User login
Patch Testing 101, Part 1: Performing the Test
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
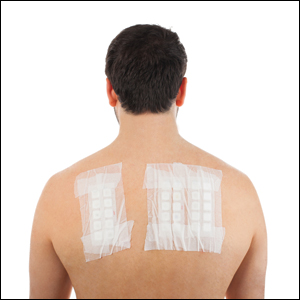
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
Our apologies, dear reader. It seems we have gotten ahead of ourselves. While we were writing about the Allergen of the Year, systemic dermatitis, and patch testing in children, we forgot to start with the basics. Let us remedy that. This is the first of a 2-part series addressing the basics of patch testing. In this article, we examine patch test systems, allergens, patch test readings, testing while on medications, and patch testing pearls and pitfalls. Let us begin!
Patch Test Systems
There are 2 patch test systems in North America: the Thin-layer Rapid Use Epicutaneous (T.R.U.E.) test (SmartPractice), which is approved by the US Food and Drug Administration for those 6 years and older, and the chamber method.
The T.R.U.E. test consists of 3 panels with 35 allergens and 1 negative control. The T.R.U.E. package insert1 describes surgical tape with individual polyester patches, each coated with an allergen film. Benefits of T.R.U.E. include ease of use (ie, easy storage and preparation, quick and straightforward application) and a readily identifiable set of allergens. The main drawback of T.R.U.E. is that only a limited number of allergens are tested, and as a result, it may miss the identification of some contact allergies. In an analysis of the 2015-2016 North American Contact Dermatitis Group (NACDG) patch test screening series, 25% to 40% of positive patch tests would have been missed if patch testing was performed with T.R.U.E. alone.2
Chamber method patch testing describes the process by which allergens are loaded into either metal or plastic chambers and then applied to the patient’s skin. The major benefit of the chamber method is that patches may be truly customized for the patient. The chamber method is time and labor intensive for patch preparation and application. Most comprehensive patch test clinics in North America use the chamber method, including the NACDG.
Patch test chambers largely can be divided into 2 categories: metal (aluminum) or plastic. Aluminum chambers, also known as Finn chambers, traditionally are used in patch testing. There are rare reports of hypersensitivity to aluminum chambers with associated diffuse positive patch test reactions,3,4 which may be more common in the pediatric population and likely is due to the fact that aluminum is present as an adjuvant in many childhood vaccines. As a precaution, some patch text experts recommend using plastic chambers in children younger than 16 to 18 years (M.R. and A.R.A., personal communication). Metal chambers require the additional application of diffusion discs for liquid allergens, and plastic chambers typically already contain the necessary diffusion discs. Finn chambers traditionally are applied with hypoallergenic porous surgical tape, but a waterproof tape also is available. To keep the chambers in place for the necessary 48 hours, additional tape may be applied over the patches.
Allergens
In patch test clinics, many dermatologists use a standard or screening allergen series. An appropriate standard series encompasses allergens that are most likely to be positive and relevant in the tested population. Some patch test experts recommend that allergens with a positive patch test frequency of greater than 0.5% to 1% should be included in a standard series.5 However, geographic differences in positive reactions can influence which allergens are appropriate to include. As a result, there is no universal standard series. Examples of standard or screening series include the American Contact Dermatitis Society (ACDS) allergen series,6 North American Baseline Series or North American 80 Comprehensive Series, European Baseline Series, NACDG series,2 and the Pediatric Baseline Series,7 as well as many other country- or region-specific series. There currently are 2 major commercial allergen distribution companies—Chemotechnique Diagnostics/Dormer Laboratories (series, individual allergens) and SmartPractice/allerGEAZE (series, individual allergens, T.R.U.E.).
In addition to a properly selected standard or screening series, supplemental patch testing with additional allergens can increase the diagnostic yield. Numerous supplemental series exist, including cosmetic, dental, textile, rubber, adhesive, plastics, and glue, among many others. In the NACDG 2015-2016 patch test cycle, it was found that 23% of 5597 patients reacted to an allergen that was not present on the NACDG screening series.2
In some situations, it is appropriate to patch test patient products, or nonstandard allergens. An abundance of caution, understanding of patch testing, and experience is necessary; for example, some chemicals are not recommended for testing, such as cleaning products, certain industrial chemicals, and those that may be carcinogens. We frequently consult De Groot’s Patch Testing8 for recommended allergen test concentrations and vehicles.
Patch Test Readings
The timing of the patch test reading is an important component of the test. Most North American comprehensive patch test clinics perform both first and delayed readings. After application, patches remain in place for 48 hours and then are removed, and a first reading is completed. Results are recorded as +/− (weak/doubtful), + (mild), ++ (strong), +++ (very strong), irritant, and negative.2 Many patch test specialists use side lighting to achieve the best reading and palpate to confirm the presence of induration; panel alignment devices commonly are utilized. There are some scenarios where shorter or longer application times are indicated, but this is beyond the scope of this article. A second, or delayed, reading should be completed 72 to 144 hours after initial application. We usually complete the delayed reading at 96 to 120 hours.
Certain patch test reactions may peak at different times, with fragrances often reacting earlier, and metals, topical antibiotics, and textile dyes reacting later.9 In the scenario of delayed peak reactions, third readings may be indicated.
Neglecting to complete a delayed reading is a potential pitfall and can increase the risk for both false-positive and false-negative reactions.10,11 In 1996, Uter et al10 published a large study of 9946 patients who were patch tested over a 4-year period. The authors compared patch test reactions at 48 and 72 hours and found that 34.5% of all positive reactions occurred at 72 hours; an additional 15.1% were positive at 96 hours. Importantly, one reading at 48 hours missed approximately one-third of positive patch test reactions, emphasizing the importance of delayed patch test readings.10 Furthermore, another study of 9997 consecutively patch tested patients examined reactions that were either negative or doubtful between days 3 or 4 and followed to see which of those reactions were positive at days 6 or 7. Of the negative reactions, the authors found that 4.4% were positive on days 6 or 7, and of the doubtful reactions, 9.1% were positive on days 6 or 7, meaning that up to 13.5% of positive reactions can be missed when a later reading is not performed.11
Medications During Patch Testing
Topical Medications
Topical medications generally can be continued during patch testing; however, patients should not apply topical medications to the patch test application site. Ideally, there should be no topical medication applied to the patch test application site for 1 to 2 weeks prior to patch test placement.12 Use of topical medications such as corticosteroids, calcineurin inhibitors, and theoretically even phosphodiesterase 4 inhibitors can not only result in suppression of positive patch test reactions but also can make patch adherence difficult.
Phototherapy
Phototherapy can result in local cutaneous immune suppression; therefore, it is recommended that it not be applied to the patch test area either during the patch test process or for 1 to 2 weeks prior to patch test application. In addition, if heat or sweating are generated during phototherapy, they can affect the success of patch testing by poor patch adherence and/or disruption of allergen distribution.
Systemic Medications
Oral antihistamines do not affect patch testing and can be continued during the patch test process.
It is ideal to avoid systemic immunomodulatory agents during the patch test process, but they occasionally are unavoidable, either because they are necessary to manage other medical conditions or because they are needed to achieve clear enough skin to proceed with patch testing. If it is required, prednisone is not recommended to exceed 10 mg daily.12,13 If intramuscular triamcinolone acetonide has been administered, patch testing should occur at least 1 month after the most recent injection.12 Oral methotrexate can probably be continued during patch testing but should be kept at the lowest possible dose and should be held during the week of testing, if possible. Adalimumab, etanercept, infliximab, and ustekinumab can be continued, as they are unlikely to interfere with patch testing.12 There are reports of positive patch test reactions on dupilumab,14,15 and some authors have described the response as variable and potentially allergen dependent.16,17 We believe that it generally is acceptable to continue dupilumab during patch testing. Data on cyclosporine during patch testing are mixed, and caution is advised as higher doses may suppress a positive patch test. Azathioprine and mycophenolate should be avoided, if possible.12
Pearls and Pitfalls
A few tips along the way can help assure your success in patch testing.
- Proper patient counseling determines a successful test. Provide your patient with verbal and written instructions about the patch test process, patch care, and any other necessary information.
- A simple sponge bath is permissible during patch testing provided the back stays dry. One of the authors (A.R.A.) advises patients to sit in a small amount of water in a bathtub to bathe, wash only the front of the body in the shower, and wash hair in the sink.
- No sweating, swimming, heavy exercise, or heavy physical labor. If your patient is planning to run a marathon the week of patch testing, they will be sorely disappointed when you tell them no sweating or showering is allowed! Patients with an occupation that requires physical labor may require a work excuse.
- Tape does not adhere to areas of the skin with excess hair. A scissor trim or electric shave will help the patches stay occluded and in place. We use an electric razor with a disposable replaceable head. A traditional straight razor should not be used, as it can increase the risk for folliculitis, which can make patch readings quite difficult.
- Securing the patches in place with an extra layer of tape provides added security. Large sheets of transparent medical dressings work particularly well for children or if there is difficulty with tape adherence.
Avoid application of patches to areas of the skin with tattoos. In theory, tattooed skin may have a decreased immune response, and tattoo pigment can obscure results.18 However, this is sometimes unavoidable, and Fowler and McTigue18 described a case of successful patch testing on a diffusely tattooed back.
- Avoid skin lesions (eg, scars, seborrheic keratoses, dermatitis) that can affect tape application, patch adherence, or patch readings.
Final Interpretation
The first step to excellent patch testing is understanding the patch test process. Patch test systems include T.R.U.E. and the chamber method. There are several allergen screening series, and the best series for each patient is determined based on geographic region, exposures, and allergen prevalence. The timing and practice of the patch test reading is vital, and physicians should be cognizant of medications and phototherapy use during the patch test process. An understanding of common pearls and pitfalls makes the difference between a good and great patch tester.
Now that you are an expert in performing the test, watch out for part 2 of this series on patch test interpretation, relevance, education, and counseling. Happy testing!
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
- T.R.U.E TEST [package insert]. Phoenix, AZ: SmartPractice; 1994.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Ward JM, Walsh RK, Bellet JS, et al. Allergic contact dermatitis to aluminum-based chambers during routine patch testing. Paper presented at: American Contact Dermatitis Society Annual Meeting; March 19, 2020; Denver, CO.
- Deleuran MG, Ahlström MG, Zachariae C, et al. Patch test reactivity to aluminum chambers. Contact Dermatitis. 2019;81:318-319.
- Bruze M, Condé-Salazar L, Goossens A, et al. European Society of Contact Dermatitis. thoughts on sensitizers in a standard patch test series. Contact Dermatitis. 1999;41:241-250.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 update. Dermatitis. 2017;28:141-143.
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- De Groot AC. Patch Testing: Test Concentrations and Vehicles for 4900 Chemicals. 4th ed. Wapserveen, The Netherlands: Acdegroot Publishing; 2018.
- Chaudhry HM, Drage LA, El-Azhary RA, et al. Delayed patch-test reading after 5 days: an update from the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28:253-260.
- Uter WJ, Geier J, Schnuch A. Good clinical practice in patch testing: readings beyond day 2 are necessary: a confirmatory analysis. Members of the Information Network of Departments of Dermatology. Am J Contact Dermat. 1996;7:231-237.
- Madsen JT, Andersen KE. Outcome of a second patch test reading of T.R.U.E. Tests® on D6/7. Contact Dermatitis. 2013;68:94-97.
- Lampel H, Atwater AR. Patch testing tools of the trade: use of immunosuppressants and antihistamines during patch testing. J Dermatol Nurses’ Assoc. 2016;8:209-211.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89.
- Hoot JW, Douglas JD, Falo LD. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164.
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162.
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121.
- Fowler JF, McTigue MK. Patch testing over tattoos. Am J Contact Dermat. 2002;13:19-20.
Practice Points
- There are 2 basic patch testing systems: the T.R.U.E. test and the chamber method.
- Patches should be applied to the upper back and should be removed after 48 hours. A delayed reading is necessary and should be performed 72 to 144 hours after placement.
- Certain oral and topical medications and phototherapy may interfere with patch test results.
Lack of Knowledge About Surgical Smoke, Masks, and Respirators Among US Dermatology Residents and Fellows in the Era of COVID-19
During dermatologic surgery, surgical smoke created by electrocautery is known to contain not only nanoparticles and carcinogenic compounds but also infectious particles.1 Poor awareness of the risks associated with breathing surgical smoke and lack of safety practices among US dermatology residents has been documented.2 In this era of the novel coronavirus disease 2019 (COVID-19) pandemic, these issues are particularly pertinent due to the theoretical risk of viral transmission through aerosolized particles. There are few studies investigating viral transmission during surgery, but large numbers of health care workers in close contact with the upper aerodigestive tract during diagnostic and therapeutic procedures have become infected with COVID-19, leading to the recommendation of added safety measures for surgeons in other fields.3 Recommendations do not yet exist for dermatologic surgeons, and it is not yet known if this population is at higher risk for COVID-19 infection due to aerosolized viral particles in the air or in surgical smoke. Nonetheless, we feel that additional safety measures during dermatologic surgery are warranted, particularly when operating on areas of higher viral load such as the nasal or oral mucosae, and understanding of safety equipment is paramount. Thus, we aimed to assess the awareness of safety measures among training dermatologists and dermatologic surgeons during the COVID-19 pandemic.
