User login
How should I treat acute agitation in pregnancy?
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20 Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20 Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death, and spontaneous abortion.1
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics, and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
FOCUS OF THE EMERGENCY EVALUATION
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania, or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing, and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine, and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania, or delirium.
NEW SYSTEM TO DETERMINE RISK DURING PREGNANCY, LACTATION
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D, and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
FIRST-GENERATION ANTIPSYCHOTICS SAFE, EFFECTIVE IN PREGNANCY
Reproductive safety of first-generation (ie, typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7 although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (eg, haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (eg, chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
NEWER ANTIPSYCHOTICS ALSO SAFE IN PREGNANCY
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
DIPHENHYDRAMINE
Recent studies of antihistamines such as diphenhydramine have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
BENZODIAZEPINES
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
STEPWISE MANAGEMENT OF AGITATION IN PREGNANCY
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death, and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints (Figure 1).4,19
Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, and the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
DRUG THERAPY RECOMMENDATIONS
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic such as olanzapine in a rapidly dissolving form or ziprasidone is another option if a rapid response is required.20 Table 1 provides a summary of pharmacotherapy recommendations.
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism, and clearance.21
Although no study to our knowledge has assessed risk associated with 1-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
PHYSICAL RESTRAINTS FOR AGITATION IN PREGNANCY
Physical restraints along with emergency medications (ie, chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as 4-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- Aftab A, Shah AA. Behavioral emergencies: special considerations in the pregnant patient. Psychiatr Clin North Am 2017; 40(3):435–448. doi:10.1016/j.psc.2017.05.017
- Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153(5):592–606. doi:10.1176/ajp.153.5.592
- Einarson A. Safety of psychotropic drug use during pregnancy: a review. MedGenMed 2005; 7(4):3. pmid:16614625
- Wilson MP, Nordstrom K, Shah AA, Vilke GM. Psychiatric emergencies in pregnant women. Emerg Med Clin North Am 2015; 33(4):841–851. doi:10.1016/j.emc.2015.07.010
- Brown HE, Stoklosa J, Freundenreich O. How to stabilize an acutely psychotic patient. Curr Psychiatry 2012; 11(12):10–16.
- US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. www.fda.gov/drugs/developmentapprovalprocess/developmentresources/labeling/ucm093307.htm. Accessed January 8, 2019.
- Brucker MC, King TL. The 2015 US Food and Drug Administration pregnancy and lactation labeling rule. J Midwifery Womens Health 2017; 62(3):308–316. doi:10.1111/jmwh.12611
- Diav-Citrin O, Shechtman S, Ornoy S, et al. Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. J Clin Psychiatry 2005; 66(3):317–322. pmid:15766297
- Galbally M, Snellen M, Power J. Antipsychotic drugs in pregnancy: a review of their maternal and fetal effects. Ther Adv Drug Saf 2014; 5(2):100–109. doi:10.1177/2042098614522682
- Kulkarni J, Storch A, Baraniuk A, Gilbert H, Gavrilidis E, Worsley R. Antipsychotic use in pregnancy. Expert Opin Pharmacother 2015; 16(9):1335–1345. doi:10.1517/14656566.2015.1041501
- Huybrechts KF, Hernández-Díaz S, Patorno E, et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73(9):938–946. doi:10.1001/jamapsychiatry.2016.1520
- Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital national pregnancy registry for atypical antipsychotics. Am J Psychiatry 2016; 173(3):263–270. doi:10.1176/appi.ajp.2015.15040506
- Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract 2013; 1(6):666–674.e1. doi:10.1016/j.jaip.2013.07.008
- Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A; National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol 2009; 85(2):137–150. doi:10.1002/bdra.20513
- Meuleman JR. Association of diphenhydramine use with adverse effects in hospitalized older patients: possible confounders. Arch Intern Med 2002; 162(6):720–721. pmid:11911733
- Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 2011; 33(1):46–48. doi:10.1016/S1701-2163(16)34772-7
- Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ 1998; 317(7162):839–843. pmid:9748174
- Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry 2013; 35(1):3–8. doi:10.1016/j.genhosppsych.2012.09.003
- Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry project BETA De-escalation Workgroup. West J Emerg Med 2012; 13(1):17–25. doi:10.5811/westjem.2011.9.6864
- Prager LM, Ivkovic A. Emergency psychiatry. In: Stern TA, Fava M, Wilens TE, Rosenbaum JF, eds. The Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:937–949.
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
A woman, age 35, with new-onset ascites
A 35-year-old woman is admitted to the hospital with a 5-day history of abdominal distention and jaundice. She reports no history of fever, chills, night sweats, abdominal pain, nausea, vomiting, diarrhea, changes in urine color, change in stool color, weight loss, weight gain, or loss of appetite.
She is petite, with a body mass index of 19.4 kg/m2. She has no known history of medical conditions or surgery and is not taking any medications. Her family history is unremarkable, and she denies current or past tobacco, alcohol, or illicit drug use.
RECENT TRAVEL
She says that during a trip to Central America several months ago, she had suffered a seizure and was taken to a local hospital, where laboratory testing revealed elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. She says that the rest of the workup at that time was normal.
About 1 week after that incident, she returned home and saw her primary care physician, who ordered further testing, which showed mild hyperbilirubinemia and mild elevation of AST and ALT levels. Her physician attributed the elevations to atovaquone, which she had been taking for malaria prophylaxis, as repeat testing 2 weeks later showed improvement in AST and ALT levels.
The patient says she returned to her normal state of health until about 5 days ago, when she noticed jaundice and abdominal distention, but without abdominal pain, dark urine, or clay-colored stools. She became concerned and went to her local hospital. Testing there noted mild elevation of AST and ALT, as well as an elevated international normalized ratio (INR) and hyperbilirubinemia. Computed tomography of the abdomen and pelvis showed hepatomegaly with possible fatty liver. Because of these results, the patient was transferred to our institution for further evaluation.
EVALUATION AT OUR INSTITUTION
On examination at our institution, she is afebrile, and vital signs are within normal ranges. She has bilateral scleral icterus and diffuse jaundice, but no other skin finding such as rash or spider angioma. She has no lymphadenopathy. Her abdomen is distended, with tense ascites, and her liver is tender to palpation. The tip of the spleen is not palpable.
The cardiovascular examination reveals no murmurs, rubs, or gallops, but she has jugular venous distention and +2 pitting edema of both lower extremities.
On respiratory examination, there is dullness to percussion, with slight crackles on auscultation at the right lung base. The neurologic examination is normal.
Table 1 shows the results of initial laboratory testing.
1. Which study would provide the most information on the cause of ascites?
- Abdominal ultrasonography
- Abdominal paracentesis with ascitic fluid analysis
- Chest radiography
- Echocardiography
- Urine protein-to-creatinine ratio
Abdominal paracentesis with ascitic fluid analysis is the essential study for any patient with clinically apparent new-onset ascites.1–3 It is the study that provides the most information on the cause of ascites.
In our patient, abdominal paracentesis yields 1,000 mL of straw-colored ascitic fluid, and analysis shows 86 nucleated cells, 28 of which are polymorphonuclear cells, and 0 red blood cells, with negative Gram stain and culture. The ascitic albumin level is 0.85 g/dL, with an ascitic protein of 1.1 g/dL.
Abdominal ultrasonography shows a diffusely echogenic liver, no focal lesions, moderate ascites, normal portal vein flow, no intrahepatic or extrahepatic biliary duct dilation, normal kidney sizes, no hydronephrosis, and no intra-abdominal mass. Chest radiography is clear with no sign of consolidation, edema, or effusion. Echocardiography shows a normal left ventricular ejection fraction with no valvular disease or pericardial effusion. A random urine protein-creatinine ratio is normal at 0.1 (reference range < 0.2).
2. What is the most likely cause of her ascites based on the workup to this point?
- Cirrhosis
- Heart failure
- Nephrotic syndrome
- Portal vein thrombus
- Abdominal malignancy
- Malaria
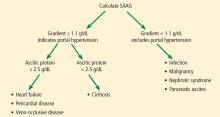
An initial approach to ascitic fluid analysis is to calculate the serum-ascites albumin gradient (SAAG). The SAAG is calculated as the serum albumin level minus the ascitic fluid albumin level.4,5 This is useful in determining the cause of the ascites (Figure 1).4,5 A gradient of 1.1 g/dL or higher indicates portal hypertension.4,5
Common causes of portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome, portal vein thrombosis), idiopathic portal fibrosis, and metastatic liver disease.5,6
If portal hypertension is present based on the SAAG, the next step is to review the ascitic protein level to help distinguish between a hepatic and a cardiac etiology of the ascites. An ascitic protein level less than 2.5 g/dL indicates a primary liver pathology (eg, cirrhosis). An ascitic protein level of 2.5 g/dL or greater typically indicates a cardiac condition (eg, heart failure, pericardial disease) with secondary congestive hepatopathy.5,6
If the SAAG is less than 1.1 g/dL, the ascites is likely not from portal hypertension. Typical causes of a low SAAG include infection, malignancy, pancreatic ascites, and nephrotic syndrome.5,6
In our patient, the SAAG is 1.35 g/dL (2.2 g/dL minus 0.85 g/dL), ie, elevated and due to portal hypertension. With an SAAG of 1.1 g/dL or greater and an ascitic fluid protein level less than 2.5 g/dL, as in our patient, the most likely cause is cirrhosis.
Heart failure is unlikely based on her normal brain natriuretic peptide level, an ascitic fluid protein level below 2.5 g/dL, and normal results on echocardiography. Nephrotic syndrome is also very unlikely based on the patient’s normal random urine protein-creatinine ratio. Portal vein thrombus and abdominal malignancy are essentially ruled out by the negative results of Doppler abdominal ultrasonography, with normal venous flow and no intra-abdominal mass and coupled with an elevated SAAG.
Although the patient has a history of travel, the incubation period for malaria would not fit the time frame of presentation. Also, she did not have typical malarial symptoms, her rapid malaria test was negative, and a peripheral blood smear for blood parasites was negative. It should be noted, however, that Plasmodium malariae infection classically presents with flulike symptoms and can resemble nephrotic syndrome, including peripheral edema, ascites, heavy proteinuria, hypoalbuminemia, and hyperlipidemia.7
3. In which patients is antibiotic prophylaxis against spontaneous bacterial peritonitis (SBP) appropriate?
- Any patient with cirrhosis
- Any patient with cirrhosis who is hospitalized
- Any patient with cirrhosis and an ascitic fluid protein level below 2.0 g/dL
- Any patient with cirrhosis and a history of SBP
Any patient with cirrhosis and a history of SBP should receive prophylactic antibiotics,8 as should any patient deemed at high risk of SBP. It is indicated in the following patients:
- Patients with cirrhosis and gastrointestinal bleeding9,10
- Patients with cirrhosis and a previous episode of SBP8
- Patients with cirrhosis and an ascitic fluid protein level less than 1.5 g/dL with either impaired renal function (creatinine ≥ 1.2 mg/dL, blood urea nitrogen level ≥ 25 mg/dL, or serum sodium ≤ 130 mmol/L) or liver failure (Child-Pugh score ≥ 9 and a bilirubin ≥ 3 mg/dL)9
- Patients with cirrhosis who are hospitalized for other reasons and have an ascitic protein level < 1.0 g/dL.9
Our patient has no signs or symptoms of gastrointestinal bleeding and no history of SBP. Her ascitic fluid protein level is 1.1 g/dL, and she has normal renal function. However, her Child-Pugh score is 12 (3 points for total bilirubin > 3 mg/dL, 3 points for serum albumin < 2.8 g/dL, 2 points for an INR 1.7 to 2.2, 3 points for moderate ascites, and 1 point for no encephalopathy), with a bilirubin of 17.0 mg/dL. Based on this, she is placed on antibiotic prophylaxis for SBP.
Our patient then undergoes an extensive workup for liver disease. Results of tests for toxins, autoimmune diseases, and inheritable diseases are all within normal limits. At this point, despite the patient’s reported negative alcohol history, our leading diagnosis is alcoholic hepatitis.
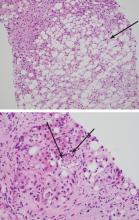
To confirm this diagnosis, she subsequently undergoes transjugular liver biopsy, considered the gold standard for the diagnosis of alcoholic hepatitis. During the procedure, the hepatic venous pressure gradient is measured at 18 mm Hg (reference range 1–5 mm Hg), suggestive of portal hypertension. The pathology study shows severe fatty change, active steatohepatitis with ballooning degeneration, easily identifiable Mallory-Denk bodies, and prominent neutrophilic infiltration, as well as extensive bridging fibrosis (Figure 2). These findings point to alcoholic hepatitis.
After the biopsy results, we speak with the patient further about her alcohol habits. At this point, she informs us that she has consumed significant amounts of alcohol since the age of 18 (6 to 12 alcoholic beverages per day, including beer and hard liquor). Therefore, based on this new information, on her jaundice and ascites, and on results of laboratory testing and biopsy, we confirmed our diagnosis of alcoholic hepatitis.
4. When is drug treatment appropriate for alcoholic hepatitis?
- Model for End-stage Liver Disease (MELD) score greater than 12
- MELD score greater than 15
- Maddrey Discriminant Function score greater than 25
- Maddrey Discriminant Function score greater than 32
- Glasgow score greater than 5
- Glasgow score greater than 7
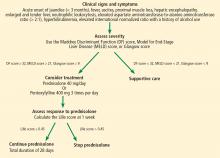
The best answer is a Maddrey Discriminant Function score greater than 32. A variety of scoring systems have been used to assess the severity of alcoholic hepatitis and to guide treatment, including the Maddrey Discriminant Function score, the MELD score, and the Glasgow score.11–16 They share similar laboratory values in their calculations, including prothrombin time (or INR) and total bilirubin.11–16 Typically, a Maddrey Discriminant Function score greater than 32, a Glasgow score of greater than 9, or a MELD score greater than 21 is used to determine whether pharmacologic treatment is indicated.11–16
The typical treatment is prednisolone or pentoxifylline.11,17–21 The Lille score is designed to help decide whether to stop corticosteroids after 1 week of administration due to lack of treatment response.22 It predicts mortality rates within 6 months; a score of 0.45 or less indicates a good prognosis, and corticosteroid therapy should continue for 28 days (Figure 3).22
Our patient’s discriminant function score is 50, her Glasgow score is 10, and her MELD score is 28; thus, she begins treatment with oral prednisolone. Her Lille score at 1 week is 0.119, indicating a good prognosis, and her corticosteroids are continued for a total of 28 days.
It should be highlighted that the most important treatment is abstinence from alcohol.11 Recent literature suggests that any benefit of prednisolone or pentoxifylline in terms of mortality rates is questionable,19–20 and there is evidence that giving both drugs simultaneously may improve mortality rates,11,21 but the evidence remains conflicting at this time.
ALCOHOLIC HEPATITIS
Alcoholic hepatitis is a clinical syndrome of jaundice and liver failure, often in the setting of heavy alcohol use for decades.11,12 The incidence is unknown, but the typical age of presentation is between 40 and 50.11,12 The chief sign is a rapid onset of jaundice (< 3 months); common signs and symptoms include fever, ascites, proximal muscle loss, and an enlarged, tender liver.12 Encephalopathy may be seen in severe alcoholic hepatitis.12
Our patient is 35 years old. She has jaundice with rapid onset, as well as ascites and a tender liver.
The diagnosis of alcoholic hepatitis must take into account the patient’s history, physical examination, and laboratory findings. Until proven otherwise, the diagnosis should be presumed in the following scenario: ascites and jaundice on examination (usually with a duration < 3 months); a history of heavy alcohol use; neutrophilic leukocytosis; an AST level that is elevated but below 300 U/L; an ALT level above the normal range but below 300 U/L; an AST-ALT ratio greater than 2; a total serum bilirubin level above 5 mg/dL; and an elevated INR.11,12 Liver biopsy is the gold standard for diagnosis. Though not routinely done because of risks associated with the procedure, it may help confirm the diagnosis if it is in question.
