User login
PPIs associated with antibiotic-resistant bacteria carriage
AMSTERDAM – The long-term safety of proton pump inhibitors has once again come into question, as they may quadruple the chance of carrying a bacterial strain highly resistant to both penicillin and cephalosporin antibiotics, a Dutch study suggested.
The observational study showed only association, not causation, according to Dr. Pepijn Huizinga of the Amphia Ziekenhuis Hospital, Breda, the Netherlands. But, he said, the association of PPIs and extended-spectrum beta-lactamase–producing enterobacteriaceae (ESBL-E) is biologically plausible, and strong enough to warrant deeper investigation. Dr. Huizinga reported the study results at the 2016 European Conference of Clinical Microbiology and Infectious Diseases.
“We are continuously exposed to ESBL-E from many sources – other humans, contaminated foods, and the environment,” Dr. Huizinga said. “The gastric acid barrier is one of the last barriers we have against developing carriage. As long as it is in the normal pH range of 1.5-3, it’s quite efficient at keeping these bacteria from entering our system. But PPIs decrease this to 3 or 4. We already know that this is associated with an increased risk of infections from campylobacteriae, salmonella, and C. [Clostridium] difficile.”
PPI use is exploding in the Netherlands, Dr. Huizinga said, following a worldwide pattern of escalating use. National statistics demonstrate a very sharp upward trend, beginning with the introduction of omeprazole in 1994. By 2013, with five PPIs on the market, there were more than 2.7 million users – 14% of the country’s adult population. About a third of people older than 65 years are using them on a daily basis, according to data from the Dutch Foundation for Pharmaceutical Statistics.
To examine the relationship, Dr. Huizinga mined data from an ESBL-E prevalence survey conducted at Amphia Ziekenhuis Hospital in 2014 and 2015. The study cohort comprised 570 adults who received a rectal culture within a day of admission. Of these, 5.4% (31) were positive for ESBL-E carriage.
He examined correlations between carriage and several patient characteristics. Women were slightly more likely to be carriers than men (6.6% vs. 4%). There was no difference in the incidence of antibiotic use on day of admission, with 6% of the positive patients taking an antibiotic and 5% not taking one. Carriers were younger than noncarriers (64 vs. 65 years). PPI use was significantly more common among those who carried ESBL-E (8.6% vs. 2.9%).
In a multivariate analysis, there were no significant associations with sex, age, or antibiotic use. PPI use conferred the only significant risk, a fourfold increase in the chance of carriage.
Dr. Huizinga noted that the model didn’t consider the length of time taking the drugs, only whether they were in use on the day of admission. Nor did the study account for any medical comorbidity.
“However,” he said, “I think we do need to consider the possibility that the frequent use of PPIs in the general population could be an important driver of the increase we are seeing in ESBL-E carriage.”
Dr. Huizinga had no relevant financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – The long-term safety of proton pump inhibitors has once again come into question, as they may quadruple the chance of carrying a bacterial strain highly resistant to both penicillin and cephalosporin antibiotics, a Dutch study suggested.
The observational study showed only association, not causation, according to Dr. Pepijn Huizinga of the Amphia Ziekenhuis Hospital, Breda, the Netherlands. But, he said, the association of PPIs and extended-spectrum beta-lactamase–producing enterobacteriaceae (ESBL-E) is biologically plausible, and strong enough to warrant deeper investigation. Dr. Huizinga reported the study results at the 2016 European Conference of Clinical Microbiology and Infectious Diseases.
“We are continuously exposed to ESBL-E from many sources – other humans, contaminated foods, and the environment,” Dr. Huizinga said. “The gastric acid barrier is one of the last barriers we have against developing carriage. As long as it is in the normal pH range of 1.5-3, it’s quite efficient at keeping these bacteria from entering our system. But PPIs decrease this to 3 or 4. We already know that this is associated with an increased risk of infections from campylobacteriae, salmonella, and C. [Clostridium] difficile.”
PPI use is exploding in the Netherlands, Dr. Huizinga said, following a worldwide pattern of escalating use. National statistics demonstrate a very sharp upward trend, beginning with the introduction of omeprazole in 1994. By 2013, with five PPIs on the market, there were more than 2.7 million users – 14% of the country’s adult population. About a third of people older than 65 years are using them on a daily basis, according to data from the Dutch Foundation for Pharmaceutical Statistics.
To examine the relationship, Dr. Huizinga mined data from an ESBL-E prevalence survey conducted at Amphia Ziekenhuis Hospital in 2014 and 2015. The study cohort comprised 570 adults who received a rectal culture within a day of admission. Of these, 5.4% (31) were positive for ESBL-E carriage.
He examined correlations between carriage and several patient characteristics. Women were slightly more likely to be carriers than men (6.6% vs. 4%). There was no difference in the incidence of antibiotic use on day of admission, with 6% of the positive patients taking an antibiotic and 5% not taking one. Carriers were younger than noncarriers (64 vs. 65 years). PPI use was significantly more common among those who carried ESBL-E (8.6% vs. 2.9%).
In a multivariate analysis, there were no significant associations with sex, age, or antibiotic use. PPI use conferred the only significant risk, a fourfold increase in the chance of carriage.
Dr. Huizinga noted that the model didn’t consider the length of time taking the drugs, only whether they were in use on the day of admission. Nor did the study account for any medical comorbidity.
“However,” he said, “I think we do need to consider the possibility that the frequent use of PPIs in the general population could be an important driver of the increase we are seeing in ESBL-E carriage.”
Dr. Huizinga had no relevant financial disclosures.
On Twitter @Alz_Gal
AMSTERDAM – The long-term safety of proton pump inhibitors has once again come into question, as they may quadruple the chance of carrying a bacterial strain highly resistant to both penicillin and cephalosporin antibiotics, a Dutch study suggested.
The observational study showed only association, not causation, according to Dr. Pepijn Huizinga of the Amphia Ziekenhuis Hospital, Breda, the Netherlands. But, he said, the association of PPIs and extended-spectrum beta-lactamase–producing enterobacteriaceae (ESBL-E) is biologically plausible, and strong enough to warrant deeper investigation. Dr. Huizinga reported the study results at the 2016 European Conference of Clinical Microbiology and Infectious Diseases.
“We are continuously exposed to ESBL-E from many sources – other humans, contaminated foods, and the environment,” Dr. Huizinga said. “The gastric acid barrier is one of the last barriers we have against developing carriage. As long as it is in the normal pH range of 1.5-3, it’s quite efficient at keeping these bacteria from entering our system. But PPIs decrease this to 3 or 4. We already know that this is associated with an increased risk of infections from campylobacteriae, salmonella, and C. [Clostridium] difficile.”
PPI use is exploding in the Netherlands, Dr. Huizinga said, following a worldwide pattern of escalating use. National statistics demonstrate a very sharp upward trend, beginning with the introduction of omeprazole in 1994. By 2013, with five PPIs on the market, there were more than 2.7 million users – 14% of the country’s adult population. About a third of people older than 65 years are using them on a daily basis, according to data from the Dutch Foundation for Pharmaceutical Statistics.
To examine the relationship, Dr. Huizinga mined data from an ESBL-E prevalence survey conducted at Amphia Ziekenhuis Hospital in 2014 and 2015. The study cohort comprised 570 adults who received a rectal culture within a day of admission. Of these, 5.4% (31) were positive for ESBL-E carriage.