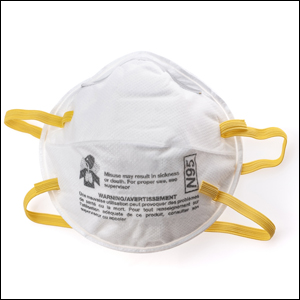
In April 2020, one of the authors (S.I.B.J.) gave a lecture to residents and fellows of accredited dermatology residency or fellowship programs in the United States on surgical masks and surgical smoke in dermatologic surgery on an online videoconferencing platform through our institution. During the lecture, participants were polled regarding their understanding of these topics. Forty-one attendees were included in this analysis, with a 100% response rate. Results showed that 54% (22/41) of respondents indicated they had not had formal lectures on surgical smoke content and management, and 51% (21/41) responded that they do not use a smoke evacuator during surgical procedures. When asked why smoke evacuators are not used, 27% (11/41) responded that the equipment is too cumbersome, 12% (5/41) reported that smoke evacuators are not available at their practice or institution, 7% did not believe that smoke evacuators are necessary, and 5% did not know they are used in dermatology. Additionally, 66% (27/41) said they had not had formal presentations on personal protective equipment (PPE) or masks, though 93% (38/41) said they wear a surgical mask during surgery. Despite the high percentage of respondents using masks, 82% (31/38) did not know what type of mask they were wearing, and the remainder wore a variety of American Society for Testing and Materials–rated (levels 1, 2, or 3) and European Standards type II (EN14683) masks. Following the presentation, 100% of respondents said they were likely to use a smoke evacuator if made available, and 100% reported that they had a better understanding of respirators and masks.
In summary, more than 50% of dermatology trainees in our study had not been formally educated regarding PPE despite its importance during the COVID-19 pandemic. The majority of respondents were unaware of the dangers of surgical smoke, and a small number of respondents believed that smoke evacuators were not necessary or did not know that they were even used in dermatology. Based on the results of this quick survey during a lecture to dermatology trainees, we believe it is important to alert educators to this knowledge gap regarding PPE in the dermatology teaching curriculum. We have shown that even a short lecture format was an effective way of disseminating information about PPE and surgical safety. We believe that safety measures are more important now during a time when risk for infection with a potentially fatal virus is high. We encourage clinical educators to emphasize the importance of personal safety to trainees during residency, especially during the COVID-19 pandemic.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467.
- Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic [published online March 31, 2020]. JAMA Otolaryngol Neck Surg. doi:10.1001/jamaoto.2020.0780.
During dermatologic surgery, surgical smoke created by electrocautery is known to contain not only nanoparticles and carcinogenic compounds but also infectious particles.1 Poor awareness of the risks associated with breathing surgical smoke and lack of safety practices among US dermatology residents has been documented.2 In this era of the novel coronavirus disease 2019 (COVID-19) pandemic, these issues are particularly pertinent due to the theoretical risk of viral transmission through aerosolized particles. There are few studies investigating viral transmission during surgery, but large numbers of health care workers in close contact with the upper aerodigestive tract during diagnostic and therapeutic procedures have become infected with COVID-19, leading to the recommendation of added safety measures for surgeons in other fields.3 Recommendations do not yet exist for dermatologic surgeons, and it is not yet known if this population is at higher risk for COVID-19 infection due to aerosolized viral particles in the air or in surgical smoke. Nonetheless, we feel that additional safety measures during dermatologic surgery are warranted, particularly when operating on areas of higher viral load such as the nasal or oral mucosae, and understanding of safety equipment is paramount. Thus, we aimed to assess the awareness of safety measures among training dermatologists and dermatologic surgeons during the COVID-19 pandemic.
In April 2020, one of the authors (S.I.B.J.) gave a lecture to residents and fellows of accredited dermatology residency or fellowship programs in the United States on surgical masks and surgical smoke in dermatologic surgery on an online videoconferencing platform through our institution. During the lecture, participants were polled regarding their understanding of these topics. Forty-one attendees were included in this analysis, with a 100% response rate. Results showed that 54% (22/41) of respondents indicated they had not had formal lectures on surgical smoke content and management, and 51% (21/41) responded that they do not use a smoke evacuator during surgical procedures. When asked why smoke evacuators are not used, 27% (11/41) responded that the equipment is too cumbersome, 12% (5/41) reported that smoke evacuators are not available at their practice or institution, 7% did not believe that smoke evacuators are necessary, and 5% did not know they are used in dermatology. Additionally, 66% (27/41) said they had not had formal presentations on personal protective equipment (PPE) or masks, though 93% (38/41) said they wear a surgical mask during surgery. Despite the high percentage of respondents using masks, 82% (31/38) did not know what type of mask they were wearing, and the remainder wore a variety of American Society for Testing and Materials–rated (levels 1, 2, or 3) and European Standards type II (EN14683) masks. Following the presentation, 100% of respondents said they were likely to use a smoke evacuator if made available, and 100% reported that they had a better understanding of respirators and masks.
In summary, more than 50% of dermatology trainees in our study had not been formally educated regarding PPE despite its importance during the COVID-19 pandemic. The majority of respondents were unaware of the dangers of surgical smoke, and a small number of respondents believed that smoke evacuators were not necessary or did not know that they were even used in dermatology. Based on the results of this quick survey during a lecture to dermatology trainees, we believe it is important to alert educators to this knowledge gap regarding PPE in the dermatology teaching curriculum. We have shown that even a short lecture format was an effective way of disseminating information about PPE and surgical safety. We believe that safety measures are more important now during a time when risk for infection with a potentially fatal virus is high. We encourage clinical educators to emphasize the importance of personal safety to trainees during residency, especially during the COVID-19 pandemic.
During dermatologic surgery, surgical smoke created by electrocautery is known to contain not only nanoparticles and carcinogenic compounds but also infectious particles.1 Poor awareness of the risks associated with breathing surgical smoke and lack of safety practices among US dermatology residents has been documented.2 In this era of the novel coronavirus disease 2019 (COVID-19) pandemic, these issues are particularly pertinent due to the theoretical risk of viral transmission through aerosolized particles. There are few studies investigating viral transmission during surgery, but large numbers of health care workers in close contact with the upper aerodigestive tract during diagnostic and therapeutic procedures have become infected with COVID-19, leading to the recommendation of added safety measures for surgeons in other fields.3 Recommendations do not yet exist for dermatologic surgeons, and it is not yet known if this population is at higher risk for COVID-19 infection due to aerosolized viral particles in the air or in surgical smoke. Nonetheless, we feel that additional safety measures during dermatologic surgery are warranted, particularly when operating on areas of higher viral load such as the nasal or oral mucosae, and understanding of safety equipment is paramount. Thus, we aimed to assess the awareness of safety measures among training dermatologists and dermatologic surgeons during the COVID-19 pandemic.
In April 2020, one of the authors (S.I.B.J.) gave a lecture to residents and fellows of accredited dermatology residency or fellowship programs in the United States on surgical masks and surgical smoke in dermatologic surgery on an online videoconferencing platform through our institution. During the lecture, participants were polled regarding their understanding of these topics. Forty-one attendees were included in this analysis, with a 100% response rate. Results showed that 54% (22/41) of respondents indicated they had not had formal lectures on surgical smoke content and management, and 51% (21/41) responded that they do not use a smoke evacuator during surgical procedures. When asked why smoke evacuators are not used, 27% (11/41) responded that the equipment is too cumbersome, 12% (5/41) reported that smoke evacuators are not available at their practice or institution, 7% did not believe that smoke evacuators are necessary, and 5% did not know they are used in dermatology. Additionally, 66% (27/41) said they had not had formal presentations on personal protective equipment (PPE) or masks, though 93% (38/41) said they wear a surgical mask during surgery. Despite the high percentage of respondents using masks, 82% (31/38) did not know what type of mask they were wearing, and the remainder wore a variety of American Society for Testing and Materials–rated (levels 1, 2, or 3) and European Standards type II (EN14683) masks. Following the presentation, 100% of respondents said they were likely to use a smoke evacuator if made available, and 100% reported that they had a better understanding of respirators and masks.
In summary, more than 50% of dermatology trainees in our study had not been formally educated regarding PPE despite its importance during the COVID-19 pandemic. The majority of respondents were unaware of the dangers of surgical smoke, and a small number of respondents believed that smoke evacuators were not necessary or did not know that they were even used in dermatology. Based on the results of this quick survey during a lecture to dermatology trainees, we believe it is important to alert educators to this knowledge gap regarding PPE in the dermatology teaching curriculum. We have shown that even a short lecture format was an effective way of disseminating information about PPE and surgical safety. We believe that safety measures are more important now during a time when risk for infection with a potentially fatal virus is high. We encourage clinical educators to emphasize the importance of personal safety to trainees during residency, especially during the COVID-19 pandemic.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467.
- Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic [published online March 31, 2020]. JAMA Otolaryngol Neck Surg. doi:10.1001/jamaoto.2020.0780.
- Georgesen C, Lipner SR. Surgical smoke: risk assessment and mitigation strategies. J Am Acad Dermatol. 2018;79:746-755.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467.
- Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic [published online March 31, 2020]. JAMA Otolaryngol Neck Surg. doi:10.1001/jamaoto.2020.0780.
Practice Points
- Harmful surgical smoke is created with electrocautery. Smoke evacuators and approved surgical masks can help mitigate the harmful effects of smoke on the health of dermatologic surgeons.
- Dermatology curricula for residents and medical students should include information on surgical smoke safety.
SHM Chapter innovations: A provider exchange program
The SHM Annual Conference is more than an educational event. It also provides an opportunity to collaborate, network and create innovative ideas to improve the quality of inpatient care.
During the 2019 Annual Conference (HM19) – the last “in-person” Annual Conference before the COVID pandemic – SHM chapter leaders from the New Mexico chapter (Krystle Apodaca) and the Wiregrass chapter (Amith Skandhan), which covers the counties of Southern Alabama and the Panhandle of Florida, met during a networking event.
As we talked, we realized the unique differences and similarities our practice settings shared. We debated the role of clinician wellbeing, quality of medical education, and faculty development on individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the Triple Aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Engagement in local SHM chapter activities promotes the efficiency of practice, a culture of wellness, and personal resilience. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of individual clinicians. During our discussion we realized that an interinstitutional exchange programs could provide a platform to exchange ideas and establish mentors.
The quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities.
Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs. Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs) but their faculty training can vary based on location.
As a young specialty, hospital medicine is still evolving and incorporating NPs/PAs and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. As chapter leaders we determined that an exchange program would afford the opportunity for visiting faculty members to experience these differences.
We shared the idea of a chapter-level exchange with SHM’s Chapter Development Committee and obtained chapter development funds to execute the event. We also requested that an SHM national board member visit during the exchange to provide insight and feedback. We researched the characteristics of individual academic HMGs and structured a faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for one week, with separate tracks for physicians and NPs/PAs, giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with the HMG and hospital leadership, to specifically address the visiting faculty’s institutional challenges.
The overall goal of the exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development and improve the quality of care. The focus of the exchange program was to share ideas and innovation, and learn the approaches to unique challenges at each institution. Out of this also grew collaboration and mentoring opportunities.
SHM’s New Mexico chapter is based in Albuquerque, a city in the desert Southwest with an ethnically diverse population of 545,000, The chapter leadership works at the University of New Mexico (UNM), a 553-bed medical center. UNM has a well-established internal medicine residency program, an academic hospitalist program, and an NP/PA fellowship program embedded within the hospital medicine department. At the time of the exchange, the HMG at UNM has 26 physicians and 9 NP/PA’s.
The SHM Wiregrass chapter is located in Dothan, Ala., a town of 80,000 near the Gulf of Mexico. Chapter leadership works at Southeast Health, a tertiary care facility with 420 beds, an affiliated medical school, and an internal medicine residency program. At the time of the exchange, the HMG at SEH has 28 physicians and 5 NP/PA’s.
These are two similarly sized hospital medicine programs, located in different geographic regions, and serving different populations. SHM board member Howard Epstein, MD, SFHM, vice president and chief medical officer of Presbyterian Healthcare Services in Albuquerque, participated on behalf of the Society when SEH faculty visited UNM. Kris Rehm, MD, SFHM, a pediatric hospitalist and the vice chair of outreach medicine at Vanderbilt University Medical Center, Nashville, came to Dothan during the faculty visit by UNM.
Two SEH faculty members, a physician and an NP, visited the University of New Mexico Hospital for one week. They participated as observers, rounding with the teams and meeting the UNM HMG leadership. The focus of the discussions included faculty education, a curriculum for quality improvement, and ways to address practice challenges. The SEH faculty also presented a QI project from their institution, and established collaborative relationships.
During the second part of the exchange, three UNM faculty members, including one physician and two NPs, visited SEH for one week. During the visit, they observed NP/PA hospitalist team models, discussed innovations, established mentoring relationships with leadership, and discussed QI projects at SEH. Additionally, the visiting UNM faculty participated in Women In Medicine events and participated as judges for a poster competition. They also had an opportunity to explore the rural landscape and visit the beach.
The evaluation process after the exchanges involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation changed our thinking as medical educators by addressing faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange program was an essential and meaningful innovation that resulted in increased SHM member engagement, crossinstitutional collaboration, networking, and mentorship.
This event created opportunities for faculty collaboration and expanded the professional network of participating institutions. The costs of the exchange were minimal given support from SHM. We believe that once the COVID pandemic has ended, this initiative has the potential to expand facilitated exchanges nationally and internationally, enhance faculty development, and improve medical education.
Dr. Apodaca is assistant professor and nurse practitioner hospitalist at the University of New Mexico. She serves as codirector of the UNM APP Hospital Medicine Fellowship and director of the APP Hospital Medicine Team. Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization.
The SHM Annual Conference is more than an educational event. It also provides an opportunity to collaborate, network and create innovative ideas to improve the quality of inpatient care.