CASE CONCLUDED
We start our patient on oral prednisolone 40 mg daily for alcoholic hepatitis. Her symptoms and laboratory testing results including bilirubin improve. Her Lille score at 7 days indicates a good prognosis, prompting continuation of corticosteroid treatment for the full 28 days.
She is referred to an outpatient alcohol rehabilitation program and has remained sober as of the last outpatient note.
Alcoholic hepatitis is extremely difficult to diagnose, and no single blood test or imaging study confirms the diagnosis. The history, physical examination findings, and laboratory findings are crucial. If the diagnosis is still in doubt, liver biopsy may help confirm the diagnosis.
- Ruyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49(6):2087–2107. doi:10.1002/hep.22853
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2(4):399–407. pmid:7095741
- Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004; 350(16):1646–1654. doi:10.1056/NEJMra035021
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117(3):215–220. pmid:1616215
- Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016; 7(3):53–56. https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/cld.537
- Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2014; 2(1):58–64. doi:10.14218/JCTH.2013.00010
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 2012; 4(1):e2012026. doi:10.4084/MJHID.2012.026
- Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988; 8(1):27–31. pmid:3257456
- Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131(4):1049–1056. doi:10.1053/j.gastro.2006.07.010
- Runyon B; The American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis: update 2012. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. Accessed September 4, 2018.
- Sidhu SS, Goyal O, Kishore H, Sidhu S. New paradigms in management of alcoholic hepatitis: a review. Hepatol Int 2017; 11(3):255–267. doi:10.1007/s12072-017-9790-5
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360(26):2758–2769. doi:10.1056/NEJMra0805786
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75(2):193–199. pmid:352788
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005; 54(8):1174–1179. doi:10.1136/gut.2004.050781
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41(2):353–358. doi:10.1002/hep.20503
- Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2. pmid:11835693
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119(6):1637–1648. pmid:11113085
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60(2):255–260. doi:10.1136/gut.2010.224097
- Thursz MR, Richardson P, Allison M, et al; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372(17):1619–1628. doi:10.1056/NEJMoa1412278
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 × 2 factorial randomised controlled trial. Health Technol Assess 2015; 19(102):1–104. doi:10.3310/hta191020
- Lee YS, Kim HJ, Kim JH, et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017; 51(4):364–377. doi:10.1097/MCG.0000000000000674
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45(6):1348–1354. doi:10.1002/hep.21607
A 35-year-old woman is admitted to the hospital with a 5-day history of abdominal distention and jaundice. She reports no history of fever, chills, night sweats, abdominal pain, nausea, vomiting, diarrhea, changes in urine color, change in stool color, weight loss, weight gain, or loss of appetite.
She is petite, with a body mass index of 19.4 kg/m2. She has no known history of medical conditions or surgery and is not taking any medications. Her family history is unremarkable, and she denies current or past tobacco, alcohol, or illicit drug use.
RECENT TRAVEL
She says that during a trip to Central America several months ago, she had suffered a seizure and was taken to a local hospital, where laboratory testing revealed elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. She says that the rest of the workup at that time was normal.
About 1 week after that incident, she returned home and saw her primary care physician, who ordered further testing, which showed mild hyperbilirubinemia and mild elevation of AST and ALT levels. Her physician attributed the elevations to atovaquone, which she had been taking for malaria prophylaxis, as repeat testing 2 weeks later showed improvement in AST and ALT levels.
The patient says she returned to her normal state of health until about 5 days ago, when she noticed jaundice and abdominal distention, but without abdominal pain, dark urine, or clay-colored stools. She became concerned and went to her local hospital. Testing there noted mild elevation of AST and ALT, as well as an elevated international normalized ratio (INR) and hyperbilirubinemia. Computed tomography of the abdomen and pelvis showed hepatomegaly with possible fatty liver. Because of these results, the patient was transferred to our institution for further evaluation.
EVALUATION AT OUR INSTITUTION
On examination at our institution, she is afebrile, and vital signs are within normal ranges. She has bilateral scleral icterus and diffuse jaundice, but no other skin finding such as rash or spider angioma. She has no lymphadenopathy. Her abdomen is distended, with tense ascites, and her liver is tender to palpation. The tip of the spleen is not palpable.
The cardiovascular examination reveals no murmurs, rubs, or gallops, but she has jugular venous distention and +2 pitting edema of both lower extremities.
On respiratory examination, there is dullness to percussion, with slight crackles on auscultation at the right lung base. The neurologic examination is normal.
Table 1 shows the results of initial laboratory testing.
1. Which study would provide the most information on the cause of ascites?
- Abdominal ultrasonography
- Abdominal paracentesis with ascitic fluid analysis
- Chest radiography
- Echocardiography
- Urine protein-to-creatinine ratio
Abdominal paracentesis with ascitic fluid analysis is the essential study for any patient with clinically apparent new-onset ascites.1–3 It is the study that provides the most information on the cause of ascites.
In our patient, abdominal paracentesis yields 1,000 mL of straw-colored ascitic fluid, and analysis shows 86 nucleated cells, 28 of which are polymorphonuclear cells, and 0 red blood cells, with negative Gram stain and culture. The ascitic albumin level is 0.85 g/dL, with an ascitic protein of 1.1 g/dL.
Abdominal ultrasonography shows a diffusely echogenic liver, no focal lesions, moderate ascites, normal portal vein flow, no intrahepatic or extrahepatic biliary duct dilation, normal kidney sizes, no hydronephrosis, and no intra-abdominal mass. Chest radiography is clear with no sign of consolidation, edema, or effusion. Echocardiography shows a normal left ventricular ejection fraction with no valvular disease or pericardial effusion. A random urine protein-creatinine ratio is normal at 0.1 (reference range < 0.2).
2. What is the most likely cause of her ascites based on the workup to this point?
- Cirrhosis
- Heart failure
- Nephrotic syndrome
- Portal vein thrombus
- Abdominal malignancy
- Malaria
An initial approach to ascitic fluid analysis is to calculate the serum-ascites albumin gradient (SAAG). The SAAG is calculated as the serum albumin level minus the ascitic fluid albumin level.4,5 This is useful in determining the cause of the ascites (Figure 1).4,5 A gradient of 1.1 g/dL or higher indicates portal hypertension.4,5
Common causes of portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome, portal vein thrombosis), idiopathic portal fibrosis, and metastatic liver disease.5,6
If portal hypertension is present based on the SAAG, the next step is to review the ascitic protein level to help distinguish between a hepatic and a cardiac etiology of the ascites. An ascitic protein level less than 2.5 g/dL indicates a primary liver pathology (eg, cirrhosis). An ascitic protein level of 2.5 g/dL or greater typically indicates a cardiac condition (eg, heart failure, pericardial disease) with secondary congestive hepatopathy.5,6
If the SAAG is less than 1.1 g/dL, the ascites is likely not from portal hypertension. Typical causes of a low SAAG include infection, malignancy, pancreatic ascites, and nephrotic syndrome.5,6
In our patient, the SAAG is 1.35 g/dL (2.2 g/dL minus 0.85 g/dL), ie, elevated and due to portal hypertension. With an SAAG of 1.1 g/dL or greater and an ascitic fluid protein level less than 2.5 g/dL, as in our patient, the most likely cause is cirrhosis.
Heart failure is unlikely based on her normal brain natriuretic peptide level, an ascitic fluid protein level below 2.5 g/dL, and normal results on echocardiography. Nephrotic syndrome is also very unlikely based on the patient’s normal random urine protein-creatinine ratio. Portal vein thrombus and abdominal malignancy are essentially ruled out by the negative results of Doppler abdominal ultrasonography, with normal venous flow and no intra-abdominal mass and coupled with an elevated SAAG.
Although the patient has a history of travel, the incubation period for malaria would not fit the time frame of presentation. Also, she did not have typical malarial symptoms, her rapid malaria test was negative, and a peripheral blood smear for blood parasites was negative. It should be noted, however, that Plasmodium malariae infection classically presents with flulike symptoms and can resemble nephrotic syndrome, including peripheral edema, ascites, heavy proteinuria, hypoalbuminemia, and hyperlipidemia.7
3. In which patients is antibiotic prophylaxis against spontaneous bacterial peritonitis (SBP) appropriate?
- Any patient with cirrhosis
- Any patient with cirrhosis who is hospitalized
- Any patient with cirrhosis and an ascitic fluid protein level below 2.0 g/dL
- Any patient with cirrhosis and a history of SBP
Any patient with cirrhosis and a history of SBP should receive prophylactic antibiotics,8 as should any patient deemed at high risk of SBP. It is indicated in the following patients:
- Patients with cirrhosis and gastrointestinal bleeding9,10
- Patients with cirrhosis and a previous episode of SBP8
- Patients with cirrhosis and an ascitic fluid protein level less than 1.5 g/dL with either impaired renal function (creatinine ≥ 1.2 mg/dL, blood urea nitrogen level ≥ 25 mg/dL, or serum sodium ≤ 130 mmol/L) or liver failure (Child-Pugh score ≥ 9 and a bilirubin ≥ 3 mg/dL)9
- Patients with cirrhosis who are hospitalized for other reasons and have an ascitic protein level < 1.0 g/dL.9
Our patient has no signs or symptoms of gastrointestinal bleeding and no history of SBP. Her ascitic fluid protein level is 1.1 g/dL, and she has normal renal function. However, her Child-Pugh score is 12 (3 points for total bilirubin > 3 mg/dL, 3 points for serum albumin < 2.8 g/dL, 2 points for an INR 1.7 to 2.2, 3 points for moderate ascites, and 1 point for no encephalopathy), with a bilirubin of 17.0 mg/dL. Based on this, she is placed on antibiotic prophylaxis for SBP.
Our patient then undergoes an extensive workup for liver disease. Results of tests for toxins, autoimmune diseases, and inheritable diseases are all within normal limits. At this point, despite the patient’s reported negative alcohol history, our leading diagnosis is alcoholic hepatitis.
To confirm this diagnosis, she subsequently undergoes transjugular liver biopsy, considered the gold standard for the diagnosis of alcoholic hepatitis. During the procedure, the hepatic venous pressure gradient is measured at 18 mm Hg (reference range 1–5 mm Hg), suggestive of portal hypertension. The pathology study shows severe fatty change, active steatohepatitis with ballooning degeneration, easily identifiable Mallory-Denk bodies, and prominent neutrophilic infiltration, as well as extensive bridging fibrosis (Figure 2). These findings point to alcoholic hepatitis.
After the biopsy results, we speak with the patient further about her alcohol habits. At this point, she informs us that she has consumed significant amounts of alcohol since the age of 18 (6 to 12 alcoholic beverages per day, including beer and hard liquor). Therefore, based on this new information, on her jaundice and ascites, and on results of laboratory testing and biopsy, we confirmed our diagnosis of alcoholic hepatitis.
4. When is drug treatment appropriate for alcoholic hepatitis?
- Model for End-stage Liver Disease (MELD) score greater than 12
- MELD score greater than 15
- Maddrey Discriminant Function score greater than 25
- Maddrey Discriminant Function score greater than 32
- Glasgow score greater than 5
- Glasgow score greater than 7
The best answer is a Maddrey Discriminant Function score greater than 32. A variety of scoring systems have been used to assess the severity of alcoholic hepatitis and to guide treatment, including the Maddrey Discriminant Function score, the MELD score, and the Glasgow score.11–16 They share similar laboratory values in their calculations, including prothrombin time (or INR) and total bilirubin.11–16 Typically, a Maddrey Discriminant Function score greater than 32, a Glasgow score of greater than 9, or a MELD score greater than 21 is used to determine whether pharmacologic treatment is indicated.11–16
The typical treatment is prednisolone or pentoxifylline.11,17–21 The Lille score is designed to help decide whether to stop corticosteroids after 1 week of administration due to lack of treatment response.22 It predicts mortality rates within 6 months; a score of 0.45 or less indicates a good prognosis, and corticosteroid therapy should continue for 28 days (Figure 3).22
Our patient’s discriminant function score is 50, her Glasgow score is 10, and her MELD score is 28; thus, she begins treatment with oral prednisolone. Her Lille score at 1 week is 0.119, indicating a good prognosis, and her corticosteroids are continued for a total of 28 days.
It should be highlighted that the most important treatment is abstinence from alcohol.11 Recent literature suggests that any benefit of prednisolone or pentoxifylline in terms of mortality rates is questionable,19–20 and there is evidence that giving both drugs simultaneously may improve mortality rates,11,21 but the evidence remains conflicting at this time.
ALCOHOLIC HEPATITIS
Alcoholic hepatitis is a clinical syndrome of jaundice and liver failure, often in the setting of heavy alcohol use for decades.11,12 The incidence is unknown, but the typical age of presentation is between 40 and 50.11,12 The chief sign is a rapid onset of jaundice (< 3 months); common signs and symptoms include fever, ascites, proximal muscle loss, and an enlarged, tender liver.12 Encephalopathy may be seen in severe alcoholic hepatitis.12
Our patient is 35 years old. She has jaundice with rapid onset, as well as ascites and a tender liver.
The diagnosis of alcoholic hepatitis must take into account the patient’s history, physical examination, and laboratory findings. Until proven otherwise, the diagnosis should be presumed in the following scenario: ascites and jaundice on examination (usually with a duration < 3 months); a history of heavy alcohol use; neutrophilic leukocytosis; an AST level that is elevated but below 300 U/L; an ALT level above the normal range but below 300 U/L; an AST-ALT ratio greater than 2; a total serum bilirubin level above 5 mg/dL; and an elevated INR.11,12 Liver biopsy is the gold standard for diagnosis. Though not routinely done because of risks associated with the procedure, it may help confirm the diagnosis if it is in question.
CASE CONCLUDED
We start our patient on oral prednisolone 40 mg daily for alcoholic hepatitis. Her symptoms and laboratory testing results including bilirubin improve. Her Lille score at 7 days indicates a good prognosis, prompting continuation of corticosteroid treatment for the full 28 days.
She is referred to an outpatient alcohol rehabilitation program and has remained sober as of the last outpatient note.
Alcoholic hepatitis is extremely difficult to diagnose, and no single blood test or imaging study confirms the diagnosis. The history, physical examination findings, and laboratory findings are crucial. If the diagnosis is still in doubt, liver biopsy may help confirm the diagnosis.
A 35-year-old woman is admitted to the hospital with a 5-day history of abdominal distention and jaundice. She reports no history of fever, chills, night sweats, abdominal pain, nausea, vomiting, diarrhea, changes in urine color, change in stool color, weight loss, weight gain, or loss of appetite.
She is petite, with a body mass index of 19.4 kg/m2. She has no known history of medical conditions or surgery and is not taking any medications. Her family history is unremarkable, and she denies current or past tobacco, alcohol, or illicit drug use.
RECENT TRAVEL
She says that during a trip to Central America several months ago, she had suffered a seizure and was taken to a local hospital, where laboratory testing revealed elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. She says that the rest of the workup at that time was normal.
About 1 week after that incident, she returned home and saw her primary care physician, who ordered further testing, which showed mild hyperbilirubinemia and mild elevation of AST and ALT levels. Her physician attributed the elevations to atovaquone, which she had been taking for malaria prophylaxis, as repeat testing 2 weeks later showed improvement in AST and ALT levels.
