User login
Prompt antidepressant treatment swiftly chops cardiovascular risk
CHICAGO – Prompt, effective treatment for depression in the primary care setting appears to swiftly reduce the elevated cardiovascular risk known to be tied to the mood disorder, Heidi Thomas May, Ph.D., reported at the annual meeting of the American College of Cardiology.
“We know that depression is a risk factor for long-term adverse cardiovascular outcomes. Our study shows that it can also have immediate effects on someone’s cardiovascular health. I think our study highlights the importance of screening for depression in the primary care setting – and if someone’s depressed, they need to be treated,” said Dr. May, a cardiovascular and genetic epidemiologist at Intermountain Medical Center in Murray, Utah.
She presented an observational study of the electronic medical records of 7,559 Intermountain Healthcare patients over age 40 years who completed the Patient Health Questionnaire-9 (PHQ-9) depression screening tool during a visit to an Intermountain primary care clinic for any reason. They completed another PHQ-9 a median of 2.7 years later. Under the Intermountain system, a PHQ-9 score of 10 or more triggers implementation of a depression treatment pathway, the specifics of which vary depending upon the severity of symptoms.
On the basis of their two PHQ-9 scores, all patients were classified into one of four groups: The “nondepressed” group of 3,286 patients had a score of 9 or less on both occasions; the “remained depressed” cohort of 1,987 patients scored 10 or more on both PHQ-9s; the “no longer depressed” group of 1,542 patients scored at least 10 but subsequently improved by at least 5 points to a score of 9 or less; and the 735 patients in the “became depressed” group first scored 9 or less on the PHQ-9 but subsequently had at least a 5-point increase to a score of 10 or more.
The subjects were then followed for major adverse cardiovascular events, or MACE – defined as a composite of death, diagnosis of coronary artery disease, acute MI, stroke, and heart failure hospitalization – for a median of 208 days after completing their second PHQ-9.
The MACE rate was 4.8% in the nondepressed group and similar at 4.6% in the “no longer depressed” group, Dr. May reported. Both groups fared significantly better than the “remained depressed” and “became depressed” groups, which had MACE rates of 6% and 6.4%, respectively.
In a multivariate regression analysis adjusted for demographics, cardiovascular risk factors, prior disease diagnoses, medications, and other potential confounders, the “remained depressed” group was 33% more likely to experience a cardiovascular event than was the nondepressed group, she said. The “became depressed” group had a 44% increase in risk, compared with the nondepressed individuals. In contrast, the MACE risk in patients in the “no longer depressed” group was not significantly different from that of patients who weren’t depressed at either time point. And the MACE risk of patients who became depressed during the course of the study was no different from that of patients who remained depressed at both time points.
This is the first study of its kind, Dr. May said. Hence, the results require confirmation, ideally in a randomized clinical trial.
She reported having no financial conflicts regarding the study, which was supported by Intermountain Healthcare.
CHICAGO – Prompt, effective treatment for depression in the primary care setting appears to swiftly reduce the elevated cardiovascular risk known to be tied to the mood disorder, Heidi Thomas May, Ph.D., reported at the annual meeting of the American College of Cardiology.
“We know that depression is a risk factor for long-term adverse cardiovascular outcomes. Our study shows that it can also have immediate effects on someone’s cardiovascular health. I think our study highlights the importance of screening for depression in the primary care setting – and if someone’s depressed, they need to be treated,” said Dr. May, a cardiovascular and genetic epidemiologist at Intermountain Medical Center in Murray, Utah.
She presented an observational study of the electronic medical records of 7,559 Intermountain Healthcare patients over age 40 years who completed the Patient Health Questionnaire-9 (PHQ-9) depression screening tool during a visit to an Intermountain primary care clinic for any reason. They completed another PHQ-9 a median of 2.7 years later. Under the Intermountain system, a PHQ-9 score of 10 or more triggers implementation of a depression treatment pathway, the specifics of which vary depending upon the severity of symptoms.
On the basis of their two PHQ-9 scores, all patients were classified into one of four groups: The “nondepressed” group of 3,286 patients had a score of 9 or less on both occasions; the “remained depressed” cohort of 1,987 patients scored 10 or more on both PHQ-9s; the “no longer depressed” group of 1,542 patients scored at least 10 but subsequently improved by at least 5 points to a score of 9 or less; and the 735 patients in the “became depressed” group first scored 9 or less on the PHQ-9 but subsequently had at least a 5-point increase to a score of 10 or more.
The subjects were then followed for major adverse cardiovascular events, or MACE – defined as a composite of death, diagnosis of coronary artery disease, acute MI, stroke, and heart failure hospitalization – for a median of 208 days after completing their second PHQ-9.
The MACE rate was 4.8% in the nondepressed group and similar at 4.6% in the “no longer depressed” group, Dr. May reported. Both groups fared significantly better than the “remained depressed” and “became depressed” groups, which had MACE rates of 6% and 6.4%, respectively.
In a multivariate regression analysis adjusted for demographics, cardiovascular risk factors, prior disease diagnoses, medications, and other potential confounders, the “remained depressed” group was 33% more likely to experience a cardiovascular event than was the nondepressed group, she said. The “became depressed” group had a 44% increase in risk, compared with the nondepressed individuals. In contrast, the MACE risk in patients in the “no longer depressed” group was not significantly different from that of patients who weren’t depressed at either time point. And the MACE risk of patients who became depressed during the course of the study was no different from that of patients who remained depressed at both time points.
This is the first study of its kind, Dr. May said. Hence, the results require confirmation, ideally in a randomized clinical trial.
She reported having no financial conflicts regarding the study, which was supported by Intermountain Healthcare.
CHICAGO – Prompt, effective treatment for depression in the primary care setting appears to swiftly reduce the elevated cardiovascular risk known to be tied to the mood disorder, Heidi Thomas May, Ph.D., reported at the annual meeting of the American College of Cardiology.
“We know that depression is a risk factor for long-term adverse cardiovascular outcomes. Our study shows that it can also have immediate effects on someone’s cardiovascular health. I think our study highlights the importance of screening for depression in the primary care setting – and if someone’s depressed, they need to be treated,” said Dr. May, a cardiovascular and genetic epidemiologist at Intermountain Medical Center in Murray, Utah.
She presented an observational study of the electronic medical records of 7,559 Intermountain Healthcare patients over age 40 years who completed the Patient Health Questionnaire-9 (PHQ-9) depression screening tool during a visit to an Intermountain primary care clinic for any reason. They completed another PHQ-9 a median of 2.7 years later. Under the Intermountain system, a PHQ-9 score of 10 or more triggers implementation of a depression treatment pathway, the specifics of which vary depending upon the severity of symptoms.
On the basis of their two PHQ-9 scores, all patients were classified into one of four groups: The “nondepressed” group of 3,286 patients had a score of 9 or less on both occasions; the “remained depressed” cohort of 1,987 patients scored 10 or more on both PHQ-9s; the “no longer depressed” group of 1,542 patients scored at least 10 but subsequently improved by at least 5 points to a score of 9 or less; and the 735 patients in the “became depressed” group first scored 9 or less on the PHQ-9 but subsequently had at least a 5-point increase to a score of 10 or more.
The subjects were then followed for major adverse cardiovascular events, or MACE – defined as a composite of death, diagnosis of coronary artery disease, acute MI, stroke, and heart failure hospitalization – for a median of 208 days after completing their second PHQ-9.
The MACE rate was 4.8% in the nondepressed group and similar at 4.6% in the “no longer depressed” group, Dr. May reported. Both groups fared significantly better than the “remained depressed” and “became depressed” groups, which had MACE rates of 6% and 6.4%, respectively.
In a multivariate regression analysis adjusted for demographics, cardiovascular risk factors, prior disease diagnoses, medications, and other potential confounders, the “remained depressed” group was 33% more likely to experience a cardiovascular event than was the nondepressed group, she said. The “became depressed” group had a 44% increase in risk, compared with the nondepressed individuals. In contrast, the MACE risk in patients in the “no longer depressed” group was not significantly different from that of patients who weren’t depressed at either time point. And the MACE risk of patients who became depressed during the course of the study was no different from that of patients who remained depressed at both time points.
This is the first study of its kind, Dr. May said. Hence, the results require confirmation, ideally in a randomized clinical trial.
She reported having no financial conflicts regarding the study, which was supported by Intermountain Healthcare.
AT ACC 16
Key clinical point: Event rate was no different in “no longer depressed” group than in “never depressed.”
Major finding: Major adverse cardiovascular events were 44% more likely in primary care patients who became depressed during a median 2.7-year period, compared with those who weren’t depressed at either time point.
Data source: An observational study of 7,550 patients screened for depression in primary care clinics.
Disclosures: The study was supported by Intermountain Healthcare. Dr. May reported having no financial conflicts of interest.
Clozapine REMS still plagued by problems
A consolidated registry system designed to increase access to the second-generation antipsychotic clozapine is plagued with glitches, delays, and excess red tape more than 8 months after its initial launch, according to clinicians and pharmacists who are attempting to use it.
The problems include psychiatrists receiving information from the registry on patients not in their care, a breach of Health Insurance Portability and Accountability Act privacy rules. And some said they fear that clozapine, the only Food and Drug Administration–approved drug for treatment-resistant schizophrenia, will end up underprescribed as a result of difficulties complying with the new registry’s demands.
The psychiatric community lauded the FDA’s decision in September 2015 to change the monitoring requirements for clozapine. One rare but dangerous adverse effect of the drug is severe neutropenia, and patients on clozapine must be monitored through regular blood screening. This means that every clozapine script requires careful coordination among the prescriber, patient, laboratory, and pharmacy.
Now, instead of looking at white blood cell counts as before, the FDA said, absolute neutrophil count (ANC) will be the lab measure used to determine whether a patient can be started or continued on clozapine, and new lowered ANC thresholds will allow more patients to be initiated. The new lowered ANC thresholds may pertain to people of African and Middle Eastern heritage with a naturally lower ANC called “benign ethnic neutropenia” and who previously might not have been able to receive the drug.
At the same time it announced the new monitoring rules, the FDA also said the six existing manufacturer registries of clozapine would be consolidated into one, called the Clozapine Risk Evaluation and Mitigation Strategies, or Clozapine REMS. The REMS is managed jointly by the manufacturers. Prescribers and pharmacies dealing with clozapine must become certified under the REMS if they wish patients to receive the drug. Though certification was supposed to have been completed by this month, the agency now is saying providers have until year-end.
REMS applauded early on
Originally, psychiatrists welcomed the news of the REMS, as it promised to make it easier for a patient to continue on clozapine even if the drug supplier changed – when transitioning from an inpatient to outpatient setting, for example.
However, when it launched in the fall of 2015, the Clozapine REMS website was full of glitches, and calls to the toll-free number weren’t picked up. “I couldn’t even get onto [the registry], as they wouldn’t answer the phone,” said Dr. Ira D. Glick, professor emeritus of psychiatry and behavioral sciences and psychopharmacology at Stanford (Calif.) University, who has prescribed clozapine for decades. More recently, he said, the response time has improved.
The FDA has acknowledged providers’ complaints, and mainly characterizes the registry’s problems as growing pains. “As with any new IT system, and large data migration and reconciliation effort, there are challenges,” an agency spokesperson said in an interview about the REMS. “Merging six previous clozapine registries was a huge undertaking for the manufacturers that encompassed merging data from over 50,000 prescribers, 28,000 pharmacies, and 200,000 patient records.”
The FDA has been working “to address the issues identified when the Clozapine REMS Program was initially implemented,” the representative said. “We believe that most of the issues have been resolved.” The FDA did not directly respond to questions about confidentiality breaches.
Providers said in interviews, however, that the issues continue, including mix-ups of confidential health information.
“I’m now registered as a provider designee for several clozapine prescribers here at my hospital,” said Megan Maroney, Pharm.D., of Monmouth Medical Center in Long Branch, N.J. “One of our prescribers also has a private practice, and those patients are popping up on my list, but they’re not my patients.” Dr. Maroney said she contacted the Clozapine REMS about the problem. “They haven’t yet come up with a good way to deal with it,” she said.
Dr. Jean-Pierre Lindenmayer, clinical professor in the department of psychiatry at New York University, said he, too, receives information about patients who are not his. “I’m still getting faxed notifications on patients I have no idea who they are, saying they’re due for a blood test. It’s incredible that they keep doing this.” The REMS never responded to his complaints about privacy breaches, he said.
Problems ‘causing confusion’
Providers say the Clozapine REMS website remains filled with glitches and that deadlines are being extended until these can be resolved. “We’re trying to follow the intended rules with the understanding that if issues come up, we’re supposed to use our clinical judgment and not delay care to the patient. But it’s causing a lot of confusion among my staff, because whenever we try to use it, we encounter some kind of glitch,” Dr. Maroney said.
Another of the providers’ key concerns is the extra red-tape burdens imposed by the REMS, which, they say, have the potential to dampen the prescribing of clozapine – the exact opposite of its intended effect.
By mandating that only registered prescribers can write for clozapine, the REMS can cause problems for hospitals. “If a patient comes in for a medical reason and happens to be on clozapine, it’s impossible for us to get our internal medicine physicians registered just so they can prescribe it for their one patient who comes through,” Dr. Maroney said. Her workaround has been to make sure all the hospital’s psychiatrists are registered and that patients on clozapine have a psych consult, regardless of the reason they’re hospitalized. This, she acknowledged, could drive up costs.
“Here at my hospital, I tend to work out the issues for my prescribers so that they can start patients and continue them, and I think we’ve been doing a pretty good job. But I wonder about places without the manpower to do that or that don’t have a psych pharmacist who can work with them,” she said.
The REMS “is a major obstacle, and it’s more complicated now than it was before,” said Dr. Lindenmayer, who also is affiliated with the Manhattan Psychiatric Center in New York, an institution that manages about 100 patients on clozapine. “The excessive registry demands, and sending doctors letters about all the potential terrible side effects, will discourage providers. Clozapine is already difficult to prescribe: You have to have a pharmacy lined up, you have to have a lab lined up, you have to have a patient that gets the prescription and the blood test in a timely manner, and you are not being reimbursed at any higher rate by having a clozapine patient.”
Dr. Glick agreed. “Clozapine is one of our best drugs, but the most difficult to manage – as it takes a lot of time and effort. This is one more step making it more complicated.”
Dr. Maroney said that at her institution, she’s already seen a chilling effect from the REMS. Recently, she said, “my prescribers and I were going over all the changes and some of them said, ‘Just forget it, I’ll put [patients] on something else.’ And I said, ‘No – the whole point of a lot of the changes was to make [clozapine] more accessible.’ ”
Dr. Lindenmayer said he considers the REMS – or at least the extra layers of bureaucracy and certification it imposes – to have been a misguided move by the FDA. “I am not aware that there have been more deaths recently due to clozapine prescribing, and haven’t seen any upsurge of morbidity and mortality in the literature, which I follow closely.” Relatively few prescribers use clozapine, he said, and those who do “are fairly careful and knowledgeable about what they’re doing. So they’re preaching to the choir,” he said.
Yet Dr. Maroney said she remains optimistic that the REMS and the providers will be able to reach common ground – eventually: “The College of Psychiatric and Neurologic Pharmacists has been communicating with the FDA to hammer out these issues. I think it should get better. I just don’t know when that will occur and how many of these issues will be completely addressed.”
The FDA spokesperson confirmed that the agency was seeking provider input to improve the Clozapine REMS, and that several changes already had been made in response to provider concerns.
A consolidated registry system designed to increase access to the second-generation antipsychotic clozapine is plagued with glitches, delays, and excess red tape more than 8 months after its initial launch, according to clinicians and pharmacists who are attempting to use it.
The problems include psychiatrists receiving information from the registry on patients not in their care, a breach of Health Insurance Portability and Accountability Act privacy rules. And some said they fear that clozapine, the only Food and Drug Administration–approved drug for treatment-resistant schizophrenia, will end up underprescribed as a result of difficulties complying with the new registry’s demands.
The psychiatric community lauded the FDA’s decision in September 2015 to change the monitoring requirements for clozapine. One rare but dangerous adverse effect of the drug is severe neutropenia, and patients on clozapine must be monitored through regular blood screening. This means that every clozapine script requires careful coordination among the prescriber, patient, laboratory, and pharmacy.
Now, instead of looking at white blood cell counts as before, the FDA said, absolute neutrophil count (ANC) will be the lab measure used to determine whether a patient can be started or continued on clozapine, and new lowered ANC thresholds will allow more patients to be initiated. The new lowered ANC thresholds may pertain to people of African and Middle Eastern heritage with a naturally lower ANC called “benign ethnic neutropenia” and who previously might not have been able to receive the drug.
At the same time it announced the new monitoring rules, the FDA also said the six existing manufacturer registries of clozapine would be consolidated into one, called the Clozapine Risk Evaluation and Mitigation Strategies, or Clozapine REMS. The REMS is managed jointly by the manufacturers. Prescribers and pharmacies dealing with clozapine must become certified under the REMS if they wish patients to receive the drug. Though certification was supposed to have been completed by this month, the agency now is saying providers have until year-end.
REMS applauded early on
Originally, psychiatrists welcomed the news of the REMS, as it promised to make it easier for a patient to continue on clozapine even if the drug supplier changed – when transitioning from an inpatient to outpatient setting, for example.
However, when it launched in the fall of 2015, the Clozapine REMS website was full of glitches, and calls to the toll-free number weren’t picked up. “I couldn’t even get onto [the registry], as they wouldn’t answer the phone,” said Dr. Ira D. Glick, professor emeritus of psychiatry and behavioral sciences and psychopharmacology at Stanford (Calif.) University, who has prescribed clozapine for decades. More recently, he said, the response time has improved.
The FDA has acknowledged providers’ complaints, and mainly characterizes the registry’s problems as growing pains. “As with any new IT system, and large data migration and reconciliation effort, there are challenges,” an agency spokesperson said in an interview about the REMS. “Merging six previous clozapine registries was a huge undertaking for the manufacturers that encompassed merging data from over 50,000 prescribers, 28,000 pharmacies, and 200,000 patient records.”
The FDA has been working “to address the issues identified when the Clozapine REMS Program was initially implemented,” the representative said. “We believe that most of the issues have been resolved.” The FDA did not directly respond to questions about confidentiality breaches.
Providers said in interviews, however, that the issues continue, including mix-ups of confidential health information.
“I’m now registered as a provider designee for several clozapine prescribers here at my hospital,” said Megan Maroney, Pharm.D., of Monmouth Medical Center in Long Branch, N.J. “One of our prescribers also has a private practice, and those patients are popping up on my list, but they’re not my patients.” Dr. Maroney said she contacted the Clozapine REMS about the problem. “They haven’t yet come up with a good way to deal with it,” she said.
Dr. Jean-Pierre Lindenmayer, clinical professor in the department of psychiatry at New York University, said he, too, receives information about patients who are not his. “I’m still getting faxed notifications on patients I have no idea who they are, saying they’re due for a blood test. It’s incredible that they keep doing this.” The REMS never responded to his complaints about privacy breaches, he said.
Problems ‘causing confusion’
Providers say the Clozapine REMS website remains filled with glitches and that deadlines are being extended until these can be resolved. “We’re trying to follow the intended rules with the understanding that if issues come up, we’re supposed to use our clinical judgment and not delay care to the patient. But it’s causing a lot of confusion among my staff, because whenever we try to use it, we encounter some kind of glitch,” Dr. Maroney said.
Another of the providers’ key concerns is the extra red-tape burdens imposed by the REMS, which, they say, have the potential to dampen the prescribing of clozapine – the exact opposite of its intended effect.
By mandating that only registered prescribers can write for clozapine, the REMS can cause problems for hospitals. “If a patient comes in for a medical reason and happens to be on clozapine, it’s impossible for us to get our internal medicine physicians registered just so they can prescribe it for their one patient who comes through,” Dr. Maroney said. Her workaround has been to make sure all the hospital’s psychiatrists are registered and that patients on clozapine have a psych consult, regardless of the reason they’re hospitalized. This, she acknowledged, could drive up costs.
“Here at my hospital, I tend to work out the issues for my prescribers so that they can start patients and continue them, and I think we’ve been doing a pretty good job. But I wonder about places without the manpower to do that or that don’t have a psych pharmacist who can work with them,” she said.
The REMS “is a major obstacle, and it’s more complicated now than it was before,” said Dr. Lindenmayer, who also is affiliated with the Manhattan Psychiatric Center in New York, an institution that manages about 100 patients on clozapine. “The excessive registry demands, and sending doctors letters about all the potential terrible side effects, will discourage providers. Clozapine is already difficult to prescribe: You have to have a pharmacy lined up, you have to have a lab lined up, you have to have a patient that gets the prescription and the blood test in a timely manner, and you are not being reimbursed at any higher rate by having a clozapine patient.”
Dr. Glick agreed. “Clozapine is one of our best drugs, but the most difficult to manage – as it takes a lot of time and effort. This is one more step making it more complicated.”
Dr. Maroney said that at her institution, she’s already seen a chilling effect from the REMS. Recently, she said, “my prescribers and I were going over all the changes and some of them said, ‘Just forget it, I’ll put [patients] on something else.’ And I said, ‘No – the whole point of a lot of the changes was to make [clozapine] more accessible.’ ”
Dr. Lindenmayer said he considers the REMS – or at least the extra layers of bureaucracy and certification it imposes – to have been a misguided move by the FDA. “I am not aware that there have been more deaths recently due to clozapine prescribing, and haven’t seen any upsurge of morbidity and mortality in the literature, which I follow closely.” Relatively few prescribers use clozapine, he said, and those who do “are fairly careful and knowledgeable about what they’re doing. So they’re preaching to the choir,” he said.
