncommon,” he said. While oral candidiasis is associated with bimekizumab, it is “easy to treat.”
Asked specifically if he will consider using bimekizumab as a first-line agent in psoriasis patients who are candidates for a biologic, Dr. Lebwohl said he would. Based on the evidence that this agent is more effective than other options and has manageable side effects, he believes it will be an important new treatment option.
Dr. Reich, Dr. Lebwohl, Dr. Gordon, and Dr. Feldman have financial relationships with multiple companies that produce therapies for psoriasis, including UCB Pharma, the sponsor of these studies.
with other biologics, according to data from two simultaneously published trials, one of which was a head-to-head comparison with ustekinumab.
In the head-to-head trial called BE VIVID, which included a placebo arm, there was a large advantage of bimekizumab over ustekinumab, a biologic that targets IL-12 and IL-23 and is approved for treating psoriasis, for both coprimary endpoints, according to a multinational group of investigators led by Kristian Reich, MD, PhD, professor of dermatology at the University Medical Center, Hamburg-Eppendorf, Germany.
The proportion of patients with skin clearance was not only greater but faster, “with responses observed after one dose,” Dr. Reich and coinvestigators reported.
The data from the BE VIVID trial was published simultaneously with the BE READY trial, which was placebo-controlled but did not include an active comparator.
Evaluated at week 16, the coprimary endpoints in both studies were skin clearance as measured by a Psoriasis Area Severity Index greater than 90% (PASI 90) and Investigators Global Assessment (IGA) score of 0 (clear) or 1 (almost clear).
In BE VIVID, 567 patients were randomized in 11 countries, including the United States. The dose of bimekizumab was 320 mg administered subcutaneously every 4 weeks. In a randomization scheme of 4:2:1, half as many patients (163) were randomized to ustekinumab (Stelara), which was administered in weight-based dosing of 45 mg or 90 mg at enrollment, at 4 weeks, and then every 12 weeks. The placebo arm had 83 patients. All were switched to bimekizumab at 16 weeks.
At week 16, PASI 90 was achieved in 85% of patients randomized to bimekizumab, compared with 50% of patients randomized to ustekinumab (P < .0001). The rate in the placebo group was 5%.
The bimekizumab advantage for an IGA response of 0 or 1 was of similar magnitude, relative to ustekinumab (84% vs. 53%; P < .0001) and placebo (5%). All secondary efficacy endpoints, such as PASI 90 at week 12 (85% vs. 44%) and PASI 100 at week 16 (59% vs. 21%), favored bimekizumab over ustekinumab.
In the BE READY trial, which evaluated the same dose and schedule of bimekizumab, the rates of PASI 90 at week 16 were 91% and 1% (P < .0001) for the experimental arm and placebo, respectively. The proportion of patients with an IGA score of 0 or 1 were 93% and 1% (P < .0001), respectively.
In BE READY, patients who achieved PASI 90 at week 16 were reallocated to receive bimekizumab every 4 weeks, bimekizumab every 8 weeks (also 320 mg), or placebo. Both schedules of bimekizumab maintained responses through week 56, according to the authors, led by Kenneth B. Gordon, MD, professor and chair of dermatology, Medical College of Wisconsin, Milwaukee.
In both trials, safety was evaluated over the first 16 weeks as well as over a subsequent maintenance period, which extended to 52 weeks in BE VIVID and 56 weeks in BE READY. For bimekizumab, oral candidiasis was the most common treatment-related adverse event. In BE VIVID, this adverse event was reported in 9% of bimekizumab patients, compared with 0% of either the ustekinumab or placebo groups, up to week 16. Out to week 52, the rates were 15% in the bimekizumab group and 1% in the ustekinumab group.
In the BE READY trial, the rates of oral candidiasis were 6% and 0% for bimekizumab and placebo, respectively, through week 16. Over the maintenance periods, the rates were 9% and 11% for the every-8-week and every-4-week doses, respectively.
Discontinuation for adverse events was not higher on bimekizumab than placebo in either trial, nor was the proportion of serious treatment-emergent adverse events.
Nevertheless, the potential for adverse events was a key part of the discussion regarding the future role of bimekizumab, if approved, in an editorial that accompanied the publication of these studies.
“Bimekizumab might be our most effective biologic for psoriasis yet,” coauthors, William W. Huang, MD, PhD, associate professor of dermatology, and Steven R. Feldman, MD, PhD, professor of dermatology, both at Wake Forest University, Winston-Salem, NC, wrote in the editorial. “If the goal of psoriasis treatment is complete clearance, bimekizumab seems like a good option from an efficacy perspective.”
However, they noted that other IL-17 blockers, like secukinumab (Cosentyx) and brodalumab (Siliq), have been associated with risks, including the development of inflammatory bowel disease. In addition to the oral candidiasis seen in the BE VIVID and BE READY trials, they cautioned that other issues might arise with longer follow-up and greater numbers of patients exposed to this therapy.
In an interview, Dr. Feldman said adequately informed patients might be willing to accept these risks for the potential of greater efficacy, but he emphasized the need for appropriate warnings and education.
“We have a lot of very good treatments that offer patients an excellent chance of an excellent outcome – treatments that have been around and in use in large numbers of people for years,” Dr. Feldman said. “Unless the doctor and patient felt strongly about the need to use this new, perhaps more potent option, I would be personally inclined to use treatment with well-established safety profiles first.”
The senior author of the BE VIVID trial, Mark Lebwohl, MD, dean for clinical therapeutics and professor of dermatology, at the Icahn School of Medicine at Mount Sinai, New York, disagreed. He acknowledged that other agents targeting IL-17 have been associated with IBD, but risk of IBD is already elevated in patients with psoriasis and the risk appears to be lower with bimekizumab relative to prior agents in this class.
“Bimekizumab has now been studied in thousands of patients over several years. We can say with support from a sizable amount of data that IBD is very uncommon,” he said. While oral candidiasis is associated with bimekizumab, it is “easy to treat.”
Asked specifically if he will consider using bimekizumab as a first-line agent in psoriasis patients who are candidates for a biologic, Dr. Lebwohl said he would. Based on the evidence that this agent is more effective than other options and has manageable side effects, he believes it will be an important new treatment option.
Dr. Reich, Dr. Lebwohl, Dr. Gordon, and Dr. Feldman have financial relationships with multiple companies that produce therapies for psoriasis, including UCB Pharma, the sponsor of these studies.
with other biologics, according to data from two simultaneously published trials, one of which was a head-to-head comparison with ustekinumab.
In the head-to-head trial called BE VIVID, which included a placebo arm, there was a large advantage of bimekizumab over ustekinumab, a biologic that targets IL-12 and IL-23 and is approved for treating psoriasis, for both coprimary endpoints, according to a multinational group of investigators led by Kristian Reich, MD, PhD, professor of dermatology at the University Medical Center, Hamburg-Eppendorf, Germany.
The proportion of patients with skin clearance was not only greater but faster, “with responses observed after one dose,” Dr. Reich and coinvestigators reported.
The data from the BE VIVID trial was published simultaneously with the BE READY trial, which was placebo-controlled but did not include an active comparator.
Evaluated at week 16, the coprimary endpoints in both studies were skin clearance as measured by a Psoriasis Area Severity Index greater than 90% (PASI 90) and Investigators Global Assessment (IGA) score of 0 (clear) or 1 (almost clear).
In BE VIVID, 567 patients were randomized in 11 countries, including the United States. The dose of bimekizumab was 320 mg administered subcutaneously every 4 weeks. In a randomization scheme of 4:2:1, half as many patients (163) were randomized to ustekinumab (Stelara), which was administered in weight-based dosing of 45 mg or 90 mg at enrollment, at 4 weeks, and then every 12 weeks. The placebo arm had 83 patients. All were switched to bimekizumab at 16 weeks.
At week 16, PASI 90 was achieved in 85% of patients randomized to bimekizumab, compared with 50% of patients randomized to ustekinumab (P < .0001). The rate in the placebo group was 5%.
The bimekizumab advantage for an IGA response of 0 or 1 was of similar magnitude, relative to ustekinumab (84% vs. 53%; P < .0001) and placebo (5%). All secondary efficacy endpoints, such as PASI 90 at week 12 (85% vs. 44%) and PASI 100 at week 16 (59% vs. 21%), favored bimekizumab over ustekinumab.
In the BE READY trial, which evaluated the same dose and schedule of bimekizumab, the rates of PASI 90 at week 16 were 91% and 1% (P < .0001) for the experimental arm and placebo, respectively. The proportion of patients with an IGA score of 0 or 1 were 93% and 1% (P < .0001), respectively.
In BE READY, patients who achieved PASI 90 at week 16 were reallocated to receive bimekizumab every 4 weeks, bimekizumab every 8 weeks (also 320 mg), or placebo. Both schedules of bimekizumab maintained responses through week 56, according to the authors, led by Kenneth B. Gordon, MD, professor and chair of dermatology, Medical College of Wisconsin, Milwaukee.
In both trials, safety was evaluated over the first 16 weeks as well as over a subsequent maintenance period, which extended to 52 weeks in BE VIVID and 56 weeks in BE READY. For bimekizumab, oral candidiasis was the most common treatment-related adverse event. In BE VIVID, this adverse event was reported in 9% of bimekizumab patients, compared with 0% of either the ustekinumab or placebo groups, up to week 16. Out to week 52, the rates were 15% in the bimekizumab group and 1% in the ustekinumab group.
In the BE READY trial, the rates of oral candidiasis were 6% and 0% for bimekizumab and placebo, respectively, through week 16. Over the maintenance periods, the rates were 9% and 11% for the every-8-week and every-4-week doses, respectively.
Discontinuation for adverse events was not higher on bimekizumab than placebo in either trial, nor was the proportion of serious treatment-emergent adverse events.
Nevertheless, the potential for adverse events was a key part of the discussion regarding the future role of bimekizumab, if approved, in an editorial that accompanied the publication of these studies.
“Bimekizumab might be our most effective biologic for psoriasis yet,” coauthors, William W. Huang, MD, PhD, associate professor of dermatology, and Steven R. Feldman, MD, PhD, professor of dermatology, both at Wake Forest University, Winston-Salem, NC, wrote in the editorial. “If the goal of psoriasis treatment is complete clearance, bimekizumab seems like a good option from an efficacy perspective.”
However, they noted that other IL-17 blockers, like secukinumab (Cosentyx) and brodalumab (Siliq), have been associated with risks, including the development of inflammatory bowel disease. In addition to the oral candidiasis seen in the BE VIVID and BE READY trials, they cautioned that other issues might arise with longer follow-up and greater numbers of patients exposed to this therapy.
In an interview, Dr. Feldman said adequately informed patients might be willing to accept these risks for the potential of greater efficacy, but he emphasized the need for appropriate warnings and education.
“We have a lot of very good treatments that offer patients an excellent chance of an excellent outcome – treatments that have been around and in use in large numbers of people for years,” Dr. Feldman said. “Unless the doctor and patient felt strongly about the need to use this new, perhaps more potent option, I would be personally inclined to use treatment with well-established safety profiles first.”
The senior author of the BE VIVID trial, Mark Lebwohl, MD, dean for clinical therapeutics and professor of dermatology, at the Icahn School of Medicine at Mount Sinai, New York, disagreed. He acknowledged that other agents targeting IL-17 have been associated with IBD, but risk of IBD is already elevated in patients with psoriasis and the risk appears to be lower with bimekizumab relative to prior agents in this class.
“Bimekizumab has now been studied in thousands of patients over several years. We can say with support from a sizable amount of data that IBD is very uncommon,” he said. While oral candidiasis is associated with bimekizumab, it is “easy to treat.”
Asked specifically if he will consider using bimekizumab as a first-line agent in psoriasis patients who are candidates for a biologic, Dr. Lebwohl said he would. Based on the evidence that this agent is more effective than other options and has manageable side effects, he believes it will be an important new treatment option.
Dr. Reich, Dr. Lebwohl, Dr. Gordon, and Dr. Feldman have financial relationships with multiple companies that produce therapies for psoriasis, including UCB Pharma, the sponsor of these studies.
FROM THE LANCET
COVID-19: Another study links colchicine to better results
The gout drug colchicine appears to lower the severity of COVID-19, a small new Brazilian study finds, adding to evidence that the familiar medication holds promise as a treatment for hospitalized patients.
Patients who received colchicine in this randomized, double-blinded, placebo-controlled clinical trial presented better evolution in terms of the need for supplemental oxygen and the length of hospitalisation. ... Colchicine was safe and well tolerated,” the study authors wrote in RMD Open. However, deaths were rare in the trial, they added, and it is impossible to “evaluate the capacity of colchicine to avoid admission to ICU and reduce mortality.”
The oral anti-inflammatory colchicine, widely used as treatment in rheumatic disease, was first approved in the United States 60 years ago. Researchers began to explore its potential as a COVID-19 treatment in the early months of the pandemic.
On Jan. 25, an international team of researchers reported in a press release – but not yet a published paper – that the drug seemed to reduce hospitalizations, mechanical ventilation, and deaths in the ColCORONA trial. Earlier, a much-smaller, randomized, open-label, Greek trial linked the drug to reduced time to clinical deterioration and hospital stay.
The Brazilian authors of the new study, led by Maria Isabel Lopes of the University of São Paulo’s Ribeirão Preto Medical School, randomly assigned 75 hospitalized patients with moderate to severe COVID-19 to colchicine or placebo. A total of 72 subjects completed the April-August 2020 trial: 36 received colchicine (typically 0.5 mg three times for 5 days, then 0.5 mg twice daily for 5 days; doses were adjusted in low-weight patients and those with chronic kidney disease). The other 36 received the placebo.
(In the United States, 0.6-mg tablets of generic colchicine cost as little as $1.90 each with free coupons, according to goodrx.com.)
The median age in the groups was similar (55 years); and the placebo group had more women (61% vs. 47% in the colchicine group, P = .34). All 72 patients received the same COVID-19 treatment at the time of the trial: azithromycin, hydroxychloroquine, and unfractionated heparin. Most patients, about two-thirds in both groups, also received methylprednisolone because they needed higher amounts of supplemental oxygen.