He examined correlations between carriage and several patient characteristics. Women were slightly more likely to be carriers than men (6.6% vs. 4%). There was no difference in the incidence of antibiotic use on day of admission, with 6% of the positive patients taking an antibiotic and 5% not taking one. Carriers were younger than noncarriers (64 vs. 65 years). PPI use was significantly more common among those who carried ESBL-E (8.6% vs. 2.9%).
In a multivariate analysis, there were no significant associations with sex, age, or antibiotic use. PPI use conferred the only significant risk, a fourfold increase in the chance of carriage.
Dr. Huizinga noted that the model didn’t consider the length of time taking the drugs, only whether they were in use on the day of admission. Nor did the study account for any medical comorbidity.
“However,” he said, “I think we do need to consider the possibility that the frequent use of PPIs in the general population could be an important driver of the increase we are seeing in ESBL-E carriage.”
Dr. Huizinga had no relevant financial disclosures.
On Twitter @Alz_Gal
AT ECCMID 2016
Key clinical point: Proton pump inhibitors may increase the risk of carrying antibiotic-resistant bacteria.
Major finding: PPIs conferred a fourfold increase in the risk of being colonized with extended-spectrum beta-lactamase–producing enterobacteriaceae.
Data source: A cross-sectional study involving 570 patients.
Disclosures: Dr. Huizinga had no relevant financial disclosures.
Coldiron Truth: Office safety
Wait, I know what you’re thinking, boring, dry, and don’t scold me. I am not going to do any of that today, and instead am going to tell you how to easily improve patient care, save money, and improve staff morale.
The easiest things to do to improve safety in the office include better communications, infection control, correct patient/site identification, dealing with emergencies, and staff safety.
Staff communication is often surprisingly poor, and physicians often assume staff can read our minds (and if they have worked for you for many years, maybe they can!). Always make sure staff – and patients – repeat back complicated instructions (better to start by making them less complicated). Never assume your patients are literate, 14% of adults cannot read, so make sure your staff goes over printed materials with the patients.
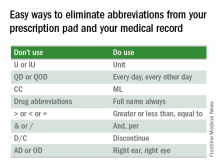
Something easy you can do is eliminate abbreviations from your prescription pad and your medical record.
Failure to follow up on path reports and lost biopsies is a frequent cause for lawsuits. You need to make sure you have a redundant system for tracking and reporting pathology results. In addition to the old reliable pathology log book, you need a sequential system ensuring that the specimen made it to the lab, that the result was generated and received, that the patient was notified, and that further action was taken if needed. If this is integrated into your electronic medical record, so much the better, but you still need a paper backup log book, “just in case.”
We all take basic infection precautions when performing procedures, including alcohol hand gel, eye protection, gloves, mask, and cleaning of surgical sites prior to excision. When there are wound infections, which should be rare, you should always culture, and if there is a cluster of infections, particularly with methicillin-resistant staphylococcus aureus (MRSA), you should consider culturing the anterior nares of the staff. Alternatively, you can preemptively treat the staff with intranasal topical mupirocin daily for 2 weeks.
Measuring the use of alcohol gel before and after staff education is an easy and beneficial quality improvement project. You and your staff are, of course, vaccinated for hepatitis B, and yearly for influenza, and you should consider PPD testing every 2 years for everyone. My asymptomatic receptionist converted, and needed prophylactic tuberculosis treatment and contact tracing.
Wrong-site surgery is a frequent problem for dermatologists. Many of our biopsies are tangential, heal almost invisibly, without marking sutures, and patients have battle-scarred skin, and are elderly. The patients may have trouble remembering or seeing where the biopsy was, so good charts and family members are helpful. Photographs of the biopsy sites can be priceless. If the site still cannot be identified, then rebiopsy, or close follow-up is indicated.
Emergencies are rare, but it is prudent to have an automatic external defibrillator in the office. These are automated, so Advanced Cardiovascular Life Support (ACLS), or even basic CPR is not required to operate them, and the survival rate after cardiac arrest increases from 2% to 50%. When it has to be replaced after 5 years, take the old one home and teach your spouse how to use it, and maybe they will.
If you do a lot of surgery you may want to get the ACLS training and buy the crash cart drug kit, but many of the drugs have become ferociously expensive, despite being generic.
Staff safety is a crucial consideration and showing concern boosts staff morale. You should dispose of sharps directly into a sharps container and remove the sharps from the tray at the end of the case. You should have a written protocol for needle sticks. I keep the red top tubes, and the first 2 days of HIV medication on site. Sometimes a patient is HIV positive, the sharp exposure is unknown (a cryostat tissue tease for example), or the patient refuses to have their blood drawn. Less obvious, but important to staff, are locks on doors, alarm systems, good parking lot lighting, and security cameras.
Office safety may seem mundane but a few simple measures can save you a fortune, boost employee morale, and most important of all, improve patient care.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics.
Wait, I know what you’re thinking, boring, dry, and don’t scold me. I am not going to do any of that today, and instead am going to tell you how to easily improve patient care, save money, and improve staff morale.
The easiest things to do to improve safety in the office include better communications, infection control, correct patient/site identification, dealing with emergencies, and staff safety.
Staff communication is often surprisingly poor, and physicians often assume staff can read our minds (and if they have worked for you for many years, maybe they can!). Always make sure staff – and patients – repeat back complicated instructions (better to start by making them less complicated). Never assume your patients are literate, 14% of adults cannot read, so make sure your staff goes over printed materials with the patients.
Something easy you can do is eliminate abbreviations from your prescription pad and your medical record.
Failure to follow up on path reports and lost biopsies is a frequent cause for lawsuits. You need to make sure you have a redundant system for tracking and reporting pathology results. In addition to the old reliable pathology log book, you need a sequential system ensuring that the specimen made it to the lab, that the result was generated and received, that the patient was notified, and that further action was taken if needed. If this is integrated into your electronic medical record, so much the better, but you still need a paper backup log book, “just in case.”
We all take basic infection precautions when performing procedures, including alcohol hand gel, eye protection, gloves, mask, and cleaning of surgical sites prior to excision. When there are wound infections, which should be rare, you should always culture, and if there is a cluster of infections, particularly with methicillin-resistant staphylococcus aureus (MRSA), you should consider culturing the anterior nares of the staff. Alternatively, you can preemptively treat the staff with intranasal topical mupirocin daily for 2 weeks.
Measuring the use of alcohol gel before and after staff education is an easy and beneficial quality improvement project. You and your staff are, of course, vaccinated for hepatitis B, and yearly for influenza, and you should consider PPD testing every 2 years for everyone. My asymptomatic receptionist converted, and needed prophylactic tuberculosis treatment and contact tracing.
Wrong-site surgery is a frequent problem for dermatologists. Many of our biopsies are tangential, heal almost invisibly, without marking sutures, and patients have battle-scarred skin, and are elderly. The patients may have trouble remembering or seeing where the biopsy was, so good charts and family members are helpful. Photographs of the biopsy sites can be priceless. If the site still cannot be identified, then rebiopsy, or close follow-up is indicated.
Emergencies are rare, but it is prudent to have an automatic external defibrillator in the office. These are automated, so Advanced Cardiovascular Life Support (ACLS), or even basic CPR is not required to operate them, and the survival rate after cardiac arrest increases from 2% to 50%. When it has to be replaced after 5 years, take the old one home and teach your spouse how to use it, and maybe they will.
If you do a lot of surgery you may want to get the ACLS training and buy the crash cart drug kit, but many of the drugs have become ferociously expensive, despite being generic.