Yet Dr. Maroney said she remains optimistic that the REMS and the providers will be able to reach common ground – eventually: “The College of Psychiatric and Neurologic Pharmacists has been communicating with the FDA to hammer out these issues. I think it should get better. I just don’t know when that will occur and how many of these issues will be completely addressed.”
The FDA spokesperson confirmed that the agency was seeking provider input to improve the Clozapine REMS, and that several changes already had been made in response to provider concerns.
A consolidated registry system designed to increase access to the second-generation antipsychotic clozapine is plagued with glitches, delays, and excess red tape more than 8 months after its initial launch, according to clinicians and pharmacists who are attempting to use it.
The problems include psychiatrists receiving information from the registry on patients not in their care, a breach of Health Insurance Portability and Accountability Act privacy rules. And some said they fear that clozapine, the only Food and Drug Administration–approved drug for treatment-resistant schizophrenia, will end up underprescribed as a result of difficulties complying with the new registry’s demands.
The psychiatric community lauded the FDA’s decision in September 2015 to change the monitoring requirements for clozapine. One rare but dangerous adverse effect of the drug is severe neutropenia, and patients on clozapine must be monitored through regular blood screening. This means that every clozapine script requires careful coordination among the prescriber, patient, laboratory, and pharmacy.
Now, instead of looking at white blood cell counts as before, the FDA said, absolute neutrophil count (ANC) will be the lab measure used to determine whether a patient can be started or continued on clozapine, and new lowered ANC thresholds will allow more patients to be initiated. The new lowered ANC thresholds may pertain to people of African and Middle Eastern heritage with a naturally lower ANC called “benign ethnic neutropenia” and who previously might not have been able to receive the drug.
At the same time it announced the new monitoring rules, the FDA also said the six existing manufacturer registries of clozapine would be consolidated into one, called the Clozapine Risk Evaluation and Mitigation Strategies, or Clozapine REMS. The REMS is managed jointly by the manufacturers. Prescribers and pharmacies dealing with clozapine must become certified under the REMS if they wish patients to receive the drug. Though certification was supposed to have been completed by this month, the agency now is saying providers have until year-end.
REMS applauded early on
Originally, psychiatrists welcomed the news of the REMS, as it promised to make it easier for a patient to continue on clozapine even if the drug supplier changed – when transitioning from an inpatient to outpatient setting, for example.
However, when it launched in the fall of 2015, the Clozapine REMS website was full of glitches, and calls to the toll-free number weren’t picked up. “I couldn’t even get onto [the registry], as they wouldn’t answer the phone,” said Dr. Ira D. Glick, professor emeritus of psychiatry and behavioral sciences and psychopharmacology at Stanford (Calif.) University, who has prescribed clozapine for decades. More recently, he said, the response time has improved.
The FDA has acknowledged providers’ complaints, and mainly characterizes the registry’s problems as growing pains. “As with any new IT system, and large data migration and reconciliation effort, there are challenges,” an agency spokesperson said in an interview about the REMS. “Merging six previous clozapine registries was a huge undertaking for the manufacturers that encompassed merging data from over 50,000 prescribers, 28,000 pharmacies, and 200,000 patient records.”
The FDA has been working “to address the issues identified when the Clozapine REMS Program was initially implemented,” the representative said. “We believe that most of the issues have been resolved.” The FDA did not directly respond to questions about confidentiality breaches.
Providers said in interviews, however, that the issues continue, including mix-ups of confidential health information.
“I’m now registered as a provider designee for several clozapine prescribers here at my hospital,” said Megan Maroney, Pharm.D., of Monmouth Medical Center in Long Branch, N.J. “One of our prescribers also has a private practice, and those patients are popping up on my list, but they’re not my patients.” Dr. Maroney said she contacted the Clozapine REMS about the problem. “They haven’t yet come up with a good way to deal with it,” she said.
Dr. Jean-Pierre Lindenmayer, clinical professor in the department of psychiatry at New York University, said he, too, receives information about patients who are not his. “I’m still getting faxed notifications on patients I have no idea who they are, saying they’re due for a blood test. It’s incredible that they keep doing this.” The REMS never responded to his complaints about privacy breaches, he said.
Problems ‘causing confusion’
Providers say the Clozapine REMS website remains filled with glitches and that deadlines are being extended until these can be resolved. “We’re trying to follow the intended rules with the understanding that if issues come up, we’re supposed to use our clinical judgment and not delay care to the patient. But it’s causing a lot of confusion among my staff, because whenever we try to use it, we encounter some kind of glitch,” Dr. Maroney said.
Another of the providers’ key concerns is the extra red-tape burdens imposed by the REMS, which, they say, have the potential to dampen the prescribing of clozapine – the exact opposite of its intended effect.
By mandating that only registered prescribers can write for clozapine, the REMS can cause problems for hospitals. “If a patient comes in for a medical reason and happens to be on clozapine, it’s impossible for us to get our internal medicine physicians registered just so they can prescribe it for their one patient who comes through,” Dr. Maroney said. Her workaround has been to make sure all the hospital’s psychiatrists are registered and that patients on clozapine have a psych consult, regardless of the reason they’re hospitalized. This, she acknowledged, could drive up costs.
“Here at my hospital, I tend to work out the issues for my prescribers so that they can start patients and continue them, and I think we’ve been doing a pretty good job. But I wonder about places without the manpower to do that or that don’t have a psych pharmacist who can work with them,” she said.
The REMS “is a major obstacle, and it’s more complicated now than it was before,” said Dr. Lindenmayer, who also is affiliated with the Manhattan Psychiatric Center in New York, an institution that manages about 100 patients on clozapine. “The excessive registry demands, and sending doctors letters about all the potential terrible side effects, will discourage providers. Clozapine is already difficult to prescribe: You have to have a pharmacy lined up, you have to have a lab lined up, you have to have a patient that gets the prescription and the blood test in a timely manner, and you are not being reimbursed at any higher rate by having a clozapine patient.”
Dr. Glick agreed. “Clozapine is one of our best drugs, but the most difficult to manage – as it takes a lot of time and effort. This is one more step making it more complicated.”
Dr. Maroney said that at her institution, she’s already seen a chilling effect from the REMS. Recently, she said, “my prescribers and I were going over all the changes and some of them said, ‘Just forget it, I’ll put [patients] on something else.’ And I said, ‘No – the whole point of a lot of the changes was to make [clozapine] more accessible.’ ”
Dr. Lindenmayer said he considers the REMS – or at least the extra layers of bureaucracy and certification it imposes – to have been a misguided move by the FDA. “I am not aware that there have been more deaths recently due to clozapine prescribing, and haven’t seen any upsurge of morbidity and mortality in the literature, which I follow closely.” Relatively few prescribers use clozapine, he said, and those who do “are fairly careful and knowledgeable about what they’re doing. So they’re preaching to the choir,” he said.
Yet Dr. Maroney said she remains optimistic that the REMS and the providers will be able to reach common ground – eventually: “The College of Psychiatric and Neurologic Pharmacists has been communicating with the FDA to hammer out these issues. I think it should get better. I just don’t know when that will occur and how many of these issues will be completely addressed.”
The FDA spokesperson confirmed that the agency was seeking provider input to improve the Clozapine REMS, and that several changes already had been made in response to provider concerns.
Health care reform 6 years out
Well, UnitedHealthcare has announced that it’s pulling out of the health insurance exchanges because of huge losses. This may be the mortal wound. It is apparent that health care reform is undergoing a slow-motion implosion. Most of the Affordable Care Act has been delayed or canceled, including the individual and employer mandates, the independent payment advisory board (thank goodness), Medicare Advantage payment cuts, the Cadillac health insurance tax, and auto enrollment. Half the insurance co-ops have failed, and the remainder are running at a deficit. The exchange-plan premiums are increasing dramatically. In fact, with 71 cancellations and delays, health care reform has already effectively been repealed.
Insurance coverage has increased by about 8% (10 million more into Medicaid, 80% of all new insureds), particularly of the poor. On the flip side, millions have lost their old insurance, now have very high deductibles, and have lost their doctors. High-deductible insurance means that patients really aren’t going to be able to use their insurance, unless they have a catastrophic event and are hospitalized.
High-deductible insurance that pays at Medicaid rates, and Medicaid, are a particularly bad mix for dermatologists. Medicaid does not even cover the cost of overhead in the office setting, and many patients cannot afford their high deductibles. Almost all of the cost-efficient in-office curative procedures we offer cost less than the deductible. We are all seeing patients delay and delay treatment until the end of the year, hoping they won’t have to meet their deductible.
Many patients, excited that they finally have health insurance, are bitterly disappointed to find that they really don’t, except for their annual physical exam and the emergency room. The doctor is put in the poisonous position of explaining insurance policy limitations, and being collection agent. Poor Medicaid patients, who get free care at the hospital, go to the emergency room for minor complaints that would be much more efficiently handled in the office setting. This clogs emergency rooms, and is ferociously expensive. This is the opposite of what health care reform was supposed to do.
Insurance premiums are rising rapidly because somebody has to pay for coverage of the millions who could not formerly qualify for health insurance because of preexisting conditions. The current exchanges allow dropping in and out of insurance coverage and, with the elimination of preexisting conditions, this allows patients to game the system and wait until they fall ill to buy insurance. Historically, almost all were in the pool of insureds and the risk was predictable.
There is no way for politicians to go back and remove millions of chronically ill from the coverage rolls. Imagine the nightly news. As a physician, it is impossible not to feel compassion for these chronically ill patients, but it would have cost a lot less to just make them eligible for Medicaid to begin with.
OK, it is easy to complain, but what are possible solutions? Patients need health care savings accounts for a sizable portion of their deductibles. Physician rosters need to be real time and accurate. Networks need to be adequate (another column, another day). Medicaid rates need to increase to Medicare levels, as they did for the initial 2-year teaser period for primary care physicians. Exchange plan payment rates need to match their commercial insurance wrappers, instead of Medicaid rates, so physicians can afford to accept them. Stricter enrollment periods are needed, so patients cannot game the system, signing up only when they get sick or want a joint replaced. If you are going to provide health insurance for all, then make sure the health insurance is real, not hollow.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Write to him at [email protected].
Well, UnitedHealthcare has announced that it’s pulling out of the health insurance exchanges because of huge losses. This may be the mortal wound. It is apparent that health care reform is undergoing a slow-motion implosion. Most of the Affordable Care Act has been delayed or canceled, including the individual and employer mandates, the independent payment advisory board (thank goodness), Medicare Advantage payment cuts, the Cadillac health insurance tax, and auto enrollment. Half the insurance co-ops have failed, and the remainder are running at a deficit. The exchange-plan premiums are increasing dramatically. In fact, with 71 cancellations and delays, health care reform has already effectively been repealed.
Insurance coverage has increased by about 8% (10 million more into Medicaid, 80% of all new insureds), particularly of the poor. On the flip side, millions have lost their old insurance, now have very high deductibles, and have lost their doctors. High-deductible insurance means that patients really aren’t going to be able to use their insurance, unless they have a catastrophic event and are hospitalized.
High-deductible insurance that pays at Medicaid rates, and Medicaid, are a particularly bad mix for dermatologists. Medicaid does not even cover the cost of overhead in the office setting, and many patients cannot afford their high deductibles. Almost all of the cost-efficient in-office curative procedures we offer cost less than the deductible. We are all seeing patients delay and delay treatment until the end of the year, hoping they won’t have to meet their deductible.
Many patients, excited that they finally have health insurance, are bitterly disappointed to find that they really don’t, except for their annual physical exam and the emergency room. The doctor is put in the poisonous position of explaining insurance policy limitations, and being collection agent. Poor Medicaid patients, who get free care at the hospital, go to the emergency room for minor complaints that would be much more efficiently handled in the office setting. This clogs emergency rooms, and is ferociously expensive. This is the opposite of what health care reform was supposed to do.
Insurance premiums are rising rapidly because somebody has to pay for coverage of the millions who could not formerly qualify for health insurance because of preexisting conditions. The current exchanges allow dropping in and out of insurance coverage and, with the elimination of preexisting conditions, this allows patients to game the system and wait until they fall ill to buy insurance. Historically, almost all were in the pool of insureds and the risk was predictable.
There is no way for politicians to go back and remove millions of chronically ill from the coverage rolls. Imagine the nightly news. As a physician, it is impossible not to feel compassion for these chronically ill patients, but it would have cost a lot less to just make them eligible for Medicaid to begin with.
OK, it is easy to complain, but what are possible solutions? Patients need health care savings accounts for a sizable portion of their deductibles. Physician rosters need to be real time and accurate. Networks need to be adequate (another column, another day). Medicaid rates need to increase to Medicare levels, as they did for the initial 2-year teaser period for primary care physicians. Exchange plan payment rates need to match their commercial insurance wrappers, instead of Medicaid rates, so physicians can afford to accept them. Stricter enrollment periods are needed, so patients cannot game the system, signing up only when they get sick or want a joint replaced. If you are going to provide health insurance for all, then make sure the health insurance is real, not hollow.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Write to him at [email protected].
Well, UnitedHealthcare has announced that it’s pulling out of the health insurance exchanges because of huge losses. This may be the mortal wound. It is apparent that health care reform is undergoing a slow-motion implosion. Most of the Affordable Care Act has been delayed or canceled, including the individual and employer mandates, the independent payment advisory board (thank goodness), Medicare Advantage payment cuts, the Cadillac health insurance tax, and auto enrollment. Half the insurance co-ops have failed, and the remainder are running at a deficit. The exchange-plan premiums are increasing dramatically. In fact, with 71 cancellations and delays, health care reform has already effectively been repealed.
Insurance coverage has increased by about 8% (10 million more into Medicaid, 80% of all new insureds), particularly of the poor. On the flip side, millions have lost their old insurance, now have very high deductibles, and have lost their doctors. High-deductible insurance means that patients really aren’t going to be able to use their insurance, unless they have a catastrophic event and are hospitalized.
High-deductible insurance that pays at Medicaid rates, and Medicaid, are a particularly bad mix for dermatologists. Medicaid does not even cover the cost of overhead in the office setting, and many patients cannot afford their high deductibles. Almost all of the cost-efficient in-office curative procedures we offer cost less than the deductible. We are all seeing patients delay and delay treatment until the end of the year, hoping they won’t have to meet their deductible.
Many patients, excited that they finally have health insurance, are bitterly disappointed to find that they really don’t, except for their annual physical exam and the emergency room. The doctor is put in the poisonous position of explaining insurance policy limitations, and being collection agent. Poor Medicaid patients, who get free care at the hospital, go to the emergency room for minor complaints that would be much more efficiently handled in the office setting. This clogs emergency rooms, and is ferociously expensive. This is the opposite of what health care reform was supposed to do.
Insurance premiums are rising rapidly because somebody has to pay for coverage of the millions who could not formerly qualify for health insurance because of preexisting conditions. The current exchanges allow dropping in and out of insurance coverage and, with the elimination of preexisting conditions, this allows patients to game the system and wait until they fall ill to buy insurance. Historically, almost all were in the pool of insureds and the risk was predictable.
There is no way for politicians to go back and remove millions of chronically ill from the coverage rolls. Imagine the nightly news. As a physician, it is impossible not to feel compassion for these chronically ill patients, but it would have cost a lot less to just make them eligible for Medicaid to begin with.
OK, it is easy to complain, but what are possible solutions? Patients need health care savings accounts for a sizable portion of their deductibles. Physician rosters need to be real time and accurate. Networks need to be adequate (another column, another day). Medicaid rates need to increase to Medicare levels, as they did for the initial 2-year teaser period for primary care physicians. Exchange plan payment rates need to match their commercial insurance wrappers, instead of Medicaid rates, so physicians can afford to accept them. Stricter enrollment periods are needed, so patients cannot game the system, signing up only when they get sick or want a joint replaced. If you are going to provide health insurance for all, then make sure the health insurance is real, not hollow.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. Write to him at [email protected].
Women reach for the top in ob.gyn.
Dr. Gloria E. Sarto was one of just six women in her medical school graduating class of 76 in 1958 – a time when many medical schools, she recalled, had quota systems for women and minorities. Later, she became the first female ob.gyn. resident at the University of Wisconsin–Madison.
“When I was interviewing for a residency position, the department chair told me ‘I’m going to treat you like one of the boys,’” the 86-year-old professor emeritus said. “And I said, ‘If you do that, it will be just fine.’”
Yet she still had to lobby sometimes for equal treatment – convincing the department chief in one instance that sleeping on a delivery table during hospital duty because there weren’t any rooms for women was not being treated “like one of the boys.” And she was often bothered by her observation that, in general, “the women [residents] weren’t noticed... they weren’t being recognized.”
Dr. Sarto has since chaired two ob.gyn. departments and was the first woman president of the American Gynecological and Obstetrical Society. Today, however, as she continues mentoring junior faculty and works to ensure the smooth succession of programs she founded, she sees a much different field – one in which women not only command more respect but where they make up a majority of ob.gyns.
Impact on women’s health
In 2014, 62% of all ob.gyns. were women (Obstet Gynecol. 2016 Jan;127[1]:148-52). The majority has been years in the making; more women than men have been entering the specialty since 1993. And if current trends continue, the percentage of women active in the specialty will only increase further. In 2010, women comprised more than 80% of all ob.gyn. residents/fellows, more than any other specialty, according to data from the Association of American Medical Colleges.
Such numerical strength is significant, but for Dr. Sarto and other leaders in the specialty who spoke about their experiences as female ob.gyns., it’s the impact that women physicians have had on women’s health that’s most important.
Dr. Sarto helped to start Lamaze classes in a hospital basement amidst widespread opposition from the male-dominated leadership and staff who felt that women didn’t need such help with labor. She also takes pride in her collaboration with Dr. Florence Haseltine, Phyllis Greenberger, and several other women to address biases in biomedical research. Their work with Congress led to a federal audit of National Institutes of Health policies and practices.
“We knew that when that report came out [in 1990], it would hit every newspaper in the country,” Dr. Sarto said. It just about did, and soon after that, the NIH Office of Research on Women’s Health was established to ensure that women were included in clinical trials and that gaps in knowledge of women’s health were addressed.
Dr. Barbara Levy, who left a private practice and two medical directorships in 2012 to become Vice President for Health Policy at the American College of Obstetricians and Gynecologists, recalls feeling early in her career that women’s health needed to be approached much more holistically.
“What I was seeing and experiencing didn’t match the textbooks,” she said. “The connection, for instance, between chronic pelvic pain and women who’d been victims of sexual abuse – there wasn’t anything in the literature. I’d see women with the same kinds of physical characteristics on their exams... and patients were willing to share with me things that they wouldn’t have been willing to share with my colleagues.”
Dr. Levy graduated from Princeton University in 1974 with the second class of admitted women, and after a year off, went west for medical school. She graduated in 1979 from the University of California, San Diego, with nine other women in a class of 110.
Her desire to care for the “whole patient” had her leaning toward family medicine until a beloved mentor, Dr. Donna Brooks, “reminded me that there were so few women to take care of women... and that as an ob.gyn. I could follow women through pregnancy, delivery, surgeries, hormone issues, and so many [other facets of their health].”
Dr. Levy, who served as president of the American Association of Gynecologic Laparoscopists in 1995, recalls a world “that was very tolerant of sexual harassment” and remembers the energy she needed to expend to be taken seriously and to correct unconscious bias.
When she applied for fellowship in the American College of Surgeons in the late 1980s, the committee members who conducted an interview “told me right away that I couldn’t expect to be a fellow if I hadn’t done my duty [serving on hospital committees],” Dr. Levy said.
“I told them I had volunteered for more than three committees every single year I’d been on staff, and had never been asked to serve,” she said. “They had no idea. Their assumption was that I had children at home and I wasn’t taking the time.”
Entering leadership
When it comes to leadership, a look at academic medicine suggests that women ob.gyns. have made significant strides. In 2013, compared with other major specialties, obstetrics and gynecology had the highest proportion of department leaders who were women. Yet the picture is mixed. According to an analysis published earlier in 2016, women in ob.gyn. and nine other major specialties “were not represented in the proportions in which they entered their fields” (Obstet Gynecol. 2016 Mar;127[3]:442-7).
Women comprised 57% of all faculty in departments of ob.gyn. in 2013. And, according to the analysis, they comprised 62% of ob.gyn. residency program directors, 30% of division directors, and 24% of department chairs.
The high numbers of women serving as residency program directors raises concern because such positions “do not result in advancement in the same way,” said Dr. Levy, adding that women have excelled in such positions and may desire them, but should be mentored early on about what tracks have the potential for upper-level leadership roles.
Dr. Maureen Phipps, who in 2013 was appointed as chair of ob.gyn. and assistant dean in the Warren Albert Medical School of Brown University in Providence, said she carries with her the fact that women are not yet proportionally represented at the upper levels. “I know that my being in this position and in other positions I’ve held is important for women to see,” she said.
Dr. Phipps graduated from the University of Vermont’s College of Medicine in 1994 as a part of a class in which men and women were fairly evenly represented. In addition to her role as department chair and assistant dean, she is also now chief of ob.gyn. at Women & Infants Hospital of Rhode Island, where she did her residency, and executive chief of ob.gyn for the Care New England Health System.
“I’ve had amazing male leaders and mentors in my career – the people who’ve gone to bat for me have been men,” said Dr. Phipps. Yet, “it’s important to have women in leadership... It’s known that we think differently and approach things differently. Having balance and a variety of different lenses will allow us to [further] grow the field.”