Patients in the colchicine group needed supplemental oxygen for less time: Their median time of need was 4.0 days (interquartile range [IQR], 2.0-6.0) vs. 6.5 days (IQR, 4.0-9.0) for the placebo group (P < .001). The median time for hospitalization was also lower at 7.0 days (IQR, 5.0–9.0) for the colchicine group vs. 9.0 (IQR, 7.0–12.0) for the placebo group (log rank test, 10.6; P = .001).
The researchers also reported the percentage of patients who needed supplemental oxygen at day 2 as 67% with colchicine vs. 86% with placebo, and at day 7 as 9% vs. 42% (log rank test, 10.6; P = .001). Two patients in the placebo group died, both from ventilator-associated pneumonia.
As for side effects, new or worsened diarrhea was reported more often in the colchicine group (17% vs. 6% with placebo), but the difference was not statistically significant (P = .26), and diarrhea was controlled via medication.
The researchers reported that limitations include the exclusion criteria and their inability to link colchicine to rates of ICU admissions and death.
The drug appears to help patients with COVID-19, the study authors wrote, by “inhibiting inflammasome, reducing neutrophil migration and activation, or preventing endothelial damage.”
A “well-conceived and well-designed” study
In an interview, NYU Langone Health rheumatologist Michael H. Pillinger, MD – an investigator with the ColCORONA trial – praised the Brazilian study. It “appears well-conceived and well-designed, and was enrolled at a rate that was greater than the sample size that was estimated to be needed based on power analysis,” he said.
The Brazilian study is small, he noted. (In contrast, the ColCORONA trial had 4,488 outpatient participants.) “This study differs from ColCORONA in several ways – the most important being that it is a study of inpatients with moderate to severe COVID (really mostly moderate),” he added. “ColCORONA is looking at a target audience that is much larger – outpatients with mild to moderate COVID with risk factors for hospitalization. Both questions are really important and certainly not mutually exclusive, since our care remains inadequate in both venues. This study also adds value in that several other studies have been conducted in hospital patients with enrollment criteria relatively similar to this one, and all showed benefit, but those were open-label or retrospective, and this is blinded and placebo-controlled.”
Using colchicine in patients with COVID-19
Should physicians turn to colchicine in patients with COVID-19? “I would rather that it still be used in the context of research until formal recommendations can be made by bodies like the NIH and CDC,” Dr. Pillinger said. “But certainly, there may be times when physicians feel compelled to treat patients off label.”
He cautioned, however, that colchicine should never be used with some other drugs. Its interaction with the antibiotic clarithromycin can be fatal, he noted. And, he said, the drug must be monitored in general since it can cause rare, severe problems.
“Overall, colchicine probably works on the overabundant inflammatory response to COVID, and it may be that it can be combined with other drugs that affect viral replication or promote immunity – e.g. vaccines,” Dr. Pillinger said. “So far, it seems as if there is no safety problem with combining colchicine with other approaches, but this has not been studied in a rigorous manner.”
Moving forward, he said, the drug’s very low price outside of the United States “could provide resource-poor countries with a way to help keep patients out of precious hospital beds – or help them go home sooner once admitted.” For now, however, “we need a large-scale inpatient study, and one is currently going on in Great Britain. We also need validation of the outpatient ColCORONA study, and studies to look at whether colchicine can work in conjunction with other strategies.”
The study was funded by grants from the São Paulo Research Foundation, Brazilian National Council for Scientific and Technological Development, and CAPES Foundation. No disclosures are reported. Dr. Pillinger reports serving as an investigator for the ColCORONA trial and receiving a unrelated investigator-initiated grant from Hikma, a colchicine manufacturer.
The gout drug colchicine appears to lower the severity of COVID-19, a small new Brazilian study finds, adding to evidence that the familiar medication holds promise as a treatment for hospitalized patients.
Patients who received colchicine in this randomized, double-blinded, placebo-controlled clinical trial presented better evolution in terms of the need for supplemental oxygen and the length of hospitalisation. ... Colchicine was safe and well tolerated,” the study authors wrote in RMD Open. However, deaths were rare in the trial, they added, and it is impossible to “evaluate the capacity of colchicine to avoid admission to ICU and reduce mortality.”
The oral anti-inflammatory colchicine, widely used as treatment in rheumatic disease, was first approved in the United States 60 years ago. Researchers began to explore its potential as a COVID-19 treatment in the early months of the pandemic.
On Jan. 25, an international team of researchers reported in a press release – but not yet a published paper – that the drug seemed to reduce hospitalizations, mechanical ventilation, and deaths in the ColCORONA trial. Earlier, a much-smaller, randomized, open-label, Greek trial linked the drug to reduced time to clinical deterioration and hospital stay.
The Brazilian authors of the new study, led by Maria Isabel Lopes of the University of São Paulo’s Ribeirão Preto Medical School, randomly assigned 75 hospitalized patients with moderate to severe COVID-19 to colchicine or placebo. A total of 72 subjects completed the April-August 2020 trial: 36 received colchicine (typically 0.5 mg three times for 5 days, then 0.5 mg twice daily for 5 days; doses were adjusted in low-weight patients and those with chronic kidney disease). The other 36 received the placebo.
(In the United States, 0.6-mg tablets of generic colchicine cost as little as $1.90 each with free coupons, according to goodrx.com.)
The median age in the groups was similar (55 years); and the placebo group had more women (61% vs. 47% in the colchicine group, P = .34). All 72 patients received the same COVID-19 treatment at the time of the trial: azithromycin, hydroxychloroquine, and unfractionated heparin. Most patients, about two-thirds in both groups, also received methylprednisolone because they needed higher amounts of supplemental oxygen.
Patients in the colchicine group needed supplemental oxygen for less time: Their median time of need was 4.0 days (interquartile range [IQR], 2.0-6.0) vs. 6.5 days (IQR, 4.0-9.0) for the placebo group (P < .001). The median time for hospitalization was also lower at 7.0 days (IQR, 5.0–9.0) for the colchicine group vs. 9.0 (IQR, 7.0–12.0) for the placebo group (log rank test, 10.6; P = .001).
The researchers also reported the percentage of patients who needed supplemental oxygen at day 2 as 67% with colchicine vs. 86% with placebo, and at day 7 as 9% vs. 42% (log rank test, 10.6; P = .001). Two patients in the placebo group died, both from ventilator-associated pneumonia.
As for side effects, new or worsened diarrhea was reported more often in the colchicine group (17% vs. 6% with placebo), but the difference was not statistically significant (P = .26), and diarrhea was controlled via medication.
The researchers reported that limitations include the exclusion criteria and their inability to link colchicine to rates of ICU admissions and death.
The drug appears to help patients with COVID-19, the study authors wrote, by “inhibiting inflammasome, reducing neutrophil migration and activation, or preventing endothelial damage.”
A “well-conceived and well-designed” study
In an interview, NYU Langone Health rheumatologist Michael H. Pillinger, MD – an investigator with the ColCORONA trial – praised the Brazilian study. It “appears well-conceived and well-designed, and was enrolled at a rate that was greater than the sample size that was estimated to be needed based on power analysis,” he said.
The Brazilian study is small, he noted. (In contrast, the ColCORONA trial had 4,488 outpatient participants.) “This study differs from ColCORONA in several ways – the most important being that it is a study of inpatients with moderate to severe COVID (really mostly moderate),” he added. “ColCORONA is looking at a target audience that is much larger – outpatients with mild to moderate COVID with risk factors for hospitalization. Both questions are really important and certainly not mutually exclusive, since our care remains inadequate in both venues. This study also adds value in that several other studies have been conducted in hospital patients with enrollment criteria relatively similar to this one, and all showed benefit, but those were open-label or retrospective, and this is blinded and placebo-controlled.”
Using colchicine in patients with COVID-19
Should physicians turn to colchicine in patients with COVID-19? “I would rather that it still be used in the context of research until formal recommendations can be made by bodies like the NIH and CDC,” Dr. Pillinger said. “But certainly, there may be times when physicians feel compelled to treat patients off label.”
He cautioned, however, that colchicine should never be used with some other drugs. Its interaction with the antibiotic clarithromycin can be fatal, he noted. And, he said, the drug must be monitored in general since it can cause rare, severe problems.
“Overall, colchicine probably works on the overabundant inflammatory response to COVID, and it may be that it can be combined with other drugs that affect viral replication or promote immunity – e.g. vaccines,” Dr. Pillinger said. “So far, it seems as if there is no safety problem with combining colchicine with other approaches, but this has not been studied in a rigorous manner.”
Moving forward, he said, the drug’s very low price outside of the United States “could provide resource-poor countries with a way to help keep patients out of precious hospital beds – or help them go home sooner once admitted.” For now, however, “we need a large-scale inpatient study, and one is currently going on in Great Britain. We also need validation of the outpatient ColCORONA study, and studies to look at whether colchicine can work in conjunction with other strategies.”
The study was funded by grants from the São Paulo Research Foundation, Brazilian National Council for Scientific and Technological Development, and CAPES Foundation. No disclosures are reported. Dr. Pillinger reports serving as an investigator for the ColCORONA trial and receiving a unrelated investigator-initiated grant from Hikma, a colchicine manufacturer.
The gout drug colchicine appears to lower the severity of COVID-19, a small new Brazilian study finds, adding to evidence that the familiar medication holds promise as a treatment for hospitalized patients.
Patients who received colchicine in this randomized, double-blinded, placebo-controlled clinical trial presented better evolution in terms of the need for supplemental oxygen and the length of hospitalisation. ... Colchicine was safe and well tolerated,” the study authors wrote in RMD Open. However, deaths were rare in the trial, they added, and it is impossible to “evaluate the capacity of colchicine to avoid admission to ICU and reduce mortality.”
The oral anti-inflammatory colchicine, widely used as treatment in rheumatic disease, was first approved in the United States 60 years ago. Researchers began to explore its potential as a COVID-19 treatment in the early months of the pandemic.
On Jan. 25, an international team of researchers reported in a press release – but not yet a published paper – that the drug seemed to reduce hospitalizations, mechanical ventilation, and deaths in the ColCORONA trial. Earlier, a much-smaller, randomized, open-label, Greek trial linked the drug to reduced time to clinical deterioration and hospital stay.
The Brazilian authors of the new study, led by Maria Isabel Lopes of the University of São Paulo’s Ribeirão Preto Medical School, randomly assigned 75 hospitalized patients with moderate to severe COVID-19 to colchicine or placebo. A total of 72 subjects completed the April-August 2020 trial: 36 received colchicine (typically 0.5 mg three times for 5 days, then 0.5 mg twice daily for 5 days; doses were adjusted in low-weight patients and those with chronic kidney disease). The other 36 received the placebo.
(In the United States, 0.6-mg tablets of generic colchicine cost as little as $1.90 each with free coupons, according to goodrx.com.)
The median age in the groups was similar (55 years); and the placebo group had more women (61% vs. 47% in the colchicine group, P = .34). All 72 patients received the same COVID-19 treatment at the time of the trial: azithromycin, hydroxychloroquine, and unfractionated heparin. Most patients, about two-thirds in both groups, also received methylprednisolone because they needed higher amounts of supplemental oxygen.
Patients in the colchicine group needed supplemental oxygen for less time: Their median time of need was 4.0 days (interquartile range [IQR], 2.0-6.0) vs. 6.5 days (IQR, 4.0-9.0) for the placebo group (P < .001). The median time for hospitalization was also lower at 7.0 days (IQR, 5.0–9.0) for the colchicine group vs. 9.0 (IQR, 7.0–12.0) for the placebo group (log rank test, 10.6; P = .001).
The researchers also reported the percentage of patients who needed supplemental oxygen at day 2 as 67% with colchicine vs. 86% with placebo, and at day 7 as 9% vs. 42% (log rank test, 10.6; P = .001). Two patients in the placebo group died, both from ventilator-associated pneumonia.
As for side effects, new or worsened diarrhea was reported more often in the colchicine group (17% vs. 6% with placebo), but the difference was not statistically significant (P = .26), and diarrhea was controlled via medication.
The researchers reported that limitations include the exclusion criteria and their inability to link colchicine to rates of ICU admissions and death.
The drug appears to help patients with COVID-19, the study authors wrote, by “inhibiting inflammasome, reducing neutrophil migration and activation, or preventing endothelial damage.”
A “well-conceived and well-designed” study
In an interview, NYU Langone Health rheumatologist Michael H. Pillinger, MD – an investigator with the ColCORONA trial – praised the Brazilian study. It “appears well-conceived and well-designed, and was enrolled at a rate that was greater than the sample size that was estimated to be needed based on power analysis,” he said.
The Brazilian study is small, he noted. (In contrast, the ColCORONA trial had 4,488 outpatient participants.) “This study differs from ColCORONA in several ways – the most important being that it is a study of inpatients with moderate to severe COVID (really mostly moderate),” he added. “ColCORONA is looking at a target audience that is much larger – outpatients with mild to moderate COVID with risk factors for hospitalization. Both questions are really important and certainly not mutually exclusive, since our care remains inadequate in both venues. This study also adds value in that several other studies have been conducted in hospital patients with enrollment criteria relatively similar to this one, and all showed benefit, but those were open-label or retrospective, and this is blinded and placebo-controlled.”
Using colchicine in patients with COVID-19
Should physicians turn to colchicine in patients with COVID-19? “I would rather that it still be used in the context of research until formal recommendations can be made by bodies like the NIH and CDC,” Dr. Pillinger said. “But certainly, there may be times when physicians feel compelled to treat patients off label.”
He cautioned, however, that colchicine should never be used with some other drugs. Its interaction with the antibiotic clarithromycin can be fatal, he noted. And, he said, the drug must be monitored in general since it can cause rare, severe problems.
“Overall, colchicine probably works on the overabundant inflammatory response to COVID, and it may be that it can be combined with other drugs that affect viral replication or promote immunity – e.g. vaccines,” Dr. Pillinger said. “So far, it seems as if there is no safety problem with combining colchicine with other approaches, but this has not been studied in a rigorous manner.”