Staff safety is a crucial consideration and showing concern boosts staff morale. You should dispose of sharps directly into a sharps container and remove the sharps from the tray at the end of the case. You should have a written protocol for needle sticks. I keep the red top tubes, and the first 2 days of HIV medication on site. Sometimes a patient is HIV positive, the sharp exposure is unknown (a cryostat tissue tease for example), or the patient refuses to have their blood drawn. Less obvious, but important to staff, are locks on doors, alarm systems, good parking lot lighting, and security cameras.
Office safety may seem mundane but a few simple measures can save you a fortune, boost employee morale, and most important of all, improve patient care.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics.
Wait, I know what you’re thinking, boring, dry, and don’t scold me. I am not going to do any of that today, and instead am going to tell you how to easily improve patient care, save money, and improve staff morale.
The easiest things to do to improve safety in the office include better communications, infection control, correct patient/site identification, dealing with emergencies, and staff safety.
Staff communication is often surprisingly poor, and physicians often assume staff can read our minds (and if they have worked for you for many years, maybe they can!). Always make sure staff – and patients – repeat back complicated instructions (better to start by making them less complicated). Never assume your patients are literate, 14% of adults cannot read, so make sure your staff goes over printed materials with the patients.
Something easy you can do is eliminate abbreviations from your prescription pad and your medical record.
Failure to follow up on path reports and lost biopsies is a frequent cause for lawsuits. You need to make sure you have a redundant system for tracking and reporting pathology results. In addition to the old reliable pathology log book, you need a sequential system ensuring that the specimen made it to the lab, that the result was generated and received, that the patient was notified, and that further action was taken if needed. If this is integrated into your electronic medical record, so much the better, but you still need a paper backup log book, “just in case.”
We all take basic infection precautions when performing procedures, including alcohol hand gel, eye protection, gloves, mask, and cleaning of surgical sites prior to excision. When there are wound infections, which should be rare, you should always culture, and if there is a cluster of infections, particularly with methicillin-resistant staphylococcus aureus (MRSA), you should consider culturing the anterior nares of the staff. Alternatively, you can preemptively treat the staff with intranasal topical mupirocin daily for 2 weeks.
Measuring the use of alcohol gel before and after staff education is an easy and beneficial quality improvement project. You and your staff are, of course, vaccinated for hepatitis B, and yearly for influenza, and you should consider PPD testing every 2 years for everyone. My asymptomatic receptionist converted, and needed prophylactic tuberculosis treatment and contact tracing.
Wrong-site surgery is a frequent problem for dermatologists. Many of our biopsies are tangential, heal almost invisibly, without marking sutures, and patients have battle-scarred skin, and are elderly. The patients may have trouble remembering or seeing where the biopsy was, so good charts and family members are helpful. Photographs of the biopsy sites can be priceless. If the site still cannot be identified, then rebiopsy, or close follow-up is indicated.
Emergencies are rare, but it is prudent to have an automatic external defibrillator in the office. These are automated, so Advanced Cardiovascular Life Support (ACLS), or even basic CPR is not required to operate them, and the survival rate after cardiac arrest increases from 2% to 50%. When it has to be replaced after 5 years, take the old one home and teach your spouse how to use it, and maybe they will.
If you do a lot of surgery you may want to get the ACLS training and buy the crash cart drug kit, but many of the drugs have become ferociously expensive, despite being generic.
Staff safety is a crucial consideration and showing concern boosts staff morale. You should dispose of sharps directly into a sharps container and remove the sharps from the tray at the end of the case. You should have a written protocol for needle sticks. I keep the red top tubes, and the first 2 days of HIV medication on site. Sometimes a patient is HIV positive, the sharp exposure is unknown (a cryostat tissue tease for example), or the patient refuses to have their blood drawn. Less obvious, but important to staff, are locks on doors, alarm systems, good parking lot lighting, and security cameras.
Office safety may seem mundane but a few simple measures can save you a fortune, boost employee morale, and most important of all, improve patient care.
Dr. Coldiron is a past president of the American Academy of Dermatology. He is currently in private practice, but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics.
Medicare delays plans for new star ratings on hospitals
Bowing to pressure from the hospital industry and Congress, the Obama administration on April 20 delayed releasing its new hospital quality rating measure just a day before its planned launch.
The new “overall hospital quality” star rating aimed to combine the government’s disparate efforts to measure hospital care into one easy-to-grasp metric. The Centers for Medicare & Medicaid Services now publishes more than 100 measures of aspects of hospital care, but many of these measures are technical and confusing since hospitals often do well on some and poorly on others. The new star rating boils 62 of the measures down into a unified rating of one to five stars, with five being the best.
But this month, 60 senators and 225 members of the House of Representatives signed letters urging the CMS to delay releasing the star ratings. “We have heard from hospitals in our districts that they do not have the necessary data to replicate or evaluate the CMS’s work to ensure that the methodology is accurate or fair,” the letter from the House members said.
In a notice sent April 20, the CMS told Congress it would delay release of the star ratings on its Hospital Compare website until July 2016. The “CMS is committed to working with hospitals and associations to provide further guidance about star ratings,” the notice said. “After the star ratings go live in their first iteration, we will refine and improve the site as we work together and gain experience.”
But in a conference call with hospital representatives, the CMS officials said they might delay release of the ratings past July if they are still analyzing or revising the methodology, according to people who participated in the call.
Mortality, readmissions, patient experience, and safety of care metrics each accounted for 22% of the star rating, while measures of effectiveness of care, timeliness of care, and efficient use of medical imaging made up 12% in total.
The hospital industry for months has been urging this delay, arguing that many of the measures will not be relevant to patients seeking a specific service. For instance, a hospital’s death rate for Medicare patients might be irrelevant for a woman trying to decide where to give birth.
The industry’s major trade groups said in a letter to the CMS that some hospitals perform poorly because their patients tend to be lower income and don’t have the support at home. Many of the nation’s most prestigious hospitals have been bracing for middling or poor ratings.
Rick Pollack, president of the American Hospital Association, said in a statement that “the delay is a necessary step as hospitals and health systems work with CMS to improve the ratings for patients, and the AHA commends CMS for their decision.”
Last year, the CMS created a star rating to represent the views of patients in surveys. Two sets of researchers recently determined that hospitals with more stars in patient experience tended to have lower death and readmission rates.
Hospital Compare received 3.7 million unique page views last year, according to an April 2016 paper published in the journal Health Affairs. The author, analyst Steven D. Findlay, called the traffic “not at a level commensurate with [the] stature and potential” of the federal government’s health care facility comparison sites.
Dr. Ashish Jha, a Harvard School of Public Health researcher, said consumers will be more likely to use the unified star ratings, but this specific mix of measures raises concerns. “The idea that dying and being readmitted to the hospital are equally important to patients seems funny to me,” he said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Bowing to pressure from the hospital industry and Congress, the Obama administration on April 20 delayed releasing its new hospital quality rating measure just a day before its planned launch.
The new “overall hospital quality” star rating aimed to combine the government’s disparate efforts to measure hospital care into one easy-to-grasp metric. The Centers for Medicare & Medicaid Services now publishes more than 100 measures of aspects of hospital care, but many of these measures are technical and confusing since hospitals often do well on some and poorly on others. The new star rating boils 62 of the measures down into a unified rating of one to five stars, with five being the best.