The patient says she returned to her normal state of health until about 5 days ago, when she noticed jaundice and abdominal distention, but without abdominal pain, dark urine, or clay-colored stools. She became concerned and went to her local hospital. Testing there noted mild elevation of AST and ALT, as well as an elevated international normalized ratio (INR) and hyperbilirubinemia. Computed tomography of the abdomen and pelvis showed hepatomegaly with possible fatty liver. Because of these results, the patient was transferred to our institution for further evaluation.
EVALUATION AT OUR INSTITUTION
On examination at our institution, she is afebrile, and vital signs are within normal ranges. She has bilateral scleral icterus and diffuse jaundice, but no other skin finding such as rash or spider angioma. She has no lymphadenopathy. Her abdomen is distended, with tense ascites, and her liver is tender to palpation. The tip of the spleen is not palpable.
The cardiovascular examination reveals no murmurs, rubs, or gallops, but she has jugular venous distention and +2 pitting edema of both lower extremities.
On respiratory examination, there is dullness to percussion, with slight crackles on auscultation at the right lung base. The neurologic examination is normal.
Table 1 shows the results of initial laboratory testing.
1. Which study would provide the most information on the cause of ascites?
- Abdominal ultrasonography
- Abdominal paracentesis with ascitic fluid analysis
- Chest radiography
- Echocardiography
- Urine protein-to-creatinine ratio
Abdominal paracentesis with ascitic fluid analysis is the essential study for any patient with clinically apparent new-onset ascites.1–3 It is the study that provides the most information on the cause of ascites.
In our patient, abdominal paracentesis yields 1,000 mL of straw-colored ascitic fluid, and analysis shows 86 nucleated cells, 28 of which are polymorphonuclear cells, and 0 red blood cells, with negative Gram stain and culture. The ascitic albumin level is 0.85 g/dL, with an ascitic protein of 1.1 g/dL.
Abdominal ultrasonography shows a diffusely echogenic liver, no focal lesions, moderate ascites, normal portal vein flow, no intrahepatic or extrahepatic biliary duct dilation, normal kidney sizes, no hydronephrosis, and no intra-abdominal mass. Chest radiography is clear with no sign of consolidation, edema, or effusion. Echocardiography shows a normal left ventricular ejection fraction with no valvular disease or pericardial effusion. A random urine protein-creatinine ratio is normal at 0.1 (reference range < 0.2).
2. What is the most likely cause of her ascites based on the workup to this point?
- Cirrhosis
- Heart failure
- Nephrotic syndrome
- Portal vein thrombus
- Abdominal malignancy
- Malaria
An initial approach to ascitic fluid analysis is to calculate the serum-ascites albumin gradient (SAAG). The SAAG is calculated as the serum albumin level minus the ascitic fluid albumin level.4,5 This is useful in determining the cause of the ascites (Figure 1).4,5 A gradient of 1.1 g/dL or higher indicates portal hypertension.4,5
Common causes of portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome, portal vein thrombosis), idiopathic portal fibrosis, and metastatic liver disease.5,6
If portal hypertension is present based on the SAAG, the next step is to review the ascitic protein level to help distinguish between a hepatic and a cardiac etiology of the ascites. An ascitic protein level less than 2.5 g/dL indicates a primary liver pathology (eg, cirrhosis). An ascitic protein level of 2.5 g/dL or greater typically indicates a cardiac condition (eg, heart failure, pericardial disease) with secondary congestive hepatopathy.5,6
If the SAAG is less than 1.1 g/dL, the ascites is likely not from portal hypertension. Typical causes of a low SAAG include infection, malignancy, pancreatic ascites, and nephrotic syndrome.5,6
In our patient, the SAAG is 1.35 g/dL (2.2 g/dL minus 0.85 g/dL), ie, elevated and due to portal hypertension. With an SAAG of 1.1 g/dL or greater and an ascitic fluid protein level less than 2.5 g/dL, as in our patient, the most likely cause is cirrhosis.
Heart failure is unlikely based on her normal brain natriuretic peptide level, an ascitic fluid protein level below 2.5 g/dL, and normal results on echocardiography. Nephrotic syndrome is also very unlikely based on the patient’s normal random urine protein-creatinine ratio. Portal vein thrombus and abdominal malignancy are essentially ruled out by the negative results of Doppler abdominal ultrasonography, with normal venous flow and no intra-abdominal mass and coupled with an elevated SAAG.
Although the patient has a history of travel, the incubation period for malaria would not fit the time frame of presentation. Also, she did not have typical malarial symptoms, her rapid malaria test was negative, and a peripheral blood smear for blood parasites was negative. It should be noted, however, that Plasmodium malariae infection classically presents with flulike symptoms and can resemble nephrotic syndrome, including peripheral edema, ascites, heavy proteinuria, hypoalbuminemia, and hyperlipidemia.7
3. In which patients is antibiotic prophylaxis against spontaneous bacterial peritonitis (SBP) appropriate?
- Any patient with cirrhosis
- Any patient with cirrhosis who is hospitalized
- Any patient with cirrhosis and an ascitic fluid protein level below 2.0 g/dL
- Any patient with cirrhosis and a history of SBP
Any patient with cirrhosis and a history of SBP should receive prophylactic antibiotics,8 as should any patient deemed at high risk of SBP. It is indicated in the following patients:
- Patients with cirrhosis and gastrointestinal bleeding9,10
- Patients with cirrhosis and a previous episode of SBP8
- Patients with cirrhosis and an ascitic fluid protein level less than 1.5 g/dL with either impaired renal function (creatinine ≥ 1.2 mg/dL, blood urea nitrogen level ≥ 25 mg/dL, or serum sodium ≤ 130 mmol/L) or liver failure (Child-Pugh score ≥ 9 and a bilirubin ≥ 3 mg/dL)9
- Patients with cirrhosis who are hospitalized for other reasons and have an ascitic protein level < 1.0 g/dL.9
Our patient has no signs or symptoms of gastrointestinal bleeding and no history of SBP. Her ascitic fluid protein level is 1.1 g/dL, and she has normal renal function. However, her Child-Pugh score is 12 (3 points for total bilirubin > 3 mg/dL, 3 points for serum albumin < 2.8 g/dL, 2 points for an INR 1.7 to 2.2, 3 points for moderate ascites, and 1 point for no encephalopathy), with a bilirubin of 17.0 mg/dL. Based on this, she is placed on antibiotic prophylaxis for SBP.
Our patient then undergoes an extensive workup for liver disease. Results of tests for toxins, autoimmune diseases, and inheritable diseases are all within normal limits. At this point, despite the patient’s reported negative alcohol history, our leading diagnosis is alcoholic hepatitis.
To confirm this diagnosis, she subsequently undergoes transjugular liver biopsy, considered the gold standard for the diagnosis of alcoholic hepatitis. During the procedure, the hepatic venous pressure gradient is measured at 18 mm Hg (reference range 1–5 mm Hg), suggestive of portal hypertension. The pathology study shows severe fatty change, active steatohepatitis with ballooning degeneration, easily identifiable Mallory-Denk bodies, and prominent neutrophilic infiltration, as well as extensive bridging fibrosis (Figure 2). These findings point to alcoholic hepatitis.
After the biopsy results, we speak with the patient further about her alcohol habits. At this point, she informs us that she has consumed significant amounts of alcohol since the age of 18 (6 to 12 alcoholic beverages per day, including beer and hard liquor). Therefore, based on this new information, on her jaundice and ascites, and on results of laboratory testing and biopsy, we confirmed our diagnosis of alcoholic hepatitis.
4. When is drug treatment appropriate for alcoholic hepatitis?
- Model for End-stage Liver Disease (MELD) score greater than 12
- MELD score greater than 15
- Maddrey Discriminant Function score greater than 25
- Maddrey Discriminant Function score greater than 32
- Glasgow score greater than 5
- Glasgow score greater than 7
The best answer is a Maddrey Discriminant Function score greater than 32. A variety of scoring systems have been used to assess the severity of alcoholic hepatitis and to guide treatment, including the Maddrey Discriminant Function score, the MELD score, and the Glasgow score.11–16 They share similar laboratory values in their calculations, including prothrombin time (or INR) and total bilirubin.11–16 Typically, a Maddrey Discriminant Function score greater than 32, a Glasgow score of greater than 9, or a MELD score greater than 21 is used to determine whether pharmacologic treatment is indicated.11–16
The typical treatment is prednisolone or pentoxifylline.11,17–21 The Lille score is designed to help decide whether to stop corticosteroids after 1 week of administration due to lack of treatment response.22 It predicts mortality rates within 6 months; a score of 0.45 or less indicates a good prognosis, and corticosteroid therapy should continue for 28 days (Figure 3).22
Our patient’s discriminant function score is 50, her Glasgow score is 10, and her MELD score is 28; thus, she begins treatment with oral prednisolone. Her Lille score at 1 week is 0.119, indicating a good prognosis, and her corticosteroids are continued for a total of 28 days.
It should be highlighted that the most important treatment is abstinence from alcohol.11 Recent literature suggests that any benefit of prednisolone or pentoxifylline in terms of mortality rates is questionable,19–20 and there is evidence that giving both drugs simultaneously may improve mortality rates,11,21 but the evidence remains conflicting at this time.
ALCOHOLIC HEPATITIS
Alcoholic hepatitis is a clinical syndrome of jaundice and liver failure, often in the setting of heavy alcohol use for decades.11,12 The incidence is unknown, but the typical age of presentation is between 40 and 50.11,12 The chief sign is a rapid onset of jaundice (< 3 months); common signs and symptoms include fever, ascites, proximal muscle loss, and an enlarged, tender liver.12 Encephalopathy may be seen in severe alcoholic hepatitis.12
Our patient is 35 years old. She has jaundice with rapid onset, as well as ascites and a tender liver.
The diagnosis of alcoholic hepatitis must take into account the patient’s history, physical examination, and laboratory findings. Until proven otherwise, the diagnosis should be presumed in the following scenario: ascites and jaundice on examination (usually with a duration < 3 months); a history of heavy alcohol use; neutrophilic leukocytosis; an AST level that is elevated but below 300 U/L; an ALT level above the normal range but below 300 U/L; an AST-ALT ratio greater than 2; a total serum bilirubin level above 5 mg/dL; and an elevated INR.11,12 Liver biopsy is the gold standard for diagnosis. Though not routinely done because of risks associated with the procedure, it may help confirm the diagnosis if it is in question.
CASE CONCLUDED
We start our patient on oral prednisolone 40 mg daily for alcoholic hepatitis. Her symptoms and laboratory testing results including bilirubin improve. Her Lille score at 7 days indicates a good prognosis, prompting continuation of corticosteroid treatment for the full 28 days.
She is referred to an outpatient alcohol rehabilitation program and has remained sober as of the last outpatient note.
Alcoholic hepatitis is extremely difficult to diagnose, and no single blood test or imaging study confirms the diagnosis. The history, physical examination findings, and laboratory findings are crucial. If the diagnosis is still in doubt, liver biopsy may help confirm the diagnosis.
- Ruyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49(6):2087–2107. doi:10.1002/hep.22853
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2(4):399–407. pmid:7095741
- Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004; 350(16):1646–1654. doi:10.1056/NEJMra035021
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117(3):215–220. pmid:1616215
- Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016; 7(3):53–56. https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/cld.537
- Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2014; 2(1):58–64. doi:10.14218/JCTH.2013.00010
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 2012; 4(1):e2012026. doi:10.4084/MJHID.2012.026
- Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988; 8(1):27–31. pmid:3257456
- Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131(4):1049–1056. doi:10.1053/j.gastro.2006.07.010
- Runyon B; The American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis: update 2012. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. Accessed September 4, 2018.
- Sidhu SS, Goyal O, Kishore H, Sidhu S. New paradigms in management of alcoholic hepatitis: a review. Hepatol Int 2017; 11(3):255–267. doi:10.1007/s12072-017-9790-5
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360(26):2758–2769. doi:10.1056/NEJMra0805786
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75(2):193–199. pmid:352788
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005; 54(8):1174–1179. doi:10.1136/gut.2004.050781
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41(2):353–358. doi:10.1002/hep.20503
- Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2. pmid:11835693
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119(6):1637–1648. pmid:11113085
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60(2):255–260. doi:10.1136/gut.2010.224097
- Thursz MR, Richardson P, Allison M, et al; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372(17):1619–1628. doi:10.1056/NEJMoa1412278
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 × 2 factorial randomised controlled trial. Health Technol Assess 2015; 19(102):1–104. doi:10.3310/hta191020
- Lee YS, Kim HJ, Kim JH, et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017; 51(4):364–377. doi:10.1097/MCG.0000000000000674
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45(6):1348–1354. doi:10.1002/hep.21607
- Ruyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009; 49(6):2087–2107. doi:10.1002/hep.22853
- Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology 1982; 2(4):399–407. pmid:7095741
- Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004; 350(16):1646–1654. doi:10.1056/NEJMra035021
- Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med 1992; 117(3):215–220. pmid:1616215
- Hernaez R, Hamilton JP. Unexplained ascites. Clin Liver Dis 2016; 7(3):53–56. https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/cld.537
- Huang LL, Xia HH, Zhu SL. Ascitic fluid analysis in the differential diagnosis of ascites: focus on cirrhotic ascites. J Clin Transl Hepatol 2014; 2(1):58–64. doi:10.14218/JCTH.2013.00010
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis 2012; 4(1):e2012026. doi:10.4084/MJHID.2012.026
- Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology 1988; 8(1):27–31. pmid:3257456
- Fernández J, Ruiz del Arbol L, Gómez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006; 131(4):1049–1056. doi:10.1053/j.gastro.2006.07.010
- Runyon B; The American Association for the Study of Liver Diseases (AASLD). Management of adult patients with ascites due to cirrhosis: update 2012. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf. Accessed September 4, 2018.
- Sidhu SS, Goyal O, Kishore H, Sidhu S. New paradigms in management of alcoholic hepatitis: a review. Hepatol Int 2017; 11(3):255–267. doi:10.1007/s12072-017-9790-5
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360(26):2758–2769. doi:10.1056/NEJMra0805786
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75(2):193–199. pmid:352788
- Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005; 54(8):1174–1179. doi:10.1136/gut.2004.050781
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41(2):353–358. doi:10.1002/hep.20503
- Sheth M, Riggs M, Patel T. Utility of the Mayo end-stage liver disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2. pmid:11835693
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119(6):1637–1648. pmid:11113085
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60(2):255–260. doi:10.1136/gut.2010.224097
- Thursz MR, Richardson P, Allison M, et al; STOPAH Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372(17):1619–1628. doi:10.1056/NEJMoa1412278
- Thursz M, Forrest E, Roderick P, et al. The clinical effectiveness and cost-effectiveness of steroids or pentoxifylline for alcoholic hepatitis (STOPAH): a 2 × 2 factorial randomised controlled trial. Health Technol Assess 2015; 19(102):1–104. doi:10.3310/hta191020
- Lee YS, Kim HJ, Kim JH, et al. Treatment of severe alcoholic hepatitis with corticosteroid, pentoxifylline, or dual therapy: a systematic review and meta-analysis. J Clin Gastroenterol 2017; 51(4):364–377. doi:10.1097/MCG.0000000000000674
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45(6):1348–1354. doi:10.1002/hep.21607
Is neuroimaging necessary to evaluate syncope?
A 40-year-old woman with a history of hypertension, who was recently started on a diuretic, presents to the emergency department after a witnessed syncopal event. She reports a prodrome of lightheadedness, nausea, and darkening of her vision that occurred a few seconds after standing, followed by loss of consciousness. She had a complete, spontaneous recovery after 10 seconds, but upon arousal she noticed she had lost bladder control.
Her blood pressure is 120/80 mm Hg supine, 110/70 mm Hg sitting, and 90/60 mm Hg standing. She has no focal neurologic deficits. The cardiac examination is normal, without murmurs, and electrocardiography shows sinus tachycardia (heart rate 110 bpm) without other abnormalities. Results of laboratory testing are unremarkable.