During the 2019 Annual Conference (HM19) – the last “in-person” Annual Conference before the COVID pandemic – SHM chapter leaders from the New Mexico chapter (Krystle Apodaca) and the Wiregrass chapter (Amith Skandhan), which covers the counties of Southern Alabama and the Panhandle of Florida, met during a networking event.
As we talked, we realized the unique differences and similarities our practice settings shared. We debated the role of clinician wellbeing, quality of medical education, and faculty development on individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the Triple Aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Engagement in local SHM chapter activities promotes the efficiency of practice, a culture of wellness, and personal resilience. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of individual clinicians. During our discussion we realized that an interinstitutional exchange programs could provide a platform to exchange ideas and establish mentors.
The quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities.
Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs. Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs) but their faculty training can vary based on location.
As a young specialty, hospital medicine is still evolving and incorporating NPs/PAs and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. As chapter leaders we determined that an exchange program would afford the opportunity for visiting faculty members to experience these differences.
We shared the idea of a chapter-level exchange with SHM’s Chapter Development Committee and obtained chapter development funds to execute the event. We also requested that an SHM national board member visit during the exchange to provide insight and feedback. We researched the characteristics of individual academic HMGs and structured a faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for one week, with separate tracks for physicians and NPs/PAs, giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with the HMG and hospital leadership, to specifically address the visiting faculty’s institutional challenges.
The overall goal of the exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development and improve the quality of care. The focus of the exchange program was to share ideas and innovation, and learn the approaches to unique challenges at each institution. Out of this also grew collaboration and mentoring opportunities.
SHM’s New Mexico chapter is based in Albuquerque, a city in the desert Southwest with an ethnically diverse population of 545,000, The chapter leadership works at the University of New Mexico (UNM), a 553-bed medical center. UNM has a well-established internal medicine residency program, an academic hospitalist program, and an NP/PA fellowship program embedded within the hospital medicine department. At the time of the exchange, the HMG at UNM has 26 physicians and 9 NP/PA’s.
The SHM Wiregrass chapter is located in Dothan, Ala., a town of 80,000 near the Gulf of Mexico. Chapter leadership works at Southeast Health, a tertiary care facility with 420 beds, an affiliated medical school, and an internal medicine residency program. At the time of the exchange, the HMG at SEH has 28 physicians and 5 NP/PA’s.
These are two similarly sized hospital medicine programs, located in different geographic regions, and serving different populations. SHM board member Howard Epstein, MD, SFHM, vice president and chief medical officer of Presbyterian Healthcare Services in Albuquerque, participated on behalf of the Society when SEH faculty visited UNM. Kris Rehm, MD, SFHM, a pediatric hospitalist and the vice chair of outreach medicine at Vanderbilt University Medical Center, Nashville, came to Dothan during the faculty visit by UNM.
Two SEH faculty members, a physician and an NP, visited the University of New Mexico Hospital for one week. They participated as observers, rounding with the teams and meeting the UNM HMG leadership. The focus of the discussions included faculty education, a curriculum for quality improvement, and ways to address practice challenges. The SEH faculty also presented a QI project from their institution, and established collaborative relationships.
During the second part of the exchange, three UNM faculty members, including one physician and two NPs, visited SEH for one week. During the visit, they observed NP/PA hospitalist team models, discussed innovations, established mentoring relationships with leadership, and discussed QI projects at SEH. Additionally, the visiting UNM faculty participated in Women In Medicine events and participated as judges for a poster competition. They also had an opportunity to explore the rural landscape and visit the beach.
The evaluation process after the exchanges involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation changed our thinking as medical educators by addressing faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange program was an essential and meaningful innovation that resulted in increased SHM member engagement, crossinstitutional collaboration, networking, and mentorship.
This event created opportunities for faculty collaboration and expanded the professional network of participating institutions. The costs of the exchange were minimal given support from SHM. We believe that once the COVID pandemic has ended, this initiative has the potential to expand facilitated exchanges nationally and internationally, enhance faculty development, and improve medical education.
Dr. Apodaca is assistant professor and nurse practitioner hospitalist at the University of New Mexico. She serves as codirector of the UNM APP Hospital Medicine Fellowship and director of the APP Hospital Medicine Team. Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization.
The SHM Annual Conference is more than an educational event. It also provides an opportunity to collaborate, network and create innovative ideas to improve the quality of inpatient care.
During the 2019 Annual Conference (HM19) – the last “in-person” Annual Conference before the COVID pandemic – SHM chapter leaders from the New Mexico chapter (Krystle Apodaca) and the Wiregrass chapter (Amith Skandhan), which covers the counties of Southern Alabama and the Panhandle of Florida, met during a networking event.
As we talked, we realized the unique differences and similarities our practice settings shared. We debated the role of clinician wellbeing, quality of medical education, and faculty development on individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the Triple Aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Engagement in local SHM chapter activities promotes the efficiency of practice, a culture of wellness, and personal resilience. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of individual clinicians. During our discussion we realized that an interinstitutional exchange programs could provide a platform to exchange ideas and establish mentors.
The quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities.
Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs. Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs) but their faculty training can vary based on location.
As a young specialty, hospital medicine is still evolving and incorporating NPs/PAs and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. As chapter leaders we determined that an exchange program would afford the opportunity for visiting faculty members to experience these differences.
We shared the idea of a chapter-level exchange with SHM’s Chapter Development Committee and obtained chapter development funds to execute the event. We also requested that an SHM national board member visit during the exchange to provide insight and feedback. We researched the characteristics of individual academic HMGs and structured a faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for one week, with separate tracks for physicians and NPs/PAs, giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with the HMG and hospital leadership, to specifically address the visiting faculty’s institutional challenges.
The overall goal of the exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development and improve the quality of care. The focus of the exchange program was to share ideas and innovation, and learn the approaches to unique challenges at each institution. Out of this also grew collaboration and mentoring opportunities.
SHM’s New Mexico chapter is based in Albuquerque, a city in the desert Southwest with an ethnically diverse population of 545,000, The chapter leadership works at the University of New Mexico (UNM), a 553-bed medical center. UNM has a well-established internal medicine residency program, an academic hospitalist program, and an NP/PA fellowship program embedded within the hospital medicine department. At the time of the exchange, the HMG at UNM has 26 physicians and 9 NP/PA’s.
The SHM Wiregrass chapter is located in Dothan, Ala., a town of 80,000 near the Gulf of Mexico. Chapter leadership works at Southeast Health, a tertiary care facility with 420 beds, an affiliated medical school, and an internal medicine residency program. At the time of the exchange, the HMG at SEH has 28 physicians and 5 NP/PA’s.
These are two similarly sized hospital medicine programs, located in different geographic regions, and serving different populations. SHM board member Howard Epstein, MD, SFHM, vice president and chief medical officer of Presbyterian Healthcare Services in Albuquerque, participated on behalf of the Society when SEH faculty visited UNM. Kris Rehm, MD, SFHM, a pediatric hospitalist and the vice chair of outreach medicine at Vanderbilt University Medical Center, Nashville, came to Dothan during the faculty visit by UNM.
Two SEH faculty members, a physician and an NP, visited the University of New Mexico Hospital for one week. They participated as observers, rounding with the teams and meeting the UNM HMG leadership. The focus of the discussions included faculty education, a curriculum for quality improvement, and ways to address practice challenges. The SEH faculty also presented a QI project from their institution, and established collaborative relationships.
During the second part of the exchange, three UNM faculty members, including one physician and two NPs, visited SEH for one week. During the visit, they observed NP/PA hospitalist team models, discussed innovations, established mentoring relationships with leadership, and discussed QI projects at SEH. Additionally, the visiting UNM faculty participated in Women In Medicine events and participated as judges for a poster competition. They also had an opportunity to explore the rural landscape and visit the beach.
The evaluation process after the exchanges involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation changed our thinking as medical educators by addressing faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange program was an essential and meaningful innovation that resulted in increased SHM member engagement, crossinstitutional collaboration, networking, and mentorship.
This event created opportunities for faculty collaboration and expanded the professional network of participating institutions. The costs of the exchange were minimal given support from SHM. We believe that once the COVID pandemic has ended, this initiative has the potential to expand facilitated exchanges nationally and internationally, enhance faculty development, and improve medical education.
Dr. Apodaca is assistant professor and nurse practitioner hospitalist at the University of New Mexico. She serves as codirector of the UNM APP Hospital Medicine Fellowship and director of the APP Hospital Medicine Team. Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization.
Colonoscopy patients may get hit with a ‘surprise bill’
A colonoscopy screening for colorectal cancer should be covered by commercial health insurance, but a new study reports that some patients receive a “surprise” bill.
The study was published online Oct. 13 as a research letter in the Annals of Internal Medicine.
Nearly 1 in 8 commercially insured patients who had an elective colonoscopy between 2012 and 2017 received an out-of-network bill, resulting in hundreds of dollars more than the typical insurance payment.
The median surprise bill was $418 (range $152-$981).
The findings are “disconcerting” say the authors, “because Section 2713 of the Patient Protection and Affordable Care Act eliminates consumer cost sharing for screening colonoscopy, and because a recent Federal Reserve study reported that 40% of Americans do not have $400 to cover unnecessary expenses.”
Most of these surprise costs were incurred from the use of out-of network anesthesiologists and pathologists, the authors note.
“Doctors need to be aware of these out-of-network bills so that patients know what to expect when they undergo these screening procedures,” said study author Karan R. Chhabra, MD, MSc, a resident in general surgery at Brigham and Women’s Hospital, Boston, Massachusetts. “Ideally, they should do their colonoscopies at facilities where all providers participate in the same major insurance plans.”
“If gastroenterologists own their endoscopy facility, this is an obvious situation in which they should not be working with anesthesiologists or pathologists who are not in the same networks as them,” he told Medscape Medical News. “And as we point out in our paper, anesthesiology and pathology review are not necessary in every single case — endoscopists can perform their own sedation, and in certain settings, lesions can be discarded without pathological examination.”
But is it really that simple for physicians to make sure that all members of the team are in-network?
It’s not simple at all, and in fact it’s a rather difficult task, said Glenn Melnick, PhD, professor and chair in health care finance at USC and director of USC’s Center for Health Financing, Policy, and Management in Los Angeles.
“It would be really difficult for Dr Smith to know that Dr Jones is out of network, so it’s really hard to hold the doctors responsible,” Melnick told Medscape Medical News. “There are so many insurers and it may be difficult to know who is in-network and who isn’t.”
In this study, anesthesiologists and pathologists were a source of surprise bills, and they are behind the scenes, he pointed out. “The patient doesn’t select them directly and there is no opportunity to even find out who they are,” said Melnick.
Most patients have no idea that there may be other doctors involved with a colonoscopy, and Melnick highlighted his own recent experience. “I just had a colonoscopy and it never would have occurred to me. It never crossed my mind to even ask who is in network and who isn’t,” he said. “And I’m an expert on this.”
“The health plan could bear some responsibility here,” Melnick commented, although he added that patients need to be informed. Patients who are undergoing an elective procedure should be told that other doctors may be involved, and then to ask if these doctors are in the network. “If enough patients do this, maybe then the gastroenterologist will use people in network,” he commented.
Details of the surprise bills
Federal regulations eliminate consumer cost-sharing when screening colonoscopies are performed in-network, but there are no stipulations regarding expenses when out-of-network providers are used, the authors note.
To investigate this issue, the authors used a claims database from a large national insurer and identified patients aged 18 to 64 years who had undergone colonoscopy between 2012 and 2017.
The analysis was limited to cases where both the facility and the endoscopist were in-network, and the colonoscopies were stratified into those with visual inspection only and those during which an intervention was done, such as a biopsy. The primary outcome measure was the prevalence of out-of-network claims when the endoscopist and facility were in-network, and the secondary outcome was the amount of the potential surprise bills, which were calculated as the total out-of network charges less the typical in-network price.
A total of 1,118,769 elective colonoscopies with in-network endoscopists and facilities were identified and of these, 12.1% (n = 135,626) were involved with out-of-network claims. Out-of network anesthesiologists accounted for 64% of cases (median potential surprise bill, $488), while out-of-network pathologists were involved in 40% of cases (median potential surprise bill, $248). The likelihood of receiving an out-of-network claim was significantly higher if an intervention was performed during colonoscopy, as compared with those without intervention (13.9% vs. 8.2%; difference, 5.7%).
If an intervention was performed, 56% of potential surprise bills involved anesthesiologists and 51% pathologists. In cases with visual inspection only, 95% of out-of-network claims involved anesthesiologists.
The authors suggest that measures that can be taken to avoid surprise bills include having endoscopists and hospitals partner with anesthesia and pathology providers who are in-network. Another cost-saving strategy is the use of endoscopist-provided sedation rather than use of deeper anesthesia, and the authors also suggest that not all low-risk polyps need to be sent for pathological evaluation.
“Providers must realize many of our patients are at risk for considerable balance bills, and therefore they should provide resources that can provide reliable estimates for out-of-pocket costs relevant to site of service,” said lead author James Scheiman, MD, a professor of medicine at the University of Virginia School of Medicine in Charlottesville.
The study was funded by the University of Michigan. Chhabra reports personal fees from Blue Cross Blue Shield of Massachusetts, Inc. Scheiman and Melnick have no disclosures.
This article first appeared on Medscape.com.
Screening colonoscopy is the most cost effective test for prevention of colorectal cancer. The American Gastroenterological Association (AGA) believes that patients should be incentivized, through the elimination of cost sharing, to use colonoscopy as a colorectal cancer screening mechanism. Additionally, the preventive screening benefit has contributed to the decline in colorectal cancer rates in our country and this benefit should be preserved in any health care reform legislation. Learn more about how AGA advocates for patients at http://ow.ly/ULCZ30rf6J8.
