User login
Cutaneous Odontogenic Sinus: An Inflammatory Mimicker of Squamous Cell Carcinoma and Epidermal Cysts
Clinical Challenge
An
Practice Gap
It is estimated that half of patients with an extraoral fistula are treated with multiple dermatologic surgical operations, radiotherapy, antibiotic therapy, and chemotherapy before the correct diagnosis is made.1 Thus, proper identification of these lesions is crucial for prognosis and treatment. The most common locations for OCSTs are the mandibular, submandibular, and cervical skin.1,2 Given these locations, patients with OCSTs commonly present to the dermatology office for evaluation. Education regarding the clinical presentation, histopathology, and proper evaluation and further referral for treatment is essential for dermatologists.
Tools and Technique for Diagnosis
We present 2 patients with OCSTs who were referred for cutaneous surgery for an SCC and epidermal cyst, but the proper diagnosis was rendered after an index of suspicion and clinicopathologic correlation led to additional testing and eventual referral for imaging.
Patient 1
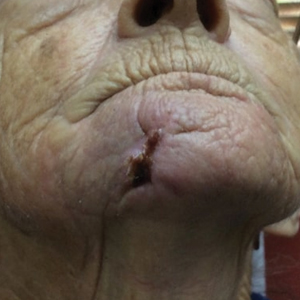
A 68-year-old woman presented for Mohs micrographic surgery (MMS) of a biopsy-proven SCC on the chin. The tumor cleared after 2 MMS stages (Figure 1A). Due to notable inflammation in each stage, the slides were sent to a pathologist who confirmed clear margins. Within 2 weeks of MMS, the wound began to dehisce (Figure 1B). The patient presented 4 months later with a crusted ulcerated nodule at the MMS site (Figure 1C). A biopsy showed likely recurrence of SCC. Upon presentation to the Mohs surgeon, the nodule felt fixed to the underlying jaw, and the patient was noted to have poor dentition. The patient was sent for computed tomography (CT), which showed focal thinning of the mandible, likely postsurgical, and clear maxillary sinuses. Due to the clinical appearance and anatomic location of the lesion, a request was made for a second read of the CT, specifically looking for an OCST at the prior surgical site. With this information, the radiologist noted an OCST extending from the mandible to the lesion, reported as a periapical lucency (representing a periapical abscess) at a mandibular tooth with a dental sinus draining into the soft tissues. The patient was started on antibiotics and referred to an oral surgeon for OCST excision.

Patient 2
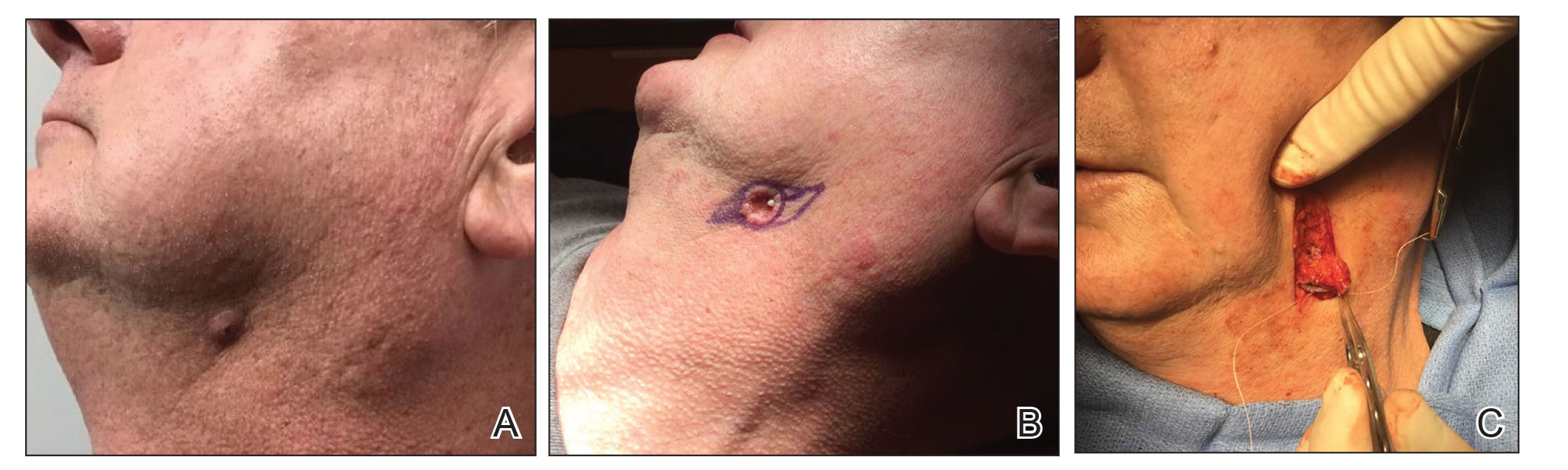
A 62-year-old man presented with an inflamed subcutaneous nodule on the left anterior neck. A biopsy showed a ruptured cyst, and the patient was referred for excision. Clinical examination revealed a subcutaneous nodule fixed to the lower portion of the mandible (Figure 2A) that exhibited a rubbery retraction when pulled (Figure 2B). After a discussion about the atypical feel and appearance of this cyst, the patient preferred to undergo excision. During excision, the lesion felt deep and fixed with retraction (Figure 2C). With intraoperative re-evaluation of the clinical scenario and location, the patient was sent for CT. The initial read noted clear maxillary and ethmoid sinuses, with no mention of an OCST. After discussing the clinical history and suspicion specifically for an OCST with the radiologist, the re-read showed notable inflammation and decay of the tooth adjacent to the area of interest. An OCST was diagnosed, and the patient was sent to an oral surgeon for excision after antibiotics were prescribed.
Practice Implications
Odontogenic cutaneous sinus tracts commonly are misdiagnosed due to variations in clinical presentations resembling more common cutaneous diagnoses, nonspecific histopathologic findings, and lack of dental symptoms or concerns about dentition. Clinically, an OCST presents as a fixed, red, crusty, nontender nodule with intermittent draining. With palpation of the involved area, the clinician may feel a cord of tissue connecting the skin lesion intraorally.2,4 A clinician should have a high index of suspicion for an OCST when evaluating fixed lesions of the lower face, jawline, and neck due to the possibility of a dental origin,1 which is important because an OCST can have similar clinical findings to lesions such as congenital fistulas, pustules, cysts, osteomyelitis, foreign-body granulomas, pyogenic granulomas, syphilis, metastatic carcinomas, basal cell carcinomas, and SCCs.2,4 A PubMed search of articles indexed for MEDLINE using the terms Mohs, MMS, chemosurgery, odontogenic sinus, odontogenic cutaneous sinus tract, and dental sinus yielded only 2 OCSTs that were referred for MMS in the last 30 years, both of which were in the nasolabial fold/medial malar cheek.2,4 Histopathologic findings of an OCST are nonspecific; a mixed or granulomatous inflammatory infiltrate, granulation tissue, and scarring can be seen.1 Pseudocarcinomatous/pseudoepitheliomatous hyperplasia of the epidermis can be seen and cause histologic misinterpretation for an SCC.2 Given that these findings are nonspecific without a clinical context, even with a histopathologic diagnosis of SCC or cyst, a clinical suspicion for an OCST should lead to an intraoral examination. Imaging can be ordered to look for an OCST in the area of interest. Although panoramic or periapical radiography with or without dental probes/radiopaque markers commonly have been used, more recent literature has suggested that CT may be superior to radiographs for making an OCST diagnosis.1,3 If imaging is not consistent with the clinically suspected OCST, we recommend directly contacting the radiologist to explain the clinical history and even refresh his/her suspicion for this diagnosis.
If a diagnosis of an OCST is made, oral antibiotics can be prescribed, though the use of antibiotics has been controversial. For severe odontogenic infections, typically beta-lactam antibiotics, cephalosporins, metronidazole, clindamycin, moxifloxacin, or erythromycin can be given for 7 days or until 3 days after symptoms have resolved.5 Although antibiotics can bring temporary resolution, it is imperative to treat the source of infection to prevent recurrence. It is crucial for these patients to be referred to an oral surgeon for evaluation and treatment of OCST by either a root canal or tooth extraction.
Final Thoughts
We present this pearl on the diagnosis and management of an OCST, also known as a dental sinus, to better assist clinicians in making this diagnosis. With an index of suspicion as well as intraoral and radiologic evaluations, a proper diagnosis may be rendered, potentially avoiding unnecessary cutaneous surgery. In addition, we highlight the importance of communication between the clinician and the radiologist to directly look for OCST in the area of concern and consider a re-read of the images when clinical suspicion does not correlate with the radiology report.
- Bai J, Ji AP, Huang MW. Submental cutaneous sinus tract of mandibular second molar origin. Int Endod J. 2014;47:1185-1191.
- Plast Reconstr Surg.
- Gregoire C. How are odontogenic infections best managed? J Can Dent Assoc. 2010;76:a37.
- Bodner L, Bar-Ziv J. Cutaneous sinus tract of dental origin—imaging with a dental CT software programme. Br J Oral Maxillofac Surg. 1998;36:311-313.
- Peermohamed S, Barber D, Kurwa H. Diagnostic challenges of cutaneous draining sinus tracts of odontogenic origin: a case report. Dermatol Surg. 2011;37:1525-1527.
Clinical Challenge
An
Practice Gap
It is estimated that half of patients with an extraoral fistula are treated with multiple dermatologic surgical operations, radiotherapy, antibiotic therapy, and chemotherapy before the correct diagnosis is made.1 Thus, proper identification of these lesions is crucial for prognosis and treatment. The most common locations for OCSTs are the mandibular, submandibular, and cervical skin.1,2 Given these locations, patients with OCSTs commonly present to the dermatology office for evaluation. Education regarding the clinical presentation, histopathology, and proper evaluation and further referral for treatment is essential for dermatologists.
Tools and Technique for Diagnosis
We present 2 patients with OCSTs who were referred for cutaneous surgery for an SCC and epidermal cyst, but the proper diagnosis was rendered after an index of suspicion and clinicopathologic correlation led to additional testing and eventual referral for imaging.
Patient 1
A 68-year-old woman presented for Mohs micrographic surgery (MMS) of a biopsy-proven SCC on the chin. The tumor cleared after 2 MMS stages (Figure 1A). Due to notable inflammation in each stage, the slides were sent to a pathologist who confirmed clear margins. Within 2 weeks of MMS, the wound began to dehisce (Figure 1B). The patient presented 4 months later with a crusted ulcerated nodule at the MMS site (Figure 1C). A biopsy showed likely recurrence of SCC. Upon presentation to the Mohs surgeon, the nodule felt fixed to the underlying jaw, and the patient was noted to have poor dentition. The patient was sent for computed tomography (CT), which showed focal thinning of the mandible, likely postsurgical, and clear maxillary sinuses. Due to the clinical appearance and anatomic location of the lesion, a request was made for a second read of the CT, specifically looking for an OCST at the prior surgical site. With this information, the radiologist noted an OCST extending from the mandible to the lesion, reported as a periapical lucency (representing a periapical abscess) at a mandibular tooth with a dental sinus draining into the soft tissues. The patient was started on antibiotics and referred to an oral surgeon for OCST excision.

Patient 2
A 62-year-old man presented with an inflamed subcutaneous nodule on the left anterior neck. A biopsy showed a ruptured cyst, and the patient was referred for excision. Clinical examination revealed a subcutaneous nodule fixed to the lower portion of the mandible (Figure 2A) that exhibited a rubbery retraction when pulled (Figure 2B). After a discussion about the atypical feel and appearance of this cyst, the patient preferred to undergo excision. During excision, the lesion felt deep and fixed with retraction (Figure 2C). With intraoperative re-evaluation of the clinical scenario and location, the patient was sent for CT. The initial read noted clear maxillary and ethmoid sinuses, with no mention of an OCST. After discussing the clinical history and suspicion specifically for an OCST with the radiologist, the re-read showed notable inflammation and decay of the tooth adjacent to the area of interest. An OCST was diagnosed, and the patient was sent to an oral surgeon for excision after antibiotics were prescribed.

Practice Implications
Odontogenic cutaneous sinus tracts commonly are misdiagnosed due to variations in clinical presentations resembling more common cutaneous diagnoses, nonspecific histopathologic findings, and lack of dental symptoms or concerns about dentition. Clinically, an OCST presents as a fixed, red, crusty, nontender nodule with intermittent draining. With palpation of the involved area, the clinician may feel a cord of tissue connecting the skin lesion intraorally.2,4 A clinician should have a high index of suspicion for an OCST when evaluating fixed lesions of the lower face, jawline, and neck due to the possibility of a dental origin,1 which is important because an OCST can have similar clinical findings to lesions such as congenital fistulas, pustules, cysts, osteomyelitis, foreign-body granulomas, pyogenic granulomas, syphilis, metastatic carcinomas, basal cell carcinomas, and SCCs.2,4 A PubMed search of articles indexed for MEDLINE using the terms Mohs, MMS, chemosurgery, odontogenic sinus, odontogenic cutaneous sinus tract, and dental sinus yielded only 2 OCSTs that were referred for MMS in the last 30 years, both of which were in the nasolabial fold/medial malar cheek.2,4 Histopathologic findings of an OCST are nonspecific; a mixed or granulomatous inflammatory infiltrate, granulation tissue, and scarring can be seen.1 Pseudocarcinomatous/pseudoepitheliomatous hyperplasia of the epidermis can be seen and cause histologic misinterpretation for an SCC.2 Given that these findings are nonspecific without a clinical context, even with a histopathologic diagnosis of SCC or cyst, a clinical suspicion for an OCST should lead to an intraoral examination. Imaging can be ordered to look for an OCST in the area of interest. Although panoramic or periapical radiography with or without dental probes/radiopaque markers commonly have been used, more recent literature has suggested that CT may be superior to radiographs for making an OCST diagnosis.1,3 If imaging is not consistent with the clinically suspected OCST, we recommend directly contacting the radiologist to explain the clinical history and even refresh his/her suspicion for this diagnosis.
If a diagnosis of an OCST is made, oral antibiotics can be prescribed, though the use of antibiotics has been controversial. For severe odontogenic infections, typically beta-lactam antibiotics, cephalosporins, metronidazole, clindamycin, moxifloxacin, or erythromycin can be given for 7 days or until 3 days after symptoms have resolved.5 Although antibiotics can bring temporary resolution, it is imperative to treat the source of infection to prevent recurrence. It is crucial for these patients to be referred to an oral surgeon for evaluation and treatment of OCST by either a root canal or tooth extraction.
Final Thoughts
We present this pearl on the diagnosis and management of an OCST, also known as a dental sinus, to better assist clinicians in making this diagnosis. With an index of suspicion as well as intraoral and radiologic evaluations, a proper diagnosis may be rendered, potentially avoiding unnecessary cutaneous surgery. In addition, we highlight the importance of communication between the clinician and the radiologist to directly look for OCST in the area of concern and consider a re-read of the images when clinical suspicion does not correlate with the radiology report.
Clinical Challenge
An
Practice Gap
It is estimated that half of patients with an extraoral fistula are treated with multiple dermatologic surgical operations, radiotherapy, antibiotic therapy, and chemotherapy before the correct diagnosis is made.1 Thus, proper identification of these lesions is crucial for prognosis and treatment. The most common locations for OCSTs are the mandibular, submandibular, and cervical skin.1,2 Given these locations, patients with OCSTs commonly present to the dermatology office for evaluation. Education regarding the clinical presentation, histopathology, and proper evaluation and further referral for treatment is essential for dermatologists.
Tools and Technique for Diagnosis
We present 2 patients with OCSTs who were referred for cutaneous surgery for an SCC and epidermal cyst, but the proper diagnosis was rendered after an index of suspicion and clinicopathologic correlation led to additional testing and eventual referral for imaging.
Patient 1
A 68-year-old woman presented for Mohs micrographic surgery (MMS) of a biopsy-proven SCC on the chin. The tumor cleared after 2 MMS stages (Figure 1A). Due to notable inflammation in each stage, the slides were sent to a pathologist who confirmed clear margins. Within 2 weeks of MMS, the wound began to dehisce (Figure 1B). The patient presented 4 months later with a crusted ulcerated nodule at the MMS site (Figure 1C). A biopsy showed likely recurrence of SCC. Upon presentation to the Mohs surgeon, the nodule felt fixed to the underlying jaw, and the patient was noted to have poor dentition. The patient was sent for computed tomography (CT), which showed focal thinning of the mandible, likely postsurgical, and clear maxillary sinuses. Due to the clinical appearance and anatomic location of the lesion, a request was made for a second read of the CT, specifically looking for an OCST at the prior surgical site. With this information, the radiologist noted an OCST extending from the mandible to the lesion, reported as a periapical lucency (representing a periapical abscess) at a mandibular tooth with a dental sinus draining into the soft tissues. The patient was started on antibiotics and referred to an oral surgeon for OCST excision.

Patient 2
A 62-year-old man presented with an inflamed subcutaneous nodule on the left anterior neck. A biopsy showed a ruptured cyst, and the patient was referred for excision. Clinical examination revealed a subcutaneous nodule fixed to the lower portion of the mandible (Figure 2A) that exhibited a rubbery retraction when pulled (Figure 2B). After a discussion about the atypical feel and appearance of this cyst, the patient preferred to undergo excision. During excision, the lesion felt deep and fixed with retraction (Figure 2C). With intraoperative re-evaluation of the clinical scenario and location, the patient was sent for CT. The initial read noted clear maxillary and ethmoid sinuses, with no mention of an OCST. After discussing the clinical history and suspicion specifically for an OCST with the radiologist, the re-read showed notable inflammation and decay of the tooth adjacent to the area of interest. An OCST was diagnosed, and the patient was sent to an oral surgeon for excision after antibiotics were prescribed.

