User login
C. difficile linked to surgery risk in pediatric Crohn’s
In pediatric Crohn’s disease, a Clostridioides difficile infection detected within the first year after diagnosis is associated with a shorter time to first bowel resection surgery, according to a study that included both a retrospective and prospective analysis. The researchers also found evidence that changes in methionine biosynthesis and depletion of beneficial bacteria may contribute to risk of surgery.
C. difficile infection (CDI) disproportionately affects individuals with inflammatory bowel disease (IBD). Pediatric IBD patients have a 34% risk of recurrent CDI infection, compared with 7.5% in the general population. Previous research found that adults with ulcerative colitis and CDI are at more risk of colectomy, but the finding has not been replicated in children.
In a study published in Inflammatory Bowel Diseases, researchers led by Jennifer Hellmann and Lee Denson of the University of Cincinnati conducted a single-center retrospective analysis of 75 pediatric Crohn’s disease patients. They also conducted a prospective study of 70 pediatric Crohn’s disease patients, using shotgun metagenome sequencing to examine the relationship between microbiota composition and C. difficile carriage or surgery history.
Nineteen percent of patients tested positive for C. difficile. Use of antibiotics was associated with C. difficile (odds ratio, 7.9; P = .02). Of patients who underwent C. difficile testing in the first year, 23 went on to have surgery: 21% who were C. difficile negative required surgery, compared with 67% of those who were positive (hazard ratio, 4.4; P = .0003). The mean time to surgery was 527 days for C. difficile–positive patients and 1,268 days for those who were negative.
A multivariate regression analysis on 54 patients with complete data sets showed that the presence of C. difficile was associated with increased risk of surgery (OR, 16.2; P = .0006). When the analysis was run on all 73 patients, using null value for missing data, the results were similar (OR, 9.17; P = .008).
Shotgun sequencing found that 47 of 114 bacterial species that were associated with the presence of C. difficile were also associated with prior surgery for Crohn’s disease. Species included some that may play a role in mucosal homeostasis, such as Bifidobacterium breve and several Alistipes and Ruminococcus species. That suggests that a reduction in the numbers of these taxa may be associated with C. difficile presence and surgical risk.
The researchers also found that methionine synthesis pathways were depressed in C. difficile–positive and surgery patients. Methionine may bolster antioxidant capacity and improve villus morphology. IBD patients with dysbiosis and those experiencing Crohn’s disease exacerbations have been shown to have decreased methionine pathway activity, suggesting methionine biosynthesis changes have clinical relevance.
The study was funded by the National Institutes of Health.
SOURCE: Hellmann J et al. Inflamm Bowel Dis. 2020. doi: 10.1093/ibd/izz263.
In pediatric Crohn’s disease, a Clostridioides difficile infection detected within the first year after diagnosis is associated with a shorter time to first bowel resection surgery, according to a study that included both a retrospective and prospective analysis. The researchers also found evidence that changes in methionine biosynthesis and depletion of beneficial bacteria may contribute to risk of surgery.
C. difficile infection (CDI) disproportionately affects individuals with inflammatory bowel disease (IBD). Pediatric IBD patients have a 34% risk of recurrent CDI infection, compared with 7.5% in the general population. Previous research found that adults with ulcerative colitis and CDI are at more risk of colectomy, but the finding has not been replicated in children.
In a study published in Inflammatory Bowel Diseases, researchers led by Jennifer Hellmann and Lee Denson of the University of Cincinnati conducted a single-center retrospective analysis of 75 pediatric Crohn’s disease patients. They also conducted a prospective study of 70 pediatric Crohn’s disease patients, using shotgun metagenome sequencing to examine the relationship between microbiota composition and C. difficile carriage or surgery history.
Nineteen percent of patients tested positive for C. difficile. Use of antibiotics was associated with C. difficile (odds ratio, 7.9; P = .02). Of patients who underwent C. difficile testing in the first year, 23 went on to have surgery: 21% who were C. difficile negative required surgery, compared with 67% of those who were positive (hazard ratio, 4.4; P = .0003). The mean time to surgery was 527 days for C. difficile–positive patients and 1,268 days for those who were negative.
A multivariate regression analysis on 54 patients with complete data sets showed that the presence of C. difficile was associated with increased risk of surgery (OR, 16.2; P = .0006). When the analysis was run on all 73 patients, using null value for missing data, the results were similar (OR, 9.17; P = .008).
Shotgun sequencing found that 47 of 114 bacterial species that were associated with the presence of C. difficile were also associated with prior surgery for Crohn’s disease. Species included some that may play a role in mucosal homeostasis, such as Bifidobacterium breve and several Alistipes and Ruminococcus species. That suggests that a reduction in the numbers of these taxa may be associated with C. difficile presence and surgical risk.
The researchers also found that methionine synthesis pathways were depressed in C. difficile–positive and surgery patients. Methionine may bolster antioxidant capacity and improve villus morphology. IBD patients with dysbiosis and those experiencing Crohn’s disease exacerbations have been shown to have decreased methionine pathway activity, suggesting methionine biosynthesis changes have clinical relevance.
The study was funded by the National Institutes of Health.
SOURCE: Hellmann J et al. Inflamm Bowel Dis. 2020. doi: 10.1093/ibd/izz263.
In pediatric Crohn’s disease, a Clostridioides difficile infection detected within the first year after diagnosis is associated with a shorter time to first bowel resection surgery, according to a study that included both a retrospective and prospective analysis. The researchers also found evidence that changes in methionine biosynthesis and depletion of beneficial bacteria may contribute to risk of surgery.
C. difficile infection (CDI) disproportionately affects individuals with inflammatory bowel disease (IBD). Pediatric IBD patients have a 34% risk of recurrent CDI infection, compared with 7.5% in the general population. Previous research found that adults with ulcerative colitis and CDI are at more risk of colectomy, but the finding has not been replicated in children.
In a study published in Inflammatory Bowel Diseases, researchers led by Jennifer Hellmann and Lee Denson of the University of Cincinnati conducted a single-center retrospective analysis of 75 pediatric Crohn’s disease patients. They also conducted a prospective study of 70 pediatric Crohn’s disease patients, using shotgun metagenome sequencing to examine the relationship between microbiota composition and C. difficile carriage or surgery history.
Nineteen percent of patients tested positive for C. difficile. Use of antibiotics was associated with C. difficile (odds ratio, 7.9; P = .02). Of patients who underwent C. difficile testing in the first year, 23 went on to have surgery: 21% who were C. difficile negative required surgery, compared with 67% of those who were positive (hazard ratio, 4.4; P = .0003). The mean time to surgery was 527 days for C. difficile–positive patients and 1,268 days for those who were negative.
A multivariate regression analysis on 54 patients with complete data sets showed that the presence of C. difficile was associated with increased risk of surgery (OR, 16.2; P = .0006). When the analysis was run on all 73 patients, using null value for missing data, the results were similar (OR, 9.17; P = .008).
Shotgun sequencing found that 47 of 114 bacterial species that were associated with the presence of C. difficile were also associated with prior surgery for Crohn’s disease. Species included some that may play a role in mucosal homeostasis, such as Bifidobacterium breve and several Alistipes and Ruminococcus species. That suggests that a reduction in the numbers of these taxa may be associated with C. difficile presence and surgical risk.
The researchers also found that methionine synthesis pathways were depressed in C. difficile–positive and surgery patients. Methionine may bolster antioxidant capacity and improve villus morphology. IBD patients with dysbiosis and those experiencing Crohn’s disease exacerbations have been shown to have decreased methionine pathway activity, suggesting methionine biosynthesis changes have clinical relevance.
The study was funded by the National Institutes of Health.
SOURCE: Hellmann J et al. Inflamm Bowel Dis. 2020. doi: 10.1093/ibd/izz263.
Fecal transplant linked to reduced C. difficile mortality
Vancomycin followed by fecal microbiota transplant (FMT) was associated with reduced Clostridioides difficile (C. diff)-related mortality in patients hospitalized with refractory severe or fulminant C. diff infection (CDI) at a single center. The improvements came after Indiana University implemented an FMT option in 2013.
About 8% of C. diff patients develop severe or fulminant CDI (SFCDI), which can lead to toxic colon and multiorgan failure. Surgery is the current recommended treatment for these patients if they are refractory to vancomycin, but 30-day mortality is above 40%. FMT is recommended for recurrent CDI, and it achieves cure rates greater than 80%, along with fewer relapses compared with anti-CDI antibiotic therapy.
FMT has been shown to be effective for SFCDI, with a 91% cure rate for serious CDI and 66% for fulminant CDI.
In the study published in the September issue of Clinical Gastroenterology and Hepatology, researchers led by Yao-Wen Cheng, MD, and Monika Fischer, MD, of Indiana University, assessed the effect of FMT on SFCDI after their institution adopted it as a treatment protocol for SFCDI. Patients could receive FMT if there was evidence that their SFCDI was refractory, or if they had two or more CDI recurrences. The treatment includes oral vancomycin and pseudomembrane-driven sequential FMT.
Two hundred five patients were admitted before FMT implementation, 225 after. Fifty patients received FMT because of refractory SFCDI. A median of two FMTs was conducted per patient. 21 other patients received FMT for nonrefractory SFCDI or other conditions, including 18 patients with multiple recurrent CDI.
Thirty-day CDI-related mortality dropped after FMT implementation (4.4% versus 10.2%; P =.02). This was true in both the fulminant subset (9.1% versus 21.3%; P =.015) and the refractory group (12.1% versus 43.2%; P < .001).
The researchers used segmented logistic regression to determine if the improved outcomes could be due to nontreatment factors that varied over time, and found that the difference in CDI-related mortality was eliminated except for refractory SFCDI patients (odds of mortality after FMT implementation, 0.09; P =.023). There was no significant difference between those receiving non-CDI antibiotics (4.8%) and those who did not (6.9%; P =.75).
FMT was associated with lower frequency of CDI-related colectomy overall (2.7% versus 6.8%; P =.041), as well as in the fulminant (5.5% versus 15.7%; P =.017) and refractory subgroups (7.6% versus 31.8%; P =.001).
The findings follow another study that showed improved 3-month mortality for FMT among patients hospitalized with severe CDI (12.1% versus 42.2%; P < .003).
The results underscore the utility of FMT for SFCDI, and suggest it might have the most benefit in refractory SFCDI. The authors believe that FMT should be an alternative to colectomy when first-line anti-CDI antibiotics are partially or completely ineffective. In the absence of FMT, patients who go on to fail vancomycin or fidaxomicin will likely continue to be managed medically, with up to 80% mortality, or through salvage colectomy, with postsurgical morality rates of 30-40%.
Although a randomized trial could answer the question of FMT efficacy more definitively, it is unlikely to be conducted for ethical reasons.
“Further investigation is required to clearly define FMT’s role and timing in the clinical course of severe and fulminant CDI. However, our study suggests that FMT should be offered to patients with severe and fulminant CDI who do not respond to a 5-day course of anti-CDI antibiotics and may be considered in lieu of or before colectomy,” the researchers wrote.
No source of funding was disclosed.
SOURCE: Cheng YW et al. Clin Gastroenterol Hepatol. 2020;18:2234-43. doi: 10.1016/j.cgh.2019.12.029.
Vancomycin followed by fecal microbiota transplant (FMT) was associated with reduced Clostridioides difficile (C. diff)-related mortality in patients hospitalized with refractory severe or fulminant C. diff infection (CDI) at a single center. The improvements came after Indiana University implemented an FMT option in 2013.
About 8% of C. diff patients develop severe or fulminant CDI (SFCDI), which can lead to toxic colon and multiorgan failure. Surgery is the current recommended treatment for these patients if they are refractory to vancomycin, but 30-day mortality is above 40%. FMT is recommended for recurrent CDI, and it achieves cure rates greater than 80%, along with fewer relapses compared with anti-CDI antibiotic therapy.
FMT has been shown to be effective for SFCDI, with a 91% cure rate for serious CDI and 66% for fulminant CDI.
In the study published in the September issue of Clinical Gastroenterology and Hepatology, researchers led by Yao-Wen Cheng, MD, and Monika Fischer, MD, of Indiana University, assessed the effect of FMT on SFCDI after their institution adopted it as a treatment protocol for SFCDI. Patients could receive FMT if there was evidence that their SFCDI was refractory, or if they had two or more CDI recurrences. The treatment includes oral vancomycin and pseudomembrane-driven sequential FMT.
Two hundred five patients were admitted before FMT implementation, 225 after. Fifty patients received FMT because of refractory SFCDI. A median of two FMTs was conducted per patient. 21 other patients received FMT for nonrefractory SFCDI or other conditions, including 18 patients with multiple recurrent CDI.
Thirty-day CDI-related mortality dropped after FMT implementation (4.4% versus 10.2%; P =.02). This was true in both the fulminant subset (9.1% versus 21.3%; P =.015) and the refractory group (12.1% versus 43.2%; P < .001).
The researchers used segmented logistic regression to determine if the improved outcomes could be due to nontreatment factors that varied over time, and found that the difference in CDI-related mortality was eliminated except for refractory SFCDI patients (odds of mortality after FMT implementation, 0.09; P =.023). There was no significant difference between those receiving non-CDI antibiotics (4.8%) and those who did not (6.9%; P =.75).
FMT was associated with lower frequency of CDI-related colectomy overall (2.7% versus 6.8%; P =.041), as well as in the fulminant (5.5% versus 15.7%; P =.017) and refractory subgroups (7.6% versus 31.8%; P =.001).
The findings follow another study that showed improved 3-month mortality for FMT among patients hospitalized with severe CDI (12.1% versus 42.2%; P < .003).
The results underscore the utility of FMT for SFCDI, and suggest it might have the most benefit in refractory SFCDI. The authors believe that FMT should be an alternative to colectomy when first-line anti-CDI antibiotics are partially or completely ineffective. In the absence of FMT, patients who go on to fail vancomycin or fidaxomicin will likely continue to be managed medically, with up to 80% mortality, or through salvage colectomy, with postsurgical morality rates of 30-40%.
Although a randomized trial could answer the question of FMT efficacy more definitively, it is unlikely to be conducted for ethical reasons.
“Further investigation is required to clearly define FMT’s role and timing in the clinical course of severe and fulminant CDI. However, our study suggests that FMT should be offered to patients with severe and fulminant CDI who do not respond to a 5-day course of anti-CDI antibiotics and may be considered in lieu of or before colectomy,” the researchers wrote.
No source of funding was disclosed.
SOURCE: Cheng YW et al. Clin Gastroenterol Hepatol. 2020;18:2234-43. doi: 10.1016/j.cgh.2019.12.029.
Vancomycin followed by fecal microbiota transplant (FMT) was associated with reduced Clostridioides difficile (C. diff)-related mortality in patients hospitalized with refractory severe or fulminant C. diff infection (CDI) at a single center. The improvements came after Indiana University implemented an FMT option in 2013.
About 8% of C. diff patients develop severe or fulminant CDI (SFCDI), which can lead to toxic colon and multiorgan failure. Surgery is the current recommended treatment for these patients if they are refractory to vancomycin, but 30-day mortality is above 40%. FMT is recommended for recurrent CDI, and it achieves cure rates greater than 80%, along with fewer relapses compared with anti-CDI antibiotic therapy.
FMT has been shown to be effective for SFCDI, with a 91% cure rate for serious CDI and 66% for fulminant CDI.
In the study published in the September issue of Clinical Gastroenterology and Hepatology, researchers led by Yao-Wen Cheng, MD, and Monika Fischer, MD, of Indiana University, assessed the effect of FMT on SFCDI after their institution adopted it as a treatment protocol for SFCDI. Patients could receive FMT if there was evidence that their SFCDI was refractory, or if they had two or more CDI recurrences. The treatment includes oral vancomycin and pseudomembrane-driven sequential FMT.
Two hundred five patients were admitted before FMT implementation, 225 after. Fifty patients received FMT because of refractory SFCDI. A median of two FMTs was conducted per patient. 21 other patients received FMT for nonrefractory SFCDI or other conditions, including 18 patients with multiple recurrent CDI.
Thirty-day CDI-related mortality dropped after FMT implementation (4.4% versus 10.2%; P =.02). This was true in both the fulminant subset (9.1% versus 21.3%; P =.015) and the refractory group (12.1% versus 43.2%; P < .001).
The researchers used segmented logistic regression to determine if the improved outcomes could be due to nontreatment factors that varied over time, and found that the difference in CDI-related mortality was eliminated except for refractory SFCDI patients (odds of mortality after FMT implementation, 0.09; P =.023). There was no significant difference between those receiving non-CDI antibiotics (4.8%) and those who did not (6.9%; P =.75).
FMT was associated with lower frequency of CDI-related colectomy overall (2.7% versus 6.8%; P =.041), as well as in the fulminant (5.5% versus 15.7%; P =.017) and refractory subgroups (7.6% versus 31.8%; P =.001).
The findings follow another study that showed improved 3-month mortality for FMT among patients hospitalized with severe CDI (12.1% versus 42.2%; P < .003).
The results underscore the utility of FMT for SFCDI, and suggest it might have the most benefit in refractory SFCDI. The authors believe that FMT should be an alternative to colectomy when first-line anti-CDI antibiotics are partially or completely ineffective. In the absence of FMT, patients who go on to fail vancomycin or fidaxomicin will likely continue to be managed medically, with up to 80% mortality, or through salvage colectomy, with postsurgical morality rates of 30-40%.
Although a randomized trial could answer the question of FMT efficacy more definitively, it is unlikely to be conducted for ethical reasons.
“Further investigation is required to clearly define FMT’s role and timing in the clinical course of severe and fulminant CDI. However, our study suggests that FMT should be offered to patients with severe and fulminant CDI who do not respond to a 5-day course of anti-CDI antibiotics and may be considered in lieu of or before colectomy,” the researchers wrote.
No source of funding was disclosed.
SOURCE: Cheng YW et al. Clin Gastroenterol Hepatol. 2020;18:2234-43. doi: 10.1016/j.cgh.2019.12.029.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Medicine and the meritocracy
Addressing systemic bias, gender inequity and discrimination
There are many challenges facing modern medicine today. Recent events have highlighted important issues affecting our society as a whole – systemic racism, sexism, and implicit bias. In medicine, we have seen a renewed focus on health equity, health disparities and the implicit systemic bias that affect those who work in the field. It is truly troubling that it has taken the continued loss of black lives to police brutality and a pandemic for this conversation to happen at every level in society.
Systemic bias is present throughout corporate America, and it is no different within the physician workforce. Overall, there has been gradual interest in promoting and teaching diversity. Institutions have been slowly creating policies and administrative positions focused on inclusion and diversity over the last decade. So has diversity training objectively increased representation and advancement of women and minority groups? Do traditionally marginalized groups have better access to health? And are women and people of color (POC) represented equally in leadership positions in medicine?
Clearly, the answers are not straightforward.
