User login
Pigmented Villonodular Synovitis of the Hip: A Systematic Review
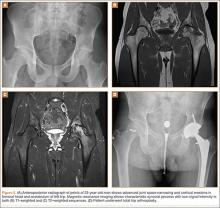
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
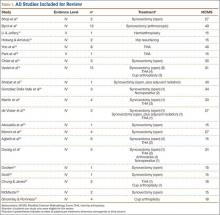
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
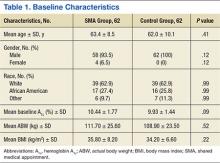
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
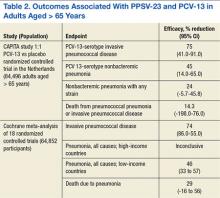
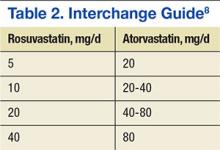
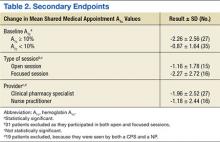
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
Pigmented villonodular synovitis (PVNS) is a rare monoarticular disorder that affects the joints, bursae, or tendon sheaths of 1.8 per million patients.1,2 PVNS is defined by exuberant proliferation of synovial villi and nodules. Although its etiology is unknown, PVNS behaves much as a neoplastic process does, with occasional chromosomal abnormalities, local tissue invasion, and the potential for malignant transformation.3,4 Radiographs show cystic erosions or joint space narrowing, and magnetic resonance imaging shows characteristic low-signal intensity (on T1- and T2-weighted sequences) because of high hemosiderin content. Biopsy remains the gold standard for diagnosis and reveals hemosiderin-laden macrophages, vascularized villi, mononuclear cell infiltration, and sporadic mitotic figures.5 Diffuse PVNS appears as a thickened synovium with matted villi and synovial folds; localized PVNS presents as a pedunculated, firm yellow nodule.6
PVNS has a predilection for large joints, most commonly the knee (up to 80% of cases) and the hip.1,2,7 Treatment strategies for knee PVNS have been well studied and, as an aggregate, show no superiority of arthroscopic or open techniques.8 The literature on hip PVNS is less abundant and more case-based, making it difficult to reach a consensus on effective treatment. Open synovectomy and arthroplasty have been the mainstays of treatment over the past 60 years, but the advent of hip arthroscopy has introduced a new treatment modality.1,9 As arthroscopic management becomes more readily available, it is important to understand and compare the effectiveness of synovectomy and arthroplasty.
We systematically reviewed the treatment modalities for PVNS of the hip to determine how synovectomy and arthroplasty compare with respect to efficacy and revision rates.
Methods
Search Strategy
We systematically reviewed the literature according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using the PRISMA checklist.10 Searches were completed in July 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Keyword selection was designed to capture all level I to V evidence English-language studies that reported clinical and/or radiographic outcomes. This was accomplished with a keyword search of all available titles and manuscript abstracts: (pigmented [Title/Abstract] AND villonodular [Title/Abstract] AND synovitis [Title/Abstract]) AND (hip [Title/Abstract]) AND (English [lang])). Abstracts from the 75 resulting studies were reviewed for exclusion criteria, which consisted of any cadaveric, biomechanical, histologic, and/or kinematic results, as well as a lack of any clinical and/or radiographic data (eg, review or technique articles). Studies were also excluded if they did not have clinical follow-up of at least 2 years. Studies not dedicated to hip PVNS specifically were not immediately excluded but were reviewed for outcomes data specific to the hip PVNS subpopulation. If a specific hip PVNS population could be distinguished from other patients, that study was included for review. If a study could not be deconstructed as such or was entirely devoted to one of our exclusion criteria, that study was excluded from our review. This initial search strategy yielded 16 studies.1,6,7,11-28
Bibliographical review of these 16 studies yielded several more for review. To ensure that no patients were counted twice, each study’s authors, data collection period, and ethnic population were reviewed and compared with those of the other studies. If there was any overlap in authorship, period, and place, only the study with the most relevant or comprehensive data was included. After accounting for all inclusion and exclusion criteria, we selected a total of 21 studies with 82 patients (86 hips) for inclusion (Figure 1).
Data Extraction
Details of study design, sample size, and patient demographics, including age, sex, and duration of symptoms, were recorded. Use of diagnostic biopsy, joint space narrowing on radiographs, treatment method, and use of radiation therapy were also abstracted. Some studies described multiple treatment methods. If those methods could not be differentiated into distinct outcomes groups, the study would have been excluded for lack of specific clinical data. Studies with sufficient data were deconstructed such that the patients from each treatment group were isolated.
Fewer than 5 studies reported physical examination findings, validated survey scores, and/or radiographic results. Therefore, the primary outcomes reported and compared between treatment groups were disease recurrence, clinical worsening defined as progressive pain or loss of function, and revision surgery. Revision surgery was subdivided into repeat synovectomy and eventual arthroplasty, arthrodesis, or revision arthroplasty. Time to revision surgery was also documented. Each study’s methodologic quality and bias were evaluated with the Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues.29 MCMS is a 15-item instrument that has been used to assess randomized and nonrandomized patient trials.30,31 It has a scaled potential score ranging from 0 to 100, with scores from 85 through 100 indicating excellent, 70 through 84 good, 55 through 69 fair, and under 55 poor.
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study reporting on a respective data point, and each mean was then weighted according to the sample size of that study. We multiplied each study’s individual mean by the number of patients enrolled in that study and divided the sum of all the studies’ weighted data points by the number of eligible patients in all relevant studies. The result is that the nonweighted means from studies with a smaller sample size did not carry as much weight as those from larger studies. We then compared 2 groups of patients: those who had only a synovectomy and those who had a combination of synovectomy and arthroplasty. The synovectomy-only group was also compared with a group that underwent total hip arthroplasty (THA) specifically (Figure 2). Groups were compared with Student t test (SPSS Version 18, IBM), and statistical significance was set at α = 0.05.
Results
Twenty-one studies (82 patients) were included in the final dataset (Table 1). Of these studies, 19 were retrospective case series (level IV evidence) in which the number of eligible hip PVNS patients ranged from 1 to 15. The other 2 studies were case reports (level V evidence). Mean (SD) MCMS was 25.0 (10.9).
Fifty-one patients (59.3%) were female. Mean (SD) age of all patients was 33.2 (12.6) years. Mean (SD) duration of symptoms was 4.2 (2.7) years. The right hip was affected in 59.5% of patients in whom laterality was documented. Sixty-eight patients (79.1%) had biopsy-proven PVNS; presence or absence of a biopsy was not documented for the other 18 patients.
Of the 82 patients in the study, 45 (54.9%) underwent synovectomy without arthroplasty. Staged radiation was used to augment the synovectomy in 2 of these 45 cases. One series in this group consisted of 15 cases of arthroscopic synovectomy.1 The 37 patients (45.1%) in the other treatment group had arthroplasty at time of synovectomy. These patients underwent 22 THAs, 8 cup arthroplasties, 2 metal-on-metal hip resurfacings, and 1 hemiarthroplasty. The remaining 4 patients were treated nonoperatively (3) or with primary arthrodesis (1).
Comparisons between the synovectomy-only and synovectomy-with-arthroplasty groups are listed in Table 2. Synovectomy patients were younger on average than arthroplasty patients, but the difference was not statistically significant (P = .28). Only 6 studies distinguished between local and diffuse PVNS histology, and the diffuse type was detected in 87.0%, with insufficient data to detect a difference between the synovectomy and arthroplasty groups. In studies with documented radiographic findings, 75.0% of patients had evidence of joint space narrowing, which was significantly (P = .03) more common in the arthroplasty group (96.7% vs 31.3%).
Mean (SD) clinical follow-up was 8.4 (5.9) years for all patients. A larger percentage of synovectomy-only patients experienced recurrence and worsened symptoms, but neither trend achieved statistical significance. The rate of eventual THA or arthrodesis after synovectomy alone was almost identical (P = .17) to the rate of revision THA in the synovectomy-with-arthroplasty group (26.2% vs 24.3%). Time to revision surgery, however, was significantly (P = .02) longer in the arthroplasty group. Two additional patients in the synovectomy-with-arthroplasty group underwent repeat synovectomy alone, but no patients in the synovectomy-only group underwent repeat synovectomy without arthroplasty.
One nonoperatively managed patient experienced symptom progression over the course of 10 years. The other 2 patients were stable after 2- and 4-year follow-up. The arthrodesis patient did not experience recurrence or have a revision operation in the 5 years after the index procedure.
Discussion
PVNS is a proliferative disorder of synovial tissue with a high risk of recurrence.15,32 Metastasis is extremely rare; there is only 1 case report of a fatality, which occurred within 42 months.12 Chiari and colleagues15 suggested that the PVNS recurrence rate is highest in the large joints. Therefore, in hip PVNS, early surgical resection is needed to limit articular destruction and the potential for recurrence. The primary treatment modalities are synovectomy alone and synovectomy with arthroplasty, which includes THA, cup arthroplasty, hip resurfacing, and hemiarthroplasty. According to our systematic review, about one-fourth of all patients in both treatment groups ultimately underwent revision surgery. Mean time to revision was significantly longer for synovectomy-with-arthroplasty patients (almost 12 years) than for synovectomy-only patients (6.5 years). One potential explanation is that arthroplasty component fixation may take longer to loosen than an inadequately synovectomized joint takes to recur. The synovectomy-only group did have a higher recurrence rate, though the difference was not statistically significant.
Open synovectomy is the most widely described technique for addressing hip PVNS. The precise pathophysiology of PVNS remains largely unknown, but most authors agree that aggressive débridement is required to halt its locally invasive course. Scott24 described the invasion of vascular foramina from synovium into bone and thought that radical synovectomy was essential to remove the stalks of these synovial villi. Furthermore, PVNS most commonly affects adults in the third through fifth decades of life,7 and many surgeons want to avoid prosthetic components (which may loosen over time) in this age group. Synovectomy, however, has persistently high recurrence rates, and, without removal of the femoral head and neck, it can be difficult to obtain adequate exposure for complete débridement. Although adjuvant external beam radiation has been used by some authors,17,19,33 its utility is unproven, and other authors have cautioned against unnecessary irradiation of reproductive organs.1,24,34
The high rates of bony involvement, joint destruction, and recurrence after synovectomy have prompted many surgeons to turn to arthroplasty. González Della Valle and colleagues18 theorized that joint space narrowing is more common in hip PVNS because of the poor distensibility of the hip capsule compared with that of the knee and other joints. In turn, bony lesions and arthritis present earlier in hip PVNS.14 Yoo and colleagues14 found a statistically significant increase in Harris Hip Scale (HHS) scores and a high rate of return to athletic activity after THA for PVNS. However, they also reported revisions for component loosening and osteolysis in 2 of 8 patients and periprosthetic osteolysis without loosening in another 2 patients. Vastel and colleagues16 similarly reported aseptic loosening of the acetabular component in half their patient cohort. No studies have determined which condition—PVNS recurrence or debris-related osteolysis—causes the accelerated loosening in this demographic.
Byrd and colleagues1 recently described use of hip arthroscopy in the treatment of PVNS. In a cohort of 13 patients, they found statistically significant improvements in HHS scores, no postoperative complications, and only 1 revision (THA 6 years after surgery). Although there is a prevailing perception that nodular (vs diffuse) PVNS is more appropriately treated with arthroscopic excision, no studies have provided data on this effect, and Byrd and colleagues1 in fact showed a trend of slightly better outcomes in diffuse cases than in nodular cases. The main challenges of hip arthroscopy are the steep learning curve and adequate exposure. Recent innovations include additional arthroscopic portals and enlarged T-capsulotomy, which may be contributing to decreased complication rates in hip arthroscopy in general.35
The limitations of this systematic review were largely imposed by the studies analyzed. The primary limitation was the relative paucity of clinical and radiographic data on hip PVNS. To our knowledge, studies on the treatment of hip PVNS have reported evidence levels no higher than IV. In addition, the studies we reviewed often had only 1 or 2 patient cases satisfying our inclusion criteria. For this reason, we included case reports, which further lowered the level of evidence of studies used. There were no consistently reported physical examination, survey, or radiographic findings that could be used to compare studies. All studies with sufficient data on hip PVNS treatment outcomes were rated poorly with the Modified Coleman Methodology Scoring system.29 Selection bias was minimized by the inclusive nature of studies with level I to V evidence, but this led to a study design bias in that most studies consisted of level IV evidence.
Conclusion
Although the hip PVNS literature is limited, our review provides insight into expected outcomes. No matter which surgery is to be performed, surgeons must counsel patients about the high revision rate. One in 4 patients ultimately undergoes a second surgery, which may be required within 6 or 7 years after synovectomy without arthroplasty. Further development and innovation in hip arthroscopy may transform the treatment of PVNS. We encourage other investigators to conduct prospective, comparative trials with higher evidence levels to assess the utility of arthroscopy and other treatment modalities.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
1. Byrd JWT, Jones KS, Maiers GP. Two to 10 years’ follow-up of arthroscopic management of pigmented villonodular synovitis in the hip: a case series. Arthroscopy. 2013;29(11):1783-1787.
2. Myers BW, Masi AT. Pigmented villonodular synovitis and tenosynovitis: a clinical epidemiologic study of 166 cases and literature review. Medicine. 1980;59(3):223-238.
3. Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the Chromosomes and Morphology (CHAMP) study group. Mod Pathol. 1999;12(6):576-579.
4. Bertoni F, Unni KK, Beabout JW, Sim FH. Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis). Am J Surg Pathol. 1997;21(2):153-163.
5. Mankin H, Trahan C, Hornicek F. Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103(5):386-389.
6. Martin RC, Osborne DL, Edwards MJ, Wrightson W, McMasters KM. Giant cell tumor of tendon sheath, tenosynovial giant cell tumor, and pigmented villonodular synovitis: defining the presentation, surgical therapy and recurrence. Oncol Rep. 2000;7(2):413-419.
7. Danzig LA, Gershuni DH, Resnick D. Diagnosis and treatment of diffuse pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1982;(168):42-47.
8. Aurégan JC, Klouche S, Bohu Y, Lefèvre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy. 2014;30(10):1327-1341.
9. Gondolph-Zink B, Puhl W, Noack W. Semiarthroscopic synovectomy of the hip. Int Orthop. 1988;12(1):31-35.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
11. Shoji T, Yasunaga Y, Yamasaki T, et al. Transtrochanteric rotational osteotomy combined with intra-articular procedures for pigmented villonodular synovitis of the hip. J Orthop Sci. 2015;20(5):943-950.
12. Li LM, Jeffery J. Exceptionally aggressive pigmented villonodular synovitis of the hip unresponsive to radiotherapy. J Bone Joint Surg Br. 2011;93(7):995-997.
13. Hoberg M, Amstutz HC. Metal-on-metal hip resurfacing in patients with pigmented villonodular synovitis: a report of two cases. Orthopedics. 2010;33(1):50-53.
14. Yoo JJ, Kwon YS, Koo KH, Yoon KS, Min BW, Kim HJ. Cementless total hip arthroplasty performed in patients with pigmented villonodular synovitis. J Arthroplasty. 2010;25(4):552-557.
15. Chiari C, Pirich C, Brannath W, Kotz R, Trieb K. What affects the recurrence and clinical outcome of pigmented villonodular synovitis? Clin Orthop Relat Res. 2006;(450):172-178.
16. Vastel L, Lambert P, De Pinieux G, Charrois O, Kerboull M, Courpied JP. Surgical treatment of pigmented villonodular synovitis of the hip. J Bone Joint Surg Am. 2005;87(5):1019-1024.
17. Shabat S, Kollender Y, Merimsky O, et al. The use of surgery and yttrium 90 in the management of extensive and diffuse pigmented villonodular synovitis of large joints. Rheumatology. 2002;41(10):1113-1118.
18. González Della Valle A, Piccaluga F, Potter HG, Salvati EA, Pusso R. Pigmented villonodular synovitis of the hip: 2- to 23-year followup study. Clin Orthop Relat Res. 2001;(388):187-199.
19. de Visser E, Veth RP, Pruszczynski M, Wobbes T, Van de Putte LB. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch Orthop Trauma Surg. 1999;119(7-8):401-404.
20. Aboulafia AJ, Kaplan L, Jelinek J, Benevenia J, Monson DK. Neuropathy secondary to pigmented villonodular synovitis of the hip. Clin Orthop Relat Res. 1996;(325):174-180.
21. Moroni A, Innao V, Picci P. Pigmented villonodular synovitis of the hip. Study of 9 cases. Ital J Orthop Traumatol. 1983;9(3):331-337.
22. Aglietti P, Di Muria GV, Salvati EA, Stringa G. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Ital J Orthop Traumatol. 1983;9(4):487-496.
23. Docken WP. Pigmented villonodular synovitis: a review with illustrative case reports. Semin Arthritis Rheum. 1979;9(1):1-22.
24. Scott PM. Bone lesions in pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50(2):306-311.
25. Chung SM, Janes JM. Diffuse pigmented villonodular synovitis of the hip joint. Review of the literature and report of four cases. J Bone Joint Surg Am. 1965;47:293-303.
26. McMaster PE. Pigmented villonodular synovitis with invasion of bone. Report of six cases. Rheumatology. 1960;42(7):1170-1183.
27. Ghormley RK, Romness JO. Pigmented villonodular synovitis (xanthomatosis) of the hip joint. Proc Staff Meet Mayo Clin. 1954;29(6):171-180.
28. Park KS, Diwanji SR, Yang HK, Yoon TR, Seon JK. Pigmented villonodular synovitis of the hip presenting as a buttock mass treated by total hip arthroplasty. J Arthroplasty. 2010;25(2):333.e9-e12.
29. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
30. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
31. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
32. Rao AS, Vigorita VJ. Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66(1):76-94.
33. Kat S, Kutz R, Elbracht T, Weseloh G, Kuwert T. Radiosynovectomy in pigmented villonodular synovitis. Nuklearmedizin. 2000;39(7):209-213.
34. Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin Orthop Relat Res. 1989;(239):154-160.
35. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
Compartment Syndrome in Children: Diagnosis and Management
Compartment syndrome (CS) is one of the true orthopedic emergencies. Identifying the high-risk patient, making a prompt diagnosis, and initiating effective treatment are the crucial steps in avoiding a poor outcome. A physician’s inability to communicate with young children can interfere with diagnosing CS in a timely fashion. Many young patients in hospitals are admitted to pediatric floors where routine orthopedic care is not the norm and staff are unfamiliar with the signs and symptoms of evolving CS. As orthopedic surgeons are often involved in caring for these patients, they should be aware of the aspects of CS that are unique to children and should be able to identify patients who are at risk and would benefit from close monitoring. In addition, given the consequences of late diagnosis, early diagnosis is important from a medicolegal standpoint. Only 44% of cases of adult and pediatric CS are decided in favor of treating physicians, compared with 75% of cases in other orthopedic malpractice claims.1,2
Risk Factors for Posttraumatic Compartment Syndrome
Supracondylar Humeral Fracture
CS is a well-described complication of this injury. CS develops in 0.1% to 0.3% of children who present with supracondylar humeral fracture.3,4 Casted elbow flexion beyond 90° and concomitant vascular injury put these children at increased risk for CS. Mubarak and Carroll5 reported 9 cases of CS in the volar compartment of the forearm after an extension-type supracondylar humeral fracture and attributed 8 of them to elbow flexion beyond 90° after closed reduction. In 29 children with supracondylar humeral fracture,Battaglia and colleagues3 found the highest compartment pressure in the deep volar compartment, especially near the fracture site, as well as a significant increase in pressure with the elbow flexed beyond 90°.
In a study of children with supracondylar humeral fracture, Choi and colleagues6 found 2 cases of CS among 9 patients who presented with a pulseless, poorly perfused hand and no cases of CS among 24 patients who presented with a pulseless but well-perfused hand.
Studies have found that a treatment delay of 8 to 12 hours did not increase the rate of CS in Gartland type 2 and type 3 fractures.7-10 The investigators in these studies did not recommend delaying treatment of patients with neurologic deficit and absent radial pulse. Ramachandran and colleagues4 reported 11 cases of CS in patients with low-energy supracondylar humeral fracture and intact radial pulse at presentation. The patients who developed CS presented with severe swelling, and their mean treatment delay was 22 hours (range, 6-64 hours). Given the data, we do not recommend delayed treatment for children with supracondylar humeral fracture and neurologic deficit or absent pulse. We do recommend close inpatient preoperative monitoring of patients with severe swelling.
CS after supracondylar humeral fracture is mostly seen in the volar compartment of the forearm, but it has also been reported in the mobile wad, the anterior arm compartment, and the posterior arm compartment.11,12
Floating Elbow
CS has been reported in children with ipsilateral humeral and forearm fractures. Blakemore and colleagues13 reported a 33% rate of CS in children with displaced distal humeral and forearm fractures. A retrospective review of 16 cases of floating elbow treated at Boston Children’s Hospital found CS in 2 patients and incipient CS in 4 of 10 patients with forearm fractures treated with closed reduction and plaster casting. There were no signs of CS in 6 patients with distal humeral and forearm fractures stabilized with Kirschner wires.14 Given the data, we do not recommend circumferential casting for forearm fractures in children with floating elbow.
Forearm Fracture
Haasbeek and Cole15 reported CS in 5 (11%) of 46 children with open forearm fracture. Yuan and colleagues16 reported CS in 3 (6%) of 50 open forearm fractures and 3 of 30 closed fractures treated with closed reduction and intramedullary nailing. They found increased risk for CS in patients with longer operative time, indicating prolonged closed manipulation of these fractures as a risk factor for CS. They did not find any cases of CS among 205 forearm fractures treated with closed reduction and casting.
Flynn and colleagues17 reported CS in 2 of 30 patients treated with intramedullary nailing within 24 hours of injury and in 0 of 73 patients treated after 24 hours.
Blackman and colleagues18 reported CS in 3 (7.7%) of 39 open forearm fractures and 0 of 74 closed fractures treated operatively. In their series, a small incision was made to facilitate reduction in 38 (51.4%) of 74 closed fractures to decrease closed manipulation and operative time. The rate of CS after intramedullary nailing of closed forearm fractures was lower in this series than in similar reports in the literature.
Reported data indicate increased risk for CS in children with open forearm fractures and fractures treated with closed reduction and intramedullary nailing, especially performed within 24 hours of injury, and prolonged closed manipulation performed during surgery. We recommend close monitoring of all children with operatively treated forearm fractures and, in particular, children with the risk factors mentioned.
Femoral Fracture
Although CS after femoral shaft fractures is not common, CS has been reported after 90/90 spica casting of femoral shaft fractures in children. Mubarak and colleagues19 reported on 9 children who developed calf CS after treatment of femoral shaft fracture in 90/90 spica casts. The technique used in 7 of the 9 reported cases involved initial application of a short leg cast and then traction applied to the leg—believed to cause impinging of the cast on the posterior compartment of the leg. The authors recommended an alternative method of applying spica casts, which is beyond the scope of this review.
Tibial Fracture
Children with tibial fracture, especially a fracture sustained in a motor vehicle accident, are at risk for CS. Hope and Cole20 found CS in 4 (4%) of 92 children with open tibial fracture.
Children with tibial tubercle fracture are at increased risk for CS because of concomitant vascular injury. Pandya and colleagues21 reported CS or vascular compromise in 4 of 40 patients with tibial tubercle fracture. We recommend close monitoring for signs of impending CS in children who present with high-energy tibial shaft fracture and tibial tubercle fracture.
Flynn and colleagues22 reported outcomes of 43 cases of acute CS of the leg in children treated at 2 pediatric trauma centers. Mean time from injury to fasciotomy was 20.5 hours (range, 3.9-118 hours). Functional outcome was excellent at time of follow-up; 41 of 43 cases had no sequelae, and the 2 patients who lost function underwent fasciotomy more than 80 hours after injury. Despite the long interval between injury and surgery, excellent results were achieved with fasciotomy, suggesting an increased potential for recovery in the pediatric population.
Mubarak23 reported on 6 cases of distal tibial physis fracture in patients who presented with severe pain and swelling of the ankle, hyposthesia of the first web space, weakness of the extensor hallucis longus and extensor digitorum communis, and pain on passive flexion of the toes. In all these patients, intramuscular pressure was more than 40 mm Hg beneath the extensor retinaculum and less than 20 mm Hg in the anterior compartment. All patients experienced prompt relief of pain and improved sensation and strength within 24 hours after release of the superior extensor retinaculum and fracture stabilization.
Miscellaneous and Nontraumatic Causes of Compartment Syndrome
Neonatal CS is very rare, and diagnosis is often missed. Neonatal CS is thought to be caused by a combination of low neonatal blood pressure and birth trauma.24 Ragland and colleagues25 reported on 24 cases of neonatal CS; in only 1 case was the diagnosis made within 24 hours.They described a “sentinel skin lesion” on the forearm of each patient as the sign of neonatal CS. Late diagnosis results in contracture and growth arrest of the involved extremity. In their series, only 1 patient underwent fasciotomy within 24 hours, and it resulted in a good functional outcome. High clinical suspicion is the key to early diagnosis and treatment of this rare pathology.
Medical problems that cause intracompartmental bleeding (hepatic failure, renal failure, leukemia, hemophilia) have been cited as causing CS.26-28 CS may be the first symptom of occult hemophilia29 Correction of the coagulation defect may take priority over surgical treatment in these cases, though the decision should be made on a case-by-case basis.26
CS in children can also be caused by snakebites. Shaw and Hosalkar30 reported on successful use of antivenin in preventing the need for surgical treatment in 16 of 19 patients with rattlesnake bites. Two patients had limited surgical débridement, and 1 underwent fasciotomy for CS. The authors recommended using antivenin to prevent CS in children with snakebites.30
Prasarn and colleagues2 reported on 12 cases of upper extremity CS in children in the absence of fractures. Of the 12 patients, 10 were managed in an intensive care unit and had an obtunded sensorium. Etiology in 7 (58%) of the 12 cases was iatrogenic (intravenous infiltration, retained phlebotomy tourniquet). In this series, 4 amputations were performed on affected extremities.
Diagnosis
Identification of evolving CS in a child is difficult because of the child’s limited ability to communicate and anxiety about being examined by a stranger. Orthopedists are trained to look for the 5 Ps (pain, paresthesia, paralysis, pallor, pulselessness) associated with CS. Examining an anxious, frightened young child is difficult, and documenting the degree of pain is not practical in a child who may not be able or willing to communicate effectively.
In a series of 33 children with CS, Bae and colleagues31 found that the 5 Ps were relatively unreliable in making a timely diagnosis. The authors also found that increased analgesic use was documented a mean of 7.3 hours before a change in vascular status and that it was a more sensitive indicator of CS in children. The resulting recommendation is that children at risk for CS be closely monitored for the 3 As (increasing analgesic requirement, anxiety, agitation).32
Regional anesthesia is used to control postoperative pain in adults and children.33,34 Injudicious use may mask the primary symptom (pain) of CS.32,35-38 Use of regional anesthesia in patients at high risk for CS is highly discouraged.
Although CS is a clinical diagnosis, compartment pressure measurements can be useful in making decisions in certain clinical scenarios. In an obtunded child or in a child with severe mental and communication disability, such a measurement can help confirm or rule out the diagnosis.
Normal compartment pressures are higher in children than in adults. Staudt and colleagues39 compared pressures in 4 lower leg compartments of 20 healthy children and 20 healthy adults. Mean pressure varied from 13.3 mm Hg to 16.6 mm Hg in children and from 5.2 mm Hg to 9.7 mm Hg in adults—indicating higher normal pressure in lower leg compartments in children.
Compartment pressures were reported highest within 5 cm of the fracture site.40 When clinically indicated, they should be measured in that area in an injured extremity. The pressure threshold that requires fasciotomy is debatable. Intracompartmental pressures of 30 to 45 mm Hg, or measurements less than 30 mm Hg of diastolic blood pressure (pressure change = diastolic blood pressure – compartment pressure), have been recommended as cutoffs by some authors.41-44 As resting normal compartment pressures are higher in children, these cutoffs cannot be used as reliably in children as in adults. Direct measurement of intracompartmental pressure is invasive and can be difficult in an agitated, awake child. The potential utility of near-infrared spectroscopy in the diagnosis of increased compartment pressure has been reported.45,46 This method uses differential light absorption properties of oxygenated hemoglobin to measure tissue ischemia—similar to the method used in pulse oximetry. Compared with pulse oximetry, near-infrared spectroscopy can sample deeper tissue (3 cm below skin level). Shuler and colleagues45 reported near-infrared spectroscopy findings for 14 adults with acute CS. Lower tissue oxygenation levels correlated with increased intracompartmental pressures, but the authors could not define a cutoff for which near-infrared spectroscopy measurements would indicate significant tissue ischemia. Use of this method in diagnosing CS in children was described in a case report.46
CS remains a clinical diagnosis. Informing family and staff about the signs and symptoms of this syndrome and closely monitoring analgesic use in these patients are crucial. Compartment pressure measurements can be used when the diagnosis is unclear, particularly in noncommunicative patients, but these values should be interpreted with caution.
Treatment
Once CS is diagnosed, emergent fasciotomy and decompression are indicated. Surgeons planning fasciotomy should be aware of the definitive treatment of the CS etiology. Treatment of clotting deficiency in cases caused by excessive bleeding, fracture fixation, and vascular repair may be indicated during fasciotomy and decompression.
Summary
Increased need for analgesics is often the first sign of CS in children and should be considered the sentinel alarm for ongoing tissue necrosis. CS remains a clinical diagnosis, and compartment pressure should be measured only as a confirmatory test in noncommunicative patients or when the diagnosis is unclear. Children with supracondylar humeral fractures, forearm fractures, tibial fractures, and medical risk factors for coagulopathy are at increased risk and should be monitored closely. When the diagnosis is made promptly and the condition is treated with fasciotomy, good long-term clinical results can be expected.
1. Bhattacharyya T, Vrahas MS. The medical-legal aspects of compartment syndrome. J Bone Joint Surg Am. 2004;86(4):864-868.
2. Prasarn ML, Ouellette EA, Livingstone A, Giuffrida AY. Acute pediatric upper extremity compartment syndrome in the absence of fracture. J Pediatr Orthop. 2009;29(3):263-268.
3. Battaglia TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment pressures in children with supracondylar fractures of the humerus. J Pediatr Orthop. 2002;22(4):431-439.
4. Ramachandran M, Skaggs DL, Crawford HA, et al. Delaying treatment of supracondylar fractures in children: has the pendulum swung too far? J Bone Joint Surg Br. 2008;90(9):1228-1233.
5. Mubarak SJ, Carroll NC. Volkmann’s contracture in children: aetiology and prevention. J Bone Joint Surg Br. 1979;61(3):285-293.
6. Choi PD, Melikian R, Skaggs DL. Risk factors for vascular repair and compartment syndrome in the pulseless supracondylar humerus fracture in children. J Pediatr Orthop. 2010;30(1):50-56.
7. Gupta N, Kay RM, Leitch K, Femino JD, Tolo VT, Skaggs DL. Effect of surgical delay on perioperative complications and need for open reduction in supracondylar humerus fractures in children. J Pediatr Orthop. 2004;24(3):245-248.
8. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
9. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
10. Mehlman CT, Strub WM, Roy DR, Wall EJ, Crawford AH. The effect of surgical timing on the perioperative complications of treatment of supracondylar humeral fractures in children. J Bone Joint Surg Am. 2001;83(3):323-327.
11. Diesselhorst MM, Deck JW, Davey JP. Compartment syndrome of the upper arm after closed reduction and percutaneous pinning of a supracondylar humerus fracture. J Pediatr Orthop. 2014;34(2):e1-e4.
12. Mai MC, Beck R, Gabriel K, Singh KA. Posterior arm compartment syndrome after a combined supracondylar humeral and capitellar fractures in an adolescent: a case report. J Pediatr Orthop. 2011;31(3):e16-e19.
13. Blakemore LC, Cooperman DR, Thompson GH, Wathey C, Ballock RT. Compartment syndrome in ipsilateral humerus and forearm fractures in children. Clin Orthop Relat Res. 2000;(376):32-38.
14. Ring D, Waters PM, Hotchkiss RN, Kasser JR. Pediatric floating elbow. J Pediatr Orthop. 2001;21(4):456-459.
15. Haasbeek JF, Cole WG. Open fractures of the arm in children. J Bone Joint Surg Br. 1995;77(4):576-581.
16. Yuan PS, Pring ME, Gaynor TP, Mubarak SJ, Newton PO. Compartment syndrome following intramedullary fixation of pediatric forearm fractures. J Pediatr Orthop. 2004;24(4):370-375.
17. Flynn JM, Jones KJ, Garner MR, Goebel J. Eleven years experience in the operative management of pediatric forearm fractures. J Pediatr Orthop. 2010;30(4):313-319.
18. Blackman AJ, Wall LB, Keeler KA, et al. Acute compartment syndrome after intramedullary nailing of isolated radius and ulna fractures in children. J Pediatr Orthop. 2014;34(1):50-54.
19. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
20. Hope PG, Cole WG. Open fractures of the tibia in children. J Bone Joint Surg Br. 1992;74(4):546-553.
21. Pandya NK, Edmonds EK, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
22. Flynn JM, Bashyal RK, Yeger-McKeever M, Garner MR, Launay F, Sponseller PD. Acute traumatic compartment syndrome of the leg in children: diagnosis and outcome. J Bone Joint Surg Am. 2011;93(10):937-941.
23. Mubarak SJ. Extensor retinaculum syndrome of the ankle after injury to the distal tibial physis. J Bone Joint Surg Br. 2002;84(1):11-14.
24. Macer GA Jr. Forearm compartment syndrome in the newborn. J Hand Surg Am. 2006;31(9):1550.
25. Ragland R 3rd, Moukoko D, Ezaki M, Carter PR, Mills J. Forearm compartment syndrome in the newborn: report of 24 cases. J Hand Surg Am. 2005;30(5):997-1003.
26. Alioglu B, Avci Z, Baskin E, Ozcay F, Tuncay IC, Ozbek N. Successful use of recombinant factor VIIa (NovoSeven) in children with compartment syndrome: two case reports. J Pediatr Orthop. 2006;26(6):815-817.
27. Lee DK, Jeong WK, Lee DH, Lee SH. Multiple compartment syndrome in a pediatric patient with CML. J Pediatr Orthop. 2011;31(8):889-892.
28. Dumontier C, Sautet A, Man M, Bennani M, Apoil A. Entrapment and compartment syndromes of the upper limb in haemophilia. J Hand Surg Br. 1994;19(4):427-429.
29. Jones G, Thompson K, Johnson M. Acute compartment syndrome after minor trauma in a patient with undiagnosed mild haemophilia B. Lancet. 2013;382(9905):1678.
30. Shaw BA, Hosalkar HS. Rattlesnake bites in children: antivenin treatment and surgical indications. J Bone Joint Surg Am. 2002;84(9):1624-1629.
31. Bae DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21(5):680-688.
32. Noonan KJ, McCarthy JJ. Compartment syndromes in the pediatric patient. J Pediatr Orthop. 2010;30(2 suppl):S96-S101.
33. Dalens B. Some current controversies in paediatric regional anaesthesia. Curr Opin Anaesthesiol. 2006;19(3):301-308.
34. Wedel DJ. Regional anesthesia and pain management: reviewing the past decade and predicting the future. Anesth Analg. 2000;90(5):1244-1245.
35. Mubarak SJ. Wilton NC. Compartment syndromes and epidural analgesia. J Pediatr Orthop. 1997;17(3):282-284.
36. Price C, Ribeiro J, Kinnebrew T. Compartment syndromes associated with postoperative epidural analgesia. A case report. J Bone Joint Surg Am. 1996;78(4):597-599.
37. Thonse R, Ashford RU, Williams TI, Harrington P. Differences in attitudes to analgesia in post-operative limb surgery put patients at risk of compartment syndrome. Injury. 2004;35(3):290-295.
38. Whitesides TE Jr. Pain: friend or foe? J Bone Joint Surg Am. 2001;83(9):1424-1425.
39. Staudt JM, Smeulders MJ, van der Horst CM. Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br. 2008;90(2):215-219.
40. Heckman MM, Whitesides TE Jr, Grewe SR, Rooks MD. Compartment pressure in association with closed tibial fractures. The relationship between tissue pressure, compartment, and the distance from the site of the fracture. J Bone Joint Surg Am. 1994;76(9):1285-1292.
41. Hargens AR, Schmidt DA, Evans KL, et al. Quantitation of skeletal-muscle necrosis in a model compartment syndrome. J Bone Joint Surg Am. 1981;63(4):631-636.
42. Heppenstall RB, Sapega AA, Scott R, et al. The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;(226):138-155.
43. McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99-104.
44. Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66(1):93-97.
45. Shuler MS, Reisman WM, Kinsey TL, et al. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92(4):863-870.
46. Tobias JD, Hoernschemeyer DG. Near-infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27(3):311-313.
Compartment syndrome (CS) is one of the true orthopedic emergencies. Identifying the high-risk patient, making a prompt diagnosis, and initiating effective treatment are the crucial steps in avoiding a poor outcome. A physician’s inability to communicate with young children can interfere with diagnosing CS in a timely fashion. Many young patients in hospitals are admitted to pediatric floors where routine orthopedic care is not the norm and staff are unfamiliar with the signs and symptoms of evolving CS. As orthopedic surgeons are often involved in caring for these patients, they should be aware of the aspects of CS that are unique to children and should be able to identify patients who are at risk and would benefit from close monitoring. In addition, given the consequences of late diagnosis, early diagnosis is important from a medicolegal standpoint. Only 44% of cases of adult and pediatric CS are decided in favor of treating physicians, compared with 75% of cases in other orthopedic malpractice claims.1,2
Risk Factors for Posttraumatic Compartment Syndrome
Supracondylar Humeral Fracture
CS is a well-described complication of this injury. CS develops in 0.1% to 0.3% of children who present with supracondylar humeral fracture.3,4 Casted elbow flexion beyond 90° and concomitant vascular injury put these children at increased risk for CS. Mubarak and Carroll5 reported 9 cases of CS in the volar compartment of the forearm after an extension-type supracondylar humeral fracture and attributed 8 of them to elbow flexion beyond 90° after closed reduction. In 29 children with supracondylar humeral fracture,Battaglia and colleagues3 found the highest compartment pressure in the deep volar compartment, especially near the fracture site, as well as a significant increase in pressure with the elbow flexed beyond 90°.
In a study of children with supracondylar humeral fracture, Choi and colleagues6 found 2 cases of CS among 9 patients who presented with a pulseless, poorly perfused hand and no cases of CS among 24 patients who presented with a pulseless but well-perfused hand.
Studies have found that a treatment delay of 8 to 12 hours did not increase the rate of CS in Gartland type 2 and type 3 fractures.7-10 The investigators in these studies did not recommend delaying treatment of patients with neurologic deficit and absent radial pulse. Ramachandran and colleagues4 reported 11 cases of CS in patients with low-energy supracondylar humeral fracture and intact radial pulse at presentation. The patients who developed CS presented with severe swelling, and their mean treatment delay was 22 hours (range, 6-64 hours). Given the data, we do not recommend delayed treatment for children with supracondylar humeral fracture and neurologic deficit or absent pulse. We do recommend close inpatient preoperative monitoring of patients with severe swelling.
CS after supracondylar humeral fracture is mostly seen in the volar compartment of the forearm, but it has also been reported in the mobile wad, the anterior arm compartment, and the posterior arm compartment.11,12
Floating Elbow
CS has been reported in children with ipsilateral humeral and forearm fractures. Blakemore and colleagues13 reported a 33% rate of CS in children with displaced distal humeral and forearm fractures. A retrospective review of 16 cases of floating elbow treated at Boston Children’s Hospital found CS in 2 patients and incipient CS in 4 of 10 patients with forearm fractures treated with closed reduction and plaster casting. There were no signs of CS in 6 patients with distal humeral and forearm fractures stabilized with Kirschner wires.14 Given the data, we do not recommend circumferential casting for forearm fractures in children with floating elbow.
Forearm Fracture
Haasbeek and Cole15 reported CS in 5 (11%) of 46 children with open forearm fracture. Yuan and colleagues16 reported CS in 3 (6%) of 50 open forearm fractures and 3 of 30 closed fractures treated with closed reduction and intramedullary nailing. They found increased risk for CS in patients with longer operative time, indicating prolonged closed manipulation of these fractures as a risk factor for CS. They did not find any cases of CS among 205 forearm fractures treated with closed reduction and casting.
Flynn and colleagues17 reported CS in 2 of 30 patients treated with intramedullary nailing within 24 hours of injury and in 0 of 73 patients treated after 24 hours.
Blackman and colleagues18 reported CS in 3 (7.7%) of 39 open forearm fractures and 0 of 74 closed fractures treated operatively. In their series, a small incision was made to facilitate reduction in 38 (51.4%) of 74 closed fractures to decrease closed manipulation and operative time. The rate of CS after intramedullary nailing of closed forearm fractures was lower in this series than in similar reports in the literature.
Reported data indicate increased risk for CS in children with open forearm fractures and fractures treated with closed reduction and intramedullary nailing, especially performed within 24 hours of injury, and prolonged closed manipulation performed during surgery. We recommend close monitoring of all children with operatively treated forearm fractures and, in particular, children with the risk factors mentioned.
Femoral Fracture
Although CS after femoral shaft fractures is not common, CS has been reported after 90/90 spica casting of femoral shaft fractures in children. Mubarak and colleagues19 reported on 9 children who developed calf CS after treatment of femoral shaft fracture in 90/90 spica casts. The technique used in 7 of the 9 reported cases involved initial application of a short leg cast and then traction applied to the leg—believed to cause impinging of the cast on the posterior compartment of the leg. The authors recommended an alternative method of applying spica casts, which is beyond the scope of this review.
Tibial Fracture
Children with tibial fracture, especially a fracture sustained in a motor vehicle accident, are at risk for CS. Hope and Cole20 found CS in 4 (4%) of 92 children with open tibial fracture.
Children with tibial tubercle fracture are at increased risk for CS because of concomitant vascular injury. Pandya and colleagues21 reported CS or vascular compromise in 4 of 40 patients with tibial tubercle fracture. We recommend close monitoring for signs of impending CS in children who present with high-energy tibial shaft fracture and tibial tubercle fracture.
Flynn and colleagues22 reported outcomes of 43 cases of acute CS of the leg in children treated at 2 pediatric trauma centers. Mean time from injury to fasciotomy was 20.5 hours (range, 3.9-118 hours). Functional outcome was excellent at time of follow-up; 41 of 43 cases had no sequelae, and the 2 patients who lost function underwent fasciotomy more than 80 hours after injury. Despite the long interval between injury and surgery, excellent results were achieved with fasciotomy, suggesting an increased potential for recovery in the pediatric population.
Mubarak23 reported on 6 cases of distal tibial physis fracture in patients who presented with severe pain and swelling of the ankle, hyposthesia of the first web space, weakness of the extensor hallucis longus and extensor digitorum communis, and pain on passive flexion of the toes. In all these patients, intramuscular pressure was more than 40 mm Hg beneath the extensor retinaculum and less than 20 mm Hg in the anterior compartment. All patients experienced prompt relief of pain and improved sensation and strength within 24 hours after release of the superior extensor retinaculum and fracture stabilization.
Miscellaneous and Nontraumatic Causes of Compartment Syndrome
Neonatal CS is very rare, and diagnosis is often missed. Neonatal CS is thought to be caused by a combination of low neonatal blood pressure and birth trauma.24 Ragland and colleagues25 reported on 24 cases of neonatal CS; in only 1 case was the diagnosis made within 24 hours.They described a “sentinel skin lesion” on the forearm of each patient as the sign of neonatal CS. Late diagnosis results in contracture and growth arrest of the involved extremity. In their series, only 1 patient underwent fasciotomy within 24 hours, and it resulted in a good functional outcome. High clinical suspicion is the key to early diagnosis and treatment of this rare pathology.
Medical problems that cause intracompartmental bleeding (hepatic failure, renal failure, leukemia, hemophilia) have been cited as causing CS.26-28 CS may be the first symptom of occult hemophilia29 Correction of the coagulation defect may take priority over surgical treatment in these cases, though the decision should be made on a case-by-case basis.26
CS in children can also be caused by snakebites. Shaw and Hosalkar30 reported on successful use of antivenin in preventing the need for surgical treatment in 16 of 19 patients with rattlesnake bites. Two patients had limited surgical débridement, and 1 underwent fasciotomy for CS. The authors recommended using antivenin to prevent CS in children with snakebites.30
Prasarn and colleagues2 reported on 12 cases of upper extremity CS in children in the absence of fractures. Of the 12 patients, 10 were managed in an intensive care unit and had an obtunded sensorium. Etiology in 7 (58%) of the 12 cases was iatrogenic (intravenous infiltration, retained phlebotomy tourniquet). In this series, 4 amputations were performed on affected extremities.
Diagnosis
Identification of evolving CS in a child is difficult because of the child’s limited ability to communicate and anxiety about being examined by a stranger. Orthopedists are trained to look for the 5 Ps (pain, paresthesia, paralysis, pallor, pulselessness) associated with CS. Examining an anxious, frightened young child is difficult, and documenting the degree of pain is not practical in a child who may not be able or willing to communicate effectively.
In a series of 33 children with CS, Bae and colleagues31 found that the 5 Ps were relatively unreliable in making a timely diagnosis. The authors also found that increased analgesic use was documented a mean of 7.3 hours before a change in vascular status and that it was a more sensitive indicator of CS in children. The resulting recommendation is that children at risk for CS be closely monitored for the 3 As (increasing analgesic requirement, anxiety, agitation).32
Regional anesthesia is used to control postoperative pain in adults and children.33,34 Injudicious use may mask the primary symptom (pain) of CS.32,35-38 Use of regional anesthesia in patients at high risk for CS is highly discouraged.
Although CS is a clinical diagnosis, compartment pressure measurements can be useful in making decisions in certain clinical scenarios. In an obtunded child or in a child with severe mental and communication disability, such a measurement can help confirm or rule out the diagnosis.
Normal compartment pressures are higher in children than in adults. Staudt and colleagues39 compared pressures in 4 lower leg compartments of 20 healthy children and 20 healthy adults. Mean pressure varied from 13.3 mm Hg to 16.6 mm Hg in children and from 5.2 mm Hg to 9.7 mm Hg in adults—indicating higher normal pressure in lower leg compartments in children.
Compartment pressures were reported highest within 5 cm of the fracture site.40 When clinically indicated, they should be measured in that area in an injured extremity. The pressure threshold that requires fasciotomy is debatable. Intracompartmental pressures of 30 to 45 mm Hg, or measurements less than 30 mm Hg of diastolic blood pressure (pressure change = diastolic blood pressure – compartment pressure), have been recommended as cutoffs by some authors.41-44 As resting normal compartment pressures are higher in children, these cutoffs cannot be used as reliably in children as in adults. Direct measurement of intracompartmental pressure is invasive and can be difficult in an agitated, awake child. The potential utility of near-infrared spectroscopy in the diagnosis of increased compartment pressure has been reported.45,46 This method uses differential light absorption properties of oxygenated hemoglobin to measure tissue ischemia—similar to the method used in pulse oximetry. Compared with pulse oximetry, near-infrared spectroscopy can sample deeper tissue (3 cm below skin level). Shuler and colleagues45 reported near-infrared spectroscopy findings for 14 adults with acute CS. Lower tissue oxygenation levels correlated with increased intracompartmental pressures, but the authors could not define a cutoff for which near-infrared spectroscopy measurements would indicate significant tissue ischemia. Use of this method in diagnosing CS in children was described in a case report.46
CS remains a clinical diagnosis. Informing family and staff about the signs and symptoms of this syndrome and closely monitoring analgesic use in these patients are crucial. Compartment pressure measurements can be used when the diagnosis is unclear, particularly in noncommunicative patients, but these values should be interpreted with caution.
