User login
Hiccups in patients with cancer often overlooked, undertreated
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF HOSPICE AND PALLIATIVE MEDICINE
Is there a doctor on the plane? Tips for providing in-flight assistance
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.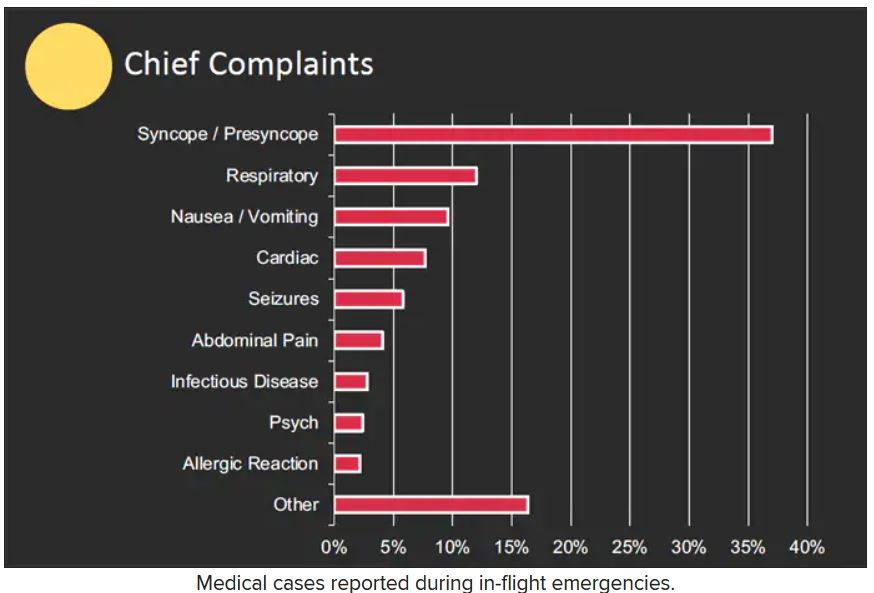
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
IBD and pregnancy: What to tell your patients
While many gastroenterologists may be comfortable with inflammatory bowel disease (IBD), most are not experts in women’s concerns about pregnancy. One study found that, although women with IBD may have concerns about the interplay of their disease and reproductive health, many have not had extensive conversations with their gastroenterologist about it. In fact, that same study found most women expect their gastroenterologist to initiate these conversations.
In this roundtable discussion, Uma Mahadevan, MD, professor of medicine and the director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco; Marla C. Dubinsky, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York; and Sunanda V. Kane, MD, professor of medicine at Mayo Clinic in Rochester, Minn., share how they respond to these questions in their clinical practice.
What should a woman with IBD who is interested in having biological children in the future be thinking about now?
Dr. Mahadevan: Because active disease is associated with lower rates of conception and higher rates of pregnancy loss, women with IBD should first ensure they are in remission. I like to document endoscopic healing with a colonoscopy or sigmoidoscopy, but, if this has been done recently, a fecal calprotectin test can be helpful.
Women with IBD, particularly those with small bowel disease, are at risk for nutritional deficiencies, so prior to conception, I also check vitamin B-12, vitamin D, and iron, and repeat as needed. Zinc and folate can be considered. Those who are underweight should work with a nutritionist to ensure adequate caloric intake.
Dr. Dubinsky: I think it’s also important to stress the importance of taking their IBD medications because they can help patients achieve and maintain disease remission. Uncontrolled inflammation is a key risk factor for spontaneous abortion in the first trimester. Medication we would use in pregnancy is not putting them at risk for spontaneous abortion or congenital anomalies, which is what mothers to be are understandably most concerned about.
I am very honest and transparent with my patients: “About the only thing I need to take care of is you. If you are good, the baby is good.”
Dr. Kane: As Dr. Mahadevan mentioned, women with IBD are at higher risk for vitamin deficiencies, so those need to be corrected before conception. If they smoke, they should stop before conceiving.
There is no increased risk of infertility unless there has been a history of abdominal surgery.
Also, if women are not actively planning on getting pregnant, that would be important to share because some gastroenterologists will avoid certain effective medications if pregnancy is a possibility.
If a woman has had surgery for her IBD, could that make it harder for her to get pregnant?
Dr. Kane: Yes, it can because scar tissue may develop within the pelvis. However, if surgery is indicated to manage a patient’s IBD, then talk to the surgeon about ways that they might be able to reduce the risk of scar tissue formation.
Dr. Dubinsky: One thing to note is that almost all the data of infertility risk and scarring are based on open surgical techniques that involve dissection of the rectum. On the other hand, we don’t yet have enough prospective data on the impact of the modern era of laparoscopic surgery to suggest whether it affects fertility. More data is needed because providers may be giving women old information that is no longer relevant in the modern era.
If a woman is experiencing IBD symptoms, should she attempt to conceive?
Dr. Kane: Gastrointestinal symptoms in patients with IBD could be from active disease but also other things, so it’s important to have a thorough check-up to assess if there is active disease or not. Active disease can (but does not always) lead to a more complicated pregnancy, and conception is not recommended while a patient has active IBD.
Dr. Dubinsky: Although some patients feel an urgency to conceive regardless of disease activity, we need to do our due diligence and explain that we need to focus on getting them into the deepest remission possible, including endoscopic findings, biomarkers, and symptoms.
The most important gift you can give your future moms is to optimize the therapy they’re on before they conceive.
Is it important for someone who’s working with a gastroenterologist and an obstetrician to also work with a maternal-fetal medicine (MFM) specialist?
Dr. Kane: Having a diagnosis of IBD makes a woman’s pregnancy “high risk” because just having the diagnosis is associated with a higher risk of prematurity and small for gestational age – but importantly, not birth defects. A woman whose IBD is in remission should still have a discussion with an MFM specialist, just so everyone is on the same page.
Dr. Dubinsky: I refer to care with MFM specialists as “tighter monitoring.” I tell my patients that MFM specialists have managed many complex pregnancies and feel confident around the safety of their medications, understand the impact of when the baby may be exposed to certain medications, and will focus on following them more closely.
What are the risks of IBD medications during pregnancy and while breastfeeding? Should women stop their medications during pregnancy and breastfeeding?
Dr. Dubinsky: Organogenesis occurs in the first 10 weeks, so any medicines that cross the placenta during that time are up for discussion and debate. Methotrexate and the newer small molecules, such as Janus kinase (JAK) inhibitors and S1P receptor modulators, do cross the placenta during the first trimester and need to be discontinued before conception, sometimes as early as 3 months before conception.
However, biologics are very large proteins and do not cross the placenta until closer to week 27. We are not advocating stopping biologics in advance of conception, or during pregnancy, or during breastfeeding. There is more risk to stopping than continuing.
Dr. Mahadevan: Methotrexate should be stopped at least 3 months prior to conception and should not be taken during pregnancy.
There are limited antibiotic safety data in pregnancy for the longer periods of time used in IBD. I generally prefer amoxicillin/clavulanic acid over ciprofloxacin or metronidazole, but short term (less than 2 weeks) use of any of those three are not contraindicated.
Mesalamine agents and thiopurine monotherapy can be continued through pregnancy and breastfeeding.
Biologic agents, such as anti–tumor necrosis factor, anti-interleukin 23, anti-integrin, and biosimilars, can be continued through pregnancy and during breastfeeding. Given limited exposure in the first trimester, there is no evidence of increased risk of birth defects. As Dr. Dubinsky pointed out, there is active transfer, particularly in the third trimester and minimal transfer in breast milk, but this has not been associated with harm.
Lastly, small molecules, such as the JAK inhibitors tofacitinib and upadacitinib, as well as ozanimod, have virtually no human safety data during pregnancy, and animal data show harm. The use of these agents in pregnancy is not recommended.
Dr. Kane: As Dr. Dubinsky stated, most of the medications our patients take are low risk to continue through pregnancy if the patients are in remission. Although a woman “in remission” on steroids is not really in remission and should not get pregnant until she is on something else.
As far as breastfeeding goes, that should be stopped if the patient is on methotrexate, cyclosporine, or certain antibiotics. If she is on more than 20 mg of prednisone, this can pass to the infant, and a mother should not breastfeed.
Women should avoid fenugreek as a lactation aid, as that contains a compound that can promote bleeding. Lactation cookies are ok.
Otherwise, there are lots of potential benefits to breastfeeding, and I encourage it.
How is a flare treated if it occurs during pregnancy?
Dr. Dubinsky: A flare during pregnancy is treated the same as a flare outside of pregnancy. We want to use noninvasive ways to confirm it, but I think we don’t need to overly investigate in most of our women. If they’re already on a biologic, you may consider changing.
Some women may need corticosteroids. It’s not our favorite move, but there is an urgency to getting a flare under control during pregnancy because of possible complications.
Dr. Mahadevan: Some of this is contingent on when during pregnancy the flare occurs. A patient who has a flare at 38 weeks’ gestation will likely proceed with delivery and the flare will be dealt with separately. Someone at 8 weeks’ gestation is at high risk for pregnancy loss, so treatment should be quick and effective.
As does Dr. Dubinsky, I do try to avoid steroids if possible. For example, I would rather start an effective biologic right away than drag out steroids to see if they will respond.
Dr. Kane: I would add that, if a mother is losing weight, she might need to be hospitalized for additional nutritional support. If surgery is necessary, we usually try to time it for the second or third trimester.
What needs to be taken into consideration regarding mode of delivery? Also, if a woman has undergone prior surgeries, do they increase the risk of delivery complications?
For ulcerative colitis, mode of delivery is based on obstetric, not gastrointestinal, variables. For Crohn’s disease, if there is evidence of perianal disease, then a cesarean is appropriate.
If there is no history of perianal disease, then delivery is based on obstetric variables.
For a woman who has a J pouch, if possible, the surgeon who created it should be contacted to ask about the technical aspects of the pouch and how it lies in the pelvis.
What’s the risk of a postpartum flare if a woman’s IBD remains in clinical remission during pregnancy?
Dr. Mahadevan: There is no increased risk of postpartum flare if a woman continues her IBD medications after delivery. Many of the reports of flare are from stopping medications (mistakenly often) to breastfeed.
Dr. Kane: As Dr. Mahadevan said, the risk of a flare is usually because a woman stops taking her medications because she thinks that medication will be passed to the infant through breastfeeding, which in most cases is not true.
Otherwise, there is not an increased risk of a flare in a 12-month period. However, it is important to monitor for symptoms after delivery; the risk of a flare is not zero.
What symptoms should women watch out for after delivery that may indicate an uptick in disease activity?
Dr. Kane: The same symptoms as before they were pregnant. Diarrhea, abdominal pain, and rectal bleeding are not normal after delivery and should be considered signs of returning disease.
As a gastroenterologist, is there any additional advice you’d offer about conception, fertility, and pregnancy when treating women with IBD?
Dr. Mahadevan: Women with IBD should, when feasible, have a planned pregnancy when in documented remission and under the care of their gastroenterologists, obstetrician, and an MFM specialist. Life happens, and this is not always possible. That said, a woman with IBD has the same chance of getting pregnant as a woman of the same age without IBD, unless she has active disease or a history of pelvic surgery. Women with IBD in remission will generally have healthy pregnancies if they continue appropriate medications.
Dr. Kane: Agreed. The majority of women with IBD will have normal, healthy pregnancies. It is important for them to not stop their IBD therapy without talking to their gastroenterologist first. Well-intentioned but ignorant obstetricians or midwives may recommend stopping, but then panic when disease flares and the mother’s health is at risk. Active inflammation is the worst enemy to a pregnancy, not active therapy.
Dr. Dubinsky: One additional thing to consider is: How do we help women with IBD who have delivered meet the needs of their family and continue to stay on their meds and be in good inflammatory control?
For example, we can give the biologic in the hospital after they’ve had a cesarean or a vaginal delivery and before they leave. We know that that is safe, giving that to them before they leave the hospital is a huge value added.
Another thing is possibly changing their infusions to home infusions. That would be helpful for the moms as well.
Dr. Mahadevan reports being a consultant for AbbVie, Janssen, Pfizer, Gilead, Bristol-Myers Squibb, Takeda, Protagonist, Prometheus, and Boehringer Ingelheim. Dr. Dubinsky is a consultant for AbbVie, Arena, Bristol-Myers Squibb, Janssen, Eli Lilly, Takeda, and Prometheus BioSciences. She is a shareholder and CEO of a publicly traded company, Trellis Health. Dr. Kane is a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, Janssen, Takeda, Seres Therapeutics, TechLab, United Healthcare, Predicta-Med, and InveniAI, and is the editor for the IBD section of UptoDate.
AGA Resource
Planning for a family can be challenging, and if your patient has IBD, there are additional factors to consider. The AGA IBD Parenthood Project is the “go-to” resource for everything patients need to know about IBD and pregnancy throughout all stages of family planning.
While many gastroenterologists may be comfortable with inflammatory bowel disease (IBD), most are not experts in women’s concerns about pregnancy. One study found that, although women with IBD may have concerns about the interplay of their disease and reproductive health, many have not had extensive conversations with their gastroenterologist about it. In fact, that same study found most women expect their gastroenterologist to initiate these conversations.
In this roundtable discussion, Uma Mahadevan, MD, professor of medicine and the director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco; Marla C. Dubinsky, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York; and Sunanda V. Kane, MD, professor of medicine at Mayo Clinic in Rochester, Minn., share how they respond to these questions in their clinical practice.
What should a woman with IBD who is interested in having biological children in the future be thinking about now?
Dr. Mahadevan: Because active disease is associated with lower rates of conception and higher rates of pregnancy loss, women with IBD should first ensure they are in remission. I like to document endoscopic healing with a colonoscopy or sigmoidoscopy, but, if this has been done recently, a fecal calprotectin test can be helpful.
Women with IBD, particularly those with small bowel disease, are at risk for nutritional deficiencies, so prior to conception, I also check vitamin B-12, vitamin D, and iron, and repeat as needed. Zinc and folate can be considered. Those who are underweight should work with a nutritionist to ensure adequate caloric intake.
Dr. Dubinsky: I think it’s also important to stress the importance of taking their IBD medications because they can help patients achieve and maintain disease remission. Uncontrolled inflammation is a key risk factor for spontaneous abortion in the first trimester. Medication we would use in pregnancy is not putting them at risk for spontaneous abortion or congenital anomalies, which is what mothers to be are understandably most concerned about.
I am very honest and transparent with my patients: “About the only thing I need to take care of is you. If you are good, the baby is good.”
Dr. Kane: As Dr. Mahadevan mentioned, women with IBD are at higher risk for vitamin deficiencies, so those need to be corrected before conception. If they smoke, they should stop before conceiving.
There is no increased risk of infertility unless there has been a history of abdominal surgery.
Also, if women are not actively planning on getting pregnant, that would be important to share because some gastroenterologists will avoid certain effective medications if pregnancy is a possibility.
If a woman has had surgery for her IBD, could that make it harder for her to get pregnant?
Dr. Kane: Yes, it can because scar tissue may develop within the pelvis. However, if surgery is indicated to manage a patient’s IBD, then talk to the surgeon about ways that they might be able to reduce the risk of scar tissue formation.
Dr. Dubinsky: One thing to note is that almost all the data of infertility risk and scarring are based on open surgical techniques that involve dissection of the rectum. On the other hand, we don’t yet have enough prospective data on the impact of the modern era of laparoscopic surgery to suggest whether it affects fertility. More data is needed because providers may be giving women old information that is no longer relevant in the modern era.
If a woman is experiencing IBD symptoms, should she attempt to conceive?
Dr. Kane: Gastrointestinal symptoms in patients with IBD could be from active disease but also other things, so it’s important to have a thorough check-up to assess if there is active disease or not. Active disease can (but does not always) lead to a more complicated pregnancy, and conception is not recommended while a patient has active IBD.
Dr. Dubinsky: Although some patients feel an urgency to conceive regardless of disease activity, we need to do our due diligence and explain that we need to focus on getting them into the deepest remission possible, including endoscopic findings, biomarkers, and symptoms.
The most important gift you can give your future moms is to optimize the therapy they’re on before they conceive.
Is it important for someone who’s working with a gastroenterologist and an obstetrician to also work with a maternal-fetal medicine (MFM) specialist?
Dr. Kane: Having a diagnosis of IBD makes a woman’s pregnancy “high risk” because just having the diagnosis is associated with a higher risk of prematurity and small for gestational age – but importantly, not birth defects. A woman whose IBD is in remission should still have a discussion with an MFM specialist, just so everyone is on the same page.
Dr. Dubinsky: I refer to care with MFM specialists as “tighter monitoring.” I tell my patients that MFM specialists have managed many complex pregnancies and feel confident around the safety of their medications, understand the impact of when the baby may be exposed to certain medications, and will focus on following them more closely.
What are the risks of IBD medications during pregnancy and while breastfeeding? Should women stop their medications during pregnancy and breastfeeding?
Dr. Dubinsky: Organogenesis occurs in the first 10 weeks, so any medicines that cross the placenta during that time are up for discussion and debate. Methotrexate and the newer small molecules, such as Janus kinase (JAK) inhibitors and S1P receptor modulators, do cross the placenta during the first trimester and need to be discontinued before conception, sometimes as early as 3 months before conception.
However, biologics are very large proteins and do not cross the placenta until closer to week 27. We are not advocating stopping biologics in advance of conception, or during pregnancy, or during breastfeeding. There is more risk to stopping than continuing.
Dr. Mahadevan: Methotrexate should be stopped at least 3 months prior to conception and should not be taken during pregnancy.
There are limited antibiotic safety data in pregnancy for the longer periods of time used in IBD. I generally prefer amoxicillin/clavulanic acid over ciprofloxacin or metronidazole, but short term (less than 2 weeks) use of any of those three are not contraindicated.
Mesalamine agents and thiopurine monotherapy can be continued through pregnancy and breastfeeding.
Biologic agents, such as anti–tumor necrosis factor, anti-interleukin 23, anti-integrin, and biosimilars, can be continued through pregnancy and during breastfeeding. Given limited exposure in the first trimester, there is no evidence of increased risk of birth defects. As Dr. Dubinsky pointed out, there is active transfer, particularly in the third trimester and minimal transfer in breast milk, but this has not been associated with harm.
Lastly, small molecules, such as the JAK inhibitors tofacitinib and upadacitinib, as well as ozanimod, have virtually no human safety data during pregnancy, and animal data show harm. The use of these agents in pregnancy is not recommended.
Dr. Kane: As Dr. Dubinsky stated, most of the medications our patients take are low risk to continue through pregnancy if the patients are in remission. Although a woman “in remission” on steroids is not really in remission and should not get pregnant until she is on something else.
As far as breastfeeding goes, that should be stopped if the patient is on methotrexate, cyclosporine, or certain antibiotics. If she is on more than 20 mg of prednisone, this can pass to the infant, and a mother should not breastfeed.
Women should avoid fenugreek as a lactation aid, as that contains a compound that can promote bleeding. Lactation cookies are ok.
Otherwise, there are lots of potential benefits to breastfeeding, and I encourage it.
How is a flare treated if it occurs during pregnancy?
Dr. Dubinsky: A flare during pregnancy is treated the same as a flare outside of pregnancy. We want to use noninvasive ways to confirm it, but I think we don’t need to overly investigate in most of our women. If they’re already on a biologic, you may consider changing.
Some women may need corticosteroids. It’s not our favorite move, but there is an urgency to getting a flare under control during pregnancy because of possible complications.
Dr. Mahadevan: Some of this is contingent on when during pregnancy the flare occurs. A patient who has a flare at 38 weeks’ gestation will likely proceed with delivery and the flare will be dealt with separately. Someone at 8 weeks’ gestation is at high risk for pregnancy loss, so treatment should be quick and effective.
As does Dr. Dubinsky, I do try to avoid steroids if possible. For example, I would rather start an effective biologic right away than drag out steroids to see if they will respond.
Dr. Kane: I would add that, if a mother is losing weight, she might need to be hospitalized for additional nutritional support. If surgery is necessary, we usually try to time it for the second or third trimester.
What needs to be taken into consideration regarding mode of delivery? Also, if a woman has undergone prior surgeries, do they increase the risk of delivery complications?
For ulcerative colitis, mode of delivery is based on obstetric, not gastrointestinal, variables. For Crohn’s disease, if there is evidence of perianal disease, then a cesarean is appropriate.