Practice Implications
Odontogenic cutaneous sinus tracts commonly are misdiagnosed due to variations in clinical presentations resembling more common cutaneous diagnoses, nonspecific histopathologic findings, and lack of dental symptoms or concerns about dentition. Clinically, an OCST presents as a fixed, red, crusty, nontender nodule with intermittent draining. With palpation of the involved area, the clinician may feel a cord of tissue connecting the skin lesion intraorally.2,4 A clinician should have a high index of suspicion for an OCST when evaluating fixed lesions of the lower face, jawline, and neck due to the possibility of a dental origin,1 which is important because an OCST can have similar clinical findings to lesions such as congenital fistulas, pustules, cysts, osteomyelitis, foreign-body granulomas, pyogenic granulomas, syphilis, metastatic carcinomas, basal cell carcinomas, and SCCs.2,4 A PubMed search of articles indexed for MEDLINE using the terms Mohs, MMS, chemosurgery, odontogenic sinus, odontogenic cutaneous sinus tract, and dental sinus yielded only 2 OCSTs that were referred for MMS in the last 30 years, both of which were in the nasolabial fold/medial malar cheek.2,4 Histopathologic findings of an OCST are nonspecific; a mixed or granulomatous inflammatory infiltrate, granulation tissue, and scarring can be seen.1 Pseudocarcinomatous/pseudoepitheliomatous hyperplasia of the epidermis can be seen and cause histologic misinterpretation for an SCC.2 Given that these findings are nonspecific without a clinical context, even with a histopathologic diagnosis of SCC or cyst, a clinical suspicion for an OCST should lead to an intraoral examination. Imaging can be ordered to look for an OCST in the area of interest. Although panoramic or periapical radiography with or without dental probes/radiopaque markers commonly have been used, more recent literature has suggested that CT may be superior to radiographs for making an OCST diagnosis.1,3 If imaging is not consistent with the clinically suspected OCST, we recommend directly contacting the radiologist to explain the clinical history and even refresh his/her suspicion for this diagnosis.
If a diagnosis of an OCST is made, oral antibiotics can be prescribed, though the use of antibiotics has been controversial. For severe odontogenic infections, typically beta-lactam antibiotics, cephalosporins, metronidazole, clindamycin, moxifloxacin, or erythromycin can be given for 7 days or until 3 days after symptoms have resolved.5 Although antibiotics can bring temporary resolution, it is imperative to treat the source of infection to prevent recurrence. It is crucial for these patients to be referred to an oral surgeon for evaluation and treatment of OCST by either a root canal or tooth extraction.
Final Thoughts
We present this pearl on the diagnosis and management of an OCST, also known as a dental sinus, to better assist clinicians in making this diagnosis. With an index of suspicion as well as intraoral and radiologic evaluations, a proper diagnosis may be rendered, potentially avoiding unnecessary cutaneous surgery. In addition, we highlight the importance of communication between the clinician and the radiologist to directly look for OCST in the area of concern and consider a re-read of the images when clinical suspicion does not correlate with the radiology report.
- Bai J, Ji AP, Huang MW. Submental cutaneous sinus tract of mandibular second molar origin. Int Endod J. 2014;47:1185-1191.
- Plast Reconstr Surg.
- Gregoire C. How are odontogenic infections best managed? J Can Dent Assoc. 2010;76:a37.
- Bodner L, Bar-Ziv J. Cutaneous sinus tract of dental origin—imaging with a dental CT software programme. Br J Oral Maxillofac Surg. 1998;36:311-313.
- Peermohamed S, Barber D, Kurwa H. Diagnostic challenges of cutaneous draining sinus tracts of odontogenic origin: a case report. Dermatol Surg. 2011;37:1525-1527.
- Bai J, Ji AP, Huang MW. Submental cutaneous sinus tract of mandibular second molar origin. Int Endod J. 2014;47:1185-1191.
- Plast Reconstr Surg.
- Gregoire C. How are odontogenic infections best managed? J Can Dent Assoc. 2010;76:a37.
- Bodner L, Bar-Ziv J. Cutaneous sinus tract of dental origin—imaging with a dental CT software programme. Br J Oral Maxillofac Surg. 1998;36:311-313.
- Peermohamed S, Barber D, Kurwa H. Diagnostic challenges of cutaneous draining sinus tracts of odontogenic origin: a case report. Dermatol Surg. 2011;37:1525-1527.
ICYMI: MSVirtual2020 Virtual Joint ACTRIMS-ECTRIMS Meeting Summary from MS Resource Center Editor in Chief, Joseph R. Berger, MD
I had the privilege of attending and speaking at the recent MSVirtual2020—the 8th Joint ACTRIMS-ECTRIMS Meeting. I came away with a wealth of knowledge, much of which can be put to immediate use in practice, and some that shows the promise of eventual clinical utility.
Dr. Helen Tremlett, PhD, kicked off the meeting with a keynote address covering her important work on the MS prodrome. The Canada research chair in neuroepidemiology and multiple sclerosis at the University of British Columbia summarized her team’s research to date and offered her thoughts on clinical implications.
Dr. Tremlett’s group has observed that in the five years before an MS symptom onset, individuals who would ultimately be diagnosed tended to experience more hospitalizations, visit their provider more, and fill more prescriptions than did those in the general population. The team dug deeper and found that these individuals experienced a range of issues prior to symptom onset, including pain, headache, migraine, fibromyalgia, irritable bowel syndrome, sleep disturbances, depression/anxiety, and dermatologic issues.
Interestingly, females in this group were less likely to become pregnant and more likely than healthy females to fill an oral contraceptive prescription, suggesting that they were trying to delay pregnancy due to these prodromal symptoms.
Dr. Tremlett noted that the more immediate implications of her group’s work are for clinical researchers, who can now use these findings to understand that there is a prodromal stage as they conduct clinical trials. The ultimate aim is to use this work to develop a diagnostic tool, but that will take more time and study.
COVID-19’s Impact on MS
The impact on COVID-19 on individuals with MS was addressed in a number of sessions. I presented data that clearly shows the risk of infection from COVID-19 is similar to that of the population at large.
- A critical evaluation of MS disease modifying therapies (DMTs) and their potential effects on COVID-19 that I published with my colleagues at the University of Pennsylvania suggested that DMTs might not increase the risk of morbidity and mortality associated with COVID-19 as some had feared. We based this conclusion on an evaluation of pathogenesis of COVID, the importance of the innate immune system in control of exposure to a novel pathogen, and the likely effects, both salutary and pernicious, of DMTs on COVID morbidity and mortality.
- Investigators from Italy looked at 232 patients from 38 centers with MS and confirmed or suspected COVID and found that the vast majority of them (96%) had mild disease consisting of no or mild pneumonia. The remainder had either severe (2%) or critical (3%) disease. These investigators have since expanded their observations and suggested that anti-CD20 monoclonal antibody treatment may be associated with a higher risk of hospitalization, though there did not appear to be an increase in the risk of death with their use. Importantly, the anti-CD20 monoclonal antibody therapies are the DMTs routinely used in patients with progressive MS, generally, the MS population at greatest risk of hospitalization with COVID-19 due to their older age, co-morbidities, and level of debility.
- Recently, French researchers evaluated 347 individuals with MS and COVID by COVID disease severity. They found that there was a higher proportion of patients with severe COVID not receiving DMT compared with individuals receiving treatment (46% and 15%, respectively).
The Increasing Importance of sNfL Concentration
Serum neurofilament light chain (sNfL) concentration continues to be a hot topic. Dr. Jens Kuhle, head of the Multiple Sclerosis Centre at the University of Basel, and colleagues have demonstrated that sNfL levels can play a role in monitoring MS treatment in practice. They evaluated more than 1000 individuals who were taking DMTs, measuring sNfL and deriving a score that reflected how participants fared relative to healthy controls of the same age. Among their findings:
- The resulting score predicted clinical events in the following year, with the effect escalating in magnitude in those whose scores were higher.
- This same predictive effect was seen with respect to future new/enlarging T2 lesions and brain volume loss.
- Score change in patients with NEDA-03 status was linked with a 37% increased risk of clinical events in the following year.
New Radiologic Techniques
Encouraging findings on new radiologic techniques were presented. I found three studies extremely informative. The first two have immediate or near-immediate clinical implications, and the third shows promise.
- In a comparison of patients with MS and healthy individuals who underwent brain 3T MRI to assess lesions and atrophy, R. Bonacchi and colleagues from Milan, Italy found that cardiovascular (CV) risk factors are linked with brain atrophy in patients with MS, even those <50 years of age. Specifically, the presence of at least two CV risk factors was linked with reduced normalized grey matter volume, white matter volume, and brain volume.
- Another comparison of individuals with MS and healthy controls—this one from O. Al-Louzi and colleagues at the National Institute of Neurological Disorders and Stroke—looked at the central vein sign (CVS) biomarker and determined that excluding lesions only if all dimensions of 3T MRI results were less than threshold (versus if any dimension was less than threshold) led to the inclusion of more CVS-positive lesions. Investigators suggested this work could lead to modified clinical guidelines.
- In an evaluation of patients with MS using 3T MRI, F. LaRosa and colleagues from Lausanne, Switzerland reported that RimNet, a prototype built upon two convolutional neural networks, was better than two alternative methods at detecting pragmatic rim lesions, which are linked with higher disease burden. Compared with expert raters, RimNet had higher sensitivity (87% vs 76%) but lower specificity (91% vs 99%).
There were many other valuable presentations at MSVirtual2020, but perhaps the most appreciated experience was the ability to hear more experts deliver their important work. Unlike a live meeting, I was able to easily attend parallel sessions and to do so at my leisure. ECTRIMS has become so big that I often left the live meeting feeling as if I missed out on a lot. Not this year. I heard almost all of it and came away with a greater appreciation of the breadth and depth of the meeting. I hope that in the future, even following the return of in-person meetings, a virtual format coexists to afford attendees and those unable to attend live the opportunity to experience the totality of the meeting.
I had the privilege of attending and speaking at the recent MSVirtual2020—the 8th Joint ACTRIMS-ECTRIMS Meeting. I came away with a wealth of knowledge, much of which can be put to immediate use in practice, and some that shows the promise of eventual clinical utility.
Dr. Helen Tremlett, PhD, kicked off the meeting with a keynote address covering her important work on the MS prodrome. The Canada research chair in neuroepidemiology and multiple sclerosis at the University of British Columbia summarized her team’s research to date and offered her thoughts on clinical implications.
Dr. Tremlett’s group has observed that in the five years before an MS symptom onset, individuals who would ultimately be diagnosed tended to experience more hospitalizations, visit their provider more, and fill more prescriptions than did those in the general population. The team dug deeper and found that these individuals experienced a range of issues prior to symptom onset, including pain, headache, migraine, fibromyalgia, irritable bowel syndrome, sleep disturbances, depression/anxiety, and dermatologic issues.
Interestingly, females in this group were less likely to become pregnant and more likely than healthy females to fill an oral contraceptive prescription, suggesting that they were trying to delay pregnancy due to these prodromal symptoms.
Dr. Tremlett noted that the more immediate implications of her group’s work are for clinical researchers, who can now use these findings to understand that there is a prodromal stage as they conduct clinical trials. The ultimate aim is to use this work to develop a diagnostic tool, but that will take more time and study.
COVID-19’s Impact on MS
The impact on COVID-19 on individuals with MS was addressed in a number of sessions. I presented data that clearly shows the risk of infection from COVID-19 is similar to that of the population at large.
- A critical evaluation of MS disease modifying therapies (DMTs) and their potential effects on COVID-19 that I published with my colleagues at the University of Pennsylvania suggested that DMTs might not increase the risk of morbidity and mortality associated with COVID-19 as some had feared. We based this conclusion on an evaluation of pathogenesis of COVID, the importance of the innate immune system in control of exposure to a novel pathogen, and the likely effects, both salutary and pernicious, of DMTs on COVID morbidity and mortality.
- Investigators from Italy looked at 232 patients from 38 centers with MS and confirmed or suspected COVID and found that the vast majority of them (96%) had mild disease consisting of no or mild pneumonia. The remainder had either severe (2%) or critical (3%) disease. These investigators have since expanded their observations and suggested that anti-CD20 monoclonal antibody treatment may be associated with a higher risk of hospitalization, though there did not appear to be an increase in the risk of death with their use. Importantly, the anti-CD20 monoclonal antibody therapies are the DMTs routinely used in patients with progressive MS, generally, the MS population at greatest risk of hospitalization with COVID-19 due to their older age, co-morbidities, and level of debility.
- Recently, French researchers evaluated 347 individuals with MS and COVID by COVID disease severity. They found that there was a higher proportion of patients with severe COVID not receiving DMT compared with individuals receiving treatment (46% and 15%, respectively).
The Increasing Importance of sNfL Concentration
Serum neurofilament light chain (sNfL) concentration continues to be a hot topic. Dr. Jens Kuhle, head of the Multiple Sclerosis Centre at the University of Basel, and colleagues have demonstrated that sNfL levels can play a role in monitoring MS treatment in practice. They evaluated more than 1000 individuals who were taking DMTs, measuring sNfL and deriving a score that reflected how participants fared relative to healthy controls of the same age. Among their findings:
- The resulting score predicted clinical events in the following year, with the effect escalating in magnitude in those whose scores were higher.
- This same predictive effect was seen with respect to future new/enlarging T2 lesions and brain volume loss.
- Score change in patients with NEDA-03 status was linked with a 37% increased risk of clinical events in the following year.
New Radiologic Techniques
Encouraging findings on new radiologic techniques were presented. I found three studies extremely informative. The first two have immediate or near-immediate clinical implications, and the third shows promise.
- In a comparison of patients with MS and healthy individuals who underwent brain 3T MRI to assess lesions and atrophy, R. Bonacchi and colleagues from Milan, Italy found that cardiovascular (CV) risk factors are linked with brain atrophy in patients with MS, even those <50 years of age. Specifically, the presence of at least two CV risk factors was linked with reduced normalized grey matter volume, white matter volume, and brain volume.
- Another comparison of individuals with MS and healthy controls—this one from O. Al-Louzi and colleagues at the National Institute of Neurological Disorders and Stroke—looked at the central vein sign (CVS) biomarker and determined that excluding lesions only if all dimensions of 3T MRI results were less than threshold (versus if any dimension was less than threshold) led to the inclusion of more CVS-positive lesions. Investigators suggested this work could lead to modified clinical guidelines.
- In an evaluation of patients with MS using 3T MRI, F. LaRosa and colleagues from Lausanne, Switzerland reported that RimNet, a prototype built upon two convolutional neural networks, was better than two alternative methods at detecting pragmatic rim lesions, which are linked with higher disease burden. Compared with expert raters, RimNet had higher sensitivity (87% vs 76%) but lower specificity (91% vs 99%).
There were many other valuable presentations at MSVirtual2020, but perhaps the most appreciated experience was the ability to hear more experts deliver their important work. Unlike a live meeting, I was able to easily attend parallel sessions and to do so at my leisure. ECTRIMS has become so big that I often left the live meeting feeling as if I missed out on a lot. Not this year. I heard almost all of it and came away with a greater appreciation of the breadth and depth of the meeting. I hope that in the future, even following the return of in-person meetings, a virtual format coexists to afford attendees and those unable to attend live the opportunity to experience the totality of the meeting.
I had the privilege of attending and speaking at the recent MSVirtual2020—the 8th Joint ACTRIMS-ECTRIMS Meeting. I came away with a wealth of knowledge, much of which can be put to immediate use in practice, and some that shows the promise of eventual clinical utility.
Dr. Helen Tremlett, PhD, kicked off the meeting with a keynote address covering her important work on the MS prodrome. The Canada research chair in neuroepidemiology and multiple sclerosis at the University of British Columbia summarized her team’s research to date and offered her thoughts on clinical implications.
Dr. Tremlett’s group has observed that in the five years before an MS symptom onset, individuals who would ultimately be diagnosed tended to experience more hospitalizations, visit their provider more, and fill more prescriptions than did those in the general population. The team dug deeper and found that these individuals experienced a range of issues prior to symptom onset, including pain, headache, migraine, fibromyalgia, irritable bowel syndrome, sleep disturbances, depression/anxiety, and dermatologic issues.
Interestingly, females in this group were less likely to become pregnant and more likely than healthy females to fill an oral contraceptive prescription, suggesting that they were trying to delay pregnancy due to these prodromal symptoms.
Dr. Tremlett noted that the more immediate implications of her group’s work are for clinical researchers, who can now use these findings to understand that there is a prodromal stage as they conduct clinical trials. The ultimate aim is to use this work to develop a diagnostic tool, but that will take more time and study.
COVID-19’s Impact on MS
The impact on COVID-19 on individuals with MS was addressed in a number of sessions. I presented data that clearly shows the risk of infection from COVID-19 is similar to that of the population at large.
- A critical evaluation of MS disease modifying therapies (DMTs) and their potential effects on COVID-19 that I published with my colleagues at the University of Pennsylvania suggested that DMTs might not increase the risk of morbidity and mortality associated with COVID-19 as some had feared. We based this conclusion on an evaluation of pathogenesis of COVID, the importance of the innate immune system in control of exposure to a novel pathogen, and the likely effects, both salutary and pernicious, of DMTs on COVID morbidity and mortality.
- Investigators from Italy looked at 232 patients from 38 centers with MS and confirmed or suspected COVID and found that the vast majority of them (96%) had mild disease consisting of no or mild pneumonia. The remainder had either severe (2%) or critical (3%) disease. These investigators have since expanded their observations and suggested that anti-CD20 monoclonal antibody treatment may be associated with a higher risk of hospitalization, though there did not appear to be an increase in the risk of death with their use. Importantly, the anti-CD20 monoclonal antibody therapies are the DMTs routinely used in patients with progressive MS, generally, the MS population at greatest risk of hospitalization with COVID-19 due to their older age, co-morbidities, and level of debility.
- Recently, French researchers evaluated 347 individuals with MS and COVID by COVID disease severity. They found that there was a higher proportion of patients with severe COVID not receiving DMT compared with individuals receiving treatment (46% and 15%, respectively).
The Increasing Importance of sNfL Concentration
Serum neurofilament light chain (sNfL) concentration continues to be a hot topic. Dr. Jens Kuhle, head of the Multiple Sclerosis Centre at the University of Basel, and colleagues have demonstrated that sNfL levels can play a role in monitoring MS treatment in practice. They evaluated more than 1000 individuals who were taking DMTs, measuring sNfL and deriving a score that reflected how participants fared relative to healthy controls of the same age. Among their findings:
- The resulting score predicted clinical events in the following year, with the effect escalating in magnitude in those whose scores were higher.
- This same predictive effect was seen with respect to future new/enlarging T2 lesions and brain volume loss.
- Score change in patients with NEDA-03 status was linked with a 37% increased risk of clinical events in the following year.
New Radiologic Techniques
Encouraging findings on new radiologic techniques were presented. I found three studies extremely informative. The first two have immediate or near-immediate clinical implications, and the third shows promise.
- In a comparison of patients with MS and healthy individuals who underwent brain 3T MRI to assess lesions and atrophy, R. Bonacchi and colleagues from Milan, Italy found that cardiovascular (CV) risk factors are linked with brain atrophy in patients with MS, even those <50 years of age. Specifically, the presence of at least two CV risk factors was linked with reduced normalized grey matter volume, white matter volume, and brain volume.