Help your patients understand what to expect when paying for their colonoscopy by sharing AGA patient education at http://ow.ly/OteU30rf6HF.
A colonoscopy screening for colorectal cancer should be covered by commercial health insurance, but a new study reports that some patients receive a “surprise” bill.
The study was published online Oct. 13 as a research letter in the Annals of Internal Medicine.
Nearly 1 in 8 commercially insured patients who had an elective colonoscopy between 2012 and 2017 received an out-of-network bill, resulting in hundreds of dollars more than the typical insurance payment.
The median surprise bill was $418 (range $152-$981).
The findings are “disconcerting” say the authors, “because Section 2713 of the Patient Protection and Affordable Care Act eliminates consumer cost sharing for screening colonoscopy, and because a recent Federal Reserve study reported that 40% of Americans do not have $400 to cover unnecessary expenses.”
Most of these surprise costs were incurred from the use of out-of network anesthesiologists and pathologists, the authors note.
“Doctors need to be aware of these out-of-network bills so that patients know what to expect when they undergo these screening procedures,” said study author Karan R. Chhabra, MD, MSc, a resident in general surgery at Brigham and Women’s Hospital, Boston, Massachusetts. “Ideally, they should do their colonoscopies at facilities where all providers participate in the same major insurance plans.”
“If gastroenterologists own their endoscopy facility, this is an obvious situation in which they should not be working with anesthesiologists or pathologists who are not in the same networks as them,” he told Medscape Medical News. “And as we point out in our paper, anesthesiology and pathology review are not necessary in every single case — endoscopists can perform their own sedation, and in certain settings, lesions can be discarded without pathological examination.”
But is it really that simple for physicians to make sure that all members of the team are in-network?
It’s not simple at all, and in fact it’s a rather difficult task, said Glenn Melnick, PhD, professor and chair in health care finance at USC and director of USC’s Center for Health Financing, Policy, and Management in Los Angeles.
“It would be really difficult for Dr Smith to know that Dr Jones is out of network, so it’s really hard to hold the doctors responsible,” Melnick told Medscape Medical News. “There are so many insurers and it may be difficult to know who is in-network and who isn’t.”
In this study, anesthesiologists and pathologists were a source of surprise bills, and they are behind the scenes, he pointed out. “The patient doesn’t select them directly and there is no opportunity to even find out who they are,” said Melnick.
Most patients have no idea that there may be other doctors involved with a colonoscopy, and Melnick highlighted his own recent experience. “I just had a colonoscopy and it never would have occurred to me. It never crossed my mind to even ask who is in network and who isn’t,” he said. “And I’m an expert on this.”
“The health plan could bear some responsibility here,” Melnick commented, although he added that patients need to be informed. Patients who are undergoing an elective procedure should be told that other doctors may be involved, and then to ask if these doctors are in the network. “If enough patients do this, maybe then the gastroenterologist will use people in network,” he commented.
Details of the surprise bills
Federal regulations eliminate consumer cost-sharing when screening colonoscopies are performed in-network, but there are no stipulations regarding expenses when out-of-network providers are used, the authors note.
To investigate this issue, the authors used a claims database from a large national insurer and identified patients aged 18 to 64 years who had undergone colonoscopy between 2012 and 2017.
The analysis was limited to cases where both the facility and the endoscopist were in-network, and the colonoscopies were stratified into those with visual inspection only and those during which an intervention was done, such as a biopsy. The primary outcome measure was the prevalence of out-of-network claims when the endoscopist and facility were in-network, and the secondary outcome was the amount of the potential surprise bills, which were calculated as the total out-of network charges less the typical in-network price.
A total of 1,118,769 elective colonoscopies with in-network endoscopists and facilities were identified and of these, 12.1% (n = 135,626) were involved with out-of-network claims. Out-of network anesthesiologists accounted for 64% of cases (median potential surprise bill, $488), while out-of-network pathologists were involved in 40% of cases (median potential surprise bill, $248). The likelihood of receiving an out-of-network claim was significantly higher if an intervention was performed during colonoscopy, as compared with those without intervention (13.9% vs. 8.2%; difference, 5.7%).
If an intervention was performed, 56% of potential surprise bills involved anesthesiologists and 51% pathologists. In cases with visual inspection only, 95% of out-of-network claims involved anesthesiologists.
The authors suggest that measures that can be taken to avoid surprise bills include having endoscopists and hospitals partner with anesthesia and pathology providers who are in-network. Another cost-saving strategy is the use of endoscopist-provided sedation rather than use of deeper anesthesia, and the authors also suggest that not all low-risk polyps need to be sent for pathological evaluation.
“Providers must realize many of our patients are at risk for considerable balance bills, and therefore they should provide resources that can provide reliable estimates for out-of-pocket costs relevant to site of service,” said lead author James Scheiman, MD, a professor of medicine at the University of Virginia School of Medicine in Charlottesville.
The study was funded by the University of Michigan. Chhabra reports personal fees from Blue Cross Blue Shield of Massachusetts, Inc. Scheiman and Melnick have no disclosures.
This article first appeared on Medscape.com.
Screening colonoscopy is the most cost effective test for prevention of colorectal cancer. The American Gastroenterological Association (AGA) believes that patients should be incentivized, through the elimination of cost sharing, to use colonoscopy as a colorectal cancer screening mechanism. Additionally, the preventive screening benefit has contributed to the decline in colorectal cancer rates in our country and this benefit should be preserved in any health care reform legislation. Learn more about how AGA advocates for patients at http://ow.ly/ULCZ30rf6J8.
Help your patients understand what to expect when paying for their colonoscopy by sharing AGA patient education at http://ow.ly/OteU30rf6HF.
A colonoscopy screening for colorectal cancer should be covered by commercial health insurance, but a new study reports that some patients receive a “surprise” bill.
The study was published online Oct. 13 as a research letter in the Annals of Internal Medicine.
Nearly 1 in 8 commercially insured patients who had an elective colonoscopy between 2012 and 2017 received an out-of-network bill, resulting in hundreds of dollars more than the typical insurance payment.
The median surprise bill was $418 (range $152-$981).
The findings are “disconcerting” say the authors, “because Section 2713 of the Patient Protection and Affordable Care Act eliminates consumer cost sharing for screening colonoscopy, and because a recent Federal Reserve study reported that 40% of Americans do not have $400 to cover unnecessary expenses.”
Most of these surprise costs were incurred from the use of out-of network anesthesiologists and pathologists, the authors note.
“Doctors need to be aware of these out-of-network bills so that patients know what to expect when they undergo these screening procedures,” said study author Karan R. Chhabra, MD, MSc, a resident in general surgery at Brigham and Women’s Hospital, Boston, Massachusetts. “Ideally, they should do their colonoscopies at facilities where all providers participate in the same major insurance plans.”
“If gastroenterologists own their endoscopy facility, this is an obvious situation in which they should not be working with anesthesiologists or pathologists who are not in the same networks as them,” he told Medscape Medical News. “And as we point out in our paper, anesthesiology and pathology review are not necessary in every single case — endoscopists can perform their own sedation, and in certain settings, lesions can be discarded without pathological examination.”
But is it really that simple for physicians to make sure that all members of the team are in-network?
It’s not simple at all, and in fact it’s a rather difficult task, said Glenn Melnick, PhD, professor and chair in health care finance at USC and director of USC’s Center for Health Financing, Policy, and Management in Los Angeles.
“It would be really difficult for Dr Smith to know that Dr Jones is out of network, so it’s really hard to hold the doctors responsible,” Melnick told Medscape Medical News. “There are so many insurers and it may be difficult to know who is in-network and who isn’t.”
In this study, anesthesiologists and pathologists were a source of surprise bills, and they are behind the scenes, he pointed out. “The patient doesn’t select them directly and there is no opportunity to even find out who they are,” said Melnick.
Most patients have no idea that there may be other doctors involved with a colonoscopy, and Melnick highlighted his own recent experience. “I just had a colonoscopy and it never would have occurred to me. It never crossed my mind to even ask who is in network and who isn’t,” he said. “And I’m an expert on this.”
“The health plan could bear some responsibility here,” Melnick commented, although he added that patients need to be informed. Patients who are undergoing an elective procedure should be told that other doctors may be involved, and then to ask if these doctors are in the network. “If enough patients do this, maybe then the gastroenterologist will use people in network,” he commented.
Details of the surprise bills
Federal regulations eliminate consumer cost-sharing when screening colonoscopies are performed in-network, but there are no stipulations regarding expenses when out-of-network providers are used, the authors note.
To investigate this issue, the authors used a claims database from a large national insurer and identified patients aged 18 to 64 years who had undergone colonoscopy between 2012 and 2017.
The analysis was limited to cases where both the facility and the endoscopist were in-network, and the colonoscopies were stratified into those with visual inspection only and those during which an intervention was done, such as a biopsy. The primary outcome measure was the prevalence of out-of-network claims when the endoscopist and facility were in-network, and the secondary outcome was the amount of the potential surprise bills, which were calculated as the total out-of network charges less the typical in-network price.
A total of 1,118,769 elective colonoscopies with in-network endoscopists and facilities were identified and of these, 12.1% (n = 135,626) were involved with out-of-network claims. Out-of network anesthesiologists accounted for 64% of cases (median potential surprise bill, $488), while out-of-network pathologists were involved in 40% of cases (median potential surprise bill, $248). The likelihood of receiving an out-of-network claim was significantly higher if an intervention was performed during colonoscopy, as compared with those without intervention (13.9% vs. 8.2%; difference, 5.7%).
If an intervention was performed, 56% of potential surprise bills involved anesthesiologists and 51% pathologists. In cases with visual inspection only, 95% of out-of-network claims involved anesthesiologists.
The authors suggest that measures that can be taken to avoid surprise bills include having endoscopists and hospitals partner with anesthesia and pathology providers who are in-network. Another cost-saving strategy is the use of endoscopist-provided sedation rather than use of deeper anesthesia, and the authors also suggest that not all low-risk polyps need to be sent for pathological evaluation.
“Providers must realize many of our patients are at risk for considerable balance bills, and therefore they should provide resources that can provide reliable estimates for out-of-pocket costs relevant to site of service,” said lead author James Scheiman, MD, a professor of medicine at the University of Virginia School of Medicine in Charlottesville.
The study was funded by the University of Michigan. Chhabra reports personal fees from Blue Cross Blue Shield of Massachusetts, Inc. Scheiman and Melnick have no disclosures.
This article first appeared on Medscape.com.
Screening colonoscopy is the most cost effective test for prevention of colorectal cancer. The American Gastroenterological Association (AGA) believes that patients should be incentivized, through the elimination of cost sharing, to use colonoscopy as a colorectal cancer screening mechanism. Additionally, the preventive screening benefit has contributed to the decline in colorectal cancer rates in our country and this benefit should be preserved in any health care reform legislation. Learn more about how AGA advocates for patients at http://ow.ly/ULCZ30rf6J8.
Help your patients understand what to expect when paying for their colonoscopy by sharing AGA patient education at http://ow.ly/OteU30rf6HF.
Rapidly developing vesicular eruption
A 23-month-old girl with a history of well-controlled atopic dermatitis was admitted to the hospital with fever and a widespread vesicular eruption of 2 days’ duration. Two days prior to admission, the patient had 3 episodes of nonbloody diarrhea and redness in the diaper area. The child’s parents reported that the red areas spread to her arms and legs later that day, and that she subsequently developed a fever, cough, and rhinorrhea. She was taken to an urgent care facility where she was diagnosed with vulvovaginitis and an upper respiratory infection; amoxicillin was prescribed. Shortly thereafter, the patient developed more lesions in and around the mouth, as well as on the trunk, prompting the parents to bring her to the emergency department.
The history revealed that the patient had spent time with her aunt and cousins who had “red spots” on their palms and soles. The patient’s sister had a flare of “cold sores,” about 2 weeks prior to the current presentation. The patient had received a varicella zoster virus (VZV) vaccine several months earlier.
Physical examination was notable for an uncomfortable infant with erythematous macules on the bilateral palms and soles and an erythematous hard palate. The child also had scattered vesicles on an erythematous base with confluent crusted plaques on her lips, perioral skin (FIGURE 1A), abdomen, back, buttocks, arms, legs (FIGURE 1B), and dorsal aspects of her hands and feet.
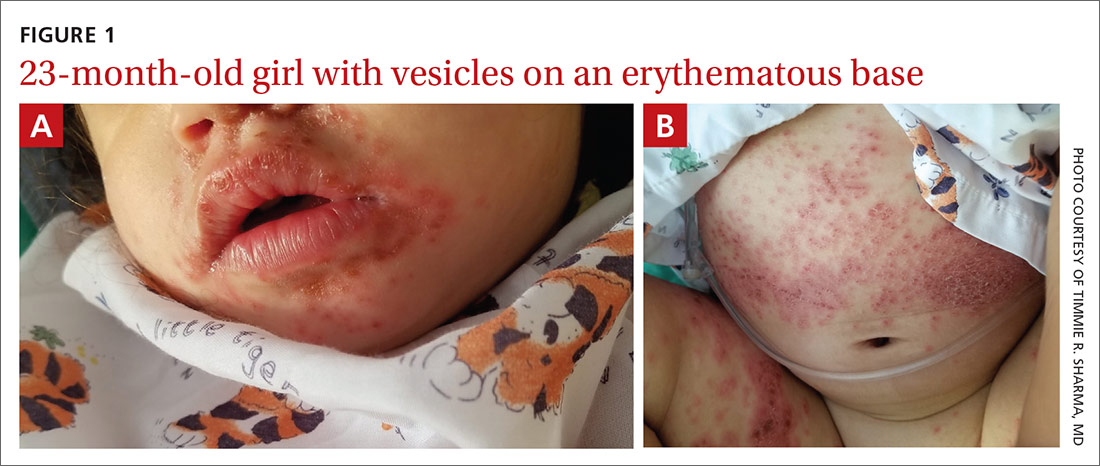
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema coxsackium
Given the history of atopic dermatitis; prodromal diarrhea/rhinorrhea; papulovesicular eruption involving areas of prior dermatitis as well as the palms, soles, and mouth; recent contacts with suspected hand-foot-mouth disease (HFMD); and history of VZV vaccination, the favored diagnosis was eczema coxsackium.