If there is no history of perianal disease, then delivery is based on obstetric variables.
For a woman who has a J pouch, if possible, the surgeon who created it should be contacted to ask about the technical aspects of the pouch and how it lies in the pelvis.
What’s the risk of a postpartum flare if a woman’s IBD remains in clinical remission during pregnancy?
Dr. Mahadevan: There is no increased risk of postpartum flare if a woman continues her IBD medications after delivery. Many of the reports of flare are from stopping medications (mistakenly often) to breastfeed.
Dr. Kane: As Dr. Mahadevan said, the risk of a flare is usually because a woman stops taking her medications because she thinks that medication will be passed to the infant through breastfeeding, which in most cases is not true.
Otherwise, there is not an increased risk of a flare in a 12-month period. However, it is important to monitor for symptoms after delivery; the risk of a flare is not zero.
What symptoms should women watch out for after delivery that may indicate an uptick in disease activity?
Dr. Kane: The same symptoms as before they were pregnant. Diarrhea, abdominal pain, and rectal bleeding are not normal after delivery and should be considered signs of returning disease.
As a gastroenterologist, is there any additional advice you’d offer about conception, fertility, and pregnancy when treating women with IBD?
Dr. Mahadevan: Women with IBD should, when feasible, have a planned pregnancy when in documented remission and under the care of their gastroenterologists, obstetrician, and an MFM specialist. Life happens, and this is not always possible. That said, a woman with IBD has the same chance of getting pregnant as a woman of the same age without IBD, unless she has active disease or a history of pelvic surgery. Women with IBD in remission will generally have healthy pregnancies if they continue appropriate medications.
Dr. Kane: Agreed. The majority of women with IBD will have normal, healthy pregnancies. It is important for them to not stop their IBD therapy without talking to their gastroenterologist first. Well-intentioned but ignorant obstetricians or midwives may recommend stopping, but then panic when disease flares and the mother’s health is at risk. Active inflammation is the worst enemy to a pregnancy, not active therapy.
Dr. Dubinsky: One additional thing to consider is: How do we help women with IBD who have delivered meet the needs of their family and continue to stay on their meds and be in good inflammatory control?
For example, we can give the biologic in the hospital after they’ve had a cesarean or a vaginal delivery and before they leave. We know that that is safe, giving that to them before they leave the hospital is a huge value added.
Another thing is possibly changing their infusions to home infusions. That would be helpful for the moms as well.
Dr. Mahadevan reports being a consultant for AbbVie, Janssen, Pfizer, Gilead, Bristol-Myers Squibb, Takeda, Protagonist, Prometheus, and Boehringer Ingelheim. Dr. Dubinsky is a consultant for AbbVie, Arena, Bristol-Myers Squibb, Janssen, Eli Lilly, Takeda, and Prometheus BioSciences. She is a shareholder and CEO of a publicly traded company, Trellis Health. Dr. Kane is a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, Janssen, Takeda, Seres Therapeutics, TechLab, United Healthcare, Predicta-Med, and InveniAI, and is the editor for the IBD section of UptoDate.
AGA Resource
Planning for a family can be challenging, and if your patient has IBD, there are additional factors to consider. The AGA IBD Parenthood Project is the “go-to” resource for everything patients need to know about IBD and pregnancy throughout all stages of family planning.
While many gastroenterologists may be comfortable with inflammatory bowel disease (IBD), most are not experts in women’s concerns about pregnancy. One study found that, although women with IBD may have concerns about the interplay of their disease and reproductive health, many have not had extensive conversations with their gastroenterologist about it. In fact, that same study found most women expect their gastroenterologist to initiate these conversations.
In this roundtable discussion, Uma Mahadevan, MD, professor of medicine and the director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco; Marla C. Dubinsky, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York; and Sunanda V. Kane, MD, professor of medicine at Mayo Clinic in Rochester, Minn., share how they respond to these questions in their clinical practice.
What should a woman with IBD who is interested in having biological children in the future be thinking about now?
Dr. Mahadevan: Because active disease is associated with lower rates of conception and higher rates of pregnancy loss, women with IBD should first ensure they are in remission. I like to document endoscopic healing with a colonoscopy or sigmoidoscopy, but, if this has been done recently, a fecal calprotectin test can be helpful.
Women with IBD, particularly those with small bowel disease, are at risk for nutritional deficiencies, so prior to conception, I also check vitamin B-12, vitamin D, and iron, and repeat as needed. Zinc and folate can be considered. Those who are underweight should work with a nutritionist to ensure adequate caloric intake.
Dr. Dubinsky: I think it’s also important to stress the importance of taking their IBD medications because they can help patients achieve and maintain disease remission. Uncontrolled inflammation is a key risk factor for spontaneous abortion in the first trimester. Medication we would use in pregnancy is not putting them at risk for spontaneous abortion or congenital anomalies, which is what mothers to be are understandably most concerned about.
I am very honest and transparent with my patients: “About the only thing I need to take care of is you. If you are good, the baby is good.”
Dr. Kane: As Dr. Mahadevan mentioned, women with IBD are at higher risk for vitamin deficiencies, so those need to be corrected before conception. If they smoke, they should stop before conceiving.
There is no increased risk of infertility unless there has been a history of abdominal surgery.
Also, if women are not actively planning on getting pregnant, that would be important to share because some gastroenterologists will avoid certain effective medications if pregnancy is a possibility.
If a woman has had surgery for her IBD, could that make it harder for her to get pregnant?
Dr. Kane: Yes, it can because scar tissue may develop within the pelvis. However, if surgery is indicated to manage a patient’s IBD, then talk to the surgeon about ways that they might be able to reduce the risk of scar tissue formation.
Dr. Dubinsky: One thing to note is that almost all the data of infertility risk and scarring are based on open surgical techniques that involve dissection of the rectum. On the other hand, we don’t yet have enough prospective data on the impact of the modern era of laparoscopic surgery to suggest whether it affects fertility. More data is needed because providers may be giving women old information that is no longer relevant in the modern era.
If a woman is experiencing IBD symptoms, should she attempt to conceive?
Dr. Kane: Gastrointestinal symptoms in patients with IBD could be from active disease but also other things, so it’s important to have a thorough check-up to assess if there is active disease or not. Active disease can (but does not always) lead to a more complicated pregnancy, and conception is not recommended while a patient has active IBD.
Dr. Dubinsky: Although some patients feel an urgency to conceive regardless of disease activity, we need to do our due diligence and explain that we need to focus on getting them into the deepest remission possible, including endoscopic findings, biomarkers, and symptoms.
The most important gift you can give your future moms is to optimize the therapy they’re on before they conceive.
Is it important for someone who’s working with a gastroenterologist and an obstetrician to also work with a maternal-fetal medicine (MFM) specialist?
Dr. Kane: Having a diagnosis of IBD makes a woman’s pregnancy “high risk” because just having the diagnosis is associated with a higher risk of prematurity and small for gestational age – but importantly, not birth defects. A woman whose IBD is in remission should still have a discussion with an MFM specialist, just so everyone is on the same page.
Dr. Dubinsky: I refer to care with MFM specialists as “tighter monitoring.” I tell my patients that MFM specialists have managed many complex pregnancies and feel confident around the safety of their medications, understand the impact of when the baby may be exposed to certain medications, and will focus on following them more closely.
What are the risks of IBD medications during pregnancy and while breastfeeding? Should women stop their medications during pregnancy and breastfeeding?
Dr. Dubinsky: Organogenesis occurs in the first 10 weeks, so any medicines that cross the placenta during that time are up for discussion and debate. Methotrexate and the newer small molecules, such as Janus kinase (JAK) inhibitors and S1P receptor modulators, do cross the placenta during the first trimester and need to be discontinued before conception, sometimes as early as 3 months before conception.
However, biologics are very large proteins and do not cross the placenta until closer to week 27. We are not advocating stopping biologics in advance of conception, or during pregnancy, or during breastfeeding. There is more risk to stopping than continuing.
Dr. Mahadevan: Methotrexate should be stopped at least 3 months prior to conception and should not be taken during pregnancy.
There are limited antibiotic safety data in pregnancy for the longer periods of time used in IBD. I generally prefer amoxicillin/clavulanic acid over ciprofloxacin or metronidazole, but short term (less than 2 weeks) use of any of those three are not contraindicated.
Mesalamine agents and thiopurine monotherapy can be continued through pregnancy and breastfeeding.
Biologic agents, such as anti–tumor necrosis factor, anti-interleukin 23, anti-integrin, and biosimilars, can be continued through pregnancy and during breastfeeding. Given limited exposure in the first trimester, there is no evidence of increased risk of birth defects. As Dr. Dubinsky pointed out, there is active transfer, particularly in the third trimester and minimal transfer in breast milk, but this has not been associated with harm.
Lastly, small molecules, such as the JAK inhibitors tofacitinib and upadacitinib, as well as ozanimod, have virtually no human safety data during pregnancy, and animal data show harm. The use of these agents in pregnancy is not recommended.
Dr. Kane: As Dr. Dubinsky stated, most of the medications our patients take are low risk to continue through pregnancy if the patients are in remission. Although a woman “in remission” on steroids is not really in remission and should not get pregnant until she is on something else.
As far as breastfeeding goes, that should be stopped if the patient is on methotrexate, cyclosporine, or certain antibiotics. If she is on more than 20 mg of prednisone, this can pass to the infant, and a mother should not breastfeed.
Women should avoid fenugreek as a lactation aid, as that contains a compound that can promote bleeding. Lactation cookies are ok.
Otherwise, there are lots of potential benefits to breastfeeding, and I encourage it.
How is a flare treated if it occurs during pregnancy?
Dr. Dubinsky: A flare during pregnancy is treated the same as a flare outside of pregnancy. We want to use noninvasive ways to confirm it, but I think we don’t need to overly investigate in most of our women. If they’re already on a biologic, you may consider changing.
Some women may need corticosteroids. It’s not our favorite move, but there is an urgency to getting a flare under control during pregnancy because of possible complications.
Dr. Mahadevan: Some of this is contingent on when during pregnancy the flare occurs. A patient who has a flare at 38 weeks’ gestation will likely proceed with delivery and the flare will be dealt with separately. Someone at 8 weeks’ gestation is at high risk for pregnancy loss, so treatment should be quick and effective.
As does Dr. Dubinsky, I do try to avoid steroids if possible. For example, I would rather start an effective biologic right away than drag out steroids to see if they will respond.
Dr. Kane: I would add that, if a mother is losing weight, she might need to be hospitalized for additional nutritional support. If surgery is necessary, we usually try to time it for the second or third trimester.
What needs to be taken into consideration regarding mode of delivery? Also, if a woman has undergone prior surgeries, do they increase the risk of delivery complications?
For ulcerative colitis, mode of delivery is based on obstetric, not gastrointestinal, variables. For Crohn’s disease, if there is evidence of perianal disease, then a cesarean is appropriate.
If there is no history of perianal disease, then delivery is based on obstetric variables.
For a woman who has a J pouch, if possible, the surgeon who created it should be contacted to ask about the technical aspects of the pouch and how it lies in the pelvis.
What’s the risk of a postpartum flare if a woman’s IBD remains in clinical remission during pregnancy?
Dr. Mahadevan: There is no increased risk of postpartum flare if a woman continues her IBD medications after delivery. Many of the reports of flare are from stopping medications (mistakenly often) to breastfeed.
Dr. Kane: As Dr. Mahadevan said, the risk of a flare is usually because a woman stops taking her medications because she thinks that medication will be passed to the infant through breastfeeding, which in most cases is not true.
Otherwise, there is not an increased risk of a flare in a 12-month period. However, it is important to monitor for symptoms after delivery; the risk of a flare is not zero.
What symptoms should women watch out for after delivery that may indicate an uptick in disease activity?
Dr. Kane: The same symptoms as before they were pregnant. Diarrhea, abdominal pain, and rectal bleeding are not normal after delivery and should be considered signs of returning disease.
As a gastroenterologist, is there any additional advice you’d offer about conception, fertility, and pregnancy when treating women with IBD?
Dr. Mahadevan: Women with IBD should, when feasible, have a planned pregnancy when in documented remission and under the care of their gastroenterologists, obstetrician, and an MFM specialist. Life happens, and this is not always possible. That said, a woman with IBD has the same chance of getting pregnant as a woman of the same age without IBD, unless she has active disease or a history of pelvic surgery. Women with IBD in remission will generally have healthy pregnancies if they continue appropriate medications.
Dr. Kane: Agreed. The majority of women with IBD will have normal, healthy pregnancies. It is important for them to not stop their IBD therapy without talking to their gastroenterologist first. Well-intentioned but ignorant obstetricians or midwives may recommend stopping, but then panic when disease flares and the mother’s health is at risk. Active inflammation is the worst enemy to a pregnancy, not active therapy.
Dr. Dubinsky: One additional thing to consider is: How do we help women with IBD who have delivered meet the needs of their family and continue to stay on their meds and be in good inflammatory control?
For example, we can give the biologic in the hospital after they’ve had a cesarean or a vaginal delivery and before they leave. We know that that is safe, giving that to them before they leave the hospital is a huge value added.
Another thing is possibly changing their infusions to home infusions. That would be helpful for the moms as well.
Dr. Mahadevan reports being a consultant for AbbVie, Janssen, Pfizer, Gilead, Bristol-Myers Squibb, Takeda, Protagonist, Prometheus, and Boehringer Ingelheim. Dr. Dubinsky is a consultant for AbbVie, Arena, Bristol-Myers Squibb, Janssen, Eli Lilly, Takeda, and Prometheus BioSciences. She is a shareholder and CEO of a publicly traded company, Trellis Health. Dr. Kane is a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, Gilead, Janssen, Takeda, Seres Therapeutics, TechLab, United Healthcare, Predicta-Med, and InveniAI, and is the editor for the IBD section of UptoDate.
AGA Resource
Planning for a family can be challenging, and if your patient has IBD, there are additional factors to consider. The AGA IBD Parenthood Project is the “go-to” resource for everything patients need to know about IBD and pregnancy throughout all stages of family planning.
Patients complain some obesity care startups offer pills, and not much else
Many Americans turn to the latest big idea to lose weight – fad diets, fitness crazes, dodgy herbs and pills, bariatric surgery, just to name a few. They’re rarely the magic solution people dream of.
Now a wave of startups offer access to a new category of drugs coupled with intensive behavioral coaching online. But already concerns are emerging.
These startups, spurred by hundreds of millions of dollars in funding from blue-chip venture capital firms, have signed up well over 100,000 patients and could reach millions more. These patients pay hundreds, if not thousands, of dollars to access new drugs, called glucagonlike peptide–1 (GLP-1) agonists, along with online coaching to encourage healthy habits.
The startups initially positioned themselves in lofty terms. “This is the last weight-loss program you’ll try,” said a 2020 marketing analysis by startup Calibrate Health, in messaging designed to reach one of its target demographics, the “working mom.” (Company spokesperson Michelle Wellington said the document does not reflect Calibrate’s current marketing strategy.)
But while doctors and patients are intrigued by the new model, some customers complain online that reality is short of the buildup: They say they got canned advice and unresponsive clinicians – and some report they couldn’t get the newest drugs.
Calibrate Health, a New York City–based startup, reported earlier in 2022 it had served 20,000 people. Another startup, Found, headquartered in San Francisco, has served 135,000 patients since July 2020, CEO Sarah Jones Simmer said in an interview. Calibrate costs patients nearly $1,600 a year, not counting the price of drugs, which can hit nearly $1,500 monthly without insurance, according to drug price savings site GoodRx. (Insurers reimburse for GLP-1agonists in limited circumstances, patients said.) Found offers a 6-month plan for nearly $600, a company spokesperson said. (That price includes generic drugs, but not the newer GLP-1 agonists, like Wegovy.)
The two companies are beneficiaries of over $200 million in combined venture funding, according to tracking by Crunchbase, a repository of venture capital investments. The firms say they’re on the vanguard of weight care, both citing the influence of biology and other scientific factors as key ingredients to their approaches.
There’s potentially a big market for these startups. Just over 4 in 10 Americans are obese, according to the Centers for Disease Control and Prevention, driving up their risk for cardiovascular conditions and type 2 diabetes. Effective medical treatments are elusive and hard to access.
Centers that provide this specialty care “are overwhelmed,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General in Boston, a teaching hospital affiliated with Harvard. Her own clinic has a wait list of 3,000.
Dr. Stanford, who said she has advised several of these telemedicine startups, is bullish on their potential.
Scott Butsch, MD, director of obesity medicine at the Cleveland Clinic, said the startups can offer care with less judgment and stigma than in-person peers. They’re also more convenient.
Dr. Butsch, who learned about the model through consultancies, patients, and colleagues, wonders whether the startups are operating “to strategically find which patients respond to which drug.” He said they should coordinate well with behavioral specialists, as antidepressants or other medications may be driving weight gain. “Obesity is a complex disease and requires treatments that match its complexity. I think programs that do not have a multidisciplinary team are less comprehensive and, in the long term, less effective.”
The startups market a two-pronged product: first, the new class of GLP-1 agonists. While these medications are effective at provoking weight loss, Wegovy, one of two in this class specifically approved for this purpose, is in short supply because of manufacturing difficulties, according to its maker, Novo Nordisk. Others in the category can be prescribed off label. But doctors generally aren’t familiar with the medications, Stanford said. In theory, the startups can bridge some of those gaps: They offer more specialized, knowledgeable clinicians.
Then there’s the other prong: behavioral changes. The companies use televisits and online messaging with nutritionists or coaches to help patients incorporate new diet and exercise habits. The weight loss figures achieved by participants in clinical trials for the new drugs – up to 15% of body mass – were tied to such changes, according to Novo Nordisk.
Social media sites are bursting with these startups’ ads, everywhere from podcasts to Instagram. A search of Meta’s ad library finds 40,000 ads on Facebook and Instagram between the two firms.
The ads complement people’s own postings on social media: Numerous Facebook groups are devoted to the new type of drugs – some even focused on helping patients manage side effects, like changes in their bowel movements. The buzz is quantifiable: On TikTok, mentions of the new GLP-1 agonists tripled from last June to this June, according to an analysis by investment bankers at Morgan Stanley.
There’s now a feverish, expectant appetite for these medications among the startups’ clientele. Patients often complained that their friends had obtained a drug they weren’t offered, recalled Alexandra Coults, a former pharmacist consultant for Found. Ms. Coults said patients may have perceived some sort of bait-and-switch when in reality clinical reasons – like drug contraindications – guide prescribing decisions.
Patient expectations influence care, Ms. Coults said. Customers came in with ideas shaped by the culture of fad diets and New Year’s resolutions. “Quite a few people would sign up for 1 month and not continue.”
In interviews with KHN and in online complaints, patients also questioned the quality of care they received. Some said intake – which began by filling out a form and proceeded to an online visit with a doctor – was perfunctory. Once medication began, they said, requests for counseling about side effects were slow to be answered.
Jess Garrant, a Found patient, recalled that after she was prescribed zonisamide, a generic anticonvulsant that has shown some ability to help with weight loss, she felt “absolutely weird.”
“I was up all night and my thoughts were racing,” she wrote in a blog post. She developed sores in her mouth.
She sought advice and help from Found physicians, but their replies “weren’t quick.” Nonemergency communications are routed through the company’s portal.
It took a week to complete a switch of medications and have a new prescription arrive at her home, she said. Meanwhile, she said, she went to an urgent care clinic for the mouth sores.
Found frequently prescribes generic medications – often off label – rather than just the new GLP-1 agonists, company executives said in an interview. Found said older generics like zonisamide are more accessible than the GLP-1 agonists advertised on social media and their own website. Both Dr. Butsch and Dr. Stanford said they’ve prescribed zonisamide successfully. Dr. Butsch said ramping up dosage rapidly can increase the risk of side effects.
But Kim Boyd, MD, chief medical officer of competitor Calibrate, said the older drugs “just haven’t worked.”
Patients of both companies have critiqued online and in interviews the startups’ behavioral care – which experts across the board maintain is integral to successful weight loss treatment. But some patients felt they simply had canned advice.
Other patients said they had ups and downs with their coaches. Dana Crom, an attorney, said she had gone through many coaches with Calibrate. Some were good, effective cheerleaders; others, not so good. But when kinks in the program arose, she said, the coach wasn’t able to help her navigate them. While the coach can report trouble with medications or the app, it appears those reports are no more effective than messages sent through the portal, Ms. Crom said.