- Another comparison of individuals with MS and healthy controls—this one from O. Al-Louzi and colleagues at the National Institute of Neurological Disorders and Stroke—looked at the central vein sign (CVS) biomarker and determined that excluding lesions only if all dimensions of 3T MRI results were less than threshold (versus if any dimension was less than threshold) led to the inclusion of more CVS-positive lesions. Investigators suggested this work could lead to modified clinical guidelines.
- In an evaluation of patients with MS using 3T MRI, F. LaRosa and colleagues from Lausanne, Switzerland reported that RimNet, a prototype built upon two convolutional neural networks, was better than two alternative methods at detecting pragmatic rim lesions, which are linked with higher disease burden. Compared with expert raters, RimNet had higher sensitivity (87% vs 76%) but lower specificity (91% vs 99%).
There were many other valuable presentations at MSVirtual2020, but perhaps the most appreciated experience was the ability to hear more experts deliver their important work. Unlike a live meeting, I was able to easily attend parallel sessions and to do so at my leisure. ECTRIMS has become so big that I often left the live meeting feeling as if I missed out on a lot. Not this year. I heard almost all of it and came away with a greater appreciation of the breadth and depth of the meeting. I hope that in the future, even following the return of in-person meetings, a virtual format coexists to afford attendees and those unable to attend live the opportunity to experience the totality of the meeting.
Systemic Racism and Health Disparities: A Statement from Editors of Family Medicine Journals
The year 2020 was marked by historic protests across the United States and the globe sparked by the deaths of George Floyd, Ahmaud Arbery, Breonna Taylor, and so many other Black people. The protests heightened awareness of racism as a public health crisis and triggered an antiracism movement. Racism is a pervasive and systemic issue that has profound adverse effects on health.1,2 Racism is associated with poorer mental and physical health outcomes and negative patient experiences in the health care system.3,4 As evidenced by the current coronavirus pandemic, race is a sociopolitical construct that continues to disadvantage Black, Latinx, Indigenous, and other People of Color.5,6,7,8 The association between racism and adverse health outcomes has been discussed for decades in the medical literature, including the family medicine literature. Today there is a renewed call to action for family medicine, a specialty that emerged as a counterculture to reform mainstream medicine,9 to both confront systemic racism and eliminate health disparities. This effort will require collaboration, commitment, education, and transformative conversations around racism, health inequity, and advocacy so that we can better serve our patients and our communities.
The editors of several North American family medicine publications have come together to address this call to action and share resources on racism across our readerships. We acknowledge those members of the family medicine scholar community who have been fighting for equity consistent with the Black Lives Matter movement by writing about racism, health inequities, and personal experiences of practicing as Black family physicians. While we recognize that much more work is needed, we want to amplify these voices. We have compiled a bibliography of scholarship generated by the family medicine community on the topic of racism in medicine.
The collection can be accessed here.
While this list is likely not complete, it does include over 250 published manuscripts and demonstrates expertise as well as a commitment to addressing these complex issues. For example, in 2016, Dr. J. Nwando Olayiwola, chair of the Department of Family Medicine at Ohio State University, wrote an essay on her experiences taking care of patients as a Black family physician.10 In January of 2019, Family Medicine published an entire issue devoted to racism in education and training.11 Dr. Eduardo Medina, a family physician and public health scholar, co-authored a call to action in 2016 for health professionals to dismantle structural racism and support Black lives to achieve health equity. His recent 2020 article builds on that theme and describes the disproportionate deaths of Black people due to racial injustice and the COVID-19 pandemic as converging public health emergencies.12,13 In the wake of these emergencies a fundamental transformation is warranted, and family physicians can play a key role.
We, the editors of family medicine journals, commit to actively examine the effects of racism on society and health and to take action to eliminate structural racism in our editorial processes. As an intellectual home for our profession, we have a unique responsibility and opportunity to educate and continue the conversation about institutional racism, health inequities, and antiracism in medicine. We will take immediate steps to enact tangible advances on these fronts. We will encourage and mentor authors from groups underrepresented in medicine. We will ensure that content includes an emphasis on cultural humility, diversity and inclusion, implicit bias, and the impact of racism on medicine and health. We will recruit editors and editorial board members from groups underrepresented in medicine. We will encourage collaboration and accountability within our specialty to confront systemic racism through content and processes in all of our individual publications. We recognize that these are small steps in an ongoing process of active antiracism, but we believe these steps are crucial. As editors in family medicine, we are committed to progress toward equity and justice.
Simultaneously published in American Family Physician, Annals of Family Medicine, Canadian Family Physician, Family Medicine, FP Essentials, FPIN/Evidence Based Practice, FPM, Journal of the American Board of Family Medicine, The Journal of Family Practice, and PRiMER.
Acknowledgement –
The authors thank Renee Crichlow, MD, Byron Jasper, MD, MPH, and Victoria Murrain, DO, for their insightful comments on this editorial.
1. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Smedley BD, Stith AY, Nelson AR, eds. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003.
2. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463.
3. Ben J, Cormack D, Harris R, Paradies Y. Racism and health service utilisation: A systematic review and meta-analysis. PLoS One. 2017;12(12):e0189900.
4. Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10(9):e0138511.
5. American Academy of Family Physicians. Institutional racism in the health care system. Published 2019. Accessed Sept. 15, 2020. https://www.aafp.org/about/policies/all/institutional-racism.html.
6. Yaya S, Yeboah H, Charles CH, Otu A, Labonte R. Ethnic and racial disparities in COVID-19-related deaths: counting the trees, hiding the forest. BMJ Glob Health. 2020;5(6):e002913.
7. Egede LE, Walker RJ. Structural Racism, Social Risk Factors, and Covid-19 — A Dangerous Convergence for Black Americans [published online ahead of print, 2020 Jul 22]. N Engl J Med. 2020;10.1056/NEJMp2023616.
8. Centers for Disease Control and Prevention. Health equity considerations and racial and ethnic minority groups. Updated July 24, 2020. Accessed Sept. 15, 2020. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html
9. Stephens GG. Family medicine as counterculture. Fam Med. 1989;21(2):103-109.
10. Olayiwola JN. Racism in medicine: shifting the power. Ann Fam Med. 2016;14(3):267-269. https://doi.org/10.1370/afm.1932.
11. Saultz J, ed. Racism. Fam Med. 2019;51(1, theme issue):1-66.
12. Hardeman RR, Medina EM, Kozhimannil KB. Structural racism and supporting black lives - the role of health professionals. N Engl J Med. 2016;375(22):2113-2115. https://doi.org/10.1056/NEJMp1609535.
13. Hardeman RR, Medina EM, Boyd RW. Stolen breaths. N Engl J Med. 2020;383(3):197-199. 10.1056/NEJMp2021072.
The year 2020 was marked by historic protests across the United States and the globe sparked by the deaths of George Floyd, Ahmaud Arbery, Breonna Taylor, and so many other Black people. The protests heightened awareness of racism as a public health crisis and triggered an antiracism movement. Racism is a pervasive and systemic issue that has profound adverse effects on health.1,2 Racism is associated with poorer mental and physical health outcomes and negative patient experiences in the health care system.3,4 As evidenced by the current coronavirus pandemic, race is a sociopolitical construct that continues to disadvantage Black, Latinx, Indigenous, and other People of Color.5,6,7,8 The association between racism and adverse health outcomes has been discussed for decades in the medical literature, including the family medicine literature. Today there is a renewed call to action for family medicine, a specialty that emerged as a counterculture to reform mainstream medicine,9 to both confront systemic racism and eliminate health disparities. This effort will require collaboration, commitment, education, and transformative conversations around racism, health inequity, and advocacy so that we can better serve our patients and our communities.
The editors of several North American family medicine publications have come together to address this call to action and share resources on racism across our readerships. We acknowledge those members of the family medicine scholar community who have been fighting for equity consistent with the Black Lives Matter movement by writing about racism, health inequities, and personal experiences of practicing as Black family physicians. While we recognize that much more work is needed, we want to amplify these voices. We have compiled a bibliography of scholarship generated by the family medicine community on the topic of racism in medicine.
The collection can be accessed here.
While this list is likely not complete, it does include over 250 published manuscripts and demonstrates expertise as well as a commitment to addressing these complex issues. For example, in 2016, Dr. J. Nwando Olayiwola, chair of the Department of Family Medicine at Ohio State University, wrote an essay on her experiences taking care of patients as a Black family physician.10 In January of 2019, Family Medicine published an entire issue devoted to racism in education and training.11 Dr. Eduardo Medina, a family physician and public health scholar, co-authored a call to action in 2016 for health professionals to dismantle structural racism and support Black lives to achieve health equity. His recent 2020 article builds on that theme and describes the disproportionate deaths of Black people due to racial injustice and the COVID-19 pandemic as converging public health emergencies.12,13 In the wake of these emergencies a fundamental transformation is warranted, and family physicians can play a key role.
We, the editors of family medicine journals, commit to actively examine the effects of racism on society and health and to take action to eliminate structural racism in our editorial processes. As an intellectual home for our profession, we have a unique responsibility and opportunity to educate and continue the conversation about institutional racism, health inequities, and antiracism in medicine. We will take immediate steps to enact tangible advances on these fronts. We will encourage and mentor authors from groups underrepresented in medicine. We will ensure that content includes an emphasis on cultural humility, diversity and inclusion, implicit bias, and the impact of racism on medicine and health. We will recruit editors and editorial board members from groups underrepresented in medicine. We will encourage collaboration and accountability within our specialty to confront systemic racism through content and processes in all of our individual publications. We recognize that these are small steps in an ongoing process of active antiracism, but we believe these steps are crucial. As editors in family medicine, we are committed to progress toward equity and justice.
Simultaneously published in American Family Physician, Annals of Family Medicine, Canadian Family Physician, Family Medicine, FP Essentials, FPIN/Evidence Based Practice, FPM, Journal of the American Board of Family Medicine, The Journal of Family Practice, and PRiMER.
Acknowledgement –
The authors thank Renee Crichlow, MD, Byron Jasper, MD, MPH, and Victoria Murrain, DO, for their insightful comments on this editorial.
The year 2020 was marked by historic protests across the United States and the globe sparked by the deaths of George Floyd, Ahmaud Arbery, Breonna Taylor, and so many other Black people. The protests heightened awareness of racism as a public health crisis and triggered an antiracism movement. Racism is a pervasive and systemic issue that has profound adverse effects on health.1,2 Racism is associated with poorer mental and physical health outcomes and negative patient experiences in the health care system.3,4 As evidenced by the current coronavirus pandemic, race is a sociopolitical construct that continues to disadvantage Black, Latinx, Indigenous, and other People of Color.5,6,7,8 The association between racism and adverse health outcomes has been discussed for decades in the medical literature, including the family medicine literature. Today there is a renewed call to action for family medicine, a specialty that emerged as a counterculture to reform mainstream medicine,9 to both confront systemic racism and eliminate health disparities. This effort will require collaboration, commitment, education, and transformative conversations around racism, health inequity, and advocacy so that we can better serve our patients and our communities.
The editors of several North American family medicine publications have come together to address this call to action and share resources on racism across our readerships. We acknowledge those members of the family medicine scholar community who have been fighting for equity consistent with the Black Lives Matter movement by writing about racism, health inequities, and personal experiences of practicing as Black family physicians. While we recognize that much more work is needed, we want to amplify these voices. We have compiled a bibliography of scholarship generated by the family medicine community on the topic of racism in medicine.
The collection can be accessed here.
While this list is likely not complete, it does include over 250 published manuscripts and demonstrates expertise as well as a commitment to addressing these complex issues. For example, in 2016, Dr. J. Nwando Olayiwola, chair of the Department of Family Medicine at Ohio State University, wrote an essay on her experiences taking care of patients as a Black family physician.10 In January of 2019, Family Medicine published an entire issue devoted to racism in education and training.11 Dr. Eduardo Medina, a family physician and public health scholar, co-authored a call to action in 2016 for health professionals to dismantle structural racism and support Black lives to achieve health equity. His recent 2020 article builds on that theme and describes the disproportionate deaths of Black people due to racial injustice and the COVID-19 pandemic as converging public health emergencies.12,13 In the wake of these emergencies a fundamental transformation is warranted, and family physicians can play a key role.
We, the editors of family medicine journals, commit to actively examine the effects of racism on society and health and to take action to eliminate structural racism in our editorial processes. As an intellectual home for our profession, we have a unique responsibility and opportunity to educate and continue the conversation about institutional racism, health inequities, and antiracism in medicine. We will take immediate steps to enact tangible advances on these fronts. We will encourage and mentor authors from groups underrepresented in medicine. We will ensure that content includes an emphasis on cultural humility, diversity and inclusion, implicit bias, and the impact of racism on medicine and health. We will recruit editors and editorial board members from groups underrepresented in medicine. We will encourage collaboration and accountability within our specialty to confront systemic racism through content and processes in all of our individual publications. We recognize that these are small steps in an ongoing process of active antiracism, but we believe these steps are crucial. As editors in family medicine, we are committed to progress toward equity and justice.
Simultaneously published in American Family Physician, Annals of Family Medicine, Canadian Family Physician, Family Medicine, FP Essentials, FPIN/Evidence Based Practice, FPM, Journal of the American Board of Family Medicine, The Journal of Family Practice, and PRiMER.
Acknowledgement –
The authors thank Renee Crichlow, MD, Byron Jasper, MD, MPH, and Victoria Murrain, DO, for their insightful comments on this editorial.
1. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Smedley BD, Stith AY, Nelson AR, eds. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003.
2. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463.
3. Ben J, Cormack D, Harris R, Paradies Y. Racism and health service utilisation: A systematic review and meta-analysis. PLoS One. 2017;12(12):e0189900.
4. Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10(9):e0138511.
5. American Academy of Family Physicians. Institutional racism in the health care system. Published 2019. Accessed Sept. 15, 2020. https://www.aafp.org/about/policies/all/institutional-racism.html.
6. Yaya S, Yeboah H, Charles CH, Otu A, Labonte R. Ethnic and racial disparities in COVID-19-related deaths: counting the trees, hiding the forest. BMJ Glob Health. 2020;5(6):e002913.
7. Egede LE, Walker RJ. Structural Racism, Social Risk Factors, and Covid-19 — A Dangerous Convergence for Black Americans [published online ahead of print, 2020 Jul 22]. N Engl J Med. 2020;10.1056/NEJMp2023616.
8. Centers for Disease Control and Prevention. Health equity considerations and racial and ethnic minority groups. Updated July 24, 2020. Accessed Sept. 15, 2020. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html
9. Stephens GG. Family medicine as counterculture. Fam Med. 1989;21(2):103-109.
10. Olayiwola JN. Racism in medicine: shifting the power. Ann Fam Med. 2016;14(3):267-269. https://doi.org/10.1370/afm.1932.
11. Saultz J, ed. Racism. Fam Med. 2019;51(1, theme issue):1-66.
12. Hardeman RR, Medina EM, Kozhimannil KB. Structural racism and supporting black lives - the role of health professionals. N Engl J Med. 2016;375(22):2113-2115. https://doi.org/10.1056/NEJMp1609535.
13. Hardeman RR, Medina EM, Boyd RW. Stolen breaths. N Engl J Med. 2020;383(3):197-199. 10.1056/NEJMp2021072.
1. Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Smedley BD, Stith AY, Nelson AR, eds. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003.
2. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463.
3. Ben J, Cormack D, Harris R, Paradies Y. Racism and health service utilisation: A systematic review and meta-analysis. PLoS One. 2017;12(12):e0189900.
4. Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10(9):e0138511.
5. American Academy of Family Physicians. Institutional racism in the health care system. Published 2019. Accessed Sept. 15, 2020. https://www.aafp.org/about/policies/all/institutional-racism.html.
6. Yaya S, Yeboah H, Charles CH, Otu A, Labonte R. Ethnic and racial disparities in COVID-19-related deaths: counting the trees, hiding the forest. BMJ Glob Health. 2020;5(6):e002913.
7. Egede LE, Walker RJ. Structural Racism, Social Risk Factors, and Covid-19 — A Dangerous Convergence for Black Americans [published online ahead of print, 2020 Jul 22]. N Engl J Med. 2020;10.1056/NEJMp2023616.
8. Centers for Disease Control and Prevention. Health equity considerations and racial and ethnic minority groups. Updated July 24, 2020. Accessed Sept. 15, 2020. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html
9. Stephens GG. Family medicine as counterculture. Fam Med. 1989;21(2):103-109.
10. Olayiwola JN. Racism in medicine: shifting the power. Ann Fam Med. 2016;14(3):267-269. https://doi.org/10.1370/afm.1932.
11. Saultz J, ed. Racism. Fam Med. 2019;51(1, theme issue):1-66.
12. Hardeman RR, Medina EM, Kozhimannil KB. Structural racism and supporting black lives - the role of health professionals. N Engl J Med. 2016;375(22):2113-2115. https://doi.org/10.1056/NEJMp1609535.
13. Hardeman RR, Medina EM, Boyd RW. Stolen breaths. N Engl J Med. 2020;383(3):197-199. 10.1056/NEJMp2021072.
What’s in a number? 697,633 children with COVID-19
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
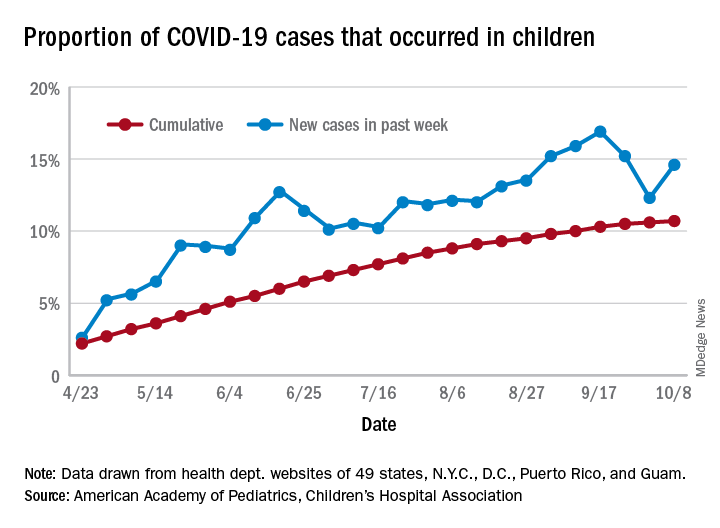
For the week, 14.6% of all COVID-19 cases reported in the United States occurred in children, after 2 consecutive weeks of declines that saw the proportion drop from 16.9% to 12.3%. The cumulative rate of child cases for the entire pandemic is 10.7%, with total child cases in the United States now up to 697,633 and cases among all ages at just over 6.5 million, the AAP and the CHA said Oct. 12 in their weekly COVID-19 report.
Nationally, there were 927 cases reported per 100,000 children as of Oct. 8, with rates at the state level varying from 176 per 100,000 in Vermont to 2,221 per 100,000 in North Dakota. Two other states were over 2,000 cases per 100,000 children: Tennessee (2,155) and South Carolina (2,116), based on data from the health departments of 49 states (New York does not report age distribution), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
Severe illness continues to be rare in children, and national (25 states and New York City) hospitalization rates dropped in the last week. The proportion of hospitalizations occurring in children slipped from a pandemic high of 1.8% the previous week to 1.7% during the week of Oct. 8, and the rate of hospitalizations for children with COVID-19 was down to 1.4% from 1.6% the week before and 1.9% on Sept. 3, the AAP and the CHA said.
Mortality data from 42 states and New York City also show a decline. For the third consecutive week, children represented just 0.06% of all COVID-19 deaths in the United States, down from a high of 0.07% on Sept. 17. Only 0.02% of all cases in children have resulted in death, and that figure has been dropping since early June, when it reached 0.06%, according to the AAP/CHA report. As of Oct. 8, there have been 115 total deaths reported in children.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