Treatment
Once CS is diagnosed, emergent fasciotomy and decompression are indicated. Surgeons planning fasciotomy should be aware of the definitive treatment of the CS etiology. Treatment of clotting deficiency in cases caused by excessive bleeding, fracture fixation, and vascular repair may be indicated during fasciotomy and decompression.
Summary
Increased need for analgesics is often the first sign of CS in children and should be considered the sentinel alarm for ongoing tissue necrosis. CS remains a clinical diagnosis, and compartment pressure should be measured only as a confirmatory test in noncommunicative patients or when the diagnosis is unclear. Children with supracondylar humeral fractures, forearm fractures, tibial fractures, and medical risk factors for coagulopathy are at increased risk and should be monitored closely. When the diagnosis is made promptly and the condition is treated with fasciotomy, good long-term clinical results can be expected.
Compartment syndrome (CS) is one of the true orthopedic emergencies. Identifying the high-risk patient, making a prompt diagnosis, and initiating effective treatment are the crucial steps in avoiding a poor outcome. A physician’s inability to communicate with young children can interfere with diagnosing CS in a timely fashion. Many young patients in hospitals are admitted to pediatric floors where routine orthopedic care is not the norm and staff are unfamiliar with the signs and symptoms of evolving CS. As orthopedic surgeons are often involved in caring for these patients, they should be aware of the aspects of CS that are unique to children and should be able to identify patients who are at risk and would benefit from close monitoring. In addition, given the consequences of late diagnosis, early diagnosis is important from a medicolegal standpoint. Only 44% of cases of adult and pediatric CS are decided in favor of treating physicians, compared with 75% of cases in other orthopedic malpractice claims.1,2
Risk Factors for Posttraumatic Compartment Syndrome
Supracondylar Humeral Fracture
CS is a well-described complication of this injury. CS develops in 0.1% to 0.3% of children who present with supracondylar humeral fracture.3,4 Casted elbow flexion beyond 90° and concomitant vascular injury put these children at increased risk for CS. Mubarak and Carroll5 reported 9 cases of CS in the volar compartment of the forearm after an extension-type supracondylar humeral fracture and attributed 8 of them to elbow flexion beyond 90° after closed reduction. In 29 children with supracondylar humeral fracture,Battaglia and colleagues3 found the highest compartment pressure in the deep volar compartment, especially near the fracture site, as well as a significant increase in pressure with the elbow flexed beyond 90°.
In a study of children with supracondylar humeral fracture, Choi and colleagues6 found 2 cases of CS among 9 patients who presented with a pulseless, poorly perfused hand and no cases of CS among 24 patients who presented with a pulseless but well-perfused hand.
Studies have found that a treatment delay of 8 to 12 hours did not increase the rate of CS in Gartland type 2 and type 3 fractures.7-10 The investigators in these studies did not recommend delaying treatment of patients with neurologic deficit and absent radial pulse. Ramachandran and colleagues4 reported 11 cases of CS in patients with low-energy supracondylar humeral fracture and intact radial pulse at presentation. The patients who developed CS presented with severe swelling, and their mean treatment delay was 22 hours (range, 6-64 hours). Given the data, we do not recommend delayed treatment for children with supracondylar humeral fracture and neurologic deficit or absent pulse. We do recommend close inpatient preoperative monitoring of patients with severe swelling.
CS after supracondylar humeral fracture is mostly seen in the volar compartment of the forearm, but it has also been reported in the mobile wad, the anterior arm compartment, and the posterior arm compartment.11,12
Floating Elbow
CS has been reported in children with ipsilateral humeral and forearm fractures. Blakemore and colleagues13 reported a 33% rate of CS in children with displaced distal humeral and forearm fractures. A retrospective review of 16 cases of floating elbow treated at Boston Children’s Hospital found CS in 2 patients and incipient CS in 4 of 10 patients with forearm fractures treated with closed reduction and plaster casting. There were no signs of CS in 6 patients with distal humeral and forearm fractures stabilized with Kirschner wires.14 Given the data, we do not recommend circumferential casting for forearm fractures in children with floating elbow.
Forearm Fracture
Haasbeek and Cole15 reported CS in 5 (11%) of 46 children with open forearm fracture. Yuan and colleagues16 reported CS in 3 (6%) of 50 open forearm fractures and 3 of 30 closed fractures treated with closed reduction and intramedullary nailing. They found increased risk for CS in patients with longer operative time, indicating prolonged closed manipulation of these fractures as a risk factor for CS. They did not find any cases of CS among 205 forearm fractures treated with closed reduction and casting.
Flynn and colleagues17 reported CS in 2 of 30 patients treated with intramedullary nailing within 24 hours of injury and in 0 of 73 patients treated after 24 hours.
Blackman and colleagues18 reported CS in 3 (7.7%) of 39 open forearm fractures and 0 of 74 closed fractures treated operatively. In their series, a small incision was made to facilitate reduction in 38 (51.4%) of 74 closed fractures to decrease closed manipulation and operative time. The rate of CS after intramedullary nailing of closed forearm fractures was lower in this series than in similar reports in the literature.
Reported data indicate increased risk for CS in children with open forearm fractures and fractures treated with closed reduction and intramedullary nailing, especially performed within 24 hours of injury, and prolonged closed manipulation performed during surgery. We recommend close monitoring of all children with operatively treated forearm fractures and, in particular, children with the risk factors mentioned.
Femoral Fracture
Although CS after femoral shaft fractures is not common, CS has been reported after 90/90 spica casting of femoral shaft fractures in children. Mubarak and colleagues19 reported on 9 children who developed calf CS after treatment of femoral shaft fracture in 90/90 spica casts. The technique used in 7 of the 9 reported cases involved initial application of a short leg cast and then traction applied to the leg—believed to cause impinging of the cast on the posterior compartment of the leg. The authors recommended an alternative method of applying spica casts, which is beyond the scope of this review.
Tibial Fracture
Children with tibial fracture, especially a fracture sustained in a motor vehicle accident, are at risk for CS. Hope and Cole20 found CS in 4 (4%) of 92 children with open tibial fracture.
Children with tibial tubercle fracture are at increased risk for CS because of concomitant vascular injury. Pandya and colleagues21 reported CS or vascular compromise in 4 of 40 patients with tibial tubercle fracture. We recommend close monitoring for signs of impending CS in children who present with high-energy tibial shaft fracture and tibial tubercle fracture.
Flynn and colleagues22 reported outcomes of 43 cases of acute CS of the leg in children treated at 2 pediatric trauma centers. Mean time from injury to fasciotomy was 20.5 hours (range, 3.9-118 hours). Functional outcome was excellent at time of follow-up; 41 of 43 cases had no sequelae, and the 2 patients who lost function underwent fasciotomy more than 80 hours after injury. Despite the long interval between injury and surgery, excellent results were achieved with fasciotomy, suggesting an increased potential for recovery in the pediatric population.
Mubarak23 reported on 6 cases of distal tibial physis fracture in patients who presented with severe pain and swelling of the ankle, hyposthesia of the first web space, weakness of the extensor hallucis longus and extensor digitorum communis, and pain on passive flexion of the toes. In all these patients, intramuscular pressure was more than 40 mm Hg beneath the extensor retinaculum and less than 20 mm Hg in the anterior compartment. All patients experienced prompt relief of pain and improved sensation and strength within 24 hours after release of the superior extensor retinaculum and fracture stabilization.
Miscellaneous and Nontraumatic Causes of Compartment Syndrome
Neonatal CS is very rare, and diagnosis is often missed. Neonatal CS is thought to be caused by a combination of low neonatal blood pressure and birth trauma.24 Ragland and colleagues25 reported on 24 cases of neonatal CS; in only 1 case was the diagnosis made within 24 hours.They described a “sentinel skin lesion” on the forearm of each patient as the sign of neonatal CS. Late diagnosis results in contracture and growth arrest of the involved extremity. In their series, only 1 patient underwent fasciotomy within 24 hours, and it resulted in a good functional outcome. High clinical suspicion is the key to early diagnosis and treatment of this rare pathology.
Medical problems that cause intracompartmental bleeding (hepatic failure, renal failure, leukemia, hemophilia) have been cited as causing CS.26-28 CS may be the first symptom of occult hemophilia29 Correction of the coagulation defect may take priority over surgical treatment in these cases, though the decision should be made on a case-by-case basis.26
CS in children can also be caused by snakebites. Shaw and Hosalkar30 reported on successful use of antivenin in preventing the need for surgical treatment in 16 of 19 patients with rattlesnake bites. Two patients had limited surgical débridement, and 1 underwent fasciotomy for CS. The authors recommended using antivenin to prevent CS in children with snakebites.30
Prasarn and colleagues2 reported on 12 cases of upper extremity CS in children in the absence of fractures. Of the 12 patients, 10 were managed in an intensive care unit and had an obtunded sensorium. Etiology in 7 (58%) of the 12 cases was iatrogenic (intravenous infiltration, retained phlebotomy tourniquet). In this series, 4 amputations were performed on affected extremities.
Diagnosis
Identification of evolving CS in a child is difficult because of the child’s limited ability to communicate and anxiety about being examined by a stranger. Orthopedists are trained to look for the 5 Ps (pain, paresthesia, paralysis, pallor, pulselessness) associated with CS. Examining an anxious, frightened young child is difficult, and documenting the degree of pain is not practical in a child who may not be able or willing to communicate effectively.
In a series of 33 children with CS, Bae and colleagues31 found that the 5 Ps were relatively unreliable in making a timely diagnosis. The authors also found that increased analgesic use was documented a mean of 7.3 hours before a change in vascular status and that it was a more sensitive indicator of CS in children. The resulting recommendation is that children at risk for CS be closely monitored for the 3 As (increasing analgesic requirement, anxiety, agitation).32
Regional anesthesia is used to control postoperative pain in adults and children.33,34 Injudicious use may mask the primary symptom (pain) of CS.32,35-38 Use of regional anesthesia in patients at high risk for CS is highly discouraged.
Although CS is a clinical diagnosis, compartment pressure measurements can be useful in making decisions in certain clinical scenarios. In an obtunded child or in a child with severe mental and communication disability, such a measurement can help confirm or rule out the diagnosis.
Normal compartment pressures are higher in children than in adults. Staudt and colleagues39 compared pressures in 4 lower leg compartments of 20 healthy children and 20 healthy adults. Mean pressure varied from 13.3 mm Hg to 16.6 mm Hg in children and from 5.2 mm Hg to 9.7 mm Hg in adults—indicating higher normal pressure in lower leg compartments in children.
Compartment pressures were reported highest within 5 cm of the fracture site.40 When clinically indicated, they should be measured in that area in an injured extremity. The pressure threshold that requires fasciotomy is debatable. Intracompartmental pressures of 30 to 45 mm Hg, or measurements less than 30 mm Hg of diastolic blood pressure (pressure change = diastolic blood pressure – compartment pressure), have been recommended as cutoffs by some authors.41-44 As resting normal compartment pressures are higher in children, these cutoffs cannot be used as reliably in children as in adults. Direct measurement of intracompartmental pressure is invasive and can be difficult in an agitated, awake child. The potential utility of near-infrared spectroscopy in the diagnosis of increased compartment pressure has been reported.45,46 This method uses differential light absorption properties of oxygenated hemoglobin to measure tissue ischemia—similar to the method used in pulse oximetry. Compared with pulse oximetry, near-infrared spectroscopy can sample deeper tissue (3 cm below skin level). Shuler and colleagues45 reported near-infrared spectroscopy findings for 14 adults with acute CS. Lower tissue oxygenation levels correlated with increased intracompartmental pressures, but the authors could not define a cutoff for which near-infrared spectroscopy measurements would indicate significant tissue ischemia. Use of this method in diagnosing CS in children was described in a case report.46
CS remains a clinical diagnosis. Informing family and staff about the signs and symptoms of this syndrome and closely monitoring analgesic use in these patients are crucial. Compartment pressure measurements can be used when the diagnosis is unclear, particularly in noncommunicative patients, but these values should be interpreted with caution.
Treatment
Once CS is diagnosed, emergent fasciotomy and decompression are indicated. Surgeons planning fasciotomy should be aware of the definitive treatment of the CS etiology. Treatment of clotting deficiency in cases caused by excessive bleeding, fracture fixation, and vascular repair may be indicated during fasciotomy and decompression.
Summary
Increased need for analgesics is often the first sign of CS in children and should be considered the sentinel alarm for ongoing tissue necrosis. CS remains a clinical diagnosis, and compartment pressure should be measured only as a confirmatory test in noncommunicative patients or when the diagnosis is unclear. Children with supracondylar humeral fractures, forearm fractures, tibial fractures, and medical risk factors for coagulopathy are at increased risk and should be monitored closely. When the diagnosis is made promptly and the condition is treated with fasciotomy, good long-term clinical results can be expected.
1. Bhattacharyya T, Vrahas MS. The medical-legal aspects of compartment syndrome. J Bone Joint Surg Am. 2004;86(4):864-868.
2. Prasarn ML, Ouellette EA, Livingstone A, Giuffrida AY. Acute pediatric upper extremity compartment syndrome in the absence of fracture. J Pediatr Orthop. 2009;29(3):263-268.
3. Battaglia TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment pressures in children with supracondylar fractures of the humerus. J Pediatr Orthop. 2002;22(4):431-439.
4. Ramachandran M, Skaggs DL, Crawford HA, et al. Delaying treatment of supracondylar fractures in children: has the pendulum swung too far? J Bone Joint Surg Br. 2008;90(9):1228-1233.
5. Mubarak SJ, Carroll NC. Volkmann’s contracture in children: aetiology and prevention. J Bone Joint Surg Br. 1979;61(3):285-293.
6. Choi PD, Melikian R, Skaggs DL. Risk factors for vascular repair and compartment syndrome in the pulseless supracondylar humerus fracture in children. J Pediatr Orthop. 2010;30(1):50-56.
7. Gupta N, Kay RM, Leitch K, Femino JD, Tolo VT, Skaggs DL. Effect of surgical delay on perioperative complications and need for open reduction in supracondylar humerus fractures in children. J Pediatr Orthop. 2004;24(3):245-248.
8. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
9. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
10. Mehlman CT, Strub WM, Roy DR, Wall EJ, Crawford AH. The effect of surgical timing on the perioperative complications of treatment of supracondylar humeral fractures in children. J Bone Joint Surg Am. 2001;83(3):323-327.
11. Diesselhorst MM, Deck JW, Davey JP. Compartment syndrome of the upper arm after closed reduction and percutaneous pinning of a supracondylar humerus fracture. J Pediatr Orthop. 2014;34(2):e1-e4.
12. Mai MC, Beck R, Gabriel K, Singh KA. Posterior arm compartment syndrome after a combined supracondylar humeral and capitellar fractures in an adolescent: a case report. J Pediatr Orthop. 2011;31(3):e16-e19.
13. Blakemore LC, Cooperman DR, Thompson GH, Wathey C, Ballock RT. Compartment syndrome in ipsilateral humerus and forearm fractures in children. Clin Orthop Relat Res. 2000;(376):32-38.
14. Ring D, Waters PM, Hotchkiss RN, Kasser JR. Pediatric floating elbow. J Pediatr Orthop. 2001;21(4):456-459.
15. Haasbeek JF, Cole WG. Open fractures of the arm in children. J Bone Joint Surg Br. 1995;77(4):576-581.
16. Yuan PS, Pring ME, Gaynor TP, Mubarak SJ, Newton PO. Compartment syndrome following intramedullary fixation of pediatric forearm fractures. J Pediatr Orthop. 2004;24(4):370-375.
17. Flynn JM, Jones KJ, Garner MR, Goebel J. Eleven years experience in the operative management of pediatric forearm fractures. J Pediatr Orthop. 2010;30(4):313-319.
18. Blackman AJ, Wall LB, Keeler KA, et al. Acute compartment syndrome after intramedullary nailing of isolated radius and ulna fractures in children. J Pediatr Orthop. 2014;34(1):50-54.
19. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
20. Hope PG, Cole WG. Open fractures of the tibia in children. J Bone Joint Surg Br. 1992;74(4):546-553.
21. Pandya NK, Edmonds EK, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
22. Flynn JM, Bashyal RK, Yeger-McKeever M, Garner MR, Launay F, Sponseller PD. Acute traumatic compartment syndrome of the leg in children: diagnosis and outcome. J Bone Joint Surg Am. 2011;93(10):937-941.
23. Mubarak SJ. Extensor retinaculum syndrome of the ankle after injury to the distal tibial physis. J Bone Joint Surg Br. 2002;84(1):11-14.
24. Macer GA Jr. Forearm compartment syndrome in the newborn. J Hand Surg Am. 2006;31(9):1550.
25. Ragland R 3rd, Moukoko D, Ezaki M, Carter PR, Mills J. Forearm compartment syndrome in the newborn: report of 24 cases. J Hand Surg Am. 2005;30(5):997-1003.
26. Alioglu B, Avci Z, Baskin E, Ozcay F, Tuncay IC, Ozbek N. Successful use of recombinant factor VIIa (NovoSeven) in children with compartment syndrome: two case reports. J Pediatr Orthop. 2006;26(6):815-817.
27. Lee DK, Jeong WK, Lee DH, Lee SH. Multiple compartment syndrome in a pediatric patient with CML. J Pediatr Orthop. 2011;31(8):889-892.
28. Dumontier C, Sautet A, Man M, Bennani M, Apoil A. Entrapment and compartment syndromes of the upper limb in haemophilia. J Hand Surg Br. 1994;19(4):427-429.
29. Jones G, Thompson K, Johnson M. Acute compartment syndrome after minor trauma in a patient with undiagnosed mild haemophilia B. Lancet. 2013;382(9905):1678.
30. Shaw BA, Hosalkar HS. Rattlesnake bites in children: antivenin treatment and surgical indications. J Bone Joint Surg Am. 2002;84(9):1624-1629.
31. Bae DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21(5):680-688.
32. Noonan KJ, McCarthy JJ. Compartment syndromes in the pediatric patient. J Pediatr Orthop. 2010;30(2 suppl):S96-S101.
33. Dalens B. Some current controversies in paediatric regional anaesthesia. Curr Opin Anaesthesiol. 2006;19(3):301-308.
34. Wedel DJ. Regional anesthesia and pain management: reviewing the past decade and predicting the future. Anesth Analg. 2000;90(5):1244-1245.
35. Mubarak SJ. Wilton NC. Compartment syndromes and epidural analgesia. J Pediatr Orthop. 1997;17(3):282-284.
36. Price C, Ribeiro J, Kinnebrew T. Compartment syndromes associated with postoperative epidural analgesia. A case report. J Bone Joint Surg Am. 1996;78(4):597-599.
37. Thonse R, Ashford RU, Williams TI, Harrington P. Differences in attitudes to analgesia in post-operative limb surgery put patients at risk of compartment syndrome. Injury. 2004;35(3):290-295.
38. Whitesides TE Jr. Pain: friend or foe? J Bone Joint Surg Am. 2001;83(9):1424-1425.
39. Staudt JM, Smeulders MJ, van der Horst CM. Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br. 2008;90(2):215-219.
40. Heckman MM, Whitesides TE Jr, Grewe SR, Rooks MD. Compartment pressure in association with closed tibial fractures. The relationship between tissue pressure, compartment, and the distance from the site of the fracture. J Bone Joint Surg Am. 1994;76(9):1285-1292.
41. Hargens AR, Schmidt DA, Evans KL, et al. Quantitation of skeletal-muscle necrosis in a model compartment syndrome. J Bone Joint Surg Am. 1981;63(4):631-636.
42. Heppenstall RB, Sapega AA, Scott R, et al. The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;(226):138-155.
43. McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99-104.
44. Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66(1):93-97.
45. Shuler MS, Reisman WM, Kinsey TL, et al. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92(4):863-870.
46. Tobias JD, Hoernschemeyer DG. Near-infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27(3):311-313.
1. Bhattacharyya T, Vrahas MS. The medical-legal aspects of compartment syndrome. J Bone Joint Surg Am. 2004;86(4):864-868.
2. Prasarn ML, Ouellette EA, Livingstone A, Giuffrida AY. Acute pediatric upper extremity compartment syndrome in the absence of fracture. J Pediatr Orthop. 2009;29(3):263-268.
3. Battaglia TC, Armstrong DG, Schwend RM. Factors affecting forearm compartment pressures in children with supracondylar fractures of the humerus. J Pediatr Orthop. 2002;22(4):431-439.
4. Ramachandran M, Skaggs DL, Crawford HA, et al. Delaying treatment of supracondylar fractures in children: has the pendulum swung too far? J Bone Joint Surg Br. 2008;90(9):1228-1233.
5. Mubarak SJ, Carroll NC. Volkmann’s contracture in children: aetiology and prevention. J Bone Joint Surg Br. 1979;61(3):285-293.
6. Choi PD, Melikian R, Skaggs DL. Risk factors for vascular repair and compartment syndrome in the pulseless supracondylar humerus fracture in children. J Pediatr Orthop. 2010;30(1):50-56.
7. Gupta N, Kay RM, Leitch K, Femino JD, Tolo VT, Skaggs DL. Effect of surgical delay on perioperative complications and need for open reduction in supracondylar humerus fractures in children. J Pediatr Orthop. 2004;24(3):245-248.
8. Iyengar SR, Hoffinger SA, Townsend DR. Early versus delayed reduction and pinning of type III displaced supracondylar fractures of the humerus in children: a comparative study. J Orthop Trauma. 1999;13(1):51-55.
9. Leet AI, Frisancho J, Ebramzadeh E. Delayed treatment of type 3 supracondylar humerus fractures in children. J Pediatr Orthop. 2002;22(2):203-207.
10. Mehlman CT, Strub WM, Roy DR, Wall EJ, Crawford AH. The effect of surgical timing on the perioperative complications of treatment of supracondylar humeral fractures in children. J Bone Joint Surg Am. 2001;83(3):323-327.
11. Diesselhorst MM, Deck JW, Davey JP. Compartment syndrome of the upper arm after closed reduction and percutaneous pinning of a supracondylar humerus fracture. J Pediatr Orthop. 2014;34(2):e1-e4.
12. Mai MC, Beck R, Gabriel K, Singh KA. Posterior arm compartment syndrome after a combined supracondylar humeral and capitellar fractures in an adolescent: a case report. J Pediatr Orthop. 2011;31(3):e16-e19.
13. Blakemore LC, Cooperman DR, Thompson GH, Wathey C, Ballock RT. Compartment syndrome in ipsilateral humerus and forearm fractures in children. Clin Orthop Relat Res. 2000;(376):32-38.
14. Ring D, Waters PM, Hotchkiss RN, Kasser JR. Pediatric floating elbow. J Pediatr Orthop. 2001;21(4):456-459.
15. Haasbeek JF, Cole WG. Open fractures of the arm in children. J Bone Joint Surg Br. 1995;77(4):576-581.
16. Yuan PS, Pring ME, Gaynor TP, Mubarak SJ, Newton PO. Compartment syndrome following intramedullary fixation of pediatric forearm fractures. J Pediatr Orthop. 2004;24(4):370-375.
17. Flynn JM, Jones KJ, Garner MR, Goebel J. Eleven years experience in the operative management of pediatric forearm fractures. J Pediatr Orthop. 2010;30(4):313-319.
18. Blackman AJ, Wall LB, Keeler KA, et al. Acute compartment syndrome after intramedullary nailing of isolated radius and ulna fractures in children. J Pediatr Orthop. 2014;34(1):50-54.
19. Mubarak SJ, Frick S, Sink E, Rathjen K, Noonan KJ. Volkmann contracture and compartment syndromes after femur fractures in children treated with 90/90 spica casts. J Pediatr Orthop. 2006;26(5):567-572.
20. Hope PG, Cole WG. Open fractures of the tibia in children. J Bone Joint Surg Br. 1992;74(4):546-553.
21. Pandya NK, Edmonds EK, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
22. Flynn JM, Bashyal RK, Yeger-McKeever M, Garner MR, Launay F, Sponseller PD. Acute traumatic compartment syndrome of the leg in children: diagnosis and outcome. J Bone Joint Surg Am. 2011;93(10):937-941.
23. Mubarak SJ. Extensor retinaculum syndrome of the ankle after injury to the distal tibial physis. J Bone Joint Surg Br. 2002;84(1):11-14.
24. Macer GA Jr. Forearm compartment syndrome in the newborn. J Hand Surg Am. 2006;31(9):1550.
25. Ragland R 3rd, Moukoko D, Ezaki M, Carter PR, Mills J. Forearm compartment syndrome in the newborn: report of 24 cases. J Hand Surg Am. 2005;30(5):997-1003.
26. Alioglu B, Avci Z, Baskin E, Ozcay F, Tuncay IC, Ozbek N. Successful use of recombinant factor VIIa (NovoSeven) in children with compartment syndrome: two case reports. J Pediatr Orthop. 2006;26(6):815-817.
27. Lee DK, Jeong WK, Lee DH, Lee SH. Multiple compartment syndrome in a pediatric patient with CML. J Pediatr Orthop. 2011;31(8):889-892.
28. Dumontier C, Sautet A, Man M, Bennani M, Apoil A. Entrapment and compartment syndromes of the upper limb in haemophilia. J Hand Surg Br. 1994;19(4):427-429.
29. Jones G, Thompson K, Johnson M. Acute compartment syndrome after minor trauma in a patient with undiagnosed mild haemophilia B. Lancet. 2013;382(9905):1678.
30. Shaw BA, Hosalkar HS. Rattlesnake bites in children: antivenin treatment and surgical indications. J Bone Joint Surg Am. 2002;84(9):1624-1629.
31. Bae DS, Kadiyala RK, Waters PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21(5):680-688.
32. Noonan KJ, McCarthy JJ. Compartment syndromes in the pediatric patient. J Pediatr Orthop. 2010;30(2 suppl):S96-S101.
33. Dalens B. Some current controversies in paediatric regional anaesthesia. Curr Opin Anaesthesiol. 2006;19(3):301-308.
34. Wedel DJ. Regional anesthesia and pain management: reviewing the past decade and predicting the future. Anesth Analg. 2000;90(5):1244-1245.
35. Mubarak SJ. Wilton NC. Compartment syndromes and epidural analgesia. J Pediatr Orthop. 1997;17(3):282-284.
36. Price C, Ribeiro J, Kinnebrew T. Compartment syndromes associated with postoperative epidural analgesia. A case report. J Bone Joint Surg Am. 1996;78(4):597-599.
37. Thonse R, Ashford RU, Williams TI, Harrington P. Differences in attitudes to analgesia in post-operative limb surgery put patients at risk of compartment syndrome. Injury. 2004;35(3):290-295.
38. Whitesides TE Jr. Pain: friend or foe? J Bone Joint Surg Am. 2001;83(9):1424-1425.
39. Staudt JM, Smeulders MJ, van der Horst CM. Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br. 2008;90(2):215-219.
40. Heckman MM, Whitesides TE Jr, Grewe SR, Rooks MD. Compartment pressure in association with closed tibial fractures. The relationship between tissue pressure, compartment, and the distance from the site of the fracture. J Bone Joint Surg Am. 1994;76(9):1285-1292.
41. Hargens AR, Schmidt DA, Evans KL, et al. Quantitation of skeletal-muscle necrosis in a model compartment syndrome. J Bone Joint Surg Am. 1981;63(4):631-636.
42. Heppenstall RB, Sapega AA, Scott R, et al. The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;(226):138-155.
43. McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99-104.
44. Rorabeck CH. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66(1):93-97.
45. Shuler MS, Reisman WM, Kinsey TL, et al. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92(4):863-870.
46. Tobias JD, Hoernschemeyer DG. Near-infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27(3):311-313.
Acute and Recurrent Bacterial Vaginosis
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in women of reproductive age, with a prevalence in North America of 29.2% among women ages 14 to 49.1-3 BV is a condition in which the normal vaginal flora are altered, primarily due to a reduction in hydrogen peroxide–producing strains of lactobacilli. This leads to an elevated vaginal pH and increased levels of proteolytic enzymes (eg, sialidase), organic acids, and volatile amines.4 This change in pH allows an overgrowth of multiple types of anaerobic, mycoplasmic, and gram-negative bacteria.
In most cases of BV, the predominant microbe is the facultative anaerobe Gardnerella vaginalis. However, evidence from recent studies of the pathogenesis of BV suggests that this bacterium forms a biofilm in the vaginal epithelium that serves as a “scaffolding” to which other bacterial species adhere in a symbiotic fashion, colonizing the vagina.5 Though asymptomatic in at least half of affected women,2,6,7 this polymicrobial condition can produce a thin, white, homogenous discharge with a distinct “fishy” odor.
The changes in the vaginal flora seen in BV are associated with serious sequelae, such as preterm delivery, spontaneous abortion, postpartum endometritis, and increased susceptibility to HIV and other sexually transmitted infections (STIs).4,8,9 The polymicrobial nature of BV and its propensity for recurrence make treatment a challenge.
PATIENT PRESENTATION/HISTORY
The most common symptom of BV is increased vaginal discharge, which usually is thin and white or dull gray.4 Some women report a strong fishy odor, especially after sex. Vaginal pain, itching, or burning may also be present, especially if the discharge is copious. Dyspareunia and dysuria are rare, but possible, symptoms. Fever, malaise, and other systemic symptoms are not associated with BV and should prompt the clinician to consider other causes. About half of women with bacterial vaginosis have no symptoms.2,6
The typical finding on physical examination is a homogeneous, off-white, creamy, malodorous discharge that adheres to the vaginal walls and pools in the vaginal vault. There are usually no or minimal signs of vaginal inflammation, and the vulva, labia, and cervix are typically normal. In some cases, BV can lead to cervicitis.6,9,10
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of BV can be made based on the history, physical examination, and microscopic examination of the vaginal discharge. Unlike with many other bacterial diseases, culture is not recommended for diagnosis of BV because many of the implicated organisms cannot be easily isolated in the laboratory, and because asymptomatic women also have small numbers of these flora in the vagina.
In 1991, Nugent et al11 described a Gram stain scoring system of vaginal smears to diagnose BV, which has a sensitivity and specificity of 96% and 96%, respectively; it remains the gold standard for diagnosis.7 However, because this method requires considerable time and skill, it is not routinely used in most clinic settings.
A widely used method of diagnosing BV is the Amsel criteria (see Table 1). The Amsel method has a sensitivity and specificity of 81% and 94%, respectively.1,12 The presence of clue cells is the most reliable indicator of BV (see figure). The positive predictive value of this test for the presence of BV is 95%.14 The Amsel method requires microscopy,4,12 which is not always available in clinics.
There are several commercially available point-of-care tests for BV that do not require microscopy. These include rapid antigen and nucleic acid amplification tests to detect elevated levels of G vaginalis, as well as tests that detect the presence of bacterial amines, elevated vaginal pH, and bacterial sialidase.4,7,15 Compared with the Nugent and Amsel methods, one test that detects elevated vaginal fluid sialidase activity was shown to have a sensitivity of 88% and specificity of 91% to 95%.4,7,15 These point-of-care tests are most effective for diagnosing BV when the vaginal pH exceeds 4.5 and when they are used in conjunction with other clinical criteria.
Continue for treatment/management >>
TREATMENT/MANAGEMENT
Treatment is recommended for women with symptoms. The established benefit of therapy in nonpregnant women is relief of vaginal symptoms and signs of infection. Other potential benefits to treatment include reduction in the risk for Chlamydia trachomatis or Neisseria gonorrhea infection, HIV, and other viral STIs. Table 2 includes the recommended and alternative treatment regimens for BV, according to the CDC’s 2015 treatment guidelines for sexually transmitted infections.7 These regimens are also recommended by the American Congress of Obstetricians and Gynecologists (ACOG).16
Treatment is also recommended for all symptomatic pregnant women. Older CDC guidelines noted a preference for oral therapy in pregnant women with BV, due to the possibility of subclinical upper genital tract infection.17 However, the 2015 CDC guidelinesstate that symptomatic pregnant women can be treated with either the oral or vaginal regimens recommended for nonpregnant women, as oral therapy has not been shown to outperform vaginal therapy in effecting cure or preventing adverse outcomes.7
PATIENT EDUCATION AND FOLLOW-UP
Patient preferences, possible adverse effects, drug interactions, and other coinfections should be considered when selecting a treatment regimen. Women should be advised to refrain from sexual intercourse or to use condoms consistently and correctly during treatment. Douching may increase the risk for relapse, and no data support its use for treatment or relief of symptoms. Follow-up visits are unnecessary unless symptoms do not resolve. Because recurrence of BV is common, however, women should be advised to return for evaluation if symptoms recur.1,8,18
Continue for when BV recurs >>
WHEN BV RECURS
Recurrence rates of 15% to 30% have been reported at three months,18,19 and of 28% when patients were tested cumulatively over six months,1 but few researchers have looked at long-term recurrence rates. In one observational study, recurrence rates of 51% were reported during a six-year follow-up period among women previously treated with oral metronidazole.20 Whether these high recurrence rates are due to treatment failure to eradicate the causative organism or to a reinfection from sexual partners remains unclear.21 Some studies have shown that treatment of male partners does not affect recurrence rates.21,22
Risk factors
Various research teams have identified risk factors associated with BV recurrence, but study results have been inconsistent. The strongest risk factor appears to be sexual activity, specifically with increased numbers of sexual partners and inconsistent condom use.1,23,24 Women who have sex with women also appear to be at increased risk for BV recurrence.9,10
BV tends to recur around the time of menstruation, and some suppressive therapies include administration of antibiotics during this time.1,8 Although reports conflict, other risk factors that may be implicated in recurrent BV include vaginal douching, cigarette smoking, and increased BMI.2,18 Use of an oral contraceptive may have a protective effect against BV recurrence.1
Caring for patients with multiple recurrences of BV can be challenging for many clinicians. Although a few studies have evaluated suppressive therapy for recurrent BV, there are no clear treatment guidelines for multiple recurrent infections. Sobel and colleagues evaluated twice-weekly use of metronidazole gel for 16 weeks and found a significant reduction in BV recurrence during treatment.25 However, there was only a 34% to 37% probability of patients’ remaining clinically cured at seven months posttreatment. Similarly, Reichman et al evaluated suppressive therapy with oral metronidazole, topical boric acid, and metronidazole gel. They found an 88% to 92% initial cure rate, but a 50% failure rate at 36 weeks’ follow-up.26
Management
Studies examining the use of probiotics for the prevention and treatment of BV have yielded mixed results. The theory is that probiotics containing lactobacillus organisms may protect women from infection by maintaining or restoring vaginal pH and preventing adhesion of bacteria to the epithelium of the vaginal walls.27 Despite the conflicting results, no adverse effects have been reported and, as a consequence, many experts recommend probiotics to reduce the risk for recurrent BV. When discussing suppressive therapy options with patients, clinicians should be mindful of the limited data and the clinically unfavorable long-term cure rates demonstrated.
In addition to treatment limitations for recurrent BV, clinicians often find it challenging to effectively address the psychosocial implications of distress, embarrassment, and lack of control that are commonly associated with recurrent BV.28 Beyond its impact on sexual activity, women have also reported refraining from their daily activities out of fear that others around them may detect their vaginal odor. Helping women take a proactive approach in the treatment and prevention of BV may ease some of this distress.
Women with recurrent BV are often eager to hear about measures they can take to reduce their risk for acute and recurrent infection. Patients should be counseled on the association of BV with douching, numerous sexual partners, unprotected sex, increased psychosocial stress, and cigarette smoking.7,18,29-31 Patients may inquire about the potential risk for BV when they use feminine hygiene spray, panty liners or pads, and underwear made from synthetic fabrics; however, one longitudinal study30 showed no association between any of these hygienic behaviors and BV.
Continue for the conclusion >>
CONCLUSION
Bacterial vaginosis is a common cause of vaginal discharge in women. Current recommendations for treatment are not very effective, with up to half of women experiencing recurrence. The likelihood of recurrence can result in significant frustration for both patient and clinician. Although recent studies have advanced our understanding of the pathophysiology of BV, further research is needed to develop more effective treatments that reduce recurrence. Addressing modifiable risk factors and considering the use of suppressive and/or probiotic therapy may improve quality of life for women affected by this condition.
REFERENCES
1. Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
2. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004: associations with symptoms, sexual behaviors, and reproductive health. Sex Trans Dis. 2007;34(11): 864-869.
3. Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey Data. Obstet Gynecol. 2007;109(1):114-120.
4. Bradshaw CS, Morton AN, Garland SM, et al. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
5. Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97.e1-e6.
6. Schwebke JR. Vaginal discharge. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. New York, NY: McGraw-Hill; 2007. http://accessmedicine.mhmedical.com/content.aspx?bookid=369&Sectionid=39914778. Accessed November 11, 2015.
7. Workowski KA, Bolan GA; CDC. Bacterial vaginosis. In: Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):69-72.
8. Hay P. Recurrent bacterial vaginosis. Curr Opin Infect Dis. 2009;22(1): 82-86.
9. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40(2):117-122.
10. Marrazzo JM, Wiesenfeld HC, Murray PG, et al. Risk factors for cervicitis among women with bacterial vaginosis. J Infect Dis. 2006;193(5):617-624.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297-301.
12. Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983; 74(1):14-22.
13. Girerd PH. Bacterial vaginosis workup (2014). http://emedicine.medscape.com/article/254342-workup. Accessed November 11, 2015.
14. Hoffman BL, Schorge JO, Schaffer JI, et al. Gynecologic infection. In: Hoffman BL, Schorge JO, Schaffer JI, et al, eds. Williams Gynecology. 2nd ed. New York, NY: McGraw-Hill; 2012.
15. Hainer BL, Gibson MV. Vaginitis: diagnosis and treatment. Am Fam Physician. 2011;83(7):807-815.
16. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists, No. 72: Vaginitis. Obstet Gynecol. 2006;107(5):1195-1206.
17. Workowski KA, Berman S; CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12): 1-110.
18. Wilson J. Managing recurrent bacterial vaginosis. Sex Transm Infect. 2004;80(1):8-11.
19. Cook RL, Redondo-Lopez V, Schmitt C, et al. Clinical, microbiological, and biochemical factors in recurrent bacterial vaginosis. J Clin Microbiol. 1992;30(4):870-877.
20. Boris J, Påhlson C, Larsson P-G. Six years observation after successful treatment of bacterial vaginosis. Infect Dis Obstetr Gynecol. 1997;5(4): 297-302.
21. Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacterial vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
22. Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11): 1478-1486.
23. Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008;47(11):1426-1435.
24. Schwebke JR, Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis. 2007;44(2):213-219.
25. Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
26. Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
27. Homayouni A, Bastani P, Ziyadi S, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18(1):79-86.
28. Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS ONE. 2013;8(9):e74378.
29. Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect. 2004;80(1):58-62.
30. Klebanoff MA, Nansel TR, Brotman RM, et al. Personal hygienic behaviors and bacterial vaginosis. Sex Transm Dis. 2010;37(2):94-99.
31. Nansel TR, Riggs MA, Yu K-F, et al. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194(2):381-386.
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in women of reproductive age, with a prevalence in North America of 29.2% among women ages 14 to 49.1-3 BV is a condition in which the normal vaginal flora are altered, primarily due to a reduction in hydrogen peroxide–producing strains of lactobacilli. This leads to an elevated vaginal pH and increased levels of proteolytic enzymes (eg, sialidase), organic acids, and volatile amines.4 This change in pH allows an overgrowth of multiple types of anaerobic, mycoplasmic, and gram-negative bacteria.
In most cases of BV, the predominant microbe is the facultative anaerobe Gardnerella vaginalis. However, evidence from recent studies of the pathogenesis of BV suggests that this bacterium forms a biofilm in the vaginal epithelium that serves as a “scaffolding” to which other bacterial species adhere in a symbiotic fashion, colonizing the vagina.5 Though asymptomatic in at least half of affected women,2,6,7 this polymicrobial condition can produce a thin, white, homogenous discharge with a distinct “fishy” odor.
The changes in the vaginal flora seen in BV are associated with serious sequelae, such as preterm delivery, spontaneous abortion, postpartum endometritis, and increased susceptibility to HIV and other sexually transmitted infections (STIs).4,8,9 The polymicrobial nature of BV and its propensity for recurrence make treatment a challenge.
PATIENT PRESENTATION/HISTORY
The most common symptom of BV is increased vaginal discharge, which usually is thin and white or dull gray.4 Some women report a strong fishy odor, especially after sex. Vaginal pain, itching, or burning may also be present, especially if the discharge is copious. Dyspareunia and dysuria are rare, but possible, symptoms. Fever, malaise, and other systemic symptoms are not associated with BV and should prompt the clinician to consider other causes. About half of women with bacterial vaginosis have no symptoms.2,6
The typical finding on physical examination is a homogeneous, off-white, creamy, malodorous discharge that adheres to the vaginal walls and pools in the vaginal vault. There are usually no or minimal signs of vaginal inflammation, and the vulva, labia, and cervix are typically normal. In some cases, BV can lead to cervicitis.6,9,10
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of BV can be made based on the history, physical examination, and microscopic examination of the vaginal discharge. Unlike with many other bacterial diseases, culture is not recommended for diagnosis of BV because many of the implicated organisms cannot be easily isolated in the laboratory, and because asymptomatic women also have small numbers of these flora in the vagina.
In 1991, Nugent et al11 described a Gram stain scoring system of vaginal smears to diagnose BV, which has a sensitivity and specificity of 96% and 96%, respectively; it remains the gold standard for diagnosis.7 However, because this method requires considerable time and skill, it is not routinely used in most clinic settings.
A widely used method of diagnosing BV is the Amsel criteria (see Table 1). The Amsel method has a sensitivity and specificity of 81% and 94%, respectively.1,12 The presence of clue cells is the most reliable indicator of BV (see figure). The positive predictive value of this test for the presence of BV is 95%.14 The Amsel method requires microscopy,4,12 which is not always available in clinics.
There are several commercially available point-of-care tests for BV that do not require microscopy. These include rapid antigen and nucleic acid amplification tests to detect elevated levels of G vaginalis, as well as tests that detect the presence of bacterial amines, elevated vaginal pH, and bacterial sialidase.4,7,15 Compared with the Nugent and Amsel methods, one test that detects elevated vaginal fluid sialidase activity was shown to have a sensitivity of 88% and specificity of 91% to 95%.4,7,15 These point-of-care tests are most effective for diagnosing BV when the vaginal pH exceeds 4.5 and when they are used in conjunction with other clinical criteria.
Continue for treatment/management >>
TREATMENT/MANAGEMENT
Treatment is recommended for women with symptoms. The established benefit of therapy in nonpregnant women is relief of vaginal symptoms and signs of infection. Other potential benefits to treatment include reduction in the risk for Chlamydia trachomatis or Neisseria gonorrhea infection, HIV, and other viral STIs. Table 2 includes the recommended and alternative treatment regimens for BV, according to the CDC’s 2015 treatment guidelines for sexually transmitted infections.7 These regimens are also recommended by the American Congress of Obstetricians and Gynecologists (ACOG).16
Treatment is also recommended for all symptomatic pregnant women. Older CDC guidelines noted a preference for oral therapy in pregnant women with BV, due to the possibility of subclinical upper genital tract infection.17 However, the 2015 CDC guidelinesstate that symptomatic pregnant women can be treated with either the oral or vaginal regimens recommended for nonpregnant women, as oral therapy has not been shown to outperform vaginal therapy in effecting cure or preventing adverse outcomes.7
PATIENT EDUCATION AND FOLLOW-UP
Patient preferences, possible adverse effects, drug interactions, and other coinfections should be considered when selecting a treatment regimen. Women should be advised to refrain from sexual intercourse or to use condoms consistently and correctly during treatment. Douching may increase the risk for relapse, and no data support its use for treatment or relief of symptoms. Follow-up visits are unnecessary unless symptoms do not resolve. Because recurrence of BV is common, however, women should be advised to return for evaluation if symptoms recur.1,8,18
Continue for when BV recurs >>
WHEN BV RECURS
Recurrence rates of 15% to 30% have been reported at three months,18,19 and of 28% when patients were tested cumulatively over six months,1 but few researchers have looked at long-term recurrence rates. In one observational study, recurrence rates of 51% were reported during a six-year follow-up period among women previously treated with oral metronidazole.20 Whether these high recurrence rates are due to treatment failure to eradicate the causative organism or to a reinfection from sexual partners remains unclear.21 Some studies have shown that treatment of male partners does not affect recurrence rates.21,22
Risk factors
Various research teams have identified risk factors associated with BV recurrence, but study results have been inconsistent. The strongest risk factor appears to be sexual activity, specifically with increased numbers of sexual partners and inconsistent condom use.1,23,24 Women who have sex with women also appear to be at increased risk for BV recurrence.9,10
BV tends to recur around the time of menstruation, and some suppressive therapies include administration of antibiotics during this time.1,8 Although reports conflict, other risk factors that may be implicated in recurrent BV include vaginal douching, cigarette smoking, and increased BMI.2,18 Use of an oral contraceptive may have a protective effect against BV recurrence.1
Caring for patients with multiple recurrences of BV can be challenging for many clinicians. Although a few studies have evaluated suppressive therapy for recurrent BV, there are no clear treatment guidelines for multiple recurrent infections. Sobel and colleagues evaluated twice-weekly use of metronidazole gel for 16 weeks and found a significant reduction in BV recurrence during treatment.25 However, there was only a 34% to 37% probability of patients’ remaining clinically cured at seven months posttreatment. Similarly, Reichman et al evaluated suppressive therapy with oral metronidazole, topical boric acid, and metronidazole gel. They found an 88% to 92% initial cure rate, but a 50% failure rate at 36 weeks’ follow-up.26
Management
Studies examining the use of probiotics for the prevention and treatment of BV have yielded mixed results. The theory is that probiotics containing lactobacillus organisms may protect women from infection by maintaining or restoring vaginal pH and preventing adhesion of bacteria to the epithelium of the vaginal walls.27 Despite the conflicting results, no adverse effects have been reported and, as a consequence, many experts recommend probiotics to reduce the risk for recurrent BV. When discussing suppressive therapy options with patients, clinicians should be mindful of the limited data and the clinically unfavorable long-term cure rates demonstrated.
In addition to treatment limitations for recurrent BV, clinicians often find it challenging to effectively address the psychosocial implications of distress, embarrassment, and lack of control that are commonly associated with recurrent BV.28 Beyond its impact on sexual activity, women have also reported refraining from their daily activities out of fear that others around them may detect their vaginal odor. Helping women take a proactive approach in the treatment and prevention of BV may ease some of this distress.
Women with recurrent BV are often eager to hear about measures they can take to reduce their risk for acute and recurrent infection. Patients should be counseled on the association of BV with douching, numerous sexual partners, unprotected sex, increased psychosocial stress, and cigarette smoking.7,18,29-31 Patients may inquire about the potential risk for BV when they use feminine hygiene spray, panty liners or pads, and underwear made from synthetic fabrics; however, one longitudinal study30 showed no association between any of these hygienic behaviors and BV.
Continue for the conclusion >>
CONCLUSION
Bacterial vaginosis is a common cause of vaginal discharge in women. Current recommendations for treatment are not very effective, with up to half of women experiencing recurrence. The likelihood of recurrence can result in significant frustration for both patient and clinician. Although recent studies have advanced our understanding of the pathophysiology of BV, further research is needed to develop more effective treatments that reduce recurrence. Addressing modifiable risk factors and considering the use of suppressive and/or probiotic therapy may improve quality of life for women affected by this condition.