But this month, 60 senators and 225 members of the House of Representatives signed letters urging the CMS to delay releasing the star ratings. “We have heard from hospitals in our districts that they do not have the necessary data to replicate or evaluate the CMS’s work to ensure that the methodology is accurate or fair,” the letter from the House members said.
In a notice sent April 20, the CMS told Congress it would delay release of the star ratings on its Hospital Compare website until July 2016. The “CMS is committed to working with hospitals and associations to provide further guidance about star ratings,” the notice said. “After the star ratings go live in their first iteration, we will refine and improve the site as we work together and gain experience.”
But in a conference call with hospital representatives, the CMS officials said they might delay release of the ratings past July if they are still analyzing or revising the methodology, according to people who participated in the call.
Mortality, readmissions, patient experience, and safety of care metrics each accounted for 22% of the star rating, while measures of effectiveness of care, timeliness of care, and efficient use of medical imaging made up 12% in total.
The hospital industry for months has been urging this delay, arguing that many of the measures will not be relevant to patients seeking a specific service. For instance, a hospital’s death rate for Medicare patients might be irrelevant for a woman trying to decide where to give birth.
The industry’s major trade groups said in a letter to the CMS that some hospitals perform poorly because their patients tend to be lower income and don’t have the support at home. Many of the nation’s most prestigious hospitals have been bracing for middling or poor ratings.
Rick Pollack, president of the American Hospital Association, said in a statement that “the delay is a necessary step as hospitals and health systems work with CMS to improve the ratings for patients, and the AHA commends CMS for their decision.”
Last year, the CMS created a star rating to represent the views of patients in surveys. Two sets of researchers recently determined that hospitals with more stars in patient experience tended to have lower death and readmission rates.
Hospital Compare received 3.7 million unique page views last year, according to an April 2016 paper published in the journal Health Affairs. The author, analyst Steven D. Findlay, called the traffic “not at a level commensurate with [the] stature and potential” of the federal government’s health care facility comparison sites.
Dr. Ashish Jha, a Harvard School of Public Health researcher, said consumers will be more likely to use the unified star ratings, but this specific mix of measures raises concerns. “The idea that dying and being readmitted to the hospital are equally important to patients seems funny to me,” he said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Bowing to pressure from the hospital industry and Congress, the Obama administration on April 20 delayed releasing its new hospital quality rating measure just a day before its planned launch.
The new “overall hospital quality” star rating aimed to combine the government’s disparate efforts to measure hospital care into one easy-to-grasp metric. The Centers for Medicare & Medicaid Services now publishes more than 100 measures of aspects of hospital care, but many of these measures are technical and confusing since hospitals often do well on some and poorly on others. The new star rating boils 62 of the measures down into a unified rating of one to five stars, with five being the best.
But this month, 60 senators and 225 members of the House of Representatives signed letters urging the CMS to delay releasing the star ratings. “We have heard from hospitals in our districts that they do not have the necessary data to replicate or evaluate the CMS’s work to ensure that the methodology is accurate or fair,” the letter from the House members said.
In a notice sent April 20, the CMS told Congress it would delay release of the star ratings on its Hospital Compare website until July 2016. The “CMS is committed to working with hospitals and associations to provide further guidance about star ratings,” the notice said. “After the star ratings go live in their first iteration, we will refine and improve the site as we work together and gain experience.”
But in a conference call with hospital representatives, the CMS officials said they might delay release of the ratings past July if they are still analyzing or revising the methodology, according to people who participated in the call.
Mortality, readmissions, patient experience, and safety of care metrics each accounted for 22% of the star rating, while measures of effectiveness of care, timeliness of care, and efficient use of medical imaging made up 12% in total.
The hospital industry for months has been urging this delay, arguing that many of the measures will not be relevant to patients seeking a specific service. For instance, a hospital’s death rate for Medicare patients might be irrelevant for a woman trying to decide where to give birth.
The industry’s major trade groups said in a letter to the CMS that some hospitals perform poorly because their patients tend to be lower income and don’t have the support at home. Many of the nation’s most prestigious hospitals have been bracing for middling or poor ratings.
Rick Pollack, president of the American Hospital Association, said in a statement that “the delay is a necessary step as hospitals and health systems work with CMS to improve the ratings for patients, and the AHA commends CMS for their decision.”
Last year, the CMS created a star rating to represent the views of patients in surveys. Two sets of researchers recently determined that hospitals with more stars in patient experience tended to have lower death and readmission rates.
Hospital Compare received 3.7 million unique page views last year, according to an April 2016 paper published in the journal Health Affairs. The author, analyst Steven D. Findlay, called the traffic “not at a level commensurate with [the] stature and potential” of the federal government’s health care facility comparison sites.
Dr. Ashish Jha, a Harvard School of Public Health researcher, said consumers will be more likely to use the unified star ratings, but this specific mix of measures raises concerns. “The idea that dying and being readmitted to the hospital are equally important to patients seems funny to me,” he said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
FROM KAISER HEALTH NEWS
Endovascular thrombectomy procedure volume for stroke may not affect outcomes
VANCOUVER – The relationship between hospitals’ procedural volume and patient outcomes that has been observed for many cardiovascular interventions and other surgeries does not hold for endovascular mechanical thrombectomy procedures for acute ischemic stroke, according to an analysis of cases during 2008-2011 in the Nationwide Inpatient Sample.
In-hospital mortality and rates for any complications were not associated with high or low endovascular mechanical thrombectomy (EMT) volume at hospitals across the United States in the analysis of 13,502 adult patients hospitalized with a primary diagnosis of acute ischemic stroke and treated with EMT, neurology resident Dr. Abhishek Lunagariya of the University of Florida, Gainesville, reported at the annual meeting of the American Academy of Neurology.
A smaller prior study of 2,749 EMTs done in 296 hospitals in 2008 showed lower mortality in high-volume hospitals that performed 10 or more of the procedures per year (J Stroke Cerebrovasc Dis. 2013 Nov; 22[8]:1263-9).
Of the 13,502 EMTs in the study, 25% occurred at low-volume hospitals performing less than 10 per year. Low-volume hospitals had higher in-hospital mortality than did higher-volume centers performing 10 or more of the procedures per year in an unadjusted comparison (26% vs. 21%). A comparison of a combined endpoint for any complications (in-hospital mortality, intracerebral hemorrhage, and vascular complications) was also significantly in favor of high-volume hospitals (34% vs. 30%).
However, in a multivariate hierarchical model, low-volume hospitals were not associated with higher in-hospital mortality (odds ratio, 0.95; 95% confidence interval, 0.74-1.23) or rate of any complications (OR, 0.96; 95% CI, 0.76-1.21). These analyses were adjusted for age, gender, ethnicity, primary payer, median household income, hospital region/teaching status/location/bed size, Charlson Comorbidity Index, calendar year, and use of intravenous tissue plasminogen activator.
Dr. Lunagariya noted that he and his associates could not adjust the comparisons for National Institutes of Health Stroke Scale scores because they are not recorded in the National Inpatient Sample. They also could not examine what happened to patients after discharge.
Dr. Lunagariya suggested a variety of possible reasons that might help to explain the lack of an association between hospital procedure volume and outcomes after adjustment: the availability of better thrombectomy devices since the smaller 2008 study, lesser operator variability, favorable patient selection, and an increased skill set of operators working at low-volume hospitals.