For the week, 14.6% of all COVID-19 cases reported in the United States occurred in children, after 2 consecutive weeks of declines that saw the proportion drop from 16.9% to 12.3%. The cumulative rate of child cases for the entire pandemic is 10.7%, with total child cases in the United States now up to 697,633 and cases among all ages at just over 6.5 million, the AAP and the CHA said Oct. 12 in their weekly COVID-19 report.
Nationally, there were 927 cases reported per 100,000 children as of Oct. 8, with rates at the state level varying from 176 per 100,000 in Vermont to 2,221 per 100,000 in North Dakota. Two other states were over 2,000 cases per 100,000 children: Tennessee (2,155) and South Carolina (2,116), based on data from the health departments of 49 states (New York does not report age distribution), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
Severe illness continues to be rare in children, and national (25 states and New York City) hospitalization rates dropped in the last week. The proportion of hospitalizations occurring in children slipped from a pandemic high of 1.8% the previous week to 1.7% during the week of Oct. 8, and the rate of hospitalizations for children with COVID-19 was down to 1.4% from 1.6% the week before and 1.9% on Sept. 3, the AAP and the CHA said.
Mortality data from 42 states and New York City also show a decline. For the third consecutive week, children represented just 0.06% of all COVID-19 deaths in the United States, down from a high of 0.07% on Sept. 17. Only 0.02% of all cases in children have resulted in death, and that figure has been dropping since early June, when it reached 0.06%, according to the AAP/CHA report. As of Oct. 8, there have been 115 total deaths reported in children.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