REFERENCES
1. Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
2. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004: associations with symptoms, sexual behaviors, and reproductive health. Sex Trans Dis. 2007;34(11): 864-869.
3. Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey Data. Obstet Gynecol. 2007;109(1):114-120.
4. Bradshaw CS, Morton AN, Garland SM, et al. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
5. Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97.e1-e6.
6. Schwebke JR. Vaginal discharge. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. New York, NY: McGraw-Hill; 2007. http://accessmedicine.mhmedical.com/content.aspx?bookid=369&Sectionid=39914778. Accessed November 11, 2015.
7. Workowski KA, Bolan GA; CDC. Bacterial vaginosis. In: Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):69-72.
8. Hay P. Recurrent bacterial vaginosis. Curr Opin Infect Dis. 2009;22(1): 82-86.
9. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40(2):117-122.
10. Marrazzo JM, Wiesenfeld HC, Murray PG, et al. Risk factors for cervicitis among women with bacterial vaginosis. J Infect Dis. 2006;193(5):617-624.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297-301.
12. Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983; 74(1):14-22.
13. Girerd PH. Bacterial vaginosis workup (2014). http://emedicine.medscape.com/article/254342-workup. Accessed November 11, 2015.
14. Hoffman BL, Schorge JO, Schaffer JI, et al. Gynecologic infection. In: Hoffman BL, Schorge JO, Schaffer JI, et al, eds. Williams Gynecology. 2nd ed. New York, NY: McGraw-Hill; 2012.
15. Hainer BL, Gibson MV. Vaginitis: diagnosis and treatment. Am Fam Physician. 2011;83(7):807-815.
16. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists, No. 72: Vaginitis. Obstet Gynecol. 2006;107(5):1195-1206.
17. Workowski KA, Berman S; CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12): 1-110.
18. Wilson J. Managing recurrent bacterial vaginosis. Sex Transm Infect. 2004;80(1):8-11.
19. Cook RL, Redondo-Lopez V, Schmitt C, et al. Clinical, microbiological, and biochemical factors in recurrent bacterial vaginosis. J Clin Microbiol. 1992;30(4):870-877.
20. Boris J, Påhlson C, Larsson P-G. Six years observation after successful treatment of bacterial vaginosis. Infect Dis Obstetr Gynecol. 1997;5(4): 297-302.
21. Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacterial vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
22. Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11): 1478-1486.
23. Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008;47(11):1426-1435.
24. Schwebke JR, Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis. 2007;44(2):213-219.
25. Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
26. Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
27. Homayouni A, Bastani P, Ziyadi S, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18(1):79-86.
28. Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS ONE. 2013;8(9):e74378.
29. Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect. 2004;80(1):58-62.
30. Klebanoff MA, Nansel TR, Brotman RM, et al. Personal hygienic behaviors and bacterial vaginosis. Sex Transm Dis. 2010;37(2):94-99.
31. Nansel TR, Riggs MA, Yu K-F, et al. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194(2):381-386.
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in women of reproductive age, with a prevalence in North America of 29.2% among women ages 14 to 49.1-3 BV is a condition in which the normal vaginal flora are altered, primarily due to a reduction in hydrogen peroxide–producing strains of lactobacilli. This leads to an elevated vaginal pH and increased levels of proteolytic enzymes (eg, sialidase), organic acids, and volatile amines.4 This change in pH allows an overgrowth of multiple types of anaerobic, mycoplasmic, and gram-negative bacteria.
In most cases of BV, the predominant microbe is the facultative anaerobe Gardnerella vaginalis. However, evidence from recent studies of the pathogenesis of BV suggests that this bacterium forms a biofilm in the vaginal epithelium that serves as a “scaffolding” to which other bacterial species adhere in a symbiotic fashion, colonizing the vagina.5 Though asymptomatic in at least half of affected women,2,6,7 this polymicrobial condition can produce a thin, white, homogenous discharge with a distinct “fishy” odor.
The changes in the vaginal flora seen in BV are associated with serious sequelae, such as preterm delivery, spontaneous abortion, postpartum endometritis, and increased susceptibility to HIV and other sexually transmitted infections (STIs).4,8,9 The polymicrobial nature of BV and its propensity for recurrence make treatment a challenge.
PATIENT PRESENTATION/HISTORY
The most common symptom of BV is increased vaginal discharge, which usually is thin and white or dull gray.4 Some women report a strong fishy odor, especially after sex. Vaginal pain, itching, or burning may also be present, especially if the discharge is copious. Dyspareunia and dysuria are rare, but possible, symptoms. Fever, malaise, and other systemic symptoms are not associated with BV and should prompt the clinician to consider other causes. About half of women with bacterial vaginosis have no symptoms.2,6
The typical finding on physical examination is a homogeneous, off-white, creamy, malodorous discharge that adheres to the vaginal walls and pools in the vaginal vault. There are usually no or minimal signs of vaginal inflammation, and the vulva, labia, and cervix are typically normal. In some cases, BV can lead to cervicitis.6,9,10
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of BV can be made based on the history, physical examination, and microscopic examination of the vaginal discharge. Unlike with many other bacterial diseases, culture is not recommended for diagnosis of BV because many of the implicated organisms cannot be easily isolated in the laboratory, and because asymptomatic women also have small numbers of these flora in the vagina.
In 1991, Nugent et al11 described a Gram stain scoring system of vaginal smears to diagnose BV, which has a sensitivity and specificity of 96% and 96%, respectively; it remains the gold standard for diagnosis.7 However, because this method requires considerable time and skill, it is not routinely used in most clinic settings.
A widely used method of diagnosing BV is the Amsel criteria (see Table 1). The Amsel method has a sensitivity and specificity of 81% and 94%, respectively.1,12 The presence of clue cells is the most reliable indicator of BV (see figure). The positive predictive value of this test for the presence of BV is 95%.14 The Amsel method requires microscopy,4,12 which is not always available in clinics.
There are several commercially available point-of-care tests for BV that do not require microscopy. These include rapid antigen and nucleic acid amplification tests to detect elevated levels of G vaginalis, as well as tests that detect the presence of bacterial amines, elevated vaginal pH, and bacterial sialidase.4,7,15 Compared with the Nugent and Amsel methods, one test that detects elevated vaginal fluid sialidase activity was shown to have a sensitivity of 88% and specificity of 91% to 95%.4,7,15 These point-of-care tests are most effective for diagnosing BV when the vaginal pH exceeds 4.5 and when they are used in conjunction with other clinical criteria.
Continue for treatment/management >>
TREATMENT/MANAGEMENT
Treatment is recommended for women with symptoms. The established benefit of therapy in nonpregnant women is relief of vaginal symptoms and signs of infection. Other potential benefits to treatment include reduction in the risk for Chlamydia trachomatis or Neisseria gonorrhea infection, HIV, and other viral STIs. Table 2 includes the recommended and alternative treatment regimens for BV, according to the CDC’s 2015 treatment guidelines for sexually transmitted infections.7 These regimens are also recommended by the American Congress of Obstetricians and Gynecologists (ACOG).16
Treatment is also recommended for all symptomatic pregnant women. Older CDC guidelines noted a preference for oral therapy in pregnant women with BV, due to the possibility of subclinical upper genital tract infection.17 However, the 2015 CDC guidelinesstate that symptomatic pregnant women can be treated with either the oral or vaginal regimens recommended for nonpregnant women, as oral therapy has not been shown to outperform vaginal therapy in effecting cure or preventing adverse outcomes.7
PATIENT EDUCATION AND FOLLOW-UP
Patient preferences, possible adverse effects, drug interactions, and other coinfections should be considered when selecting a treatment regimen. Women should be advised to refrain from sexual intercourse or to use condoms consistently and correctly during treatment. Douching may increase the risk for relapse, and no data support its use for treatment or relief of symptoms. Follow-up visits are unnecessary unless symptoms do not resolve. Because recurrence of BV is common, however, women should be advised to return for evaluation if symptoms recur.1,8,18
Continue for when BV recurs >>
WHEN BV RECURS
Recurrence rates of 15% to 30% have been reported at three months,18,19 and of 28% when patients were tested cumulatively over six months,1 but few researchers have looked at long-term recurrence rates. In one observational study, recurrence rates of 51% were reported during a six-year follow-up period among women previously treated with oral metronidazole.20 Whether these high recurrence rates are due to treatment failure to eradicate the causative organism or to a reinfection from sexual partners remains unclear.21 Some studies have shown that treatment of male partners does not affect recurrence rates.21,22
Risk factors
Various research teams have identified risk factors associated with BV recurrence, but study results have been inconsistent. The strongest risk factor appears to be sexual activity, specifically with increased numbers of sexual partners and inconsistent condom use.1,23,24 Women who have sex with women also appear to be at increased risk for BV recurrence.9,10
BV tends to recur around the time of menstruation, and some suppressive therapies include administration of antibiotics during this time.1,8 Although reports conflict, other risk factors that may be implicated in recurrent BV include vaginal douching, cigarette smoking, and increased BMI.2,18 Use of an oral contraceptive may have a protective effect against BV recurrence.1
Caring for patients with multiple recurrences of BV can be challenging for many clinicians. Although a few studies have evaluated suppressive therapy for recurrent BV, there are no clear treatment guidelines for multiple recurrent infections. Sobel and colleagues evaluated twice-weekly use of metronidazole gel for 16 weeks and found a significant reduction in BV recurrence during treatment.25 However, there was only a 34% to 37% probability of patients’ remaining clinically cured at seven months posttreatment. Similarly, Reichman et al evaluated suppressive therapy with oral metronidazole, topical boric acid, and metronidazole gel. They found an 88% to 92% initial cure rate, but a 50% failure rate at 36 weeks’ follow-up.26
Management
Studies examining the use of probiotics for the prevention and treatment of BV have yielded mixed results. The theory is that probiotics containing lactobacillus organisms may protect women from infection by maintaining or restoring vaginal pH and preventing adhesion of bacteria to the epithelium of the vaginal walls.27 Despite the conflicting results, no adverse effects have been reported and, as a consequence, many experts recommend probiotics to reduce the risk for recurrent BV. When discussing suppressive therapy options with patients, clinicians should be mindful of the limited data and the clinically unfavorable long-term cure rates demonstrated.
In addition to treatment limitations for recurrent BV, clinicians often find it challenging to effectively address the psychosocial implications of distress, embarrassment, and lack of control that are commonly associated with recurrent BV.28 Beyond its impact on sexual activity, women have also reported refraining from their daily activities out of fear that others around them may detect their vaginal odor. Helping women take a proactive approach in the treatment and prevention of BV may ease some of this distress.
Women with recurrent BV are often eager to hear about measures they can take to reduce their risk for acute and recurrent infection. Patients should be counseled on the association of BV with douching, numerous sexual partners, unprotected sex, increased psychosocial stress, and cigarette smoking.7,18,29-31 Patients may inquire about the potential risk for BV when they use feminine hygiene spray, panty liners or pads, and underwear made from synthetic fabrics; however, one longitudinal study30 showed no association between any of these hygienic behaviors and BV.
Continue for the conclusion >>
CONCLUSION
Bacterial vaginosis is a common cause of vaginal discharge in women. Current recommendations for treatment are not very effective, with up to half of women experiencing recurrence. The likelihood of recurrence can result in significant frustration for both patient and clinician. Although recent studies have advanced our understanding of the pathophysiology of BV, further research is needed to develop more effective treatments that reduce recurrence. Addressing modifiable risk factors and considering the use of suppressive and/or probiotic therapy may improve quality of life for women affected by this condition.
REFERENCES
1. Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
2. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004: associations with symptoms, sexual behaviors, and reproductive health. Sex Trans Dis. 2007;34(11): 864-869.
3. Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey Data. Obstet Gynecol. 2007;109(1):114-120.
4. Bradshaw CS, Morton AN, Garland SM, et al. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
5. Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97.e1-e6.
6. Schwebke JR. Vaginal discharge. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. New York, NY: McGraw-Hill; 2007. http://accessmedicine.mhmedical.com/content.aspx?bookid=369&Sectionid=39914778. Accessed November 11, 2015.
7. Workowski KA, Bolan GA; CDC. Bacterial vaginosis. In: Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):69-72.
8. Hay P. Recurrent bacterial vaginosis. Curr Opin Infect Dis. 2009;22(1): 82-86.
9. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40(2):117-122.
10. Marrazzo JM, Wiesenfeld HC, Murray PG, et al. Risk factors for cervicitis among women with bacterial vaginosis. J Infect Dis. 2006;193(5):617-624.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297-301.
12. Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983; 74(1):14-22.
13. Girerd PH. Bacterial vaginosis workup (2014). http://emedicine.medscape.com/article/254342-workup. Accessed November 11, 2015.
14. Hoffman BL, Schorge JO, Schaffer JI, et al. Gynecologic infection. In: Hoffman BL, Schorge JO, Schaffer JI, et al, eds. Williams Gynecology. 2nd ed. New York, NY: McGraw-Hill; 2012.
15. Hainer BL, Gibson MV. Vaginitis: diagnosis and treatment. Am Fam Physician. 2011;83(7):807-815.
16. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists, No. 72: Vaginitis. Obstet Gynecol. 2006;107(5):1195-1206.
17. Workowski KA, Berman S; CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12): 1-110.
18. Wilson J. Managing recurrent bacterial vaginosis. Sex Transm Infect. 2004;80(1):8-11.
19. Cook RL, Redondo-Lopez V, Schmitt C, et al. Clinical, microbiological, and biochemical factors in recurrent bacterial vaginosis. J Clin Microbiol. 1992;30(4):870-877.
20. Boris J, Påhlson C, Larsson P-G. Six years observation after successful treatment of bacterial vaginosis. Infect Dis Obstetr Gynecol. 1997;5(4): 297-302.
21. Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacterial vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
22. Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11): 1478-1486.
23. Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008;47(11):1426-1435.
24. Schwebke JR, Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis. 2007;44(2):213-219.
25. Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
26. Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
27. Homayouni A, Bastani P, Ziyadi S, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18(1):79-86.
28. Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS ONE. 2013;8(9):e74378.
29. Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect. 2004;80(1):58-62.
30. Klebanoff MA, Nansel TR, Brotman RM, et al. Personal hygienic behaviors and bacterial vaginosis. Sex Transm Dis. 2010;37(2):94-99.
31. Nansel TR, Riggs MA, Yu K-F, et al. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194(2):381-386.
New Developments in Adult Vaccination: Challenges and Opportunities to Protect Vulnerable Veterans From Pneumococcal Disease
Streptococcus pneumoniae (S pneumoniae), also known as pneumococcus, is a successful human pathogen with significant clinical impact that causes pneumonia and invasive infections, including bacteremia and meningitis.1 In the preantibiotic era, nearly 80% of patients with bacteremic pneumococcal pneumonia died.2 The introduction of sulfas and penicillin in the mid-20th century, subsequent refinements in antibiotic chemotherapy, and improvements in supportive care rendered pneumococcal disease readily treatable, notwithstanding the threat of antibiotic-resistant pneumococcus.3 Despite the availability of effective antibiotic therapy against S pneumoniae, pneumococcal disease remains a significant cause of morbidity and mortality among people with increased susceptibility, such as older adults and those living with chronic illness or immunosuppressive conditions. In developed countries like the U.S., where a growing portion of the population is vulnerable to S pneumoniae by virtue of their advanced age and underlying medical conditions, pneumococcal disease is still an important public health concern.4
Penumococcal Vaccines: A Long Time Coming
Vaccination against S pneumoniae has proven an efficacious strategy to reduce the morbidity and mortality associated with this pathogen.5 The original efforts to develop a pneumococcal vaccine culminated in 1945 with a vaccine containing pneumococcal capsular polysaccharides, which elicited a protective immune response among U.S. soldiers (Table 1).Subsequent investigations determined that the protective response was specific to pneumococcal disease caused by the 4 pneumococcal capsular serotypes included in the vaccine, that the carrier rate of pneumococcus with the vaccine serotypes decreased by about 50%, and that the incidence of pneumonia from the vaccine serotypes was reduced even in nonimmunized soldiers.6 These early observations remain relevant to our contemporary understanding of the impact of pneumococcal vaccination: Protection is limited to serotypes included in the vaccine; the vaccine reduces colonization; and the vaccine leads to herd immunity—the protection of unvaccinated subjects.
Despite this achievement, the use of vaccines as a strategy to combat pneumococcal disease was upstaged in the subsequent decades by the success of antibiotics. Renewed interest in pneumococcal vaccines resulted from the efforts of Robert Austrian, MD, who astutely observed that “highly effective antimicrobial drugs must be supplemented by other measures, both prophylactic and therapeutic, if the significant mortality resulting still from pneumococcal infection is to be reduced.”7
After initial studies carried out in South African gold miners, the FDA approved a pneumococcal polysaccharide vaccine (PPSV) against 23 of about 90 circulating serotypes.8 The CDC and the Advisory Committee on Immunization Practices (ACIP) initially recommended PPSV-23 for persons perceived to be at high risk for pneumococcal disease, including those with chronic diseases, immunocompromising conditions, and older adults.9
Three decades later, the analysis of a large body of available evidence demonstrated the protective effects of PPSV-23 against invasive pneumococcal disease caused by pneumococcal types included in the vaccine, especially bacteremic pneumonia (about 75% reduction, odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14-0.45).10 A perceived shortcoming of PPSV-23, which some experts dispute, is the lack of definite protection against nonbacteremic pneumococcal pneumonia.11 Studies of nursing home residents demonstrated a 50% reduction in the incidence of both pneumococcal pneumonia and all-cause pneumonia, suggesting that PPSV-23 offers protection against noninvasive pneumococcal pneumonia in specific populations.12 Additional potential limitations of PPSV-23 include reduced benefit in patients aged > 65 years and waning of protection over time.13
Related: Identification and Management of Middle East Respiratory Syndrome
Protein-Conjugate Vaccines
Clearly, the most important limitation of PPSV-23, inherent to all capsular polysaccharide vaccines, is that it does not elicit an immune response in children aged < 2 years. The successful development of a vaccine against Haemophilus influenzae type b (Hib) gave rise to a new generation of pneumococcal vaccines.14 Specifically, the Hib vaccine covalently binds, or conjugates, the capsule polysaccharide to an antigenic protein, leading to effective T-cell–mediated antibody production in infants and toddlers.
In 2000, children received the first iteration of a protein-conjugate vaccine containing the 7 most relevant pneumococcal serotypes (PCV-7).15 The effects of PCV-7 on pneumococcal disease have been extraordinary, practically eliminating infections caused by the pneumococcal serotypes included in the vaccine. Immunizing children with PCV-7 also ushered in a fundamental public health benefit for adults aged > 65 years: a reduction of nearly 90% in the incidence of pneumococcal infections caused by serotypes included in the vaccine. By eliciting mucosal immunity, which leads to decreased nasal carriage of the covered pneumococcal strains among children, PVC-7 generates herd immunity, leading to reductions in transmission, colonization, and infection with vaccine serotypes among adults.16
In 2010, a conjugate vaccine containing 13 serotypes (PCV-13) replaced PCV-7 administration for children.17,18 The PCV-13 is expected to protect children and the herd from disease caused by 6 additional pneumococcal serotypes, including those that surged as replacement strains, filling the ecologic niche created by PCV-7, such as the epidemiologically relevant serotype 19A.19,20
Immune-Compromised Adults
One of the shortcomings of PPSV-23 is its lack of efficacy in patients with advanced HIV, a group with an exquisite vulnerability to pneumococcal disease, as demonstrated by a randomized controlled trial of PPSV-23 in African patients with advanced, untreated HIV infection.21 Similarly, there is concern that patients with lymphoma, leukemia, multiple myeloma, and Hodgkin disease, also at high risk of pneumococcal infection, do not mount sufficient immune responses to polysaccharide antigens or that these responses are adversely affected by chemotherapy or immune suppressing medications. In these populations, a conjugate vaccine (PCV-7 or PCV-13) may elicit a more robust and durable immune response than a polysaccharide vaccine (PPSV-23). A randomized placebo-controlled trial demonstrated the efficacy of PCV-7 in protecting patients with advanced HIV against pneumococcal disease in Africa.22 Based in part on this observation, the ACIP now recommends the use of PCV-13 in patients with HIV and other immunosuppressing conditions, including chronic renal failure.23 A direct comparison of the relative protection of PPSV-23 vs PCV-7 in this population has not been performed.
Pneumococcal Vaccines in the Elderly
Although PPSV-23 was widely adopted in the U.S. with the intention to protect adults aged > 65 years from pneumococcal infection, this vaccine did not achieve global appeal. In the Netherlands, for instance, PPSV-23 had very low penetration and was never endorsed by public health authorities. Concerns have been issued about the decreased immunogenicity of a polysaccharide vaccine in older adults, invoking the concept of immune senescence, a term that describes a diminished capacity to mount robust immune responses due to aging.
Protein-conjugated antigens, on the other hand, can elicit a better initial immune response than a polysaccharide vaccine can in an older adult. Whether the effect is sustained and translates to better clinical outcomes remains unknown. The adoption of PCV-13 and the continued role of PPSV-23 in adults aged > 65 years have been examined carefully by experts.24,25
Dutch investigators organized the Community Acquired Pneumonia Immunization Trial in Adults (CAPITA), randomizing about 85,000 adults aged > 65 years toreceive eitherPCV-13 or a placebo.26 After 3 years of observation, the occurrence of invasive pneumococcal disease (caused by the vaccine serotypes) decreased by 85% in participants who received the vaccine compared with those who received a placebo. Additionally, the incidence of pneumococcal pneumonia (caused by the vaccine serotypes) decreased by 45% (Table 2). The findings of this trial largely inform the recommendation issued by APIC in October 2014 to administer PCV-13 vaccine to adults aged > 65 years.27
Polysaccharide Vaccine in Adults
A crucial limitation of the CAPITA study is that it does not provide a head-to-head comparison with PPSV-23. Similarly, the recommendation to use PCV-13 in patients with immune system compromising conditions did not arise from a direct comparison between PCV-13 and PPSV-23. In both groups of patients, ACIP continues to recommend PPSV-23 to extend protection to the 10 additional pneumococcal serotypes not included in PCV-13.28 Additionally, ACIP maintains its long-standing recommendation to administer PPSV-23 to adults aged < 65 years who have diabetes mellitus, or chronic liver, lung, or heart disease, or those who are tobacco smokers or misuse alcohol.
Notably, the order in which the vaccines are administered may influence their effectiveness. In adults aged 50 to 64 years, initial vaccination with PCV-13 followed by PPSV-23 lead to a better antipneumococcal immune response.14,29 Specifically, PCV-13 enhanced the response to subsequent administration of PPSV-23, whereas an initial PPSV-23 vaccine resulted in a decreased response to subsequent administration of PCV-13. After a 1-year interval between vaccines, immune titers to a second vaccination were not inferior.30 All the above considerations have shaped the ACIP recommendations for the administration of pneumococcal vaccines.27,31 Pneumococcal vaccination was a straightforward exercise when PPSV-23 was the only vaccine available for adults. The advent of PCV-13, however, renders pneumococcal immunization of adults more complicated (Figure 1).
Improving Vaccination Rates
The complex recommendations to vaccinate adults against pneumococcus may reveal obstacles to effective pneumococcal vaccination programs.32 From the perspective of health care institutions, the adoption of the new pneumococcal vaccine implies an added cost. A pneumococcal vaccination strategy that incorporates PCV-13 may be cost-effective, at least under certain parameters.33-35 There remains, however, the issue of affordability and the opportunity cost—the need to decrease funding for other health care programs to accommodate an increased budget for pneumococcal vaccination. Logistically, maintaining a reliable supply of vaccines to meet the demand of practitioners at various sites requires careful planning. Consequently, tracking vaccinated and unvaccinated patients and carefully coordinating among clinical providers, nurses, and pharmacists are essential.
From the perspective of clinical providers, an additional pneumococcal vaccine complicating the vaccination schedule for adults represents an increased burden. Providers will need information to reach their own conclusions regarding the rationale behind the development and use of pneumococcal conjugate vaccines, the existing and evolving recommendations from public health authorities, and the strengths and limitations of the evidence supporting the use of pneumococcal vaccines. Otherwise, providers may find it difficult to incorporate new data and guidelines supporting pneumococcal vaccination into their decision-making (Boxes 1-4). An additional and formidable challenge is to carve out time during an already busy clinical encounter to discuss pneumococcal vaccines and other immunizations.36
Related: The Importance of an Antimicrobial Stewardship Program
Similarly, older adults or patients with chronic health conditions may not recognize the important role that vaccines can play in their health maintenance and are likely to prioritize other issues during medical visits. It is not obvious for patients that multiple vaccines may be necessary to prevent pneumococcal disease. Furthermore, many patients, not unreasonably, may assume that their yearly influenza vaccine is sufficiently protective against pneumonia. Therefore, patients need to be educated about the rationale behind pneumococcal conjugate vaccination. Ultimately, access to immunization—the opportunity for patients to have an encounter with their providers and with the health care system that results in the administration of an appropriate vaccine—will determine whether goals for pneumococcal vaccination are achieved.
The evolving landscape for the implementation of pneumococcal vaccines creates the need to develop, implement, and refine organizational changes to adhere to the new guidelines for the use of PCV-13 and PPSV-23 vaccines. These interventions, if effective, may help improve pneumococcal vaccination coverage among adults (Table 3).
Harnessing the Power of the EMR
Interventions to improve the adherence to pneumococcal vaccination guidelines begin by identifying persons eligible for vaccination based on their age, their vaccination status (ie, persons previously unvaccinated or due for vaccination according to the recommended schedule), or the presence of medical conditions conferring high risk for pneumococcal disease. This, in turn, depends on adequate documentation of patients’ underlying medical diagnosis, as well as up-to-date records of vaccine administration to patients.
Health care systems possessing a mature and sophisticated electronic medical record (EMR), such as the VHA Computerized Patient Record System (CPRS), are in a good position to wield such information to plan, implement, and assure the quality of activities designed to improve pneumococcal vaccination rates. An analysis of the proportion of veterans in VISN 10 who received pneumococcal vaccination revealed that even with the advantages of a robust EMR and a highly developed infrastructure devoted to primary care, pneumococcal vaccine coverage remains below the 60% target goal, although well above national averages (Figure 2).37,38
Standing Orders
Standing orders make it possible for nurses and pharmacists to administer vaccines according to a preestablished protocol without a physician’s direct evaluation of each patient. Standing orders are a versatile intervention, with a record of effective implementation in both outpatient and inpatient facilities, in acute-care and long-term care facilities, and in most instances where patients interact with the health care system. Based on strong scientific evidence, ACIP recommends the adoption of standing-order programs to improve pneumococcal vaccination rates among adults.39 Indeed, standing-order programs may prove a very effective intervention to fulfill the recommendation to administer PCV-13 to adults aged > 65 years.
The Virtual Vaccination Clinic
Unfortunately, with 2 vaccines that have to be administered at different times to various groups of patients at risk, the current state of pneumococcal vaccination may be too complex to be readily reduced to a comprehensible set of standing orders. An innovative way to realize the benefit of standing orders is to target high-risk groups for pneumococcal disease who are eligible for vaccination by selecting them using the EMR and entering standing orders tailored to their specific vaccination needs. The selection of patients according to comorbidities and vaccination status and the determination of the appropriate pneumococcal vaccine takes place in the context of a “virtual” vaccination clinic.40
Enhancing Vaccinations
Further improvement in pneumococcal vaccination rates are likely to result from interventions that increase the demand for vaccines among patients and practitioners. Efforts to disseminate information and provide advice regarding pneumococcal vaccination are likely to result in patients seeking and clinicians offering the appropriate vaccine. Similarly, interventions to enhance the supply of vaccines at the point of care may reduce barriers that patients might encounter when attempting to receive vaccinations.
Another set of system-based interventions that can assist clinicians in making timely and appropriate vaccination decisions are EMR reminders, especially those targeting patients at high risk for pneumococcal disease because of underlying illnesses.41 Previous experience with pneumococcal vaccination in patients aged > 65 years indicates that EMR reminders facilitate improvements in vaccination. The involvement of a panel manager who coordinated with the primary care provider and contacted patients directly augmented the effect of the EMR reminder by 25%.42
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Patient reminder and recall systems also demonstrated effectiveness in improving immunization rates.43 In certain groups, notification of patients has been achieved through electronic methods, such as short text messaging or e-mail.44 Determining which interventions within a bundle are essential may be impossible, because the various interventions reinforce one another, and the likelihood of patients benefitting from at least one of the activities increases when multiple interventions are administered together. Therefore, the Task Force on Community Preventive Services supports combining provider reminder systems with education and other measures that encourage use of vaccines in patients and providers.45
Box answer key: 1: A; 2: A; 3: B; 4: A.
Conclusion
The increasing role of vaccines in the health maintenance of adults represents a change in paradigm for primary care and specialty providers. Physicians must assess the value and limitations of vaccines and find time to discuss immunizations with their adult patients. Health care systems can increase opportunities for vaccination and facilitate encounters that result in vaccination by educating patients and health care personnel and through the innovative use of reminders and standing orders in the EMR. Undertaking these activities may limit the burden of pneumococcal disease, an important cause of morbidity and mortality in adults that is preventable through immunization.
Acknowledgements
This work is dedicated to the memory of John M. Rieger, Information Technology Specialist at the Cleveland VAMC and Chief Master Sergeant, Air Force Reserve.
Author Disclosure
This work was supported by a research grant from Pfizer and by the Louis Stokes Cleveland VAMC, the VISN 10 Geriatric Research Education and Clinical Center, and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences of the National Institutes of Health NIH). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154.
2. Tilghman RC, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med (Chic). 1937;59(4):602-619.
3. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36(3):197-204.
4. Lexau CA, Lynfield R, Danila R, et al; Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
5. Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229-241.
6. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82(6):445-465.
7. Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759-776.
8. Austrian R. The Jeremiah Metzger Lecture: Of gold and pneumococci: a history of pneumococcal vaccines in South Africa. Trans Am Clin Climatol Assoc. 1978;89:141-161.
9. Centers for Disease Control (CDC). Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1981;30(33):410-412, 417-419.
10. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
11. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48-58.
12. Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
13. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Eng J Med. 1991;325(21):1453-1460.
14. Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52(5):633-640.
15. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
16. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Eng J Med. 2013;369(2):155-163.
17. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
18. Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children--Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258-261.
19. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics. 2014;134(2):210-217.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535-543.
21. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106-2111.
22. French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Eng J Med. 2010;362(9):812-822.
23. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-819.
24. Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55(2):259-264.
25. Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55(2):265-267.
26. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372(12):1114-1125.
27. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822-825.
28. Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55(2):255-258.
29. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594-3602.
30. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364-2374.
31. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
32. Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Am J Prev Med. 2015;pii:S0749-3797(15)00333-00335 [Epub ahead of print].
33. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011-6021.
34. Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950-3956.
35. Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804-812.
36. Kyaw MH, Greene CM, Schaffner W, et al; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med. 2006;31(4):286-292.
37. Williams WW, Lu PJ, O'Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95-102.
38. Committee on Review of Priorities in the National Vaccine Plan, Institute of Medicine. Priorities for the National Vaccine Plan. Washington, DC: National Academies Press; 2010.
39. McKibben LJ, Stange PV, Sneller VP, Strikas RA, Rodewald LE; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49(RR-1):15-16.
40. Jump RLP, Banks R, Wilson B, et al. A virtual clinic improves pneumococcal vaccination for asplenic veterans at high-risk for pneumococcal disease. Open Forum Infect Dis. In press.
41. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505-1510.
42. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558.
43. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
44. Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: a randomized controlled trial. Vaccine. 2015;33(43):5868-5872.
45. Ndiaye SM, Hopkins DP, Shefer AM, et al; Task Force on Community Preventive Services. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5)(suppl):248-279.
Streptococcus pneumoniae (S pneumoniae), also known as pneumococcus, is a successful human pathogen with significant clinical impact that causes pneumonia and invasive infections, including bacteremia and meningitis.1 In the preantibiotic era, nearly 80% of patients with bacteremic pneumococcal pneumonia died.2 The introduction of sulfas and penicillin in the mid-20th century, subsequent refinements in antibiotic chemotherapy, and improvements in supportive care rendered pneumococcal disease readily treatable, notwithstanding the threat of antibiotic-resistant pneumococcus.3 Despite the availability of effective antibiotic therapy against S pneumoniae, pneumococcal disease remains a significant cause of morbidity and mortality among people with increased susceptibility, such as older adults and those living with chronic illness or immunosuppressive conditions. In developed countries like the U.S., where a growing portion of the population is vulnerable to S pneumoniae by virtue of their advanced age and underlying medical conditions, pneumococcal disease is still an important public health concern.4
Penumococcal Vaccines: A Long Time Coming
Vaccination against S pneumoniae has proven an efficacious strategy to reduce the morbidity and mortality associated with this pathogen.5 The original efforts to develop a pneumococcal vaccine culminated in 1945 with a vaccine containing pneumococcal capsular polysaccharides, which elicited a protective immune response among U.S. soldiers (Table 1).Subsequent investigations determined that the protective response was specific to pneumococcal disease caused by the 4 pneumococcal capsular serotypes included in the vaccine, that the carrier rate of pneumococcus with the vaccine serotypes decreased by about 50%, and that the incidence of pneumonia from the vaccine serotypes was reduced even in nonimmunized soldiers.6 These early observations remain relevant to our contemporary understanding of the impact of pneumococcal vaccination: Protection is limited to serotypes included in the vaccine; the vaccine reduces colonization; and the vaccine leads to herd immunity—the protection of unvaccinated subjects.
Despite this achievement, the use of vaccines as a strategy to combat pneumococcal disease was upstaged in the subsequent decades by the success of antibiotics. Renewed interest in pneumococcal vaccines resulted from the efforts of Robert Austrian, MD, who astutely observed that “highly effective antimicrobial drugs must be supplemented by other measures, both prophylactic and therapeutic, if the significant mortality resulting still from pneumococcal infection is to be reduced.”7
After initial studies carried out in South African gold miners, the FDA approved a pneumococcal polysaccharide vaccine (PPSV) against 23 of about 90 circulating serotypes.8 The CDC and the Advisory Committee on Immunization Practices (ACIP) initially recommended PPSV-23 for persons perceived to be at high risk for pneumococcal disease, including those with chronic diseases, immunocompromising conditions, and older adults.9
Three decades later, the analysis of a large body of available evidence demonstrated the protective effects of PPSV-23 against invasive pneumococcal disease caused by pneumococcal types included in the vaccine, especially bacteremic pneumonia (about 75% reduction, odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14-0.45).10 A perceived shortcoming of PPSV-23, which some experts dispute, is the lack of definite protection against nonbacteremic pneumococcal pneumonia.11 Studies of nursing home residents demonstrated a 50% reduction in the incidence of both pneumococcal pneumonia and all-cause pneumonia, suggesting that PPSV-23 offers protection against noninvasive pneumococcal pneumonia in specific populations.12 Additional potential limitations of PPSV-23 include reduced benefit in patients aged > 65 years and waning of protection over time.13
Related: Identification and Management of Middle East Respiratory Syndrome
Protein-Conjugate Vaccines
Clearly, the most important limitation of PPSV-23, inherent to all capsular polysaccharide vaccines, is that it does not elicit an immune response in children aged < 2 years. The successful development of a vaccine against Haemophilus influenzae type b (Hib) gave rise to a new generation of pneumococcal vaccines.14 Specifically, the Hib vaccine covalently binds, or conjugates, the capsule polysaccharide to an antigenic protein, leading to effective T-cell–mediated antibody production in infants and toddlers.
In 2000, children received the first iteration of a protein-conjugate vaccine containing the 7 most relevant pneumococcal serotypes (PCV-7).15 The effects of PCV-7 on pneumococcal disease have been extraordinary, practically eliminating infections caused by the pneumococcal serotypes included in the vaccine. Immunizing children with PCV-7 also ushered in a fundamental public health benefit for adults aged > 65 years: a reduction of nearly 90% in the incidence of pneumococcal infections caused by serotypes included in the vaccine. By eliciting mucosal immunity, which leads to decreased nasal carriage of the covered pneumococcal strains among children, PVC-7 generates herd immunity, leading to reductions in transmission, colonization, and infection with vaccine serotypes among adults.16
In 2010, a conjugate vaccine containing 13 serotypes (PCV-13) replaced PCV-7 administration for children.17,18 The PCV-13 is expected to protect children and the herd from disease caused by 6 additional pneumococcal serotypes, including those that surged as replacement strains, filling the ecologic niche created by PCV-7, such as the epidemiologically relevant serotype 19A.19,20
Immune-Compromised Adults
One of the shortcomings of PPSV-23 is its lack of efficacy in patients with advanced HIV, a group with an exquisite vulnerability to pneumococcal disease, as demonstrated by a randomized controlled trial of PPSV-23 in African patients with advanced, untreated HIV infection.21 Similarly, there is concern that patients with lymphoma, leukemia, multiple myeloma, and Hodgkin disease, also at high risk of pneumococcal infection, do not mount sufficient immune responses to polysaccharide antigens or that these responses are adversely affected by chemotherapy or immune suppressing medications. In these populations, a conjugate vaccine (PCV-7 or PCV-13) may elicit a more robust and durable immune response than a polysaccharide vaccine (PPSV-23). A randomized placebo-controlled trial demonstrated the efficacy of PCV-7 in protecting patients with advanced HIV against pneumococcal disease in Africa.22 Based in part on this observation, the ACIP now recommends the use of PCV-13 in patients with HIV and other immunosuppressing conditions, including chronic renal failure.23 A direct comparison of the relative protection of PPSV-23 vs PCV-7 in this population has not been performed.
Pneumococcal Vaccines in the Elderly
Although PPSV-23 was widely adopted in the U.S. with the intention to protect adults aged > 65 years from pneumococcal infection, this vaccine did not achieve global appeal. In the Netherlands, for instance, PPSV-23 had very low penetration and was never endorsed by public health authorities. Concerns have been issued about the decreased immunogenicity of a polysaccharide vaccine in older adults, invoking the concept of immune senescence, a term that describes a diminished capacity to mount robust immune responses due to aging.
Protein-conjugated antigens, on the other hand, can elicit a better initial immune response than a polysaccharide vaccine can in an older adult. Whether the effect is sustained and translates to better clinical outcomes remains unknown. The adoption of PCV-13 and the continued role of PPSV-23 in adults aged > 65 years have been examined carefully by experts.24,25
Dutch investigators organized the Community Acquired Pneumonia Immunization Trial in Adults (CAPITA), randomizing about 85,000 adults aged > 65 years toreceive eitherPCV-13 or a placebo.26 After 3 years of observation, the occurrence of invasive pneumococcal disease (caused by the vaccine serotypes) decreased by 85% in participants who received the vaccine compared with those who received a placebo. Additionally, the incidence of pneumococcal pneumonia (caused by the vaccine serotypes) decreased by 45% (Table 2). The findings of this trial largely inform the recommendation issued by APIC in October 2014 to administer PCV-13 vaccine to adults aged > 65 years.27
Polysaccharide Vaccine in Adults
A crucial limitation of the CAPITA study is that it does not provide a head-to-head comparison with PPSV-23. Similarly, the recommendation to use PCV-13 in patients with immune system compromising conditions did not arise from a direct comparison between PCV-13 and PPSV-23. In both groups of patients, ACIP continues to recommend PPSV-23 to extend protection to the 10 additional pneumococcal serotypes not included in PCV-13.28 Additionally, ACIP maintains its long-standing recommendation to administer PPSV-23 to adults aged < 65 years who have diabetes mellitus, or chronic liver, lung, or heart disease, or those who are tobacco smokers or misuse alcohol.
Notably, the order in which the vaccines are administered may influence their effectiveness. In adults aged 50 to 64 years, initial vaccination with PCV-13 followed by PPSV-23 lead to a better antipneumococcal immune response.14,29 Specifically, PCV-13 enhanced the response to subsequent administration of PPSV-23, whereas an initial PPSV-23 vaccine resulted in a decreased response to subsequent administration of PCV-13. After a 1-year interval between vaccines, immune titers to a second vaccination were not inferior.30 All the above considerations have shaped the ACIP recommendations for the administration of pneumococcal vaccines.27,31 Pneumococcal vaccination was a straightforward exercise when PPSV-23 was the only vaccine available for adults. The advent of PCV-13, however, renders pneumococcal immunization of adults more complicated (Figure 1).
Improving Vaccination Rates
The complex recommendations to vaccinate adults against pneumococcus may reveal obstacles to effective pneumococcal vaccination programs.32 From the perspective of health care institutions, the adoption of the new pneumococcal vaccine implies an added cost. A pneumococcal vaccination strategy that incorporates PCV-13 may be cost-effective, at least under certain parameters.33-35 There remains, however, the issue of affordability and the opportunity cost—the need to decrease funding for other health care programs to accommodate an increased budget for pneumococcal vaccination. Logistically, maintaining a reliable supply of vaccines to meet the demand of practitioners at various sites requires careful planning. Consequently, tracking vaccinated and unvaccinated patients and carefully coordinating among clinical providers, nurses, and pharmacists are essential.
From the perspective of clinical providers, an additional pneumococcal vaccine complicating the vaccination schedule for adults represents an increased burden. Providers will need information to reach their own conclusions regarding the rationale behind the development and use of pneumococcal conjugate vaccines, the existing and evolving recommendations from public health authorities, and the strengths and limitations of the evidence supporting the use of pneumococcal vaccines. Otherwise, providers may find it difficult to incorporate new data and guidelines supporting pneumococcal vaccination into their decision-making (Boxes 1-4). An additional and formidable challenge is to carve out time during an already busy clinical encounter to discuss pneumococcal vaccines and other immunizations.36
Related: The Importance of an Antimicrobial Stewardship Program
Similarly, older adults or patients with chronic health conditions may not recognize the important role that vaccines can play in their health maintenance and are likely to prioritize other issues during medical visits. It is not obvious for patients that multiple vaccines may be necessary to prevent pneumococcal disease. Furthermore, many patients, not unreasonably, may assume that their yearly influenza vaccine is sufficiently protective against pneumonia. Therefore, patients need to be educated about the rationale behind pneumococcal conjugate vaccination. Ultimately, access to immunization—the opportunity for patients to have an encounter with their providers and with the health care system that results in the administration of an appropriate vaccine—will determine whether goals for pneumococcal vaccination are achieved.
The evolving landscape for the implementation of pneumococcal vaccines creates the need to develop, implement, and refine organizational changes to adhere to the new guidelines for the use of PCV-13 and PPSV-23 vaccines. These interventions, if effective, may help improve pneumococcal vaccination coverage among adults (Table 3).
Harnessing the Power of the EMR
Interventions to improve the adherence to pneumococcal vaccination guidelines begin by identifying persons eligible for vaccination based on their age, their vaccination status (ie, persons previously unvaccinated or due for vaccination according to the recommended schedule), or the presence of medical conditions conferring high risk for pneumococcal disease. This, in turn, depends on adequate documentation of patients’ underlying medical diagnosis, as well as up-to-date records of vaccine administration to patients.
Health care systems possessing a mature and sophisticated electronic medical record (EMR), such as the VHA Computerized Patient Record System (CPRS), are in a good position to wield such information to plan, implement, and assure the quality of activities designed to improve pneumococcal vaccination rates. An analysis of the proportion of veterans in VISN 10 who received pneumococcal vaccination revealed that even with the advantages of a robust EMR and a highly developed infrastructure devoted to primary care, pneumococcal vaccine coverage remains below the 60% target goal, although well above national averages (Figure 2).37,38
Standing Orders
Standing orders make it possible for nurses and pharmacists to administer vaccines according to a preestablished protocol without a physician’s direct evaluation of each patient. Standing orders are a versatile intervention, with a record of effective implementation in both outpatient and inpatient facilities, in acute-care and long-term care facilities, and in most instances where patients interact with the health care system. Based on strong scientific evidence, ACIP recommends the adoption of standing-order programs to improve pneumococcal vaccination rates among adults.39 Indeed, standing-order programs may prove a very effective intervention to fulfill the recommendation to administer PCV-13 to adults aged > 65 years.
The Virtual Vaccination Clinic
Unfortunately, with 2 vaccines that have to be administered at different times to various groups of patients at risk, the current state of pneumococcal vaccination may be too complex to be readily reduced to a comprehensible set of standing orders. An innovative way to realize the benefit of standing orders is to target high-risk groups for pneumococcal disease who are eligible for vaccination by selecting them using the EMR and entering standing orders tailored to their specific vaccination needs. The selection of patients according to comorbidities and vaccination status and the determination of the appropriate pneumococcal vaccine takes place in the context of a “virtual” vaccination clinic.40
Enhancing Vaccinations
Further improvement in pneumococcal vaccination rates are likely to result from interventions that increase the demand for vaccines among patients and practitioners. Efforts to disseminate information and provide advice regarding pneumococcal vaccination are likely to result in patients seeking and clinicians offering the appropriate vaccine. Similarly, interventions to enhance the supply of vaccines at the point of care may reduce barriers that patients might encounter when attempting to receive vaccinations.
Another set of system-based interventions that can assist clinicians in making timely and appropriate vaccination decisions are EMR reminders, especially those targeting patients at high risk for pneumococcal disease because of underlying illnesses.41 Previous experience with pneumococcal vaccination in patients aged > 65 years indicates that EMR reminders facilitate improvements in vaccination. The involvement of a panel manager who coordinated with the primary care provider and contacted patients directly augmented the effect of the EMR reminder by 25%.42
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Patient reminder and recall systems also demonstrated effectiveness in improving immunization rates.43 In certain groups, notification of patients has been achieved through electronic methods, such as short text messaging or e-mail.44 Determining which interventions within a bundle are essential may be impossible, because the various interventions reinforce one another, and the likelihood of patients benefitting from at least one of the activities increases when multiple interventions are administered together. Therefore, the Task Force on Community Preventive Services supports combining provider reminder systems with education and other measures that encourage use of vaccines in patients and providers.45
Box answer key: 1: A; 2: A; 3: B; 4: A.
Conclusion
The increasing role of vaccines in the health maintenance of adults represents a change in paradigm for primary care and specialty providers. Physicians must assess the value and limitations of vaccines and find time to discuss immunizations with their adult patients. Health care systems can increase opportunities for vaccination and facilitate encounters that result in vaccination by educating patients and health care personnel and through the innovative use of reminders and standing orders in the EMR. Undertaking these activities may limit the burden of pneumococcal disease, an important cause of morbidity and mortality in adults that is preventable through immunization.
Acknowledgements
This work is dedicated to the memory of John M. Rieger, Information Technology Specialist at the Cleveland VAMC and Chief Master Sergeant, Air Force Reserve.
Author Disclosure
This work was supported by a research grant from Pfizer and by the Louis Stokes Cleveland VAMC, the VISN 10 Geriatric Research Education and Clinical Center, and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences of the National Institutes of Health NIH). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Streptococcus pneumoniae (S pneumoniae), also known as pneumococcus, is a successful human pathogen with significant clinical impact that causes pneumonia and invasive infections, including bacteremia and meningitis.1 In the preantibiotic era, nearly 80% of patients with bacteremic pneumococcal pneumonia died.2 The introduction of sulfas and penicillin in the mid-20th century, subsequent refinements in antibiotic chemotherapy, and improvements in supportive care rendered pneumococcal disease readily treatable, notwithstanding the threat of antibiotic-resistant pneumococcus.3 Despite the availability of effective antibiotic therapy against S pneumoniae, pneumococcal disease remains a significant cause of morbidity and mortality among people with increased susceptibility, such as older adults and those living with chronic illness or immunosuppressive conditions. In developed countries like the U.S., where a growing portion of the population is vulnerable to S pneumoniae by virtue of their advanced age and underlying medical conditions, pneumococcal disease is still an important public health concern.4
Penumococcal Vaccines: A Long Time Coming
Vaccination against S pneumoniae has proven an efficacious strategy to reduce the morbidity and mortality associated with this pathogen.5 The original efforts to develop a pneumococcal vaccine culminated in 1945 with a vaccine containing pneumococcal capsular polysaccharides, which elicited a protective immune response among U.S. soldiers (Table 1).Subsequent investigations determined that the protective response was specific to pneumococcal disease caused by the 4 pneumococcal capsular serotypes included in the vaccine, that the carrier rate of pneumococcus with the vaccine serotypes decreased by about 50%, and that the incidence of pneumonia from the vaccine serotypes was reduced even in nonimmunized soldiers.6 These early observations remain relevant to our contemporary understanding of the impact of pneumococcal vaccination: Protection is limited to serotypes included in the vaccine; the vaccine reduces colonization; and the vaccine leads to herd immunity—the protection of unvaccinated subjects.