Diving into the data
A guilty pleasure of mine is to assess how diverse and inclusive an institution is by looking at the wall of pictures recognizing top leadership in hospitals. Despite women accounting for 47.9% of graduates from medical school in 2018-2019, I still see very few women or POC elevated to this level. Of the total women graduates, 22.6% were Asian, 8% were Black and 5.4% were Hispanic.
Being of Indian descent, I am a woman of color (albeit one who may not be as profoundly affected by racism in medicine as my less represented colleagues). It is especially rare for me to see someone I can identify with in the ranks of top leadership. I find encouragement in seeing any woman on any leadership board because to me, it means that there is hope. The literature seems to support this degree of disparity as well. For example, a recent analysis shows that presidential leadership in medical societies are predominantly held by men (82.6% male vs. 17.4% female). Other datasets demonstrate that only 15% of deans and interim deans are women and AAMC’s report shows that women account for only 18% of all department chairs.
Growing up, my parents fueled my interest to pursue medicine. They described it as a noble profession that rewarded true merit and dedication to the cause. However, those that have been traditionally elevated in medicine are men. If merit knows no gender, why does a gender gap exist? If merit is blind to race, why are minorities so poorly represented in the workforce (much less in leadership)? My view of the wall leaves me wondering about the role of both sexism and racism in medicine.
These visual representations of the medical culture reinforce the acceptable norms and values – white and masculine – in medicine. The feminist movement over the last several decades has increased awareness about the need for equality of the sexes. However, it was not until the concept of intersectionality was introduced by Black feminist Professor Kimberle Crenshaw, that feminism become a more inclusive term. Professor Crenshaw’s paper details how every individual has intersecting factors – race, gender, sexual identity, socioeconomic status – that create the sum of their experience be it privilege, oppression, or discrimination.
For example, a White woman has privileges that a woman of color does not. Among non-white women, race and sexual identity are confounding factors – a Black woman, a Black LGBTQ woman, and an Asian woman, for example, will not experience discrimination in the same way. The farther you deviate from the accepted norms and values, the harder it is for you to obtain support and achieve recognition.
Addressing the patriarchal structure and systemic bias in medicine
Why do patriarchal structures still exist in medicine? How do we resolve systemic bias? Addressing them in isolation – race or gender or sexual identity – is unlikely to create long-lasting change. For change to occur, organizations and individuals need to be intrinsically motivated. Creating awareness and challenging the status quo is the first step.
Over the last decade, implicit bias training and diversity training have become mandatory in various industries and states. Diversity training has grown to be a multi-billion-dollar industry that corporate America has embraced over the last several years. And yet, research shows that mandating such training may not be the most effective. To get results, organizations need to implement programs that “spark engagement, increase contact between different groups and draw on people’s desire to look good to others.”
Historically, the medical curriculum has not included a discourse on feminist theories and the advancement of women in medicine. Cultural competency training is typically offered on an annual basis once we are in the workforce, but in my experience, it focuses more on our interactions with patients and other health care colleagues, and less with regards to our physician peers and leadership. Is this enough to change deep rooted beliefs and traditions?
We can take our cue from non-medical organizations and consider changing this culture of no culture in medicine – introducing diversity task forces that hold departments accountable for recruiting and promoting women and minorities; employing diversity managers; voluntary training; cross-training to increase contact among different groups and mentoring programs that match senior leadership to women and POC. While some medical institutions have implemented some of these principles, changing century-old traditions will require embracing concepts of organizational change and every available effective tool.
Committing to change
Change is especially hard when the target outcome is not accurately quantifiable – even if you can measure attitudes, values, and beliefs, these are subject to reporting bias and tokenism. At the organizational level, change management involves employing a systematic approach to change organizational values, goals, policies, and processes.
Individual change, self-reflection, and personal growth are key components in changing culture. Reflexivity is being aware of your own values, norms, position, and power – an important concept to understand and apply in our everyday interactions. Believing that one’s class, gender, race and sexual orientation are irrelevant to their practice of medicine would not foster the change that we direly need in medicine. Rather, identifying how your own values and professional identity are shaped by your medical training, your organization and the broader cultural context are critically important to developing a greater empathic sense to motivate systemic change.
There has been valuable discussion on bottom-up changes to ensure women and POC have support, encouragement and a pathway to advance in an organization. Some of these include policy and process changes including providing flexible working conditions for women and sponsorship of women and minorities to help them navigate the barriers and microaggressions they encounter at work. While technical (policy) changes form the foundation for any organizational change, it is important to remember that the people side of change – the resistance that you encounter for any change effort in an organization – is equally important to address at the organizational level. A top-down approach is also vital to ensure that change is permanent in an organization and does not end when the individuals responsible for the change leave the organization.
Lewin’s three-stage change management model provides a framework for structural and organizational change in hospital systems. The three-stages of this model are: unfreezing, changing, and refreezing. Unfreezing is the process of determining what needs to change and obtaining leadership support. The actual change process involves getting people on board, empowering them to change and communicating with them frequently. Refreezing cements this change into the organization’s culture by providing support and training to sustain changes. Research has shown that Lewin’s change management model has applicability in the hospital setting.
Industry research in change management methodologies in the business sector has identified sponsorship by CEOs/senior management of an organization and having a structured implementation model for change management as two important factors for ensuring that change efforts are successful and sustainable.
This can be extrapolated to health care organizations – top leadership committed to changing the status quo should solidify organizational commitment by incorporating new attainable and measurable goals into their vision for the organization. Designing a phased implementation of change management methodologies should follow an open discussion to identify an organization’s weaknesses, strengths, capacity, and readiness for change. Lastly, helping busy professionals adapt to change requires innovative and continuous improvement strategies using formal, systematic tools for organization-wide strategic deployment.
Without a concrete commitment at the organizational level, programs such as diversity training may end up being band-aids on wounds that run deep.
I believe that the combination of both individual and organizational commitment to change systemic bias in medicine can be quite powerful. One without the other will fail to permanently change the system. The work to true equality – regardless of the intersecting factors of discrimination – starts with a commitment to change. We may all have different opportunities because of the inequality that is apparent in our systems today, but if we unite around the goal of a bias-free, merit-based equality, it gives us the strength we need to overcome challenges that we once thought insurmountable.
Each one of us is a leader in our own right. Speaking up for those with less power or opportunity than us and supporting talent and hard work solidifies medicine as a meritocracy. Even if the magnitude of change that we fight for may not be realized during our time in medical practice, our commitment to eradicate sexism, racism and discrimination will shape the future of medicine.
Just as our children are a legacy that we leave behind, our work in correcting bias in medicine will pave the path for a better future for the doctors of tomorrow. After all, when I think that my young daughter will be affected by what I do or do not do to address the discrimination, there is no better motivation for me to break down every barrier for her success.
Dr. Kanikkannan is a practicing hospitalist and assistant professor of medicine at Albany Medical College in Albany, NY. This article first appeared on The Hospital Leader, the official blog of SHM.
Addressing systemic bias, gender inequity and discrimination
Addressing systemic bias, gender inequity and discrimination
There are many challenges facing modern medicine today. Recent events have highlighted important issues affecting our society as a whole – systemic racism, sexism, and implicit bias. In medicine, we have seen a renewed focus on health equity, health disparities and the implicit systemic bias that affect those who work in the field. It is truly troubling that it has taken the continued loss of black lives to police brutality and a pandemic for this conversation to happen at every level in society.
Systemic bias is present throughout corporate America, and it is no different within the physician workforce. Overall, there has been gradual interest in promoting and teaching diversity. Institutions have been slowly creating policies and administrative positions focused on inclusion and diversity over the last decade. So has diversity training objectively increased representation and advancement of women and minority groups? Do traditionally marginalized groups have better access to health? And are women and people of color (POC) represented equally in leadership positions in medicine?
Clearly, the answers are not straightforward.
Diving into the data
A guilty pleasure of mine is to assess how diverse and inclusive an institution is by looking at the wall of pictures recognizing top leadership in hospitals. Despite women accounting for 47.9% of graduates from medical school in 2018-2019, I still see very few women or POC elevated to this level. Of the total women graduates, 22.6% were Asian, 8% were Black and 5.4% were Hispanic.
Being of Indian descent, I am a woman of color (albeit one who may not be as profoundly affected by racism in medicine as my less represented colleagues). It is especially rare for me to see someone I can identify with in the ranks of top leadership. I find encouragement in seeing any woman on any leadership board because to me, it means that there is hope. The literature seems to support this degree of disparity as well. For example, a recent analysis shows that presidential leadership in medical societies are predominantly held by men (82.6% male vs. 17.4% female). Other datasets demonstrate that only 15% of deans and interim deans are women and AAMC’s report shows that women account for only 18% of all department chairs.
Growing up, my parents fueled my interest to pursue medicine. They described it as a noble profession that rewarded true merit and dedication to the cause. However, those that have been traditionally elevated in medicine are men. If merit knows no gender, why does a gender gap exist? If merit is blind to race, why are minorities so poorly represented in the workforce (much less in leadership)? My view of the wall leaves me wondering about the role of both sexism and racism in medicine.
These visual representations of the medical culture reinforce the acceptable norms and values – white and masculine – in medicine. The feminist movement over the last several decades has increased awareness about the need for equality of the sexes. However, it was not until the concept of intersectionality was introduced by Black feminist Professor Kimberle Crenshaw, that feminism become a more inclusive term. Professor Crenshaw’s paper details how every individual has intersecting factors – race, gender, sexual identity, socioeconomic status – that create the sum of their experience be it privilege, oppression, or discrimination.
For example, a White woman has privileges that a woman of color does not. Among non-white women, race and sexual identity are confounding factors – a Black woman, a Black LGBTQ woman, and an Asian woman, for example, will not experience discrimination in the same way. The farther you deviate from the accepted norms and values, the harder it is for you to obtain support and achieve recognition.
Addressing the patriarchal structure and systemic bias in medicine
Why do patriarchal structures still exist in medicine? How do we resolve systemic bias? Addressing them in isolation – race or gender or sexual identity – is unlikely to create long-lasting change. For change to occur, organizations and individuals need to be intrinsically motivated. Creating awareness and challenging the status quo is the first step.
Over the last decade, implicit bias training and diversity training have become mandatory in various industries and states. Diversity training has grown to be a multi-billion-dollar industry that corporate America has embraced over the last several years. And yet, research shows that mandating such training may not be the most effective. To get results, organizations need to implement programs that “spark engagement, increase contact between different groups and draw on people’s desire to look good to others.”
Historically, the medical curriculum has not included a discourse on feminist theories and the advancement of women in medicine. Cultural competency training is typically offered on an annual basis once we are in the workforce, but in my experience, it focuses more on our interactions with patients and other health care colleagues, and less with regards to our physician peers and leadership. Is this enough to change deep rooted beliefs and traditions?
We can take our cue from non-medical organizations and consider changing this culture of no culture in medicine – introducing diversity task forces that hold departments accountable for recruiting and promoting women and minorities; employing diversity managers; voluntary training; cross-training to increase contact among different groups and mentoring programs that match senior leadership to women and POC. While some medical institutions have implemented some of these principles, changing century-old traditions will require embracing concepts of organizational change and every available effective tool.
Committing to change
Change is especially hard when the target outcome is not accurately quantifiable – even if you can measure attitudes, values, and beliefs, these are subject to reporting bias and tokenism. At the organizational level, change management involves employing a systematic approach to change organizational values, goals, policies, and processes.
Individual change, self-reflection, and personal growth are key components in changing culture. Reflexivity is being aware of your own values, norms, position, and power – an important concept to understand and apply in our everyday interactions. Believing that one’s class, gender, race and sexual orientation are irrelevant to their practice of medicine would not foster the change that we direly need in medicine. Rather, identifying how your own values and professional identity are shaped by your medical training, your organization and the broader cultural context are critically important to developing a greater empathic sense to motivate systemic change.
There has been valuable discussion on bottom-up changes to ensure women and POC have support, encouragement and a pathway to advance in an organization. Some of these include policy and process changes including providing flexible working conditions for women and sponsorship of women and minorities to help them navigate the barriers and microaggressions they encounter at work. While technical (policy) changes form the foundation for any organizational change, it is important to remember that the people side of change – the resistance that you encounter for any change effort in an organization – is equally important to address at the organizational level. A top-down approach is also vital to ensure that change is permanent in an organization and does not end when the individuals responsible for the change leave the organization.
Lewin’s three-stage change management model provides a framework for structural and organizational change in hospital systems. The three-stages of this model are: unfreezing, changing, and refreezing. Unfreezing is the process of determining what needs to change and obtaining leadership support. The actual change process involves getting people on board, empowering them to change and communicating with them frequently. Refreezing cements this change into the organization’s culture by providing support and training to sustain changes. Research has shown that Lewin’s change management model has applicability in the hospital setting.
Industry research in change management methodologies in the business sector has identified sponsorship by CEOs/senior management of an organization and having a structured implementation model for change management as two important factors for ensuring that change efforts are successful and sustainable.
This can be extrapolated to health care organizations – top leadership committed to changing the status quo should solidify organizational commitment by incorporating new attainable and measurable goals into their vision for the organization. Designing a phased implementation of change management methodologies should follow an open discussion to identify an organization’s weaknesses, strengths, capacity, and readiness for change. Lastly, helping busy professionals adapt to change requires innovative and continuous improvement strategies using formal, systematic tools for organization-wide strategic deployment.
Without a concrete commitment at the organizational level, programs such as diversity training may end up being band-aids on wounds that run deep.
I believe that the combination of both individual and organizational commitment to change systemic bias in medicine can be quite powerful. One without the other will fail to permanently change the system. The work to true equality – regardless of the intersecting factors of discrimination – starts with a commitment to change. We may all have different opportunities because of the inequality that is apparent in our systems today, but if we unite around the goal of a bias-free, merit-based equality, it gives us the strength we need to overcome challenges that we once thought insurmountable.
Each one of us is a leader in our own right. Speaking up for those with less power or opportunity than us and supporting talent and hard work solidifies medicine as a meritocracy. Even if the magnitude of change that we fight for may not be realized during our time in medical practice, our commitment to eradicate sexism, racism and discrimination will shape the future of medicine.
Just as our children are a legacy that we leave behind, our work in correcting bias in medicine will pave the path for a better future for the doctors of tomorrow. After all, when I think that my young daughter will be affected by what I do or do not do to address the discrimination, there is no better motivation for me to break down every barrier for her success.
Dr. Kanikkannan is a practicing hospitalist and assistant professor of medicine at Albany Medical College in Albany, NY. This article first appeared on The Hospital Leader, the official blog of SHM.
There are many challenges facing modern medicine today. Recent events have highlighted important issues affecting our society as a whole – systemic racism, sexism, and implicit bias. In medicine, we have seen a renewed focus on health equity, health disparities and the implicit systemic bias that affect those who work in the field. It is truly troubling that it has taken the continued loss of black lives to police brutality and a pandemic for this conversation to happen at every level in society.
Systemic bias is present throughout corporate America, and it is no different within the physician workforce. Overall, there has been gradual interest in promoting and teaching diversity. Institutions have been slowly creating policies and administrative positions focused on inclusion and diversity over the last decade. So has diversity training objectively increased representation and advancement of women and minority groups? Do traditionally marginalized groups have better access to health? And are women and people of color (POC) represented equally in leadership positions in medicine?
Clearly, the answers are not straightforward.
Diving into the data
A guilty pleasure of mine is to assess how diverse and inclusive an institution is by looking at the wall of pictures recognizing top leadership in hospitals. Despite women accounting for 47.9% of graduates from medical school in 2018-2019, I still see very few women or POC elevated to this level. Of the total women graduates, 22.6% were Asian, 8% were Black and 5.4% were Hispanic.
Being of Indian descent, I am a woman of color (albeit one who may not be as profoundly affected by racism in medicine as my less represented colleagues). It is especially rare for me to see someone I can identify with in the ranks of top leadership. I find encouragement in seeing any woman on any leadership board because to me, it means that there is hope. The literature seems to support this degree of disparity as well. For example, a recent analysis shows that presidential leadership in medical societies are predominantly held by men (82.6% male vs. 17.4% female). Other datasets demonstrate that only 15% of deans and interim deans are women and AAMC’s report shows that women account for only 18% of all department chairs.
Growing up, my parents fueled my interest to pursue medicine. They described it as a noble profession that rewarded true merit and dedication to the cause. However, those that have been traditionally elevated in medicine are men. If merit knows no gender, why does a gender gap exist? If merit is blind to race, why are minorities so poorly represented in the workforce (much less in leadership)? My view of the wall leaves me wondering about the role of both sexism and racism in medicine.
These visual representations of the medical culture reinforce the acceptable norms and values – white and masculine – in medicine. The feminist movement over the last several decades has increased awareness about the need for equality of the sexes. However, it was not until the concept of intersectionality was introduced by Black feminist Professor Kimberle Crenshaw, that feminism become a more inclusive term. Professor Crenshaw’s paper details how every individual has intersecting factors – race, gender, sexual identity, socioeconomic status – that create the sum of their experience be it privilege, oppression, or discrimination.
For example, a White woman has privileges that a woman of color does not. Among non-white women, race and sexual identity are confounding factors – a Black woman, a Black LGBTQ woman, and an Asian woman, for example, will not experience discrimination in the same way. The farther you deviate from the accepted norms and values, the harder it is for you to obtain support and achieve recognition.
Addressing the patriarchal structure and systemic bias in medicine
Why do patriarchal structures still exist in medicine? How do we resolve systemic bias? Addressing them in isolation – race or gender or sexual identity – is unlikely to create long-lasting change. For change to occur, organizations and individuals need to be intrinsically motivated. Creating awareness and challenging the status quo is the first step.
Over the last decade, implicit bias training and diversity training have become mandatory in various industries and states. Diversity training has grown to be a multi-billion-dollar industry that corporate America has embraced over the last several years. And yet, research shows that mandating such training may not be the most effective. To get results, organizations need to implement programs that “spark engagement, increase contact between different groups and draw on people’s desire to look good to others.”
Historically, the medical curriculum has not included a discourse on feminist theories and the advancement of women in medicine. Cultural competency training is typically offered on an annual basis once we are in the workforce, but in my experience, it focuses more on our interactions with patients and other health care colleagues, and less with regards to our physician peers and leadership. Is this enough to change deep rooted beliefs and traditions?
We can take our cue from non-medical organizations and consider changing this culture of no culture in medicine – introducing diversity task forces that hold departments accountable for recruiting and promoting women and minorities; employing diversity managers; voluntary training; cross-training to increase contact among different groups and mentoring programs that match senior leadership to women and POC. While some medical institutions have implemented some of these principles, changing century-old traditions will require embracing concepts of organizational change and every available effective tool.
Committing to change
Change is especially hard when the target outcome is not accurately quantifiable – even if you can measure attitudes, values, and beliefs, these are subject to reporting bias and tokenism. At the organizational level, change management involves employing a systematic approach to change organizational values, goals, policies, and processes.