Eczema coxsackium is an atypical form of HFMD that occurs in patients with a history of eczema. Classic HFMD usually is caused by coxsackievirus A16 or enterovirus 71, while atypical HFMD often is caused by coxsackievirus A6.1,2,3 Patients with HFMD present with painful oral vesicles and ulcers and a papulovesicular eruption on the palms, soles, and sometimes the buttocks and genitalia. Patients may have prodromal fever, fussiness, and diarrhea. Painful oral lesions may result in poor oral intake.1,2
Differential includes viral eruptions
Other conditions may manifest similarly to eczema coxsackium and must be ruled out before initiating proper treatment.
Eczema herpeticum (EH). In atypical HFMD, the virus can show tropism for active or previously inflamed areas of eczematous skin, leading to a widespread vesicular eruption, which can be difficult to distinguish from EH.1 Similar to EH, eczema coxsackium does not exclusively affect children with atopic dermatitis. It also has been described in adults and patients with Darier disease, incontinentia pigmenti, and epidermolytic ichthyosis.4-6
In cases of vesicular eruptions in eczema patients, it is imperative to rule out EH. One prospective study of atypical HFMD compared similarities of the conditions. Both have a predilection for mucosa during primary infection and develop vesicular eruptions on cutaneous eczematous skin.1 One key difference between eczema coxsackium and EH is that EH tends to produce intraoral vesicles beyond simple erythema; it also tends to predominate in the area of the head and neck.7
Continue to: Eczema varicellicum
Eczema varicellicum has been reported, and it has been suggested that some cases of EH may actually be caused by VZV as the 2 are clinically indistinguishable and less than half of EH cases are diagnosed with laboratory confirmation.8
Confirm Dx before you treat
To guide management, cases of suspected eczema coxsackium should be confirmed, and HSV/VZV should be ruled out.9 Testing modalities include swabbing vesicular fluid for enterovirus polymerase chain reaction (PCR) analysis (preferred modality), oropharyngeal swab up to 2 weeks after infection, or viral isolate from stool samples up to 3 months after infection.2,3
Treatment for eczema coxsackium involves supportive care such as intravenous (IV) hydration and antipyretics. Some studies show potential benefit with IV immunoglobulin in treating severe HFMD, while other studies show the exacerbation of widespread HFMD with this treatment.7,10
Prompt diagnosis and treatment for eczema coxsackium is critical to prevent unnecessary antiviral therapy and to help guide monitoring for associated morbidities including Gianotti-Crosti syndrome–like eruptions, purpuric eruptions, and onychomadesis.
Our patient. Because EH was in the differential, our patient was started on empiric IV acyclovir 10 mg/kg every 8 hours while test results were pending. In addition, she received acetaminophen, IV fluids, gentle sponge baths, and diligent emollient application. Scraping from a vesicle revealed negative herpes simplex virus 1/2 PCR, negative VZV direct fluorescent antibody, and a positive enterovirus PCR—confirming the diagnosis of eczema coxsackium. Interestingly, a viral culture was negative in our patient, consistent with prior reports of enterovirus being difficult to culture.11
With confirmation of the diagnosis of eczema coxsackium, the IV acyclovir was discontinued, and symptoms resolved after 7 days.
CORRESPONDENCE
Shane M. Swink, DO, MS, Division of Dermatology, 1200 South Cedar Crest Boulevard, Allentown, PA 18103; [email protected]
1. Neri I, Dondi A, Wollenberg A, et al. Atypical forms of hand, foot, and mouth disease: a prospective study of 47 Italian children. Pediatr Dermatol. 2016;33:429-437.
2. Nassef C, Ziemer C, Morrell DS. Hand-foot-and-mouth disease: a new look at a classic viral rash. Curr Opin Pediatr. 2015;27:486-491.
3. Horsten H, Fisker N, Bygu, A. Eczema coxsackium caused by coxsackievirus A6. Pediatr Dermatol. 2016;33:230-231.
4. Jefferson J, Grossberg A. Incontinentia pigmenti coxsackium. Pediatr Dermatol. 2016;33:E280-E281.
5. Ganguly S, Kuruvila S. Eczema coxsackium. Indian J Dermatol. 2016;61:682-683.
6. Harris P, Wang AD, Yin M, et al. Atypical hand, foot, and mouth disease: eczema coxsackium can also occur in adults. Lancet Infect Dis. 2014;14:1043.
7. Wollenberg A, Zoch C, Wetzel S, et al. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198-205.
8. Austin TA, Steele RW. Eczema varicella/zoster (varicellicum). Clin Pediatr. 2017;56:579-581.
9. Leung DYM. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
10. Cao RY, Dong DY, Liu RJ, et al. Human IgG subclasses against enterovirus type 71: neutralization versus antibody dependent enhancement of infection. PLoS One. 2013;8:E64024.
11. Mathes EF, Oza V, Frieden IJ, et al. Eczema coxsackium and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:149-157.
A 23-month-old girl with a history of well-controlled atopic dermatitis was admitted to the hospital with fever and a widespread vesicular eruption of 2 days’ duration. Two days prior to admission, the patient had 3 episodes of nonbloody diarrhea and redness in the diaper area. The child’s parents reported that the red areas spread to her arms and legs later that day, and that she subsequently developed a fever, cough, and rhinorrhea. She was taken to an urgent care facility where she was diagnosed with vulvovaginitis and an upper respiratory infection; amoxicillin was prescribed. Shortly thereafter, the patient developed more lesions in and around the mouth, as well as on the trunk, prompting the parents to bring her to the emergency department.
The history revealed that the patient had spent time with her aunt and cousins who had “red spots” on their palms and soles. The patient’s sister had a flare of “cold sores,” about 2 weeks prior to the current presentation. The patient had received a varicella zoster virus (VZV) vaccine several months earlier.
Physical examination was notable for an uncomfortable infant with erythematous macules on the bilateral palms and soles and an erythematous hard palate. The child also had scattered vesicles on an erythematous base with confluent crusted plaques on her lips, perioral skin (FIGURE 1A), abdomen, back, buttocks, arms, legs (FIGURE 1B), and dorsal aspects of her hands and feet.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema coxsackium
Given the history of atopic dermatitis; prodromal diarrhea/rhinorrhea; papulovesicular eruption involving areas of prior dermatitis as well as the palms, soles, and mouth; recent contacts with suspected hand-foot-mouth disease (HFMD); and history of VZV vaccination, the favored diagnosis was eczema coxsackium.
Eczema coxsackium is an atypical form of HFMD that occurs in patients with a history of eczema. Classic HFMD usually is caused by coxsackievirus A16 or enterovirus 71, while atypical HFMD often is caused by coxsackievirus A6.1,2,3 Patients with HFMD present with painful oral vesicles and ulcers and a papulovesicular eruption on the palms, soles, and sometimes the buttocks and genitalia. Patients may have prodromal fever, fussiness, and diarrhea. Painful oral lesions may result in poor oral intake.1,2
Differential includes viral eruptions
Other conditions may manifest similarly to eczema coxsackium and must be ruled out before initiating proper treatment.
Eczema herpeticum (EH). In atypical HFMD, the virus can show tropism for active or previously inflamed areas of eczematous skin, leading to a widespread vesicular eruption, which can be difficult to distinguish from EH.1 Similar to EH, eczema coxsackium does not exclusively affect children with atopic dermatitis. It also has been described in adults and patients with Darier disease, incontinentia pigmenti, and epidermolytic ichthyosis.4-6
In cases of vesicular eruptions in eczema patients, it is imperative to rule out EH. One prospective study of atypical HFMD compared similarities of the conditions. Both have a predilection for mucosa during primary infection and develop vesicular eruptions on cutaneous eczematous skin.1 One key difference between eczema coxsackium and EH is that EH tends to produce intraoral vesicles beyond simple erythema; it also tends to predominate in the area of the head and neck.7
Continue to: Eczema varicellicum
Eczema varicellicum has been reported, and it has been suggested that some cases of EH may actually be caused by VZV as the 2 are clinically indistinguishable and less than half of EH cases are diagnosed with laboratory confirmation.8
Confirm Dx before you treat
To guide management, cases of suspected eczema coxsackium should be confirmed, and HSV/VZV should be ruled out.9 Testing modalities include swabbing vesicular fluid for enterovirus polymerase chain reaction (PCR) analysis (preferred modality), oropharyngeal swab up to 2 weeks after infection, or viral isolate from stool samples up to 3 months after infection.2,3
Treatment for eczema coxsackium involves supportive care such as intravenous (IV) hydration and antipyretics. Some studies show potential benefit with IV immunoglobulin in treating severe HFMD, while other studies show the exacerbation of widespread HFMD with this treatment.7,10
Prompt diagnosis and treatment for eczema coxsackium is critical to prevent unnecessary antiviral therapy and to help guide monitoring for associated morbidities including Gianotti-Crosti syndrome–like eruptions, purpuric eruptions, and onychomadesis.
Our patient. Because EH was in the differential, our patient was started on empiric IV acyclovir 10 mg/kg every 8 hours while test results were pending. In addition, she received acetaminophen, IV fluids, gentle sponge baths, and diligent emollient application. Scraping from a vesicle revealed negative herpes simplex virus 1/2 PCR, negative VZV direct fluorescent antibody, and a positive enterovirus PCR—confirming the diagnosis of eczema coxsackium. Interestingly, a viral culture was negative in our patient, consistent with prior reports of enterovirus being difficult to culture.11
With confirmation of the diagnosis of eczema coxsackium, the IV acyclovir was discontinued, and symptoms resolved after 7 days.
CORRESPONDENCE
Shane M. Swink, DO, MS, Division of Dermatology, 1200 South Cedar Crest Boulevard, Allentown, PA 18103; [email protected]
A 23-month-old girl with a history of well-controlled atopic dermatitis was admitted to the hospital with fever and a widespread vesicular eruption of 2 days’ duration. Two days prior to admission, the patient had 3 episodes of nonbloody diarrhea and redness in the diaper area. The child’s parents reported that the red areas spread to her arms and legs later that day, and that she subsequently developed a fever, cough, and rhinorrhea. She was taken to an urgent care facility where she was diagnosed with vulvovaginitis and an upper respiratory infection; amoxicillin was prescribed. Shortly thereafter, the patient developed more lesions in and around the mouth, as well as on the trunk, prompting the parents to bring her to the emergency department.
The history revealed that the patient had spent time with her aunt and cousins who had “red spots” on their palms and soles. The patient’s sister had a flare of “cold sores,” about 2 weeks prior to the current presentation. The patient had received a varicella zoster virus (VZV) vaccine several months earlier.
Physical examination was notable for an uncomfortable infant with erythematous macules on the bilateral palms and soles and an erythematous hard palate. The child also had scattered vesicles on an erythematous base with confluent crusted plaques on her lips, perioral skin (FIGURE 1A), abdomen, back, buttocks, arms, legs (FIGURE 1B), and dorsal aspects of her hands and feet.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema coxsackium
Given the history of atopic dermatitis; prodromal diarrhea/rhinorrhea; papulovesicular eruption involving areas of prior dermatitis as well as the palms, soles, and mouth; recent contacts with suspected hand-foot-mouth disease (HFMD); and history of VZV vaccination, the favored diagnosis was eczema coxsackium.
Eczema coxsackium is an atypical form of HFMD that occurs in patients with a history of eczema. Classic HFMD usually is caused by coxsackievirus A16 or enterovirus 71, while atypical HFMD often is caused by coxsackievirus A6.1,2,3 Patients with HFMD present with painful oral vesicles and ulcers and a papulovesicular eruption on the palms, soles, and sometimes the buttocks and genitalia. Patients may have prodromal fever, fussiness, and diarrhea. Painful oral lesions may result in poor oral intake.1,2
Differential includes viral eruptions
Other conditions may manifest similarly to eczema coxsackium and must be ruled out before initiating proper treatment.
Eczema herpeticum (EH). In atypical HFMD, the virus can show tropism for active or previously inflamed areas of eczematous skin, leading to a widespread vesicular eruption, which can be difficult to distinguish from EH.1 Similar to EH, eczema coxsackium does not exclusively affect children with atopic dermatitis. It also has been described in adults and patients with Darier disease, incontinentia pigmenti, and epidermolytic ichthyosis.4-6
In cases of vesicular eruptions in eczema patients, it is imperative to rule out EH. One prospective study of atypical HFMD compared similarities of the conditions. Both have a predilection for mucosa during primary infection and develop vesicular eruptions on cutaneous eczematous skin.1 One key difference between eczema coxsackium and EH is that EH tends to produce intraoral vesicles beyond simple erythema; it also tends to predominate in the area of the head and neck.7
Continue to: Eczema varicellicum
Eczema varicellicum has been reported, and it has been suggested that some cases of EH may actually be caused by VZV as the 2 are clinically indistinguishable and less than half of EH cases are diagnosed with laboratory confirmation.8
Confirm Dx before you treat
To guide management, cases of suspected eczema coxsackium should be confirmed, and HSV/VZV should be ruled out.9 Testing modalities include swabbing vesicular fluid for enterovirus polymerase chain reaction (PCR) analysis (preferred modality), oropharyngeal swab up to 2 weeks after infection, or viral isolate from stool samples up to 3 months after infection.2,3
Treatment for eczema coxsackium involves supportive care such as intravenous (IV) hydration and antipyretics. Some studies show potential benefit with IV immunoglobulin in treating severe HFMD, while other studies show the exacerbation of widespread HFMD with this treatment.7,10
Prompt diagnosis and treatment for eczema coxsackium is critical to prevent unnecessary antiviral therapy and to help guide monitoring for associated morbidities including Gianotti-Crosti syndrome–like eruptions, purpuric eruptions, and onychomadesis.