Should you order neuroimaging to evaluate for syncope?
DEFINITIONS, CLASSIFICATIONS
Syncope is an abrupt loss of consciousness due to transient global cerebral hypoperfusion, with a concomitant loss of postural tone and rapid, spontaneous recovery.1 Recovery from syncope is characterized by immediate restoration of orientation and normal behavior, although the period after recovery may be accompanied by fatigue.2
The European Society of Cardiology2 has classified syncope into 3 main categories: reflex (neurally mediated) syncope, syncope due to orthostatic hypotension, and cardiac syncope. Determining the cause is critical, as this determines the prognosis.
KEYS TO THE EVALUATION
According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) and the 2009 European Society of Cardiology guidelines, the evaluation of syncope should include a thorough history, taken from the patient and witnesses, and a complete physical examination. This can identify the cause of syncope in up to 50% of cases and differentiate between cardiac and noncardiac causes. Features that point to cardiac syncope include age older than 60, male sex, known heart disease, brief prodrome, syncope during exertion or when supine, first syncopal event, family history of sudden cardiac death, and abnormal physical examination.1
Features that suggest noncardiac syncope are young age; syncope only when standing; recurrent syncope; a prodrome of nausea, vomiting, and a warm sensation; and triggers such as dehydration, pain, distressful stimulus, cough, laugh micturition, defecation, and swallowing.1
Electrocardiography should follow the history and physical examination. When done at presentation, electrocardiography is diagnostic in only about 5% of cases. However, given the importance of the diagnosis, it remains an essential part of the initial evaluation of syncope.3
If a clear cause of syncope is identified at this point, no further workup is needed, and the cause of syncope should be addressed.1 If the cause is still unclear, the ACC/AHA guidelines recommend further evaluation based on the clinical presentation and risk stratification.
WHEN TO PURSUE ADDITIONAL TESTING
Routine use of additional testing is costly; tests should be ordered on the basis of their potential diagnostic and prognostic value. Additional evaluation should follow a stepwise approach and can include targeted blood work, autonomic nerve evaluation, tilt-table testing, transthoracic echocardiography, stress testing, electrocardiographic monitoring, and electrophysiologic testing.1
Syncope is rarely a manifestation of neurologic disease, yet 11% to 58% of patients with a first episode of uncomplicated syncope undergo extensive neuroimaging with magnetic resonance imaging, computed tomography, electroencephalography (EEG), and carotid ultrasonography.4 Evidence suggests that routine neurologic testing is of limited value given its low diagnostic yield and high cost.
Epilepsy is the most common neurologic cause of loss of consciousness but is estimated to account for less than 5% of patients with syncope.5 A thorough and thoughtful neurologic history and examination is often enough to distinguish between syncope, convulsive syncope, epileptic convulsions, and pseudosyncope.
In syncope, the loss of consciousness usually occurs 30 seconds to several minutes after standing. It presents with or without a prodrome (warmth, palpitations, and diaphoresis) and can be relieved with supine positioning. True loss of consciousness usually lasts less than a minute and is accompanied by loss of postural tone, with little or no fatigue in the recovery period.6
Conversely, in convulsive syncope, the prodrome can include pallor and diaphoresis. Loss of consciousness lasts about 30 seconds but is accompanied by fixed gaze, upward eye deviation, nuchal rigidity, tonic spasms, myoclonic jerks, tonic-clonic convulsions, and oral automatisms.6
Pseudosyncope is characterized by a prodrome of lightheadedness, shortness of breath, chest pain, and tingling sensations, followed by episodes of apparent loss of consciousness that last longer than several minutes and occur multiple times a day. During these episodes, patients purposefully try to avoid trauma when they lose consciousness, and almost always keep their eyes closed, in contrast to syncopal episodes, when the eyes are open and glassy.7
ROLE OF ELECTROENCEPHALOGRAPHY
If the diagnosis remains unclear after the history and neurologic examination, EEG is recommended (class IIa, ie, reasonable, can be useful) during tilt-table testing, as it can help differentiate syncope, pseudosyncope, and epilepsy.1
In an epileptic convulsion, EEG shows epileptiform discharges, whereas in syncope, it shows diffuse brainwave slowing with delta waves and a flatline pattern. In pseudosyncope and psychogenic nonepileptic seizures, EEG shows normal activity.8
Routine EEG is not recommended if there are no specific neurologic signs of epilepsy or if the history and neurologic examination indicate syncope or pseudosyncope.1
Structural brain disease does not typically present with transient global cerebral hypoperfusion resulting in syncope, so magnetic resonance imaging and computed tomography have a low diagnostic yield. Studies have revealed that for the 11% to 58% of patients who undergo neuroimaging, it establishes a diagnosis in only 0.2% to 1%.9 For this reason and in view of their high cost, these imaging tests should not be routinely ordered in the evaluation of syncope.4,10 Similarly, carotid artery imaging should not be routinely ordered if there is no focal neurologic finding suggesting unilateral ischemia.10
CASE CONTINUED
In our 40-year-old patient, the history suggests dehydration, as she recently started taking a diuretic. Thus, laboratory testing is reasonable.
Loss of bladder control is often interpreted as a red flag for neurologic disease, but syncope can often present with urinary incontinence. Urinary incontinence may also occur in epileptic seizure and in nonepileptic events such as syncope. A pooled analysis by Brigo et al11 determined that urinary incontinence had no value in distinguishing between epilepsy and syncope. Therefore, this physical finding should not incline the clinician to one diagnosis or the other.
Given our patient’s presentation, findings on physical examination, and absence of focal neurologic deficits, she should not undergo neuroimaging for syncope evaluation. The more likely cause of her syncope is orthostatic intolerance (orthostatic hypotension or vasovagal syncope) in the setting of intravascular volume depletion, likely secondary to diuretic use. Obtaining orthostatic vital signs is mandatory, and this confirms the diagnosis.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70(5):e39–e110. doi:10.1016/j.jacc.2017.03.003
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009; 30(21):2631–2671. doi:10.1093/eurheartj/ehp298
- Mehlsen J, Kaijer MN, Mehlsen AB. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008; 10(1):91–95. doi:10.1093/europace/eum237
- Goyal N, Donnino MW, Vachhani R, Bajwa R, Ahmad T, Otero R. The utility of head computed tomography in the emergency department evaluation of syncope. Intern Emerg Med 2006; 1(2):148–150. pmid:17111790
- Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med 1983; 309(4):197–204. doi:10.1056/NEJM198307283090401
- Sheldon R. How to differentiate syncope from seizure. Cardiol Clin 2015; 33(3):377–385. doi:10.1016/j.ccl.2015.04.006
- Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014; 184:66–72. doi:10.1016/j.autneu.2014.05.003
- Mecarelli O, Pulitano P, Vicenzini E, Vanacore N, Accornero N, De Marinis M. Observations on EEG patterns in neurally-mediated syncope: an inspective and quantitative study. Neurophysiol Clin 2004; 34(5):203–207. doi:10.1016/j.neucli.2004.09.004
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 2014; 107(11):707–714. doi:10.14423/SMJ.0000000000000184
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 2010; 170(13):1150–1154. doi:10.1001/archinternmed.2010.205
- Brigo F, Nardone R Ausserer H, et al. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure 2013; 22(2):85–90. doi:10.1016/j.seizure.2012.10.011
A 40-year-old woman with a history of hypertension, who was recently started on a diuretic, presents to the emergency department after a witnessed syncopal event. She reports a prodrome of lightheadedness, nausea, and darkening of her vision that occurred a few seconds after standing, followed by loss of consciousness. She had a complete, spontaneous recovery after 10 seconds, but upon arousal she noticed she had lost bladder control.
Her blood pressure is 120/80 mm Hg supine, 110/70 mm Hg sitting, and 90/60 mm Hg standing. She has no focal neurologic deficits. The cardiac examination is normal, without murmurs, and electrocardiography shows sinus tachycardia (heart rate 110 bpm) without other abnormalities. Results of laboratory testing are unremarkable.
Should you order neuroimaging to evaluate for syncope?
DEFINITIONS, CLASSIFICATIONS
Syncope is an abrupt loss of consciousness due to transient global cerebral hypoperfusion, with a concomitant loss of postural tone and rapid, spontaneous recovery.1 Recovery from syncope is characterized by immediate restoration of orientation and normal behavior, although the period after recovery may be accompanied by fatigue.2
The European Society of Cardiology2 has classified syncope into 3 main categories: reflex (neurally mediated) syncope, syncope due to orthostatic hypotension, and cardiac syncope. Determining the cause is critical, as this determines the prognosis.
KEYS TO THE EVALUATION
According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) and the 2009 European Society of Cardiology guidelines, the evaluation of syncope should include a thorough history, taken from the patient and witnesses, and a complete physical examination. This can identify the cause of syncope in up to 50% of cases and differentiate between cardiac and noncardiac causes. Features that point to cardiac syncope include age older than 60, male sex, known heart disease, brief prodrome, syncope during exertion or when supine, first syncopal event, family history of sudden cardiac death, and abnormal physical examination.1
Features that suggest noncardiac syncope are young age; syncope only when standing; recurrent syncope; a prodrome of nausea, vomiting, and a warm sensation; and triggers such as dehydration, pain, distressful stimulus, cough, laugh micturition, defecation, and swallowing.1
Electrocardiography should follow the history and physical examination. When done at presentation, electrocardiography is diagnostic in only about 5% of cases. However, given the importance of the diagnosis, it remains an essential part of the initial evaluation of syncope.3
If a clear cause of syncope is identified at this point, no further workup is needed, and the cause of syncope should be addressed.1 If the cause is still unclear, the ACC/AHA guidelines recommend further evaluation based on the clinical presentation and risk stratification.
WHEN TO PURSUE ADDITIONAL TESTING
Routine use of additional testing is costly; tests should be ordered on the basis of their potential diagnostic and prognostic value. Additional evaluation should follow a stepwise approach and can include targeted blood work, autonomic nerve evaluation, tilt-table testing, transthoracic echocardiography, stress testing, electrocardiographic monitoring, and electrophysiologic testing.1
Syncope is rarely a manifestation of neurologic disease, yet 11% to 58% of patients with a first episode of uncomplicated syncope undergo extensive neuroimaging with magnetic resonance imaging, computed tomography, electroencephalography (EEG), and carotid ultrasonography.4 Evidence suggests that routine neurologic testing is of limited value given its low diagnostic yield and high cost.
Epilepsy is the most common neurologic cause of loss of consciousness but is estimated to account for less than 5% of patients with syncope.5 A thorough and thoughtful neurologic history and examination is often enough to distinguish between syncope, convulsive syncope, epileptic convulsions, and pseudosyncope.
In syncope, the loss of consciousness usually occurs 30 seconds to several minutes after standing. It presents with or without a prodrome (warmth, palpitations, and diaphoresis) and can be relieved with supine positioning. True loss of consciousness usually lasts less than a minute and is accompanied by loss of postural tone, with little or no fatigue in the recovery period.6
Conversely, in convulsive syncope, the prodrome can include pallor and diaphoresis. Loss of consciousness lasts about 30 seconds but is accompanied by fixed gaze, upward eye deviation, nuchal rigidity, tonic spasms, myoclonic jerks, tonic-clonic convulsions, and oral automatisms.6
Pseudosyncope is characterized by a prodrome of lightheadedness, shortness of breath, chest pain, and tingling sensations, followed by episodes of apparent loss of consciousness that last longer than several minutes and occur multiple times a day. During these episodes, patients purposefully try to avoid trauma when they lose consciousness, and almost always keep their eyes closed, in contrast to syncopal episodes, when the eyes are open and glassy.7
ROLE OF ELECTROENCEPHALOGRAPHY
If the diagnosis remains unclear after the history and neurologic examination, EEG is recommended (class IIa, ie, reasonable, can be useful) during tilt-table testing, as it can help differentiate syncope, pseudosyncope, and epilepsy.1
In an epileptic convulsion, EEG shows epileptiform discharges, whereas in syncope, it shows diffuse brainwave slowing with delta waves and a flatline pattern. In pseudosyncope and psychogenic nonepileptic seizures, EEG shows normal activity.8
Routine EEG is not recommended if there are no specific neurologic signs of epilepsy or if the history and neurologic examination indicate syncope or pseudosyncope.1
Structural brain disease does not typically present with transient global cerebral hypoperfusion resulting in syncope, so magnetic resonance imaging and computed tomography have a low diagnostic yield. Studies have revealed that for the 11% to 58% of patients who undergo neuroimaging, it establishes a diagnosis in only 0.2% to 1%.9 For this reason and in view of their high cost, these imaging tests should not be routinely ordered in the evaluation of syncope.4,10 Similarly, carotid artery imaging should not be routinely ordered if there is no focal neurologic finding suggesting unilateral ischemia.10
CASE CONTINUED
In our 40-year-old patient, the history suggests dehydration, as she recently started taking a diuretic. Thus, laboratory testing is reasonable.
Loss of bladder control is often interpreted as a red flag for neurologic disease, but syncope can often present with urinary incontinence. Urinary incontinence may also occur in epileptic seizure and in nonepileptic events such as syncope. A pooled analysis by Brigo et al11 determined that urinary incontinence had no value in distinguishing between epilepsy and syncope. Therefore, this physical finding should not incline the clinician to one diagnosis or the other.
Given our patient’s presentation, findings on physical examination, and absence of focal neurologic deficits, she should not undergo neuroimaging for syncope evaluation. The more likely cause of her syncope is orthostatic intolerance (orthostatic hypotension or vasovagal syncope) in the setting of intravascular volume depletion, likely secondary to diuretic use. Obtaining orthostatic vital signs is mandatory, and this confirms the diagnosis.
A 40-year-old woman with a history of hypertension, who was recently started on a diuretic, presents to the emergency department after a witnessed syncopal event. She reports a prodrome of lightheadedness, nausea, and darkening of her vision that occurred a few seconds after standing, followed by loss of consciousness. She had a complete, spontaneous recovery after 10 seconds, but upon arousal she noticed she had lost bladder control.
Her blood pressure is 120/80 mm Hg supine, 110/70 mm Hg sitting, and 90/60 mm Hg standing. She has no focal neurologic deficits. The cardiac examination is normal, without murmurs, and electrocardiography shows sinus tachycardia (heart rate 110 bpm) without other abnormalities. Results of laboratory testing are unremarkable.
Should you order neuroimaging to evaluate for syncope?
DEFINITIONS, CLASSIFICATIONS
Syncope is an abrupt loss of consciousness due to transient global cerebral hypoperfusion, with a concomitant loss of postural tone and rapid, spontaneous recovery.1 Recovery from syncope is characterized by immediate restoration of orientation and normal behavior, although the period after recovery may be accompanied by fatigue.2
The European Society of Cardiology2 has classified syncope into 3 main categories: reflex (neurally mediated) syncope, syncope due to orthostatic hypotension, and cardiac syncope. Determining the cause is critical, as this determines the prognosis.
KEYS TO THE EVALUATION
According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) and the 2009 European Society of Cardiology guidelines, the evaluation of syncope should include a thorough history, taken from the patient and witnesses, and a complete physical examination. This can identify the cause of syncope in up to 50% of cases and differentiate between cardiac and noncardiac causes. Features that point to cardiac syncope include age older than 60, male sex, known heart disease, brief prodrome, syncope during exertion or when supine, first syncopal event, family history of sudden cardiac death, and abnormal physical examination.1
Features that suggest noncardiac syncope are young age; syncope only when standing; recurrent syncope; a prodrome of nausea, vomiting, and a warm sensation; and triggers such as dehydration, pain, distressful stimulus, cough, laugh micturition, defecation, and swallowing.1
Electrocardiography should follow the history and physical examination. When done at presentation, electrocardiography is diagnostic in only about 5% of cases. However, given the importance of the diagnosis, it remains an essential part of the initial evaluation of syncope.3
If a clear cause of syncope is identified at this point, no further workup is needed, and the cause of syncope should be addressed.1 If the cause is still unclear, the ACC/AHA guidelines recommend further evaluation based on the clinical presentation and risk stratification.