Individual change, self-reflection, and personal growth are key components in changing culture. Reflexivity is being aware of your own values, norms, position, and power – an important concept to understand and apply in our everyday interactions. Believing that one’s class, gender, race and sexual orientation are irrelevant to their practice of medicine would not foster the change that we direly need in medicine. Rather, identifying how your own values and professional identity are shaped by your medical training, your organization and the broader cultural context are critically important to developing a greater empathic sense to motivate systemic change.
There has been valuable discussion on bottom-up changes to ensure women and POC have support, encouragement and a pathway to advance in an organization. Some of these include policy and process changes including providing flexible working conditions for women and sponsorship of women and minorities to help them navigate the barriers and microaggressions they encounter at work. While technical (policy) changes form the foundation for any organizational change, it is important to remember that the people side of change – the resistance that you encounter for any change effort in an organization – is equally important to address at the organizational level. A top-down approach is also vital to ensure that change is permanent in an organization and does not end when the individuals responsible for the change leave the organization.
Lewin’s three-stage change management model provides a framework for structural and organizational change in hospital systems. The three-stages of this model are: unfreezing, changing, and refreezing. Unfreezing is the process of determining what needs to change and obtaining leadership support. The actual change process involves getting people on board, empowering them to change and communicating with them frequently. Refreezing cements this change into the organization’s culture by providing support and training to sustain changes. Research has shown that Lewin’s change management model has applicability in the hospital setting.
Industry research in change management methodologies in the business sector has identified sponsorship by CEOs/senior management of an organization and having a structured implementation model for change management as two important factors for ensuring that change efforts are successful and sustainable.
This can be extrapolated to health care organizations – top leadership committed to changing the status quo should solidify organizational commitment by incorporating new attainable and measurable goals into their vision for the organization. Designing a phased implementation of change management methodologies should follow an open discussion to identify an organization’s weaknesses, strengths, capacity, and readiness for change. Lastly, helping busy professionals adapt to change requires innovative and continuous improvement strategies using formal, systematic tools for organization-wide strategic deployment.
Without a concrete commitment at the organizational level, programs such as diversity training may end up being band-aids on wounds that run deep.
I believe that the combination of both individual and organizational commitment to change systemic bias in medicine can be quite powerful. One without the other will fail to permanently change the system. The work to true equality – regardless of the intersecting factors of discrimination – starts with a commitment to change. We may all have different opportunities because of the inequality that is apparent in our systems today, but if we unite around the goal of a bias-free, merit-based equality, it gives us the strength we need to overcome challenges that we once thought insurmountable.
Each one of us is a leader in our own right. Speaking up for those with less power or opportunity than us and supporting talent and hard work solidifies medicine as a meritocracy. Even if the magnitude of change that we fight for may not be realized during our time in medical practice, our commitment to eradicate sexism, racism and discrimination will shape the future of medicine.
Just as our children are a legacy that we leave behind, our work in correcting bias in medicine will pave the path for a better future for the doctors of tomorrow. After all, when I think that my young daughter will be affected by what I do or do not do to address the discrimination, there is no better motivation for me to break down every barrier for her success.
Dr. Kanikkannan is a practicing hospitalist and assistant professor of medicine at Albany Medical College in Albany, NY. This article first appeared on The Hospital Leader, the official blog of SHM.
Quality measures and initiatives in private practices
It has been almost 15 years since the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy established the Task Force on Quality Endoscopy and published the first set of quality indicators for GI endoscopic procedures.
This work was motivated by two seminal reports on patient safety that fostered a demand by the public, policy makers, and payers to accurately define and measure the quality of health care services.
While the Centers for Medicare & Medicaid Services initially designated and required reporting on several basic outcome measures, leaders within the field of gastroenterology recognized the importance of developing evidence-based quality measures for our field, and specifically for endoscopic procedures.
Integrating safety measures into our daily operations has always been important, and over the years, policies have been implemented to incentivize health care providers to meet standards in everything from patient safety to patient satisfaction.
Defining quality and how to measure it
The goals of implementing quality measures within private practices include effective patient care and safety, but they also include issues like access and affordability, as well as the professionalism of your physicians and advanced practice providers.
As a larger practice, we have the resources to support a quality coordinator who spends half their time focused on quality measures. Every provider is required to complete annual education on quality parameters.
We have two committees that propose and track quality initiatives in our practice. We have one on the practice side and one for our ambulatory surgery centers (ASCs). The committees are made of physicians who have a particular interest in quality measures. On the ASC side, our ASC center director from our management partner AmSurg is also a member of the committee.
The road to improving quality within a private practice starts by defining the aspects of care that affect the quality of the patient experience.
Tracking quality in the office and in the surgery center
In our practices we have about 60 physicians. Start times and coding accuracy are good examples of what we have tracked in the past as areas of quality improvement. For instance, if only one or two providers get started late, it can cause a domino effect. Schedules get cramped, which can increase stress and possibly cause our team members to rush. Even things that seem like patient satisfaction issues can affect patient care, so it is important to make sure they are being measured.
On the ASC side, we track adenoma detection rates, colonoscopy intervals, complication rates, and many other additional criteria. As an example, when a pathology report is issued, we require our physicians to provide results to our patients within 72 hours.
Data on all providers are tabulated quarterly and then distributed to the providers in the form of a scorecard. The scorecard is then used for constructive feedback on improvements that can be made. A cumulative annual report is given to the providers, which is also incorporated into reviews. Not paying attention to quality measures can potentially have financial ramifications for providers in our group.
Find the right fit from a quality standpoint
In terms of what we are tracking, we are probably not that different from most groups of our size. Standardization will continue to increase, and it is important as an early career physician to familiarize yourself with quality measures in gastroenterology.
I often interview early career physicians who would like to join Regional GI, and the most impressive are the young men and women who ask about our processes for tracking quality measures and implementing programs geared toward improvement. If you are thinking of joining a practice, bring it up. You will be glad you did.
The interest in quality shows that you are invested in providing the best evidence-based patient care. As an independent group, this is critical because so much of what we do depends on having a track record of measurement. For instance, an ASC might not be credentialed if the quality metrics do not meet a certain threshold.
We are looking for potential partners who are seriously interested in joining us on our mission to provide the highest-quality care to our patients. After all, that is why became gastroenterologists in the first place.
Dr. Lalani serves as treasurer on the executive committee of the Digestive Health Physicians Association and is a practicing gastroenterologist at U.S. Digestive Health.
It has been almost 15 years since the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy established the Task Force on Quality Endoscopy and published the first set of quality indicators for GI endoscopic procedures.
This work was motivated by two seminal reports on patient safety that fostered a demand by the public, policy makers, and payers to accurately define and measure the quality of health care services.
While the Centers for Medicare & Medicaid Services initially designated and required reporting on several basic outcome measures, leaders within the field of gastroenterology recognized the importance of developing evidence-based quality measures for our field, and specifically for endoscopic procedures.
Integrating safety measures into our daily operations has always been important, and over the years, policies have been implemented to incentivize health care providers to meet standards in everything from patient safety to patient satisfaction.
Defining quality and how to measure it
The goals of implementing quality measures within private practices include effective patient care and safety, but they also include issues like access and affordability, as well as the professionalism of your physicians and advanced practice providers.
As a larger practice, we have the resources to support a quality coordinator who spends half their time focused on quality measures. Every provider is required to complete annual education on quality parameters.
We have two committees that propose and track quality initiatives in our practice. We have one on the practice side and one for our ambulatory surgery centers (ASCs). The committees are made of physicians who have a particular interest in quality measures. On the ASC side, our ASC center director from our management partner AmSurg is also a member of the committee.
The road to improving quality within a private practice starts by defining the aspects of care that affect the quality of the patient experience.
Tracking quality in the office and in the surgery center
In our practices we have about 60 physicians. Start times and coding accuracy are good examples of what we have tracked in the past as areas of quality improvement. For instance, if only one or two providers get started late, it can cause a domino effect. Schedules get cramped, which can increase stress and possibly cause our team members to rush. Even things that seem like patient satisfaction issues can affect patient care, so it is important to make sure they are being measured.
On the ASC side, we track adenoma detection rates, colonoscopy intervals, complication rates, and many other additional criteria. As an example, when a pathology report is issued, we require our physicians to provide results to our patients within 72 hours.
Data on all providers are tabulated quarterly and then distributed to the providers in the form of a scorecard. The scorecard is then used for constructive feedback on improvements that can be made. A cumulative annual report is given to the providers, which is also incorporated into reviews. Not paying attention to quality measures can potentially have financial ramifications for providers in our group.
Find the right fit from a quality standpoint
In terms of what we are tracking, we are probably not that different from most groups of our size. Standardization will continue to increase, and it is important as an early career physician to familiarize yourself with quality measures in gastroenterology.
I often interview early career physicians who would like to join Regional GI, and the most impressive are the young men and women who ask about our processes for tracking quality measures and implementing programs geared toward improvement. If you are thinking of joining a practice, bring it up. You will be glad you did.
The interest in quality shows that you are invested in providing the best evidence-based patient care. As an independent group, this is critical because so much of what we do depends on having a track record of measurement. For instance, an ASC might not be credentialed if the quality metrics do not meet a certain threshold.
We are looking for potential partners who are seriously interested in joining us on our mission to provide the highest-quality care to our patients. After all, that is why became gastroenterologists in the first place.
Dr. Lalani serves as treasurer on the executive committee of the Digestive Health Physicians Association and is a practicing gastroenterologist at U.S. Digestive Health.
It has been almost 15 years since the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy established the Task Force on Quality Endoscopy and published the first set of quality indicators for GI endoscopic procedures.
This work was motivated by two seminal reports on patient safety that fostered a demand by the public, policy makers, and payers to accurately define and measure the quality of health care services.
While the Centers for Medicare & Medicaid Services initially designated and required reporting on several basic outcome measures, leaders within the field of gastroenterology recognized the importance of developing evidence-based quality measures for our field, and specifically for endoscopic procedures.
Integrating safety measures into our daily operations has always been important, and over the years, policies have been implemented to incentivize health care providers to meet standards in everything from patient safety to patient satisfaction.
Defining quality and how to measure it
The goals of implementing quality measures within private practices include effective patient care and safety, but they also include issues like access and affordability, as well as the professionalism of your physicians and advanced practice providers.
As a larger practice, we have the resources to support a quality coordinator who spends half their time focused on quality measures. Every provider is required to complete annual education on quality parameters.
We have two committees that propose and track quality initiatives in our practice. We have one on the practice side and one for our ambulatory surgery centers (ASCs). The committees are made of physicians who have a particular interest in quality measures. On the ASC side, our ASC center director from our management partner AmSurg is also a member of the committee.
The road to improving quality within a private practice starts by defining the aspects of care that affect the quality of the patient experience.
Tracking quality in the office and in the surgery center
In our practices we have about 60 physicians. Start times and coding accuracy are good examples of what we have tracked in the past as areas of quality improvement. For instance, if only one or two providers get started late, it can cause a domino effect. Schedules get cramped, which can increase stress and possibly cause our team members to rush. Even things that seem like patient satisfaction issues can affect patient care, so it is important to make sure they are being measured.
On the ASC side, we track adenoma detection rates, colonoscopy intervals, complication rates, and many other additional criteria. As an example, when a pathology report is issued, we require our physicians to provide results to our patients within 72 hours.
Data on all providers are tabulated quarterly and then distributed to the providers in the form of a scorecard. The scorecard is then used for constructive feedback on improvements that can be made. A cumulative annual report is given to the providers, which is also incorporated into reviews. Not paying attention to quality measures can potentially have financial ramifications for providers in our group.
Find the right fit from a quality standpoint
In terms of what we are tracking, we are probably not that different from most groups of our size. Standardization will continue to increase, and it is important as an early career physician to familiarize yourself with quality measures in gastroenterology.
I often interview early career physicians who would like to join Regional GI, and the most impressive are the young men and women who ask about our processes for tracking quality measures and implementing programs geared toward improvement. If you are thinking of joining a practice, bring it up. You will be glad you did.
The interest in quality shows that you are invested in providing the best evidence-based patient care. As an independent group, this is critical because so much of what we do depends on having a track record of measurement. For instance, an ASC might not be credentialed if the quality metrics do not meet a certain threshold.
We are looking for potential partners who are seriously interested in joining us on our mission to provide the highest-quality care to our patients. After all, that is why became gastroenterologists in the first place.
Dr. Lalani serves as treasurer on the executive committee of the Digestive Health Physicians Association and is a practicing gastroenterologist at U.S. Digestive Health.
World Mental Health Day: Patients getting greater access
Telehealth visits allowing care to continue around the globe
Each year on Oct. 10, the world takes a moment to commemorate the significance of mental health and its impact on an individual’s life. This year, as we continue to reflect beyond World Mental Health Day, we see the world in a different light. Creating awareness for mental health issues and expanding access to psychiatric services has now become more essential than ever before.
The year 2020 will forever be known as the beginning of the “COVID era” as, unfortunately, the whole world as we know it adapts and reconstructs amid the rise of this global pandemic. This era has brought with it a wave of unemployment, social isolation, economic disaster, death, and disability. It is inevitable that such changes have brought forth perpetual fear and uncertainty, which have taken their toll not only on individuals’ physical health but largely on their mental health as well.
Factors that perpetuate deteriorating mental health include unemployment, poverty, isolation, fear and loss of loved ones – all of which have been further exacerbated globally, thanks to the current pandemic. According to the World Health Organization (WHO), 450 million people in the world suffer from mental illness, and one in four individuals are affected by mental illness in some stage of their lives. This means that mental illness accounts for 13% of the total global burden of disease.
These challenges include providing care in difficult circumstances, going to work afraid of bringing COVID-19 home, and vulnerability toward becoming mentally and physically ill. An immense sense of responsibility toward patients with mental illness, coupled with continuous fear of becoming infected with this novel virus, has made managing the mental health of our patients all the more challenging.
As a psychiatrist (A.A.M.), I have noticed a massive increase in both the incidence and prevalence of mental illness. Emergency departments are full of patients presenting with suicidal attempts/ideation. Substance abuse has increased in greater magnitude, and outpatients are presenting with escalating numbers of depression and anxiety. Relapse of symptoms among stable patients has been another major problem. Incidents of domestic violence, road rage, and impaired driving secondary to alcoholism leading to psychiatric consultations have also risen drastically.
Mental health units in hospitals are tremendously busy with scarce availability of beds. The increase in waiting times for allocation of beds has also become a major concern globally.
Governments have allocated more funds and are actively attempting to mobilize resources in the developed world. However, adapting to the circumstances has proven to be far more challenging in many regions of the developing world. To avoid personal contacts in health settings, governments have allowed virtual consultations, which has proven to be a highly commendable decision. The use of telephone and video consultations has allowed physicians, particularly psychiatrists, to continue to provide health care to their patients while maintaining social distance. Crisis services have also become far more active, which can help in alleviating mental health emergencies to a great extent.
International crisis is possible
According to the director of the World Federation for Mental Health, citing the report of World Economic Forum, mental health problems could cost the global economy up to $16 trillion between 2010 and 2030, and if this matter is not addressed, it could potentially lead to an international mental health crisis. If the pandemic continues to create such a large impact for a prolonged period of time, the state of mental health globally will continue to be a major concern.
Universal effort is imperative to strengthen the mental health service and increase our ability to provide care for vulnerable individuals. This can be achieved through collaboration with other stakeholders, the allied health sector, the WHO, and the World Bank. The efforts should be directed toward the availability of funds, mobilizing and enhancing resources and training health care and crisis workers. This focus should not only be for developed countries but also for developing countries alike because we are all suffering from the impacts of this global crisis together.
It is important to raise awareness and support one another now more than ever before as we strive to improve and strengthen our mental health on this World Mental Health Day.
Dr. Muhammad is clinical professor of psychiatry at McMaster University, Hamilton, Ont. Ms. Amin is a 5th-year MBBS student at St. George’s University Hospital in London.
Telehealth visits allowing care to continue around the globe
Telehealth visits allowing care to continue around the globe
Each year on Oct. 10, the world takes a moment to commemorate the significance of mental health and its impact on an individual’s life. This year, as we continue to reflect beyond World Mental Health Day, we see the world in a different light. Creating awareness for mental health issues and expanding access to psychiatric services has now become more essential than ever before.
The year 2020 will forever be known as the beginning of the “COVID era” as, unfortunately, the whole world as we know it adapts and reconstructs amid the rise of this global pandemic. This era has brought with it a wave of unemployment, social isolation, economic disaster, death, and disability. It is inevitable that such changes have brought forth perpetual fear and uncertainty, which have taken their toll not only on individuals’ physical health but largely on their mental health as well.
Factors that perpetuate deteriorating mental health include unemployment, poverty, isolation, fear and loss of loved ones – all of which have been further exacerbated globally, thanks to the current pandemic. According to the World Health Organization (WHO), 450 million people in the world suffer from mental illness, and one in four individuals are affected by mental illness in some stage of their lives. This means that mental illness accounts for 13% of the total global burden of disease.
These challenges include providing care in difficult circumstances, going to work afraid of bringing COVID-19 home, and vulnerability toward becoming mentally and physically ill. An immense sense of responsibility toward patients with mental illness, coupled with continuous fear of becoming infected with this novel virus, has made managing the mental health of our patients all the more challenging.
As a psychiatrist (A.A.M.), I have noticed a massive increase in both the incidence and prevalence of mental illness. Emergency departments are full of patients presenting with suicidal attempts/ideation. Substance abuse has increased in greater magnitude, and outpatients are presenting with escalating numbers of depression and anxiety. Relapse of symptoms among stable patients has been another major problem. Incidents of domestic violence, road rage, and impaired driving secondary to alcoholism leading to psychiatric consultations have also risen drastically.
Mental health units in hospitals are tremendously busy with scarce availability of beds. The increase in waiting times for allocation of beds has also become a major concern globally.
Governments have allocated more funds and are actively attempting to mobilize resources in the developed world. However, adapting to the circumstances has proven to be far more challenging in many regions of the developing world. To avoid personal contacts in health settings, governments have allowed virtual consultations, which has proven to be a highly commendable decision. The use of telephone and video consultations has allowed physicians, particularly psychiatrists, to continue to provide health care to their patients while maintaining social distance. Crisis services have also become far more active, which can help in alleviating mental health emergencies to a great extent.
International crisis is possible
According to the director of the World Federation for Mental Health, citing the report of World Economic Forum, mental health problems could cost the global economy up to $16 trillion between 2010 and 2030, and if this matter is not addressed, it could potentially lead to an international mental health crisis. If the pandemic continues to create such a large impact for a prolonged period of time, the state of mental health globally will continue to be a major concern.
Universal effort is imperative to strengthen the mental health service and increase our ability to provide care for vulnerable individuals. This can be achieved through collaboration with other stakeholders, the allied health sector, the WHO, and the World Bank. The efforts should be directed toward the availability of funds, mobilizing and enhancing resources and training health care and crisis workers. This focus should not only be for developed countries but also for developing countries alike because we are all suffering from the impacts of this global crisis together.