Our patient. Because EH was in the differential, our patient was started on empiric IV acyclovir 10 mg/kg every 8 hours while test results were pending. In addition, she received acetaminophen, IV fluids, gentle sponge baths, and diligent emollient application. Scraping from a vesicle revealed negative herpes simplex virus 1/2 PCR, negative VZV direct fluorescent antibody, and a positive enterovirus PCR—confirming the diagnosis of eczema coxsackium. Interestingly, a viral culture was negative in our patient, consistent with prior reports of enterovirus being difficult to culture.11
With confirmation of the diagnosis of eczema coxsackium, the IV acyclovir was discontinued, and symptoms resolved after 7 days.
CORRESPONDENCE
Shane M. Swink, DO, MS, Division of Dermatology, 1200 South Cedar Crest Boulevard, Allentown, PA 18103; [email protected]
1. Neri I, Dondi A, Wollenberg A, et al. Atypical forms of hand, foot, and mouth disease: a prospective study of 47 Italian children. Pediatr Dermatol. 2016;33:429-437.
2. Nassef C, Ziemer C, Morrell DS. Hand-foot-and-mouth disease: a new look at a classic viral rash. Curr Opin Pediatr. 2015;27:486-491.
3. Horsten H, Fisker N, Bygu, A. Eczema coxsackium caused by coxsackievirus A6. Pediatr Dermatol. 2016;33:230-231.
4. Jefferson J, Grossberg A. Incontinentia pigmenti coxsackium. Pediatr Dermatol. 2016;33:E280-E281.
5. Ganguly S, Kuruvila S. Eczema coxsackium. Indian J Dermatol. 2016;61:682-683.
6. Harris P, Wang AD, Yin M, et al. Atypical hand, foot, and mouth disease: eczema coxsackium can also occur in adults. Lancet Infect Dis. 2014;14:1043.
7. Wollenberg A, Zoch C, Wetzel S, et al. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198-205.
8. Austin TA, Steele RW. Eczema varicella/zoster (varicellicum). Clin Pediatr. 2017;56:579-581.
9. Leung DYM. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
10. Cao RY, Dong DY, Liu RJ, et al. Human IgG subclasses against enterovirus type 71: neutralization versus antibody dependent enhancement of infection. PLoS One. 2013;8:E64024.
11. Mathes EF, Oza V, Frieden IJ, et al. Eczema coxsackium and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:149-157.
1. Neri I, Dondi A, Wollenberg A, et al. Atypical forms of hand, foot, and mouth disease: a prospective study of 47 Italian children. Pediatr Dermatol. 2016;33:429-437.
2. Nassef C, Ziemer C, Morrell DS. Hand-foot-and-mouth disease: a new look at a classic viral rash. Curr Opin Pediatr. 2015;27:486-491.
3. Horsten H, Fisker N, Bygu, A. Eczema coxsackium caused by coxsackievirus A6. Pediatr Dermatol. 2016;33:230-231.
4. Jefferson J, Grossberg A. Incontinentia pigmenti coxsackium. Pediatr Dermatol. 2016;33:E280-E281.
5. Ganguly S, Kuruvila S. Eczema coxsackium. Indian J Dermatol. 2016;61:682-683.
6. Harris P, Wang AD, Yin M, et al. Atypical hand, foot, and mouth disease: eczema coxsackium can also occur in adults. Lancet Infect Dis. 2014;14:1043.
7. Wollenberg A, Zoch C, Wetzel S, et al. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198-205.
8. Austin TA, Steele RW. Eczema varicella/zoster (varicellicum). Clin Pediatr. 2017;56:579-581.
9. Leung DYM. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
10. Cao RY, Dong DY, Liu RJ, et al. Human IgG subclasses against enterovirus type 71: neutralization versus antibody dependent enhancement of infection. PLoS One. 2013;8:E64024.
11. Mathes EF, Oza V, Frieden IJ, et al. Eczema coxsackium and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:149-157.
Index finger plaque

The characteristic finding of small, scattered vesicular lesions on the hands that sometimes coalesce, and often are itchy or irritated led to the diagnosis of vesicular hand dermatitis, a form of eczema. It also is referred to as dyshidrotic eczema or pompholyx. (Worth noting is the fact that common warts and flat warts usually present as raised papular—not vesicular—lesions on the hands.)
The exact etiology of vesicular hand dermatitis is unknown. It is more common in women than men and often occurs in patients 20 to 40 years of age who tend to have a positive family history of eczema. It usually develops acutely and often is triggered by topical irritants or frequent hand washing. Treatment during the acute phase includes topical steroids. Avoidance of topical irritants, use of mild cleansers instead of harsh soaps, reduction of hand washing frequency (if possible), and frequent application of emollients can reduce recurrence.
This patient’s eczema had been successfully treated with betamethasone dipropionate ointment 0.05% in the past. Since she still had some at home, she was instructed to use it twice daily along with topical emmolients. She reported great improvement within 1 week.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sobering G, Dika C. Vesicular hand dermatitis. Nurse Pract. 2018;43:33-37.

The characteristic finding of small, scattered vesicular lesions on the hands that sometimes coalesce, and often are itchy or irritated led to the diagnosis of vesicular hand dermatitis, a form of eczema. It also is referred to as dyshidrotic eczema or pompholyx. (Worth noting is the fact that common warts and flat warts usually present as raised papular—not vesicular—lesions on the hands.)
The exact etiology of vesicular hand dermatitis is unknown. It is more common in women than men and often occurs in patients 20 to 40 years of age who tend to have a positive family history of eczema. It usually develops acutely and often is triggered by topical irritants or frequent hand washing. Treatment during the acute phase includes topical steroids. Avoidance of topical irritants, use of mild cleansers instead of harsh soaps, reduction of hand washing frequency (if possible), and frequent application of emollients can reduce recurrence.
This patient’s eczema had been successfully treated with betamethasone dipropionate ointment 0.05% in the past. Since she still had some at home, she was instructed to use it twice daily along with topical emmolients. She reported great improvement within 1 week.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The characteristic finding of small, scattered vesicular lesions on the hands that sometimes coalesce, and often are itchy or irritated led to the diagnosis of vesicular hand dermatitis, a form of eczema. It also is referred to as dyshidrotic eczema or pompholyx. (Worth noting is the fact that common warts and flat warts usually present as raised papular—not vesicular—lesions on the hands.)
The exact etiology of vesicular hand dermatitis is unknown. It is more common in women than men and often occurs in patients 20 to 40 years of age who tend to have a positive family history of eczema. It usually develops acutely and often is triggered by topical irritants or frequent hand washing. Treatment during the acute phase includes topical steroids. Avoidance of topical irritants, use of mild cleansers instead of harsh soaps, reduction of hand washing frequency (if possible), and frequent application of emollients can reduce recurrence.
This patient’s eczema had been successfully treated with betamethasone dipropionate ointment 0.05% in the past. Since she still had some at home, she was instructed to use it twice daily along with topical emmolients. She reported great improvement within 1 week.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sobering G, Dika C. Vesicular hand dermatitis. Nurse Pract. 2018;43:33-37.
Sobering G, Dika C. Vesicular hand dermatitis. Nurse Pract. 2018;43:33-37.
Upadacitinib more effective, less safe than abatacept for RA
Upadacitinib (Rinvoq) proved superior to abatacept in both disease activity and remission in rheumatoid arthritis patients yet led to more adverse events, according to a new study that compared the two drugs.
“Additional data from longer and larger trials are needed to better understand long-term outcomes and safety of upadacitinib as compared with other drugs for the treatment of rheumatoid arthritis,” wrote Andrea Rubbert-Roth, MD, of the Cantonal Clinic St. Gallen in St. Gallen, Switzerland, and her colleagues. The study was published in the New England Journal of Medicine.
The Food and Drug Administration approved upadacitinib for the treatment of rheumatoid arthritis in August 2019.
To compare the Janus kinase (JAK) inhibitor upadacitinib and the biologic disease-modifying antirheumatic drug (DMARD) abatacept as safe and effective treatments for RA, the researchers launched a randomized, double-blind, phase 3 clinical trial dubbed SELECT-CHOICE at 120 sites in 28 countries. All patients had moderate to severe active disease after previously having inadequate responses to at least one biologic DMARD. Slightly more than 82% of the participants were female, with a mean age of 55 years and mean RA duration of 12 years.
Patients were assigned either 15 mg of oral upadacitinib daily (n = 303) or intravenous abatacept at day 1 and weeks 2, 4, 8, 12, 16 and 20 (n = 309) with dosage tied to body weight, each in combination with stable synthetic DMARDs. Disease activity was measured after 12 weeks via the Disease Activity Score for 28 joints using C-reactive protein (DAS28-CRP). A DAS28-CRP of more than 5.1 was categorized as high disease activity, while 3.2-5.1 meant moderate disease activity, 2.6-3.2 meant low disease activity, and less than 2.6 indicated remission.
The mean DAS28-CRP at baseline was 5.70 in the upadacitinib group and 5.88 in the abatacept group. After 12 weeks, the mean change from baseline was –2.52 points and –2.00 points, respectively (difference, –0.52 points; 95% confidence interval, –0.69 to –0.35; P < .001 for noninferiority; P < .001 for superiority). In patients with a DAS28-CRP of less than 2.6, the percentage of those having remission was 30% with upadacitinib and 13.3% with abatacept (difference, 16.8 percentage points; 95% CI, 10.4 to 23.2; P < .001 for superiority).
Over the 24-week trial period, the incidence of all adverse events (209 vs. 189) and serious adverse events (10 vs. 5) was higher in the upadacitinib group than in the abatacept group. There were 23 cases of hepatic disorder with upadacitinib, compared with 5 with abatacept; 2 thromboembolic events with upadacitinib, compared with 0 with abatacept; and 2 deaths with upadacitinib, compared with 1 with abatacept.
“The thing that bothers me, actually, is the adverse events,” Daniel E. Furst, MD, professor of medicine (emeritus) and rheumatology at the University of California, Los Angeles, said in an interview. “There were a fair number of them, all of which were a little higher in upadacitinib. They certainly made very little of those.”
He noted several other concerns about the study, including a potential geographic effect stemming from 60% of the study’s centers being in South and Central America and Eastern Europe. “Those patients don’t always have very good medical care,” he said. “They have an inherent, underlying placebo response that can be much different than Western Europe and North America.”
He also questioned their choice of primary endpoint metric.
“I think a much more legitimate way at looking at remission is the CDAI [Clinical Disease Activity Index] rather than the DAS28,” he said. “The DAS28, even at its best, is low disease activity, not true remission.”
“Bottom line,” he added, “this is a legitimate study that supports previous findings. One more important thing that is overlooked, though, is an economic analysis. A true economic analysis would be very important to place this in the armamentarium.”
Study affirms upadacitinib’s place in the RA treatment pecking order
By showing that upadacitinib was not only noninferior but superior to abatacept in decreasing disease activity, Rubbert-Roth and colleagues have positioned the JAK inhibitor at “the forefront of treatment for rheumatoid arthritis,” wrote Guro L. Goll, MD, PhD, and Tore K. Kvien, MD, PhD, of Diakonhjemmet Hospital in Oslo, in an accompanying editorial.
Though the authors noted that the 24-week trial was likely too short to make meaningful assumptions about long-term outcomes, they recognized the notably improved treatment outcomes over the study period and stated the importance of “head-to-head trials ... to inform evidence-based clinical decisions.” Similar to Dr. Furst, however, they stated an interest in “detailed data on changes in the CDAI score as a continuous measure.”
They also acknowledged the significant increase in adverse events among patients in the upadacitinib group, underlining the need to learn more in forthcoming, lengthier trials. “Rheumatologists will be looking hard at future data,” they wrote, “to assess whether improved treatment outcomes justify an increased risk of adverse events.”
The study was supported by AbbVie. The authors acknowledged numerous potential conflicts of interest, including receiving research grants and fees from various pharmaceutical companies for consulting, lectures, and being on advisory boards.
SOURCE: Rubbert-Roth A et al. N Engl J Med. 2020 Oct 14. doi: 10.1056/NEJMoa2008250.
Upadacitinib (Rinvoq) proved superior to abatacept in both disease activity and remission in rheumatoid arthritis patients yet led to more adverse events, according to a new study that compared the two drugs.
“Additional data from longer and larger trials are needed to better understand long-term outcomes and safety of upadacitinib as compared with other drugs for the treatment of rheumatoid arthritis,” wrote Andrea Rubbert-Roth, MD, of the Cantonal Clinic St. Gallen in St. Gallen, Switzerland, and her colleagues. The study was published in the New England Journal of Medicine.
The Food and Drug Administration approved upadacitinib for the treatment of rheumatoid arthritis in August 2019.