WHEN TO PURSUE ADDITIONAL TESTING
Routine use of additional testing is costly; tests should be ordered on the basis of their potential diagnostic and prognostic value. Additional evaluation should follow a stepwise approach and can include targeted blood work, autonomic nerve evaluation, tilt-table testing, transthoracic echocardiography, stress testing, electrocardiographic monitoring, and electrophysiologic testing.1
Syncope is rarely a manifestation of neurologic disease, yet 11% to 58% of patients with a first episode of uncomplicated syncope undergo extensive neuroimaging with magnetic resonance imaging, computed tomography, electroencephalography (EEG), and carotid ultrasonography.4 Evidence suggests that routine neurologic testing is of limited value given its low diagnostic yield and high cost.
Epilepsy is the most common neurologic cause of loss of consciousness but is estimated to account for less than 5% of patients with syncope.5 A thorough and thoughtful neurologic history and examination is often enough to distinguish between syncope, convulsive syncope, epileptic convulsions, and pseudosyncope.
In syncope, the loss of consciousness usually occurs 30 seconds to several minutes after standing. It presents with or without a prodrome (warmth, palpitations, and diaphoresis) and can be relieved with supine positioning. True loss of consciousness usually lasts less than a minute and is accompanied by loss of postural tone, with little or no fatigue in the recovery period.6
Conversely, in convulsive syncope, the prodrome can include pallor and diaphoresis. Loss of consciousness lasts about 30 seconds but is accompanied by fixed gaze, upward eye deviation, nuchal rigidity, tonic spasms, myoclonic jerks, tonic-clonic convulsions, and oral automatisms.6
Pseudosyncope is characterized by a prodrome of lightheadedness, shortness of breath, chest pain, and tingling sensations, followed by episodes of apparent loss of consciousness that last longer than several minutes and occur multiple times a day. During these episodes, patients purposefully try to avoid trauma when they lose consciousness, and almost always keep their eyes closed, in contrast to syncopal episodes, when the eyes are open and glassy.7
ROLE OF ELECTROENCEPHALOGRAPHY
If the diagnosis remains unclear after the history and neurologic examination, EEG is recommended (class IIa, ie, reasonable, can be useful) during tilt-table testing, as it can help differentiate syncope, pseudosyncope, and epilepsy.1
In an epileptic convulsion, EEG shows epileptiform discharges, whereas in syncope, it shows diffuse brainwave slowing with delta waves and a flatline pattern. In pseudosyncope and psychogenic nonepileptic seizures, EEG shows normal activity.8
Routine EEG is not recommended if there are no specific neurologic signs of epilepsy or if the history and neurologic examination indicate syncope or pseudosyncope.1
Structural brain disease does not typically present with transient global cerebral hypoperfusion resulting in syncope, so magnetic resonance imaging and computed tomography have a low diagnostic yield. Studies have revealed that for the 11% to 58% of patients who undergo neuroimaging, it establishes a diagnosis in only 0.2% to 1%.9 For this reason and in view of their high cost, these imaging tests should not be routinely ordered in the evaluation of syncope.4,10 Similarly, carotid artery imaging should not be routinely ordered if there is no focal neurologic finding suggesting unilateral ischemia.10
CASE CONTINUED
In our 40-year-old patient, the history suggests dehydration, as she recently started taking a diuretic. Thus, laboratory testing is reasonable.
Loss of bladder control is often interpreted as a red flag for neurologic disease, but syncope can often present with urinary incontinence. Urinary incontinence may also occur in epileptic seizure and in nonepileptic events such as syncope. A pooled analysis by Brigo et al11 determined that urinary incontinence had no value in distinguishing between epilepsy and syncope. Therefore, this physical finding should not incline the clinician to one diagnosis or the other.
Given our patient’s presentation, findings on physical examination, and absence of focal neurologic deficits, she should not undergo neuroimaging for syncope evaluation. The more likely cause of her syncope is orthostatic intolerance (orthostatic hypotension or vasovagal syncope) in the setting of intravascular volume depletion, likely secondary to diuretic use. Obtaining orthostatic vital signs is mandatory, and this confirms the diagnosis.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70(5):e39–e110. doi:10.1016/j.jacc.2017.03.003
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009; 30(21):2631–2671. doi:10.1093/eurheartj/ehp298
- Mehlsen J, Kaijer MN, Mehlsen AB. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008; 10(1):91–95. doi:10.1093/europace/eum237
- Goyal N, Donnino MW, Vachhani R, Bajwa R, Ahmad T, Otero R. The utility of head computed tomography in the emergency department evaluation of syncope. Intern Emerg Med 2006; 1(2):148–150. pmid:17111790
- Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med 1983; 309(4):197–204. doi:10.1056/NEJM198307283090401
- Sheldon R. How to differentiate syncope from seizure. Cardiol Clin 2015; 33(3):377–385. doi:10.1016/j.ccl.2015.04.006
- Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014; 184:66–72. doi:10.1016/j.autneu.2014.05.003
- Mecarelli O, Pulitano P, Vicenzini E, Vanacore N, Accornero N, De Marinis M. Observations on EEG patterns in neurally-mediated syncope: an inspective and quantitative study. Neurophysiol Clin 2004; 34(5):203–207. doi:10.1016/j.neucli.2004.09.004
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 2014; 107(11):707–714. doi:10.14423/SMJ.0000000000000184
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 2010; 170(13):1150–1154. doi:10.1001/archinternmed.2010.205
- Brigo F, Nardone R Ausserer H, et al. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure 2013; 22(2):85–90. doi:10.1016/j.seizure.2012.10.011
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017; 70(5):e39–e110. doi:10.1016/j.jacc.2017.03.003
- Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009; 30(21):2631–2671. doi:10.1093/eurheartj/ehp298
- Mehlsen J, Kaijer MN, Mehlsen AB. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008; 10(1):91–95. doi:10.1093/europace/eum237
- Goyal N, Donnino MW, Vachhani R, Bajwa R, Ahmad T, Otero R. The utility of head computed tomography in the emergency department evaluation of syncope. Intern Emerg Med 2006; 1(2):148–150. pmid:17111790
- Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med 1983; 309(4):197–204. doi:10.1056/NEJM198307283090401
- Sheldon R. How to differentiate syncope from seizure. Cardiol Clin 2015; 33(3):377–385. doi:10.1016/j.ccl.2015.04.006
- Raj V, Rowe AA, Fleisch SB, Paranjape SY, Arain AM, Nicolson SE. Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci 2014; 184:66–72. doi:10.1016/j.autneu.2014.05.003
- Mecarelli O, Pulitano P, Vicenzini E, Vanacore N, Accornero N, De Marinis M. Observations on EEG patterns in neurally-mediated syncope: an inspective and quantitative study. Neurophysiol Clin 2004; 34(5):203–207. doi:10.1016/j.neucli.2004.09.004
- Johnson PC, Ammar H, Zohdy W, Fouda R, Govindu R. Yield of diagnostic tests and its impact on cost in adult patients with syncope presenting to a community hospital. South Med J 2014; 107(11):707–714. doi:10.14423/SMJ.0000000000000184
- Sclafani JJ, My J, Zacher LL, Eckart RE. Intensive education on evidence-based evaluation of syncope increases sudden death risk stratification but fails to reduce use of neuroimaging. Arch Intern Med 2010; 170(13):1150–1154. doi:10.1001/archinternmed.2010.205
- Brigo F, Nardone R Ausserer H, et al. The diagnostic value of urinary incontinence in the differential diagnosis of seizures. Seizure 2013; 22(2):85–90. doi:10.1016/j.seizure.2012.10.011
Personalizing guideline-driven cancer screening
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
The old humanities and the new science at 100: Osler’s enduring message
“Twin berries on one stem, grievous damage has been done to both in regarding the Humanities and Science in any other light than complemental.”
—Sir William Osler1
The year 2019 marks the 100th anniversary of Sir William Osler’s last public speech. Still reeling from the death of his only son in World War I, he had been asked to give the presidential inaugural address of the Classical Association at Oxford. It was the first time a physician had received the honor, and Osler took the assignment very seriously. He chose to speak about “The old humanities and the new science,” and to call for a reunification of the two fields. “Humanists have not enough Science” he warned, “and Science sadly lacks the Humanities…this unhappy divorce…should never have taken place.”1 Later, he said that it was the speech to which he had given the greatest thought and preparation. It was in fact Osler’s personal legacy: 2 months later he turned 70, and 7 months later he was dead.
Revisiting the address today, what can Osler teach the high-tech physician of today, when doctors have become “providers” and patients “consumers”? Is Osler’s message still relevant to our craft, or has he simply become an icon of professional nostalgia with little value for our times?
THE NEED FOR THE HUMANITIES IN MEDICINE
Medicine has certainly grown both powerful and successful. Yet it is also confronting hurdles that would have been unimaginable in Osler’s time. Physicians are now the professionals with the highest suicide rate,2 a burnout rate as high as 70%,3,4 rampant depression,5 dwindling empathy,6 a predominantly negative perception by the public,7,8 and a disturbing propensity to quit.9 These, of course, may just be symptoms of an increasingly meaningless environment wherein doctors have become small cogs in a medical-industrial complex they can’t control or even understand. Still, is it possible that something more personal may have been lost in the way we now select and educate physicians? Could this, in turn, make us less resilient?
In this regard, Osler’s last public speech serves as an enduring reminder of the need for the humanities in medicine. Osler not only believed it, but throughout his life never missed a chance to express in words, writings, and deeds that the humanities are indeed “the hormones” of the profession. In 1919 he warned against the risk of separating our humanistic tradition from the sciences, and urged us “to infect [anyone] with the spirit of the Humanities,” since to him that was “the greatest single gift in education.”1
Unfortunately, the humanities are slippery, not easily quantifiable, hard to define, and seemingly incompatible with an evidence-based approach. Quite understandably, today’s data-obsessed medicine views them with suspicion. But besides reminding us that in medicine not all that counts can be counted, and not all that can be counted counts, the humanities are in fact a fundamental component of the physician’s skill set.
In a multicenter survey of 5 medical schools,10 there was indeed a correlation between students’ exposure to the humanities and many of the personal qualities whose absence we lament in today’s medicine: empathy, tolerance for ambiguity, emotional intelligence, and prevention of burnout. Most significant was a strong correlation with wisdom, as measured by the 21-item Brief Wisdom Screening Scale.11 That all these traits may correlate with humanities exposure is intuitive, since the humanities not only teach tolerance and compassion, but also capture the collective experience of those who came before us. Hence, they teach us wisdom. Wisdom is not an ACGME competency, but it’s undoubtedly a prerequisite for the art of healing.12 In fact, wisdom may very well be the fundamental trait that characterizes a well-rounded physician, since it encompasses empathy, resilience, comfort with ambiguity, and the capacity to learn from the past. Not surprisingly, wisdom in the world was Osler’s closing wish in 1919.
The humanities can also nurture the very personal qualities we desire in physicians. For example, observing drama fosters empathy,13 as does taking an elective in medical humanities.14 Drawing enhances the reading of faces,15 and observing art improves the art of clinical observation.16 Reading good literature prompts better detection of emotions,17 and reflective writing improves students’ well-being.18 Playing a musical instrument reduces burnout.19 And an undergraduate major in the humanities correlates with greater tolerance for ambiguity,20 a highly desirable trait in physicians, since it means openness to new ideas and the capacity to better cope with difficult situations.21
In fact, some of the qualities fostered by the humanities even translate into better patient care. For instance, tolerance for ambiguity correlates with more positive attitudes towards patients who have frustrating complaints,22 with lower use of resources,23 and with a career choice in direct patient care.24 Hence, it has been suggested that it should be a prerequisite for medical school admission.25 Physicians’ empathy is also beneficial, since it correlates with a lower rate of complications and better outcomes in the care of diabetic patients.26 This should not come as a surprise. As Hippocrates put it 2,500 years ago, “some patients, though conscious that their condition is perilous, recover their health simply through their contentment with the goodness of the physician.”27
Lastly, studying the humanities may provide crucial antibodies against the pain and suffering that are unavoidable staples of the human condition. To paraphrase Osler, the humanities might vaccinate us against the difficulties of our profession. Hippocrates himself had suggested that “it is well to superintend the sick to make them well, to care for the healthy to keep them well, but also to care for one’s self…”27 That is why many institutions now require medical students to take humanities courses.28
MEDICINE: AN ART BASED ON SCIENCE
Yet this effort may amount to a rearguard action that arrives too late and provides too little. The humanities should probably be taught before medical school.29 After all, if it’s possible to make a scientist out of a humanist (Osler was living proof), the experience of the past decades seems to suggest that it’s considerably harder to make a humanist out of a scientist—hence the need to revisit undergraduate curricula and admission criteria to medical school, so that students can receive an adequate foundation in both arenas. Ironically, students express positive attitudes toward a liberal education and think it would actually help them as physicians.30 Yet they also understand that the selection process remains tilted towards the sciences.30–32
For Osler, scientific evidence was important but not a substitute for a humanistic approach. As he reminded students, “The practice of medicine is an art based on science,”33 whose main goals are to prevent disease, relieve suffering, and heal the sick. To do so, one ought to care more “for the individual patient than for the special features of the disease.”34 But he warned them, “It is much harder to acquire the art than the science.”35 In fact, “The practice of medicine is a calling in which your heart will be exercised equally with your head.”33 Hence the need to “cultivate equally well hearts and heads.”34 Almost foreseeing our infatuation with guidelines, he also warned against turning medicine into assembly-line work. There are “two great types of practitioners—the routinist and the rationalist,” he said in 1900, and “into the clutches of the demon routine the majority of us ultimately come.”36
Like most great people, Osler was a man of lights, shadows, and contradictions, probably not quite the saint we wish to believe. Yet he provides insights that are as valid today as they were for his own times, and possibly even more so. His 1919 speech is a paean to the humanities, but also a potential eulogy. As a Victorian physician, Osler was a blend of the new science and the old humanities. He knew that “the old art cannot possibly be replaced by, but must be absorbed in, the new science.”35 Yet he could also see the upcoming split between the two cultures, and he tried to warn us. He could in fact foresee the end of an entire way of life. As he said in his address, “there must be a very different civilization or there will be no civilization at all.”1
The crisis we face in medicine today may indeed be a symptom of a much larger cultural shift. As Osler himself put it, “The philosophies of one age have become the absurdities of the next, and the foolishness of yesterday has become the wisdom of tomorrow.”33 Like Osler, we live in times of transition that require us to act. If in 1910 Flexner gave us science,37 Osler in 1919 reminded us that medicine also needs the humanities. We ought to heed his message and reconcile the two fields. The alternative is a future full of tricorders and burned-out technicians, but sorely lacking in healers.
- Osler W. The old humanities and the new science: the presidential address delivered before the Classical Association at Oxford, May, 1919. Br Med J 1919; 2(3053):1–7. pmid:20769536
- Agerbo E, Gunnell D, Bonde JP, Mortensen PB, Nordentoft M. Suicide and occupation: the impact of socio-economic, demographic and psychiatric differences. Psychol Med 2007; 37(8):1131–1140. doi:10.1017/S0033291707000487
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc 2015; 90(12):1600–1613. doi:10.1016/j.mayocp.2015.08.023
- Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among US medical students. Ann Intern Med 2008; 149(5):334–341. pmid:18765703
- Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA 2015; 314(22):2373–2383. doi:10.1001/jama.2015.15845
- Hojat M, Mangione S, Nasca TJ, et al. An empirical study of decline in empathy in medical school. Med Educ 2004; 38(9):934–941. doi:10.1111/j.1365-2929.2004.01911.x
- Flores G. Mad scientists, compassionate healers, and greedy egotists: the portrayal of physicians in the movies. J Natl Med Assoc 2002; 94(7):635–658. pmid:12126293
- Imber JB. Trusting Doctors: The Decline of Moral Authority in American Medicine. Princeton, NJ: Princeton University Press; 2008.