It is important to raise awareness and support one another now more than ever before as we strive to improve and strengthen our mental health on this World Mental Health Day.
Dr. Muhammad is clinical professor of psychiatry at McMaster University, Hamilton, Ont. Ms. Amin is a 5th-year MBBS student at St. George’s University Hospital in London.
Each year on Oct. 10, the world takes a moment to commemorate the significance of mental health and its impact on an individual’s life. This year, as we continue to reflect beyond World Mental Health Day, we see the world in a different light. Creating awareness for mental health issues and expanding access to psychiatric services has now become more essential than ever before.
The year 2020 will forever be known as the beginning of the “COVID era” as, unfortunately, the whole world as we know it adapts and reconstructs amid the rise of this global pandemic. This era has brought with it a wave of unemployment, social isolation, economic disaster, death, and disability. It is inevitable that such changes have brought forth perpetual fear and uncertainty, which have taken their toll not only on individuals’ physical health but largely on their mental health as well.
Factors that perpetuate deteriorating mental health include unemployment, poverty, isolation, fear and loss of loved ones – all of which have been further exacerbated globally, thanks to the current pandemic. According to the World Health Organization (WHO), 450 million people in the world suffer from mental illness, and one in four individuals are affected by mental illness in some stage of their lives. This means that mental illness accounts for 13% of the total global burden of disease.
These challenges include providing care in difficult circumstances, going to work afraid of bringing COVID-19 home, and vulnerability toward becoming mentally and physically ill. An immense sense of responsibility toward patients with mental illness, coupled with continuous fear of becoming infected with this novel virus, has made managing the mental health of our patients all the more challenging.
As a psychiatrist (A.A.M.), I have noticed a massive increase in both the incidence and prevalence of mental illness. Emergency departments are full of patients presenting with suicidal attempts/ideation. Substance abuse has increased in greater magnitude, and outpatients are presenting with escalating numbers of depression and anxiety. Relapse of symptoms among stable patients has been another major problem. Incidents of domestic violence, road rage, and impaired driving secondary to alcoholism leading to psychiatric consultations have also risen drastically.
Mental health units in hospitals are tremendously busy with scarce availability of beds. The increase in waiting times for allocation of beds has also become a major concern globally.
Governments have allocated more funds and are actively attempting to mobilize resources in the developed world. However, adapting to the circumstances has proven to be far more challenging in many regions of the developing world. To avoid personal contacts in health settings, governments have allowed virtual consultations, which has proven to be a highly commendable decision. The use of telephone and video consultations has allowed physicians, particularly psychiatrists, to continue to provide health care to their patients while maintaining social distance. Crisis services have also become far more active, which can help in alleviating mental health emergencies to a great extent.
International crisis is possible
According to the director of the World Federation for Mental Health, citing the report of World Economic Forum, mental health problems could cost the global economy up to $16 trillion between 2010 and 2030, and if this matter is not addressed, it could potentially lead to an international mental health crisis. If the pandemic continues to create such a large impact for a prolonged period of time, the state of mental health globally will continue to be a major concern.
Universal effort is imperative to strengthen the mental health service and increase our ability to provide care for vulnerable individuals. This can be achieved through collaboration with other stakeholders, the allied health sector, the WHO, and the World Bank. The efforts should be directed toward the availability of funds, mobilizing and enhancing resources and training health care and crisis workers. This focus should not only be for developed countries but also for developing countries alike because we are all suffering from the impacts of this global crisis together.
It is important to raise awareness and support one another now more than ever before as we strive to improve and strengthen our mental health on this World Mental Health Day.
Dr. Muhammad is clinical professor of psychiatry at McMaster University, Hamilton, Ont. Ms. Amin is a 5th-year MBBS student at St. George’s University Hospital in London.
Low DHT linked to hip fracture in men
In older men, circulating levels of dihydrotestosterone (DHT) and sex hormone–binding globulin (SHBG) independently predict risk of hip fracture, but testosterone does not, according to a study involving more than 1,000 men.
These findings could influence clinical measurement of male hormone levels and possibly intervention for low DHT, reported lead author Emily A. Rosenberg, MD, of Brigham and Women’s Hospital in Boston and colleagues.
“Male aging is associated with a decrease in serum sex hormones, and this decline has been shown to influence bone health, although the links between androgen levels in men and bone mineral density and fracture risk remain an ongoing source of debate,” the investigators wrote in Metabolism.
According to Dr. Rosenberg and colleagues, most previous studies in this area have focused on total or bioavailable testosterone; however, DHT demonstrates greater affinity with and slower dissociation from the androgen receptor, which could translate to a more significant role in bone metabolism. With the advent of mass spectrometry–based DHT assays, it is now possible to accurately measure small concentrations of DHT in blood, they added.
Their prospective, multicenter, cohort study involved 1,128 men who were 65 years or older and without history of cardiovascular disease. Beginning in 1989-1990, participants underwent a baseline examination that included standardized medical history questionnaires, physical exam, and laboratory testing. Additional participants joined the study in 1992-1993, and in 1994-1995, a subset of participants (n = 439) underwent dual-energy x-ray absorptiometry (DXA) scanning.
Hormone assays were conducted in 2010 using frozen serum samples from 1994-1995. Testosterone and DHT were measured by liquid chromatography–tandem mass spectrometry assay, while SHBG was measured by fluoroimmunoassay.
The primary outcome, incident hip fracture, was identified from medical records through 2013. Secondary outcomes included lean body mass and bone mineral density of the hip. A variety of covariates were also recorded, including age, sex, weight, alcohol consumption, smoking status, and others.
After a median follow-up of 10.2 years (interquartile range, 5.9-15.5 years), 106 cases of hip fracture occurred, which translated to an incidence rate of 0.89 per 100 person-years. Cox regression models mutually adjusted for covariates, and the other analyses showed that each standard deviation increase in DHT correlated with a 26% decreased risk of hip fracture (adjusted hazard ratio, 0.74; 95% confidence interval, 0.55-1.00; P = .049). Conversely, each standard deviation increase in SBHG was associated with a 26% increased risk of hip fracture (aHR, 1.26; 95% CI, 1.01-1.58; P = .045). In contrast with both DHT and SBHG, testosterone was not significantly associated with the primary outcome (aHR, 1.16; 95% CI, 0.86-1.56; P = .324).
Further analysis showed that testosterone, DHT, and SBHG were not significantly associated with bone mineral density of the hip. In adjusted models, testosterone and DHT were independently associated with higher lean body mass; however, in mutually adjusted models, these associations were not statistically significant, although they remained similar and positive.
“More research is needed to determine the mechanism(s) by which DHT may affect bone health and whether interventions that regulate DHT might be used to reduce risk of hip fracture,” the investigators concluded. “While our results require confirmation, there may be a role for measurement of DHT along with testosterone when the clinical scenario requires measurement of male hormone levels.”
The study was funded by the National Heart, Lung and Blood Institute and the National Institute on Aging. The investigators reported no conflicts of interest.
SOURCE: Rosenberg EA et al. Metabolism. 2020 Oct 12. doi: 10.1016/j.metabol.2020.154399.
In older men, circulating levels of dihydrotestosterone (DHT) and sex hormone–binding globulin (SHBG) independently predict risk of hip fracture, but testosterone does not, according to a study involving more than 1,000 men.
These findings could influence clinical measurement of male hormone levels and possibly intervention for low DHT, reported lead author Emily A. Rosenberg, MD, of Brigham and Women’s Hospital in Boston and colleagues.
“Male aging is associated with a decrease in serum sex hormones, and this decline has been shown to influence bone health, although the links between androgen levels in men and bone mineral density and fracture risk remain an ongoing source of debate,” the investigators wrote in Metabolism.
According to Dr. Rosenberg and colleagues, most previous studies in this area have focused on total or bioavailable testosterone; however, DHT demonstrates greater affinity with and slower dissociation from the androgen receptor, which could translate to a more significant role in bone metabolism. With the advent of mass spectrometry–based DHT assays, it is now possible to accurately measure small concentrations of DHT in blood, they added.
Their prospective, multicenter, cohort study involved 1,128 men who were 65 years or older and without history of cardiovascular disease. Beginning in 1989-1990, participants underwent a baseline examination that included standardized medical history questionnaires, physical exam, and laboratory testing. Additional participants joined the study in 1992-1993, and in 1994-1995, a subset of participants (n = 439) underwent dual-energy x-ray absorptiometry (DXA) scanning.
Hormone assays were conducted in 2010 using frozen serum samples from 1994-1995. Testosterone and DHT were measured by liquid chromatography–tandem mass spectrometry assay, while SHBG was measured by fluoroimmunoassay.
The primary outcome, incident hip fracture, was identified from medical records through 2013. Secondary outcomes included lean body mass and bone mineral density of the hip. A variety of covariates were also recorded, including age, sex, weight, alcohol consumption, smoking status, and others.
After a median follow-up of 10.2 years (interquartile range, 5.9-15.5 years), 106 cases of hip fracture occurred, which translated to an incidence rate of 0.89 per 100 person-years. Cox regression models mutually adjusted for covariates, and the other analyses showed that each standard deviation increase in DHT correlated with a 26% decreased risk of hip fracture (adjusted hazard ratio, 0.74; 95% confidence interval, 0.55-1.00; P = .049). Conversely, each standard deviation increase in SBHG was associated with a 26% increased risk of hip fracture (aHR, 1.26; 95% CI, 1.01-1.58; P = .045). In contrast with both DHT and SBHG, testosterone was not significantly associated with the primary outcome (aHR, 1.16; 95% CI, 0.86-1.56; P = .324).
Further analysis showed that testosterone, DHT, and SBHG were not significantly associated with bone mineral density of the hip. In adjusted models, testosterone and DHT were independently associated with higher lean body mass; however, in mutually adjusted models, these associations were not statistically significant, although they remained similar and positive.
“More research is needed to determine the mechanism(s) by which DHT may affect bone health and whether interventions that regulate DHT might be used to reduce risk of hip fracture,” the investigators concluded. “While our results require confirmation, there may be a role for measurement of DHT along with testosterone when the clinical scenario requires measurement of male hormone levels.”
The study was funded by the National Heart, Lung and Blood Institute and the National Institute on Aging. The investigators reported no conflicts of interest.
SOURCE: Rosenberg EA et al. Metabolism. 2020 Oct 12. doi: 10.1016/j.metabol.2020.154399.
In older men, circulating levels of dihydrotestosterone (DHT) and sex hormone–binding globulin (SHBG) independently predict risk of hip fracture, but testosterone does not, according to a study involving more than 1,000 men.
These findings could influence clinical measurement of male hormone levels and possibly intervention for low DHT, reported lead author Emily A. Rosenberg, MD, of Brigham and Women’s Hospital in Boston and colleagues.
“Male aging is associated with a decrease in serum sex hormones, and this decline has been shown to influence bone health, although the links between androgen levels in men and bone mineral density and fracture risk remain an ongoing source of debate,” the investigators wrote in Metabolism.
According to Dr. Rosenberg and colleagues, most previous studies in this area have focused on total or bioavailable testosterone; however, DHT demonstrates greater affinity with and slower dissociation from the androgen receptor, which could translate to a more significant role in bone metabolism. With the advent of mass spectrometry–based DHT assays, it is now possible to accurately measure small concentrations of DHT in blood, they added.
Their prospective, multicenter, cohort study involved 1,128 men who were 65 years or older and without history of cardiovascular disease. Beginning in 1989-1990, participants underwent a baseline examination that included standardized medical history questionnaires, physical exam, and laboratory testing. Additional participants joined the study in 1992-1993, and in 1994-1995, a subset of participants (n = 439) underwent dual-energy x-ray absorptiometry (DXA) scanning.
Hormone assays were conducted in 2010 using frozen serum samples from 1994-1995. Testosterone and DHT were measured by liquid chromatography–tandem mass spectrometry assay, while SHBG was measured by fluoroimmunoassay.
The primary outcome, incident hip fracture, was identified from medical records through 2013. Secondary outcomes included lean body mass and bone mineral density of the hip. A variety of covariates were also recorded, including age, sex, weight, alcohol consumption, smoking status, and others.
After a median follow-up of 10.2 years (interquartile range, 5.9-15.5 years), 106 cases of hip fracture occurred, which translated to an incidence rate of 0.89 per 100 person-years. Cox regression models mutually adjusted for covariates, and the other analyses showed that each standard deviation increase in DHT correlated with a 26% decreased risk of hip fracture (adjusted hazard ratio, 0.74; 95% confidence interval, 0.55-1.00; P = .049). Conversely, each standard deviation increase in SBHG was associated with a 26% increased risk of hip fracture (aHR, 1.26; 95% CI, 1.01-1.58; P = .045). In contrast with both DHT and SBHG, testosterone was not significantly associated with the primary outcome (aHR, 1.16; 95% CI, 0.86-1.56; P = .324).
Further analysis showed that testosterone, DHT, and SBHG were not significantly associated with bone mineral density of the hip. In adjusted models, testosterone and DHT were independently associated with higher lean body mass; however, in mutually adjusted models, these associations were not statistically significant, although they remained similar and positive.
“More research is needed to determine the mechanism(s) by which DHT may affect bone health and whether interventions that regulate DHT might be used to reduce risk of hip fracture,” the investigators concluded. “While our results require confirmation, there may be a role for measurement of DHT along with testosterone when the clinical scenario requires measurement of male hormone levels.”
The study was funded by the National Heart, Lung and Blood Institute and the National Institute on Aging. The investigators reported no conflicts of interest.
SOURCE: Rosenberg EA et al. Metabolism. 2020 Oct 12. doi: 10.1016/j.metabol.2020.154399.
FROM METABOLISM
Medicare faces calls to stop physician pay cuts in E/M overhaul
A planned overhaul of reimbursement for evaluation and management (E/M) services emerged as perhaps the most contentious issue connected to Medicare’s 2021 payment policies for clinicians.
The Centers for Medicare & Medicaid Services (CMS) included the planned E/M overhaul — and accompanying offsets — in the draft 2021 physician fee schedule, released in August. The draft fee schedule drew at least 45,675 responses by October 5, the deadline for offering comments, with many of the responses addressing the E/M overhaul.
The influential Medicare Payment Advisory Commission (MedPAC) “strongly” endorsed the “budget-neutral” approach taken with the E/M overhaul. This planned reshuffling of payments is a step toward addressing a shortfall of primary care clinicians, inasmuch as it would help make this field more financially appealing, MedPAC said in an October 2 letter to CMS.
In contrast, physician organizations, including the American Medical Association (AMA), asked CMS to waive or revise the budget-neutral aspect of the E/M overhaul. Among the specialties slated for reductions are those deeply involved with the response to the pandemic, wrote James L. Madara, AMA’s chief executive officer, in an October 5 comment to CMS. Emergency medicine as a field would see a 6% cut, and infectious disease specialists, a 4% reduction.
“Payment reductions of this magnitude would be a major problem at any time, but to impose cuts of this magnitude during or immediately after the COVID-19 pandemic, including steep cuts to many of the specialties that have been on the front lines in efforts to treat patients in places with widespread infection, is unconscionable,” Madara wrote.
Madara also said specialties scheduled for payment reductions include those least able to make up for the lack of in-person care as a result of the uptick in telehealth during the pandemic.
A chart in the draft physician fee schedule (Table 90) shows reductions for many specialties that do not routinely bill for office visits. The table shows an 8% cut for anesthesiologists, a 7% cut for general surgeons, and a 6% cut for ophthalmologists. Table 90 also shows an estimated 11% reduction for radiologists and a 9% drop for pathologists.
The draft rule notes that these figures are based upon estimates of aggregate allowed charges across all services, so they may not reflect what any particular clinician might receive.
In total, Table 90 shows how the E/M changes and connected offsets would affect more than 50 fields of medicine. The proposal includes a 17% expected increase for endocrinologists and a 14% bump for those in hematology/oncology. There are expected increases of 13% for family practice and 4% for internal medicine.
This reshuffling of payments among specialties is only part of the 2021 E/M overhaul. There’s strong support for other aspects, making it unlikely that CMS would consider dropping the plan entirely.
“CMS’ new office visit policy will lead to significant administrative burden reduction and will better describe and recognize the resources involved in clinical office visits as they are performed today,” AMA’s Madara wrote in his comment.
Changes for the billing framework for E/M slated to start in 2021 are the result of substantial collaboration by an AMA-convened work group, which brought together more than 170 state medical and specialty societies, Madara said in his comment.
CMS has been developing this plan for several years. It outlined this 2021 E/M overhaul in the 2020 Medicare physician fee schedule finalized last year.
Madara urged CMS to proceed with the E/M changes but also “exercise the full breadth and depth of its administrative authority” to avoid or minimize the planned cuts.
“To be clear, we are not asking CMS to phase in implementation of the E/M changes but rather to phase in the payment reductions for certain specialties and health professionals in 2021 due to budget neutrality,” he wrote.
Other groups asking CMS to waive the budget-neutrality requirement include the American College of Physicians, the American College of Emergency Physicians, the American Society for Radiation Oncology, and the American Society of Neuroradiology.
The American Academy of Family Physicians (AAFP) asked CMS to temporarily waive the budget-neutrality requirement and pressed the agency to maintain the underlying principle of the E/M overhaul.
“Should HHS [Department of Health and Human Services] use its authority to waive budget neutrality, we also recommend that CMS finalize a reinstatement plan for the conversion factor reductions that provides physician practices with ample time to prepare and does not result in a financial cliff,” wrote John S. Cullen, MD, board chair for AAFP, in a September 28 comment to CMS.
Owing to the declaration of a public health emergency, HHS could use a special provision known as 1135 waiver authority to waive budget-neutrality requirements, Cullen wrote.
“The AAFP understands that HHS’ authority is limited by the timing of the end of the public health emergency, but we believe that this approach will provide Congress with needed time to enact an accompanying legislative solution,” he wrote.
Lawmakers weigh in
Lawmakers in both political parties have asked CMS to reconsider the offsets in the E/M overhaul.
Rep. Michael C. Burgess, MD (R-TX), who practiced as an obstetrician before joining Congress, in October introduced a bill with Rep. Bobby Rush (D-IL) that would provide for a 1-year waiver of budget-neutrality adjustments under the Medicare physician fee schedule.
Burgess and Rush were among the more than 160 members of Congress who signed a September letter to CMS asking the agency to act on its own to drop the budget-neutrality requirement. In the letter, led by Rep. Roger Marshall, MD (R-KS), the lawmakers acknowledge the usual legal requirements for CMS to offset payment increases in the physician fee schedule with cuts. But the lawmakers said the national public health emergency allows CMS to work around this.
“Given the effects of the COVID-19 pandemic, we believe you have the regulatory authority to immediately address these inequities,” the lawmakers wrote. “There is also the need to consider how the outbreak will be in the fall/winter months and if postponing certain elective procedures will go back into effect, per CMS’ recommendations.