For the week, 14.6% of all COVID-19 cases reported in the United States occurred in children, after 2 consecutive weeks of declines that saw the proportion drop from 16.9% to 12.3%. The cumulative rate of child cases for the entire pandemic is 10.7%, with total child cases in the United States now up to 697,633 and cases among all ages at just over 6.5 million, the AAP and the CHA said Oct. 12 in their weekly COVID-19 report.
Nationally, there were 927 cases reported per 100,000 children as of Oct. 8, with rates at the state level varying from 176 per 100,000 in Vermont to 2,221 per 100,000 in North Dakota. Two other states were over 2,000 cases per 100,000 children: Tennessee (2,155) and South Carolina (2,116), based on data from the health departments of 49 states (New York does not report age distribution), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
Severe illness continues to be rare in children, and national (25 states and New York City) hospitalization rates dropped in the last week. The proportion of hospitalizations occurring in children slipped from a pandemic high of 1.8% the previous week to 1.7% during the week of Oct. 8, and the rate of hospitalizations for children with COVID-19 was down to 1.4% from 1.6% the week before and 1.9% on Sept. 3, the AAP and the CHA said.
Mortality data from 42 states and New York City also show a decline. For the third consecutive week, children represented just 0.06% of all COVID-19 deaths in the United States, down from a high of 0.07% on Sept. 17. Only 0.02% of all cases in children have resulted in death, and that figure has been dropping since early June, when it reached 0.06%, according to the AAP/CHA report. As of Oct. 8, there have been 115 total deaths reported in children.
A uniquely patient-focused take on treating AML in older adults
A diagnosis of acute myeloid leukemia (AML) is particularly challenging in older adults, whose age makes them highly susceptible to the disease and treatment-related toxicity. To help patients and practitioners navigate the clinical decision-making process, the American Society of Hematology convened a panel of experts who conducted a thorough review of the literature. The result of their work can be found in a new set of guidelines for the treatment of newly diagnosed AML in older adults.
In an interview, Mikkael Sekeres, MD, chair of the ASH AML guideline panel and director of the Leukemia Program at Cleveland Clinic Taussig Cancer Institute in Cleveland, Ohio, shared the rationale behind the panel’s key recommendations and the importance of keeping the patient’s goals in mind.
Question: What is the average life expectancy of a 75-year-old developing AML compared with someone of the same age without AML?
Dr. Sekeres: A 75-year-old developing AML has an average life expectancy measured in fewer than 6 months. Somebody who is 75 without leukemia in the United States has a life expectancy that can be measured in a decade or more. AML is a really serious diagnosis when someone is older and significantly truncates expected survival.
Q: What is the median age at AML diagnosis in the United States?
Dr. Sekeres: About 67 years.
Q: What are the biological underpinnings for poor outcomes in older AML patients?
Dr. Sekeres: There are a few of them. Older adults with AML tend to have a leukemia that has evolved from a known or unknown previous bone marrow condition such as myelodysplastic syndrome. Older adults also have worse genetics driving their leukemia, which makes the leukemia cells more resistant to chemotherapy. And the leukemia cells may even have drug efflux pumps that extrude chemotherapy that tries to enter the cell. Finally, older adults are more likely to have comorbidities that make their ability to tolerate chemotherapy much lower than for younger adults.
Q: In someone who is newly diagnosed with AML, what initial options are they routinely given?
Dr. Sekeres: For someone who is older, we divide those options into three main categories.
The first is to take intensive chemotherapy, which requires a 4-6 week hospitalization and has a chance of getting somebody who is older into a remission of approximately 50%-60%. But this also carries with it significant treatment-related mortality that may be as high as 10%-20%. So I have to look my older patients in the eyes when I talk about intensive chemotherapy and say, “There is a 1 in 10 or 1 in 5 chance that you might not make it out of the hospital alive.”
The second prong is lower-dose therapy. While the more-intensive therapy requiring hospitalization does have a low, but real, chance of curing that person, less-intensive therapy is not curative. Our best hope with less-intensive therapy is that our patients enter a remission and live longer. With less-intensive therapy, the chance that someone will go into remission is probably around 20%, but again it is not curative. The flip side to that is that it improves a person’s immediate quality of life because they’re not in the hospital for 4-6 weeks.
The final prong is to discuss palliative care or hospice upfront. We designed these guidelines to be focused on a patient’s goals of therapy and to constantly revisit those goals to make sure that the treatment options we are offering are aligning with them.
Q: The panel’s first recommendation is to offer antileukemic therapy over best supportive care in patients who are appropriate candidates. Can you provide some context for this recommendation?
Dr. Sekeres: Doesn’t that strike you as funny that we even have to make a recommendation about getting chemotherapy? Some database studies conducted over the past 2 decades show that, as recently as 15 years ago, only one-third of patients who were over the age of 65 years received any type of chemotherapy for AML. More recently, as we have had a few more drugs available that allow us to use lower-dose approaches, that number has crept up to probably about 50%. We still have half the patients offered no therapy at all. So we felt that we had to deliberately make a recommendation saying that, if it aligns with the patients’ goals, they should be offered chemotherapy.
Q: The second recommendation is that patients considered candidates for intensive antileukemic therapy should receive it over less-intensive antileukemic therapy. How did you get to that recommendation?
Dr. Sekeres: There is a debate in our field about whether older adults should be offered intensive inpatient chemotherapy at all or whether we should be treating all of them with less-intensive therapy. There is not a huge amount of high-quality studies out there to answer some of these questions, in particular whether intensive chemotherapy should be recommended over less-intensive therapy. But with the available evidence, what we believe is that patients live longer if they are offered intensive antileukemic chemotherapy. So again, if it aligns with a patient’s goals, we support that patient receiving more-intensive therapy in the hospital.
Q: What does the panel recommend for patients who achieve remission after at least a single cycle of intensive antileukemic therapy and who are not candidates for allogeneic hematopoietic stem cell transplantation?
Dr. Sekeres: Once again, this may seem at first blush to be an obvious recommendation. The standard treatment of someone who is younger with AML is to offer intensive inpatient chemotherapy to induce remission. This is followed by a few cycles of chemotherapy, mostly in an outpatient setting, to consolidate that remission.
Q: What is the underlying philosophy for this approach?
Dr. Sekeres: Every time we give chemotherapy, we probably get about a 3-4 log kill of leukemia cells. Imagine when patients first present with AML, they may have 10 billion leukemia cells in their body. We are reducing that 3-4 log with the first course of chemotherapy.
When we then look at a bone marrow biopsy, it may appear to be normal. When leukemia is at a lower level in the body, we simply can’t see it using standard techniques. But that doesn’t mean the leukemia is gone. For younger patients, we give another cycle of chemotherapy, then another, then another, and then even another to reduce the number of leukemia cells left over in the body until that person has a durable remission and hopefully cure.
For someone who is older, the data are less clear. While some studies have shown that, if you give too much chemotherapy after the initial course, it doesn’t help that much, there is a paucity of studies that show that any chemotherapy at all after the first induction course is helpful. Consequently, we have to use indirect data. Older people who are long-term survivors from their acute leukemia always seem to have gotten more than one course of chemotherapy. In other words, the initial course of chemotherapy that a patient receives in the hospital isn’t enough. They should receive more than that.
Q: What about older adults with AML considered appropriate for antileukemic therapy but not for intensive antileukemic therapy?
Dr. Sekeres: This again gets to the question of what are a patient’s goals. It takes a very involved conversation with patients at the time of their AML diagnosis to determine whether they would want to pursue an aggressive approach or a less-aggressive approach. If a patients want a less-aggressive approach, and want nothing to do with a hospital stay, then they are also prioritizing initial quality of life. In this recommendation, based on existing studies, we didn’t have a preference for which of the available less-aggressive chemotherapies a person selects.
There’s also debate about what to do in those considered appropriate for antileukemic therapy, such as hypomethylating agents (azacitidine and decitabine) or low-dose cytarabine, but not for intensive antileukemic therapy. What did the available evidence seem to indicate about this issue?
There has been a lot of studies trying to add two drugs together to see if those do better than one drug alone in patients who are older and who choose less-intensive therapy. The majority of those studies have shown no advantage to getting two drugs over one drug.
Our recommendation is that in these situations a patient gets one drug, not two, but there are a couple of caveats. One caveat is that there has been a small study showing the effectiveness of one of those low-dose chemotherapies combined with the drug glasdegib. The second caveat is that there have been results presented combining one of these low-dose chemotherapies with the drug venetoclax. One of those was a negative study, and another was a positive study showing a survival advantage to the combination vs. the low-dose therapy alone. We had to couch our recommendation a little bit because we knew this other study had been presented at a conference, but it hadn’t come out in final form yet. It did recently, however, and we will now revisit this recommendation.
The other complicated aspect to this is that we weren’t 100% convinced that the combination of venetoclax with one of these lower-dose therapies is truly less-intensive therapy. We think it is starting to creep up toward more-intensive chemotherapy, even though it is commonly given to patients in the outpatient setting. It gets into the very complicated area of what are we defining as more-intensive therapy and less-intensive therapy.
Q: Is there a recommended strategy for older adults with AML who achieve a response after receiving less-intensive therapy?
Dr. Sekeres: This is also challenging because there are no randomized studies in which patients received less-intensive therapy for a finite period of time vs. receiving those therapies ad infinitum. Given the lack of data and also given a lot of anecdotal data out there about patients who stopped a certain therapy and relapsed thereafter, we recommended that patients continue the less-intensive therapy ad infinitum. So as long as they are receiving a response to that therapy, they continue on the drug.
Q: Of course, there are also unique considerations faced by older patients who are no longer receiving antileukemic therapy and have moved on to receiving end-of-life care or hospice care. What advice do the guidelines offer in this situation?
Dr. Sekeres: There are a lot of aspects of these recommendations that I think are special. The first is the focus on patient goals of care at every point in these guidelines. The second is that the guidelines follow the real disease course and a real conversation that doctors and patients have at every step of the way to help guide the decisions that have to be made in real time.
A problem we have in the United States is that once patients enter a hospice, most will not allow blood transfusions. One reason is that some say it is antithetical to their philosophy and consider it aggressive care. The second reason is that, to be completely blunt, economically it doesn’t make sense for hospices to allow blood transfusions. The amount that they are reimbursed by Medicare is much lower than the cost of receiving blood in an infusion center.
We wanted to make a clear recommendation that we consider transfusions in a patient who is in a palliative care or hospice mode to be supportive and necessary, and that these should be provided to patients even if they are in hospice and, as always, if consistent with a patient’s goals of care.
Q: How does a patient’s age inform the discussion surrounding what intensity treatment to offer?
Dr. Sekeres: With younger adults, this is not as complicated a conversation. A younger person has a better chance of being cured with intensive chemotherapy and is much more likely to tolerate that intensive chemotherapy. For someone who is younger, we offer intensive chemotherapy and the chance of going into remission is higher, at 70%-80%. The chance of dying is lower, usually less than 5%. It is an easy decision to make.
For an older adult, the risk-benefit ratio shifts and it becomes a more complicated option. Less-intensive therapy or best supportive care or hospice become viable.
Q: Are there other factors confounding the treatment decision-making process in older adults with AML that practitioners should consider?
Dr. Sekeres: Someone who is older is making a different decision than I would. I have school-aged children and believe that my job as a parent is to successfully get them to adulthood, so I would take any treatment under the sun to make sure that happens. People who have lived a longer life than I have may have children and even grandchildren who are adults, and they might have different goals of care. My goals are not going to be the same as my patient’s goals.
It is also harder because patients who are older may feel that they have lived a good life and don’t need to go through heroic measures to try to be around as long as possible, and those goals may not align with the goals of that person’s children who want their parent to be around as long as possible. One of the confounding factors in this is navigating the different goals of the different family members.
Dr. Sekeres has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A diagnosis of acute myeloid leukemia (AML) is particularly challenging in older adults, whose age makes them highly susceptible to the disease and treatment-related toxicity. To help patients and practitioners navigate the clinical decision-making process, the American Society of Hematology convened a panel of experts who conducted a thorough review of the literature. The result of their work can be found in a new set of guidelines for the treatment of newly diagnosed AML in older adults.
In an interview, Mikkael Sekeres, MD, chair of the ASH AML guideline panel and director of the Leukemia Program at Cleveland Clinic Taussig Cancer Institute in Cleveland, Ohio, shared the rationale behind the panel’s key recommendations and the importance of keeping the patient’s goals in mind.
Question: What is the average life expectancy of a 75-year-old developing AML compared with someone of the same age without AML?
Dr. Sekeres: A 75-year-old developing AML has an average life expectancy measured in fewer than 6 months. Somebody who is 75 without leukemia in the United States has a life expectancy that can be measured in a decade or more. AML is a really serious diagnosis when someone is older and significantly truncates expected survival.
Q: What is the median age at AML diagnosis in the United States?
Dr. Sekeres: About 67 years.
Q: What are the biological underpinnings for poor outcomes in older AML patients?
Dr. Sekeres: There are a few of them. Older adults with AML tend to have a leukemia that has evolved from a known or unknown previous bone marrow condition such as myelodysplastic syndrome. Older adults also have worse genetics driving their leukemia, which makes the leukemia cells more resistant to chemotherapy. And the leukemia cells may even have drug efflux pumps that extrude chemotherapy that tries to enter the cell. Finally, older adults are more likely to have comorbidities that make their ability to tolerate chemotherapy much lower than for younger adults.
Q: In someone who is newly diagnosed with AML, what initial options are they routinely given?
Dr. Sekeres: For someone who is older, we divide those options into three main categories.
The first is to take intensive chemotherapy, which requires a 4-6 week hospitalization and has a chance of getting somebody who is older into a remission of approximately 50%-60%. But this also carries with it significant treatment-related mortality that may be as high as 10%-20%. So I have to look my older patients in the eyes when I talk about intensive chemotherapy and say, “There is a 1 in 10 or 1 in 5 chance that you might not make it out of the hospital alive.”
The second prong is lower-dose therapy. While the more-intensive therapy requiring hospitalization does have a low, but real, chance of curing that person, less-intensive therapy is not curative. Our best hope with less-intensive therapy is that our patients enter a remission and live longer. With less-intensive therapy, the chance that someone will go into remission is probably around 20%, but again it is not curative. The flip side to that is that it improves a person’s immediate quality of life because they’re not in the hospital for 4-6 weeks.
The final prong is to discuss palliative care or hospice upfront. We designed these guidelines to be focused on a patient’s goals of therapy and to constantly revisit those goals to make sure that the treatment options we are offering are aligning with them.
Q: The panel’s first recommendation is to offer antileukemic therapy over best supportive care in patients who are appropriate candidates. Can you provide some context for this recommendation?
Dr. Sekeres: Doesn’t that strike you as funny that we even have to make a recommendation about getting chemotherapy? Some database studies conducted over the past 2 decades show that, as recently as 15 years ago, only one-third of patients who were over the age of 65 years received any type of chemotherapy for AML. More recently, as we have had a few more drugs available that allow us to use lower-dose approaches, that number has crept up to probably about 50%. We still have half the patients offered no therapy at all. So we felt that we had to deliberately make a recommendation saying that, if it aligns with the patients’ goals, they should be offered chemotherapy.
Q: The second recommendation is that patients considered candidates for intensive antileukemic therapy should receive it over less-intensive antileukemic therapy. How did you get to that recommendation?
Dr. Sekeres: There is a debate in our field about whether older adults should be offered intensive inpatient chemotherapy at all or whether we should be treating all of them with less-intensive therapy. There is not a huge amount of high-quality studies out there to answer some of these questions, in particular whether intensive chemotherapy should be recommended over less-intensive therapy. But with the available evidence, what we believe is that patients live longer if they are offered intensive antileukemic chemotherapy. So again, if it aligns with a patient’s goals, we support that patient receiving more-intensive therapy in the hospital.
Q: What does the panel recommend for patients who achieve remission after at least a single cycle of intensive antileukemic therapy and who are not candidates for allogeneic hematopoietic stem cell transplantation?
Dr. Sekeres: Once again, this may seem at first blush to be an obvious recommendation. The standard treatment of someone who is younger with AML is to offer intensive inpatient chemotherapy to induce remission. This is followed by a few cycles of chemotherapy, mostly in an outpatient setting, to consolidate that remission.
Q: What is the underlying philosophy for this approach?
Dr. Sekeres: Every time we give chemotherapy, we probably get about a 3-4 log kill of leukemia cells. Imagine when patients first present with AML, they may have 10 billion leukemia cells in their body. We are reducing that 3-4 log with the first course of chemotherapy.
When we then look at a bone marrow biopsy, it may appear to be normal. When leukemia is at a lower level in the body, we simply can’t see it using standard techniques. But that doesn’t mean the leukemia is gone. For younger patients, we give another cycle of chemotherapy, then another, then another, and then even another to reduce the number of leukemia cells left over in the body until that person has a durable remission and hopefully cure.
For someone who is older, the data are less clear. While some studies have shown that, if you give too much chemotherapy after the initial course, it doesn’t help that much, there is a paucity of studies that show that any chemotherapy at all after the first induction course is helpful. Consequently, we have to use indirect data. Older people who are long-term survivors from their acute leukemia always seem to have gotten more than one course of chemotherapy. In other words, the initial course of chemotherapy that a patient receives in the hospital isn’t enough. They should receive more than that.
Q: What about older adults with AML considered appropriate for antileukemic therapy but not for intensive antileukemic therapy?
Dr. Sekeres: This again gets to the question of what are a patient’s goals. It takes a very involved conversation with patients at the time of their AML diagnosis to determine whether they would want to pursue an aggressive approach or a less-aggressive approach. If a patients want a less-aggressive approach, and want nothing to do with a hospital stay, then they are also prioritizing initial quality of life. In this recommendation, based on existing studies, we didn’t have a preference for which of the available less-aggressive chemotherapies a person selects.
There’s also debate about what to do in those considered appropriate for antileukemic therapy, such as hypomethylating agents (azacitidine and decitabine) or low-dose cytarabine, but not for intensive antileukemic therapy. What did the available evidence seem to indicate about this issue?
There has been a lot of studies trying to add two drugs together to see if those do better than one drug alone in patients who are older and who choose less-intensive therapy. The majority of those studies have shown no advantage to getting two drugs over one drug.
Our recommendation is that in these situations a patient gets one drug, not two, but there are a couple of caveats. One caveat is that there has been a small study showing the effectiveness of one of those low-dose chemotherapies combined with the drug glasdegib. The second caveat is that there have been results presented combining one of these low-dose chemotherapies with the drug venetoclax. One of those was a negative study, and another was a positive study showing a survival advantage to the combination vs. the low-dose therapy alone. We had to couch our recommendation a little bit because we knew this other study had been presented at a conference, but it hadn’t come out in final form yet. It did recently, however, and we will now revisit this recommendation.
The other complicated aspect to this is that we weren’t 100% convinced that the combination of venetoclax with one of these lower-dose therapies is truly less-intensive therapy. We think it is starting to creep up toward more-intensive chemotherapy, even though it is commonly given to patients in the outpatient setting. It gets into the very complicated area of what are we defining as more-intensive therapy and less-intensive therapy.
Q: Is there a recommended strategy for older adults with AML who achieve a response after receiving less-intensive therapy?
Dr. Sekeres: This is also challenging because there are no randomized studies in which patients received less-intensive therapy for a finite period of time vs. receiving those therapies ad infinitum. Given the lack of data and also given a lot of anecdotal data out there about patients who stopped a certain therapy and relapsed thereafter, we recommended that patients continue the less-intensive therapy ad infinitum. So as long as they are receiving a response to that therapy, they continue on the drug.
Q: Of course, there are also unique considerations faced by older patients who are no longer receiving antileukemic therapy and have moved on to receiving end-of-life care or hospice care. What advice do the guidelines offer in this situation?
Dr. Sekeres: There are a lot of aspects of these recommendations that I think are special. The first is the focus on patient goals of care at every point in these guidelines. The second is that the guidelines follow the real disease course and a real conversation that doctors and patients have at every step of the way to help guide the decisions that have to be made in real time.
A problem we have in the United States is that once patients enter a hospice, most will not allow blood transfusions. One reason is that some say it is antithetical to their philosophy and consider it aggressive care. The second reason is that, to be completely blunt, economically it doesn’t make sense for hospices to allow blood transfusions. The amount that they are reimbursed by Medicare is much lower than the cost of receiving blood in an infusion center.
We wanted to make a clear recommendation that we consider transfusions in a patient who is in a palliative care or hospice mode to be supportive and necessary, and that these should be provided to patients even if they are in hospice and, as always, if consistent with a patient’s goals of care.
Q: How does a patient’s age inform the discussion surrounding what intensity treatment to offer?
Dr. Sekeres: With younger adults, this is not as complicated a conversation. A younger person has a better chance of being cured with intensive chemotherapy and is much more likely to tolerate that intensive chemotherapy. For someone who is younger, we offer intensive chemotherapy and the chance of going into remission is higher, at 70%-80%. The chance of dying is lower, usually less than 5%. It is an easy decision to make.
For an older adult, the risk-benefit ratio shifts and it becomes a more complicated option. Less-intensive therapy or best supportive care or hospice become viable.
Q: Are there other factors confounding the treatment decision-making process in older adults with AML that practitioners should consider?
Dr. Sekeres: Someone who is older is making a different decision than I would. I have school-aged children and believe that my job as a parent is to successfully get them to adulthood, so I would take any treatment under the sun to make sure that happens. People who have lived a longer life than I have may have children and even grandchildren who are adults, and they might have different goals of care. My goals are not going to be the same as my patient’s goals.
It is also harder because patients who are older may feel that they have lived a good life and don’t need to go through heroic measures to try to be around as long as possible, and those goals may not align with the goals of that person’s children who want their parent to be around as long as possible. One of the confounding factors in this is navigating the different goals of the different family members.
Dr. Sekeres has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A diagnosis of acute myeloid leukemia (AML) is particularly challenging in older adults, whose age makes them highly susceptible to the disease and treatment-related toxicity. To help patients and practitioners navigate the clinical decision-making process, the American Society of Hematology convened a panel of experts who conducted a thorough review of the literature. The result of their work can be found in a new set of guidelines for the treatment of newly diagnosed AML in older adults.
In an interview, Mikkael Sekeres, MD, chair of the ASH AML guideline panel and director of the Leukemia Program at Cleveland Clinic Taussig Cancer Institute in Cleveland, Ohio, shared the rationale behind the panel’s key recommendations and the importance of keeping the patient’s goals in mind.
Question: What is the average life expectancy of a 75-year-old developing AML compared with someone of the same age without AML?
Dr. Sekeres: A 75-year-old developing AML has an average life expectancy measured in fewer than 6 months. Somebody who is 75 without leukemia in the United States has a life expectancy that can be measured in a decade or more. AML is a really serious diagnosis when someone is older and significantly truncates expected survival.
Q: What is the median age at AML diagnosis in the United States?
Dr. Sekeres: About 67 years.
Q: What are the biological underpinnings for poor outcomes in older AML patients?
Dr. Sekeres: There are a few of them. Older adults with AML tend to have a leukemia that has evolved from a known or unknown previous bone marrow condition such as myelodysplastic syndrome. Older adults also have worse genetics driving their leukemia, which makes the leukemia cells more resistant to chemotherapy. And the leukemia cells may even have drug efflux pumps that extrude chemotherapy that tries to enter the cell. Finally, older adults are more likely to have comorbidities that make their ability to tolerate chemotherapy much lower than for younger adults.
Q: In someone who is newly diagnosed with AML, what initial options are they routinely given?
Dr. Sekeres: For someone who is older, we divide those options into three main categories.
The first is to take intensive chemotherapy, which requires a 4-6 week hospitalization and has a chance of getting somebody who is older into a remission of approximately 50%-60%. But this also carries with it significant treatment-related mortality that may be as high as 10%-20%. So I have to look my older patients in the eyes when I talk about intensive chemotherapy and say, “There is a 1 in 10 or 1 in 5 chance that you might not make it out of the hospital alive.”
The second prong is lower-dose therapy. While the more-intensive therapy requiring hospitalization does have a low, but real, chance of curing that person, less-intensive therapy is not curative. Our best hope with less-intensive therapy is that our patients enter a remission and live longer. With less-intensive therapy, the chance that someone will go into remission is probably around 20%, but again it is not curative. The flip side to that is that it improves a person’s immediate quality of life because they’re not in the hospital for 4-6 weeks.
The final prong is to discuss palliative care or hospice upfront. We designed these guidelines to be focused on a patient’s goals of therapy and to constantly revisit those goals to make sure that the treatment options we are offering are aligning with them.
Q: The panel’s first recommendation is to offer antileukemic therapy over best supportive care in patients who are appropriate candidates. Can you provide some context for this recommendation?
Dr. Sekeres: Doesn’t that strike you as funny that we even have to make a recommendation about getting chemotherapy? Some database studies conducted over the past 2 decades show that, as recently as 15 years ago, only one-third of patients who were over the age of 65 years received any type of chemotherapy for AML. More recently, as we have had a few more drugs available that allow us to use lower-dose approaches, that number has crept up to probably about 50%. We still have half the patients offered no therapy at all. So we felt that we had to deliberately make a recommendation saying that, if it aligns with the patients’ goals, they should be offered chemotherapy.
Q: The second recommendation is that patients considered candidates for intensive antileukemic therapy should receive it over less-intensive antileukemic therapy. How did you get to that recommendation?
Dr. Sekeres: There is a debate in our field about whether older adults should be offered intensive inpatient chemotherapy at all or whether we should be treating all of them with less-intensive therapy. There is not a huge amount of high-quality studies out there to answer some of these questions, in particular whether intensive chemotherapy should be recommended over less-intensive therapy. But with the available evidence, what we believe is that patients live longer if they are offered intensive antileukemic chemotherapy. So again, if it aligns with a patient’s goals, we support that patient receiving more-intensive therapy in the hospital.
Q: What does the panel recommend for patients who achieve remission after at least a single cycle of intensive antileukemic therapy and who are not candidates for allogeneic hematopoietic stem cell transplantation?
Dr. Sekeres: Once again, this may seem at first blush to be an obvious recommendation. The standard treatment of someone who is younger with AML is to offer intensive inpatient chemotherapy to induce remission. This is followed by a few cycles of chemotherapy, mostly in an outpatient setting, to consolidate that remission.
Q: What is the underlying philosophy for this approach?
Dr. Sekeres: Every time we give chemotherapy, we probably get about a 3-4 log kill of leukemia cells. Imagine when patients first present with AML, they may have 10 billion leukemia cells in their body. We are reducing that 3-4 log with the first course of chemotherapy.
When we then look at a bone marrow biopsy, it may appear to be normal. When leukemia is at a lower level in the body, we simply can’t see it using standard techniques. But that doesn’t mean the leukemia is gone. For younger patients, we give another cycle of chemotherapy, then another, then another, and then even another to reduce the number of leukemia cells left over in the body until that person has a durable remission and hopefully cure.
For someone who is older, the data are less clear. While some studies have shown that, if you give too much chemotherapy after the initial course, it doesn’t help that much, there is a paucity of studies that show that any chemotherapy at all after the first induction course is helpful. Consequently, we have to use indirect data. Older people who are long-term survivors from their acute leukemia always seem to have gotten more than one course of chemotherapy. In other words, the initial course of chemotherapy that a patient receives in the hospital isn’t enough. They should receive more than that.
Q: What about older adults with AML considered appropriate for antileukemic therapy but not for intensive antileukemic therapy?
Dr. Sekeres: This again gets to the question of what are a patient’s goals. It takes a very involved conversation with patients at the time of their AML diagnosis to determine whether they would want to pursue an aggressive approach or a less-aggressive approach. If a patients want a less-aggressive approach, and want nothing to do with a hospital stay, then they are also prioritizing initial quality of life. In this recommendation, based on existing studies, we didn’t have a preference for which of the available less-aggressive chemotherapies a person selects.
There’s also debate about what to do in those considered appropriate for antileukemic therapy, such as hypomethylating agents (azacitidine and decitabine) or low-dose cytarabine, but not for intensive antileukemic therapy. What did the available evidence seem to indicate about this issue?
There has been a lot of studies trying to add two drugs together to see if those do better than one drug alone in patients who are older and who choose less-intensive therapy. The majority of those studies have shown no advantage to getting two drugs over one drug.
Our recommendation is that in these situations a patient gets one drug, not two, but there are a couple of caveats. One caveat is that there has been a small study showing the effectiveness of one of those low-dose chemotherapies combined with the drug glasdegib. The second caveat is that there have been results presented combining one of these low-dose chemotherapies with the drug venetoclax. One of those was a negative study, and another was a positive study showing a survival advantage to the combination vs. the low-dose therapy alone. We had to couch our recommendation a little bit because we knew this other study had been presented at a conference, but it hadn’t come out in final form yet. It did recently, however, and we will now revisit this recommendation.
The other complicated aspect to this is that we weren’t 100% convinced that the combination of venetoclax with one of these lower-dose therapies is truly less-intensive therapy. We think it is starting to creep up toward more-intensive chemotherapy, even though it is commonly given to patients in the outpatient setting. It gets into the very complicated area of what are we defining as more-intensive therapy and less-intensive therapy.
Q: Is there a recommended strategy for older adults with AML who achieve a response after receiving less-intensive therapy?
Dr. Sekeres: This is also challenging because there are no randomized studies in which patients received less-intensive therapy for a finite period of time vs. receiving those therapies ad infinitum. Given the lack of data and also given a lot of anecdotal data out there about patients who stopped a certain therapy and relapsed thereafter, we recommended that patients continue the less-intensive therapy ad infinitum. So as long as they are receiving a response to that therapy, they continue on the drug.
Q: Of course, there are also unique considerations faced by older patients who are no longer receiving antileukemic therapy and have moved on to receiving end-of-life care or hospice care. What advice do the guidelines offer in this situation?
Dr. Sekeres: There are a lot of aspects of these recommendations that I think are special. The first is the focus on patient goals of care at every point in these guidelines. The second is that the guidelines follow the real disease course and a real conversation that doctors and patients have at every step of the way to help guide the decisions that have to be made in real time.
A problem we have in the United States is that once patients enter a hospice, most will not allow blood transfusions. One reason is that some say it is antithetical to their philosophy and consider it aggressive care. The second reason is that, to be completely blunt, economically it doesn’t make sense for hospices to allow blood transfusions. The amount that they are reimbursed by Medicare is much lower than the cost of receiving blood in an infusion center.
We wanted to make a clear recommendation that we consider transfusions in a patient who is in a palliative care or hospice mode to be supportive and necessary, and that these should be provided to patients even if they are in hospice and, as always, if consistent with a patient’s goals of care.
Q: How does a patient’s age inform the discussion surrounding what intensity treatment to offer?
Dr. Sekeres: With younger adults, this is not as complicated a conversation. A younger person has a better chance of being cured with intensive chemotherapy and is much more likely to tolerate that intensive chemotherapy. For someone who is younger, we offer intensive chemotherapy and the chance of going into remission is higher, at 70%-80%. The chance of dying is lower, usually less than 5%. It is an easy decision to make.
For an older adult, the risk-benefit ratio shifts and it becomes a more complicated option. Less-intensive therapy or best supportive care or hospice become viable.
Q: Are there other factors confounding the treatment decision-making process in older adults with AML that practitioners should consider?
Dr. Sekeres: Someone who is older is making a different decision than I would. I have school-aged children and believe that my job as a parent is to successfully get them to adulthood, so I would take any treatment under the sun to make sure that happens. People who have lived a longer life than I have may have children and even grandchildren who are adults, and they might have different goals of care. My goals are not going to be the same as my patient’s goals.
It is also harder because patients who are older may feel that they have lived a good life and don’t need to go through heroic measures to try to be around as long as possible, and those goals may not align with the goals of that person’s children who want their parent to be around as long as possible. One of the confounding factors in this is navigating the different goals of the different family members.
Dr. Sekeres has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
2020 Update on contraception
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.
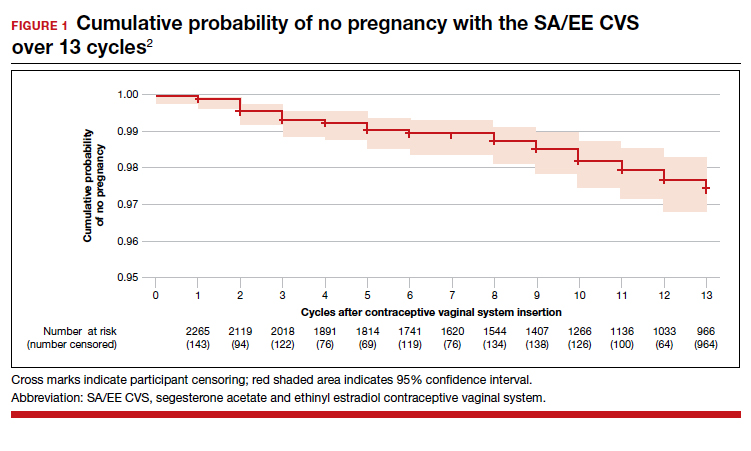
There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.
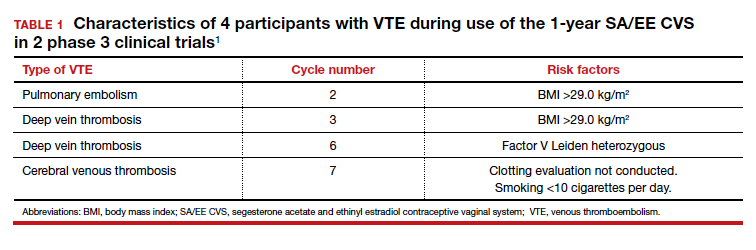
The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.
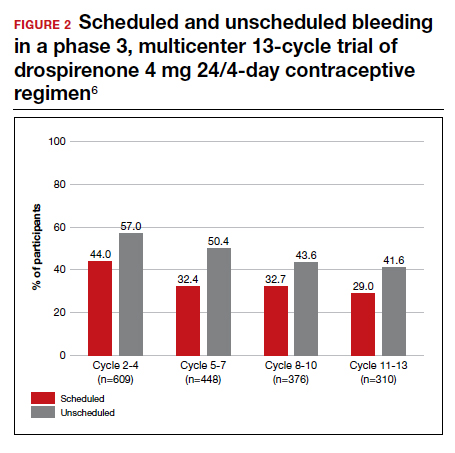
European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.
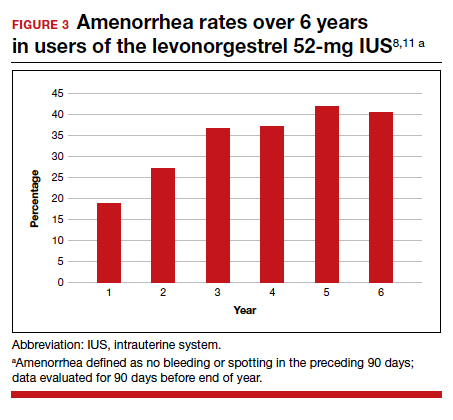
Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.