And what about when her yearlong subscription ends? Ms. Crom said she’d consider continuing with Calibrate.
Relationships with coaches, given the need to change behavior, are a critical element of the business models. Patients’ results depend “on how adherent they are to lifestyle changes,” said Found’s chief medical officer, Rehka Kumar, MD.
While the startups offer care to a larger geographic footprint, it’s not clear whether the demographics of their patient populations are different from those of the traditional bricks-and-mortar model. Calibrate’s patients are overwhelmingly White; over 8 in 10 have at least an undergraduate degree; and over 8 in 10 are women, according to the company.
And its earlier marketing strategies reflected that. The September 2020 “segmentation” document laid out three types of customers the company could hope to attract: perimenopausal or menopausal women, with income ranging from $75,000 to $150,000 a year; working mothers, with a similar income; and “men.”
Isabelle Kenyon, Calibrate’s CEO, said the company now hopes to expand its reach to partner with large employers, and that will help diversify its patients.
Patients will need to be convinced that the model – more affordable, more accessible – works for them. For her part, Ms. Garrant, who no longer is using Found, reflected on her experience, writing in her blog post that she was hoping for more follow-up and a more personal approach. “I don’t think it’s a helpful way to lose weight,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Many Americans turn to the latest big idea to lose weight – fad diets, fitness crazes, dodgy herbs and pills, bariatric surgery, just to name a few. They’re rarely the magic solution people dream of.
Now a wave of startups offer access to a new category of drugs coupled with intensive behavioral coaching online. But already concerns are emerging.
These startups, spurred by hundreds of millions of dollars in funding from blue-chip venture capital firms, have signed up well over 100,000 patients and could reach millions more. These patients pay hundreds, if not thousands, of dollars to access new drugs, called glucagonlike peptide–1 (GLP-1) agonists, along with online coaching to encourage healthy habits.
The startups initially positioned themselves in lofty terms. “This is the last weight-loss program you’ll try,” said a 2020 marketing analysis by startup Calibrate Health, in messaging designed to reach one of its target demographics, the “working mom.” (Company spokesperson Michelle Wellington said the document does not reflect Calibrate’s current marketing strategy.)
But while doctors and patients are intrigued by the new model, some customers complain online that reality is short of the buildup: They say they got canned advice and unresponsive clinicians – and some report they couldn’t get the newest drugs.
Calibrate Health, a New York City–based startup, reported earlier in 2022 it had served 20,000 people. Another startup, Found, headquartered in San Francisco, has served 135,000 patients since July 2020, CEO Sarah Jones Simmer said in an interview. Calibrate costs patients nearly $1,600 a year, not counting the price of drugs, which can hit nearly $1,500 monthly without insurance, according to drug price savings site GoodRx. (Insurers reimburse for GLP-1agonists in limited circumstances, patients said.) Found offers a 6-month plan for nearly $600, a company spokesperson said. (That price includes generic drugs, but not the newer GLP-1 agonists, like Wegovy.)
The two companies are beneficiaries of over $200 million in combined venture funding, according to tracking by Crunchbase, a repository of venture capital investments. The firms say they’re on the vanguard of weight care, both citing the influence of biology and other scientific factors as key ingredients to their approaches.
There’s potentially a big market for these startups. Just over 4 in 10 Americans are obese, according to the Centers for Disease Control and Prevention, driving up their risk for cardiovascular conditions and type 2 diabetes. Effective medical treatments are elusive and hard to access.
Centers that provide this specialty care “are overwhelmed,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General in Boston, a teaching hospital affiliated with Harvard. Her own clinic has a wait list of 3,000.
Dr. Stanford, who said she has advised several of these telemedicine startups, is bullish on their potential.
Scott Butsch, MD, director of obesity medicine at the Cleveland Clinic, said the startups can offer care with less judgment and stigma than in-person peers. They’re also more convenient.
Dr. Butsch, who learned about the model through consultancies, patients, and colleagues, wonders whether the startups are operating “to strategically find which patients respond to which drug.” He said they should coordinate well with behavioral specialists, as antidepressants or other medications may be driving weight gain. “Obesity is a complex disease and requires treatments that match its complexity. I think programs that do not have a multidisciplinary team are less comprehensive and, in the long term, less effective.”
The startups market a two-pronged product: first, the new class of GLP-1 agonists. While these medications are effective at provoking weight loss, Wegovy, one of two in this class specifically approved for this purpose, is in short supply because of manufacturing difficulties, according to its maker, Novo Nordisk. Others in the category can be prescribed off label. But doctors generally aren’t familiar with the medications, Stanford said. In theory, the startups can bridge some of those gaps: They offer more specialized, knowledgeable clinicians.
Then there’s the other prong: behavioral changes. The companies use televisits and online messaging with nutritionists or coaches to help patients incorporate new diet and exercise habits. The weight loss figures achieved by participants in clinical trials for the new drugs – up to 15% of body mass – were tied to such changes, according to Novo Nordisk.
Social media sites are bursting with these startups’ ads, everywhere from podcasts to Instagram. A search of Meta’s ad library finds 40,000 ads on Facebook and Instagram between the two firms.
The ads complement people’s own postings on social media: Numerous Facebook groups are devoted to the new type of drugs – some even focused on helping patients manage side effects, like changes in their bowel movements. The buzz is quantifiable: On TikTok, mentions of the new GLP-1 agonists tripled from last June to this June, according to an analysis by investment bankers at Morgan Stanley.
There’s now a feverish, expectant appetite for these medications among the startups’ clientele. Patients often complained that their friends had obtained a drug they weren’t offered, recalled Alexandra Coults, a former pharmacist consultant for Found. Ms. Coults said patients may have perceived some sort of bait-and-switch when in reality clinical reasons – like drug contraindications – guide prescribing decisions.
Patient expectations influence care, Ms. Coults said. Customers came in with ideas shaped by the culture of fad diets and New Year’s resolutions. “Quite a few people would sign up for 1 month and not continue.”
In interviews with KHN and in online complaints, patients also questioned the quality of care they received. Some said intake – which began by filling out a form and proceeded to an online visit with a doctor – was perfunctory. Once medication began, they said, requests for counseling about side effects were slow to be answered.
Jess Garrant, a Found patient, recalled that after she was prescribed zonisamide, a generic anticonvulsant that has shown some ability to help with weight loss, she felt “absolutely weird.”
“I was up all night and my thoughts were racing,” she wrote in a blog post. She developed sores in her mouth.
She sought advice and help from Found physicians, but their replies “weren’t quick.” Nonemergency communications are routed through the company’s portal.
It took a week to complete a switch of medications and have a new prescription arrive at her home, she said. Meanwhile, she said, she went to an urgent care clinic for the mouth sores.
Found frequently prescribes generic medications – often off label – rather than just the new GLP-1 agonists, company executives said in an interview. Found said older generics like zonisamide are more accessible than the GLP-1 agonists advertised on social media and their own website. Both Dr. Butsch and Dr. Stanford said they’ve prescribed zonisamide successfully. Dr. Butsch said ramping up dosage rapidly can increase the risk of side effects.
But Kim Boyd, MD, chief medical officer of competitor Calibrate, said the older drugs “just haven’t worked.”
Patients of both companies have critiqued online and in interviews the startups’ behavioral care – which experts across the board maintain is integral to successful weight loss treatment. But some patients felt they simply had canned advice.
Other patients said they had ups and downs with their coaches. Dana Crom, an attorney, said she had gone through many coaches with Calibrate. Some were good, effective cheerleaders; others, not so good. But when kinks in the program arose, she said, the coach wasn’t able to help her navigate them. While the coach can report trouble with medications or the app, it appears those reports are no more effective than messages sent through the portal, Ms. Crom said.
And what about when her yearlong subscription ends? Ms. Crom said she’d consider continuing with Calibrate.
Relationships with coaches, given the need to change behavior, are a critical element of the business models. Patients’ results depend “on how adherent they are to lifestyle changes,” said Found’s chief medical officer, Rehka Kumar, MD.
While the startups offer care to a larger geographic footprint, it’s not clear whether the demographics of their patient populations are different from those of the traditional bricks-and-mortar model. Calibrate’s patients are overwhelmingly White; over 8 in 10 have at least an undergraduate degree; and over 8 in 10 are women, according to the company.
And its earlier marketing strategies reflected that. The September 2020 “segmentation” document laid out three types of customers the company could hope to attract: perimenopausal or menopausal women, with income ranging from $75,000 to $150,000 a year; working mothers, with a similar income; and “men.”
Isabelle Kenyon, Calibrate’s CEO, said the company now hopes to expand its reach to partner with large employers, and that will help diversify its patients.
Patients will need to be convinced that the model – more affordable, more accessible – works for them. For her part, Ms. Garrant, who no longer is using Found, reflected on her experience, writing in her blog post that she was hoping for more follow-up and a more personal approach. “I don’t think it’s a helpful way to lose weight,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Many Americans turn to the latest big idea to lose weight – fad diets, fitness crazes, dodgy herbs and pills, bariatric surgery, just to name a few. They’re rarely the magic solution people dream of.
Now a wave of startups offer access to a new category of drugs coupled with intensive behavioral coaching online. But already concerns are emerging.
These startups, spurred by hundreds of millions of dollars in funding from blue-chip venture capital firms, have signed up well over 100,000 patients and could reach millions more. These patients pay hundreds, if not thousands, of dollars to access new drugs, called glucagonlike peptide–1 (GLP-1) agonists, along with online coaching to encourage healthy habits.
The startups initially positioned themselves in lofty terms. “This is the last weight-loss program you’ll try,” said a 2020 marketing analysis by startup Calibrate Health, in messaging designed to reach one of its target demographics, the “working mom.” (Company spokesperson Michelle Wellington said the document does not reflect Calibrate’s current marketing strategy.)
But while doctors and patients are intrigued by the new model, some customers complain online that reality is short of the buildup: They say they got canned advice and unresponsive clinicians – and some report they couldn’t get the newest drugs.
Calibrate Health, a New York City–based startup, reported earlier in 2022 it had served 20,000 people. Another startup, Found, headquartered in San Francisco, has served 135,000 patients since July 2020, CEO Sarah Jones Simmer said in an interview. Calibrate costs patients nearly $1,600 a year, not counting the price of drugs, which can hit nearly $1,500 monthly without insurance, according to drug price savings site GoodRx. (Insurers reimburse for GLP-1agonists in limited circumstances, patients said.) Found offers a 6-month plan for nearly $600, a company spokesperson said. (That price includes generic drugs, but not the newer GLP-1 agonists, like Wegovy.)
The two companies are beneficiaries of over $200 million in combined venture funding, according to tracking by Crunchbase, a repository of venture capital investments. The firms say they’re on the vanguard of weight care, both citing the influence of biology and other scientific factors as key ingredients to their approaches.
There’s potentially a big market for these startups. Just over 4 in 10 Americans are obese, according to the Centers for Disease Control and Prevention, driving up their risk for cardiovascular conditions and type 2 diabetes. Effective medical treatments are elusive and hard to access.
Centers that provide this specialty care “are overwhelmed,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General in Boston, a teaching hospital affiliated with Harvard. Her own clinic has a wait list of 3,000.
Dr. Stanford, who said she has advised several of these telemedicine startups, is bullish on their potential.
Scott Butsch, MD, director of obesity medicine at the Cleveland Clinic, said the startups can offer care with less judgment and stigma than in-person peers. They’re also more convenient.
Dr. Butsch, who learned about the model through consultancies, patients, and colleagues, wonders whether the startups are operating “to strategically find which patients respond to which drug.” He said they should coordinate well with behavioral specialists, as antidepressants or other medications may be driving weight gain. “Obesity is a complex disease and requires treatments that match its complexity. I think programs that do not have a multidisciplinary team are less comprehensive and, in the long term, less effective.”
The startups market a two-pronged product: first, the new class of GLP-1 agonists. While these medications are effective at provoking weight loss, Wegovy, one of two in this class specifically approved for this purpose, is in short supply because of manufacturing difficulties, according to its maker, Novo Nordisk. Others in the category can be prescribed off label. But doctors generally aren’t familiar with the medications, Stanford said. In theory, the startups can bridge some of those gaps: They offer more specialized, knowledgeable clinicians.
Then there’s the other prong: behavioral changes. The companies use televisits and online messaging with nutritionists or coaches to help patients incorporate new diet and exercise habits. The weight loss figures achieved by participants in clinical trials for the new drugs – up to 15% of body mass – were tied to such changes, according to Novo Nordisk.
Social media sites are bursting with these startups’ ads, everywhere from podcasts to Instagram. A search of Meta’s ad library finds 40,000 ads on Facebook and Instagram between the two firms.
The ads complement people’s own postings on social media: Numerous Facebook groups are devoted to the new type of drugs – some even focused on helping patients manage side effects, like changes in their bowel movements. The buzz is quantifiable: On TikTok, mentions of the new GLP-1 agonists tripled from last June to this June, according to an analysis by investment bankers at Morgan Stanley.
There’s now a feverish, expectant appetite for these medications among the startups’ clientele. Patients often complained that their friends had obtained a drug they weren’t offered, recalled Alexandra Coults, a former pharmacist consultant for Found. Ms. Coults said patients may have perceived some sort of bait-and-switch when in reality clinical reasons – like drug contraindications – guide prescribing decisions.
Patient expectations influence care, Ms. Coults said. Customers came in with ideas shaped by the culture of fad diets and New Year’s resolutions. “Quite a few people would sign up for 1 month and not continue.”
In interviews with KHN and in online complaints, patients also questioned the quality of care they received. Some said intake – which began by filling out a form and proceeded to an online visit with a doctor – was perfunctory. Once medication began, they said, requests for counseling about side effects were slow to be answered.
Jess Garrant, a Found patient, recalled that after she was prescribed zonisamide, a generic anticonvulsant that has shown some ability to help with weight loss, she felt “absolutely weird.”
“I was up all night and my thoughts were racing,” she wrote in a blog post. She developed sores in her mouth.
She sought advice and help from Found physicians, but their replies “weren’t quick.” Nonemergency communications are routed through the company’s portal.
It took a week to complete a switch of medications and have a new prescription arrive at her home, she said. Meanwhile, she said, she went to an urgent care clinic for the mouth sores.
Found frequently prescribes generic medications – often off label – rather than just the new GLP-1 agonists, company executives said in an interview. Found said older generics like zonisamide are more accessible than the GLP-1 agonists advertised on social media and their own website. Both Dr. Butsch and Dr. Stanford said they’ve prescribed zonisamide successfully. Dr. Butsch said ramping up dosage rapidly can increase the risk of side effects.
But Kim Boyd, MD, chief medical officer of competitor Calibrate, said the older drugs “just haven’t worked.”
Patients of both companies have critiqued online and in interviews the startups’ behavioral care – which experts across the board maintain is integral to successful weight loss treatment. But some patients felt they simply had canned advice.
Other patients said they had ups and downs with their coaches. Dana Crom, an attorney, said she had gone through many coaches with Calibrate. Some were good, effective cheerleaders; others, not so good. But when kinks in the program arose, she said, the coach wasn’t able to help her navigate them. While the coach can report trouble with medications or the app, it appears those reports are no more effective than messages sent through the portal, Ms. Crom said.
And what about when her yearlong subscription ends? Ms. Crom said she’d consider continuing with Calibrate.
Relationships with coaches, given the need to change behavior, are a critical element of the business models. Patients’ results depend “on how adherent they are to lifestyle changes,” said Found’s chief medical officer, Rehka Kumar, MD.
While the startups offer care to a larger geographic footprint, it’s not clear whether the demographics of their patient populations are different from those of the traditional bricks-and-mortar model. Calibrate’s patients are overwhelmingly White; over 8 in 10 have at least an undergraduate degree; and over 8 in 10 are women, according to the company.
And its earlier marketing strategies reflected that. The September 2020 “segmentation” document laid out three types of customers the company could hope to attract: perimenopausal or menopausal women, with income ranging from $75,000 to $150,000 a year; working mothers, with a similar income; and “men.”
Isabelle Kenyon, Calibrate’s CEO, said the company now hopes to expand its reach to partner with large employers, and that will help diversify its patients.
Patients will need to be convinced that the model – more affordable, more accessible – works for them. For her part, Ms. Garrant, who no longer is using Found, reflected on her experience, writing in her blog post that she was hoping for more follow-up and a more personal approach. “I don’t think it’s a helpful way to lose weight,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Children and COVID: Weekly cases continue to hold fairly steady
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.
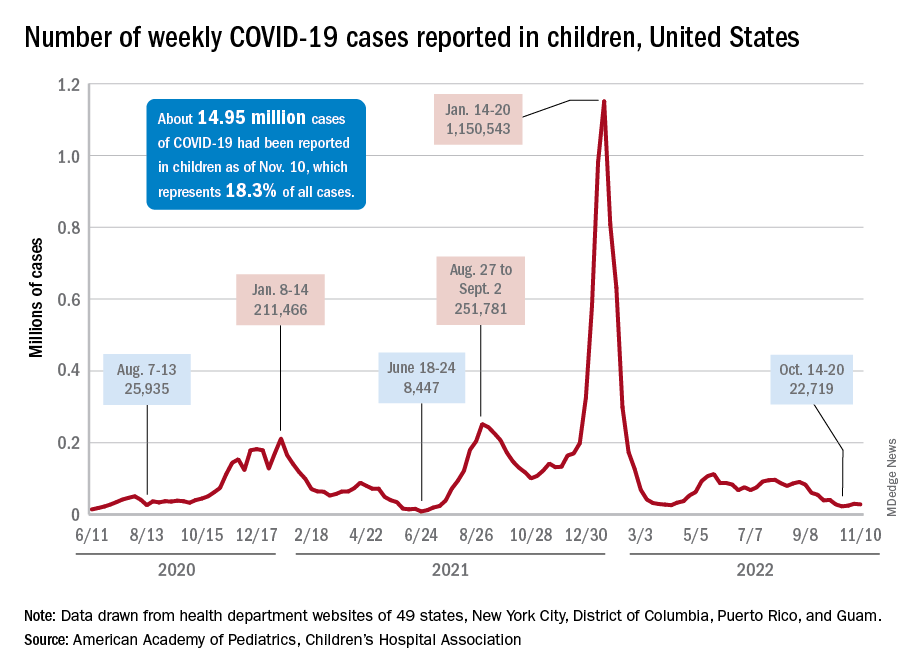
The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
Analysis affirms that giving birth protects against endometrial cancer
Compared with having no children, the risk reduction for endometrial cancer was 21% with having one child, 38% with having two, and 51% with having three, Gunn-Helen Moen, MSc, PhD, a research fellow at the University of Queensland Institute for Molecular Bioscience in St. Lucia, Australia, and the senior author of the study, said in an email.
In the United States, the prevalence of endometrial cancer is 25.7 per 100,000 women per year, with a lifetime risk of 2.8%.
Multiple observational studies have linked giving birth to risk of endometrial cancer. For the new study, Dr. Moen and her team assessed various risk factors related to ovulation and reproductive function using Mendelian randomization, an epidemiological technique that deploys genetic variants to detect cause-and-effect relationships between potentially modifiable risk factors and health outcomes in observational data.
The researcher published their findings in BMC Medicine.
Leverage genetic data
The study used detailed genetic and health data from the UK Biobank, a databank with more than half a million participants. Genetic variants related to some of the risk factors were used to assess whether the variants make people more likely to develop endometrial cancer.
Genomewide significant single-nucleotide polymorphisms (SNPs) related to number of live births, age at menopause and menarche, and body mass index (BMI) had been identified in previous studies, the researchers reported. They conducted genomewide association analyses of the databank to identify SNPs associated with years ovulating, years using the contraceptive pill, and age at last live birth.
The MR analysis showed a potential causal effect for the number of live births (inverse variance–weighted odds ratio, 0.537) and number of years ovulating (IVW OR, 1.051), in addition to known risk factors of BMI, age at menarche, and age at menopause.
A further multivariable MR analysis showed that number of births had a negative causal effect on endometrial cancer risk (OR, 0.783), independent of the causal effect of known risk factors such as BMI, age at menarche and age at menopause.
Reported limitations included being unable to perform MR analyses on some factors, such as oral contraceptive use, because of a lack of valid genetic instruments. The researchers could not perform an age adjustment at diagnosis because of a lack of data.
In addition, the genetic data came exclusively from White women of European ancestry.