Gender pay gap
Both in academic medicine and in practice, a gender pay gap still reportedly affects women physicians across the board. Various reports and analyses have shown women earning disproportionately less than their male colleagues in similar positions.
Notably, a 2011 analysis in Health Affairs found a nearly $17,000 gap between the starting salaries for men and women physicians. This differential accounted for variables such as patient care hours, practice type, and location. It is possible, the study authors reported, that practices “may now be offering greater flexibility and family-friendly attributes that are more appealing but that come at the price of commensurately lower pay” (Health Aff. 2011:30;193-201).
The American Medical Women’s Association, which promotes advocacy on a gender pay gap, said in a statement about the study, however, that “gender discrimination still exists within the echelons of medicine, and gender stereotyping frequently leads to the devaluation of women physicians.”
From her perspective, Dr. Levy said it’s “complicated” to tease apart and understand all the factors that may be involved.
The challenges of balancing work and family/caregiving and are “still really tough” for women ob.gyns., she said, especially those who want to practice obstetrics. Dr. Levy said she gave up obstetrics when it became apparent that she and her husband would need to hire an additional child care provider.
While the hospitalist-laborist model has been a valuable addition to obstetrics, Dr. Phipps said, “it’s our challenge to continue to think creatively about how we can keep clinicians engaged when they’re in the earlier parts of their careers and challenged by family responsibilities and other commitments.”
Both she and Dr. Levy emphasized their concerns about burnout and their desire to keep career satisfaction high – especially now that women are such a big part of ob.gyn. – and both spoke of the importance of making time for whatever activities help women “recharge.”
“We should be doing what we’re doing because we love it,” Dr. Levy said. “We should focus on that every day. Our patients trust us. We need to remind ourselves of what incredible connections we have.”
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
Dr. Gloria E. Sarto was one of just six women in her medical school graduating class of 76 in 1958 – a time when many medical schools, she recalled, had quota systems for women and minorities. Later, she became the first female ob.gyn. resident at the University of Wisconsin–Madison.
“When I was interviewing for a residency position, the department chair told me ‘I’m going to treat you like one of the boys,’” the 86-year-old professor emeritus said. “And I said, ‘If you do that, it will be just fine.’”
Yet she still had to lobby sometimes for equal treatment – convincing the department chief in one instance that sleeping on a delivery table during hospital duty because there weren’t any rooms for women was not being treated “like one of the boys.” And she was often bothered by her observation that, in general, “the women [residents] weren’t noticed... they weren’t being recognized.”
Dr. Sarto has since chaired two ob.gyn. departments and was the first woman president of the American Gynecological and Obstetrical Society. Today, however, as she continues mentoring junior faculty and works to ensure the smooth succession of programs she founded, she sees a much different field – one in which women not only command more respect but where they make up a majority of ob.gyns.
Impact on women’s health
In 2014, 62% of all ob.gyns. were women (Obstet Gynecol. 2016 Jan;127[1]:148-52). The majority has been years in the making; more women than men have been entering the specialty since 1993. And if current trends continue, the percentage of women active in the specialty will only increase further. In 2010, women comprised more than 80% of all ob.gyn. residents/fellows, more than any other specialty, according to data from the Association of American Medical Colleges.
Such numerical strength is significant, but for Dr. Sarto and other leaders in the specialty who spoke about their experiences as female ob.gyns., it’s the impact that women physicians have had on women’s health that’s most important.
Dr. Sarto helped to start Lamaze classes in a hospital basement amidst widespread opposition from the male-dominated leadership and staff who felt that women didn’t need such help with labor. She also takes pride in her collaboration with Dr. Florence Haseltine, Phyllis Greenberger, and several other women to address biases in biomedical research. Their work with Congress led to a federal audit of National Institutes of Health policies and practices.
“We knew that when that report came out [in 1990], it would hit every newspaper in the country,” Dr. Sarto said. It just about did, and soon after that, the NIH Office of Research on Women’s Health was established to ensure that women were included in clinical trials and that gaps in knowledge of women’s health were addressed.
Dr. Barbara Levy, who left a private practice and two medical directorships in 2012 to become Vice President for Health Policy at the American College of Obstetricians and Gynecologists, recalls feeling early in her career that women’s health needed to be approached much more holistically.
“What I was seeing and experiencing didn’t match the textbooks,” she said. “The connection, for instance, between chronic pelvic pain and women who’d been victims of sexual abuse – there wasn’t anything in the literature. I’d see women with the same kinds of physical characteristics on their exams... and patients were willing to share with me things that they wouldn’t have been willing to share with my colleagues.”
Dr. Levy graduated from Princeton University in 1974 with the second class of admitted women, and after a year off, went west for medical school. She graduated in 1979 from the University of California, San Diego, with nine other women in a class of 110.
Her desire to care for the “whole patient” had her leaning toward family medicine until a beloved mentor, Dr. Donna Brooks, “reminded me that there were so few women to take care of women... and that as an ob.gyn. I could follow women through pregnancy, delivery, surgeries, hormone issues, and so many [other facets of their health].”
Dr. Levy, who served as president of the American Association of Gynecologic Laparoscopists in 1995, recalls a world “that was very tolerant of sexual harassment” and remembers the energy she needed to expend to be taken seriously and to correct unconscious bias.
When she applied for fellowship in the American College of Surgeons in the late 1980s, the committee members who conducted an interview “told me right away that I couldn’t expect to be a fellow if I hadn’t done my duty [serving on hospital committees],” Dr. Levy said.
“I told them I had volunteered for more than three committees every single year I’d been on staff, and had never been asked to serve,” she said. “They had no idea. Their assumption was that I had children at home and I wasn’t taking the time.”
Entering leadership
When it comes to leadership, a look at academic medicine suggests that women ob.gyns. have made significant strides. In 2013, compared with other major specialties, obstetrics and gynecology had the highest proportion of department leaders who were women. Yet the picture is mixed. According to an analysis published earlier in 2016, women in ob.gyn. and nine other major specialties “were not represented in the proportions in which they entered their fields” (Obstet Gynecol. 2016 Mar;127[3]:442-7).
Women comprised 57% of all faculty in departments of ob.gyn. in 2013. And, according to the analysis, they comprised 62% of ob.gyn. residency program directors, 30% of division directors, and 24% of department chairs.
The high numbers of women serving as residency program directors raises concern because such positions “do not result in advancement in the same way,” said Dr. Levy, adding that women have excelled in such positions and may desire them, but should be mentored early on about what tracks have the potential for upper-level leadership roles.
Dr. Maureen Phipps, who in 2013 was appointed as chair of ob.gyn. and assistant dean in the Warren Albert Medical School of Brown University in Providence, said she carries with her the fact that women are not yet proportionally represented at the upper levels. “I know that my being in this position and in other positions I’ve held is important for women to see,” she said.
Dr. Phipps graduated from the University of Vermont’s College of Medicine in 1994 as a part of a class in which men and women were fairly evenly represented. In addition to her role as department chair and assistant dean, she is also now chief of ob.gyn. at Women & Infants Hospital of Rhode Island, where she did her residency, and executive chief of ob.gyn for the Care New England Health System.
“I’ve had amazing male leaders and mentors in my career – the people who’ve gone to bat for me have been men,” said Dr. Phipps. Yet, “it’s important to have women in leadership... It’s known that we think differently and approach things differently. Having balance and a variety of different lenses will allow us to [further] grow the field.”
Gender pay gap
Both in academic medicine and in practice, a gender pay gap still reportedly affects women physicians across the board. Various reports and analyses have shown women earning disproportionately less than their male colleagues in similar positions.
Notably, a 2011 analysis in Health Affairs found a nearly $17,000 gap between the starting salaries for men and women physicians. This differential accounted for variables such as patient care hours, practice type, and location. It is possible, the study authors reported, that practices “may now be offering greater flexibility and family-friendly attributes that are more appealing but that come at the price of commensurately lower pay” (Health Aff. 2011:30;193-201).
The American Medical Women’s Association, which promotes advocacy on a gender pay gap, said in a statement about the study, however, that “gender discrimination still exists within the echelons of medicine, and gender stereotyping frequently leads to the devaluation of women physicians.”
From her perspective, Dr. Levy said it’s “complicated” to tease apart and understand all the factors that may be involved.
The challenges of balancing work and family/caregiving and are “still really tough” for women ob.gyns., she said, especially those who want to practice obstetrics. Dr. Levy said she gave up obstetrics when it became apparent that she and her husband would need to hire an additional child care provider.
While the hospitalist-laborist model has been a valuable addition to obstetrics, Dr. Phipps said, “it’s our challenge to continue to think creatively about how we can keep clinicians engaged when they’re in the earlier parts of their careers and challenged by family responsibilities and other commitments.”
Both she and Dr. Levy emphasized their concerns about burnout and their desire to keep career satisfaction high – especially now that women are such a big part of ob.gyn. – and both spoke of the importance of making time for whatever activities help women “recharge.”
“We should be doing what we’re doing because we love it,” Dr. Levy said. “We should focus on that every day. Our patients trust us. We need to remind ourselves of what incredible connections we have.”
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
Dr. Gloria E. Sarto was one of just six women in her medical school graduating class of 76 in 1958 – a time when many medical schools, she recalled, had quota systems for women and minorities. Later, she became the first female ob.gyn. resident at the University of Wisconsin–Madison.
“When I was interviewing for a residency position, the department chair told me ‘I’m going to treat you like one of the boys,’” the 86-year-old professor emeritus said. “And I said, ‘If you do that, it will be just fine.’”
Yet she still had to lobby sometimes for equal treatment – convincing the department chief in one instance that sleeping on a delivery table during hospital duty because there weren’t any rooms for women was not being treated “like one of the boys.” And she was often bothered by her observation that, in general, “the women [residents] weren’t noticed... they weren’t being recognized.”
Dr. Sarto has since chaired two ob.gyn. departments and was the first woman president of the American Gynecological and Obstetrical Society. Today, however, as she continues mentoring junior faculty and works to ensure the smooth succession of programs she founded, she sees a much different field – one in which women not only command more respect but where they make up a majority of ob.gyns.
Impact on women’s health
In 2014, 62% of all ob.gyns. were women (Obstet Gynecol. 2016 Jan;127[1]:148-52). The majority has been years in the making; more women than men have been entering the specialty since 1993. And if current trends continue, the percentage of women active in the specialty will only increase further. In 2010, women comprised more than 80% of all ob.gyn. residents/fellows, more than any other specialty, according to data from the Association of American Medical Colleges.
Such numerical strength is significant, but for Dr. Sarto and other leaders in the specialty who spoke about their experiences as female ob.gyns., it’s the impact that women physicians have had on women’s health that’s most important.
Dr. Sarto helped to start Lamaze classes in a hospital basement amidst widespread opposition from the male-dominated leadership and staff who felt that women didn’t need such help with labor. She also takes pride in her collaboration with Dr. Florence Haseltine, Phyllis Greenberger, and several other women to address biases in biomedical research. Their work with Congress led to a federal audit of National Institutes of Health policies and practices.
“We knew that when that report came out [in 1990], it would hit every newspaper in the country,” Dr. Sarto said. It just about did, and soon after that, the NIH Office of Research on Women’s Health was established to ensure that women were included in clinical trials and that gaps in knowledge of women’s health were addressed.
Dr. Barbara Levy, who left a private practice and two medical directorships in 2012 to become Vice President for Health Policy at the American College of Obstetricians and Gynecologists, recalls feeling early in her career that women’s health needed to be approached much more holistically.
“What I was seeing and experiencing didn’t match the textbooks,” she said. “The connection, for instance, between chronic pelvic pain and women who’d been victims of sexual abuse – there wasn’t anything in the literature. I’d see women with the same kinds of physical characteristics on their exams... and patients were willing to share with me things that they wouldn’t have been willing to share with my colleagues.”
Dr. Levy graduated from Princeton University in 1974 with the second class of admitted women, and after a year off, went west for medical school. She graduated in 1979 from the University of California, San Diego, with nine other women in a class of 110.
Her desire to care for the “whole patient” had her leaning toward family medicine until a beloved mentor, Dr. Donna Brooks, “reminded me that there were so few women to take care of women... and that as an ob.gyn. I could follow women through pregnancy, delivery, surgeries, hormone issues, and so many [other facets of their health].”
Dr. Levy, who served as president of the American Association of Gynecologic Laparoscopists in 1995, recalls a world “that was very tolerant of sexual harassment” and remembers the energy she needed to expend to be taken seriously and to correct unconscious bias.
When she applied for fellowship in the American College of Surgeons in the late 1980s, the committee members who conducted an interview “told me right away that I couldn’t expect to be a fellow if I hadn’t done my duty [serving on hospital committees],” Dr. Levy said.
“I told them I had volunteered for more than three committees every single year I’d been on staff, and had never been asked to serve,” she said. “They had no idea. Their assumption was that I had children at home and I wasn’t taking the time.”
Entering leadership
When it comes to leadership, a look at academic medicine suggests that women ob.gyns. have made significant strides. In 2013, compared with other major specialties, obstetrics and gynecology had the highest proportion of department leaders who were women. Yet the picture is mixed. According to an analysis published earlier in 2016, women in ob.gyn. and nine other major specialties “were not represented in the proportions in which they entered their fields” (Obstet Gynecol. 2016 Mar;127[3]:442-7).
Women comprised 57% of all faculty in departments of ob.gyn. in 2013. And, according to the analysis, they comprised 62% of ob.gyn. residency program directors, 30% of division directors, and 24% of department chairs.
The high numbers of women serving as residency program directors raises concern because such positions “do not result in advancement in the same way,” said Dr. Levy, adding that women have excelled in such positions and may desire them, but should be mentored early on about what tracks have the potential for upper-level leadership roles.
Dr. Maureen Phipps, who in 2013 was appointed as chair of ob.gyn. and assistant dean in the Warren Albert Medical School of Brown University in Providence, said she carries with her the fact that women are not yet proportionally represented at the upper levels. “I know that my being in this position and in other positions I’ve held is important for women to see,” she said.
Dr. Phipps graduated from the University of Vermont’s College of Medicine in 1994 as a part of a class in which men and women were fairly evenly represented. In addition to her role as department chair and assistant dean, she is also now chief of ob.gyn. at Women & Infants Hospital of Rhode Island, where she did her residency, and executive chief of ob.gyn for the Care New England Health System.
“I’ve had amazing male leaders and mentors in my career – the people who’ve gone to bat for me have been men,” said Dr. Phipps. Yet, “it’s important to have women in leadership... It’s known that we think differently and approach things differently. Having balance and a variety of different lenses will allow us to [further] grow the field.”
Gender pay gap
Both in academic medicine and in practice, a gender pay gap still reportedly affects women physicians across the board. Various reports and analyses have shown women earning disproportionately less than their male colleagues in similar positions.
Notably, a 2011 analysis in Health Affairs found a nearly $17,000 gap between the starting salaries for men and women physicians. This differential accounted for variables such as patient care hours, practice type, and location. It is possible, the study authors reported, that practices “may now be offering greater flexibility and family-friendly attributes that are more appealing but that come at the price of commensurately lower pay” (Health Aff. 2011:30;193-201).
The American Medical Women’s Association, which promotes advocacy on a gender pay gap, said in a statement about the study, however, that “gender discrimination still exists within the echelons of medicine, and gender stereotyping frequently leads to the devaluation of women physicians.”
From her perspective, Dr. Levy said it’s “complicated” to tease apart and understand all the factors that may be involved.
The challenges of balancing work and family/caregiving and are “still really tough” for women ob.gyns., she said, especially those who want to practice obstetrics. Dr. Levy said she gave up obstetrics when it became apparent that she and her husband would need to hire an additional child care provider.
While the hospitalist-laborist model has been a valuable addition to obstetrics, Dr. Phipps said, “it’s our challenge to continue to think creatively about how we can keep clinicians engaged when they’re in the earlier parts of their careers and challenged by family responsibilities and other commitments.”
Both she and Dr. Levy emphasized their concerns about burnout and their desire to keep career satisfaction high – especially now that women are such a big part of ob.gyn. – and both spoke of the importance of making time for whatever activities help women “recharge.”
“We should be doing what we’re doing because we love it,” Dr. Levy said. “We should focus on that every day. Our patients trust us. We need to remind ourselves of what incredible connections we have.”
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
Report Shows Implanted Cardioveter-defibrillators Carries High Risk
NEW YORK (Reuters Health) - Implanted cardioverter-defibrillators (ICDs) carry a high risk of long-term complications, especially for younger patients, women, and blacks, researchers report.
Early implantation-related risks such as device malfunction are well known, but longer-term risks -- especially beyond the first year after implantation -- are poorly defined, Dr. Isuru Ranasinghe of Queen Elizabeth Hospital in South Australia and colleagues observed in an article online May 2 in Annals of
Internal Medicine.
"Knowing the long-term risks is important for patients to make an informed choice, because (implantation) is a lifelong treatment," Dr. Ranasinghe told Reuters Health by email. "Moreover, more than two-thirds of patients who receive an ICD for prevention of future events never require treatment from the ICD, although they continue to be at risk for device-related harms."
To investigate, the researchers analyzed data from the American College of Cardiology Foundation's National Cardiovascular Data Registry ICD Registry and Medicare claims to assess the long-term nonfatal risks for ICD-related complications among 114,484 patients at 1,437 U.S. centers with first-time implantations.
Implanted devices included single-chamber ICDs (19.8% of patients); dual-chamber ICDs (41.3%), and cardiac resynchronization therapy with a defibrillator (CRT-D; 38.9%).
During a median follow-up of 2.7 years, 40,072 patients died, representing 12.6 deaths per 100 patient years of followup. After accounting for the deaths, "there were 6.1 ICD-related complications per 100 patient years where the patient required an acute hospitalization or a reoperation," Dr. Ranasinghe said.
"In addition, there were 3.9 device reoperations per 100 patient years for reasons other than complications. Typically performed to replace the ICD battery, these reoperations are somewhat expected with time. Nevertheless, these surgical procedures are important for patients, as they carry a risk of patient harm," he observed.
Those more likely to experience long-term complications were women (16% higher risk), blacks (14% higher risk), and patients ages 65-69 at implantation (55% higher risk compared with those 85 and older) -- findings that require further investigation, according to Dr. Ranasinghe.
"Patients were 38% more likely to experience a complication if they had the more complex CRT-D compared with a simpler (single-chamber) device. A patient with a CRT-D device was also four times more likely to require a reoperation for reasons other than complications compared with a single-chamber ICD during the study period," he said.
Dr Ranasinghe added, "The rate of complications is substantial and higher than previously reported. The continued occurrence of complications long after the initial implantation indicates the need for vigilance and ongoing surveillance of ICD-related complications."
"There is considerable debate as to the added benefit of more complex devices compared with simpler single-chamber ICDs. Where possible, using a simpler device may reduce the risk of ICD-related harm," he said.
Dr. Paul J. Hauptman of Saint Louis University School of Medicine in Missouri, told Reuters Health by email, "The study adds to a rich literature that highlights the need for clinicians and patients to carefully consider the potential risks and benefits of implantable defibrillators and CRT devices."
"Although (this was) not a primary focus of the paper, I was struck by the 35% mortality at a median of 2.7 years (in addition to a significant ICD-related complication rate)," said Dr. Hauptman, who has done work in this area. "By linking registry and Medicare data, the authors succeed in providing meaningful insight into what happens to real patients in the real world. We would be abrogating our role as physicians if we ignore analyses like this one."
The study was funded by the American College of Cardiology Foundation's National Cardiovascular Data Registry and other organizations. Five coauthors reported disclosures.
NEW YORK (Reuters Health) - Implanted cardioverter-defibrillators (ICDs) carry a high risk of long-term complications, especially for younger patients, women, and blacks, researchers report.
Early implantation-related risks such as device malfunction are well known, but longer-term risks -- especially beyond the first year after implantation -- are poorly defined, Dr. Isuru Ranasinghe of Queen Elizabeth Hospital in South Australia and colleagues observed in an article online May 2 in Annals of
Internal Medicine.
"Knowing the long-term risks is important for patients to make an informed choice, because (implantation) is a lifelong treatment," Dr. Ranasinghe told Reuters Health by email. "Moreover, more than two-thirds of patients who receive an ICD for prevention of future events never require treatment from the ICD, although they continue to be at risk for device-related harms."
To investigate, the researchers analyzed data from the American College of Cardiology Foundation's National Cardiovascular Data Registry ICD Registry and Medicare claims to assess the long-term nonfatal risks for ICD-related complications among 114,484 patients at 1,437 U.S. centers with first-time implantations.
Implanted devices included single-chamber ICDs (19.8% of patients); dual-chamber ICDs (41.3%), and cardiac resynchronization therapy with a defibrillator (CRT-D; 38.9%).
During a median follow-up of 2.7 years, 40,072 patients died, representing 12.6 deaths per 100 patient years of followup. After accounting for the deaths, "there were 6.1 ICD-related complications per 100 patient years where the patient required an acute hospitalization or a reoperation," Dr. Ranasinghe said.
"In addition, there were 3.9 device reoperations per 100 patient years for reasons other than complications. Typically performed to replace the ICD battery, these reoperations are somewhat expected with time. Nevertheless, these surgical procedures are important for patients, as they carry a risk of patient harm," he observed.
Those more likely to experience long-term complications were women (16% higher risk), blacks (14% higher risk), and patients ages 65-69 at implantation (55% higher risk compared with those 85 and older) -- findings that require further investigation, according to Dr. Ranasinghe.