“While we understand that legislative action may also be required to address this issue, given the January 1, 2021 effective date, we would ask you to take immediate actions to delay or mitigate these cuts while allowing the scheduled increases to go into effect,” the lawmakers said in closing their letter. “This approach will give Congress sufficient time to develop a meaningful solution and to address these looming needs.”
Another option might be for CMS to preserve the budget-neutrality claim for the 2021 physician fee schedule but soften the blow on specialties, Brian Fortune, president of the consulting firm Farragut Square Group, told Medscape Medical News. A former staffer for Republican leadership in the House of Representatives, Fortune has for more than 20 years followed Medicare policy.
The agency could redo some of the assumptions used in estimating the offsets, he said, adding that in the draft rule, CMS appears to be seeking feedback that could help it with new calculations.
“CMS has been looking for a way out,” Fortune said. “CMS could remodel the assumptions, and the cuts could drop by half or more.
“The agency has several options to get creative as the need arises,” he said.
“Overvalued” vs “devalued”
In its comment to CMS, though, MedPAC argued strongly for maintaining the offsets. The commission has for several years been investigating ways to use Medicare’s payment policies as a tool to boost the ranks of clinicians who provide primary care.
A reshuffling of payments among specialties is needed to address a known imbalance in which Medicare for many years has “overvalued” procedures at the expense of other medical care, wrote Michael E. Chernew, PhD, the chairman of MedPAC, in an October 2 comment to CMS.
“Some types of services — such as procedures, imaging, and tests — experience efficiency gains over time, as advances in technology, technique, and clinical practice enable clinicians to deliver them faster,” he wrote. “However, E&M office/outpatient visits do not lend themselves to such efficiency gains because they consist largely of activities that require the clinician’s time.”
Medicare’s payment policies have thus “passively devalued” the time many clinicians spend on office visits, helping to skew the decisions of young physicians toward specialties, according to Chernew.
Reshuffling payment away from specialties that are now “overvalued” is needed to “remedy several years of passive devaluation,” he wrote.
The median income in 2018 for primary care physicians was $243,000 in 2018, whereas that of specialists such as surgeons was $426,000, Chernew said in the letter, citing MedPAC research.
These figures echo the findings of Medscape’s most recent annual physician compensation report.
As one of the largest buyers of medical services, Medicare has significant influence on the practice of medicine in the United States. In 2018 alone, Medicare directly paid $70.5 billion for clinician services. Its payment policies already may have shaped the pool of clinicians available to treat people enrolled in Medicare, which covers those aged 65 years and older, Chernew said.
“The US has over three times as many specialists as primary care physicians, which could explain why MedPAC’s annual survey of Medicare beneficiaries has repeatedly found that beneficiaries who are looking for a new physician report having an easier time finding a new specialist than a new primary care provider,” he wrote.
“Access to primary care physicians could worsen in the future as the number of primary care physicians in the US, after remaining flat for several years, has actually started to decline,” Chernew said.
This article first appeared on Medscape.com.
A planned overhaul of reimbursement for evaluation and management (E/M) services emerged as perhaps the most contentious issue connected to Medicare’s 2021 payment policies for clinicians.
The Centers for Medicare & Medicaid Services (CMS) included the planned E/M overhaul — and accompanying offsets — in the draft 2021 physician fee schedule, released in August. The draft fee schedule drew at least 45,675 responses by October 5, the deadline for offering comments, with many of the responses addressing the E/M overhaul.
The influential Medicare Payment Advisory Commission (MedPAC) “strongly” endorsed the “budget-neutral” approach taken with the E/M overhaul. This planned reshuffling of payments is a step toward addressing a shortfall of primary care clinicians, inasmuch as it would help make this field more financially appealing, MedPAC said in an October 2 letter to CMS.
In contrast, physician organizations, including the American Medical Association (AMA), asked CMS to waive or revise the budget-neutral aspect of the E/M overhaul. Among the specialties slated for reductions are those deeply involved with the response to the pandemic, wrote James L. Madara, AMA’s chief executive officer, in an October 5 comment to CMS. Emergency medicine as a field would see a 6% cut, and infectious disease specialists, a 4% reduction.
“Payment reductions of this magnitude would be a major problem at any time, but to impose cuts of this magnitude during or immediately after the COVID-19 pandemic, including steep cuts to many of the specialties that have been on the front lines in efforts to treat patients in places with widespread infection, is unconscionable,” Madara wrote.
Madara also said specialties scheduled for payment reductions include those least able to make up for the lack of in-person care as a result of the uptick in telehealth during the pandemic.
A chart in the draft physician fee schedule (Table 90) shows reductions for many specialties that do not routinely bill for office visits. The table shows an 8% cut for anesthesiologists, a 7% cut for general surgeons, and a 6% cut for ophthalmologists. Table 90 also shows an estimated 11% reduction for radiologists and a 9% drop for pathologists.
The draft rule notes that these figures are based upon estimates of aggregate allowed charges across all services, so they may not reflect what any particular clinician might receive.
In total, Table 90 shows how the E/M changes and connected offsets would affect more than 50 fields of medicine. The proposal includes a 17% expected increase for endocrinologists and a 14% bump for those in hematology/oncology. There are expected increases of 13% for family practice and 4% for internal medicine.
This reshuffling of payments among specialties is only part of the 2021 E/M overhaul. There’s strong support for other aspects, making it unlikely that CMS would consider dropping the plan entirely.
“CMS’ new office visit policy will lead to significant administrative burden reduction and will better describe and recognize the resources involved in clinical office visits as they are performed today,” AMA’s Madara wrote in his comment.
Changes for the billing framework for E/M slated to start in 2021 are the result of substantial collaboration by an AMA-convened work group, which brought together more than 170 state medical and specialty societies, Madara said in his comment.
CMS has been developing this plan for several years. It outlined this 2021 E/M overhaul in the 2020 Medicare physician fee schedule finalized last year.
Madara urged CMS to proceed with the E/M changes but also “exercise the full breadth and depth of its administrative authority” to avoid or minimize the planned cuts.
“To be clear, we are not asking CMS to phase in implementation of the E/M changes but rather to phase in the payment reductions for certain specialties and health professionals in 2021 due to budget neutrality,” he wrote.
Other groups asking CMS to waive the budget-neutrality requirement include the American College of Physicians, the American College of Emergency Physicians, the American Society for Radiation Oncology, and the American Society of Neuroradiology.
The American Academy of Family Physicians (AAFP) asked CMS to temporarily waive the budget-neutrality requirement and pressed the agency to maintain the underlying principle of the E/M overhaul.
“Should HHS [Department of Health and Human Services] use its authority to waive budget neutrality, we also recommend that CMS finalize a reinstatement plan for the conversion factor reductions that provides physician practices with ample time to prepare and does not result in a financial cliff,” wrote John S. Cullen, MD, board chair for AAFP, in a September 28 comment to CMS.
Owing to the declaration of a public health emergency, HHS could use a special provision known as 1135 waiver authority to waive budget-neutrality requirements, Cullen wrote.
“The AAFP understands that HHS’ authority is limited by the timing of the end of the public health emergency, but we believe that this approach will provide Congress with needed time to enact an accompanying legislative solution,” he wrote.
Lawmakers weigh in
Lawmakers in both political parties have asked CMS to reconsider the offsets in the E/M overhaul.
Rep. Michael C. Burgess, MD (R-TX), who practiced as an obstetrician before joining Congress, in October introduced a bill with Rep. Bobby Rush (D-IL) that would provide for a 1-year waiver of budget-neutrality adjustments under the Medicare physician fee schedule.
Burgess and Rush were among the more than 160 members of Congress who signed a September letter to CMS asking the agency to act on its own to drop the budget-neutrality requirement. In the letter, led by Rep. Roger Marshall, MD (R-KS), the lawmakers acknowledge the usual legal requirements for CMS to offset payment increases in the physician fee schedule with cuts. But the lawmakers said the national public health emergency allows CMS to work around this.
“Given the effects of the COVID-19 pandemic, we believe you have the regulatory authority to immediately address these inequities,” the lawmakers wrote. “There is also the need to consider how the outbreak will be in the fall/winter months and if postponing certain elective procedures will go back into effect, per CMS’ recommendations.
“While we understand that legislative action may also be required to address this issue, given the January 1, 2021 effective date, we would ask you to take immediate actions to delay or mitigate these cuts while allowing the scheduled increases to go into effect,” the lawmakers said in closing their letter. “This approach will give Congress sufficient time to develop a meaningful solution and to address these looming needs.”
Another option might be for CMS to preserve the budget-neutrality claim for the 2021 physician fee schedule but soften the blow on specialties, Brian Fortune, president of the consulting firm Farragut Square Group, told Medscape Medical News. A former staffer for Republican leadership in the House of Representatives, Fortune has for more than 20 years followed Medicare policy.
The agency could redo some of the assumptions used in estimating the offsets, he said, adding that in the draft rule, CMS appears to be seeking feedback that could help it with new calculations.
“CMS has been looking for a way out,” Fortune said. “CMS could remodel the assumptions, and the cuts could drop by half or more.
“The agency has several options to get creative as the need arises,” he said.
“Overvalued” vs “devalued”
In its comment to CMS, though, MedPAC argued strongly for maintaining the offsets. The commission has for several years been investigating ways to use Medicare’s payment policies as a tool to boost the ranks of clinicians who provide primary care.
A reshuffling of payments among specialties is needed to address a known imbalance in which Medicare for many years has “overvalued” procedures at the expense of other medical care, wrote Michael E. Chernew, PhD, the chairman of MedPAC, in an October 2 comment to CMS.
“Some types of services — such as procedures, imaging, and tests — experience efficiency gains over time, as advances in technology, technique, and clinical practice enable clinicians to deliver them faster,” he wrote. “However, E&M office/outpatient visits do not lend themselves to such efficiency gains because they consist largely of activities that require the clinician’s time.”
Medicare’s payment policies have thus “passively devalued” the time many clinicians spend on office visits, helping to skew the decisions of young physicians toward specialties, according to Chernew.
Reshuffling payment away from specialties that are now “overvalued” is needed to “remedy several years of passive devaluation,” he wrote.
The median income in 2018 for primary care physicians was $243,000 in 2018, whereas that of specialists such as surgeons was $426,000, Chernew said in the letter, citing MedPAC research.
These figures echo the findings of Medscape’s most recent annual physician compensation report.
As one of the largest buyers of medical services, Medicare has significant influence on the practice of medicine in the United States. In 2018 alone, Medicare directly paid $70.5 billion for clinician services. Its payment policies already may have shaped the pool of clinicians available to treat people enrolled in Medicare, which covers those aged 65 years and older, Chernew said.
“The US has over three times as many specialists as primary care physicians, which could explain why MedPAC’s annual survey of Medicare beneficiaries has repeatedly found that beneficiaries who are looking for a new physician report having an easier time finding a new specialist than a new primary care provider,” he wrote.
“Access to primary care physicians could worsen in the future as the number of primary care physicians in the US, after remaining flat for several years, has actually started to decline,” Chernew said.
This article first appeared on Medscape.com.
A planned overhaul of reimbursement for evaluation and management (E/M) services emerged as perhaps the most contentious issue connected to Medicare’s 2021 payment policies for clinicians.
The Centers for Medicare & Medicaid Services (CMS) included the planned E/M overhaul — and accompanying offsets — in the draft 2021 physician fee schedule, released in August. The draft fee schedule drew at least 45,675 responses by October 5, the deadline for offering comments, with many of the responses addressing the E/M overhaul.
The influential Medicare Payment Advisory Commission (MedPAC) “strongly” endorsed the “budget-neutral” approach taken with the E/M overhaul. This planned reshuffling of payments is a step toward addressing a shortfall of primary care clinicians, inasmuch as it would help make this field more financially appealing, MedPAC said in an October 2 letter to CMS.
In contrast, physician organizations, including the American Medical Association (AMA), asked CMS to waive or revise the budget-neutral aspect of the E/M overhaul. Among the specialties slated for reductions are those deeply involved with the response to the pandemic, wrote James L. Madara, AMA’s chief executive officer, in an October 5 comment to CMS. Emergency medicine as a field would see a 6% cut, and infectious disease specialists, a 4% reduction.
“Payment reductions of this magnitude would be a major problem at any time, but to impose cuts of this magnitude during or immediately after the COVID-19 pandemic, including steep cuts to many of the specialties that have been on the front lines in efforts to treat patients in places with widespread infection, is unconscionable,” Madara wrote.
Madara also said specialties scheduled for payment reductions include those least able to make up for the lack of in-person care as a result of the uptick in telehealth during the pandemic.
A chart in the draft physician fee schedule (Table 90) shows reductions for many specialties that do not routinely bill for office visits. The table shows an 8% cut for anesthesiologists, a 7% cut for general surgeons, and a 6% cut for ophthalmologists. Table 90 also shows an estimated 11% reduction for radiologists and a 9% drop for pathologists.
The draft rule notes that these figures are based upon estimates of aggregate allowed charges across all services, so they may not reflect what any particular clinician might receive.
In total, Table 90 shows how the E/M changes and connected offsets would affect more than 50 fields of medicine. The proposal includes a 17% expected increase for endocrinologists and a 14% bump for those in hematology/oncology. There are expected increases of 13% for family practice and 4% for internal medicine.
This reshuffling of payments among specialties is only part of the 2021 E/M overhaul. There’s strong support for other aspects, making it unlikely that CMS would consider dropping the plan entirely.
“CMS’ new office visit policy will lead to significant administrative burden reduction and will better describe and recognize the resources involved in clinical office visits as they are performed today,” AMA’s Madara wrote in his comment.
Changes for the billing framework for E/M slated to start in 2021 are the result of substantial collaboration by an AMA-convened work group, which brought together more than 170 state medical and specialty societies, Madara said in his comment.
CMS has been developing this plan for several years. It outlined this 2021 E/M overhaul in the 2020 Medicare physician fee schedule finalized last year.
Madara urged CMS to proceed with the E/M changes but also “exercise the full breadth and depth of its administrative authority” to avoid or minimize the planned cuts.
“To be clear, we are not asking CMS to phase in implementation of the E/M changes but rather to phase in the payment reductions for certain specialties and health professionals in 2021 due to budget neutrality,” he wrote.
Other groups asking CMS to waive the budget-neutrality requirement include the American College of Physicians, the American College of Emergency Physicians, the American Society for Radiation Oncology, and the American Society of Neuroradiology.
The American Academy of Family Physicians (AAFP) asked CMS to temporarily waive the budget-neutrality requirement and pressed the agency to maintain the underlying principle of the E/M overhaul.
“Should HHS [Department of Health and Human Services] use its authority to waive budget neutrality, we also recommend that CMS finalize a reinstatement plan for the conversion factor reductions that provides physician practices with ample time to prepare and does not result in a financial cliff,” wrote John S. Cullen, MD, board chair for AAFP, in a September 28 comment to CMS.
Owing to the declaration of a public health emergency, HHS could use a special provision known as 1135 waiver authority to waive budget-neutrality requirements, Cullen wrote.
“The AAFP understands that HHS’ authority is limited by the timing of the end of the public health emergency, but we believe that this approach will provide Congress with needed time to enact an accompanying legislative solution,” he wrote.
Lawmakers weigh in
Lawmakers in both political parties have asked CMS to reconsider the offsets in the E/M overhaul.
Rep. Michael C. Burgess, MD (R-TX), who practiced as an obstetrician before joining Congress, in October introduced a bill with Rep. Bobby Rush (D-IL) that would provide for a 1-year waiver of budget-neutrality adjustments under the Medicare physician fee schedule.
Burgess and Rush were among the more than 160 members of Congress who signed a September letter to CMS asking the agency to act on its own to drop the budget-neutrality requirement. In the letter, led by Rep. Roger Marshall, MD (R-KS), the lawmakers acknowledge the usual legal requirements for CMS to offset payment increases in the physician fee schedule with cuts. But the lawmakers said the national public health emergency allows CMS to work around this.
“Given the effects of the COVID-19 pandemic, we believe you have the regulatory authority to immediately address these inequities,” the lawmakers wrote. “There is also the need to consider how the outbreak will be in the fall/winter months and if postponing certain elective procedures will go back into effect, per CMS’ recommendations.
“While we understand that legislative action may also be required to address this issue, given the January 1, 2021 effective date, we would ask you to take immediate actions to delay or mitigate these cuts while allowing the scheduled increases to go into effect,” the lawmakers said in closing their letter. “This approach will give Congress sufficient time to develop a meaningful solution and to address these looming needs.”
Another option might be for CMS to preserve the budget-neutrality claim for the 2021 physician fee schedule but soften the blow on specialties, Brian Fortune, president of the consulting firm Farragut Square Group, told Medscape Medical News. A former staffer for Republican leadership in the House of Representatives, Fortune has for more than 20 years followed Medicare policy.
The agency could redo some of the assumptions used in estimating the offsets, he said, adding that in the draft rule, CMS appears to be seeking feedback that could help it with new calculations.
“CMS has been looking for a way out,” Fortune said. “CMS could remodel the assumptions, and the cuts could drop by half or more.
“The agency has several options to get creative as the need arises,” he said.
“Overvalued” vs “devalued”
In its comment to CMS, though, MedPAC argued strongly for maintaining the offsets. The commission has for several years been investigating ways to use Medicare’s payment policies as a tool to boost the ranks of clinicians who provide primary care.
A reshuffling of payments among specialties is needed to address a known imbalance in which Medicare for many years has “overvalued” procedures at the expense of other medical care, wrote Michael E. Chernew, PhD, the chairman of MedPAC, in an October 2 comment to CMS.
“Some types of services — such as procedures, imaging, and tests — experience efficiency gains over time, as advances in technology, technique, and clinical practice enable clinicians to deliver them faster,” he wrote. “However, E&M office/outpatient visits do not lend themselves to such efficiency gains because they consist largely of activities that require the clinician’s time.”
Medicare’s payment policies have thus “passively devalued” the time many clinicians spend on office visits, helping to skew the decisions of young physicians toward specialties, according to Chernew.
Reshuffling payment away from specialties that are now “overvalued” is needed to “remedy several years of passive devaluation,” he wrote.
The median income in 2018 for primary care physicians was $243,000 in 2018, whereas that of specialists such as surgeons was $426,000, Chernew said in the letter, citing MedPAC research.
These figures echo the findings of Medscape’s most recent annual physician compensation report.
As one of the largest buyers of medical services, Medicare has significant influence on the practice of medicine in the United States. In 2018 alone, Medicare directly paid $70.5 billion for clinician services. Its payment policies already may have shaped the pool of clinicians available to treat people enrolled in Medicare, which covers those aged 65 years and older, Chernew said.
“The US has over three times as many specialists as primary care physicians, which could explain why MedPAC’s annual survey of Medicare beneficiaries has repeatedly found that beneficiaries who are looking for a new physician report having an easier time finding a new specialist than a new primary care provider,” he wrote.
“Access to primary care physicians could worsen in the future as the number of primary care physicians in the US, after remaining flat for several years, has actually started to decline,” Chernew said.