There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.

The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.

European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.

Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.

There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.

The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.

European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.

Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
November 2020 – ICYMI
Gastroenterology
July 2020
Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Jonathan Gotfried et al. 2020 July;159(1):62-80. doi: 10.1053/j.gastro.2020.03.087
Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Heiko Pohl et al. 2020 July;159(1):119-28.e2. doi: 10.1053/j.gastro.2020.03.014
Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets.
Yu Tian et al. July 2020;159(1):159-168.e3. doi: 10.1053/j.gastro.2020.03.063
August 2020
Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Erica J. Brenner et al. 2020 Aug 159;(2):481-91.e3. doi: 10.1053/j.gastro.2020.05.032
Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Eli Stahl et al. 2020 Aug;159(2):549-61.e8. doi: 10.1053/j.gastro.2020.04.063
Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Alessandro Repici et al. 2020 Aug;159(2):512-20.e7. doi: 10.1053/j.gastro.2020.04.062
September 2020
Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Chun-Han Lo et al. 2020 Sept;159(3):p873-83.e1. doi: 10.1053/j.gastro.2020.05.011
Rates of incomplete resection of 1- to 20-mm colorectal polyps: A systematic review and meta-analysis. Roupen Djinbachian et al. 2020 Sept;159(3):904-14.e12. doi: 10.1053/j.gastro.2020.05.018
Clinical Gastroenterology and Hepatology
August 2020
Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Helen Burton Murray et al. 2020 Aug;18(9):1995-2002.e1. doi: 10.1016/j.cgh.2019.10.030
Ten things every gastroenterologist should know about antireflux surgery. Steven Park et al. 2020 Aug;18(9):1923-9. doi: 10.1016/j.cgh.2020.02.041
Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Boris Virine et al. 2020 Aug;18(9):2003-9. doi: 10.1016/j.cgh.2020.02.036
September 2020
Association between endoscopist annual procedure volume and colonoscopy quality: Systematic review and meta-analysis. Nauzer Forbes et al. 2020 Sept:18(10):2192-208.e12. doi: 10.1016/j.cgh.2020.03.046
Plans to reactivate gastroenterology practices following the COVID-19 pandemic: A survey of North American centers. Vladimir M. Kushnir et al on Behalf of the North American Alliance for the Study of Digestive Manifestations of COVID-19. 2020 Sept;18(10):2287-94.e1. doi: 10.1016/j.cgh.2020.05.030
Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Eduardo Vilar-Gomez et al. 2020 Sept;18(10):2305-14.e12. doi: 10.1016/j.cgh.2020.04.017
October 2020
AGA Clinical Practice Update on young adult–onset colorectal cancer diagnosis and management: Expert review. Lisa A. Boardman et al. 2020 Oct:18(11):2415-24. doi: 10.1016/j.cgh.2020.05.058
Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Helen Burton Murray et al. 2020 Oct;18(11):2471-8. doi: 10.1016/j.cgh.2019.12.030
Correction of dyssynergic defecation, but not fiber supplementation, reduces symptoms of functional dyspepsia in patients with constipation in a randomized trial. Jose-Walter Huaman et al. 2020 Oct;18(11):2463-70.e1. doi: 10.1016/j.cgh.2019.11.048
Cellular and Molecular Gastroenterology and Hepatology
A new treatment for chronic hepatitis B and D offers novel insights into obesity and hepatic steatosis. Robert Schierwagen et al. 2020;10(3):649-51. doi: 10.1016/j.jcmgh.2020.05.011
Techniques and Innovations in Gastrointestinal Endoscopy
The impact of endoscopic submucosal dissection for gastric adenocarcinomas in the United States. Shria Kumar et al. 2020 July:22(3):93-8. doi: 10.1016/j.tige.2020.03.009
Gastroenterology
July 2020
Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Jonathan Gotfried et al. 2020 July;159(1):62-80. doi: 10.1053/j.gastro.2020.03.087
Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Heiko Pohl et al. 2020 July;159(1):119-28.e2. doi: 10.1053/j.gastro.2020.03.014
Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets.
Yu Tian et al. July 2020;159(1):159-168.e3. doi: 10.1053/j.gastro.2020.03.063
August 2020
Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Erica J. Brenner et al. 2020 Aug 159;(2):481-91.e3. doi: 10.1053/j.gastro.2020.05.032
Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Eli Stahl et al. 2020 Aug;159(2):549-61.e8. doi: 10.1053/j.gastro.2020.04.063
Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Alessandro Repici et al. 2020 Aug;159(2):512-20.e7. doi: 10.1053/j.gastro.2020.04.062
September 2020
Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Chun-Han Lo et al. 2020 Sept;159(3):p873-83.e1. doi: 10.1053/j.gastro.2020.05.011
Rates of incomplete resection of 1- to 20-mm colorectal polyps: A systematic review and meta-analysis. Roupen Djinbachian et al. 2020 Sept;159(3):904-14.e12. doi: 10.1053/j.gastro.2020.05.018
Clinical Gastroenterology and Hepatology
August 2020
Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Helen Burton Murray et al. 2020 Aug;18(9):1995-2002.e1. doi: 10.1016/j.cgh.2019.10.030
Ten things every gastroenterologist should know about antireflux surgery. Steven Park et al. 2020 Aug;18(9):1923-9. doi: 10.1016/j.cgh.2020.02.041
Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Boris Virine et al. 2020 Aug;18(9):2003-9. doi: 10.1016/j.cgh.2020.02.036
September 2020
Association between endoscopist annual procedure volume and colonoscopy quality: Systematic review and meta-analysis. Nauzer Forbes et al. 2020 Sept:18(10):2192-208.e12. doi: 10.1016/j.cgh.2020.03.046
Plans to reactivate gastroenterology practices following the COVID-19 pandemic: A survey of North American centers. Vladimir M. Kushnir et al on Behalf of the North American Alliance for the Study of Digestive Manifestations of COVID-19. 2020 Sept;18(10):2287-94.e1. doi: 10.1016/j.cgh.2020.05.030
Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Eduardo Vilar-Gomez et al. 2020 Sept;18(10):2305-14.e12. doi: 10.1016/j.cgh.2020.04.017
October 2020
AGA Clinical Practice Update on young adult–onset colorectal cancer diagnosis and management: Expert review. Lisa A. Boardman et al. 2020 Oct:18(11):2415-24. doi: 10.1016/j.cgh.2020.05.058
Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Helen Burton Murray et al. 2020 Oct;18(11):2471-8. doi: 10.1016/j.cgh.2019.12.030
Correction of dyssynergic defecation, but not fiber supplementation, reduces symptoms of functional dyspepsia in patients with constipation in a randomized trial. Jose-Walter Huaman et al. 2020 Oct;18(11):2463-70.e1. doi: 10.1016/j.cgh.2019.11.048
Cellular and Molecular Gastroenterology and Hepatology
A new treatment for chronic hepatitis B and D offers novel insights into obesity and hepatic steatosis. Robert Schierwagen et al. 2020;10(3):649-51. doi: 10.1016/j.jcmgh.2020.05.011
Techniques and Innovations in Gastrointestinal Endoscopy
The impact of endoscopic submucosal dissection for gastric adenocarcinomas in the United States. Shria Kumar et al. 2020 July:22(3):93-8. doi: 10.1016/j.tige.2020.03.009
Gastroenterology
July 2020
Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Jonathan Gotfried et al. 2020 July;159(1):62-80. doi: 10.1053/j.gastro.2020.03.087
Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Heiko Pohl et al. 2020 July;159(1):119-28.e2. doi: 10.1053/j.gastro.2020.03.014
Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets.
Yu Tian et al. July 2020;159(1):159-168.e3. doi: 10.1053/j.gastro.2020.03.063
August 2020
Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Erica J. Brenner et al. 2020 Aug 159;(2):481-91.e3. doi: 10.1053/j.gastro.2020.05.032
Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Eli Stahl et al. 2020 Aug;159(2):549-61.e8. doi: 10.1053/j.gastro.2020.04.063
Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Alessandro Repici et al. 2020 Aug;159(2):512-20.e7. doi: 10.1053/j.gastro.2020.04.062
September 2020
Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Chun-Han Lo et al. 2020 Sept;159(3):p873-83.e1. doi: 10.1053/j.gastro.2020.05.011
Rates of incomplete resection of 1- to 20-mm colorectal polyps: A systematic review and meta-analysis. Roupen Djinbachian et al. 2020 Sept;159(3):904-14.e12. doi: 10.1053/j.gastro.2020.05.018
Clinical Gastroenterology and Hepatology
August 2020
Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Helen Burton Murray et al. 2020 Aug;18(9):1995-2002.e1. doi: 10.1016/j.cgh.2019.10.030
Ten things every gastroenterologist should know about antireflux surgery. Steven Park et al. 2020 Aug;18(9):1923-9. doi: 10.1016/j.cgh.2020.02.041
Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Boris Virine et al. 2020 Aug;18(9):2003-9. doi: 10.1016/j.cgh.2020.02.036
September 2020
Association between endoscopist annual procedure volume and colonoscopy quality: Systematic review and meta-analysis. Nauzer Forbes et al. 2020 Sept:18(10):2192-208.e12. doi: 10.1016/j.cgh.2020.03.046
Plans to reactivate gastroenterology practices following the COVID-19 pandemic: A survey of North American centers. Vladimir M. Kushnir et al on Behalf of the North American Alliance for the Study of Digestive Manifestations of COVID-19. 2020 Sept;18(10):2287-94.e1. doi: 10.1016/j.cgh.2020.05.030
Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Eduardo Vilar-Gomez et al. 2020 Sept;18(10):2305-14.e12. doi: 10.1016/j.cgh.2020.04.017
October 2020
AGA Clinical Practice Update on young adult–onset colorectal cancer diagnosis and management: Expert review. Lisa A. Boardman et al. 2020 Oct:18(11):2415-24. doi: 10.1016/j.cgh.2020.05.058
Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Helen Burton Murray et al. 2020 Oct;18(11):2471-8. doi: 10.1016/j.cgh.2019.12.030
Correction of dyssynergic defecation, but not fiber supplementation, reduces symptoms of functional dyspepsia in patients with constipation in a randomized trial. Jose-Walter Huaman et al. 2020 Oct;18(11):2463-70.e1. doi: 10.1016/j.cgh.2019.11.048
Cellular and Molecular Gastroenterology and Hepatology
A new treatment for chronic hepatitis B and D offers novel insights into obesity and hepatic steatosis. Robert Schierwagen et al. 2020;10(3):649-51. doi: 10.1016/j.jcmgh.2020.05.011
Techniques and Innovations in Gastrointestinal Endoscopy
The impact of endoscopic submucosal dissection for gastric adenocarcinomas in the United States. Shria Kumar et al. 2020 July:22(3):93-8. doi: 10.1016/j.tige.2020.03.009
Flu vaccine significantly cuts pediatric hospitalizations
Unlike previous studies focused on vaccine effectiveness (VE) in ambulatory care office visits, Angela P. Campbell, MD, MPH, and associates have uncovered evidence of the overall benefit influenza vaccines play in reducing hospitalizations and emergency department visits in pediatric influenza patients.
“Our data provide important VE estimates against severe influenza in children,” the researchers noted in Pediatrics, adding that the findings “provide important evidence supporting the annual recommendation that all children 6 months and older should receive influenza vaccination.”
Dr. Campbell and colleagues collected ongoing surveillance data from the New Vaccine Surveillance Network (NVSN), which is a network of pediatric hospitals across seven cities, including Kansas City, Mo.; Rochester, N.Y.; Cincinnati; Pittsburgh; Nashville, Tenn.; Houston; and Seattle. The influenza season encompassed the period Nov. 7, 2018 to June 21, 2019.
A total of 2,748 hospitalized children and 2,676 children who had completed ED visits that did not lead to hospitalization were included. Once those under 6 months were excluded, 1,792 hospitalized children were included in the VE analysis; of these, 226 (13%) tested positive for influenza infection, including 211 (93%) with influenza A viruses and 15 (7%) with influenza B viruses. Fully 1,611 of the patients (90%), had verified vaccine status, while 181 (10%) had solely parental reported vaccine status. The researchers reported 88 (5%) of the patients received mechanical ventilation and 7 (<1%) died.
Most noteworthy, They further estimated a significant reduction in hospitalizations linked to A(H3N2) and A(H1N1)pdm09 viruses, even in the presence of circulating A(H3N2) viruses that differed from the A(H3N2) vaccine component.
Studies from other countries during the same time period showed that while “significant protection against influenza-associated ambulatory care visits and hospitalizations among children infected with A(H1N1)pdm09 viruses” was observed, the same could not be said for protection against A(H3N2) viruses, which varied among pediatric outpatients in the United States (24%), in England (17% outpatient; 31% inpatient), Europe (46%), and Canada (48%). They explained that such variation in vaccine protection is multifactorial, and includes virus-, host-, and environment-related factors. They also noted that regional variations in circulating viruses, host factors including age, imprinting, and previous vaccination could explain the study’s finding of vaccine protection against both A(H1N1)pdm09 and A(H3N2) viruses.
When comparing VE estimates between ED visits and hospitalizations, the researchers observed one significant difference, that “hospitalized children likely represent more medically complex patients, with 58% having underlying medical conditions and 38% reporting at lease one hospitalization in the past year, compared with 28% and 14% respectively, among ED participants.”
Strengths of the study included the prospective multisite enrollment that provided data across diverse locations and representation from pediatric hospitalizations and ED care, which were not previously strongly represented in the literature. The single-season study with small sample size was considered a limitation, as was the inability to evaluate full and partial vaccine status. Vaccine data also were limited for many of the ED patients observed.
Dr. Campbell and colleagues did caution that while they consider their test-negative design optimal for evaluating both hospitalized and ED patients, they feel their results should not be “interpreted as VE against influenza-associated ambulatory care visits or infections that are not medically attended.”
In a separate interview, Michael E. Pichichero, MD, director of the Rochester General Hospital Research Institute and a clinical professor of pediatrics at the University of Rochester (N.Y.), observed: “There are really no surprises here. A well done contemporary study confirms again the benefits of annual influenza vaccinations for children. Viral coinfections involving SARS-CoV-2 and influenza have been reported from Australia to cause heightened illnesses. That observation provides further impetus for parents to have their children receive influenza vaccinations.”
The researchers cited multiple sources of financial support for their ongoing work, including Sanofi, Quidel, Moderna, Karius, GlaxoSmithKline, Merck, AstraZeneca, and Pfizer. Funding for this study was supported by the Centers for Disease Control and Prevention. Dr. Pichichero said he had no relevant financial disclosures.
SOURCE: Campbell AP et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1368.
Unlike previous studies focused on vaccine effectiveness (VE) in ambulatory care office visits, Angela P. Campbell, MD, MPH, and associates have uncovered evidence of the overall benefit influenza vaccines play in reducing hospitalizations and emergency department visits in pediatric influenza patients.
“Our data provide important VE estimates against severe influenza in children,” the researchers noted in Pediatrics, adding that the findings “provide important evidence supporting the annual recommendation that all children 6 months and older should receive influenza vaccination.”
Dr. Campbell and colleagues collected ongoing surveillance data from the New Vaccine Surveillance Network (NVSN), which is a network of pediatric hospitals across seven cities, including Kansas City, Mo.; Rochester, N.Y.; Cincinnati; Pittsburgh; Nashville, Tenn.; Houston; and Seattle. The influenza season encompassed the period Nov. 7, 2018 to June 21, 2019.
A total of 2,748 hospitalized children and 2,676 children who had completed ED visits that did not lead to hospitalization were included. Once those under 6 months were excluded, 1,792 hospitalized children were included in the VE analysis; of these, 226 (13%) tested positive for influenza infection, including 211 (93%) with influenza A viruses and 15 (7%) with influenza B viruses. Fully 1,611 of the patients (90%), had verified vaccine status, while 181 (10%) had solely parental reported vaccine status. The researchers reported 88 (5%) of the patients received mechanical ventilation and 7 (<1%) died.
Most noteworthy, They further estimated a significant reduction in hospitalizations linked to A(H3N2) and A(H1N1)pdm09 viruses, even in the presence of circulating A(H3N2) viruses that differed from the A(H3N2) vaccine component.
Studies from other countries during the same time period showed that while “significant protection against influenza-associated ambulatory care visits and hospitalizations among children infected with A(H1N1)pdm09 viruses” was observed, the same could not be said for protection against A(H3N2) viruses, which varied among pediatric outpatients in the United States (24%), in England (17% outpatient; 31% inpatient), Europe (46%), and Canada (48%). They explained that such variation in vaccine protection is multifactorial, and includes virus-, host-, and environment-related factors. They also noted that regional variations in circulating viruses, host factors including age, imprinting, and previous vaccination could explain the study’s finding of vaccine protection against both A(H1N1)pdm09 and A(H3N2) viruses.
When comparing VE estimates between ED visits and hospitalizations, the researchers observed one significant difference, that “hospitalized children likely represent more medically complex patients, with 58% having underlying medical conditions and 38% reporting at lease one hospitalization in the past year, compared with 28% and 14% respectively, among ED participants.”
Strengths of the study included the prospective multisite enrollment that provided data across diverse locations and representation from pediatric hospitalizations and ED care, which were not previously strongly represented in the literature. The single-season study with small sample size was considered a limitation, as was the inability to evaluate full and partial vaccine status. Vaccine data also were limited for many of the ED patients observed.
Dr. Campbell and colleagues did caution that while they consider their test-negative design optimal for evaluating both hospitalized and ED patients, they feel their results should not be “interpreted as VE against influenza-associated ambulatory care visits or infections that are not medically attended.”
In a separate interview, Michael E. Pichichero, MD, director of the Rochester General Hospital Research Institute and a clinical professor of pediatrics at the University of Rochester (N.Y.), observed: “There are really no surprises here. A well done contemporary study confirms again the benefits of annual influenza vaccinations for children. Viral coinfections involving SARS-CoV-2 and influenza have been reported from Australia to cause heightened illnesses. That observation provides further impetus for parents to have their children receive influenza vaccinations.”
The researchers cited multiple sources of financial support for their ongoing work, including Sanofi, Quidel, Moderna, Karius, GlaxoSmithKline, Merck, AstraZeneca, and Pfizer. Funding for this study was supported by the Centers for Disease Control and Prevention. Dr. Pichichero said he had no relevant financial disclosures.
SOURCE: Campbell AP et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1368.
Unlike previous studies focused on vaccine effectiveness (VE) in ambulatory care office visits, Angela P. Campbell, MD, MPH, and associates have uncovered evidence of the overall benefit influenza vaccines play in reducing hospitalizations and emergency department visits in pediatric influenza patients.
“Our data provide important VE estimates against severe influenza in children,” the researchers noted in Pediatrics, adding that the findings “provide important evidence supporting the annual recommendation that all children 6 months and older should receive influenza vaccination.”
Dr. Campbell and colleagues collected ongoing surveillance data from the New Vaccine Surveillance Network (NVSN), which is a network of pediatric hospitals across seven cities, including Kansas City, Mo.; Rochester, N.Y.; Cincinnati; Pittsburgh; Nashville, Tenn.; Houston; and Seattle. The influenza season encompassed the period Nov. 7, 2018 to June 21, 2019.
A total of 2,748 hospitalized children and 2,676 children who had completed ED visits that did not lead to hospitalization were included. Once those under 6 months were excluded, 1,792 hospitalized children were included in the VE analysis; of these, 226 (13%) tested positive for influenza infection, including 211 (93%) with influenza A viruses and 15 (7%) with influenza B viruses. Fully 1,611 of the patients (90%), had verified vaccine status, while 181 (10%) had solely parental reported vaccine status. The researchers reported 88 (5%) of the patients received mechanical ventilation and 7 (<1%) died.
Most noteworthy, They further estimated a significant reduction in hospitalizations linked to A(H3N2) and A(H1N1)pdm09 viruses, even in the presence of circulating A(H3N2) viruses that differed from the A(H3N2) vaccine component.
Studies from other countries during the same time period showed that while “significant protection against influenza-associated ambulatory care visits and hospitalizations among children infected with A(H1N1)pdm09 viruses” was observed, the same could not be said for protection against A(H3N2) viruses, which varied among pediatric outpatients in the United States (24%), in England (17% outpatient; 31% inpatient), Europe (46%), and Canada (48%). They explained that such variation in vaccine protection is multifactorial, and includes virus-, host-, and environment-related factors. They also noted that regional variations in circulating viruses, host factors including age, imprinting, and previous vaccination could explain the study’s finding of vaccine protection against both A(H1N1)pdm09 and A(H3N2) viruses.
When comparing VE estimates between ED visits and hospitalizations, the researchers observed one significant difference, that “hospitalized children likely represent more medically complex patients, with 58% having underlying medical conditions and 38% reporting at lease one hospitalization in the past year, compared with 28% and 14% respectively, among ED participants.”
Strengths of the study included the prospective multisite enrollment that provided data across diverse locations and representation from pediatric hospitalizations and ED care, which were not previously strongly represented in the literature. The single-season study with small sample size was considered a limitation, as was the inability to evaluate full and partial vaccine status. Vaccine data also were limited for many of the ED patients observed.
Dr. Campbell and colleagues did caution that while they consider their test-negative design optimal for evaluating both hospitalized and ED patients, they feel their results should not be “interpreted as VE against influenza-associated ambulatory care visits or infections that are not medically attended.”
In a separate interview, Michael E. Pichichero, MD, director of the Rochester General Hospital Research Institute and a clinical professor of pediatrics at the University of Rochester (N.Y.), observed: “There are really no surprises here. A well done contemporary study confirms again the benefits of annual influenza vaccinations for children. Viral coinfections involving SARS-CoV-2 and influenza have been reported from Australia to cause heightened illnesses. That observation provides further impetus for parents to have their children receive influenza vaccinations.”
The researchers cited multiple sources of financial support for their ongoing work, including Sanofi, Quidel, Moderna, Karius, GlaxoSmithKline, Merck, AstraZeneca, and Pfizer. Funding for this study was supported by the Centers for Disease Control and Prevention. Dr. Pichichero said he had no relevant financial disclosures.
SOURCE: Campbell AP et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1368.
FROM PEDIATRICS
AGA News
Receive $300,000 for your research in health disparities
Applications for the research scholar award are due by Nov. 9, 2020.
The American Gastroenterological Association Research Foundation is pleased to announce an important addition to its prestigious awards portfolio. The AGA Research Scholar Award in Digestive Disease Health Disparities supports early-career faculty dedicated to investigating digestive diseases or disorders that disproportionately affect racial or ethnic minority populations in North America.
Applicants must have a full-time faculty (or equivalent) position and may be performing any type of research (clinical, basic, or translational). Awardees will receive a total of $300,000 over 3 years with funding to commence in July 2021. The deadline to apply is Nov. 9, 2020.