"Patients were 38% more likely to experience a complication if they had the more complex CRT-D compared with a simpler (single-chamber) device. A patient with a CRT-D device was also four times more likely to require a reoperation for reasons other than complications compared with a single-chamber ICD during the study period," he said.
Dr Ranasinghe added, "The rate of complications is substantial and higher than previously reported. The continued occurrence of complications long after the initial implantation indicates the need for vigilance and ongoing surveillance of ICD-related complications."
"There is considerable debate as to the added benefit of more complex devices compared with simpler single-chamber ICDs. Where possible, using a simpler device may reduce the risk of ICD-related harm," he said.
Dr. Paul J. Hauptman of Saint Louis University School of Medicine in Missouri, told Reuters Health by email, "The study adds to a rich literature that highlights the need for clinicians and patients to carefully consider the potential risks and benefits of implantable defibrillators and CRT devices."
"Although (this was) not a primary focus of the paper, I was struck by the 35% mortality at a median of 2.7 years (in addition to a significant ICD-related complication rate)," said Dr. Hauptman, who has done work in this area. "By linking registry and Medicare data, the authors succeed in providing meaningful insight into what happens to real patients in the real world. We would be abrogating our role as physicians if we ignore analyses like this one."
The study was funded by the American College of Cardiology Foundation's National Cardiovascular Data Registry and other organizations. Five coauthors reported disclosures.
NEW YORK (Reuters Health) - Implanted cardioverter-defibrillators (ICDs) carry a high risk of long-term complications, especially for younger patients, women, and blacks, researchers report.
Early implantation-related risks such as device malfunction are well known, but longer-term risks -- especially beyond the first year after implantation -- are poorly defined, Dr. Isuru Ranasinghe of Queen Elizabeth Hospital in South Australia and colleagues observed in an article online May 2 in Annals of
Internal Medicine.
"Knowing the long-term risks is important for patients to make an informed choice, because (implantation) is a lifelong treatment," Dr. Ranasinghe told Reuters Health by email. "Moreover, more than two-thirds of patients who receive an ICD for prevention of future events never require treatment from the ICD, although they continue to be at risk for device-related harms."
To investigate, the researchers analyzed data from the American College of Cardiology Foundation's National Cardiovascular Data Registry ICD Registry and Medicare claims to assess the long-term nonfatal risks for ICD-related complications among 114,484 patients at 1,437 U.S. centers with first-time implantations.
Implanted devices included single-chamber ICDs (19.8% of patients); dual-chamber ICDs (41.3%), and cardiac resynchronization therapy with a defibrillator (CRT-D; 38.9%).
During a median follow-up of 2.7 years, 40,072 patients died, representing 12.6 deaths per 100 patient years of followup. After accounting for the deaths, "there were 6.1 ICD-related complications per 100 patient years where the patient required an acute hospitalization or a reoperation," Dr. Ranasinghe said.
"In addition, there were 3.9 device reoperations per 100 patient years for reasons other than complications. Typically performed to replace the ICD battery, these reoperations are somewhat expected with time. Nevertheless, these surgical procedures are important for patients, as they carry a risk of patient harm," he observed.
Those more likely to experience long-term complications were women (16% higher risk), blacks (14% higher risk), and patients ages 65-69 at implantation (55% higher risk compared with those 85 and older) -- findings that require further investigation, according to Dr. Ranasinghe.
"Patients were 38% more likely to experience a complication if they had the more complex CRT-D compared with a simpler (single-chamber) device. A patient with a CRT-D device was also four times more likely to require a reoperation for reasons other than complications compared with a single-chamber ICD during the study period," he said.
Dr Ranasinghe added, "The rate of complications is substantial and higher than previously reported. The continued occurrence of complications long after the initial implantation indicates the need for vigilance and ongoing surveillance of ICD-related complications."
"There is considerable debate as to the added benefit of more complex devices compared with simpler single-chamber ICDs. Where possible, using a simpler device may reduce the risk of ICD-related harm," he said.
Dr. Paul J. Hauptman of Saint Louis University School of Medicine in Missouri, told Reuters Health by email, "The study adds to a rich literature that highlights the need for clinicians and patients to carefully consider the potential risks and benefits of implantable defibrillators and CRT devices."
"Although (this was) not a primary focus of the paper, I was struck by the 35% mortality at a median of 2.7 years (in addition to a significant ICD-related complication rate)," said Dr. Hauptman, who has done work in this area. "By linking registry and Medicare data, the authors succeed in providing meaningful insight into what happens to real patients in the real world. We would be abrogating our role as physicians if we ignore analyses like this one."
The study was funded by the American College of Cardiology Foundation's National Cardiovascular Data Registry and other organizations. Five coauthors reported disclosures.
Silk keeps blood samples stable at high temps

Photo by Graham Colm
A new technique provides a way to keep blood samples stable for long periods at high temperatures, according to research published in PNAS.
Investigators found they could keep blood samples stable for nearly 3 months at temperatures as high as 113 degrees F by encapsulating them in air-dried silk protein.
The team believes this technique could have broad applications for clinical care and research.
“This approach should facilitate outpatient blood collection for disease screening and monitoring, particularly for underserved populations, and also serve needs of researchers and clinicians without access to centralized testing facilities,” said study author David L. Kaplan, PhD, of the Department of Biomedical Engineering at Tufts University in Medford, Massachusetts.
For this approach, Dr Kaplan and his colleagues mixed a solution or a powder of purified silk fibroin protein extracted from silkworm cocoons with blood or plasma and air-dried the mixture.
The air-dried silk films were stored at temperatures between 22 and 45 degrees C (71.6 to 113 degrees F). At set intervals, the researchers recovered the encapsulated blood samples by dissolving the films in water and analyzed them.
“We found that biomarkers could be successfully analyzed even after storage for 84 days at temperatures up to 113 degrees F,” said study author Jonathan A. Kluge, PhD, formerly of Tufts University but now at Vaxess Technologies in Cambridge, Massachusetts.
“Encapsulation of samples in silk provided better protection than the traditional approach of drying on paper, especially at these elevated temperatures, which a shipment might encounter during overseas or summer transport.”
The investigators noted that the silk-based technique requires accurate starting volumes of the blood or other specimens to be known, and salts or other buffers are needed to reconstitute samples for accurate testing of certain markers. ![]()

Photo by Graham Colm
A new technique provides a way to keep blood samples stable for long periods at high temperatures, according to research published in PNAS.
Investigators found they could keep blood samples stable for nearly 3 months at temperatures as high as 113 degrees F by encapsulating them in air-dried silk protein.
The team believes this technique could have broad applications for clinical care and research.
“This approach should facilitate outpatient blood collection for disease screening and monitoring, particularly for underserved populations, and also serve needs of researchers and clinicians without access to centralized testing facilities,” said study author David L. Kaplan, PhD, of the Department of Biomedical Engineering at Tufts University in Medford, Massachusetts.
For this approach, Dr Kaplan and his colleagues mixed a solution or a powder of purified silk fibroin protein extracted from silkworm cocoons with blood or plasma and air-dried the mixture.
The air-dried silk films were stored at temperatures between 22 and 45 degrees C (71.6 to 113 degrees F). At set intervals, the researchers recovered the encapsulated blood samples by dissolving the films in water and analyzed them.
“We found that biomarkers could be successfully analyzed even after storage for 84 days at temperatures up to 113 degrees F,” said study author Jonathan A. Kluge, PhD, formerly of Tufts University but now at Vaxess Technologies in Cambridge, Massachusetts.
“Encapsulation of samples in silk provided better protection than the traditional approach of drying on paper, especially at these elevated temperatures, which a shipment might encounter during overseas or summer transport.”
The investigators noted that the silk-based technique requires accurate starting volumes of the blood or other specimens to be known, and salts or other buffers are needed to reconstitute samples for accurate testing of certain markers. ![]()

Photo by Graham Colm
A new technique provides a way to keep blood samples stable for long periods at high temperatures, according to research published in PNAS.
Investigators found they could keep blood samples stable for nearly 3 months at temperatures as high as 113 degrees F by encapsulating them in air-dried silk protein.
The team believes this technique could have broad applications for clinical care and research.
“This approach should facilitate outpatient blood collection for disease screening and monitoring, particularly for underserved populations, and also serve needs of researchers and clinicians without access to centralized testing facilities,” said study author David L. Kaplan, PhD, of the Department of Biomedical Engineering at Tufts University in Medford, Massachusetts.
For this approach, Dr Kaplan and his colleagues mixed a solution or a powder of purified silk fibroin protein extracted from silkworm cocoons with blood or plasma and air-dried the mixture.
The air-dried silk films were stored at temperatures between 22 and 45 degrees C (71.6 to 113 degrees F). At set intervals, the researchers recovered the encapsulated blood samples by dissolving the films in water and analyzed them.
“We found that biomarkers could be successfully analyzed even after storage for 84 days at temperatures up to 113 degrees F,” said study author Jonathan A. Kluge, PhD, formerly of Tufts University but now at Vaxess Technologies in Cambridge, Massachusetts.
“Encapsulation of samples in silk provided better protection than the traditional approach of drying on paper, especially at these elevated temperatures, which a shipment might encounter during overseas or summer transport.”
The investigators noted that the silk-based technique requires accurate starting volumes of the blood or other specimens to be known, and salts or other buffers are needed to reconstitute samples for accurate testing of certain markers. ![]()
Change to EHR can increase use of generic drugs

Photo courtesy of NIH
Changing the default prescription settings in the electronic health record (EHR) can increase the prescribing rates of generic drugs, according to a study published in JAMA Internal Medicine.
The study showed an increase in generic prescribing rates from 75% to 98% when prescriptions were defaulted to a generic medication (if available) in the EHR.
To order the brand name drug, physicians had to opt out by checking a box labelled “dispense as written.”
The study, which builds upon previous research, indicates that the manner in which default options are designed and implemented can influence physician behavior.
“Many of the decisions physicians make are shifting from pen and paper to digital platforms, like the electronic health record, yet there lacks sufficient evidence on how to design choice architecture within these environments to improve healthcare value and outcomes,” said study author Mitesh S. Patel, MD, of the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
“Our results demonstrate that default options are a powerful tool for influencing physician behaviors but that they have to be well-designed to achieve the intended goals.”
For the study, Dr Patel and his colleagues tracked prescribing rates of oral medications for 10 common medical conditions across the University of Pennsylvania Health System.
Rather than changing prescription default settings to display generic names instead of brand names—a change that previously resulted in a 5% change in prescribing habits—in the new study, an opt-out checkbox was used.
When a physician prescribed a drug for a patient, the EHR would default to an equivalent generic. However, the physician could still prescribe the brand name when warranted by selecting the “dispense as written” checkbox.
The researchers compared prescribing rates between the pre-intervention period (January 2014 to October 2014) and the post-intervention period (December 2014 to June 2015).
The results showed that, during the pre-intervention period, generic drugs were prescribed 75.3% of the time, compared to 98.4% of the time during the post-intervention period (P<0.001).
The researchers said this indicates that, for most drugs, physicians specified the brand name should be prescribed only 2% of the time.
The exception to the trend was when physicians prescribed Synthroid for patients with thyroid conditions, as this brand name drug is known to have different hormone levels than its generic version, levothyroxine. In this case, physicians opted out and selected the brand name 22% of the time.
“Studies examining these seemingly minute details point to the importance of design when implementing defaults, which is something that could in result in a significant savings for patients and health systems, and hasn’t previously been examined closely in a healthcare setting,” said study author C. William Hanson, MD, of the Perelman School of Medicine.
“If a simple, low-cost change like adding an opt-out check box to prescription settings can make such a significant impact, there are likely other refinements that can be made just as easily that will also result in cost savings for patients and health systems. It’s a valuable area of research to continue exploring.” ![]()

Photo courtesy of NIH
Changing the default prescription settings in the electronic health record (EHR) can increase the prescribing rates of generic drugs, according to a study published in JAMA Internal Medicine.
The study showed an increase in generic prescribing rates from 75% to 98% when prescriptions were defaulted to a generic medication (if available) in the EHR.
To order the brand name drug, physicians had to opt out by checking a box labelled “dispense as written.”
The study, which builds upon previous research, indicates that the manner in which default options are designed and implemented can influence physician behavior.
“Many of the decisions physicians make are shifting from pen and paper to digital platforms, like the electronic health record, yet there lacks sufficient evidence on how to design choice architecture within these environments to improve healthcare value and outcomes,” said study author Mitesh S. Patel, MD, of the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
“Our results demonstrate that default options are a powerful tool for influencing physician behaviors but that they have to be well-designed to achieve the intended goals.”
For the study, Dr Patel and his colleagues tracked prescribing rates of oral medications for 10 common medical conditions across the University of Pennsylvania Health System.
Rather than changing prescription default settings to display generic names instead of brand names—a change that previously resulted in a 5% change in prescribing habits—in the new study, an opt-out checkbox was used.
When a physician prescribed a drug for a patient, the EHR would default to an equivalent generic. However, the physician could still prescribe the brand name when warranted by selecting the “dispense as written” checkbox.
The researchers compared prescribing rates between the pre-intervention period (January 2014 to October 2014) and the post-intervention period (December 2014 to June 2015).
The results showed that, during the pre-intervention period, generic drugs were prescribed 75.3% of the time, compared to 98.4% of the time during the post-intervention period (P<0.001).
The researchers said this indicates that, for most drugs, physicians specified the brand name should be prescribed only 2% of the time.
The exception to the trend was when physicians prescribed Synthroid for patients with thyroid conditions, as this brand name drug is known to have different hormone levels than its generic version, levothyroxine. In this case, physicians opted out and selected the brand name 22% of the time.
“Studies examining these seemingly minute details point to the importance of design when implementing defaults, which is something that could in result in a significant savings for patients and health systems, and hasn’t previously been examined closely in a healthcare setting,” said study author C. William Hanson, MD, of the Perelman School of Medicine.
“If a simple, low-cost change like adding an opt-out check box to prescription settings can make such a significant impact, there are likely other refinements that can be made just as easily that will also result in cost savings for patients and health systems. It’s a valuable area of research to continue exploring.” ![]()

Photo courtesy of NIH
Changing the default prescription settings in the electronic health record (EHR) can increase the prescribing rates of generic drugs, according to a study published in JAMA Internal Medicine.
The study showed an increase in generic prescribing rates from 75% to 98% when prescriptions were defaulted to a generic medication (if available) in the EHR.
To order the brand name drug, physicians had to opt out by checking a box labelled “dispense as written.”
The study, which builds upon previous research, indicates that the manner in which default options are designed and implemented can influence physician behavior.
“Many of the decisions physicians make are shifting from pen and paper to digital platforms, like the electronic health record, yet there lacks sufficient evidence on how to design choice architecture within these environments to improve healthcare value and outcomes,” said study author Mitesh S. Patel, MD, of the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
“Our results demonstrate that default options are a powerful tool for influencing physician behaviors but that they have to be well-designed to achieve the intended goals.”
For the study, Dr Patel and his colleagues tracked prescribing rates of oral medications for 10 common medical conditions across the University of Pennsylvania Health System.
Rather than changing prescription default settings to display generic names instead of brand names—a change that previously resulted in a 5% change in prescribing habits—in the new study, an opt-out checkbox was used.
When a physician prescribed a drug for a patient, the EHR would default to an equivalent generic. However, the physician could still prescribe the brand name when warranted by selecting the “dispense as written” checkbox.
The researchers compared prescribing rates between the pre-intervention period (January 2014 to October 2014) and the post-intervention period (December 2014 to June 2015).
The results showed that, during the pre-intervention period, generic drugs were prescribed 75.3% of the time, compared to 98.4% of the time during the post-intervention period (P<0.001).
The researchers said this indicates that, for most drugs, physicians specified the brand name should be prescribed only 2% of the time.
The exception to the trend was when physicians prescribed Synthroid for patients with thyroid conditions, as this brand name drug is known to have different hormone levels than its generic version, levothyroxine. In this case, physicians opted out and selected the brand name 22% of the time.
“Studies examining these seemingly minute details point to the importance of design when implementing defaults, which is something that could in result in a significant savings for patients and health systems, and hasn’t previously been examined closely in a healthcare setting,” said study author C. William Hanson, MD, of the Perelman School of Medicine.
“If a simple, low-cost change like adding an opt-out check box to prescription settings can make such a significant impact, there are likely other refinements that can be made just as easily that will also result in cost savings for patients and health systems. It’s a valuable area of research to continue exploring.” ![]()
Study suggests ticagrelor comparable to aspirin

Photo courtesy of AstraZeneca
Results of the SOCRATES trial suggest that ticagrelor is about as safe and effective as aspirin for patients with acute ischemic stroke or transient ischemic attack.
The incidence of the study’s primary endpoint—a composite of stroke, myocardial infarction, and death at 90 days—was similar between the aspirin and ticagrelor arms.
Likewise, there was no significant difference between the arms with regard to safety endpoints.
These results were published in NEJM. The trial was funded by AstraZeneca, which markets ticagrelor as Brilinta.
SOCRATES was a double-blind, controlled trial that enrolled 13,199 patients with a nonsevere ischemic stroke or high-risk transient ischemic attack. The patients had not received intravenous or intra-arterial thrombolysis and were not considered to have had a cardioembolic stroke.
Within 24 hours of symptom onset, the patients were randomized to receive ticagrelor (n=6589) or aspirin (n=6610).
Patients received ticagrelor at a loading dose of 180 mg on day 1, followed by 90 mg twice daily for days 2 through 90. Patients received aspirin at 300 mg on day 1, followed by 100 mg daily for days 2 through 90.
Overall, the differences in baseline characteristics between the treatment arms were not significant. The exceptions were the proportions of patients with a history of diabetes (higher in the ticagrelor arm) or hypertension (higher in the aspirin arm; P<0.05 for both).
The study’s primary endpoint was the occurrence of stroke, myocardial infarction, or death within 90 days. This endpoint occurred in 6.7% of patients in the ticagrelor arm and 7.5% of those in the aspirin arm. The hazard ratio (HR) was 0.89 (P=0.07).
One percent of patients in the ticagrelor arm died, as did 0.9% of patients in the aspirin arm (HR=1.18, P=0.36). The rates of myocardial infarction were 0.4% and 0.3%, respectively (HR=1.20, P=0.55).
The rates of all stroke were 5.9% and 6.8%, respectively (HR=0.86, P=0.03), and the rates of ischemic stroke were 5.8% and 6.7%, respectively (HR=0.87, P=0.046).
The researchers said the P values for all stroke and ischemic stroke were considered nonsignificant in accordance with the hierarchical testing plan used in this study.
Major bleeding occurred in 0.5% of patients in the ticagrelor arm and 0.6% of patients in the aspirin arm (HR=0.83, P=0.45).
Intracranial hemorrhage occurred in 0.2% and 0.3% of patients (HR=0.68, P=0.30), respectively. And fatal bleeding occurred in 0.1% of patients in both arms. ![]()

Photo courtesy of AstraZeneca
Results of the SOCRATES trial suggest that ticagrelor is about as safe and effective as aspirin for patients with acute ischemic stroke or transient ischemic attack.
The incidence of the study’s primary endpoint—a composite of stroke, myocardial infarction, and death at 90 days—was similar between the aspirin and ticagrelor arms.
Likewise, there was no significant difference between the arms with regard to safety endpoints.
These results were published in NEJM. The trial was funded by AstraZeneca, which markets ticagrelor as Brilinta.
SOCRATES was a double-blind, controlled trial that enrolled 13,199 patients with a nonsevere ischemic stroke or high-risk transient ischemic attack. The patients had not received intravenous or intra-arterial thrombolysis and were not considered to have had a cardioembolic stroke.
Within 24 hours of symptom onset, the patients were randomized to receive ticagrelor (n=6589) or aspirin (n=6610).
Patients received ticagrelor at a loading dose of 180 mg on day 1, followed by 90 mg twice daily for days 2 through 90. Patients received aspirin at 300 mg on day 1, followed by 100 mg daily for days 2 through 90.
Overall, the differences in baseline characteristics between the treatment arms were not significant. The exceptions were the proportions of patients with a history of diabetes (higher in the ticagrelor arm) or hypertension (higher in the aspirin arm; P<0.05 for both).
The study’s primary endpoint was the occurrence of stroke, myocardial infarction, or death within 90 days. This endpoint occurred in 6.7% of patients in the ticagrelor arm and 7.5% of those in the aspirin arm. The hazard ratio (HR) was 0.89 (P=0.07).
One percent of patients in the ticagrelor arm died, as did 0.9% of patients in the aspirin arm (HR=1.18, P=0.36). The rates of myocardial infarction were 0.4% and 0.3%, respectively (HR=1.20, P=0.55).
The rates of all stroke were 5.9% and 6.8%, respectively (HR=0.86, P=0.03), and the rates of ischemic stroke were 5.8% and 6.7%, respectively (HR=0.87, P=0.046).
The researchers said the P values for all stroke and ischemic stroke were considered nonsignificant in accordance with the hierarchical testing plan used in this study.
Major bleeding occurred in 0.5% of patients in the ticagrelor arm and 0.6% of patients in the aspirin arm (HR=0.83, P=0.45).
Intracranial hemorrhage occurred in 0.2% and 0.3% of patients (HR=0.68, P=0.30), respectively. And fatal bleeding occurred in 0.1% of patients in both arms. ![]()

Photo courtesy of AstraZeneca
Results of the SOCRATES trial suggest that ticagrelor is about as safe and effective as aspirin for patients with acute ischemic stroke or transient ischemic attack.
The incidence of the study’s primary endpoint—a composite of stroke, myocardial infarction, and death at 90 days—was similar between the aspirin and ticagrelor arms.
