User login
Assessing Treatment Delays for Vitiligo Patients: A Retrospective Chart Review
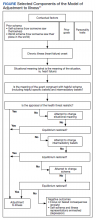
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
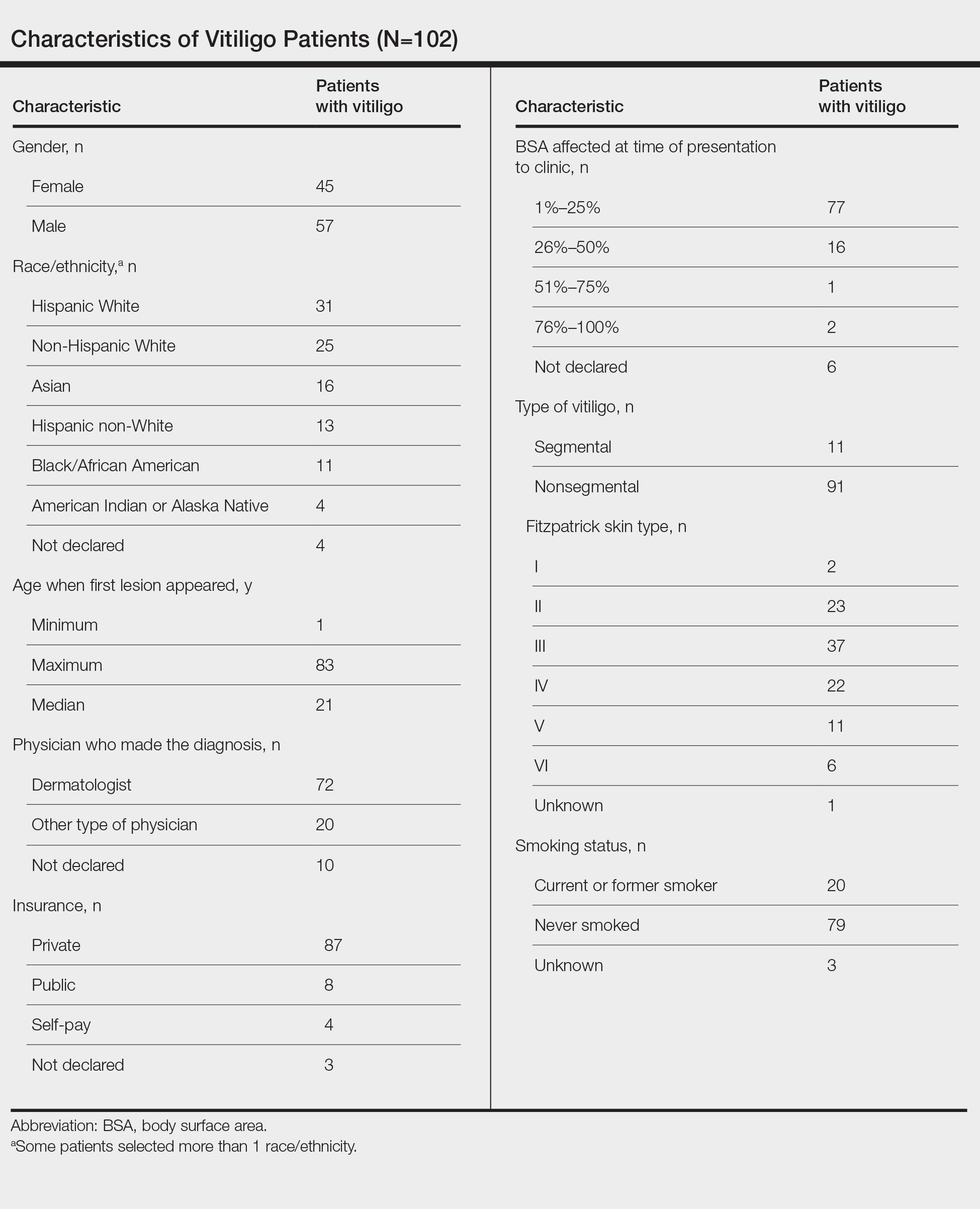
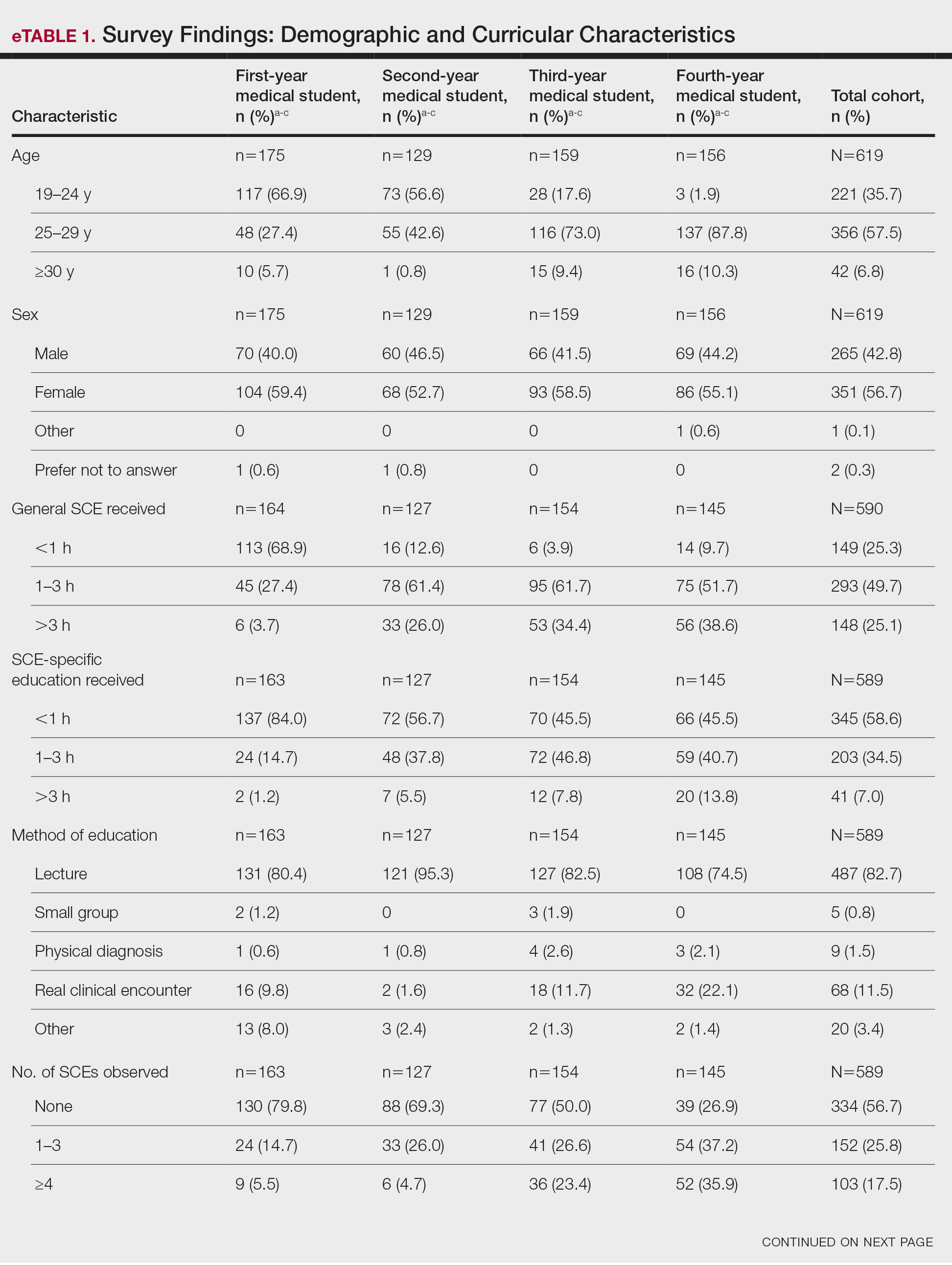
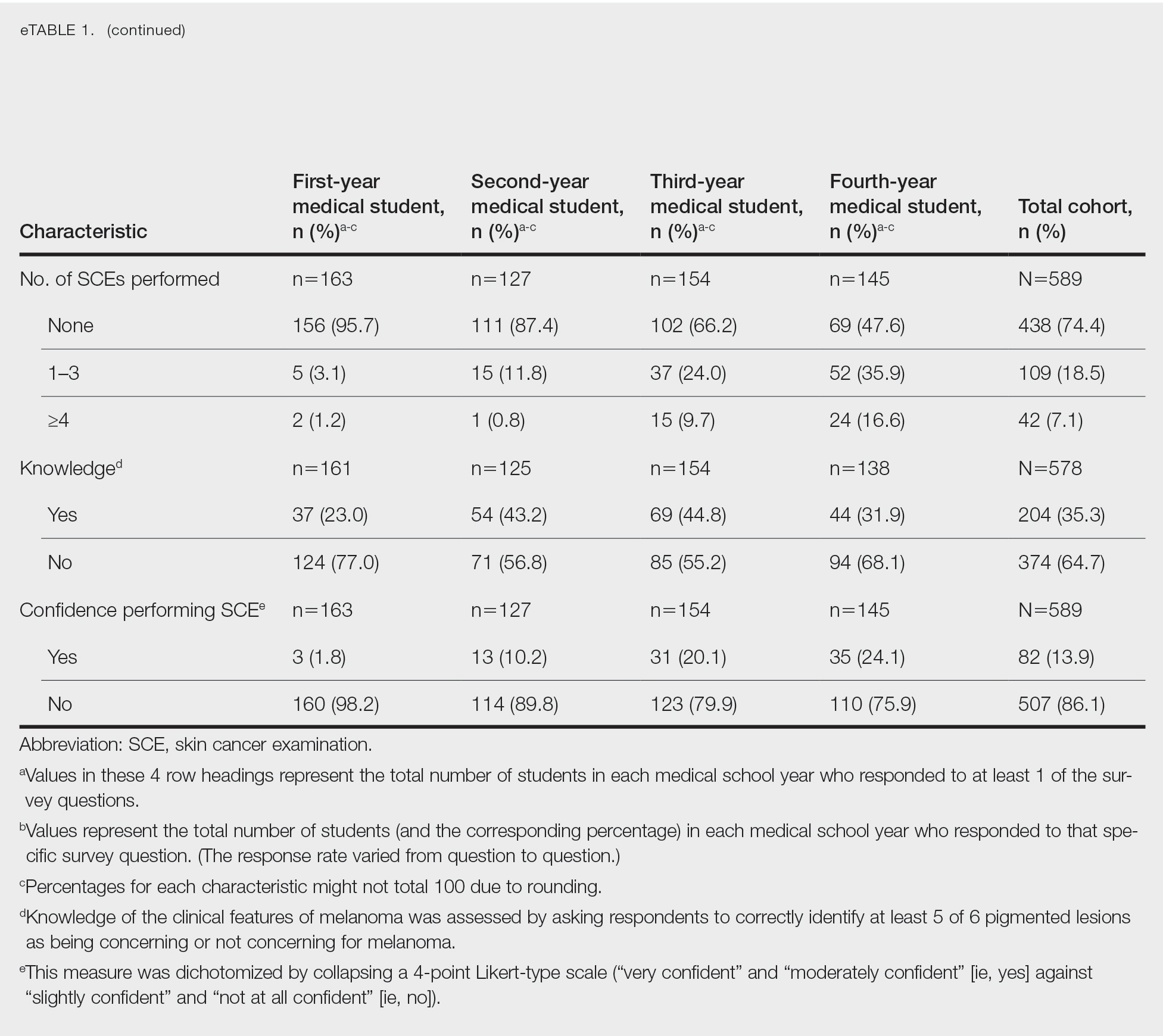
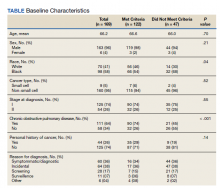
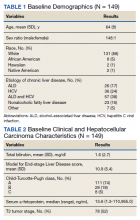
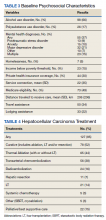
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.
Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
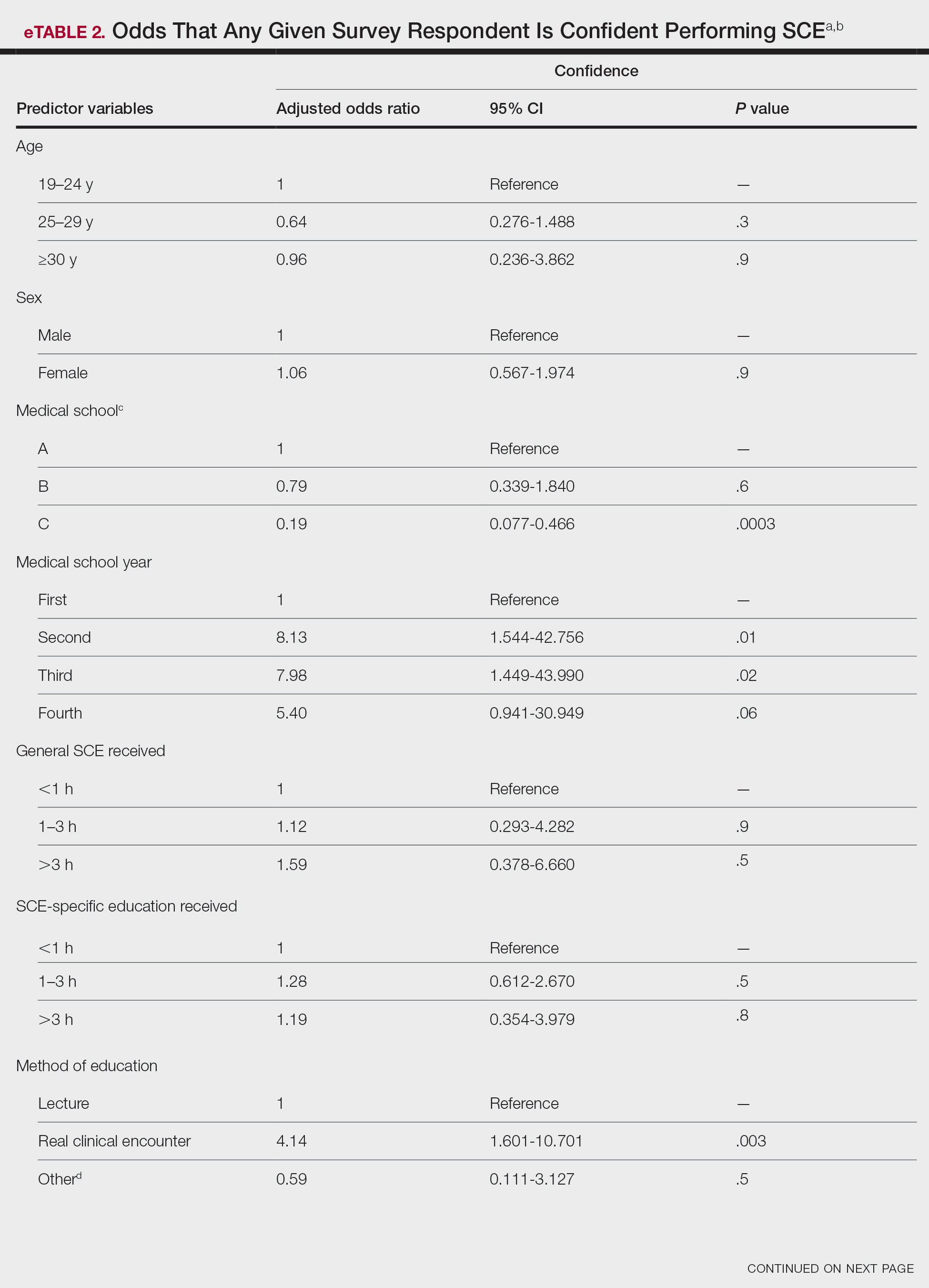
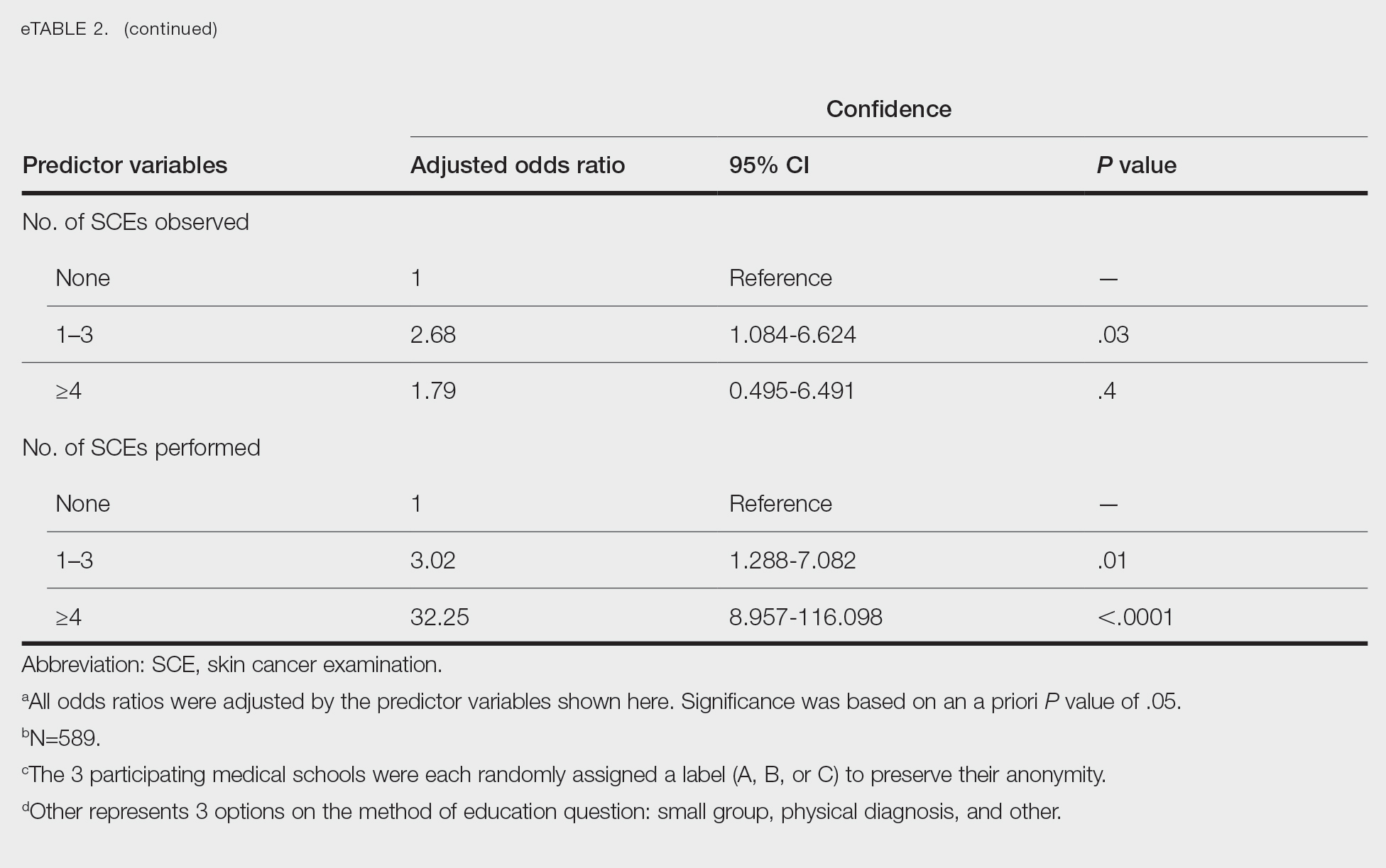
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.
Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.
Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
Practice Points
- The medical community should be aware of factors associated with delay to treatment in patients with vitiligo, such as the diagnosing physician’s specialty and patient age at disease onset.
- Race and percentage of body surface area affected at time of presentation also demonstrate trends regarding treatment delays in patients with vitiligo.
Intravenous Immunoglobulin in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia: A Pharmacoeconomic Analysis
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
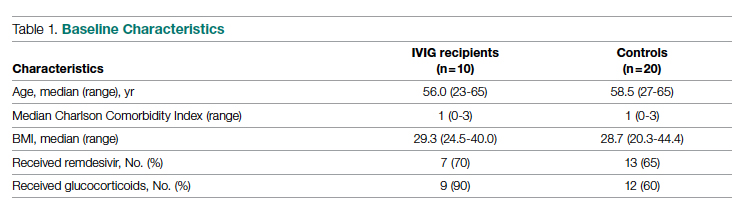
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
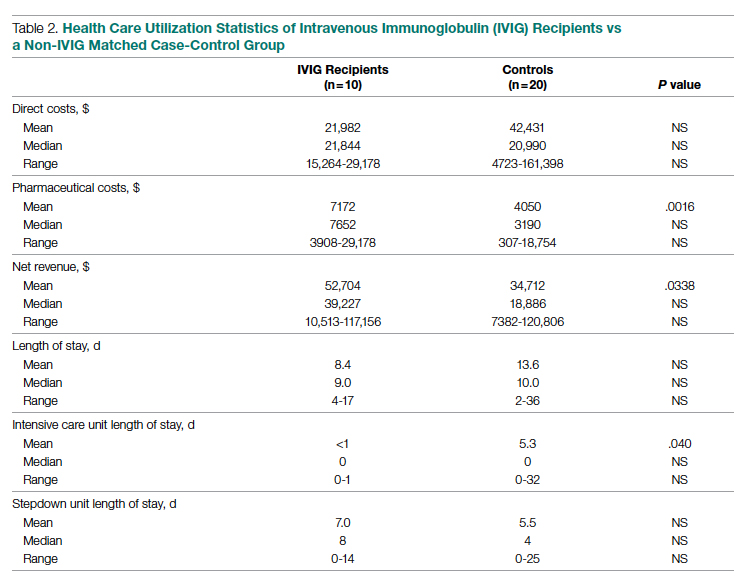
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).
Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
Skin Cancer Education in the Medical School Curriculum
To the Editor:
Skin cancer represents a notable health care burden of rising incidence.1-3 Nondermatologist health care providers play a key role in skin cancer screening through the use of skin cancer examination (SCE)1,4; however, several factors including poor diagnostic accuracy, low confidence, and lack of training have contributed to limited use of the SCE by these providers.4,5 Therefore, it is important to identify and implement changes in the medical school curriculum that can facilitate improved use of SCE in clinical practice. We sought to examine factors in the medical school curriculum that influence skin cancer education.
A voluntary electronic survey was distributed through class email and social media to all medical student classes at 4 medical schools (Figure). Responses were collected between March 2 and April 20, 2020. Survey items assessed demographics and curricular factors that influence skin cancer education.
Knowledge of the clinical features of melanoma was assessed by asking participants to correctly identify at least 5 of 6 pigmented lesions as concerning or not concerning for melanoma. Confidence in performing the SCE—the primary outcome—was measured by dichotomizing a 4-point Likert-type scale (“very confident” and “moderately confident” against “slightly confident” and “not at all confident”).
Logistic regression was used to examine curricular factors associated with confidence; descriptive statistics were used for remaining analyses. Analyses were performed using SAS 9.4 statistical software. Prior to analysis, responses from the University of South Carolina School of Medicine Greenville were excluded because the response rate was less than 20%.
The survey was distributed to 1524 students; 619 (40.6%) answered at least 1 question, with a variable response rate to each item (eTable 1). Most respondents were female (351 [56.7%]); 438 (70.8%) were White.
Most respondents said that they received 3 hours or less of general skin cancer (74.9%) or SCE-specific (93.0%) education by the end of their fourth year of medical training. Lecture was the most common method of instruction. Education was provided most often by dermatologists (48.6%), followed by general practice physicians (21.2%). Numerous (26.9%) fourth-year respondents reported that they had never observed SCE; even more (47.6%) had never performed SCE. Almost half of second- and third-year students (43.2% and 44.8%, respectively) considered themselves knowledgeable about the clinical features of melanoma, but only 31.9% of fourth-year students considered themselves knowledgeable.
Only 24.1% of fourth-year students reported confidence performing SCE (eTable 1). Students who received most of their instruction through real clinical encounters were 4.14 times more likely to be confident performing SCE than students who had been given lecture-based learning. Students who performed 1 to 3 SCE or 4 or more SCE were 3.02 and 32.25 times, respectively, more likely to be confident than students who had never performed SCE (eTable 2).
Consistent with a recent study,6 our results reflect the discrepancy between the burden and education of skin cancer. This is especially demonstrated by our cohort’s low confidence in performing SCE, a metric associated with both intention to perform and actual performance of SCE in practice.4,5 We also observed a downward trend in knowledge among students who were about to enter residency, potentially indicating the need for longitudinal training.
Given curricular time constraints, it is essential that medical schools implement changes in learning that will have the greatest impact. Although our results strongly support the efficacy of hands-on clinical training, exposure to dermatology in the second half of medical school training is limited nationwide.6 Concentrated efforts to increase clinical exposure might help prepare future physicians in all specialties to combat the burden of this disease.
Limitations of our study include the potential for selection and recall biases. Although our survey spanned multiple institutions in different regions of the United States, results might not be universally representative.
Acknowledgments—We thank Dirk Elston, MD, and Amy Wahlquist, MS (both from Charleston, South Carolina), who helped facilitate the survey on which our research is based. We also acknowledge the assistance of Philip Carmon, MD (Columbia, South Carolina); Julie Flugel (Columbia, South Carolina); Algimantas Simpson, MD (Columbia, South Carolina); Nathan Jasperse, MD (Irvine, California); Jeremy Teruel, MD (Charleston, South Carolina); Alan Snyder, MD, MSCR (Charleston, South Carolina); John Bosland (Charleston, South Carolina); and Daniel Spangler (Greenville, South Carolina).
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48:183-187. doi:10.1016/j.amepre.2014.08.036
- Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57-64. doi:10.1001/jamadermatol.2019.3353
- Lim HW, Collins SAB, Resneck JS Jr, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151-1160.e21. doi:10.1016/j.jaad.2017.03.006
- Garg A, Wang J, Reddy SB, et al; Integrated Skin Exam Consortium. Curricular factors associated with medical students’ practice of the skin cancer examination: an educational enhancement initiative by the Integrated Skin Exam Consortium. JAMA Dermatol. 2014;150:850-855. doi:10.1001/jamadermatol.2013.8723
- Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. doi:10.1001/archdermatol.2010.414
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470. doi:10.1001/jamadermatol.2020.0006
To the Editor:
Skin cancer represents a notable health care burden of rising incidence.1-3 Nondermatologist health care providers play a key role in skin cancer screening through the use of skin cancer examination (SCE)1,4; however, several factors including poor diagnostic accuracy, low confidence, and lack of training have contributed to limited use of the SCE by these providers.4,5 Therefore, it is important to identify and implement changes in the medical school curriculum that can facilitate improved use of SCE in clinical practice. We sought to examine factors in the medical school curriculum that influence skin cancer education.
A voluntary electronic survey was distributed through class email and social media to all medical student classes at 4 medical schools (Figure). Responses were collected between March 2 and April 20, 2020. Survey items assessed demographics and curricular factors that influence skin cancer education.
Knowledge of the clinical features of melanoma was assessed by asking participants to correctly identify at least 5 of 6 pigmented lesions as concerning or not concerning for melanoma. Confidence in performing the SCE—the primary outcome—was measured by dichotomizing a 4-point Likert-type scale (“very confident” and “moderately confident” against “slightly confident” and “not at all confident”).
Logistic regression was used to examine curricular factors associated with confidence; descriptive statistics were used for remaining analyses. Analyses were performed using SAS 9.4 statistical software. Prior to analysis, responses from the University of South Carolina School of Medicine Greenville were excluded because the response rate was less than 20%.
The survey was distributed to 1524 students; 619 (40.6%) answered at least 1 question, with a variable response rate to each item (eTable 1). Most respondents were female (351 [56.7%]); 438 (70.8%) were White.
Most respondents said that they received 3 hours or less of general skin cancer (74.9%) or SCE-specific (93.0%) education by the end of their fourth year of medical training. Lecture was the most common method of instruction. Education was provided most often by dermatologists (48.6%), followed by general practice physicians (21.2%). Numerous (26.9%) fourth-year respondents reported that they had never observed SCE; even more (47.6%) had never performed SCE. Almost half of second- and third-year students (43.2% and 44.8%, respectively) considered themselves knowledgeable about the clinical features of melanoma, but only 31.9% of fourth-year students considered themselves knowledgeable.