One audience member noted that some endovascular interventionalists will operate at both high-volume and low-volume hospitals and could account for some of the findings. That indeed might be happening more often and needs to happen more often, Dr. Lunagariya said in an interview, in order to combat the “common belief” that it would be better to wait for a patient to undergo the procedure at a high- rather than low-volume hospital. Patients who receive initial care for stroke at a low-volume hospital but are not stable enough or do not have enough time to be transferred could benefit from EMT if an interventionalist who performs EMT drove there instead, he said.
With even newer devices now available that are thought to be easier to use, Dr. Lunagariya suggested that the similarity in outcomes at low- and higher-volume centers may not change in updated analyses of more recent EMT procedures for ischemic stroke.
The investigators received no funding for the study, and they reported having no financial disclosures.
VANCOUVER – The relationship between hospitals’ procedural volume and patient outcomes that has been observed for many cardiovascular interventions and other surgeries does not hold for endovascular mechanical thrombectomy procedures for acute ischemic stroke, according to an analysis of cases during 2008-2011 in the Nationwide Inpatient Sample.
In-hospital mortality and rates for any complications were not associated with high or low endovascular mechanical thrombectomy (EMT) volume at hospitals across the United States in the analysis of 13,502 adult patients hospitalized with a primary diagnosis of acute ischemic stroke and treated with EMT, neurology resident Dr. Abhishek Lunagariya of the University of Florida, Gainesville, reported at the annual meeting of the American Academy of Neurology.
A smaller prior study of 2,749 EMTs done in 296 hospitals in 2008 showed lower mortality in high-volume hospitals that performed 10 or more of the procedures per year (J Stroke Cerebrovasc Dis. 2013 Nov; 22[8]:1263-9).
Of the 13,502 EMTs in the study, 25% occurred at low-volume hospitals performing less than 10 per year. Low-volume hospitals had higher in-hospital mortality than did higher-volume centers performing 10 or more of the procedures per year in an unadjusted comparison (26% vs. 21%). A comparison of a combined endpoint for any complications (in-hospital mortality, intracerebral hemorrhage, and vascular complications) was also significantly in favor of high-volume hospitals (34% vs. 30%).
However, in a multivariate hierarchical model, low-volume hospitals were not associated with higher in-hospital mortality (odds ratio, 0.95; 95% confidence interval, 0.74-1.23) or rate of any complications (OR, 0.96; 95% CI, 0.76-1.21). These analyses were adjusted for age, gender, ethnicity, primary payer, median household income, hospital region/teaching status/location/bed size, Charlson Comorbidity Index, calendar year, and use of intravenous tissue plasminogen activator.
Dr. Lunagariya noted that he and his associates could not adjust the comparisons for National Institutes of Health Stroke Scale scores because they are not recorded in the National Inpatient Sample. They also could not examine what happened to patients after discharge.
Dr. Lunagariya suggested a variety of possible reasons that might help to explain the lack of an association between hospital procedure volume and outcomes after adjustment: the availability of better thrombectomy devices since the smaller 2008 study, lesser operator variability, favorable patient selection, and an increased skill set of operators working at low-volume hospitals.
One audience member noted that some endovascular interventionalists will operate at both high-volume and low-volume hospitals and could account for some of the findings. That indeed might be happening more often and needs to happen more often, Dr. Lunagariya said in an interview, in order to combat the “common belief” that it would be better to wait for a patient to undergo the procedure at a high- rather than low-volume hospital. Patients who receive initial care for stroke at a low-volume hospital but are not stable enough or do not have enough time to be transferred could benefit from EMT if an interventionalist who performs EMT drove there instead, he said.
With even newer devices now available that are thought to be easier to use, Dr. Lunagariya suggested that the similarity in outcomes at low- and higher-volume centers may not change in updated analyses of more recent EMT procedures for ischemic stroke.
The investigators received no funding for the study, and they reported having no financial disclosures.
VANCOUVER – The relationship between hospitals’ procedural volume and patient outcomes that has been observed for many cardiovascular interventions and other surgeries does not hold for endovascular mechanical thrombectomy procedures for acute ischemic stroke, according to an analysis of cases during 2008-2011 in the Nationwide Inpatient Sample.
In-hospital mortality and rates for any complications were not associated with high or low endovascular mechanical thrombectomy (EMT) volume at hospitals across the United States in the analysis of 13,502 adult patients hospitalized with a primary diagnosis of acute ischemic stroke and treated with EMT, neurology resident Dr. Abhishek Lunagariya of the University of Florida, Gainesville, reported at the annual meeting of the American Academy of Neurology.
A smaller prior study of 2,749 EMTs done in 296 hospitals in 2008 showed lower mortality in high-volume hospitals that performed 10 or more of the procedures per year (J Stroke Cerebrovasc Dis. 2013 Nov; 22[8]:1263-9).
Of the 13,502 EMTs in the study, 25% occurred at low-volume hospitals performing less than 10 per year. Low-volume hospitals had higher in-hospital mortality than did higher-volume centers performing 10 or more of the procedures per year in an unadjusted comparison (26% vs. 21%). A comparison of a combined endpoint for any complications (in-hospital mortality, intracerebral hemorrhage, and vascular complications) was also significantly in favor of high-volume hospitals (34% vs. 30%).
However, in a multivariate hierarchical model, low-volume hospitals were not associated with higher in-hospital mortality (odds ratio, 0.95; 95% confidence interval, 0.74-1.23) or rate of any complications (OR, 0.96; 95% CI, 0.76-1.21). These analyses were adjusted for age, gender, ethnicity, primary payer, median household income, hospital region/teaching status/location/bed size, Charlson Comorbidity Index, calendar year, and use of intravenous tissue plasminogen activator.
Dr. Lunagariya noted that he and his associates could not adjust the comparisons for National Institutes of Health Stroke Scale scores because they are not recorded in the National Inpatient Sample. They also could not examine what happened to patients after discharge.
Dr. Lunagariya suggested a variety of possible reasons that might help to explain the lack of an association between hospital procedure volume and outcomes after adjustment: the availability of better thrombectomy devices since the smaller 2008 study, lesser operator variability, favorable patient selection, and an increased skill set of operators working at low-volume hospitals.
One audience member noted that some endovascular interventionalists will operate at both high-volume and low-volume hospitals and could account for some of the findings. That indeed might be happening more often and needs to happen more often, Dr. Lunagariya said in an interview, in order to combat the “common belief” that it would be better to wait for a patient to undergo the procedure at a high- rather than low-volume hospital. Patients who receive initial care for stroke at a low-volume hospital but are not stable enough or do not have enough time to be transferred could benefit from EMT if an interventionalist who performs EMT drove there instead, he said.
With even newer devices now available that are thought to be easier to use, Dr. Lunagariya suggested that the similarity in outcomes at low- and higher-volume centers may not change in updated analyses of more recent EMT procedures for ischemic stroke.
The investigators received no funding for the study, and they reported having no financial disclosures.
AT THE AAN 2016 ANNUAL MEETING
Key clinical point: Patient outcomes after endovascular mechanical thrombectomy for acute ischemic stroke do not appear to be worse at low- versus high-volume hospitals.
Major finding: In a multivariate hierarchical model, low-volume hospitals (fewer than 10 thrombectomies per year) were not associated with higher in-hospital mortality (odds ratio, 0.95; 95% confidence interval, 0.74-1.23) or rate of any complications (OR, 0.96; 95% CI, 0.76-1.21).
Data source: A retrospective review of 13,502 patients with acute ischemic stroke who underwent endovascular mechanical thrombectomy in 2008-2011.
Disclosures: The investigators received no funding for the study, and they reported having no financial disclosures.