Moving forward, he said, the drug’s very low price outside of the United States “could provide resource-poor countries with a way to help keep patients out of precious hospital beds – or help them go home sooner once admitted.” For now, however, “we need a large-scale inpatient study, and one is currently going on in Great Britain. We also need validation of the outpatient ColCORONA study, and studies to look at whether colchicine can work in conjunction with other strategies.”
The study was funded by grants from the São Paulo Research Foundation, Brazilian National Council for Scientific and Technological Development, and CAPES Foundation. No disclosures are reported. Dr. Pillinger reports serving as an investigator for the ColCORONA trial and receiving a unrelated investigator-initiated grant from Hikma, a colchicine manufacturer.
FROM RMD OPEN
Drive By Flu-FIT: CRC screening in the COVID-19 era
The model is a socially distanced version of the Flu-Fecal Immunochemical Test (Flu-FIT) program, called Drive By Flu-FIT.
The original Flu-FIT program was designed to increase access to CRC screening by offering home FIT tests to patients at the time of their annual flu shots. The program has been shown to increase CRC screening in diverse populations.
Researchers wanted to determine if a drive-by version of Flu-FIT could counteract the decrease in CRC screening seen during the pandemic, so they conducted a pilot study.
“FIT-based CRC screening overcomes many of the challenges to colonoscopy-based screening due to COVID-19, [such as] not requiring an office visit, thereby overcoming workforce disruptions and many patient concerns,” explained investigator Armenta Washington of the University of Pennsylvania, Philadelphia.
Ms. Washington presented results with Drive By Flu-FIT at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S02-04).
About the study
The pilot study of Drive By Flu-FIT was conducted in collaboration with the Einstein Healthcare Network and Enon Tabernacle Baptist Church, the largest Baptist church in the Philadelphia region.
The program enrolled community members into one of three Drive By Flu-FIT events, which took place between October and November 2020. Eligible participants were aged 45-75 years and at average risk for CRC.
Interested candidates completed eligibility, registration, and demographic questionnaires electronically prior to enrollment.
Patients who enrolled watched a 7-minute CRC educational video and completed two questionnaires – one on CRC screening knowledge and one on screening intentions – before and after watching the video.
At the events, participants remained in their cars while physicians in personal protective equipment provided instructions on how to use the FIT and how to return the completed test to a medical collection box, as well as answering questions. Participants also had the option to receive a flu vaccine at the event.
Results
Among 335 registered participants, 80 (23.9%) did not ultimately attend an event, and 63 (18.8%) were deemed ineligible.
So 192 patients attended a Drive By Flu-FIT event and received a FIT (57.3%). Patients with symptoms/signs and family history of CRC were referred for colonoscopy.
Among patients who received a FIT, the mean age was 58.9 years, 60.4% were female, 93.8% self-identified as Black, 1.6% self-identified as Hispanic, 15.5% were uninsured, and 54.6% had been previously screened for CRC.
The researchers found that scores on the knowledge questionnaire increased after the video intervention (P = .0006), as did the intention to screen scores (P = .007).
“Baseline knowledge about CRC was high, with the exception of four items related to risk factors, frequency of FIT, Lynch syndrome, and the relationship between physical activity and the risk for CRC,” Ms. Washington explained. “All knowledge scores increased after the video, except for one item related to the early discovery of CRC and its relationship to survival.”
Among the 192 participants who received a FIT, 38 (19.7%) did not return it, 141 (73.4%) had a negative FIT result, and 13 (6.7%) had a positive FIT result and were referred to colonoscopy. The colonoscopy results are pending.
“Overall, we believe that this research shows that a social-distanced, Drive By Flu-FIT program is feasible, acceptable, and effective in engaging the community in CRC education and screening during the COVID-19 pandemic,” Ms. Washington said.
During a live discussion, Ms. Washington also noted that most patients opted to receive both the FIT test and the flu vaccine.
“This was certainly great work, especially with the outreach that was done,” commented moderator Ana Maria Lopez, MD, of Sidney Kimmel Medical College, Philadelphia.
The researchers plan to use the results of this pilot study to test and evaluate a Drive By COVID-19 vaccine-FIT model in spring 2021.
Ms. Washington and Dr. Lopez disclosed no conflicts of interest. The study was supported by the National Cancer Institute. The FITs were donated by Polymedco Inc., and the flu vaccines were donated by the Philadelphia Public Health Department.
The model is a socially distanced version of the Flu-Fecal Immunochemical Test (Flu-FIT) program, called Drive By Flu-FIT.
The original Flu-FIT program was designed to increase access to CRC screening by offering home FIT tests to patients at the time of their annual flu shots. The program has been shown to increase CRC screening in diverse populations.
Researchers wanted to determine if a drive-by version of Flu-FIT could counteract the decrease in CRC screening seen during the pandemic, so they conducted a pilot study.
“FIT-based CRC screening overcomes many of the challenges to colonoscopy-based screening due to COVID-19, [such as] not requiring an office visit, thereby overcoming workforce disruptions and many patient concerns,” explained investigator Armenta Washington of the University of Pennsylvania, Philadelphia.
Ms. Washington presented results with Drive By Flu-FIT at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S02-04).
About the study
The pilot study of Drive By Flu-FIT was conducted in collaboration with the Einstein Healthcare Network and Enon Tabernacle Baptist Church, the largest Baptist church in the Philadelphia region.
The program enrolled community members into one of three Drive By Flu-FIT events, which took place between October and November 2020. Eligible participants were aged 45-75 years and at average risk for CRC.
Interested candidates completed eligibility, registration, and demographic questionnaires electronically prior to enrollment.
Patients who enrolled watched a 7-minute CRC educational video and completed two questionnaires – one on CRC screening knowledge and one on screening intentions – before and after watching the video.
At the events, participants remained in their cars while physicians in personal protective equipment provided instructions on how to use the FIT and how to return the completed test to a medical collection box, as well as answering questions. Participants also had the option to receive a flu vaccine at the event.
Results
Among 335 registered participants, 80 (23.9%) did not ultimately attend an event, and 63 (18.8%) were deemed ineligible.
So 192 patients attended a Drive By Flu-FIT event and received a FIT (57.3%). Patients with symptoms/signs and family history of CRC were referred for colonoscopy.
Among patients who received a FIT, the mean age was 58.9 years, 60.4% were female, 93.8% self-identified as Black, 1.6% self-identified as Hispanic, 15.5% were uninsured, and 54.6% had been previously screened for CRC.
The researchers found that scores on the knowledge questionnaire increased after the video intervention (P = .0006), as did the intention to screen scores (P = .007).
“Baseline knowledge about CRC was high, with the exception of four items related to risk factors, frequency of FIT, Lynch syndrome, and the relationship between physical activity and the risk for CRC,” Ms. Washington explained. “All knowledge scores increased after the video, except for one item related to the early discovery of CRC and its relationship to survival.”
Among the 192 participants who received a FIT, 38 (19.7%) did not return it, 141 (73.4%) had a negative FIT result, and 13 (6.7%) had a positive FIT result and were referred to colonoscopy. The colonoscopy results are pending.
“Overall, we believe that this research shows that a social-distanced, Drive By Flu-FIT program is feasible, acceptable, and effective in engaging the community in CRC education and screening during the COVID-19 pandemic,” Ms. Washington said.
During a live discussion, Ms. Washington also noted that most patients opted to receive both the FIT test and the flu vaccine.
“This was certainly great work, especially with the outreach that was done,” commented moderator Ana Maria Lopez, MD, of Sidney Kimmel Medical College, Philadelphia.
The researchers plan to use the results of this pilot study to test and evaluate a Drive By COVID-19 vaccine-FIT model in spring 2021.
Ms. Washington and Dr. Lopez disclosed no conflicts of interest. The study was supported by the National Cancer Institute. The FITs were donated by Polymedco Inc., and the flu vaccines were donated by the Philadelphia Public Health Department.
The model is a socially distanced version of the Flu-Fecal Immunochemical Test (Flu-FIT) program, called Drive By Flu-FIT.
The original Flu-FIT program was designed to increase access to CRC screening by offering home FIT tests to patients at the time of their annual flu shots. The program has been shown to increase CRC screening in diverse populations.
Researchers wanted to determine if a drive-by version of Flu-FIT could counteract the decrease in CRC screening seen during the pandemic, so they conducted a pilot study.
“FIT-based CRC screening overcomes many of the challenges to colonoscopy-based screening due to COVID-19, [such as] not requiring an office visit, thereby overcoming workforce disruptions and many patient concerns,” explained investigator Armenta Washington of the University of Pennsylvania, Philadelphia.
Ms. Washington presented results with Drive By Flu-FIT at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S02-04).
About the study
The pilot study of Drive By Flu-FIT was conducted in collaboration with the Einstein Healthcare Network and Enon Tabernacle Baptist Church, the largest Baptist church in the Philadelphia region.
The program enrolled community members into one of three Drive By Flu-FIT events, which took place between October and November 2020. Eligible participants were aged 45-75 years and at average risk for CRC.
Interested candidates completed eligibility, registration, and demographic questionnaires electronically prior to enrollment.
Patients who enrolled watched a 7-minute CRC educational video and completed two questionnaires – one on CRC screening knowledge and one on screening intentions – before and after watching the video.
At the events, participants remained in their cars while physicians in personal protective equipment provided instructions on how to use the FIT and how to return the completed test to a medical collection box, as well as answering questions. Participants also had the option to receive a flu vaccine at the event.
Results
Among 335 registered participants, 80 (23.9%) did not ultimately attend an event, and 63 (18.8%) were deemed ineligible.
So 192 patients attended a Drive By Flu-FIT event and received a FIT (57.3%). Patients with symptoms/signs and family history of CRC were referred for colonoscopy.
Among patients who received a FIT, the mean age was 58.9 years, 60.4% were female, 93.8% self-identified as Black, 1.6% self-identified as Hispanic, 15.5% were uninsured, and 54.6% had been previously screened for CRC.
The researchers found that scores on the knowledge questionnaire increased after the video intervention (P = .0006), as did the intention to screen scores (P = .007).
“Baseline knowledge about CRC was high, with the exception of four items related to risk factors, frequency of FIT, Lynch syndrome, and the relationship between physical activity and the risk for CRC,” Ms. Washington explained. “All knowledge scores increased after the video, except for one item related to the early discovery of CRC and its relationship to survival.”
Among the 192 participants who received a FIT, 38 (19.7%) did not return it, 141 (73.4%) had a negative FIT result, and 13 (6.7%) had a positive FIT result and were referred to colonoscopy. The colonoscopy results are pending.
“Overall, we believe that this research shows that a social-distanced, Drive By Flu-FIT program is feasible, acceptable, and effective in engaging the community in CRC education and screening during the COVID-19 pandemic,” Ms. Washington said.
During a live discussion, Ms. Washington also noted that most patients opted to receive both the FIT test and the flu vaccine.
“This was certainly great work, especially with the outreach that was done,” commented moderator Ana Maria Lopez, MD, of Sidney Kimmel Medical College, Philadelphia.
The researchers plan to use the results of this pilot study to test and evaluate a Drive By COVID-19 vaccine-FIT model in spring 2021.
Ms. Washington and Dr. Lopez disclosed no conflicts of interest. The study was supported by the National Cancer Institute. The FITs were donated by Polymedco Inc., and the flu vaccines were donated by the Philadelphia Public Health Department.
FROM AACR: COVID-19 AND CANCER 2021
U.S. COVID-19 death toll passes 450,000
The United States has now reported more than 450,000 COVID-19 deaths during the pandemic, adding 3,912 more on Wednesday, according to data from Johns Hopkins University.
Daily COVID-19 deaths still remain high in the United States, though they’ve decreased slightly from the peak of 4,466 deaths on Jan. 12.
The United States also reported more than 121,000 new COVID-19 cases on Wednesday, which is down from a peak of more than 300,000 new cases on Tuesday. In total, more than 26.5 million people in the United States have been diagnosed with COVID-19, making up a quarter of the 104.5 million cases reported worldwide.
The 7-day average for COVID-19 hospitalizations and deaths continues to decline, according to the COVID Tracking Project. The 7-day average for hospitalizations is around 96,500, and the 7-day average for deaths is about 3,000. With the exception of Vermont, all states and territories have reported declines or no changes in their hospitalizations and deaths.
“We have seen the 7-day average for new deaths decrease for over a week. At the same time, states are reporting an average of 3,000 people dying per day,” the COVID Tracking Project wrote in a post on Twitter. “The data is hopeful and devastating.”
More than 2.2 million COVID-19 deaths have been reported worldwide. The United States continues to report the most deaths, followed by Brazil with 227,500, Mexico with 161,200, and India with 154,700 deaths.
The U.S. COVID-19 death toll could reach 496,000-534,000 by the end of February, according to a new forecast by the CDC, which includes models from 36 national groups. Deaths will likely decrease during the next 4 weeks, with about 11,300-22,600 deaths possibly reported during the last week of February.
The 534,000 total would equal about 1 death for every minute of the pandemic, according to CNN, given that the first U.S. death was reported on Feb. 29 last year.
A version of this article first appeared on WebMD.com.
The United States has now reported more than 450,000 COVID-19 deaths during the pandemic, adding 3,912 more on Wednesday, according to data from Johns Hopkins University.
Daily COVID-19 deaths still remain high in the United States, though they’ve decreased slightly from the peak of 4,466 deaths on Jan. 12.
The United States also reported more than 121,000 new COVID-19 cases on Wednesday, which is down from a peak of more than 300,000 new cases on Tuesday. In total, more than 26.5 million people in the United States have been diagnosed with COVID-19, making up a quarter of the 104.5 million cases reported worldwide.
The 7-day average for COVID-19 hospitalizations and deaths continues to decline, according to the COVID Tracking Project. The 7-day average for hospitalizations is around 96,500, and the 7-day average for deaths is about 3,000. With the exception of Vermont, all states and territories have reported declines or no changes in their hospitalizations and deaths.