Only 24.1% of fourth-year students reported confidence performing SCE (eTable 1). Students who received most of their instruction through real clinical encounters were 4.14 times more likely to be confident performing SCE than students who had been given lecture-based learning. Students who performed 1 to 3 SCE or 4 or more SCE were 3.02 and 32.25 times, respectively, more likely to be confident than students who had never performed SCE (eTable 2).
Consistent with a recent study,6 our results reflect the discrepancy between the burden and education of skin cancer. This is especially demonstrated by our cohort’s low confidence in performing SCE, a metric associated with both intention to perform and actual performance of SCE in practice.4,5 We also observed a downward trend in knowledge among students who were about to enter residency, potentially indicating the need for longitudinal training.
Given curricular time constraints, it is essential that medical schools implement changes in learning that will have the greatest impact. Although our results strongly support the efficacy of hands-on clinical training, exposure to dermatology in the second half of medical school training is limited nationwide.6 Concentrated efforts to increase clinical exposure might help prepare future physicians in all specialties to combat the burden of this disease.
Limitations of our study include the potential for selection and recall biases. Although our survey spanned multiple institutions in different regions of the United States, results might not be universally representative.
Acknowledgments—We thank Dirk Elston, MD, and Amy Wahlquist, MS (both from Charleston, South Carolina), who helped facilitate the survey on which our research is based. We also acknowledge the assistance of Philip Carmon, MD (Columbia, South Carolina); Julie Flugel (Columbia, South Carolina); Algimantas Simpson, MD (Columbia, South Carolina); Nathan Jasperse, MD (Irvine, California); Jeremy Teruel, MD (Charleston, South Carolina); Alan Snyder, MD, MSCR (Charleston, South Carolina); John Bosland (Charleston, South Carolina); and Daniel Spangler (Greenville, South Carolina).
To the Editor:
Skin cancer represents a notable health care burden of rising incidence.1-3 Nondermatologist health care providers play a key role in skin cancer screening through the use of skin cancer examination (SCE)1,4; however, several factors including poor diagnostic accuracy, low confidence, and lack of training have contributed to limited use of the SCE by these providers.4,5 Therefore, it is important to identify and implement changes in the medical school curriculum that can facilitate improved use of SCE in clinical practice. We sought to examine factors in the medical school curriculum that influence skin cancer education.
A voluntary electronic survey was distributed through class email and social media to all medical student classes at 4 medical schools (Figure). Responses were collected between March 2 and April 20, 2020. Survey items assessed demographics and curricular factors that influence skin cancer education.
Knowledge of the clinical features of melanoma was assessed by asking participants to correctly identify at least 5 of 6 pigmented lesions as concerning or not concerning for melanoma. Confidence in performing the SCE—the primary outcome—was measured by dichotomizing a 4-point Likert-type scale (“very confident” and “moderately confident” against “slightly confident” and “not at all confident”).
Logistic regression was used to examine curricular factors associated with confidence; descriptive statistics were used for remaining analyses. Analyses were performed using SAS 9.4 statistical software. Prior to analysis, responses from the University of South Carolina School of Medicine Greenville were excluded because the response rate was less than 20%.
The survey was distributed to 1524 students; 619 (40.6%) answered at least 1 question, with a variable response rate to each item (eTable 1). Most respondents were female (351 [56.7%]); 438 (70.8%) were White.
Most respondents said that they received 3 hours or less of general skin cancer (74.9%) or SCE-specific (93.0%) education by the end of their fourth year of medical training. Lecture was the most common method of instruction. Education was provided most often by dermatologists (48.6%), followed by general practice physicians (21.2%). Numerous (26.9%) fourth-year respondents reported that they had never observed SCE; even more (47.6%) had never performed SCE. Almost half of second- and third-year students (43.2% and 44.8%, respectively) considered themselves knowledgeable about the clinical features of melanoma, but only 31.9% of fourth-year students considered themselves knowledgeable.
Only 24.1% of fourth-year students reported confidence performing SCE (eTable 1). Students who received most of their instruction through real clinical encounters were 4.14 times more likely to be confident performing SCE than students who had been given lecture-based learning. Students who performed 1 to 3 SCE or 4 or more SCE were 3.02 and 32.25 times, respectively, more likely to be confident than students who had never performed SCE (eTable 2).
Consistent with a recent study,6 our results reflect the discrepancy between the burden and education of skin cancer. This is especially demonstrated by our cohort’s low confidence in performing SCE, a metric associated with both intention to perform and actual performance of SCE in practice.4,5 We also observed a downward trend in knowledge among students who were about to enter residency, potentially indicating the need for longitudinal training.
Given curricular time constraints, it is essential that medical schools implement changes in learning that will have the greatest impact. Although our results strongly support the efficacy of hands-on clinical training, exposure to dermatology in the second half of medical school training is limited nationwide.6 Concentrated efforts to increase clinical exposure might help prepare future physicians in all specialties to combat the burden of this disease.
Limitations of our study include the potential for selection and recall biases. Although our survey spanned multiple institutions in different regions of the United States, results might not be universally representative.
Acknowledgments—We thank Dirk Elston, MD, and Amy Wahlquist, MS (both from Charleston, South Carolina), who helped facilitate the survey on which our research is based. We also acknowledge the assistance of Philip Carmon, MD (Columbia, South Carolina); Julie Flugel (Columbia, South Carolina); Algimantas Simpson, MD (Columbia, South Carolina); Nathan Jasperse, MD (Irvine, California); Jeremy Teruel, MD (Charleston, South Carolina); Alan Snyder, MD, MSCR (Charleston, South Carolina); John Bosland (Charleston, South Carolina); and Daniel Spangler (Greenville, South Carolina).
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48:183-187. doi:10.1016/j.amepre.2014.08.036
- Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57-64. doi:10.1001/jamadermatol.2019.3353
- Lim HW, Collins SAB, Resneck JS Jr, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151-1160.e21. doi:10.1016/j.jaad.2017.03.006
- Garg A, Wang J, Reddy SB, et al; Integrated Skin Exam Consortium. Curricular factors associated with medical students’ practice of the skin cancer examination: an educational enhancement initiative by the Integrated Skin Exam Consortium. JAMA Dermatol. 2014;150:850-855. doi:10.1001/jamadermatol.2013.8723
- Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. doi:10.1001/archdermatol.2010.414
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470. doi:10.1001/jamadermatol.2020.0006
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48:183-187. doi:10.1016/j.amepre.2014.08.036
- Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57-64. doi:10.1001/jamadermatol.2019.3353
- Lim HW, Collins SAB, Resneck JS Jr, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151-1160.e21. doi:10.1016/j.jaad.2017.03.006
- Garg A, Wang J, Reddy SB, et al; Integrated Skin Exam Consortium. Curricular factors associated with medical students’ practice of the skin cancer examination: an educational enhancement initiative by the Integrated Skin Exam Consortium. JAMA Dermatol. 2014;150:850-855. doi:10.1001/jamadermatol.2013.8723
- Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. doi:10.1001/archdermatol.2010.414
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470. doi:10.1001/jamadermatol.2020.0006
Practice Points
- Nondermatologist practitioners play a notable role in mitigating the health care burden of skin cancer by screening with the skin cancer examination.
- Exposure to the skin cancer examination should occur during medical school prior to graduates’ entering diverse specialties.
- Most medical students received relatively few hours of skin cancer education, and many never performed or even observed a skin cancer examination prior to graduating medical school.
- Increasing hands-on training and clinical exposure during medical school is imperative to adequately prepare future physicians.
Applicability of the USPSTF Lung Cancer Screening Guidelines in a Predominantly Black Veteran Population
Lung cancer is the leading cause of cancer death in the United States.1 The 2011 National Lung Screening Trial (NLST) demonstrated that low-dose computed tomography (LDCT) screening provided a 20% relative reduction in lung cancer–specific mortality.2 Based on these findings, the United States Preventive Services Task Force (USPSTF) published lung cancer screening guidelines in 2013 recommending an annual LDCT of the thorax in patients aged 55 to 80 years with a 30 pack-year smoking history and who currently smoke or quit within the past 15 years.
In 2021, the USPSTF updated its recommendations by reducing the qualifications for annual screening to a 20 pack-year smoking history.3 The updated guidelines recognized the increased risk of lung cancer for Black individuals.4,5 Evidence suggests the 2013 screening criteria was too conservative for this population.6,7
Similarly, US Department of Veteran Affairs (VA) patients are a population at higher risk for lung cancer due to a male predominance, presence of comorbidities, exposure to carcinogenic agents, and possibly a higher prevalence of tobacco smoking.8 This study sought to examine the applicability of the USPSTF guidelines in a VA health care system with a predominantly Black population.
Methods
A retrospective chart review of adult patients who were diagnosed and treated with early-stage small cell or non–small cell lung cancer (stage I or II) was performed within the Southeast Louisiana Veterans Health Care System (SLVHCS) in New Orleans. The review used data from the VA Cancer Registry from January 1, 2005, through December 31, 2017. Patients were grouped by whether they met 2013 USPSTF screening criteria at time of diagnosis vs those that did not. Data collected included type and stage of lung cancer at time of diagnosis, context of diagnosis (incidental, screening, symptomatic), diagnostic method, smoking history, and presence of chronic obstructive pulmonary disease (COPD). Patients without a clear smoking history documented in the health record were excluded.
Statistical analyses were performed with GraphPad Prism 8.0. Student t test and Fischer exact test were performed for most of the statistical analyses, with differences between groups noted to be statistically significant at a P < .05.
Results
A total of 182 patient charts were reviewed and 13 patients were excluded for missing information related to the USPSTF screening criteria. Of the 169 patients included, 122 (72%) met USPSTF screening criteria while 47 (28%) patients did not. The reasons for not meeting screening criteria were 14 patients were too young at and 9 patients were too old at time of diagnosis, 7 had a < 20 pack-year smoking history, 7 patients had quit > 15 years previously, and 12 patients met multiple exclusion criteria. The study population was 96% male and there was an overall predominance of Black patients (58%) within the sample (Table).
There was a significantly higher proportion of Black patients in the group that did not meet screening criteria compared with the group that met screening criteria (68% vs 54%, P = .04). Cancer type and stage at diagnosis were similar in both patient populations. There was a statistically significant difference in COPD diagnosis between the groups, with a larger proportion of COPD patients in the met screening criteria group (74% vs 45%, P < .001). The mean smoking history was 61.4 pack-years in the met criteria group and 43.3 pack-years in the did not meet criteria group.
Five additional patients in the group that did not meet the 2013 USPSTF screening criteria would have met criteria if the 2021 USPSTF guidelines were applied. All 5 were Black patients. Using the 2021 guidelines, Black patients would have made up 56% of the patients who met screening criteria and 54% of the patients who did not meet screening criteria at time of diagnosis.
Discussion
This study sought to determine the hypothetical effectiveness of national lung cancer screening guidelines in detecting early-stage lung cancer for a high-risk population. Patients diagnosed with early-stage lung cancer were selected as these patients have improved outcomes with treatment, and thus would theoretically benefit from early detection through screening. As expected, the study population had a majority of Black veterans (58%), with a higher proportion of Black patients in the did not meet screening criteria group compared with the met screening criteria group (68% vs 54%, P = .04). This difference highlights the concern that Black individuals were being underscreened with the 2013 USPSTF guidelines.7 This is not all surprising as the NLST, from which the initial screening guidelines were based, included a majority White population with only 4.4% of their population being Black.2 The USPSTF also cites the NELSON trial as evidence to support annual lung cancer screening, a trial that was performed in the Netherlands with a very different population compared with that of southeast Louisiana.9
Given concern that the old criteria were underscreening certain populations, the updated 2021 USPSTF guidelines sought to expand the screening population. In this study, the implementation of these new guidelines resulted in more Black patients meeting screening criteria.
Racial and ethnic disparities in health care in the US are no secret, as Black individuals consistently have increased disease and death rates, higher rates of unemployment, and decreased access to preventive medical care compared to White individuals.10 Despite the updated USPSTF guidelines, additional modifications to the screening criteria could improve the ability to identify high-risk patients. A modified model using data from the Prostate, Lung, Colorectal, and Ovarian Screening Trial (PLCO) incorporating COPD history, race and ethnicity, and personal history of cancer increased the sensitivity for high-risk Black ever-smokers.11 Additional models and analyses also support the utility of incorporating race and ethnicity in lung cancer screening criteria.7,12 Using race and ethnicity to guide screening criteria for cancer is not unheard of; in 2017, the US Multi-Society Task Force recommended that Black individuals start colon cancer screening at age 45 years rather than the typical age of 50 years, before updating the guidelines again in 2021 to recommend that all adults start at age 45 years.13,14
Limitations
This study had the inherent weakness of being a retrospective study at a single institution. Additionally, the 7th edition of the International Association for the Study of Lung Cancer was published in 2010, during the 2005 to 2017 time frame from which our data was collected, leading to possible inconsistencies in staging between patients before and after 2010.15 However, these changes in staging are unlikely to significantly impact the results for in this study, since the vast majority of the patients diagnosed with lung cancer stage I or II before 2010 would still be in the those 2 stages in the 2010 edition. Finally, specific to our patient population, it was often difficult to ascertain an accurate smoking history for each patient, especially in the early years of the data set, likely due to the disruption of care caused by Hurricane Katrina.
Conclusions
In this retrospective study performed at the SLVHCS in New Orleans, a significantly higher proportion of Black patients compared with White patients with early-stage lung cancer did not meet the 2013 USPSTF lung cancer screening guidelines at time of diagnosis, highlighting the concern that this population was being underscreened. These findings demonstrate the challenges and failures of applying national guidelines to a unique, high-risk population. An individualized, risk-based screening model incorporating race and ethnicity could be more effective at diagnosing early-stage lung cancer and requires more investigation. Centralized lung cancer screening programs within the VA system could also be beneficial for early detection and treatment, as well as provide insight into the increased risk within the veteran population.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. doi:10.3322/caac.21590
2. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa110287
3. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
4. Jonas DE, Reuland DS, Reddy SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(10):971-987. doi:10.1001/jama.2021.0377
5. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi:10.1056/NEJMoa033250
6. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211-233. doi:10.3322/caac.21555
7. Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi:10.1001/jamaoncol.2019.1402
8. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147-149. doi:10.1007/s11606-009-1160-0
9. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
10. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. 2000;21(4):75-90.
11. Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk prediction model versus United States Preventive Services Task Force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15(11):1738-1747. doi:10.1016/j.jtho.2020.08.006
12. Ten Haaf K, Bastani M, Cao P, et al. A comparative modeling analysis of risk-based lung cancer screening strategies. J Natl Cancer Inst. 2020;112(5):466-479. doi:10.1093/jnci/djz164
13. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
14. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
15. Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012;4(4):128-134. doi:10.4329/wjr.v4.i4.128
Lung cancer is the leading cause of cancer death in the United States.1 The 2011 National Lung Screening Trial (NLST) demonstrated that low-dose computed tomography (LDCT) screening provided a 20% relative reduction in lung cancer–specific mortality.2 Based on these findings, the United States Preventive Services Task Force (USPSTF) published lung cancer screening guidelines in 2013 recommending an annual LDCT of the thorax in patients aged 55 to 80 years with a 30 pack-year smoking history and who currently smoke or quit within the past 15 years.
In 2021, the USPSTF updated its recommendations by reducing the qualifications for annual screening to a 20 pack-year smoking history.3 The updated guidelines recognized the increased risk of lung cancer for Black individuals.4,5 Evidence suggests the 2013 screening criteria was too conservative for this population.6,7
Similarly, US Department of Veteran Affairs (VA) patients are a population at higher risk for lung cancer due to a male predominance, presence of comorbidities, exposure to carcinogenic agents, and possibly a higher prevalence of tobacco smoking.8 This study sought to examine the applicability of the USPSTF guidelines in a VA health care system with a predominantly Black population.
Methods
A retrospective chart review of adult patients who were diagnosed and treated with early-stage small cell or non–small cell lung cancer (stage I or II) was performed within the Southeast Louisiana Veterans Health Care System (SLVHCS) in New Orleans. The review used data from the VA Cancer Registry from January 1, 2005, through December 31, 2017. Patients were grouped by whether they met 2013 USPSTF screening criteria at time of diagnosis vs those that did not. Data collected included type and stage of lung cancer at time of diagnosis, context of diagnosis (incidental, screening, symptomatic), diagnostic method, smoking history, and presence of chronic obstructive pulmonary disease (COPD). Patients without a clear smoking history documented in the health record were excluded.
Statistical analyses were performed with GraphPad Prism 8.0. Student t test and Fischer exact test were performed for most of the statistical analyses, with differences between groups noted to be statistically significant at a P < .05.
Results
A total of 182 patient charts were reviewed and 13 patients were excluded for missing information related to the USPSTF screening criteria. Of the 169 patients included, 122 (72%) met USPSTF screening criteria while 47 (28%) patients did not. The reasons for not meeting screening criteria were 14 patients were too young at and 9 patients were too old at time of diagnosis, 7 had a < 20 pack-year smoking history, 7 patients had quit > 15 years previously, and 12 patients met multiple exclusion criteria. The study population was 96% male and there was an overall predominance of Black patients (58%) within the sample (Table).
There was a significantly higher proportion of Black patients in the group that did not meet screening criteria compared with the group that met screening criteria (68% vs 54%, P = .04). Cancer type and stage at diagnosis were similar in both patient populations. There was a statistically significant difference in COPD diagnosis between the groups, with a larger proportion of COPD patients in the met screening criteria group (74% vs 45%, P < .001). The mean smoking history was 61.4 pack-years in the met criteria group and 43.3 pack-years in the did not meet criteria group.
Five additional patients in the group that did not meet the 2013 USPSTF screening criteria would have met criteria if the 2021 USPSTF guidelines were applied. All 5 were Black patients. Using the 2021 guidelines, Black patients would have made up 56% of the patients who met screening criteria and 54% of the patients who did not meet screening criteria at time of diagnosis.
Discussion
This study sought to determine the hypothetical effectiveness of national lung cancer screening guidelines in detecting early-stage lung cancer for a high-risk population. Patients diagnosed with early-stage lung cancer were selected as these patients have improved outcomes with treatment, and thus would theoretically benefit from early detection through screening. As expected, the study population had a majority of Black veterans (58%), with a higher proportion of Black patients in the did not meet screening criteria group compared with the met screening criteria group (68% vs 54%, P = .04). This difference highlights the concern that Black individuals were being underscreened with the 2013 USPSTF guidelines.7 This is not all surprising as the NLST, from which the initial screening guidelines were based, included a majority White population with only 4.4% of their population being Black.2 The USPSTF also cites the NELSON trial as evidence to support annual lung cancer screening, a trial that was performed in the Netherlands with a very different population compared with that of southeast Louisiana.9
Given concern that the old criteria were underscreening certain populations, the updated 2021 USPSTF guidelines sought to expand the screening population. In this study, the implementation of these new guidelines resulted in more Black patients meeting screening criteria.
Racial and ethnic disparities in health care in the US are no secret, as Black individuals consistently have increased disease and death rates, higher rates of unemployment, and decreased access to preventive medical care compared to White individuals.10 Despite the updated USPSTF guidelines, additional modifications to the screening criteria could improve the ability to identify high-risk patients. A modified model using data from the Prostate, Lung, Colorectal, and Ovarian Screening Trial (PLCO) incorporating COPD history, race and ethnicity, and personal history of cancer increased the sensitivity for high-risk Black ever-smokers.11 Additional models and analyses also support the utility of incorporating race and ethnicity in lung cancer screening criteria.7,12 Using race and ethnicity to guide screening criteria for cancer is not unheard of; in 2017, the US Multi-Society Task Force recommended that Black individuals start colon cancer screening at age 45 years rather than the typical age of 50 years, before updating the guidelines again in 2021 to recommend that all adults start at age 45 years.13,14
Limitations
This study had the inherent weakness of being a retrospective study at a single institution. Additionally, the 7th edition of the International Association for the Study of Lung Cancer was published in 2010, during the 2005 to 2017 time frame from which our data was collected, leading to possible inconsistencies in staging between patients before and after 2010.15 However, these changes in staging are unlikely to significantly impact the results for in this study, since the vast majority of the patients diagnosed with lung cancer stage I or II before 2010 would still be in the those 2 stages in the 2010 edition. Finally, specific to our patient population, it was often difficult to ascertain an accurate smoking history for each patient, especially in the early years of the data set, likely due to the disruption of care caused by Hurricane Katrina.
Conclusions
In this retrospective study performed at the SLVHCS in New Orleans, a significantly higher proportion of Black patients compared with White patients with early-stage lung cancer did not meet the 2013 USPSTF lung cancer screening guidelines at time of diagnosis, highlighting the concern that this population was being underscreened. These findings demonstrate the challenges and failures of applying national guidelines to a unique, high-risk population. An individualized, risk-based screening model incorporating race and ethnicity could be more effective at diagnosing early-stage lung cancer and requires more investigation. Centralized lung cancer screening programs within the VA system could also be beneficial for early detection and treatment, as well as provide insight into the increased risk within the veteran population.
Lung cancer is the leading cause of cancer death in the United States.1 The 2011 National Lung Screening Trial (NLST) demonstrated that low-dose computed tomography (LDCT) screening provided a 20% relative reduction in lung cancer–specific mortality.2 Based on these findings, the United States Preventive Services Task Force (USPSTF) published lung cancer screening guidelines in 2013 recommending an annual LDCT of the thorax in patients aged 55 to 80 years with a 30 pack-year smoking history and who currently smoke or quit within the past 15 years.
In 2021, the USPSTF updated its recommendations by reducing the qualifications for annual screening to a 20 pack-year smoking history.3 The updated guidelines recognized the increased risk of lung cancer for Black individuals.4,5 Evidence suggests the 2013 screening criteria was too conservative for this population.6,7
Similarly, US Department of Veteran Affairs (VA) patients are a population at higher risk for lung cancer due to a male predominance, presence of comorbidities, exposure to carcinogenic agents, and possibly a higher prevalence of tobacco smoking.8 This study sought to examine the applicability of the USPSTF guidelines in a VA health care system with a predominantly Black population.
Methods
A retrospective chart review of adult patients who were diagnosed and treated with early-stage small cell or non–small cell lung cancer (stage I or II) was performed within the Southeast Louisiana Veterans Health Care System (SLVHCS) in New Orleans. The review used data from the VA Cancer Registry from January 1, 2005, through December 31, 2017. Patients were grouped by whether they met 2013 USPSTF screening criteria at time of diagnosis vs those that did not. Data collected included type and stage of lung cancer at time of diagnosis, context of diagnosis (incidental, screening, symptomatic), diagnostic method, smoking history, and presence of chronic obstructive pulmonary disease (COPD). Patients without a clear smoking history documented in the health record were excluded.
Statistical analyses were performed with GraphPad Prism 8.0. Student t test and Fischer exact test were performed for most of the statistical analyses, with differences between groups noted to be statistically significant at a P < .05.
Results
A total of 182 patient charts were reviewed and 13 patients were excluded for missing information related to the USPSTF screening criteria. Of the 169 patients included, 122 (72%) met USPSTF screening criteria while 47 (28%) patients did not. The reasons for not meeting screening criteria were 14 patients were too young at and 9 patients were too old at time of diagnosis, 7 had a < 20 pack-year smoking history, 7 patients had quit > 15 years previously, and 12 patients met multiple exclusion criteria. The study population was 96% male and there was an overall predominance of Black patients (58%) within the sample (Table).
There was a significantly higher proportion of Black patients in the group that did not meet screening criteria compared with the group that met screening criteria (68% vs 54%, P = .04). Cancer type and stage at diagnosis were similar in both patient populations. There was a statistically significant difference in COPD diagnosis between the groups, with a larger proportion of COPD patients in the met screening criteria group (74% vs 45%, P < .001). The mean smoking history was 61.4 pack-years in the met criteria group and 43.3 pack-years in the did not meet criteria group.
Five additional patients in the group that did not meet the 2013 USPSTF screening criteria would have met criteria if the 2021 USPSTF guidelines were applied. All 5 were Black patients. Using the 2021 guidelines, Black patients would have made up 56% of the patients who met screening criteria and 54% of the patients who did not meet screening criteria at time of diagnosis.
Discussion
This study sought to determine the hypothetical effectiveness of national lung cancer screening guidelines in detecting early-stage lung cancer for a high-risk population. Patients diagnosed with early-stage lung cancer were selected as these patients have improved outcomes with treatment, and thus would theoretically benefit from early detection through screening. As expected, the study population had a majority of Black veterans (58%), with a higher proportion of Black patients in the did not meet screening criteria group compared with the met screening criteria group (68% vs 54%, P = .04). This difference highlights the concern that Black individuals were being underscreened with the 2013 USPSTF guidelines.7 This is not all surprising as the NLST, from which the initial screening guidelines were based, included a majority White population with only 4.4% of their population being Black.2 The USPSTF also cites the NELSON trial as evidence to support annual lung cancer screening, a trial that was performed in the Netherlands with a very different population compared with that of southeast Louisiana.9
Given concern that the old criteria were underscreening certain populations, the updated 2021 USPSTF guidelines sought to expand the screening population. In this study, the implementation of these new guidelines resulted in more Black patients meeting screening criteria.
Racial and ethnic disparities in health care in the US are no secret, as Black individuals consistently have increased disease and death rates, higher rates of unemployment, and decreased access to preventive medical care compared to White individuals.10 Despite the updated USPSTF guidelines, additional modifications to the screening criteria could improve the ability to identify high-risk patients. A modified model using data from the Prostate, Lung, Colorectal, and Ovarian Screening Trial (PLCO) incorporating COPD history, race and ethnicity, and personal history of cancer increased the sensitivity for high-risk Black ever-smokers.11 Additional models and analyses also support the utility of incorporating race and ethnicity in lung cancer screening criteria.7,12 Using race and ethnicity to guide screening criteria for cancer is not unheard of; in 2017, the US Multi-Society Task Force recommended that Black individuals start colon cancer screening at age 45 years rather than the typical age of 50 years, before updating the guidelines again in 2021 to recommend that all adults start at age 45 years.13,14
Limitations
This study had the inherent weakness of being a retrospective study at a single institution. Additionally, the 7th edition of the International Association for the Study of Lung Cancer was published in 2010, during the 2005 to 2017 time frame from which our data was collected, leading to possible inconsistencies in staging between patients before and after 2010.15 However, these changes in staging are unlikely to significantly impact the results for in this study, since the vast majority of the patients diagnosed with lung cancer stage I or II before 2010 would still be in the those 2 stages in the 2010 edition. Finally, specific to our patient population, it was often difficult to ascertain an accurate smoking history for each patient, especially in the early years of the data set, likely due to the disruption of care caused by Hurricane Katrina.
Conclusions
In this retrospective study performed at the SLVHCS in New Orleans, a significantly higher proportion of Black patients compared with White patients with early-stage lung cancer did not meet the 2013 USPSTF lung cancer screening guidelines at time of diagnosis, highlighting the concern that this population was being underscreened. These findings demonstrate the challenges and failures of applying national guidelines to a unique, high-risk population. An individualized, risk-based screening model incorporating race and ethnicity could be more effective at diagnosing early-stage lung cancer and requires more investigation. Centralized lung cancer screening programs within the VA system could also be beneficial for early detection and treatment, as well as provide insight into the increased risk within the veteran population.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. doi:10.3322/caac.21590
2. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa110287
3. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
4. Jonas DE, Reuland DS, Reddy SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(10):971-987. doi:10.1001/jama.2021.0377
5. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi:10.1056/NEJMoa033250
6. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211-233. doi:10.3322/caac.21555
7. Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi:10.1001/jamaoncol.2019.1402
8. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147-149. doi:10.1007/s11606-009-1160-0
9. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
10. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. 2000;21(4):75-90.
11. Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk prediction model versus United States Preventive Services Task Force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15(11):1738-1747. doi:10.1016/j.jtho.2020.08.006
12. Ten Haaf K, Bastani M, Cao P, et al. A comparative modeling analysis of risk-based lung cancer screening strategies. J Natl Cancer Inst. 2020;112(5):466-479. doi:10.1093/jnci/djz164
13. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
14. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
15. Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012;4(4):128-134. doi:10.4329/wjr.v4.i4.128
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. doi:10.3322/caac.21590
2. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi:10.1056/NEJMoa110287
3. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
4. Jonas DE, Reuland DS, Reddy SM, et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325(10):971-987. doi:10.1001/jama.2021.0377
5. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi:10.1056/NEJMoa033250
6. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211-233. doi:10.3322/caac.21555
7. Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi:10.1001/jamaoncol.2019.1402
8. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147-149. doi:10.1007/s11606-009-1160-0
9. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
10. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. 2000;21(4):75-90.
11. Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk prediction model versus United States Preventive Services Task Force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15(11):1738-1747. doi:10.1016/j.jtho.2020.08.006
12. Ten Haaf K, Bastani M, Cao P, et al. A comparative modeling analysis of risk-based lung cancer screening strategies. J Natl Cancer Inst. 2020;112(5):466-479. doi:10.1093/jnci/djz164
13. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
14. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
15. Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012;4(4):128-134. doi:10.4329/wjr.v4.i4.128
Psychosocial Barriers and Their Impact on Hepatocellular Carcinoma Care in US Veterans: Tumor Board Model of Care
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
Hepatocellular carcinoma (HCC) remains a major global health problem and is the third leading cause of cancer-related mortality worldwide.1 Management of HCC is complex; as it largely occurs in the background of chronic liver disease, its management must simultaneously address challenges related to the patient’s tumor burden, as well as their underlying liver dysfunction and performance status. HCC is universally fatal without treatment, with a 5-year survival < 10%.2 However, if detected early HCC is potentially curable, with treatments such as hepatic resection, ablation, and/or liver transplantation, which are associated with 5-year survival rates as high as 70%.2 HCC-specific palliative treatments, including intra-arterial therapies (eg, trans-arterial chemoembolization, radioembolization) and systemic chemotherapy, have also been shown to prolong survival in patients with advanced HCC. Therefore, a key driver of patient survival is receipt of HCC-specific therapy.
There is rising incidence and mortality related to HCC in the US veteran population, largely attributed to acquisition of chronic hepatitis C virus (HCV) infection decades prior.3 There is also a high prevalence of psychosocial barriers in this population, such as low socioeconomic status, homelessness, alcohol and substance use disorders, and psychiatric disorders which can negatively influence receipt of medical treatment, including cancer care.4,5 Given the complexity of managing HCC, as well as the plethora of potential treatment options available, it is widely accepted that a multidisciplinary team approach, such as the multidisciplinary tumor board (MDTB) provides optimal care to patients with HCC.2,6 The aim of the present study was to identify in a population of veterans diagnosed with HCC the prevalence of psychosocial barriers to care and assess their impact and the role of an MDTB on receipt of HCC-specific care.
Methods
In June 2007, a joint institutional MDTB was established for patients with primary liver tumors receiving care at the William S. Middleton Memorial Veterans’ Hospital (WSMMVH) in Madison, Wisconsin. As we have described elsewhere, individual cases with their corresponding imaging studies were reviewed at a weekly conference attended by transplant hepatologists, medical oncologists, hepatobiliary and transplant surgeons, pathologists, diagnostic and interventional radiologists, and nurse coordinators.6 Potential therapies offered included surgical resection, liver transplantation (LT), thermal ablation, intra-arterial therapies (chemo and/or radioembolization), systemic chemotherapy, stereotactic radiation, and best supportive care. Decisions regarding the appropriate treatment modality were made based on patient factors, review of their cross-sectional imaging studies and/or histopathology, and context of their underlying liver dysfunction. The tumor board discussion was summarized in meeting minutes as well as tumor board encounters recorded in each patient’s health record. Although patients with benign tumors were presented at the MDTB, only patients with a diagnosis of HCC were included in this study.
A database analysis was conducted of all veteran patients with HCC managed through the WSMMVH MDTB, since its inception up to December 31, 2016, with follow-up until December 31, 2018. Data for analysis included demographics, laboratory parameters at time of diagnosis and treatment, imaging findings, histopathology and/or surgical pathology, treatment rendered, and follow-up information. The primary outcome measured in this study included receipt of any therapy and secondarily, patient survival.
Discrete variables were analyzed with χ2 statistics or Fisher exact test and continuous variables with the student t test. Multivariable analyses were carried out with logistic regression. Variables with a P < .05 were considered statistically significant. Analyses were carried out using IBM SPSS v24.0.
As a quality-improvement initiative for the care and management of veterans with HCC, this study was determined to be exempt from review by the WSMMVH and University of Wisconsin School of Medicine and Public Health Institutional Review Board.
Results
From January 1, 2007, through December 31, 2016, 149 patients with HCC were managed through the MDTB. Baseline demographic data, Model for End-stage Liver Disease (MELD) score and Child-Turcotte-Pugh class, and baseline HCC characteristics of the cohort are shown in Tables 1 and 2.
There was a high prevalence of psychosocial barriers in our study cohort, including alcohol or substance use disorder, mental illness diagnosis, and low socioeconomic status (Table 3). The mean distance traveled to WSMMVH for HCC-specific care was 206 km. Fifty patients in the cohort utilized travel assistance and 33 patients accessed lodging assistance.
HCC Treatments
There was a high rate of receipt of treatment in our study cohort with 127 (85%) patients receiving at least one HCC-specific therapy. Care was individualized and coordinated through our institutional MDTB, with both curative and palliative treatment modalities utilized (Table 4).
Curative treatment, which includes LT, ablation, or resection, was offered to 78 (52%) patients who were within T2 stage. Of these 78 patients who were potential candidates for LT as a curative treatment for HCC, 31 were not deemed suitable transplant candidates. Psychosocial barriers precluded consideration for LT in 7 of the 31 patients due to active substance use, homelessness in 1 patient, and severe mental illness in 3 patients. Medical comorbidities, advanced patient age, and patient preference accounted for the remainder.
In a univariate analysis of the cohort of 149 patients, factors that decreased the likelihood of receipt of curative HCC therapy included T2 stage or higher at diagnosis and a diagnosis of depression, whereas provision for lodging was associated with increased likelihood of receiving HCC-specific care (Table 5). Other factors that influenced receipt of any treatment included patient’s MELD score, total bilirubin, and serum α-fetoprotein, a surrogate marker for tumor stage. In the multivariable analysis, predictors of receiving curative therapy included absence of substance use, within T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. The presence of psychosocial barriers apart from substance use did not predict a lower chance of receiving curative HCC therapy (including homelessness, distance traveled to center, mental health disorder, and low income).
Median survival was 727 (95% CI, 488-966) days from diagnosis. Survival from HCC diagnosis in study cohort was 72% at 1 year, 50% at 2 years, 39% at 3 years, and 36% at 5 years. Death occurred in 71 (48%) patients; HCC accounted for death in 52 (73%) patients, complications of end-stage liver disease in 13 (18%) patients, and other causes for the remainder of patients.
Discussion
Increases in prevalence and mortality related to cirrhosis and HCC have been reported among the US veteran population.3 This is in large part attributable to the burden of chronic HCV infection in this population. As mirrored in the US population in general, we may be at a turning point regarding the gradual increase in prevalence in HCC.7 The prevalence of cirrhosis and viral-related HCC related to HCV infection will decline with availability of effective antiviral therapy. Alcoholic liver disease remains a main etiological factor for development of cirrhosis and HCC. Nonalcoholic fatty liver disease is becoming a more prevalent cause for development of cirrhosis, indication for liver transplantation, and development of HCC, and indeed may lead to HCC even in the absence of cirrhosis.8
HCC remains a challenging clinical problem.2 As the vast majority of cases arise in the context of cirrhosis, management of HCC not only must address the cancer stage at diagnosis, but also the patient’s underlying liver dysfunction and performance status. Receipt of HCC-specific therapy is a key driver of patient outcome, with curative therapies available for those diagnosed with early-stage disease. We and others have shown that a multidisciplinary approach to coordinate, individualize, and optimize care for these complex patients can improve the rate of treatment utilization, reduce treatment delays, and improve patient survival.6,9,10
Patient psychosocial barriers, such as low socioeconomic status, homelessness, alcohol and substance use, and psychiatric disorders, are more prevalent among the veteran population and have the potential to negatively influence successful health care delivery. One retrospective study of 100 veterans at a US Department of Veterans Affairs (VA) medical center treated for HCC from 2009 to 2014 showed a majority of the patients lived on a meager income, a high prevalence of homelessness, substance use history in 96% of their cohort, and psychiatric illness in 65% of patients.11 Other studies have documented similar findings in the veteran population, with alcohol, substance use, as well as other uncontrolled comorbidities as barriers to providing care, such as antiviral therapy for chronic HCV infection.12
Herein, we present a cohort of veterans with HCC managed through our MDTB from 2007 to 2016, for whom chronic HCV infection and/or alcoholic liver disease were the main causes of cirrhosis. Our cohort had a high burden of alcohol and substance use disorders while other psychiatric illnesses were also common. Our cohort includes patients who were poor, and even some veterans who lacked a stable home. This profile of poverty and social deprivation among veterans is matched in national data.13-15 Using a tumor board model of nurse navigation and multidisciplinary care, we were able to provide travel and lodging assistance to 50 (34%) and 33 (22%) patients, respectively, in order to facilitate their care.
Our data demonstrate that the impact of psychosocial barriers on our capacity to deliver care varies with the nature of the treatment under consideration: curative vs cancer control. For example, active substance use disorder, homelessness, and severe established mental illness were often considered insurmountable when the treatment in question was LT. Nevertheless, despite the high prevalence in our study group of barriers, such as lack of transport while living far from a VA medical center, or alcohol use disorder, a curative treatment with either LT, tumor ablation, or resection could be offered to over half of our cohort. When noncurative therapies are included, most patients (85%) received HCC-specific care, with good relative survival.
Our reported high receipt of HCC-specific care and patient survival is in contrast to previously reported low rates of HCC-specific care in in a national survey of management of 1296 veteran patients infected with HCV who developed HCC from 1998 to 2006. In this population, HCC-specific treatment was provided to 34%.16 However our data are consistent with our previously published data of patients with HCC managed through an institutional MDTB.6 Indeed, as shown by a univariate analysis in our present study, individualizing care to address modifiable patient barriers, such as providing provisions for lodging if needed, was associated with an increased likelihood of receiving HCC-specific care. On the other hand, advanced tumor stage (> T2) at diagnosis and a diagnosis of depression, which was the most common psychiatric diagnosis in our cohort, were both associated with decreased likelihood of receiving HCC-specific care. Clinical factors such as MELD score, total bilirubin, and serum AFP all affected the likelihood of providing HCC-specific care. In a multivariate analysis, factors that predicted ability to receive curative therapy included absence of substance use, T2 stage of tumor, and Child-Turcotte-Pugh class A cirrhosis. This is expected as patients with HCC within T2 stage (or Milan criteria) with compensated cirrhosis are most likely to receive curative therapies, such as resection, ablation, or LT.2
Conclusions
Our study demonstrates a high burden of psychosocial challenges in veterans with HCC. These accounted for a significant barrier to receive HCC-specific care. Despite the presence of these patient barriers, high rates of HCC-specific treatment are attainable through individualization and coordination of patient care in the context of a MDTB model with nurse navigation. Provision of targeted social support to ameliorate these modifiable factors improves patient outcomes.
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4-13. doi:10.1002/hep.31288.
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750. doi:10.1002/hep.29913
3. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-e18. doi:10.1053/j.gastro.2015.07.056
4. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. doi:10.1001/archinte.158.6.626
5. Slind LM, Keating TM, Fisher AG, Rose TG. A patient navigation model for veterans traveling for cancer care. Fed Pract. 2016;33(suppl 1):40S-45S.
6. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845-849. doi:10.1097/MCG.0000000000000825
7. White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152(4):812-820.e5. doi:10.1053/j.gastro.2016.11.020
8. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828-1837.e2. doi:10.1053/j.gastro.2018.08.024
9. Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287-1295. doi:10.1245/s10434-013-3413-8
10. Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10(6):405-411. doi:10.1080/13651820802356572
11. Hwa KJ, Dua MM, Wren SM, Visser BC. Missing the obvious: psychosocial obstacles in veterans with hepatocellular carcinoma. HPB (Oxford). 2015;17(12):1124-1129. doi:10.1111/hpb.12508
12. Taylor J, Carr-Lopez S, Robinson A, et al. Determinants of treatment eligibility in veterans with hepatitis C viral infection. Clin Ther. 2017;39(1):130-137. doi:10.1016/j.clinthera.2016.11.019
13. Fargo J, Metraux S, Byrne T, et al. Prevalence and risk of homelessness among US veterans. Prev Chronic Dis. 2012;9:E45.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among US veterans. Epidemiol Rev. 2015;37:177-195. doi:10.1093/epirev/mxu004
15. Tsai J, Link B, Rosenheck RA, Pietrzak RH. Homelessness among a nationally representative sample of US veterans: prevalence, service utilization, and correlates. Soc Psychiatry Psychiatr Epidemiol. 2016;51(6):907-916. doi:10.1007/s00127-016-1210-y
16. Davila JA, Kramer JR, Duan Z, et al. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57(5):1858-1868. doi:10.1002/hep.26287
Stories of the Heart: Illness Narratives of Veterans Living With Heart Failure
Heart failure (HF) is a costly and burdensome illness and is the top reason for hospital admissions for the US Department of Veterans Affairs (VA) and Medicare.1 The cost of HF to the United States is estimated to grow to $3 billion annually by 2030.2 People living with HF have a high symptom burden and poor quality of life.3,4 Symptoms include shortness of breath, fatigue, depression, and decreases in psychosocial, existential, and spiritual well-being.5-9
Veterans in the US are a unique cultural group with distinct contextual considerations around their experiences.10 Different groups of veterans require unique cultural considerations, such as the experiences of veterans who served during the Vietnam war and during Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF). The extent of unmet needs of people living with HF, the number of veterans living with this illness, and the unique contextual components related to living with HF among veterans require further exploration into this illness experience for this distinct population. Research should explore innovative ways of managing both the number of people living with the illness and the significant impact of HF in people’s lives due to the high symptom burden and poor quality of life.3
This study used the model of adjustment to illness to explore the psychosocial adjustment to illness and the experience of US veterans living with HF, with a focus on the domains of meaning creation, self-schema, and world schema.11 The model of adjustment to illness describes how people learn to adjust to living with an illness, which can lead to positive health outcomes. Meaning creation is defined as the process in which people create meaning from their experience living with illness. Self-schema is how people living with illness see themselves, and world schema is how people living with chronic illness see their place in the world. These domains shift as part of the adjustment to living with an illness described in this model.11 This foundation allowed the investigators to explore the experience of living with HF among veterans with a focus on these domains. Our study aimed to cocreate illness narratives among veterans living with HF and to explore components of psychosocial adjustment informed by the model.
Methods
This study used narrative inquiry with a focus on illness narratives.12-17 Narrative inquiry as defined by Catherine Riessman involves the generation of socially constructed and cocreated meanings between the researcher and narrator. The researcher is an active participant in narrative creation as the narrator chooses which events to include in the stories based on the social, historical, and cultural context of both the narrator (study participant) and audience (researcher). Riessman describes the importance of contextual factors and meaning creation as an important aspect of narrative inquiry.12-14,16,17 It is important in narrative inquiry to consider how cultural, social, and historical factors influence narrative creation, constriction, and/or elimination.
This study prospectively created and collected data at a single time point. Semi-structured interviews explored psychosocial adjustment for people living with HF using an opening question modified from previous illness narrative research: Why do you think you got heart failure?18 Probes included the domains of psychosocial adjustment informed by the model of adjustment to illness domains (Figure). Emergent probes were used to illicit additional data around psychosocial adjustment to illness. Data were created and collected in accordance with narrative inquiry during the cocreation of the illness narratives between the researcher and study participants. This interview guide was tested by the first author in preliminary work to prepare for this study.
Allowing for emergent probes and acknowledging the role of the researcher as audience is key to the cocreation of narratives using this methodological framework. Narrators shape their narrative with the audience in mind; they cocreate their narrative with their audience using this type of narrative inquiry.12,16 What the narrator chooses to include and exclude from their story provides a window into how they see themselves and their world.19 Audio recordings were used to capture data, allowing for the researcher to take contemporaneous notes exploring contextual considerations to the narrative cocreation process and to be used later in analysis. Analytic notes were completed during the interviews as well as later in analysis as part of the contextual reflection.
Setting
Research was conducted in the Rocky Mountain Regional VA Medical Center, Aurora, Colorado. Participants were recruited through the outpatient cardiology clinic where the interviews also took place. This study was approved through the Colorado Institutional Review Board and Rocky Mountain Regional VA Medical Center (IRB: 19-1064). Participants were identified by the treating cardiologist who was a part of the study team. Interested veterans were introduced to the first author who was stationed in an empty clinic room. The study cardiologist screened to ensure all participants were ≥ 18 years of age and had a diagnosis of HF for > 1 year. Persons with an impairment that could interfere with their ability to construct a narrative were also excluded.
Recruitment took place from October 2019 to January 2020. Three veterans refused participation. Five study participants provided informed consent and were enrolled and interviewed. All interviews were completed in the clinic at the time of consent per participant preference. One-hour long semi-structured interviews were conducted and audio recorded. A demographic form was administered at the end of each interview to capture contextual data. The researcher also kept a reflexive journal and audit trail.
Narrative Analysis
Riessman described general steps to conduct narrative analysis, including transcription, narrative clean-up, consideration of contextual factors, exploration of thematic threads, consideration of larger social narratives, and positioning.12 The first author read transcripts while listening to the audio recordings to ensure accuracy. With narrative clean-up each narrative was organized to cocreate overall meaning, changed to protect anonymity, and refined to only include the illness narrative. For example, if a narrator told a story about childhood and then later in the interview remembered another detail to add to their story, narrative clean-up reordered events to make cohesive sense of the story. Demographic, historical, cultural, and social contexts of both the narrator and audience were reflected on during analysis to explore how these components may have shaped and influenced cocreation. Context was also considered within the larger VA setting.
Emergent themes were explored for convergence, divergence, and points of tension within and across each narrative. Larger social narratives were also considered for their influence on possible inclusion/exclusion of experience, such as how gender identity may have influenced study participants’ descriptions of their roles in social systems. These themes and narratives were then shared with our team, and we worked through decision points during the analysis process and discussed interpretation of the data to reach consensus.
Results
Five veterans living with HF were recruited and consented to participate in the study. Demographics of the participants and first author are included in the Table. Five illness narratives were cocreated, entitled: Blame the Cheese: Frank’s Illness Narrative; Love is Love: Bob’s Illness Narrative; The Brighter Things in Life is My Family: George’s Illness Narrative; We Never Know When Our Time is Coming: Bill’s Illness Narrative; and A Dream Deferred: Henry’s Illness Narrative.
Each narrative was explored focusing on the domains of the model of adjustment to illness. An emergent theme was also identified with multiple subthemes: being a veteran is unique. Related subthemes included: financial benefits, intersectionality of government and health care, the intersectionality of masculinity and military service, and the dichotomy of military experience.
The search for meaning creation after the experience of chronic illness emerged across interviews. One example of meaning creation was in Frank's illness narrative. Frank was unsure why he got HF: “Probably because I ate too much cheese…I mean, that’s gotta be it. It can’t be anything else.” By tying HF to his diet, he found meaning through his health behaviors.
Model of Adjustment to Illness
The narratives illustrate components of the model of adjustment to illness and describe how each of the participants either shifted their self-schema and world schema or reinforced their previously established schemas. It also demonstrates how people use narratives to create meaning and illness understanding from their illness experience, reflecting, and emphasizing different parts creating meaning from their experience.
A commonality across the narratives was a shift in self-schema, including the shift from being a provider to being reliant on others. In accordance with the dominant social narrative around men as providers, each narrator talked about their identity as a provider for themselves and their families. Often keeping their provider identity required modifications of the definition, from physical abilities and employment to financial security and stability. George made all his health care decisions based on his goal of providing for his family and protecting them from having to care for him: “I’m always thinking about the future, always trying to figure out how my family, if something should happen to me, how my family would cope, and how my family would be able to support themselves.” Bob’s health care goals were to stay alive long enough for his wife to get financial benefits as a surviving spouse: “That’s why I’m trying to make everything for her, you know. I’m not worried about myself. I’m not. Her I am, you know. And love is love.” Both of their health care decisions are shaped by their identity as a provider shifting to financial support.
Some narrators changed the way they saw their world, or world schema, while others felt their illness experience just reinforced the way they had already experienced the world. Frank was able to reprioritize what was important to him after his diagnosis and accept his own mortality: “I might as well chill out, no more stress, and just enjoy things ’cause you could die…” For Henry, getting HF was only part of the experience of systemic oppression that had impacted his and his family’s lives for generations. He saw how his oppression by the military and US government led to his father’s exposure to chemicals that Henry believed he inherited and caused his illness. Henry’s illness experience reinforced his distrust in the institutions that were oppressed him and his family.
Veteran Status
Being a veteran in the Veterans Health Administration (VHA) system impacted how a narrative understanding of illness was created. Veterans are a unique cultural population with aspects of their illness experience that are important to understand.10 Institutions such as the VA also enable and constrain components of narrative creation.20 The illness narratives in this study were cocreated within the institutional setting of the VA. Part of the analysis included exploring how the institutional setting impacted the narrative creation. Emergent subthemes of the uniqueness of the veteran experience include financial benefits, intersectionality of government and health care, intersectionality of masculinity and military service, and the dichotomy of military experience.
In the US it is unique to the VA that the government both treats and assesses the severity of medical conditions to determine eligibility for health care and financial benefits. The VA’s financial benefits are intended to help compensate veterans who are experiencing illness as a result of their military experience.21 However, because the VA administers them the Veterans Benefits Administration and the VHA, veterans see both as interconnected. The perceived tie between illness severity and financial compensation could influence or bias how veterans understand their illness severity and experience. This may inadvertently encourage veterans to see their illness as being tied to their military service. This shaping of narratives should be considered as a contextual component as veterans obtain financial compensation and health insurance from the same larger organization that provides their health care and management.
George was a young man who during his service had chest pains and felt tired during physical training. He was surprised when his cardiologist explained his heart was enlarged. “All I know is when I initially joined the military, I was perfectly fine, you know, and when I was in the military, graduating, all that stuff, there was a glitch on the [electrocardiogram] they gave me after one day of doing [physical training] and then they’re like, oh, that’s fine. Come to find out it was mitral valve prolapse. And the doctors didn’t catch it then.” George feels the stress of the military caused his heart problems: “It wasn’t there before… so I’d have to say the strain from the military had to have caused it.” George’s medical history noted that he has a genetic connective tissue disorder that can lead to HF and likely was underlying cause of his illness. This example of how George pruned his narrative experience to highlight the cause as his military experience instead of a genetic disorder could have multiple financial and health benefits. The financial incentive for George to see his illness as caused by his military service could potentially bias his illness narrative to find his illness cause as tied to his service.
Government/Health Care Intersectionality
Veterans who may have experienced trust-breaking events with the government, like Agent Orange exposure or intergenerational racial trauma, may apply that experience to all government agencies. Bob felt the government had purposefully used him to create a military weapon. The army “knew I was angry and they used that for their advantage,” he said. Bob learned that he was exposed to Agent Orange in Vietnam, which is presumed to be associated with HF. Bob felt betrayed that the VHA had not figured out his health problems earlier. “I didn’t know anything about it until 6 months ago… Our government knew about it when they used it, and they didn’t care. They just wanted to win the war, and a whole lot of GIs like me suffered because of that, and I was like my government killed me? And I was fighting for them?”
Henry learned to distrust the government and the health care system because of a long history of systematic oppression and exploitation. These institutions’ erosion of trust has impact beyond the trust-breaking event itself but reverberates into how communities view organizations and institutions for generations. For Black Americans, who have historically been experimented on without consent by the US government and health care systems, this can make it especially hard to trust and build working relationships with those institutions. Health care professionals (HCPs) need to build collaborative partnerships with patients to provide effective care while understanding why some patients may have difficulty trusting health care systems, especially government-led systems.
The nature of HF as an illness can also make it difficult to predict and manage.22 This uncertainty and difficulty in managing HF can make it especially hard for people to establish trust with their HCPs whom they want to see as experts in their illness. HCPs in these narratives were often portrayed as incompetent or neglectful. The unpredictable nature of the illness itself was not reflected in the narrator’s experience.
Masculinity/Military Service Intersectionality
For the veteran narrators, tied into the identity of being a provider are social messages about masculinity. There is a unique intersectionality of being a man, the military culture, and living with chronic illnesses. Dominant social messages around being a man include being tough, not expressing emotion, self-reliance, and having power. This overlaps with social messages on military culture, including self-reliance, toughness, persistence in the face of adversity, limited expression of emotions, and the recognition of power and respect.23
People who internalize these social messages on masculinity may be less likely to access mental health treatment.23 This stigmatizing barrier to mental health treatment could impact how positive narratives are constructed around the experience of chronic illness for narrators who identify as masculine. Military and masculine identity could exclude or constrain stories about a veteran who did not “solider on” or who had to rely on others in a team to get things done. This shift can especially impact veterans experiencing chronic illnesses like HF, which often impact their physical abilities. Veterans may feel pressured to think of and portray themselves as being strong by limiting their expression of pain and other symptoms to remain in alignment with the dominant narrative. By not being open about the full experience of their illness both positive and negative, veterans may have unaddressed aspects of their illness experience or HCPs may not be able have all the information they need before the concern becomes a more serious health problem.
Dichotomy of Military Experience
Some narrators in this study talked about their military experience as both traumatic and beneficial. These dichotomous viewpoints can be difficult for veterans to construct a narrative understanding around. How can an inherently painful potentially traumatic experience, such as war, have benefits? This way of looking at the world may require a large narrative shift in their world and self-schemas to accept.
Bob hurt people in Vietnam as part of his job. “I did a lot of killing.” Bob met a village elder who stopped him from hurting people in the village and “in my spare time, I would go back to the village and he would teach me, how to be a better man,” Bob shared. “He taught me about life and everything, and he was awesome, just to this day, he’s like a father to me.” Bob tried to change his life and learned how to live a life full of love and care because of his experience in Vietnam. Though Bob hurt a lot of people in Vietnam, which still haunts him, he found meaning through his life lessons from the village elder. “I’m ashamed of what I did in Vietnam. I did some really bad stuff, but ever since then, I’ve always tried to do good to help people.”
Discussion
Exploring a person’s illness experience from a truly holistic pathway allows HCPs to see how the ripples of illness echo into the interconnection of surrounding systems and even across time. These stories suggest that veterans may experience their illness and construct their illness narratives based on the distinct contextual considerations of veteran culture.10 Research exploring how veterans see their illness and its potential impact on their health care access and choices could benefit from exploration into narrative understanding and meaning creation as a potentially contributing factor to health care decision making. As veterans are treated across health care systems, this has implications not only for VHA care, but community care as well.
These narratives also demonstrate how veterans create health care goals woven into their narrative understanding of their illness and its cause, lending insight into understanding health care decision making. This change in self-schema shapes how veterans see themselves and their role which shapes other aspects of their health care. These findings also contribute to our understanding of meaning creation. By exploring meaning making and narrative understanding, this work adds to our knowledge of the importance of spirituality as a component of the holistic experience of illness. There have been previous studies exploring the spiritual aspects of HF and the importance of meaning making.24,25 Exploring meaning making as an aspect of illness narratives can have important implications. Future research could explore the connections between meaning creation and illness narratives.
Limitations
The sample of veterans who participated in this study and are not generalizable to all veteran populations. The sample also only reflects people who were willing to participate and may exclude experience of people who may not have felt comfortable talking to a VA employee about their experience. It is also important to note that the small sample size included primarily male and White participants. In narrative inquiry, the number of participants is not as essential as diving into the depth of the interviews with the participants.
It is also important to note the position of the interviewer. As a White cisgender, heterosexual, middle-aged, middle class female who was raised in rural Kansas in a predominantly Protestant community, the positionality of the interviewer as a cocreator of the data inherently shaped and influenced the narratives created during this study. This contextual understanding of narratives created within the research relationship is an essential component to narrative inquiry and understanding.
Conclusions
Exploring these veterans’ narrative understanding of their experience of illness has many potential implications for health care systems, HCPs, and our military and veteran populations described in this article. Thinking about how the impact of racism, the influence of incentives to remain ill, and the complex intersection of identity and health brings light to how these domains may influence how people see themselves and engage in health care. These domains from these stories of the heart may help millions of people living with chronic illnesses like HF to not only live with their illness but inform how their experience is shaped by the systems surrounding them, including health care, government, and systems of power and oppression.
1. Ashton CM, Bozkurt B, Colucci WB, et al. Veterans Affairs quality enhancement research initiative in chronic heart failure. Medical care. 2000;38(6):I-26-I-37.
2. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi:10.1161/CIR.0000000000000350
3. Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35(6):594-603. doi:10.1016/j.jpainsymman.2007.06.007
4. Zambroski CH. Qualitative analysis of living with heart failure. Heart Lung. 2003;32(1):32-40. doi:10.1067/mhl.2003.10
5. Walthall H, Jenkinson C, Boulton M. Living with breathlessness in chronic heart failure: a qualitative study. J Clin Nurs. 2017;26(13-14):2036-2044. doi:10.1111/jocn.13615
6. Francis GS, Greenberg BH, Hsu DT, et al. ACCF/AHA/ACP/HFSA/ISHLT 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant: a report of the ACCF/AHA/ACP Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2010;56(5):424-453. doi:10.1016/j.jacc.2010.04.014
7. Rumsfeld JS, Havranek E, Masoudi FA, et al. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol. 2003;42(10):1811-1817. doi:10.1016/j.jacc.2003.07.013
8. Leeming A, Murray SA, Kendall M. The impact of advanced heart failure on social, psychological and existential aspects and personhood. Eur J Cardiovasc Nurs. 2014;13(2):162-167. doi:10.1177/1474515114520771
9. Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643-648. doi:10.1016/j.cardfail.2007.05.005
10. Weiss E, Coll JE. The influence of military culture and veteran worldviews on mental health treatment: practice implications for combat veteran help-seeking and wellness. Int J Health, Wellness Society. 2011;1(2):75-86. doi:10.18848/2156-8960/CGP/v01i02/41168
11. Sharpe L, Curran L. Understanding the process of adjustment to illness. Soc Sci Med. 2006;62(5):1153-1166. doi:10.1016/j.socscimed.2005.07.010
12. Riessman CK. Narrative Methods for the Human Sciences. SAGE Publications; 2008.
13. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qualitative Research. 2003;3(1):5-33. doi:10.1177/146879410300300101
14. Riessman CK. Strategic uses of narrative in the presentation of self and illness: a research note. Soc Sci Med. 1990;30(11):1195-1200. doi:10.1016/0277-9536(90)90259-U
15. Riessman CK. Analysis of personal narratives. In: Handbook of Interview Research. Sage; 2002:695-710.
16. Riessman CK. Illness Narratives: Positioned Identities. Invited Annual Lecture. Cardiff University. May 2002. Accessed April 14 2022. https://www.researchgate.net/publication/241501264_Illness_Narratives_Positioned_Identities
17. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qual Res. 2003;3(1):5-33. doi:10.1177/146879410300300101
18. Williams G. The genesis of chronic illness: narrative re‐construction. Sociol Health Illn. 1984;6(2):175-200. doi:10.1111/1467-9566.ep10778250
19. White M, Epston D. Narrative Means to Therapeutic Ends. WW Norton & Company; 1990.
20. Burchardt M. Illness Narratives as Theory and Method. SAGE Publications; 2020.
21. Sayer NA, Spoont M, Nelson D. Veterans seeking disability benefits for post-traumatic stress disorder: who applies and the self-reported meaning of disability compensation. Soc Sci Med. 2004;58(11):2133-2143. doi:10.1016/j.socscimed.2003.08.009
22. Winters CA. Heart failure: living with uncertainty. Prog Cardiovasc Nurs. 1999;14(3):85.
23. Plys E, Smith R, Jacobs ML. Masculinity and military culture in VA hospice and palliative care: a narrative review with clinical recommendations. J Palliat Care. 2020;35(2):120-126. doi:10.1177/0825859719851483
24. Johnson LS. Facilitating spiritual meaning‐making for the individual with a diagnosis of a terminal illness. Counseling and Values. 2003;47(3):230-240. doi:10.1002/j.2161-007X.2003.tb00269.x
25. Shahrbabaki PM, Nouhi E, Kazemi M, Ahmadi F. Defective support network: a major obstacle to coping for patients with heart failure: a qualitative study. Glob Health Action. 2016;9:30767. Published 2016 Apr 1. doi:10.3402/gha.v9.30767
Heart failure (HF) is a costly and burdensome illness and is the top reason for hospital admissions for the US Department of Veterans Affairs (VA) and Medicare.1 The cost of HF to the United States is estimated to grow to $3 billion annually by 2030.2 People living with HF have a high symptom burden and poor quality of life.3,4 Symptoms include shortness of breath, fatigue, depression, and decreases in psychosocial, existential, and spiritual well-being.5-9
Veterans in the US are a unique cultural group with distinct contextual considerations around their experiences.10 Different groups of veterans require unique cultural considerations, such as the experiences of veterans who served during the Vietnam war and during Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF). The extent of unmet needs of people living with HF, the number of veterans living with this illness, and the unique contextual components related to living with HF among veterans require further exploration into this illness experience for this distinct population. Research should explore innovative ways of managing both the number of people living with the illness and the significant impact of HF in people’s lives due to the high symptom burden and poor quality of life.3
This study used the model of adjustment to illness to explore the psychosocial adjustment to illness and the experience of US veterans living with HF, with a focus on the domains of meaning creation, self-schema, and world schema.11 The model of adjustment to illness describes how people learn to adjust to living with an illness, which can lead to positive health outcomes. Meaning creation is defined as the process in which people create meaning from their experience living with illness. Self-schema is how people living with illness see themselves, and world schema is how people living with chronic illness see their place in the world. These domains shift as part of the adjustment to living with an illness described in this model.11 This foundation allowed the investigators to explore the experience of living with HF among veterans with a focus on these domains. Our study aimed to cocreate illness narratives among veterans living with HF and to explore components of psychosocial adjustment informed by the model.
Methods
This study used narrative inquiry with a focus on illness narratives.12-17 Narrative inquiry as defined by Catherine Riessman involves the generation of socially constructed and cocreated meanings between the researcher and narrator. The researcher is an active participant in narrative creation as the narrator chooses which events to include in the stories based on the social, historical, and cultural context of both the narrator (study participant) and audience (researcher). Riessman describes the importance of contextual factors and meaning creation as an important aspect of narrative inquiry.12-14,16,17 It is important in narrative inquiry to consider how cultural, social, and historical factors influence narrative creation, constriction, and/or elimination.
This study prospectively created and collected data at a single time point. Semi-structured interviews explored psychosocial adjustment for people living with HF using an opening question modified from previous illness narrative research: Why do you think you got heart failure?18 Probes included the domains of psychosocial adjustment informed by the model of adjustment to illness domains (Figure). Emergent probes were used to illicit additional data around psychosocial adjustment to illness. Data were created and collected in accordance with narrative inquiry during the cocreation of the illness narratives between the researcher and study participants. This interview guide was tested by the first author in preliminary work to prepare for this study.
Allowing for emergent probes and acknowledging the role of the researcher as audience is key to the cocreation of narratives using this methodological framework. Narrators shape their narrative with the audience in mind; they cocreate their narrative with their audience using this type of narrative inquiry.12,16 What the narrator chooses to include and exclude from their story provides a window into how they see themselves and their world.19 Audio recordings were used to capture data, allowing for the researcher to take contemporaneous notes exploring contextual considerations to the narrative cocreation process and to be used later in analysis. Analytic notes were completed during the interviews as well as later in analysis as part of the contextual reflection.
Setting
Research was conducted in the Rocky Mountain Regional VA Medical Center, Aurora, Colorado. Participants were recruited through the outpatient cardiology clinic where the interviews also took place. This study was approved through the Colorado Institutional Review Board and Rocky Mountain Regional VA Medical Center (IRB: 19-1064). Participants were identified by the treating cardiologist who was a part of the study team. Interested veterans were introduced to the first author who was stationed in an empty clinic room. The study cardiologist screened to ensure all participants were ≥ 18 years of age and had a diagnosis of HF for > 1 year. Persons with an impairment that could interfere with their ability to construct a narrative were also excluded.
Recruitment took place from October 2019 to January 2020. Three veterans refused participation. Five study participants provided informed consent and were enrolled and interviewed. All interviews were completed in the clinic at the time of consent per participant preference. One-hour long semi-structured interviews were conducted and audio recorded. A demographic form was administered at the end of each interview to capture contextual data. The researcher also kept a reflexive journal and audit trail.
Narrative Analysis
Riessman described general steps to conduct narrative analysis, including transcription, narrative clean-up, consideration of contextual factors, exploration of thematic threads, consideration of larger social narratives, and positioning.12 The first author read transcripts while listening to the audio recordings to ensure accuracy. With narrative clean-up each narrative was organized to cocreate overall meaning, changed to protect anonymity, and refined to only include the illness narrative. For example, if a narrator told a story about childhood and then later in the interview remembered another detail to add to their story, narrative clean-up reordered events to make cohesive sense of the story. Demographic, historical, cultural, and social contexts of both the narrator and audience were reflected on during analysis to explore how these components may have shaped and influenced cocreation. Context was also considered within the larger VA setting.
Emergent themes were explored for convergence, divergence, and points of tension within and across each narrative. Larger social narratives were also considered for their influence on possible inclusion/exclusion of experience, such as how gender identity may have influenced study participants’ descriptions of their roles in social systems. These themes and narratives were then shared with our team, and we worked through decision points during the analysis process and discussed interpretation of the data to reach consensus.
Results
Five veterans living with HF were recruited and consented to participate in the study. Demographics of the participants and first author are included in the Table. Five illness narratives were cocreated, entitled: Blame the Cheese: Frank’s Illness Narrative; Love is Love: Bob’s Illness Narrative; The Brighter Things in Life is My Family: George’s Illness Narrative; We Never Know When Our Time is Coming: Bill’s Illness Narrative; and A Dream Deferred: Henry’s Illness Narrative.
Each narrative was explored focusing on the domains of the model of adjustment to illness. An emergent theme was also identified with multiple subthemes: being a veteran is unique. Related subthemes included: financial benefits, intersectionality of government and health care, the intersectionality of masculinity and military service, and the dichotomy of military experience.
The search for meaning creation after the experience of chronic illness emerged across interviews. One example of meaning creation was in Frank's illness narrative. Frank was unsure why he got HF: “Probably because I ate too much cheese…I mean, that’s gotta be it. It can’t be anything else.” By tying HF to his diet, he found meaning through his health behaviors.
Model of Adjustment to Illness
The narratives illustrate components of the model of adjustment to illness and describe how each of the participants either shifted their self-schema and world schema or reinforced their previously established schemas. It also demonstrates how people use narratives to create meaning and illness understanding from their illness experience, reflecting, and emphasizing different parts creating meaning from their experience.
A commonality across the narratives was a shift in self-schema, including the shift from being a provider to being reliant on others. In accordance with the dominant social narrative around men as providers, each narrator talked about their identity as a provider for themselves and their families. Often keeping their provider identity required modifications of the definition, from physical abilities and employment to financial security and stability. George made all his health care decisions based on his goal of providing for his family and protecting them from having to care for him: “I’m always thinking about the future, always trying to figure out how my family, if something should happen to me, how my family would cope, and how my family would be able to support themselves.” Bob’s health care goals were to stay alive long enough for his wife to get financial benefits as a surviving spouse: “That’s why I’m trying to make everything for her, you know. I’m not worried about myself. I’m not. Her I am, you know. And love is love.” Both of their health care decisions are shaped by their identity as a provider shifting to financial support.
Some narrators changed the way they saw their world, or world schema, while others felt their illness experience just reinforced the way they had already experienced the world. Frank was able to reprioritize what was important to him after his diagnosis and accept his own mortality: “I might as well chill out, no more stress, and just enjoy things ’cause you could die…” For Henry, getting HF was only part of the experience of systemic oppression that had impacted his and his family’s lives for generations. He saw how his oppression by the military and US government led to his father’s exposure to chemicals that Henry believed he inherited and caused his illness. Henry’s illness experience reinforced his distrust in the institutions that were oppressed him and his family.
Veteran Status
Being a veteran in the Veterans Health Administration (VHA) system impacted how a narrative understanding of illness was created. Veterans are a unique cultural population with aspects of their illness experience that are important to understand.10 Institutions such as the VA also enable and constrain components of narrative creation.20 The illness narratives in this study were cocreated within the institutional setting of the VA. Part of the analysis included exploring how the institutional setting impacted the narrative creation. Emergent subthemes of the uniqueness of the veteran experience include financial benefits, intersectionality of government and health care, intersectionality of masculinity and military service, and the dichotomy of military experience.
In the US it is unique to the VA that the government both treats and assesses the severity of medical conditions to determine eligibility for health care and financial benefits. The VA’s financial benefits are intended to help compensate veterans who are experiencing illness as a result of their military experience.21 However, because the VA administers them the Veterans Benefits Administration and the VHA, veterans see both as interconnected. The perceived tie between illness severity and financial compensation could influence or bias how veterans understand their illness severity and experience. This may inadvertently encourage veterans to see their illness as being tied to their military service. This shaping of narratives should be considered as a contextual component as veterans obtain financial compensation and health insurance from the same larger organization that provides their health care and management.
George was a young man who during his service had chest pains and felt tired during physical training. He was surprised when his cardiologist explained his heart was enlarged. “All I know is when I initially joined the military, I was perfectly fine, you know, and when I was in the military, graduating, all that stuff, there was a glitch on the [electrocardiogram] they gave me after one day of doing [physical training] and then they’re like, oh, that’s fine. Come to find out it was mitral valve prolapse. And the doctors didn’t catch it then.” George feels the stress of the military caused his heart problems: “It wasn’t there before… so I’d have to say the strain from the military had to have caused it.” George’s medical history noted that he has a genetic connective tissue disorder that can lead to HF and likely was underlying cause of his illness. This example of how George pruned his narrative experience to highlight the cause as his military experience instead of a genetic disorder could have multiple financial and health benefits. The financial incentive for George to see his illness as caused by his military service could potentially bias his illness narrative to find his illness cause as tied to his service.
Government/Health Care Intersectionality
Veterans who may have experienced trust-breaking events with the government, like Agent Orange exposure or intergenerational racial trauma, may apply that experience to all government agencies. Bob felt the government had purposefully used him to create a military weapon. The army “knew I was angry and they used that for their advantage,” he said. Bob learned that he was exposed to Agent Orange in Vietnam, which is presumed to be associated with HF. Bob felt betrayed that the VHA had not figured out his health problems earlier. “I didn’t know anything about it until 6 months ago… Our government knew about it when they used it, and they didn’t care. They just wanted to win the war, and a whole lot of GIs like me suffered because of that, and I was like my government killed me? And I was fighting for them?”
Henry learned to distrust the government and the health care system because of a long history of systematic oppression and exploitation. These institutions’ erosion of trust has impact beyond the trust-breaking event itself but reverberates into how communities view organizations and institutions for generations. For Black Americans, who have historically been experimented on without consent by the US government and health care systems, this can make it especially hard to trust and build working relationships with those institutions. Health care professionals (HCPs) need to build collaborative partnerships with patients to provide effective care while understanding why some patients may have difficulty trusting health care systems, especially government-led systems.
The nature of HF as an illness can also make it difficult to predict and manage.22 This uncertainty and difficulty in managing HF can make it especially hard for people to establish trust with their HCPs whom they want to see as experts in their illness. HCPs in these narratives were often portrayed as incompetent or neglectful. The unpredictable nature of the illness itself was not reflected in the narrator’s experience.
Masculinity/Military Service Intersectionality
For the veteran narrators, tied into the identity of being a provider are social messages about masculinity. There is a unique intersectionality of being a man, the military culture, and living with chronic illnesses. Dominant social messages around being a man include being tough, not expressing emotion, self-reliance, and having power. This overlaps with social messages on military culture, including self-reliance, toughness, persistence in the face of adversity, limited expression of emotions, and the recognition of power and respect.23
People who internalize these social messages on masculinity may be less likely to access mental health treatment.23 This stigmatizing barrier to mental health treatment could impact how positive narratives are constructed around the experience of chronic illness for narrators who identify as masculine. Military and masculine identity could exclude or constrain stories about a veteran who did not “solider on” or who had to rely on others in a team to get things done. This shift can especially impact veterans experiencing chronic illnesses like HF, which often impact their physical abilities. Veterans may feel pressured to think of and portray themselves as being strong by limiting their expression of pain and other symptoms to remain in alignment with the dominant narrative. By not being open about the full experience of their illness both positive and negative, veterans may have unaddressed aspects of their illness experience or HCPs may not be able have all the information they need before the concern becomes a more serious health problem.
Dichotomy of Military Experience
Some narrators in this study talked about their military experience as both traumatic and beneficial. These dichotomous viewpoints can be difficult for veterans to construct a narrative understanding around. How can an inherently painful potentially traumatic experience, such as war, have benefits? This way of looking at the world may require a large narrative shift in their world and self-schemas to accept.
Bob hurt people in Vietnam as part of his job. “I did a lot of killing.” Bob met a village elder who stopped him from hurting people in the village and “in my spare time, I would go back to the village and he would teach me, how to be a better man,” Bob shared. “He taught me about life and everything, and he was awesome, just to this day, he’s like a father to me.” Bob tried to change his life and learned how to live a life full of love and care because of his experience in Vietnam. Though Bob hurt a lot of people in Vietnam, which still haunts him, he found meaning through his life lessons from the village elder. “I’m ashamed of what I did in Vietnam. I did some really bad stuff, but ever since then, I’ve always tried to do good to help people.”
Discussion
Exploring a person’s illness experience from a truly holistic pathway allows HCPs to see how the ripples of illness echo into the interconnection of surrounding systems and even across time. These stories suggest that veterans may experience their illness and construct their illness narratives based on the distinct contextual considerations of veteran culture.10 Research exploring how veterans see their illness and its potential impact on their health care access and choices could benefit from exploration into narrative understanding and meaning creation as a potentially contributing factor to health care decision making. As veterans are treated across health care systems, this has implications not only for VHA care, but community care as well.
These narratives also demonstrate how veterans create health care goals woven into their narrative understanding of their illness and its cause, lending insight into understanding health care decision making. This change in self-schema shapes how veterans see themselves and their role which shapes other aspects of their health care. These findings also contribute to our understanding of meaning creation. By exploring meaning making and narrative understanding, this work adds to our knowledge of the importance of spirituality as a component of the holistic experience of illness. There have been previous studies exploring the spiritual aspects of HF and the importance of meaning making.24,25 Exploring meaning making as an aspect of illness narratives can have important implications. Future research could explore the connections between meaning creation and illness narratives.
Limitations
The sample of veterans who participated in this study and are not generalizable to all veteran populations. The sample also only reflects people who were willing to participate and may exclude experience of people who may not have felt comfortable talking to a VA employee about their experience. It is also important to note that the small sample size included primarily male and White participants. In narrative inquiry, the number of participants is not as essential as diving into the depth of the interviews with the participants.
It is also important to note the position of the interviewer. As a White cisgender, heterosexual, middle-aged, middle class female who was raised in rural Kansas in a predominantly Protestant community, the positionality of the interviewer as a cocreator of the data inherently shaped and influenced the narratives created during this study. This contextual understanding of narratives created within the research relationship is an essential component to narrative inquiry and understanding.
Conclusions
Exploring these veterans’ narrative understanding of their experience of illness has many potential implications for health care systems, HCPs, and our military and veteran populations described in this article. Thinking about how the impact of racism, the influence of incentives to remain ill, and the complex intersection of identity and health brings light to how these domains may influence how people see themselves and engage in health care. These domains from these stories of the heart may help millions of people living with chronic illnesses like HF to not only live with their illness but inform how their experience is shaped by the systems surrounding them, including health care, government, and systems of power and oppression.
Heart failure (HF) is a costly and burdensome illness and is the top reason for hospital admissions for the US Department of Veterans Affairs (VA) and Medicare.1 The cost of HF to the United States is estimated to grow to $3 billion annually by 2030.2 People living with HF have a high symptom burden and poor quality of life.3,4 Symptoms include shortness of breath, fatigue, depression, and decreases in psychosocial, existential, and spiritual well-being.5-9
Veterans in the US are a unique cultural group with distinct contextual considerations around their experiences.10 Different groups of veterans require unique cultural considerations, such as the experiences of veterans who served during the Vietnam war and during Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF). The extent of unmet needs of people living with HF, the number of veterans living with this illness, and the unique contextual components related to living with HF among veterans require further exploration into this illness experience for this distinct population. Research should explore innovative ways of managing both the number of people living with the illness and the significant impact of HF in people’s lives due to the high symptom burden and poor quality of life.3
This study used the model of adjustment to illness to explore the psychosocial adjustment to illness and the experience of US veterans living with HF, with a focus on the domains of meaning creation, self-schema, and world schema.11 The model of adjustment to illness describes how people learn to adjust to living with an illness, which can lead to positive health outcomes. Meaning creation is defined as the process in which people create meaning from their experience living with illness. Self-schema is how people living with illness see themselves, and world schema is how people living with chronic illness see their place in the world. These domains shift as part of the adjustment to living with an illness described in this model.11 This foundation allowed the investigators to explore the experience of living with HF among veterans with a focus on these domains. Our study aimed to cocreate illness narratives among veterans living with HF and to explore components of psychosocial adjustment informed by the model.
Methods
This study used narrative inquiry with a focus on illness narratives.12-17 Narrative inquiry as defined by Catherine Riessman involves the generation of socially constructed and cocreated meanings between the researcher and narrator. The researcher is an active participant in narrative creation as the narrator chooses which events to include in the stories based on the social, historical, and cultural context of both the narrator (study participant) and audience (researcher). Riessman describes the importance of contextual factors and meaning creation as an important aspect of narrative inquiry.12-14,16,17 It is important in narrative inquiry to consider how cultural, social, and historical factors influence narrative creation, constriction, and/or elimination.
This study prospectively created and collected data at a single time point. Semi-structured interviews explored psychosocial adjustment for people living with HF using an opening question modified from previous illness narrative research: Why do you think you got heart failure?18 Probes included the domains of psychosocial adjustment informed by the model of adjustment to illness domains (Figure). Emergent probes were used to illicit additional data around psychosocial adjustment to illness. Data were created and collected in accordance with narrative inquiry during the cocreation of the illness narratives between the researcher and study participants. This interview guide was tested by the first author in preliminary work to prepare for this study.
Allowing for emergent probes and acknowledging the role of the researcher as audience is key to the cocreation of narratives using this methodological framework. Narrators shape their narrative with the audience in mind; they cocreate their narrative with their audience using this type of narrative inquiry.12,16 What the narrator chooses to include and exclude from their story provides a window into how they see themselves and their world.19 Audio recordings were used to capture data, allowing for the researcher to take contemporaneous notes exploring contextual considerations to the narrative cocreation process and to be used later in analysis. Analytic notes were completed during the interviews as well as later in analysis as part of the contextual reflection.
Setting
Research was conducted in the Rocky Mountain Regional VA Medical Center, Aurora, Colorado. Participants were recruited through the outpatient cardiology clinic where the interviews also took place. This study was approved through the Colorado Institutional Review Board and Rocky Mountain Regional VA Medical Center (IRB: 19-1064). Participants were identified by the treating cardiologist who was a part of the study team. Interested veterans were introduced to the first author who was stationed in an empty clinic room. The study cardiologist screened to ensure all participants were ≥ 18 years of age and had a diagnosis of HF for > 1 year. Persons with an impairment that could interfere with their ability to construct a narrative were also excluded.
Recruitment took place from October 2019 to January 2020. Three veterans refused participation. Five study participants provided informed consent and were enrolled and interviewed. All interviews were completed in the clinic at the time of consent per participant preference. One-hour long semi-structured interviews were conducted and audio recorded. A demographic form was administered at the end of each interview to capture contextual data. The researcher also kept a reflexive journal and audit trail.
Narrative Analysis
Riessman described general steps to conduct narrative analysis, including transcription, narrative clean-up, consideration of contextual factors, exploration of thematic threads, consideration of larger social narratives, and positioning.12 The first author read transcripts while listening to the audio recordings to ensure accuracy. With narrative clean-up each narrative was organized to cocreate overall meaning, changed to protect anonymity, and refined to only include the illness narrative. For example, if a narrator told a story about childhood and then later in the interview remembered another detail to add to their story, narrative clean-up reordered events to make cohesive sense of the story. Demographic, historical, cultural, and social contexts of both the narrator and audience were reflected on during analysis to explore how these components may have shaped and influenced cocreation. Context was also considered within the larger VA setting.
Emergent themes were explored for convergence, divergence, and points of tension within and across each narrative. Larger social narratives were also considered for their influence on possible inclusion/exclusion of experience, such as how gender identity may have influenced study participants’ descriptions of their roles in social systems. These themes and narratives were then shared with our team, and we worked through decision points during the analysis process and discussed interpretation of the data to reach consensus.
Results
Five veterans living with HF were recruited and consented to participate in the study. Demographics of the participants and first author are included in the Table. Five illness narratives were cocreated, entitled: Blame the Cheese: Frank’s Illness Narrative; Love is Love: Bob’s Illness Narrative; The Brighter Things in Life is My Family: George’s Illness Narrative; We Never Know When Our Time is Coming: Bill’s Illness Narrative; and A Dream Deferred: Henry’s Illness Narrative.
Each narrative was explored focusing on the domains of the model of adjustment to illness. An emergent theme was also identified with multiple subthemes: being a veteran is unique. Related subthemes included: financial benefits, intersectionality of government and health care, the intersectionality of masculinity and military service, and the dichotomy of military experience.
The search for meaning creation after the experience of chronic illness emerged across interviews. One example of meaning creation was in Frank's illness narrative. Frank was unsure why he got HF: “Probably because I ate too much cheese…I mean, that’s gotta be it. It can’t be anything else.” By tying HF to his diet, he found meaning through his health behaviors.
Model of Adjustment to Illness
The narratives illustrate components of the model of adjustment to illness and describe how each of the participants either shifted their self-schema and world schema or reinforced their previously established schemas. It also demonstrates how people use narratives to create meaning and illness understanding from their illness experience, reflecting, and emphasizing different parts creating meaning from their experience.
A commonality across the narratives was a shift in self-schema, including the shift from being a provider to being reliant on others. In accordance with the dominant social narrative around men as providers, each narrator talked about their identity as a provider for themselves and their families. Often keeping their provider identity required modifications of the definition, from physical abilities and employment to financial security and stability. George made all his health care decisions based on his goal of providing for his family and protecting them from having to care for him: “I’m always thinking about the future, always trying to figure out how my family, if something should happen to me, how my family would cope, and how my family would be able to support themselves.” Bob’s health care goals were to stay alive long enough for his wife to get financial benefits as a surviving spouse: “That’s why I’m trying to make everything for her, you know. I’m not worried about myself. I’m not. Her I am, you know. And love is love.” Both of their health care decisions are shaped by their identity as a provider shifting to financial support.
Some narrators changed the way they saw their world, or world schema, while others felt their illness experience just reinforced the way they had already experienced the world. Frank was able to reprioritize what was important to him after his diagnosis and accept his own mortality: “I might as well chill out, no more stress, and just enjoy things ’cause you could die…” For Henry, getting HF was only part of the experience of systemic oppression that had impacted his and his family’s lives for generations. He saw how his oppression by the military and US government led to his father’s exposure to chemicals that Henry believed he inherited and caused his illness. Henry’s illness experience reinforced his distrust in the institutions that were oppressed him and his family.
Veteran Status
Being a veteran in the Veterans Health Administration (VHA) system impacted how a narrative understanding of illness was created. Veterans are a unique cultural population with aspects of their illness experience that are important to understand.10 Institutions such as the VA also enable and constrain components of narrative creation.20 The illness narratives in this study were cocreated within the institutional setting of the VA. Part of the analysis included exploring how the institutional setting impacted the narrative creation. Emergent subthemes of the uniqueness of the veteran experience include financial benefits, intersectionality of government and health care, intersectionality of masculinity and military service, and the dichotomy of military experience.
In the US it is unique to the VA that the government both treats and assesses the severity of medical conditions to determine eligibility for health care and financial benefits. The VA’s financial benefits are intended to help compensate veterans who are experiencing illness as a result of their military experience.21 However, because the VA administers them the Veterans Benefits Administration and the VHA, veterans see both as interconnected. The perceived tie between illness severity and financial compensation could influence or bias how veterans understand their illness severity and experience. This may inadvertently encourage veterans to see their illness as being tied to their military service. This shaping of narratives should be considered as a contextual component as veterans obtain financial compensation and health insurance from the same larger organization that provides their health care and management.