To compare the Janus kinase (JAK) inhibitor upadacitinib and the biologic disease-modifying antirheumatic drug (DMARD) abatacept as safe and effective treatments for RA, the researchers launched a randomized, double-blind, phase 3 clinical trial dubbed SELECT-CHOICE at 120 sites in 28 countries. All patients had moderate to severe active disease after previously having inadequate responses to at least one biologic DMARD. Slightly more than 82% of the participants were female, with a mean age of 55 years and mean RA duration of 12 years.
Patients were assigned either 15 mg of oral upadacitinib daily (n = 303) or intravenous abatacept at day 1 and weeks 2, 4, 8, 12, 16 and 20 (n = 309) with dosage tied to body weight, each in combination with stable synthetic DMARDs. Disease activity was measured after 12 weeks via the Disease Activity Score for 28 joints using C-reactive protein (DAS28-CRP). A DAS28-CRP of more than 5.1 was categorized as high disease activity, while 3.2-5.1 meant moderate disease activity, 2.6-3.2 meant low disease activity, and less than 2.6 indicated remission.
The mean DAS28-CRP at baseline was 5.70 in the upadacitinib group and 5.88 in the abatacept group. After 12 weeks, the mean change from baseline was –2.52 points and –2.00 points, respectively (difference, –0.52 points; 95% confidence interval, –0.69 to –0.35; P < .001 for noninferiority; P < .001 for superiority). In patients with a DAS28-CRP of less than 2.6, the percentage of those having remission was 30% with upadacitinib and 13.3% with abatacept (difference, 16.8 percentage points; 95% CI, 10.4 to 23.2; P < .001 for superiority).
Over the 24-week trial period, the incidence of all adverse events (209 vs. 189) and serious adverse events (10 vs. 5) was higher in the upadacitinib group than in the abatacept group. There were 23 cases of hepatic disorder with upadacitinib, compared with 5 with abatacept; 2 thromboembolic events with upadacitinib, compared with 0 with abatacept; and 2 deaths with upadacitinib, compared with 1 with abatacept.
“The thing that bothers me, actually, is the adverse events,” Daniel E. Furst, MD, professor of medicine (emeritus) and rheumatology at the University of California, Los Angeles, said in an interview. “There were a fair number of them, all of which were a little higher in upadacitinib. They certainly made very little of those.”
He noted several other concerns about the study, including a potential geographic effect stemming from 60% of the study’s centers being in South and Central America and Eastern Europe. “Those patients don’t always have very good medical care,” he said. “They have an inherent, underlying placebo response that can be much different than Western Europe and North America.”
He also questioned their choice of primary endpoint metric.
“I think a much more legitimate way at looking at remission is the CDAI [Clinical Disease Activity Index] rather than the DAS28,” he said. “The DAS28, even at its best, is low disease activity, not true remission.”
“Bottom line,” he added, “this is a legitimate study that supports previous findings. One more important thing that is overlooked, though, is an economic analysis. A true economic analysis would be very important to place this in the armamentarium.”
Study affirms upadacitinib’s place in the RA treatment pecking order
By showing that upadacitinib was not only noninferior but superior to abatacept in decreasing disease activity, Rubbert-Roth and colleagues have positioned the JAK inhibitor at “the forefront of treatment for rheumatoid arthritis,” wrote Guro L. Goll, MD, PhD, and Tore K. Kvien, MD, PhD, of Diakonhjemmet Hospital in Oslo, in an accompanying editorial.
Though the authors noted that the 24-week trial was likely too short to make meaningful assumptions about long-term outcomes, they recognized the notably improved treatment outcomes over the study period and stated the importance of “head-to-head trials ... to inform evidence-based clinical decisions.” Similar to Dr. Furst, however, they stated an interest in “detailed data on changes in the CDAI score as a continuous measure.”
They also acknowledged the significant increase in adverse events among patients in the upadacitinib group, underlining the need to learn more in forthcoming, lengthier trials. “Rheumatologists will be looking hard at future data,” they wrote, “to assess whether improved treatment outcomes justify an increased risk of adverse events.”
The study was supported by AbbVie. The authors acknowledged numerous potential conflicts of interest, including receiving research grants and fees from various pharmaceutical companies for consulting, lectures, and being on advisory boards.
SOURCE: Rubbert-Roth A et al. N Engl J Med. 2020 Oct 14. doi: 10.1056/NEJMoa2008250.
Upadacitinib (Rinvoq) proved superior to abatacept in both disease activity and remission in rheumatoid arthritis patients yet led to more adverse events, according to a new study that compared the two drugs.
“Additional data from longer and larger trials are needed to better understand long-term outcomes and safety of upadacitinib as compared with other drugs for the treatment of rheumatoid arthritis,” wrote Andrea Rubbert-Roth, MD, of the Cantonal Clinic St. Gallen in St. Gallen, Switzerland, and her colleagues. The study was published in the New England Journal of Medicine.
The Food and Drug Administration approved upadacitinib for the treatment of rheumatoid arthritis in August 2019.
To compare the Janus kinase (JAK) inhibitor upadacitinib and the biologic disease-modifying antirheumatic drug (DMARD) abatacept as safe and effective treatments for RA, the researchers launched a randomized, double-blind, phase 3 clinical trial dubbed SELECT-CHOICE at 120 sites in 28 countries. All patients had moderate to severe active disease after previously having inadequate responses to at least one biologic DMARD. Slightly more than 82% of the participants were female, with a mean age of 55 years and mean RA duration of 12 years.
Patients were assigned either 15 mg of oral upadacitinib daily (n = 303) or intravenous abatacept at day 1 and weeks 2, 4, 8, 12, 16 and 20 (n = 309) with dosage tied to body weight, each in combination with stable synthetic DMARDs. Disease activity was measured after 12 weeks via the Disease Activity Score for 28 joints using C-reactive protein (DAS28-CRP). A DAS28-CRP of more than 5.1 was categorized as high disease activity, while 3.2-5.1 meant moderate disease activity, 2.6-3.2 meant low disease activity, and less than 2.6 indicated remission.
The mean DAS28-CRP at baseline was 5.70 in the upadacitinib group and 5.88 in the abatacept group. After 12 weeks, the mean change from baseline was –2.52 points and –2.00 points, respectively (difference, –0.52 points; 95% confidence interval, –0.69 to –0.35; P < .001 for noninferiority; P < .001 for superiority). In patients with a DAS28-CRP of less than 2.6, the percentage of those having remission was 30% with upadacitinib and 13.3% with abatacept (difference, 16.8 percentage points; 95% CI, 10.4 to 23.2; P < .001 for superiority).
Over the 24-week trial period, the incidence of all adverse events (209 vs. 189) and serious adverse events (10 vs. 5) was higher in the upadacitinib group than in the abatacept group. There were 23 cases of hepatic disorder with upadacitinib, compared with 5 with abatacept; 2 thromboembolic events with upadacitinib, compared with 0 with abatacept; and 2 deaths with upadacitinib, compared with 1 with abatacept.
“The thing that bothers me, actually, is the adverse events,” Daniel E. Furst, MD, professor of medicine (emeritus) and rheumatology at the University of California, Los Angeles, said in an interview. “There were a fair number of them, all of which were a little higher in upadacitinib. They certainly made very little of those.”
He noted several other concerns about the study, including a potential geographic effect stemming from 60% of the study’s centers being in South and Central America and Eastern Europe. “Those patients don’t always have very good medical care,” he said. “They have an inherent, underlying placebo response that can be much different than Western Europe and North America.”
He also questioned their choice of primary endpoint metric.
“I think a much more legitimate way at looking at remission is the CDAI [Clinical Disease Activity Index] rather than the DAS28,” he said. “The DAS28, even at its best, is low disease activity, not true remission.”
“Bottom line,” he added, “this is a legitimate study that supports previous findings. One more important thing that is overlooked, though, is an economic analysis. A true economic analysis would be very important to place this in the armamentarium.”
Study affirms upadacitinib’s place in the RA treatment pecking order
By showing that upadacitinib was not only noninferior but superior to abatacept in decreasing disease activity, Rubbert-Roth and colleagues have positioned the JAK inhibitor at “the forefront of treatment for rheumatoid arthritis,” wrote Guro L. Goll, MD, PhD, and Tore K. Kvien, MD, PhD, of Diakonhjemmet Hospital in Oslo, in an accompanying editorial.
Though the authors noted that the 24-week trial was likely too short to make meaningful assumptions about long-term outcomes, they recognized the notably improved treatment outcomes over the study period and stated the importance of “head-to-head trials ... to inform evidence-based clinical decisions.” Similar to Dr. Furst, however, they stated an interest in “detailed data on changes in the CDAI score as a continuous measure.”
They also acknowledged the significant increase in adverse events among patients in the upadacitinib group, underlining the need to learn more in forthcoming, lengthier trials. “Rheumatologists will be looking hard at future data,” they wrote, “to assess whether improved treatment outcomes justify an increased risk of adverse events.”
The study was supported by AbbVie. The authors acknowledged numerous potential conflicts of interest, including receiving research grants and fees from various pharmaceutical companies for consulting, lectures, and being on advisory boards.
SOURCE: Rubbert-Roth A et al. N Engl J Med. 2020 Oct 14. doi: 10.1056/NEJMoa2008250.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Upadacitinib decreased disease activity but was associated with more serious adverse events, compared with abatacept, over a 24-week trial period.
Major finding: After 12 weeks, the mean change from baseline in the DAS28-CRP was –2.52 points with upadacitinib and –2.00 points with abatacept (difference, –0.52 points; 95% CI, –0.69 to –0.35; P < .001).
Study details: A randomized, double-blind, phase 3 clinical trial of RA patients who had previous inadequate responses to at least one biologic DMARD.
Disclosures: The study was supported by AbbVie. The authors acknowledged numerous potential conflicts of interest, including receiving research grants and fees from various pharmaceutical companies for consulting, lectures, and being on advisory boards.
Source: Rubbert-Roth A et al. N Engl J Med. 2020 Oct 14. doi: 10.1056/NEJMoa2008250
New technologies show promise for treating pigmented lesions
carry a higher risk for postinflammatory hyperpigmentation than intense pulsed light or the long-pulsed laser, according to Mathew M. Avram, MD, JD.
For treating melanosomes with selective photothermolysis, some of the peak wavelengths include 532 nm, 694 nm, 755 nm, and 1064 nm, Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during the virtual annual Masters of Aesthetics Symposium. “The ideal target is fair skin with a dark, pigmented lesion,” he said. “That way you’re going to get energy focused to the melanin that’s in the lesion itself.”
Q-switched and picosecond lasers are effective for pigmented lesions. These employ as much energy as the city of Boston for 20-30 billionths of a second, or 750 picoseconds. “This raises the temperature to 1,000° C in that time, which produces the characteristic epidermal whitening,” he said. “This targets pigment cells only, whether it’s exogenous or endogenous pigment.”
Benign pigmented lesions amenable to the Q-switched nanosecond and picosecond laser include lentigines and nevus of Ota/Ito. The mechanism of action for clinical lightening is fragmentation and release of melanin-laden cells and the gradual uptake and removal of fragments by activated macrophages into lymphatic vessels. “For effective results, do not blindly memorize settings or replicate recommended settings from a colleague or a device manufacturer,” advised Dr. Avram, who practiced law prior to becoming a physician. “Some lasers are not externally calibrated, so what you have to do is pay attention to the laser endpoint, which in this case is epidermal whitening. Tissue ‘splatter’ is an unsafe endpoint and may lead to scarring. Safe and unsafe laser endpoints and close clinical observation are the best means to avoid complications and get the best results for your patients. The key finding is the endpoint, not the energy settings.”
Pigmented lesions that should not be treated with a laser include atypical nevi, lentigo maligna, and other forms of melanoma. “When in doubt, perform a biopsy,” he said. “Regardless of who referred the case, you are liable if you treat a melanoma with a laser. This is not only misdiagnosis but it probably delays diagnosis as well. If you cannot recognize basis pigmented lesion morphology, do not treat pigmented lesions. At some point, it’s going to catch up with you.”
Patients with more pigment to their skin face a higher risk for postinflammatory hyperpigmentation, Dr. Avram continued. While longer pulsed lasers produce less hyperpigmentation, they’re also less effective at getting rid of lesions. “You can combine a long-pulsed laser with fractional resurfacing or IPL [intense pulsed light] to optimize improvement,” he said. “If you don’t have two lasers to use, you can just use a longer-pulsed laser. The desired treatment endpoint for this approach is an ashen gray appearance.” Options include a 532-nm Nd:YAG laser with or without cooling, a 595-nm pulsed dye laser without cooling, and a 755-nm alexandrite laser without cooling.
One advance in the treatment of seborrheic keratoses is Nano-Pulse Stimulation (NPS), a novel technology being developed by Pulse Biosciences. With this approach, nanosecond electrical energy pulses cause internal organelle disruption, which leads to regulated cell death. “The cell-specific effect is nonthermal, as a typical nano-pulse delivers 0.1 joules of energy distributed in a volume of tissue,” Dr. Avram said. Early human studies established safe doses and validation of mechanism hypothesis for benign-lesion efficacy. “What you have are tiny nanopores that allow calcium ions to flow into the cell,” he explained. “The nanopores in the endoplasmic reticulum allow calcium ions to flow out of the endoplasmic reticulum, stressing it. These nanopores in the mitochondria disrupt the ability to generate energy, and the cell dies.”
Histology has revealed that within days the procedure causes regulated cell death with no thermal effects. The ability of NPS energy to clear seborrheic keratoses (SK) was confirmed in a study of 58 subjects who had 174 SK lesions treated. The majority of SKs (82%) were rated as clear or mostly clear 106 days post treatment. All results reflected a single treatment session.