“We have seen the 7-day average for new deaths decrease for over a week. At the same time, states are reporting an average of 3,000 people dying per day,” the COVID Tracking Project wrote in a post on Twitter. “The data is hopeful and devastating.”
More than 2.2 million COVID-19 deaths have been reported worldwide. The United States continues to report the most deaths, followed by Brazil with 227,500, Mexico with 161,200, and India with 154,700 deaths.
The U.S. COVID-19 death toll could reach 496,000-534,000 by the end of February, according to a new forecast by the CDC, which includes models from 36 national groups. Deaths will likely decrease during the next 4 weeks, with about 11,300-22,600 deaths possibly reported during the last week of February.
The 534,000 total would equal about 1 death for every minute of the pandemic, according to CNN, given that the first U.S. death was reported on Feb. 29 last year.
A version of this article first appeared on WebMD.com.
The United States has now reported more than 450,000 COVID-19 deaths during the pandemic, adding 3,912 more on Wednesday, according to data from Johns Hopkins University.
Daily COVID-19 deaths still remain high in the United States, though they’ve decreased slightly from the peak of 4,466 deaths on Jan. 12.
The United States also reported more than 121,000 new COVID-19 cases on Wednesday, which is down from a peak of more than 300,000 new cases on Tuesday. In total, more than 26.5 million people in the United States have been diagnosed with COVID-19, making up a quarter of the 104.5 million cases reported worldwide.
The 7-day average for COVID-19 hospitalizations and deaths continues to decline, according to the COVID Tracking Project. The 7-day average for hospitalizations is around 96,500, and the 7-day average for deaths is about 3,000. With the exception of Vermont, all states and territories have reported declines or no changes in their hospitalizations and deaths.
“We have seen the 7-day average for new deaths decrease for over a week. At the same time, states are reporting an average of 3,000 people dying per day,” the COVID Tracking Project wrote in a post on Twitter. “The data is hopeful and devastating.”
More than 2.2 million COVID-19 deaths have been reported worldwide. The United States continues to report the most deaths, followed by Brazil with 227,500, Mexico with 161,200, and India with 154,700 deaths.
The U.S. COVID-19 death toll could reach 496,000-534,000 by the end of February, according to a new forecast by the CDC, which includes models from 36 national groups. Deaths will likely decrease during the next 4 weeks, with about 11,300-22,600 deaths possibly reported during the last week of February.
The 534,000 total would equal about 1 death for every minute of the pandemic, according to CNN, given that the first U.S. death was reported on Feb. 29 last year.
A version of this article first appeared on WebMD.com.
Trends in Risk-Adjusted 28-Day Mortality Rates for Patients Hospitalized with COVID-19 in England
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 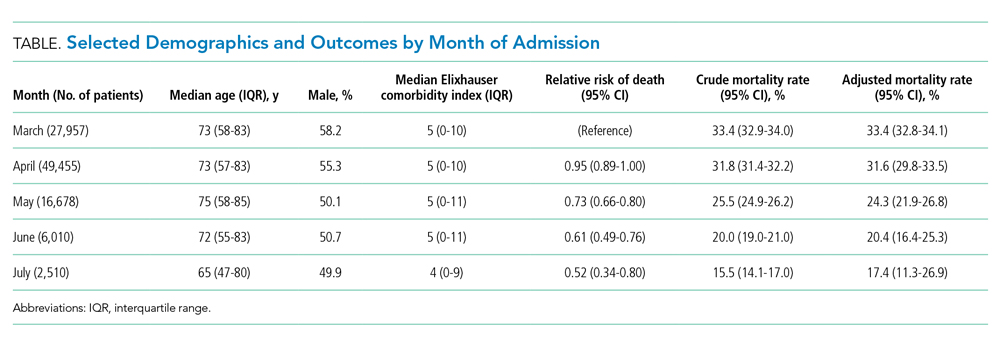
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.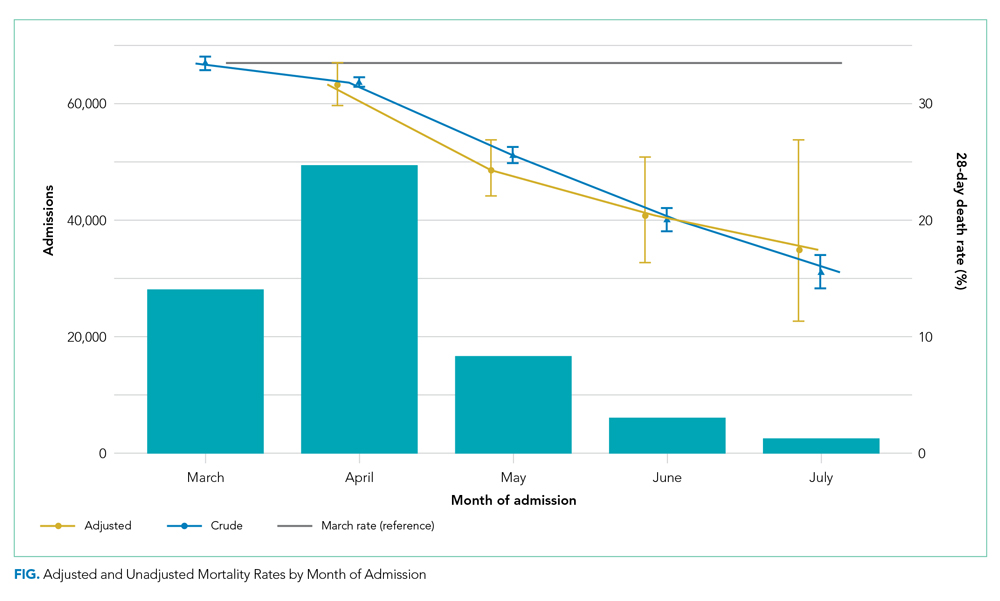
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
The early phase of the coronavirus disease 2019 (COVID-19) pandemic in the United Kingdom (UK) was characterized by uncertainty as clinicians grappled to understand and manage an unfamiliar disease that affected very high numbers of patients amid radically evolving working environments, with little evidence to support their efforts. Early reports indicated high mortality in patients hospitalized with COVID-19.
As the disease became better understood, treatment evolved and the mortality appears to have decreased. For example, two recent papers, a national study of critical care patients in the UK and a single-site study from New York, have demonstrated a significant reduction in adjusted mortality between the pre- and post-peak periods.1,2 However, the UK study was restricted to patients receiving critical care, potentially introducing bias due to varying critical care admission thresholds over time, while the single-site US study may not be generalizable. Moreover, both studies measured only in-hospital mortality. It remains uncertain therefore whether overall mortality has decreased on a broad scale after accounting for changes in patient characteristics.
The aim of this study was to use a national dataset to assess the
METHODS
We conducted a retrospective, secondary analysis of English National Health Services (NHS) hospitals’ admissions of patients at least 18 years of age between March 1 and July 31, 2020. Data were obtained from the Hospital Episode Statistics (HES) admitted patient care dataset.3 This is an administrative dataset that contains data on diagnoses and procedures as well as organizational characteristics and patient demographics for all NHS activity in England. We included all patients with an International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis of U07.1 (COVID-19, virus identified) and U07.2 (COVID-19, virus not identified).
The primary outcome of death within 28 days of admission was obtained by linking to the Civil Registrations (Deaths) - Secondary Care Cut - Information dataset, which includes the date, place, and cause of death from the Office for National Statistics4 and which was complete through September 31, 2020. The time horizon of 28 days from admission was chosen to approximate the Public Health England definition of a death from COVID-19 as being within 28 days of testing positive.5 We restricted our analysis to emergency admissions of persons age >18 years. If a patient had multiple emergency admissions, we restricted our analysis to the first admission to ensure comparability across hospitalizations and to best represent outcomes from the earliest onset of COVID-19.
We estimated a modified Poisson regression6 to predict death at 28 days, with month of admission, region, source of admission, age, deprivation, gender, ethnic group, and the 29 comorbidities in the Elixhauser comorbidity measure as variables in the regression.7 The derivation of each of these variables from the HES dataset is shown in Appendix Table 1.
Deprivation was measured by the Index of Multiple Deprivation, a methodology used widely within the UK to classify relative deprivation.8 To control for clustering, hospital system (known as Trust) was added as a random effect. Robust errors were estimated using the sandwich package.9 Modified Poisson regression was chosen in preference to the more common logistic regression because the coefficients can be interpreted as relative risks and not odds ratios. The model was fitted using R, version 4.0.3, geepack library.10 We carried out three sensitivity analyses, restricting to laboratory-confirmed COVID-19, length of stay ≥3 days, and primary respiratory disease.
For each month, we obtained a standardized mortality ratio (SMR) by fixing the month to the reference month of March 2020 and repredicting the outcome using the existing model. We calculated the ratio of the sum of observed and expected deaths (obtained from the model) in each month, comparing observed deaths to the number we would have expected had those patients been hospitalized in March. We then multiplied each period’s SMR by the March crude mortality to generate monthly adjusted mortality rates. We calculated Poisson confidence intervals around the SMR and used these to obtain confidence intervals for the adjusted rate. The binomial exact method was used to obtain confidence intervals for the crude rate. Multicollinearity was assessed using both the variance inflation factor (VIF) and the condition number test.7 All analyses used two-sided statistical tests, and we considered a P value < .05 to be statistically significant without adjustment for multiple testing. The study was exempt from UK National Research Ethics Committee approval because it involved secondary analysis of anonymized data.
RESULTS
The dataset included 115,643 emergency admissions from 179 healthcare systems, of which 103,202 were first admissions eligible for inclusion. A total of 592 patients were excluded due to missing demographic data (0.5%), resulting in 102,610 admissions included in the analysis. Peak hospitalizations occurred in late March to mid April, accounting for 44% of the hospitalizations (Table). Median length of stay for patients who died was 7 days (interquartile range, 3-12). The median age and number of Elixhauser comorbidities decreased in July. The proportion of men decreased between May and July. 
The modified Poisson regression had a C statistic of 0.743 (95% CI, 0.740-0.746) (Appendix Table 4). The VIF and condition number test found no evidence of multicollinearity.11
Adjusted mortality decreased each month, from 33.4% in March to 17.4% in July (Figure). The relative risk of death declined progressively to a minimum of 0.52 (95% CI, 0.34-0.80) in July, compared to March.
Admission from another hospital and being female were associated with reduced risk of death. Admission from a skilled nursing facility and being >75 years were associated with increased risk of death. Ten of the 29 Elixhauser comorbidities were associated with increased risk of mortality (cardiac arrhythmia, peripheral vascular disease, other neurologic disorders, renal failure, lymphoma, metastatic cancer, solid tumor without metastasis, coagulopathy, fluid and electrolyte disorders, and anemia). Deprivation and ethnic group were not associated with death among hospitalized patients.
DISCUSSION
Our study of all emergency hospital admissions in England during the first wave of the COVID-19 pandemic demonstrated that, even after adjusting for patient comorbidity and risk factors, the mortality rate decreased by approximately half over the first 5 months. Although the demographics of hospitalized patients changed over that period (with both the median age and the number of comorbidities decreasing), this does not fully explain the decrease in mortality. It is therefore likely that the decrease is due, at least in part, to an improvement in treatment and/or a reduction in hospital strain.
For example, initially the use of corticosteroids was controversial, in part due to previous experience with severe acute respiratory syndrome and Middle East respiratory syndrome (in which a Cochrane review demonstrated no benefit but potential harm). However, this changed as a result of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,12 which showed a significant survival benefit.One of the positive defining characteristics of the COVID-19 pandemic has been the intensive collaborative research effort combined with the rapid dissemination and discussion of new management protocols. The RECOVERY trial randomly assigned >11,000 participants in just 3 months, amounting to approximately 15% of all patients hospitalized with COVID-19 in the UK. Its results were widely publicized via professional networks and rapidly adopted into widespread clinical practice.
Examples of other changes include a higher threshold for mechanical ventilation (and a lower threshold for noninvasive ventilation), increased clinician experience, and, potentially, a reduced viral load arising from increased social distancing and mask wearing. Finally, the hospitals and staff themselves were under enormous physical and mental strain in the early months from multiple factors, including unfamiliar working environments, the large-scale redeployment of inexperienced staff, and very high numbers of patients with an unfamiliar disease. These factors all lessened as the initial peak passed. It is therefore likely that the reduction in adjusted mortality we observed arises from a combination of all these factors, as well as other incremental benefits.
The factors associated with increased mortality risk in our study (increasing age, male gender, certain comorbidities, and frailty [with care home residency acting as a proxy in our study]) are consistent with multiple previous reports. Although not the focus of our analysis, we found no effect of ethnicity or deprivation on mortality. This is consistent with many US studies that demonstrate that the widely reported effect of these factors is likely due to differences in exposure to the disease. Once patients are hospitalized, adjusted mortality risks are similar across ethnic groups and deprivation levels.
The strengths of this study include complete capture of hospitalizations across all hospitals and areas in England. Likewise, linking the hospital data to death data from the Office for National Statistics allows complete capture of outcomes, irrespective of where the patient died. This is a significant strength compared to prior studies, which only included in-hospital mortality. Our results are therefore likely robust and a true observation of the mortality trend.
Limitations include the lack of physiologic and laboratory data; having these would have allowed us to adjust for disease severity on admission and strengthened the risk stratification. Likewise, although the complete national coverage is overall a significant strength, aggregating data from numerous areas that might be at different stages of local outbreaks, have different management strategies, and have differing data quality introduces its own biases.
Furthermore, these results predate the second wave in the UK, so we cannot distinguish whether the reduced mortality is due to improved treatment, a seasonal effect, evolution of the virus itself, or a reduction in the strain on hospitals.
CONCLUSION
This nationwide study indicates that, even after accounting for changing patient characteristics, the mortality of patients hospitalized with COVID-19 in England decreased significantly as the outbreak progressed. This is likely due to a combination of incremental treatment improvements.