This award is just one example of how AGA is helping to improve patient care for those who need it most. Support AGA Giving Day and learn more about the AGA Equity Project – a multiyear effort spanning all aspects of our organization to achieve equity and eradicate disparities in digestive diseases.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, the digestive disease community has connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Virtual 2021 Crohn’s & Colitis Congress® now open for registration
Help forge the roadmap to advance prevention, treatments, and cures for all patients living with inflammatory bowel disease (IBD).
Join the Crohn’s & Colitis Foundation, AGA, and a true community of friends and colleagues at the premier conference on IBD. The fourth annual Crohn’s & Colitis Congress®, taking place virtually Jan. 21-24, 2021, is now open for registration.
The 2021 Crohn’s & Colitis Congress virtual experience will look a little different but will still bring you all the benefits and quality programming you have come to expect. The Congress will offer 4 days of learning, with more than 100 speakers and more than 200 expected abstracts – all from the safety of your home or work. Now at an even more affordable price, access from anywhere, and the ability to hear from the top leaders in the IBD field – this is a unique opportunity to join us as we come together virtually.
By moving our event online, we can now pass on greater savings to you. Registration for the conference provides you with substantial savings over last year and access to all sessions and networking opportunities. This virtual experience will bring our community of IBD professionals together in an engaging, interactive setting which will include breakout rooms, receptions, and much more.
The 2021 congress committee chair David T. Rubin, MD, AGAF, University of Chicago, and cochair Bruce E. Sands, MD, MS, AGAF, Icahn School of Medicine at Mount Sinai, New York, lead a faculty that includes thought leaders in the fields of GI, research investigation, surgery, pediatrics, advanced practice, IBD nursing, diet and nutrition, mental health, radiology, pathology, and more.
Register and get inspired to improve skills and patient outcomes, learn practical information you can immediately implement, hear what’s on the horizon in potential IBD treatments, discover fresh perspectives from multidisciplinary faculty and attendees.
You don’t want to miss the 2021 Crohn’s & Colitis Congress, connecting virtually on Jan. 21-24, 2021.
Register today to save before the early bird deadline of Friday, Nov. 6.
Learn more, submit an abstract, and register by visiting crohnscolitiscongress.org.
AGA releases largest real-world report on safety and effectiveness of fecal microbiota transplantation
About 90% of patients tracked in the AGA FMT National Registry were cured of Clostridioides difficile infection with few serious side effects.
AGA has released the first results from the NIH-funded AGA Fecal Microbiota Transplantation (FMT) National Registry, the largest real-world study on the safety and effectiveness of FMT. Published in Gastroenterology, the registry reported that FMT led to a cure of C. difficile infection in 90% of patients across 20 North American FMT practice sites. Few serious side effects were reported.
“While the value of fecal microbiota transplantation for treating recurrent C. difficile infection is clear from research studies, the potential long-term consequences of altering a patient’s gut microbiota are not fully known,” says Colleen R. Kelly, MD, AGAF, associate professor of medicine at Brown University, Providence, R.I. and coprincipal investigator of the AGA FMT National Registry. “Releasing the initial results of the AGA FMT National Registry is an important step toward understanding the true risks and benefits of microbiota therapeutics in a real-world setting.”
This new report details effectiveness and safety outcomes from the first 259 patients enrolled in the registry between December 2017 and September 2019. Almost all participants received FMT using an unknown donor from stool banks. The most common method of FMT delivery was colonoscopy followed by upper endoscopy. Of the 222 participants who returned for the 1-month follow-up, 200 participants (90%) had their C. difficile infection cured with 197 of those requiring only a single FMT. Infections were reported in 11 participants, but only 2 were thought to be possibly related to the procedure. FMT response was deemed durable, with recurrence of C. difficile infection in the 6 months after successful FMT occurring in only 4% of participants. This data includes patients with comorbidities, such as IBD and immunocompromised status, who are typically excluded from FMT clinical trials.
“These initial results show a high success rate of FMT in the real-world setting. We’ll continue to track these patients for 10 years to assess long-term safety, which will be critical to determining the full safety profile of FMT,” added Dr. Kelly.
AGA raises concerns about recent executive order
We are speaking out to ensure a brighter and more equitable future.
AGA is concerned by the Executive Order on Combating Race and Sex Stereotyping issued on Sept. 22, 2020. This order, while confirming that training of the federal workforce to create an inclusive workspace is beneficial, also leads to a misguided perception of the purpose and outcomes of this type of training. In addition, it may have unintended ramifications for institutions receiving federal research funding.
We believe it is critical and necessary to understand both the positive and negative realities of our nation’s history, so that together we can forge forward into a brighter, and more equitable future.
As highlighted in AGA’s commentary published in Gastroenterology, AGA believes that equity is defined by fair treatment, access, opportunity, and advancement for all, acknowledging that there are historically underserved and underrepresented populations. Equity requires identifying and eliminating barriers that have created unbalanced conditions and prevented the full participation of some groups in order to provide equal opportunity for all groups.
By default, teaching and practicing equity, diversity and inclusion aims not to place any group above or below any other group, or to create division. It rather seeks to achieve fairness and understanding, and fully recognize the dignity of all groups, identities, and individuals.
AGA stands with the Association of American Medical Colleges in our commitment to being a diverse, inclusive, equitable, and antiracist organization.
Our commitment to this issue is manifest in the AGA Equity Project.
Receive $300,000 for your research in health disparities
Applications for the research scholar award are due by Nov. 9, 2020.
The American Gastroenterological Association Research Foundation is pleased to announce an important addition to its prestigious awards portfolio. The AGA Research Scholar Award in Digestive Disease Health Disparities supports early-career faculty dedicated to investigating digestive diseases or disorders that disproportionately affect racial or ethnic minority populations in North America.
Applicants must have a full-time faculty (or equivalent) position and may be performing any type of research (clinical, basic, or translational). Awardees will receive a total of $300,000 over 3 years with funding to commence in July 2021. The deadline to apply is Nov. 9, 2020.
This award is just one example of how AGA is helping to improve patient care for those who need it most. Support AGA Giving Day and learn more about the AGA Equity Project – a multiyear effort spanning all aspects of our organization to achieve equity and eradicate disparities in digestive diseases.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, the digestive disease community has connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Virtual 2021 Crohn’s & Colitis Congress® now open for registration
Help forge the roadmap to advance prevention, treatments, and cures for all patients living with inflammatory bowel disease (IBD).
Join the Crohn’s & Colitis Foundation, AGA, and a true community of friends and colleagues at the premier conference on IBD. The fourth annual Crohn’s & Colitis Congress®, taking place virtually Jan. 21-24, 2021, is now open for registration.
The 2021 Crohn’s & Colitis Congress virtual experience will look a little different but will still bring you all the benefits and quality programming you have come to expect. The Congress will offer 4 days of learning, with more than 100 speakers and more than 200 expected abstracts – all from the safety of your home or work. Now at an even more affordable price, access from anywhere, and the ability to hear from the top leaders in the IBD field – this is a unique opportunity to join us as we come together virtually.
By moving our event online, we can now pass on greater savings to you. Registration for the conference provides you with substantial savings over last year and access to all sessions and networking opportunities. This virtual experience will bring our community of IBD professionals together in an engaging, interactive setting which will include breakout rooms, receptions, and much more.
The 2021 congress committee chair David T. Rubin, MD, AGAF, University of Chicago, and cochair Bruce E. Sands, MD, MS, AGAF, Icahn School of Medicine at Mount Sinai, New York, lead a faculty that includes thought leaders in the fields of GI, research investigation, surgery, pediatrics, advanced practice, IBD nursing, diet and nutrition, mental health, radiology, pathology, and more.
Register and get inspired to improve skills and patient outcomes, learn practical information you can immediately implement, hear what’s on the horizon in potential IBD treatments, discover fresh perspectives from multidisciplinary faculty and attendees.
You don’t want to miss the 2021 Crohn’s & Colitis Congress, connecting virtually on Jan. 21-24, 2021.
Register today to save before the early bird deadline of Friday, Nov. 6.
Learn more, submit an abstract, and register by visiting crohnscolitiscongress.org.
AGA releases largest real-world report on safety and effectiveness of fecal microbiota transplantation
About 90% of patients tracked in the AGA FMT National Registry were cured of Clostridioides difficile infection with few serious side effects.
AGA has released the first results from the NIH-funded AGA Fecal Microbiota Transplantation (FMT) National Registry, the largest real-world study on the safety and effectiveness of FMT. Published in Gastroenterology, the registry reported that FMT led to a cure of C. difficile infection in 90% of patients across 20 North American FMT practice sites. Few serious side effects were reported.
“While the value of fecal microbiota transplantation for treating recurrent C. difficile infection is clear from research studies, the potential long-term consequences of altering a patient’s gut microbiota are not fully known,” says Colleen R. Kelly, MD, AGAF, associate professor of medicine at Brown University, Providence, R.I. and coprincipal investigator of the AGA FMT National Registry. “Releasing the initial results of the AGA FMT National Registry is an important step toward understanding the true risks and benefits of microbiota therapeutics in a real-world setting.”
This new report details effectiveness and safety outcomes from the first 259 patients enrolled in the registry between December 2017 and September 2019. Almost all participants received FMT using an unknown donor from stool banks. The most common method of FMT delivery was colonoscopy followed by upper endoscopy. Of the 222 participants who returned for the 1-month follow-up, 200 participants (90%) had their C. difficile infection cured with 197 of those requiring only a single FMT. Infections were reported in 11 participants, but only 2 were thought to be possibly related to the procedure. FMT response was deemed durable, with recurrence of C. difficile infection in the 6 months after successful FMT occurring in only 4% of participants. This data includes patients with comorbidities, such as IBD and immunocompromised status, who are typically excluded from FMT clinical trials.
“These initial results show a high success rate of FMT in the real-world setting. We’ll continue to track these patients for 10 years to assess long-term safety, which will be critical to determining the full safety profile of FMT,” added Dr. Kelly.
AGA raises concerns about recent executive order
We are speaking out to ensure a brighter and more equitable future.
AGA is concerned by the Executive Order on Combating Race and Sex Stereotyping issued on Sept. 22, 2020. This order, while confirming that training of the federal workforce to create an inclusive workspace is beneficial, also leads to a misguided perception of the purpose and outcomes of this type of training. In addition, it may have unintended ramifications for institutions receiving federal research funding.
We believe it is critical and necessary to understand both the positive and negative realities of our nation’s history, so that together we can forge forward into a brighter, and more equitable future.
As highlighted in AGA’s commentary published in Gastroenterology, AGA believes that equity is defined by fair treatment, access, opportunity, and advancement for all, acknowledging that there are historically underserved and underrepresented populations. Equity requires identifying and eliminating barriers that have created unbalanced conditions and prevented the full participation of some groups in order to provide equal opportunity for all groups.
By default, teaching and practicing equity, diversity and inclusion aims not to place any group above or below any other group, or to create division. It rather seeks to achieve fairness and understanding, and fully recognize the dignity of all groups, identities, and individuals.
AGA stands with the Association of American Medical Colleges in our commitment to being a diverse, inclusive, equitable, and antiracist organization.
Our commitment to this issue is manifest in the AGA Equity Project.
Receive $300,000 for your research in health disparities
Applications for the research scholar award are due by Nov. 9, 2020.
The American Gastroenterological Association Research Foundation is pleased to announce an important addition to its prestigious awards portfolio. The AGA Research Scholar Award in Digestive Disease Health Disparities supports early-career faculty dedicated to investigating digestive diseases or disorders that disproportionately affect racial or ethnic minority populations in North America.
Applicants must have a full-time faculty (or equivalent) position and may be performing any type of research (clinical, basic, or translational). Awardees will receive a total of $300,000 over 3 years with funding to commence in July 2021. The deadline to apply is Nov. 9, 2020.
This award is just one example of how AGA is helping to improve patient care for those who need it most. Support AGA Giving Day and learn more about the AGA Equity Project – a multiyear effort spanning all aspects of our organization to achieve equity and eradicate disparities in digestive diseases.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, the digestive disease community has connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Virtual 2021 Crohn’s & Colitis Congress® now open for registration
Help forge the roadmap to advance prevention, treatments, and cures for all patients living with inflammatory bowel disease (IBD).
Join the Crohn’s & Colitis Foundation, AGA, and a true community of friends and colleagues at the premier conference on IBD. The fourth annual Crohn’s & Colitis Congress®, taking place virtually Jan. 21-24, 2021, is now open for registration.
The 2021 Crohn’s & Colitis Congress virtual experience will look a little different but will still bring you all the benefits and quality programming you have come to expect. The Congress will offer 4 days of learning, with more than 100 speakers and more than 200 expected abstracts – all from the safety of your home or work. Now at an even more affordable price, access from anywhere, and the ability to hear from the top leaders in the IBD field – this is a unique opportunity to join us as we come together virtually.
By moving our event online, we can now pass on greater savings to you. Registration for the conference provides you with substantial savings over last year and access to all sessions and networking opportunities. This virtual experience will bring our community of IBD professionals together in an engaging, interactive setting which will include breakout rooms, receptions, and much more.
The 2021 congress committee chair David T. Rubin, MD, AGAF, University of Chicago, and cochair Bruce E. Sands, MD, MS, AGAF, Icahn School of Medicine at Mount Sinai, New York, lead a faculty that includes thought leaders in the fields of GI, research investigation, surgery, pediatrics, advanced practice, IBD nursing, diet and nutrition, mental health, radiology, pathology, and more.
Register and get inspired to improve skills and patient outcomes, learn practical information you can immediately implement, hear what’s on the horizon in potential IBD treatments, discover fresh perspectives from multidisciplinary faculty and attendees.
You don’t want to miss the 2021 Crohn’s & Colitis Congress, connecting virtually on Jan. 21-24, 2021.
Register today to save before the early bird deadline of Friday, Nov. 6.
Learn more, submit an abstract, and register by visiting crohnscolitiscongress.org.
AGA releases largest real-world report on safety and effectiveness of fecal microbiota transplantation
About 90% of patients tracked in the AGA FMT National Registry were cured of Clostridioides difficile infection with few serious side effects.
AGA has released the first results from the NIH-funded AGA Fecal Microbiota Transplantation (FMT) National Registry, the largest real-world study on the safety and effectiveness of FMT. Published in Gastroenterology, the registry reported that FMT led to a cure of C. difficile infection in 90% of patients across 20 North American FMT practice sites. Few serious side effects were reported.
“While the value of fecal microbiota transplantation for treating recurrent C. difficile infection is clear from research studies, the potential long-term consequences of altering a patient’s gut microbiota are not fully known,” says Colleen R. Kelly, MD, AGAF, associate professor of medicine at Brown University, Providence, R.I. and coprincipal investigator of the AGA FMT National Registry. “Releasing the initial results of the AGA FMT National Registry is an important step toward understanding the true risks and benefits of microbiota therapeutics in a real-world setting.”
This new report details effectiveness and safety outcomes from the first 259 patients enrolled in the registry between December 2017 and September 2019. Almost all participants received FMT using an unknown donor from stool banks. The most common method of FMT delivery was colonoscopy followed by upper endoscopy. Of the 222 participants who returned for the 1-month follow-up, 200 participants (90%) had their C. difficile infection cured with 197 of those requiring only a single FMT. Infections were reported in 11 participants, but only 2 were thought to be possibly related to the procedure. FMT response was deemed durable, with recurrence of C. difficile infection in the 6 months after successful FMT occurring in only 4% of participants. This data includes patients with comorbidities, such as IBD and immunocompromised status, who are typically excluded from FMT clinical trials.
“These initial results show a high success rate of FMT in the real-world setting. We’ll continue to track these patients for 10 years to assess long-term safety, which will be critical to determining the full safety profile of FMT,” added Dr. Kelly.
AGA raises concerns about recent executive order
We are speaking out to ensure a brighter and more equitable future.
AGA is concerned by the Executive Order on Combating Race and Sex Stereotyping issued on Sept. 22, 2020. This order, while confirming that training of the federal workforce to create an inclusive workspace is beneficial, also leads to a misguided perception of the purpose and outcomes of this type of training. In addition, it may have unintended ramifications for institutions receiving federal research funding.
We believe it is critical and necessary to understand both the positive and negative realities of our nation’s history, so that together we can forge forward into a brighter, and more equitable future.
As highlighted in AGA’s commentary published in Gastroenterology, AGA believes that equity is defined by fair treatment, access, opportunity, and advancement for all, acknowledging that there are historically underserved and underrepresented populations. Equity requires identifying and eliminating barriers that have created unbalanced conditions and prevented the full participation of some groups in order to provide equal opportunity for all groups.
By default, teaching and practicing equity, diversity and inclusion aims not to place any group above or below any other group, or to create division. It rather seeks to achieve fairness and understanding, and fully recognize the dignity of all groups, identities, and individuals.
AGA stands with the Association of American Medical Colleges in our commitment to being a diverse, inclusive, equitable, and antiracist organization.
Our commitment to this issue is manifest in the AGA Equity Project.
These images of diabetic retinopathy tell the story better
I read, with great interest, Dr. Farford’s thorough review article “Diabetic retinopathy: the FP’s role in preserving vision” (J Fam Pract. 2020;69:120-126). I am a family physician with ophthalmology training. For more than 20 years, I have regularly performed dilated eye exams and reviewed nonmydriatic fundus photos for uninsured patients with diabetic retinopathy (DR) at the community health clinic where I work. The burden of visual loss from poorly controlled diabetes is staggering.
I do, however, want to point out some inaccuracies in the labeling of 2 of the photos included in Table 1.
- The photo labeled “Severe NPDR [nonproliferative DR]”—Figure 1A—actually shows an eye that has been treated with panretinal photocoagulation (multiple laser scars present in all quadrants) with nice regression of DR. Along the superior temporal arcade there is fibrosis, which likely represents regression of vitreal neovascularization or resolution of vitreal hemorrhage. There is little apparent active DR in this photo. The caption indicated the presence of intraretinal microvascular abnormalities; however, while these abnormalities may be present, they are not evident due to the photo resolution.
- The photo labeled “Proliferative diabetic retinopathy”—Figure 2a—does not show evidence of neovascularization of the disc or the retina. This photo would be more accurately labeled “severe DR with likely clinically significant macular edema.”
The 2 photos shown here, from my photo collection, are perhaps more instructive:
- FIGURE 1B is an example of severe NPDR and maculopathy (this eye has undergone previous panretinal photocoagulation, a treatment option for severe NPDR and proliferative DR [defined as new vessel growth or neovascularization]).
- FIGURE 2b is an example of proliferative DR with vitreal hemorrhage that can lead to irreversible visual loss via traction retinal detachment.
I appreciate your efforts in publishing Dr. Farford’s article. DR is a broad, complicated topic, and this informative article will help many FPs.
Kenneth Libre, MD
Central City Community Health Center
Salt Lake City, UT
I read, with great interest, Dr. Farford’s thorough review article “Diabetic retinopathy: the FP’s role in preserving vision” (J Fam Pract. 2020;69:120-126). I am a family physician with ophthalmology training. For more than 20 years, I have regularly performed dilated eye exams and reviewed nonmydriatic fundus photos for uninsured patients with diabetic retinopathy (DR) at the community health clinic where I work. The burden of visual loss from poorly controlled diabetes is staggering.
I do, however, want to point out some inaccuracies in the labeling of 2 of the photos included in Table 1.
- The photo labeled “Severe NPDR [nonproliferative DR]”—Figure 1A—actually shows an eye that has been treated with panretinal photocoagulation (multiple laser scars present in all quadrants) with nice regression of DR. Along the superior temporal arcade there is fibrosis, which likely represents regression of vitreal neovascularization or resolution of vitreal hemorrhage. There is little apparent active DR in this photo. The caption indicated the presence of intraretinal microvascular abnormalities; however, while these abnormalities may be present, they are not evident due to the photo resolution.
- The photo labeled “Proliferative diabetic retinopathy”—Figure 2a—does not show evidence of neovascularization of the disc or the retina. This photo would be more accurately labeled “severe DR with likely clinically significant macular edema.”
The 2 photos shown here, from my photo collection, are perhaps more instructive:
- FIGURE 1B is an example of severe NPDR and maculopathy (this eye has undergone previous panretinal photocoagulation, a treatment option for severe NPDR and proliferative DR [defined as new vessel growth or neovascularization]).
- FIGURE 2b is an example of proliferative DR with vitreal hemorrhage that can lead to irreversible visual loss via traction retinal detachment.