Despite this achievement, the use of vaccines as a strategy to combat pneumococcal disease was upstaged in the subsequent decades by the success of antibiotics. Renewed interest in pneumococcal vaccines resulted from the efforts of Robert Austrian, MD, who astutely observed that “highly effective antimicrobial drugs must be supplemented by other measures, both prophylactic and therapeutic, if the significant mortality resulting still from pneumococcal infection is to be reduced.”7
After initial studies carried out in South African gold miners, the FDA approved a pneumococcal polysaccharide vaccine (PPSV) against 23 of about 90 circulating serotypes.8 The CDC and the Advisory Committee on Immunization Practices (ACIP) initially recommended PPSV-23 for persons perceived to be at high risk for pneumococcal disease, including those with chronic diseases, immunocompromising conditions, and older adults.9
Three decades later, the analysis of a large body of available evidence demonstrated the protective effects of PPSV-23 against invasive pneumococcal disease caused by pneumococcal types included in the vaccine, especially bacteremic pneumonia (about 75% reduction, odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14-0.45).10 A perceived shortcoming of PPSV-23, which some experts dispute, is the lack of definite protection against nonbacteremic pneumococcal pneumonia.11 Studies of nursing home residents demonstrated a 50% reduction in the incidence of both pneumococcal pneumonia and all-cause pneumonia, suggesting that PPSV-23 offers protection against noninvasive pneumococcal pneumonia in specific populations.12 Additional potential limitations of PPSV-23 include reduced benefit in patients aged > 65 years and waning of protection over time.13
Related: Identification and Management of Middle East Respiratory Syndrome
Protein-Conjugate Vaccines
Clearly, the most important limitation of PPSV-23, inherent to all capsular polysaccharide vaccines, is that it does not elicit an immune response in children aged < 2 years. The successful development of a vaccine against Haemophilus influenzae type b (Hib) gave rise to a new generation of pneumococcal vaccines.14 Specifically, the Hib vaccine covalently binds, or conjugates, the capsule polysaccharide to an antigenic protein, leading to effective T-cell–mediated antibody production in infants and toddlers.
In 2000, children received the first iteration of a protein-conjugate vaccine containing the 7 most relevant pneumococcal serotypes (PCV-7).15 The effects of PCV-7 on pneumococcal disease have been extraordinary, practically eliminating infections caused by the pneumococcal serotypes included in the vaccine. Immunizing children with PCV-7 also ushered in a fundamental public health benefit for adults aged > 65 years: a reduction of nearly 90% in the incidence of pneumococcal infections caused by serotypes included in the vaccine. By eliciting mucosal immunity, which leads to decreased nasal carriage of the covered pneumococcal strains among children, PVC-7 generates herd immunity, leading to reductions in transmission, colonization, and infection with vaccine serotypes among adults.16
In 2010, a conjugate vaccine containing 13 serotypes (PCV-13) replaced PCV-7 administration for children.17,18 The PCV-13 is expected to protect children and the herd from disease caused by 6 additional pneumococcal serotypes, including those that surged as replacement strains, filling the ecologic niche created by PCV-7, such as the epidemiologically relevant serotype 19A.19,20
Immune-Compromised Adults
One of the shortcomings of PPSV-23 is its lack of efficacy in patients with advanced HIV, a group with an exquisite vulnerability to pneumococcal disease, as demonstrated by a randomized controlled trial of PPSV-23 in African patients with advanced, untreated HIV infection.21 Similarly, there is concern that patients with lymphoma, leukemia, multiple myeloma, and Hodgkin disease, also at high risk of pneumococcal infection, do not mount sufficient immune responses to polysaccharide antigens or that these responses are adversely affected by chemotherapy or immune suppressing medications. In these populations, a conjugate vaccine (PCV-7 or PCV-13) may elicit a more robust and durable immune response than a polysaccharide vaccine (PPSV-23). A randomized placebo-controlled trial demonstrated the efficacy of PCV-7 in protecting patients with advanced HIV against pneumococcal disease in Africa.22 Based in part on this observation, the ACIP now recommends the use of PCV-13 in patients with HIV and other immunosuppressing conditions, including chronic renal failure.23 A direct comparison of the relative protection of PPSV-23 vs PCV-7 in this population has not been performed.
Pneumococcal Vaccines in the Elderly
Although PPSV-23 was widely adopted in the U.S. with the intention to protect adults aged > 65 years from pneumococcal infection, this vaccine did not achieve global appeal. In the Netherlands, for instance, PPSV-23 had very low penetration and was never endorsed by public health authorities. Concerns have been issued about the decreased immunogenicity of a polysaccharide vaccine in older adults, invoking the concept of immune senescence, a term that describes a diminished capacity to mount robust immune responses due to aging.
Protein-conjugated antigens, on the other hand, can elicit a better initial immune response than a polysaccharide vaccine can in an older adult. Whether the effect is sustained and translates to better clinical outcomes remains unknown. The adoption of PCV-13 and the continued role of PPSV-23 in adults aged > 65 years have been examined carefully by experts.24,25
Dutch investigators organized the Community Acquired Pneumonia Immunization Trial in Adults (CAPITA), randomizing about 85,000 adults aged > 65 years toreceive eitherPCV-13 or a placebo.26 After 3 years of observation, the occurrence of invasive pneumococcal disease (caused by the vaccine serotypes) decreased by 85% in participants who received the vaccine compared with those who received a placebo. Additionally, the incidence of pneumococcal pneumonia (caused by the vaccine serotypes) decreased by 45% (Table 2). The findings of this trial largely inform the recommendation issued by APIC in October 2014 to administer PCV-13 vaccine to adults aged > 65 years.27
Polysaccharide Vaccine in Adults
A crucial limitation of the CAPITA study is that it does not provide a head-to-head comparison with PPSV-23. Similarly, the recommendation to use PCV-13 in patients with immune system compromising conditions did not arise from a direct comparison between PCV-13 and PPSV-23. In both groups of patients, ACIP continues to recommend PPSV-23 to extend protection to the 10 additional pneumococcal serotypes not included in PCV-13.28 Additionally, ACIP maintains its long-standing recommendation to administer PPSV-23 to adults aged < 65 years who have diabetes mellitus, or chronic liver, lung, or heart disease, or those who are tobacco smokers or misuse alcohol.
Notably, the order in which the vaccines are administered may influence their effectiveness. In adults aged 50 to 64 years, initial vaccination with PCV-13 followed by PPSV-23 lead to a better antipneumococcal immune response.14,29 Specifically, PCV-13 enhanced the response to subsequent administration of PPSV-23, whereas an initial PPSV-23 vaccine resulted in a decreased response to subsequent administration of PCV-13. After a 1-year interval between vaccines, immune titers to a second vaccination were not inferior.30 All the above considerations have shaped the ACIP recommendations for the administration of pneumococcal vaccines.27,31 Pneumococcal vaccination was a straightforward exercise when PPSV-23 was the only vaccine available for adults. The advent of PCV-13, however, renders pneumococcal immunization of adults more complicated (Figure 1).
Improving Vaccination Rates
The complex recommendations to vaccinate adults against pneumococcus may reveal obstacles to effective pneumococcal vaccination programs.32 From the perspective of health care institutions, the adoption of the new pneumococcal vaccine implies an added cost. A pneumococcal vaccination strategy that incorporates PCV-13 may be cost-effective, at least under certain parameters.33-35 There remains, however, the issue of affordability and the opportunity cost—the need to decrease funding for other health care programs to accommodate an increased budget for pneumococcal vaccination. Logistically, maintaining a reliable supply of vaccines to meet the demand of practitioners at various sites requires careful planning. Consequently, tracking vaccinated and unvaccinated patients and carefully coordinating among clinical providers, nurses, and pharmacists are essential.
From the perspective of clinical providers, an additional pneumococcal vaccine complicating the vaccination schedule for adults represents an increased burden. Providers will need information to reach their own conclusions regarding the rationale behind the development and use of pneumococcal conjugate vaccines, the existing and evolving recommendations from public health authorities, and the strengths and limitations of the evidence supporting the use of pneumococcal vaccines. Otherwise, providers may find it difficult to incorporate new data and guidelines supporting pneumococcal vaccination into their decision-making (Boxes 1-4). An additional and formidable challenge is to carve out time during an already busy clinical encounter to discuss pneumococcal vaccines and other immunizations.36
Related: The Importance of an Antimicrobial Stewardship Program
Similarly, older adults or patients with chronic health conditions may not recognize the important role that vaccines can play in their health maintenance and are likely to prioritize other issues during medical visits. It is not obvious for patients that multiple vaccines may be necessary to prevent pneumococcal disease. Furthermore, many patients, not unreasonably, may assume that their yearly influenza vaccine is sufficiently protective against pneumonia. Therefore, patients need to be educated about the rationale behind pneumococcal conjugate vaccination. Ultimately, access to immunization—the opportunity for patients to have an encounter with their providers and with the health care system that results in the administration of an appropriate vaccine—will determine whether goals for pneumococcal vaccination are achieved.
The evolving landscape for the implementation of pneumococcal vaccines creates the need to develop, implement, and refine organizational changes to adhere to the new guidelines for the use of PCV-13 and PPSV-23 vaccines. These interventions, if effective, may help improve pneumococcal vaccination coverage among adults (Table 3).
Harnessing the Power of the EMR
Interventions to improve the adherence to pneumococcal vaccination guidelines begin by identifying persons eligible for vaccination based on their age, their vaccination status (ie, persons previously unvaccinated or due for vaccination according to the recommended schedule), or the presence of medical conditions conferring high risk for pneumococcal disease. This, in turn, depends on adequate documentation of patients’ underlying medical diagnosis, as well as up-to-date records of vaccine administration to patients.
Health care systems possessing a mature and sophisticated electronic medical record (EMR), such as the VHA Computerized Patient Record System (CPRS), are in a good position to wield such information to plan, implement, and assure the quality of activities designed to improve pneumococcal vaccination rates. An analysis of the proportion of veterans in VISN 10 who received pneumococcal vaccination revealed that even with the advantages of a robust EMR and a highly developed infrastructure devoted to primary care, pneumococcal vaccine coverage remains below the 60% target goal, although well above national averages (Figure 2).37,38
Standing Orders
Standing orders make it possible for nurses and pharmacists to administer vaccines according to a preestablished protocol without a physician’s direct evaluation of each patient. Standing orders are a versatile intervention, with a record of effective implementation in both outpatient and inpatient facilities, in acute-care and long-term care facilities, and in most instances where patients interact with the health care system. Based on strong scientific evidence, ACIP recommends the adoption of standing-order programs to improve pneumococcal vaccination rates among adults.39 Indeed, standing-order programs may prove a very effective intervention to fulfill the recommendation to administer PCV-13 to adults aged > 65 years.
The Virtual Vaccination Clinic
Unfortunately, with 2 vaccines that have to be administered at different times to various groups of patients at risk, the current state of pneumococcal vaccination may be too complex to be readily reduced to a comprehensible set of standing orders. An innovative way to realize the benefit of standing orders is to target high-risk groups for pneumococcal disease who are eligible for vaccination by selecting them using the EMR and entering standing orders tailored to their specific vaccination needs. The selection of patients according to comorbidities and vaccination status and the determination of the appropriate pneumococcal vaccine takes place in the context of a “virtual” vaccination clinic.40
Enhancing Vaccinations
Further improvement in pneumococcal vaccination rates are likely to result from interventions that increase the demand for vaccines among patients and practitioners. Efforts to disseminate information and provide advice regarding pneumococcal vaccination are likely to result in patients seeking and clinicians offering the appropriate vaccine. Similarly, interventions to enhance the supply of vaccines at the point of care may reduce barriers that patients might encounter when attempting to receive vaccinations.
Another set of system-based interventions that can assist clinicians in making timely and appropriate vaccination decisions are EMR reminders, especially those targeting patients at high risk for pneumococcal disease because of underlying illnesses.41 Previous experience with pneumococcal vaccination in patients aged > 65 years indicates that EMR reminders facilitate improvements in vaccination. The involvement of a panel manager who coordinated with the primary care provider and contacted patients directly augmented the effect of the EMR reminder by 25%.42
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Patient reminder and recall systems also demonstrated effectiveness in improving immunization rates.43 In certain groups, notification of patients has been achieved through electronic methods, such as short text messaging or e-mail.44 Determining which interventions within a bundle are essential may be impossible, because the various interventions reinforce one another, and the likelihood of patients benefitting from at least one of the activities increases when multiple interventions are administered together. Therefore, the Task Force on Community Preventive Services supports combining provider reminder systems with education and other measures that encourage use of vaccines in patients and providers.45
Box answer key: 1: A; 2: A; 3: B; 4: A.
Conclusion
The increasing role of vaccines in the health maintenance of adults represents a change in paradigm for primary care and specialty providers. Physicians must assess the value and limitations of vaccines and find time to discuss immunizations with their adult patients. Health care systems can increase opportunities for vaccination and facilitate encounters that result in vaccination by educating patients and health care personnel and through the innovative use of reminders and standing orders in the EMR. Undertaking these activities may limit the burden of pneumococcal disease, an important cause of morbidity and mortality in adults that is preventable through immunization.
Acknowledgements
This work is dedicated to the memory of John M. Rieger, Information Technology Specialist at the Cleveland VAMC and Chief Master Sergeant, Air Force Reserve.
Author Disclosure
This work was supported by a research grant from Pfizer and by the Louis Stokes Cleveland VAMC, the VISN 10 Geriatric Research Education and Clinical Center, and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences of the National Institutes of Health NIH). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154.
2. Tilghman RC, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med (Chic). 1937;59(4):602-619.
3. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36(3):197-204.
4. Lexau CA, Lynfield R, Danila R, et al; Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
5. Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229-241.
6. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82(6):445-465.
7. Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759-776.
8. Austrian R. The Jeremiah Metzger Lecture: Of gold and pneumococci: a history of pneumococcal vaccines in South Africa. Trans Am Clin Climatol Assoc. 1978;89:141-161.
9. Centers for Disease Control (CDC). Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1981;30(33):410-412, 417-419.
10. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
11. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48-58.
12. Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
13. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Eng J Med. 1991;325(21):1453-1460.
14. Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52(5):633-640.
15. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
16. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Eng J Med. 2013;369(2):155-163.
17. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
18. Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children--Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258-261.
19. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics. 2014;134(2):210-217.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535-543.
21. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106-2111.
22. French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Eng J Med. 2010;362(9):812-822.
23. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-819.
24. Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55(2):259-264.
25. Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55(2):265-267.
26. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372(12):1114-1125.
27. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822-825.
28. Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55(2):255-258.
29. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594-3602.
30. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364-2374.
31. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
32. Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Am J Prev Med. 2015;pii:S0749-3797(15)00333-00335 [Epub ahead of print].
33. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011-6021.
34. Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950-3956.
35. Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804-812.
36. Kyaw MH, Greene CM, Schaffner W, et al; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med. 2006;31(4):286-292.
37. Williams WW, Lu PJ, O'Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95-102.
38. Committee on Review of Priorities in the National Vaccine Plan, Institute of Medicine. Priorities for the National Vaccine Plan. Washington, DC: National Academies Press; 2010.
39. McKibben LJ, Stange PV, Sneller VP, Strikas RA, Rodewald LE; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49(RR-1):15-16.
40. Jump RLP, Banks R, Wilson B, et al. A virtual clinic improves pneumococcal vaccination for asplenic veterans at high-risk for pneumococcal disease. Open Forum Infect Dis. In press.
41. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505-1510.
42. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558.
43. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
44. Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: a randomized controlled trial. Vaccine. 2015;33(43):5868-5872.
45. Ndiaye SM, Hopkins DP, Shefer AM, et al; Task Force on Community Preventive Services. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5)(suppl):248-279.
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154.
2. Tilghman RC, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med (Chic). 1937;59(4):602-619.
3. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36(3):197-204.
4. Lexau CA, Lynfield R, Danila R, et al; Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
5. Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229-241.
6. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82(6):445-465.
7. Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759-776.
8. Austrian R. The Jeremiah Metzger Lecture: Of gold and pneumococci: a history of pneumococcal vaccines in South Africa. Trans Am Clin Climatol Assoc. 1978;89:141-161.
9. Centers for Disease Control (CDC). Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1981;30(33):410-412, 417-419.
10. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
11. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48-58.
12. Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
13. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Eng J Med. 1991;325(21):1453-1460.
14. Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52(5):633-640.
15. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
16. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Eng J Med. 2013;369(2):155-163.
17. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
18. Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children--Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258-261.
19. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics. 2014;134(2):210-217.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535-543.
21. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106-2111.
22. French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Eng J Med. 2010;362(9):812-822.
23. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-819.
24. Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55(2):259-264.
25. Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55(2):265-267.
26. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372(12):1114-1125.
27. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822-825.
28. Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55(2):255-258.
29. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594-3602.
30. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364-2374.
31. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
32. Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Am J Prev Med. 2015;pii:S0749-3797(15)00333-00335 [Epub ahead of print].
33. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011-6021.
34. Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950-3956.
35. Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804-812.
36. Kyaw MH, Greene CM, Schaffner W, et al; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med. 2006;31(4):286-292.
37. Williams WW, Lu PJ, O'Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95-102.
38. Committee on Review of Priorities in the National Vaccine Plan, Institute of Medicine. Priorities for the National Vaccine Plan. Washington, DC: National Academies Press; 2010.
39. McKibben LJ, Stange PV, Sneller VP, Strikas RA, Rodewald LE; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49(RR-1):15-16.
40. Jump RLP, Banks R, Wilson B, et al. A virtual clinic improves pneumococcal vaccination for asplenic veterans at high-risk for pneumococcal disease. Open Forum Infect Dis. In press.
41. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505-1510.
42. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558.
43. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
44. Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: a randomized controlled trial. Vaccine. 2015;33(43):5868-5872.
45. Ndiaye SM, Hopkins DP, Shefer AM, et al; Task Force on Community Preventive Services. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5)(suppl):248-279.
Therapeutic Interchange From Rosuvastatin to Atorvastatin in a Veteran Population
Patients with known cardiovascular (CV) disease are at greater risk for CV events.1 Hydroxymethylglutaryl-CoA (HMG Co-A) reductase inhibitors, or statins, have been shown to reduce CV events and to reduce all-cause mortality.1,2 Thus, these agents should be a standard approach to secondary prevention of CV events.1,2 Although the main function of statins is to lower total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels, trials have shown that other lipid-lowering agents have reduced the incidence of CV events but have failed to show any difference in mortality.1 Thus, the therapeutic effects of statins may be a result of “pleiotropic” effects in addition to a reduction in LDL-C.3
As a result, prescribing practices and professional society guidelines have deferred to statins as a first-line choice for lipid-lowering therapy.1 Although each statin varies in its ability to lower LDL-C and inhibit HMG Co-A reductase, as a class, statins have been proven to be safe and efficacious in reducing LDL-C, decreasing risk of coronary artery disease, and decreasing mortality.
The 2013 American College of Cardiology (ACC) and American Heart Association (AHA) guideline on the treatment of blood cholesterol to reduce CV risk in adults resulted in a major shift in clinical practice recommendations. The focus of treatment has changed from LDL-C and TC goals to stratifying patients to either high-intensity or moderate-intensity statin therapy, based on their comorbidities and risk of atherosclerotic CV disease (ASCVD).1 Primary prevention of CV disease has been proposed for patients with diabetes mellitus aged 40 to 75 years, familial hypercholesterolemia (LDL-C > 190 mg/dL), and for patients with an ASCVD risk score > 7.5%. Secondary prevention has been proposed for all patients with a history of ASCVD. Among the available choices for intensive statin therapy, the 2 most potent regimens are atorvastatin (40-80 mg) and rosuvastatin (20-40 mg) daily. The ACC/AHA guideline recommends high-potency therapy with either rosuvastatin or atorvastatin with equal preference.1
Related: New Incentives for Helping Prevent Heart Disease
Statin therapy is generally well tolerated; however, the use of statins is not without risk of adverse drug reactions (ADRs). Skeletal muscle discomfort has been reported in 4% to 10% of patients taking either atorvastatin or rosuvastatin.4,5 Liver enzyme abnormalities are less common, having been reported in only about 2% to 3% of patients taking either atorvastatin or rosuvastatin.4,5 Although muscle-related intolerance and liver enzyme abnormalities are considered to be class effects, research speculates that the therapeutic and safety effects of statins may differ, based on tissue solubility.2,6 Variability in the myotoxic and hepatotoxic effects of statins has been attributed to differences in tissue solubility, hypothesizing that lipophilic statins are more easily taken up into myocytes and hepatocytes, resulting in an increase in toxic effects.2,6
This study assessed differences in therapeutic and safety endpoints resulting from the recent interchange from rosuvastatin to atorvastatin within the North Florida/South Georgia Veterans Health System (NF/SGVHS). Although both these agents are high-potency HMG Co-A reductase inhibitors and share a mechanism of action, they have pharmacokinetic differences, including a key difference in tissue solubility (Table 1).
With the availability of low-cost generic atorvastatin in early 2012, the VA Medical Advisory Panel and Pharmacy Benefits Management (VA MAP/PBM) added atorvastatin to the VA National Formulary as the preferred high-potency statin.7,8 Before October 2012, rosuvastatin had been the preferred high-potency statin within the VA. With support from VA MAP/PBM leadership, NF/SGVHS instituted an interchange from rosuvastatin to atorvastatin for cost-savings purposes.9
Before the interchange, the records of patients were reviewed to determine whether justification existed for continued use of rosuvastatin. Patients were converted to atorvastatin if deemed appropriate and received education and consultation through direct patient contact or a letter regarding the interchange. Justifications for continued use of rosuvastatin included documentation of an atorvastatin ADR, active liver disease, or patients taking cyclosporine or certain protease inhibitors.8
The objective of this retrospective evaluation was to assess the efficacy and safety of the interchange from rosuvastatin to atorvastatin within NF/SGVHS. The results of this review are helpful to confirm the efficacy and safety of the interchange and identify any differences in efficacy and safety that may have occurred as a result of the interchange to a different high-potency statin. For this review, statin efficacy was assessed via review of pre- and postinterchange lipid panel values, assessing for a significant difference between equipotent atorvastatin and rosuvastatin therapy. Similarly, safety was assessed by analysis of pre- and postinterchange liver enzyme panels and assessing for significant differences as a result of the interchange.
Methods
The therapeutic interchange was conducted within the NF/SGVHS, which provides patient care at hospitals in Gainesville, Florida, and Lake City, Florida, and 11 outpatient clinics located throughout North Florida and South Georgia. Like other VA facilities, NF/SGVHS uses Computerized Patient Records System (CPRS) to electronically integrate all clinical patient information, including medical progress notes, consults, admission and discharge summaries, allergies and ADRs, patient problem lists (diagnoses), vital signs, medication orders, and laboratory test results. Approval to conduct this study was granted by the University of Florida Investigational Review Board and the Research and Development Committee at NF/SGVHS.
Therapeutic Interchange Process
Interchange from rosuvastatin to atorvastatin was expected to provide about $643,000 annually in drug cost savings to NF/SGVHS while providing equivalent therapy. The cost for a 30-day supply of rosuvastatin was about $22.56 at the time of interchange, and a 30-day supply of generic atorvastatin was $1.77.The interchange from rosuvastatin to atorvastatin was approved by the Pharmacy and Therapeutics Committee Meeting on August 8, 2012, and began shortly thereafter. Interchanges were halted temporarily in November 2012 due to a shortage of manufacturer supply, but the process fully resumed in January 2013 once the drug shortage resolved. Direction was provided to VA facilities by a guidance letter issued through PBM leadership.8 The interchange used a standard interchange guide to complete the process (Table 2).
Posttherapeutic Interchange Analysis
Researchers conducted an internal pharmacy computerizedprescription records search to identify VA outpatients who were converted from rosuvastatin to atorvastatin from February 1, 2012, to August 1, 2013. A total of 202 patients were randomly selected and included in this retrospective chart review. Investigators analyzed data points for safety and efficacy, including liver function tests (LFTs), lipid panels, and ADR reports. This information was obtained from laboratory data, vital signs, allergy information, ADR data, and progress notes using CPRS. Two sets of laboratory data were obtained for research purposes, the most recent laboratory values pre-interchange and the most recent laboratory values postinterchange to atorvastatin.
Investigators determined whether patients were converted to an equivalent dose of atorvastatin through an assessment of the most recent dosage of rosuvastatin before the interchange and the dosage of atorvastatin postinterchange. Researchers also analyzed refill history and interacting medications to assess possible confounding factors. Medication adherence was assessed via refill history. Medication adherence was defined as a medication possession ratio of at least 70%, which correlated to receipt of 3 or more 90-day supplies in the year prior to interchange. The above data were collected via retrospective chart review and entered into a spreadsheet.
Statistics
All identifying information was removed from the data set prior to statistical analysis. The data analysis for this project was generated using SAS/STAT software, version 9.3 of the SAS System for Linux x64 (SAS Institute, Cary, North Carolina).
Researchers assumed an equal variance in preinterchange and postinterchange lipid and liver panel values. Preinterchange and postinterchange lipid values (LDL-C, HDL-C, total cholesterol [TC], and triglycerides [TGs]) and liver values (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], and creatinine phosphokinase [CPK]) were analyzed by paired t test. All values were reported as mean (SD), and significance was defined as P < .05.
Results
More than 6,000 veterans within the NF/SGVHS were identified as eligible for the interchange. Of those who were converted, 202 patient records were randomly selected and reviewed. Patient population characteristics are summarized in Table 3. Most patients were aged > 65 years (61.4%) with an average body mass index (BMI) of 32.4. Most patients were converted to the correct corresponding dose of atorvastatin (82.7%) and achieved adherence with statin therapy (84.2%). There was no difference in pre- and postinterchange adherence detected as a result of this review.
Related: New Guideline on Dyslipidemia: Less Is More
Adverse drug reactions were documented in 16 cases, accounting for 8% of the study population. The most commonly reported ADR was myalgia or arthralgia, which was found in 10 cases (5%). Other ADRs identified in this retrospective review included treatment failure, nausea, vomiting, abdominal discomfort, abnormal liver enzymes, nasopharyngitis, and pruritis (Table 4). Of note, treatment failure was determined on a case-by-case basis but was generally defined as a failure to reach LDL-C goal (< 100 mg/dL or < 70 mg/dL), despite titration of atorvastatin. Interacting medications were identified in 30.7% of patients; however, no reported ADRs were associated with interacting medications. The most common drug interaction was concomitant niacin, followed by antiarrhythmics (ie, amiodarone, diltiazem, etc), and fibrates (ie, gemfibrozil, fenofibrate). All potentially interacting medications identified in this retrospective chart review are compiled in Table 5.
No significant difference between mean pre- and postinterchange lipid panel values was identified in this retrospective chart review (Table 6). In addition, no significant difference was detected in pre- and post-interchange AST, ALT, and CPK values (Table 7). However, a statistically significant increase in ALP was detected, with a mean ALP of 73.33 IU/L prior to interchange and 83.64 IU/L postinterchange (P < .0001).
Discussion
The goal of this retrospective observation was to ensure that safety and efficacy were not compromised as a result of this cost-saving therapeutic interchange. No differences in liver enzymes (safety) and lipid control (effectiveness) were observed in this study. There were no statistically significant changes to the lipid panel or liver panel detected with the exception of ALP. The reason for this statistically significant increase is unknown; however, it may support the hypothesis of variation in hepatocellular effects between the statins due to lipophilic properties.3,6 In general, liver enzymes can be affected by extrahepatic functions. Serum ALP and other liver enzymes can be affected by bone disease, abdominal adiposity, alcoholism, and other concomitant diseases.10 No comorbid conditions were assessed, thus differences in liver enzymes may not be fully attributable to statin therapy. This retrospective review found no clinically significant effect correlated with the increase in ALP.
The results of this analysis are congruent with similar therapeutic interchange studies, which resulted in cost savings without compromising safety or efficacy.9,11 Unlike other therapeutic interchange studies, this study analyzed both safety and lipid-lowering efficacy outcomes, instead of focusing solely on changes in LDL-C lowering, total cost savings, and/or adherence.12,13 By including the entire lipid panel and liver panel into the review, this study conducted a more inclusive review of interchangeability with statins, addressing issues such as HDL-C lowering, TG changes, and liver enzyme fluctuation on conversion. There had not been a sufficient time to assess efficacy in terms of CV outcomes.
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Two adverse events alluded to therapeutic failure as a reason for discontinuing atorvastatin. In the previous ATP III lipid guidelines, therapeutic failure was achieved when patients did not reach their LDL-C goals despite appropriate titration of statin therapy.2 However, the ACC/AHA lipid guidelines have done away with lipid goals as a measurement of treatment therapy, focusing rather on evidence-based high- or moderate-intensity statin therapy that has been proven in clinical trials to reduce mortality and CV events.1 Although measurement of efficacy via lipid panel values is no longer a guideline recommendation, the results of this chart review have shown no difference in lipid values as a result of the interchange, confirming the interchangeability of rosuvastatin and atorvastatin at their equivalent doses.
Limitations
The interchange of rosuvastatin to atorvastatin was a policy change affecting all patients within the NF/SGVHS. In order to reflect true population data and more accurately predict the effects of such a policy change, this study used intention-to-treat analysis, including all patients, even patients who were found to be nonadherent. This study is also limited by sample size (N = 202). Additionally, the generalizability of these findings may be limited. The study population was mostly males aged > 65 years with an average BMI of 32.4. Researchers did not compile comorbidity, race, or concomitant medication data. Additionally, the duration of statin therapy prior to laboratory value collection was undefined.
A retrospective chart review lends itself to limitations in data collection. Medication adherence is a factor that is assumed to have a significant effect on the results of this interchange. In this review, adherence was assessed via refill history. Researchers were unable to confirm actual consumption of the medication.
Additionally, researchers did not analyze comorbid conditions, which may have had an effect on lipid panel and liver panel values. For those veterans who discontinued atorvastatin therapy, the reason for discontinuation was often not documented. Thus, researchers were unable to assess reasons for discontinuation.
Conclusion
The results generated from a review of the therapeutic exchange of rosuvastatin to atorvastatin within a veteran population affirm that the interchange was not associated with any differences in safety or lipid control, but did result in significant drug cost savings. This study provides support for health care systems considering therapeutic interchange with high-intensity statins safely and effectively.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida. The authors would like to acknowledge Kim Hoang, PharmD, for her contributions to this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
2. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
3. McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26(4 suppl 3):III32-III38.
4. Pfizer. Lipitor [package insert]. New York, NY: Pfizer; 2015.
5. Crestor [package insert]. Wilmington, DE:AstraZeneca; 2015.
6. Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116(6):408-416.
7. FDA approves first generic version of cholesterol-lowering drug Lipitor [news release]. U.S. Food and Drug Administration; November 30, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm281817.htm. Accessed November 11, 2015.
8. U.S. Department of Veterans Affairs. VHA Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. Interchange to generic atorvastatin-recommendations. Atorvastatin Conversion Guidance. 2012. U.S. Department of Veterans Affairs intranet website. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Therapeutic%20Interchange%20Guidance/Atorvastatin%20Conversion%20Guidance%20(PBM-MAP-VPE)-Final.docx. Accessed November 16, 2015.
9. Pratt DS, Kaplan MM. Evaluation of Liver Function. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw Hill Professional Publishing; 2011:2527-2530.
10. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy . 2001;21(9):1130-1139.
11. Billups SJ, Plushner SL, Olson KI, Koehler TJ, Kerzee J. Clinical and economic outcomes of conversion of simvastatin to lovastatin in a group-model health maintenance organization. J Manag Care Pharm. 2005;11(8):681-686.
12. Schachtner JM, Guharoy R, Medicis JJ, Newman N, Speizer R. Prevalence and cost savings of therapeutic interchange among U.S. hospitals. Am J Health Syst Pharm. 2002;59(6):529-533.
13. Hilleman DE, Wurdeman RL, Lenz TL. Therapeutic change of HMG-CoA reductase inhibitors in patients with coronary artery disease. Pharmacotherapy. 2001;21(4):410-415.
Patients with known cardiovascular (CV) disease are at greater risk for CV events.1 Hydroxymethylglutaryl-CoA (HMG Co-A) reductase inhibitors, or statins, have been shown to reduce CV events and to reduce all-cause mortality.1,2 Thus, these agents should be a standard approach to secondary prevention of CV events.1,2 Although the main function of statins is to lower total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels, trials have shown that other lipid-lowering agents have reduced the incidence of CV events but have failed to show any difference in mortality.1 Thus, the therapeutic effects of statins may be a result of “pleiotropic” effects in addition to a reduction in LDL-C.3
As a result, prescribing practices and professional society guidelines have deferred to statins as a first-line choice for lipid-lowering therapy.1 Although each statin varies in its ability to lower LDL-C and inhibit HMG Co-A reductase, as a class, statins have been proven to be safe and efficacious in reducing LDL-C, decreasing risk of coronary artery disease, and decreasing mortality.
The 2013 American College of Cardiology (ACC) and American Heart Association (AHA) guideline on the treatment of blood cholesterol to reduce CV risk in adults resulted in a major shift in clinical practice recommendations. The focus of treatment has changed from LDL-C and TC goals to stratifying patients to either high-intensity or moderate-intensity statin therapy, based on their comorbidities and risk of atherosclerotic CV disease (ASCVD).1 Primary prevention of CV disease has been proposed for patients with diabetes mellitus aged 40 to 75 years, familial hypercholesterolemia (LDL-C > 190 mg/dL), and for patients with an ASCVD risk score > 7.5%. Secondary prevention has been proposed for all patients with a history of ASCVD. Among the available choices for intensive statin therapy, the 2 most potent regimens are atorvastatin (40-80 mg) and rosuvastatin (20-40 mg) daily. The ACC/AHA guideline recommends high-potency therapy with either rosuvastatin or atorvastatin with equal preference.1
Related: New Incentives for Helping Prevent Heart Disease
Statin therapy is generally well tolerated; however, the use of statins is not without risk of adverse drug reactions (ADRs). Skeletal muscle discomfort has been reported in 4% to 10% of patients taking either atorvastatin or rosuvastatin.4,5 Liver enzyme abnormalities are less common, having been reported in only about 2% to 3% of patients taking either atorvastatin or rosuvastatin.4,5 Although muscle-related intolerance and liver enzyme abnormalities are considered to be class effects, research speculates that the therapeutic and safety effects of statins may differ, based on tissue solubility.2,6 Variability in the myotoxic and hepatotoxic effects of statins has been attributed to differences in tissue solubility, hypothesizing that lipophilic statins are more easily taken up into myocytes and hepatocytes, resulting in an increase in toxic effects.2,6
This study assessed differences in therapeutic and safety endpoints resulting from the recent interchange from rosuvastatin to atorvastatin within the North Florida/South Georgia Veterans Health System (NF/SGVHS). Although both these agents are high-potency HMG Co-A reductase inhibitors and share a mechanism of action, they have pharmacokinetic differences, including a key difference in tissue solubility (Table 1).
With the availability of low-cost generic atorvastatin in early 2012, the VA Medical Advisory Panel and Pharmacy Benefits Management (VA MAP/PBM) added atorvastatin to the VA National Formulary as the preferred high-potency statin.7,8 Before October 2012, rosuvastatin had been the preferred high-potency statin within the VA. With support from VA MAP/PBM leadership, NF/SGVHS instituted an interchange from rosuvastatin to atorvastatin for cost-savings purposes.9
Before the interchange, the records of patients were reviewed to determine whether justification existed for continued use of rosuvastatin. Patients were converted to atorvastatin if deemed appropriate and received education and consultation through direct patient contact or a letter regarding the interchange. Justifications for continued use of rosuvastatin included documentation of an atorvastatin ADR, active liver disease, or patients taking cyclosporine or certain protease inhibitors.8
The objective of this retrospective evaluation was to assess the efficacy and safety of the interchange from rosuvastatin to atorvastatin within NF/SGVHS. The results of this review are helpful to confirm the efficacy and safety of the interchange and identify any differences in efficacy and safety that may have occurred as a result of the interchange to a different high-potency statin. For this review, statin efficacy was assessed via review of pre- and postinterchange lipid panel values, assessing for a significant difference between equipotent atorvastatin and rosuvastatin therapy. Similarly, safety was assessed by analysis of pre- and postinterchange liver enzyme panels and assessing for significant differences as a result of the interchange.
Methods
The therapeutic interchange was conducted within the NF/SGVHS, which provides patient care at hospitals in Gainesville, Florida, and Lake City, Florida, and 11 outpatient clinics located throughout North Florida and South Georgia. Like other VA facilities, NF/SGVHS uses Computerized Patient Records System (CPRS) to electronically integrate all clinical patient information, including medical progress notes, consults, admission and discharge summaries, allergies and ADRs, patient problem lists (diagnoses), vital signs, medication orders, and laboratory test results. Approval to conduct this study was granted by the University of Florida Investigational Review Board and the Research and Development Committee at NF/SGVHS.
Therapeutic Interchange Process
Interchange from rosuvastatin to atorvastatin was expected to provide about $643,000 annually in drug cost savings to NF/SGVHS while providing equivalent therapy. The cost for a 30-day supply of rosuvastatin was about $22.56 at the time of interchange, and a 30-day supply of generic atorvastatin was $1.77.The interchange from rosuvastatin to atorvastatin was approved by the Pharmacy and Therapeutics Committee Meeting on August 8, 2012, and began shortly thereafter. Interchanges were halted temporarily in November 2012 due to a shortage of manufacturer supply, but the process fully resumed in January 2013 once the drug shortage resolved. Direction was provided to VA facilities by a guidance letter issued through PBM leadership.8 The interchange used a standard interchange guide to complete the process (Table 2).
Posttherapeutic Interchange Analysis
Researchers conducted an internal pharmacy computerizedprescription records search to identify VA outpatients who were converted from rosuvastatin to atorvastatin from February 1, 2012, to August 1, 2013. A total of 202 patients were randomly selected and included in this retrospective chart review. Investigators analyzed data points for safety and efficacy, including liver function tests (LFTs), lipid panels, and ADR reports. This information was obtained from laboratory data, vital signs, allergy information, ADR data, and progress notes using CPRS. Two sets of laboratory data were obtained for research purposes, the most recent laboratory values pre-interchange and the most recent laboratory values postinterchange to atorvastatin.
Investigators determined whether patients were converted to an equivalent dose of atorvastatin through an assessment of the most recent dosage of rosuvastatin before the interchange and the dosage of atorvastatin postinterchange. Researchers also analyzed refill history and interacting medications to assess possible confounding factors. Medication adherence was assessed via refill history. Medication adherence was defined as a medication possession ratio of at least 70%, which correlated to receipt of 3 or more 90-day supplies in the year prior to interchange. The above data were collected via retrospective chart review and entered into a spreadsheet.
Statistics
All identifying information was removed from the data set prior to statistical analysis. The data analysis for this project was generated using SAS/STAT software, version 9.3 of the SAS System for Linux x64 (SAS Institute, Cary, North Carolina).
Researchers assumed an equal variance in preinterchange and postinterchange lipid and liver panel values. Preinterchange and postinterchange lipid values (LDL-C, HDL-C, total cholesterol [TC], and triglycerides [TGs]) and liver values (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], and creatinine phosphokinase [CPK]) were analyzed by paired t test. All values were reported as mean (SD), and significance was defined as P < .05.
Results
More than 6,000 veterans within the NF/SGVHS were identified as eligible for the interchange. Of those who were converted, 202 patient records were randomly selected and reviewed. Patient population characteristics are summarized in Table 3. Most patients were aged > 65 years (61.4%) with an average body mass index (BMI) of 32.4. Most patients were converted to the correct corresponding dose of atorvastatin (82.7%) and achieved adherence with statin therapy (84.2%). There was no difference in pre- and postinterchange adherence detected as a result of this review.
Related: New Guideline on Dyslipidemia: Less Is More
Adverse drug reactions were documented in 16 cases, accounting for 8% of the study population. The most commonly reported ADR was myalgia or arthralgia, which was found in 10 cases (5%). Other ADRs identified in this retrospective review included treatment failure, nausea, vomiting, abdominal discomfort, abnormal liver enzymes, nasopharyngitis, and pruritis (Table 4). Of note, treatment failure was determined on a case-by-case basis but was generally defined as a failure to reach LDL-C goal (< 100 mg/dL or < 70 mg/dL), despite titration of atorvastatin. Interacting medications were identified in 30.7% of patients; however, no reported ADRs were associated with interacting medications. The most common drug interaction was concomitant niacin, followed by antiarrhythmics (ie, amiodarone, diltiazem, etc), and fibrates (ie, gemfibrozil, fenofibrate). All potentially interacting medications identified in this retrospective chart review are compiled in Table 5.
No significant difference between mean pre- and postinterchange lipid panel values was identified in this retrospective chart review (Table 6). In addition, no significant difference was detected in pre- and post-interchange AST, ALT, and CPK values (Table 7). However, a statistically significant increase in ALP was detected, with a mean ALP of 73.33 IU/L prior to interchange and 83.64 IU/L postinterchange (P < .0001).
Discussion
The goal of this retrospective observation was to ensure that safety and efficacy were not compromised as a result of this cost-saving therapeutic interchange. No differences in liver enzymes (safety) and lipid control (effectiveness) were observed in this study. There were no statistically significant changes to the lipid panel or liver panel detected with the exception of ALP. The reason for this statistically significant increase is unknown; however, it may support the hypothesis of variation in hepatocellular effects between the statins due to lipophilic properties.3,6 In general, liver enzymes can be affected by extrahepatic functions. Serum ALP and other liver enzymes can be affected by bone disease, abdominal adiposity, alcoholism, and other concomitant diseases.10 No comorbid conditions were assessed, thus differences in liver enzymes may not be fully attributable to statin therapy. This retrospective review found no clinically significant effect correlated with the increase in ALP.
The results of this analysis are congruent with similar therapeutic interchange studies, which resulted in cost savings without compromising safety or efficacy.9,11 Unlike other therapeutic interchange studies, this study analyzed both safety and lipid-lowering efficacy outcomes, instead of focusing solely on changes in LDL-C lowering, total cost savings, and/or adherence.12,13 By including the entire lipid panel and liver panel into the review, this study conducted a more inclusive review of interchangeability with statins, addressing issues such as HDL-C lowering, TG changes, and liver enzyme fluctuation on conversion. There had not been a sufficient time to assess efficacy in terms of CV outcomes.
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Two adverse events alluded to therapeutic failure as a reason for discontinuing atorvastatin. In the previous ATP III lipid guidelines, therapeutic failure was achieved when patients did not reach their LDL-C goals despite appropriate titration of statin therapy.2 However, the ACC/AHA lipid guidelines have done away with lipid goals as a measurement of treatment therapy, focusing rather on evidence-based high- or moderate-intensity statin therapy that has been proven in clinical trials to reduce mortality and CV events.1 Although measurement of efficacy via lipid panel values is no longer a guideline recommendation, the results of this chart review have shown no difference in lipid values as a result of the interchange, confirming the interchangeability of rosuvastatin and atorvastatin at their equivalent doses.
Limitations
The interchange of rosuvastatin to atorvastatin was a policy change affecting all patients within the NF/SGVHS. In order to reflect true population data and more accurately predict the effects of such a policy change, this study used intention-to-treat analysis, including all patients, even patients who were found to be nonadherent. This study is also limited by sample size (N = 202). Additionally, the generalizability of these findings may be limited. The study population was mostly males aged > 65 years with an average BMI of 32.4. Researchers did not compile comorbidity, race, or concomitant medication data. Additionally, the duration of statin therapy prior to laboratory value collection was undefined.
A retrospective chart review lends itself to limitations in data collection. Medication adherence is a factor that is assumed to have a significant effect on the results of this interchange. In this review, adherence was assessed via refill history. Researchers were unable to confirm actual consumption of the medication.
Additionally, researchers did not analyze comorbid conditions, which may have had an effect on lipid panel and liver panel values. For those veterans who discontinued atorvastatin therapy, the reason for discontinuation was often not documented. Thus, researchers were unable to assess reasons for discontinuation.
Conclusion
The results generated from a review of the therapeutic exchange of rosuvastatin to atorvastatin within a veteran population affirm that the interchange was not associated with any differences in safety or lipid control, but did result in significant drug cost savings. This study provides support for health care systems considering therapeutic interchange with high-intensity statins safely and effectively.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida. The authors would like to acknowledge Kim Hoang, PharmD, for her contributions to this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Patients with known cardiovascular (CV) disease are at greater risk for CV events.1 Hydroxymethylglutaryl-CoA (HMG Co-A) reductase inhibitors, or statins, have been shown to reduce CV events and to reduce all-cause mortality.1,2 Thus, these agents should be a standard approach to secondary prevention of CV events.1,2 Although the main function of statins is to lower total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels, trials have shown that other lipid-lowering agents have reduced the incidence of CV events but have failed to show any difference in mortality.1 Thus, the therapeutic effects of statins may be a result of “pleiotropic” effects in addition to a reduction in LDL-C.3
As a result, prescribing practices and professional society guidelines have deferred to statins as a first-line choice for lipid-lowering therapy.1 Although each statin varies in its ability to lower LDL-C and inhibit HMG Co-A reductase, as a class, statins have been proven to be safe and efficacious in reducing LDL-C, decreasing risk of coronary artery disease, and decreasing mortality.
The 2013 American College of Cardiology (ACC) and American Heart Association (AHA) guideline on the treatment of blood cholesterol to reduce CV risk in adults resulted in a major shift in clinical practice recommendations. The focus of treatment has changed from LDL-C and TC goals to stratifying patients to either high-intensity or moderate-intensity statin therapy, based on their comorbidities and risk of atherosclerotic CV disease (ASCVD).1 Primary prevention of CV disease has been proposed for patients with diabetes mellitus aged 40 to 75 years, familial hypercholesterolemia (LDL-C > 190 mg/dL), and for patients with an ASCVD risk score > 7.5%. Secondary prevention has been proposed for all patients with a history of ASCVD. Among the available choices for intensive statin therapy, the 2 most potent regimens are atorvastatin (40-80 mg) and rosuvastatin (20-40 mg) daily. The ACC/AHA guideline recommends high-potency therapy with either rosuvastatin or atorvastatin with equal preference.1
Related: New Incentives for Helping Prevent Heart Disease
Statin therapy is generally well tolerated; however, the use of statins is not without risk of adverse drug reactions (ADRs). Skeletal muscle discomfort has been reported in 4% to 10% of patients taking either atorvastatin or rosuvastatin.4,5 Liver enzyme abnormalities are less common, having been reported in only about 2% to 3% of patients taking either atorvastatin or rosuvastatin.4,5 Although muscle-related intolerance and liver enzyme abnormalities are considered to be class effects, research speculates that the therapeutic and safety effects of statins may differ, based on tissue solubility.2,6 Variability in the myotoxic and hepatotoxic effects of statins has been attributed to differences in tissue solubility, hypothesizing that lipophilic statins are more easily taken up into myocytes and hepatocytes, resulting in an increase in toxic effects.2,6
This study assessed differences in therapeutic and safety endpoints resulting from the recent interchange from rosuvastatin to atorvastatin within the North Florida/South Georgia Veterans Health System (NF/SGVHS). Although both these agents are high-potency HMG Co-A reductase inhibitors and share a mechanism of action, they have pharmacokinetic differences, including a key difference in tissue solubility (Table 1).