‘A personal choice’
Other investigators have hypothesized that the protective effect of childbirth may be caused by shedding of malignant and premalignant endometrial cells during and after childbirth and exposure to high levels of progesterone in late stages of pregnancy, the research team noted.
Dr. Moen said, based on the results, physicians might consider number of childbirths in assessing a patient’s risk of endometrial cancer.
However, Britton Trabert, MSPH, MS, PhD, an epidemiologist and assistant professor of obstetrics and gynecology at the University of Utah, Salt Lake City, said it’s unlikely the findings will affect clinical practice given that they “largely replicate well-characterized endometrial cancer risk associations.”
“Pregnancy and childbirth are a personal choice and is not largely regarded as a modifiable factor for cancer prevention,” said Dr. Trabert, who was not involved in the study.
The study’s investigators reported funding from the governments of Australia, Norway and the United Kingdom and the British Heart Foundation. No financial conflicts of interest were reported. Dr. Trabert reported no relevant financial interests.
Compared with having no children, the risk reduction for endometrial cancer was 21% with having one child, 38% with having two, and 51% with having three, Gunn-Helen Moen, MSc, PhD, a research fellow at the University of Queensland Institute for Molecular Bioscience in St. Lucia, Australia, and the senior author of the study, said in an email.
In the United States, the prevalence of endometrial cancer is 25.7 per 100,000 women per year, with a lifetime risk of 2.8%.
Multiple observational studies have linked giving birth to risk of endometrial cancer. For the new study, Dr. Moen and her team assessed various risk factors related to ovulation and reproductive function using Mendelian randomization, an epidemiological technique that deploys genetic variants to detect cause-and-effect relationships between potentially modifiable risk factors and health outcomes in observational data.
The researcher published their findings in BMC Medicine.
Leverage genetic data
The study used detailed genetic and health data from the UK Biobank, a databank with more than half a million participants. Genetic variants related to some of the risk factors were used to assess whether the variants make people more likely to develop endometrial cancer.
Genomewide significant single-nucleotide polymorphisms (SNPs) related to number of live births, age at menopause and menarche, and body mass index (BMI) had been identified in previous studies, the researchers reported. They conducted genomewide association analyses of the databank to identify SNPs associated with years ovulating, years using the contraceptive pill, and age at last live birth.
The MR analysis showed a potential causal effect for the number of live births (inverse variance–weighted odds ratio, 0.537) and number of years ovulating (IVW OR, 1.051), in addition to known risk factors of BMI, age at menarche, and age at menopause.
A further multivariable MR analysis showed that number of births had a negative causal effect on endometrial cancer risk (OR, 0.783), independent of the causal effect of known risk factors such as BMI, age at menarche and age at menopause.
Reported limitations included being unable to perform MR analyses on some factors, such as oral contraceptive use, because of a lack of valid genetic instruments. The researchers could not perform an age adjustment at diagnosis because of a lack of data.
In addition, the genetic data came exclusively from White women of European ancestry.
‘A personal choice’
Other investigators have hypothesized that the protective effect of childbirth may be caused by shedding of malignant and premalignant endometrial cells during and after childbirth and exposure to high levels of progesterone in late stages of pregnancy, the research team noted.
Dr. Moen said, based on the results, physicians might consider number of childbirths in assessing a patient’s risk of endometrial cancer.
However, Britton Trabert, MSPH, MS, PhD, an epidemiologist and assistant professor of obstetrics and gynecology at the University of Utah, Salt Lake City, said it’s unlikely the findings will affect clinical practice given that they “largely replicate well-characterized endometrial cancer risk associations.”
“Pregnancy and childbirth are a personal choice and is not largely regarded as a modifiable factor for cancer prevention,” said Dr. Trabert, who was not involved in the study.
The study’s investigators reported funding from the governments of Australia, Norway and the United Kingdom and the British Heart Foundation. No financial conflicts of interest were reported. Dr. Trabert reported no relevant financial interests.
Compared with having no children, the risk reduction for endometrial cancer was 21% with having one child, 38% with having two, and 51% with having three, Gunn-Helen Moen, MSc, PhD, a research fellow at the University of Queensland Institute for Molecular Bioscience in St. Lucia, Australia, and the senior author of the study, said in an email.
In the United States, the prevalence of endometrial cancer is 25.7 per 100,000 women per year, with a lifetime risk of 2.8%.
Multiple observational studies have linked giving birth to risk of endometrial cancer. For the new study, Dr. Moen and her team assessed various risk factors related to ovulation and reproductive function using Mendelian randomization, an epidemiological technique that deploys genetic variants to detect cause-and-effect relationships between potentially modifiable risk factors and health outcomes in observational data.
The researcher published their findings in BMC Medicine.
Leverage genetic data
The study used detailed genetic and health data from the UK Biobank, a databank with more than half a million participants. Genetic variants related to some of the risk factors were used to assess whether the variants make people more likely to develop endometrial cancer.
Genomewide significant single-nucleotide polymorphisms (SNPs) related to number of live births, age at menopause and menarche, and body mass index (BMI) had been identified in previous studies, the researchers reported. They conducted genomewide association analyses of the databank to identify SNPs associated with years ovulating, years using the contraceptive pill, and age at last live birth.
The MR analysis showed a potential causal effect for the number of live births (inverse variance–weighted odds ratio, 0.537) and number of years ovulating (IVW OR, 1.051), in addition to known risk factors of BMI, age at menarche, and age at menopause.
A further multivariable MR analysis showed that number of births had a negative causal effect on endometrial cancer risk (OR, 0.783), independent of the causal effect of known risk factors such as BMI, age at menarche and age at menopause.
Reported limitations included being unable to perform MR analyses on some factors, such as oral contraceptive use, because of a lack of valid genetic instruments. The researchers could not perform an age adjustment at diagnosis because of a lack of data.
In addition, the genetic data came exclusively from White women of European ancestry.
‘A personal choice’
Other investigators have hypothesized that the protective effect of childbirth may be caused by shedding of malignant and premalignant endometrial cells during and after childbirth and exposure to high levels of progesterone in late stages of pregnancy, the research team noted.
Dr. Moen said, based on the results, physicians might consider number of childbirths in assessing a patient’s risk of endometrial cancer.
However, Britton Trabert, MSPH, MS, PhD, an epidemiologist and assistant professor of obstetrics and gynecology at the University of Utah, Salt Lake City, said it’s unlikely the findings will affect clinical practice given that they “largely replicate well-characterized endometrial cancer risk associations.”
“Pregnancy and childbirth are a personal choice and is not largely regarded as a modifiable factor for cancer prevention,” said Dr. Trabert, who was not involved in the study.
The study’s investigators reported funding from the governments of Australia, Norway and the United Kingdom and the British Heart Foundation. No financial conflicts of interest were reported. Dr. Trabert reported no relevant financial interests.
FROM BMC MEDICINE
Teclistamab for MM: Lifesaver or 'cause of death'?
Following “unprecedented” results in a phase 1/2 study, teclistamab (Tecvayli, Janssen Biotech) received accelerated approval from the Food and Drug Administration for adults with relapsed/refractory multiple myeloma who had received at least four lines of therapy. Typically, patients in this situation have just a few weeks to live.
This is “unprecedented” said Nikhil Munshi, MD, professor of medicine at Harvard Medical School, Boston, who was not involved with the study. “Pomalidomide got approved with 30% response rate, carfilzomib got approved with 29% response rate, selinexor got approved with 31% response rate and so on and on. ... So here is teclistamab with [this] response rate in patients having five, six lines of treatment. ...[It’s] going to be so much in demand because it’s a great drug.”
The first cut of the data appeared in the New England Journal of Medicine.
At the 6-month mark, 90.6% of patients who responded had no progression of their disease, and at 9 months, 66.5% of patients were still holding steady.
Senior investigator in the trial, Saad Usmani, MD, of Memorial Sloan Kettering Cancer Center, New York, said: “What was most striking was the high response rates and the durability of response.”
Dr. Usmani said ease of administration was the other aspect of teclistamab that impressed him. The drug is given by subcutaneous injection weekly after a short ramp-up period.
He contrasted this regimen with that of chimeric antigen receptor (CAR) T-cell therapy, the only alternative with similar efficacy in such sick patients: “I can prescribe [teclistamab] today, and my patient gets it tomorrow,” Dr. Usmani said. “With CAR T, I prescribe today and it will take 4-6 weeks for us to collect T cells and another 6-7 weeks for the product to come back.” Dr. Usmani said many patients die before CAR T reaches them.
Community oncology will benefit greatly from teclistamab, especially patients for whom CAR T isn’t feasible, said Kashyap Patel, MD, president of the Community Oncology Alliance. “My patients are most of them underserved minority-class populations with myeloma, and they cannot travel many miles to go to a CAR T center. With sub[cutaneous] injection, the patient can have [teclistamab] administered in their doctor’s office and continue to live their normal life.”
However, how should the wider oncology community make sense of a drug approval based solely on response in a single-arm, phase 1/2 study, with no survival data?
Dr. Patel said, “Phase 1 plus phase 2 data is probably a little bit quick, but time will tell eventually.” He cited melflufen as a cautionary tale: a product given accelerated approval for multiple myeloma, then withdrawn when new data showed that it increased the risk of death.
When Dr. Munshi was asked about trial design for accelerated approvals, he responded, “you are touching a topic very close to my heart, a topic of great significance currently.”
He went on to say that overall survival (OS) is no longer a viable trial endpoint in diseases like multiple myeloma for several reasons. Most significantly, he noted: “Survival has gone up to 10 or 15 years [so] today, if you randomize between one [drug] versus another, there are going to be seven or eight more treatments before the patient dies.”
Similarly, progression-free survival (PFS) in multiple myeloma is now as much as 5 years, Dr. Munshi said. “Do we want a patient to wait 5 years to get a very good new drug?”
For these reasons and others, Dr, Munshi observed, myeloma researchers are increasingly relying on a surrogate called “negative minimal residual disease” (negative MRD) – in other words, a situation in which myeloma cells can no longer be detected in the bone marrow. MRD is hunted out using next-generation flow or next-generation sequencing of myeloma-cell DNA from bone-marrow aspirate to levels as low as 1 in 100,000 or 1,000,000 cells.
In 2020, Dr. Munshi and colleagues published a large meta-analysis showing that a negative MRD in a patient with multiple myeloma was significantly prognostic for both progression-free survival (hazard ratio, 0.33; P < .001) and overall survival (HR, 0.45; P < .001). The team concluded: “MRD can fulfill all the prerequisites to be a clinically valid surrogate biomarker for PFS and OS in [multiple myeloma].”
In MajesTEC-1 overall, 26.7% of patients on teclistamab had no signs of residual disease at a threshold of 1 in 100,000. Among patients who showed a “complete response” by International Myeloma Working Group criteria, 46% had no residual disease.
Dr. Munshi stressed that such patients are not necessarily “cured.” It will take a few more years to prove that. He noted: “Simply, physiologically, [negative MRD] means that if a patient has one [myeloma] cell in a million, that cell is going to take a much longer time to grow up to be myeloma.”
On Nov. 8 and 9, the FDA and the International Myeloma Society held a workshop to discuss the vexed question of surrogate endpoints and single-arm studies for drug approvals entitled the “Future of Drug Development in Multiple Myeloma.” Dr. Munshi was cochair.
A panelist at the meeting who was a senior investigator in the MajesTEC-1 trial, Ajai Chari, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, summed up the dilemma: “No one disagrees that randomized studies are the best way of doing things. The question is, if you’re a patient who’s exhausted all available therapies, do you have that time to wait? ... The role of accelerated approval is to get the drug to the patient faster. But what does it not pick up? How do we make these accelerated approvals more meaningful and not have to retract for safety?”
Jonathon Vallejo, also on the panel, agreed that safety was the key worry. The ideal scenario for accelerated approval would be a drug that was better than available therapy, and “in some sense, it’s much safer.” However, such situations are rare.
“Most of the time, we don’t have these products that come in that have no toxicity signals,” he said. “So one thing we have to think carefully about in the single-arm trial setting is, what are the toxicities? How do they stack up?”
Dr. Chari said that, for his part, he wanted to see more transparency around “cause of death” in all studies that lead to accelerated approvals. He said he was “tired” of seeing a death labeled as “not attributed” to the drug by the investigator or the drug company.
“Let me decide. Show me the deaths, and show me the myeloma status at that point,” Dr. Chari said. “That’s a signal – if you’re a responding patient and dying, then the FDA should be a little bit more cautious.”
The FDA has added a boxed warning to the teclistamab product information concerning cytokine-release syndrome and neurologic toxicity.
Cytokine-release syndrome, the most common side effect overall, showed up in 72% of patients, typically 2 days after the first step-up dose.
Neurologic toxicity occurred in 57% of patients, including headache (25%), motor dysfunction (16%), sensory neuropathy (15%) and encephalopathy (13%). About 6%of patients developed a serious, life-threatening neurologic condition called immune effector cell–associated neurotoxicity syndrome.
Overall, serious adverse reactions occurred in 54% of participants in MajesTEC-1, and 5% of people in the trial died from adverse reactions during the study, most commonly infections.
Because of its safety profile, teclistamab is available only through a restricted program called TECVAYLI Risk Evaluation and Mitigation Strategy.
The continued approval of teclistamab for this indication “may be contingent upon verification and description of clinical benefit in confirmatory trials,” according to the FDA.
To that end, eight more studies of teclistamab are underway, aiming for approximately 1,300 multiple myeloma patients around the world. Three of these trials are in newly diagnosed patients. Four more studies are planned to come online in the next 3 months, raising the final tally of patients testing out teclistamab to approximately 4,700. The trials will look at teclistamab in sequence or in combination with standards such as bortezomib and pomalidomide. All studies are open label.
Dr. Patel believes that, until these trials say otherwise, the benefits of teclistamab outweigh the risks. “I’m very happy we have one more option in this space, particularly the fourth or fifth line for patients who want to continue to fight the disease,” Dr. Patel concluded.
Dr. Munshi disclosed advisory board/consultant work for Adaptive, Abbvie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Karyopharm, Legend, Millennium, Novartis, Oncopep, and Pfizer and is the scientific founder of Oncopep and DCT. The 2020 meta-analysis by Dr. Munshi and colleagues was funded by Janssen-Cilag. Dr. Patel declared funding from Janssen for a diversity-equity initiative and membership of the South Carolina Medicaid P & T Committee. Dr. Usmani declared conflicts of interest with Amgen, BMS/Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, Abbvie, Genentech, Gilead, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio.
Following “unprecedented” results in a phase 1/2 study, teclistamab (Tecvayli, Janssen Biotech) received accelerated approval from the Food and Drug Administration for adults with relapsed/refractory multiple myeloma who had received at least four lines of therapy. Typically, patients in this situation have just a few weeks to live.
This is “unprecedented” said Nikhil Munshi, MD, professor of medicine at Harvard Medical School, Boston, who was not involved with the study. “Pomalidomide got approved with 30% response rate, carfilzomib got approved with 29% response rate, selinexor got approved with 31% response rate and so on and on. ... So here is teclistamab with [this] response rate in patients having five, six lines of treatment. ...[It’s] going to be so much in demand because it’s a great drug.”
The first cut of the data appeared in the New England Journal of Medicine.
At the 6-month mark, 90.6% of patients who responded had no progression of their disease, and at 9 months, 66.5% of patients were still holding steady.
Senior investigator in the trial, Saad Usmani, MD, of Memorial Sloan Kettering Cancer Center, New York, said: “What was most striking was the high response rates and the durability of response.”
Dr. Usmani said ease of administration was the other aspect of teclistamab that impressed him. The drug is given by subcutaneous injection weekly after a short ramp-up period.
He contrasted this regimen with that of chimeric antigen receptor (CAR) T-cell therapy, the only alternative with similar efficacy in such sick patients: “I can prescribe [teclistamab] today, and my patient gets it tomorrow,” Dr. Usmani said. “With CAR T, I prescribe today and it will take 4-6 weeks for us to collect T cells and another 6-7 weeks for the product to come back.” Dr. Usmani said many patients die before CAR T reaches them.
Community oncology will benefit greatly from teclistamab, especially patients for whom CAR T isn’t feasible, said Kashyap Patel, MD, president of the Community Oncology Alliance. “My patients are most of them underserved minority-class populations with myeloma, and they cannot travel many miles to go to a CAR T center. With sub[cutaneous] injection, the patient can have [teclistamab] administered in their doctor’s office and continue to live their normal life.”
However, how should the wider oncology community make sense of a drug approval based solely on response in a single-arm, phase 1/2 study, with no survival data?
Dr. Patel said, “Phase 1 plus phase 2 data is probably a little bit quick, but time will tell eventually.” He cited melflufen as a cautionary tale: a product given accelerated approval for multiple myeloma, then withdrawn when new data showed that it increased the risk of death.
When Dr. Munshi was asked about trial design for accelerated approvals, he responded, “you are touching a topic very close to my heart, a topic of great significance currently.”
He went on to say that overall survival (OS) is no longer a viable trial endpoint in diseases like multiple myeloma for several reasons. Most significantly, he noted: “Survival has gone up to 10 or 15 years [so] today, if you randomize between one [drug] versus another, there are going to be seven or eight more treatments before the patient dies.”
Similarly, progression-free survival (PFS) in multiple myeloma is now as much as 5 years, Dr. Munshi said. “Do we want a patient to wait 5 years to get a very good new drug?”
For these reasons and others, Dr, Munshi observed, myeloma researchers are increasingly relying on a surrogate called “negative minimal residual disease” (negative MRD) – in other words, a situation in which myeloma cells can no longer be detected in the bone marrow. MRD is hunted out using next-generation flow or next-generation sequencing of myeloma-cell DNA from bone-marrow aspirate to levels as low as 1 in 100,000 or 1,000,000 cells.
In 2020, Dr. Munshi and colleagues published a large meta-analysis showing that a negative MRD in a patient with multiple myeloma was significantly prognostic for both progression-free survival (hazard ratio, 0.33; P < .001) and overall survival (HR, 0.45; P < .001). The team concluded: “MRD can fulfill all the prerequisites to be a clinically valid surrogate biomarker for PFS and OS in [multiple myeloma].”
In MajesTEC-1 overall, 26.7% of patients on teclistamab had no signs of residual disease at a threshold of 1 in 100,000. Among patients who showed a “complete response” by International Myeloma Working Group criteria, 46% had no residual disease.
Dr. Munshi stressed that such patients are not necessarily “cured.” It will take a few more years to prove that. He noted: “Simply, physiologically, [negative MRD] means that if a patient has one [myeloma] cell in a million, that cell is going to take a much longer time to grow up to be myeloma.”
On Nov. 8 and 9, the FDA and the International Myeloma Society held a workshop to discuss the vexed question of surrogate endpoints and single-arm studies for drug approvals entitled the “Future of Drug Development in Multiple Myeloma.” Dr. Munshi was cochair.
A panelist at the meeting who was a senior investigator in the MajesTEC-1 trial, Ajai Chari, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, summed up the dilemma: “No one disagrees that randomized studies are the best way of doing things. The question is, if you’re a patient who’s exhausted all available therapies, do you have that time to wait? ... The role of accelerated approval is to get the drug to the patient faster. But what does it not pick up? How do we make these accelerated approvals more meaningful and not have to retract for safety?”
Jonathon Vallejo, also on the panel, agreed that safety was the key worry. The ideal scenario for accelerated approval would be a drug that was better than available therapy, and “in some sense, it’s much safer.” However, such situations are rare.
“Most of the time, we don’t have these products that come in that have no toxicity signals,” he said. “So one thing we have to think carefully about in the single-arm trial setting is, what are the toxicities? How do they stack up?”
Dr. Chari said that, for his part, he wanted to see more transparency around “cause of death” in all studies that lead to accelerated approvals. He said he was “tired” of seeing a death labeled as “not attributed” to the drug by the investigator or the drug company.
“Let me decide. Show me the deaths, and show me the myeloma status at that point,” Dr. Chari said. “That’s a signal – if you’re a responding patient and dying, then the FDA should be a little bit more cautious.”
The FDA has added a boxed warning to the teclistamab product information concerning cytokine-release syndrome and neurologic toxicity.
Cytokine-release syndrome, the most common side effect overall, showed up in 72% of patients, typically 2 days after the first step-up dose.