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
1. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2020;16(2):90-92. https://doi.org/10.12788/jhm.3552
2. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021;49(2):209-214. https://doi.org/10.1097/CCM.0000000000004747
3. NHS Digital. Hospital Episode Statistics Data Dictionary. Published March 2018. Accessed October 15, 2020. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/hospital-episode-statistics-data-dictionary
4. NHS Digital. HES-ONS Linked Mortality Data Dictionary. Accessed October 15, 2020. https://digital.nhs.uk/binaries/content/assets/legacy/word/i/p/hes-ons_linked_mortality_data_dictionary_-_mar_20181.docx
5. Public Health England. Technical summary: Public Health England data series on deaths in people with COVID-19. Accessed November 11, 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/916035/RA_Technical_Summary_-_PHE_Data_Series_COVID_19_Deaths_20200812.pdf
6. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. https://doi.org/10.1093/aje/kwh090
7. van Walraven C, Austin PC, Jennings A, et al. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org /10.1097/MLR.0b013e31819432e5
8. Ministry of Housing Communities & Local Government. The English Indices of Deprivation 2019 (IoD2019). Published September 26, 2020. Accessed January 15, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf
9. Zeileis A. Object-oriented computation of sandwich estimators. J Stat Software. 2006;16:1-16. https://doi.org/10.18637/jss.v016.i09
10. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Software. 2006;15:1-11. https://doi.org/10.18637/jss.v015.i02
11. Belsley DA, Kuh E, Welsch RE. Diagnostics: Identifying Influential Data and Sources of Collinearity. John Wiley & Sons; 1980.
12. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. https://doi.org/10.1056/NEJMoa2021436
© 2021 Society of Hospital Medicine
Higher dietary fiber tied to lower depression risk in young women
Higher fiber intake may be associated with decreased risk of depression in premenopausal women, new research suggests.
Investigators analyzed data from close to 6,000 pre- and postmenopausal women. They found that, in premenopausal women, dietary fiber intake was higher among those without depression versus their counterparts with the disorder in a dose-dependent manner. However, there appeared to be no relationship between higher fiber intake and depression risk in postmenopausal women.
“We think the most important finding of our study is that dietary fiber intake was inversely associated with depression in premenopausal but not postmenopausal women,” lead author Yunsun Kim, MD, resident, department of family medicine, Chung-Ang University Hospital, Seoul, South Korea, said in an interview.
“We hope that the findings of this study could form the basis of future investigations to determine the causal relationship between dietary fiber intake and depression,” she added.
The study was published online Dec. 21, 2020, in Menopause.
Gut-brain interaction
The prevalence of depression is twice as high in women, compared with men, which may be attributable to a number of factors, including hormonal status – especially during menstruation and menopause, the authors wrote.
Previous research suggests a potential association between dietary fiber and depression in premenopausal women and between estrogen and gut microbiota. Fiber intake has an impact on gut microbiota, Dr. Kim said.
“We are motivated by the fact that depression provokes disease burden internationally and we would like to find modifiable factors that could prevent depression, especially in women, who are more vulnerable to depression,” she noted.
To investigate, the researchers drew on data from the Korea National Health and Nutrition Examination Survey for 2014, 2016, and 2018. Of the total number of women who met inclusion criteria (n = 5807; mean age, 47.11), roughly half were premenopausal and half were postmenopausal (n = 2,949 [mean age, 36.23 years] and n = 2,868 [mean age, 62.73], respectively).
Dietary fiber intake was assessed using the 24-hour dietary recall method, while depression was assessed using the Patient Health Questionnaire-9. The researchers used the Dietary Reference Intakes for Koreans to define a sufficient intake of dietary fiber (i.e., 12 g/1,000 kcal).
Covariates included chronic diseases, body mass index, medications, smoking status, alcohol use, physical activity, and sociodemographic factors.
When the researchers looked at all participants, they found that the estimated mean dietary fiber intake was significantly higher in women without depression, compared with those with depression (14.07 g/1,000 kcal/d; 95% confidence interval, 13.85-14.29 vs. 12.67 g/1,000 kcal/d; 95% CI, 11.79-13.55; P = .003).
Although the relationship remained significant in premenopausal women, it lost significance in postmenopausal women.
A 5% decrease in the prevalence of depression in premenopausal (but not postmenopausal) women was found in those with an increased intake of dietary fiber – i..e, there was a 1-g increase for every 1,000 kcal of daily energy intake, after adjusting for potential confounders in premenopausal women (OR, 0.949; 95% CI, 0.906-0.993]).
“The inverse relationship between dietary fiber intake and depression could be explained by the gut-brain interactions,” said Dr. Kim.
“Changes in the gut microbiota composition may affect neurotransmission and various neuropsychiatric phenomena in the brain,” she said, noting that previous studies have suggested that dietary fiber intake “may modulate the richness and diversity of the gut microbiota, and this change may promote brain health by affecting neurotransmission.”
Because postmenopausal women experience estrogen depletion, “the decreased interaction between estrogen and the gut microbiota may be related to the insignificant association between dietary fiber intake and depression in postmenopausal women,” she said.
Despite the lack of a significant association between postmenopausal depression and fiber intake, Dr. Kim said she “advises middle-aged women to have dietary fiber–rich diets, regardless of their menopausal status.”
Link between food and mood
In a comment, Stephanie S. Faubion, MD, MBA, a professor and chair of the department of medicine and the Penny and Bill George director of the Mayo Clinic’s Center for Women’s Health in Rochester, Minn., noted the study was cross-sectional and therefore the direction of the association could not be determined and “causality cannot be assumed.”
It is possible that “depressed women are less likely to eat fiber than women without depression. For example, a depressed woman may be more likely to sit on the couch eating Cheetos than shopping for and preparing a healthy meal,” said Dr. Faubion, who is also the medical director of the North American Menopause Society and was not involved with the study.
She noted that other potential confounders, including access to fresh fruits and vegetables or geographic locations could also “impact the findings and .”
Nevertheless, the study does “add to the body of evidence suggesting a link between diet and overall health, including brain health,” Dr. Faubion said.
One take-home message for practicing clinicians is that a healthy diet that includes fiber may benefit women (and men) for a number of reasons and “appears to be linked to mood.”
More research is needed “to determine the pathophysiologic mechanisms (such as potential brain-gut connection that involves the microbiome) that may explain this association,” Dr. Faubion added.
No source of funding listed. Dr. Kim and coauthors, as well as Dr. Faubion, disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher fiber intake may be associated with decreased risk of depression in premenopausal women, new research suggests.
Investigators analyzed data from close to 6,000 pre- and postmenopausal women. They found that, in premenopausal women, dietary fiber intake was higher among those without depression versus their counterparts with the disorder in a dose-dependent manner. However, there appeared to be no relationship between higher fiber intake and depression risk in postmenopausal women.
“We think the most important finding of our study is that dietary fiber intake was inversely associated with depression in premenopausal but not postmenopausal women,” lead author Yunsun Kim, MD, resident, department of family medicine, Chung-Ang University Hospital, Seoul, South Korea, said in an interview.
“We hope that the findings of this study could form the basis of future investigations to determine the causal relationship between dietary fiber intake and depression,” she added.
The study was published online Dec. 21, 2020, in Menopause.
Gut-brain interaction
The prevalence of depression is twice as high in women, compared with men, which may be attributable to a number of factors, including hormonal status – especially during menstruation and menopause, the authors wrote.
Previous research suggests a potential association between dietary fiber and depression in premenopausal women and between estrogen and gut microbiota. Fiber intake has an impact on gut microbiota, Dr. Kim said.
“We are motivated by the fact that depression provokes disease burden internationally and we would like to find modifiable factors that could prevent depression, especially in women, who are more vulnerable to depression,” she noted.
To investigate, the researchers drew on data from the Korea National Health and Nutrition Examination Survey for 2014, 2016, and 2018. Of the total number of women who met inclusion criteria (n = 5807; mean age, 47.11), roughly half were premenopausal and half were postmenopausal (n = 2,949 [mean age, 36.23 years] and n = 2,868 [mean age, 62.73], respectively).
Dietary fiber intake was assessed using the 24-hour dietary recall method, while depression was assessed using the Patient Health Questionnaire-9. The researchers used the Dietary Reference Intakes for Koreans to define a sufficient intake of dietary fiber (i.e., 12 g/1,000 kcal).
Covariates included chronic diseases, body mass index, medications, smoking status, alcohol use, physical activity, and sociodemographic factors.
When the researchers looked at all participants, they found that the estimated mean dietary fiber intake was significantly higher in women without depression, compared with those with depression (14.07 g/1,000 kcal/d; 95% confidence interval, 13.85-14.29 vs. 12.67 g/1,000 kcal/d; 95% CI, 11.79-13.55; P = .003).
Although the relationship remained significant in premenopausal women, it lost significance in postmenopausal women.
A 5% decrease in the prevalence of depression in premenopausal (but not postmenopausal) women was found in those with an increased intake of dietary fiber – i..e, there was a 1-g increase for every 1,000 kcal of daily energy intake, after adjusting for potential confounders in premenopausal women (OR, 0.949; 95% CI, 0.906-0.993]).
“The inverse relationship between dietary fiber intake and depression could be explained by the gut-brain interactions,” said Dr. Kim.
“Changes in the gut microbiota composition may affect neurotransmission and various neuropsychiatric phenomena in the brain,” she said, noting that previous studies have suggested that dietary fiber intake “may modulate the richness and diversity of the gut microbiota, and this change may promote brain health by affecting neurotransmission.”
Because postmenopausal women experience estrogen depletion, “the decreased interaction between estrogen and the gut microbiota may be related to the insignificant association between dietary fiber intake and depression in postmenopausal women,” she said.
Despite the lack of a significant association between postmenopausal depression and fiber intake, Dr. Kim said she “advises middle-aged women to have dietary fiber–rich diets, regardless of their menopausal status.”
Link between food and mood
In a comment, Stephanie S. Faubion, MD, MBA, a professor and chair of the department of medicine and the Penny and Bill George director of the Mayo Clinic’s Center for Women’s Health in Rochester, Minn., noted the study was cross-sectional and therefore the direction of the association could not be determined and “causality cannot be assumed.”
It is possible that “depressed women are less likely to eat fiber than women without depression. For example, a depressed woman may be more likely to sit on the couch eating Cheetos than shopping for and preparing a healthy meal,” said Dr. Faubion, who is also the medical director of the North American Menopause Society and was not involved with the study.
She noted that other potential confounders, including access to fresh fruits and vegetables or geographic locations could also “impact the findings and .”
Nevertheless, the study does “add to the body of evidence suggesting a link between diet and overall health, including brain health,” Dr. Faubion said.
One take-home message for practicing clinicians is that a healthy diet that includes fiber may benefit women (and men) for a number of reasons and “appears to be linked to mood.”
More research is needed “to determine the pathophysiologic mechanisms (such as potential brain-gut connection that involves the microbiome) that may explain this association,” Dr. Faubion added.
No source of funding listed. Dr. Kim and coauthors, as well as Dr. Faubion, disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher fiber intake may be associated with decreased risk of depression in premenopausal women, new research suggests.
Investigators analyzed data from close to 6,000 pre- and postmenopausal women. They found that, in premenopausal women, dietary fiber intake was higher among those without depression versus their counterparts with the disorder in a dose-dependent manner. However, there appeared to be no relationship between higher fiber intake and depression risk in postmenopausal women.
“We think the most important finding of our study is that dietary fiber intake was inversely associated with depression in premenopausal but not postmenopausal women,” lead author Yunsun Kim, MD, resident, department of family medicine, Chung-Ang University Hospital, Seoul, South Korea, said in an interview.
“We hope that the findings of this study could form the basis of future investigations to determine the causal relationship between dietary fiber intake and depression,” she added.
The study was published online Dec. 21, 2020, in Menopause.
Gut-brain interaction
The prevalence of depression is twice as high in women, compared with men, which may be attributable to a number of factors, including hormonal status – especially during menstruation and menopause, the authors wrote.
Previous research suggests a potential association between dietary fiber and depression in premenopausal women and between estrogen and gut microbiota. Fiber intake has an impact on gut microbiota, Dr. Kim said.
“We are motivated by the fact that depression provokes disease burden internationally and we would like to find modifiable factors that could prevent depression, especially in women, who are more vulnerable to depression,” she noted.
To investigate, the researchers drew on data from the Korea National Health and Nutrition Examination Survey for 2014, 2016, and 2018. Of the total number of women who met inclusion criteria (n = 5807; mean age, 47.11), roughly half were premenopausal and half were postmenopausal (n = 2,949 [mean age, 36.23 years] and n = 2,868 [mean age, 62.73], respectively).
Dietary fiber intake was assessed using the 24-hour dietary recall method, while depression was assessed using the Patient Health Questionnaire-9. The researchers used the Dietary Reference Intakes for Koreans to define a sufficient intake of dietary fiber (i.e., 12 g/1,000 kcal).
Covariates included chronic diseases, body mass index, medications, smoking status, alcohol use, physical activity, and sociodemographic factors.
When the researchers looked at all participants, they found that the estimated mean dietary fiber intake was significantly higher in women without depression, compared with those with depression (14.07 g/1,000 kcal/d; 95% confidence interval, 13.85-14.29 vs. 12.67 g/1,000 kcal/d; 95% CI, 11.79-13.55; P = .003).
Although the relationship remained significant in premenopausal women, it lost significance in postmenopausal women.
A 5% decrease in the prevalence of depression in premenopausal (but not postmenopausal) women was found in those with an increased intake of dietary fiber – i..e, there was a 1-g increase for every 1,000 kcal of daily energy intake, after adjusting for potential confounders in premenopausal women (OR, 0.949; 95% CI, 0.906-0.993]).
“The inverse relationship between dietary fiber intake and depression could be explained by the gut-brain interactions,” said Dr. Kim.
“Changes in the gut microbiota composition may affect neurotransmission and various neuropsychiatric phenomena in the brain,” she said, noting that previous studies have suggested that dietary fiber intake “may modulate the richness and diversity of the gut microbiota, and this change may promote brain health by affecting neurotransmission.”