Another novel treatment, “cryomodulation,” a technology being developed by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry Chan, MD, PhD, expresses cold-induced change to the skin as a way to pause melanin production. “You get melanin production paused but melanocyte function is preserved,” Dr. Avram explained. “There is a normal epidermal barrier and no persistent inflammatory response, so there’s no hyperpigmentation.” He characterized it as an ease-of-use clinical procedure for treating benign lesions in all skin types. A mask is applied to confine freezing to the desired treatment area, and hydrated gauze is used to help facilitate ice crystal propagation. A prototype of the device features a parameter selection based on lesion type, anatomical location, and skin type. “It uses between 107 and 166 kJ/m2 of extracted energy, and you take photos at baseline and follow-up,” he said. “You get 2-3 days of redness, darkening, and swelling. It’s well tolerated, with minimal discomfort. There’s no long-term dyschromia. This is nice, because patients have little, if any, downtime.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
carry a higher risk for postinflammatory hyperpigmentation than intense pulsed light or the long-pulsed laser, according to Mathew M. Avram, MD, JD.
For treating melanosomes with selective photothermolysis, some of the peak wavelengths include 532 nm, 694 nm, 755 nm, and 1064 nm, Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during the virtual annual Masters of Aesthetics Symposium. “The ideal target is fair skin with a dark, pigmented lesion,” he said. “That way you’re going to get energy focused to the melanin that’s in the lesion itself.”
Q-switched and picosecond lasers are effective for pigmented lesions. These employ as much energy as the city of Boston for 20-30 billionths of a second, or 750 picoseconds. “This raises the temperature to 1,000° C in that time, which produces the characteristic epidermal whitening,” he said. “This targets pigment cells only, whether it’s exogenous or endogenous pigment.”
Benign pigmented lesions amenable to the Q-switched nanosecond and picosecond laser include lentigines and nevus of Ota/Ito. The mechanism of action for clinical lightening is fragmentation and release of melanin-laden cells and the gradual uptake and removal of fragments by activated macrophages into lymphatic vessels. “For effective results, do not blindly memorize settings or replicate recommended settings from a colleague or a device manufacturer,” advised Dr. Avram, who practiced law prior to becoming a physician. “Some lasers are not externally calibrated, so what you have to do is pay attention to the laser endpoint, which in this case is epidermal whitening. Tissue ‘splatter’ is an unsafe endpoint and may lead to scarring. Safe and unsafe laser endpoints and close clinical observation are the best means to avoid complications and get the best results for your patients. The key finding is the endpoint, not the energy settings.”
Pigmented lesions that should not be treated with a laser include atypical nevi, lentigo maligna, and other forms of melanoma. “When in doubt, perform a biopsy,” he said. “Regardless of who referred the case, you are liable if you treat a melanoma with a laser. This is not only misdiagnosis but it probably delays diagnosis as well. If you cannot recognize basis pigmented lesion morphology, do not treat pigmented lesions. At some point, it’s going to catch up with you.”
Patients with more pigment to their skin face a higher risk for postinflammatory hyperpigmentation, Dr. Avram continued. While longer pulsed lasers produce less hyperpigmentation, they’re also less effective at getting rid of lesions. “You can combine a long-pulsed laser with fractional resurfacing or IPL [intense pulsed light] to optimize improvement,” he said. “If you don’t have two lasers to use, you can just use a longer-pulsed laser. The desired treatment endpoint for this approach is an ashen gray appearance.” Options include a 532-nm Nd:YAG laser with or without cooling, a 595-nm pulsed dye laser without cooling, and a 755-nm alexandrite laser without cooling.
One advance in the treatment of seborrheic keratoses is Nano-Pulse Stimulation (NPS), a novel technology being developed by Pulse Biosciences. With this approach, nanosecond electrical energy pulses cause internal organelle disruption, which leads to regulated cell death. “The cell-specific effect is nonthermal, as a typical nano-pulse delivers 0.1 joules of energy distributed in a volume of tissue,” Dr. Avram said. Early human studies established safe doses and validation of mechanism hypothesis for benign-lesion efficacy. “What you have are tiny nanopores that allow calcium ions to flow into the cell,” he explained. “The nanopores in the endoplasmic reticulum allow calcium ions to flow out of the endoplasmic reticulum, stressing it. These nanopores in the mitochondria disrupt the ability to generate energy, and the cell dies.”
Histology has revealed that within days the procedure causes regulated cell death with no thermal effects. The ability of NPS energy to clear seborrheic keratoses (SK) was confirmed in a study of 58 subjects who had 174 SK lesions treated. The majority of SKs (82%) were rated as clear or mostly clear 106 days post treatment. All results reflected a single treatment session.
Another novel treatment, “cryomodulation,” a technology being developed by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry Chan, MD, PhD, expresses cold-induced change to the skin as a way to pause melanin production. “You get melanin production paused but melanocyte function is preserved,” Dr. Avram explained. “There is a normal epidermal barrier and no persistent inflammatory response, so there’s no hyperpigmentation.” He characterized it as an ease-of-use clinical procedure for treating benign lesions in all skin types. A mask is applied to confine freezing to the desired treatment area, and hydrated gauze is used to help facilitate ice crystal propagation. A prototype of the device features a parameter selection based on lesion type, anatomical location, and skin type. “It uses between 107 and 166 kJ/m2 of extracted energy, and you take photos at baseline and follow-up,” he said. “You get 2-3 days of redness, darkening, and swelling. It’s well tolerated, with minimal discomfort. There’s no long-term dyschromia. This is nice, because patients have little, if any, downtime.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
carry a higher risk for postinflammatory hyperpigmentation than intense pulsed light or the long-pulsed laser, according to Mathew M. Avram, MD, JD.
For treating melanosomes with selective photothermolysis, some of the peak wavelengths include 532 nm, 694 nm, 755 nm, and 1064 nm, Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said during the virtual annual Masters of Aesthetics Symposium. “The ideal target is fair skin with a dark, pigmented lesion,” he said. “That way you’re going to get energy focused to the melanin that’s in the lesion itself.”
Q-switched and picosecond lasers are effective for pigmented lesions. These employ as much energy as the city of Boston for 20-30 billionths of a second, or 750 picoseconds. “This raises the temperature to 1,000° C in that time, which produces the characteristic epidermal whitening,” he said. “This targets pigment cells only, whether it’s exogenous or endogenous pigment.”
Benign pigmented lesions amenable to the Q-switched nanosecond and picosecond laser include lentigines and nevus of Ota/Ito. The mechanism of action for clinical lightening is fragmentation and release of melanin-laden cells and the gradual uptake and removal of fragments by activated macrophages into lymphatic vessels. “For effective results, do not blindly memorize settings or replicate recommended settings from a colleague or a device manufacturer,” advised Dr. Avram, who practiced law prior to becoming a physician. “Some lasers are not externally calibrated, so what you have to do is pay attention to the laser endpoint, which in this case is epidermal whitening. Tissue ‘splatter’ is an unsafe endpoint and may lead to scarring. Safe and unsafe laser endpoints and close clinical observation are the best means to avoid complications and get the best results for your patients. The key finding is the endpoint, not the energy settings.”
Pigmented lesions that should not be treated with a laser include atypical nevi, lentigo maligna, and other forms of melanoma. “When in doubt, perform a biopsy,” he said. “Regardless of who referred the case, you are liable if you treat a melanoma with a laser. This is not only misdiagnosis but it probably delays diagnosis as well. If you cannot recognize basis pigmented lesion morphology, do not treat pigmented lesions. At some point, it’s going to catch up with you.”
Patients with more pigment to their skin face a higher risk for postinflammatory hyperpigmentation, Dr. Avram continued. While longer pulsed lasers produce less hyperpigmentation, they’re also less effective at getting rid of lesions. “You can combine a long-pulsed laser with fractional resurfacing or IPL [intense pulsed light] to optimize improvement,” he said. “If you don’t have two lasers to use, you can just use a longer-pulsed laser. The desired treatment endpoint for this approach is an ashen gray appearance.” Options include a 532-nm Nd:YAG laser with or without cooling, a 595-nm pulsed dye laser without cooling, and a 755-nm alexandrite laser without cooling.
One advance in the treatment of seborrheic keratoses is Nano-Pulse Stimulation (NPS), a novel technology being developed by Pulse Biosciences. With this approach, nanosecond electrical energy pulses cause internal organelle disruption, which leads to regulated cell death. “The cell-specific effect is nonthermal, as a typical nano-pulse delivers 0.1 joules of energy distributed in a volume of tissue,” Dr. Avram said. Early human studies established safe doses and validation of mechanism hypothesis for benign-lesion efficacy. “What you have are tiny nanopores that allow calcium ions to flow into the cell,” he explained. “The nanopores in the endoplasmic reticulum allow calcium ions to flow out of the endoplasmic reticulum, stressing it. These nanopores in the mitochondria disrupt the ability to generate energy, and the cell dies.”
Histology has revealed that within days the procedure causes regulated cell death with no thermal effects. The ability of NPS energy to clear seborrheic keratoses (SK) was confirmed in a study of 58 subjects who had 174 SK lesions treated. The majority of SKs (82%) were rated as clear or mostly clear 106 days post treatment. All results reflected a single treatment session.
Another novel treatment, “cryomodulation,” a technology being developed by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry Chan, MD, PhD, expresses cold-induced change to the skin as a way to pause melanin production. “You get melanin production paused but melanocyte function is preserved,” Dr. Avram explained. “There is a normal epidermal barrier and no persistent inflammatory response, so there’s no hyperpigmentation.” He characterized it as an ease-of-use clinical procedure for treating benign lesions in all skin types. A mask is applied to confine freezing to the desired treatment area, and hydrated gauze is used to help facilitate ice crystal propagation. A prototype of the device features a parameter selection based on lesion type, anatomical location, and skin type. “It uses between 107 and 166 kJ/m2 of extracted energy, and you take photos at baseline and follow-up,” he said. “You get 2-3 days of redness, darkening, and swelling. It’s well tolerated, with minimal discomfort. There’s no long-term dyschromia. This is nice, because patients have little, if any, downtime.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
FROM MOA 2020
Urethral bulking agents for SUI: Rethinking their indications
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.
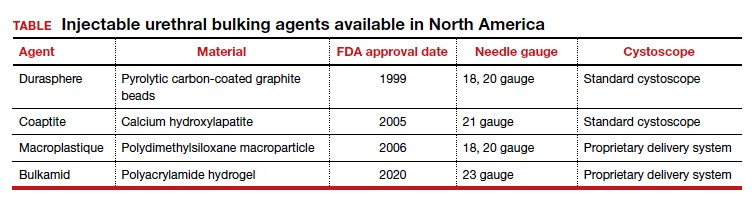
Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4
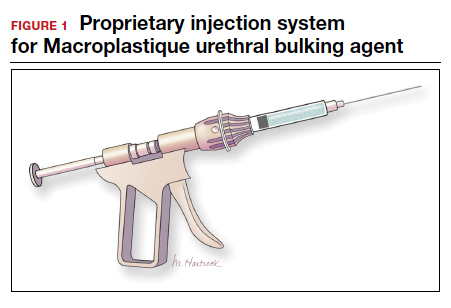

After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).
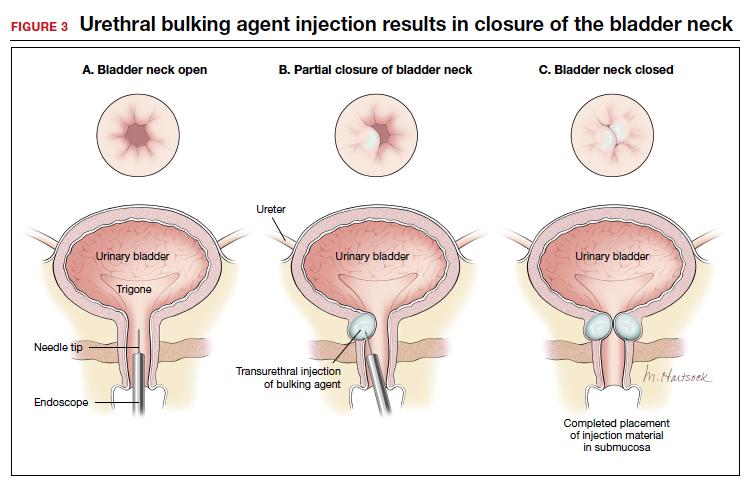
Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.

Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4


After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).

Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.

Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4


After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).

Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
Does early introduction of peanuts to an infant’s diet reduce the risk for peanut allergy?
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
EVIDENCE-BASED ANSWER:
Probably not, unless the child has severe eczema or egg allergy. In a general pediatric population, introducing peanuts early (at age 3 to 6 months) doesn’t appear to alter rates of subsequent peanut allergy compared with introduction after age 6 months (strength of recommendation [SOR]: B, randomized clinical trial [RCT] using multiple potential food allergens).
In children with severe eczema, egg allergy, or both, however, the risk for a peanut allergy is 12% to 24% lower when peanut-containing foods are introduced at age 4 to 11 months than after age 1 year. Early introduction of peanuts is associated with about 1 additional mild virus-associated syndrome (upper respiratory infection [URI], exanthem, conjunctivitis, or gastroenteritis) per patient (SOR: B, RCT).
Introducing peanuts before age 1 year is recommended for atopic children without evidence of pre-existing peanut allergy; an earlier start, at age 4 to 6 months, is advised for infants with severe eczema or egg allergy (SOR: C, expert opinion).