- Krauthammer, C. Why doctors quit. The Washington Post. May 28, 2015. https://www.washingtonpost.com/opinions/why-doctors-quit/2015/05/28/1e9d8e6e-056f-11e5-a428-c984eb077d4e_story.html?utm_term=.aa8804a518db. Accessed March 4, 2019.
- Mangione S, Chakraborti C, Staltari G, et al. Medical students' exposure to the humanities correlates with positive personal qualities and reduced burnout: a multi-institutional US survey. J Gen Intern Med 2018; 33(5):628–634. doi:10.1007/s11606-017-4275-8
- Glück J, König S, Naschenweng K, et al. How to measure wisdom: content, reliability, and validity of five measures. Front Psychol 2013; 4:405. doi:10.3389/fpsyg.2013.00405
- Papagiannis A. Eliot’s triad: information, knowledge and wisdom in medicine. Hektoen International. Spring 2014. https://hekint.org/2017/01/29/eliots-triad-information-knowledge-and-wisdom-in-medicine. Accessed March 4, 2019.
- Hojat M, Axelrod D, Spandorfer J, Mangione S. Enhancing and sustaining empathy in medical students. Med Teach 2013; 35(12):996–1001. doi:10.3109/0142159X.2013.802300
- Graham J, Benson LM, Swanson J, Potyk D, Daratha K, Roberts K. Medical humanities coursework is associated with greater measured empathy in medical students. Am J Med 2016; 129(12):1334–1337. doi:10.1016/j.amjmed.2016.08.005
- Brechet C, Baldy R, Picard D. How does Sam feel? Children's labelling and drawing of basic emotions. Br J Dev Psychol 2009; 27(Pt 3):587–606. pmid:19994570
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med 2008; 23(7):991–997. doi:10.1007/s11606-008-0667-0
- Kidd DC, Castano E. Reading literary fiction improves theory of mind. Science 2013; 342(6156):377–380. doi:10.1126/science.1239918
- Shapiro J, Kasman D, Shafer A. Words and wards: a model of reflective writing and its uses in medical education. J Med Humanit 2006; 27(4):231–244. doi:10.1007/s10912-006-9020-y
- Bittman BB, Snyder C, Bruhn KT, et al. Recreational music-making: an integrative group intervention for reducing burnout and improving mood states in first year associate degree nursing students: insights and economic impact. Int J Nurs Educ Scholarsh 2004;1:Article12. doi:10.2202/1548-923x.1044
- DeForge BR, Sobal J. Intolerance of ambiguity in students entering medical school. Soc Sci Med 1989; 28(8):869–874. pmid:2705020
- Ghosh AK. Understanding medical uncertainty: a primer for physicians. J Assoc Physicians India 2004; 52:739–742. pmid:15839454
- Merrill JM, Camacho Z, Laux LF, Thornby JI, Vallbona C. How medical school shapes students’ orientation to patients’ psychological problems. Acad Med 1991; 66(9 suppl):S4–S6. pmid:1930523
- Allison JJ, Kiefe CI, Cook EF, Gerrity MS, Orav EJ, Centor R. The association of physician attitudes about uncertainty and risk taking with resource use in a Medicare HMO. Med Decis Making 1998; 18(3):320–329. doi:10.1177/0272989X9801800310
- Gerrity MS, Earp JAL, DeVilles RF, DW Light. Uncertainty and professional work: perceptions of physicians in clinical practice. Am J Sociol 1992; 97(4):1022–1051. https://www.jstor.org/stable/2781505. Accessed March 6, 2019.
- Geller G. Tolerance for ambiguity: an ethics-based criterion for medical student selection. Acad Med 2013; 88(5):581–584. doi:10.1097/ACM.0b013e31828a4b8e
- Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011; 86(3):359–364. doi:10.1097/ACM.0b013e3182086fe1
- Hippocrates. Precepts. Section 8, Part VI. Perseus Digital Library. http://perseus.uchicago.edu/perseus-cgi/citequery3.pl?dbname=GreekFeb2011&getid=1&query=Hipp.%20Praec.%208. Accessed March 4, 2019.
- Kidd MG, Connor JT. Striving to do good things: teaching humanities in Canadian medical schools. J Med Humanit 2008; 29(1):45–54. doi:10.1007/s10912-007-9049-6
- Thomas L. Notes of a biology-watcher. How to fix the premedical curriculum. N Engl J Med 1978; 298(21):1180–1181. doi:10.1056/NEJM197805252982106
- Simmons A. Beyond the premedical syndrome: premedical student attitudes toward liberal education and implications for advising. NACADA Journal 2005; 25(1):64–73.
- Kumar B, Swee ML and Suneja M. The premedical curriculum: we can do better for future physicians. South Med J 2017; 110(8):538–539. doi:10.14423/SMJ.0000000000000683
- Gunderman RB, Kanter SL. Perspective: “how to fix the premedical curriculum” revisited. Acad Med 2008; 83(12):1158–1161. doi:10.1097/ACM.0b013e31818c6515
- Osler W. Aequanimitas with Other Addresses to Medical Students, Nurses and Practitioners of Medicine. Philadelphia, PA: Blakiston; 1904.
- Osler W. Address to the students of the Albany Medical College. Albany Med Ann 1899; 20:307–309.
- Osler W. The reserves of life. St Mary’s Hosp Gaz 1907; 13:95–98.
- Osler W. An address on the importance of post-graduate study. Delivered at the opening of the Museums of the Medical Graduates College and Polyclinic, July 4th, 1900. Br Med J 1900; 2(2063):73–75. pmid:20759107
- Flexner A. Medical Education in the United States and Canada. New York, The Carnegie Foundation 1910.
“Twin berries on one stem, grievous damage has been done to both in regarding the Humanities and Science in any other light than complemental.”
—Sir William Osler1
The year 2019 marks the 100th anniversary of Sir William Osler’s last public speech. Still reeling from the death of his only son in World War I, he had been asked to give the presidential inaugural address of the Classical Association at Oxford. It was the first time a physician had received the honor, and Osler took the assignment very seriously. He chose to speak about “The old humanities and the new science,” and to call for a reunification of the two fields. “Humanists have not enough Science” he warned, “and Science sadly lacks the Humanities…this unhappy divorce…should never have taken place.”1 Later, he said that it was the speech to which he had given the greatest thought and preparation. It was in fact Osler’s personal legacy: 2 months later he turned 70, and 7 months later he was dead.
Revisiting the address today, what can Osler teach the high-tech physician of today, when doctors have become “providers” and patients “consumers”? Is Osler’s message still relevant to our craft, or has he simply become an icon of professional nostalgia with little value for our times?
THE NEED FOR THE HUMANITIES IN MEDICINE
Medicine has certainly grown both powerful and successful. Yet it is also confronting hurdles that would have been unimaginable in Osler’s time. Physicians are now the professionals with the highest suicide rate,2 a burnout rate as high as 70%,3,4 rampant depression,5 dwindling empathy,6 a predominantly negative perception by the public,7,8 and a disturbing propensity to quit.9 These, of course, may just be symptoms of an increasingly meaningless environment wherein doctors have become small cogs in a medical-industrial complex they can’t control or even understand. Still, is it possible that something more personal may have been lost in the way we now select and educate physicians? Could this, in turn, make us less resilient?
In this regard, Osler’s last public speech serves as an enduring reminder of the need for the humanities in medicine. Osler not only believed it, but throughout his life never missed a chance to express in words, writings, and deeds that the humanities are indeed “the hormones” of the profession. In 1919 he warned against the risk of separating our humanistic tradition from the sciences, and urged us “to infect [anyone] with the spirit of the Humanities,” since to him that was “the greatest single gift in education.”1
Unfortunately, the humanities are slippery, not easily quantifiable, hard to define, and seemingly incompatible with an evidence-based approach. Quite understandably, today’s data-obsessed medicine views them with suspicion. But besides reminding us that in medicine not all that counts can be counted, and not all that can be counted counts, the humanities are in fact a fundamental component of the physician’s skill set.
In a multicenter survey of 5 medical schools,10 there was indeed a correlation between students’ exposure to the humanities and many of the personal qualities whose absence we lament in today’s medicine: empathy, tolerance for ambiguity, emotional intelligence, and prevention of burnout. Most significant was a strong correlation with wisdom, as measured by the 21-item Brief Wisdom Screening Scale.11 That all these traits may correlate with humanities exposure is intuitive, since the humanities not only teach tolerance and compassion, but also capture the collective experience of those who came before us. Hence, they teach us wisdom. Wisdom is not an ACGME competency, but it’s undoubtedly a prerequisite for the art of healing.12 In fact, wisdom may very well be the fundamental trait that characterizes a well-rounded physician, since it encompasses empathy, resilience, comfort with ambiguity, and the capacity to learn from the past. Not surprisingly, wisdom in the world was Osler’s closing wish in 1919.
The humanities can also nurture the very personal qualities we desire in physicians. For example, observing drama fosters empathy,13 as does taking an elective in medical humanities.14 Drawing enhances the reading of faces,15 and observing art improves the art of clinical observation.16 Reading good literature prompts better detection of emotions,17 and reflective writing improves students’ well-being.18 Playing a musical instrument reduces burnout.19 And an undergraduate major in the humanities correlates with greater tolerance for ambiguity,20 a highly desirable trait in physicians, since it means openness to new ideas and the capacity to better cope with difficult situations.21
In fact, some of the qualities fostered by the humanities even translate into better patient care. For instance, tolerance for ambiguity correlates with more positive attitudes towards patients who have frustrating complaints,22 with lower use of resources,23 and with a career choice in direct patient care.24 Hence, it has been suggested that it should be a prerequisite for medical school admission.25 Physicians’ empathy is also beneficial, since it correlates with a lower rate of complications and better outcomes in the care of diabetic patients.26 This should not come as a surprise. As Hippocrates put it 2,500 years ago, “some patients, though conscious that their condition is perilous, recover their health simply through their contentment with the goodness of the physician.”27
Lastly, studying the humanities may provide crucial antibodies against the pain and suffering that are unavoidable staples of the human condition. To paraphrase Osler, the humanities might vaccinate us against the difficulties of our profession. Hippocrates himself had suggested that “it is well to superintend the sick to make them well, to care for the healthy to keep them well, but also to care for one’s self…”27 That is why many institutions now require medical students to take humanities courses.28
MEDICINE: AN ART BASED ON SCIENCE
Yet this effort may amount to a rearguard action that arrives too late and provides too little. The humanities should probably be taught before medical school.29 After all, if it’s possible to make a scientist out of a humanist (Osler was living proof), the experience of the past decades seems to suggest that it’s considerably harder to make a humanist out of a scientist—hence the need to revisit undergraduate curricula and admission criteria to medical school, so that students can receive an adequate foundation in both arenas. Ironically, students express positive attitudes toward a liberal education and think it would actually help them as physicians.30 Yet they also understand that the selection process remains tilted towards the sciences.30–32
For Osler, scientific evidence was important but not a substitute for a humanistic approach. As he reminded students, “The practice of medicine is an art based on science,”33 whose main goals are to prevent disease, relieve suffering, and heal the sick. To do so, one ought to care more “for the individual patient than for the special features of the disease.”34 But he warned them, “It is much harder to acquire the art than the science.”35 In fact, “The practice of medicine is a calling in which your heart will be exercised equally with your head.”33 Hence the need to “cultivate equally well hearts and heads.”34 Almost foreseeing our infatuation with guidelines, he also warned against turning medicine into assembly-line work. There are “two great types of practitioners—the routinist and the rationalist,” he said in 1900, and “into the clutches of the demon routine the majority of us ultimately come.”36
Like most great people, Osler was a man of lights, shadows, and contradictions, probably not quite the saint we wish to believe. Yet he provides insights that are as valid today as they were for his own times, and possibly even more so. His 1919 speech is a paean to the humanities, but also a potential eulogy. As a Victorian physician, Osler was a blend of the new science and the old humanities. He knew that “the old art cannot possibly be replaced by, but must be absorbed in, the new science.”35 Yet he could also see the upcoming split between the two cultures, and he tried to warn us. He could in fact foresee the end of an entire way of life. As he said in his address, “there must be a very different civilization or there will be no civilization at all.”1
The crisis we face in medicine today may indeed be a symptom of a much larger cultural shift. As Osler himself put it, “The philosophies of one age have become the absurdities of the next, and the foolishness of yesterday has become the wisdom of tomorrow.”33 Like Osler, we live in times of transition that require us to act. If in 1910 Flexner gave us science,37 Osler in 1919 reminded us that medicine also needs the humanities. We ought to heed his message and reconcile the two fields. The alternative is a future full of tricorders and burned-out technicians, but sorely lacking in healers.
“Twin berries on one stem, grievous damage has been done to both in regarding the Humanities and Science in any other light than complemental.”
—Sir William Osler1
The year 2019 marks the 100th anniversary of Sir William Osler’s last public speech. Still reeling from the death of his only son in World War I, he had been asked to give the presidential inaugural address of the Classical Association at Oxford. It was the first time a physician had received the honor, and Osler took the assignment very seriously. He chose to speak about “The old humanities and the new science,” and to call for a reunification of the two fields. “Humanists have not enough Science” he warned, “and Science sadly lacks the Humanities…this unhappy divorce…should never have taken place.”1 Later, he said that it was the speech to which he had given the greatest thought and preparation. It was in fact Osler’s personal legacy: 2 months later he turned 70, and 7 months later he was dead.
Revisiting the address today, what can Osler teach the high-tech physician of today, when doctors have become “providers” and patients “consumers”? Is Osler’s message still relevant to our craft, or has he simply become an icon of professional nostalgia with little value for our times?
THE NEED FOR THE HUMANITIES IN MEDICINE
Medicine has certainly grown both powerful and successful. Yet it is also confronting hurdles that would have been unimaginable in Osler’s time. Physicians are now the professionals with the highest suicide rate,2 a burnout rate as high as 70%,3,4 rampant depression,5 dwindling empathy,6 a predominantly negative perception by the public,7,8 and a disturbing propensity to quit.9 These, of course, may just be symptoms of an increasingly meaningless environment wherein doctors have become small cogs in a medical-industrial complex they can’t control or even understand. Still, is it possible that something more personal may have been lost in the way we now select and educate physicians? Could this, in turn, make us less resilient?
In this regard, Osler’s last public speech serves as an enduring reminder of the need for the humanities in medicine. Osler not only believed it, but throughout his life never missed a chance to express in words, writings, and deeds that the humanities are indeed “the hormones” of the profession. In 1919 he warned against the risk of separating our humanistic tradition from the sciences, and urged us “to infect [anyone] with the spirit of the Humanities,” since to him that was “the greatest single gift in education.”1
Unfortunately, the humanities are slippery, not easily quantifiable, hard to define, and seemingly incompatible with an evidence-based approach. Quite understandably, today’s data-obsessed medicine views them with suspicion. But besides reminding us that in medicine not all that counts can be counted, and not all that can be counted counts, the humanities are in fact a fundamental component of the physician’s skill set.