Because postmenopausal women experience estrogen depletion, “the decreased interaction between estrogen and the gut microbiota may be related to the insignificant association between dietary fiber intake and depression in postmenopausal women,” she said.
Despite the lack of a significant association between postmenopausal depression and fiber intake, Dr. Kim said she “advises middle-aged women to have dietary fiber–rich diets, regardless of their menopausal status.”
Link between food and mood
In a comment, Stephanie S. Faubion, MD, MBA, a professor and chair of the department of medicine and the Penny and Bill George director of the Mayo Clinic’s Center for Women’s Health in Rochester, Minn., noted the study was cross-sectional and therefore the direction of the association could not be determined and “causality cannot be assumed.”
It is possible that “depressed women are less likely to eat fiber than women without depression. For example, a depressed woman may be more likely to sit on the couch eating Cheetos than shopping for and preparing a healthy meal,” said Dr. Faubion, who is also the medical director of the North American Menopause Society and was not involved with the study.
She noted that other potential confounders, including access to fresh fruits and vegetables or geographic locations could also “impact the findings and .”
Nevertheless, the study does “add to the body of evidence suggesting a link between diet and overall health, including brain health,” Dr. Faubion said.
One take-home message for practicing clinicians is that a healthy diet that includes fiber may benefit women (and men) for a number of reasons and “appears to be linked to mood.”
More research is needed “to determine the pathophysiologic mechanisms (such as potential brain-gut connection that involves the microbiome) that may explain this association,” Dr. Faubion added.
No source of funding listed. Dr. Kim and coauthors, as well as Dr. Faubion, disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cervical cancer screening: Should my practice switch to primary HPV testing?
How should I be approaching cervical cancer screening: with primary human papillomavirus (HPV) testing, or cotesting? We get this question all the time from clinicians. Although they have heard of the latest cervical cancer screening guidelines for stand-alone “primary” HPV testing, they are still ordering cervical cytology (Papanicolaou, or Pap, test) for women aged 21 to 29 years and cotesting (cervical cytology with HPV testing) for women with a cervix aged 30 and older.
Changes in cervical cancer testing guidance
Cervical cancer occurs in more than 13,000 women in the United States annually.1 High-risk types of HPV—the known cause of cervical cancer—also cause a large majority of cancers of the anus, vagina, vulva, and oropharynx.2
Cervical cancer screening programs in the United States have markedly decreased the incidence of and mortality from cervical cancer since introduction of the Pap smear in the 1950s. In 2000, HPV testing was approved by the US Food and Drug Administration (FDA) as a reflex test to a Pap smear result of atypical squamous cells of undetermined significance (ASC-US). HPV testing was then approved for use with cytology as a cotest in 2003 and subsequently as a primary stand-alone test in 2014.
Recently, the American Cancer Society (ACS) released new cervical screening guidelines that depart from prior guidelines.3 They recommend not to screen 21- to 24-year-olds and to start screening at age 25 until age 65 with the preferred strategy of primary HPV testing every 5 years, using an FDA-approved HPV test. Alternative screening strategies are cytology (Pap) every 3 years or cotesting every 5 years.
The 2018 US Preventive Services Task Force (USPSTF) guidelines differ from the ACS guidelines. The USPSTF recommends cytology every 3 years as the preferred method for women with a cervix who are aged 21 to 29 years and, for women with a cervix who are aged 30 to 65 years, the option for cytology every 3 years, primary HPV testing every 5 years, or cotesting every 5 years (TABLE).4
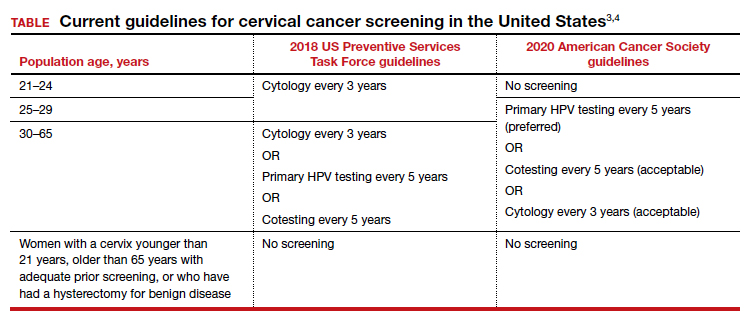
Why the reluctance to switch to HPV testing?
Despite FDA approval in 2014 for primary HPV testing and concurrent professional society guidance to use this testing strategy in women with a cervix who are aged 25 years and older, few practices in the United States have switched over to primary HPV testing for cervical cancer screening.5,6 Several reasons underlie this inertia:
- Many practices currently use HPV tests that are not FDA approved for primary HPV testing.
- Until recently, national screening guidelines did not recommend primary HPV testing as the preferred testing strategy.
- Long-established guidance on the importance of regular cervical cytology screening promoted by the ACS and others (which especially impacts women with a cervix older than age 50 who guide their younger daughters) will rely on significant re-education to move away from the established “Pap smear” cultural icon to a new approach.
- Last but not least, companies that manufacture HPV tests and laboratories integrated to offer such tests not yet approved for primary screening are promoting reliance on the prior proven cotest strategy. They have lobbied to preserve cotesting as a primary test, with some laboratory database studies showing gaps in detection with HPV test screening alone.7-9
Currently, the FDA-approved HPV tests for primary HPV screening include the Cobas HPV test (Roche) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Both are DNA tests for 14 high-risk types of HPV that include genotyping for HPV 16 and 18.
Continue to: Follow the evidence...
Follow the evidence
Several trials in Europe and Canada provide supporting evidence for primary HPV testing, and many European countries have moved to primary HPV testing as their preferred screening method.10,11 The new ACS guidelines put us more in sync with the rest of the world, where HPV testing is the dominant strategy.
It is true that doing additional tests will find more disease; cotesting has been shown to very minimally increase detection of cervical intraepithelial neoplasia grade 2/3 (CIN 2/3) compared with HPV testing alone, but it incurs many more costs and procedures.12 The vast majority of cervical cancer is HPV positive, and cytology still can be used as a triage to primary HPV screening until tests with better sensitivity and/or specificity (such as dual stain and methylation) can be employed to reduce unnecessary “false-positive” driven procedures.
As mentioned, many strong forces are trying to keep cotesting as the preferred strategy. It is important for clinicians to recognize the corporate investment into screening platforms, relationships, and products that underlie some of these efforts so as not to be unfairly influenced by their lobbying. Data from well-conducted, high-quality studies should be the evidence on which one bases a cervical cancer screening strategy.
Innovation catalyzes change
We acknowledge that it is difficult to give up something you have been doing for decades, so there is natural resistance by both patients and clinicians to move the Pap smear into a secondary role. But the data support primary HPV testing as the best screening option from a public health perspective.
At some point, hopefully soon, primary HPV testing will receive approval for self-sampling; this has the potential to reach patients in rural or remote locations who may otherwise not get screened for cervical cancer.13
The 2019 risk-based management guidelines from the ASCCP (American Society for Colposcopy and Cervical Pathology) also incorporate the use of HPV-based screening and surveillance after abnormal tests or colposcopy. Therefore, switching to primary HPV screening will not impact your ability to follow patients appropriately based on clinical guidelines.
Our advice to clinicians is to switch to primary HPV screening now if possible. If that is not feasible, continue your current strategy until you can make the change. And, of course, we recommend that you implement an HPV vaccination program in your practice to maximize primary prevention of HPV-related cancers. ●
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30.
- Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers–United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- US Preventive Services Task Force; Curry SJ, KristAH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337.
- Cooper CP, Saraiya M. Cervical cancer screening intervals preferred by US women. Am J Prev Med. 2018;55:389-394.
- Austin RM, Onisko A, Zhao C. Enhanced detection of cervical cancer and precancer through use of imaged liquid-based cytology in routine cytology and HPV cotesting. Am J Clin Pathol. 2018;150:385-392.
- Kaufman HW, Alagia DP, Chen Z, et al. Contributions of liquid-based (Papanicolaou) cytology and human papillomavirus testing in cotesting for detection of cervical cancer and precancer in the United States. Am J Clin Pathol. 2020;154:510-516.
- Blatt AJ, Kennedy R, Luff RD, et al. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:282-288.
- Ronco G, Dillner J, Elfstrom KM, et al; International HPV Screening Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Kim JJ, Burger EA, Regan C, et al. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320:706-714.
- Arbyn M, Smith SB, Temin S, et al; on behalf of the Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
How should I be approaching cervical cancer screening: with primary human papillomavirus (HPV) testing, or cotesting? We get this question all the time from clinicians. Although they have heard of the latest cervical cancer screening guidelines for stand-alone “primary” HPV testing, they are still ordering cervical cytology (Papanicolaou, or Pap, test) for women aged 21 to 29 years and cotesting (cervical cytology with HPV testing) for women with a cervix aged 30 and older.
Changes in cervical cancer testing guidance
Cervical cancer occurs in more than 13,000 women in the United States annually.1 High-risk types of HPV—the known cause of cervical cancer—also cause a large majority of cancers of the anus, vagina, vulva, and oropharynx.2
Cervical cancer screening programs in the United States have markedly decreased the incidence of and mortality from cervical cancer since introduction of the Pap smear in the 1950s. In 2000, HPV testing was approved by the US Food and Drug Administration (FDA) as a reflex test to a Pap smear result of atypical squamous cells of undetermined significance (ASC-US). HPV testing was then approved for use with cytology as a cotest in 2003 and subsequently as a primary stand-alone test in 2014.
Recently, the American Cancer Society (ACS) released new cervical screening guidelines that depart from prior guidelines.3 They recommend not to screen 21- to 24-year-olds and to start screening at age 25 until age 65 with the preferred strategy of primary HPV testing every 5 years, using an FDA-approved HPV test. Alternative screening strategies are cytology (Pap) every 3 years or cotesting every 5 years.
The 2018 US Preventive Services Task Force (USPSTF) guidelines differ from the ACS guidelines. The USPSTF recommends cytology every 3 years as the preferred method for women with a cervix who are aged 21 to 29 years and, for women with a cervix who are aged 30 to 65 years, the option for cytology every 3 years, primary HPV testing every 5 years, or cotesting every 5 years (TABLE).4

Why the reluctance to switch to HPV testing?
Despite FDA approval in 2014 for primary HPV testing and concurrent professional society guidance to use this testing strategy in women with a cervix who are aged 25 years and older, few practices in the United States have switched over to primary HPV testing for cervical cancer screening.5,6 Several reasons underlie this inertia:
- Many practices currently use HPV tests that are not FDA approved for primary HPV testing.
- Until recently, national screening guidelines did not recommend primary HPV testing as the preferred testing strategy.
- Long-established guidance on the importance of regular cervical cytology screening promoted by the ACS and others (which especially impacts women with a cervix older than age 50 who guide their younger daughters) will rely on significant re-education to move away from the established “Pap smear” cultural icon to a new approach.
- Last but not least, companies that manufacture HPV tests and laboratories integrated to offer such tests not yet approved for primary screening are promoting reliance on the prior proven cotest strategy. They have lobbied to preserve cotesting as a primary test, with some laboratory database studies showing gaps in detection with HPV test screening alone.7-9
Currently, the FDA-approved HPV tests for primary HPV screening include the Cobas HPV test (Roche) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Both are DNA tests for 14 high-risk types of HPV that include genotyping for HPV 16 and 18.
Continue to: Follow the evidence...
Follow the evidence
Several trials in Europe and Canada provide supporting evidence for primary HPV testing, and many European countries have moved to primary HPV testing as their preferred screening method.10,11 The new ACS guidelines put us more in sync with the rest of the world, where HPV testing is the dominant strategy.
It is true that doing additional tests will find more disease; cotesting has been shown to very minimally increase detection of cervical intraepithelial neoplasia grade 2/3 (CIN 2/3) compared with HPV testing alone, but it incurs many more costs and procedures.12 The vast majority of cervical cancer is HPV positive, and cytology still can be used as a triage to primary HPV screening until tests with better sensitivity and/or specificity (such as dual stain and methylation) can be employed to reduce unnecessary “false-positive” driven procedures.
As mentioned, many strong forces are trying to keep cotesting as the preferred strategy. It is important for clinicians to recognize the corporate investment into screening platforms, relationships, and products that underlie some of these efforts so as not to be unfairly influenced by their lobbying. Data from well-conducted, high-quality studies should be the evidence on which one bases a cervical cancer screening strategy.
Innovation catalyzes change
We acknowledge that it is difficult to give up something you have been doing for decades, so there is natural resistance by both patients and clinicians to move the Pap smear into a secondary role. But the data support primary HPV testing as the best screening option from a public health perspective.
At some point, hopefully soon, primary HPV testing will receive approval for self-sampling; this has the potential to reach patients in rural or remote locations who may otherwise not get screened for cervical cancer.13
The 2019 risk-based management guidelines from the ASCCP (American Society for Colposcopy and Cervical Pathology) also incorporate the use of HPV-based screening and surveillance after abnormal tests or colposcopy. Therefore, switching to primary HPV screening will not impact your ability to follow patients appropriately based on clinical guidelines.
Our advice to clinicians is to switch to primary HPV screening now if possible. If that is not feasible, continue your current strategy until you can make the change. And, of course, we recommend that you implement an HPV vaccination program in your practice to maximize primary prevention of HPV-related cancers. ●
How should I be approaching cervical cancer screening: with primary human papillomavirus (HPV) testing, or cotesting? We get this question all the time from clinicians. Although they have heard of the latest cervical cancer screening guidelines for stand-alone “primary” HPV testing, they are still ordering cervical cytology (Papanicolaou, or Pap, test) for women aged 21 to 29 years and cotesting (cervical cytology with HPV testing) for women with a cervix aged 30 and older.