With the availability of low-cost generic atorvastatin in early 2012, the VA Medical Advisory Panel and Pharmacy Benefits Management (VA MAP/PBM) added atorvastatin to the VA National Formulary as the preferred high-potency statin.7,8 Before October 2012, rosuvastatin had been the preferred high-potency statin within the VA. With support from VA MAP/PBM leadership, NF/SGVHS instituted an interchange from rosuvastatin to atorvastatin for cost-savings purposes.9
Before the interchange, the records of patients were reviewed to determine whether justification existed for continued use of rosuvastatin. Patients were converted to atorvastatin if deemed appropriate and received education and consultation through direct patient contact or a letter regarding the interchange. Justifications for continued use of rosuvastatin included documentation of an atorvastatin ADR, active liver disease, or patients taking cyclosporine or certain protease inhibitors.8
The objective of this retrospective evaluation was to assess the efficacy and safety of the interchange from rosuvastatin to atorvastatin within NF/SGVHS. The results of this review are helpful to confirm the efficacy and safety of the interchange and identify any differences in efficacy and safety that may have occurred as a result of the interchange to a different high-potency statin. For this review, statin efficacy was assessed via review of pre- and postinterchange lipid panel values, assessing for a significant difference between equipotent atorvastatin and rosuvastatin therapy. Similarly, safety was assessed by analysis of pre- and postinterchange liver enzyme panels and assessing for significant differences as a result of the interchange.
Methods
The therapeutic interchange was conducted within the NF/SGVHS, which provides patient care at hospitals in Gainesville, Florida, and Lake City, Florida, and 11 outpatient clinics located throughout North Florida and South Georgia. Like other VA facilities, NF/SGVHS uses Computerized Patient Records System (CPRS) to electronically integrate all clinical patient information, including medical progress notes, consults, admission and discharge summaries, allergies and ADRs, patient problem lists (diagnoses), vital signs, medication orders, and laboratory test results. Approval to conduct this study was granted by the University of Florida Investigational Review Board and the Research and Development Committee at NF/SGVHS.
Therapeutic Interchange Process
Interchange from rosuvastatin to atorvastatin was expected to provide about $643,000 annually in drug cost savings to NF/SGVHS while providing equivalent therapy. The cost for a 30-day supply of rosuvastatin was about $22.56 at the time of interchange, and a 30-day supply of generic atorvastatin was $1.77.The interchange from rosuvastatin to atorvastatin was approved by the Pharmacy and Therapeutics Committee Meeting on August 8, 2012, and began shortly thereafter. Interchanges were halted temporarily in November 2012 due to a shortage of manufacturer supply, but the process fully resumed in January 2013 once the drug shortage resolved. Direction was provided to VA facilities by a guidance letter issued through PBM leadership.8 The interchange used a standard interchange guide to complete the process (Table 2).
Posttherapeutic Interchange Analysis
Researchers conducted an internal pharmacy computerizedprescription records search to identify VA outpatients who were converted from rosuvastatin to atorvastatin from February 1, 2012, to August 1, 2013. A total of 202 patients were randomly selected and included in this retrospective chart review. Investigators analyzed data points for safety and efficacy, including liver function tests (LFTs), lipid panels, and ADR reports. This information was obtained from laboratory data, vital signs, allergy information, ADR data, and progress notes using CPRS. Two sets of laboratory data were obtained for research purposes, the most recent laboratory values pre-interchange and the most recent laboratory values postinterchange to atorvastatin.
Investigators determined whether patients were converted to an equivalent dose of atorvastatin through an assessment of the most recent dosage of rosuvastatin before the interchange and the dosage of atorvastatin postinterchange. Researchers also analyzed refill history and interacting medications to assess possible confounding factors. Medication adherence was assessed via refill history. Medication adherence was defined as a medication possession ratio of at least 70%, which correlated to receipt of 3 or more 90-day supplies in the year prior to interchange. The above data were collected via retrospective chart review and entered into a spreadsheet.
Statistics
All identifying information was removed from the data set prior to statistical analysis. The data analysis for this project was generated using SAS/STAT software, version 9.3 of the SAS System for Linux x64 (SAS Institute, Cary, North Carolina).
Researchers assumed an equal variance in preinterchange and postinterchange lipid and liver panel values. Preinterchange and postinterchange lipid values (LDL-C, HDL-C, total cholesterol [TC], and triglycerides [TGs]) and liver values (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], and creatinine phosphokinase [CPK]) were analyzed by paired t test. All values were reported as mean (SD), and significance was defined as P < .05.
Results
More than 6,000 veterans within the NF/SGVHS were identified as eligible for the interchange. Of those who were converted, 202 patient records were randomly selected and reviewed. Patient population characteristics are summarized in Table 3. Most patients were aged > 65 years (61.4%) with an average body mass index (BMI) of 32.4. Most patients were converted to the correct corresponding dose of atorvastatin (82.7%) and achieved adherence with statin therapy (84.2%). There was no difference in pre- and postinterchange adherence detected as a result of this review.
Related: New Guideline on Dyslipidemia: Less Is More
Adverse drug reactions were documented in 16 cases, accounting for 8% of the study population. The most commonly reported ADR was myalgia or arthralgia, which was found in 10 cases (5%). Other ADRs identified in this retrospective review included treatment failure, nausea, vomiting, abdominal discomfort, abnormal liver enzymes, nasopharyngitis, and pruritis (Table 4). Of note, treatment failure was determined on a case-by-case basis but was generally defined as a failure to reach LDL-C goal (< 100 mg/dL or < 70 mg/dL), despite titration of atorvastatin. Interacting medications were identified in 30.7% of patients; however, no reported ADRs were associated with interacting medications. The most common drug interaction was concomitant niacin, followed by antiarrhythmics (ie, amiodarone, diltiazem, etc), and fibrates (ie, gemfibrozil, fenofibrate). All potentially interacting medications identified in this retrospective chart review are compiled in Table 5.
No significant difference between mean pre- and postinterchange lipid panel values was identified in this retrospective chart review (Table 6). In addition, no significant difference was detected in pre- and post-interchange AST, ALT, and CPK values (Table 7). However, a statistically significant increase in ALP was detected, with a mean ALP of 73.33 IU/L prior to interchange and 83.64 IU/L postinterchange (P < .0001).
Discussion
The goal of this retrospective observation was to ensure that safety and efficacy were not compromised as a result of this cost-saving therapeutic interchange. No differences in liver enzymes (safety) and lipid control (effectiveness) were observed in this study. There were no statistically significant changes to the lipid panel or liver panel detected with the exception of ALP. The reason for this statistically significant increase is unknown; however, it may support the hypothesis of variation in hepatocellular effects between the statins due to lipophilic properties.3,6 In general, liver enzymes can be affected by extrahepatic functions. Serum ALP and other liver enzymes can be affected by bone disease, abdominal adiposity, alcoholism, and other concomitant diseases.10 No comorbid conditions were assessed, thus differences in liver enzymes may not be fully attributable to statin therapy. This retrospective review found no clinically significant effect correlated with the increase in ALP.
The results of this analysis are congruent with similar therapeutic interchange studies, which resulted in cost savings without compromising safety or efficacy.9,11 Unlike other therapeutic interchange studies, this study analyzed both safety and lipid-lowering efficacy outcomes, instead of focusing solely on changes in LDL-C lowering, total cost savings, and/or adherence.12,13 By including the entire lipid panel and liver panel into the review, this study conducted a more inclusive review of interchangeability with statins, addressing issues such as HDL-C lowering, TG changes, and liver enzyme fluctuation on conversion. There had not been a sufficient time to assess efficacy in terms of CV outcomes.
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Two adverse events alluded to therapeutic failure as a reason for discontinuing atorvastatin. In the previous ATP III lipid guidelines, therapeutic failure was achieved when patients did not reach their LDL-C goals despite appropriate titration of statin therapy.2 However, the ACC/AHA lipid guidelines have done away with lipid goals as a measurement of treatment therapy, focusing rather on evidence-based high- or moderate-intensity statin therapy that has been proven in clinical trials to reduce mortality and CV events.1 Although measurement of efficacy via lipid panel values is no longer a guideline recommendation, the results of this chart review have shown no difference in lipid values as a result of the interchange, confirming the interchangeability of rosuvastatin and atorvastatin at their equivalent doses.
Limitations
The interchange of rosuvastatin to atorvastatin was a policy change affecting all patients within the NF/SGVHS. In order to reflect true population data and more accurately predict the effects of such a policy change, this study used intention-to-treat analysis, including all patients, even patients who were found to be nonadherent. This study is also limited by sample size (N = 202). Additionally, the generalizability of these findings may be limited. The study population was mostly males aged > 65 years with an average BMI of 32.4. Researchers did not compile comorbidity, race, or concomitant medication data. Additionally, the duration of statin therapy prior to laboratory value collection was undefined.
A retrospective chart review lends itself to limitations in data collection. Medication adherence is a factor that is assumed to have a significant effect on the results of this interchange. In this review, adherence was assessed via refill history. Researchers were unable to confirm actual consumption of the medication.
Additionally, researchers did not analyze comorbid conditions, which may have had an effect on lipid panel and liver panel values. For those veterans who discontinued atorvastatin therapy, the reason for discontinuation was often not documented. Thus, researchers were unable to assess reasons for discontinuation.
Conclusion
The results generated from a review of the therapeutic exchange of rosuvastatin to atorvastatin within a veteran population affirm that the interchange was not associated with any differences in safety or lipid control, but did result in significant drug cost savings. This study provides support for health care systems considering therapeutic interchange with high-intensity statins safely and effectively.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida. The authors would like to acknowledge Kim Hoang, PharmD, for her contributions to this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
2. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
3. McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26(4 suppl 3):III32-III38.
4. Pfizer. Lipitor [package insert]. New York, NY: Pfizer; 2015.
5. Crestor [package insert]. Wilmington, DE:AstraZeneca; 2015.
6. Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116(6):408-416.
7. FDA approves first generic version of cholesterol-lowering drug Lipitor [news release]. U.S. Food and Drug Administration; November 30, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm281817.htm. Accessed November 11, 2015.
8. U.S. Department of Veterans Affairs. VHA Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. Interchange to generic atorvastatin-recommendations. Atorvastatin Conversion Guidance. 2012. U.S. Department of Veterans Affairs intranet website. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Therapeutic%20Interchange%20Guidance/Atorvastatin%20Conversion%20Guidance%20(PBM-MAP-VPE)-Final.docx. Accessed November 16, 2015.
9. Pratt DS, Kaplan MM. Evaluation of Liver Function. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw Hill Professional Publishing; 2011:2527-2530.
10. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy . 2001;21(9):1130-1139.
11. Billups SJ, Plushner SL, Olson KI, Koehler TJ, Kerzee J. Clinical and economic outcomes of conversion of simvastatin to lovastatin in a group-model health maintenance organization. J Manag Care Pharm. 2005;11(8):681-686.
12. Schachtner JM, Guharoy R, Medicis JJ, Newman N, Speizer R. Prevalence and cost savings of therapeutic interchange among U.S. hospitals. Am J Health Syst Pharm. 2002;59(6):529-533.
13. Hilleman DE, Wurdeman RL, Lenz TL. Therapeutic change of HMG-CoA reductase inhibitors in patients with coronary artery disease. Pharmacotherapy. 2001;21(4):410-415.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
2. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
3. McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26(4 suppl 3):III32-III38.
4. Pfizer. Lipitor [package insert]. New York, NY: Pfizer; 2015.
5. Crestor [package insert]. Wilmington, DE:AstraZeneca; 2015.
6. Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116(6):408-416.
7. FDA approves first generic version of cholesterol-lowering drug Lipitor [news release]. U.S. Food and Drug Administration; November 30, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm281817.htm. Accessed November 11, 2015.
8. U.S. Department of Veterans Affairs. VHA Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. Interchange to generic atorvastatin-recommendations. Atorvastatin Conversion Guidance. 2012. U.S. Department of Veterans Affairs intranet website. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Therapeutic%20Interchange%20Guidance/Atorvastatin%20Conversion%20Guidance%20(PBM-MAP-VPE)-Final.docx. Accessed November 16, 2015.
9. Pratt DS, Kaplan MM. Evaluation of Liver Function. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw Hill Professional Publishing; 2011:2527-2530.
10. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy . 2001;21(9):1130-1139.
11. Billups SJ, Plushner SL, Olson KI, Koehler TJ, Kerzee J. Clinical and economic outcomes of conversion of simvastatin to lovastatin in a group-model health maintenance organization. J Manag Care Pharm. 2005;11(8):681-686.
12. Schachtner JM, Guharoy R, Medicis JJ, Newman N, Speizer R. Prevalence and cost savings of therapeutic interchange among U.S. hospitals. Am J Health Syst Pharm. 2002;59(6):529-533.
13. Hilleman DE, Wurdeman RL, Lenz TL. Therapeutic change of HMG-CoA reductase inhibitors in patients with coronary artery disease. Pharmacotherapy. 2001;21(4):410-415.
Treatment Failure With Atorvastatin After Change From Rosuvastatin to Atorvastatin
Due to the growing number of drug shortages and increasing cost of medications, large-scale formulary conversions are becoming more common and necessary for pharmacies to stay within their budget allocations. Statins are widely recognized as first-line therapy for cholesterol lowering and have been proven to reduce cardiovascular morbidity and mortality.1-5 In addition to statin therapy, weight loss and lifestyle changes are often necessary to meet optimum low-density lipoprotein cholesterol (LDL-C) goals.6 According to the Adult Treatment Panel III (ATP III) guidelines, the optimum LDL-C for each patient varies based on the presence of coronary artery disease (CAD), CAD risk equivalents, and other risk factors.7 Patients with CAD or CAD risk equivalents have an LDL-C goal of < 100 mg/dL. Those with multiple risk factors have an LDL-C goal of < 130 mg/dL, and those with 0 to 1 risk factors have an LDL-C goal of < 160 mg/dL.1-3,7,8
Numerous trials have compared the safety and efficacy of the 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors, stating that rosuvastatin 5 to 10 mg per day is equivalent to 20 mg per day of atorvastatin in terms of its ability to lower LDL-C levels.7,9-14 The LUNAR (Limiting Under treatment of lipids in Acute coronary syndrome with Rosuvastatin) study compared the efficacy of rosuvastatin with that of atorvastatin in decreasing LDL-C in patients with acute coronary syndrome.8 Rosuvastatin 40 mg was significantly more successful in lowering LDL-C and increasing high-density lipoprotein cholesterol (HDL-C) compared with atorvastatin 80-mg daily therapy.
In October 2012, the national VA Pharmacy Benefits Management (PBM) Services released guidance regarding the conversion from rosuvastatin to atorvastatin, including the dosing conversion (Table 1), stating that rosuvastatin 5 mg daily should be considered equivalent and converted to atorvastatin 20 mg daily. In general, adverse events (AEs), such as increased liver enzymes, myopathies, and increased creatinine phosphokinase (CPK), are considered a class effect of the statins.13,14
Related: Statins showed no benefit in reducing risk of recurrent VTE
The recent 2013 American College of Cardiology/American Heart Association (ACC/AHA) Blood Cholesterol Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults has identified a large number of patients as candidates for high-intensity statins, which the authors defined as atorvastatin and rosuvastatin.4 These guidelines do not recommend LDL-C goals and instead use a risk calculator to determine which intensity of statin therapy is appropriate for certain patients. This change in practice will lead to a higher volume of high-intensity statin prescriptions and higher drug costs for some medical centers. Given the volume of prescriptions and the increased use of large-scale formulary conversions to reduce costs, more research is warranted to ensure equivalent dosing. With more data available and equivalent dosing defined, pharmacists may be better able to improve clinical results in patients that are included in these large-scale formulary conversions.
Methods
A retrospective chart review was performed on all LDL-C levels in patients receiving atorvastatin therapy due to the formulary conversion from rosuvastatin to atorvastatin at the Huntington VAMC (HVAMC) in Huntington, West Virginia, per the guidance published by the national VA PBM Services. The number of patients not at their LDL-C goal (as defined by ATP III guidelines) as a result of atorvastatin therapy was determined. Furthermore, AEs due to atorvastatin therapy such as increased liver enzymes, myopathy, and increased CPK were identified and analyzed.
The primary endpoint of this study focused on the rate of treatment failure in LDL-C reduction with atorvastatin treatment in patients previously at their LDL-C goal, as defined by the ATP III guidelines, with rosuvastatin therapy. Secondary endpoints included rate of AEs due to atorvastatin therapy, percentage increase in CPK and liver enzymes as a result of atorvastatin therapy, and percentage LDL-C, HDL-C, total cholesterol (TC), and triglyceride (TGs) changes since conversion from rosuvastatin to atorvastatin.
Patients were included in the study if they were aged 18 to 89 years, previously at LDL-C goal with rosuvastatin therapy for at least 3 months, had never previously received atorvastatin, were converted to atorvastatin therapy as a result of a large-scale formulary conversion at HVAMC, and had a fasting lipid panel completed 1 to 6 months after conversion. Patients were excluded from the study if they received other lipid-lowering medications (eg, bile acid sequestrants, fibrates, niacin, or ezetimibe) in the 12 months before or after receiving statin therapy, had previously documented AEs (eg, myopathy, increased liver enzymes, increased CPK as a result of statin therapy, or history of known homozygous familial hypercholesterolemia) current active liver disease (ALT > 2x ULN [upper limit of normal]), unexplained CPK ≥ 3x ULN, serum creatinine (SCr) > 2 mg/dL, or history of alcohol or drug abuse within the last 5 years.
Results
Three hundred twenty-three patients were identified and reviewed as converted from rosuvastatin to atorvastatin during the study period with no prior use of atorvastatin. Of the 323 charts that were reviewed, 195 patients met the study inclusion criteria and were analyzed for rate of treatment failure in terms of lipid goals and rate of AEs. Twenty of 195 patients (10.3%) were no longer at their LDL-C goal after conversion from rosuvastatin to atorvastatin. Of those 195 patients, 29 (14.9%) experienced an adverse drug reaction (ADR) as a result of atorvastatin treatment that was severe enough to result in discontinuation of the drug and switching the patient back to the originally prescribed dose of rosuvastatin. Figure 1 illustrates the number of patients and documented atorvastatin ADRs. The most common ADR documented to atorvastatin was myalgias (8.2%).
The average change in lipid levels was calculated and atorvastatin therapy was found to result in clinically insignificant changes to the lipid panel (Table 2). A 2-tailed paired t test was used to assess the statistical significance of these changes. Atorvastatin therapy resulted in an average decrease of LDL-C by 5.0 mg/dL (P < .01) in comparison to previous therapy with equivalent rosuvastatin dose. Other noted changes to lipid profile after formulary conversion included TG reduction by 2 mg/dL (P = .69), TC increased by 0.58 mg/dL (P = .80), and HDL-C reduction by 4.66 mg/dL (P < .01).
Although the decrease in LDL-C and HDL-C as a result of the formulary change was found to be statistically significant, they are not thought to result in a clinical difference. Clinically and statistically insignificant changes in liver enzymes and CPK were also discovered as a result of atorvastatin therapy conversion (Table 3). Atorvastatin therapy resulted in an averaged decrease of aspartate aminotransferase by 2.2 IU/L (P = .19) and an increase in alanine aminotransferase by 1.4 IU/L (P = .47). Average change in CPK was -6 IU/L (P = 89).
Related: New Guideline on Dyslipidemia: Less Is More
Discussion
Lipid levels were found to be mostly unchanged and remained at therapy goals, demonstrating use and appropriate equivalent dosing of rosuvastatin and atorvastatin following the formulary conversion defined in Table 1. Documented ADRs were minimal, indicating the ingredient conversion was well tolerated overall by our patients. Following the release of the 2013ACC/AHA guidelines, many patients in the VA required treatment with high-potency statins such as rosuvastatin and atorvastatin. Given the volume of statin prescriptions in the VA and the significant potential for providing the most cost-efficient lipid therapy (Table 4), the formulary conversion from rosuvastatin to atorvastatin was warranted.
Limitations
There are several limitations for extrapolating results from this study to the general population. Due to the retrospective design of the study, no formal assessment of adherence was conducted. A prospective trial, with a researcher monitoring refills and tablet counts would be more accurate to ensure patients adherence with statin therapy.
Many of the patients that were included in the formulary conversion did not have follow-up laboratory work at the time of this study and were therefore excluded, leading to a smaller study population. A larger study population with a similar study design may be able to detect more significant correlations.
There are also several potential complications in regard to formulary conversions, including the inability to ensure the exact period that the patient switched therapies. During the conversion of rosuvastatin to atorvastatin at HVAMC, an attempt was made to minimize this confounder by converting patients on request for a refill of their rosuvastatin therapy. Last, all 185 patients that were studied were male; therefore, it is difficult to extrapolate these results for female patients.
Conclusions
This study shows that the conversion of patients from rosuvastatin therapy to atorvastatin was effective when it targeted a specific LDL-C goal or specific reduction in LDL-C. A similar conversion would likely lead to lower drug costs for many other health systems. Additional studies will be necessary given the recent changes in the national lipid guidelines. Furthermore, studies will be needed to assess concrete clinical endpoints (cardiovascular mortality, all-cause mortality, and cardiac events).
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Krasuski RA, Doeppenschmidt D, Henry JS, et al. Conversion to atorvastatin in patients intolerant or refractory to simvastatin therapy: the CAPISH study. Mayo Clin Proc. 2005;80(9):1163-1168.
2. Clearfield MB, Amerena J, Bassand JP, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high-risk patients with hypercholesterolemia--Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials. 2006;7:35.
3. Park JS, Kim YJ, Choi JY, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010;25(1):27-35.
4. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
5. Schuster H, Barter PJ, Stender S, et al; Effective Reductions in Cholesterol Using Rosuvastatin Therapy I study group. Effects of switching statin on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) study. Am Heart J. 2004;147(4):705-713.
6. Ballantyne CM, Bertolami M, Garcia HR, et al. Achieving LDL cholesterol, non-HDL cholesterol and apolipoprotein B target levels in high-risk patients: Measuring effective Reductions in Cholesterol Using Rosuvastatin therapY (MERCURY) II. Am Heart J. 2006;151(5):975.e1-975.e9.
7. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
8. Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid-modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study). Am J Cardiol. 2012;109(9);1239-1246.
9. Takagi H, Niwa M, Mizuno Y, Yamamoto H, Goto SN, Umemoto T. Effects of rosuvastatin versus atorvastatin on small dense low-density lipoprotein: a meta-analysis of randomized trials. Heart Vessels. 2014;29(3);287-299.
10. Fox KM, Gandhi SK, Ohsfeldt RL, Davidson MH. Comparison of low-density lipoprotein cholesterol reduction after switching patients on other statins to rosuvastatin or simvastatin in a real-world clinical practice setting. Am J Manag Care. 2007;13(suppl 10):S270-S275.
11. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy. 2001;21(9):1130-1139.
12. Bullano MF, Kamat S, Wertz DA, et al. Effectiveness of rosuvastatin versus atorvastatin in reducing lipid levels and achieving low-density-lipoprotein cholesterol goals in a usual care setting. Am J Health Syst Pharm. 2007;64(3):276-284.
13. Palmer MK, Nicholls SJ, Lundman P, Barter PJ, Karison BW. Achievement of LDL-C goals depends on baseline LDL-C and choice and dose of statin: an analysis from the VOYAGER database. Eur J Prev Cardiol. 2013;20(6):1080-1087.
14. Berne C, Siewert-Delle A; URANUS study investigators. Comparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: results from the URANUS study. Cardiovasc Diabetol. 2005;4:7.
Due to the growing number of drug shortages and increasing cost of medications, large-scale formulary conversions are becoming more common and necessary for pharmacies to stay within their budget allocations. Statins are widely recognized as first-line therapy for cholesterol lowering and have been proven to reduce cardiovascular morbidity and mortality.1-5 In addition to statin therapy, weight loss and lifestyle changes are often necessary to meet optimum low-density lipoprotein cholesterol (LDL-C) goals.6 According to the Adult Treatment Panel III (ATP III) guidelines, the optimum LDL-C for each patient varies based on the presence of coronary artery disease (CAD), CAD risk equivalents, and other risk factors.7 Patients with CAD or CAD risk equivalents have an LDL-C goal of < 100 mg/dL. Those with multiple risk factors have an LDL-C goal of < 130 mg/dL, and those with 0 to 1 risk factors have an LDL-C goal of < 160 mg/dL.1-3,7,8
Numerous trials have compared the safety and efficacy of the 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors, stating that rosuvastatin 5 to 10 mg per day is equivalent to 20 mg per day of atorvastatin in terms of its ability to lower LDL-C levels.7,9-14 The LUNAR (Limiting Under treatment of lipids in Acute coronary syndrome with Rosuvastatin) study compared the efficacy of rosuvastatin with that of atorvastatin in decreasing LDL-C in patients with acute coronary syndrome.8 Rosuvastatin 40 mg was significantly more successful in lowering LDL-C and increasing high-density lipoprotein cholesterol (HDL-C) compared with atorvastatin 80-mg daily therapy.
In October 2012, the national VA Pharmacy Benefits Management (PBM) Services released guidance regarding the conversion from rosuvastatin to atorvastatin, including the dosing conversion (Table 1), stating that rosuvastatin 5 mg daily should be considered equivalent and converted to atorvastatin 20 mg daily. In general, adverse events (AEs), such as increased liver enzymes, myopathies, and increased creatinine phosphokinase (CPK), are considered a class effect of the statins.13,14
Related: Statins showed no benefit in reducing risk of recurrent VTE
The recent 2013 American College of Cardiology/American Heart Association (ACC/AHA) Blood Cholesterol Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults has identified a large number of patients as candidates for high-intensity statins, which the authors defined as atorvastatin and rosuvastatin.4 These guidelines do not recommend LDL-C goals and instead use a risk calculator to determine which intensity of statin therapy is appropriate for certain patients. This change in practice will lead to a higher volume of high-intensity statin prescriptions and higher drug costs for some medical centers. Given the volume of prescriptions and the increased use of large-scale formulary conversions to reduce costs, more research is warranted to ensure equivalent dosing. With more data available and equivalent dosing defined, pharmacists may be better able to improve clinical results in patients that are included in these large-scale formulary conversions.
Methods
A retrospective chart review was performed on all LDL-C levels in patients receiving atorvastatin therapy due to the formulary conversion from rosuvastatin to atorvastatin at the Huntington VAMC (HVAMC) in Huntington, West Virginia, per the guidance published by the national VA PBM Services. The number of patients not at their LDL-C goal (as defined by ATP III guidelines) as a result of atorvastatin therapy was determined. Furthermore, AEs due to atorvastatin therapy such as increased liver enzymes, myopathy, and increased CPK were identified and analyzed.
The primary endpoint of this study focused on the rate of treatment failure in LDL-C reduction with atorvastatin treatment in patients previously at their LDL-C goal, as defined by the ATP III guidelines, with rosuvastatin therapy. Secondary endpoints included rate of AEs due to atorvastatin therapy, percentage increase in CPK and liver enzymes as a result of atorvastatin therapy, and percentage LDL-C, HDL-C, total cholesterol (TC), and triglyceride (TGs) changes since conversion from rosuvastatin to atorvastatin.
Patients were included in the study if they were aged 18 to 89 years, previously at LDL-C goal with rosuvastatin therapy for at least 3 months, had never previously received atorvastatin, were converted to atorvastatin therapy as a result of a large-scale formulary conversion at HVAMC, and had a fasting lipid panel completed 1 to 6 months after conversion. Patients were excluded from the study if they received other lipid-lowering medications (eg, bile acid sequestrants, fibrates, niacin, or ezetimibe) in the 12 months before or after receiving statin therapy, had previously documented AEs (eg, myopathy, increased liver enzymes, increased CPK as a result of statin therapy, or history of known homozygous familial hypercholesterolemia) current active liver disease (ALT > 2x ULN [upper limit of normal]), unexplained CPK ≥ 3x ULN, serum creatinine (SCr) > 2 mg/dL, or history of alcohol or drug abuse within the last 5 years.
Results
Three hundred twenty-three patients were identified and reviewed as converted from rosuvastatin to atorvastatin during the study period with no prior use of atorvastatin. Of the 323 charts that were reviewed, 195 patients met the study inclusion criteria and were analyzed for rate of treatment failure in terms of lipid goals and rate of AEs. Twenty of 195 patients (10.3%) were no longer at their LDL-C goal after conversion from rosuvastatin to atorvastatin. Of those 195 patients, 29 (14.9%) experienced an adverse drug reaction (ADR) as a result of atorvastatin treatment that was severe enough to result in discontinuation of the drug and switching the patient back to the originally prescribed dose of rosuvastatin. Figure 1 illustrates the number of patients and documented atorvastatin ADRs. The most common ADR documented to atorvastatin was myalgias (8.2%).
The average change in lipid levels was calculated and atorvastatin therapy was found to result in clinically insignificant changes to the lipid panel (Table 2). A 2-tailed paired t test was used to assess the statistical significance of these changes. Atorvastatin therapy resulted in an average decrease of LDL-C by 5.0 mg/dL (P < .01) in comparison to previous therapy with equivalent rosuvastatin dose. Other noted changes to lipid profile after formulary conversion included TG reduction by 2 mg/dL (P = .69), TC increased by 0.58 mg/dL (P = .80), and HDL-C reduction by 4.66 mg/dL (P < .01).
Although the decrease in LDL-C and HDL-C as a result of the formulary change was found to be statistically significant, they are not thought to result in a clinical difference. Clinically and statistically insignificant changes in liver enzymes and CPK were also discovered as a result of atorvastatin therapy conversion (Table 3). Atorvastatin therapy resulted in an averaged decrease of aspartate aminotransferase by 2.2 IU/L (P = .19) and an increase in alanine aminotransferase by 1.4 IU/L (P = .47). Average change in CPK was -6 IU/L (P = 89).
Related: New Guideline on Dyslipidemia: Less Is More
Discussion
Lipid levels were found to be mostly unchanged and remained at therapy goals, demonstrating use and appropriate equivalent dosing of rosuvastatin and atorvastatin following the formulary conversion defined in Table 1. Documented ADRs were minimal, indicating the ingredient conversion was well tolerated overall by our patients. Following the release of the 2013ACC/AHA guidelines, many patients in the VA required treatment with high-potency statins such as rosuvastatin and atorvastatin. Given the volume of statin prescriptions in the VA and the significant potential for providing the most cost-efficient lipid therapy (Table 4), the formulary conversion from rosuvastatin to atorvastatin was warranted.
Limitations
There are several limitations for extrapolating results from this study to the general population. Due to the retrospective design of the study, no formal assessment of adherence was conducted. A prospective trial, with a researcher monitoring refills and tablet counts would be more accurate to ensure patients adherence with statin therapy.
Many of the patients that were included in the formulary conversion did not have follow-up laboratory work at the time of this study and were therefore excluded, leading to a smaller study population. A larger study population with a similar study design may be able to detect more significant correlations.
There are also several potential complications in regard to formulary conversions, including the inability to ensure the exact period that the patient switched therapies. During the conversion of rosuvastatin to atorvastatin at HVAMC, an attempt was made to minimize this confounder by converting patients on request for a refill of their rosuvastatin therapy. Last, all 185 patients that were studied were male; therefore, it is difficult to extrapolate these results for female patients.
Conclusions
This study shows that the conversion of patients from rosuvastatin therapy to atorvastatin was effective when it targeted a specific LDL-C goal or specific reduction in LDL-C. A similar conversion would likely lead to lower drug costs for many other health systems. Additional studies will be necessary given the recent changes in the national lipid guidelines. Furthermore, studies will be needed to assess concrete clinical endpoints (cardiovascular mortality, all-cause mortality, and cardiac events).
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Due to the growing number of drug shortages and increasing cost of medications, large-scale formulary conversions are becoming more common and necessary for pharmacies to stay within their budget allocations. Statins are widely recognized as first-line therapy for cholesterol lowering and have been proven to reduce cardiovascular morbidity and mortality.1-5 In addition to statin therapy, weight loss and lifestyle changes are often necessary to meet optimum low-density lipoprotein cholesterol (LDL-C) goals.6 According to the Adult Treatment Panel III (ATP III) guidelines, the optimum LDL-C for each patient varies based on the presence of coronary artery disease (CAD), CAD risk equivalents, and other risk factors.7 Patients with CAD or CAD risk equivalents have an LDL-C goal of < 100 mg/dL. Those with multiple risk factors have an LDL-C goal of < 130 mg/dL, and those with 0 to 1 risk factors have an LDL-C goal of < 160 mg/dL.1-3,7,8
Numerous trials have compared the safety and efficacy of the 3-hydroxy-3-methylglutaryl-coenzyme A inhibitors, stating that rosuvastatin 5 to 10 mg per day is equivalent to 20 mg per day of atorvastatin in terms of its ability to lower LDL-C levels.7,9-14 The LUNAR (Limiting Under treatment of lipids in Acute coronary syndrome with Rosuvastatin) study compared the efficacy of rosuvastatin with that of atorvastatin in decreasing LDL-C in patients with acute coronary syndrome.8 Rosuvastatin 40 mg was significantly more successful in lowering LDL-C and increasing high-density lipoprotein cholesterol (HDL-C) compared with atorvastatin 80-mg daily therapy.
In October 2012, the national VA Pharmacy Benefits Management (PBM) Services released guidance regarding the conversion from rosuvastatin to atorvastatin, including the dosing conversion (Table 1), stating that rosuvastatin 5 mg daily should be considered equivalent and converted to atorvastatin 20 mg daily. In general, adverse events (AEs), such as increased liver enzymes, myopathies, and increased creatinine phosphokinase (CPK), are considered a class effect of the statins.13,14
Related: Statins showed no benefit in reducing risk of recurrent VTE
The recent 2013 American College of Cardiology/American Heart Association (ACC/AHA) Blood Cholesterol Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults has identified a large number of patients as candidates for high-intensity statins, which the authors defined as atorvastatin and rosuvastatin.4 These guidelines do not recommend LDL-C goals and instead use a risk calculator to determine which intensity of statin therapy is appropriate for certain patients. This change in practice will lead to a higher volume of high-intensity statin prescriptions and higher drug costs for some medical centers. Given the volume of prescriptions and the increased use of large-scale formulary conversions to reduce costs, more research is warranted to ensure equivalent dosing. With more data available and equivalent dosing defined, pharmacists may be better able to improve clinical results in patients that are included in these large-scale formulary conversions.
Methods
A retrospective chart review was performed on all LDL-C levels in patients receiving atorvastatin therapy due to the formulary conversion from rosuvastatin to atorvastatin at the Huntington VAMC (HVAMC) in Huntington, West Virginia, per the guidance published by the national VA PBM Services. The number of patients not at their LDL-C goal (as defined by ATP III guidelines) as a result of atorvastatin therapy was determined. Furthermore, AEs due to atorvastatin therapy such as increased liver enzymes, myopathy, and increased CPK were identified and analyzed.
The primary endpoint of this study focused on the rate of treatment failure in LDL-C reduction with atorvastatin treatment in patients previously at their LDL-C goal, as defined by the ATP III guidelines, with rosuvastatin therapy. Secondary endpoints included rate of AEs due to atorvastatin therapy, percentage increase in CPK and liver enzymes as a result of atorvastatin therapy, and percentage LDL-C, HDL-C, total cholesterol (TC), and triglyceride (TGs) changes since conversion from rosuvastatin to atorvastatin.
Patients were included in the study if they were aged 18 to 89 years, previously at LDL-C goal with rosuvastatin therapy for at least 3 months, had never previously received atorvastatin, were converted to atorvastatin therapy as a result of a large-scale formulary conversion at HVAMC, and had a fasting lipid panel completed 1 to 6 months after conversion. Patients were excluded from the study if they received other lipid-lowering medications (eg, bile acid sequestrants, fibrates, niacin, or ezetimibe) in the 12 months before or after receiving statin therapy, had previously documented AEs (eg, myopathy, increased liver enzymes, increased CPK as a result of statin therapy, or history of known homozygous familial hypercholesterolemia) current active liver disease (ALT > 2x ULN [upper limit of normal]), unexplained CPK ≥ 3x ULN, serum creatinine (SCr) > 2 mg/dL, or history of alcohol or drug abuse within the last 5 years.
Results
Three hundred twenty-three patients were identified and reviewed as converted from rosuvastatin to atorvastatin during the study period with no prior use of atorvastatin. Of the 323 charts that were reviewed, 195 patients met the study inclusion criteria and were analyzed for rate of treatment failure in terms of lipid goals and rate of AEs. Twenty of 195 patients (10.3%) were no longer at their LDL-C goal after conversion from rosuvastatin to atorvastatin. Of those 195 patients, 29 (14.9%) experienced an adverse drug reaction (ADR) as a result of atorvastatin treatment that was severe enough to result in discontinuation of the drug and switching the patient back to the originally prescribed dose of rosuvastatin. Figure 1 illustrates the number of patients and documented atorvastatin ADRs. The most common ADR documented to atorvastatin was myalgias (8.2%).
The average change in lipid levels was calculated and atorvastatin therapy was found to result in clinically insignificant changes to the lipid panel (Table 2). A 2-tailed paired t test was used to assess the statistical significance of these changes. Atorvastatin therapy resulted in an average decrease of LDL-C by 5.0 mg/dL (P < .01) in comparison to previous therapy with equivalent rosuvastatin dose. Other noted changes to lipid profile after formulary conversion included TG reduction by 2 mg/dL (P = .69), TC increased by 0.58 mg/dL (P = .80), and HDL-C reduction by 4.66 mg/dL (P < .01).
Although the decrease in LDL-C and HDL-C as a result of the formulary change was found to be statistically significant, they are not thought to result in a clinical difference. Clinically and statistically insignificant changes in liver enzymes and CPK were also discovered as a result of atorvastatin therapy conversion (Table 3). Atorvastatin therapy resulted in an averaged decrease of aspartate aminotransferase by 2.2 IU/L (P = .19) and an increase in alanine aminotransferase by 1.4 IU/L (P = .47). Average change in CPK was -6 IU/L (P = 89).
Related: New Guideline on Dyslipidemia: Less Is More
Discussion
Lipid levels were found to be mostly unchanged and remained at therapy goals, demonstrating use and appropriate equivalent dosing of rosuvastatin and atorvastatin following the formulary conversion defined in Table 1. Documented ADRs were minimal, indicating the ingredient conversion was well tolerated overall by our patients. Following the release of the 2013ACC/AHA guidelines, many patients in the VA required treatment with high-potency statins such as rosuvastatin and atorvastatin. Given the volume of statin prescriptions in the VA and the significant potential for providing the most cost-efficient lipid therapy (Table 4), the formulary conversion from rosuvastatin to atorvastatin was warranted.
Limitations
There are several limitations for extrapolating results from this study to the general population. Due to the retrospective design of the study, no formal assessment of adherence was conducted. A prospective trial, with a researcher monitoring refills and tablet counts would be more accurate to ensure patients adherence with statin therapy.
Many of the patients that were included in the formulary conversion did not have follow-up laboratory work at the time of this study and were therefore excluded, leading to a smaller study population. A larger study population with a similar study design may be able to detect more significant correlations.
There are also several potential complications in regard to formulary conversions, including the inability to ensure the exact period that the patient switched therapies. During the conversion of rosuvastatin to atorvastatin at HVAMC, an attempt was made to minimize this confounder by converting patients on request for a refill of their rosuvastatin therapy. Last, all 185 patients that were studied were male; therefore, it is difficult to extrapolate these results for female patients.
Conclusions
This study shows that the conversion of patients from rosuvastatin therapy to atorvastatin was effective when it targeted a specific LDL-C goal or specific reduction in LDL-C. A similar conversion would likely lead to lower drug costs for many other health systems. Additional studies will be necessary given the recent changes in the national lipid guidelines. Furthermore, studies will be needed to assess concrete clinical endpoints (cardiovascular mortality, all-cause mortality, and cardiac events).
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Krasuski RA, Doeppenschmidt D, Henry JS, et al. Conversion to atorvastatin in patients intolerant or refractory to simvastatin therapy: the CAPISH study. Mayo Clin Proc. 2005;80(9):1163-1168.
2. Clearfield MB, Amerena J, Bassand JP, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high-risk patients with hypercholesterolemia--Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials. 2006;7:35.
3. Park JS, Kim YJ, Choi JY, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010;25(1):27-35.
4. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
5. Schuster H, Barter PJ, Stender S, et al; Effective Reductions in Cholesterol Using Rosuvastatin Therapy I study group. Effects of switching statin on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) study. Am Heart J. 2004;147(4):705-713.
6. Ballantyne CM, Bertolami M, Garcia HR, et al. Achieving LDL cholesterol, non-HDL cholesterol and apolipoprotein B target levels in high-risk patients: Measuring effective Reductions in Cholesterol Using Rosuvastatin therapY (MERCURY) II. Am Heart J. 2006;151(5):975.e1-975.e9.
7. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
8. Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid-modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study). Am J Cardiol. 2012;109(9);1239-1246.
9. Takagi H, Niwa M, Mizuno Y, Yamamoto H, Goto SN, Umemoto T. Effects of rosuvastatin versus atorvastatin on small dense low-density lipoprotein: a meta-analysis of randomized trials. Heart Vessels. 2014;29(3);287-299.
10. Fox KM, Gandhi SK, Ohsfeldt RL, Davidson MH. Comparison of low-density lipoprotein cholesterol reduction after switching patients on other statins to rosuvastatin or simvastatin in a real-world clinical practice setting. Am J Manag Care. 2007;13(suppl 10):S270-S275.
11. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy. 2001;21(9):1130-1139.
12. Bullano MF, Kamat S, Wertz DA, et al. Effectiveness of rosuvastatin versus atorvastatin in reducing lipid levels and achieving low-density-lipoprotein cholesterol goals in a usual care setting. Am J Health Syst Pharm. 2007;64(3):276-284.
13. Palmer MK, Nicholls SJ, Lundman P, Barter PJ, Karison BW. Achievement of LDL-C goals depends on baseline LDL-C and choice and dose of statin: an analysis from the VOYAGER database. Eur J Prev Cardiol. 2013;20(6):1080-1087.
14. Berne C, Siewert-Delle A; URANUS study investigators. Comparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: results from the URANUS study. Cardiovasc Diabetol. 2005;4:7.
1. Krasuski RA, Doeppenschmidt D, Henry JS, et al. Conversion to atorvastatin in patients intolerant or refractory to simvastatin therapy: the CAPISH study. Mayo Clin Proc. 2005;80(9):1163-1168.
2. Clearfield MB, Amerena J, Bassand JP, et al. Comparison of the efficacy and safety of rosuvastatin 10 mg and atorvastatin 20 mg in high-risk patients with hypercholesterolemia--Prospective study to evaluate the Use of Low doses of the Statins Atorvastatin and Rosuvastatin (PULSAR). Trials. 2006;7:35.
3. Park JS, Kim YJ, Choi JY, et al. Comparative study of low doses of rosuvastatin and atorvastatin on lipid and glycemic control in patients with metabolic syndrome and hypercholesterolemia. Korean J Intern Med. 2010;25(1):27-35.
4. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2889-2934.
5. Schuster H, Barter PJ, Stender S, et al; Effective Reductions in Cholesterol Using Rosuvastatin Therapy I study group. Effects of switching statin on achievement of lipid goals: Measuring Effective Reductions in Cholesterol Using Rosuvastatin Therapy (MERCURY I) study. Am Heart J. 2004;147(4):705-713.
6. Ballantyne CM, Bertolami M, Garcia HR, et al. Achieving LDL cholesterol, non-HDL cholesterol and apolipoprotein B target levels in high-risk patients: Measuring effective Reductions in Cholesterol Using Rosuvastatin therapY (MERCURY) II. Am Heart J. 2006;151(5):975.e1-975.e9.
7. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
8. Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid-modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study). Am J Cardiol. 2012;109(9);1239-1246.
9. Takagi H, Niwa M, Mizuno Y, Yamamoto H, Goto SN, Umemoto T. Effects of rosuvastatin versus atorvastatin on small dense low-density lipoprotein: a meta-analysis of randomized trials. Heart Vessels. 2014;29(3);287-299.
10. Fox KM, Gandhi SK, Ohsfeldt RL, Davidson MH. Comparison of low-density lipoprotein cholesterol reduction after switching patients on other statins to rosuvastatin or simvastatin in a real-world clinical practice setting. Am J Manag Care. 2007;13(suppl 10):S270-S275.
11. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy. 2001;21(9):1130-1139.
12. Bullano MF, Kamat S, Wertz DA, et al. Effectiveness of rosuvastatin versus atorvastatin in reducing lipid levels and achieving low-density-lipoprotein cholesterol goals in a usual care setting. Am J Health Syst Pharm. 2007;64(3):276-284.
13. Palmer MK, Nicholls SJ, Lundman P, Barter PJ, Karison BW. Achievement of LDL-C goals depends on baseline LDL-C and choice and dose of statin: an analysis from the VOYAGER database. Eur J Prev Cardiol. 2013;20(6):1080-1087.
14. Berne C, Siewert-Delle A; URANUS study investigators. Comparison of rosuvastatin and atorvastatin for lipid lowering in patients with type 2 diabetes mellitus: results from the URANUS study. Cardiovasc Diabetol. 2005;4:7.
Implications of Vancomycin Troughs Drawn Earlier Than Current Guidelines
Vancomycin was isolated in the 1950s, but due to impurities causing adverse events and semisynthetic penicillin production, its use was greatly reduced.1,2 However, this medication gained in popularity 30 years later as a first-line treatment for methicillin-resistant Staphylococcus aureus infections.
In 2009 the Infectious Diseases Society of America (IDSA), American Society of Health System Pharmacists, and Society of Infectious Diseases Pharmacists developed a consensus review of the therapeutic monitoring and dosing of vancomycin in adult patients.3 Trough serum concentration levels are recommended as the most accurate and convenient method to monitor vancomycin. Per IDSA guidelines, an optimal trough is intended to be high enough to clear infections (> 10 mg/L) and prevent the development of vancomycin intermediate and resistant bacteria. Troughs should be obtained just before the next dose in steady-state conditions (starting just before the fourth dose) in patients with normal renal function.
Since the development of these guidelines, vancomycin trough levels are often drawn early.4-7 This may lead to an overestimation of the true trough concentration. A study by Morrison and colleagues in Boston, Massachusetts, found that 41.3% of vancomycin troughs were drawn early, and this resulted in statistically significant increases in the vancomycin concentrations, the rate of vancomycin regimen adjustments (decrease, discontinuation, or holding of dose), and the repeat vancomycin level orders compared with correctly timed troughs.5 It was noted by the study authors that lowering the daily dose of vancomycin based on early trough levels could lead to an underdosing of vancomycin and an increase in intermediate or resistant bacteria.