In a multicenter survey of 5 medical schools,10 there was indeed a correlation between students’ exposure to the humanities and many of the personal qualities whose absence we lament in today’s medicine: empathy, tolerance for ambiguity, emotional intelligence, and prevention of burnout. Most significant was a strong correlation with wisdom, as measured by the 21-item Brief Wisdom Screening Scale.11 That all these traits may correlate with humanities exposure is intuitive, since the humanities not only teach tolerance and compassion, but also capture the collective experience of those who came before us. Hence, they teach us wisdom. Wisdom is not an ACGME competency, but it’s undoubtedly a prerequisite for the art of healing.12 In fact, wisdom may very well be the fundamental trait that characterizes a well-rounded physician, since it encompasses empathy, resilience, comfort with ambiguity, and the capacity to learn from the past. Not surprisingly, wisdom in the world was Osler’s closing wish in 1919.
The humanities can also nurture the very personal qualities we desire in physicians. For example, observing drama fosters empathy,13 as does taking an elective in medical humanities.14 Drawing enhances the reading of faces,15 and observing art improves the art of clinical observation.16 Reading good literature prompts better detection of emotions,17 and reflective writing improves students’ well-being.18 Playing a musical instrument reduces burnout.19 And an undergraduate major in the humanities correlates with greater tolerance for ambiguity,20 a highly desirable trait in physicians, since it means openness to new ideas and the capacity to better cope with difficult situations.21
In fact, some of the qualities fostered by the humanities even translate into better patient care. For instance, tolerance for ambiguity correlates with more positive attitudes towards patients who have frustrating complaints,22 with lower use of resources,23 and with a career choice in direct patient care.24 Hence, it has been suggested that it should be a prerequisite for medical school admission.25 Physicians’ empathy is also beneficial, since it correlates with a lower rate of complications and better outcomes in the care of diabetic patients.26 This should not come as a surprise. As Hippocrates put it 2,500 years ago, “some patients, though conscious that their condition is perilous, recover their health simply through their contentment with the goodness of the physician.”27
Lastly, studying the humanities may provide crucial antibodies against the pain and suffering that are unavoidable staples of the human condition. To paraphrase Osler, the humanities might vaccinate us against the difficulties of our profession. Hippocrates himself had suggested that “it is well to superintend the sick to make them well, to care for the healthy to keep them well, but also to care for one’s self…”27 That is why many institutions now require medical students to take humanities courses.28
MEDICINE: AN ART BASED ON SCIENCE
Yet this effort may amount to a rearguard action that arrives too late and provides too little. The humanities should probably be taught before medical school.29 After all, if it’s possible to make a scientist out of a humanist (Osler was living proof), the experience of the past decades seems to suggest that it’s considerably harder to make a humanist out of a scientist—hence the need to revisit undergraduate curricula and admission criteria to medical school, so that students can receive an adequate foundation in both arenas. Ironically, students express positive attitudes toward a liberal education and think it would actually help them as physicians.30 Yet they also understand that the selection process remains tilted towards the sciences.30–32
For Osler, scientific evidence was important but not a substitute for a humanistic approach. As he reminded students, “The practice of medicine is an art based on science,”33 whose main goals are to prevent disease, relieve suffering, and heal the sick. To do so, one ought to care more “for the individual patient than for the special features of the disease.”34 But he warned them, “It is much harder to acquire the art than the science.”35 In fact, “The practice of medicine is a calling in which your heart will be exercised equally with your head.”33 Hence the need to “cultivate equally well hearts and heads.”34 Almost foreseeing our infatuation with guidelines, he also warned against turning medicine into assembly-line work. There are “two great types of practitioners—the routinist and the rationalist,” he said in 1900, and “into the clutches of the demon routine the majority of us ultimately come.”36
Like most great people, Osler was a man of lights, shadows, and contradictions, probably not quite the saint we wish to believe. Yet he provides insights that are as valid today as they were for his own times, and possibly even more so. His 1919 speech is a paean to the humanities, but also a potential eulogy. As a Victorian physician, Osler was a blend of the new science and the old humanities. He knew that “the old art cannot possibly be replaced by, but must be absorbed in, the new science.”35 Yet he could also see the upcoming split between the two cultures, and he tried to warn us. He could in fact foresee the end of an entire way of life. As he said in his address, “there must be a very different civilization or there will be no civilization at all.”1
The crisis we face in medicine today may indeed be a symptom of a much larger cultural shift. As Osler himself put it, “The philosophies of one age have become the absurdities of the next, and the foolishness of yesterday has become the wisdom of tomorrow.”33 Like Osler, we live in times of transition that require us to act. If in 1910 Flexner gave us science,37 Osler in 1919 reminded us that medicine also needs the humanities. We ought to heed his message and reconcile the two fields. The alternative is a future full of tricorders and burned-out technicians, but sorely lacking in healers.
- Osler W. The old humanities and the new science: the presidential address delivered before the Classical Association at Oxford, May, 1919. Br Med J 1919; 2(3053):1–7. pmid:20769536
- Agerbo E, Gunnell D, Bonde JP, Mortensen PB, Nordentoft M. Suicide and occupation: the impact of socio-economic, demographic and psychiatric differences. Psychol Med 2007; 37(8):1131–1140. doi:10.1017/S0033291707000487
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc 2015; 90(12):1600–1613. doi:10.1016/j.mayocp.2015.08.023
- Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among US medical students. Ann Intern Med 2008; 149(5):334–341. pmid:18765703
- Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA 2015; 314(22):2373–2383. doi:10.1001/jama.2015.15845
- Hojat M, Mangione S, Nasca TJ, et al. An empirical study of decline in empathy in medical school. Med Educ 2004; 38(9):934–941. doi:10.1111/j.1365-2929.2004.01911.x
- Flores G. Mad scientists, compassionate healers, and greedy egotists: the portrayal of physicians in the movies. J Natl Med Assoc 2002; 94(7):635–658. pmid:12126293
- Imber JB. Trusting Doctors: The Decline of Moral Authority in American Medicine. Princeton, NJ: Princeton University Press; 2008.
- Krauthammer, C. Why doctors quit. The Washington Post. May 28, 2015. https://www.washingtonpost.com/opinions/why-doctors-quit/2015/05/28/1e9d8e6e-056f-11e5-a428-c984eb077d4e_story.html?utm_term=.aa8804a518db. Accessed March 4, 2019.
- Mangione S, Chakraborti C, Staltari G, et al. Medical students' exposure to the humanities correlates with positive personal qualities and reduced burnout: a multi-institutional US survey. J Gen Intern Med 2018; 33(5):628–634. doi:10.1007/s11606-017-4275-8
- Glück J, König S, Naschenweng K, et al. How to measure wisdom: content, reliability, and validity of five measures. Front Psychol 2013; 4:405. doi:10.3389/fpsyg.2013.00405
- Papagiannis A. Eliot’s triad: information, knowledge and wisdom in medicine. Hektoen International. Spring 2014. https://hekint.org/2017/01/29/eliots-triad-information-knowledge-and-wisdom-in-medicine. Accessed March 4, 2019.
- Hojat M, Axelrod D, Spandorfer J, Mangione S. Enhancing and sustaining empathy in medical students. Med Teach 2013; 35(12):996–1001. doi:10.3109/0142159X.2013.802300
- Graham J, Benson LM, Swanson J, Potyk D, Daratha K, Roberts K. Medical humanities coursework is associated with greater measured empathy in medical students. Am J Med 2016; 129(12):1334–1337. doi:10.1016/j.amjmed.2016.08.005
- Brechet C, Baldy R, Picard D. How does Sam feel? Children's labelling and drawing of basic emotions. Br J Dev Psychol 2009; 27(Pt 3):587–606. pmid:19994570
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med 2008; 23(7):991–997. doi:10.1007/s11606-008-0667-0
- Kidd DC, Castano E. Reading literary fiction improves theory of mind. Science 2013; 342(6156):377–380. doi:10.1126/science.1239918
- Shapiro J, Kasman D, Shafer A. Words and wards: a model of reflective writing and its uses in medical education. J Med Humanit 2006; 27(4):231–244. doi:10.1007/s10912-006-9020-y
- Bittman BB, Snyder C, Bruhn KT, et al. Recreational music-making: an integrative group intervention for reducing burnout and improving mood states in first year associate degree nursing students: insights and economic impact. Int J Nurs Educ Scholarsh 2004;1:Article12. doi:10.2202/1548-923x.1044
- DeForge BR, Sobal J. Intolerance of ambiguity in students entering medical school. Soc Sci Med 1989; 28(8):869–874. pmid:2705020
- Ghosh AK. Understanding medical uncertainty: a primer for physicians. J Assoc Physicians India 2004; 52:739–742. pmid:15839454
- Merrill JM, Camacho Z, Laux LF, Thornby JI, Vallbona C. How medical school shapes students’ orientation to patients’ psychological problems. Acad Med 1991; 66(9 suppl):S4–S6. pmid:1930523
- Allison JJ, Kiefe CI, Cook EF, Gerrity MS, Orav EJ, Centor R. The association of physician attitudes about uncertainty and risk taking with resource use in a Medicare HMO. Med Decis Making 1998; 18(3):320–329. doi:10.1177/0272989X9801800310
- Gerrity MS, Earp JAL, DeVilles RF, DW Light. Uncertainty and professional work: perceptions of physicians in clinical practice. Am J Sociol 1992; 97(4):1022–1051. https://www.jstor.org/stable/2781505. Accessed March 6, 2019.
- Geller G. Tolerance for ambiguity: an ethics-based criterion for medical student selection. Acad Med 2013; 88(5):581–584. doi:10.1097/ACM.0b013e31828a4b8e
- Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011; 86(3):359–364. doi:10.1097/ACM.0b013e3182086fe1
- Hippocrates. Precepts. Section 8, Part VI. Perseus Digital Library. http://perseus.uchicago.edu/perseus-cgi/citequery3.pl?dbname=GreekFeb2011&getid=1&query=Hipp.%20Praec.%208. Accessed March 4, 2019.
- Kidd MG, Connor JT. Striving to do good things: teaching humanities in Canadian medical schools. J Med Humanit 2008; 29(1):45–54. doi:10.1007/s10912-007-9049-6
- Thomas L. Notes of a biology-watcher. How to fix the premedical curriculum. N Engl J Med 1978; 298(21):1180–1181. doi:10.1056/NEJM197805252982106
- Simmons A. Beyond the premedical syndrome: premedical student attitudes toward liberal education and implications for advising. NACADA Journal 2005; 25(1):64–73.
- Kumar B, Swee ML and Suneja M. The premedical curriculum: we can do better for future physicians. South Med J 2017; 110(8):538–539. doi:10.14423/SMJ.0000000000000683
- Gunderman RB, Kanter SL. Perspective: “how to fix the premedical curriculum” revisited. Acad Med 2008; 83(12):1158–1161. doi:10.1097/ACM.0b013e31818c6515
- Osler W. Aequanimitas with Other Addresses to Medical Students, Nurses and Practitioners of Medicine. Philadelphia, PA: Blakiston; 1904.
- Osler W. Address to the students of the Albany Medical College. Albany Med Ann 1899; 20:307–309.
- Osler W. The reserves of life. St Mary’s Hosp Gaz 1907; 13:95–98.
- Osler W. An address on the importance of post-graduate study. Delivered at the opening of the Museums of the Medical Graduates College and Polyclinic, July 4th, 1900. Br Med J 1900; 2(2063):73–75. pmid:20759107
- Flexner A. Medical Education in the United States and Canada. New York, The Carnegie Foundation 1910.
- Osler W. The old humanities and the new science: the presidential address delivered before the Classical Association at Oxford, May, 1919. Br Med J 1919; 2(3053):1–7. pmid:20769536
- Agerbo E, Gunnell D, Bonde JP, Mortensen PB, Nordentoft M. Suicide and occupation: the impact of socio-economic, demographic and psychiatric differences. Psychol Med 2007; 37(8):1131–1140. doi:10.1017/S0033291707000487
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc 2015; 90(12):1600–1613. doi:10.1016/j.mayocp.2015.08.023
- Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among US medical students. Ann Intern Med 2008; 149(5):334–341. pmid:18765703
- Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA 2015; 314(22):2373–2383. doi:10.1001/jama.2015.15845
- Hojat M, Mangione S, Nasca TJ, et al. An empirical study of decline in empathy in medical school. Med Educ 2004; 38(9):934–941. doi:10.1111/j.1365-2929.2004.01911.x
- Flores G. Mad scientists, compassionate healers, and greedy egotists: the portrayal of physicians in the movies. J Natl Med Assoc 2002; 94(7):635–658. pmid:12126293
- Imber JB. Trusting Doctors: The Decline of Moral Authority in American Medicine. Princeton, NJ: Princeton University Press; 2008.
- Krauthammer, C. Why doctors quit. The Washington Post. May 28, 2015. https://www.washingtonpost.com/opinions/why-doctors-quit/2015/05/28/1e9d8e6e-056f-11e5-a428-c984eb077d4e_story.html?utm_term=.aa8804a518db. Accessed March 4, 2019.
- Mangione S, Chakraborti C, Staltari G, et al. Medical students' exposure to the humanities correlates with positive personal qualities and reduced burnout: a multi-institutional US survey. J Gen Intern Med 2018; 33(5):628–634. doi:10.1007/s11606-017-4275-8
- Glück J, König S, Naschenweng K, et al. How to measure wisdom: content, reliability, and validity of five measures. Front Psychol 2013; 4:405. doi:10.3389/fpsyg.2013.00405
- Papagiannis A. Eliot’s triad: information, knowledge and wisdom in medicine. Hektoen International. Spring 2014. https://hekint.org/2017/01/29/eliots-triad-information-knowledge-and-wisdom-in-medicine. Accessed March 4, 2019.
- Hojat M, Axelrod D, Spandorfer J, Mangione S. Enhancing and sustaining empathy in medical students. Med Teach 2013; 35(12):996–1001. doi:10.3109/0142159X.2013.802300
- Graham J, Benson LM, Swanson J, Potyk D, Daratha K, Roberts K. Medical humanities coursework is associated with greater measured empathy in medical students. Am J Med 2016; 129(12):1334–1337. doi:10.1016/j.amjmed.2016.08.005
- Brechet C, Baldy R, Picard D. How does Sam feel? Children's labelling and drawing of basic emotions. Br J Dev Psychol 2009; 27(Pt 3):587–606. pmid:19994570
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med 2008; 23(7):991–997. doi:10.1007/s11606-008-0667-0
- Kidd DC, Castano E. Reading literary fiction improves theory of mind. Science 2013; 342(6156):377–380. doi:10.1126/science.1239918
- Shapiro J, Kasman D, Shafer A. Words and wards: a model of reflective writing and its uses in medical education. J Med Humanit 2006; 27(4):231–244. doi:10.1007/s10912-006-9020-y
- Bittman BB, Snyder C, Bruhn KT, et al. Recreational music-making: an integrative group intervention for reducing burnout and improving mood states in first year associate degree nursing students: insights and economic impact. Int J Nurs Educ Scholarsh 2004;1:Article12. doi:10.2202/1548-923x.1044
- DeForge BR, Sobal J. Intolerance of ambiguity in students entering medical school. Soc Sci Med 1989; 28(8):869–874. pmid:2705020
- Ghosh AK. Understanding medical uncertainty: a primer for physicians. J Assoc Physicians India 2004; 52:739–742. pmid:15839454
- Merrill JM, Camacho Z, Laux LF, Thornby JI, Vallbona C. How medical school shapes students’ orientation to patients’ psychological problems. Acad Med 1991; 66(9 suppl):S4–S6. pmid:1930523
- Allison JJ, Kiefe CI, Cook EF, Gerrity MS, Orav EJ, Centor R. The association of physician attitudes about uncertainty and risk taking with resource use in a Medicare HMO. Med Decis Making 1998; 18(3):320–329. doi:10.1177/0272989X9801800310
- Gerrity MS, Earp JAL, DeVilles RF, DW Light. Uncertainty and professional work: perceptions of physicians in clinical practice. Am J Sociol 1992; 97(4):1022–1051. https://www.jstor.org/stable/2781505. Accessed March 6, 2019.