George was a young man who during his service had chest pains and felt tired during physical training. He was surprised when his cardiologist explained his heart was enlarged. “All I know is when I initially joined the military, I was perfectly fine, you know, and when I was in the military, graduating, all that stuff, there was a glitch on the [electrocardiogram] they gave me after one day of doing [physical training] and then they’re like, oh, that’s fine. Come to find out it was mitral valve prolapse. And the doctors didn’t catch it then.” George feels the stress of the military caused his heart problems: “It wasn’t there before… so I’d have to say the strain from the military had to have caused it.” George’s medical history noted that he has a genetic connective tissue disorder that can lead to HF and likely was underlying cause of his illness. This example of how George pruned his narrative experience to highlight the cause as his military experience instead of a genetic disorder could have multiple financial and health benefits. The financial incentive for George to see his illness as caused by his military service could potentially bias his illness narrative to find his illness cause as tied to his service.
Government/Health Care Intersectionality
Veterans who may have experienced trust-breaking events with the government, like Agent Orange exposure or intergenerational racial trauma, may apply that experience to all government agencies. Bob felt the government had purposefully used him to create a military weapon. The army “knew I was angry and they used that for their advantage,” he said. Bob learned that he was exposed to Agent Orange in Vietnam, which is presumed to be associated with HF. Bob felt betrayed that the VHA had not figured out his health problems earlier. “I didn’t know anything about it until 6 months ago… Our government knew about it when they used it, and they didn’t care. They just wanted to win the war, and a whole lot of GIs like me suffered because of that, and I was like my government killed me? And I was fighting for them?”
Henry learned to distrust the government and the health care system because of a long history of systematic oppression and exploitation. These institutions’ erosion of trust has impact beyond the trust-breaking event itself but reverberates into how communities view organizations and institutions for generations. For Black Americans, who have historically been experimented on without consent by the US government and health care systems, this can make it especially hard to trust and build working relationships with those institutions. Health care professionals (HCPs) need to build collaborative partnerships with patients to provide effective care while understanding why some patients may have difficulty trusting health care systems, especially government-led systems.
The nature of HF as an illness can also make it difficult to predict and manage.22 This uncertainty and difficulty in managing HF can make it especially hard for people to establish trust with their HCPs whom they want to see as experts in their illness. HCPs in these narratives were often portrayed as incompetent or neglectful. The unpredictable nature of the illness itself was not reflected in the narrator’s experience.
Masculinity/Military Service Intersectionality
For the veteran narrators, tied into the identity of being a provider are social messages about masculinity. There is a unique intersectionality of being a man, the military culture, and living with chronic illnesses. Dominant social messages around being a man include being tough, not expressing emotion, self-reliance, and having power. This overlaps with social messages on military culture, including self-reliance, toughness, persistence in the face of adversity, limited expression of emotions, and the recognition of power and respect.23
People who internalize these social messages on masculinity may be less likely to access mental health treatment.23 This stigmatizing barrier to mental health treatment could impact how positive narratives are constructed around the experience of chronic illness for narrators who identify as masculine. Military and masculine identity could exclude or constrain stories about a veteran who did not “solider on” or who had to rely on others in a team to get things done. This shift can especially impact veterans experiencing chronic illnesses like HF, which often impact their physical abilities. Veterans may feel pressured to think of and portray themselves as being strong by limiting their expression of pain and other symptoms to remain in alignment with the dominant narrative. By not being open about the full experience of their illness both positive and negative, veterans may have unaddressed aspects of their illness experience or HCPs may not be able have all the information they need before the concern becomes a more serious health problem.
Dichotomy of Military Experience
Some narrators in this study talked about their military experience as both traumatic and beneficial. These dichotomous viewpoints can be difficult for veterans to construct a narrative understanding around. How can an inherently painful potentially traumatic experience, such as war, have benefits? This way of looking at the world may require a large narrative shift in their world and self-schemas to accept.
Bob hurt people in Vietnam as part of his job. “I did a lot of killing.” Bob met a village elder who stopped him from hurting people in the village and “in my spare time, I would go back to the village and he would teach me, how to be a better man,” Bob shared. “He taught me about life and everything, and he was awesome, just to this day, he’s like a father to me.” Bob tried to change his life and learned how to live a life full of love and care because of his experience in Vietnam. Though Bob hurt a lot of people in Vietnam, which still haunts him, he found meaning through his life lessons from the village elder. “I’m ashamed of what I did in Vietnam. I did some really bad stuff, but ever since then, I’ve always tried to do good to help people.”
Discussion
Exploring a person’s illness experience from a truly holistic pathway allows HCPs to see how the ripples of illness echo into the interconnection of surrounding systems and even across time. These stories suggest that veterans may experience their illness and construct their illness narratives based on the distinct contextual considerations of veteran culture.10 Research exploring how veterans see their illness and its potential impact on their health care access and choices could benefit from exploration into narrative understanding and meaning creation as a potentially contributing factor to health care decision making. As veterans are treated across health care systems, this has implications not only for VHA care, but community care as well.
These narratives also demonstrate how veterans create health care goals woven into their narrative understanding of their illness and its cause, lending insight into understanding health care decision making. This change in self-schema shapes how veterans see themselves and their role which shapes other aspects of their health care. These findings also contribute to our understanding of meaning creation. By exploring meaning making and narrative understanding, this work adds to our knowledge of the importance of spirituality as a component of the holistic experience of illness. There have been previous studies exploring the spiritual aspects of HF and the importance of meaning making.24,25 Exploring meaning making as an aspect of illness narratives can have important implications. Future research could explore the connections between meaning creation and illness narratives.
Limitations
The sample of veterans who participated in this study and are not generalizable to all veteran populations. The sample also only reflects people who were willing to participate and may exclude experience of people who may not have felt comfortable talking to a VA employee about their experience. It is also important to note that the small sample size included primarily male and White participants. In narrative inquiry, the number of participants is not as essential as diving into the depth of the interviews with the participants.
It is also important to note the position of the interviewer. As a White cisgender, heterosexual, middle-aged, middle class female who was raised in rural Kansas in a predominantly Protestant community, the positionality of the interviewer as a cocreator of the data inherently shaped and influenced the narratives created during this study. This contextual understanding of narratives created within the research relationship is an essential component to narrative inquiry and understanding.
Conclusions
Exploring these veterans’ narrative understanding of their experience of illness has many potential implications for health care systems, HCPs, and our military and veteran populations described in this article. Thinking about how the impact of racism, the influence of incentives to remain ill, and the complex intersection of identity and health brings light to how these domains may influence how people see themselves and engage in health care. These domains from these stories of the heart may help millions of people living with chronic illnesses like HF to not only live with their illness but inform how their experience is shaped by the systems surrounding them, including health care, government, and systems of power and oppression.
1. Ashton CM, Bozkurt B, Colucci WB, et al. Veterans Affairs quality enhancement research initiative in chronic heart failure. Medical care. 2000;38(6):I-26-I-37.
2. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi:10.1161/CIR.0000000000000350
3. Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35(6):594-603. doi:10.1016/j.jpainsymman.2007.06.007
4. Zambroski CH. Qualitative analysis of living with heart failure. Heart Lung. 2003;32(1):32-40. doi:10.1067/mhl.2003.10
5. Walthall H, Jenkinson C, Boulton M. Living with breathlessness in chronic heart failure: a qualitative study. J Clin Nurs. 2017;26(13-14):2036-2044. doi:10.1111/jocn.13615
6. Francis GS, Greenberg BH, Hsu DT, et al. ACCF/AHA/ACP/HFSA/ISHLT 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant: a report of the ACCF/AHA/ACP Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2010;56(5):424-453. doi:10.1016/j.jacc.2010.04.014
7. Rumsfeld JS, Havranek E, Masoudi FA, et al. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol. 2003;42(10):1811-1817. doi:10.1016/j.jacc.2003.07.013
8. Leeming A, Murray SA, Kendall M. The impact of advanced heart failure on social, psychological and existential aspects and personhood. Eur J Cardiovasc Nurs. 2014;13(2):162-167. doi:10.1177/1474515114520771
9. Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643-648. doi:10.1016/j.cardfail.2007.05.005
10. Weiss E, Coll JE. The influence of military culture and veteran worldviews on mental health treatment: practice implications for combat veteran help-seeking and wellness. Int J Health, Wellness Society. 2011;1(2):75-86. doi:10.18848/2156-8960/CGP/v01i02/41168
11. Sharpe L, Curran L. Understanding the process of adjustment to illness. Soc Sci Med. 2006;62(5):1153-1166. doi:10.1016/j.socscimed.2005.07.010
12. Riessman CK. Narrative Methods for the Human Sciences. SAGE Publications; 2008.
13. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qualitative Research. 2003;3(1):5-33. doi:10.1177/146879410300300101
14. Riessman CK. Strategic uses of narrative in the presentation of self and illness: a research note. Soc Sci Med. 1990;30(11):1195-1200. doi:10.1016/0277-9536(90)90259-U
15. Riessman CK. Analysis of personal narratives. In: Handbook of Interview Research. Sage; 2002:695-710.
16. Riessman CK. Illness Narratives: Positioned Identities. Invited Annual Lecture. Cardiff University. May 2002. Accessed April 14 2022. https://www.researchgate.net/publication/241501264_Illness_Narratives_Positioned_Identities
17. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qual Res. 2003;3(1):5-33. doi:10.1177/146879410300300101
18. Williams G. The genesis of chronic illness: narrative re‐construction. Sociol Health Illn. 1984;6(2):175-200. doi:10.1111/1467-9566.ep10778250
19. White M, Epston D. Narrative Means to Therapeutic Ends. WW Norton & Company; 1990.
20. Burchardt M. Illness Narratives as Theory and Method. SAGE Publications; 2020.
21. Sayer NA, Spoont M, Nelson D. Veterans seeking disability benefits for post-traumatic stress disorder: who applies and the self-reported meaning of disability compensation. Soc Sci Med. 2004;58(11):2133-2143. doi:10.1016/j.socscimed.2003.08.009
22. Winters CA. Heart failure: living with uncertainty. Prog Cardiovasc Nurs. 1999;14(3):85.
23. Plys E, Smith R, Jacobs ML. Masculinity and military culture in VA hospice and palliative care: a narrative review with clinical recommendations. J Palliat Care. 2020;35(2):120-126. doi:10.1177/0825859719851483
24. Johnson LS. Facilitating spiritual meaning‐making for the individual with a diagnosis of a terminal illness. Counseling and Values. 2003;47(3):230-240. doi:10.1002/j.2161-007X.2003.tb00269.x
25. Shahrbabaki PM, Nouhi E, Kazemi M, Ahmadi F. Defective support network: a major obstacle to coping for patients with heart failure: a qualitative study. Glob Health Action. 2016;9:30767. Published 2016 Apr 1. doi:10.3402/gha.v9.30767
1. Ashton CM, Bozkurt B, Colucci WB, et al. Veterans Affairs quality enhancement research initiative in chronic heart failure. Medical care. 2000;38(6):I-26-I-37.
2. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi:10.1161/CIR.0000000000000350
3. Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35(6):594-603. doi:10.1016/j.jpainsymman.2007.06.007
4. Zambroski CH. Qualitative analysis of living with heart failure. Heart Lung. 2003;32(1):32-40. doi:10.1067/mhl.2003.10
5. Walthall H, Jenkinson C, Boulton M. Living with breathlessness in chronic heart failure: a qualitative study. J Clin Nurs. 2017;26(13-14):2036-2044. doi:10.1111/jocn.13615
6. Francis GS, Greenberg BH, Hsu DT, et al. ACCF/AHA/ACP/HFSA/ISHLT 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant: a report of the ACCF/AHA/ACP Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2010;56(5):424-453. doi:10.1016/j.jacc.2010.04.014
7. Rumsfeld JS, Havranek E, Masoudi FA, et al. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol. 2003;42(10):1811-1817. doi:10.1016/j.jacc.2003.07.013
8. Leeming A, Murray SA, Kendall M. The impact of advanced heart failure on social, psychological and existential aspects and personhood. Eur J Cardiovasc Nurs. 2014;13(2):162-167. doi:10.1177/1474515114520771
9. Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643-648. doi:10.1016/j.cardfail.2007.05.005
10. Weiss E, Coll JE. The influence of military culture and veteran worldviews on mental health treatment: practice implications for combat veteran help-seeking and wellness. Int J Health, Wellness Society. 2011;1(2):75-86. doi:10.18848/2156-8960/CGP/v01i02/41168
11. Sharpe L, Curran L. Understanding the process of adjustment to illness. Soc Sci Med. 2006;62(5):1153-1166. doi:10.1016/j.socscimed.2005.07.010
12. Riessman CK. Narrative Methods for the Human Sciences. SAGE Publications; 2008.
13. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qualitative Research. 2003;3(1):5-33. doi:10.1177/146879410300300101
14. Riessman CK. Strategic uses of narrative in the presentation of self and illness: a research note. Soc Sci Med. 1990;30(11):1195-1200. doi:10.1016/0277-9536(90)90259-U
15. Riessman CK. Analysis of personal narratives. In: Handbook of Interview Research. Sage; 2002:695-710.
16. Riessman CK. Illness Narratives: Positioned Identities. Invited Annual Lecture. Cardiff University. May 2002. Accessed April 14 2022. https://www.researchgate.net/publication/241501264_Illness_Narratives_Positioned_Identities
17. Riessman CK. Performing identities in illness narrative: masculinity and multiple sclerosis. Qual Res. 2003;3(1):5-33. doi:10.1177/146879410300300101
18. Williams G. The genesis of chronic illness: narrative re‐construction. Sociol Health Illn. 1984;6(2):175-200. doi:10.1111/1467-9566.ep10778250
19. White M, Epston D. Narrative Means to Therapeutic Ends. WW Norton & Company; 1990.
20. Burchardt M. Illness Narratives as Theory and Method. SAGE Publications; 2020.
21. Sayer NA, Spoont M, Nelson D. Veterans seeking disability benefits for post-traumatic stress disorder: who applies and the self-reported meaning of disability compensation. Soc Sci Med. 2004;58(11):2133-2143. doi:10.1016/j.socscimed.2003.08.009
22. Winters CA. Heart failure: living with uncertainty. Prog Cardiovasc Nurs. 1999;14(3):85.
23. Plys E, Smith R, Jacobs ML. Masculinity and military culture in VA hospice and palliative care: a narrative review with clinical recommendations. J Palliat Care. 2020;35(2):120-126. doi:10.1177/0825859719851483
24. Johnson LS. Facilitating spiritual meaning‐making for the individual with a diagnosis of a terminal illness. Counseling and Values. 2003;47(3):230-240. doi:10.1002/j.2161-007X.2003.tb00269.x
25. Shahrbabaki PM, Nouhi E, Kazemi M, Ahmadi F. Defective support network: a major obstacle to coping for patients with heart failure: a qualitative study. Glob Health Action. 2016;9:30767. Published 2016 Apr 1. doi:10.3402/gha.v9.30767
Is There a Relationship Between Facility Peer Review Findings and Quality in the Veterans Health Administration?
Hospital leaders report the most common aim of peer review (PR) is to improve quality and patient safety, thus it is a potentially powerful quality improvement (QI) driver.1 “When conducted systematically and credibly, peer review for quality management can result in both short-term and long-term improvements in patient care by revealing areas for improvement in the provision of care,” Veterans Health Administration (VHA) Directive 1190 states. “This ultimately contributes to organizational improvements.” At the same time, there are anecdotal concerns that PR may be used punitively and driven by case outcomes rather than by accepted best practices supporting QI.
Studies of the PR process suggest these concerns are valid. A key tenet of QI is standardization. PR is problematic in that regard; studies show poor interrater reliability for judgments on care, as well as hindsight bias—the fact that raters are strongly influenced by the outcome of care, not the process of care.2-5 There are concerns that case selection or review process when not standardized may be wielded as punitive too.6 In this study, we sought to identify the relationship between PR findings and subsequent institution quality metrics. If PR does lead to an improvement in quality, or if quality concerns are managed within the PR committee, it should be possible to identify a measurable relationship between the PR process and a facility’s subsequent quality measures.
A handful of studies describe the association between PR and quality of care. Itri and colleagues noted that random, not standardized PR in radiology does not achieve reductions in diagnostic error rate.7 However, adoption of just culture principles in PR resulted in a significant improvement in facility leaders’ self-reports of quality measures at surveyed institutions.8 The same author reported that increases in PR standardization and integration with performance improvement activities could explain up to 18% of objective quality measure variation.9
We sought to determine whether a specific aspect of the PR process, the PR committee judgment of quality of care by clinicians, was related to medical center quality in a cross-sectional study of 136 Veterans Health Administration (VHA) medical centers. The VHA is a good source of study because there are standardized PR processes and training for committee members and reviewers. Our hypothesis was that medical centers with a higher number of Level 2 (“most experienced and competent clinicians might have managed the case differently”) and Level 3 (“most experienced and competent providers would have managed the case differently”) PR findings would also have lower quality metric scores for processes and outcomes of care.
Methods
We used PR data from fiscal year 2018 and 2019. VHA PR data are available quarterly and are self-reported by each facility to the VHA Office of Clinical Risk Management. These data are broken down by facility. The following data, when available in both fiscal years 2018 and 2019, were used for this analysis: percent and number of PR that are ranked as level 1, 2, or 3; medical center group (MCG) acuity measure assigned by the VHA (1 is highest, 3 is lowest); and number of PR per 100,000 unique veteran encounters in 2019. Measures of facility quality are drawn from Strategic Analytics for Improvement and Learning (SAIL) data from 2019, which are available quarterly by facility and are rolling for 12 months. SAIL measures processes and outcomes of care. Table 1 indicates which measures are focused on outcomes vs quality processes.
SAS Version 9.2 was used to perform statistical analyses. We used Spearman correlation to estimate the PR and quality relationship.
Results
There were 136 facilities with 2 years of PR data available. The majority of these facilities (89) were highest complexity MCG 1 facilities; 19 were MCG 2, and 28 were MCG 3. Of 13,515 PRs, most of the 9555 PR findings were level 1 (70.7%). The between-facility range of level 2 and 3 findings was large, varying from 3.5% to nearly 70% in 2019 (Table 2). Findings were similar in 2018; facilities level 2 and 3 ratings ranged from 3.6% to 73.5% of all PR findings.
There was no correlation between most quality measures and facility PR findings (Table 3). The only exception was for Global Measures (GM90), an inpatient process of care measure. Unexpectedly, the correlation was positive—facilities with a higher percentage of level 2 and 3 PR findings had better inpatient processes of care SAIL score. The strongest correlation was between 2018 and 2019 PR findings.
Discussion
We hypothesized that a high percentage of level 2 and 3 PR findings would be negatively associated with objective facility measures of care processes in SAIL but we did not see this association. The only quality measure associated with PR findings was GM90, a score of inpatient care processes. However, the association was positive, with better performance associated with more level 2 and 3 PR findings.
The best predictor of the proportion of a facility’s PR findings is the previous year’s PR findings. With an R = 0.59, the previous year findings explain about 35% of the variability in level assignment. Our analysis may describe a new bias in PR, in which committees consistently assign either low or high proportions of level 2 and 3 findings. This correlation could be due to individual PR committee culture or composition, but it does not relate to objective quality measures.
Strengths
For this study we use objective measures of PR processes, the assignment of levels of care.
Limitations
Facilities self-report PR outcomes, so there could be errors in reporting. In addition, this study was cross sectional and not longitudinal and it is possible that change in quality measures over time are correlated with PR findings. Future studies using the VHA PR and SAIL data could evaluate whether changes over time, and perhaps in response to level 2 and 3 findings, would be a more sensitive indicator of the impact of the PR process on quality metrics. Future studies could incorporate the relationship between findings from the All Employee Survey, which is conducted annually, such as psychologic safety, as well as the distance the facility has gone on the high reliability organization journey, with PR findings and SAIL metrics. Finally, PR is focused on the practice of an individual clinician, while SAIL quality metrics reflect facility performance. Interventions possibly stay at the clinician level and do not drive subsequent QI processes.
What does this mean for PR? Since the early 1990s, there have been exhortations from experts to improve PR, by adopting a QI model, or for a deeper integration of PR and QI.1,2,10 Just culture tools, which include QI, are promoted as a means to improve PR.8,11,12 Other studies show PR remains problematic in terms of standardization, incorporation of best practices, redesigning systems of care, or demonstrable improvements to facility safety and care quality.1,4,6,8 Several publications have described interventions to improve PR. Deyo-Svedson discussed a program with standardized training and triggers, much like VHA.13 Itri and colleagues standardized PR in radiology to target areas of known diagnostic error, as well as use the issues assessed in PR to perform QI and education. One example of a successful QI effort involved changing the radiology reporting template to make sure areas that are prone to diagnostic error are addressed.7
Conclusions
Since 35% of PR level variance is correlated with prior year’s results, PR committees should look at increased standardization in reviews and findings. We endorse a strong focus on standardization, application of just culture tools to case reviews, and tighter linkage between process and outcome metrics measured by SAIL and PR case finding. Studies should be performed to pilot interventions to improve the linkage between PR and quality, so that greater and faster gains can be made in quality processes and, leading from this, outcomes. Additionally, future research should investigate why some facilities consistently choose higher or lower PR ratings.
Acknowledgments
We acknowledge Dr. George “Web” Ross for his helpful edits.
1. Edwards MT. In pursuit of quality and safety: an 8-year study of clinical peer review best practices in US hospitals. Int J Qual Health Care. 2018;30(8):602-607. doi:10.1093/intqhc/mzy069
2. Dans PE. Clinical peer Review: burnishing a tarnished icon. Ann Intern Med. 1993;118(7):566-568. doi:10.7326/0003-4819-118-7-199304010-00014
3. Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958-960. doi:10.1001/jama.1992.03480070074034
4. Swaroop R. Disrupting physician clinical practice peer review. Perm J. 2019;23:18-207. doi:10.7812/TPP/18-207
5. Caplan RA, Posner KL, Cheney FW. Effect of outcome on physician judgments of appropriateness of care. JAMA. 1991;265(15):1957–1960. doi:10.1001/jama.1991.03460150061024
6. Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20(21):6357-6363. doi:10.3748/wjg.v20.i21.6357
7. Itri JN, Donithan A, Patel SH. Random versus nonrandom peer review: a case for more meaningful peer review. J Am Coll Radiol. 2018;15(7):1045-1052. doi:10.1016/j.jacr.2018.03.054
8. Edwards MT. An assessment of the impact of just culture on quality and safety in US hospitals. Am J Med Qual. 2018; 33(5):502-508. doi:10.1177/1062860618768057
9. Edwards MT. The objective impact of clinical peer review on hospital quality and safety. Am J Med Qual. 2011;26(2);110-119. doi:10.1177/1062860610380732
10. Berwick DM. Peer review and quality management: are they compatible?. QRB Qual Rev Bull. 1990;16(7):246-251. doi:10.1016/s0097-5990(16)30377-3
11. Volkar JK, Phrampus P, English D, et al. Institution of just culture physician peer review in an academic medical center. J Patient Saf. 2021;17(7):e689-e693. doi:10.1097/PTS.0000000000000449
12. Burns J, Miller T, Weiss JM, Erdfarb A, Silber D, Goldberg-Stein S. Just culture: practical implementation for radiologist peer review. J Am Coll Radiol. 2019;16(3):384-388. doi:10.1016/j.jacr.2018.10.021
13. Deyo-Svendsen ME, Phillips MR, Albright JK, et al. A systematic approach to clinical peer review in a critical access hospital. Qual Manag Health Care. 2016;25(4):213-218. doi:10.1097/QMH.0000000000000113
Hospital leaders report the most common aim of peer review (PR) is to improve quality and patient safety, thus it is a potentially powerful quality improvement (QI) driver.1 “When conducted systematically and credibly, peer review for quality management can result in both short-term and long-term improvements in patient care by revealing areas for improvement in the provision of care,” Veterans Health Administration (VHA) Directive 1190 states. “This ultimately contributes to organizational improvements.” At the same time, there are anecdotal concerns that PR may be used punitively and driven by case outcomes rather than by accepted best practices supporting QI.
Studies of the PR process suggest these concerns are valid. A key tenet of QI is standardization. PR is problematic in that regard; studies show poor interrater reliability for judgments on care, as well as hindsight bias—the fact that raters are strongly influenced by the outcome of care, not the process of care.2-5 There are concerns that case selection or review process when not standardized may be wielded as punitive too.6 In this study, we sought to identify the relationship between PR findings and subsequent institution quality metrics. If PR does lead to an improvement in quality, or if quality concerns are managed within the PR committee, it should be possible to identify a measurable relationship between the PR process and a facility’s subsequent quality measures.
A handful of studies describe the association between PR and quality of care. Itri and colleagues noted that random, not standardized PR in radiology does not achieve reductions in diagnostic error rate.7 However, adoption of just culture principles in PR resulted in a significant improvement in facility leaders’ self-reports of quality measures at surveyed institutions.8 The same author reported that increases in PR standardization and integration with performance improvement activities could explain up to 18% of objective quality measure variation.9
We sought to determine whether a specific aspect of the PR process, the PR committee judgment of quality of care by clinicians, was related to medical center quality in a cross-sectional study of 136 Veterans Health Administration (VHA) medical centers. The VHA is a good source of study because there are standardized PR processes and training for committee members and reviewers. Our hypothesis was that medical centers with a higher number of Level 2 (“most experienced and competent clinicians might have managed the case differently”) and Level 3 (“most experienced and competent providers would have managed the case differently”) PR findings would also have lower quality metric scores for processes and outcomes of care.
Methods
We used PR data from fiscal year 2018 and 2019. VHA PR data are available quarterly and are self-reported by each facility to the VHA Office of Clinical Risk Management. These data are broken down by facility. The following data, when available in both fiscal years 2018 and 2019, were used for this analysis: percent and number of PR that are ranked as level 1, 2, or 3; medical center group (MCG) acuity measure assigned by the VHA (1 is highest, 3 is lowest); and number of PR per 100,000 unique veteran encounters in 2019. Measures of facility quality are drawn from Strategic Analytics for Improvement and Learning (SAIL) data from 2019, which are available quarterly by facility and are rolling for 12 months. SAIL measures processes and outcomes of care. Table 1 indicates which measures are focused on outcomes vs quality processes.
SAS Version 9.2 was used to perform statistical analyses. We used Spearman correlation to estimate the PR and quality relationship.
Results
There were 136 facilities with 2 years of PR data available. The majority of these facilities (89) were highest complexity MCG 1 facilities; 19 were MCG 2, and 28 were MCG 3. Of 13,515 PRs, most of the 9555 PR findings were level 1 (70.7%). The between-facility range of level 2 and 3 findings was large, varying from 3.5% to nearly 70% in 2019 (Table 2). Findings were similar in 2018; facilities level 2 and 3 ratings ranged from 3.6% to 73.5% of all PR findings.
There was no correlation between most quality measures and facility PR findings (Table 3). The only exception was for Global Measures (GM90), an inpatient process of care measure. Unexpectedly, the correlation was positive—facilities with a higher percentage of level 2 and 3 PR findings had better inpatient processes of care SAIL score. The strongest correlation was between 2018 and 2019 PR findings.
Discussion
We hypothesized that a high percentage of level 2 and 3 PR findings would be negatively associated with objective facility measures of care processes in SAIL but we did not see this association. The only quality measure associated with PR findings was GM90, a score of inpatient care processes. However, the association was positive, with better performance associated with more level 2 and 3 PR findings.
The best predictor of the proportion of a facility’s PR findings is the previous year’s PR findings. With an R = 0.59, the previous year findings explain about 35% of the variability in level assignment. Our analysis may describe a new bias in PR, in which committees consistently assign either low or high proportions of level 2 and 3 findings. This correlation could be due to individual PR committee culture or composition, but it does not relate to objective quality measures.
Strengths
For this study we use objective measures of PR processes, the assignment of levels of care.
Limitations
Facilities self-report PR outcomes, so there could be errors in reporting. In addition, this study was cross sectional and not longitudinal and it is possible that change in quality measures over time are correlated with PR findings. Future studies using the VHA PR and SAIL data could evaluate whether changes over time, and perhaps in response to level 2 and 3 findings, would be a more sensitive indicator of the impact of the PR process on quality metrics. Future studies could incorporate the relationship between findings from the All Employee Survey, which is conducted annually, such as psychologic safety, as well as the distance the facility has gone on the high reliability organization journey, with PR findings and SAIL metrics. Finally, PR is focused on the practice of an individual clinician, while SAIL quality metrics reflect facility performance. Interventions possibly stay at the clinician level and do not drive subsequent QI processes.