Neurologic toxicity occurred in 57% of patients, including headache (25%), motor dysfunction (16%), sensory neuropathy (15%) and encephalopathy (13%). About 6%of patients developed a serious, life-threatening neurologic condition called immune effector cell–associated neurotoxicity syndrome.
Overall, serious adverse reactions occurred in 54% of participants in MajesTEC-1, and 5% of people in the trial died from adverse reactions during the study, most commonly infections.
Because of its safety profile, teclistamab is available only through a restricted program called TECVAYLI Risk Evaluation and Mitigation Strategy.
The continued approval of teclistamab for this indication “may be contingent upon verification and description of clinical benefit in confirmatory trials,” according to the FDA.
To that end, eight more studies of teclistamab are underway, aiming for approximately 1,300 multiple myeloma patients around the world. Three of these trials are in newly diagnosed patients. Four more studies are planned to come online in the next 3 months, raising the final tally of patients testing out teclistamab to approximately 4,700. The trials will look at teclistamab in sequence or in combination with standards such as bortezomib and pomalidomide. All studies are open label.
Dr. Patel believes that, until these trials say otherwise, the benefits of teclistamab outweigh the risks. “I’m very happy we have one more option in this space, particularly the fourth or fifth line for patients who want to continue to fight the disease,” Dr. Patel concluded.
Dr. Munshi disclosed advisory board/consultant work for Adaptive, Abbvie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Karyopharm, Legend, Millennium, Novartis, Oncopep, and Pfizer and is the scientific founder of Oncopep and DCT. The 2020 meta-analysis by Dr. Munshi and colleagues was funded by Janssen-Cilag. Dr. Patel declared funding from Janssen for a diversity-equity initiative and membership of the South Carolina Medicaid P & T Committee. Dr. Usmani declared conflicts of interest with Amgen, BMS/Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, Abbvie, Genentech, Gilead, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio.
Following “unprecedented” results in a phase 1/2 study, teclistamab (Tecvayli, Janssen Biotech) received accelerated approval from the Food and Drug Administration for adults with relapsed/refractory multiple myeloma who had received at least four lines of therapy. Typically, patients in this situation have just a few weeks to live.
This is “unprecedented” said Nikhil Munshi, MD, professor of medicine at Harvard Medical School, Boston, who was not involved with the study. “Pomalidomide got approved with 30% response rate, carfilzomib got approved with 29% response rate, selinexor got approved with 31% response rate and so on and on. ... So here is teclistamab with [this] response rate in patients having five, six lines of treatment. ...[It’s] going to be so much in demand because it’s a great drug.”
The first cut of the data appeared in the New England Journal of Medicine.
At the 6-month mark, 90.6% of patients who responded had no progression of their disease, and at 9 months, 66.5% of patients were still holding steady.
Senior investigator in the trial, Saad Usmani, MD, of Memorial Sloan Kettering Cancer Center, New York, said: “What was most striking was the high response rates and the durability of response.”
Dr. Usmani said ease of administration was the other aspect of teclistamab that impressed him. The drug is given by subcutaneous injection weekly after a short ramp-up period.
He contrasted this regimen with that of chimeric antigen receptor (CAR) T-cell therapy, the only alternative with similar efficacy in such sick patients: “I can prescribe [teclistamab] today, and my patient gets it tomorrow,” Dr. Usmani said. “With CAR T, I prescribe today and it will take 4-6 weeks for us to collect T cells and another 6-7 weeks for the product to come back.” Dr. Usmani said many patients die before CAR T reaches them.
Community oncology will benefit greatly from teclistamab, especially patients for whom CAR T isn’t feasible, said Kashyap Patel, MD, president of the Community Oncology Alliance. “My patients are most of them underserved minority-class populations with myeloma, and they cannot travel many miles to go to a CAR T center. With sub[cutaneous] injection, the patient can have [teclistamab] administered in their doctor’s office and continue to live their normal life.”
However, how should the wider oncology community make sense of a drug approval based solely on response in a single-arm, phase 1/2 study, with no survival data?
Dr. Patel said, “Phase 1 plus phase 2 data is probably a little bit quick, but time will tell eventually.” He cited melflufen as a cautionary tale: a product given accelerated approval for multiple myeloma, then withdrawn when new data showed that it increased the risk of death.
When Dr. Munshi was asked about trial design for accelerated approvals, he responded, “you are touching a topic very close to my heart, a topic of great significance currently.”
He went on to say that overall survival (OS) is no longer a viable trial endpoint in diseases like multiple myeloma for several reasons. Most significantly, he noted: “Survival has gone up to 10 or 15 years [so] today, if you randomize between one [drug] versus another, there are going to be seven or eight more treatments before the patient dies.”
Similarly, progression-free survival (PFS) in multiple myeloma is now as much as 5 years, Dr. Munshi said. “Do we want a patient to wait 5 years to get a very good new drug?”
For these reasons and others, Dr, Munshi observed, myeloma researchers are increasingly relying on a surrogate called “negative minimal residual disease” (negative MRD) – in other words, a situation in which myeloma cells can no longer be detected in the bone marrow. MRD is hunted out using next-generation flow or next-generation sequencing of myeloma-cell DNA from bone-marrow aspirate to levels as low as 1 in 100,000 or 1,000,000 cells.
In 2020, Dr. Munshi and colleagues published a large meta-analysis showing that a negative MRD in a patient with multiple myeloma was significantly prognostic for both progression-free survival (hazard ratio, 0.33; P < .001) and overall survival (HR, 0.45; P < .001). The team concluded: “MRD can fulfill all the prerequisites to be a clinically valid surrogate biomarker for PFS and OS in [multiple myeloma].”
In MajesTEC-1 overall, 26.7% of patients on teclistamab had no signs of residual disease at a threshold of 1 in 100,000. Among patients who showed a “complete response” by International Myeloma Working Group criteria, 46% had no residual disease.
Dr. Munshi stressed that such patients are not necessarily “cured.” It will take a few more years to prove that. He noted: “Simply, physiologically, [negative MRD] means that if a patient has one [myeloma] cell in a million, that cell is going to take a much longer time to grow up to be myeloma.”
On Nov. 8 and 9, the FDA and the International Myeloma Society held a workshop to discuss the vexed question of surrogate endpoints and single-arm studies for drug approvals entitled the “Future of Drug Development in Multiple Myeloma.” Dr. Munshi was cochair.
A panelist at the meeting who was a senior investigator in the MajesTEC-1 trial, Ajai Chari, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, summed up the dilemma: “No one disagrees that randomized studies are the best way of doing things. The question is, if you’re a patient who’s exhausted all available therapies, do you have that time to wait? ... The role of accelerated approval is to get the drug to the patient faster. But what does it not pick up? How do we make these accelerated approvals more meaningful and not have to retract for safety?”
Jonathon Vallejo, also on the panel, agreed that safety was the key worry. The ideal scenario for accelerated approval would be a drug that was better than available therapy, and “in some sense, it’s much safer.” However, such situations are rare.
“Most of the time, we don’t have these products that come in that have no toxicity signals,” he said. “So one thing we have to think carefully about in the single-arm trial setting is, what are the toxicities? How do they stack up?”
Dr. Chari said that, for his part, he wanted to see more transparency around “cause of death” in all studies that lead to accelerated approvals. He said he was “tired” of seeing a death labeled as “not attributed” to the drug by the investigator or the drug company.
“Let me decide. Show me the deaths, and show me the myeloma status at that point,” Dr. Chari said. “That’s a signal – if you’re a responding patient and dying, then the FDA should be a little bit more cautious.”
The FDA has added a boxed warning to the teclistamab product information concerning cytokine-release syndrome and neurologic toxicity.
Cytokine-release syndrome, the most common side effect overall, showed up in 72% of patients, typically 2 days after the first step-up dose.
Neurologic toxicity occurred in 57% of patients, including headache (25%), motor dysfunction (16%), sensory neuropathy (15%) and encephalopathy (13%). About 6%of patients developed a serious, life-threatening neurologic condition called immune effector cell–associated neurotoxicity syndrome.
Overall, serious adverse reactions occurred in 54% of participants in MajesTEC-1, and 5% of people in the trial died from adverse reactions during the study, most commonly infections.
Because of its safety profile, teclistamab is available only through a restricted program called TECVAYLI Risk Evaluation and Mitigation Strategy.
The continued approval of teclistamab for this indication “may be contingent upon verification and description of clinical benefit in confirmatory trials,” according to the FDA.
To that end, eight more studies of teclistamab are underway, aiming for approximately 1,300 multiple myeloma patients around the world. Three of these trials are in newly diagnosed patients. Four more studies are planned to come online in the next 3 months, raising the final tally of patients testing out teclistamab to approximately 4,700. The trials will look at teclistamab in sequence or in combination with standards such as bortezomib and pomalidomide. All studies are open label.
Dr. Patel believes that, until these trials say otherwise, the benefits of teclistamab outweigh the risks. “I’m very happy we have one more option in this space, particularly the fourth or fifth line for patients who want to continue to fight the disease,” Dr. Patel concluded.
Dr. Munshi disclosed advisory board/consultant work for Adaptive, Abbvie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Karyopharm, Legend, Millennium, Novartis, Oncopep, and Pfizer and is the scientific founder of Oncopep and DCT. The 2020 meta-analysis by Dr. Munshi and colleagues was funded by Janssen-Cilag. Dr. Patel declared funding from Janssen for a diversity-equity initiative and membership of the South Carolina Medicaid P & T Committee. Dr. Usmani declared conflicts of interest with Amgen, BMS/Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, Abbvie, Genentech, Gilead, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Total replacement and fusion yield similar outcomes for ankle osteoarthritis
Ankle osteoarthritis remains a cause of severe pain and disability. Patients are treated nonoperatively if possible, but surgery is often needed for individuals with end-stage disease, wrote Andrew Goldberg, MBBS, of University College London and colleagues in the Annals of Internal Medicine.
“Most patients with ankle arthritis respond to nonoperative treatments, such as weight loss, activity modification, support braces, and analgesia, [but] once the disease has progressed to end-stage osteoarthritis, the main surgical treatments are total ankle re-placement or ankle arthrodesis,” Dr. Goldberg said, in an interview.
In the new study, patients were randomized to receive either a total ankle replacement (TAR) or ankle fusion (AF).
“We showed that, in both treatment groups the clinical scores improved hugely, by more than three times the minimal clinically important difference,” Dr. Goldberg said in an interview.
“Although the ankle replacement arm improved, on average, by more than an extra 4 points over ankle fusion, this was not considered clinically or statistically significant,” he said.
The study is the first randomized trial to show high-quality and robust results, he noted, and findings support data from previous studies.
“Although both TAR and ankle fusion have been shown to be effective, they are very different treatments, with one fusing the bones so that there is no ankle joint movement, and the other replacing the joint with the aim of retaining ankle joint movement. It is difficult for a patient to know which treatment is more suitable for them, with most seeking guidance from their surgeon,” he said.
Generating high-quality evidence
The study, a randomized, multicenter, open-label trial known as TARVA (Total Ankle Replacement Versus Ankle Arthrodesis), aimed to compare the clinical effectiveness of the two existing publicly funded U.K. treatment options, the authors wrote.
Patients were recruited at 17 U.K. centers between March 6, 2015, and Jan. 10, 2019. The study enrolled 303 adults aged 50-85 years with end-stage ankle osteoarthritis. The mean age of the participants was 68 years; 71% were men. A total of 137 TAR patients and 144 ankle fusion patients completed their surgeries with clinical scores available for analysis. Baseline characteristics were mainly similar between the groups.
Blinding was not possible because of the nature of the procedures, but the surgeons who screened the patients were not aware of the randomization allocations, the researchers noted. A total of 33 surgeons participated in the trial, with a median number of seven patients per surgeon during the study period.
For TAR, U.K. surgeons use both two-component, fixed-bearing and three-component, mobile-bearing implants, the authors write. Ankle fusion was done using the surgeon’s usual technique of either arthroscopic-assisted or open ankle fusion.
The primary outcome was the change in the Manchester–Oxford Foot Questionnaire walking/standing (MOXFQ-W/S) domain scores from baseline to 52 weeks after surgery. The MOXFQ-W/S uses a scale of 0-100, with lower scores representing better outcomes. Secondary outcomes included change in the MOXFQ-W/S scores at 26 weeks after surgery, as well as measures of patient quality of life.
No statistically significant difference
Overall, the mean MOXFQ-W/S scores improved significantly from baseline to 52 weeks for both groups, with average improvements of 49.9 in the TAR group and 44.4 points in the AF group. The average scores at 52 weeks were 31.4 in the TAR group and 36.8 in the AF group.
The adjusted difference in score change from baseline was –5.56, showing a slightly greater degree of improvement with TAR, but this difference was not clinically or statistically significant, the researchers noted.
Adverse event numbers were similar for both procedures, with 54% of TAR patients and 53% of AF patients experiencing at least 1 adverse event during the study period. Of those, 18% of TAR patients and 24% of AF patients experienced at least 1 serious adverse event.
However, the TAR patients experienced a higher rate of wound healing complications and nerve injuries, while thromboembolism was higher in the AF patients, the researchers noted.
A prespecified subgroup analysis of patients with osteoarthritis in adjacent joints suggested a greater improvement in TAR, compared with AF, a difference that increased when fixed-bearing TAR was compared with AF, the authors wrote.
“This reinforces previous reports that suggest that the presence of adjacent joint arthritis may be an indication for ankle replacement over AF,” the authors wrote in their discussion.
“Many of these patients did not have any symptoms in the adjacent joints,” they noted.
“The presence of adjacent joint arthritis, meaning the wear and tear of the joints around the ankle joint, seemed to favor ankle replacement,” Dr. Goldberg said. Approximately 30 joints in the foot continue to move after the ankle is fused, and if these adjacent joints are not healthy before surgery [as was the case in 42% of the study patients], the results of fusion were less successful, he explained.
A post hoc analysis between TAR subtypes showed that patients who had fixed-bearing TAR had significantly greater improvements, compared with AF patients, but this difference was not observed in patients who had mobile-bearing TAR, the researchers noted.
Dr. Goldberg said it was surprising “that, in a separate analysis, we found that the fixed-bearing ankle replacement patients [who accounted for half of the implants used] improved by a much greater difference when compared to ankle fusion.”
The study findings were limited by several factors including the short follow-up and study design that allowed surgeons to choose any implant and technique, the researchers noted.
Other limitations include a lack of data on cost-effectiveness and the impact of comorbidities on outcomes, they wrote. However, the study is the first completed multicenter randomized controlled trial to compare TAR and AF procedures for end-stage ankle osteoarthritis and shows that both yield similar clinical improvements, they concluded.
Data can inform treatment discussion
The take-home messages for clinicians are that both ankle replacement and ankle fusion are effective treatments that improve patients’ quality of life, and it is important to establish the health of adjacent joints before making treatment recommendations, Dr. Goldberg said.
“Careful counseling on the relative risks of each procedure should be part of the informed consent process,” he added. Ideally, all patients seeking surgical care for ankle arthritis should have a choice between ankle replacement and ankle fusion, but sometimes there is inequity of provision of the two treatments, he noted.
“We now encourage all surgeons to work in ankle arthritis networks so that every patient, no matter where they live, can have choice about the best treatment for them,” he said.
Researchers met the challenge of surgical RCT
Randomized trials of surgical interventions are challenging to conduct, and therefore limited, wrote Bruce Sangeorzan, MD, of the University of Washington, Seattle, and colleagues in an accompanying editorial. However, the new study was strengthened by the inclusion of 17 centers for heterogeneity of implant type and surgeon experience level, the editorialists said in the Annals of Internal Medicine.
The study is especially important, because ankle arthritis treatment is very understudied, compared with hip and knee arthritis, but it has a similar impact on activity, editorial coauthor Dr. Sangeorzan said in an interview.
“Randomized controlled trials are the gold standard for comparing medical therapies,” he said, “but they are very difficult to do in surgical treatments, particularly when the two treatments can be differentiated, in this case by movement of the ankle.”
In addition, there is a strong placebo effect attached to interventions, Dr. Sangeorzan noted. “Determining best-case treatment relies on prospective research, preferably randomized. Since both ankle fusion and ankle replacement are effective therapies, a prospective randomized trial is the best way to help make treatment decisions,” he said.
The current study findings are not surprising, but they are preliminary, and 1 year of follow-up is not enough to determine effectiveness, Dr. Sangeorzan emphasized. However, “the authors have done the hard work of randomizing the patients and collecting the data, and the patients can now be followed for a longer time,” he said.
“In addition, the trial was designed with multiple secondary outcome measures, so the data can be matched up with larger trials that were not randomized to identify key elements of success for each procedure,” he noted.
The key message for clinicians is that ankle arthritis has a significant impact on patients’ lives, but there are two effective treatments that can reduce the impact of the disease, said Dr. Sangeorzan. “The data suggest that there are differences in implant design and differences in comorbidities that should influence decision-making,” he added.
Additional research is needed in the form of a longer study duration with larger cohorts, said Dr. Sangeorzan. In particular, researchers need to determine what comorbidities might drive patients to one type of care vs. another, he said. “The suggestion that [patients receiving implants with two motion segments have better outcomes than those receiving implants with a one-motion segment] also deserves further study,” he added.
The research was supported by the UK National Institute for Health and Care Research Health Technology Assessment Programme. The trial was sponsored by University College London. Dr. Goldberg disclosed grant support from NIHR HTA, as well as financial relationships with companies including Stryker, Paragon 28, and stock options with Standing CT Company, Elstree Waterfront Outpatients, and X Bolt Orthopedics.
The editorialists had no financial conflicts to disclose.
Ankle osteoarthritis remains a cause of severe pain and disability. Patients are treated nonoperatively if possible, but surgery is often needed for individuals with end-stage disease, wrote Andrew Goldberg, MBBS, of University College London and colleagues in the Annals of Internal Medicine.
“Most patients with ankle arthritis respond to nonoperative treatments, such as weight loss, activity modification, support braces, and analgesia, [but] once the disease has progressed to end-stage osteoarthritis, the main surgical treatments are total ankle re-placement or ankle arthrodesis,” Dr. Goldberg said, in an interview.
In the new study, patients were randomized to receive either a total ankle replacement (TAR) or ankle fusion (AF).
“We showed that, in both treatment groups the clinical scores improved hugely, by more than three times the minimal clinically important difference,” Dr. Goldberg said in an interview.
“Although the ankle replacement arm improved, on average, by more than an extra 4 points over ankle fusion, this was not considered clinically or statistically significant,” he said.
The study is the first randomized trial to show high-quality and robust results, he noted, and findings support data from previous studies.
“Although both TAR and ankle fusion have been shown to be effective, they are very different treatments, with one fusing the bones so that there is no ankle joint movement, and the other replacing the joint with the aim of retaining ankle joint movement. It is difficult for a patient to know which treatment is more suitable for them, with most seeking guidance from their surgeon,” he said.
Generating high-quality evidence
The study, a randomized, multicenter, open-label trial known as TARVA (Total Ankle Replacement Versus Ankle Arthrodesis), aimed to compare the clinical effectiveness of the two existing publicly funded U.K. treatment options, the authors wrote.
Patients were recruited at 17 U.K. centers between March 6, 2015, and Jan. 10, 2019. The study enrolled 303 adults aged 50-85 years with end-stage ankle osteoarthritis. The mean age of the participants was 68 years; 71% were men. A total of 137 TAR patients and 144 ankle fusion patients completed their surgeries with clinical scores available for analysis. Baseline characteristics were mainly similar between the groups.
Blinding was not possible because of the nature of the procedures, but the surgeons who screened the patients were not aware of the randomization allocations, the researchers noted. A total of 33 surgeons participated in the trial, with a median number of seven patients per surgeon during the study period.
For TAR, U.K. surgeons use both two-component, fixed-bearing and three-component, mobile-bearing implants, the authors write. Ankle fusion was done using the surgeon’s usual technique of either arthroscopic-assisted or open ankle fusion.
The primary outcome was the change in the Manchester–Oxford Foot Questionnaire walking/standing (MOXFQ-W/S) domain scores from baseline to 52 weeks after surgery. The MOXFQ-W/S uses a scale of 0-100, with lower scores representing better outcomes. Secondary outcomes included change in the MOXFQ-W/S scores at 26 weeks after surgery, as well as measures of patient quality of life.