Likewise, there was no significant difference between the arms with regard to safety endpoints.
These results were published in NEJM. The trial was funded by AstraZeneca, which markets ticagrelor as Brilinta.
SOCRATES was a double-blind, controlled trial that enrolled 13,199 patients with a nonsevere ischemic stroke or high-risk transient ischemic attack. The patients had not received intravenous or intra-arterial thrombolysis and were not considered to have had a cardioembolic stroke.
Within 24 hours of symptom onset, the patients were randomized to receive ticagrelor (n=6589) or aspirin (n=6610).
Patients received ticagrelor at a loading dose of 180 mg on day 1, followed by 90 mg twice daily for days 2 through 90. Patients received aspirin at 300 mg on day 1, followed by 100 mg daily for days 2 through 90.
Overall, the differences in baseline characteristics between the treatment arms were not significant. The exceptions were the proportions of patients with a history of diabetes (higher in the ticagrelor arm) or hypertension (higher in the aspirin arm; P<0.05 for both).
The study’s primary endpoint was the occurrence of stroke, myocardial infarction, or death within 90 days. This endpoint occurred in 6.7% of patients in the ticagrelor arm and 7.5% of those in the aspirin arm. The hazard ratio (HR) was 0.89 (P=0.07).
One percent of patients in the ticagrelor arm died, as did 0.9% of patients in the aspirin arm (HR=1.18, P=0.36). The rates of myocardial infarction were 0.4% and 0.3%, respectively (HR=1.20, P=0.55).
The rates of all stroke were 5.9% and 6.8%, respectively (HR=0.86, P=0.03), and the rates of ischemic stroke were 5.8% and 6.7%, respectively (HR=0.87, P=0.046).
The researchers said the P values for all stroke and ischemic stroke were considered nonsignificant in accordance with the hierarchical testing plan used in this study.
Major bleeding occurred in 0.5% of patients in the ticagrelor arm and 0.6% of patients in the aspirin arm (HR=0.83, P=0.45).
Intracranial hemorrhage occurred in 0.2% and 0.3% of patients (HR=0.68, P=0.30), respectively. And fatal bleeding occurred in 0.1% of patients in both arms. ![]()
Vaccine can protect some adults from malaria long-term

Photo by James Gathany
An experimental vaccine can protect some healthy adults from malaria infection long-term, according to a phase 1 study.
Researchers tested the vaccine, PfSPZ, by exposing malaria-naïve adults to mosquitoes infected with the parasite Plasmodium falciparum.
Six of the 11 subjects who received PfSPZ at the optimal dose and schedule were free of malaria parasites after they were exposed to the mosquitoes at 21 weeks after immunization.
Five of these subjects were still free of malaria parasites after they were exposed to the mosquitoes at 59 weeks after immunization.
In addition, the researchers said the vaccine was well-tolerated.
“It is now clear that administering the PfSPZ vaccine intravenously confers long-term, sterile protection in a small number of subjects, which has not been achieved with other current vaccine approaches,” said Robert A. Seder, MD, of the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland.
“Based on the favorable safety profile, we’re testing higher doses in larger trials to see if even greater protection can be achieved long-term against other P falciparum strains different than the vaccine strain.”
Dr Seder and his colleagues reported the results of the current trial in Nature Medicine.
The PfSPZ vaccine was developed and produced by Sanaria Inc., with support from several Small Business Innovation Research awards from the National Institute of Allergy and Infectious Diseases.
The PfSPZ vaccine is composed of live but weakened P falciparum sporozoites. Previous research showed the vaccine to be protective 3 weeks after immunization.
In this trial, researchers assessed if protection could last for 5 months to a year. The trial enrolled 101 healthy adults, ages 18 to 45, who had never had malaria.
Fifty-seven subjects were scheduled to receive the PfSPZ vaccine, and 32 were not vaccinated. Vaccine recipients were divided into several groups to assess the roles of the route of administration, dose, and number of immunizations in conferring short- and long-term protection against malaria.
To evaluate how well the PfSPZ vaccine prevented malaria infection, all subjects were exposed at varying times to the bites of mosquitoes carrying the same P falciparum strain from which the PfSPZ vaccine was derived—a process known as controlled human malaria infection (CHMI).
Fifty-five subjects completed all their scheduled vaccinations, and 52 of these subjects underwent at least 1 CHMI.
Safety
Seventy-two percent of the vaccinated subjects (n=41) did not have any solicited adverse events at the injection site after any vaccination. Twenty-six percent (n=15) had mild symptoms (pain and redness), and 1 patient (2%) had a moderate symptom (pain).
Fifty-six percent of vaccinated subjects (n=32) did not have solicited systemic adverse events.
Thirty-three percent (n=19) had mild systemic symptoms (malaise, myalgia, headache, chills, nausea, temperature, and joint pain), and 11% (n=6) had moderate systemic symptoms (malaise, myalgia, headache, chills, nausea, and joint pain).
There were no serious adverse events attributed to vaccination.
Efficacy
The researchers found the efficacy of the PfSPZ vaccine depended on the dose given, the number of vaccinations received, and the route of administration. A higher dose, a higher number of doses, and intravenous (IV) administration were all associated with increased efficacy.
The estimated vaccine efficacy against CHMI at 3 weeks after immunization was 24% with 3 doses of 2.7×105 PfSPZ IV, compared to 73% with 4 doses of 2.7×105 PfSPZ IV.
The estimated vaccine efficacy against CHMI at 21 weeks was 25% with 4 or 5 doses of 1.35×105 PfSPZ IV, compared to 55% with 4 doses of 2.7×105 PfSPZ IV.
In other words, after 4 immunizations with PfSPZ at 2.7×105 IV, 6 of 11 (55%) vaccinated subjects remained without parasitemia following CHMI at 21 weeks after immunization.
Five of the subjects without parasitemia underwent CHMI again at 59 weeks, and none developed parasitemia.
Based on these results, the researchers hypothesize that further increasing the dose of PfSPZ will increase the magnitude and durability of efficacy. They said ongoing studies using 4.5×105 to 2.7×106 PfSPZ per dose are assessing this for homologous CHMI, heterologous CHMI, and natural exposure in all age groups. ![]()

Photo by James Gathany
An experimental vaccine can protect some healthy adults from malaria infection long-term, according to a phase 1 study.
Researchers tested the vaccine, PfSPZ, by exposing malaria-naïve adults to mosquitoes infected with the parasite Plasmodium falciparum.
Six of the 11 subjects who received PfSPZ at the optimal dose and schedule were free of malaria parasites after they were exposed to the mosquitoes at 21 weeks after immunization.
Five of these subjects were still free of malaria parasites after they were exposed to the mosquitoes at 59 weeks after immunization.
In addition, the researchers said the vaccine was well-tolerated.
“It is now clear that administering the PfSPZ vaccine intravenously confers long-term, sterile protection in a small number of subjects, which has not been achieved with other current vaccine approaches,” said Robert A. Seder, MD, of the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland.
“Based on the favorable safety profile, we’re testing higher doses in larger trials to see if even greater protection can be achieved long-term against other P falciparum strains different than the vaccine strain.”
Dr Seder and his colleagues reported the results of the current trial in Nature Medicine.
The PfSPZ vaccine was developed and produced by Sanaria Inc., with support from several Small Business Innovation Research awards from the National Institute of Allergy and Infectious Diseases.
The PfSPZ vaccine is composed of live but weakened P falciparum sporozoites. Previous research showed the vaccine to be protective 3 weeks after immunization.
In this trial, researchers assessed if protection could last for 5 months to a year. The trial enrolled 101 healthy adults, ages 18 to 45, who had never had malaria.
Fifty-seven subjects were scheduled to receive the PfSPZ vaccine, and 32 were not vaccinated. Vaccine recipients were divided into several groups to assess the roles of the route of administration, dose, and number of immunizations in conferring short- and long-term protection against malaria.
To evaluate how well the PfSPZ vaccine prevented malaria infection, all subjects were exposed at varying times to the bites of mosquitoes carrying the same P falciparum strain from which the PfSPZ vaccine was derived—a process known as controlled human malaria infection (CHMI).
Fifty-five subjects completed all their scheduled vaccinations, and 52 of these subjects underwent at least 1 CHMI.
Safety
Seventy-two percent of the vaccinated subjects (n=41) did not have any solicited adverse events at the injection site after any vaccination. Twenty-six percent (n=15) had mild symptoms (pain and redness), and 1 patient (2%) had a moderate symptom (pain).
Fifty-six percent of vaccinated subjects (n=32) did not have solicited systemic adverse events.
Thirty-three percent (n=19) had mild systemic symptoms (malaise, myalgia, headache, chills, nausea, temperature, and joint pain), and 11% (n=6) had moderate systemic symptoms (malaise, myalgia, headache, chills, nausea, and joint pain).
There were no serious adverse events attributed to vaccination.
Efficacy
The researchers found the efficacy of the PfSPZ vaccine depended on the dose given, the number of vaccinations received, and the route of administration. A higher dose, a higher number of doses, and intravenous (IV) administration were all associated with increased efficacy.
The estimated vaccine efficacy against CHMI at 3 weeks after immunization was 24% with 3 doses of 2.7×105 PfSPZ IV, compared to 73% with 4 doses of 2.7×105 PfSPZ IV.
The estimated vaccine efficacy against CHMI at 21 weeks was 25% with 4 or 5 doses of 1.35×105 PfSPZ IV, compared to 55% with 4 doses of 2.7×105 PfSPZ IV.
In other words, after 4 immunizations with PfSPZ at 2.7×105 IV, 6 of 11 (55%) vaccinated subjects remained without parasitemia following CHMI at 21 weeks after immunization.
Five of the subjects without parasitemia underwent CHMI again at 59 weeks, and none developed parasitemia.
Based on these results, the researchers hypothesize that further increasing the dose of PfSPZ will increase the magnitude and durability of efficacy. They said ongoing studies using 4.5×105 to 2.7×106 PfSPZ per dose are assessing this for homologous CHMI, heterologous CHMI, and natural exposure in all age groups. ![]()

Photo by James Gathany
An experimental vaccine can protect some healthy adults from malaria infection long-term, according to a phase 1 study.
Researchers tested the vaccine, PfSPZ, by exposing malaria-naïve adults to mosquitoes infected with the parasite Plasmodium falciparum.
Six of the 11 subjects who received PfSPZ at the optimal dose and schedule were free of malaria parasites after they were exposed to the mosquitoes at 21 weeks after immunization.
Five of these subjects were still free of malaria parasites after they were exposed to the mosquitoes at 59 weeks after immunization.
In addition, the researchers said the vaccine was well-tolerated.
“It is now clear that administering the PfSPZ vaccine intravenously confers long-term, sterile protection in a small number of subjects, which has not been achieved with other current vaccine approaches,” said Robert A. Seder, MD, of the National Institute of Allergy and Infectious Diseases in Bethesda, Maryland.
“Based on the favorable safety profile, we’re testing higher doses in larger trials to see if even greater protection can be achieved long-term against other P falciparum strains different than the vaccine strain.”
Dr Seder and his colleagues reported the results of the current trial in Nature Medicine.
The PfSPZ vaccine was developed and produced by Sanaria Inc., with support from several Small Business Innovation Research awards from the National Institute of Allergy and Infectious Diseases.
The PfSPZ vaccine is composed of live but weakened P falciparum sporozoites. Previous research showed the vaccine to be protective 3 weeks after immunization.
In this trial, researchers assessed if protection could last for 5 months to a year. The trial enrolled 101 healthy adults, ages 18 to 45, who had never had malaria.
Fifty-seven subjects were scheduled to receive the PfSPZ vaccine, and 32 were not vaccinated. Vaccine recipients were divided into several groups to assess the roles of the route of administration, dose, and number of immunizations in conferring short- and long-term protection against malaria.
To evaluate how well the PfSPZ vaccine prevented malaria infection, all subjects were exposed at varying times to the bites of mosquitoes carrying the same P falciparum strain from which the PfSPZ vaccine was derived—a process known as controlled human malaria infection (CHMI).
Fifty-five subjects completed all their scheduled vaccinations, and 52 of these subjects underwent at least 1 CHMI.
Safety
Seventy-two percent of the vaccinated subjects (n=41) did not have any solicited adverse events at the injection site after any vaccination. Twenty-six percent (n=15) had mild symptoms (pain and redness), and 1 patient (2%) had a moderate symptom (pain).
Fifty-six percent of vaccinated subjects (n=32) did not have solicited systemic adverse events.
Thirty-three percent (n=19) had mild systemic symptoms (malaise, myalgia, headache, chills, nausea, temperature, and joint pain), and 11% (n=6) had moderate systemic symptoms (malaise, myalgia, headache, chills, nausea, and joint pain).
There were no serious adverse events attributed to vaccination.
Efficacy
The researchers found the efficacy of the PfSPZ vaccine depended on the dose given, the number of vaccinations received, and the route of administration. A higher dose, a higher number of doses, and intravenous (IV) administration were all associated with increased efficacy.
The estimated vaccine efficacy against CHMI at 3 weeks after immunization was 24% with 3 doses of 2.7×105 PfSPZ IV, compared to 73% with 4 doses of 2.7×105 PfSPZ IV.
The estimated vaccine efficacy against CHMI at 21 weeks was 25% with 4 or 5 doses of 1.35×105 PfSPZ IV, compared to 55% with 4 doses of 2.7×105 PfSPZ IV.
In other words, after 4 immunizations with PfSPZ at 2.7×105 IV, 6 of 11 (55%) vaccinated subjects remained without parasitemia following CHMI at 21 weeks after immunization.
Five of the subjects without parasitemia underwent CHMI again at 59 weeks, and none developed parasitemia.
Based on these results, the researchers hypothesize that further increasing the dose of PfSPZ will increase the magnitude and durability of efficacy. They said ongoing studies using 4.5×105 to 2.7×106 PfSPZ per dose are assessing this for homologous CHMI, heterologous CHMI, and natural exposure in all age groups. ![]()
Analysis of Hospitalist Discontinuity
Studies examining the importance of continuity of care have shown that patients who maintain a continuous relationship with a single physician have improved outcomes.[1, 2] However, most of these studies were performed in the outpatient, rather than the inpatient setting. With over 35 million patients admitted to hospitals in 2013, along with the significant increase in hospital discontinuity over recent years, the impact of inpatient continuity of care on quality outcomes and patient satisfaction is becoming increasingly relevant.[3, 4]
Service handoffs, when a physician hands over treatment responsibility for a panel of patients and is not expected to return, are a type of handoff that contributes to inpatient discontinuity. In particular, service handoffs between hospitalists are an especially common and inherently risky type of transition, as there is a severing of an established relationship during a patient's hospitalization. Unfortunately, due to the lack of evidence on the effects of service handoffs, current guidelines are limited in their recommendations.[5] Whereas several recent studies have begun to explore the effects of these handoffs, no prior study has examined this issue from a patient's perspective.[6, 7, 8]
Patients are uniquely positioned to inform us about their experiences in care transitions. Furthermore, with patient satisfaction now affecting Medicare reimbursement rates, patient experiences while in the hospital are becoming even more significant.[9] Despite this emphasis toward more patient‐centered care, no study has explored the hospitalized patient's experience with hospitalist service handoffs. Our goal was to qualitatively assess the hospitalized patients' experiences with transitions between hospitalists to develop a conceptual model to inform future work on improving inpatient transitions of care.
METHODS
Sampling and Recruitment
We conducted bedside interviews of hospitalized patients at an urban academic medical center from October 2014 through December 2014. The hospitalist service consists of a physician and an advanced nurse practitioner (ANP) who divide a panel of patients that consist of general medicine and subspecialty patients who are often comanaged with hepatology, oncology, and nephrology subspecialists. We performed a purposive selection of patients who could potentially comment on their experience with a hospitalist service transition using the following method: 48 hours after a service handoff (ie, an outgoing physician completing 1 week on service, then transfers the care of the patient to a new oncoming hospitalist), oncoming hospitalists were approached and asked if any patient on their service had experienced a service handoff and still remained in the hospital. A 48‐hour time period was chosen to give the patients time to familiarize themselves with their new hospitalist, allowing them to properly comment on the handoff. Patients who were managed by the ANP, who were non‐English speaking, or who were deemed to have an altered mental status based on clinical suspicion by the interviewing physician (C.M.W.) were excluded from participation. Following each weekly service transition, a list of patients who met the above criteria was collected from 4 nonteaching hospitalist services, and were approached by the primary investigator (C.M.W.) and asked if they would be willing to participate. All patients were general medicine patients and no exclusions were made based on physical location within the hospital. Those who agreed provided signed written consent prior to participation to allow access to the electronic health records (EHRs) by study personnel.
Data Collection
Patients were administered a 9‐question, semistructured interview that was informed by expert opinion and existing literature, which was developed to elicit their perspective regarding their transition between hospitalists.[10, 11] No formal changes were made to the interview guide during the study period, and all patients were asked the same questions. Outcomes from interim analysis guided further questioning in subsequent interviews so as to increase the depth of patient responses (ie, Can you explain your response in greater depth?). Prior to the interview, patients were read a description of a hospitalist, and were reminded which hospitalists had cared for them during their stay (see Supporting Information, Appendix 1, in the online version of this article). If family members or a caregiver were present at the time of interview, they were asked not to comment. No repeat interviews were carried out.
All interviews were performed privately in single‐occupancy rooms, digitally recorded using an iPad (Apple, Cupertino, CA) and professionally transcribed verbatim (Rev, San Francisco, CA). All analysis was performed using MAXQDA Software (VERBI Software GmbH, Berlin, Germany). We obtained demographic information about each patient through chart review
Data Analysis
Grounded theory was utilized, with an inductive approach with no a priori hypothesis.[12] The constant comparative method was used to generate emerging and reoccurring themes.[13] Units of analysis were sentences and phrases. Our research team consisted of 4 academic hospitalists, 2 with backgrounds in clinical medicine, medical education, and qualitative analysis (J.M.F., V.M.A.), 1 as a clinician (C.M.W.), and 1 in health economics (D.O.M.). Interim analysis was performed on a weekly basis (C.M.W.), during which time a coding template was created and refined through an iterative process (C.M.W., J.M.F.). All disagreements in coded themes were resolved through group discussion until full consensus was reached. Each week, responses were assessed for thematic saturation.[14] Interviews were continued if new themes arose during this analysis. Data collection was ended once we ceased to extract new topics from participants. A summary of all themes was then presented to a group of 10 patients who met the same inclusion criteria for respondent validation and member checking. All reporting was performed within the Standards for Reporting Qualitative Research, with additional guidance derived from the Consolidated Criteria for Reporting Qualitative Research.[15, 16] The University of Chicago Institutional Review Board approved this protocol.