This article first appeared on Medscape.com.
Irritated Pigmented Plaque on the Scalp
The Diagnosis: Clonal Melanoacanthoma
Melanoacanthoma (MA) is an extremely rare, benign, epidermal tumor histologically characterized by keratinocytes and large, pigmented, dendritic melanocytes. These lesions are loosely related to seborrheic keratoses, and the term was first coined by Mishima and Pinkus1 in 1960. It is estimated that the lesion occurs in only 5 of 500,000 individuals and tends to occur in older, light-skinned individuals.2 The majority are slow growing and are present on the head, neck, or upper extremities; however, similar lesions also have been reported on the oral mucosa.3 Melanoacanthomas range in size from 2×2 to 15×15 cm; are clinically pigmented; and present as either a papule, plaque, nodule, or horn.2
Classic histologic findings of MA include papillomatosis, acanthosis, and hyperkeratosis with heavily pigmented dendritic melanocytes diffusely dispersed throughout all layers of the seborrheic keratosis-like epidermis.3 Other features include keratin-filled pseudocysts, Langerhans cells, reactive spindling of keratinocytes, and an inflammatory infiltrate. In our case, the classic histologic findings also were architecturally arranged in oval to round clones within the epidermis (quiz images 1 and 2). A MART-1 (melanoma antigen recognized by T cells) immunostain was obtained that highlighted the numerous but benign-appearing, dendritic melanocytes (quiz image 2 [inset]). A dual MART-1/Ki67 immunostain later was obtained and demonstrated a negligible proliferation index within the dendritic melanocytes. Therefore, the diagnosis of clonal MA was rendered. This formation of epidermal clones also is called the Borst-Jadassohn phenomenon, which rarely occurs in MAs. This subtype is important to recognize because the clonal pattern can more closely mimic malignant neoplasms such as melanoma.
Hidroacanthoma simplex is an intraepidermal variant of eccrine poroma. It is a rare entity that typically occurs in the extremities of women as a hyperkeratotic plaque. These typically clonal epidermal tumors may be heavily pigmented and rarely contain dendritic melanocytes; therefore, they may be confused with MA. However, classic histology will reveal an intraepidermal clonal proliferation of bland, monotonous, cuboidal cells with ample pink cytoplasm, as well as occasional cuticle-lined ducts (Figure 1).4 These ducts will highlight with carcinoembryonic antigen and epithelial membrane antigen immunostaining.
Malignant melanoma typically presents as a growing pigmented lesion and therefore can clinically mimic MA. Histologically, MA could be confused with melanoma due to the increased number of melanocytes plus the appearance of pagetoid spread resulting from the diffuse presence of melanocytes throughout the neoplasm. However, histologic assessment of melanoma should reveal cytologic atypia such as nuclear enlargement, hyperchromasia, molding, pleomorphism, and mitotic activity (Figure 2). Architectural atypia such as poor lateral circumscription of melanocytes, confluence and pagetoid spread of nondendritic atypical junctional melanocytes, production of pigment in deep dermal nests of melanocytes, and lack of maturation and dispersion of dermal melanocytes also should be seen.5 Unlike a melanocytic neoplasm, true melanocytic nests are not seen in MA, and the melanocytes are bland, normal-appearing but heavily pigmented, dendritic melanocytes. Electron microscopy has shown a defect in the transfer of melanin from these highly dendritic melanocytes to the keratinocytes.6

Similar to melanoma, seborrheic keratosis presents as a pigmented growing lesion; therefore, definitive diagnosis often is achieved via skin biopsy. Classic histologic findings include acanthotic or exophytic epidermal growth with a dome-shaped configuration containing multiple cornified hornlike cysts (Figure 3).7 Multiple keratin plugs and variably sized concentric keratin islands are common features. There may be varying degrees of melanin pigment deposition among the proliferating cells, and clonal formation may occur. Melanocyte-specific special stains and immunostains can be used to differentiate MA from seborrheic keratosis by highlighting numerous dendritic melanocytes diffusely spread throughout the epidermis in MA vs a normal distribution of occasional junctional melanocytes in seborrheic keratosis.2,8
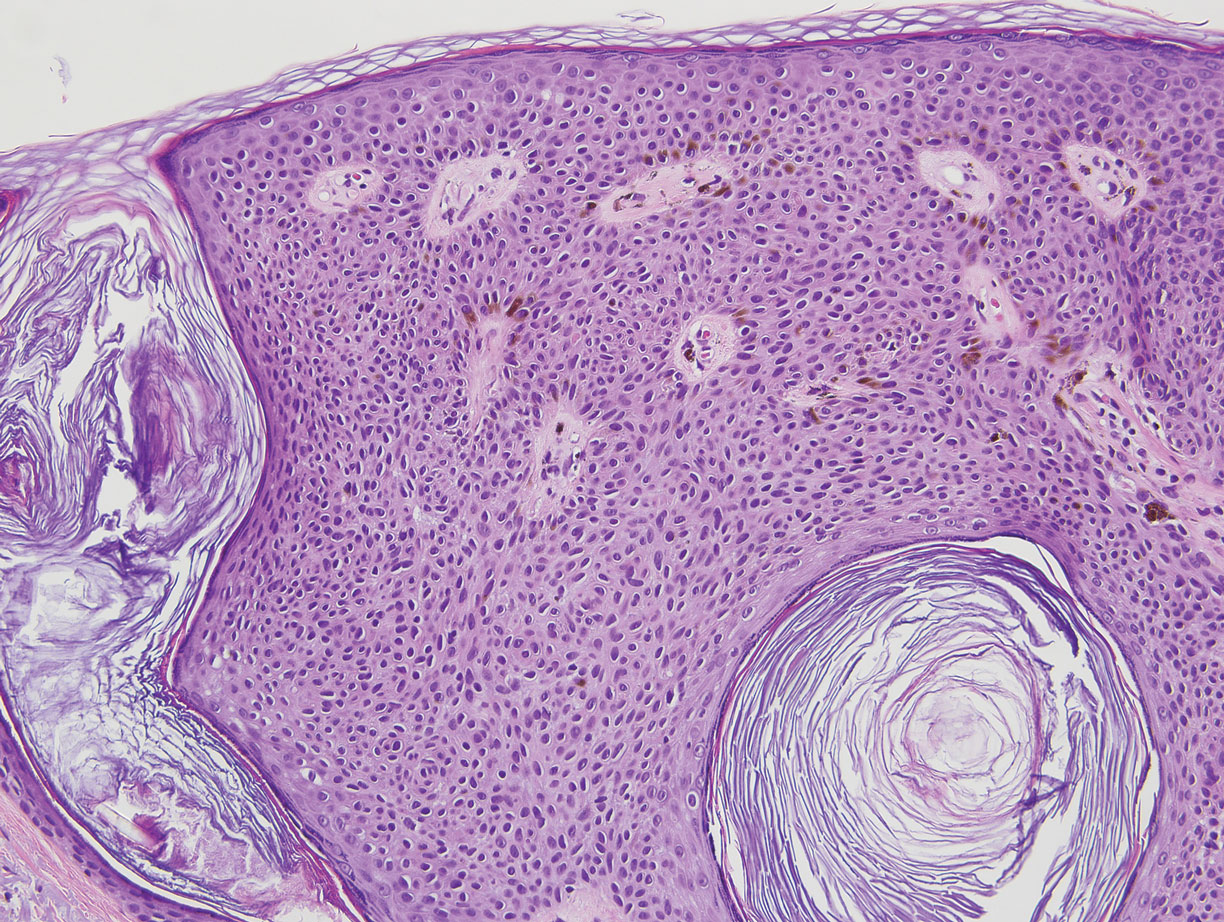
Squamous cell carcinoma in situ presents histologically with cytologically atypical keratinocytes encompassing the full thickness of the epidermis and sometimes crushing the basement membrane zone (Figure 4). There is a loss of the granular layer and overlying parakeratosis that often spares the adnexal ostial epithelium.9 Clonal formation can occur as well as increased pigment production. In comparison, bland keratinocytes are seen in MA.
Establishing the diagnosis of MA based on clinical features alone can be difficult. Dermoscopy can prove to be useful and typically will show a sunburst pattern with ridges and fissures.2 However, seborrheic keratoses and melanomas can have similar dermoscopic findings10; therefore, a biopsy often is necessary to establish the diagnosis.
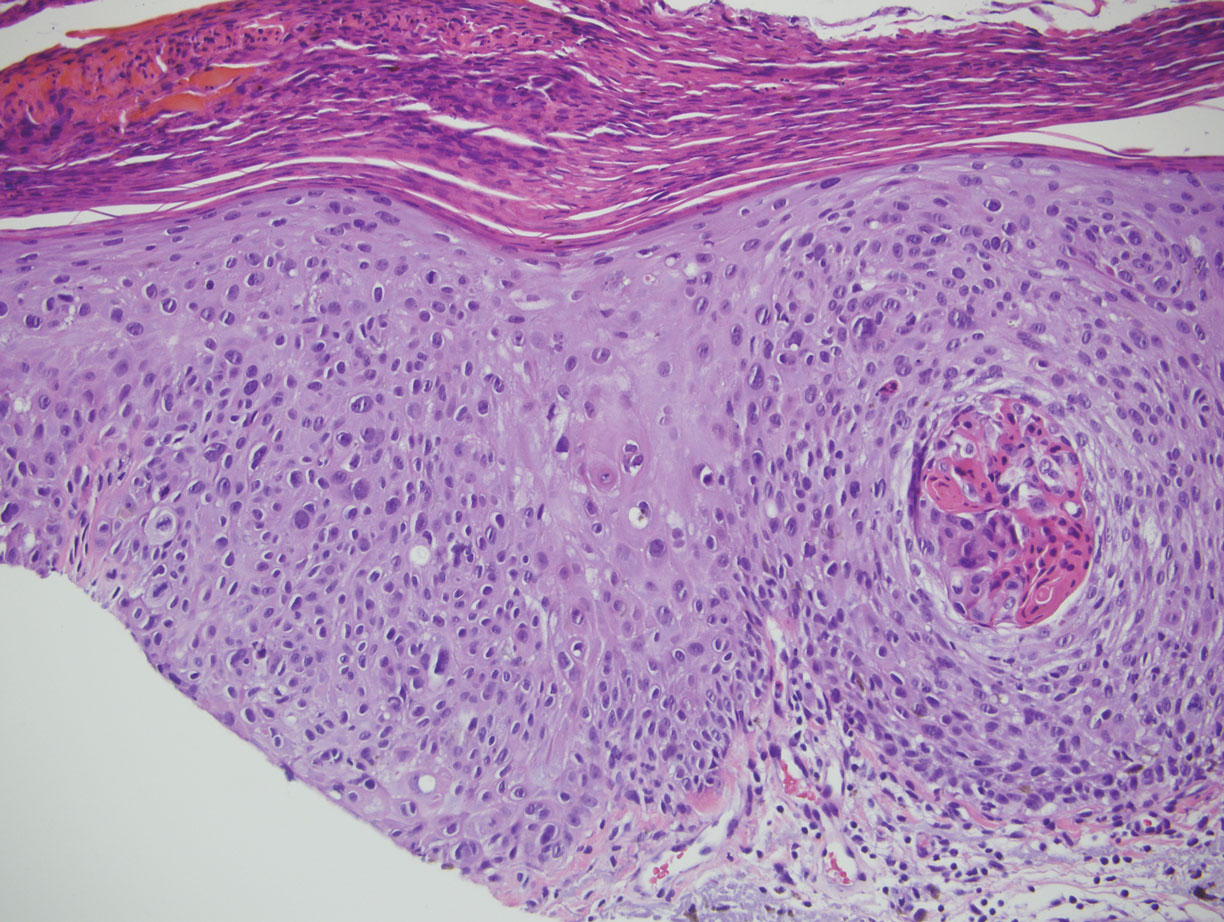
- Mishima Y, Pinkus H. Benign mixed tumor of melanocytes and malpighian cells: melanoacanthoma: its relationship to Bloch's benign non-nevoid melanoepithelioma. Arch Dermatol. 1960;81:539-550.
- Gutierrez N, Erickson C P, Calame A, et al. Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019;11:E4998.
- Fornatora ML, Reich RF, Haber S, et al. Oral melanoacanthoma: a report of 10 cases, review of literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol. 2003;25:12-15.
- Rahbari H. Hidroacanthoma simplex--a review of 15 cases. Br J Dermatol. 1983;109:219-225.
- Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19:S34-S40.
- Mishra DK, Jakati S, Dave TV, et al. A rare pigmented lesion of the eyelid. Int J Trichol. 2019;11:167-169.
- Greco MJ, Mahabadi N, Gossman W. Seborrheic keratosis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK545285/. Accessed September 18, 2020.
- Kihiczak G, Centurion SA, Schwartz RA, et al. Giant cutaneous melanoacanthoma. Int J Dermatol. 2004;43:936-937.
- Morais P, Schettini A, Junior R. Pigmented squamous cell carcinoma: a case report and importance of differential diagnosis. An Bras Dermatol. 2018;93:96-98.
- Chung E, Marqhoob A, Carrera C, et al. Clinical and dermoscopic features of cutaneous melanoacanthoma. JAMA Dermatol. 2015;151:1129-1130.
The Diagnosis: Clonal Melanoacanthoma
Melanoacanthoma (MA) is an extremely rare, benign, epidermal tumor histologically characterized by keratinocytes and large, pigmented, dendritic melanocytes. These lesions are loosely related to seborrheic keratoses, and the term was first coined by Mishima and Pinkus1 in 1960. It is estimated that the lesion occurs in only 5 of 500,000 individuals and tends to occur in older, light-skinned individuals.2 The majority are slow growing and are present on the head, neck, or upper extremities; however, similar lesions also have been reported on the oral mucosa.3 Melanoacanthomas range in size from 2×2 to 15×15 cm; are clinically pigmented; and present as either a papule, plaque, nodule, or horn.2
Classic histologic findings of MA include papillomatosis, acanthosis, and hyperkeratosis with heavily pigmented dendritic melanocytes diffusely dispersed throughout all layers of the seborrheic keratosis-like epidermis.3 Other features include keratin-filled pseudocysts, Langerhans cells, reactive spindling of keratinocytes, and an inflammatory infiltrate. In our case, the classic histologic findings also were architecturally arranged in oval to round clones within the epidermis (quiz images 1 and 2). A MART-1 (melanoma antigen recognized by T cells) immunostain was obtained that highlighted the numerous but benign-appearing, dendritic melanocytes (quiz image 2 [inset]). A dual MART-1/Ki67 immunostain later was obtained and demonstrated a negligible proliferation index within the dendritic melanocytes. Therefore, the diagnosis of clonal MA was rendered. This formation of epidermal clones also is called the Borst-Jadassohn phenomenon, which rarely occurs in MAs. This subtype is important to recognize because the clonal pattern can more closely mimic malignant neoplasms such as melanoma.
Hidroacanthoma simplex is an intraepidermal variant of eccrine poroma. It is a rare entity that typically occurs in the extremities of women as a hyperkeratotic plaque. These typically clonal epidermal tumors may be heavily pigmented and rarely contain dendritic melanocytes; therefore, they may be confused with MA. However, classic histology will reveal an intraepidermal clonal proliferation of bland, monotonous, cuboidal cells with ample pink cytoplasm, as well as occasional cuticle-lined ducts (Figure 1).4 These ducts will highlight with carcinoembryonic antigen and epithelial membrane antigen immunostaining.
Malignant melanoma typically presents as a growing pigmented lesion and therefore can clinically mimic MA. Histologically, MA could be confused with melanoma due to the increased number of melanocytes plus the appearance of pagetoid spread resulting from the diffuse presence of melanocytes throughout the neoplasm. However, histologic assessment of melanoma should reveal cytologic atypia such as nuclear enlargement, hyperchromasia, molding, pleomorphism, and mitotic activity (Figure 2). Architectural atypia such as poor lateral circumscription of melanocytes, confluence and pagetoid spread of nondendritic atypical junctional melanocytes, production of pigment in deep dermal nests of melanocytes, and lack of maturation and dispersion of dermal melanocytes also should be seen.5 Unlike a melanocytic neoplasm, true melanocytic nests are not seen in MA, and the melanocytes are bland, normal-appearing but heavily pigmented, dendritic melanocytes. Electron microscopy has shown a defect in the transfer of melanin from these highly dendritic melanocytes to the keratinocytes.6


Similar to melanoma, seborrheic keratosis presents as a pigmented growing lesion; therefore, definitive diagnosis often is achieved via skin biopsy. Classic histologic findings include acanthotic or exophytic epidermal growth with a dome-shaped configuration containing multiple cornified hornlike cysts (Figure 3).7 Multiple keratin plugs and variably sized concentric keratin islands are common features. There may be varying degrees of melanin pigment deposition among the proliferating cells, and clonal formation may occur. Melanocyte-specific special stains and immunostains can be used to differentiate MA from seborrheic keratosis by highlighting numerous dendritic melanocytes diffusely spread throughout the epidermis in MA vs a normal distribution of occasional junctional melanocytes in seborrheic keratosis.2,8

Squamous cell carcinoma in situ presents histologically with cytologically atypical keratinocytes encompassing the full thickness of the epidermis and sometimes crushing the basement membrane zone (Figure 4). There is a loss of the granular layer and overlying parakeratosis that often spares the adnexal ostial epithelium.9 Clonal formation can occur as well as increased pigment production. In comparison, bland keratinocytes are seen in MA.
Establishing the diagnosis of MA based on clinical features alone can be difficult. Dermoscopy can prove to be useful and typically will show a sunburst pattern with ridges and fissures.2 However, seborrheic keratoses and melanomas can have similar dermoscopic findings10; therefore, a biopsy often is necessary to establish the diagnosis.

The Diagnosis: Clonal Melanoacanthoma
Melanoacanthoma (MA) is an extremely rare, benign, epidermal tumor histologically characterized by keratinocytes and large, pigmented, dendritic melanocytes. These lesions are loosely related to seborrheic keratoses, and the term was first coined by Mishima and Pinkus1 in 1960. It is estimated that the lesion occurs in only 5 of 500,000 individuals and tends to occur in older, light-skinned individuals.2 The majority are slow growing and are present on the head, neck, or upper extremities; however, similar lesions also have been reported on the oral mucosa.3 Melanoacanthomas range in size from 2×2 to 15×15 cm; are clinically pigmented; and present as either a papule, plaque, nodule, or horn.2
Classic histologic findings of MA include papillomatosis, acanthosis, and hyperkeratosis with heavily pigmented dendritic melanocytes diffusely dispersed throughout all layers of the seborrheic keratosis-like epidermis.3 Other features include keratin-filled pseudocysts, Langerhans cells, reactive spindling of keratinocytes, and an inflammatory infiltrate. In our case, the classic histologic findings also were architecturally arranged in oval to round clones within the epidermis (quiz images 1 and 2). A MART-1 (melanoma antigen recognized by T cells) immunostain was obtained that highlighted the numerous but benign-appearing, dendritic melanocytes (quiz image 2 [inset]). A dual MART-1/Ki67 immunostain later was obtained and demonstrated a negligible proliferation index within the dendritic melanocytes. Therefore, the diagnosis of clonal MA was rendered. This formation of epidermal clones also is called the Borst-Jadassohn phenomenon, which rarely occurs in MAs. This subtype is important to recognize because the clonal pattern can more closely mimic malignant neoplasms such as melanoma.