What does this mean for PR? Since the early 1990s, there have been exhortations from experts to improve PR, by adopting a QI model, or for a deeper integration of PR and QI.1,2,10 Just culture tools, which include QI, are promoted as a means to improve PR.8,11,12 Other studies show PR remains problematic in terms of standardization, incorporation of best practices, redesigning systems of care, or demonstrable improvements to facility safety and care quality.1,4,6,8 Several publications have described interventions to improve PR. Deyo-Svedson discussed a program with standardized training and triggers, much like VHA.13 Itri and colleagues standardized PR in radiology to target areas of known diagnostic error, as well as use the issues assessed in PR to perform QI and education. One example of a successful QI effort involved changing the radiology reporting template to make sure areas that are prone to diagnostic error are addressed.7
Conclusions
Since 35% of PR level variance is correlated with prior year’s results, PR committees should look at increased standardization in reviews and findings. We endorse a strong focus on standardization, application of just culture tools to case reviews, and tighter linkage between process and outcome metrics measured by SAIL and PR case finding. Studies should be performed to pilot interventions to improve the linkage between PR and quality, so that greater and faster gains can be made in quality processes and, leading from this, outcomes. Additionally, future research should investigate why some facilities consistently choose higher or lower PR ratings.
Acknowledgments
We acknowledge Dr. George “Web” Ross for his helpful edits.
Hospital leaders report the most common aim of peer review (PR) is to improve quality and patient safety, thus it is a potentially powerful quality improvement (QI) driver.1 “When conducted systematically and credibly, peer review for quality management can result in both short-term and long-term improvements in patient care by revealing areas for improvement in the provision of care,” Veterans Health Administration (VHA) Directive 1190 states. “This ultimately contributes to organizational improvements.” At the same time, there are anecdotal concerns that PR may be used punitively and driven by case outcomes rather than by accepted best practices supporting QI.
Studies of the PR process suggest these concerns are valid. A key tenet of QI is standardization. PR is problematic in that regard; studies show poor interrater reliability for judgments on care, as well as hindsight bias—the fact that raters are strongly influenced by the outcome of care, not the process of care.2-5 There are concerns that case selection or review process when not standardized may be wielded as punitive too.6 In this study, we sought to identify the relationship between PR findings and subsequent institution quality metrics. If PR does lead to an improvement in quality, or if quality concerns are managed within the PR committee, it should be possible to identify a measurable relationship between the PR process and a facility’s subsequent quality measures.
A handful of studies describe the association between PR and quality of care. Itri and colleagues noted that random, not standardized PR in radiology does not achieve reductions in diagnostic error rate.7 However, adoption of just culture principles in PR resulted in a significant improvement in facility leaders’ self-reports of quality measures at surveyed institutions.8 The same author reported that increases in PR standardization and integration with performance improvement activities could explain up to 18% of objective quality measure variation.9
We sought to determine whether a specific aspect of the PR process, the PR committee judgment of quality of care by clinicians, was related to medical center quality in a cross-sectional study of 136 Veterans Health Administration (VHA) medical centers. The VHA is a good source of study because there are standardized PR processes and training for committee members and reviewers. Our hypothesis was that medical centers with a higher number of Level 2 (“most experienced and competent clinicians might have managed the case differently”) and Level 3 (“most experienced and competent providers would have managed the case differently”) PR findings would also have lower quality metric scores for processes and outcomes of care.
Methods
We used PR data from fiscal year 2018 and 2019. VHA PR data are available quarterly and are self-reported by each facility to the VHA Office of Clinical Risk Management. These data are broken down by facility. The following data, when available in both fiscal years 2018 and 2019, were used for this analysis: percent and number of PR that are ranked as level 1, 2, or 3; medical center group (MCG) acuity measure assigned by the VHA (1 is highest, 3 is lowest); and number of PR per 100,000 unique veteran encounters in 2019. Measures of facility quality are drawn from Strategic Analytics for Improvement and Learning (SAIL) data from 2019, which are available quarterly by facility and are rolling for 12 months. SAIL measures processes and outcomes of care. Table 1 indicates which measures are focused on outcomes vs quality processes.
SAS Version 9.2 was used to perform statistical analyses. We used Spearman correlation to estimate the PR and quality relationship.
Results
There were 136 facilities with 2 years of PR data available. The majority of these facilities (89) were highest complexity MCG 1 facilities; 19 were MCG 2, and 28 were MCG 3. Of 13,515 PRs, most of the 9555 PR findings were level 1 (70.7%). The between-facility range of level 2 and 3 findings was large, varying from 3.5% to nearly 70% in 2019 (Table 2). Findings were similar in 2018; facilities level 2 and 3 ratings ranged from 3.6% to 73.5% of all PR findings.
There was no correlation between most quality measures and facility PR findings (Table 3). The only exception was for Global Measures (GM90), an inpatient process of care measure. Unexpectedly, the correlation was positive—facilities with a higher percentage of level 2 and 3 PR findings had better inpatient processes of care SAIL score. The strongest correlation was between 2018 and 2019 PR findings.
Discussion
We hypothesized that a high percentage of level 2 and 3 PR findings would be negatively associated with objective facility measures of care processes in SAIL but we did not see this association. The only quality measure associated with PR findings was GM90, a score of inpatient care processes. However, the association was positive, with better performance associated with more level 2 and 3 PR findings.
The best predictor of the proportion of a facility’s PR findings is the previous year’s PR findings. With an R = 0.59, the previous year findings explain about 35% of the variability in level assignment. Our analysis may describe a new bias in PR, in which committees consistently assign either low or high proportions of level 2 and 3 findings. This correlation could be due to individual PR committee culture or composition, but it does not relate to objective quality measures.
Strengths
For this study we use objective measures of PR processes, the assignment of levels of care.
Limitations
Facilities self-report PR outcomes, so there could be errors in reporting. In addition, this study was cross sectional and not longitudinal and it is possible that change in quality measures over time are correlated with PR findings. Future studies using the VHA PR and SAIL data could evaluate whether changes over time, and perhaps in response to level 2 and 3 findings, would be a more sensitive indicator of the impact of the PR process on quality metrics. Future studies could incorporate the relationship between findings from the All Employee Survey, which is conducted annually, such as psychologic safety, as well as the distance the facility has gone on the high reliability organization journey, with PR findings and SAIL metrics. Finally, PR is focused on the practice of an individual clinician, while SAIL quality metrics reflect facility performance. Interventions possibly stay at the clinician level and do not drive subsequent QI processes.
What does this mean for PR? Since the early 1990s, there have been exhortations from experts to improve PR, by adopting a QI model, or for a deeper integration of PR and QI.1,2,10 Just culture tools, which include QI, are promoted as a means to improve PR.8,11,12 Other studies show PR remains problematic in terms of standardization, incorporation of best practices, redesigning systems of care, or demonstrable improvements to facility safety and care quality.1,4,6,8 Several publications have described interventions to improve PR. Deyo-Svedson discussed a program with standardized training and triggers, much like VHA.13 Itri and colleagues standardized PR in radiology to target areas of known diagnostic error, as well as use the issues assessed in PR to perform QI and education. One example of a successful QI effort involved changing the radiology reporting template to make sure areas that are prone to diagnostic error are addressed.7
Conclusions
Since 35% of PR level variance is correlated with prior year’s results, PR committees should look at increased standardization in reviews and findings. We endorse a strong focus on standardization, application of just culture tools to case reviews, and tighter linkage between process and outcome metrics measured by SAIL and PR case finding. Studies should be performed to pilot interventions to improve the linkage between PR and quality, so that greater and faster gains can be made in quality processes and, leading from this, outcomes. Additionally, future research should investigate why some facilities consistently choose higher or lower PR ratings.
Acknowledgments
We acknowledge Dr. George “Web” Ross for his helpful edits.
1. Edwards MT. In pursuit of quality and safety: an 8-year study of clinical peer review best practices in US hospitals. Int J Qual Health Care. 2018;30(8):602-607. doi:10.1093/intqhc/mzy069
2. Dans PE. Clinical peer Review: burnishing a tarnished icon. Ann Intern Med. 1993;118(7):566-568. doi:10.7326/0003-4819-118-7-199304010-00014
3. Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958-960. doi:10.1001/jama.1992.03480070074034
4. Swaroop R. Disrupting physician clinical practice peer review. Perm J. 2019;23:18-207. doi:10.7812/TPP/18-207
5. Caplan RA, Posner KL, Cheney FW. Effect of outcome on physician judgments of appropriateness of care. JAMA. 1991;265(15):1957–1960. doi:10.1001/jama.1991.03460150061024
6. Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20(21):6357-6363. doi:10.3748/wjg.v20.i21.6357
7. Itri JN, Donithan A, Patel SH. Random versus nonrandom peer review: a case for more meaningful peer review. J Am Coll Radiol. 2018;15(7):1045-1052. doi:10.1016/j.jacr.2018.03.054
8. Edwards MT. An assessment of the impact of just culture on quality and safety in US hospitals. Am J Med Qual. 2018; 33(5):502-508. doi:10.1177/1062860618768057
9. Edwards MT. The objective impact of clinical peer review on hospital quality and safety. Am J Med Qual. 2011;26(2);110-119. doi:10.1177/1062860610380732
10. Berwick DM. Peer review and quality management: are they compatible?. QRB Qual Rev Bull. 1990;16(7):246-251. doi:10.1016/s0097-5990(16)30377-3
11. Volkar JK, Phrampus P, English D, et al. Institution of just culture physician peer review in an academic medical center. J Patient Saf. 2021;17(7):e689-e693. doi:10.1097/PTS.0000000000000449
12. Burns J, Miller T, Weiss JM, Erdfarb A, Silber D, Goldberg-Stein S. Just culture: practical implementation for radiologist peer review. J Am Coll Radiol. 2019;16(3):384-388. doi:10.1016/j.jacr.2018.10.021
13. Deyo-Svendsen ME, Phillips MR, Albright JK, et al. A systematic approach to clinical peer review in a critical access hospital. Qual Manag Health Care. 2016;25(4):213-218. doi:10.1097/QMH.0000000000000113
1. Edwards MT. In pursuit of quality and safety: an 8-year study of clinical peer review best practices in US hospitals. Int J Qual Health Care. 2018;30(8):602-607. doi:10.1093/intqhc/mzy069
2. Dans PE. Clinical peer Review: burnishing a tarnished icon. Ann Intern Med. 1993;118(7):566-568. doi:10.7326/0003-4819-118-7-199304010-00014
3. Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958-960. doi:10.1001/jama.1992.03480070074034
4. Swaroop R. Disrupting physician clinical practice peer review. Perm J. 2019;23:18-207. doi:10.7812/TPP/18-207
5. Caplan RA, Posner KL, Cheney FW. Effect of outcome on physician judgments of appropriateness of care. JAMA. 1991;265(15):1957–1960. doi:10.1001/jama.1991.03460150061024
6. Vyas D, Hozain AE. Clinical peer review in the United States: history, legal development and subsequent abuse. World J Gastroenterol. 2014;20(21):6357-6363. doi:10.3748/wjg.v20.i21.6357
7. Itri JN, Donithan A, Patel SH. Random versus nonrandom peer review: a case for more meaningful peer review. J Am Coll Radiol. 2018;15(7):1045-1052. doi:10.1016/j.jacr.2018.03.054
8. Edwards MT. An assessment of the impact of just culture on quality and safety in US hospitals. Am J Med Qual. 2018; 33(5):502-508. doi:10.1177/1062860618768057
9. Edwards MT. The objective impact of clinical peer review on hospital quality and safety. Am J Med Qual. 2011;26(2);110-119. doi:10.1177/1062860610380732
10. Berwick DM. Peer review and quality management: are they compatible?. QRB Qual Rev Bull. 1990;16(7):246-251. doi:10.1016/s0097-5990(16)30377-3
11. Volkar JK, Phrampus P, English D, et al. Institution of just culture physician peer review in an academic medical center. J Patient Saf. 2021;17(7):e689-e693. doi:10.1097/PTS.0000000000000449
12. Burns J, Miller T, Weiss JM, Erdfarb A, Silber D, Goldberg-Stein S. Just culture: practical implementation for radiologist peer review. J Am Coll Radiol. 2019;16(3):384-388. doi:10.1016/j.jacr.2018.10.021
13. Deyo-Svendsen ME, Phillips MR, Albright JK, et al. A systematic approach to clinical peer review in a critical access hospital. Qual Manag Health Care. 2016;25(4):213-218. doi:10.1097/QMH.0000000000000113
BRAF V600E Expression in Primary Melanoma and Its Association With Death: A Population-Based, Retrospective, Cross-Sectional Study
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.
Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).
BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.
Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).
BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
Approximately 50% of melanomas contain BRAF mutations, which occur in a greater proportion of melanomas found on sites of intermittent sun exposure.1BRAF-mutated melanomas have been associated with high levels of early-life ambient UV exposure, especially between ages 0 and 20 years.2 In addition, studies have shown that BRAF-mutated melanomas commonly are found on the trunk and extremities.1-3BRAF mutations also have been associated with younger age, superficial spreading subtype and low tumor thickness, absence of dermal melanocyte mitosis, low Ki-67 score, low phospho-histone H3 score, pigmented melanoma, advanced melanoma stage, and conjunctival melanoma.4-7BRAF mutations are found more frequently in metastatic melanoma lesions than primary melanomas, suggesting that BRAF mutations may be acquired during metastasis.8 Studies have shown different conclusions on the effect of BRAF mutation on melanoma-related death.5,9,10
The aim of this study was to identify trends in BRAF V600E–mutated melanoma according to age, sex, and melanoma-specific survival among Olmsted County, Minnesota, residents with a first diagnosis of melanoma at 18 to 60 years of age.
Methods
In total, 638 patients aged 18 to 60 years who resided in Olmsted County and had a first lifetime diagnosis of cutaneous melanoma between 1970 and 2009 were retrospectively identified as a part of the Rochester Epidemiology Project (REP). The REP is a health records linkage system that encompasses almost all sources of medical care available to the local population of Olmsted County.11 This study was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota).
Of the 638 individuals identified in the REP, 536 had been seen at Mayo Clinic and thus potentially had tissue blocks available for the study of BRAF mutation expression. Of these 536 patients, 156 did not have sufficient residual tissue available. As a result, 380 (60%) of the original 638 patients had available blocks with sufficient tissue for immunohistochemical analysis of BRAF expression. Only primary cutaneous melanomas were included in the present study.
All specimens were reviewed by a board-certified dermatopathologist (J.S.L.) for appropriateness of inclusion, which involved confirmation of the diagnosis of melanoma, histologic type of melanoma, and presence of sufficient residual tissue for immunohistochemical stains.
All specimens were originally diagnosed as malignant melanoma at the time of clinical care by at least 2 board-certified dermatopathologists. For the purposes of this study, all specimens were rereviewed for diagnostic accuracy. We required that specimens exhibit severe cytologic and architectural atypia as well as other features favoring melanoma, such as consumption of rete pegs, pagetosis, confluence of junctional melanocytes, evidence of regression, lack of maturation of melanocytes with descent into the dermis, or mitotic figures among the dermal melanocyte population.
The available tissue blocks were retrieved, sectioned, confirmed as melanoma, and stained with a mouse antihuman BRAF V600E monoclonal antibody (clone VE1; Spring Bioscience) to determine the presence of a BRAF V600E mutation. BRAF staining was evaluated in conjunction with a review of the associated slides stained with hematoxylin and eosin. Cytoplasmic staining of melanocytes for BRAF was graded as negative, focal or partial positive (<50% of tumor), or diffuse positive (>50% of tumor)(Figure 1). When a melanoma arose in association with a nevus, we considered only the melanoma component for BRAF staining. We categorized the histologic type as superficial spreading, nodular, or lentigo maligna, and the location as head and neck, trunk, or extremities.
Patient characteristics and survival outcomes were gathered through the health record and included age, Breslow thickness, location, decade of diagnosis, histologic type, stage (ie, noninvasive, invasive, or advanced), and follow-up. Pathologic stage 0 was considered noninvasive; stages IA and IB, invasive; and stages IIA or higher, advanced.
Statistical Analysis—Comparisons between the group of patients in the study (n=380) and the group of patients excluded for the reasons stated above (n=258) as well as associations of mutant BRAF status (positive [partial positive and diffuse positive] vs negative) with patient age (young adults [age range, 18–39 years] and middle-aged adults [age range, 40–60 years]), sex, decade of diagnosis, location, histologic type, and stage were evaluated with Wilcoxon rank sum, χ2, Fisher exact, or Cochran-Armitage trend tests. Disease-specific survival and overall survival rates were estimated with the Kaplan-Meier method, and the duration of follow-up was calculated from the date of melanoma diagnosis to the date of death or the last follow-up. Associations of mutant BRAF expression status with death from melanoma and death from any cause were evaluated with Cox proportional hazard regression models and summarized with hazard ratio (HR) and 95% CI. Survival analyses were limited to patients with invasive or advanced disease. Statistical analyses were performed with SAS statistical software (SAS version 9.4). All tests were 2-sided, and P<.05 was considered statistically significant.
Results
Clinical and Tumor Characteristics—Of the 380 tissue specimens that underwent BRAF V600E analysis, 247 had negative staining; 106 had diffuse strong staining; and 27 had focal or partial staining. In total, 133 (35%) were positive, either partially or diffusely. The median age for patients who had negative staining was 45 years; for those with positive staining, it was 41 years (P=.07).
The patients who met inclusion criteria (n=380) were compared with those who were excluded (n=258)(eTable 1). The groups were similar on the basis of sex; age; and melanoma location, stage, and histologic subtype. However, some evidence showed that patients included in the study received the diagnosis of melanoma more recently (1970-1989, 13.2%; 1990-1999, 28.7%; 2000-2009, 58.2%) than those who were excluded (1970-1989, 24.7%; 1990-1999, 23.5%; 2000-2009, 51.8%)(P=.02).
BRAF V600E expression was more commonly found in superficial spreading (37.7%) and nodular melanomas (35.0%) than in situ melanomas (17.1%)(P=.01). Other characteristics of BRAF V600E expression are described in eTable 2. Overall, invasive and advanced melanomas were significantly more likely to harbor BRAF V600E expression than noninvasive melanomas (39.6% and 37.9%, respectively, vs 17.9%; P=.003). However, advanced melanomas more commonly expressed BRAF positivity among women, and invasive melanomas more commonly expressed BRAF positivity among men (eTable 2).
Survival—Survival analyses were limited to 297 patients with confirmed invasive or advanced disease. Of these, 180 (61%) had no BRAF V600E staining; 25 (8%) had partial staining; and 92 (31%) had diffuse positive staining. In total, 117 patients (39%) had a BRAF-mutated melanoma.
Among the patients still alive, the median (interquartile range [IQR]) duration of follow-up was 10.2 (7.0-16.8) years. Thirty-nine patients with invasive or advanced disease had died of any cause at a median (IQR) of 3.0 (1.3-10.2) years after diagnosis. In total, 26 patients died of melanoma at a median (IQR) follow-up of 2.5 (1.3-7.4) years after diagnosis. Eight women and 18 men died of malignant melanoma. Five deaths occurred because of malignant melanoma among patients aged 18 to 39 years, and 21 occurred among patients aged 40 to 60 years. In the 18- to 39-year-old group, all 5 deaths were among patients with a BRAF-positive melanoma. Estimated disease-specific survival rate (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis was 94% (91%-97%; 243), 91% (87%-95%; 142), 89% (85%-94%; 87), and 88% (83%-93%; 45), respectively.
In a univariable analysis, the HR for association of positive mutant BRAF expression with death of malignant melanoma was 1.84 (95% CI, 0.85-3.98; P=.12). No statistically significant interaction was observed between decade of diagnosis and BRAF expression (P=.60). However, the interaction between sex and BRAF expression was significant (P=.04), with increased risk of death from melanoma among women with BRAF-mutated melanoma (HR, 10.88; 95% CI, 1.34-88.41; P=.026) but not among men (HR 1.02; 95% CI, 0.40-2.64; P=.97)(Figures 2A and 2B). The HR for death from malignant melanoma among young adults aged 18 to 39 years with a BRAF-mutated melanoma was 16.4 (95% CI, 0.81-330.10; P=.068), whereas the HR among adults aged 40 to 60 years with a BRAF-mutated melanoma was 1.24 (95% CI, 0.52-2.98; P=.63)(Figures 2C and 2D).
BRAF V600E expression was not significantly associated with death from any cause (HR, 1.39; 95% CI, 0.74-2.61; P=.31) or with decade of diagnosis (P=.13). Similarly, BRAF expression was not associated with death from any cause according to sex (P=.31). However, a statistically significant interaction was seen between age at diagnosis and BRAF expression (P=.003). BRAF expression was significantly associated with death from any cause for adults aged 18 to 39 years (HR, 9.60; 95% CI, 1.15-80.00; P=.04). In comparison, no association of BRAF expression with death was observed for adults aged 40 to 60 years (HR, 0.99; 95% CI, 0.48-2.03; P=.98).
Comment
We found that melanomas with BRAF mutations were more likely in advanced and invasive melanoma. The frequency of BRAF mutations among melanomas that were considered advanced was higher in women than men. Although the number of deaths was limited, women with a melanoma with BRAF expression were more likely to die of melanoma, young adults with a BRAF-mutated melanoma had an almost 10-fold increased risk of dying from any cause, and middle-aged adults showed no increased risk of death. These findings suggest that young adults who are genetically prone to a BRAF-mutated melanoma could be at a disadvantage for all-cause mortality. Although this finding was significant, the 95% CI was large, and further studies would be warranted before sound conclusions could be made.
Melanoma has been increasing in incidence across all age groups in Olmsted County over the last 4 decades.12-14 However, our results show that the percentage of BRAF-mutated melanomas in this population has been stable over time, with no statistically significant difference by age or sex. Other confounding factors may have an influence, such as increased rates of early detection and diagnosis of melanoma in contemporary times. Our data suggest that patients included in the BRAF-mutation analysis study had received the diagnosis of melanoma more recently than those who were excluded from the study, which could be due to older melanomas being less likely to have adequate tissue specimens available for immunohistochemical staining/evaluation.
Prior research has shown that BRAF-mutated melanomas typically occur on the trunk and are more likely in individuals with more than 14 nevi on the back.2 In the present cohort, BRAF-positive melanomas had a predisposition toward the trunk but also were found on the head, neck, and extremities—areas that are more likely to have long-term sun damage. One suggestion is that 2 distinct pathways for melanoma development exist: one associated with a large number of melanocytic nevi (that is more prone to genetic mutations in melanocytes) and the other associated with long-term sun exposure.15,16 The combination of these hypotheses suggests that individuals who are prone to the development of large numbers of nevi may require sun exposure for the initial insult, but the development of melanoma may be carried out by other factors after this initial sun exposure insult, whereas individuals without large numbers of nevi who may have less genetic risk may require continued long-term sun exposure for melanoma to develop.17
Our study had limitations, including the small numbers of deaths overall and cause-specific deaths of metastatic melanoma, which limited our ability to conduct more extensive multivariable modeling. Also, the retrospective nature and time frame of looking back 4 decades did not allow us to have information sufficient to categorize some patients as having dysplastic nevus syndrome or not, which would be a potentially interesting variable to include in the analysis. Because the number of deaths in the 18- to 39-year-old cohort was only 5, further statistical comparison regarding tumor type and other variables pertaining to BRAF positivity were not possible. In addition, our data were collected from patients residing in a single geographic county (Olmsted County, Minnesota), which may limit generalizability. Lastly, BRAF V600E mutations were identified through immunostaining only, not molecular data, so it is possible some patients had false-negative immunohistochemistry findings and thus were not identified.
Conclusion
BRAF-mutated melanomas were found in 35% of our cohort, with no significant change in the percentage of melanomas with BRAF V600E mutations over the last 4 decades in this population. In addition, no differences or significant trends existed according to sex and BRAF-mutated melanoma development. Women with BRAF-mutated melanomas were more likely to die of metastatic melanoma than men, and young adults with BRAF-mutated melanomas had a higher all-cause mortality risk. Further research is needed to decipher what effect BRAF-mutated melanomas have on metastasis and cause-specific death in women as well as all-cause mortality in young adults.
Acknowledgment—The authors are indebted to Scientific Publications, Mayo Clinic (Rochester, Minnesota).
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
- Grimaldi AM, Cassidy PB, Leachmann S, et al. Novel approaches in melanoma prevention and therapy. Cancer Treat Res. 2014;159: 443-455.
- Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16:991-997.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-2147.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 2015;1:359-368.
- Liu W, Kelly JW, Trivett M, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900-905.
- Kim SY, Kim SN, Hahn HJ, et al. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J Am Acad Dermatol. 2015;72:1036-1046.e2.
- Larsen AC, Dahl C, Dahmcke CM, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016;94:463-470.
- Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res. 2004;10:1753-1757.
- Heppt MV, Siepmann T, Engel J, et al. Prognostic significance of BRAF and NRAS mutations in melanoma: a German study from routine care. BMC Cancer. 2017;17:536.
- Mar VJ, Liu W, Devitt B, et al. The role of BRAF mutations in primary melanoma growth rate and survival. Br J Dermatol. 2015;173:76-82.
- Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202-1213.
- Reed KB, Brewer JD, Lohse CM, et al. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87:328-334.
- Olazagasti Lourido JM, Ma JE, Lohse CM, et al. Increasing incidence of melanoma in the elderly: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2016;91:1555-1562.
- Lowe GC, Saavedra A, Reed KB, et al. Increasing incidence of melanoma among middle-aged adults: an epidemiologic study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:52-59.
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843-848.
- Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
- Olsen CM, Zens MS, Green AC, et al. Biologic markers of sun exposure and melanoma risk in women: pooled case-control analysis. Int J Cancer. 2011;129:713-723.
Practice Points
- Approximately 50% of melanomas contain BRAF mutations; the effects on survival are unclear.
- Women with BRAF-mutated melanoma are at increased risk for death from melanoma.
- BRAF expression is associated with death of any cause for adults aged 18 to 39 years.