No statistically significant difference
Overall, the mean MOXFQ-W/S scores improved significantly from baseline to 52 weeks for both groups, with average improvements of 49.9 in the TAR group and 44.4 points in the AF group. The average scores at 52 weeks were 31.4 in the TAR group and 36.8 in the AF group.
The adjusted difference in score change from baseline was –5.56, showing a slightly greater degree of improvement with TAR, but this difference was not clinically or statistically significant, the researchers noted.
Adverse event numbers were similar for both procedures, with 54% of TAR patients and 53% of AF patients experiencing at least 1 adverse event during the study period. Of those, 18% of TAR patients and 24% of AF patients experienced at least 1 serious adverse event.
However, the TAR patients experienced a higher rate of wound healing complications and nerve injuries, while thromboembolism was higher in the AF patients, the researchers noted.
A prespecified subgroup analysis of patients with osteoarthritis in adjacent joints suggested a greater improvement in TAR, compared with AF, a difference that increased when fixed-bearing TAR was compared with AF, the authors wrote.
“This reinforces previous reports that suggest that the presence of adjacent joint arthritis may be an indication for ankle replacement over AF,” the authors wrote in their discussion.
“Many of these patients did not have any symptoms in the adjacent joints,” they noted.
“The presence of adjacent joint arthritis, meaning the wear and tear of the joints around the ankle joint, seemed to favor ankle replacement,” Dr. Goldberg said. Approximately 30 joints in the foot continue to move after the ankle is fused, and if these adjacent joints are not healthy before surgery [as was the case in 42% of the study patients], the results of fusion were less successful, he explained.
A post hoc analysis between TAR subtypes showed that patients who had fixed-bearing TAR had significantly greater improvements, compared with AF patients, but this difference was not observed in patients who had mobile-bearing TAR, the researchers noted.
Dr. Goldberg said it was surprising “that, in a separate analysis, we found that the fixed-bearing ankle replacement patients [who accounted for half of the implants used] improved by a much greater difference when compared to ankle fusion.”
The study findings were limited by several factors including the short follow-up and study design that allowed surgeons to choose any implant and technique, the researchers noted.
Other limitations include a lack of data on cost-effectiveness and the impact of comorbidities on outcomes, they wrote. However, the study is the first completed multicenter randomized controlled trial to compare TAR and AF procedures for end-stage ankle osteoarthritis and shows that both yield similar clinical improvements, they concluded.
Data can inform treatment discussion
The take-home messages for clinicians are that both ankle replacement and ankle fusion are effective treatments that improve patients’ quality of life, and it is important to establish the health of adjacent joints before making treatment recommendations, Dr. Goldberg said.
“Careful counseling on the relative risks of each procedure should be part of the informed consent process,” he added. Ideally, all patients seeking surgical care for ankle arthritis should have a choice between ankle replacement and ankle fusion, but sometimes there is inequity of provision of the two treatments, he noted.
“We now encourage all surgeons to work in ankle arthritis networks so that every patient, no matter where they live, can have choice about the best treatment for them,” he said.
Researchers met the challenge of surgical RCT
Randomized trials of surgical interventions are challenging to conduct, and therefore limited, wrote Bruce Sangeorzan, MD, of the University of Washington, Seattle, and colleagues in an accompanying editorial. However, the new study was strengthened by the inclusion of 17 centers for heterogeneity of implant type and surgeon experience level, the editorialists said in the Annals of Internal Medicine.
The study is especially important, because ankle arthritis treatment is very understudied, compared with hip and knee arthritis, but it has a similar impact on activity, editorial coauthor Dr. Sangeorzan said in an interview.
“Randomized controlled trials are the gold standard for comparing medical therapies,” he said, “but they are very difficult to do in surgical treatments, particularly when the two treatments can be differentiated, in this case by movement of the ankle.”
In addition, there is a strong placebo effect attached to interventions, Dr. Sangeorzan noted. “Determining best-case treatment relies on prospective research, preferably randomized. Since both ankle fusion and ankle replacement are effective therapies, a prospective randomized trial is the best way to help make treatment decisions,” he said.
The current study findings are not surprising, but they are preliminary, and 1 year of follow-up is not enough to determine effectiveness, Dr. Sangeorzan emphasized. However, “the authors have done the hard work of randomizing the patients and collecting the data, and the patients can now be followed for a longer time,” he said.
“In addition, the trial was designed with multiple secondary outcome measures, so the data can be matched up with larger trials that were not randomized to identify key elements of success for each procedure,” he noted.
The key message for clinicians is that ankle arthritis has a significant impact on patients’ lives, but there are two effective treatments that can reduce the impact of the disease, said Dr. Sangeorzan. “The data suggest that there are differences in implant design and differences in comorbidities that should influence decision-making,” he added.
Additional research is needed in the form of a longer study duration with larger cohorts, said Dr. Sangeorzan. In particular, researchers need to determine what comorbidities might drive patients to one type of care vs. another, he said. “The suggestion that [patients receiving implants with two motion segments have better outcomes than those receiving implants with a one-motion segment] also deserves further study,” he added.
The research was supported by the UK National Institute for Health and Care Research Health Technology Assessment Programme. The trial was sponsored by University College London. Dr. Goldberg disclosed grant support from NIHR HTA, as well as financial relationships with companies including Stryker, Paragon 28, and stock options with Standing CT Company, Elstree Waterfront Outpatients, and X Bolt Orthopedics.
The editorialists had no financial conflicts to disclose.
Ankle osteoarthritis remains a cause of severe pain and disability. Patients are treated nonoperatively if possible, but surgery is often needed for individuals with end-stage disease, wrote Andrew Goldberg, MBBS, of University College London and colleagues in the Annals of Internal Medicine.
“Most patients with ankle arthritis respond to nonoperative treatments, such as weight loss, activity modification, support braces, and analgesia, [but] once the disease has progressed to end-stage osteoarthritis, the main surgical treatments are total ankle re-placement or ankle arthrodesis,” Dr. Goldberg said, in an interview.
In the new study, patients were randomized to receive either a total ankle replacement (TAR) or ankle fusion (AF).
“We showed that, in both treatment groups the clinical scores improved hugely, by more than three times the minimal clinically important difference,” Dr. Goldberg said in an interview.
“Although the ankle replacement arm improved, on average, by more than an extra 4 points over ankle fusion, this was not considered clinically or statistically significant,” he said.
The study is the first randomized trial to show high-quality and robust results, he noted, and findings support data from previous studies.
“Although both TAR and ankle fusion have been shown to be effective, they are very different treatments, with one fusing the bones so that there is no ankle joint movement, and the other replacing the joint with the aim of retaining ankle joint movement. It is difficult for a patient to know which treatment is more suitable for them, with most seeking guidance from their surgeon,” he said.
Generating high-quality evidence
The study, a randomized, multicenter, open-label trial known as TARVA (Total Ankle Replacement Versus Ankle Arthrodesis), aimed to compare the clinical effectiveness of the two existing publicly funded U.K. treatment options, the authors wrote.
Patients were recruited at 17 U.K. centers between March 6, 2015, and Jan. 10, 2019. The study enrolled 303 adults aged 50-85 years with end-stage ankle osteoarthritis. The mean age of the participants was 68 years; 71% were men. A total of 137 TAR patients and 144 ankle fusion patients completed their surgeries with clinical scores available for analysis. Baseline characteristics were mainly similar between the groups.
Blinding was not possible because of the nature of the procedures, but the surgeons who screened the patients were not aware of the randomization allocations, the researchers noted. A total of 33 surgeons participated in the trial, with a median number of seven patients per surgeon during the study period.
For TAR, U.K. surgeons use both two-component, fixed-bearing and three-component, mobile-bearing implants, the authors write. Ankle fusion was done using the surgeon’s usual technique of either arthroscopic-assisted or open ankle fusion.
The primary outcome was the change in the Manchester–Oxford Foot Questionnaire walking/standing (MOXFQ-W/S) domain scores from baseline to 52 weeks after surgery. The MOXFQ-W/S uses a scale of 0-100, with lower scores representing better outcomes. Secondary outcomes included change in the MOXFQ-W/S scores at 26 weeks after surgery, as well as measures of patient quality of life.
No statistically significant difference
Overall, the mean MOXFQ-W/S scores improved significantly from baseline to 52 weeks for both groups, with average improvements of 49.9 in the TAR group and 44.4 points in the AF group. The average scores at 52 weeks were 31.4 in the TAR group and 36.8 in the AF group.
The adjusted difference in score change from baseline was –5.56, showing a slightly greater degree of improvement with TAR, but this difference was not clinically or statistically significant, the researchers noted.
Adverse event numbers were similar for both procedures, with 54% of TAR patients and 53% of AF patients experiencing at least 1 adverse event during the study period. Of those, 18% of TAR patients and 24% of AF patients experienced at least 1 serious adverse event.
However, the TAR patients experienced a higher rate of wound healing complications and nerve injuries, while thromboembolism was higher in the AF patients, the researchers noted.
A prespecified subgroup analysis of patients with osteoarthritis in adjacent joints suggested a greater improvement in TAR, compared with AF, a difference that increased when fixed-bearing TAR was compared with AF, the authors wrote.
“This reinforces previous reports that suggest that the presence of adjacent joint arthritis may be an indication for ankle replacement over AF,” the authors wrote in their discussion.
“Many of these patients did not have any symptoms in the adjacent joints,” they noted.
“The presence of adjacent joint arthritis, meaning the wear and tear of the joints around the ankle joint, seemed to favor ankle replacement,” Dr. Goldberg said. Approximately 30 joints in the foot continue to move after the ankle is fused, and if these adjacent joints are not healthy before surgery [as was the case in 42% of the study patients], the results of fusion were less successful, he explained.
A post hoc analysis between TAR subtypes showed that patients who had fixed-bearing TAR had significantly greater improvements, compared with AF patients, but this difference was not observed in patients who had mobile-bearing TAR, the researchers noted.
Dr. Goldberg said it was surprising “that, in a separate analysis, we found that the fixed-bearing ankle replacement patients [who accounted for half of the implants used] improved by a much greater difference when compared to ankle fusion.”
The study findings were limited by several factors including the short follow-up and study design that allowed surgeons to choose any implant and technique, the researchers noted.
Other limitations include a lack of data on cost-effectiveness and the impact of comorbidities on outcomes, they wrote. However, the study is the first completed multicenter randomized controlled trial to compare TAR and AF procedures for end-stage ankle osteoarthritis and shows that both yield similar clinical improvements, they concluded.
Data can inform treatment discussion
The take-home messages for clinicians are that both ankle replacement and ankle fusion are effective treatments that improve patients’ quality of life, and it is important to establish the health of adjacent joints before making treatment recommendations, Dr. Goldberg said.
“Careful counseling on the relative risks of each procedure should be part of the informed consent process,” he added. Ideally, all patients seeking surgical care for ankle arthritis should have a choice between ankle replacement and ankle fusion, but sometimes there is inequity of provision of the two treatments, he noted.
“We now encourage all surgeons to work in ankle arthritis networks so that every patient, no matter where they live, can have choice about the best treatment for them,” he said.
Researchers met the challenge of surgical RCT
Randomized trials of surgical interventions are challenging to conduct, and therefore limited, wrote Bruce Sangeorzan, MD, of the University of Washington, Seattle, and colleagues in an accompanying editorial. However, the new study was strengthened by the inclusion of 17 centers for heterogeneity of implant type and surgeon experience level, the editorialists said in the Annals of Internal Medicine.
The study is especially important, because ankle arthritis treatment is very understudied, compared with hip and knee arthritis, but it has a similar impact on activity, editorial coauthor Dr. Sangeorzan said in an interview.
“Randomized controlled trials are the gold standard for comparing medical therapies,” he said, “but they are very difficult to do in surgical treatments, particularly when the two treatments can be differentiated, in this case by movement of the ankle.”
In addition, there is a strong placebo effect attached to interventions, Dr. Sangeorzan noted. “Determining best-case treatment relies on prospective research, preferably randomized. Since both ankle fusion and ankle replacement are effective therapies, a prospective randomized trial is the best way to help make treatment decisions,” he said.
The current study findings are not surprising, but they are preliminary, and 1 year of follow-up is not enough to determine effectiveness, Dr. Sangeorzan emphasized. However, “the authors have done the hard work of randomizing the patients and collecting the data, and the patients can now be followed for a longer time,” he said.
“In addition, the trial was designed with multiple secondary outcome measures, so the data can be matched up with larger trials that were not randomized to identify key elements of success for each procedure,” he noted.
The key message for clinicians is that ankle arthritis has a significant impact on patients’ lives, but there are two effective treatments that can reduce the impact of the disease, said Dr. Sangeorzan. “The data suggest that there are differences in implant design and differences in comorbidities that should influence decision-making,” he added.
Additional research is needed in the form of a longer study duration with larger cohorts, said Dr. Sangeorzan. In particular, researchers need to determine what comorbidities might drive patients to one type of care vs. another, he said. “The suggestion that [patients receiving implants with two motion segments have better outcomes than those receiving implants with a one-motion segment] also deserves further study,” he added.
The research was supported by the UK National Institute for Health and Care Research Health Technology Assessment Programme. The trial was sponsored by University College London. Dr. Goldberg disclosed grant support from NIHR HTA, as well as financial relationships with companies including Stryker, Paragon 28, and stock options with Standing CT Company, Elstree Waterfront Outpatients, and X Bolt Orthopedics.
The editorialists had no financial conflicts to disclose.
Watching violent TV in preschool linked with emotional, behavioral issues at age 12
Preschoolers who watch violent television are more likely to have emotional and behavioral issues at the age of 12, according to investigators.
These findings align with previous studies that have shown the negative effects of watching violent content, reinforcing the importance of restricting childhood screen time, lead author Linda S. Pagani, PhD, of Université de Montréal and colleagues reported.
Past research measured the immediate or short-term effects of seeing violent media. This study examined how TV violence could be leading to issues almost a decade later, the investigators wrote in the Journal of Developmental & Behavioral Pediatrics.
Their study looked at 1,976 children from the Quebec Longitudinal Study of Child Development, a random representative cohort of boys and girls followed since their births in 1997 and 1998.
At the cohort study follow-ups at ages 3.5 and 4.5 years, the parents of these children reported if their kids watched violent TV, showing that about half of them were exposed. At age 12, the same children were scored by their teachers on a range of psychosocial outcomes, including emotional distress, inattentive behavior, disorderly behavior, social withdrawal, classroom engagement, and overall academic achievement. At this second time point, the children also scored themselves on their own academic motivation and confidence in writing.
To adjust for other factors that could be playing a role, the investigators accounted for participant characteristics at various ages between 5 months and 12 years, as well as differences in parenting styles, home environment, and socioeconomic status.
Dr. Pagani noted that these were not “garden-variety” statistical techniques.
“We did them in such a way that we set ourselves up for not finding results,” Dr. Pagani said in an interview. “That’s why this is really interesting.”
She and her colleagues found that watching TV violence during preschool was significantly associated with multiple negative outcomes at age 12.
For girls, negative outcomes included greater emotional distress, less classroom engagement, lower academic achievement, and less academic motivation. Boys showed greater emotional distress, decreased attention, disorderly behavior, social withdrawal, less classroom engagement, lower academic achievement, and less academic motivation.
“As expected, early screen violence exposure seems to come at a cost,” the investigators wrote.
Seeing TV through a child’s eyes
According to Dr. Pagani, many parents think that TV shows watched by preschoolers – like cartoons – are harmless, but these parents need to understand that the brains of children are not yet fully developed.
“The kid has an interpretation that’s very concrete,” Dr. Pagani said. “They don’t have abstract thinking.”
Because of this, kids who see “good guys” beating up “bad guys” don’t understand that the violence is comical and justified; they just see violence being used to address social disagreement, Dr. Pagani said. This leads children to believe that violence is an acceptable way to solve problems in daily life. Children are also more likely to see hostility in others when it isn’t present, leading to conflict.
Although the natural response to these findings is to restrict childhood exposure to violent content, this may be easier said than done, the investigators noted, particularly because TV is no longer the only screen in the home, as it was when this study began. Nowadays, parents need to monitor multiple devices, including smartphones, tablets, and computers, all of which may negatively impact normal brain development.
“People think this technology is innocuous,” Dr. Pagani said. “We are asleep at the wheel.”
She advised parents to wake up and follow the World Health Organization guidelines for sedentary screen time. The guidelines call for no screen time at all until a child is at least 2 years old, and then less than 1 hour per day until age 5.
“It’s the parents who should be in charge,” she said. “They’re the ones who have the cognitive ability to make decisions for their children.”
Choosing quality time over screen time
Loredana Marchica, PhD, of Montreal Children’s Hospital and McGill University, also in Montreal, expressed confidence in the study findings, because the results line up with past research, and because the investigators accounted for other explanations.
There is a “very strong probability” that watching violent TV in preschool leads to psychological issues down the line, Dr. Marchica said.
If a child is exposed to violent content, then parents should help children understand the difference between what happens in TV shows and real life, she added, as this can reduce negative effects on behavior.
“Parents need to explain that it’s a TV show,” Dr. Marchica said. “It’s not real, and if [that violent act] happened in real life, it would actually hurt a person.”
In addition to limiting screen time and explaining any violent content, she encouraged parents to spend quality time with their children, especially during the preschool years.
“Those are the years to fortify the attachment you have with that child,” Dr. Marchica said. “Even 15 minutes a day of quality, interactive play time can make such a difference in their development, their imagination, and their social engagement and abilities.”
Parents should also try to have conversations with their young children, she said, noting that it’s okay to share personal feelings, as this teaches kids how to manage their own emotions.
“Not everything is wonderful in life, and we’re allowed to talk about that,” Dr. Marchica said. “[Parents can say,] ‘Mommy had a bad day today. This bad thing happened. But here’s what I did to make myself feel better.’ ”
Dr. Pagani and coauthors termed their findings “robust,” but also cautioned that, in their correlational study, TV violence cannot be interpreted as causal. In other limitations, they noted that the study relies on a single parent-reported item that yielded a low rate of reported exposure. Or the findings could result from other things, such as family chaos or parenting style or something else.
The longitudinal study was supported by Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, and others. The investigators and Dr. Marchica reported no relevant conflicts of interest.
Preschoolers who watch violent television are more likely to have emotional and behavioral issues at the age of 12, according to investigators.
These findings align with previous studies that have shown the negative effects of watching violent content, reinforcing the importance of restricting childhood screen time, lead author Linda S. Pagani, PhD, of Université de Montréal and colleagues reported.
Past research measured the immediate or short-term effects of seeing violent media. This study examined how TV violence could be leading to issues almost a decade later, the investigators wrote in the Journal of Developmental & Behavioral Pediatrics.
Their study looked at 1,976 children from the Quebec Longitudinal Study of Child Development, a random representative cohort of boys and girls followed since their births in 1997 and 1998.
At the cohort study follow-ups at ages 3.5 and 4.5 years, the parents of these children reported if their kids watched violent TV, showing that about half of them were exposed. At age 12, the same children were scored by their teachers on a range of psychosocial outcomes, including emotional distress, inattentive behavior, disorderly behavior, social withdrawal, classroom engagement, and overall academic achievement. At this second time point, the children also scored themselves on their own academic motivation and confidence in writing.
To adjust for other factors that could be playing a role, the investigators accounted for participant characteristics at various ages between 5 months and 12 years, as well as differences in parenting styles, home environment, and socioeconomic status.
Dr. Pagani noted that these were not “garden-variety” statistical techniques.
“We did them in such a way that we set ourselves up for not finding results,” Dr. Pagani said in an interview. “That’s why this is really interesting.”
She and her colleagues found that watching TV violence during preschool was significantly associated with multiple negative outcomes at age 12.
For girls, negative outcomes included greater emotional distress, less classroom engagement, lower academic achievement, and less academic motivation. Boys showed greater emotional distress, decreased attention, disorderly behavior, social withdrawal, less classroom engagement, lower academic achievement, and less academic motivation.
“As expected, early screen violence exposure seems to come at a cost,” the investigators wrote.
Seeing TV through a child’s eyes
According to Dr. Pagani, many parents think that TV shows watched by preschoolers – like cartoons – are harmless, but these parents need to understand that the brains of children are not yet fully developed.
“The kid has an interpretation that’s very concrete,” Dr. Pagani said. “They don’t have abstract thinking.”
Because of this, kids who see “good guys” beating up “bad guys” don’t understand that the violence is comical and justified; they just see violence being used to address social disagreement, Dr. Pagani said. This leads children to believe that violence is an acceptable way to solve problems in daily life. Children are also more likely to see hostility in others when it isn’t present, leading to conflict.