Changes in cervical cancer testing guidance
Cervical cancer occurs in more than 13,000 women in the United States annually.1 High-risk types of HPV—the known cause of cervical cancer—also cause a large majority of cancers of the anus, vagina, vulva, and oropharynx.2
Cervical cancer screening programs in the United States have markedly decreased the incidence of and mortality from cervical cancer since introduction of the Pap smear in the 1950s. In 2000, HPV testing was approved by the US Food and Drug Administration (FDA) as a reflex test to a Pap smear result of atypical squamous cells of undetermined significance (ASC-US). HPV testing was then approved for use with cytology as a cotest in 2003 and subsequently as a primary stand-alone test in 2014.
Recently, the American Cancer Society (ACS) released new cervical screening guidelines that depart from prior guidelines.3 They recommend not to screen 21- to 24-year-olds and to start screening at age 25 until age 65 with the preferred strategy of primary HPV testing every 5 years, using an FDA-approved HPV test. Alternative screening strategies are cytology (Pap) every 3 years or cotesting every 5 years.
The 2018 US Preventive Services Task Force (USPSTF) guidelines differ from the ACS guidelines. The USPSTF recommends cytology every 3 years as the preferred method for women with a cervix who are aged 21 to 29 years and, for women with a cervix who are aged 30 to 65 years, the option for cytology every 3 years, primary HPV testing every 5 years, or cotesting every 5 years (TABLE).4

Why the reluctance to switch to HPV testing?
Despite FDA approval in 2014 for primary HPV testing and concurrent professional society guidance to use this testing strategy in women with a cervix who are aged 25 years and older, few practices in the United States have switched over to primary HPV testing for cervical cancer screening.5,6 Several reasons underlie this inertia:
- Many practices currently use HPV tests that are not FDA approved for primary HPV testing.
- Until recently, national screening guidelines did not recommend primary HPV testing as the preferred testing strategy.
- Long-established guidance on the importance of regular cervical cytology screening promoted by the ACS and others (which especially impacts women with a cervix older than age 50 who guide their younger daughters) will rely on significant re-education to move away from the established “Pap smear” cultural icon to a new approach.
- Last but not least, companies that manufacture HPV tests and laboratories integrated to offer such tests not yet approved for primary screening are promoting reliance on the prior proven cotest strategy. They have lobbied to preserve cotesting as a primary test, with some laboratory database studies showing gaps in detection with HPV test screening alone.7-9
Currently, the FDA-approved HPV tests for primary HPV screening include the Cobas HPV test (Roche) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Both are DNA tests for 14 high-risk types of HPV that include genotyping for HPV 16 and 18.
Continue to: Follow the evidence...
Follow the evidence
Several trials in Europe and Canada provide supporting evidence for primary HPV testing, and many European countries have moved to primary HPV testing as their preferred screening method.10,11 The new ACS guidelines put us more in sync with the rest of the world, where HPV testing is the dominant strategy.
It is true that doing additional tests will find more disease; cotesting has been shown to very minimally increase detection of cervical intraepithelial neoplasia grade 2/3 (CIN 2/3) compared with HPV testing alone, but it incurs many more costs and procedures.12 The vast majority of cervical cancer is HPV positive, and cytology still can be used as a triage to primary HPV screening until tests with better sensitivity and/or specificity (such as dual stain and methylation) can be employed to reduce unnecessary “false-positive” driven procedures.
As mentioned, many strong forces are trying to keep cotesting as the preferred strategy. It is important for clinicians to recognize the corporate investment into screening platforms, relationships, and products that underlie some of these efforts so as not to be unfairly influenced by their lobbying. Data from well-conducted, high-quality studies should be the evidence on which one bases a cervical cancer screening strategy.
Innovation catalyzes change
We acknowledge that it is difficult to give up something you have been doing for decades, so there is natural resistance by both patients and clinicians to move the Pap smear into a secondary role. But the data support primary HPV testing as the best screening option from a public health perspective.
At some point, hopefully soon, primary HPV testing will receive approval for self-sampling; this has the potential to reach patients in rural or remote locations who may otherwise not get screened for cervical cancer.13
The 2019 risk-based management guidelines from the ASCCP (American Society for Colposcopy and Cervical Pathology) also incorporate the use of HPV-based screening and surveillance after abnormal tests or colposcopy. Therefore, switching to primary HPV screening will not impact your ability to follow patients appropriately based on clinical guidelines.
Our advice to clinicians is to switch to primary HPV screening now if possible. If that is not feasible, continue your current strategy until you can make the change. And, of course, we recommend that you implement an HPV vaccination program in your practice to maximize primary prevention of HPV-related cancers. ●
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30.
- Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers–United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- US Preventive Services Task Force; Curry SJ, KristAH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337.
- Cooper CP, Saraiya M. Cervical cancer screening intervals preferred by US women. Am J Prev Med. 2018;55:389-394.
- Austin RM, Onisko A, Zhao C. Enhanced detection of cervical cancer and precancer through use of imaged liquid-based cytology in routine cytology and HPV cotesting. Am J Clin Pathol. 2018;150:385-392.
- Kaufman HW, Alagia DP, Chen Z, et al. Contributions of liquid-based (Papanicolaou) cytology and human papillomavirus testing in cotesting for detection of cervical cancer and precancer in the United States. Am J Clin Pathol. 2020;154:510-516.
- Blatt AJ, Kennedy R, Luff RD, et al. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:282-288.
- Ronco G, Dillner J, Elfstrom KM, et al; International HPV Screening Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Kim JJ, Burger EA, Regan C, et al. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320:706-714.
- Arbyn M, Smith SB, Temin S, et al; on behalf of the Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30.
- Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers–United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- US Preventive Services Task Force; Curry SJ, KristAH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337.
- Cooper CP, Saraiya M. Cervical cancer screening intervals preferred by US women. Am J Prev Med. 2018;55:389-394.
- Austin RM, Onisko A, Zhao C. Enhanced detection of cervical cancer and precancer through use of imaged liquid-based cytology in routine cytology and HPV cotesting. Am J Clin Pathol. 2018;150:385-392.
- Kaufman HW, Alagia DP, Chen Z, et al. Contributions of liquid-based (Papanicolaou) cytology and human papillomavirus testing in cotesting for detection of cervical cancer and precancer in the United States. Am J Clin Pathol. 2020;154:510-516.
- Blatt AJ, Kennedy R, Luff RD, et al. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:282-288.
- Ronco G, Dillner J, Elfstrom KM, et al; International HPV Screening Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Kim JJ, Burger EA, Regan C, et al. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320:706-714.
- Arbyn M, Smith SB, Temin S, et al; on behalf of the Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
Cesarean myomectomy: Safe operation or surgical folly?

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
How does long-term OC use affect breast, ovarian, and endometrial cancer risk?
Karlsson T, Johansson T, Hoguland J, et al. Time-dependent effects of oral contraceptive use on breast, ovarian and endometrial cancers. Cancer Research. 2020;canres.2476.2020. doi:10.1158/0008-5472.CAN-20-2476.
EXPERT COMMENTARY
The long-term effects of OC use on gynecologic and breast cancers has been uncertain, with different reports yielding conflicting findings. To assess the time-dependent and long-term associations between OC use and the risk of breast, ovarian, and endometrial cancer in women born between 1939 and 1970, Karlsson and colleagues used data from the UK Biobank (which includes a large cross-sectional cohort of individuals recruited between 2006 and 2010) and national databases.
Details of the study
A total of 256,661 women were included in this study. Of these, 82% (210,443) had used or were currently using OC (ever-users) and 18% (46,218) had never used OC (never-users). There were 17,739; 1,966; and 2,462 cases of breast, ovarian, and endometrial cancer, respectively, identified.
In analyses adjusted for 10 parameters, the ORs for ovarian (OR, 0.72) and endometrial cancer (OR, 0.68) were lower among ever-users of OC compared with never-users (P<.05). However, the OR for breast cancer (OR, 1.02) was similar among ever-users and never-users of OC (P>.05).
Among women followed to age 55, results were similar for the 2 gynecologic cancers but were significantly higher for breast cancer (OR, 1.10; P<.05). With 20 or more years of OC use, greater prevention of ovarian (OR, 0.60) and, particularly, endometrial cancer (OR, 0.36) was observed (P<.05). However, the risk of breast cancer was similar in never-users and long-term users of OC.
Study strengths and limitations
A strength of this study is that, compared with most previous studies, it had a much longer follow-up period.
The authors noted, however, that among the potential limitations in the study design was the fact that only 6% of participants invited to the UK Biobank volunteered to participate in the study. This may have resulted in participation bias within the cohort, reflecting a healthier cohort that is not representative of the overall population. ●
These study findings from a large cross-sectional cohort by Karlsson and colleagues suggest that controversy regarding the association of breast cancer with OC use may reflect different study methodologies with respect to timing. The authors note that while the lifetime risk of breast cancer may not differ between OC ever-users and never-users, there appears to be a transient elevated risk associated with OC use. By contrast, OC use, particularly when used long-term, appears to “dramatically” reduce the risk of ovarian and endometrial cancer, according to the study authors.
ANDREW M. KAUNITZ, MD
Karlsson T, Johansson T, Hoguland J, et al. Time-dependent effects of oral contraceptive use on breast, ovarian and endometrial cancers. Cancer Research. 2020;canres.2476.2020. doi:10.1158/0008-5472.CAN-20-2476.
EXPERT COMMENTARY
The long-term effects of OC use on gynecologic and breast cancers has been uncertain, with different reports yielding conflicting findings. To assess the time-dependent and long-term associations between OC use and the risk of breast, ovarian, and endometrial cancer in women born between 1939 and 1970, Karlsson and colleagues used data from the UK Biobank (which includes a large cross-sectional cohort of individuals recruited between 2006 and 2010) and national databases.
Details of the study
A total of 256,661 women were included in this study. Of these, 82% (210,443) had used or were currently using OC (ever-users) and 18% (46,218) had never used OC (never-users). There were 17,739; 1,966; and 2,462 cases of breast, ovarian, and endometrial cancer, respectively, identified.
In analyses adjusted for 10 parameters, the ORs for ovarian (OR, 0.72) and endometrial cancer (OR, 0.68) were lower among ever-users of OC compared with never-users (P<.05). However, the OR for breast cancer (OR, 1.02) was similar among ever-users and never-users of OC (P>.05).
Among women followed to age 55, results were similar for the 2 gynecologic cancers but were significantly higher for breast cancer (OR, 1.10; P<.05). With 20 or more years of OC use, greater prevention of ovarian (OR, 0.60) and, particularly, endometrial cancer (OR, 0.36) was observed (P<.05). However, the risk of breast cancer was similar in never-users and long-term users of OC.
Study strengths and limitations
A strength of this study is that, compared with most previous studies, it had a much longer follow-up period.
The authors noted, however, that among the potential limitations in the study design was the fact that only 6% of participants invited to the UK Biobank volunteered to participate in the study. This may have resulted in participation bias within the cohort, reflecting a healthier cohort that is not representative of the overall population. ●
These study findings from a large cross-sectional cohort by Karlsson and colleagues suggest that controversy regarding the association of breast cancer with OC use may reflect different study methodologies with respect to timing. The authors note that while the lifetime risk of breast cancer may not differ between OC ever-users and never-users, there appears to be a transient elevated risk associated with OC use. By contrast, OC use, particularly when used long-term, appears to “dramatically” reduce the risk of ovarian and endometrial cancer, according to the study authors.
ANDREW M. KAUNITZ, MD
Karlsson T, Johansson T, Hoguland J, et al. Time-dependent effects of oral contraceptive use on breast, ovarian and endometrial cancers. Cancer Research. 2020;canres.2476.2020. doi:10.1158/0008-5472.CAN-20-2476.
EXPERT COMMENTARY
The long-term effects of OC use on gynecologic and breast cancers has been uncertain, with different reports yielding conflicting findings. To assess the time-dependent and long-term associations between OC use and the risk of breast, ovarian, and endometrial cancer in women born between 1939 and 1970, Karlsson and colleagues used data from the UK Biobank (which includes a large cross-sectional cohort of individuals recruited between 2006 and 2010) and national databases.
Details of the study
A total of 256,661 women were included in this study. Of these, 82% (210,443) had used or were currently using OC (ever-users) and 18% (46,218) had never used OC (never-users). There were 17,739; 1,966; and 2,462 cases of breast, ovarian, and endometrial cancer, respectively, identified.
In analyses adjusted for 10 parameters, the ORs for ovarian (OR, 0.72) and endometrial cancer (OR, 0.68) were lower among ever-users of OC compared with never-users (P<.05). However, the OR for breast cancer (OR, 1.02) was similar among ever-users and never-users of OC (P>.05).
Among women followed to age 55, results were similar for the 2 gynecologic cancers but were significantly higher for breast cancer (OR, 1.10; P<.05). With 20 or more years of OC use, greater prevention of ovarian (OR, 0.60) and, particularly, endometrial cancer (OR, 0.36) was observed (P<.05). However, the risk of breast cancer was similar in never-users and long-term users of OC.
Study strengths and limitations
A strength of this study is that, compared with most previous studies, it had a much longer follow-up period.
The authors noted, however, that among the potential limitations in the study design was the fact that only 6% of participants invited to the UK Biobank volunteered to participate in the study. This may have resulted in participation bias within the cohort, reflecting a healthier cohort that is not representative of the overall population. ●
These study findings from a large cross-sectional cohort by Karlsson and colleagues suggest that controversy regarding the association of breast cancer with OC use may reflect different study methodologies with respect to timing. The authors note that while the lifetime risk of breast cancer may not differ between OC ever-users and never-users, there appears to be a transient elevated risk associated with OC use. By contrast, OC use, particularly when used long-term, appears to “dramatically” reduce the risk of ovarian and endometrial cancer, according to the study authors.