RESULTS
In total, 43 eligible patients were recruited, and 40 (93%) agreed to participate. Interviewed patients were between 51 and 65 (39%) years old, had a mean age of 54.5 (15) years, were predominantly female (65%), African American (58%), had a median length of stay at the time of interview of 6.5 days (interquartile range [IQR]: 48), and had an average of 2.0 (IQR: 13) hospitalists oversee their care at the time of interview (Table 1). Interview times ranged from 10:25 to 25:48 minutes, with an average of 15:32 minutes.
| Value | |
|---|---|
| |
| Response rate, n (%) | 40/43 (93) |
| Age, mean SD | 54.5 15 |
| Sex, n (%) | |
| Female | 26 (65) |
| Male | 14 (35) |
| Race, n (%) | |
| African American | 23 (58) |
| White | 16 (40) |
| Hispanic | 1 (2) |
| Median LOS at time of interview, d (IQR) | 6.5 (48) |
| Median no. of hospitalists at time of interview, n (IQR) | 2.0 (13) |
We identified 6 major themes on patient perceptions of hospitalist service handoffs including (1) physician‐patient communication, (2) transparency in the hospitalist transition process, (3) indifference toward the hospitalist transition, (4) hospitalist‐subspecialist communication, (5) recognition of new opportunities due to a transition, and (6) hospitalists' bedside manner (Table 2).
| Themes | Subthemes | Representative Quotes |
|---|---|---|
| Physician‐patient communication | Patients dislike redundant communication with oncoming hospitalist. | I mean it's just you always have to explain your situation over and over and over again. (patient 14) |
| When I said it once already, then you're repeating it to another doctor. I feel as if that hospitalist didn't talk to the other hospitalist. (patient 7) | ||
| Poor communication can negatively affect the doctor‐patient relationship. | They don't really want to explain things. They don't think I'll understand. I think & yeah, I'm okay. You don't even have to put it in layman's terms. I know medical. I'm in nursing school. I have a year left. But even if you didn't know that, I would still hope you would try to tell me what was going on instead of just doing it in your head, and treating it. (patient 2) | |
| I mean it's just you always have to explain your situation over and over and over again. After a while you just stop trusting them. (patient 20) | ||
| Good communication can positively affect the doctor‐patient relationship. | Just continue with the communication, the open communication, and always stress to me that I have a voice and just going out of their way to do whatever they can to help me through whatever I'm going through. (patient 1) | |
| Transparency in transition | Patients want to be informed prior to a service changeover. | I think they should be told immediately, even maybe given prior notice, like this may happen, just so you're not surprised when it happens. (patient 15) |
| When the doctor approached me, he let me know that he wasn't going to be here the next day and there was going to be another doctor coming in. That made me feel comfortable. (patient 9) | ||
| Patients desire a more formalized process in the service changeover. | People want things to be consistent. People don't like change. They like routine. So, if he's leaving, you're coming on, I'd like for him to bring you in, introduce you to me, and for you just assure me that I'll take care of you. (patient 4) | |
| Just like when you get a new medication, you're given all this information on it. So when you get a new hospitalist, shouldn't I get all the information on them? Like where they went to school, what they look like. (patient 23) | ||
| Patients want clearer definition of the roles the physicians will play in their care. | The first time I was hospitalized for the first time I had all these different doctors coming in, and I had the residency, and the specialists, and the department, and I don't know who's who. What I asked them to do is when they come in the room, which they did, but introduce it a little more for me. Write it down like these are the special team and these are the doctors because even though they come in and give me their name, I have no idea what they're doing. (patient 5) | |
| Someone should explain the setup and who people are. Someone would say, Okay when you're in a hospital this is your [doctor's] role. Like they should have booklets and everything. (patient 19) | ||
| Indifference toward transition | Many patients have trust in service changeovers. | [S]o as long as everybody's on board and communicates well and efficiently, I don't have a problem with it. (patient 6) |
| To me, it really wasn't no preference, as long as I was getting the care that I needed. (patient 21) | ||
| It's not a concern as long as they're on the same page. (patient 17) | ||
| Hospitalist‐specialist communication | Patients are concerned about communication between their hospitalist and their subspecialists. | The more cooks you get in the kitchen, the more things get to get lost, so I'm always concerned that they're not sharing the same information, especially when you're getting asked the same questions that you might have just answered the last hour ago. (patient 9) |
| I don't know if the hospitalist are talking to them [subspecialist]. They haven't got time. (patient 35) | ||
| Patients place trust in the communication between hospitalist and subspecialist. | I think among the teams themselveswhich is my pain doctor, Dr. K's group, the oncology group itself, they switch off and trade with each other and they all speak the same language so that works out good. (patient 3) | |
| Lack of interprofessional communication can lead to patient concern. | I was afraid that one was going to drop the ball on something and not pass something on, or you know. (patient 11) | |
| I had numerous doctors who all seemed to not communicate with each other at all or did so by email or whatever. They didn't just sit down together and say we feel this way and we feel that way. I didn't like that at all. (patient 10) | ||
| New opportunities due to transition | Patients see new doctor as opportunity for medical reevaluation. | I see it as two heads are better than one, three heads are better than one, four heads are better than one. When people put their heads together to work towards a common goal, especially when they're, you know, people working their craft, it can't be bad. (patient 9) |
| I finally got my ears looked atbecause I've asked to have my ears looked at since Mondayand the new doc is trying to make an effort to look at them. (patient 39) | ||
| Patients see service changeover as an opportunity to form a better personal relationship. | Having a new hospitalist it gives you opportunity for a new beginning. (patient 11) | |
| Bedside manner | Good bedside manner can assist in a service changeover. | Some of them are all business‐like but some of them are, Well how do you feel today? Hi, how are you? So this made a little difference. You feel more comfortable. You're going to be more comfortable with them. Their bedside manner helps. (patient 16) |
| It's just like when a doctor sits down and talks to you, they just seem more relaxed and more .... I know they're very busy and they have lots of things to do and other patients to see, but while they're in there with you, you know, you don't get too much time with them. So bedside manner is just so important. (patient 24) | ||
| Poor bedside manner can be detrimental in transition. | [B]ecause they be so busy they claim they don't have time just to sit and talk to a patient, and make sure they all right. (patient 17) |
Physician‐Patient Communication
Communication between the physician and the patient was an important element in patients' assessment of their experience. Patient's tended to divide physician‐patient communication into 2 categories: good communication, which consisted of open communication (patient 1) and patient engagement, and bad communication, which was described as physicians not sharing information or taking the time to explain the course of care in words that I'll understand (patient 2). Patients also described dissatisfaction with redundant communication between multiple hospitalists and the frustration of often having to describe their clinical course to multiple providers.
Transparency in Communication
The desire to have greater transparency in the handoff process was another common theme. This was likely due to the fact that 34/40 (85%) of surveyed patients were unaware that a service changeover had ever taken place. This lack of transparency was viewed to have further downstream consequences as patients stated that there should be a level of transparency, and when it's not, then there is always trust issues (patient 1). Upon further questioning as to how to make the process more transparent, many patients recommended a formalized, face‐to‐face introduction involving the patient and both hospitalists, in which the outgoing hospitalist would, bring you [oncoming hospitalist] in, and introduce you to me (patient 4).
Patients often stated that given the large spectrum of physicians they might encounter during their stay (ie, medical student, resident, hospitalist attending, subspecialty fellow, subspecialist attending), clearer definitions of physicians' roles are needed.
Hospitalist‐Specialist Communication
Concern about the communication between their hospitalist and subspecialist was another predominant theme. Conflicting and unclear directions from multiple services were especially frustrating, as a patient stated, One guy took me off this pill, the other guy wants me on that pill, I'm like okay, I can't do both (patient 8). Furthermore, a subset of patients referenced their subspecialist as their primary care provider and preferred their subspecialist for guidance in their hospital course, rather than their hospitalist. This often appeared in cases where the patient had an established relationship with the subspecialist prior to their hospitalization.
New Opportunities Due to Transition
Patients expressed positive feelings toward service handoffs by viewing the transition as an opportunity for medical reevaluation by a new physician. Patients told of instances in which a specific complaint was not being addressed by the first physician, but would be addressed by the second (oncoming) physician. A commonly expressed idea was that the oncoming physician might know something that he [Dr. B] didn't know, and since Dr. B was only here for a week, why not give him [oncoming hospitalist] a chance (patient 10). Patients would also describe the transition as an opportunity to form, and possibly improve, therapeutic alliances with a new hospitalist.
Bedside Manner
Bedside manner was another commonly mentioned thematic element. Patients were often quick to forget prior problems or issues that they may have suffered because of the transition if the oncoming physician was perceived to have a good bedside manner, often described as someone who formally introduced themselves, was considered relaxed, and would take the time to sit and talk with the patient. As a patient put it, [S]he sat down and got to know meand asked me what I wanted to do (patient 12). Conversely, patients described instances in which a perceived bad bedside manner led to a poor relationship between the physician and the patient, in which trust and comfort (patient 11) were sacrificed.
Indifference Toward Transition
In contrast to some of the previous findings, which called for improved interactions between physicians and patients, we also discovered a theme of indifference toward the transition. Several patients stated feelings of trust with the medical system, and were content with the service changeover as long as they felt that their medical needs were being met. Patients also tended to express a level of acceptance with the transition, and tended to believe that this was the price we pay for being here [in the hospital] (patient 7).
Conceptual Model
Following the collection and analysis of all patient responses, all themes were utilized to construct the ideal patient‐centered service handoff. The ideal transition describes open lines of communication between all involved parties, is facilitated by multiple modalities, such as the EHRs and nursing staff, and recognizes the patient as the primary stakeholder (Figure 1).
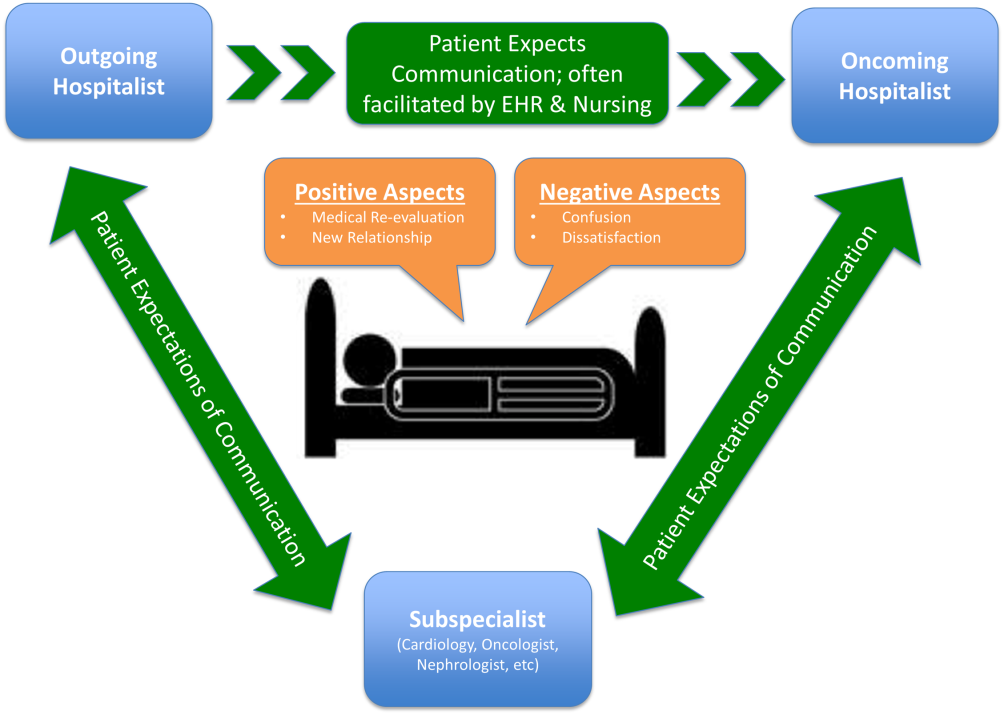
DISCUSSION
To our knowledge, this is the first qualitative investigation of the hospitalized patient's experience with service handoffs between hospitalists. The patient perspective adds a personal and first‐hand description of how fragmented care may impact the hospitalized patient experience.
Of the 6 themes, communication was found to be the most pertinent to our respondents. Because much of patient care is an inherently communicative activity, it is not surprising that patients, as well as patient safety experts, have focused on communication as an area in need of improvement in transition processes.[17, 18] Moreover, multiple medical societies have directly called for improvements within this area, and have specifically recommended clear and direct communication of treatment plans between the patient and physician, timely exchange of information, and knowledge of who is primarily in charge of the patients care.[11] Not surprisingly, each of these recommendations appears to be echoed by our participants. This theme is especially important given that good physician‐patient communication has been noted to be a major goal in achieving patient‐centered care, and has been positively correlated to medication adherence, patient satisfaction, and physical health outcomes.[19, 20, 21, 22, 23]
Although not a substitute for face‐to‐face interactions, other communication interventions between physicians and patients should be considered. For example, get to know me posters placed in patient rooms have been shown to encourage communication between patients and physicians.[24] Additionally, physician face cards have been used to improve patients' abilities to identify and clarify physicians' roles in patient care.[25] As a patient put it, If they got a new one [hospitalist], just as if I got a new medicationprint out information on themlike where they went to med school, and stuff(patient 13). These modalities may represent highly implementable, cost‐effective adjuncts to current handoff methods that may improve lines of communication between physicians and patients.
In addition to the importance placed on physician‐patient communication, interprofessional communication between hospitalists and subspecialists was also highly regarded. Studies have shown that practice‐based interprofessional communication, such as daily interdisciplinary rounds and the use of external facilitators, can improve healthcare processes and outcomes.[26] However, these interventions must be weighed with the many conflicting factors that both hospitalists and subspecialists face on daily basis, including high patient volumes, time limitations, patient availability, and scheduling conflicts.[27] None the less, the strong emphasis patients placed on this line of communication highlights this domain as an area in which hospitalist and subspecialist can work together for systematic improvement.
Patients also recognized the complexity of the transfer process between hospitalists and called for improved transparency. For example, patients repeatedly requested to be informed prior to any changes in their hospitalists, a request that remains consistent with current guidelines.[11] There also existed a strong desire for a more formalized process of transitioning between hospitalists, which often described a handoff procedure that would occur at the patient's bedside. This desire seems to be mirrored in the data that show that patients prefer to interact with their care team at the bedside and report higher satisfaction when they are involved with their care.[28, 29] Unfortunately, this desire for more direct interaction with physicians runs counter to the current paradigm of patient care, where most activities on rounds do not take place at the bedside.[30]
In contrast to patient's calls for improved transparency, an equally large portion of patients expressed relative indifference to the transition. Whereas on the surface this may seem salutary, some studies suggest that a lack of patient activation and engagement may have adverse effects toward patients' overall care.[31] Furthermore, others have shown evidence of better healthcare experiences, improved health outcomes, and lower costs in patients who are more active in their care.[30, 31] Altogether, this suggests that despite some patients' indifference, physicians should continue to engage patients in their hospital care.[32]
Although prevailing sentiments among patient safety advocates are that patient handoffs are inherently dangerous and place patients at increased risk of adverse events, patients did not always share this concern. A frequently occurring theme was that the transition is an opportunity for medical reevaluation or the establishment of a new, possibly improved therapeutic alliance. Recognizing this viewpoint offers oncoming hospitalists the opportunity to focus on issues that the patient may have felt were not being properly addressed with their prior physician.
Finally, although our conceptual model is not a strict guideline, we believe that any future studies should consider this framework when constructing interventions to improve service‐level handoffs. Several interventions already exist. For instance, educational interventions, such as patient‐centered interviewing, have been shown to improve patient satisfaction, compliance with medications, lead to fewer lawsuits, and improve health outcomes.[33, 34, 35] Additional methods of keeping the patient more informed include physician face sheets and performance of the handoff at the patient's bedside. Although well known in nursing literature, the idea of physicians performing handoffs at the patient's bedside is a particularly patient‐centric process.[36] This type of intervention may have the ability to transform the handoff from the current state of a 2‐way street, in which information is passed between 2 hospitalists, to a 3‐way stop, in which both hospitalists and the patient are able to communicate at this critical junction of care.
Although our study does offer new insight into the effects of discontinuous care, its exploratory nature does have limitations. First, being performed at a single academic center limits our ability to generalize our findings. Second, perspectives of those who did not wish to participate, patients' family members or caregivers, and those who were not queried, could highly differ from those we interviewed. Additionally, we did not collect data on patients' diagnoses or reason for admission, thus limiting our ability to assess if certain diagnosis or subpopulations predispose patients to experiencing a service handoff. Third, although our study was restricted to English‐speaking patients only, we must consider that non‐English speakers would likely suffer from even greater communication barriers than those who took part in our study. Finally, our interviews and data analysis were conducted by hospitalists, which could have subconsciously influenced the interview process, and the interpretation of patient responses. However, we tried to mitigate these issues by having the same individual interview all participants, by using an interview guide to ensure cross‐cohort consistency, by using open‐ended questions, and by attempting to give patients every opportunity to express themselves.
CONCLUSIONS
From a patients' perspective, inpatient service handoffs are often opaque experiences that are highlighted by poor communication between physicians and patients. Although deficits in communication and transparency acted as barriers to a patient‐centered handoff, physicians should recognize that service handoffs may also represent opportunities for improvement, and should focus on these domains when they start on a new service.
Disclosures
All funding for this project was provided by the Section of Hospital Medicine at The University of Chicago Medical Center. The data from this article were presented at the Society of Hospital Medicine Annual Conference, National Harbor, March 31, 2015, and at the Society of General Internal Medicine National Meeting in Toronto, Canada, April 23, 2015. The authors report that no conflicts of interest, financial or otherwise, exist.
- , , , , , . Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA. 2009;301(16):1671–1680.
- , , , et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879–1885.
- Agency for Healthcare Research and Quality. HCUPnet: a tool for identifying, tracking, and analyzing national hospital statistics. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=82B37DA366A36BAD6(8):438–444.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335–338.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10(3):147–151.
- Agency for Healthcare Research and Quality. HCAHPS Fact Sheet. CAHPS Hospital Survey August 2013. Available at: http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed February 2, 2015.
- , , , et al. A conceptual framework for studying the safety of transitions in emergency care. In: Henriksen K, Battles JB, Marks ES, eds. Advances in Patient Safety: From Research to Implementation. Rockville, MD: Agency for Healthcare Research and Quality; 2005:309–321. Concepts and Methodology; vol 2. Available at: http://www.ncbi.nlm.nih.gov/books/NBK20522. Accessed January 15, 2015.
- , , , et al. Transitions of care consensus policy statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , . Grounded theory in medical education research: AMEE guide no. 70. Med Teach. 2012;34(10):850–861.
- . A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36(4):391–409.
- . The significance of saturation. Qual Health Res. 1995;5(2):147–149.
- , , , , . Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245–1251.
- , , . Consolidated criteria for reporting qualitative research (COREQ): a 32‐item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- The Joint Commission. Hot Topics in Healthcare, Issue 2. Transitions of care: the need for collaboration across entire care continuum. Available at: http://www.jointcommission.org/assets/1/6/TOC_Hot_Topics.pdf. Accessed April 9, 2015.
- , . Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47(8):826–834.
- , . Impediments to adherence to post myocardial infarction medications. Curr Cardiol Rep. 2013;15(1):322.
- , , , , . Medical specialists' patient‐centered communication and patient‐reported outcomes. Med Care. 2007;45(4):330–339.
- , , , . Does doctor‐patient communication affect patient satisfaction with hospital care? Results of an analysis with a novel instrumental variable. Health Serv Res. 2008;43(5 pt 1):1505–1519.
- , , . Patient‐centredness in chronic illness: what is it and does it matter? Patient Educ Couns. 2003;51(3):197–206.
- , , , et al. Merging cultures: palliative care specialists in the medical intensive care unit. Crit Care Med. 2006;34(11 suppl):S388–S393.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;(3):CD000072.
- , , , et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326–334.
- , , , , . The effect of bedside case presentations on patients' perceptions of their medical care. N Engl J Med. 1997;336(16):1150–1155.
- , , , . Patient‐centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040–1047.
- , , , et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084–1089.
- , . What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32(2):207–214.
- , , , , . When patient activation levels change, health outcomes and costs change, too. Health Aff Proj Hope. 2015;34(3):431–437.
- , , , et al. Evidence‐based guidelines for teaching patient‐centered interviewing. Patient Educ Couns. 2000;39(1):27–36.
- , , . Meta‐analysis of correlates of provider behavior in medical encounters. Med Care. 1988;26(7):657–675.
- , . Characteristics of potential plaintiffs in malpractice litigation. Ann Intern Med. 1994;120(9):792–798.
- , , , , . Bedside shift reports: what does the evidence say? J Nurs Adm. 2014;44(10):541–545.
Studies examining the importance of continuity of care have shown that patients who maintain a continuous relationship with a single physician have improved outcomes.[1, 2] However, most of these studies were performed in the outpatient, rather than the inpatient setting. With over 35 million patients admitted to hospitals in 2013, along with the significant increase in hospital discontinuity over recent years, the impact of inpatient continuity of care on quality outcomes and patient satisfaction is becoming increasingly relevant.[3, 4]
Service handoffs, when a physician hands over treatment responsibility for a panel of patients and is not expected to return, are a type of handoff that contributes to inpatient discontinuity. In particular, service handoffs between hospitalists are an especially common and inherently risky type of transition, as there is a severing of an established relationship during a patient's hospitalization. Unfortunately, due to the lack of evidence on the effects of service handoffs, current guidelines are limited in their recommendations.[5] Whereas several recent studies have begun to explore the effects of these handoffs, no prior study has examined this issue from a patient's perspective.[6, 7, 8]
Patients are uniquely positioned to inform us about their experiences in care transitions. Furthermore, with patient satisfaction now affecting Medicare reimbursement rates, patient experiences while in the hospital are becoming even more significant.[9] Despite this emphasis toward more patient‐centered care, no study has explored the hospitalized patient's experience with hospitalist service handoffs. Our goal was to qualitatively assess the hospitalized patients' experiences with transitions between hospitalists to develop a conceptual model to inform future work on improving inpatient transitions of care.
METHODS
Sampling and Recruitment
We conducted bedside interviews of hospitalized patients at an urban academic medical center from October 2014 through December 2014. The hospitalist service consists of a physician and an advanced nurse practitioner (ANP) who divide a panel of patients that consist of general medicine and subspecialty patients who are often comanaged with hepatology, oncology, and nephrology subspecialists. We performed a purposive selection of patients who could potentially comment on their experience with a hospitalist service transition using the following method: 48 hours after a service handoff (ie, an outgoing physician completing 1 week on service, then transfers the care of the patient to a new oncoming hospitalist), oncoming hospitalists were approached and asked if any patient on their service had experienced a service handoff and still remained in the hospital. A 48‐hour time period was chosen to give the patients time to familiarize themselves with their new hospitalist, allowing them to properly comment on the handoff. Patients who were managed by the ANP, who were non‐English speaking, or who were deemed to have an altered mental status based on clinical suspicion by the interviewing physician (C.M.W.) were excluded from participation. Following each weekly service transition, a list of patients who met the above criteria was collected from 4 nonteaching hospitalist services, and were approached by the primary investigator (C.M.W.) and asked if they would be willing to participate. All patients were general medicine patients and no exclusions were made based on physical location within the hospital. Those who agreed provided signed written consent prior to participation to allow access to the electronic health records (EHRs) by study personnel.
Data Collection
Patients were administered a 9‐question, semistructured interview that was informed by expert opinion and existing literature, which was developed to elicit their perspective regarding their transition between hospitalists.[10, 11] No formal changes were made to the interview guide during the study period, and all patients were asked the same questions. Outcomes from interim analysis guided further questioning in subsequent interviews so as to increase the depth of patient responses (ie, Can you explain your response in greater depth?). Prior to the interview, patients were read a description of a hospitalist, and were reminded which hospitalists had cared for them during their stay (see Supporting Information, Appendix 1, in the online version of this article). If family members or a caregiver were present at the time of interview, they were asked not to comment. No repeat interviews were carried out.