Impact of the COVID-19 Pandemic on Characteristics of Cutaneous Tumors Treated by Mohs Micrographic Surgery
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
The COVID-19 pandemic has brought about unprecedented changes and challenges to medical practice, including new public health measure legislation, local and national medical authority recommendations, nursing home and other ancillary health center protocols, and novel clinical decision-making considerations.1-3 In July 2020, the American Academy of Dermatology (AAD) addressed the changing landscape in dermatologic surgery, in part, by publishing recommendations on practice protocols during the COVID-19 pandemic.4 The guidelines recommended deferred treatment of superficial basal cell carcinomas (BCCs) for 6 months and all other BCC subtypes for 3 to 6 months. Furthermore, the guidelines recommended deferring treatment of all actinic keratoses and squamous cell carcinomas (SCCs) in situ “for now.” Squamous cell carcinoma treatment was to be guided by prognostic variables, such as location, size, depth, differentiation, perineural or lymphovascular invasion, recurrence, and immunosuppression. The guidelines recommended melanoma in situ (MIS) treatment be deferred for 3 months and invasive melanoma with histologic clearance obtained on excisional biopsy for 3 months. Other general recommendations included triaging clinics, rebooking according to clinical priority, using telehealth where possible, screening patients for COVID-19 signs and symptoms, staggering appointment times, spacing patient chairs, limiting support persons to 1, removing possible sources of infection in the waiting room, ensuring all patients sanitized their hands on arrival, rationing personal protective equipment, considering N95 masks for periorificial surgery, and using dissolving sutures to minimize multiple presentations.4
The American College of Mohs Surgery (ACMS), with guidance from its sister societies and the National Comprehensive Cancer Network, also communicated COVID-19–related recommendations to its members via intermittent newsletters during the initial peak of the pandemic in March and June 2020.5 General social distancing and office recommendations were similar to those released by the AAD. Recommendations for skin cancer treatment included deferring all BCCs for up to 3 months, with exceptions for highly symptomatic cancers and those with potential for substantial rapid growth. Squamous cell carcinoma in situ and small, well-differentiated SCCs were deferred, with priority placed on SCCs that were rapidly enlarging, poorly differentiated, demonstrated perineural invasion, were ulcerated, or were symptomatic. Patients with major risk factors were prioritized for treatment. Melanoma in situ was deferred for 2 to 3 months.5
State-level guidance from the Texas Dermatological Society (TDS) communicated in April 2020 stated that skin cancers with a potential for rapid progression and metastasis, such as melanoma and SCC, may require treatment as determined by the physician.6 The potential risk of serious adverse medical outcomes from not treating these cancers should be carefully documented. General practice measures for preventing the spread of COVID-19 were also recommended.6
In the setting of emerging novel recommendations, the practice of Mohs micrographic surgery (MMS) was notably impacted by the COVID-19 pandemic. According to one survey study from the United Kingdom conducted in April and May 2020, 49% of MMS services ceased and 36% were reduced during the infancy of the COVID-19 pandemic.7 Mohs micrographic surgery was largely suspended because of a lack of personal protective equipment and safety concerns, according to respondents. Additionally, respondents reported 77% of departments experienced redeployment of physicians and nurses to intensive care and medical wards. Thirty-five percent reported a reduction in the proportion of flaps/grafts to primary closures performed, 74% reported a decrease in outside referrals for repair by other specialties, 81% reported increased usage of dissolvable sutures, and 29% reported an increase in prophylactic antibiotic prescriptions.7 Another study from Italy reported a 46.5% reduction in dermatologic surgeries performed during the initial lockdown of the COVID-19 pandemic. Patients canceled 52.9% of procedures, and 12.5% were cancelled because of confirmed or suspected COVID-19 infection.8 Patient perceptions of MMS have also been impacted by the COVID-19 pandemic. According to a survey study of patients in the United Kingdom undergoing MMS during the pandemic, 47% were worried the hospital would cancel their surgery, 54% were anxious about using public transportation to attend their appointment, 30% were concerned about transmitting COVID-19 to household or family members, and 19% were worried about their ability to socially distance in the hospital.9
Evidence is also emerging that suggests the potential negative impact of the COVID-19 pandemic on morbidity and mortality outcomes in patients with skin cancer. One European study found an increase in Breslow thickness in primary melanomas diagnosed following the initial COVID-19 lockdown (0.88-mm average thickness prelockdown vs 1.96-mm average thickness postlockdown).10 An Italian study observed similar results—an increase in median Breslow thickness during the initial COVID-19 lockdown period of 0.5 mm from 0.4 mm during the prelockdown time period.11 Also providing evidence for potentially poor patient outcomes, one study modeled the impact of backlog in cutaneous melanoma referrals in the United Kingdom on patient survival and predicted 138 attributable lives lost for a 1-month delay and 1171 lives lost for a 6-month delay. The model further predicted a 3.1% to 12.5% reduction in 10-year net survival incurred from a 3-month delay in melanoma treatment, with the largest reduction seen in the patient population older than 80 years.12
Although the COVID-19 pandemic has been observed to impact MMS practice, patient perceptions, and clinical outcomes, it is unknown how the COVID-19 pandemic and corresponding rapidly evolving recommendations in dermatologic surgery have impacted the characteristics of cutaneous tumors treated by MMS.
Our study sought to determine the characteristics of skin cancers treated by MMS during the peak of government-mandated medical practice restrictions and business shutdowns in response to the COVID-19 pandemic and to compare them with characteristics of skin cancers treated during a prepandemic control period.
Methods
A retrospective chart review was conducted with approval from our institutional review board at the University of Texas Medical Branch (Galveston, Texas). Included in the chart review were all cutaneous malignancies treated by MMS at our outpatient, office-based surgical center from March 15, 2020, to April 30, 2020; this period corresponded to the peak of the COVID-19–related government-mandated medical and business shutdowns in our geographic region (southeast Texas). All cases performed were in compliance with national- and state-level guidance. Data were also collected for all cutaneous malignancies treated by MMS at our office from March 15, 2019, to April 30, 2019, as well as March 15, 2018, to April 30, 2018; these periods represented prepandemic control periods.
Data were collected for 516 surgeries performed on 458 patients and included patient age, preoperative clinical size, postoperative defect size, number of Mohs stages to achieve clearance, MMS appropriate use criteria (AUC) location (categorized as high-, medium-, or low-risk tumor location),13 and tumor type (categorized as BCC, SCC, or MIS). All variables were examined for unusual or missing values. Five patients with rare tumor types were observed and removed from the data set.
Statistical Analysis—An a priori power analysis for a power set at 0.85 determined sample sizes of 105 per group. Bivariate analyses were performed to compare variables for patients undergoing MMS during the pandemic vs prepandemic periods. Continuous outcome variables—Mohs stages, preoperative size, postoperative size, and patient age—were categorized for the analysis. Preoperative tumor size was dichotomized, with less than 2 cm2 as the referent category vs 2 cm2 or greater, and postoperative defect size was dichotomized with less than 3.6 cm2 as the referent category vs 3.6 cm2 or greater. Mohs stage was dichotomized as 1 stage (referent) vs more than 1 stage, and patient age was dichotomized as younger than 65 years (referent) vs 65 years or older.
Multivariate analyses were also performed to compare preoperative and postoperative sizes for patients undergoing MMS during the pandemic vs prepandemic periods, controlling for Mohs AUC location. Bivariate unadjusted and multivariate analyses were performed using a GENMOD logistic regression procedure in SAS (SAS Institute) to account for correlation in clustered data because a patient could be included for more than 1 surgery in the data set. Data were analyzed using SAS 9.4 for Windows. Because outcome variables tended to be skewed and not distributed normally, outcome variables were recorded as medians with interquartile ranges where possible to give a more accurate representation of the data than could be demonstrated with means with standard deviations.
Results
One hundred thirty-eight skin cancers were treated during the COVID-19 pandemic from March 15, 2020, to April 30, 2020, and 378 skin cancers were treated during the prepandemic control periods of March 15, 2019, to April 30, 2019, and March 15, 2018, to April 30, 2018. Tumor type treated during the pandemic period was more likely to be SCC or MIS (representing generally more severe tumor types) vs BCC when compared with the prepandemic periods, with an odds ratio (OR) of 1.763 (95% CI, 1.17-2.66). This outcome was statistically significant (P=.01).
Tumors treated during the pandemic period were more likely to have necessitated more than one Mohs stage for clearance compared to the prepandemic periods, though this difference was not statistically significant (OR, 1.461; 95% CI, 0.97-2.19; P=.056). Neither AUC location of treated tumors nor age were significantly different between prepandemic and pandemic periods (P=.58 and P=.84, respectively). Table 1 includes all bivariate analysis results.
Additionally, although mean preoperative and postoperative sizes were larger for each AUC location during the pandemic vs prepandemic periods, these differences did not reach statistical significance on multivariate analysis (P=.71 and P=.50, respectively)(Table 2).
Comment
Our practice has followed best practice guidelines dictated by our governing professional societies during the COVID-19 pandemic in the treatment of skin cancers by MMS, specifically highly symptomatic BCCs (in accordance with ACMS guidance), SCCs with high-risk features (in accordance with AAD, ACMS, and TDS guidance), and tumors with high risk for progression and metastasis such as melanomas (in accordance with TDS guidance). Melanoma in situ was also treated during the COVID-19 pandemic in accordance with the latter TDS guidance, particularly in light of the potential for upstaging to melanoma following resection (a phenomenon demonstrated to occur in 5%–29% of biopsied MIS lesions).14
In following best practice guidelines, our results suggested tumors treated by MMS were more severe, as evidenced by a statistically significant higher proportion of SCC and MIS tumors (representing more severe tumor types) vs BCC when compared to the prepandemic period. Supporting this conclusion, we observed larger pretreatment and posttreatment tumor sizes for all AUC locations and more tumors necessitating 2 or more stages for clearance during the pandemic vs prepandemic periods, though these differences did not reach statistical significance. We postulate these findings may be attributed to allocation of finite medical resources to the treatment of larger and more aggressive skin cancers. Additionally, these findings may be explained, in part, by limitations on patient case load imposed by social distancing measures and governing body regulations in effect during the study period, including those put forth by the AAD, ACMS, and TDS. Of note, our practice observed no hospitalizations or 911 calls during the studied period. This suggests no allocation of precious hospital resources away from patients with COVID-19 in our treatment of high-risk skin cancers.
The changing characteristics of cutaneous tumors treated by MMS during the pandemic are of clinical relevance. Larger postoperative wound sizes as observed during the pandemic, albeit not statistically significant, presumably affect reconstructive decisions. With larger wounds tending to necessitate repair by techniques higher on the reconstructive ladder, greater patient morbidity and cost are expected.15 As the cost-effectiveness of dermatology services remains a critical issue, this is an area ripe for future follow-up research. Furthermore, our observation that tumors tended to necessitate 2 or more stages for clearance during the pandemic more often than prepandemic periods, though not statistically significant, presumably affected operating times. Longer operating times during the pandemic may be of importance when making clinical decisions for patients for whom limiting health care exposure may be of particular concern. With more SCC and MIS tumors being treated relative to BCCs during the pandemic, one might expect greater size and severity of the BCCs we observe in the proceeding months to years.
As the ongoing COVID-19 pandemic continues to impact the landscape of cutaneous oncology, the need for adaptability is imperative. With 3- and 6-month skin cancer treatment deferrals lapsed, uncertainty surrounds ideal management of existing and new skin cancers arising during the pandemic. This study adds to a growing body of literature elucidating the impact of the COVID-19 pandemic on MMS practice; however, further studies and a tincture of time are needed to guide future best practice standards.
Acknowledgment—The authors acknowledge Gwen Baillargeon, MS (Galveston, Texas), who was the statistician for this article.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
- Gostin LO, Hodge JH. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. JAMA. 2020;323:131-32.
- Barnett ML, Grabowski DC. Nursing homes are ground zero for COVID-19 pandemic. JAMA Health Forum. 2020;1:E200369.
- Perlis RH. Exercising heart and head in managing coronavirus disease 2019 in Wuhan. JAMA Netw Open. 2020;3:E204006.
- Sarkissian SA, Kim L, Veness M, et al. Recommendations on dermatologic surgery during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:29-30.
- Billingsley EM. President’s message: COVID-19 (coronavirus) preparedness. American College of Mohs Surgery. March 30, 2020. Accessed April 14, 2022. https://www.mohscollege.org/UserFiles/AM20/Member%20Alert/COVIDAlert3March20.pdf
- Texas Dermatological Society Board of Directors. TDS Best Practice Recommendations—COVID-19. TDS Board Message. Texas Dermatologic Society. April 7, 2020.
- Nicholson P, Ali FR, Mallipeddi R. Impact of COVID‐19 on Mohs micrographic surgery: UK‐wide survey and recommendations for practice. Clin Exp Dermatol. 2020;45:901-902.
- Gironi LC, Boggio P, Giorgione R, et al. The impact of COVID-19 pandemics on dermatologic surgery: real-life data from the Italian Red-Zone [published online July 7, 2020]. J Dermatol Treat. doi:10.1080/09546634.2020.1789044
- Nicholson P, Ali FR, Craythorne E, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180.
- Ricci F, Fania L, Paradisi A, et al. Delayed melanoma diagnosis in the COVID-19 era: increased breslow thickness in primary melanomas seen after the COVID-19 lockdown. J Eur Acad Dermatol Venereol. 2020;34:E778-E779.
- Gualdi G, Porreca A, Amoruso GF, et al. The effect of the COVID-19 lockdown on melanoma diagnosis in Italy. Clin Dermatol. 2021;39:911-919.
- Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035-1044.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Higgins HW, Lee KC, Galan A, et al. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73:193-203.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698-703.
Practice Points
- Mohs surgeons should follow best practice guidelines dictated by our governing professional societies in selecting skin cancers for treatment by Mohs micrographic surgery (MMS) during the COVID-19 pandemic and beyond.
- The COVID-19 pandemic has impacted the characteristics of skin cancers treated by MMS, largely driven by new guidelines.
- Changing characteristics of skin cancers treated by MMS are of clinical significance, potentially affecting the extent of reconstructive surgery, cost, operating time, and future tumor characteristics.
Impact of Clinical Pharmacists on Access to Care in an Epilepsy Clinic
Epilepsy affects about 1% of the world population and is one of the most burdensome in terms of disability-adjusted life-years.1,2 Veterans are at increased risk of developing epilepsy when compared with the general population due to a variety of factors, including a higher frequency of traumatic brain injuries.3 A recent study from the US Centers for Disease Control and Prevention found that veterans who developed epilepsy during their service not only had a higher rate of mental and physical comorbidities, but also were 2.6 times more likely to die compared with veterans without epilepsy.4
Oral antiseizure medications (ASM) remain the mainstay of outpatient epilepsy treatment. Patterns of ASM use are complex within the US Department of Veterans Affairs (VA) patient population, particularly within patients at the Epilepsy Centers of Excellence (ECoE). For example, many patients are transitioned from older ASMs with greater adverse effects (AEs) to better tolerated newer generation ASMs or polytherapy regimens with complex pharmacokinetic profiles and drug interactions.5 Multiple factors are considered when choosing an ASM, including age, sex, epilepsy/seizure type, comorbidities, past medication trials, AEs, and drug interactions. The complex pharmacologic profile of both older and newer ASMs can confound the optimal management of epilepsy, and suboptimal management can lead to neurologic, psychological, physical, and social consequences, including sudden unexplained death in epilepsy.6,7 Psychiatric and behavioral problems are seen in up to 30% of patients with newly diagnosed epilepsy and 50% in those with pharmacoresistant epilepsy.8 Early screening, detection, and treatment for psychiatric comorbidities are an integral part of evidence-based care in epilepsy.
Being familiar with ASM AEs and comorbid conditions such as anxiety and depression can allow for quick identification and intervention to improve safety and quality of life. A 2007 population-based study found that measures of suicidality had a strong association with epilepsy, and performing mental health screenings, such as the Patient Health Questionnaire (PHQ-9), Generalized Anxiety Disorder Screener (GAD-7), and the Brief Irritability Test (BITe), can assist in identifying those patients at risk.9
During the COVID-19 pandemic, it has become increasingly clear that the health care sector is facing increasing pressure. The combination of patient acuity as well as critical health care professional (HCP) shortages may be of particular concern in certain specialty clinics where access to practitioners may already be limited. While this is a multifaceted problem, a pragmatic approach would be to increase the use of clinicians, such as clinical pharmacist practitioners (CPPs).
The William S. Middleton Memorial Veterans Hospital (WSMVH) in Madison, Wisconsin, is 1 of 17 VA ECoE sites. The VA ECoE provides high-quality, comprehensive epilepsy evaluation and care to veterans. In fiscal year (FY) 2020, the 17 sites provided care to 5544 veterans.10 The WSMVH epilepsy clinic sees about 400 veterans each year, receiving referrals from other VA medical centers, and prescribes ASMs, neuromodulation devices, and resective surgeries for epilepsy. The multidisciplinary team consists of an epileptologist, neurophysiology fellow, psychiatrist, nurse practitioner, CPP, and neurology residents. The WSMVH epilepsy clinic has employed CPPs at their highest level of clinical practice authority since 1991.
The WSMVH epilepsy clinic is open 4 hours once weekly. The clinic offers fourteen 30-minute appointment slots either in person or via telehealth. The epileptologist reviews patient charts prior to clinic and assigns each patient to the appropriate HCP. When making the determination to assign a patient to a CPP or pharmacy resident, the epileptologist considers current treatment response, mental health issues as well as medication-related concerns (eg, potential pharmacokinetic/pharmacodynamic interactions, AEs, adherence). The CPP can independently lead routine follow-up appointments and address acute as well as ongoing ASM therapy needs. Pharmacy residents are fully integrated into the clinic workflow, seeing assigned patients independently when appropriate but ensuring that each patient has access to either the epileptologist, CPP, or psychiatrist prior to finalizing the treatment plan. The epilepsy clinic rotation is required for first-year pharmacy residents and is an elective rotation in the second year.
While this level of service has been in place at WSMVH for more than 3 decades, a systematic evaluation on workload and clinical impact has not been conducted.11 The purpose of this analysis is to evaluate and quantify the breadth and impact of CPPs in this specialty setting. The WSMVH/University of Wisconsin-Madison institutional review board deemed this quality improvement study exempt from review.
Methods
This study was a single-center, retrospective, quality improvement project evaluating the impact of a CPP and clinical pharmacy resident have within the WSMVH epilepsy clinic on access to epilepsy care and medication management. The secondary outcomes were the types of interventions made by the CPP and mental health screening performed.
Between October 2019 and May 2021, 591 appointments were scheduled at the epilepsy clinic for medical, psychiatry, neurosurgery, and pharmacy residents; the epileptologist; CPP; psychiatrist; epilepsy fellow; or nurse practitioner. A retrospective chart review of the 446 patients seen by either a CPP or clinical pharmacy resident from October 2017 to June 2021 assessed pharmacist-led interventions made during each appointment. The following treatment interventions were assessed: medication initiations/discontinuations, dose changes, and nonpharmacologic interventions, including education. Additionally, any mental health screenings completed, consultations to other specialties placed, or laboratory tests ordered were documented.
Results
In the epilepsy clinic, 591 appointments were completed from October 1, 2019, to May 31, 2021. Of those appointments, 255 (43.2%) were led by pharmacists; 156 (26.4%) by pharmacy residents and 99 (16.8%) by CPPs (16.8%) (Table 1). Appointments held by other HCPs included 139 (23.5%) by nurse practitioner, 108 (18.3%) by the attending epileptologist, 41 (6.9%) by fellows, 22 (3.7%) by psychiatrists, 19 (3.2) by medical residents, 4 (0.7%) by neurosurgery residents, and 3 (0.5%) by psychiatry residents. Medication interventions included 55 (11.8%) dose increases, 52 (11.1%) medication initiations, and 32 (6.9%) dose decreases (Table 2). Mental health screening was conducted for 229 (49.1%) patients with PHQ-9, 225 (48.3%) with GAD-7, and 111 (23.8) with BITe. Some veterans received multiple screeners at a clinic visit, and others received none (most commonly during telephone follow-up appointments). The mean time spent with each patient was 27 minutes.
Discussion
Within the private sector, access to a neurologist or epileptologist is limited, and the US Health Resources and Services Administration National Center for Workforce Analysis projected that the demand for these specialists would exceed supply by 2025.12 In 2017, Kobau and colleagues found that only 1 in 10 adults with epilepsy saw a neurologist within the year, similar to previous years. As demand for specialty care exceeds capacity, additional members of the health care team are needed to ensure timely, effective, and safe care for patients with epilepsy.
One way to increase health care access is to use an interdisciplinary model of care, integrating pharmacists in the management of epilepsy in collaboration with other HCPs, a strategy that has been endorsed by the American Epilepsy Society (AES).13 As experts in pharmacotherapy, pharmacists can uniquely provide medication management for this complex disease as ASMs continue to remain the first-line treatment.14
In addition to increased demand for specialty services, there also is an increase in health care spending with a push to limit additional spending. In 2016, despite similar health care use in other high-income countries, health care costs are approximately twice as much in the US, mostly driven by prices of pharmaceuticals and administrative costs.15 Bond and colleagues evaluated 9380 Medicare patients with epilepsy or seizure disorders throughout US hospitals in 1998.16 They found that hospitals without pharmacist-managed ASM therapy had Medicare charges that were 11.2% higher than hospitals with pharmacist-managed therapy. Many factors contribute to the rise in cost, including an increase in laboratory charges for serum drug assays, legal litigations related to drug AEs, and an increase in hospital length of stay (about 14 additional days). Similar to pharmacist-managed anticoagulation, vancomycin, and aminoglycoside therapy, direct involvement of pharmacists with ASM management decreases health care costs.14
The American Academy of Neurology (AAN) developed 8 epilepsy quality measures: seizure type and frequency, etiology or epilepsy syndrome, review of electroencephalogram and imaging findings, counseling of ASM AEs, consideration of surgical treatment of intractable epilepsy, epilepsy-specific safety issues, and counseling for women of childbearing potential on contraception and pregnancy. These measures serve as a guide for evidence-based therapy and standardization of epilepsy care.17 Additionally, bone health, depression, and awareness of sudden unexplained death in epilepsy are increasing in importance when providing quality epilepsy care. Wasade and colleagues surveyed Michigan neurologists and found that only 37% of the respondents addressed ASM AEs at every clinic visit. They also found that just 26% of responding neurologists inquire about depression at every clinic visit, and 17% inquire only once a year. In our practice, screening for depression, suicidality, and counseling on ASM AEs are routinely provided by CPPs during each clinic visit.
Within the VA, CPPs are granted a scope of practice that allows them to perform comprehensive medication management, including but not limited to, prescribing medication regimens, ordering laboratory tests and diagnostic studies, and performing physical assessments. In our practice, the most common interventions made by CPPs were patient-focused counseling, bone health screening, mental health triage and referral, and ASM regimen adjustments. Assessment of ASM adherence also was noted to be an active area of CPP-patient engagement. These most common interventions align well with the AAN quality measures. It is now well recognized that nonadherence in patients with epilepsy not only can lead to loss of seizure control, but injury and death as well.18,19 Malek and colleagues found that patients with epilepsy who are nonadherent to their ASM regimens have a 3-times greater risk of mortality compared with those who were adherent.20 Adherence to the appropriate medication regimen in epilepsy can result in seizure-freedom in 70% of patients; therefore, exploring nonadherence in this population is crucial.21
The COVID-19 pandemic precipitated changes to the health care industry, including the heavy reliance on telehealth. Following the Wisconsin stay-at-home order on March 25, 2020, all nonessential face-to-face appointments at the WSMVH halted. The epilepsy clinic transitioned the majority of appointments to either telephone or VA Video Connect (VVC), which is a program on the veteran’s computer, tablet, or mobile device upon which the appointment is held. Although it became more challenging to obtain a mental health screening during virtual appointments and the frequency did decrease, patients were asked for a subjective report of their mood during each telephone or video appointment. The AES has since put forth a statement of support for the continuation of telehealth following the COVID-19 pandemic due to the flexibility that telehealth provides people with epilepsy. Additionally, the AES taskforce provided suggestions for continued pharmacist engagement within the epilepsy care team, including the triaging of patients, management of ASMs, and involvement in the delivery of telehealth.
Limitations
There is limited research available on the impact that a CPP has on medication management and access to care within an epilepsy clinic, especially those with a scope of practice. One limitation of this retrospective chart review is that the appropriateness of each medication intervention was not assessed; therefore, the impact of each intervention was not captured. Additionally, this single-site study of veterans may not reflect the general population. However, we believe that this model could be adapted to nonspecialty neurology practices. Of note the scope of this study did not include a comparison of medication interventions for the other specialties within the clinic.
Conclusions
The integration of a CPP and pharmacy residents into the WSMVH epilepsy clinic has allowed for greater and more timely access to care, managing 43.2% of all patients within the clinic during the study. Pharmacy scope of practice allows for collaborative autonomy with ASM adjustments and for the epileptologist time to focus on higher acuity cases. In settings where pharmacists do not have prescriptive status, medication management services, such as comprehensive medication reviews, identifying drug-drug and drug-disease interactions, recognizing adherence barriers, and medication safety surveillance, can still be performed to improve management of epilepsy.
Acknowledgments
Ellina S. Seckel, PharmD, BCACP, DPLA; Anita Kashyap, PharmD, BCACP; Brooke Keenan, NP; Leigh Heffner, PharmD
1. Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5(6):a022426. doi:10.1101/cshperspect.a022426
2. GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165-176. doi:10.1001/jamaneurol.2020.4152
3. Rehman R, Kelly PR, Husain AM, Tran TT. Characteristics of veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762. doi:10.1682/JRRD.2014.10.0241
4. Pugh MJ, Van Cott AC, Amuan M, et al. Epilepsy among Iraq and Afghanistan War veterans - United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1224-1227. doi:10.15585/mmwr.mm6544a5
5. Rohde NN, Baca CB, Van Cott AC, Parko KL, Amuan ME, Pugh MJ. Antiepileptic drug prescribing patterns in Iraq and Afghanistan war veterans with epilepsy. Epilepsy Behav. 2015;46:133-139. doi:10.1016/j.yebeh.2015.03.027
6. Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59-70. doi:10.1016/j.yebeh.2014.05.031
7. Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075-1088. doi:10.1016/S1474-4422(16)30158-2
8. Tolchin B, Hirsch LJ, LaFrance WC Jr. Neuropsychiatric aspects of epilepsy. Psychiatr Clin North Am. 2020;43(2):275-290. doi:10.1016/j.psc.2020.02.002
9. Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53(6):1095-1103. doi:10.1111/j.1528-1167.2012.03500.x
10. US Department of Veterans Affairs. Epilepsy Centers of Excellence. Annual report fiscal year 2020. Accessed March 11, 2022. https://www.epilepsy.va.gov/docs/ECoENational_AnnualReportFY20_web_508c.pdf
11. Fogg A, Staufenberg EF, Small I, Bhattacharya D. An exploratory study of primary care pharmacist-led epilepsy consultations. Int J Pharm Pract. 2012;20(5):294-302. doi:10.1111/j.2042-7174.2012.00207.x
12. Kobau R, Sapkota S, Pennell PB, Croft JB. Epilepsy by the numbers - from the US Centers for Disease Control and Prevention: six in 10 adults with active epilepsy saw a neurologist or epilepsy specialist in the past year, United States, 2017. Epilepsy Behav. 2020;112:107348. doi:10.1016/j.yebeh.2020.107348
13. Shawahna R. Development of key performance indicators to capture in measuring the impact of pharmacists in caring for patients with epilepsy in primary healthcare: A Delphi consensual study. Epilepsy Behav. 2019;98(pt A):129-138. doi:10.1016/j.yebeh.2019.07.034
14. Asadi-Pooya AA, Beniczky S, Rubboli G, Sperling MR, Rampp S, Perucca E. A pragmatic algorithm to select appropriate antiseizure medications in patients with epilepsy. Epilepsia. 2020;61(8):1668-1677. doi:10.1111/epi.16610
15. Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA. 2018;319(10):1024-1039. doi:10.1001/jama.2018.1150
16. Bond CA, Raehl CL. Clinical and economic outcomes of pharmacist-managed aminoglycoside or vancomycin therapy. Am J Health Syst Pharm. 2005;62(15):1596-1605. doi:10.2146/ajhp040555
17. Wasade VS, Spanaki M, Iyengar R, Barkley GL, Schultz L. AAN Epilepsy Quality Measures in clinical practice: a survey of neurologists. Epilepsy Behav. 2012;24(4):468-473. doi:10.1016/j.yebeh.2012.05.017
18. Hovinga CA, Asato MR, Manjunath R, et al. Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy Behav. 2008;13(2):316-322. doi:10.1016/j.yebeh.2008.03.009
19. Faught RE, Weiner JR, Guérin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501-509. doi:10.1111/j.1528-1167.2008.01794.x
20. Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507-515. doi:10.1111/ane.12703
21. O’ Rourke G, O’ Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160-168. doi:10.1016/j.seizure.2016.12.006
Epilepsy affects about 1% of the world population and is one of the most burdensome in terms of disability-adjusted life-years.1,2 Veterans are at increased risk of developing epilepsy when compared with the general population due to a variety of factors, including a higher frequency of traumatic brain injuries.3 A recent study from the US Centers for Disease Control and Prevention found that veterans who developed epilepsy during their service not only had a higher rate of mental and physical comorbidities, but also were 2.6 times more likely to die compared with veterans without epilepsy.4
Oral antiseizure medications (ASM) remain the mainstay of outpatient epilepsy treatment. Patterns of ASM use are complex within the US Department of Veterans Affairs (VA) patient population, particularly within patients at the Epilepsy Centers of Excellence (ECoE). For example, many patients are transitioned from older ASMs with greater adverse effects (AEs) to better tolerated newer generation ASMs or polytherapy regimens with complex pharmacokinetic profiles and drug interactions.5 Multiple factors are considered when choosing an ASM, including age, sex, epilepsy/seizure type, comorbidities, past medication trials, AEs, and drug interactions. The complex pharmacologic profile of both older and newer ASMs can confound the optimal management of epilepsy, and suboptimal management can lead to neurologic, psychological, physical, and social consequences, including sudden unexplained death in epilepsy.6,7 Psychiatric and behavioral problems are seen in up to 30% of patients with newly diagnosed epilepsy and 50% in those with pharmacoresistant epilepsy.8 Early screening, detection, and treatment for psychiatric comorbidities are an integral part of evidence-based care in epilepsy.