Although the natural response to these findings is to restrict childhood exposure to violent content, this may be easier said than done, the investigators noted, particularly because TV is no longer the only screen in the home, as it was when this study began. Nowadays, parents need to monitor multiple devices, including smartphones, tablets, and computers, all of which may negatively impact normal brain development.
“People think this technology is innocuous,” Dr. Pagani said. “We are asleep at the wheel.”
She advised parents to wake up and follow the World Health Organization guidelines for sedentary screen time. The guidelines call for no screen time at all until a child is at least 2 years old, and then less than 1 hour per day until age 5.
“It’s the parents who should be in charge,” she said. “They’re the ones who have the cognitive ability to make decisions for their children.”
Choosing quality time over screen time
Loredana Marchica, PhD, of Montreal Children’s Hospital and McGill University, also in Montreal, expressed confidence in the study findings, because the results line up with past research, and because the investigators accounted for other explanations.
There is a “very strong probability” that watching violent TV in preschool leads to psychological issues down the line, Dr. Marchica said.
If a child is exposed to violent content, then parents should help children understand the difference between what happens in TV shows and real life, she added, as this can reduce negative effects on behavior.
“Parents need to explain that it’s a TV show,” Dr. Marchica said. “It’s not real, and if [that violent act] happened in real life, it would actually hurt a person.”
In addition to limiting screen time and explaining any violent content, she encouraged parents to spend quality time with their children, especially during the preschool years.
“Those are the years to fortify the attachment you have with that child,” Dr. Marchica said. “Even 15 minutes a day of quality, interactive play time can make such a difference in their development, their imagination, and their social engagement and abilities.”
Parents should also try to have conversations with their young children, she said, noting that it’s okay to share personal feelings, as this teaches kids how to manage their own emotions.
“Not everything is wonderful in life, and we’re allowed to talk about that,” Dr. Marchica said. “[Parents can say,] ‘Mommy had a bad day today. This bad thing happened. But here’s what I did to make myself feel better.’ ”
Dr. Pagani and coauthors termed their findings “robust,” but also cautioned that, in their correlational study, TV violence cannot be interpreted as causal. In other limitations, they noted that the study relies on a single parent-reported item that yielded a low rate of reported exposure. Or the findings could result from other things, such as family chaos or parenting style or something else.
The longitudinal study was supported by Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, and others. The investigators and Dr. Marchica reported no relevant conflicts of interest.
Preschoolers who watch violent television are more likely to have emotional and behavioral issues at the age of 12, according to investigators.
These findings align with previous studies that have shown the negative effects of watching violent content, reinforcing the importance of restricting childhood screen time, lead author Linda S. Pagani, PhD, of Université de Montréal and colleagues reported.
Past research measured the immediate or short-term effects of seeing violent media. This study examined how TV violence could be leading to issues almost a decade later, the investigators wrote in the Journal of Developmental & Behavioral Pediatrics.
Their study looked at 1,976 children from the Quebec Longitudinal Study of Child Development, a random representative cohort of boys and girls followed since their births in 1997 and 1998.
At the cohort study follow-ups at ages 3.5 and 4.5 years, the parents of these children reported if their kids watched violent TV, showing that about half of them were exposed. At age 12, the same children were scored by their teachers on a range of psychosocial outcomes, including emotional distress, inattentive behavior, disorderly behavior, social withdrawal, classroom engagement, and overall academic achievement. At this second time point, the children also scored themselves on their own academic motivation and confidence in writing.
To adjust for other factors that could be playing a role, the investigators accounted for participant characteristics at various ages between 5 months and 12 years, as well as differences in parenting styles, home environment, and socioeconomic status.
Dr. Pagani noted that these were not “garden-variety” statistical techniques.
“We did them in such a way that we set ourselves up for not finding results,” Dr. Pagani said in an interview. “That’s why this is really interesting.”
She and her colleagues found that watching TV violence during preschool was significantly associated with multiple negative outcomes at age 12.
For girls, negative outcomes included greater emotional distress, less classroom engagement, lower academic achievement, and less academic motivation. Boys showed greater emotional distress, decreased attention, disorderly behavior, social withdrawal, less classroom engagement, lower academic achievement, and less academic motivation.
“As expected, early screen violence exposure seems to come at a cost,” the investigators wrote.
Seeing TV through a child’s eyes
According to Dr. Pagani, many parents think that TV shows watched by preschoolers – like cartoons – are harmless, but these parents need to understand that the brains of children are not yet fully developed.
“The kid has an interpretation that’s very concrete,” Dr. Pagani said. “They don’t have abstract thinking.”
Because of this, kids who see “good guys” beating up “bad guys” don’t understand that the violence is comical and justified; they just see violence being used to address social disagreement, Dr. Pagani said. This leads children to believe that violence is an acceptable way to solve problems in daily life. Children are also more likely to see hostility in others when it isn’t present, leading to conflict.
Although the natural response to these findings is to restrict childhood exposure to violent content, this may be easier said than done, the investigators noted, particularly because TV is no longer the only screen in the home, as it was when this study began. Nowadays, parents need to monitor multiple devices, including smartphones, tablets, and computers, all of which may negatively impact normal brain development.
“People think this technology is innocuous,” Dr. Pagani said. “We are asleep at the wheel.”
She advised parents to wake up and follow the World Health Organization guidelines for sedentary screen time. The guidelines call for no screen time at all until a child is at least 2 years old, and then less than 1 hour per day until age 5.
“It’s the parents who should be in charge,” she said. “They’re the ones who have the cognitive ability to make decisions for their children.”
Choosing quality time over screen time
Loredana Marchica, PhD, of Montreal Children’s Hospital and McGill University, also in Montreal, expressed confidence in the study findings, because the results line up with past research, and because the investigators accounted for other explanations.
There is a “very strong probability” that watching violent TV in preschool leads to psychological issues down the line, Dr. Marchica said.
If a child is exposed to violent content, then parents should help children understand the difference between what happens in TV shows and real life, she added, as this can reduce negative effects on behavior.
“Parents need to explain that it’s a TV show,” Dr. Marchica said. “It’s not real, and if [that violent act] happened in real life, it would actually hurt a person.”
In addition to limiting screen time and explaining any violent content, she encouraged parents to spend quality time with their children, especially during the preschool years.
“Those are the years to fortify the attachment you have with that child,” Dr. Marchica said. “Even 15 minutes a day of quality, interactive play time can make such a difference in their development, their imagination, and their social engagement and abilities.”
Parents should also try to have conversations with their young children, she said, noting that it’s okay to share personal feelings, as this teaches kids how to manage their own emotions.
“Not everything is wonderful in life, and we’re allowed to talk about that,” Dr. Marchica said. “[Parents can say,] ‘Mommy had a bad day today. This bad thing happened. But here’s what I did to make myself feel better.’ ”
Dr. Pagani and coauthors termed their findings “robust,” but also cautioned that, in their correlational study, TV violence cannot be interpreted as causal. In other limitations, they noted that the study relies on a single parent-reported item that yielded a low rate of reported exposure. Or the findings could result from other things, such as family chaos or parenting style or something else.
The longitudinal study was supported by Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, and others. The investigators and Dr. Marchica reported no relevant conflicts of interest.
FROM THE JOURNAL OF DEVELOPMENTAL & BEHAVIORAL PEDIATRICS
Glioblastoma spreading strategies discovered
Every year, around 7,000 people in Germany develop a brain tumor, and around half of those cases involve a glioblastoma, a particularly aggressive form of the disease. Glioblastomas are incurable, but advances are being made in both diagnostics and therapy.
This news organization spoke to Wolfgang Wick, MD, medical director of the neurologic clinic at UKHD, about how glioblastomas are treated; the role that vaccinations, recombinant proteins, and parvoviruses play; and what therapeutic approaches might be derived from the discovery of this method by which glioblastomas spread.
Question: Glioblastomas spread through the brain like a fungal network. So how would a glioblastoma currently be treated? The tumor can only be partially removed through surgery.
Answer: Nevertheless, glioblastoma would be operated on. This would have a significant effect. Relieving the strain of the main tumor mass, without generating a new deficit, is prognostically very good for the patient concerned. However, surgery on glioblastoma is never curative.
The reason a cure is not possible is down to the special form and spread of the glioblastoma. Nevertheless, an operation helps. This seems to be because removing the main tumor mass maybe has a positive immunological effect. But it may also be connected to the tumor’s network communication. The surgical intervention stimulates the network by increasing resistance.
If the main tumor mass is decreased through a surgical procedure, this results in an at least temporarily improved starting position for the patient until the mass regenerates. This could also be connected to the fact that tumor communication is not unregulated but is rather in accordance with a certain hierarchy and order, which requires a certain structure and mass.
The other aspect is that support can be requested via this communication. You can imagine that a cell connected to another cell via a conduit receives help from this other cell in the form of organelles by exchanging ions and that, for example, stress or toxicity can be much better balanced out in large networks than in small networks. That means that external attacks, such as a surgical intervention, can be much better balanced by a well-organized network than by isolated cells.
Resistance to chemotherapy
Q: How do irradiation and chemotherapy rank in the treatment of glioblastomas?
A: Irradiation is another therapeutic approach. It causes cells to be stuck in the growth phase of the cell cycle. The cells are not killed through radiation, but they are practically halted. And this arrest of the cell cycle is often sufficient to help people with glioblastomas for a very long time. But the same is true for irradiation as for surgery. This deep network of cells cannot be addressed.
Attempts have been made in the past to reduce the radiation dose to the extent that the brain is no longer damaged by it, but this low dose was then not sufficient to exert any control. If you want to control the tumor, the dose must be high and the volume must be correspondingly low, since there is a clear limit.
Every patient is offered alkylating chemotherapy. At the moment, just one substance is used here in the primary therapy: temozolomide. The problem with this is that two-thirds of tumors in all cells exhibit a resistance to this alkylating chemotherapy, which means that the efficacy of this therapy is highly limited in two-thirds of patients.
In the one-third of patients in whom this resistance is not present, the chemotherapy works fairly well. But even then, it is unfortunately only a matter of time until there is a relapse or disease progression. In my practice, this has always been the case, but there are people who have been living with this disease for 20 years now. There seem to be tumor cells that calmly and silently survive this phase of chemotherapy and then restart the cell cycle at some point.
Q: What do you think of alternating electric fields as a therapy option?
A: Therapy with alternating electric fields is currently being used and offered to patients. This means that patients who have survived well through radiochemotherapy should also be offered treatment with alternating electric fields.
However, what happens in this process is not as well understood as with other therapies. It is assumed that the cell cycle, i.e., cell division, is altered by disrupting the mitotic spindle. But you can imagine, and this is now speculation, but quite sound speculation I believe, that alternating electric fields also cause a certain amount of confusion in the previously described networks. But this still needs to be investigated in more detail.
It is not implausible. We know that such alternating electric fields disturb the organization of cell organelles. And we also know that for this communication, we need fairly good order and also organization. This would definitely be a starting point on the way to understanding why this therapy potentially shows a certain effect in some patients.
Nerve cell precursors
Q: Scientists from the UKHD and the DKFZ have discovered a new glioblastoma spreading strategy and have learned that the tumor cells imitate the properties and movement patterns of nerve cells. They are labeling the results a “milestone in the field of cancer neuroscience.” Could you explain a bit more?
A: Glioblastoma does not grow on its own as a solid mass, but instead, the entire brain is affected by the disease. The question of how the tumor’s individual cells move the main tumor mass from afar, how they get there, how they continue to be supplied, and what their interaction partners are – an entirely new light has been shed on all of this in our work.
The development of tumor cell mobility has been recognized as a remnant of brain development. The tumor cells have retained properties that the precursor cells for nervous-system development require for an organized nervous system to emerge from just a few cells. This means that the tumor cells copy or eventually retain properties of the nerve-cell precursors that, unlike mature nerve cells, are mobile to a fairly high degree.
Mobility here means that it can advance along a network, despite said network being very densely packed. This also means that certain processes, such as releasing and then continuing to move again, must function and that the communication regarding the original disease must be maintained.
First, we understand what the different glioblastoma cell types do, which molecular properties are associated with which behaviors, and which cell type (namely the swarming cells) is responsible for the invasive tumor growth. In contrast, the network-forming cell type, which only develops from these, is responsible for the resistance.
Interrupting communication
Q: Which starting points for new therapies do you see?
A: In terms of new therapies, these movement phenomena are one good starting point. The other starting point – I find this one much more interesting – is that the programming steps that these tumor cells use [are] no longer needed. This is because our mature nervous system no longer requires this program, which was necessary for the mobility of cells in development.
Our central nervous system exhibits little cell movement. This is to do with programs of nervous-system development that are switched off in the mature nervous system. But they are then reactivated or remain active in the tumor cells. This process reveals potential starting points for therapy.
Addressing the movement of cells, that has been investigated for the last 20 years, but it seems to have an extraordinarily high number of side effects, because these movement mechanisms are also important for other, healthy cells in the body. For example, digestive mechanisms and other proliferation mechanisms, on mucous membranes, in the blood system, in the bone marrow, are then affected and no longer function.
There is another possible approach: the more-or-less specific interaction between the nerve cells and the tumor cells also offers starting points for therapies, from our point of view. The key word is epilepsy treatment. We know that people with brain tumors suffer badly, or worse than usual, from epileptic seizures. This was often regarded purely as a pressure problem. There is a disruptive element in the brain, and this causes the electrical activity in the brain to become disorganized. For some people, this can lead to seizures in certain situations.
The communication between tumor cells and nerve cells takes place via transmission substances, e.g., through the neurotransmitter glutamate. Now you can consider whether a “surplus” of communication, such as an excessively strong stimulus, can trigger epileptic seizures.
In this work, we demonstrate that by interrupting this communication, we can also prevent the movement of these cells and the growth, the proliferation, of these cells.
Q: What is the significance of parvoviruses for therapy?
A: The major topic for cancer is immunotherapy. And one option for performing immunotherapies lies with viruses. Parvoviruses are a plausible therapy for proliferating cells.
Parvoviruses are usually administered locally. This means that a surgical cavity is infected with the viruses and the tumor cells that remain after an operation will then hopefully be killed off by these viruses.
This is the first step and the immediate effect of virus therapy. The attempt is made to kill off cells in the same way as with a medication. The advantage of viruses is the high specificity, i.e., only dividing cells will be attacked. In addition, parvoviruses are so small that they can also spread well and circulate through the brain.
The second reason for immunotherapy is that when killing off cells with viruses, antigens are often released that otherwise would not be, depending on the virus. But it’s the case with parvoviruses. They integrate with the virus’s genetic material. When cells rupture, certain proteins are then revealed, hybrids of viruses and the human genome, and these are attractive to the immune system.
There is a whole range of studies on this subject. However, there are currently no randomized studies that directly compare the therapies. But the expectation is that the use of parvoviruses could be a good addition to therapy.
One limitation that should be mentioned is that the use of viruses may be beneficial for some patients, but it will not have an effect in every patient. What is exciting about parvoviruses is that these viruses can be injected via the bloodstream and still achieve a good effect in the brain.
Protein APG101
Q: How relevant is the recombinant protein APG101 to therapy?
A: APG101 is a protein that simulates the cell-death receptor CD95 and binds with a stable antibody fragment. By doing so, it blocks the signaling pathway between CD95 ligand and receptor. The interaction between the CD95 ligand and the CD95 receptor activates an intracellular signaling pathway, which in turn stimulates the invasive growth and migration of tumor cells.
APG101 blocks the CD95 ligand and thereby prevents the activation of the CD95 signaling pathway, which leads to a reduction in the invasive cell growth and migration.
Apoptosis, programmed cell death, is a system we have used throughout our evolution to kill off the cell components we no longer need. During tumor development, this system is perverted, so to speak. Here, the stimulation of this system does not actually lead to cell death but rather to cell movement (i.e., to cell mobility). And in principle, APG101 blocks this mobility.
To date, I only know of three studies in which the medication has been used for tumors. One study was published 8 years ago. We demonstrated that we can achieve a relatively good effect with APG101 in connection with repeat irradiation, compared with repeat irradiation alone. We consider this effect to most likely be due to this influence on cell mobility.
There is a study on primary therapy: a four-arm study by the Neuro-Oncological Working Group. The results are still not available, however. In addition, a study on primary therapy with APG101 is currently being conducted in China. It is investigating whether the mechanism of action influences mobility. Whether it will be pushed through as therapy remains to be seen.
Vaccinations and antigens
Q: Vaccinations are of course a part of immunotherapy. What is their status?
A: We are looking at the IDH1 protein, which is present in mutated form in a group of brain tumors, as a very good target for a vaccine. The reason is that the protein is present in its mutated form in every cell of the tumor but not in healthy cells. That is a prerequisite for immunotherapy.
We started a study with peptides a few years ago. These peptides are injected under the skin on the stomach and leg. They cause an immune response systemically and in the brain tumor. This immune response may cause an inflammatory reaction (we can demonstrate this inflammatory reaction). And in this noncontrolled study, the approach was successful, at least compared to historical controls. There is no randomized study with treatment-naive control patients.
However, we are cautious because we know that peptide, unlike CAR T cells or RNA-based vaccines, for example, only triggers a relatively small immune response in many patients. The scale of the immune response is important, rather than the specificity. The scale is probably not large enough in most patients for a long-term effect to be expected.
But there are exceptions. Patients we vaccinated many years ago still have a very remarkable immune status. But we also have patients in whom an immune status cannot even be seen anymore, after just a short period of time.
Therefore, our aim is to perform the immune strategy with more effective, stronger measures – not more specific, but stronger. Unfortunately, it is often the case with glioblastomas that there is not a single antigen that can be vaccinated against. Instead, a relatively large cocktail is needed, which unfortunately also often varies from patient to patient. The conditions are difficult.
Q: You mentioned that glioblastomas can be classified into subgroups. Does this improve the prognosis?
A: Yes, in certain subgroups the prognosis improves. That is the case with those usually very small groups that are molecularly well defined. I believe that by better understanding the individual groups, we have succeeded in making major progress in those groups. But where there is light, there is also shadow. We know that there are many groups with which we have not achieved a great deal.
Fundamental research leads to a better understanding, and the next step in this is to be able to adapt the therapy. Instead of it being one therapy for everyone, it will become a part of various differing therapies for these quite different groups. We are making a lot of progress with individual groups. But unfortunately, we have not come quite as far as we want with many patients.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
Every year, around 7,000 people in Germany develop a brain tumor, and around half of those cases involve a glioblastoma, a particularly aggressive form of the disease. Glioblastomas are incurable, but advances are being made in both diagnostics and therapy.
This news organization spoke to Wolfgang Wick, MD, medical director of the neurologic clinic at UKHD, about how glioblastomas are treated; the role that vaccinations, recombinant proteins, and parvoviruses play; and what therapeutic approaches might be derived from the discovery of this method by which glioblastomas spread.
Question: Glioblastomas spread through the brain like a fungal network. So how would a glioblastoma currently be treated? The tumor can only be partially removed through surgery.
Answer: Nevertheless, glioblastoma would be operated on. This would have a significant effect. Relieving the strain of the main tumor mass, without generating a new deficit, is prognostically very good for the patient concerned. However, surgery on glioblastoma is never curative.
The reason a cure is not possible is down to the special form and spread of the glioblastoma. Nevertheless, an operation helps. This seems to be because removing the main tumor mass maybe has a positive immunological effect. But it may also be connected to the tumor’s network communication. The surgical intervention stimulates the network by increasing resistance.
If the main tumor mass is decreased through a surgical procedure, this results in an at least temporarily improved starting position for the patient until the mass regenerates. This could also be connected to the fact that tumor communication is not unregulated but is rather in accordance with a certain hierarchy and order, which requires a certain structure and mass.
The other aspect is that support can be requested via this communication. You can imagine that a cell connected to another cell via a conduit receives help from this other cell in the form of organelles by exchanging ions and that, for example, stress or toxicity can be much better balanced out in large networks than in small networks. That means that external attacks, such as a surgical intervention, can be much better balanced by a well-organized network than by isolated cells.
Resistance to chemotherapy
Q: How do irradiation and chemotherapy rank in the treatment of glioblastomas?