BRCA1 mutations reduce ovarian reserve
Women with mutations in the BRCA1 gene are likely to have significantly reduced ovarian reserve, according to Dr. Kelly-Anne Phillips and her associates.
After adjusting for age, the average anti-Müllerian hormone concentration for the 172 BRCA1 mutation carriers tested was about 25% less than the 216 noncarriers tested. This difference remained after adjusting for oral contraceptive pill use at time of blood draw, BMI at cohort entry, cigarette smoking ever, length of time from blood draw to analysis, or exclusion of current oral contraceptive users and women who reported they were postmenopausal. The odds ratio for mutation carriers of having an AMH concentration in the lowest quartile was 1.84.
Average AMH concentration in women with BRCA2 mutations did not differ from women who do not carry the mutation. The odds ratio of having an AMH concentration in the lowest quartile for BRCA2 carriers was 0.87.
“Low AMH concentrations have not been shown to affect natural fecundability in young women but are associated with reduced fecundability in older women in their 30s,” the investigators wrote. “The reduced concentrations of AMH observed in this study were equivalent, for example, to a 2-year age increase for a woman in her mid 30s. Thus it is possible that the findings of our study might not translate to clinically relevant fertility implications for younger women, but may be important for the subgroup of BRCA1 mutation carriers who wish to conceive in their late 30s or 40s when fertility is reduced even in the general population.”
Find the full study in Human Reproduction (doi: 10.1093/humrep/dew044).
Women with mutations in the BRCA1 gene are likely to have significantly reduced ovarian reserve, according to Dr. Kelly-Anne Phillips and her associates.
After adjusting for age, the average anti-Müllerian hormone concentration for the 172 BRCA1 mutation carriers tested was about 25% less than the 216 noncarriers tested. This difference remained after adjusting for oral contraceptive pill use at time of blood draw, BMI at cohort entry, cigarette smoking ever, length of time from blood draw to analysis, or exclusion of current oral contraceptive users and women who reported they were postmenopausal. The odds ratio for mutation carriers of having an AMH concentration in the lowest quartile was 1.84.
Average AMH concentration in women with BRCA2 mutations did not differ from women who do not carry the mutation. The odds ratio of having an AMH concentration in the lowest quartile for BRCA2 carriers was 0.87.
“Low AMH concentrations have not been shown to affect natural fecundability in young women but are associated with reduced fecundability in older women in their 30s,” the investigators wrote. “The reduced concentrations of AMH observed in this study were equivalent, for example, to a 2-year age increase for a woman in her mid 30s. Thus it is possible that the findings of our study might not translate to clinically relevant fertility implications for younger women, but may be important for the subgroup of BRCA1 mutation carriers who wish to conceive in their late 30s or 40s when fertility is reduced even in the general population.”
Find the full study in Human Reproduction (doi: 10.1093/humrep/dew044).
Women with mutations in the BRCA1 gene are likely to have significantly reduced ovarian reserve, according to Dr. Kelly-Anne Phillips and her associates.
After adjusting for age, the average anti-Müllerian hormone concentration for the 172 BRCA1 mutation carriers tested was about 25% less than the 216 noncarriers tested. This difference remained after adjusting for oral contraceptive pill use at time of blood draw, BMI at cohort entry, cigarette smoking ever, length of time from blood draw to analysis, or exclusion of current oral contraceptive users and women who reported they were postmenopausal. The odds ratio for mutation carriers of having an AMH concentration in the lowest quartile was 1.84.
Average AMH concentration in women with BRCA2 mutations did not differ from women who do not carry the mutation. The odds ratio of having an AMH concentration in the lowest quartile for BRCA2 carriers was 0.87.
“Low AMH concentrations have not been shown to affect natural fecundability in young women but are associated with reduced fecundability in older women in their 30s,” the investigators wrote. “The reduced concentrations of AMH observed in this study were equivalent, for example, to a 2-year age increase for a woman in her mid 30s. Thus it is possible that the findings of our study might not translate to clinically relevant fertility implications for younger women, but may be important for the subgroup of BRCA1 mutation carriers who wish to conceive in their late 30s or 40s when fertility is reduced even in the general population.”
Find the full study in Human Reproduction (doi: 10.1093/humrep/dew044).
FROM HUMAN REPRODUCTION
VP Biden to AACR: Help me help you
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
FROM THE AACR ANNUAL MEETING
Neoadjuvant chemotherapy improves survival for some with pancreatic cancer
MONTREAL – For individuals with stage III pancreatic cancer, neoadjuvant therapy may improve survival, compared with surgery-first treatment. “This study supports, however does not prove, the hypothesis that early and continued control of occult metastatic disease prolongs survival in surgical patients,” said Dr. Christopher Shubert.
Dr. Shubert and his colleagues used an intention-to-treat (ITT) analysis to compare overall survival (OS) for 377 patients who were to receive neoadjuvant chemotherapy with 216 patients who received surgery first. Median OS for the neoadjuvant group was 20.7 months, compared with 13.7 months for the surgery-first group (log-rank P less than .0001).
This study was the first to utilize national data to look at how patients who received neoadjuvant chemotherapy for stage III pancreatic cancer fared, when compared with those treated with a surgery-first approach, Dr. Shubert, a general surgery resident at the Mayo Clinic in Rochester, Minn., said at the annual meeting of the Central Surgical Association.
Stage III pancreatic cancer (T4, any N, M0) means that the cancer has invaded the celiac trunk, or there is superior mesenteric artery involvement, he noted.
Using data from the National Cancer Database from 2002 to 2011, the investigators identified patients with clinical stage III pancreatic adenocarcinoma of the head or body of the pancreas. The ITT neoadjuvant therapy cohort included all patients whose treatment recommendations included curative-intent surgery and neoadjuvant chemotherapy, regardless of what therapies the patients received. The surgery-first cohort included those who were recommended to receive adjuvant therapy.
A total of 593 patients were identified, of whom 377 (63.5%) were in the neoadjuvant group. Of these, 104 (27.6%) were lost to presurgical attrition. The surgery-first group included 216 patients (36.3%), 30 (13.9%) of whom did not receive the intended adjuvant chemotherapy. Comparing the two ITT groups yielded an adjusted hazard ratio of 0.68 (P = .001).
A secondary aim of the study was to see which aspects of therapy, and which pathologic features, were associated with longer OS. The addition of postsurgical therapy was associated with additional survival benefit (31.6 vs. 22.6 months for no postsurgical therapy; HR, 0.60; P = .002). Node-negative and R0 status were both also significantly increased among those receiving neoadjuvant chemotherapy, and both disease characteristics were associated with increased OS, he reported.
Dr. Shubert said that study limitations included its review of a prospective database. Also, investigators could not determine the type or duration of chemotherapy; they also were unable to tell when systemic chemotherapy plus chemoradiation or just chemoradiation alone had been used. No recurrence data were available, and all cases were grouped under one procedure code, limiting information about vascular resections.
“Neoadjuvant therapy may offer survival advantages compared to a surgery-first approach,” said Dr. Shubert, and control of small occult metastases may be the mechanism behind this advantage. Still to be determined, however, are the optimal type, duration, and sequencing of neoadjuvant chemotherapy and chemoradiation, he said.
Dr. Shubert reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – For individuals with stage III pancreatic cancer, neoadjuvant therapy may improve survival, compared with surgery-first treatment. “This study supports, however does not prove, the hypothesis that early and continued control of occult metastatic disease prolongs survival in surgical patients,” said Dr. Christopher Shubert.