I appreciate your efforts in publishing Dr. Farford’s article. DR is a broad, complicated topic, and this informative article will help many FPs.
Kenneth Libre, MD
Central City Community Health Center
Salt Lake City, UT
I read, with great interest, Dr. Farford’s thorough review article “Diabetic retinopathy: the FP’s role in preserving vision” (J Fam Pract. 2020;69:120-126). I am a family physician with ophthalmology training. For more than 20 years, I have regularly performed dilated eye exams and reviewed nonmydriatic fundus photos for uninsured patients with diabetic retinopathy (DR) at the community health clinic where I work. The burden of visual loss from poorly controlled diabetes is staggering.
I do, however, want to point out some inaccuracies in the labeling of 2 of the photos included in Table 1.
- The photo labeled “Severe NPDR [nonproliferative DR]”—Figure 1A—actually shows an eye that has been treated with panretinal photocoagulation (multiple laser scars present in all quadrants) with nice regression of DR. Along the superior temporal arcade there is fibrosis, which likely represents regression of vitreal neovascularization or resolution of vitreal hemorrhage. There is little apparent active DR in this photo. The caption indicated the presence of intraretinal microvascular abnormalities; however, while these abnormalities may be present, they are not evident due to the photo resolution.
- The photo labeled “Proliferative diabetic retinopathy”—Figure 2a—does not show evidence of neovascularization of the disc or the retina. This photo would be more accurately labeled “severe DR with likely clinically significant macular edema.”
The 2 photos shown here, from my photo collection, are perhaps more instructive:
- FIGURE 1B is an example of severe NPDR and maculopathy (this eye has undergone previous panretinal photocoagulation, a treatment option for severe NPDR and proliferative DR [defined as new vessel growth or neovascularization]).
- FIGURE 2b is an example of proliferative DR with vitreal hemorrhage that can lead to irreversible visual loss via traction retinal detachment.

I appreciate your efforts in publishing Dr. Farford’s article. DR is a broad, complicated topic, and this informative article will help many FPs.
Kenneth Libre, MD
Central City Community Health Center
Salt Lake City, UT