A: Irradiation is another therapeutic approach. It causes cells to be stuck in the growth phase of the cell cycle. The cells are not killed through radiation, but they are practically halted. And this arrest of the cell cycle is often sufficient to help people with glioblastomas for a very long time. But the same is true for irradiation as for surgery. This deep network of cells cannot be addressed.
Attempts have been made in the past to reduce the radiation dose to the extent that the brain is no longer damaged by it, but this low dose was then not sufficient to exert any control. If you want to control the tumor, the dose must be high and the volume must be correspondingly low, since there is a clear limit.
Every patient is offered alkylating chemotherapy. At the moment, just one substance is used here in the primary therapy: temozolomide. The problem with this is that two-thirds of tumors in all cells exhibit a resistance to this alkylating chemotherapy, which means that the efficacy of this therapy is highly limited in two-thirds of patients.
In the one-third of patients in whom this resistance is not present, the chemotherapy works fairly well. But even then, it is unfortunately only a matter of time until there is a relapse or disease progression. In my practice, this has always been the case, but there are people who have been living with this disease for 20 years now. There seem to be tumor cells that calmly and silently survive this phase of chemotherapy and then restart the cell cycle at some point.
Q: What do you think of alternating electric fields as a therapy option?
A: Therapy with alternating electric fields is currently being used and offered to patients. This means that patients who have survived well through radiochemotherapy should also be offered treatment with alternating electric fields.
However, what happens in this process is not as well understood as with other therapies. It is assumed that the cell cycle, i.e., cell division, is altered by disrupting the mitotic spindle. But you can imagine, and this is now speculation, but quite sound speculation I believe, that alternating electric fields also cause a certain amount of confusion in the previously described networks. But this still needs to be investigated in more detail.
It is not implausible. We know that such alternating electric fields disturb the organization of cell organelles. And we also know that for this communication, we need fairly good order and also organization. This would definitely be a starting point on the way to understanding why this therapy potentially shows a certain effect in some patients.
Nerve cell precursors
Q: Scientists from the UKHD and the DKFZ have discovered a new glioblastoma spreading strategy and have learned that the tumor cells imitate the properties and movement patterns of nerve cells. They are labeling the results a “milestone in the field of cancer neuroscience.” Could you explain a bit more?
A: Glioblastoma does not grow on its own as a solid mass, but instead, the entire brain is affected by the disease. The question of how the tumor’s individual cells move the main tumor mass from afar, how they get there, how they continue to be supplied, and what their interaction partners are – an entirely new light has been shed on all of this in our work.
The development of tumor cell mobility has been recognized as a remnant of brain development. The tumor cells have retained properties that the precursor cells for nervous-system development require for an organized nervous system to emerge from just a few cells. This means that the tumor cells copy or eventually retain properties of the nerve-cell precursors that, unlike mature nerve cells, are mobile to a fairly high degree.
Mobility here means that it can advance along a network, despite said network being very densely packed. This also means that certain processes, such as releasing and then continuing to move again, must function and that the communication regarding the original disease must be maintained.
First, we understand what the different glioblastoma cell types do, which molecular properties are associated with which behaviors, and which cell type (namely the swarming cells) is responsible for the invasive tumor growth. In contrast, the network-forming cell type, which only develops from these, is responsible for the resistance.
Interrupting communication
Q: Which starting points for new therapies do you see?
A: In terms of new therapies, these movement phenomena are one good starting point. The other starting point – I find this one much more interesting – is that the programming steps that these tumor cells use [are] no longer needed. This is because our mature nervous system no longer requires this program, which was necessary for the mobility of cells in development.
Our central nervous system exhibits little cell movement. This is to do with programs of nervous-system development that are switched off in the mature nervous system. But they are then reactivated or remain active in the tumor cells. This process reveals potential starting points for therapy.
Addressing the movement of cells, that has been investigated for the last 20 years, but it seems to have an extraordinarily high number of side effects, because these movement mechanisms are also important for other, healthy cells in the body. For example, digestive mechanisms and other proliferation mechanisms, on mucous membranes, in the blood system, in the bone marrow, are then affected and no longer function.
There is another possible approach: the more-or-less specific interaction between the nerve cells and the tumor cells also offers starting points for therapies, from our point of view. The key word is epilepsy treatment. We know that people with brain tumors suffer badly, or worse than usual, from epileptic seizures. This was often regarded purely as a pressure problem. There is a disruptive element in the brain, and this causes the electrical activity in the brain to become disorganized. For some people, this can lead to seizures in certain situations.
The communication between tumor cells and nerve cells takes place via transmission substances, e.g., through the neurotransmitter glutamate. Now you can consider whether a “surplus” of communication, such as an excessively strong stimulus, can trigger epileptic seizures.
In this work, we demonstrate that by interrupting this communication, we can also prevent the movement of these cells and the growth, the proliferation, of these cells.
Q: What is the significance of parvoviruses for therapy?
A: The major topic for cancer is immunotherapy. And one option for performing immunotherapies lies with viruses. Parvoviruses are a plausible therapy for proliferating cells.
Parvoviruses are usually administered locally. This means that a surgical cavity is infected with the viruses and the tumor cells that remain after an operation will then hopefully be killed off by these viruses.
This is the first step and the immediate effect of virus therapy. The attempt is made to kill off cells in the same way as with a medication. The advantage of viruses is the high specificity, i.e., only dividing cells will be attacked. In addition, parvoviruses are so small that they can also spread well and circulate through the brain.
The second reason for immunotherapy is that when killing off cells with viruses, antigens are often released that otherwise would not be, depending on the virus. But it’s the case with parvoviruses. They integrate with the virus’s genetic material. When cells rupture, certain proteins are then revealed, hybrids of viruses and the human genome, and these are attractive to the immune system.
There is a whole range of studies on this subject. However, there are currently no randomized studies that directly compare the therapies. But the expectation is that the use of parvoviruses could be a good addition to therapy.
One limitation that should be mentioned is that the use of viruses may be beneficial for some patients, but it will not have an effect in every patient. What is exciting about parvoviruses is that these viruses can be injected via the bloodstream and still achieve a good effect in the brain.
Protein APG101
Q: How relevant is the recombinant protein APG101 to therapy?
A: APG101 is a protein that simulates the cell-death receptor CD95 and binds with a stable antibody fragment. By doing so, it blocks the signaling pathway between CD95 ligand and receptor. The interaction between the CD95 ligand and the CD95 receptor activates an intracellular signaling pathway, which in turn stimulates the invasive growth and migration of tumor cells.
APG101 blocks the CD95 ligand and thereby prevents the activation of the CD95 signaling pathway, which leads to a reduction in the invasive cell growth and migration.
Apoptosis, programmed cell death, is a system we have used throughout our evolution to kill off the cell components we no longer need. During tumor development, this system is perverted, so to speak. Here, the stimulation of this system does not actually lead to cell death but rather to cell movement (i.e., to cell mobility). And in principle, APG101 blocks this mobility.
To date, I only know of three studies in which the medication has been used for tumors. One study was published 8 years ago. We demonstrated that we can achieve a relatively good effect with APG101 in connection with repeat irradiation, compared with repeat irradiation alone. We consider this effect to most likely be due to this influence on cell mobility.
There is a study on primary therapy: a four-arm study by the Neuro-Oncological Working Group. The results are still not available, however. In addition, a study on primary therapy with APG101 is currently being conducted in China. It is investigating whether the mechanism of action influences mobility. Whether it will be pushed through as therapy remains to be seen.
Vaccinations and antigens
Q: Vaccinations are of course a part of immunotherapy. What is their status?
A: We are looking at the IDH1 protein, which is present in mutated form in a group of brain tumors, as a very good target for a vaccine. The reason is that the protein is present in its mutated form in every cell of the tumor but not in healthy cells. That is a prerequisite for immunotherapy.
We started a study with peptides a few years ago. These peptides are injected under the skin on the stomach and leg. They cause an immune response systemically and in the brain tumor. This immune response may cause an inflammatory reaction (we can demonstrate this inflammatory reaction). And in this noncontrolled study, the approach was successful, at least compared to historical controls. There is no randomized study with treatment-naive control patients.
However, we are cautious because we know that peptide, unlike CAR T cells or RNA-based vaccines, for example, only triggers a relatively small immune response in many patients. The scale of the immune response is important, rather than the specificity. The scale is probably not large enough in most patients for a long-term effect to be expected.
But there are exceptions. Patients we vaccinated many years ago still have a very remarkable immune status. But we also have patients in whom an immune status cannot even be seen anymore, after just a short period of time.
Therefore, our aim is to perform the immune strategy with more effective, stronger measures – not more specific, but stronger. Unfortunately, it is often the case with glioblastomas that there is not a single antigen that can be vaccinated against. Instead, a relatively large cocktail is needed, which unfortunately also often varies from patient to patient. The conditions are difficult.
Q: You mentioned that glioblastomas can be classified into subgroups. Does this improve the prognosis?
A: Yes, in certain subgroups the prognosis improves. That is the case with those usually very small groups that are molecularly well defined. I believe that by better understanding the individual groups, we have succeeded in making major progress in those groups. But where there is light, there is also shadow. We know that there are many groups with which we have not achieved a great deal.
Fundamental research leads to a better understanding, and the next step in this is to be able to adapt the therapy. Instead of it being one therapy for everyone, it will become a part of various differing therapies for these quite different groups. We are making a lot of progress with individual groups. But unfortunately, we have not come quite as far as we want with many patients.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
Every year, around 7,000 people in Germany develop a brain tumor, and around half of those cases involve a glioblastoma, a particularly aggressive form of the disease. Glioblastomas are incurable, but advances are being made in both diagnostics and therapy.
This news organization spoke to Wolfgang Wick, MD, medical director of the neurologic clinic at UKHD, about how glioblastomas are treated; the role that vaccinations, recombinant proteins, and parvoviruses play; and what therapeutic approaches might be derived from the discovery of this method by which glioblastomas spread.
Question: Glioblastomas spread through the brain like a fungal network. So how would a glioblastoma currently be treated? The tumor can only be partially removed through surgery.
Answer: Nevertheless, glioblastoma would be operated on. This would have a significant effect. Relieving the strain of the main tumor mass, without generating a new deficit, is prognostically very good for the patient concerned. However, surgery on glioblastoma is never curative.
The reason a cure is not possible is down to the special form and spread of the glioblastoma. Nevertheless, an operation helps. This seems to be because removing the main tumor mass maybe has a positive immunological effect. But it may also be connected to the tumor’s network communication. The surgical intervention stimulates the network by increasing resistance.
If the main tumor mass is decreased through a surgical procedure, this results in an at least temporarily improved starting position for the patient until the mass regenerates. This could also be connected to the fact that tumor communication is not unregulated but is rather in accordance with a certain hierarchy and order, which requires a certain structure and mass.
The other aspect is that support can be requested via this communication. You can imagine that a cell connected to another cell via a conduit receives help from this other cell in the form of organelles by exchanging ions and that, for example, stress or toxicity can be much better balanced out in large networks than in small networks. That means that external attacks, such as a surgical intervention, can be much better balanced by a well-organized network than by isolated cells.
Resistance to chemotherapy
Q: How do irradiation and chemotherapy rank in the treatment of glioblastomas?
A: Irradiation is another therapeutic approach. It causes cells to be stuck in the growth phase of the cell cycle. The cells are not killed through radiation, but they are practically halted. And this arrest of the cell cycle is often sufficient to help people with glioblastomas for a very long time. But the same is true for irradiation as for surgery. This deep network of cells cannot be addressed.
Attempts have been made in the past to reduce the radiation dose to the extent that the brain is no longer damaged by it, but this low dose was then not sufficient to exert any control. If you want to control the tumor, the dose must be high and the volume must be correspondingly low, since there is a clear limit.
Every patient is offered alkylating chemotherapy. At the moment, just one substance is used here in the primary therapy: temozolomide. The problem with this is that two-thirds of tumors in all cells exhibit a resistance to this alkylating chemotherapy, which means that the efficacy of this therapy is highly limited in two-thirds of patients.
In the one-third of patients in whom this resistance is not present, the chemotherapy works fairly well. But even then, it is unfortunately only a matter of time until there is a relapse or disease progression. In my practice, this has always been the case, but there are people who have been living with this disease for 20 years now. There seem to be tumor cells that calmly and silently survive this phase of chemotherapy and then restart the cell cycle at some point.
Q: What do you think of alternating electric fields as a therapy option?
A: Therapy with alternating electric fields is currently being used and offered to patients. This means that patients who have survived well through radiochemotherapy should also be offered treatment with alternating electric fields.
However, what happens in this process is not as well understood as with other therapies. It is assumed that the cell cycle, i.e., cell division, is altered by disrupting the mitotic spindle. But you can imagine, and this is now speculation, but quite sound speculation I believe, that alternating electric fields also cause a certain amount of confusion in the previously described networks. But this still needs to be investigated in more detail.
It is not implausible. We know that such alternating electric fields disturb the organization of cell organelles. And we also know that for this communication, we need fairly good order and also organization. This would definitely be a starting point on the way to understanding why this therapy potentially shows a certain effect in some patients.
Nerve cell precursors
Q: Scientists from the UKHD and the DKFZ have discovered a new glioblastoma spreading strategy and have learned that the tumor cells imitate the properties and movement patterns of nerve cells. They are labeling the results a “milestone in the field of cancer neuroscience.” Could you explain a bit more?
A: Glioblastoma does not grow on its own as a solid mass, but instead, the entire brain is affected by the disease. The question of how the tumor’s individual cells move the main tumor mass from afar, how they get there, how they continue to be supplied, and what their interaction partners are – an entirely new light has been shed on all of this in our work.
The development of tumor cell mobility has been recognized as a remnant of brain development. The tumor cells have retained properties that the precursor cells for nervous-system development require for an organized nervous system to emerge from just a few cells. This means that the tumor cells copy or eventually retain properties of the nerve-cell precursors that, unlike mature nerve cells, are mobile to a fairly high degree.
Mobility here means that it can advance along a network, despite said network being very densely packed. This also means that certain processes, such as releasing and then continuing to move again, must function and that the communication regarding the original disease must be maintained.
First, we understand what the different glioblastoma cell types do, which molecular properties are associated with which behaviors, and which cell type (namely the swarming cells) is responsible for the invasive tumor growth. In contrast, the network-forming cell type, which only develops from these, is responsible for the resistance.
Interrupting communication
Q: Which starting points for new therapies do you see?
A: In terms of new therapies, these movement phenomena are one good starting point. The other starting point – I find this one much more interesting – is that the programming steps that these tumor cells use [are] no longer needed. This is because our mature nervous system no longer requires this program, which was necessary for the mobility of cells in development.
Our central nervous system exhibits little cell movement. This is to do with programs of nervous-system development that are switched off in the mature nervous system. But they are then reactivated or remain active in the tumor cells. This process reveals potential starting points for therapy.
Addressing the movement of cells, that has been investigated for the last 20 years, but it seems to have an extraordinarily high number of side effects, because these movement mechanisms are also important for other, healthy cells in the body. For example, digestive mechanisms and other proliferation mechanisms, on mucous membranes, in the blood system, in the bone marrow, are then affected and no longer function.
There is another possible approach: the more-or-less specific interaction between the nerve cells and the tumor cells also offers starting points for therapies, from our point of view. The key word is epilepsy treatment. We know that people with brain tumors suffer badly, or worse than usual, from epileptic seizures. This was often regarded purely as a pressure problem. There is a disruptive element in the brain, and this causes the electrical activity in the brain to become disorganized. For some people, this can lead to seizures in certain situations.
The communication between tumor cells and nerve cells takes place via transmission substances, e.g., through the neurotransmitter glutamate. Now you can consider whether a “surplus” of communication, such as an excessively strong stimulus, can trigger epileptic seizures.
In this work, we demonstrate that by interrupting this communication, we can also prevent the movement of these cells and the growth, the proliferation, of these cells.
Q: What is the significance of parvoviruses for therapy?
A: The major topic for cancer is immunotherapy. And one option for performing immunotherapies lies with viruses. Parvoviruses are a plausible therapy for proliferating cells.
Parvoviruses are usually administered locally. This means that a surgical cavity is infected with the viruses and the tumor cells that remain after an operation will then hopefully be killed off by these viruses.
This is the first step and the immediate effect of virus therapy. The attempt is made to kill off cells in the same way as with a medication. The advantage of viruses is the high specificity, i.e., only dividing cells will be attacked. In addition, parvoviruses are so small that they can also spread well and circulate through the brain.
The second reason for immunotherapy is that when killing off cells with viruses, antigens are often released that otherwise would not be, depending on the virus. But it’s the case with parvoviruses. They integrate with the virus’s genetic material. When cells rupture, certain proteins are then revealed, hybrids of viruses and the human genome, and these are attractive to the immune system.
There is a whole range of studies on this subject. However, there are currently no randomized studies that directly compare the therapies. But the expectation is that the use of parvoviruses could be a good addition to therapy.
One limitation that should be mentioned is that the use of viruses may be beneficial for some patients, but it will not have an effect in every patient. What is exciting about parvoviruses is that these viruses can be injected via the bloodstream and still achieve a good effect in the brain.
Protein APG101
Q: How relevant is the recombinant protein APG101 to therapy?
A: APG101 is a protein that simulates the cell-death receptor CD95 and binds with a stable antibody fragment. By doing so, it blocks the signaling pathway between CD95 ligand and receptor. The interaction between the CD95 ligand and the CD95 receptor activates an intracellular signaling pathway, which in turn stimulates the invasive growth and migration of tumor cells.
APG101 blocks the CD95 ligand and thereby prevents the activation of the CD95 signaling pathway, which leads to a reduction in the invasive cell growth and migration.
Apoptosis, programmed cell death, is a system we have used throughout our evolution to kill off the cell components we no longer need. During tumor development, this system is perverted, so to speak. Here, the stimulation of this system does not actually lead to cell death but rather to cell movement (i.e., to cell mobility). And in principle, APG101 blocks this mobility.
To date, I only know of three studies in which the medication has been used for tumors. One study was published 8 years ago. We demonstrated that we can achieve a relatively good effect with APG101 in connection with repeat irradiation, compared with repeat irradiation alone. We consider this effect to most likely be due to this influence on cell mobility.
There is a study on primary therapy: a four-arm study by the Neuro-Oncological Working Group. The results are still not available, however. In addition, a study on primary therapy with APG101 is currently being conducted in China. It is investigating whether the mechanism of action influences mobility. Whether it will be pushed through as therapy remains to be seen.
Vaccinations and antigens
Q: Vaccinations are of course a part of immunotherapy. What is their status?
A: We are looking at the IDH1 protein, which is present in mutated form in a group of brain tumors, as a very good target for a vaccine. The reason is that the protein is present in its mutated form in every cell of the tumor but not in healthy cells. That is a prerequisite for immunotherapy.
We started a study with peptides a few years ago. These peptides are injected under the skin on the stomach and leg. They cause an immune response systemically and in the brain tumor. This immune response may cause an inflammatory reaction (we can demonstrate this inflammatory reaction). And in this noncontrolled study, the approach was successful, at least compared to historical controls. There is no randomized study with treatment-naive control patients.
However, we are cautious because we know that peptide, unlike CAR T cells or RNA-based vaccines, for example, only triggers a relatively small immune response in many patients. The scale of the immune response is important, rather than the specificity. The scale is probably not large enough in most patients for a long-term effect to be expected.
But there are exceptions. Patients we vaccinated many years ago still have a very remarkable immune status. But we also have patients in whom an immune status cannot even be seen anymore, after just a short period of time.
Therefore, our aim is to perform the immune strategy with more effective, stronger measures – not more specific, but stronger. Unfortunately, it is often the case with glioblastomas that there is not a single antigen that can be vaccinated against. Instead, a relatively large cocktail is needed, which unfortunately also often varies from patient to patient. The conditions are difficult.
Q: You mentioned that glioblastomas can be classified into subgroups. Does this improve the prognosis?
A: Yes, in certain subgroups the prognosis improves. That is the case with those usually very small groups that are molecularly well defined. I believe that by better understanding the individual groups, we have succeeded in making major progress in those groups. But where there is light, there is also shadow. We know that there are many groups with which we have not achieved a great deal.
Fundamental research leads to a better understanding, and the next step in this is to be able to adapt the therapy. Instead of it being one therapy for everyone, it will become a part of various differing therapies for these quite different groups. We are making a lot of progress with individual groups. But unfortunately, we have not come quite as far as we want with many patients.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.