Related: IDWEEK: Antibiotic ‘time-out’ cut vancomycin use
The prevalence and implications of early trough samples have been measured at only 1 facility, and it is unknown whether these data can be reproduced elsewhere.5 Thus, this study sought to determine the prevalence and corresponding clinical actions of early trough levels at the Captain James A. Lovell Federal Health Care Center (JALFHCC). This is a unique facility that in 2010 combined a VA hospital with a DoD hospital. This facility cares for 67,000 military and retiree beneficiaries each year from southwestern Wisconsin and northwestern Illinois.The primary objective of this study was to measure the rate of early troughs drawn and their resultant effect on vancomycin regimens compared with correctly timed troughs. Secondarily, this study sought to compare the rate of repeated vancomycin trough levels in early vs correctly timed measurements.
Methods
This retrospective cohort analysis compared the outcomes of early and correctly timed vancomycin troughs. This study was approved by the Edward Hines, Jr. VA Hospital and JALFHCC Institutional Review Board. Veteran patients aged ≥ 18 years, hospitalized at JALFHCC, and receiving IV vancomycin at dosing intervals of 8, 12, 24, and 48 hours with measured trough levels between July 1, 2009, and July 1, 2013, were included in this study. Patients were excluded from analysis if vancomycin was given at any schedule other than the previously stated frequencies, they received hemodialysis during the treatment period, or their insurance coverage was through TRICARE (these patients had either active-duty or retired active-duty status).
Potentially eligible patients were identified via a Computerized Patient Records System (CPRS) search for laboratory vancomycin level measurements. The search supplied the researcher with the patient name, vancomycin level date and time, type of vancomycin level (trough or random), and vancomycin concentration. With this information, further data were gathered through CPRS: demographics, type of clinical infection, desired trough level (inferred if not listed in CPRS note), and vancomycin administration time (through the bar code medication administration system [BCMA] in CPRS). This analysis was of troughs, and multiple troughs may have originated from the same patient.
An early trough was defined as a trough taken more than 2 hours earlier than the next theoretical administration time or anytime before the third dose. After a trough was determined to be early or on time, the clinical actions taken during the dosing interval following sample collection were documented. A dose was considered to be held if stated in the BCMA or in a CPRS provider note. A dose was considered to be decreased with a change in frequency or strength that resulted in an overall daily dose decrease. A recollected vancomycin trough was counted within 24 hours of the trough or per a note in CPRS. Finally, observations that noted trends in vancomycin trough management were recorded.
The chi-square test with a significance criterion of 0.05 was used to compare early and on time troughs. Based on the results from the Boston, Massachusetts, study and 1 other study, about 780 vancomycin troughs would be required to meet significance in the primary outcome.5,6
Results
A total of 474 patient charts were reviewed, and 278 met inclusion criteria (196 were excluded). Of the included patients, 799 trough levels were analyzed. Of these, 377 (42.2%) were drawn early. There was no significant difference in the baseline characteristics of the early group vs the correctly timed group (Table 1). Of the early troughs, 190 (56.3%) were drawn prior to the third dose of vancomycin. It was observed that a large portion of these troughs occurred after a vancomycin dose adjustment.
Clinical actions taken after sampling occurred at a rate of 14.5% in the early group and 22.9% in the correctly timed group (P = .003; Table 2). Early troughs led to a 7.7% rate of trough recollection, which was significantly greater than the 1.5% rate in the correctly timed group (P < .001). An analysis of each factor resulting in a clinical action illustrated that the rates of daily dose decrease and discontinued dose were similar between the groups (Table 3). However, the rate of held doses was 8.3% in the early group and 17.1% in the correctly timed group.
This research process yielded some observations. Occasionally a trough was drawn after vancomycin therapy was discontinued and when there was no concern for nephrotoxicity. After the guidelines were published, providers continued to document in CPRS notes to check troughs before the third dose. This incidence decreased over time. Troughs were taken often in patients who were receiving a short course of therapy or who were hemodynamically stable. Finally, documentation of vancomycin regimen changes occasionally did not match the record in the BCMA (in these situations, the BCMA record was used for this study).
Related: Assessment of High Staphylococcus aureus MIC and Poor Patient Outcomes
Discussion
A large portion of trough levels at the JALFHCC were drawn early and did not adhere to the 2009 consensus guidelines. The rates of early troughs in this study and in the Boston, Massachusetts, study are similar.5 However, the 2 studies differed in 1 significant aspect: Clinical actions were taken less often in the early group at JALFHCC, whereas they were taken more often in the early group in the Boston, Massachusetts, study. This dissimilarity could be attributed to a difference in software between the hospitals. In the previous study, trough levels and the time that they were drawn were not displayed together. Thus, clinicians may have been less likely to gauge whether a trough was early. Since this information is available at the JALFHCC, clinicians may have been aware that the trough was early and avoided adjusting treatment (such as holding a dose, as illustrated in the data) based on a falsely elevated trough. This point is further supported by significantly greater amounts of recollected troughs in the early group, suggesting an understanding that the trough was early.
The low trough recollection rate of 7.7% of all early samples could be due to several factors that would prevent a trough redraw. First, medication discontinuation resulting from course completion or sensitivity results would not require further trough monitoring. Second, practitioners may assess the early sample as insignificantly different from a correctly timed one and elect not to redraw the trough. Sometimes a trough was drawn at the correct time, but the time was recorded incorrectly. In this situation, a new trough level would not be necessary. Finally, a lack of sufficient staffing during nights and weekends may result in a delay in interpreting results leading to a missed opportunity for recollection. Additionally, some troughs may not have been redrawn based on a practitioner’s opinion that a trough was not significantly early and did not represent skewed results. Sometimes an incorrect recording of trough draw time reflected that it was taken after vancomycin dosing when it was not.
Specific observations regarding the timing of the trough indicate other possible concerns and areas for improvement. First, providers must cancel future trough orders concurrently with canceling treatment. Second, at the time of publication of the consensus, some providers were slow adopters of the new guidelines. Finally, the IDSA guidelines state that frequent monitoring for short course, lower intensity therapy, or in patients who are hemodynamically stable is not recommended.3 However, troughs were sometimes measured 2 to 3 times weekly in these patients.
Related: Results mixed in hospital efforts to tackle antimicrobial resistance
The data and observations lead to the conclusion that although providers may be able to discern between early and correctly timed troughs, they were not consistently adherent to the 2009 IDSA guidelines. It has been shown that pharmacy involvement of Medicare patients with infections in the intensive care unit has led to better clinical and monetary outcomes.8 Therefore, continued efforts by clinical pharmacists to monitor trough timing can be used to improve adherence and decrease costs (each trough is estimated to cost $16.97).
A study conducted in Australia demonstrated that pharmacist-led education of vancomycin dosing and monitoring (including when to measure a trough level) among prescribers and nurses led to improved adherence to the current guidelines and a greater number of patients treated within desired therapeutic ranges.9 In addition, a small study at the Atlanta VAMC in Georgia demonstrated that education of nurses, lab personnel, residents, ward clerks, and pharmacists led to a greater number of appropriately timed vancomycin and aminoglycoside levels.10 Thus, an interdisciplinary review of the current IDSA guidelines and review on the publication of the anticipated updated vancomycin guidelines should be provided to hospital personnel to aid in adoption of current dosing and monitoring recommendations.11
Limitations
This study is limited by the 4-year span of time that it encompassed, which may give a skewed depiction of current practices. Another limitation is that patients with fluctuating renal function were included in the analysis. Instead of selecting a random level order, a trough level order was sometimes selected for these patients. This could lead to a lower actual rate of early troughs. A third limitation is that this was a small and unblinded study. Also, the actual trough levels and the resulting changes that were made to specific regimens were not recorded. Thus, these data do not indicate whether the changes that were made reflected guideline recommendations. Finally, some clinical actions were taken after the dosing interval following the trough. This was often a result of off-hours lab results or waiting on attending physician or infectious disease guidance. These data were not included in the analysis.
Conclusion
Vancomycin troughs were often drawn too early and resulted in an increased rate of trough recollection. In an attempt to improve adherence to the current and the upcoming revised version of the IDSA consensus statement, it is recommended to educate and reeducate providers through interdisciplinary-led review sessions.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Moellering RC Jr. Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42(suppl 1):S3-S4.
2. Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(suppl 1):S5-S12.
3. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29(11):1275-1279.
4. Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. hospitals. Pharmacotherapy. 2013;33(12):1256-1263.
5. Morrison AP, Melanson SEF, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early? Frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472-478.
6. Traugott KA, Maxwell PR, Green K, Frei C, Lewis JS 2nd. Effects of therapeutic drug monitoring criteria in a computerized prescriber-order-entry system on the appropriateness of vancomycin level orders. Am J Health Syst Pharm. 2011;68(4):347-352.
7. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801-806.
8. MacLaren R, Bond CA, Martin SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36(12):3184-3189.
9. Phillips CJ, Doan H, Quinn S, Kirkpatrick CM, Gordon DL, Doogue MP. An educational intervention to improve vancomycin prescribing and monitoring. Int J Antimicrob Agents. 2013;41(4):393-394.
10. Carroll DJ, Austin GE, Stajich GV, Miyrhaya RK, Murphy JE, Ward ES. Effect of education on the appropriateness of serum drug concentration determination. Ther Drug Monit. 1992;14(1):81-84.
11. Infectious Diseases Society of America (IDSA). IDSA practice guidelines: antimicrobial agent use. IDSA Website. 2015. http://www.idsociety.org/Antimicrobial_Agents. Accessed November 16, 2015.
Vancomycin was isolated in the 1950s, but due to impurities causing adverse events and semisynthetic penicillin production, its use was greatly reduced.1,2 However, this medication gained in popularity 30 years later as a first-line treatment for methicillin-resistant Staphylococcus aureus infections.
In 2009 the Infectious Diseases Society of America (IDSA), American Society of Health System Pharmacists, and Society of Infectious Diseases Pharmacists developed a consensus review of the therapeutic monitoring and dosing of vancomycin in adult patients.3 Trough serum concentration levels are recommended as the most accurate and convenient method to monitor vancomycin. Per IDSA guidelines, an optimal trough is intended to be high enough to clear infections (> 10 mg/L) and prevent the development of vancomycin intermediate and resistant bacteria. Troughs should be obtained just before the next dose in steady-state conditions (starting just before the fourth dose) in patients with normal renal function.
Since the development of these guidelines, vancomycin trough levels are often drawn early.4-7 This may lead to an overestimation of the true trough concentration. A study by Morrison and colleagues in Boston, Massachusetts, found that 41.3% of vancomycin troughs were drawn early, and this resulted in statistically significant increases in the vancomycin concentrations, the rate of vancomycin regimen adjustments (decrease, discontinuation, or holding of dose), and the repeat vancomycin level orders compared with correctly timed troughs.5 It was noted by the study authors that lowering the daily dose of vancomycin based on early trough levels could lead to an underdosing of vancomycin and an increase in intermediate or resistant bacteria.
Related: IDWEEK: Antibiotic ‘time-out’ cut vancomycin use
The prevalence and implications of early trough samples have been measured at only 1 facility, and it is unknown whether these data can be reproduced elsewhere.5 Thus, this study sought to determine the prevalence and corresponding clinical actions of early trough levels at the Captain James A. Lovell Federal Health Care Center (JALFHCC). This is a unique facility that in 2010 combined a VA hospital with a DoD hospital. This facility cares for 67,000 military and retiree beneficiaries each year from southwestern Wisconsin and northwestern Illinois.The primary objective of this study was to measure the rate of early troughs drawn and their resultant effect on vancomycin regimens compared with correctly timed troughs. Secondarily, this study sought to compare the rate of repeated vancomycin trough levels in early vs correctly timed measurements.
Methods
This retrospective cohort analysis compared the outcomes of early and correctly timed vancomycin troughs. This study was approved by the Edward Hines, Jr. VA Hospital and JALFHCC Institutional Review Board. Veteran patients aged ≥ 18 years, hospitalized at JALFHCC, and receiving IV vancomycin at dosing intervals of 8, 12, 24, and 48 hours with measured trough levels between July 1, 2009, and July 1, 2013, were included in this study. Patients were excluded from analysis if vancomycin was given at any schedule other than the previously stated frequencies, they received hemodialysis during the treatment period, or their insurance coverage was through TRICARE (these patients had either active-duty or retired active-duty status).
Potentially eligible patients were identified via a Computerized Patient Records System (CPRS) search for laboratory vancomycin level measurements. The search supplied the researcher with the patient name, vancomycin level date and time, type of vancomycin level (trough or random), and vancomycin concentration. With this information, further data were gathered through CPRS: demographics, type of clinical infection, desired trough level (inferred if not listed in CPRS note), and vancomycin administration time (through the bar code medication administration system [BCMA] in CPRS). This analysis was of troughs, and multiple troughs may have originated from the same patient.
An early trough was defined as a trough taken more than 2 hours earlier than the next theoretical administration time or anytime before the third dose. After a trough was determined to be early or on time, the clinical actions taken during the dosing interval following sample collection were documented. A dose was considered to be held if stated in the BCMA or in a CPRS provider note. A dose was considered to be decreased with a change in frequency or strength that resulted in an overall daily dose decrease. A recollected vancomycin trough was counted within 24 hours of the trough or per a note in CPRS. Finally, observations that noted trends in vancomycin trough management were recorded.
The chi-square test with a significance criterion of 0.05 was used to compare early and on time troughs. Based on the results from the Boston, Massachusetts, study and 1 other study, about 780 vancomycin troughs would be required to meet significance in the primary outcome.5,6
Results
A total of 474 patient charts were reviewed, and 278 met inclusion criteria (196 were excluded). Of the included patients, 799 trough levels were analyzed. Of these, 377 (42.2%) were drawn early. There was no significant difference in the baseline characteristics of the early group vs the correctly timed group (Table 1). Of the early troughs, 190 (56.3%) were drawn prior to the third dose of vancomycin. It was observed that a large portion of these troughs occurred after a vancomycin dose adjustment.
Clinical actions taken after sampling occurred at a rate of 14.5% in the early group and 22.9% in the correctly timed group (P = .003; Table 2). Early troughs led to a 7.7% rate of trough recollection, which was significantly greater than the 1.5% rate in the correctly timed group (P < .001). An analysis of each factor resulting in a clinical action illustrated that the rates of daily dose decrease and discontinued dose were similar between the groups (Table 3). However, the rate of held doses was 8.3% in the early group and 17.1% in the correctly timed group.
This research process yielded some observations. Occasionally a trough was drawn after vancomycin therapy was discontinued and when there was no concern for nephrotoxicity. After the guidelines were published, providers continued to document in CPRS notes to check troughs before the third dose. This incidence decreased over time. Troughs were taken often in patients who were receiving a short course of therapy or who were hemodynamically stable. Finally, documentation of vancomycin regimen changes occasionally did not match the record in the BCMA (in these situations, the BCMA record was used for this study).
Related: Assessment of High Staphylococcus aureus MIC and Poor Patient Outcomes
Discussion
A large portion of trough levels at the JALFHCC were drawn early and did not adhere to the 2009 consensus guidelines. The rates of early troughs in this study and in the Boston, Massachusetts, study are similar.5 However, the 2 studies differed in 1 significant aspect: Clinical actions were taken less often in the early group at JALFHCC, whereas they were taken more often in the early group in the Boston, Massachusetts, study. This dissimilarity could be attributed to a difference in software between the hospitals. In the previous study, trough levels and the time that they were drawn were not displayed together. Thus, clinicians may have been less likely to gauge whether a trough was early. Since this information is available at the JALFHCC, clinicians may have been aware that the trough was early and avoided adjusting treatment (such as holding a dose, as illustrated in the data) based on a falsely elevated trough. This point is further supported by significantly greater amounts of recollected troughs in the early group, suggesting an understanding that the trough was early.
The low trough recollection rate of 7.7% of all early samples could be due to several factors that would prevent a trough redraw. First, medication discontinuation resulting from course completion or sensitivity results would not require further trough monitoring. Second, practitioners may assess the early sample as insignificantly different from a correctly timed one and elect not to redraw the trough. Sometimes a trough was drawn at the correct time, but the time was recorded incorrectly. In this situation, a new trough level would not be necessary. Finally, a lack of sufficient staffing during nights and weekends may result in a delay in interpreting results leading to a missed opportunity for recollection. Additionally, some troughs may not have been redrawn based on a practitioner’s opinion that a trough was not significantly early and did not represent skewed results. Sometimes an incorrect recording of trough draw time reflected that it was taken after vancomycin dosing when it was not.
Specific observations regarding the timing of the trough indicate other possible concerns and areas for improvement. First, providers must cancel future trough orders concurrently with canceling treatment. Second, at the time of publication of the consensus, some providers were slow adopters of the new guidelines. Finally, the IDSA guidelines state that frequent monitoring for short course, lower intensity therapy, or in patients who are hemodynamically stable is not recommended.3 However, troughs were sometimes measured 2 to 3 times weekly in these patients.
Related: Results mixed in hospital efforts to tackle antimicrobial resistance
The data and observations lead to the conclusion that although providers may be able to discern between early and correctly timed troughs, they were not consistently adherent to the 2009 IDSA guidelines. It has been shown that pharmacy involvement of Medicare patients with infections in the intensive care unit has led to better clinical and monetary outcomes.8 Therefore, continued efforts by clinical pharmacists to monitor trough timing can be used to improve adherence and decrease costs (each trough is estimated to cost $16.97).
A study conducted in Australia demonstrated that pharmacist-led education of vancomycin dosing and monitoring (including when to measure a trough level) among prescribers and nurses led to improved adherence to the current guidelines and a greater number of patients treated within desired therapeutic ranges.9 In addition, a small study at the Atlanta VAMC in Georgia demonstrated that education of nurses, lab personnel, residents, ward clerks, and pharmacists led to a greater number of appropriately timed vancomycin and aminoglycoside levels.10 Thus, an interdisciplinary review of the current IDSA guidelines and review on the publication of the anticipated updated vancomycin guidelines should be provided to hospital personnel to aid in adoption of current dosing and monitoring recommendations.11
Limitations
This study is limited by the 4-year span of time that it encompassed, which may give a skewed depiction of current practices. Another limitation is that patients with fluctuating renal function were included in the analysis. Instead of selecting a random level order, a trough level order was sometimes selected for these patients. This could lead to a lower actual rate of early troughs. A third limitation is that this was a small and unblinded study. Also, the actual trough levels and the resulting changes that were made to specific regimens were not recorded. Thus, these data do not indicate whether the changes that were made reflected guideline recommendations. Finally, some clinical actions were taken after the dosing interval following the trough. This was often a result of off-hours lab results or waiting on attending physician or infectious disease guidance. These data were not included in the analysis.
Conclusion
Vancomycin troughs were often drawn too early and resulted in an increased rate of trough recollection. In an attempt to improve adherence to the current and the upcoming revised version of the IDSA consensus statement, it is recommended to educate and reeducate providers through interdisciplinary-led review sessions.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Vancomycin was isolated in the 1950s, but due to impurities causing adverse events and semisynthetic penicillin production, its use was greatly reduced.1,2 However, this medication gained in popularity 30 years later as a first-line treatment for methicillin-resistant Staphylococcus aureus infections.
In 2009 the Infectious Diseases Society of America (IDSA), American Society of Health System Pharmacists, and Society of Infectious Diseases Pharmacists developed a consensus review of the therapeutic monitoring and dosing of vancomycin in adult patients.3 Trough serum concentration levels are recommended as the most accurate and convenient method to monitor vancomycin. Per IDSA guidelines, an optimal trough is intended to be high enough to clear infections (> 10 mg/L) and prevent the development of vancomycin intermediate and resistant bacteria. Troughs should be obtained just before the next dose in steady-state conditions (starting just before the fourth dose) in patients with normal renal function.
Since the development of these guidelines, vancomycin trough levels are often drawn early.4-7 This may lead to an overestimation of the true trough concentration. A study by Morrison and colleagues in Boston, Massachusetts, found that 41.3% of vancomycin troughs were drawn early, and this resulted in statistically significant increases in the vancomycin concentrations, the rate of vancomycin regimen adjustments (decrease, discontinuation, or holding of dose), and the repeat vancomycin level orders compared with correctly timed troughs.5 It was noted by the study authors that lowering the daily dose of vancomycin based on early trough levels could lead to an underdosing of vancomycin and an increase in intermediate or resistant bacteria.
Related: IDWEEK: Antibiotic ‘time-out’ cut vancomycin use
The prevalence and implications of early trough samples have been measured at only 1 facility, and it is unknown whether these data can be reproduced elsewhere.5 Thus, this study sought to determine the prevalence and corresponding clinical actions of early trough levels at the Captain James A. Lovell Federal Health Care Center (JALFHCC). This is a unique facility that in 2010 combined a VA hospital with a DoD hospital. This facility cares for 67,000 military and retiree beneficiaries each year from southwestern Wisconsin and northwestern Illinois.The primary objective of this study was to measure the rate of early troughs drawn and their resultant effect on vancomycin regimens compared with correctly timed troughs. Secondarily, this study sought to compare the rate of repeated vancomycin trough levels in early vs correctly timed measurements.
Methods
This retrospective cohort analysis compared the outcomes of early and correctly timed vancomycin troughs. This study was approved by the Edward Hines, Jr. VA Hospital and JALFHCC Institutional Review Board. Veteran patients aged ≥ 18 years, hospitalized at JALFHCC, and receiving IV vancomycin at dosing intervals of 8, 12, 24, and 48 hours with measured trough levels between July 1, 2009, and July 1, 2013, were included in this study. Patients were excluded from analysis if vancomycin was given at any schedule other than the previously stated frequencies, they received hemodialysis during the treatment period, or their insurance coverage was through TRICARE (these patients had either active-duty or retired active-duty status).
Potentially eligible patients were identified via a Computerized Patient Records System (CPRS) search for laboratory vancomycin level measurements. The search supplied the researcher with the patient name, vancomycin level date and time, type of vancomycin level (trough or random), and vancomycin concentration. With this information, further data were gathered through CPRS: demographics, type of clinical infection, desired trough level (inferred if not listed in CPRS note), and vancomycin administration time (through the bar code medication administration system [BCMA] in CPRS). This analysis was of troughs, and multiple troughs may have originated from the same patient.
An early trough was defined as a trough taken more than 2 hours earlier than the next theoretical administration time or anytime before the third dose. After a trough was determined to be early or on time, the clinical actions taken during the dosing interval following sample collection were documented. A dose was considered to be held if stated in the BCMA or in a CPRS provider note. A dose was considered to be decreased with a change in frequency or strength that resulted in an overall daily dose decrease. A recollected vancomycin trough was counted within 24 hours of the trough or per a note in CPRS. Finally, observations that noted trends in vancomycin trough management were recorded.
The chi-square test with a significance criterion of 0.05 was used to compare early and on time troughs. Based on the results from the Boston, Massachusetts, study and 1 other study, about 780 vancomycin troughs would be required to meet significance in the primary outcome.5,6
Results
A total of 474 patient charts were reviewed, and 278 met inclusion criteria (196 were excluded). Of the included patients, 799 trough levels were analyzed. Of these, 377 (42.2%) were drawn early. There was no significant difference in the baseline characteristics of the early group vs the correctly timed group (Table 1). Of the early troughs, 190 (56.3%) were drawn prior to the third dose of vancomycin. It was observed that a large portion of these troughs occurred after a vancomycin dose adjustment.
Clinical actions taken after sampling occurred at a rate of 14.5% in the early group and 22.9% in the correctly timed group (P = .003; Table 2). Early troughs led to a 7.7% rate of trough recollection, which was significantly greater than the 1.5% rate in the correctly timed group (P < .001). An analysis of each factor resulting in a clinical action illustrated that the rates of daily dose decrease and discontinued dose were similar between the groups (Table 3). However, the rate of held doses was 8.3% in the early group and 17.1% in the correctly timed group.
This research process yielded some observations. Occasionally a trough was drawn after vancomycin therapy was discontinued and when there was no concern for nephrotoxicity. After the guidelines were published, providers continued to document in CPRS notes to check troughs before the third dose. This incidence decreased over time. Troughs were taken often in patients who were receiving a short course of therapy or who were hemodynamically stable. Finally, documentation of vancomycin regimen changes occasionally did not match the record in the BCMA (in these situations, the BCMA record was used for this study).
Related: Assessment of High Staphylococcus aureus MIC and Poor Patient Outcomes
Discussion
A large portion of trough levels at the JALFHCC were drawn early and did not adhere to the 2009 consensus guidelines. The rates of early troughs in this study and in the Boston, Massachusetts, study are similar.5 However, the 2 studies differed in 1 significant aspect: Clinical actions were taken less often in the early group at JALFHCC, whereas they were taken more often in the early group in the Boston, Massachusetts, study. This dissimilarity could be attributed to a difference in software between the hospitals. In the previous study, trough levels and the time that they were drawn were not displayed together. Thus, clinicians may have been less likely to gauge whether a trough was early. Since this information is available at the JALFHCC, clinicians may have been aware that the trough was early and avoided adjusting treatment (such as holding a dose, as illustrated in the data) based on a falsely elevated trough. This point is further supported by significantly greater amounts of recollected troughs in the early group, suggesting an understanding that the trough was early.
The low trough recollection rate of 7.7% of all early samples could be due to several factors that would prevent a trough redraw. First, medication discontinuation resulting from course completion or sensitivity results would not require further trough monitoring. Second, practitioners may assess the early sample as insignificantly different from a correctly timed one and elect not to redraw the trough. Sometimes a trough was drawn at the correct time, but the time was recorded incorrectly. In this situation, a new trough level would not be necessary. Finally, a lack of sufficient staffing during nights and weekends may result in a delay in interpreting results leading to a missed opportunity for recollection. Additionally, some troughs may not have been redrawn based on a practitioner’s opinion that a trough was not significantly early and did not represent skewed results. Sometimes an incorrect recording of trough draw time reflected that it was taken after vancomycin dosing when it was not.
Specific observations regarding the timing of the trough indicate other possible concerns and areas for improvement. First, providers must cancel future trough orders concurrently with canceling treatment. Second, at the time of publication of the consensus, some providers were slow adopters of the new guidelines. Finally, the IDSA guidelines state that frequent monitoring for short course, lower intensity therapy, or in patients who are hemodynamically stable is not recommended.3 However, troughs were sometimes measured 2 to 3 times weekly in these patients.
Related: Results mixed in hospital efforts to tackle antimicrobial resistance
The data and observations lead to the conclusion that although providers may be able to discern between early and correctly timed troughs, they were not consistently adherent to the 2009 IDSA guidelines. It has been shown that pharmacy involvement of Medicare patients with infections in the intensive care unit has led to better clinical and monetary outcomes.8 Therefore, continued efforts by clinical pharmacists to monitor trough timing can be used to improve adherence and decrease costs (each trough is estimated to cost $16.97).
A study conducted in Australia demonstrated that pharmacist-led education of vancomycin dosing and monitoring (including when to measure a trough level) among prescribers and nurses led to improved adherence to the current guidelines and a greater number of patients treated within desired therapeutic ranges.9 In addition, a small study at the Atlanta VAMC in Georgia demonstrated that education of nurses, lab personnel, residents, ward clerks, and pharmacists led to a greater number of appropriately timed vancomycin and aminoglycoside levels.10 Thus, an interdisciplinary review of the current IDSA guidelines and review on the publication of the anticipated updated vancomycin guidelines should be provided to hospital personnel to aid in adoption of current dosing and monitoring recommendations.11
Limitations
This study is limited by the 4-year span of time that it encompassed, which may give a skewed depiction of current practices. Another limitation is that patients with fluctuating renal function were included in the analysis. Instead of selecting a random level order, a trough level order was sometimes selected for these patients. This could lead to a lower actual rate of early troughs. A third limitation is that this was a small and unblinded study. Also, the actual trough levels and the resulting changes that were made to specific regimens were not recorded. Thus, these data do not indicate whether the changes that were made reflected guideline recommendations. Finally, some clinical actions were taken after the dosing interval following the trough. This was often a result of off-hours lab results or waiting on attending physician or infectious disease guidance. These data were not included in the analysis.
Conclusion
Vancomycin troughs were often drawn too early and resulted in an increased rate of trough recollection. In an attempt to improve adherence to the current and the upcoming revised version of the IDSA consensus statement, it is recommended to educate and reeducate providers through interdisciplinary-led review sessions.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Moellering RC Jr. Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42(suppl 1):S3-S4.
2. Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(suppl 1):S5-S12.
3. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29(11):1275-1279.
4. Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. hospitals. Pharmacotherapy. 2013;33(12):1256-1263.
5. Morrison AP, Melanson SEF, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early? Frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472-478.
6. Traugott KA, Maxwell PR, Green K, Frei C, Lewis JS 2nd. Effects of therapeutic drug monitoring criteria in a computerized prescriber-order-entry system on the appropriateness of vancomycin level orders. Am J Health Syst Pharm. 2011;68(4):347-352.
7. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801-806.
8. MacLaren R, Bond CA, Martin SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36(12):3184-3189.
9. Phillips CJ, Doan H, Quinn S, Kirkpatrick CM, Gordon DL, Doogue MP. An educational intervention to improve vancomycin prescribing and monitoring. Int J Antimicrob Agents. 2013;41(4):393-394.
10. Carroll DJ, Austin GE, Stajich GV, Miyrhaya RK, Murphy JE, Ward ES. Effect of education on the appropriateness of serum drug concentration determination. Ther Drug Monit. 1992;14(1):81-84.
11. Infectious Diseases Society of America (IDSA). IDSA practice guidelines: antimicrobial agent use. IDSA Website. 2015. http://www.idsociety.org/Antimicrobial_Agents. Accessed November 16, 2015.
1. Moellering RC Jr. Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42(suppl 1):S3-S4.
2. Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(suppl 1):S5-S12.
3. Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29(11):1275-1279.
4. Davis SL, Scheetz MH, Bosso JA, Goff DA, Rybak MJ. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: a cross-sectional survey of U.S. hospitals. Pharmacotherapy. 2013;33(12):1256-1263.
5. Morrison AP, Melanson SEF, Carty MG, Bates DW, Szumita PM, Tanasijevic MJ. What proportion of vancomycin trough levels are drawn too early? Frequency and impact on clinical actions. Am J Clin Pathol. 2012;137(3):472-478.
6. Traugott KA, Maxwell PR, Green K, Frei C, Lewis JS 2nd. Effects of therapeutic drug monitoring criteria in a computerized prescriber-order-entry system on the appropriateness of vancomycin level orders. Am J Health Syst Pharm. 2011;68(4):347-352.
7. Melanson SE, Mijailovic AS, Wright AP, Szumita PM, Bates DW, Tanasijevic MJ. An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 2013;140(6):801-806.
8. MacLaren R, Bond CA, Martin SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36(12):3184-3189.
9. Phillips CJ, Doan H, Quinn S, Kirkpatrick CM, Gordon DL, Doogue MP. An educational intervention to improve vancomycin prescribing and monitoring. Int J Antimicrob Agents. 2013;41(4):393-394.
10. Carroll DJ, Austin GE, Stajich GV, Miyrhaya RK, Murphy JE, Ward ES. Effect of education on the appropriateness of serum drug concentration determination. Ther Drug Monit. 1992;14(1):81-84.
11. Infectious Diseases Society of America (IDSA). IDSA practice guidelines: antimicrobial agent use. IDSA Website. 2015. http://www.idsociety.org/Antimicrobial_Agents. Accessed November 16, 2015.
Shared Medical Appointments and Their Effects on Achieving Diabetes Mellitus Goals in a Veteran Population
In 2012, 9.3% of the U.S. population had diabetes mellitus (DM).1 According to the American Diabetes Association, in 2012, the total cost of diagnosed DM in the U.S. was $245 billion.2 Diabetes mellitus is a leading cause of blindness, end-stage renal disease, and amputation in the U.S.3 Up to 80% of patients with DM will develop or die of macrovascular disease, such as heart attack or stroke.3
Diabetes mellitus is a chronic disease of epidemic proportion with management complexity that threatens to overwhelm providers in the acute care and primary care settings. Limited specialist availability and increased wait times continue to afflict the VA health care system, prompting efforts to increase health care provider (HCP) access and improve clinic efficiency.4 One of the methods proposed to increase HCP access and maximize clinic efficiency is the shared medical appointment (SMA).5,6
The SMA was designed to improve access and quality of care through enhanced education and support. With the number of people living with chronic diseases on the rise, the current patient-provider model is unrealistic in today’s health care environment. Shared medical appointments offer a unique format for providing evidence-based chronic disease management in which patients and a multidisciplinary team of providers collaborate toward education, discussion, and medication management in a supportive environment.7 Research has suggested that SMAs are a successful way to manage type 2 DM (T2DM).8,9 However, there is uncertainty regarding the optimal model design. The goals of this study were to evaluate whether the diabetes SMA at the Adam Benjamin, Jr. (ABJ) community-based outpatient clinic (CBOC) was an effective practice model for achieving improvements in glycemic control and to use subgroup analyses to elucidate unique characteristics about SMAs that may have been correlated with clinical success. This study may provide valuable information for other facilities considering SMAs.
Overview
The Jesse Brown VAMC (JBVAMC) and the ABJ CBOC implemented a T2DM-focused SMA in 2011. The ABJ CBOC multidisciplinary SMA team consisted of a medical administration service clerk, a registered dietician, a certified DM educator, a registered nurse, a nurse practitioner (NP), and a clinical pharmacy specialist (CPS). This team collaborated to deliver high-quality care to patients with poorly controlled T2DM to improve their glycemic control as well as clinical knowledge of their disease. A private conference room at the ABJ CBOC served as the location for the SMAs. This room was divided into 2 adjacent areas: One area with tables was organized in a semicircle to promote group discussion as well as minimize isolated conversations; the other area had computer terminals to facilitate individualized medication management. Other equipment included a scale for obtaining patient weights and various audio-visual devices.
The ABJ CBOC offered monthly SMAs. The team made several attempts to maximize SMA show rates, as previous studies indicated that low SMA show rates were a barrier to success.3,4,7-9 One review reported no-show rates as high as 70% in certain group visit models.4 About 2 weeks prior to a session, prospective SMA patients received automated and customized preappointment letters. Automated and customized phone call reminders were made to prospective SMA patients a few days before each session. As many as 18 patients participated in a single ABJ SMA.
The ABJ SMAs lasted from 60 to 90 minutes, depending on the level of patient participation and the size of the group. The first half of the SMA was dedicated to a group discussion, which involved the SMA team, the patient, and the patient’s family (if desired). The topic of conversation was typically guided by patient curiosity and knowledge deficits in a spontaneous and free-flowing manner; for this reason, these sessions were considered to be open.
The team also engaged in more structured focused sessions, which limited the spontaneous flow of conservation and narrowed the scope to provide targeted education about various aspects of T2DM care. During focused sessions, services such as dental, optometry, podiatry, MOVE! (a VA self-management weight reduction program), and nutrition also participated. Focused sessions addressed topics such as hypoglycemia management, eating around the holidays, sick-day management of T2DM, grocery shopping, exercise, oral health, eye care, and foot care. The specialty services were encouraged to be creative and interactive during the SMA. Many of these services used supportive literature, demonstrations, diagrams, and props to enrich the educational experience. Group discussion typically lasted 30 to 40 minutes; after which patients met individually with either a CPS or NP for medication management.
Medication management focused on optimizing T2DM therapy (both oral and injectable) to improve glycemic control. Interventions outside of T2DM therapy (eg, cholesterol, hypertension, and other risk reduction modalities) were not made, due to time constraints. Once a patient demonstrated improved working knowledge of T2DM and a clinically significant reduction in their glycosylated hemoglobin A1c (A1c) they were discharged from SMAs at the discretion of the SMA team. There was no set minimum or maximum duration for the SMAs.
Methods
This study was a retrospective chart review conducted at the JBVAMC and was approved by the institutional review board and the research and development committee. Patient confidentiality was maintained by identifying patients by means other than name or unique identifiers. Protected health information was accessible only by the aforementioned investigators. There was no direct patient contact during this study.
Patient lists were generated from the computerized patient record system (CPRS). Patients were tracked up to 6 months after SMA discharge or until the last SMA in which they participated. The control group was matched according to location, age, glycemic control, and time. The control group never attended an ABJ SMA but may have received regular care through their primary care provider, CPS, or endocrinologist. Prospective control group patients were randomized and reviewed sequentially to obtain the matched cohort.
The study took place at ABJ, an outpatient clinic serving veterans in northwest Indiana and surrounding areas. Inclusion criteria for the SMA group were patients with T2DM, aged ≥ 45 years, with an A1c ≥ 8.5% seen at ABJ for T2DM from May 1, 2011, to June 30, 2013. The control group included patients with T2DM, aged ≥ 45 years, with an A1c > 9% who never attended SMAs but may have received regular care at ABJ during the study period. The SMA group’s inclusion criteria threshold for A1c was lower in order to maximize sample size. The control group’s inclusion criteria threshold for A1c was higher due to use of a default reminder report called “A1c > 9%” to generate patient lists. Patients were excluded from the study if they did not meet inclusion criteria.
Baseline datum was the most recent parameter available in CPRS prior to enrollment. The endpoint datum was the parameter nearest the time of SMA discharge or the first available parameter within 6 months from the date of discharge. In the control group, the baseline datum was the initial parameter during the study period and the endpoint datum was the closest measure to 4 months after baseline. Four months was chosen to allow for at least 1 A1c measurement during the study period. In addition, it was estimated (prior to collecting any data) that 4 months was the average time a patient participated in SMAs. Serial A1c measurements were defined as values obtained at SMA discharge and 3- and 6-months postdischarge. These parameters were used to evaluate the sustainability of improvements in glycemic control. All values falling outside of these defined parameters were excluded.
Related: Experiences of Veterans With Diabetes From Shared Medical Appointments
The data analysis compared A1c change from baseline to endpoint for the SMA and control groups. Data collection included baseline characteristics, SMA show rate, number of SMA patients seen by a CPS or NP, number and type of SMA interventions made by a CPS or NP, and the number and type of non-SMA interventions made during the study period. Intervention types were medications: added, discontinued, or titrated; and other, defined as referrals made to specialty services (such as dental, optometry, and podiatry).
Secondary endpoints included the number of SMAs and glycemic improvement, SMA format style (open- vs focused session) and glycemic improvement, SMA provider (CPS vs NP) and glycemic improvement, the change in A1c stratified by baseline A1c (A1c ≥ 10% vs < 10%), the change in actual body weight (ABW) and body mass index (BMI), and maintenance of A1c (3- and 6-months postdischarge).
The primary endpoint was evaluated using a 2-sample Student t test. Secondary endpoints were evaluated using the independent t test. Statistical significance was defined as P < .05.
Results
A total of 129 unique patients were scheduled for SMAs, 62 of which met inclusion criteria and were included in the SMA group. During enrollment, 67 patients were excluded: 55 never participated in SMAs, 6 had baseline A1c values < 8.5%, 4 had insufficient data, and 2 were aged < 45 years. A total of 29 SMAs were conducted during the study period, and patients attended an average of 3.15 ± 2.14 (SD) SMAs. The average attendance at each SMA was 7.1 ± 2.62 (SD) patients. For the control group, 754 unique patients were identified and randomized. A total of 90 charts were sequentially reviewed in order to obtain the 62 patients for the control group.
Baseline characteristics were balanced between groups. However, there were more women in the SMA group vs the control group (Table 1). Within the control group, there were a total of 107 appointments that addressed T2DM, which averaged 1.72 ± 1.51 (SD) appointments per patient. The total number of interventions made in the SMA group was 192: 64.6% (124) by a CPS and 35.4% (68) by a NP. For the CPS, the most frequent intervention was medication titration (69.5%), followed by other (23.5%), medication addition (4%), and medication discontinuation (3%). Of note, 53.2% (33) of the SMA patients were seen an average of 1.2 times by non-SMA providers. The SMA patients had a total of 45 non-SMA interventions (0.73 per patient) during the study period.
For the primary endpoint, the SMA group had a 1.48% ± 0.02 (SD) reduction in A1c compared with a 0.6% ± 0.02 (SD) decrease in the control group (P = .01). When evaluating mean changes in A1c by the number of SMAs attended, it was noted that participation in ≥ 6 SMAs led to the greatest reduction in A1c of 2.08%. In the SMA group, it was noted that patients with higher A1c values at baseline demonstrated greater improvements in glycemic control compared with patients with lower baseline A1c values. The mean change in A1c, stratified by baseline A1c, was -2.26% for those with baseline A1c values ≥ 10% and -0.87% for those with baseline A1c values < 10%.
In evaluating the format style, open- vs focused-session, it was observed that participation in focused sessions led to greater improvements in glycemic control. Furthermore, when stratified by provider, greater improvements in glycemic control were demonstrated when medication management was completed by a CPS vs a NP (Table 2). The average number of interventions per SMA patient was 3.1 ± 2.22 (SD). For the control group, the total number of interventions made was 86, with an average of 1.37 ± 1.51 (SD) per patient. The overall show rate was 49% ± 16 (SD), 52% ± 16 (SD) for open visits, and 46% ± 15 (SD) for focused visits. The mean change in ABW and BMI from baseline to endpoint was no different between the SMA and control groups (Table 3). The SMA group participants demonstrated a decrease in A1c at 3 months postdischarge, and a moderate increase in A1c was noted at 6 months postdischarge.
Discussion
Shared medical appointments provide an effective alternative to standards of care in order to obtain improvements in glycemic control. Consistent with previous studies, this study reported significant improvements in glycemic control in the SMA group vs the control group. This study also elucidated unique characteristics about SMAs that may have been correlated with clinical success.
Although the greatest improvements in glycemic control were noted for those who participated in ≥ 6 SMAs, it was observed that participation in only 1 SMA also led to improvements. For a site with limited staff and a high volume of patients waiting to participate in SMAs, it may be mutually beneficial to offer only 1 SMA per patient. In addition, patients with ≥ 10% A1c at baseline demonstrated greater improvements in glycemic control compared to those with < 10% A1c at baseline. The reasons the higher baseline A1c subgroup responded to interventions more robustly are unclear and likely multifactorial. Nonetheless, factors such as psychosocial influences (eg, peer pressure to get healthy) may have increased motivation to prevent complications and improved medication adherence in the setting of closer follow-up. Additionally, hyperresponsiveness to drug therapy may have played a role. Regardless, for new SMA programs interested in making an immediate impact, it may be advantageous to initially select patients with very poorly controlled DM.
A unique aspect of the ABJ SMA was the variety of focused sessions offered. Previous studies did not demonstrate such a variety of focused sessions, nor did they evaluate the impact of a focused visit on the patient’s T2DM control. Participation in focused ABJ SMA sessions may have led to improved T2DM control, which may be attributed to the value patients assigned to specialty care and an increased motivation to get healthy.
Related: SGLT2 Inhibitors for Type 2 Diabetes Mellitus Treatment
Another factor that may have led to improved T2DM control was CPS involvement with medication management. The presence of a NP was highly valued, both from a group discussion and medication management standpoint; still, it is a good idea to involve a CPS who has a strong command of DM pharmacotherapy. One shortcoming of this SMA program was the inability for patients to maintain glycemic improvements 6 months after discharge. This pitfall was likely the result of suboptimal coordination of care after SMA discharge and may be avoided by asking the medical administration service clerk to promptly schedule discharged SMA patients for a general medicine clinic T2DM follow-up.The SMA patients had more T2DM interventions within the same time frame compared with the control patients. Although not causative, the increased number of interventions in addition to the bolstered support of the SMA may have correlated with glycemic improvements.
An important finding of this study was the SMA show rate and how it compared with attendance rates found in other group models. The favorable ABJ SMA show rate could have been due to the rigorous attention paid to reminder letters and phone calls. The literature has not established a standard approach to increasing SMA show rates; however, the current data suggest that increased reminders may have increased attendance.
Limitations
This study had several limitations. The external validity was weakened by the modest sample size and the homogenous baseline characteristics of those enrolled. Another limitation was inconsistent documentation of laboratory parameters. The inability to obtain A1c values exactly at enrollment and discharge could have potentially skewed the results. In addition, incomplete documentation of interventions for dual-care patients (ie, those who obtained care outside of the VA) was an unavoidable challenge. Last, this study did not perform an assessment of SMA patient satisfaction, cost-benefit, or safety.
Conclusion
The ABJ SMA was an effective addition to standards of care in order to achieve improvements in glycemic control in a veteran population with poorly controlled T2DM. Furthermore,the data suggest that a successful program should be multidisciplinary, select poorly controlled patients, offer focused sessions, have a CPS participate in medication management, and encourage patients to complete ≥ 6 sessions. Future studies should be conducted to include more diverse patients to see whether the efficacy of this SMA format is maintained.
A safety analysis should also be conducted to ensure that the SMA format is not only effective, but also a safe means to manage medical conditions. In addition, the scope of the ABJ SMAs should be expanded to allow for evaluation of other diseases. An evaluation of patient satisfaction and cost-benefit could provide additional support for the implementation of SMAs, as improvements in quality of life and cost savings are endpoints to be desired.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimate of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
3. Kirsh SR, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16(5):349-353.
4. Jaber R, Braksmajer A, Trilling JS. Group visits: a qualitative review of current research. J Am Board Fam Med. 2006;19(3):276-290.
5. Klein S. The Veterans Health Administration: implementing patient-centered medical homes in the nation's largest integrated delivery system. Commonwealth Fund Website. http://www.common wealthfund.org/publications/case-studies/2011 /sep/va-medical-homes. Published September 2011. Accessed November 11, 2015.
6. U.S. Department of Veterans Affairs. VA Shared Medical Appointments for Patients With Diabetes: Maximizing Patient & Provider Expertise to Strengthen Patient Management. Washington, DC: U.S. Department of Veterans Affairs; 2008.
7. Bronson DL, Maxwell RA. Shared medical appointments: increasing patient access without increasing physician hours. Cleve Clin J Med. 2004;71(5):369-370, 372, 374 passim.
8. Cohen LB, Taveira T, Khatana SA, Dooley AG, Pirraglia PA, Wu WC. Pharmacist-led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes. Diabetes Educ. 2011;37(6):801-812.
9. Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med. 2011;171(5):453-459.
In 2012, 9.3% of the U.S. population had diabetes mellitus (DM).1 According to the American Diabetes Association, in 2012, the total cost of diagnosed DM in the U.S. was $245 billion.2 Diabetes mellitus is a leading cause of blindness, end-stage renal disease, and amputation in the U.S.3 Up to 80% of patients with DM will develop or die of macrovascular disease, such as heart attack or stroke.3
Diabetes mellitus is a chronic disease of epidemic proportion with management complexity that threatens to overwhelm providers in the acute care and primary care settings. Limited specialist availability and increased wait times continue to afflict the VA health care system, prompting efforts to increase health care provider (HCP) access and improve clinic efficiency.4 One of the methods proposed to increase HCP access and maximize clinic efficiency is the shared medical appointment (SMA).5,6
The SMA was designed to improve access and quality of care through enhanced education and support. With the number of people living with chronic diseases on the rise, the current patient-provider model is unrealistic in today’s health care environment. Shared medical appointments offer a unique format for providing evidence-based chronic disease management in which patients and a multidisciplinary team of providers collaborate toward education, discussion, and medication management in a supportive environment.7 Research has suggested that SMAs are a successful way to manage type 2 DM (T2DM).8,9 However, there is uncertainty regarding the optimal model design. The goals of this study were to evaluate whether the diabetes SMA at the Adam Benjamin, Jr. (ABJ) community-based outpatient clinic (CBOC) was an effective practice model for achieving improvements in glycemic control and to use subgroup analyses to elucidate unique characteristics about SMAs that may have been correlated with clinical success. This study may provide valuable information for other facilities considering SMAs.