Hidroacanthoma simplex is an intraepidermal variant of eccrine poroma. It is a rare entity that typically occurs in the extremities of women as a hyperkeratotic plaque. These typically clonal epidermal tumors may be heavily pigmented and rarely contain dendritic melanocytes; therefore, they may be confused with MA. However, classic histology will reveal an intraepidermal clonal proliferation of bland, monotonous, cuboidal cells with ample pink cytoplasm, as well as occasional cuticle-lined ducts (Figure 1).4 These ducts will highlight with carcinoembryonic antigen and epithelial membrane antigen immunostaining.
Malignant melanoma typically presents as a growing pigmented lesion and therefore can clinically mimic MA. Histologically, MA could be confused with melanoma due to the increased number of melanocytes plus the appearance of pagetoid spread resulting from the diffuse presence of melanocytes throughout the neoplasm. However, histologic assessment of melanoma should reveal cytologic atypia such as nuclear enlargement, hyperchromasia, molding, pleomorphism, and mitotic activity (Figure 2). Architectural atypia such as poor lateral circumscription of melanocytes, confluence and pagetoid spread of nondendritic atypical junctional melanocytes, production of pigment in deep dermal nests of melanocytes, and lack of maturation and dispersion of dermal melanocytes also should be seen.5 Unlike a melanocytic neoplasm, true melanocytic nests are not seen in MA, and the melanocytes are bland, normal-appearing but heavily pigmented, dendritic melanocytes. Electron microscopy has shown a defect in the transfer of melanin from these highly dendritic melanocytes to the keratinocytes.6


Similar to melanoma, seborrheic keratosis presents as a pigmented growing lesion; therefore, definitive diagnosis often is achieved via skin biopsy. Classic histologic findings include acanthotic or exophytic epidermal growth with a dome-shaped configuration containing multiple cornified hornlike cysts (Figure 3).7 Multiple keratin plugs and variably sized concentric keratin islands are common features. There may be varying degrees of melanin pigment deposition among the proliferating cells, and clonal formation may occur. Melanocyte-specific special stains and immunostains can be used to differentiate MA from seborrheic keratosis by highlighting numerous dendritic melanocytes diffusely spread throughout the epidermis in MA vs a normal distribution of occasional junctional melanocytes in seborrheic keratosis.2,8

Squamous cell carcinoma in situ presents histologically with cytologically atypical keratinocytes encompassing the full thickness of the epidermis and sometimes crushing the basement membrane zone (Figure 4). There is a loss of the granular layer and overlying parakeratosis that often spares the adnexal ostial epithelium.9 Clonal formation can occur as well as increased pigment production. In comparison, bland keratinocytes are seen in MA.
Establishing the diagnosis of MA based on clinical features alone can be difficult. Dermoscopy can prove to be useful and typically will show a sunburst pattern with ridges and fissures.2 However, seborrheic keratoses and melanomas can have similar dermoscopic findings10; therefore, a biopsy often is necessary to establish the diagnosis.

- Mishima Y, Pinkus H. Benign mixed tumor of melanocytes and malpighian cells: melanoacanthoma: its relationship to Bloch's benign non-nevoid melanoepithelioma. Arch Dermatol. 1960;81:539-550.
- Gutierrez N, Erickson C P, Calame A, et al. Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019;11:E4998.
- Fornatora ML, Reich RF, Haber S, et al. Oral melanoacanthoma: a report of 10 cases, review of literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol. 2003;25:12-15.
- Rahbari H. Hidroacanthoma simplex--a review of 15 cases. Br J Dermatol. 1983;109:219-225.
- Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19:S34-S40.
- Mishra DK, Jakati S, Dave TV, et al. A rare pigmented lesion of the eyelid. Int J Trichol. 2019;11:167-169.
- Greco MJ, Mahabadi N, Gossman W. Seborrheic keratosis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK545285/. Accessed September 18, 2020.
- Kihiczak G, Centurion SA, Schwartz RA, et al. Giant cutaneous melanoacanthoma. Int J Dermatol. 2004;43:936-937.
- Morais P, Schettini A, Junior R. Pigmented squamous cell carcinoma: a case report and importance of differential diagnosis. An Bras Dermatol. 2018;93:96-98.
- Chung E, Marqhoob A, Carrera C, et al. Clinical and dermoscopic features of cutaneous melanoacanthoma. JAMA Dermatol. 2015;151:1129-1130.
- Mishima Y, Pinkus H. Benign mixed tumor of melanocytes and malpighian cells: melanoacanthoma: its relationship to Bloch's benign non-nevoid melanoepithelioma. Arch Dermatol. 1960;81:539-550.
- Gutierrez N, Erickson C P, Calame A, et al. Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019;11:E4998.
- Fornatora ML, Reich RF, Haber S, et al. Oral melanoacanthoma: a report of 10 cases, review of literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol. 2003;25:12-15.
- Rahbari H. Hidroacanthoma simplex--a review of 15 cases. Br J Dermatol. 1983;109:219-225.
- Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19:S34-S40.
- Mishra DK, Jakati S, Dave TV, et al. A rare pigmented lesion of the eyelid. Int J Trichol. 2019;11:167-169.
- Greco MJ, Mahabadi N, Gossman W. Seborrheic keratosis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK545285/. Accessed September 18, 2020.
- Kihiczak G, Centurion SA, Schwartz RA, et al. Giant cutaneous melanoacanthoma. Int J Dermatol. 2004;43:936-937.
- Morais P, Schettini A, Junior R. Pigmented squamous cell carcinoma: a case report and importance of differential diagnosis. An Bras Dermatol. 2018;93:96-98.
- Chung E, Marqhoob A, Carrera C, et al. Clinical and dermoscopic features of cutaneous melanoacanthoma. JAMA Dermatol. 2015;151:1129-1130.
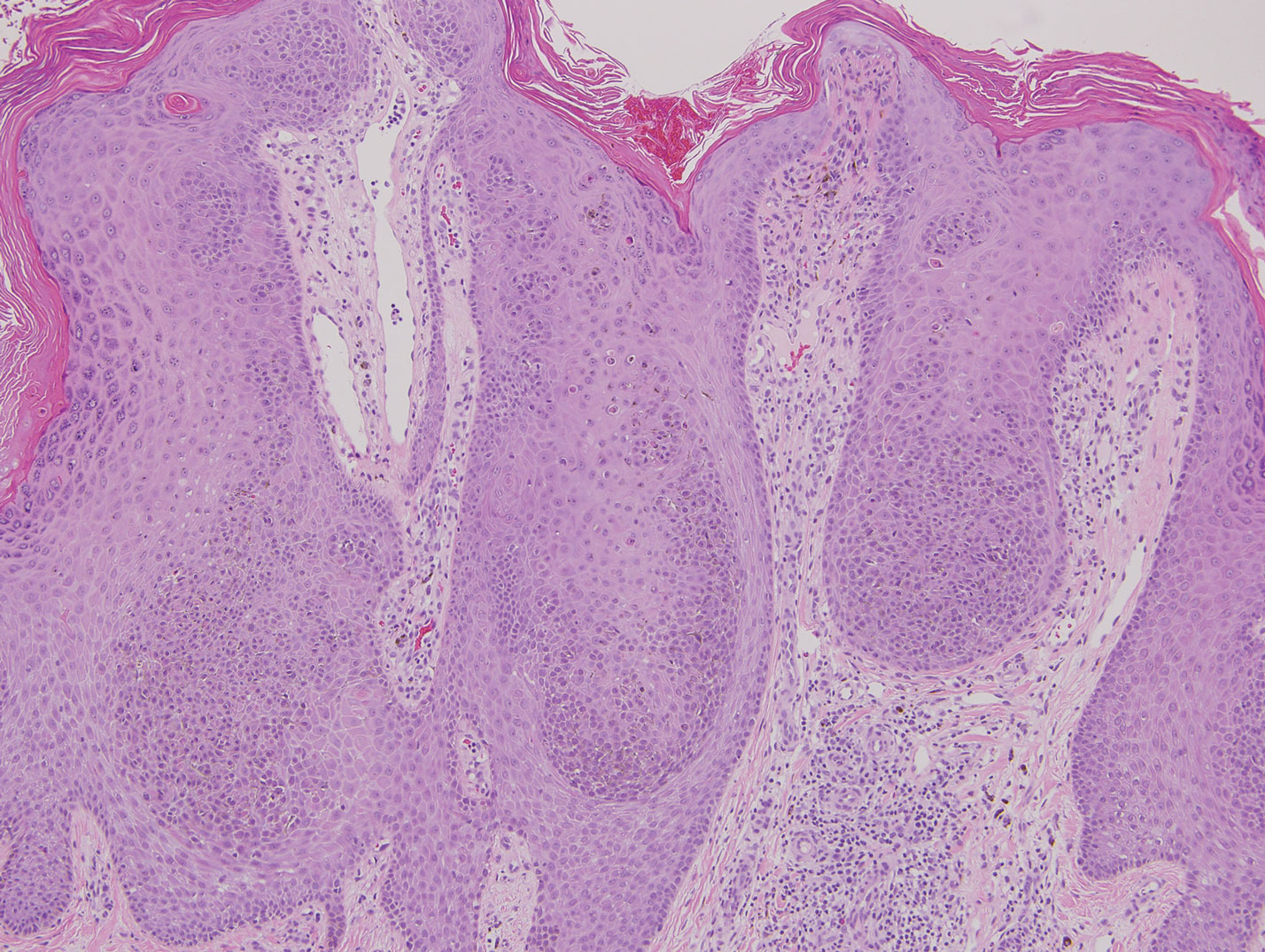
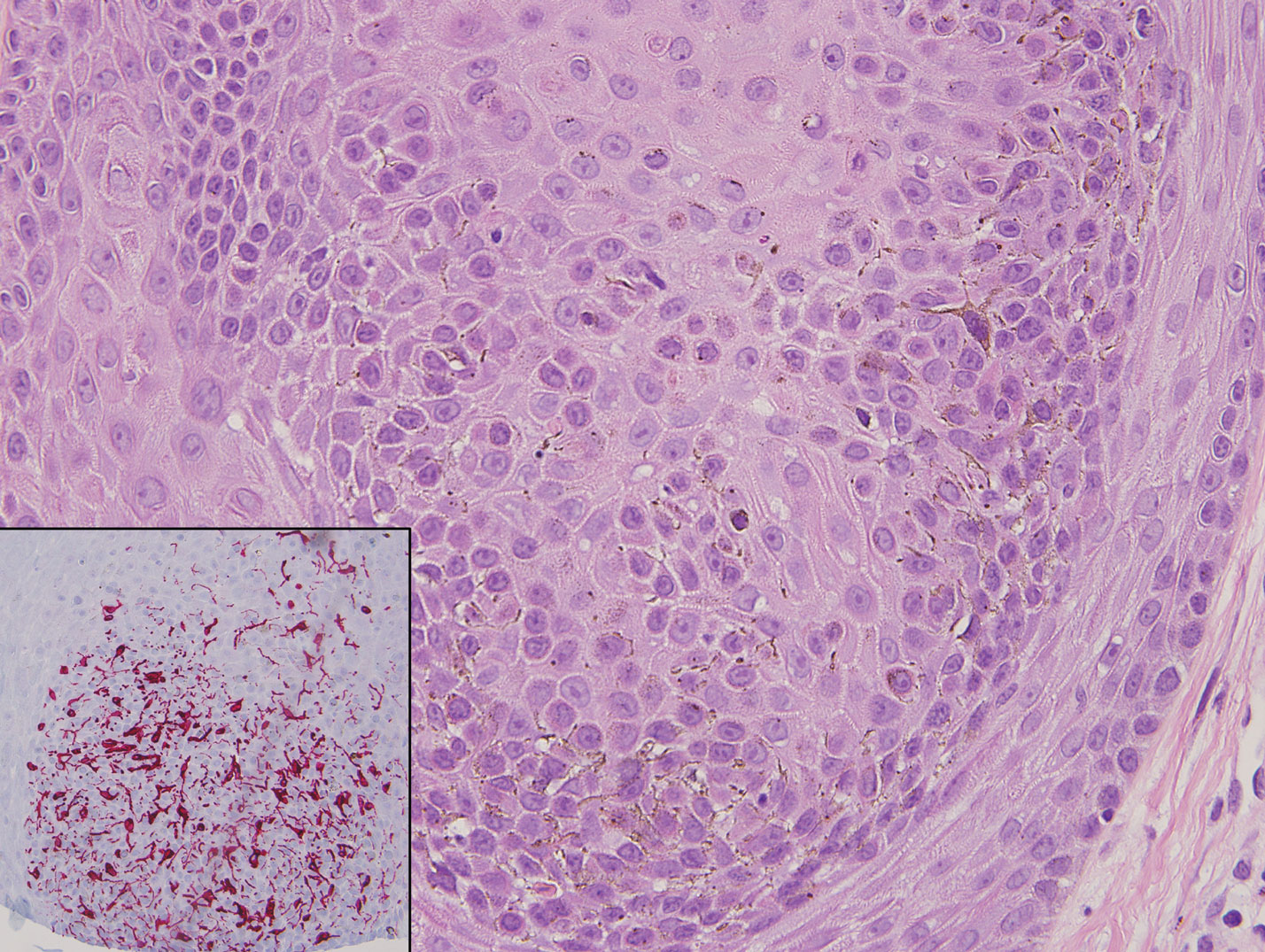
A 49-year-old man with light brown skin and no history of skin cancer presented with a pruritic lesion on the scalp of 3 years’ duration. Physical examination revealed a 7×3-cm, brown, mammillated plaque on the left parietal scalp. A shave biopsy of the scalp lesion was performed.
Influenza Vaccination Recommendations During Use of Select Immunosuppressants for Psoriasis
A 42-year-old woman with psoriasis presents for a checkup at the dermatology clinic. Her psoriasis has been fairly stable on methotrexate with no recent flares. She presents her concern of the coronavirus pandemic continuing into the flu season and mentions she would like to minimize her chances of having a respiratory illness. The influenza vaccine has just become available, and she inquires when she can get the vaccine and whether it will interfere with her treatment. What are your recommendations for the patient?
Psoriasis is an immune-mediated, inflammatory skin condition stemming from hyperproliferation of keratinocytes that classically involves erythematous skin plaques with overlying scale. Treatment options vary widely and include topical modalities, phototherapy, immunosuppressants, and biologic agents. Selection of treatment largely depends on the severity and extent of body surface area involvement; systemic therapy generally is indicated when the affected body surface area is greater than 5% to 10%. In patients on systemic therapy, increased susceptibility to infection is a priority concern for prescribing physicians. In the context of continuing immunosuppressive medications, vaccines that reduce susceptibility to infectious diseases can play an important role in reducing morbidity and mortality for these patients; however, an important consideration is that in patients with chronic conditions and frequent hospital visits, vaccines may be administered by various clinicians who may not be familiar with the management of immunosuppressive treatments. It is pivotal for prescribing dermatologists to provide appropriate vaccination instructions for the patient and any future clinicians to ensure vaccine efficacy in these patients.
The intramuscular influenza vaccine is a killed vaccine that is administered annually and has been shown to be safe for use in both immunocompetent and immunocompromised patients.1,2 Despite its safety, questions remain regarding the efficacy of vaccines while a patient is unable to mount a normal immune response and whether the treatment must be altered to maximize immunogenicity. The common systemic treatment options for psoriasis and any recommendations that can be made regarding administration of the influenza vaccine in that context are outlined in the Table. Given the sparsity of clinical data measuring vaccine immunogenicity in patients with psoriasis, vaccine guidelines are drawn from patients with various conditions who are receiving the same dose of medication as indicated for psoriasis.

Immunosuppressants and biologics commonly are used in dermatology for the management of many conditions, including psoriasis. As flu season approaches in the setting of a global pandemic, it is critical to understand the effects of commonly used psoriasis medications on the influenza vaccine. Through a brief review of the latest data concerning their interactions, dermatologists will be able to provide appropriate recommendations that maximize a patient’s immune response to the vaccine while minimizing adverse effects from holding medication.
- Zbinden D, Manuel O. Influenza vaccination in immunocompromised patients: efficacy and safety. Immunotherapy. 2014;6:131-139.
- Milanovic M, Stojanovich L, Djokovic A, et al. Influenza vaccination in autoimmune rheumatic disease patients. Tohoku J Exp Med. 2013;229:29-34.
- Dengler TJ, Strnad N, Bühring I, et al. Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplantation. 1998;66:1340-1347.
- Willcocks LC, Chaudhry AN, Smith JC, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant. 2007;7:2006-2011.
- Chioato A, Noseda E, Stevens M, et al. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19:1597-1602.
- Richi P, Martín MD, de Ory F, et al. Secukinumab does not impair the immunogenic response to the influenza vaccine in patients. RMD Open. 2019;5:e001018.
- Furer V, Zisman D, Kaufman I, et al. Immunogenicity and safety of vaccination against seasonal influenza vaccine in patients with psoriatic arthritis treated with secukinumab. Vaccine. 2020;38:847-851.
- Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2014;66:1016-1026.
- Park JK, Choi Y, Winthrop KL, et al. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann Rheum Dis. 2019;78:1283-1284.
- Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76:1559-1565.
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898-904.
- Shirai S, Hara M, Sakata Y, et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24:1082-1091.
- Fomin I. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF blockers. Ann Rheum Dis. 2006;65:191-194.
- Bosaeed M, Kumar D. Seasonal influenza vaccine in immunocompromised persons. Hum Vaccin Immunother. 2018;14:1311-1322.
- Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272-279.
A 42-year-old woman with psoriasis presents for a checkup at the dermatology clinic. Her psoriasis has been fairly stable on methotrexate with no recent flares. She presents her concern of the coronavirus pandemic continuing into the flu season and mentions she would like to minimize her chances of having a respiratory illness. The influenza vaccine has just become available, and she inquires when she can get the vaccine and whether it will interfere with her treatment. What are your recommendations for the patient?
Psoriasis is an immune-mediated, inflammatory skin condition stemming from hyperproliferation of keratinocytes that classically involves erythematous skin plaques with overlying scale. Treatment options vary widely and include topical modalities, phototherapy, immunosuppressants, and biologic agents. Selection of treatment largely depends on the severity and extent of body surface area involvement; systemic therapy generally is indicated when the affected body surface area is greater than 5% to 10%. In patients on systemic therapy, increased susceptibility to infection is a priority concern for prescribing physicians. In the context of continuing immunosuppressive medications, vaccines that reduce susceptibility to infectious diseases can play an important role in reducing morbidity and mortality for these patients; however, an important consideration is that in patients with chronic conditions and frequent hospital visits, vaccines may be administered by various clinicians who may not be familiar with the management of immunosuppressive treatments. It is pivotal for prescribing dermatologists to provide appropriate vaccination instructions for the patient and any future clinicians to ensure vaccine efficacy in these patients.