Being familiar with ASM AEs and comorbid conditions such as anxiety and depression can allow for quick identification and intervention to improve safety and quality of life. A 2007 population-based study found that measures of suicidality had a strong association with epilepsy, and performing mental health screenings, such as the Patient Health Questionnaire (PHQ-9), Generalized Anxiety Disorder Screener (GAD-7), and the Brief Irritability Test (BITe), can assist in identifying those patients at risk.9
During the COVID-19 pandemic, it has become increasingly clear that the health care sector is facing increasing pressure. The combination of patient acuity as well as critical health care professional (HCP) shortages may be of particular concern in certain specialty clinics where access to practitioners may already be limited. While this is a multifaceted problem, a pragmatic approach would be to increase the use of clinicians, such as clinical pharmacist practitioners (CPPs).
The William S. Middleton Memorial Veterans Hospital (WSMVH) in Madison, Wisconsin, is 1 of 17 VA ECoE sites. The VA ECoE provides high-quality, comprehensive epilepsy evaluation and care to veterans. In fiscal year (FY) 2020, the 17 sites provided care to 5544 veterans.10 The WSMVH epilepsy clinic sees about 400 veterans each year, receiving referrals from other VA medical centers, and prescribes ASMs, neuromodulation devices, and resective surgeries for epilepsy. The multidisciplinary team consists of an epileptologist, neurophysiology fellow, psychiatrist, nurse practitioner, CPP, and neurology residents. The WSMVH epilepsy clinic has employed CPPs at their highest level of clinical practice authority since 1991.
The WSMVH epilepsy clinic is open 4 hours once weekly. The clinic offers fourteen 30-minute appointment slots either in person or via telehealth. The epileptologist reviews patient charts prior to clinic and assigns each patient to the appropriate HCP. When making the determination to assign a patient to a CPP or pharmacy resident, the epileptologist considers current treatment response, mental health issues as well as medication-related concerns (eg, potential pharmacokinetic/pharmacodynamic interactions, AEs, adherence). The CPP can independently lead routine follow-up appointments and address acute as well as ongoing ASM therapy needs. Pharmacy residents are fully integrated into the clinic workflow, seeing assigned patients independently when appropriate but ensuring that each patient has access to either the epileptologist, CPP, or psychiatrist prior to finalizing the treatment plan. The epilepsy clinic rotation is required for first-year pharmacy residents and is an elective rotation in the second year.
While this level of service has been in place at WSMVH for more than 3 decades, a systematic evaluation on workload and clinical impact has not been conducted.11 The purpose of this analysis is to evaluate and quantify the breadth and impact of CPPs in this specialty setting. The WSMVH/University of Wisconsin-Madison institutional review board deemed this quality improvement study exempt from review.
Methods
This study was a single-center, retrospective, quality improvement project evaluating the impact of a CPP and clinical pharmacy resident have within the WSMVH epilepsy clinic on access to epilepsy care and medication management. The secondary outcomes were the types of interventions made by the CPP and mental health screening performed.
Between October 2019 and May 2021, 591 appointments were scheduled at the epilepsy clinic for medical, psychiatry, neurosurgery, and pharmacy residents; the epileptologist; CPP; psychiatrist; epilepsy fellow; or nurse practitioner. A retrospective chart review of the 446 patients seen by either a CPP or clinical pharmacy resident from October 2017 to June 2021 assessed pharmacist-led interventions made during each appointment. The following treatment interventions were assessed: medication initiations/discontinuations, dose changes, and nonpharmacologic interventions, including education. Additionally, any mental health screenings completed, consultations to other specialties placed, or laboratory tests ordered were documented.
Results
In the epilepsy clinic, 591 appointments were completed from October 1, 2019, to May 31, 2021. Of those appointments, 255 (43.2%) were led by pharmacists; 156 (26.4%) by pharmacy residents and 99 (16.8%) by CPPs (16.8%) (Table 1). Appointments held by other HCPs included 139 (23.5%) by nurse practitioner, 108 (18.3%) by the attending epileptologist, 41 (6.9%) by fellows, 22 (3.7%) by psychiatrists, 19 (3.2) by medical residents, 4 (0.7%) by neurosurgery residents, and 3 (0.5%) by psychiatry residents. Medication interventions included 55 (11.8%) dose increases, 52 (11.1%) medication initiations, and 32 (6.9%) dose decreases (Table 2). Mental health screening was conducted for 229 (49.1%) patients with PHQ-9, 225 (48.3%) with GAD-7, and 111 (23.8) with BITe. Some veterans received multiple screeners at a clinic visit, and others received none (most commonly during telephone follow-up appointments). The mean time spent with each patient was 27 minutes.
Discussion
Within the private sector, access to a neurologist or epileptologist is limited, and the US Health Resources and Services Administration National Center for Workforce Analysis projected that the demand for these specialists would exceed supply by 2025.12 In 2017, Kobau and colleagues found that only 1 in 10 adults with epilepsy saw a neurologist within the year, similar to previous years. As demand for specialty care exceeds capacity, additional members of the health care team are needed to ensure timely, effective, and safe care for patients with epilepsy.
One way to increase health care access is to use an interdisciplinary model of care, integrating pharmacists in the management of epilepsy in collaboration with other HCPs, a strategy that has been endorsed by the American Epilepsy Society (AES).13 As experts in pharmacotherapy, pharmacists can uniquely provide medication management for this complex disease as ASMs continue to remain the first-line treatment.14
In addition to increased demand for specialty services, there also is an increase in health care spending with a push to limit additional spending. In 2016, despite similar health care use in other high-income countries, health care costs are approximately twice as much in the US, mostly driven by prices of pharmaceuticals and administrative costs.15 Bond and colleagues evaluated 9380 Medicare patients with epilepsy or seizure disorders throughout US hospitals in 1998.16 They found that hospitals without pharmacist-managed ASM therapy had Medicare charges that were 11.2% higher than hospitals with pharmacist-managed therapy. Many factors contribute to the rise in cost, including an increase in laboratory charges for serum drug assays, legal litigations related to drug AEs, and an increase in hospital length of stay (about 14 additional days). Similar to pharmacist-managed anticoagulation, vancomycin, and aminoglycoside therapy, direct involvement of pharmacists with ASM management decreases health care costs.14
The American Academy of Neurology (AAN) developed 8 epilepsy quality measures: seizure type and frequency, etiology or epilepsy syndrome, review of electroencephalogram and imaging findings, counseling of ASM AEs, consideration of surgical treatment of intractable epilepsy, epilepsy-specific safety issues, and counseling for women of childbearing potential on contraception and pregnancy. These measures serve as a guide for evidence-based therapy and standardization of epilepsy care.17 Additionally, bone health, depression, and awareness of sudden unexplained death in epilepsy are increasing in importance when providing quality epilepsy care. Wasade and colleagues surveyed Michigan neurologists and found that only 37% of the respondents addressed ASM AEs at every clinic visit. They also found that just 26% of responding neurologists inquire about depression at every clinic visit, and 17% inquire only once a year. In our practice, screening for depression, suicidality, and counseling on ASM AEs are routinely provided by CPPs during each clinic visit.
Within the VA, CPPs are granted a scope of practice that allows them to perform comprehensive medication management, including but not limited to, prescribing medication regimens, ordering laboratory tests and diagnostic studies, and performing physical assessments. In our practice, the most common interventions made by CPPs were patient-focused counseling, bone health screening, mental health triage and referral, and ASM regimen adjustments. Assessment of ASM adherence also was noted to be an active area of CPP-patient engagement. These most common interventions align well with the AAN quality measures. It is now well recognized that nonadherence in patients with epilepsy not only can lead to loss of seizure control, but injury and death as well.18,19 Malek and colleagues found that patients with epilepsy who are nonadherent to their ASM regimens have a 3-times greater risk of mortality compared with those who were adherent.20 Adherence to the appropriate medication regimen in epilepsy can result in seizure-freedom in 70% of patients; therefore, exploring nonadherence in this population is crucial.21
The COVID-19 pandemic precipitated changes to the health care industry, including the heavy reliance on telehealth. Following the Wisconsin stay-at-home order on March 25, 2020, all nonessential face-to-face appointments at the WSMVH halted. The epilepsy clinic transitioned the majority of appointments to either telephone or VA Video Connect (VVC), which is a program on the veteran’s computer, tablet, or mobile device upon which the appointment is held. Although it became more challenging to obtain a mental health screening during virtual appointments and the frequency did decrease, patients were asked for a subjective report of their mood during each telephone or video appointment. The AES has since put forth a statement of support for the continuation of telehealth following the COVID-19 pandemic due to the flexibility that telehealth provides people with epilepsy. Additionally, the AES taskforce provided suggestions for continued pharmacist engagement within the epilepsy care team, including the triaging of patients, management of ASMs, and involvement in the delivery of telehealth.
Limitations
There is limited research available on the impact that a CPP has on medication management and access to care within an epilepsy clinic, especially those with a scope of practice. One limitation of this retrospective chart review is that the appropriateness of each medication intervention was not assessed; therefore, the impact of each intervention was not captured. Additionally, this single-site study of veterans may not reflect the general population. However, we believe that this model could be adapted to nonspecialty neurology practices. Of note the scope of this study did not include a comparison of medication interventions for the other specialties within the clinic.
Conclusions
The integration of a CPP and pharmacy residents into the WSMVH epilepsy clinic has allowed for greater and more timely access to care, managing 43.2% of all patients within the clinic during the study. Pharmacy scope of practice allows for collaborative autonomy with ASM adjustments and for the epileptologist time to focus on higher acuity cases. In settings where pharmacists do not have prescriptive status, medication management services, such as comprehensive medication reviews, identifying drug-drug and drug-disease interactions, recognizing adherence barriers, and medication safety surveillance, can still be performed to improve management of epilepsy.
Acknowledgments
Ellina S. Seckel, PharmD, BCACP, DPLA; Anita Kashyap, PharmD, BCACP; Brooke Keenan, NP; Leigh Heffner, PharmD
Epilepsy affects about 1% of the world population and is one of the most burdensome in terms of disability-adjusted life-years.1,2 Veterans are at increased risk of developing epilepsy when compared with the general population due to a variety of factors, including a higher frequency of traumatic brain injuries.3 A recent study from the US Centers for Disease Control and Prevention found that veterans who developed epilepsy during their service not only had a higher rate of mental and physical comorbidities, but also were 2.6 times more likely to die compared with veterans without epilepsy.4
Oral antiseizure medications (ASM) remain the mainstay of outpatient epilepsy treatment. Patterns of ASM use are complex within the US Department of Veterans Affairs (VA) patient population, particularly within patients at the Epilepsy Centers of Excellence (ECoE). For example, many patients are transitioned from older ASMs with greater adverse effects (AEs) to better tolerated newer generation ASMs or polytherapy regimens with complex pharmacokinetic profiles and drug interactions.5 Multiple factors are considered when choosing an ASM, including age, sex, epilepsy/seizure type, comorbidities, past medication trials, AEs, and drug interactions. The complex pharmacologic profile of both older and newer ASMs can confound the optimal management of epilepsy, and suboptimal management can lead to neurologic, psychological, physical, and social consequences, including sudden unexplained death in epilepsy.6,7 Psychiatric and behavioral problems are seen in up to 30% of patients with newly diagnosed epilepsy and 50% in those with pharmacoresistant epilepsy.8 Early screening, detection, and treatment for psychiatric comorbidities are an integral part of evidence-based care in epilepsy.
Being familiar with ASM AEs and comorbid conditions such as anxiety and depression can allow for quick identification and intervention to improve safety and quality of life. A 2007 population-based study found that measures of suicidality had a strong association with epilepsy, and performing mental health screenings, such as the Patient Health Questionnaire (PHQ-9), Generalized Anxiety Disorder Screener (GAD-7), and the Brief Irritability Test (BITe), can assist in identifying those patients at risk.9
During the COVID-19 pandemic, it has become increasingly clear that the health care sector is facing increasing pressure. The combination of patient acuity as well as critical health care professional (HCP) shortages may be of particular concern in certain specialty clinics where access to practitioners may already be limited. While this is a multifaceted problem, a pragmatic approach would be to increase the use of clinicians, such as clinical pharmacist practitioners (CPPs).
The William S. Middleton Memorial Veterans Hospital (WSMVH) in Madison, Wisconsin, is 1 of 17 VA ECoE sites. The VA ECoE provides high-quality, comprehensive epilepsy evaluation and care to veterans. In fiscal year (FY) 2020, the 17 sites provided care to 5544 veterans.10 The WSMVH epilepsy clinic sees about 400 veterans each year, receiving referrals from other VA medical centers, and prescribes ASMs, neuromodulation devices, and resective surgeries for epilepsy. The multidisciplinary team consists of an epileptologist, neurophysiology fellow, psychiatrist, nurse practitioner, CPP, and neurology residents. The WSMVH epilepsy clinic has employed CPPs at their highest level of clinical practice authority since 1991.
The WSMVH epilepsy clinic is open 4 hours once weekly. The clinic offers fourteen 30-minute appointment slots either in person or via telehealth. The epileptologist reviews patient charts prior to clinic and assigns each patient to the appropriate HCP. When making the determination to assign a patient to a CPP or pharmacy resident, the epileptologist considers current treatment response, mental health issues as well as medication-related concerns (eg, potential pharmacokinetic/pharmacodynamic interactions, AEs, adherence). The CPP can independently lead routine follow-up appointments and address acute as well as ongoing ASM therapy needs. Pharmacy residents are fully integrated into the clinic workflow, seeing assigned patients independently when appropriate but ensuring that each patient has access to either the epileptologist, CPP, or psychiatrist prior to finalizing the treatment plan. The epilepsy clinic rotation is required for first-year pharmacy residents and is an elective rotation in the second year.
While this level of service has been in place at WSMVH for more than 3 decades, a systematic evaluation on workload and clinical impact has not been conducted.11 The purpose of this analysis is to evaluate and quantify the breadth and impact of CPPs in this specialty setting. The WSMVH/University of Wisconsin-Madison institutional review board deemed this quality improvement study exempt from review.
Methods
This study was a single-center, retrospective, quality improvement project evaluating the impact of a CPP and clinical pharmacy resident have within the WSMVH epilepsy clinic on access to epilepsy care and medication management. The secondary outcomes were the types of interventions made by the CPP and mental health screening performed.
Between October 2019 and May 2021, 591 appointments were scheduled at the epilepsy clinic for medical, psychiatry, neurosurgery, and pharmacy residents; the epileptologist; CPP; psychiatrist; epilepsy fellow; or nurse practitioner. A retrospective chart review of the 446 patients seen by either a CPP or clinical pharmacy resident from October 2017 to June 2021 assessed pharmacist-led interventions made during each appointment. The following treatment interventions were assessed: medication initiations/discontinuations, dose changes, and nonpharmacologic interventions, including education. Additionally, any mental health screenings completed, consultations to other specialties placed, or laboratory tests ordered were documented.
Results
In the epilepsy clinic, 591 appointments were completed from October 1, 2019, to May 31, 2021. Of those appointments, 255 (43.2%) were led by pharmacists; 156 (26.4%) by pharmacy residents and 99 (16.8%) by CPPs (16.8%) (Table 1). Appointments held by other HCPs included 139 (23.5%) by nurse practitioner, 108 (18.3%) by the attending epileptologist, 41 (6.9%) by fellows, 22 (3.7%) by psychiatrists, 19 (3.2) by medical residents, 4 (0.7%) by neurosurgery residents, and 3 (0.5%) by psychiatry residents. Medication interventions included 55 (11.8%) dose increases, 52 (11.1%) medication initiations, and 32 (6.9%) dose decreases (Table 2). Mental health screening was conducted for 229 (49.1%) patients with PHQ-9, 225 (48.3%) with GAD-7, and 111 (23.8) with BITe. Some veterans received multiple screeners at a clinic visit, and others received none (most commonly during telephone follow-up appointments). The mean time spent with each patient was 27 minutes.
Discussion
Within the private sector, access to a neurologist or epileptologist is limited, and the US Health Resources and Services Administration National Center for Workforce Analysis projected that the demand for these specialists would exceed supply by 2025.12 In 2017, Kobau and colleagues found that only 1 in 10 adults with epilepsy saw a neurologist within the year, similar to previous years. As demand for specialty care exceeds capacity, additional members of the health care team are needed to ensure timely, effective, and safe care for patients with epilepsy.
One way to increase health care access is to use an interdisciplinary model of care, integrating pharmacists in the management of epilepsy in collaboration with other HCPs, a strategy that has been endorsed by the American Epilepsy Society (AES).13 As experts in pharmacotherapy, pharmacists can uniquely provide medication management for this complex disease as ASMs continue to remain the first-line treatment.14
In addition to increased demand for specialty services, there also is an increase in health care spending with a push to limit additional spending. In 2016, despite similar health care use in other high-income countries, health care costs are approximately twice as much in the US, mostly driven by prices of pharmaceuticals and administrative costs.15 Bond and colleagues evaluated 9380 Medicare patients with epilepsy or seizure disorders throughout US hospitals in 1998.16 They found that hospitals without pharmacist-managed ASM therapy had Medicare charges that were 11.2% higher than hospitals with pharmacist-managed therapy. Many factors contribute to the rise in cost, including an increase in laboratory charges for serum drug assays, legal litigations related to drug AEs, and an increase in hospital length of stay (about 14 additional days). Similar to pharmacist-managed anticoagulation, vancomycin, and aminoglycoside therapy, direct involvement of pharmacists with ASM management decreases health care costs.14
The American Academy of Neurology (AAN) developed 8 epilepsy quality measures: seizure type and frequency, etiology or epilepsy syndrome, review of electroencephalogram and imaging findings, counseling of ASM AEs, consideration of surgical treatment of intractable epilepsy, epilepsy-specific safety issues, and counseling for women of childbearing potential on contraception and pregnancy. These measures serve as a guide for evidence-based therapy and standardization of epilepsy care.17 Additionally, bone health, depression, and awareness of sudden unexplained death in epilepsy are increasing in importance when providing quality epilepsy care. Wasade and colleagues surveyed Michigan neurologists and found that only 37% of the respondents addressed ASM AEs at every clinic visit. They also found that just 26% of responding neurologists inquire about depression at every clinic visit, and 17% inquire only once a year. In our practice, screening for depression, suicidality, and counseling on ASM AEs are routinely provided by CPPs during each clinic visit.
Within the VA, CPPs are granted a scope of practice that allows them to perform comprehensive medication management, including but not limited to, prescribing medication regimens, ordering laboratory tests and diagnostic studies, and performing physical assessments. In our practice, the most common interventions made by CPPs were patient-focused counseling, bone health screening, mental health triage and referral, and ASM regimen adjustments. Assessment of ASM adherence also was noted to be an active area of CPP-patient engagement. These most common interventions align well with the AAN quality measures. It is now well recognized that nonadherence in patients with epilepsy not only can lead to loss of seizure control, but injury and death as well.18,19 Malek and colleagues found that patients with epilepsy who are nonadherent to their ASM regimens have a 3-times greater risk of mortality compared with those who were adherent.20 Adherence to the appropriate medication regimen in epilepsy can result in seizure-freedom in 70% of patients; therefore, exploring nonadherence in this population is crucial.21
The COVID-19 pandemic precipitated changes to the health care industry, including the heavy reliance on telehealth. Following the Wisconsin stay-at-home order on March 25, 2020, all nonessential face-to-face appointments at the WSMVH halted. The epilepsy clinic transitioned the majority of appointments to either telephone or VA Video Connect (VVC), which is a program on the veteran’s computer, tablet, or mobile device upon which the appointment is held. Although it became more challenging to obtain a mental health screening during virtual appointments and the frequency did decrease, patients were asked for a subjective report of their mood during each telephone or video appointment. The AES has since put forth a statement of support for the continuation of telehealth following the COVID-19 pandemic due to the flexibility that telehealth provides people with epilepsy. Additionally, the AES taskforce provided suggestions for continued pharmacist engagement within the epilepsy care team, including the triaging of patients, management of ASMs, and involvement in the delivery of telehealth.
Limitations
There is limited research available on the impact that a CPP has on medication management and access to care within an epilepsy clinic, especially those with a scope of practice. One limitation of this retrospective chart review is that the appropriateness of each medication intervention was not assessed; therefore, the impact of each intervention was not captured. Additionally, this single-site study of veterans may not reflect the general population. However, we believe that this model could be adapted to nonspecialty neurology practices. Of note the scope of this study did not include a comparison of medication interventions for the other specialties within the clinic.
Conclusions
The integration of a CPP and pharmacy residents into the WSMVH epilepsy clinic has allowed for greater and more timely access to care, managing 43.2% of all patients within the clinic during the study. Pharmacy scope of practice allows for collaborative autonomy with ASM adjustments and for the epileptologist time to focus on higher acuity cases. In settings where pharmacists do not have prescriptive status, medication management services, such as comprehensive medication reviews, identifying drug-drug and drug-disease interactions, recognizing adherence barriers, and medication safety surveillance, can still be performed to improve management of epilepsy.
Acknowledgments
Ellina S. Seckel, PharmD, BCACP, DPLA; Anita Kashyap, PharmD, BCACP; Brooke Keenan, NP; Leigh Heffner, PharmD
1. Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5(6):a022426. doi:10.1101/cshperspect.a022426
2. GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165-176. doi:10.1001/jamaneurol.2020.4152
3. Rehman R, Kelly PR, Husain AM, Tran TT. Characteristics of veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762. doi:10.1682/JRRD.2014.10.0241
4. Pugh MJ, Van Cott AC, Amuan M, et al. Epilepsy among Iraq and Afghanistan War veterans - United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1224-1227. doi:10.15585/mmwr.mm6544a5
5. Rohde NN, Baca CB, Van Cott AC, Parko KL, Amuan ME, Pugh MJ. Antiepileptic drug prescribing patterns in Iraq and Afghanistan war veterans with epilepsy. Epilepsy Behav. 2015;46:133-139. doi:10.1016/j.yebeh.2015.03.027
6. Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59-70. doi:10.1016/j.yebeh.2014.05.031
7. Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075-1088. doi:10.1016/S1474-4422(16)30158-2
8. Tolchin B, Hirsch LJ, LaFrance WC Jr. Neuropsychiatric aspects of epilepsy. Psychiatr Clin North Am. 2020;43(2):275-290. doi:10.1016/j.psc.2020.02.002
9. Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53(6):1095-1103. doi:10.1111/j.1528-1167.2012.03500.x
10. US Department of Veterans Affairs. Epilepsy Centers of Excellence. Annual report fiscal year 2020. Accessed March 11, 2022. https://www.epilepsy.va.gov/docs/ECoENational_AnnualReportFY20_web_508c.pdf
11. Fogg A, Staufenberg EF, Small I, Bhattacharya D. An exploratory study of primary care pharmacist-led epilepsy consultations. Int J Pharm Pract. 2012;20(5):294-302. doi:10.1111/j.2042-7174.2012.00207.x
12. Kobau R, Sapkota S, Pennell PB, Croft JB. Epilepsy by the numbers - from the US Centers for Disease Control and Prevention: six in 10 adults with active epilepsy saw a neurologist or epilepsy specialist in the past year, United States, 2017. Epilepsy Behav. 2020;112:107348. doi:10.1016/j.yebeh.2020.107348
13. Shawahna R. Development of key performance indicators to capture in measuring the impact of pharmacists in caring for patients with epilepsy in primary healthcare: A Delphi consensual study. Epilepsy Behav. 2019;98(pt A):129-138. doi:10.1016/j.yebeh.2019.07.034
14. Asadi-Pooya AA, Beniczky S, Rubboli G, Sperling MR, Rampp S, Perucca E. A pragmatic algorithm to select appropriate antiseizure medications in patients with epilepsy. Epilepsia. 2020;61(8):1668-1677. doi:10.1111/epi.16610
15. Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA. 2018;319(10):1024-1039. doi:10.1001/jama.2018.1150
16. Bond CA, Raehl CL. Clinical and economic outcomes of pharmacist-managed aminoglycoside or vancomycin therapy. Am J Health Syst Pharm. 2005;62(15):1596-1605. doi:10.2146/ajhp040555
17. Wasade VS, Spanaki M, Iyengar R, Barkley GL, Schultz L. AAN Epilepsy Quality Measures in clinical practice: a survey of neurologists. Epilepsy Behav. 2012;24(4):468-473. doi:10.1016/j.yebeh.2012.05.017
18. Hovinga CA, Asato MR, Manjunath R, et al. Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy Behav. 2008;13(2):316-322. doi:10.1016/j.yebeh.2008.03.009
19. Faught RE, Weiner JR, Guérin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501-509. doi:10.1111/j.1528-1167.2008.01794.x
20. Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507-515. doi:10.1111/ane.12703
21. O’ Rourke G, O’ Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160-168. doi:10.1016/j.seizure.2016.12.006
1. Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5(6):a022426. doi:10.1101/cshperspect.a022426
2. GBD 2017 US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165-176. doi:10.1001/jamaneurol.2020.4152
3. Rehman R, Kelly PR, Husain AM, Tran TT. Characteristics of veterans diagnosed with seizures within Veterans Health Administration. J Rehabil Res Dev. 2015;52(7):751-762. doi:10.1682/JRRD.2014.10.0241
4. Pugh MJ, Van Cott AC, Amuan M, et al. Epilepsy among Iraq and Afghanistan War veterans - United States, 2002-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1224-1227. doi:10.15585/mmwr.mm6544a5
5. Rohde NN, Baca CB, Van Cott AC, Parko KL, Amuan ME, Pugh MJ. Antiepileptic drug prescribing patterns in Iraq and Afghanistan war veterans with epilepsy. Epilepsy Behav. 2015;46:133-139. doi:10.1016/j.yebeh.2015.03.027
6. Laxer KD, Trinka E, Hirsch LJ, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59-70. doi:10.1016/j.yebeh.2014.05.031
7. Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15(10):1075-1088. doi:10.1016/S1474-4422(16)30158-2
8. Tolchin B, Hirsch LJ, LaFrance WC Jr. Neuropsychiatric aspects of epilepsy. Psychiatr Clin North Am. 2020;43(2):275-290. doi:10.1016/j.psc.2020.02.002
9. Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53(6):1095-1103. doi:10.1111/j.1528-1167.2012.03500.x
10. US Department of Veterans Affairs. Epilepsy Centers of Excellence. Annual report fiscal year 2020. Accessed March 11, 2022. https://www.epilepsy.va.gov/docs/ECoENational_AnnualReportFY20_web_508c.pdf
11. Fogg A, Staufenberg EF, Small I, Bhattacharya D. An exploratory study of primary care pharmacist-led epilepsy consultations. Int J Pharm Pract. 2012;20(5):294-302. doi:10.1111/j.2042-7174.2012.00207.x
12. Kobau R, Sapkota S, Pennell PB, Croft JB. Epilepsy by the numbers - from the US Centers for Disease Control and Prevention: six in 10 adults with active epilepsy saw a neurologist or epilepsy specialist in the past year, United States, 2017. Epilepsy Behav. 2020;112:107348. doi:10.1016/j.yebeh.2020.107348
13. Shawahna R. Development of key performance indicators to capture in measuring the impact of pharmacists in caring for patients with epilepsy in primary healthcare: A Delphi consensual study. Epilepsy Behav. 2019;98(pt A):129-138. doi:10.1016/j.yebeh.2019.07.034
14. Asadi-Pooya AA, Beniczky S, Rubboli G, Sperling MR, Rampp S, Perucca E. A pragmatic algorithm to select appropriate antiseizure medications in patients with epilepsy. Epilepsia. 2020;61(8):1668-1677. doi:10.1111/epi.16610
15. Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA. 2018;319(10):1024-1039. doi:10.1001/jama.2018.1150
16. Bond CA, Raehl CL. Clinical and economic outcomes of pharmacist-managed aminoglycoside or vancomycin therapy. Am J Health Syst Pharm. 2005;62(15):1596-1605. doi:10.2146/ajhp040555
17. Wasade VS, Spanaki M, Iyengar R, Barkley GL, Schultz L. AAN Epilepsy Quality Measures in clinical practice: a survey of neurologists. Epilepsy Behav. 2012;24(4):468-473. doi:10.1016/j.yebeh.2012.05.017
18. Hovinga CA, Asato MR, Manjunath R, et al. Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physicians. Epilepsy Behav. 2008;13(2):316-322. doi:10.1016/j.yebeh.2008.03.009
19. Faught RE, Weiner JR, Guérin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009;50(3):501-509. doi:10.1111/j.1528-1167.2008.01794.x
20. Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507-515. doi:10.1111/ane.12703
21. O’ Rourke G, O’ Brien JJ. Identifying the barriers to antiepileptic drug adherence among adults with epilepsy. Seizure. 2017;45:160-168. doi:10.1016/j.seizure.2016.12.006