All interviews were performed privately in single‐occupancy rooms, digitally recorded using an iPad (Apple, Cupertino, CA) and professionally transcribed verbatim (Rev, San Francisco, CA). All analysis was performed using MAXQDA Software (VERBI Software GmbH, Berlin, Germany). We obtained demographic information about each patient through chart review
Data Analysis
Grounded theory was utilized, with an inductive approach with no a priori hypothesis.[12] The constant comparative method was used to generate emerging and reoccurring themes.[13] Units of analysis were sentences and phrases. Our research team consisted of 4 academic hospitalists, 2 with backgrounds in clinical medicine, medical education, and qualitative analysis (J.M.F., V.M.A.), 1 as a clinician (C.M.W.), and 1 in health economics (D.O.M.). Interim analysis was performed on a weekly basis (C.M.W.), during which time a coding template was created and refined through an iterative process (C.M.W., J.M.F.). All disagreements in coded themes were resolved through group discussion until full consensus was reached. Each week, responses were assessed for thematic saturation.[14] Interviews were continued if new themes arose during this analysis. Data collection was ended once we ceased to extract new topics from participants. A summary of all themes was then presented to a group of 10 patients who met the same inclusion criteria for respondent validation and member checking. All reporting was performed within the Standards for Reporting Qualitative Research, with additional guidance derived from the Consolidated Criteria for Reporting Qualitative Research.[15, 16] The University of Chicago Institutional Review Board approved this protocol.
RESULTS
In total, 43 eligible patients were recruited, and 40 (93%) agreed to participate. Interviewed patients were between 51 and 65 (39%) years old, had a mean age of 54.5 (15) years, were predominantly female (65%), African American (58%), had a median length of stay at the time of interview of 6.5 days (interquartile range [IQR]: 48), and had an average of 2.0 (IQR: 13) hospitalists oversee their care at the time of interview (Table 1). Interview times ranged from 10:25 to 25:48 minutes, with an average of 15:32 minutes.
| Value | |
|---|---|
| |
| Response rate, n (%) | 40/43 (93) |
| Age, mean SD | 54.5 15 |
| Sex, n (%) | |
| Female | 26 (65) |
| Male | 14 (35) |
| Race, n (%) | |
| African American | 23 (58) |
| White | 16 (40) |
| Hispanic | 1 (2) |
| Median LOS at time of interview, d (IQR) | 6.5 (48) |
| Median no. of hospitalists at time of interview, n (IQR) | 2.0 (13) |
We identified 6 major themes on patient perceptions of hospitalist service handoffs including (1) physician‐patient communication, (2) transparency in the hospitalist transition process, (3) indifference toward the hospitalist transition, (4) hospitalist‐subspecialist communication, (5) recognition of new opportunities due to a transition, and (6) hospitalists' bedside manner (Table 2).
| Themes | Subthemes | Representative Quotes |
|---|---|---|
| Physician‐patient communication | Patients dislike redundant communication with oncoming hospitalist. | I mean it's just you always have to explain your situation over and over and over again. (patient 14) |
| When I said it once already, then you're repeating it to another doctor. I feel as if that hospitalist didn't talk to the other hospitalist. (patient 7) | ||
| Poor communication can negatively affect the doctor‐patient relationship. | They don't really want to explain things. They don't think I'll understand. I think & yeah, I'm okay. You don't even have to put it in layman's terms. I know medical. I'm in nursing school. I have a year left. But even if you didn't know that, I would still hope you would try to tell me what was going on instead of just doing it in your head, and treating it. (patient 2) | |
| I mean it's just you always have to explain your situation over and over and over again. After a while you just stop trusting them. (patient 20) | ||
| Good communication can positively affect the doctor‐patient relationship. | Just continue with the communication, the open communication, and always stress to me that I have a voice and just going out of their way to do whatever they can to help me through whatever I'm going through. (patient 1) | |
| Transparency in transition | Patients want to be informed prior to a service changeover. | I think they should be told immediately, even maybe given prior notice, like this may happen, just so you're not surprised when it happens. (patient 15) |
| When the doctor approached me, he let me know that he wasn't going to be here the next day and there was going to be another doctor coming in. That made me feel comfortable. (patient 9) | ||
| Patients desire a more formalized process in the service changeover. | People want things to be consistent. People don't like change. They like routine. So, if he's leaving, you're coming on, I'd like for him to bring you in, introduce you to me, and for you just assure me that I'll take care of you. (patient 4) | |
| Just like when you get a new medication, you're given all this information on it. So when you get a new hospitalist, shouldn't I get all the information on them? Like where they went to school, what they look like. (patient 23) | ||
| Patients want clearer definition of the roles the physicians will play in their care. | The first time I was hospitalized for the first time I had all these different doctors coming in, and I had the residency, and the specialists, and the department, and I don't know who's who. What I asked them to do is when they come in the room, which they did, but introduce it a little more for me. Write it down like these are the special team and these are the doctors because even though they come in and give me their name, I have no idea what they're doing. (patient 5) | |
| Someone should explain the setup and who people are. Someone would say, Okay when you're in a hospital this is your [doctor's] role. Like they should have booklets and everything. (patient 19) | ||
| Indifference toward transition | Many patients have trust in service changeovers. | [S]o as long as everybody's on board and communicates well and efficiently, I don't have a problem with it. (patient 6) |
| To me, it really wasn't no preference, as long as I was getting the care that I needed. (patient 21) | ||
| It's not a concern as long as they're on the same page. (patient 17) | ||
| Hospitalist‐specialist communication | Patients are concerned about communication between their hospitalist and their subspecialists. | The more cooks you get in the kitchen, the more things get to get lost, so I'm always concerned that they're not sharing the same information, especially when you're getting asked the same questions that you might have just answered the last hour ago. (patient 9) |
| I don't know if the hospitalist are talking to them [subspecialist]. They haven't got time. (patient 35) | ||
| Patients place trust in the communication between hospitalist and subspecialist. | I think among the teams themselveswhich is my pain doctor, Dr. K's group, the oncology group itself, they switch off and trade with each other and they all speak the same language so that works out good. (patient 3) | |
| Lack of interprofessional communication can lead to patient concern. | I was afraid that one was going to drop the ball on something and not pass something on, or you know. (patient 11) | |
| I had numerous doctors who all seemed to not communicate with each other at all or did so by email or whatever. They didn't just sit down together and say we feel this way and we feel that way. I didn't like that at all. (patient 10) | ||
| New opportunities due to transition | Patients see new doctor as opportunity for medical reevaluation. | I see it as two heads are better than one, three heads are better than one, four heads are better than one. When people put their heads together to work towards a common goal, especially when they're, you know, people working their craft, it can't be bad. (patient 9) |
| I finally got my ears looked atbecause I've asked to have my ears looked at since Mondayand the new doc is trying to make an effort to look at them. (patient 39) | ||
| Patients see service changeover as an opportunity to form a better personal relationship. | Having a new hospitalist it gives you opportunity for a new beginning. (patient 11) | |
| Bedside manner | Good bedside manner can assist in a service changeover. | Some of them are all business‐like but some of them are, Well how do you feel today? Hi, how are you? So this made a little difference. You feel more comfortable. You're going to be more comfortable with them. Their bedside manner helps. (patient 16) |
| It's just like when a doctor sits down and talks to you, they just seem more relaxed and more .... I know they're very busy and they have lots of things to do and other patients to see, but while they're in there with you, you know, you don't get too much time with them. So bedside manner is just so important. (patient 24) | ||
| Poor bedside manner can be detrimental in transition. | [B]ecause they be so busy they claim they don't have time just to sit and talk to a patient, and make sure they all right. (patient 17) |
Physician‐Patient Communication
Communication between the physician and the patient was an important element in patients' assessment of their experience. Patient's tended to divide physician‐patient communication into 2 categories: good communication, which consisted of open communication (patient 1) and patient engagement, and bad communication, which was described as physicians not sharing information or taking the time to explain the course of care in words that I'll understand (patient 2). Patients also described dissatisfaction with redundant communication between multiple hospitalists and the frustration of often having to describe their clinical course to multiple providers.
Transparency in Communication
The desire to have greater transparency in the handoff process was another common theme. This was likely due to the fact that 34/40 (85%) of surveyed patients were unaware that a service changeover had ever taken place. This lack of transparency was viewed to have further downstream consequences as patients stated that there should be a level of transparency, and when it's not, then there is always trust issues (patient 1). Upon further questioning as to how to make the process more transparent, many patients recommended a formalized, face‐to‐face introduction involving the patient and both hospitalists, in which the outgoing hospitalist would, bring you [oncoming hospitalist] in, and introduce you to me (patient 4).
Patients often stated that given the large spectrum of physicians they might encounter during their stay (ie, medical student, resident, hospitalist attending, subspecialty fellow, subspecialist attending), clearer definitions of physicians' roles are needed.
Hospitalist‐Specialist Communication
Concern about the communication between their hospitalist and subspecialist was another predominant theme. Conflicting and unclear directions from multiple services were especially frustrating, as a patient stated, One guy took me off this pill, the other guy wants me on that pill, I'm like okay, I can't do both (patient 8). Furthermore, a subset of patients referenced their subspecialist as their primary care provider and preferred their subspecialist for guidance in their hospital course, rather than their hospitalist. This often appeared in cases where the patient had an established relationship with the subspecialist prior to their hospitalization.
New Opportunities Due to Transition
Patients expressed positive feelings toward service handoffs by viewing the transition as an opportunity for medical reevaluation by a new physician. Patients told of instances in which a specific complaint was not being addressed by the first physician, but would be addressed by the second (oncoming) physician. A commonly expressed idea was that the oncoming physician might know something that he [Dr. B] didn't know, and since Dr. B was only here for a week, why not give him [oncoming hospitalist] a chance (patient 10). Patients would also describe the transition as an opportunity to form, and possibly improve, therapeutic alliances with a new hospitalist.
Bedside Manner
Bedside manner was another commonly mentioned thematic element. Patients were often quick to forget prior problems or issues that they may have suffered because of the transition if the oncoming physician was perceived to have a good bedside manner, often described as someone who formally introduced themselves, was considered relaxed, and would take the time to sit and talk with the patient. As a patient put it, [S]he sat down and got to know meand asked me what I wanted to do (patient 12). Conversely, patients described instances in which a perceived bad bedside manner led to a poor relationship between the physician and the patient, in which trust and comfort (patient 11) were sacrificed.
Indifference Toward Transition
In contrast to some of the previous findings, which called for improved interactions between physicians and patients, we also discovered a theme of indifference toward the transition. Several patients stated feelings of trust with the medical system, and were content with the service changeover as long as they felt that their medical needs were being met. Patients also tended to express a level of acceptance with the transition, and tended to believe that this was the price we pay for being here [in the hospital] (patient 7).
Conceptual Model
Following the collection and analysis of all patient responses, all themes were utilized to construct the ideal patient‐centered service handoff. The ideal transition describes open lines of communication between all involved parties, is facilitated by multiple modalities, such as the EHRs and nursing staff, and recognizes the patient as the primary stakeholder (Figure 1).

DISCUSSION
To our knowledge, this is the first qualitative investigation of the hospitalized patient's experience with service handoffs between hospitalists. The patient perspective adds a personal and first‐hand description of how fragmented care may impact the hospitalized patient experience.
Of the 6 themes, communication was found to be the most pertinent to our respondents. Because much of patient care is an inherently communicative activity, it is not surprising that patients, as well as patient safety experts, have focused on communication as an area in need of improvement in transition processes.[17, 18] Moreover, multiple medical societies have directly called for improvements within this area, and have specifically recommended clear and direct communication of treatment plans between the patient and physician, timely exchange of information, and knowledge of who is primarily in charge of the patients care.[11] Not surprisingly, each of these recommendations appears to be echoed by our participants. This theme is especially important given that good physician‐patient communication has been noted to be a major goal in achieving patient‐centered care, and has been positively correlated to medication adherence, patient satisfaction, and physical health outcomes.[19, 20, 21, 22, 23]
Although not a substitute for face‐to‐face interactions, other communication interventions between physicians and patients should be considered. For example, get to know me posters placed in patient rooms have been shown to encourage communication between patients and physicians.[24] Additionally, physician face cards have been used to improve patients' abilities to identify and clarify physicians' roles in patient care.[25] As a patient put it, If they got a new one [hospitalist], just as if I got a new medicationprint out information on themlike where they went to med school, and stuff(patient 13). These modalities may represent highly implementable, cost‐effective adjuncts to current handoff methods that may improve lines of communication between physicians and patients.
In addition to the importance placed on physician‐patient communication, interprofessional communication between hospitalists and subspecialists was also highly regarded. Studies have shown that practice‐based interprofessional communication, such as daily interdisciplinary rounds and the use of external facilitators, can improve healthcare processes and outcomes.[26] However, these interventions must be weighed with the many conflicting factors that both hospitalists and subspecialists face on daily basis, including high patient volumes, time limitations, patient availability, and scheduling conflicts.[27] None the less, the strong emphasis patients placed on this line of communication highlights this domain as an area in which hospitalist and subspecialist can work together for systematic improvement.
Patients also recognized the complexity of the transfer process between hospitalists and called for improved transparency. For example, patients repeatedly requested to be informed prior to any changes in their hospitalists, a request that remains consistent with current guidelines.[11] There also existed a strong desire for a more formalized process of transitioning between hospitalists, which often described a handoff procedure that would occur at the patient's bedside. This desire seems to be mirrored in the data that show that patients prefer to interact with their care team at the bedside and report higher satisfaction when they are involved with their care.[28, 29] Unfortunately, this desire for more direct interaction with physicians runs counter to the current paradigm of patient care, where most activities on rounds do not take place at the bedside.[30]
In contrast to patient's calls for improved transparency, an equally large portion of patients expressed relative indifference to the transition. Whereas on the surface this may seem salutary, some studies suggest that a lack of patient activation and engagement may have adverse effects toward patients' overall care.[31] Furthermore, others have shown evidence of better healthcare experiences, improved health outcomes, and lower costs in patients who are more active in their care.[30, 31] Altogether, this suggests that despite some patients' indifference, physicians should continue to engage patients in their hospital care.[32]
Although prevailing sentiments among patient safety advocates are that patient handoffs are inherently dangerous and place patients at increased risk of adverse events, patients did not always share this concern. A frequently occurring theme was that the transition is an opportunity for medical reevaluation or the establishment of a new, possibly improved therapeutic alliance. Recognizing this viewpoint offers oncoming hospitalists the opportunity to focus on issues that the patient may have felt were not being properly addressed with their prior physician.
Finally, although our conceptual model is not a strict guideline, we believe that any future studies should consider this framework when constructing interventions to improve service‐level handoffs. Several interventions already exist. For instance, educational interventions, such as patient‐centered interviewing, have been shown to improve patient satisfaction, compliance with medications, lead to fewer lawsuits, and improve health outcomes.[33, 34, 35] Additional methods of keeping the patient more informed include physician face sheets and performance of the handoff at the patient's bedside. Although well known in nursing literature, the idea of physicians performing handoffs at the patient's bedside is a particularly patient‐centric process.[36] This type of intervention may have the ability to transform the handoff from the current state of a 2‐way street, in which information is passed between 2 hospitalists, to a 3‐way stop, in which both hospitalists and the patient are able to communicate at this critical junction of care.
Although our study does offer new insight into the effects of discontinuous care, its exploratory nature does have limitations. First, being performed at a single academic center limits our ability to generalize our findings. Second, perspectives of those who did not wish to participate, patients' family members or caregivers, and those who were not queried, could highly differ from those we interviewed. Additionally, we did not collect data on patients' diagnoses or reason for admission, thus limiting our ability to assess if certain diagnosis or subpopulations predispose patients to experiencing a service handoff. Third, although our study was restricted to English‐speaking patients only, we must consider that non‐English speakers would likely suffer from even greater communication barriers than those who took part in our study. Finally, our interviews and data analysis were conducted by hospitalists, which could have subconsciously influenced the interview process, and the interpretation of patient responses. However, we tried to mitigate these issues by having the same individual interview all participants, by using an interview guide to ensure cross‐cohort consistency, by using open‐ended questions, and by attempting to give patients every opportunity to express themselves.
CONCLUSIONS
From a patients' perspective, inpatient service handoffs are often opaque experiences that are highlighted by poor communication between physicians and patients. Although deficits in communication and transparency acted as barriers to a patient‐centered handoff, physicians should recognize that service handoffs may also represent opportunities for improvement, and should focus on these domains when they start on a new service.
Disclosures
All funding for this project was provided by the Section of Hospital Medicine at The University of Chicago Medical Center. The data from this article were presented at the Society of Hospital Medicine Annual Conference, National Harbor, March 31, 2015, and at the Society of General Internal Medicine National Meeting in Toronto, Canada, April 23, 2015. The authors report that no conflicts of interest, financial or otherwise, exist.
Studies examining the importance of continuity of care have shown that patients who maintain a continuous relationship with a single physician have improved outcomes.[1, 2] However, most of these studies were performed in the outpatient, rather than the inpatient setting. With over 35 million patients admitted to hospitals in 2013, along with the significant increase in hospital discontinuity over recent years, the impact of inpatient continuity of care on quality outcomes and patient satisfaction is becoming increasingly relevant.[3, 4]
Service handoffs, when a physician hands over treatment responsibility for a panel of patients and is not expected to return, are a type of handoff that contributes to inpatient discontinuity. In particular, service handoffs between hospitalists are an especially common and inherently risky type of transition, as there is a severing of an established relationship during a patient's hospitalization. Unfortunately, due to the lack of evidence on the effects of service handoffs, current guidelines are limited in their recommendations.[5] Whereas several recent studies have begun to explore the effects of these handoffs, no prior study has examined this issue from a patient's perspective.[6, 7, 8]
Patients are uniquely positioned to inform us about their experiences in care transitions. Furthermore, with patient satisfaction now affecting Medicare reimbursement rates, patient experiences while in the hospital are becoming even more significant.[9] Despite this emphasis toward more patient‐centered care, no study has explored the hospitalized patient's experience with hospitalist service handoffs. Our goal was to qualitatively assess the hospitalized patients' experiences with transitions between hospitalists to develop a conceptual model to inform future work on improving inpatient transitions of care.
METHODS
Sampling and Recruitment
We conducted bedside interviews of hospitalized patients at an urban academic medical center from October 2014 through December 2014. The hospitalist service consists of a physician and an advanced nurse practitioner (ANP) who divide a panel of patients that consist of general medicine and subspecialty patients who are often comanaged with hepatology, oncology, and nephrology subspecialists. We performed a purposive selection of patients who could potentially comment on their experience with a hospitalist service transition using the following method: 48 hours after a service handoff (ie, an outgoing physician completing 1 week on service, then transfers the care of the patient to a new oncoming hospitalist), oncoming hospitalists were approached and asked if any patient on their service had experienced a service handoff and still remained in the hospital. A 48‐hour time period was chosen to give the patients time to familiarize themselves with their new hospitalist, allowing them to properly comment on the handoff. Patients who were managed by the ANP, who were non‐English speaking, or who were deemed to have an altered mental status based on clinical suspicion by the interviewing physician (C.M.W.) were excluded from participation. Following each weekly service transition, a list of patients who met the above criteria was collected from 4 nonteaching hospitalist services, and were approached by the primary investigator (C.M.W.) and asked if they would be willing to participate. All patients were general medicine patients and no exclusions were made based on physical location within the hospital. Those who agreed provided signed written consent prior to participation to allow access to the electronic health records (EHRs) by study personnel.
Data Collection
Patients were administered a 9‐question, semistructured interview that was informed by expert opinion and existing literature, which was developed to elicit their perspective regarding their transition between hospitalists.[10, 11] No formal changes were made to the interview guide during the study period, and all patients were asked the same questions. Outcomes from interim analysis guided further questioning in subsequent interviews so as to increase the depth of patient responses (ie, Can you explain your response in greater depth?). Prior to the interview, patients were read a description of a hospitalist, and were reminded which hospitalists had cared for them during their stay (see Supporting Information, Appendix 1, in the online version of this article). If family members or a caregiver were present at the time of interview, they were asked not to comment. No repeat interviews were carried out.
All interviews were performed privately in single‐occupancy rooms, digitally recorded using an iPad (Apple, Cupertino, CA) and professionally transcribed verbatim (Rev, San Francisco, CA). All analysis was performed using MAXQDA Software (VERBI Software GmbH, Berlin, Germany). We obtained demographic information about each patient through chart review
Data Analysis
Grounded theory was utilized, with an inductive approach with no a priori hypothesis.[12] The constant comparative method was used to generate emerging and reoccurring themes.[13] Units of analysis were sentences and phrases. Our research team consisted of 4 academic hospitalists, 2 with backgrounds in clinical medicine, medical education, and qualitative analysis (J.M.F., V.M.A.), 1 as a clinician (C.M.W.), and 1 in health economics (D.O.M.). Interim analysis was performed on a weekly basis (C.M.W.), during which time a coding template was created and refined through an iterative process (C.M.W., J.M.F.). All disagreements in coded themes were resolved through group discussion until full consensus was reached. Each week, responses were assessed for thematic saturation.[14] Interviews were continued if new themes arose during this analysis. Data collection was ended once we ceased to extract new topics from participants. A summary of all themes was then presented to a group of 10 patients who met the same inclusion criteria for respondent validation and member checking. All reporting was performed within the Standards for Reporting Qualitative Research, with additional guidance derived from the Consolidated Criteria for Reporting Qualitative Research.[15, 16] The University of Chicago Institutional Review Board approved this protocol.