The intramuscular influenza vaccine is a killed vaccine that is administered annually and has been shown to be safe for use in both immunocompetent and immunocompromised patients.1,2 Despite its safety, questions remain regarding the efficacy of vaccines while a patient is unable to mount a normal immune response and whether the treatment must be altered to maximize immunogenicity. The common systemic treatment options for psoriasis and any recommendations that can be made regarding administration of the influenza vaccine in that context are outlined in the Table. Given the sparsity of clinical data measuring vaccine immunogenicity in patients with psoriasis, vaccine guidelines are drawn from patients with various conditions who are receiving the same dose of medication as indicated for psoriasis.

Immunosuppressants and biologics commonly are used in dermatology for the management of many conditions, including psoriasis. As flu season approaches in the setting of a global pandemic, it is critical to understand the effects of commonly used psoriasis medications on the influenza vaccine. Through a brief review of the latest data concerning their interactions, dermatologists will be able to provide appropriate recommendations that maximize a patient’s immune response to the vaccine while minimizing adverse effects from holding medication.
A 42-year-old woman with psoriasis presents for a checkup at the dermatology clinic. Her psoriasis has been fairly stable on methotrexate with no recent flares. She presents her concern of the coronavirus pandemic continuing into the flu season and mentions she would like to minimize her chances of having a respiratory illness. The influenza vaccine has just become available, and she inquires when she can get the vaccine and whether it will interfere with her treatment. What are your recommendations for the patient?
Psoriasis is an immune-mediated, inflammatory skin condition stemming from hyperproliferation of keratinocytes that classically involves erythematous skin plaques with overlying scale. Treatment options vary widely and include topical modalities, phototherapy, immunosuppressants, and biologic agents. Selection of treatment largely depends on the severity and extent of body surface area involvement; systemic therapy generally is indicated when the affected body surface area is greater than 5% to 10%. In patients on systemic therapy, increased susceptibility to infection is a priority concern for prescribing physicians. In the context of continuing immunosuppressive medications, vaccines that reduce susceptibility to infectious diseases can play an important role in reducing morbidity and mortality for these patients; however, an important consideration is that in patients with chronic conditions and frequent hospital visits, vaccines may be administered by various clinicians who may not be familiar with the management of immunosuppressive treatments. It is pivotal for prescribing dermatologists to provide appropriate vaccination instructions for the patient and any future clinicians to ensure vaccine efficacy in these patients.
The intramuscular influenza vaccine is a killed vaccine that is administered annually and has been shown to be safe for use in both immunocompetent and immunocompromised patients.1,2 Despite its safety, questions remain regarding the efficacy of vaccines while a patient is unable to mount a normal immune response and whether the treatment must be altered to maximize immunogenicity. The common systemic treatment options for psoriasis and any recommendations that can be made regarding administration of the influenza vaccine in that context are outlined in the Table. Given the sparsity of clinical data measuring vaccine immunogenicity in patients with psoriasis, vaccine guidelines are drawn from patients with various conditions who are receiving the same dose of medication as indicated for psoriasis.

Immunosuppressants and biologics commonly are used in dermatology for the management of many conditions, including psoriasis. As flu season approaches in the setting of a global pandemic, it is critical to understand the effects of commonly used psoriasis medications on the influenza vaccine. Through a brief review of the latest data concerning their interactions, dermatologists will be able to provide appropriate recommendations that maximize a patient’s immune response to the vaccine while minimizing adverse effects from holding medication.
- Zbinden D, Manuel O. Influenza vaccination in immunocompromised patients: efficacy and safety. Immunotherapy. 2014;6:131-139.
- Milanovic M, Stojanovich L, Djokovic A, et al. Influenza vaccination in autoimmune rheumatic disease patients. Tohoku J Exp Med. 2013;229:29-34.
- Dengler TJ, Strnad N, Bühring I, et al. Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplantation. 1998;66:1340-1347.
- Willcocks LC, Chaudhry AN, Smith JC, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant. 2007;7:2006-2011.
- Chioato A, Noseda E, Stevens M, et al. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19:1597-1602.
- Richi P, Martín MD, de Ory F, et al. Secukinumab does not impair the immunogenic response to the influenza vaccine in patients. RMD Open. 2019;5:e001018.
- Furer V, Zisman D, Kaufman I, et al. Immunogenicity and safety of vaccination against seasonal influenza vaccine in patients with psoriatic arthritis treated with secukinumab. Vaccine. 2020;38:847-851.
- Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2014;66:1016-1026.
- Park JK, Choi Y, Winthrop KL, et al. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann Rheum Dis. 2019;78:1283-1284.
- Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76:1559-1565.
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898-904.
- Shirai S, Hara M, Sakata Y, et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24:1082-1091.
- Fomin I. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF blockers. Ann Rheum Dis. 2006;65:191-194.
- Bosaeed M, Kumar D. Seasonal influenza vaccine in immunocompromised persons. Hum Vaccin Immunother. 2018;14:1311-1322.
- Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272-279.
- Zbinden D, Manuel O. Influenza vaccination in immunocompromised patients: efficacy and safety. Immunotherapy. 2014;6:131-139.
- Milanovic M, Stojanovich L, Djokovic A, et al. Influenza vaccination in autoimmune rheumatic disease patients. Tohoku J Exp Med. 2013;229:29-34.
- Dengler TJ, Strnad N, Bühring I, et al. Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplantation. 1998;66:1340-1347.
- Willcocks LC, Chaudhry AN, Smith JC, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant. 2007;7:2006-2011.
- Chioato A, Noseda E, Stevens M, et al. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19:1597-1602.
- Richi P, Martín MD, de Ory F, et al. Secukinumab does not impair the immunogenic response to the influenza vaccine in patients. RMD Open. 2019;5:e001018.
- Furer V, Zisman D, Kaufman I, et al. Immunogenicity and safety of vaccination against seasonal influenza vaccine in patients with psoriatic arthritis treated with secukinumab. Vaccine. 2020;38:847-851.
- Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2014;66:1016-1026.
- Park JK, Choi Y, Winthrop KL, et al. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann Rheum Dis. 2019;78:1283-1284.
- Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76:1559-1565.
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898-904.
- Shirai S, Hara M, Sakata Y, et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24:1082-1091.
- Fomin I. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF blockers. Ann Rheum Dis. 2006;65:191-194.
- Bosaeed M, Kumar D. Seasonal influenza vaccine in immunocompromised persons. Hum Vaccin Immunother. 2018;14:1311-1322.
- Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272-279.
Practice Points
- Patients receiving methotrexate appear to benefit from suspending treatment for 2 weeks following influenza vaccination, as it maximizes the seroprotective response.
- Patients receiving tumor necrosis factor α inhibitors and low-dose IL-17 inhibitors have an unaltered humoral response to vaccination and attain protection equal to that of the general population.
- Patients treated with cyclosporine should be closely monitored for influenza symptoms even after vaccination, as approximately half of patients do not achieve a seroprotective response.
- Consider the increased risk for psoriatic flare during treatment suspension and the possibility of failed seroprotection, warranting close monitoring and clinical judgement tailored to each individual.
Tobacco-free homes yield more tobacco-free youth
Tsu-Suan Wu and Benjamin W. Chaffee, DDS, PhD, of the University of California, San Francisco, advised in their study in Pediatrics.
Previous studies have shown that children who grow up in a nonsmoking household are less likely to begin smoking themselves, and active parental engagement in interventions shows promise overall in protecting children from drug, alcohol, and illicit drug use. Households with rigid rules against smoking offer a deterrent for children who might otherwise be tempted, the researchers noted.
Other studies have shown that while youth smoking is on the decline, use of noncigarette products is increasing sharply. The inconspicuous appearance and attractive scents these delivery devices afford make it easier to conceal them from parents.
In the current study, using data from the Population Assessment of Tobacco and Health (PATH) Study involving 23,170 parents and youth ages 9 and up, Mr. Wu and Dr. Chaffee sought to assess to what extent parents had knowledge or suspicions of tobacco use and also to evaluate the association between youth initiating tobacco use and the establishment of household rules and engaging in regular conversation about tobacco.
Study results revealed in three of the four groups evaluated that youth were most likely to engage in using several different types of tobacco (polytobacco) products; in the fourth group, e-cigarette use was most common. Among polytobacco users, fully 77%-80% reported cigarette usage.
Parental knowledge and actions
Overall, Mr. Wu and Dr. Chaffee “identified substantial lapses in parents’ awareness of their children’s tobacco use.” Parents were most likely to register awareness when their children smoked cigarettes; half as many parents were aware or suspected use when noncigarette products were used.
Parents who had heightened awareness about possible tobacco usage tended to be the child’s mother, had completed lower levels of education, parented children who were older, male and non-Hispanic, and lived with a tobacco user.
Noteworthy was the growing percentage of parents who report awareness or suspicions of cigarette usage – approximately 70% – compared with previous study findings – about 40%. The researchers speculated that this increase could be directly tied to growing social concern regarding youth smoking. Unfortunately, parents will continue to be challenged to keep up with constantly changing e-cigarette designs in maintaining their awareness, Mr. Wu and Dr. Chaffee noted.
Establishing strict household rules was found to be more effective than just talking with youth about usage, which half of the youth reported their parents did. At all time points, the risk of tobacco initiation was 20%-26% lower for children who lived in a house with strict household rules forbidding any tobacco use by anyone. The researchers observed that success with the household rules method was best achieved with children at younger ages.
The study did not measure the quality or frequency of antitobacco conversations but it should not be concluded definitively that all parental communication is unhelpful, the researchers cautioned.
To their knowledge, this study is the first to analyze the effects of household antitobacco strategies on discouraging initiation the use of tobacco and other smoking products as well as assessing parental awareness surrounding tobacco usage among youth.
What to tell parents
In a separate interview, Kelly Curran, MD, MA, assistant professor of pediatrics at the University of Oklahoma, Oklahoma City, commented on the explosive growth of e-cigarette use in the last 7 years.
What makes e-cigs so difficult to detect is that they “can resemble common objects such as flash drives or pens, and as a result, can often be hidden or overlooked by parents,” noted Dr. Curran.
The most important message for parents from this study is that they have the potential to have a large impact in the prevention of tobacco initiation, she said. “This effort requires parents to ‘walk the walk’ instead of just ‘talking the talk.”
As the study revealed, simply talking to teens about not using tobacco products doesn’t decrease use, but “creating strict household rules around no tobacco use for all visitors and inhabitants has a significant impact in decreasing youth tobacco initiation – by nearly 25%,” she added. “When counseling patients and families about tobacco prevention, clinicians should encourage them to create a tobacco-free home.”
The study was funded by a National Institutes of Health grant and the Delta Dental Community Care Foundation. The authors have no relevant financial disclosures. Dr. Curran, who is a member of the Pediatric News editorial advisory board, said she had no relevant financial disclosures.
SOURCE: Wu T-S and Chaffee BW. Pediatrics 2020 October. doi: 10.1542/peds.2019-4034.
Tsu-Suan Wu and Benjamin W. Chaffee, DDS, PhD, of the University of California, San Francisco, advised in their study in Pediatrics.
Previous studies have shown that children who grow up in a nonsmoking household are less likely to begin smoking themselves, and active parental engagement in interventions shows promise overall in protecting children from drug, alcohol, and illicit drug use. Households with rigid rules against smoking offer a deterrent for children who might otherwise be tempted, the researchers noted.
Other studies have shown that while youth smoking is on the decline, use of noncigarette products is increasing sharply. The inconspicuous appearance and attractive scents these delivery devices afford make it easier to conceal them from parents.
In the current study, using data from the Population Assessment of Tobacco and Health (PATH) Study involving 23,170 parents and youth ages 9 and up, Mr. Wu and Dr. Chaffee sought to assess to what extent parents had knowledge or suspicions of tobacco use and also to evaluate the association between youth initiating tobacco use and the establishment of household rules and engaging in regular conversation about tobacco.
Study results revealed in three of the four groups evaluated that youth were most likely to engage in using several different types of tobacco (polytobacco) products; in the fourth group, e-cigarette use was most common. Among polytobacco users, fully 77%-80% reported cigarette usage.
Parental knowledge and actions
Overall, Mr. Wu and Dr. Chaffee “identified substantial lapses in parents’ awareness of their children’s tobacco use.” Parents were most likely to register awareness when their children smoked cigarettes; half as many parents were aware or suspected use when noncigarette products were used.
Parents who had heightened awareness about possible tobacco usage tended to be the child’s mother, had completed lower levels of education, parented children who were older, male and non-Hispanic, and lived with a tobacco user.
Noteworthy was the growing percentage of parents who report awareness or suspicions of cigarette usage – approximately 70% – compared with previous study findings – about 40%. The researchers speculated that this increase could be directly tied to growing social concern regarding youth smoking. Unfortunately, parents will continue to be challenged to keep up with constantly changing e-cigarette designs in maintaining their awareness, Mr. Wu and Dr. Chaffee noted.
Establishing strict household rules was found to be more effective than just talking with youth about usage, which half of the youth reported their parents did. At all time points, the risk of tobacco initiation was 20%-26% lower for children who lived in a house with strict household rules forbidding any tobacco use by anyone. The researchers observed that success with the household rules method was best achieved with children at younger ages.
The study did not measure the quality or frequency of antitobacco conversations but it should not be concluded definitively that all parental communication is unhelpful, the researchers cautioned.
To their knowledge, this study is the first to analyze the effects of household antitobacco strategies on discouraging initiation the use of tobacco and other smoking products as well as assessing parental awareness surrounding tobacco usage among youth.
What to tell parents
In a separate interview, Kelly Curran, MD, MA, assistant professor of pediatrics at the University of Oklahoma, Oklahoma City, commented on the explosive growth of e-cigarette use in the last 7 years.
What makes e-cigs so difficult to detect is that they “can resemble common objects such as flash drives or pens, and as a result, can often be hidden or overlooked by parents,” noted Dr. Curran.
The most important message for parents from this study is that they have the potential to have a large impact in the prevention of tobacco initiation, she said. “This effort requires parents to ‘walk the walk’ instead of just ‘talking the talk.”
As the study revealed, simply talking to teens about not using tobacco products doesn’t decrease use, but “creating strict household rules around no tobacco use for all visitors and inhabitants has a significant impact in decreasing youth tobacco initiation – by nearly 25%,” she added. “When counseling patients and families about tobacco prevention, clinicians should encourage them to create a tobacco-free home.”
The study was funded by a National Institutes of Health grant and the Delta Dental Community Care Foundation. The authors have no relevant financial disclosures. Dr. Curran, who is a member of the Pediatric News editorial advisory board, said she had no relevant financial disclosures.
SOURCE: Wu T-S and Chaffee BW. Pediatrics 2020 October. doi: 10.1542/peds.2019-4034.
Tsu-Suan Wu and Benjamin W. Chaffee, DDS, PhD, of the University of California, San Francisco, advised in their study in Pediatrics.
Previous studies have shown that children who grow up in a nonsmoking household are less likely to begin smoking themselves, and active parental engagement in interventions shows promise overall in protecting children from drug, alcohol, and illicit drug use. Households with rigid rules against smoking offer a deterrent for children who might otherwise be tempted, the researchers noted.
Other studies have shown that while youth smoking is on the decline, use of noncigarette products is increasing sharply. The inconspicuous appearance and attractive scents these delivery devices afford make it easier to conceal them from parents.
In the current study, using data from the Population Assessment of Tobacco and Health (PATH) Study involving 23,170 parents and youth ages 9 and up, Mr. Wu and Dr. Chaffee sought to assess to what extent parents had knowledge or suspicions of tobacco use and also to evaluate the association between youth initiating tobacco use and the establishment of household rules and engaging in regular conversation about tobacco.
Study results revealed in three of the four groups evaluated that youth were most likely to engage in using several different types of tobacco (polytobacco) products; in the fourth group, e-cigarette use was most common. Among polytobacco users, fully 77%-80% reported cigarette usage.
Parental knowledge and actions
Overall, Mr. Wu and Dr. Chaffee “identified substantial lapses in parents’ awareness of their children’s tobacco use.” Parents were most likely to register awareness when their children smoked cigarettes; half as many parents were aware or suspected use when noncigarette products were used.
Parents who had heightened awareness about possible tobacco usage tended to be the child’s mother, had completed lower levels of education, parented children who were older, male and non-Hispanic, and lived with a tobacco user.
Noteworthy was the growing percentage of parents who report awareness or suspicions of cigarette usage – approximately 70% – compared with previous study findings – about 40%. The researchers speculated that this increase could be directly tied to growing social concern regarding youth smoking. Unfortunately, parents will continue to be challenged to keep up with constantly changing e-cigarette designs in maintaining their awareness, Mr. Wu and Dr. Chaffee noted.
Establishing strict household rules was found to be more effective than just talking with youth about usage, which half of the youth reported their parents did. At all time points, the risk of tobacco initiation was 20%-26% lower for children who lived in a house with strict household rules forbidding any tobacco use by anyone. The researchers observed that success with the household rules method was best achieved with children at younger ages.
The study did not measure the quality or frequency of antitobacco conversations but it should not be concluded definitively that all parental communication is unhelpful, the researchers cautioned.
To their knowledge, this study is the first to analyze the effects of household antitobacco strategies on discouraging initiation the use of tobacco and other smoking products as well as assessing parental awareness surrounding tobacco usage among youth.
What to tell parents
In a separate interview, Kelly Curran, MD, MA, assistant professor of pediatrics at the University of Oklahoma, Oklahoma City, commented on the explosive growth of e-cigarette use in the last 7 years.
What makes e-cigs so difficult to detect is that they “can resemble common objects such as flash drives or pens, and as a result, can often be hidden or overlooked by parents,” noted Dr. Curran.
The most important message for parents from this study is that they have the potential to have a large impact in the prevention of tobacco initiation, she said. “This effort requires parents to ‘walk the walk’ instead of just ‘talking the talk.”
As the study revealed, simply talking to teens about not using tobacco products doesn’t decrease use, but “creating strict household rules around no tobacco use for all visitors and inhabitants has a significant impact in decreasing youth tobacco initiation – by nearly 25%,” she added. “When counseling patients and families about tobacco prevention, clinicians should encourage them to create a tobacco-free home.”
The study was funded by a National Institutes of Health grant and the Delta Dental Community Care Foundation. The authors have no relevant financial disclosures. Dr. Curran, who is a member of the Pediatric News editorial advisory board, said she had no relevant financial disclosures.
SOURCE: Wu T-S and Chaffee BW. Pediatrics 2020 October. doi: 10.1542/peds.2019-4034.
FROM PEDIATRICS