- Geller G. Tolerance for ambiguity: an ethics-based criterion for medical student selection. Acad Med 2013; 88(5):581–584. doi:10.1097/ACM.0b013e31828a4b8e
- Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011; 86(3):359–364. doi:10.1097/ACM.0b013e3182086fe1
- Hippocrates. Precepts. Section 8, Part VI. Perseus Digital Library. http://perseus.uchicago.edu/perseus-cgi/citequery3.pl?dbname=GreekFeb2011&getid=1&query=Hipp.%20Praec.%208. Accessed March 4, 2019.
- Kidd MG, Connor JT. Striving to do good things: teaching humanities in Canadian medical schools. J Med Humanit 2008; 29(1):45–54. doi:10.1007/s10912-007-9049-6
- Thomas L. Notes of a biology-watcher. How to fix the premedical curriculum. N Engl J Med 1978; 298(21):1180–1181. doi:10.1056/NEJM197805252982106
- Simmons A. Beyond the premedical syndrome: premedical student attitudes toward liberal education and implications for advising. NACADA Journal 2005; 25(1):64–73.
- Kumar B, Swee ML and Suneja M. The premedical curriculum: we can do better for future physicians. South Med J 2017; 110(8):538–539. doi:10.14423/SMJ.0000000000000683
- Gunderman RB, Kanter SL. Perspective: “how to fix the premedical curriculum” revisited. Acad Med 2008; 83(12):1158–1161. doi:10.1097/ACM.0b013e31818c6515
- Osler W. Aequanimitas with Other Addresses to Medical Students, Nurses and Practitioners of Medicine. Philadelphia, PA: Blakiston; 1904.
- Osler W. Address to the students of the Albany Medical College. Albany Med Ann 1899; 20:307–309.
- Osler W. The reserves of life. St Mary’s Hosp Gaz 1907; 13:95–98.
- Osler W. An address on the importance of post-graduate study. Delivered at the opening of the Museums of the Medical Graduates College and Polyclinic, July 4th, 1900. Br Med J 1900; 2(2063):73–75. pmid:20759107
- Flexner A. Medical Education in the United States and Canada. New York, The Carnegie Foundation 1910.
Rapidly progressive pleural effusion
To the Editor: Regarding the article about a man with rapidly progressive pleural effusion by Zoumot et al in the January 2019 issue,1 there was some inconsistency between the teaching points and the actions taken.
Question 1 asked what was the most likely cause of the patient’s pleuritic chest pain. Pulmonary embolism was an unlikely diagnosis, given the patient’s presentation and his normal D-dimer level, which the text acknowledges, but then proceeds to state that computed tomographic angiography of the chest was done anyway.
After pleural effusion was diagnosed, question 2 asked what was the best management strategy for the patient at that time. The best management strategy was to give oral antibiotics with close follow-up because the patient was at low risk of a poor outcome, but he was advised to be admitted for intravenous antibiotics anyway.
I’m not quite sure of the point of the didactic exercise when actions are not consistent with the analytic rationale for testing and treatment.
- Zoumot Z, Wahla AS, Farha S. Rapidly progressive pleural effusion. Cleve Clin J Med 2019; 86(1):21–27. doi:10.3949/ccjm.86a.18067
To the Editor: Regarding the article about a man with rapidly progressive pleural effusion by Zoumot et al in the January 2019 issue,1 there was some inconsistency between the teaching points and the actions taken.
Question 1 asked what was the most likely cause of the patient’s pleuritic chest pain. Pulmonary embolism was an unlikely diagnosis, given the patient’s presentation and his normal D-dimer level, which the text acknowledges, but then proceeds to state that computed tomographic angiography of the chest was done anyway.
After pleural effusion was diagnosed, question 2 asked what was the best management strategy for the patient at that time. The best management strategy was to give oral antibiotics with close follow-up because the patient was at low risk of a poor outcome, but he was advised to be admitted for intravenous antibiotics anyway.
I’m not quite sure of the point of the didactic exercise when actions are not consistent with the analytic rationale for testing and treatment.
To the Editor: Regarding the article about a man with rapidly progressive pleural effusion by Zoumot et al in the January 2019 issue,1 there was some inconsistency between the teaching points and the actions taken.
Question 1 asked what was the most likely cause of the patient’s pleuritic chest pain. Pulmonary embolism was an unlikely diagnosis, given the patient’s presentation and his normal D-dimer level, which the text acknowledges, but then proceeds to state that computed tomographic angiography of the chest was done anyway.
After pleural effusion was diagnosed, question 2 asked what was the best management strategy for the patient at that time. The best management strategy was to give oral antibiotics with close follow-up because the patient was at low risk of a poor outcome, but he was advised to be admitted for intravenous antibiotics anyway.
I’m not quite sure of the point of the didactic exercise when actions are not consistent with the analytic rationale for testing and treatment.
- Zoumot Z, Wahla AS, Farha S. Rapidly progressive pleural effusion. Cleve Clin J Med 2019; 86(1):21–27. doi:10.3949/ccjm.86a.18067
- Zoumot Z, Wahla AS, Farha S. Rapidly progressive pleural effusion. Cleve Clin J Med 2019; 86(1):21–27. doi:10.3949/ccjm.86a.18067
In reply: Rapidly progressive pleural effusion
In Reply: We thank Dr. Davidson for his comments. Indeed, the teaching points may appear inconsistent with the actual patient journey in this case. In the real world, physicians from different teams and specialties are involved in the care of a patient, and medical practice may not strictly adhere to guidelines.
In question 1, the emergency department physician decided to proceed with computed tomographic pulmonary angiography to rule out pulmonary embolism. Based on best practice guidelines, pulmonary angiography was not indicated, as the clinical pretest probability of pulmonary embolism was low, supported by the patient’s negative D-dimer test. When we wrote the article, as we already had the scan, we used it to support the learning points in terms of findings on computed tomography at the early stage of a developing empyema, and also to support that the scan was in fact not indicated (not the other way around).
As for question 2, specific data-driven guidelines do not exist on how best to manage patients with bronchopneumonia with an early evolving parapneumonic effusion. In the text that follows question 2, we stated that management as an inpatient or outpatient would have been reasonable. Although we considered the patient at low risk for a poor outcome, we offered inpatient admission at the time for better control of his severe pleuritic pain (this could have been made clearer in the text), as well as close monitoring of his evolving parapneumonic effusion, and we do not believe that this contradicts the teaching points of this case.
In Reply: We thank Dr. Davidson for his comments. Indeed, the teaching points may appear inconsistent with the actual patient journey in this case. In the real world, physicians from different teams and specialties are involved in the care of a patient, and medical practice may not strictly adhere to guidelines.
In question 1, the emergency department physician decided to proceed with computed tomographic pulmonary angiography to rule out pulmonary embolism. Based on best practice guidelines, pulmonary angiography was not indicated, as the clinical pretest probability of pulmonary embolism was low, supported by the patient’s negative D-dimer test. When we wrote the article, as we already had the scan, we used it to support the learning points in terms of findings on computed tomography at the early stage of a developing empyema, and also to support that the scan was in fact not indicated (not the other way around).
As for question 2, specific data-driven guidelines do not exist on how best to manage patients with bronchopneumonia with an early evolving parapneumonic effusion. In the text that follows question 2, we stated that management as an inpatient or outpatient would have been reasonable. Although we considered the patient at low risk for a poor outcome, we offered inpatient admission at the time for better control of his severe pleuritic pain (this could have been made clearer in the text), as well as close monitoring of his evolving parapneumonic effusion, and we do not believe that this contradicts the teaching points of this case.
In Reply: We thank Dr. Davidson for his comments. Indeed, the teaching points may appear inconsistent with the actual patient journey in this case. In the real world, physicians from different teams and specialties are involved in the care of a patient, and medical practice may not strictly adhere to guidelines.
In question 1, the emergency department physician decided to proceed with computed tomographic pulmonary angiography to rule out pulmonary embolism. Based on best practice guidelines, pulmonary angiography was not indicated, as the clinical pretest probability of pulmonary embolism was low, supported by the patient’s negative D-dimer test. When we wrote the article, as we already had the scan, we used it to support the learning points in terms of findings on computed tomography at the early stage of a developing empyema, and also to support that the scan was in fact not indicated (not the other way around).
As for question 2, specific data-driven guidelines do not exist on how best to manage patients with bronchopneumonia with an early evolving parapneumonic effusion. In the text that follows question 2, we stated that management as an inpatient or outpatient would have been reasonable. Although we considered the patient at low risk for a poor outcome, we offered inpatient admission at the time for better control of his severe pleuritic pain (this could have been made clearer in the text), as well as close monitoring of his evolving parapneumonic effusion, and we do not believe that this contradicts the teaching points of this case.
Metformin for type 2 diabetes
To the Editor: I enjoyed reading “Should metformin be used in every patient with type 2 diabetes” by Makin and Lansang in the January 2019 issue.1
I just wanted to point out that metformin is a frequent cause of low serum vitamin B12 levels, and serum vitamin B12 levels should be monitored intermittently in patients using metformin.
- Makin V, Lansang MC. Should metformin be used in every patient with type 2 diabetes? Cleve Clin J Med 2019; 86(1):17–20. doi:10.3949/ccjm.86a.18039
To the Editor: I enjoyed reading “Should metformin be used in every patient with type 2 diabetes” by Makin and Lansang in the January 2019 issue.1
I just wanted to point out that metformin is a frequent cause of low serum vitamin B12 levels, and serum vitamin B12 levels should be monitored intermittently in patients using metformin.
To the Editor: I enjoyed reading “Should metformin be used in every patient with type 2 diabetes” by Makin and Lansang in the January 2019 issue.1
I just wanted to point out that metformin is a frequent cause of low serum vitamin B12 levels, and serum vitamin B12 levels should be monitored intermittently in patients using metformin.
- Makin V, Lansang MC. Should metformin be used in every patient with type 2 diabetes? Cleve Clin J Med 2019; 86(1):17–20. doi:10.3949/ccjm.86a.18039
- Makin V, Lansang MC. Should metformin be used in every patient with type 2 diabetes? Cleve Clin J Med 2019; 86(1):17–20. doi:10.3949/ccjm.86a.18039
In reply: Metformin for type 2 diabetes
In Reply: We thank Dr. Moskowitz for his kind comments. We agree about the need for assessing vitamin B12 levels during chronic metformin use.
Secondary analysis of patients in the Diabetes Prevention Program Outcomes Study showed a higher incidence of combined low and low-normal vitamin B12 deficiency in users assigned to the metformin group compared with those assigned to the placebo group at the 5-year and 13-year marks after randomization.1 Post hoc analysis of patients in the Hyperinsulinemia: the Outcome of Its Metabolic Effects trial also showed lower levels of vitamin B12 and higher levels of methylmalonic acid associated with significant worsening of a validated neuropathy score in metformin users.2
The mechanism behind the development of vitamin B12 deficiency is not completely understood but could possibly be alterations in intestinal mobility, bacterial overgrowth, or calcium-dependent uptake by ileal cells of the vitamin B12-intrinsic factor complex.3
Our electronic medical record has a built-in tool that suggests checking vitamin B12 whenever a patient requests metformin refills. There are no current guidelines on the need for baseline testing of the vitamin B12 level. The American Diabetes Association recommends periodic measurement of vitamin B12 levels, possibly yearly, in metformin users and more often if there are symptoms indicative of deficiency.4
- Aroda VR, Edelstein SL, Goldberg RB, et al; Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2019; 101(4):1754–1761. doi:10.1210/jc.2015-3754
- Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: post hoc analysis of a randomized controlled 4.3 year trial. J Diabetes Complications 2018; 32(2):171–178. doi:10.1016/j.jdiacomp.2017.11.001
- Liu KW, Dai LK, Jean W. Metformin-related vitamin B12 deficiency. Age Ageing 2006; 35(2):200–201. doi:10.1093/ageing/afj042
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019; 42(suppl 1):S90–S102. doi:10.2337/dc19-S009
In Reply: We thank Dr. Moskowitz for his kind comments. We agree about the need for assessing vitamin B12 levels during chronic metformin use.
Secondary analysis of patients in the Diabetes Prevention Program Outcomes Study showed a higher incidence of combined low and low-normal vitamin B12 deficiency in users assigned to the metformin group compared with those assigned to the placebo group at the 5-year and 13-year marks after randomization.1 Post hoc analysis of patients in the Hyperinsulinemia: the Outcome of Its Metabolic Effects trial also showed lower levels of vitamin B12 and higher levels of methylmalonic acid associated with significant worsening of a validated neuropathy score in metformin users.2
The mechanism behind the development of vitamin B12 deficiency is not completely understood but could possibly be alterations in intestinal mobility, bacterial overgrowth, or calcium-dependent uptake by ileal cells of the vitamin B12-intrinsic factor complex.3
Our electronic medical record has a built-in tool that suggests checking vitamin B12 whenever a patient requests metformin refills. There are no current guidelines on the need for baseline testing of the vitamin B12 level. The American Diabetes Association recommends periodic measurement of vitamin B12 levels, possibly yearly, in metformin users and more often if there are symptoms indicative of deficiency.4
In Reply: We thank Dr. Moskowitz for his kind comments. We agree about the need for assessing vitamin B12 levels during chronic metformin use.
Secondary analysis of patients in the Diabetes Prevention Program Outcomes Study showed a higher incidence of combined low and low-normal vitamin B12 deficiency in users assigned to the metformin group compared with those assigned to the placebo group at the 5-year and 13-year marks after randomization.1 Post hoc analysis of patients in the Hyperinsulinemia: the Outcome of Its Metabolic Effects trial also showed lower levels of vitamin B12 and higher levels of methylmalonic acid associated with significant worsening of a validated neuropathy score in metformin users.2
The mechanism behind the development of vitamin B12 deficiency is not completely understood but could possibly be alterations in intestinal mobility, bacterial overgrowth, or calcium-dependent uptake by ileal cells of the vitamin B12-intrinsic factor complex.3
Our electronic medical record has a built-in tool that suggests checking vitamin B12 whenever a patient requests metformin refills. There are no current guidelines on the need for baseline testing of the vitamin B12 level. The American Diabetes Association recommends periodic measurement of vitamin B12 levels, possibly yearly, in metformin users and more often if there are symptoms indicative of deficiency.4
- Aroda VR, Edelstein SL, Goldberg RB, et al; Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2019; 101(4):1754–1761. doi:10.1210/jc.2015-3754
- Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: post hoc analysis of a randomized controlled 4.3 year trial. J Diabetes Complications 2018; 32(2):171–178. doi:10.1016/j.jdiacomp.2017.11.001
- Liu KW, Dai LK, Jean W. Metformin-related vitamin B12 deficiency. Age Ageing 2006; 35(2):200–201. doi:10.1093/ageing/afj042
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019; 42(suppl 1):S90–S102. doi:10.2337/dc19-S009
- Aroda VR, Edelstein SL, Goldberg RB, et al; Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2019; 101(4):1754–1761. doi:10.1210/jc.2015-3754
- Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: post hoc analysis of a randomized controlled 4.3 year trial. J Diabetes Complications 2018; 32(2):171–178. doi:10.1016/j.jdiacomp.2017.11.001
- Liu KW, Dai LK, Jean W. Metformin-related vitamin B12 deficiency. Age Ageing 2006; 35(2):200–201. doi:10.1093/ageing/afj042
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019; 42(suppl 1):S90–S102. doi:10.2337/dc19-S009
Click for Credit: Suicide in Medicaid youth; persistent back pain; more
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019