Dr. Shubert and his colleagues used an intention-to-treat (ITT) analysis to compare overall survival (OS) for 377 patients who were to receive neoadjuvant chemotherapy with 216 patients who received surgery first. Median OS for the neoadjuvant group was 20.7 months, compared with 13.7 months for the surgery-first group (log-rank P less than .0001).
This study was the first to utilize national data to look at how patients who received neoadjuvant chemotherapy for stage III pancreatic cancer fared, when compared with those treated with a surgery-first approach, Dr. Shubert, a general surgery resident at the Mayo Clinic in Rochester, Minn., said at the annual meeting of the Central Surgical Association.
Stage III pancreatic cancer (T4, any N, M0) means that the cancer has invaded the celiac trunk, or there is superior mesenteric artery involvement, he noted.
Using data from the National Cancer Database from 2002 to 2011, the investigators identified patients with clinical stage III pancreatic adenocarcinoma of the head or body of the pancreas. The ITT neoadjuvant therapy cohort included all patients whose treatment recommendations included curative-intent surgery and neoadjuvant chemotherapy, regardless of what therapies the patients received. The surgery-first cohort included those who were recommended to receive adjuvant therapy.
A total of 593 patients were identified, of whom 377 (63.5%) were in the neoadjuvant group. Of these, 104 (27.6%) were lost to presurgical attrition. The surgery-first group included 216 patients (36.3%), 30 (13.9%) of whom did not receive the intended adjuvant chemotherapy. Comparing the two ITT groups yielded an adjusted hazard ratio of 0.68 (P = .001).
A secondary aim of the study was to see which aspects of therapy, and which pathologic features, were associated with longer OS. The addition of postsurgical therapy was associated with additional survival benefit (31.6 vs. 22.6 months for no postsurgical therapy; HR, 0.60; P = .002). Node-negative and R0 status were both also significantly increased among those receiving neoadjuvant chemotherapy, and both disease characteristics were associated with increased OS, he reported.
Dr. Shubert said that study limitations included its review of a prospective database. Also, investigators could not determine the type or duration of chemotherapy; they also were unable to tell when systemic chemotherapy plus chemoradiation or just chemoradiation alone had been used. No recurrence data were available, and all cases were grouped under one procedure code, limiting information about vascular resections.
“Neoadjuvant therapy may offer survival advantages compared to a surgery-first approach,” said Dr. Shubert, and control of small occult metastases may be the mechanism behind this advantage. Still to be determined, however, are the optimal type, duration, and sequencing of neoadjuvant chemotherapy and chemoradiation, he said.
Dr. Shubert reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – For individuals with stage III pancreatic cancer, neoadjuvant therapy may improve survival, compared with surgery-first treatment. “This study supports, however does not prove, the hypothesis that early and continued control of occult metastatic disease prolongs survival in surgical patients,” said Dr. Christopher Shubert.
Dr. Shubert and his colleagues used an intention-to-treat (ITT) analysis to compare overall survival (OS) for 377 patients who were to receive neoadjuvant chemotherapy with 216 patients who received surgery first. Median OS for the neoadjuvant group was 20.7 months, compared with 13.7 months for the surgery-first group (log-rank P less than .0001).
This study was the first to utilize national data to look at how patients who received neoadjuvant chemotherapy for stage III pancreatic cancer fared, when compared with those treated with a surgery-first approach, Dr. Shubert, a general surgery resident at the Mayo Clinic in Rochester, Minn., said at the annual meeting of the Central Surgical Association.
Stage III pancreatic cancer (T4, any N, M0) means that the cancer has invaded the celiac trunk, or there is superior mesenteric artery involvement, he noted.
Using data from the National Cancer Database from 2002 to 2011, the investigators identified patients with clinical stage III pancreatic adenocarcinoma of the head or body of the pancreas. The ITT neoadjuvant therapy cohort included all patients whose treatment recommendations included curative-intent surgery and neoadjuvant chemotherapy, regardless of what therapies the patients received. The surgery-first cohort included those who were recommended to receive adjuvant therapy.
A total of 593 patients were identified, of whom 377 (63.5%) were in the neoadjuvant group. Of these, 104 (27.6%) were lost to presurgical attrition. The surgery-first group included 216 patients (36.3%), 30 (13.9%) of whom did not receive the intended adjuvant chemotherapy. Comparing the two ITT groups yielded an adjusted hazard ratio of 0.68 (P = .001).
A secondary aim of the study was to see which aspects of therapy, and which pathologic features, were associated with longer OS. The addition of postsurgical therapy was associated with additional survival benefit (31.6 vs. 22.6 months for no postsurgical therapy; HR, 0.60; P = .002). Node-negative and R0 status were both also significantly increased among those receiving neoadjuvant chemotherapy, and both disease characteristics were associated with increased OS, he reported.
Dr. Shubert said that study limitations included its review of a prospective database. Also, investigators could not determine the type or duration of chemotherapy; they also were unable to tell when systemic chemotherapy plus chemoradiation or just chemoradiation alone had been used. No recurrence data were available, and all cases were grouped under one procedure code, limiting information about vascular resections.
“Neoadjuvant therapy may offer survival advantages compared to a surgery-first approach,” said Dr. Shubert, and control of small occult metastases may be the mechanism behind this advantage. Still to be determined, however, are the optimal type, duration, and sequencing of neoadjuvant chemotherapy and chemoradiation, he said.
Dr. Shubert reported no relevant disclosures.
On Twitter @karioakes
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Overall survival in stage III pancreatic cancer was better with neoadjuvant chemotherapy.
Major finding: Median survival with neoadjuvant chemotherapy was 20.7 months, compared with 13.7 months for surgery-first patients.
Data source: Review of stage III pancreatic cancer patients in the prospective National Cancer Database, comparing 377 patients recommended to receive neoadjuvant chemotherapy with 216 patients who were to receive surgery first.
Disclosures: Dr. Shubert reported no relevant disclosures.
SHM Launches Enhanced SHM Learning Portal
You asked, we listened: Introducing the enhanced SHM Learning Portal!
The SHM Learning Portal, the online learning home for hospitalists with all eLearning initiatives in one place, just launched a brand-new responsive design in March 2016. Feedback gathered by the Learning Portal team in the summer and fall of 2015 was used to develop a more user-friendly design aimed at reducing the time it takes to discover and access a growing catalog of educational content.
Mobile enhancements now allow for easy access and navigation on the go. Check out the new design for yourself at www.shmlearningportal.org.
You asked, we listened: Introducing the enhanced SHM Learning Portal!
The SHM Learning Portal, the online learning home for hospitalists with all eLearning initiatives in one place, just launched a brand-new responsive design in March 2016. Feedback gathered by the Learning Portal team in the summer and fall of 2015 was used to develop a more user-friendly design aimed at reducing the time it takes to discover and access a growing catalog of educational content.
Mobile enhancements now allow for easy access and navigation on the go. Check out the new design for yourself at www.shmlearningportal.org.
You asked, we listened: Introducing the enhanced SHM Learning Portal!
The SHM Learning Portal, the online learning home for hospitalists with all eLearning initiatives in one place, just launched a brand-new responsive design in March 2016. Feedback gathered by the Learning Portal team in the summer and fall of 2015 was used to develop a more user-friendly design aimed at reducing the time it takes to discover and access a growing catalog of educational content.
Mobile enhancements now allow for easy access and navigation on the go. Check out the new design for yourself at www.shmlearningportal.org.