Overview
The Jesse Brown VAMC (JBVAMC) and the ABJ CBOC implemented a T2DM-focused SMA in 2011. The ABJ CBOC multidisciplinary SMA team consisted of a medical administration service clerk, a registered dietician, a certified DM educator, a registered nurse, a nurse practitioner (NP), and a clinical pharmacy specialist (CPS). This team collaborated to deliver high-quality care to patients with poorly controlled T2DM to improve their glycemic control as well as clinical knowledge of their disease. A private conference room at the ABJ CBOC served as the location for the SMAs. This room was divided into 2 adjacent areas: One area with tables was organized in a semicircle to promote group discussion as well as minimize isolated conversations; the other area had computer terminals to facilitate individualized medication management. Other equipment included a scale for obtaining patient weights and various audio-visual devices.
The ABJ CBOC offered monthly SMAs. The team made several attempts to maximize SMA show rates, as previous studies indicated that low SMA show rates were a barrier to success.3,4,7-9 One review reported no-show rates as high as 70% in certain group visit models.4 About 2 weeks prior to a session, prospective SMA patients received automated and customized preappointment letters. Automated and customized phone call reminders were made to prospective SMA patients a few days before each session. As many as 18 patients participated in a single ABJ SMA.
The ABJ SMAs lasted from 60 to 90 minutes, depending on the level of patient participation and the size of the group. The first half of the SMA was dedicated to a group discussion, which involved the SMA team, the patient, and the patient’s family (if desired). The topic of conversation was typically guided by patient curiosity and knowledge deficits in a spontaneous and free-flowing manner; for this reason, these sessions were considered to be open.
The team also engaged in more structured focused sessions, which limited the spontaneous flow of conservation and narrowed the scope to provide targeted education about various aspects of T2DM care. During focused sessions, services such as dental, optometry, podiatry, MOVE! (a VA self-management weight reduction program), and nutrition also participated. Focused sessions addressed topics such as hypoglycemia management, eating around the holidays, sick-day management of T2DM, grocery shopping, exercise, oral health, eye care, and foot care. The specialty services were encouraged to be creative and interactive during the SMA. Many of these services used supportive literature, demonstrations, diagrams, and props to enrich the educational experience. Group discussion typically lasted 30 to 40 minutes; after which patients met individually with either a CPS or NP for medication management.
Medication management focused on optimizing T2DM therapy (both oral and injectable) to improve glycemic control. Interventions outside of T2DM therapy (eg, cholesterol, hypertension, and other risk reduction modalities) were not made, due to time constraints. Once a patient demonstrated improved working knowledge of T2DM and a clinically significant reduction in their glycosylated hemoglobin A1c (A1c) they were discharged from SMAs at the discretion of the SMA team. There was no set minimum or maximum duration for the SMAs.
Methods
This study was a retrospective chart review conducted at the JBVAMC and was approved by the institutional review board and the research and development committee. Patient confidentiality was maintained by identifying patients by means other than name or unique identifiers. Protected health information was accessible only by the aforementioned investigators. There was no direct patient contact during this study.
Patient lists were generated from the computerized patient record system (CPRS). Patients were tracked up to 6 months after SMA discharge or until the last SMA in which they participated. The control group was matched according to location, age, glycemic control, and time. The control group never attended an ABJ SMA but may have received regular care through their primary care provider, CPS, or endocrinologist. Prospective control group patients were randomized and reviewed sequentially to obtain the matched cohort.
The study took place at ABJ, an outpatient clinic serving veterans in northwest Indiana and surrounding areas. Inclusion criteria for the SMA group were patients with T2DM, aged ≥ 45 years, with an A1c ≥ 8.5% seen at ABJ for T2DM from May 1, 2011, to June 30, 2013. The control group included patients with T2DM, aged ≥ 45 years, with an A1c > 9% who never attended SMAs but may have received regular care at ABJ during the study period. The SMA group’s inclusion criteria threshold for A1c was lower in order to maximize sample size. The control group’s inclusion criteria threshold for A1c was higher due to use of a default reminder report called “A1c > 9%” to generate patient lists. Patients were excluded from the study if they did not meet inclusion criteria.
Baseline datum was the most recent parameter available in CPRS prior to enrollment. The endpoint datum was the parameter nearest the time of SMA discharge or the first available parameter within 6 months from the date of discharge. In the control group, the baseline datum was the initial parameter during the study period and the endpoint datum was the closest measure to 4 months after baseline. Four months was chosen to allow for at least 1 A1c measurement during the study period. In addition, it was estimated (prior to collecting any data) that 4 months was the average time a patient participated in SMAs. Serial A1c measurements were defined as values obtained at SMA discharge and 3- and 6-months postdischarge. These parameters were used to evaluate the sustainability of improvements in glycemic control. All values falling outside of these defined parameters were excluded.
Related: Experiences of Veterans With Diabetes From Shared Medical Appointments
The data analysis compared A1c change from baseline to endpoint for the SMA and control groups. Data collection included baseline characteristics, SMA show rate, number of SMA patients seen by a CPS or NP, number and type of SMA interventions made by a CPS or NP, and the number and type of non-SMA interventions made during the study period. Intervention types were medications: added, discontinued, or titrated; and other, defined as referrals made to specialty services (such as dental, optometry, and podiatry).
Secondary endpoints included the number of SMAs and glycemic improvement, SMA format style (open- vs focused session) and glycemic improvement, SMA provider (CPS vs NP) and glycemic improvement, the change in A1c stratified by baseline A1c (A1c ≥ 10% vs < 10%), the change in actual body weight (ABW) and body mass index (BMI), and maintenance of A1c (3- and 6-months postdischarge).
The primary endpoint was evaluated using a 2-sample Student t test. Secondary endpoints were evaluated using the independent t test. Statistical significance was defined as P < .05.
Results
A total of 129 unique patients were scheduled for SMAs, 62 of which met inclusion criteria and were included in the SMA group. During enrollment, 67 patients were excluded: 55 never participated in SMAs, 6 had baseline A1c values < 8.5%, 4 had insufficient data, and 2 were aged < 45 years. A total of 29 SMAs were conducted during the study period, and patients attended an average of 3.15 ± 2.14 (SD) SMAs. The average attendance at each SMA was 7.1 ± 2.62 (SD) patients. For the control group, 754 unique patients were identified and randomized. A total of 90 charts were sequentially reviewed in order to obtain the 62 patients for the control group.
Baseline characteristics were balanced between groups. However, there were more women in the SMA group vs the control group (Table 1). Within the control group, there were a total of 107 appointments that addressed T2DM, which averaged 1.72 ± 1.51 (SD) appointments per patient. The total number of interventions made in the SMA group was 192: 64.6% (124) by a CPS and 35.4% (68) by a NP. For the CPS, the most frequent intervention was medication titration (69.5%), followed by other (23.5%), medication addition (4%), and medication discontinuation (3%). Of note, 53.2% (33) of the SMA patients were seen an average of 1.2 times by non-SMA providers. The SMA patients had a total of 45 non-SMA interventions (0.73 per patient) during the study period.
For the primary endpoint, the SMA group had a 1.48% ± 0.02 (SD) reduction in A1c compared with a 0.6% ± 0.02 (SD) decrease in the control group (P = .01). When evaluating mean changes in A1c by the number of SMAs attended, it was noted that participation in ≥ 6 SMAs led to the greatest reduction in A1c of 2.08%. In the SMA group, it was noted that patients with higher A1c values at baseline demonstrated greater improvements in glycemic control compared with patients with lower baseline A1c values. The mean change in A1c, stratified by baseline A1c, was -2.26% for those with baseline A1c values ≥ 10% and -0.87% for those with baseline A1c values < 10%.
In evaluating the format style, open- vs focused-session, it was observed that participation in focused sessions led to greater improvements in glycemic control. Furthermore, when stratified by provider, greater improvements in glycemic control were demonstrated when medication management was completed by a CPS vs a NP (Table 2). The average number of interventions per SMA patient was 3.1 ± 2.22 (SD). For the control group, the total number of interventions made was 86, with an average of 1.37 ± 1.51 (SD) per patient. The overall show rate was 49% ± 16 (SD), 52% ± 16 (SD) for open visits, and 46% ± 15 (SD) for focused visits. The mean change in ABW and BMI from baseline to endpoint was no different between the SMA and control groups (Table 3). The SMA group participants demonstrated a decrease in A1c at 3 months postdischarge, and a moderate increase in A1c was noted at 6 months postdischarge.
Discussion
Shared medical appointments provide an effective alternative to standards of care in order to obtain improvements in glycemic control. Consistent with previous studies, this study reported significant improvements in glycemic control in the SMA group vs the control group. This study also elucidated unique characteristics about SMAs that may have been correlated with clinical success.
Although the greatest improvements in glycemic control were noted for those who participated in ≥ 6 SMAs, it was observed that participation in only 1 SMA also led to improvements. For a site with limited staff and a high volume of patients waiting to participate in SMAs, it may be mutually beneficial to offer only 1 SMA per patient. In addition, patients with ≥ 10% A1c at baseline demonstrated greater improvements in glycemic control compared to those with < 10% A1c at baseline. The reasons the higher baseline A1c subgroup responded to interventions more robustly are unclear and likely multifactorial. Nonetheless, factors such as psychosocial influences (eg, peer pressure to get healthy) may have increased motivation to prevent complications and improved medication adherence in the setting of closer follow-up. Additionally, hyperresponsiveness to drug therapy may have played a role. Regardless, for new SMA programs interested in making an immediate impact, it may be advantageous to initially select patients with very poorly controlled DM.
A unique aspect of the ABJ SMA was the variety of focused sessions offered. Previous studies did not demonstrate such a variety of focused sessions, nor did they evaluate the impact of a focused visit on the patient’s T2DM control. Participation in focused ABJ SMA sessions may have led to improved T2DM control, which may be attributed to the value patients assigned to specialty care and an increased motivation to get healthy.
Related: SGLT2 Inhibitors for Type 2 Diabetes Mellitus Treatment
Another factor that may have led to improved T2DM control was CPS involvement with medication management. The presence of a NP was highly valued, both from a group discussion and medication management standpoint; still, it is a good idea to involve a CPS who has a strong command of DM pharmacotherapy. One shortcoming of this SMA program was the inability for patients to maintain glycemic improvements 6 months after discharge. This pitfall was likely the result of suboptimal coordination of care after SMA discharge and may be avoided by asking the medical administration service clerk to promptly schedule discharged SMA patients for a general medicine clinic T2DM follow-up.The SMA patients had more T2DM interventions within the same time frame compared with the control patients. Although not causative, the increased number of interventions in addition to the bolstered support of the SMA may have correlated with glycemic improvements.
An important finding of this study was the SMA show rate and how it compared with attendance rates found in other group models. The favorable ABJ SMA show rate could have been due to the rigorous attention paid to reminder letters and phone calls. The literature has not established a standard approach to increasing SMA show rates; however, the current data suggest that increased reminders may have increased attendance.
Limitations
This study had several limitations. The external validity was weakened by the modest sample size and the homogenous baseline characteristics of those enrolled. Another limitation was inconsistent documentation of laboratory parameters. The inability to obtain A1c values exactly at enrollment and discharge could have potentially skewed the results. In addition, incomplete documentation of interventions for dual-care patients (ie, those who obtained care outside of the VA) was an unavoidable challenge. Last, this study did not perform an assessment of SMA patient satisfaction, cost-benefit, or safety.
Conclusion
The ABJ SMA was an effective addition to standards of care in order to achieve improvements in glycemic control in a veteran population with poorly controlled T2DM. Furthermore,the data suggest that a successful program should be multidisciplinary, select poorly controlled patients, offer focused sessions, have a CPS participate in medication management, and encourage patients to complete ≥ 6 sessions. Future studies should be conducted to include more diverse patients to see whether the efficacy of this SMA format is maintained.
A safety analysis should also be conducted to ensure that the SMA format is not only effective, but also a safe means to manage medical conditions. In addition, the scope of the ABJ SMAs should be expanded to allow for evaluation of other diseases. An evaluation of patient satisfaction and cost-benefit could provide additional support for the implementation of SMAs, as improvements in quality of life and cost savings are endpoints to be desired.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
In 2012, 9.3% of the U.S. population had diabetes mellitus (DM).1 According to the American Diabetes Association, in 2012, the total cost of diagnosed DM in the U.S. was $245 billion.2 Diabetes mellitus is a leading cause of blindness, end-stage renal disease, and amputation in the U.S.3 Up to 80% of patients with DM will develop or die of macrovascular disease, such as heart attack or stroke.3
Diabetes mellitus is a chronic disease of epidemic proportion with management complexity that threatens to overwhelm providers in the acute care and primary care settings. Limited specialist availability and increased wait times continue to afflict the VA health care system, prompting efforts to increase health care provider (HCP) access and improve clinic efficiency.4 One of the methods proposed to increase HCP access and maximize clinic efficiency is the shared medical appointment (SMA).5,6
The SMA was designed to improve access and quality of care through enhanced education and support. With the number of people living with chronic diseases on the rise, the current patient-provider model is unrealistic in today’s health care environment. Shared medical appointments offer a unique format for providing evidence-based chronic disease management in which patients and a multidisciplinary team of providers collaborate toward education, discussion, and medication management in a supportive environment.7 Research has suggested that SMAs are a successful way to manage type 2 DM (T2DM).8,9 However, there is uncertainty regarding the optimal model design. The goals of this study were to evaluate whether the diabetes SMA at the Adam Benjamin, Jr. (ABJ) community-based outpatient clinic (CBOC) was an effective practice model for achieving improvements in glycemic control and to use subgroup analyses to elucidate unique characteristics about SMAs that may have been correlated with clinical success. This study may provide valuable information for other facilities considering SMAs.
Overview
The Jesse Brown VAMC (JBVAMC) and the ABJ CBOC implemented a T2DM-focused SMA in 2011. The ABJ CBOC multidisciplinary SMA team consisted of a medical administration service clerk, a registered dietician, a certified DM educator, a registered nurse, a nurse practitioner (NP), and a clinical pharmacy specialist (CPS). This team collaborated to deliver high-quality care to patients with poorly controlled T2DM to improve their glycemic control as well as clinical knowledge of their disease. A private conference room at the ABJ CBOC served as the location for the SMAs. This room was divided into 2 adjacent areas: One area with tables was organized in a semicircle to promote group discussion as well as minimize isolated conversations; the other area had computer terminals to facilitate individualized medication management. Other equipment included a scale for obtaining patient weights and various audio-visual devices.
The ABJ CBOC offered monthly SMAs. The team made several attempts to maximize SMA show rates, as previous studies indicated that low SMA show rates were a barrier to success.3,4,7-9 One review reported no-show rates as high as 70% in certain group visit models.4 About 2 weeks prior to a session, prospective SMA patients received automated and customized preappointment letters. Automated and customized phone call reminders were made to prospective SMA patients a few days before each session. As many as 18 patients participated in a single ABJ SMA.
The ABJ SMAs lasted from 60 to 90 minutes, depending on the level of patient participation and the size of the group. The first half of the SMA was dedicated to a group discussion, which involved the SMA team, the patient, and the patient’s family (if desired). The topic of conversation was typically guided by patient curiosity and knowledge deficits in a spontaneous and free-flowing manner; for this reason, these sessions were considered to be open.
The team also engaged in more structured focused sessions, which limited the spontaneous flow of conservation and narrowed the scope to provide targeted education about various aspects of T2DM care. During focused sessions, services such as dental, optometry, podiatry, MOVE! (a VA self-management weight reduction program), and nutrition also participated. Focused sessions addressed topics such as hypoglycemia management, eating around the holidays, sick-day management of T2DM, grocery shopping, exercise, oral health, eye care, and foot care. The specialty services were encouraged to be creative and interactive during the SMA. Many of these services used supportive literature, demonstrations, diagrams, and props to enrich the educational experience. Group discussion typically lasted 30 to 40 minutes; after which patients met individually with either a CPS or NP for medication management.
Medication management focused on optimizing T2DM therapy (both oral and injectable) to improve glycemic control. Interventions outside of T2DM therapy (eg, cholesterol, hypertension, and other risk reduction modalities) were not made, due to time constraints. Once a patient demonstrated improved working knowledge of T2DM and a clinically significant reduction in their glycosylated hemoglobin A1c (A1c) they were discharged from SMAs at the discretion of the SMA team. There was no set minimum or maximum duration for the SMAs.
Methods
This study was a retrospective chart review conducted at the JBVAMC and was approved by the institutional review board and the research and development committee. Patient confidentiality was maintained by identifying patients by means other than name or unique identifiers. Protected health information was accessible only by the aforementioned investigators. There was no direct patient contact during this study.
Patient lists were generated from the computerized patient record system (CPRS). Patients were tracked up to 6 months after SMA discharge or until the last SMA in which they participated. The control group was matched according to location, age, glycemic control, and time. The control group never attended an ABJ SMA but may have received regular care through their primary care provider, CPS, or endocrinologist. Prospective control group patients were randomized and reviewed sequentially to obtain the matched cohort.
The study took place at ABJ, an outpatient clinic serving veterans in northwest Indiana and surrounding areas. Inclusion criteria for the SMA group were patients with T2DM, aged ≥ 45 years, with an A1c ≥ 8.5% seen at ABJ for T2DM from May 1, 2011, to June 30, 2013. The control group included patients with T2DM, aged ≥ 45 years, with an A1c > 9% who never attended SMAs but may have received regular care at ABJ during the study period. The SMA group’s inclusion criteria threshold for A1c was lower in order to maximize sample size. The control group’s inclusion criteria threshold for A1c was higher due to use of a default reminder report called “A1c > 9%” to generate patient lists. Patients were excluded from the study if they did not meet inclusion criteria.
Baseline datum was the most recent parameter available in CPRS prior to enrollment. The endpoint datum was the parameter nearest the time of SMA discharge or the first available parameter within 6 months from the date of discharge. In the control group, the baseline datum was the initial parameter during the study period and the endpoint datum was the closest measure to 4 months after baseline. Four months was chosen to allow for at least 1 A1c measurement during the study period. In addition, it was estimated (prior to collecting any data) that 4 months was the average time a patient participated in SMAs. Serial A1c measurements were defined as values obtained at SMA discharge and 3- and 6-months postdischarge. These parameters were used to evaluate the sustainability of improvements in glycemic control. All values falling outside of these defined parameters were excluded.
Related: Experiences of Veterans With Diabetes From Shared Medical Appointments
The data analysis compared A1c change from baseline to endpoint for the SMA and control groups. Data collection included baseline characteristics, SMA show rate, number of SMA patients seen by a CPS or NP, number and type of SMA interventions made by a CPS or NP, and the number and type of non-SMA interventions made during the study period. Intervention types were medications: added, discontinued, or titrated; and other, defined as referrals made to specialty services (such as dental, optometry, and podiatry).
Secondary endpoints included the number of SMAs and glycemic improvement, SMA format style (open- vs focused session) and glycemic improvement, SMA provider (CPS vs NP) and glycemic improvement, the change in A1c stratified by baseline A1c (A1c ≥ 10% vs < 10%), the change in actual body weight (ABW) and body mass index (BMI), and maintenance of A1c (3- and 6-months postdischarge).
The primary endpoint was evaluated using a 2-sample Student t test. Secondary endpoints were evaluated using the independent t test. Statistical significance was defined as P < .05.
Results
A total of 129 unique patients were scheduled for SMAs, 62 of which met inclusion criteria and were included in the SMA group. During enrollment, 67 patients were excluded: 55 never participated in SMAs, 6 had baseline A1c values < 8.5%, 4 had insufficient data, and 2 were aged < 45 years. A total of 29 SMAs were conducted during the study period, and patients attended an average of 3.15 ± 2.14 (SD) SMAs. The average attendance at each SMA was 7.1 ± 2.62 (SD) patients. For the control group, 754 unique patients were identified and randomized. A total of 90 charts were sequentially reviewed in order to obtain the 62 patients for the control group.
Baseline characteristics were balanced between groups. However, there were more women in the SMA group vs the control group (Table 1). Within the control group, there were a total of 107 appointments that addressed T2DM, which averaged 1.72 ± 1.51 (SD) appointments per patient. The total number of interventions made in the SMA group was 192: 64.6% (124) by a CPS and 35.4% (68) by a NP. For the CPS, the most frequent intervention was medication titration (69.5%), followed by other (23.5%), medication addition (4%), and medication discontinuation (3%). Of note, 53.2% (33) of the SMA patients were seen an average of 1.2 times by non-SMA providers. The SMA patients had a total of 45 non-SMA interventions (0.73 per patient) during the study period.
For the primary endpoint, the SMA group had a 1.48% ± 0.02 (SD) reduction in A1c compared with a 0.6% ± 0.02 (SD) decrease in the control group (P = .01). When evaluating mean changes in A1c by the number of SMAs attended, it was noted that participation in ≥ 6 SMAs led to the greatest reduction in A1c of 2.08%. In the SMA group, it was noted that patients with higher A1c values at baseline demonstrated greater improvements in glycemic control compared with patients with lower baseline A1c values. The mean change in A1c, stratified by baseline A1c, was -2.26% for those with baseline A1c values ≥ 10% and -0.87% for those with baseline A1c values < 10%.
In evaluating the format style, open- vs focused-session, it was observed that participation in focused sessions led to greater improvements in glycemic control. Furthermore, when stratified by provider, greater improvements in glycemic control were demonstrated when medication management was completed by a CPS vs a NP (Table 2). The average number of interventions per SMA patient was 3.1 ± 2.22 (SD). For the control group, the total number of interventions made was 86, with an average of 1.37 ± 1.51 (SD) per patient. The overall show rate was 49% ± 16 (SD), 52% ± 16 (SD) for open visits, and 46% ± 15 (SD) for focused visits. The mean change in ABW and BMI from baseline to endpoint was no different between the SMA and control groups (Table 3). The SMA group participants demonstrated a decrease in A1c at 3 months postdischarge, and a moderate increase in A1c was noted at 6 months postdischarge.
Discussion
Shared medical appointments provide an effective alternative to standards of care in order to obtain improvements in glycemic control. Consistent with previous studies, this study reported significant improvements in glycemic control in the SMA group vs the control group. This study also elucidated unique characteristics about SMAs that may have been correlated with clinical success.
Although the greatest improvements in glycemic control were noted for those who participated in ≥ 6 SMAs, it was observed that participation in only 1 SMA also led to improvements. For a site with limited staff and a high volume of patients waiting to participate in SMAs, it may be mutually beneficial to offer only 1 SMA per patient. In addition, patients with ≥ 10% A1c at baseline demonstrated greater improvements in glycemic control compared to those with < 10% A1c at baseline. The reasons the higher baseline A1c subgroup responded to interventions more robustly are unclear and likely multifactorial. Nonetheless, factors such as psychosocial influences (eg, peer pressure to get healthy) may have increased motivation to prevent complications and improved medication adherence in the setting of closer follow-up. Additionally, hyperresponsiveness to drug therapy may have played a role. Regardless, for new SMA programs interested in making an immediate impact, it may be advantageous to initially select patients with very poorly controlled DM.
A unique aspect of the ABJ SMA was the variety of focused sessions offered. Previous studies did not demonstrate such a variety of focused sessions, nor did they evaluate the impact of a focused visit on the patient’s T2DM control. Participation in focused ABJ SMA sessions may have led to improved T2DM control, which may be attributed to the value patients assigned to specialty care and an increased motivation to get healthy.
Related: SGLT2 Inhibitors for Type 2 Diabetes Mellitus Treatment
Another factor that may have led to improved T2DM control was CPS involvement with medication management. The presence of a NP was highly valued, both from a group discussion and medication management standpoint; still, it is a good idea to involve a CPS who has a strong command of DM pharmacotherapy. One shortcoming of this SMA program was the inability for patients to maintain glycemic improvements 6 months after discharge. This pitfall was likely the result of suboptimal coordination of care after SMA discharge and may be avoided by asking the medical administration service clerk to promptly schedule discharged SMA patients for a general medicine clinic T2DM follow-up.The SMA patients had more T2DM interventions within the same time frame compared with the control patients. Although not causative, the increased number of interventions in addition to the bolstered support of the SMA may have correlated with glycemic improvements.
An important finding of this study was the SMA show rate and how it compared with attendance rates found in other group models. The favorable ABJ SMA show rate could have been due to the rigorous attention paid to reminder letters and phone calls. The literature has not established a standard approach to increasing SMA show rates; however, the current data suggest that increased reminders may have increased attendance.
Limitations
This study had several limitations. The external validity was weakened by the modest sample size and the homogenous baseline characteristics of those enrolled. Another limitation was inconsistent documentation of laboratory parameters. The inability to obtain A1c values exactly at enrollment and discharge could have potentially skewed the results. In addition, incomplete documentation of interventions for dual-care patients (ie, those who obtained care outside of the VA) was an unavoidable challenge. Last, this study did not perform an assessment of SMA patient satisfaction, cost-benefit, or safety.
Conclusion
The ABJ SMA was an effective addition to standards of care in order to achieve improvements in glycemic control in a veteran population with poorly controlled T2DM. Furthermore,the data suggest that a successful program should be multidisciplinary, select poorly controlled patients, offer focused sessions, have a CPS participate in medication management, and encourage patients to complete ≥ 6 sessions. Future studies should be conducted to include more diverse patients to see whether the efficacy of this SMA format is maintained.
A safety analysis should also be conducted to ensure that the SMA format is not only effective, but also a safe means to manage medical conditions. In addition, the scope of the ABJ SMAs should be expanded to allow for evaluation of other diseases. An evaluation of patient satisfaction and cost-benefit could provide additional support for the implementation of SMAs, as improvements in quality of life and cost savings are endpoints to be desired.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimate of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
3. Kirsh SR, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16(5):349-353.
4. Jaber R, Braksmajer A, Trilling JS. Group visits: a qualitative review of current research. J Am Board Fam Med. 2006;19(3):276-290.
5. Klein S. The Veterans Health Administration: implementing patient-centered medical homes in the nation's largest integrated delivery system. Commonwealth Fund Website. http://www.common wealthfund.org/publications/case-studies/2011 /sep/va-medical-homes. Published September 2011. Accessed November 11, 2015.
6. U.S. Department of Veterans Affairs. VA Shared Medical Appointments for Patients With Diabetes: Maximizing Patient & Provider Expertise to Strengthen Patient Management. Washington, DC: U.S. Department of Veterans Affairs; 2008.
7. Bronson DL, Maxwell RA. Shared medical appointments: increasing patient access without increasing physician hours. Cleve Clin J Med. 2004;71(5):369-370, 372, 374 passim.
8. Cohen LB, Taveira T, Khatana SA, Dooley AG, Pirraglia PA, Wu WC. Pharmacist-led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes. Diabetes Educ. 2011;37(6):801-812.
9. Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med. 2011;171(5):453-459.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimate of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
3. Kirsh SR, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16(5):349-353.
4. Jaber R, Braksmajer A, Trilling JS. Group visits: a qualitative review of current research. J Am Board Fam Med. 2006;19(3):276-290.
5. Klein S. The Veterans Health Administration: implementing patient-centered medical homes in the nation's largest integrated delivery system. Commonwealth Fund Website. http://www.common wealthfund.org/publications/case-studies/2011 /sep/va-medical-homes. Published September 2011. Accessed November 11, 2015.
6. U.S. Department of Veterans Affairs. VA Shared Medical Appointments for Patients With Diabetes: Maximizing Patient & Provider Expertise to Strengthen Patient Management. Washington, DC: U.S. Department of Veterans Affairs; 2008.
7. Bronson DL, Maxwell RA. Shared medical appointments: increasing patient access without increasing physician hours. Cleve Clin J Med. 2004;71(5):369-370, 372, 374 passim.
8. Cohen LB, Taveira T, Khatana SA, Dooley AG, Pirraglia PA, Wu WC. Pharmacist-led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes. Diabetes Educ. 2011;37(6):801-812.
9. Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med. 2011;171(5):453-459.
Stroke Risk
ALCOHOL CONSUMPTION AND STROKE RISK IN MIDLIFE
Jones SB, Loehr L, Avery CL, et al. Midlife alcohol consumption and the risk of stroke in the Atherosclerosis Risk in Communities study. Stroke. 2015;46(11):3124-3130.
Light-to-moderate alcohol consumption at midlife was not associated with reduced stroke risk compared with abstention over 20 years of follow-up in a study of 12,433 never and current drinkers ages 45 to 65. An increased risk for both stroke and intracerebral hemorrhage (ICH) was observed with heavier consumption, as well as moderate intake for ICH. There were 773 ischemic strokes and 81 ICH incidents over follow-up. Study details included
• For ICH, light and moderate alcohol consumption was not associated with incidence (hazard ratios [HRs], 0.98, 1.06, 0.84).
• Heavier drinking was associated with a 31% increased rate relative to abstention (HR, 1.31).
• For ICH, moderate to heavy (HR, 1.99), but not light, consumption increased incidence.
Continue for spicy foods and mortality >>
SPICY FOODS AND MORTALITY
Lv J, Qi L, Yu C, et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study. BMJ. 2015;351:h3942.
Consumption of spicy foods was inversely associated with total and certain cause-specific mortality, independent of other risk factors for death (eg, cancer, heart disease, and stroke), according to a population-based cohort study of 199,293 men and 288,082 women ages 30 to 79 in China. Specifically:
• Spicy food consumption showed highly consistent inverse associations with total mortality among both men and women.
• Compared with those who ate spicy foods less than once per week, adjusted hazard ratios for death were 0.90, 0.86, and 0.86 for those who ate spicy food on one or two; three to five; or six or seven days per week.
• A 14% relative risk reduction in total mortality was shown among those who consumed spicy foods on six or seven days per week.
COMMENTARY
This is the type of study that I look forward to and use as an argument to defend my eating habits. Previous studies have shown that chocolate consumption decreases blood pressure and mortality.1-3 Pistachio consumption has been associated with beneficial effects on glucose metabolism and insulin resistance.4 This study makes me think of suggesting to a local restaurant a new health food, based on the evidence, that I would suggest they call the “Skolnik Meal”: a spicy, chocolate pistachio taco.
1. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide. JAMA. 2007;298(1):49-60.
2. Taubert D, Roesen R, Schomig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167(7):626-634.
3. Buijsse B, Feskens EJM, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med. 2006;166:411-417.
4. Hernandez-Alonso P, Salas-Salvado J, Baldrich-Mora M, et al. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care. 2014;37:1–8.
Continue for FDA warns of heart attack and stroke risk of NSAIDs >>
FDA WARNS OF HEART ATTACK AND STROKE RISK OF NSAIDs
FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. www.fda.gov/Drugs/DrugSafety/ucm451800.htm. Accessed November 19, 2015.
The FDA has added heart attack and stroke warnings to OTC nonaspirin NSAIDs. The warning is similar to that added to prescription NSAIDs in 2005.
People who have cardiovascular disease, particularly those who recently had a heart attack or cardiac bypass surgery, are at the greatest risk for cardiovascular adverse events associated with NSAIDs, according to the FDA announcement.
While people who have already had a heart attack are most vulnerable for having another or for dying of heart attack–related causes if treated with NSAIDs, everyone may be at risk—even those without an underlying risk for CVD.
COMMENTARY
This new statement was based on a comprehensive review of observational studies and clinical trials published since the FDA boxed warning appeared in 2005. The primary changes reflect that the risk for heart attack or stroke can occur as early as the first few weeks of using an NSAID, and the risk increases with longer use and higher doses. The estimates of the level of increased risk range from 10% to more than 50%, depending on the medication and the doses studied. The relative increase in risk is similar for patients with and without heart disease, but of course the likelihood of an adverse event is higher in patients with existent heart disease because of the increased prevalence of cardiovascular outcomes. NSAIDs also increase the risk for congestive heart failure.
Continue for childhood arterial ischemic stroke triggers >>
CHILDHOOD ARTERIAL ISCHEMIC STROKE TRIGGERS
Fullerton HJ, Hills NK, Elkind MS, et al. Infection, vaccination, and childhood arterial ischemic stroke: Results of the VIPS study. Neurology. 2015; 85(17):1459-1466.
Infection may act as a trigger for childhood arterial ischemic stroke (AIS), while routine vaccinations appear protective, according to a study of 355 patients (ages 29 days to 18 years) with AIS and 354 controls. Researchers found:
• Infection in the week prior to stroke was reported in 18% of cases versus 3% of controls, conferring a 6.3-fold increased risk for AIS.
• Upper respiratory infections were the most common.
• Prevalence of preceding infection was similar across arteriopathic, cardioembolic, and idiopathic stroke types.
• Use of vasoactive cold medications was similarly low in both groups.
• Children with some/few/no vaccinations were at higher stroke risk than those receiving all or most immunizations.
• In an age-adjusted model, independent risk factors for AIS included infection in the prior week, undervaccination, black race (compared to white), and rural residence.
Continue for comparing stroke risk scores for patients with AF >>
COMPARING STROKE RISK SCORES FOR PATIENTS WITH AF
van den Ham HA, Klungel OH, Singer DE, et al. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol. 2015;66(17):1851-1859.
The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score more accurately identified patients with atrial fibrillation (AF) who were at low risk for stroke than did the CHA2DS2-VASc score, which assigned the same patients to higher-risk categories, in a study of 60,594 patients with AF. Researchers found:
• Event rates for moderate- and high-risk categories for CHA2DS2-VASc were lower than those of the ATRIA and CHADS2.
• Age and previous stroke most strongly predicted ischemic stroke.
• C statistics for the full point scores were 0.70 for the ATRIA risk score, 0.68 for CHADS2, and 0.68 for CHA2DS2-VASc risk score.
• Net reclassification improvement was 0.23 for the ATRIA compared with CHA2DS2-VASc.
• Reclassifying patients with very low stroke risk and AF could prevent overuse of anticoagulants.
Continue for risk of thromboembolism after ICH: Is resumption of warfarin therapy safe? >>
RISK OF THROMBOEMBOLISM AFTER ICH: IS RESUMPTION OF WARFARIN THERAPY SAFE?
Witt DM, Clark NP, Martinez K, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for intracranial hemorrhage. Thromb Res. 2015;136(5):1040-1044.
Patients resuming warfarin therapy following warfarin-associated intracranial hemorrhage (ICH) appeared not to be at increased risk for recurrent ICH but instead tended toward reduced thrombosis and all-cause mortality in a cohort study of 160 individuals discharged from the hospital following warfarin-related index of ICH. In the study, 33.8% of patients resumed warfarin therapy and 66.2% did not. Researchers found:
• Recurrent ICH occurred in a numerically greater, but statistically nonsignificant, proportion of patients who did not resume warfarin therapy (7.6% vs 3.7%).
• Patients who did not resume warfarin had three-fold higher (12.3% vs 3.7%) and approximately two-fold higher (31.3% vs. 18.5%) rates of thrombosis and all-cause mortality during follow-up.
Continue for lipid lowering drugs and stroke risk >>
LIPID LOWERING DRUGS AND STROKE RISK
Alpérovitch A, Kurth T, Bertrand M, et al. Primary prevention with lipid lowering drugs and long term risk of vascular events in older people: population based cohort study. BMJ. 2015;350:h2335.
Statins and fibrates may reduce stroke risk by 30% in older adults with no history of vascular events, according to a population-based cohort study of 7,484 patients.
In a random sample of community-dwelling adults ages 65 and older, investigators calculated hazard ratios for use of any lipid-lowering drug, and for statin and fibrates separately, and found:
• Users of lipid-lowering drugs were at decreased risk for stroke compared with nonusers (hazard ratio [HR], 0.66).
• Statin users were at similarly decreased risk (HR, 0.68).
• Fibrate users were also at decreased risk (HR, 0.66).
• There was no association between lipid-lowering drug use and coronary heart disease (HR, 1.12).
COMMENTARY
This study supports the use of LDL cholesterol–lowering medication as primary prevention in older individuals to reduce the risk for stroke. The mean age in this study was 74. Previously, the PROSPER study—the only randomized study of older individuals with high vascular risk—showed that pravastatin reduced the risk for coronary disease but not for stroke.1 This study gives support that lowering cholesterol in older patients with elevated cholesterol can decrease their risk for stroke.
1. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk for vascular disease (PROSPER). Lancet. 2002;360;1623-1630.
Continue for metabolic syndrome and diabetes in stroke recurrence >>
METABOLIC SYNDROME AND DIABETES IN STROKE RECURRENCE
Zhu S, McClure LA, Lau H, et al. Recurrent vascular events in lacunar stroke patients with metabolic syndrome and/or diabetes. Neurology. 2015 Aug 21. [Epub ahead of print]
Metabolic syndrome (METS) and diabetes mellitus (DM) were significant comorbid conditions in lacunar stroke patients, and they were associated with stroke recurrence, according to a study of 3,020 patients with lacunar strokes. Researchers found:
• 25% of patients had METS only, 6% had DM only, 32% had both conditions, and 37% had neither.
• Over a median 3.8 years of follow-up, there were 274 recurrent strokes and 74 myocardial infarctions (MIs).
• Among 240 recurrent ischemic strokes, 56% were lacunar.
• The hazard ratios for any recurrent stroke (HR, 1.7) or lacunar stroke (HR, 2.4) were significantly higher for those with concurrent METS and DM than for those who had neither.
• Risk for incident MI was higher in patients with DM (HR, 2.8) or concurrent DM and METS (HR, 2.6).
ALCOHOL CONSUMPTION AND STROKE RISK IN MIDLIFE
Jones SB, Loehr L, Avery CL, et al. Midlife alcohol consumption and the risk of stroke in the Atherosclerosis Risk in Communities study. Stroke. 2015;46(11):3124-3130.
Light-to-moderate alcohol consumption at midlife was not associated with reduced stroke risk compared with abstention over 20 years of follow-up in a study of 12,433 never and current drinkers ages 45 to 65. An increased risk for both stroke and intracerebral hemorrhage (ICH) was observed with heavier consumption, as well as moderate intake for ICH. There were 773 ischemic strokes and 81 ICH incidents over follow-up. Study details included
• For ICH, light and moderate alcohol consumption was not associated with incidence (hazard ratios [HRs], 0.98, 1.06, 0.84).
• Heavier drinking was associated with a 31% increased rate relative to abstention (HR, 1.31).
• For ICH, moderate to heavy (HR, 1.99), but not light, consumption increased incidence.
Continue for spicy foods and mortality >>
SPICY FOODS AND MORTALITY
Lv J, Qi L, Yu C, et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study. BMJ. 2015;351:h3942.
Consumption of spicy foods was inversely associated with total and certain cause-specific mortality, independent of other risk factors for death (eg, cancer, heart disease, and stroke), according to a population-based cohort study of 199,293 men and 288,082 women ages 30 to 79 in China. Specifically:
• Spicy food consumption showed highly consistent inverse associations with total mortality among both men and women.
• Compared with those who ate spicy foods less than once per week, adjusted hazard ratios for death were 0.90, 0.86, and 0.86 for those who ate spicy food on one or two; three to five; or six or seven days per week.
• A 14% relative risk reduction in total mortality was shown among those who consumed spicy foods on six or seven days per week.
COMMENTARY
This is the type of study that I look forward to and use as an argument to defend my eating habits. Previous studies have shown that chocolate consumption decreases blood pressure and mortality.1-3 Pistachio consumption has been associated with beneficial effects on glucose metabolism and insulin resistance.4 This study makes me think of suggesting to a local restaurant a new health food, based on the evidence, that I would suggest they call the “Skolnik Meal”: a spicy, chocolate pistachio taco.
1. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide. JAMA. 2007;298(1):49-60.
2. Taubert D, Roesen R, Schomig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167(7):626-634.
3. Buijsse B, Feskens EJM, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med. 2006;166:411-417.
4. Hernandez-Alonso P, Salas-Salvado J, Baldrich-Mora M, et al. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care. 2014;37:1–8.
Continue for FDA warns of heart attack and stroke risk of NSAIDs >>
FDA WARNS OF HEART ATTACK AND STROKE RISK OF NSAIDs
FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. www.fda.gov/Drugs/DrugSafety/ucm451800.htm. Accessed November 19, 2015.
The FDA has added heart attack and stroke warnings to OTC nonaspirin NSAIDs. The warning is similar to that added to prescription NSAIDs in 2005.
People who have cardiovascular disease, particularly those who recently had a heart attack or cardiac bypass surgery, are at the greatest risk for cardiovascular adverse events associated with NSAIDs, according to the FDA announcement.
While people who have already had a heart attack are most vulnerable for having another or for dying of heart attack–related causes if treated with NSAIDs, everyone may be at risk—even those without an underlying risk for CVD.
COMMENTARY
This new statement was based on a comprehensive review of observational studies and clinical trials published since the FDA boxed warning appeared in 2005. The primary changes reflect that the risk for heart attack or stroke can occur as early as the first few weeks of using an NSAID, and the risk increases with longer use and higher doses. The estimates of the level of increased risk range from 10% to more than 50%, depending on the medication and the doses studied. The relative increase in risk is similar for patients with and without heart disease, but of course the likelihood of an adverse event is higher in patients with existent heart disease because of the increased prevalence of cardiovascular outcomes. NSAIDs also increase the risk for congestive heart failure.
Continue for childhood arterial ischemic stroke triggers >>
CHILDHOOD ARTERIAL ISCHEMIC STROKE TRIGGERS
Fullerton HJ, Hills NK, Elkind MS, et al. Infection, vaccination, and childhood arterial ischemic stroke: Results of the VIPS study. Neurology. 2015; 85(17):1459-1466.
Infection may act as a trigger for childhood arterial ischemic stroke (AIS), while routine vaccinations appear protective, according to a study of 355 patients (ages 29 days to 18 years) with AIS and 354 controls. Researchers found:
• Infection in the week prior to stroke was reported in 18% of cases versus 3% of controls, conferring a 6.3-fold increased risk for AIS.
• Upper respiratory infections were the most common.
• Prevalence of preceding infection was similar across arteriopathic, cardioembolic, and idiopathic stroke types.
• Use of vasoactive cold medications was similarly low in both groups.
• Children with some/few/no vaccinations were at higher stroke risk than those receiving all or most immunizations.
• In an age-adjusted model, independent risk factors for AIS included infection in the prior week, undervaccination, black race (compared to white), and rural residence.
Continue for comparing stroke risk scores for patients with AF >>
COMPARING STROKE RISK SCORES FOR PATIENTS WITH AF
van den Ham HA, Klungel OH, Singer DE, et al. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol. 2015;66(17):1851-1859.
The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score more accurately identified patients with atrial fibrillation (AF) who were at low risk for stroke than did the CHA2DS2-VASc score, which assigned the same patients to higher-risk categories, in a study of 60,594 patients with AF. Researchers found:
• Event rates for moderate- and high-risk categories for CHA2DS2-VASc were lower than those of the ATRIA and CHADS2.
• Age and previous stroke most strongly predicted ischemic stroke.
• C statistics for the full point scores were 0.70 for the ATRIA risk score, 0.68 for CHADS2, and 0.68 for CHA2DS2-VASc risk score.
• Net reclassification improvement was 0.23 for the ATRIA compared with CHA2DS2-VASc.
• Reclassifying patients with very low stroke risk and AF could prevent overuse of anticoagulants.
Continue for risk of thromboembolism after ICH: Is resumption of warfarin therapy safe? >>
RISK OF THROMBOEMBOLISM AFTER ICH: IS RESUMPTION OF WARFARIN THERAPY SAFE?
Witt DM, Clark NP, Martinez K, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for intracranial hemorrhage. Thromb Res. 2015;136(5):1040-1044.
Patients resuming warfarin therapy following warfarin-associated intracranial hemorrhage (ICH) appeared not to be at increased risk for recurrent ICH but instead tended toward reduced thrombosis and all-cause mortality in a cohort study of 160 individuals discharged from the hospital following warfarin-related index of ICH. In the study, 33.8% of patients resumed warfarin therapy and 66.2% did not. Researchers found:
• Recurrent ICH occurred in a numerically greater, but statistically nonsignificant, proportion of patients who did not resume warfarin therapy (7.6% vs 3.7%).
• Patients who did not resume warfarin had three-fold higher (12.3% vs 3.7%) and approximately two-fold higher (31.3% vs. 18.5%) rates of thrombosis and all-cause mortality during follow-up.
Continue for lipid lowering drugs and stroke risk >>
LIPID LOWERING DRUGS AND STROKE RISK
Alpérovitch A, Kurth T, Bertrand M, et al. Primary prevention with lipid lowering drugs and long term risk of vascular events in older people: population based cohort study. BMJ. 2015;350:h2335.
Statins and fibrates may reduce stroke risk by 30% in older adults with no history of vascular events, according to a population-based cohort study of 7,484 patients.
In a random sample of community-dwelling adults ages 65 and older, investigators calculated hazard ratios for use of any lipid-lowering drug, and for statin and fibrates separately, and found:
• Users of lipid-lowering drugs were at decreased risk for stroke compared with nonusers (hazard ratio [HR], 0.66).
• Statin users were at similarly decreased risk (HR, 0.68).
• Fibrate users were also at decreased risk (HR, 0.66).
• There was no association between lipid-lowering drug use and coronary heart disease (HR, 1.12).
COMMENTARY
This study supports the use of LDL cholesterol–lowering medication as primary prevention in older individuals to reduce the risk for stroke. The mean age in this study was 74. Previously, the PROSPER study—the only randomized study of older individuals with high vascular risk—showed that pravastatin reduced the risk for coronary disease but not for stroke.1 This study gives support that lowering cholesterol in older patients with elevated cholesterol can decrease their risk for stroke.
1. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk for vascular disease (PROSPER). Lancet. 2002;360;1623-1630.
Continue for metabolic syndrome and diabetes in stroke recurrence >>
METABOLIC SYNDROME AND DIABETES IN STROKE RECURRENCE
Zhu S, McClure LA, Lau H, et al. Recurrent vascular events in lacunar stroke patients with metabolic syndrome and/or diabetes. Neurology. 2015 Aug 21. [Epub ahead of print]
Metabolic syndrome (METS) and diabetes mellitus (DM) were significant comorbid conditions in lacunar stroke patients, and they were associated with stroke recurrence, according to a study of 3,020 patients with lacunar strokes. Researchers found:
• 25% of patients had METS only, 6% had DM only, 32% had both conditions, and 37% had neither.
• Over a median 3.8 years of follow-up, there were 274 recurrent strokes and 74 myocardial infarctions (MIs).
• Among 240 recurrent ischemic strokes, 56% were lacunar.
• The hazard ratios for any recurrent stroke (HR, 1.7) or lacunar stroke (HR, 2.4) were significantly higher for those with concurrent METS and DM than for those who had neither.
• Risk for incident MI was higher in patients with DM (HR, 2.8) or concurrent DM and METS (HR, 2.6).
ALCOHOL CONSUMPTION AND STROKE RISK IN MIDLIFE
Jones SB, Loehr L, Avery CL, et al. Midlife alcohol consumption and the risk of stroke in the Atherosclerosis Risk in Communities study. Stroke. 2015;46(11):3124-3130.
Light-to-moderate alcohol consumption at midlife was not associated with reduced stroke risk compared with abstention over 20 years of follow-up in a study of 12,433 never and current drinkers ages 45 to 65. An increased risk for both stroke and intracerebral hemorrhage (ICH) was observed with heavier consumption, as well as moderate intake for ICH. There were 773 ischemic strokes and 81 ICH incidents over follow-up. Study details included
• For ICH, light and moderate alcohol consumption was not associated with incidence (hazard ratios [HRs], 0.98, 1.06, 0.84).
• Heavier drinking was associated with a 31% increased rate relative to abstention (HR, 1.31).
• For ICH, moderate to heavy (HR, 1.99), but not light, consumption increased incidence.
Continue for spicy foods and mortality >>
SPICY FOODS AND MORTALITY
Lv J, Qi L, Yu C, et al. Consumption of spicy foods and total and cause specific mortality: population based cohort study. BMJ. 2015;351:h3942.