ANDREW M. KAUNITZ, MD
Treating PPH: A novel vacuum-induced hemorrhage control device
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5
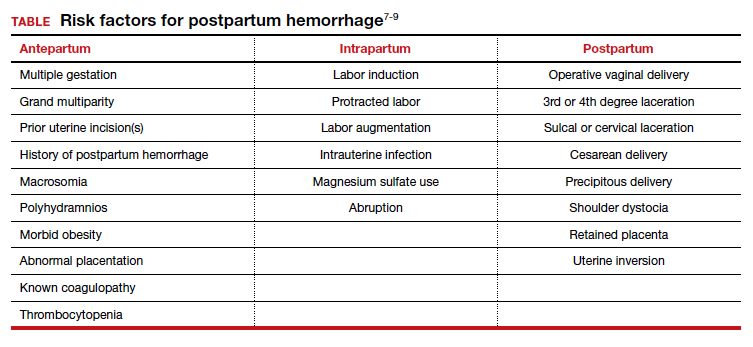
Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).
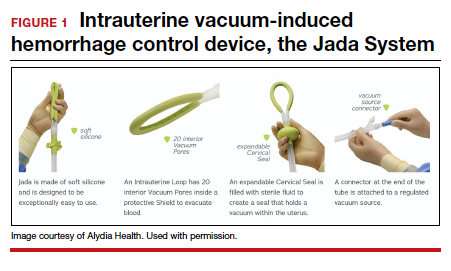
Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.
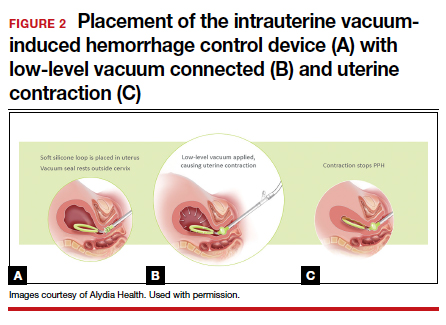
CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5

Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).

Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.

CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
Postpartum hemorrhage (PPH) continues to be a leading cause of maternal morbidity and mortality both worldwide and in the United States.1-3 A PPH is defined as the cumulative blood loss of 1,000 mL or more, or blood loss accompanied by signs or symptoms of hypovolemia, within 24 hours following the birth process (including intrapartum loss).4
Approximately 70% to 80% of hemorrhages are due to abnormal uterine tone.5 Bimanual massage and medical management, the primary treatments for uterine atony, attempt to restore the normal uterine tone that compresses the vessels in the placental implantation site and limits bleeding. For women in whom the primary treatments are not effective, only uterine compression sutures in a laparotomy can achieve physiologic contracture of the uterus. The second-line treatment option, intrauterine tamponade, places pressure over the placental implantation site while distending the uterus.
In October 2020, the US Food and Drug Administration (FDA) granted clearance to a novel device that offers an alternative treatment option. The Jada System (Alydia Health), an intrauterine vacuum-induced hemorrhage control device, is placed in the uterus and uses wall suction to induce physiologic contraction of the uterus to control bleeding.6
In this article, within the context of a case vignette, we discuss the recent study on the Jada System and how this device can be used in the management of PPH.6
CASE Woman with PPH history fears repeat hemorrhage
Ms. B. is a 25-year-old woman (G2P1) who presents for prenatal care at 10 weeks’ gestation. Her medical history is significant for asthma and PPH after her first delivery. When you review her prior delivery records, you learn that she had a protracted labor and delivered a healthy 10 lb 8 oz baby boy after 3 hours of pushing. After delivery, she received postpartum intravenous oxytocin followed by intramuscular uterotonics when her bleeding was heavy during her laceration repair. Her estimated blood loss at delivery was 600 mL. The team was called back to her bedside for the continued bleeding. Uterine atony was diagnosed. Although she received additional uterotonics, the bleeding continued. An intrauterine tamponade balloon was placed, and the bleeding ultimately was controlled. The total estimated blood loss (EBL) was 2.5 L, and the patient then was transfused with 2 U of packed red blood cells.
Currently, Ms. B. is very worried about having another hemorrhage as the bleeding terrified her and her partner, disrupted breastfeeding initiation while the tamponade was in place, and made her anxious about having another baby.
What steps would you take to prepare for a potential PPH in this patient?
Risk factors
While PPH often is unpredictable, many risk factors have been identified (TABLE).7-9 Some risk factors are present during the antepartum period while others arise during labor. In some cases, obstetric clinicians may be able to intervene during prenatal care, such as by giving iron supplementation to address anemia. Other factors, however, are not modifiable, including multiparity, polyhydramnios, and multiple gestations. On presentation to the labor unit, new risk factors may arise, such as magnesium sulfate use, chorioamnionitis, protracted labor, or the need for general anesthesia. In addition, the presence of a fibroid uterus or a uterine inversion can impede effective uterine contractions.5

Various tools are available for assessing these risk factors on admission, during labor, and after delivery, such as the AWHONN postpartum hemorrhage risk assessment table and the CMQCC obstetric hemorrhage toolkit.10,11
Continue to: CASE continued Patient’s history reveals risk factors...
CASE continued Patient’s history reveals risk factors
You review with Ms. B. that she had several risk factors present during labor. She had a large baby and a protracted labor. Knowing her history in this pregnancy will allow the clinical team to be prepared for a potential recurrent hemorrhage and to respond proactively to bleeding.
Consider the management options
The initial treatment for PPH includes bimanual massage, oxytocin, and other uterotonics (methylergonovine, 15-methyl prostaglandin F2α, and misoprostol). While various algorithms are available on the order of treatment, a single agent has not been shown superior to others.12 The antifibrinolytic medication tranexamic acid also was shown to reduce the risk of death from obstetric hemorrhage in the international WOMAN trial.13
While these agents often are used simultaneously to achieve hemostasis, their systemic effects are associated with contraindications. Specifically, F2α prostaglandins cannot be used in patients with asthma or active hepatic, pulmonary, or cardiac disease. Ergot derivatives cannot be used in patients with hypertension, pre-eclampsia, or cardiovascular disease. Given the rising rate of medical comorbidities during pregnancy, such contraindications limit the treatment options for many patients.
In cases in which medical management is not sufficient or is contraindicated for controlling hemorrhage, second-line treatment includes the use of tamponade techniques, such as intrauterine packing or balloons. The tamponade applies pressure directly to the placental implantation site for 12 to 24 hours, which allows time for the uterus to contract and return to normal tone. While this method may seem counterintuitive to achieving uterine tone, studies suggest a success rate between 75% and 86% with balloon tamponade.12
Third-line treatment options are increasingly invasive but should be used to prevent further maternal morbidity and mortality. These include uterine artery embolization and surgery. Uterine artery embolization is an option for a stable patient at a center with available interventional radiology services. If embolization is either not successful or not available, an exploratory laparotomy should be performed. Uterine compression sutures can be placed along with vascular ligation sutures of the uterine arteries (O’Leary sutures) and the hypogastric arteries. If all other methods have failed, a hysterectomy is the definitive treatment for hemorrhage.
CASE continued Patient desires an alternative to tamponade if needed
Following your visit, Ms. B. has an ultrasound scan that shows a dichorionic diamniotic twin pregnancy. She also has a microcytic anemia. After you discuss iron supplementation with the patient, she asks if there are any other options should medical management fail in the event of a recurrent hemorrhage. While intrauterine tamponade balloon did treat her hemorrhage, she was not happy with the length of time it had to remain in place, the discomfort while it was used, and the disruption to her planned recovery. You inform her of a new treatment option available for PPH, a vacuum-induced hemorrhage control device that was recently FDA cleared.
Continue to: New device controls bleeding fast...
New device controls bleeding fast
In 2020, D’Alton and colleagues reported on their multicenter, prospective single-arm treatment study on the effectiveness and safety of an intrauterine vacuum-induced hemorrhage control device.6 This device, the Jada System, uses low-level vacuum to induce uterine contraction to control bleeding from uterine atony. The prospective study, which followed a 2016 feasibility study, enrolled more than 100 women at 12 centers across the United States.6,14 Women were eligible to participate if they delivered at a gestational age of 34 weeks or later and had an EBL between 500 and 1,000 mL after a vaginal delivery or an EBL between 1,000 and 1,500 mL after a cesarean delivery.
Treatment with the vacuum device was successful in 94% (100/106, 95% confidence interval, 88%–98%) of women, and definitive control of abnormal bleeding was achieved in a median of 3 minutes (interquartile range [IQR], 2.0–5.0) after connection to the vacuum device.6
CASE continued Patient has questions
Your patient expresses interest in this device, but she wants to understand how it works. Would it require transfer to another unit or prolonged monitoring?
How the device works
Compared with intrauterine tamponade balloon devices, which apply pressure by distending the uterus, the Jada System applies low-level intrauterine vacuum to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve hemostasis.6 The device is made of medical-grade silicone. Its distal end, which is placed in the uterus, is an elliptical loop. The loop’s inner surface contains 20 vacuum pores protected by a shield that facilitate creation of a vacuum within the uterine cavity. The loop is soft and smooth to limit the chance of tissue damage during insertion, treatment, and removal of the device. The device’s proximal end has a vacuum connector. The vacuum source is hospital-grade wall suction, but a portable vacuum source also can be used (FIGURE 1).

Prior to placing the device, a manual sweep of the uterine cavity is performed. If needed, ultrasonography can be used with the manual sweep to ensure that there is no retained placental tissue or clot. The loop of the Jada System is then inserted in the uterine cavity, and the circular cervical seal, just outside the external cervical os, is filled with sterile water.
Low-level vacuum (80 ± 10 mm Hg) is applied so that pooled blood is evacuated from the uterus as it collapses (FIGURE 2). The volume of any ongoing bleeding is measured in the suction tubing while the uterine response to treatment can be palpated. Once there is no bleeding without any need for further treatment, the device should remain in the uterus for at least 1 hour. The suction is then turned off, and bleeding is monitored for 30 minutes. If bleeding remains controlled, the device can be removed.

CASE continued The question of complications
Ms. B. is concerned about safety and asks about potential complications with the device’s use.
Safety findings
In the prospective study and FDA review, the device was deemed safe. There were 8 possibly related adverse events (endometritis, laceration disruption, and vaginal infection), which all resolved without serious clinical sequelae. Forty women (38%) received a blood transfusion, but only 5 required 4 U or more of red blood cells.6
Continue to: CASE continued What do other physicians think?...
CASE continued What do other physicians think?
Your patient is curious about the time it takes for the device to work and whether other clinicians like using this new device for hemorrhage treatment.
Duration of treatment
The times to achieve uterine collapse and control of hemorrhage are both relatively short. In the prospective study, the initial collapse of the uterus took a median of 1 minute (IQR, 1–2 min) from the time of vacuum connection.6 Bleeding was controlled in less than 5 minutes in 82% of women, with an overall median time of 3 minutes (IQR, 2–5 min). The median duration of vacuum treatment was 144.0 minutes (IQR, 85.8–295.8 min), which includes the required minimum of 60 minutes for vacuum treatment time and 30 minutes of observation without the vacuum connected but with the device still in place.6
When polled, the majority of clinicians—98%—reported that the intrauterine vacuum-induced hemorrhage control device was easy to use, and 97% would recommend its use for future patients.6
Further, recognizing the device’s potential, the Cleveland Clinic cited it as one of the top 10 health care innovations for 2021 for offering a low-tech and minimally invasive tool for obstetric clinicians.15
CASE continued Final questions
Ms. B. thanks you for the information and asks, should she know anything else about the device?
Vacuum device vs other treatments
The study by D’Alton and colleagues was a single-arm treatment trial that did not directly compare the effectiveness of the device with that of other PPH treatment options, such as balloon tamponade.6 At this point, we know that clinicians can safely and quickly use the device to treat uterine atony, but we do not know if it is superior to other treatments for PPH.
Key takeaways
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When first-line uterotonics fail, obstetric clinicians previously had only balloon tamponade or invasive procedures to treat patients. The novel intrauterine vacuum-induced hemorrhage control device takes a new approach that simulates the physiologic process of uterine contractions. The device can rapidly and effectively control abnormal postpartum uterine bleeding. More studies are needed, however, to compare the device’s effectiveness with that of other PPH treatments and to consider its use in women with more severe degrees of postpartum hemorrhage as well as its cost-effectiveness. ●
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323-e333.
- Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029-1036.
- Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. http://www .cdc.gov/reproductivehealth/maternalinfanthealth /severematernalmorbidity.html. Accessed November 6, 2020.
- Menard MK, Main EK, Currigan SM. Executive summary of the reVITALize initiative: standardizing obstetric data definitions. Obstet Gynecol. 2014;124:150-153.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
- D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136:882-891.
- Mavrides E, Allard S, Chandraharan E, et al; on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum hemorrhage. BJOG. 2016;124:e106-e149.
- Lyndon A, Lagrew D, Shields L, et al. Improving health care response to obstetric hemorrhage, version 2.0 (California Maternal Quality Care Collaborative Toolkit to Transform Maternity Care). Developed under contract #11-10006 with the California Department of Public Health; Maternal, Child and Adolescent Health Division; Published by the California Maternal Quality Care Collaborative, March 17, 2015.
- Main EK, Goffman D, Scavone BM, et al; National Partnership for Maternal Safety; Council on Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Obstet Gynecol. 2015;126:155-162.
- AWHONN Postpartum Hemorrhage Project. Postpartum hemorrhage (PPH) risk assessment table 1.0. https:// mygnosis.com/Content/Chunks/3504/assets/pdfs/PPH _Risk_Assessment_Table-7-17-15.pdf. Accessed November 15, 2020.
- Bingham D, Melsop K, Main E. CMQCC obstetric hemorrhage toolkit: hospital level implementation guide. 2010. California Maternal Quality Care Collaborative (CMQCC). Palo Alto, CA: Stanford University. https://www.cmqcc.org/resource/1489 /download. Accessed November 15, 2020.
- Likis FE, Sathe NA, Morgans AK, et al. Management of postpartum hemorrhage. Comparative effectiveness review no. 151. AHRQ publication no. 15-EHC013-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstet Gynecol. 2016;128:33-36.
- Cleveland Clinic Innovations. Cleveland Clinic unveils top 10 medical innovations for 2021. October 6, 2020. https:// innovations.clevelandclinic.org/Programs/Top-10-Medical -Innovations/Top-10-for-2021. Accessed November 6, 2020.