Survey Helps Assess Engagement of Your Hospital Medicine Group
Engaged hospitalists drive quality care, and SHM has the tools to help you assess the level of engagement of hospitalists in your hospital medicine group. SHM offered a Hospitalist Engagement Benchmarking Service in 2015 and analyzed engagement of approximately 1,500 hospitalists. The survey can help open conversations about everything from relationships with the C-suite to sustaining teamwork.
Help ensure hospitalists are engaged in your hospital medicine group by registering now for the next cohort of the Hospitalist Engagement Benchmarking Service at www.hospitalmedicine.org/engage.
Engaged hospitalists drive quality care, and SHM has the tools to help you assess the level of engagement of hospitalists in your hospital medicine group. SHM offered a Hospitalist Engagement Benchmarking Service in 2015 and analyzed engagement of approximately 1,500 hospitalists. The survey can help open conversations about everything from relationships with the C-suite to sustaining teamwork.
Help ensure hospitalists are engaged in your hospital medicine group by registering now for the next cohort of the Hospitalist Engagement Benchmarking Service at www.hospitalmedicine.org/engage.
Engaged hospitalists drive quality care, and SHM has the tools to help you assess the level of engagement of hospitalists in your hospital medicine group. SHM offered a Hospitalist Engagement Benchmarking Service in 2015 and analyzed engagement of approximately 1,500 hospitalists. The survey can help open conversations about everything from relationships with the C-suite to sustaining teamwork.
Help ensure hospitalists are engaged in your hospital medicine group by registering now for the next cohort of the Hospitalist Engagement Benchmarking Service at www.hospitalmedicine.org/engage.
Predicting Tongue Cancer Recurrence
For patients with squamous cell carcinoma (SCC) of the tongue, recurrence is common and closely associated with survival, say researchers from Capital Medical University, Beijing. The researchers conducted a retrospective study of 204 patients with SCC of the tongue that aimed to identify the factors that predict relapse and prognosis.
Related: IBD and the Risk of Oral Cancer
In an earlier study, the researchers assessed the best indications for neck dissection and the prognostic factors of oral SCC. Their results showed that middle-late oral SCC is an indication for simultaneous neck dissection, even in patients whose nodes are clear. But few studies have focused on the outcomes of treatment after different surgical approaches, such as en bloc resection and discontinuous resection. En bloc dissection requires continuity between the tumor and the level I neck nodes, and the technique involves removal of the sublingual gland and the mylohyoid muscle, as well as the associated sublingual nodes.
Related: A Team Approach to Nonmelanotic Skin Cancer Procedures
Within the median follow-up of 82 months, 59 patients died (29%), 4 from non-cancer causes. Two patients in the en bloc group and 12 in the control group (discontinuous resection) had relapses. Ten of the 14 in this group had recurrences within a year of the first operation. Nine patients had salvage operations; however, only 2 experienced a successful outcome.
Surgical technique and pathological nodal (pN) status independently predicted both 5-year recurrence and disease-specific survival.
Related: Nivolumab Approved for Expanded Indication
The analysis showed a “dramatic” decrease in the 5-year disease-specific survival if the cancer recurred after the primary operation (no recurrence, 77% vs recurrence, 14%). Patients in the en bloc group “could expect an obviously lower” 5-year recurrence rate (2%, compared with 11% in the control group). They also had a better 5-year disease-specific survival (80%, versus 66%). An aggressive pN status was closely correlated with recurrence.
Source:
Feng Z, Xu QS, Qin LZ, et al. Br J Oral Maxillofac Surg. 2016;54(1):88-93.
doi: 10.1016/j.bjoms.2015.09.024
For patients with squamous cell carcinoma (SCC) of the tongue, recurrence is common and closely associated with survival, say researchers from Capital Medical University, Beijing. The researchers conducted a retrospective study of 204 patients with SCC of the tongue that aimed to identify the factors that predict relapse and prognosis.
Related: IBD and the Risk of Oral Cancer
In an earlier study, the researchers assessed the best indications for neck dissection and the prognostic factors of oral SCC. Their results showed that middle-late oral SCC is an indication for simultaneous neck dissection, even in patients whose nodes are clear. But few studies have focused on the outcomes of treatment after different surgical approaches, such as en bloc resection and discontinuous resection. En bloc dissection requires continuity between the tumor and the level I neck nodes, and the technique involves removal of the sublingual gland and the mylohyoid muscle, as well as the associated sublingual nodes.
Related: A Team Approach to Nonmelanotic Skin Cancer Procedures
Within the median follow-up of 82 months, 59 patients died (29%), 4 from non-cancer causes. Two patients in the en bloc group and 12 in the control group (discontinuous resection) had relapses. Ten of the 14 in this group had recurrences within a year of the first operation. Nine patients had salvage operations; however, only 2 experienced a successful outcome.
Surgical technique and pathological nodal (pN) status independently predicted both 5-year recurrence and disease-specific survival.
Related: Nivolumab Approved for Expanded Indication
The analysis showed a “dramatic” decrease in the 5-year disease-specific survival if the cancer recurred after the primary operation (no recurrence, 77% vs recurrence, 14%). Patients in the en bloc group “could expect an obviously lower” 5-year recurrence rate (2%, compared with 11% in the control group). They also had a better 5-year disease-specific survival (80%, versus 66%). An aggressive pN status was closely correlated with recurrence.
Source:
Feng Z, Xu QS, Qin LZ, et al. Br J Oral Maxillofac Surg. 2016;54(1):88-93.
doi: 10.1016/j.bjoms.2015.09.024
For patients with squamous cell carcinoma (SCC) of the tongue, recurrence is common and closely associated with survival, say researchers from Capital Medical University, Beijing. The researchers conducted a retrospective study of 204 patients with SCC of the tongue that aimed to identify the factors that predict relapse and prognosis.
Related: IBD and the Risk of Oral Cancer
In an earlier study, the researchers assessed the best indications for neck dissection and the prognostic factors of oral SCC. Their results showed that middle-late oral SCC is an indication for simultaneous neck dissection, even in patients whose nodes are clear. But few studies have focused on the outcomes of treatment after different surgical approaches, such as en bloc resection and discontinuous resection. En bloc dissection requires continuity between the tumor and the level I neck nodes, and the technique involves removal of the sublingual gland and the mylohyoid muscle, as well as the associated sublingual nodes.
Related: A Team Approach to Nonmelanotic Skin Cancer Procedures
Within the median follow-up of 82 months, 59 patients died (29%), 4 from non-cancer causes. Two patients in the en bloc group and 12 in the control group (discontinuous resection) had relapses. Ten of the 14 in this group had recurrences within a year of the first operation. Nine patients had salvage operations; however, only 2 experienced a successful outcome.
Surgical technique and pathological nodal (pN) status independently predicted both 5-year recurrence and disease-specific survival.
Related: Nivolumab Approved for Expanded Indication
The analysis showed a “dramatic” decrease in the 5-year disease-specific survival if the cancer recurred after the primary operation (no recurrence, 77% vs recurrence, 14%). Patients in the en bloc group “could expect an obviously lower” 5-year recurrence rate (2%, compared with 11% in the control group). They also had a better 5-year disease-specific survival (80%, versus 66%). An aggressive pN status was closely correlated with recurrence.
Source:
Feng Z, Xu QS, Qin LZ, et al. Br J Oral Maxillofac Surg. 2016;54(1):88-93.
doi: 10.1016/j.bjoms.2015.09.024