Consumption of spicy foods was inversely associated with total and certain cause-specific mortality, independent of other risk factors for death (eg, cancer, heart disease, and stroke), according to a population-based cohort study of 199,293 men and 288,082 women ages 30 to 79 in China. Specifically:
• Spicy food consumption showed highly consistent inverse associations with total mortality among both men and women.
• Compared with those who ate spicy foods less than once per week, adjusted hazard ratios for death were 0.90, 0.86, and 0.86 for those who ate spicy food on one or two; three to five; or six or seven days per week.
• A 14% relative risk reduction in total mortality was shown among those who consumed spicy foods on six or seven days per week.
COMMENTARY
This is the type of study that I look forward to and use as an argument to defend my eating habits. Previous studies have shown that chocolate consumption decreases blood pressure and mortality.1-3 Pistachio consumption has been associated with beneficial effects on glucose metabolism and insulin resistance.4 This study makes me think of suggesting to a local restaurant a new health food, based on the evidence, that I would suggest they call the “Skolnik Meal”: a spicy, chocolate pistachio taco.
1. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide. JAMA. 2007;298(1):49-60.
2. Taubert D, Roesen R, Schomig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167(7):626-634.
3. Buijsse B, Feskens EJM, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med. 2006;166:411-417.
4. Hernandez-Alonso P, Salas-Salvado J, Baldrich-Mora M, et al. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care. 2014;37:1–8.
Continue for FDA warns of heart attack and stroke risk of NSAIDs >>
FDA WARNS OF HEART ATTACK AND STROKE RISK OF NSAIDs
FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. www.fda.gov/Drugs/DrugSafety/ucm451800.htm. Accessed November 19, 2015.
The FDA has added heart attack and stroke warnings to OTC nonaspirin NSAIDs. The warning is similar to that added to prescription NSAIDs in 2005.
People who have cardiovascular disease, particularly those who recently had a heart attack or cardiac bypass surgery, are at the greatest risk for cardiovascular adverse events associated with NSAIDs, according to the FDA announcement.
While people who have already had a heart attack are most vulnerable for having another or for dying of heart attack–related causes if treated with NSAIDs, everyone may be at risk—even those without an underlying risk for CVD.
COMMENTARY
This new statement was based on a comprehensive review of observational studies and clinical trials published since the FDA boxed warning appeared in 2005. The primary changes reflect that the risk for heart attack or stroke can occur as early as the first few weeks of using an NSAID, and the risk increases with longer use and higher doses. The estimates of the level of increased risk range from 10% to more than 50%, depending on the medication and the doses studied. The relative increase in risk is similar for patients with and without heart disease, but of course the likelihood of an adverse event is higher in patients with existent heart disease because of the increased prevalence of cardiovascular outcomes. NSAIDs also increase the risk for congestive heart failure.
Continue for childhood arterial ischemic stroke triggers >>
CHILDHOOD ARTERIAL ISCHEMIC STROKE TRIGGERS
Fullerton HJ, Hills NK, Elkind MS, et al. Infection, vaccination, and childhood arterial ischemic stroke: Results of the VIPS study. Neurology. 2015; 85(17):1459-1466.
Infection may act as a trigger for childhood arterial ischemic stroke (AIS), while routine vaccinations appear protective, according to a study of 355 patients (ages 29 days to 18 years) with AIS and 354 controls. Researchers found:
• Infection in the week prior to stroke was reported in 18% of cases versus 3% of controls, conferring a 6.3-fold increased risk for AIS.
• Upper respiratory infections were the most common.
• Prevalence of preceding infection was similar across arteriopathic, cardioembolic, and idiopathic stroke types.
• Use of vasoactive cold medications was similarly low in both groups.
• Children with some/few/no vaccinations were at higher stroke risk than those receiving all or most immunizations.
• In an age-adjusted model, independent risk factors for AIS included infection in the prior week, undervaccination, black race (compared to white), and rural residence.
Continue for comparing stroke risk scores for patients with AF >>
COMPARING STROKE RISK SCORES FOR PATIENTS WITH AF
van den Ham HA, Klungel OH, Singer DE, et al. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol. 2015;66(17):1851-1859.
The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) risk score more accurately identified patients with atrial fibrillation (AF) who were at low risk for stroke than did the CHA2DS2-VASc score, which assigned the same patients to higher-risk categories, in a study of 60,594 patients with AF. Researchers found:
• Event rates for moderate- and high-risk categories for CHA2DS2-VASc were lower than those of the ATRIA and CHADS2.
• Age and previous stroke most strongly predicted ischemic stroke.
• C statistics for the full point scores were 0.70 for the ATRIA risk score, 0.68 for CHADS2, and 0.68 for CHA2DS2-VASc risk score.
• Net reclassification improvement was 0.23 for the ATRIA compared with CHA2DS2-VASc.
• Reclassifying patients with very low stroke risk and AF could prevent overuse of anticoagulants.
Continue for risk of thromboembolism after ICH: Is resumption of warfarin therapy safe? >>
RISK OF THROMBOEMBOLISM AFTER ICH: IS RESUMPTION OF WARFARIN THERAPY SAFE?
Witt DM, Clark NP, Martinez K, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for intracranial hemorrhage. Thromb Res. 2015;136(5):1040-1044.
Patients resuming warfarin therapy following warfarin-associated intracranial hemorrhage (ICH) appeared not to be at increased risk for recurrent ICH but instead tended toward reduced thrombosis and all-cause mortality in a cohort study of 160 individuals discharged from the hospital following warfarin-related index of ICH. In the study, 33.8% of patients resumed warfarin therapy and 66.2% did not. Researchers found:
• Recurrent ICH occurred in a numerically greater, but statistically nonsignificant, proportion of patients who did not resume warfarin therapy (7.6% vs 3.7%).
• Patients who did not resume warfarin had three-fold higher (12.3% vs 3.7%) and approximately two-fold higher (31.3% vs. 18.5%) rates of thrombosis and all-cause mortality during follow-up.
Continue for lipid lowering drugs and stroke risk >>
LIPID LOWERING DRUGS AND STROKE RISK
Alpérovitch A, Kurth T, Bertrand M, et al. Primary prevention with lipid lowering drugs and long term risk of vascular events in older people: population based cohort study. BMJ. 2015;350:h2335.
Statins and fibrates may reduce stroke risk by 30% in older adults with no history of vascular events, according to a population-based cohort study of 7,484 patients.
In a random sample of community-dwelling adults ages 65 and older, investigators calculated hazard ratios for use of any lipid-lowering drug, and for statin and fibrates separately, and found:
• Users of lipid-lowering drugs were at decreased risk for stroke compared with nonusers (hazard ratio [HR], 0.66).
• Statin users were at similarly decreased risk (HR, 0.68).
• Fibrate users were also at decreased risk (HR, 0.66).
• There was no association between lipid-lowering drug use and coronary heart disease (HR, 1.12).
COMMENTARY
This study supports the use of LDL cholesterol–lowering medication as primary prevention in older individuals to reduce the risk for stroke. The mean age in this study was 74. Previously, the PROSPER study—the only randomized study of older individuals with high vascular risk—showed that pravastatin reduced the risk for coronary disease but not for stroke.1 This study gives support that lowering cholesterol in older patients with elevated cholesterol can decrease their risk for stroke.
1. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk for vascular disease (PROSPER). Lancet. 2002;360;1623-1630.
Continue for metabolic syndrome and diabetes in stroke recurrence >>
METABOLIC SYNDROME AND DIABETES IN STROKE RECURRENCE
Zhu S, McClure LA, Lau H, et al. Recurrent vascular events in lacunar stroke patients with metabolic syndrome and/or diabetes. Neurology. 2015 Aug 21. [Epub ahead of print]
Metabolic syndrome (METS) and diabetes mellitus (DM) were significant comorbid conditions in lacunar stroke patients, and they were associated with stroke recurrence, according to a study of 3,020 patients with lacunar strokes. Researchers found:
• 25% of patients had METS only, 6% had DM only, 32% had both conditions, and 37% had neither.
• Over a median 3.8 years of follow-up, there were 274 recurrent strokes and 74 myocardial infarctions (MIs).
• Among 240 recurrent ischemic strokes, 56% were lacunar.
• The hazard ratios for any recurrent stroke (HR, 1.7) or lacunar stroke (HR, 2.4) were significantly higher for those with concurrent METS and DM than for those who had neither.
• Risk for incident MI was higher in patients with DM (HR, 2.8) or concurrent DM and METS (HR, 2.6).
Gut Grief: The Truth About Gluten Sensitivity
IN THIS ARTICLE
• So what is gluten?
• Selected symptoms of celiac disease
• Selected foods and products containing gluten
Gluten has become a dietary pariah (see “So What Is Gluten?"). The American public’s enthusiasm for a gluten-free diet has spurred a gluten-free food industry that has grown, on average, 34% per year since 2009, with annual sales predicted to reach an impressive $15.5 billion by 2016.1 This trend coincides with the national media’s intense focus on gluten sensitivity (GS), as well as best-selling books such as Wheat Belly and Grain Brain.2,3
Gluten-free food products, once relegated to boutique food shops and limited shelf space, now fill sections in large grocery and drugstore chains. Many restaurants have added gluten-free items to their menus (although gluten-free Big Macs have been available in Finland for more than 20 years).4 Only celiac disease (CD), which affects approximately 1% of the American population, requires strict gluten avoidance; yet more than 30% of US adults report having reduced their gluten intake, most claiming they did so to promote a “healthier” diet or support weight loss.1
PREVALENCE AND PATHOLOGY OF GS DISORDERS
Gluten sensitivity, once used to denote CD alone, now includes a group of gluten-intolerant conditions unrelated to CD—primarily nonceliac gluten sensitivity (NCGS) and wheat allergy (WA)—although the nomenclature is likely to change. While these disorders differ in underlying pathogenesis, each demonstrates a resolution of symptoms when the patient is placed on a gluten-free diet. Of these GS disorders, only NCGS lacks clarity with regard to incidence, diagnosis, and pathology.5
Celiac Disease
Celiac disease is an autoimmune, T-cell–activated disease that manifests in genetically susceptible individuals (with gene variants HLA-DQ2 and HLA-DQ8); it can occur at any age. The incidence of CD in the US has increased from 1 in 500 in 1974 to a current estimate of 1 in 100, although many with CD are believed to be undiagnosed.4,6
CD is the only autoimmune disease for which a trigger is known: gluten. Suspicion for CD should be heightened if the patient or a family member has a history of autoimmune disease. Nearly one-quarter of patients with CD will develop an autoimmune thyroid disorder.7
In CD, a significant enteropathy occurs in response to gluten intake, characterized by inflammation of the proximal small intestine. Individuals with CD produce tissue transglutaminase (tTG) or transglutaminase 2 (TG2) autoantibodies, resulting in gluten-specific CD4+ Th1 T-cell activation and an immune response that causes an upregulation of zonulin.8 Zonulin, a protein that modulates the permeability of the intestinal mucosal wall, is believed to play a role in “leaky gut syndrome” and autoimmune disease. The upregulation of zonulin in CD creates a disruption of the intestinal mucosal lining, causing villous mucosal atrophy and impairment of intestinal permeability and absorption.9
Nonceliac Gluten Sensitivity
NCGS is a poorly understood condition first described in the 1980s and recently “rediscovered” as a gluten-related disorder.10 Its actual prevalence is unknown because of unclear diagnostic criteria but is likely much higher than that of CD.1,4 Unlike CD, there does not appear to be a genetic predisposition for NCGS, nor is it believed to be an autoimmune disorder. However, research does suggest that NCGS may increase the risk for autoimmune diseases, such as Hashimoto thyroiditis. NCGS can occur at any age but appears more commonly in adults than children, and in women than men.4
A small but meticulous 2013 study raised doubt about NCGS as a specific gluten-related disorder.11 The results suggested that NCGS should be viewed as a variant of irritable bowel syndrome (IBS), not triggered by gluten but by poorly absorbed carbohydrates found in wheat known as fructans and galactans, and perhaps by other foods containing fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPS). It is believed those with diarrhea-prone IBS are particularly sensitive to gluten.11 As a result, ardent claims of NCGS and improved health with gluten-free diets in those without CD are often discounted.
More recent research findings refute this conjecture, suggesting that NCGS is likely a reaction to other proteins within the gluten family, such as beta-, gamma-, or omega-gliadin, glutenin, wheat germ agglutinin, gluteomorphin, and deamidated gliadin. Development of GS is believed to be triggered by such factors as intestinal infections, altered microbiota, or food additives.4
In any event, the pathogenesis of NCGS remains unclear, and it does not present with the diagnostic antibodies or inflammatory enteropathy seen in patients with CD. Despite this, NCGS does present with gastrointestinal (GI) and extra-intestinal symptoms similar to those of CD.
Wheat Allergies
Wheat is frequently implicated in food allergies, especially in infants and children. The incidence of WA is not known, although up to 4% of adults and 6% of children are estimated to have food allergies. In WA, there is an IgE antibody–mediated reaction to one or more of the wheat proteins (albumin, gliadin, globulin, gluten) that occurs within minutes to hours after exposure to the offending food. Many children with IgE-mediated allergies may “outgrow” them with time.12
Continue for the clinical presentation of GS >>
CLINICAL PRESENTATION OF GS
Although CD is a disorder associated with the GI system, the “classic” GI symptoms of bloating, flatulence, diarrhea, and/or constipation are often absent (silent CD), especially in older individuals. It is for this reason that the diagnosis of CD is easily missed.
Delaying diagnosis can have serious health consequences, as CD is associated with significant morbidities, such as malnutrition (worse in children), iron-deficiency anemia, neuropsychiatric aberrations (depression, anxiety, attention-deficit, and cerebral ataxia), osteoporosis, lymphoma, and death (see Table 1).4,13 CD may also present with dermatitis herpetiformis, a chronic vesicular rash, seen most often in adult males.
The role of gluten in the development of autism spectrum disorders or schizophrenia, though not proven, remains hotly debated, especially as close biochemical links are now recognized between the gut and the brain. It is clear, however, that gluten intake in severely gluten-sensitive individuals can directly affect mood and brain function. Most CD-associated morbidities will resolve after one year of complete gluten avoidance.1,13
Prominent symptoms of NCGS occur soon after gluten ingestion and disappear within days to weeks of gluten avoidance. The classic NCGS presentation combines IBS-like symptoms, such as abdominal cramps, bloating, diarrhea, and constipation, with systemic manifestations that include “brain fog,” fatigue, headache, joint and muscle pain, peripheral numbness, skin rash, aphthous stomatitis, anemia, and depression or anxiety. As with CD, GI symptoms usually predominate in children and abate with gluten avoidance.14,15
Allergic reactions to wheat will present within minutes to two hours of wheat exposure and may manifest with pruritic rash, hives, swelling of the lips or tongue, rhinitis, abdominal cramps, vomiting, diarrhea, constipation, and/or anaphylaxis. Subtle reactions may make diagnosis difficult.12
DIAGNOSTIC STUDIES FOR GS
The effectiveness of diagnostic testing for CD has been well established. Testing for antitissue transglutaminase antibodies (tTG-IgA) is the preferred laboratory test for CD, with a sensitivity of 93%, specificity of 98%, and few false-negative results. The endomysial antibody (EMA-IgA) test, though highly specific for CD, lacks the sensitivity of tTG-IgA. Newer antibody tests, such as deamidated gliadin peptide IgA and IgG, have not proven superior in detecting CD. Genetic testing for HLA-DQ2 and HLA-DQ8 may also be performed, but many people carry the gene without ever developing CD.13
To improve the reliability of CD antibody tests, the patient should have consumed gluten regularly for at least one month prior to testing. If the patient has been on a gluten-free diet for several weeks, then a gluten challenge should be done: The patient would be instructed to consume at least 3 g/d of gluten (two slices of bread) for a minimum of two weeks (versus eight weeks in previous protocols), after which the celiac antibody tests would be repeated.16
If these antibody test results are negative but the suspicion for CD remains high, an endoscopy with a duodenal biopsy should be performed. The appearance of villous atrophy would confirm the diagnosis of CD.1,13,16
Unlike CD, there are currently no reliable diagnostic tests for NCGS, although some researchers suggest testing for IgG antigliadin antibodies (AGA); NCGS is currently a diagnosis of exclusion.7 In NCGS, celiac antibodies will be negative and the duodenal biopsy will demonstrate only mild inflammation without the mucosal atrophy of CD. As with CD, patients affected by NCGS will also test negative for the wheat allergy IgE response.
Another option is a gluten challenge. The patient is instructed to follow a gluten-free diet for six weeks and monitor for NCGS symptoms. If symptoms abate, a gluten-containing diet is then reintroduced and the patient is evaluated for the reemergence of NCGS symptoms. If symptoms are not reduced with a gluten-free diet, NCGS may be excluded. Newer GS laboratory tests will emerge that can assay more forms of gliadin antibodies, possibly aiding in NCGS diagnosis.4,14
To make a diagnosis of WA, skin prick tests and allergen-specific IgE testing are used, along with a medical history, clinical presentation, and possibly a food challenge.
Continue for management of GS >>
MANAGEMENT OF GS
The hallmark treatment for GS, regardless of its causative factor, is a strict gluten-free diet (GFD). For patients with CD, a 100% GFD is recommended for life. It is not yet known whether this lifelong duration is necessary for those with NCGS and WA, or if there is a safe threshold in these patients for gluten consumption. It is helpful for patients to keep a food diary, noting what they eat and how that affects the appearance or attenuation of symptoms.
Transitioning to a gluten-free lifestyle can be confusing, frustrating, and expensive for patients. Removing gluten from the diet is also challenging, as wheat is the predominant grain consumed in this country. Barley and rye (less so oats) also contain gluten, leaving limited alternatives, like amaranth, corn, quinoa, rice, and tapioca. Unlike CD and NCGS, WA requires only elimination of wheat-containing products; thus, it may not be necessary for affected patients to avoid barley and rye.1,4
Extensive patient education is important for success. Referral to a knowledgeable nutritionist is ideal but not always practical. Lists of foods to avoid on a gluten-free diet are readily available, but important points should be stressed, including how to read food labels. For example, the term wheat-free does not mean gluten-free (see Table 2).1,17 As of August 2014, the food industry, by law, can only claim a product is “gluten-free” if it contains no more than 20 parts per million (ppm) of gluten.1
Due to malabsorption issues, it is recommended that patients with CD be monitored for micronutrient deficiencies (ie, iron, B1, B6, B12, and zinc), and osteopenia/osteoporosis (dual-energy x-ray absorptiometry [DEXA] at the time of diagnosis) and be offered fertility counseling. What patients with GS need most of all are informed, caring providers to help guide them through diagnosis and treatment.6,13
Continue for the conclusion >>
CONCLUSION
Gluten-free diets are increasing in popularity, and many people who do not have CD claim improved health and vitality when they avoid gluten. Much is known about the incidence and pathogenesis of the gluten-associated disorders of CD and WA. Far less is known about the controversial disorder of NCGS. The symptoms and morbidities associated with NCGS have been well documented and present a curious mix of CD and IBS, yet neither condition fully accounts for the pathogenesis of NCGS. While CD is linked to more serious morbidities (including death if the disease is not readily diagnosed), NCGS and WA do produce significant manifestations and risks.
Research into NCGS remains limited and conflicting, and biomarkers for the disorder are not yet known. Unsupported or not, many patients attribute mood disorders, pain, and chronic ills to gluten intake and seek input from their health care providers. Rather than dismiss their claims, clinicians can provide pertinent instructions on a gluten-free lifestyle and healthy diet, and encourage the use of food diaries to document food-symptom associations. Gluten sensitivities are not benign and “going gluten-free” may be of great benefit for many patients with GS. That’s a fact.
REFERENCES
1. Capili B, Chang M, Anastasi JK. Nonceliac gluten sensitivity—is it really the gluten? J Nurs Pract. 2014;10(9):666-673.
2. Davis W. Wheat Belly: Lose the Wheat, Lose the Weight, and Find Your Path Back to Health. Emmaus, PA: Rodale Books; 2011.
3. Perlmutter D. Grain Brain: The Surprising Truth about Wheat, Carbs, and Sugar—Your Brain’s Silent Killers. New York, NY: Little, Brown and Company; 2013.
4. Brown AC. Gluten sensitivity: problems of an emerging condition separate from celiac disease. Expert Rev Gastroenterol Hepatol. 2012;6(1): 43-55.
5. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012 Feb 7;10:13.
6. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656-676.
7. Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Non-celiac gluten sensitivity: literature review. J Am Coll Nutr. 2014;33(1):39-54.
8. Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):728-736.
9. Fasano A, Sapone A, Zevallos V, Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148(6):1195-1204.
10. Catassi C, Bai JC, Bonaz B, Boouma G. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5(10):3839-3853.
11. Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320-328.
12. Guandalini S, Newland C. Differentiating food allergies from food intolerances. Curr Gastroenterol Rep. 2011;13(5):426-434.
13. Scanlon SA, Murray JA. Update on celiac disease—etiology, differential diagnosis, drug targets, and management devices. Clin Exp Gastroenterol. 2011;4:297-311.
14. Catassi C, Elli L, Bonaz B, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno Experts’ Criteria. Nutrients. 2015;7(6):4966-4977.
15. Peters SL, Biesiekierski JR, Yelland GW,et al. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity—an exploratory clinical study. Aliment Pharmacol Ther. 2014:39(10): 1104–1112.
16. Leffler D, Schuppen D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62(7):996-1004.
17. Celiac Disease Foundation. Sources of gluten (2015). https://celiac.org/live-gluten-free/glutenfreediet/sources-of-gluten. Accessed November 24, 2015.
IN THIS ARTICLE
• So what is gluten?
• Selected symptoms of celiac disease
• Selected foods and products containing gluten
Gluten has become a dietary pariah (see “So What Is Gluten?"). The American public’s enthusiasm for a gluten-free diet has spurred a gluten-free food industry that has grown, on average, 34% per year since 2009, with annual sales predicted to reach an impressive $15.5 billion by 2016.1 This trend coincides with the national media’s intense focus on gluten sensitivity (GS), as well as best-selling books such as Wheat Belly and Grain Brain.2,3
Gluten-free food products, once relegated to boutique food shops and limited shelf space, now fill sections in large grocery and drugstore chains. Many restaurants have added gluten-free items to their menus (although gluten-free Big Macs have been available in Finland for more than 20 years).4 Only celiac disease (CD), which affects approximately 1% of the American population, requires strict gluten avoidance; yet more than 30% of US adults report having reduced their gluten intake, most claiming they did so to promote a “healthier” diet or support weight loss.1
PREVALENCE AND PATHOLOGY OF GS DISORDERS
Gluten sensitivity, once used to denote CD alone, now includes a group of gluten-intolerant conditions unrelated to CD—primarily nonceliac gluten sensitivity (NCGS) and wheat allergy (WA)—although the nomenclature is likely to change. While these disorders differ in underlying pathogenesis, each demonstrates a resolution of symptoms when the patient is placed on a gluten-free diet. Of these GS disorders, only NCGS lacks clarity with regard to incidence, diagnosis, and pathology.5
Celiac Disease
Celiac disease is an autoimmune, T-cell–activated disease that manifests in genetically susceptible individuals (with gene variants HLA-DQ2 and HLA-DQ8); it can occur at any age. The incidence of CD in the US has increased from 1 in 500 in 1974 to a current estimate of 1 in 100, although many with CD are believed to be undiagnosed.4,6
CD is the only autoimmune disease for which a trigger is known: gluten. Suspicion for CD should be heightened if the patient or a family member has a history of autoimmune disease. Nearly one-quarter of patients with CD will develop an autoimmune thyroid disorder.7
In CD, a significant enteropathy occurs in response to gluten intake, characterized by inflammation of the proximal small intestine. Individuals with CD produce tissue transglutaminase (tTG) or transglutaminase 2 (TG2) autoantibodies, resulting in gluten-specific CD4+ Th1 T-cell activation and an immune response that causes an upregulation of zonulin.8 Zonulin, a protein that modulates the permeability of the intestinal mucosal wall, is believed to play a role in “leaky gut syndrome” and autoimmune disease. The upregulation of zonulin in CD creates a disruption of the intestinal mucosal lining, causing villous mucosal atrophy and impairment of intestinal permeability and absorption.9
Nonceliac Gluten Sensitivity
NCGS is a poorly understood condition first described in the 1980s and recently “rediscovered” as a gluten-related disorder.10 Its actual prevalence is unknown because of unclear diagnostic criteria but is likely much higher than that of CD.1,4 Unlike CD, there does not appear to be a genetic predisposition for NCGS, nor is it believed to be an autoimmune disorder. However, research does suggest that NCGS may increase the risk for autoimmune diseases, such as Hashimoto thyroiditis. NCGS can occur at any age but appears more commonly in adults than children, and in women than men.4
A small but meticulous 2013 study raised doubt about NCGS as a specific gluten-related disorder.11 The results suggested that NCGS should be viewed as a variant of irritable bowel syndrome (IBS), not triggered by gluten but by poorly absorbed carbohydrates found in wheat known as fructans and galactans, and perhaps by other foods containing fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPS). It is believed those with diarrhea-prone IBS are particularly sensitive to gluten.11 As a result, ardent claims of NCGS and improved health with gluten-free diets in those without CD are often discounted.
More recent research findings refute this conjecture, suggesting that NCGS is likely a reaction to other proteins within the gluten family, such as beta-, gamma-, or omega-gliadin, glutenin, wheat germ agglutinin, gluteomorphin, and deamidated gliadin. Development of GS is believed to be triggered by such factors as intestinal infections, altered microbiota, or food additives.4
In any event, the pathogenesis of NCGS remains unclear, and it does not present with the diagnostic antibodies or inflammatory enteropathy seen in patients with CD. Despite this, NCGS does present with gastrointestinal (GI) and extra-intestinal symptoms similar to those of CD.
Wheat Allergies
Wheat is frequently implicated in food allergies, especially in infants and children. The incidence of WA is not known, although up to 4% of adults and 6% of children are estimated to have food allergies. In WA, there is an IgE antibody–mediated reaction to one or more of the wheat proteins (albumin, gliadin, globulin, gluten) that occurs within minutes to hours after exposure to the offending food. Many children with IgE-mediated allergies may “outgrow” them with time.12
Continue for the clinical presentation of GS >>
CLINICAL PRESENTATION OF GS
Although CD is a disorder associated with the GI system, the “classic” GI symptoms of bloating, flatulence, diarrhea, and/or constipation are often absent (silent CD), especially in older individuals. It is for this reason that the diagnosis of CD is easily missed.
Delaying diagnosis can have serious health consequences, as CD is associated with significant morbidities, such as malnutrition (worse in children), iron-deficiency anemia, neuropsychiatric aberrations (depression, anxiety, attention-deficit, and cerebral ataxia), osteoporosis, lymphoma, and death (see Table 1).4,13 CD may also present with dermatitis herpetiformis, a chronic vesicular rash, seen most often in adult males.
The role of gluten in the development of autism spectrum disorders or schizophrenia, though not proven, remains hotly debated, especially as close biochemical links are now recognized between the gut and the brain. It is clear, however, that gluten intake in severely gluten-sensitive individuals can directly affect mood and brain function. Most CD-associated morbidities will resolve after one year of complete gluten avoidance.1,13
Prominent symptoms of NCGS occur soon after gluten ingestion and disappear within days to weeks of gluten avoidance. The classic NCGS presentation combines IBS-like symptoms, such as abdominal cramps, bloating, diarrhea, and constipation, with systemic manifestations that include “brain fog,” fatigue, headache, joint and muscle pain, peripheral numbness, skin rash, aphthous stomatitis, anemia, and depression or anxiety. As with CD, GI symptoms usually predominate in children and abate with gluten avoidance.14,15
Allergic reactions to wheat will present within minutes to two hours of wheat exposure and may manifest with pruritic rash, hives, swelling of the lips or tongue, rhinitis, abdominal cramps, vomiting, diarrhea, constipation, and/or anaphylaxis. Subtle reactions may make diagnosis difficult.12
DIAGNOSTIC STUDIES FOR GS
The effectiveness of diagnostic testing for CD has been well established. Testing for antitissue transglutaminase antibodies (tTG-IgA) is the preferred laboratory test for CD, with a sensitivity of 93%, specificity of 98%, and few false-negative results. The endomysial antibody (EMA-IgA) test, though highly specific for CD, lacks the sensitivity of tTG-IgA. Newer antibody tests, such as deamidated gliadin peptide IgA and IgG, have not proven superior in detecting CD. Genetic testing for HLA-DQ2 and HLA-DQ8 may also be performed, but many people carry the gene without ever developing CD.13
To improve the reliability of CD antibody tests, the patient should have consumed gluten regularly for at least one month prior to testing. If the patient has been on a gluten-free diet for several weeks, then a gluten challenge should be done: The patient would be instructed to consume at least 3 g/d of gluten (two slices of bread) for a minimum of two weeks (versus eight weeks in previous protocols), after which the celiac antibody tests would be repeated.16
If these antibody test results are negative but the suspicion for CD remains high, an endoscopy with a duodenal biopsy should be performed. The appearance of villous atrophy would confirm the diagnosis of CD.1,13,16
Unlike CD, there are currently no reliable diagnostic tests for NCGS, although some researchers suggest testing for IgG antigliadin antibodies (AGA); NCGS is currently a diagnosis of exclusion.7 In NCGS, celiac antibodies will be negative and the duodenal biopsy will demonstrate only mild inflammation without the mucosal atrophy of CD. As with CD, patients affected by NCGS will also test negative for the wheat allergy IgE response.
Another option is a gluten challenge. The patient is instructed to follow a gluten-free diet for six weeks and monitor for NCGS symptoms. If symptoms abate, a gluten-containing diet is then reintroduced and the patient is evaluated for the reemergence of NCGS symptoms. If symptoms are not reduced with a gluten-free diet, NCGS may be excluded. Newer GS laboratory tests will emerge that can assay more forms of gliadin antibodies, possibly aiding in NCGS diagnosis.4,14
To make a diagnosis of WA, skin prick tests and allergen-specific IgE testing are used, along with a medical history, clinical presentation, and possibly a food challenge.
Continue for management of GS >>
MANAGEMENT OF GS
The hallmark treatment for GS, regardless of its causative factor, is a strict gluten-free diet (GFD). For patients with CD, a 100% GFD is recommended for life. It is not yet known whether this lifelong duration is necessary for those with NCGS and WA, or if there is a safe threshold in these patients for gluten consumption. It is helpful for patients to keep a food diary, noting what they eat and how that affects the appearance or attenuation of symptoms.
Transitioning to a gluten-free lifestyle can be confusing, frustrating, and expensive for patients. Removing gluten from the diet is also challenging, as wheat is the predominant grain consumed in this country. Barley and rye (less so oats) also contain gluten, leaving limited alternatives, like amaranth, corn, quinoa, rice, and tapioca. Unlike CD and NCGS, WA requires only elimination of wheat-containing products; thus, it may not be necessary for affected patients to avoid barley and rye.1,4
Extensive patient education is important for success. Referral to a knowledgeable nutritionist is ideal but not always practical. Lists of foods to avoid on a gluten-free diet are readily available, but important points should be stressed, including how to read food labels. For example, the term wheat-free does not mean gluten-free (see Table 2).1,17 As of August 2014, the food industry, by law, can only claim a product is “gluten-free” if it contains no more than 20 parts per million (ppm) of gluten.1
Due to malabsorption issues, it is recommended that patients with CD be monitored for micronutrient deficiencies (ie, iron, B1, B6, B12, and zinc), and osteopenia/osteoporosis (dual-energy x-ray absorptiometry [DEXA] at the time of diagnosis) and be offered fertility counseling. What patients with GS need most of all are informed, caring providers to help guide them through diagnosis and treatment.6,13
Continue for the conclusion >>
CONCLUSION
Gluten-free diets are increasing in popularity, and many people who do not have CD claim improved health and vitality when they avoid gluten. Much is known about the incidence and pathogenesis of the gluten-associated disorders of CD and WA. Far less is known about the controversial disorder of NCGS. The symptoms and morbidities associated with NCGS have been well documented and present a curious mix of CD and IBS, yet neither condition fully accounts for the pathogenesis of NCGS. While CD is linked to more serious morbidities (including death if the disease is not readily diagnosed), NCGS and WA do produce significant manifestations and risks.
Research into NCGS remains limited and conflicting, and biomarkers for the disorder are not yet known. Unsupported or not, many patients attribute mood disorders, pain, and chronic ills to gluten intake and seek input from their health care providers. Rather than dismiss their claims, clinicians can provide pertinent instructions on a gluten-free lifestyle and healthy diet, and encourage the use of food diaries to document food-symptom associations. Gluten sensitivities are not benign and “going gluten-free” may be of great benefit for many patients with GS. That’s a fact.
REFERENCES
1. Capili B, Chang M, Anastasi JK. Nonceliac gluten sensitivity—is it really the gluten? J Nurs Pract. 2014;10(9):666-673.
2. Davis W. Wheat Belly: Lose the Wheat, Lose the Weight, and Find Your Path Back to Health. Emmaus, PA: Rodale Books; 2011.
3. Perlmutter D. Grain Brain: The Surprising Truth about Wheat, Carbs, and Sugar—Your Brain’s Silent Killers. New York, NY: Little, Brown and Company; 2013.
4. Brown AC. Gluten sensitivity: problems of an emerging condition separate from celiac disease. Expert Rev Gastroenterol Hepatol. 2012;6(1): 43-55.
5. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012 Feb 7;10:13.
6. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656-676.
7. Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Non-celiac gluten sensitivity: literature review. J Am Coll Nutr. 2014;33(1):39-54.
8. Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):728-736.
9. Fasano A, Sapone A, Zevallos V, Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148(6):1195-1204.
10. Catassi C, Bai JC, Bonaz B, Boouma G. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5(10):3839-3853.
11. Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320-328.
12. Guandalini S, Newland C. Differentiating food allergies from food intolerances. Curr Gastroenterol Rep. 2011;13(5):426-434.
13. Scanlon SA, Murray JA. Update on celiac disease—etiology, differential diagnosis, drug targets, and management devices. Clin Exp Gastroenterol. 2011;4:297-311.
14. Catassi C, Elli L, Bonaz B, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno Experts’ Criteria. Nutrients. 2015;7(6):4966-4977.
15. Peters SL, Biesiekierski JR, Yelland GW,et al. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity—an exploratory clinical study. Aliment Pharmacol Ther. 2014:39(10): 1104–1112.
16. Leffler D, Schuppen D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62(7):996-1004.
17. Celiac Disease Foundation. Sources of gluten (2015). https://celiac.org/live-gluten-free/glutenfreediet/sources-of-gluten. Accessed November 24, 2015.
IN THIS ARTICLE
• So what is gluten?
• Selected symptoms of celiac disease
• Selected foods and products containing gluten
Gluten has become a dietary pariah (see “So What Is Gluten?"). The American public’s enthusiasm for a gluten-free diet has spurred a gluten-free food industry that has grown, on average, 34% per year since 2009, with annual sales predicted to reach an impressive $15.5 billion by 2016.1 This trend coincides with the national media’s intense focus on gluten sensitivity (GS), as well as best-selling books such as Wheat Belly and Grain Brain.2,3
Gluten-free food products, once relegated to boutique food shops and limited shelf space, now fill sections in large grocery and drugstore chains. Many restaurants have added gluten-free items to their menus (although gluten-free Big Macs have been available in Finland for more than 20 years).4 Only celiac disease (CD), which affects approximately 1% of the American population, requires strict gluten avoidance; yet more than 30% of US adults report having reduced their gluten intake, most claiming they did so to promote a “healthier” diet or support weight loss.1
PREVALENCE AND PATHOLOGY OF GS DISORDERS
Gluten sensitivity, once used to denote CD alone, now includes a group of gluten-intolerant conditions unrelated to CD—primarily nonceliac gluten sensitivity (NCGS) and wheat allergy (WA)—although the nomenclature is likely to change. While these disorders differ in underlying pathogenesis, each demonstrates a resolution of symptoms when the patient is placed on a gluten-free diet. Of these GS disorders, only NCGS lacks clarity with regard to incidence, diagnosis, and pathology.5
Celiac Disease
Celiac disease is an autoimmune, T-cell–activated disease that manifests in genetically susceptible individuals (with gene variants HLA-DQ2 and HLA-DQ8); it can occur at any age. The incidence of CD in the US has increased from 1 in 500 in 1974 to a current estimate of 1 in 100, although many with CD are believed to be undiagnosed.4,6
CD is the only autoimmune disease for which a trigger is known: gluten. Suspicion for CD should be heightened if the patient or a family member has a history of autoimmune disease. Nearly one-quarter of patients with CD will develop an autoimmune thyroid disorder.7
In CD, a significant enteropathy occurs in response to gluten intake, characterized by inflammation of the proximal small intestine. Individuals with CD produce tissue transglutaminase (tTG) or transglutaminase 2 (TG2) autoantibodies, resulting in gluten-specific CD4+ Th1 T-cell activation and an immune response that causes an upregulation of zonulin.8 Zonulin, a protein that modulates the permeability of the intestinal mucosal wall, is believed to play a role in “leaky gut syndrome” and autoimmune disease. The upregulation of zonulin in CD creates a disruption of the intestinal mucosal lining, causing villous mucosal atrophy and impairment of intestinal permeability and absorption.9
Nonceliac Gluten Sensitivity
NCGS is a poorly understood condition first described in the 1980s and recently “rediscovered” as a gluten-related disorder.10 Its actual prevalence is unknown because of unclear diagnostic criteria but is likely much higher than that of CD.1,4 Unlike CD, there does not appear to be a genetic predisposition for NCGS, nor is it believed to be an autoimmune disorder. However, research does suggest that NCGS may increase the risk for autoimmune diseases, such as Hashimoto thyroiditis. NCGS can occur at any age but appears more commonly in adults than children, and in women than men.4
A small but meticulous 2013 study raised doubt about NCGS as a specific gluten-related disorder.11 The results suggested that NCGS should be viewed as a variant of irritable bowel syndrome (IBS), not triggered by gluten but by poorly absorbed carbohydrates found in wheat known as fructans and galactans, and perhaps by other foods containing fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPS). It is believed those with diarrhea-prone IBS are particularly sensitive to gluten.11 As a result, ardent claims of NCGS and improved health with gluten-free diets in those without CD are often discounted.
More recent research findings refute this conjecture, suggesting that NCGS is likely a reaction to other proteins within the gluten family, such as beta-, gamma-, or omega-gliadin, glutenin, wheat germ agglutinin, gluteomorphin, and deamidated gliadin. Development of GS is believed to be triggered by such factors as intestinal infections, altered microbiota, or food additives.4
In any event, the pathogenesis of NCGS remains unclear, and it does not present with the diagnostic antibodies or inflammatory enteropathy seen in patients with CD. Despite this, NCGS does present with gastrointestinal (GI) and extra-intestinal symptoms similar to those of CD.
Wheat Allergies
Wheat is frequently implicated in food allergies, especially in infants and children. The incidence of WA is not known, although up to 4% of adults and 6% of children are estimated to have food allergies. In WA, there is an IgE antibody–mediated reaction to one or more of the wheat proteins (albumin, gliadin, globulin, gluten) that occurs within minutes to hours after exposure to the offending food. Many children with IgE-mediated allergies may “outgrow” them with time.12
Continue for the clinical presentation of GS >>
CLINICAL PRESENTATION OF GS
Although CD is a disorder associated with the GI system, the “classic” GI symptoms of bloating, flatulence, diarrhea, and/or constipation are often absent (silent CD), especially in older individuals. It is for this reason that the diagnosis of CD is easily missed.
Delaying diagnosis can have serious health consequences, as CD is associated with significant morbidities, such as malnutrition (worse in children), iron-deficiency anemia, neuropsychiatric aberrations (depression, anxiety, attention-deficit, and cerebral ataxia), osteoporosis, lymphoma, and death (see Table 1).4,13 CD may also present with dermatitis herpetiformis, a chronic vesicular rash, seen most often in adult males.
The role of gluten in the development of autism spectrum disorders or schizophrenia, though not proven, remains hotly debated, especially as close biochemical links are now recognized between the gut and the brain. It is clear, however, that gluten intake in severely gluten-sensitive individuals can directly affect mood and brain function. Most CD-associated morbidities will resolve after one year of complete gluten avoidance.1,13
Prominent symptoms of NCGS occur soon after gluten ingestion and disappear within days to weeks of gluten avoidance. The classic NCGS presentation combines IBS-like symptoms, such as abdominal cramps, bloating, diarrhea, and constipation, with systemic manifestations that include “brain fog,” fatigue, headache, joint and muscle pain, peripheral numbness, skin rash, aphthous stomatitis, anemia, and depression or anxiety. As with CD, GI symptoms usually predominate in children and abate with gluten avoidance.14,15
Allergic reactions to wheat will present within minutes to two hours of wheat exposure and may manifest with pruritic rash, hives, swelling of the lips or tongue, rhinitis, abdominal cramps, vomiting, diarrhea, constipation, and/or anaphylaxis. Subtle reactions may make diagnosis difficult.12
DIAGNOSTIC STUDIES FOR GS
The effectiveness of diagnostic testing for CD has been well established. Testing for antitissue transglutaminase antibodies (tTG-IgA) is the preferred laboratory test for CD, with a sensitivity of 93%, specificity of 98%, and few false-negative results. The endomysial antibody (EMA-IgA) test, though highly specific for CD, lacks the sensitivity of tTG-IgA. Newer antibody tests, such as deamidated gliadin peptide IgA and IgG, have not proven superior in detecting CD. Genetic testing for HLA-DQ2 and HLA-DQ8 may also be performed, but many people carry the gene without ever developing CD.13
To improve the reliability of CD antibody tests, the patient should have consumed gluten regularly for at least one month prior to testing. If the patient has been on a gluten-free diet for several weeks, then a gluten challenge should be done: The patient would be instructed to consume at least 3 g/d of gluten (two slices of bread) for a minimum of two weeks (versus eight weeks in previous protocols), after which the celiac antibody tests would be repeated.16
If these antibody test results are negative but the suspicion for CD remains high, an endoscopy with a duodenal biopsy should be performed. The appearance of villous atrophy would confirm the diagnosis of CD.1,13,16
Unlike CD, there are currently no reliable diagnostic tests for NCGS, although some researchers suggest testing for IgG antigliadin antibodies (AGA); NCGS is currently a diagnosis of exclusion.7 In NCGS, celiac antibodies will be negative and the duodenal biopsy will demonstrate only mild inflammation without the mucosal atrophy of CD. As with CD, patients affected by NCGS will also test negative for the wheat allergy IgE response.
Another option is a gluten challenge. The patient is instructed to follow a gluten-free diet for six weeks and monitor for NCGS symptoms. If symptoms abate, a gluten-containing diet is then reintroduced and the patient is evaluated for the reemergence of NCGS symptoms. If symptoms are not reduced with a gluten-free diet, NCGS may be excluded. Newer GS laboratory tests will emerge that can assay more forms of gliadin antibodies, possibly aiding in NCGS diagnosis.4,14
To make a diagnosis of WA, skin prick tests and allergen-specific IgE testing are used, along with a medical history, clinical presentation, and possibly a food challenge.
Continue for management of GS >>
MANAGEMENT OF GS
The hallmark treatment for GS, regardless of its causative factor, is a strict gluten-free diet (GFD). For patients with CD, a 100% GFD is recommended for life. It is not yet known whether this lifelong duration is necessary for those with NCGS and WA, or if there is a safe threshold in these patients for gluten consumption. It is helpful for patients to keep a food diary, noting what they eat and how that affects the appearance or attenuation of symptoms.
Transitioning to a gluten-free lifestyle can be confusing, frustrating, and expensive for patients. Removing gluten from the diet is also challenging, as wheat is the predominant grain consumed in this country. Barley and rye (less so oats) also contain gluten, leaving limited alternatives, like amaranth, corn, quinoa, rice, and tapioca. Unlike CD and NCGS, WA requires only elimination of wheat-containing products; thus, it may not be necessary for affected patients to avoid barley and rye.1,4
Extensive patient education is important for success. Referral to a knowledgeable nutritionist is ideal but not always practical. Lists of foods to avoid on a gluten-free diet are readily available, but important points should be stressed, including how to read food labels. For example, the term wheat-free does not mean gluten-free (see Table 2).1,17 As of August 2014, the food industry, by law, can only claim a product is “gluten-free” if it contains no more than 20 parts per million (ppm) of gluten.1
Due to malabsorption issues, it is recommended that patients with CD be monitored for micronutrient deficiencies (ie, iron, B1, B6, B12, and zinc), and osteopenia/osteoporosis (dual-energy x-ray absorptiometry [DEXA] at the time of diagnosis) and be offered fertility counseling. What patients with GS need most of all are informed, caring providers to help guide them through diagnosis and treatment.6,13
Continue for the conclusion >>
CONCLUSION
Gluten-free diets are increasing in popularity, and many people who do not have CD claim improved health and vitality when they avoid gluten. Much is known about the incidence and pathogenesis of the gluten-associated disorders of CD and WA. Far less is known about the controversial disorder of NCGS. The symptoms and morbidities associated with NCGS have been well documented and present a curious mix of CD and IBS, yet neither condition fully accounts for the pathogenesis of NCGS. While CD is linked to more serious morbidities (including death if the disease is not readily diagnosed), NCGS and WA do produce significant manifestations and risks.
Research into NCGS remains limited and conflicting, and biomarkers for the disorder are not yet known. Unsupported or not, many patients attribute mood disorders, pain, and chronic ills to gluten intake and seek input from their health care providers. Rather than dismiss their claims, clinicians can provide pertinent instructions on a gluten-free lifestyle and healthy diet, and encourage the use of food diaries to document food-symptom associations. Gluten sensitivities are not benign and “going gluten-free” may be of great benefit for many patients with GS. That’s a fact.
REFERENCES
1. Capili B, Chang M, Anastasi JK. Nonceliac gluten sensitivity—is it really the gluten? J Nurs Pract. 2014;10(9):666-673.
2. Davis W. Wheat Belly: Lose the Wheat, Lose the Weight, and Find Your Path Back to Health. Emmaus, PA: Rodale Books; 2011.
3. Perlmutter D. Grain Brain: The Surprising Truth about Wheat, Carbs, and Sugar—Your Brain’s Silent Killers. New York, NY: Little, Brown and Company; 2013.
4. Brown AC. Gluten sensitivity: problems of an emerging condition separate from celiac disease. Expert Rev Gastroenterol Hepatol. 2012;6(1): 43-55.
5. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012 Feb 7;10:13.
6. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656-676.
7. Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Non-celiac gluten sensitivity: literature review. J Am Coll Nutr. 2014;33(1):39-54.
8. Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):728-736.
9. Fasano A, Sapone A, Zevallos V, Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148(6):1195-1204.
10. Catassi C, Bai JC, Bonaz B, Boouma G. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5(10):3839-3853.
11. Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145(2):320-328.
12. Guandalini S, Newland C. Differentiating food allergies from food intolerances. Curr Gastroenterol Rep. 2011;13(5):426-434.
13. Scanlon SA, Murray JA. Update on celiac disease—etiology, differential diagnosis, drug targets, and management devices. Clin Exp Gastroenterol. 2011;4:297-311.
14. Catassi C, Elli L, Bonaz B, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno Experts’ Criteria. Nutrients. 2015;7(6):4966-4977.
15. Peters SL, Biesiekierski JR, Yelland GW,et al. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity—an exploratory clinical study. Aliment Pharmacol Ther. 2014:39(10): 1104–1112.
16. Leffler D, Schuppen D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62(7):996-1004.
17. Celiac Disease Foundation. Sources of gluten (2015). https://celiac.org/live-gluten-free/glutenfreediet/sources-of-gluten. Accessed November 24, 2015.