RESULTS
In total, 43 eligible patients were recruited, and 40 (93%) agreed to participate. Interviewed patients were between 51 and 65 (39%) years old, had a mean age of 54.5 (15) years, were predominantly female (65%), African American (58%), had a median length of stay at the time of interview of 6.5 days (interquartile range [IQR]: 48), and had an average of 2.0 (IQR: 13) hospitalists oversee their care at the time of interview (Table 1). Interview times ranged from 10:25 to 25:48 minutes, with an average of 15:32 minutes.
| Value | |
|---|---|
| |
| Response rate, n (%) | 40/43 (93) |
| Age, mean SD | 54.5 15 |
| Sex, n (%) | |
| Female | 26 (65) |
| Male | 14 (35) |
| Race, n (%) | |
| African American | 23 (58) |
| White | 16 (40) |
| Hispanic | 1 (2) |
| Median LOS at time of interview, d (IQR) | 6.5 (48) |
| Median no. of hospitalists at time of interview, n (IQR) | 2.0 (13) |
We identified 6 major themes on patient perceptions of hospitalist service handoffs including (1) physician‐patient communication, (2) transparency in the hospitalist transition process, (3) indifference toward the hospitalist transition, (4) hospitalist‐subspecialist communication, (5) recognition of new opportunities due to a transition, and (6) hospitalists' bedside manner (Table 2).
| Themes | Subthemes | Representative Quotes |
|---|---|---|
| Physician‐patient communication | Patients dislike redundant communication with oncoming hospitalist. | I mean it's just you always have to explain your situation over and over and over again. (patient 14) |
| When I said it once already, then you're repeating it to another doctor. I feel as if that hospitalist didn't talk to the other hospitalist. (patient 7) | ||
| Poor communication can negatively affect the doctor‐patient relationship. | They don't really want to explain things. They don't think I'll understand. I think & yeah, I'm okay. You don't even have to put it in layman's terms. I know medical. I'm in nursing school. I have a year left. But even if you didn't know that, I would still hope you would try to tell me what was going on instead of just doing it in your head, and treating it. (patient 2) | |
| I mean it's just you always have to explain your situation over and over and over again. After a while you just stop trusting them. (patient 20) | ||
| Good communication can positively affect the doctor‐patient relationship. | Just continue with the communication, the open communication, and always stress to me that I have a voice and just going out of their way to do whatever they can to help me through whatever I'm going through. (patient 1) | |
| Transparency in transition | Patients want to be informed prior to a service changeover. | I think they should be told immediately, even maybe given prior notice, like this may happen, just so you're not surprised when it happens. (patient 15) |
| When the doctor approached me, he let me know that he wasn't going to be here the next day and there was going to be another doctor coming in. That made me feel comfortable. (patient 9) | ||
| Patients desire a more formalized process in the service changeover. | People want things to be consistent. People don't like change. They like routine. So, if he's leaving, you're coming on, I'd like for him to bring you in, introduce you to me, and for you just assure me that I'll take care of you. (patient 4) | |
| Just like when you get a new medication, you're given all this information on it. So when you get a new hospitalist, shouldn't I get all the information on them? Like where they went to school, what they look like. (patient 23) | ||
| Patients want clearer definition of the roles the physicians will play in their care. | The first time I was hospitalized for the first time I had all these different doctors coming in, and I had the residency, and the specialists, and the department, and I don't know who's who. What I asked them to do is when they come in the room, which they did, but introduce it a little more for me. Write it down like these are the special team and these are the doctors because even though they come in and give me their name, I have no idea what they're doing. (patient 5) | |
| Someone should explain the setup and who people are. Someone would say, Okay when you're in a hospital this is your [doctor's] role. Like they should have booklets and everything. (patient 19) | ||
| Indifference toward transition | Many patients have trust in service changeovers. | [S]o as long as everybody's on board and communicates well and efficiently, I don't have a problem with it. (patient 6) |
| To me, it really wasn't no preference, as long as I was getting the care that I needed. (patient 21) | ||
| It's not a concern as long as they're on the same page. (patient 17) | ||
| Hospitalist‐specialist communication | Patients are concerned about communication between their hospitalist and their subspecialists. | The more cooks you get in the kitchen, the more things get to get lost, so I'm always concerned that they're not sharing the same information, especially when you're getting asked the same questions that you might have just answered the last hour ago. (patient 9) |
| I don't know if the hospitalist are talking to them [subspecialist]. They haven't got time. (patient 35) | ||
| Patients place trust in the communication between hospitalist and subspecialist. | I think among the teams themselveswhich is my pain doctor, Dr. K's group, the oncology group itself, they switch off and trade with each other and they all speak the same language so that works out good. (patient 3) | |
| Lack of interprofessional communication can lead to patient concern. | I was afraid that one was going to drop the ball on something and not pass something on, or you know. (patient 11) | |
| I had numerous doctors who all seemed to not communicate with each other at all or did so by email or whatever. They didn't just sit down together and say we feel this way and we feel that way. I didn't like that at all. (patient 10) | ||
| New opportunities due to transition | Patients see new doctor as opportunity for medical reevaluation. | I see it as two heads are better than one, three heads are better than one, four heads are better than one. When people put their heads together to work towards a common goal, especially when they're, you know, people working their craft, it can't be bad. (patient 9) |
| I finally got my ears looked atbecause I've asked to have my ears looked at since Mondayand the new doc is trying to make an effort to look at them. (patient 39) | ||
| Patients see service changeover as an opportunity to form a better personal relationship. | Having a new hospitalist it gives you opportunity for a new beginning. (patient 11) | |
| Bedside manner | Good bedside manner can assist in a service changeover. | Some of them are all business‐like but some of them are, Well how do you feel today? Hi, how are you? So this made a little difference. You feel more comfortable. You're going to be more comfortable with them. Their bedside manner helps. (patient 16) |
| It's just like when a doctor sits down and talks to you, they just seem more relaxed and more .... I know they're very busy and they have lots of things to do and other patients to see, but while they're in there with you, you know, you don't get too much time with them. So bedside manner is just so important. (patient 24) | ||
| Poor bedside manner can be detrimental in transition. | [B]ecause they be so busy they claim they don't have time just to sit and talk to a patient, and make sure they all right. (patient 17) |
Physician‐Patient Communication
Communication between the physician and the patient was an important element in patients' assessment of their experience. Patient's tended to divide physician‐patient communication into 2 categories: good communication, which consisted of open communication (patient 1) and patient engagement, and bad communication, which was described as physicians not sharing information or taking the time to explain the course of care in words that I'll understand (patient 2). Patients also described dissatisfaction with redundant communication between multiple hospitalists and the frustration of often having to describe their clinical course to multiple providers.
Transparency in Communication
The desire to have greater transparency in the handoff process was another common theme. This was likely due to the fact that 34/40 (85%) of surveyed patients were unaware that a service changeover had ever taken place. This lack of transparency was viewed to have further downstream consequences as patients stated that there should be a level of transparency, and when it's not, then there is always trust issues (patient 1). Upon further questioning as to how to make the process more transparent, many patients recommended a formalized, face‐to‐face introduction involving the patient and both hospitalists, in which the outgoing hospitalist would, bring you [oncoming hospitalist] in, and introduce you to me (patient 4).
Patients often stated that given the large spectrum of physicians they might encounter during their stay (ie, medical student, resident, hospitalist attending, subspecialty fellow, subspecialist attending), clearer definitions of physicians' roles are needed.
Hospitalist‐Specialist Communication
Concern about the communication between their hospitalist and subspecialist was another predominant theme. Conflicting and unclear directions from multiple services were especially frustrating, as a patient stated, One guy took me off this pill, the other guy wants me on that pill, I'm like okay, I can't do both (patient 8). Furthermore, a subset of patients referenced their subspecialist as their primary care provider and preferred their subspecialist for guidance in their hospital course, rather than their hospitalist. This often appeared in cases where the patient had an established relationship with the subspecialist prior to their hospitalization.
New Opportunities Due to Transition
Patients expressed positive feelings toward service handoffs by viewing the transition as an opportunity for medical reevaluation by a new physician. Patients told of instances in which a specific complaint was not being addressed by the first physician, but would be addressed by the second (oncoming) physician. A commonly expressed idea was that the oncoming physician might know something that he [Dr. B] didn't know, and since Dr. B was only here for a week, why not give him [oncoming hospitalist] a chance (patient 10). Patients would also describe the transition as an opportunity to form, and possibly improve, therapeutic alliances with a new hospitalist.
Bedside Manner
Bedside manner was another commonly mentioned thematic element. Patients were often quick to forget prior problems or issues that they may have suffered because of the transition if the oncoming physician was perceived to have a good bedside manner, often described as someone who formally introduced themselves, was considered relaxed, and would take the time to sit and talk with the patient. As a patient put it, [S]he sat down and got to know meand asked me what I wanted to do (patient 12). Conversely, patients described instances in which a perceived bad bedside manner led to a poor relationship between the physician and the patient, in which trust and comfort (patient 11) were sacrificed.
Indifference Toward Transition
In contrast to some of the previous findings, which called for improved interactions between physicians and patients, we also discovered a theme of indifference toward the transition. Several patients stated feelings of trust with the medical system, and were content with the service changeover as long as they felt that their medical needs were being met. Patients also tended to express a level of acceptance with the transition, and tended to believe that this was the price we pay for being here [in the hospital] (patient 7).
Conceptual Model
Following the collection and analysis of all patient responses, all themes were utilized to construct the ideal patient‐centered service handoff. The ideal transition describes open lines of communication between all involved parties, is facilitated by multiple modalities, such as the EHRs and nursing staff, and recognizes the patient as the primary stakeholder (Figure 1).

DISCUSSION
To our knowledge, this is the first qualitative investigation of the hospitalized patient's experience with service handoffs between hospitalists. The patient perspective adds a personal and first‐hand description of how fragmented care may impact the hospitalized patient experience.
Of the 6 themes, communication was found to be the most pertinent to our respondents. Because much of patient care is an inherently communicative activity, it is not surprising that patients, as well as patient safety experts, have focused on communication as an area in need of improvement in transition processes.[17, 18] Moreover, multiple medical societies have directly called for improvements within this area, and have specifically recommended clear and direct communication of treatment plans between the patient and physician, timely exchange of information, and knowledge of who is primarily in charge of the patients care.[11] Not surprisingly, each of these recommendations appears to be echoed by our participants. This theme is especially important given that good physician‐patient communication has been noted to be a major goal in achieving patient‐centered care, and has been positively correlated to medication adherence, patient satisfaction, and physical health outcomes.[19, 20, 21, 22, 23]
Although not a substitute for face‐to‐face interactions, other communication interventions between physicians and patients should be considered. For example, get to know me posters placed in patient rooms have been shown to encourage communication between patients and physicians.[24] Additionally, physician face cards have been used to improve patients' abilities to identify and clarify physicians' roles in patient care.[25] As a patient put it, If they got a new one [hospitalist], just as if I got a new medicationprint out information on themlike where they went to med school, and stuff(patient 13). These modalities may represent highly implementable, cost‐effective adjuncts to current handoff methods that may improve lines of communication between physicians and patients.
In addition to the importance placed on physician‐patient communication, interprofessional communication between hospitalists and subspecialists was also highly regarded. Studies have shown that practice‐based interprofessional communication, such as daily interdisciplinary rounds and the use of external facilitators, can improve healthcare processes and outcomes.[26] However, these interventions must be weighed with the many conflicting factors that both hospitalists and subspecialists face on daily basis, including high patient volumes, time limitations, patient availability, and scheduling conflicts.[27] None the less, the strong emphasis patients placed on this line of communication highlights this domain as an area in which hospitalist and subspecialist can work together for systematic improvement.
Patients also recognized the complexity of the transfer process between hospitalists and called for improved transparency. For example, patients repeatedly requested to be informed prior to any changes in their hospitalists, a request that remains consistent with current guidelines.[11] There also existed a strong desire for a more formalized process of transitioning between hospitalists, which often described a handoff procedure that would occur at the patient's bedside. This desire seems to be mirrored in the data that show that patients prefer to interact with their care team at the bedside and report higher satisfaction when they are involved with their care.[28, 29] Unfortunately, this desire for more direct interaction with physicians runs counter to the current paradigm of patient care, where most activities on rounds do not take place at the bedside.[30]
In contrast to patient's calls for improved transparency, an equally large portion of patients expressed relative indifference to the transition. Whereas on the surface this may seem salutary, some studies suggest that a lack of patient activation and engagement may have adverse effects toward patients' overall care.[31] Furthermore, others have shown evidence of better healthcare experiences, improved health outcomes, and lower costs in patients who are more active in their care.[30, 31] Altogether, this suggests that despite some patients' indifference, physicians should continue to engage patients in their hospital care.[32]
Although prevailing sentiments among patient safety advocates are that patient handoffs are inherently dangerous and place patients at increased risk of adverse events, patients did not always share this concern. A frequently occurring theme was that the transition is an opportunity for medical reevaluation or the establishment of a new, possibly improved therapeutic alliance. Recognizing this viewpoint offers oncoming hospitalists the opportunity to focus on issues that the patient may have felt were not being properly addressed with their prior physician.
Finally, although our conceptual model is not a strict guideline, we believe that any future studies should consider this framework when constructing interventions to improve service‐level handoffs. Several interventions already exist. For instance, educational interventions, such as patient‐centered interviewing, have been shown to improve patient satisfaction, compliance with medications, lead to fewer lawsuits, and improve health outcomes.[33, 34, 35] Additional methods of keeping the patient more informed include physician face sheets and performance of the handoff at the patient's bedside. Although well known in nursing literature, the idea of physicians performing handoffs at the patient's bedside is a particularly patient‐centric process.[36] This type of intervention may have the ability to transform the handoff from the current state of a 2‐way street, in which information is passed between 2 hospitalists, to a 3‐way stop, in which both hospitalists and the patient are able to communicate at this critical junction of care.
Although our study does offer new insight into the effects of discontinuous care, its exploratory nature does have limitations. First, being performed at a single academic center limits our ability to generalize our findings. Second, perspectives of those who did not wish to participate, patients' family members or caregivers, and those who were not queried, could highly differ from those we interviewed. Additionally, we did not collect data on patients' diagnoses or reason for admission, thus limiting our ability to assess if certain diagnosis or subpopulations predispose patients to experiencing a service handoff. Third, although our study was restricted to English‐speaking patients only, we must consider that non‐English speakers would likely suffer from even greater communication barriers than those who took part in our study. Finally, our interviews and data analysis were conducted by hospitalists, which could have subconsciously influenced the interview process, and the interpretation of patient responses. However, we tried to mitigate these issues by having the same individual interview all participants, by using an interview guide to ensure cross‐cohort consistency, by using open‐ended questions, and by attempting to give patients every opportunity to express themselves.
CONCLUSIONS
From a patients' perspective, inpatient service handoffs are often opaque experiences that are highlighted by poor communication between physicians and patients. Although deficits in communication and transparency acted as barriers to a patient‐centered handoff, physicians should recognize that service handoffs may also represent opportunities for improvement, and should focus on these domains when they start on a new service.
Disclosures
All funding for this project was provided by the Section of Hospital Medicine at The University of Chicago Medical Center. The data from this article were presented at the Society of Hospital Medicine Annual Conference, National Harbor, March 31, 2015, and at the Society of General Internal Medicine National Meeting in Toronto, Canada, April 23, 2015. The authors report that no conflicts of interest, financial or otherwise, exist.
- , , , , , . Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA. 2009;301(16):1671–1680.
- , , , et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879–1885.
- Agency for Healthcare Research and Quality. HCUPnet: a tool for identifying, tracking, and analyzing national hospital statistics. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=82B37DA366A36BAD6(8):438–444.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335–338.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10(3):147–151.
- Agency for Healthcare Research and Quality. HCAHPS Fact Sheet. CAHPS Hospital Survey August 2013. Available at: http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed February 2, 2015.
- , , , et al. A conceptual framework for studying the safety of transitions in emergency care. In: Henriksen K, Battles JB, Marks ES, eds. Advances in Patient Safety: From Research to Implementation. Rockville, MD: Agency for Healthcare Research and Quality; 2005:309–321. Concepts and Methodology; vol 2. Available at: http://www.ncbi.nlm.nih.gov/books/NBK20522. Accessed January 15, 2015.
- , , , et al. Transitions of care consensus policy statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , . Grounded theory in medical education research: AMEE guide no. 70. Med Teach. 2012;34(10):850–861.
- . A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36(4):391–409.
- . The significance of saturation. Qual Health Res. 1995;5(2):147–149.
- , , , , . Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245–1251.
- , , . Consolidated criteria for reporting qualitative research (COREQ): a 32‐item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- The Joint Commission. Hot Topics in Healthcare, Issue 2. Transitions of care: the need for collaboration across entire care continuum. Available at: http://www.jointcommission.org/assets/1/6/TOC_Hot_Topics.pdf. Accessed April 9, 2015.
- , . Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47(8):826–834.
- , . Impediments to adherence to post myocardial infarction medications. Curr Cardiol Rep. 2013;15(1):322.
- , , , , . Medical specialists' patient‐centered communication and patient‐reported outcomes. Med Care. 2007;45(4):330–339.
- , , , . Does doctor‐patient communication affect patient satisfaction with hospital care? Results of an analysis with a novel instrumental variable. Health Serv Res. 2008;43(5 pt 1):1505–1519.
- , , . Patient‐centredness in chronic illness: what is it and does it matter? Patient Educ Couns. 2003;51(3):197–206.
- , , , et al. Merging cultures: palliative care specialists in the medical intensive care unit. Crit Care Med. 2006;34(11 suppl):S388–S393.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;(3):CD000072.
- , , , et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326–334.
- , , , , . The effect of bedside case presentations on patients' perceptions of their medical care. N Engl J Med. 1997;336(16):1150–1155.
- , , , . Patient‐centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040–1047.
- , , , et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084–1089.
- , . What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32(2):207–214.
- , , , , . When patient activation levels change, health outcomes and costs change, too. Health Aff Proj Hope. 2015;34(3):431–437.
- , , , et al. Evidence‐based guidelines for teaching patient‐centered interviewing. Patient Educ Couns. 2000;39(1):27–36.
- , , . Meta‐analysis of correlates of provider behavior in medical encounters. Med Care. 1988;26(7):657–675.
- , . Characteristics of potential plaintiffs in malpractice litigation. Ann Intern Med. 1994;120(9):792–798.
- , , , , . Bedside shift reports: what does the evidence say? J Nurs Adm. 2014;44(10):541–545.
- , , , , , . Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA. 2009;301(16):1671–1680.
- , , , et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879–1885.
- Agency for Healthcare Research and Quality. HCUPnet: a tool for identifying, tracking, and analyzing national hospital statistics. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=82B37DA366A36BAD6(8):438–444.
- , , , , , . Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , , . The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335–338.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- , , , et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10(3):147–151.
- Agency for Healthcare Research and Quality. HCAHPS Fact Sheet. CAHPS Hospital Survey August 2013. Available at: http://www.hcahpsonline.org/files/August_2013_HCAHPS_Fact_Sheet3.pdf. Accessed February 2, 2015.
- , , , et al. A conceptual framework for studying the safety of transitions in emergency care. In: Henriksen K, Battles JB, Marks ES, eds. Advances in Patient Safety: From Research to Implementation. Rockville, MD: Agency for Healthcare Research and Quality; 2005:309–321. Concepts and Methodology; vol 2. Available at: http://www.ncbi.nlm.nih.gov/books/NBK20522. Accessed January 15, 2015.
- , , , et al. Transitions of care consensus policy statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , . Grounded theory in medical education research: AMEE guide no. 70. Med Teach. 2012;34(10):850–861.
- . A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36(4):391–409.
- . The significance of saturation. Qual Health Res. 1995;5(2):147–149.
- , , , , . Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245–1251.
- , , . Consolidated criteria for reporting qualitative research (COREQ): a 32‐item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- The Joint Commission. Hot Topics in Healthcare, Issue 2. Transitions of care: the need for collaboration across entire care continuum. Available at: http://www.jointcommission.org/assets/1/6/TOC_Hot_Topics.pdf. Accessed April 9, 2015.
- , . Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47(8):826–834.
- , . Impediments to adherence to post myocardial infarction medications. Curr Cardiol Rep. 2013;15(1):322.
- , , , , . Medical specialists' patient‐centered communication and patient‐reported outcomes. Med Care. 2007;45(4):330–339.
- , , , . Does doctor‐patient communication affect patient satisfaction with hospital care? Results of an analysis with a novel instrumental variable. Health Serv Res. 2008;43(5 pt 1):1505–1519.
- , , . Patient‐centredness in chronic illness: what is it and does it matter? Patient Educ Couns. 2003;51(3):197–206.
- , , , et al. Merging cultures: palliative care specialists in the medical intensive care unit. Crit Care Med. 2006;34(11 suppl):S388–S393.
- , , , et al. Improving inpatients' identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613–619.
- , , . Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;(3):CD000072.
- , , , et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326–334.
- , , , , . The effect of bedside case presentations on patients' perceptions of their medical care. N Engl J Med. 1997;336(16):1150–1155.
- , , , . Patient‐centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040–1047.
- , , , et al. Attending rounds in the current era: what is and is not happening. JAMA Intern Med. 2013;173(12):1084–1089.
- , . What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32(2):207–214.
- , , , , . When patient activation levels change, health outcomes and costs change, too. Health Aff Proj Hope. 2015;34(3):431–437.
- , , , et al. Evidence‐based guidelines for teaching patient‐centered interviewing. Patient Educ Couns. 2000;39(1):27–36.
- , , . Meta‐analysis of correlates of provider behavior in medical encounters. Med Care. 1988;26(7):657–675.
- , . Characteristics of potential plaintiffs in malpractice litigation. Ann Intern Med. 1994;120(9):792–798.
- , , , , . Bedside shift reports: what does the evidence say? J Nurs Adm. 2014;44(10):541–545.